Abstract
Resourceful beyond-graphene two-dimensional (2D) carbon crystals have been proposed/synthesized; however, the fundamental knowledge of their melting thermodynamics remains lacking. Here, the structural and thermodynamic properties of nine contemporary 2D carbon crystals upon heating are investigated using first-principle-based ReaxFF molecular dynamics simulations. Those 2D carbon crystals show distinct evolution of energetic and Lindemann index that distinguish their thermal stabilities. There are two or three critical temperatures at which structural transformation occurs for non-hexagon-contained 2D carbon allotropes. Analysis of polygons reveals that non-hexagon-contained 2D carbon crystals show thermally induced hex-graphene transitions via mechanisms such as bond rotations, dissociation, and reformation of bonds. The study provides new insights into the thermodynamics and pyrolysis chemistry of 2D carbon materials, as well as structural transitions, which is of great importance in the synthesis and application of 2D materials in high-temperature processing and environment.
1 Introduction
Carbon is a unique element because it plays a crucial role in the chemistry of living things. It has an electron configuration of 1s22s22p2, enabling to form diverse sp n (n = 1, 2, 3)-hybrid bonds. As a result, a variety of carbon allotropes form, for example, three-dimensional (3D) diamond and graphite found since prehistoric times and low-dimensional carbon buckyballs [1], carbine [2], carbon nanotubes (CNTs) [3], and graphene [4] discovered in the last decades.
Remarkably, graphene ushers a new era of two-dimensional (2D) materials. It is a quasi 2D monolayered structure of carbon atoms that has attracted particular attention due to its unique electronic properties [5], as well as its outstanding strength, elasticity, and flexibility [6]. As an example, due to the fact that rather high energy of around 5.0 eV is required to break a sp2-hybrid C–C bond of graphene [7], it exhibits excellent resistance to mechanical and thermal actions suggesting its extremely high fusing degree. This mainly stems from the arrangement of the sp2-hybrid carbon atoms in a hexagonal honeycomb structure [8]. Beyond graphene, there are other 2D materials such as 2D MoS2 [9,10,11,12,13,14,15] and 2D SiC [16] that have also attracted great attentions due to their unique mechanical properties [12,17,18], electrical conductivity [9], and thermal stability [16,19].
Recently, a large number of 2D periodic carbon allotropes consisting of diverse carbon polygons have been predicted. For instance, Terrones et al. [20] proposed three 2D carbon allotropes, named rectangular haeckelite (R-haeckelite), hexagonal haeckelite (H-haeckelite), and oblique haeckelite (O-haeckelite), respectively. The three haeckelites are composed of sp2-carbon pentagons, hexagons, and heptagons, but show different arrangements of those polygons. Wang et al. [21] designed another 2D carbon allotrope, termed as phagraphene, also composed of pentagons, hexagons, and heptagons. Mandal et al. [22] and Sharma et al. [23] predicted two 2D carbon crystals consisting of pentagons, hexagons, and octagons, termed as HOP-graphene and penta-hexoctite [21,22,23,24], respectively. In addition, two other 2D carbon crystals, named T-graphene and S-graphene comprising tetragons and octagons, have been proposed [24,25]. Recently, Zhang et al. [26] refreshed a novel 2D carbon crystal from T12-carbon, termed as penta-graphene, which consists only of pentagonal rings. Interestingly, carbon atoms in penta-graphene are sp2- or sp3-hybridized, while the abovementioned 2D carbon allotropes only contain sp2-carbon atoms.
Moreover, the properties of those 2D carbon allotropes have also been investigated. Electronically, the family of haeckelites, phagraphene, HOP-graphene, penta-hexoctite, and T-graphene exhibits metallic behavior [20,21,23], while S-graphene shows semi-metallic properties [27,28,29]. Intriguingly, unlike graphene, penta-graphene is an indirect semiconductor with a band gap of 3.25 eV [26]. Mechanically, they show high in-plane stiffness and tensile strength [20,23,30,31,32]. As an example, penta-graphene possesses in-plane Young’s modulus of around 263 GPa nm which is over two-thirds of that of graphene (345 GPa nm) [26,33]. More interestingly, penta-graphene exhibits in-plane negative Poisson’s ratio, namely auxetic behavior, originating from planar tension-induced de-wrinkling mechanism [23,26,34].
Besides, the behaviors of carbon crystals at elevated temperatures are a crucial piece of knowledge for their formation mechanisms and applications; however, they are still very limited [29,35]. As is known, the thermal characteristics of materials are greatly dominated by the atomic structural arrangements [36]. Understanding the thermodynamics and structural transition of 2D carbon crystals is critical for developing novel carbon-based platforms for emerging nanotechnological applications in hash conditions such as high-temperature processing and environments. To this end, the objective of this work is to reveal the thermal properties of the abovementioned 2D carbon allotropes (Figure 1) with distinct atomic arrangements in terms of melting and thermodynamics by first-principle-based ReaxFF molecular dynamics (MD) simulations. This study provides critical insights into the melting thermodynamics and pyrolysis chemistry of 2D carbon crystals, which sheds light on the synthesis and applications of 2D carbon allotropes and offers guidance for heat-resistant composite designs by 2D carbon crystals.
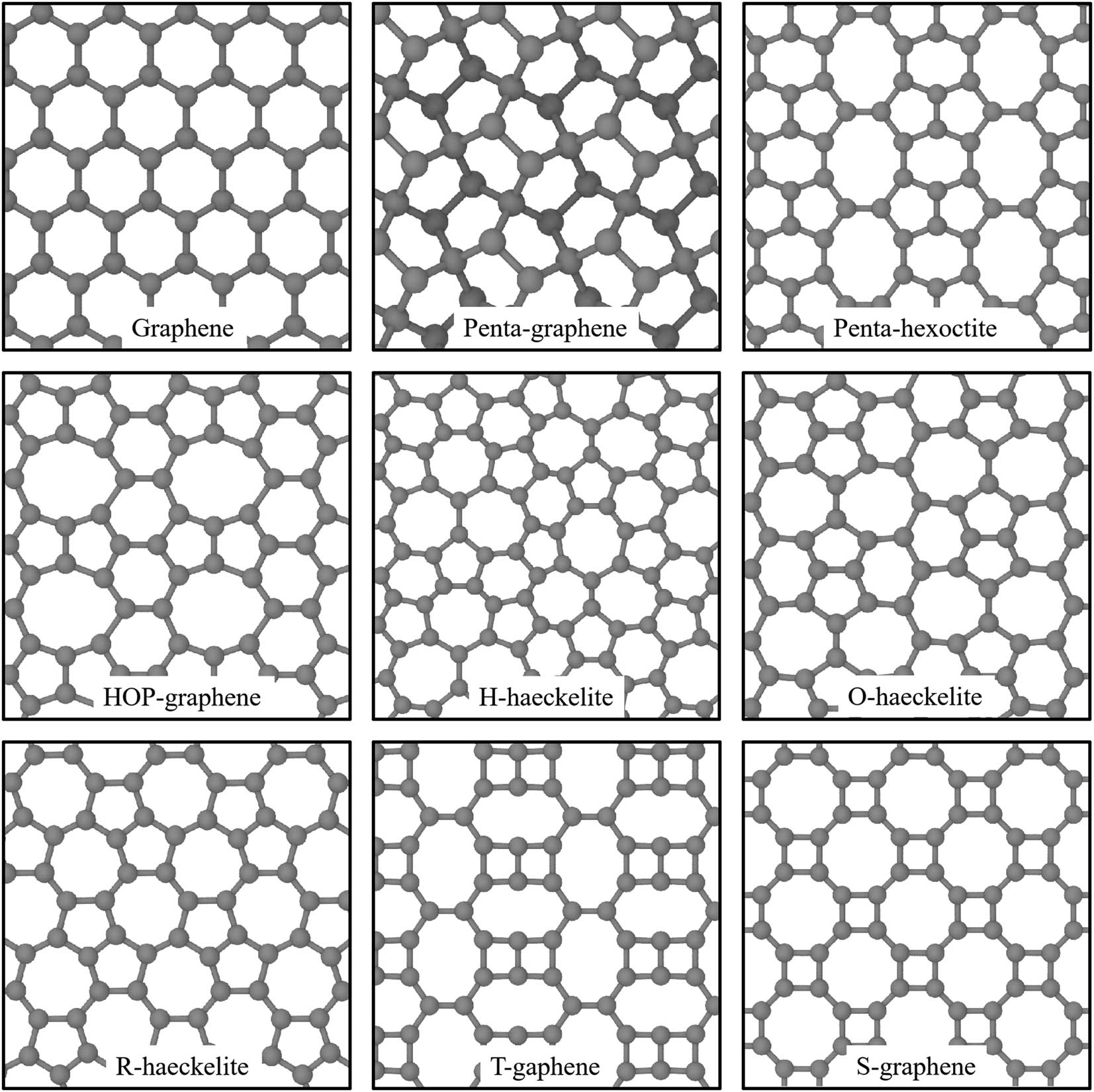
Top views of atomic models of graphene, penta-graphene, penta-hexoctite, HOP-graphene, H-haeckelite, O-haeckelite, R-haeckelite, T-graphene, and S-graphene.
2 Methodology
2.1 Forcefield
In this study, the first-principle-based ReaxFF potential [37] is used to describe the interatomic interactions of 2D carbon allotropes. This ReaxFF forcefield comprises of three potential terms, namely bond-order-dependent covalent interaction, nonbonded standard Coulomb and Morse interaction terms. As a result, such forcefield is capable of mimicking chemical reactions of simulation systems during MD calculations, such as dissociation and formation of covalent bonds. Moreover, previous studies showed that the ReaxFF potential could accurately predict the chemical and mechanical behavior of various carbon-based structures [8,38,39,40,41,42]. Here, the version of ReaxFFC-2013 potential developed by Srinivasan et al. [43] is adopted to predict the thermal properties of graphene and its allotropes. The ReaxFFC-2013 was developed on the basis of DFT data for equations of state of sp2-graphite and sp3-diamond, as well as formation energies of a variety of defects in graphene, fullerene, and amorphous carbon phases [43]. As a result, the ReaxFFC-2013 is capable of mimicking the chemistry and dynamics of carbon condensed phases, for example, thermal decompositions and C–C bond formation of hydrocarbons like graphene and fullerene. The similarity between graphene and its allotropes (penta-graphene, penta-hexoctite, HOP-graphene, H-haeckelite, O-haeckelite, R-haeckelite, T-graphene, and S-graphene) considered in this study indicates that the ReaxFFC-2013 potential should be capable of predicting their thermal and chemical properties.
2.2 MD simulations
Initially, each 2D carbon crystal is quasi-statically relaxed to a local minimum-energy configuration through the conjugate gradient method, where the convergence tolerances of energy and force are 1.0 × 10−4 kcal/mol and 1.0 × 10−4 kcal/(mol Å), respectively. Subsequently, the as-optimized sample is relaxed for 10 ps at zero pressure and temperature of 10 K under NPT (constant number of particles, constant volume, and constant temperature) ensemble. Lastly, the as-relaxed sample is gradually heated to an extremely high temperature of 7,000 K from 10 K by 7,000,000 MD steps, namely a constant heating rate of around 0.00986 K/fs. Periodic boundary conditions (PBCs) are imposed in the two planar directions of 2D carbon crystals to mimic infinite sheet, while non-PBC is applied in the off-plane direction. Such settings avoid any spurious boundary effects. The velocity–Verlet integration algorithm with a small timestep of 0.1 fs is used to integrate Newton’s motions in the MD simulations. All MD calculations are carried out using the Large-scale Atomic-Molecular Massively Parallel Simulator software package.
2.3 Lindemann index (LI)
It is well-known that the root-mean-square relative bond-length variance, termed as LI [44,45], is a reasonable and effective method to estimate the melting temperature of diverse materials, such as 3D bulk materials, nanoclusters [46], and 2D systems [47]. In this study, the distance-fluctuation of the LI is adopted to identify the melting temperature of our investigated 2D carbon crystals. For a system composed of N atoms, the local LI for the ith atom in the system is calculated as follows [48,49]:
and the average LI of the system is determined by
where
2.4 Polygon statistics
For structural characterization of 2D carbon crystals upon heating, their microstructure features of carbon polygons can be examined by exact geometric methods for capturing structural transformations. In this study, by means of the “shortest path ring” algorithm developed by Franzblau [50], a variety of carbon polygons ranging from trigon to decagon in our investigated 2D carbon structures upon heating are recognized on the assumption that the minimum and maximum carbon–carbon bond distances are 1.2 and 1.9 Å [8,38], respectively.
3 Results and discussion
3.1 Energetics
Figure 2 shows the evolution of potential energy per atom (E) of the nine 2D carbon crystals as a function of temperature (T). Apparently, all 2D carbon structures show unique E–T curves. For graphene, the curve is described by that E increases linearly at low T region, but starts to positively deviate from the linear behavior approaching T of around 5,000 K, and finally rises sharply when over around 5,600 K. For structures of penta-hexoctite, the haeckelite family, and T-graphene, they show similar nonlinear characteristics of E–T curves. Within low T regions, E increases linearly with increasing T. Upon heating at intermediate T of around 2,000–3,500 K (depending on the type of 2D carbon structures), E starts to negatively deviate the linear behavior, conflicting with the case of graphene. When T is increased to critical values, there is a turning point in E. Above which, E of H- and R-haeckelites becomes much less increased with increasing T, while penta-hexoctite, O-haeckelite, and T-graphene show a sharp decrease in the E within finite T regimes. Those indicate the occurrence of large-scale structural transformations. Thereafter, E increases nonlinearly for those structures within finite T regimes, followed by rapid increases in the E at high T. As for the 2D carbon crystal of HOP-graphene, its curve is primarily characterized by a number of turning points within the T region of around 2,400–4,600 K, implying step-by-step structural transformations. With regard to 2D carbon crystals of penta-graphene and S-graphene who possess the highest E at ground state; however, their E–T curves are highlighted by double drops of E at two critical T. This signifies the thermally induced occurrence of two obvious structural transitions in penta-graphene and S-graphene.
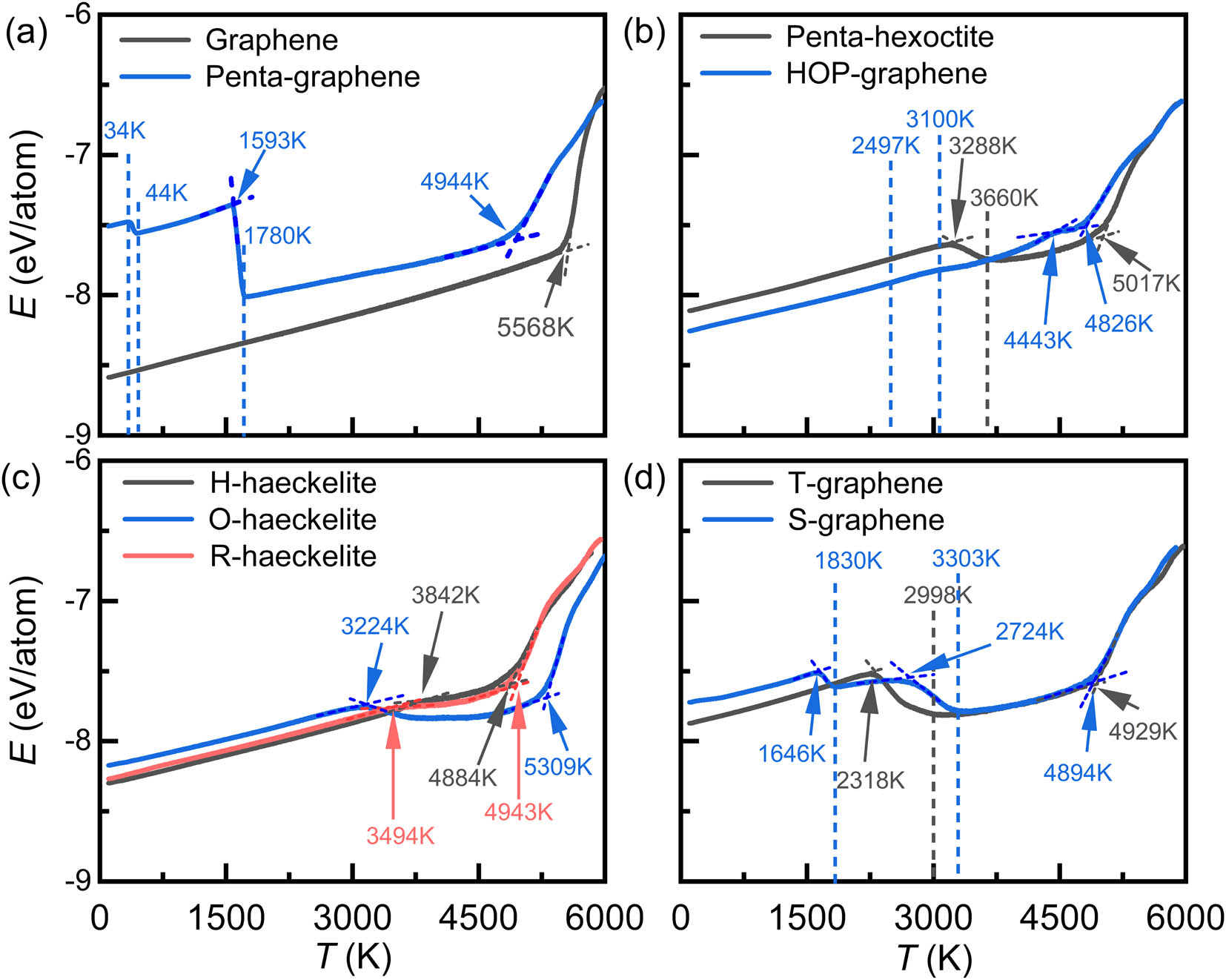
Variations in the potential energy (E) per atom with temperature for 2D carbon crystals of (a) graphene and penta-graphene; (b) penta-hexoctite and HOP-graphene; (c) H-, O-, and R-haeckelites; and (d) T-graphene and S-graphene, respectively.
3.2 LI
LI is a critical indicator in revealing the microstructural changes of materials. Figure 3 shows the average LI of the nine 2D carbon allotropes as a function of T. Based on the characteristics of LI–T curves, three groups of 2D carbon allotropes can be roughly classified. The first group can be represented by graphene, HOP-graphene and H-haeckelite. For this group, the LI–T curves are smooth throughout and are described by that the LI increases linearly with low T region, but then positively deviates from the linear behavior and eventually increases steeply. In terms of the intermediate deviation region, they are sorted as H-haeckelite > HOP-graphene > graphene. The second group consists of 2D carbon crystals of penta-hexoctite, O-haeckelite, R-haeckelite, T- and S-graphene. The LI–T curves of this group are primarily characterized by that there are two critical T at which the increase in LI becomes much more significant. This suggests that those 2D carbon crystals undergo two distinct thermally induced structural changes during the whole heating process. Remarkably, S-graphene shows fluctuation in the LI within the intermediate T region, indicating complex structural transformations. The last group is represented by a 2D carbon crystal of penta-graphene. The LI–T curve of penta-graphene is characterized by four distinct stages in the change of LI. In stage I, LI linearly decreases within the extremely low T region. In stage II, reduction in the LI becomes less pronounced. In stage III, LI suddenly increases, indicating the occurrence of large-scale structural transformations. In the last stage, LI increases nonlinearly and the increase in the LI becomes more significant with increasing T, which is similar to the whole curves of the first group.
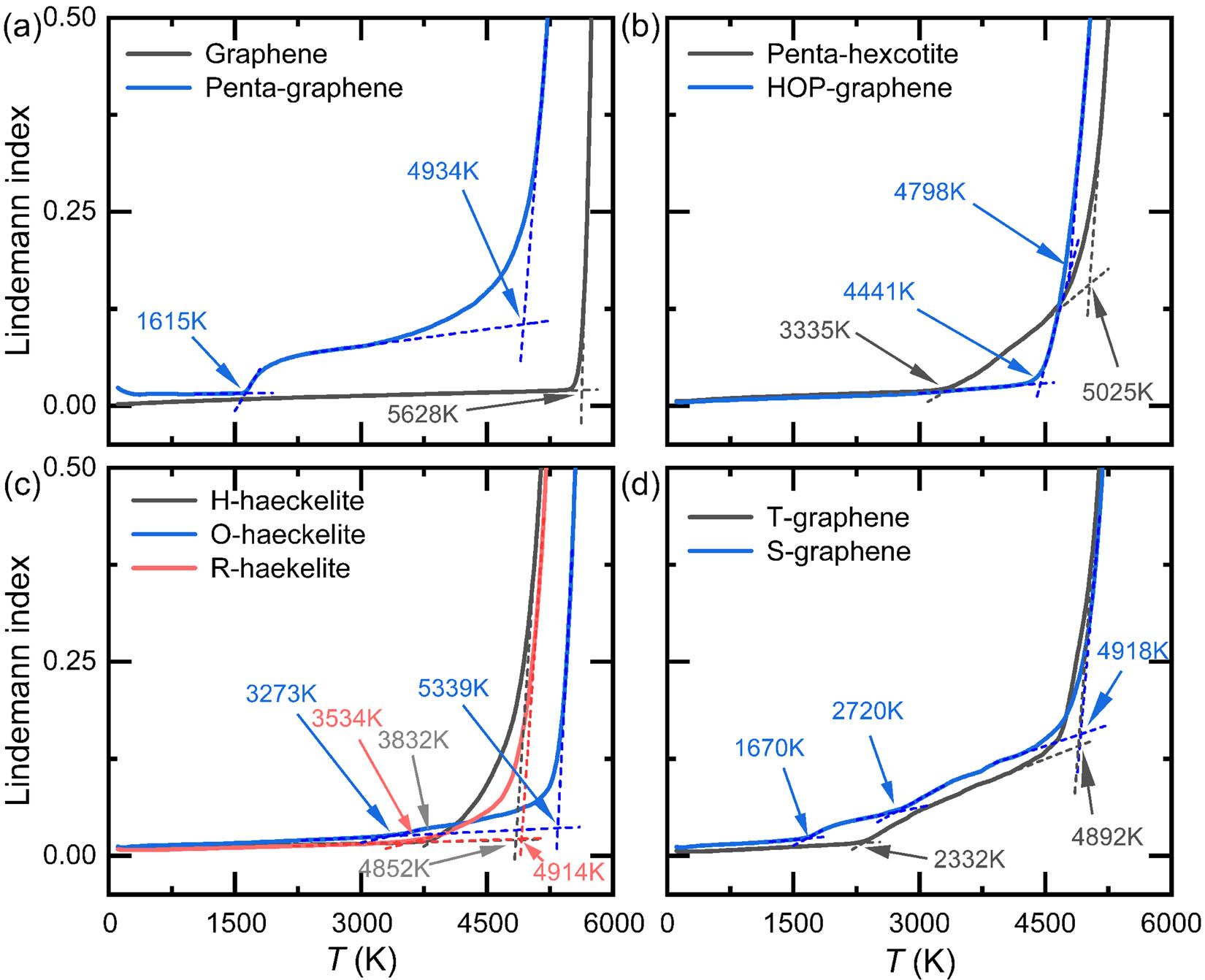
Temperature dependence of average LI for (a) graphene and penta-graphene; (b) penta-hexoctite and HOP-graphene; (c) H-, O-, and R-haeckelites; and (d) T- and S-graphene, respectively.
3.3 Critical temperatures for structural destabilizations
As a result of the unique composition and arrangement of carbon polygons in the 2D carbon crystals, 1–3 inflection points that correspond to critical temperatures (T c) can be identified from the nonlinear E–T and LI–T curves depending on the structural type. The critical temperature (T c) of the 2D carbon crystals is quantitatively determined by the intersection T of the E–T and LI–T linear-part curves, as indicated by the dash lines in Figures 2 and 3. In this study, the last T c is referred to as the melting temperature (T m) of 2D carbon crystals. Previous studies showed that the LI of nanoparticles and homopolymers at melting temperature (T m) varies around 0.03–0.05 [48]. In our study of hexagon-dominated graphene, the value of LI is found to be 0.03 at T m, similar to hexagon-dominated one-dimensional CNTs [51], indicating the accuracy of T m by LI–T curve. Table 1 lists the T c of the nine 2D carbon crystals obtained from the E–T and LI–T curves. As is seen, graphene shows only one T c (T m) of around 5,628 and 5,568 K using the LI–T and E–T curves, respectively. Our predicted T m of graphene falls in the regime determined by previous studies [52,53,54] that reported the minimum and maximum T m of around 4,510 and 7,750 K, respectively, suggesting the reliability in predicting T m of 2D carbon crystals. As for penta-hexoctite, HOP-graphene, and H-, O,- and R-haeckelite, they exhibit two T c. The first T c varies from around 2,300 to 4,500 K, and in terms of the first T c, they are ranked as HOP-graphene > H-haeckelite > R-haeckelite > penta-hexoctite > O-haeckelite > T-graphene. This implies that tetragon in 2D carbon crystals is critical to reducing thermal stability. Whereas in terms of the second T c, they are sorted as O-haeckelite > penta-hexoctite > R-haeckelite > T-graphene > H-haeckelite > HOP-graphene, with minimum/maximum T c of around 4,798/5,339 K. Apparently, H-, R-, and O-haeckelites show distinct T c, although they are composed of pentagons, hexagons, and heptagons, indicative of the critical role of arrangement of polygons in T m of 2D carbon structures. More intriguingly, penta- and S-graphene present three T c, suggesting their complex structural transformations upon heating. In a nutshell, non-hexagon-contained 2D carbon crystals show low T m than hex-graphene and complex pyrolysis chemistry.
Critical temperatures of 2D carbon crystals using LI and potential energy
| 2D carbon | T c (K) | ΔT (K) | |
|---|---|---|---|
| By Lindemann index (LI) | By potential energy (E) | ||
| Graphene | 5,628 | 5,568 | 60 |
| Penta-graphene | 34/1,615/4,934 | 35/1,593/4,944 | 1/22/−10 |
| Penta-hexoctite | 3,335/5,025 | 3,288/5,017 | 47/8 |
| HOP-graphene | 4,441/4,798 | 4,443/4,826 | −2/−28 |
| H-haeckelite | 3,832/4,852 | 3,842/4,884 | −11/−32 |
| O-haeckelite | 3,273/5,339 | 3,224/5,309 | 49/30 |
| R-haeckelite | 3,534/4,914 | 3,494/4,943 | 40/29 |
| T-graphene | 2,332/4,892 | 2,318/4,924 | 14/32 |
| S-graphene | 1,670/2,720/4,918 | 1,646/2,724/4,894 | 24/−4/24 |
3.4 Thermally induced hex-graphene transitions
To quantitively understand their thermal instability, the number of various polygons in those 2D carbon crystals subjected to the heating process is countered, as shown in Figure 4. For graphene as reference (Figure 4a), the ring number – T curve is mainly featured with initial long stable plateau and then rapid deep drop of the hexagon number at high T, accompanied by slight increases of non-hexagons. This indicates that graphene is thermally destabilized primarily by the dissociation of hexagons.
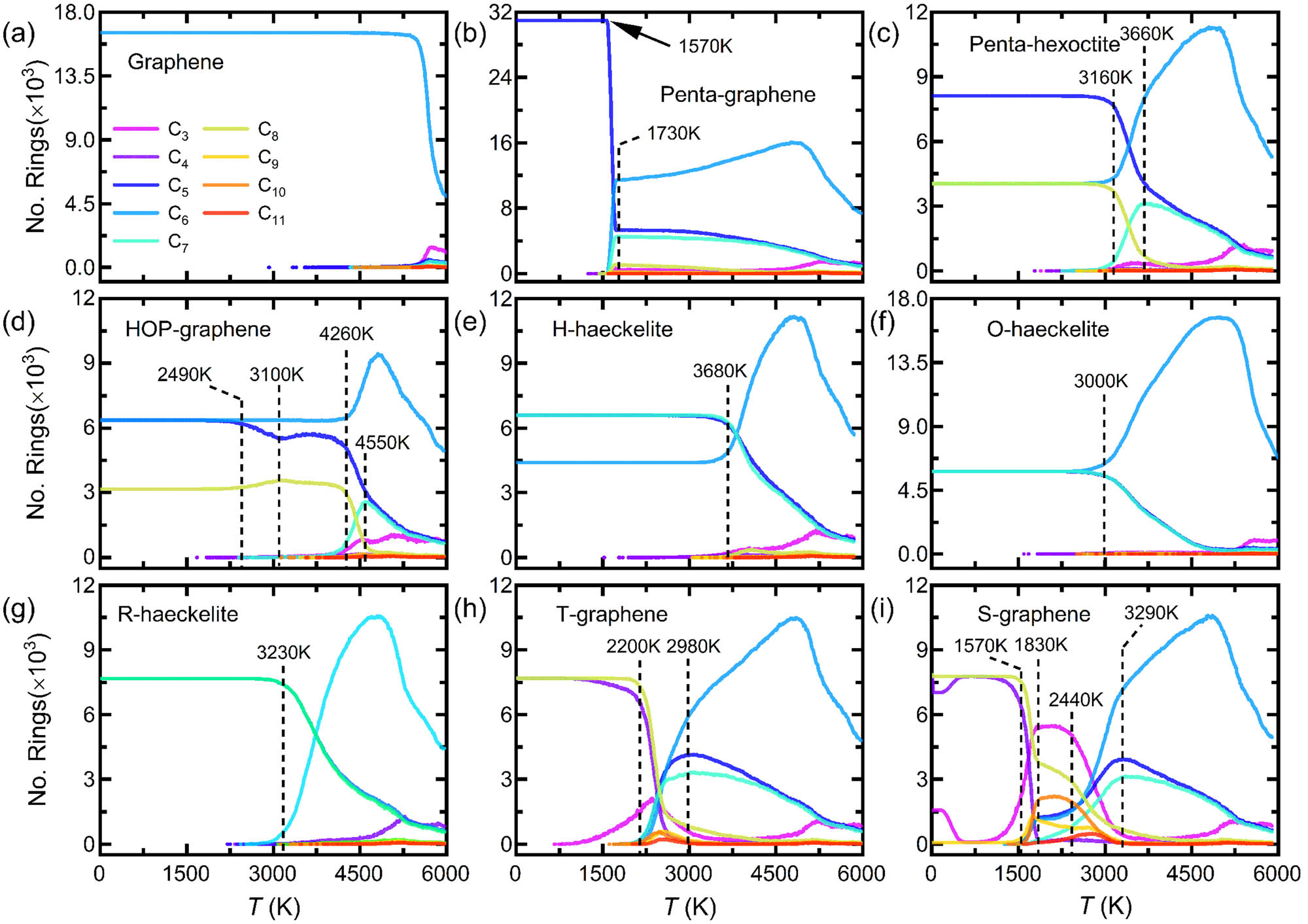
Temperature dependence of the number of various polygons in (a) graphene, (b) penta-graphene, (c) penta-hexoctite, (d) HOP-graphene, (e) H-haeckelite, (f) O-haeckelite, (g) R-haeckelite, (h) T-graphene, and (i) S-graphene, respectively. C n (n = 3, 4 5, …, 11) indicates n-membered ring.
For other 2D carbon crystals, there are unique variations in the number of polygons with temperature, strikingly differing from that in graphene. For penta-graphene (Figure 4b), its ring number – T curves are mainly characterized by that, as the heating T is over around 1,570 K, there is a sudden drop of the pentagon, accompanied by a sharp rising in the number of hexagon and heptagon, signifying instantaneous large-scale structural transformations from pentagon to hexagon + heptagon. With a further increase of T, there is a crossover in the number of hexagons, while the numbers of pentagon and heptagon monotonically decrease.
For penta-hexoctite (Figure 4c), it is observed from the curves that above critical T, the numbers of pentagon and octagon monotonically reduce, while the numbers of hexagon and heptagon initially increase but then decrease at high temperatures. Note that the transition in the change of the number of hexagon and heptagon occurs at different T. With regard to HOP-graphene (Figure 4d), it is uniquely found from the curves that above around 2,400 K, a two-step reduction in the number of pentagon is identified, whereas for the number of octagon, it initially increases, but then monotonically decreases. Concerning the haeckelite-based structures (Figure 4e–g) subjected to heating of high T, they are primarily involved in the nonlinear changes of the number of hexagon, pentagon, and heptagon, similar to the case of penta-graphene. Moreover, they show almost synchronous changes in the number of pentagon and heptagon, indicating the formation of pentagon–heptagon pair defects.
Remarkably, as displayed in Figure 4h and i, as a result of their composition of energetically unfavorable tetragon and octagon, T- and S-graphene show more complex changes in the polygons than other 2D carbon crystals. For example, above the critical temperature, besides the significant increases in the number of hexagons, pentagons, and heptagons, there is a considerable formation of trigon and nonagon as the rapid reduction in the number of native tetragon and octagon. Additionally, the number of the newly formed pentagon is larger than that of the newly formed heptagon, indicating that they are not purely pentagon–heptagon pair defects.
In a nutshell, excluding graphene, as indicated by the maximum peak of hexagons in the curves of Figure 4, non-hexagons contained 2D carbon crystals subjected to critical elevated T are dominated by the formation of hexagons (over 80%). This clearly demonstrates that thermally induced hex-graphene transitions are a key roadmap in the course of the pyrolysis of non-hexagon-contained 2D carbon crystals.
3.5 Atomistic origins in the thermally-induced structural transformations
To reveal their atomic origins in the thermally induced structural transformations, the evolution of molecular configurations of those 2D carbon crystals subjected to heating is captured. Based on the formation mechanisms of local polygons and the characteristics of microstructural changes, those 2D carbon crystals can be classified into several groups.
3.5.1 Graphene
Figure 5 shows the top-viewed snapshots of graphene at different T. Upon heating at the low T region, graphene is thermally characterized by local out-of-plane displacements, resulting in wrinkling morphology. At 5,000 K, graphene is an imperfect crystal with nucleation of a variety of non-hexagonal defects varying from tetragon to decagon. As the heating T reaches 5,400 K, more non-hexagons are generated and well-distributed in graphene. Once the heating T increases, a number of voids nucleate at non-hexagonal defects and further grow, resulting in the melting of graphene.
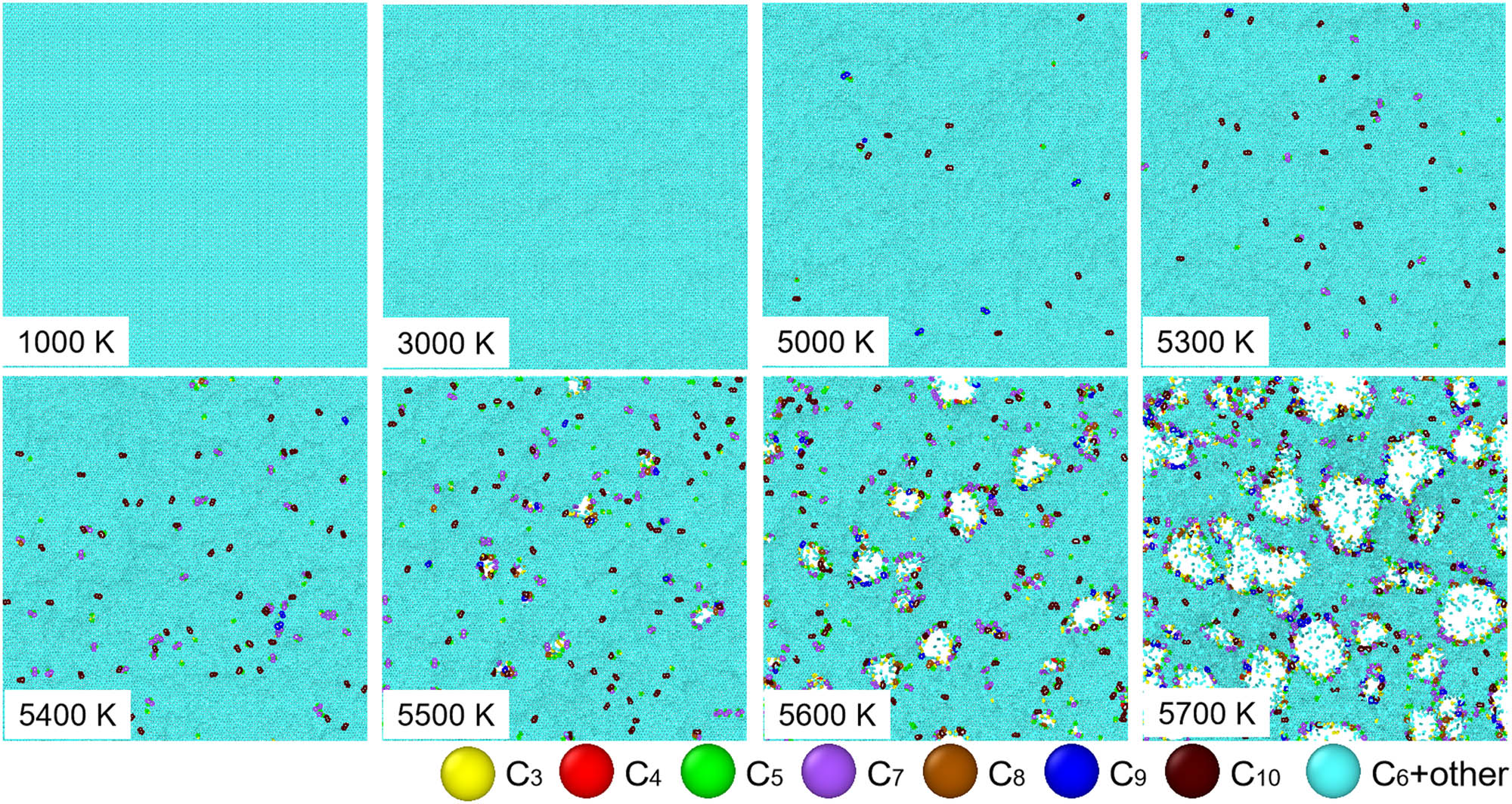
Top views of atomic structure of 2D graphene subjected to heating at eight different T. For enhanced observation of the thermally induced structural transformations, carbon atoms belonging to different polygonal rings from trigon to decagon are color-highlighted.
3.5.2 Penta-graphene
Figure 6a presents the top views of penta-graphene at eight different T. Note that at an extremely low T of around 34 K, penta-graphene is globally structurally transformed via sudden changes in bond configurations, resulting in global in-plane expansion and off-plane contraction in penta-graphene. At 1,580 K, sp2 + sp3 mixed penta-graphene is thermally destabilized by several local nucleations of sp2-polygons (mainly sp2-pentagon, sp2-hexagon, and sp2-heptagon). As illustrated in Figure 6b, nucleation of an sp2-hexagon occurs by dissociation of C(1)–C(2), C(3)–C(4), and C(1)–C(5) bonds and formation of C(1)–C(3) and C(1)–C(4) bonds, while an sp2-heptagon nucleates via breaking C(6)–C(7) and C(8)–C(9) bonds and formation of C(7)–C(8) bond. However, nucleation of a local polygon rapidly proceeds to cover all the surface of penta-graphene as the heating T approaches 1,730 K, achieving a second large-scale structural transformation to form an sp2-carbon sheet, consistent with the previous study [55]. With further increase of the heating T to around 4,900 K, the structural change is dominated by large-scale structural transitions of topological motifs from pentagon and heptagon to hexagon through the roadmap of dissociation of three bonds and formation of two bonds involved in a pentagon as indicated in Figure 6b. Finally, over 4,900 K, similar to graphene, the destabilization of the as-formed sheet through nucleation of voids and their growth due to thermal disintegration, resulting in the melting of penta-graphene.
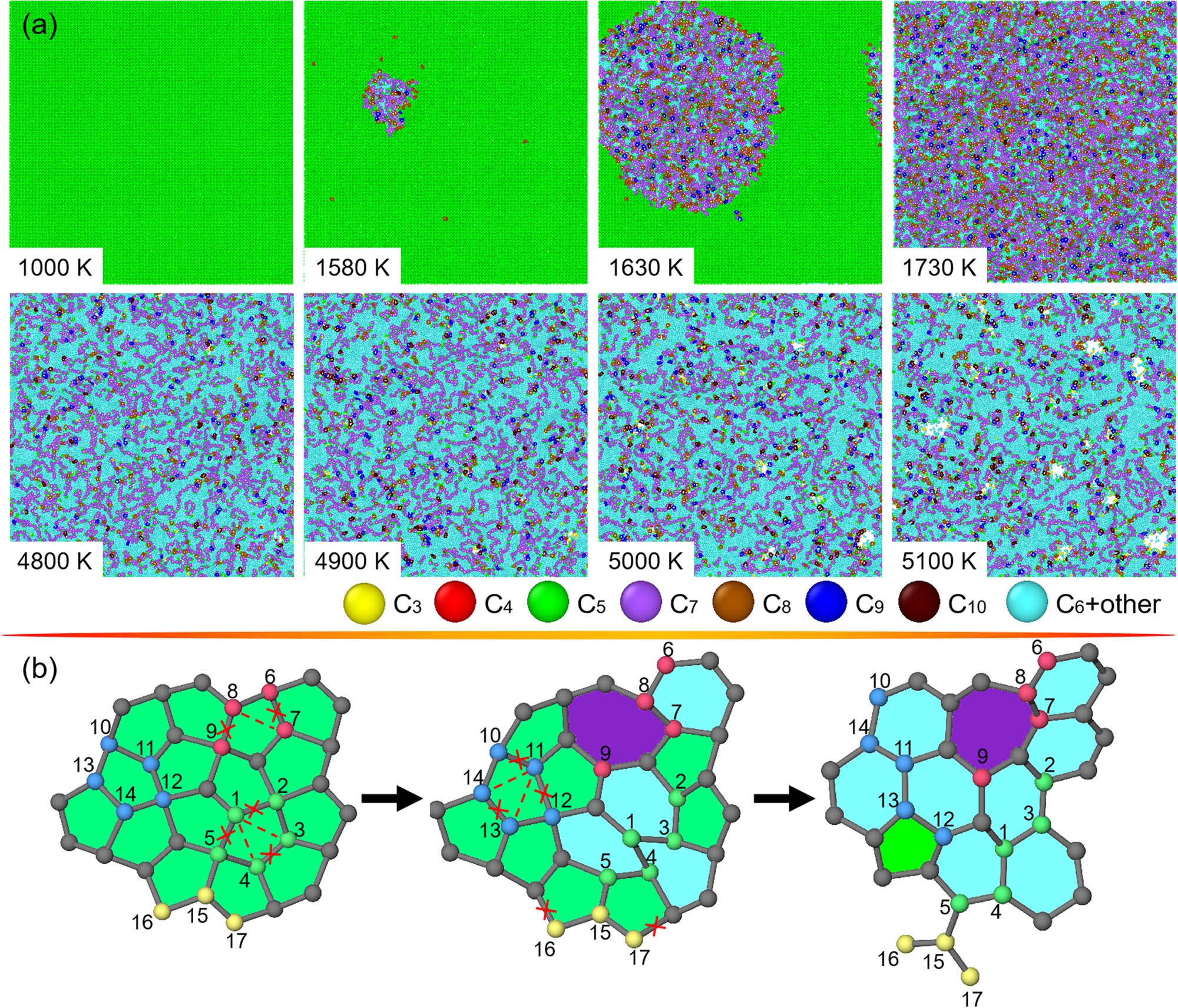
Structural changes in penta-graphene subjected to heating. (a) Top views of penta-graphene at T varying from 1,000 to 5,100 K. For clarification of microstructural changes, carbon atoms in recognized polygonal rings from trigon to decagon are differently rendered. (b) Atomic illustration of nucleation of hexagon and heptagon from pentagon in the local region of penta-graphene, in which carbon atoms are number-ranked for identifying the microstructural transitions.
3.5.3 Penta-hexoctite and HOP-graphene
Figure 7a and Figure S1a show snapshots of pentagon + hexagon + octagon contained penta-hexoctite and HOP-graphene subjected to heating, respectively. As is observed, both structures are thermally destabilized by the following stages. Initially, well-distributed local nucleation of non-native polygons occurs. However, the critical heating T of HOP-graphene responsible for such an event of microstructural change is higher than that of penta-hexoctite. In this stage, as a result of the structural changes, thermally induced buckling occurs in penta-hexoctite, as indicated by the inset of the snapshot at 3,500 K. Second, the thermally induced structural changes are dominated by the formation of hexagon accompanied by the local nucleation of non-native polygons. Third, as-nucleated pentagon and heptagon co-rearrange to form hexagons. In those above stages, “bond rotation” is the key mechanism in the large-scale structural transitions, as illustrated in Figure 7b and Figure S1b. As an example, the structural transitions of penta-hexoctite result from stepwise rotations of bonds shared by pentagon–pentagon, heptagon–octagon, and octagon–octagon, whereas for HOP-graphene, they are mainly from stepwise rotation of bonds shared by hexagon–hexagon and hexagon–heptagon. Lastly, as-formed sheets primarily composed of hexagons are thermally disintegrated at several sites.
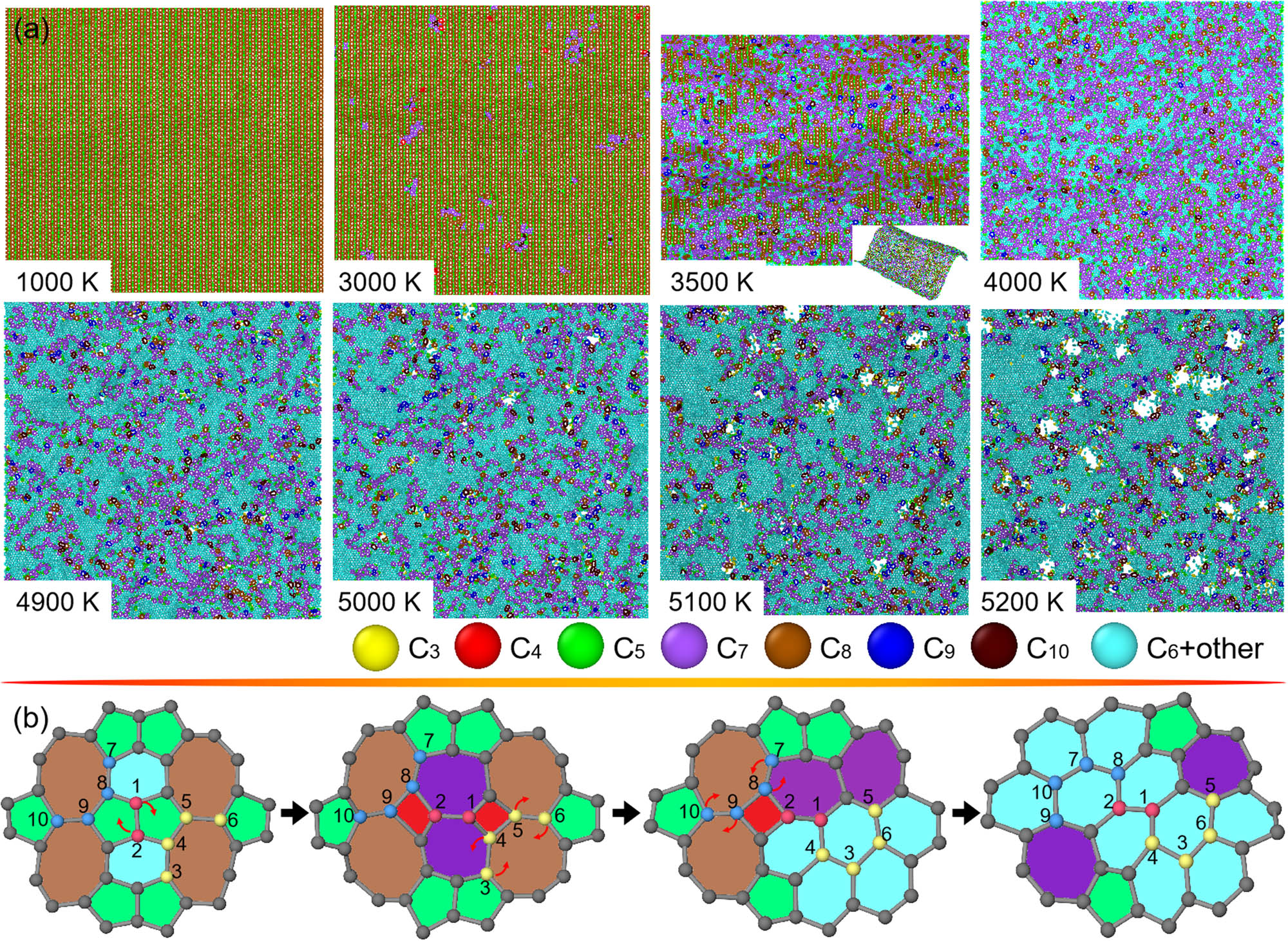
Structural changes in penta-hexoctite under heating. (a) Top-viewed snapshots of penta-hexoctite at T from around 1,000–5,200 K. For eye-catching the microstructural changes, carbon atoms in polygonal rings from trigon to decagon are color-painted. (b) Atomic illustration of nucleation of hexagons from pentagons, hexagons, and octagons in a zoomed-in region of penta-hexoctite. Specifically, carbon atoms are number-ranked and arrows are marked for explaining the origins of microstructural transitions.
3.5.4 H-, O- and R-haeckelites
Figure 8a and Figures (S2a and S3a) display top views of pentagon + hexagon + heptagon-dominated O-, H-, and R-haeckelites at different T, respectively. As is indicated, the thermally induced structural transformations of O-, H-, and R-haeckelites are described by initial scattered nucleation of hexagons, large-scale nucleation of hexagons, and disintegration. By comparison, O- and R-haeckelites show a higher rapid rate in the nucleation of hexagons than H-haeckelite during the heating process. As the nucleation of hexagons terminates, O- and R-haeckelites contain over 95% hexagon in the structures, indicating excellent thermally induced hex-graphene transition for their 2D crystals.
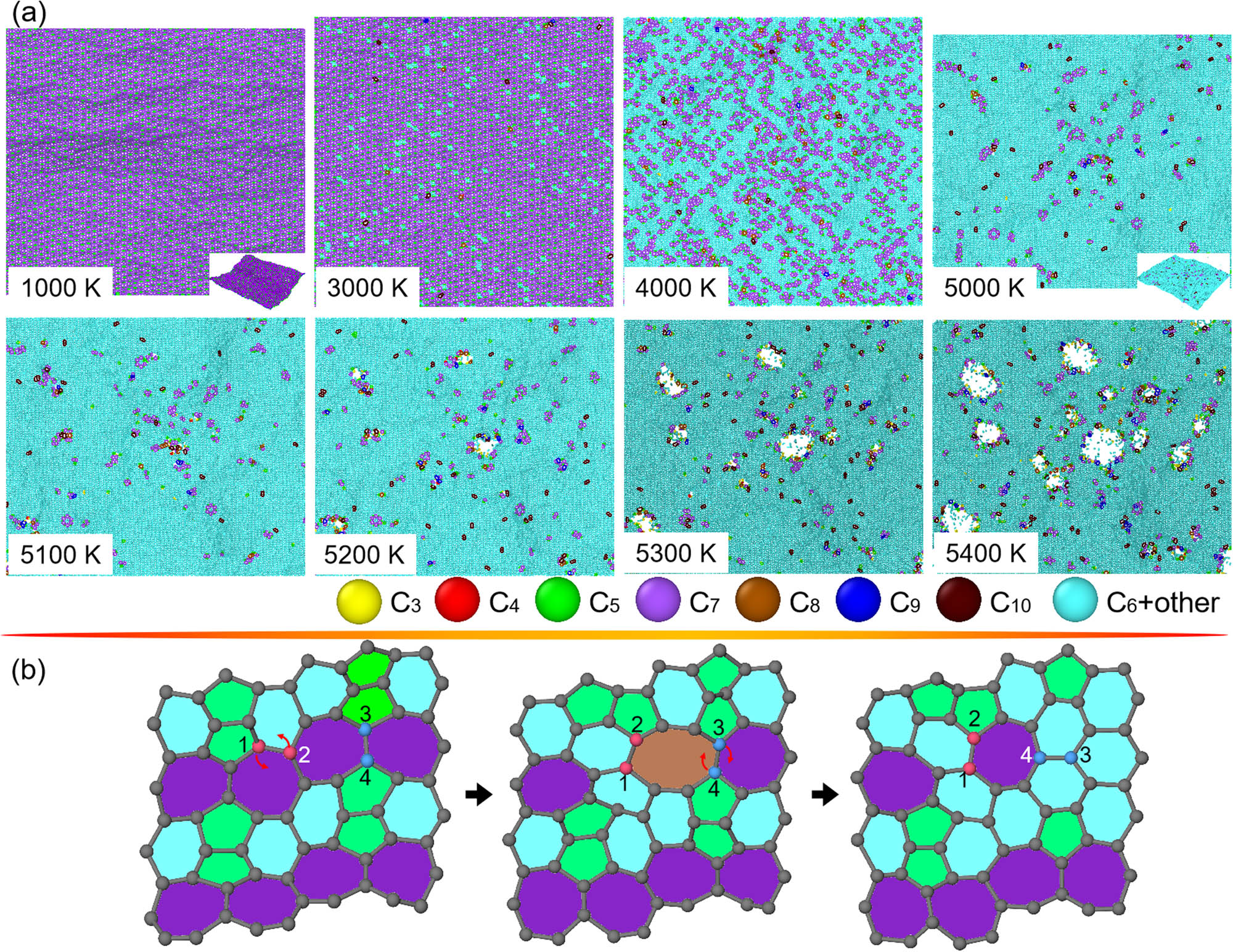
Structural changes in O-haeckelite upon heating. (a) Top views of O-haeckelite as the heating T increases from around 1,000 to 5,400 K. into melting phase. For eye-catching the thermally induced structural transformation in the O-haeckelite, carbon atoms in polygonal rings from trigon to decagon are differently rendered. (b) Atomic illustration of nucleation of hexagons from pentagons, hexagons, and heptagons in a local region of O-haeckelite. Particularly, some carbon atoms are number-ranked, and arrows are marked for revealing the origins of microstructural transitions.
Figure 8b and Figures (S2b and S3b) show their representative local structural changes during the heating process. Similar to penta-hexoctite and HOP-graphene, the microstructural transitions in the family of haeckelites are mainly dominated by the “bond rotation” mechanisms. For example, in O-haeckelite, the structural transformations result from two-step rotation of bonds shared by hexagon–heptagon and heptagon–octagon, whereas in H-/R-haeckelite, they are mainly from one-step rotation of bonds shared by hexagon–heptagon/heptagon–heptagon. Such difference stems from the distinct arrangement of pentagons, hexagons, and heptagons in the sheets.
3.5.5 S-graphene and T-graphene
Figure 9a and Figure S4a display snapshots of tetragon + octagon dominated S- and T-graphene at different T, respectively. Because of the inhomogeneous arrangement of tetragons and octagons in the sheet, S-graphene shows strong thermally induced buckling morphology at low T regions, while T-graphene remains flat morphology at low T region due to its homogeneous arrangement of tetragons and octagons. As a result of their energetically unfavorable configurations of tetragon and octagon, they show scattered nucleation in the sheets at relatively low T. With increasing T, in comparison with other non-hexagon contained 2D carbon crystals, more complex polygons forms. At high T, both structures tend to form hexagon-dominated stable sheets, followed by thermal disintegration, similar to other 2D carbon crystals.
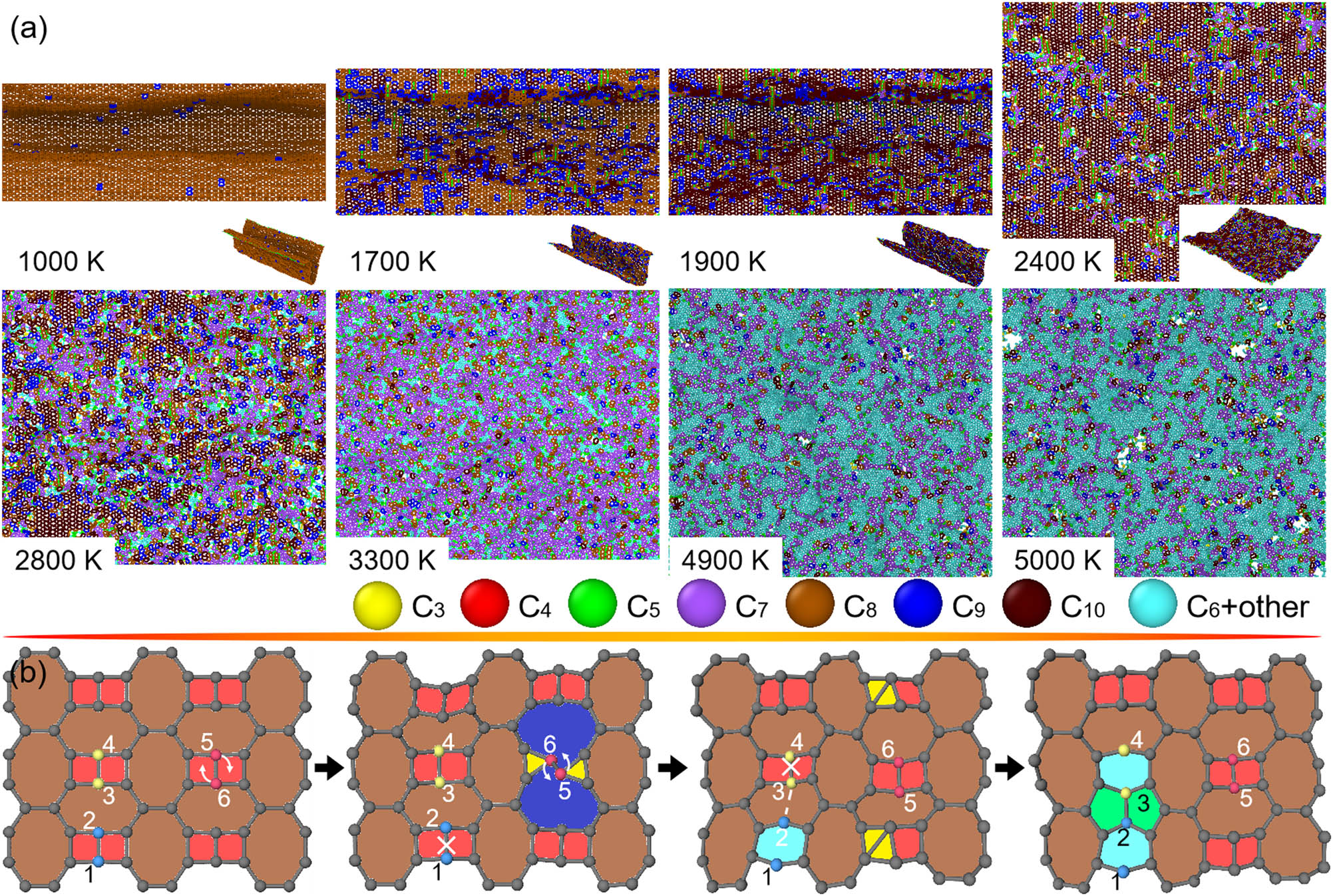
Structural changes in S-graphene subjected to heating. (a) Top views of O-haeckelite as the heating T increases from around 1,000 to 5,400 K into melting phase. For eye-catching the thermally induced structural transformation in the O-haeckelite, carbon atoms in polygonal rings from trigon to decagon are differently rendered. (b) Atomic illustration of nucleation of hexagons from pentagons, hexagons, and heptagons in a local region of O-haeckelite. Particularly, some carbon atoms are number-ranked and arrows are marked for revealing the origins of microstructural transitions.
Figure 9b and Figure S4b show the representative snapshots of local structures of S- and T-graphene during the structural changes, respectively. As is illustrated, for S-graphene, the initial structural transformations are primarily described by rotation and dissociation of bonds shared by tetragon–tetragon and bond formation in the octagon, resulting in the formation of trigon, pentagon, hexagon, and nonagon in the sheets. As a result, the microstructures in S- and T-graphene becomes close to other 2D carbon crystals. Therefore, the following structural transformations of S- and T-graphene are analogous to multi-polygon-dominated 2D carbon crystals.
4 Conclusion
In summary, the melting thermodynamic behaviors of graphene and its contemporary allotropes are comprehensively investigated by conducting first-principle-based ReaxFF MD simulations. All graphene allotropes show unique nonlinearity in the LI–T and E–T curves that reflect their distinct thermal properties. Differing from that of hexagon-dominated graphene, the melting behaviors of investigated graphene allotropes are primarily characterized by multiple phase transformations at critical temperatures that are greatly dictated by the polygonal compositions and arrangement. At low heating T regions, graphene, H-, O-, and R-haeckelites that are mainly composed of pentagon, hexagon, and heptagon are thermally stable structures, while penta-graphene, penta-hexoctite, HOP-, T-, and S-graphene show apparent local structural transformations. Upon heating at moderate T regions, all graphene allotropes containing non-hexagonal defects transform into hexagon-dominated structures via distinct mechanisms that are also controlled by their unique microstructural features such as polygonal compositions and arrangement. This indicates that thermally induced hex-graphene transitions are a key roadmap in the course of the pyrolysis of graphene allotropes. As they are heated at high T, similar to graphene, the thermodynamic behaviors of graphene allotropes are characterized by the nucleation and growth of nanovoids as a result of thermal disintegration.
-
Funding information: This work is financially supported by the National Natural Science Foundation of China (Grant Nos. 12172314, 11772278, and 11904300), the Jiangxi Provincial Outstanding Young Talents Program (Grant No. 20192BCBL23029), the Fundamental Research Funds for the Central Universities (Xiamen University: Grant No. 20720210025), Y. Yu, and Z. Xu from Information and Network Center of Xiamen University for the help with the high-performance computing and the Norwegian Metacenter for Computational Science (NOTUR NN9110 K and NN9391 K.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: Authors state no conflict of interest.
References
[1] Howard JB, McKinnon JT, Makarovsky Y, Lafleur AL, Johnson ME. Fullerenes C60 and C70 in flames. Nature. 1991;352:139–41.10.1038/352139a0Search in Google Scholar
[2] Cataldo F. From dicopper acetylide to carbyne. Polym Int. 1999;48:15–22.10.1002/(SICI)1097-0126(199901)48:1<15::AID-PI85>3.0.CO;2-#Search in Google Scholar
[3] Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–8.10.1038/354056a0Search in Google Scholar
[4] Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, et al. Electric field effect in atomically thin carbon films. Science. 2004;306:666–9.10.1126/science.1102896Search in Google Scholar
[5] Novoselov KS, Geim AK, Morozov SV, Jiang D, Katsnelson MI, Grigorieva IV, et al. Two-dimensional gas of massless Dirac fermions in graphene. Nature. 2005;438:197–200.10.1038/nature04233Search in Google Scholar
[6] Galashev AE, Rakhmanova OR. Mechanical and thermal stability of graphene and graphene-based materials. Physics-Uspekhi. 2014;57:970–89.10.3367/UFNe.0184.201410c.1045Search in Google Scholar
[7] Openov LA, Podlivaev AI. On graphene melting. Phys Solid State. 2016;58:847–52.10.1134/S1063783416040168Search in Google Scholar
[8] Sui C, Zhao Y, Zhang Z, He J, Zhang Z, He X, et al. Morphology-controlled tensile mechanical characteristics in graphene allotropes. Acs Omega. 2017;2:3977–88.10.1021/acsomega.7b00732Search in Google Scholar
[9] Li Y, Chen P, Zhang C, Peng J, Gao F, Liu H. Molecular dynamics simulation on the buckling of single-layer MoS2 sheet with defects under uniaxial compression. Compl Mater Sci. 2019;162:116–23.10.1016/j.commatsci.2019.02.043Search in Google Scholar
[10] Han Y, Chen P, Zhu J, Liu H, Zhang Y. Mechanical behavior of single layer MoS2 sheets with aligned defects under uniaxial tension. J Appl Phys. 2021;130:130.10.1063/5.0061556Search in Google Scholar
[11] Li Y, Chen P, Liu H, Peng J, Luo N. The buckling behavior of single-layer MoS2 sheets on silica substrates. J Appl Phys. 2021;129:129.10.1063/5.0030528Search in Google Scholar
[12] Yang L, Liu J, Lin Y, Xu K, Cao X, Zhang Z, et al. Strengthening and weakening by dislocations in monolayer MoS2. Chem Mater. 2021;33:8758–67.10.1021/acs.chemmater.1c02797Search in Google Scholar
[13] Cao P, Wu J. Self-assembly of MoS2 monolayer sheets by desulfurization. Langmuir. 2021;37:4971–83.10.1021/acs.langmuir.1c00369Search in Google Scholar PubMed
[14] Xu K, Liang T, Zhang Z, Cao X, Han M, Wei N, et al. Grain boundary and misorientation angle-dependent thermal transport in single-layer MoS2. Nanoscale. 2022;14:1241–9. 10.1039/D1NR05113J.Search in Google Scholar PubMed
[15] Wu J, Cao P, Zhang Z, Ning F, Zheng S-S, He J, et al. Grain-size-controlled mechanical properties of polycrystalline monolayer MoS2. Nano Lett. 2018;18:1543–52.10.1021/acs.nanolett.7b05433Search in Google Scholar PubMed
[16] Van Hoang V. Melting and pre-melting of two-dimensional crystalline SiC nanoribbons. Physica E Low Dimens Syst Nanostruct. 2021;137:115012. 10.1016/j.physe.2021.115012 Search in Google Scholar
[17] Wu J, Gong H, Zhang Z, He J, Ariza P, Ortiz M, et al. Topology and polarity of dislocation cores dictate the mechanical strength of monolayer MoS2. Appl Mater Today. 2019;15:34–42.10.1016/j.apmt.2018.12.019Search in Google Scholar
[18] Qin J-K, Sui C, Qin Z, Wu J, Guo H, Zhen L, et al. Mechanical anisotropy in two-dimensional selenium atomic layers. Nano Lett. 2021;21:8043–50.10.1021/acs.nanolett.1c02294Search in Google Scholar PubMed
[19] Fang B, Ning F, Ou W, Wang D, Zhang Z, Yu Y, et al. The dynamic behavior of gas hydrate dissociation by heating in tight sandy reservoirs: a molecular dynamics simulation study. Fuel. 2019;258:116106.10.1016/j.fuel.2019.116106Search in Google Scholar
[20] Terrones H, Terrones M, Hernandez E, Grobert N, Charlier JC, Ajayan PM. New metallic allotropes of planar and tubular carbon. Phys Rev Lett. 2000;84:1716–9.10.1103/PhysRevLett.84.1716Search in Google Scholar PubMed
[21] Wang Z, Zhou XF, Zhang X, Zhu Q, Dong H, Zhao M, et al. Phagraphene: a low-energy graphene allotrope composed of 5-6-7 carbon rings with distorted dirac cones. Nano Lett. 2015;15:6182–6.10.1021/acs.nanolett.5b02512Search in Google Scholar PubMed
[22] Mandal B, Sarkar S, Pramanik A, Sarkar P. Theoretical prediction of a new two-dimensional carbon allotrope and NDR behaviour of its one-dimensional derivatives. Phys Chem Chem Phys. 2013;15:21001–6.10.1039/c3cp53390eSearch in Google Scholar PubMed
[23] Sharma BR, Manjanath A, Singh AK. Pentahexoctite: a new two-dimensional allotrope of carbon. Sci Rep-Uk. 2014;4:7164.10.1038/srep07164Search in Google Scholar PubMed PubMed Central
[24] Liu Y, Wang G, Huang QS, Guo LW, Chen XL. Structural and electronic properties of T graphene: a two-dimensional carbon allotrope with tetrarings. Phys Rev Lett. 2012;108:225505.10.1103/PhysRevLett.108.225505Search in Google Scholar PubMed
[25] Xu LC, Wang RZ, Miao MS, Wei XL, Chen YP, Yan H, et al. Two dimensional Dirac carbon allotropes from graphene. Nanoscale. 2014;6:1113–8.10.1039/C3NR04463GSearch in Google Scholar
[26] Zhang SH, Zhou J, Wang Q, Chen XS, Kawazoe Y, Jena P. Penta-graphene: a new carbon allotrope. P Natl Acad Sci USA. 2015;112:2372–7.10.1073/pnas.1416591112Search in Google Scholar PubMed PubMed Central
[27] Majidi R. Electronic properties of edge functionalized S-graphene nanoribbons. Solid State Commun. 2021;330:114286.10.1016/j.ssc.2021.114286Search in Google Scholar
[28] Halterman K, Valls OT, Alidoust M. Characteristic energies, transition temperatures, and switching effects in clean S|N|S graphene nanostructures. Phys Rev B. 2011;84:064509.10.1103/PhysRevB.84.064509Search in Google Scholar
[29] Nath S, Bandyopadhyay A, Datta S, Uddin MM, Jana D. Electronic and optical properties of non-hexagonal Dirac material S-graphene sheet and nanoribbons. Physica E Low Dimens Syst Nanostruct. 2020;120:114087.10.1016/j.physe.2020.114087Search in Google Scholar
[30] Sun J, Guo YG, Wang Q, Kawazoe Y. Thermal transport properties of penta-graphene with grain boundaries. Carbon. 2019;145:445–51.10.1016/j.carbon.2019.01.015Search in Google Scholar
[31] Winczewski S, Rybicki J. Anisotropic mechanical behavior and auxeticity of penta-graphene: Molecular statics/molecular dynamics studies. Carbon. 2019;146:572–87.10.1016/j.carbon.2019.02.042Search in Google Scholar
[32] Wong EW, Sheehan PE, Lieber CM. Nanobeam mechanics: elasticity, strength, and toughness of nanorods and nanotubes. Science. 1997;277:1971–5.10.1126/science.277.5334.1971Search in Google Scholar
[33] Winczewski S, Shaheen MY, Rybicki J. Interatomic potential suitable for the modeling of penta-graphene: Molecular statics/molecular dynamics studies. Carbon. 2018;126:165–75.10.1016/j.carbon.2017.10.002Search in Google Scholar
[34] Sun H, Mukherjee S, Singh CV. Mechanical properties of monolayer penta-graphene and phagraphene: a first-principles study. Phys Chem Chem Phys. 2016;18:26736–42.10.1039/C6CP04595BSearch in Google Scholar PubMed
[35] Fomin YD, Brazhkin VV. Comparative study of melting of graphite and graphene. Carbon. 2020;157:767–78.10.1016/j.carbon.2019.10.065Search in Google Scholar
[36] Andrievski RA. Review of thermal stability of nanomaterials. J Mater Sci. 2013;49:1449–60.10.1007/s10853-013-7836-1Search in Google Scholar
[37] Chenoweth K, van Duin AC, Goddard 3rd WA. ReaxFF reactive force field for molecular dynamics simulations of hydrocarbon oxidation. J Phys Chem A. 2008;112:1040–53.10.1021/jp709896wSearch in Google Scholar PubMed
[38] Zhao H, Shi Q, Han Z, Gong H, Zhang Z, Wu S, et al. Anomalous thermal stability in supergiant onion-like carbon fullerenes. Carbon. 2018;138:243–56.10.1016/j.carbon.2018.06.012Search in Google Scholar
[39] Feng C, Xu J, Zhang Z, Wu J. Morphology- and dehydrogenation-controlled mechanical properties in diamond nanothreads. Carbon. 2017;124:9–22.10.1016/j.carbon.2017.08.015Search in Google Scholar
[40] Fu Y, Wu J, Xiao S, Liu S, Zhang Z, He J. Tensile mechanical characteristics of ultra-thin carbon sulfur nanothreads in orientational order. Carbon. 2021;184:146–55.10.1016/j.carbon.2021.08.006Search in Google Scholar
[41] Han S, Li X, Nie F, Zheng M, Liu X, Guo L. Revealing the initial chemistry of soot nanoparticle formation by ReaxFF molecular dynamics simulations. Energy Fuel. 2017;31:8434–44.10.1021/acs.energyfuels.7b01194Search in Google Scholar
[42] Fu Y, Xu K, Wu J, Zhang Z, He J. The effects of morphology and temperature on the tensile characteristics of carbon nitride nanothreads. Nanoscale. 2020;12:12462–75.10.1039/D0NR03206ASearch in Google Scholar PubMed
[43] Srinivasan SG, van Duin AC, Ganesh P. Development of a ReaxFF potential for carbon condensed phases and its application to the thermal fragmentation of a large fullerene. J Phys Chem A. 2015;119:571–80.10.1021/jp510274eSearch in Google Scholar PubMed
[44] March NH, Tosi MP. Introduction to liquid state physics. World Scientific Publishing Company; 2002. 10.1142/4717.Search in Google Scholar
[45] Ziman J. Principles of the theory of solids. Am J Phys. 1979;33:349.10.1017/CBO9781139644075Search in Google Scholar
[46] Singh SK, Neek-Amal M, Peeters FM. Melting of graphene clusters. Phys Rev B. 2013;87:134103.10.1103/PhysRevB.87.134103Search in Google Scholar
[47] Tranh DTN, Van Hoang V, Thi Thu Hanh T. Molecular dynamics simulation of melting of 2D glassy monatomic system. Mater Rez Express. 2018;5:015205.10.1088/2053-1591/aaa7a5Search in Google Scholar
[48] Zhou YQ, Karplus M, Ball KD, Berry RS. The distance fluctuation criterion for melting: Comparison of square-well and Morse potential models for clusters and homopolymers. J Chem Phys. 2002;116:2323–9.10.1063/1.1426419Search in Google Scholar
[49] Ding F, Bolton K, Rosen A. Molecular dynamics study of the surface melting of iron clusters. Eur Phys J D. 2005;34:275–7.10.1140/epjd/e2005-00157-xSearch in Google Scholar
[50] Franzblau DS. Computation of ring statistics for network models of solids. Phys Rev B. 1991;44:4925–30.10.1103/PhysRevB.44.4925Search in Google Scholar PubMed
[51] Zhang KW, Stocks GM, Zhong JX. Melting and premelting of carbon nanotubes. Nanotechnology. 2007;18:285703.10.1088/0957-4484/18/28/285703Search in Google Scholar
[52] Los JH, Zakharchenko KV, Katsnelson MI, Fasolino A. Melting temperature of graphene. Phys Rev B. 2015;91:045415.10.1103/PhysRevB.91.045415Search in Google Scholar
[53] Zakharchenko KV, Fasolino A, Los JH, Katsnelson MI. Melting of graphene: from two to one dimension. J Phys-Condens Mat. 2011;23:202202.10.1088/0953-8984/23/20/202202Search in Google Scholar PubMed
[54] Hoang VV, Cam Tuyen LT, Dong TQ. Stages of melting of graphene model in two-dimensional space. Philo Mag. 2016;96:1993–2008.10.1080/14786435.2016.1185183Search in Google Scholar
[55] Cranford SW. When is 6 less than 5? Penta- to hexa-graphene transition. Carbon. 2016;96:421–8.10.1016/j.carbon.2015.09.092Search in Google Scholar
© 2022 Ran Fu et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption

