Abstract
Heat, ultrasonic, microwave, and external energy are the essential conditions in the conventional preparation of nano-cupric oxide (CuO) from copper hydroxide (Cu(OH)2) precursor. In this work, CuSO4 aqueous solution (0.02 mol) was gradually added dropwise into the methanol/sodium hydroxide (NaOH/CH3OH) solution (0.04 mol) in 20 min at normal temperature and then constantly stirred the black solution for about 5 min; nano-CuO was synthesized. The as-prepared CuO had a high purity and a regular nanosize of 4–10 nm. What is more, the by-product sodium sulfate (Na2SO4) could be separated using a high-speed centrifuge, indicating that the methanol could be conveniently recycled; thereby, an environmentally friendly sustainable route of the preparation of nano-CuO was developed. In addition, the as-prepared nano-CuO was melt-compounded with polyamide 6 to produce fiber composites. The results showed that the nano-CuO was uniformly dispersed in PA6 fiber composites and presented an excellent antibacterial performance. Most importantly, the function of methanol in the dehydration process was revealed.
1 Introduction
Copper oxide (CuO), as an important metal oxide, has gained an ever-increasing interest and a wide range of applications [1,2,3,4,5]. Except for the common use in rayon, stained glass, and ceramics [6,7,8,9,10], it also has been widely used in antifouling paints for boats, inks, and antimicrobial coatings [11,12]. In addition, CuO nanocrystalline particle is the basis of some high-Tc superconductors, thereby attracting much attention in magnetic storage media, solar energy transformation, electronics, and catalysis [13,14,15,16].
Traditionally, physical gas phase synthesis and chemical low-temperature hydrothermal solution method are commonly used for the growth of CuO nanostructure assemblies [17,18,19]. However, the commercial potential of gas phase-grown CuO nanostructures was restricted owing to the high investment for equipment, as well as the high-energy consumption [13]. Compared with the physical technique method, chemical techniques exhibit indisputable advantages, because the chemical techniques can produce CuO with high purity and small particle size with lower energy consumption and costs. Over the years, various chemical techniques, such as hydrothermal method [20,21,22], sol–gel method [23], sonochemical method [24,25], microwave [26], and so on methods, were explored to prepare CuO. Howbeit, a number of studies have disclosed that to impel the copper hydroxide (Cu(OH)2) precursor to transform into CuO via chemical methods, an external driving force, such as heat, ultrasonic, and microwave, was essential.
As is known to all of our knowledge, there lacks study focused on the one-pot preparation of CuO at room temperature using convenient facilities [27]. Some researchers claimed to notice that, given a long enough time, Cu(OH)2 could partially transform into CuO at room temperature [28,29]. Cudennec et al. reported that in the presence of hydroxide ions (OH−), the mentioned transformation would become very fast, for the reason that the divalent copper ions were dissolved under the form of tetrahy-droxocuprate(ii) anions (Cu(OH)4 2−) [30]. Later, Zhao and Zhao further developed the method to produce CuO at room temperature using Cu(OA)2 precipitates prepared by the reaction of sodium oleate and copper sulfate as precursors [31].
As well as known, it took at least 3 h to obtain CuO at room temperature using the conventional synthesis methods. Besides, few individuals are concerned about the recycle of the solvent used in the preparation of CuO. Herein, in this work, we report a novel chemical method for the preparation of CuO nanoparticles by simply adding copper sulfate (CuSO4) aqueous solution into methanol/sodium hydroxide (CH3OH/NaOH) solution. By this means, CuO particles with regular nanosize could be obtained conveniently in one step at room temperature in 20–30 min, which significantly reduced the preparation time compared to that of the published work. What is more, the by-product sodium sulfate (Na2SO4) could be separated using a high-speed centrifuge, which allowed the recovery and repeated circle of methanol to be realized, so that a sustainable method for preparing nano-CuO was proposed. In addition, the as-prepared CuO was then blended with polymer to produce antibacterial fiber composites. The results referred that the composite had a superior antibacterial performance and good spinnability. Most importantly, using a high-speed centrifuge to separate the water-soluble by-product sodium sulfate from its aqueous solution will have far-reaching theoretical direction and application implications.
2 Materials and methods
2.1 Materials
Analytical grade CuSO4, NaOH, and ether (C2H5OC2H5) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Analytical grade CH3OH was obtained from Tianjin Comeo Chemical Reagent Co., Ltd. (Tianjin, China). PA6 (η r = 2.48, M n = 22,500) was purchased from Xinhui Meida Nylon Chemical Co., Ltd. (China). Escherichia coli (TOP10) and Staphylococcus aureus (CMCC26003) were bought from the Guangdong Institute of Microbiology (China). The other chemical agents are analytic pure.
2.2 Methods
2.2.1 Preparation of nano-CuO
First, 1.6 g (0.04 mol) of NaOH was dissolved in 100 ml of CH3OH in a 400-ml beaker (Figure 1a). Then, 3.82 g of CuSO4 (0.02 mol) was fully dissolved in 40 ml of deionized water in a 100-ml beaker (Figure 1b). Following that, the CuSO4 aqueous solution was gradually added dropwise into the above-mentioned NaOH/CH3OH solution for 20 min. Later, constantly stirred the black solution at normal temperature for about 5 min to ensure the completion of the transformation (Figure 1c). After the reaction, the black suspension was filtrated to separate CuO. The as-prepared CuO (Figure 1d) was washed with deionized water and ethyl ether thrice and then dried at 50°C for 24 h in a vacuum oven.
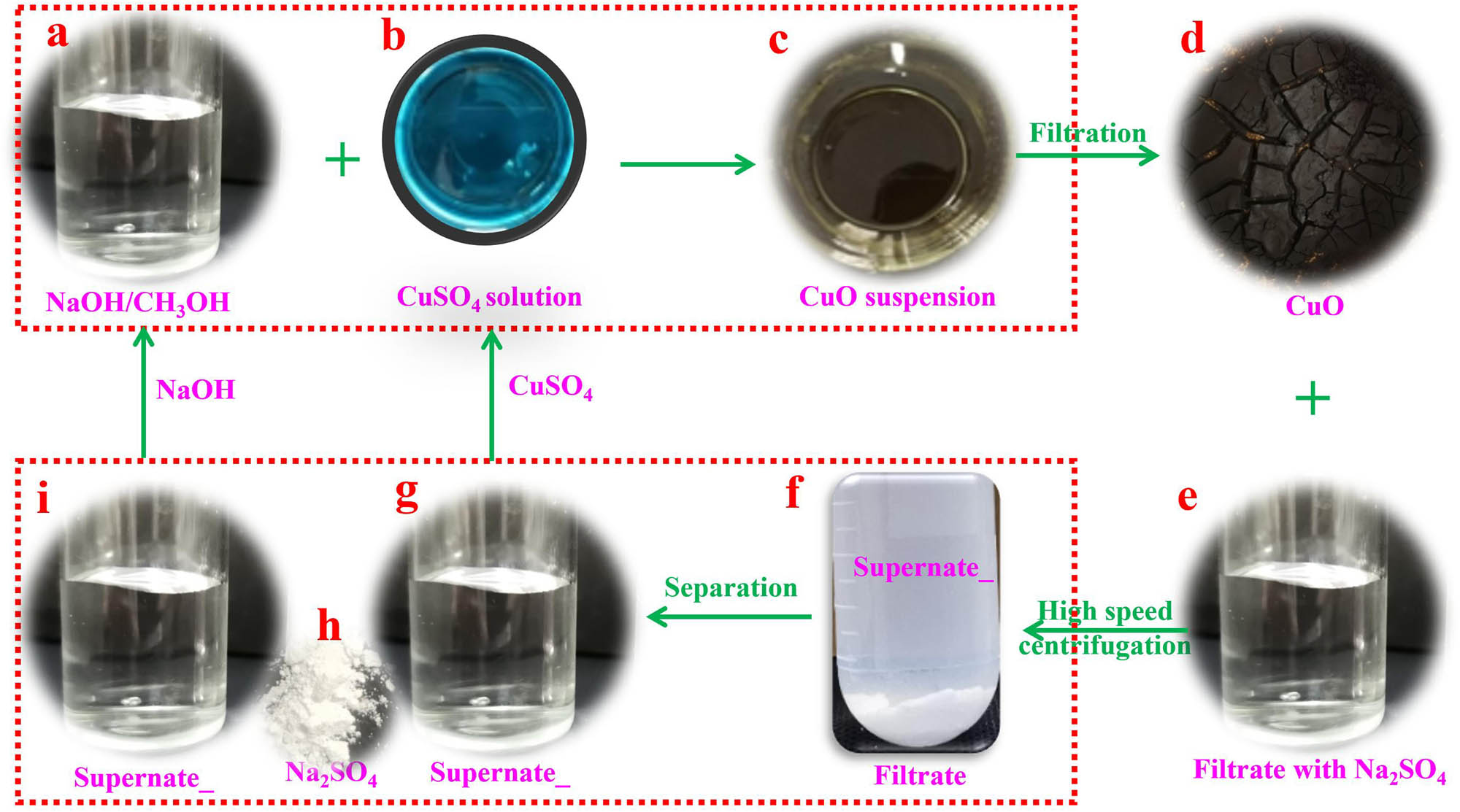
The preparation process of nano-CuO at room temperature and the recycle of CH3OH.
2.2.2 Recovery of CH3OH and cycle preparation of nano-CuO
When the above-mentioned preparation process was ended, CuO was first separated through filtration (Figure 1d and e) and then used a high-speed centrifuge to remove the by-product Na2SO4 under a rotating speed of 10,000 rpm (Figure 1f). Following that, the transparent CH3OH aqueous solution was equally divided into two groups (Figure 1g and i). Afterwards, 1.6 g of NaOH was added to one of them, and 3.82 g of CuSO4 was added to another group. Through duplicating the preparation process at normal temperature (Figure 1c), nano-CuO precipitates were obtained again.
2.2.3 Preparation of PA6-CuO composites and fibers
After fully dried, CuO precipitates and PA6 pellets were mixed and then melt-extruded by a co-rotating twin-screw extruder (SHJ-20, Nanjing Giant Co., China). The length and diameter of the extruder are 800 and 20 mm, respectively. The temperature for the I, II, III, IV, and V zones of the extruder was 180, 235, 235, 238, and 240°C, respectively. By varying the adding mass ratio of PA6 and nano-CuO, batches of PA6-CuO composites were produced. The concentration of Cu2+ in PA6 was 0, 0.12, 0.24, 0.36, and 0.6 wt%, respectively, and pure PA6 was used as a comparison.
Later, some of the selected PA6-CuO composites were further fabricated into yarns by melt-spinning with a spinning speed of 800 m/min under the temperature of 260°C.
2.2.4 Antibacterial tests
To evaluate the bactericidal activity of PA6-CuO fiber composites on E. coli (TOP10) and S. aureus (CMCC26003), the colony-forming count method was adopted, and Gram-negative and Gram-positive bacteria were used as the model, respectively. After 18–20 h of incubation, sterile phosphate buffer saline (1×) was used to serially dilute the initially cultured bacteria of E. coli and S. aureus to 1 × 106–5 × 106 CFU/ml. 5 ml of bacterial solution and 70 ml of buffer solution were subsequently poured into a conical flask in which 1 g of the as-prepared sample was previously placed. Following that, the incubation was executed in a shaking incubator at 37°C and 220 rpm for 24 h. Then, 100 µl of each bacterial suspension was spread on a Luria–Bertani (LB) solid agar plate. After incubating the agar plates in an incubator for 24 h at 37°C, the living bacterial colonies were finally counted.
2.2.5 Characterizations
A typical Fourier transform infrared spectrometer (FTIR) analysis was performed on a WQF-200 spectrometer (Beijing Second Optical Instrument Factory) using a solid potassium bromide method. The recorded range of the spectra was 4,000–400 cm−1.
The particle sizes of nano-CuO particles were observed by transmission electron microscopy (TEM, 200CX, JEOL, Japan). An S-4800 field emission scanning electron microscope was used to investigate the PA6-CuO fibers’ morphology, as well as the dispersion of nano-CuO in the composites, the samples were coated with conductive gold before a test.
PANalytical X’Pert Pro multipurpose powder diffractometer with nickel-filtered Cu-Ka radiation (λ = 1.54 Å) was adopted to execute the X-ray diffraction (XRD) tests conducted at 45 kV and 40 mA. The sweeping signals from 5° < 2θ < 80° were collected, and the scan rate is 2°(2θ)/min. The chemical composition was analyzed by X-ray photoelectron spectroscopy (XPS). X-ray photoelectron spectroscopy profiles were collected on a K-Alpha+ (Thermo Fisher Scientific, USA) apparatus using monochromatic Al Kα (1486.6 eV) radiation as the radiation source.
3 Results and discussion
3.1 The effect of the adding sequence on the purity of nano-CuO
Figure 2 shows the complete experimental procedures for the preparation of nano-CuO in our lab. As we all know, the reaction of equivalent CuSO4 and NaOH aqueous solution produced only blue copper hydroxide (Cu(OH)2) at room temperature. Blue powders were generated by adding CH3OH into CuSO4 aqueous solution. Putting solid CuSO4 into CH3OH at room temperature also produced blue powders; however, the powders were actually water-insoluble copper sulfate basic crystals with regular shape (Figure S1).
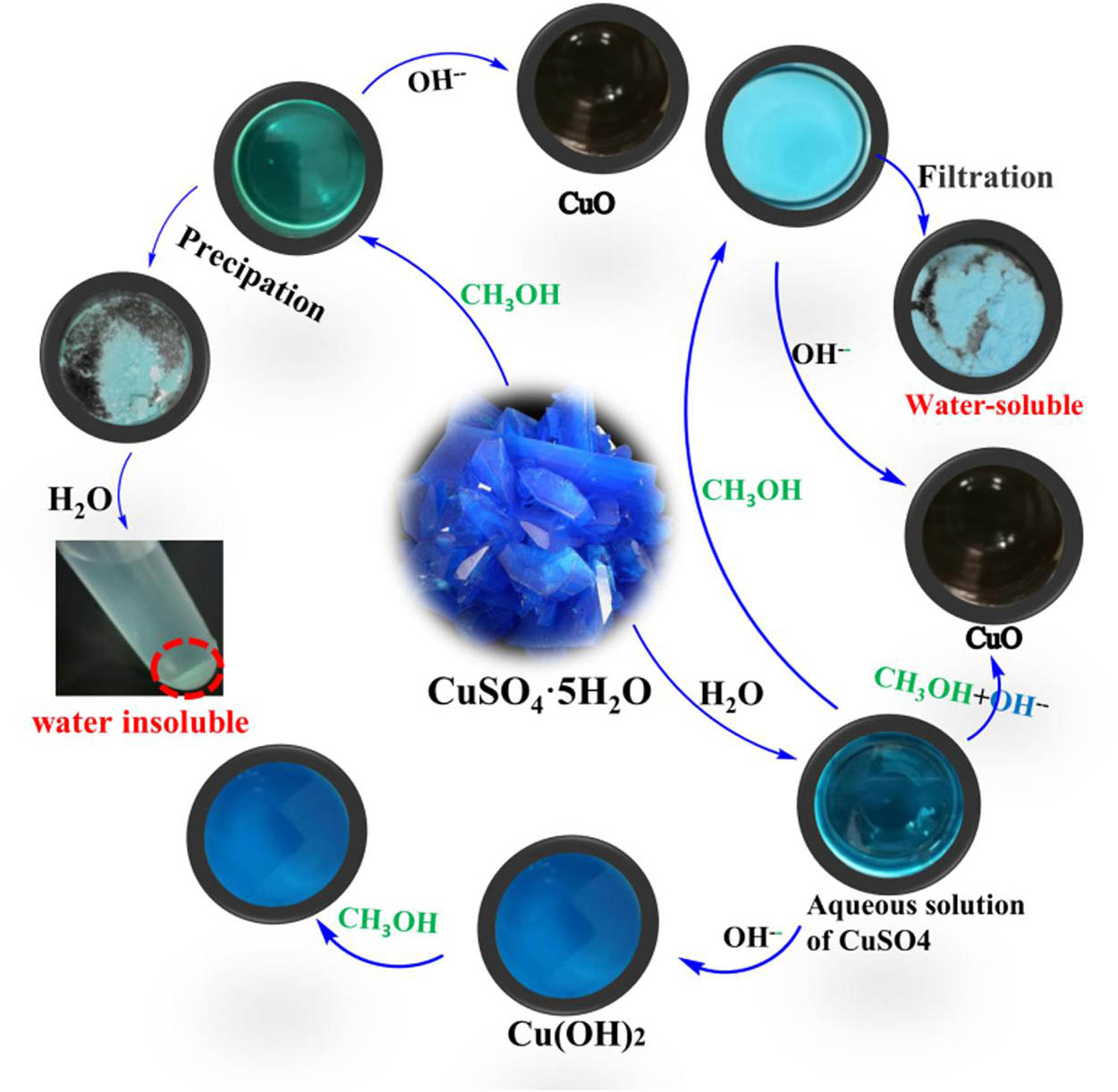
The complete experimental procedures in lab.
Interestingly, when CuSO4 aqueous solution was added dropwise into CH3OH/NaOH solution under room temperature, some black precipitates were produced immediately; these black precipitates were later confirmed to be nano-CuO. Adding other copper aqueous solution like copper chloride into NaOH/CH3OH solution, or using ethanol, ethylene glycol to substitute CH3OH, black precipitates were also obtained under room temperature (Figure S2). Moreover, using other water-soluble hydroxides instead of NaOH also achieved a similar result in this work (Figure S3). In sum, strong alkaline and adequate alcohol is in favor of the rapid preparation of CuO at room temperature.
Herein, a CuSO4 aqueous solution was prepared by adding 0.02 mol of CuSO4 into 40 ml of water. Then, the CuSO4 aqueous solution was added to a series of NaOH/methanol solution to further investigate the generation of CuO at room temperatures. As shown in Figure 3, it is clear to see that the adding mass of NaOH and CH3OH had a strong effect on the purity of CuO. XRD results indicated that adding equivalent CuSO4 aqueous solution into NaOH aqueous solution, it only produced blue Cu(OH)2 (Figure 3a). However, it tended to produce a mixture of CuO and Cu(OH)2 if we added dropwise 0.02 mol of CuSO4 aqueous solution into NaOH/methanol (NaOH is 0.2 and 0.3 mol in this case). Interestingly, if we repeated this process in a very short time, a green mixture of Cu(OH)2 and columnar sodium copper alum (Na2(CuSO4)2·2H2O) (Figure 3b and c, Figure S4) was obtained. That is, pure CuO could not be obtained under an improper molar ratio of NaOH and CuSO4. When an equivalent amount of CuSO4 was added dropwise into NaOH/CH3OH solution (prepared by 0.04 mol of NaOH and 25, 50, and 100 ml of CH3OH), pure CuO (Figure 3d–f) could be obtained at room temperature. The results inferred that except for the NaOH and methanol, the adding speed also had an influence on the generation of CuO. What is more, the variation of the reaction temperature (0–30°C) had an influence on the reaction speed; however, it did not affect the purity of nano-CuO.

The effect of adding content of NaOH and CH3OH on the purity of CuO.
3.2 Characterization of nano-CuO
The composition of the product shown in Figure 3f was analyzed by XRD and FTIR analysis. Figure 4 presents the XRD and FTIR patterns of the sample before washing. The characteristic 2θ of 32.5°, 39.0°, 48.8°, 61.6°, 66.3°, and 68.1° is assigned to the (110), (200), (−202), (−113), (−311), and (220) crystal faces, respectively, of the CuO standard map card (JCPDS file: 72-0629); the 2θ locates at 19.0°, 29.0°, 32.1°, 38.6°, 49.5°, 59.5°, and 71.4° are the characteristic peaks of Na2SO4 (JCPDS file: 74-1738) and are assigned to the (111), (004), (131), (222), (151), (333), and (119) crystal faces, respectively. The above results show that the obtained sample is a mixture of CuO and Na2SO4.

Characterizations of the as-prepared CuO nanoparticles before washing: (a) XRD spectra and (b) FTIR spectra.
Figure 4b presents the FTIR spectrum of the black precipitates. The absorption peak around 3,400 cm−1 is attributed to the stretching vibration of −OH, indicating the existence of hydroxyl groups. The stretching vibration peaks of −CH3 correspond to 2,925 and 2,854 cm−1. 1,117 cm−1 is the C–O stretching vibration peak of CH3OH, and the characteristic peaks of CuO bond are located at 861, 626, and 548 cm−1, respectively. The infrared analysis results show that the product contains hydroxyl, methyl, and Cu–O bonds. Therefore, we conjecture that part of the CH3OH was absorbed on the surface of CuO.
The absorbed methanol and water-soluble Na2SO4 in the black precipitates were then removed by further water and ether washing. The pure black powders were further characterized by FTIR, XRD, XPS, and inductively coupled plasma optical emission spectrometer (ICP-OES). As shown in Figure 5a, the FTIR peaks at 828, 626 and 548 cm−1 are attributed to nano-CuO. The absorption peak at 3,407 cm−1 is assigned to the −OH stretching vibration, owing to the absorption of water during the test. In addition, due to the large proportion of surface atoms in the nano-CuO crystals, the stretching vibration of the hanging bond on the vertical surface became very active, resulting in a sharp characteristic peak at 1,550 cm−1. Changing the adding sequence could result in an incomplete reaction and leave some Cu(OH)2 in CuO, multi −OH stretching vibrations were observed around 3,500 cm−1 (Figure S4).

Characterizations of as-prepared CuO nanoparticles after washing: (a) FTIR pattern and (b) XRD pattern; high-resolution XPS spectra of (c) Cu LMM2 and (d) Cu2p, and (e) O1s; and (f) wide X-ray photoelectron spectra.
The XRD pattern of the black powders after water and ether washing is presented in Figure 5b. The intensities and positions of the peaks are in good agreement with the values of the literature and the data from JCPDS file: 72-0629 [19,26]. Based on the diffraction peaks, the as-prepared CuO can be indexed to the single-phase CuO with a monoclinic structure. What is more, the broadening of all recorded peaks in the XPS spectrum indicates that there were nanoscale crystallites existed in the structure [19]. In addition, XRD results prove that there are no other impurities in the as-prepared CuO. The average size of the CuO nanoparticles was further calculated according to the Debye–Scherer formula, which is about 6 nm.
The phase purity and chemical composition of the black powders were further investigated by XPS. The CuLm2 scan curve (Figure 5c) indicates that there is a peak of 569.4 eV, which proves that there is only Cu(ii) in the products. Furthermore, the peaks at 932.2 and 952.9 eV in Figure 5d are attributed to the Cu2p3/2 and Cu2p1/2, respectively. In addition, the satellite peaks are also produced by Cu2+. In Figure 5f, there are two O1s peaks in the O1s core-level spectrum: the one at 530 eV is in agreement with O2− in CuO and another one at 531.6 eV is attributed to O adsorbed on the surface of the rectangular-shaped structure of CuO [32,33,34]. As shown in Figure 5e, the wide survey scan spectrum of the sample presents only CuO and C peaks. Thus, XPS results proved that the sample was composed of CuO. The ICP-OES result further proved that the content of Cu2+ was 79.67%, which is essentially consistent with the theoretic content in pure CuO.
TEM results show that the CuO particles have a regular nanosize. The average length and thickness of the particles are around 20 and 5 nm, respectively, which is highly consistent with the result calculated by the Debye–Scherer formula (Figure 6a–c). Further, a high-resolution transmission electron microscope indicates the main crystal surface (111) of CuO, and the spacing of the lattice fringe is 0.215 nm (Figure 6d, Figure S5). Obviously, the size of the as-prepared CuO particles is significantly smaller than that of the nanoleaves with lengths of 500 nm and widths of 200 nm synthesized by a similar method [31].

TEM (a–c) and HTEM (d) images of the as-prepared CuO nanoparticles.
Combined with the results of the upper experiments, we denote that by simply dropping CuSO4 into CH3OH/NaOH solution in a suitable ratio, pure nano-CuO could be obtained under room temperature in short time.
3.3 Presumable mechanism of preparation of CuO
Cudennec et al. proposed a probable mechanism for the transformation of Cu(OH)2 to CuO in an alkaline solution, but they neglected the dehydration step of
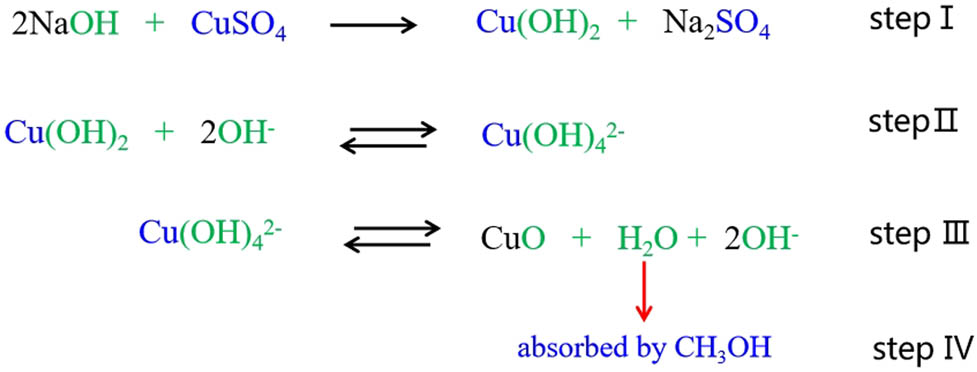
Possible mechanism for preparation of nano-CuO.

Diagrammatic sketch of methanol dehydration.
In the process of dropping, with the addition of CuSO4 aqueous solution, the transparent NaOH/CH3OH solution became turquoise and then turned blackish green
Figure 8b presents the influence of the mass of CH3OH on the dehydration. When there is excessive CH3OH in the reaction system, the released water is quickly absorbed by CH3OH owing to the hydrogen bonding, which is in favor of the removal of water and promotes the generation of CuO. When there is very little CH3OH and excessive water in the reaction system, where the CH3OH has been previously combined with water by hydrogen bonding; thus, it was unfavorable to the dehydration of
3.4 Recovery of CH3OH and cycle preparation of nano-CuO
There are four components, including CuO, Na2SO4, CH3OH, and water, that existed in the solution after the reaction. This raises an issue on the separation of the by-product Na2SO4 and the recycling of the solvent, or else it does not meet the principle of sustainable development. Therefore, a green sustainable route for the cyclic utilization of solvent was designed as described in the experiment part: the as-prepared CuO and the by-product Na2SO4 were separated successively from the solution by a physic filtration followed by a high-speed centrifugation so that the recovery of CH3OH/water solution was realized.
Commonly, Na2SO4 exists in water in the form of Na+ and SO4 2− (Figure 9a), while in the CH3OH/water solution, Na+ and SO4 2− tend to form Na2SO4 because it has very low solubility in CH3OH (Figure 9b). At the function of high-speed centrifugation, Na2SO4 molecules were forced to move to the outside of the CH3OH/water solution interface quickly (Figure 9c) and deposited on the bottom of the tube (Figure 9d). The XRD result further proved that the white deposited powders are Na2SO4 (Figure S6). In addition, barium chloride was added into the supernate, there was no precipitate formed in the supernate, which proved that the Na2SO4 had been completely removed.

Diagrammatic sketch of separation of the by-product Na2SO4 under high-speed centrifugation.
In this case, the by-product Na2SO4 could be removed in 5 min by high-speed centrifugation with a total power of 700 kW/h. The energy consumption is around 225 kJ, which is a little higher than that of the traditional evaporation method to remove the water and methanol (202.66 kJ). The detailed calculation of the energy consumption for evaporation is shown in SI. Considering that the high-speed centrifugation can save more time and is more convenient to operate compared to evaporation, therefore, high-speed centrifugation was adopted to recover methanol in this work.
Before the first reaction, the CH3OH/NaOH solution had an initial pH value of 13.68. Normally, the solution should be neutral at the end of the reaction of an equivalent amount of NaOH and CuSO4. However, it can be seen from Figure 10 and Table S1 that at the end of each circulation, the pH value of the supernate fluctuated around 11.2, referring that there was plenty of OH− in the solution and could be the most definitive evidence for the transformation of

Solution pH in each preparation step.
In all, the CH3OH aqueous solution was reused 15 times in this work. TEM (Figure 11, Figure S7) and XRD tests were conducted to explore the physicochemical properties of the as-prepared CuO in each round. TEM results show that the CuO particles prepared in the first three rounds of recycling have a regular size with a length and thickness of about 20 and 5 nm, respectively (Figure 11a–c), which is similar to that of the initial sample as shown in Figure 6. With the increase in cycle rounds, the length of the as-prepared CuO particles gradually increased from 20 to 50 nm. Accordingly, the thickness of the particles increased to 10 nm (Figure 11d–f). The results implied that the as-prepared particles still retained nanoscale size and integrity with the circulation of methanol, which guaranteed the realization of the reuse of methanol so that it has great meaning of resource environmental protection.

TEM morphologies of cycle-prepared nano-CuO.
The XRD spectra of the seven selected CuO samples are shown in Figure 12. The peak position, as well as the shape of the 2θ for the selected samples, is identical to each other. The 2θ locates at 32.5°, 35.6°, 38.8°, 48.8°, 61.6°, and 83.1° and is assigned to the (110), (−111), (111), (−202), (−113), and (222) crystal planes, respectively, which is in perfect agreement with the CuO standard map card (JCPDS file: 72-0629). The above results refer that the CH3OH solution can be reused and has little effect on the physical properties of the as-prepared CuO.
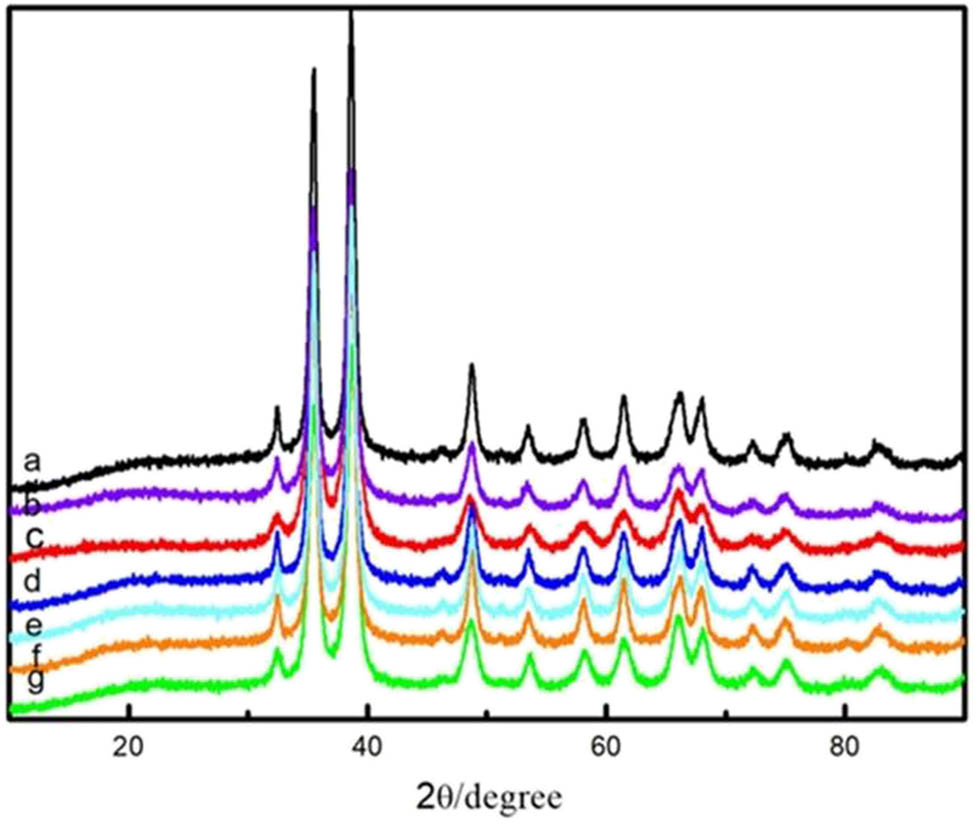
XRD pattern: (a) CuO prepared at the first time and (b–g) CuO obtained in the 1st, 2nd, 4th, 7th, 11th, and 14th cycles.
During the separation process, a trace loss of wet nano-CuO powders was inevitable because they tended to stick to the paper filter, thus leading to a lower yield of CuO compared to the theoretical calculated value (0.02 mol, equivalent to 1.5909 g). As shown in Table 1, it is clear to see that the average yield of CuO is about 1.5508 g, which is equivalent to about 97.5% of the theoretical value of 1.5909 g. The XPS patterns of the selected samples (1st, 2nd, 4th, 7th, 11th, and 14th) are very similar to that of Figure 2. The data shown in Table 1 also prove that the as-prepared CuO is able to adsorb CH3OH during preparation. The average adsorption amount of CH3OH reached 0.0146 g. This value gradually increased to 0.1068 g if we did not separate the as-prepared CuO from the clear liquid in time.
The mass of CuO obtained at each round
| Cycle round | M CuO after WD washing (g) | M CuO after washing with ether (g) | Adsorbed methanol (g) | Percentage of methanol adsorbed (%) | Yield of CuO (%) |
|---|---|---|---|---|---|
| 1st preparation | 1.6151 | 1.5566 | 0.0585 | 3.62 | 97.84 |
| The 1st cycle | 1.6132 | 1.5405 | 0.0727 | 4.51 | 96.83 |
| The 2nd cycle | 1.6356 | 1.5389 | 0.0967 | 5.91 | 96.73 |
| The 3rd cycle | 1.6689 | 1.5621 | 0.1068 | 6.40 | 98.19 |
| The 4th cycle | 1.5801 | 1.5432 | 0.0369 | 2.34 | 97.00 |
| The 5th cycle | 1.6490 | 1.5725 | 0.0765 | 4.64 | 98.84 |
| The 6th cycle | 1.6115 | 1.5738 | 0.0377 | 2.34 | 98.93 |
| The 7th cycle | 1.6085 | 1.5468 | 0.0617 | 3.84 | 97.23 |
| The 8th cycle | 1.5740 | 1.5386 | 0.0354 | 2.25 | 96.71 |
| The 9th cycle | 1.5674 | 1.5479 | 0.0195 | 1.24 | 97.30 |
| The 10th cycle | 1.5811 | 1.5502 | 0.0309 | 1.95 | 97.44 |
| The 11th cycle | 1.6008 | 1.5259 | 0.0749 | 4.68 | 95.91 |
| The 12th cycle | 1.5788 | 1.5642 | 0.0146 | 0.92 | 98.32 |
| The 13th cycle | 1.6444 | 1.5527 | 0.0917 | 5.58 | 97.60 |
| The 14th cycle | 1.6090 | 1.5645 | 0.0445 | 2.77 | 98.34 |
| The 15th cycle | 1.5985 | 1.5351 | 0.0634 | 3.97 | 96.49 |
| Average value | 1.6085 | 1.5508 | 0.0577 | 3.56 | 97.48 |
| Standard deviation | 0.0293 | 0.0139 | 0.0277 | 1.6634 | 0.8716 |
3.5 Antibacterial performance and spinnability of PA6-CuO composites
Most published literature has disclosed that CuO had a positive impact in improving the antibacterial performance of polymer. Considering that, in recent years, the commercial demand for antibacterial PA6 fiber composite materials has developed fast [35,36,37], thereby, the antibacterial performance of the as-prepared nano-CuO in PA6 polymer was especially evaluated according to the national standard GB/T 20944.3 2008. Figure 13a indicates that pure PA6 was easy to be contaminated by bacteria; E. coli and S. aureus in the discs grew confluently so that the number of bacteria was countless. As the adding content of CuO increased to 0.12 wt%, the reproduction of S. aureus and E. coli was significantly inhibited and the numbers decreased to less than 106 and 30, respectively (Figure 13b). The results also prove that the antibacterial performance of the as-prepared CuO against E. coli is more effective than that of S. aureus, this is because the Gram-positive bacteria have a thicker cell wall than that of Gram-negative, so the Cu2+ provides stronger antibacterial activity on E. coli [38,39,40]. Gladly, the bactericidal rate of the PA6-CuO composite reached 100% when added 0.24 wt% Cu2+; there was no living colony in the disc. It infers that by adding a tiny amount of the as-prepared nano-CuO into PA6 polymer, the released Cu2+ is enough to kill all bacterium, thus effectively saving the dosing of the antibacterial agent, which is highly similar to our previous work [37].
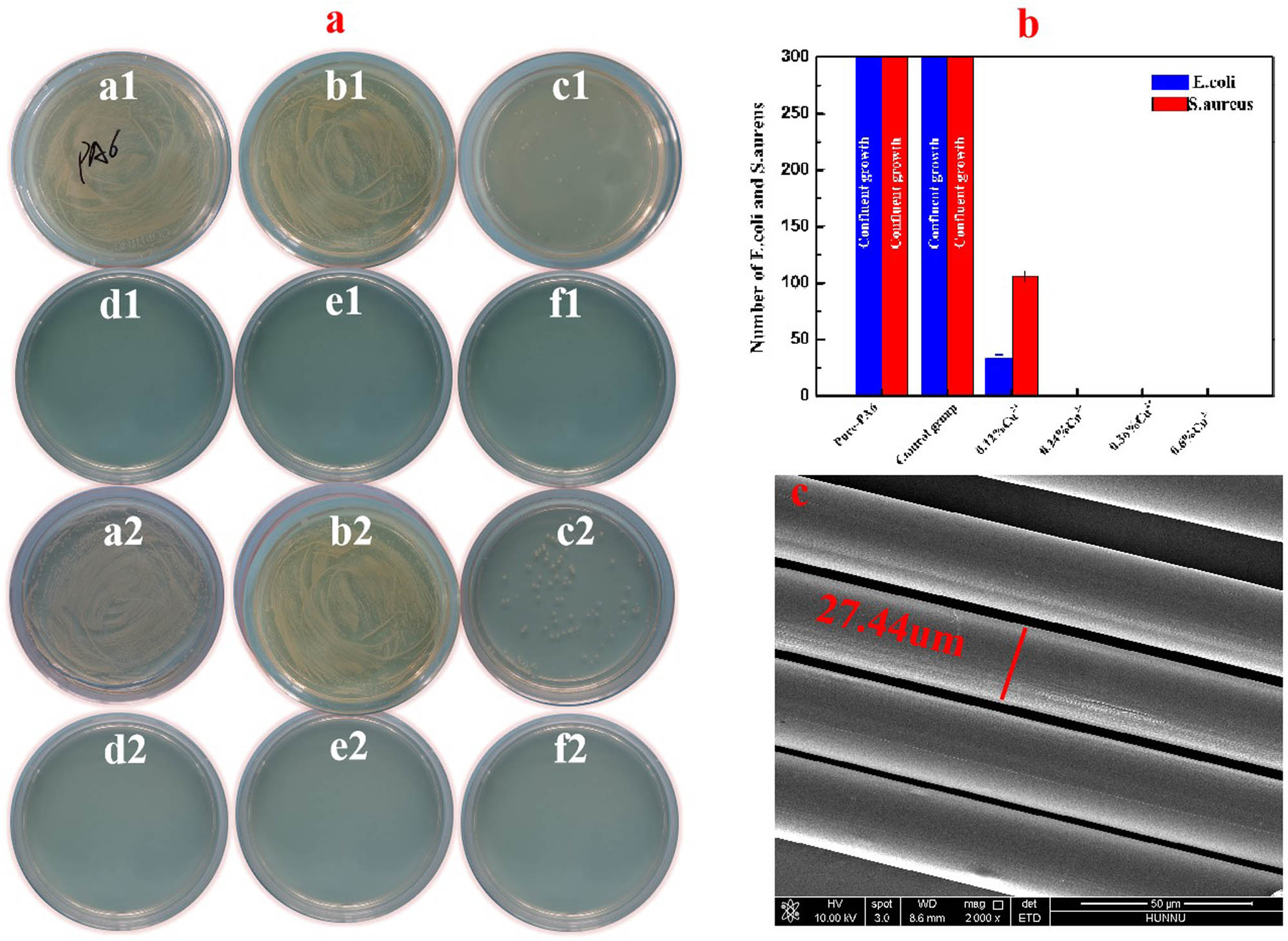
Antibacterial performance of PA6-CuO composites with different copper contents: (a–f) Cu2+ containing 0 (pure PA6), 0 (control group), 0.12, 0.24, 0.36, and 0.6 wt%, respectively; (b) bacterial number; and (c) spinnability of fiber size.
The spinnability of composite is a key feature to evaluate whether it is suitable to produce fiber composite or not. In this work, the PA6-CuO was further spun at a speed of 800 m/min, with a drawing ratio of 3.5. As shown in Figure 13c, the surface of PA6-CuO fiber appeared highly smooth under a scanning electron microscope. There was not any aggregated particle article being found even when adding 1 wt% CuO which far exceeds the minimum adding content of CuO which meets the antibacterial requirement. What is more, the fibers produced under a drawing ratio of 3.5 showed an average diameter of 27.44 µm. The smooth and regular morphology of PA6-CuO fiber clearly infers that the nano-CuO is highly compatible with the PA6 matrix and has good spinnability superior to that of reported results [28,41]. Besides, the refraction and the reflection of light were changed by the addition of nano-CuO, thus inducing variable optical effects on the fiber composite; as a result, the manufactured fibers appear silvery-white rather than the expected black. To sum up, the PA6-CuO composite has good spinnability when producing fine silk through a continuous spinning process and gives the fiber materials superior antibacterial properties, which implies that it has a potential application in the field of the fiber industry.
4 Conclusions
In this work, by simply adding dropwise CuSO4 aqueous solution into NaOH/CH3OH solution at room temperatures (0–30°C), CuO nanoparticles with a controllable size were obtained in 25 min. The average length and thickness of the particles are around 20 and 5 nm, respectively. More importantly, agent methanol could accelerate the dehydration of the
Acknowledgments
The authors acknowledge the support of the Open Foundation of National & Local Joint Engineering Laboratory for New Petro-chemical Materials and Fine Utilization of Resources (grant number KF201804), Hunan Normal University, and the Open Fund of State Key Laboratory of Biobased Fiber Manufacturing Technology (grant number SKL202210), China Textile Academy. Special thanks to Prof. Xiaoyi Yi (Central South University, China) for guidance to remove the methanol by ether wash.
-
Funding information: This research was supported by the Open Foundation of National & Local Joint Engineering Laboratory for New Petro-chemical Materials and Fine Utilization of Resources (grant number KF201804), Hunan Normal University, and the Open Fund of State Key Laboratory of Biobased Fiber Manufacturing Technology (grant number SKL202210), China Textile Academy.
-
Author contributions: Yongseng Niu: experiments, methodology, writing – original draft, Boren Xu: experiments, measurements; Xin Yi: experiments; Xi Wang: supervision, investigation, reviewing and editing; Chunwang Yi: supervision, investigation, reviewing and correcting the draft, writing point to point response. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Porkovich A, Ziadi Z, Kumar P, Kioseoglou J, Jian N, Weng L, et al. In situ observation of metal to metal oxide progression: A study of charge transfer phenomenon at Ru–CuO interfaces. ACS Nano. 2019;13:12425–37.10.1021/acsnano.9b06224Search in Google Scholar PubMed
[2] Guo A, Zhou Q, Bao Y, Qian F, Zhou X. Prochloraz alone or in combination with nano-CuO promotes the conjugative transfer of antibiotic resistance genes between Escherichia coli in pure water. J Hazard Mater. 2020;424:127761.10.1016/j.jhazmat.2021.127761Search in Google Scholar PubMed
[3] Naseer A, Ali S, Haider W. Powder injection molded nano copper oxide grafted graphene reinforced copper matrix composites. Powder Technol. 2022;397:117101.10.1016/j.powtec.2021.117101Search in Google Scholar
[4] Mazurkow JM, Yuzbasi NS, Domagala KW, Pfeiffer S, Kata D, Graule T. Nano-sized copper (Oxide) on alumina granules for water filtration: Effect of copper oxidation state on virus removal performance. Env Sci Technol. 2019;54:1214–22.10.1021/acs.est.9b05211Search in Google Scholar PubMed
[5] Zhang Q, Zhang K, Xu D, Yang G, Huang H, Nie F, et al. CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog Mater Sci. 2014;60:208–337.10.1016/j.pmatsci.2013.09.003Search in Google Scholar
[6] Seo SD, Jin YH, Lee SH, Shim HW, Kim DW. Low-temperature synthesis of CuO-interlaced nanodiscs for lithium ion battery electrodes. Nanoscale Res Lett. 2011;6:397.10.1186/1556-276X-6-397Search in Google Scholar PubMed PubMed Central
[7] Bu YYI. Novel all solution processed heterojunction using p-type cupric oxide and n-type zinc oxide nanowires for solar cell applications. Ceram Int. 2013;39:8073–8.10.1016/j.ceramint.2013.03.079Search in Google Scholar
[8] Naatz H, Lin S, Li R, Jiang W, Ji Z, Chang CH, et al. Safe-by-design CuO nanoparticles via Fe-doping, Cu–O bond length variation, and biological assessment in cells and zebrafish embryos. ACS Nano. 2017;11:501–15.10.1021/acsnano.6b06495Search in Google Scholar PubMed PubMed Central
[9] Duan Y, Liu X, Han L, Asahina S, Xu D, Cao Y, et al. Optically active chiral CuO “nanoflowers”. J Am Chem Soc. 2014;136:7193–6.10.1021/ja500197eSearch in Google Scholar PubMed
[10] Liu Y, Liu X, Qiu K, Cheng J, Wang W, Yan H, et al. Facile synthesis of graphene-like copper oxide nanofilms with enhanced electrochemical and photocatalytic properties in energy and environmental applications. ACS Appl Mater Inter. 2015;7:9682–90.10.1021/acsami.5b01451Search in Google Scholar PubMed
[11] Paglia F, Vak D, Van Embden J. Photonic sintering of copper through the controlled reduction of printed CuO nanocrystals. ACS Appl Mater Inter. 2015;7:25473–8.10.1021/acsami.5b08430Search in Google Scholar PubMed
[12] Perelshtein I, Applerot G, Perkas N. CuO-cotton nanocomposite: Formation, morphology, and antibacterial activity. Surf Coat Tech. 2009;204:54–7.10.1016/j.surfcoat.2009.06.028Search in Google Scholar
[13] Wang H, Xu JZ, Zhu JJ, Chen HY. Preparation of CuO nanoparticles by microwave irradiation. J Cryst Growth. 2002;244:88–94.10.1016/S0022-0248(02)01571-3Search in Google Scholar
[14] Lin XZ, Liu P, Yu JM. Synthesis of CuO nanocrystals and sequential assembly of nanostructures with shape-dependent optical absorption upon laser ablation in liquid. J Phys Chem C. 2009;113:17543–7.10.1021/jp907237qSearch in Google Scholar
[15] Yin M, Wu CK, Lou YB. Copper oxide nanocrystals. J Am Chem Soc. 2005;127:9506–11.10.1021/ja050006uSearch in Google Scholar PubMed
[16] Pfeifer P, Schubert K, Emig G. Preparation of copper catalyst washcoats for methanol steam reforming in microchannels based on nanoparticles. Appl Catal A-Gen. 2005;286:175–85.10.1016/j.apcata.2005.02.036Search in Google Scholar
[17] Bouzit SE, Boualy B, Firdoussi L. Fast room temperature solution-phase approach for selective synthesis of nanostructured Cu(OH)2, Cu2O and CuO. Int Res J Pure Appl Chem. 2015;8:157–64.10.9734/IRJPAC/2015/17920Search in Google Scholar
[18] Hu M, He J, Yang M. Controlled synthesis of nanostructured copper oxides through an elaborate solution route. J Nanosci Nanotechnol. 2016;16:783.10.1166/jnn.2016.10681Search in Google Scholar PubMed
[19] Dar MA, Ahsanulhaq Q, Kim YS, Sohn JM, Kim WB, Shin HS. Versatile synthesis of rectangular shaped nanobat-like CuO nanostructures by hydrothermal method; structural properties and growth mechanism. Appl Surf Sci. 2009;255:6279–84.10.1016/j.apsusc.2009.02.002Search in Google Scholar
[20] Zhuang Z, Peng Q, Li Y. Controlled synthesis of semiconductor nanostructures in the liquid phase. Chem Soc Rev. 2011;40:5492–513.10.1039/c1cs15095bSearch in Google Scholar PubMed
[21] Ozga M, Kaszewski J, Seweryn A, Sybilski P, Godlewski M, Witkowski BS. Ultra-fast growth of copper oxide (II) thin films using hydrothermal method. Mat Sci Semicon Proc. 2020;120:105279.10.1016/j.mssp.2020.105279Search in Google Scholar
[22] Chauhan D, Satsangi VR, Dass S. Preparation and characterization of nanostructured CuO thin films for photoelectrochemical splitting of water. Bull Mater Sci. 2006;29(7):709–16.Search in Google Scholar
[23] Kim DS, Kim JC, Kim BK. One-pot low-temperature sonochemical synthesis of CuO nanostructures and their electrochemical properties. Ceram Int. 2016;42:19454–60.10.1016/j.ceramint.2016.09.044Search in Google Scholar
[24] Chen TW, Rajaji U, Chen SM. Facile synthesis of copper(II) oxide nanospheres covered on functionalized multiwalled carbon nanotubes modified electrode as rapid electrochemical sensing platform for super-sensitive detection of antibiotic. Ultrason Sonochem. 2019;58:104596.10.1016/j.ultsonch.2019.05.013Search in Google Scholar PubMed
[25] Saadatian MH, Shahverdizadeh GH, Babazadeh M, Edjlali L, Es’haghi M. The effect of ultrasonic irradiation power and initial concentration on the particle size of nano copper(II) coordination polymer: Precursors for preparation of CuO nanostructures. J Polym Res. 2022;29:57.10.1007/s10965-022-02913-xSearch in Google Scholar
[26] Zhu HT, Zhang CY, Tang YM, Wang JX. Novel synthesis and thermal conductivity of CuO Nanofluid. J Phys Chem C. 2007;111:1646–50.10.1021/jp065926tSearch in Google Scholar
[27] Amaniampong PN, Trinh QT, Varghese JJ. Unraveling the mechanism of the oxidation of glycerol to dicarboxylic acids over a sonochemically synthesized copper oxide catalyst. Green Chem. 2018;20:2730–41.10.1039/C8GC00961ASearch in Google Scholar
[28] Lu C, Qi L, Yang J. Simple template-free solution route for the controlled synthesis of Cu(OH)2 and CuO nanostructures. J Phys Chem B. 2004;108:17825–31.10.1021/jp046772pSearch in Google Scholar
[29] Jia W, Reitz E, Sun H. From Cu2(OH)3Cl to nanostructured sisal-like Cu(OH)2 and CuO: Synthesis and characterization. J Appl Phys. 2009;105:064917.10.1063/1.3097286Search in Google Scholar
[30] Cudennec Y, Lecerf A. The transformation of Cu(OH)2 into CuO, revisited. Solid State Sci. 2003;5:1471–4.10.1016/j.solidstatesciences.2003.09.009Search in Google Scholar
[31] Zhao Y, Zhao J. Room temperature synthesis of 2D CuO nanoleaves in aqueous solution. Nanotechnology. 2011;22:115604.10.1088/0957-4484/22/11/115604Search in Google Scholar PubMed
[32] Francisco MSP, Mastelaro VR, Nascente PAP. Activity and characterization by XPS, HR-TEM, Raman spectroscopy, and BET surface area of CuO/CeO2-TiO2 catalysts. J Phys Chem B. 2001;105:10515–22.10.1021/jp0109675Search in Google Scholar
[33] Chen YZ, Liaw BJ, Chen HC. Selective oxidation of CO in excess hydrogen over CuO/CexZr1-xO2 catalysts. Int J Hydrog Energ. 2006;31:427–35.10.1016/j.ijhydene.2005.11.004Search in Google Scholar
[34] Sarkar D, Khan GG, Singh AK. High-performance pseudocapacitor electrodes based on α-Fe2O3/MnO2 core–shell nanowire heterostructure arrays. J Phys Chem C. 2013;117:15523–31.10.1021/jp4039573Search in Google Scholar
[35] Chen J, Gong CH, Yang C, Yi CW. Flexible preparation of polyamide-6 based thermoplastic elastomers via amide exchange. J Mater Sci. 2021;56:12018–29.10.1007/s10853-021-06057-zSearch in Google Scholar
[36] Zhang YJ, Zheng W, Xiao YJ, Yi CW. Preparation of high-efficient flame retardant PA6 via DOPO-ITA initiated ring-opening polymerization of caprolactam. J Polym Sci. 2024;62:547–53.10.1002/pol.20230521Search in Google Scholar
[37] Xu BR, Yi CW. Preparation of nano-CuO@BaSO 4 under room temperatures and its application in PA6 composites. J Reinf Plast Comp. 2024. 10.1177/07316844241236871.Search in Google Scholar
[38] Pant HR, Pant DR, Pandeya G, Panthi KT, Nam ST, Hong CS, et al. Characterization andm antibacterial properties of Ag NPs loaded nylon-6 nanocomposite prepared by one-step electrospinning process. Colloid Surf A. 2012;395:94–9.10.1016/j.colsurfa.2011.12.011Search in Google Scholar
[39] Kara S, Ureyen ME, Erdogan UH. Structural and antibacterial properties of PP/CuO composite filaments having different cross sectional shapes. Int Polym Proc. 2016;31:398–409.10.3139/217.3159Search in Google Scholar
[40] Ahmad MM, Kot HM, Mushtaq S, Waheed-Ur-Rehman M, Maghanga CM, Alam MW. Green synthesis of Mn + Cu bimetallic nanoparticles using Vinca rosea extract and their antioxidant, antibacterial, and catalytic activities. Crystals. 2022;12(1):72.10.3390/cryst12010072Search in Google Scholar
[41] Zhang W, Xu B, Gong C. Antibacterial and anti-flaming PA6 composite with metathetically prepared nano AgCl@ BaSO4 co-precipitates. Front Chem Sci Eng 2021;15:340–50.10.1007/s11705-020-1942-9Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy

