Abstract
Bi2S3 nanostructures with various morphologies were synthesized through hydrothermal vulcanization at different sulfur precursor (thiourea) concentrations. A 100 nm thick sputter-deposited Bi2O3 thin-film layer on a fluorine-doped tin oxide glass substrate was used as a sacrificial template layer. The etching of the Bi2O3 sacrificial template layer and the regrowth of Bi2S3 crystallites during hydrothermal vulcanization produced the different Bi2S3 nanostructure morphologies. The lowest sulfur precursor concentration (0.01 M) induced the formation of Bi2S3 nanosheets, whereas the Bi2S3 nanoribbons and nanowires were formed with increased sulfur precursor concentrations of 0.03 and 0.1 M, respectively. These results indicate that sputter-deposited Bi2O3 thin-film layers can be effectively used to form low-dimensional Bi2S3 crystals with controllable morphologies. Among the various Bi2S3 samples, the Bi2S3 nanosheets exhibited superior photoactive ability. The higher active surface area, surface defect density, light absorption capacity, and photo-induced charge separation ability of Bi2S3 nanosheets explain their superior photoelectrocatalytic degradation ability of rhodamine B dyes.
1 Introduction
Bi2S3 is characterized by high photoconductivity, a small direct energy gap (1.3–1.7 eV), and a large visible light absorption coefficient; moreover, it is photostable in a semiconductor–electrolyte configuration. Due to these characteristics, low-dimensional Bi2S3 has become one of the most innovative materials used in research on applications of photocatalysts, photoresponsive devices, and photoelectrochemistry [1,2]. The photoactive performances of photosensitive materials are dependent on the materials’ size, shape, composition, crystallinity, and structure [3,4]. Therefore, the synthesis route and process parameters are key to synthesizing low-dimensional Bi2S3 crystals with desirable properties. Several methods have been adopted to prepare Bi2S3 crystals, including hydrothermal and solvothermal processing, the sol-gel process, and vulcanization [5,6,7,8].
Semiconductor crystal synthesis in an aqueous solution is generally considered a promising approach because it is a simple, low-cost, and low-temperature process with easy process control. Moreover, thermodynamics and kinetic obstacles are major factors affecting the morphology and defect density of as-grown nanomaterials; therefore, simple process parameter control is especially crucial in synthesizing nanomaterials with a highly reproducible crystal quality. Among the various chemical solution methods for the synthesis of Bi2S3 crystals, the hydrothermal method is an excellent option based on the aforementioned criteria [9]. When used in photoresponsive devices, the as-grown nanomaterials tend to firmly align on the substrates. The traditional hydrothermal route for synthesizing most nanomaterial systems usually requires a homoseed or heteroseed layer or execution under specific harsh parameters to obtain the as-grown nanomaterials with specific crystal alignments and to firmly attach them to the required substrate for applications in devices [10,11]. A hydrothermal-assisted vulcanization method has also been adopted to synthesize sulfide or oxide–sulfide composite systems using a pregrown oxide sacrificial template layer on the substrate in the vulcanization process [12,13]. Therefore, the integration of hydrothermal and vulcanization processes to synthesize Bi2S3 crystals firmly aligned on the substrates is also a promising approach for device applications. Hydrothermal vulcanization has been used to synthesize Bi2S3–BiVO4 microspheres by forming Bi2S3 on hydrothermally prepared BiVO4 crystals [14]. Moreover, single-crystal Bi2S3 nanorods and nanosheet-decorated Bi2O3 microtubes have been prepared using the same sacrificial template synthesis method [15]. However, the use of hydrothermal vulcanization with a sacrificial template in the synthesis of single-phase Bi2S3 crystals with a high growth density that are firmly aligned in a specific orientation on the substrate has not been systematically investigated so far. In situ growth of nanostructures that are firmly attached to a specific substrate facilitates their direct application in devices and eliminates the complicated procedures required for secondary processing and integration.
In addition, the relationship between the structural evolution and photoactive properties of single-phase Bi2S3 crystals synthesized through hydrothermal vulcanization with a suitable oxide sacrificial template has not been investigated. In this study, hydrothermal vulcanization was used to grow Bi2S3 crystals on a substrate using a sputter-deposited Bi2O3 thin-film sacrificial layer. Through the adjustment of the concentration of the sulfur source during hydrothermal vulcanization, different pure Bi2S3 nanostructures on the substrate were successfully synthesized. The microstructure-dependent photoactive properties were also measured to evaluate these nanostructures’ suitability for use in photoactive nanodevices.
2 Experiments
2.1 Preparation of Bi2S3 crystals
First, a 100 nm thick Bi2O3 thin-film layer was grown on a fluorine-doped tin oxide (FTO) glass substrate by radio frequency sputtering. A metallic bismuth disc of 2 inches diameter was used as the target. The working pressure is 20 mtorr, sputtering power is fixed at 30 W, and the ratio of argon to oxygen (Ar/O2) is fixed at 1:1 during the thin-film sputtering process. The substrate temperature was maintained at 425°C, and the sputtering duration was 50 min. Second, the as-prepared Bi2O3 thin-film layer underwent the hydrothermal vulcanization reaction to form Bi2S3 crystals. Thiourea (TU) powder was dissolved in distilled water, and TU aqueous solutions with molar concentrations of 0.01, 0.03, and 0.1 M were prepared for different sulfur sources to vulcanize the Bi2O3 thin-film layer. Then, 12.5 mL of the TU aqueous solution with various concentrations was transferred into Teflon-lined stainless steel autoclaves and the Bi2O3 thin-film layer/FTO substrates are immersed in the solution for the hydrothermal reaction. The hydrothermal reaction was conducted at 180°C for 3 h. After the hydrothermal reaction, the autoclave was cooled to room temperature naturally. The final products were washed with deionized water several times and dried in air at 60°C. Notably, no other bismuth source was added in the reaction solution during the hydrothermal vulcanization process in addition to the bismuth source from the Bi2O3 thin-film sacrificial layer.
2.1.1 Materials analysis
The crystal structure of various Bi2S3 crystals was characterized by X-ray diffraction (XRD; Bruker D2 PHASER) with monochromatic Cu-Kα radiation. The surface morphology of the samples was investigated through a field emission scanning electron microscope (SEM:JSM-7900F, JEOL). A high-resolution transmission electron microscope equipped with a 1024 × 1024 CCD camera (HRTEM; Philips Tecnai F20 G2) was operated at 200 kV to study the microstructures of the samples. X-ray photoelectron spectroscopy (XPS ULVAC-PHI, PHI 5000 VersaProbe) with Al Kα X-rays was used to detect the element binding state of the samples. The absorption spectra of various samples were obtained using an UV-Vis spectrophotometer (Jasco V750). The photoelectrochemical (PEC) performance, electrochemical impedance (EIS), and PEC degradation were conducted with a potentiostat (SP150, BioLogic). The active area of the sample used as a working electrode in this study was 1.0 cm2. Saturated Ag/AgCl (in saturated KCl) and platinum (Pt) wire were used as the reference electrode and counter electrode, respectively, in the electrochemical system. A 0.2 M Na2SO4 aqueous solution was used as the electrolyte. A 100 W Xenon lamp was used as the light source during the photoexcitation experiments. In the PEC-degradation experiment, rhodamine B (RhB) solution (10−5 M) was used as the target degraded pollutant. The residual concentration of RhB solution after different PEC-degradation processes was analyzed with a UV-vis spectrophotometer. The degradation level of RhB solution PEC-degraded by the Bi2S3 photocatalysts was defined as C/C o, C o and C being the initial RhB concentration and RhB concentration at reaction time t, respectively.
3 Results and discussion
The morphologies of Bi2O3 thin-film templates used in the various vulcanization processes are presented in the SEM images. In Figure 1(a), distinct sheet-like crystals distributed on a large scale were observed on the Bi2O3 thin-film template vulcanized with the lowest sulfur precursor concentration (0.01 M). The interconnected nanosheets were uniformly distributed on the substrate, forming an orderly array with a highly open structure. These sheet-like structures had the thickness of approximately 31–54 nm and a diameter of approximately 1.6–2.8 μm. When the concentration of the sulfur precursor was increased to 0.03 M, no sheet-like crystals were observed; in contrast, ultrathin, ribbon-like nanostructures appeared as shown in Figure 1(b). The surfaces of the ribbon-like crystals were smooth, and their periphery was straight; moreover, the nanoribbons covered the substrate completely and uniformly. The average length of the synthesized nanoribbons was approximately 2.1–3 μm, and their width was approximately 400–500 nm. Figure 1(c) presents the morphology of the product formed using the highest concentration of the sulfur precursor (0.1 M). Uniform nanowires can be clearly distinguished in the SEM image, and the nanowires have a homogeneous size with an average diameter of approximately 100 nm and average length of 1 μm. The SEM images reveal that after hydrothermal vulcanization processes with different sulfur concentrations, the Bi2O3 thin-film templates formed nanostructures with various morphologies. During the hydrothermal vulcanization reaction, the Bi2O3 thin-film template layer reacted with S2− ions from the added TU to form Bi2S3 crystals. The Bi2O3 thin-film crystals acted as the sacrificial layer and acted as a Bi3+ source for the formation of the Bi2S3 crystals during the hydrothermal vulcanization through the reactions proposed in Wu et al.’s work [16]. Ion exchange, in which the surface oxygen ions of the Bi2O3 thin-film template were replaced by sulfur ions, occurred during the hydrothermal vulcanization. Furthermore, the Bi3 + ions reacted with S2− ions to form the Bi2S3 crystallites. Bi2S3 is much less soluble than Bi2O3; therefore, Bi2O3 can spontaneously undergo a sulfidation reaction with S2− and perform anion exchange even in a low-temperature hydrothermal vulcanization process [17]. In this study, the amount of Bi3+ ions in the hydrothermal vulcanization process was fixed because the Bi2O3 thin-film template amount in the various vulcanization processes was the same; however, the S2− sources differed due to the different added amounts of TU. The addition of different S2− precursor concentrations in the hydrothermal vulcanization might have dominated the etching rate of the Bi2O3 thin-film template and controlled the formation rate of the Bi2S3 crystallites at the time of the synthetic reaction. Therefore, various morphologies of the Bi2S3 products were formed on the Bi2O3 thin-film template after vulcanization with different sulfur precursor concentrations. The etching of the Bi2O3 thin-film sacrificial layer and formation of Bi2S3 crystallites of various morphologies during different vulcanization processes are shown in Figure 1(d). A similar vulcanization process using BiVO4 to form Bi2S3 crystals has also been used to synthesize a BiVO4–Bi2S3 nanowire composite [18].

SEM micrographs of Bi2S3 crystals formed with Bi2O3 thin-film templates from various vulcanization processes: (a) 0.01 M TU, (b) 0.03 M TU, and (c) 0.1 M TU. (d) Illustration of formation mechanisms of Bi2S3 crystals via Bi2O3 thin-film template treated with various vulcanization processes. The Bi2S3 crystallographic plane has been marked in red.
Figure 2(a)–(c) show the XRD patterns of Bi2O3 thin-film templates treated with various hydrothermal vulcanization processes. In addition to the Bragg reflections from the FTO substrate, all samples showed distinct Bragg reflections originating from the crystallographic planes of the orthorhombic Bi2S3 structure according to JCPDS No. 17-0320 [19]. Notably, no impurities, such as Bi2O3 and Bi, were detected, which indicates that a high-purity Bi2S3 phase was synthesized via the given vulcanization processes. Furthermore, the Bragg reflections are narrow and sharp, indicating that the Bi2S3 products are of high crystallinity. All the Bi2S3 samples show the intense (130) and (310) Bragg reflections. This crystallographic feature of the as-synthesized Bi2S3 samples is in agreement with the XRD results of hydrothermally derived ribbon-like Bi2S3 crystals [20].
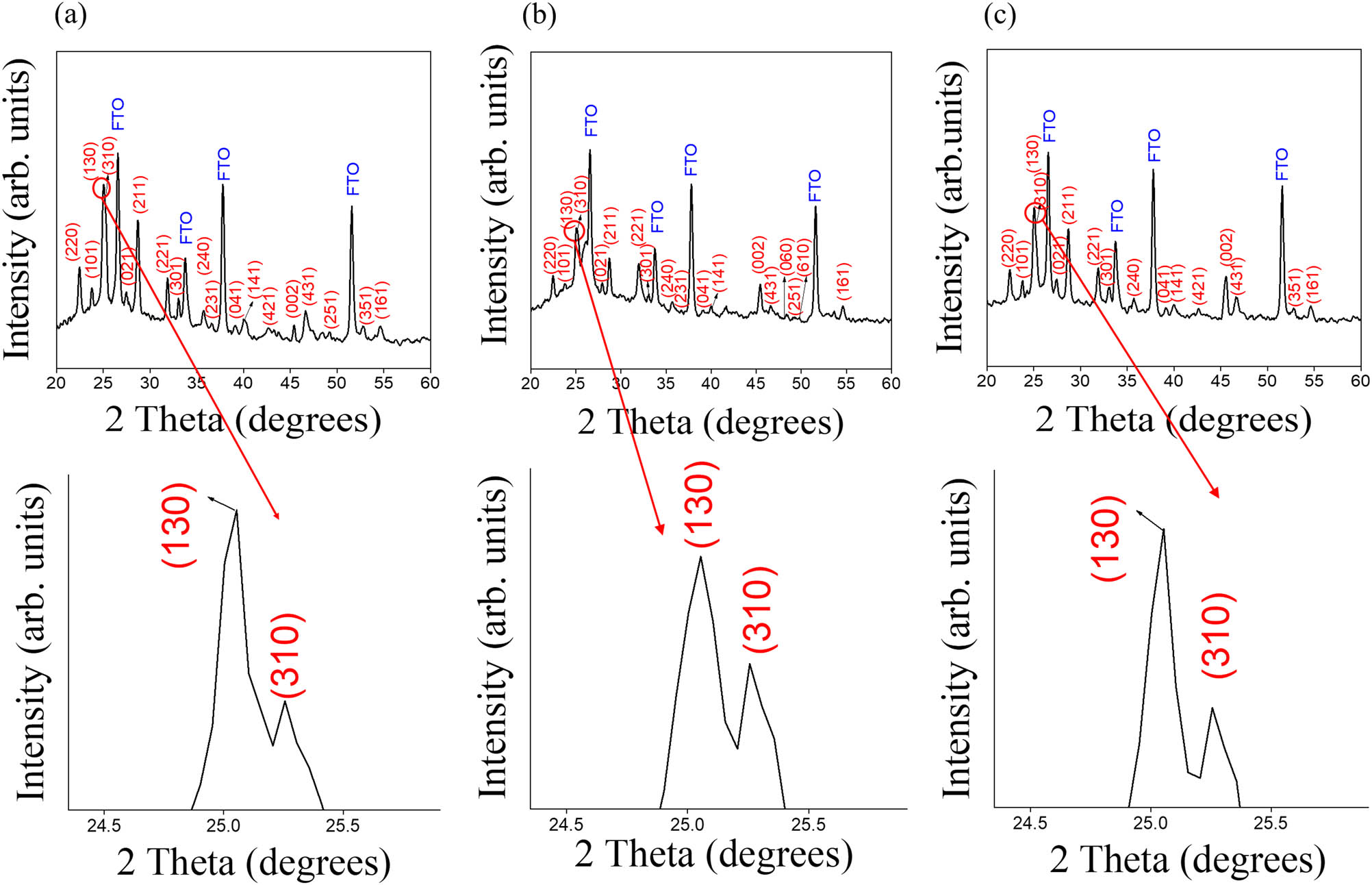
XRD patterns of Bi2S3 crystals formed with Bi2O3 thin-film templates from various vulcanization processes: (a) 0.01 M TU, (b) 0.03 M TU, and (c) 0.1 M TU. The enlarged XRD plots with 2θ at around 25o are also shown for clarity.
Figure 3(a) shows a low-magnification TEM micrograph of a Bi2S3 nanosheet. The nanosheet exhibits a slightly rugged peripheral morphology and grows into a rectangular shape. The width of the sheet is approximately 1.4 μm, and the length is approximately 1.6 μm. Moreover, many irregular rippling stripes appeared on the surface of the sheet. Here, the appearance of ripples might indicate the existence of surface defects on the surface of the as-synthesized Bi2S3 nanosheet. The formation of similar surface ripples of the materials associated with crystal defects has been proposed previously [21]. Figure 3(b) demonstrates a HRTEM micrograph taken from the local region (marked with yellow circle) of the nanosheet. Vaisible lattice fringes with distinct and ordered arrangement in the local region are observed in the HRTEM output, which reveals its crystalline nature. In addition, some messy lattice images exist in the local regions of the HRTEM output, supporting reasonably that crystal defects are present in the Bi2S3 nanosheets. The lattice spacing of approximately 0.35 nm in the HRTEM output was indexed as the interplanar spacing of (130) of the orthorhombic structured Bi2S3. A corresponding selected area electron diffraction (SAED) pattern of a single nanosheet is shown in Figure 3(c). The sharp, bright, and ordered spots corroborate that the nanosheet was highly crystalline. The spots in the SAED pattern could readily be indexed to (130) and (211), the crystallographic planes were determined to correspond to the orthorhombic Bi2S3 phase. The phase purity and chemical composition of the product were confirmed by energy-dispersive X-ray spectroscopy analysis. Figure 3(d) displays Bi and S are the main constituent elements in the sample except for the observed signals of Cu and C that originated from the copper grid. The molar ratio of Bi to S is estimated to be approximately 2:3 (Bi: 38.6%, S: 61.4%), which is close to the standard stoichiometric composition of the Bi2S3 phase. Figure 3(e) presents a low-magnification TEM micrograph of a Bi2S3 nanoribbon. Figure 3(e) confirms that the Bi2S3 ribbon structure has a width of approximately 410 nm, which is consistent with the result of SEM observations. The Bi2S3 ribbon exhibits a flat, side-wall morphology and grows into a rectangular shape. Notably, the top region of the ribbon consisted of three facets, and the angle between the adjacent facets is approximately 158.8°. Moreover, the grayscale contrast reveals that the side-wall regions of the ribbon structure are thinner than the core region. More evidence on its structure and crystallinity was obtained from the HRTEM output (Figure 3(f)). Visible lattice fringes with a neat arrangement are displayed in Figure 3(f). It can be observed that the ordered lattice spacing is approximately 0.37 nm, corresponding to the interplanar distance of Bi2S3 (101). The SAED pattern in Figure 3(g) organized with sharp and orderly arranged spots evidences to the orthorhombic Bi2S3 phase of the ribbon structure; moreover, it indicates that the ribbon has single crystallinity. Furthermore, the compositional purity of the Bi2S3 ribbon structure is evident in Figure 3(h), and the molar ratio of Bi to S is estimated to be approximately 2:3 (Bi: 36.6%, S: 63.4%) herein. Figure 3(i) shows the low-magnification TEM micrograph of a Bi2S3 nanowire. It is observed that the length of these nanowires has a large distribution whereas their diameter has a relatively narrow distribution. The mean diameter of the Bi2S3 nanowire was measured to be approximately 70 nm. In contrast to the ribbon structure, a two-facet structure was observed in the top region of the nanowire, and the angle between these two crystal facets was approximately 136.5°. The highly crystalline nature of the nanowire is clear from Figure 3(j). The clear lattice spacing of 0.39 nm is attributed to the interplanar distance of Bi2S3 (220). A corresponding SAED pattern of a single nanowire was further examined and its unique, sharp diffraction pattern (Figure 3(k)) could readily be indexed to (002), and (220) on two of the spots, which confirms that the nanowire is a single crystal and might have a [001] growth direction. This is in agreement with previous finding for Bi2S3 nanorods synthesized via a hydrothermal method [22]. The composition of the Bi2S3 nanowire is shown in Figure 3(l). A stoichiometric composition of the Bi2S3 nanowire was used in this study.

(a) Low-magnification TEM image of the Bi2S3 nanosheet. (b) HRTEM image of the Bi2S3 nanosheet taken from the local region. (c) SAED pattern of the Bi2S3 nanosheet. (d) EDS spectra of the Bi2S3 nanosheet. (e) Low-magnification TEM image of the Bi2S3 nanoribbon. (f) HRTEM image of the Bi2S3 nanoribbon taken from the local region. (g) SAED pattern of the Bi2S3 nanoribbon. (h) EDS spectra of the Bi2S3 nanoribbon. (i) Low-magnification TEM image of the Bi2S3 nanowire. (j) HRTEM image of the Bi2S3 nanowire taken from the local region. (k) SAED pattern of the Bi2S3 nanowire. (l) EDS spectra of the Bi2S3 nanowire.
Figure 4(a)–(c) presents the narrow scan XPS spectra of the Bi 4f and S 2p regions taken from different Bi2S3 samples. Figure 4(a)–(c) show two predominant peaks centered at the range of 158.6–159.0 eV and 163.8–164.1 eV, and these two strong peaks can be assigned to the binding energies of Bi 4f7/2 and Bi 4f5/2, respectively. The binding energy feature of the bismuth herein closely matched with the Bi3+ in Bi2S3 crystals [23]. Moreover, the nearly symmetric shape of the Bi 4f peaks without distinct distortions at their shoulders excludes the presence of metallic Bi and Bi2O3, and therefore Bio binding energies appeared at 162.4 and 157.1 eV and Bi3+ binding energies of Bi 4f7/2 and Bi 4f5/2, respectively, appeared at 161.5 and 166.0 eV [23]. The spin–orbit doublet of S 2p spectra overlaps with the bottom regions of the Bi 4f spectra. The asymmetric S 2p spectra consist of two asymmetric peaks corresponding to S 2p3/2 and S 2p1/2. The more intense subpeak centered at 161.1 eV is ascribed to S 2p3/2, and by contrast, the peak with a lower intensity located at approximately 161.9 eV is assigned as S 2p1/2 in Bi2S3. The binding energies of the S 2p doublet signals herein prove that sulfur exists in the S2− state in the products. This is in agreement with the sulfur binding states in Bi2S3 nanowires synthesized via hydrothermal vulcanization [24]. Notably, the Bi 4f binding energy of Bi2S3 nanosheets exhibited a higher value in comparison with that of the Bi2S3 nanoribbons and nanowires (Figure 4(d)). This might be associated with the occurrence of more surface bismuth defects in the Bi2S3 nanosheets that affects the Bi 4f binding energy shifted to a higher binding energy [25,26]. The surface defects of semiconductors might act as charge traps and can prevent electron–hole recombination, thereby helping to improve the photocatalytic activity of semiconductors [27]. Furthermore, the representative O 1s spectrum of Bi2S3 nanosheets is shown in Figure 4(e). It has been shown that O 1s spectrum of Bi2S3/Bi2O3 composites has an asymmetric O 1s peak feature [28]. In that work, the binding energies of 531.0 and 529.6 eV could be attributed to the H–O bond of the oxygen-containing species adsorbed on the surface and the Bi–O bond in the Bi2O3 crystal lattice, respectively. In contrast, the O 1s peak of Bi2S3 nanosheets herein has a symmetric feature and it centered at approximately 531.4 eV (calibrated with a C signal). This binding energy matches the bonding energy associated with the surface-adsorbed oxygen-containing species.

Narrow scan XPS spectra of the Bi 4f and S 2p regions taken from different Bi2S3 products: (a) nanosheet, (b) nanoribbon, and (c) nanowire. (d) Comparison of Bi 4f core-level binding energy of all products. (e) The representative O 1s spectrum of Bi2S3 nanosheets. The red line is the fitted result.
Figure 5(a) shows the representative UV-Vis absorption spectra of various Bi2S3 samples. The Bi2S3 products herein showed a good, correspondingly wide absorption range in the visible light region. Similarly, the hydrothermally derived Bi2S3 nanorods confirm the narrow band gap nature of the Bi2S3, and it could absorb the spectra of the visible light region and show excellent photocatalytic performance in previous reported work [29]. Notably, the nanosheet-like Bi2S3 crystals exhibit more intense light absorption intensity in the visible light region among various samples, indicating that the free-standing surface topography and large diameter of the sheet crystal are beneficial for improving the light capture efficiency. In contrast, the ribbon-like Bi2S3 crystals possess lower absorption intensity in the visible light region. This may be due to the ribbon structures mostly lying flat on the substrate and in such a morphology cannot effectively harvest the reflectance lights, further losing the light harvesting ability. This phenomenon has been confirmed in the differences in the light harvesting ability of chemical vapor-deposited titanium sulfide sheets with a flat stacked structure and a three-dimensional clustered appearance. Comparatively, the sheet-like crystals that are flat on the substrate have a smaller light absorption capacity [30]. In addition, it is known that the optical absorption properties of semiconductors are closely related to their optical energy band gap. Figure 5(b)–(d) shows the band gap energy evaluation of various Bi2S3 samples according to the conversion of the Kubelka–Munk function [4]. Bi2S3 is supposed to exhibit a direct transition in the band gap measurement. Therefore, its optical band gap is deduced from the following conversion equation by considering the direct transitions for Bi2S3:

(a) Optical absorption spectra of all Bi2S3 products. Band gap evolution of various Bi2S3 products: (b) nanosheet, (c) nanoribbon, and (d) nanowire.
The electrochemical active surface area (ECSA) of various Bi2S3 crystals is further studied herein. ECSA is closely related to the number of surface active sites, which is effectively evaluated by the double-layer capacitance (C
dl), and C
dl is directly proportional to ECSA [32]. The cyclic voltammetry (CV) curves with different scan rates (20–100 mV/s) of various Bi2S3 samples in the region of non-Faradaic potentials (−0.15 to 0 V vs Ag/AgCl) are shown in Figure 6(a)–(c). It can be seen that the CV curves of all Bi2S3 samples are typical in a rectangular shape between the measured potential region between −0.15 and 0 V, revealing a behavior of electrical double-layered capacitance [33]. As displayed in Figure 6(d), the C
dl values of various samples from CV curves can be evaluated from the linear relationship plot of the current density (

CV curves at various scan rates of different Bi2S3 products: (a) nanosheet, (b) nanoribbon, and (c) nanowire. (d) The fitted C dl results of various Bi2S3 products.
Figure 7(a) displays the transient photocurrent responses of various Bi2S3 photoelectrodes under chopped illumination at 1 V vs Ag/AgCl. All the photoelectrodes exhibited a prompt response to the light under the test conditions. The photocurrent density of each sample remained stable after several cycles. The photocurrent density of the Bi2S3 nanosheet-based (0.083 mA/cm2) photoelectrode was higher than that of the nanowire-based photoelectrode (0.036 mA/cm2) and that of the nanoribbon-based photoelectrode (0.026 mA/cm2). As photocurrent density increased, the separation efficiency of the photoinduced charge carriers in the semiconductors also increased, indicating the higher photocatalytic performance of semiconductors [35,36]. The higher photoresponse performance of the Bi2S3 nanosheet-based photoelectrode in this study might be associated with several factors. First, a smaller band gap energy in a semiconductor allows the semiconductor to absorb more light, and more electron–hole pairs can therefore be generated, thus producing greater photocurrent density [37]. This corresponds to the previous optical absorption analysis result in which the Bi2S3 nanosheet structure exhibited the highest light absorption, highest light-harvesting ability, and smallest energy gap among various samples. Second, according to earlier ECSA measurements, the Bi2S3 nanosheet has the highest electrochemically active surface area. A high electrochemically active surface area can improve PEC performance [38]. Third, in the preceding microstructural analysis, the Bi2S3 nanosheet exhibited a wrinkled surface appearance, and in the HRTEM, it exhibited greater disorder and more irregular lattice stripe arrangements than the other samples. These phenomena increase the possibility of defects in nanosheets. Crystal defects in a nanosheet make the recombination of the photogenerated electron–hole pairs more difficult; therefore, a higher photocurrent value might be obtained. Improved PEC performance has also been exhibited by In2S3 nanoplates with a high surface defect density [39]. Figure 7(b) presents the Nyquist plots of various Bi2S3 photoelectrodes; these plots are used to analyze the interface charge transfer characteristics of the prepared samples. A smaller Nyquist curve radius indicates a lower charge transfer resistance in the electrode–electrolyte interface, a higher reaction rate on the photoelectrode, and a higher separation efficiency of photogenerated electrons and holes in the semiconductor [40]. All the Nyquist plots of the Bi2S3 photoelectrodes exhibit typical semicircular arcs with different curve radii. Furthermore, the plot of the Bi2S3 nanosheet-based photoelectrode contains the smallest semicircular radius among those of various samples. Although the semicircular radius of the Bi2S3 nanoribbon-based photoelectrode’s Nyquist plot is comparable to that of the nanowire-based photoelectrode, the plot of the nanoribbon-based photoelectrode has a slightly larger semicircular radius than that of the nanowire-based photoelectrode. The Nyquist plots indicate that the Bi2S3 nanosheet-based photoelectrode exhibits the smallest charge transfer resistance, the most effective photogenerated electron–hole pair separation, and the fastest interface charge transfer among the various Bi2S3 samples. Conversely, the Bi2S3 nanoribbon-based photoelectrode has the largest charge transfer resistance and the slowest interface charge transfer among the samples. This preliminary result is consistent with the transient photocurrent response results displayed in Figure 7(a). Figure 7(c) presents the possible equivalent circuits for a quantitative analysis of the interfacial charge transfer ability of the various Bi2S3 photoelectrodes. A Randles equivalent circuit model comprising a series-connected resistor (R s) and a parallel circuit (R ct − C dl) in the electrochemical half-cell was employed [41]. In the illustration, R s represents solution resistance, C dl represents the double-layer capacitor, and R ct represents the charge transfer resistance. According to the fitting results of Nyquist data, the R ct values of the nanosheet-, nanoribbon-, and nanowire-based photoelectrodes were approximately 1,041, 1,979, and 1,768 Ω, respectively. According to these results, the Bi2S3 nanosheet-based photoelectrode exhibited the lowest charge transfer resistance among the various photoelectrodes.
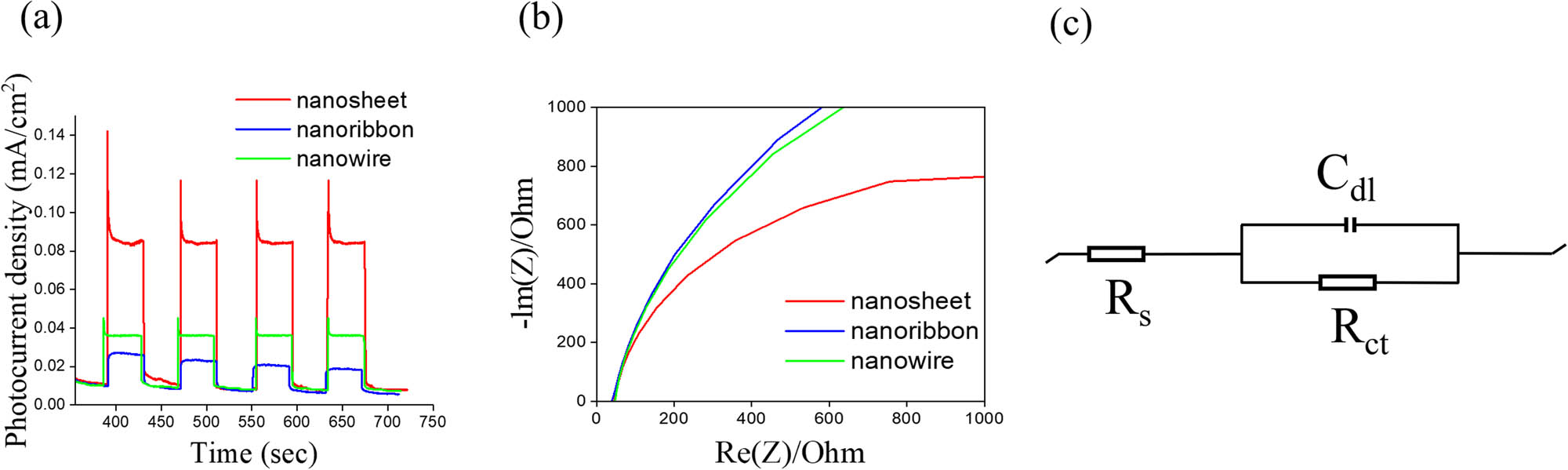
(a) Photocurrent density versus time curves of various Bi2S3 photoelectrodes under chopped illumination at 1 V. (b) Nyquist plots of various Bi2S3 photoelectrodes under irradiation. (c) The possible equivalent circuit used for R ct evaluation of various Bi2S3 photoelectrodes.
The electronic properties of the various Bi2S3 photoelectrodes are presented in the Mott–Schottky (M–S) plots (Figure 8). All the Bi2S3 photoelectrodes exhibited a positive slope in the M–S curves, reflecting the n-type nature of Bi2S3. The flat band potential (E fb) of the various Bi2S3 samples can be obtained from the tangent to the focal point of the X axis at 1/C 2 = 0 in Figure 8, according to the M–S equation [42]. The E fb values of the various Bi2S3 samples were approximately −0.254, −0.232, and −0.239 eV relative to normal hydrogen electrode (NHE) for the Bi2S3 nanosheet-, nanoribbon-, and nanowire-based photoelectrodes, respectively. The negative shift of the E fb of an n-type semiconductor is closely related to its active surface area. For example, the E fb values of the hydrothermally derived Bi2S3 nanorods differed from those of the nanoflowers. The V fb value of the Bi2S3 nanoflowers with a larger active surface area was more negative than that of the Bi2S3 nanorods [43]. Moreover, electrochemically deposited ZnO nanopillars and nanotubes also exhibited different E fb; the ZnO nanotubes with a larger surface area exhibited a significant negative shift in E fb with reference to that of the ZnO nanopillars in the M–S measurements [44]. These examples indicate that a larger surface area corresponds to the presence of more active sites on the surface of a semiconductor, which promotes the transfer of more carriers on the surface of the sample and on the electrolyte, causing a change in the Fermi level of the semiconductor. As indicated in Figure 8, the E fb of the Bi2S3 nanosheet-based photoelectrode exhibited a large negative shift with reference to that of other samples; this finding is consistent with the results of the previous surface active site analysis and of those in other published works [43,44].

M–S plots of various Bi2S3 products: (a) nanosheet, (b) nanoribbon, and (c) nanowire.
Figure 9(a)–(c) presents the absorbance spectra intensity variations of an RhB solution with the Bi2S3 nanosheet, nanoribbon, and nanowire photocatalysts, respectively, under the given PEC-degradation conditions. The decreased absorbance spectra intensity of the RhB solution with irradiation time indicated that all the Bi2S3 photocatalysts exhibited effective PEC-degradation of the RhB solution. Figure 9(d) summarizes the variation in C/C o concentrations with respect to irradiation time at an applied bias potential of 1.0 V. After 80 min of irradiation, the Bi2S3 nanosheets, nanoribbons, and nanowires degraded approximately 89.2, 66.3, and 72.6% of the RhB solution, respectively, indicating that the Bi2S3 nanosheets degrade RhB most effectively under the same PEC-degradation conditions. Figure 9(e) displays the coloration variation of the RhB solution PEC degraded by the Bi2S3 nanosheets under different irradiation durations. The pink RhB solution gradually became transparent during 80 min of PEC degradation, indicating that the Bi2S3 nanosheets effectively degraded the RhB over time. Figure 9(f) presents the kinetic linear simulation curves of the PEC degradation of the RhB solution by various Bi2S3 samples according to a pseudo first-order kinetic model [38]. The rate constant k was evaluated to be 0.0204 min−1 for the Bi2S3 nanosheets, 0.0135 min−1 for the nanoribbons, and 0.0159 min−1 for the nanowires. The rate constant k of the Bi2S3 nanosheets was the highest, whereas that of the Bi2S3 nanoribbons was the smallest, indicating that the nanosheet-structured Bi2S3 exhibited superior PEC performance.

The irradiation–duration-dependent absorption intensity variation of the RhB solution with various Bi2S3 photocatalysts at 1 V: (a) nanosheet, (b) nanoribbon, and (c) nanowire. (d) C/C o versus irradiation duration plot with various Bi2S3 photocatalysts at 1 V. (e) Decoloration of RhB solution with Bi2S3 nanosheets after different PEC-degradation durations. (f) ln (C o/C) versus irradiation duration plot with various Bi2S3 photocatalysts at 1 V.
The possible PEC-degradation mechanism of the Bi2S3 toward RhB solution is illustrated in Figure 10. The E
g (i.e., energy gap) values of the Bi2S3 nanosheets, nanoribbons, and nanowires were 1.41, 1.47, and 1.46 eV, respectively. The conduction band (CB) edge energy of n-type semiconductors is often approximately 0.1 eV more negative than the semiconductor’s flat band potential [17]. Therefore, the CB values of the Bi2S3 nanosheets, nanoribbons, and nanowires can be estimated as −0.354, −0.332, and −0.339 eV (vs NHE), respectively. Furthermore, the valence band (VB) potential of a semiconductor can be calculated according to the equation

The possible PEC-degradation mechanism of RhB solution with Bi2S3 photocatalysts.
The superior PEC degradation ability of Bi2S3 nanosheet photocatalysts among various Bi2S3 photocatalysts can be attributed to several factors, according to the preceding analyses. A photocatalyst’s active surface area affects its degradation ability by allowing for greater contact with electrolytes and dyes [52]. Furthermore, the superior light-trapping ability and smaller band gap of Bi2S3 nanosheets, as compared with those of the other Bi2S3 samples, also contribute to their efficient PEC degradation of RhB solutions [53]. The desirable photocatalytic properties of photocatalysts have also been associated with surface defects, which act as charge traps and can effectively prevent photoinduced electrons and holes from recombining [27]. As described by Xu et al., the higher surface defect density in the hydrothermally synthesized BiOCl nanosheets promotes superior photocatalytic performance relative to that of the less-defective nanosheets [25]. Similarly, the abundant defect structure of the Bi2S3 nanosheets contributes to its higher PEC-degradation efficiency compared with those among the various other samples. These results indicate that a Bi2O3 thin-film layer used to form free-standing Bi2S3 crystals in a flaky structure through hydrothermal vulcanization can be effectively used to degrade organic pollutants.
4 Conclusion
Bi2S3 nanostructures with different morphologies, including nanosheets, nanoribbons, and nanowires, can be synthesized using hydrothermal vulcanization. These Bi2S3 nanostructures can be firmly grown on FTO glass substrates using a Bi2O3 sacrificial thin-film layer during the hydrothermal vulcanization process. The formation of Bi2S3 nanostructures involves the etching of the Bi2O3 sacrificial thin-film layer and the regrowth of Bi2S3 crystallites. The Bi2S3 nanosheets exhibit photoactive performance superior to that of Bi2S3 crystals with other morphologies. This can be attributed to the fact that Bi2S3 nanosheets have a larger active surface active area, higher surface defect density, higher light absorption capacity, and lower recombination rate of photogenerated charges. Finally, comparison of PEC degradation of RhB solutions indicates that the ability of Bi2S3 nanosheets to degrade RhB dyes is superior to that of other Bi2S3 products. This study therefore demonstrated a promising approach to in situ growth of Bi2S3 nanostructures firmly aligned on an FTO substrate to be used in various PEC applications.
-
Funding information: This research was funded by the Ministry of Science and Technology of Taiwan. Grant No. MOST 108-2221-E-019-034-MY3.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Li Y, Wei F, Ma Y, Zhang H, Gao Z, Dai L, et al. Selected-control hydrothermal synthesis and photoresponse properties of Bi2S3 micro/nanocrystals. CrystEngComm. 2013;15:6611–6.10.1039/c3ce40435hSearch in Google Scholar
[2] Yu H, Wang J, Wang T, Yu H, Yang J, Liu G, et al. Scalable colloidal synthesis of uniform Bi2S3 nanorods as sensitive materials for visible-light photodetectors. CrystEngComm. 2017;19:727–33.10.1039/C6CE01879CSearch in Google Scholar
[3] Liang YC, Chung CC, Lin TY, Cheng YR. Synthesis and microstructure-dependent photoactivated properties of three-dimensional cadmium sulfide crystals. J Alloy Compd. 2016;688:769–75.10.1016/j.jallcom.2016.07.108Search in Google Scholar
[4] Liang YC, Lung TW. Growth of hydrothermally derived CdS-based nanostructures with various crystal features and photoactivated properties. Nanoscale Res Lett. 2016;11:264–74.10.1186/s11671-016-1490-xSearch in Google Scholar PubMed PubMed Central
[5] Sun B, Feng T, Dong J, Li X, Liu X, Wu J, et al. Green synthesis of bismuth sulfide nanostructures with tunable morphologies and robust photoelectrochemical performance. CrystEngComm. 2019;21:1474–81.10.1039/C8CE02089BSearch in Google Scholar
[6] Hui Y, Dijie H, Zhonghong J, Yong D. Preparation and optical properties of Bi2S3 microcrystallite doped glass and thin film by the sol-gel process. J Sol-Gel Sci Technol. 1994;3:235–9.10.1007/BF00486562Search in Google Scholar
[7] Liu Z, Peng S, Xie Q, Hu Z, Yang Y, Zhang S, et al. large-scale synthesis of ultralong Bi2S3 nanoribbons via a solvothermal process. Adv Mater. 2003;15:936–40.10.1002/adma.200304693Search in Google Scholar
[8] Kim JH, Ma A, Jung H, Kim HY, Choe HR, Kim YH, et al. In situ growth of the Bi2S3 nanowire array on the bi2moo6 film for an improved photoelectrochemical performance. ACS Omega. 2019;4:17359–65.10.1021/acsomega.9b02111Search in Google Scholar PubMed PubMed Central
[9] Warule SS, Chaudhari NS, Kale BB, Pandiraj S, Khare RT, More MA. Controlled synthesis of aligned Bi2S3 nanowires, sharp apex nanowires and nanobelts with its morphology dependent field emission investigations. CrystEngComm. 2013;15:890–6.10.1039/C2CE26588ESearch in Google Scholar
[10] Liang YC, Hunga CS. Effects of sputtering deposited homoseed layer microstructures on crystal growth behavior and photoactivity of chemical route-derived WO3 nanorods. CrystEngComm. 2019;21:5779–88.10.1039/C9CE00779BSearch in Google Scholar
[11] Liang YC, Liu YC, Hung CS. Sputtering control of Ag2O decoration configurations on ZnO nanorods and their surface arrangement effects on photodegradation ability toward methyl orange. Nanotechnology. 2019;30:495701.10.1088/1361-6528/ab40ddSearch in Google Scholar PubMed
[12] Liang YC, Xu NC. Synthesis of TiO2–ZnS nanocomposites via sacrificial template sulfidation and their ethanol gas-sensing performance. RSC Adv. 2018;8:22437–46.10.1039/C8RA04157ASearch in Google Scholar
[13] Liang YC, Wanga CC. Surface crystal feature-dependent photoactivity of ZnO–ZnS composite rods via hydrothermal sulfidation. RSC Adv. 2018;8:5063–70.10.1039/C7RA13061ASearch in Google Scholar PubMed PubMed Central
[14] Ma DK, Guan ML, Liu SS, Zhang YQ, Zhang CW, Hea YX, et al. Controlled synthesis of olive-shaped Bi2S3/BiVO4 microspheres through a limited chemical conversion route and enhanced visible-light-responding photocatalytic activity. Dalton Trans. 2012;41:5581–6.10.1039/c2dt30099kSearch in Google Scholar PubMed
[15] Chen L, He J, Yuan Q, Liu Y, Au CT, Yin SF. Environmentally benign synthesis of branched Bi2O3–Bi2S3 photocatalysts by an etching and re-growth method. J Mater Chem A. 2015;3:1096–102.10.1039/C4TA05346JSearch in Google Scholar
[16] Wu J, Qin F, Cheng G, Li H, Zhang J, Xie Y, et al. Large-scale synthesis of bismuth sulfide nanorods by microwave irradiation. J Alloy Compd. 2011;509:2116–26.10.1016/j.jallcom.2010.10.160Search in Google Scholar
[17] Kim JH, Lim T, Park JY, Ma A, Jung H, Kim HY, et al. Understanding and improving photoelectrochemical performance of Bi2O3/Bi2S3 composite. N J Chem. 2019;43:11893–902.10.1039/C9NJ02913CSearch in Google Scholar
[18] Hong C, Kim YI, Seo JH, Kim JH, Ma A, Lim YJ, et al. Comprehensive study of growth mechanism and photoelectrochemical activity of BiVO4/Bi2S3 nanowire composite. ACS Appl Mater Interfaces. 2020;12:39713–9.10.1021/acsami.0c07577Search in Google Scholar PubMed
[19] Jia T, Wang X, Long F, Li J, Kang Z, Fu F, et al. Facile synthesis, characterization, and visible-light photocatalytic activities of 3D hierarchical Bi2S3 architectures assembled by nanoplatelets. Crystals. 2016;6(11):140.10.3390/cryst6110140Search in Google Scholar
[20] Li C, Kan H, Luo J, Fu C, Zhou J, Liu X, et al. A high performance surface acoustic wave visible light sensor using novel materials: Bi2S3 nanobelts. RSC Adv. 2020;10:8936–40.10.1039/C9RA08848BSearch in Google Scholar PubMed PubMed Central
[21] Soileau M. Ripple structures associated with ordered surface defects in dielectrics. IEEE J Quantum Electron. 1984;20:464–7.10.1109/JQE.1984.1072422Search in Google Scholar
[22] Phuruangrat A, Thongtem T, Thongtem S. Characterization of Bi2S3 nanorods and nano-structured flowers prepared by a hydrothermal method. Mater Lett. 2009;63:1496–8.10.1016/j.matlet.2009.03.051Search in Google Scholar
[23] Miniach E, Gryglewicz G. Solvent-controlled morphology of bismuth sulfide for supercapacitor applications. J Mater Sci. 2018;53:16511–23.10.1007/s10853-018-2785-3Search in Google Scholar
[24] Xiao L, Zhang X, Yan G. Diatomite-Bi2S3 composite photocatalyst: enhanced photocatalytic performance for visible light reduction of Cr(vi). Mater Res Express. 2019;6:065902.10.1088/2053-1591/ab086dSearch in Google Scholar
[25] Xu J, Teng Y, Teng F. effect of surface defect states on valence band and charge separation and transfer efficiency. Sci Rep. 2016;6:32457.10.1038/srep32457Search in Google Scholar PubMed PubMed Central
[26] Wang J, Jiang W, Liu D, Wei Z, Zhu Y. photocatalytic performance enhanced via surface bismuth vacancy of Bi6S2O15 core/shell nanowires. Appl Catal B: Environ. 2015;176–177:306–14.10.1016/j.apcatb.2015.04.022Search in Google Scholar
[27] Cheng H, Selloni A. Surface and subsurface oxygen vacancies in anatase TiO2 and differences with rutile. Phys Rev B. 2009;79:092101.10.1103/PhysRevB.79.092101Search in Google Scholar
[28] Chang F, Peng S, Yan W, Yang C, Li S, Liu X. A novel and facile procedure to decorate Bi2O3 with Bi2S3 nanocrystals: composites synthesis, analyses, and photocatalytic performance assessment. Colloids Surf A: Physicochem Eng Asp. 2021;610:125640.10.1016/j.colsurfa.2020.125640Search in Google Scholar
[29] Masoud SN, Zeynab B, Omid A, Elahe K, Mostafa H. Hydrothermal synthesis of bismuth sulfide (Bi2S3) nanorods: bismuth(III) monosalicylate precursor in the presence of thioglycolic acid. J Cluster Sci. 2013;24:349–63.10.1007/s10876-012-0520-9Search in Google Scholar
[30] Talib M, Tabassum R, Islam SS, Mishra P. Influence of growth temperature on titanium sulphide nanostructures: from trisulphide nanosheets and nanoribbons to disulphide nanodiscs. RSC Adv. 2019;9:645–57.10.1039/C8RA08181FSearch in Google Scholar
[31] Meng S, Ogawa T, Okumura H, Ishihara KN. Enhanced photocatalytic activity of BiVO4/Bi2S3/SnS2 heterojunction under visible light. Catalysts. 2020;10(11):1294.10.3390/catal10111294Search in Google Scholar
[32] Liang YC, Hsu YU. enhanced sensing ability of brush-like Fe2O3–ZnO nanostructures towards NO2 gas via manipulating material synergistic effect. Int J Mol Sci. 2021;22:6884–95.10.3390/ijms22136884Search in Google Scholar PubMed PubMed Central
[33] McCrory CCL, Jung S, Peters JC, Jaramillo TF. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J Am Chem Soc. 2013;135(45):16977–87.10.1021/ja407115pSearch in Google Scholar PubMed
[34] Tang Y, Fang X, Zhang X, Fernandes G, Yan Y, Yan D, et al. A space-confined earth-abundant bifunctional electrocatalyst for high-efficiency water splitting. ACS Appl Mater Interfaces. 2017;9(42):36762–71.10.1021/acsami.7b10338Search in Google Scholar PubMed
[35] Liang YC, Yang HC. Boosting photoresponsive ability of WO3–Bi2O3 nanocomposite rods via annealing-induced intrinsic precipitation of nanosized Bi particles. Nanotechnology. 2021;32:315701.10.1088/1361-6528/abfabfSearch in Google Scholar PubMed
[36] Liang YC, Chou YH. Matrix phase induced boosting photoactive performance of ZnO nanowire turf-coated Bi2O3 plate composites. J Am Ceram Soc. 2021;1–13.10.1111/jace.17928Search in Google Scholar
[37] Mohamedkhair AK, Drmosh QA, Qamar M, Yamani ZH. Tuning structural properties of WO3 thin films for photoelectrocatalytic water oxidation. Catalysts. 2021;11(3):381.10.3390/catal11030381Search in Google Scholar
[38] Liang YC, Zhao WC. crystal growth and design of disk/filament ZnO-decorated 1D TiO2 composite ceramics for photoexcited device applications. Nanomaterials. 2021;11:667.10.3390/nano11030667Search in Google Scholar PubMed PubMed Central
[39] Gao Y, Zhang S, Bu X, Tian Y. surface defect engineering via acid treatment improving photoelectrocatalysis of −In2S3 nanoplates for water splitting. Catal Today. 2019;327:271–8.10.1016/j.cattod.2018.04.039Search in Google Scholar
[40] Han M, Jia J. The interlace of Bi2S3 nanowires with TiO2 nanorods: an effective strategy for high photoelectrochemical performance. J Colloid Interface Sci. 2016;481:91–9.10.1016/j.jcis.2016.07.045Search in Google Scholar PubMed
[41] Santana J, Andaluz ME, Villon G, Qi Y, Li T, Andersson M. Temperature impact on the internal resistance of a polymer electrolyte fuel cell considering the electrochemical impedance spectroscopy diagnosis. ECS Trans. 2020;96:183–90.10.1149/09601.0183ecstSearch in Google Scholar
[42] Wang Q, Liu Z, Jin R, Wang Y, Gao S. SILAR preparation of Bi2S3 nanoparticles sensitized TiO2 nanotube arrays for efficient solar cells and photocatalysts. Sep Purif Technol. 2019;210:798–803.10.1016/j.seppur.2018.08.050Search in Google Scholar
[43] Sharma S, Kumar D, Khare N. Plasmonic Ag nanoparticles decorated Bi2S3 nanorods and nanoflowers: their comparative assessment for photoelectrochemical water splitting. Int J Hydrog Energy. 2019;44:3538–52.10.1016/j.ijhydene.2018.11.238Search in Google Scholar
[44] Rokade A, Rondiya S, Sharma V, Prasad M, Pathan H, Jadkar S. Electrochemical synthesis of 1D ZnO nanoarchitectures and their role in efficient photoelectrochemical splitting of water. J Solid State Electrochem. 2017;21:2639–48.10.1007/s10008-016-3427-9Search in Google Scholar
[45] Li S, Wang Z, Xie X, Liang G, Cai X, Zhang X, et al. Fabrication of vessel–like biochar–based heterojunction photocatalyst Bi2S3/BiOBr/BC for diclofenac removal under visible LED light irradiation: Mechanistic investigation and intermediates analysis. J Hazard Mater. 2020;391:121407.10.1016/j.jhazmat.2019.121407Search in Google Scholar PubMed
[46] Li J, li M, Jin Z. Rational design of a cobalt sulfide/bismuth sulfide S-scheme heterojunction for efficient photocatalytic hydrogen evolution. J Colloid Interface Sci. 2021;592:237–48.10.1016/j.jcis.2021.02.053Search in Google Scholar PubMed
[47] Hu T, Dai K, Zhang J, Zhu G, Liang C. One-pot synthesis of step-scheme Bi2S3/porous g-C3N4 heterostructure for enhanced photocatalytic performance. Mater Lett. 2019;257:126740.10.1016/j.matlet.2019.126740Search in Google Scholar
[48] Chen Y, Wang G, Li H, Zhang F, Jiang H, Tian G. Controlled synthesis and exceptional photoelectrocatalytic properties of Bi2S3/MoS2/Bi2MoO6 ternary hetero-structured porous film. J Colloid Interface Sci. 2019;555:214–23.10.1016/j.jcis.2019.07.097Search in Google Scholar PubMed
[49] Zheng X, Li D, Li X, Yu L, Wang P, Zhang X, et al. Photoelectrocatalytic degradation of rhodamine B on TiO2 photonic crystals. Phys Chem Chem Phys. 2014;16:15299–306.10.1039/C4CP01888ESearch in Google Scholar
[50] Xue C, Yan X, Dingb S, Yang G. Monodisperse Ag–AgBr nanocrystals anchored on one-dimensional TiO2 nanotubes with efficient plasmon-assisted photocatalytic performance. RSC Adv. 2016;6:68653–62.10.1039/C6RA13269CSearch in Google Scholar
[51] Lin HP, Lee WW, Huang ST, Chen LW, Yeh TW, Fu JY, et al. Controlled hydrothermal synthesis of PbBiO2Br/BiOBr heterojunction with enhanced visible-driven-light photocatalytic activities. J Mol Catal A: Chem. 2016;417:168–83.10.1016/j.molcata.2016.03.021Search in Google Scholar
[52] Yao Y, Li K, Chen S, Jia J, Wang Y, Wang H. Decolorization of Rhodamine B in a thin-film photoelectrocatalytic (PEC) reactor with slant-placed TiO2 nanotubes electrode. Chem Eng J. 2012;187:29–35.10.1016/j.cej.2012.01.056Search in Google Scholar
[53] Shi R, Xu T, Yan L, Zhu Y, Zhou J. Enhancement of visible light photocatalysis performances of Bi2MoS2O4 nanoplates. Catal Sci Technol. 2013;3:1757–64.10.1039/c3cy20853bSearch in Google Scholar
© 2022 Yuan-Chang Liang and Tsun-Hsuan Li, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption

