Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
-
Ahmed A. H. Abdellatif
, Riaz A. Khan
Abstract
Drug uptake and distribution through cell–receptor interactions are of prime interest in reducing the adverse effects and increasing the therapeutic effectiveness of delivered formulations. This study aimed to formulate silver nanoparticles (AgNPs) conjugated to somatostatin analogs for specific delivery through somatostatin receptors (SSTRs) expressed on cells and by nebulizing the prepared AgNPs formulations into lung cells for in vivo application. AgNPs were prepared using the citrate reduction method, yielding AgNPs–CTT, which was further chemically conjugated to octreotide (OCT) to form AgNPs–OCT through an amide linkage. The AgNPs–OCT formulation was coated using alginate to yield a carrier, AgNPs–OCT–Alg, feasible for drug delivery through nebulization. AgNPs were uniform in size with an acceptable range of zeta potential. Furthermore, the concentrations of AgNP formulations were found safe for the model cell lines used, and cell proliferation was significantly reduced in a dose-dependent manner (p < 0.05). In the healthy lung tissues, AgNPs–OCT–Alg accumulated at a concentration of 0.416 ± 5.7 mg/kgtissue, as determined via inductively coupled plasma optical emission spectrometry. This study established the accumulation of AgNPs, specifically the AgNPs–OCT–Alg, in lung tissues, and substantiated the active, specific, and selective targeting of SSTRs at pulmonary sites. The anticancer efficacy of the formulations was in vitro tested and confirmed in the MCF-7 cell lines. Owing to the delivery suitability and cytotoxic effects of the AgNPs–OCT–Alg formulation, it is a potential drug delivery formulation for lung cancer therapy in the future.
1 Introduction
It is difficult to diagnose lung cancer because it has no early observable symptoms. Thus, the tumor is typically advanced at the time of primary diagnosis and continues to progress [1,2]. Currently, although radiotherapeutic and chemotherapeutic interventions are available as part of lung cancer treatment modalities, their harmful effects on normal and other noncancerous cells have limited their role, and research has been conducted to determine alternative treatment approaches [3]. These limitations and applications to treatment, through radio-oncologic modalities, have also led to dose perturbations with uncertain modulations during drug and radiological dose delivery. However, the side effects of these treatment modalities have not yet rendered them ineffective; therefore, even with putative damage to normal or non-cancerous cells, studies on improving the efficacy and drug- and radiation-dose controls are under development. Although the effectiveness of a drug increases as its dose increases, the increase in the dose is limited by the inherent toxicity of the drug and the radio-intervention, leading to undesired physiological and morphological changes and the destruction of normal cells [4]. The inhalation route of drug delivery is considered an effective strategy for the treatment of lung diseases [5]. This route of drug administration shows favorable advantages in targeted organs such as the lungs because the drug can reach the sites directly through inhalation, provided that the carrier-based formulation and the drug to be delivered are feasible for inhalation. Both show receptivity to diseased tissue parts. In this case, the direct delivery of the drug to the lungs has the advantage of lowering the required dose of the drug, which would otherwise have been higher and required more frequent administrations. It also specifically targets cancer sites and reduces the toxic effects on normal/noncancerous lung cells, which is feasible [6].
Breast cancer is the commonest cancer among women and the leading cause of cancer-related deaths worldwide. Breast cancer is initiated when the cells of the breast undergo uncontrolled division. Symptoms of breast disease include breast masses, changes in the volume of the breast, breast discomfort, and nipple discharge. A hard, painless mass in one of the nipples is the most common symptom of breast disease in males [7]. Currently, available treatment modalities for breast cancer include radiotherapy and chemotherapy; however, radiotherapy damages normal cells in addition to cancer cells. Chemotherapy can also be administered after surgery and radiotherapy [3]. However, cancer chemotherapy is limited due to its toxicity to normal cells. Moreover, increasing the dose can increase the effectiveness of chemotherapy [4]. However, many new drugs result in severe forms of toxicity, mainly in the form of myelo-suppression [8]. Delivering cytotoxic agents directly to tumor cells is more beneficial in terms of dose increase, reduction in the occurrence of peripheral toxicity, and improvement of therapeutic efficiency. Active targeting is considered an effective strategy for curing breast cancer. Further, there is a need to reduce the systemic side effects of parenteral and oral chemotherapy by using site-specific formulas.
Silver nanoparticles (AgNPs) are considered one of the most promising nanostructures. Their favorable physicochemical characteristics and bioactive properties have been found to be appropriate for several biomedical applications in vitro and in vivo. Intense interest in AgNPs and their derivatives has improved the treatment choices for currently available oncological interventions [9]. AgNPs have many applications in the medical, biomedical, and biotechnical fields, including cancer cell targeting therapy, and several other biomedical treatment arenas [10]. Although all studied AgNPs were previously formulated only for inhalation as a passive targeting strategy or to determine their cytotoxicity [11,12,13], AgNPs can be applied in active targeting via receptors. Moreover, ligand-coated AgNPs can target specific receptors in the human body, such as somatostatin (SST) receptors (SSTRs), which are among the most abundant receptors expressed by lung tumor cells [14]. High- and low-affinity SSTRs have been identified in numerous cancer cells, such as those of the lung, ovaries, pancreas, colorectum, and prostate [15,16]. Furthermore, SST analogs, such as octreotide (OCT) and vapreotide (VAP), have also been shown to have high and specific affinities to SSTR subtypes 2 and 5 [17,18]. OCT binds directly to receptors on tumor cells, as a majority of human tumor cells, either benign or malignant, usually express SSTRs [19]. Nilsson et al. have reported that numerous normal and infected human cells overexpress SSTRs. This makes SSTRs (particularly, SSTR2) feasible and promising target ligands using OCT or VAP-conjugated nanoparticulate delivery systems to reduce side effects and increase the accumulation of drugs in tumor cells [20]. Dasgupta et al. confirmed the delivery of OCT to cancer cells that express SSTRs, while Norenberg et al. reported that SST analogs widely used to target cancer cells express SSTRs for therapy with 90Y or 177Lu radionuclides [21].
Currently, no studies have reported the use of an AgNPs-conjugated SST analog OCT to target lung cancer via inhalation. This study aimed to develop a simple, noninvasive, aerosol-based, site-specific method to deliver a payload (drug) to the lung tissues for delivery by nebulization for in vivo inhalation and subsequently targeted delivery.
2 Methods
2.1 Materials
Silver nitrate (AgNO3), tri-sodium citrate (TSC), sodium alginate (Alg), sodium chloride, potassium chloride, potassium dihydrogen phosphate, sodium hydroxide, and disodium hydrogen phosphate were purchased from El Nasr Pharmaceuticals Chemicals Company (Abu Zaabal, Cairo, Egypt). OCT acetate, Gibco Roswell Park Memorial Institute (RPMI) 1640 medium with 10% fetal bovine serum (FBS), and antibodies were purchased from Santa Cruz Biotechnology Inc. (Heidelberg, Germany). The tumor necrosis factor (TNF)-α inhibitor was purchased from Calbiochem (San Diego, CA, USA). Kits for cytokine-specific enzyme-linked immunosorbent assays (ELISAs) were purchased from R&D Systems, Inc. (St. Paul, MN, USA). The Michigan Cancer Foundation-7 (MCF-7) cell line was obtained from Vacsera (Dokki, Giza, Egypt). All chemicals were of analytical grade.
2.2 Synthesis of silver citrate nanoparticles by the reduction technique
Citrate-reduced and capped AgNPs (AgNPs–CTT) were prepared using the citrate reduction method following a previously reported protocol [22] with some modifications using 100 mL of aqueous AgNO3 (molecular weight, 169.87; 0.05 mol; 8.4935 g) solution. Briefly, 28 mg of AgNO3 was diluted in 50 mL of Millipore-purified water (stock solution I). The stock solution I (6.5 mL) was diluted to 100 mL with Millipore water. The solution was stirred vigorously at 6,000 rpm to 100°C. About 2 mL of 2% TSC solution was slowly and carefully added to the boiling AgNO3 solution in an Erlenmeyer flask and allowed to continue boiling for 15 min. The colorless solution changed to yellow, bright red, red, and brown, indicating completion of the reaction after 15 min. The AgNPs–CTTs were stored at room temperature (27°C) in amber-colored bottles. The particles were purified by centrifugation at 1,200 rpm for 6 min to remove large particulate matter.
2.3 Preparation of AgNPs–CTT capped with OCT and wrapped with alginate
The OCT was selectively conjugated to AgNPs–CTT through its N-terminus [23]. The AgNPs–CTT formed at a concentration of 0.1 mM was reacted with a two-fold excess of OCT. Then, 100 µL of 0.2 mM OCT was added to the reaction mixture, followed by the addition of 0.15 mM N-(3-dimethylaminopropyl)-N′-ethyl carbodiimide hydrochloride (EDC) and 0.2 mM N-hydroxysuccinimide, and stirred for an additional 3 h at room temperature. The final product, AgNPs–OCT, was purified by centrifugation at 1,200 rpm for 5 min to remove large particulate matter [24]. The AgNPs–OCT formulation was optimized for lung delivery by stirring AgNPs through dispersion in a sodium alginate aqueous solution (1%), which generated a coat of alginate around the AgNPs–OCT. The formulated AgNPs–OCT–Alg was prepared to enhance AgNPs–OCT delivery into the lungs through inhalation.
We calculated the amount of Ag+ transformed to Ag0, which represents the percent yield of the produced AgNPs, using the previously described approach with some minor modifications [25,26]. Inductively coupled plasma optical emission spectrometry (JY-70 PLUS, Jobin Yvon Instruments SA Inc., Edison, NJ, USA) was used to calculate the amount of Ag0 in all AgNP formulas. Each formula was centrifuged at 1,200 rpm for 5 min in order to separate the large particulate and remove the impurities. The supernatant was evaluated by ICP-OES. The quantity of silver Ag0 was measured in the supernatant and then divided by the initial concentration of AgNO3. 5 Each solution was scanned times. The % yield of the produced AgNPs was calculated by dividing the obtained concentration by the initial concentration for AgNO3 using the following equation:
2.4 Size and zeta potential measurements
A Malvern Zetasizer Nano 6.01 analyzer (Malvern Instruments GmbH, Herrenberg, Germany) was used to measure the particle size, polydispersity index, and zeta (ζ) potential. Sampling times were automatically set on the instrument. Three measurements were permitted for every 10 subruns. All calculations were performed according to previously described protocols [14,27].
2.5 UV-Vis spectroscopy
The absorption spectra of AgNPs–CTT, AgNPs–OCT, and AgNPs–OCT–Alg formulations were recorded. The absorption spectrum for each formulation was scanned in the range of 300–700 nm in a 2/cm plastic cuvette using the Uvikon-941 spectrophotometer (Kontron Device GmbH, Herrenberg, Germany). Surface plasmon resonance (SPR) peaks were obtained using Microsoft Excel for Macintosh 2019 (Microsoft Corporation, Redmond, WA, USA) [14].
2.6 Cell culture
2.6.1 Toxicity study
MCF-7 cells were grown in the Gibco RPMI 1640 medium containing 10% FBS and 1% streptomycin and penicillin at 37°C in 5% carbon dioxide, as described previously [28,29]. Toxicity studies were performed on the optimized formulations, and the results were compared with those obtained among the control cells. The cells were treated with various concentrations (0.1–10 μM) of AgNPs–CTT [30], AgNPs–OCT, and AgNPs–OCT–Alg for 24 h. The concentrations of all formulas were calculated depending on the molecular weight of silver. Cytotoxicity was examined using a kit (Promega, Madison, WI, USA), as previously described [31].
2.6.2 TNF-α ELISA
After pretreatment with different doses of AgNPs–CTT, AgNPs–OCT, and AgNPs–OCT–Alg (0.1–10 µM) for 2 h, MCF-7 cells were examined. The TNF-α content in the culture medium was quantified using a TNF-α-specific ELISA kit according to the manufacturer’s instructions (R&D Systems Inc., Minneapolis, MN, USA) [30].
2.6.3 Cell uptake and displacement studies
The uptake and transport of AgNPs–CTT, AgNPs–OCT, and AgNPs–OCT–Alg in MCF-7 cells were investigated to determine the internalization of SSTR-based AgNPs–OCT into the cells. Furthermore, the AgNPs coupled with OCT and alginate ensured targeting of the nanoformulation, while the alginate wrapping ensured its feasibility for nebulization. A competitive replacement experiment was performed by incubating the AgNPs–OCT for 1 h at 37°C [32]. In brief, MCF-7 cells (1 × 107/mL) were placed in 35 mm culture dishes containing Dulbecco’s modified Eagle’s medium and incubated at 37°C overnight. The cells were incubated with 10 µM each of AgNPs–CTT, AgNPs–OCT, and AgNPs–OCT–Alg. MCF-7 cells were used as a model for SSTR expression [33]. Moreover, the groups that received AgNPs–OCT and AgNPs–OCT–Alg were also incubated with 100 µM free OCT 30 min prior to incubation with the nanoparticles, which led to receptor saturation with OCT via competitive replacement. This step aimed to confirm that AgNPs–OCT and AgNPs–OCT–Alg could be displaced from SSTRs by free OCT. The cells were then washed twice with Dulbecco’s phosphate-buffered saline, centrifuged at 300 g (180.5°C) for 10 min, and placed in 5 mL glass vials. ICP-OES was used to assess the concentration of silver ions (Ag+) [34,35]. Briefly, each AgNP sample (0.5 mL) was mixed with 0.2 mL of freshly prepared aqua regia and diluted to 5 mL with Millipore water. ICP-OES was performed using a JY-70 PLUS (Jobin Yvon Instruments SA Inc.) to determine the quantity of Ag+ in the digested cells. Ag+ concentrations of 1, 10, and 100 ppm were used as standards. The concentration of AgNPs in solution was calculated by dividing the number of silver atoms per particle by the estimated concentration of Ag+ [14,36]. The ICP-OES principle is used to determine the quantity of metals in a sample by measuring the amount of light emitted at different wavelengths [37]. The amount of Ag+ was calculated using a Microsoft Excel sheet, as reported previously [35].
2.7 Experimental animals
The animal experiments were conducted at the Pharmacology Research Laboratory, College of Pharmacy, Qassim University (Buraydah, Saudi Arabia). The study protocol was approved by the Institutional Animal Ethics Committee Deanship of Scientific Research, Qassim University, Saudi Arabia (approval number: 2019-2-2-I-5551). A histamine chamber (Plexiglas) was prepared (with the following dimensions: length 32 cm, width 15 cm, and height 28 cm) to expose four rats to the prepared AgNP formulations. The nebulizer inlet carried AgNPs to the rats, and the outlet tube maintained the optimum pressure inside the chamber for inhalation. Millipore water was used to prepare all nebulizing solutions. This experiment was carried out on 12 Sprague–Dawley rats of both sexes obtained from the College of Pharmacy Animal House. The rats were 7 weeks old, with body weights of 200–250 g, and were provided a rodent chow diet and water ad libitum. All rats were acclimatized to the laboratory environment in clean cages for 5 days and maintained daily with a 10–14 h photoperiod. The rats were nebulized using a System LS 290 aerosol nebulizer (System Assistance Medical SAS, Villeneuve-sur-Lot, France). This system used room air with appropriate pressures and temperatures to vaporize solutions into a micro-fine aerosol mist that flowed into the tubes of the Plexiglas chamber for the animals to inhale. The rats were distributed equally into groups (the control, AgNPs–CTT, and AgNPs–OCT–Alg groups). Rats in the control group were nebulized with Millipore water, those in the AgNPs–CTT group with AgNPs–CTT, and those in the AgNPs–OCT–Alg group with AgNPs–OCT–Alg. The concentrations of the nanoformulations were 10 µM each. The total dose of each formulation was 1.27 mg/kg [38], and 50 mL of each formulation was used for nebulization at a rate of 5 mL/h. In each experiment, the nebulizer dosing cup was filled with 10 mL of liquid and nebulized until it dried out; the nebulization was continued for 3 days (within 10 h/day). On the same day, lung tissues were excised and processed [39,40].
2.7.1 Analysis of the biodistribution of the AgNPs–OCT–Alg
The quantity of Ag+ was analyzed using ICP-OES to determine the amount of inhaled AgNPs–OCT. Lung tissues were homogenized in 86% nitric acid and hydrochloric acid. The homogenized tissue was diluted with phosphate-buffered saline (pH 7.4). The AgNPs–OCT concentration was determined by quantifying the amount of Ag+ using the ICP-OES technique according to a protocol described in previous studies [41,42].
2.7.2 Ultrastructural examination by transmission electron microscopy (TEM)
Lung specimens were excised and processed (fixed in 2.5% glutaraldehyde for 2 h, post-fixed in 1% osmium tetroxide for 1 h, dehydrated in ethanol, cleared in propylene oxide, and embedded in epoxy resin). After thermal polymerization, ultrathin sections were cut with an ultramicrotome and stained with uranyl acetate and lead citrate [43]. The grids of the ultrathin sections were examined and photographed using a transmission electron microscope (JEOL 1010; JEOL Ltd, Tokyo, Japan) at the Electron Microscopy Unit of the College of Applied Medical Sciences (Qassim University) to identify the ultrastructural details of the alveolar cells [44]. The nanoparticles were electron-dense with characteristic round shapes and equal in size.
2.7.3 Statistical analysis
The data are presented as the mean of at least three experiments with their standard deviations. Comparisons of single or multiple samples were performed using t-tests. The analyses were performed using SPSS Statistics version 27 (IBM Corporation, Armonk, NY, USA). Analysis items with p < 0.05 were considered statistically significant.
3 Results
The AgNPs–CTT formulation was prepared and conjugated to OCT to enable it to specifically target SSTR2. The prepared AgNPs–CTT–OCT or AgNPs–OCT was coated with alginate (via a 1% aqueous sodium alginate solution) to yield AgNPs–OCT–Alg for easy delivery to the lungs by inhalation in rat models in a histamine chamber (Figure 1a–c) (Plexiglas). The nebulizer inlet transported the AgNPs–OCT–Alg formulation to the rats (Figure 1b and c). The prepared AgNPs–CTTs were uniformly shaped and brown in color. The brown color turned darker after conjugating the AgNPs–CTT with OCT. The AgNPs–CTT was coupled with OCT at its N-terminus, forming an amide bond between the OCT and the citrate carboxyl group of the AgNPs–CTT. The reaction can be progressed at a slightly acidic or neutral pH to avoid reaction with the lysine side-chain amino group (NH2) of OCT, which (lysine-NH2) is essential for activity (Figure 1d) [17,45].
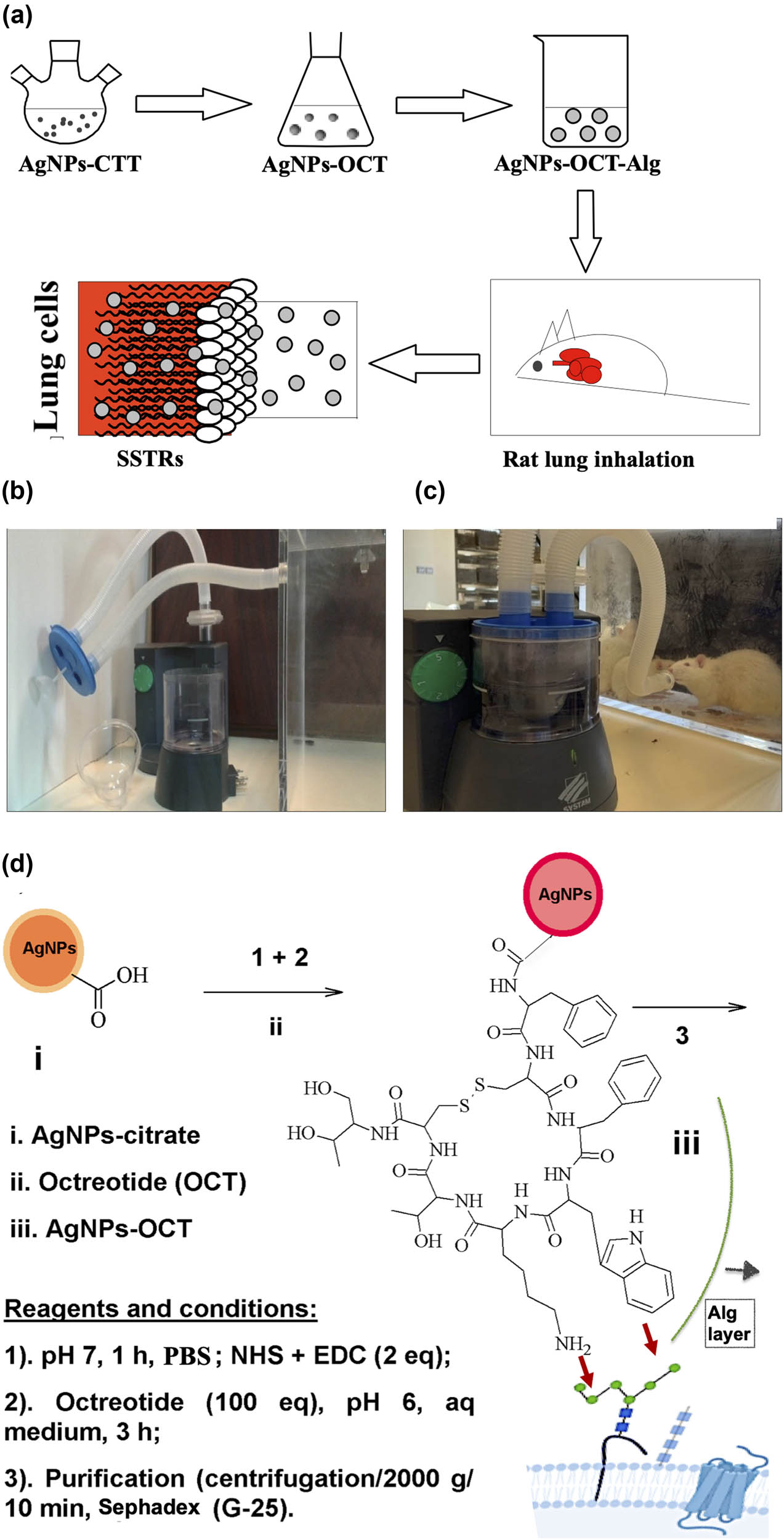
(a) Schematic diagram of AgNPs–OCT–Alg preparation and delivery to lung cells. (b and c) Nebulization of AgNPs–OCT–Alg among rats. (d) Schematic diagram of AgNPs–OCT synthesis; the AgNPs–OCT formed were coated with alginate for delivery to the lung cells and the proposed manner in which OCT fits into the SSTRs by interactions between lysine and phenylalanine.
Table 1 shows the initial Ag+ and Ag0 concentrations in AgNPs following NP formation, as measured by ICP-OES. The yield% was calculated for Ag0 synthesized in the AgNPs–CTT, AgNPs–OCT, AgNPs–OCT–Alg to be 2.82 ± 0.016 mg/100 mL (≈ 77.9%), 2.43 ± 0.21 mg/100 mL (≈ 67.12%), and 2.166 ± 0.19 mg/100 mL (≈ 59.83%), respectively.
The yield percentage of Ag° in AgNPs after conversion of Ag+ to Ag0
| Sample | Concentration (mg/100 mL) | Yield % (conversion%) |
|---|---|---|
| Ag+ (initial concentration) | 3.62 ± 0.1 | — |
| AgNPs–CTT | 2.82 ± 0.016 | 77.9 |
| AgNPs–OCT | 2.43 ± 0.21 | 67.12 |
| AgNPs–OCT–Alg | 2.166 ± 0.19 | 59.83 |
3.1 Size, ζ potential, and surface morphology
A dynamic light scattering zetasizer nano was used to record the sizes of the AgNPs–CTT, AgNPs–OCT, and AgNPs–OCT–Alg, which were determined to be 22.77 ± 1.1, 78.77 ± 2.3, and 155.99 ± 5.2 nm, respectively. The ζ potentials of the three formulations had surface charge values of −40.17 ± 2.0, −14.13 ± 1.3, and +16.17 ± 1.3 mV, respectively (Figure 2).
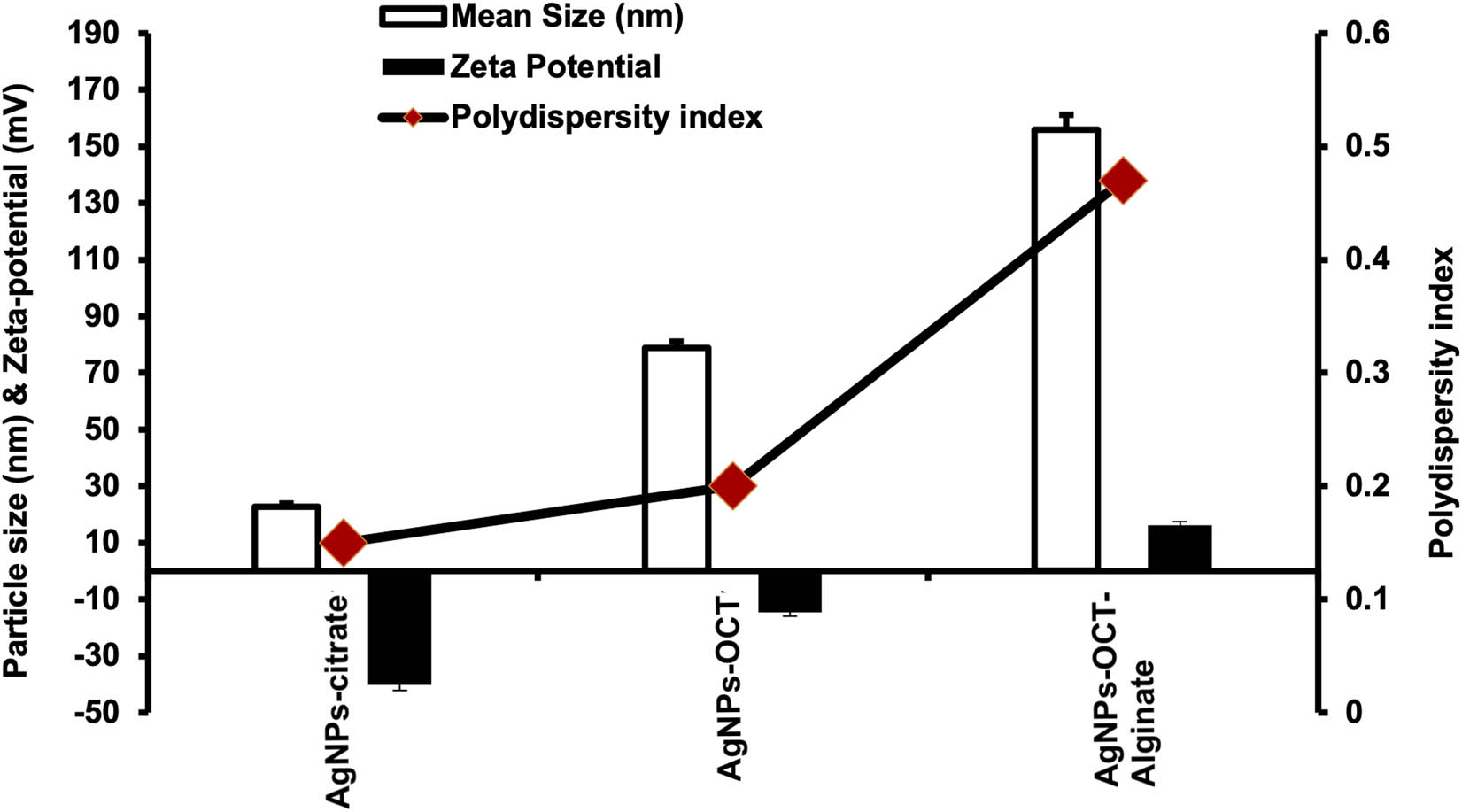
Size distributions (nm), ζ potentials (mV), and polydispersity indices of the AgNPs–CTT, AgNPs–OCT, and AgNPs–OCT–Alg.
3.2 Ultraviolet-visible spectroscopy
Ultraviolet-visible spectroscopy (UV-VIS) was used to record the wavelengths of maximum absorption (λ max) of the AgNPs–CTT, AgNPs–OCT, and AgNPs–OCT–Alg, which were determined as 430.7 ± 2.99, 447.8 ± 2.93, and 450.8 ± 3.5 nm, respectively. The UV-VIS spectrum of the AgNPs–CTT showed redshifts of 17 and 3 nm in the SPR peaks for both AgNPs–OCT and AgNPs–OCT–Alg, respectively (Figure 3) [35].
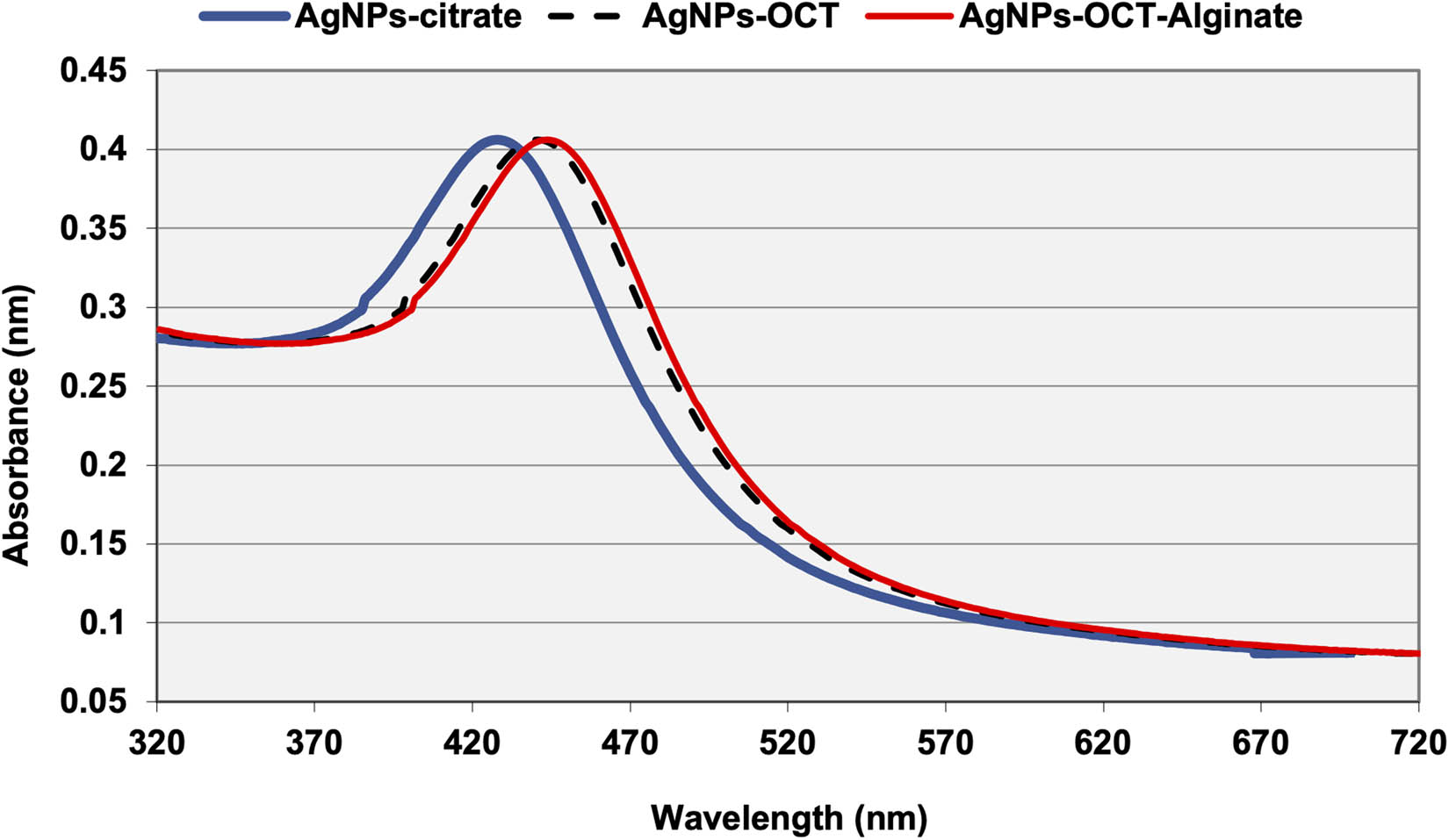
UV-Vis spectra of AgNPs–CTT, AgNPs–OCT and AgNPs–OCT–Alg. The AgNPs–OCT and AgNPs–OCT–Alg showed redshifts in their SPR peaks.
3.3 In vitro treatment of MCF-7 cells with AgNP formulations
The cytotoxic effects of the AgNPs–CTT, AgNPs–OCT, and AgNPs–OCT–Alg on MCF-7 cells were evaluated using different concentrations (0.1–10 µM) of the nanoparticle formulations. The extent of cancer cell proliferation significantly decreased after treatment with different AgNP formulations compared to the proliferation of control cells (Figure 4). In the model cytotoxicity evaluation experiment involving the MCF-7 cell lines, the results showed that treatment of the breast cancer cell lines with AgNPs–CTT, AgNPs–OCT, and AgNPs–OCT–Alg significantly reduced cell proliferation in a concentration-dependent manner (Figure 4). The degree of proliferation was significantly reduced when MCF-7 cells were treated with each of the AgNP formulations for 24 h. The AgNPs–OCT–Alg formulation was more efficient in reducing cell proliferation than the AgNPs–OCT, and AgNPs–CTT formulations after 24 h of incubation (Figure 4). Treatment of the MCF-7 cell lines with 5–10 μM of each of the AgNP formulations revealed that treatment with AgNPs–OCT and AgNPs–OCT–Alg inhibited the proliferation of all cells compared to the cells treated with only the AgNPs–CTT [46].
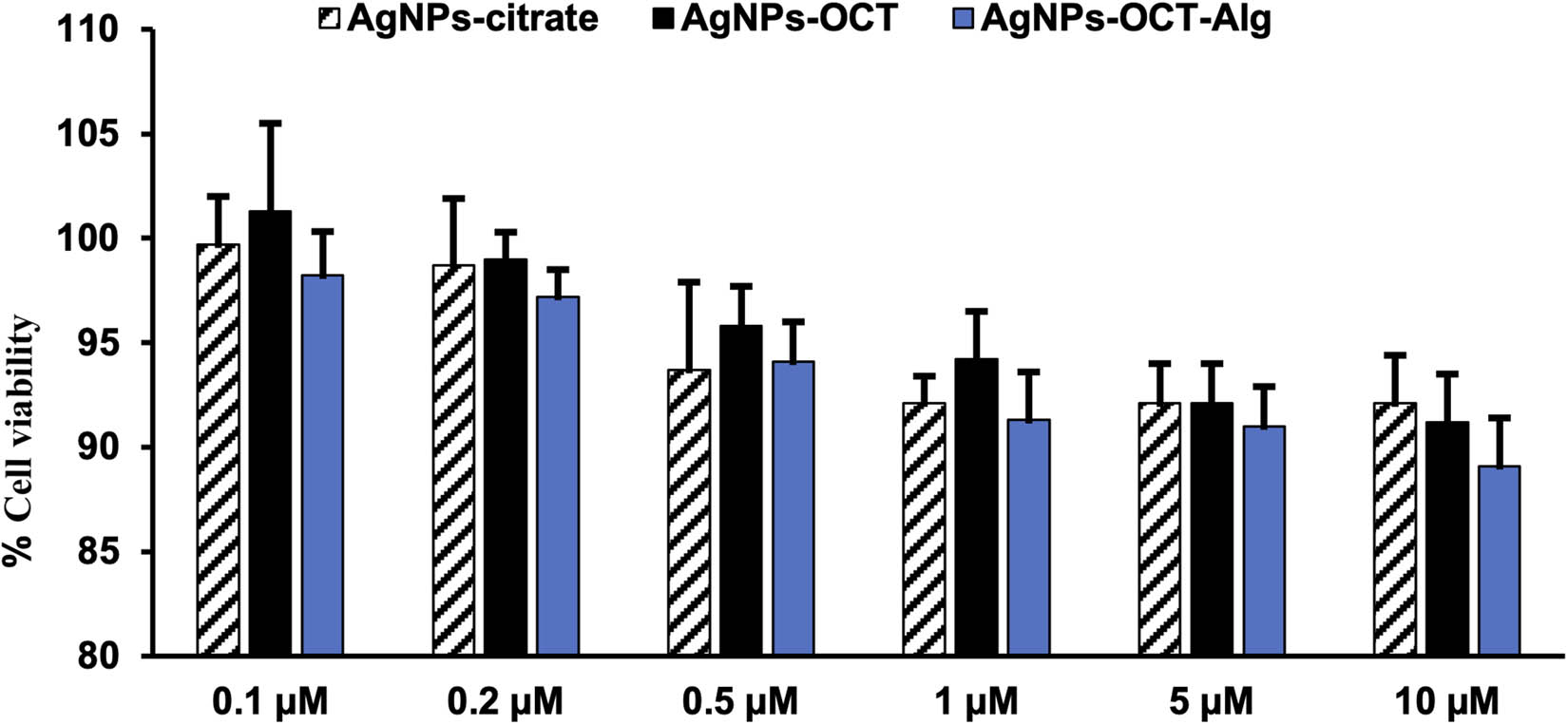
Effects of different AgNP formulations on MCF-7 cell proliferation after treatment with different concentrations (0.1–5 μM) of AgNP formulations. The results are representative (mean ± standard error of the mean) of triplicate experiments; data without an asterisk differ, *p < 0.05 versus untreated cells (control cells). All formulations showed no toxicity, while concentrations of 1–5 µM showed significant inhibition of cell proliferation (p < 0.05).
In addition, the protein levels of TNF-α were determined via ELISA. Treatment with 0.1–10 μM of AgNP preparations significantly decreased the production of TNF-α in the MCF-7 cell lines compared with control cells (Figure 5). The cell cultures treated with 5–10 µM each of the AgNP formulations showed significant suppression (p < 0.05). The level of TNF-α production was higher in the 0.1 µM group than in the control group. The levels of TNF-α decreased significantly in the cells treated with 5 and 10 µM each of the AgNPs–CTT, AgNPs–OCT, and AgNPs–OCT–Alg (p < 0.05) formulations compared with the cells in the control group. These results confirmed that the nanoparticles could be used as cancer formulations at concentrations >5 µM.
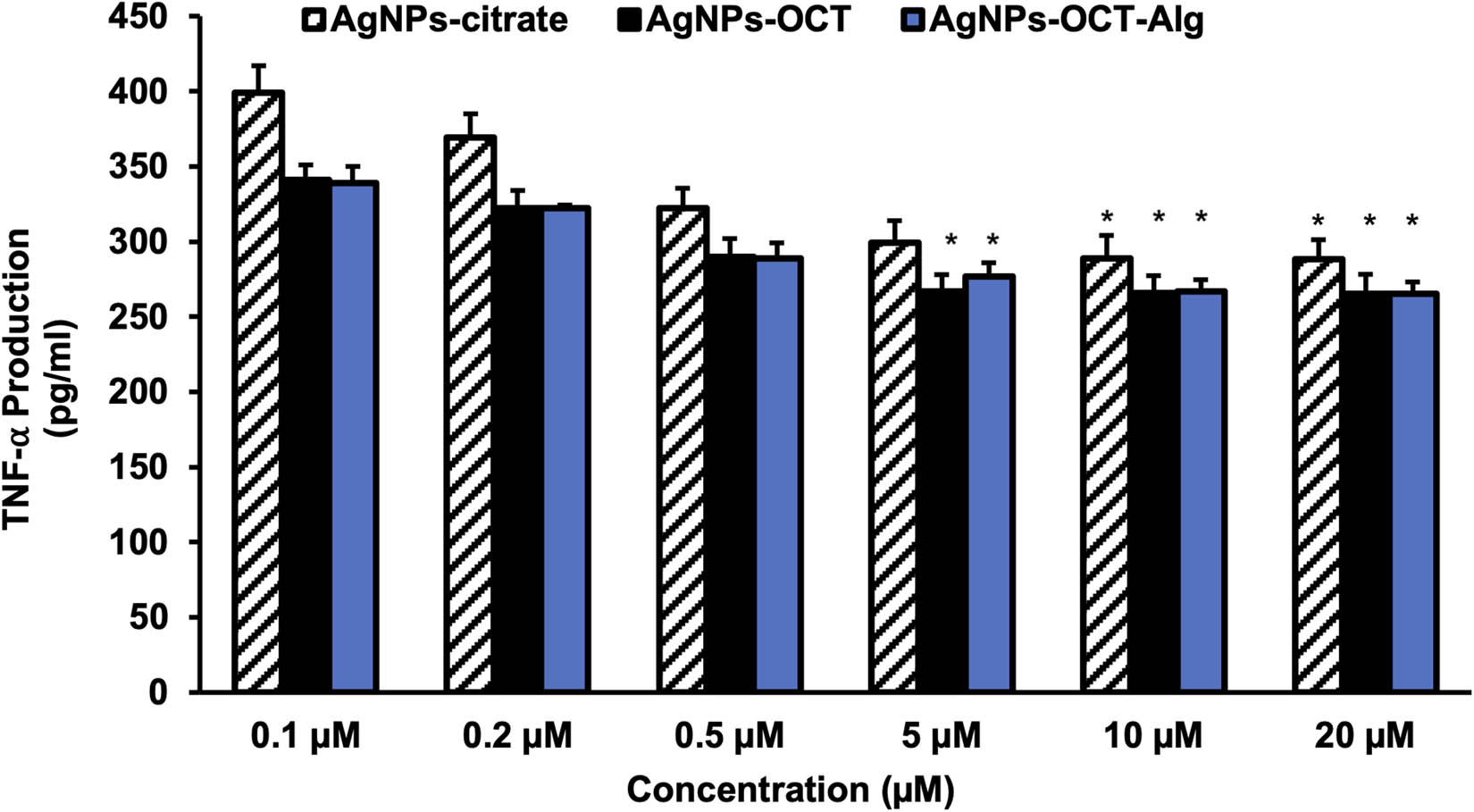
Production of TNF-α after treatment with AgNP formulations in MCF-7 cells. The results are representative (mean ± standard error of the mean) of triplicate experiments. Data without an asterisk differ and concentrations of 5–10 µM showed significant TNF-α inhibition (p < 0.05). *p < 0.05 versus untreated cells (control cells).
Moreover, ICP-OES showed higher levels of internalization of AgNPs–OCT also into the MCF-7 cells. The cellular uptakes of 10 µM formulations of AgNPs–CTT, AgNPs–OCT, and AgNPs–OCT–Alg were determined via ICP-OES after 60 min of incubation. The control MCF-7 cell lines showed negligible quantities of internalized AgNPs, and AgNPs–CTT showed nonsignificant levels of random internalization (p > 0.05). AgNPs–OCT showed more significant levels of internalization at 387 ± 23.6 × 103 per cell (p < 0.001). The amount of accumulated AgNPs decreased to 243 ± 12.6 × 103 in the cells treated with AgNPs–OCT–Alg (p < 0.05). Furthermore, the amounts of internalized AgNPs decreased to 156.88 ± 21.4 × 103 and 126 ± 11.7 × 103 in the AgNPs–OCT and AgNPs–OCT–Alg treated groups, respectively (p < 0.001), when the receptors were occupied by 100 µM of free OCT (Figure 6).
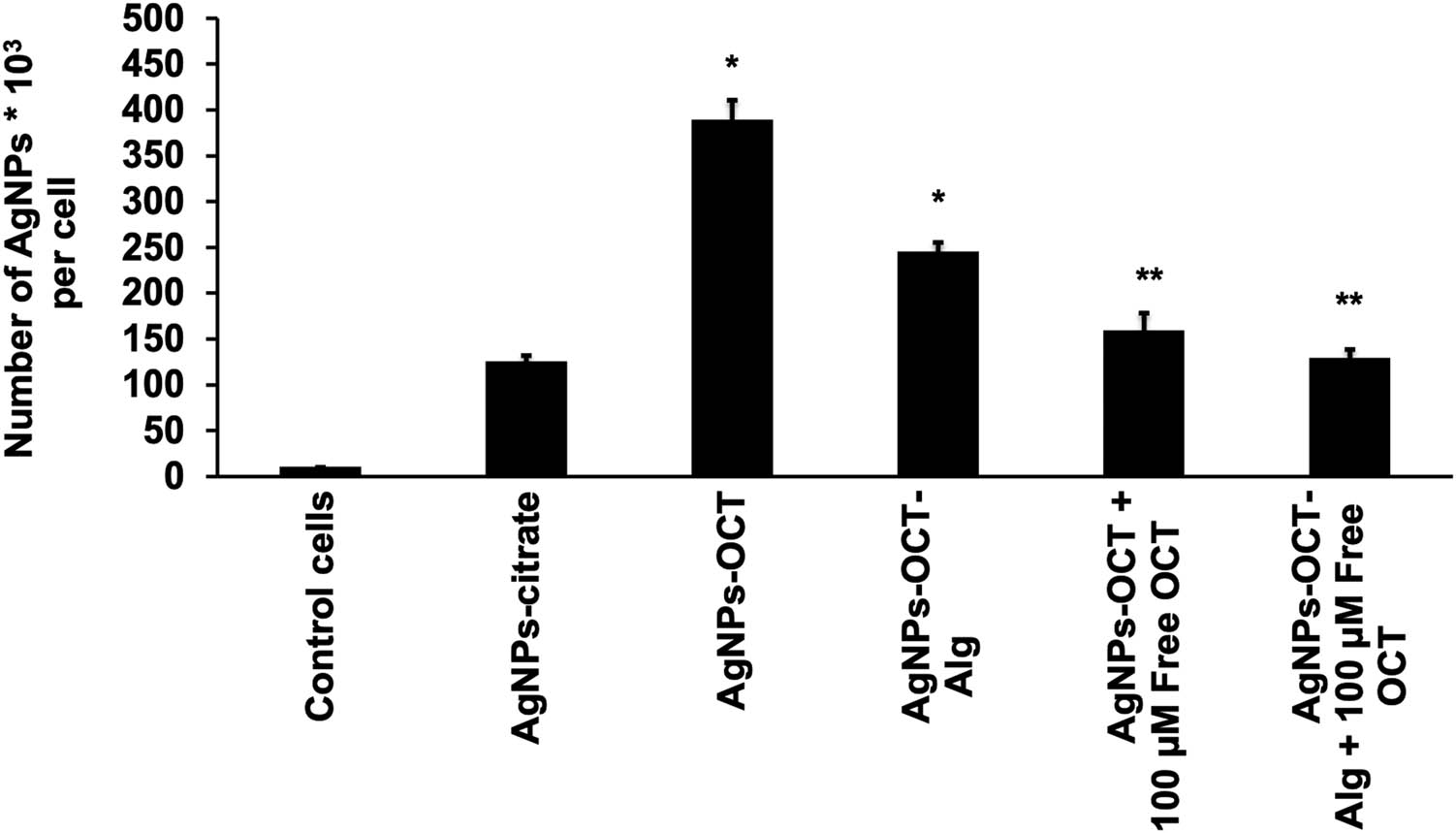
Cellular uptake of the AgNPs–CTT, AgNPs–OCT, and AgNPs–OCT–Alg was determined by ICP-OES and cell counting after 60 min of incubation. The administered concentrations of AgNP formulations were 10 µM. *Statistically significant (p ≤ 0.05; analysis of variance/Tukey’s test). The AgNPs–OCT and AgNPs–OCT–Alg specifically targeted SSTR2, while the level of internalization was decreased because SSTR2 was occupied by free OCT. *p < 0.05 versus untreated cells (control cells), **p < 0.001 versus the group of AgNPs–OCT and AgNPs–OCT–Alg.
3.4 In vivo inhalation of AgNP formulations
The images of inhaled AgNPs in different groups are presented as photomicrographs from lung sections stained with toluidine blue (Figure 7a–c). Figure 7b shows an image of inhaled AgNPs–CTT (arrow) and Figure 7c shows an image of inhaled AgNPs–OCT–Alg (arrow). Also, Figure 7d–f presents the TEM photomicrographs of the ultrathin lung sections. Figure 7e presents an image of inhaled AgNPs–CTT (arrow) and Figure 7f shows an image of inhaled AgNPs–OCT–Alg (arrow). Figure 7g shows the proportions of inhaled AgNPs–CTT, AgNPs–OCT, and AgNPs–OCT–Alg as determined via ICP-OES. Figure 7a shows the absence of inhaled AgNPs in the lungs; Figure 7b shows an image with a low amount of inhaled AgNPs–CTT (red arrow), while Figure 7c shows an image with a significant amount of inhaled AgNPs–OCT–Alg (red arrow) (p < 0.05). The results were confirmed by TEM photomicrographs of ultrathin lung sections (Figure 7d–f). Figure 7d shows the absence of inhaled AgNPs, Figure 7e displays a low amount of inhaled AgNPs–CTT (red arrow), while Figure 7f shows a higher amount of inhaled AgNPs–OCT–Alg (red arrow), which confirms our hypothesis. In addition, the nanoparticles shown in Figure 7e and f were smaller than the artifacts appearing as irregular black spots (blue arrows) in Figure 7d. These results were also confirmed by ICP-OES, which determined the amount of inhaled AgNPs in lung cells. Figure 7g demonstrates the proportion of inhaled AgNPs–CTT, AgNPs–OCT, and AgNPs–OCT–Alg as determined by ICP-OES. The proportion of inhaled AgNPs–OCT–Alg was 0.416 ± 5.7 mg/kgtissue, which confirmed active targeting of the lung tissue, while the proportion of inhaled AgNPs–CTT was 0.199 ± 3.21 mg/kgtissue, which indicated nonspecific uptake leading to Ag+ accumulation. ICP-OES showed that AgNPs–OCT–Alg inhalation led to a higher amount of Ag+ accumulation compared with AgNPs–CTT inhalation (p < 0.001; analysis of variance/Tukey’s post-hoc test). The AgNPs–CTT, AgNPs–OCT, and AgNPs–OCT–Alg formulations were all inhaled after nebulization, and the lung tissues were histologically examined for the presence of AgNPs. Nonspecific and random accumulation of AgNPs–CTT in the lung occurred due to rapid uptake by alveolar cells [47].
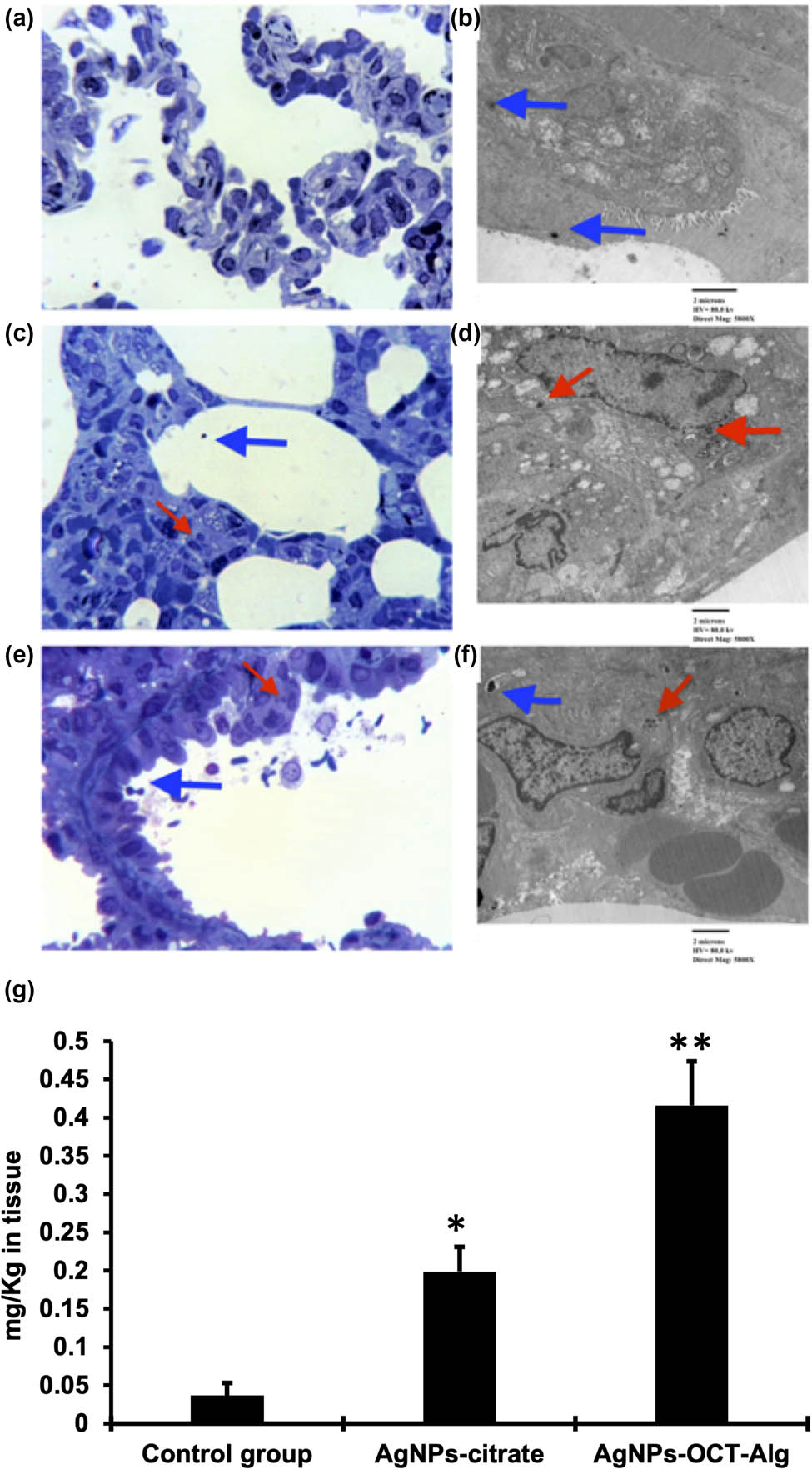
(a–c) Photomicrographs of lung sections stained with toluidine blue. Panel b shows inhaled AgNPs–CTT (red arrow) and panel c shows inhaled AgNPs–OCT–Alg (red arrow); (d–f) transmission electron microscopy (TEM) photomicrographs of ultrathin lung sections; the irregularly shaped, comparatively larger black spots are artifacts shown by blue arrows; (e) inhaled AgNPs–CTT (red arrow); (f) inhaled AgNPs–OCT–Alg (red arrow); (g) the proportions of inhaled AgNPs–CTT, AgNPs–OCT, and AgNPs–OCT–Alg as determined by ICP-OES. The concentrations of AgNPs were 10 µM. *p < 0.05 versus control group, **p < 0.001 versus AgNPs–CTT group and control group.
4 Discussion
In the present study, we modified a previously described chemical reduction technique to synthesize citrate-reduced and capped nanoparticles (AgNPs–CTT) [48]. Due to the reduction of Ag+, neutral silver atoms were formed by the nucleation of Ag+ [49] according to the redox equation, based on the reduction potential of the bulk starting material, as shown below:
Seeding and nucleus production were more likely to occur when Ag+ was used at low concentrations (sub-millimolar range), as used in this study. Constant stirring of the reaction solution was necessary to prevent collision and the formation of silver nuclei in the solution. Temperature and pH are also important factors in the preparation; however, they also affect synthesis and product yields, resulting in changes in the nanoparticle size, charge, and polydispersity [36]. The reduced AgNPs–CTT provided strongly reactive, thermodynamically stable, fully reduced, appropriately citrate-capped AgNPs with high antioxidant potential, functional, specific, and ready-to-conjugate nanopreparations. The reduction process yielded characteristically superior quality by designing nanoparticles for further use in the preparation. Bulk materials are very sluggish in reacting and producing conjugates, have been improved to highly chemically reactive, energy-absorbing nanopreparations, and have provided the functional material for further use in preparing desired nanoparticles with site-specific targeting. The yield% was calculated for Ag0 synthesized in the AgNPs–CTT, AgNPs–OCT, AgNPs–OCT–Alg using ICP-OES to be 2.82 ± 0.016 mg/100 mL (≈ 77.9%), 2.43 ± 0.21 mg/100 mL (≈ 67.12%), and 2.166 ± 0.19 mg/100 mL (≈ 59.83%), respectively. Although the yield percentage does not reach 100% with the current synthesis method, while an acceptable yield percentage was attained for all kinds of AgNPs. Inconsistencies between reported and theoretical Ag inputs can also be ascribed to Ag+ or AgNPs adsorption on centrifuge tubes or pipettes, volume loss, and transportation during sample transfer for dilution and quantification [25].
Nanoparticle delivery to the lungs suffers from two significant drawbacks, one of which occurs prior to administration and the other after administration. First, nanoparticle aggregation occurs more frequently in an aqueous medium after long-term storage. Second, the smaller-sized nanoparticles are quickly exhaled unless they are delivered and deposited deep inside the lungs [50,51]. The AgNPs–OCT–Alg was designed as a formulation carrier to overcome the problems associated with pulmonary delivery and the aggregation of nanoparticles to achieve better delivery and deposition in the lungs. The formulated nanoparticles incorporated an alginate layer, which acted as an outer coat and worked as a better carrier than the nanoparticulate formulations, AgNPs–CTT and AgNPs–OCT, which are easily exhaled upon administration among the animals. The alginate-coated AgNPs–OCT forming AgNPs–OCT–Ag was a facile delivery carrier for nebulization. There was no chemical bonding between the outer alginate coating and the nanoparticles themselves, as the alginate layer was merely a physical wrap around the nano-entity. Due to the weight and size of the AgNPs–OCT–Alg, it was successfully delivered into the lungs without exhalation, which was observed with the noncoated AgNPs–CTT and AgNPs–OCT formulations [52]. Moreover, the alginate wrapping was soluble in aqueous media. The alginate wrappings of AgNPs–OCT–Alg only served as a medium for effective delivery and dispersion. Nonetheless, the fragility of the alginate coating was demonstrated by the UV absorption maxima for AgNPs–OCT and AgNPs–OCT–Alg. Both of these formulations showed near similar values at 447.8 ± 2.93 and 450.8 ± 3.5 nm (Figure 3), respectively, wherein the λ max determined via UV-VIS of both the nanoparticle formulations, AgNPs–CTT and AgNPs–OCT, was nearly unaffected by the alginate coating, with a 3 nm shift of the absorption maxima. The alginate wrapped around the nanoparticles easily and quickly dissipated into the aqueous-based media in the lungs of the animal models. The AgNPs–CTT and the AgNPs–OCT–Alg treatment groups were compared to confirm the feasibility of nonspecific and specific delivery of the carrier-based formulations. The alginate coating was only used to facilitate the inhalation of the AgNPs–OCT formulation, which was not effectively inhaled. The alginate layer (in the AgNPs–OCT–Alg formulation) facilitated delivery through the inhalation route and nearly diminished the exhalation of the AgNPs–OCT formulation. The alginate wrapping was replaced in aqueous media, both in the in vivo (inhalation among the rats) and in vitro (MCF7 cancer cells) models, and resulted in internalization at higher levels than the non-OCT citrate-capped formulation (AgNPs–CTT) (Figure 8). The SPR redshifts were observed due to the coupling of AgNPs–CTT with the OCT and alginate wrapping of the AgNPs–OCT. The optical properties of all AgNPs depended on the diameters of the preparations, and the peaks shifted toward longer wavelengths (redshift); therefore, the large-sized particles had larger optical cross-sections, and their albedo (the reflected fraction of SPR) increased similar to the redshifts with the increased size [35]. In the case of the AgNPs–OCT–Alg, the redshift was 3 nm, which signified the least changes in the SPR shifts (nearly no structural change, except noncovalent coating between the AgNPs–OCT and the AgNPs–OCT–Alg). The final products were citrate-capped nanoparticles (AgNPs–CTT), OCT-chemically-conjugated-to-citrate-nanoparticles (AgNPs–OCT), and the alginate-wrapped AgNPs–OCT–Ag. The absorption for the covalently linked alginate was much higher. However, in this case, the AgNPs–OCT was treated in an alginate solution, dried to produce the alginate-wrapped nanoparticles (a thin coating/film around the AgNPs–OCT) as AgNPs–OCT–Alg, and the chemically conjugated (absorption being higher) and nonconjugated (with no chemical bond) formulations showed comparatively low levels of absorption. As the nanoparticles were in aqueous media, alginate displacement yielded very little change in the absorption, as in this spectrum recording event, alginate was displaced with enough water in the medium. All other UV recordings were also performed using water as the medium. All three preparations were also of different colors. The smaller difference of 3 nm was a result of the effect of alginate. UV spectra were recorded for the AgNPs–OCT–Alg product, a characterized material with size, charge, and shape specifications. The small shift (redshift) of <3 nm was in agreement with the layer-by-layer coating of AgNPs reported by Detsri et al. [53].
Schematic representation of the different types of AgNPs applied in in vitro and in vivo experiments.
Therefore, due to nanoparticles, AgNPs–OCT–Alg increased in size and was found to be more effectively delivered into the lungs through inhalation. As reported previously, for a carrier of suitable (nano-metric) size, entrapment in the lungs is better for achieving pulmonary deposition (Figure 9) [54]. The size of the AgNPs–OCT–Alg was intended to be increased, and the size of AgNPs increased from 22.77 ± 1.1 nm for the AgNPs–CTT to 78.77 ± 2.3 nm for the AgNPs–OCT. Finally, the coated nanoformulation, AgNPs–OCT–Alg, resulted in an average size of 155.99 ± 5.2 nm, which could be effectively delivered to the lungs. The alginate coating has been proposed to be dissolved after formulation delivery since there is no chemical bonding between the alginate and the surface-located OCT of the AgNPs–OCT, except for the physical wrappings of the alginate coating around the AgNPs–OCT. However, a chemical bond forms between the AgNPs–CTT and OCT to produce AgNPs–OCT from the AgNPs–CTT based carboxylate and the NH2 group of the OCT forming an amide linkage, which is stable enough for delivery and deposition [45,55]. The Ag+ internalizations of the formulations, AgNPs–CTT and AgNPs–OCT–Alg, were compared in terms of site-specific delivery and Ag+ internalizations by the alginate-coated nanoparticles and noncoated, non-OCT-capped AgNPs–CTT. There was no need to compare the AgNPs–CTT and AgNPs–OCT, as the OCT coating itself was target-specific (targeting SSTRs) and was expected to yield higher levels of internalization of Ag+.
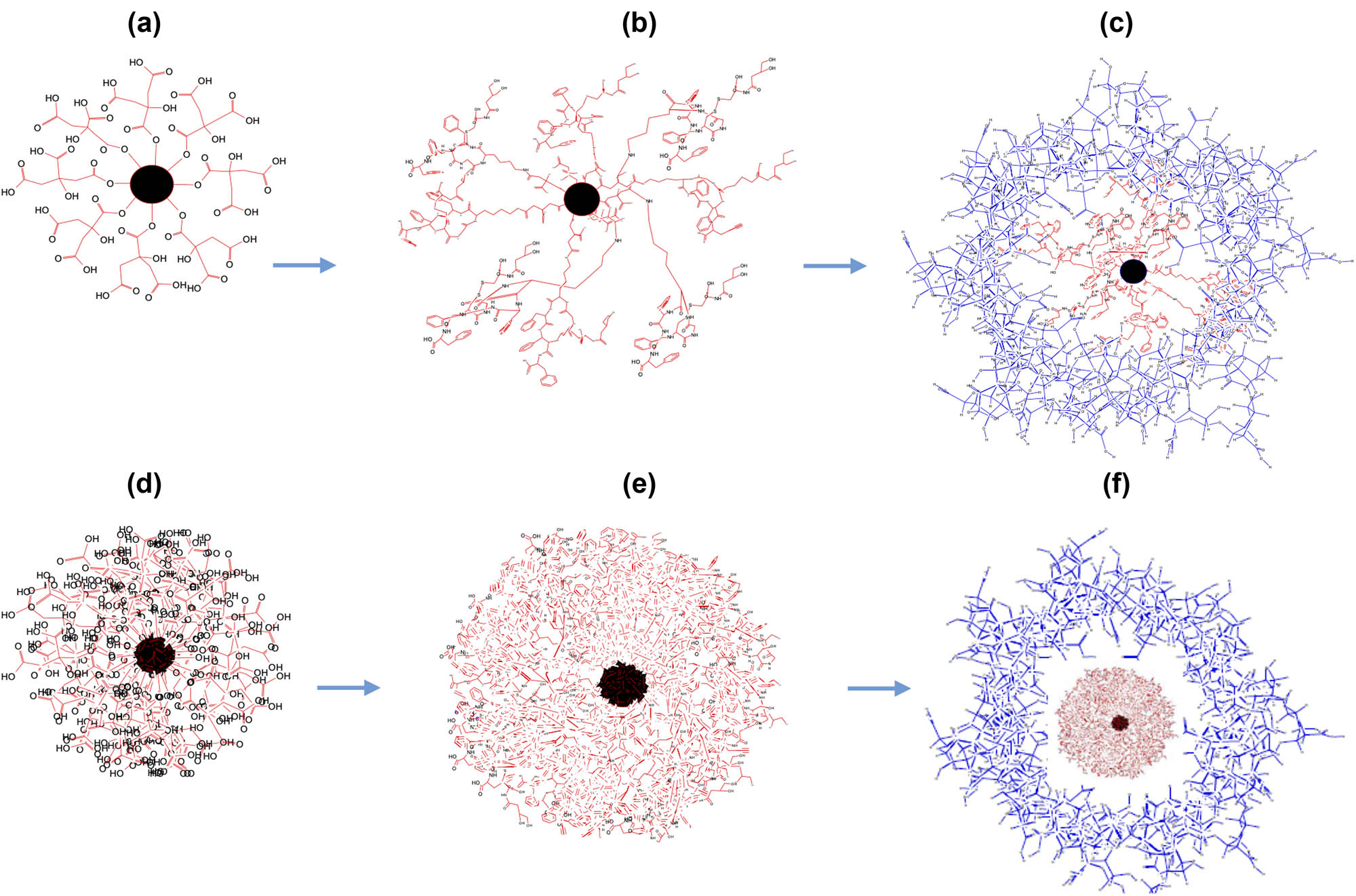
Structural representations of the AgNP nanocarriers; (a–c) 2D renderings, (a) AgNPs–CTT (citrate bound to the nanoparticle, nanoparticle depicted in black). (b) AgNPs–OCT (OCT covalently bound to citrate, CTT). (c) AgNPs–OCT–Alg (no chemical bonding; instead, physical wrapping of the alginate around the AgNPs–OCT. Alg: alginate, coatings shown in blue). 3D renderings of the AgNPs. (d–f) 3D renderings, (d) AgNPs–CTT. (e) AgNPs–CTT–OCT. (f) AgNPs–OCT–Alg. Alginate coatings are not chemically bound. The core of the formulated AgNPs–OCT is shown in red, while the surrounding alginate wrapping is shown in blue.
The AgNPs–OCT–Alg could be considered as an innovative therapy for lung diseases, as it imparts SSTR analogs and several drugs with the potential for delivery due to the capacity for attachment to the AgNPs–CTT nanopreparation. All types of AgNP formulations (AgNPs–CTT, AgNPs–OCT, and AgNPs–OCT–Alg) were successfully prepared, characterized, and biologically tested in the present study. The conjugation of the AgNPs–CTT with OCT and alginate coatings were analyzed and confirmed through spectrophotometric analyses, morphological observations, and ζ potential measurements. The AgNPs–OCT formulation was optimized for lung delivery through dispersion in a sodium alginate aqueous solution (1%) as a coat around the AgNPs–OCT. The successful coupling of AgNPs–CTT with OCT and alginate coating to form AgNPs–OCT–Alg showed a surface charge of −40.17 ± 2 mV, with an increase to −14.13 ± 1.3 mV for AgNPs–CTT, and AgNPs–CTT–OCT (AgNPs–OCT), to a positive (inverted sign) potential of +16.17 ± 1.3 mV upon alginate treatment. The changes in value and reversal in the ζ potential sign confirmed the successful coupling of OCT and coating of alginate onto the surface of the AgNPs–CTT and the AgNPs–OCT wrappings, respectively. The AgNPs–OCT preparation showed higher stability due to its high ζ potential [56]. These results were in agreement with the results obtained by Lu et al., who established that distinct stability is achievable when the ζ potential values are approximately within +10 to −10 mV [57]. Initial preparation involving aqueous media reaction between TSC and AgNO3 produced AgNPs–CTT, which were brown and produced the characteristic SPR absorption bands [58].
Figure 9 presents plausible 2D and 3D shapes, physical, and chemical bonding representations of the AgNPs–CTT, AgNPs–OCT, and AgNPs–OCT–Alg nanopreparations throughout their stages of preparation and developmental processes (Figure 9a–f). The present study aimed to actively target the alginate-coated AgNPs (AgNPs–CTT–OCT–Alg or AgNPs–OCT–Alg) to SSTRs using the surface-attached ligand of SSTR (OCT) as the site-directed specific ligand for active and accurate delivery to the lung tissue.
To study the cellular uptake and displacement of all formulated AgNPs, we used MCF-7 cells as a model cell line that express SSTRs, which specifically express SSTRs as studied previously at our laboratory [59]. The expression of SSTR2 in MCF-7 cells was confirmed by binding with OCT-conjugated fluorescein isothiocyanate, using BON-1 cells as positive controls [44]. Besides, the mRNA level of SSTR2, as determined by reverse transcription polymerase chain reaction assay, was significantly higher in MCF-7 cells than in BON-1 cells as positive controls. The results confirmed that the AgNPs–OCT–Alg was effective in delivering the formulation to cell lines under in vitro conditions, and successfully and effectively delivered the AgNPs–OCT–Alg formulation to effectively deposit into the lung tissues within the optimal concentration limits, between 5 and 10 µM, for in vivo anticancer activity in rat models. The lowest concentration that induced anticancer activity in vivo has also been investigated [46,60]. The half-maximal inhibitory concentration (IC50) values were observed at 5–10 and 10 µM under in vitro and in vivo conditions, respectively. Moreover, the IC50 for AgNPs–CTT was also found to be less than 20 µM [30]. As TNF-α promotes the proliferation of cancer cells [61], it was also observed that AgNPs–OCT significantly inhibited TNF-α production. Moreover, the cells were affected by the minimum concentration of AgNPs–OCT. A higher concentration of all AgNP preparations (10 µM) was used to assess their antiproliferative activity, as suggested by Xu et al. [62]. The researchers confirmed that the toxicity of AgNPs–CTT increased at concentrations >10 µg/mL, while AgNPs–CTT (5 μg/mL) tended to show comparatively reduced levels of cytotoxicity. Moreover, ICP-OES showed higher levels of internalization of AgNPs in the form of AgNPs–OCT in in vitro tests due to the direct interaction between OCT and SSTR2 expressed on the surfaces of the MCF-7 cells. In addition, the weak interactions between AgNPs–OCT–Alg and MCF-7 cells due to alginate coating on the surface of the nanoparticles initially formed a barrier between the OCT binding sites and SSTR2.
The selectivity for SSTR2 was confirmed based on the occupation of the receptors with 100 µM free OCT, and the binding of the nanoparticulate formulations to the receptor was observed to be significantly reduced under these conditions [59,63,64]. Moreover, these results showed lower levels of internalization of the nanoparticle formulations in the cells, as binding was reduced in the presence of free OCT, which preferentially and quickly blocked and occupied the SSTR2. The AgNP formulations were applied at concentrations described in a previous report published by Xu et al. [62]. The levels of TNF-α also showed a marked decline, as determined via ELISA. The AgNP formulations at concentrations of 0.1–10 μM significantly decreased TNF-α production in MCF-7 cells. Moreover, the 10 µM AgNP formulations showed the most significant levels of suppression of TNF-α, with no additional decrease in TNF-α production at higher nanoparticle concentrations [30]. This significant decline in TNF-α production indicated that AgNPs–OCT could be an effective material to target cancer cells, particularly, breast cancer cells, while decreasing TNF-α production. The results obtained also showed promise in targeting breast cancer since the AgNPs–OCT and AgNPs–OCT–Alg were better designed for active targeting with an increased cellular accumulation of nanoparticles. Active targeting of breast cancer is a promising strategy for efficient drug delivery [65]. Many target sites can be reached in breast tissue for site-specific and active targeting [65]. SSTR2 and SSTR5 are expressed in neuroendocrine breast cancer and are considered potential targets for the treatment of breast cancer in the future [66,67]. Li et al. [68] reported a novel active targeting approach established on L-type amino acid transporter 1 (LAT1) expressed in a range of cancers. Poly(lactic-co-glycolic acid) nanoparticles have also been investigated among MCF-7 cells. Compared to unmodified nanoparticles, LAT1-targeting nanoparticles showed a considerable increase in cellular uptake and cytotoxicity.
The AgNPs–OCT–Alg formulation is stabilized primarily by the adsorption of alginate (Alg) onto the surface of AgNPs–OCT [69]. The produced nanomaterials (AgNPs–CTT and AgNPs–OCT) are comparatively smaller in size and lighter in weight, which implies that upon inhalation, these AgNPs preparations will be either partially or completely exhaled without deposition into the lungs. The alginate coating provides additional increases in layering, weightage, thickness, and size to yield a larger and effective nanoscale carrier (approximately, 155.99 nm) with good flow properties to target the required regions in the lungs and with minimum or no exhalation of the formulation. After accumulating in the lungs, the AgNPs–OCT–Alg attaches to the surface of the pulmonary alveoli, and the coated nanoparticle formulation is presented in the vicinity of the epithelial cells for delivery into the lung cells [70]. These sizes of deposition are in agreement with the report published by Brown [71], which stated that particle deposition in the respiratory tract occurs mainly by diffusion, sedimentation, and impaction. The total amount deposited in the respiratory tract follows a “U-shaped” curve, with a minimum deposition of nanoparticles with sizes ranging between 0.1 and 1.0 μm, with enhanced diffusive deposition. Han et al. [72], formulated gold nanoparticles (AuNPs) and studied the effect of size on the biodistribution of AuNPs among Sprague–Dawley rats via inhalation. The researchers used small AuNPs (with diameters of 13–105 nm and concentrations of 12.8–13.7 µg/m). The animals were exposed to AuNPs for 5 days (6 h/day). They found that the exposed rats exhibited no toxic responses to AuNPs. Regardless of size, both small and large AuNPs were deposited in the lungs of rats. The biodistribution of small AuNPs from the lungs to secondary target organs was significantly higher when compared to those of large AuNPs. Moreover, nanoparticles with diameters smaller than 100 nm were also probably deposited [73,74].
Upon reaching the lung tissues, the alginate layer (Alg) is expected to dissolve slowly in the presence of aqueous media based on the presence of water/moisture, swelling, and removal of the alginate coating, and AgNPs–OCT is released from the wrapped coating of alginate. The AgNPs–OCT escapes the initial mucociliary clearance of the lungs and crosses the epithelial layer to target lung cancer through OCT, and cancer cell surfaces present SSTR interactions for deposition deep in the lung tissues. Alginate, a highly hydrophilic ionic material (in the form of Alg), is expected to dissolve within a short time, as recorded in the UV-VIS absorption experiments as well as in the tests of the accumulation of AgNPs in nanoformulation, wherein the coated material accumulated to a lesser extent than did the free OCT.
Healthy rats were used for the in vivo study as reported to express the SSTR2 [75], which was our target to prove. The OCT ligand-coated AgNPs–OCT was capable of specifically targeting SSTRs located in the lung tissues. The ability of these AgNP formulations to target SSTRs expressed in lungs is also potentially beneficial in the future treatment of other cancers that express SSTRs. The results obtained in targeting the lungs in the present study also corroborated a previous finding that SST analogs coated with AuNPs target SSTR-expressing cancer cells [14]. Other studies also provided details on VAP-coated quantum dots (QDs) and showed that the formulated VAP-QDs can be delivered to BON-1 cells overexpressing SSTR2 [17]. SST has a longer peptide sequence, while OCT has a shorter sequence that is feasible in many ways for conjugation, targeting, and delivery. OCT also selectively binds to SSTR2 [76]. Biomechanical considerations for current targeting by OCT have been proposed, wherein a binding interface has been depicted that exhibits both hydrophobic and hydrophilic interactions between the peptide residues. Ala-Gly-*Cys(cyclo)-Lys-Asn-Phe-Trp-Lys-Thr-Phe-Ser-*Cys-(*cyclo)-OH is the amino acid sequence of SSTR2 that is involved in binding with the Phe-Lys residues of OCT. A preliminary binding model of the interaction between the SSTR and OCT is shown in Figure 1. The pharmacophore-containing residues Phe-Trp-Lys-Thr of the SSTR bind with the incoming ligand to exert its biological activity. A parallel and detailed mechanistic study of the binding interactions is available [77].
The AgNPs–CTT, AgNPs–OCT, and AgNPs–OCT–Alg formulations were inhaled via nebulization at a dose for each formulation 1.27 mg/kg. This dose was selected based on the results of in vitro cytotoxicity and previously reported results of in vivo experiments [12,38,78,79]. All formulations were used in the liquid form to be nebulized and not in dry form, as the dried form of AgNPs has the potential for aggregation and cannot be resuspended. Freeze-drying of AgNPs induces aggregation [80]. The animals successfully inhaled the formulated AgNPs without experiencing drowsiness or coma. The AgNPs–OCT–Alg was more viscous than the other formulas but was successfully nebulized. To determine the amounts of all AgNPs, the lungs in each group of animals were digested and examined for the inhalation of AgNPs. Upon pulmonary delivery, the AgNPs–CTT accumulated in the lungs due to rapid lymphatic uptake through nonspecific binding interactions, while the AgNPs–OCT and AgNPs–OCT–Alg formulations accumulated via specific and targeted delivery. The proportion of inhaled AgNPs–OCT–Alg was 0.416 ± 5.7 mg/kgtissue, which confirmed the successful active targeting of the lung tissue, while only 0.199 ± 3.21 mg/kgtissue was detected for AgNPs–CTT, indicating nonspecific uptake of Ag+. The lungs showed a higher accumulation of AgNPs–OCT and AgNPs–OCT–Alg due to the expression of SSTRs on the lung cells, which specifically bound to the AgNPs-conjugated OCT [47,81,82]. This form of targeting is active, specific, and selective for lung cancer cells, such as those of non-small cell lung cancer. The AgNPs–OCT–Alg was internalized via active targeting, while a parallel report described the internalization of AgNPs into A549 human alveolar cells via nonspecific targeting [83].
However, numerous new drugs have shown high levels of cytotoxicity, mainly due to myelosuppression. Indeed, the delivery of cytotoxic agents that can selectively target tumor cells is useful in terms of controlling concentration by way of frequency and quantity. A reduction in peripheral toxicity also improves the therapeutic efficacy of chemotherapeutic agents [8]. Previous studies have reported a number of approaches to targeted drug delivery through specific tags on delivery carriers or at the site, compatible with the double-ended molecular structure. Reubi demonstrated that certain SST analogs labeled with radionuclides (111In, 90Y, 177Lu, and 68Ga) and injected intravenously in mice resulted in reductions in tumor masses [84]. Laznickova et al. used materials labeled with radioactive compounds to target SST analogs for diagnostic imaging and the treatment of tumors expressing SSTRs [85]. Moreover, it has also been reported that nanoparticulate formulations provide controlled and sustained drug release in the lung tissue, which supplements reduce the frequency of dosing and complement patient compliance. These approaches of targeted delivery, particularly, delivery to the lungs, further provided impetus to our proposal for the alginate-masked and OCT-mediated site-specific and well-targeted delivery of the lungs mediated by SSTRs.
In conclusion, the functionalized AgNPs were successfully produced using the citrate reduction method, and the synthesized AgNPs–CTT was chemically conjugated with OCT to target SSTRs, particularly, the SSTR2. We developed and evaluated a formulation (AgNPs–OCT–Alg) that was capable of delivery to and internalization in the healthy lung tissues through the nebulization route in the animal model. An array of better prospects exists for the AgNPs–OCT–Alg formulation to serve as a specific, active, site-directed, and on-site method of drug delivery by using the currently presented noninvasive drug delivery method, which is plausible and holds further promise. The prepared AgNPs–OCT–Alg successfully targeted healthy lung tissues in vivo in a rat model, and in in vitro condition in the model breast cancer cell line, MCF-7, through binding to SSTRs. We analytically confirmed that the AgNPs–CTT was coated with OCT and the alginate wrappings. The presence of OCT in the nanoformulations, AgNPs–OCT and AgNPs–OCT–Alg, assisted in on-site delivery and cell targeting. MCF-7 cells showed specific interactions with the prepared AgNPs–OCT–Alg formulation. Moreover, the AgNPs–OCT and AgNPs–OCT–Alg exhibited higher internalization in the lung tissues due to the facile inhalation strategy and subsequent internalization. The in vivo experiments also exhibited high levels of biodistribution and high concentrations of AgNPs in the lung tissue, as confirmed by the presence of Ag+ in ICP-OES analyses of several lung tissue samples obtained from various animal experimental groups treated with different nanoformulations and their varying dose concentrations. Further studies are needed for future lung cancer targeting. Also, more studies of animal lung cancer models are required to reflect the accuracy of active targeting of SSTRs by the AgNPs–OCT–Alg in lung tissues and lung cancer.
Acknowledgements
The researchers would like to thank the Deanship of Scientific Research, Qassim University, for supporting this project.
-
Funding information: The researchers gratefully acknowledge Qassim University, represented by the Deanship of Scientific Research, on the financial support for this research under the grant number pharmacy-2019-2-2-I-5551 during the academic years 2019/2021.
-
Author contributions: Conceptualization: A.A.H.A.; Data curation: A.A.H.A., A.H.A., A.A., O.A.R., and A.M.M.; formal analysis: A.A.H.A., R.A.K., and A.M.M.; funding acquisition: A.A.H.A., A.H.A., and O.A.R.; investigation: A.A.H.A., S.M.S., M.A., O.A.R., and A.M.M; methodology: A.A.H.A., S.M.S., A.M.M., and A.M.M.; project administration: A.A.H.A., A.H.A., A.A., and M.A.; resources: A.A.H.A., A.A., and A.M.M.; software: A.A.H.A., M.A., R.A.K., and A.M.M.; supervision: A.A.H.A., A.A., M.A., and O.A.R.; validation: A.A.H.A. and R.A.K.; visualization, A.H.A., and A.M.M.; writing–original draft: A.A.H.A., R.A.K., A.A., S.M.S., and A.M.M.; writing–review and editing: A.A.H.A., R.A.K., and A.M.M. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Alghamdi HI, Alshehri AF, Farhat GN. An overview of mortality & predictors of small-cell and non-small cell lung cancer among Saudi patients. J Epidemiol Glob Health. 2018;7(Suppl 1):S1–6.10.1016/j.jegh.2017.09.004Suche in Google Scholar PubMed PubMed Central
[2] Vergnenegre A, Chouaid C. Review of economic analyses of treatment for non-small-cell lung cancer (NSCLC). Expert Rev Pharmacoecon Outcomes Res. 2018;18(5):519–28.10.1080/14737167.2018.1485099Suche in Google Scholar PubMed
[3] Rosenberg SA, Niglio SA, Jo VY, Goydos JS. Interdigitating dendritic cell sarcoma presenting in the skin: diagnosis and the role of surgical resection, chemotherapy and radiotherapy in management. Rare Tumors. 2014;6(4):5573.10.4081/rt.2014.5573Suche in Google Scholar PubMed PubMed Central
[4] Doroshow JH. Redox modulation of chemotherapy-induced tumor cell killing and normal tissue toxicity. J Natl Cancer Inst. 2006;98(4):223–5.10.1093/jnci/djj065Suche in Google Scholar PubMed
[5] Garbuzenko OB, Mainelis G, Taratula O, Minko T. Inhalation treatment of lung cancer: the influence of composition, size and shape of nanocarriers on their lung accumulation and retention. Cancer Biol Med. 2014;11(1):44–55.Suche in Google Scholar
[6] Cooper A, Potter T, Luker T. Prediction of efficacious inhalation lung doses via the use of in silico lung retention quantitative structure-activity relationship models and in vitro potency screens. Drug Metab Dispos. 2010;38(12):2218–25.10.1124/dmd.110.034462Suche in Google Scholar PubMed
[7] Alotaibi RM, Rezk HR, Juliana CI, Guure C. Breast cancer mortality in Saudi Arabia: modelling observed and unobserved factors. PLoS One. 2018;13(10):e0206148.10.1371/journal.pone.0206148Suche in Google Scholar PubMed PubMed Central
[8] Gupta V, Liu YY. New insights on glucosylceramide synthase in cancer drug resistance and myelosuppression. Biochem Pharmacol (Los Angel). 2013;2:120.Suche in Google Scholar
[9] AnnuAhmed, S, Kaur G, Sharma P, Singh S, Ikram S. Evaluation of the antioxidant, antibacterial and anticancer (lung cancer cell line A549) activity of Punica granatum mediated silver nanoparticles. Toxicol Res (Camb). 2018;7(5):923–30.10.1039/C8TX00103KSuche in Google Scholar
[10] Burdusel AC, Gherasim O, Grumezescu AM, Mogoantă L, Ficai A, Andronescu E. Biomedical applications of silver nanoparticles: an up-to-date overview. Nanomaterials (Basel). 2018;8(9):681.10.3390/nano8090681Suche in Google Scholar PubMed PubMed Central
[11] Kim JK, Kim HP, Park JD, Ahn K, Kim WY, Gulumian M, et al. Lung retention and particokinetics of silver and gold nanoparticles in rats following subacute inhalation co-exposure. Part Fibre Toxicol. 2021;18(1):5.10.1186/s12989-021-00397-zSuche in Google Scholar PubMed PubMed Central
[12] Jo MS, Kim JK, Kim Y, Kim HP, Kim HS, Ahn K, et al. Mode of silver clearance following 28-day inhalation exposure to silver nanoparticles determined from lung burden assessment including post-exposure observation periods. Arch Toxicol. 2020;94(3):773–84.10.1007/s00204-020-02660-2Suche in Google Scholar PubMed
[13] Braakhuis HM, Cassee FR, Fokkens PH, de la Fonteyne LJ, Oomen AG, Krystek P, et al. Identification of the appropriate dose metric for pulmonary inflammation of silver nanoparticles in an inhalation toxicity study. Nanotoxicology. 2016;10(1):63–73.10.3109/17435390.2015.1012184Suche in Google Scholar
[14] Abdellatif AA, Zayed G, El-Bakry A, Zaky A, Saleem IY, Tawfeek HM. Novel gold nanoparticles coated with somatostatin as a potential delivery system for targeting somatostatin receptors. Drug Dev Ind Pharm. 2016;42(11):1782–91.10.3109/03639045.2016.1173052Suche in Google Scholar PubMed
[15] Lehman JM, Hoeksema MD, Staub J, Qian J, Harris B, Callison JC, et al. Somatostatin receptor 2 signaling promotes growth and tumor survival in small-cell lung cancer. Int J Cancer. 2019;144(5):1104–14.10.1002/ijc.31771Suche in Google Scholar PubMed PubMed Central
[16] Stumpf C, Kaemmerer D, Neubauer E, Sanger J, Schulz S, Lupp A. Somatostatin and CXCR4 expression patterns in adenocarcinoma and squamous cell carcinoma of the lung relative to small cell lung cancer. J Cancer Res Clin Oncol. 2018;144(10):1921–32.10.1007/s00432-018-2722-5Suche in Google Scholar PubMed
[17] Abdellatif AAH, Abou-Taleb HA, Abd El Ghany AA, Lutz I, Bouazzaoui A. Targeting of somatostatin receptors expressed in blood cells using quantum dots coated with vapreotide. Saudi Pharm J. 2018;26(8):1162–9.10.1016/j.jsps.2018.07.004Suche in Google Scholar PubMed PubMed Central
[18] Abdellatif AAH, Aldalaen SM, Faisal W, Tawfeek HM. Somatostatin receptors as a new active targeting sites for nanoparticles. Saudi Pharm J. 2018;26(7):1051–9.10.1016/j.jsps.2018.05.014Suche in Google Scholar PubMed PubMed Central
[19] McMahon KA, Zajicek H, Li WP, Peyton MJ, Minna JD, Hernandez VJ, et al. SRBC/cavin-3 is a caveolin adapter protein that regulates caveolae function. Embo J. 2009;28(8):1001–15.10.1038/emboj.2009.46Suche in Google Scholar PubMed PubMed Central
[20] Nilsson O, Kölby L, Wängberg B, Wigander A, Billig H, William-Olsson L, et al. Comparative studies on the expression of somatostatin receptor subtypes, outcome of octreotide scintigraphy and response to octreotide treatment in patients with carcinoid tumours. Br J Cancer. 1998;77(4):632–7.10.1038/bjc.1998.101Suche in Google Scholar PubMed PubMed Central
[21] Nayak T, Norenberg J, Anderson T, Atcher R. A comparison of high- versus low-linear energy transfer somatostatin receptor targeted radionuclide therapy in vitro. Cancer Biother Radiopharm. 2005;20(1):52–7.10.1089/cbr.2005.20.52Suche in Google Scholar PubMed
[22] Romer I, White TA, Baalousha M, Chipman K, Viant MR, Lead JR. Aggregation and dispersion of silver nanoparticles in exposure media for aquatic toxicity tests. J Chromatogr A. 2011;1218(27):4226–33.10.1016/j.chroma.2011.03.034Suche in Google Scholar PubMed
[23] Na DH, Murty SB, Lee KC, Thanoo BC, DeLuca PP. Preparation and stability of poly(ethylene glycol) (PEG)ylated octreotide for application to microsphere delivery. AAPS PharmSciTech. 2003;4(4):E72.10.1208/pt040472Suche in Google Scholar PubMed PubMed Central
[24] Updegrove TB, Correia JJ, Chen YF, Terry C, Wartell RM. The stoichiometry of the Escherichia coli Hfq protein bound to RNA. Rna-a Publication of the Rna Society. 2011;17(3):489–500.10.1261/rna.2452111Suche in Google Scholar PubMed PubMed Central
[25] Rahman A, Kumar S, Bafana A, Dahoumane SA, Jeffryes C. Biosynthetic conversion of Ag(+) to highly stable Ag(0) nanoparticles by wild type and cell wall deficient strains of Chlamydomonas reinhardtii. Molecules. 2019;24(1):98.10.3390/molecules24010098Suche in Google Scholar PubMed PubMed Central
[26] Abdellatif AAH, Alturki HNH, Tawfeek HM. Different cellulosic polymers for synthesizing silver nanoparticles with antioxidant and antibacterial activities. Sci Rep. 2021;11(1):84.10.1038/s41598-020-79834-6Suche in Google Scholar PubMed PubMed Central
[27] Abdellatif AAH, El Hamd MA, Salman KH, Abd-El-Rahim AM, El-Maghrabey M, Tawfeek HM. Integrative physicochemical and HPLC assessment studies for the inclusion of lornoxicam in buffalo’s milk fat globules as a potential carrier delivery system for lipophilic drugs. Microchem J. 2020;152:104321.10.1016/j.microc.2019.104321Suche in Google Scholar
[28] Rasheed Z, Rasheed N, Abdulmonem WA, Khan MI. MicroRNA-125b-5p regulates IL-1beta induced inflammatory genes via targeting TRAF6-mediated MAPKs and NF-kappaB signaling in human osteoarthritic chondrocytes. Sci Rep. 2019;9(1):6882.10.1038/s41598-019-42601-3Suche in Google Scholar PubMed PubMed Central
[29] Rasheed Z, Al-Shobaili HA, Rasheed N, Mahmood A, Khan MI. MicroRNA-26a-5p regulates the expression of inducible nitric oxide synthase via activation of NF-kappaB pathway in human osteoarthritis chondrocytes. Arch Biochem Biophys. 2016;594:61–7.10.1016/j.abb.2016.02.003Suche in Google Scholar PubMed
[30] Abdellatif AAH, Rasheed Z, Alhowail AH, Alqasoumi A, Alsharidah M, Khan RA, et al. Silver citrate nanoparticles inhibit PMA-induced TNFalpha expression via deactivation of NF-kappaB activity in human cancer cell-lines, MCF-7. Int J Nanomedicine. 2020;15:8479–93.10.2147/IJN.S274098Suche in Google Scholar PubMed PubMed Central
[31] Rasheed Z, Rasheed N, Al-Shaya O. Epigallocatechin-3-O-gallate modulates global microRNA expression in interleukin-1beta-stimulated human osteoarthritis chondrocytes: potential role of EGCG on negative co-regulation of microRNA-140-3p and ADAMTS5. Eur J Nutr. 2018;57(3):917–28.10.1007/s00394-016-1375-xSuche in Google Scholar PubMed
[32] Pollinger K, Hennig R, Breunig M, Tessmar J, Ohlmann A, Tamm ER, et al. Kidney podocytes as specific targets for cyclo(RGDfC)-modified nanoparticles. Small. 2012;8(21):3368–75.10.1002/smll.201200733Suche in Google Scholar PubMed
[33] Hennig R, Pollinger K, Tessmar J, Goepferich A. Multivalent targeting of AT1receptors with angiotensin II-functionalized nanoparticles. J Drug Targeting. 2015;23(7–8):681–9.10.3109/1061186X.2015.1035276Suche in Google Scholar PubMed
[34] Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6(4):662–8.10.1021/nl052396oSuche in Google Scholar
[35] Elbakry A, Zaky A, Liebkl R, Rachel R, Goepferich A, Breunig M. Layer-by-layer assembled gold nanoparticles for siRNA delivery. Nano Lett. 2009;9(5):2059–64.10.1021/nl9003865Suche in Google Scholar
[36] Abdellatif AAH, Tawfeek HM. Development and evaluation of fluorescent gold nanoparticles. Drug Dev Ind Pharm. 2018;44(10):1679–84.10.1080/03639045.2018.1483400Suche in Google Scholar
[37] Sneddon J, Vincent MD. ICP-OES and ICP-MS for the determination of metals: application to oysters. Analytical Letters. 2008;41(8):1291–303.10.1080/00032710802013991Suche in Google Scholar
[38] Tiwari DK, Jin T, Behari J. Dose-dependent in-vivo toxicity assessment of silver nanoparticle in Wistar rats. Toxicol Mech Methods. 2011;21(1):13–24.10.3109/15376516.2010.529184Suche in Google Scholar
[39] Dufort S, Bianchi A, Henry M, Lux F, Le Duc G, Josserand V, et al. Nebulized gadolinium-based nanoparticles: a theranostic approach for lung tumor imaging and radiosensitization. Small. 2015;11(2):215–21.10.1002/smll.201401284Suche in Google Scholar
[40] Martin AR, Thompson RB, Finlay WH. MRI measurement of regional lung deposition in mice exposed nose-only to nebulized superparamagnetic iron oxide nanoparticles. J Aerosol Med Pulm Drug Deliv. 2008;21(4):335–42.10.1089/jamp.2008.0698Suche in Google Scholar
[41] Lee RF, Pickering WF. Effect of precipitate and complex formation on the determination of silver by atomic-absorption spectroscopy. Talanta. 1971;18(11):1083–94.10.1016/0039-9140(71)80220-5Suche in Google Scholar
[42] Yuan JJ, Xie YZ, Han C, Sun W, Zhang K, Zhao J, et al. Determination of trace element silver in animal serum, tissues and organs by microwave digestion-ICP-MS. Guang Pu Xue Yu Guang Pu Fen Xi. 2014;34(9):2533–7.Suche in Google Scholar
[43] Helal OK, Mousa AM, Kandeel N. Effect of antox on hippocampal structure in male albino rats exposed to lead toxicity. Egyptian J Histol. 2011;34(4):808–17.10.1097/01.EHX.0000407618.33328.f2Suche in Google Scholar
[44] Alshora D, Ibrahim M, Elzayat E, Almeanazel OT, Alanazi F. Defining the process parameters affecting the fabrication of rosuvastatin calcium nanoparticles by planetary ball mill. Int J Nanomed. 2019;14:4625–36.10.2147/IJN.S207301Suche in Google Scholar PubMed PubMed Central
[45] Abdellatif AAH, Hennig R, Pollinger K, Tawfeek HM, Bouazzaoui A, Goepferich A. Fluorescent nanoparticles coated with a somatostatin analogue target blood monocyte for efficient leukaemia treatment. Pharm Res. 2020;37(11):217.10.1007/s11095-020-02938-1Suche in Google Scholar PubMed
[46] Sato I, Umemura M, Mitsudo K, Fukumura H, Kim JH, Hoshino Y, et al. Simultaneous hyperthermia-chemotherapy with controlled drug delivery using single-drug nanoparticles. Sci Rep. 2016;6:24629.10.1038/srep24629Suche in Google Scholar PubMed PubMed Central
[47] Zahr AS, Davis CA, Pishko MV. Macrophage uptake of core-shell nanoparticles surface modified with poly(ethylene glycol). Langmuir. 2006;22(19):8178–85.10.1021/la060951bSuche in Google Scholar PubMed
[48] Afshinnia K, Baalousha M. Effect of phosphate buffer on aggregation kinetics of citrate-coated silver nanoparticles induced by monovalent and divalent electrolytes. Sci Total Environ. 2017;581–582:268–76.10.1016/j.scitotenv.2016.12.117Suche in Google Scholar PubMed
[49] Khan Z, Al-Thabaiti SA, Obaid AY, Al-Youbi AO. Preparation and characterization of silver nanoparticles by chemical reduction method. Colloids Surf B Biointerfaces. 2011;82(2):513–7.10.1016/j.colsurfb.2010.10.008Suche in Google Scholar PubMed
[50] Li HY, Zhang F. Preparation of spray-dried nanoparticles for efficient drug delivery to the lungs. Methods Mol Biol. 2020;2118:139–45.10.1007/978-1-0716-0319-2_10Suche in Google Scholar PubMed
[51] Ullrich SJ, Freedman-Weiss M, Ahle S, Mandl HK, Piotrowski-Daspit AS, Roberts K, et al. Nanoparticles for delivery of agents to fetal lungs. Acta Biomater. 2021;123:346–53.10.1016/j.actbio.2021.01.024Suche in Google Scholar PubMed PubMed Central
[52] Lokugamage MP, Vanover D, Beyersdorf J, Hatit MZC, Rotolo L, Echeverri ES, et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat Biomed Eng. 2021;5(9):1059–68.10.1038/s41551-021-00786-xSuche in Google Scholar PubMed
[53] Detsri E, Kamhom K, Ruen-ngam D. Layer-by-layer deposition of green synthesised silver nanoparticles on polyester air filters and its antimicrobial activity. J Exp Nanosci. 2016;11(12):930–9.10.1080/17458080.2016.1181277Suche in Google Scholar
[54] Ali ME, Lamprecht A. Spray freeze drying for dry powder inhalation of nanoparticles. Eur J Pharm Biopharm. 2014;87(3):510–7.10.1016/j.ejpb.2014.03.009Suche in Google Scholar PubMed
[55] Abdellatif AAH. Identification of somatostatin receptors using labeled PEGylated octreotide, as an active internalization. Drug Dev Ind Pharm. 2019;45(10):1707–15.10.1080/03639045.2019.1656735Suche in Google Scholar PubMed
[56] Salvioni L, Galbiati E, Collico V, Alessio G, Avvakumova S, Corsi F, et al. Negatively charged silver nanoparticles with potent antibacterial activity and reduced toxicity for pharmaceutical preparations. Int J Nanomedicine. 2017;12:2517–30.10.2147/IJN.S127799Suche in Google Scholar PubMed PubMed Central
[57] Lu GW, Gao P. Emulsions and microemulsions for topical and transdermal drug delivery. In: Handbook of Non-Invasive Drug Delivery Systems. The Netherlands: Elsevier; 2010. p. 59–94.10.1016/B978-0-8155-2025-2.10003-4Suche in Google Scholar
[58] Ranoszek-Soliwoda K, Tomaszewska E, Socha E, Krzyczmonik P, Ignaczak A, Orlowski P, et al. The role of tannic acid and sodium citrate in the synthesis of silver nanoparticles. J Nanopart Res. 2017;19(8):273.10.1007/s11051-017-3973-9Suche in Google Scholar PubMed PubMed Central
[59] Abdellatif A. Octreotide labelled fluorescein isothiocyanate for identification of somatostatin receptor subtype 2. Biochem Physiol. 2015;4(183):2.10.4172/2168-9652.1000183Suche in Google Scholar
[60] Fernandes JC, Wang H, Jreyssaty C, Benderdour M, Lavigne P, Qiu X, et al. Bone-protective effects of nonviral gene therapy with folate-chitosan DNA nanoparticle containing interleukin-1 receptor antagonist gene in rats with adjuvant-induced arthritis. Mol Ther. 2008;16(7):1243–51.10.1038/mt.2008.99Suche in Google Scholar PubMed
[61] Wang XH, Hong X, Zhu L, Wang YT, Bao JP, Liu L, et al. Tumor necrosis factor alpha promotes the proliferation of human nucleus pulposus cells via nuclear factor-kappaB, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase. Exp Biol Med (Maywood). 2015;240(4):411–7.10.1177/1535370214554533Suche in Google Scholar PubMed PubMed Central
[62] Xu Y, Wang L, Bai R, Zhang T, Chen C. Silver nanoparticles impede phorbol myristate acetate-induced monocyte-macrophage differentiation and autophagy. Nanoscale. 2015;7(38):16100–9.10.1039/C5NR04200CSuche in Google Scholar
[63] Abdellatif A. Targeting of somatostatin receptors using quantum dots nanoparticles decorated with octreotide. J Nanomed Nanotechnol S. 2015;6:2.10.4172/2157-7439.S6-005Suche in Google Scholar
[64] Abdellatif AAH, Rasoul SAEl, Osman S. Gold nanoparticles decorated with octreotide for somatostatin receptors targeting. J Pharm Sci & Res. 2015;7(1):14–20.Suche in Google Scholar
[65] Shirshahi V, Shamsipour F, Zarnani AH, Verdi J, Saber R. Active targeting of HER2-positive breast cancer cells by Herceptin-functionalized organically modified silica nanoparticles. Cancer Nanotechnol. 2013;4(1–3):27–37.10.1007/s12645-013-0035-6Suche in Google Scholar PubMed PubMed Central
[66] Terlević R, Perić Balja M, Tomas D, Skenderi F, Krušlin B, Vranic S, et al. Somatostatin receptor SSTR2A and SSTR5 expression in neuroendocrine breast cancer. Ann Diagn Pathol. 2019;38:62–6.10.1016/j.anndiagpath.2018.11.002Suche in Google Scholar
[67] Kumar U, Grigorakis SI, Watt HL, Sasi R, Snell L, Watson P, et al. Somatostatin receptors in primary human breast cancer: quantitative analysis of mRNA for subtypes 1--5 and correlation with receptor protein expression and tumor pathology. Breast Cancer Res Treat. 2005;92(2):175–86.10.1007/s10549-005-2414-0Suche in Google Scholar
[68] Li L, Di X, Wu M, Sun Z, Zhong L, Wang Y, et al. Targeting tumor highly-expressed LAT1 transporter with amino acid-modified nanoparticles: toward a novel active targeting strategy in breast cancer therapy. Nanomedicine. 2017;13(3):987–98.10.1016/j.nano.2016.11.012Suche in Google Scholar
[69] Bhunchu S, Rojsitthisak P. Biopolymeric alginate-chitosan nanoparticles as drug delivery carriers for cancer therapy. Pharmazie. 2014;69(8):563–70.Suche in Google Scholar
[70] Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55(3):329–47.10.1016/S0169-409X(02)00228-4Suche in Google Scholar
[71] Brown JS. Deposition of particles. In: Comparative biology of the normal lung. The Netherlands: Elsevier; 2015. p. 513–36.10.1016/B978-0-12-404577-4.00027-8Suche in Google Scholar
[72] Han SG, Lee JS, Ahn K, Kim YS, Kim JK, Lee JH, et al. Size-dependent clearance of gold nanoparticles from lungs of Sprague-Dawley rats after short-term inhalation exposure. Arch Toxicol. 2015;89(7):1083–94.10.1007/s00204-014-1292-9Suche in Google Scholar PubMed
[73] Morfeld P, Treumann S, Ma-Hock L, Bruch J, Landsiedel R. Deposition behavior of inhaled nanostructured TiO2 in rats: fractions of particle diameter below 100 nm (nanoscale) and the slicing bias of transmission electron microscopy. Inhal Toxicol. 2012;24(14):939–51.10.3109/08958378.2012.738256Suche in Google Scholar PubMed
[74] Lee JH, Kim YS, Song KS, Ryu HR, Sung JH, Park JD, et al. Biopersistence of silver nanoparticles in tissues from Sprague-Dawley rats. Part Fibre Toxicol. 2013;10:36.10.1186/1743-8977-10-36Suche in Google Scholar PubMed PubMed Central
[75] Han L, Ravoori M, Wu G, Sakai R, Yan S, Singh S, et al. Somatostatin receptor type 2-based reporter expression after plasmid-based in vivo gene delivery to non-small cell lung cancer. Mol Imaging. 2013;12(7):1–10.10.2310/7290.2013.00060Suche in Google Scholar
[76] Watt HL, Kharmate G, Kumar U. Biology of somatostatin in breast cancer. Mol Cell Endocrinol. 2008;286(1–2):251–61.10.1016/j.mce.2008.01.006Suche in Google Scholar PubMed
[77] Nagarajan SK, Babu S, Sohn H, Madhavan T. Molecular-level understanding of the somatostatin receptor 1 (SSTR1)-ligand binding: a structural biology study based on computational methods. ACS Omega. 2020;5(33):21145–61.10.1021/acsomega.0c02847Suche in Google Scholar PubMed PubMed Central
[78] Jasim R, Schneider EK, Han M, Azad MAK, Hussein M, Nowell C, et al. A fresh shine oncystic fibrosis inhalation therapy: antimicrobial synergy of polymyxin B in combination with silver nanoparticles. J Biomed Nanotechnol. 2017;13(4):447–57.10.1166/jbn.2017.2355Suche in Google Scholar PubMed
[79] Seiffert J, Buckley A, Leo B, Martin NG, Zhu J, Dai R, et al. Pulmonary effects of inhalation of spark-generated silver nanoparticles in Brown-Norway and Sprague-Dawley rats. Respir Res. 2016;17(1):85.10.1186/s12931-016-0407-7Suche in Google Scholar PubMed PubMed Central
[80] Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv Drug Deliv Rev. 2006;58(15):1688–713.10.1016/j.addr.2006.09.017Suche in Google Scholar PubMed
[81] Lapa C, Hänscheid H, Wild V, Pelzer T, Schirbel A, Werner RA, et al. Somatostatin receptor expression in small cell lung cancer as a prognostic marker and a target for peptide receptor radionuclide therapy. Oncotarget. 2016;7(15):20033–40.10.18632/oncotarget.7706Suche in Google Scholar PubMed PubMed Central
[82] Kanakis G, Grimelius L, Spathis A, Tringidou R, Rassidakis GZ, Öberg K, et al. Expression of somatostatin receptors 1–5 and dopamine receptor 2 in lung carcinoids: implications for a therapeutic role. Neuroendocrinology. 2015;101(3):211–22.10.1159/000381061Suche in Google Scholar PubMed
[83] Foldbjerg R, Dang DA, Autrup H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch Toxicol. 2011;85(7):743–50.10.1007/s00204-010-0545-5Suche in Google Scholar PubMed
[84] Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev. 2003;24(4):389–427.10.1210/er.2002-0007Suche in Google Scholar PubMed
[85] Petrik M, Laznickova A, Laznicek M, Zalutsky MR. Radiolabelling of glucose-Tyr3-octreotate with 125I and analysis of its metabolism in rats: comparison with radiolabelled DOTA-Tyr3-octreotate. Anticancer Res. 2007;27(6B):3941–6.Suche in Google Scholar
© 2022 Ahmed A. H. Abdellatif et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption

