Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
Abstract
The development of a facile approach to simultaneously detect and separate Hg(ii) ions in an aqueous solution is a challenging topic in the chemosensing field. Herein, we focus on constructing the Hg(ii)-sensitive fluorescence “turn-on”-type composite materials by using halloysite nanotube (HNT) as substrate. Two types of HNTs-based chemosensors, HNTs-PHT and HNTs-BP, were developed in this study, which exhibit Hg(ii)-sensitive fluorescence “turn on” behavior by forming interparticle and intraparticle excimers, respectively. Fortunately, HNTs-PHT is able to effectively restrict the solvent relaxation of π–π* transition and make it a better detection tool in aqueous solution than HNTs-BP. The addition of Hg(ii) can trigger a dramatical increase at 469 nm in emission curves of HNTs-PHT, which cannot exhibit emission behaviors without the addition of Hg(ii). Furthermore, the formation of interparticle excimers makes Hg(ii) serve as a crosslinker to aggregate HNTs-PHT into precipitations. Following this way, Hg(ii) ions can be facilely removed from the water via a simple filtration or centrifugation approach. The as-prepared HNTs-PHT shows high specificity and precision in simultaneously detecting and separating Hg(ii) without the recourse to energy consumption, which will give a novel insight to deal with heavy metal pollution.
1 Introduction
The exceeded hazard of metal salts, especially the heavy metal ions in water, is an increasingly serious problem in the environmental protection field around the world in recent years [1,2]. The nonbiodegradable nature, bioaccumulation, and high toxicity give rise to severe health risks for humans [3,4]. Among the various heavy transition metals, mercury (Hg) is serving as the main role in heavy metal pollution, which has been reported to be usually involved in wastewater [5]. It should be noted that Hg is highly toxic that even 10–6 M can also result in irretrievable damages to health, even death [6,7]. A prime challenge for researchers is, therefore, to develop a facile, versatile, specific and sensitive system for simultaneous detection and separation of Hg(ii) ions in water.
Up to now, some analytical methods have been built for detecting Hg(ii) ions, including localized surface plasmon resonance (LSPR) [8], high-performance liquid chromatography (HPLC) [9] and electrochemistry technique [10,11]. The fluorescence-based method shows promising advantages including naked eye visualization capability, high selectivity and facile operation when used in the detection of metal ions [12,13]. It is worth noting that planar-conjugated molecules, the main source of fluorescent chromophores, often show poor water solubility and aggregate to form π–π accumulations in water [14]. So, some unexpected phenomena usually take place when dissolving or dispersing the planar-conjugated molecules in water, e.g. aggregation-caused quenching, formation of excimers, large fluctuation in fluorescence intensity, and poor reproducibility [15,16]. At present, the use of planar-conjugated molecules as detection tools is mainly limited to organic solvents or mixing solvents. Very few cases can be used in the pure water phase, which seriously reduces their practical application level. Functionalization of the planar-conjugated molecules with hydrophilic groups can improve the water solubility but may be not effective to solve the solvent relaxation problems of π–π* transition in water. The amphipathic molecules are easy to form micelles and also contribute to the unexpected influence on fluorescence behaviors [17,18]. These problems inspired us to explore novel detection systems with good feasibility in water.
There are some studies reported in the literature regarding Hg(ii) detection and removal based on physically immobilizing organic ligands onto nanomaterials aiming to meet the application in an aqueous solution [19,20]. However, the physical immobilization usually suffers from serious stability problems. Our hypothesis is to covalently functionalize the chemosensor on the surface of nanoparticles with good water dispersion and surface charge repulsion [21], which is quite beneficial to overcome the stability problems in physically immobilized nanocomposites.
In this way, the self-aggregation behavior and solvent relaxation of the π–π* transition can be effectively suppressed. Otherwise, the formation of interparticle excimers makes the Hg(ii) ions act as crosslinking points and drive the nanoparticles into precipitation, meeting the requirement of Hg(ii) separation. Halloysite nanotube (HNT) [22,23], a natural tubular nanomaterial, was selected as the substrate for the construction of the smart tool for simultaneous detection and separation of Hg(ii) ions. Benefiting from the hollow tubular structure, HNTs exhibit lower relative density and thereby better water dispersion than other commonly used nanoparticles such as silica [24,25]. The self-aggregation behavior, usually taking place in nanoparticles, is effectively suppressed based on the external charge distribution [26]. Other favorable merits including good biocompatibility [27,28], nontoxicity [29], non-degradation [30], hydrophilic [31] and low-cost [32] also make HNTs promising materials in the field of environmental protection [33,34,35,36], biomaterial [37,38,39,40], catalyst [41,42,43], battery [44], sensor [45,46], and coating [47]. Herein, the HNTs-based smart tool for the simultaneous detection and separation of Hg(ii) ions was facilely developed by anchoring the pyrene-containing chemosensor on the surface of HNTs, which exhibit an obvious “turn on” response on fluorescence after the addition of Hg(ii) in water. Moreover, the Hg(ii)-chelated aggregation would enlarge the particle sizes and result in macroscopic precipitations, which can serve as a facile separation approach without the recourse to energy consumption. As a result, a smart tool for simultaneously detecting and separating Hg(ii) in aqueous media was developed, which may pave a new path for managing metal ions not merely Hg(ii). Further studies will focus on exploiting more HNT-based detection systems with more simplified processes.
2 Experimental
2.1 Materials
HNTs were obtained from GuangZhou Shinshi Metallurgy and Chemical Co., Ltd., and purified before use [48]. Aminated HNTs (HNTs-NH2) and acyl chloride-bearing HNTs (HNTs-COCl) were prepared according to our previous work [49,50]. 4-Bromothiophene-2-carbaldehyde (A1), hydrazine hydrate, o-nitrophenol (B1), 1,3-dibromo-2-propanol (B2), 1-pyrenealdehyde (A3) and anhydrous SnCl2 in the analytical grade were purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd. 4-(Formylphenyl)boronic acid (A5) was purchased from Soochiral Chemical Science & Technology Co., Ltd. Oxalyl chloride and KH550® were obtained from Energy-Chemical.
2.2 Synthesis and preparation
2.2.1 Synthesis of A2
4-Bromothiophene-2-carbaldehyde (A1, 1.00 g, 5.24 mmol), MgSO4 (1.26 g, 10.48 mmol) and hydrazine hydrate (1.00 mL) were dissolved in ethanol (30 mL) and heated at 80°C under N2 atmosphere for 6 h. Then, the solution was cooled to room temperature and extracted three times with dichloromethane and saturated NaCl solution. After removal of the solvent in a vacuum, the residue was chromatographically purified on silica gel eluting with dichloromethane to afford A2 as a white solid (1.05 g, 98%). 1H NMR (400 MHz, CDCl3) δ (ppm): 7.80 (s, 1H), 7.11 (s, 1H), 6.94 (s, 1H), 5.55 (s, 2H). 13C NMR (100 MHz, CDCl3) δ (ppm): 181.84, 144.04, 137.97, 132.26, 111.51. MS (ESI): m/z: calcd for C5H5BrN2S: 205.07 [M]+; found: 206.86 [M + 2H]+.
2.2.2 Synthesis of A4
Compound A2 (0.70 g, 3.41 mmol), 1-pyrenealdehyde (A3, 0.86 g, 3.75 mmol) and MgSO4 (0.82 g, 6.82 mmol) were dissolved in absolute ethanol (30 mL) and stirred at 80°C under N2 atmosphere for 15 h. Then, the solution was cooled to room temperature and extracted for three times with dichloromethane and saturated NaCl solution. After removal of the solvent, the residue was chromatographically purified on silica gel eluting with petroleum ether/dichloromethane (3:1, v/v) to afford A4 as a faint yellow solid (0.98 g, 69%). 1H NMR (400 MHz, d 6-DMSO) δ (ppm): 9.67 (s, 1H), 9.14 (d, J = 6.4 Hz, 1H), 9.04 (s, 1H), 8.69 (d, J = 5.6 Hz, 1H), 8.42–8.35 (m, 4H), 8.32 (d, J = 6.0 Hz, 1H), 8.25 (d, J = 6.0 Hz, 1H), 8.15 (t, J = 10.4 Hz, 1H), 7.96 (s, 1H), 7.75 (s, 1H). 13C NMR (100 MHz, d 6-DMSO) δ (ppm): 161.96, 155.98, 155.60, 140.41, 140.01, 135.98, 135.82, 133.57, 131.28, 130.63, 130.42, 129.73, 129.66, 129.14, 128.91, 127.91, 127.34, 127.26, 126.97, 126.72, 126.62, 125.66, 124.60, 124.11, 123.82, 110.29. MS (ESI): m/z: calcd for C22H13BrN2S: 417.32 [M]+; found: 418.72 [M+H]+.
2.2.3 Synthesis of A6
Compound A4 (0.30 g, 0.72 mmol), (4-formylphenyl)boronic acid (A5, 0.13 g, 0.86 mmol), K2CO3 (0.6 g, 4.34 mmol) and Pd(PPh3)4 (8 mg, 0.007 mmol) in H2O (4 mL) and tetrahydrofuran (THF) (30 mL) were carefully degassed. The mixture was heated by microwave reactor at 65°C and stirred under N2 atmosphere for 2.5 h. After cooling to room temperature, saturated NaCl solution and dichloromethane (2:1, v/v) were added. The organic layer was separated and then stirred with anhydrous Na2SO4. After filtration and removal of the solvent in vacuum, the residue was chromatographically purified on silica gel eluting with petroleum ether/dichloromethane (2:1, v/v) to afford A6 as a faint yellow solid (302 mg, 95%). 1H NMR (400 MHz, d 6-DMSO) δ (ppm): 10.05 (s, 1H), 9.73 (s, 1H), 9.16 (d, J = 9.6 Hz, 1H), 9.13 (s, 1H), 8.74 (d, J = 8.4 Hz, 1H), 8.42 (m, 5H), 8.33 (s, 1H), 8.27 (d, J = 8.4 Hz, 2H), 8.17 (t, J = 15.6 Hz, 1H), 8.01 (s, 4H). MS (ESI): m/z: calcd for C29H18N2OS: 442.54 [M]+; found: 442.86 [M]+; FTIR: 3,447, 3,103, 3,039, 3,955, 2,921, 2,848, 2,760, 1,918, 1,875, 1,685, 1,600, 1,308, 1,294, 1,249, 1,231, 1,225, 1,211, 1,187, 1,171, 937, 901, 849, 830, 785, 716, 681, 660, 624, 612, 547, 535, 509, 499.
2.2.4 Synthesis of B3
O-Nitrophenol (B1, 3.00 g, 21.56 mmol), 1,3-dibromo-2-propanol (B2, 1.90 g, 8.72 mmol) and KOH (3.00 g, 17.86 mmol) were dissolved in dimethyl sulfoxide (15 mL) under N2 atmosphere. The solution was heated at 90°C for 4 h. After cooling to room temperature, the solution was extracted three times with dichloromethane and saturated NaCl solution. The organic layer was collected and then stirred with anhydrous Na2SO4. After filtration and removal of the solvent in a vacuum, the residue was chromatographically purified on silica gel eluting with dichloromethane to afford B3 as a faint yellow solid (85%). 1H NMR (400 MHz, CDCl3) δ (ppm): 7.88 (d, J = 8.0 Hz, 2H), 7.56 (t, J = 16.0 Hz, 2H), 7.16 (d, J = 8.0 Hz, 2H), 7.06 (t, J = 16.0 Hz, 2H), 4.43 (m, 2H), 4.34 (m, 2H), 3.04 (br, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 151.98, 139.60, 134.73, 125.95, 121.11, 115.06, 69.56, 68.01. MS (ESI): m/z: calcd for C15H14N2O7: 334.28 [M]+; found: 335.00 [M+H]+.
2.2.5 Synthesis of B4
Compound B3 (1.50 g, 4.49 mmol) was dissolved in an ethanol/THF mixed solution (5:1, v/v) with the addition of an HCl solution containing anhydrous SnCl2. The system was carefully degassed and heated at 80°C for 8 h under a nitrogen atmosphere. After cooling to room temperature, saturated NaCl solution and dichloromethane (2:1, v/v) were added. The organic layer was separated and then stirred with anhydrous Na2SO4. After filtration and removal of the solvent, the residue was chromatographically purified on silica gel eluting with dichloromethane to afford B4 as a brown viscous liquid (1.11 g, 90%). 1H NMR (400 MHz, CDCl3) δ (ppm): 6.89–6.75 (m, 8H), 4.41 (m, 1H), 4.17 (m, 4H), 3.86 (s, 5H). 13C NMR (100 MHz, CDCl3) δ (ppm): 148.98, 135.76, 122.02, 118.99, 115.19, 111.35, 70.02, 68.89. MS (ESI): m/z: calcd for C15H18N2O3: 274.32 [M]+; found: 275.04 [M+H]+.
2.2.6 Synthesis of B5
Compound B4 (0.60 g, 2.19 mmol), 1-pyrenealdehyde (A3, 1.11 g, 4.81 mmol) and MgSO4 (3.87 g, 8.76 mmol) were dissolved in absolute ethanol (50 mL) and stirred at 80°C under N2 atmosphere for 15 h. Then, the solution was cooled to room temperature and extracted for three times with dichloromethane and saturated NaCl solution. After removal of the solvent, the residue was chromatographically purified on silica gel eluting with petroleum ether/dichloromethane (2:1, v/v) to afford B5 as a yellow solid (0.92 g, 60%). 1H NMR (400 MHz, d 6-DMSO) δ (ppm): 9.52 (s, 2H), 9.27 (d, J = 9.2 Hz, 2H), 8.64 (d, J = 8.0 Hz, 2H), 8.36 (d, J = 7.6 Hz, 2H), 8.28 (d, J = 7.6 Hz, 6H), 8.20–8.16 (m, 4H), 8.10 (t, J = 15.2 Hz, 2H), 7.28 (d, J = 9.2 Hz, 2H), 7.11–6.99 (m, 6H), 5.47 (d, J = 4.0 Hz, 1H), 4.35–4.26 (m, 5H). 13C NMR (100 MHz, d 6-DMSO) δ (ppm): 163.65, 155.25, 144.64, 134.36, 131.24, 130.24, 129.40, 129.33, 129.03, 127.86, 127.65, 127.03, 126.68, 126.35, 125.40, 124.51, 124.13, 123.77, 122.33, 121.62, 116.86, 116.24, 115.27, 72.90, 68.33. MS (ESI): m/z: calcd for C49H34N2O3: 698.82 [M]+; found: 698.49 [M]+; FTIR: 3,351, 3,044, 2,923, 2,868, 1,922, 1,801, 1,674, 1,610, 1,595, 1,579, 1,538, 1,506, 1,486, 1,448, 1,382, 1,241, 1,188, 1,111, 1,033, 898, 847, 819, 747, 712, 679, 607.
2.2.7 Preparation of HNTs-PHT
Compound A6 (80 mg, 0.18 mmol) and anhydrous MgSO4 (65 mg, 0.54 mmol) were dissolved into 20 mL of ethanol. Then, 200 mg of HNTs-NH2 was added to the solution. The mixture was refluxed for 8 h and then centrifuged to collect the residue. The residue was purified by washing with water, ethanol and dichloromethane. After drying in a vacuum, the pyrene-functionalized product HNTs-PHT was achieved as a yellow solid (160 mg).
2.2.8 Preparation of HNTs-BP
HNTs-COCl (270 mg), compound B5 (80 mg) and triethylamine (800 μL) in 5 mL of DMSO were vigorously stirred at 50°C under an N2 atmosphere. After cooling to ambient temperature, the suspension was centrifuged to collect the residue. The residue was then purified by washing with water and ethanol. After drying in a vacuum, the bispyrene-containing product HNTs-PB was achieved as a yellow solid (260 mg).
2.3 Characterizations
Transmission electron microscopy (TEM) observations and energy-dispersive spectrometer (EDS) mapping were performed on a field emission transmission electron microscope (Tecnai G2 F20 S-TWIN, FEI). Attenuated total refraction FTIR spectra were recorded on a Thermo Fisher Scientific NICOTET IS10 FTIR spectrophotometer in the region of 4,000–400 cm−1. NMR data were recorded on a WNMR spectrometer or a Bruker Advance III spectrometer (400 MHz). X-ray photoelectron spectroscopy (XPS) data were collected via a Thermo Scientific ESCALab 250Xi XPS equipped with 200 W monochromated Al Ka radiation. Thermogravimetric analysis (TGA) was performed on a Pyris1 TGA instrument under the nitrogen atmosphere. MS analyses were obtained by using a Bruker Daltonics Autoflex III. Turbidity test and fluorescence experiments were conducted on a Shimadzu UV-Vis spectrometer model UV-2550 and a Shimadzu RF-5301PC photometer, respectively.
3 Results and discussion
3.1 Synthesis and structural characterization
Two types of HNTs-based chemosensors were developed for simultaneously detecting and separating Hg(ii) ions (Scheme 1). As for Type I, HNTs-PHT was prepared by anchoring aldehyde-containing pyrene derivative (A6) on the surface of aminated HNTs (HNTs-NH2) via a typical Schiff base reaction, in which “P” represents “pyrene,” “H” represents “hydrazone” and “T” represents thiophene. The pyrene–hydrazone–thiophene moiety in obtained HNTs-PHT can serve as an ionophore to Hg(ii) ion and trigger a “turn on” fluorescence by the formation of interparticle excimer. As for the other type of HNTs-based chemosensor, HNTs-BP was synthesized by treating acyl chloride-bearing HNTs (HNTs-COCl) with bispyrene-based chemosensor (B5) via the reaction between –COCl and –OH groups, in which the “BP” is abbreviated for bispyrene. The nitrogen and oxygen atoms in the backbone of B5 moiety are able to chelate with Hg(ii) ions with the formation of intraparticle excimer. The reactants were kept in high concentrations in the above-mentioned Schiff base reaction and esterification reaction aiming to afford desirable grafting degrees. The as-obtained HNTs-PHT and HNTs-BP were carefully characterized by FTIR, TGA, solid-state NMR and XPS.
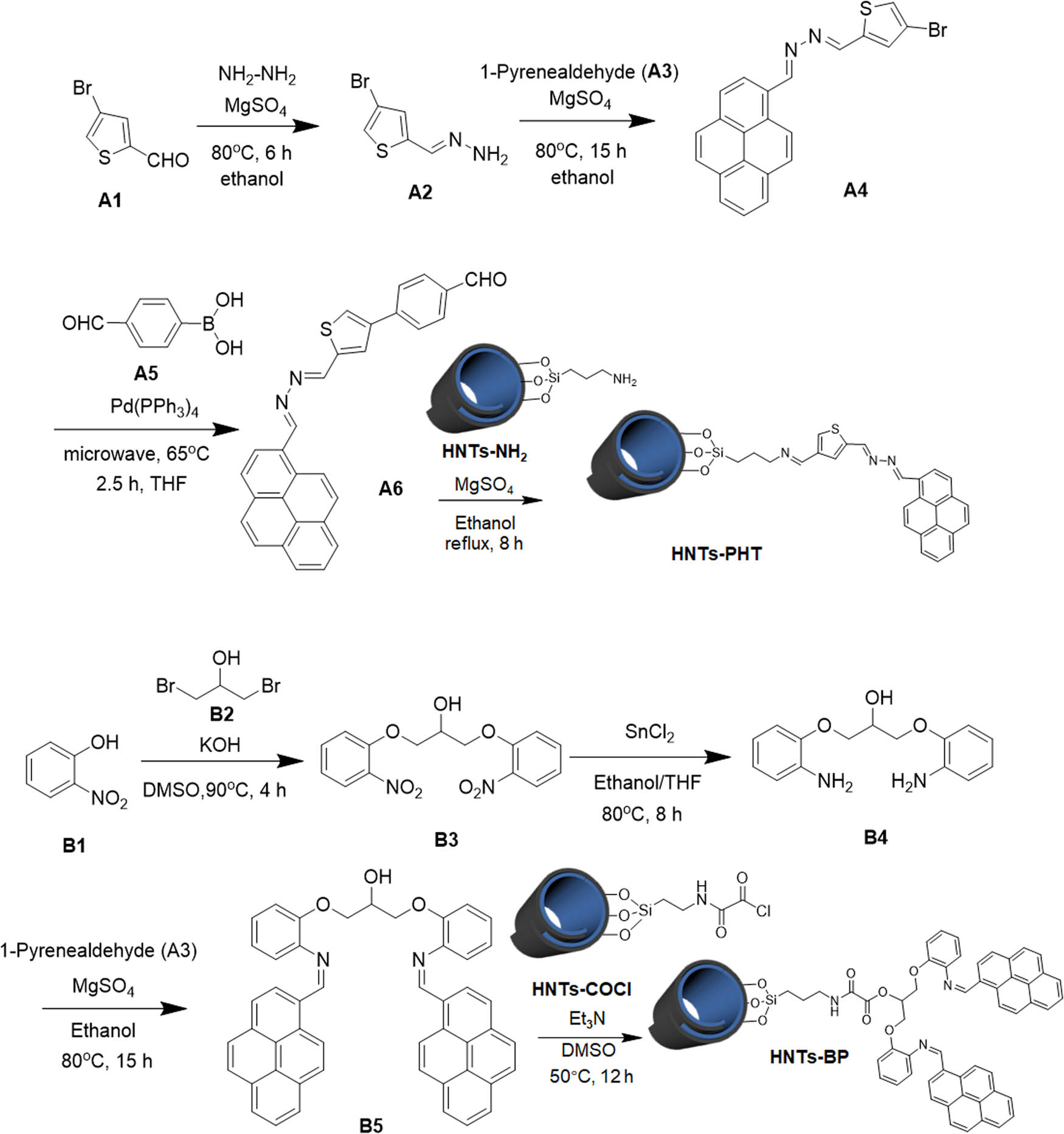
Synthetic approaches to HNTs-PHT and HNTs-BP.
The FTIR spectrum of HNTs features three distinct peaks at 3,701, 3,625 and 1,030 cm–1 in Figure S18. The stretching vibration around 1,030 cm−1 should be attributed to the in-plane Si–O–Si. Owing to the curly and multilayer character of HNTs, the Al–OH groups are categorized into two different kinds. As a result, the band in the FTIR spectrum of HNTs divides into two peaks. The peak at 3,701 cm–1 should be attributed to the stretching vibrations of Al–OH on the lumen surface, meanwhile, the peak at 3,625 cm–1 can be ascribed to the Al–OH groups inside the multilayered structure. It should be noted that the peaks assigned to Al–OH groups and Si–O–Si groups can also be detected after the amination approach, Schiff base reaction and esterification reaction, suggesting that the aluminosilicate composites did not undergo serious damage in the modification processes.
13C solid-state NMR spectra of HNTs-PHT and HNTs-BP are shown in Figure 1, in which the peaks have been completed and interpreted. Original HNTs cannot display signals in the 13C solid-state NMR spectrum, which has been evidenced in our previous study [51]. So, the peaks in HNTs-PHT and HNTs-BP should be caused by the carbons in A6 and B5 moieties, respectively. As for HNTs-PHT, the carbons in –C═N– units give the signals in high-frequency regions, e.g. 159.90 and 148.46 ppm. The strong peaks located at 131.11, 128.27 and 122.38 ppm should be assigned to the carbons in pyrene groups and partly carbons in thiophene moiety. The peaks less than 40 ppm should be attributed to the carbons in saturated alkyl chains. The 13C solid-state NMR spectrum of HNTs-BP also displays the peaks at 159.42, 153.86, 14.54, 128.75, 124.47 and 108.88 ppm, which should be assigned to carbons in unsaturated groups, including benzene unit and pyrene groups. The weak peak at 64.01 ppm is caused by the carbons in saturated units near oxygen atoms. The NMR results match well with the 13C NMR data in Figure S11.
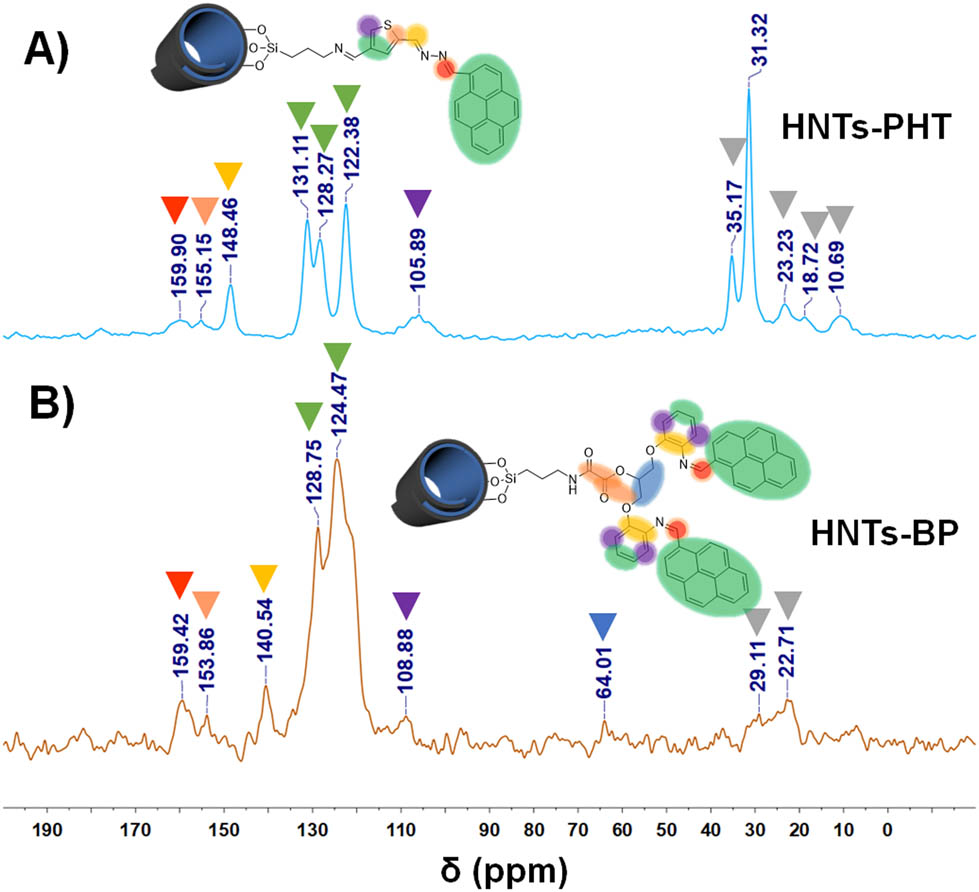
13C solid-state NMR spectra of HNTs-PHT (a) and HNTs-BP (b).
To further reveal the composition of HNTs-PHT and HNTs-BP, XPS analyses were conducted and are summarized in Figure 2. Figure 2a shows the XPS curves of HNTs, HNTs-PHT and HNTs-BP. The XPS spectrum of original HNTs shows the existence of aluminum (Al 2s and Al 2p) and silicon (Si 2s and Si 2p) in the range from 154 to 75 eV, matching well with the components in aluminosilicate clays. The strongest peak can be found at 532 eV, which should be assigned to O 1s signal. Regions from 290 to 280 eV relating to C 1s are expanded in Figure 2b. Original HNTs display a weak C 1s signal, which may be caused by the naturally curly process. HNTs-PHT and HNTs-BP show much stronger intensity for the C 1s peak at 284.8, which should be attributed to the anchoring of the chemosensors onto the HNTs. The presence of unsaturated groups contributes to the peaks around 288.5 eV.
![Figure 2
XPS studies [(a) XPS curves of HNTs, HNTs-PHT and HNTs-BP; (b) C 1s regions of HNTs-PHT and HNTs-BP; (c) N 1s regions of HNTs-PHT and HNTs-BP; and (d) S 2p regions of HNTs-PHT].](/document/doi/10.1515/ntrev-2022-0119/asset/graphic/j_ntrev-2022-0119_fig_002.jpg)
XPS studies [(a) XPS curves of HNTs, HNTs-PHT and HNTs-BP; (b) C 1s regions of HNTs-PHT and HNTs-BP; (c) N 1s regions of HNTs-PHT and HNTs-BP; and (d) S 2p regions of HNTs-PHT].
Figure 2c shows the expanded regions from 411 to 394 eV relating to N 1s regions. The N 1s signal is undetected in original HNTs. Because of the presence of nitrogen in A6 and B5 moieties, both HNTs-PHT and HNTs-BP display N 1s peaks in the XPS curves. The difference in peak value and peak pattern can be clearly found between HNTs-PHT and HNTs-BP. As for HNTs-BP, nitrogen can be found in –NH– and –N═C– groups. The presence of –C═N–N═C– gives rise to a more complex peak pattern in HNTs-PHT. Moreover, the presence of S can be evidenced by the S 2p peak at 164.1 eV in Figure 2d. The XPS observations, as well as NMR findings, are in agreement with the expected structures of HNTs-PHT and HNTs-BP. Furthermore, the grafting degrees were calculated from the TGA curves depicted in Figure 3. The grafting degrees are 6.2, 9.2 and 9.8% for HNTs-NH2, HNTs-PHT and HNTs-BP, respectively.
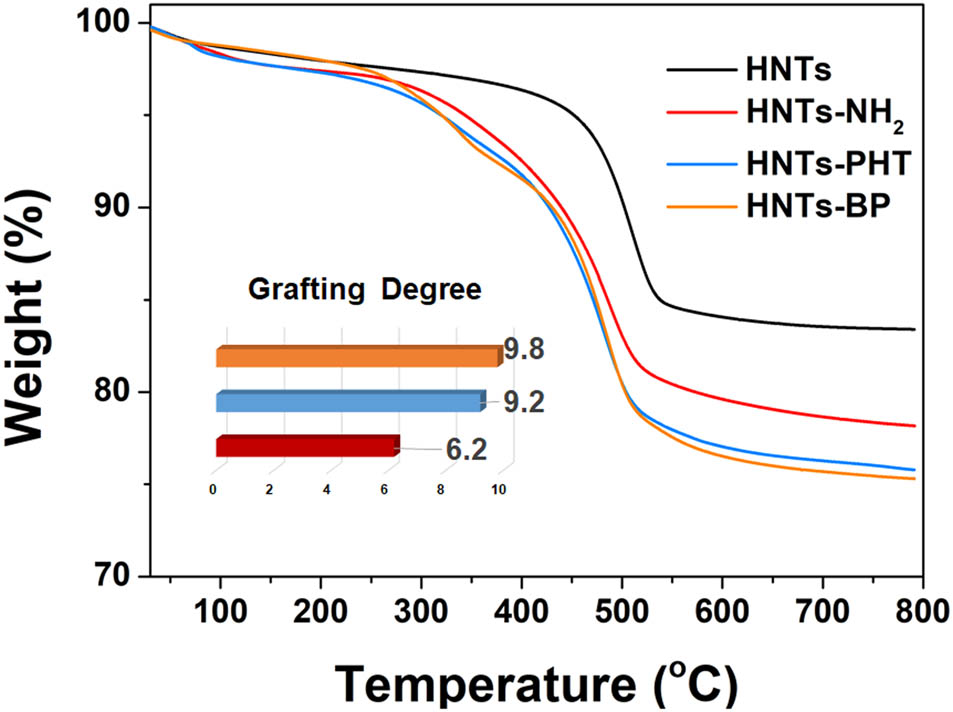
TGA curves of HNTs, HNTs-NH2, HNTs-PHT and HNTs-BP.
3.2 Optical properties and the detection properties upon Hg(ii)
The fluorescent emission behavior of HNTs-BP was evaluated in water, which displayed an unexpected emission peak at 469 nm (Figure 4a) with the fluorescence quantum yield (Φ) at 0.06. Pyrene usually displays its emission peak at ca. 430 nm, while the emission peak of 469 nm should be attributed to the solvent effect rather than the monomer emission. Generally, pyrene is very sensitive to the polarity of the solvent [52,53]. In nonpolar solvents, e.g. THF and CH2Cl2, the fluorescence signal originates from pyrene only for monomer emission. In addition to the monomer emission, the formation of intense excimer emission can take place in polar solvents, due to the solvent relaxation of the π–π* transition. Hence, the titration of HNTs-BP with polarity was conducted by preparing the stock solution of HNTs-BP in only THF and, then, varying the water concentration from 0 to 90%. HNTs-BP does not show any excimer emission in only THF solvent (Figure S20). With the gradual addition of water, the fluorescence intensity increases significantly with obvious bathochromic shifts. The results show that the intramolecular rotation of the pyrene platform is restricted, which results in the formation of excimers without recourse to Hg(ii). After the addition of Hg(ii), the fluorescence intensity at 469 nm was increased by ca. 2-fold without any bathochromic or hypsochromic shift, in which the Φ increased to 0.09. In this case, Hg(ii) can chelate with N and O atoms in HNTs-BP and force the pyrene platform to form more excimers (Figure 4b). However, the strong background fluorescent emission and the inconspicuous difference in fluorescence intensity make HNTs-BP not a desirable detection tool for Hg(ii).
![Figure 4
Study on luminescence properties. [(a) Fluorescence emission spectra of HNTs-BP with (iv) or without (iii) the addition of Hg(ii); (b) mechanism of the Hg(ii)-triggered intraparticle excimer in case of HNTs-BP; (c) fluorescence emission spectra of HNTs-PHT with (ii) or without (i) the addition of Hg(ii); and (d) mechanism of the Hg(ii)-triggered interparticle excimer in case of HNTs-PHT].](/document/doi/10.1515/ntrev-2022-0119/asset/graphic/j_ntrev-2022-0119_fig_004.jpg)
Study on luminescence properties. [(a) Fluorescence emission spectra of HNTs-BP with (iv) or without (iii) the addition of Hg(ii); (b) mechanism of the Hg(ii)-triggered intraparticle excimer in case of HNTs-BP; (c) fluorescence emission spectra of HNTs-PHT with (ii) or without (i) the addition of Hg(ii); and (d) mechanism of the Hg(ii)-triggered interparticle excimer in case of HNTs-PHT].
For the other case, when HNTs-PHT dispersed into water, non-fluorescent emission behavior can be observed (Φ < 0.001) (iii in Figure 4c). The solvent relaxation of the π–π* transition was effectively reduced. The N and S atoms in HNTs-PHT are able to chelate with Hg(ii) and force the pyrene moieties to afford interparticle excimers, which can cause a dramatical enhancement of fluorescence. Following this way, a remarkable fluorescence “turn on” behavior from nonfluorescent to strong fluorescent (iv in Figure 4c) can be found with the addition of Hg(ii) with an increased Φ value at 0.07. The remarkable fluorescence enhancement at 469 nm matches well with the proposed mechanism in Figure 4d. As a result, HNTs-PHT shows a much better detection performance in an aqueous solution than HNTs-BP.
To evaluate the detection accuracy and sensitivity of HNTs-PHT to Hg(ii), an aqueous solution with different quantities of Hg(ii) was added to HNTs-PHT aqueous suspension to reveal the relationship between fluorescence intensity and the concentration of Hg(ii). Plot of fluorescence intensity (I x ) of HNTs-BP aqueous suspension vs the concentration of Hg(ii) ([Hg(ii)]) was recorded. The fitting results indicate that the linear regression for I x and [Hg(ii)] exhibit a good linear correlation coefficient (R > 0.99). A good linearity relationship was achieved within the range from 1.0 × 10−5 to 1.0 × 10−4 M (Figure S21).
Some studies have shown that some other metal ions are also able to chelate with O, N or S atoms [54,55,56,57]. Therefore, the specificity of HNTs-PHT to Hg(ii) over commonly used ions, including Zn2+, Pb2+, Ni+, Na+, Mn2+, Mg2+, K+, Fe2+, Cu2+, Ba2+ and Al3+, was also investigated (the concentration is equal to 1 × 10−5 M, details are shown in Figure 5). Upon individual addition of a wide range of the abovementioned metal ions as their
![Figure 5
Fluorescence spectra of HNTs-PHT in aqueous solution ([c] = 1.0 mg/mL) with the addition of Hg2+, Zn2+, Pb2+, Ni+, Na+, Mn2+, Mg2+, K+, Fe2+, Cu2+, Ba2+ and Al3+ (the concentration of each ion is equal to 1 × 10−5 M).](/document/doi/10.1515/ntrev-2022-0119/asset/graphic/j_ntrev-2022-0119_fig_005.jpg)
Fluorescence spectra of HNTs-PHT in aqueous solution ([c] = 1.0 mg/mL) with the addition of Hg2+, Zn2+, Pb2+, Ni+, Na+, Mn2+, Mg2+, K+, Fe2+, Cu2+, Ba2+ and Al3+ (the concentration of each ion is equal to 1 × 10−5 M).
3.3 Absorption studies
The absorption behaviors of HNTs-based chemosensors on Hg(ii) were confirmed by TGA curves. The weight (%) at 790°C for HNTs-PHT and HNTs-BP in TGA curves is 75.8 and 75.3%, respectively. After treating with excess Hg(ClO4)2, the Hg(ii)-chelated products, HNTs-PHT-Hg(ii) and HNTs-BP-Hg(ii), were also examined by TGA. The weight (%) at 790°C for HNTs-PHT-Hg(ii) and HNTs-BP-Hg(ii) in TGA curves increased to 78.2 and 77.5%, respectively, suggesting the absorption of Hg(ii). The absorption capacity of HNTs-PHT and HNTs-BP toward Hg(ii) is calculated as ca. 110 and 95 mg/g. HNTs-PHT shows a higher absorption capacity than that of HNTs-NP. Otherwise, the dynamic adsorption behaviors of HNTs-PHT and HNTs-BP displayed distinct differences. The interaction of HNTs-based chemosensors and Hg(ii) was investigated based on a turbidimetric assay method, which is widely used to evaluate the interactions of carbohydrates with proteins in the literature [58]. Plots of absorbance at 550 nm vs the time after the addition of Hg(ii) were recorded and are shown in Figure S22. A completely different behavior can be tracked between HNTs-PHT and HNTs-BP, which should be caused by the difference in the formation between interparticle and intraparticle excimers. The intraparticle excimer cannot give rise to the changes in particle sizes. So, the chelation of HNTs-BP with Hg(ii) cannot raise any significant changes in the absorbance values. The removal efficiency of HNTs-PHT toward Hg(ii) in an aqueous solution was evaluated by adding 5.0 mg of HNTs-PHT into 1.0 mL of Hg(ii) solution with different concentrations at 1 × 10−3, 1 × 10−4 and 1 × 10−5 M. The removal efficiency follows a concertation-dependent manner, which was calculated ad 72, 85 and 91%, respectively.
As for HNTs-PHT, the formation of interparticle excimers makes Hg(ii) ions serve as crosslinkers to aggregate the particles. As a result, a dramatic increase in absorbance values can be clearly found in HNTs-PHT after the formation of interparticle excimers, suggesting the formation of precipitations. Therefore, the separation of Hg(ii) was achieved by a simple precipitation approach without power consumption, when using HNTs-PHT as the adsorbent. TEM observations were conducted to further characterize the as-obtained precipitations. Figure 6b reveals that the HNTs-PHT is the monodisperse cylindrical-shaped nanotube with an open-ended lumen. After chelating with Hg(ii), the aggregates can be clearly observed in the TEM image (Figure 6c) of HNTs-PHT-Hg(ii). The corresponding elemental maps displaced in Figure 6d–g illustrate the distribution of the detected elements in EDS analysis, including Al (blue in Figure 6d), Hg (yellow in Figure 6e), O (purple in Figure 6f) and Si (green in Figure 6g). The mixture of distribution of the abovementioned elements is shown in Figure 6h. The signals from Al, O and Si mappings show their elemental distribution in the nanotube-based aggregation, which is consistence with the chemical composition of HNTs. The presence of Hg and the highly coincided distribution with the Al, O and Si indicate that the aggregates should be caused by Hg(ii) and thereby results in the macroscopic precipitations. Moreover, the Hg(ii) ions can be dissociated from HNTs-PHT-Hg(ii) with the addition of cysteamine, which endow HNTs-PHT a potential recyclable material for detecting and separating the Hg(ii) (Figure 6i).
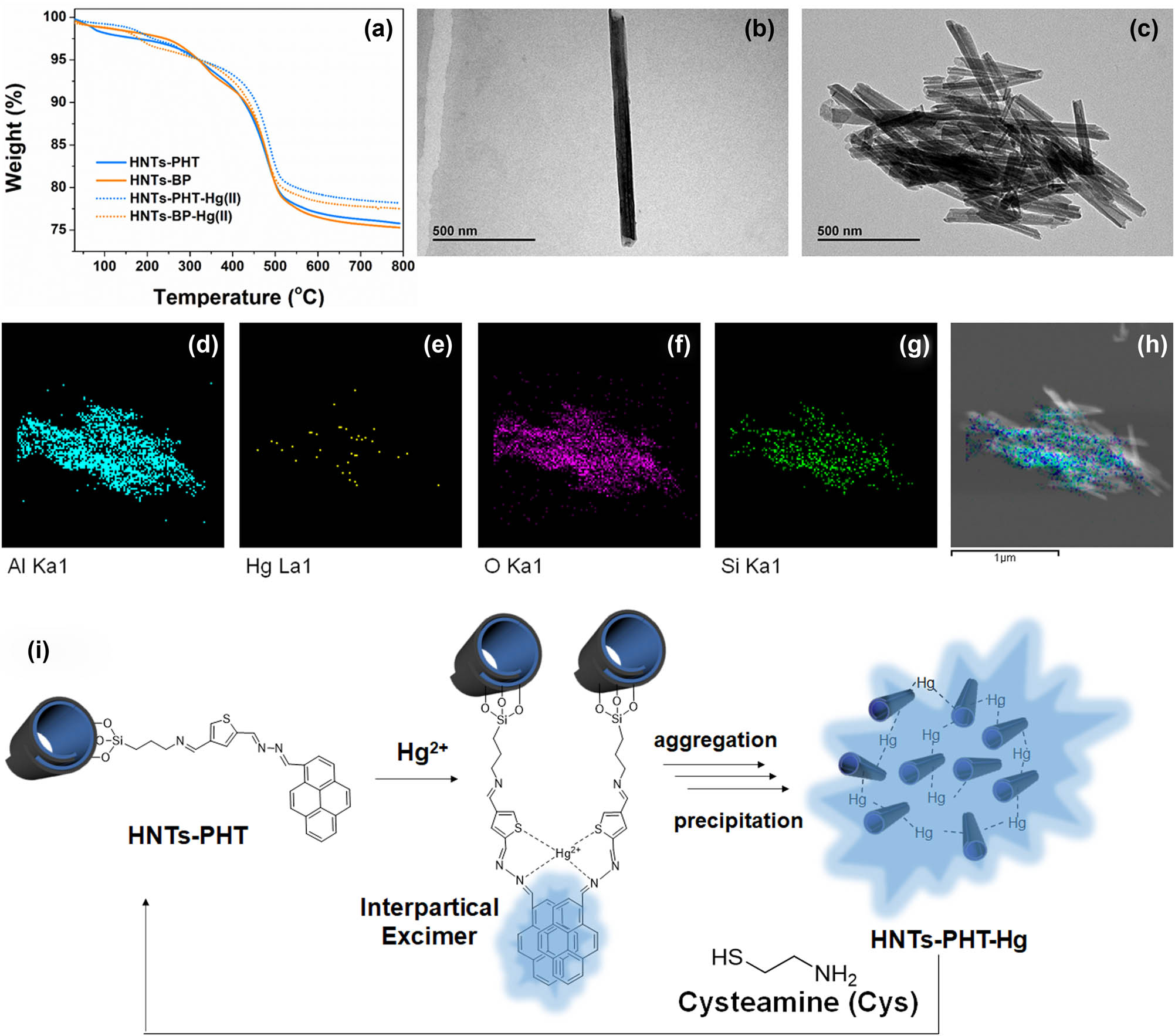
Absorption studies. [(a) TGA curves of HNTs-PHT, HNTs-BP, HNTs-PHT-Hg(ii) and HNTs-BP-Hg(ii); (b) TEM image of HNTs-PHT; (c) TEM image of HNTs-PHT-Hg(ii); (d) EDS mapping of Al; (e) EDS mapping of Hg; (f) EDS mapping of O; (g) EDS mapping of Si; (h) mixture of the above-mentioned elements; and (i) aggregation and recycling mechanism for as-obtained HNTs-PHT-Hg(ii).
4 Conclusion
We have developed two types of HNTs-based chemosensors, HNTs-PHT and HNTs-BP, for simultaneously detecting and separating Hg(ii) ions. The structural characterizations, including FTIR, solid-state NMR, XPS and TGA, demonstrated the successful modifications. The chemosensors have been covalently linked onto HNTs with good stability. Both HNTs-PHT and HNTs-BP are able to detect Hg(ii) ions via a “turn on” behavior on fluorescence, in which HNTs-PHT can effectively restrict the solvent relaxation of π–π* transition and make it a better detection tool in aqueous solution than HNTs-BP. The aqueous solution of HNTs-PHT displayed no emission behavior (Φ < 0.001), while a significant increase in emission behavior (Φ = 0.07) can be detected. HNTs-PHT shows highly specific, precise and sensitive responses to Hg(ii) in an aqueous system as compared to the literature. Moreover, HNTs-PHT exhibited a high absorption capacity upon Hg(ii) at ca. 110 mg/g, in which the Hg(ii) ions serve as crosslinking agents to aggregate HNTs-PHT into macroscopical precipitations. The established simultaneously detecting and separating approach upon Hg(ii) ions by HNTs-PHT gives a novel insight on dealing with a mercury pollution problem in water and widen the insight of environmental remediation.
NMR data, FTIR spectra, fluorescence spectra of HNTs-PB as a function of increasing water content, linear regression data and turbidity results are included in the Supporting information.
-
Funding information: This work was financially supported by the National Natural Science Foundation of China (Grant No. 22102045) and the Key Project from the Natural Science Foundation of Hebei Province (No. B2020201072).
-
Author contributions: The manuscript was written through contributions of all authors. Conceptualization: Y. Wu, X. Ba and H. Zhang; investigation and methodology: H. Fan, P. Jia and Z. Su; data curation: Y. Wu and H. Zhang; writing – original draft: H. Zhang; writing – review & editing: Y. Wu. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
Reference
[1] Peng Y, Huang H, Zhang Y, Kang C, Chen S, Song L, et al. A versatile MOF-based trap for heavy metal ion capture and dispersion. Nat Commun. 2018;9(1):187.10.1038/s41467-017-02600-2Suche in Google Scholar PubMed PubMed Central
[2] Tang Z, Qiu Z, Lu S, Shi X. Functionalized layered double hydroxide applied to heavy metal ions absorption: a review. Nanotechnol Rev. 2020;9(1):800–19.10.1515/ntrev-2020-0065Suche in Google Scholar
[3] Xu J, Liu C, Hsu P-C, Zhao J, Wu T, Tang J, et al. Remediation of heavy metal contaminated soil by asymmetrical alternating current electrochemistry. Nat Commun. 2019;10(1):2440.10.1038/s41467-019-10472-xSuche in Google Scholar PubMed PubMed Central
[4] Yuan X, Xue N, Han Z. A meta-analysis of heavy metals pollution in farmland and urban soils in China over the past 20 years. J Environ Sci. 2021;101:217–26.10.1016/j.jes.2020.08.013Suche in Google Scholar PubMed
[5] Khanam R, Kumar A, Nayak AK, Shahid M, Tripathi R, Vijayakumar S, et al. Metal(loid)s (As, Hg, Se, Pb and Cd) in paddy soil: Bioavailability and potential risk to human health. Sci Total Environ. 2020;699:134330.10.1016/j.scitotenv.2019.134330Suche in Google Scholar PubMed
[6] Cooke CA, Martinez-Cortizas A, Bindler R, Gustin MS. Environmental archives of atmospheric Hg deposition – a review. Sci Total Environ. 2020;709:134800.10.1016/j.scitotenv.2019.134800Suche in Google Scholar PubMed
[7] Dong L, Wang H, Huang Y, Zha J, Cheng H, Liu L, et al. γ-Fe2O3 decorated attapulgite composite modified with CuCl2 as magnetically separable sorbents for Hg-0 removal from coal combustion flue gas. Chem Eng J. 2021;408:127888.10.1016/j.cej.2020.127888Suche in Google Scholar
[8] Sadani K, Nag P, Mukherji S. LSPR based optical fiber sensor with chitosan capped gold nanoparticles on BSA for trace detection of Hg (II) in water, soil and food samples. Biosens Bioelectron. 2019;134:90–6.10.1016/j.bios.2019.03.046Suche in Google Scholar PubMed
[9] De Gregori I, Quiroz W, Pinochet H, Pannier F, Potin-Gautier M. Simultaneous speciation analysis of Sb(III), Sb(V) and (CH3)3SbCl2 by high performance liquid chromatography-hydride generation-atomic fluorescence spectrometry detection (HPLC-HG-AFS): application to antimony speciation in sea water. J Chromatogr A. 2005;1091(1–2):94–101.10.1016/j.chroma.2005.07.060Suche in Google Scholar PubMed
[10] Su X, Kushima A, Halliday C, Zhou J, Li J, Hatton TA. Electrochemically-mediated selective capture of heavy metal chromium and arsenic oxyanions from water. Nat Commun. 2018;9:4701.10.1038/s41467-018-07159-0Suche in Google Scholar PubMed PubMed Central
[11] Sengupta P, Pramanik K, Sarkar P. Simultaneous detection of trace Pb(II), Cd(II) and Hg(II) by anodic stripping analyses with glassy carbon electrode modified by solid phase synthesized iron-aluminate nano particles. Sens Actuators B Chem. 2021;329:129052.10.1016/j.snb.2020.129052Suche in Google Scholar
[12] Haldar U, Lee H-i. BODIPY-derived polymeric chemosensor appended with thiosemicarbazone units for the simultaneous detection and separation of Hg(II) ions in pure aqueous media. ACS Appl Mater Interfaces. 2019;11(14):13685–93.10.1021/acsami.9b00408Suche in Google Scholar PubMed
[13] Zhou L, Lin Y, Huang Z, Ren J, Qu X. Carbon nanodots as fluorescence probes for rapid, sensitive, and label-free detection of Hg2+ and biothiols in complex matrices. Chem Commun. 2012;48(8):1147–9.10.1039/C2CC16791CSuche in Google Scholar PubMed
[14] Gale PA, Caltagirone C. Anion sensing by small molecules and molecular ensembles. Chem Soc Rev. 2015;44(13):4212–27.10.1039/C4CS00179FSuche in Google Scholar PubMed
[15] Yuan W, Lu P, Chen S, Lam JWY, Wang Z, Liu Y, et al. Changing the behavior of chromophores from aggregation-caused quenching to aggregation-induced emission: development of highly efficient light emitters in the solid state. Adv Mater. 2010;22(19):2159–63.10.1002/adma.200904056Suche in Google Scholar PubMed
[16] Wang D, Tang B. Aggregation-induced emission luminogens for activity-based sensing. Acc Chem Res. 2019;52(9):2559–70.10.1021/acs.accounts.9b00305Suche in Google Scholar PubMed
[17] Aathimanikandan SV, Savariar EN, Thayumanavan S. Temperature-sensitive dendritic micelles. J Am Chem Soc. 2005;127(42):14922–29.10.1021/ja054542ySuche in Google Scholar PubMed
[18] Nakano M, Fukuda M, Kudo T, Miyazaki M, Wada Y, Matsuzaki N, et al. Static and dynamic properties of phospholipid bilayer nanodiscs. J Am Chem Soc. 2009;131(23):8308–12.10.1021/ja9017013Suche in Google Scholar PubMed
[19] Awual MR. Novel nanocomposite materials for efficient and selective mercury ions capturing from wastewater. Chem Eng J. 2017;307:456–65.10.1016/j.cej.2016.08.108Suche in Google Scholar
[20] Abbas K, Znad H, Awual MR. A ligand anchored conjugate adsorbent for effective mercury(II) detection and removal from aqueous media. Chem Eng J. 2018;334:432–43.10.1016/j.cej.2017.10.054Suche in Google Scholar
[21] Liu J, Hui D, Lau D. Two-dimensional nanomaterial-based polymer composites: fundamentals and applications. Nanotechnol Rev. 2022;11(1):770–92.10.1515/ntrev-2022-0041Suche in Google Scholar
[22] Glotov A, Vutolkina A, Pimerzin A, Vinokurov V, Lvov Y. Clay nanotube-metal core/shell catalysts for hydroprocesses. Chem Soc Rev. 2021;50(16):9240–77.10.1039/D1CS00502BSuche in Google Scholar
[23] Cheng C, Song W, Zhao Q, Zhang H. Halloysite nanotubes in polymer science: purification, characterization, modification and applications. Nanotechnol Rev. 2020;9(1):323–44.10.1515/ntrev-2020-0024Suche in Google Scholar
[24] Zhao S, Yuan Y, Yu Q, Niu B, Liao J, Guo Z, et al. A dual-surface amidoximated halloysite nanotube for high-efficiency economical uranium extraction from seawater. Angew Chem Int Ed. 2019;58(42):14979–85.10.1002/anie.201908762Suche in Google Scholar PubMed
[25] Pierchala MK, Kadumudi FB, Mehrali M, Zsurzsan T-G, Kempen PJ, Serdeczny MP, et al. Soft electronic materials with combinatorial properties generated via mussel-inspired chemistry and halloysite nanotube reinforcement. ACS Nano. 2021;15(6):9531–49.10.1021/acsnano.0c09204Suche in Google Scholar PubMed
[26] Feng X, Liu D, Yan B, Shao M, Hao Z, Yuan G, et al. Highly active PdO/Mn3O4/CeO2 nanocomposites supported on one dimensional halloysite nanotubes for photoassisted thermal catalytic methane combustion. Angew Chem Int Ed. 2021;60(34):18552–6.10.1002/anie.202107226Suche in Google Scholar PubMed
[27] Fakhrullina G, Khakimova E, Akhatova F, Lazzara G, Parisi F, Fakhrullin R. Selective antimicrobial effects of curcumin@halloysite nanoformulation: a Caenorhabditis Elegans study. ACS Appl Mater Interfaces. 2019;11(26):23050–64.10.1021/acsami.9b07499Suche in Google Scholar PubMed
[28] Luo X, Zhang J, Wu Y-P, Yang X, Kuang X-P, Li W-X, et al. Multifunctional HNT@Fe3O4@PPy@DOX nanoplatform for effective chemo-photothermal combination therapy of breast cancer with MR imaging. ACS Biomater Sci Eng. 2020;6(6):3361–74.10.1021/acsbiomaterials.9b01709Suche in Google Scholar PubMed
[29] Long Z, Wu Y-P, Gao H-Y, Zhang J, Ou X, He R-R, et al. In vitro and in vivo toxicity evaluation of halloysite nanotubes. J Mater Chem B. 2018;6(44):7204–16.10.1039/C8TB01382ASuche in Google Scholar
[30] Lisuzzo L, Cavallaro G, Milioto S, Lazzara G. Halloysite nanotubes as nanoreactors for heterogeneous micellar catalysis. J Colloid Interface Sci. 2022;608:424–34.10.1016/j.jcis.2021.09.146Suche in Google Scholar PubMed
[31] Lvov Y, Shchukin D, Mohwald H, Price R. Halloysite clay nanotubes for controlled release of protective agents. ACS Nano. 2008;2(5):814–20.10.1021/nn800259qSuche in Google Scholar PubMed
[32] Lisuzzo L, Cavallaro G, Milioto S, Lazzara G. Halloysite nanotubes filled with MgO for paper reinforcement and deacidification. Appl Clay Sci. 2021;213:106231.10.1016/j.clay.2021.106231Suche in Google Scholar
[33] Yu L, Wang H, Zhang Y, Zhang B, Liu J. Recent advances in halloysite nanotube derived composites for water treatment. Environ Sci Nano. 2016;3(1):28–44.10.1039/C5EN00149HSuche in Google Scholar
[34] Zhang Y, Wang H, Liu J, Hou J, Zhang Y. Enzyme-embedded metal-organic framework membranes on polymeric substrates for efficient CO2 capture. J Mater Chem A. 2017;5(37):19954–62.10.1039/C7TA03719HSuche in Google Scholar
[35] Zahidah KA, Kakooei S, Ismail MC, Raja PB. Halloysite nanotubes as nanocontainer for smart coating application: a review. Prog Org Coat. 2017;111:175–85.10.1016/j.porgcoat.2017.05.018Suche in Google Scholar
[36] Wei Y, Yuan P, Liu D, Losic D, Tan D, Chen F, et al. Activation of natural halloysite nanotubes by introducing lanthanum oxycarbonate nanoparticles via co-calcination for outstanding phosphate removal. Chem Commun. 2019;55(14):2110–3.10.1039/C8CC10314CSuche in Google Scholar
[37] Zhang J, Luo X, Wu Y, Wu F, Li Y, He R, et al. Rod in Tube: a novel nanoplatform for highly effective chemo-photothermal combination therapy toward breast cancer. ACS Appl Mater Interfaces. 2019;11(4):3690–703.10.1021/acsami.8b17533Suche in Google Scholar PubMed
[38] Wu S, Qiu M, Guo B, Zhang L, Lvov YM. Nanodot-loaded clay nanotubes as green and sustained radical scavengers for elastomer. ACS Sustain Chem Eng. 2017;5(2):1775–83.10.1021/acssuschemeng.6b02523Suche in Google Scholar
[39] Li L, Zhou Y, Gao R, Liu X, Du H, Zhang J, et al. Naturally occurring nanotube with surface modification as biocompatible, target-specific nanocarrier for cancer phototherapy. Biomaterials. 2019;190–191:86–96.10.1016/j.biomaterials.2018.10.046Suche in Google Scholar PubMed
[40] Fan L, Zhang J, Wang A. In situ generation of sodium alginate/hydroxyapatite/halloysite nanotubes nanocomposite hydrogel beads as drug-controlled release matrices. J Mater Chem B. 2013;1(45):6261–70.10.1039/c3tb20971gSuche in Google Scholar PubMed
[41] Ouyang J, Zhao Z, Yang H, Zhang Y, Tang A. Large-scale synthesis of sub-micro sized halloysite-composed cza with enhanced catalysis performances. Appl Clay Sci. 2018;152:221–9.10.1016/j.clay.2017.11.015Suche in Google Scholar
[42] Sadjadi S, Lazzara G, Malmir M, Heravi MM. Pd nanoparticles immobilized on the poly-dopamine decorated halloysite nanotubes hybridized with N-doped porous carbon monolayer: a versatile catalyst for promoting Pd catalyzed reactions. J Catal. 2018;366:245–57.10.1016/j.jcat.2018.08.013Suche in Google Scholar
[43] Sidorenko AY, Kravtsova AV, Aho A, Heinmaa I, Warna J, Pazniak H, et al. Highly selective Prins reaction over acid-modified halloysite nanotubes for synthesis of isopulegol-derived 2H-chromene compounds. J Catal. 2019;374:360–77.10.1016/j.jcat.2019.05.009Suche in Google Scholar
[44] Cao X, Sun Y, Sun Y, Xie D, Li H, Liu M. Conductive halloysite clay nanotubes for high performance sodium ion battery cathode. Appl Clay Sci. 2021;213:106265.10.1016/j.clay.2021.106265Suche in Google Scholar
[45] Ghanei-Motlagh M, Taher MA. A novel electrochemical sensor based on silver/halloysite nanotube/molybdenum disulfide nanocomposite for efficient nitrite sensing. Biosens Bioelectron. 2018;109:279–85.10.1016/j.bios.2018.02.057Suche in Google Scholar PubMed
[46] Shao L, Wang X, Yang B, Wang Q, Tian Q, Ji Z, et al. A highly sensitive ascorbic acid sensor based on hierarchical polyaniline coated halloysite nanotubes prepared by electrophoretic deposition. Electrochim Acta. 2017;255:286–97.10.1016/j.electacta.2017.09.178Suche in Google Scholar
[47] Feng Y, Luo X, Wu F, Liu H, Liang E, He R, et al. Systematic studies on blood coagulation mechanisms of halloysite nanotubes-coated PET dressing as superior topical hemostatic agent. Chem Eng J. 2022;428:132049.10.1016/j.cej.2021.132049Suche in Google Scholar
[48] Cheng C, Gao Y, Song W, Zhao Q, Zhang H, Zhang H. Halloysite nanotube-based H2O2-responsive drug delivery system with a turn on effect on fluorescence for real-time monitoring. Chem Eng J. 2020;380:122474.10.1016/j.cej.2019.122474Suche in Google Scholar
[49] Zhao Q, Jiang H, Tang B, Wu Y, Ba X, Zhang H. Chemosensor-anchored halloysite nanotubes for detection and removal of hypochlorite in water. ACS Appl Nano Mater. 2021;4(7):7435–42.10.1021/acsanm.1c01432Suche in Google Scholar
[50] Su Z, Zhang H, Gao Y, Huo L, Wu Y, Ba X. Coumarin-anchored halloysite nanotubes for highly selective detection and removal of Zn(II). Chem Eng J. 2020;393:124695.10.1016/j.cej.2020.124695Suche in Google Scholar
[51] Liu F, Bai L, Zhang H, Song H, Hu L, Wu Y, et al. Smart H2O2-responsive drug delivery system made by halloysite nanotubes and carbohydrate polymers. ACS Appl Mater Interfaces. 2017;9(37):31626–33.10.1021/acsami.7b10867Suche in Google Scholar PubMed
[52] Sarkar S, Roy S, Saha RN, Panja SS. Thiophene appended dual fluorescent sensor for detection of Hg2+ and cysteamine. J Fluoresc. 2018;28(1):427–37.10.1007/s10895-017-2204-1Suche in Google Scholar PubMed
[53] Zhou Y, Zhu C, Gao X, You X, Yao C. Hg2+-selective ratiometric and “Off-On” chemosensor based on the azadiene-pyrene derivative. Org Lett. 2010;12(11):2566–9.10.1021/ol1007636Suche in Google Scholar PubMed
[54] Awual MR, Hasan MM. A ligand based innovative composite material for selective lead(II) capturing from wastewater. J Mol Liq. 2019;294:111679.10.1016/j.molliq.2019.111679Suche in Google Scholar
[55] Islam MA, Angove MJ, Morton DW, Pramanik BK, Awual MR. A mechanistic approach of chromium (VI) adsorption onto manganese oxides and boehmite. J Environ Chem Eng. 2020;8(2):103515.10.1016/j.jece.2019.103515Suche in Google Scholar
[56] Awual MR. An efficient composite material for selective lead(II) monitoring and removal from wastewater. J Environ Chem Eng. 2019;7(3):103087.10.1016/j.jece.2019.103087Suche in Google Scholar
[57] Awual MR. Novel ligand functionalized composite material for efficient copper(II) capturing from wastewater sample. Compos Part B Eng. 2019;172:387–96.10.1016/j.compositesb.2019.05.103Suche in Google Scholar
[58] Chen Y, Chen G, Stenzel MH. Synthesis and lectin recognition of glyco star polymers prepared by “clicking” thiocarbohydrates onto a reactive scaffold. Macromolecules. 2010;43(19):8109–14.10.1021/ma100919xSuche in Google Scholar
© 2022 Haiyun Fan et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption

