Chemical composition’s effect on Solanum nigrum Linn.’s antioxidant capacity and erythrocyte protection: Bioactive components and molecular docking analysis
-
Abdelatif Aouadi
, Djamila Hamada Saoud
and Fatma Mohamed Abd El-Mordy
Abstract
Oxidative stress has been widely believed to be the mechanism responsible for developing diseases such as arthritis, asthma, dementia, and aging. Solanum nigrum Linn. is a common edible medicinal herb that belongs to the family Solanaceae which has more than 180 chemical components that have so far been discovered. The main bioactive components of these are steroidal saponins, alkaloids, phenols, and polysaccharides. This article presents comparative phytochemical profiling including total phenolic, total flavonoid, alkaloid, proanthocyanidins, tannin, and vitamin C contents of three Algerian S. nigrum samples collected from three different locations in the Algerian desert. Additionally, the potential antioxidant activity of the three samples was assessed by 2,2-diphenyl-1-picrylhydrazyl, ferric reducing antioxidant power, and oxidative hemolysis inhibition assay. Moreover, the correlation between the major phenolic phytoconstituents previously reported and isolated from the plant and antioxidant activity has also been done by in silico molecular docking. Ten bioactive compounds were docked with selected proteins, arachidonate-5-lipoxygenase (PDB: 6n2w) and cytochrome c peroxidase (PDB: 2x08), to check their affinity with binding sites of these proteins for the possible mechanism of action. The docking scores suggest that S. nigrum’s quercetin and kaempferol may play a significant role in its antioxidant action.
Graphical abstract

1 Introduction
Reactive oxygen species (ROS) and reactive nitrogen species are generated by natural biological processes as well as by external factors such as radiation, pollution, cigarette smoke, and medication [1]. These two classes of reactive species break down carbohydrates, lipids, proteins, and nucleic acids [2]. Although these reactive species play advantageous physiological roles at low to moderate concentrations, they are harmful and lead to oxidative stress at higher quantities [3]. Oxidative stress is worsened by high levels of free radical production and insufficient endogenous antioxidant defense in the human body. This leads to cellular damage, ageing, diabetes, cancer, neurological illness, and cardiovascular disease [4]. Therefore, exogenous dietary antioxidants can attenuate or stop this oxidative stress-induced degenerative illness. Numerous bioactive substances that can be derived from plants or found in functional meals are being studied for their potential to treat serious, complex disorders including cancer [5]. There are two types of antioxidants: synthetic antioxidants and natural antioxidants. Propyl gallate, tributyl hydroquinone, butylated hydroxyl toluene, and butylated hydroxyl anisole are among the first set of chemicals that harm humans’ livers and cause cancer [6]. The recent focus of the food and pharmaceutical industries has been to replace these synthetic antioxidants with natural antioxidants that have beneficial medical, nutritional, and safe properties. The body is protected from free radicals by natural antioxidants that have been taken from plant sources that are high in phenolic, flavonoids, vitamin C, carotenoids, tannins, and proanthocyanins [7]. These plant sources include edible wild plants, which may contain undiscovered nutritional and bioactive components that can reduce oxidative stress [8]. Natural chemicals derived from plants are viewed as superior substitutes to manufactured medications since they are cheaper, less harmful, and less expensive [9]. The existence and quantity of phytochemicals like alkaloids, tannins, flavonoids, and phenolic compounds that these plants synthesize are what make them helpful as therapeutic species [10].
Solanum nigrum (L.) is a herbaceous plant which is widespread around the world and also a great source of phytochemicals [11]. Black nightshade is the English name for S. nigrum L., while Makoi, Kachcipandu, Munatakali, Piludi, and Kamuni are the Hindi, Gujrati, Telugu, Tamil, and Marathi names for the plant [12]. S. nigrum has naturalized in Africa despite being native to Eurasia and imported to the Americas, Australasia, and South Africa. It is now a part of the flora known for several health advantages [13]. This plant has a long history of use in the traditional treatment and prevention of numerous illnesses. It has long been connected to analgesic properties and the treatment of tracheitis, cancer, liver damage, mastitis, and aerodontalgia [14]. Despite the plant’s numerous health benefits, it is only accessible in the wild, and its population is rapidly falling due to human activity, including habitat modification, chemical eradication, displacement for the cultivation of farmer’s chosen field crops, and others [14].
Many steroidal glycosides, steroidal alkaloids, steroidal oligoglycosides, such as solamargine, solasonine, solavilline, and solanine, as well as steroidal saponins and glycoprotein, as well as a number of polyphenolic compounds, including gallic acid, protocatechuic acid, catechin, caffeic acid, epicatechin, rutin, and naringenin, have been previously reported in S. nigrum L., which possess strong antioxidant, anticancer activity, and HIV reverse transcriptase inhibitors [15].
Many studies have reported that there is a direct relationship between biological activities and therapeutic properties of plants [16], and it is also understood that the biological attributes of the plant could be linked to the available resources in the soil for plant uptake. However, no report has been made on the influence of soil texture types on the phytochemical and antioxidant properties of S. nigrum. Thus, this study therefore hypothesized that modulation of soil texture does influence the phytochemical constituents and antioxidant properties of S. nigrum cultivated in glasshouses.
The aim of this study was to investigate the total phenolic content (TPC), total flavonoid content (TFC), and vitamin C content of three Algerian S. nigrum samples collected from three different places. Furthermore, the study determines the alkaloid, proanthocyanidins, and tannin contents in the three different plant samples. This phytochemical investigation was followed by assessing its potential antioxidant activity by 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP), and oxidative hemolysis inhibition assay. Moreover, the correlation between phytoconstituents reported in the plant and antioxidant activity has been done by in silico molecular docking.
2 Materials and methods
2.1 Plant material
S. nigrum L. was collected in the summer of 2022 from eastern Algeria, from three sites listed in Table 1. S. nigrum L. was identified by Pr. Chehma A. (Ouargla University) and a voucher specimen (SN-12-22) was placed in the Laboratory of Process Engineering, Faculty of Applied Sciences, Kasdi Merbah University, Ouargla, Algeria. Then, the plant samples were dried, crushed, and kept away from moisture and light.
Sites of plant collection
| Collection area | Plant organ | Abbreviation |
|---|---|---|
| Tebessa | Fruits | TF |
| Leaves | TL | |
| Stem | TS | |
| Biskra | Fruits | BF |
| Leaves | BL | |
| Stem | BS | |
| El-Oued | Fruits | EF |
| Leaves | EL | |
| Stem | ES |
2.2 Plant extraction
The plants were completely dried using a freeze dryer (Alpha 1-4 LD plus, Christ, Osterode, Germany). The dried powdered plant samples were extracted using a modified version of the method of Aouadi et al. [17], with some modifications. About 0.5 g of each sample was soaked for 24 h with 50 mL of methanol (99.8% absolute methanol) in a capped bottle and shaken in an orbital shaker at 180 rpm at room temperature. The agitated sample was centrifuged at 10,000 rpm for 10 min and the supernatant was recovered.
2.3 Phytochemical profiling
2.3.1 Determination of TPC
Using a modified version of Aouadi et al.’s 2003 approach, the total phenols in the methanol extract were assessed spectrophotometrically using Folin–Ciocalteu reagent [16]. About 5 mL of Folin–Ciocalteu reagent that had been diluted 1:9 with water was added to the extracts. About 4 mL of sodium carbonate (75 g/L) was added to this mixture, vortexed for 10 s, and then left to stand for 30 min at 40°C to develop color. The UV–Vis 3000 PC spectrophotometer was used to measure the absorbance at 765 nm. The extracts’ TPC was calculated as mg of tannic acid equivalents (TAE) per gram of dry sample weight (mg/g). The experiment was conducted in triplicate and the results were expressed as mean standard deviation (SD) values. The TPC was calculated as TAE by the following equation:
where T is the TPC in mg/g of the extracts as TAE, C is the concentration of tannic acid established from the calibration curve in mg/mL, V is the volume of the extract solution in mL, and M is the weight of the extract in g.
2.3.2 Determination of TFC
The TFCs of all the extracts were quantified following the protocol outlined by Hailong et al. [18]. Initially, 700 μL of all the plant extracts were taken in different test tubes. To each extract, 2 mL of distilled water was added. Then, 150 μL of NaNO2 was added to each test tube followed by incubation at room temperature for 6 min. After incubation, 150 μL of AlCl3 (10%) was added to each test tube. The test tubes were incubated for 6 min at room temperature. Then, 2 mL of 4% NaOH was added to all the test tubes which were made up to 5 mL using distilled water. The contents in all the test tubes were vortexed well and they were allowed to stand for 15 min at room temperature. The presence of flavonoids was read with a spectrophotometer at 510 nm. The level of flavonoids was calculated as milligram catechin equivalent per gram (mg CE/g).
2.3.3 Determination of alkaloid content
Crude samples of S. nigrum extracts were subjected to quantitative analysis using a modified version of Harborne’s (1980) methodology [19]. Five grams of the S. nigrum sample was mixed with 20 mL of 10% acetic acid (Ac) prepared in ethanol, then capped and allowed to sit for 4 hours. The combination was filtered, and the filtrate was then concentrated in a water bath to a quarter of its original volume. Drop by drop, concentrated ammonium hydroxide was added to the extract until the precipitation was complete. The entire solution was left to settle and washed with diluted ammonium hydroxide before being re-filtered. The weight of the dried residue was determined, and the percentage composition was calculated using the equation:
2.3.4 Determination of proanthocyanidin content
The total proanthocyanidin content of S. nigrum was determined using the procedure reported by Zhang et al. [20]. A total of 3.0 mL of (4% v/v) vanillin methanol solution, 1.5 mL of hydrochloric acid, and 0.5 mL of the extract at 0.1 mg/mL were added together and vortexed. The combination was left at room temperature for 15 min, after which the absorbance at 500 nm was determined. Total proanthocyanidin content was calculated as catechin equivalents (CEs) (mg/g) using the equation from the calibration curve: Y = 0.5825x, R 2 = 0.9277, where x is the concentration of the CE and Y is the absorbance.
2.3.5 Determination of tannin contents
Tannin content was determined in plants from different soil types using the method of Irina et al. [21] with some modifications. A mass of 0.20 g of S. nigrum methanol extract was added to 20 mL of 50% methanol, vortexed vigorously, and incubated in the water bath for 1 h at 80°C and filtered. The filtrate was poured in 20 mL of distilled water. About 2.5 mL of Folin-Denis reagent was then mixed with 10 mL of 17% aqueous NaCO3, mixed with the filtrate, and made up to 100 mL with distilled water. The mixture was allowed to react for 20 min. At the end of the reaction, there was a bluish-green color that indicated the presence of tannins. Absorption of the tannic standard solution and the samples were then measured at 706 nm. Results were expressed in mg/g of TAE to the calibration curve: Y = 0.0593x0.0485, R 2 = 0.9826, where x is the absorbance and Y is the TAE.
2.4 Determination of vitamin C content
The vitamin C content was determined according to the official methods of analysis [22], 2,6-dichloroindophenol titration method 967.21. Briefly, a 0.1 g powder sample was extracted with 40 mL of 15 g of metaphosphoric acid (HPO3) and mixed with 40 mL of Ac in 500 mL of deionized H2O. The extracted sample was filtered using the Whatman number one filter paper. The filtrated sample was titrated using an indophenol solution made by dissolving 50 mg of 2,6-dichloroindophenol sodium salt and 42 mg of NaHCO3 in 200 mL of deionized water. The mixture was filtered through Whatman number one filter paper into the amber bottle and stored in a refrigerator until use.
The standard solution of vitamin C was prepared by transferring 50 mg of vitamin C into 50 mL flask and diluted to volume using freshly prepared HPO3(Ac). The vitamin C content was calculated according to the following equation:
where A = volume in mL of the 2,6-dichloroindophenol sodium salt solution used for the sample; B = volume in mL of the 2,6-dichloroindophenol sodium salt solution used for the blank; C = mass in mg of l ascorbic acid equivalent to 1.0 mL of standard indophenol solution; S = weight of a sample taken (g); 40 = volume of extract; and 10 = volume of extract used for the determination.
2.5 Antioxidant activity
2.5.1 Determination of antioxidant activity by DPPH assay
The determination of DPPH stable radical scavenging activities of the extracts and standards was evaluated based on the method described by Aouadi et al. [23]. Extracts (1 mL) of different concentrations (0.2–0.56 mg/mL) made by reconstituting in respective solvents were added to DPPH solution (5 mL, 0.1 mM) in methanol and vortexed. After 20 min of reaction at 25°C, the absorbance was measured at 517 nm against a blank (methanol) in a UV–Vis spectrophotometer (Shimadzu UV-2401PC). Methanolic DPPH solution (5 mL) without antioxidants was used as a control. The DPPH scavenging activity of the extract was expressed as IC50 (inhibitory concentration), that is, the concentration of the extract at which DPPH radicals were quenched by 50%. Ascorbic acid was used as the standard antioxidant. The percentage quenching of DPPH was calculated as follows:
2.5.2 Determination of antioxidant activity by FRAP
The FRAP analysis was conducted based on a modified method of Carducci et al. [24]. Briefly, the FRAP reagent was made by mixing acetate buffer (pH 3.6, 300 mL), 10 mMol/L 2,4,6-tri-2-pyridyl-s-triazine, and 20 mmol/L FeCl3 at a ratio of 10:1:1 (v/v/v). Then, 150 μL of the methanol extract solution of S. nigrum was mixed with 4.5 mL of the FRAP reagent, and the mixture was incubated in a water bath at 37°C for 10 min. The absorbance of the reacted mixture was measured by the UV–Vis spectrophotometer (Shimadzu UV-2401PC) at 593 nm, and the FRAP of each sample was calculated using a calibration curve with Fe2 (Sigma-Aldrich Co, USA) as the standard.
2.5.3 Oxidative hemolysis inhibition assay
This test is used to determine the ability of the plant extracts to protect the erythrocyte blood cells from damage or disruption of the cell membrane after exposing them to oxidative stress and free radicals by measuring the percentage of dissolved erythrocytes [25]. According to Chouikh et al. [26], a volume of 40 µL of human erythrocytes was mixed with 2 mL of different concentrations (0.25–1 mg/mL) of S. nigrum extract and conserved for 5 min at 37°C. Then, we added 40 µL of H2O2 (30 × 10−3 M), 40 µL of FeCl3 (80 × 10−3 M), and 40 µL of ascorbic acid solution (50 × 10−3 M), respectively. After 1 h of incubation at 37°C, the mixture was centrifuged with 700 rot/min for 10 min. The absorbance of the supernatant was read at λ = 540 nm. The percentage of hemolysis was determined using the following formula:
2.6 Docking study
The docking analysis was performed with the Autodock vina 4.2 software. The derivatives were subsequently submitted to energy minimization using the default MMFF94x force. The three-dimensional conformations of these compounds were acquired through the application of a conformer search methodology. The lipoxygenase (LOX) and peroxidase enzymes were obtained from the Protein Data Bank (PDB) with the corresponding identification codes 6n2w and 2x08, respectively. Water molecules were eliminated from the software, and any hydrogen atoms that were absent were replaced to guarantee the protein structure maintained accurate ionization states. We utilized the Biovia discovery-studio 2021 visualizer to examine the representation of protein–ligand interactions in the active site of the complex. In conclusion, the H bonds, hydrophobic, and Pi–Pi interactions are regarded as the most significant ones [27,28].
2.7 Statistical analysis
The data presented represent the average of three replicates with SD.
A multiway analysis of variance was applied to the results and mean comparisons were performed using Tukey’s multiple range test with SPSS version 20.0 (Statistical Package for the Social Sciences, Inc., Chicago, IL, USA). Significance was considered at p < 0.05. To determine the relationship between the chemical content of plants and the relationship between the chemical content of plants and antioxidants or other biological activities, the linear correlation coefficient was calculated. For exploratory data analysis, the results were processed through one of the multivariate analysis techniques, principal component analysis (PCA). PCA was performed using XLSTAT (version 2020.1, Addinsoft, Pearson edition, Waltman, MA, USA) to enhance discrimination between the studied parameters.
A multiway analysis of variance (ANOVA) was utilized to evaluate the effects of multiple factors and their interactions on the outcome variables simultaneously. This statistical method allows researchers to determine the individual impact of each factor on the dependent variable, while also identifying how different factors interact with one another. By accounting for multiple sources of variation, multiway ANOVA increases the statistical power, enhancing the ability to detect significant differences. Additionally, this approach helps control for confounding variables, thereby reducing potential bias and leading to more accurate and reliable conclusions.
3 Results and discussion
3.1 Quantitative estimation of TPC
Phenolic compounds are known to be potent chain-breaking antioxidants that serve as free radical terminators, which may directly contribute to antioxidative action. These chemicals are very significant plant ingredients, and their radical scavenging ability is related to the presence of hydroxyl groups in them. Extracts with significant antioxidant activity have a high phenolic content in general. Polyphenols are the most abundant plant chemicals with antioxidant activity due to their redox characteristics; they are vital in absorbing and neutralizing free radicals, quenching singlet and triplet O2, and degrading peroxides. Plants in phenols have antioxidant, anti-mutagenic, and anti-cancer effects [29]. The TPC of three different samples of S. nigrum L. fruits, leaves, and stem was determined by the Folin–Ciocalteu method [30]. The TPC of extracts ranged from 11.3 to 109.2 mg of gallic acid equivalent (GAE)/g on a dry weight (dw) basis (Table 2). TPC levels were highest in the Biskra fruit (BF) extract of S. nigrum, and lowest in El-Oued leaf (EL) extract of S. nigrum (Figure 1a).
Total phenolic, total flavonoid, and phytochemical profile of the wild Solanum nigrum L. collected from three different locations in Algerian desert
| S. nigrum extract | TPC (mg GAE/g) | TFC (mg CE/g) | Tannin content (mg TE/g) | Proanthocyanidin content (mg CE/g) | Vitamin C (mg/100 g) | Alkaloids (%) |
|---|---|---|---|---|---|---|
| TF | 79.87 ± 0.10c | 58.23 ± 0.12cd | 93.37 ± 0.14b | 861.33 ± 0.10c | 32.27 ± 0.12c | 37.67 ± 0.12d |
| TL | 17.6 ± 0.09h | 29.07 ± 0.12f | 56.30 ± 0.05e | 557.77 ± 0.17f | 18.37 ± 0.12fg | 24.80 ± 0.14h |
| TS | 53.43 ± 0.12f | 19.77 ± 0.12h | 40.07 ± 0.17g | 220.30 ± 0.09i | 15.33 ± 0.12h | 22.23 ± 0.11i |
| BF | 109.17 ± 0.17a | 88.33 ± 0.12a | 97.17 ± 0.12a | 889.67 ± 0.07a | 47.07 ± 0.12a | 50.97 ± 0.19ab |
| BL | 40.1 ± 0.14g | 51.23 ± 0.07e | 74.47 ± 0.10c | 697.57 ± 0.07de | 34.77 ± 0.12b | 35.20 ± 0.19e |
| BS | 76.30 ± 0.09d | 22.47 ± 0.12g | 29.73 ± 0.07h | 331.67 ± 0.07h | 27.40 ± 0.14d | 26.70 ± 0.09g |
| EF | 83.47 ± 0.17b | 74.30 ± 0.08b | 92.83 ± 0.17b | 881.43 ± 0.14ab | 34.17 ± 0.17b | 46.33 ± 0.15c |
| EL | 11.33 ± 0.12i | 59.67 ± 0.12c | 64.43 ± 0.14d | 708.37 ± 0.14d | 21.63 ± 0.10e | 51.03 ± 0.14a |
| ES | 63.67 ± 0.15e | 19.63 ± 0.10h | 42.77 ± 0.14f | 490.30 ± 0.12g | 19.90 ± 0.17f | 33.47 ± 0.19f |
Values with different superscript letters are statistically different.

(a) TPC and (b) TFC of S. nigrum extracts.
3.2 Quantitative estimation of TFC
Flavonoids are likely the most important natural phenolics due to their wide range of chemical and biological activity, including antioxidant and free radical scavenging characteristics. Flavonoids have been described as antioxidants, scavengers of a wide range of ROS, and regulators of lipid peroxidation, as well as potential therapeutic agents against a wide range of disorders [29]. The TFC of the nine S. nigrum extracts was expressed as mg CE/g dw extract. The TFC of extracts ranged from 19.7 to 88.3 mg CE/g (Table 2). BF and El-Oued fruit (EF) extracts of S. nigrum contained the most TFC, whereas El-Oued stem (ES) extract and Tebessa stem (TS) extract contained the least (Figure 1).
3.3 Quantitative estimation of proanthocyanidin content
Proanthocyanidins are flavan-3-ol-based tannins that are condensed or nonhydrolyzable. These chemicals are extensively spread throughout nature, including vegetables, fruits, and plants. Proanthocyanidins have been shown to have potent free-radical scavenging and antioxidative properties. Many significant biological actions, such as anticancer, antiviral, and anti-inflammatory, have been reported [31]. Total proanthocyanidin content was calculated as CE (mg/g) as listed in Table 2. The study results for proanthocyanidin concentration of S. nigrum L. extracts ranged from 220.3 to 889.7 mg CE/g. Fruits of S. nigrum L. had the highest proanthocyanidin concentration, followed by S. nigrum L. leaves, while S. nigrum L. stem had the lowest as shown in Figure 2a.

(a) Proanthocyanidin content and (b) tannin content in S. nigrum extracts.
3.4 Quantitative estimation of tannin content
Tannins are classified as polyphenols due to the abundance of phenolic rings in their structures. These natural compounds can be found in plant tissues such as bark, fruit, and wood and can be extracted from these sources using water [32]. The tannin content of S. nigrum L. was expressed in mg/g of TAE. From Table 2, tannin content was highest in the fruits of S. nigrum L. rather than leaves and stem. Fruits from Biskra region (BF) were the richest followed by fruits from Tebessa (TF) and El-Oued (EF) regions. While the stem of S. nigrum L. from Tebessa (TS) contains the lowest concentration of tannins (Figure 2b).
3.5 Quantitative estimation of alkaloid content
Alkaloids have always piqued the curiosity of scientists due to their good or bad impacts on humans. Surprisingly, many alkaloids can act as both anti-oxidants and pro-oxidants depending on the circumstances [33]. The results showed that S. nigrum leaf from El-Oued region (EL) had the maximum alkaloid concentration (51.1%), followed by S. nigrum fruits from Biskra region (BF) (50.9%) and S. nigrum fruits from El-Oued region (EF) (46.2%). Stem of S. nigrum collected from Tebessa region (TS) has the lowest alkaloid concentration (22.1%) (Figure 3).

Alkaloids content of S. nigrum extract.
3.6 Quantitative estimation of vitamin C content
As demonstrated in Table 2, the vitamin C content of three different samples of S. nigrum L. collected from Tebessa, Biskra, and El-Oued differed somewhat. The vitamin C content of the nine extracts ranged from 15.3 mg/100 g (dw) in the stem extract of S. nigrum collected from Tebessa (TS) to 47.1 mg/100 g (dw) in the fruit extract of S. nigrum collected from BF. The maximum vitamin C concentration was identified in the fruits of S. nigrum collected from BF, followed by S. nigrum fruits collected from El-Oued (EF), while the lowest quantity was found in the stems collected from TS (Figure 4).

Vitamin C content of S. nigrum extracts.
3.7 PCA
Using PCA, we were able to acquire a more in-depth understanding of the link between the overall amount of various chemical components that were computed for each of the nine extracts. Through the use of this study, the purpose was to shed light on the relationships that exist between the nine plant extracts’ six primary components, which are as follows: TPC, TFC, tannin, proanthocyanidins, vitamin C, and alkaloids. Figure 5 and Table 3 illustrate this point.

Biplot PCA of TPC, TFC, tannin, proanthocyanidins, vitamin C, and alkaloids for the nine plant extracts. The percentages of PC1 and PC2 represent the largest and second largest variance in the data on the X-axis and Y-axis, respectively.
Correlation matrix between chemical content of plant extracts
| TPC | TFC | Tannin | Proanthocyanidins | Vitamin C | Alkaloids | |
|---|---|---|---|---|---|---|
| TPC | 1 | 0.40335 | 0.3869 | 0.29468 | 0.68767 | 0.24912 |
| TFC | 0.40335 | 1 | 0.92158 | 0.90794 | 0.82593 | 0.88579 |
| Tannin | 0.3869 | 0.92158 | 1 | 0.95028 | 0.76529 | 0.72839 |
| Proanthocyanidins | 0.29468 | 0.90794 | 0.95028 | 1 | 0.73841 | 0.81997 |
| Vitamin C | 0.68767 | 0.82593 | 0.76529 | 0.73841 | 1 | 0.63174 |
| Alkaloids | 0.24912 | 0.88579 | 0.72839 | 0.81997 | 0.63174 | 1 |
With a correlation value of 0.68767, the total polyphenol content was shown to have a substantial positive link with vitamin C. On the other hand, the correlations with the remaining chemicals ranged from weak to moderate. The flavonoid content had a substantial and positive association with all of the components, with the correlation coefficients being 0.92158, 0.3869, and 0.90794 for TPC, tannin, proanthocyanidins, vitamin C, and alkaloids, respectively (as shown in Table 3). It was also found that the flavonoid content was favorably connected with all of the components.
Figure 5 demonstrates that a very significant positive correlation ratio was found between proanthocyanidin and tannin and TFC. This connection was documented and demonstrated by the structural inputs. The results of the study are presented in Table 4, which shows that PC1 had a significant positive correlation with TFC (r = 0.45874), but PC2 only had a high and positive correlation with TPC (r = 0.83556) and vitamin C (r = 0.35783).
Correlation between variables (chemical content) and factors for plant extracts
| Coefficients of PC1 | Coefficients of PC2 | |
|---|---|---|
| TPC | 0.24891 | 0.83556 |
| TFC | 0.45874 | −0.13163 |
| Tannin | 0.44199 | −0.12841 |
| Proanthocyanidin | 0.4412 | −0.2493 |
| Vitamin C | 0.41827 | 0.33783 |
| Alkaloids | 0.4033 | −0.30288 |
The results of Tukey’s test (Table 2) confirm that there are large variations between the harvest area and the plant part with regard to their effectiveness in extracting the active substances studied, as it is clear that the BF sample is the best sample. Therefore, the Tebessa region is the best harvesting area, and the leaf part is the best part of the plant.
3.8 Antioxidant activity
3.8.1 Determination of antioxidant activity by DPPH assay
The ability of S. nigrum L. extracts to bleach the stable DPPH radical was used to evaluate their free radical-scavenging activity. All extracts quenched the DPPH-radical at all concentrations tested. The half-maximal inhibitory concentration (IC50) is used in the DPPH assay. The IC50 value is defined as the crude extract’s inhibitory concentration that scavenges 50% of ROS or inhibits the oxidation process by 50% [34]. The concentration-inhibition activity curve was used to calculate it. IC50 is proportional to antioxidant capacity, and a lower IC50 value indicates greater antioxidant activity. S. nigrum Tebessa fruits (TF; 0.0067), S. nigrum BF (0.0069), S. nigrum Tebessa leaves (TL; 0.0085), and S. nigrum EF (0.0088) had the lowest IC50 values (g/mL), whereas S. nigrum TS (0.0129) had the highest (Table 5 and Figure 6). This experiment proved that S. nigrum L. has antioxidant characteristics.
Antioxidant activity of different S. nigrum L. extracts
| Sample | DPPH | FRAP (mM of Fe2+/g) | Hemolysis assay |
|---|---|---|---|
| IC50 | AEAC | Protection (%) | |
| TF | 0.007 ± 0.001ab | 257.43 ± 0.11ab | 55.95 ± 0.14de |
| TL | 0.009 ± 0.000c | 192.73 ± 0.19g | 39.24 ± 0.21i |
| TS | 0.013 ± 0.002de | 199.87 ± 0.28e | 50.27 ± 0.12g |
| BF | 0.006 ± 0.001a | 211.13 ± 0.03c | 67.84 ± 0.25b |
| BL | 0.012 ± 0.002d | 260.87 ± 0.21a | 44.98 ± 0.33h |
| BS | 0.014 ± 0.002e | 196.83 ± 2.06ef | 57.43 ± 0.11d |
| EF | 0.008 ± 0.001bc | 203.70 ± 0.17d | 60.65 ± 0.67c |
| EL | 0.013 ± 0.002de | 152.10 ± 0.12h | 28.12 ± 0.03j |
| ES | 0.021 ± 0.001f | 107.83 ± 0.21i | 52.75 ± 0.17f |
| Ascorbic acid | 0.013 ± 0.002de | — | 72.07 ± 0.33a |
Values with different superscript letters are statistically different.

DPPH quenching activity of nine extracts from wild S. nigrum L. collected from three different regions in Algeria.
Furthermore, the decrease in DPPH uptake in the presence of antioxidant standards can be used to calculate the binding constant and free energy, whereas the shift in wavelength values can be used to determine the mode of interaction; these findings were inspired by the study of drug molecule binding to DNA [35]. Increased reaction time between the samples and DPPH in the same solvent leads to a significant overall drop in absorbance. Based on this lack of absorbance, the binding constant has been calculated using the Benesi–Hildebrand equation, which is used to quantify an anticancer drug’s intrinsic binding constant/binding constant with DNA [36].
where K is the binding constant,
Binding constant calculation for S. nigrum L. different extracts
| S. nigrum | Equation | R 2 | K (L/mol) | ∆G (kJ/mol) |
|---|---|---|---|---|
| TF | y = −0.015x − 0.293 | 0.88 | 19.53 | −7.37 |
| TL | y = −0.019x − 0.422 | 0.89 | 22.21 | −7.69 |
| TS | y = −0.03x − 1.44 | 0.91 | 48 | −9.6 |
| BF | y = −0.0088x − 0.2862 | 0.996 | 32.52 | −8.63 |
| BL | y = −0.01944x − 0.034 | 0.87 | 1.74 | −1.37 |
| BS | y = −0.0151x − 0.7292 | 0.982 | 48.29 | −9.61 |
| EF | y = −0.0087x − 0.5426 | 0.998 | 62.37 | −10.25 |
| EL | y = −0.0124x − 0.674 | 0.990 | 54.35 | −9.9 |
| ES | y = −0.022x – 0.7 | 0.898 | 31.81 | −8.58 |
3.8.2 Determination of antioxidant activity by FRAP
In comparison to other assays that evaluate free radical inhibition, the FRAP test is the only one that directly analyses antioxidants (or reductants) in a sample [37]. The FRAP assay values listed in Table 5 represent the concentration of electron-donating antioxidants associated with the reduction of ferric iron (Fe3+) to ferrous ion (Fe2+) [38]. Biskra leaf extract of S. nigrum L. showed higher FRAP (BL; 260.9) than TF (257.34) and BF (211.18). All samples had significantly different FRAPs with values ranging from 107.85 to 260.9 mM Fe2+/g (dw) (Figure 7).

Antioxidant activity of S. nigrum L. extracts by FRAP.
3.8.3 PCA
PCA was used to get a more in-depth understanding of the connection that exists between the total content of various chemical components that were computed for each of the nine extracts and the antioxidant activity of DPPH and FRAP. The purpose of this investigation was to shed light on the relationships that exist between the nine plant extracts’ six primary constituents, which are respectively referred to as TPC, TFC, tannin, proanthocyanidins, vitamin C, and alkaloids. These are the antioxidant activity of DPPH and FRAP, which may be found in Figure 8 and Table 7, respectively.

Biplot PCA of TPC, TFC, tannin, proanthocyanidins, vitamin C, and alkaloids for the nine plant extracts and antioxidant activity. The percentages PC1 and PC2 represent the largest and second-largest variances in the data on the X-axis and Y-axis, respectively.
Correlation matrix between chemical content and antioxidant activity of plant extracts
| TPC | TFC | Tannin | Proanthocyanidin | Vitamin C | Alkaloids | DPPH | FRAP | |
|---|---|---|---|---|---|---|---|---|
| TPC | 1 | 0.40335 | 0.3869 | 0.29468 | 0.68767 | 0.24912 | −0.29836 | 0.23047 |
| TFC | 0.40335 | 1 | 0.92158 | 0.90794 | 0.82593 | 0.88579 | −0.71371 | 0.37253 |
| Tannin | 0.3869 | 0.92158 | 1 | 0.95028 | 0.76529 | 0.72839 | −0.76237 | 0.51368 |
| Proanthocyanidin | 0.29468 | 0.90794 | 0.95028 | 1 | 0.73841 | 0.81997 | −0.63373 | 0.34385 |
| Vitamin C | 0.68767 | 0.82593 | 0.76529 | 0.73841 | 1 | 0.63174 | −0.60105 | 0.52347 |
| Alkaloids | 0.24912 | 0.88579 | 0.72839 | 0.81997 | 0.63174 | 1 | −0.35403 | −0.02724 |
| DPPH | −0.29836 | −0.71371 | −0.76237 | −0.63373 | −0.60105 | −0.35403 | 1 | −0.70951 |
| FRAP | 0.23047 | 0.37253 | 0.51368 | 0.34385 | 0.52347 | −0.02724 | −0.70951 | 1 |
According to the findings of the statistical analysis that was carried out, which are included in the table, the correlation between all chemical compounds and the outcomes of the DPPH test was found to be negative. This is because the results of the antioxidant activity of the DPPH test were found to be more effective when the IC50 concentration was lower.
Table 8 shows that all compounds had a very strong correlation, as the concentration of the compounds increased, the concentration of IC50 decreased, as the correlation coefficients for the six compounds TPC, TFC, tannin, proanthocyanidins, vitamin C, and alkaloids were −0.29836, −0.71371, −0.76237, −0.63373, −0.60105, and −0.35403, respectively (as shown in Table 7).
Correlation between variables (chemical content and antioxidant activity) and factors for plant extracts
| Coefficients of PC1 | Coefficients of PC2 | |
|---|---|---|
| TPC | 0.225 | −0.14526 |
| TFC | 0.42079 | 0.17815 |
| Tannin | 0.41825 | 0.02589 |
| Proanthocyanidin | 0.40089 | 0.20898 |
| Vitamin C | 0.3898 | −0.07314 |
| Alkaloids | 0.33449 | 0.53723 |
| DPPH | −0.34301 | 0.36903 |
| FRAP | 0.23489 | −0.68751 |
Table 8 illustrates the high connection that exists between chemical compounds and the outcomes of the antioxidant activity test using the FRAP test. The relationship between the two groups was found to be satisfactory and positive. This is because the mechanism of the chemicals in this antioxidant test is responsible for this positive and satisfied association. Vitamin C and tannin had the highest correlation with the FRAP test, with the correlation coefficients being 0.52347 and 0.51368, respectively. The remaining compounds had a moderate to strong correlation, with the correlation coefficients for TPC, TFC, and proanthocyanidin being 0.23047, 0.37253, and 0.34385, respectively. Vitamin C and tannin produced the strongest correlation. The correlation coefficient for the alkaloids molecule was −0.02724, which indicates that the inverse correlation was not very strong because of this.
Strong positive correlations with TFC, tannin, and proanthocyanidin were found, and the structural inputs shown in Figure 8 provided further evidence that these correlations are strong. According to the data presented in Table 8, F1 exhibited a high positive correlation with TFC (r = 0.41825), tannin (r = 0.41825), and proanthocyanidin (r = 0.40089).
The results of Tukey’s test (Table 5) confirm that there are large variations between the harvest area and the plant part with regard to their effectiveness in antioxidant activity, as it is clear that the BF sample is the best sample. Therefore, the Tebessa region is the best harvesting area, and the leaf part is the best part of the plant.
3.9 Oxidative hemolysis inhibition assay
Oxidative hemolysis inhibition assay is based on antioxidants’ ability to prevent free radical-induced membrane damage in erythrocytes. The benefit of this approach is that it employs peroxyl radicals as pro-oxidants and erythrocytes as oxidizable targets, resulting in results that represent biologically meaningful antioxidant micro localization and radical-scavenging activity [39]. The percentage of hemolysis was calculated as in Table 5 and the hemolysis % is inversely related to the antioxidant activity of the extract. The highest percentage indicates lower antioxidant activity, as seen in Table 5 and Figure 9, showing that EF extract of S. nigrum L. has the lowest percentage 14.6 which is just above the percentage of the reference antioxidant drug (ascorbic acid; 12.07), followed by TF (17.9%) and TS (18.2). The highest percentage of hemolysis was recorded by stem extract of the S. nigrum L. sample collected from the El-Oued region.

Anti-hemolytic activity of S. nigrum L. extracts.
3.9.1 PCA
PCA was used to acquire a more profound comprehension of the connection that exists between the total content of various chemical components that were computed for each of the nine extracts and the hemolysis test. By examining the nine plant extracts and the hemolysis test, this investigation aimed to shed light on the relationships that exist between the six primary components, which are as follows: TPC, TFC, tannin, proanthocyanidins, vitamin C, and alkaloids. Figure 10 and Table 9 present the results of this investigation. According to the findings of the statistical analysis that was carried out, which are presented in the table, the correlation between all of the chemical compounds and the outcomes of the hemolysis test was a strong and positive correlation. This indicates that the percentage of protection increases in proportion to the concentration of chemical compounds. The correlation coefficient for TPC was 0.97698, which indicates that the concentration of TPC plays a very significant influence on the protection rate. Table 9 demonstrates that the correlation between TPC and the protection rate was extremely high, and it was very near to 1. The correlation coefficient for vitamin C was 0.62896, and the correlation coefficient for alkaloids was 0.09513. TFC, tannin, and proanthocyanidin each had a correlation value of 0.29827, 0.3147, and 0.20262, respectively, falling somewhere in the middle of the moderate to strong range. The coefficient of correlation for alkaloids was 0.09513, which indicates that the connection was very weak despite the fact that it was positive. A significant positive connection with TPC was found to exist, and this finding is supported by the structural inputs shown in Figure 10. PC1 was found to have a strong positive relationship with TFC (r = 0.28985), and PC2 was found to have a strong positive relationship with TFC (r = 0.57856). This information is shown in Table 10.

Biplot PCA of TPC, TFC, tannin, proanthocyanidins, vitamin C, and alkaloids for the nine plant extracts and hemolysis test. The percentages of PC1 and PC2 represent the largest and second largest variance in the data on the X-axis and Y-axis, respectively.
Correlation matrix between chemical content and hemolysis test of plant extracts
| TPC | TFC | Tannin | Proanthocyanidin | Vitamin C | Alkaloids | Hemolysis | |
|---|---|---|---|---|---|---|---|
| TPC | 1 | 0.40335 | 0.3869 | 0.29468 | 0.68767 | 0.24912 | 0.97698 |
| TFC | 0.40335 | 1 | 0.92158 | 0.90794 | 0.82593 | 0.88579 | 0.29827 |
| Tannin | 0.3869 | 0.92158 | 1 | 0.95028 | 0.76529 | 0.72839 | 0.3147 |
| Proanthocyanidin | 0.29468 | 0.90794 | 0.95028 | 1 | 0.73841 | 0.81997 | 0.20262 |
| Vitamin C | 0.68767 | 0.82593 | 0.76529 | 0.73841 | 1 | 0.63174 | 0.62896 |
| Alkaloids | 0.24912 | 0.88579 | 0.72839 | 0.81997 | 0.63174 | 1 | 0.09513 |
| Hemolysis | 0.97698 | 0.29827 | 0.3147 | 0.20262 | 0.62896 | 0.09513 | 1 |
Correlation between variables (chemical content and hemolysis test) and factors for plant extracts
| Coefficients of PC1 | Coefficients of PC2 | |
|---|---|---|
| TPC | 0.28985 | 0.57856 |
| TFC | 0.43579 | −0.195 |
| Tannin | 0.42136 | −0.17682 |
| Proanthocyanidin | 0.41333 | −0.26925 |
| Vitamin C | 0.42136 | 0.14588 |
| Alkaloids | 0.37215 | −0.31254 |
| Hemolysis | 0.24829 | 0.63602 |
The Tukey test results (Table 5 and Figure 9) confirm that there are significant differences between the harvest area and the plant part with regard to their effectiveness in protecting red blood cells, and it is also clear that the BF sample is the best sample. Therefore, the Tebessa region is considered the best area for harvesting, and the leafy part is the best part of the plant
4 Correlation between phenolic phytochemicals of S. nigrum and it’s antioxidant activity via In-sillico docking studies
The aim of this part of study was to investigate the correlation of the phenolic chemical composition of S. nigrum L. with their antioxidant activity. Fifteen polyphenolic compounds were previously reported as the major isolated and identified compound from S. nigrum L. methanol extract as reported by Chen et al. [40], which are listed in Table S1. Based on the physicochemical and ADME properties of major phenolic compounds reports in S. nigrum L., in silico docking study was performed on only ten compounds to determine the potential antioxidant mechanism of action.
4.1 In silico evaluation of physicochemical and ADME properties of major phenolic compound reports in S. nigrum L.
A computational study of major phenolic compounds [1–14] presented in the S. nigrum L. plant was performed to evaluate physicochemical and ADME properties using SwissADME [41]. With respect to physicochemical properties (Table 11), all the reported compounds have zero violation of the Lipinski’s rule for oral drugs, except for compounds 2–5 and 14, which have two violations and more. So, we pursue studies on the other ten compounds (1, 6–13, and 15). All the topological polar surface areas (TPSA) of the chosen ten compounds are less than 131.36 A0 2. Additionally, absorption (% ABS) was estimated by using the equation% ABS = 109 – (0.345 × TPSA) [42] and found that the calculated% ABS of those compounds ranged between 63.68 and 89.15%, demonstrating that those compounds may have the required cell membrane permeability and bioavailability. All ten compounds have rotatable bonds between 1 and 2, which indicate molecular flexibility to their biotarget. Regarding pharmacokinetics, it was found that the chosen ten compounds have high gastrointestinal absorption and have no permeation to the blood–brain barrier except for 4-hydroxybenzoic acid (compound 11) and 4-hydroxycinnamic acid (compound 15), which have high GIT absorption and can pass blood–brain barrier. Thus, ensuring that almost all compounds will have low to no central nervous system side effects. Bioavailability is an index of the amount of drug present in the plasma and is considered the most crucial factor affecting absorption. Interestingly, all the compounds were found to have high bioavailability scores ranging from 0.55 to 0.85.
Physicochemical properties of major phenolic compounds previously isolated from Solanum nigrum L. based on Lipinskí’s rule of five, TPSA, % ABS, and number of rotatable bonds
| No. | Comp. name | HBD | HBA | MlogP | MW | Lipinski’s violations | TPSA | % ABS | No. of rot. bonds |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Quercetin | 5 | 7 | −0.56 | 302.24 | 0 | 131.36 | 63.68 | 1 |
| 2 | Quercitrin | 7 | 11 | −1.84 | 448.38 | 2 | 190.28 | 43.35 | 3 |
| 3 | Isoquercitrin | 8 | 12 | −2.59 | 464.38 | 2 | 210.51 | 36.37 | 4 |
| 4 | Quercetin-3-gentiobioside | 11 | 17 | −4.62 | 626.52 | 3 | 289.66 | 9.06 | 7 |
| 5 | 6-Hydroxyluteolin 7-sophoroside | 11 | 17 | −4.62 | 626.52 | 3 | 289.66 | 9.06 | 7 |
| 6 | Kaempferol | 4 | 6 | −0.03 | 286.24 | 0 | 111.13 | 70.66 | 1 |
| 7 | Gallic acid | 4 | 5 | −0.16 | 170.18 | 0 | 97.99 | 75.19 | 1 |
| 8 | 2,4-Dihydroxybenzoic acid | 3 | 4 | 0.4 | 154.12 | 0 | 77.76 | 82.17 | 1 |
| 9 | Protocatechuic acid | 3 | 4 | 0.4 | 154.12 | 0 | 77.76 | 82.17 | 1 |
| 10 | Vanillic acid | 2 | 4 | 0.74 | 168.15 | 0 | 66.76 | 85.96 | 2 |
| 11 | 4-Hydroxybenzoic acid | 2 | 3 | 0.99 | 138.12 | 0 | 57.53 | 89.15 | 1 |
| 12 | 2,5-Dihydroxybenzoic acid (gentisic acid) | 3 | 4 | 0.4 | 154.12 | 0 | 77.76 | 82.17 | 1 |
| 13 | Caffeic acid | 3 | 4 | 0.7 | 180.16 | 0 | 77.76 | 82.17 | 2 |
| 14 | Chlorogenic acid | 6 | 9 | −1.05 | 354.31 | 1 | 164.75 | 52.16 | 5 |
| 15 | 4-Hydroxycinnamic acid | 2 | 3 | 1.28 | 164.16 | 0 | 57.53 | 89.15 | 2 |
4.2 In silico antioxidant docking study
Docking seeks to appropriately assess the strength of binding by accurately predicting a ligand’s shape inside the confines of a binding pocket. The ten bioactive compounds quercetin, kaempferol, gallic acid, 2,4-dihydroxybenzoic acid, protocatechuic acid, vanillic acid, 4-hydroxybenzoic acid, 2,5-dihydroxybenzoic acid (gentisic acid), caffeic acid, and 4-hydroxycinnamic acid were docked with selected proteins, arachidonate-5-lipoxygenase (5-LOX) (PDB: 6n2w) [3] and cytochrome c peroxidase (PDB: 2x08), to check their affinity with the binding sites of these proteins for the possible mechanism of action. According to the docking scores, quercetin and kaempferol of S. nigrum L. may have a crucial role as antioxidant. Our results are shown in Table 12, Tables S2 and S3, Figures 11–18, and Figures S1 and S2.
Docking scores and binding interactions of selected phenolic compounds
| Com. no. | LOX | Peroxidase | ||||
|---|---|---|---|---|---|---|
| Docking score (kcal/mol) | Type of interaction (interacting amino acids) | Distance (A0) | Docking score (kcal/mol) | Type of interaction (interacting amino acids) | Distance (A0) | |
| NDGA | −11.01 | Hydrogen bond1(His372) | 2.93 | |||
| Hydrogen bond1(Arg596) | 3.08 | |||||
| Hydrogen bond1(His600) | 3.10 | |||||
| Hydrogen bond1(Ile406) | 3.37 | |||||
| Pi-anion(Ile673) | 4.68 | |||||
| Pi–Pi T-shaped(Phe359) | 5.11 | |||||
| Alkyl(Ala603) | 4.39 | |||||
| Pi-Alkyl(Ala410) | 4.63 | |||||
| Ascorbic acid | −9.71 | Hydrogen bond1(Val45) | 2.77 | |||
| Hydrogen bond1(Arg184) | 3.32 | |||||
| Hydrogen bond1(Arg184) | 2.79 | |||||
| Hydrogen bond1(Gly41) | 2.69 | |||||
| Hydrogen bond1(Asp37) | 2.59 | |||||
| Hydrogen bond1(Gly41) | 3.34 | |||||
| Quercetin | −11.86 | Hydrogen bond1(Thr364) | 2.95 | −14.82 | Hydrogen bond1(Lys179) | 3.28 |
| Hydrogen bond1(Arg596) | 2.99 | Hydrogen bond1(Ser185) | 2.91 | |||
| Hydrogen bond1(His600) | 2.91 | Hydrogen bond1(Ala174) | 3.33 | |||
| Hydrogen bond2(His432) | 3.70 | Hydrogen bond1(Lys179) | 3.20 | |||
| Pi–Pi T-shaped(Phe359) | 5.30 | Pi-cation(Arg48) | 4.23 | |||
| Pi–Pi T-shaped(Trp599) | 5.36 | Hydrogen Bond3(Trp51) | 3.35 | |||
| Pi-Alkyl(Ala603) | 4.37 | Pi–Pi T-shaped(His175) | 4.48 | |||
| Pi–Pi T-shaped(Phe191 | 5.57 | |||||
| Amide-Pi stacked(Val47/Arg48) | 5.20 | |||||
| Pi-alkyl(Arg48) | 3.96 | |||||
| Pi-alkyl(Arg48) | 3.98 | |||||
| Pi-alkyl(Ala174) | 4.49 | |||||
| Kaempferol | −11.11 | Hydrogen bond1(His600) | 3.36 | −14.27 | Hydrogen bond1(Lys179) | 3.26 |
| Hydrogen bond1(His600) | 2.94 | Hydrogen bond1(His181) | 3.34 | |||
| Hydrogen bond1(Gln363) | 3.02 | Hydrogen bond1(Ala174) | 3.34 | |||
| Hydrogen bond2(His600) | 3.64 | Hydrogen bond1(Lys179) | 3.07 | |||
| Pi–Pi T-shaped(Phe359) | 5.10 | Pi-cation(Arg48) | 4.25 | |||
| Pi–Pi T-shaped(Trp599) | 5.50 | Hydrogen bond3(Trp51) | 3.36 | |||
| Pi-alkyl(Ala603) | 5.02 | Pi–Pi T-shaped(His175) | 4.45 | |||
| Pi–Pi T-shaped(Phe191) | 5.53 | |||||
| Amide-Pi stacked(Val47/Arg48) | 5.25 | |||||
| Pi-alkyl(Arg48) | 3.97 | |||||
| Pi-alkyl(Arg48) | 3.96 | |||||
| Pi-alkyl(Ala174) | 4.57 | |||||
| Gallic acid | −10.72 | Hydrogen bond1(Arg596) | 3.06 | −10.56 | Hydrogen bond1(Arg48) | 3.16 |
| Hydrogen bond1(Arg596) | 3.04 | Hydrogen bond1(Trp51) | 3.07 | |||
| Hydrogen Bond1(Gln363) | 2.98 | Hydrogen bond1(Lys179) | 3.26 | |||
| Pi–Pi T-shaped(Phe359) | 5.37 | Hydrogen bond1(Lys179) | 2.98 | |||
| Pi-Alkyl(Ala603) | 5.14 | Hydrogen bond1(Lys179) | 3.07 | |||
| Hydrogen bond1(Pro44)) | 3.24 | |||||
| Pi–Pi T-shaped(Phe191) | 5.45 | |||||
| Pi-Alkyl(Arg48) | 4.02 | |||||
| 2,4-Dihydroxybenzoic acid | −11.15 | Hydrogen bond1(Gln557) | 3.10 | −10.98 | Hydrogen bond1(Lys179) | 2.96 |
| Hydrogen bond1(Arg596) | 3.11 | Hydrogen bond1(Arg184) | 3.00 | |||
| Hydrogen bond1(Arg596) | 3.34 | Hydrogen bond1(Arg184) | 3.20 | |||
| Hydrogen bond1(Arg596) | 3.17 | Hydrogen bond1(Gly41)) | 2.50 | |||
| Hydrogen bond1(Gln363) | 2.86 | Pi-Cation(His181) | 4.74 | |||
| Hydrogen bond1(His600) | 3.12 | Pi–Pi T-shaped(His181) | 4.79 | |||
| Pi–Pi T-shaped(Phe359) | 5.26 | Pi-Alkyl(Pro44) | 4.63 | |||
| Pi-Alkyl(Ala603) | 4.90 | Pi-Alkyl(Val45) | 4.55 | |||
| Protocatechuic acid | −10.36 | Hydrogen bond1(Arg596) | 3.01 | −12.10 | Hydrogen bond1(Arg48) | 2.92 |
| Hydrogen bond1(Arg596) | 3.04 | Hydrogen bond1(Arg184) | 2.71 | |||
| Hydrogen bond1(Gln359) | 2.89 | Hydrogen Bond2(Thr180) | 3.79 | |||
| Pi–Pi T-shaped(Phe359) | 5.23 | Pi-cation(Arg48) | 2.93 | |||
| Pi-alkyl(Ala603) | 5.15 | Pi-alkyl(Arg184) | 4.70 | |||
| Vanillic acid | −9.19 | Hydrogen bond1(Arg596) | 3.12 | −10.81 | Hydrogen bond1(His181) | 2.91 |
| Hydrogen bond1(Arg596) | 3.19 | Hydrogen bond1(Ala83) | 2.73 | |||
| Hydrogen bond1(His600) | 2.91 | Hydrogen bond2(Arg184) | 3.27 | |||
| Hydrogen bond1(Gln363) | 3.00 | Hydrogen bond4(Arg184) | 3.67 | |||
| Hydrogen bond2(Arg596) | 3.38 | Pi-Alkyl(Arg48) | 4.90 | |||
| Pi–Pi T-shaped(Phe359) | 5.41 | Pi-Alkyl(Arg184) | 5.46 | |||
| Pi-Alkyl(Ala603) | 4.75 | |||||
| 4-Hydroxybenzoic acid | −11.10 | Hydrogen bond1(Gln557) | 3.24 | −9.92 | Hydrogen bond1(Lys179) | 2.96 |
| Hydrogen bond1(Arg596) | 2.96 | Hydrogen bond1(Arg184) | 3.02 | |||
| Hydrogen bond1(Arg596) | 3.20 | Hydrogen bond1(Arg184) | 3.20 | |||
| Hydrogen bond1(His600) | 2.89 | Hydrogen bond1(Gly41) | 3.13 | |||
| Hydrogen bond1(Gln363) | 2.80 | Hydrogen bond1(Lys179) | 3.10 | |||
| Pi–Pi T-shaped(Phe359) | 5.12 | Pi-cation(His181) | 4.71 | |||
| Pi-Alkyl(Ala603) | 4.59 | Pi–Pi T-shaped(His181) | 4.77 | |||
| Pi-Alkyl(Pro44) | 4.62 | |||||
| Pi-Alkyl(Val45) | 4.61 | |||||
| 2,5-Dihydroxybenzoic acid (gentisic acid) | −10.74 | Hydrogen bond1(Arg596) | 3.20 | −11.49 | Hydrogen bond1(His181) | 2.79 |
| Hydrogen bond1(Arg596) | 3.09 | Hydrogen bond1(Lys179) | 2.58 | |||
| Hydrogen bond1(Gln363) | 3.00 | Hydrogen bond1(Ala83) | 2.51 | |||
| Hydrogen bond1(His600) | 3.02 | Hydrogen bond1(Arg184) | 3.37 | |||
| Pi–Pi T-shaped(Phe359) | 5.34 | Hydrogen bond1(Ala83) | 2.51 | |||
| Pi-Alkyl(Ala603) | 4.65 | Hydrogen bond2(Thr180) | 3.16 | |||
| Hydrogen bond2(Ser185) | 3.22 | |||||
| Hydrogen bond4(Arg184) | 3.93 | |||||
| Pi-Alkyl(Arg184) | 4.26 | |||||
| Caffeic acid | −10.75 | Hydrogen bond1(Thr364) | 3.29 | −11.18 | Pi-Cation(His175) | 4.81 |
| Hydrogen bond1(his432) | 3.06 | Pi–Pi T-shaped(His175) | 3.86 | |||
| Hydrogen bond1(Arg596) | 3.26 | Pi-Alkyl(Arg48) | 5.34 | |||
| Hydrogen bond1(Arg596) | 3.38 | |||||
| Hydrogen bond1(His600) | 3.15 | |||||
| Pi–Pi T-shaped (Phe359) | 5.57 | |||||
| 4-Hydroxycinnamic acid | −8.69 | Hydrogen bond1(His432) | 3.18 | −10.90 | Hydrogen bond1(Ser185) | 2.99 |
| Hydrogen bond1(Gln363) | 2.92 | Hydrogen bond1(Pro44) | 3.10 | |||
| Hydrogen bond1(His600) | 3.35 | Hydrogen bond2(Arg184) | 3.77 | |||
| Pi-Alkyl (Agr48) | 4.08 | |||||
1Conventional hydrogen bond.
2Carbon hydrogen bond.
3Pi-donor hydrogen bond.
4Pi-cation; Pi-donor hydrogen bond.
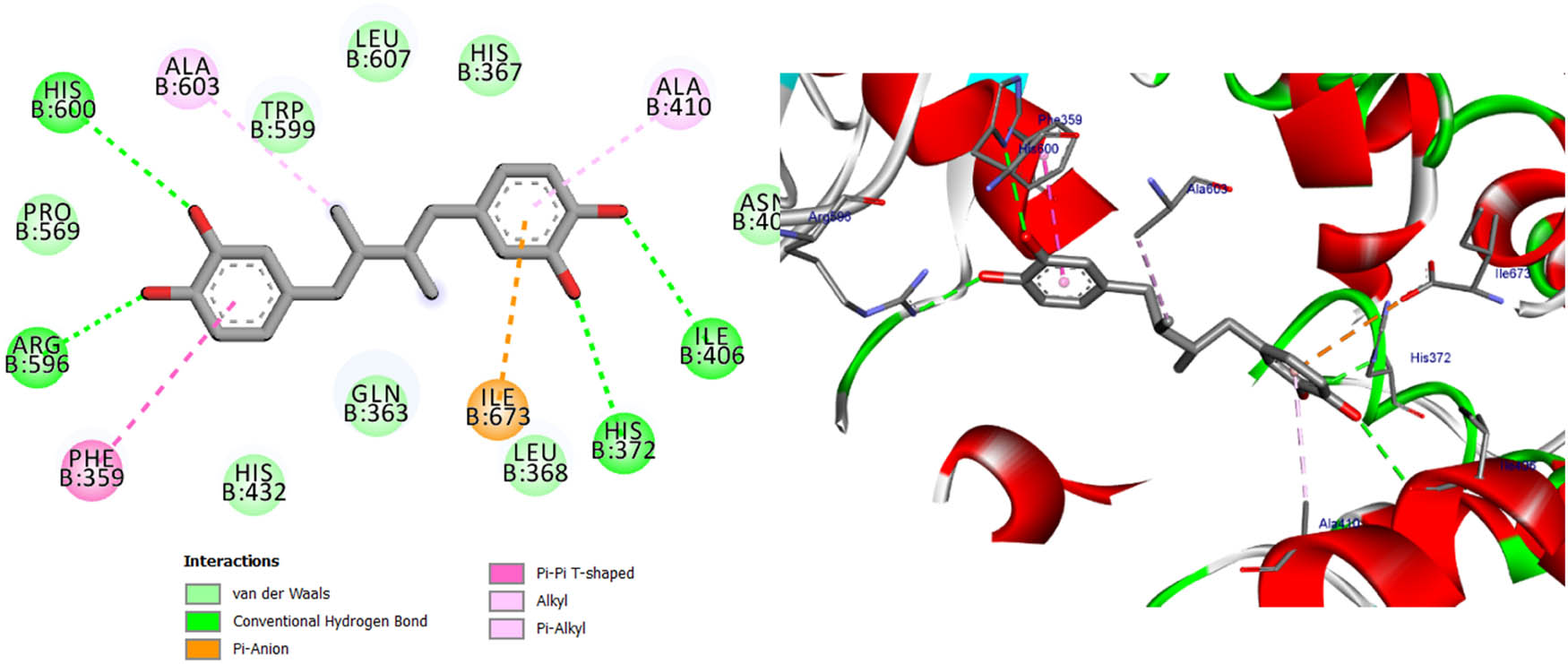
Two- and three-dimensional protein/ligand interaction between bioactive compound NDGA, and arachidonate-5-LOX (ALOX5, PDB: 6n2w).

Two- and three-dimensional protein/ligand interaction between bioactive compound Quercetin, and arachidonate-5-LOX (ALOX5, PDB: 6n2w).
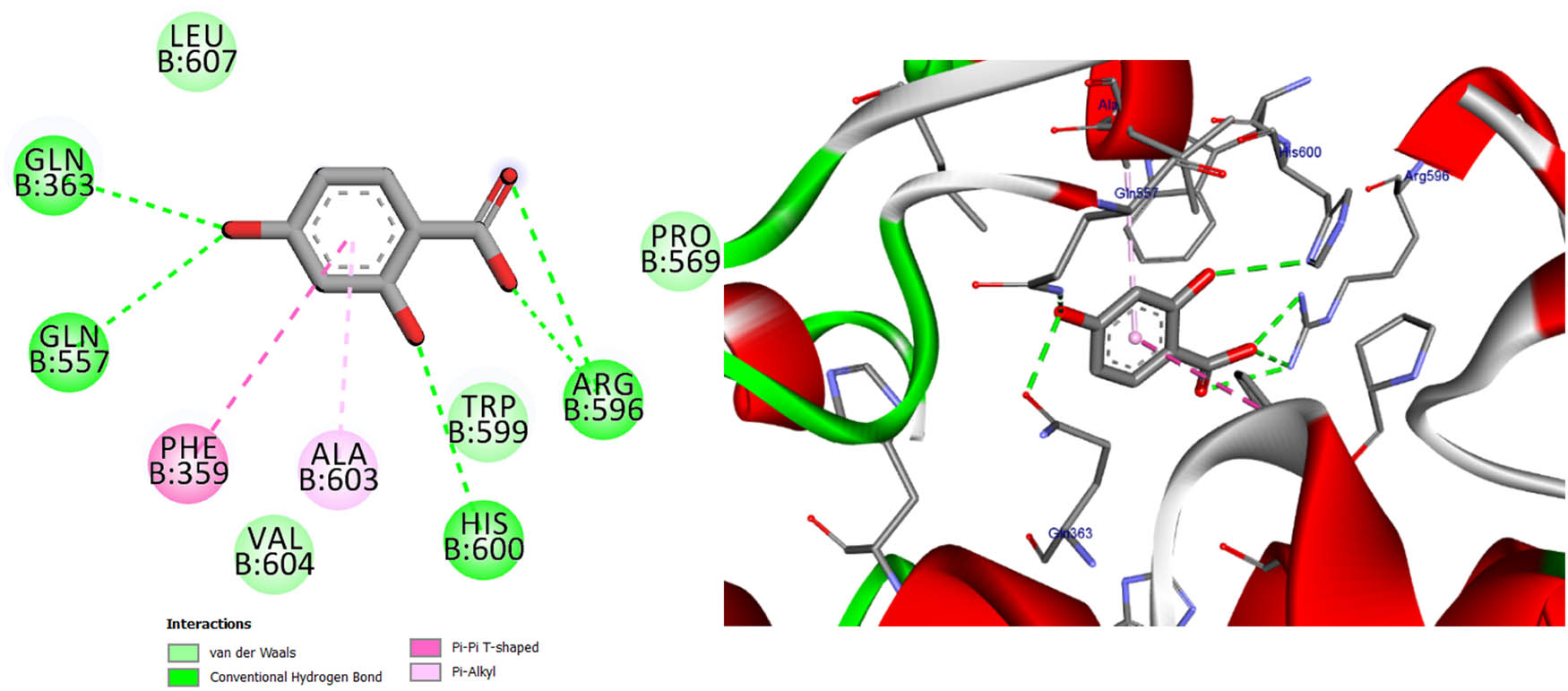
Two- and three-dimensional protein/ligand interaction between 2,4-dihydroxybenzoic acid and arachidonate-5-LOX (ALOX5, PDB: 6n2w).

Two- and three-dimensional protein/ligand interaction between kaempferol and arachidonate-5-LOX (ALOX5, PDB: 6n2w).

Two- and three-dimensional protein/ligand interaction between ascorbic acid and cytochrome c peroxidase (PDB: 2x08).

Two- and three-dimensional protein/ligand interaction between quercetin and cytochrome c peroxidase (PDB: 2x08).

Two- and three-dimensional protein/ligand interaction between kaempferol and cytochrome c peroxidase (PDB: 2x08).

Two- and three-dimensional protein/ligand interaction between protocatechuic acid and cytochrome c peroxidase (PDB: 2x08).
4.2.1 Docking into LOX
LOXs are a class of enzymes responsible for facilitating the oxidation process of polyunsaturated fatty acids and lipids that possess a cis, cis-l.4-pentadiene structure [43]. LOXs are enzymes involved in the oxidation of lipids, and their role in the generation of oxidized lipids within atherosclerotic lesions is widely acknowledged. The enzyme known as arachidonate 5-LOX is of significant importance in the process of leukotriene production. The excessive activation of the 5-LOX pathway leads to the production of an abundance of leukotrienes and lipoxins. These bioactive molecules possess high cytotoxic properties and play a significant role in various pathophysiological conditions such as cancer, psoriasis, and atherosclerosis [44].
The involvement of oxidative stress has been suggested in the pathophysiology of various diseases. The inhibition of 5-LOX has been shown to provide cellular protection against oxidative stress [45]. The active site of 5-LOX consists of certain amino acid residues, namely His367, His372, His550, Leu673, Gln363, Ala410, His432, Gln557, and His600 [46]. The co-crystallized ligand NDGA was re-docked into its corresponding enzyme, resulting in a measured root mean square deviation (RMSD) of 0.62 Å between the docked and co-crystallized ligand. This finding indicates the validity of the docking approach employed. The ligand NDGA, which was co-crystalized, exhibits a docking score of −11.11 kcal/mol. It forms hydrogen bonds with amino acid residues His372, Arg596, His600, and Ile406. Additionally, it engaged in four hydrophobic interactions with Ile673, Phe359, Ala603, and Ala410, as depicted in Figure 11. All the compounds that were chosen for this study exhibit binding within the active site of 5-LOX in a manner similar to that of the co-crystal ligand, NDGA, as depicted in Figure 1. In addition, the binding energy of the ten compounds exhibited a range of −8.69 to −11.86 kcal/mol. The majority of compounds exhibited a Pi–Pi T-shaped link with Phe359, a Pi-Alkyl bond with Ala603, and one or more hydrogen bonds with Gln363, Arg596, and His600, as indicated in Table 12 and Table S2. We conducted a search to identify the optimal conformations that exhibited the lowest binding energy (∆G, kcal/mol) for quercetin and 2,4-dihydroxybenzoic acid. In Table 12, it can be observed that quercetin had a binding energy of −11.86 kcal/mol, whereas 2,4-dihydroxybenzoic acid displayed a binding value of −11.15 kcal/mol. Both compounds exhibited hydrogen bond interactions with specific amino acid residues, namely Arg596 and His600, in the inhibition of 5-LOX. For quercetin, the hydrogen bond distances were measured at 2.99 Å for Arg596 and 2.91 Å for His600. In the case of 2,4-dihydroxybenzoic acid, the hydrogen bond distances were recorded at 3.11, 3.34, and 3.34 Å for Arg596 and 3.12 Å for His600. These findings are depicted in Figures 12 and 14. Furthermore, it was seen that quercetin formed two hydrogen connections with Thr364 and His432, as well as a Pi–Pi T-shaped interaction with Trp599. In contrast, it was shown that 2,4-dihydroxybenzoic acid established a total of two hydrogen bonds with Gln557 and Gln363. Kaempferol had a docking score equivalent to that of NDGA (−11.11 kcal/mol). Its stabilization was facilitated by three hydrogen bonding interactions involving the 4-hydroxyl group and the amino acid His600. In addition, it was shown that the compound is known as Kaempferol, while in the form of quercitrin, exhibited the formation of two Pi–Pi T-shaped connections with amino acid residues Phe359 and Trp599, as well as a Pi-Alkyl link with Ala603 (as depicted in Figure 14).
4.2.2 Docking into cytochrome c peroxidase enzyme
In addition to its primary function, cytochrome c peroxidase has the ability to facilitate the oxidation of several substrate molecules in the presence of hydrogen peroxide (H2O2). The peroxidase activity has garnered significant attention owing to its involvement in the process of apoptosis [47]. The binding scores of our candidates (−9.92, −14.82 kcal/mol) were found to be highly promising when compared to the binding score of ascorbic acid (−9.71 kcal/mol), as presented in Table 12. Based on the obtained data, it may be inferred that the aforementioned compounds exhibit stabilization within the binding pocket of cytochrome c peroxidase. The RMSD between the original and docked poses of ascorbic acid in the peroxidase enzyme (PDB: 2x08) was determined to be 0.20 Å. Furthermore, the stabilization of ascorbic acid occurred within the binding pocket of cytochrome c peroxidase through the formation of six hydrogen bonds with specific amino acid residues. These residues include Val45 (at a distance of 2.77 Å), Gly41 (at distances of 2.69 and 3.34 Å), Asp37 (at a distance of 2.59 Å), and Arg184 (at distances of 3.32 and 2.79 Å) (refer to Figure 15). The stabilization of candidates inside the binding pocket was achieved through their interaction with essential amino acids, as illustrated in Table 12 and Table S3. The docking scores of quercetin and kaempferol were found to be −14.82 and −14.27 kcal/mol, respectively. These compounds exhibited strong binding affinities to important amino acids, namely Lys179, Ala174, and Trp51, through the formation of five hydrogen bonds. Additionally, they formed three Pi-Alkyl bonds with Arg48 and Ala174, and two Pi–Pi T-shaped bonds with His175 and Phe191. Furthermore, a Pi-cation bond was observed between these compounds and Arg48, with a distance of 4.23 Å. Additionally, it was observed that quercetin and kaempferol exhibited an Amid-Pi stacking interaction with Val47 and Arg48 at a distance of 5.20 Å, as depicted in Figures 16 and 17. The binding of protocatechuic acid occurred by the formation of three hydrogen bonds with Arg48, Arg184, and Thr180, at distances of 2.92, 2.71, and 3.79 Å, respectively. Further, it was observed that protocatechuic acid formed a Pi-cation bond with Arg48 at a distance of 2.93 Å and a Pi-alkyl bond with Arg184 at a distance of 4.70 Å, as depicted in Figure 18. The binding energy value of gentisic acid was determined to be −11.49 kcal/mol. This compound was found to be bound to crucial amino acid residues, including Lys179, Arg184, His181, Ala83, Thr180, and Ser185, through a total of eight hydrogen bonds, as indicated in Table S3. Ultimately, the majority of the compounds were bound by one or more H-bonds and/or hydrophobic bonds to the vital amino acids Arg48, Lys179, and Arg184. The receptor interactions of substances with the (2×08) protein were analyzed in both two-dimensional (2D) and three-dimensional (3D) formats. The results of these analyses are shown in Table 12. Additionally, for more comprehensive examination, the corresponding figures illustrating these interactions may be found in the Supplementary Materials, specifically in Table S3.
5 Conclusions
In conclusion, this study highlights the potential of S. nigrum Linn. as a valuable natural source of antioxidants. The comparative phytochemical profiling revealed the presence of various bioactive components, including steroidal saponins, alkaloids, flavonoids, phenols, and proanthocyanidins, in the Algerian S. nigrum samples. The assessment of antioxidant activity through DPPH, FRAP, and oxidative hemolysis inhibition assays demonstrated the substantial antioxidant potential of the three samples. These findings support the notion that S. nigrum can effectively scavenge free radicals and mitigate oxidative stress.
Furthermore, the in silico molecular docking analysis provided insights into the potential mechanism of action of S. nigrum’s antioxidant activity. The docking scores indicated a significant affinity for the bioactive compounds, particularly quercetin, and kaempferol, with the binding sites of selected proteins, arachidonate-5-lipoxygenase, and cytochrome c peroxidase. This suggests that these compounds may contribute significantly to the antioxidant action of S. nigrum.
Overall, the comprehensive investigation of the phytochemical composition, antioxidant activity, and molecular docking analysis enhances our understanding of the therapeutic potential of S. nigrum in combating oxidative stress-related diseases such as arthritis, asthma, dementia, and aging. The findings of this study provide a foundation for further research and development of antioxidant-based therapies utilizing natural sources like S. nigrum for improved healthcare and well-being.
-
Funding information: Authors state no funding involved.
-
Author contributions: Conceptualization: A.A. and F.M.A.; methodology: A.A., Kh.A., H.Z., F.M.A., and M.H.I.; software: A.A., M.H.I., A.V.A., and M.M.; validation: D.H., A.R., and M.M.; formal analysis: Kh.A., H.Z., M.M., and A.A.; investigation: A.A., F.M.A., and M.H.I.; resources: D.H., A.R., and M.M.; data curation: A.A. and M.H.I.; writing – original draft preparation: F.M.A., A.A., M.H.I., and M.M.; writing – review and editing: F.M.A., A.A., A.V.A., and M.H.I.; and funding acquisition. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Tanabe S, O’Brien J, Tollefsen KE, Kim Y, Chauhan V, Yauk C, et al. Reactive oxygen species in the adverse outcome pathway framework: Toward creation of harmonized consensus key events. Front Toxicol. 2022;4:887135.10.3389/ftox.2022.887135Search in Google Scholar PubMed PubMed Central
[2] Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94(2):329–54.10.1152/physrev.00040.2012Search in Google Scholar PubMed PubMed Central
[3] Singh P, Mahadi F, Roy A, Sharma PB. Reactive oxygen species, reactive nitrogen species and antioxidants in etiopathogenesis of diabetes mellitus type-2. Indian J Clin Biochem. 2009;24:324–42.10.1007/s12291-009-0062-6Search in Google Scholar PubMed PubMed Central
[4] Adebiyi OE, Olayemi FO, Ning-Hua T, Guang-Zhi ZB. Sciences A. In vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewia carpinifolia. Beni-Suef Univ J Basic Appl Sci. 2017;6(1):10–4.10.1016/j.bjbas.2016.12.003Search in Google Scholar
[5] Maruca A, Catalano R, Bagetta D, Mesiti F, Ambrosio FA, Romeo I, et al. The mediterranean diet as source of bioactive compounds with multi-targeting anti-cancer profile. Eur J Med Chem. 2019;181:111579.10.1016/j.ejmech.2019.111579Search in Google Scholar PubMed
[6] Alam MK, Rana ZH, Islam SN, Akhtaruzzaman MJP. Total phenolic content and antioxidant activity of methanolic extract of selected wild leafy vegetables grown in Bangladesh: A cheapest source of antioxidants. Potravinarstvo. 2019;13:1.10.5219/1107Search in Google Scholar
[7] Baba SA, Malik SA. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J Taibah Univ Sci. 2015;9(4):449–54.10.1016/j.jtusci.2014.11.001Search in Google Scholar
[8] Sharifi-Rad M, Anil Kumar NV, Zucca P, Varoni EM, Dini L, Panzarini E, et al. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front Physiol. 2020;11:694.10.3389/fphys.2020.00694Search in Google Scholar PubMed PubMed Central
[9] Krishnaiah D, Sarbatly R, Nithyanandam RJF. Processing b. A review of the antioxidant potential of medicinal plant species. Food Bioprod Process. 2011;89(3):217–33.10.1016/j.fbp.2010.04.008Search in Google Scholar
[10] Yu M, Gouvinhas I, Rocha J, Barros A. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci Rep. 2021;11(1):10041.10.1038/s41598-021-89437-4Search in Google Scholar PubMed PubMed Central
[11] Emmanuela N, Muhammad DR, Iriawati, Wijaya CH, Ratnadewi YMD, Takemori H, et al. Isolation of plant-derived exosome-like nanoparticles (PDENs) from Solanum nigrum L. berries and Their Effect on interleukin-6 expression as a potential anti-inflammatory agent. PLoS One. 2024;19(1):e0296259.10.1371/journal.pone.0296259Search in Google Scholar PubMed PubMed Central
[12] Guo T, He D, Liu Y, Li J, Wang F. Lanthanum promotes Solanum nigrum L. growth and phytoremediation of cadmium and lead through endocytosis: Physiological and biochemical response, heavy metal uptake and visualization. Sci Total Environ. 2024;912:168915.10.1016/j.scitotenv.2023.168915Search in Google Scholar PubMed
[13] Dai G, Shi H, McBride MB, Fu H, Li Z, Wang X, et al. Biogeochemical processes in heterogeneous soil-Solanum nigrum L. system control lead partitioning: Roles of strengite and oxalated zero-valent iron nanoparticle. J Clean Prod. 2024;450:141993.10.1016/j.jclepro.2024.141993Search in Google Scholar
[14] Wang X, Sun Z, Wang X, Li M, Zhou B, Zhang X. Solanum nigrum L. berries extract ameliorated the alcoholic liver injury by regulating gut microbiota, lipid metabolism, inflammation, and oxidative stress. Food Res Int. 2024;188:114489.10.1016/j.foodres.2024.114489Search in Google Scholar PubMed
[15] Pradhan S, Huidrom P. Antimicrobial properties and phytochemical analysis of some medicinal plants: A review. Int J Sci Res. 2024;13:508–11.10.21275/SR24105144616Search in Google Scholar
[16] Aouadi A, Saoud DH, Rebiai A, ABD FM, Hadjadj S, Mustafa AA. Impact of different extraction solvents and concentrations on the total phenolics content and bioactivity of the Algerian lemongrass (Cymbopogon citratus) extracts. Ovidius Univ Ann Chem. 2024;35:16–26.10.2478/auoc-2024-0003Search in Google Scholar
[17] Aouadi A, Saoud DH, Bouafia A, Mohammed HA, Gamal HG, Achouri A, et al. Unveiling the antioxidant power: synthesis and characterization of lemon and orange peel-derived carbon quantum dots with exceptional free radical scavenging activity. Biomass Convers Biorefin. 2024;33:1–14.10.1007/s13399-024-05765-1Search in Google Scholar
[18] Hailong M, Rui B, Xiao W, Hongli Y. Content determination of alkaloids and catalpol in different proportions of Qianjin Huanglian Pills. Her Med. 2024;43(4):601–6.Search in Google Scholar
[19] Zhao X, Ren D, Jin R, Chen W, Xu L, Guo D, et al. Development of UHPLC-Q-exactive Orbitrap/MS technique for determination of proanthocyanidins (PAs) monomer composition content in persimmon. Plants. 2024;13(11):1440.10.3390/plants13111440Search in Google Scholar PubMed PubMed Central
[20] Zhang P, Wu Q, Wang Y, Huang Y, Xie M, Fan LJL. Rapid detection of tannin content in wine grapes using hyperspectral technology. Life. 2024;14(3):416.10.3390/life14030416Search in Google Scholar PubMed PubMed Central
[21] Irina Z, Irina P, Dmitriy E, Inessa P, Alla C, Alena P, et al. Assessment of vitamin-and mineral-content stability of tomato fruits as a potential raw material to produce functional food. Funct Foods Health Dis. 2024;14(1):14–32.10.31989/ffhd.v14i1.1259Search in Google Scholar
[22] Aouadi A, Hamada Saud D, Rebiai A, Achouri A, Benabdesselam S, Mohamed Abd El-Mordy F, et al. Introducing the antibacterial and photocatalytic degradation potentials of biosynthesized chitosan, chitosan–ZnO, and chitosan–ZnO/PVP nanoparticles. Sci Rep. 2024;14(1):14753.10.1038/s41598-024-65579-zSearch in Google Scholar PubMed PubMed Central
[23] Aouadi A, Saoud DH, Laouini SE, Rebiai A, Achouri A, Mohammed HA, et al. Synergistic chitin-zinc nanocomposites from shrimp shell waste: characterization, antioxidant, and antibacterial properties. Biomass Convers Biorefinery. 2023;27:1–17.10.1007/s13399-023-05237-ySearch in Google Scholar
[24] Carducci M, Whitcombe A, Rovetini L, Massai L, Keeley AJ, de Silva TI, et al. Development and characterization of a hemolysis inhibition assay to determine functionality of anti-Streptolysin O antibodies in human sera. J Immunol Methods. 2024;526:113618.10.1016/j.jim.2024.113618Search in Google Scholar PubMed PubMed Central
[25] Hussain S, Zahid A, Imran M, Massey S, Riaz M, Sagir M, et al. Unveiling the chemical profile, synergistic antibacterial and hemolytic effects of Cymbopogon citratus and Tachyspermum ammi leaves. Biocatal Agric Biotechnol. 2024;58:103221.10.1016/j.bcab.2024.103221Search in Google Scholar
[26] Chouikh A, Alia F, Neffar S, Rebiai A, Adjal EH, Chefrour A. Evaluation of phenolic contents (quantitative and qualitative) and antioxidant activities in different physiological phases of Genista saharae Coss. & Dur. growing in the Sahara of Algeria. Fascicula Biologie. 2018;25(2):83–9.Search in Google Scholar
[27] Ismail M, Husseiny E, Ibrahim M. Mimicry of sorafenib: novel diarylureas as VEGFR2 inhibitors and apoptosis inducers in breast cancer. 2023.10.1039/D3NJ01638BSearch in Google Scholar
[28] Harras M, Sabour R, Farghaly T, Ibrahim M. Drug repurposing approach in developing new furosemide analogs as antimicrobial candidates and Anti-PBP: design, synthesis, and molecular docking. Bioorg Chem. 2023;137:106585.10.1016/j.bioorg.2023.106585Search in Google Scholar PubMed
[29] Shanmugapriya K, Saravana P, Payal H, Mohammed SP. Binnie WJIJoP, Sciences P. Antioxidant activity, total phenolic and flavonoid contents of Artocarpus heterophyllus and Manilkara zapota seeds and its reduction potential. Int J Pharm Pharm Sci. 2011;3(5):256–60.Search in Google Scholar
[30] Singleton VL, Orthofer R, Lamuela-Raventós RM. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in enzymology. 299. Academic press; 1999. p. 152–78.10.1016/S0076-6879(99)99017-1Search in Google Scholar
[31] Pareek K, Jagasia PV. Determination of the antioxidant content in selected medicinal plants of Kalyan and Kolhapur. Int Res J Adv Eng Manag (IRJAEM). 2024;2(3):290–8.10.47392/IRJAEM.2024.0043Search in Google Scholar
[32] Realini FM, Escobedo VM, Ueno AC, Bastías DA, Schardl CL, Biganzoli F, et al. Anti-herbivory defences delivered by Epichloë fungal endophytes: a quantitative review of alkaloid concentration variation among hosts and plant parts. Ann Botany. 2024;133(4):509–20.10.1093/aob/mcae014Search in Google Scholar PubMed PubMed Central
[33] Arar A. Determination of the Phytochemical, Antioxidant and Antibacterial activitiesof Arbutus andrachne L. Methanolic Leaf and Fruit Extract. Hebron University DSpace Repository; 2024.Search in Google Scholar
[34] Baliyan S, Mukherjee R, Priyadarshini A, Vibhuti A, Gupta A, Pandey RP, et al. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules. 2022;27(4):1326.10.3390/molecules27041326Search in Google Scholar PubMed PubMed Central
[35] Lu X, Zhu K, Zhang M, Liu H, Kang J. Voltammetric studies of the interaction of transition-metal complexes with DNA. J Biochem Bioph Meth. 2002;52(3):189–200.10.1016/S0165-022X(02)00074-XSearch in Google Scholar
[36] Benesi HA, Hildebrand J. A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J Am Chem Soc. 1949;71(8):2703–7.10.1021/ja01176a030Search in Google Scholar
[37] Payne AC, Mazzer A, Clarkson GJ, Taylor G. Antioxidant assays - consistent findings from FRAP and ORAC reveal a negative impact of organic cultivation on antioxidant potential in spinach but not watercress or rocket leaves. Food Sci Nutr. 2013;1(6):439–44.10.1002/fsn3.71Search in Google Scholar PubMed PubMed Central
[38] Kebal L, Djebli N, Pokajewicz K, Mostefa N, Wieczorek PP. Antioxidant activity and effectiveness of fig extract in counteracting carbon tetrachloride-induced oxidative damage in rats. Molecules (Basel, Switz). 2024;29(9):1997.10.3390/molecules29091997Search in Google Scholar PubMed PubMed Central
[39] El Kamari F, Zouirech O, Metouekel A, Bouslamti M, Maliki I, El Moussaoui A, et al. Chemical Profiling and antioxidant, antimicrobial, and hemolytic properties of Euphorbia calyptrata (l.) Essential oils: in Vitro and in Silico analysis. ChemistryOpen. 2024;13:e202300243.10.1002/open.202300243Search in Google Scholar PubMed PubMed Central
[40] Chen X, Dai X, Liu Y, Yang Y, Yuan L, He X, et al. Solanum nigrum Linn.: an insight into current research on traditional uses, phytochemistry, and pharmacology. Front Pharmacol. 2022;13:918071.10.3389/fphar.2022.918071Search in Google Scholar PubMed PubMed Central
[41] Daina A, Michielin O, Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717.10.1038/srep42717Search in Google Scholar PubMed PubMed Central
[42] Zhao YH, Abraham MH, Le J, Hersey A, Luscombe CN, Beck G, et al. Rate-limited steps of human oral absorption and QSAR studies. Pharm Res. 2002;19(10):1446–57.10.1023/A:1020444330011Search in Google Scholar
[43] Singh P, Arif Y, Miszczuk E, Bajguz A, Hayat S. Specific roles of lipoxygenases in development and responses to stress in plants. Plants. 2022;11:979.10.3390/plants11070979Search in Google Scholar PubMed PubMed Central
[44] Mashima R, Okuyama T. The role of lipoxygenases in pathophysiology; new Insights and future Perspectives. Redox Biol. 2015;6:979. REDOXD1500117.10.1016/j.redox.2015.08.006Search in Google Scholar PubMed PubMed Central
[45] Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, et al. Oxidative Stress: harms and benefits for human health. Oxid Med Cell Longev. 2017;2017:1–13.10.1155/2017/8416763Search in Google Scholar PubMed PubMed Central
[46] Liyanaarachchi G, Perera A, Samarasekera J, Mahanama R, Hemalal P, Dlamini S, et al. Bioactive constituents isolated from the Sri Lankan endemic plant Artocarpus nobilis and their potential to use in novel cosmeceuticals. Ind Crop Products. 2022;184:115076.10.1016/j.indcrop.2022.115076Search in Google Scholar
[47] Yin V, Shaw G, Konermann L. Cytochrome c as a peroxidase: activation of the pre-catalytic native state by H2O2-induced covalent modifications. J Am Chem Soc. 2017;139(44):15701–9.10.1021/jacs.7b07106Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Constitutive and evoked release of ATP in adult mouse olfactory epithelium
- LARP1 knockdown inhibits cultured gastric carcinoma cell cycle progression and metastatic behavior
- PEGylated porcine–human recombinant uricase: A novel fusion protein with improved efficacy and safety for the treatment of hyperuricemia and renal complications
- Research progress on ocular complications caused by type 2 diabetes mellitus and the function of tears and blepharons
- The role and mechanism of esketamine in preventing and treating remifentanil-induced hyperalgesia based on the NMDA receptor–CaMKII pathway
- Brucella infection combined with Nocardia infection: A case report and literature review
- Detection of serum interleukin-18 level and neutrophil/lymphocyte ratio in patients with antineutrophil cytoplasmic antibody-associated vasculitis and its clinical significance
- Ang-1, Ang-2, and Tie2 are diagnostic biomarkers for Henoch-Schönlein purpura and pediatric-onset systemic lupus erythematous
- PTTG1 induces pancreatic cancer cell proliferation and promotes aerobic glycolysis by regulating c-myc
- Role of serum B-cell-activating factor and interleukin-17 as biomarkers in the classification of interstitial pneumonia with autoimmune features
- Effectiveness and safety of a mumps containing vaccine in preventing laboratory-confirmed mumps cases from 2002 to 2017: A meta-analysis
- Low levels of sex hormone-binding globulin predict an increased breast cancer risk and its underlying molecular mechanisms
- A case of Trousseau syndrome: Screening, detection and complication
- Application of the integrated airway humidification device enhances the humidification effect of the rabbit tracheotomy model
- Preparation of Cu2+/TA/HAP composite coating with anti-bacterial and osteogenic potential on 3D-printed porous Ti alloy scaffolds for orthopedic applications
- Aquaporin-8 promotes human dermal fibroblasts to counteract hydrogen peroxide-induced oxidative damage: A novel target for management of skin aging
- Current research and evidence gaps on placental development in iron deficiency anemia
- Single-nucleotide polymorphism rs2910829 in PDE4D is related to stroke susceptibility in Chinese populations: The results of a meta-analysis
- Pheochromocytoma-induced myocardial infarction: A case report
- Kaempferol regulates apoptosis and migration of neural stem cells to attenuate cerebral infarction by O‐GlcNAcylation of β-catenin
- Sirtuin 5 regulates acute myeloid leukemia cell viability and apoptosis by succinylation modification of glycine decarboxylase
- Apigenin 7-glucoside impedes hypoxia-induced malignant phenotypes of cervical cancer cells in a p16-dependent manner
- KAT2A changes the function of endometrial stromal cells via regulating the succinylation of ENO1
- Current state of research on copper complexes in the treatment of breast cancer
- Exploring antioxidant strategies in the pathogenesis of ALS
- Helicobacter pylori causes gastric dysbacteriosis in chronic gastritis patients
- IL-33/soluble ST2 axis is associated with radiation-induced cardiac injury
- The predictive value of serum NLR, SII, and OPNI for lymph node metastasis in breast cancer patients with internal mammary lymph nodes after thoracoscopic surgery
- Carrying SNP rs17506395 (T > G) in TP63 gene and CCR5Δ32 mutation associated with the occurrence of breast cancer in Burkina Faso
- P2X7 receptor: A receptor closely linked with sepsis-associated encephalopathy
- Probiotics for inflammatory bowel disease: Is there sufficient evidence?
- Identification of KDM4C as a gene conferring drug resistance in multiple myeloma
- Microbial perspective on the skin–gut axis and atopic dermatitis
- Thymosin α1 combined with XELOX improves immune function and reduces serum tumor markers in colorectal cancer patients after radical surgery
- Highly specific vaginal microbiome signature for gynecological cancers
- Sample size estimation for AQP4-IgG seropositive optic neuritis: Retinal damage detection by optical coherence tomography
- The effects of SDF-1 combined application with VEGF on femoral distraction osteogenesis in rats
- Fabrication and characterization of gold nanoparticles using alginate: In vitro and in vivo assessment of its administration effects with swimming exercise on diabetic rats
- Mitigating digestive disorders: Action mechanisms of Mediterranean herbal active compounds
- Distribution of CYP2D6 and CYP2C19 gene polymorphisms in Han and Uygur populations with breast cancer in Xinjiang, China
- VSP-2 attenuates secretion of inflammatory cytokines induced by LPS in BV2 cells by mediating the PPARγ/NF-κB signaling pathway
- Factors influencing spontaneous hypothermia after emergency trauma and the construction of a predictive model
- Long-term administration of morphine specifically alters the level of protein expression in different brain regions and affects the redox state
- Application of metagenomic next-generation sequencing technology in the etiological diagnosis of peritoneal dialysis-associated peritonitis
- Clinical diagnosis, prevention, and treatment of neurodyspepsia syndrome using intelligent medicine
- Case report: Successful bronchoscopic interventional treatment of endobronchial leiomyomas
- Preliminary investigation into the genetic etiology of short stature in children through whole exon sequencing of the core family
- Cystic adenomyoma of the uterus: Case report and literature review
- Mesoporous silica nanoparticles as a drug delivery mechanism
- Dynamic changes in autophagy activity in different degrees of pulmonary fibrosis in mice
- Vitamin D deficiency and inflammatory markers in type 2 diabetes: Big data insights
- Lactate-induced IGF1R protein lactylation promotes proliferation and metabolic reprogramming of lung cancer cells
- Meta-analysis on the efficacy of allogeneic hematopoietic stem cell transplantation to treat malignant lymphoma
- Mitochondrial DNA drives neuroinflammation through the cGAS-IFN signaling pathway in the spinal cord of neuropathic pain mice
- Application value of artificial intelligence algorithm-based magnetic resonance multi-sequence imaging in staging diagnosis of cervical cancer
- Embedded monitoring system and teaching of artificial intelligence online drug component recognition
- Investigation into the association of FNDC1 and ADAMTS12 gene expression with plumage coloration in Muscovy ducks
- Yak meat content in feed and its impact on the growth of rats
- A rare case of Richter transformation with breast involvement: A case report and literature review
- First report of Nocardia wallacei infection in an immunocompetent patient in Zhejiang province
- Rhodococcus equi and Brucella pulmonary mass in immunocompetent: A case report and literature review
- Downregulation of RIP3 ameliorates the left ventricular mechanics and function after myocardial infarction via modulating NF-κB/NLRP3 pathway
- Evaluation of the role of some non-enzymatic antioxidants among Iraqi patients with non-alcoholic fatty liver disease
- The role of Phafin proteins in cell signaling pathways and diseases
- Ten-year anemia as initial manifestation of Castleman disease in the abdominal cavity: A case report
- Coexistence of hereditary spherocytosis with SPTB P.Trp1150 gene variant and Gilbert syndrome: A case report and literature review
- Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells
- Exploratory evaluation supported by experimental and modeling approaches of Inula viscosa root extract as a potent corrosion inhibitor for mild steel in a 1 M HCl solution
- Imaging manifestations of ductal adenoma of the breast: A case report
- Gut microbiota and sleep: Interaction mechanisms and therapeutic prospects
- Isomangiferin promotes the migration and osteogenic differentiation of rat bone marrow mesenchymal stem cells
- Prognostic value and microenvironmental crosstalk of exosome-related signatures in human epidermal growth factor receptor 2 positive breast cancer
- Circular RNAs as potential biomarkers for male severe sepsis
- Knockdown of Stanniocalcin-1 inhibits growth and glycolysis in oral squamous cell carcinoma cells
- The expression and biological role of complement C1s in esophageal squamous cell carcinoma
- A novel GNAS mutation in pseudohypoparathyroidism type 1a with articular flexion deformity: A case report
- Predictive value of serum magnesium levels for prognosis in patients with non-small cell lung cancer undergoing EGFR-TKI therapy
- HSPB1 alleviates acute-on-chronic liver failure via the P53/Bax pathway
- IgG4-related disease complicated by PLA2R-associated membranous nephropathy: A case report
- Baculovirus-mediated endostatin and angiostatin activation of autophagy through the AMPK/AKT/mTOR pathway inhibits angiogenesis in hepatocellular carcinoma
- Metformin mitigates osteoarthritis progression by modulating the PI3K/AKT/mTOR signaling pathway and enhancing chondrocyte autophagy
- Evaluation of the activity of antimicrobial peptides against bacterial vaginosis
- Atypical presentation of γ/δ mycosis fungoides with an unusual phenotype and SOCS1 mutation
- Analysis of the microecological mechanism of diabetic kidney disease based on the theory of “gut–kidney axis”: A systematic review
- Omega-3 fatty acids prevent gestational diabetes mellitus via modulation of lipid metabolism
- Refractory hypertension complicated with Turner syndrome: A case report
- Interaction of ncRNAs and the PI3K/AKT/mTOR pathway: Implications for osteosarcoma
- Association of low attenuation area scores with pulmonary function and clinical prognosis in patients with chronic obstructive pulmonary disease
- Long non-coding RNAs in bone formation: Key regulators and therapeutic prospects
- The deubiquitinating enzyme USP35 regulates the stability of NRF2 protein
- Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as potential diagnostic markers for rebleeding in patients with esophagogastric variceal bleeding
- G protein-coupled receptor 1 participating in the mechanism of mediating gestational diabetes mellitus by phosphorylating the AKT pathway
- LL37-mtDNA regulates viability, apoptosis, inflammation, and autophagy in lipopolysaccharide-treated RLE-6TN cells by targeting Hsp90aa1
- The analgesic effect of paeoniflorin: A focused review
- Chemical composition’s effect on Solanum nigrum Linn.’s antioxidant capacity and erythrocyte protection: Bioactive components and molecular docking analysis
- Knockdown of HCK promotes HREC cell viability and inner blood–retinal barrier integrity by regulating the AMPK signaling pathway
- The role of rapamycin in the PINK1/Parkin signaling pathway in mitophagy in podocytes
- Laryngeal non-Hodgkin lymphoma: Report of four cases and review of the literature
- Clinical value of macrogenome next-generation sequencing on infections
- Overview of dendritic cells and related pathways in autoimmune uveitis
- TAK-242 alleviates diabetic cardiomyopathy via inhibiting pyroptosis and TLR4/CaMKII/NLRP3 pathway
- Hypomethylation in promoters of PGC-1α involved in exercise-driven skeletal muscular alterations in old age
- Profile and antimicrobial susceptibility patterns of bacteria isolated from effluents of Kolladiba and Debark hospitals
- The expression and clinical significance of syncytin-1 in serum exosomes of hepatocellular carcinoma patients
- A histomorphometric study to evaluate the therapeutic effects of biosynthesized silver nanoparticles on the kidneys infected with Plasmodium chabaudi
- PGRMC1 and PAQR4 are promising molecular targets for a rare subtype of ovarian cancer
- Analysis of MDA, SOD, TAOC, MNCV, SNCV, and TSS scores in patients with diabetes peripheral neuropathy
- SLIT3 deficiency promotes non-small cell lung cancer progression by modulating UBE2C/WNT signaling
- The relationship between TMCO1 and CALR in the pathological characteristics of prostate cancer and its effect on the metastasis of prostate cancer cells
- Heterogeneous nuclear ribonucleoprotein K is a potential target for enhancing the chemosensitivity of nasopharyngeal carcinoma
- PHB2 alleviates retinal pigment epithelium cell fibrosis by suppressing the AGE–RAGE pathway
- Anti-γ-aminobutyric acid-B receptor autoimmune encephalitis with syncope as the initial symptom: Case report and literature review
- Comparative analysis of chloroplast genome of Lonicera japonica cv. Damaohua
- Human umbilical cord mesenchymal stem cells regulate glutathione metabolism depending on the ERK–Nrf2–HO-1 signal pathway to repair phosphoramide mustard-induced ovarian cancer cells
- Electroacupuncture on GB acupoints improves osteoporosis via the estradiol–PI3K–Akt signaling pathway
- Renalase protects against podocyte injury by inhibiting oxidative stress and apoptosis in diabetic nephropathy
- Review: Dicranostigma leptopodum: A peculiar plant of Papaveraceae
- Combination effect of flavonoids attenuates lung cancer cell proliferation by inhibiting the STAT3 and FAK signaling pathway
- Renal microangiopathy and immune complex glomerulonephritis induced by anti-tumour agents: A case report
- Correlation analysis of AVPR1a and AVPR2 with abnormal water and sodium and potassium metabolism in rats
- Gastrointestinal health anti-diarrheal mixture relieves spleen deficiency-induced diarrhea through regulating gut microbiota
- Myriad factors and pathways influencing tumor radiotherapy resistance
- Exploring the effects of culture conditions on Yapsin (YPS) gene expression in Nakaseomyces glabratus
- Screening of prognostic core genes based on cell–cell interaction in the peripheral blood of patients with sepsis
- Coagulation factor II thrombin receptor as a promising biomarker in breast cancer management
- Ileocecal mucinous carcinoma misdiagnosed as incarcerated hernia: A case report
- Methyltransferase like 13 promotes malignant behaviors of bladder cancer cells through targeting PI3K/ATK signaling pathway
- The debate between electricity and heat, efficacy and safety of irreversible electroporation and radiofrequency ablation in the treatment of liver cancer: A meta-analysis
- ZAG promotes colorectal cancer cell proliferation and epithelial–mesenchymal transition by promoting lipid synthesis
- Baicalein inhibits NLRP3 inflammasome activation and mitigates placental inflammation and oxidative stress in gestational diabetes mellitus
- Impact of SWCNT-conjugated senna leaf extract on breast cancer cells: A potential apoptotic therapeutic strategy
- MFAP5 inhibits the malignant progression of endometrial cancer cells in vitro
- Major ozonated autohemotherapy promoted functional recovery following spinal cord injury in adult rats via the inhibition of oxidative stress and inflammation
- Axodendritic targeting of TAU and MAP2 and microtubule polarization in iPSC-derived versus SH-SY5Y-derived human neurons
- Differential expression of phosphoinositide 3-kinase/protein kinase B and Toll-like receptor/nuclear factor kappa B signaling pathways in experimental obesity Wistar rat model
- The therapeutic potential of targeting Oncostatin M and the interleukin-6 family in retinal diseases: A comprehensive review
- BA inhibits LPS-stimulated inflammatory response and apoptosis in human middle ear epithelial cells by regulating the Nf-Kb/Iκbα axis
- Role of circRMRP and circRPL27 in chronic obstructive pulmonary disease
- Investigating the role of hyperexpressed HCN1 in inducing myocardial infarction through activation of the NF-κB signaling pathway
- Characterization of phenolic compounds and evaluation of anti-diabetic potential in Cannabis sativa L. seeds: In vivo, in vitro, and in silico studies
- Quantitative immunohistochemistry analysis of breast Ki67 based on artificial intelligence
- Ecology and Environmental Science
- Screening of different growth conditions of Bacillus subtilis isolated from membrane-less microbial fuel cell toward antimicrobial activity profiling
- Degradation of a mixture of 13 polycyclic aromatic hydrocarbons by commercial effective microorganisms
- Evaluation of the impact of two citrus plants on the variation of Panonychus citri (Acari: Tetranychidae) and beneficial phytoseiid mites
- Prediction of present and future distribution areas of Juniperus drupacea Labill and determination of ethnobotany properties in Antalya Province, Türkiye
- Population genetics of Todarodes pacificus (Cephalopoda: Ommastrephidae) in the northwest Pacific Ocean via GBS sequencing
- A comparative analysis of dendrometric, macromorphological, and micromorphological characteristics of Pistacia atlantica subsp. atlantica and Pistacia terebinthus in the middle Atlas region of Morocco
- Macrofungal sporocarp community in the lichen Scots pine forests
- Assessing the proximate compositions of indigenous forage species in Yemen’s pastoral rangelands
- Food Science
- Gut microbiota changes associated with low-carbohydrate diet intervention for obesity
- Reexamination of Aspergillus cristatus phylogeny in dark tea: Characteristics of the mitochondrial genome
- Differences in the flavonoid composition of the leaves, fruits, and branches of mulberry are distinguished based on a plant metabolomics approach
- Investigating the impact of wet rendering (solventless method) on PUFA-rich oil from catfish (Clarias magur) viscera
- Non-linear associations between cardiovascular metabolic indices and metabolic-associated fatty liver disease: A cross-sectional study in the US population (2017–2020)
- Knockdown of USP7 alleviates atherosclerosis in ApoE-deficient mice by regulating EZH2 expression
- Utility of dairy microbiome as a tool for authentication and traceability
- Agriculture
- Enhancing faba bean (Vicia faba L.) productivity through establishing the area-specific fertilizer rate recommendation in southwest Ethiopia
- Impact of novel herbicide based on synthetic auxins and ALS inhibitor on weed control
- Perspectives of pteridophytes microbiome for bioremediation in agricultural applications
- Fertilizer application parameters for drip-irrigated peanut based on the fertilizer effect function established from a “3414” field trial
- Improving the productivity and profitability of maize (Zea mays L.) using optimum blended inorganic fertilization
- Application of leaf multispectral analyzer in comparison to hyperspectral device to assess the diversity of spectral reflectance indices in wheat genotypes
- Animal Sciences
- Knockdown of ANP32E inhibits colorectal cancer cell growth and glycolysis by regulating the AKT/mTOR pathway
- Development of a detection chip for major pathogenic drug-resistant genes and drug targets in bovine respiratory system diseases
- Exploration of the genetic influence of MYOT and MB genes on the plumage coloration of Muscovy ducks
- Transcriptome analysis of adipose tissue in grazing cattle: Identifying key regulators of fat metabolism
- Comparison of nutritional value of the wild and cultivated spiny loaches at three growth stages
- Transcriptomic analysis of liver immune response in Chinese spiny frog (Quasipaa spinosa) infected with Proteus mirabilis
- Disruption of BCAA degradation is a critical characteristic of diabetic cardiomyopathy revealed by integrated transcriptome and metabolome analysis
- Plant Sciences
- Effect of long-term in-row branch covering on soil microorganisms in pear orchards
- Photosynthetic physiological characteristics, growth performance, and element concentrations reveal the calcicole–calcifuge behaviors of three Camellia species
- Transcriptome analysis reveals the mechanism of NaHCO3 promoting tobacco leaf maturation
- Bioinformatics, expression analysis, and functional verification of allene oxide synthase gene HvnAOS1 and HvnAOS2 in qingke
- Water, nitrogen, and phosphorus coupling improves gray jujube fruit quality and yield
- Improving grape fruit quality through soil conditioner: Insights from RNA-seq analysis of Cabernet Sauvignon roots
- Role of Embinin in the reabsorption of nucleus pulposus in lumbar disc herniation: Promotion of nucleus pulposus neovascularization and apoptosis of nucleus pulposus cells
- Revealing the effects of amino acid, organic acid, and phytohormones on the germination of tomato seeds under salinity stress
- Combined effects of nitrogen fertilizer and biochar on the growth, yield, and quality of pepper
- Comprehensive phytochemical and toxicological analysis of Chenopodium ambrosioides (L.) fractions
- Impact of “3414” fertilization on the yield and quality of greenhouse tomatoes
- Exploring the coupling mode of water and fertilizer for improving growth, fruit quality, and yield of the pear in the arid region
- Metagenomic analysis of endophytic bacteria in seed potato (Solanum tuberosum)
- Antibacterial, antifungal, and phytochemical properties of Salsola kali ethanolic extract
- Exploring the hepatoprotective properties of citronellol: In vitro and in silico studies on ethanol-induced damage in HepG2 cells
- Enhanced osmotic dehydration of watermelon rind using honey–sucrose solutions: A study on pre-treatment efficacy and mass transfer kinetics
- Effects of exogenous 2,4-epibrassinolide on photosynthetic traits of 53 cowpea varieties under NaCl stress
- Comparative transcriptome analysis of maize (Zea mays L.) seedlings in response to copper stress
- An optimization method for measuring the stomata in cassava (Manihot esculenta Crantz) under multiple abiotic stresses
- Fosinopril inhibits Ang II-induced VSMC proliferation, phenotype transformation, migration, and oxidative stress through the TGF-β1/Smad signaling pathway
- Antioxidant and antimicrobial activities of Salsola imbricata methanolic extract and its phytochemical characterization
- Bioengineering and Biotechnology
- Absorbable calcium and phosphorus bioactive membranes promote bone marrow mesenchymal stem cells osteogenic differentiation for bone regeneration
- New advances in protein engineering for industrial applications: Key takeaways
- An overview of the production and use of Bacillus thuringiensis toxin
- Research progress of nanoparticles in diagnosis and treatment of hepatocellular carcinoma
- Bioelectrochemical biosensors for water quality assessment and wastewater monitoring
- PEI/MMNs@LNA-542 nanoparticles alleviate ICU-acquired weakness through targeted autophagy inhibition and mitochondrial protection
- Unleashing of cytotoxic effects of thymoquinone-bovine serum albumin nanoparticles on A549 lung cancer cells
- Erratum
- Erratum to “Investigating the association between dietary patterns and glycemic control among children and adolescents with T1DM”
- Erratum to “Activation of hypermethylated P2RY1 mitigates gastric cancer by promoting apoptosis and inhibiting proliferation”
- Retraction
- Retraction to “MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB”
- Retraction to “A data mining technique for detecting malignant mesothelioma cancer using multiple regression analysis”
- Special Issue on Advances in Neurodegenerative Disease Research and Treatment
- Transplantation of human neural stem cell prevents symptomatic motor behavior disability in a rat model of Parkinson’s disease
- Special Issue on Multi-omics
- Inflammasome complex genes with clinical relevance suggest potential as therapeutic targets for anti-tumor drugs in clear cell renal cell carcinoma
- Gastroesophageal varices in primary biliary cholangitis with anti-centromere antibody positivity: Early onset?
Articles in the same Issue
- Biomedical Sciences
- Constitutive and evoked release of ATP in adult mouse olfactory epithelium
- LARP1 knockdown inhibits cultured gastric carcinoma cell cycle progression and metastatic behavior
- PEGylated porcine–human recombinant uricase: A novel fusion protein with improved efficacy and safety for the treatment of hyperuricemia and renal complications
- Research progress on ocular complications caused by type 2 diabetes mellitus and the function of tears and blepharons
- The role and mechanism of esketamine in preventing and treating remifentanil-induced hyperalgesia based on the NMDA receptor–CaMKII pathway
- Brucella infection combined with Nocardia infection: A case report and literature review
- Detection of serum interleukin-18 level and neutrophil/lymphocyte ratio in patients with antineutrophil cytoplasmic antibody-associated vasculitis and its clinical significance
- Ang-1, Ang-2, and Tie2 are diagnostic biomarkers for Henoch-Schönlein purpura and pediatric-onset systemic lupus erythematous
- PTTG1 induces pancreatic cancer cell proliferation and promotes aerobic glycolysis by regulating c-myc
- Role of serum B-cell-activating factor and interleukin-17 as biomarkers in the classification of interstitial pneumonia with autoimmune features
- Effectiveness and safety of a mumps containing vaccine in preventing laboratory-confirmed mumps cases from 2002 to 2017: A meta-analysis
- Low levels of sex hormone-binding globulin predict an increased breast cancer risk and its underlying molecular mechanisms
- A case of Trousseau syndrome: Screening, detection and complication
- Application of the integrated airway humidification device enhances the humidification effect of the rabbit tracheotomy model
- Preparation of Cu2+/TA/HAP composite coating with anti-bacterial and osteogenic potential on 3D-printed porous Ti alloy scaffolds for orthopedic applications
- Aquaporin-8 promotes human dermal fibroblasts to counteract hydrogen peroxide-induced oxidative damage: A novel target for management of skin aging
- Current research and evidence gaps on placental development in iron deficiency anemia
- Single-nucleotide polymorphism rs2910829 in PDE4D is related to stroke susceptibility in Chinese populations: The results of a meta-analysis
- Pheochromocytoma-induced myocardial infarction: A case report
- Kaempferol regulates apoptosis and migration of neural stem cells to attenuate cerebral infarction by O‐GlcNAcylation of β-catenin
- Sirtuin 5 regulates acute myeloid leukemia cell viability and apoptosis by succinylation modification of glycine decarboxylase
- Apigenin 7-glucoside impedes hypoxia-induced malignant phenotypes of cervical cancer cells in a p16-dependent manner
- KAT2A changes the function of endometrial stromal cells via regulating the succinylation of ENO1
- Current state of research on copper complexes in the treatment of breast cancer
- Exploring antioxidant strategies in the pathogenesis of ALS
- Helicobacter pylori causes gastric dysbacteriosis in chronic gastritis patients
- IL-33/soluble ST2 axis is associated with radiation-induced cardiac injury
- The predictive value of serum NLR, SII, and OPNI for lymph node metastasis in breast cancer patients with internal mammary lymph nodes after thoracoscopic surgery
- Carrying SNP rs17506395 (T > G) in TP63 gene and CCR5Δ32 mutation associated with the occurrence of breast cancer in Burkina Faso
- P2X7 receptor: A receptor closely linked with sepsis-associated encephalopathy
- Probiotics for inflammatory bowel disease: Is there sufficient evidence?
- Identification of KDM4C as a gene conferring drug resistance in multiple myeloma
- Microbial perspective on the skin–gut axis and atopic dermatitis
- Thymosin α1 combined with XELOX improves immune function and reduces serum tumor markers in colorectal cancer patients after radical surgery
- Highly specific vaginal microbiome signature for gynecological cancers
- Sample size estimation for AQP4-IgG seropositive optic neuritis: Retinal damage detection by optical coherence tomography
- The effects of SDF-1 combined application with VEGF on femoral distraction osteogenesis in rats
- Fabrication and characterization of gold nanoparticles using alginate: In vitro and in vivo assessment of its administration effects with swimming exercise on diabetic rats
- Mitigating digestive disorders: Action mechanisms of Mediterranean herbal active compounds
- Distribution of CYP2D6 and CYP2C19 gene polymorphisms in Han and Uygur populations with breast cancer in Xinjiang, China
- VSP-2 attenuates secretion of inflammatory cytokines induced by LPS in BV2 cells by mediating the PPARγ/NF-κB signaling pathway
- Factors influencing spontaneous hypothermia after emergency trauma and the construction of a predictive model
- Long-term administration of morphine specifically alters the level of protein expression in different brain regions and affects the redox state
- Application of metagenomic next-generation sequencing technology in the etiological diagnosis of peritoneal dialysis-associated peritonitis
- Clinical diagnosis, prevention, and treatment of neurodyspepsia syndrome using intelligent medicine
- Case report: Successful bronchoscopic interventional treatment of endobronchial leiomyomas
- Preliminary investigation into the genetic etiology of short stature in children through whole exon sequencing of the core family
- Cystic adenomyoma of the uterus: Case report and literature review
- Mesoporous silica nanoparticles as a drug delivery mechanism
- Dynamic changes in autophagy activity in different degrees of pulmonary fibrosis in mice
- Vitamin D deficiency and inflammatory markers in type 2 diabetes: Big data insights
- Lactate-induced IGF1R protein lactylation promotes proliferation and metabolic reprogramming of lung cancer cells
- Meta-analysis on the efficacy of allogeneic hematopoietic stem cell transplantation to treat malignant lymphoma
- Mitochondrial DNA drives neuroinflammation through the cGAS-IFN signaling pathway in the spinal cord of neuropathic pain mice
- Application value of artificial intelligence algorithm-based magnetic resonance multi-sequence imaging in staging diagnosis of cervical cancer
- Embedded monitoring system and teaching of artificial intelligence online drug component recognition
- Investigation into the association of FNDC1 and ADAMTS12 gene expression with plumage coloration in Muscovy ducks
- Yak meat content in feed and its impact on the growth of rats
- A rare case of Richter transformation with breast involvement: A case report and literature review
- First report of Nocardia wallacei infection in an immunocompetent patient in Zhejiang province
- Rhodococcus equi and Brucella pulmonary mass in immunocompetent: A case report and literature review
- Downregulation of RIP3 ameliorates the left ventricular mechanics and function after myocardial infarction via modulating NF-κB/NLRP3 pathway
- Evaluation of the role of some non-enzymatic antioxidants among Iraqi patients with non-alcoholic fatty liver disease
- The role of Phafin proteins in cell signaling pathways and diseases
- Ten-year anemia as initial manifestation of Castleman disease in the abdominal cavity: A case report
- Coexistence of hereditary spherocytosis with SPTB P.Trp1150 gene variant and Gilbert syndrome: A case report and literature review
- Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells
- Exploratory evaluation supported by experimental and modeling approaches of Inula viscosa root extract as a potent corrosion inhibitor for mild steel in a 1 M HCl solution
- Imaging manifestations of ductal adenoma of the breast: A case report
- Gut microbiota and sleep: Interaction mechanisms and therapeutic prospects
- Isomangiferin promotes the migration and osteogenic differentiation of rat bone marrow mesenchymal stem cells
- Prognostic value and microenvironmental crosstalk of exosome-related signatures in human epidermal growth factor receptor 2 positive breast cancer
- Circular RNAs as potential biomarkers for male severe sepsis
- Knockdown of Stanniocalcin-1 inhibits growth and glycolysis in oral squamous cell carcinoma cells
- The expression and biological role of complement C1s in esophageal squamous cell carcinoma
- A novel GNAS mutation in pseudohypoparathyroidism type 1a with articular flexion deformity: A case report
- Predictive value of serum magnesium levels for prognosis in patients with non-small cell lung cancer undergoing EGFR-TKI therapy
- HSPB1 alleviates acute-on-chronic liver failure via the P53/Bax pathway
- IgG4-related disease complicated by PLA2R-associated membranous nephropathy: A case report
- Baculovirus-mediated endostatin and angiostatin activation of autophagy through the AMPK/AKT/mTOR pathway inhibits angiogenesis in hepatocellular carcinoma
- Metformin mitigates osteoarthritis progression by modulating the PI3K/AKT/mTOR signaling pathway and enhancing chondrocyte autophagy
- Evaluation of the activity of antimicrobial peptides against bacterial vaginosis
- Atypical presentation of γ/δ mycosis fungoides with an unusual phenotype and SOCS1 mutation
- Analysis of the microecological mechanism of diabetic kidney disease based on the theory of “gut–kidney axis”: A systematic review
- Omega-3 fatty acids prevent gestational diabetes mellitus via modulation of lipid metabolism
- Refractory hypertension complicated with Turner syndrome: A case report
- Interaction of ncRNAs and the PI3K/AKT/mTOR pathway: Implications for osteosarcoma
- Association of low attenuation area scores with pulmonary function and clinical prognosis in patients with chronic obstructive pulmonary disease
- Long non-coding RNAs in bone formation: Key regulators and therapeutic prospects
- The deubiquitinating enzyme USP35 regulates the stability of NRF2 protein
- Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as potential diagnostic markers for rebleeding in patients with esophagogastric variceal bleeding
- G protein-coupled receptor 1 participating in the mechanism of mediating gestational diabetes mellitus by phosphorylating the AKT pathway
- LL37-mtDNA regulates viability, apoptosis, inflammation, and autophagy in lipopolysaccharide-treated RLE-6TN cells by targeting Hsp90aa1
- The analgesic effect of paeoniflorin: A focused review
- Chemical composition’s effect on Solanum nigrum Linn.’s antioxidant capacity and erythrocyte protection: Bioactive components and molecular docking analysis
- Knockdown of HCK promotes HREC cell viability and inner blood–retinal barrier integrity by regulating the AMPK signaling pathway
- The role of rapamycin in the PINK1/Parkin signaling pathway in mitophagy in podocytes
- Laryngeal non-Hodgkin lymphoma: Report of four cases and review of the literature
- Clinical value of macrogenome next-generation sequencing on infections
- Overview of dendritic cells and related pathways in autoimmune uveitis
- TAK-242 alleviates diabetic cardiomyopathy via inhibiting pyroptosis and TLR4/CaMKII/NLRP3 pathway
- Hypomethylation in promoters of PGC-1α involved in exercise-driven skeletal muscular alterations in old age
- Profile and antimicrobial susceptibility patterns of bacteria isolated from effluents of Kolladiba and Debark hospitals
- The expression and clinical significance of syncytin-1 in serum exosomes of hepatocellular carcinoma patients
- A histomorphometric study to evaluate the therapeutic effects of biosynthesized silver nanoparticles on the kidneys infected with Plasmodium chabaudi
- PGRMC1 and PAQR4 are promising molecular targets for a rare subtype of ovarian cancer
- Analysis of MDA, SOD, TAOC, MNCV, SNCV, and TSS scores in patients with diabetes peripheral neuropathy
- SLIT3 deficiency promotes non-small cell lung cancer progression by modulating UBE2C/WNT signaling
- The relationship between TMCO1 and CALR in the pathological characteristics of prostate cancer and its effect on the metastasis of prostate cancer cells
- Heterogeneous nuclear ribonucleoprotein K is a potential target for enhancing the chemosensitivity of nasopharyngeal carcinoma
- PHB2 alleviates retinal pigment epithelium cell fibrosis by suppressing the AGE–RAGE pathway
- Anti-γ-aminobutyric acid-B receptor autoimmune encephalitis with syncope as the initial symptom: Case report and literature review
- Comparative analysis of chloroplast genome of Lonicera japonica cv. Damaohua
- Human umbilical cord mesenchymal stem cells regulate glutathione metabolism depending on the ERK–Nrf2–HO-1 signal pathway to repair phosphoramide mustard-induced ovarian cancer cells
- Electroacupuncture on GB acupoints improves osteoporosis via the estradiol–PI3K–Akt signaling pathway
- Renalase protects against podocyte injury by inhibiting oxidative stress and apoptosis in diabetic nephropathy
- Review: Dicranostigma leptopodum: A peculiar plant of Papaveraceae
- Combination effect of flavonoids attenuates lung cancer cell proliferation by inhibiting the STAT3 and FAK signaling pathway
- Renal microangiopathy and immune complex glomerulonephritis induced by anti-tumour agents: A case report
- Correlation analysis of AVPR1a and AVPR2 with abnormal water and sodium and potassium metabolism in rats
- Gastrointestinal health anti-diarrheal mixture relieves spleen deficiency-induced diarrhea through regulating gut microbiota
- Myriad factors and pathways influencing tumor radiotherapy resistance
- Exploring the effects of culture conditions on Yapsin (YPS) gene expression in Nakaseomyces glabratus
- Screening of prognostic core genes based on cell–cell interaction in the peripheral blood of patients with sepsis
- Coagulation factor II thrombin receptor as a promising biomarker in breast cancer management
- Ileocecal mucinous carcinoma misdiagnosed as incarcerated hernia: A case report
- Methyltransferase like 13 promotes malignant behaviors of bladder cancer cells through targeting PI3K/ATK signaling pathway
- The debate between electricity and heat, efficacy and safety of irreversible electroporation and radiofrequency ablation in the treatment of liver cancer: A meta-analysis
- ZAG promotes colorectal cancer cell proliferation and epithelial–mesenchymal transition by promoting lipid synthesis
- Baicalein inhibits NLRP3 inflammasome activation and mitigates placental inflammation and oxidative stress in gestational diabetes mellitus
- Impact of SWCNT-conjugated senna leaf extract on breast cancer cells: A potential apoptotic therapeutic strategy
- MFAP5 inhibits the malignant progression of endometrial cancer cells in vitro
- Major ozonated autohemotherapy promoted functional recovery following spinal cord injury in adult rats via the inhibition of oxidative stress and inflammation
- Axodendritic targeting of TAU and MAP2 and microtubule polarization in iPSC-derived versus SH-SY5Y-derived human neurons
- Differential expression of phosphoinositide 3-kinase/protein kinase B and Toll-like receptor/nuclear factor kappa B signaling pathways in experimental obesity Wistar rat model
- The therapeutic potential of targeting Oncostatin M and the interleukin-6 family in retinal diseases: A comprehensive review
- BA inhibits LPS-stimulated inflammatory response and apoptosis in human middle ear epithelial cells by regulating the Nf-Kb/Iκbα axis
- Role of circRMRP and circRPL27 in chronic obstructive pulmonary disease
- Investigating the role of hyperexpressed HCN1 in inducing myocardial infarction through activation of the NF-κB signaling pathway
- Characterization of phenolic compounds and evaluation of anti-diabetic potential in Cannabis sativa L. seeds: In vivo, in vitro, and in silico studies
- Quantitative immunohistochemistry analysis of breast Ki67 based on artificial intelligence
- Ecology and Environmental Science
- Screening of different growth conditions of Bacillus subtilis isolated from membrane-less microbial fuel cell toward antimicrobial activity profiling
- Degradation of a mixture of 13 polycyclic aromatic hydrocarbons by commercial effective microorganisms
- Evaluation of the impact of two citrus plants on the variation of Panonychus citri (Acari: Tetranychidae) and beneficial phytoseiid mites
- Prediction of present and future distribution areas of Juniperus drupacea Labill and determination of ethnobotany properties in Antalya Province, Türkiye
- Population genetics of Todarodes pacificus (Cephalopoda: Ommastrephidae) in the northwest Pacific Ocean via GBS sequencing
- A comparative analysis of dendrometric, macromorphological, and micromorphological characteristics of Pistacia atlantica subsp. atlantica and Pistacia terebinthus in the middle Atlas region of Morocco
- Macrofungal sporocarp community in the lichen Scots pine forests
- Assessing the proximate compositions of indigenous forage species in Yemen’s pastoral rangelands
- Food Science
- Gut microbiota changes associated with low-carbohydrate diet intervention for obesity
- Reexamination of Aspergillus cristatus phylogeny in dark tea: Characteristics of the mitochondrial genome
- Differences in the flavonoid composition of the leaves, fruits, and branches of mulberry are distinguished based on a plant metabolomics approach
- Investigating the impact of wet rendering (solventless method) on PUFA-rich oil from catfish (Clarias magur) viscera
- Non-linear associations between cardiovascular metabolic indices and metabolic-associated fatty liver disease: A cross-sectional study in the US population (2017–2020)
- Knockdown of USP7 alleviates atherosclerosis in ApoE-deficient mice by regulating EZH2 expression
- Utility of dairy microbiome as a tool for authentication and traceability
- Agriculture
- Enhancing faba bean (Vicia faba L.) productivity through establishing the area-specific fertilizer rate recommendation in southwest Ethiopia
- Impact of novel herbicide based on synthetic auxins and ALS inhibitor on weed control
- Perspectives of pteridophytes microbiome for bioremediation in agricultural applications
- Fertilizer application parameters for drip-irrigated peanut based on the fertilizer effect function established from a “3414” field trial
- Improving the productivity and profitability of maize (Zea mays L.) using optimum blended inorganic fertilization
- Application of leaf multispectral analyzer in comparison to hyperspectral device to assess the diversity of spectral reflectance indices in wheat genotypes
- Animal Sciences
- Knockdown of ANP32E inhibits colorectal cancer cell growth and glycolysis by regulating the AKT/mTOR pathway
- Development of a detection chip for major pathogenic drug-resistant genes and drug targets in bovine respiratory system diseases
- Exploration of the genetic influence of MYOT and MB genes on the plumage coloration of Muscovy ducks
- Transcriptome analysis of adipose tissue in grazing cattle: Identifying key regulators of fat metabolism
- Comparison of nutritional value of the wild and cultivated spiny loaches at three growth stages
- Transcriptomic analysis of liver immune response in Chinese spiny frog (Quasipaa spinosa) infected with Proteus mirabilis
- Disruption of BCAA degradation is a critical characteristic of diabetic cardiomyopathy revealed by integrated transcriptome and metabolome analysis
- Plant Sciences
- Effect of long-term in-row branch covering on soil microorganisms in pear orchards
- Photosynthetic physiological characteristics, growth performance, and element concentrations reveal the calcicole–calcifuge behaviors of three Camellia species
- Transcriptome analysis reveals the mechanism of NaHCO3 promoting tobacco leaf maturation
- Bioinformatics, expression analysis, and functional verification of allene oxide synthase gene HvnAOS1 and HvnAOS2 in qingke
- Water, nitrogen, and phosphorus coupling improves gray jujube fruit quality and yield
- Improving grape fruit quality through soil conditioner: Insights from RNA-seq analysis of Cabernet Sauvignon roots
- Role of Embinin in the reabsorption of nucleus pulposus in lumbar disc herniation: Promotion of nucleus pulposus neovascularization and apoptosis of nucleus pulposus cells
- Revealing the effects of amino acid, organic acid, and phytohormones on the germination of tomato seeds under salinity stress
- Combined effects of nitrogen fertilizer and biochar on the growth, yield, and quality of pepper
- Comprehensive phytochemical and toxicological analysis of Chenopodium ambrosioides (L.) fractions
- Impact of “3414” fertilization on the yield and quality of greenhouse tomatoes
- Exploring the coupling mode of water and fertilizer for improving growth, fruit quality, and yield of the pear in the arid region
- Metagenomic analysis of endophytic bacteria in seed potato (Solanum tuberosum)
- Antibacterial, antifungal, and phytochemical properties of Salsola kali ethanolic extract
- Exploring the hepatoprotective properties of citronellol: In vitro and in silico studies on ethanol-induced damage in HepG2 cells
- Enhanced osmotic dehydration of watermelon rind using honey–sucrose solutions: A study on pre-treatment efficacy and mass transfer kinetics
- Effects of exogenous 2,4-epibrassinolide on photosynthetic traits of 53 cowpea varieties under NaCl stress
- Comparative transcriptome analysis of maize (Zea mays L.) seedlings in response to copper stress
- An optimization method for measuring the stomata in cassava (Manihot esculenta Crantz) under multiple abiotic stresses
- Fosinopril inhibits Ang II-induced VSMC proliferation, phenotype transformation, migration, and oxidative stress through the TGF-β1/Smad signaling pathway
- Antioxidant and antimicrobial activities of Salsola imbricata methanolic extract and its phytochemical characterization
- Bioengineering and Biotechnology
- Absorbable calcium and phosphorus bioactive membranes promote bone marrow mesenchymal stem cells osteogenic differentiation for bone regeneration
- New advances in protein engineering for industrial applications: Key takeaways
- An overview of the production and use of Bacillus thuringiensis toxin
- Research progress of nanoparticles in diagnosis and treatment of hepatocellular carcinoma
- Bioelectrochemical biosensors for water quality assessment and wastewater monitoring
- PEI/MMNs@LNA-542 nanoparticles alleviate ICU-acquired weakness through targeted autophagy inhibition and mitochondrial protection
- Unleashing of cytotoxic effects of thymoquinone-bovine serum albumin nanoparticles on A549 lung cancer cells
- Erratum
- Erratum to “Investigating the association between dietary patterns and glycemic control among children and adolescents with T1DM”
- Erratum to “Activation of hypermethylated P2RY1 mitigates gastric cancer by promoting apoptosis and inhibiting proliferation”
- Retraction
- Retraction to “MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB”
- Retraction to “A data mining technique for detecting malignant mesothelioma cancer using multiple regression analysis”
- Special Issue on Advances in Neurodegenerative Disease Research and Treatment
- Transplantation of human neural stem cell prevents symptomatic motor behavior disability in a rat model of Parkinson’s disease
- Special Issue on Multi-omics
- Inflammasome complex genes with clinical relevance suggest potential as therapeutic targets for anti-tumor drugs in clear cell renal cell carcinoma
- Gastroesophageal varices in primary biliary cholangitis with anti-centromere antibody positivity: Early onset?

