Abstract
The development of advanced composite materials based on polyoxometalates (POMs) and fibers has attracted significant attention due to their combination of the unique chemical reactivity of POMs and the flexible wearable properties of fiber materials. The exceptional properties exhibited by those resultant composites have been widely employed in catalysis and optical sensors. In this article, we aim to provide an overview of progress on POMs functionalized fiber materials involving the preparation methods, namely, electrostatic spinning and layer-by-layer self-assembly methods, as well as the developments in the fields of catalysis, photochromism, and photoluminescence. Current applications are critically assessed and promising future target systems are discussed.
Graphical abstract
In this review, polyoxometalate-based fiber materials, including preparation and application, are introduced, showing promising performances in catalysis and optical property.
1 Introduction
A recent trend in molecular and material sciences is the development of multi-functional materials that meet societal needs in catalysis, material science, energy-related sciences, and magnetism [1–3]. It has been widely recognized that functional fibers offer additional value through effective enhancement. As such, fiber in the new era should not only meet the fundamental needs of consumers but also possess muti-functionals, such as photochromic property, anti-UV, and anti-bacterial [4,5]. Generally speaking, these significant functions are achieved by incorporating functional molecules on or within fibers or embedding relevant components. Among the suitable and tunable components considered by researchers, the resort to structurally defined inorganic building blocks appears as a convenient and feasible route to better control their dispersion in the hybrid system.
Polyoxometalates (POMs) are a class of discrete anionic molecular metal oxides of early transition metals (e.g., V, Mo, W, Nb, Ta), usually constructed via the condensation of metal oxide polyhedrons in a corner-, edge-, and even face-sharing manner [6]. POM family that structurally and compositionally can be broken into two subcategories according to whether they contain heteroatoms, namely, isopolyanions and heteropolyanions. Currently, POMs have six basic structures: Keggin, Dawson, Anderson, Lindqvist, Waugh, and Silverton (Figure 1). POMs continue to attract the attention of research groups due to their intriguing chemical and physical features, allowing them to be employed in various research fields such as catalysis, and molecular electronics [7,8]. Several excellent reviews describe the state-of-the-art cluster designing and redox behavior characterization of POM for redox flow batteries [9], application of POMs in chemiresistive gas sensors [10], discussing synthetic methodologies of POM-based inorganic-organic hybrids for heterogeneous catalysts [11], and so on. Nevertheless, study on fiber as organic polymers and vehicle is limited. In particular, Carraro and Gross have described in their review paper that the hybrid materials are based on the embedding of organically modified transition metal oxoclusters or POMs into polymers for functional applications [12]. Snider and Hill detailed the emerging field of functionalized reactive polymers for the elimination of CWAs including POMs as catalytic components [13].
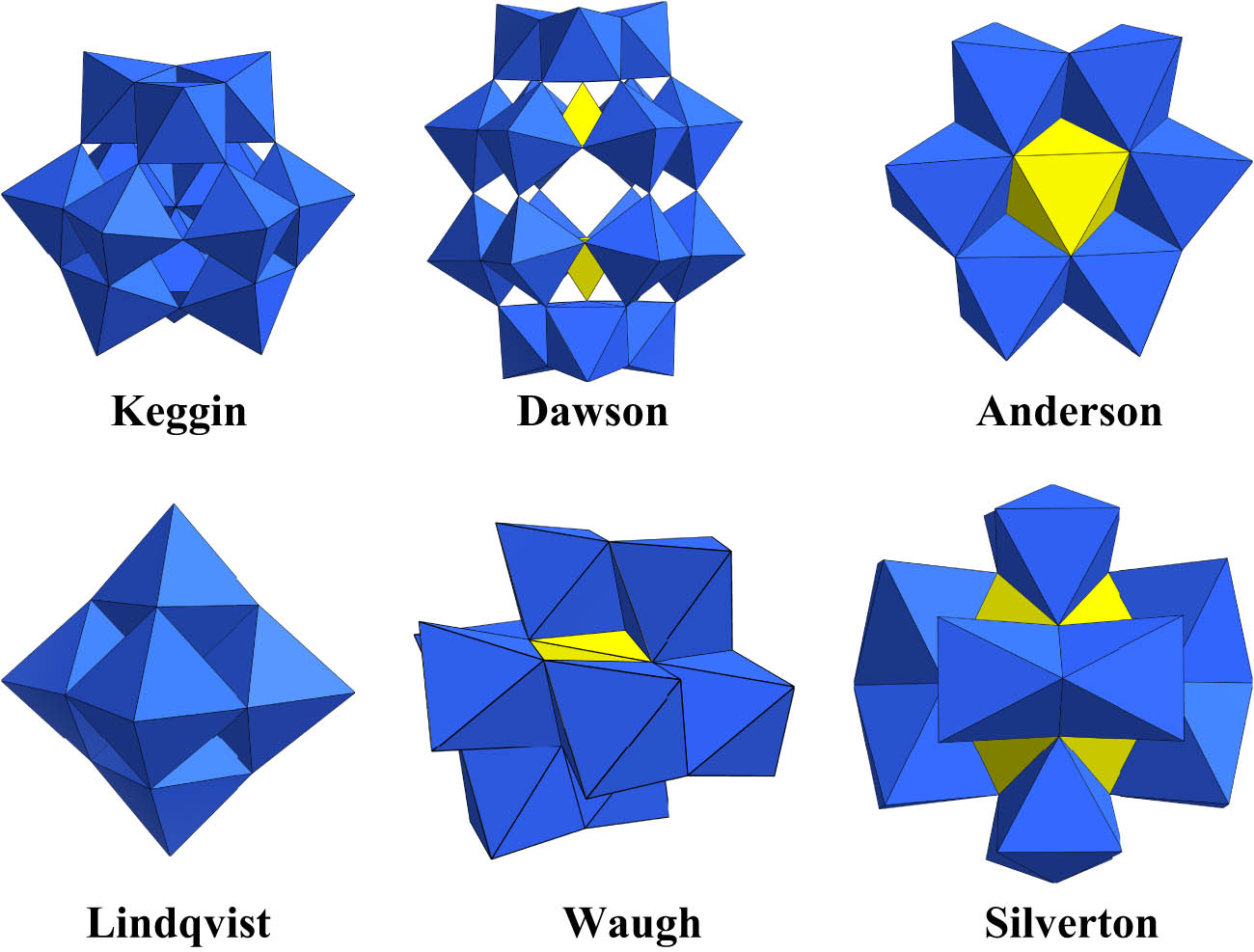
Polyhedral representation of six kinds of POM architectures.
Here, we will develop the discussion on composite materials combining POMs and fiber, focusing on the rational design and properties of hybrids achieved through two classical methods. These hybrids are further discussed in the light of multi-functional assemblies and materials; in particular those displaying encouraging results in catalysis and optical property, in the light of current challenges, such as multi-functional and stable material. Two main pathways for the preparation of the POM/fiber composites can be identified; their main advantages and disadvantages together with potential future directions are described. In the first part, we focus on the formation of hybrids through electrostatic spinning with different polymers: polyacrylamide (PAM), polyvinylpyrrolidone (PVP), polyacrylonitrile (PAN), polyvinyl alcohol (PVA), and polylactic acid (PLA). In the second part, we consider hybrids synthesized by layer-by-layer (LBL) self-assembly. In fact, there are differences, and relation as well, between them. Besides, the properties and applications of these systems are further highlighted in sole section, and their potential in future developments, within this emerging area at the interface of material and molecular sciences, are put into perspective.
2 Synthetic strategy
With the continuous advancement of science and technology, the research on the application of POM-based composites is experiencing rapid growth. Fiber, due to its morphology, structure, and properties, emerges as one of the most promising carriers for constructing functionalized fiber materials with POMs. The current preparation methods for this type of composite materials are primarily categorized into two approaches: electrostatic spinning method and LBL self-assembly method.
2.1 Electrostatic spinning method
The electrospinning technique is regarded as one of the most promising methods for nanofiber preparation due to its inherent advantages, including a simple and efficient process, high production yield, and rapid repetition rate [14]. Driven by the research on inorganic functional fibers and inorganic–organic hybrid fiber materials, pure POM nanofibers or POM-based nanocomposite fibers are synthesized using electrostatic spinning technology to showcase the role of POMs through fiber morphology. The current practice involves the utilization of organic polymers, with the specific preparation process outlined as follows (Figure 2): POMs are blended with a polymer to create an electrospinning solution, which is subsequently loaded into a syringe equipped with a copper needle. The copper needle and the aluminum foil receiving device are connected to the positive and negative terminals of a high-voltage power source, respectively. Under a specific voltage, the electrospinning solution is ejected from the copper needle and deposited on receiving device at an appropriate distance from the needle tip, resulting in the formation of nanofibers. The distance must be sufficient to allow for solidification and subsequent collection on the receiving device during the fine-flow injection process.
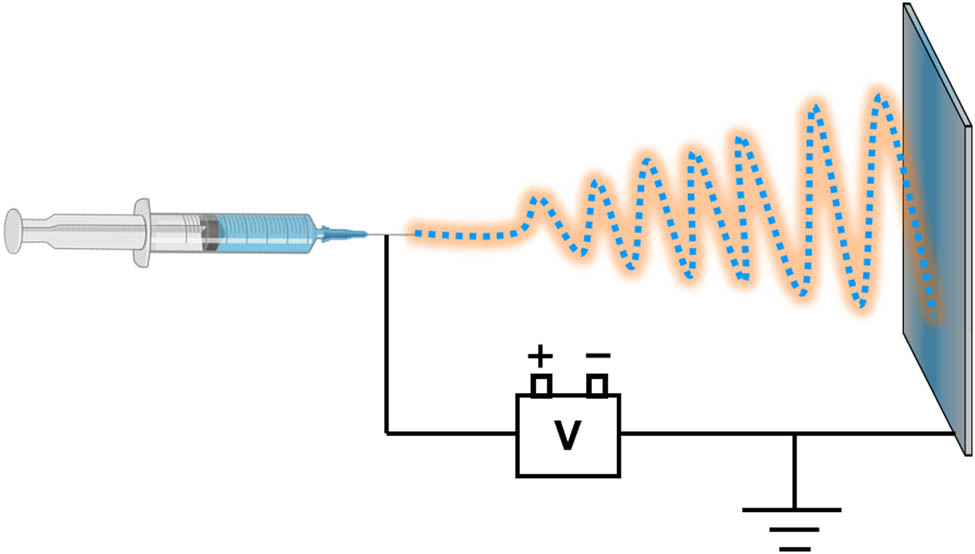
Diagram schematic of electrospinning device.
The common polymers used as POM carriers include PAM, PVP, PAN, PVA, PLA, etc. (Figure 3). Among them, PVA is applied most in many studies. In 2004 and 2007, Gong et al. reported PW12/PVA, SiW11V/PVA, SiW9V3/PVA, and P2W17V/PVA (the abbreviations are shown in Table S1) composite fibers furnished from spinning solution including different POMs and PVA, respectively. The results indicated that POMs are helpful in the crosslinking and in decreasing the swelling degree of PVA [15,16]. Later, EuPW11 and EuPW10-1 were dissolved in PVA to weave different fibers, which exhibited excellent photoluminescence [17]. Taking into account the ability to store and release electrons of POM-based materials under specific conditions, POMs are regarded as excellent candidates for the design of composite hybrid materials which exhibit interesting cooperative effects for behaving as electrode. Subsequently, Gong et al. reported POM-based composite nanofiber-modified electrode. At first, P2W18-1 was dissolved in PVA solution resulting in electrospinning solution. Then, the spun fibers were collected on the aluminum foil covered on the surface of ITO electrode. The formation of modified electrode was achieved by removing the aluminum foil [18]. Additionally, such a nitrite sensor was developed since P2W18-1 had the performance of electrocatalytic reduction of NO2− .
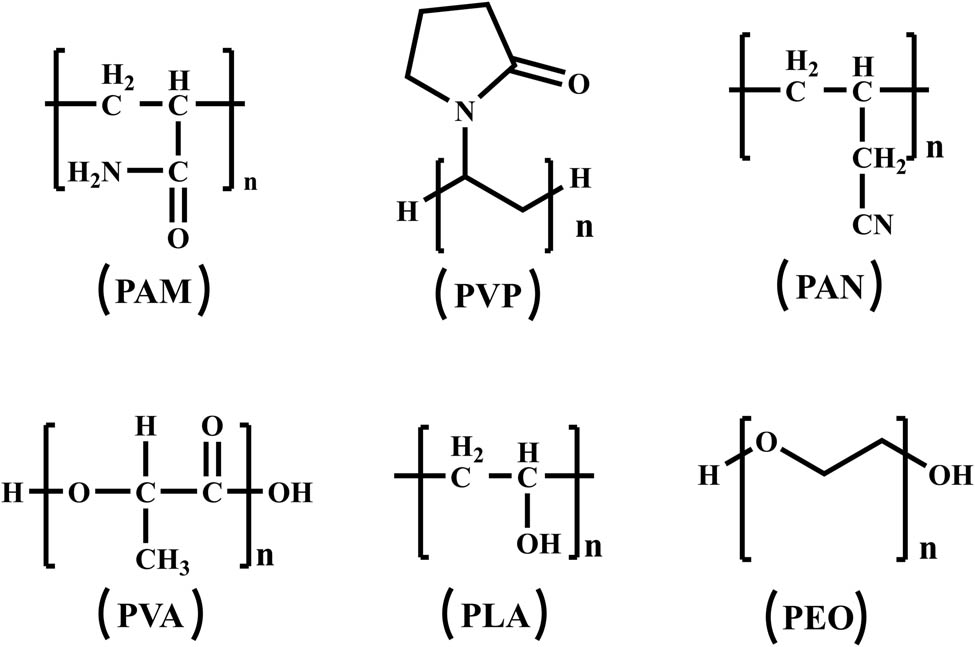
Polymer structure formula commonly used for POMs carriers.
In 2006, Peng et al. obtained PVA/SiW11Co-P2O7 composite fiber, and the fiber underwent calcine to form thin fiber with 250 nm and maintained intact Keggin structure. Interestingly, the fiber showed thermal stability and color change performance [19]. Based on this, other polymers were used to prepare POM-based fiber materials with various properties. For example, Song et al. reported soft EuW10-2/PAN composite film by blending EuW10-2 and PAN [20] (Figure 4), while Qi et al. obtained hydrophobic PLA nanofibers blended with P2W18-1, OXA and PLA [21]. Furthermore, combined with hydrophilic PAN nanofibers containing PSP, double-layer Janus nanofiber was prepared with the help of hydrophobic PLA. Owing to the difference in wettability between them, excess exudate could be drained around the wound.
![Figure 4
(a) Electrospinning process of the EuW10/PAN nanocomposite films. (b) 23% EuW10/PAN nanocomposite film exposed to HCl/NH3. (c) Luminescent intensity at 616 nm of the nanocomposite film as a function of exposure cycles. (d) Luminescent intensity exposed to H2S/NH3. Reproduced from ref. [20].](/document/doi/10.1515/ntrev-2023-0199/asset/graphic/j_ntrev-2023-0199_fig_004.jpg)
(a) Electrospinning process of the EuW10/PAN nanocomposite films. (b) 23% EuW10/PAN nanocomposite film exposed to HCl/NH3. (c) Luminescent intensity at 616 nm of the nanocomposite film as a function of exposure cycles. (d) Luminescent intensity exposed to H2S/NH3. Reproduced from ref. [20].
The loading of POMs onto the polymer by electrostatic spinning technology can prevent the POMs from changing into heterogeneous microphase and mass aggregation, which is an effective and simple method to avoid the phase separation of POMs in the polymer matrix. In 2006, Zhang et al. prepared PVA/EuW10-3 composite fibers by electrostatic spinning. TEM showed that EuW10-3 particles with diameters of several nanometers to tens of nanometers were dispersed in the composite fibers [22]. These mono-disperse nanoparticles prevented the phase separation of EuW10-3 from PVA matrix, making the inorganic component and polymer component good compatibility. In addition, POMs can be loaded on SiO2 or other organic microporous polymers. In 2019, Wu et al. reported Ni(CH3COO)2 and Keggin-type SiW12 in PVA solution for electrospinning [23]. The product was carbonized in N2, resulting in modified carbon nanofibers (SiWNi-CNFs) after decomposition and recombination. Besides, SiWNi-CNFs showed excellent electrocatalytic hydrogen evolution performance. In 2021, the PMo12, TiO2 (100 nm), and SiO2 (12 nm) were dispersed in PVA solution to form electrospinning solution for further electrostatic spinning [24]. PMo12/TiO2/SiO2 nanofibers were obtained by calcination, which were further mixed into polyvinylidene fluoride to obtain a film with excellent photocatalytic properties. In 2022, Farha et al. prepared functional microporous polymer PIM-1-AO with carboxylic acid and amide oxime groups, and then acetonitrile suspension of PW12, PIM-1-AO, and DMF were mixed to make electrospinning solution for the formation of PIM-POM porous fiber pad through electrostatic spinning [25]. Additionally, PIM-POM had catalytic oxidation effect on CEES in the presence of H2O2. In 2020, Semnani et al. reported solvent-free nanofiber electrolyte, which was prepared using polyethylene oxide (PEO) as polymer matrix, lithium perchlorate as salt, ethyl carbonate as plasticizer, and Keggin-type POMs (Cu-POM@Ru-rGO, Ni-POM@Ru-rGO, CoPOM@Ru-rGO) as filler [26]. Compared with polymer electrolytes obtained by melting and solution spinning, electrospinning electrolytes had excellent ionic conductivity and were good candidates for solvo-free electrolytes for lithium-ion batteries (Figure 5).
![Figure 5
Electrospinning set-up. Reproduced from ref. [26].](/document/doi/10.1515/ntrev-2023-0199/asset/graphic/j_ntrev-2023-0199_fig_005.jpg)
Electrospinning set-up. Reproduced from ref. [26].
Although electrostatic spinning technology is a very classical and practical method to yield nanofibers, it requires some special equipment and rigorous operating conditions, such as air humidity and spinning voltage, which affect the spinning effect. Consequently, inadequate control of operational conditions can lead to the emergence of issues.
2.2 LBL self-assembly method
LBL self-assembly method, known as chemical deposition technique, is that POMs or other materials are deposited on the fiber LBL, so that POMs can make full use of the fiber performance (Figure 6). Electrostatic interaction is the main driving force for deposition alternately. The details are as follows: first, the substrate with charge (negative charge as an example) is immersed in the solution with opposite charge. Owing to the electrostatic action, the particles with opposite charge will be adsorbed on the surface of substrate, making the substrate with positive charge. The materials were washed to remove the physical adsorption particles. Then, the substrate is dipped into a solution with negatively charged particles to assemble another layer of negatively charged particles on the surface of the substrate. By repeating the above process, the multilayer film can be obtained by alternating deposition.

Diagram schematic of LBL self-assembly.
In 2005, Ding et al. reported a composite cellulose acetate (CA) material by LBL self-assembly technology, employing positively charged polyacrylamide hydrochloride (PAH) and negatively charged SiW12 [27]. After high-temperature calcination, pure Keggin-type SiW12 nanotubes with wall thickness of about 50 nm were obtained (Figure 7). Furthermore, the investigation and application of fibers and nanotubes with nanostructured features have been in various fields including catalysis, conduction, photochromism, and electrochromism.
![Figure 7
(a) Fabrication of POM nanotubes via LBL coating and thermal removal of the electrospun nanofiber. (b) SEM images of (PAH2.5/H4SiW12O402.5)5 film-coated fibers. (c) SEM images of (PAH2.5/H4SiW12O402.5)5 film-coated fibers calcined at 380°C. Reproduced from ref. [27].](/document/doi/10.1515/ntrev-2023-0199/asset/graphic/j_ntrev-2023-0199_fig_007.jpg)
(a) Fabrication of POM nanotubes via LBL coating and thermal removal of the electrospun nanofiber. (b) SEM images of (PAH2.5/H4SiW12O402.5)5 film-coated fibers. (c) SEM images of (PAH2.5/H4SiW12O402.5)5 film-coated fibers calcined at 380°C. Reproduced from ref. [27].
Additionally, the combination of electrospinning technology and LBL self-assembly technology is also an effective method to prepare POMs functionalized fiber materials. In 2006, Dong et al. successfully fabricated PVA nanofibers using electrospinning technology, which was applied on ITO electrode [28]. Then, PAH and P2Mo18 with opposite charge were sequentially deposited on the composite electrode PVA/ITO, resulting in the formation of a multilayer film composed of P2Mo18/PAH. Interestingly, the electrospun PVA nanofiber can be used as a self-assembly platform because of its large surface area and the negative charge generated by the dissociation of –CH2OH in PVA. As mentioned above, Zhou et al. prepared negatively charged polyacrylic acid (PAA)/PVA/PW12 nanofibers by electrospinning, followed by the deposition of negatively charged PW12, positively charged polyethylenimide (PEI) and Ag+ using LBL self-assembly technology [29]. The electrostatic forces between the cationic compounds (such as PEI or Ag+) and the PAA/PVA/PW12 substrate facilitate the attraction of negatively charged PW12 to form a double ultra-thin layer or multilayer film on the substrate. Finally, NaBH4 was employed to reduce Ag+, resulting in Ag-NPs deposited on the multilayer film. Besides, the results demonstrated that the Ag-(PEI) n (PW12) n film exhibited remarkable photocatalytic activity toward MB (Figure 8). Moreover, the self-assembly of multilayer films can be achieved through hydrogen bonding as the driving force. In 2020, decatungstate (DTS) and PEI were deposited on linen fabric alternately, resulting in the formation of a multilayer film through repeated dipping [30]. Linen, being a cellulose fiber, possesses a large number of hydroxyl groups, which have the capability to establish hydrogen bonds with amino or imino groups on PEI. Meanwhile, the amino groups in PEI can also form hydrogen bonds with oxygen atoms in DTS (Figure 9). In the same year, the group employed a similar method to fabricate PAA and DTS multilayers on cotton fabrics [31].
![Figure 8
(a) LBL self-assembly process by depositing polycation and polyanion onto the PAA/PVA/PW12 fiber. (b) Photocatalytic degradation curves for different LBL films. (c) Cycle testing of photocatalytic activity of Ag-(PEI)2(PW12)2 film. Reproduced from ref. [29].](/document/doi/10.1515/ntrev-2023-0199/asset/graphic/j_ntrev-2023-0199_fig_008.jpg)
(a) LBL self-assembly process by depositing polycation and polyanion onto the PAA/PVA/PW12 fiber. (b) Photocatalytic degradation curves for different LBL films. (c) Cycle testing of photocatalytic activity of Ag-(PEI)2(PW12)2 film. Reproduced from ref. [29].
![Figure 9
(a) LBL self-assembly preparation of composite fabric. (b) Mechanism scheme of LBL self-assembly to prepare composite fabric. Reproduced from ref. [30].](/document/doi/10.1515/ntrev-2023-0199/asset/graphic/j_ntrev-2023-0199_fig_009.jpg)
(a) LBL self-assembly preparation of composite fabric. (b) Mechanism scheme of LBL self-assembly to prepare composite fabric. Reproduced from ref. [30].
In a word, LBL assembly technology offers straightforward equipment and operation, along with a wide range of driving forces (such as electrostatic force, hydrogen bond, coordination, covalent, and other secondary forces) for device formation. Besides, there is an abundant of substances available for film formation including POMs, carbon nanotubes [32,33], proteins [34], etc. Additionally, the size and shape of the substrate do not impose limitations on these formations; furthermore, precise control over composition and structure of the film at the nanometer level can be achieved. The resulting films exhibit stable mechanical and chemical properties. However, it should be noted that various factors affecting film stability need to be considered in LBL technology, such as solvent type, ionic strength, solution concentration, temperature, and pH value of the system, making it necessary to continuously improve and study this technique. In fact, there are other methods in this field, such as sol–gel method [35], dry-jet wet spinning [36], deposition method [37], blending method [38–40], and covalent binding method [41,42]. Among them, deposition method and blending method are applied in many fields.
3 Applications
3.1 Catalysis
Based on their inherent redox activity, POMs can be employed for a wide range of storage and transfer electrons. The remarkable stability of POMs, coupled with their versatile synthetic tunability, renders them highly attractive for investigating charge during light absorption. These general features of POMs, combined with alterable acid-base properties, make them valuable catalysts. Fiber materials have gained significant attention across various fields due to their unique nanosized structures with exceptional flexibility features. In recent years, researchers have begun exploiting the synergistic effects between POMs and fiber materials by developing a range of composite materials to address the pressing global energy challenges. Currently, research efforts focusing on POM-functionalized fiber materials primarily revolve around oxidation catalysis, photocatalysis, and electrocatalysis.
3.1.1 Oxidation catalysis
Oxidation is one of the most important chemical processes, often requiring catalysts. POMs are preponderant catalysts due to their noteworthy redox properties, strong persistence against oxidants, and environmental compatibility. Meanwhile, POM anions with high electron density can donate electrons to other acceptors, while accepting electrons into their vacant orbitals. Thus, POMs exhibit unique redox stability and reversibility. When reduced, POMs can be re-oxidized back to their original state by oxides such as O2. In 2011, Cavaco-Paulo et al. found that the laccase-POM system (LMS) could effectively catalyze the oxidative polymerization of catechol for in situ dyeing of linen fabric with improved dyeing and washable properties [43] (Figure 10). Inspired by this work, our group reported the one-step one-bath method for in situ dyeing of cotton fabric using MnV13-catalyzed catechol polymerization [44]. Through comprehensive analysis of various factors, it was found that the increase of POM concentration led to continuous enhancement in dyeing depth on treated cotton fabric while minimizing color loss during friction. The results indicated that POMs promoted the fixation level of the colorant.
![Figure 10
(a) UV–Vis spectra of single reaction solutions monitored with acetate buffer. (b) UV–Vis spectra of the oxidized solutions after incubation at 50°C for 2 h. (c) Mechanism of catechol polymerization in LMS. Reproduced from ref. [43].](/document/doi/10.1515/ntrev-2023-0199/asset/graphic/j_ntrev-2023-0199_fig_010.jpg)
(a) UV–Vis spectra of single reaction solutions monitored with acetate buffer. (b) UV–Vis spectra of the oxidized solutions after incubation at 50°C for 2 h. (c) Mechanism of catechol polymerization in LMS. Reproduced from ref. [43].
In 2013, Dong et al. reported a new catalytic membrane composed of PV2Mo10 attached to a PVDF hollow fiber membrane [36]. This membrane exhibited efficient oxidation and degradation of phenol in wastewater at room temperature using air as a green oxidant. Notably, the catalytic degradation efficiency was improved by reducing the air pressure above the water surface. In 2016, Allen et al. reported a CA/PEO (polyethylene oxide) nanofiber membrane grafted with PV2Mo10 [45]. The grafted nanofiber membrane demonstrated remarkable capability for oxidizing and degrading organophosphorus or other toxic substances. The reduced POMs were oxidized to the original state by any oxides such as O2 in the air. Therefore, the cellulose POM-grafted membrane exhibited the self-decontamination ability for many cycles.
As known, Ru-based compounds are considered promising oxidation catalysts due to their unique redox ability. POMs with more than one Ru atom generally have better catalytic oxidation performance than those with only one Ru atom, facilitating the formation of peroxy or oxygen complex and promoting selective oxidation. In 2016, Gamelas et al. reported a cellulose fiber/silica material loaded with Ru-POM for the decomposition of formaldehyde through its oxidation catalytic capacity of Ru-POM [46].
Compared to traditional hydrodesulfurization methods, oxidative desulfurization is particularly appealing due to its mild conditions, high desulfurization rate, and simple process. POMs are ideal candidates to address this challenge as their highly redox-active metals. In 2019, Zhao et al. synthesized Co2+-PMo12-based MOF-199 [47], which was then loaded on the CA fiber for oxidative desulfurization. Due to the large pore size of CA fiber, the specific surface area of POMs in polar reaction system can be increased while its solubility is reduced. Besides, the catalytic oxidation desulfurization effect was better than that of Co2+-PMo12/MOF. Subsequently, Zhao et al. synthesized a new type of inorganic-organic hybrid PMo6W6@MOF@CA fiber [48]. The oxidative desulfurization efficiency achieved 99.23% at 313 K with O2 as oxidant. And the catalyst was easily recovered after drying at 323 K and reused more than 10 times.
3.1.2 Photocatalysis
POMs exhibit oxygen pπ to metal dπ, ligand to metal charge transfer (LMCT) transitions with absorbance maximum in the UV spectral region between 200 and 400 nm. Exciting into the LMCT, the resulting CT excited state consists of a reduced metal center and cation radical at the oxygen sites. In general, the reactivity of cation radicals is regarded to be the origin of the photocatalytic ability of the POMs. Specifically, there are two main mechanisms of POMs as a photocatalyst: (1) POMs have a band gap similar to the valence band and the conduction band in semiconductors. Under UV irradiation, the electrons on O 2p in POMs transferred to transition metal M 5d orbital transition, corresponding to photogenerated holes and photoelectrons. (2) Under light irradiation, POM LMCT excited states can result in photocatalysis of substrate through direct electron transfer or electron coupled atom transfer, thus leading to reduction of POM.
TiO2, being the earliest semiconductor material in photocatalysis, imposes limitations on the utilization of visible light due to its inherent properties. The short lifetime, low mobility, and easy recombination of photogenerated carriers produced by TiO2 necessitate a deeper understanding of sensitizer photophysics. POMs serve as excellent electron acceptors, effectively suppressing the recombination of photogenerated carriers and facilitating their separation and migration by capturing photogenerated electrons on the semiconductorduction band. Therefore, combining with POMs provides a feasible method to address the inherent limitations of TiO2, thereby enhancing its overall photocatalytic performance. Due to the wide band gap of POMs and TiO2, the POMs-TiO2 system can only generate an optical response in the near ultraviolet region. For example, Guo et al. have designed a new type of photoreactor consisting of Ag/PW12/TiO2 film-coated optical fiber bundles [49]. The surface plasmon resonance effect of Ag nanoparticles was used to broaden the spectral absorption of TiO2 and effectively inhibited the recombination of photogenerated electron-hole. Because of the synergetic photocatalysis effect between POMs and TiO2 as well as the surface plasmon resonance effect of Ag, the photocatalytic effect of optical fiber-photocatalyst film was more excellent. The results showed that the combination of fiber and Ag/PW12/TiO2 film enhanced the photocatalytic degradation of RhB and 4-NP in aqueous solution under natural light irradiation (Figure 11). Wang et al. then prepared a new type of PMo12-TiO2 composite nanofiber loaded with Ag-NPs [50]. The photocatalytic experiment revealed that the PMo12/TiO2/Ag catalyst showed high efficiency and stable activity for the photodegradation of methyl orange under visible light (λ > 420 nm). In the process of photocatalysis, PMo12 was reduced to heteropoly blue undergoing light irradiation, which enhanced the visible light absorption of PMo12/TiO2/Ag composite material and significantly increased the density of photogenerated carriers. At the same time, the loaded Ag-NPs increased the photogenerated electron-hole separation, thus improving the photocatalytic activity of PMo12/TiO2/Ag composites. In 2021, Mahmoodi et al. reported a mixed matrix membrane formed by PMo12/TiO2/SiO2 nanofibers and PVDF, showing excellent photocatalytic degradation activity for methylene blue and humic acid. In the report, PMo12 was used to eliminate photocatalytic defects in TiO2 [24].
![Figure 11
(a) Ag/H3PW12O40/TiO2-coated optical fiber photoreactor system. Photocatalytic activity of various fibers toward the degradation of RhB (b and c) and 4-NP (d and e). Reproduced from ref. [49].](/document/doi/10.1515/ntrev-2023-0199/asset/graphic/j_ntrev-2023-0199_fig_011.jpg)
(a) Ag/H3PW12O40/TiO2-coated optical fiber photoreactor system. Photocatalytic activity of various fibers toward the degradation of RhB (b and c) and 4-NP (d and e). Reproduced from ref. [49].
Driven by the HOMO-LUMO orbitals in POM, analogous to photogenerated electron–hole pairs, POMs are employed independently for photocatalysis. In 2014, Meng et al. have studied a new green catalytic membrane grafted polyionic liquid PVBMC onto polypropylene (PP) non-woven fabric and was further combined with PMo12 [51]. In such a light-driven system, the membrane exhibited excellent catalytic performance for acid orange 7 with a degradation rate of 95% under the irradiation of two 55 W fluorescent lamps. Besides, the catalyst showed good reusability and stability. In 2017, Zhou et al. reported multilayer catalytic films formed by PW12, PEI, and Ag-NPs deposited onto PAA/PVA/PW12 nanofiber substrates [29]. Experiments have shown that the photocatalytic activity of negatively charged surface (PEI) n (PW12) n on MB was higher than that of positively charged surface (PEI) n (PW12) n−1. Because negatively charged (PEI) n (PW12) n films can not only absorb positively charged MB due to strong electrostatic attraction but also improve the degradation rate of MB solution owing to the top layer PW12 as a photocatalyst. PW12 was always covered by PEI as the number of layer (PEI-PW12 as a layer) increased, which reduced the photocatalytic activity of the catalyst. By comparison, (PEI)2(PW12)2 had the best catalytic effect. Nevertheless, the photocatalytic activity of (PEI)2(PW12)2 film was still not excellent. To further enhance electron transfer and improve MB degradation rate, Ag-NPs were deposited on (PEI)2(PW12)2. Apparently, this is a catalysis-promoting process and is advantageous to photocatalytic activity, because Ag-NPs can effectively capture photoelectrons, and the double-layer structure is helpful in interfacial charge transfer. Therefore, the photocatalytic activity of Ag-(PEI) n (PW12) n film is much higher than that of (PEI) n (PW12) n film.
In terms of composite material, the combination of POMs with fiber supports provides POMs with much larger surface areas, which may increase their catalytic activities by providing large contact areas between the catalysts and substrates for the surface-mediated electron-transfer reactions. For example, activated carbon fiber (ACF) as a photosensitizer can improve the light response of POMs in the visible light region. Besides, ACF exhibits high conductivity and facilitates efficient transmission of photogenerated electrons to prevent recombination with photogenerated holes. Additionally, its large surface area and exceptional absorption capabilities make it an ideal carrier for composite preparation. Due to the presence of a large number of active groups on ACF surface, stable composite materials can be formed with POMs. In 2015, Xu et al. prepared ZnSiW11NB/ACF composite materials [40]. Contrast the photocatalytic activity of ZnSiW11NB with ZnSiW11NB/ACF on the degradation of RhB in aqueous solution, it was found that excess ACF could promote the recombination of photo-generated electron–hole pairs and reduced the photocatalytic efficiency. When ZnSiW11NB:ACF was 100:1, the photogenerated electron–hole pairs was separated effectively and had excellent catalytic activity for RhB degradation. The enhancement of photocatalytic activity was attributed to the synergistic effect between ZnSiW11NB and ACF (Figure 12).
![Figure 12
(a) Ball-and-stick of ZnSiW11. (b) SEM of ZnSiW11. (c) Tauc plots of ZnSiW11NB/ACF. (d) photocurrent spectra of ZnSiW11NB and ZnSiW11NB/ACF under visible light. (e) IPCE of ZnSiW11NB and ZnSiW11NB/ACF; (f) EIS of ZnSiW11NB and ZnSiW11NB/ACF. Reproduced from ref. [40].](/document/doi/10.1515/ntrev-2023-0199/asset/graphic/j_ntrev-2023-0199_fig_012.jpg)
(a) Ball-and-stick of ZnSiW11. (b) SEM of ZnSiW11. (c) Tauc plots of ZnSiW11NB/ACF. (d) photocurrent spectra of ZnSiW11NB and ZnSiW11NB/ACF under visible light. (e) IPCE of ZnSiW11NB and ZnSiW11NB/ACF; (f) EIS of ZnSiW11NB and ZnSiW11NB/ACF. Reproduced from ref. [40].
3.1.3 Electrocatalysis
Owing to the high oxidation states of metal in metal-oxygen polyhedra of MO x , POM anions undergo several rapid one- or two-electron reversible reductions, and even irreversible multi-electron reductions accompanied by decomposition. In 2012, Gong et al. reported an ITO electrode on which PVA/P2W18 covered through electrospinning [18]. After thermal crosslinking, P2W18 composite nanofibers would not dissolve in aqueous solution even after 24 h. It ensured the electrochemical stability of P2W18 composite nanofibers modified ITO electrode and its good electrocatalytic activity for nitrite reduction in acidic solution.
However, the properties of POMs used for loading, such as electrical conductivity, also have a certain impact on the electrocatalytic performance of modified electrodes. In 2006, Dong et al. reported modified ITO electrodes, which were formed by depositing P2Mo18/PAH multilayer films on PVA nanofibers. However, the poor conductivity of PVA nanofibers resulted in a low peak current of P2Mo18, which limited the application of modified electrodes [28]. To increase the conductivity, subsequently, Gong et al. combined P2W18 with chitosan nanofibers to modify the ITO electrode and the composite electrode, exhibiting excellent electrocatalytic reduction of NO2‒ [52]. As known, chitosan has a high positive charge density, providing support for the adsorption of P2W18 ions. Notice that chitosan is stable in the common potential range, and the complex formed with chitosan can ensure high proton conductivity. Thus, the electrocatalytic activity of cationic polyelectrolyte chitosan nanofibers is better than that of the modified electrode. In addition, in 2018, Anderson-type CoMo6 had been applied to produce vertically arranged oxidation CoS2-MoS2 (CoMoS) hetero-film on conductive and flexible carbon fiber cloth [53]. Besides, the O-CoMoS hetero-film was used as anode and cathode for the electrocatalyse water decomposition. Under 10 mA current density, only 1.6 V of low voltage was needed for 10 h catalysis, with high electrocatalytic decomposition water activity (Figure 13).
![Figure 13
(a) Synthesis of as-prepared heteronanosheets arrays grown on carbon fiber cloth and overall water splitting. (b) HER polarization curves of metal sulfides nanosheet arrays. (c) Tafel slopes of O-CoMoS, O-NiMoS, and O-FeMoS arrays. Reproduced from ref. [53].](/document/doi/10.1515/ntrev-2023-0199/asset/graphic/j_ntrev-2023-0199_fig_013.jpg)
(a) Synthesis of as-prepared heteronanosheets arrays grown on carbon fiber cloth and overall water splitting. (b) HER polarization curves of metal sulfides nanosheet arrays. (c) Tafel slopes of O-CoMoS, O-NiMoS, and O-FeMoS arrays. Reproduced from ref. [53].
POMs can also improve the reaction performance of hydrogen evolution and oxygen evolution of electrolytic water. In 2019, Wu et al. prepared carbon nanofibers (SiWNi-CNFs) modified by SiO2/WO3/NiWO4 composites. The experimental results showed that SiWNi-CNFs haved more efficient electrocatalytic hydrogen evolution (HER) performance [23]. Because the SiO2/WO3/NiWO4 composites modified on the surface of carbon nanofibers can produce a large number of active centers through the synergistic action of each component. Meanwhile, the close combination of ternary oxides and carbon nanofibers can accelerate the electron transfer of active components in carbonization and improve the stability of active components to prevent them from gathering. In 2022, Haider et al. loaded Co containing POMs Co4POM onto carbon nanofiber tubes (CNTFs) generating Co4POM@CNTF, which could be used for electrocatalytic oxygen evolution reaction [39]. CNTF is an excellent electronic medium and highly conductive carrier with efficient electron transfer and high electrochemical activity. Co4POM can be used as the catalytic site of OER through self-activation to produce cobalt oxide or hydroxide in alkaline medium.
3.2 Photochromism
Photochromism refers to the change of molecular structure under the irradiation of visible light, ultraviolet light, infrared light, etc., leading to the corresponding color change. Besides, most of these changes are obvious, rapid, and generally reversible. In general, photochromic materials can be classified into two categories, inorganic and organic photochromic materials. The synthesis of organic photochromic materials is typically challenging, expensive, high toxicity, and poor light fatigue resistance. In contrast, inorganic photochromic materials such as POMs can address the limitations associated with organic counterparts by virtue of their reversible redox-based photochromic mechanism. The photochromic mechanism of POMs is as follows. Due to the photoexcitation, LMCT results in the transfer of a hydrogen proton from the nitrogen of the ligand to the bridge oxygen atom of POMs. Meanwhile, interaction between O → W LMCT transfer and nonbond electrons on the nitrogen site forms a charge transfer complex (N → O), leaving a hole at the oxygen site. Owing to the transfer of hydrogen protons, the hexavalent W or Mo is reduced to pentavalent. The bands in the visible or near-infrared regions are mainly derived from the intervalence charge transfer transition filled 5 d1 electrons and d–d transitions. Meanwhile, there may be occurrence of delocalized electron on N⋯O bond or radical on amine molecule along with an unpaired electron on metal atom. Moreover, fading of colored POMs is caused by the reverse reaction.
The photochromic property of POMs enables them to be used as photoresponsive switches. Especially, the research and development of photoresponsive intelligent textiles is expected to develop low-cost and practical photochromic textiles. In 2005, Gong et al. prepared PVA/SiW12 microfine composite fiber [54]. Under ultraviolet light, the electron transfer occured between PVA and SiW12. SiW12 was reduced through single-electron reaction while PVA was oxidized. The color of the fiber changed from white to blue, and the color faded without light. In 2006, Dong et al. made photochromic films by depositing P2Mo18/PAH on PVA nanofibers [28]. In the presence of UV light, P2Mo18 was reduced, that is, Mo(vi) was reduced to Mo(v), and charge transfer complex was formed between PAH and P2Mo18, producing the color of nanofibers gradually changing from light yellow to dark blue.
In 2018, Freire et al. introduced the prepared SiO2@C18-PMo12-NPs hybrid nanomaterials into PP fiber to prepare a new type of photoresponsive PP fiber. Under UV light, Mo(vi) was reduced to Mo(v), and the corresponding fiber color changed from the initial yellow-green to blue after 30 min irradiation. After oxygen bleaching, Mo(v) was oxidized to Mo(vi) again, and the color was changed from blue to yellow-green [55]. In 2020, Yu et al. obtained a composite fabric by loading (DTS/PEI) n onto linen fabric through hydrogen bond [30]. Under UV irradiation, the electron on the amino group of PEI was transferred to W6+ through hydrogen bond. Correspondingly, colorless W6+ was reduced to blue W5+. The composite fabric showed rapid and obvious color changes in 5 min, and it would be oxidized and faded exposure without UV light for 5 h. Meanwhile, the composite fabric showed excellent stability. After 15 times of reduction–oxidation process, the color proportion of the material had only decreased by about 10%, indicating good resistance to optical fatigue of composite fabric. In the same year, an analog with cotton fabric was reported by them [30]. With the enhancement of UV irradiation, the conversion rate of W6+ increased, so did the photochromic speed of the fabric. Therefore, the composite fabric could be used for visual and rapid detection of different UV intensity. Likewise, the blue composite fabric could be oxidized and faded in the air for 2 h. In 2022, our group obtained the composite fabric with DTS and cationic-modified cotton fabric [56]. Under low-power UV light (5 W) irradiation, the composite fabric would produce color difference changes (white gradually turns to blue) within 5 s, showing fast light response. When the UV light was removed, the blue color could fade. Besides, as the increase of temperature, the fading time was shortened. In addition, the composite fabric after chemical modification still maintained its original moisture absorption and mechanical properties (Figure 14).
![Figure 14
(a) Cyclability of the composite fabric. (b) Mechanism of photochromism. (c) Simple model showing the electronic transitions in the POMs containing electron donor and acceptor. (d) Schematic diagram of the color change of the composite fabric. Reproduced from ref. [56].](/document/doi/10.1515/ntrev-2023-0199/asset/graphic/j_ntrev-2023-0199_fig_014.jpg)
(a) Cyclability of the composite fabric. (b) Mechanism of photochromism. (c) Simple model showing the electronic transitions in the POMs containing electron donor and acceptor. (d) Schematic diagram of the color change of the composite fabric. Reproduced from ref. [56].
3.3 Photoluminescence
Photoluminescence, is the phenomenon of emitting energy in the form of light radiation after absorbing energy, and is commonly. The property makes it suitable for applications in light-emitting sensors. At present, extensive research has been conducted on photoluminescence properties of lanthanide-based POMs. Therefore, combining lanthanide POMs with the fibers enables the development of flexible composite materials with fluorescence properties.
In 2009, Gong et al. prepared EuPW11/PVA and EuW10/PVA photoluminescent composite microfiber pads [17]. They found that different polyoxotungstate ligands exhibited different luminescence behaviors in composite microfibers. In 2013, Song et al. developed EuW10/PAN nanofiber composite membranes [20]. Upon light stimulation, the nanofiber films showed strong Eu3+ red light emission and demonstrated sensitivity toward acidic and alkaline gases. In the presence of HCl and H2S acid gas, ‒C≡N‒H…O‒W could be formed between the −CN of PAN and the O atom of WO6 in EuW10/PAN nanofiber membrane. The hydrogen bonding prevented the energy transfer of the intramolecular luminescence resonance between WO6 and Eu3+ ions, therefore hindering sufficient energy release to activate photoluminescence in acid gas environments. Under SO2 atmosphere, SO2 reacted with H2O to produce weak electrolyte H2SO3, which ionized into H+ and HSO3‒, thus resulting in the protonation of −CN in PAN and hindering the energy transfer mentioned above. Therefore, photochromism did not occur. However, when the EuW10/PAN nanofiber membrane was exposed to alkaline gas such as NH3 again after exposure to acid gas, the photoluminescence phenomenon was restored because of complete deprotonation of −CN groups. Besides, the relative luminescence intensity was even stronger than that of the original membrane. Therefore, owing to its high sensitivity and adjustable luminescence signal, EuW10/PAN nanofiber membrane could be used as a pH-responsive luminescence switch.
Additionally, owing to the strong absorption in the range of 200–400 nm, POMs endow a new functionality on fiber. Recently, we reported an anti-UV blocker compatible with cotton fabric by simple covalent bond self-assembly [57]. The as-designed composite fabric showed excellent UV blocking property with a UPF value of 397.19, superior to original cotton (7.09).
4 Conclusion and outlook
To summarize, the design, synthesis, and reactivity of novel POM/fiber composite materials based on two methods, namely, electrostatic spinning method and LBL self-assembly, have been discussed in detail. Apparently, the structure and performance of composite materials are improved by combining the superiority between POMs and fibers. Furthermore, this study highlights various applications such as catalysts, luminous sensors, and health monitoring. Fiber materials based on POMs possess several unique advantages: first, POMs with diverse architectures facilitate the structural regulation of composite materials for achieving different characteristics; second, the excellent coordination ability of POMs enables the combination with other functional molecules; and third, POMs are of great importance in enhancing the analysis of catalytic mechanism and photoelectric property theory.
There is no doubt that POM/fiber composite materials exhibit remarkable diversity and their unique properties led to new discoveries. Although there has been considerable progress in POMs functionalized fiber materials, there remain several crucial challenges that need to be addressed, and the following points may need to be focused on: 1) overcoming the limitations of POMs with stable structures and exceptional performance by developing new POMs with simplified synthesis processes, cost-effectiveness, and superior properties. Similarly, the synthesis and design of fibers with specialized characteristics contribute to the preparation of functional devices; 2) in terms of composite material, electrostatic spinning method, and LBL are employed. Electrostatic spinning needs some special equipment, and operating conditions, such as humidity of the air, spinning voltage, and solvents, which can affect the effect of spinning and stability of POMs. The LBL self-assembly method mainly relies on electrostatic interaction; therefore, it is necessary to enhance the durability of constructed functional fiber materials while also exploring novel theories and solutions. Therefore, it is necessary to comprehensively consider the use of functional fiber materials and to select the appropriate POMs and fabric type. Besides, it is important to solve the problem of combining POMs with the base material, so as to ensure the robustness and persistence of materials; 3) fiber or textiles possess softness and breathability as inherent characteristics which may be affected during the process of functionalization. Therefore, when developing POM-functionalized fiber materials, how to maintain the original flexibility and air permeability of fiber under the premise of ensuring excellent performance? Or whether other processes such as dyeing and functionalization of fibers interacting with each other should be considered.
Acknowledgments
The authors would like to express their heartfelt gratitude to all the individuals for their expertise throughout all aspects of our study and contribution to writing the manuscript. The authors are truly grateful to all of you.
-
Funding information: The authors gratefully acknowledge the financial support from the horizontal cooperation project of Nantong University (22ZH660).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Tong Y, Liu J, Wang L, Su B-J, Wu K-H, Juang J-Y, et al. Carbon-shielded single-atom alloy material family for multi-functional electrocatalysis. Adv Funct Mater. 2022;32:22056.10.1002/adfm.202205654Suche in Google Scholar
[2] Li R, Li N, Hou J, Yu Y, Liang L, Yan B, et al. Aquatic environment remediation by atomic layer deposition-based multi functional materials: A review. J Hazard Mater. 2021;402:123513.10.1016/j.jhazmat.2020.123513Suche in Google Scholar PubMed
[3] Deng N, Li Y, Li Q, Zeng Q, Luo S, Wang H, et al. Multi-functional yolk-shell structured materials and their applications for high-performance lithium ion battery and lithium sulfur battery. Energy Storage Mater. 2022;53:684–743.10.1016/j.ensm.2022.08.003Suche in Google Scholar
[4] Wen Y, Jian M, Huang J, Luo J, Qian L, Zhang J. Carbonene fibers: toward next-generation fiber materials. Nano Lett. 2022;22:6035–47.10.1021/acs.nanolett.1c04878Suche in Google Scholar PubMed
[5] Chen C, Feng J, Li J, Guo Y, Shi X, Peng H. Functional fiber materials to smart fiber devices. Chem Rev. 2023;123:613–62.10.1021/acs.chemrev.2c00192Suche in Google Scholar PubMed
[6] Liu J-X, Zhang X-B, Li Y-L, Huang S-L, Yang G-Y. Polyoxometalate functionalized architectures. Coord Chem Rev. 2020;414:213260.10.1016/j.ccr.2020.213260Suche in Google Scholar
[7] Yang Z-X, Gong F, Lin D, Huo Y. Recent advances in polyoxometalate-based single-molecule magnets. Coord Chem Rev. 2023;492:215205.10.1016/j.ccr.2023.215205Suche in Google Scholar
[8] Li H, Zheng L, Lu Q, Li Z, Wang X. A monolayer crystalline covalent network of polyoxometalate clusters. Sci Adv. 2023;9:eadi6595.10.1126/sciadv.adi6595Suche in Google Scholar PubMed PubMed Central
[9] Han Y, Lan J, Li K, Yang L, Zhu C, Chen J. The cluster design and redox behavior characterization of polyoxometalates for redox flow batteries. Chem-Asian J. 2022;17:e202200950.10.1002/asia.202200950Suche in Google Scholar PubMed
[10] Song P, Wang T. Application of polyoxometalates in chemiresistive gas sensors: a review. ACS Sens. 2022;7:3634–43.10.1021/acssensors.2c02341Suche in Google Scholar PubMed
[11] Najafi M. Polyoxometalate-based inorganic-organic hybrids as heterogeneous catalysts for asymmetric and tandem reactions. ChemCatChem. 2023;15:e202201045.10.1002/cctc.202201045Suche in Google Scholar
[12] Carraro M, Gross S. Hybrid materials based on the embedding of organically modified transition metal oxoclusters or polyoxometalates into polymers for functional applications: A review. Materials. 2014;7:3956–89.10.3390/ma7053956Suche in Google Scholar PubMed PubMed Central
[13] Snider VG, Hill CL. Functionalized reactive polymers for the removal of chemical warfare agents: A review. J Hazard Mater. 2023;442:130015.10.1016/j.jhazmat.2022.130015Suche in Google Scholar PubMed
[14] Zhang C-L, Yu S-H. Spraying functional fibres by electrospinning. Mater Horiz. 2016;3:266–9.10.1039/C6MH00045BSuche in Google Scholar
[15] Gong J, Shao C, Pan Y, Gao F, Qu L. Preparation, characterization and swelling behavior of H3PW12O40/poly(vinyl alcohol) fiber aggregates produced by an electrospinning method. Mater Chem Phys. 2004;86:156–60.10.1016/j.matchemphys.2004.02.007Suche in Google Scholar
[16] Yin R, Guan X-H, Gong J, Qu L-Y. Evaluation of swelling capacity of poly(vinyl alcohol) fibrous mats dealt with polyoxometalate containing vanadium. J Appl Polym Sci. 2007;106:1677–82.10.1002/app.26801Suche in Google Scholar
[17] Sun Y, Shan Y, Cui X, Li B, Gong J, Su Z, et al. Investigation on fluorescence properties of ultrafifine PVA fiber mats-contained polyoxometalate with different molecular structure. J Appl Polym Sci. 2009;113:1369–74.10.1002/app.30086Suche in Google Scholar
[18] Cao F, Guo S, Ma H, Gong J. ITO electrode modified by α-K6[P2W18O62] hybrid nanofibers for nitrite determination. Electroanalysis. 2012;24:418–24.10.1002/elan.201100613Suche in Google Scholar
[19] Yu H, Peng J, Guan H, Xin Z, Pan Y, Gong J, et al. Electrospinning preparation and characterization of polyoxometalate fibers. Chin Sci Bull. 2006;51:906–10.10.1007/s11434-006-0906-xSuche in Google Scholar
[20] Wang X, Wang J, Tsunashima R, Pan K, Cao B, Song Y-F. Electrospun self-supporting nanocomposite films of Na9[EuW10O36]·32H2O/PAN as pH-modulated luminescent switch. Ind Eng Chem Res. 2013;52:2598–603.10.1021/ie302712sSuche in Google Scholar
[21] Zhang X, Lv R, Chen L, Sun R, Zhang Y, Sheng R, et al. A multifunctional janus electrospun nanofiber dressing with biofluid draining, monitoring, and antibacterial properties for wound healing. ACS Appl Mater Interfaces. 2022;14:12984–3000.10.1021/acsami.1c22629Suche in Google Scholar PubMed
[22] Lu X, Liu X, Wang L, Zhang W, Wang C. Fabrication of luminescent hybrid fibres based on the encapsulation of polyoxometalate into polymer matrices. Nanotechnology. 2006;17:3048–53.10.1088/0957-4484/17/12/039Suche in Google Scholar
[23] Zhu M, Sun J, Li C, Han C, Shan Y, He J, et al. Electrospun SiO2/WO3/NiWO4 decorated carbon nanofibers for an efficient electrocatalytic hydrogen evolution. Fullerenes Nanotubes Carbon Nanostruct. 2019;27:506–13.10.1080/1536383X.2019.1609950Suche in Google Scholar
[24] Samadi Mollayousefi H, Fallah Shojaei A, Mahmoodi NA. Preparation, characterization, and performance study of PVDF nanocomposite contained hybrid nanostructure TiO2-POM used as a photocatalytic membrane. Iran J Chem Chem Eng. 2021;40:35–47.Suche in Google Scholar
[25] Jung D, Su S, Syed ZH, Atilgan A, Wang X, Sha F, et al. A catalytically accessible polyoxometalate in a porous fiber for degradation of a mustard gas simulant. ACS Appl Mater Inter. 2022;14:16687–93.10.1021/acsami.2c01584Suche in Google Scholar PubMed
[26] Banitaba SN, Semnani D, Heydari-Soureshjani E, Rezaei B, Ensafi AA, Taghipour-Jahromi A. Novel electrospun polymer electrolytes incorporated with Keggin-type hetero polyoxometalate fillers as solvent-free electrolytes for lithium ion batteries. Polym Int. 2020;69:675–87.10.1002/pi.6001Suche in Google Scholar
[27] Ding B, Gong J, Kim J, Shiratori S. Polyoxometalate nanotubes from layer-by-layer coating and thermal removal of electrospun nanofibres. Nanotechnology. 2005;16:785–90.10.1088/0957-4484/16/6/028Suche in Google Scholar
[28] Yang G, Gong H, Yang R, Guo H, Wang Y, Liu B, et al. Modification of electrode surface through electrospinning followed by self-assembly multilayer film of polyoxometalate and its photochromic. Electrochem Commum. 2006;8:790–6.10.1016/j.elecom.2006.03.019Suche in Google Scholar
[29] Sui C, Wang C, Wang Z, Xu Y, Gong E, Cheng T, et al. Different coating on electrospun nanofiber via layer-by-layer self-assembly for their photocatalytic activities. Colloid Surf A. 2017;529:425–33.10.1016/j.colsurfa.2017.06.030Suche in Google Scholar
[30] Bao B, Fan J, Wang W, Yu D. Novel linen/polyethyleneimine/sodium decadecanate photochromic fabric prepared by layer-by-layer self-assembly method. Cellulose. 2020;27:6591–602.10.1007/s10570-020-03234-2Suche in Google Scholar
[31] Bao B, Fan J, Wang Z, Wang Y, Wang W, Qin X, et al. Sodium deca-tungstate/polyacrylic acid self-assembled flexible wearable photochromic composite fabric for solar UV detector. Compos Part B-Eng. 2020;202:108464.10.1016/j.compositesb.2020.108464Suche in Google Scholar
[32] Shim BS, Podsiadlo P, Lilly DG, Agarwal A, Lee J, Tang Z, et al. Nanostructured thin films made by dewetting method of layer-by-layer assembly. Nano Lett. 2007;7:3266–73.10.1021/nl071245dSuche in Google Scholar PubMed
[33] Roh S-H. Layer-by-layer self-assembled carbon nanotube electrode for microbial fuel cells application. J Nanosci Nanotechnol. 2013;13:4158–61.10.1166/jnn.2013.7021Suche in Google Scholar PubMed
[34] Temmerman M-LD, Demeester J, Vos FD, Smedt SD. Encapsulation performance of layer-by-layer microcapsules for proteins. Biomacromolecules. 2011;12:1283–9.10.1021/bm101559wSuche in Google Scholar PubMed
[35] Naddaf E, Ebrahimi M, Es’haghi Z, Bamoharram FF. Application of carbon nanotubes modified with a Keggin polyoxometalate as a new sorbent for the hollow-fiber micro-solid-phase extraction of trace naproxen in hair samples with fluorescence spectrophotometry using factorial experimental design. J Sep Sci. 2015;38:2348–56.10.1002/jssc.201401459Suche in Google Scholar PubMed
[36] Yao L, Zhang L-Z, Wang R, Loh CH, Dong Z-L. Fabrication of catalytic membrane contactors based on polyoxometalates and polyvinylidene fluoride intended for degrading phenol in wastewater under mild conditions. Sep Purif Technol. 2013;118:162–9.10.1016/j.seppur.2013.06.029Suche in Google Scholar
[37] Xu L, Boring E, Hill CL. Polyoxometalate-modified fabrics: New catalytic materials for low-temperature aerobic oxidation. J Catal. 2000;195:394–405.10.1006/jcat.2000.3008Suche in Google Scholar
[38] Serenjeh FN, Hashemi P, Rasolzadeh F, Farhadi S, Hoseini A-A. Magnetic fiber headspace solid-phase microextraction of Ferulago angulata volatile components using Preyssler-type polyoxometalate/metal-organic framework/silica aerogel sorbent. Food Chem. 2022;373:131423.10.1016/j.foodchem.2021.131423Suche in Google Scholar PubMed
[39] Tariq I, Asghar MA, Ali A, Badshah A, Abbas SM, Iqbal W, et al. Surface reconstruction of cobalt-based polyoxometalate and CNT fiber composite for efficient oxygen evolution reaction. Catalysts. 2022;12:1242.10.3390/catal12101242Suche in Google Scholar
[40] Lu T, Xu X, Li H, Li Z, Zhang X, Ou J, et al. The loading of coordination complex modified polyoxometalate nanobelts on activated carbon fiber: A feasible strategy to obtain visible light active and highly efficient polyoxometalate based photocatalysts. Dalton Trans. 2015;44:2267–75.10.1039/C4DT03092CSuche in Google Scholar PubMed
[41] Lange LE, Obendorf SK. Functionalization of cotton fiber by partial etherification and self-assembly of polyoxometalate encapsulated in Cu3(BTC)2 metal-organic framework. ACS Appl Mater Interfaces. 2015;7:3974–80.10.1021/am506510qSuche in Google Scholar PubMed
[42] Yao L, Zhang L, Zhang Y, Wang R, Wongchitphimon S, Dong Z. Self-assembly of rare-earth Anderson polyoxometalates on the surface of imide polymeric hollow fiber membranes potentially for organic pollutant degradation. Sep Purif Technol. 2015;151:155–64.10.1016/j.seppur.2015.05.045Suche in Google Scholar
[43] Kim S, Silva C, Evtuguin DV, Gamelas JAF, Cavaco-Paulo A. Polyoxometalate/laccase-mediated oxidative polymerization of catechol for textile dyeing. Appl Microbiol Biot. 2011;89:981–7.10.1007/s00253-010-2932-5Suche in Google Scholar PubMed
[44] Liang Z, Peng J, Liang J, Song Y, Jia W, Mao Q. Polymerization of catechol employing polyoxovanadate as biomimetic models catalyze for textile dyeing. Fiber Polym. 2022;23:3380–5.10.1007/s12221-022-4671-5Suche in Google Scholar
[45] Allen NE, Obendorf SK, Fan J. Polyoxometalate (POM) grafted grooved nanofibrous membranes for improved self-Decontamination. RSC Adv. 2016;6:85985–93.10.1039/C6RA04456ESuche in Google Scholar
[46] Gamelas JAF, Oliveira F, Evtyugina MG, Portugal I, Evtuguin DV. Catalytic oxidation of formaldehyde by ruthenium multisubstituted tungstosilicic polyoxometalate supported on cellulose/silica hybrid. Appl Catal A-Gen. 2016;509:8–16.10.1016/j.apcata.2015.10.003Suche in Google Scholar
[47] Li S-W, Gao R-M, Zhao J. Different supports of modified heteropolyacid for ultra-deep oxidative desulfurization: a newly easy shaped catalyst and the DFT cluster model study. Fuel. 2019;237:840–50.10.1016/j.fuel.2018.10.061Suche in Google Scholar
[48] Li J, Yang Z, Hu G, Zhao J. Heteropolyacid supported MOF fibers for oxidative desulfurization of fuel. Chem Eng J. 2020;388:124325.10.1016/j.cej.2020.124325Suche in Google Scholar
[49] Zhang S, Chen L, Liu H, Guo W, Yang Y, Guo Y, et al. Design of H3PW12O40/TiO2 and Ag/H3PW12O40/TiO2 film-coated optical fiber photoreactor for the degradation of aqueous rhodamine B and 4-nitrophenol under simulated sunlight irradiation. Chem Eng J. 2012;200:300–9.10.1016/j.cej.2012.06.060Suche in Google Scholar
[50] Shi H, Yu Y, Zhang Y, Feng X, Zhao X, Tan H, et al. Polyoxometalate/TiO2/Ag composite nanofibers with enhanced photocatalytic performance under visible light. Appl Catal B-Environ. 2018;221:280–9.10.1016/j.apcatb.2017.09.027Suche in Google Scholar
[51] Ma S, Meng J, Li J, Zhang Y, Ni L. Synthesis of catalytic polypropylene membranes enabling visible-light-driven photocatalytic degradation of dyes in water. J Membr Sci. 2014;453:221–9.10.1016/j.memsci.2013.11.021Suche in Google Scholar
[52] Shan Y, Yang G, Jia Y, Gong J, Su Z, Qu L. ITO electrode modified with chitosan nanofibers loading polyoxometalate by one step self-assembly method and its electrocatalysis. Electrochem Commun. 2007;9:2224–8.10.1016/j.elecom.2007.06.028Suche in Google Scholar
[53] Hou J, Zhang B, Li Z, Cao S, Sun Y, Wu Y, et al. Vertically aligned oxygenated–CoS2–MoS2 heteronanosheet architecture from polyoxometalate for efficient and stable overall water splitting. ACS Catal. 2018;8:4612–21.10.1021/acscatal.8b00668Suche in Google Scholar
[54] Yang G, Pan Y, Gao F, Gong J, Cui X, Shao C, et al. A novel photochromic PVA fiber aggregates contained H4SiW12O40. Mater Lett. 2005;59:450–5.10.1016/j.matlet.2004.09.043Suche in Google Scholar
[55] Pinto TV, Fernandes DM, Guedes A, Cardoso N, Duraes NF, Silva C, et al. Photochromic polypropylene fibers based on UV-responsive silica@phosphomolybdate nanoparticles through melt spinning technology. Chem Eng J. 2018;350:856–66.10.1016/j.cej.2018.05.155Suche in Google Scholar
[56] Liang Z, Cheng H, Wang H, Zhong L, Mao Q, Zhang D. Obtaining reversible, durable, high-contrast photochromism and antibacterial properties in a flexible, wearable fiber using nanometer-sized polyoxotungstate. ACS Appl Nano Mater. 2022;5:19043–52.10.1021/acsanm.2c05008Suche in Google Scholar
[57] Cheng H, Zhong L, Jia W, Zhang X, Mao Q, Liang Z. In Situ Functionalization of cellulose with polyoxometalate for UV-resistant performance. J Nat Fibers. 2023;20:2137619.10.1080/15440478.2022.2137619Suche in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy
Artikel in diesem Heft
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy

