Exploratory evaluation supported by experimental and modeling approaches of Inula viscosa root extract as a potent corrosion inhibitor for mild steel in a 1 M HCl solution
-
Mohamed Adil Mahraz
, Rajae Salim
, Mohammed Kara
, Riaz Ullah
Abstract
The corrosion of metals poses a threat to the economy, the environment, and human health due to undesirable reactions and contaminated products. Corrosion inhibitors, including natural products, can play a key role in protecting metallic materials, especially under challenging conditions. In this study, the roots of the Inula viscosa plant were examined for their ability to act as corrosion inhibitors in a 1 M hydrochloric acid (HCl) solution. Different extracts of the plant were evaluated for their corrosion inhibition capacity in a 1 M HCl solution. The effectiveness of different plant extracts was assessed, including an aqueous extract, an ethanolic extract, and a combined water–ethanol extract. Compounds present in the roots of Inula viscosa were identified using high-performance liquid chromatography. The electrochemical properties of the extracts were studied using various techniques such as open circuit potential, electrochemical impedance spectroscopy, and potentiodynamic polarization. Additionally, surface analysis after immersion was performed using scanning electron microscopy. Electrochemical data revealed that Inula viscosa root (IVR) extracts acted as mixed-type corrosion inhibitors with pronounced cathodic characteristics. The inhibitory efficiency was closely related to the concentration of Inula viscosa (I. viscosa), showing a significant increase with higher concentrations. This resulted in a decrease in corrosion current and an increase in polarization resistance. Notably, inhibitory efficiency reached high levels, up to 97.7% in mixed extract which represents a mixture between water and ethanol. In our study, it was observed that the mixed extract (water + ethanol) allowed for a greater corrosion inhibition compared to the other solvents studied, 97.7%. Surface analyses confirmed the formation of an organic film layer on the steel surface, attributed to the bonding of functional groups and heteroatoms in I. viscosa components. Therefore, this study paves the way for the potential integration of I. viscosa as a promising corrosion inhibition material, offering durable protection against steel corrosion and opening avenues for various related applications.
1 Introduction
Substances like HCl and H2SO4 are essential in various industrial processes such as cleaning, descaling, pickling, and more [1]. However, in sectors where mild steel (MS) is a major infrastructure, corrosion rates are accelerated in acidic environments, leading to annual losses of billions of dollars. Preventing the contact between acid and metal is of paramount importance. Organic inhibitors, when applied in low concentrations, create a barrier against acid contact, thus reducing corrosion rates [2]. Depending on the chosen extraction or synthesis method, inhibition can take both organic and inorganic forms. It is crucial to consider the inherent properties of these inhibitors. Biodegradability is fundamental, ensuring corrosion inhibitors can naturally degrade through environmental processes, reducing their long-term impact. Non-toxicity is also essential to ensure that inhibitors do not pose risks to human health or result in harmful pollution.
The availability of inhibitors is another critical consideration. Ideally, the materials needed for inhibitor production should be easily accessible, enabling their widespread application across various industries. Furthermore, environmental sustainability is paramount when selecting inhibitors, as minimizing the impact on ecosystems and maintaining a balance between industrial needs and nature preservation is necessary. The importance of considering these factors when selecting and applying corrosion inhibitors cannot be overstated [3,4]. Considering these aspects is essential to ensure effective corrosion protection while minimizing adverse environmental and human health effects. Currently, the market offers a diverse range of corrosion inhibitors, but many come with a high price tag and are highly toxic, posing risks to human health and the environment [5,6]. Therefore, there is an urgent need to develop green corrosion inhibitors that exhibit strong corrosion inhibition effects and are cost-effective, intending to replace the toxic corrosion inhibitors on the market. Addressing this issue has led to increased attention to green corrosion inhibitors. These inhibitors encompass amino acids, proteins, plant extracts, cellulose, starch, and more [7,8,9]. As a result, research into plant-based inhibitors for green corrosion chemistry is gaining popularity. Natural compounds are well-suited to act as acid corrosion inhibitors due to their cost-effectiveness [10,11,12,13]. Organic molecules containing heteroatoms such as N, S, and O tend to be present on the metal surface, adsorbed at active sites, forming a thin protective layer that reduces the passage of corrosive substances through the metal [14,15,16,17]. The components of natural products that adhere to the metal surface are influenced by various factors, including (a) the chemical composition of the inhibitors, (b) the nature of the surface, and (c) the metal’s charge. Compounds derived from plants containing heterocyclic components like steroids, alkaloids, and flavonoids have been studied for their effective corrosion inhibition properties [18,19,20,21]. Numerous studies have been conducted on the use of plant extracts to prevent corrosion [22,23,24,25,26,27,32,33,34,35].
A complex assortment of secondary metabolites has previously been identified in I. viscosa, including flavonoids such as sakuranetin, 7-O-methylaromadendrine, and 3-O-acetylpadmatine, sesquiterpene lactones, acids such as inuviscolide, tomentosine, and their derivatives, carabrone, ilicic acid, costic acid, and their derivatives, as well as phenolic acid derivatives such as caffeoylquinic acid, dicaffeoylquinic acid, and their derivatives, glycolipids such as inugalactolipid A, and triterpenoids [36].
Inula viscosa L. (I. viscosa) (Asteraceae) is a medicinal plant widely used in traditional medicine. The growth of this plant depends on environmental conditions, including light energy, water, and soil nutrients. The Inula genus comprises more than 100 species [37] and is commonly found in the Mediterranean basin. In Morocco, Inula viscosa (L.) Aiton, also known as Dittrichia viscosa (L.), locally referred to as “Bageraman,” is a perennial Mediterranean herbaceous plant belonging to the Asteraceae family. It has a history of being used in folk medicine to treat animal wounds and has been employed to address various health concerns in different regions. North African traditional medicine has been used to address diabetes and inflammation [38]. In Jordan, it has been utilized to treat tuberculosis anemia and as a poultice for rheumatic pains [39]. It has been known in Spain for its antiseptic properties, for managing skin inflammations, and for treating gastro-duodenal disorders [40,41]. Scientific research conducted on this plant has demonstrated its high antioxidant, antimicrobial, and antifungal activity [42,43,44,45,46,47].
Research on corrosion inhibition of Inula viscosa root (IVR) parts holds promising potential across various industries. Chemical compounds found in these roots have demonstrated corrosion inhibitory properties, offering opportunities for eco-friendly and efficient applications. Understanding these mechanisms could reduce maintenance costs and environmental impacts associated with metal corrosion.
2 Materials and methods
2.1 Preparation of MS
This study focused on material selection, with MS as the primary choice. MS is widely used in various industries due to its cost-effectiveness and good conductivity. While other elements can be present, carbon significantly influences the steel’s properties. Please refer to Table 1 for a detailed chemical breakdown of MS’s composition.
Mass fraction of the chemical composition of the steel components used
| Elements | Fe | Si | C | Mn | S | P | Al |
|---|---|---|---|---|---|---|---|
| Mass (%) | 99.21 | 0.38 | 0.21 | 0.05 | 0.05 | 0.09 | 0.01 |
The MS specimens used in the test underwent mechanical polishing with sandpaper on various cores. Subsequently, impurities were removed from the sample by immersing it in distilled water, followed by degreasing with an analytical solution of acetone, and finally, they were dried before each trial. The choice of hydrochloric acid (HCl) is justified by its versatility, particularly its common use in the acid pickling of steel.
2.2 Electrolytic medium
In this study, the corrosive solution of interest is a HCl solution with a specific molarity. This solution is prepared by diluting HCl with distilled water, with the original HCl having a concentration of 37%. The resulting solution has a specific gravity of 1.19. The inhibitors used in this context are prepared with 1 M HCl and range in content from 0.25 to 1 g/L.
2.3 Sample collection and authentication
I. viscosa plants were gathered based on ethnopharmacological insights from communities near the Moulay Yaâcoub in Morocco (Figure 1). This collection was carried out in collaboration with local authorities and in full accordance with the United Nations Convention on Biodiversity. The invaluable assistance of a traditional healer played a crucial role throughout this process. Professor Amina Bari, a botanist at the Faculty of Science, Dhar Mahraz, Fes, confirmed the plant material’s identity. Furthermore, a reference specimen (212As02 Iv 001) [48] was officially archived in the herbarium of the Botany Department.

I. viscosa plant (a) and powder (b).
2.4 Inhibitor’s preparation
The extraction method is critical to ensure that the desired components are extracted from the complex natural matrix without damaging them. A wide range of techniques have been reported, and the results of this review have shown the use of both conventional and unconventional methods for extracting bioactive compounds. The commonly reported extraction methods were maceration and Soxhlet [49–51].
The roots of the Inula viscosa plant are dried and uniformly ground in a mortar to obtain a fine powder. In total, 200 g of I. viscosa plant root powder was extracted with 500 mL of water and placed in a container under continuous agitation for 72 h. The mixture was then filtered through a filter paper (Whatman No. 2, 125 mm) to separate the water-based solvent contained in the root extract. The remaining solvent was removed using a rotary evaporator at temperatures above 90°C under low pressure. After the solvent was evaporated using the rotary evaporator, the sample was transferred to an oven maintained at a constant temperature. This additional step allowed for the elimination of any residual water molecules, ensuring the purity of the extract. The final result, obtained after this drying step in the oven, is referred to as the IVR water extract, ready to be used in our study. The resulting aqueous extract was then stored at 4°C. Ethanol extracts were obtained by subjecting 150 g of root powder material from the I. viscosa plant to a Soxhlet extraction process for 6 h, using approximately 100 mL of solvent. Subsequently, the filtered extracts were evaporated under vacuum using a rotary evaporator at temperatures exceeding 40°C until completely dried. These concentrated extracts were then stored as residues at 4°C. Mixed extracts (70 mL of ethanol + 30 mL of water) were obtained by subjecting 200 g of root powder material from the I. viscosa plant to a Soxhlet extraction process for 6 h, using approximately 300 mL of solvent. Subsequently, the filtered extracts were evaporated under vacuum using a rotary evaporator at temperatures exceeding 40°C until completely dried. These concentrated extracts were then stored as residues at 4°C.
2.5 HPLC-DAD analysis
Extracts from the roots of the I. viscosa plant, including the ethanolic extract, aqueous extract, and mixed extract, were prepared at a concentration of 50 mg/mL, after being prepared, 0.45 μm microfilters were used to filter these extracts. Phenolic chemicals were identified and quantified using high-performance liquid chromatography (HPLC) in conjunction with a UV detector that operated between 210 and 400 nm in wavelength. Specifically, a reverse-phase C18 column (250 × 4 mm, 5 μm) was filled with 40 µL of sample injection. 20% B for 0–25 min, 100% B for 25–30 min, and 20% B for 30–35 min were the elution gradients that were used. For sample elution, mobile phases A (water/0.5% phosphoric acid) and B (methanol) were used at a flow rate of 1 mL/min. A constant temperature of 40°C was used for the separating procedure. The processes used for compound identification followed the guidelines provided by earlier research [52,53,54].
2.6 Electrochemical technique
The electrochemical evaluations were carried out using a Versa-STAT 4 potentiostat controlled by Versa-Studio software. A small glass cell with three distinct electrodes was utilized for this procedure. In this cell, the specimen under investigation (MS 1 cm²) acted as the working electrode, while the Ag/AgCl electrode was the reference electrode. Additionally, a counter electrode was created using platinum wire. The test sample, characterized by a rectangular shape with a surface area of 1 cm², was immersed in the sample preparation for 30 min to establish an open circuit potential (OCP).
The corrosion current density values were measured under two different conditions: without the presence of the inhibitor and with the inhibitor
Furthermore, graphs depicting the potentiodynamic polarization (PDP) curves were generated by altering the potential within the range of ±250 mV/Ag/AgCl, with a scanning rate of 1 mV/s. The study involving electrochemical impedance spectroscopy (EIS) was also carried out across a frequency range from 100 mHz to 100 kHz at the OCP, using an alternating waveform with an amplitude of 10 mV [55].
2.7 Surface analysis
The Scanning electron microscopy (SEM)-Energy-dispersive X-ray (EDX) spectroscopy was used to analyze the structure and arrangement of the surface of MS samples under two different conditions: the first condition without the inhibitor and the second condition with an inhibitor at a concentration of 1 g/L [52,56,57]. This examination was conducted using an environmental SEM. Specifically, the QUANTA 200 model, equipped with an EDX probe, operates at a 15 kV accelerating voltage. This technique allowed for the examination of the elemental composition of the MS surface and provided insights into its surface morphology and any changes that occurred in the presence of the inhibitors.
2.8 Quantum chemical calculations
The theoretical study is very interesting and useful in the corrosion inhibition field to correlate the reactivity of the obtained molecule structures with their inhibition performance. Therefore, Density Functional Theory (DFT) calculations were executed at B3LYP/6-311G (d, p) using Gaussian 09 and GaussView 5.0.8 software. The calculations were executed in water to approach the experimental conditions. Various global descriptors such as E HOMO, E LUMO, energy gap (ΔE gap), global hardness (η), global softness (σ), electronegativity (χ), and the fraction of electrons transferred (ΔN 110) were extracted using the following equations, where ΦFe/110 is 4.82 eV and ηFe(110) = 0 eV:
Monte Carlo (MC) simulation was carried out to gain insight into the adsorption approach of the tested molecular structures and their interactions with the iron surface. The simulated system considered the inhibitor molecules, 180 water molecules, the acidic solution (6H3O+ and 6Cl− ions), and the steel surface composed of iron atoms. All system components under study were optimized using a COMPASS force field before performing the simulation using the Adsorption Locator module in Materials Studio 7.0 software. Additionally, the Fe (110) crystal was constructed with a 30 Å edge to provide sufficient depth and then enlarged to draw a supercell (10 × 10).
3 Results and discussion
3.1 HPLC analysis
The analysis of extracts from the root part of the I. viscosa plant using HPLC revealed a high content of bioactive molecules, including flavonoids, polyphenols, and phenolic acids. The results of this analysis are presented in Table 2 and Figure 2, providing a detailed identification of compounds in the three studied extracts. The ethanolic extract of the root part highlighted the predominance of bioactive molecules such as catechin, vanillin, and quercetin 3-O-β-d-glucoside. The mixed extract (water + ethanol) showed a significant concentration of syringic acid, 3-hydroxybenzoic acid, and catechin. Finally, the aqueous extract exhibited an abundance of gallic acid, 4-hydroxybenzoic acid, and catechin. The analysis of the chromatograms indicates a diversity in the chemical composition of the various extracts while underscoring the presence of the same bioactive molecules in several of them, with varying concentrations depending on the solvent used. Previous studies have also confirmed the presence of compounds similar to those discovered in our research. Ten distinct phenolic compounds were identified in I. viscosa according to an investigation reviewed by Ozkan et al. These include gallic acid, caffeic acid, rutin, luteolin, kaempferol, rosmarinic acid, myricetin, quercetin, coumarin, and apigenin [58]. 21 different polyphenols are visible in the polyphenolic profile of the EtOAc extract of I. viscosa, which was determined through analysis. Five of these are flavonoids, namely, derivatives of quercetin, luteolin, naringin, and apigenin; the other four are phenolic acids, specifically caffeic acid, galloylquinic acid, and two isomers of di-O-caffeoylquinic acid [59]. Based on the information gathered from the analysis of phenolic compounds, it was discovered that I. viscosa plants have high levels of hyperoside, protocatechuic acid, and chloridric acid [60].
Chromatographic analysis using HPLC of the compounds identified within the various extracts obtained from the roots of the I. viscosa plant
| No. | Standards | Tr (min) | Ethanol extract | Mixed extract (ethanol + water) | Extract water |
|---|---|---|---|---|---|
| 1 | Gallic acid | 3.26 | ND | 5.88 | 23.44 |
| 2 | NI | 3.69 | ND | 2.35 | 8.50 |
| 3 | Catechin | 3.92 | ND | ND | 10.84 |
| 4 | Salicylic acid | 4.38 | ND | ND | 5.85 |
| 5 | NI | 6.55 | ND | ND | 2.92 |
| 6 | NI | 7.97 | ND | 2.16 | ND |
| 7 | Caffeic acid | 9.02 | ND | ND | 2.74 |
| 8 | NI | 9.64 | ND | 1.31 | ND |
| 9 | 4-hydroxybenzoic acid | 10.01 | ND | ND | 10.77 |
| 10 | Catechin hydrate | 10.56 | ND | 10.52 | 3.83 |
| 11 | Syringic acid | 10.93 | ND | 41.19 | 8.21 |
| 12 | NI | 11.27 | 6.79 | ND | 3.69 |
| 13 | 3-hydroxybenzoic acid | 11.54 | ND | 27.32 | 8.79 |
| 14 | Vanillic acid | 11.73 | ND | ND | 6.72 |
| 15 | Vanillin | 12.57 | 6.84 | 2.36 | ND |
| 16 | NI | 12.94 | 5.72 | 2.46 | ND |
| 17 | Naringin | 13.00 | 3.01 | 4.44 | ND |
| 18 | Cinnamic acid | 14.44 | 2.81 | ND | 3.71 |
| 19 | Ferulic acid | 14.83 | 2.52 | ND | ND |
| 20 | p-coumaric acid | 15.24 | 16.54 | ND | ND |
| 21 | Sinapic acid | 15.43 | 29.97 | ND | ND |
| 22 | Succinic acid | 15.78 | 4.13 | ND | ND |
| 23 | Quercetin 3-O-β-d-glucoside | 16.11 | 10.55 | ND | ND |
| 24 | Rutin | 16.25 | 3.28 | ND | ND |
| 25 | Quercetin | 17.08 | 2.19 | ND | ND |
| 26 | Kaempferol | 17.307 | 2.77 | ND | ND |
| 27 | Apigenin | 17.532 | 2.88 | ND | ND |
ND: non detected; NI: not identified.
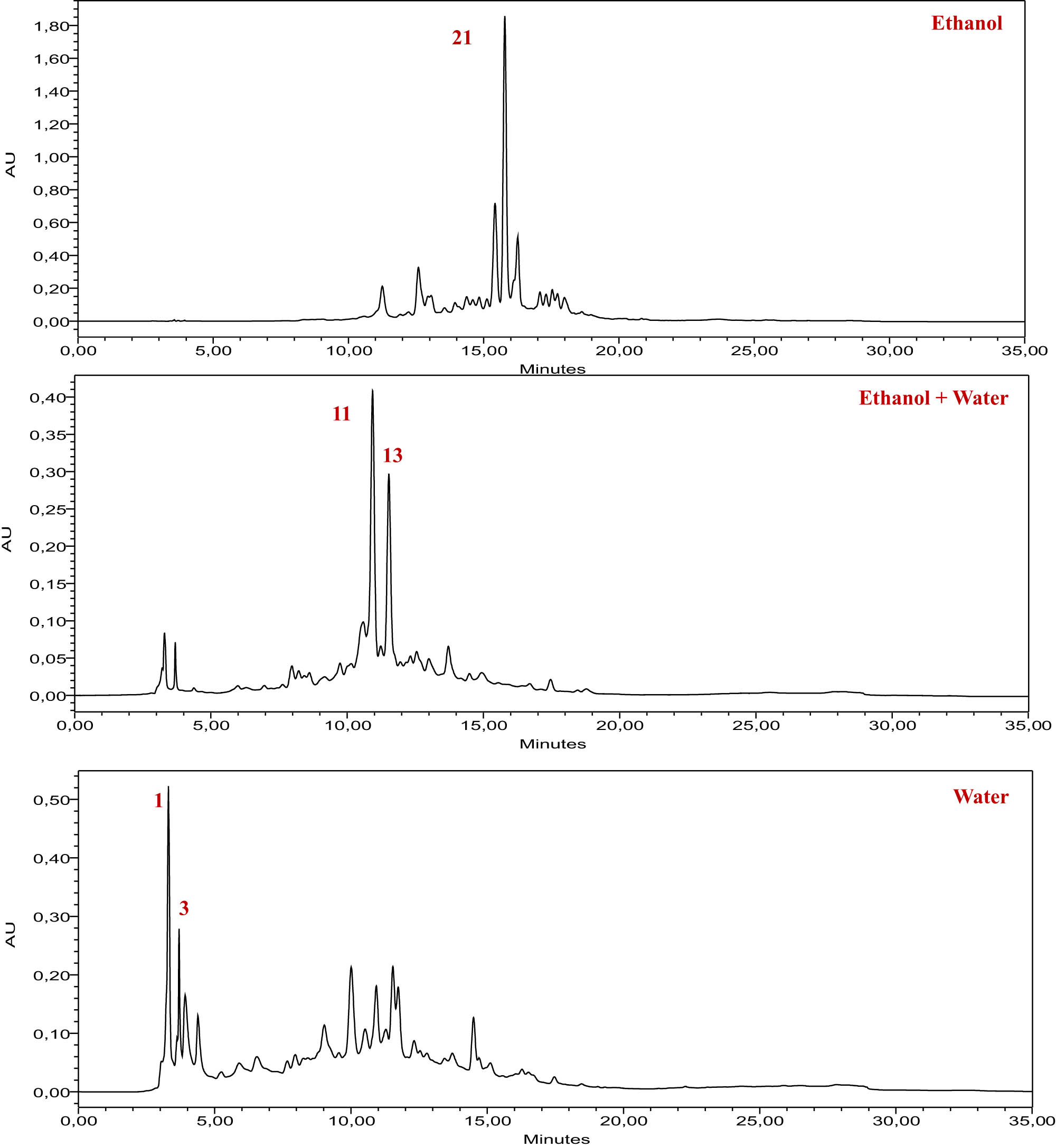
HPLC-DAD chromatogram of extracts from the root part of the I. viscosa plant, recorded at a wavelength of 320 nm, using the standards mentioned in the table above.
3.2 Electrochemical study
It is imperative to determine the period during which the electrode must remain immersed in the electrolyte to achieve the electrochemical equilibrium of the solution. Given that the corrosion potential is primarily determined by the stability of the working electrode in the electrolyte, it is crucial to establish the required duration for the corrosion potential to reach stabilization. This study estimated that an immersion period of 30 min is necessary to achieve a 99% stabilization of the corrosion potential when immersing an iron metal plate in a molar HCl solution.
3.2.1 OCP monitoring
This straightforward approach provides initial insights into the underlying mechanisms occurring at the metal and electrolyte interface [61]. Particular emphasis was placed on ensuring the OCP stability before initiating each polarization and impedance cycle. The change in OCP for the MS electrode is observed over time in the corrosive environment. This evolution is shown in Figure 3, both in the absence and presence of 1 g/L of I. viscosa plant extract at 298 K. In the presence of inhibitors, a slight shift towards more negative potential values is noticeable, indicating the primary impact of these compounds on the cathodic reactions. Additionally, 30 min is sufficient to reach the equilibrium potential.
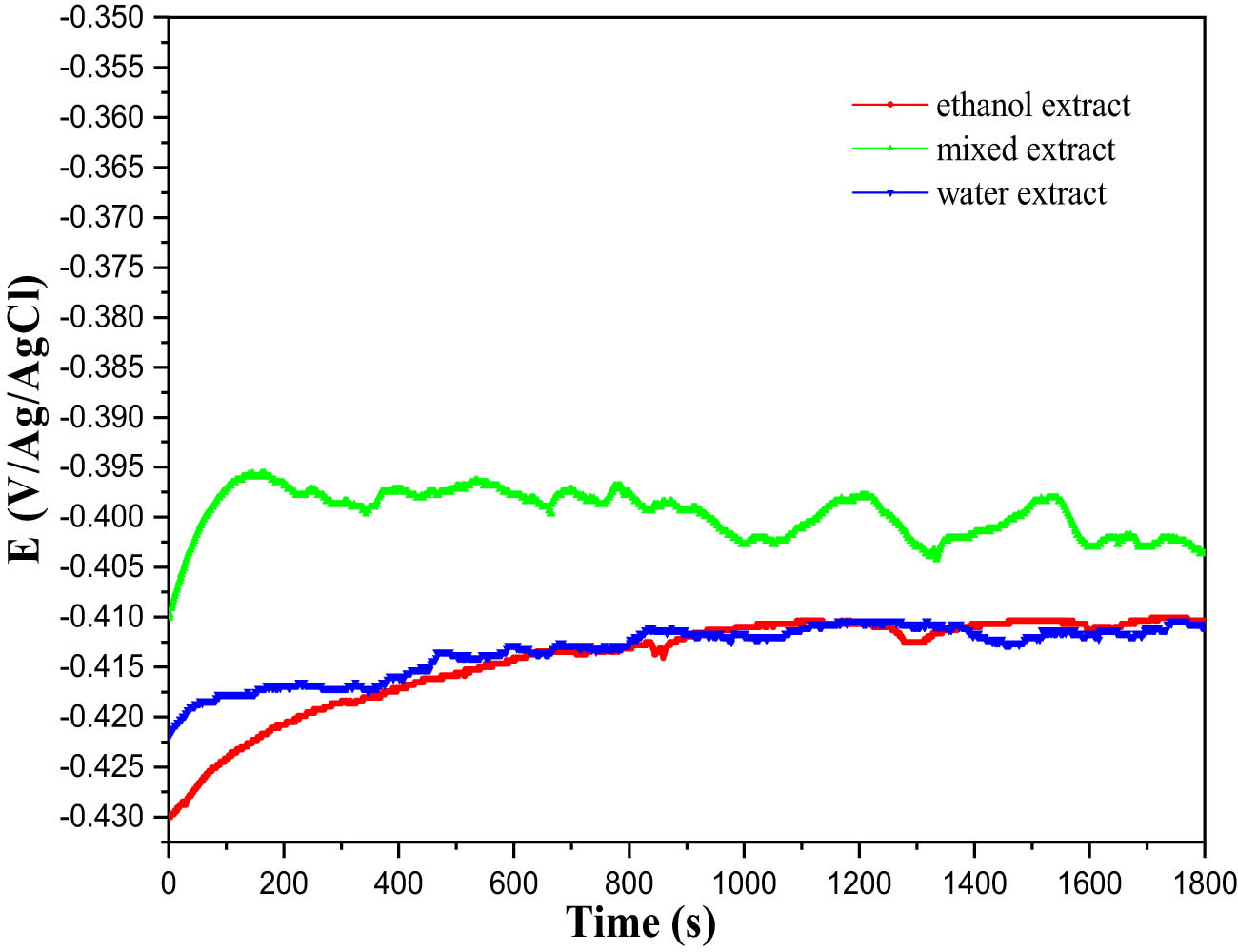
The progression of the OCP of MS in a 1 M HCl solution at 298 K, both with and without the presence of 1 g/L of the different inhibitor solutions under investigation.
3.2.2 Polarization curves (steady-state electrochemical method)
Electrochemical parameters such as cathodic slopes ß c corrosion potential, corrosion current densities, etc., were carefully selected and organized in Table 3. Polarization curves depicting the behavior of MSs in an acidic environment, both without and with the various detected inhibitors, are shown in Figure 4. The corrosion inhibition efficiency values (IE%) were calculated using the following equation:
Electrochemical parameters were derived from PDP curves for MS corrosion in a 1 M HCl solution at 298 K, with varying concentrations of IVR
| Conc. | −E corr | i corr | −β c | η PDP | |
|---|---|---|---|---|---|
| (g/L) | (mV/Ag/AgCl) | (µA cm−2) | (mV dec−1) | (%) | |
| 1 M HCl | — | 498 | 983 | 140 | — |
| Ethanol | 1 | 405 | 51 | 128 | 94.8 |
| 0.8 | 407 | 61 | 127 | 93.7 | |
| 0.6 | 409 | 74 | 126 | 92.4 | |
| 0.4 | 400 | 103 | 133 | 89.5 | |
| 0.2 | 410 | 212 | 134 | 78.4 | |
| Mixed (water + ethanol) | 1 | 390 | 22 | 128 | 97.7 |
| 0.8 | 397 | 31 | 130 | 96.8 | |
| 0.6 | 409 | 37 | 131 | 96.2 | |
| 0.4 | 413 | 63 | 129 | 93.5 | |
| 0.2 | 414 | 155 | 118 | 84.2 | |
| 1 | 404 | 46 | 126 | 95.3 | |
| Water | 0.8 | 401 | 57 | 134 | 94.2 |
| 0.6 | 402 | 67 | 135 | 93.1 | |
| 0.4 | 409 | 121 | 136 | 87.6 | |
| 0.2 | 411 | 215 | 135 | 78.1 |
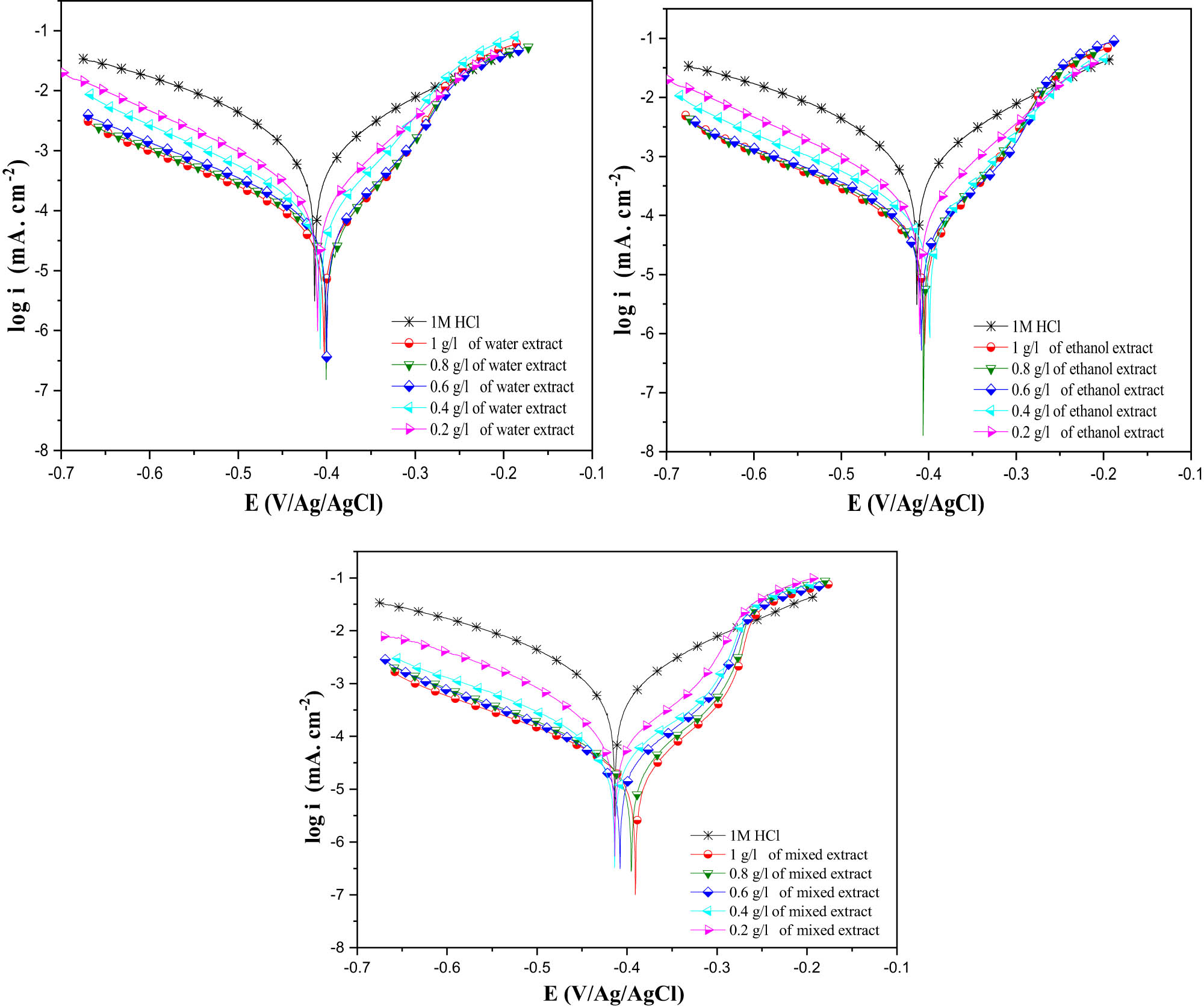
PDP curves for MS in 1 M HCl with and without different IVR concentrations at 298 K.
Stationary electrochemical techniques were employed to assess the effectiveness of I. viscosa plant extracts obtained using water, a mixture of ethanol and water, and ethanol alone. These techniques mainly involve the analysis of curves describing the current/voltage relationships under potentiodynamic conditions at anodic and cathodic potentials.
Figure 4 illustrates the polarization curves for cathodic and anodic reactions in a 1 M HCl solution containing MS. Measurements were conducted at 298 K, including scenarios with and without the tested inhibitors at different concentrations, ranging from 0.25 to 1 g/L, at 25°C. The observed balancing in all samples can be attributed to the formation of an iron oxide/hydroxide layer on the surface of MS in the absence of inhibiting substances. However, in a 1 M HCl solution with an inhibitor, this stabilization results from the adsorption of inhibitor molecules on the MS surface. Table 3 illustrates the evolution of the corrosion rate of MS in HCl solutions with and without the inhibitor. The results indicate that current density (i corr) decreases progressively with increasing IVR inhibitor concentrations in the different extracts studied [62]. For instance, in the IVR mixed extract, when the inhibitor concentration is 0.2 g/L, the current density is 155 µA cm −2, but after an increase in the inhibitor concentration to 1 g/L, there is a decrease in current density of almost 22 µA cm −2. Using the PDP technique, the inhibitory power increases with the concentration, reaching 97.7% with the mixed extract and 94.8% with the ethanolic extract, and 95.3% with the water extract when the inhibitor concentration is 1 g/L.
The presence of the inhibitor influences both anodic and cathodic current densities. Cathodic curves exhibit similarities among all samples, indicating an unchanged cathodic reduction mechanism and hydrogen reduction controlled by pure activation kinetics. Furthermore, anodic curves are affected by the presence of the IVR extract in an acidic environment, altering the iron dissolution mechanism and forming a new protective layer. Electrochemical parameters were determined through polarization curve analysis, including cathodic slope (β c), corrosion potential (E corr), inhibition efficiency (η PDP), and corrosion current density (i corr), as summarized in Table 3. It is important to emphasize that the shift in the corrosion potential (E corr) is less than 85 mV, indicating that the IVR extract mitigates the corrosive effects of HCl through a mixed-type mechanism, as the E corr differences fall within the ±85 mV range [63–67].
3.2.3 EIS
The appearance of offensive chloride ions in the solution increases the speed of degradation of the MS surface by increasing the disintegration of the anode component, as described by the reactions involving Cl− and Fe. To gain a deeper understanding of the kinetic behavior at the metal/solution interface, we notice two different cases. When the inhibitor does not exist, the impedance spectrum appears as a small half-circle, with a time constant observed in the Bode diagram. This result confirms the rapid decomposition of iron as well as the release of hydrogen. Conversely, in the second case, a high-frequency capacitive loop was evident when various IVR extracts were added to the 1 M HCl solution. Furthermore, increasing the concentration of different IVR extracts resulted in the enlargement of the semicircle. This observation suggests that the inhibitor influences the charge transfer mechanism between the metal and the solution and the adsorption of inhibitor molecules.
The specific phenomenon observed on the surface of MS in the impedance diagram of solid-state electrodes is known as frequency dispersion. This phenomenon can be attributed to several factors, including surface heterogeneity [68], the presence of impurities or dislocations [69], surface fragility, dispersion of active centers, and the formation of porous protective layers [70]. In our study of Bode diagram, we observed that in the absence of inhibitor, the impedance amplitude may be lower, indicating more active corrosion and less effective protection of the metal surface. Additionally, the peak of impedance amplitude may be located at a different frequency compared to the system with inhibitor, reflecting differences in ongoing electrochemical processes.
Conversely, after the addition of inhibitor at different concentrations, we observed an increase in impedance amplitude at certain frequencies, indicating a reduction in corrosion rate and improved protection of the metal surface. Furthermore, the peak of impedance amplitude may shift to lower or higher frequencies with the addition of inhibitor, suggesting a change in the dominant corrosion mechanism or in the adsorption processes of the inhibitor onto the metal surface.
In the context of this study, the circuit model shown in Figure 5, which includes elements like solution resistance (R s), polarization resistance (R p), and double layer capacitance (C dl), is insufficient for accurately calculating the parameters of EIS. This model is designed for homogeneous systems. A more appropriate model should be employed to account for the observed frequency dispersion, including a frequency dispersion element like the constant phase element (CPE). The CPE parameter is calculated using an appropriate equation (equation (7)) [71,72].
where Q represents the amplitude of the CPE, i represents the imaginary number of the CPE, ω represents the rotating frequency, and n represents the experimental coefficient.
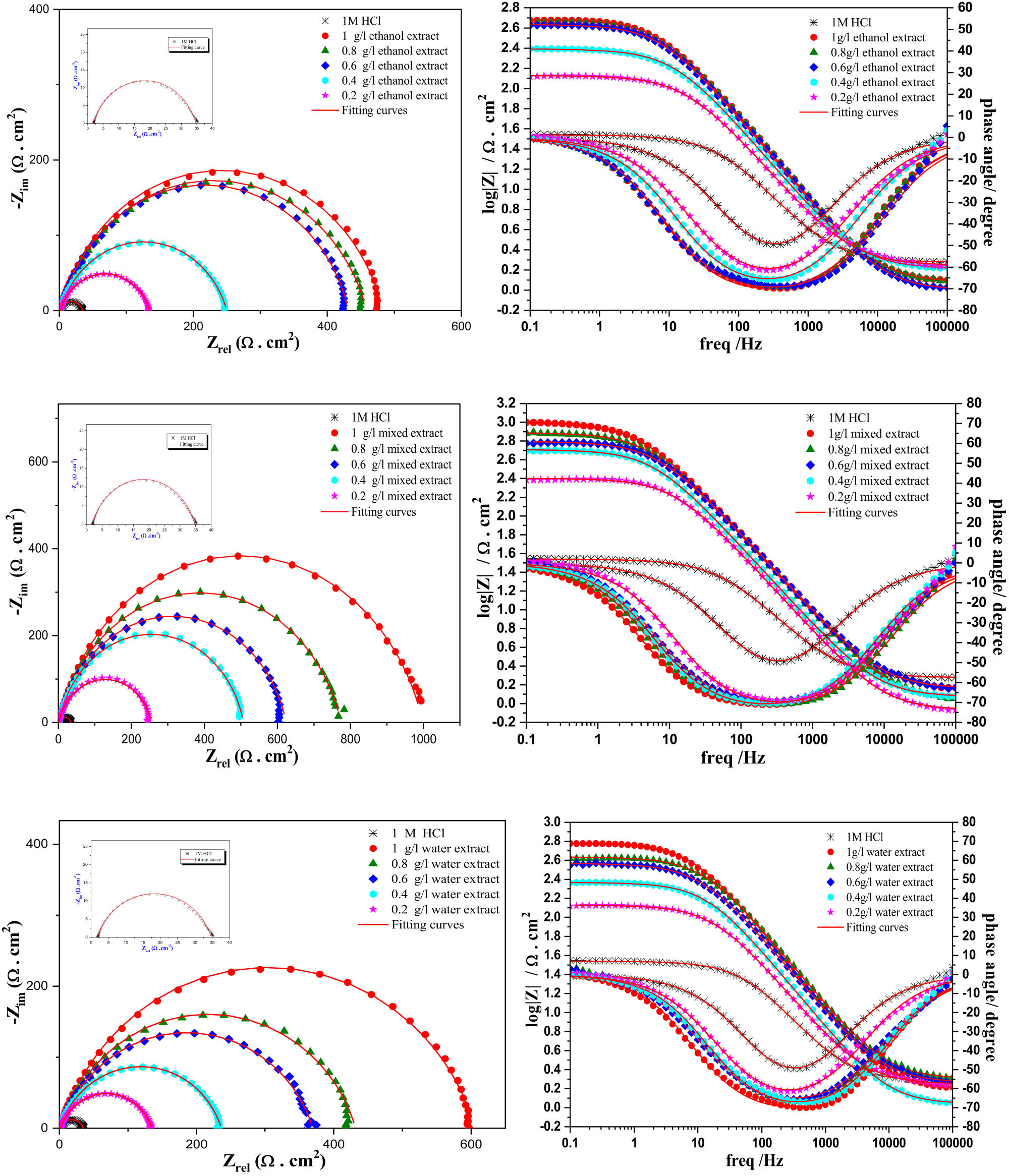
Nyquist and Bode diagrams for inhibitors extracted from the roots of I. viscosa plants at 298 K at different concentrations.
Electrolytic data, such as solution resistance (R s), charge transfer resistance (R ct), CPE and double layer capacitance (C dl), are presented in Table 4 (Figure 6).
where R ct(inh) is the inhibitor charge transfer resistance and R ct is the charge transfer resistance of 1 M HCl.
EIS values for MS in corrosive media 1 M HCl without and with inhibitor IVR extracts
| Conc. | R s | R ct | C dl | n dl | Q | Ɵ | ƞ imp | |
|---|---|---|---|---|---|---|---|---|
| (g/L) | (Ω cm2) | (Ω cm2) | (µF cm−2) | (%) | ||||
| 1 M HCl | — | 1.76 | 33.2 | 89.10 | 0.784 | 312.7 | — | — |
| Ethanol | 1 | 1.20 | 479.1 | 43.03 | 0.837 | 80.75 | 0.930 | 93.0 |
| 0.8 | 1.18 | 453.3 | 44.46 | 0.829 | 86.41 | 0.926 | 92.6 | |
| 0.6 | 0.99 | 431.5 | 46.78 | 0.827 | 91.58 | 0.923 | 92.3 | |
| 0.4 | 1.63 | 245.7 | 52.78 | 0.823 | 113.7 | 0.864 | 86.4 | |
| 0.2 | 1.76 | 132.9 | 64.54 | 0.806 | 162.0 | 0.750 | 75.0 | |
| Mixed (water + ethanol) | 1 | 1.44 | 996.7 | 40.96 | 0.836 | 69.22 | 0.966 | 96.6 |
| 0.8 | 1.10 | 772.9 | 41.08 | 0.840 | 71.35 | 0.957 | 95.7 | |
| 0.6 | 1.44 | 631.4 | 42.49 | 0.846 | 74.18 | 0.947 | 94.7 | |
| 0.4 | 1.18 | 511.1 | 51.26 | 0.844 | 90.47 | 0.935 | 93.5 | |
| 0.2 | 0.85 | 254.4 | 54.08 | 0.843 | 105.40 | 0.869 | 86.9 | |
| Water | 1 | 1.56 | 595.3 | 29.83 | 0.816 | 62.64 | 0.944 | 94.4 |
| 0.8 | 2.01 | 429.7 | 30.16 | 0.817 | 66.66 | 0.922 | 92.2 | |
| 0.6 | 1.82 | 367.8 | 38.34 | 0.805 | 87.93 | 0.909 | 90.9 | |
| 0.4 | 1.08 | 232.1 | 48.72 | 0.825 | 106.7 | 0.856 | 85.6 | |
| 0.2 | 1.75 | 133.1 | 64.59 | 0.806 | 162.0 | 0.678 | 75.0 |
Bold values show maximum percentages of corrosion inhibition.
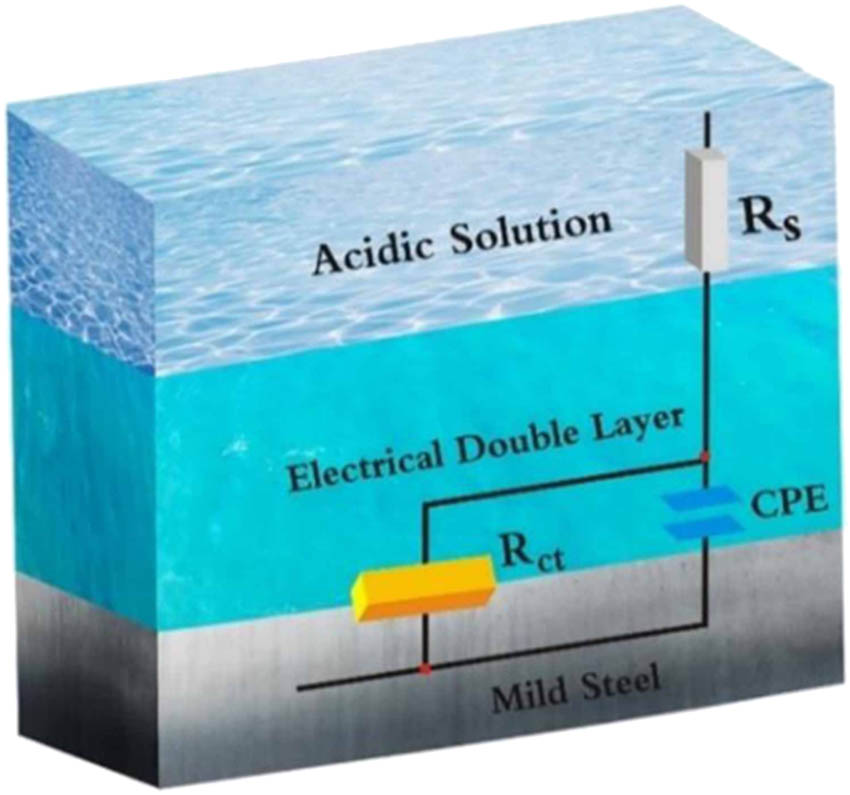
Equivalent circuit used to fit electrochemical data.
Solution resistance (R s) is an important measure in electrolytic systems as it represents the resistance to electrical conduction offered by the solution itself. This value is essential for assessing the electrical conductivity of the medium and understanding how ions move through the solution. A high R s may indicate low solution conductivity, which can influence ongoing electrochemical processes. For instance, in the context of corrosion inhibitor studies, a higher R s may suggest reduced ion mobility in the solution, which can impact the effectiveness of corrosion inhibition. In summary, the R s value in Table 4, ranging from a minimum of 0.85 to a maximum of 2.01, provides valuable insight into the electrical conductivity of the system and its involvement in the studied electrochemical processes.
According to the data presented in Table 4, it can be observed that the values of ndl are significantly higher than those of the control sample (blank). This observation suggests that the presence of inhibitor in the chloridric acid solution reduces the irregularity of the MS surface [73]. This reduction is probably attributed to the formation of an organic film on the steel surface. Additionally, the phase shift values (n) when inhibitors are introduced are very close to unity. This observation implies that the electric double layer formed by the adsorption of IVR bioactive molecules at the metal-electrolyte interfaces behaves like a pseudo-capacitor. The most evident observation lies in the low values of solution resistance, approximately 1–2 Ω cm2, and charge transfer resistance, around 33.2 Ω cm2, observed in a corrosive environment, confirming the rapidity of the corrosion processes [74]. In comparison, significantly higher values of solution resistance and charge transfer resistance have been recorded, confirming that the addition of corrosion inhibitors increased resistance against corrosion processes [75].
In our study, it is noticeable that the R ct (charge transfer resistance) value for the blank (absence of inhibitor) is 33.2 Ω cm². Conversely, after the addition of inhibitors, a remarkable increase in R ct is observed, ranging from a maximum of 996.7 Ω cm2 to a minimum of 132.9 Ω cm2. This variation in R ct is linked to the variation in IVR extract and the concentration of each IVR extract. While the values of the double-layer capacitance (C dl) decrease, in our case, in the absence of inhibitor, the value of C dl is 89.10 µF cm−2. After the addition of inhibitor, a rapid decrease in double-layer capacitance is observed, reaching a maximum value of 64.59 µF cm−2 and a minimum value of 29.83 µF cm−2. This phenomenon leads to a reduction in the rate of charge and discharge at the interface between the working electrode and the solution. The roughness of the MS surface and the corrosive environment of the CPE contribute systematically to the formation of a double layer [76].
The EIS parameters obtained confirmed that the corrosion inhibition efficiency was higher with various IVR extracts and different concentrations, reaching maximum values of up to 96.6% in the mixed extract (ethanol + water). This can be explained by solvent interaction: Water and ethanol are polar solvents, meaning they have the ability to interact with both polar and non-polar molecules. This interaction can facilitate the dissolution and dispersion of inhibitor molecules, leading to better coverage and adherence to the metal surface, thus improving inhibition performance. Additionally, synergistic effects may also be at play: The combination of water and ethanol can create synergistic effects that enhance inhibition performance beyond what would be achieved with either solvent alone. These synergistic effects could result from changes in the composition of the mixture, leading to enhanced interactions between the components [77].
3.2.4 Isotherm adsorption
In order to ascertain accessible information regarding the adsorption capabilities of inhibitors derived from the roots of the I. viscosa plant, the outcomes of experimental EIS data were assessed through parallel lines corresponding to diverse adsorption isotherms (Langmuir, Temkin, Frumkin, Freundlich, Flory-Huggin, and El-Awady), employing linear equations (Table 5) [78].
Linear equations for isotherms
| Isotherms | Linear equations | Descriptions |
|---|---|---|
| Langmuir |
|
K: Adsorption coefficient, C inh: Inhibitor concentration, ϴ: Inhibitor retention rate. |
| El-Awady |
|
1/y represents the quantity of water molecules removed by a single molecule of the inhibitor compound. |
| Flory-Huggins |
|
X represents the number of adsorbed H2O molecules substituted by inhibitor compounds. |
| Freundlich |
|
Z, where 0 < Z < 1, indicates that the adsorption of the inhibitor on the metal surface is facile |
| When Z = 1, it denotes a moderate adsorption of the inhibitor on the metal surface | ||
| If Z > 1, it implies a challenging adsorption behavior of the inhibitor | ||
| Frumkin |
|
a represents the interaction factors between adsorbed molecules, which can involve either repulsive or attractive forces |
| Temkin |
|
a represents the coefficient for the interaction, whether it is repulsion or attraction, among adsorbed compounds |
The adsorption process of various extracts from the roots of the I. viscosa plant was examined using different isothermal models. From the data presented in Table 6 and Figure 7, it is observed that the correlation coefficient values were close to 1 in the Langmuir model, except for the Frumkin model, which showed moderate correlation coefficients varying with solvent variation (min 0.16–max 0.59). Hence, given the adsorption constants, it can be suggested that the adsorption phenomenon of the studied inhibitors can be better represented by the Langmuir model for the different extracts studied. It is noteworthy that the Langmuir model exhibits a very high correlation coefficient of 0.99 and a slope near to unity. The Freundlich model is excluded due to its very low K values, despite having a high correlation coefficient, with the adsorption values of the studied inhibitors being far from the fitting. In the El-Awady model, a very strong correlation coefficient is observed along with
Key parameters were obtained by testing different isothermal models
| Isotherms | Inhibitors | R² | Parameters | K |
|
|
|---|---|---|---|---|---|---|
| Freundlich | Ethanol | 0.95 | z | 7.26 | 0.95 | −17.02 |
| Mixed | 0.96 | 15.66 | 0.97 | −17.06 | ||
| Water | 0.98 | 7.00 | 0.95 | −17.02 | ||
| El-Awady | 1/y | |||||
| Ethanol | 0.97 | 1.02 | 16.6 | −24.09 | ||
| Mixed | 0.99 | 1.15 | 47.1 | −26.67 | ||
| Water | 0.99 | 0.94 | 16.2 | −24.02 | ||
| Frumkin | a | |||||
| Ethanol | 0.16 | −0.16 | 8.40 × 10−2 | −10.98 | ||
| Mixed | 0.59 | 0.84 | 6.69 × 10−3 | −4.71 | ||
| Water | 0.53 | −0.22 | 9.50 × 10−2 | −11.29 | ||
| Florry-Huggins | x | |||||
| Ethanol | 0.96 | 0.96 | 14.6 | −23.77 | ||
| Mixed | 0.99 | 1.14 | 46.8 | −26.66 | ||
| Water | 0.99 | 0.93 | 13.4 | −23.56 | ||
| Temkin | Methanol | a | ||||
| Ethanol | 0.95 | −4.31 | 3.78 × 103 | −37.55 | ||
| Mixed | 0.96 | −8.54 | 1.66 × 107 | −58.34 | ||
| Water | 0.98 | −4.14 | 2.76 × 103 | −36.77 | ||
| Langmuir | Slope | |||||
| Ethanol | 0.99 | 1.009 | 17.1 | −24.17 | ||
| Mixed | 0.99 | 1.009 | 36.9 | −26.07 | ||
| Water | 0.99 | 0.992 | 14.6 | −23.77 | ||
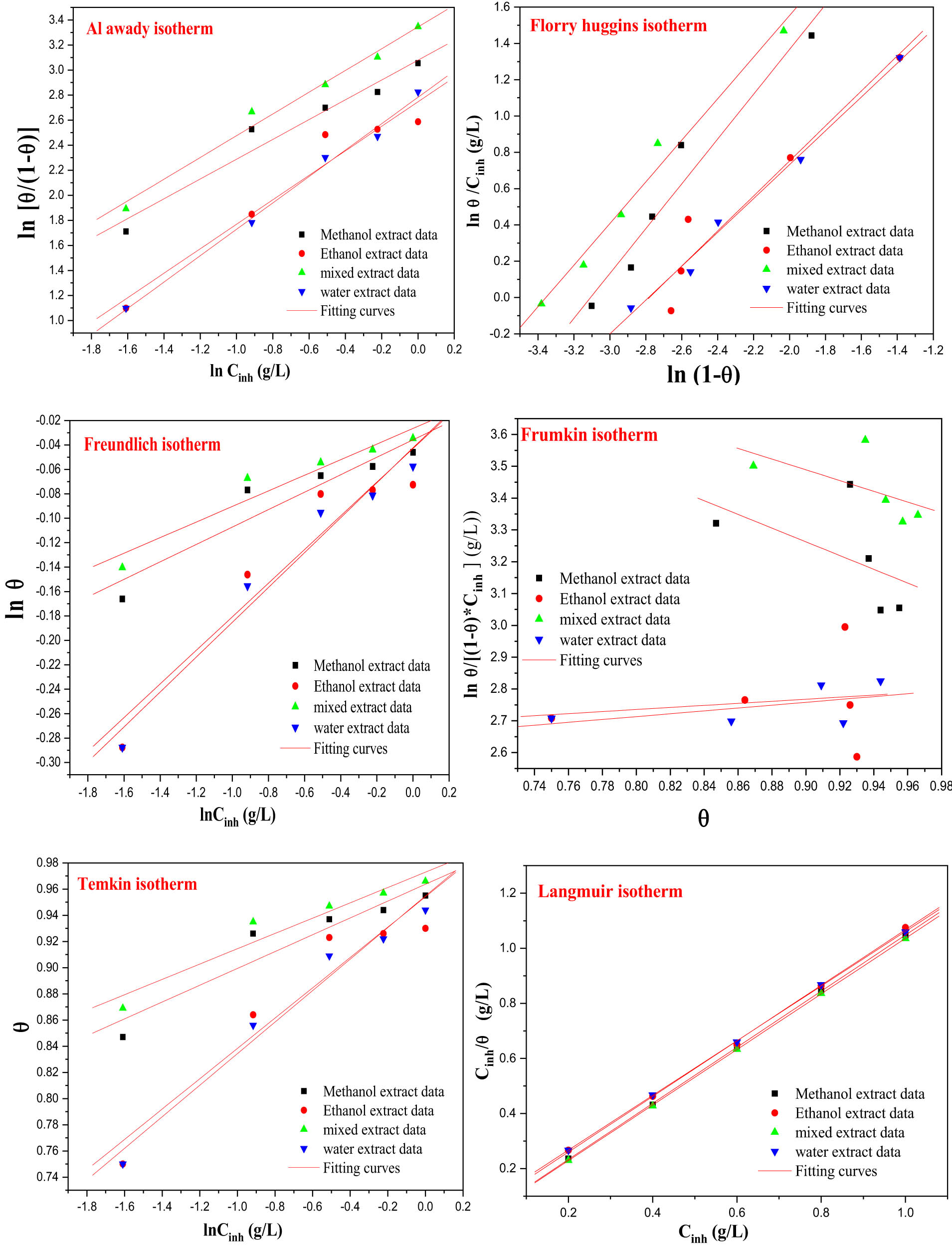
Different isothermal models for MS in a pickling medium containing different concentrations of IVR extract at 298 K.
3.3 Surface morphological studies
Figure 8 illustrates the morphological attributes of MS after a 6-h immersion in 1 M HCl, both in the absence and presence of a 1 g/L concentration of various root extracts from the I. viscosa plant. In Figure 8(1), the topography of the sample surface is corroded after immersion in 1 M HCl at 298 K for 6 h, resulting in irregular surfaces due to the effects of corrosion. Conversely, samples immersed in 1 M HCl containing concentrations of 1 g/L ethanol, mixed extract, and aqueous inhibitors of the root of the I. viscosa plant (Figure 8(2)–(4)) show smoother surfaces. This smoothness implies that the primary inhibitors are absorbed by the metal surface, generating a protective layer that shields the metal from the corrosive environment, thus preserving it from the damaging effects of corrosion [80].
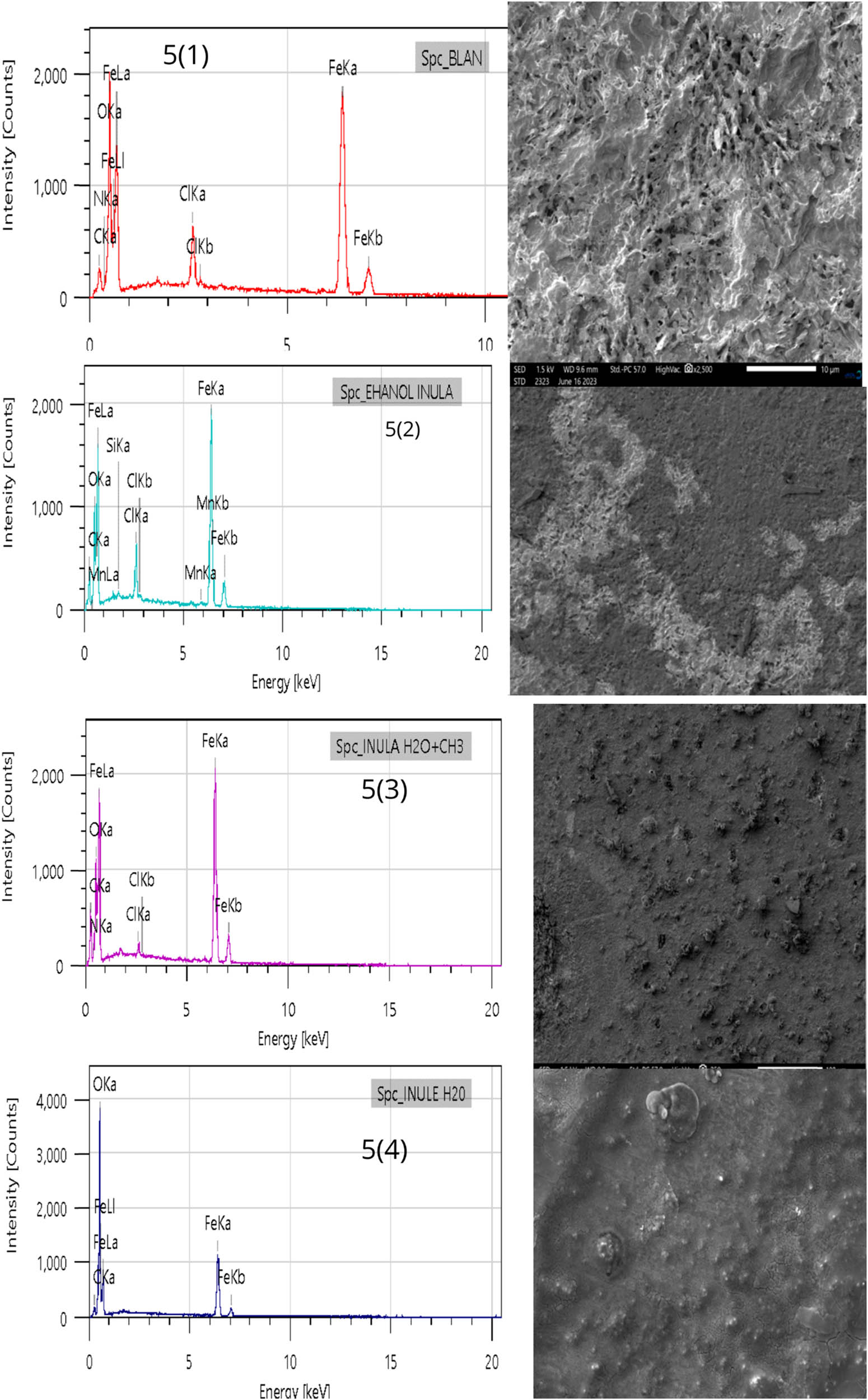
SEM/EDX images of MS in a molar HCl solution under investigation, both without and with the presence of inhibitors, following 6 h of immersion.
Table 7 presents the weight proportions of various elements obtained through EDX analysis of the steel surface in a 1 M HCl solution. These measurements were conducted at a temperature of 298 K, both in the presence and absence of inhibitors at a concentration of 1 g/L. The experimental EDX spectrum of the MS sample reveals characteristic peaks for its fundamental components, including silicon (Si), aluminum (Al), carbon (C), manganese (Mn), chromium (Cr), and iron. When the sample is immersed in the uninhibited solution, the mass percentages for MS indicate a significant presence of chloride (Cl) and oxygen (O) elements. These elements are attributed to the formation of corrosion products on the steel surface. However, with the introduction of inhibitors, these rates decrease, thus demonstrating the protective effectiveness of these antioxidants on the steel surface against corrosive attacks. They contribute to reducing chloride ion penetration and preventing iron oxidation at the metal surface [49].
Mass percentage of various constituents obtained from the EDX spectroscopy analysis of the steel surface was determined in a 1 M HCl solution at a temperature of 298 K, both in the presence and absence of inhibitors at a concentration of 1 g/L
| Element | wt% of MS with HCl | wt% of MS with ethanol inhibitors | wt% of weight MS with mixed inhibitors | wt% of weight MS with water inhibitors |
|---|---|---|---|---|
| C | 4.74 ± 0.06 | 7.70 ± 0.07 | 8.57 ± 0.07 | 3.73 ± 0.05 |
| N | — | — | — | — |
| O | 13.75 ± 0.11 | 6.80 ± 0.08 | 6.99 ± 0.08 | 5.98 ± 0.17 |
| Cl | 3.96 ± 0.06 | 1.2 ± 0.06 | 0.90 ± 0.04 | 0.82 ± 0.04 |
| Fe | 77.55 ± 0.51 | 80.54 ± 0.51 | 83.55 ± 0.51 | 65.14 ± 0.53 |
| Mn | — | 0.74 ± 0.08 | — | — |
| Si | — | 0.25 ± 0.03 | — | — |
3.4 Result of theoretical methods
The quantum calculations help us understand and classify the reactivity of the molecules used as corrosion inhibitors and their interaction with the MS surface. Therefore, before the calculations, the predicted protonated form of the studied molecules was tested using MarvinSketch software [81,82]. The species distribution vs pH axes is presented in Figure 9. The theoretical calculations were conducted in the neutral state in the aqueous phase, and their descriptors were extracted and presented in Table 8. From the protonation results, it can be observed that all the molecules showed a high distribution of the neutral form in the acidic zone. Therefore, it can be suggested that these neutral forms are the predicted forms that can exist in the studied acidic solution (1 M HCl).
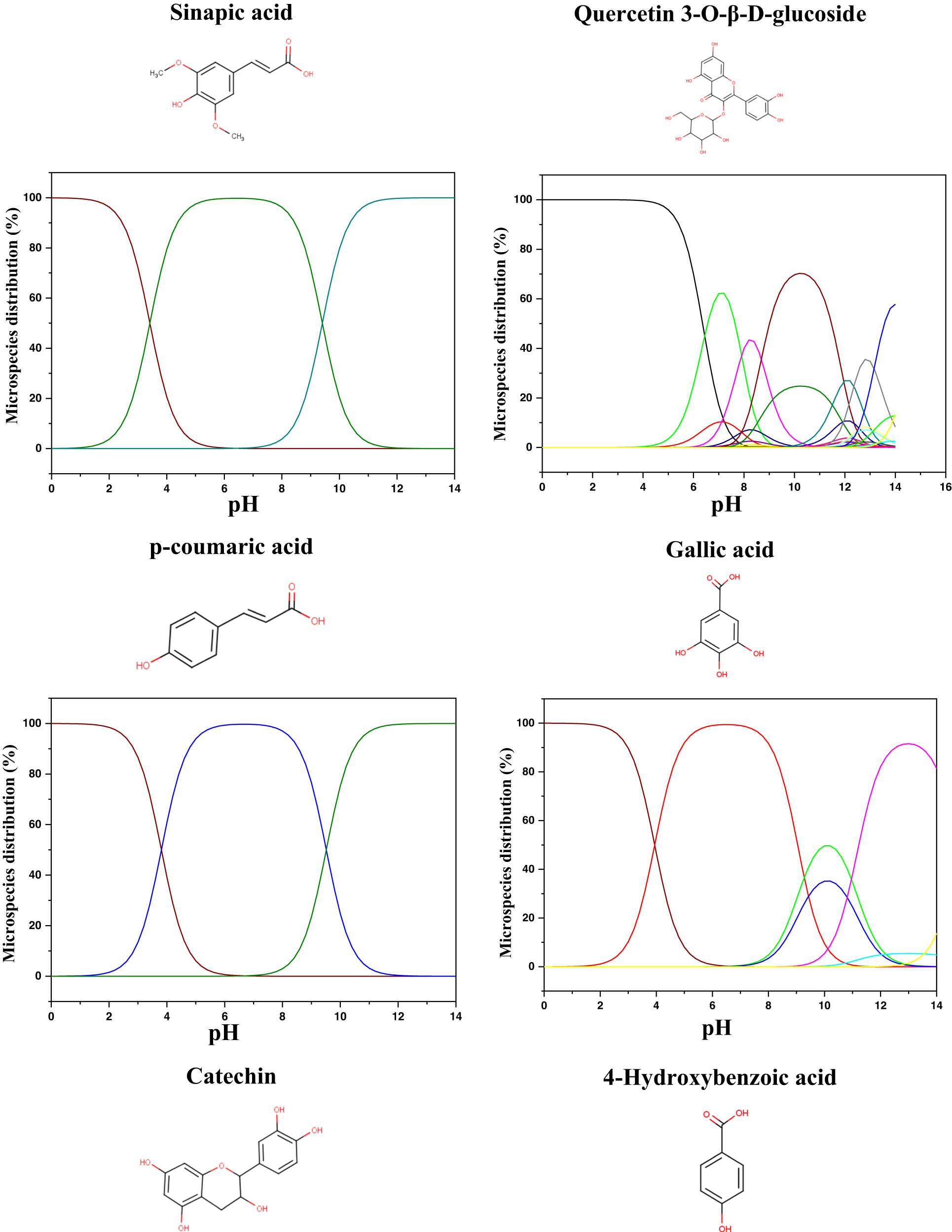
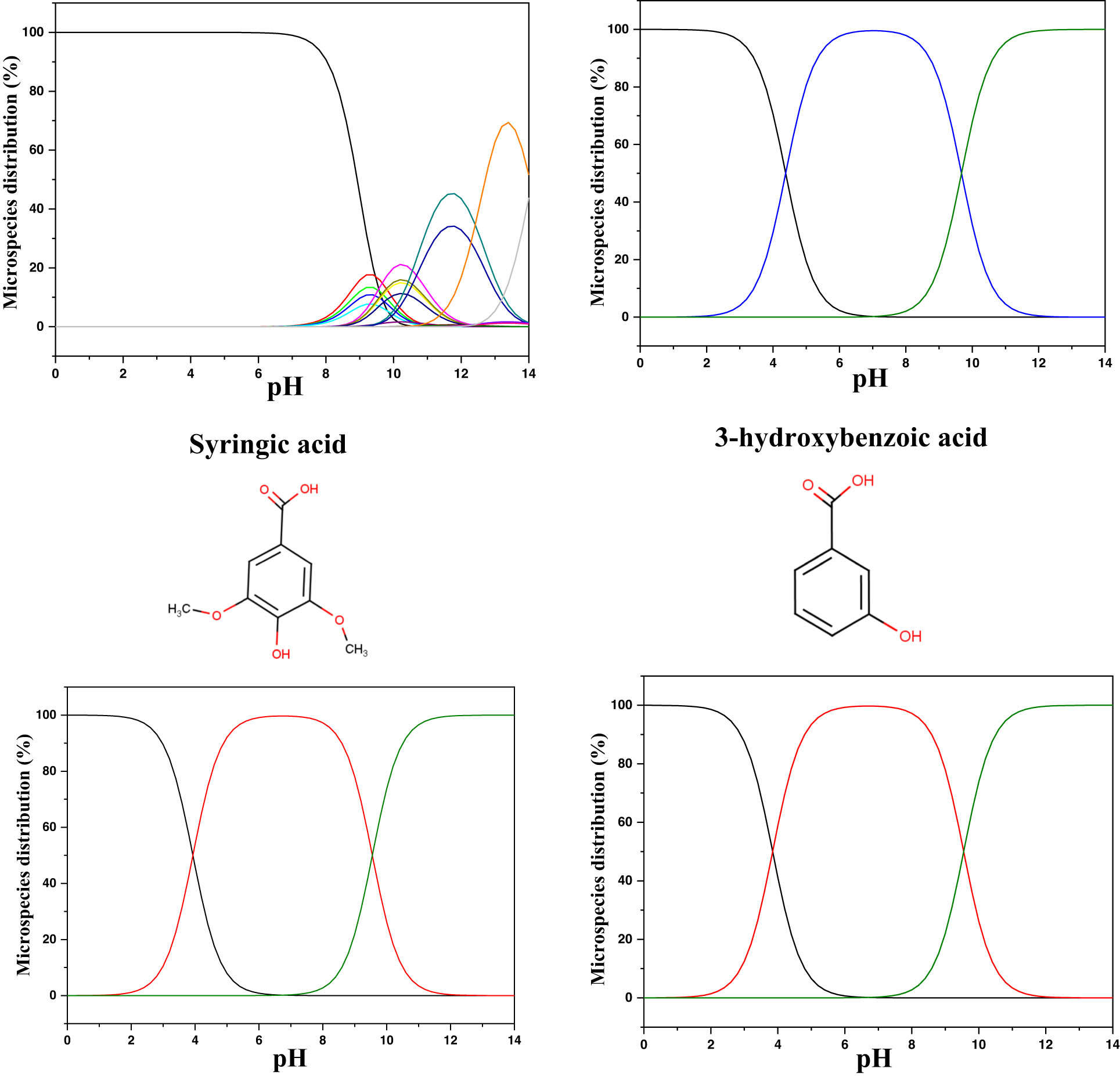
Microspecies distribution vs pH.
Descriptors of reactivity for the neutral form of the studied molecules in aqueous phase
| Descriptors | E HOMO (eV) | E LUMO (eV) | ΔE gap (eV) | Ƞ (eV) | σ (eV−1) | χ (eV) | ΔE back-donation | ΔN Fe/110 | |
|---|---|---|---|---|---|---|---|---|---|
| Ethanol | Sinapic acid | −5.9855 | −2.0335 | 3.9519 | 1.9759 | 0.5060 | 4.0095 | −0.4939 | 0.2050 |
| Quercetin 3-O-β-d-glucoside | −5.8726 | −1.8335 | 4.0390 | 2.0195 | 0.4951 | 3.8530 | −0.5048 | 0.2393 | |
| p-coumaric acid | −6.2266 | −1.9965 | 4.2301 | 2.1150 | 0.4728 | 4.1115 | −0.5287 | 0.1674 | |
| Water | Gallic acid | −6.2824 | −1.4305 | 4.8518 | 2.4259 | 0.4122 | 3.8564 | −0.6064 | 0.1985 |
| 4-Hydroxybenzoic acid | −6.6778 | −1.3986 | 5.2791 | 2.6395 | 0.3788 | 4.0382 | −0.6598 | 0.1480 | |
| Catechin | −6.0320 | −0.4378 | 5.5942 | 2.7971 | 0.3575 | 3.2349 | −0.6992 | 0.2833 | |
| Water + ethanol | Syringic acid | −6.2843 | −1.4523 | 4.8320 | 2.4160 | 0.4139 | 3.8683 | −0.6040 | 0.1969 |
| 3-Hydroxybenzoic acid | −6.5861 | −1.6572 | 4.9289 | 2.4644 | 0.4057 | 4.1216 | −0.6161 | 0.1416 | |
| Catechin | −6.0320 | −0.4378 | 5.5942 | 2.7971 | 0.3575 | 3.2349 | −0.6992 | 0.2833 |
3.4.1 DFT calculations
DFT plays a crucial role in understanding the inhibitory efficiency of the molecules found in the extract when adsorbed onto the MS surface. The adsorption process relies on donor-acceptor correlations governed by the electronic organization of the molecules. The electron-dense regions of these extract molecules can potentially transport their charges to the metal-liquid contact zone, especially towards the unsaturated orbitals of iron atoms. Figure 10 illustrates the electronic structure of the extracted molecules, highlighting the distribution of the Highest Occupied Molecular Orbital (HOMO) and Lowest Unoccupied Molecular Orbital (LUMO) for various molecules from the IVR extract. The graphs reveal that the HOMO orbital is primarily localized on the benzene function, methoxy function, and alcohol function for molecules such as sinapic acid, quercetin 3-O-β-d-glucoside, p-coumaric acid, gallic acid, 4-hydroxybenzoic acid, catechin, syringic acid, and 3-hydroxybenzoic acid. Simultaneously, the HOMO orbital is distributed across the entire surface of the molecule. Therefore, it can be concluded that the HOMO orbital contains π electrons from benzene functions and non-bonding electron pairs from heteroatoms, making them available for interaction with metal orbitals at the interface.
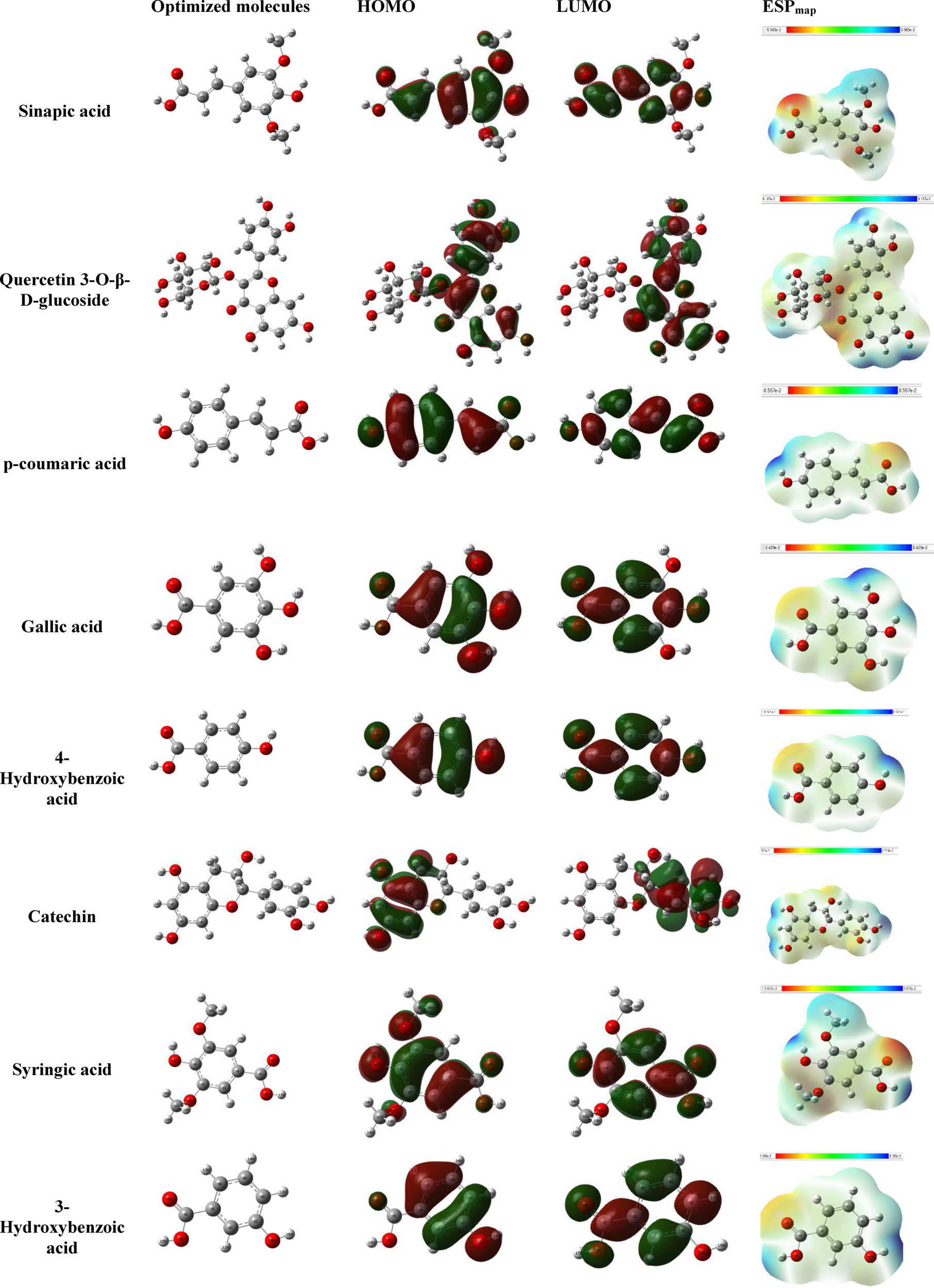
Optimized structure, LUMO, HOMO densities, and ESP map of detected molecules.
In addition, LUMO orbital is graphically located on the benzene function and the oxygen/carbon atoms. Consequently, the molecules of the extracts can be adsorbed on the surface of iron by accepting the electrons located in the orbitals occupied by the iron atoms [65].
Table 8 lists data regarding the fundamental characteristics of neutral forms within the predominant structure found in each IVR extract. These parameters encompass the estimation of HOMO, the quantum levels of LUMO, dipole moment values, and the energy gap (ΔE gap), which serve as indicators of the anticorrosion potential of the extracted molecules [66].
The lowest ΔE gap values for IVR molecules (ethanolic extract) are as follows: 3.9519 eV for sinapic acid, 4.0390 eV for quercetin 3-O-β-d-glucoside, and 4.2301 eV for p-coumaric acid. On the other hand, the minimal ΔE gap values for IVR molecules (aqueous extract) are 4.8518 eV for gallic acid, 5.2791 eV for 4-hydroxybenzoic acid, and 5.5942 eV for catechin. As for the minimal ΔE gap values for molecules in the mixed IVR extract, they are, respectively, 4.8320 eV for syringic acid, 4.9289 eV for 3-hydroxybenzoic acid, and 5.5942 eV for catechin. These results suggest that the majority of molecules present in each extract exhibit remarkable anticorrosion reactivity.
Based on the chemical hardness/softness descriptors, it can be observed that Sinapic acid molecule has a high reactivity compared to the other components in the ethanolic extract. For the aqueous extract, the Gallic acid molecule showed a small hardness value and a high softness value compared to the other components that mostly existed in this extract, verifying the high reactivity of Gallic acid molecule. In addition, syringic acid molecule is the high reactive molecule in the mixed IVR extract. This observation leads us to suppose that the studied molecule can offer electrons to the metal surface, facilizing their adsorption onto the metal surface. Moreover, all the studied molecules showed a negative sign in ΔE back-donation parameter, indicating the adsorption of these particles into the steel surface, and offering electrons to the d vacuum orbital of iron atoms. Furthermore, the electronegativity values results are gone with the same trend as the other parameters, confirming the reactivity order of the studied components.
The number of transferred electrons (ΔN Fe/110) can be considered as an indicator of the capacity to donate electrons. Referring to Table 8, it is evident that the ΔN Fe/110 values are positive, indicating that these molecules act as electron donors.
3.4.2 MC simulation
A MC simulation was performed on Fe 110/200 H2O systems to enhance our comprehension of how molecular structures interact with the iron surface. The most stable adsorption configuration of the inhibitors under investigation is illustrated in Figure 11, presenting both a top view and a side view.
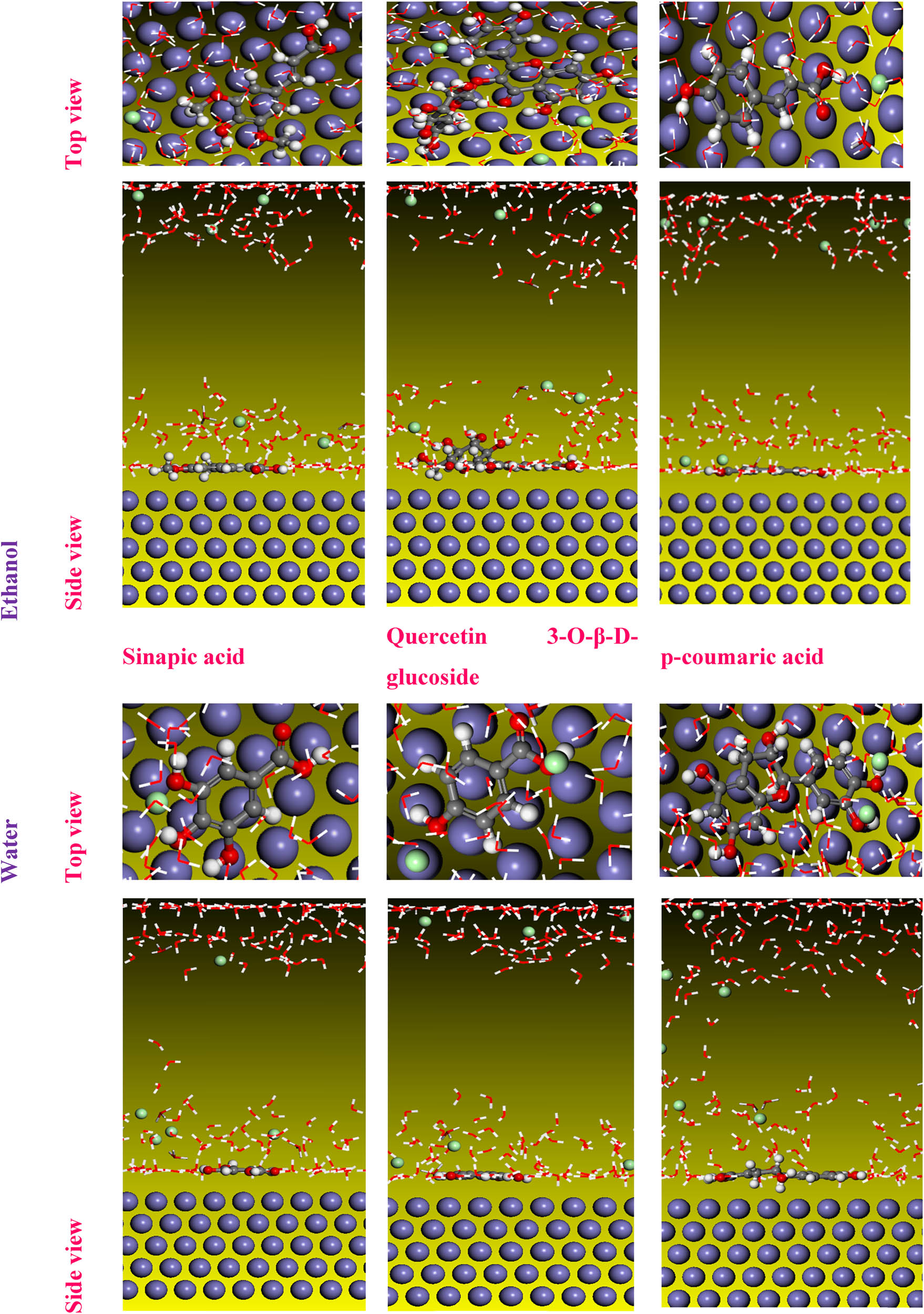
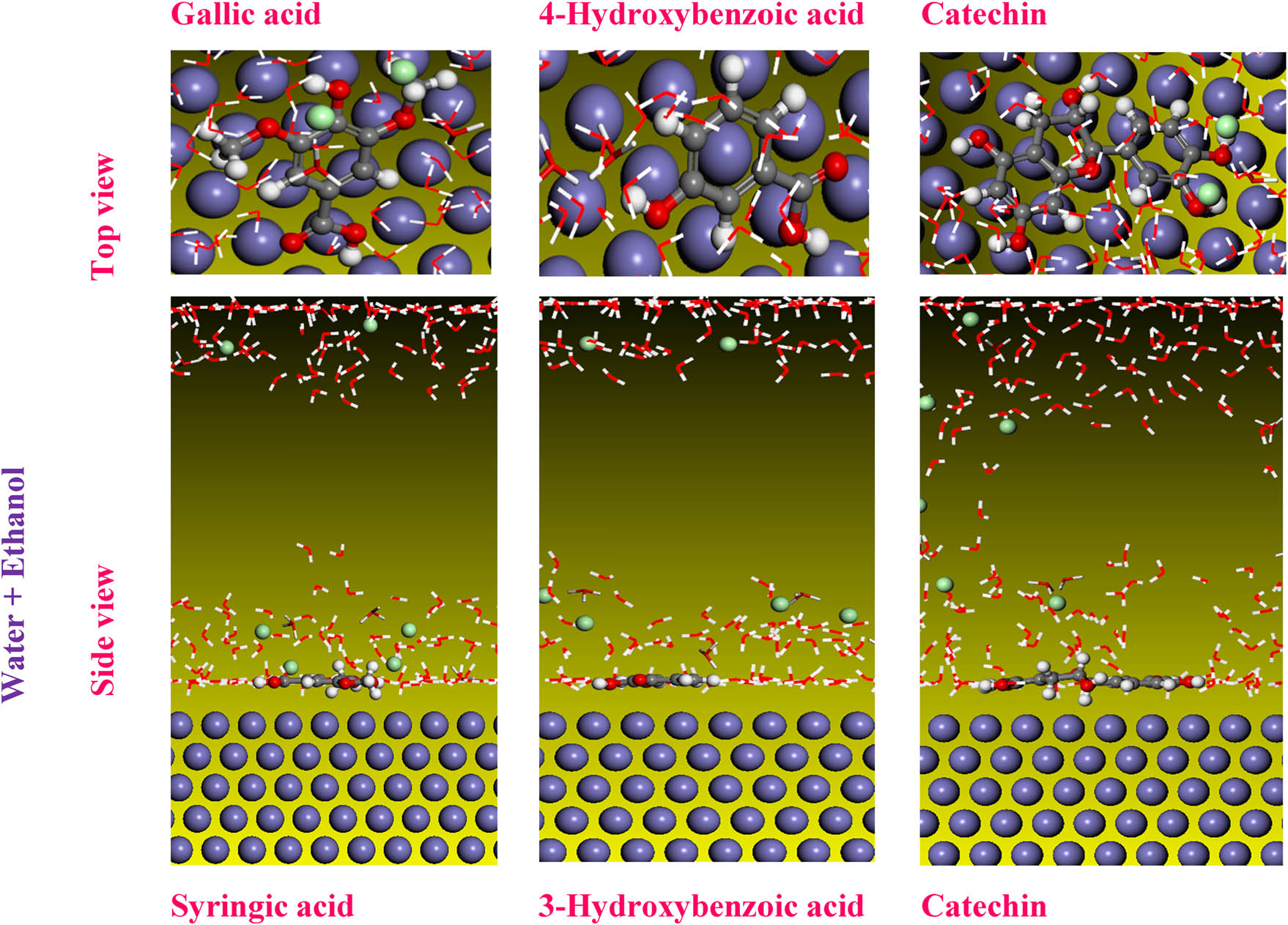
Top and side views of the stable configuration for the studied molecules in the DFT study.
The inhibitory performance of the examined chemical structures has previously conducted, appearing that the sinapic acid structure exhibits higher reactivity compared to quercetin 3-O-β-d-glucoside and p-coumaric acid in an ethanolic extract. Moreover, gallic acid exhibits significant reactivity compared to other structures in a water extract. In contrast, syringic acid is more reactive than other structures in an IVR extract, representing a mixture of water and ethanol. The simulation interface containing the Fe 110 inhibitor and 200 water molecules shows that the structures found are adsorbed concerning the iron surface layer.
It can be observed that the absorption of p-coumaric acid increases with the rise in concentration, indicating a stronger interaction with the medium at higher concentrations. In contrast, in the water extract, the catechin molecule showed some inclination compared to 4-hydroxybenzoic acid and gallic acid, which can explain the high adsorption of these molecules in the steel surface. The results of the mixed extract show that catechin is also adsorbed with fewer reactive sites compared to 3-hydroxybenzoic acid and syringic acid [83].
Furthermore, based on the absolute value of E ads, the adsorption energy follows the following order: sinapic acid > quercetin 3-O-β-d-glucoside > p-coumaric acid in the ethanolic IVR extract. In the water IVR extract: gallic acid > 4-hydroxybenzoic acid > catechin. Finally, syringic acid > 3-hydroxybenzoic acid > catechin in the mixed IVR extract. This order validates the results of the DFT calculations (Table 9). These results indicated that the adsorption energy of water is very low compared to the other molecular forms studied, which allows us to conclude their migration from the surface of iron [74].
MC simulation parameters
| Solvant | Molecules | E ads | Inhibitor: dE ad/dN i | Rigid adsorption energy | Deformation energy | H2O: dE ad/dN i | H3O+: dE ad/dN i | Cl−: dE ad/dN i |
|---|---|---|---|---|---|---|---|---|
| Ethanol | Sinapic acid | −3855.70 | −158.51 | −3990.86 | 135.16 | −12.86 | −147.40 | −143.63 |
| Quercetin 3-O-β-d-glucoside | −3781.43 | −210.18 | −3919.55 | 138.12 | −11.87 | −126.26 | −138.87 | |
| p-Coumaric acid | −3759.53 | −119.11 | −3890.15 | 130.62 | −10.38 | −147.85 | −152.96 | |
| Water | Gallic acid | −3713.40 | −118.20 | −3853.04 | 139.64 | −8.05 | −138.61 | −146.11 |
| 4-Hydroxybenzoic acid | −3666.35 | −104.90 | −3801.91 | 135.56 | −12.72 | −143.30 | −163.31 | |
| Catechin | −3490.41 | −192.30 | −3620.79 | 130.37 | −12.91 | −148.52 | −124.30 | |
| Water + ethanol | Syringic acid | −3748.77 | −144.59 | −3886.1 | 137.33 | −13.37 | −149.59 | −153.08 |
| 3-Hydroxybenzoic acid | −3709.56 | −102.36 | −3845.48 | 135.92 | −12.14 | −149.03 | −149.90 | |
| Catechin | −3490.41 | −192.30 | −3620.79 | 130.37 | −12.91 | −148.52 | −124.30 |
4 Conclusion
The corrosion of materials remains a significant global issue, with substantial costs and implications for material longevity. Large corporations are actively seeking effective and sustainable solutions to mitigate or reduce material corrosion. In this scientific study, we have demonstrated that the roots of the I. viscosa plant, found in the Moulay Yaâcoub province of Morocco, can efficiently inhibit corrosion in various extracts, particularly in the mixed extract composed of water and ethanol, achieving a remarkable inhibition rate of 96.6%. We observed that the combination of these two solvents in the IVR extract yields excellent corrosion inhibition efficiency. Analyses conducted using EDX and SEM techniques on the various studied extracts have revealed a significant reduction in the dissolution of MS, owing to the formation of a protective and preservative surface layer. Additionally, HPLC analyses identified three major molecules in each studied extract. Notably, HPLC results for the mixed extract enabled the identification of three predominant molecules: syringic acid, 3-hydroxybenzoic acid, and catechin, which contribute to outstanding corrosion inhibition compared to other studied extracts. Therefore, considering the adsorption constants, we suggest that the adsorption phenomenon of the studied inhibitors can be better represented by the Langmuir model for different extracts. It should be noted that the Langmuir model exhibits a very high correlation coefficient of 0.99. Finally, the application of DFT and classical MC simulation methods has established a strong correlation between experimental data and theoretical predictions, thereby enhancing our understanding of the underlying mechanisms.
Acknowledgements
Authors wish to thank Researchers Supporting Project number (RSP2024R346) at King Saud University, Riyadh Saudi Arabia for financial support.
-
Funding information: This research paper was Supported by Project number (RSP2024R346) at King Saud University, Riyadh Saudi Arabia for financial support.
-
Author contributions: M.A.D., R.S., and M.K. – conceptualization; M.A.D., A.A., R.U., and A.B. – formal analysis; M.A.D., M.K., H.F., and E.L. – investigation; M.A.D., A.B., A.L., and M.K. – writing – original draft; M.A.D., M.K., A.M.I., and B.H. – software; M.A.D., M.K., A.A., and H.F. – writing – review and editing; and Z.R. and M.T. – supervision. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Wang Q, Tan B, Bao H, Xie Y, Mou Y, Li P, et al. Evaluation of Ficus tikoua leaves extract as an eco-friendly corrosion inhibitor for carbon steel in HCl media. Bioelectrochemistry. 2019;128:49–55. 10.1016/j.bioelechem.2019.03.001.Search in Google Scholar PubMed
[2] Nikpour S, Ramezanzadeh M, Bahlakeh G, Ramezanzadeh B, Mahdavian M. Eriobotrya japonica Lindl leaves extract application for effective corrosion mitigation of mild steel in HCl solution: Experimental and computational studies. Constr Build Mater. 2019;220:161–76. 10.1016/j.conbuildmat.2019.06.005.Search in Google Scholar
[3] Fernandes CM, Ferreira Fagundes TS, Escarpini dos Santos N, Shewry de M. Rocha T, Garrett R, Borges RM, et al. Ircinia strobilina crude extract as corrosion inhibitor for mild steel in acid medium. Electrochim Acta. 2019;312:137–48. 10.1016/j.electacta.2019.04.148.Search in Google Scholar
[4] Umoren SA, Solomon MM, Obot IB, Suleiman RK. A critical review on the recent studies on plant biomaterials as corrosion inhibitors for industrial metals. J Ind Eng Chim. 2019;76:91–115. 10.1016/j.jiec.2019.03.057.Search in Google Scholar
[5] Wang N, Liu X, Jiang D. Preparation of Isoetes Sinensis Extract as a Green Corrosion Inhibitor for Q235 Carbon Steel in Hydrochloric Acid. Int J Electrochem Sci. 2022;17:221–66.10.20964/2022.12.67Search in Google Scholar
[6] Alrefaee SH, Yop Rhee K, Verma C, Quraishi MA, Ebenso EE. Challenges and advantages of using plant extract as inhibitors in modern corrosion inhibition systems: Recent advancements. J Mol Liq. 2021;321:114666. 10.1016/j.molliq.2020.114666.Search in Google Scholar
[7] Hossain N, Chowdhury MA, Kchaou M. An overview of green corrosion inhibitors for sustainable and environment friendly industrial development: an overview of green corrosion inhibitors for sustainable and environment friendly industrial development. J Adhes Sci Technol. 2021;35(7):673–90. 10.1080/01694243.2020.1816793.Search in Google Scholar
[8] Assouguem A, Lahlali R, Joutei AB, Kara M, Bari A, Aberkani K, et al. Assessing Panonychus ulmi (Acari: Tetranychidae) infestations and their key predators on Malus domestica Borkh in Varied Ecological Settings. Agronomy. 2024;14(3):457.10.3390/agronomy14030457Search in Google Scholar
[9] Majd M, Asaldoust S, Bahlakeh G, Ramezanzadeh B, Ramezanzadeh M. Green method for effective mitigation of carbon steel corrosion in a 1 M HCl medium protected by an aqueous extract of Primula vulgaris flower via experimental MC/MD simulation at the atomic level and theoretical DFT elucidation at the electronic level DFT theoretical elucidation. J Mol Liquide. 2019;284:658–74. 10.1016/j.molliq.2019.04.037.Search in Google Scholar
[10] Lgaza H, Bhat KS, Salghi R, Shubhalaxmi S, Jodeh M, Algarra B, et al. Overview of the corrosion inhibition behavior of three chalcone derivatives for mild steel in hydrochloric acid solution. J Mol Liquide. 2017;238:71–83. 10.1016/j.molliq.2017.04.124.Search in Google Scholar
[11] Zunita M, JuliyusKevin Y. Ionic liquids as corrosion inhibitors: from research and development to commercialization. Results Eng. 2022;15:100562. 10.1016/j.rineng.2022.100562.Search in Google Scholar
[12] Ali I, Khan M. Study of the inhibitory effect of carbendazim on carbon steel corrosion in 0.50 M HCl solutions. Int J Electrochem Sci. 2017;12:2285–96. 10.20974/2017.03.14.Search in Google Scholar
[13] Patel N, Hadlicka J, Beranek P, Salghi R, Bouya H, Ismat HA, et al. Inhibition of steel corrosion by various parts of Rotula aquatic plant extracts in H2SO4 solutions. Port Électrochim Acta. 2014;32:395–403. 10.4152/pea.201406395.Search in Google Scholar
[14] Bahramim M, Hosseini S, Pilvar P. Experimental and theoretical study of organic compounds as inhibitors of mild steel corrosion in sulfuric acid media. Corros Sci. 2010;52(9):2793–803. 10.1016/j.corsci.2010.04.024.Search in Google Scholar
[15] Assouguem A, Joutei AB, Lahlali R, Kara M, Bari A, Ali EA, et al. Evaluation of the impact of two citrus plants on the variation of Panonychus citri (Acari: Tetranychidae) and beneficial phytoseiid mites. Open Life Sci. 2024;19(1):1–10.10.1515/biol-2022-0837Search in Google Scholar PubMed PubMed Central
[16] Liu F, Du M, Zhang J, Qiu M. Electrochemical behavior of Q235 steel in carbon dioxide-saturated salt water based on a new imidazoline inhibitor. Corros Sci. 2009;51:102–9. 10.1016/j.corsci.2008.09.036.Search in Google Scholar
[17] Musa A, Kadhum A, Mohamad A, Takriff M. Experimental and theoretical study on the inhibition performance of triazole compounds for mild steel corrosion. Corros Sci. 2010;52:3331–40. 10.1016/j.corsci.2010.06.002.Search in Google Scholar
[18] Alrefaee SH, Rhee KY, Verma C, Quraishi MA, Eno EE. Challenges and advantages of using plant extract as inhibitors in modern corrosion inhibition systems: Recent advancements. J Mol Liq. 2021;321:114666. 10.1016/j.molliq.2020.114666.Search in Google Scholar
[19] Norman S, Wahyuadi J, Rini R, Daniel SF. Development of environmentally friendly corrosion inhibitor from the extract of areca flower for mild steel in acidic media, East. EurJ Enterp. 2020;2(6):104–45. 10.15587/1729-4061.2020.197875.Search in Google Scholar
[20] Verma C, Alfantazi A, Quraishi MA, Rhee KY. Are extracts really green substitutes for traditional toxic corrosion inhibitors? Challenges beyond origin and availability. Sustain Chem Pharm. 2023;31:100943. 10.1016/j.scp.2022.100943.Search in Google Scholar
[21] Hossain A, Chowdhury MA, Rana M, Hassan M, Islam S. Terminalia arjuna leaves extract as green corrosion inhibitor for mild steel in HCl solution. Results Eng. 2022;14:100438. 10.1016/j.rineng.2022.100438.Search in Google Scholar
[22] Dehghani A, Ghahremani P, Mostafatabar AH, Ramezanzadeh B. Plant extracts: likely alternatives to traditional inhibitors for controlling alloy corrosion against acidic media – A review. Conv Bioréf. 2022;14:7467–86. 10.1007/s13399-022-02893-4.Search in Google Scholar
[23] Ahmed ESJ, Ganesh GM. A comprehensive overview on corrosion in RCC and its prevention using various green corrosion inhibitors. Build Mater Sci. 2022;12:1682. 10.3390/buildings12101682.Search in Google Scholar
[24] Zakeri L, Bahmani E, Aghdam AS. Plant extracts as sustainable and green corrosion inhibitors for protection of ferrous metals in corrosive media: A mini review. Corros Commun. 2022;5:25–38. 10.1016/j.corcom.2022.03.002.Search in Google Scholar
[25] Fazal BR, Becker T, Kinsella B, Lepkova K. A review of plant extracts as green corrosion inhibitors for CO2 corrosion of carbon steel. Mater Sci. 2022;5:5. 10.1038/s41529-021-00201-5.Search in Google Scholar
[26] Kaur J, Daksh N, Saxena A. Corrosion inhibition applications of natural and eco-friendly corrosion inhibitors on steel in the acidic environment: An overview. Arab J Sci Eng. 2022;47:57–74. 10.1007/s13369-021-05699-0.Search in Google Scholar
[27] Kaya F, Solmaz R, Geçibesler İH. The use of methanol extract of Rheum Ribes (Işgın) flower as a natural and promising corrosion inhibitor for mild steel protection in 1 M HCl solution. J Ind Eng Chem. 2023;122:102–17. 10.1016/j.jiec.2023.02.013.Search in Google Scholar
[28] Naderi R, Bautista A, Velasco F, Soleimani M, Pourfath M. Use of licorice plant extract for controlling corrosion of steel rebar in chloride-polluted concrete pore solution. J Mol Liq. 2022;346:117–856. 10.1016/j.molliq.2021.117856.Search in Google Scholar
[29] Rasaq OM, Kingsley OU, Kelvin OY, Tien-Chien J. Sustainable approach for corrosion control in mild steel using plant-based inhibitors: A review. Mater Today Sustainability. 2023;22:100373. 10.1016/j.mtsust.2023.100373.Search in Google Scholar
[30] Huang L, Chen WQ, Wang SS, Zhao Q, Li HJ, Wu YC. Starch, cellulose and plant extracts as green inhibitors of metal corrosion: a review. Env Chem Lett. 2022;20:3235–64. 10.1007/s10311-022-01400-5.Search in Google Scholar
[31] Deyab MA, Mohsen Q, Guo L. Aesculus hippocastanum seeds extract as eco-friendly corrosion inhibitor for desalination plants: Experimental and theoretical studies. J Mol Liq. 2022;361:119–594. 10.1016/j.molliq.2022.119594.Search in Google Scholar
[32] Gabsi M, Ferkous H, Delimi A, Boublia A, Boulechfar C, Kahlouche A, et al. The curious case of polyphenols as green corrosion inhibitors: a review on their extraction, design, and applications. Env Sci Pollut Res. 2023;30:59081–105. 10.1007/s11356-023-26753-4.Search in Google Scholar PubMed
[33] Kusumaningrum I, Soenoko R, Siswanto E, Gapsari F. Investigation of Artocarpus Heteropyllus peel extract as non-toxic corrosion inhibitor for pure copper protection in nitric acid. Case Stud Chem Env Eng. 2022;6:100–223. 10.1016/j.cscee.2022.100223.Search in Google Scholar
[34] Daoudi W, El Aatiaoui A, Dagdag O, Zaidi K, Haldhar R, Kim SC, et al. Anti-corrosion coating formation by a biopolymeric extract of artemisia herba-alba plant: Experimental and theoretical investigations. Coatings. 2023;13:611. 10.3390/coatings13030611.Search in Google Scholar
[35] Thakur A, Kaya S, Abousalem AS, Sharma S, Ganjoo R, Assad H, et al. Computational and experimental studies on the corrosion inhibition performance of an aerial extract of Cnicus Benedictus weed on the acidic corrosion of mild steel. Process Saf Env Prot. 2022;161:801–18. 10.1016/j.psep.2022.03.082.Search in Google Scholar
[36] Fantana G, Roccaz LS, Passannantiy S, Pia Paternostro M. Sesquiterpene compounds from Inula viscosa. Nat Prod Rés. 2007;21(9):824–7.10.1080/14786410701415681Search in Google Scholar PubMed
[37] Rechek H, Haouat A, Hamaidia K, Pinto D, Boudiar T, Válega M, et al. Inula viscosa (L.) Aiton Ethanolic Extract Inhibits the Growth of Human AGS and A549 Cancer Cell Lines. Chem Biodivers. 2023;20(3):e202200890.10.1002/cbdv.202200890Search in Google Scholar PubMed
[38] Nisreen MA, Abdulazim SS, Hameed AA. Effects of Inula viscosa leaf extracts on abortion and implantation in rats. J Ethnopharmacol. 2001;77:117–21. 10.1016/S0378-8741(01)00261-6.Search in Google Scholar
[39] Jeddi M, Benziane OZ, Shita R, Fikri-Benbrahim K. Focus on the ethnobotany of north Moroccan sage, false yellowhead, and carrot: insights into their pharmacological potential. J Herbmed Pharmacol. 2023;12(4):536548. 10.34172/jhp.2023.46047.Search in Google Scholar
[40] Seca AM, Grigore A, Pinto DC, Silva AM. The genus Inula and their metabolites: From ethnopharmacological to medicinal uses. J Ethnopharmacol. 2014;154:286–310. 10.1016/j.jep.2014.04.010.Search in Google Scholar PubMed
[41] Lyoussi B, Derwich E. Phytochemical characterization, antioxidant activity, and in vitro investigation of antimicrobial potential of Dittrichia viscosa L. leaf extracts against nosocomial infections. Sheng Tai Xue Bao. 2022;42:661–9.10.1016/j.chnaes.2021.09.021Search in Google Scholar
[42] Mssillou I, Agour A, Allali A, Saghrouchni H, Bourhia M, El Moussaoui A, et al. Antioxidant, antimicrobial, and insecticidal properties of a chemically characterized essential oil from the leaves of Dittrichia viscosa L. Molecules. 2022;27:2282. 10.3390/molecules27072282.Search in Google Scholar PubMed PubMed Central
[43] Rhimi W, Salem IB, Iatta R, Chaabane H, Saidi M, Boulila M, et al. Dittrichia viscosa L. leaves lipid extract: An unexploited source of essential fatty acids and tocopherols with antifungal and anti-inflammatory properties. Ind Crop Prod. 2018;113:196–201.10.1016/j.indcrop.2018.01.032Search in Google Scholar
[44] Vuko E, Dunkić V, Maravić A, Ruščić M, Nazlić M, Radan M, et al. Not only a weed plant biological activities of essential oil and hydrosol of Dittrichia viscosa (L.) greuter. Plants. 2021;10:1837. 10.3390/plants10091837.Search in Google Scholar PubMed PubMed Central
[45] Bentarhlia N, Kartah BE, Fadil M, Harkaoui SE, Matthäus B, Abboussi O, et al. Exploring the wound-healing and antimicrobial potential of Dittrichia viscosa L lipidic extract: Chemical composition and in vivo evaluation. Fitoterapia. 2023;172:105707. 10.1016/j.fitote.2023.105707.Search in Google Scholar PubMed
[46] Okka EZ, Tongur T, Aytas T, Yılmaz M, Topel Ö, Sahin R. Green synthesis and the formation kinetics of silver nanoparticles in aqueous Inula viscosa extract. Optik. 2023;294:171–487. 10.1016/j.ijleo.2023.171487.Search in Google Scholar
[47] Bouslamti M, Loukili EH, Elrherabi A, El Moussaoui A, Chebaibi M, Bencheikh N, et al. Phenolic profile, inhibition of α-amylase and α-glucosidase enzymes, and antioxidant properties of Solanum elaeagnifolium Cav. (Solanaceae): In vitro and in silico investigations. Processes. 2023;11:1384.10.3390/pr11051384Search in Google Scholar
[48] Fernine Y, Salim R, Arrousse N, Haldhar R, El Hajjaji F, Kim S, et al. Anti-corrosion performance of Ocimum basilicum seed extract as environmentally friendly inhibitors for mild steel in HCl solution: Evaluations of electrochemical, EDX, DFT and Monte Carlo. JMolLiq. 2022;355:118–867. 10.1016/j.molliq.2022.118867.Search in Google Scholar
[49] Eroglu E, Girgin SN. A unique phenolic extraction method from olive oil macerate of Hypericum perforatum using DMSO: Assessment of in vitro anticancer activity, LC-MS/MS profile, total phenolic content and antioxidant capacity. SAfrJBot. 2021;139:6–11. 10.1016/j.sajb.2021.01.015.Search in Google Scholar
[50] Luis JCR, López G, Durán-Aranguren D, Isabela Q, Chiara C, Rocío C. Compressed fluids and Soxhlet extraction for the valorization of compounds from Colombian cashew (Anacardium occidentale) nut shells aimed at a cosmetic application. J Supercrit Fluids. 2023;192:105808. 10.1016/j.supflu.2022.105808.Search in Google Scholar
[51] Loukili EH, Bouchal B, Bouhrim M, Abrigach F, Genva M, Kahina Z, et al. Chemical composition, antibacterial, antifungal and antidiabetic activities of ethanolic extracts of Opuntia dillenii fruits collected from Morocco. J Food Qual. 2022;947–1239.10.1155/2022/9471239Search in Google Scholar
[52] Azzouni D, Haldhar R, Salim R, Ech-chihbi E, Mrani SA, Rais Z, et al. Adsorption treatment residues as novel ecological corrosion inhibitors applied to mild steel in a molar hydrochloric acid medium: Experimental studies and surface characterization. Mater Today Commun. 2023;35:106181. 10.1016/j.mtcomm.2023.106181.Search in Google Scholar
[53] Ouahabi S, Loukili EH, Daoudi NE, Chebaibi M, Ramdani M, Rahhou I, et al. Study of the phytochemical composition, antioxidant properties, and in vitro anti-diabetic efficacy of gracilaria bursa-pastoris extracts. Mar Drugs. 2023;21:372.10.3390/md21070372Search in Google Scholar PubMed PubMed Central
[54] Mahraz AM, Elhachmia C, Zakia R, Taleb M. Medicinal plants of Moulay Yaâcoub Province in Morocco: An ethnobotanical and biodiversity survey. Trop J Nat Prod. 2023;7(8):3590–3601. 10.26538/tjnpr/v7i8.3. https://tjnpr.org/index.php/home/article/view/2396.Search in Google Scholar
[55] Şenol ZM, Messaoudi NE, Fernine Y, Keskin ZS. Bioremoval of rhodamine B dye from aqueous solution by using agricultural solid waste (almond shell): experimental and DFT modeling studies. Biomass Conv Bioref. 2023;1:350–60. 10.1007/s13399-023-03781-1.Search in Google Scholar
[56] ELHajjaji F, Salim R, Ech-chihbi E, Titi A, Messali M, Kaya S, et al. New imidazolium ionic liquids as ecofriendly corrosion inhibitors for mild steel in hydrochloric acid (1 M): Experimental and theoretical approach. JTaiwan InstChem. 2021;123:346–62. 10.1016/j.jtice.2021.05.005.Search in Google Scholar
[57] Ouass A, Galai M, Ouakki M, Ech-Chihbi E, Kadiri L, Hsissou R, et al. Poly (sodium acrylate) and Poly (acrylic acidsodium) as an eco-friendly corrosion inhibitor of mild steel in normal hydrochloric acid: Experimental, spectroscopic and theoretical approach. J Appl Electrochem. 2021;51(7):1009–32. 10.100710800-021-01556-y.Search in Google Scholar
[58] Ozkan E, Karakas FP, Yildirim AB, Tas I, Eker I, Yavuz MZ, et al. Promising medicinal plant Inula viscosa L.: Antiproliferative, antioxidant, antibacterial and phenolic profiles. Prog Nutr. 2019;21(3):652–61.Search in Google Scholar
[59] Asraoui F, Kounnoun A, Cacciola F, El Mansouri F, Kabach I, Oulad El Majdoub Y, et al. Phytochemical profile, antioxidant capacity, α-amylase and α-glucosidase inhibitory potential of wild Moroccan Inula viscosa (L.) aiton leaves. Molecules. 2021;26(11):3134.10.3390/molecules26113134Search in Google Scholar PubMed PubMed Central
[60] Tepe HD, Uğurlu A, Yazgan İ. Determination of phenolic compounds, organic volatile molecules and anti-cancer properties in Inula viscosa L., Viscum Album L. and Raphanus Sativus L. Sakarya Univ J Sci. 2021;25(3):647–62.10.16984/saufenbilder.742432Search in Google Scholar
[61] Hajjaji FE, Ech-Chihbi E, Salim R, Titi A, Messali M, El Ibrahimi B, et al. A detailed electronic-scale DFT modeling/MD simulation, electrochemical and surface morphological explorations of imidazolium-based ionic liquids as sustainable and non-toxic corrosion inhibitors for mild steel in 1 M HCl. Mater Sci Engineering: B. 2023;289:116232.10.1016/j.mseb.2022.116232Search in Google Scholar
[62] Al Jahdaly BA, Maghraby RY, Ibrahim AH, Shouier KR, Alturki AM, El-Shabasy RM. Role of green chemistry in sustainable corrosion inhibition: a review on recent developments. Mater Today Sustainability. 2022;20:100242. 10.1016/j.mtsust.2022.100242.Search in Google Scholar
[63] Hbika A, Bouyanzer A, Jalal M, Setti N, Loukili E, Aouniti A, et al. The inhibiting effect of aqueous extracts of Artemisia Absinthium L. (Wormwood) on the corrosion of mild steel in HCl 1 M. Anal Bioanal Electrochem. 2023;15(1):17–35.Search in Google Scholar
[64] Ech-chihbi E, Salim R, Ouakki M, Koudad M, Guo L, Azam M, et al. Corrosion resistance assessment of copper, mild steel, and aluminum alloy 2024-T3 in acidic solution by a novel imidazothiazole derivative. Mater Today Sustainability. 2023;24:100524.10.1016/j.mtsust.2023.100524Search in Google Scholar
[65] Chen G, Liu Y, Fan B, Liu Z, Zhao J, Fu Z. Accurate clarification on assembling and protective mechanisms of β-cyclodextrin/benzothiazole complex for copper in marine environment. J Mol Liq. 2024;394:123764.10.1016/j.molliq.2023.123764Search in Google Scholar
[66] Kumar D, Vinay J, Beena R. Unravelling the mechanisms of corrosion inhibition of iron by henna extract: A density functional theory study. Corros Sci. 2018;142:102–9.10.1016/j.corsci.2018.07.011Search in Google Scholar
[67] Salim R, Adardour M, Ettahiri W, Ech-chihbi E, Hammouti B, Azam M, et al. Computational and electrochemistry of effective triazolyl-benzimidazolone inhibitors in aggressive environment. Sustain Mater Technol. 2024;39:e00862.10.1016/j.susmat.2024.e00862Search in Google Scholar
[68] Lu W, Ronald LE, Bernhard W. Corrosion protection of mild steel by coatings containing polyaniline. Synth Met. 1995;71(1–3):2163–6.10.1016/0379-6779(94)03204-JSearch in Google Scholar
[69] Bentiss F, Traisnel M, Lagrenée M. Inhibitor effects of triazole derivatives on corrosion of mild steel in acidic media. Br Corros J. 2000;35:315–20. https://api.semanticscholar.org/CorpusID:95370242.10.1179/000705900101501326Search in Google Scholar
[70] El Kacimi Y, Touir R, Alaoui K, Touhami ME. Recent progress and comprehension of corrosion of steels in the hot-dip galvanizing industry. Corros Sci. 2023;81–106.10.1201/9781003328513-3Search in Google Scholar
[71] Sriti Eljazi J, Selmi S, Zarroug Y, Wesleti I, Aouini B, Jallouli S, et al. Essential oil composition, phenolic compound, and antioxidant potential of Inula viscosa as affected by extraction process. Int J Food Prop. 2018;21(1):2309–19.10.1080/10942912.2018.1517782Search in Google Scholar
[72] Murulana LC, Kabanda M, Ebenso E. Experimental and theoretical studies on the inhibition of mild steel corrosion by certain sulfonamides in aqueous HCl. RSC Adv. 2015;5(36):28743–61.10.1039/C4RA11414KSearch in Google Scholar
[73] Nahlé A, Salim R, Hajjaji FE, Ech-Chihbi E, Titi A, Messali M, et al. Experimental and theoretical approach for novel imidazolium ionic liquids as Smart Corrosion inhibitors for mild steel in 1.0 M hydrochloric acid. Arab J Chem. 2022;15(8):103967.10.1016/j.arabjc.2022.103967Search in Google Scholar
[74] Arrousse N, Fernine Y, Al-Zaqri N, Boshaala A, Ech-Chihbi E, Salim R, et al. Thiophene derivatives as corrosion inhibitors for 2024-T3 aluminum alloy in hydrochloric acid medium. RSC Adv. 2022;12(17):10321–35. 10.1039/d2ra00185c.Search in Google Scholar PubMed PubMed Central
[75] Gerengi H, Mielniczek M, Gece G, Solomon MM. Experimental and quantum chemical evaluation of 8-hydroxyquinoline as a corrosion inhibitor for copper in 0.1 M HCl. Ind Eng Chem Res. 2016;55:9614–24.10.1021/acs.iecr.6b02414Search in Google Scholar
[76] Arrousse N, Salim R, Bousraf. FZ, Ech-chihbi E, Hammouti B, Abdellaoui A, et al. Experimental and theoretical study of xanthene derivatives as corrosion inhibitor for mild steel in hydrochloric acid solution. J Appl Electrochem. 2022;52:1275–94. 10.1007/s10800-022-01705-x.Search in Google Scholar
[77] Aslam J, Mobin M, Huda, Aslam A, Aslam R. Performance d’inhibition de la corrosion des multi-phytoconstituants de l’extrait d'écorce d’eucalyptus sur la corrosion de l’acier doux dans une solution à 5 % de HCl. Int J Env Sci Technologie. 2023;20:2441–54. 10.1007/s13762-022-04152-5.Search in Google Scholar
[78] Vaszilcsin CG, Putz MV, Kellenberger A, Dan ML. On the evaluation of metal-corrosion inhibitor interactions by adsorption isotherms. J Mol Struct. 2023;1286:135–643. 10.1016/j.molstruc.2023.135643.Search in Google Scholar
[79] Alaoui Mrani S, Ech-chihbi E, Arrousse N, Rais Z, El Hajjaji F, El Abiad C, et al. DFT and electrochemical investigations on the corrosion inhibition of mild steel by novel schiff’s base derivatives in 1 M HCl solution. Arab J Sci Eng. 2021;46:5691–707. 10.1007/s13369-020-05229-4.Search in Google Scholar
[80] Gori A, Boucherle B, Rey A, Rome M, Fuzzati N, Peuchmaur M. Development of an innovative maceration technique to optimize extraction and phase partition of natural products. Fitoterapia. 2021;148:104798. 10.1016/j.fitote.2020.104798.Search in Google Scholar PubMed
[81] Logiciel MarvinSketch, version: 18.22, ChemAxon Ltd.; 2018.Search in Google Scholar
[82] Arrousse N, Salim R, Benhiba F, Mabrouk EH, Abdelaoui A, El Hajjaji F, et al. Insight into the corrosion inhibition property of two new soluble and non-toxic xanthenbenzoate derivatives. J Mol Liq. 2021;338:116–610.10.1016/j.molliq.2021.116610Search in Google Scholar
[83] Rehman A, Nazir G, Heo K, Hussain S, Ikram M, Akhter Z, et al. A focused review on lignocellulosic biomass-derived porous carbons for effective pharmaceuticals removal: Current trends, challenges and future prospects. Sep Purif Technol. 2024;330:125356. 10.1016/j.seppur.2023.125356.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Constitutive and evoked release of ATP in adult mouse olfactory epithelium
- LARP1 knockdown inhibits cultured gastric carcinoma cell cycle progression and metastatic behavior
- PEGylated porcine–human recombinant uricase: A novel fusion protein with improved efficacy and safety for the treatment of hyperuricemia and renal complications
- Research progress on ocular complications caused by type 2 diabetes mellitus and the function of tears and blepharons
- The role and mechanism of esketamine in preventing and treating remifentanil-induced hyperalgesia based on the NMDA receptor–CaMKII pathway
- Brucella infection combined with Nocardia infection: A case report and literature review
- Detection of serum interleukin-18 level and neutrophil/lymphocyte ratio in patients with antineutrophil cytoplasmic antibody-associated vasculitis and its clinical significance
- Ang-1, Ang-2, and Tie2 are diagnostic biomarkers for Henoch-Schönlein purpura and pediatric-onset systemic lupus erythematous
- PTTG1 induces pancreatic cancer cell proliferation and promotes aerobic glycolysis by regulating c-myc
- Role of serum B-cell-activating factor and interleukin-17 as biomarkers in the classification of interstitial pneumonia with autoimmune features
- Effectiveness and safety of a mumps containing vaccine in preventing laboratory-confirmed mumps cases from 2002 to 2017: A meta-analysis
- Low levels of sex hormone-binding globulin predict an increased breast cancer risk and its underlying molecular mechanisms
- A case of Trousseau syndrome: Screening, detection and complication
- Application of the integrated airway humidification device enhances the humidification effect of the rabbit tracheotomy model
- Preparation of Cu2+/TA/HAP composite coating with anti-bacterial and osteogenic potential on 3D-printed porous Ti alloy scaffolds for orthopedic applications
- Aquaporin-8 promotes human dermal fibroblasts to counteract hydrogen peroxide-induced oxidative damage: A novel target for management of skin aging
- Current research and evidence gaps on placental development in iron deficiency anemia
- Single-nucleotide polymorphism rs2910829 in PDE4D is related to stroke susceptibility in Chinese populations: The results of a meta-analysis
- Pheochromocytoma-induced myocardial infarction: A case report
- Kaempferol regulates apoptosis and migration of neural stem cells to attenuate cerebral infarction by O‐GlcNAcylation of β-catenin
- Sirtuin 5 regulates acute myeloid leukemia cell viability and apoptosis by succinylation modification of glycine decarboxylase
- Apigenin 7-glucoside impedes hypoxia-induced malignant phenotypes of cervical cancer cells in a p16-dependent manner
- KAT2A changes the function of endometrial stromal cells via regulating the succinylation of ENO1
- Current state of research on copper complexes in the treatment of breast cancer
- Exploring antioxidant strategies in the pathogenesis of ALS
- Helicobacter pylori causes gastric dysbacteriosis in chronic gastritis patients
- IL-33/soluble ST2 axis is associated with radiation-induced cardiac injury
- The predictive value of serum NLR, SII, and OPNI for lymph node metastasis in breast cancer patients with internal mammary lymph nodes after thoracoscopic surgery
- Carrying SNP rs17506395 (T > G) in TP63 gene and CCR5Δ32 mutation associated with the occurrence of breast cancer in Burkina Faso
- P2X7 receptor: A receptor closely linked with sepsis-associated encephalopathy
- Probiotics for inflammatory bowel disease: Is there sufficient evidence?
- Identification of KDM4C as a gene conferring drug resistance in multiple myeloma
- Microbial perspective on the skin–gut axis and atopic dermatitis
- Thymosin α1 combined with XELOX improves immune function and reduces serum tumor markers in colorectal cancer patients after radical surgery
- Highly specific vaginal microbiome signature for gynecological cancers
- Sample size estimation for AQP4-IgG seropositive optic neuritis: Retinal damage detection by optical coherence tomography
- The effects of SDF-1 combined application with VEGF on femoral distraction osteogenesis in rats
- Fabrication and characterization of gold nanoparticles using alginate: In vitro and in vivo assessment of its administration effects with swimming exercise on diabetic rats
- Mitigating digestive disorders: Action mechanisms of Mediterranean herbal active compounds
- Distribution of CYP2D6 and CYP2C19 gene polymorphisms in Han and Uygur populations with breast cancer in Xinjiang, China
- VSP-2 attenuates secretion of inflammatory cytokines induced by LPS in BV2 cells by mediating the PPARγ/NF-κB signaling pathway
- Factors influencing spontaneous hypothermia after emergency trauma and the construction of a predictive model
- Long-term administration of morphine specifically alters the level of protein expression in different brain regions and affects the redox state
- Application of metagenomic next-generation sequencing technology in the etiological diagnosis of peritoneal dialysis-associated peritonitis
- Clinical diagnosis, prevention, and treatment of neurodyspepsia syndrome using intelligent medicine
- Case report: Successful bronchoscopic interventional treatment of endobronchial leiomyomas
- Preliminary investigation into the genetic etiology of short stature in children through whole exon sequencing of the core family
- Cystic adenomyoma of the uterus: Case report and literature review
- Mesoporous silica nanoparticles as a drug delivery mechanism
- Dynamic changes in autophagy activity in different degrees of pulmonary fibrosis in mice
- Vitamin D deficiency and inflammatory markers in type 2 diabetes: Big data insights
- Lactate-induced IGF1R protein lactylation promotes proliferation and metabolic reprogramming of lung cancer cells
- Meta-analysis on the efficacy of allogeneic hematopoietic stem cell transplantation to treat malignant lymphoma
- Mitochondrial DNA drives neuroinflammation through the cGAS-IFN signaling pathway in the spinal cord of neuropathic pain mice
- Application value of artificial intelligence algorithm-based magnetic resonance multi-sequence imaging in staging diagnosis of cervical cancer
- Embedded monitoring system and teaching of artificial intelligence online drug component recognition
- Investigation into the association of FNDC1 and ADAMTS12 gene expression with plumage coloration in Muscovy ducks
- Yak meat content in feed and its impact on the growth of rats
- A rare case of Richter transformation with breast involvement: A case report and literature review
- First report of Nocardia wallacei infection in an immunocompetent patient in Zhejiang province
- Rhodococcus equi and Brucella pulmonary mass in immunocompetent: A case report and literature review
- Downregulation of RIP3 ameliorates the left ventricular mechanics and function after myocardial infarction via modulating NF-κB/NLRP3 pathway
- Evaluation of the role of some non-enzymatic antioxidants among Iraqi patients with non-alcoholic fatty liver disease
- The role of Phafin proteins in cell signaling pathways and diseases
- Ten-year anemia as initial manifestation of Castleman disease in the abdominal cavity: A case report
- Coexistence of hereditary spherocytosis with SPTB P.Trp1150 gene variant and Gilbert syndrome: A case report and literature review
- Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells
- Exploratory evaluation supported by experimental and modeling approaches of Inula viscosa root extract as a potent corrosion inhibitor for mild steel in a 1 M HCl solution
- Imaging manifestations of ductal adenoma of the breast: A case report
- Gut microbiota and sleep: Interaction mechanisms and therapeutic prospects
- Isomangiferin promotes the migration and osteogenic differentiation of rat bone marrow mesenchymal stem cells
- Prognostic value and microenvironmental crosstalk of exosome-related signatures in human epidermal growth factor receptor 2 positive breast cancer
- Circular RNAs as potential biomarkers for male severe sepsis
- Knockdown of Stanniocalcin-1 inhibits growth and glycolysis in oral squamous cell carcinoma cells
- The expression and biological role of complement C1s in esophageal squamous cell carcinoma
- A novel GNAS mutation in pseudohypoparathyroidism type 1a with articular flexion deformity: A case report
- Predictive value of serum magnesium levels for prognosis in patients with non-small cell lung cancer undergoing EGFR-TKI therapy
- HSPB1 alleviates acute-on-chronic liver failure via the P53/Bax pathway
- IgG4-related disease complicated by PLA2R-associated membranous nephropathy: A case report
- Baculovirus-mediated endostatin and angiostatin activation of autophagy through the AMPK/AKT/mTOR pathway inhibits angiogenesis in hepatocellular carcinoma
- Metformin mitigates osteoarthritis progression by modulating the PI3K/AKT/mTOR signaling pathway and enhancing chondrocyte autophagy
- Evaluation of the activity of antimicrobial peptides against bacterial vaginosis
- Atypical presentation of γ/δ mycosis fungoides with an unusual phenotype and SOCS1 mutation
- Analysis of the microecological mechanism of diabetic kidney disease based on the theory of “gut–kidney axis”: A systematic review
- Omega-3 fatty acids prevent gestational diabetes mellitus via modulation of lipid metabolism
- Refractory hypertension complicated with Turner syndrome: A case report
- Interaction of ncRNAs and the PI3K/AKT/mTOR pathway: Implications for osteosarcoma
- Association of low attenuation area scores with pulmonary function and clinical prognosis in patients with chronic obstructive pulmonary disease
- Long non-coding RNAs in bone formation: Key regulators and therapeutic prospects
- The deubiquitinating enzyme USP35 regulates the stability of NRF2 protein
- Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as potential diagnostic markers for rebleeding in patients with esophagogastric variceal bleeding
- G protein-coupled receptor 1 participating in the mechanism of mediating gestational diabetes mellitus by phosphorylating the AKT pathway
- LL37-mtDNA regulates viability, apoptosis, inflammation, and autophagy in lipopolysaccharide-treated RLE-6TN cells by targeting Hsp90aa1
- The analgesic effect of paeoniflorin: A focused review
- Chemical composition’s effect on Solanum nigrum Linn.’s antioxidant capacity and erythrocyte protection: Bioactive components and molecular docking analysis
- Knockdown of HCK promotes HREC cell viability and inner blood–retinal barrier integrity by regulating the AMPK signaling pathway
- The role of rapamycin in the PINK1/Parkin signaling pathway in mitophagy in podocytes
- Laryngeal non-Hodgkin lymphoma: Report of four cases and review of the literature
- Clinical value of macrogenome next-generation sequencing on infections
- Overview of dendritic cells and related pathways in autoimmune uveitis
- TAK-242 alleviates diabetic cardiomyopathy via inhibiting pyroptosis and TLR4/CaMKII/NLRP3 pathway
- Hypomethylation in promoters of PGC-1α involved in exercise-driven skeletal muscular alterations in old age
- Profile and antimicrobial susceptibility patterns of bacteria isolated from effluents of Kolladiba and Debark hospitals
- The expression and clinical significance of syncytin-1 in serum exosomes of hepatocellular carcinoma patients
- A histomorphometric study to evaluate the therapeutic effects of biosynthesized silver nanoparticles on the kidneys infected with Plasmodium chabaudi
- PGRMC1 and PAQR4 are promising molecular targets for a rare subtype of ovarian cancer
- Analysis of MDA, SOD, TAOC, MNCV, SNCV, and TSS scores in patients with diabetes peripheral neuropathy
- SLIT3 deficiency promotes non-small cell lung cancer progression by modulating UBE2C/WNT signaling
- The relationship between TMCO1 and CALR in the pathological characteristics of prostate cancer and its effect on the metastasis of prostate cancer cells
- Heterogeneous nuclear ribonucleoprotein K is a potential target for enhancing the chemosensitivity of nasopharyngeal carcinoma
- PHB2 alleviates retinal pigment epithelium cell fibrosis by suppressing the AGE–RAGE pathway
- Anti-γ-aminobutyric acid-B receptor autoimmune encephalitis with syncope as the initial symptom: Case report and literature review
- Comparative analysis of chloroplast genome of Lonicera japonica cv. Damaohua
- Human umbilical cord mesenchymal stem cells regulate glutathione metabolism depending on the ERK–Nrf2–HO-1 signal pathway to repair phosphoramide mustard-induced ovarian cancer cells
- Electroacupuncture on GB acupoints improves osteoporosis via the estradiol–PI3K–Akt signaling pathway
- Renalase protects against podocyte injury by inhibiting oxidative stress and apoptosis in diabetic nephropathy
- Review: Dicranostigma leptopodum: A peculiar plant of Papaveraceae
- Combination effect of flavonoids attenuates lung cancer cell proliferation by inhibiting the STAT3 and FAK signaling pathway
- Renal microangiopathy and immune complex glomerulonephritis induced by anti-tumour agents: A case report
- Correlation analysis of AVPR1a and AVPR2 with abnormal water and sodium and potassium metabolism in rats
- Gastrointestinal health anti-diarrheal mixture relieves spleen deficiency-induced diarrhea through regulating gut microbiota
- Myriad factors and pathways influencing tumor radiotherapy resistance
- Exploring the effects of culture conditions on Yapsin (YPS) gene expression in Nakaseomyces glabratus
- Screening of prognostic core genes based on cell–cell interaction in the peripheral blood of patients with sepsis
- Coagulation factor II thrombin receptor as a promising biomarker in breast cancer management
- Ileocecal mucinous carcinoma misdiagnosed as incarcerated hernia: A case report
- Methyltransferase like 13 promotes malignant behaviors of bladder cancer cells through targeting PI3K/ATK signaling pathway
- The debate between electricity and heat, efficacy and safety of irreversible electroporation and radiofrequency ablation in the treatment of liver cancer: A meta-analysis
- ZAG promotes colorectal cancer cell proliferation and epithelial–mesenchymal transition by promoting lipid synthesis
- Baicalein inhibits NLRP3 inflammasome activation and mitigates placental inflammation and oxidative stress in gestational diabetes mellitus
- Impact of SWCNT-conjugated senna leaf extract on breast cancer cells: A potential apoptotic therapeutic strategy
- MFAP5 inhibits the malignant progression of endometrial cancer cells in vitro
- Major ozonated autohemotherapy promoted functional recovery following spinal cord injury in adult rats via the inhibition of oxidative stress and inflammation
- Axodendritic targeting of TAU and MAP2 and microtubule polarization in iPSC-derived versus SH-SY5Y-derived human neurons
- Differential expression of phosphoinositide 3-kinase/protein kinase B and Toll-like receptor/nuclear factor kappa B signaling pathways in experimental obesity Wistar rat model
- The therapeutic potential of targeting Oncostatin M and the interleukin-6 family in retinal diseases: A comprehensive review
- BA inhibits LPS-stimulated inflammatory response and apoptosis in human middle ear epithelial cells by regulating the Nf-Kb/Iκbα axis
- Role of circRMRP and circRPL27 in chronic obstructive pulmonary disease
- Investigating the role of hyperexpressed HCN1 in inducing myocardial infarction through activation of the NF-κB signaling pathway
- Characterization of phenolic compounds and evaluation of anti-diabetic potential in Cannabis sativa L. seeds: In vivo, in vitro, and in silico studies
- Quantitative immunohistochemistry analysis of breast Ki67 based on artificial intelligence
- Ecology and Environmental Science
- Screening of different growth conditions of Bacillus subtilis isolated from membrane-less microbial fuel cell toward antimicrobial activity profiling
- Degradation of a mixture of 13 polycyclic aromatic hydrocarbons by commercial effective microorganisms
- Evaluation of the impact of two citrus plants on the variation of Panonychus citri (Acari: Tetranychidae) and beneficial phytoseiid mites
- Prediction of present and future distribution areas of Juniperus drupacea Labill and determination of ethnobotany properties in Antalya Province, Türkiye
- Population genetics of Todarodes pacificus (Cephalopoda: Ommastrephidae) in the northwest Pacific Ocean via GBS sequencing
- A comparative analysis of dendrometric, macromorphological, and micromorphological characteristics of Pistacia atlantica subsp. atlantica and Pistacia terebinthus in the middle Atlas region of Morocco
- Macrofungal sporocarp community in the lichen Scots pine forests
- Assessing the proximate compositions of indigenous forage species in Yemen’s pastoral rangelands
- Food Science
- Gut microbiota changes associated with low-carbohydrate diet intervention for obesity
- Reexamination of Aspergillus cristatus phylogeny in dark tea: Characteristics of the mitochondrial genome
- Differences in the flavonoid composition of the leaves, fruits, and branches of mulberry are distinguished based on a plant metabolomics approach
- Investigating the impact of wet rendering (solventless method) on PUFA-rich oil from catfish (Clarias magur) viscera
- Non-linear associations between cardiovascular metabolic indices and metabolic-associated fatty liver disease: A cross-sectional study in the US population (2017–2020)
- Knockdown of USP7 alleviates atherosclerosis in ApoE-deficient mice by regulating EZH2 expression
- Utility of dairy microbiome as a tool for authentication and traceability
- Agriculture
- Enhancing faba bean (Vicia faba L.) productivity through establishing the area-specific fertilizer rate recommendation in southwest Ethiopia
- Impact of novel herbicide based on synthetic auxins and ALS inhibitor on weed control
- Perspectives of pteridophytes microbiome for bioremediation in agricultural applications
- Fertilizer application parameters for drip-irrigated peanut based on the fertilizer effect function established from a “3414” field trial
- Improving the productivity and profitability of maize (Zea mays L.) using optimum blended inorganic fertilization
- Application of leaf multispectral analyzer in comparison to hyperspectral device to assess the diversity of spectral reflectance indices in wheat genotypes
- Animal Sciences
- Knockdown of ANP32E inhibits colorectal cancer cell growth and glycolysis by regulating the AKT/mTOR pathway
- Development of a detection chip for major pathogenic drug-resistant genes and drug targets in bovine respiratory system diseases
- Exploration of the genetic influence of MYOT and MB genes on the plumage coloration of Muscovy ducks
- Transcriptome analysis of adipose tissue in grazing cattle: Identifying key regulators of fat metabolism
- Comparison of nutritional value of the wild and cultivated spiny loaches at three growth stages
- Transcriptomic analysis of liver immune response in Chinese spiny frog (Quasipaa spinosa) infected with Proteus mirabilis
- Disruption of BCAA degradation is a critical characteristic of diabetic cardiomyopathy revealed by integrated transcriptome and metabolome analysis
- Plant Sciences
- Effect of long-term in-row branch covering on soil microorganisms in pear orchards
- Photosynthetic physiological characteristics, growth performance, and element concentrations reveal the calcicole–calcifuge behaviors of three Camellia species
- Transcriptome analysis reveals the mechanism of NaHCO3 promoting tobacco leaf maturation
- Bioinformatics, expression analysis, and functional verification of allene oxide synthase gene HvnAOS1 and HvnAOS2 in qingke
- Water, nitrogen, and phosphorus coupling improves gray jujube fruit quality and yield
- Improving grape fruit quality through soil conditioner: Insights from RNA-seq analysis of Cabernet Sauvignon roots
- Role of Embinin in the reabsorption of nucleus pulposus in lumbar disc herniation: Promotion of nucleus pulposus neovascularization and apoptosis of nucleus pulposus cells
- Revealing the effects of amino acid, organic acid, and phytohormones on the germination of tomato seeds under salinity stress
- Combined effects of nitrogen fertilizer and biochar on the growth, yield, and quality of pepper
- Comprehensive phytochemical and toxicological analysis of Chenopodium ambrosioides (L.) fractions
- Impact of “3414” fertilization on the yield and quality of greenhouse tomatoes
- Exploring the coupling mode of water and fertilizer for improving growth, fruit quality, and yield of the pear in the arid region
- Metagenomic analysis of endophytic bacteria in seed potato (Solanum tuberosum)
- Antibacterial, antifungal, and phytochemical properties of Salsola kali ethanolic extract
- Exploring the hepatoprotective properties of citronellol: In vitro and in silico studies on ethanol-induced damage in HepG2 cells
- Enhanced osmotic dehydration of watermelon rind using honey–sucrose solutions: A study on pre-treatment efficacy and mass transfer kinetics
- Effects of exogenous 2,4-epibrassinolide on photosynthetic traits of 53 cowpea varieties under NaCl stress
- Comparative transcriptome analysis of maize (Zea mays L.) seedlings in response to copper stress
- An optimization method for measuring the stomata in cassava (Manihot esculenta Crantz) under multiple abiotic stresses
- Fosinopril inhibits Ang II-induced VSMC proliferation, phenotype transformation, migration, and oxidative stress through the TGF-β1/Smad signaling pathway
- Antioxidant and antimicrobial activities of Salsola imbricata methanolic extract and its phytochemical characterization
- Bioengineering and Biotechnology
- Absorbable calcium and phosphorus bioactive membranes promote bone marrow mesenchymal stem cells osteogenic differentiation for bone regeneration
- New advances in protein engineering for industrial applications: Key takeaways
- An overview of the production and use of Bacillus thuringiensis toxin
- Research progress of nanoparticles in diagnosis and treatment of hepatocellular carcinoma
- Bioelectrochemical biosensors for water quality assessment and wastewater monitoring
- PEI/MMNs@LNA-542 nanoparticles alleviate ICU-acquired weakness through targeted autophagy inhibition and mitochondrial protection
- Unleashing of cytotoxic effects of thymoquinone-bovine serum albumin nanoparticles on A549 lung cancer cells
- Erratum
- Erratum to “Investigating the association between dietary patterns and glycemic control among children and adolescents with T1DM”
- Erratum to “Activation of hypermethylated P2RY1 mitigates gastric cancer by promoting apoptosis and inhibiting proliferation”
- Retraction
- Retraction to “MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB”
- Retraction to “A data mining technique for detecting malignant mesothelioma cancer using multiple regression analysis”
- Special Issue on Advances in Neurodegenerative Disease Research and Treatment
- Transplantation of human neural stem cell prevents symptomatic motor behavior disability in a rat model of Parkinson’s disease
- Special Issue on Multi-omics
- Inflammasome complex genes with clinical relevance suggest potential as therapeutic targets for anti-tumor drugs in clear cell renal cell carcinoma
- Gastroesophageal varices in primary biliary cholangitis with anti-centromere antibody positivity: Early onset?
Articles in the same Issue
- Biomedical Sciences
- Constitutive and evoked release of ATP in adult mouse olfactory epithelium
- LARP1 knockdown inhibits cultured gastric carcinoma cell cycle progression and metastatic behavior
- PEGylated porcine–human recombinant uricase: A novel fusion protein with improved efficacy and safety for the treatment of hyperuricemia and renal complications
- Research progress on ocular complications caused by type 2 diabetes mellitus and the function of tears and blepharons
- The role and mechanism of esketamine in preventing and treating remifentanil-induced hyperalgesia based on the NMDA receptor–CaMKII pathway
- Brucella infection combined with Nocardia infection: A case report and literature review
- Detection of serum interleukin-18 level and neutrophil/lymphocyte ratio in patients with antineutrophil cytoplasmic antibody-associated vasculitis and its clinical significance
- Ang-1, Ang-2, and Tie2 are diagnostic biomarkers for Henoch-Schönlein purpura and pediatric-onset systemic lupus erythematous
- PTTG1 induces pancreatic cancer cell proliferation and promotes aerobic glycolysis by regulating c-myc
- Role of serum B-cell-activating factor and interleukin-17 as biomarkers in the classification of interstitial pneumonia with autoimmune features
- Effectiveness and safety of a mumps containing vaccine in preventing laboratory-confirmed mumps cases from 2002 to 2017: A meta-analysis
- Low levels of sex hormone-binding globulin predict an increased breast cancer risk and its underlying molecular mechanisms
- A case of Trousseau syndrome: Screening, detection and complication
- Application of the integrated airway humidification device enhances the humidification effect of the rabbit tracheotomy model
- Preparation of Cu2+/TA/HAP composite coating with anti-bacterial and osteogenic potential on 3D-printed porous Ti alloy scaffolds for orthopedic applications
- Aquaporin-8 promotes human dermal fibroblasts to counteract hydrogen peroxide-induced oxidative damage: A novel target for management of skin aging
- Current research and evidence gaps on placental development in iron deficiency anemia
- Single-nucleotide polymorphism rs2910829 in PDE4D is related to stroke susceptibility in Chinese populations: The results of a meta-analysis
- Pheochromocytoma-induced myocardial infarction: A case report
- Kaempferol regulates apoptosis and migration of neural stem cells to attenuate cerebral infarction by O‐GlcNAcylation of β-catenin
- Sirtuin 5 regulates acute myeloid leukemia cell viability and apoptosis by succinylation modification of glycine decarboxylase
- Apigenin 7-glucoside impedes hypoxia-induced malignant phenotypes of cervical cancer cells in a p16-dependent manner
- KAT2A changes the function of endometrial stromal cells via regulating the succinylation of ENO1
- Current state of research on copper complexes in the treatment of breast cancer
- Exploring antioxidant strategies in the pathogenesis of ALS
- Helicobacter pylori causes gastric dysbacteriosis in chronic gastritis patients
- IL-33/soluble ST2 axis is associated with radiation-induced cardiac injury
- The predictive value of serum NLR, SII, and OPNI for lymph node metastasis in breast cancer patients with internal mammary lymph nodes after thoracoscopic surgery
- Carrying SNP rs17506395 (T > G) in TP63 gene and CCR5Δ32 mutation associated with the occurrence of breast cancer in Burkina Faso
- P2X7 receptor: A receptor closely linked with sepsis-associated encephalopathy
- Probiotics for inflammatory bowel disease: Is there sufficient evidence?
- Identification of KDM4C as a gene conferring drug resistance in multiple myeloma
- Microbial perspective on the skin–gut axis and atopic dermatitis
- Thymosin α1 combined with XELOX improves immune function and reduces serum tumor markers in colorectal cancer patients after radical surgery
- Highly specific vaginal microbiome signature for gynecological cancers
- Sample size estimation for AQP4-IgG seropositive optic neuritis: Retinal damage detection by optical coherence tomography
- The effects of SDF-1 combined application with VEGF on femoral distraction osteogenesis in rats
- Fabrication and characterization of gold nanoparticles using alginate: In vitro and in vivo assessment of its administration effects with swimming exercise on diabetic rats
- Mitigating digestive disorders: Action mechanisms of Mediterranean herbal active compounds
- Distribution of CYP2D6 and CYP2C19 gene polymorphisms in Han and Uygur populations with breast cancer in Xinjiang, China
- VSP-2 attenuates secretion of inflammatory cytokines induced by LPS in BV2 cells by mediating the PPARγ/NF-κB signaling pathway
- Factors influencing spontaneous hypothermia after emergency trauma and the construction of a predictive model
- Long-term administration of morphine specifically alters the level of protein expression in different brain regions and affects the redox state
- Application of metagenomic next-generation sequencing technology in the etiological diagnosis of peritoneal dialysis-associated peritonitis
- Clinical diagnosis, prevention, and treatment of neurodyspepsia syndrome using intelligent medicine
- Case report: Successful bronchoscopic interventional treatment of endobronchial leiomyomas
- Preliminary investigation into the genetic etiology of short stature in children through whole exon sequencing of the core family
- Cystic adenomyoma of the uterus: Case report and literature review
- Mesoporous silica nanoparticles as a drug delivery mechanism
- Dynamic changes in autophagy activity in different degrees of pulmonary fibrosis in mice
- Vitamin D deficiency and inflammatory markers in type 2 diabetes: Big data insights
- Lactate-induced IGF1R protein lactylation promotes proliferation and metabolic reprogramming of lung cancer cells
- Meta-analysis on the efficacy of allogeneic hematopoietic stem cell transplantation to treat malignant lymphoma
- Mitochondrial DNA drives neuroinflammation through the cGAS-IFN signaling pathway in the spinal cord of neuropathic pain mice
- Application value of artificial intelligence algorithm-based magnetic resonance multi-sequence imaging in staging diagnosis of cervical cancer
- Embedded monitoring system and teaching of artificial intelligence online drug component recognition
- Investigation into the association of FNDC1 and ADAMTS12 gene expression with plumage coloration in Muscovy ducks
- Yak meat content in feed and its impact on the growth of rats
- A rare case of Richter transformation with breast involvement: A case report and literature review
- First report of Nocardia wallacei infection in an immunocompetent patient in Zhejiang province
- Rhodococcus equi and Brucella pulmonary mass in immunocompetent: A case report and literature review
- Downregulation of RIP3 ameliorates the left ventricular mechanics and function after myocardial infarction via modulating NF-κB/NLRP3 pathway
- Evaluation of the role of some non-enzymatic antioxidants among Iraqi patients with non-alcoholic fatty liver disease
- The role of Phafin proteins in cell signaling pathways and diseases
- Ten-year anemia as initial manifestation of Castleman disease in the abdominal cavity: A case report
- Coexistence of hereditary spherocytosis with SPTB P.Trp1150 gene variant and Gilbert syndrome: A case report and literature review
- Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells
- Exploratory evaluation supported by experimental and modeling approaches of Inula viscosa root extract as a potent corrosion inhibitor for mild steel in a 1 M HCl solution
- Imaging manifestations of ductal adenoma of the breast: A case report
- Gut microbiota and sleep: Interaction mechanisms and therapeutic prospects
- Isomangiferin promotes the migration and osteogenic differentiation of rat bone marrow mesenchymal stem cells
- Prognostic value and microenvironmental crosstalk of exosome-related signatures in human epidermal growth factor receptor 2 positive breast cancer
- Circular RNAs as potential biomarkers for male severe sepsis
- Knockdown of Stanniocalcin-1 inhibits growth and glycolysis in oral squamous cell carcinoma cells
- The expression and biological role of complement C1s in esophageal squamous cell carcinoma
- A novel GNAS mutation in pseudohypoparathyroidism type 1a with articular flexion deformity: A case report
- Predictive value of serum magnesium levels for prognosis in patients with non-small cell lung cancer undergoing EGFR-TKI therapy
- HSPB1 alleviates acute-on-chronic liver failure via the P53/Bax pathway
- IgG4-related disease complicated by PLA2R-associated membranous nephropathy: A case report
- Baculovirus-mediated endostatin and angiostatin activation of autophagy through the AMPK/AKT/mTOR pathway inhibits angiogenesis in hepatocellular carcinoma
- Metformin mitigates osteoarthritis progression by modulating the PI3K/AKT/mTOR signaling pathway and enhancing chondrocyte autophagy
- Evaluation of the activity of antimicrobial peptides against bacterial vaginosis
- Atypical presentation of γ/δ mycosis fungoides with an unusual phenotype and SOCS1 mutation
- Analysis of the microecological mechanism of diabetic kidney disease based on the theory of “gut–kidney axis”: A systematic review
- Omega-3 fatty acids prevent gestational diabetes mellitus via modulation of lipid metabolism
- Refractory hypertension complicated with Turner syndrome: A case report
- Interaction of ncRNAs and the PI3K/AKT/mTOR pathway: Implications for osteosarcoma
- Association of low attenuation area scores with pulmonary function and clinical prognosis in patients with chronic obstructive pulmonary disease
- Long non-coding RNAs in bone formation: Key regulators and therapeutic prospects
- The deubiquitinating enzyme USP35 regulates the stability of NRF2 protein
- Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as potential diagnostic markers for rebleeding in patients with esophagogastric variceal bleeding
- G protein-coupled receptor 1 participating in the mechanism of mediating gestational diabetes mellitus by phosphorylating the AKT pathway
- LL37-mtDNA regulates viability, apoptosis, inflammation, and autophagy in lipopolysaccharide-treated RLE-6TN cells by targeting Hsp90aa1
- The analgesic effect of paeoniflorin: A focused review
- Chemical composition’s effect on Solanum nigrum Linn.’s antioxidant capacity and erythrocyte protection: Bioactive components and molecular docking analysis
- Knockdown of HCK promotes HREC cell viability and inner blood–retinal barrier integrity by regulating the AMPK signaling pathway
- The role of rapamycin in the PINK1/Parkin signaling pathway in mitophagy in podocytes
- Laryngeal non-Hodgkin lymphoma: Report of four cases and review of the literature
- Clinical value of macrogenome next-generation sequencing on infections
- Overview of dendritic cells and related pathways in autoimmune uveitis
- TAK-242 alleviates diabetic cardiomyopathy via inhibiting pyroptosis and TLR4/CaMKII/NLRP3 pathway
- Hypomethylation in promoters of PGC-1α involved in exercise-driven skeletal muscular alterations in old age
- Profile and antimicrobial susceptibility patterns of bacteria isolated from effluents of Kolladiba and Debark hospitals
- The expression and clinical significance of syncytin-1 in serum exosomes of hepatocellular carcinoma patients
- A histomorphometric study to evaluate the therapeutic effects of biosynthesized silver nanoparticles on the kidneys infected with Plasmodium chabaudi
- PGRMC1 and PAQR4 are promising molecular targets for a rare subtype of ovarian cancer
- Analysis of MDA, SOD, TAOC, MNCV, SNCV, and TSS scores in patients with diabetes peripheral neuropathy
- SLIT3 deficiency promotes non-small cell lung cancer progression by modulating UBE2C/WNT signaling
- The relationship between TMCO1 and CALR in the pathological characteristics of prostate cancer and its effect on the metastasis of prostate cancer cells
- Heterogeneous nuclear ribonucleoprotein K is a potential target for enhancing the chemosensitivity of nasopharyngeal carcinoma
- PHB2 alleviates retinal pigment epithelium cell fibrosis by suppressing the AGE–RAGE pathway
- Anti-γ-aminobutyric acid-B receptor autoimmune encephalitis with syncope as the initial symptom: Case report and literature review
- Comparative analysis of chloroplast genome of Lonicera japonica cv. Damaohua
- Human umbilical cord mesenchymal stem cells regulate glutathione metabolism depending on the ERK–Nrf2–HO-1 signal pathway to repair phosphoramide mustard-induced ovarian cancer cells
- Electroacupuncture on GB acupoints improves osteoporosis via the estradiol–PI3K–Akt signaling pathway
- Renalase protects against podocyte injury by inhibiting oxidative stress and apoptosis in diabetic nephropathy
- Review: Dicranostigma leptopodum: A peculiar plant of Papaveraceae
- Combination effect of flavonoids attenuates lung cancer cell proliferation by inhibiting the STAT3 and FAK signaling pathway
- Renal microangiopathy and immune complex glomerulonephritis induced by anti-tumour agents: A case report
- Correlation analysis of AVPR1a and AVPR2 with abnormal water and sodium and potassium metabolism in rats
- Gastrointestinal health anti-diarrheal mixture relieves spleen deficiency-induced diarrhea through regulating gut microbiota
- Myriad factors and pathways influencing tumor radiotherapy resistance
- Exploring the effects of culture conditions on Yapsin (YPS) gene expression in Nakaseomyces glabratus
- Screening of prognostic core genes based on cell–cell interaction in the peripheral blood of patients with sepsis
- Coagulation factor II thrombin receptor as a promising biomarker in breast cancer management
- Ileocecal mucinous carcinoma misdiagnosed as incarcerated hernia: A case report
- Methyltransferase like 13 promotes malignant behaviors of bladder cancer cells through targeting PI3K/ATK signaling pathway
- The debate between electricity and heat, efficacy and safety of irreversible electroporation and radiofrequency ablation in the treatment of liver cancer: A meta-analysis
- ZAG promotes colorectal cancer cell proliferation and epithelial–mesenchymal transition by promoting lipid synthesis
- Baicalein inhibits NLRP3 inflammasome activation and mitigates placental inflammation and oxidative stress in gestational diabetes mellitus
- Impact of SWCNT-conjugated senna leaf extract on breast cancer cells: A potential apoptotic therapeutic strategy
- MFAP5 inhibits the malignant progression of endometrial cancer cells in vitro
- Major ozonated autohemotherapy promoted functional recovery following spinal cord injury in adult rats via the inhibition of oxidative stress and inflammation
- Axodendritic targeting of TAU and MAP2 and microtubule polarization in iPSC-derived versus SH-SY5Y-derived human neurons
- Differential expression of phosphoinositide 3-kinase/protein kinase B and Toll-like receptor/nuclear factor kappa B signaling pathways in experimental obesity Wistar rat model
- The therapeutic potential of targeting Oncostatin M and the interleukin-6 family in retinal diseases: A comprehensive review
- BA inhibits LPS-stimulated inflammatory response and apoptosis in human middle ear epithelial cells by regulating the Nf-Kb/Iκbα axis
- Role of circRMRP and circRPL27 in chronic obstructive pulmonary disease
- Investigating the role of hyperexpressed HCN1 in inducing myocardial infarction through activation of the NF-κB signaling pathway
- Characterization of phenolic compounds and evaluation of anti-diabetic potential in Cannabis sativa L. seeds: In vivo, in vitro, and in silico studies
- Quantitative immunohistochemistry analysis of breast Ki67 based on artificial intelligence
- Ecology and Environmental Science
- Screening of different growth conditions of Bacillus subtilis isolated from membrane-less microbial fuel cell toward antimicrobial activity profiling
- Degradation of a mixture of 13 polycyclic aromatic hydrocarbons by commercial effective microorganisms
- Evaluation of the impact of two citrus plants on the variation of Panonychus citri (Acari: Tetranychidae) and beneficial phytoseiid mites
- Prediction of present and future distribution areas of Juniperus drupacea Labill and determination of ethnobotany properties in Antalya Province, Türkiye
- Population genetics of Todarodes pacificus (Cephalopoda: Ommastrephidae) in the northwest Pacific Ocean via GBS sequencing
- A comparative analysis of dendrometric, macromorphological, and micromorphological characteristics of Pistacia atlantica subsp. atlantica and Pistacia terebinthus in the middle Atlas region of Morocco
- Macrofungal sporocarp community in the lichen Scots pine forests
- Assessing the proximate compositions of indigenous forage species in Yemen’s pastoral rangelands
- Food Science
- Gut microbiota changes associated with low-carbohydrate diet intervention for obesity
- Reexamination of Aspergillus cristatus phylogeny in dark tea: Characteristics of the mitochondrial genome
- Differences in the flavonoid composition of the leaves, fruits, and branches of mulberry are distinguished based on a plant metabolomics approach
- Investigating the impact of wet rendering (solventless method) on PUFA-rich oil from catfish (Clarias magur) viscera
- Non-linear associations between cardiovascular metabolic indices and metabolic-associated fatty liver disease: A cross-sectional study in the US population (2017–2020)
- Knockdown of USP7 alleviates atherosclerosis in ApoE-deficient mice by regulating EZH2 expression
- Utility of dairy microbiome as a tool for authentication and traceability
- Agriculture
- Enhancing faba bean (Vicia faba L.) productivity through establishing the area-specific fertilizer rate recommendation in southwest Ethiopia
- Impact of novel herbicide based on synthetic auxins and ALS inhibitor on weed control
- Perspectives of pteridophytes microbiome for bioremediation in agricultural applications
- Fertilizer application parameters for drip-irrigated peanut based on the fertilizer effect function established from a “3414” field trial
- Improving the productivity and profitability of maize (Zea mays L.) using optimum blended inorganic fertilization
- Application of leaf multispectral analyzer in comparison to hyperspectral device to assess the diversity of spectral reflectance indices in wheat genotypes
- Animal Sciences
- Knockdown of ANP32E inhibits colorectal cancer cell growth and glycolysis by regulating the AKT/mTOR pathway
- Development of a detection chip for major pathogenic drug-resistant genes and drug targets in bovine respiratory system diseases
- Exploration of the genetic influence of MYOT and MB genes on the plumage coloration of Muscovy ducks
- Transcriptome analysis of adipose tissue in grazing cattle: Identifying key regulators of fat metabolism
- Comparison of nutritional value of the wild and cultivated spiny loaches at three growth stages
- Transcriptomic analysis of liver immune response in Chinese spiny frog (Quasipaa spinosa) infected with Proteus mirabilis
- Disruption of BCAA degradation is a critical characteristic of diabetic cardiomyopathy revealed by integrated transcriptome and metabolome analysis
- Plant Sciences
- Effect of long-term in-row branch covering on soil microorganisms in pear orchards
- Photosynthetic physiological characteristics, growth performance, and element concentrations reveal the calcicole–calcifuge behaviors of three Camellia species
- Transcriptome analysis reveals the mechanism of NaHCO3 promoting tobacco leaf maturation
- Bioinformatics, expression analysis, and functional verification of allene oxide synthase gene HvnAOS1 and HvnAOS2 in qingke
- Water, nitrogen, and phosphorus coupling improves gray jujube fruit quality and yield
- Improving grape fruit quality through soil conditioner: Insights from RNA-seq analysis of Cabernet Sauvignon roots
- Role of Embinin in the reabsorption of nucleus pulposus in lumbar disc herniation: Promotion of nucleus pulposus neovascularization and apoptosis of nucleus pulposus cells
- Revealing the effects of amino acid, organic acid, and phytohormones on the germination of tomato seeds under salinity stress
- Combined effects of nitrogen fertilizer and biochar on the growth, yield, and quality of pepper
- Comprehensive phytochemical and toxicological analysis of Chenopodium ambrosioides (L.) fractions
- Impact of “3414” fertilization on the yield and quality of greenhouse tomatoes
- Exploring the coupling mode of water and fertilizer for improving growth, fruit quality, and yield of the pear in the arid region
- Metagenomic analysis of endophytic bacteria in seed potato (Solanum tuberosum)
- Antibacterial, antifungal, and phytochemical properties of Salsola kali ethanolic extract
- Exploring the hepatoprotective properties of citronellol: In vitro and in silico studies on ethanol-induced damage in HepG2 cells
- Enhanced osmotic dehydration of watermelon rind using honey–sucrose solutions: A study on pre-treatment efficacy and mass transfer kinetics
- Effects of exogenous 2,4-epibrassinolide on photosynthetic traits of 53 cowpea varieties under NaCl stress
- Comparative transcriptome analysis of maize (Zea mays L.) seedlings in response to copper stress
- An optimization method for measuring the stomata in cassava (Manihot esculenta Crantz) under multiple abiotic stresses
- Fosinopril inhibits Ang II-induced VSMC proliferation, phenotype transformation, migration, and oxidative stress through the TGF-β1/Smad signaling pathway
- Antioxidant and antimicrobial activities of Salsola imbricata methanolic extract and its phytochemical characterization
- Bioengineering and Biotechnology
- Absorbable calcium and phosphorus bioactive membranes promote bone marrow mesenchymal stem cells osteogenic differentiation for bone regeneration
- New advances in protein engineering for industrial applications: Key takeaways
- An overview of the production and use of Bacillus thuringiensis toxin
- Research progress of nanoparticles in diagnosis and treatment of hepatocellular carcinoma
- Bioelectrochemical biosensors for water quality assessment and wastewater monitoring
- PEI/MMNs@LNA-542 nanoparticles alleviate ICU-acquired weakness through targeted autophagy inhibition and mitochondrial protection
- Unleashing of cytotoxic effects of thymoquinone-bovine serum albumin nanoparticles on A549 lung cancer cells
- Erratum
- Erratum to “Investigating the association between dietary patterns and glycemic control among children and adolescents with T1DM”
- Erratum to “Activation of hypermethylated P2RY1 mitigates gastric cancer by promoting apoptosis and inhibiting proliferation”
- Retraction
- Retraction to “MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB”
- Retraction to “A data mining technique for detecting malignant mesothelioma cancer using multiple regression analysis”
- Special Issue on Advances in Neurodegenerative Disease Research and Treatment
- Transplantation of human neural stem cell prevents symptomatic motor behavior disability in a rat model of Parkinson’s disease
- Special Issue on Multi-omics
- Inflammasome complex genes with clinical relevance suggest potential as therapeutic targets for anti-tumor drugs in clear cell renal cell carcinoma
- Gastroesophageal varices in primary biliary cholangitis with anti-centromere antibody positivity: Early onset?

