Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
-
Xiuren Ni
, Guoyun Zhou
Abstract
Cobalt is a promising material for electronic interconnections in the post-Moore law period. However, the vertical cobalt pillar is not fully compatible with the current electroplating-involved manufacturing process due to hydrogen evolution at the cathode and poor throwing power of the products. In this article, electrodeposition with multiple organic additives was employed to realize the fabrication of cobalt pillars. Electrochemical measurements were used to investigate the depolarization of 3-mercapto-1-propane sulfonate sulfonic acid (MPS) and the polarization of the polyvinylpyrrolidone (PVP) during cobalt electrodeposition. Notably, the competitive adsorption between MPS and PVP was verified and discussed in cobalt electrodeposition. In order to understand the adsorption and functional groups of the additives, quantum chemical calculations were performed to simulate the distribution of electrostatic potential and molecular orbital energy of the additives. Accordingly, the thiol group of MPS and the amide group of PVP were speculated to be the molecular adsorption sites in cobalt electrodeposition. The mechanism including three stages was proposed for cobalt pillar electrodeposition in solution with MPS and PVP. The electrodeposition of practical cobalt pillars with a depth of 50 µm and diameters of 60, 80, and 100 µm was successfully achieved by electroplating experiments, thereby promoting the application of metal cobalt for electronic packaging.
1 Introduction
With the booming development in the miniaturization of electronic products in the 5G era, the system-in-package technology and chip interconnection have reached the scale of a single micron in recent years [1,2]. However, copper as one of the major conductors can hardly fulfill all the needs of functions including low resistance and capacitance (RC) delay and low bulk resistivity for signal transmission embedded in chips because of the inherent long electron mean free path, high thermal expansion coefficient and low effective resistance at the contact interface of copper [3,4,5,6,7,8,9]. Cobalt as an alternative conductor is an excellent substitute for copper in particular electronic applications [10,11,12,13]. It has been widely attempted to use cobalt as a magnetic alloy material for miniaturized magnetic electronic components, memory devices, and other special apparatus [14]. As a good candidate of alternative metal in the post-Moore law period, cobalt exhibits several advantages such as the low electron mean free path and low thermal expansion coefficient [15,16]. In this way, it is reasonable to assume that the cobalt structures from the compatible electroplating process will promote the productivity and reliability of cobalt formation. Electronic interconnection in the vertical section can be realized by metal pillars in three-dimensional (3D) packaging. However, conventional pillar electrodeposition has not been widely implemented for cobalt yet because of the hydrogen evolution and poor electrodeposited throwing power [17,18]. In the cobalt pillar electrodeposition, the growth of cobalt along the wall of the hole is faster than that in the middle, leading to the overgrowth outside of the hole without enough cobalt deposition in the middle.
It is well-known that organic additives can effectively improve the quality of electroplating, especially in coating uniformity and filling completeness. Additive mixtures facilitate the super-filling of holes by adjusting the overpotential and the kinetics of plating in damascene copper electrodeposition [19]. The accelerator and various levelers were investigated in copper micro-via filling, and the convection-dependent adsorption of additives was proposed [20,21]. In cobalt electrodeposition, the cobalt nucleation and the morphology of nanograin were also affected by organic additives and electrolytes [22,23]. The group of additives for metal electrodeposition mainly contains accelerators and inhibitors. The selective adsorption (such as convection adsorption) of accelerators and inhibitors causes a higher deposition rate at the bottom of the hole than the rate outside the hole, leading to a complete hole filling [24]. The accelerator for metal electrodeposition, such as 3-mercapto-1-propane sulfonate sulfonic acid (MPS), is a crucial additive in the copper hole filling [25]. Previous studies indicated that MPS molecules adsorbed on the surface of the copper layer through the sulfhydryl group, while the sulfonic acid group captured copper ions in solution to accelerate the deposition of copper [26]. Cobalt performs close electron-donating and -accepting ability similar to copper so similar additives could be chosen during electrodeposition. However, current studies on the mechanism of MPS are still insufficient to explain cobalt pillar electrodeposition and the cobalt hole filling. On the other hand, inhibitors are usually necessary for metal electrodeposition. The inhibitor molecules can selectively adsorb on the surface of the metal layer or generate complex with metal ions to improve the quality of the metal coating and electrodeposition efficiency [27]. For instance, polyvinylpyrrolidone (PVP) undergoes a convection-dependent adsorption inhibition effect on cobalt electrodeposition [28]. The PVP molecule has great absorption at sites with strong convection (such as the upper part of the hole). In contrast, the sites with weak convection (such as the bottom of the hole) has relatively low PVP absorption, thereby making PVP a suitable additive in the hole filling for cobalt electrodeposition [28]. However, PVP brings an inherent suppressing effect on the overall deposition rate and the quality of the deposited cobalt layer, so the practical application of PVP is hindered in the manufacture of cobalt devices. In this article, competitive adsorption of MPS and PVP in cobalt electrodeposition was proposed first and cobalt pillar was successfully realized. Notably, the morphology of cobalt particles was modified and the uniformity of the cobalt layer was improved.
In this work, the polarization of PVP and depolarization of MPS in cobalt electrodeposition were investigated by the electrochemical method. The corresponding competitive adsorption of MPS and PVP was discussed during cobalt pillar electrodeposition. Quantum chemical calculations for simulation on the distribution of electrostatic potential and molecular orbital energy helps to explain the adsorption behavior of the additives. The mechanism of three stages was proposed for the electrodeposition of the cobalt pillar. Furthermore, cobalt pillar electrodeposition was successfully carried out in solution with MPS and PVP by galvanostatic electrodeposition.
2 Experimental study
2.1 Electrochemical measurements
The electrochemical actions of MPS and PVP were analyzed by cyclic voltammetry (CV) and the galvanostatic method (GM). Metrohm electrochemical workstation (PGSTT302N) was used with a 5 mm-diameter platinum rotating disk electrode (Pt-RDE), a Pt rod, and a saturated Hg/Hg2SO4 reference electrode (0.615 V vs S.H.E.). The CV tests were conducted in a cell with 200 mL of electrolyte containing 0.3 mol/L cobalt sulfate heptahydrate (CoSO4·7H2O), 0.57 mol/L boric acid (H3BO3), and 0.84 mmol/L cobalt chloride hexahydrate (CoCl2·6H2O), labeled as solution I. The solution with 0.3 mol/L CoSO4·7H2O, 0.57 mol/L H3BO3, 0.84 mmol/L CoCl2·6H2O, and 7 mg/L MPS was labeled as solution II. GM tests were performed in a cell containing 0.3 mol/L CoSO4·7H2O and 0.57 mol/L H3BO3.
2.2 Quantum chemical calculation
The quantum chemical calculation is a useful method in the simulation of interface interaction to analyze the highest occupied molecular orbital (HOMO), lowest unoccupied molecular orbital (LUMO), and molecule electrostatic potential (ESP) of PVP containing six monomers [29,30,31,32,33,34]. Gaussian 09 package was used to calculate the orbital energy and electrostatic potential [35,36]. The density functional theory (DFT) optimization was chosen in quantum chemical calculations. The settings for the Gaussian calculation include the B3LYP method in an aqueous solution, lanl2dz, and 6-311G+(d,p) settings [37,38,39,40,41]. The energy gap
2.3 Electrodeposition
The cobalt electrodeposition was conducted in a 500 mL bath with a direct current from the DC power supply (INTERLOCK IPD-3305SLU). Cobalt electrodeposition was performed in the solution containing 0.3 mol/L CoSO4·7H2O, 0.57 mol/L H3BO3, and 0.84 mmol/L CoCl2·6H2O. PVP (100 g/L) and 1 g/L MPS were prepared in advance for the injection in the electroplating solution before electrodeposition. RuO2-coated titanium was connected to the cathode of the power and 2 L/min airflow was used to improve the convection in the electroplating solution. Cobalt was electrodeposited on the copper layer on a printed circuit board (PCB) with photopolymer film after imaging transfer at a current density of 10 mA/cm2. The depth of the hole in the photopolymer film was 50 µm and three different diameters of the holes were 100, 80, and 60 µm, respectively. A scanning electron microscope (SEM, HITACHI S3400) was used to analyze the morphology, while a metallographic microscope (ASIDA-JX23RT, China) was used to observe the top view and cross-section of the cobalt pillar. The crystal phases of the coating were measured by an X-ray diffractometer (Rigaku MINIFLEX 600). The roughness tests of the coating were observed by a 3D measuring laser microscope (Olympus S4100) and the contact angle was measured by a contact angle tester (JY-PHa, China).
3 Results and discussion
3.1 Competitive adsorption of MPS and PVP
The effect of MPS on cobalt electrodeposition was investigated to observe the electrochemical behavior with electrochemical measurements. As shown in Figure 1a, the oxidation peak current density (from 1.72 to 2.08 A/dm2) and the cobalt oxidation area increases when 7 mg/L MPS is added to solution I. The change of the peak current density and oxidation area indicates the promotion of the cobalt electroplating process. In addition, the potential during cobalt deposition is shifted positively, revealing that MPS with a depolarization effect accelerates the electrodeposition of cobalt. With the addition of MPS, the stripping peak current density further increases to enhance the depolarization behavior during cobalt electrodeposition. The GM tests were also employed to verify the depolarization effect of MPS in cobalt electrodeposition (Figure 1b). As shown in Figure 1b, the potential shifted positively from −1.476 V to −1.362 V after the addition of 7 mg/L MPS. This potential pattern indicates that MPS has a significant acceleration effect on cobalt electroplating.
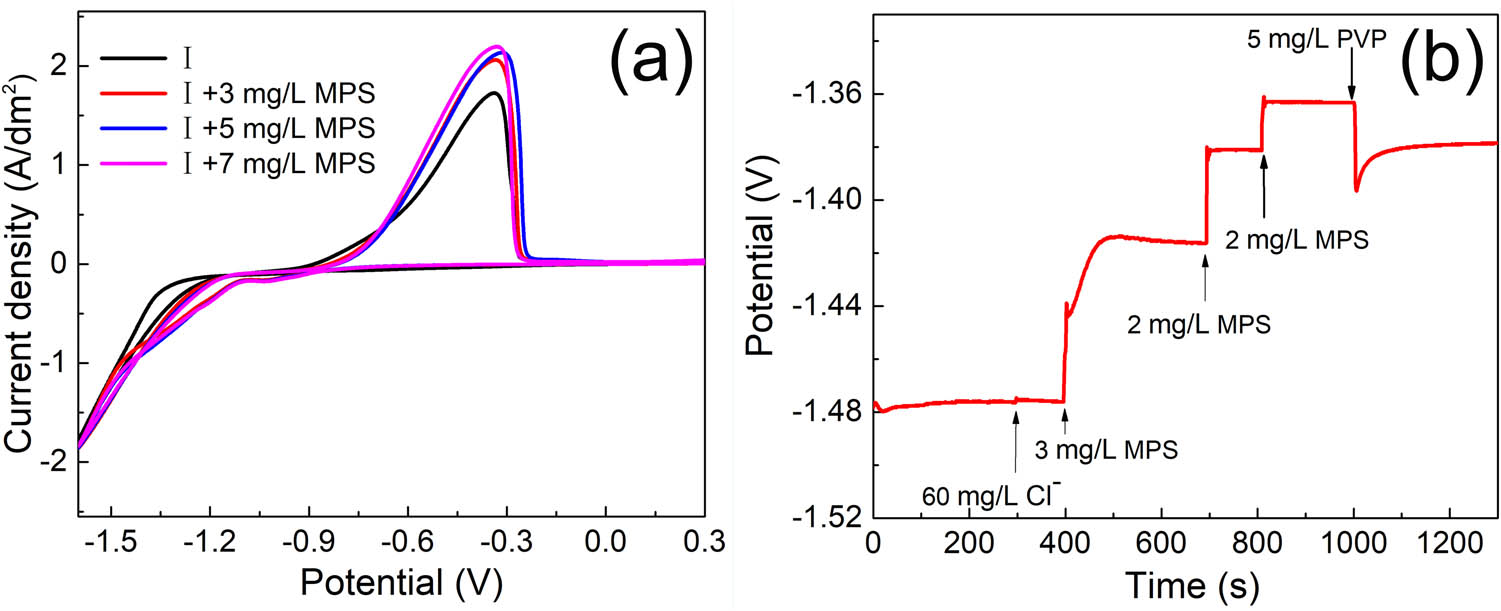
CV plots of cobalt plating solutions with MPS (a) Time–potential curve with the addition of PVP and MPS (b). The basic solution in (a) contains 0.3 mol/L CoSO4·7H2O, 0.84 mmol/L CoCl2·6H2O and 0.57 mol/L H3BO3. The basic solution in (b) contains 0.3 mol/L CoSO4·7H2O and 0.57 mol/L H3BO3.
The electrochemical performance of the solution with MPS and PVP is further investigated to analyze the competitive adsorption behavior of MPS and PVP. The inhibitive effect and adsorption behavior of PVP in cobalt electrodeposition has been reported in the previous work and the oxidation peak current density reduces obviously with the addition of PVP in the solution without MPS [28]. In this work, as shown in Figure 1b, the inhibition behavior of PVP is also observed in the solution with MPS because the potential sharply dropped by 33 mV after the addition of 5 mg/L PVP. In addition, as presented in Figure 2a, the oxidation peak current density decreases when 5 mg/L PVP is injected into the plating solution with 7 mg/L MPS. In contrast, the decrease of the peak current density in the previous work is higher in the plating solution without MPS [28]. Overall, solution II containing both 7 mg/L MPS and 5 mg/L PVP still exhibits an inhibition behavior on cobalt electrodeposition although the inhibition of PVP is attenuated by MPS. Meantime, in Figure 2b, a current density of −1.6 V for the solution with the addition of both MPS and PVP is generally lower than the one with only MPS or without additives. The above changes of the oxidation peak current density, deposition potential, and electrodeposition current density are attributed to the dynamic adsorption status of MPS and PVP molecules in the plating solution. In the first stage, the adsorption spontaneously occurred after MPS was added to the solution. In the presence of MPS in the solution, the sulfhydryl groups for the transfer of electrons and sulfonate groups for capturing the metal cations synergistically contribute to accelerating the deposition of metal cations [42,43]. However, the adsorption sites of MPS are partially replaced with PVP when PVP is added in the solution with MPS because PVP is attracted to the cathode surface and weakens the acceleration level of MPS. In the previous work, convection-dependent adsorption of PVP was verified to cause the diverged deposition rate between the bottom and the outside of the holes [28]. Therefore, the cobalt deposition rate on the outside of the hole could be relatively inhibited by PVP. Meanwhile, MPS, as a relatively small molecule, is able to diffuse into the hole easily and accumulate in the bottom of the hole. As a result, the deposition rate of cobalt increases at the bottom of the hole.
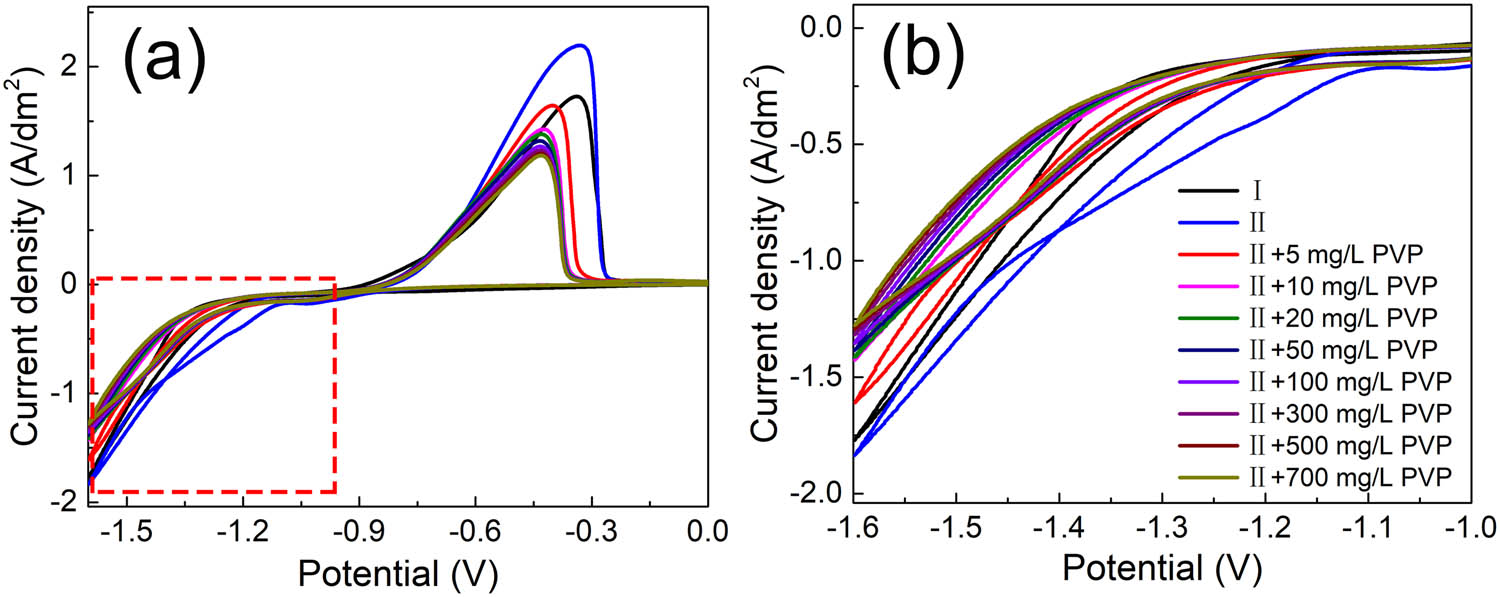
CV plots of cobalt plating solutions (a and b). Panel (b) is an enlarged view of the potential in Figure 1a from −1.6 to −1.0 V. The solution in this work contains 0.3 mol/L CoSO4·7H2O, 0.84 mmol/L CoCl2·6H2O, 0.57 mol/L H3BO3, and 7 mg/L MPS, labeled as solution II.
3.2 Quantum chemical calculation
The quantum chemical calculation was considered to illustrate the adsorption and predict the potential adsorption sites of MPS and PVP. At first, the molecule was constructed. Then, the molecule was input into the Gaussian and calculated the structure optimization. The calculation results were displayed after visualization processing in Gauss View. The molecular frontier orbital theory claims that the energy value of HOMO and LUMO for the molecules are the dominant factors in chemical reactions [37]. Furthermore, the HOMO energy value (E HOMO) of a molecule suggests the tendency to lose electrons when the LUMO energy value (E LUMO) represents the tendency of accepting electrons. The molecule with higher E HOMO and E LUMO shows stronger electron-donating ability and stronger electron-accepting ability, respectively [44]. In this work, the calculation results in Figure 3 presented the status of HOMO and LUMO of PVP containing six monomers. As shown in Figure 3, E HOMO and E LUMO values of PVP are −6.623 and −0.343 eV, respectively. Similar simulated values have been reported in another work for PVP (four monomers) [28]. The distribution of HOMO and LUMO orbitals of PVP in Figure 3a and c implies that the amide group of PVP mainly contributes to the HOMO. Amide groups in PVP are probably the electrophilic attack site for adsorption on the surface of the cobalt cathode.
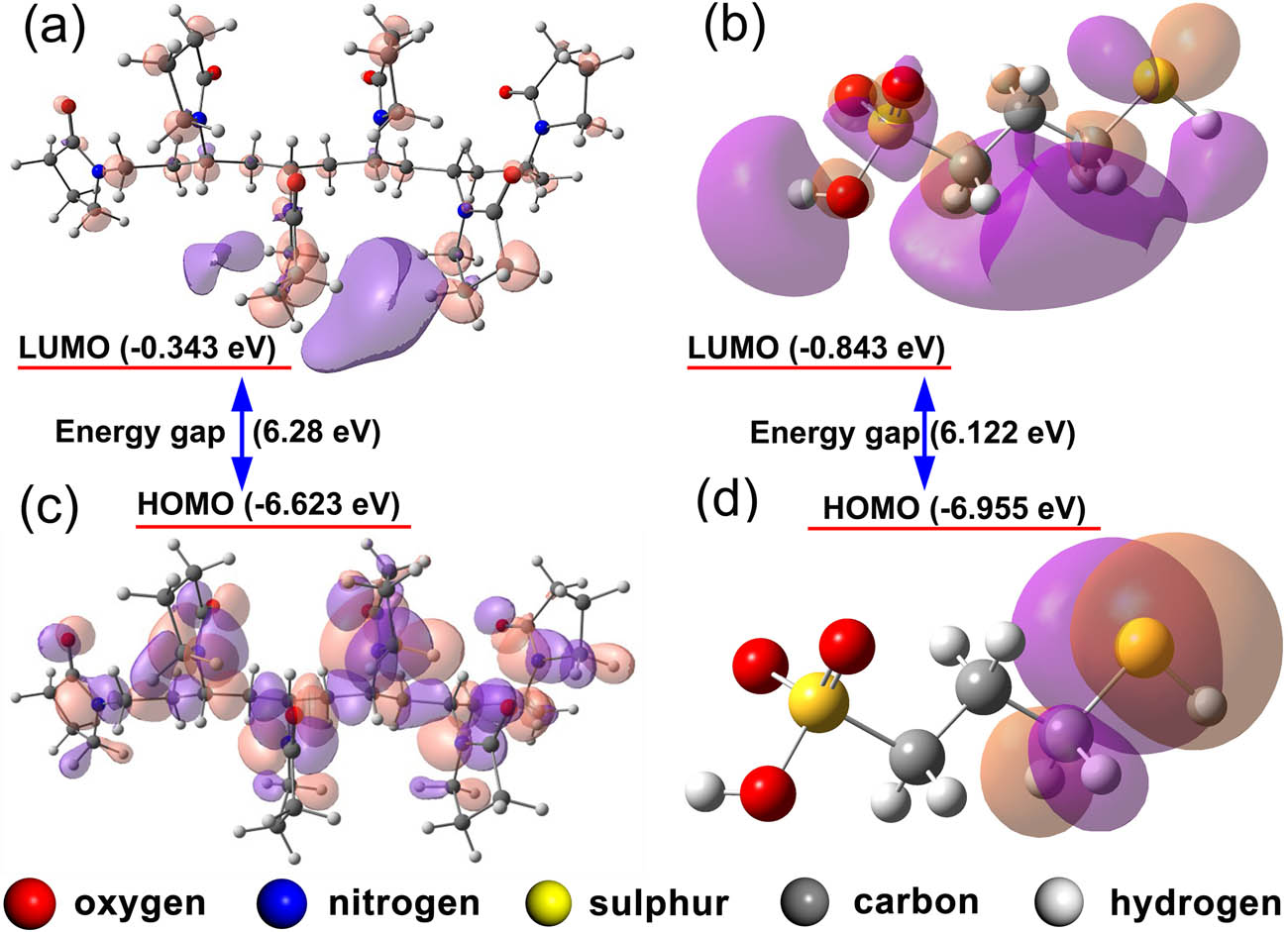
The simulated LUMO of PVP (a) and MPS (b). The simulated HOMO of PVP (c) and MPS (d).
Multiple studies have reported that MPS molecules adsorb on the surface of the copper layer through the sulfhydryl groups, and the sulfonic acid group captures copper ions in solution to accelerate the deposition [45,46]. HOMO and LUMO of MPS were calculated in this work, and the energy values of HOMO and LUMO for MPS were −6.955 and −0.843 eV, respectively. The HOMO status of MPS in Figure 3d indicates that the thiol group of MPS possesses the major space of HOMO so the thiol group is probably the electrophilic attack site. Notably, the value of the energy gap of PVP (6.28 eV) is close to that of MPS (6.122 eV), indicating similar chemical reactivities of PVP and MPS on the surface of cobalt. The computational result is consistent with the fact that MPS and PVP sustain competitive adsorption in cobalt electrodeposition.
The influence of functional groups of MPS and PVP on the cobalt electrodeposition was also illustrated by ESP results to understand the mechanism of adsorption and polarization of additives as displayed in Figure 4. The results of the ESP calculation graphically present the electron density contribution of groups to determine the possible chemical reaction sites. Figure 4a shows that the PVP containing six monomers has the lowest ESP value of −69.65 kJ/mol and a highest ESP value of 46.89 kJ/mol. A lower ESP value means higher electron density so the electron density distribution of PVP illustrates that amide groups with a high electron density contribute to the probable adsorption site during cobalt electrodeposition. However, the MPS molecule shows the lowest ESP value of −32.74 kJ/mol and a highest ESP value of 59.01 kJ/mol as shown in Figure 4b. Thus, the thiol group of MPS with a high electron density could lead to the favorable site for the electrophilic attack reaction, so the thiol group is probably the adsorption site during cobalt electrodeposition.
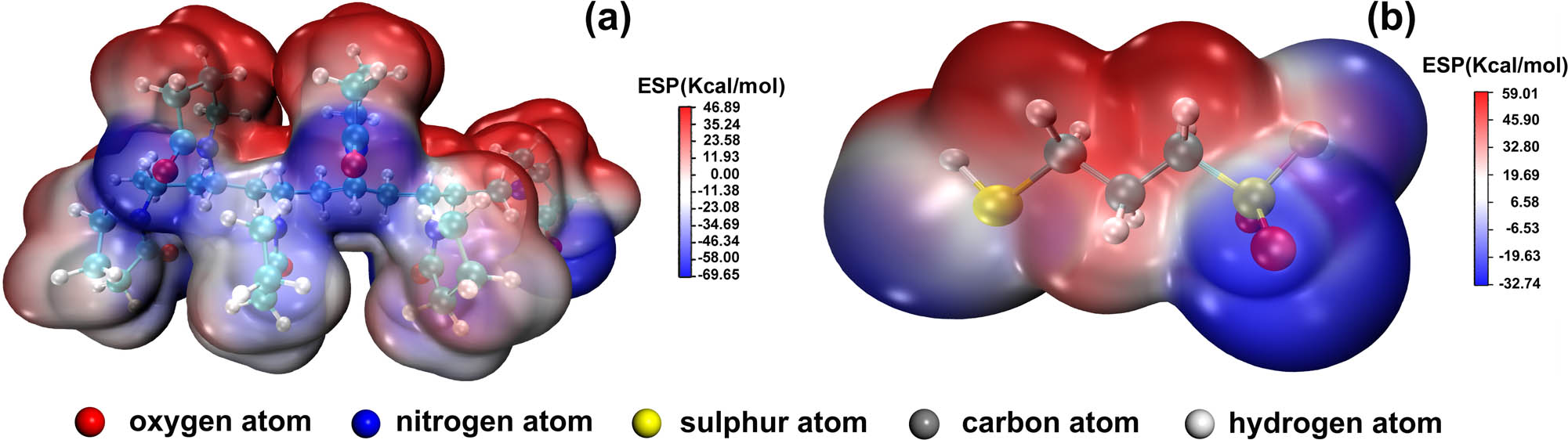
Electrostatic potential distribution on the surface of molecular van der Waals of PVP containing six monomers (a) and MPS (b).
Based on the above electrochemical characterization and quantum chemical calculation, the mechanism including three stages is proposed for the cobalt pillar electrodeposition in Figure 5. In the first stage, cobalt atoms are electrodeposited on the bottom of the hole and then grow along the wall of the hole with the formation of the cobalt nanoparticles layer. In a solution with MPS and PVP, cobalt deposition is suppressed on the upper part of the hole but the rate of cobalt atom deposition is promoted on the bottom of the hole. In the second stage, MPS and PVP molecules both adsorb to the cobalt surface in the presence of chloride ions. In this way, PVP suppresses the growth of cobalt, especially in the area with stronger convection when MPS and PVP are added to the plating solution. In the third stage, the deposition of cobalt on the upper side of the hole is inhibited more than that in the hole. In fact, MPS with a small molecule structure easily diffuses into the hole so that it accelerates the deposition process of cobalt. As a result, on the upper side of the hole, cobalt deposition is suppressed but on the bottom cobalt growth is accelerated, thereby leading to the balanced deposition rate and successful filling for regular manufacture.

The strategy of three stages for the pillar electrodeposition in solution with MPS and PVP.
3.3 Cobalt electrodeposition
With the aid of MPS and PVP, cobalt pillars are achieved by cobalt hole super-filling (Figure 6a and b). The cobalt electrodeposition was conducted in the bath and the cobalt pillar was fabricated in the solution with competitive adsorption of MPS and PVP (Figures S1 and S2). The cross-sectional slice and top view of the cobalt pillar were observed by a metallographic microscope. As displayed in Figure 6c, the diameter of the filled hole is 100 µm. In the case without additives, it is very hard to fully fill the hole that a large amount of cobalt is only electrodeposited on the upper edge (Figure 6d). The failed filling is caused by the stronger convection outside the hole of the photopolymer so that the cobalt deposition outside the hole is faster than that inside the hole and results in a conformal deposition. With the addition of 7 mg/L MPS and 300 mg/L PVP, the hole of the photopolymer is successfully filled to generate cobalt pillars. In cobalt pillar electrodeposition, 7 mg/L MPS was added in solution considering the depolarization effect of MPS and loss of MPS. PVP (300 mg/L) was chosen in order to ensure the effect of polarization and improve the morphology of the electrodeposited cobalt as a surfactant.
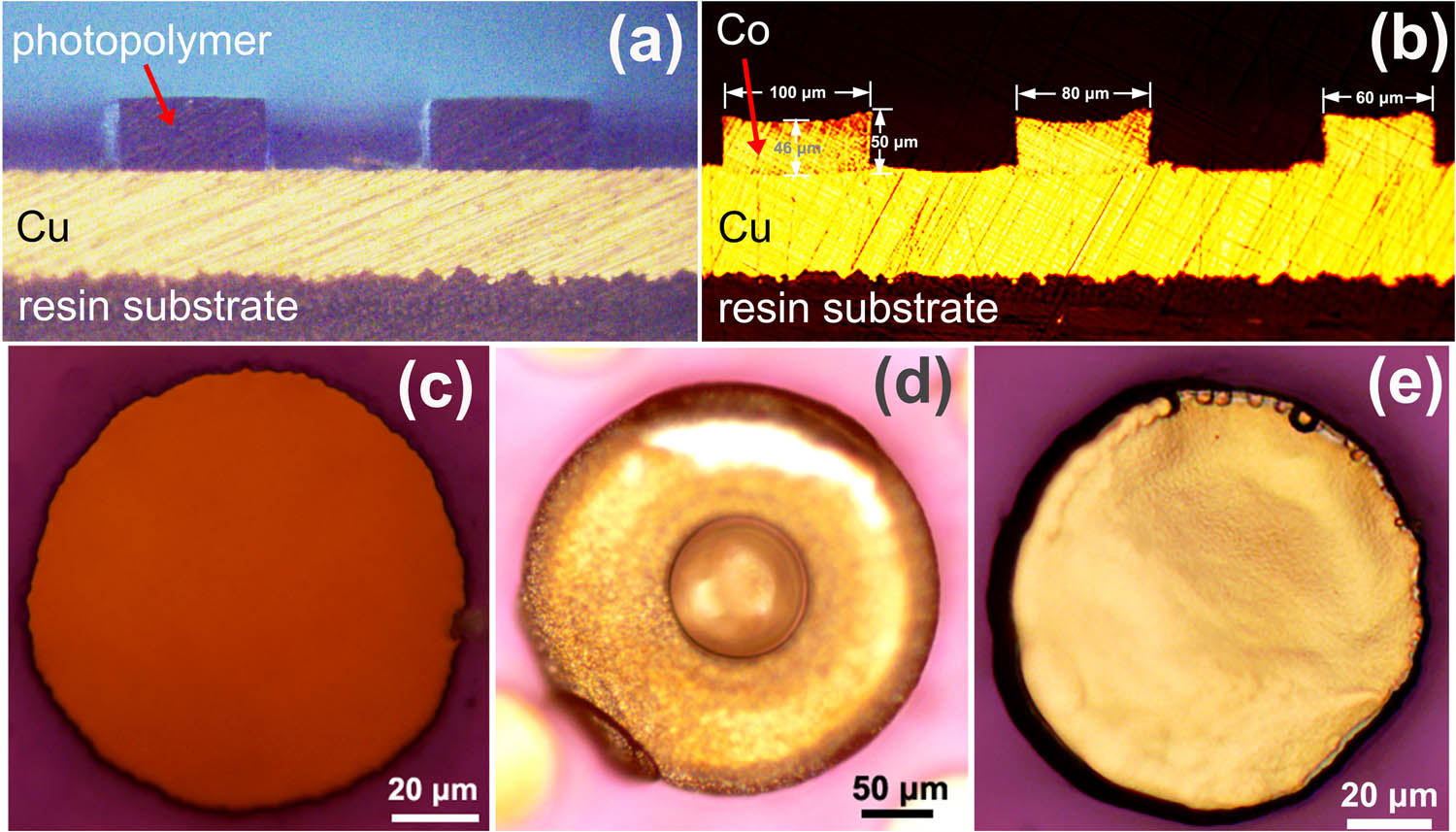
Cross-sectional slice of the holes before electrodeposition (a) and the electrodeposited cobalt-filled holes (b). Top view of the holes before cobalt electrodeposition (c). Top view of the hole after cobalt electrodeposition in solution without additives (d) and with 7 mg/L MPS and 300 mg/L PVP (e).
The top view of the cobalt pillar in Figure 6e and the cross-sectional view of the pillar in Figure 6b both verify that the holes are filled without any overgrowth of cobalt extending outside the holes, thereby leading to the balanced deposition rate and successful filling for the regular manufacture.
Besides, MPS and PVP have a considerable effect on the crystallization and morphology of the electrodeposited cobalt. As illustrated in Figure 7a, the solution without additives leads to scattered cobalt deposition, and the nanoparticles on the cobalt surface cover a wide range of sizes. In the solution with only 7 mg/L MPS, the result in Figure 7b indicates that MPS improves the formation of large size cobalt particles due to the acceleration of MPS in cobalt electrodeposition. As shown in Figure 7c, in the solution with only 300 mg/L PVP, the electrodeposited cobalt particles are refined to generate the flocculent structure on the surface. Notably, with the addition of MPS and PVP (Figure 7d), the cobalt particles turn to be electrodeposited compactly and uniformly because nano cobalt particles gradually grow to form the cobalt pillar. XRD patterns in Figure 7e show that (101̄0), (112̄1), (101̄1), (224̄0), and (314̄1) directions are observed for the cobalt growth with a hexagonal structure. In the solution with only PVP, cobalt electrodeposition is effectively inhibited wherein the XRD pattern of the copper substrate also appears. The XRD results indicate that MPS enhances the diffraction peak intensity of the cobalt 224̄0 direction and PVP reduces it. The introduction of MPS accelerated the growth of the 224̄0 crystal plane, while PVP inhibited the growth of the 224̄0 surface due to the adsorption of MPS and PVP in the above discussion.

SEM images of the electrodeposited cobalt in solution without additives (a) and with only 7 mg/L MPS (b), only 300 mg/L PVP (c), 7 mg/L MPS, and 300 mg/L PVP (d). The XRD pattern of the electrodeposited cobalt (e). 3D roughness tests of the electroplated cobalt surface and the contact angle images of the plating solution on the electrodeposited cobalt without additives (f and h) and with 7 mg/L MPS and 300 mg/L PVP (g and i).
The addition of two additives could synergistically make the cobalt surface finer and uniform. The uniformity of the electrodeposited cobalt surface was further examined by 3D roughness tests. The value of S a represents the arithmetic mean of the height values of all the peaks and troughs and a lower S a value indicates a better uniformity of the surface. As shown in Figure 7f and g, the S a value of the electrodeposited cobalt in solution with additives is significantly lower than the one without additives, verifying that the coexistence of MPS and PVP promotes uniformity of the cobalt surface. In addition, the wettability of the plated metal surface was evaluated. In Figure 7h and i, the contact angle of the solution on the cobalt substrate without additives is more than 75° and the surface contact angle is less than 36° with the addition of MPS and PVP. The wettability of the plating solution on the cobalt surface was substantially improved, which might be attributed to the interfacial reaction and hydrogen release in cobalt electrodeposition [47]. In summary, the additives effectively improved the uniformity of the electrodeposited cobalt and make the cobalt layer finer.
4 Conclusion
MPS and PVP as additives exhibit the function of acceleration and inhibition during cobalt electrodeposition, respectively. They undergo competitive adsorption behavior in cobalt electrodeposition and some adsorption sites of MPS are replaced by PVP. The adsorption site was calculated by quantum chemical calculations. The amide groups of PVP and thiol groups of MPS are probably the adsorption sites in cobalt electrodeposition. Besides, the amide groups of PVP mainly contribute to the HOMO orbital as a probable main electrophilic attack region. The cobalt pillars with different diameters are successfully fabricated with the use of competitive adsorption between MPS and PVP. In this way, the additives of cobalt electrodeposition can effectively control the process of electrodeposition and a uniform cobalt deposition could be obtained. Cobalt electrodeposition has great potential in system-in-package, and this work facilitates the theoretical research and application of cobalt pillar in electronic interconnection. Synthesis of additives and more theoretical studies focusing on the working mechanism of the additives in cobalt electrodeposition are expected in the future.
Acknowledgements
The authors gratefully acknowledge the support of the National Natural Science Foundation of China (Nos. 51801018 and 61974020). The work is also supported by the Innovation Team Project of Zhuhai City (No. ZH0405190005PWC), and the projects of Sci & Tech planning of Guangdong Province (No. 2019B090910003) and Zhuhai City (No. ZH01084702180040HJL).
-
Funding information: National Natural Science Foundation of China (Nos. 51801018 and 61974020). The work is also supported by Innovation Team Project of Zhuhai city (No. ZH0405190005PWC), and the projects of Sci & Tech planning of Guangdong Province (No. 2019B090910003) and Zhuhai City (No. ZH01084702180040HJL).
-
Author contributions: All authors have accepted responsibility for the entire content of this article and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statements: All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
[1] Hao HL, Hui D, Lau D. Material advancement in technological development for the 5G wireless communications. Nanotechnol Rev. 2020;9(1):683–99.10.1515/ntrev-2020-0054Suche in Google Scholar
[2] Shang ZW, Hsu HH, Zheng ZW, Cheng CH. Progress and challenges in p-type oxide-based thin film transistors. Nanotechnol Rev. 2019;8(1):422–43.10.1515/ntrev-2019-0038Suche in Google Scholar
[3] Gall D. Electron mean free path in elemental metals. J Appl Phys. 2016;119(8):1–5.10.1063/1.4942216Suche in Google Scholar
[4] Huang Q, Lyons TW, Sides WD. Electrodeposition of cobalt for interconnect application: effect of dimethylglyoxime. J Electrochem Soc. 2016;163(13):D715–21.10.1149/2.1111613jesSuche in Google Scholar
[5] Steinhögl W, Schindler G, Steinlesberger G, Engelhardt M. Size-dependent resistivity of metallic wires in the mesoscopic range. Phys Rev B. 2002;66(7):075414.10.1103/PhysRevB.66.075414Suche in Google Scholar
[6] Shin H-AS, Kim B-J, Kim J-H, Hwang S-H, Budiman AS, Son H-Y, et al. Microstructure evolution and defect formation in cu through-silicon vias (TSVs) during thermal annealing. J Electron Mater. 2012;41(4):712–9.10.1007/s11664-012-1943-7Suche in Google Scholar
[7] Chen SH, Fu SL, Liang D, Chen XH, Mi XJ, Liu P, et al. Preparation and properties of 3D interconnected CNTs/Cu composites. Nanotechnol Rev. 2020;9(1):146–54.10.1515/ntrev-2020-0013Suche in Google Scholar
[8] Zhang XH, Zhang Y, Tian BH, Song KX, Liu P, Jia YL, et al. Review of nano-phase effects in high strength and conductivity copper alloys. Nanotechnol Rev. 2019;8(1):383–95.10.1515/ntrev-2019-0034Suche in Google Scholar
[9] Feng J, Liang SH, Guo XH, Zhang Y, Song KX. Electrical conductivity anisotropy of copper matrix composites reinforced with SiC whiskers. Nanotechnol Rev. 2019;8(1):285–92.10.1515/ntrev-2019-0027Suche in Google Scholar
[10] Beyne E. 3D system integration technologies. 2006 International Symposium on VLSI Technology, Systems, and Applications. Hsinchu: IEEE; 2006. p. 19–27.10.1109/VTSA.2006.251113Suche in Google Scholar
[11] Fischer AC, Forsberg F, Lapisa M, Bleiker SJ, Stemme G, Roxhed N, et al. Integrating MEMS and ICs. Microsyst Nanoeng. 2015;1(1):1–16.10.1038/micronano.2015.5Suche in Google Scholar
[12] Hu Y, Deb S, Li D, Huang Q. Effects of organic additives on the impurity and grain structure of electrodeposited cobalt. Electrochim Acta. 2021;368(13):1–8.10.1016/j.electacta.2020.137594Suche in Google Scholar
[13] Hu Y, Huang Q. Oscillatory behavior in cobalt electrodeposition with 3-mercapto-1-propanesulfonate. J Phys Chem C. 2020;124(39):21608–16.10.1021/acs.jpcc.0c06877Suche in Google Scholar
[14] Luo X, Chen CY, Chang TFM, Sone M, Zhang Q, Zhang JZ. The Structure and micro-mechanical properties of electrodeposited cobalt films by micro-compression test. J Electrochem Soc. 2021;168(10):1–7.10.1149/1945-7111/ac2be9Suche in Google Scholar
[15] Wu J, Wafula F, Branagan S, Suzuki H, van Eisden J. Mechanism of cobalt bottom-up filling for advanced node interconnect metallization. J Electrochem Soc. 2018;166(1):D3136–41.10.1149/2.0161901jesSuche in Google Scholar
[16] Kelly J, Chen JHC, Huang H, Hu CK, Liniger E, Patlolla R et al. Experimental study of nanoscale co damascene BEOL interconnect structures. 2016 IEEE International Interconnect Technology Conference/Advanced Metallization Conference. IEEE International Interconnect Technology Conference IITC; 2016. p. 40–2.10.1109/IITC-AMC.2016.7507673Suche in Google Scholar
[17] Rigsby MA, Brogan LJ, Doubina NV, Liu YH, Opocensky EC, Spurlin TA, et al. The critical role of pH gradient formation in driving superconformal cobalt deposition. J Electrochem Soc. 2018;166(1):D3167–74.10.1149/2.0211901jesSuche in Google Scholar
[18] Kang J, Sung M, Byun J, Kwon OJ, Kim JJ. Superconformal cobalt electrodeposition with a hydrogen evolution reaction suppressing additive. J Electrochem Soc. 2020;167(16):1–7.10.1149/1945-7111/abd3b9Suche in Google Scholar
[19] Vereecken PM, Binstead RA, Deligianni H, Andricacos PC. The chemistry of additives in damascene copper plating. Ibm J Res Dev. 2005;49(1):3–18.10.1147/rd.491.0003Suche in Google Scholar
[20] Dow WP, Li CC, Lin MW, Su GW, Huang CC. Copper fill of microvia using a thiol-modified Cu seed layer and various levelers. J Electrochem Soc. 2009;156(8):D314–20.10.1149/1.3147273Suche in Google Scholar
[21] Dow WP, Huang HS, Yen MY, Huang HC. Influence of convection-dependent adsorption of additives on microvia filling by copper electroplating. J Electrochem Soc. 2005;152(6):C425–34.10.1149/1.1901670Suche in Google Scholar
[22] Hu Y, Lyons T, Huang Q. Influence of furil dioxime on cobalt electrochemical nucleation and growth. J Electrochem Soc. 2020;167(2):1–11.10.1149/1945-7111/ab69fbSuche in Google Scholar
[23] Kong DL, Zheng Z, Meng FY, Li N, Li DY. Electrochemical nucleation and growth of cobalt from methanesulfonic acid electrolyte. J Electrochem Soc. 2018;165(16):D783–9.10.1149/2.0191816jesSuche in Google Scholar
[24] Chan PF, Chiu YD, Dow WP, Krug K, Lee YL, Yau SL. Use of 3,3-thiobis(1-propanesulfonate) to accelerate microvia filling by copper electroplating. J Electrochem Soc. 2013;160(12):D3271–7.10.1149/2.047312jesSuche in Google Scholar
[25] Dow WP, Huang HS, Yen MY, Chen HH. Roles of chloride ion in microvia filling by copper electrodeposition-II. Studies using EPR and galvanostatic measurements. J Electrochem Soc. 2005;152(2):C77–88.10.1149/1.1849935Suche in Google Scholar
[26] Chiu YD, Dow WP. Accelerator screening by cyclic voltammetry for microvia filling by copper electroplating. J Electrochem Soc. 2013;160(12):D3021–7.10.1149/2.006312jesSuche in Google Scholar
[27] Jovic VD, Jovic GM. Copper electrodeposition from a copper acid baths in the presence of PEG and NaCl. J Serbian Chem Soc. 2001;66(11–12):935–52.10.2298/JSC0112935JSuche in Google Scholar
[28] Ni XR, Chen YM, Jin XF, Wang C, Huang YZ, Hong Y, et al. Investigation of polyvinylpyrrolidone as an inhibitor for trench super-filling of cobalt electrodeposition. J Taiwan Inst Chem Eng. 2020;112:232–9.10.1016/j.jtice.2020.06.010Suche in Google Scholar
[29] Jiang Q, Tallury SS, Qiu YP, Pasquinelli MA. Interfacial characteristics of a carbon nanotube-polyimide nanocomposite by molecular dynamics simulation. Nanotechnol Rev. 2020;9(1):136–45.10.1515/ntrev-2020-0012Suche in Google Scholar
[30] Khan S, Nandi CK. Optimizing the underlying parameters for protein-nanoparticle interaction: advancement in theoretical simulation. Nanotechnol Rev. 2014;3(4):347–59.10.1515/ntrev-2014-0002Suche in Google Scholar
[31] Saraireh SA, Altarawneh M, Tarawneh MA. Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface. Nanotechnol Rev. 2021;10(1):719–27.10.1515/ntrev-2021-0051Suche in Google Scholar
[32] Hassan J, Diamantopoulos G, Homouz D, Papavassiliou G. Water inside carbon nanotubes: structure and dynamics. Nanotechnol Rev. 2016;5(3):341–54.10.1515/ntrev-2015-0048Suche in Google Scholar
[33] Wang CH, Chen Q, Guo TT, Zhang L. Preparation and adsorption properties of nano-graphene oxide/tourmaline composites. Nanotechnol Rev. 2021;10(1):1812–26.10.1515/ntrev-2021-0113Suche in Google Scholar
[34] Kim S-H, Zhang Y, Lee J-H, Lee S-Y, Kim Y-H, Rhee KY, et al. A study on interfacial behaviors of epoxy/graphene oxide derived from pitch-based graphite fibers. Nanotechnol Rev. 2021;10(1):1827–37.10.1515/ntrev-2021-0111Suche in Google Scholar
[35] Lu T, Chen FW. Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem. 2012;33(5):580–92.10.1002/jcc.22885Suche in Google Scholar PubMed
[36] Premkumar S, Jawahar A, Mathavan T, Dhas MK, Sathe VG, Benial AMF. DFT calculation and vibrational spectroscopic studies of 2-(tert-butoxycarbonyl(Boc)-amino)-5-bromopyridine. Spectrochim Acta Part A-Mol Biomol Spectrosc. 2014;129:74–83.10.1016/j.saa.2014.02.147Suche in Google Scholar PubMed
[37] Lee C, Yang W, Parr RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B Condens Matter. 1988;37(2):785–9.10.1103/PhysRevB.37.785Suche in Google Scholar
[38] Lai Z, Wang S, Wang C, Hong Y, Zhou G, Chen Y, et al. A comparison of typical additives for copper electroplating based on theoretical computation. Comput Mater Sci. 2018;147:95–102.10.1016/j.commatsci.2017.11.049Suche in Google Scholar
[39] Murray JS, Politzer P. The electrostatic potential: an overview. Wiley Interdiscip Rev Comput Mol Sci. 2011;1(2):153–63.10.1002/wcms.19Suche in Google Scholar
[40] Hackett JC. Chemical reactivity theory: a density functional view. J Am Chem Soc. 2010;132(21):7558.10.1021/ja1030744Suche in Google Scholar
[41] Wang C, An MZ, Yang PX, Zhang JQ. Prediction of a new leveler (N-butyl-methyl piperidinium bromide) for through-hole electroplating using molecular dynamics simulations. Electrochem Commun. 2012;18:104–7.10.1016/j.elecom.2012.02.028Suche in Google Scholar
[42] Kreider A, Barkey DP, Wong EH. On the displacement of adsorbed polyethylene glycol by 3-mercapto-1-propanesulfonate during copper electrodeposition. J Electrochem Soc. 2014;161(12):D663–5.10.1149/2.0371412jesSuche in Google Scholar
[43] Zhong Q, Gu M, Li QA. Studies on the influence of sodium 3-mercaptopropanesulphonate additives on copper electrodeposition. Acta Chim Sin. 2010;68(17):1707–12.Suche in Google Scholar
[44] Gece G. The use of quantum chemical methods in corrosion inhibitor studies. Corros Sci. 2008;50(11):2981–92.10.1016/j.corsci.2008.08.043Suche in Google Scholar
[45] Tu HL, Yen PY, Wu HL, Chen S, Vogel W, Yau S, et al. In situ STM of 3-mercaptopropanesulfonate adsorbed on Pt(111) electrode and its effect on the electrodeposition of copper. J Electrochem Soc. 2010;157(4):D206–10.10.1149/1.3295713Suche in Google Scholar
[46] Dow WP, Chiu YD, Yen MY. Microvia filling by Cu electroplating over a Au seed layer modified by a disulfide. J Electrochem Soc. 2009;156(4):D155–67.10.1149/1.3078407Suche in Google Scholar
[47] Tarasevich YI. The surface energy of hydrophilic and hydrophobic adsorbents. Colloid J. 2007;69(2):212–20.10.1134/S1061933X0702010XSuche in Google Scholar
© 2022 Xiuren Ni et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption

