Abstract
Rheumatoid arthritis (RA) is a persistent inflammatory illness that causes joint destruction and dysfunction due to the activation of macrophages and the generation of reactive oxygen species. Current therapy choices frequently limit the effectiveness of targeting the inflammatory areas. To reduce inflammation and oxidative stress in RA, this research will create and assess multifunctional nanoparticles that selectively target inflammatory cells and deliver therapeutic medicines. Tannic acid, ferric chloride hexahydrate, methotrexate (MTX), and bovine serum albumin were conjugated using sonication and centrifugation to create the nanoparticles. Folic acid was added to improve the ability to target. Transmission electron microscopy, dynamic light scattering (DLS), UV-vis spectroscopy, and in vitro release experiments were used to characterize the nanoparticles. RAW 264.7 macrophage cells were used to test the cellular uptake of the nanoparticles using confocal microscopy and fluorescence-activated cell sorting (FACS). TFMBP-FA achieved 65.56%, and TFMBP reached 68.96%, indicating a high drug delivery rate for the synthesized nanoparticles. Confocal microscopy showed that the TFMBP-FA group had a greater density of fluorescent markers, indicating that the cells effectively targeted and absorbed the inflammatory environment. These results imply that the created nanoparticles may improve how medications are delivered during RA therapy.
Abbreviations and expansions
- RA
-
Rheumatoid arthritis
- MTX
-
Methotrexate
- DLS
-
dynamic light scattering
- TEM
-
transmission electron microscopy
- FACS
-
fluorescence-activated cell sorting
- RAW
-
RAW 264.7 – A macrophage cell line used in the experiment
- TFMBP-FA
-
TA-Fe3+-MTX@BSA-PEG-FA – Tannic Acid-Ferric Chloride-Methotrexate@Bovine Serum Albumin-Polyethylene Glycol-Folic Acid
- DMARD
-
disease-modifying antirheumatic drugs
- NSAID
-
non-steroidal anti-inflammatory drugs
- GC
-
glucocorticoids
- RONS
-
reactive nitrogen and oxygen species
- ELVIS
-
extravasation through leaky vasculature and subsequent inflammatory cell-mediated sequestration
- FA
-
folic acid
- TA
-
tannin acid
- MOF
-
meta-organic framework
- BSA
-
bovine serum albumin
- ROS
-
reactive oxygen species
- EDC
-
ethylcarbodiimide hydrochloride
- DMEM
-
Dulbecco’s modified eagle medium
- PBS
-
phosphate-buffered saline
- NHS
-
N-Hydroxysuccinimide
- NaHCO3
-
sodium bicarbonate
- CCK-8
-
cell counting kit-8
- HUVEC
-
human umbilical vein endothelial cells
- FBS
-
fetal bovine serum
- mPEG
-
methoxy polyethylene glycol
- PDI
-
polydispersity index
- FT-IR
-
Fourier transform infrared
- UV-Vis
-
Ultraviolet-Visible spectroscopy
- IVRT
-
in vitro release test
- VICTOR TM X4
-
a multilabel plate reader purchased from Perkin Elmer Inc
- LPS
-
lipopolysaccharides
- DCFH-DA
-
dichlorofluorescein diacetate – a fluorescent probe used for detecting reactive oxygen species
- ELISA
-
enzyme-linked immunosorbent assay
- FACS
-
fluorescence-activated cell sorting
- qPCR
-
quantitative PCR
- SD
-
standard deviation
1 Introduction
Rheumatoid arthritis (RA) is an autoimmune disease that can lead to dysfunction and deformity in a patient’s joints. RA spreads worldwide, affecting approximately 0.5–1% of people in the United States and Europe [1]. RA is a long-term autoimmune condition that destroys, swells, and inflames joints. Immune cells and pro-inflammatory cytokines are essential players in the inflammatory response directed toward the synovium. Therapeutic approaches to address chronic inflammation and joint deformities include immune cell modification, cytokine suppression, and antioxidants. DMARDs, including biologics, corticosteroids, and NSAIDs, are the current therapy for RA. Although DMARDs delay the course of the disease but can have serious adverse effects, NSAIDs relieve symptoms. Biologic DMARDs are costly and need parenteral administration, which limits accessibility and adherence. However, they target specific immunological pathways. Around 1.3 million adults in the United States suffer from RA, and it has become one of the most common diseases that cause disability. RA is more prevalent in females, and the majority of patients diagnosed with RA are between the ages of 30 and 50 [2]. Its pathological features include synovial hyperplasia, angiogenesis, and bone and cartilage destruction, and it often occurs with symptoms including fever, fatigue, stiffness, deformation, and pain in one or more joints. RA is an incurable disease, and in later stages, patients experience constant pain and face heavy economic burdens. Patients’ living conditions are greatly affected by the disease. Therefore, finding a treatment that can restrict the progression of RA in its early stages is essential to its treatment [3,4]. With their effective and targeted delivery mechanism, the suggested nanoparticles seek to enhance the available RA therapies. Encapsulating DMARDs such as methotrexate can be released selectively at inflamed joints while preventing degradation.
Nowadays, the treatment of RA mainly focuses on medicines, physical therapies, and surgeries, which can only relieve the pain temporarily. Medicines like nonsteroidal antiinflammatory drugs (NSAIDs) and disease-modifying antirheumatic drugs (DMARDs), glucocorticoids (GCs), and biological agents are widely used [5]. Because they block COX enzymes, NSAIDs can treat pain and inflammation in RA patients. However, prolonged use of NSAIDs can lead to kidney damage, gastrointestinal ulcers, and cardiovascular problems. The medicine Disease modifying anti-rheumatic drugs (DMARDs), such as methotrexate, target specific immunological pathways; nonetheless, they might have adverse consequences, such as organ damage and immunosuppression, which call for ongoing monitoring. Even though the medicines do have effects on RA, they cannot eliminate inflammation and often come with side effects. Some side effects include diseases affecting the lungs, heart, digestive system, liver, pancreas, and nerve system, which can be potentially fatal [6]. Indirect delivery to the inflamed joints triggers severe side effects, and the tissues get injured when the medicine is delivered to noninflammatory organs.
The past research shows that inflammation plays a vital role in the initiation of RA, and immune cells, inflammatory factors, and reactive nitrogen and oxygen species (RONS) are strongly related to each other in the development of RA [7]. Immune cells detect the healthy tissues as a negative stimulation and start inflammation. Immune cells like macrophages change to M1 polarized phenotype due to the acidic environment in the inflamed joint. M1-type macrophages release abundant inflammatory factors (TNF-α and IL-1β), which are small proteins that can activate the signaling pathway of RONS [8]. Excess RONS increases the immune cells, further enhancing the vicious cycle of RA. RONS can also deteriorate the symptoms. It can stimulate osteoclasts, synovial fibroblasts, and epithelial cells, which are the cells that cause bone erosions, synovitis, and angiogenesis [9]. Therefore, RONS and inflammatory factors are essential targets in the treatment, and conversion of M1-type macrophage is also a strategy in the therapy.
Nanotechnology has been widely used in every aspect of life, and nanoparticles have also been proven to treat various diseases effectively. Due to their small size and specific properties, nanoparticles have been considered a promising strategy for RA therapy. An autoimmune reaction that results in persistent inflammation and joint damage is called RA. By delivering anti-inflammatory medications to inflammatory joints, blocking immune cell activation, and altering the cytokine milieu, nanoparticles can target these pathways and lessen inflammation and joint degradation. For RA therapies, multifunctional nanoparticles provide targeted distribution, regulated release, multifunctionality, and fewer dosing cycles. They can be designed to improve patient compliance, keep therapeutic doses at optimal levels, and incorporate imaging agents for effectiveness monitoring. Nanoparticles can be either passively or positively accumulated in the inflamed joint. Extravasation through leaky vasculature and subsequent inflammatory cell-mediated sequestration (ELVIS) affects the accumulation of small-size nanoparticles in the joint. Also, M1-type macrophages have overexpressed receptors on the cell membrane. For example, the folate receptor in the membrane is overexpressed, so the nanoparticles decorated with folic acid (FA) would actively accumulate in a place with many M1 macrophages. Active and passive targeting help nanoparticles directly deliver to the inflamed joint, leading to a high therapeutic effect and a low risk of getting side effects. Integrating medicinal, diagnostic, and targeted functions into a single nanoscale entity is known as multifunctional nanoparticles, an emerging area of nanotechnology. They reduce adverse effects, increase specificity, and improve therapy effectiveness. The synthesis process includes conjugation or encapsulation of the medicinal substance, surface changes, and core components.
Tannin acid (TA) is a type of polyphenol and is an FDA-approved medicine today. Tannin acid has an abundant trihydroxybenzene group, which enables TA to develop versatile functions [10,11,12]. TA can attenuate oxidative stress and eliminate RONS due to its effect on antioxidative activity. Metal-organic frameworks (MOFs) consist of metal clusters and organic ligands to form one-, two-, or three-dimensional structures [13]. TA and iron(III) chloride MOFs can build the structure of the experimental nanoparticle from the bottom up. Bovine serum albumin (BSA) is the globulin from bovine serum coated around the nanoparticles, and it can increase the solubility and stability of nanoparticles [14,15]. Inside the tannin nanoparticles, a type of DMARDS medicine, methotrexate (MTX), will be loaded.
MTX would be released after the nanoparticles are either passive or actively accumulated in the inflamed joint. Direct delivery of MTX can reduce inflammation by getting rid of reactive oxygen species (ROS) and RNS and can reduce the risk of side effects [16]. The nanoparticles can reduce the RONS and inflammatory factors in the joint, decreasing the amount of M1-type macrophage and relieving inflammation [17,18,19]. Therefore, the nanoparticles demonstrate an effective treatment for RA therapy. RA is exacerbated by reactive oxygen and nitrogen species (RONS), which lead to tissue damage, persistent inflammation, and inflammation. Antioxidants, anti-inflammatory medications, and nanoparticles are therapeutic approaches that lower inflammation and oxidative stress. Effective treatment plans for RA have been made possible by our growing understanding of the disease’s pathophysiology; nonetheless, issues with systemic toxicity, bioavailability, and insufficient response still exist. Nanomedicines have been created to overcome these drawbacks with their controlled characteristics, customized drug release patterns, and active targeting. Recent developments in antigen-specific tolerance, pro-resolving therapy, and immunometabolism modulation in nanomedicines for RA treatment are covered in this review [20]. Two chronic inflammatory joint illnesses are osteoarthritis and RA. Pain relief and cartilage repair are achieved using conventional medications.
However, conventional medications have drawbacks, including the cartilage barrier and quick drug elimination. Controlled drug release, extended retention times, and improved penetration into joint tissue are some ways that nanoparticles might increase the effectiveness of intra-articular injections. With an emphasis on tissue/cell targeting and controlled drug release, this review examines nanoparticle-based therapeutics for managing OA and RA [21]. This study investigates the viability of predicting cardiovascular risk in people with RA using long-term blood samples. Assessing biomarker stability and lipid profiles integrates conventional risk factors with markers unique to RA. Disease activity indexes and cardiovascular outcomes are studied using longitudinal data analysis. By filling scientific gaps and emphasizing clinical translation, the project seeks to enhance patient outcomes, boost cardiovascular risk prediction, and enable customized medicines [22].
2 Materials and methods
2.1 Materials and cells
2.1.1 Materials
Tannin acid (TA) was obtained from Shanghai Titan Scientific Co., Ltd. (CAS: 1401-55-4), and iron(iii) chloride hexahydrate (FeCl3·6H2O) was from Sigma-Aldrich Co., Ltd. (CAS: 10025-77-1). BSA was purchased from Merck Science and Technology Co., Ltd. (CAS: 9048-46-8), and methotrexate (MTX) was purchased from Adamas Pharmaceutical Co., Ltd. (CAS: 59-05-2). 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) were gained from Tokyo Chemical Industry Co., Ltd. with (CAS: 25952-53-8) and (CAS: 6066-82-6). FA-PEG2000-NH2 and mPEG2000-NH2 were purchased from Ponsure Biological Co., Ltd. Dulbecco’s modified eagle medium (DMEM) and phosphate-buffered saline (PBS) were obtained from Thermo Fisher Scientific Inc. Reagents employed in the manufacture of nanoparticles are NHS and EDC. ToEDC activates carboxyl groups with amines to couple them and produce amide bonds. NHS produces a stable intermediate, which improves this coupling efficiency. The creation of nanoparticles requires both reagents. Sodium bicarbonate (NaHCO3) and N-hydroxysuccinimide (NHS) esters were purchased from Shanghai Saint-Bio Co., Ltd. TMB ELISA substrate solution was from Beyotime Co., Ltd. CCK-8 cell proliferation and cytotoxicity detection kit was purchased from Dalian Bergolin Co., Ltd. The CCK-8 cell proliferation and cytotoxicity detection kit from Dalian Bergolin Co., Ltd. assesses the safety and biocompatibility for possible therapeutic applications and evaluates the viability and cytotoxicity of cells exposed to nanoparticles. This allows comparison across experimental conditions and the determination of cytotoxic effects.
2.1.2 Cells
The macrophage cell line (RAW 264.7) and human umbilical vein endothelial cells (HUVEC) used in the experiment were from the Shanghai Cell Centre of the Chinese Academy of Sciences. RAW 264.7 and HUVEC were cultured in DMEM medium composed of 10% fetal bovine serum (FBS) and 1% streptomycin and penicillin. The cells were stored in a CO2 incubator purchased from Thermo Fisher Scientific Inc.
2.2 Synthesis of TA-Fe3+-MTX@BSA
Using an electronic balance, 102.5 mg of 95% tannic acid (TA), 100.6 mg of 98% ferric chloride hexahydrate (FeCl3·6H2O), 40.6 mg of 98% BSA, and 5.1 mg MTX were weighed. TA and FeCl3·6H2O were dissolved in 10 mL of deionized water and BSA was dissolved in 5 mL. MTX was added into a 10 mg/mL ferric chloride solution and reacted for 10 min using an ultrasonic reactor. To treat RA, methotrexate (MTX) and ferric chloride are mixed to form a stable, targeted nanoparticle delivery system. This combination guarantees that MTX is selectively administered to inflamed joints, improving its stability and solubility, which increases efficacy and decreases adverse effects. After the reaction, 10 mg/mL of TA solution was added, stirred, and reacted for 10 min using an ultrasonic reactor. After that, 8 mg/mL of BSA solution was added and reacted for 10 min. The overnight reaction was carried out under ice bath conditions for 24 h. With minimum equipment needed, overnight ice bath reactions provide temperature control, stability, yield, purity, practicality, safety, and enhancement of selectivity and stability while minimizing the danger of overheating and avoiding decomposition. Samples were collected by centrifugation at 14,000 rpm for 15 min and then cleaned with deionized water three times. A drying machine dried the samples, and the nanoparticle TA-Fe³⁺-MTX@BSA was obtained (Figure 1). To preserve the uniformity, stability, biocompatibility, and purity of the nanoparticles, avoid aggregation, and improve performance, the sample must be cleaned three times with deionized water following centrifugation.

Synthesis of TA-Fe3+-MTX@BSA-PEG-FA and TA-Fe3+-MTX@BSA-mPEG.
2.3 Synthesis of TA-Fe3+-MTX@BSA-mPEG/TA-Fe3+-MTX@BSA-PEG-FA
Two nanoparticles with and without folic acid would be synthesized separately. A magnetic stirrer works well for nanoparticle mixes because of its practical efficiency, repeatability, uniform mixing, and temperature control. Even component distribution is guaranteed, reagent interaction is maximized, temperature is maintained consistently, stirring speeds may be adjusted, and contamination hazards are reduced. The capacity to prevent aggregation, stabilize surface chemistry, employ moderate functionalization techniques, and inherent stability all contribute to the shape maintenance of nanoparticles. Surface treatments that provide a hydration layer, stop aggregation, and improve stability include BSA and PEG. Two portions of TA-Fe³⁺-MTX@BSA weighed about the same mass (25.6 and 25.0 mg), and the nanoparticles were put into two separate bottles. Two portions of EDC (25.1 and 25.3 mg) and two portions of NHS (25.3 and 25.8 mg) were added to the bottle. A magnetic stirrer was used to stir the two bottles of mixture for 30 min. A total of 10.3 mg of FA-PEG2000-NH2 and 10.2 mg of mPEG2000-NH2 were weighed and dissolved with deionized water in separate centrifuge tubes. To synthesize TFMBP-FA and TFMBP, FA-PEG2000-NH2 solution and mPEG2000-NH2 solution were mixed with TFMBP in separate bottles. After 3 h of reaction, the samples were collected thrice by centrifugation at 14,000 rpm for 15 min. Finally, the nanoparticles were dried and collected with 10.1 mg TFMBP and 11.8 mg TFMBP-FA. The yield rate for TFMBP was 40.4%, and that for TFMBP-FA was 47.2%.
2.4 Preparation and characterizations of TA-Fe3+-MTX@BSA-PEG-FA
The nanoparticles TFMBP-FA and TFMBP were synthesized using the aforementioned procedures. After TFMB was synthesized by conjugating tannin acid, FeCl3·6H2O, BSA, and MTX, two nanoparticles were created separately by adding FA-PEG2000-NH2 and mPEG2000-NH2. FA-PEG2000-NH2 and mPEG2000-NH2 are nanoparticles that target cells that express the folate receptor, thereby improving medication delivery. Active targeting, improved drug specificity, stability, solubility, and dual functionalization are among the benefits they provide, giving designers more freedom to create nanoparticles with various therapeutic effects and processes. The successful conjugation of the nanoparticles was confirmed by transmission electron microscopy (TEM), DLS, UV-vis, FT-IR characterization, and in vitro release test. Numerous tests, including TEM, DLS, UV-vis, FT-IR, and in vitro release experiments, visually assess the size, shape, morphology, aggregation, optical characteristics, and molecular structure to confirm the production of nanoparticles. Their effectiveness as drug delivery devices is ensured by the fact that these tests also yield data on hydrodynamic diameter and surface charge for stability evaluation.
TEM examined the feature and surface morphology. The size of the particles, zeta potential, and polydispersity index (PDI) were measured using dynamic light scattering (DLS). One method for determining a nanoparticle’s size and zeta potential is DLS. A detector is used to analyze the light scattered off the nanoparticles by a laser beam. The hydrodynamic diameter may be computed using the zeta potential and diffusion coefficient. DLS analyzes hydrodynamic diameter and particle size distribution, utilizing scattered light intensity variations. This technique is vital for formulation development, stability studies, and quality control in nanoparticle characterization. The method applies the Stokes–Einstein equation and the polydispersity index to analyze particle size distribution. The UV-vis and infrared absorbance spectra were determined by UV-Vis spectroscopy and infrared FT-IR spectroscopy. FT-IR spectroscopy and UV-Vis spectroscopy are two methods that provide complimentary data in various scientific domains. FT-IR concentrates on vibrational transitions in the infrared spectrum, whereas UV-Vis concentrates on electronic transitions in the ultraviolet and visible areas. Also, the drug delivery system’s safety, efficacy, and quality were assessed by an in vitro release test (IVRT).
2.5 In vitro cytotoxicity study
RAW 264.7 and HUVEC cells were seeded in a 96-well plate at 5,000 cells/healthy density and cultured in a humidified incubator at 37°C with 5% CO2 for 24 h (the cell density reached approximately 70%). Nanoparticles are trained by incubating them at certain temperatures and times to enable effective drug encapsulation and functionalization while regulating their physicochemical characteristics. This optimizes the stability, size, and drug-loading efficiency of the nanoparticles. The cell density is measured using a cell counting board. Meanwhile, the medium with cell solutions is prepared and diluted to different concentrations (0, 50, 100, 150, 200, 250, and 300 µg/mL). Then, the old cell culture medium is discarded in the healthy plate, 100 μL of the culture is added to the aforementioned medium containing synthesized nanoparticle materials at different concentrations, culture medium with nontargeting nanomaterials is added to the control group, and neither cells nor materials are added to the blank group. After the cells have grown in the incubator for 24 h, the old medium is discarded and it is replaced with a fresh medium containing 10% CCK-8 reagent. A total of 10 μL is added to each well, and the plates are incubated for 1 h in the incubator. A microplate reader is used to measure the absorbance of the culture medium in each well at a wavelength of 450 nm. The method of measuring absorbance at 450 nm is a sensitive, specific, and standardized approach to measuring cell viability. It is also connected with forming formazan dye and yields quantitative data that are easy to interpret, repeatable, and compatible with various tests. Four wells were set up as parallel samples for each concentration of the experimental, control, and blank groups. The VICTOR TM X4 multilabel plate reader was purchased from Perkin Elmer Inc.
2.6 Cellular uptake study
Raw 264.7 cells were seeded in a 96-well plate and cultured in a 5% CO2 incubator. A total of 10 μL of fluorescent conjugation (NHS-ester) and 50 μL of 1 M NaHCO3 were added to the nanoparticles separately. The mixtures were stirred in an essential condition for 2 h and then centrifuged for 15 min at 14,000 rpm. A total of 100 μL RAW 264.7, 3,900 μL DMEM, 1 μL LPS, and 0.1 μL of the nanoparticles were added to each well in the plate. 500 μL PBS was added to the solution, and the solution in each well was removed and placed into a 1.5 mL microcentrifuge tube, followed by centrifugation for 3 min at 1,200 rpm. Finally, 1 mL of PBS was added to each centrifuge tube, and each tube was analyzed with flow cytometry. The confocal microscopy images were taken with Alexa Fluorescence 488.
2.7 RONS scavenging activity assay
The RONS scavenging activity assay improves sensitivity and accuracy by eliminating excess DCFH-DA from cells. To achieve consistency and trustworthy data for assessing RONS scavenging activity, this minimizes interference, removes nonspecific binding, decreases background noise, and guarantees that the fluorescence is from intracellular RONS. RAW 264.7 cells were collected and re-suspended, adjusted to a density of 106 cells/mL, and seeded into 3.5 cm cell culture dishes according to the tested group (LPS+, TFMBP, LPS+TFMBP, TFMBP-FA, LPS+TFMBP-FA). A total of 10 μL of LPS and 2 μL of the nanoparticles were added into the dishes and placed in the 5% CO2 37°C incubator for 24 h. Each cell culture dish was stained with 5 µM of ROS probe DCFH-DA and cultured in an incubator under the same conditions for 20 min. Then, the cell culture dishes were washed three times with PBS to remove excess DCFH-DA outside the cells. Finally, a scanning laser confocal microscope was applied to observe and record the samples. High-resolution imaging is possible using scanning laser confocal microscopes, capturing minute details for analysis and depth perception. They employ fluorescent labeling, high-contrast pictures, and quantitative analysis to evaluate the quantity and distribution of nanoparticles. They record time-lapse videos for live cell imaging and temporal dynamics.
2.8 Inflammatory factors scavenging study
All reagents and samples returned to room temperature (18–25°C) before use. RAW 264.7 was seeded into 3.5 cm cell culture dishes according to the tested group (LPS+, TFMBP, LPS+TFMBP, TFMBP-FA, LPS+TFMBP-FA). A total of 10 μL of LPS and 2 μL of the nanoparticles were added into the dishes and placed in the 5% CO2 37°C incubator for 24 h. In a 96-well plate, two precoated plates were prepared, and 100 μL of cell solution was added to each well. The plate was wrapped with tape and placed in the fridge overnight. The solution in the well was discarded and washed four times with ELISA wash buffer. Each well was washed with a wash buffer (300 µL) using a multichannel pipette. After the last wash, the residual wash buffer was removed by pipetting or pouring. The plate was turned upside down, and it was blot dried with a clean paper towel. 100 μL of the prepared TNF-α and IL-1β detection antibody was added to each well. The wells were covered and incubated for 30 min at room temperature. 100 μL of TMB one-step substrate reagent was added to each well. The wells were covered and incubated in the dark at room temperature for 10 min with gentle shaking. The solution on the plate turned blue. 100 μL of stop solution (H2SO4) was added to each well, and the solution turned yellow. A microplate reader at 450 absorbance was used to examine the plate.
3 Results and discussions
3.1 Synthesis of TA-Fe3+-MTX@BSA-PEG-FA
Nontargeting material TA-Fe3+-MTX@BSA (TFMB) was synthesized by conjugating tannin acid, ferric chloride hexahydrate, MTX, and BSA through sonication and centrifugation. The nanoparticle TA-Fe3+-MTX@BSA-PEG-FA (TFMBP-FA) was synthesized by combining folic acid with TFMB, and NHS and EDC were used to help conjugation. Two different kinds of nanoparticles were created: TA-Fe3+-MTX@BSA-PEG-FA and TA-Fe3+-MTX@BSA-mPEG. The folic acid-containing nanoparticles were designed to improve macrophage targeting in inflammatory joints by binding to overexpressed folate receptors. The successful synthesis of the nanoparticle was qualified by TEM. From TEM graphs, the polydisperse nanoparticles formed various distributions through overlapping due to their small size and formed a web structure (Figure 3a and b). mPEG2000-NH2 was attached to the surface of the nanoparticles and formed TA-Fe3+-MTX@BSA-PEG (TFMBP). By adding folic acid, the polydisperse arrangement of TFMBP-FA is retained in a web structure (Figure 3c and d). With or without folic acid, the nanoparticles maintained the same shapes, affirming the basic structure of the nanoparticle. Compared to nontargeted nanoparticles and conventional treatments, the study demonstrates that multifunctional nanoparticles can enhance methotrexate delivery and efficacy in RA therapy. Targeted folic acid nanoparticles demonstrated superior accumulation in inflamed joints, reduced systemic toxicity, and improved therapeutic outcomes.
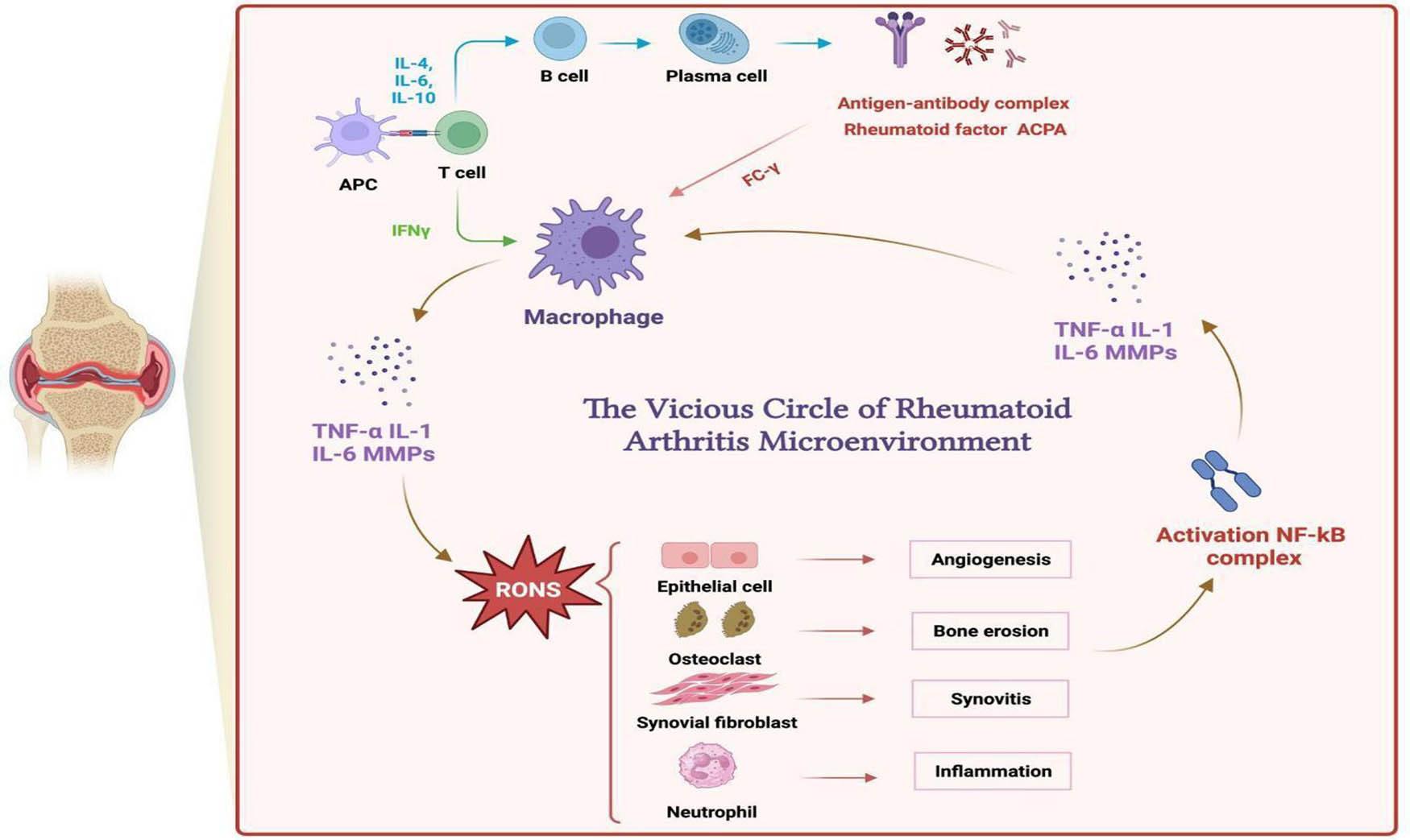
Effect of RONS in inflamed joints and the vicious circle of RA.

TEM micrographs of (a) TFMBP (scale bar = 500 nm) and (b) TFMBP (scale bar = 200 nm). TEM micrographs of (c) TFMBP-FA (scale bar = 500 nm) and (d) TFMBP-FA (scale bar = 200 nm).
3.2 Preparation and characterization of TA-Fe3+-MTX@BSA-PEG-FA
Both TFMBP and TFMBP-FA were measured using dynamic light catering (DLS). DLS measures the size and zeta potential of the nanoparticles, and the zeta potential indicates the stability of the particles [23]. When a zeta potential that is close to 0 (e.g. ± 5 mV) would be considered unstable, and as the absolute value increases, the particle becomes more stable. A nanoparticle with zeta potential ± 10 mV is considered charged neutral. A negative zeta potential value indicates that the material would not combine with other proteins and molecules, which helped the active targeting [24]. TFMBP had an average size of 171.7 ± 1.13 nm, and its zeta potential was −5.24 (Figure 4a and c). TFMBP-FA’s size average was 286.5 ± 1.89 nm, and its zeta potential was −12.4 (Figure 4b and c). TFMBP-FA had a relatively larger size compared to TFMBP. The conjugation of folic acid on the nanoparticle’s surface increased the size. TFMBP-FA’s zeta potential was ∼10 mV, and TFMBP’s zeta potential was ∼5 mV (Figure 4c). The change in the zeta potential shows that folic acid lowered the zeta potential, which led to higher stability and better biological application.

Dynamic light scattering graphs represent (a) TFMBP size and (b) TFMBP-FA size. (c) Zeta potential and (d) the polydispersity index (PDI) of TFMBP and TFMBP-FA.
The polydispersity index (PDI) displays the heterogeneity of the particles based on their size [25]. When the index is closer to 0.0, the size distribution is more petite and uniform. TFMBP has a PDI of 0.157, and TFMBP-FA has a PDI of 0.133 (Figure 4d). TFMBP-FA has a smaller PDI than TFMBP, which indicates that the size of TFMBP-FA is approximately uniform. By adding the folic acid group, the size of the nanoparticle increased, the PDI decreased, and the stability of the material also increased. Therefore, the nanoparticles were proved to be suitable for biological application.
TFMBP and TFMBP-FA showed a solid absorbance to the ultraviolet light, which has a peak absorbance at ∼300 nm wavelength (Figure 5a). The wavelength of UV or visible light absorbed or transmitted through the particle was collected and compared by ultraviolet-visible spectroscopy.

(a) Ultraviolet-visible (UV-vis) spectroscopy analysis of TFMBP and TFMBP-FA. (b) Fourier transform infrared spectroscopic (FTIR) analysis of TFMBP and TFMBP-FA.
Nanoparticles were characterized by Fourier transform infrared (FTIR) spectroscopy, and their absorbance to infrared radiation by FTIR spectroscopy was tested [26]. FTIR spectroscopy is a method that helps detect chemical bonds and functional groups to characterize nanoparticles. A suspension or pellet must be prepared to measure infrared radiation. The data must then be converted into an infrared spectrum with particular chemical vibration peaks. FTIR spectroscopy is used to determine molecular structures and identify chemical species. It is also used to study nanoparticles’ surface effect and identify the functional groups’ presence [27]. The characteristic absorbance peaks for TFMBP-FA at 945 cm−1 (attributed to the N–H motions in FA), 1,480 cm−1 (absorption band of the phenyl ring in FA), and ∼1,900 cm−1 (amide bond, H–N–C═O bending vibrations from FA) correspondingly appeared in the TFMBP nanoparticle (Figure 5b).
The nanoparticles were characterized by an in vitro release test (IVRT), and the examination is to assess the safety, efficacy, and quality of nanoparticle-based drug (MTX) delivery systems [28]. IVRT can reflect the combined effects of several physicochemical characteristics, particle or droplet size, viscosity, microstructure arrangement of the material, and state of aggregation of the dosage form [29]. The standard curves were measured at five different MTX concentrations. The peak absorbance of various MTX concentrations was at 390 nm (Figure 6a). As the MTX concentration increases, the absorbance of UV light also increases (Figure 6b). According to the Beer-Lambert Law, absorbance and route length are closely correlated with MTX concentration. In the UV spectrum, MTX exhibits distinct absorption peaks at about 300 nm. Higher absorbance results from more molecules absorbing UV light as MTX concentration rises. This connection makes accurate measurement and medication release monitoring possible. The R 2 value is 0.9999, which shows the data and equation fit properly. The R 2 value shows the correlation between the data and the line equation. According to the standard curve equation y = 14.05798x + 0.00267, the drug delivery rate for TFMBP-FA is 65.56%, and the drug delivery rate for TFMBP is 68.96%, which indicates that the nanoparticles would be able to be delivered to the inflammation place with enough MTX.

(a) Absorbance vs wavelength graph of five different concentrations of MTX and (b) standard curve of drug release.
3.3 In vitro cellular uptake
The cellular uptake of TFMBP-FA and TFMBP was assessed by using the RAW 264.7 cells. The cells were divided into six different experimental groups: LPS−, LPS+, TFMBP, LPS+TFMBP, TFMBP-FA, and LPS+TFMBP-FA. Lipopolysaccharides (LPS) can transform M0 macrophage to M1 type macrophage, which can mimic the inflammatory environment in the experiment. The cellular uptake of the materials was measured by fluorescence-activated cell sorting (FACS) and confocal microscopy because both techniques used lasers as part of the measurement, and fluorescent conjugation (NHS-ester) was added to the nanoparticles. Among the confocal microscopy images, both TFMBP and TFMBP-FA images show the abundant presentation of the fluorescent markers, indicating that the cells can take the nanoparticles (Figure 7a and b). In the TFMBP-FA images, the fluorescent markers have a higher density, which shows the effectiveness of FA, which functions as an actively targeted material. Increased fluorescence marker density in TFMBP-FA pictures enhances signal strength, sensitivity, and nanoparticle identification, allowing for more precise quantification and lucid visualization. However, it also brings significant difficulties, such as improved photobleaching and background fluorescence, which call for optimization to strike a compromise between signal strength and noise. The blank group did not present any fluorescent markers because they did not add any materials. Assuring fluorescence specificity, removing artifacts, and bolstering the validity of findings by proving rigorous experimental design and effects attributable to experimental variables are just a few reasons the statement highlights the importance of the blank group (Figure 7a and b).
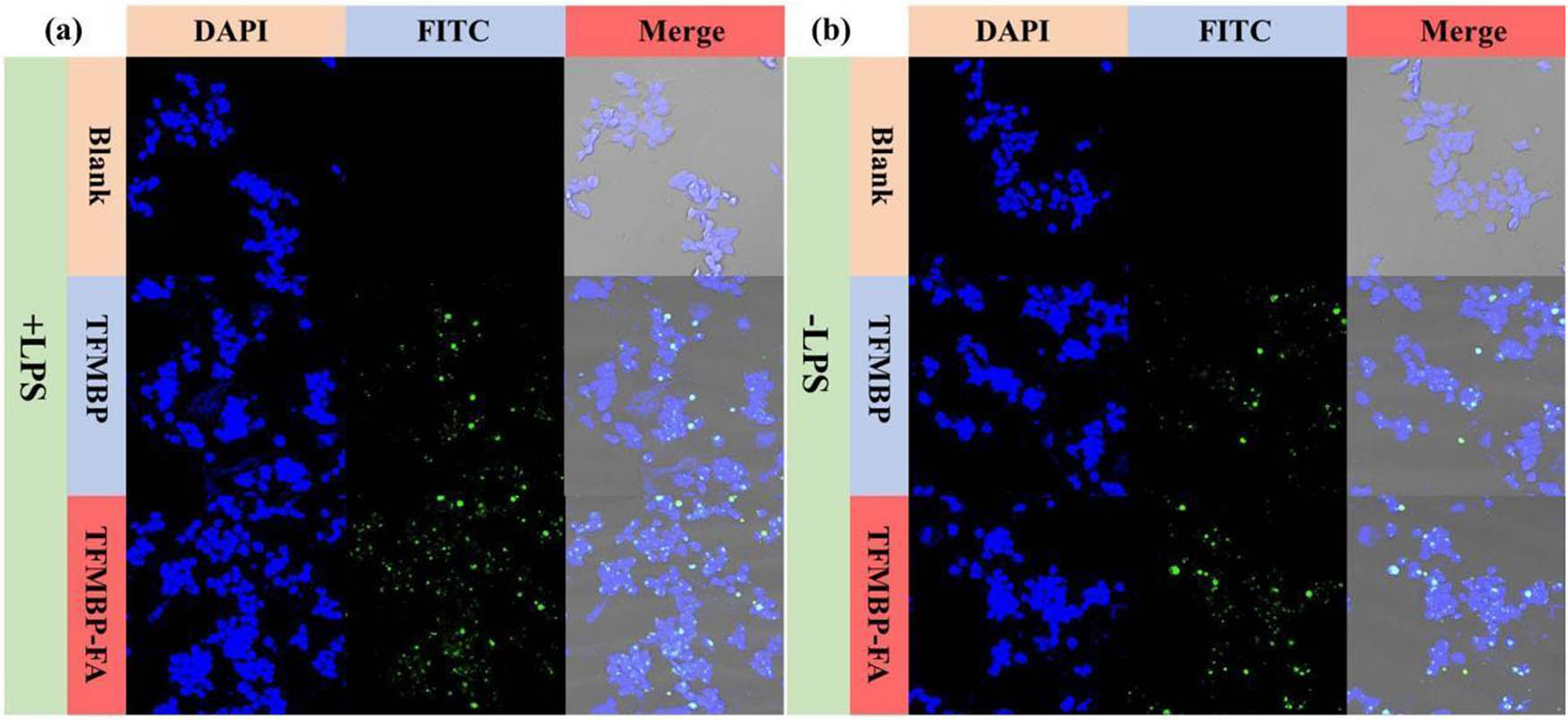
Fluorescent confocal microscopy images of (a) RAW 264.7 cells treated with TFMBP and TFMBP-FA and (b) activated RAW 264.7 cells treated with TFMBP and TFMBP-FA.
According to the data from FACS, the fluorescence intensity was differentiated due to the materials they added. TFMBP-FA and LPS+TFMBP-FA had a fluorescence intensity of ∼105; TFMBP and LPS+TFMBP had a fluorescence intensity of ∼104; and LPS− and LPS+ had a fluorescence intensity of ∼103 (Figure 8). The nanoparticles with folic acid had the highest value among the groups, whether with LPS+ or LPS−. Nanoparticles with folic acid can be better uptake by the cells, as proved in the data, and the active targeting capability of TFMBP-FA is demonstrated.

Flow cytometry images of RAW 264.7 cells and activated RAW 264.7 cells treated with TFMBP or TFMBP-FA.
3.4 In vitro cytotoxicity
The cell counting kit-8 (CCK-8) was used to measure the cell viability and cytotoxicity of the material, which is essential to maintaining cell proliferation and cellular health [30]. CCK-8, under the condition of existing electron carriers, is reduced by the dehydrogenase inside the cell into soluble formazan that dyes the culture solution orange. The quantity of formazan produced is directly proportional to the number of living cells.
The cytotoxicity of the material TFMBP-FA was tested by RAW 264.7 and HUVEC cells. In HUVEC cells and RAW 264.7 with normal and activated macrophages, the cell viability demonstrated a concentration-dependent trend; as the concentration increased, the cell viability decreased. In HUVEC cells, the cell viability is above 100% when both materials are at 50 mM (Figure 9a). The cell viability of TFMBP-FA was significantly higher than TFMBP until 150 mM. In RAW 264.7 cells with normal macrophages, the cell viability of TFMBP-FA at 50, 100, 150, and 200 mM were ∼90% (Figure 9b), and the rate of TFMBP decreased gradually. Starting at 100 mM, the cell viability rates of TFMBP-FA were higher than TFMBP. In RAW 264.7 cells with activated macrophages, cell viability was above 50% at 50 mM for both nanoparticles (Figure 9c). Compared to the cell viability in normal and activated macrophages, cells with activated macrophages had a higher viability rate. Because tannin acid and MTX would react with RONS and inflammatory factors, the reaction eliminates the remainder of the nanoparticles (Figure 10). Fewer nanoparticle portions would remain, leading to lower cytotoxicity to the cells. 300 mM had the lowest viability rate, and 50 mM had the highest (∼100 mM) in three experiments, which is suitable for biological application based on safety and biosecurity. Therefore, 50 mM was used as the standard concentration in the later experiment.

In vitro, cytotoxicity test of (a) HUVEC cells, (b) RAW 264.7 cells, and (c) activated RAW 264.7 cells in the presence of six different concentrations of TFMBP and TFMBP-FA.

Schematic illustration of the synthesis of TFMBP and its application in RA therapy.
3.5 ROS scavenging capability
During inflammation, immune cells release inflammatory factors (cytokines) to activate various oxidases that produce a large amount of ROS [31]. The capability of TFMBP-FA to eliminate ROS was tested by a ROS detection kit (ROS Assay Kit), which uses the fluorescent probe DCFH-DA for ROS detection. DCFH-DA itself has no fluorescence and can freely pass through the cell membrane, and after entering the cell, it can be hydrolyzed by intracellular esterases to produce DCFH. However, DCFH cannot penetrate the cell membrane through DCFH, making it easy for probes to be loaded into cells. Reactive oxygen species in the cell can oxidize nonfluorescent DCF to form fluorescent DCF. The level of the fluorescence of DCF is directly related to the cell’s ROS level [32].
RAW 264.7 cells were used and divided into six experimental groups. In the confocal images, LPS+, the activated macrophages, had significant bright and intense fluorescence (Figure 11a). LPS−, TFMBP-FA with activated macrophages, and TFMBP and TFMBP-FA with normal macrophages had barely visible fluorescence and low DCF density. TFMBP with activated macrophages had more visible fluorescence and low but relatively higher DCF density than LPS (Figure 11a). The significant high and low fluorescence intensities of LPS+TFMBP and LPS+TFMBP-FA were also displayed in Figure 11b. In LPS−, the subtle fluorescence intensity indicates the ROS originally contained in normal macrophages. In the inflammatory M1 macrophages, both TFMBP and TFMBP-FA had significant differences with LPS+, indicating the nanoparticles could eliminate ROS and turn M1 macrophage into M2 macrophage. The fluorescence intensity of LPS+TFMBP-FA was lower than LPS+TFMBP. Active targeting by folic acid increases the uptake of nanoparticles by the cells; increasing the uptake would eliminate more ROS. TFMBP could not eliminate the ROS in those cells that did not take in nanoparticles.

(a) Fluorescent confocal microscopy images of RAW 264.7 cells and activated RAW 264.7 cells with different treatments and then stained with ROS fluorescent probe DCFH-DA. (b) Quantified fluorescence intensity from flow cytometry. Statistics are reported as mean ± standard deviation (SD), n = 3. ***p < 0.001.
3.6 Anti-inflammation of TA-Fe3+-MTX@BSA-PEG-FA
Inflammatory factors like cytokines deteriorate inflammation and symptoms of RA. The capability of TFMBP-FA to reduce the inflammatory factors was tested using a quantitative PCR (qPCR) and enzyme-linked immunosorbent assay (ELISA) kit [33]. ELISA kit is used to detect the antibody of TNF-α and IL-1β in the cell solution. For accurate detection, measurement, and confirmation of anti-inflammatory effects, the IL-1 detection antibody is employed in inflammation scavenging research. It accurately measures IL-1β levels, enabling thorough examination and cross-sample comparison, and improves sensitivity and specificity with the slightest disturbance. RAW 264.7 cells were used for the experiment and separated into five experimental groups (LPS+, TFMBP, LPS+TFMBP, TFMBP-FA, and LPS+TFMBP-FA). The antibodies in the ELISA kit would bind to TNF-α and IL-1β, which would turn yellow after adding TMB ELISA substrate solution and H2SO4. The expression level of the antibody was indirectly related to the capability to eliminate inflammatory factors.
In the experiment of TNF-α, LPS+’s relative expression level of inflammatory factors reaches 90. LPS+TFMBP and LPS+TFMBP-FA reach the relative expression level of ∼10. After using the nanoparticle, the expression level decreases nine times (Figure 12a). Similarly, in the IL-1β experiment, the relative expression level of LPS+ was around 90. In TFMBP with LPS, the relative expression level was about 15, 6 times lower than LPS+. The relative expression level of inflammatory factors was even lower in the LPS+TFMBP-FA group, which is around 5. LPS+ was 18 times larger than that (Figure 12b). After being treated with nanoparticles, the expression levels of the inflammatory factors decreased significantly. Lower levels of inflammatory factors would gradually reduce the vicious cycle of the RA microenvironment (Figure 2) [34]. The change in the expression level of inflammatory factors before (LPS+) and after (LPS+TFMBP-FA) the treatment of nanoparticles proved to have a therapeutic effect on RA. The data suggest that downregulated inflammatory cytokines indicate that TFMBP-FA nanoparticles have an inhibitory effect on inflammatory pathways and can alleviate inflammatory symptoms.

The mRNA levels of (a) TNF-α in activated RAW 264.7 cells treated with LPS+, TFMBP, LPS+TFMBP, TFMBP-FA, and LPS+TFMBP-FA, and (b) IL-1β in activated RAW 264.7 cells treated with LPS+, TFMBP, LPS+TFMBP, TFMBP-FA, and LPS+TFMBP-FA. Statistics are reported as mean ± standard deviation (SD), n = 3. ***p < 0.001.
4 Conclusions
In summary, TFMBP-FA was developed and expected to be an effective new drug in targeted RA therapy. TFMBP-FA can reduce inflammation by eliminating ROS and decreasing inflammatory factor levels. The statistical data showed that the tannin acid released by the nanoparticle plays a vital role in the treatment, which can remove ROS to relieve the three main pathological features. As an anti-inflammation medicine, MTX can be directly delivered to the inflamed joints to reduce the inflammatory factors, which is a critical step in alleviating inflammation. FA anchored around the surface enables nanoparticles to be actively targeted to M1-type macrophages in the inflamed joints. Active targeting enabled MTX to be directly sent to joint inflammation, reducing the interaction with other organs and diminishing the side effects. TFMBP-FA was developed to have surface stability and homogeneity, which enable biological application with high biosafety. The cytotoxicity was low at 50 mM with a cell viability rate of 99%, which proved the safety of TFMBP-FA. In future studies, animal experiments can be done on inflamed mice. The animal experiment evaluate the therapeutic efficiency and long-term toxicology of the nanoparticle. By continuing the exploration, this innovative treatment holds the potential to provide new hope in the battle against RA.
Acknowledgments
I would like to express my sincere appreciation to everyone who helped me, as their contributions and support have greatly enhanced the quality and rigor of this research. First and foremost, I am grateful to my primary advisor Dr. Xu for his unwavering guidance, insights, and constant encouragement throughout the research period. His expertise and wisdom were invaluable, always assisting me in overcoming obstacles during the most challenging phases of the project. Also, I would like to express my gratitude to my school advisor Mrs. Aidoo for all the support, instructions, and encouragement I received throughout this difficult project. I am grateful to the Suzhou Institute of Nano-Tech and Nano-Bionics and The Spence School for offering facilities and resources for this project. Their support facilitated the smooth execution of the research. I extend my appreciation to my friends, who have been supportive throughout and provided a stimulating academic environment. Their encouragement was immensely motivating during my challenging research journey. Lastly, I am thankful to my family for their understanding, encouragement, and support.
-
Funding information: The author states no funding involved.
-
Author contributions: Xinran Ma designed the framework, analyzed the performance, validated the results, and wrote the article. The author has accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The author states no conflict of interest.
References
[1] Xu Y, Wu Q. Prevalence trend and disparities in rheumatoid arthritis among US adults, 2005–2018. J Clin Med. 2021;10(15):3289.10.3390/jcm10153289Suche in Google Scholar PubMed PubMed Central
[2] Smolen JS, Aletaha D. Challenges of predicting treatment response in patients with rheumatoid arthritis. Nat Clin Pract Rheumatol. 2005;1(2):62–3.10.1038/ncprheum0050Suche in Google Scholar PubMed
[3] Cush JJ. Rheumatoid arthritis: Early diagnosis and treatment. Rheum Dis Clin North Am. 2022;48(2):537–47.10.1016/j.rdc.2022.02.010Suche in Google Scholar PubMed
[4] Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet. 2017;389(10086):2338–48.10.1016/S0140-6736(17)31491-5Suche in Google Scholar PubMed
[5] Abbasi M, Mousavi MJ, Jamalzehi S, Alimohammadi R, Bezvan MH, Mohammadi H, et al. Strategies toward rheumatoid arthritis therapy: The old and the new. J Cell Physiol. 2019;234(7):10018–31.10.1002/jcp.27860Suche in Google Scholar PubMed
[6] England BR, Thiele GM, Anderson DR, Mikuls TR. Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. Bmj. 2018;361:k1036.10.1136/bmj.k1036Suche in Google Scholar PubMed PubMed Central
[7] Zhu Y, Zhao T, Liu M, Wang S, Liu S, Yang Y, et al. Rheumatoid arthritis microenvironment insights into treatment effect of nanomaterials. Nano Today. 2022;42:101358.10.1016/j.nantod.2021.101358Suche in Google Scholar
[8] Yang Y, Guo L, Wang Z, Liu P, Liu X, Ding J, et al. Targeted silver nanoparticles for rheumatoid arthritis therapy via macrophage apoptosis and Re-polarization. Biomaterials. 2021;264:120390.10.1016/j.biomaterials.2020.120390Suche in Google Scholar PubMed
[9] Zhao J, Jiang P, Guo S, Schrodi SJ, He D. Apoptosis, autophagy, NETosis, necroptosis, and pyroptosis mediated programmed cell death as targets for innovative therapy in rheumatoid arthritis. Front Immunol. 2021;12:809806.10.3389/fimmu.2021.809806Suche in Google Scholar PubMed PubMed Central
[10] Pucci C, Martinelli C, De Pasquale D, Battaglini M, di Leo N, Degl’Innocenti A, et al. Tannic acid–iron complex-based nanoparticles as a novel tool against oxidative stress. ACS Appl Mater Interfaces. 2022;14(14):15927–41.10.1021/acsami.1c24576Suche in Google Scholar PubMed PubMed Central
[11] Yu M, Sun X, Dai X, Gu C, Gu M, Wang A, et al. Effects of tannic acid on antioxidant activity and ovarian development in adolescent and adult female Brandt’s voles. Reprod Sci. 2021;28:2839–46.10.1007/s43032-021-00578-3Suche in Google Scholar PubMed
[12] Li M, Liu P, Xue Y, Liang Y, Shi J, Han X, et al. Tannic acid attenuates hepatic oxidative stress, apoptosis and inflammation by activating the Keap1‑Nrf2/ARE signaling pathway in arsenic trioxide‑toxicated rats. Oncol Rep. 2020;44(5):2306–16.10.3892/or.2020.7764Suche in Google Scholar PubMed
[13] Guo L, Zhong S, Liu P, Guo M, Ding J, Zhou W. Radicals scavenging MOFs enabling targeting delivery of siRNA for rheumatoid arthritis therapy. Small. 2022;18(27):2202604.10.1002/smll.202202604Suche in Google Scholar PubMed
[14] Brzezicka KA, Arlian BM, Wang S, Olmer M, Lotz M, Paulson JC. Suppression of autoimmune rheumatoid arthritis with hybrid nanoparticles that induce B and T cell tolerance to self-antigen. ACS Nano. 2022;16(12):20206–21.10.1021/acsnano.2c05643Suche in Google Scholar PubMed
[15] Xie L, Zhang N, Zhang Q, Li C, Sandhu AF, Williams G, III, et al. Inflammatory factors and amyloid β-induced microglial polarization promote inflammatory crosstalk with astrocytes. Aging (Albany NY). 2020;12(22):22538.10.18632/aging.103663Suche in Google Scholar PubMed PubMed Central
[16] Yan F, Zhong Z, Wang Y, Feng Y, Mei Z, Li H, et al. Exosome-based biomimetic nanoparticles targeted to inflamed joints for enhanced treatment of rheumatoid arthritis. J Nanobiotechnol. 2020;18:1–5.10.1186/s12951-020-00675-6Suche in Google Scholar PubMed PubMed Central
[17] Li H, Feng Y, Zheng X, Jia M, Mei Z, Wang Y, et al. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. J Controll Release. 2022;341:16–30.10.1016/j.jconrel.2021.11.019Suche in Google Scholar PubMed
[18] Lv R, Bao Q, Li Y. Regulation of M1‑type and M2‑type macrophage polarization in RAW264. 7 cells by Galectin‑9. Mol Med Rep. 2017;16(6):9111–9.10.3892/mmr.2017.7719Suche in Google Scholar PubMed
[19] Solomon DH, Giles JT, Liao KP, Ridker PM, Rist PM, Glynn RJ, et al. Reducing cardiovascular risk with immunomodulators: A randomised active comparator trial among patients with rheumatoid arthritis. Ann Rheum Dis. 2023;82(3):324–30.10.1136/ard-2022-223302Suche in Google Scholar PubMed PubMed Central
[20] Wang Q, Qin X, Fang J, Sun X. Nanomedicines for the treatment of rheumatoid arthritis: State of art and potential therapeutic strategies. Acta Pharm Sin B. 2021;11(5):1158–74.10.1016/j.apsb.2021.03.013Suche in Google Scholar PubMed PubMed Central
[21] Wen J, Li H, Dai H, Hua S, Long X, Li H, et al. Intra-articular nanoparticles based therapies for osteoarthritis and rheumatoid arthritis management. Mater Today Bio. 2023;19:100597.10.1016/j.mtbio.2023.100597Suche in Google Scholar PubMed PubMed Central
[22] Veerappermal Devarajan M. Assessing long-term serum sample viability for cardiovascular risk prediction in rheumatoid arthritis. Int J Inf Technol Comput Eng. 2020;8(2):2347–57.Suche in Google Scholar
[23] Zinke M, Lejeune M, Mechaly A, Bardiaux B, Boneca IG, Delepelaire P, et al. Ton motor conformational switch and peptidoglycan role in bacterial nutrient uptake. Nat Commun. 2024;15(1):331.10.1038/s41467-023-44606-zSuche in Google Scholar PubMed PubMed Central
[24] Clogston JD, Patri AK. Zeta potential measurement. Characterization of nanoparticles intended for drug delivery. Methods in Molecular Biology. Humana Press; 2011. p. 63–70.10.1007/978-1-60327-198-1_6Suche in Google Scholar PubMed
[25] Clayton KN, Salameh JW, Wereley ST, Kinzer-Ursem TL. Physical characterization of nanoparticle size and surface modification using particle scattering diffusometry. Biomicrofluidics. 2016;10:054107.10.1063/1.4962992Suche in Google Scholar PubMed PubMed Central
[26] Kiefer J, Grabow J, Kurland HD, Müller FA. Characterization of nanoparticles by solvent infrared spectroscopy. Anal Chem. 2015;87(24):12313–7.10.1021/acs.analchem.5b03625Suche in Google Scholar PubMed
[27] Ramachandran VS, Beaudoin JJ. Handbook of analytical techniques in concrete science and technology: principles, techniques and applications. Norwich, New York, USA: Elsevier; 2000.Suche in Google Scholar
[28] Shah VP, Miron DS, Rădulescu FȘ, Cardot JM, Maibach HI. In vitro release test (IVRT): Principles and applications. Int J Pharm. 2022;626:122159.10.1016/j.ijpharm.2022.122159Suche in Google Scholar PubMed
[29] Barbillon G. Plasmonics and its applications. Materials. 2019;12(9):1502.10.3390/ma12091502Suche in Google Scholar PubMed PubMed Central
[30] Yang X, Zhong Y, Wang D, Lu Z. A simple colorimetric method for viable bacteria detection based on cell counting Kit-8. Anal Methods. 2021;13(43):5211–5.10.1039/D1AY01624ESuche in Google Scholar PubMed
[31] Fedr R, Kahounová Z, Remšík J, Reiterová M, Kalina T, Souček K. Variability of fluorescence intensity distribution measured by flow cytometry is influenced by cell size and cell cycle progression. Sci Rep. 2023;13(1):4889.10.1038/s41598-023-31990-1Suche in Google Scholar PubMed PubMed Central
[32] Gao C, Wang Q, Ding Y, Kwong CH, Liu J, Xie B, et al. Targeted therapies of inflammatory diseases with intracellularly gelated macrophages in mice and rats. Nat Commun. 2024;15(1):328.10.1038/s41467-023-44662-5Suche in Google Scholar PubMed PubMed Central
[33] Mousa ZS, Abdulamir AS. Application and validation of SARS-CoV-2 RBD neutralizing ELISA assay. Arch Razi Inst. 2022;77(1):391–402.Suche in Google Scholar
[34] Choy EH, Kavanaugh AF, Jones SA. The problem of choice: Current biologic agents and future prospects in RA. Nat Rev Rheumatol. 2013;9(3):154–63.10.1038/nrrheum.2013.8Suche in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy
Artikel in diesem Heft
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy

