Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
-
Ashour M. Ahmed
, Mervat Nasr
, Shaymaa Sayed
Abstract
In this study, sodium titanate (ST)/iron oxide (Fe2O3) was successfully prepared as a novel binary photocatalyst for the first time to enhance the photocatalytic activity. The prepared photocatalyst was used in the photodegradation of methylene blue (MB) dye under sunlight and a tungsten lamp. The green synthesis method using orange peel extract was employed to prepare Fe2O3, while the hydrothermal method was used to synthesize ST. To achieve optimal photocatalytic efficiency, the loading of Fe2O3 onto ST was carefully controlled. The average crystallite size of ST, Fe2O3, and ST@Fe2O3 (with a 1:1 wt% ratio) was 999.8, 81.9, and 104 nm, respectively, using the Williamson–Hall (W–H) model. Optical analysis revealed that ST@Fe2O3 had a smaller direct bandgap (2.54 eV) compared to ST@0.3 Fe2O3 (2.70 eV) and ST@0.5 Fe2O3 (3.24 eV). The photodegradation of MB was analyzed considering the weight of the photocatalyst, the irradiation time, and the dye concentration. In-depth explanations of stability and kinetic models were also provided. Remarkably, the ST@Fe2O3 photocatalyst demonstrated superior performance compared to the other evaluated photocatalysts, completely degrading MB dye within just 60 min of solar light exposure. Incorporating Fe2O3 into ST effectively reduces the recombination of photo-produced electron/hole (e/h) pairs and broadens the response range of the solar spectrum. Based on these findings, ST@Fe2O3 appears to have a promising future as a practical photocatalyst for degrading various dye pollutants in wastewater.
Graphical abstract
1 Introduction
Organic dyes are considered one of the main sources of water pollution [1,2]. These dyes release harmful byproducts into wastewater, causing a multitude of issues and risks to plants, animals, and human life [3]. Methylene blue (MB) is used for various industrial purposes, such as dyeing cotton, coloring paper, and hair coloring agents [4]. However, this dye is highly carcinogenic and can stimulate the growth of malignant cells that lead to tumors [5]. Its presence in water can result in respiratory problems, vomiting, diarrhea, and gastritis infections, as well as damage to the ocular and nervous systems. Therefore, it is crucial to develop advanced methods to remove these hazardous pollutants from the water [6,7]. Recently, researchers have shown interest in using semiconductor-based photocatalytic reactions as a potential method for wastewater treatment [8,9]. In this regard, sodium titanate (ST) material has attracted more interest owing to its layered microstructures, high surface area, and quantum size effects. The presence of defects from sodium vacancies makes ST (Na2Ti3O7) p-type semiconductors [10]. It exhibits an absorption band in the ultraviolet (UV) region [11]. This absorption is due to the TiO6 octahedra and is related to the electronic shifts from the valence band (VB) to the conduction band (CB). Furthermore, ST possesses ion-exchange capabilities and photoelectric properties [12,13]. However, the pure ST (Na2Ti3O7) suffers from low absorption ability of light in the visible region [14]. Additionally, it has low electrical conductivity and poor structural stability, which further limits its photocatalytic activity [15,16]. Extensive research has been conducted to address these challenges and enhance the photoactivity of ST. One promising approach involves the creation of heterojunctions or nanocomposites, which can improve light absorption and facilitate the separation of photogenerated charges. The synergistic effects at the interfaces of these materials can contribute to more efficient utilization of sunlight and enhance the overall photocatalytic performance of ST [17,18]. For example, Iani et al. reported that the Na2Ti3O7/H2Ti3O7 heterojunction nanotube photocatalyst shows 96.4% degradation of Rhodamine B after 120 min [12]. Lei et al. reported that ST@GO exhibits the maximum photocatalytic efficiency for degrading MB (97.5%) after 90 min under UV radiation [13]. Mohanty et al. demonstrated that titanate nanotubes show 95% dye removal after 2 h [19]. Unfortunately, the performance of the photodegradation process in these works is still poor.
Iron oxide (Fe2O3; hematite) is a metal oxide with a small bandgap energy and high absorption of solar radiation in the visible range. Fe2O3 is an n-type semiconductor due to vacancies in the VB and a CB occupied by electrons [20]. Additionally, Fe2O3 is biocompatible, non-toxic, photo-stable, and low-cost [21]. However, the fast recombination rate of photogenerated electron–hole pairs makes it hard for Fe2O3 to break down photochemically. The development of photocatalysts employing plant materials, plant fruit waste, and plant extracts is the most recent environmentally friendly research trend [22]. By using aqueous orange peel extract, known for its strong reducing properties, Fe2O3 can be fabricated rapidly and in an environmentally friendly manner.
Based on the above survey, it can be expected that combining Fe2O3 with ST semiconductors can increase the efficiency of the photodegradation process of MB dye. This study introduces a novel approach by developing an ST@xFe2O3 photocatalyst for the first time. The photocatalyst was synthesized using a cost-effective method. Adjusting the ratios of ST and Fe2O3 successfully leads to improved light absorption and minimized recombination rates of the photo-generated electron/hole pairs, resulting in enhanced efficiency in the photodegradation process. The findings from this study hold promising implications for the design and development of ST@Fe2O3 photocatalysts in diverse environmental and industrial applications.
2 Experimental details
2.1 Materials
The chemicals used in this study were sourced from multiple suppliers. Ferric chloride hexahydrate (FeCl3·6H2O) with a purity of 99% and titanium dioxide (TiO2) with a purity of 99% were purchased from Sigma-Aldrich Company, USA. Sodium hydroxide (NaOH) with a purity of 96%, hydrochloric acid 36% (w/w) in aqueous solution, and ultra-high purity MB dye were obtained from Alfa Aesar Company, UK. All chemicals were utilized in their original form without undergoing any additional processing.
2.2 Chemicals and orange-peel extract preparation
Fresh orange peels that had just been picked were gathered, well cleaned with distilled water (DW), and then the peel was cut into little fragments. About 50 g of freshly orange peel was boiled in 250 mL of DW in a conical flask at 75°C for 25 min on a hot-plate device (Brandmart, Mienta-HP41425A). The filter paper (Ahlstrom-Munksjö Scientific, Sweden) was used to separate the hot material. The resulting extract from orange peels was used to create Fe2O3 photocatalysts.
2.3 Orange peel-based green synthesis of Fe2O3 photocatalyst
Fe2O3 photocatalysts were synthesized by the green method. Briefly, 5 mL of freshly prepared orange peel extract was mixed with 50 mL of ferric chloride (1 M) under constant stirring for 20 min at 55°C until the solution became brownish-colored as a result of the reduction. Drop by drop, 50 mL of NaOH (1 M) was added to the mixture. The color change from brownish to black serves as evidence that Fe2O3 photocatalysts are developing [23]. The solid pellets of Fe2O3 were obtained by centrifuging the colloidal solution at 3,000 rpm for 10 min in test tubes. After that, the solid pellets were washed several times with DW and then filtered. The yield of solid Fe2O3 was dried at 70°C in an oven for 10 h, followed by calcination at 550°C in a muffle furnace for 2 h.
2.4 Preparation of ST
The ST photocatalyst was prepared using an alkaline hydrothermal process. About 5 g of TiO2 photocatalyst was mixed with 250 mL of NaOH (10 M) and stirred vigorously for 40 min till a milky white suspension solution was formed. Then, the obtained suspension solution was placed in a 1 L autoclave and heated to 150°C for 1 day in a furnace for hydrothermal treatment. The autoclave cooled to normal temperature after the hydrothermal process was completed, and the subsequent white product was removed. To get rid of the unreacted NaOH, the resultant white precipitate was repeatedly filtered and washed with DW. Ultimately, the white photocatalyst generated was dried for 5 h at 80°C.
2.5 Synthesis of ST@Fe2O3 photocatalyst
The ST@Fe2O3 photocatalyst was fabricated by mixing different weight ratios of fabricated Fe2O3 with ST photocatalysts. Both these ingredients were initially mixed thoroughly for 30 min using a mortar and pestle. The mixed oxides were ultrasonically dispersed in 50 mL of DW for 2 h, and then they were stirred for 4 h to create an aqueous dispersion. The precipitation that was left over after filtering was gathered and dried for 10 h at 70°C in an oven. The final obtained photocatalysts were labeled as ST@xFe2O3, where (x = 0.3, 0.5, and 1) were devoted to the different weights loaded with Fe2O3.
2.6 Photocatalyst characterization
An X-ray diffraction (XRD) device was used with a Cu-K radiation source (λ = 1.54 Å) at 40 kV to find the phase composition and structure. The scanning electron microscopy (SEM) device technique was used to study the morphologies of Fe2O3, ST, and ST@xFe2O3 photocatalysts attached to an energy-dispersive X-ray (EDX) unit to examine the chemical compositions of fabricated photocatalysts and their ratio. The optical properties of the synthesized photocatalysts were investigated with a UV–Vis double-beam spectrophotometer. The textural properties of the photocatalysts were determined by the Brunauer–Emmett–Teller (BET) technique (Micromeritics Gemini 2375).
2.7 Photocatalysis measurements
The photocatalytic activity of fabricated Fe2O3, ST, and ST@xFe2O3 photocatalysts was evaluated via the degradation of MB solution as a model pollutant under solar light. The initial concentration of MB dye was 5 ppm with a pH of 7. The mixture was stirred for 20 min in dark conditions before the photocatalytic process to ensure adsorption–desorption equilibrium. After that, solution photocatalysts were taken every regular time interval for periods up to 1 h, centrifuged, and then used to calculate absorbance by using the spectrophotometer device technique (λ ranged from 600 to 700 nm). The typical absorption peak of MB dye is located at nearly 664 nm [24]. All photocatalytic experiments were achieved during the day, from 9 a.m. to 3 p.m., at Beni-Suef city (Egypt), with a temperature of 25°C and clear skies. The solar radiation is about 6 kW/m2. For comparison, the MB decomposition was checked below the tungsten lamp (400 W). The measurements were made concerning the photocatalyst dose, illumination period, and beginning MB concentration. Additionally, the stability of the optimum fabricated sample was investigated for the tenth photodegradation cycle at various exposure periods. All photocatalytic measurements were repeated three independent times, and average values were presented with statistical error bars.
3 Results and discussions
3.1 Characterization of the fabricated photocatalysts
3.1.1 Structural study exploration
Figure 1 displays the XRD patterns of Fe2O3, ST, and ST@Fe2O3 photocatalysts. The XRD pattern (Figure 1(a)) indicates the hexagonal structure of Fe2O3 (JCPDS No. 96-900-8878) [25,26]. The characteristic peaks of Fe2O3 are located at 2θ = 24.119°, 33.125°, 35.588°, 40.848°, 49.389°, 53.999°, 62.455°, 63.943°, and 75.339° in association with planes (012), (104), (110), (113), (024), (116), (214), (300), and (220), respectively. The chart of XRD for pure Na2Ti3O7 completely corresponded to the monoclinic phase structure (Card No. 96-231-0332), as shown in Figure 1(b) [27,28]. The main characteristic peaks of monoclinic ST are positioned at 2θ = 34.168°, 34.524°, 35.243°, 38.112°, 39.922°, 41.448°, 48.262°, and 57.768°. These peaks corresponded to the Miller indices

XRD charts of (a) Fe2O3, (b) ST, and (c) ST@Fe2O3.
When a photocatalyst undergoes deformation, it introduces microstrain, a lattice distortion that leads to the broadening of the diffraction peaks in XRD. The broadening of XRD peaks is primarily influenced by both the size and microstrain in the photocatalyst. However, the Scherrer equation only considers the size effect and does not account for microstrain in peak broadening. To obtain a more accurate evaluation of microstrain and size, the Williamson–Hall (W–H) model is employed. The W–H model is a superior method for determining the crystallite size (D) and microstrain (ε) in nanoparticles as it incorporates both size and strain effects [30]. The following equation can represent the W–H model:
Here, β represents the corrected FWHM associated with the (hkl) Bragg’s peak, θ denotes Bragg’s angle, and λ represents the wavelength of X-ray.
The FWHM of all diffraction peaks was corrected to exclude instrumental broadening contributions. When the microstrain is close to zero or very small, the W–H equation simplifies to the Scherrer equation. The analysis of the W–H model was performed using the X’Pert HighScore Plus software, as shown in Figure 2. This software is widely recognized as an ideal tool for crystallographic analysis and understanding powder diffraction patterns. The average crystallite sizes were calculated to be 1,341 Å for Fe2O3, 601 Å for ST, and 775 Å for ST@Fe2O3 using the W–H model. Correspondingly, the microstrain (%) values were found to be 0.20, 0.03, and 0.02 for Fe2O3, ST, and ST@Fe2O3, respectively.

W–H plots of the (a) Fe2O3, (b) ST, and (c) ST@Fe2O3.
The crystal cell parameters play a crucial role in understanding materials’ properties by providing essential insights into their structural characteristics. These parameters were calculated using the Unit Cell software based on the Bragg peaks. For the hexagonal Fe2O3 photocatalyst, XRD measurements yielded lattice parameters of a = b = 5.03640 Å and c = 13.7750 Å, which agree with the literature [31,32]. The measurement uncertainty (sigma) for these parameters is 0.00082 and 0.00436, respectively. Similarly, the lattice parameters were determined for the monoclinic ST photocatalyst as a = 9.07631 Å, b = 3.59237 Å, and c = 9.51687 Å, with an angle beta = 107.3180°. The measurement uncertainties (sigmas) are 0.00188, 0.00128, 0.00288, and 0.01963, respectively.
3.1.2 Surface morphological evaluation
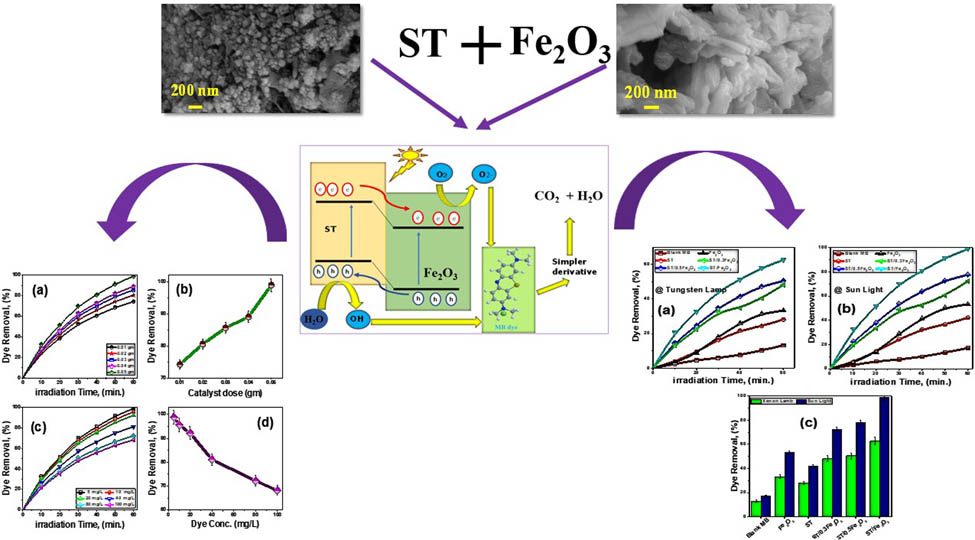
The surface morphology of fabricated photocatalysts was analyzed by the SEM technique. Figure 3 shows an SEM top view of the green-synthesized Fe2O3, ST, and ST@Fe2O3 photocatalysts. From Figure 3(a), Fe2O3 appears as a porous network with abundant micro- and mesoporous structures. The high-magnification SEM of Fe2O3, Figure 3(b), shows that the greenly synthesized Fe2O3 has a nano/mesoporous structure with a random distribution of agglomerated photocatalysts. Figure 3(c) reveals that the fabricated ST is a small photocatalyst agglomerated together, forming nanoclusters. These photocatalysts are spherical with different crystallites, as shown in the high-magnification SEM (Figure 3(d)). The average size is nearly 60 nm. ST@Fe2O3 photocatalyst has two phases of flower-like features and nanorods in agglomerated form, as shown in Figures 3(e) and (f). The change in the morphology of ST@Fe2O3 compared with pure ST and pure Fe2O3 indicates a significant interaction between ST and Fe2O3.

SEM images at different magnifications of the fabricated (a, b) Fe2O3, (c, d) ST, and (e, f) ST@Fe2O3.
3.1.3 EDX analysis
The EDX approach is commonly employed to ascertain the compositional constituents’ chemical makeup and quantitative ratios. The EDX of fabricated photocatalysts was achieved at an applied bias of 15 kV. Figure 4 displays the EDX spectrum for (a) Fe2O3, (b) ST, and (c) ST@Fe2O3. For Fe2O3 (Figure 4(a)), two signals are detected for iron (Fe) with mass fractions of 45.66% and oxygen (O) with mass fractions of 54.34%. Within the EDX’s detection range, no traces of elements with impurities like Na or C were discovered. This shows that the manufactured Fe2O3’s high purity and good crystallization correlate well with the XRD data. For ST (Figure 4(b)), there are signs for sodium (Na), titanium (Ti), and oxygen (O). Also, no impurities have been observed in the EDX spectrum, confirming the purity and crystallization of the fabricated ST by the alkaline hydrothermal method. For ST@Fe2O3 (Figure 4(c)), four signals are detected for Na, Ti, Fe, and O.

EDX spectra of the fabricated (a) Fe2O3, (b) ST, and (c) ST@Fe2O3.
3.1.4 Optical analysis
Optical analysis plays a crucial role in understanding the photocatalytic activity reaction. For a material to function effectively as a photocatalyst, its band gap (E g) must be equal to or smaller than the energy of the incident light. When a photocatalyst absorbs light, it generates electron/hole pairs that participate in the photodegradation of organic dyes. If the band gap is too large, the absorbed light lacks sufficient energy to excite electrons from the VB to the CB. As a result, the photocatalyst cannot generate charge carriers, which limits its ability to catalyze reactions. Therefore, the intensity of absorption and the energy gaps of the direct and indirect bands of the photocatalyst directly affect the efficiency of dye degradation. Calculating both types of band gaps provides valuable insights into the electronic properties of photocatalysts and the underlying energy transfer processes. This understanding enhances knowledge of the mechanisms involved in photocatalysis and facilitates the optimization of photocatalyst materials. Visible light is particularly important because it accounts for approximately 45% of solar irradiation, compared to less than 5% for UV light. Thus, adjusting the bandgap of the photocatalyst to the visible range is advantageous. It enables efficient light harvesting by utilizing abundant visible light and achieving a high visible absorption coefficient.
The ultraviolet–visible (UV–Vis) optical absorption spectra of the fabricated photocatalysts are presented, as shown in Figure 5(a). The absorption test was measured in the wavelength range of 200–1,000 nm. It can be noted that the strong absorption band of ST is located in the ultraviolet region near 295 nm, as displayed in Figure 5(a). The UV absorption band of ST is allocated to Ti particles in the octahedral matrix (TiO6) [33]. Then, the absorption of this photocatalyst decreases as the wavelength of incident light increases toward the visible region. Hence, the ST can absorb light at ultraviolet wavelengths while exhibiting no discernible spectral response to the visible region.

(a) Absorbance bands and (b) comparison of the direct and indirect bandgap of all fabricated photocatalysts.
The absorption spectrum of the Fe2O3 photocatalyst displays a comprehensive absorption in the visible region. The absorption band of Fe2O3 is caused by both direct transitions and the spin-forbidden excitations of indirect transitions [34]. While Fe2O3 is effective at absorbing light in visible light, the high recombination rate of the photogenerated e/h pairs in Fe2O3 has a negative impact on photodegradation. Due to loading Fe2O3 over ST, the color of ST, ST@0.3 Fe2O3, ST@0.5 Fe2O3, and ST@Fe2O3 photocatalysts changes from white to bright red, which confirms the change in absorption and bandgap of the fabricated ST@xFe2O3 photocatalysts. In the case of the ST@Fe2O3 photocatalyst, increasing the Fe2O3 loading up to 1 wt% resulted in a gradual shift of the absorption edge towards longer wavelengths in the visible range. This shift enhances the solar light absorptivity of the ST@Fe2O3 photocatalyst within the visible light spectrum, surpassing that of other photocatalysts, as depicted in Figure 5(a). This can be attributed to the overlapping bands and interactions between ST and Fe2O3, which lead to alterations in the electronic structure of the ST@Fe2O3 photocatalyst [35,36]. Additionally, the incorporation of Fe2O3 generates intermediate states between the VB and CB of ST, further enhancing the absorption of solar light by the ST@Fe2O3 photocatalyst [37].
The UV–Vis data were used to calculate the direct and indirect bandgap of photocatalysts based on the Tauc equation [38]:
where α, E p, and A o are the coefficients of absorption, the energy (eV) of incident photons, and constants linked to the electrons and hole effective mass. E g is the optical bandgap of photocatalysts. The values m = 0.5 and 2, respectively, represent direct or indirect interband transitions [39]. The Tauc curves for the direct and indirect bandgaps of all photocatalysts are given in Figure S2. The calculated values of the bandgap are presented in Figure 5(b).
The ST has a wide bandgap, mainly in the UV region. The direct bandgap fits of Fe2O3 and ST are 2,73 and 3.03 eV, respectively, which is consistent with earlier studies [33,40]. With an increase in the weight ratio of loaded Fe2O3 over ST (from 0.3 to 1), the direct band gap changed. The bandgap obtained from the direct gap shows ST@Fe2O3 possesses a relatively smaller bandgap (2.54 eV) compared to ST@0.5 Fe2O3 (3.24 eV) and ST@0.3 Fe2O3 (2.70 eV). The direct transition correlates with the
The estimated indirect Eg of the Fe2O3, ST, ST@0.3Fe2O3, ST@0.5 Fe2O3, and ST@Fe2O3 photocatalysts, respectively, are 1.5, 3.02, 2.51, 2.28, and 2.05 eV. Based on these optical data, the ST@Fe2O3 photocatalyst demonstrated lower direct and indirect bandgaps along with high absorption of visible light. This characteristic facilitated the generation of photoinduced charge carriers. Consequently, it is expected that the ST@Fe2O3 photocatalyst will play a significant role in the photocatalytic degradation process [42].
3.1.5 BET analysis
The textural properties of photocatalysts were assessed using the BET technique, with surface area and mean pore diameter as the key parameters. Figure 6 displays the nitrogen adsorption and desorption isotherm curves for ST, Fe2O3, and ST@Fe2O3 photocatalysts. The measured BET surface areas were found to be 14.14 m2/g for ST, 4.38 m2/g for Fe2O3, and notably higher at 24.28 m2/g for ST@Fe2O3. This significant increase in surface area suggests an augmented availability of active sites for reactions in the ST@Fe2O3 photocatalyst. In terms of pore diameter, ST exhibited 9.85 nm, Fe2O3 had 11.07 nm, and ST@Fe2O3 demonstrated 9.76 nm. These results underscore the improved textural properties of the ST@Fe2O3 photocatalyst, highlighting its potential for diverse applications in the field of photocatalysis.

Nitrogen adsorption-desorption isotherm of ST, Fe2O3, and ST@Fe2O3 photocatalysts.
4 Photocatalytic measurements
When photons with suitable energy strike a semiconductor material known as ST@Fe2O3 photocatalyst, electrons become excited and move to the CB, leaving behind positively charged holes in the VB. The photogenerated electrons have a high reduction potential, whereas these holes have a high oxidation potential. The holes can rejoin with adsorbed H2O on the surface of ST@Fe2O3 and produce free radicals. Meanwhile, the electrons reduce O2 to superoxide anion-free radicals. Because of their potent oxidation capability, the free radicals in the solution can disrupt the bonds that bind organic pigments. As a result, the dye present in wastewater is decomposed into harmless compounds such as H2O and CO2.
The percentage of dye removal (η%) was estimated based on the change in absorbance over time. Achieving complete dye removal (η = 100%) in a short time is crucial for efficient wastewater treatment. Using the following equation (3) [43], one may estimate the dye elimination by tracking the decline in the dye’s absorption maximum at λ = 664 nm
where
4.1 Effect of the ratio of Fe2O3 on the ST@Fe2O3 photocatalyst
The influence of Fe2O3 on the photodegradation performance features of the ST@Fe2O3 photocatalyst was investigated. The photocatalytic activities of six photocatalysts, including the blank MB (dye without catalysis), Fe2O3, ST, ST@0.3 Fe2O3, ST@0.5 Fe2O3, and ST@Fe2O3, were evaluated for the degradation of MB by ordinary sunlight and a tungsten lamp. All photocatalysts were exposed to light for approximately 60 min at room temperature. The initial dye concentration and catalysis mass were 5 ppm and 50 mg, respectively. The pH of the medium was approximately 7.
Figure 7 presents the time-dependent percentage of dye removal (η%) for different photocatalysts. The data show that for all photocatalysts, the proportion of dye elimination increases with increasing irradiation time. The self-degradation of MB without any catalyst is low. Additionally, ST and Fe2O3 exhibit low photocatalytic activity due to the high bandgap of ST and the limited portion of UV light capable of generating e/h pairs within the material. Turki et al. have investigated the impact of Na+ as a recombination center in ST, which can decrease its photocatalytic efficiency [44]. The rapid recombination of photogenerated carriers in Fe2O3 also reduces its photocatalytic activity. However, the hybridization of ST with Fe2O3 demonstrates effective photocatalytic behavior. Among all the photocatalysts, ST@Fe2O3 exhibits the highest rate of decreasing absorption under the tungsten lamp. This finding is consistent with the optical data obtained. After 60 min of tungsten lamp irradiation, ST and ST@Fe2O3 exhibit dye removal efficiencies of approximately 50 and 60%, respectively, using an initial 5 ppm MB dye concentration.

The dye removal versus irradiation time for all photocatalysts (a) under a tungsten lamp, (b) under sunlight, and (c) the dye removal after 60 min. Error bars refer to the standard deviation of triplicate measurements.
Under sunlight conditions, as displayed in Figure 7(b), the fraction of dye removal is higher compared to that under tungsten lamps for the same exposure time. This is because the emitted intensity from tungsten lamps is centered in the UV region. Sunlight encompasses a broader spectrum of light, including UV, visible, and NIR. This improves the excitation of the photocatalyst and generates more free radicals. These free radicals react with the dye molecules and facilitate their degradation. In other words, the increased production of free radicals under sunlight leads to fast dye degradation in less time compared to tungsten lamps.
Figure 7(c) shows that the ST@Fe2O3 photocatalyst exhibits the highest MB degradation percentage (100%) after 60 min of reaction under sunlight. In contrast, Fe2O3, ST, ST@0.5 Fe2O3, and ST@0.3 Fe2O3 photocatalysts remove 40, 50, 80, and 70% of MB after 60 min of exposure to sunlight, respectively. The maximum removal efficiency achieved through the adsorption process was approximately 9% after 30 min in complete darkness, as seen in Figure S3. The ST@Fe2O3 photocatalyst outperforms other photocatalysts due to its favorable characteristics. It possesses a lower band gap, a higher surface area, and good crystallinity, as confirmed in the optical, BET, and XRD analyses. The lower band gap allows for the efficient generation of charge carriers within the visible light region. The higher surface area provides numerous contact points for pollutant degradation and increased adsorption of dye molecules. The good crystallinity further facilitates the transfer of photocarriers to the catalyst’s surface. Additionally, the higher number of accessible active sites on the ST@Fe2O3 photocatalyst’s surface contributes to the accelerated degradation of the MB dye. Collectively, these advantageous features synergistically contribute to the superior performance of the ST@Fe2O3 photocatalyst in effectively degrading contaminants.
4.2 The total organic carbon
Total organic carbon (TOC) content was measured to evaluate the mineralization of MB via the process of photocatalytic activity. TOC is an important metric for measuring the level of organic pollution in water. To determine the TOC, the dye solution was combined with potassium hydrogen phthalate, phosphoric acid, and sodium persulfate. The mixture was then subjected to analysis using a TOC analyzer device (Sievers InnovOx ES). The findings showed that using ST, Fe2O3, and ST@Fe2O3 as photocatalysts, respectively, reduced TOC by 2.33, 2.17, and 0.55. A lower TOC value in the water sample suggests a lower concentration of organic pollutants, indicating a more significant reduction in organic pollution. Among the tested photocatalysts, the ST@Fe2O3 photocatalyst exhibited the highest degree of mineralization for MB, indicating its exceptional effectiveness in converting MB into CO2 and H2O compared to the other photocatalysts.
4.3 Effect of MB concentration and catalyst quantity
From a practical standpoint, it is crucial to look at how catalyst dose and starting dye concentration affect degradation efficiency to assess photocatalytic performance for the ST@Fe2O3 nanohybrid. Figure 6(a) and (b) demonstrate the impact of the photocatalyst dose on the degradation proficiency of the dye. After 60 min, the percentage of MB degradation increased dramatically from 75 to 100% when the dosage of the ST@Fe2O3 catalyst was raised from 10 to 50 mg. This is because higher charges of ST@Fe2O3 are more favorable for the photo-reduction of MB. Increasing the amount of ST@Fe2O3 also makes it easier for more organic waste to stick to the photocatalyst’s surface. Furthermore, a higher number of accessible active sites on the ST@Fe2O3 photocatalyst’s surface would cause the MB dye to degrade more quickly. In other words, the removal percentage of the dye increased as the catalyst weight increased. These findings are consistent with numerous previous studies [29,45].
Figure 8(c) and (d) indicates that the MB degradation ratio decreased as the original MB dye concentration was increased. In particular, the degradation efficiencies after 60 min were 100, 95, 85, 75, and 70%, respectively, when the starting MB concentrations were 5, 20, 40, 80, and 100 ppm. This decline in decomposition efficacy can be ascribed to the increase in the molecule number of MB at higher concentrations, while the free radicals (

The dye removal under sunlight (a, b) catalyst dose (c, d) dye concertation. Error bars refer to the standard deviation of triplicate measurements.
4.4 Effect of temperature and starting pH
The impact of variation temperature on the degradation of MB dye was examined from 25 to 50°C as depicted in Figure 9(a). The results revealed that the catalytic performance of ST@Fe2O3 increases with temperature [48,49]. Photocatalysis is typically regarded as a temperature-independent process. However, increasing the temperature has been found to have beneficial effects on the photocatalytic degradation of MB dye. The temperature rise enhances the adsorption of reactants by increasing the kinetic energy of molecules, facilitating their interaction with the photocatalyst. Additionally, higher temperatures promote the reaction of oxidation species with dye molecules, leading to more efficient degradation.

Effects of (a) temperature and (b) pH values on the dye removal process of MB dye under sunlight illumination for 50 min (reaction conditions: dye concentration = 50 mL, 5 mg/L, ST@Fe2O3 photocatalyst weight 50 mg).
The starting pH of the MB dye solution has a significant influence on the photodegradation efficiency. Figure 9(a) demonstrates the impact of the initial pH on the removal of MB dye using the ST@Fe2O3 photocatalyst. The experiment was conducted with a solution volume of 50 mL containing an initial dye concentration of 5 mg/L and a photocatalyst mass of 5 mg. The solution was subjected to sunlight irradiation for 50 minutes at 25 C. To adjust the pH of the solution, 0.1 M NaOH and 0.1 M HCl were employed. The results showed that higher pH values led to lower percentages of MB dye removal [45]. Specifically, the percentages of MB dye removal were 100, 90.1, and 69.5% at pH values of 4, 7, and 10, respectively. The pH value affects both the surface charge properties of the photocatalyst and the ionization of the MB dye [50]. The surface charge properties of the photocatalyst play a crucial role in the adsorption of MB dye molecules, while the ionization of the dye affects its interaction with the photocatalyst surface. At pH 4, the highest percentage of MB dye removal (100%) was achieved, indicating that this pH condition favored the photocatalytic degradation process.
4.5 Reusability and stability of ST@Fe2O3 photocatalyst
The reusability and stability of a photocatalyst are critical for practical products. The high reusability of the photocatalyst is particularly beneficial in industrial settings. The capability of the catalyst to be recycled several times is a crucial consideration for the cost-effective removal of toxic organic pollutants. Therefore, the photocatalyst with the best performance (ST@Fe2O3) was selected for reusability evaluations under identical conditions. The photodegradation experiment of MB (5 ppm) in the occurrence of ST@Fe2O3 (50 mg) was repeated several times when exposed to sunlight for 60 min. After each photodegradation experiment, the ST@Fe2O3 photocatalyst was centrifuged to extract it from the reaction solution, followed by a DW wash and an 11 h drying period at 70°C. The ST@Fe2O3 catalyst was then reused for the following experiments under the same reaction environment parameters.
Figure S4 illustrates the dye removal efficiency observed in ten repeated experiments. The efficiency gradually decreases from 100 to 79% as the catalyst is used successively for the first through tenth runs. The reduction in the catalytic activity of ST@Fe2O3 can be attributed to two possible factors: the leaching of Ti–Fe components or the poisoning effect caused by the accumulation of dye molecules on the surface of ST@Fe2O3 [51]. This demonstrated that the ST@Fe2O3 catalyst has high stability with low photocatalytic efficiency within ten consecutive runs of MB photodegradation. This designates that the ST@Fe2O3 photocatalyst is highly effective for the photocatalytic degradation of wastewater with excellent stability.
4.6 Kinetic modeling of photodegradation
Understanding the efficiency of a catalyst in breaking down MB dye is crucial as it directly impacts the development and enhancement of photocatalytic processes. Kinetic models provide a quantitative evaluation of the proficiency of the photocatalyst and can help in the design and optimization of photocatalytic systems. The choice of kinetic model depends on the reaction parameters and the character of the photocatalyst used. Kinetic models can also provide insights into the mechanisms of the photocatalytic reaction. In addition, kinetic models can be used to predict the degradation rate of the dye under different reaction conditions. This can be useful in optimizing the reaction parameters for maximum efficiency. Hence, the kinetic models are important tools in the evaluation of the performance efficiency of photocatalysts.
The kinetic models for the photodegradation of MB dye across all photocatalysts under sunlight irradiation were studied. These models include the zero-, first-, and second-order replicas, which can be, respectively, described by the next equations
where
Figure 10 shows graphs of linear fits for the three kinetic models depicting of

Fit of the degradation records with the (a) zero-order, (b) first-order, and (c) second-order kinetic model.
Constraints of the zero-, first-, and second-order kinetic representations for dye decomposition by ST@Fe2O3 photocatalyst
| Fe2O3 | ST | ST@0.3 Fe2O3 | ST@0.5 Fe2O3 | ST@Fe2O3 | ||
|---|---|---|---|---|---|---|
| Zero-order | K 0 | −0.01 | −0.01 | −0.020 | −0.02 | −0.020 |
| R 2 | 0.968 | 0.982 | 0.953 | 0.948 | 0.931 | |
| First-order | K 1 | 0.014 | 0.009 | 0.020 | 0.025 | 0.066 |
| R 2 | 0.967 | 0.989 | 0.978 | 0.998 | 0.846 | |
| Second-order | K 2 | 0.014 | 0.008 | 0.026 | 0.039 | 0.728 |
| R 2 | 0.950 | 0.985 | 0.903 | 0.952 | 0.362 |
Table 1 reveals that the first-order kinetic model for the ST photocatalyst had the lowest rate constant value (k 1) of 0.009 min−1, while the kinetic second-order model for the ST@Fe2O3 photocatalyst had the highest rate constant value (k 2) of 0.728 min−1. For the three kinetic models, the degradation rate constant gradually increased with an increase in the weight ratio of the Fe2O3 photocatalyst in the ST@Fe2O3 photocatalyst. Kushwaha et al. noted that the reason for this rise in the rate constant was the production of additional HO˙ radicals during the breakdown of MB dye [51].
The ST@Fe2O3 photocatalyst demonstrated the fastest rate of reaction among the photocatalysts within the same kinetic model. For the three-order kinetic models, the corresponding k values for ST@Fe2O3 were k 0 = 0.023, k 1 = 0.066, and k 2 = 0.728 min−1. In the zero-order model, the reaction constant of ST@Fe2O3 was found to be 2.2 times greater than that of ST. This suggests that there is a chance that the ST@Fe2O3 catalyst will have a much stronger synergistic impact [47].
The regression coefficient (R 2) value for the zero-order reaction kinetics for ST@Fe2O3 was observed to be higher than those of the first-order and second-order kinetics. A high R 2 value of 0.931 specified that the data fitted in the zero-order equation was relative to another model (Table 1). Finally, the study indicates that the zero-order kinetic model and the MB dye degradation rates for the ST@Fe2O3 photocatalyst agree.
The ST@Fe2O3 photocatalyst, with its higher surface area and smaller band gap compared to the other two photocatalysts. This higher activity of ST@Fe2O3 results in a faster reaction rate compared to ST@0.5 Fe2O3 and ST@0.3 Fe2O3. The increased loading of Fe2O3 in ST@Fe2O3 provides a greater number of active sites for the photocatalytic reaction. Consequently, a higher concentration of dye contaminants can be degraded simultaneously, leading to a more pronounced zero-order kinetic behavior. In contrast, ST@0.5 Fe2O3 and ST@0.3 Fe2O3, with their lower Fe2O3 loading, exhibit a lower number of active sites, leading to a first-order kinetic behavior where the reaction rate depends on the concentration of the dye molecules.
4.7 Photoelectrochemical measurements
The photoelectrochemical properties of ST, Fe2O3, and ST@Fe2O3 were investigated using an OrigaFlex potentiostat. These measurements were conducted at room temperature under illumination from a solar simulator Xe-lamp. The photoelectrochemical measurements provided valuable insights into the dynamics of charge carrier generation and recombination within the materials. The photocurrent density analysis involved applying a potential ranging from −1 to 1 V in a 0.1 M sodium thiosulfate (Na2S2O3) solution, as depicted in Figure 11(a). Additionally, electrochemical impedance (EIS) analysis was performed in the frequency range of 0.01 Hz to 100 kHz, as illustrated in Figure 11(b). The results revealed that the ST@Fe2O3 material exhibited high photocurrent and good conductivity. These findings suggest that ST@Fe2O3 shows promise in enhancing dye photodegradation processes.

The photoelectrochemical properties of ST, Fe2O3, and ST@Fe2O3 (a) photocurrent density-potential and (b) EIS spectrum.
4.8 Photocatalytic mechanism
Figure 12 illustrates a schematic diagram that explains the photocatalytic mechanism and charge transfer based on ST@Fe2O3 nanostructures. The efficiency of the photocatalytic process is influenced by various factors such as dye adsorption on the photocatalyst surface, band-gap energy, surface area, crystallinity, and the rate of electron–hole recombination [52].

Schematic diagram of charge transfer in an ST@Fe2O3 photocatalyst under visible light illumination.
When ST and Fe2O3 photocatalysts are exposed to light energy, they undergo photoexcitation, resulting in the movement of electrons from the VB to the CB, generating positive holes and negative electrons. These electron–hole pairs play a crucial role in the degradation of dyes. However, the limited UV light absorption of ST and the rapid recombination of charge carriers in Fe2O3 present challenges and restrict their practical applications.
To overcome these challenges, the incorporation of Fe2O3 into ST to form ST@Fe2O3 photocatalysts proves advantageous. The interaction between ST and Fe2O3 leads to the creation of a p-ST/n-Fe2O3 heterojunction structure. This structure generates a built-in electric field and a depleted layer at the interface of the p-ST/n-Fe2O3 heterojunction. Consequently, band bending and modification of the electronic structure occur, reducing the band gap and enhancing light absorption capabilities. Moreover, the synergistic effect of the ST@Fe2O3 configuration and the modified surface morphology results in increased surface roughness, creating a greater number of active sites for the effective adsorption of MB dye. In the ST@Fe2O3 configuration, electrons migrate from the CB of ST to the CB of Fe2O3, while the holes move in the opposite direction, accumulating on the VB of ST. This spatial separation of electron–hole pairs significantly enhances the efficiency of the photocatalytic reaction [53]. These combined factors contribute to the observed enhanced photocatalytic activity of the ST@Fe2O3 photocatalyst material.
The positive holes react with water or hydroxyl ions, resulting in the generation of reactive hydroxyl radicals [54]. Simultaneously, the electrons can reduce oxygen, forming superoxide anions. These reactive species then react with organic dye compounds adsorbed on the surface of the photocatalyst, facilitating the oxidation of the dye molecules and enabling complete mineralization. Consequently, the dye molecules are broken down into carbon dioxide, water, and inorganic nitrogen compounds, including nitrate ions.
5 Conclusions
In summary, we successfully synthesized ST@Fe2O3 photocatalysts with different Fe2O3 weight ratios was achieved for the first time. For every photocatalyst, structural investigation was carried out using XRD, SEM, and EDX. The optical band gap, both direct and indirect, was also computed. The ST@Fe2O3 showed the best photocatalytic activity among the several photocatalysts when it came to breaking down MB in an aqueous solution when exposed to sunlight. The full photodegradation (100%) was achieved after 60 min. Due to the synergistic effects at the heterogeneous interfaces, the ST@Fe2O3 photocatalyst’s improved photocatalytic behavior was attributed to increased light absorption and the promotion of photogenerated charge separation. A novel ST@Fe2O3 catalyst with numerous uses in wastewater treatment and environmental remediation is presented in this work.
Acknowledgments
The authors acknowledge the support and funding by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-RG23130).
-
Funding information: This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-RG23130).
-
Author contributions: A. M. Ahmed: conceptualization, methodology, formal analysis, investigation, and writing. M. Nasr, M. Zayed, and M. J. Aljaafreh: conceptualization, methodology, formal analysis, investigation, and writing – review and editing. S. Sayed, H. Hamdy, and M. Shaban: methodology, investigation, formal analysis. M. Marashdeh and Mohannad AL-Hmoud: investigation, validation, and formal analysis. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Barzinjy AA, Hamad SM, Aydın S, Ahmed MH, Hussain FHS. Green and eco-friendly synthesis of Nickel oxide nanoparticles and its photocatalytic activity for methyl orange degradation. J Mater Sci Mater Electron. 2020;31:11303–16. 10.1007/S10854-020-03679-Y.Search in Google Scholar
[2] Iqbal MS, Aslam AA, Iftikhar R, Junaid M, Imran SM, Nazir MS, et al. The potential of functionalized graphene-based composites for removing heavy metals and organic pollutants. J Water Process Eng. 2023;53:103809. 10.1016/J.JWPE.2023.103809.Search in Google Scholar
[3] Naikwade AG, Jagadale MB, Kale DP, Gophane AD, Garadkar KM, Rashinkar GS. Photocatalytic degradation of methyl orange by magnetically retrievable supported ionic liquid phase photocatalyst. ACS Omega. 2020;5:131–44. 10.1021/ACSOMEGA.9B02040.Search in Google Scholar PubMed PubMed Central
[4] Khan I, Saeed K, Zekker I, Zhang B, Hendi AH, Ahmad A, et al. Review on methylene blue: Its properties, uses, toxicity and photodegradation. Water. 2022;14:242. 10.3390/W14020242.Search in Google Scholar
[5] Al-Ansari MM, Li Z, Masood A, Rajaselvam J. Decolourization of azo dye using a batch bioreactor by an indigenous bacterium Enterobacter aerogenes ES014 from the waste water dye effluent and toxicity analysis. Environ Res. 2022;205:112189. 10.1016/J.ENVRES.2021.112189.Search in Google Scholar PubMed
[6] Pang AL, Arsad A, Ahmad Zaini MA, Garg R, Saqlain Iqbal M, Pal U, et al. A comprehensive review on photocatalytic removal of heavy metal ions by polyaniline-based nanocomposites. Chem Eng Commun. 2024;211:275–99. 10.1080/00986445.2023.2227568.Search in Google Scholar
[7] Shubha JP, Adil SF, Khan M, Hatshan MR, Khan A. Facile fabrication of a ZnO/Eu2O3/NiO-based ternary heterostructure nanophotocatalyst and its application for the degradation of methylene blue. ACS Omega. 2021;6:3866–74.10.1021/acsomega.0c05670Search in Google Scholar PubMed PubMed Central
[8] Ardani MR, Pang AL, Pal U, Haniff MASM, Ismail AG, Hamzah AA, et al. Ultrasonic-assisted of TiO2-MWCNT nanocomposite with advanced photocatalytic efficiency for elimination of dye pollutions. Diam Relat Mater. 2023;137:110066. 10.1016/J.DIAMOND.2023.110066.Search in Google Scholar
[9] Li X, Wang W, Dong F, Zhang Z, Han L, Luo X, et al. Recent advances in noncontact external-field-assisted photocatalysis: from fundamentals to applications. ACS Catal. 2021;11:4739–69. 10.1021/ACSCATAL.0C05354.Search in Google Scholar
[10] Abass SAH, Seriani N. Structural and electronic properties of Na2Ti3O7 and H2Ti3O7. Phys Status Solidi. 2018;255:1700612. 10.1002/PSSB.201700612.Search in Google Scholar
[11] Zhang HM, Cheng K, Ji YL, Liu XL, Li LS, Zhang XT, et al. Preparation of sodium titanate nanotubes modified by CdSe quantum dots and their photovoltaic characteristics. Sci China Ser B Chem. 2008;51:976–82. 10.1007/S11426-008-0023-6/METRICS.Search in Google Scholar
[12] Iani IM, Teodoro V, Marana NL, Coleto U, Sambrano JR, Simões AZ, et al. Cation-exchange mediated synthesis of hydrogen and sodium titanates heterojunction: Theoretical and experimental insights toward photocatalyic mechanism. Appl Surf Sci. 2021;538:148137. 10.1016/j.apsusc.2020.148137.Search in Google Scholar
[13] Lei X, Li X, Ruan Z, Zhang T, Pan F, Li Q, et al. Adsorption-photocatalytic degradation of dye pollutant in water by graphite oxide grafted titanate nanotubes. J Mol Liq. 2018;266:122–31. 10.1016/j.molliq.2018.06.053.Search in Google Scholar
[14] Mozia S, Borowiak-Paleń E, Przepiórski J, Grzmil B, Tsumura T, Toyoda M, et al. Physico-chemical properties and possible photocatalytic applications of titanate nanotubes synthesized via hydrothermal method. J Phys Chem Solids. 2010;71:263–72. 10.1016/J.JPCS.2009.12.074.Search in Google Scholar
[15] Choi YS, Costa SIR, Tapia-Ruiz N, Scanlon DO. Intrinsic defects and their role in the phase transition of Na-Ion anode Na2Ti3O7. ACS Appl Energy Mater. 2023;6:484–95. 10.1021/ACSAEM.2C03466.Search in Google Scholar PubMed PubMed Central
[16] Chen Q, Zhang M, Li J, Zhang G, Xin Y, Chai C. Construction of immobilized 0D/1D heterostructure photocatalyst Au/CuS/CdS/TiO2 NBs with enhanced photocatalytic activity towards moxifloxacin degradation. Chem Eng J. 2020;389:124476. 10.1016/J.CEJ.2020.124476.Search in Google Scholar
[17] Charate S, Shinde S, Kondawar S, Desai U, Wadgaonkar P, Rode C. Role of preparation parameters of Cu–Zn mixed oxide catalyst in solvent free glycerol carbonylation with urea. J Indian Chem Soc. 2021;98:100090. 10.1016/J.JICS.2021.100090.Search in Google Scholar
[18] Low J, Yu J, Jaroniec M, Wageh S, Al-Ghamdi AA. Heterojunction Photocatalysts. Adv Mater. 2017;29:1601694. 10.1002/ADMA.201601694.Search in Google Scholar
[19] Mohanty S, Moulick S, Maji SK. Adsorption/photodegradation of crystal violet (basic dye) from aqueous solution by hydrothermally synthesized titanate nanotube (TNT). J Water Process Eng. 2020;37:101428. 10.1016/j.jwpe.2020.101428.Search in Google Scholar
[20] Wu H, Wu G, Wang L. Peculiar porous α-Fe2O3, γ-Fe2O3 and Fe3O4 nanospheres: Facile synthesis and electromagnetic properties. Powder Technol. 2015;269:443–51. 10.1016/J.POWTEC.2014.09.045.Search in Google Scholar
[21] Hitam CNC, Jalil AA. A review on exploration of Fe2O3 photocatalyst towards degradation of dyes and organic contaminants. J Environ Manage. 2020;258:110050.10.1016/j.jenvman.2019.110050Search in Google Scholar PubMed
[22] Joseph A, Vijayanandan A. Photocatalysts synthesized via plant mediated extracts for degradation of organic compounds: A review of formation mechanisms and application in wastewater treatment. Sustain Chem Pharm. 2021;22:100453. 10.1016/J.SCP.2021.100453.Search in Google Scholar
[23] Bashir M, Ali S, Farrukh MA. Green synthesis of Fe2O3 nanoparticles from orange peel extract and a study of its antibacterial activity. J Korean Phys Soc. 2020;76:848–54. 10.3938/JKPS.76.848/METRICS.Search in Google Scholar
[24] Długosz O, Szostak K, Banach M. Photocatalytic properties of zirconium oxide–zinc oxide nanoparticles synthesised using microwave irradiation. Appl Nanosci. 2020;10:941–54. 10.1007/S13204-019-01158-3/TABLES/6.Search in Google Scholar
[25] Lubis S, Maulana I, Sheilatina S, Mahyuni L. Preparation and photocatalytic activity of hematite from iron sand modified ZnO for indigo carmine degradation. J Phys Conf Ser. 2019;1402:055077. 10.1088/1742-6596/1402/5/055077.Search in Google Scholar
[26] Shashanka R. Investigation of optical and thermal properties of CuO and ZnO nanoparticles prepared by Crocus Sativus (Saffron) flower extract. J Iran Chem Soc. 2021;18:415–27. 10.1007/S13738-020-02037-3/FIGURES/11.Search in Google Scholar
[27] Kumari P, Li Y, Boston R. An ionic liquid synthesis route for mixed-phase sodium titanate (Na2Ti3O7 and Na2Ti6O13) rods as an anode for sodium-ion batteries. Nanoscale. 2023;15:12087–94. 10.1039/D3NR00639E.Search in Google Scholar PubMed
[28] Al-Senani GM, Nasr M, Zayed M, Ali SS, Alshaikh H, Abd El-Salam HM, et al. Fabrication of PES modified by TiO2/Na2Ti3O7 nanocomposite mixed-matrix woven membrane for enhanced performance of forward osmosis: Influence of membrane orientation and feed solutions. Membranes. 2023;13:654. 10.3390/MEMBRANES13070654.Search in Google Scholar
[29] Mohamed F, Hassaballa S, Shaban M, Ahmed AM. Highly efficient photocatalyst fabricated from the chemical recycling of iron waste and natural zeolite for super dye degradation. Nanomaterials. 2022;12:235. 10.3390/NANO12020235/S1.Search in Google Scholar
[30] Abdel-Rahman AS, Sabry YA. An approach to the micro-strain distribution inside nanoparticle structure. Int J Non Linear Mech. 2024;161:104670. 10.1016/J.IJNONLINMEC.2024.104670.Search in Google Scholar
[31] Demianets LN, Pouchko SV, Gaynutdinov RV. Fe2O3 single crystals: Hydrothermal growth, crystal chemistry and growth morphology. J Cryst Growth. 2003;259:165–78. 10.1016/S0022-0248(03)01586-0.Search in Google Scholar
[32] Hill AH, Jiao F, Bruce PG, Harrison A, Kockelmann W, Ritter C. Neutron diffraction study of mesoporous and bulk hematite, α-Fe2O3. Chem Mater. 2008;20:4891–9. 10.1021/CM800009S.Search in Google Scholar
[33] Capel-Sanchez MC, Campos-Martin JM, Fierro JLG, de Frutos MP, Polo AP. Effective alkene epoxidation with dilute hydrogen peroxide on amorphous silica-supported titanium catalysts. Chem Commun. 2000;10:855–6. 10.1039/B000929F.Search in Google Scholar
[34] Shaban M, Binsabt M, Ahmed AM, Mohamed F. Recycling rusty iron with natural zeolite heulandite to create a unique nanocatalyst for green hydrogen production. Nanomaterials. 2021;11:3445. 10.3390/NANO11123445/S1.Search in Google Scholar
[35] Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13:2638–50. 10.1039/C1GC15386B.Search in Google Scholar
[36] Sreethawong T, Ngamsinlapasathian S, Yoshikawa S. Surfactant-aided sol-gel synthesis of mesoporous-assembled TiO2-NiO mixed oxide nanocrystals and their photocatalytic azo dye degradation activity. Chem Eng J. 2012;192:292–300. 10.1016/j.cej.2012.04.006.Search in Google Scholar
[37] Chkirida S, Zari N, Achour R, Qaiss A, Bouhfid R. Effect of iron doped titanium oxide encapsulated in alginate on photocatalytic activity for the removal of dye pollutants. RSC Adv. 2020;10:22311–7. 10.1039/D0RA02898C.Search in Google Scholar
[38] Al-Senani GM, Zayed M, Nasr M, Ali SS, Shaban M, Mohamed F. Flexible electrode based on PES/GO mixed matrix woven membrane for efficient photoelectrochemical water splitting application. Membranes. 2023;13:653. 10.3390/MEMBRANES13070653.Search in Google Scholar
[39] Roy R, Dutta A. Structural, optical, electrical, and dielectric relaxation properties of rare earth containing sodium bismuth titanate Na0.5Bi0.5TiO3 perovskite: Effect of ionic radius. J Rare Earths. 2023;42(2):383–91. 10.1016/J.JRE.2023.04.011.Search in Google Scholar
[40] Souza FL, Lopes KP, Nascente PAP, Leite ER. Nanostructured hematite thin films produced by spin-coating deposition solution: Application in water splitting. Sol Energy Mater Sol Cell. 2009;93:362–8. 10.1016/J.SOLMAT.2008.11.049.Search in Google Scholar
[41] Duret A, Grätzel M. Visible light-induced water oxidation on mesoscopic α-Fe2O3 films made by ultrasonic spray pyrolysis. J Phys Chem B. 2005;109:17184–91. 10.1021/JP044127C.Search in Google Scholar
[42] Ahmed AM, Mohamed F, Ashraf AM, Shaban M, Aslam Parwaz Khan A, Asiri AM. Enhanced photoelectrochemical water splitting activity of carbon nanotubes@TiO2 nanoribbons in different electrolytes. Chemosphere. 2020;238:124554. 10.1016/j.chemosphere.2019.124554.Search in Google Scholar PubMed
[43] Shaban M, Ahmed AM, Shehata N, Betiha MA, Rabie AM. Ni-doped and Ni/Cr co-doped TiO2 nanotubes for enhancement of photocatalytic degradation of methylene blue. J Colloid Interface Sci. 2019;555:31–41. 10.1016/j.jcis.2019.07.070.Search in Google Scholar PubMed
[44] Turki A, Kochkar H, Guillard C, Berhault G, Ghorbel A. Effect of Na content and thermal treatment of titanate nanotubes on the photocatalytic degradation of formic acid. Appl Catal B Env. 2013;138–139:401–15. 10.1016/J.APCATB.2013.03.020.Search in Google Scholar
[45] Shaban M, Hamd A, Amin RR, Abukhadra MR, Khalek AA, Khan A, et al. Preparation and characterization of MCM-48/nickel oxide composite as an efficient and reusable catalyst for the assessment of photocatalytic activity. Environ Sci Pollut Res. 2020;27:32670–82. 10.1007/S11356-020-09431-7.Search in Google Scholar
[46] Hassena H. Photocatalytic degradation of methylene blue by using Al2O3/Fe2O3 nano composite under visible light. Mod Chem Appl. 2016;4:176. 10.4172/2329-6798.1000176.Search in Google Scholar
[47] Nuengmatcha P, Kuyyogsuy A, Porrawatkul P, Pimsen R, Chanthai S, Nuengmatcha P. Efficient degradation of dye pollutants in wastewater via photocatalysis using a magnetic zinc oxide/graphene/iron oxide-based catalyst. Water Sci Eng. 2023;16(3):243–51. 10.1016/J.WSE.2023.01.004.Search in Google Scholar
[48] Barka N, Qourzal S, Assabbane A, Nounah A, Ait-Ichou Y. Photocatalytic degradation of an azo reactive dye, Reactive Yellow 84, in water using an industrial titanium dioxide coated media. Arab J Chem. 2010;3:279–83. 10.1016/J.ARABJC.2010.06.016.Search in Google Scholar
[49] Siddique M, Khan NM, Saeed M. Photocatalytic activity of bismuth ferrite nanoparticles synthesized via sol-gel route. Z fur Phys Chem. 2019;233:595–607. 10.1515/ZPCH-2018-1225.Search in Google Scholar
[50] Isai KA, Shrivastava VS. Photocatalytic degradation of methylene blue using ZnO and 2%Fe–ZnO semiconductor nanomaterials synthesized by sol–gel method: a comparative study. SN Appl Sci. 2019;1:1–11. 10.1007/S42452-019-1279-5.Search in Google Scholar
[51] Kushwaha R, Garg S, Bajpai S, Giri AS. Degradation of Nile blue sulphate dye onto iron oxide nanoparticles: Kinetic study, identification of reaction intermediates, and proposed mechanistic pathways. Asia-Pacific J Chem Eng. 2018;13:e2200. 10.1002/APJ.2200.Search in Google Scholar
[52] Fu H, Sun S, Yang X, Li W, An X, Zhang H, et al. A facile coating method to construct uniform porous α-Fe2O3@TiO2 core-shell nanostructures with enhanced solar light photocatalytic activity. Powder Technol. 2018;328:389–96. 10.1016/J.POWTEC.2018.01.067.Search in Google Scholar
[53] Vinosel VM, Anand S, Janifer MA, Pauline S, Dhanavel S, Praveena P, et al. Preparation and performance of Fe3O4/TiO2 nanocomposite with enhanced photo-Fenton activity for photocatalysis by facile hydrothermal method. Appl Phys A Mater Sci Process. 2019;125:1–13. 10.1007/S00339-019-2622-9.Search in Google Scholar
[54] Mohamed MM, Bayoumy WA, Goher ME, Abdo MH, Mansour El-Ashkar TY. Optimization of α-Fe2O3@Fe3O4 incorporated N-TiO2 as super effective photocatalysts under visible light irradiation. Appl Surf Sci. 2017;412:668–82. 10.1016/J.APSUSC.2017.03.200.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy

