Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
-
Yixi Wang
und Zhengyong Li
Abstract
Extracellular vesicles (EVs), products released by cells in multiple biological activities, are currently widely accepted as functional particles and intercellular communicators. From the orthodox perspective, EVs derived from apoptotic cells (apoEVs) are responsible for cell debris clearance, while recent studies have demonstrated that apoEVs participate in tissue regeneration. However, the underlying mechanisms and particular functions in tissue regeneration promotion of apoEVs remain ambiguous. Some molecules active during apoptosis also function in tissue regeneration triggered by apoptosis, such as caspases. ApoEVs are generated in the process of apoptosis, carrying cell contents to manifest biological effects and possess biomarkers to target phagocytes. The regenerative effect of apoEVs might be due to their abilities to facilitate cell proliferation and regulate inflammation. Such regenerative effect has been observed in various tissues, including skin, bone, cardiovascular system, and kidneys. Engineered apoEVs are produced to amplify the biological benefits of apoEVs, rendering them optional for drug delivery. Meanwhile, challenges exist in thorough mechanistic exploration and standardization of production. In this review, we discussed the link between apoptosis and regeneration, current comprehension of the origination and investigation strategies of apoEVs, and mechanisms in tissue regeneration of apoEVs and their applications. Challenges and prospects are also addressed here.
Abbreviations
- AiP
-
apoptosis-induced compensatory proliferation
- ApoEVs
-
extracellular vesicles derived from apoptotic cells
- BMM
-
bone marrow macrophages
- DLS
-
dynamic light scattering
- EVs
-
extracellular vesicles
- FACS
-
Fluorescence activated cell sorting
- HUVEC
-
human umbilical vein endothelial cells
- MSCs
-
mesenchymal stem cells
- MSNs
-
mesoporous silica nanoparticles
- PtdSer
-
phospholipid phosphatidylserine
- ROS
-
oxygen species
- SEM
-
scanning electron microscope
- TEM
-
transmission electron microscope
- UV
-
ultraviolet
1 Introduction
Extracellular vesicles (EVs) are micro/nanosized vesicles containing small particles with lipid membranes secreted by cells in multiple biological activities. EVs serve as information communicators of cells [1,2], which were formerly considered cell debris. According to biogenesis and size, EVs can be roughly classified as exosomes (endosome origin, 30–100 nm in diameter), microvesicles (plasma origin, 50–1,000 nm), and apoptotic bodies (apoptotic cell origin, 1–5 µm) [3,4]. EVs of various sizes from apoptotic cells have been studied over the last several decades and they are not just confined to apoptotic bodies. As a result, apoptotic vesicles are used to refer to all types of EVs derived from apoptotic cells (apoEVs) [5,6,7].
Apoptosis is a highly ordinated general physiological process that occurs every second throughout the body, resulting in the death of billions of cells every day [8,9]. Different from necrosis, apoptosis is unlikely to cause a traumatic defect or aberrant tissue shrinkage. As a result, apoptosis is well-known to play a role in tissue homeostasis and life cycle regulation, although there are still many unsolved mysteries in the field [10]. In recent years, tissue renewal, such as epithelium, skin, and liver renewal, has been widely described as one of the major biological effects mediated by apoptosis [11,12,13]. ApoEVs are products in the apoptotic process. Traditionally, the uptake of apoEVs by phagocytes was thought to be the end of apoptosis, which biochemically signals the death of cells, but new studies suggest that their fates include transfer and reuse [14,15,16,17]. Apoptosis-like regeneration properties have also been demonstrated in the application of apoEVs, which come in a variety of sizes and are produced through different apoptotic pathways. Recent studies indicate that apoEVs might promote regeneration in tissue maintenance, including skin, bone, cardiovascular system, and kidneys, by functioning bioactive effects through recruitment of targeted cells and delivery of biomolecules [18,19,20,21,22]. To better utilize and amplify the therapeutic properties of apoEVs, engineered methods implicating targeting, releasing, and retention have been investigated [23,24]. Figure 1 demonstrates the production, isolation, functions, and applications of apoEVs. The culture medium of apoptotic cells was collected and centrifuged to obtain apoEVs. By recruiting targeting cells and delivering biomolecules, apoEVs promote cell proliferation and regulate inflammation, thus being applied in tissue regeneration. ApoEVs are modified to function in broader fields as well. Recent studies have discussed the functions and applications of apoEVs in immune regulation, inflammation mediation, mesenchymal stem cell (MSC) transplantation, and tumor progression [3,25,26,27,28]. Nonetheless, a few documents have comprehensively discussed the regenerative effect of apoEVs and analyzed the underlying mechanisms. In this review, the mechanisms of apoEVs in tissue regeneration are addressed here, along with their applications in both medicine and engineering. We hope this work will provide an indicative view of the mechanisms in tissue regeneration of apoEVs and inspiration for novel application of apoEVs in the future.
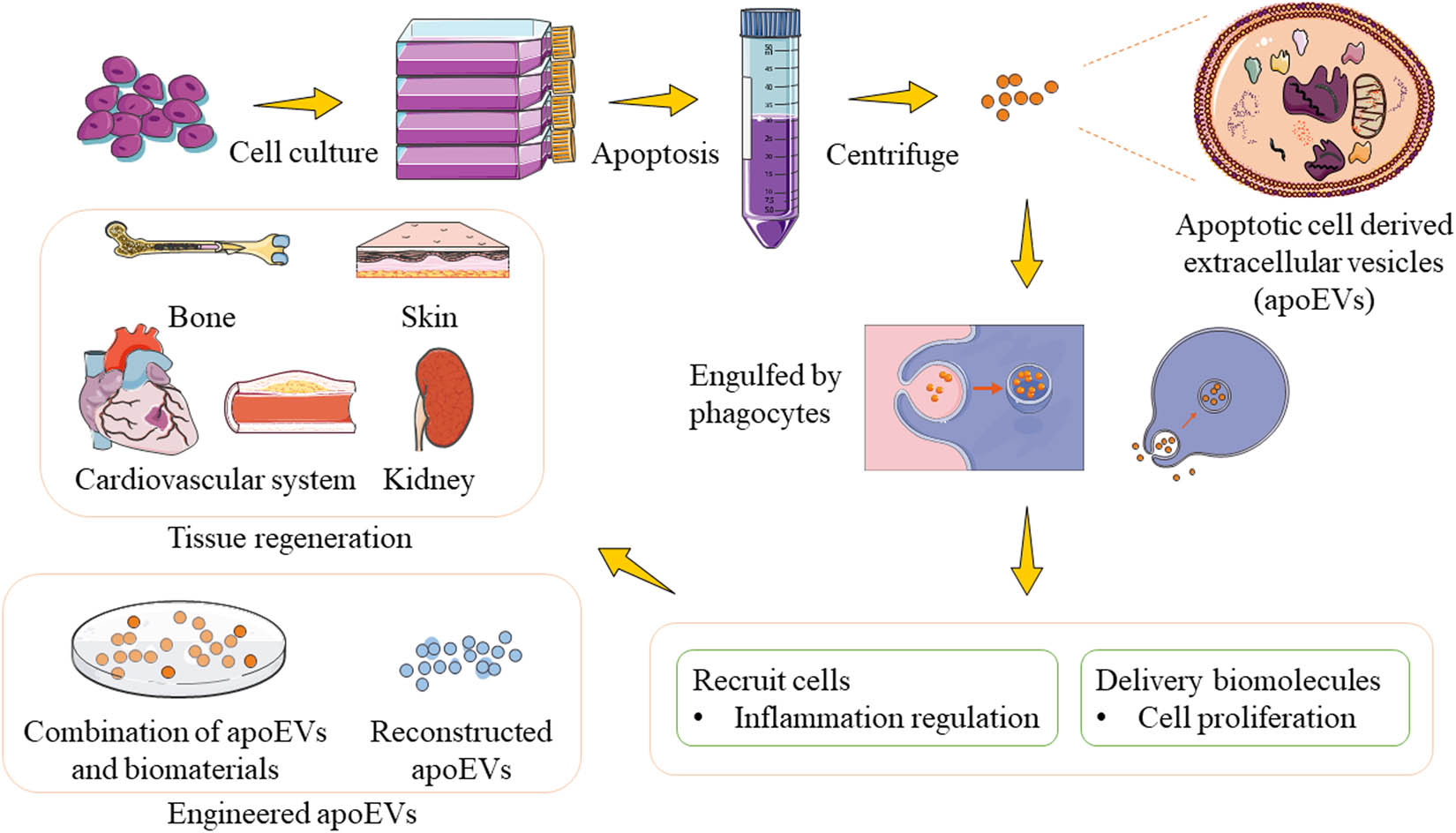
The production, isolation, functions and applications of apoptotic cell-derived extracellular vesicles (apoEVs). The culture medium of apoptotic cells was collected and centrifuged to obtain apoEVs. By recruiting targeting cells and delivering biomolecules, apoEVs promote cell proliferation and regulate inflammation, thus being applied in tissue regeneration. ApoEVs are modified to function in broader fields as well.
2 Apoptosis and regeneration
2.1 Apoptosis with caspases
Since the term “apoptosis” was proposed for the first time by John Foxton Ross Kerr and his colleagues at the University of Queensland [29], it has been proven to participate in various physiological processes, including development, aging, immune homeostasis, and tissue integrity maintenance [3,16,30]. Apoptosis, the most prominent pattern of programmed cell death, is characterized by several morphological changes, involving cell shrinkage, cytoplasmic and nuclear condensation, membrane blebbing, and the subsequent formation of apoptotic bodies.
Apoptosis, a conserved physiological process, can typically be triggered by the intrinsic pathway and the extrinsic pathway. A general summary is addressed here to clarify the processes of apoptosis. Caspases are fundamental participants in both pathways of apoptosis. The intrinsic pathway of apoptosis is closely associated with mitochondrial regulation, in which stimuli (for example, DNA damage) generate biochemical changes inside the cells. Stimuli elicit the dissipation of mitochondrial transmembrane potential, thus forming mitochondrial permeability transition pore on the outer membrane and leaking proapoptotic mitochondrial contents. Cytochrome c is then released into the cytoplasm to induce oligomerization of apoptotic peptide activating factor 1, which recruits procaspase-9 and forms apoptosome, the activator of caspase-9. Subsequently, activated caspase-9 in turn cleaves response caspases, activating caspase cascade reactions [16,31,32]. The extrinsic pathway of apoptosis initiates with the activation of death receptors. Upon contact with extracellular ligands, mainly TNF and Fas, the death receptors on the cell membrane transfer death signals toward the cytoplasm. The binding of ligands and receptors initiates the recruitment of Fas-associated death domain protein, causing autocleavage of procaspase-8. The activated caspase-8 then cleaves procaspase-3 to yield activated caspase-3 responsible for executing the downstream degradation process [16,30,32].
2.2 Regeneration with apoptosis
The mechanism of apoptosis is much more sophisticated than the brief sketch above, concerning a series of interactions and cascade signaling pathways, in which caspases have a well-defined role. However, increasing evidence implies the function of caspases in tissue repair and regeneration [33]. Dead cells are replaced by compensatory proliferated cells to maintain tissue homeostasis. Apoptotic cells deliver mitogenic signals to trigger the compensatory proliferation of neighboring cells, which is so-called “apoptosis-induced compensatory proliferation (AiP)” [11,34].
Caspases, the initiator and effector of apoptosis, are the major activators of AiP [11]. In Drosophila, cell death was blocked by the effector caspase inhibitor p53 even with high expression of the initiator caspase Dronc, rendering Dronc functioning apart from apoptosis [35,36]. Actually, Dronc activated AiP via the JNK signaling pathway to express Wingless and Decapentaplegic mitogens, triggering compensatory proliferation of surrounding cells [34,37]. In Dronc-induced AiP, extracellular reactive oxygen species (eROS) produced by NADPH oxidase Duox activated macrophage-like hemocytes, which in turn amplified the JNK signal by TNF ortholog Eiger, thus promoting epithelial cell growth [11,12]. The effector caspases DrICE and Dcp-1 induced AiP in the eye tissue via Hedgehog signaling [36,38]. In some other species, such as Xenopus tadpole and Hydra, ROS are pivotal compartments for AiP. Profuse ROS at the wound activated Wnt/β-catenin signaling and downstream fgf20, initiating active cell proliferation in tail regeneration of Xenopus tadpole [39]. In Hydra, ROS produced immediately after injury elicited MAPK signaling [11] and Wnt3 [40] in head regeneration.
In mammals, regeneration due to AiP is also linked to caspases. In mice, the lack of caspase-3 or caspase-7 leads to deficiency in the skin and liver regeneration [13]. The cleaved caspase-3 and caspase-7 initiate calcium-independent phospholipase A2 to upregulate the expression of arachidonic acid, which further mediates the production of prostaglandin E2, facilitating tissue repair and stem cell proliferation [13]. The activation of caspase-3 enlarged YAP-dependent organs, while inhibition of caspase-3 attenuated cell proliferation and diminished the sebaceous gland [41]. Some mediate evidence also proves the function of caspases in regeneration. The self-renewal property of embryonic stem cells was remarkably inhibited when lacking caspase-3 [42]. Deficient caspase-3 in mice resulted in the impaired differentiation of bone marrow stem cells and decreased bone density by the activation of the TGF-β/Smad2 signaling pathway [43,44], which was ameliorated by secretion from apoptotic MSCs [44]. In conclusion, participants in apoptosis, such as caspases and ROS, function in tissue renewal, coupling cell death, and regeneration.
3 Apoptotic extracellular vesicles (apoEVs)
Extracellular vesicles derived from apoptotic cells (apoEVs), the products of the programmed cell clearance process, are intercellular signal transporters harboring biomolecules from dying cells, which were previously recognized as cell debris [8,25]. Traditionally, apoEVs are indicated to be apoptotic bodies with a diameter of 1–5 µm [8,29,32]. Some smaller EVs are also generated in apoptosis, described as apoptotic microvesicles (apoMVs, 50–1,000 nm) [4,7,28,44] and apoptotic exosomes (apoExos) (<150 nm) [6,45], because their size resembles microvesicles and exosomes released by viable cells [46]. Since the conventional identification of subtypes of apoEVs is currently ambiguous [25], apoEVs could be a general description of micro- to nanoscale vesicles [47]. Here, we demonstrated several representative morphologies of apoEVs (see Figure 2).
![Figure 2
Several representative images of apoEVs. (a) Scanning electron microscopy (SEM) image of MSC-derived apoEVs. Scale bar, 1 µm [18]. (b) Transmission electron microscopy (TEM) image of MSC-derived apoEVs. Scale bar, 125 nm [68]. (c) Live differential inference contrast (DIC) microscopy image showing generation of apoEVs from THP‐1 cells undergo UV-induced apoptosis. Scale bar, 5 or 10 µm as indicated [5].](/document/doi/10.1515/ntrev-2022-0052/asset/graphic/j_ntrev-2022-0052_fig_002.jpg)
Several representative images of apoEVs. (a) Scanning electron microscopy (SEM) image of MSC-derived apoEVs. Scale bar, 1 µm [18]. (b) Transmission electron microscopy (TEM) image of MSC-derived apoEVs. Scale bar, 125 nm [68]. (c) Live differential inference contrast (DIC) microscopy image showing generation of apoEVs from THP‐1 cells undergo UV-induced apoptosis. Scale bar, 5 or 10 µm as indicated [5].
3.1 Biogenesis and characteristics
Nowadays, the biogenesis of apoEVs is described as three sequential well-coordinated steps with corresponding morphological changes: plasma membrane blebbing, thin membrane protrusions formation, and fragmentation [8,25]. In the formation of membrane protrusions, different protrusions might be generated, including microtubule spike, apoptopodia, and beaded apoptopodia. However, for some specific cells, not every step is necessary for the biogenesis of apoEVs (Figure 3). Apoptotic membrane blebbing, the earliest morphological change in apoptosis, is driven by cytoskeleton collapse and increased hydrostatic pressure [32]. Caspases and various protein kinases regulate this mechanism, preventing apoptosis when they are inhibited [31]. Rho-associated protein kinase 1, a target of active caspase-3, induces phosphorylation of the myosin light chain (MLC), causing actomyosin contraction [32]. MLC kinase facilitates the assignment of degraded chromatin into blebs [48]. Serine/threonine LIM domain kinase 1 inhibits the actin-binding protein cofilin to promote actin polymerization [49]. This membrane blebbing is known as fundamental for membrane protrusions, but some cells undergo different membrane deformation in this process, indicating diverse mechanisms in addition to blebbing solely in apoEVs formation (Figure 3). Apoptotic A431 epithelial cells formed a rigid membrane protrusion, the microtubule spike, in the process of apoptosis without blebbing [50]. Human Jurkat T cells generated apoptopodia following blebbing, which is regulated by the caspase-activated four-pass transmembrane pannexin 1 channel, a negative regulator of apoptosis [51]. Recently, a novel “beads-on-a-string” type of membrane protrusion in apoptosis was described in apoptotic human THP-1 cells and monocytes. This beaded apoptopodia forms abundant apoEVs (approximately 10–20) with diameters of 1–4 µm due to the formation of protrusions on the “string” with a length eight times that of the dying cell, rendering it much more efficient than the previous two patterns of membrane protrusion [52]. After the formation of blebs and/or membrane protrusions, fragmentation is responsible for the drop of single apoEV (Figure 3). While the specific mechanism or molecules mediating this process are currently unclear now, shear force in circulation or intercellular physical force of cell disassembly might be involved [8].
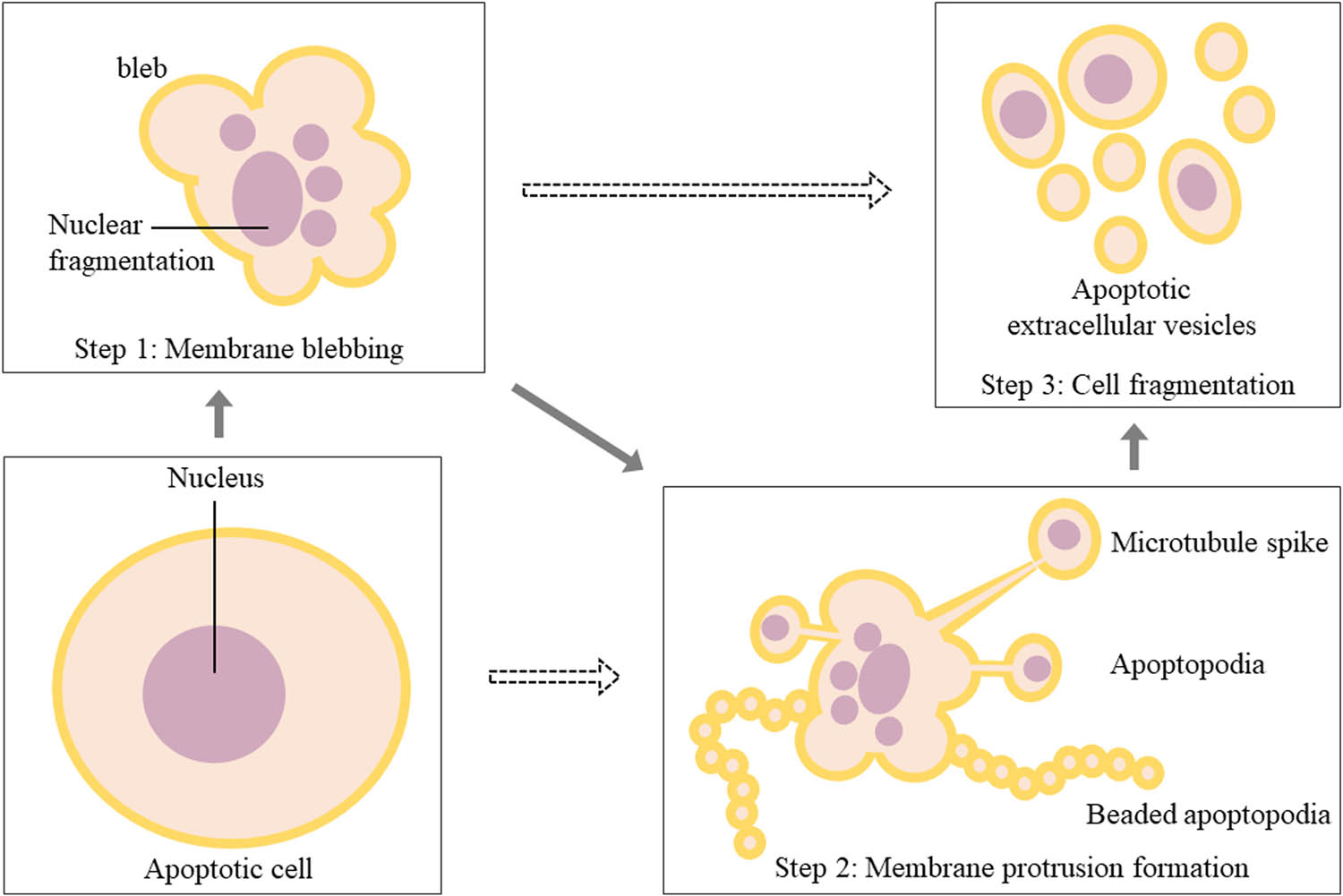
Morphological changes of apoptotic cells in the biogenesis of apoEVs. It could be described as three steps with corresponding morphological changes: plasma membrane blebbing, membrane protrusions formation, and cell fragmentation. In step 2, different protrusions might be generated, including microtubule spike, apoptopodia, and beaded apoptopodia. Dotted arrows mean that apoEVs can be generated by some specific cells without step 1 or step 2.
By the end of apoptosis, “find-me” and “eat-me” signals are indispensable for the attraction and clearance of phagocytes [53], avoiding cell content leakage and secondary necrosis [32]. The “find-me” signal is a chemo-attractive gradient generated by the released soluble molecules in apoptosis, such as ATP, UTP [54], and CX3CL1/fractalkine [55], which was also found to be associated with apoEVs [25,56]. Some receptor proteins in the membrane of phagocytes, the G protein-coupled receptor G2A, for example, induce the recognition of “find-me” signals, even though the precise pathway of this process and quantified attractive gradient remain to be elucidated [57]. The most universally studied “eat-me” signal is the exposure of phospholipid phosphatidylserine (PtdSer) [55], anchoring on the surface of apoEVs. In the membrane of healthy cells, PtdSer is strictly confined to the inner side of the bilayer. While undergoing apoptosis, PtdSer flips from the inner of the lipid bilayer of the plasma membrane to the outer layer during blebbing, which is induced by caspase-activated scramblases, a set of phospholipid translocases [57]. The appearance of PtdSer on the outer layer in both apoptotic cells and apoEVs transmits the dying signal, inducing recognition and clearance of phagocytes. In addition, ICAM-3, a confirmed mediator of apoptotic cell clearance, was also present on the surface of apoEVs to attract macrophages [58]. Notably, apoEVs seem to recruit phagocytes selectively, which might be due to content diversity caused by different parental cells and specific physiological and pathological processes. Adipocyte-derived apoEVs recruited macrophages rather than neutrophils [59]. However, neutrophils’ infiltration was observed in mice, in which larger apoEVs (1–3 µm in diameter) attracted neutrophils, whereas smaller ones (<1 µm) could not. Inflammatory chemokine IL-1 appeared in larger apoEVs but not in smaller ones to induce sterile neutrophil inflammation, which might originate from the nuclear IL-1α precursor that translocated into the nucleus during apoptosis [60]. The equilibrium between apoEVs and phagocytes is indispensable for homeostasis. Secondary necrosis might occur when massive apoptosis and subsequent excessive apoEVs containing inflammatory factors overwhelm the clearance ability of phagocytes or when the function or quantity of phagocytes is impaired in diseases.
In most studies, apoEVs were described as bioactive carriers transporting functional molecules [3,16,31], including DNA debris [61], RNA [21,62,63,64], and proteins [65,66]; yet, cargo differences among each group of apoEVs exist widely. The proteomes of healthy and cirrhotic human biliary epithelial cells showed a huge divergence in the shotgun proteomics of apoEVs [65]. Furthermore, exosome-like apoEVs carried more active 20S proteasome core than that in apoBDs, which is responsible for their immunogenic activity [45]. More interestingly, DNA and RNA could not be packed into apoEVs of HL-60 cells simultaneously. Over 90% of apoEVs containing RNA were free of DNA, and vice versa [62]. Additionally, nuclear material was absent in beaded apoptopodia [52], suggesting that some bioactive molecules may enter apoEVs with tropism. Different groups of apoEVs possess diverse properties and functions due to their distinct biogenesis processes and unequal contents that result from different parental cells and their previous physiological and pathological changes.
3.2 Production and identification
Technically, it is necessary for researchers to study apoEVs in a relatively uniform condition to acquire convincing information, whereas cells in a general culture condition are less likely to undergo apoptosis under control. Thus, some stimuli of apoptosis in specific physiological or pathological conditions have been applied in studies to produce apoEVs. Here, we reviewed production and isolation methods in recent years studying apoEVs, along with their documented times, donor cells, and experimental models (Table 1). The most universally used stimulus of apoEVs is staurosporine [18,19,23,64,67,68], a potent inhibitor of protein kinase C, which induces apoptosis by activating caspase-3 and suppressing the AKT/MAPK pathway [69,70]. Serum starvation might affect the cell cycle and promote apoptosis [71]. Exposure to gamma rays or ultraviolet radiation injured the stability of nucleic acids [67,72,73], which also appeared when applying some chemotherapeutic drugs, such as cisplatin [67]. For some specific cells, the corresponding stimulus was applied. Alendronate was used to produce apoEVs from osteoclasts due to endoplasmic reticulum stress in osteoclast precursors caused by alendronate [74].
Production and isolation strategies of apoEVs in recent years
| Ref | Origin of apoEVs | Inducer of apoptosis | Models | Isolation strategy |
|---|---|---|---|---|
| Hristov et al. [118] | HUVEC | Serum starvation | In vitro: epithelial progenitor cell | 16,000g* |
| Zernecke et al. [21] | HUVEC; human atherosclerotic plaque material, mouse whole blood | Serum starvation | In vitro: HUVEC | 16,000g for supernatant; FACS for tissue and blood |
| In vivo: rat | ||||
| Zhu et al. [63] | BMM | Lipopolysaccharide | In vitro: A549 epithelial cell | 2,000g |
| Shen et al. [72] | T cell, neutrophil | UV exposure | In vitro: Th cell | 100,000g |
| Liu et al. [44] | MSC | Staurosporine | In vitro: HUVEC | 1 and 5 µm filters followed by 2,000g |
| In vivo: mice | ||||
| Brock et al. [75] | Epithelal stem cell | Metronidazole | In vivo: zebrafish | — |
| Hardy et al. [64] | HUVEC | Serum starvation | — | 50,000g for apoBDs and 200,000g for apoExos |
| Chen et al. [73] | Primary murine thymocytes, Jurkat cell | Gamma ray or UV exposure, serum starvation | In vitro: macrophage | 180,000g |
| In vivo: mice | ||||
| CoralGarcía-Pastor et al. [67] | HK-2 cell | Cisplatin, UV exposure | In vitro: HK-2 cell | 5,000g |
| Ma et al. [20] | BMM, osteoclast | Alendronate | In vitro: MC3T3-E1 cell | 1 and 5 µm filters followed by 3,500g |
| Tyukavin et al. [90] | Cardiomyocyte, fibroblast | Serum starvation | In vivo: rat | 16,500g |
| Liu et al. [15] | MSC | Staurosporine | In vitro: HUVEC | 16,000g |
| In vivo: rat | ||||
| Ma et al. [19] | Osteoclast | Staurosporine | In vitro: endothelial cell | 3,000g |
| In vivo: mice | ||||
| Dou et al. [23] | T cell, HUVEC | Staurosporine | In vitro: macrophage | 1,000g |
| In vivo: mice | ||||
| Liu et al. [18] | MSC | Staurosporine | In vitro: macrophage | 16,000g |
| In vivo: mice | ||||
| Zheng et al. [68] | MSC | Staurosporine | In vitro: macrophage | 16,000g |
| In vivo: mice |
*Centrifuge speed; HUVEC: human umbilical vein endothelial cells; FACS: fluorescence activated cell sorting; BMM: bone marrow macrophages; UV: ultraviolet; MSC: mesenchymal stem cells.
For isolation of apoEVs, apoMVs, a subtype of apoEVs resembling microvesicles in size [4], were obtained in a centrifuge at 16,000–50,000g. ApoBDs and apoExos were isolated in a centrifuge at 1,000–5,000g and 100,000–200,000g, relatively. The size and morphology were observed and determined by dynamic light scattering (DLS) analysis, scanning electron microscope (SEM), transmission electron microscope (TEM), or confocal microscope. Fluorescence-activated cell sorting (FACS) was used to quantify and purify apoEVs. Several molecules were detected to identify apoEVs. The most widely accepted biomarker is Annexin V, a calcium-binding protein of the annexin superfamily, which can interact with the “eat-me” signal PtdSer. Thrombospondin and complement protein C3b, the products of membrane changes during apoptosis, are also well-accepted biomarkers. Cleaved caspase-3 and C1q were also applied in some cases. Nuclear granularity and hypochromicity were detected by propidium iodide but their quantities were not equal and were even absent in some apoEVs [5,15,18,19,20,21,23,44,63,64,67,68,72,73,75].
However, it is noteworthy that the current classification is based on size while ignoring their biogenesis in cells and the contents of each group. Besides, apoEVs derived from different cells might differ in size, and apoEVs would collapse over time in vitro [5]. Actually, the current studies are less likely to identify and analyze the subtype properties of apoEVs utilized in experiments, rendering disturbances from different groups inevitable. It is pivotal to further analyze the contents and properties and uncover the key proportion of each group of apoEVs, which contributes to standardizing protocols for isolation and identification, thus obtaining rigorous scientific results with diminished confounding.
4 Functions of apoEVs in tissue regeneration
ApoEVs regulate cell function by transferring various and abundant cargo, which involves many bioactive factors participating in tissue repair and regeneration. ApoEVs derived from different parental cells might differ in content and affect cell activities via diverse pathways. In tissue regeneration, the beneficial effects of apoEVs have been studied and discussed. Figure 4 demonstrates the main mechanisms of apoEVs in tissue regeneration, including promoting the proliferation of neighboring cells by compensatory proliferation signals and remote cells by transferring therapeutic molecules and suppressing inflammation by regulating macrophage polarization (Figure 4).

The main mechanisms of apoEVs in tissue regeneration, including promoting the proliferation of neighboring cells by compensatory proliferation signals and remote cells by transferring therapeutic molecules, and suppressing inflammation by regulating macrophage polarization.
4.1 Promoting cell proliferation
Tissue integrity is maintained in the homeostasis of dynamic cell death and compensatory proliferation. Apoptosis has been implied to activate compensatory proliferation signaling, and hence, dying cells mediate compensatory proliferation in adjacent cells [11,13,30,34,76,77], which is also called AiP [11,34]. ApoEVs, the product of dying cells, seem to inherit such a compensatory proliferation function. ApoEVs produced by different apoptotic stimuli were reported to trigger neighboring cell proliferation in both transformed and primary cells upon contact via compensatory proliferation signaling [78], which is related to CrkI, a type of protein responsible for cell proliferation and cytokinesis [79,80] (Figure 4). Epithelial stem cell-released apoEVs also triggered cell proliferation of standby healthy stem cells in such a compensatory proliferation way, maintaining regeneration of the epithelium [75]. In addition to the near approach, apoEVs were also implicated in promoting cell proliferation in remote sites. ApoEVs from macrophages delivering miR-221/222 induced lung epithelial cell growth by suppressing the expression of cyclin-dependent kinase inhibitor 1B, a tumor suppressor controlling cell cycle at G1 [63] (Figure 4). Circulating apoEVs from MSCs transferring ubiquitin ligase RNF146 and miR-328-3p recovered the self-renewal and differentiation properties of distant impaired MSCs [44] (Figure 4). Intriguingly, but predictably, the Wnt/β-catenin signaling pathway participated closely in cell growth and renewal promoted by apoEVs [44,75,78]. Focusing on miRNAs and proteins modulating the Wnt pathway in apoEVs could be a possible way to elucidate the mechanisms by which apoEVs promote tissue regeneration.
4.2 Regulating inflammation
Inappropriate inflammation impedes repair and regeneration progress in multiple diseases [81,82,83,84]. ApoEVs are capable of attenuating inflammation by modulating the cell activities of inflammatory cells, thereby smoothing the tissue regeneration process. Suppression of inflammatory cytokines, including MCP-1 and IL-6, caused by upregulated TSG-6 was observed after treatment with apoptotic MSCs [85] (Figure 4). TSG-6 has been reported to reduce proinflammatory signals from macrophages [86] and inhibit the activation of macrophages [87]. Furthermore, apoEVs have been implicated in modulating macrophage polarization both in vivo and in vitro. In mouse models, apoEVs from MSCs promoted polarization of anti-inflammatory M2 macrophages, whereby they increased the anti-inflammatory cytokines IL-10 and TGF-β and enhanced the migration and proliferation of fibroblasts [18] (Figure 4). Biomimic apoEVs were taken up more effectively by activated M1 macrophages and led to more intensive M2 macrophage polarization, ameliorating inflammation by upregulating anti-inflammatory cytokines [88]. In addition, apoEVs derived from activated T cells were likely to possess trophic properties to inflammatory sites similar to that of their parental cells, meanwhile, promoting M2 macrophage polarization [23]. In short, apoEVs help to recover a healthy microenvironment of repair and regeneration by ameliorating excessive local inflammation.
Besides all the above, many other intricate mechanisms contribute to the regeneration function of apoEVs. ApoEVs from MSCs promoted the proliferation of endothelial cells [15] and endothelial progenitor cells [89], thus inducing angiogenesis. ApoEVs derived from different parental cells manifested specific functions in regeneration resembling their parental cells, which was observed in preosteoclasts and mature osteoclasts [19,20], cardiomyocytes [90,91], and proximal tubular cells [67]. From this perspective, stem cells might be a promising origin of apoEVs due to their intrinsic regenerative properties. However, it is difficult to draw a definite conclusion from these scattered studies since there is a little convincing result indicating the direct correlation between the regenerative property of apoEVs and specific mechanisms.
5 Therapeutic effects of apoEVs in regeneration
In recent years, researchers have probed and utilized the regeneration ability of apoEVs in numerous tissue regeneration processes. Here, we summarize and discuss the promotion of repair and regeneration of the skin, bone, heart, and kidneys by apoEVs (Figure 5).
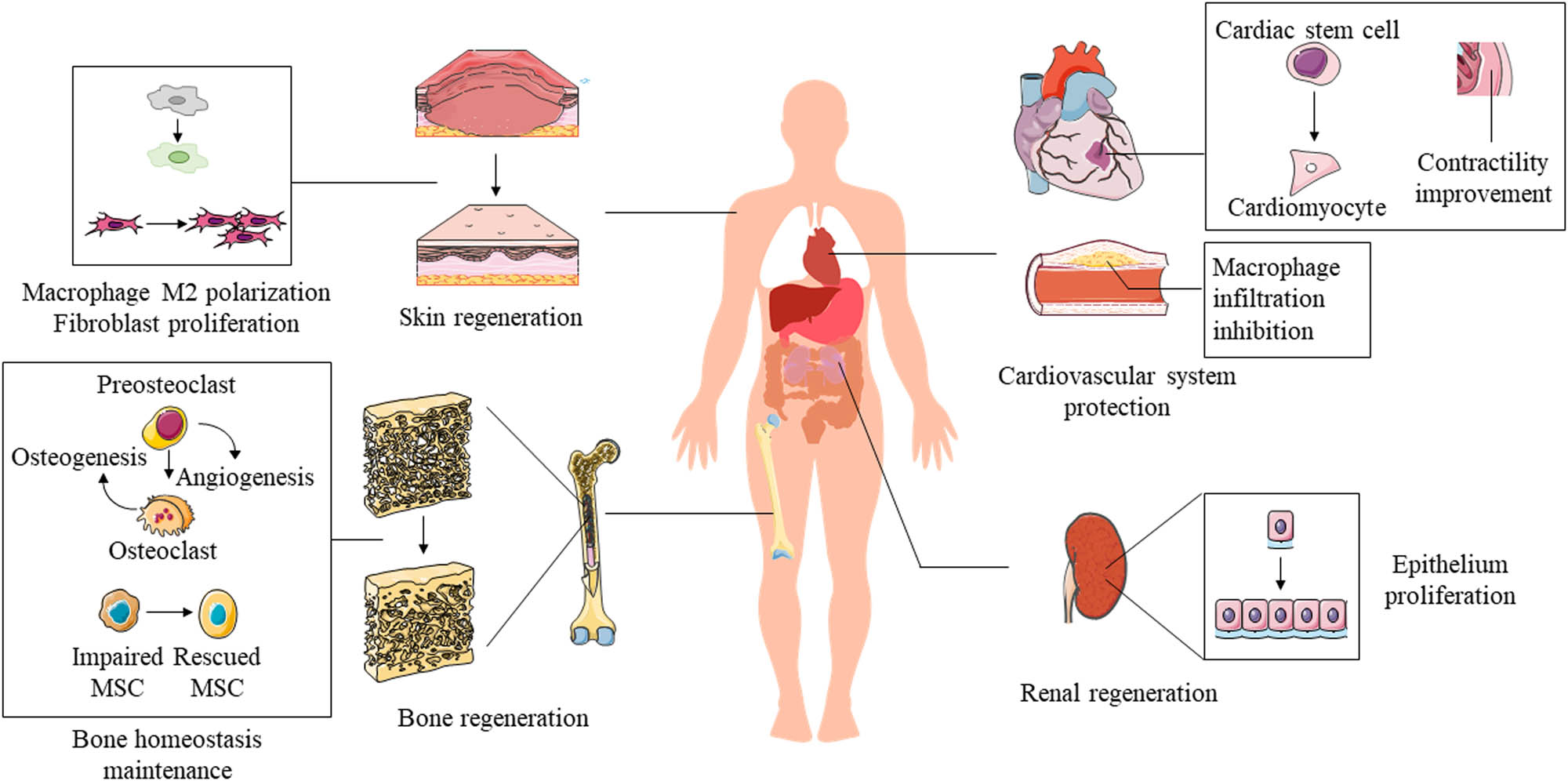
Therapeutic effects of apoEVs in regeneration of the skin, bone, cardiovascular system, and kidneys.
5.1 ApoEVs in skin regeneration
ApoEVs produced by MSCs facilitated wound healing as MSCs [18,85,92]. ApoEVs induced M2 macrophage polarization, the anti-inflammatory subtype of macrophages, and sequentially promoted the viability of fibroblasts, accelerating cutaneous wound repair [18] (Figure 5). Furthermore, apoEV mimics carrying miR-21/curcumin boosted M2 polarization [23], indicating that the membrane of apoEVs and these therapeutic factors cooperated in skin regeneration. Taking advantage of the macrophage targeting property, apoEVs delivering antibiotic vancomycin relieved intracellular methicillin-resistant Staphylococcus aureus infection of macrophages [24]. Remaining dormant in macrophages, intracellular Staphylococcus aureus escape from the immune system and antibiotics, causing persistent cutaneous infection, which results in impaired wound healing and skin regeneration [93]. Moreover, after the apoptotic induction of TNF-α, apoEVs enveloping more IL-1RA were released by MSCs, promoting gingival and skin regeneration through the Fas/Fap-1/Cav-1 axis [92]. Reduced hypertrophic scar formation in the process of skin regeneration was also observed after treatment with TSG-6 secreted by apoptotic human MSCs [85]. TSG-6, a protective factor in the inflammatory response, has been reported to be one of the dominant mediators in the regenerative ability of MSCs [94].
5.2 ApoEVs in bone regeneration
ApoEVs produced by osteoclasts also manifested similar biological effects as parental cells [19,20]. Preosteoclast-derived apoEVs promoted angiogenesis, while mature osteoclast-apoEVs promoted osteogenesis, similar to perspective parental cells both in bioinformatics analysis and in vivo [19]. Mechanistically, mature osteoclast-derived apoEVs induced the differentiation of osteoblasts that mediate osteogenesis by activating receptor activators of NF-κB ligand reverse signaling [20,95], coupling bone resorption to formation (Figure 5). Notably, MSCs released apoEVs rescued the osteopenia phenotype in both apoptosis-deficient mice and ovariectomized mice by restoring stem cell properties of impaired MSCs (Figure 5). The uptake in the circulation of ubiquitin ligase RNF146 and miR-328-3p carried by apoEVs activated the Wnt/β-catenin pathway [44], a conservative signaling pathway crucial in the development and tissue regeneration [96,97]. These results demonstrate the indispensable role of apoEVs in regulating bone remodeling and maintaining bone homeostasis.
5.3 ApoEVs in the cardiovascular system
ApoEVs derived from different donor cells provide varied biological benefits for cardiovascular system protection and regeneration. Endothelial apoEVs deliver miR-126 ameliorated atherosclerosis and plaque in vivo by reducing macrophage infiltration and apoptotic cells. Endothelial apoEVs upregulated the expression of chemokine CXCL12 and mobilized Sca-1+ progenitor cells, thus inhibiting CXCR4-dependent atheroprogression [21]. Similarly, an apoEV biomimetic liposome enhanced the stability of atherosclerotic plaques, which means a lower possibility of plaque rupture and subsequent thrombosis by improving macrophage-induced inflammation to protect the cardiovascular system [88] (Figure 5). In another aspect, MSC-derived apoEVs enhanced angiogenesis and cardiac function recovery in myocardial infarction mice, which was due to recipient endothelial cells activating autophagy [15]. Moreover, apoEVs derived from cardiomyocytes regulated the differentiation of cardiac stem cells to cardiomyocyte precursors and improved the contractility of the myocardium [90,91], while apoEVs released by fibroblast produced no such effect [90] (Figure 5).
5.4 ApoEVs in renal regeneration
In renal regeneration, a novel type of apoEVs containing CrkI has been described, inducing mitosis and proliferation of nearly 100% analyzed recipient cells including renal parietal epithelial cells [78]. Podocyte-derived apoEVs containing CrkI might promote regeneration of injured tubular epithelial cells [22] (Figure 5). Surprisingly, apoEVs released by the first apoptosis in proximal tubular cells (PTCs) caused by cisplatin mediated the secondary apoptosis of recipient PTCs, while apoEVs produced by this secondary apoptosis stimulated tubular cell proliferation [67]. These controversial effects of apoEVs derived from different apoptosis processes might be explained by the propagation of toxic effects of cisplatin or apoptotic stimulus of Fas ligand that was probably expressed on apoEVs [98]. Nevertheless, specific comparison experiments concerning apoEVs induced by cisplatin and other stimuli, and the interaction and homeostasis between apoptosis and regeneration of PTCs need to be addressed further. In other words, apoEVs from diverse origins may have distinct therapeutic effects or play different roles in different biological processes.
6 Engineered apoEVs
EVs have been reported to possess tropism targeting a specific tissue or cell [99,100,101,102,103], known as the “homing effect” [101,102,103]. Moreover, EVs show natural advantages as drug delivery systems over synthetic nanoparticles, with the ability to cross biological barriers efficiently and interact with the plasma membrane via ligand/receptor responses, while possessing low immunogenicity and toxicity [100,104,105,106,107]. These intrinsic properties of apoEVs qualify them as optimal bioactive materials to be modified for tissue regeneration, causing more precise tissue targeting, enhanced inflammation modulation, and longer drug retention. Thus, apart from the direct utilization of natural apoEVs, modified apoEVs or biomimetic apoEVs are also a concern.
The unique identifying signal of apoEVs for phagocytes has piqued the interest of researchers in harnessing their targeting ability. ApoEV biomimicking liposomes were constructed by connecting PtdSer onto the surface of liposomes [88], inspired by the “eat-me” signal from the exposed PtdSer of apoEVs. The PtdSer-modified liposomes effectively escaped from clearance by the reticuloendothelial system owing to PEGylation [88,108]. Compared with liposomes, the modified liposomes that targeted M1 macrophages were engulfed more effectively as expected. As a sign of suppressed inflammation, inhibited M1 polarization and motivated M2 polarization were observed from increased anti-inflammatory cytokines such as IL-4 and IL-10 and decreased proinflammatory cytokines such as IL-1β, IL-6, and TNF-α [88] (Table 2). In addition, apoEVs derived from some kinds of cells might inherit the particular targeting property of their parental cells [109], such as leukocytes. Taking this into consideration, apoEVs released by T cells were manipulated to target inflammatory sites and then macrophages [23]. In this research, natural membranes of T cell-derived apoEVs without cargos were obtained and then encapsulated mesoporous silica nanoparticles (MSNs) that were preloaded with anti-inflammatory molecules. These chimeric apoEVs showed comparable CD44 and Mac-1 protein expression to T cells, which were responsible for recognition between inflamed endothelium and leukocytes [110,111], thus displaying inflammation targeting capacity both in vitro and in colitis models (Table 2). Promoted macrophage-specific phagocytose and M2 polarization was observed as well [23]. Similarly, cancer cell-derived apoEVs were reconstructed for targeting the same type of cancer cells as well as targeting macrophages [24].
Modification of engineered apoEVs in recent years
| Methods of modification | Forms of modification | Purposes of modification | Functional parts | Results of modification |
|---|---|---|---|---|
| Connecting PtdSer onto the surface of liposomes | Liposomes with “eat-me” signal of apoEVs | Targeting property for macrophages | PtdSer on the surface | M2 macropahge polarization; inflammation amelioration |
| Co-bathing in sonication of the membrane of apoEVs and MSNs | Chimeric T-cell apoEVs with MSNs loading anti-inflammatory molecules | Targeting property for inflammatory sites | CD44 and Mac-1 | Inflammation amelioration |
| Freezing and thawing apoEVs with vancomycin | Reconstructed apoEVs carrying vancomycin | Delivering drugs into cells | Lipid membrane of apoEVs; vancomycin | Antibacterial effect inside macrophages |
| Transient transfection of miRNA/siRNA into donor cells | apoEVs containing miRNA transfected | Loading and delivering miRNA into apoEVs | Lipid membrane of apoEVs; therapeutic miRNAs | Proved possibility of loading miRNA into apoEVs |
| Embedding apoEVs into PF-127 hydrogel | Combination of apoVEs and bioactive scaffolds | Longer drug retention | Sustained release of apoEVs in PF-127 hydrogel | Improved skin regeneration |
PtdSer: phosphatidylserine; MSN: mesoporous silica nanoparticles; miRNA: micro-RNA; siRNA: silencing RNA.
Combined with their targeting property dictated by their membrane markers and cargo content [105], the natural lipid membrane potentialized apoEVs as drug delivers. Co-bathing in sonication is one of the common strategies to load therapeutic molecules into apoEVs [23] (Table 2). To boost the efficiency and stability of drug loading, therapeutic molecules could be preloaded into MSNs, an outstanding nanoplatform as a drug carrier [112,113], by electrostatic interactions [23] or undergoing a freeze–thaw process bathing with apoEVs [24]. By a mechanically extruding technique, apoEVs were processed into a relatively uniform size of 80–150 nm and thereby obtained an enhanced encapsulation efficiency of vancomycin [24] (Table 2). Otherwise, genetic manipulation can also be considered. Transient transfection into donor cells of therapeutic miRNA could also be transferred into apoEVs successfully [68], providing another cargo loading approach (Table 2).
Another drug delivery strategy is loading apoEVs into materials or scaffolds with biocompatibility, not concerning the targeting property or membrane superiority, but aiming to achieve longer drug retention and controlled release. When embedded into PF-127 hydrogel, apoEVs showed the fastest skin regeneration [18] (Table 2). Although a concentration–time curve is lacking in this research, a compelling retention time and promoted regeneration ability of other EVs combined with biomaterials have been provided [114,115,116]. In general, the combination of apoEVs and biomaterials is an attractive method with the potential for sustainable therapy.
7 Challenges, prospects, and conclusions
ApoEVs, the products, and meanwhile the information disseminators, seem to inherit the therapeutic role of apoptosis in tissue regeneration, changing death to another beginning of life. Different biogenesis pathways of apoEVs seem to be linked to cell types. To obtain information on the reason why different biogenesis patterns of apoEVs occur, investigating the functions of cells undergoing diverse apoptotic ways and respective apoEVs might be a possible direction. Moreover, morphological diversities in different biogenesis patterns of apoEVs might contribute to the clearance of apoptotic cells. For example, suppressed blebbing in Jurkat T cells caused inhibited uptake by macrophages in cell disassembly [117]. The interaction between microtubule spikes of A431 epithelial cells and phagocytes was reduced when the formation of microtubule spikes was inhibited [50]. Although a conclusive explanation for such diverse patterns still needs to be clarified, diversity among the contents of apoEVs originating from different sources exists. For example, apoEVs undergoing “beads-on-string” apoptosis contain no nuclear material, unlike other groups of apoEVs [52]. In addition, research has seldom compared the underlying discrepancies in different production protocols or differences among subtypes of apoEVs. In limited literature that analyzed the content diversity of apoEVs, different attractive abilities for neutrophils were demonstrated in various groups of apoEVs. The group containing inflammatory chemokines caused secondary inflammation, which we will not anticipate to occur in tissue regeneration. This means that it is not that more apoEVs there are, the better the regenerative effect, but the appropriate quantity and prompt processing of phagocytes, along with the optimal group of apoEVs, or the specific contents of apoEVs; in another word, matter more. Figuring out these puzzles is fundamental to probe into the particular portion of apoEVs functioning directly or mediately as key therapeutics in different diseases, thus further formulating instructive protocols to study or manufacture in the future, aiming at specific pathological situations.
In conclusion, apoptosis, the silent closure of one cell, and the initiation of other cells play pivotal roles in tissue integrity maintenance. ApoEVs may promote tissue regeneration, including the tissue of skin, bone, cardiovascular system, and kidneys, by activating targeted recipient cells or transferring biomolecules (DNA, RNA, proteins) to facilitate proliferation and regulate inflammation, both locally and remotely. Nevertheless, the reason why apoEVs could be generated in different patterns in vivo, and the connection between different biogenesis patterns and the properties of the respective apoEVs remain unclear. Controlling the distribution and release of biomolecules or drugs carried by apoEVs is also important for therapeutic usage, for which manipulation by engineered means and combination with biomaterials can be optional. There is still a long way to utilize the regenerative and therapeutic functions of apoEVs, and many comprehensive research studies need to be finished.
-
Funding information: This work was partially supported by the National Natural Science Foundation of China (31971251), National Key Research and Development Program of China (No. 2018YFC1106800), China National Key R&D Program during the 13th Five-year Plan Period, West China Hospital Sichuan University (No. ZYGD21001), Sichuan Province Science & Technology Department Projects (No. 2020YFS0082), Scientific Research Projects of Sichuan Health Commission (No. 19PJ097), and The “111” Project (No. B16033).
-
Author contributions: Yixi Wang and Haider Mohammed Khan contributed equally to this work. Conceptualization: Z.Z., Z.L., and C.Z. methodology: Y.W. and H.M.K. validation: Y.W., Z.Z., and Z.L. investigation: Y.W., X.L., P.T., P.S., and X.G. resources: Y.C. and S.L. data curation: H.L. original draft preparation: Y.W. review and editing: H.M.K. and C.Z. visualization: X.L., Z.C., and H.L. supervision: C.Z. project administration: Z.Z. Funding acquisition: Z.Z, Z.L., and C.Z. ALl authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164(6):1226–32.10.1016/j.cell.2016.01.043Suche in Google Scholar PubMed
[2] Cheng AN, Cheng LC, Kuo CL, Lo YK, Chou HY, Chen CH, et al. Mitochondrial Lon-induced mtDNA leakage contributes to PD-L1-mediated immunoescape via STING-IFN signaling and extracellular vesicles. J Immunother Cancer. 2020;8(2):e001372.10.1136/jitc-2020-001372Suche in Google Scholar PubMed PubMed Central
[3] Fu Y, Sui B, Xiang L, Yan X, Wu D, Shi S, et al. Emerging understanding of apoptosis in mediating mesenchymal stem cell therapy. Cell Death Dis. 2021;12(6):596.10.1038/s41419-021-03883-6Suche in Google Scholar PubMed PubMed Central
[4] Karpman D, Ståhl AL, Arvidsson I. Extracellular vesicles in renal disease. Nat Rev Nephrol. 2017;13(9):545–62.10.1038/nrneph.2017.98Suche in Google Scholar PubMed
[5] Poon IKH, Parkes MAF, Jiang L, Atkin-Smith GK, Tixeira R, Gregory CD, et al. Moving beyond size and phosphatidylserine exposure: evidence for a diversity of apoptotic cell-derived extracellular vesicles in vitro. J Extracell Vesicles. 2019;8(1):1608786.10.1080/20013078.2019.1608786Suche in Google Scholar PubMed PubMed Central
[6] Pavlyukov MS, Yu H, Bastola S, Minata M, Shender VO, Lee Y, et al. Apoptotic cell-derived extracellular vesicles promote malignancy of glioblastoma via intercellular transfer of splicing factors. Cancer Cell. 2018;34(1):119–35.e10.10.1016/j.ccell.2018.05.012Suche in Google Scholar PubMed PubMed Central
[7] Gao Y, Zhang H, Zhou N, Xu P, Wang J, Gao Y, et al. Methotrexate-loaded tumour-cell-derived microvesicles can relieve biliary obstruction in patients with extrahepatic cholangiocarcinoma. Nat Biomed Eng. 2020;4(7):743–53.10.1038/s41551-020-0583-0Suche in Google Scholar PubMed
[8] Atkin-Smith GK, Poon IKH. Disassembly of the dying: mechanisms and functions. Trends Cell Biol. 2017;27(2):151–62.10.1016/j.tcb.2016.08.011Suche in Google Scholar PubMed
[9] Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9(3):231–41.10.1038/nrm2312Suche in Google Scholar PubMed
[10] King KL, Cidlowski JA. Cell cycle regulation and apoptosis. Annu Rev Physiol. 1998;60:601–17.10.1146/annurev.physiol.60.1.601Suche in Google Scholar PubMed
[11] Diwanji N, Bergmann A. An unexpected friend - ROS in apoptosis-induced compensatory proliferation: Implications for regeneration and cancer. Semin Cell Dev Biol. 2018;80:74–82.10.1016/j.semcdb.2017.07.004Suche in Google Scholar PubMed PubMed Central
[12] Fogarty CE, Diwanji N, Lindblad JL, Tare M, Amcheslavsky A, Makhijani K, et al. Extracellular reactive oxygen species drive apoptosis-induced proliferation via drosophila macrophages. Curr Biol. 2016;26(5):575–84.10.1016/j.cub.2015.12.064Suche in Google Scholar PubMed PubMed Central
[13] Li F, Huang Q, Chen J, Peng Y, Roop DR, Bedford JS, et al. Apoptotic cells activate the “phoenix rising” pathway to promote wound healing and tissue regeneration. Sci Signal. 2010;3(110):ra13.10.1126/scisignal.2000634Suche in Google Scholar PubMed PubMed Central
[14] Samos J, García-Olmo DC, Picazo MG, Rubio-Vitaller A, García-Olmo D. Circulating nucleic acids in plasma/serum and tumor progression: are apoptotic bodies involved? An experimental study in a rat cancer model. Ann N Y Acad Sci. 2006;1075:165–73.10.1196/annals.1368.022Suche in Google Scholar PubMed
[15] Liu H, Liu S, Qiu X, Yang X, Bao L, Pu F, et al. Donor MSCs release apoptotic bodies to improve myocardial infarction via autophagy regulation in recipient cells. Autophagy. 2020;16(12):2140–55.10.1080/15548627.2020.1717128Suche in Google Scholar PubMed PubMed Central
[16] Xu X, Lai Y, Hua ZC. Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci Rep. 2019;39(1):BSR20180992.10.1042/BSR20180992Suche in Google Scholar PubMed PubMed Central
[17] Battistelli M, Falcieri E. Apoptotic bodies: particular extracellular vesicles involved in intercellular communication. Biology (Basel). 2020;9(1):21.10.1201/9781003180449-20Suche in Google Scholar
[18] Liu J, Qiu X, Lv Y, Zheng C, Dong Y, Dou G, et al. Apoptotic bodies derived from mesenchymal stem cells promote cutaneous wound healing via regulating the functions of macrophages. Stem Cell Res Ther. 2020;11(1):507.10.1186/s13287-020-02014-wSuche in Google Scholar PubMed PubMed Central
[19] Ma Q, Liang M, Limjunyawong N, Dan Y, Xing J, Li J, et al. Osteoclast-derived apoptotic bodies show extended biological effects of parental cell in promoting bone defect healing. Theranostics. 2020;10(15):6825–38.10.7150/thno.45170Suche in Google Scholar PubMed PubMed Central
[20] Ma Q, Liang M, Wu Y, Ding N, Duan L, Yu T, et al. Mature osteoclast-derived apoptotic bodies promote osteogenic differentiation via RANKL-mediated reverse signaling. J Biol Chem. 2019;294(29):11240–7.10.1074/jbc.RA119.007625Suche in Google Scholar PubMed PubMed Central
[21] Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2(100):ra81.10.1126/scisignal.2000610Suche in Google Scholar PubMed
[22] Bussolati B, Camussi G. Renal injury: early apoptotic extracellular vesicles in injury and repair. Nat Rev Nephrol. 2017;13(9):523–4.10.1038/nrneph.2017.117Suche in Google Scholar PubMed
[23] Dou G, Tian R, Liu X, Yuan P, Ye Q, Liu J, et al. Chimeric apoptotic bodies functionalized with natural membrane and modular delivery system for inflammation modulation. Sci Adv. 2020;6(30):eaba2987.10.1126/sciadv.aba2987Suche in Google Scholar PubMed PubMed Central
[24] Bose RJC, Tharmalingam N, Garcia Marques FJ, Sukumar UK, Natarajan A, Zeng Y, et al. Reconstructed apoptotic bodies as targeted “nano decoys” to treat intracellular bacterial infections within macrophages and cancer cells. ACS Nano. 2020;14(5):5818–35.10.1021/acsnano.0c00921Suche in Google Scholar PubMed PubMed Central
[25] Caruso S, Poon IKH. Apoptotic cell-derived extracellular vesicles: more than just debris. Front Immunol. 2018;9:1486.10.3389/fimmu.2018.01486Suche in Google Scholar PubMed PubMed Central
[26] Arienti S, Barth ND, Dorward DA, Rossi AG, Dransfield I. Regulation of apoptotic cell clearance during resolution of inflammation. Front Pharmacol. 2019;10:891.10.3389/fphar.2019.00891Suche in Google Scholar PubMed PubMed Central
[27] Grant LR, Milic I, Devitt A. Apoptotic cell-derived extracellular vesicles: structure-function relationships. Biochem Soc Trans. 2019;47(2):509–16.10.1042/BST20180080Suche in Google Scholar PubMed
[28] Muhsin-Sharafaldine MR, McLellan AD. Tumor-derived apoptotic vesicles: with death they do part. Front Immunol. 2018;9:957.10.3389/fimmu.2018.00957Suche in Google Scholar PubMed PubMed Central
[29] Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–57.10.1038/bjc.1972.33Suche in Google Scholar PubMed PubMed Central
[30] Fuchs Y, Steller H. Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat Rev Mol Cell Biol. 2015;16(6):329–44.10.1038/nrm3999Suche in Google Scholar PubMed PubMed Central
[31] Li M, Liao L, Tian W. Extracellular vesicles derived from apoptotic cells: an essential link between death and regeneration. Front Cell Dev Biol. 2020;8:573511.10.3389/fcell.2020.573511Suche in Google Scholar PubMed PubMed Central
[32] Zhang Y, Chen X, Gueydan C, Han J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018;28(1):9–21.10.1038/cr.2017.133Suche in Google Scholar PubMed PubMed Central
[33] Hounsell C, Fan Y. The duality of caspases in cancer, as told through the fly. Int J Mol Sci. 2021;22(16):8927.10.3390/ijms22168927Suche in Google Scholar PubMed PubMed Central
[34] Shalini S, Dorstyn L, Dawar S, Kumar S. Old, new and emerging functions of caspases. Cell Death Differ. 2015;22(4):526–39.10.1038/cdd.2014.216Suche in Google Scholar PubMed PubMed Central
[35] Fogarty CE, Bergmann A. Killers creating new life: caspases drive apoptosis-induced proliferation in tissue repair and disease. Cell Death Differ. 2017;24(8):1390–400.10.1038/cdd.2017.47Suche in Google Scholar PubMed PubMed Central
[36] Kondo S, Senoo-Matsuda N, Hiromi Y, Miura M. DRONC coordinates cell death and compensatory proliferation. Mol Cell Biol. 2006;26(19):7258–68.10.1128/MCB.00183-06Suche in Google Scholar PubMed PubMed Central
[37] Wells BS, Yoshida E, Johnston LA. Compensatory proliferation in Drosophila imaginal discs requires Dronc-dependent p53 activity. Curr Biol. 2006;16(16):1606–15.10.1016/j.cub.2006.07.046Suche in Google Scholar PubMed PubMed Central
[38] Fan Y, Bergmann A. Distinct mechanisms of apoptosis-induced compensatory proliferation in proliferating and differentiating tissues in the Drosophila eye. Dev Cell. 2008;14(3):399–410.10.1016/j.devcel.2008.01.003Suche in Google Scholar PubMed PubMed Central
[39] Love NR, Chen Y, Ishibashi S, Kritsiligkou P, Lea R, Koh Y, et al. Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nat Cell Biol. 2013;15(2):222–8.10.1038/ncb2659Suche in Google Scholar PubMed PubMed Central
[40] Chera S, Ghila L, Dobretz K, Wenger Y, Bauer C, Buzgariu W, et al. Apoptotic cells provide an unexpected source of Wnt3 signaling to drive hydra head regeneration. Dev Cell. 2009;17(2):279–89.10.1016/j.devcel.2009.07.014Suche in Google Scholar PubMed
[41] Yosefzon Y, Soteriou D, Feldman A, Kostic L, Koren E, Brown S, et al. Caspase-3 regulates YAP-dependent cell proliferation and organ size. Mol Cell. 2018;70(4):573–87.e4.10.1016/j.molcel.2018.04.019Suche in Google Scholar PubMed
[42] Fujita J, Crane AM, Souza MK, Dejosez M, Kyba M, Flavell RA, et al. Caspase activity mediates the differentiation of embryonic stem cells. Cell Stem Cell. 2008;2(6):595–601.10.1016/j.stem.2008.04.001Suche in Google Scholar PubMed PubMed Central
[43] Miura M, Chen XD, Allen MR, Bi Y, Gronthos S, Seo BM, et al. A crucial role of caspase-3 in osteogenic differentiation of bone marrow stromal stem cells. J Clin Invest. 2004;114(12):1704–13.10.1172/JCI20427Suche in Google Scholar PubMed PubMed Central
[44] Liu D, Kou X, Chen C, Liu S, Liu Y, Yu W, et al. Circulating apoptotic bodies maintain mesenchymal stem cell homeostasis and ameliorate osteopenia via transferring multiple cellular factors. Cell Res. 2018;28(9):918–33.10.1038/s41422-018-0070-2Suche in Google Scholar PubMed PubMed Central
[45] Dieudé M, Bell C, Turgeon J, Beillevaire D, Pomerleau L, Yang B, et al. The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection. Sci Transl Med. 2015;7(318):318ra200.10.1126/scitranslmed.aac9816Suche in Google Scholar PubMed
[46] Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750.10.1080/20013078.2018.1535750Suche in Google Scholar PubMed PubMed Central
[47] Baxter AA. Stoking the fire: how dying cells propagate inflammatory signalling through extracellular vesicle trafficking. Int J Mol Sci. 2020;21(19):7256.10.3390/ijms21197256Suche in Google Scholar PubMed PubMed Central
[48] Zirngibl M, Fürnrohr BG, Janko C, Munoz LE, Voll RE, Gregory CD, et al. Loading of nuclear autoantigens prototypically recognized by systemic lupus erythematosus sera into late apoptotic vesicles requires intact microtubules and myosin light chain kinase activity. Clin Exp Immunol. 2015;179(1):39–49.10.1111/cei.12342Suche in Google Scholar PubMed PubMed Central
[49] Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, et al. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393(6687):805–9.10.1038/31729Suche in Google Scholar PubMed
[50] Moss DK, Betin VM, Malesinski SD, Lane JD. A novel role for microtubules in apoptotic chromatin dynamics and cellular fragmentation. J Cell Sci. 2006;119(Pt 11):2362–74.10.1242/jcs.02959Suche in Google Scholar PubMed PubMed Central
[51] Poon IK, Chiu YH, Armstrong AJ, Kinchen JM, Juncadella IJ, Bayliss DA, et al. Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature. 2014;507(7492):329–34.10.1038/nature13147Suche in Google Scholar PubMed PubMed Central
[52] Atkin-Smith GK, Tixeira R, Paone S, Mathivanan S, Collins C, Liem M, et al. A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat Commun. 2015;6:7439.10.1038/ncomms8439Suche in Google Scholar PubMed PubMed Central
[53] Ando H, Yoshimoto S, Yoshida M, Shimoda N, Tadokoro R, Kohda H, et al. Dermal fibroblasts internalize phosphatidylserine-exposed secretory melanosome clusters and apoptotic melanocytes. Int J Mol Sci. 2020;21(16):5789.10.3390/ijms21165789Suche in Google Scholar PubMed PubMed Central
[54] Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467(7317):863–7.10.1038/nature09413Suche in Google Scholar PubMed PubMed Central
[55] Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol. 2013;5(1):a008748.10.1101/cshperspect.a008748Suche in Google Scholar PubMed PubMed Central
[56] Truman LA, Ford CA, Pasikowska M, Pound JD, Wilkinson SJ, Dumitriu IE, et al. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 2008;112(13):5026–36.10.1182/blood-2008-06-162404Suche in Google Scholar PubMed
[57] Lemke G. How macrophages deal with death. Nat Rev Immunol. 2019;19(9):539–49.10.1038/s41577-019-0167-ySuche in Google Scholar PubMed PubMed Central
[58] Torr EE, Gardner DH, Thomas L, Goodall DM, Bielemeier A, Willetts R, et al. Apoptotic cell-derived ICAM-3 promotes both macrophage chemoattraction to and tethering of apoptotic cells. Cell Death Differ. 2012;19(4):671–9.10.1038/cdd.2011.167Suche in Google Scholar PubMed PubMed Central
[59] Eguchi A, Mulya A, Lazic M, Radhakrishnan D, Berk MP, Povero D, et al. Microparticles release by adipocytes act as “find-me” signals to promote macrophage migration. PLoS One. 2015;10(4):e0123110.10.1371/journal.pone.0123110Suche in Google Scholar PubMed PubMed Central
[60] Berda-Haddad Y, Robert S, Salers P, Zekraoui L, Farnarier C, Dinarello CA, et al. Sterile inflammation of endothelial cell-derived apoptotic bodies is mediated by interleukin-1α. Proc Natl Acad Sci U S A. 2011;108(51):20684–9.10.1073/pnas.1116848108Suche in Google Scholar PubMed PubMed Central
[61] Holmgren L, Szeles A, Rajnavölgyi E, Folkman J, Klein G, Ernberg I, et al. Horizontal transfer of DNA by the uptake of apoptotic bodies. Blood. 1999;93(11):3956–63.10.1182/blood.V93.11.3956.411k05_3956_3963Suche in Google Scholar
[62] Halicka HD, Bedner E, Darzynkiewicz Z. Segregation of RNA and separate packaging of DNA and RNA in apoptotic bodies during apoptosis. Exp Cell Res. 2000;260(2):248–56.10.1006/excr.2000.5027Suche in Google Scholar PubMed
[63] Zhu Z, Zhang D, Lee H, Menon AA, Wu J, Hu K, et al. Macrophage-derived apoptotic bodies promote the proliferation of the recipient cells via shuttling microRNA-221/222. J Leukoc Biol. 2017;101(6):1349–59.10.1189/jlb.3A1116-483RSuche in Google Scholar PubMed PubMed Central
[64] Hardy MP, Audemard É, Migneault F, Feghaly A, Brochu S, Gendron P, et al. Apoptotic endothelial cells release small extracellular vesicles loaded with immunostimulatory viral-like RNAs. Sci Rep. 2019;9(1):7203.10.1038/s41598-019-43591-ySuche in Google Scholar PubMed PubMed Central
[65] Lleo A, Zhang W, McDonald WH, Seeley EH, Leung PS, Coppel RL, et al. Shotgun proteomics: identification of unique protein profiles of apoptotic bodies from biliary epithelial cells. Hepatology. 2014;60(4):1314–23.10.1002/hep.27230Suche in Google Scholar PubMed PubMed Central
[66] Turiák L, Misják P, Szabó TG, Aradi B, Pálóczi K, Ozohanics O, et al. Proteomic characterization of thymocyte-derived microvesicles and apoptotic bodies in BALB/c mice. J Proteomics. 2011;74(10):2025–33.10.1016/j.jprot.2011.05.023Suche in Google Scholar PubMed
[67] García-Pastor C, Blázquez-Serra R, Bosch RJ, Lucio Cazaña FJ, Fernández-Martínez AB. Apoptosis and cell proliferation in proximal tubular cells exposed to apoptotic bodies. Novel pathophysiological implications in cisplatin-induced renal injury. Biochim Biophys Acta Mol Basis Dis. 2019;1865(9):2504–15.10.1016/j.bbadis.2019.06.008Suche in Google Scholar PubMed
[68] Zheng C, Sui B, Zhang X, Hu J, Chen J, Liu J, et al. Apoptotic vesicles restore liver macrophage homeostasis to counteract type 2 diabetes. J Extracell Vesicles. 2021;10(7):e12109.10.1002/jev2.12109Suche in Google Scholar PubMed PubMed Central
[69] Schwarz N, Tumpara S, Wrenger S, Ercetin E, Hamacher J, Welte T, et al. Alpha1-antitrypsin protects lung cancer cells from staurosporine-induced apoptosis: the role of bacterial lipopolysaccharide. Sci Rep. 2020;10(1):9563.10.1038/s41598-020-66825-wSuche in Google Scholar PubMed PubMed Central
[70] Ding Y, Wang B, Chen X, Zhou Y, Ge J. Staurosporine suppresses survival of HepG2 cancer cells through Omi/HtrA2-mediated inhibition of PI3K/Akt signaling pathway. Tumour Biol. 2017;39(3):1010428317694317.10.1177/1010428317694317Suche in Google Scholar PubMed
[71] Huang Y, Fu Z, Dong W, Zhang Z, Mu J, Zhang J. Serum starvation-induces down-regulation of Bcl-2/Bax confers apoptosis in tongue coating-related cells in vitro. Mol Med Rep. 2018;17(4):5057–64.10.3892/mmr.2018.8512Suche in Google Scholar PubMed PubMed Central
[72] Shen G, Krienke S, Schiller P, Nießen A, Neu S, Eckstein V, et al. Microvesicles released by apoptotic human neutrophils suppress proliferation and IL-2/IL-2 receptor expression of resting T helper cells. Eur J Immunol. 2017;47(5):900–10.10.1002/eji.201546203Suche in Google Scholar PubMed
[73] Chen H, Kasagi S, Chia C, Zhang D, Tu E, Wu R, et al. Extracellular vesicles from apoptotic cells promote TGFβ production in macrophages and suppress experimental colitis. Sci Rep. 2019;9(1):5875.10.1038/s41598-019-42063-7Suche in Google Scholar PubMed PubMed Central
[74] Ding N, Liu C, Yao L, Bai Y, Cheng P, Li Z, et al. Alendronate induces osteoclast precursor apoptosis via peroxisomal dysfunction mediated ER stress. J Cell Physiol. 2018;233(9):7415–23.10.1002/jcp.26587Suche in Google Scholar PubMed
[75] Brock CK, Wallin ST, Ruiz OE, Samms KM, Mandal A, Sumner EA, et al. Stem cell proliferation is induced by apoptotic bodies from dying cells during epithelial tissue maintenance. Nat Commun. 2019;10(1):1044.10.1038/s41467-019-09010-6Suche in Google Scholar PubMed PubMed Central
[76] Eisenhoffer GT, Loftus PD, Yoshigi M, Otsuna H, Chien CB, Morcos PA, et al. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature. 2012;484(7395):546–9.10.1038/nature10999Suche in Google Scholar PubMed PubMed Central
[77] Fan Y, Bergmann A. Apoptosis-induced compensatory proliferation. The cell is dead. Long live the Cell! Trends Cell Biol. 2008;18(10):467–73.10.1016/j.tcb.2008.08.001Suche in Google Scholar PubMed PubMed Central
[78] Gupta KH, Goldufsky JW, Wood SJ, Tardi NJ, Moorthy GS, Gilbert DZ, et al. Apoptosis and compensatory proliferation signaling are coupled by CrkI-containing microvesicles. Dev Cell. 2017;41(6):674–84.e5.10.1016/j.devcel.2017.05.014Suche in Google Scholar PubMed PubMed Central
[79] Shafikhani SH, Mostov K, Engel J. Focal adhesion components are essential for mammalian cell cytokinesis. Cell Cycle. 2008;7(18):2868–76.10.4161/cc.7.18.6674Suche in Google Scholar PubMed PubMed Central
[80] Shafikhani SH, Engel J. Pseudomonas aeruginosa type III-secreted toxin ExoT inhibits host-cell division by targeting cytokinesis at multiple steps. Proc Natl Acad Sci U S A. 2006;103(42):15605–10.10.1073/pnas.0605949103Suche in Google Scholar PubMed PubMed Central
[81] Przekora A. A concise review on tissue engineered artificial skin grafts for chronic wound treatment: can we reconstruct functional skin tissue in vitro? Cells. 2020;9(7):1622.10.3390/cells9071622Suche in Google Scholar PubMed PubMed Central
[82] Howard EE, Pasiakos SM, Blesso CN, Fussell MA, Rodriguez NR. Divergent roles of inflammation in skeletal muscle recovery from injury. Front Physiol. 2020;11:87.10.3389/fphys.2020.00087Suche in Google Scholar PubMed PubMed Central
[83] Harrell CR, Markovic BS, Fellabaum C, Arsenijevic A, Volarevic V. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomed Pharmacother. 2019;109:2318–26.10.1016/j.biopha.2018.11.099Suche in Google Scholar PubMed
[84] Mountziaris PM, Spicer PP, Kasper FK, Mikos AG. Harnessing and modulating inflammation in strategies for bone regeneration. Tissue Eng Part B Rev. 2011;17(6):393–402.10.1089/ten.teb.2011.0182Suche in Google Scholar PubMed PubMed Central
[85] Liu S, Jiang L, Li H, Shi H, Luo H, Zhang Y, et al. Mesenchymal stem cells prevent hypertrophic scar formation via inflammatory regulation when undergoing apoptosis. J Invest Dermatol. 2014;134(10):2648–57.10.1038/jid.2014.169Suche in Google Scholar PubMed
[86] Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood. 2011;118(2):330–8.10.1182/blood-2010-12-327353Suche in Google Scholar PubMed PubMed Central
[87] Qi Y, Jiang D, Sindrilaru A, Stegemann A, Schatz S, Treiber N, et al. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin wounds. J Invest Dermatol. 2014;134(2):526–37.10.1038/jid.2013.328Suche in Google Scholar PubMed
[88] Wu Y, Zhang Y, Dai L, Wang Q, Xue L, Su Z, et al. An apoptotic body-biomimic liposome in situ upregulates anti-inflammatory macrophages for stabilization of atherosclerotic plaques. J Control Release. 2019;316:236–49.10.1016/j.jconrel.2019.10.043Suche in Google Scholar PubMed
[89] Chen HH, Lin KC, Wallace CG, Chen YT, Yang CC, Leu S, et al. Additional benefit of combined therapy with melatonin and apoptotic adipose-derived mesenchymal stem cell against sepsis-induced kidney injury. J Pineal Res. 2014;57(1):16–32.10.1111/jpi.12140Suche in Google Scholar PubMed
[90] Tyukavin AI, Belostotskaya GB, Zakharov E, Ivkin DY, Rad’ko SV, Knyazev NA, et al. Apoptotic bodies of cardiomyocytes and fibroblasts - regulators of directed differentiation of heart stem cells. Bull Exp Biol Med. 2020;170(1):112–7.10.1007/s10517-020-05015-0Suche in Google Scholar PubMed
[91] Tyukavin AI, Belostotskaya GB, Golovanova TA, Galagudza MM, Zakharov EA, Burkova NV, et al. Stimulation of proliferation and differentiation of rat resident myocardial cells with apoptotic bodies of cardiomyocytes. Bull Exp Biol Med. 2015;159(1):138–41.10.1007/s10517-015-2909-6Suche in Google Scholar PubMed
[92] Kou X, Xu X, Chen C, Sanmillan ML, Cai T, Zhou Y, et al. The Fas/Fap-1/Cav-1 complex regulates IL-1RA secretion in mesenchymal stem cells to accelerate wound healing. Sci Transl Med. 2018;10(432):eaai8524.10.1126/scitranslmed.aai8524Suche in Google Scholar PubMed PubMed Central
[93] Huitema L, Phillips T, Alexeev V, Tomic-Canic M, Pastar I, Igoucheva O. Intracellular escape strategies of Staphylococcus aureus in persistent cutaneous infections. Exp Dermatol. 2020;30(10):1428–39.10.1111/exd.14235Suche in Google Scholar PubMed PubMed Central
[94] Yang H, Feng R, Fu Q, Xu S, Hao X, Qiu Y, et al. Human induced pluripotent stem cell-derived mesenchymal stem cells promote healing via TNF-α-stimulated gene-6 in inflammatory bowel disease models. Cell Death Dis. 2019;10(10):718.10.1038/s41419-019-1957-7Suche in Google Scholar PubMed PubMed Central
[95] Ikebuchi Y, Aoki S, Honma M, Hayashi M, Sugamori Y, Khan M, et al. Coupling of bone resorption and formation by RANKL reverse signalling. Nature. 2018;561(7722):195–200.10.1038/s41586-018-0482-7Suche in Google Scholar PubMed
[96] Huang P, Yan R, Zhang X, Wang L, Ke X, Qu Y. Activating Wnt/β-catenin signaling pathway for disease therapy: Challenges and opportunities. Pharmacol Ther. 2019;196:79–90.10.1016/j.pharmthera.2018.11.008Suche in Google Scholar PubMed
[97] Schunk SJ, Floege J, Fliser D, Speer T. WNT-β-catenin signalling - a versatile player in kidney injury and repair. Nat Rev Nephrol. 2021;17(3):172–84.10.1038/s41581-020-00343-wSuche in Google Scholar PubMed
[98] Singh P, Goel H, Husain M, Lan X, Mikulak J, Malthotra A, et al. Tubular cell HIV-entry through apoptosed CD4 T cells: a novel pathway. Virology. 2012;434(1):68–77.10.1016/j.virol.2012.09.009Suche in Google Scholar PubMed PubMed Central
[99] Kooijmans SAA, Schiffelers RM, Zarovni N, Vago R. Modulation of tissue tropism and biological activity of exosomes and other extracellular vesicles: New nanotools for cancer treatment. Pharmacol Res. 2016;111:487–500.10.1016/j.phrs.2016.07.006Suche in Google Scholar PubMed
[100] Murphy DE, de Jong OG, Brouwer M, Wood MJ, Lavieu G, Schiffelers RM, et al. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019;51(3):1–12.10.1038/s12276-019-0223-5Suche in Google Scholar PubMed PubMed Central
[101] Tran PH, Xiang D, Nguyen TN, Tran TT, Chen Q, Yin W, et al. Aptamer-guided extracellular vesicle theranostics in oncology. Theranostics. 2020;10(9):3849–66.10.7150/thno.39706Suche in Google Scholar PubMed PubMed Central
[102] Park EJ, Prajuabjinda O, Soe ZY, Darkwah S, Appiah MG, Kawamoto E, et al. Exosomal regulation of lymphocyte homing to the gut. Blood Adv. 2019;3(1):1–11.10.1182/bloodadvances.2018024877Suche in Google Scholar PubMed PubMed Central
[103] Li K, Chen Y, Li A, Tan C, Liu X. Exosomes play roles in sequential processes of tumor metastasis. Int J Cancer. 2019;144(7):1486–95.10.1002/ijc.31774Suche in Google Scholar PubMed
[104] Tran PHL, Xiang D, Tran TTD, Yin W, Zhang Y, Kong L, et al. Exosomes and Nanoengineering: A Match Made for Precision Therapeutics. Adv Mater. 2020;32(18):e1904040.10.1002/adma.201904040Suche in Google Scholar PubMed
[105] Elsharkasy OM, Nordin JZ, Hagey DW, de Jong OG, Schiffelers RM, Andaloussi SE, et al. Extracellular vesicles as drug delivery systems: Why and how? Adv Drug Deliv Rev. 2020;159:332–43.10.1016/j.addr.2020.04.004Suche in Google Scholar PubMed
[106] Zangabad PS, Mirkiani S, Shahsavari S, Masoudi B, Masroor M, Hamed H, et al. Stimulus-responsive liposomes as smart nanoplatforms for drug delivery applications. Nanotechnol Rev. 2018;7(1):95–122.10.1515/ntrev-2017-0154Suche in Google Scholar PubMed PubMed Central
[107] Shannahan J. The biocorona: a challenge for the biomedical application of nanoparticles. Nanotechnol Rev. 2017;6(4):345–53.10.1515/ntrev-2016-0098Suche in Google Scholar PubMed PubMed Central
[108] Gajbhiye KR, Pawar A, Mahadik KR, Gajbhiye V. PEGylated nanocarriers: a promising tool for targeted delivery to the brain. Colloids Surf B Biointerfaces. 2020;187:110770.10.1016/j.colsurfb.2019.110770Suche in Google Scholar PubMed
[109] Wang Q, Ren Y, Mu J, Egilmez NK, Zhuang X, Deng Z, et al. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites. Cancer Res. 2015;75(12):2520–9.10.1158/0008-5472.CAN-14-3095Suche in Google Scholar PubMed PubMed Central
[110] Jin K, Luo Z, Zhang B, Pang Z. Biomimetic nanoparticles for inflammation targeting. Acta Pharm Sin B. 2018;8(1):23–33.10.1016/j.apsb.2017.12.002Suche in Google Scholar PubMed PubMed Central
[111] Johnson P, Ruffell B. CD44 and its role in inflammation and inflammatory diseases. Inflamm Allergy Drug Targets. 2009;8(3):208–20.10.2174/187152809788680994Suche in Google Scholar PubMed
[112] Li T, Shi S, Goel S, Shen X, Xie X, Chen Z, et al. Recent advancements in mesoporous silica nanoparticles towards therapeutic applications for cancer. Acta Biomater. 2019;89:1–13.10.1016/j.actbio.2019.02.031Suche in Google Scholar PubMed
[113] Castillo RR, Lozano D, González B, Manzano M, Izquierdo-Barba I, Vallet-Regí M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery: an update. Expert Opin Drug Deliv. 2019;16(4):415–39.10.1080/17425247.2019.1598375Suche in Google Scholar PubMed PubMed Central
[114] Huang CC, Kang M, Shirazi S, Lu Y, Cooper LF, Gajendrareddy P, et al. 3D encapsulation and tethering of functionally engineered extracellular vesicles to hydrogels. Acta Biomater. 2021;126:199–210.10.1016/j.actbio.2021.03.030Suche in Google Scholar PubMed PubMed Central
[115] Tao SC, Guo SC, Li M, Ke QF, Guo YP, Zhang CQ. Chitosan wound dressings incorporating exosomes derived from MicroRNA-126-overexpressing synovium mesenchymal stem cells provide sustained release of exosomes and heal full-thickness skin defects in a diabetic rat model. Stem Cells Transl Med. 2017;6(3):736–47.10.5966/sctm.2016-0275Suche in Google Scholar PubMed PubMed Central
[116] Cunnane EM, Lorentz KL, Ramaswamy AK, Gupta P, Mandal BB, O’Brien FJ, et al. Extracellular vesicles enhance the remodeling of cell-free silk vascular scaffolds in rat aortae. ACS Appl Mater Interfaces. 2020;12(24):26955–65.10.1021/acsami.0c06609Suche in Google Scholar PubMed
[117] Witasp E, Uthaisang W, Elenström-Magnusson C, Hanayama R, Tanaka M, Nagata S, et al. Bridge over troubled water: milk fat globule epidermal growth factor 8 promotes human monocyte-derived macrophage clearance of non-blebbing phosphatidylserine-positive target cells. Cell Death Differ. 2007;14(5):1063–5.10.1038/sj.cdd.4402096Suche in Google Scholar PubMed
[118] Hristov M, Erl W, Linder S, Weber PC. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood. 2004;104(9):2761–6.10.1182/blood-2003-10-3614Suche in Google Scholar PubMed
© 2022 Yixi Wang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption

