Abstract
Neuroinflammation is pivotal in the development of neuropathic pain (NeP). While mitochondrial deoxyribonucleic acid (mtDNA) and cyclic GMP-AMP synthase (cGAS) are recognized for inducing inflammation in various neurological disorders, their involvement in NeP remains ambiguous. In this study, we examined: (1) the changes in mtDNA and cGAS in mice with NeP induced by chronic constriction injury (CCI) of the sciatic nerve, whether mtDNA triggers inflammation via the cGAS signaling; (2) the effects of RU.521, a cGAS antagonist, on CCI-induced nociception (allodynia and hyperalgesia) and relative inflammatory protein expression; (3) the activation of microglia and the cGAS-IFN pathway mediated by mtDNA in BV2 cell; (4) the effect of RU.521 on mtDNA-induced inflammatory response in BV2 cells. Results revealed reduced mtDNA levels in the sciatic nerve but increased levels in the spinal cord of CCI mice, along with elevated cGAS expression and inflammatory factors. RU.521 alleviated nociceptive behaviors in CCI mice, possibly by normalizing cGAS levels and suppressing inflammation. Neuron-derived mtDNA provoked cellular activation and upregulated cGAS signaling in BV2 cells. Additionally, RU.521 and DNase I effectively inhibited cGAS-induced inflammation. These findings underscore the critical role of mtDNA accumulation and mtDNA-mediated cGAS signaling in NeP development after peripheral nerve injury.
Graphical abstract
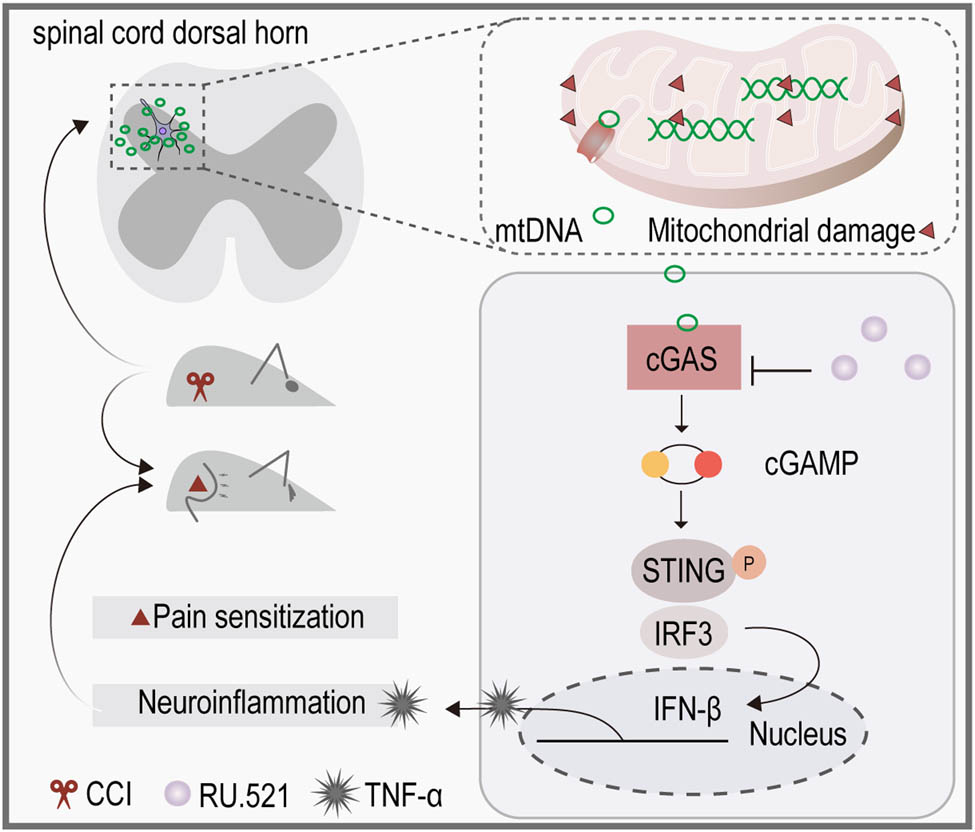
1 Introduction
Neuropathic pain (NeP) presents as a prevalent and formidable clinical challenge, characterized by clinical manifestations such as allodynia and hyperalgesia, impacting approximately 10% of the world population [1,2]. Nevertheless, its underlying causes remain poorly understood. Our earlier research has substantiated that mitochondrial dysfunction contributes to the advancement of NeP by promoting neuroinflammation and oxidative stress [3]. Additionally, enhancing mitochondrial autophagy can alleviate hyperalgesia by reducing neuroinflammation [4,5]. However, the specific mechanism by which mitochondrial dysfunction triggers neuroinflammation in the NeP remains to be further elucidated.
Recently, increasing evidence suggests that mitochondrial deoxyribonucleic acid (mtDNA) plays a significant role in the pathogenesis of various neurological diseases [6,7], including amyotrophic lateral sclerosis [8], multiple sclerosis (MS) [9], and ischemic stroke [10]. Besides, previous studies have demonstrated that elevated reactive oxygen species (ROS) not only impair mitochondrial function but also induce oxidative stress reactions that damage various biomolecules such as DNA, RNA, lipids, and proteins [6,11]. The oxidative stress leads to the release of mtDNA into the cytoplasm and intercellular space, thereby initiating immune responses [12]. However, the impact of mtDNA on NeP and the possible mechanisms involved warrant further investigation.
The mtDNA fragments can directly bind to pattern recognition receptors on the cellular membrane, initiating the signaling cascade, and activating immune-related genes [12,13]. In addition, the release of mtDNA also activates the Cyclic GMP-AMP synthase (cGAS) pathway [14,15]. The cGAS is a DNA sensor present in the cytoplasm and on the plasma membrane [16], it is capable of binding to mtDNA as a damage-associated molecular pattern due to its distinctive molecular structure. Then their combination catalyzes ATP and GTP to generate the secondary messenger 2′–3′ cyclic GMP-AMP (2′–3′ cGAMP), facilitating the translocation and phosphorylation of the adaptor protein known as stimulator of interferon genes (STING). Subsequently, the activated STING triggers the activation of TGFβ-activated kinase 1 and the transcription factor interferon regulatory factor 3 (IRF3), which play important roles in the innate immune system’s response [17,18]. This chain of events ultimately leads to the enhancement of type I interferon (IFN) response, resulting in inflammation in response to viral and microbial infections [19,20]. Even though the activated cGAS exhibits obvious immunomodulatory effects, it is still unknown whether mtDNA triggers inflammatory responses through the cGAS signaling pathway in NeP.
In this study, we assessed alterations in mtDNA and cGAS levels and the relevant inflammatory responses in the spinal cord and/or sciatic nerve of NeP mice induced by chronic constriction injury of the sciatic nerve (CCI) and explored the antinociceptive effect of the administration of cGAS inhibitor. Subsequently, we conducted in vitro experiments to appraise the impact of neuron-derived mtDNA on microglial activation, and whether the inhibition of cGAS could mitigate the inflammatory response.
2 Materials and methods
2.1 Animals
C57BL6/J male mice (8–12 weeks of age) were purchased from Hunan SJA Laboratory Animal Co., Ltd (Hunan, China). All experimental procedures were approved by the Laboratory Animal Welfare and Ethics Committee of the Army Medical University and followed the principle of minimizing animal suffering, as few as possible the animals used.
-
Ethical approval: The research related to animal use has been complied with all the relevant national regulations and institutional policies for the care and use of animals, and has been approved by the Laboratory Animal Welfare and Ethics Committee of the Army Medical University.
2.2 Induction of NeP
Mice were subjected to CCI surgery as previously described [3]. Briefly, under isoflurane (2%–4%) anesthesia, the right common sciatic nerve branches (close to the trifurcation into the sural, peroneal, and tibial nerve) were exposed and ligated with 4–0 cotton sutures. Mice without nerve ligation as the sham-operated group. Then, these CCI mice were divided into three groups: 1, 2, and 3 weeks.
2.3 Experimental process
The mice treated with the cGAS inhibitor RU.521 (Med Chem Express, USA) were randomly divided into four groups: (1) sham-operated; (2) CCI: CCI surgery group; (3) CCI + saline: CCI mice were intraperitoneally injected with 0.9% saline daily for 5 days; and (4) RU.521-treated CCI: CCI mice received intraperitoneal injection of RU.521 (5 mg/kg/day) for five consecutive days after 10 days of sciatic nerve ligation, and the determination of pain threshold was performed before administration each time. as depicted in Figure 1 (Figure 1). The dose and manner of injection of the drug were based on previous studies and our preliminary experiments [21]. Experimental mice were euthanized after completing treatment and tests on day 14.

Timeline of RU.521 injection. The arrows indicate intraperitoneal injection of saline or RU.521 at a dose of 5 mg/kg/day, with thermal latency and mechanical thresholds measured before each injection, and euthanasia after testing on day 14.
2.4 Mechanical allodynia threshold and thermal latency measurement
The mechanical allodynia threshold of each group of mice was measured at 1 day before surgery and 3, 7, 14, and 21 days after surgery. Mice were placed independently in a cage for 30 min of acclimatization before measurement. Utilized von Frey filament to vertically stimulate the ipsilateral hind paw of mice, and recorded a positive response when the mouse withdraws its hind paw [22]. Measure again after 5 min, and the average value of the three measurements was taken as the paw-withdrawal mechanical threshold (PWMT).
The Hargreaves test was used to quantify the thermal threshold of radiant thermal stimulation in the hind paws of mice [22]. The radiation source was placed under the animal and aimed at the plantar, and the duration required for the mouse to withdraw its paw from the thermal stimulus was recorded as the paw withdrawal thermal latency (PWTL).
2.5 Cell culture and treatments
The mouse microglia (BV2) and neurons (Neuro2) were purchased from Procell Life Science & Technology Co., Ltd (Wuhan, China). Cells were cultured in DMEM medium (C11995500BT, Gibco, Thermo Fisher Scientific, USA) with 10% fetal bovine serum (9006-53-5, Biological Industries, Israel) and 1% penicillin/streptomycin (15140122, Gibco, Thermo Fisher Scientific, USA), incubated at 37°C with 5% CO2 and (Thermo Fisher Scientific, USA). Upon reaching a confluence exceeding 70%, BV2 cells were treated with mtDNA isolated from Neuro2 cells.
2.6 mtDNA isolation and transfection
According to the manufacturer’s instructions, Neuro2 cell mtDNA was isolated with the Mitochondrial DNA Isolation Kit (ab65321, Abcam, Britain). Briefly, cells were collected. The cells were resuspended in 1× cytoplasmic extraction buffer and incubated on ice for 15 min. The cells were homogenized and transferred to a centrifuge at 1,200×g for 10 min at 4°C. The supernatant was removed and resuspended pellets in 1× cytosolic extraction buffer and centrifuged again. The pellets (isolated mitochondria) were resuspended and lysed in mitochondrial lysis buffer on ice for 10 min, added to the enzyme mixture, and incubated for 60 min at 50°C. Subsequently, the samples were centrifuged and resuspended the particles (mtDNA) with Tris-EDTA (TE) buffer. The concentration of mtDNA was measured with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific Rockford, USA).
According to the manufacturer’s instructions, Neuro2-derived mtDNA was transfected into BV2 cells using Attractene Transfection Reagent (301005, Qiagen, Germany). In brief, cells were seeded in six-well plates and reached a confluence of 70%. The mtDNA was diluted in TE buffer and mixed with serum-free cell culture medium to the desired concentration for the experiment and added the Attractene Transfection Reagent to the Eppendorf tube to promote the formation of transfection complexes. After vortexing, the samples were incubated at room temperature for 15 min. The transfection complexes were added to the plates and rotated to ensure uniform distribution.
2.7 Detection of mtDNA and cytosolic DNA with staining
The sciatic nerve and spinal cord of each group of mice were collected rapidly and preserved at −80°C. DNA was extracted according to the TIANamp Genomic DNA Kit (DP304, TIANGEN, Germany) manual. Quantitative PCR (qPCR) analysis was performed on the extracted DNA using primers specific for nuclear DNA (18S rDNA) and mitochondrial DNA (CO1 and ND1). The abundance of nuclear DNA, as detected by the 18S rDNA primers, was utilized as the internal control for standardizing mtDNA quantification. The primers used in this study are shown in Table 1.
The primer sequences used for real-time PCR assay
| Gene (mouse) | Forward primer sequence (5′–3′) | Reverse primer sequence (5′–3′) |
|---|---|---|
| IFN-beta | GAGGAAAGATTGACGTGGGAGAT | AGTCCGCCTCTGATGCTTAAAG |
| TNF-α | GCCTCCCTCTCATCAGTTCTATG | ACCTGGGAGTAGACAAGGTACAA |
| Actin, beta | ACTGTCGAGTCGCGTCC | CTGACCCATTCCCACCATCA |
| TLR9 | ATGCCTTCGTGGTGTTCGAT | TCTCGGTCCTCCAGACACAA |
| cGAS | TCAGCTACCAAGATGCTGTCAA | AGTGTTACAGCAGGGCTTCC |
| NLRP3 | TATCTCTCCCGCATCTCCATTTG | GCGTTCCTGTCCTTGATAGAGTA |
| 18S rDNA | CCTGAGAAACGGCTACCACATC | CACCAGACTTGCCCTCCA |
| mt-CO1 | GCATCTGTTCTGATTCTTTGGGCAC | GTGGTGGGCTCATACAATAAAGCC |
| mt-ND1 | CGGCCCATTCGCGTTATTC | GATCGTAACGGAAGCGTGGA |
| Tert | CACGTACTGTATCCGCCAGT | TGCACTGGCATCTGAATCCT |
Double-labeled immunofluorescence was used to show mtDNA released into the cytoplasm. The cells were incubated with 400 nM MitoTracker Red Stock Solution (M7512, MitoTracker™ Red CMXRos-Special Package, Invitrogen, USA) for 15–45 min under appropriate growth conditions in the dark. After the mitochondria were labeled with a MitoTracker dye, cells were washed in fresh pre-warmed growth medium and fixed with 4% paraformaldehyde for 15 min. Cell permeabilization and blocking were performed sequentially, as described in immunofluorescence staining. After incubation overnight at 4°C with the double-stranded DNA (dsDNA) primary antibody (1:100, sc-58749, Santa Cruz, CA, USA), the FITC-conjugated secondary antibody (ab150115, Abcam, Britain) was incubated for 1 h at room temperature. Finally, the cells were stained with DAPI and observed under Laser confocal microscopy (LSM780, ZEISS, Germany).
2.8 Quantitative polymerase chain reaction (qPCR) analysis
RNA was extracted from tissues or cells using RNA-Quick Purification kits (RN001, ES Science, Shanghai, China) and reverse transcribed into cDNA using Fast All-in-One RT Kit (RT001, ES Science, Shanghai, China) according to the instruction manual. The qPCR experiment was conducted in 10 μl of reaction mixtures containing 0.2 μl of forward primer, 0.2 μl of reverse primer, 1 μl of cDNA, 5 μl of the PowerUp™SYBR™ Green Master Mix (A25742, Thermo Fisher Scientific, USA), and 3.6 μl of RNase free ddH2O and quantified using a real-time PCR instrument (BIO-Rad, USA). As a part of the thermal cycling parameters, UDGase activation was performed for 2 min at 50°C and predenaturation for 2 min at 95°C, followed by 40 cycles for 1 s at 95°C and for 30 s at 60°C. To determine relative expression levels, the mRNA levels of each sample were normalized to those of Actin, beta mRNA by the 2−ΔΔCt method. Primer synthesis was performed by Beijing Tsingke Biotech Co., Ltd. (Beijing, China). The primers used in this study are shown in Table 1.
2.9 Western blotting analysis
Tissues and BV2 cells were collected and lysed in RIPA Lysis Buffer (P0013B, Beyotime, Shanghai, China) containing protease and phosphatase inhibitors for 15 min on ice; the mixture was centrifuged at 10,000×g for 15 min. The lysates were heated with sodium dodecyl sulfate-polyacrylamide gel electrophoresis Sample Loading Buffer 5× (P0015, Beyotime, Shanghai, China) for 10 min at 100°C. Then, the protein samples were electrophoresed on 10% polyacrylamide gradient SDS gels (PG212, Epizyme, Shanghai, China) and transferred onto polyvinylidene fluoride membranes (66543, Millipore, Billerica, MA, USA). The membranes were blocked in 5% bovine serum albumin (ST023-50g, Beyotime, Shanghai, China) and incubated overnight with primary antibodies against cGAS (31659, CST, MA, USA, 1:1,000), phosphorylated-STING (p-STING) (72971, CST, 1:1,000), STING (13647, CST, 1:1,000), ionized calcium-binding adaptor molecule 1 (IBA1) (382207, ZEN-BIOSCIENCE, Chengdu, China, 1:1,000), IRF3 (381333, ZEN-BIOSCIENCE, 1:1,000), GAPDH (60004-1-Ig, Proteintech, Wuhan, China, 1:5,000), and tubulin (66031-1-Ig, Proteintech, 1:5,000). Next, HRP-conjugated secondary antibodies were incubated for 1 h at room temperature. The immunoblots were observed in BeyoECL Moon (P0018FS, Beyotime, Shanghai, China) by BIO-Rad gel imaging instrument and quantified by ImageJ software (National Institutes of Health, USA).
2.10 Immunofluorescence staining
For fluorescence staining of tissue samples, freeze slices of mouse spinal dorsal cords at L3-L5 and sciatic nerve were used to permeabilize with 0.3% TritonX-100 and blocked with 10% goat-derived serum for 1 h at room temperature. Then, the slices were incubated overnight at 4°C with primary antibodies against dsDNA (sc-58749, Santa Cruz, USA, 1:100), cGAS (A23846, Abclonal, Wuhan, China, 1:100), GFAP (250027, ZEN-BIOSCIENCE, Chengdu, China, 1:100), Neuron (222545, ZEN-BIOSCIENCE, 1:100), and IBA1 (17198, CST, USA, 1:100). CoraLite488-conjugated Goat Anti-Mouse, CoraLite594-conjugated Goat Anti-Mouse, and CoraLite594-conjugated Goat Anti-Rabbit (1:100, Proteintech, Wuhan, China) antibodies were incubated for 1 h at room temperature. These slices were covered with DAPI (C1002, Beyotime, Shanghai, China) and photographed under Laser confocal microscopy (LSM780, ZEISS, Germany).
For fluorescence staining of cells, BV2 microglia were seeded in confocal dishes, fixed in 4% paraformaldehyde for 15 min, and permeabilized with 0.3% TritonX-100 for 20 min at room temperature. After blocking with 5% goat-derived serum, cells were incubated overnight with IRF3 (ZEN-BIOSCIENCE, Chengdu, China), IBA1 (1:100, Cell Signaling Technology, USA), and p-STING (PA5-105674, Thermo Fisher Scientific, USA, 1:100). Then incubated with CoraLite488-conjugated Goat Anti-Mouse, CoraLite594-conjugated Goat Anti-Rabbit, or Alexa Fluor® 488 Goat Anti-Rabbit (1:100, Proteintech, Wuhan, China) antibodies for 1 h at room temperature, and DAPI staining solution was used for nuclear staining. Cells were observed under Laser confocal microscopy.
2.11 ROS determination
BV2 cells were seeded in 12-well plates and transfected mtDNA at different times. The DCFH-DA ROS probe was used to detect the production of intracellular ROS by the ROS Assay Kit (CA1410, Solarbio, Beijing, China), according to the manufacturer’s instructions.
2.12 Nitrate/nitrite measurement
BV2 cells seeded in six-well plates were transfected with mtDNA at 70% confluence. After stimulation ended, the cell culture supernatants were collected at 0, 3, 6, 12, and 24 h respectively, for the determination of Nitrate/Nitrite by the Total Nitric Oxide Assay Kit (S0023, Beyotime, Shanghai, China), according to the manufacturer’s instructions.
2.13 Statistical analysis
Statistical analysis was performed using GraphPad Prism 9.0 (San Diego, CA, USA). All data resulted from at least three independent experiments (n = 3) and presented as mean ± SEM. Before statistical analysis, All datasets were subjected to normality and variance homogeneity tests. When the data do not conform to the positive distribution or the variance is uneven, a nonparametric test is used. In addition, the same time point values of PWMT and PWTL among groups were analyzed by the application of one-way analysis of variance (ANOVA) with Tukey’s post hoc test. The remaining data were analyzed using either Student’s t-test or one-way ANOVA. The statistical significance was set to P < 0.05.
3 Results
3.1 CCI surgery downregulated mtDNA in the injured peripheral nerve and upregulated mtDNA in the spinal cord of CCI mice
As shown, on postoperative day 7, both PWMT and PWTL values of CCI mice were significantly lower compared to the sham-operated group (Figure 2a). DNA was extracted from the proximal and distal ends of the ligated nerve in mice, and qPCR was performed using CO1 and ND1 primers to assess mtDNA content. The results demonstrated a progressive decline in mtDNA content within the distal sciatic nerve of CCI mice, with a more pronounced decrease observed at each time point in the distal end compared to the proximal end (Figure 2b). Conversely, an increase in mtDNA content was observed in the spinal cord of CCI mice on the 14th day following CCI surgery (Figure 2c). Subsequently, we conducted co-immunostaining of dsDNA and the nucleus to further examine the changes in the spinal cord of CCI mice. In the sham-operated group, the spinal cord showed a well-defined texture with dsDNA staining largely overlapping with the nucleus. In contrast, the spinal cord tissue of CCI mice showed morphological blurring and disorganization, accompanied by numerous small extracellular dsDNA particles (Figure 2d), consistent with the qPCR results. Collectively, the data revealed a decrease in mtDNA content in the sciatic nerve and an increase in the spinal cord of CCI mice.
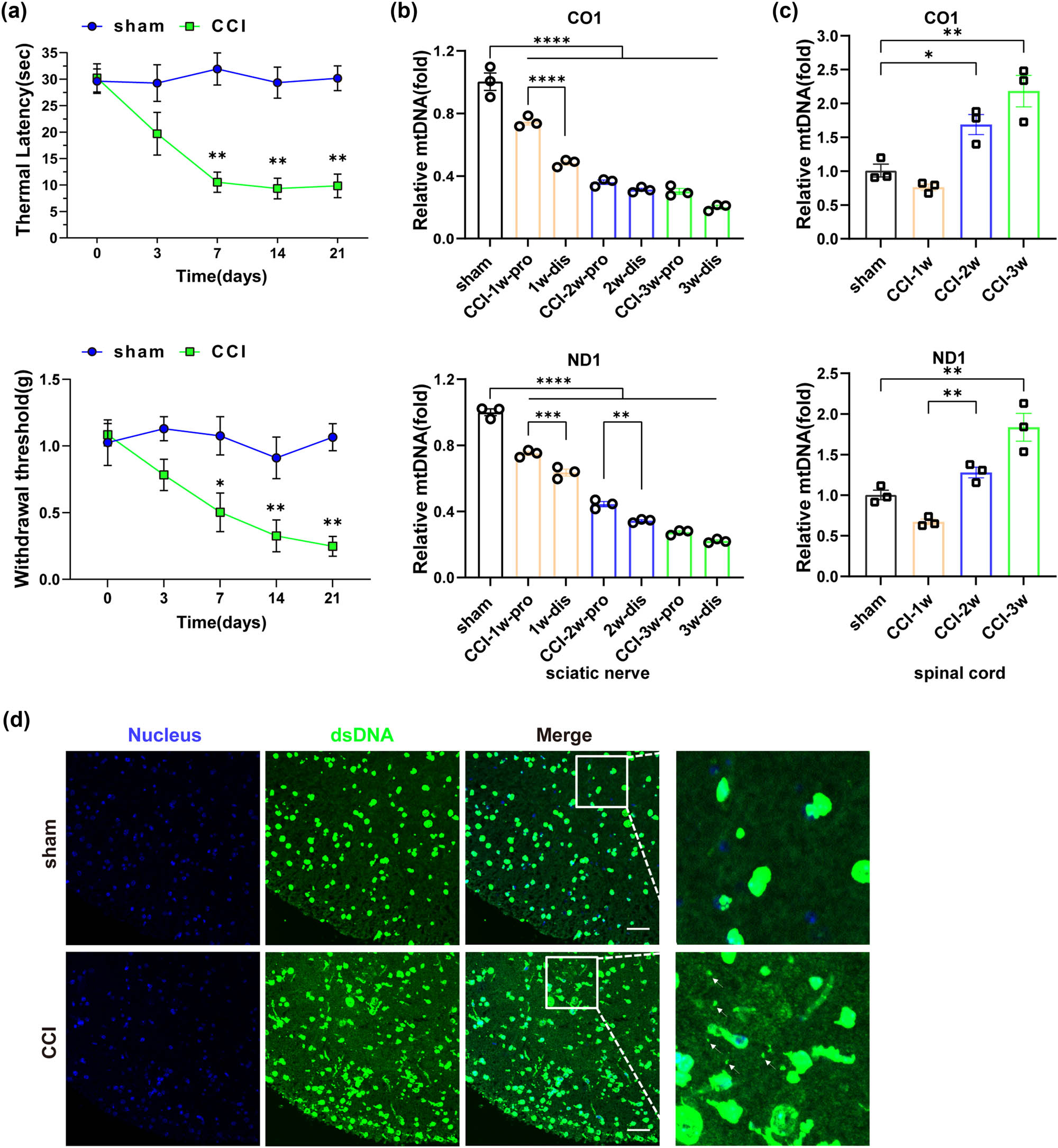
Changes in mtDNA content occurred in CCI mice following peripheral nerve injury. (a) Changes in mechanical threshold and thermal latency in mice (n = 3, *P < 0.05, **P < 0.01 compared to the sham group, the same time point values of PWMT and PWTL among groups were analyzed by the application of one-way ANOVA with Tukey’s post hoc test). (b) Changes in mtDNA content in the sciatic nerve by qPCR (n = 3, **P < 0.01, ***P <0.001, ****P < 0.0001, comparisons between the proximal and distal ends of the sciatic nerve within the same time group were performed using unpaired two-tailed t-test). (c) Changes in mtDNA content in the spinal cord by qPCR (n = 3, ***p = 0.001, ****P < 0.0001, using one-way ANOVA followed by Turkey’s test). (d) dsDNA in the spinal cord tissue of mice in each group was detected by immunofluorescent double-labeling with dsDNA labeled with anti-dsDNA (green), and nucleus labeled with DAPI (blue). The white arrow indicated free dsDNA, scale bar = 50 μM, and the framed image was amplified by four times.
3.2 Activation of cGAS-IFN signaling in the spinal cord of CCI mice
Considering the significant increase in mtDNA in the spinal cord of CCI mice observed 14 days post-CCI surgery, we next began our investigation by quantifying the mRNA expression levels of receptor molecules associated with mtDNA at this time point, specifically TLR9, NLRP3, and cGAS. As shown, the expression levels of all these receptors were upregulated after surgery in the CCI group (Figure 3a). cGAS, a more specific intracellular DNA receptor, was examined in the spinal cord of CCI mice and showed significant upregulation in the second week after surgery (Figure 3b). We then performed immunofluorescence staining analysis to investigate the colocalization of cGAS within neurons, microglia, and astrocytes in the spinal cord. The results revealed a higher expression of cGAS in microglia compared to astrocytes and neurons (Figure 3c), aligning with the findings of Sun [21]. In addition, the expression of IBA1 in the spinal cord was increased (Figure 3b). Meanwhile, an increase in the mRNA expression of interferon-β (IFN-β) (Figure 3d), a molecule that plays a crucial role in facilitating endogenous immune responses and pro-inflammatory reactions. Similarly, upregulation of mRNA expression of the inflammatory mediator tumor necrosis factor-α (TNF-α) was observed in the spinal cord of CCI mice by qPCR analysis (Figure 3e). To sum up, these data suggested the upregulation of mtDNA-related receptors and the associated inflammatory response after CCI surgery, while highlighting the increased presence of cGAS protein in microglia.
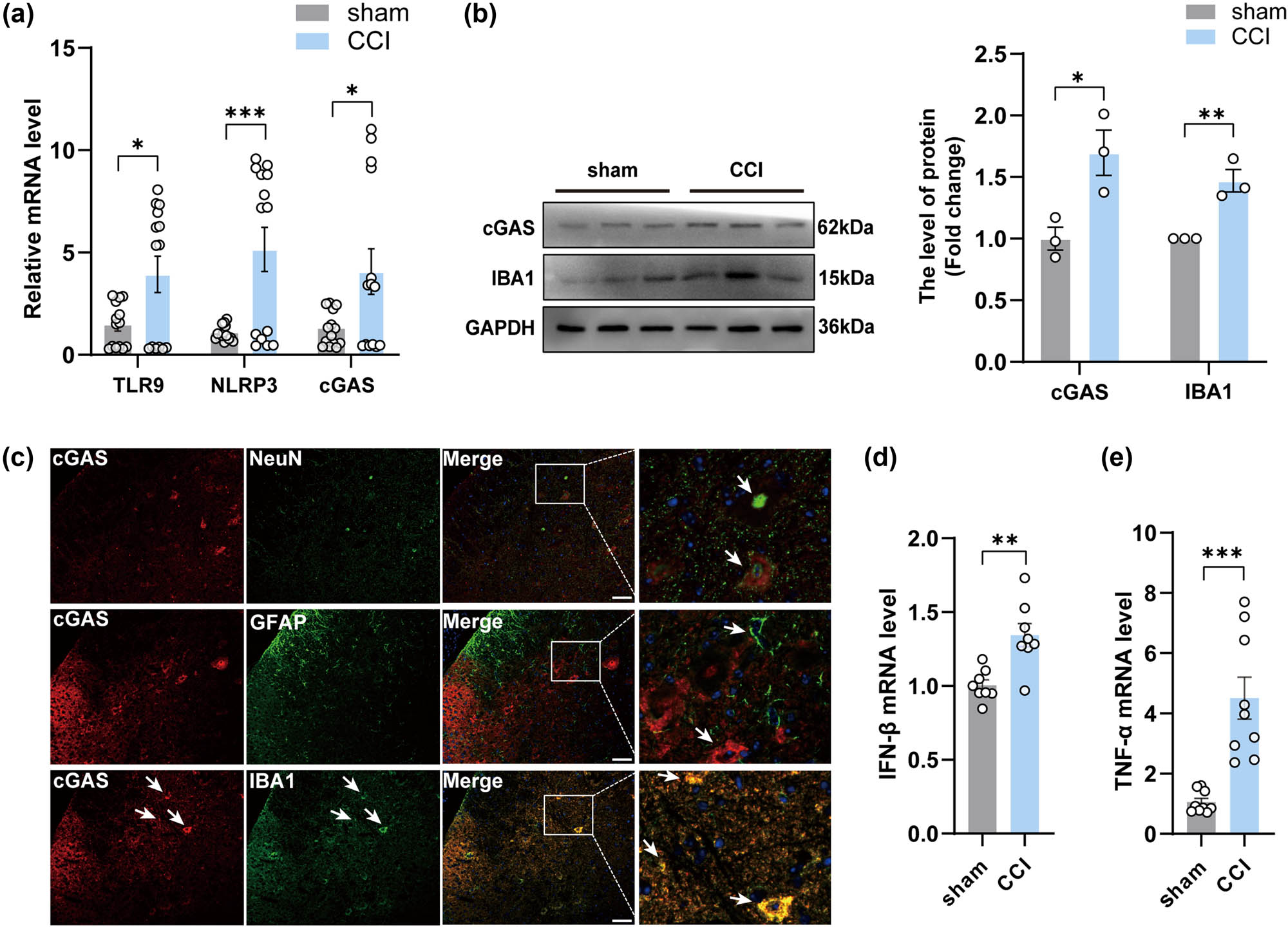
The activation of cGAS-IFN signaling occurred in the spinal cord of CCI mice. (a, d, and e) The mRNA expression levels of TLR9, NLRP3,cGAS, IFN-β and TNF-α in the spinal cord of groups (n = 3, **P < 0.01, ***P < 0.001, using unpaired two-tailed t-test). (b) The protein levels and quantification of cGAS in the spinal cord of mice in each group (n = 3, *P < 0.05, **P < 0.01 compared to the sham group, using unpaired two-tailed t-test). (c) Double immunofluorescence labeling of cGAS with GFAP (an astrocyte marker), NeuN (a neuronal marker), or IBA1 (a microglia marker) in the spinal cord of mice after CCI. scale bar = 50 μM, the framed image was amplified by four times.
3.3 cGAS inhibitors reversed decreased pain threshold and spinal cord inflammation in CCI mice
Studies have demonstrated that activation of the cGAS signaling induces the release of inflammatory factors and interferons. In addition, given the significant elevation of cGAS in the spinal cord of CCI mice observed in the above results, we investigated whether RU.521, a cGAS inhibitor, has therapeutic effects in NeP, and the mechanisms implicated. As depicted in the figure (Figure 4d), on the first day after injection (Day 11), there was no discernible improvement in thermal and mechanical allodynia in the CCI + RU.521 group. However, after three consecutive days of treatment, RU.521 partially reversed the decrease in PWMT and PWTL in the intervention group when compared to the untreated groups (CCI group and CCI + saline group). Pain thresholds in the saline-treated CCI mice group were not significantly different from those in the untreated CCI mice group. In addition, administration of RU.521 effectively suppressed the cGAS-IFN signaling pathway in CCI mice by inhibiting the expression of cGAS, phosphorylation of STING, IRF3, and IFN-β, which was confirmed by following the Western blotting and qPCR test (Figure 4a and b). Besides, inhibition of cGAS also led to a reduction in the expression of TNF-α (Figure 4c). Additionally, the alterations in fluorescence intensity observed in IBA1 staining indicated a significant reversal of microglial activation (Figure 4e). These data demonstrated that RU.521 effectively ameliorated allodynia and hyperalgesia in mice with NeP, indicating the involvement of the cGAS-IFN pathway in the pathogenesis of NeP.
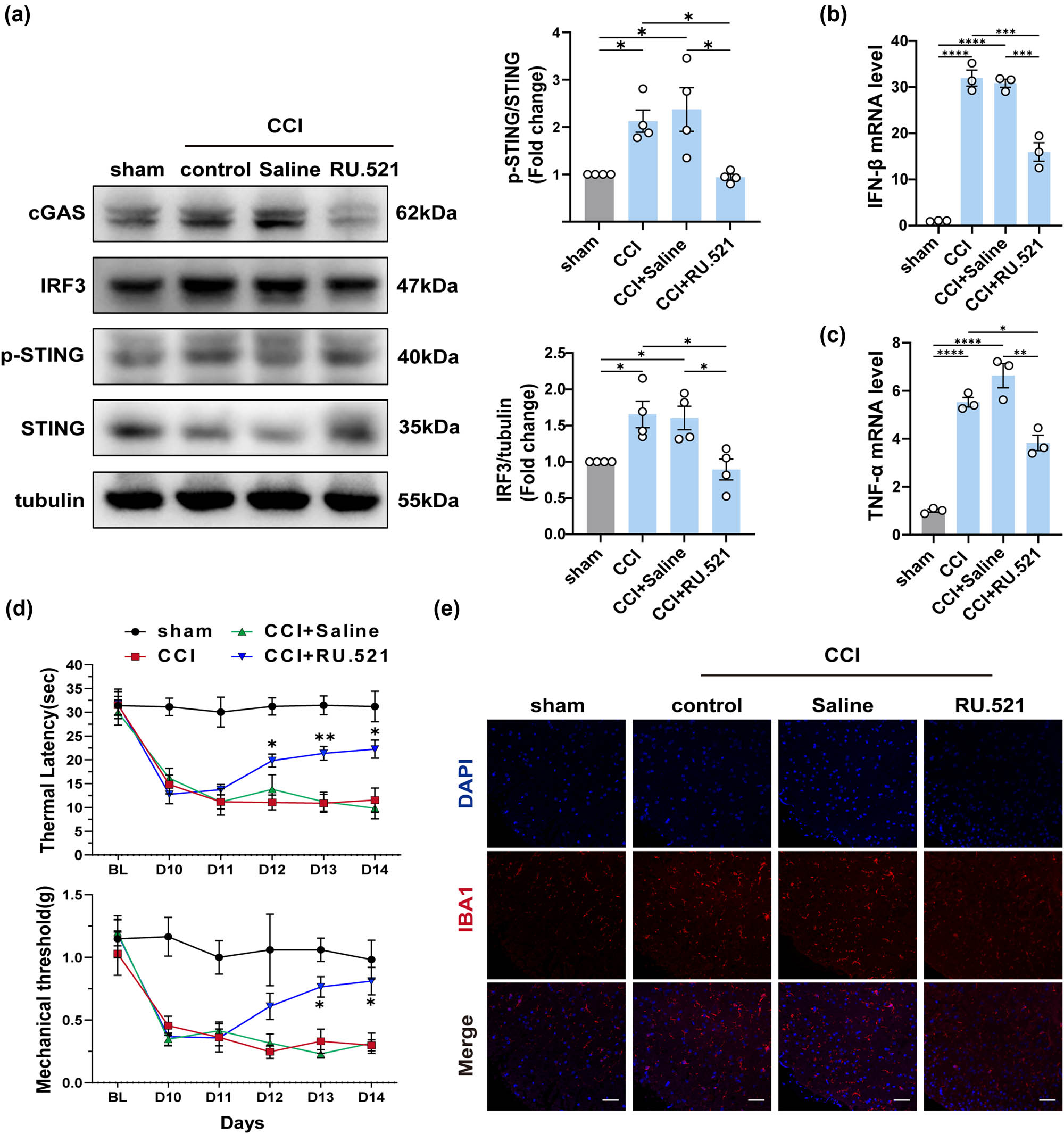
The pain threshold of mice with peripheral nerve damage is reversed by cGAS inhibitors. (a) The protein levels and quantification of cGAS, STING, IRF3, and p-STING in the spinal cord of each group (n = 4, *P < 0.05, **P < 0.01, using one-way ANOVA followed by Turkey’s test). (b and c) The mRNA levels of IFN-β and TNF-α in the spinal cord of mice in each group (n = 4, **P < 0.01, ***P < 0.001, ****P < 0.0001, using one-way ANOVA followed by Turkey’s test). (d) Thermal latency and mechanical thresholds were measured in each group of mice (n = 4, *P < 0.05, **P < 0.01 compared to the sham group, the same time point values of PWMT and PWTL among groups were analyzed by the application of one-way ANOVA with Tukey’s post hoc test). (e) Representative immunofluorescence images of IBA1 in the spinal cord of each group, n = 4, scale bar = 50 μM.
3.4 Neuron-derived mtDNA activated BV2 microglial cells and promoted the release of inflammatory factors
In view of the diversity of cells in the spinal cord of mice, and the uncontrollability of in vivo experiments, it was not possible to accurately assess the effect of mtDNA on microglia, so we embarked on an in vitro investigation using BV2 cells. After confirming that the stimulated cells release mtDNA into the cytoplasm or outside the cell (Figure S1). We isolated and purified mtDNA from neuro2 cells, transfected it into BV2 cells, and then detected the expression of IBA1 in BV2 cells following mtDNA transfection [23,24]. As shown, an initial upregulation of IBA1 was observed at the outset of transfection and confirmed by western blotting (Figure 5a). This was followed by a gradual increase in the concentration of TNF-α in the cell supernatant (Figure 5b). Meanwhile, ROS in the cell supernatant increased progressively over time (Figure 5c). In parallel, there was an increase in NO production, which is also a marker of microglial activation (Figure 5d). In summary, these findings suggested that mtDNA, when it enters BV2 cells, triggers the activation of these cells and the subsequent release of inflammatory factors.
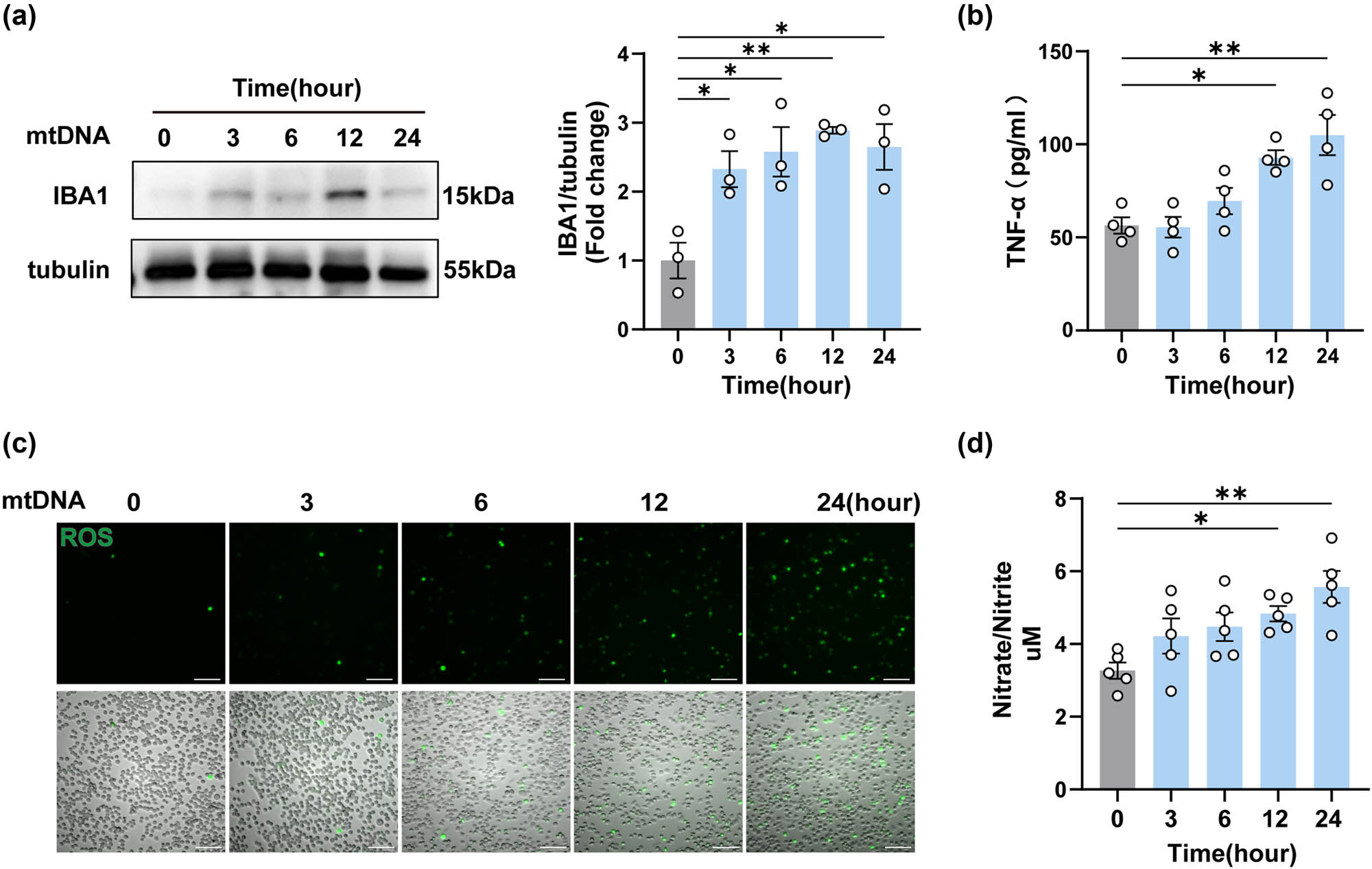
Neuron-derived mtDNA activated BV2 microglial cells and promoted the release of inflammatory factors. (a) The protein levels and quantification of IBA1 after mtDNA treatment on BV2 cells for different times (n = 3, *P < 0.05, **P < 0.01 compared to the 0-h group, using one-way ANOVA followed by Turkey’s test). (b) TNF-α in cell supernatants was detected by ELISA (n = 4, *P < 0.05, **P < 0.01, using one-way ANOVA followed by Turkey’s test). (c) Representative oxidative stress images of mtDNA-treated BV2 cells at different times, n = 3, scale bar, 100 μM. (d) The release levels of NO were detected in BV2 cells after mtDNA treatment (n = 5, *P < 0.05, **P < 0.01, using one-way ANOVA followed by Turkey’s test).
3.5 Neuron-derived mtDNA activated the cGAS-IFN signal pathway in BV2 cells
To further reveal the underlying pro-inflammatory mechanism of neuron-derived mtDNA. We closely monitored the expression of cGAS protein after mtDNA transfection. Notably, there was a substantial activation of cGAS protein expression after transfection, and this activation exhibited a positive correlation with both the concentration of transfected mtDNA and the duration of transfection (Figure 6a and b). Moreover, we investigated the downstream elements of the cGAS signaling pathway at 0, 3, 6, 12, and 24 h in BV2 cells (Figure 6c). Notably, an interesting trend emerged: while the expression of STING showed a decrease, its phosphorylation state (p-STING) exhibited an increase. This was accompanied by elevated expression of IRF3 and IFN-β (Figure 6d). These results demonstrated that neuron-derived mtDNA activated the cGAS-IFN signaling pathway in BV2 cells.
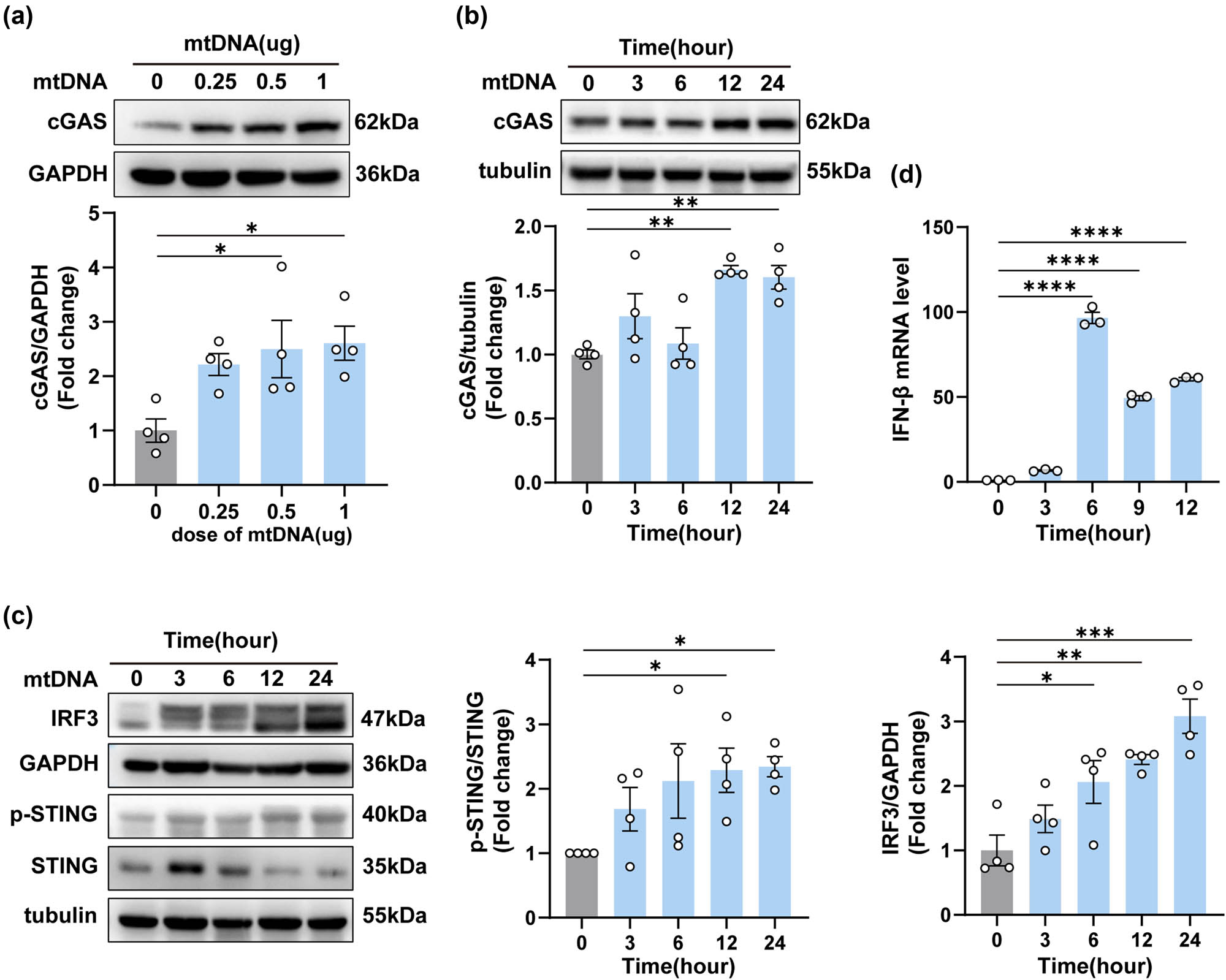
Neuron-derived mtDNA activated the cGAS-IFN signaling pathway in BV2 cells. (a) The protein levels and quantification of cGAS at different doses after the transfection of mtDNA (n = 3, *P < 0.05, using one-way ANOVA followed by Turkey’s test). (b and c) The protein levels and quantification of cGAS, IRF3, p-STING, and STING at different times after the transfection of mtDNA (n = 3, *P < 0.05, **P < 0.01, ***P < 0.001 compared to the 0-h group). (d) The mRNA expression levels of IFN-β (*P < 0.05, ****P < 0.0001, using one-way ANOVA followed by Turkey’s test).
3.6 cGAS inhibitors blocked mtDNA-induced inflammation in BV2 cells
To further confirm the role of cGAS in mtDNA-induced neuroinflammation, we used RU.521 to block the cGAS signaling pathway in BV2 cells. DNase I, an endonuclease, was employed to evaluate the efficacy of RU.521 by directly eliminating mtDNA [25,26]. Notably, there was a significant upsurge in cGAS expression in mtDNA-treated BV2 cells over 12 h. Pre-treatment with 10 μM RU.521 for 2 h effectively reversed the increase in cGAS expression (Figure 7a). We then detected the expression of phosphorylated STING and IRF3 in mtDNA-treated BV2 cells, comparing those pretreated or not with RU.521 or DNase I, by Western blotting and immunostaining (Figure 7a–c). Concurrently, we assessed the activated BV2 cells through immunofluorescence staining for IBA1 (Figure 7c). As shown, the degree of reduction in expression of phosphorylated STING, IRF3, and fluorescence intensity of IBA1 in mtDNA + RU.521 group was similar to the effect observed when mtDNA was hydrolyzed by DNase I before transfection. Notably, the mRNA expression of IFN-β (Figure 7d) and the release of TNF-α in cell supernatants in the mtDNA + RU.521 group also showed a significant reduction (Figure 7e). In light of these findings, it is reasonable to conclude that the inhibition of cGAS effectively attenuates mtDNA-induced inflammation in BV2 cells.
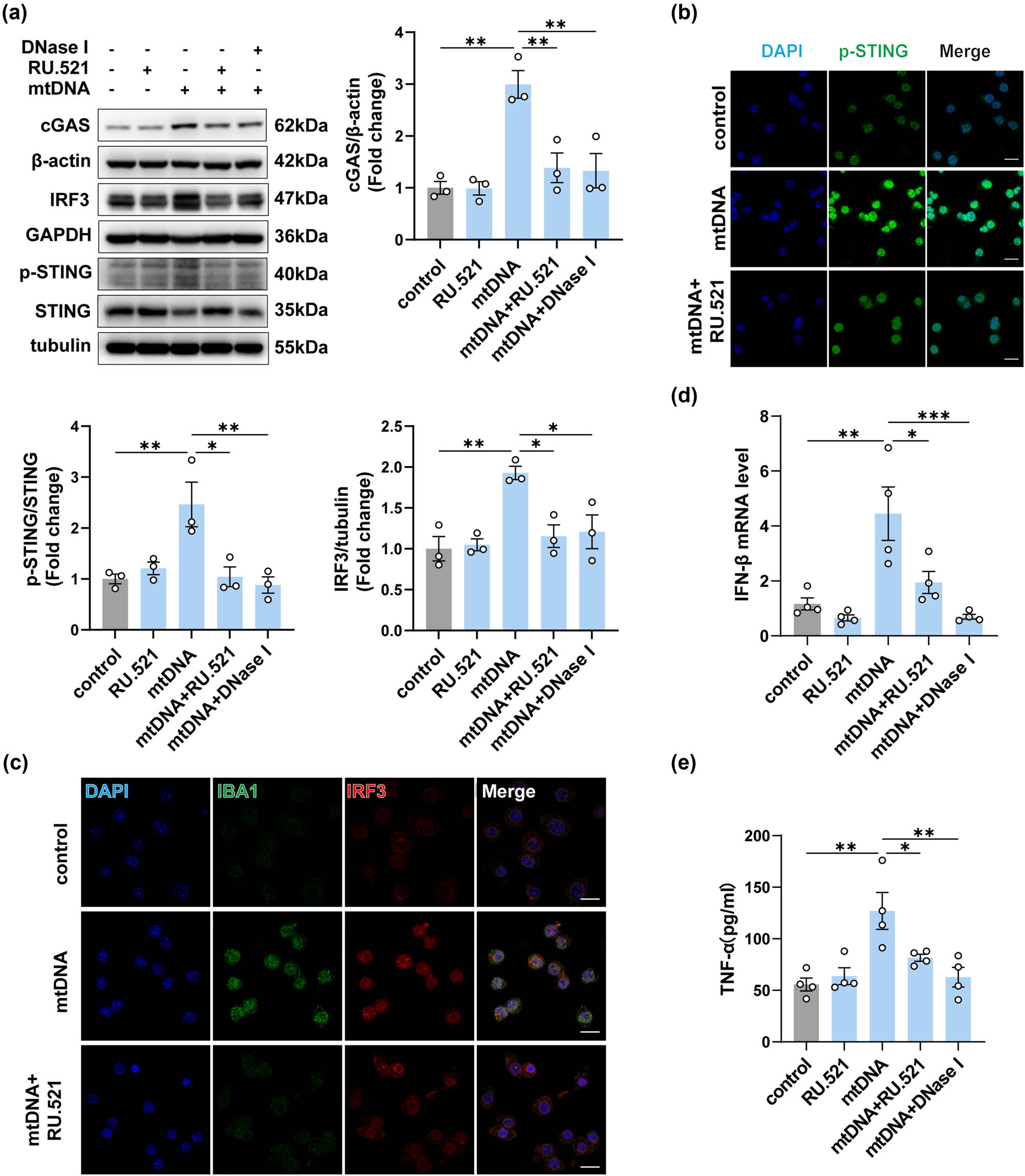
Inhibition of cGAS attenuated the inflammation in BV2 cells induced by neuron-derived mtDNA. (a) The protein levels and quantification of cGAS, STING, p-STING, and IRF3 in mtDNA-treated BV2 cells with or without intervention (n = 3, *P < 0.05, **P < 0.01, ***P < 0.001 compared to the control group). (b and c) Representative immunofluorescence images of IBA1, IRF3, and p-STING in each group, n = 3, scale bar = 20 μM. (d) The mRNA expression levels of IFN-β (n = 3, *P < 0.05, ***P < 0.001, using one-way ANOVA followed by Turkey’s test). (e) TNF-α in cell supernatants was detected by ELISA (n = 4, *P < 0.05, **P < 0.01, using one-way ANOVA followed by Turkey’s test).
4 Discussion
In this study, we demonstrated the elevation of mtDNA, cGAS signaling, activated microglia, and inflammatory mediators in the spinal cord of CCI mice, while the opposite trend was observed in the sciatic nerve. Inhibition of cGAS effectively alleviated allodynia and hyperalgesia in CCI mice and also attenuated the cGAS-induced inflammatory response in the spinal cord. Furthermore, our results confirmed that mtDNA triggers microglial activation and upregulates cGAS and its downstream inflammatory mediators in BV2 cells. Importantly, these responses are reversible upon administration of DNase I or cGAS inhibitors.
The release of mtDNA from neurons and glial cells has been extensively implicated in various neurological disorders [8,27,28]. In this study, the detection results of mtDNA in the sciatic nerve of CCI mice showed a significant decrease compared to sham-operated mice, and the extent of the decrease showed an increasing trend over time (Figure 2b), reflecting the temporal course of the decrease in pain threshold. This highlights the important role of mitochondrial dysfunction in nerve damage as well. It has been reported that stimulation of mitochondria after neuronal injury can cause mitochondrial damage, resulting in the release or degradation of mitochondrial contents (including mtDNA), and even directly lead to neuronal death under overstimulation [29]. The comparison between the distal group and the proximal group at the same time point after nerve ligation confirmed that the distal group may experience a greater reduction in mtDNA due to more severe cellular energy metabolism damage. We also examined the changes in mtDNA content in the spinal cord of CCI mice. Interestingly, mtDNA content in the spinal cord of CCI mice began to increase on day 14 after surgery, and the increase was most significant on day 21. The trend of increased mtDNA in the spinal cord is consistent with the pain threshold curve in mice, suggesting a possible regulatory role of mtDNA in NeP.
In subsequent cell experiments, we further confirmed that neuro2 cells can release a substantial amount of mtDNA into the cytoplasm and cell supernatant upon stimulation (Figure S1). Thus, the source of increased mtDNA in the spinal cord of CCI mice does not exclude the possibility of the release from neurons within the spinal cord following peripheral nerve damage. Besides, MS is characterized by elevated mtDNA levels and a robust inflammatory response [30]. This is consistent with our observation of increased mtDNA in the spinal cord of CCI mice. Nevertheless, real-time tracking is essential to confirm the source of elevated mtDNA in the spinal dorsal. More research is needed in the future. Moreover, previous studies have indicated a reduction in mtDNA detected in the cerebrospinal fluid during the early stages of Alzheimer’s and Parkinson’s diseases [30]. This contrasts with our findings of increased mtDNA levels in the spinal cord of CCI mice, which may be attributed to differences in experimental models and detection timing. In general, understanding the distinct impacts of mtDNA in different diseases remains a critical challenge that requires further investigation.
Microglia, acting as the vanguard of immune defense during nerve inflammation and injury [31], also contribute significantly to neuroinflammation, particularly as prolonged nociceptive stimulation promotes the creation of an inflammatory microenvironment [32,33]. Moreover, the release of inflammatory mediators from the affected tissue, such as IFN, NF-κB, and TNF-α, culminates in the sustained onset of pain [34]. Thus, in our in vitro experiments, we employed microglia as the recipient cells to delve deeper into the potential pro-inflammatory effects of mtDNA-induced activation of cGAS signaling in these cells. Our data are consistent with prior research [10]. Additionally, the utilization of RU.521 inhibited the cGAS-IFN signaling pathway in CCI mice, diminished spinal neuroinflammation, and further relieved NeP in CCI mice. This suggests that mtDNA is involved in the development of neuroinflammation by activating microglia.
It is worth mentioning that heightened levels of mitochondrial-derived RNA fragments in the blood of stroke patients have been observed to modulate gene expression and immune response in CD14 monocytes, exerting a significant influence on both central and peripheral immune responses via cholinergic pathways [35]. Thus, it is plausible that mtDNA could initiate additional signaling cascades and potentially induce inflammation in the spinal cord or periphery through alternative cell types.
While TLR9 and NLRP3 show significant increases, the role of cGAS, a cellular DNA sensor, has been extensively documented in neurological injury and neurodegenerative diseases, yet remains understudied in NeP [36,37]. Therefore, we intend to further investigate whether cGAS has an effect on NeP and its mechanism of action. When cGAS interacts with mtDNA, it catalyzes the formation of cGAMP, a secondary messenger that transmits signals, activating downstream signaling molecules such as STING and IRF3 [38]. This, in turn, triggers the transcription of the type I interferon pathway and the induction of innate immunity [39]. This is consistent with the findings in our study, and the application of RU.521 effectively reduces downstream protein levels of p-STING and IRF3. Our findings suggest that mtDNA likely contributes to neuroinflammation by upregulating cGAS signaling in NeP models.
Finally, some investigators have found that small fragments of mitochondria-derived RNA can target cholinergic mRNA and control cholinergic responses like miRNAs, which are involved in neuroinflammation mediated by a variety of neurological diseases [40,41]. In addition, a large number of non-coding RNAs, including lncRNAs, circRNAs, and miRNAs, have been reported to alleviate neuroinflammation and nociceptive hypersensitivity caused by nerve injury by modulating gene expression in damaged nerves and are potential therapeutic targets for NeP [42]. Mitochondrial DNA also functions as a derivative of mitochondria. These findings imply the existence of numerous unexplored aspects of mtDNA involvement in the nervous system, which necessitate further exploration in future studies.
5 Conclusion
The results of our study reveal that peripheral nerve injury leads to the accumulation of mtDNA and activation of cGAS-IFN signaling in the spinal cord of CCI mice. This activation subsequently increases mRNA transcription associated with the type I interferon response, leading to the induction of inflammation in mice. These results clearly validate the occurrence of mtDNA-mediated cGAS-IFN signaling activation as a regulatory mechanism in the inflammatory processes associated with NeP. The confirmation of the above mechanism provides new therapeutic targets for the treatment of NeP.
Acknowledgments
The authors sincerely thank all those who provided assistance during the research process.
-
Funding information: This research has been granted by Chongqing Natural Science Foundation (CSTB2023NSCQ-MSX0599 and CSTB2023NSCQ-MSX0683) and National Natural Science Foundation of China (81870883).
-
Author contributions: P.H.H., L.L., Y.H.C., and J.C. designed the experiments. L.L. and P.H.H. performed the experiments. Y.P.L. and D.Z. analyzed the data. P.H.H. prepared the manuscript with contributions from all authors.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Bannister K, Sachau J, Baron R, Dickenson AH. Neuropathic pain: mechanism-based therapeutics. Annu Rev Pharmacol. 2020;60:257–74.10.1146/annurev-pharmtox-010818-021524Search in Google Scholar PubMed
[2] Szok D, Tajti J, Nyari A, Vecsei L. Therapeutic approaches for peripheral and central neuropathic pain. Behav Neurol. 2019;2019:8685954.10.1155/2019/8685954Search in Google Scholar PubMed PubMed Central
[3] Mu Y, Mei Y, Chen Y, Li Y, Zhu D, Cui J, et al. Perisciatic nerve dexmedetomidine alleviates spinal oxidative stress and improves peripheral mitochondrial dynamic equilibrium in a neuropathic pain mouse model in an AMPK-dependent manner. Dis Markers. 2022;2022:1–14.10.1155/2022/6889676Search in Google Scholar PubMed PubMed Central
[4] D’Amico D, Olmer M, Fouassier AM, Valdes P, Andreux PA, Rinsch C, et al. Urolithin A improves mitochondrial health, reduces cartilage degeneration, and alleviates pain in osteoarthritis. Aging Cell. 2022;21(8):e13662.10.1111/acel.13662Search in Google Scholar PubMed PubMed Central
[5] Cheng XT, Huang N, Sheng ZH. Programming axonal mitochondrial maintenance and bioenergetics in neurodegeneration and regeneration. Neuron. 2022;110(12):1899–923.10.1016/j.neuron.2022.03.015Search in Google Scholar PubMed PubMed Central
[6] Zhao Y, Liu B, Xu L, Yu S, Fu J, Wang J, et al. ROS-induced mtDNA release: the emerging messenger for communication between neurons and innate immune cells during neurodegenerative disorder progression. Antioxidants-Basel. 2021;10(12):1917.10.3390/antiox10121917Search in Google Scholar PubMed PubMed Central
[7] Hinkle JT, Patel J, Panicker N, Karuppagounder SS, Biswas D, Belingon B, et al. Sting mediates neurodegeneration and neuroinflammation in nigrostriatal alpha-synucleinopathy. Proc Natl Acad Sci USA. 2022;119(15):e2118819119.10.1073/pnas.2118819119Search in Google Scholar PubMed PubMed Central
[8] Yu C, Davidson S, Harapas CR, Hilton JB, Mlodzianoski MJ, Laohamonthonkul P, et al. TDP-43 triggers mitochondrial DNA release via mPTP to activate cGAS/STING in ALS. Cell. 2020;183(3):636–49.10.1016/j.cell.2020.09.020Search in Google Scholar PubMed PubMed Central
[9] Pavlovic I, Zjukovskaja C, Nazir FH, Muller M, Wiberg A, Burman J. Cerebrospinal fluid mtDNA concentrations are increased in multiple sclerosis and were normalized after intervention with autologous hematopoietic stem cell transplantation. Mult Scler Relat Dis. 2024;84:105482.10.1016/j.msard.2024.105482Search in Google Scholar PubMed
[10] Liao Y, Cheng J, Kong X, Li S, Li X, Zhang M, et al. HDAC3 inhibition ameliorates ischemia/reperfusion-induced brain injury by regulating the microglial cGAS-STING pathway. Theranostics. 2020;10(21):9644–62.10.7150/thno.47651Search in Google Scholar PubMed PubMed Central
[11] Czarny P, Wigner P, Galecki P, Sliwinski T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog Neuro-Psychopharmacol. 2018;80(Pt C):309–21.10.1016/j.pnpbp.2017.06.036Search in Google Scholar PubMed
[12] Lin MM, Liu N, Qin ZH, Wang Y. Mitochondrial-derived damage-associated molecular patterns amplify neuroinflammation in neurodegenerative diseases. Acta Pharmacol Sin. 2022;43(10):2439–47.10.1038/s41401-022-00879-6Search in Google Scholar PubMed PubMed Central
[13] Liu J, Jia Z, Gong W. Circulating mitochondrial DNA stimulates innate immune signaling pathways to mediate acute kidney injury. Front Immunol. 2021;12:680648.10.3389/fimmu.2021.680648Search in Google Scholar PubMed PubMed Central
[14] Huang LS, Hong Z, Wu W, Xiong S, Zhong M, Gao X, et al. mtDNA activates cGAS signaling and suppresses the YAP-mediated endothelial cell proliferation program to promote inflammatory injury. Immunity. 2020;52(3):475–86.10.1016/j.immuni.2020.02.002Search in Google Scholar PubMed PubMed Central
[15] Liu Z, Wang M, Wang X, Bu Q, Wang Q, Su W, et al. XBP1 deficiency promotes hepatocyte pyroptosis by impairing mitophagy to activate mtDNA-cGAS-STING signaling in macrophages during acute liver injury. Redox Biol. 2022;52:102305.10.1016/j.redox.2022.102305Search in Google Scholar PubMed PubMed Central
[16] Zhao B, Xu P, Rowlett CM, Jing T, Shinde O, Lei Y, et al. The molecular basis of tight nuclear tethering and inactivation of cGAS. Nature. 2020;587(7835):673–7.10.1038/s41586-020-2749-zSearch in Google Scholar PubMed PubMed Central
[17] Tumburu L, Ghosh-Choudhary S, Seifuddin FT, Barbu EA, Yang S, Ahmad MM, et al. Circulating mitochondrial DNA is a proinflammatory DAMP in sickle cell disease. Blood. 2021;137(22):3116–26.10.1182/blood.2020009063Search in Google Scholar PubMed PubMed Central
[18] De Gaetano A, Solodka K, Zanini G, Selleri V, Mattioli AV, Nasi M, et al. Molecular mechanisms of mtDNA-mediated inflammation. Cells-Basel. 2021;10(11):2898.10.3390/cells10112898Search in Google Scholar PubMed PubMed Central
[19] Cheng AN, Cheng L, Kuo C, Lo YK, Chou H, Chen C, et al. Mitochondrial Lon-induced mtDNA leakage contributes to PD-L1–mediated immunoescape via STING-IFN signaling and extracellular vesicles. J Immunother Cancer. 2020;8(2):e001372.Search in Google Scholar
[20] Cheng AN, Cheng LC, Kuo CL, Lo YK, Chou HY, Chen CH, et al. Mitochondrial Lon-induced mtDNA leakage contributes to PD-L1-mediated immunoescape via STING-IFN signaling and extracellular vesicles. J Immunother Cancer. 2020;8(2):e001372.10.1136/jitc-2020-001372Search in Google Scholar PubMed PubMed Central
[21] Wu W, Zhang X, Wang S, Li T, Hao Q, Li S, et al. Pharmacological inhibition of the cGAS-STING signaling pathway suppresses microglial M1-polarization in the spinal cord and attenuates neuropathic pain. Neuropharmacology. 2022;217:109206.10.1016/j.neuropharm.2022.109206Search in Google Scholar PubMed
[22] Tai LW, Hung VK, Mei W, Qiu Q, Chung SK, Cheung CW. Effects of repeated central administration of endothelin type A receptor antagonist on the development of neuropathic pain in rats. Biomed Res Int. 2013;2013:529871.10.1155/2013/529871Search in Google Scholar PubMed PubMed Central
[23] Guo Y, Gu R, Gan D, Hu F, Li G, Xu G. Mitochondrial DNA drives noncanonical inflammation activation via cGAS–STING signaling pathway in retinal microvascular endothelial cells. Cell Commun Signal. 2020;18(1):172.10.1186/s12964-020-00637-3Search in Google Scholar PubMed PubMed Central
[24] Hu H, Zhao R, He Q, Cui C, Song J, Guo X, et al. cGAS‐STING mediates cytoplasmic mitochondrial‐DNA‐induced inflammatory signal transduction during accelerated senescence of pancreatic β‐cells induced by metabolic stress. Faseb J. 2022;36(5):e22266.10.1096/fj.202101988RSearch in Google Scholar PubMed
[25] Li Y, Chen H, Yang Q, Wan L, Zhao J, Wu Y, et al. Increased Drp1 promotes autophagy and ESCC progression by mtDNA stress mediated cGAS-STING pathway. J Exp Clin Cancer Res. 2022;41(1):76.10.1186/s13046-022-02262-zSearch in Google Scholar PubMed PubMed Central
[26] Yan X, Yao C, Fang C, Han M, Gong C, Hu D, et al. Rocaglamide promotes the infiltration and antitumor immunity of NK cells by activating cGAS-STING signaling in non-small cell lung cancer. Int J Biol Sci. 2022;18(2):585–98.10.7150/ijbs.65019Search in Google Scholar PubMed PubMed Central
[27] Nasi M, De Gaetano A, Bianchini E, De Biasi S, Gibellini L, Neroni A, et al. Mitochondrial damage-associated molecular patterns stimulate reactive oxygen species production in human microglia. Mol Cell neurosci. 2020;108:103538.10.1016/j.mcn.2020.103538Search in Google Scholar PubMed
[28] Li Q, Cao Y, Dang C, Han B, Han R, Ma H, et al. Inhibition of double‐strand DNA‐sensing cGAS ameliorates brain injury after ischemic stroke. Embo Mol Med. 2020;12(4):e11002.10.15252/emmm.201911002Search in Google Scholar PubMed PubMed Central
[29] Dai C, Guo Y, Chu X. Neuropathic pain: the dysfunction of Drp1, mitochondria, and ROS homeostasis. Neurotox Res. 2020;38(3):553–63.10.1007/s12640-020-00257-2Search in Google Scholar PubMed
[30] Pinti M, Ferraro D, Nasi M. Microglia activation: a role for mitochondrial DNA? Neural Regen Res. 2021;16(12):2393–4.10.4103/1673-5374.313034Search in Google Scholar PubMed PubMed Central
[31] Nishihara T, Tanaka J, Sekiya K, Nishikawa Y, Abe N, Hamada T, et al. Chronic constriction injury of the sciatic nerve in rats causes different activation modes of microglia between the anterior and posterior horns of the spinal cord. Neurochem Int. 2020;134:104672.10.1016/j.neuint.2020.104672Search in Google Scholar PubMed
[32] Tsuda M, Masuda T, Kohno K. Microglial diversity in neuropathic pain. Trends Neurosci. 2023;46(7):597–610.10.1016/j.tins.2023.05.001Search in Google Scholar PubMed
[33] Ji A, Xu J. Neuropathic pain: biomolecular intervention and imaging via targeting microglia activation. Biomolecules. 2021;11(9):1343.10.3390/biom11091343Search in Google Scholar PubMed PubMed Central
[34] Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiol Rev. 2021;101(1):259–301.10.1152/physrev.00045.2019Search in Google Scholar PubMed
[35] Winek K, Lobentanzer S, Nadorp B, Dubnov S, Dames C, Jagdmann S, et al. Transfer RNA fragments replace microRNA regulators of the cholinergic poststroke immune blockade. Proc Natl Acad Sci USA. 2020;117(51):3260616.10.1073/pnas.2013542117Search in Google Scholar PubMed PubMed Central
[36] Skopelja-Gardner S, An J, Elkon KB. Role of the cGAS-STING pathway in systemic and organ-specific diseases. Nat Rev Nephrol. 2022;18(9):558–72.10.1038/s41581-022-00589-6Search in Google Scholar PubMed PubMed Central
[37] Chen K, Lai C, Su Y, Bao WD, Yang LN, Xu PP, et al. cGAS-STING-mediated IFN-I response in host defense and neuroinflammatory diseases. Curr Neuropharmacol. 2022;20(2):362–71.10.2174/1570159X19666210924110144Search in Google Scholar PubMed PubMed Central
[38] Oduro PK, Zheng X, Wei J, Yang Y, Wang Y, Zhang H, et al. The cGAS-STING signaling in cardiovascular and metabolic diseases: Future novel target option for pharmacotherapy. Acta Pharm Sin B. 2022;12(1):50–75.10.1016/j.apsb.2021.05.011Search in Google Scholar PubMed PubMed Central
[39] He B, Yu H, Liu S, Wan H, Fu S, Liu S, et al. Mitochondrial cristae architecture protects against mtDNA release and inflammation. Cell Rep. 2022;41(10):111774.10.1016/j.celrep.2022.111774Search in Google Scholar PubMed
[40] Shulman D, Dubnov S, Zorbaz T, Madrer N, Paldor I, Bennett DA, et al. Sex-specific declines in cholinergic-targeting tRNA fragments in the nucleus accumbens in Alzheimer’s disease. Alzheimers Dement. 2023;19(11):5159–72.10.1002/alz.13095Search in Google Scholar PubMed PubMed Central
[41] Paldor I, Madrer N, Vaknine TS, Shulman D, Greenberg DS, Soreq H. Cerebrospinal fluid and blood profiles of transfer RNA fragments show age, sex, and Parkinson’s disease-related changes. J Neurochem. 2023;164(5):671–83.10.1111/jnc.15723Search in Google Scholar PubMed
[42] Song G, Yang Z, Guo J, Zheng Y, Su X, Wang X. Interactions among lncRNAs/circRNAs, miRNAs, and mRNAs in neuropathic pain. Neurotherapeutics. 2020;17(3):917–31.10.1007/s13311-020-00881-ySearch in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Constitutive and evoked release of ATP in adult mouse olfactory epithelium
- LARP1 knockdown inhibits cultured gastric carcinoma cell cycle progression and metastatic behavior
- PEGylated porcine–human recombinant uricase: A novel fusion protein with improved efficacy and safety for the treatment of hyperuricemia and renal complications
- Research progress on ocular complications caused by type 2 diabetes mellitus and the function of tears and blepharons
- The role and mechanism of esketamine in preventing and treating remifentanil-induced hyperalgesia based on the NMDA receptor–CaMKII pathway
- Brucella infection combined with Nocardia infection: A case report and literature review
- Detection of serum interleukin-18 level and neutrophil/lymphocyte ratio in patients with antineutrophil cytoplasmic antibody-associated vasculitis and its clinical significance
- Ang-1, Ang-2, and Tie2 are diagnostic biomarkers for Henoch-Schönlein purpura and pediatric-onset systemic lupus erythematous
- PTTG1 induces pancreatic cancer cell proliferation and promotes aerobic glycolysis by regulating c-myc
- Role of serum B-cell-activating factor and interleukin-17 as biomarkers in the classification of interstitial pneumonia with autoimmune features
- Effectiveness and safety of a mumps containing vaccine in preventing laboratory-confirmed mumps cases from 2002 to 2017: A meta-analysis
- Low levels of sex hormone-binding globulin predict an increased breast cancer risk and its underlying molecular mechanisms
- A case of Trousseau syndrome: Screening, detection and complication
- Application of the integrated airway humidification device enhances the humidification effect of the rabbit tracheotomy model
- Preparation of Cu2+/TA/HAP composite coating with anti-bacterial and osteogenic potential on 3D-printed porous Ti alloy scaffolds for orthopedic applications
- Aquaporin-8 promotes human dermal fibroblasts to counteract hydrogen peroxide-induced oxidative damage: A novel target for management of skin aging
- Current research and evidence gaps on placental development in iron deficiency anemia
- Single-nucleotide polymorphism rs2910829 in PDE4D is related to stroke susceptibility in Chinese populations: The results of a meta-analysis
- Pheochromocytoma-induced myocardial infarction: A case report
- Kaempferol regulates apoptosis and migration of neural stem cells to attenuate cerebral infarction by O‐GlcNAcylation of β-catenin
- Sirtuin 5 regulates acute myeloid leukemia cell viability and apoptosis by succinylation modification of glycine decarboxylase
- Apigenin 7-glucoside impedes hypoxia-induced malignant phenotypes of cervical cancer cells in a p16-dependent manner
- KAT2A changes the function of endometrial stromal cells via regulating the succinylation of ENO1
- Current state of research on copper complexes in the treatment of breast cancer
- Exploring antioxidant strategies in the pathogenesis of ALS
- Helicobacter pylori causes gastric dysbacteriosis in chronic gastritis patients
- IL-33/soluble ST2 axis is associated with radiation-induced cardiac injury
- The predictive value of serum NLR, SII, and OPNI for lymph node metastasis in breast cancer patients with internal mammary lymph nodes after thoracoscopic surgery
- Carrying SNP rs17506395 (T > G) in TP63 gene and CCR5Δ32 mutation associated with the occurrence of breast cancer in Burkina Faso
- P2X7 receptor: A receptor closely linked with sepsis-associated encephalopathy
- Probiotics for inflammatory bowel disease: Is there sufficient evidence?
- Identification of KDM4C as a gene conferring drug resistance in multiple myeloma
- Microbial perspective on the skin–gut axis and atopic dermatitis
- Thymosin α1 combined with XELOX improves immune function and reduces serum tumor markers in colorectal cancer patients after radical surgery
- Highly specific vaginal microbiome signature for gynecological cancers
- Sample size estimation for AQP4-IgG seropositive optic neuritis: Retinal damage detection by optical coherence tomography
- The effects of SDF-1 combined application with VEGF on femoral distraction osteogenesis in rats
- Fabrication and characterization of gold nanoparticles using alginate: In vitro and in vivo assessment of its administration effects with swimming exercise on diabetic rats
- Mitigating digestive disorders: Action mechanisms of Mediterranean herbal active compounds
- Distribution of CYP2D6 and CYP2C19 gene polymorphisms in Han and Uygur populations with breast cancer in Xinjiang, China
- VSP-2 attenuates secretion of inflammatory cytokines induced by LPS in BV2 cells by mediating the PPARγ/NF-κB signaling pathway
- Factors influencing spontaneous hypothermia after emergency trauma and the construction of a predictive model
- Long-term administration of morphine specifically alters the level of protein expression in different brain regions and affects the redox state
- Application of metagenomic next-generation sequencing technology in the etiological diagnosis of peritoneal dialysis-associated peritonitis
- Clinical diagnosis, prevention, and treatment of neurodyspepsia syndrome using intelligent medicine
- Case report: Successful bronchoscopic interventional treatment of endobronchial leiomyomas
- Preliminary investigation into the genetic etiology of short stature in children through whole exon sequencing of the core family
- Cystic adenomyoma of the uterus: Case report and literature review
- Mesoporous silica nanoparticles as a drug delivery mechanism
- Dynamic changes in autophagy activity in different degrees of pulmonary fibrosis in mice
- Vitamin D deficiency and inflammatory markers in type 2 diabetes: Big data insights
- Lactate-induced IGF1R protein lactylation promotes proliferation and metabolic reprogramming of lung cancer cells
- Meta-analysis on the efficacy of allogeneic hematopoietic stem cell transplantation to treat malignant lymphoma
- Mitochondrial DNA drives neuroinflammation through the cGAS-IFN signaling pathway in the spinal cord of neuropathic pain mice
- Application value of artificial intelligence algorithm-based magnetic resonance multi-sequence imaging in staging diagnosis of cervical cancer
- Embedded monitoring system and teaching of artificial intelligence online drug component recognition
- Investigation into the association of FNDC1 and ADAMTS12 gene expression with plumage coloration in Muscovy ducks
- Yak meat content in feed and its impact on the growth of rats
- A rare case of Richter transformation with breast involvement: A case report and literature review
- First report of Nocardia wallacei infection in an immunocompetent patient in Zhejiang province
- Rhodococcus equi and Brucella pulmonary mass in immunocompetent: A case report and literature review
- Downregulation of RIP3 ameliorates the left ventricular mechanics and function after myocardial infarction via modulating NF-κB/NLRP3 pathway
- Evaluation of the role of some non-enzymatic antioxidants among Iraqi patients with non-alcoholic fatty liver disease
- The role of Phafin proteins in cell signaling pathways and diseases
- Ten-year anemia as initial manifestation of Castleman disease in the abdominal cavity: A case report
- Coexistence of hereditary spherocytosis with SPTB P.Trp1150 gene variant and Gilbert syndrome: A case report and literature review
- Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells
- Exploratory evaluation supported by experimental and modeling approaches of Inula viscosa root extract as a potent corrosion inhibitor for mild steel in a 1 M HCl solution
- Imaging manifestations of ductal adenoma of the breast: A case report
- Gut microbiota and sleep: Interaction mechanisms and therapeutic prospects
- Isomangiferin promotes the migration and osteogenic differentiation of rat bone marrow mesenchymal stem cells
- Prognostic value and microenvironmental crosstalk of exosome-related signatures in human epidermal growth factor receptor 2 positive breast cancer
- Circular RNAs as potential biomarkers for male severe sepsis
- Knockdown of Stanniocalcin-1 inhibits growth and glycolysis in oral squamous cell carcinoma cells
- The expression and biological role of complement C1s in esophageal squamous cell carcinoma
- A novel GNAS mutation in pseudohypoparathyroidism type 1a with articular flexion deformity: A case report
- Predictive value of serum magnesium levels for prognosis in patients with non-small cell lung cancer undergoing EGFR-TKI therapy
- HSPB1 alleviates acute-on-chronic liver failure via the P53/Bax pathway
- IgG4-related disease complicated by PLA2R-associated membranous nephropathy: A case report
- Baculovirus-mediated endostatin and angiostatin activation of autophagy through the AMPK/AKT/mTOR pathway inhibits angiogenesis in hepatocellular carcinoma
- Metformin mitigates osteoarthritis progression by modulating the PI3K/AKT/mTOR signaling pathway and enhancing chondrocyte autophagy
- Evaluation of the activity of antimicrobial peptides against bacterial vaginosis
- Atypical presentation of γ/δ mycosis fungoides with an unusual phenotype and SOCS1 mutation
- Analysis of the microecological mechanism of diabetic kidney disease based on the theory of “gut–kidney axis”: A systematic review
- Omega-3 fatty acids prevent gestational diabetes mellitus via modulation of lipid metabolism
- Refractory hypertension complicated with Turner syndrome: A case report
- Interaction of ncRNAs and the PI3K/AKT/mTOR pathway: Implications for osteosarcoma
- Association of low attenuation area scores with pulmonary function and clinical prognosis in patients with chronic obstructive pulmonary disease
- Long non-coding RNAs in bone formation: Key regulators and therapeutic prospects
- The deubiquitinating enzyme USP35 regulates the stability of NRF2 protein
- Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as potential diagnostic markers for rebleeding in patients with esophagogastric variceal bleeding
- G protein-coupled receptor 1 participating in the mechanism of mediating gestational diabetes mellitus by phosphorylating the AKT pathway
- LL37-mtDNA regulates viability, apoptosis, inflammation, and autophagy in lipopolysaccharide-treated RLE-6TN cells by targeting Hsp90aa1
- The analgesic effect of paeoniflorin: A focused review
- Chemical composition’s effect on Solanum nigrum Linn.’s antioxidant capacity and erythrocyte protection: Bioactive components and molecular docking analysis
- Knockdown of HCK promotes HREC cell viability and inner blood–retinal barrier integrity by regulating the AMPK signaling pathway
- The role of rapamycin in the PINK1/Parkin signaling pathway in mitophagy in podocytes
- Laryngeal non-Hodgkin lymphoma: Report of four cases and review of the literature
- Clinical value of macrogenome next-generation sequencing on infections
- Overview of dendritic cells and related pathways in autoimmune uveitis
- TAK-242 alleviates diabetic cardiomyopathy via inhibiting pyroptosis and TLR4/CaMKII/NLRP3 pathway
- Hypomethylation in promoters of PGC-1α involved in exercise-driven skeletal muscular alterations in old age
- Profile and antimicrobial susceptibility patterns of bacteria isolated from effluents of Kolladiba and Debark hospitals
- The expression and clinical significance of syncytin-1 in serum exosomes of hepatocellular carcinoma patients
- A histomorphometric study to evaluate the therapeutic effects of biosynthesized silver nanoparticles on the kidneys infected with Plasmodium chabaudi
- PGRMC1 and PAQR4 are promising molecular targets for a rare subtype of ovarian cancer
- Analysis of MDA, SOD, TAOC, MNCV, SNCV, and TSS scores in patients with diabetes peripheral neuropathy
- SLIT3 deficiency promotes non-small cell lung cancer progression by modulating UBE2C/WNT signaling
- The relationship between TMCO1 and CALR in the pathological characteristics of prostate cancer and its effect on the metastasis of prostate cancer cells
- Heterogeneous nuclear ribonucleoprotein K is a potential target for enhancing the chemosensitivity of nasopharyngeal carcinoma
- PHB2 alleviates retinal pigment epithelium cell fibrosis by suppressing the AGE–RAGE pathway
- Anti-γ-aminobutyric acid-B receptor autoimmune encephalitis with syncope as the initial symptom: Case report and literature review
- Comparative analysis of chloroplast genome of Lonicera japonica cv. Damaohua
- Human umbilical cord mesenchymal stem cells regulate glutathione metabolism depending on the ERK–Nrf2–HO-1 signal pathway to repair phosphoramide mustard-induced ovarian cancer cells
- Electroacupuncture on GB acupoints improves osteoporosis via the estradiol–PI3K–Akt signaling pathway
- Renalase protects against podocyte injury by inhibiting oxidative stress and apoptosis in diabetic nephropathy
- Review: Dicranostigma leptopodum: A peculiar plant of Papaveraceae
- Combination effect of flavonoids attenuates lung cancer cell proliferation by inhibiting the STAT3 and FAK signaling pathway
- Renal microangiopathy and immune complex glomerulonephritis induced by anti-tumour agents: A case report
- Correlation analysis of AVPR1a and AVPR2 with abnormal water and sodium and potassium metabolism in rats
- Gastrointestinal health anti-diarrheal mixture relieves spleen deficiency-induced diarrhea through regulating gut microbiota
- Myriad factors and pathways influencing tumor radiotherapy resistance
- Exploring the effects of culture conditions on Yapsin (YPS) gene expression in Nakaseomyces glabratus
- Screening of prognostic core genes based on cell–cell interaction in the peripheral blood of patients with sepsis
- Coagulation factor II thrombin receptor as a promising biomarker in breast cancer management
- Ileocecal mucinous carcinoma misdiagnosed as incarcerated hernia: A case report
- Methyltransferase like 13 promotes malignant behaviors of bladder cancer cells through targeting PI3K/ATK signaling pathway
- The debate between electricity and heat, efficacy and safety of irreversible electroporation and radiofrequency ablation in the treatment of liver cancer: A meta-analysis
- ZAG promotes colorectal cancer cell proliferation and epithelial–mesenchymal transition by promoting lipid synthesis
- Baicalein inhibits NLRP3 inflammasome activation and mitigates placental inflammation and oxidative stress in gestational diabetes mellitus
- Impact of SWCNT-conjugated senna leaf extract on breast cancer cells: A potential apoptotic therapeutic strategy
- MFAP5 inhibits the malignant progression of endometrial cancer cells in vitro
- Major ozonated autohemotherapy promoted functional recovery following spinal cord injury in adult rats via the inhibition of oxidative stress and inflammation
- Axodendritic targeting of TAU and MAP2 and microtubule polarization in iPSC-derived versus SH-SY5Y-derived human neurons
- Differential expression of phosphoinositide 3-kinase/protein kinase B and Toll-like receptor/nuclear factor kappa B signaling pathways in experimental obesity Wistar rat model
- The therapeutic potential of targeting Oncostatin M and the interleukin-6 family in retinal diseases: A comprehensive review
- BA inhibits LPS-stimulated inflammatory response and apoptosis in human middle ear epithelial cells by regulating the Nf-Kb/Iκbα axis
- Role of circRMRP and circRPL27 in chronic obstructive pulmonary disease
- Investigating the role of hyperexpressed HCN1 in inducing myocardial infarction through activation of the NF-κB signaling pathway
- Characterization of phenolic compounds and evaluation of anti-diabetic potential in Cannabis sativa L. seeds: In vivo, in vitro, and in silico studies
- Quantitative immunohistochemistry analysis of breast Ki67 based on artificial intelligence
- Ecology and Environmental Science
- Screening of different growth conditions of Bacillus subtilis isolated from membrane-less microbial fuel cell toward antimicrobial activity profiling
- Degradation of a mixture of 13 polycyclic aromatic hydrocarbons by commercial effective microorganisms
- Evaluation of the impact of two citrus plants on the variation of Panonychus citri (Acari: Tetranychidae) and beneficial phytoseiid mites
- Prediction of present and future distribution areas of Juniperus drupacea Labill and determination of ethnobotany properties in Antalya Province, Türkiye
- Population genetics of Todarodes pacificus (Cephalopoda: Ommastrephidae) in the northwest Pacific Ocean via GBS sequencing
- A comparative analysis of dendrometric, macromorphological, and micromorphological characteristics of Pistacia atlantica subsp. atlantica and Pistacia terebinthus in the middle Atlas region of Morocco
- Macrofungal sporocarp community in the lichen Scots pine forests
- Assessing the proximate compositions of indigenous forage species in Yemen’s pastoral rangelands
- Food Science
- Gut microbiota changes associated with low-carbohydrate diet intervention for obesity
- Reexamination of Aspergillus cristatus phylogeny in dark tea: Characteristics of the mitochondrial genome
- Differences in the flavonoid composition of the leaves, fruits, and branches of mulberry are distinguished based on a plant metabolomics approach
- Investigating the impact of wet rendering (solventless method) on PUFA-rich oil from catfish (Clarias magur) viscera
- Non-linear associations between cardiovascular metabolic indices and metabolic-associated fatty liver disease: A cross-sectional study in the US population (2017–2020)
- Knockdown of USP7 alleviates atherosclerosis in ApoE-deficient mice by regulating EZH2 expression
- Utility of dairy microbiome as a tool for authentication and traceability
- Agriculture
- Enhancing faba bean (Vicia faba L.) productivity through establishing the area-specific fertilizer rate recommendation in southwest Ethiopia
- Impact of novel herbicide based on synthetic auxins and ALS inhibitor on weed control
- Perspectives of pteridophytes microbiome for bioremediation in agricultural applications
- Fertilizer application parameters for drip-irrigated peanut based on the fertilizer effect function established from a “3414” field trial
- Improving the productivity and profitability of maize (Zea mays L.) using optimum blended inorganic fertilization
- Application of leaf multispectral analyzer in comparison to hyperspectral device to assess the diversity of spectral reflectance indices in wheat genotypes
- Animal Sciences
- Knockdown of ANP32E inhibits colorectal cancer cell growth and glycolysis by regulating the AKT/mTOR pathway
- Development of a detection chip for major pathogenic drug-resistant genes and drug targets in bovine respiratory system diseases
- Exploration of the genetic influence of MYOT and MB genes on the plumage coloration of Muscovy ducks
- Transcriptome analysis of adipose tissue in grazing cattle: Identifying key regulators of fat metabolism
- Comparison of nutritional value of the wild and cultivated spiny loaches at three growth stages
- Transcriptomic analysis of liver immune response in Chinese spiny frog (Quasipaa spinosa) infected with Proteus mirabilis
- Disruption of BCAA degradation is a critical characteristic of diabetic cardiomyopathy revealed by integrated transcriptome and metabolome analysis
- Plant Sciences
- Effect of long-term in-row branch covering on soil microorganisms in pear orchards
- Photosynthetic physiological characteristics, growth performance, and element concentrations reveal the calcicole–calcifuge behaviors of three Camellia species
- Transcriptome analysis reveals the mechanism of NaHCO3 promoting tobacco leaf maturation
- Bioinformatics, expression analysis, and functional verification of allene oxide synthase gene HvnAOS1 and HvnAOS2 in qingke
- Water, nitrogen, and phosphorus coupling improves gray jujube fruit quality and yield
- Improving grape fruit quality through soil conditioner: Insights from RNA-seq analysis of Cabernet Sauvignon roots
- Role of Embinin in the reabsorption of nucleus pulposus in lumbar disc herniation: Promotion of nucleus pulposus neovascularization and apoptosis of nucleus pulposus cells
- Revealing the effects of amino acid, organic acid, and phytohormones on the germination of tomato seeds under salinity stress
- Combined effects of nitrogen fertilizer and biochar on the growth, yield, and quality of pepper
- Comprehensive phytochemical and toxicological analysis of Chenopodium ambrosioides (L.) fractions
- Impact of “3414” fertilization on the yield and quality of greenhouse tomatoes
- Exploring the coupling mode of water and fertilizer for improving growth, fruit quality, and yield of the pear in the arid region
- Metagenomic analysis of endophytic bacteria in seed potato (Solanum tuberosum)
- Antibacterial, antifungal, and phytochemical properties of Salsola kali ethanolic extract
- Exploring the hepatoprotective properties of citronellol: In vitro and in silico studies on ethanol-induced damage in HepG2 cells
- Enhanced osmotic dehydration of watermelon rind using honey–sucrose solutions: A study on pre-treatment efficacy and mass transfer kinetics
- Effects of exogenous 2,4-epibrassinolide on photosynthetic traits of 53 cowpea varieties under NaCl stress
- Comparative transcriptome analysis of maize (Zea mays L.) seedlings in response to copper stress
- An optimization method for measuring the stomata in cassava (Manihot esculenta Crantz) under multiple abiotic stresses
- Fosinopril inhibits Ang II-induced VSMC proliferation, phenotype transformation, migration, and oxidative stress through the TGF-β1/Smad signaling pathway
- Antioxidant and antimicrobial activities of Salsola imbricata methanolic extract and its phytochemical characterization
- Bioengineering and Biotechnology
- Absorbable calcium and phosphorus bioactive membranes promote bone marrow mesenchymal stem cells osteogenic differentiation for bone regeneration
- New advances in protein engineering for industrial applications: Key takeaways
- An overview of the production and use of Bacillus thuringiensis toxin
- Research progress of nanoparticles in diagnosis and treatment of hepatocellular carcinoma
- Bioelectrochemical biosensors for water quality assessment and wastewater monitoring
- PEI/MMNs@LNA-542 nanoparticles alleviate ICU-acquired weakness through targeted autophagy inhibition and mitochondrial protection
- Unleashing of cytotoxic effects of thymoquinone-bovine serum albumin nanoparticles on A549 lung cancer cells
- Erratum
- Erratum to “Investigating the association between dietary patterns and glycemic control among children and adolescents with T1DM”
- Erratum to “Activation of hypermethylated P2RY1 mitigates gastric cancer by promoting apoptosis and inhibiting proliferation”
- Retraction
- Retraction to “MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB”
- Retraction to “A data mining technique for detecting malignant mesothelioma cancer using multiple regression analysis”
- Special Issue on Advances in Neurodegenerative Disease Research and Treatment
- Transplantation of human neural stem cell prevents symptomatic motor behavior disability in a rat model of Parkinson’s disease
- Special Issue on Multi-omics
- Inflammasome complex genes with clinical relevance suggest potential as therapeutic targets for anti-tumor drugs in clear cell renal cell carcinoma
- Gastroesophageal varices in primary biliary cholangitis with anti-centromere antibody positivity: Early onset?
Articles in the same Issue
- Biomedical Sciences
- Constitutive and evoked release of ATP in adult mouse olfactory epithelium
- LARP1 knockdown inhibits cultured gastric carcinoma cell cycle progression and metastatic behavior
- PEGylated porcine–human recombinant uricase: A novel fusion protein with improved efficacy and safety for the treatment of hyperuricemia and renal complications
- Research progress on ocular complications caused by type 2 diabetes mellitus and the function of tears and blepharons
- The role and mechanism of esketamine in preventing and treating remifentanil-induced hyperalgesia based on the NMDA receptor–CaMKII pathway
- Brucella infection combined with Nocardia infection: A case report and literature review
- Detection of serum interleukin-18 level and neutrophil/lymphocyte ratio in patients with antineutrophil cytoplasmic antibody-associated vasculitis and its clinical significance
- Ang-1, Ang-2, and Tie2 are diagnostic biomarkers for Henoch-Schönlein purpura and pediatric-onset systemic lupus erythematous
- PTTG1 induces pancreatic cancer cell proliferation and promotes aerobic glycolysis by regulating c-myc
- Role of serum B-cell-activating factor and interleukin-17 as biomarkers in the classification of interstitial pneumonia with autoimmune features
- Effectiveness and safety of a mumps containing vaccine in preventing laboratory-confirmed mumps cases from 2002 to 2017: A meta-analysis
- Low levels of sex hormone-binding globulin predict an increased breast cancer risk and its underlying molecular mechanisms
- A case of Trousseau syndrome: Screening, detection and complication
- Application of the integrated airway humidification device enhances the humidification effect of the rabbit tracheotomy model
- Preparation of Cu2+/TA/HAP composite coating with anti-bacterial and osteogenic potential on 3D-printed porous Ti alloy scaffolds for orthopedic applications
- Aquaporin-8 promotes human dermal fibroblasts to counteract hydrogen peroxide-induced oxidative damage: A novel target for management of skin aging
- Current research and evidence gaps on placental development in iron deficiency anemia
- Single-nucleotide polymorphism rs2910829 in PDE4D is related to stroke susceptibility in Chinese populations: The results of a meta-analysis
- Pheochromocytoma-induced myocardial infarction: A case report
- Kaempferol regulates apoptosis and migration of neural stem cells to attenuate cerebral infarction by O‐GlcNAcylation of β-catenin
- Sirtuin 5 regulates acute myeloid leukemia cell viability and apoptosis by succinylation modification of glycine decarboxylase
- Apigenin 7-glucoside impedes hypoxia-induced malignant phenotypes of cervical cancer cells in a p16-dependent manner
- KAT2A changes the function of endometrial stromal cells via regulating the succinylation of ENO1
- Current state of research on copper complexes in the treatment of breast cancer
- Exploring antioxidant strategies in the pathogenesis of ALS
- Helicobacter pylori causes gastric dysbacteriosis in chronic gastritis patients
- IL-33/soluble ST2 axis is associated with radiation-induced cardiac injury
- The predictive value of serum NLR, SII, and OPNI for lymph node metastasis in breast cancer patients with internal mammary lymph nodes after thoracoscopic surgery
- Carrying SNP rs17506395 (T > G) in TP63 gene and CCR5Δ32 mutation associated with the occurrence of breast cancer in Burkina Faso
- P2X7 receptor: A receptor closely linked with sepsis-associated encephalopathy
- Probiotics for inflammatory bowel disease: Is there sufficient evidence?
- Identification of KDM4C as a gene conferring drug resistance in multiple myeloma
- Microbial perspective on the skin–gut axis and atopic dermatitis
- Thymosin α1 combined with XELOX improves immune function and reduces serum tumor markers in colorectal cancer patients after radical surgery
- Highly specific vaginal microbiome signature for gynecological cancers
- Sample size estimation for AQP4-IgG seropositive optic neuritis: Retinal damage detection by optical coherence tomography
- The effects of SDF-1 combined application with VEGF on femoral distraction osteogenesis in rats
- Fabrication and characterization of gold nanoparticles using alginate: In vitro and in vivo assessment of its administration effects with swimming exercise on diabetic rats
- Mitigating digestive disorders: Action mechanisms of Mediterranean herbal active compounds
- Distribution of CYP2D6 and CYP2C19 gene polymorphisms in Han and Uygur populations with breast cancer in Xinjiang, China
- VSP-2 attenuates secretion of inflammatory cytokines induced by LPS in BV2 cells by mediating the PPARγ/NF-κB signaling pathway
- Factors influencing spontaneous hypothermia after emergency trauma and the construction of a predictive model
- Long-term administration of morphine specifically alters the level of protein expression in different brain regions and affects the redox state
- Application of metagenomic next-generation sequencing technology in the etiological diagnosis of peritoneal dialysis-associated peritonitis
- Clinical diagnosis, prevention, and treatment of neurodyspepsia syndrome using intelligent medicine
- Case report: Successful bronchoscopic interventional treatment of endobronchial leiomyomas
- Preliminary investigation into the genetic etiology of short stature in children through whole exon sequencing of the core family
- Cystic adenomyoma of the uterus: Case report and literature review
- Mesoporous silica nanoparticles as a drug delivery mechanism
- Dynamic changes in autophagy activity in different degrees of pulmonary fibrosis in mice
- Vitamin D deficiency and inflammatory markers in type 2 diabetes: Big data insights
- Lactate-induced IGF1R protein lactylation promotes proliferation and metabolic reprogramming of lung cancer cells
- Meta-analysis on the efficacy of allogeneic hematopoietic stem cell transplantation to treat malignant lymphoma
- Mitochondrial DNA drives neuroinflammation through the cGAS-IFN signaling pathway in the spinal cord of neuropathic pain mice
- Application value of artificial intelligence algorithm-based magnetic resonance multi-sequence imaging in staging diagnosis of cervical cancer
- Embedded monitoring system and teaching of artificial intelligence online drug component recognition
- Investigation into the association of FNDC1 and ADAMTS12 gene expression with plumage coloration in Muscovy ducks
- Yak meat content in feed and its impact on the growth of rats
- A rare case of Richter transformation with breast involvement: A case report and literature review
- First report of Nocardia wallacei infection in an immunocompetent patient in Zhejiang province
- Rhodococcus equi and Brucella pulmonary mass in immunocompetent: A case report and literature review
- Downregulation of RIP3 ameliorates the left ventricular mechanics and function after myocardial infarction via modulating NF-κB/NLRP3 pathway
- Evaluation of the role of some non-enzymatic antioxidants among Iraqi patients with non-alcoholic fatty liver disease
- The role of Phafin proteins in cell signaling pathways and diseases
- Ten-year anemia as initial manifestation of Castleman disease in the abdominal cavity: A case report
- Coexistence of hereditary spherocytosis with SPTB P.Trp1150 gene variant and Gilbert syndrome: A case report and literature review
- Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells
- Exploratory evaluation supported by experimental and modeling approaches of Inula viscosa root extract as a potent corrosion inhibitor for mild steel in a 1 M HCl solution
- Imaging manifestations of ductal adenoma of the breast: A case report
- Gut microbiota and sleep: Interaction mechanisms and therapeutic prospects
- Isomangiferin promotes the migration and osteogenic differentiation of rat bone marrow mesenchymal stem cells
- Prognostic value and microenvironmental crosstalk of exosome-related signatures in human epidermal growth factor receptor 2 positive breast cancer
- Circular RNAs as potential biomarkers for male severe sepsis
- Knockdown of Stanniocalcin-1 inhibits growth and glycolysis in oral squamous cell carcinoma cells
- The expression and biological role of complement C1s in esophageal squamous cell carcinoma
- A novel GNAS mutation in pseudohypoparathyroidism type 1a with articular flexion deformity: A case report
- Predictive value of serum magnesium levels for prognosis in patients with non-small cell lung cancer undergoing EGFR-TKI therapy
- HSPB1 alleviates acute-on-chronic liver failure via the P53/Bax pathway
- IgG4-related disease complicated by PLA2R-associated membranous nephropathy: A case report
- Baculovirus-mediated endostatin and angiostatin activation of autophagy through the AMPK/AKT/mTOR pathway inhibits angiogenesis in hepatocellular carcinoma
- Metformin mitigates osteoarthritis progression by modulating the PI3K/AKT/mTOR signaling pathway and enhancing chondrocyte autophagy
- Evaluation of the activity of antimicrobial peptides against bacterial vaginosis
- Atypical presentation of γ/δ mycosis fungoides with an unusual phenotype and SOCS1 mutation
- Analysis of the microecological mechanism of diabetic kidney disease based on the theory of “gut–kidney axis”: A systematic review
- Omega-3 fatty acids prevent gestational diabetes mellitus via modulation of lipid metabolism
- Refractory hypertension complicated with Turner syndrome: A case report
- Interaction of ncRNAs and the PI3K/AKT/mTOR pathway: Implications for osteosarcoma
- Association of low attenuation area scores with pulmonary function and clinical prognosis in patients with chronic obstructive pulmonary disease
- Long non-coding RNAs in bone formation: Key regulators and therapeutic prospects
- The deubiquitinating enzyme USP35 regulates the stability of NRF2 protein
- Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as potential diagnostic markers for rebleeding in patients with esophagogastric variceal bleeding
- G protein-coupled receptor 1 participating in the mechanism of mediating gestational diabetes mellitus by phosphorylating the AKT pathway
- LL37-mtDNA regulates viability, apoptosis, inflammation, and autophagy in lipopolysaccharide-treated RLE-6TN cells by targeting Hsp90aa1
- The analgesic effect of paeoniflorin: A focused review
- Chemical composition’s effect on Solanum nigrum Linn.’s antioxidant capacity and erythrocyte protection: Bioactive components and molecular docking analysis
- Knockdown of HCK promotes HREC cell viability and inner blood–retinal barrier integrity by regulating the AMPK signaling pathway
- The role of rapamycin in the PINK1/Parkin signaling pathway in mitophagy in podocytes
- Laryngeal non-Hodgkin lymphoma: Report of four cases and review of the literature
- Clinical value of macrogenome next-generation sequencing on infections
- Overview of dendritic cells and related pathways in autoimmune uveitis
- TAK-242 alleviates diabetic cardiomyopathy via inhibiting pyroptosis and TLR4/CaMKII/NLRP3 pathway
- Hypomethylation in promoters of PGC-1α involved in exercise-driven skeletal muscular alterations in old age
- Profile and antimicrobial susceptibility patterns of bacteria isolated from effluents of Kolladiba and Debark hospitals
- The expression and clinical significance of syncytin-1 in serum exosomes of hepatocellular carcinoma patients
- A histomorphometric study to evaluate the therapeutic effects of biosynthesized silver nanoparticles on the kidneys infected with Plasmodium chabaudi
- PGRMC1 and PAQR4 are promising molecular targets for a rare subtype of ovarian cancer
- Analysis of MDA, SOD, TAOC, MNCV, SNCV, and TSS scores in patients with diabetes peripheral neuropathy
- SLIT3 deficiency promotes non-small cell lung cancer progression by modulating UBE2C/WNT signaling
- The relationship between TMCO1 and CALR in the pathological characteristics of prostate cancer and its effect on the metastasis of prostate cancer cells
- Heterogeneous nuclear ribonucleoprotein K is a potential target for enhancing the chemosensitivity of nasopharyngeal carcinoma
- PHB2 alleviates retinal pigment epithelium cell fibrosis by suppressing the AGE–RAGE pathway
- Anti-γ-aminobutyric acid-B receptor autoimmune encephalitis with syncope as the initial symptom: Case report and literature review
- Comparative analysis of chloroplast genome of Lonicera japonica cv. Damaohua
- Human umbilical cord mesenchymal stem cells regulate glutathione metabolism depending on the ERK–Nrf2–HO-1 signal pathway to repair phosphoramide mustard-induced ovarian cancer cells
- Electroacupuncture on GB acupoints improves osteoporosis via the estradiol–PI3K–Akt signaling pathway
- Renalase protects against podocyte injury by inhibiting oxidative stress and apoptosis in diabetic nephropathy
- Review: Dicranostigma leptopodum: A peculiar plant of Papaveraceae
- Combination effect of flavonoids attenuates lung cancer cell proliferation by inhibiting the STAT3 and FAK signaling pathway
- Renal microangiopathy and immune complex glomerulonephritis induced by anti-tumour agents: A case report
- Correlation analysis of AVPR1a and AVPR2 with abnormal water and sodium and potassium metabolism in rats
- Gastrointestinal health anti-diarrheal mixture relieves spleen deficiency-induced diarrhea through regulating gut microbiota
- Myriad factors and pathways influencing tumor radiotherapy resistance
- Exploring the effects of culture conditions on Yapsin (YPS) gene expression in Nakaseomyces glabratus
- Screening of prognostic core genes based on cell–cell interaction in the peripheral blood of patients with sepsis
- Coagulation factor II thrombin receptor as a promising biomarker in breast cancer management
- Ileocecal mucinous carcinoma misdiagnosed as incarcerated hernia: A case report
- Methyltransferase like 13 promotes malignant behaviors of bladder cancer cells through targeting PI3K/ATK signaling pathway
- The debate between electricity and heat, efficacy and safety of irreversible electroporation and radiofrequency ablation in the treatment of liver cancer: A meta-analysis
- ZAG promotes colorectal cancer cell proliferation and epithelial–mesenchymal transition by promoting lipid synthesis
- Baicalein inhibits NLRP3 inflammasome activation and mitigates placental inflammation and oxidative stress in gestational diabetes mellitus
- Impact of SWCNT-conjugated senna leaf extract on breast cancer cells: A potential apoptotic therapeutic strategy
- MFAP5 inhibits the malignant progression of endometrial cancer cells in vitro
- Major ozonated autohemotherapy promoted functional recovery following spinal cord injury in adult rats via the inhibition of oxidative stress and inflammation
- Axodendritic targeting of TAU and MAP2 and microtubule polarization in iPSC-derived versus SH-SY5Y-derived human neurons
- Differential expression of phosphoinositide 3-kinase/protein kinase B and Toll-like receptor/nuclear factor kappa B signaling pathways in experimental obesity Wistar rat model
- The therapeutic potential of targeting Oncostatin M and the interleukin-6 family in retinal diseases: A comprehensive review
- BA inhibits LPS-stimulated inflammatory response and apoptosis in human middle ear epithelial cells by regulating the Nf-Kb/Iκbα axis
- Role of circRMRP and circRPL27 in chronic obstructive pulmonary disease
- Investigating the role of hyperexpressed HCN1 in inducing myocardial infarction through activation of the NF-κB signaling pathway
- Characterization of phenolic compounds and evaluation of anti-diabetic potential in Cannabis sativa L. seeds: In vivo, in vitro, and in silico studies
- Quantitative immunohistochemistry analysis of breast Ki67 based on artificial intelligence
- Ecology and Environmental Science
- Screening of different growth conditions of Bacillus subtilis isolated from membrane-less microbial fuel cell toward antimicrobial activity profiling
- Degradation of a mixture of 13 polycyclic aromatic hydrocarbons by commercial effective microorganisms
- Evaluation of the impact of two citrus plants on the variation of Panonychus citri (Acari: Tetranychidae) and beneficial phytoseiid mites
- Prediction of present and future distribution areas of Juniperus drupacea Labill and determination of ethnobotany properties in Antalya Province, Türkiye
- Population genetics of Todarodes pacificus (Cephalopoda: Ommastrephidae) in the northwest Pacific Ocean via GBS sequencing
- A comparative analysis of dendrometric, macromorphological, and micromorphological characteristics of Pistacia atlantica subsp. atlantica and Pistacia terebinthus in the middle Atlas region of Morocco
- Macrofungal sporocarp community in the lichen Scots pine forests
- Assessing the proximate compositions of indigenous forage species in Yemen’s pastoral rangelands
- Food Science
- Gut microbiota changes associated with low-carbohydrate diet intervention for obesity
- Reexamination of Aspergillus cristatus phylogeny in dark tea: Characteristics of the mitochondrial genome
- Differences in the flavonoid composition of the leaves, fruits, and branches of mulberry are distinguished based on a plant metabolomics approach
- Investigating the impact of wet rendering (solventless method) on PUFA-rich oil from catfish (Clarias magur) viscera
- Non-linear associations between cardiovascular metabolic indices and metabolic-associated fatty liver disease: A cross-sectional study in the US population (2017–2020)
- Knockdown of USP7 alleviates atherosclerosis in ApoE-deficient mice by regulating EZH2 expression
- Utility of dairy microbiome as a tool for authentication and traceability
- Agriculture
- Enhancing faba bean (Vicia faba L.) productivity through establishing the area-specific fertilizer rate recommendation in southwest Ethiopia
- Impact of novel herbicide based on synthetic auxins and ALS inhibitor on weed control
- Perspectives of pteridophytes microbiome for bioremediation in agricultural applications
- Fertilizer application parameters for drip-irrigated peanut based on the fertilizer effect function established from a “3414” field trial
- Improving the productivity and profitability of maize (Zea mays L.) using optimum blended inorganic fertilization
- Application of leaf multispectral analyzer in comparison to hyperspectral device to assess the diversity of spectral reflectance indices in wheat genotypes
- Animal Sciences
- Knockdown of ANP32E inhibits colorectal cancer cell growth and glycolysis by regulating the AKT/mTOR pathway
- Development of a detection chip for major pathogenic drug-resistant genes and drug targets in bovine respiratory system diseases
- Exploration of the genetic influence of MYOT and MB genes on the plumage coloration of Muscovy ducks
- Transcriptome analysis of adipose tissue in grazing cattle: Identifying key regulators of fat metabolism
- Comparison of nutritional value of the wild and cultivated spiny loaches at three growth stages
- Transcriptomic analysis of liver immune response in Chinese spiny frog (Quasipaa spinosa) infected with Proteus mirabilis
- Disruption of BCAA degradation is a critical characteristic of diabetic cardiomyopathy revealed by integrated transcriptome and metabolome analysis
- Plant Sciences
- Effect of long-term in-row branch covering on soil microorganisms in pear orchards
- Photosynthetic physiological characteristics, growth performance, and element concentrations reveal the calcicole–calcifuge behaviors of three Camellia species
- Transcriptome analysis reveals the mechanism of NaHCO3 promoting tobacco leaf maturation
- Bioinformatics, expression analysis, and functional verification of allene oxide synthase gene HvnAOS1 and HvnAOS2 in qingke
- Water, nitrogen, and phosphorus coupling improves gray jujube fruit quality and yield
- Improving grape fruit quality through soil conditioner: Insights from RNA-seq analysis of Cabernet Sauvignon roots
- Role of Embinin in the reabsorption of nucleus pulposus in lumbar disc herniation: Promotion of nucleus pulposus neovascularization and apoptosis of nucleus pulposus cells
- Revealing the effects of amino acid, organic acid, and phytohormones on the germination of tomato seeds under salinity stress
- Combined effects of nitrogen fertilizer and biochar on the growth, yield, and quality of pepper
- Comprehensive phytochemical and toxicological analysis of Chenopodium ambrosioides (L.) fractions
- Impact of “3414” fertilization on the yield and quality of greenhouse tomatoes
- Exploring the coupling mode of water and fertilizer for improving growth, fruit quality, and yield of the pear in the arid region
- Metagenomic analysis of endophytic bacteria in seed potato (Solanum tuberosum)
- Antibacterial, antifungal, and phytochemical properties of Salsola kali ethanolic extract
- Exploring the hepatoprotective properties of citronellol: In vitro and in silico studies on ethanol-induced damage in HepG2 cells
- Enhanced osmotic dehydration of watermelon rind using honey–sucrose solutions: A study on pre-treatment efficacy and mass transfer kinetics
- Effects of exogenous 2,4-epibrassinolide on photosynthetic traits of 53 cowpea varieties under NaCl stress
- Comparative transcriptome analysis of maize (Zea mays L.) seedlings in response to copper stress
- An optimization method for measuring the stomata in cassava (Manihot esculenta Crantz) under multiple abiotic stresses
- Fosinopril inhibits Ang II-induced VSMC proliferation, phenotype transformation, migration, and oxidative stress through the TGF-β1/Smad signaling pathway
- Antioxidant and antimicrobial activities of Salsola imbricata methanolic extract and its phytochemical characterization
- Bioengineering and Biotechnology
- Absorbable calcium and phosphorus bioactive membranes promote bone marrow mesenchymal stem cells osteogenic differentiation for bone regeneration
- New advances in protein engineering for industrial applications: Key takeaways
- An overview of the production and use of Bacillus thuringiensis toxin
- Research progress of nanoparticles in diagnosis and treatment of hepatocellular carcinoma
- Bioelectrochemical biosensors for water quality assessment and wastewater monitoring
- PEI/MMNs@LNA-542 nanoparticles alleviate ICU-acquired weakness through targeted autophagy inhibition and mitochondrial protection
- Unleashing of cytotoxic effects of thymoquinone-bovine serum albumin nanoparticles on A549 lung cancer cells
- Erratum
- Erratum to “Investigating the association between dietary patterns and glycemic control among children and adolescents with T1DM”
- Erratum to “Activation of hypermethylated P2RY1 mitigates gastric cancer by promoting apoptosis and inhibiting proliferation”
- Retraction
- Retraction to “MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB”
- Retraction to “A data mining technique for detecting malignant mesothelioma cancer using multiple regression analysis”
- Special Issue on Advances in Neurodegenerative Disease Research and Treatment
- Transplantation of human neural stem cell prevents symptomatic motor behavior disability in a rat model of Parkinson’s disease
- Special Issue on Multi-omics
- Inflammasome complex genes with clinical relevance suggest potential as therapeutic targets for anti-tumor drugs in clear cell renal cell carcinoma
- Gastroesophageal varices in primary biliary cholangitis with anti-centromere antibody positivity: Early onset?


