Abstract
Cyclodextrin (CD)-block copolymer hybrid systems have recently received great attention from the pharmaceutical and materials research community because this combination can result in new biomaterials and supramolecular structures, which possess the physicochemical and thermotropic properties of both classes of materials. Different structures of CD-block copolymer systems have been described (i.e., micelles, vehicles, core-shell structures, nanospheres, and membranes) and they can encapsulate active pharmaceutical ingredients or other bioactive compounds. The aim of this review is to summarize several examples, the properties, the morphological and physicochemical characteristics, the added value, the techniques used for their preparation and characterization, as well as the limitations of CD-block copolymer systems. Taking into consideration the wide variety of block copolymers and CD materials and the expected beneficial characteristics/behavior following their complexation, we could suggest them as new-generation formulations in the upcoming years.
Graphical abstract

Abbreviations
- APIs
-
active pharmaceutical ingredients
- BM
-
benzimidazole
- CDs
-
cyclodextrins
- CMC
-
critical micelle concentration
- DOX
-
doxorubicin
- DM-β-CD
-
heptakis-(2,6-O-dimethyl)-β-CD
- EMA
-
European medical agency
- EPR
-
enhanced permeability and retention effect
- FA-PEL-CD
-
folate-poly(ethylene glycol)-poly(d,l-lactide)-β-CD
- HP-β-CD
-
hydroxypropyl-β-CD
- HP-γ-CD
-
hydroxypropyl-γ-CD
- mPEG-b-PLA
-
methoxy poly(ethylene glycol)-b-poly(lactide)
- MNPs
-
magnetic nanoparticles
- M-β-CD
-
methyl-β-CD
- NPs
-
nanoparticles
- NSs
-
nanospheres
- LCST
-
critical solution temperature
- PAA
-
poly(acrylic acid)
- PBLA
-
poly(b-benzyl-l-aspartate)
- PCL
-
poly(ε-caprolactone)
- PDI
-
polydispersity index
- PDMAEMA
-
poly(2-(dimethylamino) ethyl methacrylate)
- PEG
-
polyethylene glycol
- PEO
-
poly(ethylene oxide)
- PHB
-
poly[(R)-3-hydroxybutyrate]
- PLGA
-
poly(lactic-co-glycolic acid)
- PPEGA
-
poly(polyethylene glycol methyl ether acrylate)
- PPO
-
poly(propylene oxide)
- PS-b-P4VP
-
polystyrene-block-poly(4-vinylpyridine)
- PST
-
poly(styrene)
- PVA
-
polyvinyl acetate
- PVCL
-
polyvinyl caprolactam
- SBE-β-CD
-
sulfobutylether β-CD sodium salt
- SMGels
-
supramolecular hydrogels
- tNEA
-
trans-N-(2-ethoxy-1,3-dioxan-5-yl) acrylamide
- US-FDA
-
U.S. Food and Drug Administration
1 Introduction
Cyclodextrins (CDs) are a group of cyclic oligosaccharides and they possess molecular structures with a configuration of a truncated cone [1], consisting of a hydrophobic interior environment and hydrophilic exterior surfaces. This configuration permits them to accommodate diverse molecules into their cavities. Therefore, they can form inclusion (host–guest) complexes with noncovalent interactions, without complex chemical reactions, encapsulating poorly water-soluble compounds to improve their solubility in water and their stability, as well as to diminish the side reactions of drugs to a certain extent [2]. A large gamut of interactions between guests and host molecules contribute to the formation of complexes, like hydrophobic interactions, hydrogen bonds, van der Waals forces, and size effects [3,4].
Concerning the other substances of hybrid systems, polymers have many potentials as excipients for the pharmaceutical industry. A significant application of polymer nanoparticulate platforms is the design of innovative delivery systems for the transport of Active Pharmaceutical Ingredients (APIs) and nucleic acids. However, some factors compromise the use of polymers and they must be considered for the application of polymers in clinical practice, including the possible toxicity due to the use of cationic polymers, physicochemical instability of drug-containing systems which show changes in the size distribution and nanoparticle size of polymer-based formulations, system stability problems in the human body, reduced ability for the transfection of cell-target, and high expenses for scale up production of the final formulation [5]. The selection of the most appropriate polymer for creating a novel drug delivery platform is associated with the way of administration, the physicochemical profile of the incorporated drug, the properties of polymers utilized, the interactions between the different materials of the composed platforms, as well as several other factors [6].
Research over the past years led to the identification of amphiphilic block copolymers as potent therapeutic moieties for curing challenging human diseases, that tend to self-assemble in aqueous media leading to a plethora of nano-assemblies [7]. Thus, they can encapsulate hydrophobic compounds such as drugs or other bioactive molecules into their cavity. The aim of this review is to investigate the potential of CD-block copolymer hybrid systems. To the best of the authors’ knowledge, this is the first report in the literature where these drug delivery platforms and applications are addressed.
2 Methodology of literature review
A comprehensive search and review of literature concerning CD-block copolymer systems were conducted across multiple platforms, including PubMed, Medline, Scopus, and Web of Science, as well as through the consideration of abstract presentations at international conferences. The publications from 2006 to 2024 served as a comprehensive guide for the systematic investigation of CD-block copolymer systems. The search was based on the following terms: polymer-based nanocarriers, block copolymer, CD, drug delivery carriers, and hybrid systems. The authors performed the literature search excluding the studies with full text unavailable and publication language other than English.
3 Cyclodextrins systems
3.1 CDs
CDs, also called cycloamyloses, cyclomaltoses, or Schardinger dextrins [8], due to their backbone consisting of sugars, are a group of cyclic oligosaccharides composed of six (α-CD), seven (β-CD), or eight (γ-CD) D(+)-glucopyranose units, linked by α-1,4 glycosidic [9], shaping supramolecular structures with a toroidal configuration [1]. Due to the formation of sugars, the secondary 2- and 3-hydroxy groups are extended from the broader edge, whereas the primary 6-hydroxy groups are located at the narrower edge [10]. CDs contain hydrophobic central inner cavities, surrounded by hydrophilic outer surfaces (Figure 1a) [11]. Their shape permits them to accommodate diverse molecules. Therefore, they can create inclusion (host-guest) complexes through noncovalent interactions, without complex chemical reactions, enabling the encapsulation of hydrophobic compounds (Figure 1b). This encapsulation serves the purpose of enhancing water solubility and stability of the compounds, as well as decreasing the side reactions of drugs to a certain extent [2].

(a) Structure of CDs. OH1: primary 6-hydroxy groups; OH2: secondary 2-, 3-hydroxy groups and (b) schematic configuration of inclusion complex formation (1:1) between CD and a guest compound.
CDs are natural products, which are formed by the enzymatic degradation of starch, catalyzed by CD glucanotransferases from various bacteria like Bacillus strains. Larger CDs, containing 9 (δ-CD), 10 (ε-CD), 11 (ζ-CD), 12 (η-CD), and 13 (θ-CD) glucopyranose units have been reported, but they do not have applications in the pharmaceutical industry [3]. A wide variety of interactions contributes to the formation of guest-host complexes, such as hydrogen bonds, hydrophobic interactions, van der Waals forces, and size effects [3,4].
The most commonly abundant CD in medicinal and pharmaceutical industries is β-CD [12]. The beneficial size of its cavity permits the accommodation of many bioactive compounds with a molecular weight of 200–800 g/mol, whereas the easy production process, availability in pure form, low cost, efficient formation of inclusion complexes, ability to enhance the bioavailability of drugs, and ameliorated toxicological profile [3,13,23] are some of the advantages that make β-CD the most frequently used CD in pharmaceutical applications.
3.2 CD derivatives
Despite the inherent hydrophilicity of natural CDs, their solubility in water remains inadequate, particularly in the case of β-CD. This deficiency can be attributed to the extremely strong intermolecular hydrogen bonding of CD in the crystalline state [10]. To overcome this obstacle, scientists have designed and developed various hydrophilic, lipophilic, and ionic derivatives of CDs which show superior inclusion capacity, specific water solubility, improved complexation efficacy, greater physicochemical properties, and lower toxicity in comparison with the natural ones [3]. The enhancement of natural CDs’ solubility could be achieved by the substitution of hydroxy groups using a wide variety of reagents, even lipophilic entities like those found in randomly methylated β-CD (M-β-CD) [14]. The improved water solubility is attributed to the random substitution process, which transforms the crystalline solids of natural CDs into physically stable, amorphous mixtures of isomers [14].
The number of CD derivatives is approximately more than 11,000. According to a search in SciFinder®, there are at least 2,000 different substituted derivatives for α-CD, more than 8,000 for β-CD and almost 1,000 for γ-CD [15]. For instance, the replacement of natural β-CD and γ-CD by propylene oxide leads to the formation of hydroxypropylated CD derivatives [16]. Hydroxypropyl-β-CD (HP-β-CD), heptakis-(2,6-O-dimethyl) β-CD, hydroxypropyl-γ-CD (HP-γ-CD), randomly M-β-CD, sulfobutylether β-CD sodium salt (SBE-β-CD), and the branched CDs such as glucosyl-β-CD, are some synthesized derivatives of high pharmaceutical interest [17].
3.3 Applications of CDs in the pharmaceutical industry
CDs are useful as one of the most common drug carriers [15]. In the pharmaceutical industry, the most widely highlighted application is the use of CDs for the formulation of hydrophobic drugs with insufficient bioavailability [11], increasing their poor aqueous solubility and physicochemical stability [18]. Their cavity is a substrate, which interacts with non-covalent bonds with drug molecules. As CDs act like molecular encapsulants, they modify dramatically the chemical, physical, and biological properties of the encapsulated organic molecules [19]. Also, CDs can be modified to CDs with specific features, such as hydrophobically-modified CDs to decrease the aqueous solubility of hydrophilic compounds, as well as peracylated CDs, characterized by high solubility in organic solvents, such as ethanol and acetone, and low solubility in water, that are used to reduce the water-solubility, prolong the elimination half-life, and permit favorable release properties through the formation of water-insoluble drug/CD complexes [18].
They can also act as flavor masking agents of unsavory drugs and reduce nasal mucosa damage, as well as irritation of the eye, skin, and gastrointestinal tract, because of the embedment of the drug molecules in CD cavity, making them useful tools for ameliorating drug delivery through the nasal, ocular, and pulmonary routes of administration [3,16]. Indeed, the progressive release of the drug from the complex with a slow rate permits the maintenance of free drug’s concentration below the levels that could be vexatious for the mucosa [20]. Furthermore, CDs can also prevent the interactions between drugs, or between drugs and excipients, and prevent the conversion of liquid compounds into amorphous or microcrystalline powder [21]. Another noteworthy application of CD complexes is their ability to separate enantiomers of drugs, obtaining the enantiomer with the most favorable properties [22]. Last but not least, CDs play a crucial role in facilitating the dissolution and delivery of hydrophobic drugs across lipophilic biological membrane barriers [23]. The chemical structure, hydrophilicity, and high molecular weight of CDs give them the ability to act as alternative penetration enhancers by concentrating the drug on the surface of the cell membrane without disturbing the integrity of the biological membrane [23,24].
3.4 Methods of creating drug-CD inclusion complexes
Solid drug-CD complexes can be performed using the following main techniques: 1. physical mixing; 2. kneading; 3. freeze–drying; 4. co-evaporation [13], as well as other methods and techniques such as co-precipitation, heating, damp mixing, extrusion, dry mixing, paste complexation, and slurry complexation [25]. Regarding the main techniques used, at physical mixing, the drug and CD are homogenously blended by using a mortar and pestle. The interactions developed between the API and CD molecules due to the mechanical stirring facilitate the formation of the inclusion complex. The kneading method represents a modification of the physical mixing approach wherein a minor quantity of co-solvent is introduced into the drug-CD blend during the kneading process to augment the formation of the complex. This leads to the creation of a uniform paste, which is subsequently dried to yield a finely powdered product. Within the freeze–drying technique, the drug and CD are dissolved in a solvent. Following this, the solution undergoes freezing, and in the presence of a vacuum, the solvent is effectively removed, resulting in the formation of the inclusion complex. In the co-evaporation technique, the drug is dissolved within an appropriate solvent, followed by the addition of a CD solution. This combined liquid mixture is subsequently exposed to an evaporation process, leading to the formation of the inclusion complex through precipitation. The used method determines the physicochemical characteristics of the complexes [25].
3.5 Medicinal products with CDs
A large gamut of pharmaceutical products containing CDs are currently undergoing clinical trials or have already been introduced to the global market [26]. These medicines are given using different ways of administration, like oral, nasal, pulmonary, rectal, dermal, ocular, and parenteral routes (Figure 2) [27]. According to the European Medicines Agency (EMA), some examples of the use of CDs in medicinal products on the European market include β-CD in brivaracetam film-coated tablets [28] and in glucagon nasal powder [29]. HP-β-CD has been used in the authorized Jcovden vaccine against Adenovirus type 26 encoding the SARS-CoV-2 spike glycoprotein [30], in cladribine tablets [31], in hard capsules of fingolimod [32] and Larotrectinib [33] and in film-coated capsules of letermovir [34]. SBE-β-CD has also been utilized in many marketed medicinal products in Europe. For example, it is contained in aripiprazole tablets [35], in carfilzomib powder for solution for infusion [36], in the veterinary tablets of citrate monohydrate [37], in concentrate for solution for infusion products of posaconazole [38], remdesivir [39], and voriconazole [40], as well as in film-coated voriconazole tablets [41]. Table 1 presents the authorized medical products in the European market that contain CDs (API, type of CD, trade name, pharmaceutical form, manufacturing company, EMA product number, and date of marketing authorization).
![Figure 2
Routes of administration of cyclodextrin-drug inclusion complexes based on EMA [27].](/document/doi/10.1515/ntrev-2023-0204/asset/graphic/j_ntrev-2023-0204_fig_002.jpg)
Routes of administration of cyclodextrin-drug inclusion complexes based on EMA [27].
Authorized medical products in the European market that contain CDs
| API | CD | Trade name | Pharmaceutical form | Company | EMA product number | Date of marketing authorization | Ref. |
|---|---|---|---|---|---|---|---|
| Adenovirus type 26 encoding the SARS-CoV-2 spike glycoprotein | HP-β-CD | Jcovden (previously COVID-19 Vaccine Janssen) | Suspension for injection | Janssen-Cilag International NV | EMEA/H/C/005737 | 11/03/2021 | [30] |
| Aripiprazole | SBE-β-CD | ABILIFY | Tablets | Otsuka Pharmaceutical Netherlands B.V. | EMEA/H/C/000471 | 04/06/2004 | [35] |
| Brivaracetam | β-CD | Briviact (in Italy: Nubriveo) | Film-coated tablets | UCB Pharma SA | EMEA/H/C/003898 | 13/01/2016 | [28] |
| Carfilzomib | SBE-β-CD | Kyprolis | Powder for solution for infusion | Amgen Europe | EMEA/H/C/003790 | 19/11/2015 | [36] |
| Citrate monohydrate | SBE-β-CD | Cerenia | Tablets (veterinary) | Zoetis Belgium SA | EMEA/V/C/000106 | 28/09/2006 | [37] |
| Cladribine | HP-β-CD | Mavenclad | Tablets | Merck Europe B.V. | EMEA/H/C/004230 | 22/08/2017 | [31] |
| Fingolimod | HP-β-CD | Gilenya | Hard capsules | Merck Europe B.V. | EMEA/H/C/004230 | 22/08/2017 | [32] |
| Glucagon | β-CD | Baqsimi | Nasal powder in single-dose container | Eli Lilly Nederland B.V. | EMEA/H/C/003848 | 16/12/2019 | [29] |
| Larotrectinib sulfate | HP-β-CD | Vitrakvi | Hard capsules | Bayer AG | EMEA/H/C/004919 | 19/09/2019 | [33] |
| Letermovir | HP-β-CD | Prevymis | Film-coated tablets | Merck Sharp & Dohme B.V. | EMEA/H/C/004536 | 08/01/2018 | [34] |
| Posaconazole | SBE-β-CD | Noxafil | Concentrate for solution for infusion | Merck Sharp and Dohme B.V. | EMEA/H/C/000610 | 25/10/2005 | [38] |
| Remdesivir | SBE-β-CD | Veklury | Powder for concentrate for solution for infusion | Gilead Sciences Ireland UC | EMEA/H/C/005622 | 03/07/2020 | [39] |
| Voriconazole | SBE-β-CD | VFEND | Film-coated tablets | Pfizer Europe MA EEIG | EMEA/H/C/000387 | 19/03/2002 | [41] |
| Voriconazole | SBE-β-CD | Voriconazole Hikma | Powder for solution for infusion | Hikma Farmaceutica (Portugal) S.A. | EMEA/H/C/003737 | 27/05/2015 | [40] |
3.6 Safety of the use of CD based drug delivery systems in clinical applications
Safety is a factor of paramount importance and should be taken into consideration for the incorporation of CDs in pharmaceutical formulations. The route of administration is the most crucial parameter which affects the toxicity or safety of these products.
First, the administration of CDs products by the oral route does not cause any irradiated effects because these substances are not absorbable due to their high hydrophilicity and their large configuration [27]. According to EMA, high doses of CD products administered orally could lead to gastrointestinal disorders in animals [27,42]. Nevertheless, it is noticed that pure compounds can be used as food additives as they are considered safe [42].
Concerning dermal products, natural CDs present a safety profile at low concentrations (up to 0.1%), whereas some CD derivatives (e.g., HP-β-CD) are used as excipients in cosmetics due to good skin tolerability [42,43].
Furthermore, it has been reported that suppositories including a high quantity of β-CD (230 mg) and 12% HP-β-CD do not induce damage in the rectal epithelium of humans and rabbits, respectively. Nevertheless, α-CD affects the rectal mucosa in the opposite way [42,43].
Regarding the nasal and pulmonary CDs administered, EMA [42] and Asai et al. [44] have demonstrated that amount of CD solutions lower than 10% do not cause degradation of the nasal mucosal tissue.
As CDs are capable of acting as alternative penetration enhancers, they can be incorporated into ocular products to improve drug penetration into the eye. Concentrations of α-CD and randomly M-β-CD up to 4 and 5%, respectively, can cause epithelial toxicity in the cornea of rabbits. On the other hand, concentrations of 10% SBE-β-CD and 12.5% HP-β-CD are well tolerable by the corneal surface of rabbit eyes [42,43].
Most intravenously administered CDs are excreted through urine rapidly. EMA and US Food and Drug Administration (US-FDA) do not permit the use of medicinal products of α-CD, β-CD, and randomly M-β-CD given intravenously because even in relatively low doses they are considered nephrotoxic. On the contrary, two modified derivatives of β-CD, HP-β-CD, and SBE-β-CD are used as excipients in parenteral products as they are considered safe even in relatively high doses. According to EMA, the evaluation of two approved products revealed the safety of these derivatives at the dose of 250 mg/kg/day over a period of 3 weeks and 6 months for HP-β-CD and SBE-β-CD, respectively [27]. Several studies have indicated the severe hemolytic effect that the administration of CDs can cause which is correlated with their capability to solubilize the lipids of cellular membranes, like cholesterol [43]. Leroy-Lechat and co-workers listed the CDs in accordance with their augmented in vitro hemolytic activity. More specifically, they reported that β-CD influenced the erythrocytes collected from human and P388 murine leukemic cells with the most negative effect, followed in order by α-CD, HP-β-CD, γ-CD, HP-γ-CD, and HP-α-CD [45].
4 Polymers: Copolymers-block copolymers
Polymers are high molecular weight substances made up of recurring units known as monomers, which link together in elongated chains. Polymers can be composed of identical monomers, forming homopolymers, or a combination of various monomers, known as copolymers. Several different types of copolymers can arise from the diverse orientations and structures of monomers along the polymer chain [5,46]. The four principal copolymer structures are illustrated in Figure 3, and they can assume the subsequent configurations:
Random copolymers: In which the different monomer units are organized in a random sequence along the linear polymer chain.
Alternating copolymers: In which the different monomer units are arranged in a repeating pattern of the different monomers along the linear polymer chain.
Block copolymers: These are composed of separate monomer blocks which are linked in a linear chain. Depending on the number of blocks they contain they can be categorized as:
Diblock copolymers: Contain two different blocks (AB)
Triblock copolymers: Contain three different blocks (ABA or BAB)
Multiblock copolymers: Contain more than three different blocks (ABC, ABCDAC)
Graft copolymers: Involve a main monomer that constitutes the central chain, accompanied by a different monomer that is attached as side chains along the central chain.

The diverse categories of copolymers: (a) Random copolymer; (b) alternating copolymer; (c) block ((a) diblock, (b) triblock, and (c) multiblock copolymer) and (d) Graft copolymer.
Polymers-based nanotheranostic platforms have attracted the interest of polymer scientists for many decades [47,48]. It is a fast-growing field that provides significant therapeutic solutions for many diseases [48,49]. The capability of different architectures of copolymers to self-assemble in various nanostructures and morphologies makes them potential multifunctional candidates as drug, gene, and bio-imaging carriers [50–52]. The self-assembly of them in aqueous solutions can provide interesting structures, including, uni- and multi-molecular micelles, vesicles, polymersomes, and nanogels. These structures have the capacity to encapsulate hydrophobic molecules such as drugs or other bioactive compounds within their hydrophobic domains [53–55].
The ability of block copolymers to self-assemble in aqueous media has gained considerable attention [56]. This phenomenon can yield ordered nanostructures in a wide range of morphologies such as spheres, cylinders, vesicles, micelles, and many other complex assemblies. The properties of these polymeric aggregates provide potential applications in many fields. Drug encapsulation and delivery are facilitated by the amphiphilicity of block copolymers in solution [57].
4.1 Methods of creating polymer-based drug delivery systems
The incompatibility between their different components results in their self-assembly into polymeric micelles or polymeric aggregates [58]. The encapsulation of hydrophobic drugs in the micellar hydrophobic core enhances its solubility in water [59]. Many drug encapsulation protocols have been created for the delivery of hydrophobic drugs. Among these, two main methods have been established to create drug-polymer systems: the thin film hydration protocol and the dialysis method. These techniques represent the principal approaches for encapsulating drugs within the polymeric core [58,60].
4.2 Studies on polymer-based drug delivery systems
Qiao et al. [61] reported an interesting thermo-sensitive diblock copolymer system as a co-loaded carrier. The block copolymer was prepared by atom transfer radical polymerization of trans-N-(2-ethoxy-1,3-dioxan-5-yl) acrylamide (tNEA) and poly(ethylene oxide)45 (PEO)45 as a macroinitiator. PEO45-b-PtNEA n , (n = 22, 44, 63, 91, and 172) at low temperatures was soluble in water, while at above 37°C, this polymeric system formed aggregates with different morphologies by changing the chain length of PtNEA each time. Specifically, the formation of spherical aggregates gradually changed into polymersomes with rod-like structure at the intermediate PtNEA length. Both NPs formed were stable at physiological conditions and were able to be co-loaded efficiently by the hydrophobic drug doxorubicin (DOX), and water-soluble fluorescein isothiocyanate-lysozyme. The drug release profiles revealed that at acidic environment, a greater drug release takes place than at physiological conditions. Furthermore, DOX-loaded carriers demonstrated an excellent cytotoxicity against HepG2 cells. These novel co-loaded spherical and rod-like NPs could be a promising candidate for diseases such as cancer [61].
Liu and co-workers [62] developed a novel pH-sensitive poly(ε-caprolactone-b-2-(N,N-dimethylamino)ethyl methacrylate) amphiphilic dendritic star-block copolymer system by ring-opening polymerization and atom transfer radical polymerization. The study of these copolymers in aqueous media revealed their self-organization into unimolecular or multimolecular micelles with sizes around 28 and 190 nm, respectively. Dynamic light scattering technique exhibits bimodal size distribution. Camptothecin was used as a model drug for its encapsulation in the hydrophobic environment of the micelles. The drug release profiles showed different release rates by altering the buffer conditions. Specifically, under acidic conditions, the drug was released more quickly and to a greater extent than under physiological conditions. Also, the cytotoxicity studies corroborated the low level of cytotoxicity of these micelles. Confocal laser scanning microscopy was able to determine the penetration of the drug-loaded NPs into the cell membrane and those being internalized into cytoplasm and cell nucleus of HeLa cells. These pH-responsive micelles could be promising candidates as drug delivery carriers [62].
Another interesting work for the treatment of cancer was reported by Yang’s team [63]. An amphiphilic star-shaped polycarbonate/polyethylene glycol (PEG) copolymer system was constructed by ring-opening polymerization, forming micelles which easily converted to vesicles in aqueous media for the effective delivery of the anticancer drug, DOX. DOX encapsulation was achieved by the dialysis method and DOX-loaded vesicles exhibited high drug loading capacity (22.5% in weight) for DOX. Subsequently, DOX-loaded vesicles were inserted into 4T1 tumor-bearing mice and presented an increased accumulation in tumor tissues due to the improved permeation and retention effect. An important observation was that the DOX-loaded vesicles showed a higher tumor growth inhibition than free DOX without side effects. All these features, give the possibility to utilize these vesicles as potential candidates for cancer treatment [63].
Mandal et al. [64] fabricated a series of dual stimuli responsive spherical nanogels with different sets of star block copolymers based on pentaerythritol-poly(ε-caprolactone)-b-poly(acrylic acid) by using ring-opening and atom transfer radical polymerization. These dually responsive (pH and magneto) nanogels have been synthesized in order to incorporate the magnetic NPs (MNPs) Fe3O4 via hydrogen bonding interactions of amine groups of MNPs and that of carboxylic groups of the poly(acrylic acid) (PAA) components of the copolymers. Dynamic light scattering method determined the hydrodynamic radius of these nanogels in the range of 65–616 nm. These nanogels were used for the encapsulation of an anticancer and hydrophobic drug, DOX. The drug release profiles revealed that a greater amount (73%) of encapsulated DOX was released at the physiological pH of the cancer cells, while it increased to 76% when an external magnetic field was applied. The MNPs/DOX-loaded nanogels presented selectively great cell uptake when the magnetic field was applied which enhanced its magnetic targeting ability. In vitro cytotoxicity studies exhibited high toxicity of DOX-loaded nanogels against the C6 glioma cell lines [64].
Wang’s group [65] reported a brush-shaped poly[styrene-b-poly(polyethylene glycol methyl ether acrylate)] (PST-b-PPEGA) block copolymer and poly(PEGA) homopolymer containing a –C12H25 end group constructed by reversible addition fragmentation chain transfer polymerization. Polystyrene was the hydrophobic component of the formed micelles and polyPEG was the hydrophilic corona. These micelles were designed for the encapsulation of ruthenium(ii) complex anticancer drug in aqueous media. Cytotoxicity investigations of these loaded micelles enhanced the drug’s toxicity to human liver cancer cells (SK-HEP-1) by 3-fold under dark and 12-fold under light. Furthermore, both blank and drug-loaded polymeric micelles presented no toxicity to the normal cells, indicating their important role in the treatment of cancer [65].
Bai et al. [66] synthesized two pH-responsive drug delivery systems containing fluorescent properties by atom transfer radical polymerization. They developed poly(ethylene glycol)-b-poly(2-(diisopropylamino) ethyl methacrylate-co-dithiomaleimide) and poly(ethylene glycol)-b-poly(2-(dibutylamino) ethyl methacrylate-co-dithiomaleimide) amphiphilic block copolymers. The copolymerization of dithiomaleimide monomer within pH-responsive polymers led to an enhanced efficacy for cell imaging and an improved drug release profile. These polymeric systems formed micelles in aqueous solutions which were used as drug vehicles for the encapsulation of the anticancer drug, DOX. Both systems showed a greater DOX release profile in acidic environments than at physiological conditions. Also, the poly(ethylene glycol)-b-poly(2-(diisopropylamino) ethyl methacrylate-co-dithiomaleimide) exhibits a better antitumor effect than the polymer with poly(2-(dibutylamino) ethyl methacrylate) responsive component. Both systems, revealed significant biocompatibilities, as almost 85% of cells survive in the presence of these fluorescent micelles at a concentration of up to 200 μg/mL [66].
Recently Mandal and co-workers [67] reported the same nanogel system as mentioned above but in its more advanced form. The current responsive system has an extra stimulus for more efficiency and targeted drug delivery. Specifically, they synthesized an amphiphilic pentaerythritol-poly(ε-caprolactone)-b-poly(acrylic acid) triple-responsive (pH, magneto, redox) block copolymer which formed nanogel. The PAA monomer is assumed to participate in the reaction with 2-hydroxyethyldisulfide generating redox and pH sensitive nanogel. The spherical nanogels were able to form nanosized particles (120 nm) and encapsulate DOX. Drug release studies revealed their responsive character, under the participation of all three stimuli. Especially, at physiological conditions, the nanogel showed low percentage of drug release, while, at acidic conditions the release of DOX improved significantly. Furthermore, the DOX release was significantly reinforced to 65% in 1 h at acidic conditions in the presence of 10 mM glutathione and external magnetic field [67].
4.3 Safety of polymer-based drug delivery systems
As mentioned above, safety and biocompatibility are crucial parameters, which should be taken into account for the involvement of block copolymers in pharmaceutical formulations. The design of block copolymers aims to adjust the in vivo pharmacokinetics, stability, and distribution profiles of drug-loaded block copolymers. Several systems based on block copolymers are currently undergoing clinical studies. However, an important challenge in the research and development of these materials is to specify the physicochemical characteristics that influence the properties of the drug cargo in vivo.
Poloxamers also known as “Pluronics” are amphiphilic triblock copolymers consisting of a central hydrophobic chain of poly(propylene oxide) (PPO) and hydrophilic chains of PEO [68]. These block copolymers are the most studied materials used to form drug-loaded polymeric systems for drug delivery, as they are commercially available and biocompatible with low toxicity [69,70]. However, there are some limitations related to the inability of these polymers to encapsulate large amounts of hydrophobic drugs, as well as their high critical micelle concentrations (CMC), which lead to the low stability of micelles and their increased dissociation when diluted in the blood stream during intravenous injection [71].
One way to improve the stability of formed micelles is to mix poloxamers with a more hydrophobic copolymer which can significantly improve their stability and thus the bioavailability of encapsulated drugs. A characteristic example was reported by Mu et al. [72]. Specifically, they mixed poloxamer copolymers L61, L62, and P85 each with methoxy poly(ethylene glycol)-b-poly(lactide) (mPEG-b-PLA) that presented lower CMC at different ratios. The acquired data revealed sizes at nanoscale for all mixed systems in a range from 19.6 to 28.5 nm, in contrast to pure poloxamers forming large particles of approximately 1,000 nm. The anticancer drug docetaxel was entrapped in these mixed NPs and both in vitro and in vivo results revealed superior effectiveness and bioavailability compared to the commercial formulation, Taxotere.
Another interesting work to enhance the CMC, in vivo bioavailability, and drug loading capacity was reported by Lo and co-workers [73]. They tried to control the low critical solution temperature (LCST) of mixed micelles. Methoxy poly(ethylene glycol)-block-poly(N-n-propylacrylamide-co-vinylimidazole) is a temperature-responsive copolymer and it presents LCST around 31°C. Above LCST, this copolymer forms micelles. Below LCST, the polymeric micelles dissociated as poly(N-n-propylacrylamide-co-vinylimidazole) and becomes hydrophilic. Thus, there is a chance of premature release of the drug when the drug is encapsulated in the copolymer and its storage is at a temperature below LCST. By mixing the copolymer with mPEG-b-PLA of low CMC, the mobility of the temperature-responsive copolymer was limited due to the interactions with PLA block, thus lowering the LCST. Therefore, stable mixed micelles were able to form at room temperature and in dilute solutions.
Popovici et al. [74] reported the fabrication of polymeric micelles for the encapsulation and delivery of 1-(5′-nitrobenzimidazole-2′-yl-sulphonyl-acetyl)-4-aryl-thiosemicarbazide to address bacterial oral diseases. Notably, they created poloxamer 407/API micellar systems characterized by a particle size of approximately 20 nm and physical stability at the refrigerator temperature without significant modifications of zeta-potential value. These systems demonstrated a remarkable encapsulation efficiency of 85% at a polymer/API ratio of 10/1, exhibiting both hemocompatibility and cytocompatibility. Significantly, the drug-loaded poloxamer micelles demonstrated enhanced antimicrobial activity against Staphylococcus aureus and Escherichia coli, highlighting the potential of these nanoscale formulations to overcome the challenges posed by multidrug-resistant bacterial infections [74].
5 CD-block copolymer drug delivery systems
5.1 Exploring the synergistic potential of hybrid CD-block copolymer systems
From the technological point of view, polymers and, consequently, CDs-block copolymer NPs offer design versatility [75]. First, there are different types (compositions and architectures) of polymers and CDs and consequently, different types of NPs can be prepared (i.e., micelles, polymersomes, hydrogels, polymeric NPs, hybrid particles, etc.).
This review article recapitulates the potential applications of CD-block copolymer mixed systems in different drug delivery platforms. In this part, we present and analyze different examples from the recent literature. Figure 4 illustrates the combination of block copolymers with CDs for the formation of a drug delivery system.
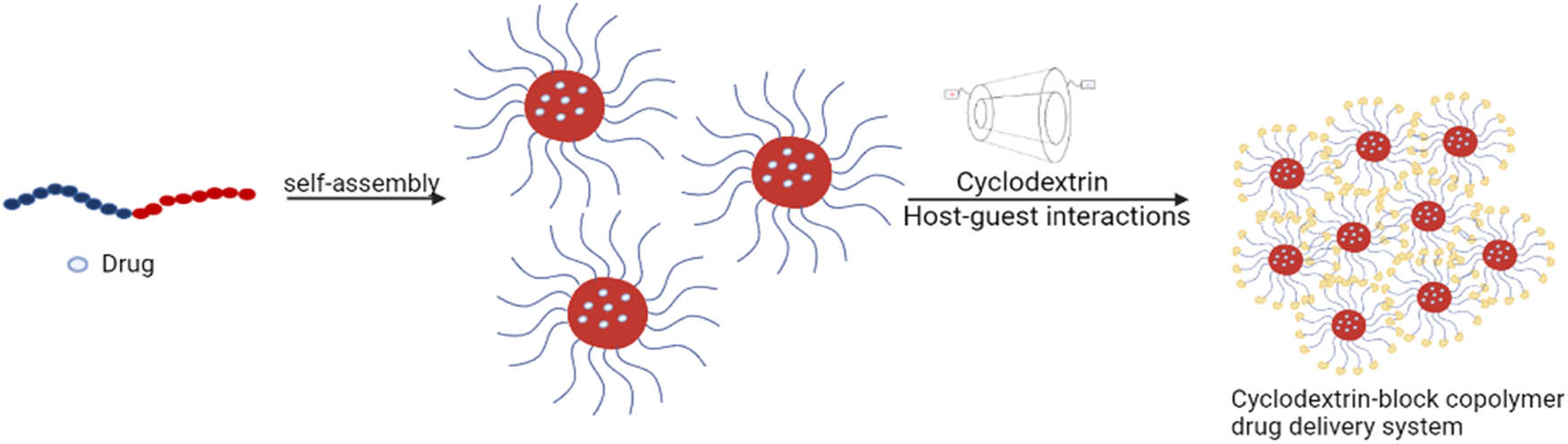
Illustration of a drug delivery system formed after the combination of CDs with block copolymers.
First, DOX has gained great interest as the drug model in many studies. Poudel et al. [76] fabricated a hydrogel exploiting the interactions developed between the α-CD and the amphiphilic PEG-b-PLA block copolymer micelles as a potent injectable DOX carrier for tumor treatment. More specifically, PEG-b-PLA micelles were created through hydrophobic interactions in an aqueous environment, followed by the formation of polypseudorotaxane microcrystals via host–guest interactions between α-CD and PEG chains. The prepared micelles presented spherical structure with their hydrodynamic diameter ranging from 40–80 nm. Results obtained by rheological studies indicated that the higher concentration of α-CD led to faster gel formation and improved viscosity. Moreover, altering the proportion of α-CD in the hydrogel formulation resulted in controllable and sustained drug release. The shear-thinning and self-healing behaviors of the composed hydrogel and the long-term superior inhibition efficacy against cancer cells in vitro were also demonstrated. Therefore, the great biocompatibility and excellent rheological properties of hydrogel based on PEG-b-PLA block copolymer micelles and α-CD created a better profile of the formulation for application in cancer therapy [76].
A new generation of non-viral gene carrier, the amphiphilic star-like polymer was proposed by Cheng et al. to facilitate immunotherapy [77]. The core of the system was comprised of a biocompatible β-CD core, and hydrophobic poly(ε-caprolactone) (PCL) segments and the extending hydrophilic cationic poly(2-(dimethylamino) ethyl methacrylate) (PDMAEMA) arms were the main components of the shell. The prepared spherical NPs could condense plasmid DNA, forming stable polymeric micelles with particle size around 220 nm, narrow size distribution, positive zeta potential values, and the absence of obvious agglomeration phenomena. Owing to the micelle formation by PCL segments, it was proven to have enhanced stability after complexation with genes, lower toxicity, and greater condensation ability of plasmid DNA than the system without PCL chains. Using the most widely utilized nonviral gene transfection reagent lipofectamine as a reference, they showed superior transfection efficiency of more than 10.8%. Finally, the capability of the system to deliver genes and hydrophobic drugs, such as dexamethasone into macrophages was proven, and consequently reduce inflammatory reactions, making it a high-promising system for immune cell therapy [77].
Zhou’ s team [78] prepared supramolecular micelles combining PDMAEMA based on β-CD (β-CD-(PDMAEMA)7) and benzimidazole (BM) modified PCL through the host-guest recognition between β-CD and BM (Figure 5). The hydrophobic complex of β-CD and the modified drug resided preferably in the core, while the pH-sensitive PDMAEMA acted as the shell, forming micelles with regular spherical configuration. The entrapment efficiency of drug molecules like DOX was proven since drug-loading content and entrapment efficiency values ranged up to 40 and 86%, respectively. Owing to the presence of pH-sensitive PDMAEMA, supramolecular micelles exhibited pH-sensitivity, leading to different results under neutral and acidic conditions as the drug release could be accelerated with the decrease in pH and simultaneously with the increase in temperature. Thus, the above properties, in combination with the excellent anticancer activity, offer great prospects to supramolecular micelles as nanocarriers of anticancer drugs [78].
![Figure 5
Formation of supramolecular micelles from β-CD-(PDMAEMA)7 star polymer. Adopted from Zhou et al. [78].](/document/doi/10.1515/ntrev-2023-0204/asset/graphic/j_ntrev-2023-0204_fig_005.jpg)
Formation of supramolecular micelles from β-CD-(PDMAEMA)7 star polymer. Adopted from Zhou et al. [78].
A similar study was conducted by Cui and co-workers [79], who developed supramolecular assemblies through inclusion complexation between β-CD and BM after the combination of chitosan-graft-β-CD with BM end functionalized PEG-b-PCL block copolymer. PCL segments and β-CD cavity functioned as the hydrophobic membrane of the complex vehicles, while the corona consisted of hydrophilic PEG chains and chitosan. The complex vehicle size was around 100 nm and they could efficiently host both hydrophilic DOX and hydrophobic curcumin. The drug loading content was up to 38.4 and 20.2% for DOX and curcumin, respectively. As it was mentioned in the previous study [78], due to the sensitivity of host–guest interactions between β-CD and BM, the release of both drugs from supramolecular micelles could be controlled by altering the surrounding temperature and pH conditions. More specifically, at neutral pH conditions, BM has a hydrophobic character and could be incorporated into CD cavity. In contrast, acidic pH leads to the protonation of BM and therefore to the dissociation of the complex. Consistent with the results taken by cytotoxicity assays showed that the prepared drug-loaded vesicles retained higher inhibition of proliferation efficiency than free drugs. As a result, these dual drug carriers could have good prospects as intelligent tools for anticancer drug delivery [79].
A novel vector based on folate-conjugated amphiphilic copolymer folate-poly(ethylene glycol)-poly(d,l-lactide)-β-CD (FA-PEL-CD) was synthesized by Zhang et al. [80]. Results demonstrated the ability of PEL-CD and FA-PEL-CD to form stable micelles at low concentrations, due to their low colloidal micelles concentrations. The particle size of polymeric systems was around 100–150 nm, whereas that of DOX-loaded micelles ranged from 100 to 200 nm, presenting smooth exterior part and low size distribution. Treatment with FA-PEL-CD/DOX micelles induced significantly superior tumor growth inhibition (86%) compared to treatment with the same dose of micelles without the conjunction of folate (73%). Thus, this innovative micellar drug delivery system is suggested to be a potential nanocarrier for targeted DOX delivery, with high accumulation of the drug in tumor sites, ameliorated anticancer activity and alleviated toxic side effects in healthy tissues [80].
Gao et al. [81] prepared stimuli-responsive complex micelles with core-shell morphology driven by host-guest interactions between β-CD containing block copolymer PEG-b-PCD and BM modified PCL (Figure 6). Complex micelles presented as regular spheres with narrow size distribution and their diameter was around 255 nm. DOX was used as a model drug and the study demonstrated the high encapsulation efficiency of micelles for DOX, which was up to 74.77%. In vitro drug release studies revealed a significantly faster release of DOX under mildly acidic environments or high temperatures compared to neutral environments and lower temperatures. These polymeric micelles represent a potential new smart drug carrier for long-term controlled release of drugs as guest molecules [81].
![Figure 6
Formation of complex micelles for drug loading. Adopted from Gao et al. [81].](/document/doi/10.1515/ntrev-2023-0204/asset/graphic/j_ntrev-2023-0204_fig_006.jpg)
Formation of complex micelles for drug loading. Adopted from Gao et al. [81].
Another promising candidate for the cure of cancer is a micellar drug delivery system developed by Song et al. [82]. They formed non-covalently connected micelles consisting of a hydrophobic, poly(N-isopropylacrylamide) core and hydrophilic PEG corona, whereas a well-defined β-CD-based poly(N-isopropylacrylamide) star host polymer with a degree of polymerization of 21 per arm and adamantyl-containing PEG guest polymer was synthesized (Figure 7). Subsequently, the host polymer and the guest polymer formed a pseudo-block copolymer via inclusion complexation between β-CD core of the host polymer and adamantyl-moiety of the guest polymer. Poly(N-isopropylacrylamide) is a widely used thermoresponsive polymer which is soluble in water below its LCST (32°C) and it experiences a reversible, abrupt coil-to-globule phase transition above its LCST [83,84]. The thermoresponsive properties of the micellar drug delivery system were identified. More specifically, the pseudo-block copolymer was hydrophilic and soluble in aqueous media at room temperature (below the LCST), while the hydrophobic character of the poly(N-isopropylacrylamide) host polymer was prevailing at body temperature, leading to micelles formation. At the same conditions and at the concentration of 0.2 mg/mL, there were micelles formed with particle size around 282 nm, which was a suitable size for drug carriers. Afterwards, Song et al. loaded DOX into the interior of the micelles, and they achieved a loading efficiency of 17%. In vitro cell cytotoxicity studies demonstrated good biocompatibility, enhanced antitumor activity and ability to circumvent the multidrug resistance in cancer cells. All these properties permitted this thermoresponsive platform to deliver successfully and to achieve desired therapeutic effects of the incorporated guest-molecule [82].
![Figure 7
Illustration of the formation of β-CD-(poly(N-isopropylacrylamide))4/adamantyl-containing PEG supramolecular pseudo-block copolymer via host–guest interactions, followed by the thermoresponsive micellization while encapsulating drug molecules into the core, forming drug-loaded micelles for drug delivery and overcoming drug resistance. Adopted from Song et al. [82].](/document/doi/10.1515/ntrev-2023-0204/asset/graphic/j_ntrev-2023-0204_fig_007.jpg)
Illustration of the formation of β-CD-(poly(N-isopropylacrylamide))4/adamantyl-containing PEG supramolecular pseudo-block copolymer via host–guest interactions, followed by the thermoresponsive micellization while encapsulating drug molecules into the core, forming drug-loaded micelles for drug delivery and overcoming drug resistance. Adopted from Song et al. [82].
To combine both drug release advantages of physical cross-linked injectable hydrogels and NPs, Zhu et al. [85] presented a new type of self-assembled supramolecular hydrogels composed of cisplatin-coordinated polymeric micelles poly(ethylene glycol)-b-poly(acrylic acid) (PEG-b-PAA) and α-CD which is a promising injectable system for sustained delivery of cisplatin in cancer therapy. The creation of a network construction was accomplished due to the cooperative interactions between platinum(ii) atoms and PAA blocks, as well as the complexation of PEG chains with α-CDs. These composite hydrogelated micelles provide indispensable cross-links, the micellar cores, as an integral part of the network structure in comparison with other hydrogel/micelle and hydrogel/microsphere systems. The research of Zhu et al. indicated that the gelation properties could be tuned by altering concentrations of the polymers and cisplatin, as well as by adding PEG homopolymers or poloxamer copolymers. Finally, the thixotropic behavior, reversibility, and relatively high cytotoxicity to human bladder carcinoma EJ cells of cisplatin-coordinated PEG-b-PAA/α-CD supramolecular hydrogels offer them great potential for applications in sustained delivery of cisplatin by injection [85].
Xu et al. [86] presented unimolecular micelles of star-like amphiphilic polymers consisting of a β-CD core, from which 21 hydrophobic PLA arms and hydrophilic PEG arms were grafted sequentially. This cooperation effect of complexation resulted in the formation of robust, monodisperse unimolecular micelles that were uniform and stable in aqueous solution. The composed biocompatible micelles showed little cytotoxicity against HeLa and HEK 293T cells accompanied by their efficient ability to encapsulate hydrophobic anticancer agents, such as DOX, in the hydrophobic domain of PLA and to demonstrate pH-controlled release behavior. Thus, the unimolecular micelles formed by this star-like β-CD-PLA-PEG constitute a new promising drug delivery system for a wide variety of bioactive agents [86].
Vega et al. [87] examined the influence of HP-β-CD as a cryoprotectant to poly(lactic-co-glycolic acid) (PLGA)-PEG diblock copolymer nanospheres (NSs) incorporating flurbiprofen and they evaluated the effect of freeze–drying and γ-irradiation sterilization on the properties of NSs. It was demonstrated that HP-β-CD offered efficient cryoprotection to maintain the NSs unaffected and γ-irradiation was an appropriate way that permitted the sterilization of the formulations for ophthalmic administration preventing the development of contaminants. All formulations prepared were characterized by suitable particle size (less than 250 nm) with a narrow particle size distribution for ocular administration. In vitro drug release studies showed that the presence of HP-β-CD led to the reduction in the burst effect, providing an enhanced sustained release of the drug, whereas a significant reduction in the flurbiprofen transcorneal permeation of NSs containing HP-β-CD was observed in the in vitro results. Finally, Vega et al. suggested that NSs penetrated corneal epithelium through a transcellular pathway. They showed that γ-irradiated NSs stored as freeze-dried powders maintained their initial characteristics and were stable for 3 months of storage in the aqueous form at 4°C [87].
Highly auspicious nanovehicles for targeted anticancer therapy were the innovative amphiphilic star-shaped reductive stimulus-responsive nanocarriers which were composed of M-β-CD conjugated with mPEG-b-PCL and hydrosulfonyls through disulfide links (Figure 8) [88]. The hydrophobic cores of assemblies were mainly composed of methyl groups and PCL, while the extending PEG chains were the main component of the shell. The resultant NSs had core-shell structure and micellar properties and their particle size ranged from 50 to 70 nm, permitting the entrapment of hydrophobic drugs like DOX in the inner cavity. Therefore, Li et al. developed amphiphilic drug delivery platforms which displayed excellent drug loading capacity, targeted uptake in cancer cells, good biocompatibility in vivo, controlled drug release under reductive conditions, superior anticancer performance, high tumor clearance with minimal toxicity to normal cells, making them promising candidates which should be further evaluated in clinical practice [88].
![Figure 8
Detailed synthetic routes of a series of CD-block copolymers complex. Adopted from Li et al. [88].](/document/doi/10.1515/ntrev-2023-0204/asset/graphic/j_ntrev-2023-0204_fig_008.jpg)
Detailed synthetic routes of a series of CD-block copolymers complex. Adopted from Li et al. [88].
Çirpanli et al. designed, developed, and compared thoroughly polymeric and amphiphilic CD NPs for the incorporation and delivery of the hydrophobic drug camptothecin, using amphiphilic β-CDs (6-O-Capro-β-CD, β-CDC6, HP-β-CD), PLGA, or PCL. Particle size was less than 275 nm and polydispersity index (PDI) less than 0.2, whereas drug-loading values of amphiphilic CD NPs were higher than those of PLGA and PCL polymeric NPs. Furthermore, a superior controlled release profile was observed with a duration of 12 days for amphiphilic CD NPs in comparison with the duration of 2 days for polymeric NPs. Finally, CD NPs showed higher anticancer efficacy than PLGA or PCL NPs loaded with camptothecin. These NPs were characterized by their small size, stability, effectiveness, safety, syringeability, and membrane filterability, representing a new generation carrier system for the effective delivery of hydrophobic APIs with a similar hydrophobic profile to camptothecin [89].
Aiming to improve the encapsulation efficiency of relatively hydrophilic sepiapterin drug within the lipophilic cavity of methoxy-PEG-PCL NPs, Kuplennik and Sosnik used 2,3,6-triacetyl-β-CD for the complexation and augmentation of hydrophobicity of sepiapterin [18]. They used two different methods for the achievement of nanoencapsulation: drying with copolymer and preparation of a sepiapterin/2,3,6-triacetyl-β-CD complex by the spray-drying technique. By means of the nano-precipitation method between the sepiapterin/2,3,6-triacetyl-β-CD spray-dried complex and methoxy-PEG-PCL solution at the appropriate molar ratio, they obtained NPs with a particle size of 74–75 nm and SD = 21–22 nm. The encapsulation efficiency (85%) and drug loading (2.6%) values were significantly higher than those of the pristine API (14 and 0.6%, respectively), whereas sepiapterin release from NPs exhibited a relatively low burst effect of 20%. Overall, the obtained results pave the way for the use of this innovative strategy for nanoencapsulation of hydrophilic drugs, such as sepiapterin [18].
Li et al. [90] created NPs complexes by combining PLGA polymer with 2-HP-β-CD to accomplish the therapeutic efficacy and improve the bioavailability and ocular delivery of triamcinolone acetonide, which is a highly lipophilic drug. The composed systems had spherical and uniform dispersion of NPs. Their average particle size was less than 200 nm, proving their ability to carry drugs around the cornea and deliver them to the posterior segment of the eye, whereas ζ-potential measurements demonstrated negative values. The cumulative permeation amount, loading capacity, and entrapment efficiency values were significantly higher than that of the PLGA NPs, proving that the presence of CD enhanced the entrapment efficiency due to the increase in drug’s solubility and system’s stability [90].
Supramolecular hydrogels (SMGels) are multifunctional systems, formed by CDs and polymers achieving the establishment of a three dimensional solid-like network through intermolecular non-covalent bonds that are capable to absorb large amounts of water [9]. Due to their advantageous biocompatibility and their capacity to absorb large amounts of water, they have been widely investigated for biomedical applications and particularly for the controlled and sustained release of the encapsulated bioactive compounds [91].
SMGel formed by triblock PCL-PEG-PCL copolymer and γ-CD was investigated for injectable delivery of insulin by Khodaverdi et al. [9]. The formulation of a hydrogel loaded with insulin was prepared by mixing an aqueous solution of copolymer and drug with an aqueous γ-CD solution in less than a minute. The result was the formation of a hydrogel with a three-dimensional porous foam-like structure, thixotropic behavior, good water-swelling ability in distilled water, and excellent syringeability. In vitro release studies demonstrated that insulin-loaded SMGel has a continuous release profile of above 80% of the drug over a 20 days period, whereas insulin maintained its native state after formulating and releasing from the SMGel. In other words, the prepared, biocompatible, biodegradable formulation could be a novel carrier for injectable sustained release of insulin [9].
In the same manner, the same research group developed self-assembled SMGels through inclusion complexation of PCL-PEG-PCL micelles and γ-CD for the controlled release of dexamethasone [92]. Many formulations with different ratios of components were tested. Among them, only the aqueous solution containing 10 and 20% (w/v), and the one containing 2.5 and 25% (w/v) of copolymer and γ-CD, respectively, formed hydrogels at 25°C. They used the first one which was formed successfully in just 4 s. The SMGel showed a porous, foam-like structure and good water-swelling ability because its weight increased 1.5 times after 15 min. It also demonstrated shear-thinning and thixotropic behavior, making it capable to absorb large amounts of water, resulting in the increase in the system’s biocompatibility. Results of in vitro release studies indicated the slow-release rate of dexamethasone from the SMGel, with a slight burst release. The greater the amount of dexamethasone was, the significantly higher the rate of release, whereas SMGel degradation occurred after more than 3 weeks. The aforementioned results suggested the potential use of the prepared hydrogel as a sustained-release system for dexamethasone [92].
A new supramolecular hydrogel self-assembled between α-CD and a biodegradable poly(ethylene oxide)-poly[(R)-3-hydroxybutyrate]-poly(ethylene oxide) (PEO-PHB-PEO) triblock copolymer was developed by Li et al. (Figure 9) [93]. The interactions that took place between copolymer segments with α-CD led to the formation of a self-assembled SMGel that produced a strong macromolecular network. The composition, molecular weight, and chemical structure of the copolymers could be modified to ameliorate the hydrogel properties. The in vitro release kinetics studies indicated the potential of hydrogels for long-term sustained controlled release of drugs, whereas rheological experiments demonstrated that it was thixotropic and reversible, holding great potential as injectable drug delivery systems of macromolecular drugs [93].
![Figure 9
(a) The structure of α-CD, (b) the schematic illustrations of the proposed structures of a-CD/PEO-PHB-PEO inclusion complex, and (c) a-CD/PEO-PHB-PEO supramolecular hydrogel. Adopted from Li et al. [93].](/document/doi/10.1515/ntrev-2023-0204/asset/graphic/j_ntrev-2023-0204_fig_009.jpg)
(a) The structure of α-CD, (b) the schematic illustrations of the proposed structures of a-CD/PEO-PHB-PEO inclusion complex, and (c) a-CD/PEO-PHB-PEO supramolecular hydrogel. Adopted from Li et al. [93].
The potential of the innovative CD-functionalized asymmetric block copolymer films was demonstrated by the work of Huang and co-workers [94]. They combined the amphiphilic polystyrene-block-poly(4-vinylpyridine) (PS-b-P4VP) block copolymer with β-CD, to construct highly adsorptive, asymmetric membranes, that were characterized by a thin layer of highly ordered and uniform cylindrical nano-channels on top of a non-ordered macroporous sponge-like layer. The membranes showed excellent biocompatibility and outstanding adhesion capability to various kinds of substrates, which was of paramount importance for application in medical devices, whereas the cytotoxicity assay results confirmed their biocompatibility and safe application, showing minimal cytotoxicity. The relatively thick (about 50 μm) macroporous sponge-like sublayer acted as a reservoir for the drug molecules. Moreover, the supramolecular interaction of CD and the spongy highly porous sublayer of the membranes provide them a large space to load in a high extent the model drug, triclosan. Drug release lasted for more than 2 weeks without an initial burst effect, showing a pH-responsive release behavior and a significant antibacterial effect against Escherichia coli due to the triclosan release. As such, from all we mentioned, it is obvious that the isoporous block copolymer membranes could serve as a novel high-capacity reservoir for drug delivery [94].
Another new generation nanocarrier for the delivery of lipophilic drugs was developed by Zhang et al. [95]. They synthesized double hydrophilic copolymers (PEG-b-PCDs) consisting of one PEG block and a block containing β-CD units, which in the presence of the hydrophobic poly(b-benzyl l-aspartate) (PBLA) formed complex assemblies with a core-shell structure due to host–guest interactions between β-CD and hydrophobic benzyl group. The hydrophobic core composed of PBLA homopolymer was surrounded by hydrophilic PEG chains at the outer surface of the corona. It was found that the increase in PBLA’s molecular weight resulted in a decrease in particle size of the assemblies. Finally, they proved that the prepared assemblies maintain their stability during long-term storage and their reconstitution ability after freeze–drying [95].
As mentioned before, poloxamers are amphiphilic triblock copolymers that have received approval for pharmaceutical application by the US-FDA [96]. These polymers have gained high scientific interest due to their high degree of biocompatibility and their self-assembly behavior, forming several structures, from micelles to polymersomes and polymeric particles, which are ideal for drug delivery purposes. They are commonly utilized to increase the solubilization of some hydrophobic drugs [96] and can also be modified for bioconjugation purposes [97]. In the existing literature, numerous studies explore the synergy effect of poloxamers and CDs to formulate hybrid drug delivery systems.
An attractive drug delivery vehicle for targeted delivery of DOX to human breast tumors was developed by the combination of poloxamer 407 with surface modification of aptamer AS1411 and β-CD linked PEG-b-PLA (Figure 10) [97]. This novel binary system loaded with DOX exhibited enhanced antitumor activity and decreased induction of cardiotoxicity in comparison to the non-AS1411-modified system. Also, it showed a long-circulation profile in vivo with a longer half-life, larger AUC, and slower clearance when compared to free DOX, permitting it to accumulate more efficiently within the tumor tissues via the enhanced permeability and retention (EPR) effect and through the selective binding to receptors on MCF-7 cells. Results indicated a dependence of in vitro drug release on pH conditions, as the amount of released DOX from micelles and pH values were inversely related. Overall, this multifunctional composite micelle modified with aptamer AS1411 is suitable for DOX loading with efficient capacity, enhanced micelle stability, precise and effective tumor targeting, greater cellular uptake, prolonged circulation time in blood, better accumulation in tumor, increased anticancer activity, and alleviated side effects compared to free DOX [97].
![Figure 10
Schematic diagram of the multifunctional composite micelles modified by aptamer AS1411 and the mechanism of interaction between nucleolin receptors and aptamer AS1411 ligands. Adopted from Li et al. [97].](/document/doi/10.1515/ntrev-2023-0204/asset/graphic/j_ntrev-2023-0204_fig_010.jpg)
Schematic diagram of the multifunctional composite micelles modified by aptamer AS1411 and the mechanism of interaction between nucleolin receptors and aptamer AS1411 ligands. Adopted from Li et al. [97].
In another report, Jansook et al. formulated CD-poloxamer P407 micelles as self-assembled nanocarriers for dexamethasone and amphotericin B [98]. The formation of inclusion complex occurred by entering the PPO part into the γ-CD cavity. The presence of a more hydrophilic γ-CD derivative, HP-γ-CD, resulted in the improvement of drug solubility, reduction in the crystallinity of CD-poloxamer aggregates, and avoidance of hemolytic effect, making the ternary system appropriate for ophthalmic drug delivery. In their study, they tested two different model drugs: dexamethasone, a small hydrophobic drug molecule, and amphotericin B, a large amphiphilic drug. Results indicated that dexamethasone and other similar molecules in combination with the ternary system might form large complex aggregates with improved water solubility, in contrast to drugs like amphotericin B, which did not hold great potential for delivery with these nanocarriers due to low drug aqueous solubility occurring by the competitive effect. Also, they observed that the surface tension of the formulations and poloxamer P407 concentration were inversely related, whereas higher P407 amounts in the formulations led to the higher formation of complex aggregates with a slower drug release rate. Consequently, adjusting the molar ratios and concentrations of the used components could lead to a desirable ocular drug formulation development [98].
Mixed micelles of Soluplus, that is polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol (PVCL-PVA-PEG) graft copolymer with amphiphilic characteristics, and poloxamer P103 and their combination with α-CD could lead to the formation of polypseudorotaxanes [99]. The aforementioned hybrid system was developed as a suitable platform to regulate drug release and improve the ocular permeability of poorly soluble drugs, like natamycin. Zeta potential showed slight negative values, whereas the particle size of mixed micelles (150–110 nm) was significantly higher than poloxamer P103 micelles (16–20 nm) and Soluplus micelles (90–103 nm), indicating that the large PPO segment of poloxamer P103 inserted into the Soluplus cores. Mixed micelles increased natamycin solubility up to 2.77-fold. Overall, the results of this study indicated that mixed micelles and their related polypseudorotaxanes can be used for the controlled release of drugs with relatively low water aqueous solubility, permitting middle diffusion of drug, cornea, and sclera accumulation, and sclera permeability coefficients, without damaging effects on biocompatibility [99].
Ni et al. [100] developed a novel SMGel based on self-assembly between poloxamer triblock copolymers and α-CD. They concluded that the gelation was induced by the complex formation between the PEO segments of the PEO-PPO-PEO triblock copolymer and α-CD, and the further self-assembly of the partially formed inclusion complexes. The gelation process could be aided by the addition of α-CD, reducing the concentration of the copolymer needed. The hydrogels were found to be thixotropic and reversible and can be applied as a promising injectable drug delivery system [100].
Zafar et al. [101] used HP-β-CD and poloxamer 188 to incorporate genistein into binary (genistein:HP-β-CD, 1:1) and ternary (genistein:HP-β-CD:poloxamer188, 1:1:0.5) inclusion complexes. The obtained results of the study revealed a remarkably greater release profile of genistein from inclusion complexes. Furthermore, the existence of poloxamer 188 in the ternary complex permitted them to exhibit a significant enhancement in dissolution rate in comparison to the binary complexes. There was also observed an increase in activity and a significantly higher concentration-dependent cytotoxicity to cancer cells in ternary genistein-inclusion complex than in pure API, whereas the crystal structure of genistein converted to an amorphous structure with a rough and irregular shape in genistein ternary systems. Consequently, the ternary system could be a promising way for ameliorating the solubility profile of genistein, making it a promising candidate for the cure of breast cancer [101].
Nogueiras-Nieto et al. [102] showed that the incorporation of poloxamer 407 into the drug-CD complexes aqueous solutions involved competition with studied drugs, triamcinolone acetonide and ciclopirox olamine, for the cavity of the CD molecule. They used two hydrophilic derivatives of β-CD, HP-β-CD, and partially M-β-CD. This competitive mechanism of action led to the displacement of the drug from the CD cavity and the formation of soluble polypseudorotaxanes that stack forming nanorods, which had a crucial impact on the release of the drugs studied. Therefore, noteworthy changes in the release rate and the solubility properties of the systems have taken place, depending on the stability of the drug-CD inclusion complex and on CD concentration in the bulk solution. Thus, the formation of polypseudorotaxanes can be employed to modulate drug-controlled release from thermosensitive hydrogels [102].
Simões et al. [103] formulated a syringeable gel formulation for sustained delivery of vancomycin, taking advantage of the capability of poloxamer 407 to form supramolecular gels with α-CD. The results of the study showed that the augmentation of α-CD concentration led to faster gel formation. Other crucial parameters that affect remarkably not only the gel rate formation but also the physical stability of the prepared systems were the molar ratio of the components and the storage temperature. Indeed, the gels composed of a low proportion of α-CD showed phase separation at 4 or 37°C, whereas the systems containing 6.5% copolymer and at least 5% α-CD at 20°C appeared to have the most physical stability. Also, they reported that the proportion of 6.5–13% triblock copolymer and 5–7% α-CD appeared to be the most adequate for the preparation of injectable drug depots for the local treatment of infections against Staphylococcus aureus. From all taken results, they suggested that the most promising formulation was that which contained 13% poloxamer 407 and 5% α-CD, since it was characterized by high viscoelastic behavior, stability at least for 1 month, rapid gel formation, easy syringeability, and controlled drug release for several days [103].
The combination of poloxamer 407 with M-β-CD or HP-β-CD as components of drug delivery systems for possible brain targeting after nasal administration of ropinirole has also been reported in our recent article [104]. The findings of our study demonstrated the existence of a range of interactions between the compounds, likely due to the creation of an inclusion complex. The NPs presented spherical shape and were stable during the 1 month stability assessment. In vitro cytotoxicity evaluation on HEK293 cell lines revealed that the prepared systems were biocompatible at low concentrations. In vitro diffusion experiments revealed that more than 90% of the loading dose was released up to 4 h, and ex vivo permeation experiments through rabbit nasal mucosa showed enhanced permeability of RH up to 50% compared to the API solution. Thus, these NPs have potential for efficient drug delivery, specifically for nose-to-brain delivery of the antiparkinsonian drug, ropinirole [104].
Lin et al. [105] formulated a thermoresponsive gel containing the triblock copolymers poloxamer 407 and poloxamer 188, as well as PEG 6000 and HP-β-CD for the intranasal administration of rhynchophylline for managing Parkinson’s disease. The use of polymers prompts the transformation of gel from the solution state to the semi-solid state after intranasal administration. The pharmacokinetic analysis revealed that the system’s components could serve as permeation enhancers of rhynchophylline through nasal mucosa. Indeed, in vivo studies in mice revealed that this nasally administered formulation enhanced the bioavailability and brain targeting of rhynchophylline, improved the damaged dopaminergic neurons caused by Parkinson’s disease and offered neuroprotection and recovery of motor behavior [105].
5.2 Drug molecules encapsulation in CD-block copolymer delivery systems
The combination of block copolymers with CDs can result in new biomaterials and supramolecular structures, which possess the physicochemical and thermotropic properties of both classes of materials. A significant application of their combination is the design of innovative delivery systems for the transport of genes and APIs. In the literature, there is available a large gamut of APIs that can be encapsulated into these systems, such as amphotericin B, camptothecin, ciclopirox olamine, cisplatin, curcumin, dexamethasone, doxorubicin, insulin, flurbiprofen, genistein, natamycin, rhynchophylline, ropinirole, sepiapterin, triamcinolone acetonide, triclosan, and vancomycin. Table 2 presents some examples of CD-block copolymer formulations from the literature, showcasing their added value alongside the corresponding encapsulated molecules and the related diseases they are used for.
CD-block copolymer formulations, their added value, and corresponding encapsulated molecules and associated diseases
| Formulation | Block copolymer | CD | APIs | Disease | Added value | Ref. |
|---|---|---|---|---|---|---|
| Micelles | PEG-b-PLA | α-CD | Doxorubicin | Cervical cancer |
|
[76] |
| NPs/micelles | PCL-b-PDMAEMA | β-CD |
|
— |
|
[77] |
| Micelles |
|
Doxorubicin | Cancer |
|
[78] | |
| Supramolecular assemblies | PEG-PCL | β-CD |
|
Cancer |
|
[79] |
| Micelles | PEG-PLA | β-CD | Doxorubicin | Cancer |
|
[80] |
| Micelles | PEG-PCL | β-CD | Doxorubicin | Cancer |
|
[81] |
| Micelles |
|
β-CD | Doxorubicin | Cancer |
|
[82] |
| Hydrogelated micelles | PEG-b-PAA | α-CD | Cisplatin | Cancer |
|
[85] |
| Micelles | PLA-PEG | β-CD | Doxorubicin | Cancer |
|
[86] |
| Nanospheres | PLGA-PEG | HP-β-CD | Flurbiprofen | Inflammatory diseases concerning ocular structures |
|
[87] |
| Nanospheres | PEG-PCL | M-β-CD | Doxorubicin | Cancer |
|
[88] |
| NPs |
|
|
Camptothecin | Cancer |
|
[89] |
| NPs | Methoxy-PEG-PCL | 2,3,6-triacetyl-β-CD | Sepiapterin | Tetrahydrobiopterin deficiency |
|
[18] |
| NPs | PLGA | HP-β-CD | Triamcinolone acetonide | Inflammatory diseases |
|
[90] |
| SMGel/Vehicles | PCL-PEG-PCL | γ-CD | Insulin | Diabetes mellitus |
|
[9] |
| SMGel/micelles | PCL-PEG-PCL | γ-CD | Dexamethasone | Inflammation in response to infectious, immunological, mechanical, and chemical stimuli |
|
[92] |
| SMGel/micelles | PEO-PHB-PEO | α-CD | Macromolecular drugs | — |
|
[93] |
| Films/membranes | PS-b-P4VP | β-CD | Triclosan | Antibacterial |
|
[94] |
| Core-shell structure |
|
β-CD | Doxorubicin | Cancer |
|
[95] |
| Micelles |
|
β-CD | Doxorubicin | Breast cancer |
|
[97] |
| Micelles | Poloxamer 407 (PEO-PPO-PEO) |
|
|
Eye inflammation |
|
[98] |
| Micelles |
|
α-CD | Natamycin | Fungal keratitis |
|
[99] |
| SMGel | Poloxamer (PEO-PPO-PEO) | α-CD | Injectable drug | — |
|
[100] |
| Inclusion complexes | Poloxamer 188 (PEO-PPO-PEO) | HP-β-CD | Genistein | Breast cancer |
|
[101] |
| Polypseudorotaxanes | Poloxamer 407 (PEO-PPO-PEO) |
|
|
|
|
[102] |
| SMGel | Poloxamer 407 (PEO-PPO-PEO) | α-CD | Vancomycin | Activity against Staphylococcus aureus |
|
[103] |
| NPs | Poloxamer 407 (PEO-PPO-PEO) |
|
Ropinirole | Parkinson’s disease |
|
[104] |
| SMGel/micelles |
|
HP-β-CD | Rhynchophylline | Parkinson’s disease |
|
[105] |
5.3 Preparation techniques and physicochemical characterization of CD-block copolymer based drug delivery systems
As it has been described in the literature, a wide spectrum of techniques has been conducted for the preparation and characterization of CD-block copolymer-based drug delivery systems. More specifically, these techniques include the co-lyophilization method, co-solvent evaporation method, nanoprecipitation method, ring-opening polymerization technique with microwave irradiation, oil/water method, cold method, dropwise mixing, and magnetic stirring. Regarding the characterization of the CD-block copolymer based drug delivery systems, differential scanning calorimetry has been used for the evaluation of the physicochemical state and thermal transitions of the pristine compounds, their combination, and the whole system, as well as the interactions between them. Furthermore, dynamic light scattering, electrophoretic light scattering, static light scattering, scanning electron microscope analysis, transmission electron microscopy, high-performance liquid chromatography, proton nuclear magnetic resonance and carbon-13 nuclear magnetic resonance, Fourier transform infrared-attenuated total reflection, UV-vis and fluorescence spectroscopy, and X-ray powder diffraction have been used to extract information on structural, morphological, and physicochemical properties of the aforementioned systems.
5.4 Challenges in developing and scaling up CD-block copolymer hybrid systems at the industrial level
We have described several examples of hybrid systems containing CDs and block copolymers that have appeared in the literature. The findings of these studies were very optimistic, indicating the added value and promising prospects for fabricating CD-block copolymer-based platforms in the field of drug delivery applications. However, to the best of our knowledge, there is no CD-block copolymer product that has been approved by the EMA or the US-FDA, nor has it been investigated in clinical trials, and for this purpose, this review could be a roadmap for the further development of these hybrid platforms, summarizing their properties, added value, and limitations in clinical translation.
Some important issues prevent their investigation in clinical trials and further scale-up at the industrial level. First of all, the development of polymer therapeutics faces a challenge in synthesizing new polymers with minimal nanotoxicity and low immunogenicity [75,106].
Moreover, an extensive physicochemical and morphological characterization is crucial for the development of all nanomedicine products. According to EMA [107], the identification of critical quality attributes of block copolymer micelle products is essential for ensuring Pharmaceutical Quality. For block copolymer micelle products, these attributes include their mean particle size, size distribution, morphology, zeta potential value, CMC, critical association concentration, drug loading, surface properties, chemical structure, in vitro stability and in vitro degradation of the block copolymer micelle in plasma and/or relevant media, in vitro release of the API from the block copolymer micelle product in plasma and/or relevant media, as well as the properties related to the manufacturing process (validated process for reconstitution and for ensuring sterility) and properties related to the in vivo behavior (osmolarity, fraction of active substance that is surface-associated, release rate and place of active substance release, degradation rate and place of degradation). Consequently, the elevated cost of the techniques employed for this purpose, in comparison to those used for other pharmaceutical formulations, poses a significant obstacle for pharmaceutical companies [75,106,108].
The CD-block copolymer is a hybrid drug delivery system that behaves as a new material. For this reason, the degree of complexity of this new hybrid material is higher than that of each material separately. The techniques used during the pre-formulation, formulation, stability, and scale-up studies are separate, with limited access for the industrial units in some cases. The last increase is also the cost of the clinical translation of these hybrid platforms; even their added value is very important for the efficacy of the medicine.
Pharmaceutical industries also necessitate scientists, including chemists and formulation scientists, with training and significant experience in the synthesis and characterization of materials and the further design and development of CD-block copolymer formulations. Simultaneously, academics should collaborate with them to facilitate knowledge transfer from academic institutions to pharmaceutical companies [75,109].
Finally, the regulatory landscape is not very clear containing grey areas for the nanoformulations, and this is an additional obstacle to the preparation and scale-up of CD-block copolymer hybrid nanosystems. To overcome all these limitations, the scientific community should consider them as opportunities for research and collaboration.
6 Conclusion
This article presents a comprehensive review of CD-block copolymer systems. Important information about the components of these hybrid formulations, as well as several examples from the relevant literature on these mixed systems are analyzed in this review article. The combination of CDs with block copolymers into hybrid formulations can result in new biomaterials and supramolecular structures, which encompass the physicochemical and thermotropic properties of both classes of materials. Despite the fact that there is no CD-block copolymer product that has been authorized yet, the added value of these systems is well established in the literature. Finally, considering the diversity of block copolymers and CD materials, and the potential benefits of their mixing/complexation, we could suggest them as promising candidates for new-generation formulations in the upcoming future.
Acknowledgments
Elmina Saitani would like to acknowledge Bodossaki Foundation for the PhD scholarship.
-
Funding information: The publication of the article in OA model was financially supported by HEAL-Link.
-
Author contributions: Conceptualization: N.P., S.P., and G.V.; writing – original draft preparation: E.-M.S. and D.S.; writing – review and editing: N.P., S.P., and G.V.; supervision: N.P., S.P., and G.V. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Valero M, Grillo I, Dreiss CA. Rupture of pluronic micelles by di-methylated beta-cyclodextrin is not due to polypseudorotaxane formation. J Phys Chem B. 2012;116(4):1273–81.10.1021/jp210439nSuche in Google Scholar PubMed
[2] Zhang D, Lv P, Zhou C, Zhao Y, Liao X, Yang B. Cyclodextrin-based delivery systems for cancer treatment. Mater Sci Eng C Mater Biol Appl. 2019;96:872–86.10.1016/j.msec.2018.11.031Suche in Google Scholar PubMed
[3] Lachowicz M, Stanczak A, Kolodziejczyk M. Characteristic of Cyclodextrins: Their role and use in the pharmaceutical technology. Curr Drug Targets. 2020;21(14):1495–510.Suche in Google Scholar
[4] Paul R, Paul S. Synergistic host-guest hydrophobic and hydrogen bonding interactions in the complexation between endo-functionalized molecular tube and strongly hydrophilic guest molecules in aqueous solution. Phys Chem Chem Phys. 2018;20(24):16540–50.10.1039/C8CP01502CSuche in Google Scholar PubMed
[5] Pippa N, Pispas S, Demetzos C. Polymer self-assembled nanostructures as innovative drug nanocarrier platforms. Curr Pharm Des. 2016;22(19):2788–95.10.2174/1381612822666160217141232Suche in Google Scholar PubMed
[6] Pippa N, Naziris N, Stellas D, Massala C, Zouliati K, Pispas S, et al. PEO-b-PCL grafted niosomes: The cooperativilty of amphiphilic components and their properties in vitro and in vivo. Colloids Surf B Biointerfaces. 2019;177:338–45.10.1016/j.colsurfb.2019.01.036Suche in Google Scholar PubMed
[7] Pispas S, Sarantopoulou E. Self-assembly in mixed aqueous solutions of amphiphilic block copolymers and vesicle-forming surfactant. Langmuir: ACS J Surf Colloids. 2007;23(14):7484–90.10.1021/la700342sSuche in Google Scholar PubMed
[8] van de Manakker F, Vermonden T, van Nostrum CF, Hennink WE. Cyclodextrin-based polymeric materials: synthesis, properties, and pharmaceutical/biomedical applications. Biomacromolecules. 2009;10(12):3157–75.10.1021/bm901065fSuche in Google Scholar PubMed
[9] Khodaverdi E, Heidari Z, Tabassi SA, Tafaghodi M, Alibolandi M, Tekie FS, et al. Injectable supramolecular hydrogel from insulin-loaded triblock PCL-PEG-PCL copolymer and gamma-cyclodextrin with sustained-release property. AAPS PharmSciTech. 2015;16(1):140–9.10.1208/s12249-014-0198-4Suche in Google Scholar PubMed PubMed Central
[10] Haley RM, Gottardi R, Langer R, Mitchell MJ. Cyclodextrins in drug delivery: applications in gene and combination therapy. Drug Delivery Transl Res. 2020;10(3):661–77.10.1007/s13346-020-00724-5Suche in Google Scholar PubMed PubMed Central
[11] Cirpanli Y, Bilensoy E, Lale Doğan A, Caliş S. Comparative evaluation of polymeric and amphiphilic cyclodextrin nanoparticles for effective camptothecin delivery. Eur J Pharm Biopharm: Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2009;73(1):82–9.Suche in Google Scholar
[12] Lala R, Thorat A, Gargote CS. Current trends in β-cyclodextrin based drug delivery systems. Int J Res Ayur Pharm. 2011;2:1520–6.Suche in Google Scholar
[13] Doile MM, Fortunato KA, Schmucker IC, Schucko SK, Silva MA, Rodrigues PO. Physicochemical properties and dissolution studies of dexamethasone acetate-beta-cyclodextrin inclusion complexes produced by different methods. AAPS PharmSciTech. 2008;9(1):314–21.10.1208/s12249-008-9042-zSuche in Google Scholar PubMed PubMed Central
[14] Loftsson T, Jarho P, Másson M, Järvinen T. Cyclodextrins in drug delivery. Expert Opin Drug Delivery. 2005;2(2):335–51.10.1517/17425247.2.1.335Suche in Google Scholar PubMed
[15] Řezanka M. Synthesis of substituted cyclodextrins. Environ Chem Lett. 2018;17:49–63.10.1007/s10311-018-0779-7Suche in Google Scholar
[16] Jansook P, Ogawa N, Loftsson T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int J Pharm. 2018;535(1–2):272–84.10.1016/j.ijpharm.2017.11.018Suche in Google Scholar PubMed
[17] Messner M, Kurkov SV, Jansook P, Loftsson T. Self-assembled cyclodextrin aggregates and nanoparticles. Int J Pharm. 2010;387(1–2):199–208.10.1016/j.ijpharm.2009.11.035Suche in Google Scholar PubMed
[18] Kuplennik N, Sosnik A. Enhanced nanoencapsulation of sepiapterin within PEG-PCL nanoparticles by complexation with triacetyl-beta cyclodextrin. Molecules. 2019;24(15).10.3390/molecules24152715Suche in Google Scholar PubMed PubMed Central
[19] Karathanos V, Mourtzinos I, Yannakopoulou K, Andrikopoulos N. Study of the solubility, antioxidant activity and structure of inclusion complex of vanillin with β-cyclodextrin. Food Chem. 2007;101:652–8.10.1016/j.foodchem.2006.01.053Suche in Google Scholar
[20] Tiwari G, Tiwari R, Rai AK. Cyclodextrins in delivery systems: Applications. J Pharm Bioallied Sci. 2010;2(2):72–9.10.4103/0975-7406.67003Suche in Google Scholar PubMed PubMed Central
[21] Rasheed A, Ashok K, Sravanthi S. Cyclodextrins as drug carrier molecule: A review. Sci Pharm. 2008;76:567–98.10.3797/scipharm.0808-05Suche in Google Scholar
[22] Charumanee S, Okonogi S, Sirithunyalug J, Wolschann P, Viernstein H. Effect of cyclodextrin types and co-solvent on solubility of a poorly water soluble drug. Sci Pharm. 2016;84(4):694–704.10.3390/scipharm84040694Suche in Google Scholar PubMed PubMed Central
[23] de Araujo DR, Tsuneda SS, Cereda CM, Carvalho FDGF, Preté PS, Fernandes SA, et al. Development and pharmacological evaluation of ropivacaine-2-hydroxypropyl-beta-cyclodextrin inclusion complex. Eur J Pharm Sci. 2008;33(1):60–71.10.1016/j.ejps.2007.09.010Suche in Google Scholar PubMed
[24] Lachowicz M, Stańczak A, Kołodziejczyk M. Characteristic of cyclodextrins: Their role and use in the pharmaceutical technology. Curr Drug Targets. 2020;21(14):1495–510.10.2174/1389450121666200615150039Suche in Google Scholar PubMed
[25] Ghosh A, Biswas S, Ghosh T. Preparation and evaluation of silymarin β-cyclodextrin molecular inclusion complexes. J Young Pharm. 2011;3:205–10.10.4103/0975-1483.83759Suche in Google Scholar PubMed PubMed Central
[26] Kali G, Haddadzadegan S, Bernkop-Schnürch A. Cyclodextrins and derivatives in drug delivery: New developments, relevant clinical trials, and advanced products. Carbohydr Polym. 2024;324:121500.10.1016/j.carbpol.2023.121500Suche in Google Scholar PubMed
[27] European Medicines Agency. Questions and answers on cyclodextrins used as excipients in medicinal products for human use: EMA; 2017. https://www.ema.europa.eu/en/documents/report/cyclodextrins-used-excipients-report-published-support-questions-answers-cyclodextrins-used_en.pdf.Suche in Google Scholar
[28] European Medicines Agency. Briviact (in Italy: Nubriveo): EMA; 2024. https://www.ema.europa.eu/en/medicines/human/EPAR/briviact-italy-nubriveo.Suche in Google Scholar
[29] European Medicines Agency. Baqsimi: EMA; 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/baqsimi#ema-inpage-item-product-info.Suche in Google Scholar
[30] European Medicines Agency. Jcovden (previously COVID-19 Vaccine Janssen): EMA; 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/jcovden-previously-covid-19-vaccine-janssen.Suche in Google Scholar
[31] European Medicines Agency. Mavenclad: EMA; 2024. https://www.ema.europa.eu/en/medicines/human/EPAR/mavenclad.Suche in Google Scholar
[32] European Medicines Agency. Gilenya: EMA; 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/gilenya.Suche in Google Scholar
[33] European Medicines Agency. Vitrakvi: EMA; 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/vitrakvi.Suche in Google Scholar
[34] Agency EM Prevymis: EMA; 2024. https://www.ema.europa.eu/en/medicines/human/EPAR/prevymis.Suche in Google Scholar
[35] European Medicines Agency. Abilify: EMA; 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/abilify.Suche in Google Scholar
[36] European Medicines Agency. Kyprolis: EMA; 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/kyprolis.Suche in Google Scholar
[37] European Medicines Agency. Cerenia: EMA; 2021. https://www.ema.europa.eu/en/medicines/veterinary/EPAR/cerenia.Suche in Google Scholar
[38] European Medicines Agency. Noxafil: EMA; 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/noxafil.Suche in Google Scholar
[39] European Medicines Agency. Veklury: EMA; 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/veklury.Suche in Google Scholar
[40] European Medicines Agency. Voriconazole Hikma (previously Voriconazole Hospira): EMA; 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/voriconazole-hikma-previously-voriconazole-hospira.Suche in Google Scholar
[41] European Medicines Agency. Vfend: EMA; 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/vfend.Suche in Google Scholar
[42] European Medicines Agency. Background review for cyclodextrins used as excipients: EMA; 2014. Available from: European Medicines Agency.Suche in Google Scholar
[43] Irie T, Uekama K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J Pharm Sci. 1997;86(2):147–62.10.1021/js960213fSuche in Google Scholar PubMed
[44] Asai K, Morishita M, Katsuta H, Hosoda S, Shinomiya K, Noro M, et al. The effects of water-soluble cyclodextrins on the histological integrity of the rat nasal mucosa. Int J Pharm. 2002;246(1–2):25–35.10.1016/S0378-5173(02)00345-9Suche in Google Scholar PubMed
[45] Leroy-Lechat F, Wouessidjewe D, Andreux J-P, Puisieux F, Duchêne D. Evaluation of the cytotoxicity of cyclodextrins and hydroxypropylated derivatives. Int J Pharmaceutics. 1994;101(1):97–103.10.1016/0378-5173(94)90080-9Suche in Google Scholar
[46] Eisenberg A. Block copolymers: Synthetic strategies, physical properties, and applications by Nikos Hadjichristidis and Stergios Pispas (University of Athens) and George Floudas (University of Ioannina). Hoboken, NJ: John Wiley and Sons, Inc.; 2003. J Am Chem Soc. 2003;125(37):11453–4.10.1021/ja025357cSuche in Google Scholar
[47] McCarthy JR. The future of theranostic nanoagents. Nanomed (London, Engl). 2009;4(7):693–5.10.2217/nnm.09.58Suche in Google Scholar PubMed
[48] Lammers T, Kiessling F, Hennink WE, Storm G. Nanotheranostics and image-guided drug delivery: current concepts and future directions. Mol pharmaceutics. 2010;7(6):1899–912.10.1021/mp100228vSuche in Google Scholar PubMed
[49] Teruya K, Doh-Ura K. Therapeutic development of polymers for prion disease. Cell Tissue Res. 2023;392(1):349–65.10.1007/s00441-022-03604-1Suche in Google Scholar PubMed
[50] Chroni A, Forys A, Sentoukas T, Trzebicka B, Pispas S. Poly[(vinyl benzyl trimethylammonium chloride)]-based nanoparticulate copolymer structures encapsulating insulin. Eur Polym J. 2022;169:111158.10.1016/j.eurpolymj.2022.111158Suche in Google Scholar
[51] Selianitis D, Pispas S. Multi-responsive poly(oligo(ethylene glycol)methyl methacrylate)-co-poly(2-(diisopropylamino)ethyl methacrylate) hyperbranched copolymers via reversible addition fragmentation chain transfer polymerization. Polym Chem. 2021;12(45):6582–93.10.1039/D1PY01320CSuche in Google Scholar
[52] Selianitis D, Forys A, Trzebicka B, Alemayehu A, Tyrpekl V, Pispas S. Amphiphilic P(OEGMA-co-DIPAEMA) hyperbranched copolymer/magnetic nanoparticle hybrid nanostructures by co-assembly. Nanomanufacturing. 2022;2.10.3390/nanomanufacturing2010004Suche in Google Scholar
[53] Luk BT, Zhang L. Current advances in polymer-based nanotheranostics for cancer treatment and diagnosis. ACS Appl Mater & Interfaces. 2014;6(24):21859–73.10.1021/am5036225Suche in Google Scholar PubMed PubMed Central
[54] Preman NK, Jain S, Johnson RP. “Smart” polymer nanogels as pharmaceutical carriers: A versatile platform for programmed delivery and diagnostics. ACS Omega. 2021;6(8):5075–90.10.1021/acsomega.0c05276Suche in Google Scholar PubMed PubMed Central
[55] Jain JP, Ayen WY, Kumar N. Self assembling polymers as polymersomes for drug delivery. Curr Pharm Des. 2011;17(1):65–79.10.2174/138161211795049822Suche in Google Scholar PubMed
[56] Kuperkar K, Atanase LI, Bahadur A, Crivei IC, Bahadur P. Degradable polymeric bio(nano)materials and their biomedical applications: A comprehensive overview and recent updates. Polymers. 2024;16(2):206.10.3390/polym16020206Suche in Google Scholar PubMed PubMed Central
[57] Feng H, Lu X, Wang W, Kang NG, Mays JW. Block copolymers: Synthesis, self-assembly, and applications. Polymers. 2017;9(10):494.10.3390/polym9100494Suche in Google Scholar PubMed PubMed Central
[58] Zhulina EB, Borisov OV. Theory of block polymer micelles: Recent advances and current challenges. Macromolecules. 2012;45(11):4429–40.10.1021/ma300195nSuche in Google Scholar
[59] Skandalis A, Selianitis D, Sory DR, Rankin SM, Jones JR, Pispas S. Poly(2-(dimethylamino) ethyl methacrylate)-b-poly(lauryl methacrylate)-b-poly(oligo ethylene glycol methacrylate) triblock terpolymer micelles as drug delivery carriers for curcumin. J Appl Polym Sci. 2022;139(38):e52899.10.1002/app.52899Suche in Google Scholar
[60] Son GM, Kim HY, Ryu JH, Chu CW, Kang DH, Park SB, et al. Self-assembled polymeric micelles based on hyaluronic acid-g-poly(d,l-lactide-co-glycolide) copolymer for tumor targeting. Int J Mol Sci. 2014;15(9):16057–68.10.3390/ijms150916057Suche in Google Scholar PubMed PubMed Central
[61] Qiao H, Huang Z, Ren X, Liu S, Zhang Y, Qi X, et al. Self-powered photodetectors based on 2D materials. 2020;8(1):1900765.10.1002/adom.201900765Suche in Google Scholar
[62] Liu Y, Li J, Ren J, Lin C, Leng J. Preparation and in vitro pH-responsive drug release of amphiphilic dendritic star-block copolymer complex micelles. Mater Lett. 2014;127:8–11.10.1016/j.matlet.2014.04.070Suche in Google Scholar
[63] Yang C, Liu SQ, Venkataraman S, Gao SJ, Ke X, Chia XT, et al. Structure-directing star-shaped block copolymers: supramolecular vesicles for the delivery of anticancer drugs. J Controlled Release: Off J Controlled Rel Soc. 2015;208:93–105.10.1016/j.jconrel.2015.03.027Suche in Google Scholar PubMed
[64] Mandal P, Maji S, Panja S, Bajpai OP, Maiti TK, Chattopadhyay S. Magnetic particle ornamented dual stimuli responsive nanogel for controlled anticancer drug delivery. N J Chem. 2019;43(7):3026–37.10.1039/C8NJ04841JSuche in Google Scholar
[65] Wang D, Wang J, Huang H, Zhao Z, Gunatillake PA, Hao X. Brush-shaped RAFT polymer micelles as nanocarriers for a ruthenium(ii) complex photodynamic anticancer drug. Eur Polym J. 2019;113:267–75.10.1016/j.eurpolymj.2019.01.074Suche in Google Scholar
[66] Bai T, Shao D, Chen J, Li Y, Xu BB, Kong J. pH-responsive dithiomaleimide-amphiphilic block copolymer for drug delivery and cellular imaging. J Colloid Interface Sci. 2019;552:439–47.10.1016/j.jcis.2019.05.074Suche in Google Scholar PubMed
[67] Mandal P, Panja S, Banerjee SL, Ghorai SK, Maji S, Maiti TK, et al. Magnetic particle anchored reduction and pH responsive nanogel for enhanced intracellular drug delivery. Eur Polym J. 2020;129:109638.10.1016/j.eurpolymj.2020.109638Suche in Google Scholar
[68] Khaliq NU, Park DY, Yun BM, Yang DH, Jung YW, Seo JH, et al. Pluronics: Intelligent building units for targeted cancer therapy and molecular imaging. Int J Pharm. 2019;556:30–44.10.1016/j.ijpharm.2018.11.064Suche in Google Scholar PubMed
[69] Doğan A, Yalvaç ME, Şahin F, Kabanov AV, Palotás A, Rizvanov AA. Differentiation of human stem cells is promoted by amphiphilic pluronic block copolymers. Int J Nanomed. 2012;7:4849–60.10.2147/IJN.S31949Suche in Google Scholar PubMed PubMed Central
[70] Pitto-Barry A, Barry NPE. Pluronic® block-copolymers in medicine: from chemical and biological versatility to rationalisation and clinical advances. Polym Chem. 2014;5(10):3291–7.10.1039/C4PY00039KSuche in Google Scholar
[71] Kabanov AV, Batrakova EV, Alakhov VY. Pluronic block copolymers for overcoming drug resistance in cancer. Adv Drug Deliv Rev. 2002;54(5):759–79.10.1016/S0169-409X(02)00047-9Suche in Google Scholar PubMed
[72] Mu CF, Balakrishnan P, Cui FD, Yin YM, Lee YB, Choi HG, et al. The effects of mixed MPEG-PLA/Pluronic copolymer micelles on the bioavailability and multidrug resistance of docetaxel. Biomaterials. 2010;31(8):2371–9.10.1016/j.biomaterials.2009.11.102Suche in Google Scholar PubMed
[73] Lo CL, Lin SJ, Tsai HC, Chan WH, Tsai CH, Cheng CH, et al. Mixed micelle systems formed from critical micelle concentration and temperature-sensitive diblock copolymers for doxorubicin delivery. Biomaterials. 2009;30(23-24):3961–70.10.1016/j.biomaterials.2009.04.002Suche in Google Scholar PubMed
[74] Popovici C, Popa M, Sunel V, Atanase LI, Ichim DL. Drug delivery systems based on pluronic micelles with antimicrobial activity. Polymers. 2022;14(15):3007.10.3390/polym14153007Suche in Google Scholar PubMed PubMed Central
[75] Pippa N, Gazouli M, Pispas S. Recent advances and future perspectives in polymer-based nanovaccines. Vaccines. 2021;9(6):558.10.3390/vaccines9060558Suche in Google Scholar PubMed PubMed Central
[76] Poudel AJ, He F, Huang L, Xiao L, Yang G. Supramolecular hydrogels based on poly (ethylene glycol)-poly (lactic acid) block copolymer micelles and α-cyclodextrin for potential injectable drug delivery system. Carbohydr Polym. 2018;194:69–79.10.1016/j.carbpol.2018.04.035Suche in Google Scholar PubMed
[77] Cheng H, Fan X, Wu C, Wang X, Wang LJ, Loh XJ, et al. Cyclodextrin-based star-like amphiphilic cationic polymer as a potential pharmaceutical carrier in macrophages. Macromol Rapid Commun. 2019;40(5):e1800207.10.1002/marc.201800207Suche in Google Scholar PubMed
[78] Zhou Z, Guo F, Wang N, Meng M, Li G. Dual pH-sensitive supramolecular micelles from star-shaped PDMAEMA based on β-cyclodextrin for drug release. Int J Biol Macromolecules. 2018;116:911–9.10.1016/j.ijbiomac.2018.05.092Suche in Google Scholar PubMed
[79] Cui X, Wang N, Wang H, Li G, Tao Q. pH sensitive supramolecular vesicles from cyclodextrin graft copolymer and benzimidazole ended block copolymer as dual drug carriers. Int J Polym Mater Polym Biomater. 2019;68(12):733–40.10.1080/00914037.2018.1493686Suche in Google Scholar
[80] Zhang L, Lu J, Jin Y, Qiu L. Folate-conjugated beta-cyclodextrin-based polymeric micelles with enhanced doxorubicin antitumor efficacy. Colloids Surf B Biointerfaces. 2014;122:260–9.10.1016/j.colsurfb.2014.07.005Suche in Google Scholar PubMed
[81] Gao Y, Li G, Zhou Z, Guo L, Liu X. Supramolecular assembly of poly(β-cyclodextrin) block copolymer and benzimidazole-poly(ε-caprolactone) based on host-guest recognition for drug delivery. Colloids Surf B Biointerfaces. 2017;160:364–71.10.1016/j.colsurfb.2017.09.047Suche in Google Scholar PubMed
[82] Song X, Zhu JL, Wen Y, Zhao F, Zhang ZX, Li J. Thermoresponsive supramolecular micellar drug delivery system based on star-linear pseudo-block polymer consisting of β-cyclodextrin-poly(N-isopropylacrylamide) and adamantyl-poly(ethylene glycol). J Colloid Interface Sci. 2017;490:372–9.10.1016/j.jcis.2016.11.056Suche in Google Scholar PubMed
[83] Chee CK, Rimmer S, Soutar I, Swanson L. Fluorescence investigations of the thermally induced conformational transition of poly(N-isopropylacrylamide). Polymer. 2001;42(12):5079–87.10.1016/S0032-3861(00)00821-1Suche in Google Scholar
[84] Maeda Y, Higuchi T, Ikeda I. Change in hydration state during the coil-globule transition of aqueous solutions of poly(af-isopropylacrylamide) as evidenced by FTIR spectroscopy. Langmuir: ACS J Surf Colloids. 2000;16(19):7503–9.10.1021/la0001575Suche in Google Scholar
[85] Zhu W, Li Y, Liu L, Chen Y, Wang C, Xi F. Supramolecular hydrogels from cisplatin-loaded block copolymer nanoparticles and α-cyclodextrins with a stepwise delivery property. Biomacromolecules. 2010;11(11):3086–92.10.1021/bm100889jSuche in Google Scholar PubMed
[86] Xu Z, Liu S, Liu H, Yang C, Kang Y, Wang M. Unimolecular micelles of amphiphilic cyclodextrin-core star-like block copolymers for anticancer drug delivery. Chem Commun. 2015;51(87):15768–71.10.1039/C5CC02743HSuche in Google Scholar
[87] Vega E, Egea MA, Calpena AC, Espina M, García ML. Role of hydroxypropyl-β-cyclodextrin on freeze-dried and gamma-irradiated PLGA and PLGA-PEG diblock copolymer nanospheres for ophthalmic flurbiprofen delivery. Int J Nanomed. 2012;7:1357–71.10.2147/IJN.S28481Suche in Google Scholar PubMed PubMed Central
[88] Li X, Liu H, Li J, Deng Z, Li L, Liu J, et al. Micelles via self-assembly of amphiphilic beta-cyclodextrin block copolymers as drug carrier for cancer therapy. Colloids Surf B Biointerfaces. 2019;183:110425.10.1016/j.colsurfb.2019.110425Suche in Google Scholar PubMed
[89] Çirpanli Y, Bilensoy E, Lale Doğan A, Çaliş S. Comparative evaluation of polymeric and amphiphilic cyclodextrin nanoparticles for effective camptothecin delivery. Eur J Pharm Biopharm. 2009;73(1):82–9.10.1016/j.ejpb.2009.04.013Suche in Google Scholar PubMed
[90] Li F, Wen Y, Zhang Y, Zheng K, Ban J, Xie Q, et al. Characterisation of 2-HP-β-cyclodextrin-PLGA nanoparticle complexes for potential use as ocular drug delivery vehicles. Artif Cell Nanomed Biotechnol. 2019;47(1):4097–108.10.1080/21691401.2019.1683567Suche in Google Scholar PubMed
[91] Liao Y, Zhang X, Li C, Huang Y, Lei M, Yan M, et al. Inclusion complexes of HP-beta-cyclodextrin with agomelatine: Preparation, characterization, mechanism study and in vivo evaluation. Carbohydr Polym. 2016;147:415–25.10.1016/j.carbpol.2016.04.022Suche in Google Scholar PubMed
[92] Khodaverdi E, Gharechahi M, Alibolandi M, Tekie FS, Khashyarmanesh BZ, Hadizadeh F. Self-assembled supramolecular hydrogel based on PCL-PEG-PCL triblock copolymer and gamma-cyclodextrin inclusion complex for sustained delivery of dexamethasone. Int J Pharm Investig. 2016;6(2):78–85.10.4103/2230-973X.177809Suche in Google Scholar PubMed PubMed Central
[93] Li J, Li X, Ni X, Wang X, Li H, Leong KW. Self-assembled supramolecular hydrogels formed by biodegradable PEO-PHB-PEO triblock copolymers and alpha-cyclodextrin for controlled drug delivery. Biomaterials. 2006;27(22):4132–40.10.1016/j.biomaterials.2006.03.025Suche in Google Scholar PubMed
[94] Huang T, Manchanda P, Zhang L, Shekhah O, Khashab NM, Eddaoudi M, et al. Cyclodextrin-functionalized asymmetric block copolymer films as high-capacity reservoir for drug delivery. J Membr Sci. 2019;584:1–8.10.1016/j.memsci.2019.04.039Suche in Google Scholar
[95] Zhang J, Ma PX. Core-shell structured nanoassemblies based on beta-cyclodextrin containing block copolymer and poly(beta-benzyl l-aspartate) via host-guest complexation. Polym (Guildf). 2011;52(21):4928–37.10.1016/j.polymer.2011.08.030Suche in Google Scholar PubMed PubMed Central
[96] Wang H, Williams GR, Wu J, Wu J, Niu S, Xie X, et al. Pluronic F127-based micelles for tumor-targeted bufalin delivery. Int J Pharm. 2019;559:289–98.10.1016/j.ijpharm.2019.01.049Suche in Google Scholar PubMed
[97] Li X, Yu Y, Ji Q, Qiu L. Targeted delivery of anticancer drugs by aptamer AS1411 mediated Pluronic F127/cyclodextrin-linked polymer composite micelles. Nanomed: Nanotechnol Biol Med. 2015;11(1):175–84.10.1016/j.nano.2014.08.013Suche in Google Scholar PubMed
[98] Jansook P, Pichayakorn W, Muankaew C, Loftsson T. Cyclodextrin-poloxamer aggregates as nanocarriers in eye drop formulations: Dexamethasone and amphotericin B. Drug Dev Ind Pharm. 2016;42(9):1446–54.10.3109/03639045.2016.1141932Suche in Google Scholar PubMed
[99] Lorenzo-Veiga B, Sigurdsson HH, Loftsson T, Alvarez-Lorenzo C. Cyclodextrin–amphiphilic copolymer supramolecular assemblies for the ocular delivery of natamycin. Nanomaterials (Basel, Switz). 2019;9(5):745.10.3390/nano9050745Suche in Google Scholar PubMed PubMed Central
[100] Ni X, Cheng A, Li J. Supramolecular hydrogels based on self-assembly between PEO-PPO-PEO triblock copolymers and alpha-cyclodextrin. J Biomed Mater Res A. 2009;88(4):1031–6.10.1002/jbm.a.31906Suche in Google Scholar PubMed
[101] Zafar A, Alruwaili NK, Imam SS, Alsaidan OA, Alkholifi FK, Alharbi KS, et al. Formulation of genistein-HP beta cyclodextrin-poloxamer 188 ternary inclusion complex: Solubility to cytotoxicity assessment. Pharmaceutics. 2021;13(12):1997.10.3390/pharmaceutics13121997Suche in Google Scholar PubMed PubMed Central
[102] Nogueiras-Nieto L, Sobarzo-Sanchez E, Gomez-Amoza JL, Otero-Espinar FJ. Competitive displacement of drugs from cyclodextrin inclusion complex by polypseudorotaxane formation with poloxamer: implications in drug solubilization and delivery. Eur J Pharm Biopharm: Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2012;80(3):585–95.10.1016/j.ejpb.2011.12.001Suche in Google Scholar PubMed
[103] Simoes SM, Veiga F, Torres-Labandeira JJ, Ribeiro AC, Sandez-Macho MI, Concheiro A, et al. Syringeable Pluronic-alpha-cyclodextrin supramolecular gels for sustained delivery of vancomycin. Eur J Pharm Biopharm: Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2012;80(1):103–12.10.1016/j.ejpb.2011.09.017Suche in Google Scholar PubMed
[104] Saitani EM, Pippa N, Perinelli DR, Forys A, Papakyriakopoulou P, Lagopati N, et al. Fabricating polymer/surfactant/cyclodextrin hybrid particles for possible nose-to-brain delivery of ropinirole hydrochloride: In vitro and ex vivo evaluation. Int J Mol Sci. 2024;25(2):1162.10.3390/ijms25021162Suche in Google Scholar PubMed PubMed Central
[105] Lin H, Xie L, Lv L, Chen J, Feng F, Liu W, et al. Intranasally administered thermosensitive gel for brain-targeted delivery of rhynchophylline to treat Parkinson’s disease. Colloids Surf B, Biointerfaces. 2023;222:113065.10.1016/j.colsurfb.2022.113065Suche in Google Scholar PubMed
[106] Siegrist S, Cörek E, Detampel P, Sandström J, Wick P, Huwyler J. Preclinical hazard evaluation strategy for nanomedicines. Nanotoxicology. 2019;13(1):73–99.10.1080/17435390.2018.1505000Suche in Google Scholar PubMed
[107] European Medicines Agency. Development of block-copolymer-micelle medicinal products - Scientific guideline: EMA; 2013. https://www.ema.europa.eu/en/development-block-copolymer-micelle-medicinal-products-scientific-guideline.Suche in Google Scholar
[108] Swierczewska M, Crist RM, McNeil SE. Evaluating nanomedicines: Obstacles and advancements. In: McNeil SE, editor. Characterization of nanoparticles intended for drug delivery. New York, NY: Springer New York; 2018. 3–16.10.1007/978-1-4939-7352-1_1Suche in Google Scholar PubMed
[109] Chountoulesi M, Selianitis D, Pispas S, Pippa N. Recent advances on PEO-PCL block and graft copolymers as nanocarriers for drug delivery applications. Mater (Basel, Switz). 2023;16(6):2298.10.3390/ma16062298Suche in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy
Artikel in diesem Heft
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy

