Abstract
To investigate the vaginal microbiota signature of patients with gynecologic cancer and evaluate its diagnostic biomarker potential. We incorporated vaginal 16S rRNA-seq data from 529 women and utilized VSEARCH to analyze the raw data. α-Diversity was evaluated utilizing the Chao1, Shannon, and Simpson indices, and β-diversity was evaluated through principal component analysis using Bray-Curtis distances. Linear discriminant analysis effect size (LEfSe) was utilized to determine species differences between groups. A bacterial co-abundance network was constructed utilizing Spearman correlation analysis. A random forest model of gynecologic tumor risk based on genus was constructed and validated to test its diagnostic efficacy. In gynecologic cancer patients, vaginal α-diversity was significantly greater than in controls, and vaginal β-diversity was significantly separated from that of controls; there was no correlation between these characteristics and menopause status among the subject women. Women diagnosed with gynecological cancer exhibited a reduction in the abundance of vaginal Firmicutes and Lactobacillus, while an increase was observed in the proportions of Bacteroidetes, Proteobacteria, Prevotella, Streptococcus, and Anaerococcus. A random forest model constructed based on 56 genus achieved high accuracy (area under the curve = 84.96%) in gynecological cancer risk prediction. Furthermore, there were discrepancies observed in the community complexity of co-abundance networks between gynecologic cancer patients and the control group. Our study provides evidence that women with gynecologic cancer have a unique vaginal flora structure and microorganisms may be involved in the gynecologic carcinogenesis process. A gynecological cancer risk prediction model based on characteristic genera has good diagnostic value.
1 Introduction
Gynecological cancers are a serious threat to women’s health and have a high incidence and mortality rate worldwide [1]. Gynecologic malignancies manifest in the female reproductive organs, with cervical cancer, ovarian cancer, and endometrial cancer being the most prevalent. With the development of next-generation sequencing technologies, the vaginal microbiome, as a uniquely female flora, is increasingly recognized as being able to influence gynecological carcinogenesis [2–5].
Microorganisms are associated with 20% of human malignancies [6]. The role of microorganisms in cancer development is complex, as both cancer-promoting and anticancer functions are attributed to this. The local microbiota forms an important part of the tumor microenvironment. Microbiome alterations causing dysbiosis resulting in impaired barrier function, triggering inflammatory responses, leading to immune dysregulation, and metabolic disturbances may directly or indirectly modulate carcinogenesis, progression, and therapeutic efficacy [7]. The notion that infection and inflammation are risk factors for gynecologic malignancies [8–10] dates back to the 1990s. The preponderance of the colonizing environment in the vaginal region confers vaginal flora a more pronounced influence on female reproductive organs in comparison to other microbiota. The unorthodox composition of vaginal microbiota has the potential to induce carcinogenic changes at the local immunopathological level [11]. Lactobacillus is the hallmark microorganism in vaginal flora studies, and its predominance consistently indicates the health of the female reproductive system [12]. Lactobacillus maintains a healthy female reproductive system and a low vaginal pH by manufacturing lactic acid in defense against pathogens. The dysbiosis of the vaginal microbiota and the migration of invading pathogens along the genital tract may likewise induce pathological processes [13], and this process brings the pathogen and the resulting pathological response closer to the reproductive organs, further acting as a pro-cancer factor. Previous research has shown that microorganisms can enter the uterus through the cervix [14,15]. Furthermore, a noteworthy correlation has been identified between the microbiomes of the fallopian tubes, ovaries, vagina, and cervix [16], which has been found to impact pregnancy outcomes and gynecological diseases [17,18].
Despite the rapid growth of gynecologic cancer microbiome studies in recent years, most of them are limited to the analysis of female cancer patients with the same cancer type and the same region, the consistency of results is not strong. In addition, gynecological cancers are often difficult to detect at an early stage [19,20], and precise and scientific means of early screening are still lacking. In this study, we collected data (n = 529) from multiple projects of different gynecologic cancers, analyzed the vaginal microbiome of women with cancer in a multidimensional manner.
2 Method
2.1 Public data collection
We collected data from projects published on PubMed.gov containing 16S rRNA-seq for all gynecologic types of cancer (cervical, ovarian, endometrial, squamous-cell, uterine, etc.) as well as healthy populations. Ten projects with accessible sample metadata and high-throughput sequencing are included in this study. Raw sequencing data for these projects were downloaded from the National Library of Medicine and the European Nucleotide Archive via the SRA tool using the identifiers: PRJNA836143 by Mayo Clinic, PRJNA662091 [21] by Jacobson et al., PRJNA481576 [22] by Walsh et al., PRJNA758386 [23] by Gressel et al., PRJNA295859 [16] by Walther-António et al., PRJNA448161 [24] by Tsementzi et al., PRJNA725946 [25] by Fan et al., PRJNA518153 [8] by Ilhan et al., PRJNA415526 [26] by Chen et al., and PRJNA687644 [27] by Li et al.
2.2 Data pre-processing
VSERACH (v2.18.0) was utilized to filter the 16S rRNA data from ten projects for data merging, barcode and primer removal, quality control, etc., and the ten cohorts of clean data were merged again, and then the feature table and representative sequences were obtained through redundancy and noise reduction. Paired sequences with 97% homology to operational classification units (OTUs) were selected using the upsee-out algorithm in VSERACH to select OTUs with a mean relative abundance greater than 1 in 10,000. Using QIIME, species were labeled using a pre-trained plain Bayesian classifier by searching the Greengenes (http://greengenes.secondgenome.com/) database using default parameters (https://github.com/QIIME2/q2-feature-classifier).
2.3 Analysis of microbial composition and diversity
α-Diversity (Chao1, Shannon, Simpson) was assessed using the “Vegan” package (v2.5-7) running in R software v4.0.2. β-Diversity was assessed by principal component analysis (PCA) based on Bray–Curtis distance in the USEARCH (v11) platform. Statistical significance was assessed by analysis of similarity (ANOSIM). Species difference analysis was performed using the Wilcox test with a critical value of log value >2.0 and P < 0.01. A linear discriminant analysis (LDA) effect size (LEfSe) was used for species difference analysis at the phylum and genus level to find the biomarkers, with an LDA threshold of 2. Venn diagrams were plotted for marker comparisons.
2.4 Random forest model construction
We used vaginal microbial 16S rRNA-seq data to construct random forests based on genus, and the models were constructed using the “RandomForest” R package. Specifically, 70% of the data were randomly selected as the training dataset to train and construct the model, and the rest were used as the data in the validation dataset to verify the accuracy of the model. The “pROC” package was used to plot the subject operating characteristic (ROC) curve, and area under receiver operating characteristic (AUC) analysis was used to validate the fitted model.
2.5 Random forest model validation
To test the generalizability and robustness of the model, we performed study-to-study transfer validation and leave-one-dataset-out (LODO) validation on the entire sample based on the methods of previous researchers [28]. In the study-to-study transfer validation, we trained the classifier in a single study and evaluated its performance. Meanwhile, we applied a nested cross-validation procedure to the training studies to calculate the within-study accuracy. In LODO validation, the data from one study is set as the test set, while the data from all the remaining studies are combined as the training set.
2.6 Co-abundance network analysis
Correlation relationships between vaginal microorganisms in women with gynecological cancer were determined by network analysis. Taxa were represented by different node colors, node degrees by node sizes, and correlations by the thickness of the connecting lines. Associations between taxa were calculated by Spearman correlation to generate the network and visualized using Gephi (v0.9). Only connections that were significantly (P < 0.05) strongly correlated (≥0.7) are shown in the graph.
2.7 Statistical analyses
Statistical analyses were carried out in QIIME and in R packages (v4.0.2). The data of age and BMI are expressed as a mean ± standard deviation. P < 0.05 was considered significant.
3 Result
3.1 Characteristics of the data
In this study, we gathered ten gynecologic cancer vaginal microbiome 16S rRNA-seq data to assess vaginal microbiome differences between gynecologic cancer patients and healthy women, construct a gynecologic cancer risk prediction model, and identify biomarkers specific to the three most prevalent gynecologic cancers (cervical cancer, ovarian cancer, and endometrial cancer). We obtained a total of 529 samples from the vagina (Table 1), including the Gynecological_cancer (Gynca) group (n = 348) and the Control group (n = 181), which were subsequently grouped by cancer type, including the Cervical_cancer group (n = 161), the Ovarian_cancer group (n = 71), the Endometrial_cancer group (n = 101), and Normal group (n = 181).
Details of the Bioprojects included in this study
| Bioproject | Year | Location | Sample data | Age | HPV | BMI |
|---|---|---|---|---|---|---|
| PRJNA836143 | 2022 | USA | OC (n = 26) | — | — | — |
| PRJNA662091 | 2020 | USA | OC (n = 45) | — | — | — |
| PRJNA481576 | 2018 | USA | EC (n = 66) | 61.8 ± 10.3 | — | 35.2 |
| Normal (n = 5) | — | — | ||||
| PRJNA758386 | 2021 | USA | EC (n = 20) | — | — | — |
| PRJNA295859 | 2015 | USA | EC (n = 15) | — | — | — |
| PRJNA448161 | 2018 | USA | CC (n = 12) | — | — | — |
| UC (n = 14) | ||||||
| SCC (n = 1) | ||||||
| Normal (n = 30) | ||||||
| PRJNA725946 | 2021 | China | CC (n = 65) | 48.65 ± 6.87 | HPV− (n = 2) | 24.95 ± 4.23 |
| HPV+ (n = 63) | ||||||
| Normal (n = 54) | 46.81 ± 8.15 | HPV− (n = 7) | 24.57 ± 3.43 | |||
| HPV+ (n = 47) | ||||||
| PRJNA518153 | 2019 | USA | CC (n = 10) | — | — | — |
| Normal (n = 18) | ||||||
| PRJNA415526 | 2017 | China | CC (n = 9) | 56.11 ± 9.02 | — | 23.99 ± 0.68 |
| Normal (n = 68) | 43.00 ± 8.69 | — | 22.94 ± 2.74 | |||
| PRJNA687644 | 2020 | China | CC (n = 65) | — | — | — |
| Normal (n = 6) |
3.2 Female menopausal status and gynecological cancer
Gynecologic malignancies are commonly observed in women who have completed menopause and perimenopause, according to previous research [29,30]. To mitigate the potential confounding influence of menopausal status on the results, we subjected gynecologic cancer samples categorized as premenopausal and postmenopausal and compared microbiome variations. We found a difference in Firmicutes and Lactobacillus abundance between postmenopausal and premenopausal women in terms of species composition (Figure 1a–c), and a decrease in Firmicutes and Lactobacillus dominance in the postmenopausal group is now a consensus trend of postmenopausal vaginal microbial alteration. Interestingly, however, the vaginal microbiome of premenopausal and postmenopausal women did not differ significantly in α-diversity and β-diversity (P = 0.611) (Figure 1d–f), and the Wilcox test did not show the presence of significant differences (Figure 1g).

Microbial composition and difference analysis of vaginal samples in Pre_menopausal group and Post_menopausal group. (a) Stacked graph of species composition at the phylum level. (b) Stacked graph of species composition at the genus level. (c) Circle graph of species composition at the genus level. (d) Box graphs of measures of α-diversity (Chao1 index, Simpson index, and Shannon index) indices of microbial OTUs. (e) PCA based on Bray–Curtis distances for all samples from Pre_menopausal and Post_menopausal gynecological cancer patients. Ellipses represented 95% confidence level. (f) R and P values for β-diversity based on Bray-Curtis distances calculated using the ANOSIM analysis. The closer the R value was to 1, the greater the differences between the groups than the differences within groups; the smaller the R value, the less significant the differences between the groups. P < 0.05 showed high reliability of the test. (g) Wilcox test was used to analyze the differences between the groups. The green dots indicate that the differences are not significant.
3.3 Gynecologic cancer patients have unique vaginal microbiome characteristics
3.3.1 Alterations in the composition of vaginal microflora associated with Gynca
Following the exclusion of the influence of menopausal status on vaginal microbiology, we compiled all data to investigate the variations in the vaginal microbiome of women with gynecological cancer from different perspectives. Figure 2a and b shows the ten most abundant taxa at the phylum and genus levels, respectively. The abundance of Lactobacillus, Gardnerella, and Prevotella was found to be preponderant at the genus level, constituting over 50% of the overall abundance. We observed a decrease in the proportion of Firmicutes, Actinobacteria, and Lactobacillus and an increase in the proportion of Bacteroidetes, Proteobacteria, Prevotella, Streptococcus, and Anaerococcus in the Gynca group. This is a significant sign of vaginal flora dysbiosis. By comparing groups using Chao1, Shannon, Simpson vaginal microbial α-diversity indices, we demonstrated that the abundance and diversity of vaginal bacteria were substantially greater in women with gynecological cancer compared to the Control group (Figure 2c–e). By Bray-Curtis PCA, the results showed that the vaginal microbiota composition was distinguished between the Gynca and Control groups (P < 0.001) (Figure 2f and g). In addition, Wilcox test at the ASV level identified 111 ASVs with significantly different abundance, with 77 and 34 ASVs enriched in the Gynca and Control groups, respectively (Figure 2h). Finally, based on LEfSe analysis performed at the phylum and genus level, we found that Proteobacteria, Bacteroidetes, Prevotella, Rhodococcus, Wolbachia, and Bacteroides were enriched in the Gynca group; while in the Control group, they were enriched by Firmicutes, Lactobacillus, Atopobium, etc. (Figure 2i and j). Women with gynecologic cancers exhibit a more pronounced reduction in the healthy vaginal microbiome, and dysbiosis of the vaginal flora is a frequently observed characteristic.

Microbial composition and difference analysis of vaginal samples in Gynca group and Control group. (a) Stacked graph of species composition at the phylum level. (b) Stacked graph of species composition at the genus level. (c–e) Box graphs of measures of α-diversity indices of microbial OTUs. (f) PCA based on Bray–Curtis distances for all samples from Gynca and Control groups. (g) R and P values for β-diversity based on Bray-Curtis distances. (h) Wilcox test was used to analyze the differences between the groups. Green and red dots indicate significantly different results, where green indicates the abundance dominance in the Control group, and red indicates the abundance dominance in Gynca group. (i) Histogram of differential enrichment phylum between Gynca and Control groups. (j) Histograms of differentially enriched genera between Gynca and Control groups. Gynca, gynecological cancer.
3.3.2 Vaginal microbial differences in three types of Gynca
Additional examination of the vaginal microbiomes of cervical, ovarian, and endometrial cancer samples revealed that, in line with the overall analysis, the vaginal microbiomes of the three gynecologic cancer patients generally exhibited a greater variety and abundance, distinguishing them significantly from those of healthy samples (Figure 3a–f). Species composition tended to be disordered (depletion of Firmicutes, Lactobacillus, and enrichment of unconventional vaginal microbiome members), with depletion of Lactobacillus in all three gynecologic cancer groups exceeding half that of the Normal group (Figure 3g–i).

Microbial composition and differential analysis of three major gynecologic cancers (cervical, ovarian, and endometrial cancers). (a) Box graphs of the measured α-diversity indices of microbial OTUs between Cervical_cancer and Normal groups. (b) Box graphs of the measured α-diversity indices of microbial OTUs between Ovarian_cancer and Normal groups. (c) Box graphs of the measured α-diversity indices of microbial OTUs between Endometrial_cancer and Normal groups. (d) PCA based on Bray–Curtis distances for all samples from Cervical_cancer and Normal groups. (e) PCA based on Bray–Curtis distances for all samples from Ovarian_cancer and Normal groups. (f) PCA based on Bray–Curtis distances for all samples from Endometrial_cancer and Normal groups. (g) Stacked graph of species composition at the genus level between Cervical_cancer group and Normal group. (h) Stacked graph of species composition at the genus level between Ovarian_cancer group and Normal group. (i) Stacked graph of species composition at the genus level between Endometrial_cancer group and Normal group.
3.4 Mining of potential biomarkers for gynecological cancer and construction of risk prediction models
To evaluate whether vaginal characteristic genus can be used to identify gynecological cancer patients, we constructed a 10-fold cross-validated random forest classifier. The ROC showed an AUC of 84.96% for the random forest model based on 56 characteristic genera (Figure 4a, Table 2). Figure 4c illustrates a notable rate of overlap between the characteristic genus and the differential genus derived from LEfSe analysis. This finding provides additional support for the notion that alterations in the vaginal microbiome may contribute to the development of gynecologic cancer.

Performance of genera in early screening and diagnosis of gynecological cancer using ROC curve analysis. (a) Gynca subjects and Control group vaginal genera were tested by ROC analysis. (b) Heat map showing AUROC values for models constructed using genus characteristics in each cohort of the vaginal gynecological cancer prediction model. (c) Overlap of random forest characteristic genus with LEfSe analysis of differential genus in the Venn diagram. Gynca, Gynecological cancer.
Microbial markers involved in random forest construction
| Biomarkers | Control | Gynca | Mean decrease accuracy | Mean decrease Gini |
|---|---|---|---|---|
| f_Verrucomicrobiaceae_g_Akkermansia | 8.130625837 | 7.981390228 | 10.1693016 | 0.349836829 |
| f_Veillonellaceae_g_Dialister | 1.194255489 | −3.412856386 | −2.000844476 | 0.108540437 |
| f_Tissierellaceae_g_WAL_1855D | 5.171008761 | −0.499699073 | 3.932924102 | 0.217222638 |
| f_Tissierellaceae_g_Peptoniphilus | 3.014947767 | 1.390877945 | 3.75357992 | 0.164203009 |
| f_Tissierellaceae_g_Finegoldia | −3.800142148 | 6.002905321 | 4.55298823 | 0.16260623 |
| f_Tissierellaceae_g_Anaerococcus | 10.99707312 | 6.095641166 | 12.85108446 | 0.682133506 |
| f_Tissierellaceae_g_1_68 | 12.60067787 | 1.76911911 | 10.86663237 | 0.666871707 |
| f_Thermaceae_g_Thermus | 3.002369856 | 6.858691272 | 7.842029517 | 0.1392703 |
| f_Streptococcaceae_g_Streptococcus | 25.53643998 | 12.84395156 | 24.02786147 | 1.702967976 |
| f_Staphylococcaceae_g_Staphylococcus | 8.165846361 | 10.74503856 | 12.73640691 | 0.370328825 |
| f_Ruminococcaceae_g_Faecalibacterium | 8.384700377 | −1.606223498 | 6.64500351 | 0.213810796 |
| f_Rhizobiaceae_g_Agrobacterium | 30.12847811 | 27.84012109 | 32.0959227 | 2.106384822 |
| f_Pseudomonadaceae_g_Pseudomonas | 5.457975748 | 7.565552243 | 8.677567931 | 0.295668966 |
| f_Propionibacteriaceae_g_Propionibacterium | 8.569740784 | 5.573278911 | 10.60137601 | 0.509739228 |
| f_Prevotellaceae_g_Prevotella | −2.228729226 | 5.620292466 | 5.003047691 | 0.169860197 |
| f_Porphyromonadaceae_g_Porphyromonas | 9.323467961 | 4.08158266 | 9.467854282 | 0.450804465 |
| f_Peptostreptococcaceae_g_Peptostreptococcus | −1.596133316 | 2.941351965 | 2.1828139 | 0.114553903 |
| f_Peptococcaceae_g_Peptococcus | 3.532271109 | 0.85300622 | 3.091388947 | 0.282730238 |
| f_Oxalobacteraceae_g_Ralstonia | 55.28656736 | 54.64730039 | 56.56342649 | 10.78604609 |
| f_Nocardiaceae_g_Rhodococcus | 11.84416411 | 14.89354745 | 17.33426406 | 0.878281431 |
| f_Mycoplasmataceae_g_Ureaplasma | 10.69172362 | 8.78887713 | 10.94887106 | 0.493423031 |
| f_Moraxellaceae_g_Acinetobacter | 5.535186843 | 8.424077552 | 8.950990882 | 0.329059814 |
| f_Leptotrichiaceae_g_Sneathia | 3.735190693 | 6.562793874 | 6.49505965 | 0.246821973 |
| f_Lactobacillaceae_g_Lactobacillus | 40.81643571 | 34.75834302 | 41.04815989 | 4.24709247 |
| f_Lachnospiraceae_g_Shuttleworthia | 11.71829449 | 14.85827269 | 14.94916199 | 0.610181563 |
| f_Halomonadaceae_g_Halomonas | 8.928191068 | 5.918939411 | 8.978388464 | 0.243580244 |
| f_Fusobacteriaceae_g_Fusobacterium | 8.302472812 | 3.115664493 | 8.618932924 | 0.453598561 |
| f_Enterobacteriaceae_g_Escherichia | 7.042481937 | 9.216263298 | 11.29157555 | 0.647092168 |
| f_Enterobacteriaceae_g_Enterobacter | 5.698888967 | 8.091642991 | 9.169832362 | 0.170686966 |
| f_Corynebacteriaceae_g_Corynebacterium | −1.863373546 | 5.029912164 | 4.18831727 | 0.08922379 |
| f_Coriobacteriaceae_g_Unassigned | 1.372108039 | 4.223483687 | 4.26937606 | 0.064650275 |
| f_Coriobacteriaceae_g_Atopobium | 27.1097741 | 27.04100508 | 29.82148678 | 1.797738189 |
| f_Comamonadaceae_g_Pelomonas | 44.56984894 | 44.61587745 | 46.85293505 | 5.855587147 |
| f_Comamonadaceae_g_Acidovorax | 6.266470723 | 6.450580023 | 7.966480421 | 0.165877918 |
| f_Clostridiaceae_g_SMB53 | 9.394722859 | 7.520454855 | 10.30003086 | 0.489248715 |
| f_Caulobacteraceae_g_Unassigned | 26.24904703 | 25.51608685 | 30.13620118 | 1.8349312 |
| f_Campylobacteraceae_g_Campylobacter | 11.65098856 | 4.929140317 | 11.62204257 | 0.474756115 |
| f_Brucellaceae_g_Ochrobactrum | 10.50976271 | 13.87811985 | 14.70803387 | 0.66962882 |
| f_Bifidobacteriaceae_g_Gardnerella | 8.785600024 | 8.146163905 | 10.87443683 | 0.44674851 |
| f_Bacteroidaceae_g_Bacteroides | 7.461755521 | 4.978492048 | 9.614458661 | 0.454329199 |
| f_Veillonellaceae_g_Megasphaera | −1.675168596 | 0.22605679 | −1.098843325 | 0.0686129 |
| f_Lachnospiraceae_g_Moryella | 1.123339558 | 1.165443468 | 2.195355928 | 0.197778515 |
| f_Bifidobacteriaceae_g_Alloscardovia | 12.55915455 | 13.55590274 | 14.82657561 | 0.490900345 |
| f_Actinomycetaceae_g_Actinomyces | 3.708156379 | −4.987815709 | −1.35495428 | 0.114286338 |
| f_Shewanellaceae_g_Shewanella | 8.952987617 | 7.082627587 | 8.993314118 | 0.153189286 |
| f_Propionibacteriaceae_g_Propionimicrobium | 4.010861158 | −0.783109657 | 3.093319864 | 0.040338255 |
| f_Rickettsiaceae_g_Wolbachia | 6.315602866 | 5.806839567 | 7.358207092 | 0.182713441 |
| f_Actinomycetaceae_g_Mobiluncus | 5.185292613 | 0.76711354 | 4.444137075 | 0.107299151 |
| f_Enterococcaceae_g_Enterococcus | 0.855180178 | 3.922466182 | 3.771787352 | 0.153734201 |
| f_Aerococcaceae_g_Aerococcus | −0.149417976 | −0.58248759 | −0.480383343 | 0.053894346 |
| f_Clostridiaceae_g_Clostridium | 1.121653282 | 0.624245003 | 1.06339699 | 0.08292002 |
| f_Carnobacteriaceae_g_Granulicatella | 0.957878156 | 2.326026794 | 2.685557543 | 0.044026949 |
| f_Bifidobacteriaceae_g_Bifidobacterium | −2.926525324 | −2.111394511 | −3.246347418 | 0.055627807 |
| f_Desulfovibrionaceae_g_Bilophila | 6.631996477 | 3.748598764 | 6.296820874 | 0.181776495 |
| f_Comamonadaceae_g_Delftia | 9.253710827 | 4.388970065 | 8.636540815 | 0.219687561 |
| f_S24_7_g_Unassigned | −1.536439334 | 1.104572592 | −0.02063218 | 0.036908881 |
We performed study-to-study transfer validation and LODO validation to test model robustness. In the gynecologic cancer model, the AUC for study-to-study metastasis validation ranged from 0.59 to 0.98, with a mean of 0.77. The AUC for the LODO analysis ranged from 0.61 to 0.76 (mean AUC = 0.70) (Figure 4b).
3.5 Differences in the vaginal microbiome of women with cervical, ovarian, and endometrial cancers
To compare the variations in vaginal microbiome among the three distinct categories of gynecological cancers, we pooled all samples and analyzed them for different cancer types. Significant variations in the composition of vaginal microorganisms among women diagnosed with cervical, ovarian, and endometrial cancer are readily apparent (Figure 5a–c). Firmicutes abundance decreased sequentially in endometrial, cervical, and ovarian cancers, while Proteobacteria increased. Notably, Lactobacillus abundance was essentially the same in the three groups of gynecologic cancers. All three indices of α-diversity in cervical and ovarian cancers were significantly different, while Endometrial_cancer group was not significantly different from the other groups (Figure 5d). In addition, PCA of vaginal microorganisms showed significant separation of the three groups (Figure 5e and f). Additionally, LEfSe analysis was conducted to discern the distinctive microorganisms within the groups (Figure 5g and h). We found that Firmicutes and Lactobacillus were significantly enriched in the Endometrial_cancer group; Halomonas, Escherichia, and Staphylococcus in the Ovarian_cancer group; and Gardnerella, Rhodococcus, and Pseudomonas in the Cervical_cancer group.

Analysis of vaginal microbial composition and differential between cervical, ovarian, and endometrial cancers. (a) Stacked graph of species composition at the phylum level. (b) Stacked graph of species composition at the genus level. (c) Circle graph of species composition at the genus level. (d) Box graphs of measures of α-diversity indices of microbial OTUs. (e) PCA based on Bray–Curtis distances for all samples from CC, EC, and OC patients. (f) R and P values for β-diversity based on Bray-Curtis distances. (g) Histogram of differential enrichment phylum among CC, OC, and EC groups. (h) Histogram of differential enrichment genus among CC, OC, and EC groups. CC, cervical_cancer; OC, ovarian cancer; EC, endometrial cancer.
3.6 Vaginas of gynecologic cancer patients show unique microbial interactions
To investigate the vaginal microbial interaction relationships potential alterations in gynecological cancer patients, we constructed a co-abundance network based on the species annotation outcomes of the Gynca and Control groups, respectively. We utilized Spearman correlation coefficient to evaluate the relative abundance relationships of microorganisms at the level of genus. The findings unveiled that the Gynca group exhibited a greater degree of network complexity, suggesting that vaginal microbial interactions were more robust in cases of gynecological cancer as opposed to the Control group (Figure 6a and b). In addition, the key hubs of the Gynca group were Mesorhizobium, Wolbachia, Akkermansia, Ochrobactrum, and Peptoniphilus, most of which are members of Proteobacteria, Firmicutes biomarkers, suggesting a change in the relevance of the differential genus to other microbial members, which may be one of the cancer causative factors.
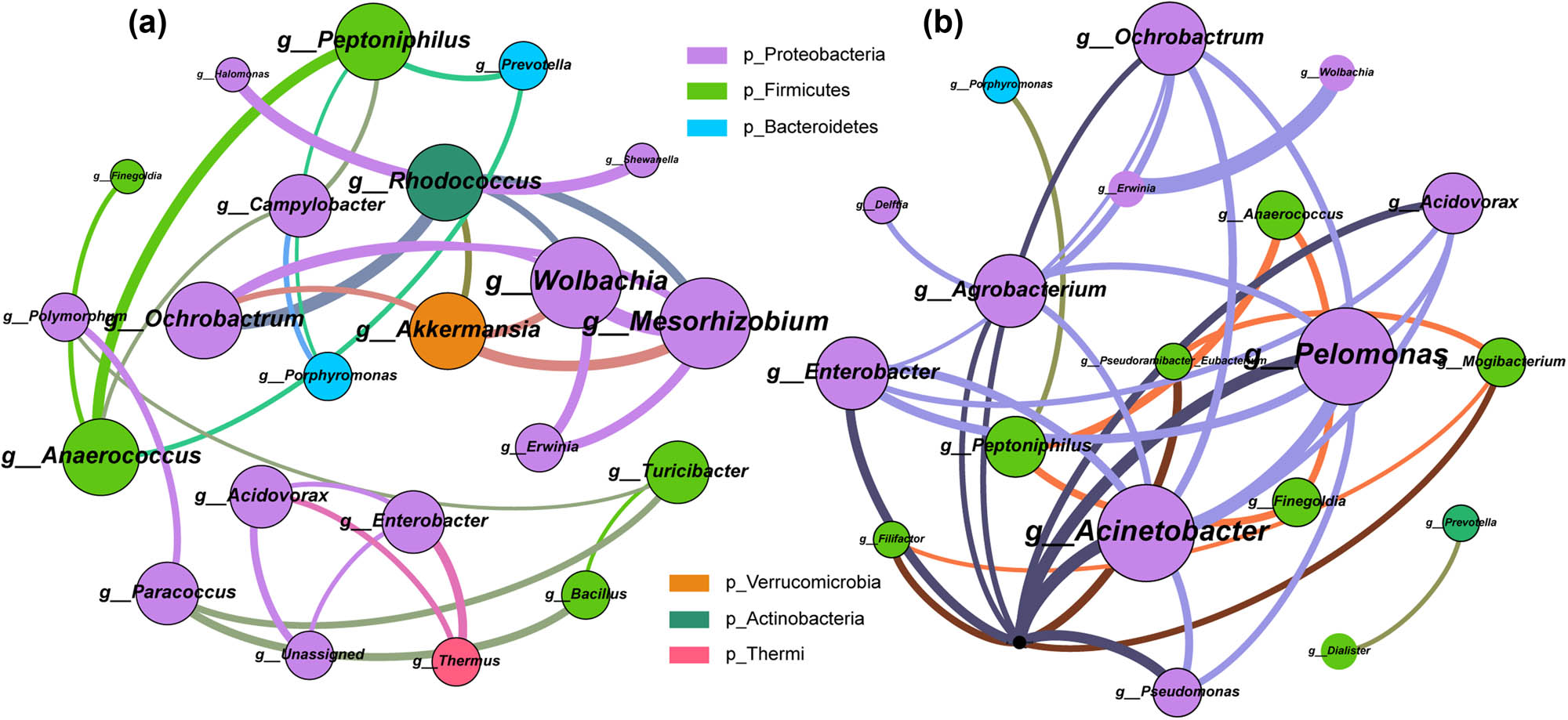
Analysis of vaginal microbial co-abundance network between Gynca and Control groups. The color of nodes indicates different phylum, node size represents node degree, connecting line indicates the interaction between genera, and the width of connecting line represents correlation. (a) Gynca group vaginal microbial co-abundance network. (b) Control group vaginal microbial co-abundance network.
4 Discussion
Gynecologic cancers pose a significant health risk to women, particularly during menopause and perimenopause. The significant association between infection and vaginal microorganisms is its primary trigger. The prevalence of cervical cancer has declined due to technological advancements that have made human papillomavirus (HPV) screening and the HPV vaccine more accessible [31]. However, there are still no efficacious preventive and therapeutic measures in place for endometrial and ovarian malignancies [32]. Large regional differences and late detection period are the main features of gynecologic cancers, and the patients with advanced cancer have a dismal prognosis. Although there is no shortage of studies related to dysbiosis affecting cancer development, most of them focus on single cancer types and specific regions. A comprehensive analysis based on a large sample size and multiple projects is necessary to explore the microbial structure of cancer risk. Therefore, we compiled vaginal microbial 16S rRNA-seq data from various gynecological cancers, including cervical, ovarian, endometrial, and other gynecological cancers. Following the exclusion of potential confounding variables based on menopausal status, a comprehensive analysis was conducted on the vaginal microbiome specific to gynecological cancer in females, as well as the patterns of flora across various forms of cancer.
In general, gynecologic cancers are more prevalent in postmenopausal and perimenopausal women, and the vaginal microbiome in postmenopausal women undergoes substantial alterations, according to previous research. Therefore, we formulated the hypothesis that the vaginal microbiome of gynecologic cancer patients might be independently influenced by menopausal status, which could compromise the validity of the current findings. Through a comparative analysis of the vaginal microbiomes of premenopausal and postmenopausal women with gynecologic cancers, we demonstrated that while minor variations in species composition existed, the overall differences were not significant. This reveals that the previous study and the subsequent analysis we performed could exclude microbiome interference from the menopausal status of the samples and the results were reliable. Variability in the outcomes of a limited number of studies may be attributable to sample and sample size variation. It is worth noting, however, that the examination of disparities during menopause was restricted to the studies incorporated in this analysis and thus was unable to ascertain a correlation between gynecologic cancer incidence and menopausal status.
Gynecologic carcinogenesis is a consequence of multiple pathological pathways. Combined with previous studies and our results, the vaginal microbiome of women with gynecological cancer is specific. A prevailing pattern is observed wherein diversity increases, Firmicutes decreased, Lactobacillus dominance was lost and pathogenic and conditionally pathogenic bacteria are enriched. The microbiome is thought to alter host immunity and influence cancer development and treatment outcomes [33]. Deviations in the composition of vaginal microbial have the potential to generate an inflammatory milieu [34] that facilitates tissue damage and contributes to disease progression and progression. Indicators of vaginal dysbiosis frequently include an enrichment of non-Lactobacillus bacteria, including conditionally pathogenic and pathogenic bacteria, and a decline in the prevalence of Lactobacillus. It is suggested that the protective effect of Lactobacillus on the vaginal environment, local infection, and inflammation caused by unconventional vaginal microbial enrichment may be a risk factor for gynecological cancer. Gardnerella, Prevotella, Actinomyces, Porphyromonas, Anaerococcus, Peptostreptococcus, Streptococcus, and Pseudomonas are emerging pathogens in the clinic due to their close association with pathological processes such as bacterial vaginitis, HPV infections, and precancerous lesions. These microorganisms stimulate innate immune responses in vaginal epithelial cells, resulting in the local production of cytokines and defensins. [35–41]. As an illustration, the presence and changes of group B Streptococcus, which is a member of Streptococcus, in the vagina can cause shedding of vaginal epithelial cells to induce rising infection and inflammation [42], stimulate host immune response [43], and is detected at a higher rate in older women [44]. Group B Streptococcus colonization is impacted by dysbiosis of the vaginal flora, altered vaginal pH, decreased abundance of Lactobacillus, and increased non-commensal bacteria. In addition, exposure to G. vaginalis encourages the colonization of the vagina by group B Streptococcus [45]. Gardnerella and other vaginal non-commensal bacteria in the Gynca group increased in parallel with the proportion of Streptococcus, whereas Lactobacillus abundance decreased, as demonstrated by our findings. In addition to this, pro-cancer effects of the microbiota include genotox release, inhibition of apoptosis, stimulation of proliferation, and promotion of genomic instability [46]. Campylobacter enriched in the Gynca group is closely related to gastrointestinal diseases [47], revealing potential migration and exchange of gut-vaginal flora. In addition, Campylobacter jejuni is capable of producing a genotoxin (cytolethal distending toxin) that acts as a tumorigenic agent [48]. Fusobacterium nucleatum affects cancer cell proliferation by promoting genomic instability and mutations. Consistent with the findings of previous research, our analysis of vaginal flora of patients with three prevalent gynecologic malignancies revealed that the distribution of pathogenic bacteria differed among the different cancer types. Significantly, pathogenic bacteria enriched in the endometrium, including Prevotella, Atopobium, Anaerococcus, Porphyromonas, and Peptoniphilus, were also enriched in the vagina of patients with endometrial cancer, were also enriched in the endometrium [3], revealing a potential correlation between pathogenic bacterial enrichment and carcinogenesis.
Previously, machine learning-based microbiome approaches were also employed to detect patients with gynecologic cancer-related conditions. In 2020, Kang et al. conducted an analysis of the stool microbiome associated with early invasive cervical cancer and distinguished patients based on a machine learning classifier model with an AUC of 0.913 [49]. Subsequently, in 2021, Li et al. investigated endometrial cancer using microbiome and transcriptomic techniques and revealed that microbial markers Prevotella and serum D-dimer, which are fibrin degradation products showed high potential (AUC = 0.86) for predicting endometrial carcinogenesis [50]. Notwithstanding the high predictive value shown by the above models, their brief sample sizes and superficial analysis constitute certain limitations. In addition, the application of machine learning model construction extended to the prediction of chemotherapy resistance and the detection of precancerous lesions. Lee and colleagues’ model for identification of cervical intraepithelial neoplasia based on the vaginal microbiome with 33 characteristic genera had an AUC of 0.952 [51]; Gong et al. showed an AUC of 0.909 [52] for an random forest model for predicting chemotherapy resistance in ovarian cancer based on gut microbes; Wang et al. distinguished neoadjuvant chemotherapy response in locally advanced cervical cancer based on Bacteroides with an AUC of 0.84 [53]. Random forest models constructed using machine learning based on the microbiome can contribute to the construction of predictive diagnostic models for future diseases. Besides the obvious advantages of non-invasive, inexpensive, and highly acceptable, it can also advance the time to early screening and diagnosis to a certain extent, which is of great value for diseases whose prognosis is affected by patients staging.
One notable characteristic that sets this study apart from others is its comprehensive examination of gynecological malignancies, including the dynamic vaginal microbiome. Numerous factors are known to influence the human microbiome, and even minor modifications to the human body can result in substantial alterations to the microbiome. Our sample comprised individuals hailing from different countries, ethnicities, with different lifestyles and significantly different physical conditions, and we could not exclude the interference of these factors in the results. Nevertheless, the generalizability of the acquired results is beyond dispute. Because of the complexity and diversity of the samples included, the results are representative of a wide range of people. The sample size is large enough to dilute to some extent the effect of excessively varied data and extreme data on the results. In addition, we have compared the microbiomes of three types of gynecologic cancers, making the results both more applicable and specific, enabling analysis of differences across populations and regions. Our results contribute to future development and research on non-invasive screening, decrease the financial burden of screening for a broader spectrum of women, mitigate regional disparities, enable gynecologic cancers to be identified more accurately at an earlier stage, enhance the efficacy of subsequent therapies, and contribute to the global cancer burden.
Nonetheless, this study is not devoid of limitations; the precision of 16S rRNA-seq analysis at the species level is low, and we were unable to acquire outcomes pertaining to vaginal microbial species, while the metabolism and effect of distinct species may have large differences, constituting the source of error in our findings. Furthermore, the data we pooled were characterized by too few controls, and the introduction of external controls could potentially introduce additional errors.
In light of current results and trends, it is imperative that women of reproductive age undergo routine vaginal examinations. Early detection and intervention for gynecologic cancer among women will be one of the most effective cancer control strategies of the future. Cancer triggers are diverse and the microbiome is equally influenced by multiple factors. Further research should follow up with patients and prospectively capture multiple indicators of their age, ethnicity, disease history, sexual life, and diet. Avoid errors in results due to non-cancerous factors. How to organically combine microbiome screening with other tests to achieve non-invasive, accurate, and efficient, should also be the focus of future research.
5 Conclusion
In summary, we demonstrated that women with gynecologic cancer have a unique vaginal microbiome. The dysbiotic vaginal flora of gynecologic cancer patients is generally characterized by increased diversity and abundance, loss of dominance of Lactobacillus, and an increase in conditionally pathogenic bacteria. These variants can predict unique interactions between the host and certain bacteria or metabolites, which may help to explore the pathogenesis of gynecological cancers in the future.
-
Funding information: This work was supported by National Nature Science Foundation of China (82373113, X.J.), Shenyang Breast Cancer Clinical Medical Research Center (2020-48-3-1, S.T.), Liaoning Cancer Hospital Yangtse River Scholars Project (S.T., X.J.), LiaoNing Revitalization Talents Program (XLYC1907160, X.J.), Beijing Medical Award Foundation (YXJL-2020-0941-0752, S.T.), Wu Jieping Medical Foundation (320.6750.2020-12-21,320.6750.2020-6-30, S.T.), and the Fundamental Research Funds for the Central Universities (202229, S.T.; 202230, X.J.).
-
Author contributions: Data curation: Mengzhen Han and Na Wang; project administration: Wenjie Han; validation: Junnan Xu; visualization: Xiaolin Liu; writing – original draft: Mengzhen Han; writing – review & editing: Tao Sun.
-
Conflict of interest: X.L. is employed by Liaoning Microhealth Biotechnology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48.Search in Google Scholar
[2] Jarrett OD, Srinivasan S, Richardson BA, Fiedler T, Wallis JM, Kinuthia J, et al. Specific vaginal bacteria are associated with an increased risk of Trichomonas vaginalis acquisition in women. J Infect Dis. 2019;220(9):1503–10.Search in Google Scholar
[3] White BA, Creedon DJ, Nelson KE, Wilson BA. The vaginal microbiome in health and disease. Trends Endocrinol Metab. 2011;22(10):389–93.Search in Google Scholar
[4] Torok MR, Miller WC, Hobbs MM, Macdonald PD, Leone PA, Schwebke JR, et al. The association between Trichomonas vaginalis infection and level of vaginal lactobacilli, in nonpregnant women. J Infect Dis. 2007;196(7):1102–7.Search in Google Scholar
[5] Laniewski P, Ilhan ZE, Herbst-Kralovetz MM. The microbiome and gynaecological cancer development, prevention and therapy. Nat Rev Urol. 2020;17(4):232–50.Search in Google Scholar
[6] de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13(6):607–15.Search in Google Scholar
[7] Wong-Rolle A, Wei HK, Zhao C, Jin C. Unexpected guests in the tumor microenvironment: microbiome in cancer. Protein Cell. 2021;12(5):426–35.Search in Google Scholar
[8] Ilhan ZE, Laniewski P, Thomas N, Roe DJ, Chase DM, Herbst-Kralovetz MM. Deciphering the complex interplay between microbiota, HPV, inflammation and cancer through cervicovaginal metabolic profiling. EBioMedicine. 2019;44:675–90.Search in Google Scholar
[9] Xie X, Yang M, Ding Y, Chen J. Microbial infection, inflammation and epithelial ovarian cancer. Oncol Lett. 2017;14(2):1911–9.Search in Google Scholar
[10] Lu W, He F, Lin Z, Liu S, Tang L, Huang Y, et al. Dysbiosis of the endometrial microbiota and its association with inflammatory cytokines in endometrial cancer. Int J Cancer. 2021;148(7):1708–16.Search in Google Scholar
[11] Ramchander NC, Crosbie EJ. The vaginal microbiome and gynaecological cancer: exercise caution when considering causation. BJOG. 2018;125(3):316.Search in Google Scholar
[12] Zheng N, Guo R, Wang J, Zhou W, Ling Z. Contribution of Lactobacillus iners to vaginal health and diseases: a systematic review. Front Cell Infect Microbiol. 2021;11:792787.Search in Google Scholar
[13] Baker JM, Chase DM, Herbst-Kralovetz MM. Uterine microbiota: residents, tourists, or invaders? Front Immunol. 2018;9:208.Search in Google Scholar
[14] Hansen LK, Becher N, Bastholm S, Glavind J, Ramsing M, Kim CJ, et al. The cervical mucus plug inhibits, but does not block, the passage of ascending bacteria from the vagina during pregnancy. Acta Obstet Gynecol Scand. 2014;93(1):102–8.Search in Google Scholar
[15] Zervomanolakis I, Ott HW, Hadziomerovic D, Mattle V, Seeber BE, Virgolini I, et al. Physiology of upward transport in the human female genital tract. Ann N Y Acad Sci. 2007;1101:1–20.Search in Google Scholar
[16] Walther-António MR, Chen J, Multinu F, Hokenstad A, Distad TJ, Cheek EH, et al. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med. 2016;8(1):122.Search in Google Scholar
[17] Morikawa A, Kawabata A, Shirahige K, Akiyama T, Okamoto A, Sutani T. Altered cervicovaginal microbiota in premenopausal ovarian cancer patients. Gene. 2022;811:146083.Search in Google Scholar
[18] Noda-Nicolau NM, Tantengco OAG, Polettini J, Silva MC, Bento GFC, Cursino GC, et al. Genital mycoplasmas and biomarkers of inflammation and their association with spontaneous preterm birth and preterm prelabor rupture of membranes: a systematic review and meta-analysis. Front Microbiol. 2022;13:859732.Search in Google Scholar
[19] Matulonis UA, Sood AK, Fallowfield L, Howitt BE, Sehouli J, Karlan BY. Ovarian cancer. Nat Rev Dis Primers. 2016;2:16061.Search in Google Scholar
[20] Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet (London, Engl). 2005;366(9484):491–505.Search in Google Scholar
[21] Jacobson D, Moore K, Gunderson C, Rowland M, Austin R, Honap TP, et al. Shifts in gut and vaginal microbiomes are associated with cancer recurrence time in women with ovarian cancer. PeerJ. 2021;9:e11574.Search in Google Scholar
[22] Walsh DM, Hokenstad AN, Chen J, Sung J, Jenkins GD, Chia N, et al. Postmenopause as a key factor in the composition of the endometrial cancer microbiome (ECbiome). Sci Rep. 2019;9(1):19213.Search in Google Scholar
[23] Gressel GM, Usyk M, Frimer M, Kuo DYS, Burk RD. Characterization of the endometrial, cervicovaginal and anorectal microbiota in post-menopausal women with endometrioid and serous endometrial cancers. PLoS One. 2021;16(11):e0259188.Search in Google Scholar
[24] Tsementzi D, Pena-Gonzalez A, Bai J, Hu YJ, Patel P, Shelton J, et al. Comparison of vaginal microbiota in gynecologic cancer patients pre- and post-radiation therapy and healthy women. Cancer Med. 2020;9(11):3714–24.Search in Google Scholar
[25] Fan Q, Wu Y, Li M, An F, Yao L, Wang M, et al. Lactobacillus spp. create a protective micro-ecological environment through regulating the core fucosylation of vaginal epithelial cells against cervical cancer. Cell Death Dis. 2021;12(12):1094.Search in Google Scholar
[26] Chen Y, Qiu X, Wang W, Li D, Wu A, Hong Z, et al. Human papillomavirus infection and cervical intraepithelial neoplasia progression are associated with increased vaginal microbiome diversity in a Chinese cohort. BMC Infect Dis. 2020;20(1):629.Search in Google Scholar
[27] Jiang L, Li B, Zhang Y, Ma S, Liu C, Liang F, et al. Influence of pelvic intensity-modulated radiation therapy with concurrent cisplatin-based chemotherapy of cervical cancer on the vaginal microbiome. Front Oncol. 2021;11:615439.Search in Google Scholar
[28] Wu Y, Jiao N, Zhu R, Zhang Y, Wu D, Wang AJ, et al. Identification of microbial markers across populations in early detection of colorectal cancer. Nat Commun. 2021;12(1):3063.Search in Google Scholar
[29] Salehi F, Dunfield L, Phillips KP, Krewski D, Vanderhyden BC. Risk factors for ovarian cancer: an overview with emphasis on hormonal factors. J Toxicol Env Health B Crit Rev. 2008;11(3–4):301–21.Search in Google Scholar
[30] Wu Y, Sun W, Liu H, Zhang D. Age at menopause and risk of developing endometrial cancer: a meta-analysis. Biomed Res Int. 2019;2019:8584130.Search in Google Scholar
[31] Brisson M, Kim JJ, Canfell K, Drolet M, Gingras G, Burger EA, et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395(10224):575–90.Search in Google Scholar
[32] Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet (London, Engl). 2022;399(10333):1412–28.Search in Google Scholar
[33] Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018;33(4):570–80.Search in Google Scholar
[34] Manzanares-Leal GL, Coronel-Martinez JA, Rodriguez-Morales M, Rangel-Cuevas I, Bustamante-Montes LP, Sandoval-Trujillo H, et al. Preliminary identification of the aerobic cervicovaginal microbiota in Mexican women with cervical cancer as the first step towards metagenomic studies. Front Cell Infect Microbiol. 2022;12:838491.Search in Google Scholar
[35] Bedir Demirdag T, Ozkaya-Parlakay A, Bayrakdar F, Gulhan B, Kanik Yuksek S, Suzuk Yildiz S, et al. An outbreak of Ralstonia pickettii bloodstream infection among pediatric leukemia patients. J Microbiol Immunol Infect. 2022;55(1):80–5.Search in Google Scholar
[36] Thoma B, Straube E, Scholz HC, Al Dahouk S, Zoller L, Pfeffer M, et al. Identification and antimicrobial susceptibilities of Ochrobactrum spp. Int J Med Microbiol. 2009;299(3):209–20.Search in Google Scholar
[37] Ryan MP, Adley CC. Ralstonia spp.: emerging global opportunistic pathogens. Eur J Clin Microbiol Infect Dis. 2014;33(3):291–304.Search in Google Scholar
[38] Zhang Z, Li T, Zhang D, Zong X, Bai H, Bi H, et al. Distinction between vaginal and cervical microbiota in high-risk human papilloma virus-infected women in China. BMC Microbiol. 2021;21(1):90.Search in Google Scholar
[39] Wang L, Yang J, Su H, Shi L, Chen B, Zhang S. Endometrial microbiota from endometrial cancer and paired pericancer tissues in postmenopausal women: differences and clinical relevance. Menopause. 2022;29(10):1168–75.Search in Google Scholar
[40] Mitra A, MacIntyre DA, Ntritsos G, Smith A, Tsilidis KK, Marchesi JR, et al. The vaginal microbiota associates with the regression of untreated cervical intraepithelial neoplasia 2 lesions. Nat Commun. 2020;11(1):1999.Search in Google Scholar
[41] Lin W, Zhang Q, Chen Y, Dong B, Xue H, Lei H, et al. Changes of the vaginal microbiota in HPV infection and cervical intraepithelial neoplasia: a cross-sectional analysis. Sci Rep. 2022;12(1):2812.Search in Google Scholar
[42] Vornhagen J, Armistead B, Santana-Ufret V, Gendrin C, Merillat S, Coleman M, et al. Group B streptococcus exploits vaginal epithelial exfoliation for ascending infection. J Clin Invest. 2018;128(5):1985–99.Search in Google Scholar
[43] Patras KA, Rosler B, Thoman ML, Doran KS. Characterization of host immunity during persistent vaginal colonization by Group B Streptococcus. Mucosal Immunol. 2015;8(6):1339–48.Search in Google Scholar
[44] Baldan R, Droz S, Casanova C, Knabben L, Huang DJ, Brulisauer C, et al. Group B streptococcal colonization in elderly women. BMC Infect Dis. 2021;21(1):408.Search in Google Scholar
[45] Gilbert NM, Foster LR, Cao B, Yin Y, Mysorekar IU, Lewis AL. Gardnerella vaginalis promotes group B Streptococcus vaginal colonization, enabling ascending uteroplacental infection in pregnant mice. Am J Obstet Gynecol. 2021;224(5):530.e1-e17.Search in Google Scholar
[46] Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13(11):800–12.Search in Google Scholar
[47] Kaakoush NO, Castano-Rodriguez N, Mitchell HM, Man SM. Global epidemiology of campylobacter infection. Clin Microbiol Rev. 2015;28(3):687–720.Search in Google Scholar
[48] He Z, Gharaibeh RZ, Newsome RC, Pope JL, Dougherty MW, Tomkovich S, et al. Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin. Gut. 2019;68(2):289–300.Search in Google Scholar
[49] Kang GU, Jung DR, Lee YH, Jeon SY, Han HS, Chong GO, et al. Dynamics of fecal microbiota with and without invasive cervical cancer and its application in early diagnosis. Cancers (Basel). 2020;12(12):3800.Search in Google Scholar
[50] Li C, Gu Y, He Q, Huang J, Song Y, Wan X, et al. Integrated analysis of microbiome and transcriptome data reveals the interplay between commensal bacteria and fibrin degradation in endometrial cancer. Front Cell Infect Microbiol. 2021;11:748558.Search in Google Scholar
[51] Lee YH, Kang GU, Jeon SY, Tagele SB, Pham HQ, Kim MS, et al. Vaginal microbiome-based bacterial signatures for predicting the severity of cervical intraepithelial neoplasia. Diagnostics (Basel). 2020;10(12):1013.Search in Google Scholar
[52] Gong TT, He XH, Gao S, Wu QJ. Application of machine learning in prediction of chemotherapy resistant of ovarian cancer based on gut microbiota. J Cancer. 2021;12(10):2877–85.Search in Google Scholar
[53] Wang Z, Xiao R, Huang J, Qin X, Hu D, Guo E, et al. The diversity of vaginal microbiota predicts neoadjuvant chemotherapy responsiveness in locally advanced cervical cancer. Microb Ecol. 2022;84(1):302–13.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Constitutive and evoked release of ATP in adult mouse olfactory epithelium
- LARP1 knockdown inhibits cultured gastric carcinoma cell cycle progression and metastatic behavior
- PEGylated porcine–human recombinant uricase: A novel fusion protein with improved efficacy and safety for the treatment of hyperuricemia and renal complications
- Research progress on ocular complications caused by type 2 diabetes mellitus and the function of tears and blepharons
- The role and mechanism of esketamine in preventing and treating remifentanil-induced hyperalgesia based on the NMDA receptor–CaMKII pathway
- Brucella infection combined with Nocardia infection: A case report and literature review
- Detection of serum interleukin-18 level and neutrophil/lymphocyte ratio in patients with antineutrophil cytoplasmic antibody-associated vasculitis and its clinical significance
- Ang-1, Ang-2, and Tie2 are diagnostic biomarkers for Henoch-Schönlein purpura and pediatric-onset systemic lupus erythematous
- PTTG1 induces pancreatic cancer cell proliferation and promotes aerobic glycolysis by regulating c-myc
- Role of serum B-cell-activating factor and interleukin-17 as biomarkers in the classification of interstitial pneumonia with autoimmune features
- Effectiveness and safety of a mumps containing vaccine in preventing laboratory-confirmed mumps cases from 2002 to 2017: A meta-analysis
- Low levels of sex hormone-binding globulin predict an increased breast cancer risk and its underlying molecular mechanisms
- A case of Trousseau syndrome: Screening, detection and complication
- Application of the integrated airway humidification device enhances the humidification effect of the rabbit tracheotomy model
- Preparation of Cu2+/TA/HAP composite coating with anti-bacterial and osteogenic potential on 3D-printed porous Ti alloy scaffolds for orthopedic applications
- Aquaporin-8 promotes human dermal fibroblasts to counteract hydrogen peroxide-induced oxidative damage: A novel target for management of skin aging
- Current research and evidence gaps on placental development in iron deficiency anemia
- Single-nucleotide polymorphism rs2910829 in PDE4D is related to stroke susceptibility in Chinese populations: The results of a meta-analysis
- Pheochromocytoma-induced myocardial infarction: A case report
- Kaempferol regulates apoptosis and migration of neural stem cells to attenuate cerebral infarction by O‐GlcNAcylation of β-catenin
- Sirtuin 5 regulates acute myeloid leukemia cell viability and apoptosis by succinylation modification of glycine decarboxylase
- Apigenin 7-glucoside impedes hypoxia-induced malignant phenotypes of cervical cancer cells in a p16-dependent manner
- KAT2A changes the function of endometrial stromal cells via regulating the succinylation of ENO1
- Current state of research on copper complexes in the treatment of breast cancer
- Exploring antioxidant strategies in the pathogenesis of ALS
- Helicobacter pylori causes gastric dysbacteriosis in chronic gastritis patients
- IL-33/soluble ST2 axis is associated with radiation-induced cardiac injury
- The predictive value of serum NLR, SII, and OPNI for lymph node metastasis in breast cancer patients with internal mammary lymph nodes after thoracoscopic surgery
- Carrying SNP rs17506395 (T > G) in TP63 gene and CCR5Δ32 mutation associated with the occurrence of breast cancer in Burkina Faso
- P2X7 receptor: A receptor closely linked with sepsis-associated encephalopathy
- Probiotics for inflammatory bowel disease: Is there sufficient evidence?
- Identification of KDM4C as a gene conferring drug resistance in multiple myeloma
- Microbial perspective on the skin–gut axis and atopic dermatitis
- Thymosin α1 combined with XELOX improves immune function and reduces serum tumor markers in colorectal cancer patients after radical surgery
- Highly specific vaginal microbiome signature for gynecological cancers
- Sample size estimation for AQP4-IgG seropositive optic neuritis: Retinal damage detection by optical coherence tomography
- The effects of SDF-1 combined application with VEGF on femoral distraction osteogenesis in rats
- Fabrication and characterization of gold nanoparticles using alginate: In vitro and in vivo assessment of its administration effects with swimming exercise on diabetic rats
- Mitigating digestive disorders: Action mechanisms of Mediterranean herbal active compounds
- Distribution of CYP2D6 and CYP2C19 gene polymorphisms in Han and Uygur populations with breast cancer in Xinjiang, China
- VSP-2 attenuates secretion of inflammatory cytokines induced by LPS in BV2 cells by mediating the PPARγ/NF-κB signaling pathway
- Factors influencing spontaneous hypothermia after emergency trauma and the construction of a predictive model
- Long-term administration of morphine specifically alters the level of protein expression in different brain regions and affects the redox state
- Application of metagenomic next-generation sequencing technology in the etiological diagnosis of peritoneal dialysis-associated peritonitis
- Clinical diagnosis, prevention, and treatment of neurodyspepsia syndrome using intelligent medicine
- Case report: Successful bronchoscopic interventional treatment of endobronchial leiomyomas
- Preliminary investigation into the genetic etiology of short stature in children through whole exon sequencing of the core family
- Cystic adenomyoma of the uterus: Case report and literature review
- Mesoporous silica nanoparticles as a drug delivery mechanism
- Dynamic changes in autophagy activity in different degrees of pulmonary fibrosis in mice
- Vitamin D deficiency and inflammatory markers in type 2 diabetes: Big data insights
- Lactate-induced IGF1R protein lactylation promotes proliferation and metabolic reprogramming of lung cancer cells
- Meta-analysis on the efficacy of allogeneic hematopoietic stem cell transplantation to treat malignant lymphoma
- Mitochondrial DNA drives neuroinflammation through the cGAS-IFN signaling pathway in the spinal cord of neuropathic pain mice
- Application value of artificial intelligence algorithm-based magnetic resonance multi-sequence imaging in staging diagnosis of cervical cancer
- Embedded monitoring system and teaching of artificial intelligence online drug component recognition
- Investigation into the association of FNDC1 and ADAMTS12 gene expression with plumage coloration in Muscovy ducks
- Yak meat content in feed and its impact on the growth of rats
- A rare case of Richter transformation with breast involvement: A case report and literature review
- First report of Nocardia wallacei infection in an immunocompetent patient in Zhejiang province
- Rhodococcus equi and Brucella pulmonary mass in immunocompetent: A case report and literature review
- Downregulation of RIP3 ameliorates the left ventricular mechanics and function after myocardial infarction via modulating NF-κB/NLRP3 pathway
- Evaluation of the role of some non-enzymatic antioxidants among Iraqi patients with non-alcoholic fatty liver disease
- The role of Phafin proteins in cell signaling pathways and diseases
- Ten-year anemia as initial manifestation of Castleman disease in the abdominal cavity: A case report
- Coexistence of hereditary spherocytosis with SPTB P.Trp1150 gene variant and Gilbert syndrome: A case report and literature review
- Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells
- Exploratory evaluation supported by experimental and modeling approaches of Inula viscosa root extract as a potent corrosion inhibitor for mild steel in a 1 M HCl solution
- Imaging manifestations of ductal adenoma of the breast: A case report
- Gut microbiota and sleep: Interaction mechanisms and therapeutic prospects
- Isomangiferin promotes the migration and osteogenic differentiation of rat bone marrow mesenchymal stem cells
- Prognostic value and microenvironmental crosstalk of exosome-related signatures in human epidermal growth factor receptor 2 positive breast cancer
- Circular RNAs as potential biomarkers for male severe sepsis
- Knockdown of Stanniocalcin-1 inhibits growth and glycolysis in oral squamous cell carcinoma cells
- The expression and biological role of complement C1s in esophageal squamous cell carcinoma
- A novel GNAS mutation in pseudohypoparathyroidism type 1a with articular flexion deformity: A case report
- Predictive value of serum magnesium levels for prognosis in patients with non-small cell lung cancer undergoing EGFR-TKI therapy
- HSPB1 alleviates acute-on-chronic liver failure via the P53/Bax pathway
- IgG4-related disease complicated by PLA2R-associated membranous nephropathy: A case report
- Baculovirus-mediated endostatin and angiostatin activation of autophagy through the AMPK/AKT/mTOR pathway inhibits angiogenesis in hepatocellular carcinoma
- Metformin mitigates osteoarthritis progression by modulating the PI3K/AKT/mTOR signaling pathway and enhancing chondrocyte autophagy
- Evaluation of the activity of antimicrobial peptides against bacterial vaginosis
- Atypical presentation of γ/δ mycosis fungoides with an unusual phenotype and SOCS1 mutation
- Analysis of the microecological mechanism of diabetic kidney disease based on the theory of “gut–kidney axis”: A systematic review
- Omega-3 fatty acids prevent gestational diabetes mellitus via modulation of lipid metabolism
- Refractory hypertension complicated with Turner syndrome: A case report
- Interaction of ncRNAs and the PI3K/AKT/mTOR pathway: Implications for osteosarcoma
- Association of low attenuation area scores with pulmonary function and clinical prognosis in patients with chronic obstructive pulmonary disease
- Long non-coding RNAs in bone formation: Key regulators and therapeutic prospects
- The deubiquitinating enzyme USP35 regulates the stability of NRF2 protein
- Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as potential diagnostic markers for rebleeding in patients with esophagogastric variceal bleeding
- G protein-coupled receptor 1 participating in the mechanism of mediating gestational diabetes mellitus by phosphorylating the AKT pathway
- LL37-mtDNA regulates viability, apoptosis, inflammation, and autophagy in lipopolysaccharide-treated RLE-6TN cells by targeting Hsp90aa1
- The analgesic effect of paeoniflorin: A focused review
- Chemical composition’s effect on Solanum nigrum Linn.’s antioxidant capacity and erythrocyte protection: Bioactive components and molecular docking analysis
- Knockdown of HCK promotes HREC cell viability and inner blood–retinal barrier integrity by regulating the AMPK signaling pathway
- The role of rapamycin in the PINK1/Parkin signaling pathway in mitophagy in podocytes
- Laryngeal non-Hodgkin lymphoma: Report of four cases and review of the literature
- Clinical value of macrogenome next-generation sequencing on infections
- Overview of dendritic cells and related pathways in autoimmune uveitis
- TAK-242 alleviates diabetic cardiomyopathy via inhibiting pyroptosis and TLR4/CaMKII/NLRP3 pathway
- Hypomethylation in promoters of PGC-1α involved in exercise-driven skeletal muscular alterations in old age
- Profile and antimicrobial susceptibility patterns of bacteria isolated from effluents of Kolladiba and Debark hospitals
- The expression and clinical significance of syncytin-1 in serum exosomes of hepatocellular carcinoma patients
- A histomorphometric study to evaluate the therapeutic effects of biosynthesized silver nanoparticles on the kidneys infected with Plasmodium chabaudi
- PGRMC1 and PAQR4 are promising molecular targets for a rare subtype of ovarian cancer
- Analysis of MDA, SOD, TAOC, MNCV, SNCV, and TSS scores in patients with diabetes peripheral neuropathy
- SLIT3 deficiency promotes non-small cell lung cancer progression by modulating UBE2C/WNT signaling
- The relationship between TMCO1 and CALR in the pathological characteristics of prostate cancer and its effect on the metastasis of prostate cancer cells
- Heterogeneous nuclear ribonucleoprotein K is a potential target for enhancing the chemosensitivity of nasopharyngeal carcinoma
- PHB2 alleviates retinal pigment epithelium cell fibrosis by suppressing the AGE–RAGE pathway
- Anti-γ-aminobutyric acid-B receptor autoimmune encephalitis with syncope as the initial symptom: Case report and literature review
- Comparative analysis of chloroplast genome of Lonicera japonica cv. Damaohua
- Human umbilical cord mesenchymal stem cells regulate glutathione metabolism depending on the ERK–Nrf2–HO-1 signal pathway to repair phosphoramide mustard-induced ovarian cancer cells
- Electroacupuncture on GB acupoints improves osteoporosis via the estradiol–PI3K–Akt signaling pathway
- Renalase protects against podocyte injury by inhibiting oxidative stress and apoptosis in diabetic nephropathy
- Review: Dicranostigma leptopodum: A peculiar plant of Papaveraceae
- Combination effect of flavonoids attenuates lung cancer cell proliferation by inhibiting the STAT3 and FAK signaling pathway
- Renal microangiopathy and immune complex glomerulonephritis induced by anti-tumour agents: A case report
- Correlation analysis of AVPR1a and AVPR2 with abnormal water and sodium and potassium metabolism in rats
- Gastrointestinal health anti-diarrheal mixture relieves spleen deficiency-induced diarrhea through regulating gut microbiota
- Myriad factors and pathways influencing tumor radiotherapy resistance
- Exploring the effects of culture conditions on Yapsin (YPS) gene expression in Nakaseomyces glabratus
- Screening of prognostic core genes based on cell–cell interaction in the peripheral blood of patients with sepsis
- Coagulation factor II thrombin receptor as a promising biomarker in breast cancer management
- Ileocecal mucinous carcinoma misdiagnosed as incarcerated hernia: A case report
- Methyltransferase like 13 promotes malignant behaviors of bladder cancer cells through targeting PI3K/ATK signaling pathway
- The debate between electricity and heat, efficacy and safety of irreversible electroporation and radiofrequency ablation in the treatment of liver cancer: A meta-analysis
- ZAG promotes colorectal cancer cell proliferation and epithelial–mesenchymal transition by promoting lipid synthesis
- Baicalein inhibits NLRP3 inflammasome activation and mitigates placental inflammation and oxidative stress in gestational diabetes mellitus
- Impact of SWCNT-conjugated senna leaf extract on breast cancer cells: A potential apoptotic therapeutic strategy
- MFAP5 inhibits the malignant progression of endometrial cancer cells in vitro
- Major ozonated autohemotherapy promoted functional recovery following spinal cord injury in adult rats via the inhibition of oxidative stress and inflammation
- Axodendritic targeting of TAU and MAP2 and microtubule polarization in iPSC-derived versus SH-SY5Y-derived human neurons
- Differential expression of phosphoinositide 3-kinase/protein kinase B and Toll-like receptor/nuclear factor kappa B signaling pathways in experimental obesity Wistar rat model
- The therapeutic potential of targeting Oncostatin M and the interleukin-6 family in retinal diseases: A comprehensive review
- BA inhibits LPS-stimulated inflammatory response and apoptosis in human middle ear epithelial cells by regulating the Nf-Kb/Iκbα axis
- Role of circRMRP and circRPL27 in chronic obstructive pulmonary disease
- Investigating the role of hyperexpressed HCN1 in inducing myocardial infarction through activation of the NF-κB signaling pathway
- Characterization of phenolic compounds and evaluation of anti-diabetic potential in Cannabis sativa L. seeds: In vivo, in vitro, and in silico studies
- Quantitative immunohistochemistry analysis of breast Ki67 based on artificial intelligence
- Ecology and Environmental Science
- Screening of different growth conditions of Bacillus subtilis isolated from membrane-less microbial fuel cell toward antimicrobial activity profiling
- Degradation of a mixture of 13 polycyclic aromatic hydrocarbons by commercial effective microorganisms
- Evaluation of the impact of two citrus plants on the variation of Panonychus citri (Acari: Tetranychidae) and beneficial phytoseiid mites
- Prediction of present and future distribution areas of Juniperus drupacea Labill and determination of ethnobotany properties in Antalya Province, Türkiye
- Population genetics of Todarodes pacificus (Cephalopoda: Ommastrephidae) in the northwest Pacific Ocean via GBS sequencing
- A comparative analysis of dendrometric, macromorphological, and micromorphological characteristics of Pistacia atlantica subsp. atlantica and Pistacia terebinthus in the middle Atlas region of Morocco
- Macrofungal sporocarp community in the lichen Scots pine forests
- Assessing the proximate compositions of indigenous forage species in Yemen’s pastoral rangelands
- Food Science
- Gut microbiota changes associated with low-carbohydrate diet intervention for obesity
- Reexamination of Aspergillus cristatus phylogeny in dark tea: Characteristics of the mitochondrial genome
- Differences in the flavonoid composition of the leaves, fruits, and branches of mulberry are distinguished based on a plant metabolomics approach
- Investigating the impact of wet rendering (solventless method) on PUFA-rich oil from catfish (Clarias magur) viscera
- Non-linear associations between cardiovascular metabolic indices and metabolic-associated fatty liver disease: A cross-sectional study in the US population (2017–2020)
- Knockdown of USP7 alleviates atherosclerosis in ApoE-deficient mice by regulating EZH2 expression
- Utility of dairy microbiome as a tool for authentication and traceability
- Agriculture
- Enhancing faba bean (Vicia faba L.) productivity through establishing the area-specific fertilizer rate recommendation in southwest Ethiopia
- Impact of novel herbicide based on synthetic auxins and ALS inhibitor on weed control
- Perspectives of pteridophytes microbiome for bioremediation in agricultural applications
- Fertilizer application parameters for drip-irrigated peanut based on the fertilizer effect function established from a “3414” field trial
- Improving the productivity and profitability of maize (Zea mays L.) using optimum blended inorganic fertilization
- Application of leaf multispectral analyzer in comparison to hyperspectral device to assess the diversity of spectral reflectance indices in wheat genotypes
- Animal Sciences
- Knockdown of ANP32E inhibits colorectal cancer cell growth and glycolysis by regulating the AKT/mTOR pathway
- Development of a detection chip for major pathogenic drug-resistant genes and drug targets in bovine respiratory system diseases
- Exploration of the genetic influence of MYOT and MB genes on the plumage coloration of Muscovy ducks
- Transcriptome analysis of adipose tissue in grazing cattle: Identifying key regulators of fat metabolism
- Comparison of nutritional value of the wild and cultivated spiny loaches at three growth stages
- Transcriptomic analysis of liver immune response in Chinese spiny frog (Quasipaa spinosa) infected with Proteus mirabilis
- Disruption of BCAA degradation is a critical characteristic of diabetic cardiomyopathy revealed by integrated transcriptome and metabolome analysis
- Plant Sciences
- Effect of long-term in-row branch covering on soil microorganisms in pear orchards
- Photosynthetic physiological characteristics, growth performance, and element concentrations reveal the calcicole–calcifuge behaviors of three Camellia species
- Transcriptome analysis reveals the mechanism of NaHCO3 promoting tobacco leaf maturation
- Bioinformatics, expression analysis, and functional verification of allene oxide synthase gene HvnAOS1 and HvnAOS2 in qingke
- Water, nitrogen, and phosphorus coupling improves gray jujube fruit quality and yield
- Improving grape fruit quality through soil conditioner: Insights from RNA-seq analysis of Cabernet Sauvignon roots
- Role of Embinin in the reabsorption of nucleus pulposus in lumbar disc herniation: Promotion of nucleus pulposus neovascularization and apoptosis of nucleus pulposus cells
- Revealing the effects of amino acid, organic acid, and phytohormones on the germination of tomato seeds under salinity stress
- Combined effects of nitrogen fertilizer and biochar on the growth, yield, and quality of pepper
- Comprehensive phytochemical and toxicological analysis of Chenopodium ambrosioides (L.) fractions
- Impact of “3414” fertilization on the yield and quality of greenhouse tomatoes
- Exploring the coupling mode of water and fertilizer for improving growth, fruit quality, and yield of the pear in the arid region
- Metagenomic analysis of endophytic bacteria in seed potato (Solanum tuberosum)
- Antibacterial, antifungal, and phytochemical properties of Salsola kali ethanolic extract
- Exploring the hepatoprotective properties of citronellol: In vitro and in silico studies on ethanol-induced damage in HepG2 cells
- Enhanced osmotic dehydration of watermelon rind using honey–sucrose solutions: A study on pre-treatment efficacy and mass transfer kinetics
- Effects of exogenous 2,4-epibrassinolide on photosynthetic traits of 53 cowpea varieties under NaCl stress
- Comparative transcriptome analysis of maize (Zea mays L.) seedlings in response to copper stress
- An optimization method for measuring the stomata in cassava (Manihot esculenta Crantz) under multiple abiotic stresses
- Fosinopril inhibits Ang II-induced VSMC proliferation, phenotype transformation, migration, and oxidative stress through the TGF-β1/Smad signaling pathway
- Antioxidant and antimicrobial activities of Salsola imbricata methanolic extract and its phytochemical characterization
- Bioengineering and Biotechnology
- Absorbable calcium and phosphorus bioactive membranes promote bone marrow mesenchymal stem cells osteogenic differentiation for bone regeneration
- New advances in protein engineering for industrial applications: Key takeaways
- An overview of the production and use of Bacillus thuringiensis toxin
- Research progress of nanoparticles in diagnosis and treatment of hepatocellular carcinoma
- Bioelectrochemical biosensors for water quality assessment and wastewater monitoring
- PEI/MMNs@LNA-542 nanoparticles alleviate ICU-acquired weakness through targeted autophagy inhibition and mitochondrial protection
- Unleashing of cytotoxic effects of thymoquinone-bovine serum albumin nanoparticles on A549 lung cancer cells
- Erratum
- Erratum to “Investigating the association between dietary patterns and glycemic control among children and adolescents with T1DM”
- Erratum to “Activation of hypermethylated P2RY1 mitigates gastric cancer by promoting apoptosis and inhibiting proliferation”
- Retraction
- Retraction to “MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB”
- Retraction to “A data mining technique for detecting malignant mesothelioma cancer using multiple regression analysis”
- Special Issue on Advances in Neurodegenerative Disease Research and Treatment
- Transplantation of human neural stem cell prevents symptomatic motor behavior disability in a rat model of Parkinson’s disease
- Special Issue on Multi-omics
- Inflammasome complex genes with clinical relevance suggest potential as therapeutic targets for anti-tumor drugs in clear cell renal cell carcinoma
- Gastroesophageal varices in primary biliary cholangitis with anti-centromere antibody positivity: Early onset?
Articles in the same Issue
- Biomedical Sciences
- Constitutive and evoked release of ATP in adult mouse olfactory epithelium
- LARP1 knockdown inhibits cultured gastric carcinoma cell cycle progression and metastatic behavior
- PEGylated porcine–human recombinant uricase: A novel fusion protein with improved efficacy and safety for the treatment of hyperuricemia and renal complications
- Research progress on ocular complications caused by type 2 diabetes mellitus and the function of tears and blepharons
- The role and mechanism of esketamine in preventing and treating remifentanil-induced hyperalgesia based on the NMDA receptor–CaMKII pathway
- Brucella infection combined with Nocardia infection: A case report and literature review
- Detection of serum interleukin-18 level and neutrophil/lymphocyte ratio in patients with antineutrophil cytoplasmic antibody-associated vasculitis and its clinical significance
- Ang-1, Ang-2, and Tie2 are diagnostic biomarkers for Henoch-Schönlein purpura and pediatric-onset systemic lupus erythematous
- PTTG1 induces pancreatic cancer cell proliferation and promotes aerobic glycolysis by regulating c-myc
- Role of serum B-cell-activating factor and interleukin-17 as biomarkers in the classification of interstitial pneumonia with autoimmune features
- Effectiveness and safety of a mumps containing vaccine in preventing laboratory-confirmed mumps cases from 2002 to 2017: A meta-analysis
- Low levels of sex hormone-binding globulin predict an increased breast cancer risk and its underlying molecular mechanisms
- A case of Trousseau syndrome: Screening, detection and complication
- Application of the integrated airway humidification device enhances the humidification effect of the rabbit tracheotomy model
- Preparation of Cu2+/TA/HAP composite coating with anti-bacterial and osteogenic potential on 3D-printed porous Ti alloy scaffolds for orthopedic applications
- Aquaporin-8 promotes human dermal fibroblasts to counteract hydrogen peroxide-induced oxidative damage: A novel target for management of skin aging
- Current research and evidence gaps on placental development in iron deficiency anemia
- Single-nucleotide polymorphism rs2910829 in PDE4D is related to stroke susceptibility in Chinese populations: The results of a meta-analysis
- Pheochromocytoma-induced myocardial infarction: A case report
- Kaempferol regulates apoptosis and migration of neural stem cells to attenuate cerebral infarction by O‐GlcNAcylation of β-catenin
- Sirtuin 5 regulates acute myeloid leukemia cell viability and apoptosis by succinylation modification of glycine decarboxylase
- Apigenin 7-glucoside impedes hypoxia-induced malignant phenotypes of cervical cancer cells in a p16-dependent manner
- KAT2A changes the function of endometrial stromal cells via regulating the succinylation of ENO1
- Current state of research on copper complexes in the treatment of breast cancer
- Exploring antioxidant strategies in the pathogenesis of ALS
- Helicobacter pylori causes gastric dysbacteriosis in chronic gastritis patients
- IL-33/soluble ST2 axis is associated with radiation-induced cardiac injury
- The predictive value of serum NLR, SII, and OPNI for lymph node metastasis in breast cancer patients with internal mammary lymph nodes after thoracoscopic surgery
- Carrying SNP rs17506395 (T > G) in TP63 gene and CCR5Δ32 mutation associated with the occurrence of breast cancer in Burkina Faso
- P2X7 receptor: A receptor closely linked with sepsis-associated encephalopathy
- Probiotics for inflammatory bowel disease: Is there sufficient evidence?
- Identification of KDM4C as a gene conferring drug resistance in multiple myeloma
- Microbial perspective on the skin–gut axis and atopic dermatitis
- Thymosin α1 combined with XELOX improves immune function and reduces serum tumor markers in colorectal cancer patients after radical surgery
- Highly specific vaginal microbiome signature for gynecological cancers
- Sample size estimation for AQP4-IgG seropositive optic neuritis: Retinal damage detection by optical coherence tomography
- The effects of SDF-1 combined application with VEGF on femoral distraction osteogenesis in rats
- Fabrication and characterization of gold nanoparticles using alginate: In vitro and in vivo assessment of its administration effects with swimming exercise on diabetic rats
- Mitigating digestive disorders: Action mechanisms of Mediterranean herbal active compounds
- Distribution of CYP2D6 and CYP2C19 gene polymorphisms in Han and Uygur populations with breast cancer in Xinjiang, China
- VSP-2 attenuates secretion of inflammatory cytokines induced by LPS in BV2 cells by mediating the PPARγ/NF-κB signaling pathway
- Factors influencing spontaneous hypothermia after emergency trauma and the construction of a predictive model
- Long-term administration of morphine specifically alters the level of protein expression in different brain regions and affects the redox state
- Application of metagenomic next-generation sequencing technology in the etiological diagnosis of peritoneal dialysis-associated peritonitis
- Clinical diagnosis, prevention, and treatment of neurodyspepsia syndrome using intelligent medicine
- Case report: Successful bronchoscopic interventional treatment of endobronchial leiomyomas
- Preliminary investigation into the genetic etiology of short stature in children through whole exon sequencing of the core family
- Cystic adenomyoma of the uterus: Case report and literature review
- Mesoporous silica nanoparticles as a drug delivery mechanism
- Dynamic changes in autophagy activity in different degrees of pulmonary fibrosis in mice
- Vitamin D deficiency and inflammatory markers in type 2 diabetes: Big data insights
- Lactate-induced IGF1R protein lactylation promotes proliferation and metabolic reprogramming of lung cancer cells
- Meta-analysis on the efficacy of allogeneic hematopoietic stem cell transplantation to treat malignant lymphoma
- Mitochondrial DNA drives neuroinflammation through the cGAS-IFN signaling pathway in the spinal cord of neuropathic pain mice
- Application value of artificial intelligence algorithm-based magnetic resonance multi-sequence imaging in staging diagnosis of cervical cancer
- Embedded monitoring system and teaching of artificial intelligence online drug component recognition
- Investigation into the association of FNDC1 and ADAMTS12 gene expression with plumage coloration in Muscovy ducks
- Yak meat content in feed and its impact on the growth of rats
- A rare case of Richter transformation with breast involvement: A case report and literature review
- First report of Nocardia wallacei infection in an immunocompetent patient in Zhejiang province
- Rhodococcus equi and Brucella pulmonary mass in immunocompetent: A case report and literature review
- Downregulation of RIP3 ameliorates the left ventricular mechanics and function after myocardial infarction via modulating NF-κB/NLRP3 pathway
- Evaluation of the role of some non-enzymatic antioxidants among Iraqi patients with non-alcoholic fatty liver disease
- The role of Phafin proteins in cell signaling pathways and diseases
- Ten-year anemia as initial manifestation of Castleman disease in the abdominal cavity: A case report
- Coexistence of hereditary spherocytosis with SPTB P.Trp1150 gene variant and Gilbert syndrome: A case report and literature review
- Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells
- Exploratory evaluation supported by experimental and modeling approaches of Inula viscosa root extract as a potent corrosion inhibitor for mild steel in a 1 M HCl solution
- Imaging manifestations of ductal adenoma of the breast: A case report
- Gut microbiota and sleep: Interaction mechanisms and therapeutic prospects
- Isomangiferin promotes the migration and osteogenic differentiation of rat bone marrow mesenchymal stem cells
- Prognostic value and microenvironmental crosstalk of exosome-related signatures in human epidermal growth factor receptor 2 positive breast cancer
- Circular RNAs as potential biomarkers for male severe sepsis
- Knockdown of Stanniocalcin-1 inhibits growth and glycolysis in oral squamous cell carcinoma cells
- The expression and biological role of complement C1s in esophageal squamous cell carcinoma
- A novel GNAS mutation in pseudohypoparathyroidism type 1a with articular flexion deformity: A case report
- Predictive value of serum magnesium levels for prognosis in patients with non-small cell lung cancer undergoing EGFR-TKI therapy
- HSPB1 alleviates acute-on-chronic liver failure via the P53/Bax pathway
- IgG4-related disease complicated by PLA2R-associated membranous nephropathy: A case report
- Baculovirus-mediated endostatin and angiostatin activation of autophagy through the AMPK/AKT/mTOR pathway inhibits angiogenesis in hepatocellular carcinoma
- Metformin mitigates osteoarthritis progression by modulating the PI3K/AKT/mTOR signaling pathway and enhancing chondrocyte autophagy
- Evaluation of the activity of antimicrobial peptides against bacterial vaginosis
- Atypical presentation of γ/δ mycosis fungoides with an unusual phenotype and SOCS1 mutation
- Analysis of the microecological mechanism of diabetic kidney disease based on the theory of “gut–kidney axis”: A systematic review
- Omega-3 fatty acids prevent gestational diabetes mellitus via modulation of lipid metabolism
- Refractory hypertension complicated with Turner syndrome: A case report
- Interaction of ncRNAs and the PI3K/AKT/mTOR pathway: Implications for osteosarcoma
- Association of low attenuation area scores with pulmonary function and clinical prognosis in patients with chronic obstructive pulmonary disease
- Long non-coding RNAs in bone formation: Key regulators and therapeutic prospects
- The deubiquitinating enzyme USP35 regulates the stability of NRF2 protein
- Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as potential diagnostic markers for rebleeding in patients with esophagogastric variceal bleeding
- G protein-coupled receptor 1 participating in the mechanism of mediating gestational diabetes mellitus by phosphorylating the AKT pathway
- LL37-mtDNA regulates viability, apoptosis, inflammation, and autophagy in lipopolysaccharide-treated RLE-6TN cells by targeting Hsp90aa1
- The analgesic effect of paeoniflorin: A focused review
- Chemical composition’s effect on Solanum nigrum Linn.’s antioxidant capacity and erythrocyte protection: Bioactive components and molecular docking analysis
- Knockdown of HCK promotes HREC cell viability and inner blood–retinal barrier integrity by regulating the AMPK signaling pathway
- The role of rapamycin in the PINK1/Parkin signaling pathway in mitophagy in podocytes
- Laryngeal non-Hodgkin lymphoma: Report of four cases and review of the literature
- Clinical value of macrogenome next-generation sequencing on infections
- Overview of dendritic cells and related pathways in autoimmune uveitis
- TAK-242 alleviates diabetic cardiomyopathy via inhibiting pyroptosis and TLR4/CaMKII/NLRP3 pathway
- Hypomethylation in promoters of PGC-1α involved in exercise-driven skeletal muscular alterations in old age
- Profile and antimicrobial susceptibility patterns of bacteria isolated from effluents of Kolladiba and Debark hospitals
- The expression and clinical significance of syncytin-1 in serum exosomes of hepatocellular carcinoma patients
- A histomorphometric study to evaluate the therapeutic effects of biosynthesized silver nanoparticles on the kidneys infected with Plasmodium chabaudi
- PGRMC1 and PAQR4 are promising molecular targets for a rare subtype of ovarian cancer
- Analysis of MDA, SOD, TAOC, MNCV, SNCV, and TSS scores in patients with diabetes peripheral neuropathy
- SLIT3 deficiency promotes non-small cell lung cancer progression by modulating UBE2C/WNT signaling
- The relationship between TMCO1 and CALR in the pathological characteristics of prostate cancer and its effect on the metastasis of prostate cancer cells
- Heterogeneous nuclear ribonucleoprotein K is a potential target for enhancing the chemosensitivity of nasopharyngeal carcinoma
- PHB2 alleviates retinal pigment epithelium cell fibrosis by suppressing the AGE–RAGE pathway
- Anti-γ-aminobutyric acid-B receptor autoimmune encephalitis with syncope as the initial symptom: Case report and literature review
- Comparative analysis of chloroplast genome of Lonicera japonica cv. Damaohua
- Human umbilical cord mesenchymal stem cells regulate glutathione metabolism depending on the ERK–Nrf2–HO-1 signal pathway to repair phosphoramide mustard-induced ovarian cancer cells
- Electroacupuncture on GB acupoints improves osteoporosis via the estradiol–PI3K–Akt signaling pathway
- Renalase protects against podocyte injury by inhibiting oxidative stress and apoptosis in diabetic nephropathy
- Review: Dicranostigma leptopodum: A peculiar plant of Papaveraceae
- Combination effect of flavonoids attenuates lung cancer cell proliferation by inhibiting the STAT3 and FAK signaling pathway
- Renal microangiopathy and immune complex glomerulonephritis induced by anti-tumour agents: A case report
- Correlation analysis of AVPR1a and AVPR2 with abnormal water and sodium and potassium metabolism in rats
- Gastrointestinal health anti-diarrheal mixture relieves spleen deficiency-induced diarrhea through regulating gut microbiota
- Myriad factors and pathways influencing tumor radiotherapy resistance
- Exploring the effects of culture conditions on Yapsin (YPS) gene expression in Nakaseomyces glabratus
- Screening of prognostic core genes based on cell–cell interaction in the peripheral blood of patients with sepsis
- Coagulation factor II thrombin receptor as a promising biomarker in breast cancer management
- Ileocecal mucinous carcinoma misdiagnosed as incarcerated hernia: A case report
- Methyltransferase like 13 promotes malignant behaviors of bladder cancer cells through targeting PI3K/ATK signaling pathway
- The debate between electricity and heat, efficacy and safety of irreversible electroporation and radiofrequency ablation in the treatment of liver cancer: A meta-analysis
- ZAG promotes colorectal cancer cell proliferation and epithelial–mesenchymal transition by promoting lipid synthesis
- Baicalein inhibits NLRP3 inflammasome activation and mitigates placental inflammation and oxidative stress in gestational diabetes mellitus
- Impact of SWCNT-conjugated senna leaf extract on breast cancer cells: A potential apoptotic therapeutic strategy
- MFAP5 inhibits the malignant progression of endometrial cancer cells in vitro
- Major ozonated autohemotherapy promoted functional recovery following spinal cord injury in adult rats via the inhibition of oxidative stress and inflammation
- Axodendritic targeting of TAU and MAP2 and microtubule polarization in iPSC-derived versus SH-SY5Y-derived human neurons
- Differential expression of phosphoinositide 3-kinase/protein kinase B and Toll-like receptor/nuclear factor kappa B signaling pathways in experimental obesity Wistar rat model
- The therapeutic potential of targeting Oncostatin M and the interleukin-6 family in retinal diseases: A comprehensive review
- BA inhibits LPS-stimulated inflammatory response and apoptosis in human middle ear epithelial cells by regulating the Nf-Kb/Iκbα axis
- Role of circRMRP and circRPL27 in chronic obstructive pulmonary disease
- Investigating the role of hyperexpressed HCN1 in inducing myocardial infarction through activation of the NF-κB signaling pathway
- Characterization of phenolic compounds and evaluation of anti-diabetic potential in Cannabis sativa L. seeds: In vivo, in vitro, and in silico studies
- Quantitative immunohistochemistry analysis of breast Ki67 based on artificial intelligence
- Ecology and Environmental Science
- Screening of different growth conditions of Bacillus subtilis isolated from membrane-less microbial fuel cell toward antimicrobial activity profiling
- Degradation of a mixture of 13 polycyclic aromatic hydrocarbons by commercial effective microorganisms
- Evaluation of the impact of two citrus plants on the variation of Panonychus citri (Acari: Tetranychidae) and beneficial phytoseiid mites
- Prediction of present and future distribution areas of Juniperus drupacea Labill and determination of ethnobotany properties in Antalya Province, Türkiye
- Population genetics of Todarodes pacificus (Cephalopoda: Ommastrephidae) in the northwest Pacific Ocean via GBS sequencing
- A comparative analysis of dendrometric, macromorphological, and micromorphological characteristics of Pistacia atlantica subsp. atlantica and Pistacia terebinthus in the middle Atlas region of Morocco
- Macrofungal sporocarp community in the lichen Scots pine forests
- Assessing the proximate compositions of indigenous forage species in Yemen’s pastoral rangelands
- Food Science
- Gut microbiota changes associated with low-carbohydrate diet intervention for obesity
- Reexamination of Aspergillus cristatus phylogeny in dark tea: Characteristics of the mitochondrial genome
- Differences in the flavonoid composition of the leaves, fruits, and branches of mulberry are distinguished based on a plant metabolomics approach
- Investigating the impact of wet rendering (solventless method) on PUFA-rich oil from catfish (Clarias magur) viscera
- Non-linear associations between cardiovascular metabolic indices and metabolic-associated fatty liver disease: A cross-sectional study in the US population (2017–2020)
- Knockdown of USP7 alleviates atherosclerosis in ApoE-deficient mice by regulating EZH2 expression
- Utility of dairy microbiome as a tool for authentication and traceability
- Agriculture
- Enhancing faba bean (Vicia faba L.) productivity through establishing the area-specific fertilizer rate recommendation in southwest Ethiopia
- Impact of novel herbicide based on synthetic auxins and ALS inhibitor on weed control
- Perspectives of pteridophytes microbiome for bioremediation in agricultural applications
- Fertilizer application parameters for drip-irrigated peanut based on the fertilizer effect function established from a “3414” field trial
- Improving the productivity and profitability of maize (Zea mays L.) using optimum blended inorganic fertilization
- Application of leaf multispectral analyzer in comparison to hyperspectral device to assess the diversity of spectral reflectance indices in wheat genotypes
- Animal Sciences
- Knockdown of ANP32E inhibits colorectal cancer cell growth and glycolysis by regulating the AKT/mTOR pathway
- Development of a detection chip for major pathogenic drug-resistant genes and drug targets in bovine respiratory system diseases
- Exploration of the genetic influence of MYOT and MB genes on the plumage coloration of Muscovy ducks
- Transcriptome analysis of adipose tissue in grazing cattle: Identifying key regulators of fat metabolism
- Comparison of nutritional value of the wild and cultivated spiny loaches at three growth stages
- Transcriptomic analysis of liver immune response in Chinese spiny frog (Quasipaa spinosa) infected with Proteus mirabilis
- Disruption of BCAA degradation is a critical characteristic of diabetic cardiomyopathy revealed by integrated transcriptome and metabolome analysis
- Plant Sciences
- Effect of long-term in-row branch covering on soil microorganisms in pear orchards
- Photosynthetic physiological characteristics, growth performance, and element concentrations reveal the calcicole–calcifuge behaviors of three Camellia species
- Transcriptome analysis reveals the mechanism of NaHCO3 promoting tobacco leaf maturation
- Bioinformatics, expression analysis, and functional verification of allene oxide synthase gene HvnAOS1 and HvnAOS2 in qingke
- Water, nitrogen, and phosphorus coupling improves gray jujube fruit quality and yield
- Improving grape fruit quality through soil conditioner: Insights from RNA-seq analysis of Cabernet Sauvignon roots
- Role of Embinin in the reabsorption of nucleus pulposus in lumbar disc herniation: Promotion of nucleus pulposus neovascularization and apoptosis of nucleus pulposus cells
- Revealing the effects of amino acid, organic acid, and phytohormones on the germination of tomato seeds under salinity stress
- Combined effects of nitrogen fertilizer and biochar on the growth, yield, and quality of pepper
- Comprehensive phytochemical and toxicological analysis of Chenopodium ambrosioides (L.) fractions
- Impact of “3414” fertilization on the yield and quality of greenhouse tomatoes
- Exploring the coupling mode of water and fertilizer for improving growth, fruit quality, and yield of the pear in the arid region
- Metagenomic analysis of endophytic bacteria in seed potato (Solanum tuberosum)
- Antibacterial, antifungal, and phytochemical properties of Salsola kali ethanolic extract
- Exploring the hepatoprotective properties of citronellol: In vitro and in silico studies on ethanol-induced damage in HepG2 cells
- Enhanced osmotic dehydration of watermelon rind using honey–sucrose solutions: A study on pre-treatment efficacy and mass transfer kinetics
- Effects of exogenous 2,4-epibrassinolide on photosynthetic traits of 53 cowpea varieties under NaCl stress
- Comparative transcriptome analysis of maize (Zea mays L.) seedlings in response to copper stress
- An optimization method for measuring the stomata in cassava (Manihot esculenta Crantz) under multiple abiotic stresses
- Fosinopril inhibits Ang II-induced VSMC proliferation, phenotype transformation, migration, and oxidative stress through the TGF-β1/Smad signaling pathway
- Antioxidant and antimicrobial activities of Salsola imbricata methanolic extract and its phytochemical characterization
- Bioengineering and Biotechnology
- Absorbable calcium and phosphorus bioactive membranes promote bone marrow mesenchymal stem cells osteogenic differentiation for bone regeneration
- New advances in protein engineering for industrial applications: Key takeaways
- An overview of the production and use of Bacillus thuringiensis toxin
- Research progress of nanoparticles in diagnosis and treatment of hepatocellular carcinoma
- Bioelectrochemical biosensors for water quality assessment and wastewater monitoring
- PEI/MMNs@LNA-542 nanoparticles alleviate ICU-acquired weakness through targeted autophagy inhibition and mitochondrial protection
- Unleashing of cytotoxic effects of thymoquinone-bovine serum albumin nanoparticles on A549 lung cancer cells
- Erratum
- Erratum to “Investigating the association between dietary patterns and glycemic control among children and adolescents with T1DM”
- Erratum to “Activation of hypermethylated P2RY1 mitigates gastric cancer by promoting apoptosis and inhibiting proliferation”
- Retraction
- Retraction to “MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB”
- Retraction to “A data mining technique for detecting malignant mesothelioma cancer using multiple regression analysis”
- Special Issue on Advances in Neurodegenerative Disease Research and Treatment
- Transplantation of human neural stem cell prevents symptomatic motor behavior disability in a rat model of Parkinson’s disease
- Special Issue on Multi-omics
- Inflammasome complex genes with clinical relevance suggest potential as therapeutic targets for anti-tumor drugs in clear cell renal cell carcinoma
- Gastroesophageal varices in primary biliary cholangitis with anti-centromere antibody positivity: Early onset?

