Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
-
Reda A. Mahmoud
, Wael A. Abdelhafez
, Gamal M. Zayed
, Saleh A. El-Rasoul
Abstract
Cilostazol, an anti-platelet aggregation medicine, is also known to have vasodilation properties and is commonly used for treating muscle soreness and cramps by increasing the muscle oxygen supply. The medication has limited oral bioavailability, is prone to pre-systemic metabolism, and is poorly soluble in aqueous media. A transdermal administration was planned to increase the drug’s solubility and therapeutic efficacy. The current work intended to develop cilostazol niosome-loaded transdermal gel, which was prepared, and with the use of Fourier transform infrared and differential scanning calorimetry analyses, drug-excipient interactions were observed. The medication was formulated utilizing Carbopol-934, Pluronic-F-127, and HPMC gel bases for the transdermally delivered niosomal gels. The produced niosomes had a maximum percentage of drug entrapment at 96.4%, with a particle size of 102 ± 11.30 nm and polydispersity index of 0.29 ± 0.069. The highest percentage of the medication that was entrapped was 96.4%, and the Carbopol-934 gel basis released the major part of the drug under in vitro conditions. A maximum transdermal flux was recorded at 3850.92 μg after 4 h, indicating a 10% increase in cilostazol permeation through rat skin. The flux rate for the niosomal preparation containing the drug ranged from 14.85 to 28.02 μg/cm2 h−1. In comparison to the pure cilostazol-loaded gels, the pharmacokinetics investigation showed that the niosomal gel formulations had considerably greater C max, T max, and AUC0. The niosomes loaded with cilostazol exhibited greater solubility, higher bioavailability, and improved effectiveness. Better therapeutic results may be achieved with systemic and site-directed delivery of cilostazol using the designed transdermal niosomal gel with appropriate molecular tagging modification/(s).
1 Introduction
Cilostazol (CLZ, Figure 1, CAS # 73963-72-1, IUPAC name, 6-[4-(1-cyclohexyl-1H-tetrazol-5-y1)-butoxy]-3,4-dihydro-2(1H)-quinolinone; C20H27N5O2, MW 369.21), an anti-platelet aggregating agent, is reported to possess vasodilatory and selective inhibitory effects on human cyclic nucleotide phosphodiesterase-III [1]. The inhibition of PDE works by stopping cAMP from being degraded, which raises the level of cAMP in platelets and blood vessels, and inhibits platelet aggregation with vascular dilation [2]. Besides, the drug limits the uptake of adenosine, an effect that augments its ability to inhibit platelet aggregation. Furthermore, the administration of CLZ raises the blood flow and oxygen supply to the muscle to relieve muscle pain and cramps that occur during exercise and walking [3].
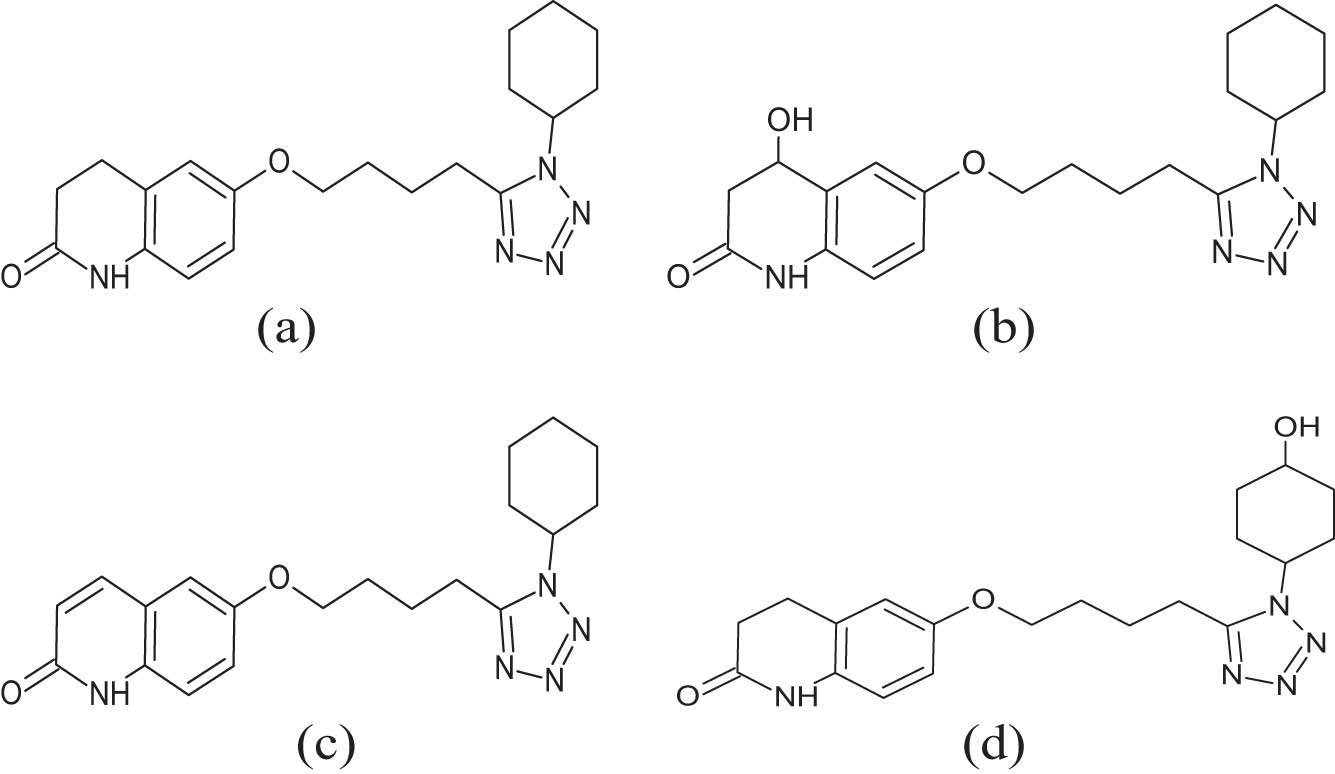
Cilostazol structure and its metabolites: (a) cilostazol, (b) 4-hydroxy cilostazol, (c) 3,4-dehydro cilostazol, and (d) cyclohexan-4′-ol cilostazol.
According to the biopharmaceutical classification system, CLZ is categorized under class II drug, which indicated that the drug possesses relatively lower solubility and higher permeability, thereby affecting the dissolution rate of CLZ and reducing its uptake after oral administration [4]. The low oral bioavailability (∼56%) of the drug is mostly attributed to its poor solubility, low dissolution rate, metabolism in plasma, and hepatic pre-systemic conversion by hepatic enzymes [5]. Based on these facts, it was speculated that enhancing the aqueous solubility and changing the oral administration route of CLZ affect the low bioavailability scenario and may prevent presystemic metabolism, improving the drug’s bioavailability and therapeutic efficacy. However, the CLZ interactions with CYP3A4 and CYP2C19, the two isoenzymes belonging to the cytochrome P450 family, produced active metabolites (Figure 1), of which one metabolite (C) contributed to at least 50% of the pharmacological (PDE-III inhibitions) activity after the drug administration [6,7]. In addition, the drugs that are known to inhibit CYP3A4, e.g., ketoconazole, omeprazole, itraconazole, diltiazem, and erythromycin, are also known to interact with the CLZ. For example, omeprazole, a proton pump inhibitor, inhibits CYP2C19, which increases the exposure of the active metabolites of cilostazol to further interactions by the CYP system [8,9]. Moreover, the grapefruit juice, an acknowledged CYP3A4 inhibitor, has been reported to increase the effects of CLZ [10], which is achieved through expanding the drug’s maximum concentration to ∼50% [11]. Nonetheless, the CLZ has its drawbacks, and the side effects it produces include chest fluttering, diarrhea, headache, pounding heartbeat, chest pain, passing out feeling with being light-headed, fever, chills, mouth and throat sores, bruising, uncommon bleeding, red or purple spots under the skin, allergic reactions with troubled breathing, face, lips, and tongue swellings, trouble in the throat, low blood pressure, dizziness, nausea, blurry vision, fainting, and occurrence of thrombocytopenia, or leukopenia [12,13]. The hazard ratio varied between 0.94 (95% confidence interval [CI], 0.64–1.39) and 0.99 (95% CI, 0.52–1.88) for the drug and placebo under different circumstances, wherein the long-term drug adherence was poor at 60%. Serious bleeding and deaths were reported under treatment and within the dose discontinuation period of 1 month [14]. Unfortunately, the acute and chronic toxicity data in humans are not available. However, the oral lethal dose in rats and mice was found to be above 5 g/kg, while in dogs, it was >2 g/kg. Nonetheless, the orally delivered CLZ overdose expressed symptoms of severe headache, diarrhea, hypotension, tachycardia, cardiac arrhythmia, suspected liver impact, and renal impairment (CrCl < 25 mL/min) in humans under treatment [15,16]. The biological half-life of the drug is 11–13 h, and the overdosed patients required supportive treatment, since the drug is at high concentrations, 95–98%, as protein-bound. The drug is reported to be excreted through urine (70%) and the fecal route (20%). Also, it was found that the drug was unlikely to be efficiently removed through hemodialysis, or peritoneal dialysis, or the emptying of the stomach through gastric lavage internment [17].
The CLZ adverse effects, suspected toxicity, and severe overdose reactions to patients, together with the median, 11–13 h, biological half-life, prompted to suggest taking an alternative route of drug administration. Considering the hepatic enzyme interactions, as well as blood-based metabolism of the drug while delivering it under the oral route, it was envisioned to adopt the topical delivery of the drug, which is considered comparatively safe with controlled and sustained dose release with diminished and supposedly with no adverse reactions, which conceptually produces less toxicity responsible for uncomfortable and harmful allergic reactions. Consequently, the administered drug was supposed to provide lowered discomfort to patients. Therefore, the CLZ delivery was adopted through the skin, first on an experimental basis under in vitro conditions, and then the observations were extended with modifications under in vivo conditions. The skin being the largest organ of the human body offered many advantages and opportunities for delivery of the drug. Earlier studies have indicated that the benefits of drug delivery through skin include lower fluctuations in plasma levels of the drug, thereby, to an extent, protecting the drugs from first-pass metabolism. The skin through delivery also localizes the needed high concentration of the drug in the affected areas, thereby achieving certain levels of site specificity. In addition to reducing systemic adverse effects, the transdermal route of delivery is also reported to enhance patient compliance [18,19], which is specifically needed in the case of CLZ, since patients’ compliance for oral delivery has at the maximum at 60% of the individuals under treatment. These patients’ compliance statistics, also include the alarming deaths of patients within 1 month of the drug’s, CLZ, postdiscontinuation period. However, within this context, it is further imperative to consider the inherently restricted permeability of the skin as another drawback. Also, the drug’s passage through the skin layers is dependent on the drug’s physico-chemical properties, and earlier studies have suggested that the stratum corneum, the upper layer of the skin, acts as the principal obstruction to the movements of drug molecules unless the drug molecules are under small molecular weight category (under 500 amu) and are lipophilic [20]. With sight on improvement in the drug’s penetration through this remarkable skin barrier, the modifications to reach the delivery site, across the skin, are among one of the desired possibilities that can be achieved through chemical, physico-chemical, and physico-mechanical transformations of the drugs, and drug’s formulation modules and these kinds of structural and physical alterations for efficient transdermal carrierability of the drug have been previously employed [21]. Studies have shown a number of other techniques for improving transdermal delivery, including delivery through microneedles, iontophoresis, sonophoresis, and electroporation [22]. None of these techniques have been widely used due to several issues, including poor efficacy, skin irritability, application difficulty, high expense, and patient preference [21,22,23,24,25]. According to earlier reports, lipid-based nanocarriers, such as microemulsions, liposomes, niosomes, and several other types of nano-vesicles, including solid lipid nanoparticles, which are considered as low-risk delivery options, are available [26,27]. Furthermore, the lipoid constituents, and varying of the surfactants in formulating the delivery module, have provided feasible formulations fit for skin delivery. Nonionic surfactants, i.e., cholesterol, and molecular inducers of charge self-assemble into bilayer structures, and they generate niosomes, a kind of vesicular drug delivery system that traps aqueous media inside. These entities are structurally comparable to the liposomes and have comparable in vitro and in vivo effectiveness. The polar groups in niosomes are in touch with the aqueous space in the central core, while the hydrophobic tail’s bilayer composition is insulated and is present on the outskirts of the vesicles [28]. To circumvent the drawbacks of CLZ oral delivery, the current work intended to investigate the applicability of CLZ-loaded niosomal systems as a transdermal formula. The size, shape, effectiveness of the entrapment, in vitro transdermal permeation, and in vivo pharmacokinetic examinations of the niosomal formulation of CLZ’s as a drug delivery module were addressed. Moreover, the choice of in vivo model for the CLZ drug delivery was selected, since several in vitro studies have been reported on different aspects of this drug’s delivery, including its bioavailability studies, delivery through drug-inclusion complex formation, delivery self-emulsifying formulation, and finally the absorption modeling simulations for effective designing of the intended drug formulation. The drug’s bioavailability through the oral route was the major issue together with the safe and efficient delivery of the drug [29,30,31]. In this context of drawbacks, and previous approaches in drug delivery modules for the CLZ, the in vivo approach was also considered a better model to study the safe drug release, and an outline was developed, which included the pharmacokinetics study of the transdermally released drug, CLZ.
2 Materials and methods
2.1 Materials
CLZ was donated by SEDICO Pharmaceuticals, Egypt. Methanol, KH2PO4, and Na2HPO4 were obtained from El Nasr Pharmaceutical Chemical Co., Cairo, Egypt. Cholesterol, Span 20, 40, and 60, and Tween 60, 80, Carbopol-934, Pluronic-F-127, HPMC, and triethanolamine were purchased from Sigma Chem. Co., St. Louis, MO, USA.
2.2 Preparation of CLZ-loaded niosomes by ether injection method
By using an ether injection approach, drug-loaded niosomes, with the properties shown in Table 1, were produced. Accurately weighed quantities of nonionic surfactant, i.e., Span 20, Span 40, Span 60, Tween 60, and Tween 80, and cholesterol (different cholesterol/surfactant molar ratios) were used in diethyl ether (6 mL). The preceding solution was mixed with CLZ in methanol (2 mL), and the resulting combination was carefully injected using a micro syringe at a rate of 1 mL/min into 10 mL of phosphate buffer saline (PBS, pH 6.8). The dispersion was heated to 60 ± 5°C with continuous stirring on a magnetic stirrer [32]. Under the conditions, the organic solvents were evaporated, resulting in the formation of CLZ-loaded niosomes.
Composition of the prepared CLZ-loaded niosomes
| Preparation | Drug (mg) | Surfactant | Molar ratios | |
|---|---|---|---|---|
| Cholesterol | Surfactant | |||
| F1 | 10 | Span 20 | 1 | 0.5 |
| F2 | 10 | Span 20 | 1 | 1 |
| F3 | 10 | Span 20 | 1 | 1.5 |
| F4 | 10 | Tween 60 | 1 | 0.5 |
| F5 | 10 | Tween 60 | 1 | 1 |
| F6 | 10 | Tween 60 | 1 | 1.5 |
| F7 | 10 | Tween 80 | 1 | 0.5 |
| F8 | 10 | Tween 80 | 1 | 1 |
| F9 | 10 | Tween 80 | 1 | 1.5 |
| F10 | 10 | Span 60 | 1 | 0.5 |
| F11 | 10 | Span 60 | 1 | 1 |
| F12 | 10 | Span 60 | 1 | 1.5 |
| F13 | 10 | Span 40 | 1 | 0.5 |
| F14 | 10 | Span 40 | 1 | 1 |
| F15 | 10 | Span 40 | 1 | 1.5 |
2.3 Characterization of the prepared CLZ niosomes
2.3.1 Niosomal size and polydispersity index (PDI)
The size of the prepared niosomal formulations was measured using the dynamic light scattering technique. Using a zeta sizer ZS 90 (Malvern Instruments, Worcestershire, United Kingdom), the niosomes’ particle size and zeta potential were observed at room temperature (25°C). Before measurement, the samples were diluted with 1:1, methanol: water. The generated CLZ niosomal formulations’ particle size homogeneity and PDI were also measured [33].
2.3.2 Drug entrapment efficiency
A definite volume of the niosomal dispersion was centrifuged at high speed to precipitate the niosomes and obtain a clear supernatant. The precipitated niosomes were washed 3× with 50% aqueous methanol. The collected supernatant (1 mL) was mixed with 9 mL of 50% aqueous-methanol, and using a UV spectrophotometer, absorbance was measured at 258 nm wavelength for the determination of CLZ quantity in the supernatant. Equation (1) is used to determine the drug entrapment efficiency (DEE) [34,35].
C t and C f referred to the concentrations of total CLZ, and free CLZ (in the supernatant), respectively.
2.4 In vitro drug release test
The in vitro release experiments of CLZ from niosomal formulations were carried out using a standard cellophane membrane. In brief, an open-ended glass tube with a piece of wetted cellophane membrane (2.65 cm2, surface area), which was wrapped over one end and secured with a rubber band, was used. In a 0.5 L beaker, the tube was submerged in PBS (200 mL) that had a pH of 6.8 and was kept under shaking (50 rpm) at 37°C [36]. The cellophane membrane was located slightly below the surface of the buffer in the tube and held vertically inside the beaker. The tube contained niosomal preparation equal to 5 mg of CLZ. Free CLZ, 5 mg, was suspended in 1 mL distilled water, fixed inside the release tube, and acted as a control for drug release. At predetermined time intervals, and to maintain a consistent volume, 5 mL aliquots of the released media were taken out and replaced with an equivalent volume (5 mL) of fresh PBS. Using a UV spectrophotometer, at a wavelength of 258 nm, the absorbance of the samples was measured in comparison to the blank, and the quantity of the released drug was calculated. The mean value of three releases of the same experiment were recorded.
2.5 Fourier transform infrared (FT-IR) spectroscopic analysis
Infrared spectroscopic analyses for CLZ, excipients, and selected niosomal formula were recorded by FT-IR- 476 (Shimadzu Kyoto, Japan) instrument. KBr was added to the dry test sample before being compacted into a solid disc. Spectra were recorded in the IR region 400–4,000 cm−1.
2.6 Differential scanning calorimetry scanning
DSC analyses were conducted using a differential scanning calorimeter calibrated with indium (DSC-50, Shimadzu Co., Japan). Samples of CLZ, surfactant, cholesterol, their physical mixtures, and CLZ-loaded niosomal formulations were analyzed. The thermograms were taken at a 10°C/min rate. The temperature range for the scanning of each sample was 20–380°C.
2.7 SEM scanning
Surface morphology of CLZ niosomal formulations was observed through scanning electron micrograph, using the SEM instrument (JSM-5400LVSEM, Jeol, Japan). One drop of niosomal formulation, placed on a transparent glass stub, was air dried and coated with Polaron-E-5100 sputter coater, and SEMs were obtained.
2.8 Stability studies
For 6 months, stability investigations of selected CLZ niosomal formulations were conducted at 4 and 25°C to observe the changes within the chosen parameters. To prevent any possible interactions between the niosomal formulations and the glass of the utilized vials, which may impair the study, the formulations were stored in boron glass vials. Each month, the evaluated CLZ niosomal formulations were examined for any physical alterations in parameters, including color changes, vesicular size change, DEE, and any in vitro drug release.
2.9 Preparation of CLZ niosomal gels
The following gel bases, Carbopol-934 (G1), Pluronic-F-127 (G2), and HPMC (G3) were selected to observe the delivery of CLZ at concentrations of 1, 30, and 3%, respectively. The gelling ingredients were dissolved in distilled water to create the bases, which were then given a 24-h hydration time. The selected CLZ-loaded niosomal dispersion (F10) was centrifuged at 10,000 rpm at 4°C for 45 min to obtain the niosomal vesicles. These vesicles (F10) were then incorporated into aqueous dispersions of Carbopol-934 (CLZ-G1-F10), Pluronic-F-127 (CLZ-G2-F10), and HPMC (CLZ-G3-F10). The resultant semi-solid dispersions were slowly triturated and handled to avoid entrapment of air bubbles. In the case of the G1 gel base (Carbopol-934), the dispersion was neutralized by triethanolamine (5%, w/v) to obtain the viscous, translucent gel.
2.10 Characterization of the prepared CLZ niosomal gel drug contents
In 100 mL of PBS (pH 6.8), 500 mg of prepared CLZ niosomal gel, precisely weighed and shaken, was taken, and a clear solution was produced when the mixture was mechanically shaken for 30 min. After filtering the resultant solution, the quantity of the medication was measured at 258 nm on a UV instrument using PBS (pH 6.8) as a reference.
2.11 Viscosity determination
By using a Brookfield viscometer (DV-III ultra-viscometer, Brookfield Engineering Laboratories, INC, Stoughton, USA), the viscosity of the generated CLZ gel compositions was assessed. The samples’ viscosities were measured using spindle number 4, which revolved at 50 rpm, with 20 g of each gel charged in a beaker [37].
2.12 In vitro release of CLZ from niosomes-loaded gels
The effects of compositional variation on release rates were examined under in vitro conditions for various formulations. The tests were conducted by placing 3 g of each gel into the dialysis bag, bags closed with clips and immersed in 250 mL PBS (pH 6.8), which were kept at 37°C. A magnetic stirrer was used to stir the beaker’s contents at ∼100 rpm. Aliquots of samples were taken at predetermined intervals and were promptly replaced with an equivalent quantity of fresh fluid. The withdrawn samples were analyzed by UV spectrophotometer at 258 nm wavelength for determination of the quantity of the released drug, CLZ.
2.13 Kinetic studies of the in vitro drug release
All the prepared niosome gel formulations’ drug release mechanisms were examined. In vitro, release data were subjected to regression analysis using zero-order, first-order, and Higuchi-diffusion models [38].
2.14 Ex vivo permeation of CLZ from niosomal gel
CLZ penetration from the prepared gels was examined through a 3.65 cm2 patch of rat abdomen skin using a Franz diffusion cell. The receptor cells containing 20 mL of diffusion medium (PBS, pH 6.8) were kept at 37°C and were shaken constantly at 300 rpm using a horizontal water bath shaker. On the shaved rat abdomen skin in the donor area, CLZ niosomal gel preparations containing niosomal formulations corresponding to 5 mg of CLZ, or pure drug, were applied. For 12 h, 1 mL sample was taken at regular intervals and subjected to spectrophotometric analysis to determine the quantity of the CLZ released. To maintain optimal sink conditions and maintain a consistent volume of the diffusion, an equivalent volume of fresh diffusion medium was introduced into the receptor cells. The mean was obtained after each experiment was run in triplicate.
2.15 Pharmacokinetics of CLZ from drug-loaded niosomal gel
The procedure, in brief, included loading Carbopol-934 gel preparation with CLZ niosomal formulation equivalent to 1% drug (CLZ-G1-F10) and pure drug (1%, w/w) of a Carbopol-934 gel formulation (CLZ-G0). The ethical committee, national legislation, and university codes of conduct regulations for the care of experimental animals were followed. The animal experiments were approved by the Zagazig University, with approval No. ZU-IACUC/3/E/861/2021. The rats were divided into three groups (n = 3): group 1 was treated with CLZ niosomal gel (CLZ-G1-F10), group 2 was treated with pure CLZ gel (CLZ-G0), and group 3 did not receive any treatment (control group). The rats fasted during the night with free access to water till the drug was administered [39]. On the next day, groups 1 and 2 of the rats, CLZ niosomal gels, and pure CLZ gels, respectively, were treated with 100 mg gel containing 1 mg of CLZ (equivalent to 3 mg/kg). Blood samples were taken after 1, 2, 3, 4, 6, 8, 10, and 12 h from the ear vein of the animal under light ether anesthesia and were analyzed for drug content using HPLC.
2.16 Blood samples’ treatment and HPLC assay
Collection of blood samples (1 mL) was performed from the rat’s ear vein in heparinized tubes, which were centrifuged at 5,000 rpm for 15 min. A 5 mL polypropylene centrifuge tube with a flattened tip was filled with 0.5 mL of methyl tertiary butyl ether, and 0.2 mL of the separated plasma before the tube was vortexed for 1 min at a high speed. Immediately afterward, the sample was agitated on a revolving shaker at 50 revolutions per minute for 3 min. The transparent layer was transferred with a measured pipette to a temporary glass tube and evaporated over a moderate flow of dry nitrogen gas after the sample was centrifuged for 20 min @2,000 rpm, at 4°C temperature. Dried residue, 20 µL, in mobile phase combination, was injected into the HPLC. The mobile phase for HPLC analysis was composed of 50 portions of CH3CN, 20 portions of acetate buffer (50 mM, pH 5.0), and 30 portions of H2O (ratio of 50:20:30, v/v). On the C18 column (Phenomenex, 250 4.60 mm, 0.5 mm particle size), filtered samples of CLZ were analyzed at a rate of 1.5 mL/min. The CLZ detection wavelength was 258 nm. The extraction recovery was calculated by comparing the assay results of plasma, spiked with CLZ, at three concentrations of 0.5, 1, and 1.5 μg/mL, with the same concentrations dissolved in methanol. CLZ’s pharmacokinetic characteristics, C max (the maximum plasma concentration), and T max (the time to reach the peak concentration) were observed using the plasma concentration plotted against time. The absorption rate constant (K abs), elimination rate constant (K el), and t 1/2 (half-life) were also calculated.
2.17 Coagulation measurements
The rats were fasted overnight, and the blood samples were drawn in EDTA-tubes 2, 6, 12, 18, 24, 36, and 48 h after the application of the pure drug, and the niosomal CLZ formulations for PT and APTT (prothrombin time and activated partial thromboplastin time) [40].
2.18 Bleeding time (BT) and clotting time (CT)
The BT was measured according to the described procedure [41]. The rat’s tail was heated for 1 min in a water bath at 40°C, and before it was dried, with a knife, a tiny incision was created in the tail’s center. When the first drop contacted the circular filter paper, the bleeding process was counted to begin. Up until the bleeding stopped, it was checked every 30 s.
For measuring CT, a glass microhematocrit capillary was used to hold a 25 μL sample of capillary blood. Once the blood initially came into touch with the glass capillary tube, the chronometer was activated. When the blood stopped flowing (the endpoint), the capillary tube was tilted alternatively to +60° and −60° angles concerning the horizontal plane. The two tube markings were left to flow by gravity between them [42].
2.19 Prothrombin time and activated partial thromboplastin time (PTT and APTT)
Partial thromboplastin time (PTT), a frequently used test for coagulation, and APTT, quantifying the combined activities of the majority of coagulation components in intrinsic and extrinsic blood coagulation cascade pathways, were performed. The PTT measured the time it took to clot after the tissue factor was added (PT and APTT). A Systemex CA-5000 Coagulation Analyzer was used to measure CTs [43].
2.20 Statistical analysis
Using SPSS v.24 software, the data were subjected to analysis of variance at the 0.05 level of statistical significance. The information was displayed as mean ± SE. Every experiment was run in triplicate.
3 Results and discussion
3.1 CLZ structure and characterization of the prepared CLZ niosomes: size and polydispersity indices
The CLZ chemical structure possessed a quinolinone alkaloid molecular skeleton, with cyclohexyl moiety attached to the tetrazole ring, which bridged to the quinolinone through a butoxy group (Figure 1). The common structural features for the activity elicitation needed to possess a strong dipole producer, adjacent acid proton, short alkyl chain attached to the heterocyclic ring, an electron-rich center with a hydrogen bond acceptor, and a nearly flat molecular geometry. These structural features contributed to the structural stability of the CLZ molecule under different bio-conditions and made the molecule susceptible to hydroxylation and dehydrogenative transformations under the hepatic and other metabolites forming conditions [6,7,44]. The niosomal preparation using different types and ratios of the surfactants with the drug and cholesterol (both components’ weight ratios were kept constant) produced niosomes of different sizes. The increase in the niosome sizes was observed for certain types of surfactants. The use of nonionic surfactant’s physico-chemical properties, the lipophilic/hydrophilic character, molecular size, the presence of polar and non-polar groups, together with the alkyl chain length and their disposition in the molecular structure of the surfactant, methods of preparation, and the niosome formation parameters, including the surfactants’ HLB values, are among the main factors in the niosomal size variations during their preparations. Prepared formulations, F1–F9, had progressively increased in size. For the formulations F10–F15, there was a drop in the size of the formulation F10 as compared to the formulation F9, but the sizes increased gradually from formulations F10 to F15, thereby suggesting the roles of the molecular and physico-chemical characteristics of the surfactant, the interactions of the preparative components of the formulating niosomes, lamellar layers’ formation, and the lipophilic nature of the surfactants playing their respective parts in size determinations. Changes in surfactants and the ratio of their use were also among the deciding factors in the niosome size determinations. Surfactants, Span 20, Tween 60, and 80 in different but constant ratios of 0.5, 1, and 1.5 were utilized for the preparations of F1–F9 formulations, while Span 60 and 40 were used for preparations of F10-F15 formulations of niosomes. For these preparations, the surfactant–cholesterol ratios were kept constant at 0.5, 1, and 1.5 for all the used surfactant types. For both the size increment trends, F1–F9, and F10–F15, the size increase from F1–F9, and F10–F15 was attributed to the use of different span surfactant types, and the most hydrophilic surfactant, Tween 60, was primarily the major cause. The size differences were also thought to be a culmination of unilamellar and multilamellar niosome formations, the multilamellar vesicles being larger. However, current preparations had provided over 100 nm-sized niosomes, all being multilamellar in their built. The surfactant effect played its main part in deciding the size increments of the niosomal formulations. The observations suggested that the hydrophobicity of the surfactant, and the HLB values had a significant impact on the vesicle size. The hydrophobicity of the surfactant increased with an increase in the alkyl chain length, which lowered the HLB score. The lowest vesicle sizes were observed in niosomes that were prepared using Span 60, and that may be due to the lowest level of HLB value of the employed surfactant [45]. Niosome size and PDI of the prepared CLZ-loaded niosomal vesicles are presented in Table 2 and Figure 2. The particle size of the obtained vesicles averaged from 102 to 550 nm for all niosomal preparations. The HLB value of the utilized surfactant determined the size of the vesicles. The vesicle surface energy and water absorption into the niosomal bilayer increased as the hydrophilic monomers of the surfactant increased, which may have also contributed to the vesicle’s growth in size. These findings also indicated that the size of the niosomal vesicles decreased with the decreasing HLB values. The size of the developed CLZ-loaded niosomal formulations also grew as the cholesterol content arose. The niosomal preparations with CLZ revealed a limited peak in their size distribution, which indicated that the currently used technique for the niosome preparation (Ether injection method) produced comparatively homogeneous niosomal vesicles. The PDI for the most tested niosomal formulations was around 0.3, suggesting a monodisperse vesicle condition [46,47,48].
Entrapment efficiencies, vesicle sizes, and PDI of CLZ-loaded niosomes
| Preparation | EE | Rank | Vesicle size (nm) | Rank | PDI | Rank |
|---|---|---|---|---|---|---|
| F1 | 62.6 ± 1.5 | 8 | 214 ± 16.5 | 7 | 0.46 ± 0.111 | 11 |
| F2 | 60.2 ± 1.2 | 9 | 291 ± 9.05 | 8 | 0.4 ± 0.028 | 9 |
| F3 | 56.7 ± 1.4 | 11 | 306 ± 11.23 | 9 | 0.13 ± 0.240 | 2 |
| F4 | 42.4 ± 2.4 | 13 | 326 ± 8.45 | 10 | 0.29 ± 0.099 | 8 |
| F5 | 36.2 ± 1.9 | 14 | 365 ± 12.15 | 11 | 0.2 ± 0.100 | 5 |
| F6 | 29.9 ± 1.4 | 15 | 385 ± 8.37 | 12 | 0.2 ± 0.156 | 5 |
| F7 | 68.1 ± 3.1 | 7 | 424 ± 10.11 | 13 | 0.68 ± 0.105 | 14 |
| F8 | 48.7 ± 1.3 | 12 | 477 ± 9.55 | 14 | 0.75 ± 0.201 | 14 |
| F9 | 57.3 ± 1.5 | 10 | 550 ± 7.98 | 15 | 0.17 ± 0.194 | 4 |
| F10 | 96.4 ± 2.6 | 1 | 102 ± 11.30 | 1 | 0.10 ± 0.069 | 1 |
| F11 | 85.8 ± 1.3 | 3 | 122 ± 13.15 | 2 | 0.28 ± 0.111 | 7 |
| F12 | 91.3 ± 1.7 | 2 | 132 ± 17.56 | 3 | 0.25 ± 0.102 | 6 |
| F13 | 79.5 ± 2.3 | 5 | 149 ± 11.26 | 4 | 0.15 ± 0.094 | 3 |
| F14 | 73.9 ± 1.8 | 6 | 192 ± 10.90 | 5 | 0.66 ± 0.127 | 12 |
| F15 | 80.5 ± 2.7 | 4 | 209 ± 11.45 | 6 | 0.45 ± 0.106 | 10 |

Sizes of different CLZ-loaded niosomal formulations (inset figure: size distributions of CLZ niosomal formula, F10).
3.2 Drug entrapment efficiency of CLZ-loaded niosomes
The DEE results of the prepared CLZ-loaded niosomal formulations are presented in Figure 3. The results showed that the DEE for the prepared niosomes using Span® (especially Span 60) was higher than those prepared using the Tween® as a surfactant, which could be due to the lipophilic nature of both the Span (low HLB) used. In contrast, due to the hydroxyl groups and ether oxygen-based functional groups in their chemical structures, Tweens® behaved as hydrophilic surfactants (with high HLB, and had high aqueous solubility, as compared to the hydrophobic Span® surfactants). As a result, in an aqueous media, the Tweens® did not properly form the niosomal vesicular structures. However, since Span® surfactants are lipophilic (low HLB), they formed the niosomal vesicles and entrapped higher concentrations of the drug, CLZ [49]. An increase in the Spans® and Tweens® concentrations resulted in lowering the entrapment efficiency, which was attributed to the decreased effects of cholesterol ratio in the preparative constituents of the niosomes, and played its role in stabilizing the prepared niosomal vesicles. According to previous reports, the quantity of lipophilic drug that is integrated into the niosomal bilayers would increase in entrapment with a lower HLB value surfactant [50]. Niosomal formulation, F10, containing Span 60 displayed maximum DEE (96.4 ± 2.6%), an impressive higher DEE than other formulations containing Span 40 and Span 20. Span 60 had a longer alkyl chain moiety in its structure as compared to the Span 40 and Span 20, which provided higher entrapment efficiency for the drug, which was also demonstrated earlier [51].
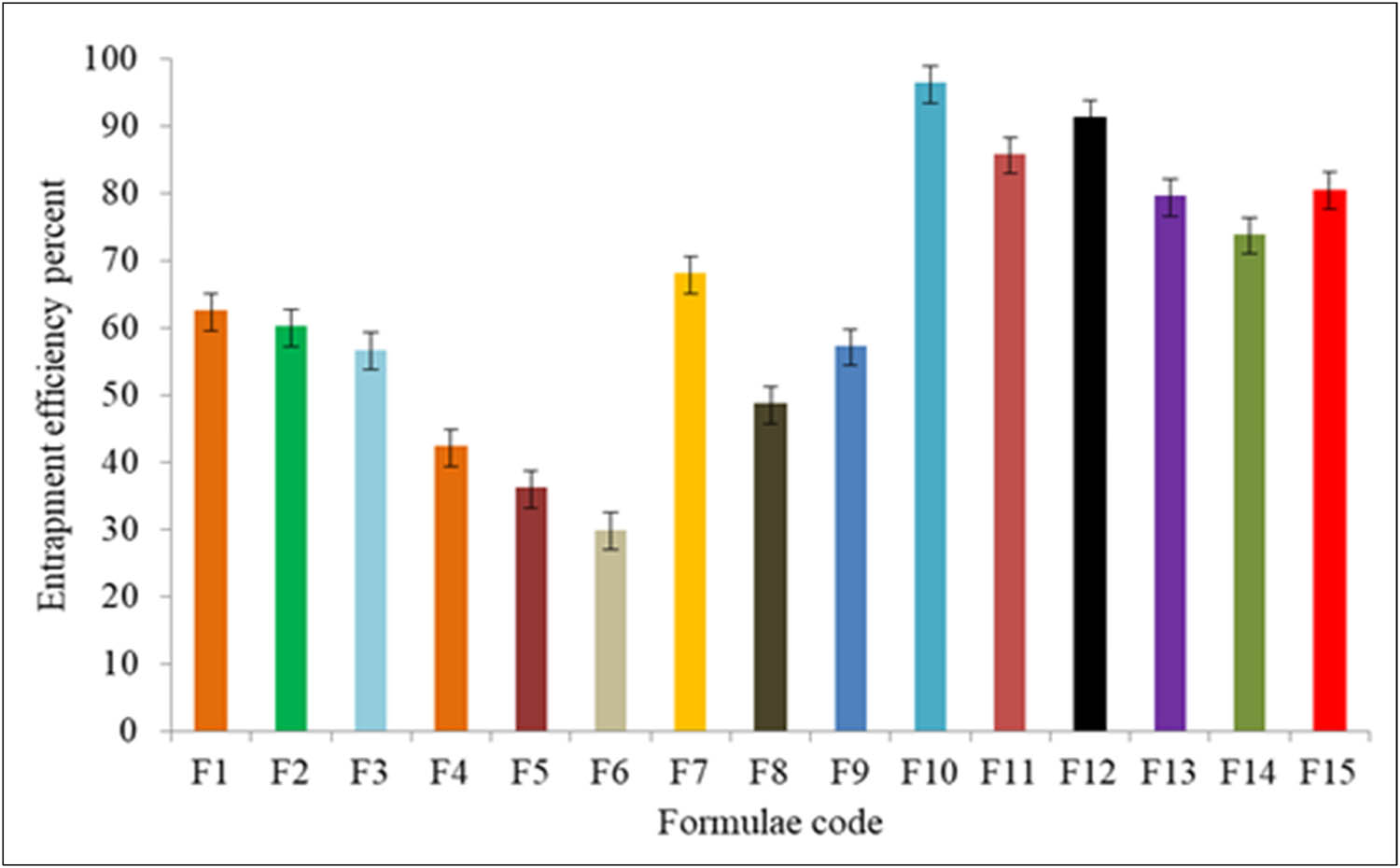
Entrapment efficiency (%) of the CLZ-loaded niosomes.
3.3 In vitro release of CLZ from niosomes
The in vitro release of CLZ from niosomal formulation was carried out by the diffusion method using a semi-permeable cellophane membrane (molecular cut-off range of ∼12,000). The results are illustrated in Figures 4 and 5. The highest drug, CLZ, release rate after 24 h (84.11 ± 4.06%) was observed in the case of F10 containing Span 60, with a ratio equal to (1:0.5) cholesterol/surfactant presence, while the slowest release was noticed from the niosomal formulation, F9 (27.58 ± 2.55%). The ongoing results indicated that the CLZ niosomal formulations had sustained release patterns, which were extended for a comparatively longer period. It was detected that in vitro CLZ release from the niosomal formulations containing different Spans® (20, 40, and 80) was sharply increased, and it was higher than the formulations containing CLZ from different formulations containing Tweens® (Tween 60 and 80). In addition, it was noted that the drug release rate slowed down (with an increase in size) when the surfactant’s contents were also higher. These results demonstrated the impact of Spans’ alkyl chain length on the niosomes’ stability and quantity of the CLZ released [52,53]. The stability and size quotients of the niosomes and the use of the surfactants, i.e., non-ionic and highly lipophilic, in nature provided comparatively higher drug entrapments as well as higher drug release [54]. The drug release from the niosomes prepared from Tween® surfactants fell behind the Span® surfactants-based niosome formulations.
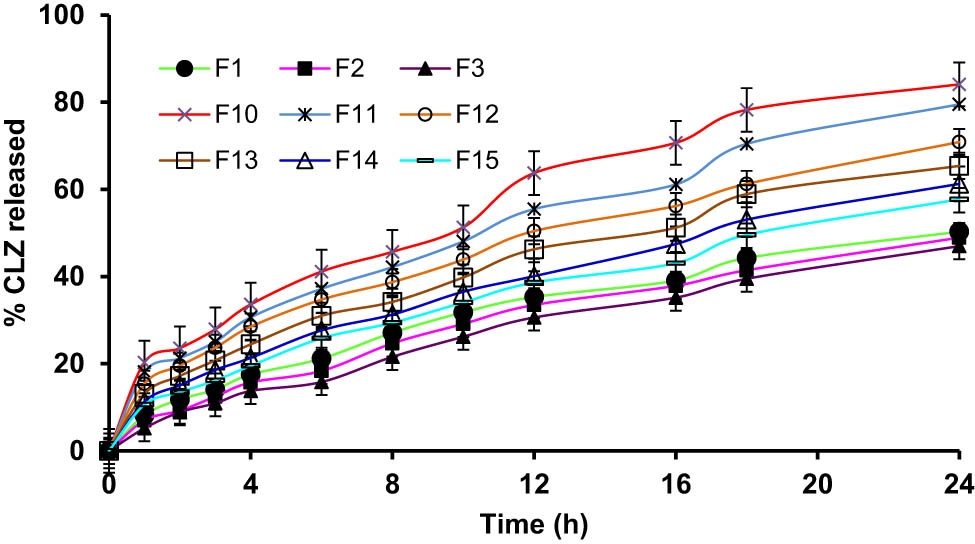
Cumulative percent of CLZ quantities released from niosomes prepared from different types of Spans®.
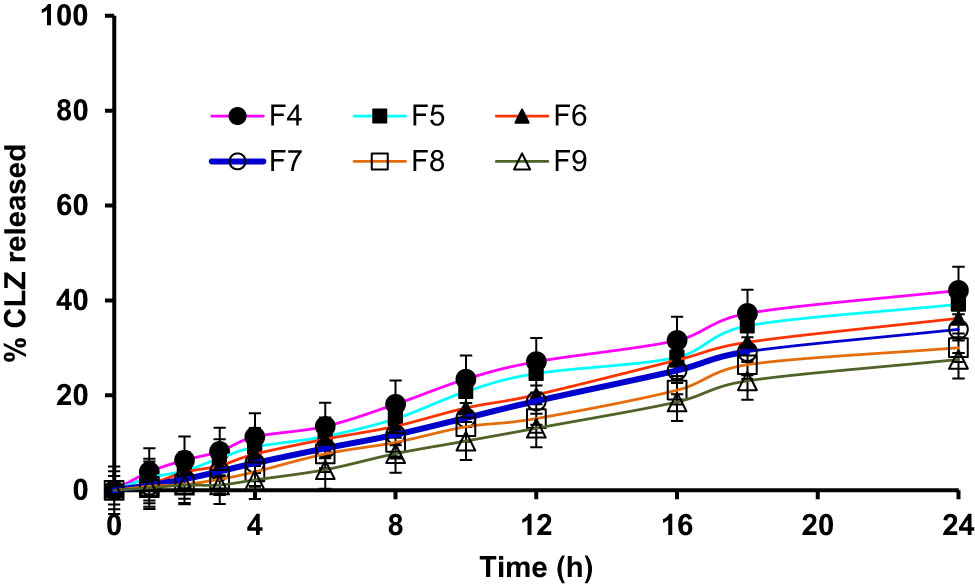
Cumulative percent of CLZ quantity released from niosomes containing different types of Tweens®.
3.4 FT-IR spectroscopic analysis
FT-IR spectra of the ingredients of CLZ-loaded niosomes formulations were recorded and are illustrated in Figure 6. The characteristic peaks in the FT-IR spectrum of the CLZ, cholesterol, and Span® 60 were comparable to those described in the literature, which indicated the purity of the samples suitable for niosomal formulations. The CH stretching at 2950 cm−1, aromatic ether peak at 1,196 cm−1, and NH peak of the quinolinone moiety showed between 3100 and 3400 cm−1. These exhibited peaks were assigned for the drug, CLZ [53]. FT-IR spectrum of the physical mixture of components showed a nearly similar peak pattern (spectrum D). The FT-IR spectra of CLZ niosomal formulations (F10) (spectrum E) showed major absorption peaks with nearly similar peak patterns, thereby indicating the niosomal preparation (Figure 6).
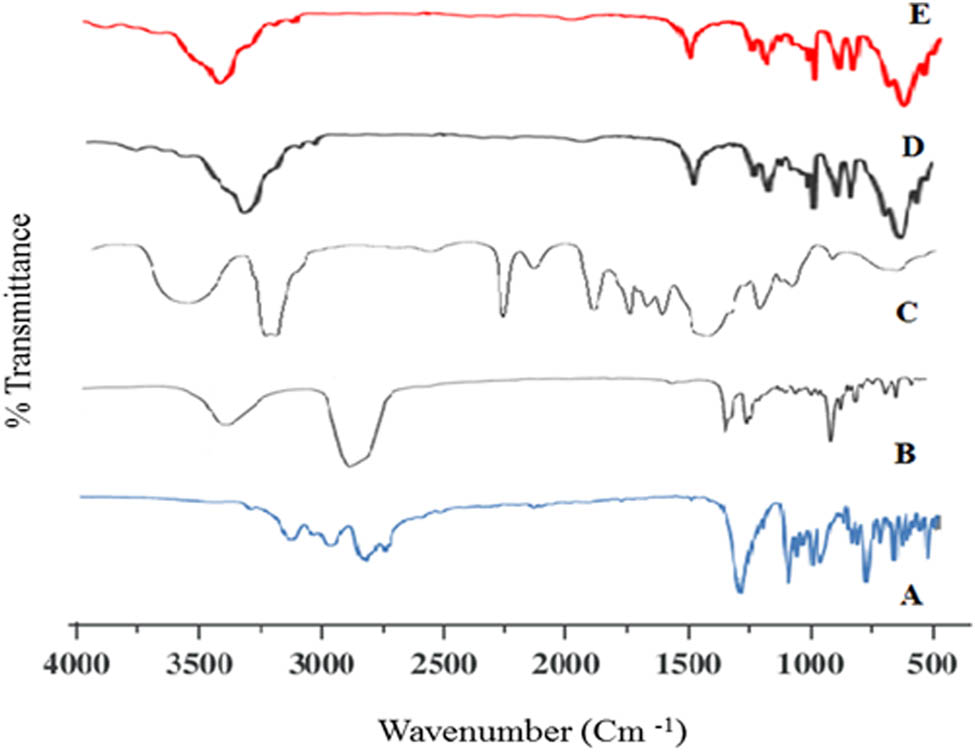
FT-IR spectra of (A) CLZ, (B) cholesterol, (C) Span 60, (D) physical mixture of the ingredients, and (E) CLZ-loaded niosomal formulation (F10).
3.5 DSC scanning
DSC thermograms of CLZ, cholesterol, Span 60, nonloaded drug niosomes, and CLZ-loaded niosomal formulation (F10) are presented in Figure 7. The thermogram of CLZ (Scan A) revealed a clear endothermic peak at 159–161°C, which is the melting point of the drug. The thermal transitions of the cholesterol monohydrate are known to be below 100°C (Scan B). The conversion of cholesterol monohydrate to anhydrous cholesterol is known as cholesterol transition, which happens at 70°C. The endothermic peak (Scan C) for Span 60 was seen at 35°C, which matches the transition temperature for Span-60 hydrate [55]. The DSC thermograms of drug-free niosomal formula (Scan D) indicated a shift in the transition endotherm of Span 60–86°C. The melting endotherm of the cholesterol also appeared to have diminished in intensity and shifted on the thermogram. On the other hand, the melting endotherm of CLZ was shifted to 98°C on the thermogram of the niosomal formulation (Scan E). In addition, it demonstrated a reduced intensity of the cholesterol endotherm, showing that the medication was distributed uniformly in the niosomal formulation [56].
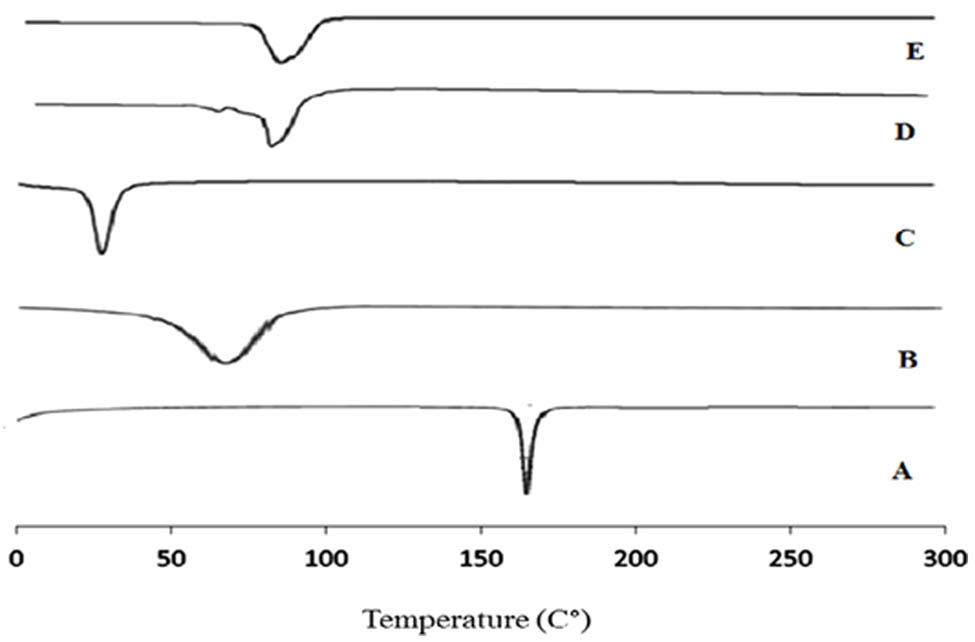
DSC thermograms of (A) CLZ, (B) cholesterol, (C) Span 60, (D) nonloaded drug niosomes, and (E) CLZ-loaded niosomal formulation (F10).
3.6 Scanning electron microscopy
SEM image of the selected niosomal formulation loaded with CLZ (F10) showed a nearly spherical shape. The prepared niosomes also indicated a closed vesicular structure with smooth surfaces and no aggregation. The SEM image demonstrated differently sized CLZ-loaded niosomal vesicles, which were produced using the ether injection method (Figure 8).
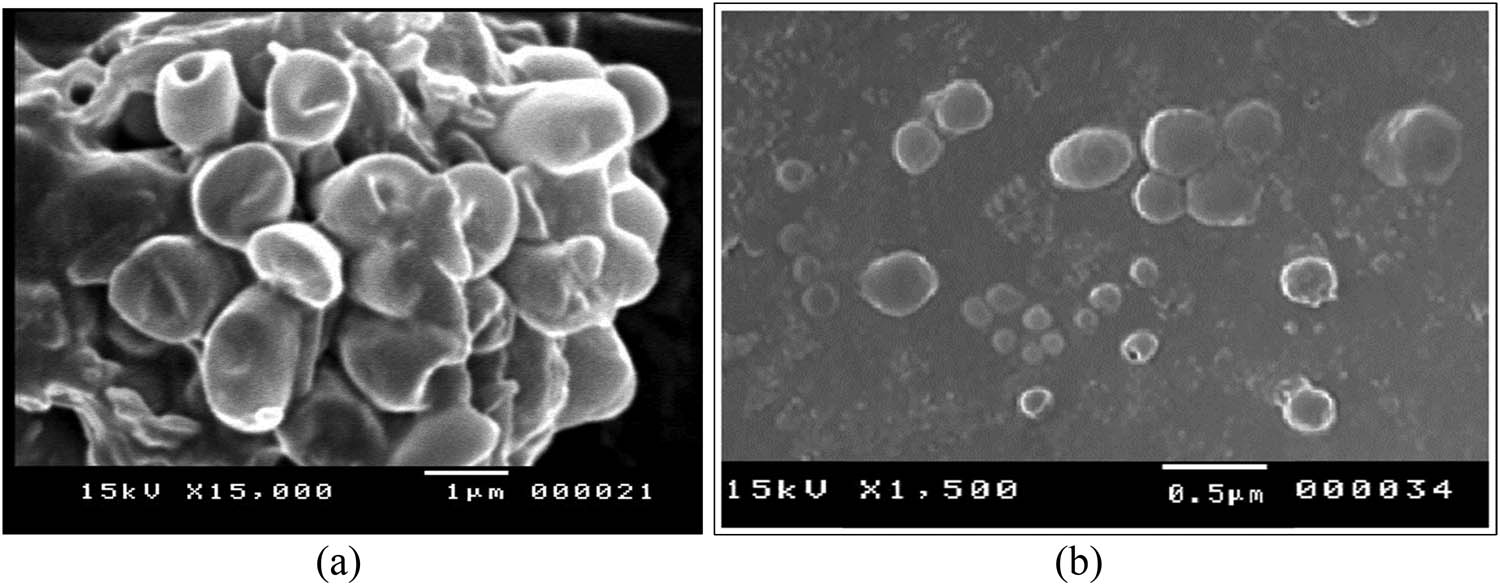
SEM of the (a) empty niosomes and (b) CLZ-loaded niosomes, formulation (F10).
3.7 Physical stability of the selected niosomal formulation (F10)
Physical stability studies of the selected CLZ-loaded niosomes formulation were conducted at two different temperatures, 4°C and 25°C. In the refrigerator (4°C), the stored CLZ-loaded niosomes (formulation, F10) prepared with Span 60 surfactant (at a ratio of 0.5:1, surfactant: cholesterol) containing 10 mg CLZ were stable during the storage period (6 months). During this storage time, the drug was not leaked from the stored niosomes. The color of the niosomal dispersion also did not change, and the vesicles particle sizes were also not observed to be changed noticeably. At room temperature (25°C), the stored niosomes preparation (F10) was not stable during the time (6 months). The instability was caused due to drug leaking from the stored CLZ-loaded niosomes, and the niosomal suspension’s color was also noticed to have changed with the size increment of the vesicles. The hydrated bilayer niosomal vesicles are thermodynamically unstable and present a metastable condition [57]. The niosomal vesicular systems kept in aqueous dispersion tend to clump and fuse, and as a result of these changes, the encapsulated drug leaks during storage. The cholesterol’s capacity to stabilize membranes may explain the CLZ niosomal formula (F10) stability, whereas the breakdown and/or clumping of the prepared niosomal vesicles can be related to the loss of niosomal vesicles’ density in the suspension media. A minimal loss of CLZ was seen during the cold storage (4°C), which may have happened because of the rigidification of the niosomal vesicles at low temperature, which decreased the permeability of the drug, CLZ, across the membrane [58]. Accordingly, the stability study results indicated that the prepared niosomal vesicular systems were stable at 4°C as compared to the storage at 25°C in terms of mean vesicle size, the entrapped concentration of the drug, and percent cumulative drug released after 24 h (Table 3).
Stability studies
| Characteristics | Time lapse | At 25 ± 2°C | At 4 ± 1°C |
|---|---|---|---|
| Vesicle size (nm) | At 0 month | 107 ± 9.45 | 107 ± 9.45 |
| After 1 month | 188 ± 10.21 | 120 ± 7.33 | |
| After 3 months | 270 ± 8.92 | 145 ± 8.12 | |
| After 6 months | 385 ± 11.25 | 193 ± 9.44 | |
| % DEE | At 0 month | 95.23 ± 3.20 | 95.23 ± 3.20 |
| After 1 month | 80.61 ± 2.55 | 91.88 ± 3.24 | |
| After 3 months | 69.28 ± 4.12 | 87.65 ± 2.81 | |
| After 6 months | 46.66 ± 3.05 | 83.90 ± 3.11 | |
| Cumulative % release after 24 h | At 0 month | 87.23 ± 2.59 | 87.23 ± 2.59 |
| After 1 month | 93.52 ± 3.2 | 89.41 ± 3.82 | |
| After 3 months | 95.89 ± 2.30 | 90.66 ± 2.25 | |
| After 6 months | 98.25 ± 3.44 | 91.92 ± 3.06 |
3.8 Preparation of different CLZ gel formulations
Three types of gels were prepared, namely, Carbopol-934, 1% gel (CLZ-G1-F10), Pluronic F-127, 30% gel (CLZ-G2-F10), and HPMC-based 3% gel (CLZ-G3-F10). The results of the drug content assays confirmed nonsignificant variations between the theoretical and actual CLZ contents in the prepared niosomal gels (94.16–97.54%). The gel formulations were kept in brown-colored glass vials (120 mL) in the refrigerator until further investigation. The viscosities of the prepared CLZ gel formulations ranged between 7945.34 and 5097.53 cps (Table 4), which was considered ideal since they offered excellent in vitro drug release from the gel.
Drug content and viscosity values of CLZ different gel formulations
| Preparation | Drug contents (%) | Viscosity (CPS) |
|---|---|---|
| CLZ-G1-F10 | 96.21 ± 3.38 | 7945.34 ± 99.43 |
| CLZ-G2-F10 | 97.54 ± 5.87 | 6360.11 ± 174.78 |
| CLZ-G3-F10 | 94.16 ± 3.74 | 5097.53 ± 112.30 |
3.9 In vitro CLZ release from gel bases containing CLZ-loaded niosomal formula through cellophane membranes
The in vitro CLZ release profile obtained from various niosomal gel formulations is shown in Figure 9. It was observed that only about 54.44 to 70.90% of the encapsulated CLZ was released during 12 h from different niosomal gel formulations. The in vitro release of CLZ was significantly impacted by the changes in the gelling agent/gelling points. As a result, the increased viscosity of CLZ-G1-F10, as compared to other gel formulations, showed in vitro CLZ release slower for the Carbopol 934 gel. In contrast, the niosomal HPMC gel formulation (CLZ-G3-F10) exhibited the highest drug release (70.90 ± 2.22%) as compared with the other two gel formulations. The highest drug release from HPMC gel may be linked to lower viscosity of CLZ-G3-F10 [59]. The quantity of CLZ released from different gel formulations was, for CLZ-G3-F10 > CLZ-G2-F10 > CLZ-G1-F10. Accordingly, the transdermal gel formulation CLZ-G1-F10 was selected for further skin permeation study in comparison to other gel formulations containing pure CLZ with similar levels of the gelling agent.
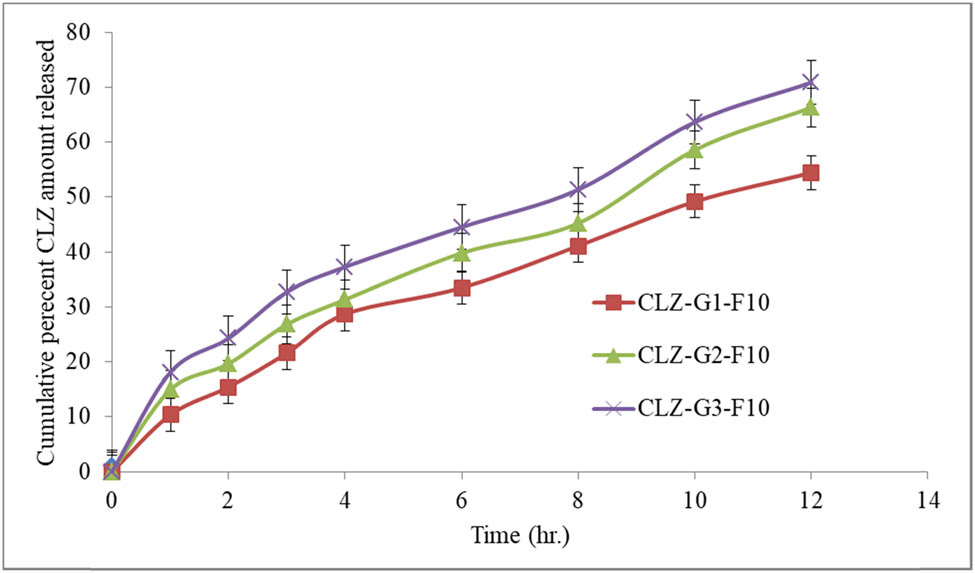
Percent cumulative CLZ quantity released through cellophane membrane from different gel formulations.
3.10 Kinetic studies of in vitro drug release
To validate the model that described the CLZ release, an in vitro release of CLZ from various gel bases loaded with the drug niosomal formula (F10) was conducted using zero-order, first-order, and Higuchi diffusion models, together with the Korsmeyer-Peppas equation. The correlation coefficient (R2) value was used to determine the suitable release model. The release profiles of CLZ from all studied gel formulations in the consecutive release profile corresponded best to the Higuchi-diffusion model (Table 5).
Kinetic parameters of the in vitro release profiles of CLZ from different gel formulations
| Preparation | Parameters | Zero order | First order | Diffusion | Mechanism |
|---|---|---|---|---|---|
| CLZ-G1-F10 | a | 6.329 | 1.384 | −4.366 | Diffusion |
| b | 4.291 | 0.046 | 16.268 | ||
| r | 0.983 | 0.313 | 0.990 | ||
| K (min−1) | 4.291 | 0.106 | 16.268 | ||
| t 1/2 (min) | 11.651 | 6.511 | 9.446 | ||
| CLZ-G2-F10 | a | 8.048 | 1.386 | −4.283 | Diffusion |
| b | 5.007 | 0.037 | 18.909 | ||
| r | 0.985 | 0.257 | 0.987 | ||
| K (min−1) | 5.007 | 0.086 | 18.909 | ||
| t 1/2 (min) | 9.985 | 8.046 | 6.992 | ||
| CLZ-G3-F10 | a | 11.342 | 1.373 | −2.298 | Diffusion |
| b | 5.235 | 0.033 | 20.143 | ||
| r | 0.973 | 0.230 | 0.995 | ||
| K (min−1) | 5.235 | 0.076 | 20.143 | ||
| t 1/2 (min) | 9.550 | 9.148 | 6.162 |
3.11 Ex vivo skin permeation studies
Figure 10 represented the ex vivo skin permeation of the drug, CLZ, from the CLZ niosomal gel-based formulation, (CLZ-G1-F10), as compared to the plain CLZ gel (CLZ-G0). The percentage quantity of the CLZ permeated after 12 h from the CLZ-G0 was found to be at 60.47 ± 3.44%, which was smaller than the percentage of CLZ that seeped from the CLZ niosomal gel (CLZ-G1-F10) (71.34 ± 3.12%). The Transdermal flux (J) values for the CLZ-G1-F10 and CLZ-G0 were found to be at 27.107 and 26.318 μg/cm2 h−1, respectively. The obtained data revealed the potential of CLZ niosomal gel (CLZ-G1-F10) to remain at the targeted site for a considerable duration of time, which may increase the patients’ compliance [60].
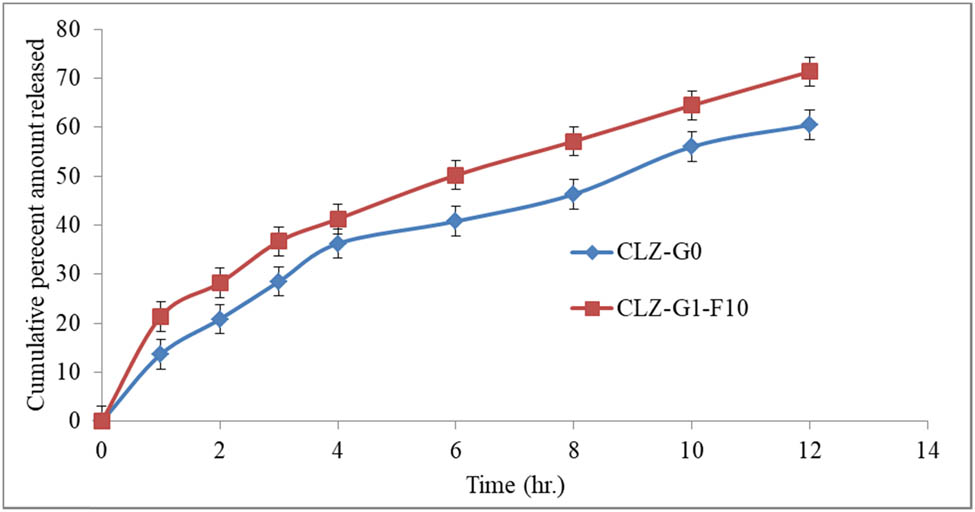
Percent cumulative CLZ released through abdominal rat skin after 12 h of duration from CLZ-G0 and CLZ-G1-F10.
The kinetics of the CLZ permeated from the niosomal gel-based formulation (CLZ-G1-F10) across the skin showed a Higuchi diffusion release profile by which it can be inferred that the CLZ niosomal gel combined with hydrophilic Carbopol-934 gel shall release adequate quantities of the drug [61].
3.12 In vivo pharmacokinetics parameters study
Figure 11 illustrated the CLZ plasma concentration profiles versus the time after topical application of the CLZ-G0 and CLZ-G1-F10 gel formulations. Table 6 lists the corresponding pharmacokinetic parameters (C max, T max, AUC0–12, AUC0–∞, CLT, and t 1/2) of CLZ from these formulations. The peak plasma CLZ concentration (C max), after applying the gel base containing CLZ-loaded niosomal formulation, CLZ-G1-F10 (3850.92 ± 207.50 ng/mL), was significantly (p < 0.005) higher than that obtained after the topical administration of the CLZ-G0 preparation (1250.46 ± 99.56 ng/mL). The investigated pure CLZ gel showed a shorter T max (3 h) than that obtained for CLZ niosomal gel formulation (4 h). The stratum corneum layer of the rat skin’s barrier capacity and the continuous release of CLZ from the prepared niosomal transdermal gel formulations were the reasons for the variations in the T max values. The CLZ was released instantly from the pure drug gel formulation [62]. Moreover, the AUC0–12 and AUC0–end values of CLZ-G1-F10 formulation were 28992.16 ng h/mL and 35508.72 ng h/mL, respectively, which, when compared with the same parameters, were observed for CLZ-G0 to be at 8488.825 and 5312.3 ng h/mL, respectively, and these values were substantially different (p < 0.005).
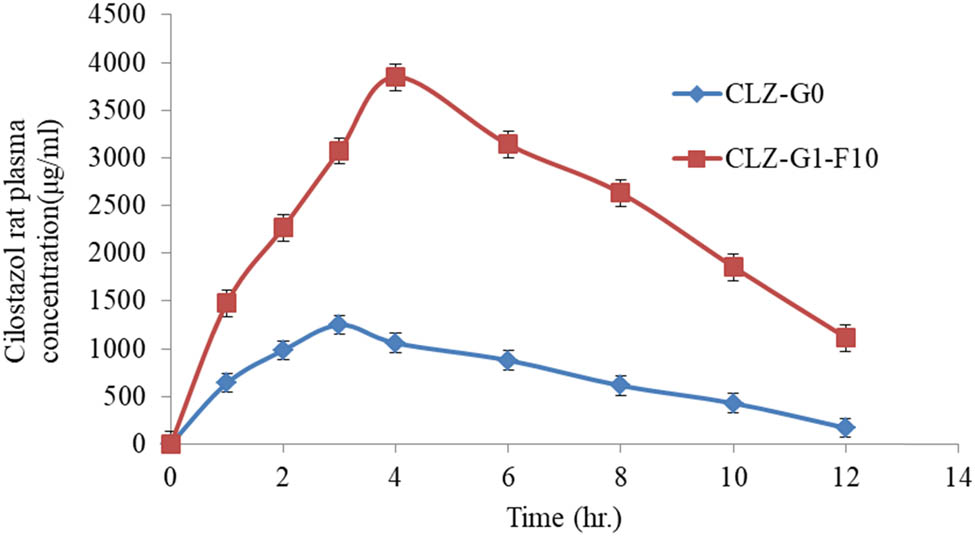
Mean plasma concentration-time profiles of CLZ after topical administration of CLZ-G0 and CLZ-G1-F10 gel formulations.
Pharmacokinetic parameters of CLZ after transdermal application of CLZ-G0 and CLZ-G1-F10 gel formulations
| Parameters | Applied formulations | |
|---|---|---|
| CLZ-G0 | CLZ-G1-F10 | |
| Dose | 3 mg/kg | 3 mg/kg |
| C max (ng/mL) | 1250.46 ± 99.56 | 3850.92 ± 207.50 |
| T max (h) | 3 ± 0.88 | 4 ± 1.06 |
| K abs (h−1) | 0.5350 ± 0.06 | 0.4005 ± 0.03 |
| t 1/2 (abs) (h) | 1.2952 ± 0.33 | 1.7301 ± 0.041 |
| K el (h−1) | 0.2185 ± 0.071 | 0.17193 ± 0.05 |
| t 1/2 (el) (h) | 3.1712 ± 0.553 | 4.0306 ± 0.794 |
| AUC0–12 (ng h/mL) | 8488.825 ± 238.69 | 28992.16 ± 156.99 |
| AUC0–end (ng h/mL) | 53125.3 ± 352.78 | 35508.72 ± 278.48 |
| MRT (h) | 5.7319 ± 1.14 | 6.9553 ± 1.89 |
| ClT (mL/min) | 0.000404 ± 0.0009 | 0.000105 ± 0.0006 |
These results confirmed that the enhanced CLZ bioavailability could be obtained from topically routed niosomal gel formulations, as observed throughout the evaluation period (12 h). The considerably enhanced CLZ bioavailability through transdermal niosomal gel formulations may be a result of the hepatic enzymes’ avoidance of CLZ metabolism. The elimination half-lives (t 1/2) of CLZ-G1-F10 (4.0306 h) were significantly higher than that of the pure CLZ gel (CLZ-G0) (3.171 h). This occurred as a result of the niosomal gel formulations’ longer-lasting CLZ release profile.
3.13 Coagulation profile measurements
The effects of CLZ on bleeding time (BT), and on ex vivo APTT and PT anti-platelet effects could be accompanied by adverse effects, such as, prolonged BT, and impaired blood coagulation. First, the effects of gel base containing pure CLZ (CLZ-G0) formulation, and niosomal CLZ formula (CLZ-G1-F10), on bleeding and CT, were determined under in vivo conditions, using male Wister rats.
A significant increase in bleeding and CTs was observed at all the tested time durations and compared with the control group. The observations are presented in Table 7. Pure CLZ gel (CLZ-G0) and the niosomal CLZ gel (CLZ-G1-F10) showed no effects on the PT and on the APTT, as they did not increase the risk of platelet inhibitor-induced bleeding effects in the experiments.
Effects of pure CLZ gel (CLZ-G0), and CLZ niosomal gel (CLZ-G1-F10) on CT, BT, PT, and APTT in male Wister rats at different times after administration, as compared to the control (values are mean ± S.D)
| Groups (time collected) | Blood CT (s) | BT (s) | Prothrombin time (s) | Activated partial thromboplastin time | |
|---|---|---|---|---|---|
| Control | 121 ± 3.15e | 90 ± 2.75f | 24.52 ± 0.75 | 16.80 ± 0.37 | |
| 2 h | CLZ-G0 | 215 ± 4.25a | 162 ± 2.36a | 26.50 ± 0.73 | 17.28 ± 0.25 |
| CLZ-G1-F10 | 211 ± 5.36a | 160 ± 3.40a | 25.81 ± 0.69 | 16.43 ± 0.18 | |
| 6 h | CLZ-G0 | 201 ± 3.85a | 154 ± 2.10a | 29.61 ± 0.40 | 17.93 ± 0.97 |
| CLZ-G1-F10 | 199 ± 4.17a | 151 ± 3.24a | 27.37 ± 0.64 | 17.52 ± 0.45 | |
| 12 h | CLZ-G0 | 193 ± 5.23a | 149 ± 3.01b | 22.86 ± 0.28 | 16.31 ± 0.78 |
| CLZ-G1-F10 | 191 ± 4.57a | 145 ± 2.85b | 23.95 ± 0.57 | 15.94 ± 0.93 | |
| 18 h | CLZ-G0 | 177 ± 4.69b | 130 ± 1.78c | 26.59 ± 0.24 | 17.64 ± 0.28 |
| CLZ-G1-F10 | 174 ± 3.70b | 127 ± 2.59c | 29.16 ± 0.24 | 18.39 ± 0.74 | |
| 24 h | CLZ-G0 | 155 ± 2.83c | 118 ± 2.24c | 28.70 ± 0.58 | 17.67 ± 0.34 |
| CLZ-G1-F10 | 151 ± 1.92c | 115 ± 1.36c | 30.06 ± 0.17 | 17.90 ± 0.86 | |
| 36 h | CLZ-G0 | 143 ± 1.54c | 107 ± 1.91d | 27.27 ± 0.78 | 17.95 ± 0.43 |
| CLZ-G1-F10 | 140 ± 1.96c | 103 ± 2.98d | 26.76 ± 0.35 | 17.40 ± 0.87 | |
| 48 h | CLZ-G0 | 124 ± 1.73e | 94 ± 1.94f | 27.94 ± 0.73 | 18.21 ± 0.58 |
| CLZ-G1-F10 | 136 ± 2.04d | 98 ± 2.08e | 26.43 ± 0.31 | 18.94 ± 0.72 | |
| F-Test | ** | ** | NS+ | NS+ | |
+NS, not significant, **Not conducted. The mean values that do not share a superscript letter (a to f) in the respective columns are significantly different.
4 Conclusions
The niosomes loaded with CLZ were successfully prepared using the ether injection approach, and high entrapment efficiencies were observed. The examinations of the electron micrographs demonstrated the production of approximately spherical and multilamellar niosomal vesicles, which were observed to be stable at low temperature (4°C), which confirmed the stable shelf life of the CLZ niosomal product. Compared to the niosomal gel formulation that contained CLZ, the pure CLZ gel exhibited a shorter T max (the time to reach the peak concentration in the plasma). Moreover, without changing the prothrombin and APTTs, the drug-loaded niosomal gel formulation demonstrated a notable increase in bleeding and CTs. Future action is required to take advantage of the potential for continued usage of the CLZ niosome formulation, particularly in clinical settings, and in future clinical development for patients.
Acknowledgments
The authors are thankful to the Deanship of Graduate Studies and Scientific Research at the University of Bisha for supporting this work through the Fast-Track Research Support Program.
-
Funding information: This work was supported by the Deanship of Scientific Research at the University of Bisha through the Fast-Track Research Support Program.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Manolis AA, Manolis TA, Melita H, Mikhailidis DP, Manolis AS. Update on cilostazol: A critical review of its antithrombotic and cardiovascular actions and its clinical applications. J Clin Pharmacol. 2022;62(3):320–58. 10.1002/jcph.1988.Search in Google Scholar PubMed
[2] Gresele P, Momi S, Falcinelli E. Anti‐platelet therapy: Phosphodiesterase inhibitors. Br J Clin Pharmacol. 2011;72:634–46.10.1111/j.1365-2125.2011.04034.xSearch in Google Scholar PubMed PubMed Central
[3] Abdelkader H, Alani AW, Alany RG. Recent advances in non-ionic surfactant vesicles (niosomes): self-assembly, fabrication, characterization, drug delivery applications, and limitations. Drug Deliv. 2014;21:87–100.10.3109/10717544.2013.838077Search in Google Scholar PubMed
[4] Biju S, Talegaonkar S, Mishra P, Khar R. Vesicular systems: An overview. Indian J Pharm Sci. 2006;68:141–53.10.4103/0250-474X.25707Search in Google Scholar
[5] Hua S. Lipid-based nano-delivery systems for skin delivery of drugs and bioactives. Front Pharmacol. 2015;6:219–24.10.3389/fphar.2015.00219Search in Google Scholar PubMed PubMed Central
[6] Abbas R, Chow CP, Browder NJ, Thacker D, Bramer SL, Fu CJ, et al. In vitro metabolism and interaction of cilostazol with human hepatic cytochrome P450 isoforms. Hum Exp Toxicol. 2000;19(3):178–84. 10.1191/096032700678827717.Search in Google Scholar PubMed
[7] Bhatt NM, Chavada VD, Patel DP, Sharma P, Sanyal M, Shrivastav PS. Determination of cilostazol and its active metabolite 3,4-dehydro cilostazol from small plasma volume by UPLC-MS/MS. J Pharm Anal. 2015 Feb;5(1):1–11. 10.1016/j.jpha.2014.08.001.Search in Google Scholar PubMed PubMed Central
[8] Cilostazol: Official FDA information, side effects, and uses. Drugs.com; February 2008. Retrieved 2024-04-22.Search in Google Scholar
[9] USFDA. Drug Development and Drug Interactions: Table of substrates, inhibitors and inducers. Food and Drug Administration; Retrieved 2024-04-29.Search in Google Scholar
[10] Taniguchi K, Ohtani H, Ikemoto T, Miki A, Hori S, Sawada Y. Possible case of potentiation of the antiplatelet effect of cilostazol by grapefruit juice. J Clin Pharm Therapeutics. 2007;32(5):457–9. 10.1111/j.1365-2710.2007.00844.x.Search in Google Scholar PubMed
[11] Cilostazol: Official USFDA information, side effects, and uses. Drugs.com. February 2008. Retrieved 2024-04-22.Search in Google Scholar
[12] Cilostazol: Uses, side effects, dosage & reviews (goodrx.com).Search in Google Scholar
[13] Cilostazol (Oral Route) Side Effects – Mayo Clinic.Search in Google Scholar
[14] Hiatt WR, Money SR, Brass EP. Long-term safety of cilostazol in patients with peripheral artery disease: the CASTLE study (Cilostazol: A Study in Long-term Effects). J Vasc Surg. 2008 Feb;47(2):330–6. 10.1016/j.jvs.2007.10.009.Search in Google Scholar PubMed
[15] Rogers KC, Oliphant CS, Finks SW. Clinical efficacy and safety of cilostazol: a critical review of the literature. Drugs. 2015 Mar;75(4):377–95. 10.1007/s40265-015-0364-3.Search in Google Scholar PubMed
[16] Pratt CM. Analysis of the cilostazol safety database. Am J Cardiology. 2001 Jun;87(12):28–33. 10.1016/S0002-9149(01)01719-2.Search in Google Scholar PubMed
[17] Frontera JA, Lewin JJ, Rabinstein AA, Aisiku IP, Alexandrov AW, Cook AM, et al. Guideline for reversal of antithrombotics in intracranial hemorrhage: A statement for healthcare professionals from the neurocritical care society and society of critical care medicine. Neurocrit Care. 2016;24(1):6–46.10.1007/s12028-015-0222-xSearch in Google Scholar PubMed
[18] Abdellatif AA, Zayed GM, Kamel H, Mohamed AG, Arafa WM, Khatib AM, et al. A novel controlled release microsponges containing Albendazole against Haemonchus contortus in experimentally infected goats. J Drug Delivery Sci Tech. 2018;46:469–76. 10.1016/j.jddst.2017.10.022.Search in Google Scholar
[19] Verma P, Pathak K. Therapeutic and cosmeceutical potential of ethosomes: An overview. J Adv Pharm Technol. 2010;1:274–82. 10.4103/0110-5558.72415.Search in Google Scholar PubMed PubMed Central
[20] Rajera R, Nagpal K, Singh SK, Mishra DN. Niosomes: a controlled and novel drug delivery system. Biol Pharm Bull. 2011;34:945–53.10.1248/bpb.34.945Search in Google Scholar PubMed
[21] Rajera R, Nagpal K, Singh SK, Mishra DN. An overview on niosomes: a drug nanocarrier. DDIPIJ. 2018;1:143–51. 10.1248/bpb.34.945.Search in Google Scholar
[22] Akhtar N, Singh V, Yusuf M, Khan RA. Non-invasive drug delivery technology: development and current status of transdermal drug delivery devices, techniques, and biomedical applications. Biomed Tech (Berl). 2020 May 26;65(3):243–72. 10.1515/bmt-2019-0019.Search in Google Scholar PubMed
[23] Vyas SP, Khar RK. Targeted and controlled drug delivery: Novel carrier systems. Darya Ganj, Delhi, India: CBS Publishers & Distributors; 2004.Search in Google Scholar
[24] Mahale N, Thakkar P, Mali R, Walunj DR, Chaudhari SR. Niosomes: Novel sustained release nonionic stable vesicular systems-An overview. Adv Colloid Interface Sci. 2012;183:46–54. 10.1248/bpb.34.945.Search in Google Scholar
[25] Mohammed HA, Khan RA, Singh V, Yusuf M, Akhtar N, Sulaiman GM, et al. Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development. Nanotechnol Rev. 2023;12(1):20220517.10.1515/ntrev-2022-0517Search in Google Scholar
[26] Abdellatif AAH, Mohammed HA, Khan RA, Singh V, Bouazzaoui A, Yusuf M, et al. Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity. Nanotechnol Rev. 2021;10(1):1493–559. 10.1515/ntrev-2021-0096.Search in Google Scholar
[27] Bawazeer S, El-Telbany DFA, Al-Sawahli MM, Zayed G, Keed AA, Abdelaziz AE, et al. Effect of nanostructured lipid carriers on transdermal delivery of tenoxicam in irradiated rats. Drug Deliv. 2020;27:1218–30. 10.1080/10717544.2020.1803448.Search in Google Scholar PubMed PubMed Central
[28] Keshav J. Niosomes as a potential carrier system: A review. Inter J Pharm Chem Bio Sci. 2015;5(4):947–59.Search in Google Scholar
[29] Patel SG, Rajput SJ. Enhancement of oral bioavailability of cilostazol by forming its inclusion complexes. AAPS PharmSciTech. 2009;10(2):660–9. 10.1208/s12249-009-9249-7.Search in Google Scholar PubMed PubMed Central
[30] Wannas A, Maraie NK. Characterization and optimization of oral solid supersaturable self-emulsifying drug delivery system of cilostazol. Res J Pharm Technol. 2021;14(6):1–6. 10.52711/0974-360X.2021.00586.Search in Google Scholar
[31] Lu W, Pengfei Z, Ting L, Dandan Y, Qianqian J, Jinliang C, et al. Physiologically based absorption modeling to predict the bioequivalence of two cilostazol formulations. Clin Transl Sci. 2023;16(11):2323–30. 10.1111/cts.13633.Search in Google Scholar PubMed PubMed Central
[32] Hao Y, Zhao F, Li N, Yang Y. Studies on a high encapsulation of colchicine by a niosome system. Inter J Pharm. 2002;244:73–80. 10.1016/s0378-5173(02)00301-0.Search in Google Scholar PubMed
[33] Srinivas S, Kumar YA, Hemanth A, Anitha M. Preparation and evaluation of niosomes containing aceclofenac. Dig J Nanomater Bios. 2010;5:249–54.Search in Google Scholar
[34] Sankhyan A, Pawar P. Recent trends in niosome as vesicular drug delivery system. J Appl Pharm Sci. 2012;2:20–32. 10.7324/JAPS.2012.2625.Search in Google Scholar
[35] Dahiya NK, Rao R, Nanda S. Preparation and characterization techniques in niosomal vesicular systems-A review. J Pharm Biomed Sci. 2011;5:1–8.Search in Google Scholar
[36] Zayed GM, Kamal I, Abdelhafez WA, Alsharif F, Amin MA, Shaykoon MS, et al. Effect of chemical binding of doxorubicin hydrochloride to gold nanoparticles, versus electrostatic adsorption, on the in vitro drug release and cytotoxicity to breast cancer cells. Pharm Res. 2018;35(6):1–14.10.1007/s11095-018-2393-6Search in Google Scholar PubMed
[37] Mahmoud DB, Shukr MH, Bendas ER. In vitro and in vivo evaluation of self-nanoemulsifying drug delivery systems of cilostazol for oral and parenteral administration. Inter J Pharm. 2014;476:60–9. 10.1007/s11095-018-2393-6.Search in Google Scholar
[38] Nvs M, Saini A. Niosomes: A novel drug delivery system. Int J Res Pharm Chem. 2011;1:498–511.10.4103/2231-0738.77539Search in Google Scholar
[39] Shahiwala A, Misra A. Studies in topical application of niosomally entrapped nimesulide. J Pharm Sci. 2002;5:220–5.Search in Google Scholar
[40] Lenders JW, Willemsen JJ, Eisenhofer G, Ross HA, Pacak K, Timmers HJ, et al. Is supine rest necessary before blood sampling for plasma metanephrines? Clin Chem. 2007;53:352–4. 10.1373/clinchem.2006.076489.Search in Google Scholar PubMed
[41] Martin AC, Le Bonniec B, Fischer AM, Marchand-Leroux C, Gaussem P, Samama CM, et al. Evaluation of recombinant activated factor VII, prothrombin complex concentrate, and fibrinogen concentrate to reverse apixaban in a rabbit model of bleeding and thrombosis. Inter J Cardiol. 2013;168:4228–33. 10.1016/j.ijcard.2013.07.152.Search in Google Scholar PubMed
[42] Garcia-Manzano A, Gonzalez-Llaven J, Lemini C, Rubio-Poo C. Standardization of rat blood clotting tests with reagents used for humans. Proceedings of the Western Pharmacology Society. Seattle, WA, USA: 2001.Search in Google Scholar
[43] Jiskani A, Memon S, Naseem L. Prothrombin time (PT), activated partial thromboplastin time (APTT) and international normalized ratio (INR) as predictive factors of coagulopathy in newly diagnosed hypertensive patients. Hematol Transfus Int J. 2017;4:84–8. 10.1007/s40200-018-0347-5.Search in Google Scholar PubMed PubMed Central
[44] Fossa P, Boggia R, Mosti L. Toward the identification of the cardiac cGMP inhibited-phosphodiesterase catalytic site. J Comput Mol Des. 1998;12(4):361–72.10.1023/A:1007928412086Search in Google Scholar
[45] Naggar V, El Sallam A. Proniosomes as a stable carrier for oral acyclovir: Formulation and physicochemical characterization. Am J Sci. 2012;8:1–13.Search in Google Scholar
[46] Zhai Y, Zhai G. Advances in lipid-based colloid systems as a drug carrier for topic delivery. J Control Rel. 2014;193:90–9. 10.1016/j.jconrel.2014.05.054.Search in Google Scholar PubMed
[47] Solanki AB, Parikh JR, Parikh RH, Patel MR. Evaluation of different compositions of niosomes to optimize aceclofenac transdermal delivery. Asian J Pharm Sci. 2010;5:87–95.Search in Google Scholar
[48] Boddu SH. Formulation, and delivery of drugs for macular edema and retinoblastoma; synthesis and in vitro characterization of doxorubicin-loaded surface modified nanoparticles using PLGA-PEG-FOL polymer. Kansas City, MO, USA: University of Missouri-Kansas; 2010.Search in Google Scholar
[49] Benson HA. Transdermal drug delivery: Penetration enhancement techniques. Curr Drug Deliv. 2005;2(1):23–33.10.2174/1567201052772915Search in Google Scholar PubMed
[50] Abu-Elyazid SK, Kassem AA, Samy AM, Gomaa ME. Evaluation of skin permeation and pharmacological effects of tenoxicam nanoemulsión in topical formulations. Asian J Pharm Health Sci. 2011;1:99–105.Search in Google Scholar
[51] Gravett D, Takacs-Cox A, Toleikis PA, Maiti A, Embree L. Tissue reactive compounds and compositions and uses thereof. IFI CLAIMS Patent Services, Barcelona, Spain, 2004.Search in Google Scholar
[52] Patel KK, Kumar P, Thakkar HP. Formulation of niosomal gel for enhanced transdermal lopinavir delivery and its comparative evaluation with ethosomal gel. AAPS PharmSciTech. 2012;13:1502–10.10.1208/s12249-012-9871-7Search in Google Scholar PubMed PubMed Central
[53] Shilakari GA, Asthana A, Singh D, Sharma PK. Etodolac containing topical niosomal gel: formulation development and evaluation. J Drug Deliv. 2016;2016:1–8.10.1155/2016/9324567Search in Google Scholar PubMed PubMed Central
[54] Shreya A, Managuli RS, Menon J, Kondapalli L, Hegde AR, Avadhani K, et al. Nano-transfersomal formulations for transdermal delivery of asenapine maleate: In vitro and in vivo performance evaluations. J Liposome Res. 2016;26:221–32. 10.3109/08982104.2015.1098659.Search in Google Scholar PubMed
[55] El-Say KM, Abd-Allah FI, Lila AE, Hassan AE-S, Kassem AEA. Diacerein niosomal gel for topical delivery: Development, in vitro and in vivo assessment. J Liposome Res. 2016;26(1):57–68. 10.3109/08982104.2015.1029495.Search in Google Scholar PubMed
[56] Samanidou V, Demetriou C, Papadoyannis I. Direct determination of four fluoroquinolones, enoxacin, norfloxacin, ofloxacin, and ciprofloxacin, in pharmaceuticals and blood serum by HPLC. Anal Bioanal Chem. 2003;375:623–9.10.1007/s00216-003-1749-9Search in Google Scholar PubMed
[57] Gomes ML, Klein T, Simionatto M, Nadal JM, Zanin SM, Borsato DM, et al. A simple RP-HPLC/UV method for determination of cilostazol in polymeric nanoparticles suspensions: Development and validation. Lat Am J Pharm. 2015;34:803–9.Search in Google Scholar
[58] Velam V, Yalavarthi PR, Sundaresan C, Vandana K, Dudala TB, Kodavatikanti H, et al. In vitro and in vivo assessment of piroxicam incorporated Aloe vera transgel. Int J Pharm Investig. 2013;3:212–6. 10.4103/2230-973X.121303.Search in Google Scholar PubMed PubMed Central
[59] Lee D, Lim LA, Jang SB, Lee YJ, Chung JY, Choi JR, et al. Pharmacokinetic comparison of sustained-and immediate-release oral formulations of cilostazol in healthy Korean subjects: A randomized, open-label, 3-part, sequential, 2-period, crossover, single-dose, food-effect, and multiple-dose study. Clin Ther. 2011;33:2038–53. 10.1016/j.clinthera.2011.10.024.Search in Google Scholar PubMed
[60] Yoshioka C, Ito Y, Nagai N. Enhanced percutaneous absorption of Cilostazol nanocrystals using aqueous gel patch systems and clarification of the absorption mechanism. Exp Ther Med. 2018;15:3501–8. 10.3892/etm.2018.5820.Search in Google Scholar PubMed PubMed Central
[61] Nam K-Y, Cho SM, Choi YW, Park C, Meghani NM, Park J-B, et al. Double controlled release of highly insoluble cilostazol using surfactant-driven pH-dependent and pH-independent polymeric blends and in vivo bioavailability in beagle dogs. Int J Pharm. 2019;10:284–90. 10.1016/j.ijpharm.2019.01.004.Search in Google Scholar PubMed
[62] Aymard G, Warot D, Démolis P, Giudicelli JF, Lechat P, Le Guern ME, et al. Comparative pharmacokinetics and pharmacodynamics of intravenous and oral nefopam in healthy volunteers, Pharmacol Toxicol. 2003;92:279–86. 10.1034/j.1600-0773.2003.920605.x.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy

