Abstract
Capturing and detecting Fe3+ ions in aqueous solution is of great significance in biological systems as well as the water treatment industry. Herein, pyrene-modified cellulose nanocrystal (CNC-1-Pyr) acting as a fluorescent probe was prepared by a one-step esterification reaction, which shows geometry relaxation under UV-light excitation. Experiments and density functional theory-based simulations revealed that the structural geometry relaxation is controlled by the electron excitation and fluorescence emission. The S1 state of CNC-1-Pyr provides a conformation match for coordination with Fe3+ under the excitation of UV light, facilitating the detecting and capturing of Fe3+ efficiently.
1 Introduction
Iron, as an irreplaceable role, has long been widely distributed in natural environment and organisms [1,2]. It is of importance to achieve highly sensitive and specific detection of Fe3+, which is closely associated with some key issues such as product quality and sewage discharge. Various techniques involving voltammetric determination [3], inductively coupled plasma-mass spectrometry [4], atomic absorption spectrometry [5], and chemical probes [6–9] have been employed for detecting Fe3+. Among these strategies, the fluorescent probes gain considerable attention because of its advantages in superior selectivity, high sensitivity, and rapid response [10,11]. Once contacting with target ions, the designed probes show phenomena such as isomer tautomerism, electron charge transfer [12], and formation or fracture of dynamic chemical bonds [13], which lead to the change of fluorescence spectrum [14–17]. Therefore, it is available for identifying the target ions, indicating a device-independent method for the qualitative detection of Fe3+.
Pyrene and its derivatives have been proved to be superior candidates for designing fluorescent probes [18]. Benefiting from their intrinsic structures of polycyclic aromatic hydrocarbons, these probes have advantages in high quantum yield and long fluorescence lifetime [19–22]. Moreover, the emitted fluorescence locates in the visible bands, facilitating the observation of sensing signals. However, the aromatic structure of pyrene results in poor solubility in aqueous solution. These aggregated pyrene molecules lost the photoluminescence ability, known as the aggregation caused fluorescence quenching [23]. Thus, pyrene and its derivatives are always chemically modified for achieving applications as fluorescent probes. For example, pyrene was grafted onto cellulose nanocrystals (CNCs) to achieve sensitive detection for distinguishing Fe3+ [24]. Actually, it is a two-stage detection process, including capturing Fe3+ and subsequent fluorescence quenching. The latter has been empirically attributed to electron transfer-induced quenching and few discussions talking about the former.
In this study, we synthesized CNC-1-Pyr as a fluorescent probe by one-step esterification. Considering the fact that light-excited molecules always undergo geometry relaxations, we speculated that the excited-state geometry relaxation of CNC-1-Pyr from S0 to S1 state may affect their efficiency in capturing Fe3+. A series of experiments and simulations were performed to investigate the geometry relaxation under UV-light excitation, further exploring their influence on capturing and detecting Fe3+. We believe that this will bring to light the potential mechanisms responsible for the special complex of excited fluorescent molecules with ions, such as Fe3+.
2 Experimental methods
2.1 Materials
CNCs were purchased from Science-K Nanotechnology Co., Ltd. 1-Pyrene carboxylic acid (1-Pyr) was purchased from Macklin (Shanghai, China). N,N-Dimethylformamide (DMF) and kinds of metal salts (including LiCl, NaCl, KCl, NiCl2, NH4Cl, CoCl2·6H2O, BaCl2, ZnCl2, FeCl2·4H2O, FeCl3·6H2O, CuCl2·2H2O, MnCl2·4H2O, SnCl2, Mg(NO3)2·6H2O, Pb(NO3)2, CaCl2, AlSO4·18H2O, and K2Cr2O7) were supplied by Chengdu Kelong Chemical Reagent Co. (Chengdu, China). N,N′-Dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP) were provided by Aladdin (Shanghai, China). All the reagents and solvents were of analytical grades and used as received.
2.2 Synthesis of pyrene-modified CNCs
The pyrene-modified CNCs were prepared by a one-step esterification reaction between 1-Pyr and CNCs. Generally, CNCs (1 g) and 1-Pyr (0.09 g) were added into anhydrous DMF (80 mL) with ultrasonication to achieve a homogeneous suspension. Then, 0.53 g of DCC and 1.21 g of DMAP were added to the suspension. It was heated to 155°C and kept for 2 h with continuous magnetic stirring. All these processes were performed avoiding light. After the reaction, the suspension was cooled down to room temperature. The precipitates were separated by vacuum filtration and then washed with DMF and water three times to remove the unreacted 1-Pyr. The obtained products were dispersed into water and dialyzed against water for 1 week. Finally, they were centrifuged concentrated (8,000 rpm, 10 min) followed by freeze-drying to obtain white powder referred to as CNC-1-Pyr.
2.3 Characterizations
The morphologies of raw material CNCs and CNC-1-Pyr were observed by transmission scanning electron microscope (TEM; Thermo scientific, USA). Fourier transform infrared (FT-IR; Bruker, Germany) spectra were collected on a Tensor II, with a spectral resolution of 2 cm−1 in the range of 4,000–400 cm−1. The C element variation during the synthesis process was analyzed by X-ray photoelectron spectroscopy (XPS; Thermo scientific, USA). Raman (RENISHAW; InVia, England) spectra were recorded with a spectral resolution of 2 cm−1. The excitation wavelength used for the test was 532 nm. The crystal structures were studied by X-ray diffraction (XRD; Empyrean, Netherlands) with Cu Kα radiation. The UV-visible absorbance spectra and fluorescence emission spectra were collected from U-3310 (UV-Vis, SHIMADZU, Japan) and F-2700 spectrometers (FLS; Hitachi, Japan), respectively.
2.4 Detection of Fe3+ aqueous solution
CNC-1-Pyr solution of 0.5 mL (0.05 mg/mL) was mixed with 0.5 mL Fe3+ aqueous solution. The Fe3+ aqueous solution was prepared by dissolving a given mass of FeCl3·6H2O into deionized water. The concentration of Fe3+ (labeled as c(Fe3+)) is in the range of 0–90,000 μmol/L. Fluorescence emission spectra of the mixed solution were characterized to investigate the relationships between fluorescence intensities and c(Fe3+). Some other kinds of metal salts including LiCl, NaCl, KCl, NiCl2, NH4Cl, CoCl2·6H2O, BaCl2, ZnCl2, FeCl2·4H2O, FeCl3·6H2O, CuCl2·2H2O, MnCl2·4H2O, SnCl2, Mg(NO3)2·6H2O, Pb(NO3)2, CaCl2, AlSO4·18H2O, K2Cr2O7, and their mixture with Fe3+ were subjected to a similar operation, for investigating the specific detection ability of CNC-1-Pyr. The limit of detection (LOD) [12] is calculated based on the three times standard deviation method. The formula for calculating LOD is described in the following equation:
where σ stands for the standard deviation of the fluorescence emission intensity of blank CNC-1-Pyr and m is the slope of the linear fits drawn by the fluorescence intensity (420 nm) as a function of Fe3+ concentration.
2.5 Computational details
The molecular models including CNC, 1-Pyr, CNC-1-Pyr, and their complexes were constructed in Gauss View 5.0. Structural optimizations of these models were first performed based on the density functional theory (DFT). The single-point calculations for the optimized models were subsequently implemented to obtain their molecular orbitals and density of states (DOS). Their electron excitation was calculated based on time-dependent-DFT (TD-DFT). All simulations were implemented by Gaussian 16 program [25] using PBE1PBE exchange-correlation functional in conjunction with 6-311g** basis set. The wave functions were handled with Multiwfn 3.8 [26]. Molecular models and orbitals were rendered by Visual Molecular Dynamics software [27].
3 Results and discussion
First, the pyrene-modified CNCs were prepared, taking advantage of the abundant hydroxyl groups in the intrinsic cellulose molecule [28,29]. 1-Pyrene carboxylic acids (1-Pyr) were covalently grafted onto the surface of CNCs by a one-step esterification reaction. The grafting ratio was calculated based on the UV-Vis colorimetric method [30] (details in Supporting Information) to be 3.89 wt% (Figure S1). TEM images show that the surface grafting did not affect the original spindle-shaped morphologies of CNCs (Figure 1a and b). FT-IR spectrum of CNCs, depicted in Figure 1c, displays the typical absorption peaks of natural cellulose [31]. However, a newly emerging peak observed at around 1,670 cm−1 in the CNC-1-Pyr spectrum (Figure 1d), when de-convoluted, shows the presence of C═O stretching vibration from the ester bonds. Raman shift at around 1650 cm−1 (Figure 1e) was also observed in the spectrum of CNC-1-Pyr, which is induced by the conjugated benzene rings in 1-Pyr [32]. Moreover, explicit blue shifts are observed in Figure S2a for the –CH2– stretching vibration in the modified CNCs with respect to the original one. Moreover, the symmetrical vibration of C–H shifts from 2,851 cm−1 in the original CNCs to 2,854 cm−1 in the modified one, whereas the antisymmetric one shifts from 2,902 to 2923 cm−1. As the –CH2– group is adjacent to the formed ester bond, the presence of C═O moiety reinforced the C–H bonds in –CH2– by conjugating the π-electron clouds from pyrene, as illustrated in Figure S2b.
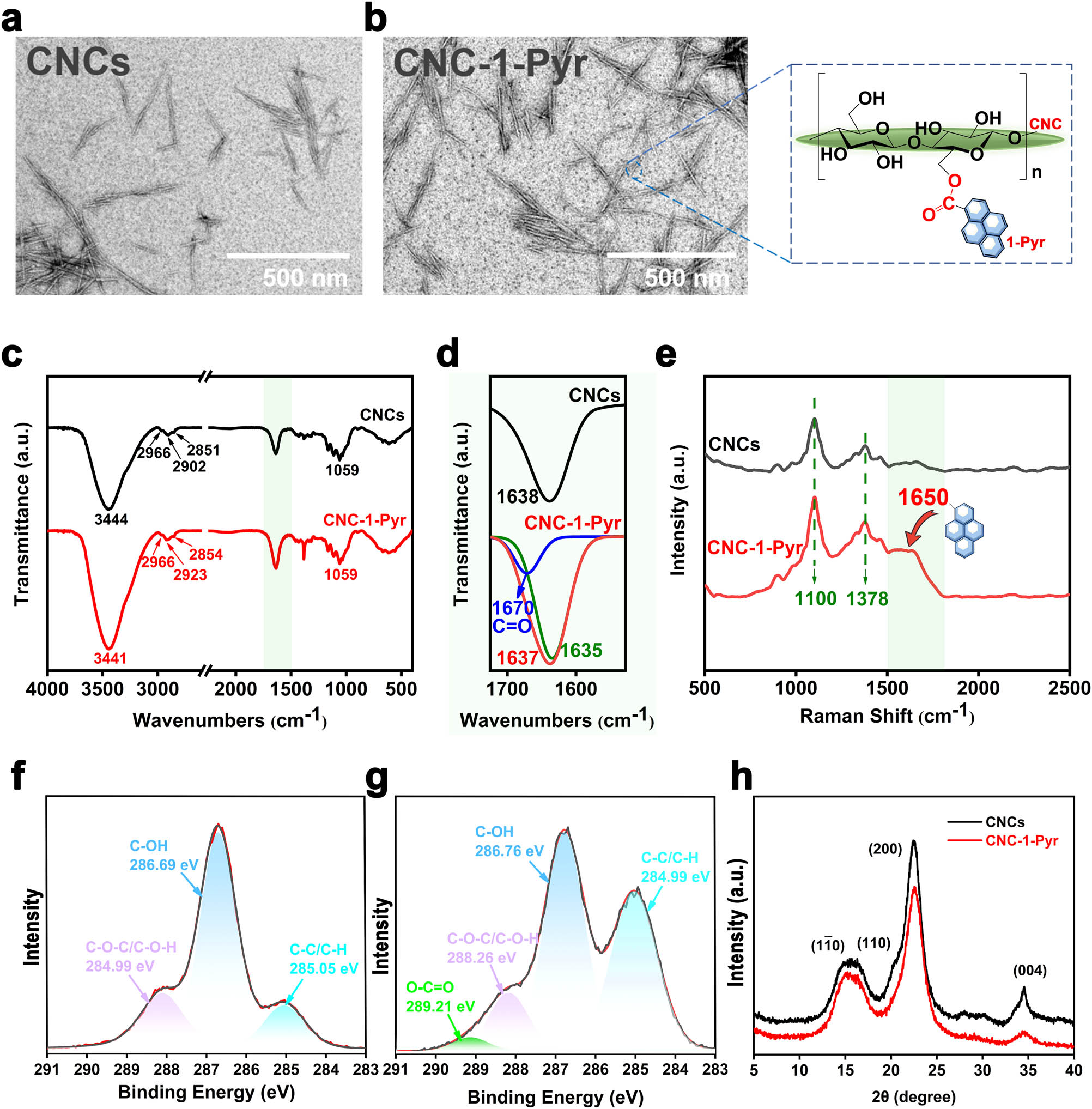
Structural characterizations of pyrene-modified CNCs: (a and b) TEM images of CNCs and CNC-1-Pyr, (c) FT-IR spectra of CNCs and CNC-1-Pyr, (d) enlarged absorption peaks in a range of 1,530–1,730 cm−1 as marked in (c), (e) Raman spectra of CNCs and CNC-1-Pyr (f and g) high-resolution XPS of C 1s in (f) CNCs, (g) CNC-1-Pyr, and (h) XRD of CNCs and CNC-1-Pyr.
XPS characterization provides additional evidence for the esterification. Compared with the raw material CNCs, emerging of a new peak located at a binding energy of 289.21 eV in CNC-1-Pyr was attributed to the C═O bond in the ester group (Figure 1f and g). The C–C/C–H area ratio of de-convoluted peaks increased from 14 to 39% after the grafting also proves the esterification grafting of 1-Pyr onto CNCs.
The crystal structure of raw material CNCs and fluorescent probe CNC-1-Pyr was characterized by XRD, as shown in Figure 1h. Pyrene-modified CNCs present a similar diffraction pattern to CNCs. The peaks located at 2θ = 15.2°, 17°, 22.5°, and 34.5° correspond to the crystal planes (1
The optical absorption and photoluminescence of pyrene-modified CNCs were then evaluated. Figure 2a and f presents the digital photos of CNCs, 1-Pyr, and CNC-1-Pyr when exposed either to natural or UV light. Their UV-vis and fluorescence emission spectra are presented in Figure 2g and h, respectively. Due to the presence of abundant hydrophilic hydroxyls and ether bonds, CNCs can be easily dispersed in aqueous medium. The transparent solution of CNCs displays hardly any excitation or fluorescence emission peak in the tested wavelength range (Figure 2g1 and h1 ). As for 1-Pyr, the three major adsorption peaks located at about 240.0, 275.5, and 341.5 nm (Figure 2g2 ) were attributed to the electronic transition around the Fermi level, as depicted by the simulation results (Figure S3). The subsequent de-excitation resulted in fluorescence emission, as detected in Figure 2h2 . However, the low solubility of 1-Pyr results in the formation of aggregates in the aqueous solution, consequently, leading to inhomogeneous fluorescent solution (Figure 2c and d). The shoulder peaks observed in UV-vis spectra may also originate from the presence of these aggregates. After grafting, the pyrene-modified CNCs still hold an excellent dispersion ability in the aqueous medium. The slightly blue shift of the electronic excitation peaks (Figure 2g3 ) occurred due to the enlarged gap (Figure S4) between the highest occupied molecular orbital and the lowest unoccupied molecular orbital. Bright and homogeneous blue fluorescence was observed from the CNC-1-Pyr solution, as displayed in Figure 2f. Furthermore, the fluorescence emission spectra shown in Figure 2h3 exhibit characteristic bands located at 387.4, 406.8, and 433.4 nm, which attributed to the fluorescent molecule 1-pyr. This is consistent with the above structural characterization results, and the esterification reaction was successfully carried out.
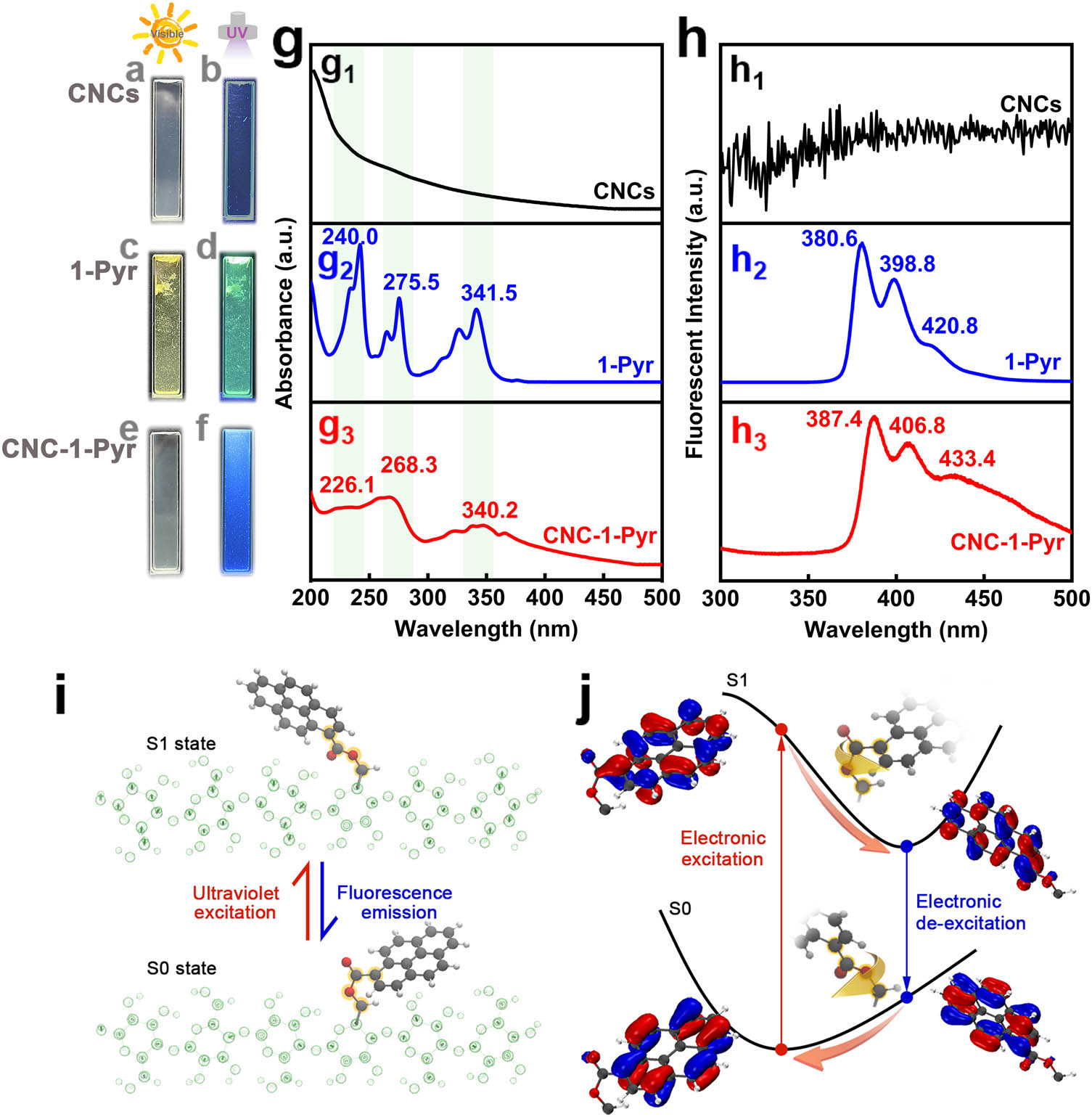
Fluorescence emission of CNCs, 1-Pyr, CNC-1-Pyr, and excited-state geometry relaxation in CNC-1-Pyr: (a–f) digital photos of (a and b) CNCs, (c and d) 1-Pyr, and (e and f) CNC-1-Pyr under (a, c, and e) natural light or (b, d, and f) UV light. (g) UV-vis spectra and (h) fluorescence emission spectra of (g1 and h1) CNCs, (g2 and h2) 1-Pyr, and (g3 and h3) CNC-1-Pyr. (i) Stable conformation of CNC-1-Pyr in S0 and S1 state. (j) Transformation of electronic and molecular structures along S0/S1 potential energy surface during excitation, relaxation, and de-excitation.
DFT-based simulations were performed to investigate the photoluminescence and quenching behavior of the interaction between CNC-1-Pyr and Fe3+. First, the molecular structures of CNC-1-Pyr in the ground state (S0 state) and the first excited state (S1 state) are presented in Figure 2i. The cellulose molecule chains shown here are rendered using “glass bubble” models. One can notice that there is a conspicuous geometry relaxation during UV-light excitation and fluorescence emission, which is dominated by the rotation of the ester bonds. It is well known that the rotation of C–C and C–O bonds in an ester bond is generally permissible. However, distinct steric hindrance may exist during the rotation process due to the existence of the large pyrene groups. Our simulation results calculated based on TD-DFT demonstrate that the rotation of ester bonds in CNC-1-Pyr is allowed thermodynamically. The rotation of dihedral angle reaches up to −174° when excited from S0 to S1 state. Furthermore, molecular orbitals around the Fermi level are presented in Figure 2j to illustrate the structural relaxation process. A local excitation dominated by π → π* transition is recognized during the UV-induced electronic excitation. The excited electron clouds diffuse around the ester bond, resulting in the geometry relaxation along with the S1 potential energy surface. The ester bond consequently rotates to achieve a low-energy conformation, driving the swing of the pyrene segment. A similar phenomenon occurs during the electronic de-excitation throughout the fluorescence emission process.
It is wondered whether metal ions can affect the fluorescence property of CNC-1-Pyr. So various kinds of metal ions including Li+, Na+, K+, Ni+,
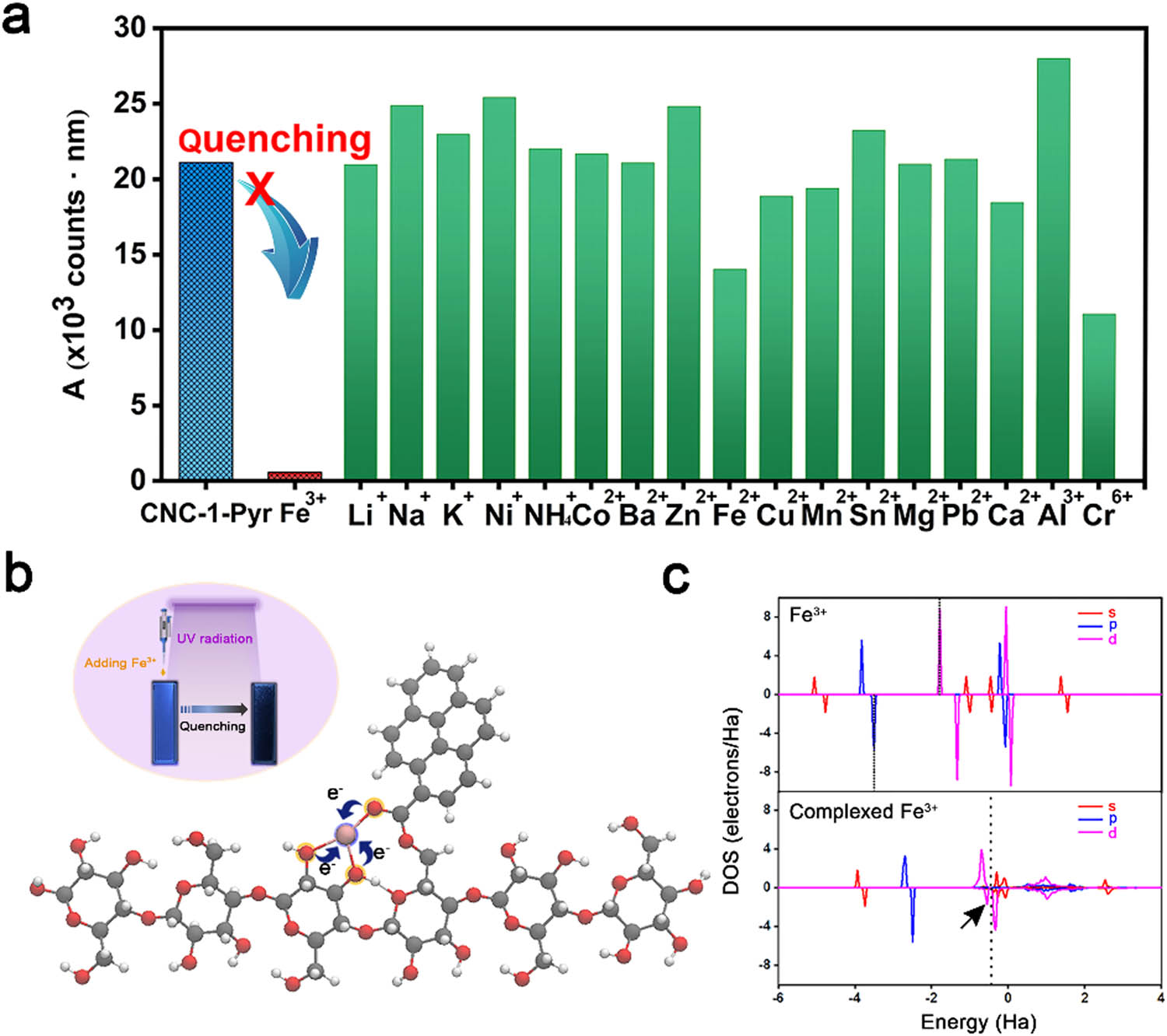
Fluorescence quenching of CNC-1-Pyr for specific detection of Fe3+: (a) integral area of emission peaks calculated from the fluorescence emission spectra, (b) stable conformation of CNC-1-Pyr after complexing with Fe3+. The inset is the digital photos of the CNC-1-Pyr solution before and after fluorescence quenching. (c) DOS of free Fe3+ and Fe3+ @CNC-1-Pyr complex. The dotted lines refer to the Fermi levels. The arrow points out the captured electron from adjacent O atoms.
The CNC-1-Pyr solution displays a broad linear response range from 1 to 1,000 μmol/L (Figure 4b) for quantitative detection of Fe3+. The linear regression equation is fitting to be I 0/I = 0.0048 C + 1.0525 (R = 0.9964). Thus, the quenching constant K SV is calculated to be 4,800 mol/L and the combination ratio of CNC-1-Pyr and Fe3+ is about 1:1. In addition, the LOD is as low as 0.3374 μmol/L with a signal-to-noise ratio of 3. As compared to recently reported fluorescent probes or chemical sensors (Table S1), CNC-1-Pyr possesses a large detection range and relatively high detection sensitivity, indicating its application potential for specific detection Fe3+ in the aqueous solution.
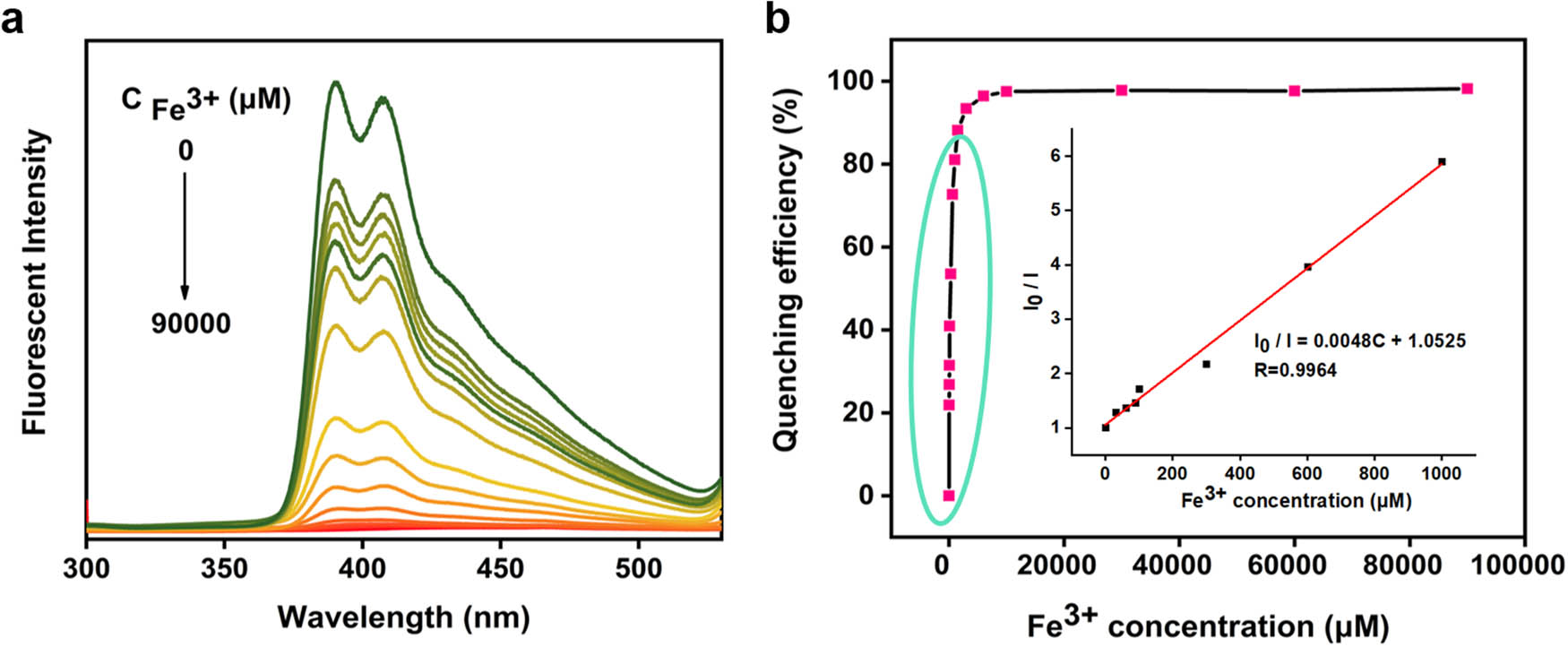
Linear range and the LOD of CNC-1-Pyr solution for quantitative detection of Fe3+: (a) fluorescence emission spectra of CNC-1-Pyr solution mixing with Fe3+ solution and (b) calculated quenching efficiency of CNC-1-Pyr solution, the inset is Stern–Volmer plot of the quenching behavior.
To reveal the mechanism of specific quenching, the conformation of the optimized Fe3+ @CNC-1-Pyr complex is exhibited in Figure 3b. The Fe3+ is found to be triangularly anchored by the synergistic effect of the carbonyl group in the ester bond and the hydroxyl groups in the cellulose molecule. According to the DOS of free Fe3+ and Fe3+ @CNC-1-Pyr complex shown in Figure 3c, the half-full 3d orbit in Fe3+ captures an additional electron from the adjacent O atoms, as marked by the arrow, which indicates the formation of the coordination complex between CNC-1-Pyr and Fe3+. Therefore, the mechanism of identifying Fe3+ ions can be reasonably explained by the electron transfer process occurring between the excited pyrene groups and Fe3+ ions [6,7,34–36]. In addition, the higher the Fe3+ ion concentration, the greater the redox reaction between the two, and the fluorescence intensity quenching clearer consequently.
Further experiments demonstrate that there are two possible routes for the complexing of Fe3+ and CNC-1-Pyr, as shown in Figure 5. The first case, if CNC-1-Pyr is primarily excited into the S1 state by UV irradiation, and there has an immediate fluorescence quench of the mix solution (Fe3+ and CNC-1-Pyr), followed by quick precipitation of CNC-1-Pyr in the next 10 min (Figure 5b1 and 4). The red arrows in Figure 5b point out the complex precipitation. On the other hand, almost no changes can be observed after adding Fe3+ without UV irradiation (natural light), as shown in Figure 5b5 and 8. Note that the Fe3+ complexation by CNC-1-Pyr does occur under natural light because it is a thermodynamically favorable process, but this process seems to be relatively slow since there is no obvious phenomenon of precipitation even after observation continued for 10 min. The obvious distinction between the two paths is shown in time-lapse videos in the Supporting Information. The differences may originate from significant differences in molecular conformations between S0 and S1 states. We notice that the excited S1 state of CNC-1-Pyr and the complex of Fe3+ @CNC-1-Pyr are similar. The underlying mechanisms are believed to be that the excited S1 state is more likely to be complex with Fe3+ (details in Figure S7), and then forming a stable structure. As for the S0 state, however, a noteworthy structural relaxation is necessary before the possible complexing with Fe3+. Therefore, the excitation of UV light seems quite important for generating ion traps for perfectly capturing Fe3+ sensitively and specifically.
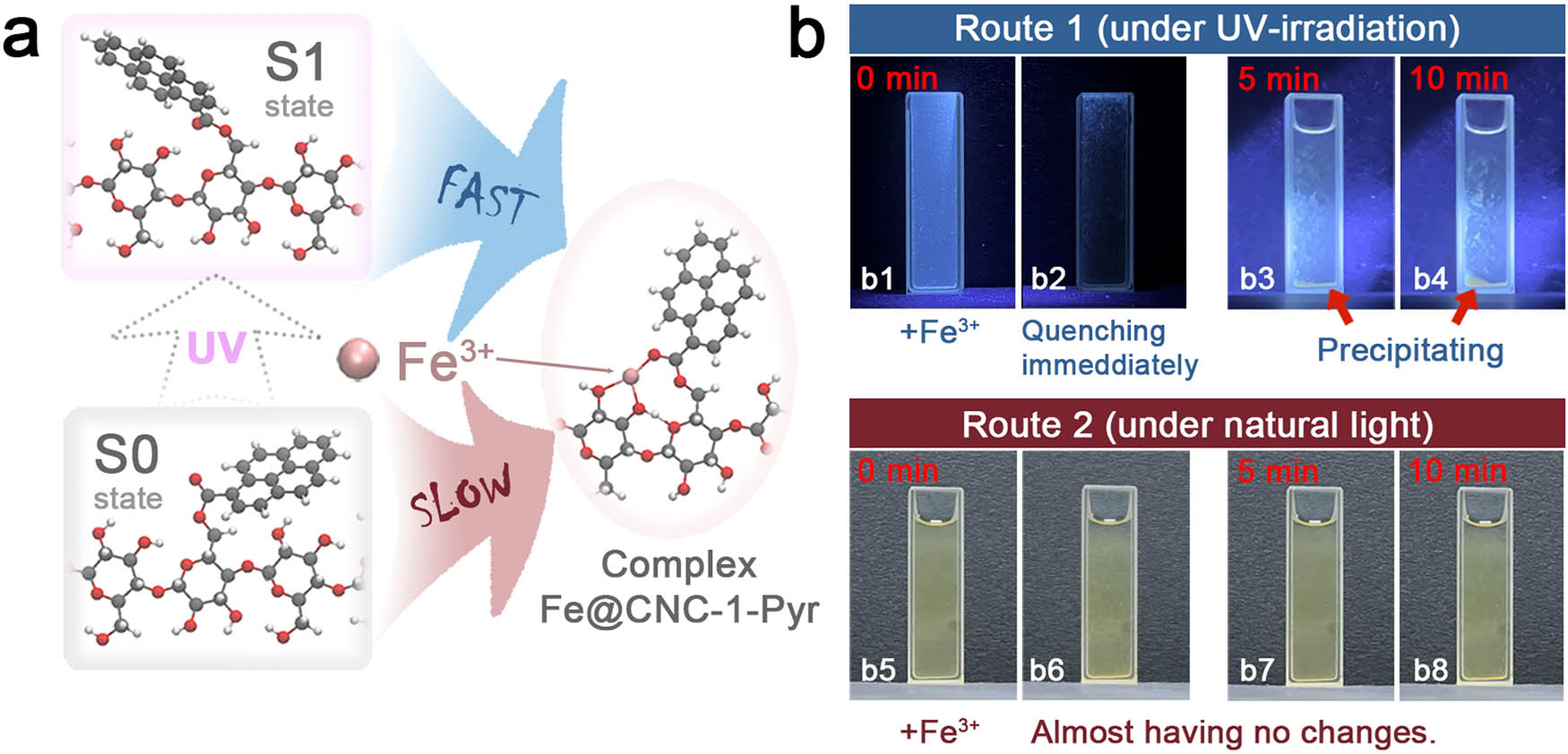
Excited-state geometry relaxation of CNC-1-Pyr for capturing and detecting Fe3+: (a) reaction path for capturing Fe3+ and (b) digital photos of CNC-1-Pyr solution mixing with Fe3+ solution under (b1–b4) UV light and (b5–b8) natural light.
4 Conclusions
In summary, pyrene-modified CNCs are prepared by one-step esterification, exhibiting potential in detecting and capturing Fe3+. In addition, the plenty of hydroxyl groups on the surface of CNC-1-Pyr make them disperse well in water. The results of simulation and experiments show that a remarkable geometry relaxation of CNC-1-Pyr based on the rotation of ester bonds occurs under UV-light excitation. The excited S1 state seems quite suitable for capturing Fe3+, leading to obvious fluorescence quench. This unique feature endows CNC-1-Pyr dual function of selectively detecting and efficiently capturing Fe3+. The LOD of Fe3+ is as low as 0.3374 μmol/L and there is good linear relationship between the maximum fluorescent intensity and the concentration of Fe3+ in the range of 1–1,000 μmol/L. Besides, this unusual phenomenon may have important implications for the further exploration of the possible photo-isomerization effect and mechanism of CNC-1-Pyr or similar structures.
Acknowledgments
The authors express their gratitude to Edit Springs (https://www.editsprings.cn/) for the expert linguistics provided.
-
Funding information: The authors acknowledge the financial support provided by the National Natural Science Foundation of China (No. 51772251) and the Science and Technology Planning Project of Sichuan Province (Nos. 2021ZYD0053, 2020YFN0150 and 2020ZDZX0008).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
[1] PhilipAisen P, ALeibold E. Iron metabolism. Curr Opin Chem Biol. 1999;3(2):200–6.10.1016/S1367-5931(99)80033-7Suche in Google Scholar
[2] Guo Q, Li L, Hou S, Yuan Z, Li C, Zhang W, et al. The role of iron in cancer progression. Front Oncol. 2021;11:778492.10.3389/fonc.2021.778492Suche in Google Scholar PubMed PubMed Central
[3] Lee S, Bong S, Ha J, Kwak M, Park S-K, Piao Y. Electrochemical deposition of bismuth on activated graphene-nafion composite for anodic stripping voltammetric determination of trace heavy metals. Sens Actuators B Chem. 2015;215:62–9.10.1016/j.snb.2015.03.032Suche in Google Scholar
[4] Aydin FA, Soylak M. Separation, preconcentration and inductively coupled plasma-mass spectrometric (ICP-MS) determination of thorium(IV), titanium(IV), iron(III), lead(II) and chromium(III) on 2-nitroso-1-naphthol impregnated MCI GEL CHP20P resin. J Haz Mat. 2010;173(1–3):669–74.10.1016/j.jhazmat.2009.08.137Suche in Google Scholar PubMed
[5] Špirić Z, Vučković I, Stafilov T, Kušan V, Frontasyeva M. Air pollution study in croatia using moss biomonitoring and ICP-AES and AAS analytical techniques. Arch Environ Con Tox. 2013;65(1):33–46.10.1007/s00244-013-9884-6Suche in Google Scholar PubMed
[6] Nawaz H, Tian W, Zhang J, Jia R, Chen Z, Zhang J. Cellulose-based sensor containing phenanthroline for the highly selective and rapid detection of Fe2+ ions with naked eye and fluorescent dual modes. ACS Appl Mater Inter. 2018;10(2):2114–21.10.1021/acsami.7b17342Suche in Google Scholar PubMed
[7] Park S-H, Kwon N, Lee J-H, Yoon J, Shin I. Synthetic ratiometric fluorescent probes for detection of ions. Chem Soc Rev. 2020;49(1):143–79.10.1039/C9CS00243JSuche in Google Scholar
[8] Rajar K, Alveroglu E. CNTs based water soluble fluorescent sensor for selective detection of Fe3+ ion. Mater Res Bull. 2020;124:110748.10.1016/j.materresbull.2019.110748Suche in Google Scholar
[9] Zhou Y, Zheng H, Kravchenko II, Valentine J. Flat optics for image differentiation. Nat Photonics. 2020;14(5):316–23.10.1038/s41566-020-0591-3Suche in Google Scholar
[10] Murugan B, Sagadevan S, Fatimah I, Oh WC, Hossain MA, Johan MR. Smart stimuli-responsive nanocarriers for the cancer therapy-nanomedicine. Nanotechnol Rev. 2021;10(1):933–53.10.1515/ntrev-2021-0067Suche in Google Scholar
[11] Visconti P, Primiceri P, Fazio Rd, Strafella L, Ficarella A, Carlucci AP. Light-induced ignition of carbon nanotubes and energetic nano-materials: a review on methods and advanced technical solutions for nanoparticles-enriched fuels combustions. Rev Adv Mater Sci. 2020;59(1):26–46.10.1515/rams-2020-0010Suche in Google Scholar
[12] Song RY, Zhang Q, Chu YL, Zhang L, Dai HQ, Wu WB. Fluorescent cellulose nanocrystals for the detection of lead ions in complete aqueous solution. Cellulose. 2019;26(18):9553–65.10.1007/s10570-019-02760-ySuche in Google Scholar
[13] Weishaupt R, Siqueira G, Schubert M, Kämpf MM, Zimmermann T, Maniura-Weber K, et al. A protein-nanocellulose paper for sensing copper ions at the nano- to micromolar level. Adv Funct Mater. 2017;27(4):1604291.10.1002/adfm.201604291Suche in Google Scholar
[14] Hou P, Wang J, Fu S, Liu L, Chen S. A new turn-on fluorescent probe with ultra-large fluorescence enhancement for detection of hydrogen polysulfides based on dual quenching strategy. Spectrochim Acta A Mol Biomol Spectrosc. 2019;213:342–6.10.1016/j.saa.2019.01.081Suche in Google Scholar PubMed
[15] Sîrbu E, Eyley S, Thielemans W. Coumarin and carbazole fluorescently modified cellulose nanocrystals using a one-step esterification procedure. Can J Chem Eng. 2016;94(11):2186–94.10.1002/cjce.22624Suche in Google Scholar
[16] Tian H, Dai Y, Fu W, Liu H, Li M, Lv M, et al. Dansyl-modified carbon dots with dual-emission for pH sensing, Fe3+ ion detection and fluorescent ink. RSC Adv. 2020;10(61):36971–9.10.1039/D0RA06097FSuche in Google Scholar
[17] Tian W, Zhang J, Yu J, Wu J, Nawaz H, Zhang J, et al. Cellulose-based solid fluorescent materials. Adv Opt Mater. 2016;4(12):2044–50.10.1002/adom.201600500Suche in Google Scholar
[18] Ayyavoo K, Velusamy P. Pyrene based materials as fluorescent probes in chemical and biological fields. N J Chem. 2021;45(25):10997–1017.10.1039/D1NJ00158BSuche in Google Scholar
[19] Usman K, Islam A, Ullah Shah SH, Javaid K, Amin A, Mustafa Z, et al. Fluorescent pyrene-imidazole material for deep-blue organic light-emitting devices. Opt Mater. 2021;121:111582.10.1016/j.optmat.2021.111582Suche in Google Scholar
[20] Bai CB, Xu P, Zhang J, Qiao R, Chen MY, Mei MY, et al. Long-wavelength fluorescent chemosensors for Hg2+ based on pyrene. ACS Omega. 2019;4(11):14621–5.10.1021/acsomega.9b02078Suche in Google Scholar PubMed PubMed Central
[21] Zhou Y, Kravchenko II, Wang H, Zheng H, Gu G, Valentine J. Multifunctional metaoptics based on bilayer metasurfaces. Light: Sci Appl. 2019;8(1):80.10.1038/s41377-019-0193-3Suche in Google Scholar
[22] Zhou Y, He X-T, Zhao F-L, Dong J-W. Proposal for achieving in-plane magnetic mirrors by silicon photonic crystals. Opt Lett. 2016;41(10):2209–12.10.1364/OL.41.002209Suche in Google Scholar
[23] Ye X, Wang HY, Yu LS, Zhou JP. Aggregation-induced emission (AIE)-labeled cellulose nanocrystals for the detection of nitrophenolic explosives in aqueous solutions. Nanomaterials. 2019;9(5):707.10.3390/nano9050707Suche in Google Scholar
[24] Zhang L, Li Q, Zhou J, Zhang L. Synthesis and photophysical behavior of pyrene-bearing cellulose nanocrystals for Fe3+ sensing. Macromol Chem Phys. 2012;213(15):1612–7.10.1002/macp.201200233Suche in Google Scholar
[25] Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, et al. Gaussian 16 Rev. C.01. Wallingford, CT; 2016.Suche in Google Scholar
[26] Lu T, Chen FW. Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem. 2012;33(5):580–92.10.1002/jcc.22885Suche in Google Scholar
[27] Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph Model. 1996;14(1):33–8.10.1016/0263-7855(96)00018-5Suche in Google Scholar
[28] Yu T, Soomro SA, Huang F, Wei W, Wang B, Zhou Z, et al. Naturally or artificially constructed nanocellulose architectures for epoxy composites: a reviews. Rev Adv Mater Sci. 2020;9(1):1643–59.10.1515/ntrev-2020-0116Suche in Google Scholar
[29] Chanda S, Bajwa DS. A review of current physical techniques for dispersion of cellulose nanomaterials in polymer matrices. Rev Adv Mater Sci. 2021;60(1):325–41.10.1515/rams-2021-0023Suche in Google Scholar
[30] Tian W, Zhang J, Yu J, Wu J, Zhang J, He J, et al. Phototunable full-color emission of cellulose-based dynamic fluorescent materials. Adv Funct Mater. 2018;28(9):1703548.10.1002/adfm.201703548Suche in Google Scholar
[31] Li W, Tan L, Fan QD, Wei W, Zhou ZW. Effect of storage time and temperature on dissolved state of cellulose in TBAH-based solvents and mechanical property of regenerated films. Rev Adv Mater Sci. 2021;60(1):466–78.10.1515/rams-2021-0034Suche in Google Scholar
[32] Zeng YL, Jiang L, Cai XY, Li Y. Identification of the characteristic vibrations for 16 PAHs based on Raman spectrum. Spectrosc Spect Anal. 2014;34(11):2999–3004.Suche in Google Scholar
[33] Thompson L, Azadmanjiri J, Nikzad M, Sbarski I, Wang J, Yu A. Cellulose nanocrystals: production, functionalization and advanced applications. Rev Adv Mater Sci. 2019;58(1):1–16.10.1515/rams-2019-0001Suche in Google Scholar
[34] Zhang YJ, Ma XZ, Gan L, Xia T, Shen J, Huang J. Fabrication of fluorescent cellulose nanocrystal via controllable chemical modification towards selective and quantitative detection of Cu(II) ion. Cellulose. 2018;25(10):5831–42.10.1007/s10570-018-1995-9Suche in Google Scholar
[35] Zhang H, Chen G, Yu B, Cong H. Emerging advanced nanomaterials for cancer photothermal therapy. Rev Adv Mater Sci. 2018;53(2):131–46.10.1515/rams-2018-0010Suche in Google Scholar
[36] Yang X, Zhang D, Ye Y, Zhao Y. Recent advances in multifunctional fluorescent probes for viscosity and analytes. Coord Chem Rev. 2022;453:214336.10.1016/j.ccr.2021.214336Suche in Google Scholar
© 2022 Ling Tan et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption

