Abstract
Pharmaceutical scientists have long struggled to develop reliable and efficient systems of administering insulin orally due to multiple barriers, including stomach acidity, enzymatic degradation, and mucus barriers. However, various strategies were developed to avoid insulin degradation in the gastrointestinal tract (GIT) and promote membrane permeability and biological activity. Among these strategies, chitosan polymer-based carriers are widely researched due to their ability to protect insulin in the alimentary canal and deliver it effectively through the intestinal mucosa, improving its bioavailability. To improve chitosan properties, chemical and physical modifications have been developed, and recently, nanoparticles, microparticles, and beads of chitosan exhibited potential systems for oral insulin delivery (OID). This review facilitates an outline of the types of diabetes mellitus, insulin biosynthesis, and gastrointestinal barriers against oral insulin. Moreover, the limitations of subcutaneous insulin delivery and alternative routes of administration are also discussed. As an ideal and most convenient oral administration route, the challenges of safe insulin delivery through the GIT and strategies to elevate its bioavailability are highlighted. In addition, this review focuses on recent advancements in chitosan based carriers for OID and their potential future applications.
Graphical Abstract
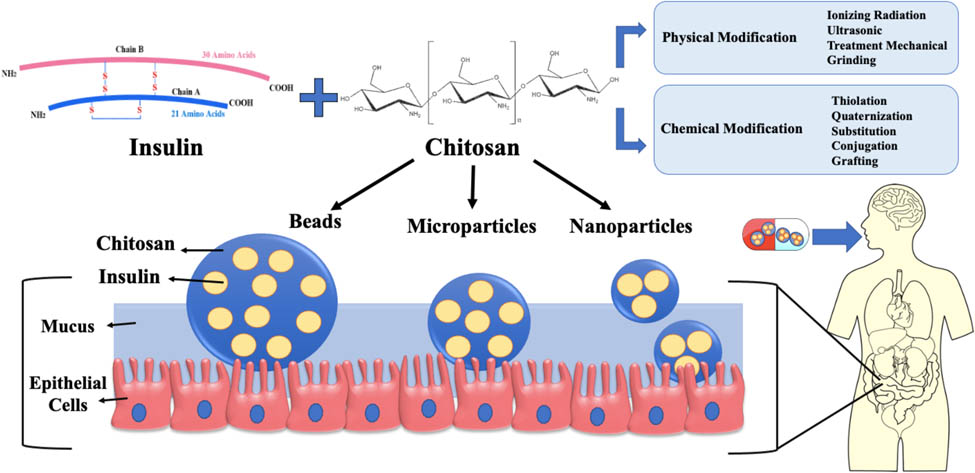
1 Introduction
Diabetes mellitus (DM) is a chronic endocrine disorder characterized by increased blood glucose levels due to impaired insulin synthesis caused by reduced or failure in pancreatic insulin secretion and/or low cell sensitivity toward insulin [1]. DM can primarily be diagnosed by the high level of blood or plasma glucose. Diabetes can also be detected if fasting plasma glucose level of 126 mg/dL or a 2 h plasma glucose level of 200 mg/dL with nonspecific symptoms are observed [2]. Diabetes can manifest as a contributing factor for triggering syndromes such as Turner syndrome, Prader-Willi syndrome, Friedreich ataxia, Alström syndrome, Klinefelter syndrome, Bardet-Biedl syndrome, Berardinelli-Seip syndrome, and Down syndrome [3]. Also, stress, war trauma, glucocorticoids, surgery, thiazides, starvation, infections, high levels of epinephrine, glucagon, and growth hormone are additional triggers that may lead to an autoimmune reaction that results in DM [3].
According to the International Diabetic Federation (IDF), 643 million adults will have diabetes by 2030, up from an estimated 537 million in 2021 [4]. The high complications and prevalence of diabetes are responsible for around three million deaths yearly worldwide, according to the World Health Organization [5]. Subcutaneous injections are still the most common approach to deliver insulin regularly. However, this route has several disadvantages, including reduced patient compliance due to pain, needle phobia, hypoglycemic episodes, and allergic reactions [6]. The convenience of administering insulin through the oral route is frequently acknowledged as the preferred method, although it confronts major obstacles of limited intestinal penetration and low insulin stability in the gastrointestinal tract (GIT). Thus, during the past 10 years, the emphasis on encapsulating insulin in polymer-based carriers has been proposed as a viable technique for improving insulin oral bioavailability by overcoming the orally associated limitations [7]. In addition, therapeutic proteins and peptides are increasingly being delivered orally using several hydrophilic mucoadhesive vehicle types like nanoparticles (NPs), microparticles (MPs), and beads as delivery systems with different advanced preparation methods [8,9,10,11]. These carriers showed improved strategy for attaching to the gut wall and increasing the residence time in the intestine, thereby boosting the drug's bioavailability, offering advantages over other drug delivery methods [12,13]. As a natural polysaccharide polymer, chitosan is considered the most common mucoadhesive polymer for delivering proteins and peptides orally. It is non-toxic, inexpensive, biodegradable, biocompatible, easy to process, and can be digested by colonic microbial enzymes. Furthermore, the availability of amine groups in its chemical structure makes it a perfect candidate for various modifications, conferring it a superior polymer compared to other polysaccharides [14,15].
Chitosan-based oral delivery systems, like NPs, MPs, and beads, have been explored as a potential option for delivering insulin orally in recent years. This comprehensive review will address the challenges and transport mechanisms associated with developing such systems for oral insulin delivery (OID). Moreover, the aim of this review is to provide a detailed description of the use of chitosan in designing OID systems, focusing specifically on recent strategies to enhance chitosan through various modifications. In addition, methods used for preparing chitosan carriers are discussed in detail.
2 DM
2.1 Type 1 DM (T1DM)
T1DM accounts for 5–10% of people with diabetes, characterized by damaged pancreatic beta cells and reduced or even terminated insulin production (Figure 1). It results from the cellular-mediated death of pancreatic beta-cells, which can be due to autoimmune or environmental-related factors [16]. The autoimmune destruction includes autoantibodies of islet cells, insulin, glutamic acid decarboxylase (GAD65), and tyrosine phosphatases IA-2 and IA-2β [17,18]. Studies on environmental-related factors have shown that diabetes prevalence varied among people of the same ethnic group living in different geographical areas (e.g., Finland vs Estonia). In Estonia (Baltic area), the risk of developing T1DM was one-third of that in Finland. In addition, exposure to antigenic substances at a young age is also thought to play an influential role in disease development [19]. For blood glucose management and control in type 1 diabetic patients, several daily exogenous insulin injections are necessary [6,20].
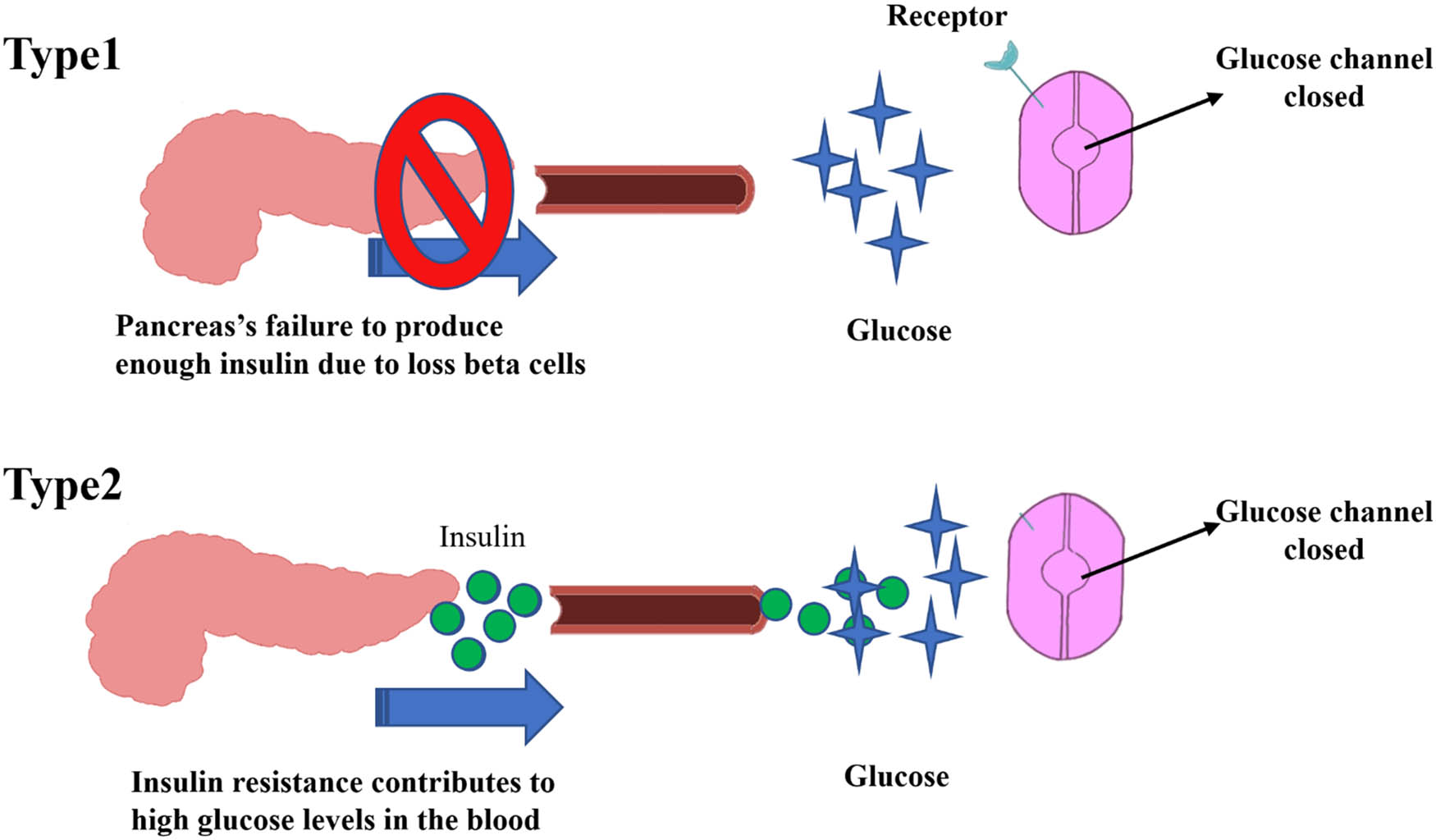
Schematic presentation of insulin fate in T1DM and T2DM.
2.2 Type 2 DM (T2DM)
T2DM is also one of the most frequent metabolic disorders globally, and its occurrence is commonly caused by two main factors: (1) failure to respond to insulin by insulin-sensitive tissues and (2) impairment of insulin secretion and action by pancreatic β-cells (Figure 1) [21,22]. Impaired resistance to insulin action occurs when target cells cannot respond to the circulating insulin [23]. T2DM patients also have a significant risk of infections brought on by an abnormal immune system, impaired glucose control, and diabetic neuropathy. These are either common mild infections such as external otitis, cystitis, pneumonia, enteric infections, appendicitis, and peritonitis or rare severe infections like emphysematous pyelonephritis [24]. Regarding signaling insulin pathways, T2DM is a complex disorder influencing several metabolic pathways [25]. When glucose reaches physiological amounts, it stimulates the β-cells to produce and release insulin, facilitating glucose uptake by muscle, adipose tissue, liver, and brain. Insulin also stimulates the synthesis of glycogen, proteins, and lipogenesis while suppressing hepatic gluconeogenesis production [26]. Physiologically, insulin has various hormonal effects in addition to its well-known characteristic of reducing blood sugar, explaining its impact on different tissues. The first step in the insulin signaling process is binding to the receptors that initiate a sequence of phosphorylation processes where intracellular protein substrates are activated to induce signaling cascades. Later, phosphatidylinositol 3-kinase (PI3K) triggers the activation of protein kinase B (PKB), known as AKT. In target cells, except for hepatocytes, which mainly express the non-insulin-regulated glucose transporter 2 (GLUT2), translocation of GLUT4 to the plasma membrane, glycogen synthase, and a variety of other enzymes are then triggered through the action of insulin. Mitogen-activated protein kinase activity, which encourages protein translocation, gene expression, and cell proliferation, also impacts the insulin signaling pathway. Ultimately, the metabolism of lipids, proteins, and carbohydrates is significantly regulated by insulin [21].
2.3 Insulin biosynthesis
Insulin is synthesized by converting insulin mRNA into preproinsulin, a single-chain molecule that serves as an inactive insulin precursor. The composition of preproinsulin consists of a single peptide that includes insulin B-chain, C-peptide, and insulin A-chain (Figure 2) [27]. Once the single-chain molecule is secreted to the endoplasmic reticulum, proinsulin is created by eliminating the signal peptide through signal peptidase. Proinsulin comprises three unique chains consisting of an amino-terminal B chain, a C-peptide, and a carboxy-terminal A chain, forming 86 amino acids. Proinsulin is recognized as the prohormone precursor to mature insulin. Proinsulin is transformed into insulin in the endoplasmic reticulum by cutting out the C-peptide by specific endopeptidases (Figure 2) [28]. Finally, the C-peptide and insulin are transported to the Golgi for packaging into secretory granules and stored in the cytoplasm [29].
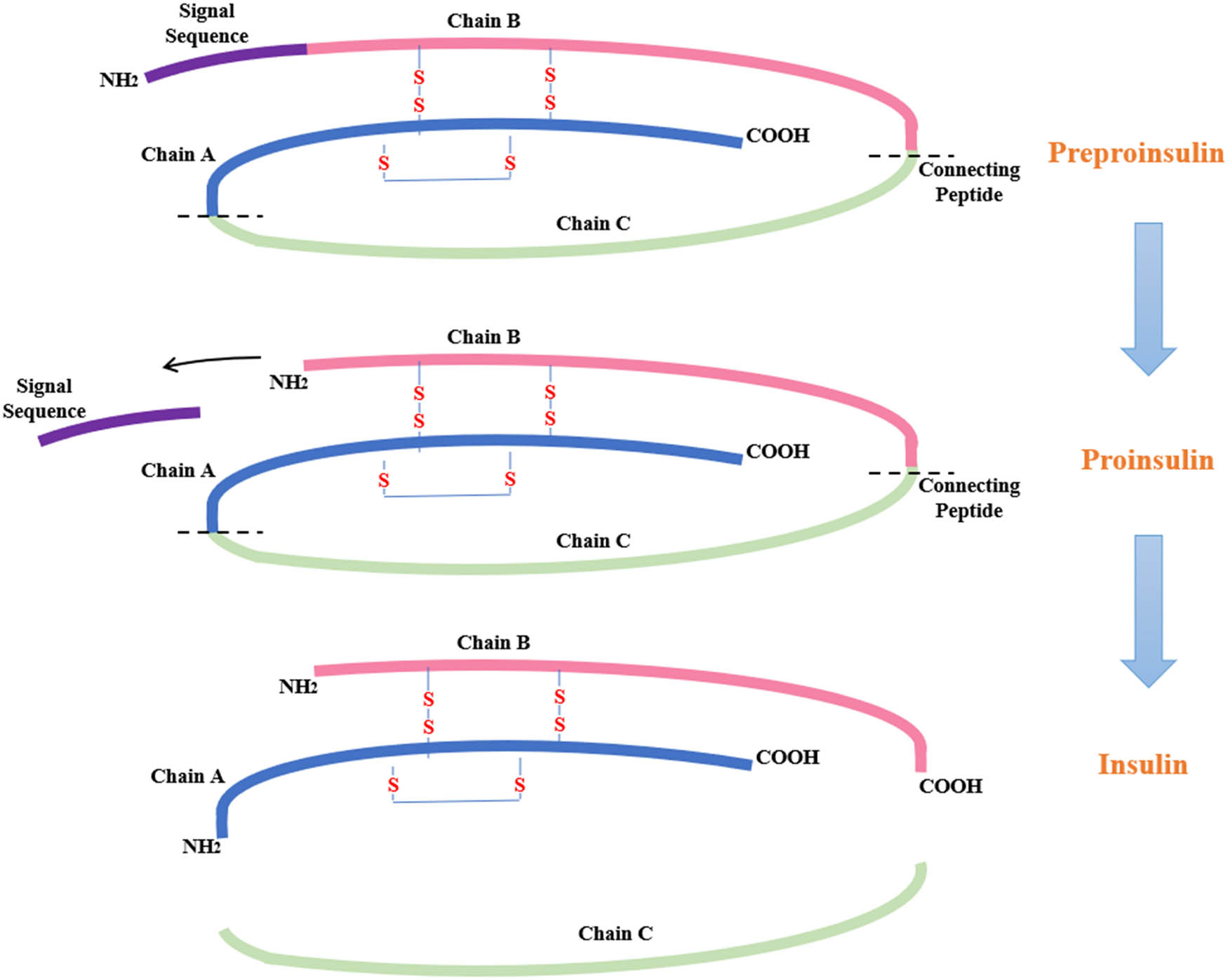
Biosynthesis of insulin.
3 Current insulin administration
The GIT has a hostile and harsh environment that can cause insulin degradation [30]. To avoid this degradation, insulin is usually administered through subcutaneous injections that offer rapid onset of action and high bioavailability [31]. Patients commonly receive insulin injections directly into the subcutaneous fat layer with a lower absorption rate due to reduced vascularity. Although there has been a significant improvement in various techniques for subcutaneous insulin injections, this technique can still lead to several issues, including patient discomfort, poor compliance, nonadherence, infections, lipid deposits at the injection site, and local hypertrophy [32]. In addition, as this route is invasive, the subcutaneous injection formulations might not be the best choice when multiple injections are needed per day. In fact, it is reported that hypoglycemia can occur when multiple injections are given leading to poor control of blood glucose and thus putting patients at high risk of low sugar complications. Therefore, alternative administration routes, such as oral, buccal, nasal, peritoneal, and transdermal, have recently gained significant attention [33,34].
4 Non-oral insulin delivery
Insulin has various duration effects, including rapid, short, intermediate, and long-acting [35]. Also, different insulins, including basal, prandial, and premixed, are administered through a pump, syringe, pen, and prefilled pen. There are long-acting analogues for basal insulins (first generation: detemir and glargine 100 I/mL; second generation: glargine 300 U/mL and Degludec) as well as human intermediate-acting insulin (neutral protamine Hagedorn insulin, for example). The basal short-and rapid-acting insulin analogues (e.g., Aspart, Lispro, and Glulisine) and prandial insulin require frequent daily injections. The pre-mixed insulin analogues combine slow- and rapid-acting insulin in varying ratios to replicate the prandial and basal effects of insulin production in a single dose, to reduce the dose frequency of intermediate/rapid-acting insulin, hence improving the patients' convenience. However, combining rapid and intermediate-acting insulins is significantly challenging as basal insulin is not miscible with other insulins; thus, part of rapid-acting insulin should contain protamine to convert it into intermediate-acting insulin [36].
Besides injection, non-OID, such as pulmonary and nasal, was developed, considering the large surface area of the respiratory, pulmonary, and nasal pathways. However, there are some safety concerns as these routes can allow the entry of exogenous allergens into the lung and have an irreversible alteration of the epithelial cell membrane [37]. The nasal administration possesses a highly vascularized absorption region (150 cm2) that allows rapid circulatory drugs [38]. This facilitates direct protein transfer into the blood circulation, thus avoiding first-pass hepatic and gut metabolism [39]. Nevertheless, a local burning sensation in the nose is a common side effect after administering the drug through the nasal. Although intranasal insulin was widely employed in clinical trials, only a few studies reported satisfactory results [40].
Transdermal insulin can bypass the enzymatic and chemical degradation in the GIT. This approach can also provide a sustained release with maintained therapeutic concentration for an extended period, allowing for better glycemic control. However, the skin's protective properties are still the main challenge for transdermal insulin delivery [41]. Finally, buccal mucosa has received considerable attention due to its visibility, resilience, saliva-protected, and highly vascular properties. However, buccal administration has numerous intrinsic limitations, one of which is the limited permeability of neutral lipids across the surface layers of the epithelium [42].
5 Oral insulin delivery
Although the subcutaneous route overcomes the first-pass effect, it can lead to peripheral hyperinsulinemia [43,44]. Alternatively, oral insulin administration is the most convenient delivery route as it is cost-effective, safe, and painless [45]. Oral insulin passes the liver via the portal vein, reducing hyperinsulinemia and increasing cells' sensitivity to insulin, hence utilizing blood glucose more effectively [44]. Moreover, the GIT cavities offer a large surface area for enhanced drug absorption that mimic the physiological insulin pathway, improving glucose homeostasis [46]. Furthermore, OID can improve insulin portal levels while lowering peripheral hyperinsulinemia linked to retinopathy and neuropathy in other administration routes [18,47]. However, OID is highly challenging in delivering insulin effectively and efficiently, which is the primary research highlighted in recent years [48]. For high molecular weight hydrophilic macromolecules like insulin, poor intestinal absorption and enzymatic degradation negate insulin bioavailability and hepatic metabolism [49]. Although many approaches are developed to deliver insulin orally, the absorption rate and the capacity to penetrate the gut lining vary significantly [50].
5.1 Barriers to OID in the stomach and small intestine
As shown in Figure 3, the stomach, which contains high gastric acid, comprises the most significant chemical barrier to insulin absorption. Gastric acid is a digestive fluid containing hydrochloric acid secreted by the parietal cells of gastric glands in the stomach, resulting in a highly acidic environment (pH 1∼2). Moreover, the pepsin enzyme in the stomach degrades and proteolyzes the free insulin. As a result, shielding insulin from both acids and enzymes is a significant step in aiding to deliver oral insulin efficiently [51]. Among several available strategies, pH-triggered release approaches are used to overcome stomach acidity. pH-responsive carriers such as hydrogel demonstrated enhanced drug oral delivery and controlled release in the intestine [52]. Pancreatic enzymes like lipase, elastase, chymotrypsin, trypsin, and carboxypeptidases (A and B) can degrade proteins transiting the small intestine and lumen [53]. The most common strategy for suppressing these enzymes is by including enzyme inhibitors in drug formulation, such as non-amino acids, amino acids, modified amino acids, peptides, and modified peptides that inactivate target enzymes by reversibly or irreversibly attaching to the particular sites of the enzymes [54].
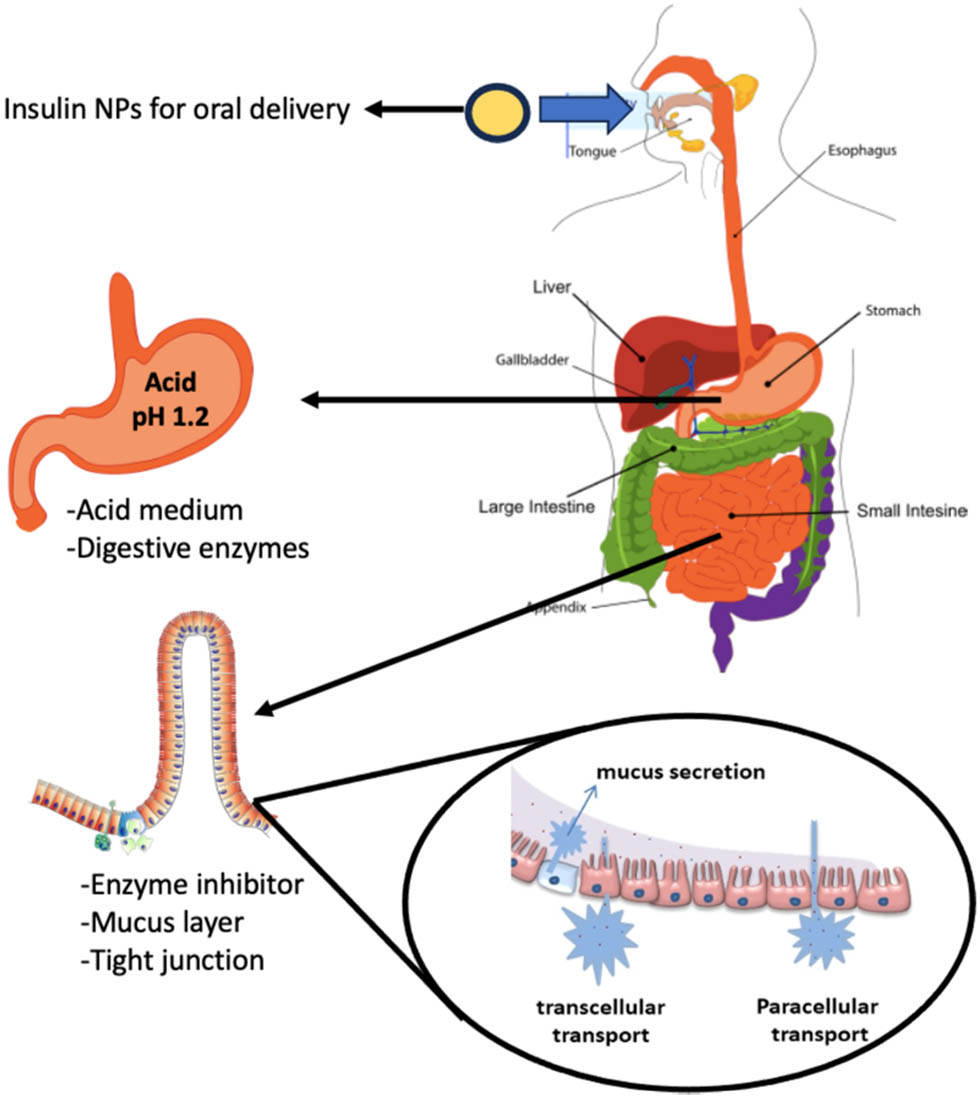
The GI barriers for OID.
Furthermore, delivering insulin orally faces physical barriers such as the mucous layer, intestinal epithelium and tight junctions. Mucus is the first obstacle against polypeptides that can serve as diffusional and enzymatic barriers. Mucus, an adhesive and viscoelastic hydrogel covers the GIT epithelial cells and exhibits a strong capacity to trap unknown particles by physicochemical forces like hydrogen bonds, electrostatic interactions, and hydrophobic forces. Eventually, these trapped particles are removed from the body via a natural mucociliary clearance pathway [55]. Also, mucus interacts electrostatically with positively charged drugs and proteins due to its negative charge [47].
The intestine has a layer of epithelial cells protected by a mucus layer, the major component of the intestinal mucosal barrier. Before oral insulin reaches the bloodstream, it must pass through the intestinal epithelium via two pathways, transcellular or paracellular. Most oral medications are absorbed transcellular, which involves intermembrane transport, endocytosis fusion, and adsorption [56]. At the same time, hydrophilic molecules are preferentially transported by the paracellular pathway regulated by tight junctions between the epithelial cells. The absorption of these molecules is regulated by the junctional complexes, including adherens junctions (AJs), desmosomes, and tight junctions (TJs). TJs consist of peripheral membrane proteins (zonula occludens (ZO)-1, ZO-2), regulatory proteins, and transmembrane proteins (occludins and claudins), where TJs are the apical-most adhesive complexes that primarily close the intercellular space. AJs are required for their assembly and are found below the TJs. Together with desmosomes, it provides strong adhesive bonds to retain the integrity of the epithelium. AJs and TJs are connected to the prejunctional ring of myosin and actin that allows the control of junctions via the cytoskeleton [57].
6 Oral delivery of insulin via colon targeting
As OID faces numerous challenges, the colon-targeted protein drug delivery systems were intensively investigated due to the neutral pH (∼7.5), minimal proteolytic activity, and prolonged residence duration [58]. The large intestinal tract comprises the cecum, colon, rectum, and anus, with the colon being the largest at roughly 1.5 m in length. Generally, the high water-absorbing capacity of the colon confers it more viscous than the upper GIT parts. Besides, the lumen walls of the colon are covered by a thick mucus layer that consists of mucin glycoprotein, lipids, water, and inorganic ions, which reduce drug dissolution and absorption [59]. Therefore, the colon poses a potential target site for the oral delivery of insulin.
6.1 Challenges in colon targeting insulin delivery
Although the colon delivery system can potentially be used for macromolecule administration, several obstacles complicate oral drug delivery to the colon. For example, the burst drug release, drug degradation in the stomach, the variation in pH in different GIT regions, mucus entrapment, and systematic jejunum [60] make the colon-target delivery system more challenging. The human digestive tract is complex, and several factors should be considered in designing a targeted colonic drug delivery system, including the pH, mucus barrier, and colonic microbiota [61].
6.2 pH
Although varying pH values along the GIT can complicate colon-targeted delivery systems, the differences in pH ranges can help control drug release time. Colon-targeted delivery systems use a pH-sensitive polymer as a coating agent to cover the entire drug surface that is insoluble in acidic pH but soluble in neutral pH or slightly alkaline [62]. pH-sensitive polymers enable shielding the drug while in the upper region of the GIT and suppressing the encapsulant release before approaching the colon. The coating may consist of single or multiple enteric polymers of pH-dependent or a mixture with pH-independent polymer [61]. In addition, a formulation based on one or a pair of enteric polymers with different pH-dependent solubility profiles can be formulated for colon drug delivery [63].
6.3 Mucus barrier
Another barrier that influences the absorption of drugs in colon delivery is the hydrogel layer, composed of large mucin glycoproteins, known as mucus. Mucus protects the epithelium from mechanical damage, traps and prevents infections from accessing the epithelial cells, and lubricates the chyme. Conventional drug delivery methods can also stick to the GI mucosal layers before being removed in the feces, shortening the sustained local drug release period and resulting in unsatisfactory therapeutic effects [60]. However, the mucus can benefit specific delivery systems by prolonging the drug residence time [64]. While high molecular weight proteins and peptides may not effectively penetrate through the mucus, drug molecules with hydrophilicity, net-neutral surface charge, and smaller hydrodynamic size can negate adhesion, resist mucosal enzymatic action, and circumvent steric hindrance [65].
6.4 Colonic microbiota
In the digestive tract, the anaerobic and aerobic bacteria constitute the most significant number of bacteria in the colon. These bacteria have around 400 types with a concentration of 1,000 CFU/mL [66] that produce some biomolecules and metabolize drugs [67]. Polysaccharides are prone to anaerobic bacterial metabolism in the colon while resisting the intestinal and gastric enzymes. Moreover, colonic enzymes can mediate drug biotransformation that results in inactive, active, or harmful metabolites, whereas bacterial drug metabolism can cause toxicity. An active metabolite formed by colonic drug metabolism is a typical “prodrug approach” for colon-specific drug delivery systems [60]. As a result, while designing a medication delivery system for the colon, it is critical to consider therapeutic formulations based on bacterial enzymes [68].
7 Polymers-based carrier for OID
Over the last decade, several research studies based on polymeric carriers to improve the delivery of insulin orally have been conducted [69]. Various polymers were investigated to determine the possible applications, emphasizing natural polymers. Natural polymers are preferred over artificial counterparts regarding biocompatibility, accessibility, and ease of modification. Hence, various functional groups can be integrated into the native natural polymers, providing additional physicochemical properties.
Monosaccharide chains are linked by hydroxyl, carboxyl, and amino groups to produce polysaccharides, typically extracted from organic materials, marine plants, and exogenous bacterial metabolites. Polysaccharides are hydrophilic, stable, non-toxic, readily biodegradable, and can encapsulate, immobilize, and release various active substances under controlled conditions [70]. Polysaccharides such as chitosan, pectin, alginate, starch, and dextran were extensively employed as encapsulation matrices [71,72].
In recent years, introducing polymeric NPs/MPs as carriers to deliver insulin orally has gained significant interest [73]. Numerous biodegradable and non-biodegradable polymers were studied, but the latter pose issues with toxicity, removal challenges, and the inability to achieve sustained release of insulin. Biodegradable polymeric particles separate the drug from the surrounding medium, shielding the peptide from peptidases and facilitating enterocyte uptake. After oral administration, polymeric particles gradually degrade depending on the nature of the polymer, providing a sustained and controlled drug release.
8 Chitosan-based polymer for oral insulin drug delivery
The mucoadhesive polymer-based drug delivery can boost absorption and bioavailability by extending drug retention at the absorption site. Ideally, the mucoadhesive polymer should attach to the mucosa before penetrating the mucus layer. Protein drug delivery systems based on mucoadhesive property have been developed using polymers such as chitosan, alginate, and many others [74]. Other properties of these polymers, including gastro-resistance, degradability by colonic microbiota, and pH responsiveness, provide micro- and nano-based drug delivery systems several advantages in overcoming barriers to oral drug administration and targeting specific absorption sites [75]. Chitosan is considered one of the most effective polymers in developing oral drug administration systems among all available mucoadhesive polymers. Chitosan is a positively charged, non-toxic, biodegradable, biocompatible, and mucoadhesive polymer produced by the hydrolysis of crab or shrimp chitin [10].
8.1 Physicochemical properties of chitosan
Chitosan, a polysaccharide, comprises repeating d-glucosamine and N-acetyl-d-glucosamine blocks (Figure 4). Following deacetylation, each chitosan subunit includes a primary amine group (pK a = 6.5) and two hydroxyl groups that can be conveniently modified depending on the purpose of application. At pH < 6.5, chitosan amine groups are protonated to NH3+, conferring chitosan cationic and soluble in an acidic medium. The degree of deacetylation and molecular weight of chitosan significantly impact its solubility [76].
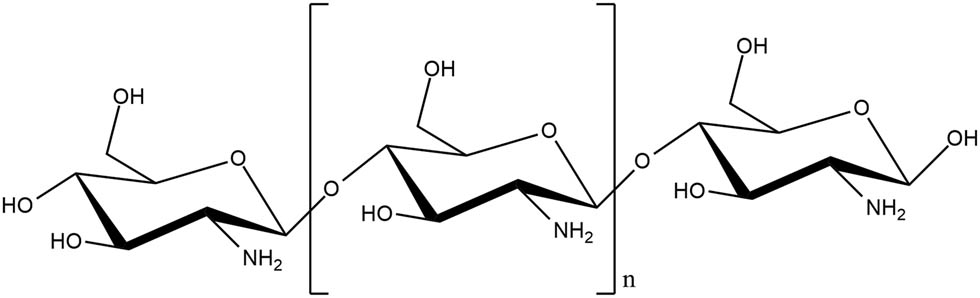
Chemical structure of chitosan.
Chitosan gains its mucoadhesive properties through ionic interactions between its cationic groups and the anionic nature of the mucous layer. These characteristics promote its adhesion into the GITs, retaining the encapsulant for a prolonged time to reach a sustained release profile [77]. The mucoadhesive property is vital in developing drug delivery systems that improve drug targeting, controlled drug release, effectiveness, and bioavailability [78]. Furthermore, chitosan can form gels in acidic pH that can be utilized as carriers for slow drug release. Moreover, it can be cross-linked with covalent bonds through the reaction between the negative phosphate groups of sodium triphosphate (TPP) (non-toxic) and the amino groups of chitosan [79]. Pharmaceutically, chitosan is employed in several applications, such as clinical biomedicine, drug delivery systems, tissue engineering, and taste masking [80]. Recently, chitosan has attracted wider attention in developing micro- and nano-carriers as oral delivery systems due to the modifiable physicochemical characteristics of its backbone.
8.2 Drug release from chitosan matrix
Recent studies have employed chitosan in drug delivery systems for various applications as described in (Table 1). Like other dosage forms, chitosan-based drug delivery systems rely on the physicochemical properties of the encapsulant. In addition, drug delivery in these systems depends on chitosan properties such as swelling, adhesion to mucus layer, and gel-forming ability in different body fluids with various ion concentrations and pH, as well as the presence of excipients and co-polymers in drug formulations. The release of encapsulated drug from chitosan hydrogel is affected by diffusion, swelling, erosion, and biodegradation [81].
Chitosan applications for colon targeted drug delivery
| Formulation | Active pharmaceutical ingredients (APIs) | Delivery system | Approaches for CDDS | Degradation mechanism | Ref. |
|---|---|---|---|---|---|
| Chitosan/pectin | Curcumin | Modified citrus pectinate-chitosan NPs | Drug delivery to the colon using a pH-sensitive polymer-coated carrier | Mucoadhesiveness | [123] |
| Chitosan/kappa-carrageenan/alginate | 5-Fluorouracil | pH-sensitive bilayered chitosan/kappa-carrageenan microbeads/alginate | Drug delivery to the colon using a pH-sensitive polymer-coated carrier | pH-responsive | [124] |
| Chitosan/alginate | Interleukin-1RA | Chitosan/alginate microcapsules | Drug delivery to the colon using a pH-sensitive polymer-coated carrier | pH-responsive | [125] |
| Chitosan/pectin | Insulin | Chitosan-pectin NP colon-specific drug delivery | Drug delivery to the colon using a pH-sensitive polymer-coated carrier | pH-responsive | [126] |
| Chitosan-heparin | Oligonucleotides | Chitosan-heparin NP colon-specific drug delivery | Drug delivery to the colon using a pH-sensitive polymer-coated carrier | pH-responsive | [126] |
| Fucoidan/chitosan | Quercetin | Fucoidan-chitosan NP colon-specific drug delivery | Drug delivery to the colon using a pH-sensitive polymer-coated carrier | pH-responsive | [127] |
| Alginate-chitosan | BSA | Alginate-chitosan NP colon-specific drug delivery | Microbially triggered drug delivery to the colon | Enzymatic | [128] |
| Chitosan | Resveratrol | NP colon-specific drug delivery | Microbially triggered drug delivery to the colon | Enzymatic sensitive | [129,130,131] |
| Pectinate-chitosan | Curcumin | Modified citrus pectinate-chitosan nanoparticle | Microbially triggered drug delivery to the colon | Enzyme sensitivity and mucoadhesiveness | [123,132] |
| Resveratrol | Chitosan-zinc-pectinate-polyethene glycol NPs | Drug delivery to the colon using a pH-sensitive polymer-coated carrier | |||
| Thiolated chitosan | Sitagliptin | NP colon-specific drug delivery | Drug delivery to the colon using a pH-sensitive polymer-coated carrier | Mucoadhesiveness | [133] |
| Chitosan | Insulin | NP colon-specific drug delivery | Drug delivery to the colon using a pH-sensitive polymer-coated carrier Microbially triggered drug delivery to the colon | Enzymatic, pH-responsive | [130] |
| TDF | |||||
| Chitosan + mucin | Insulin | NP colon-specific drug delivery | Drug delivery to the colon using-sensitivities polymer-coated carrier | Mucoadhesiveness | [134] |
| Chitosan/ alginate | Naringenin | NP colon-specific drug delivery | Drug delivery to the colon using a pH-sensitive polymer-coated carrier | pH-responsive | [135] |
| PEGylated chitosan | Rosuvastatin | NP colon-specific drug delivery | Drug delivery to the colon using a pH-sensitive polymer-coated carrier | pH-responsive | [136] |
| Succinyl chitosan/alginate | Quercetin | NP colon-specific drug delivery | Drug delivery to the colon using a pH-sensitive polymer-coated carrier | pH-responsive | [137] |
| Chitosan/ alginate | Lovastatin | NP colon-specific drug delivery | Drug delivery to the colon using a pH-sensitive polymer-coated carrier | pH-responsive | [138] |
| Chitosan anti-biopeptides | Lovastatin | NP colon-specific drug delivery | Microbially triggered drug delivery to the colon | Enzymatic responsive | [139] |
| Chitosan-modified | Curcumin | NP colon-specific drug delivery | Ligand/receptor-mediated drug delivery system | Epithelium | [140] |
| Chitosan/alginate | Capecitabine | Chitosan succinate-sodium alginate beads | Drug delivery to the colon using a pH-sensitive polymer-coated carrier, microbially triggered drug delivery to the colon | pH-responsive, enzyme-sensitive, and mucoadhesiveness | [141] |
| Alginate/chitosan/Konjac glucomannan | Ciprofloxacin | Chitosan coated konjac glucomannan/sodium alginate/graphene oxide microspheres | Drug delivery to the colon using a pH-sensitive polymer-coated carrier, microbially triggered drug delivery to the colon | pH-responsive, enzyme sensitive, and mucoadhesiveness | [124] |
| Chitosan/nutriose | Quercetin | PEG-containing vesicles coated with chitosan/ nutriose | Drug delivery to the colon using a pH-sensitive polymer-coated carrier, microbially triggered drug delivery to the colon | Enzyme sensitivity and mucoadhesiveness | [142] |
A cationic polymer like chitosan can promote polyelectrolyte complexation with an anionic polymer like pectin to control the drug release with the required protection. In coacervation with pectin, chitosan helps encapsulate anionic drugs like bovine serum albumin. The prepared particles showed an enhanced pH-sensitive drug release profile, whereas the results of the animal studies on rats showed that 72.6% of physiologically active anti-A/B toxin immunoglobin of egg yolk (IgY) was released preferentially in the colon [82]. Several studies demonstrated the colon-targeting capability of chitosan-calcium pectinate microbeads compositions with a limited drug release in the stomach and small intestine and a controlled release in the colon [83]. Hence, a chitosan-based hydrogel system is a promising candidate for developing oral colon insulin delivery [84].
8.3 Chitosan limitation
The increased swelling of chitosan in aqueous environments can cause a rapid premature drug release. Moreover, chitosan-intrinsic properties, such as low mechanical resistance and high matrix porosity, can impact its application in drug delivery [85]. Furthermore, chitosan has weak acid resistance, and its solubility is poor in physiological fluids (pH around 7.4) due to its weak basic nature with pK a between 6.2 and 7 [86]. To overcome these limitations, chitosan is commonly derivatized and/or modified physically or chemically [81].
9 Applications of chitosan systems for OID
The management of diabetes, a global health concern, has long been dominated by traditional insulin administration methods, primarily injections [87]. However, the search for non-invasive alternatives has led to significant advancements in drug delivery systems. The advancements in OID through chitosan systems represent a significant breakthrough in the landscape of diabetes management. The multifaceted contributions of chitosan, encompassing biocompatibility, mucoadhesive properties, controlled release mechanisms, and protection against enzymatic degradation, position it as a versatile and effective platform for ensuring successful oral delivery of insulin. As a protein drug, the orally administered insulin must pass through many physiological barriers, such as GIT, mucus layer, intestinal epithelium, then finally reach the circulatory system [88]. Thus far, oral absorption of insulin remains a major scientific challenge. First, oral insulin must be effectively transported along the GIT tract without being degraded by acidic conditions in the stomach and proteases in the GIT. Second, insulin is a hydrophilic protein, which is difficult to be encapsulated in a hydrophobic macromolecular carrier. Third, the bioavailability of untreated insulin is extremely low due to the first-pass effect in the liver. Additionally, transit time is another factor that affects oral delivery and bioavailability of drugs to the colon. The normal transit time in the small intestine is approximately 4 h, with an inter-individual variability of 2–6 h; that of colon, however, is relatively variable, ranging from 6 to 70 h [89]. Chitosan polymer and its derivatives were used in several OID systems due to their properties such as mucoadhesive property, controlled release, and protection against enzymatic degradation.
9.1 Mucoadhesive property
Chitosan's mucoadhesive properties as illustrated in Figure 5 add another layer of sophistication to its role in OID [90]. In the GIT, the mucosal lining presents a formidable barrier that drugs must overcome for efficient absorption. Chitosan's mucoadhesiveness allows the encapsulant to interact with the mucosal layer, extending the residence time of the drug delivery system. This positive impact is attributed to its ability to open the tight junctions between epithelial cells, facilitating the transport of insulin through well-organized epithelial layers. Insulin is a peptide hormone known for its poor oral bioavailability, the ability to adhere to the mucosal surface is particularly advantageous [91]. The prolonged contact facilitated by chitosan enhances the absorption of insulin, contributing to its therapeutic effectiveness. This mucoadhesive feature positions chitosan as a key player in addressing one of the longstanding challenges in OID. Importantly, chitosan serves as a permeation enhancer without causing significant harm to the mucosa. Chitosan has demonstrated effectiveness in various oral insulin formulations as a transmucosal delivery polymer, with the goal of eliminating the need for injections over time. Both chitosan and insulin have been incorporated into various forms such as liquid mixtures, NPs, and MPs and others. Additionally, a range of chitosan derivatives are utilized as mucoadhesive, encapsulation polymers, stabilizers, and permeation enhancers [92,93,94,95,96].

Illustrates the mucoadhesive property of chitosan and transferring the encapsulated insulin to the blood stream.
Chitosan, a positively charged polymer, holds promise for OID due to its modifiable nature through various chemical reactions. However, in its original form, chitosan's effectiveness as an absorption enhancer for insulin in the intestinal tract can be compromised. To address this, several chemical modifications such as chitosan thiolation, conjugation, and grafting have been applied. While possessing excellent mucoadhesive characteristics, they also exhibit permeation-enhancing effects, the capacity to inhibit efflux pumps, and the ability to form in situ gels [97,98,99].
A previous conducted study involved chitosan NPs suspended in deionized water for OID. The obtained SPECT/CT images highlighted a notable presence of chitosan nanoparticles, marked with 99mTc, persisting in the stomach for an extended duration post oral administration. To address potential insulin release from chitosan NPs, a strategic step was taken. The chitosan NPs were subjected to lyophilization, and the resulting dried NPs were encapsulated in a gelatin capsule featuring an enteric polymer coating (Eudragit L100-55). This enteric polymer exhibits pH sensitivity, ensuring resilience in the acidic gastric environment while readily dissolving in the mildly acidic to neutral conditions of the small intestine. This ingenious approach serves a dual purpose – preventing premature insulin release in the stomach and promoting enhanced absorption on the small intestine's surface. Ultimately, this methodology aims to maximize the bioavailability of insulin delivered through chitosan NPs [100,101].
9.2 Controlled release mechanisms
Chitosan’s versatility is further evident in its ability to be tailored for controlled release, a critical aspect of optimizing OID [102]. Various formulation techniques, including microencapsulation and NP design, allow researchers to modulate the release kinetics of insulin from chitosan-based systems. Controlled release mechanisms are paramount for achieving sustained and prolonged insulin delivery, mimicking the physiological secretion profile of endogenous insulin. This not only enhances therapeutic efficacy but also contributes to better glycemic control for individuals with diabetes. The controlled release capabilities of chitosan systems represent a leap forward in the precision and customization of OID strategies [103].
Quaternized derivatives of chitosan (QCs) have expanded the utility of chitosan for effective protein and peptide delivery in the neutral or weakly alkaline pH of the small intestine. Recently, chitosan nanoparticles (CS-NPs) were prepared using synthesized diethylmethyl chitosan (DEMC) and trimethyl chitosan (TMC) through the ionotropic gelation method or polyelectrolyte complex (PEC). Similarly, another study utilized newly synthesized tri-ethyl chitosan (TEC) and DMEC for NP preparation. In both investigations, insulin-loaded NPs were orally delivered to the colon. The encapsulation efficiency of insulin in positively charged CS-derivatized NPs ranged from approximately 70% (TMC, DEMC) to 90% (TEC, DMEC) when employing the PEC method. This high encapsulation efficiency is attributed to electrostatic interactions between the negatively charged acidic groups of insulin and the positively charged amino groups of CS derivatives. NPs derived from TEC and DMEC demonstrated sustained insulin release over 5 h, exhibiting minimal burst release. However, no significant deviation in the release profile was observed in phosphate-buffered saline (PBS) at different pH levels (6.8 and 7.4). Importantly, the derivatives exhibited higher insulin release compared to CS-NPs alone, as the quaternized derivatives showed enhanced solubility at neutral and alkaline pH levels [104,105,106,107].
9.3 Protection against enzymatic degradation
Enzymatic degradation in the GIT poses a significant threat to the stability of therapeutic proteins like insulin. Chitosan, acting as a protective barrier, shields insulin from enzymatic breakdown, ensuring that a substantial amount of the active drug reaches the bloodstream intact. This protective mechanism is crucial for maintaining the potency and bioavailability of insulin during its journey through the digestive system. The enzymatic protection offered by chitosan represents a strategic advantage in OID, addressing a fundamental challenge that has impeded the development of effective oral protein therapies [108].
Pai et al. have reported a method of preparing lipid NPs with enhanced physical stability via the incorporation of chitosan into the lipid NPs. The selection of the chitosan-lipid system depends on the drug's affinity to the matrix. Chitosan-lipid system and drug of similar affinities are selected to enhance the association efficiency of drug. The insulin is complexed with sodium docusate in formulation using the chitosan-monoolein system. The process of complexation removes or alleviates the charge density of insulin thereby increasing its affinity for lipophilic carrier. The formation of NPs comprising insulin-sodium docusate complex embedded in chitosan–lipid matrix is expected to increase the resistance of matrix and insulin against enzymatic degradation following its administration orally [109].
10 Modification of chitosan for colon targeting
By modifying its backbone of hydroxyl and amine groups, the properties of chitosan, such as mucoadhesion, stability, swelling degree, complexation ability, and solubility, can be modulated for specific applications. The main techniques commonly used in modifying chitosan include curing, blending or graft co-polymerization [110]. While in curing technique, complex methods such as electrochemical, ultraviolet radiation processing, and thermal methods are needed to convert polymers into a solidified mass by forming three-dimensional bonds within a polymer network, blending is accomplished by mixing two or more polymers. In comparison, graft co-polymerization involves chitosan modification through covalent chemical bonding with grafting agents [111].
10.1 Physical modification
Chitosan modified by ionizing radiation, ultrasonic treatment, and mechanical grinding to design various shapes like gel particles, NPs, and sponge materials has shown great applicability. The most preferred technique endowing chitosan with desirable properties in practical application is polymer blending. Good mechanical and chemical properties have been reported when chitosan is blended with synthetic polymers such as polyvinyl chloride and polyvinyl alcohol. Moreover, physically crosslinked hydrogels are desired over chemically modified ones because of the uncomplicated formation of the physical polymeric network [112]. Different combinations of polysaccharides and polymers have been developed to improve colon-targeted drug delivery, as shown in Table 1.
10.2 Chemical modifications
Various modifications have been attempted to overcome absorption and enzymatic barriers of GIT by alteration of the polymer functional groups of chitosan. The type of chemical change is influenced mainly by the structural modifications involved in a given formulation, such as thiolation, quaternization, substitution, conjugation, and grafting [15].
10.2.1 Thiolation
Thiolation is a process where thiol groups are grafted to the backbone of the polymers using various types of thiolating agents such as thioglycolic acid, isopropyl-S-acetylthioacetimidate, 2-iminothiolane or 4-thiobutylamidine, cysteine, N-acetyl cysteine, and glutathione. The mucoadhesion property of chitosan can greatly be enhanced by means of thiolation through binding covalently to the mucosal layer as the thiol moiety reacts with the mucin-glycoprotein of the GIT by disulfide bond formation. Therefore, chitosan strongly binds to the mucosal layer and interferes with drug absorption while reducing drug diffusion [113,114]. In addition to its strong mucoadhesive ability, thiolated chitosan has permeability-boosting capabilities, block efflux pumps, and in situ gelling properties [115].
10.2.2 Quaternization
This method raises the pK a of chitosan by adding a quaternary ammonium side chain, which changes the main amino group into a quaternary ammonium group. There are three ways to quaternate chitosan: direct quaternary ammonium substitution, epoxy derivative open loop, and N-alkylation [116]. The quaternization method can raise the solubility of chitosan at gastric pH when the hydrophobic functional groups are linked to the quaternized polymer or when adopting additional enteric coating [117,118].
10.2.3 Substitution
In this method, the functional groups are grafted to the hydroxyl group's oxygen or chitosan's primary amino nitrogen. The hydrophobicity of chitosan is increased when the hydrogen of amino groups is replaced with a long-chain acyl moiety, thus resulting in improved resistance to enzymatic actions and mucosal permeability [119].
10.2.4 Conjugation
In this approach, either chelating moieties are inserted into the system, or the extension of the pK a of chitosan takes place. Polymer-ethylene glycol tetraacetic acid (EGTA) conjugation can result in calcium chelation in the vicinity of the oral insulin systems in the GIT. Calcium ions have a major role in enzymatic degradation in the GIT and the generation of the apical junctions along the mucosal layer, and these ions are required for the thermodynamic stability of proteolytic enzymes such as trypsin and chymotrypsin [98]. Therefore, chelating of Ca2+ ions can synergistically lower the enzymatic effects and enhance mucosal absorption. Similarly, conjugating glutamine with chitosan can change the pK a of chitosan from 6.5 to 9.13, leading to positive charge retention, even in natural pH regions like the intestine or colon [15,120].
10.2.5 Grafting
Grafting is a method that copolymerizes a polymer into another polymer with negated effects on the original characteristics. Grafting was used in OID systems due to chitosan's inability to be ionically complexed with cationic or non-ionic polymers such as polymethacrylic acid, which can enhance mucoadhesion [99]. Four grafting methods are primarily used in grafting chitosan: free radical-mediated grafting, electrochemical methods, radiation, and enzyme-catalyzed grafting. The grafting efficiency may differ according to the procedure parameters and method [121]. Nevertheless, grafting chitosan with polymethacrylic acid (incorporating N-vinyl pyrrolidone) increases hydrophilicity and poor mucin interaction with the formulation. This phenomenon can be explained by the fact that high levels of mucin adsorption have a detrimental effect on mucoadhesive delivery systems due to particle entrapment on the mucosal surface, which is eliminated by the dynamic nature of mucus [15,122].
The functional amine –NH2 groups of chitosan provide a reaction site to facilitate binding with other active agents in developing different pharmaceutical applications, where they can react with citrates, phosphates, and sulfates, improving drug encapsulation efficiency and stability. For instance, the solubility of chitosan in intestinal media is improved by producing quaternized chitosan (N-trimethyl chitosan chloride). Trimethyl chitosan with a degree of quaternization in the range of 40–60% increases the intestinal permeability of hydrophilic macromolecular drugs. Moreover, formulating NPs with thiolated chitosan can further increase the mucoadhesiveness of chitosan [111].
10.2.6 Future perspectives
The future of OID through chitosan systems presents a promising frontier with the exploration of modified colon delivery strategies. Considering the challenges associated with insulin's transit through the GIT, including enzymatic degradation and poor bioavailability, focusing on colon-specific delivery mechanisms becomes crucial. Tailoring chitosan-based formulations for targeted insulin release in the colon could significantly enhance bioavailability, ensuring optimal therapeutic outcomes. Chitosan's unique properties, encompassing biocompatibility, mucoadhesion, and controlled release mechanisms, position it as a versatile platform. Specifically, in the context of modified colon delivery, the focus could be on optimizing chitosan carriers to ensure targeted insulin loading in the colon region. Chitosan modifications from different CS derivatives and CS complexes, such as N-sulphation, trimethyl CS, and diethylmethyl CS emerge as valuable carrier to enhance better insulin association efficiency and loading capacity in the intricate environment of the intestinal tract [109,118]. Looking forward, future perspectives might entail delving into advanced microencapsulation integration to fine-tune chitosan NPs, enabling breakthroughs in insulin bioavailability. Additionally, a shift towards personalized medicine approaches, tailoring chitosan carriers based on individual patient characteristics, could bring about more customized and effective strategies. The development of smart delivery systems responsive to specific GI conditions represent another intriguing avenue. Overall, this evolving landscape signifies a paradigm shift in diabetes care, offering a patient-friendly and efficient alternative to conventional insulin administration. Continuous innovation, interdisciplinary collaboration, and successful clinical translation will be pivotal in realizing the full potential of chitosan systems for OID in the modified colon context.
11 Preparation methods of chitosan NPs
Several types of NPs loaded with insulin for oral delivery by combining various materials with chitosan and chitosan modifications, including natural polymers, synthetic polymers, lipids, metals, and proteins have been developed (Table 2). Effective NP systems must be stable, non-toxic, biodegradable, non-thrombogenic, non-inflammatory, and nonimmunogenic and should escape their reticuloendothelial system. Furthermore, nanocarriers can be modified with particular ligands that target the receptors on the surface of epithelial cells to enhance peptides and protein uptake through oral administration.
Formulations of insulin chitosan NPs
| Formulation | Method of preparation | Chitosan concentration (% w/v) | Size (mean value ± SD) (nm) | Zeta potential (mV) | Encapsulation efficiency (%) | Loading capacity (%) | Release profile | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Chitosan-coated mPEG-b-PLGA NPs | Double-emulsion (water-in-oil-in-water) solvent evaporation method | 0.5 | 224.4 ± 13.8 | +13.7 ± 1.6 | 55.2 ± 7.0 | 4.9 ± 0.7 | pH 1.2 13.91% | [155] | |
| pH 6.8 38% | |||||||||
| 4 h | |||||||||
| Polyurethane–alginate/chitosan core-shell NPs | Polyelectrolyte complexation method | 1 | 156.12 | 38.5 | 90 | 12 | pH 1.2 15.77 2 h | [156] | |
| pH 6.8 50% 10 h | |||||||||
| pH7.4 100% 20 h | |||||||||
| pH-responsive carboxymethylated iota-carrageenan/chitosan NPs | Polyelectrolyte complexation method | 0.1 | 613 ± 41 | 52.5 ± 0.5 | 86.9 ± 2.6 | 10.7 ± 0.6 | pH 1.2: 5% 2 h | [157] | |
| pH: 6.8: 87% 12 h | |||||||||
| Insulin-loaded chitosan-NPs | Complex coacervation | 0.5 | 534 ± 24 | 14.57 ± 1.1 | 79.96 ± 3.96 | — | pH 2 13% 10 h | [9] | |
| pH 6.8 88% 10 h | |||||||||
| Insulin-loaded NPs | Self-gelation method | 2 | 479.6 | 22.1–31.2 | 88.6 | — | pH 1.2: 7% 4 h | [134] | |
| pH 7.4: 80 % 10 h | |||||||||
| Insulin-loaded trimethyl chitosan-fucoidan NPs | Simple PEC | 0.2 | 256.7 ± 4.9 | 26.5 ± 1.1 | 56.4 ± 4.3 | 8.6 ± 2.2 | SGF 38.3 | [158] | |
| SBF 75.4 | |||||||||
| Chitosan/lecithin liposomal nanovesicles | PECs associated with lecithin liposomes | — | 105 ± 17 | −30 | 20 | — | PBS 6.8: 80% after 20 min | [159] | |
| Insulin loaded chitosan-TPP NPs | Flash nano-complexation using a multi-inlet vortex mixer | — | 46.2 ± 2.7 | 9.4 ± 1.2 | 91.0 ± 1.7 | 27.5 ± 0.4 | pH 2.5: 55% | [131] | |
| pH 6.6: 10% | |||||||||
| pH 7:23% | |||||||||
| after 1 h | |||||||||
| Insulin-loaded chitosan nanoparticles (INS-CS-NPs) | Ionic gelation | 0.3 | 356.5 ± 43.4 | 46.5 | 78.3% | — | pH 2.5: 40% | [160] | |
| pH 6.8: 50% | |||||||||
| 2 h | |||||||||
| Chitosan-alginate (CS/ALG) NPs encapsulated in insulin | Polyelectrolyte complexation | — | 216 | +3.89 | 78.3 | — | pH 1.2: 26.7% 2 h | [161] | |
| pH 6.8: 79% 12 h | |||||||||
| pH 7.4: 84% 12 h | |||||||||
Polyglutamic acid (PGA) is commonly applied in chitosan functionalization to enhance insulin uptake by amino acid transporters and calcium-sensing receptors present in the intestinal epithelium, as reported by Urimi et al. [143]. They have formulated chitosan-PGA insulin NPs with particle size of 210 ± 2.8 nm, zeta potential of 18.1 ± 0.14 mV, entrapment efficiency of 85.9% ± 0.28%, and sustained release profile at different pH conditions. Cellular uptake analysis showed a threefold higher uptake in the Caco-2 cell line, and the blood glucose study in diabetic animals reported low levels for almost 24 h. In another study, the preparation of insulin NPs by self-gelation technique involved natural polymers like chitosan and snail mucin [134]. The resulting NPs were smooth with an average particle size of 504.1 nm, a positive surface charge at 31.2 mV, low polydispersity index (PDI), encapsulation efficiency of 92.5%, and controlled release for 8 h. The in vivo study on diabetic rats demonstrated that oral insulin NPs lowered blood glucose levels more efficiently than the free oral insulin solution.
Chitosan NPs can be prepared using various methods such as coacervation, emulsion cross-linking, ionic gelation, reverse microemulsion, and spray-drying methods. The choice of preparation method depends on various factors, such as the intended application and desired properties of the chitosan NPs [144,145]. Several formulation and process parameters should be optimized to achieve superior characteristics of the NPs that suit the intended objectives.
11.1 Coacervation
Coacervation is a separation process induced by a change in the media environment, such as ionic strength, temperature, solubility, or pH, under a controlled condition. NPs are formed due to water deficit or dehydration mechanism after liquid desolvation or salt’s addition into the reaction medium (Figure 6) [146]. For polysaccharides to develop coacervate droplets, chitosan solution must first be blown into an alkali solution such as NaOH, methanol, or ethane-di-amine using a compressed air nozzle. Then, the formed particles are centrifuged, followed by successive washing with cold and hot water before introducing the crosslinking agent for further hardening [32].
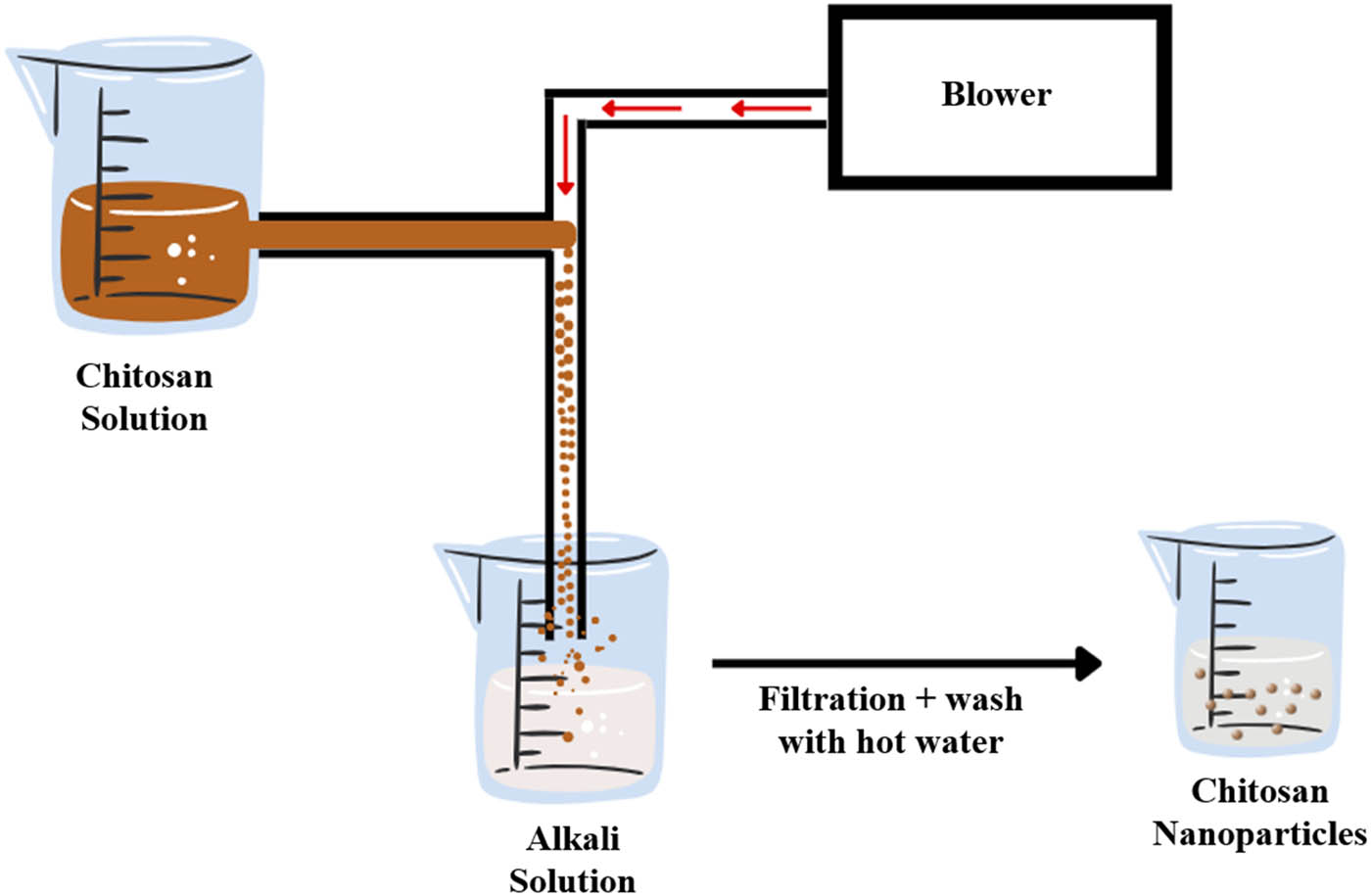
Schematic presentation of the coacervation separation process.
Oral insulin chitosan-Dz13Scr NPs were fabricated by means of a complex coacervation method [147]. The produced NPs showed enhanced stability in the presence of stomach acid, and in the presence of alkaline conditions, a biphasic release behavior of the drug was observed, with an initial burst release followed by a more regulated release. In addition, the study reported that the NPs formulation was shown a glucose uptake into in C2C12 cell line. The NPs are prepared at low temperatures using aqueous solutions; hence coacervation method is safe and appropriate for temperature-sensitive drugs, including proteins. However, some drawbacks are associated with this method, such as poor encapsulation efficiency and system instabilities [148].
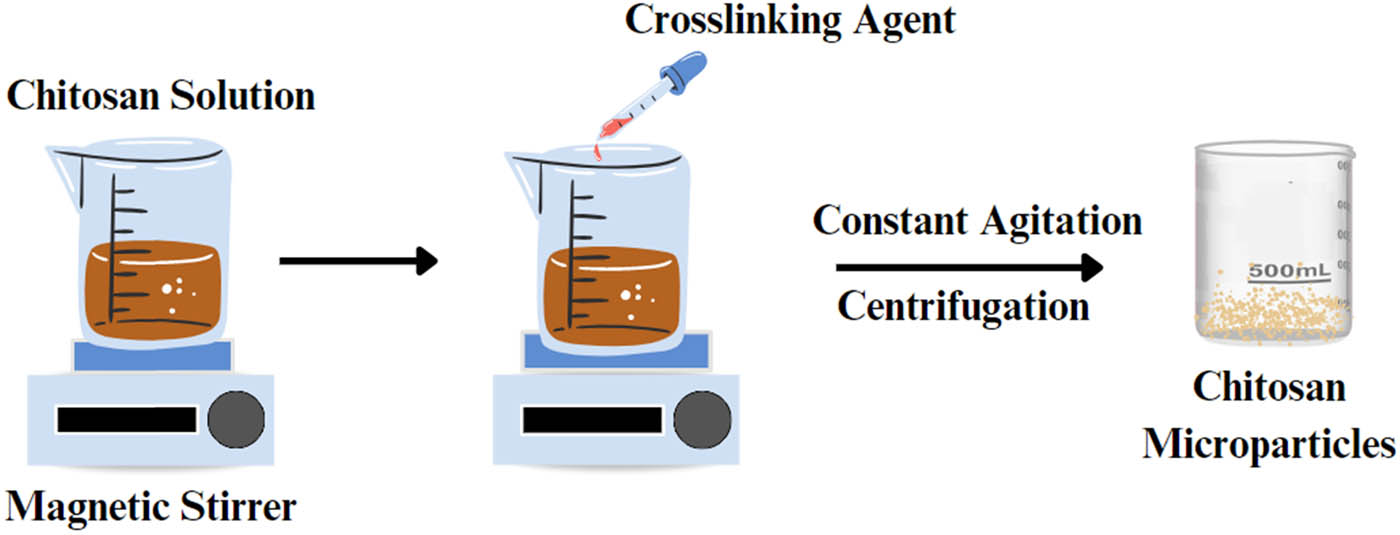
11.2 Emulsion crosslinking
It is one of the most straightforward techniques that can encapsulate liquid and solid materials to produce various particle sizes [149]. It involves crosslinking the functional groups of the coating material with the crosslinking agent. This approach entails water formation in oil emulsion (W/O) through emulsifying the coating solution in the oil phase, which can be stabilized using appropriate surfactants. Once a stable emulsion is established, a crosslinking agent such as TPP is introduced to solidify the emulsion droplets (Figure 7) [32]. This method is simple to scale up and suitable to encapsulate hydrophobic drugs, while the drawbacks include high-shear and incorporation of organic solvents and surfactants [6]. In these cross-linked emulsions, a sustained release of insulin can be achieved by varying the ratio of polymer to surfactants. For example, it was reported that a microemulsion of insulin administrated orally showed slower rate of glucose depletion but a more sustained release of insulin compared to solution and subcutaneous formulations. In another study, it was reported that insulin delivery was controlled using a various ratio of mucin to Tween® 80 in oil/water microemulsions [150].
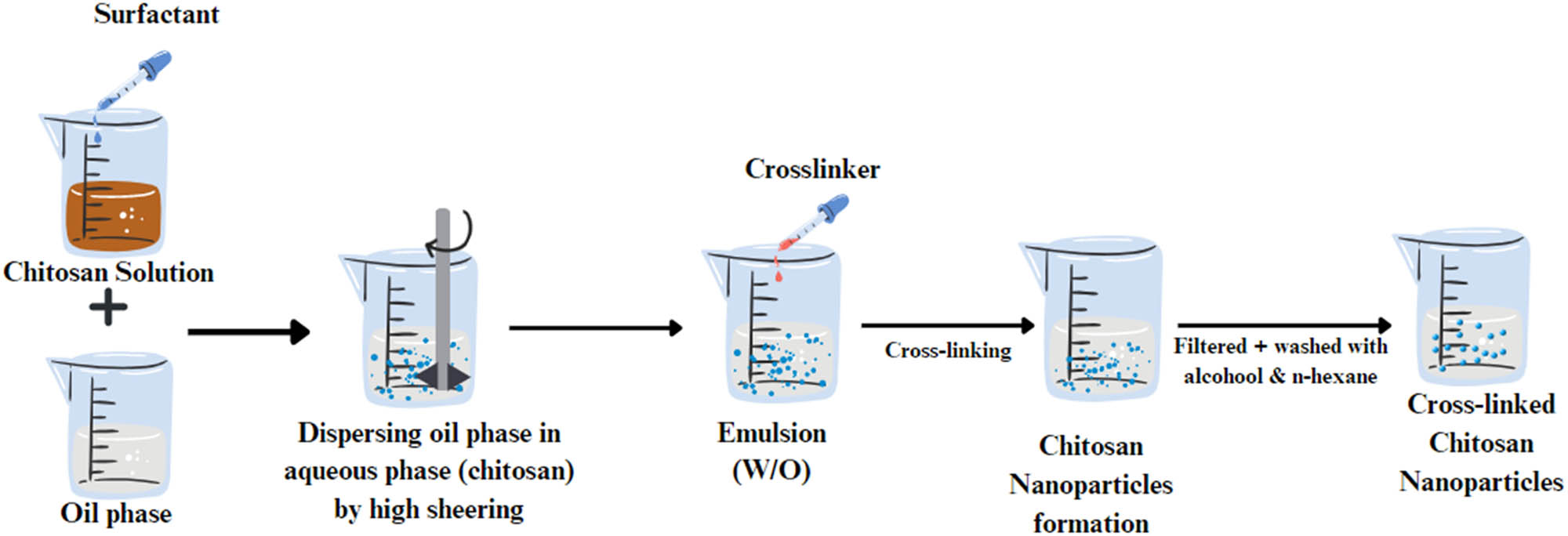
Schematic presentation of the emulsion crosslinking process.
Fonte and his team 2011 fabricated a type of solid lipid nanoparticle (SLN) containing insulin and a chitosan coating. To prepare these SLNs, they used a technique that involved creating a double emulsion of water and oil and then applying chitosan to the surface of the SLNs. The optimized formulation showed a particle size of around 450 nm with a positively charged surface. A greater extent of NPs permeation was reported after adding chitosan. The hypoglycemic effect of diabetic rats was observed for 24 h with relative pharmacological bioavailability of around 17% (oral absorption of insulin is improved by SLNs coated with chitosan) [151].
11.3 Ionic gelation
The approach of this method is based on electrostatic interaction between different charged polymers under mechanical stirring. In this method, various chitosan carriers including beads, microbeads, MPs, and NPs can be produced. This is achieved by adding a crosslinker solution, such as TPP, dropwise to a chitosan solution under constant high stirring. This process causes chitosan to undergo a gel ionization process by the electrostatic interaction between the polyanion groups of the TPP and the cationic amino groups of chitosan, resulting in NPs formation (Figure 8). Then, the prepared particles can be harvested by precipitation centrifugation techniques.
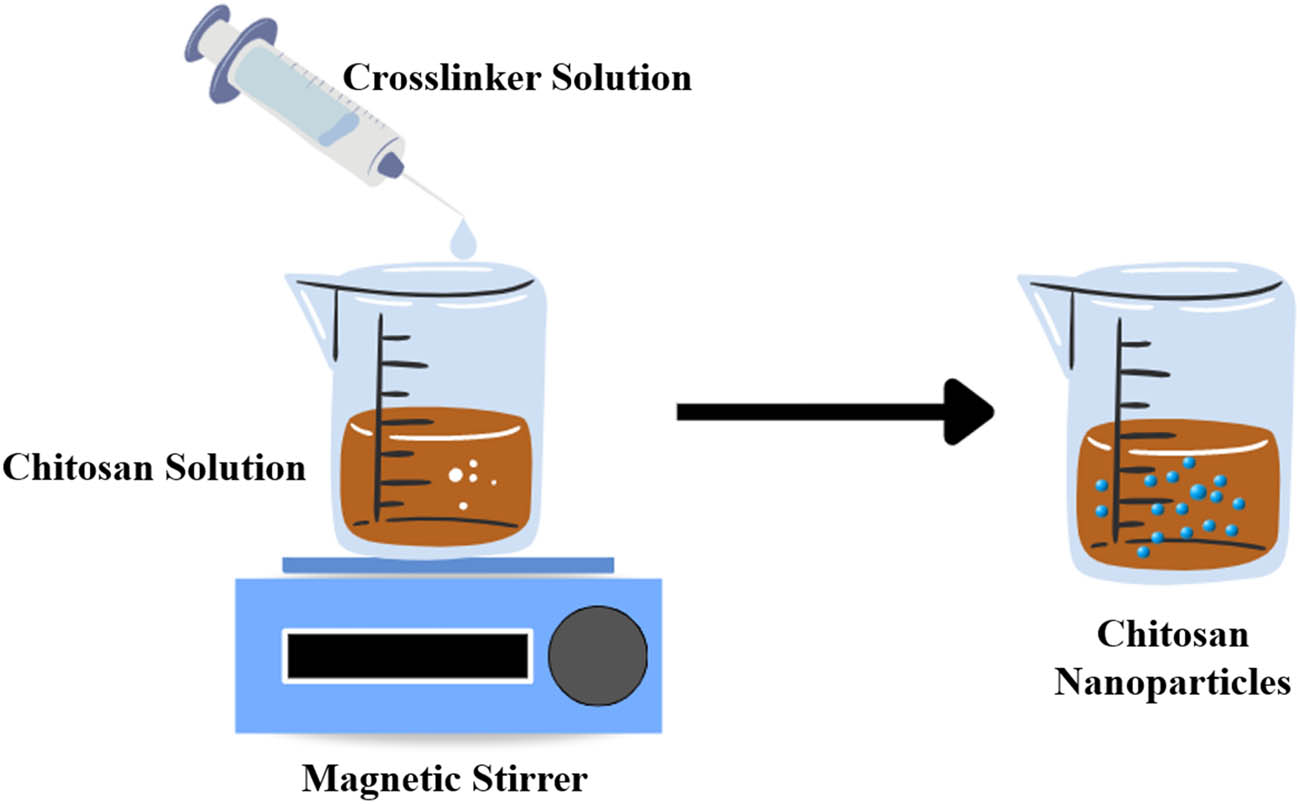
Schematic presentation of the ionic gelation process.
In the ionic crosslinking arrangement, the main types of interactions are H-links and T-links. An H-link occurs when O– and NH3+ molecules interact in the same plane. Conversely, a T-link happens when a non-binding oxygen atom and NH3+ molecules located in different planes interact with each other [152]. The polyanion (negative charge), including sulfate salts (Mg, Na, or hexadecyl-, lauryl-, octyl-, cetostearyl-, chloride salts (Cu, Zn, Ba, Ca, Mg, and Co), polyphosphate salts (pyro-, tri-, tetra-, octal-, and hexameter-), ferricyanide salts, and ferrocyanide were used as a crosslinker for the synthesis of chitosan NPs. Chitosan polymer and TPP can be seen as a very well-known pair of counter ions in this method [152]. Ionic gelation consists of a simple preparation procedure that can be carried out under mild conditions, using cheap equipment and free of organic solvents. However, the disadvantage of this method is the high PDI of the prepared NPs [6]. The synthesis of chitosan NPs is influenced by chitosan concentration, crosslinker concentration, chitosan molecular weight, CS/TPP ratio, drug concentration, pH of the preparation medium, stirring rate, and crosslinking time [152].
Mahdizadeh Barzoki et al. have developed and optimized a novel oral insulin NP delivery system using thiolated N-dimethyl ethyl chitosan (DMEC-Cys) conjugate by means of an ionic gelation method [153]. The particle size measured 148 nm with a PDI of 0.26, a zeta potential of 15.5 mV, and an encapsulation efficiency of 97.56%. The release of insulin amounted to approximately 13.2% and 11.42% in pH 6.8 and 7.4 media, respectively. El Leithy et al. have designed a system for controlling glucose levels in rats for an extended time using insulin folate-chitosan NPs [154]. The NPs were positively charged and had a particle size of 288 ± 5.11 nm, with an entrapment efficiency of 80%. In these NPs, the stability of insulin when exposed to GI enzymes was enhanced, leading to a release of less than 10% at pH 1.2 and a release of 38.92 ± 4.52% over 8 hours at pH 7.4.
In another study, Xu et al. have developed a new system for delivering insulin orally, called NiM (NPs in MPs), which addresses the challenges posed by GI barriers [69]. It comprises a three-dimensional system of biocompatible chitosan, alginate, and casein. Initially, alginate/chitosan NPs were prepared by ionic gelation and complexation method, followed by coating with casein to form NiM, which helps to maintain insulin stability and physiological activity in the stomach. The NiM was stable in GI with an entrapment efficiency of 51.1%, while the in vitro insulin release was 13.5% in the simulated gastric fluid after 2 h followed by a slow release of 57.4% in simulated intestinal fluid over 10 h [69].
12 Preparation methods of chitosan MPs
MPs have the potential to provide an extensive reservoir for drug-coated NPs to exhibit a stimulus release at the target site. In the context of targeting the colon region, an oral system employing NiM can effectively safeguard the encapsulated drug in NPs from chemical and enzymatic degradation effects during GI transit while also reducing or eliminating the burst drug release in the upper GI and maximize the release of the encapsulant by intestinal or colon’s stimuli. For example, a recent study reported Green Fluorescent Protein NPs encapsulated in alginate/chitosan MPs for colon-specific release and stomach transit [10,60]. Table 3 represents examples of insulin chitosan MP formulations.
Formulations of insulin chitosan MPs
| Formulation | Method of preparation | Chitosan concentration (% w/v) | Size (mean value ± SD) (µm) | Encapsulation efficiency (%) | Loading capacity (%) | Release profile | Ref. |
|---|---|---|---|---|---|---|---|
| PA-crosslinked chitosan microsphere | Ionic crosslinking with PA | — | 663 ± 40 | 97.1 | 7.6 ± 0.3 | pH 1.2: 33% 2 h | [163] |
| pH 6.8: 70% 4 h | |||||||
| Insulin-loaded chitosan microspheres along with polyvinyl alcohol | Spray drying | 1.0 | 1.116 ± 0.507 | — | 4.3 ± 02 | pH 6.5: 30% 2 h | [176] |
| Insulin-loaded water-soluble chitosan-MPs | Polyelectrolyte complexation | — | 0.292 | 48.28 ± 0.90 | 9.52 ± 1.34 | pH 1.2: 50% 2 h | [177] |
| pH 7.4: 50% 4 h | |||||||
| Insulin-loaded N-trimethyl chitosan MPs | Supercritical fluid-assisted atomization | — | 1.34 | 9.24 | 9.24 ± 4.7 | — | [178] |
| Insulin-loaded chitosan MPs | multiple emulsion technique | 2.0 | 37.5 ± 0.3 | 80.1 ± 0.1 | 28.0 ± 0.0 | — | [179] |
| Chitosan-pectin Np and MP | Electrostatic self-assembly | 0.5 | 0.240–1.900 | 62.0% | pH 1.2: 13% 2 h | [128] | |
| pH 6.8: 80% 2 h |
In 2020, a group of researchers applied polysaccharides to fabricate an effective system for oral delivery consisting of hydrogel MPs to control the release and enhance paracellular and transcellular insulin absorption. In their study, carboxymethyl chitosan hydrogels were grafted by carboxymethyl βcyclodextrin (CMCD-g-CMCs) using carbodiimide as a crosslinker. The insulin released in the acidic medium (SGF, pH = 1.2) was just 8% after 2 h. After changing the release medium to pH = 6.8, the rate of insulin release was significantly raised to around 55% after 2 h, while it reached 70% in the medium with pH = 7.4 after 4 h [162].
Kim et al., have prepared chitosan insulin microspheres using ionic crosslinking with phytic acid (PA). The microspheres showed a good encapsulation efficiency (97.1%), with a diameter of 663.3 μm, and the amount of insulin released in a gastric digestion medium was 67.0% after 2 h. The pharmacological bioavailability study was at 10.6%, significantly decreasing blood glucose levels [163].
12.1 Electrospray
In the electrospray process, a high voltage is applied to a solution containing the material of interest, typically a polymer or a drug. As chitosan solution is ejected through a stainless steel needle or multiple needles, the electrostatic forces cause the solution to form cone-shaped droplets, known as Taylor cones (Figure 9). As the voltage increases, the droplet's surface tension decreases until it reaches a critical point, and the droplet is ejected from the needle as a highly charged jet toward a collector. The extruded particles away from the needle are mono-positively charged and tend to homogenously distribute in the collecting solution [164]. The basic experimental setup mainly consists of a syringe pump, a metal nozzle connected to a high-voltage power source, and a collector. A transparent electrospray chamber is recommended to determine the electric field focus, temperature, humidity, and atmospheric pressure. This method is influenced mainly by the solution flow rate, applied electric voltage, and the solution properties such as viscosity, surface tension, and electrical conductivity [165]. Electrospray is a simple and low-cost technique that can control the particle size (usually this technique can produce particles with their sizes ranging from nanometers to several micrometers depending on the flow rate and the voltage applied) and yield using low amounts of solvents [166,167]. Even though it is employed to produce a variety of sizes based on the experimental set-up, MP preparation is more efficient concerning yield and time compared to smaller sizes since the flow rate and particle size are directly proportional [168].
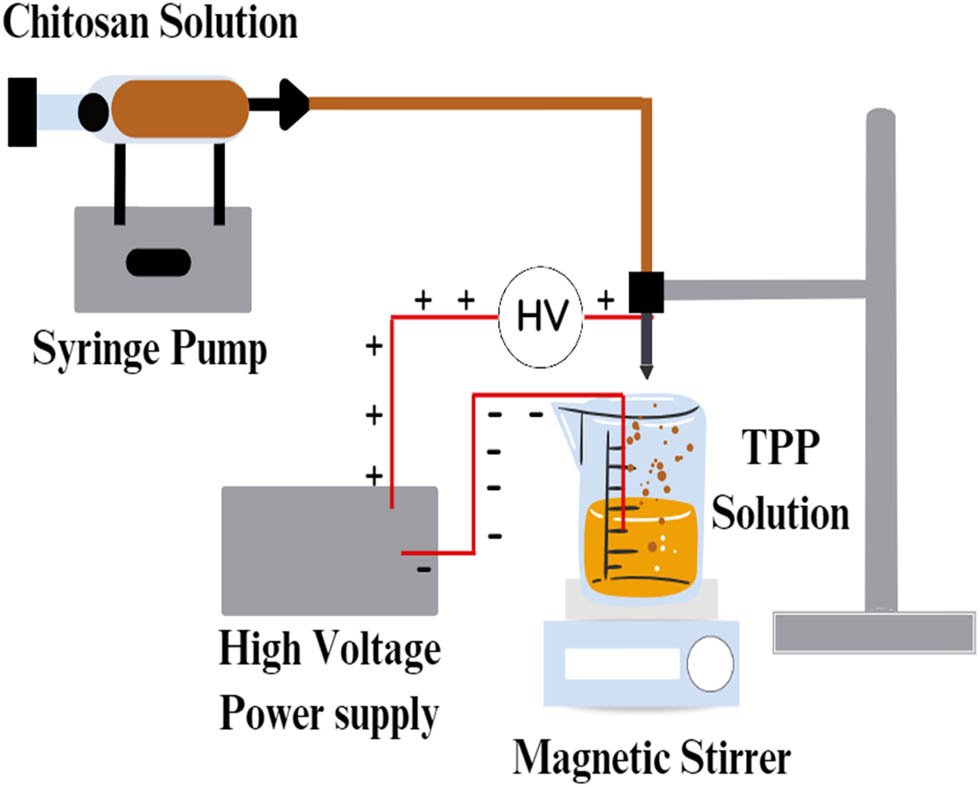
Schematic presentation of electrospray process.
12.2 Membrane emulsification
This process involves injecting a dispersed phase (aqueous) into a continuous phase (immiscible liquid or pre-emulsified mixture) through a microporous membrane that leads to the production of droplets at the continuous/membrane phase interface [169]. Chitosan solution is used as a dispersed phase, while an oil-soluble emulsifier is a constant phase. Nitrogen gas is used to press the chitosan solution through the pores of a membrane wall into the oil phase, forming a W/O emulsion with homogeneous droplet sizes with the presence of an emulsifier [170]. Solidifying the droplets can be achieved using a conventional stepwise crosslinking method with a crosslinking agent such as TPP or glutaraldehyde, as shown in Figure 10 [171]. This method easily controls the size of chitosan microspheres with a good PDI. The interfacial tension between the membrane and the dispersed phase must be high to form microspheres of uniform size [31,32].
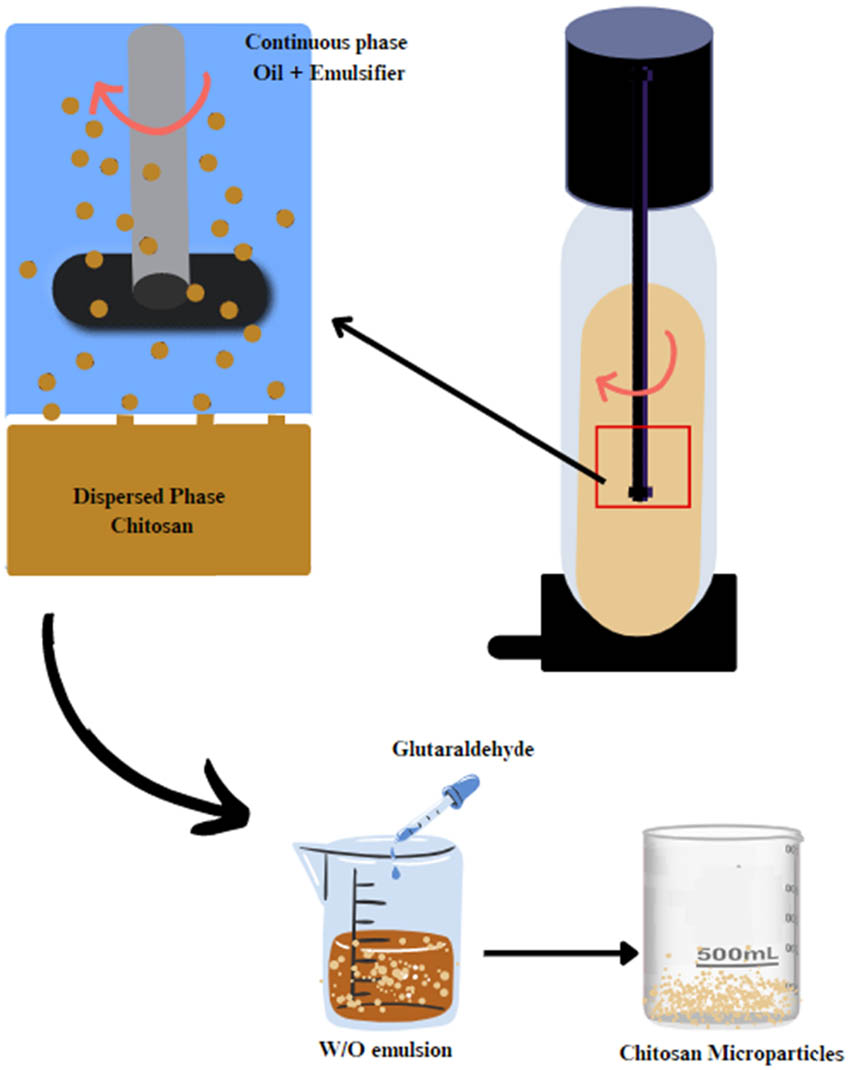
Schematic representation of the membrane emulsification process.
In a previous study, researchers used a membrane emulsification technique and Ca2+ ion and polymer (chitosan) solidification to prepare alginate-chitosan microspheres. These microspheres were then used to load insulin, where the effect of various loading methods on loading efficiency and immunological activity was examined. The findings showed that insulin-loaded microspheres prepared using the chitosan solidification process had improved loading efficiency (56.7%) and remarkable activity maintenance of insulin (99.4%). The release profile showed that 32% of the loaded insulin was released into simulated GI media. In addition, a continuous insulin release was recorded in pH conditions similar to the blood environment for 14 days. An in vivo study using diabetic rats showed a stable reduction in blood glucose levels for approximately 60 h [172].
Another study described an innovative approach for delivering insulin orally using hollow quaternized chitosan microspheres that uses the SPG membrane emulsification process and glutaraldehyde cross-linking procedure. These microspheres had uniform size, autofluorescence, and structural properties that helped maintain insulin bioactivity. In addition, the particle size was around 7.5 μm, which was small enough to be absorbed by the GIT and target the reticuloendothelial system after oral administration was achieved. Unlike conventional methods, the SPG membrane technique produced microspheres with a narrow size range and low coefficient of variation [173].
12.3 Microfluidic technology
Microfluidic technology is a device used to fabricate uniform emulsion droplets. This technique involves a delicate balance between various forces acting on the fluid flow, such as the periodic breakup of the dispersed phase into the continuous phase, mainly driven by viscous force, inertial forces, buoyancy, and interfacial forces. Conventional methods for forming emulsions typically involve droplet breakup using high-pressure homogenization and/or ultrasonication and rotary agitation, resulting in highly polydisperse droplet sizes due to the nonuniform shear stresses applied [33,34]. The preparation of MPs using the microfluidic method involves two steps, the formation of emulsion, where the polymeric or monomer fluid emulsifies to form the emulsion droplets in the channels, and the polymerization or curing of the droplets in situ to obtain microspheres (Figure 11). The curing methods generally involve freezing, solvent evaporation, and polymerization [165]. Compared to other methods, the microfluidic method produces MPs with a unified size and narrow size distribution, good process repeatability, and a more stable yield. However, microspheres produced by microfluidics are compact, with a relatively small specific surface area and slow adsorption rate, resulting in a low adsorption capacity [168]. Moreover, other microfluidic limitations include low flow rate, low yield, and clogging of channels due to the adhesion of small particles [169].
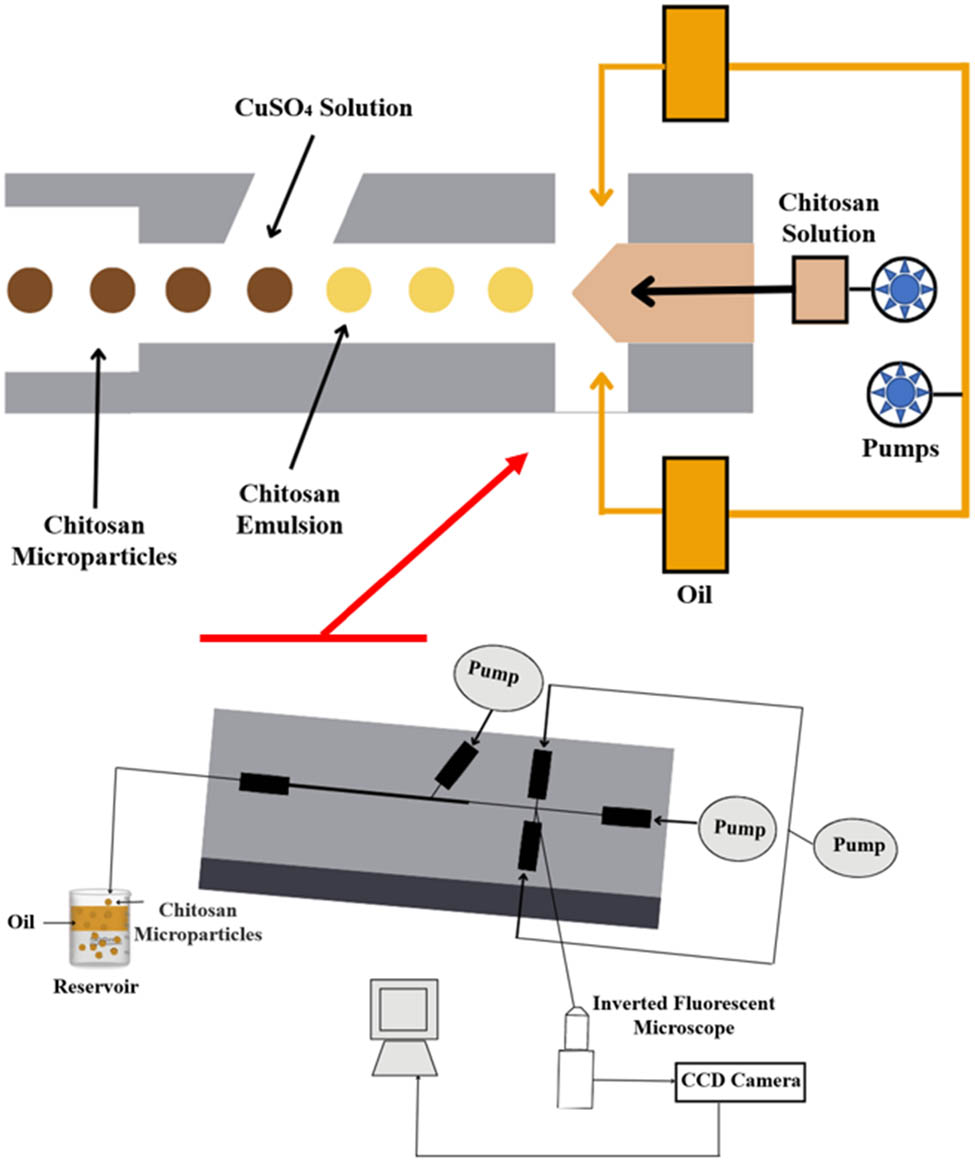
Schematic representation of the microfluidic method.
12.4 Reverse micellar method
Reverse micelles are droplets of an aqueous phase stabilized through a surface-active agent (surfactant) in an organic phase. They are formed when organic and aqueous phases are mixed in the presence of a surfactant (Figure 12). The efficiency of the approach and the stability of the prepared reverse micelles mainly depends on the processing parameters, the physicochemical properties, and the ratios of the components and surfactants used [170]. Employing chitosan with low molecular weight is superior for controlling MP size and size distribution. However, the main limitation of this method is the usage of organic solvents and the collection of the MPs [171].
Schematic representation of the reverse micellar method.
12.5 Sieving
In the sieving method, a chitosan solution is pre-crosslinked and then pushed under a certain pressure to pass through a sieve with a suitable mesh size to form chitosan MPs (Figure 13). The homogeneous pore size of the sieve produces MPs with a very narrow size distribution. To remove any unreacted excess glutaraldehyde (crosslinker), particles need to be washed with NaOH and dried in an oven at 40°C overnight [32]. This method is characterized by easy scalability and preparation, but the MPs produced possess irregular shapes [31].
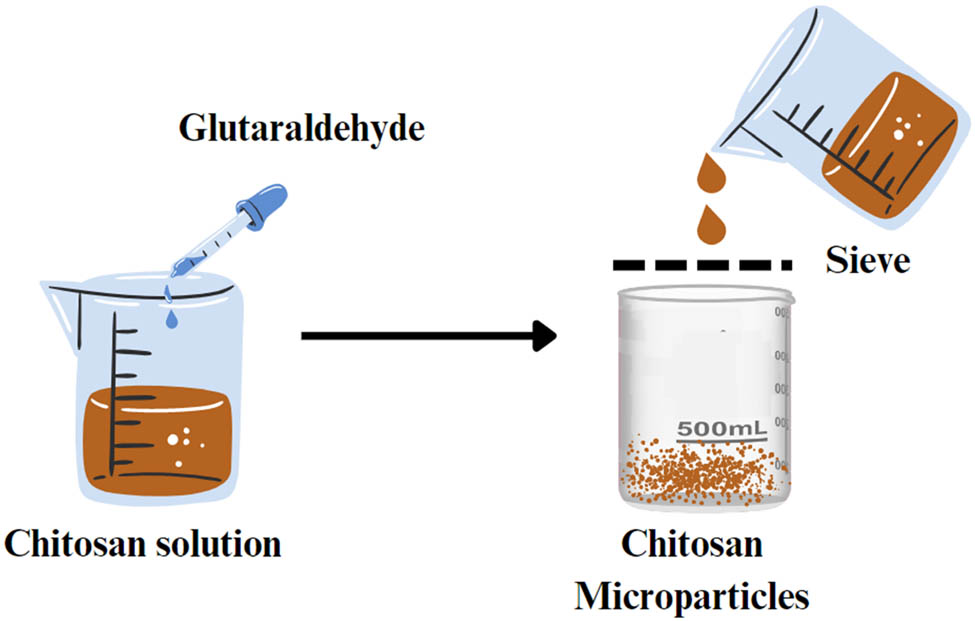
Schematic representation of the sieving method.
12.6 Spray drying
Spray drying is a common method for producing granules, agglomerates, or even powder from a mixture of excipients and APIs. The method involves spraying suspensions or solutions into a closed chamber through a nozzle. The resulting atomized droplets undergo rapid evaporation drying using a hot air flow at constant pressure and temperature. The final dried particles are separated from the drying gas and collected in a collection vessel (Figure 14) [32]. According to Shehata and Ibrahima, this method can produce spherical-shaped spray-dried particles with low values of the angle of repose, which leads to good flowability during capsule filling or tablet compression. However, a loss in yield was reported due to powder sticking on the scraper and electrode in the cyclone and the collection chamber [174]. Insulin-chitosan MPs- or NPs can be produced through this method by optimizing the processing parameters such as nozzle size, inlet and outlet temperature, feed flow rate, the concentration of the insulin-chitosan solution, air flow rate, and atomizer [175]. Table 3 summarizes chitosan MPs prepared using different methods and techniques for OID.
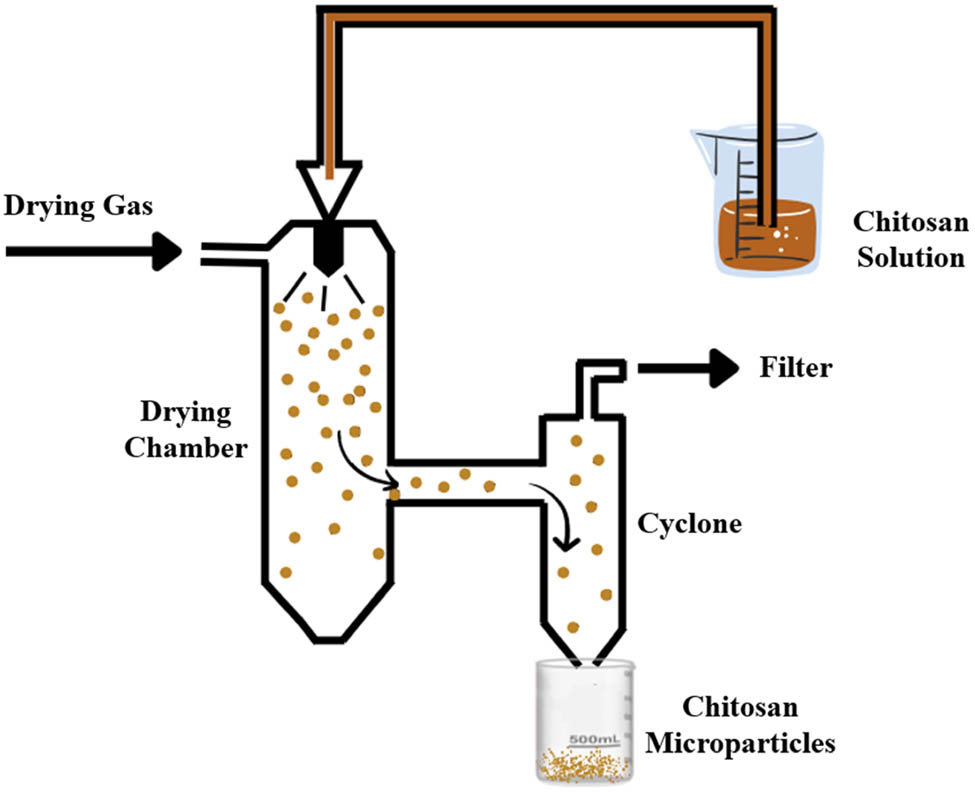
Schematic representation of spray drying method.
13 Preparation methods of chitosan beads
Chitosan hydrogel beads are three-dimensional networks of chitosan chains crosslinked with different chemical agents or by physical means. These beads have drawn much interest in drug delivery because of their unique qualities, including biocompatibility, biodegradability, and the capacity to expand and release medications under controlled conditions [180]. Chitosan hydrogel beads can absorb much water and swell in aqueous environments. Several parameters, such as temperature, pH, and degree of cross-linking, influence the swelling behavior of these beads. The swelling capacity of chitosan hydrogel beads can be modulated by altering the degree of crosslinking, and it is generally inversely proportional to the degree of cross-linking.
Furthermore, the swelling behavior of chitosan hydrogel beads depends on the surrounding media's pH, where the beads swell more at higher pH values [181]. Chitosan beads have been widely used in drug delivery applications due to their ability to control drug release. The drug release behavior from chitosan hydrogel beads is determined by several factors, including the degree of crosslinking, size and shape of the beads, drug solubility, and pH of the release medium [182]. Several parameters of the chitosan hydrogel bead preparation process can affect the physicochemical properties of the beads, including chitosan concentration, crosslinking agent concentration, pH of the reaction medium, and reaction time. The choice of these parameters can affect the degree of crosslinking, the size and morphology of the beads, and their swelling and drug-release properties. Therefore, optimizing these parameters is essential for obtaining chitosan hydrogel beads with desirable properties for specific applications [183].
13.1 Ionic gelation for beads preparation
Ionic gelation is commonly used to form beads where chitosan is dissolved in an acidic solution to form a polycationic solution. Then, the chitosan solution is dropped into an alkaline coagulating solvent that instantaneously forms spherical beads (Figure 15). However, chitosan beads' mechanical strength and acid resistance properties usually require optimization to meet specific purposes. In order to enhance the mechanical stability and adsorption capacity of the beads, cross-linking agents like glutaraldehyde, glycol diglycidyl, and epichlorohydrin can be used [184].
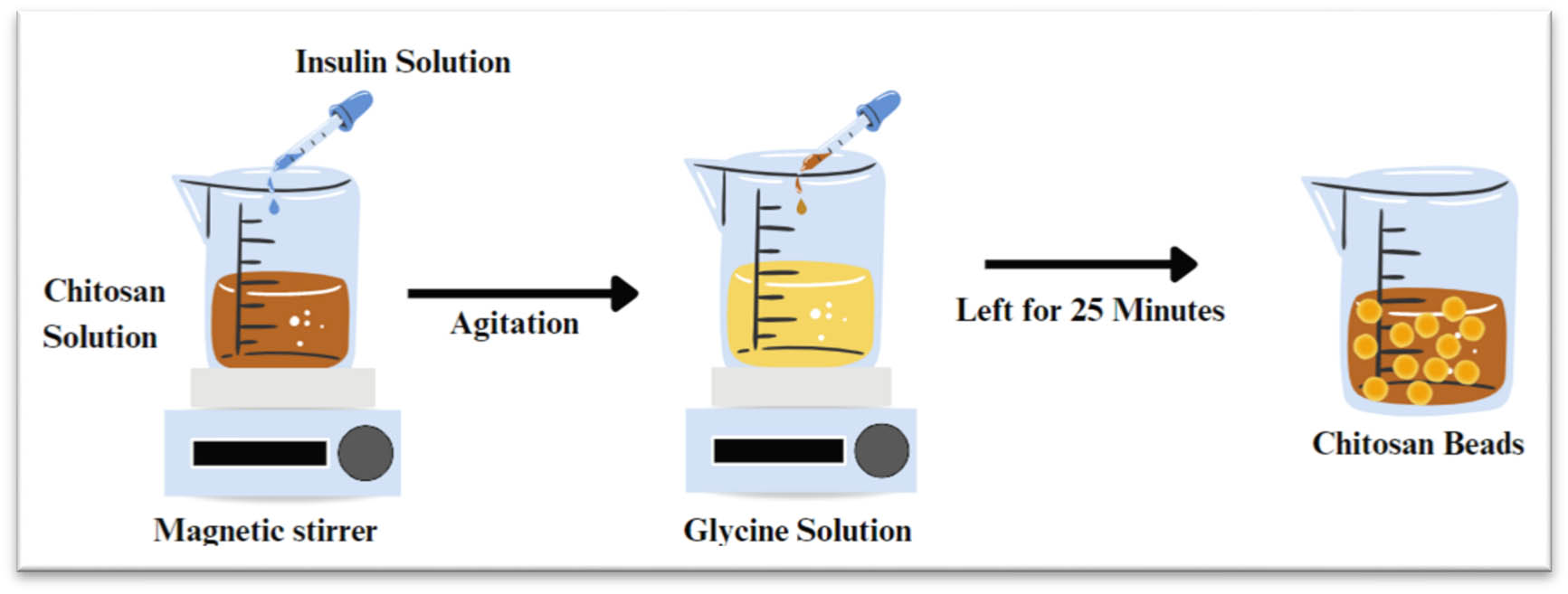
Schematic representation of ionic gelation method.
In a study conducted by Kofuji et al. insulin chitosan gel beads using the ionic gelation method were reported by dropping insulin suspension into chitosan solution with agitation. Then, the mixed solution was dropped slowly into an aqueous glycine solution and left under gentle stirring for 25 min at room temperature, and the beads were then formed spontaneously [185]. Ionic gelation in producing beads or microbeads is considered a preferred method due to its lower cost, flexibility in producing particles with various ranges of size, high to medium drug encapsulation efficiency, good stability, and controlled drug release. However, it shows limitations in the concentration range of the polymer [186].
13.2 Simultaneous crosslinking
Simultaneous crosslinking is a one-step method for preparing chitosan beads by covalent cross-linking and precipitation or solubilization in an alkaline aqueous medium (Figure 16) [187]. This method can prepare chitosan beads or hydrogels due to the cross-linked hydrophilic polymer nature and can absorb large amounts of biological fluids or water. Crosslinking between polymer chains provides benefits such as increased elasticity, polymer insolubility, reduced viscosity, increased strength, and reduced melting point [187,188]. However, the main concern with this method is its lower reactivity due to many functional groups being buried in the polymer matrix [189]. A study conducted by Barreiro-Iglesias et al. found that chitosan beads can be prepared by mixing the solutions of chitosan and glutaraldehyde (as a condensing agent) in purified water for a few seconds and then adding them dropwise to NaOH solution at 20 or 37°C [187].
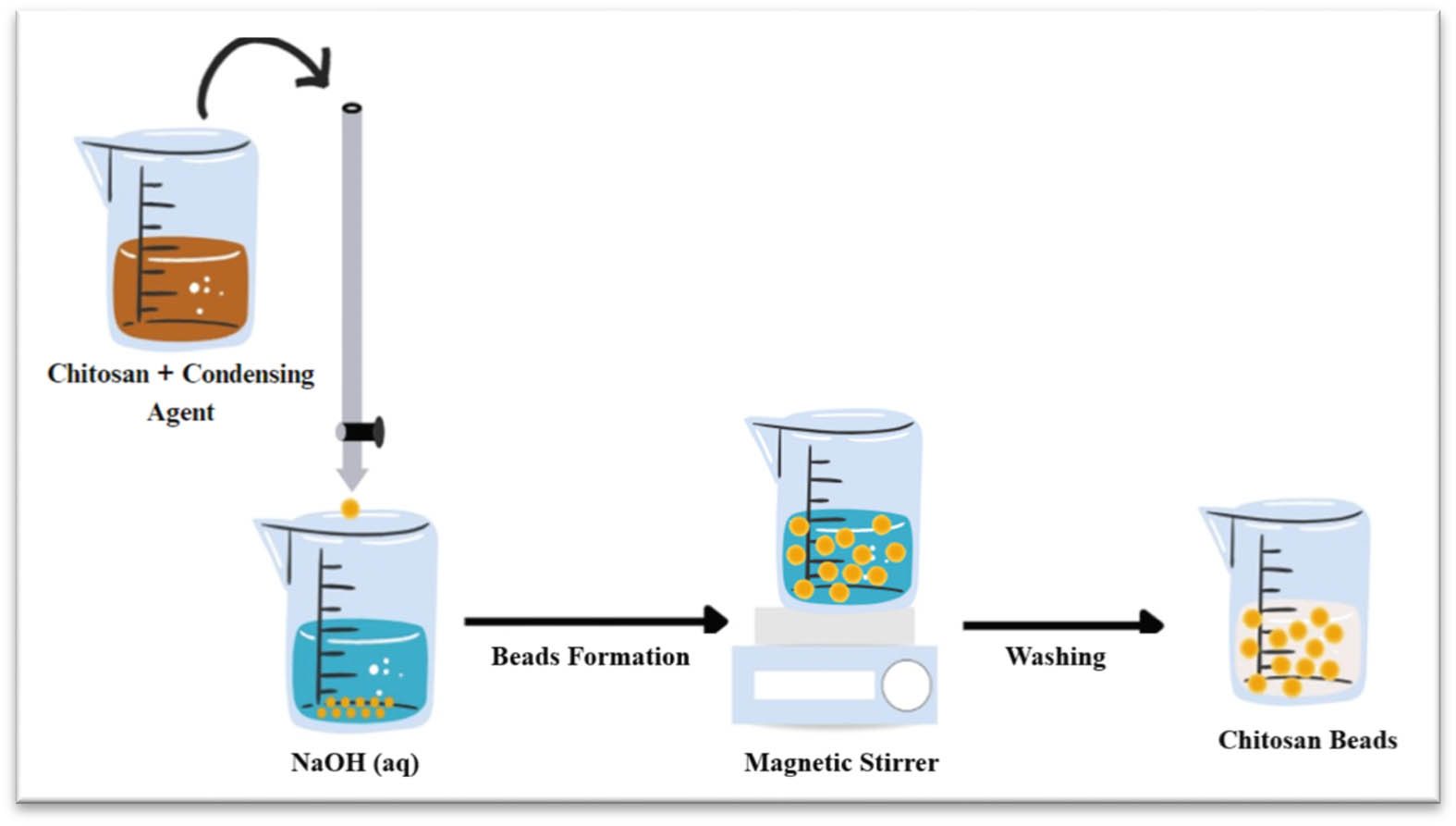
Schematic representation of simultaneous crosslinking method.
14 Chitosan as coating material for colon insulin delivery
Chitosan has been widely used as a surface coating and particle-forming polymer. This coating was widely employed with many types of NPs like lipid NPs, metal-based NPs, and polymeric NPs. It is used for mucoadhesive enhancement, tissue penetration property, drug release, bioavailability improvement of drug-loaded, and stability control of chitosan-coated drugs [190]. In some studies, chitosan and chitosan derivatives were employed as coating materials to protect insulin NPs by increasing the residence time of NPs in intestinal mucosa and avoiding premature insulin release in the gastric medium [191]. Other than being covalently attached to chitosan, the drugs are also dispersed in the chitosan matrix, released by controlled and slow diffusion through/from the chitosan. The enzymatic action of colonic microflora enables chitosan to release the entrapped drug, specifically within the colon, like, β-glycosidase while retaining the matrix integrity in the upper GIT [192].
15 Future perspectives of chitosan-based formulations
The challenges associated with OID have provided a significant opportunity for research initiatives. Addressing issues such as the acidic environment of the stomach, enzymatic degradation, and the mucus barrier in the GIT has been a substantial point of investigation. Chitosan, as a polysaccharide polymer, exhibits notable characteristics including biocompatibility, biodegradability, and low toxicity. These attributes make chitosan as a promising material for the development of formulations aimed at overcoming challenges linked to OID. As research advances, the exploration of chitosan-based formulations continues to hold potential for transforming oral insulin drug delivery systems. Thus, chitosan-based formulations emerge as promising approaches for OID.
Insulin has been formulated for oral delivery in various chitosan-based formulations utilizing microencapsulation approaches, including NPs, MPs, and relatively large matrices such as beads. Microencapsulation, employing chitosan as a wall material, has resulted in a promising OID and colon-targeting system. Future perspectives of chitosan-based formulations for OID may focus on enhancing its bioavailability and increasing protection in the GIT. Achieving these objectives could involve physical and chemical modifications of the wall material, combining chitosan with other functional polymers, or refining the fabrication methods. Furthermore, formulating complex systems, such as microencapsulating chitosan-insulin NPs/MPs within larger delivery systems, holds potential for overcoming challenges related to insulin bioavailability and achieving a successful controlled release profile. This approach requires further exploration through biological studies, followed by subsequent clinical trials, to comprehensively assess its efficacy and safety in practical applications.
16 Conclusion
The current insulin administration route has several limitations, including the need for frequent injections, which can lead to poor patient compliance. OID represents an attractive alternative to current methods, but various barriers limit its success. Chitosan-based polymers are a promising carrier matrix for OID due to biocompatibility, biodegradability, and low toxicity. Physical and chemical modifications of chitosan can further improve its efficacy in insulin delivery by increasing its solubility, mucosal permeability, and improving insulin protection in the GIT. Modification techniques such as thiolation, substitution, conjugation, and grafting can further enhance the ability of chitosan to protect insulin and promote its release. Various chitosan carriers, such as NPs, MPs, and beads, have been developed to enhance OID, and many of them exhibited promising results for oral colon delivery. These carriers have shown great potential in overcoming the challenges of oral insulin delivery, including acidity, enzymatic degradation, and mucus barrier and attempted to improve colon insulin absorption. Numerous preparation methods, including coacervation, emulsion crosslinking, ionic gelation, electrospray, and spray drying, can produce all sorts of chitosan carriers for drug delivery with enhanced physicochemical properties. While there are still challenges in improving oral insulin bioavailability with a controlled insulin release profile, chitosan-based carriers have shown promising results in preclinical studies and represent a promising avenue for developing an OID system.
Acknowledgments
The authors wish to thank Universiti Sultan Zainal Abidin and University of Birmingham for the support.
-
Funding information: This research was funded by the Malaysian Ministry of Higher Education (MOHE) Fundamental Research Grant Scheme (FRGS), grant number FRGS/1/2022/SKK16/UNISZA/03/2, project code RR451.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Mukhtar Y, Yunusa U, A. M. G. A modern overview on diabetes mellitus: A chronic endocrine disorder. Eur J Biol. 2019 Oct;5.10.47672/ejb.409Search in Google Scholar
[2] Egan AM, Dinneen SF. What is diabetes? Medicine. 2019;47(1):1–4.10.1016/j.mpmed.2018.10.002Search in Google Scholar
[3] Enrique B, Zangen D, Abedrahim W, Katz J. Type 1 diabetes mellitus (juvenile diabetes) - A review for the pediatric oral health provider. J Clin Pediatr Dent. 2019;43(6):417–23.10.17796/1053-4625-43.6.10Search in Google Scholar PubMed
[4] Scholtz S, Becker M, MacMorris L, Langenbucher A. Diabetes. A new life. Curiosities in medicine [Internet]; 2023. p. 65–8. [cited 2023 May 4]. https://link.springer.com/chapter/10.1007/978-3-031-14002-0_18.10.1007/978-3-031-14002-0_18Search in Google Scholar
[5] Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011 Dec 1;94(3):311–21.10.1016/j.diabres.2011.10.029Search in Google Scholar PubMed
[6] Seyam S, Nordin NA, Alfatama M. Recent progress of chitosan and chitosan derivatives-based nanoparticles : Pharmaceutical perspectives of oral insulin delivery. Pharmaceuticals. 2020;13(10):307. 10.3390/ph13100307.Search in Google Scholar PubMed PubMed Central
[7] Fonte P, Araújo F, Silva C, Pereira C, Reis S, Santos HA, et al. Polymer-based nanoparticles for oral insulin delivery: Revisited approaches. Biotechnol Adv. 2015 Nov 1;33(6):1342–54.10.1016/j.biotechadv.2015.02.010Search in Google Scholar PubMed
[8] Barbosa FC, da Silva MC, da Silva HN, Albuquerque D, Gomes AAR, Silva SM, et al. Progress in the development of chitosan based insulin delivery systems: A systematic literature review. Polymers. 2020 Oct 27;12(11):2499. [cited 2023 May 4] https://www.mdpi.com/2073-4360/12/11/2499/htm.10.3390/polym12112499Search in Google Scholar PubMed PubMed Central
[9] Wong CY, Al-Salami H, Dass CR. Fabrication techniques for the preparation of orally administered insulin nanoparticles. J Drug Target. 2020 Sep 2;1:1–46. [cited 2020 Oct 28] https://www.tandfonline.com/doi/abs/10.1080/1061186X.2020.1817042.Search in Google Scholar
[10] Wong CY, Al-Salami H, Dass CR. Microparticles, microcapsules and microspheres: A review of recent developments and prospects for oral delivery of insulin. Int J Pharm. 2018 Feb;537(1–2):223–44.10.1016/j.ijpharm.2017.12.036Search in Google Scholar PubMed
[11] Zhang Z, Zhang R, Zou L, McClements DJ. Protein encapsulation in alginate hydrogel beads: Effect of pH on microgel stability, protein retention and protein release. Food Hydrocoll. 2016 Jul 1;58:308–15.10.1016/j.foodhyd.2016.03.015Search in Google Scholar
[12] Czuba E, Diop M, Mura C, Schaschkow A, Langlois A, Bietiger W, et al. Oral insulin delivery, the challenge to increase insulin bioavailability: Influence of surface charge in nanoparticle system. Int J Pharm. 2018;542(1–2):47–55. 10.1016/j.ijpharm.2018.02.045.Search in Google Scholar PubMed
[13] Sorasitthiyanukarn FN, Muangnoi C, Rojsitthisak P, Rojsitthisak P. Chitosan-alginate nanoparticles as effective oral carriers to improve the stability, bioavailability, and cytotoxicity of curcumin diethyl disuccinate. Carbohydr Polym. 2021 Mar 15;256:117426.10.1016/j.carbpol.2020.117426Search in Google Scholar PubMed
[14] Muñoz Ruiz GA, Fabio H, Corrales Z. Chitosan, chitosan derivatives and their biomedical applications. In: Biological activities and application of marine polysaccharides [Internet]. London, United Kingdom: IntechOpen; 2017. [cited 2022 May 17] https://www.intechopen.com/chapters/53455.10.5772/66527Search in Google Scholar
[15] Al Rubeaan K, Rafiullah M, Jayavanth S. Oral insulin delivery systems using chitosan-based formulation: A review. Expert Opin Drug Deliv. 2016 Feb;13(2):223–37.10.1517/17425247.2016.1107543Search in Google Scholar PubMed
[16] Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. 2017;66(2):241–55.10.2337/db16-0806Search in Google Scholar PubMed PubMed Central
[17] Katsarou A, Gudbjörnsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, et al. Type 1 diabetes mellitus. Nat Rev Dis Primers. 2017;3(1):1–17.10.1038/nrdp.2017.16Search in Google Scholar PubMed
[18] Wong CY, Martinez J, Dass CR. Oral delivery of insulin for treatment of diabetes: status quo, challenges and opportunities. J Pharm Pharmacology. 2016;68:1093–108.10.1111/jphp.12607Search in Google Scholar PubMed
[19] Acharjee S, Ghosh B, Al-Dhubiab BE, Nair AB. Understanding type 1 diabetes: etiology and models. Can J Diabetes. 2013;37(4):269–76.10.1016/j.jcjd.2013.05.001Search in Google Scholar PubMed
[20] Chellappan DK, Sivam NS, Teoh KX, Leong WP, Fui TZ, Chooi K, et al. Gene therapy and type 1 diabetes mellitus. Biomed Pharmacother. 2018;108:1188–200.10.1016/j.biopha.2018.09.138Search in Google Scholar PubMed
[21] Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21(17):6275.10.3390/ijms21176275Search in Google Scholar PubMed PubMed Central
[22] Song M, Wang H, Chen K, Zhang S, Yu L, Elshazly EH, et al. Oral insulin delivery by carboxymethyl-β-cyclodextrin-grafted chitosan nanoparticles for improving diabetic treatment. Artif Cell Nanomed Biotechnol. 2018 Nov 12;46(sup 3):S774–82 [cited 2023 Apr 29] https://pubmed.ncbi.nlm.nih.gov/30280608/.10.1080/21691401.2018.1511575Search in Google Scholar PubMed
[23] Goyal R, Jialal I. Type 2 diabetes. Vol. 3; 2023. p. 8–17. StatPearls. http://www.ncbi.nlm.nih.gov/pubmed/29219149.Search in Google Scholar
[24] Pivari F, Mingione A, Brasacchio C, Soldati L. Curcumin and type 2 diabetes mellitus: Prevention and treatment. Nutrients. 2019;11:1837.10.3390/nu11081837Search in Google Scholar PubMed PubMed Central
[25] Goetzman ES, Gong Z, Schiff M, Wang Y, Muzumdar RH. Metabolic pathways at the crossroads of diabetes and inborn errors. J Inherit Metab Dis. 2018 Jan 26;41(1):5–17. http://doi.wiley.com/10.1007/s10545-017-0091-x.10.1007/s10545-017-0091-xSearch in Google Scholar PubMed PubMed Central
[26] Xie J, Li A, Li J. Advances in pH-sensitive polymers for smart insulin delivery. Macromol Rapid Commun. 2017;38(23):1–14.10.1002/marc.201700413Search in Google Scholar PubMed
[27] Vladisavljević GT. Structured microparticles with tailored properties produced by membrane emulsification. Adv Colloid Interface Sci. 2015 Nov;225:53–87.10.1016/j.cis.2015.07.013Search in Google Scholar PubMed
[28] Niu R, Yang Y, Wang S, Zhou X, Luo S, Zhang C, et al. Chitosan microparticle-based immunoaffinity chromatography supports prepared by membrane emulsification technique: Characterization and application. Int J Biol Macromol. 2019 Jun 15;131:1147–54.10.1016/j.ijbiomac.2019.04.064Search in Google Scholar PubMed
[29] Gabriel Paulraj M, Ignacimuthu S, Gandhi MR, Shajahan A, Ganesan P, Packiam SM, et al. Comparative studies of tripolyphosphate and glutaraldehyde cross-linked chitosan-botanical pesticide nanoparticles and their agricultural applications. Int J Biol Macromol. 2017 Nov 1;104:1813–9.10.1016/j.ijbiomac.2017.06.043Search in Google Scholar PubMed
[30] Wang LY, Ma GH, Su ZG. Preparation of uniform sized chitosan microspheres by membrane emulsification technique and application as a carrier of protein drug. J Controlled Rel. 2005 Aug;106(1–2):62–75.10.1016/j.jconrel.2005.04.005Search in Google Scholar PubMed
[31] Mitra A, Dey B. Chitosan microspheres in novel drug delivery systems. Indian J Pharm Sci. 2011 Jul;73(4):355 [cited 2022 Oct 26] /pmc/articles/PMC3374549/.Search in Google Scholar
[32] Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J Controlled Rel. 2004 Nov;100(1):5–28.10.1016/j.jconrel.2004.08.010Search in Google Scholar PubMed
[33] Lee TY, Choi TM, Shim TS, Frijns RAM, Kim SH. Microfluidic production of multiple emulsions and functional microcapsules. Lab Chip. 2016;16(18):3415–40.10.1039/C6LC00809GSearch in Google Scholar PubMed
[34] Seiffert S. Functional microgels tailored by droplet‐based microfluidics. Macromol Rapid Commun. 2011;32(20):1600–9.10.1002/marc.201100342Search in Google Scholar PubMed
[35] Sunny A, Mateti UV, Kellarai A, Shetty S, Rafikahmed SR, Sirimalla S, et al. Knowledge, attitude, and practice on insulin administration among diabetic patients and their caregivers–Cross-sectional study. Clin Epidemiol Glob Health. 2021;12:100860.10.1016/j.cegh.2021.100860Search in Google Scholar
[36] Sharma M, Sharma R, Jain DK, Saraf A. Enhancement of oral bioavailability of poorly water soluble carvedilol by chitosan nanoparticles: Optimization and pharmacokinetic study. Int J Biol Macromol. 2019;135:246–60.10.1016/j.ijbiomac.2019.05.162Search in Google Scholar PubMed
[37] Ghadiri M, Young PM, Traini D. Strategies to enhance drug absorption via nasal and pulmonary routes. Pharmaceutics. 2019 Mar;11(3):113.10.3390/pharmaceutics11030113Search in Google Scholar PubMed PubMed Central
[38] Kaneko K, Osman N, Carini V, Scagnetti G, Saleem I. Overview of the advantages and disadvantages of different mucosal sites for the delivery of nanoparticles. Mucosal Delivery Drugs Biologics Nanopart. 2020;41:61.10.1007/978-3-030-35910-2_3Search in Google Scholar
[39] Bahman F, Greish K, Taurin S. Nanotechnology in insulin delivery for management of diabetes. Pharm Nanotechnol. 2019;7(2):113–28.10.2174/2211738507666190321110721Search in Google Scholar PubMed
[40] Zaric BL, Obradovic M, Sudar-Milovanovic E, Nedeljkovic J, Lazic V, Isenovic ER. Drug delivery systems for diabetes treatment. Curr Pharm Des. 2019;25(2):166–73.10.2174/1381612825666190306153838Search in Google Scholar PubMed
[41] Zhang Y, Yu J, Kahkoska AR, Wang J, Buse JB, Gu Z. Advances in transdermal insulin delivery. Adv Drug Deliv Rev. 2019;139:51–70.10.1016/j.addr.2018.12.006Search in Google Scholar PubMed PubMed Central
[42] Yang Y, Guo Y, Xu Y, Meng Y, Zhang X, Xia X, et al. Factors affecting the buccal delivery of deformable nanovesicles based on insulin–phospholipid complex: An in vivo investigation. Drug Deliv. 2020;27(1):900–8.10.1080/10717544.2020.1778814Search in Google Scholar PubMed PubMed Central
[43] Heinemann L, Jacques Y. Oral insulin and buccal insulin: A critical reappraisal. J Diabetes Sci Technol. 2009 May 1;3(3):568–84.10.1177/193229680900300323Search in Google Scholar PubMed PubMed Central
[44] Gregory JM, Cherrington AD, Moore DJ. The peripheral peril: Injected insulin induces insulin insensitivity in type 1 diabetes. Diabetes. 2020 May 1;69(5):837–47.10.2337/dbi19-0026Search in Google Scholar PubMed PubMed Central
[45] Lee SH, Back SY, Song JG, Han HK. Enhanced oral delivery of insulin via the colon-targeted nanocomposite system of organoclay/glycol chitosan/Eudragit®S100. J Nanobiotechnol. 2020;18(1):104.10.1186/s12951-020-00662-xSearch in Google Scholar PubMed PubMed Central
[46] Macedo A, Filipe P, Thomé NG, Vieira J, Oliveira C, Teodósio C, et al. A brief overview of the oral delivery of insulin as an alternative to the parenteral delivery. Curr Mol Med. 2020 Jan 21;20(2):134–43.10.2174/1566524019666191010095522Search in Google Scholar PubMed
[47] Gedawy A, Martinez J, Al-Salami H, Dass CR. Oral insulin delivery: Existing barriers and current counter-strategies. J Pharm Pharmacology. 2018;70(2):197–213.10.1111/jphp.12852Search in Google Scholar PubMed
[48] Homayun B, Lin X, Choi HJ. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics. 2019 Mar;11:129.10.3390/pharmaceutics11030129Search in Google Scholar PubMed PubMed Central
[49] Fonte P, Araújo F, Reis S, Sarmento B. Oral insulin delivery: How far are we? J Diabetes Sci Technol. 2013 Mar;7(2):520–31, http://journals.sagepub.com/doi/10.1177/193229681300700228.10.1177/193229681300700228Search in Google Scholar PubMed PubMed Central
[50] Alejandro Juárez B, Fleischmann-de la Parra P, Rayo-Mercado KTE, Ponce-Lopez T. Comparison between insulin delivery methods: subcutaneous, inhaled, oral, and buccal. Proc Sci Res Univ Anáhuac Multidiscip J Healthc. 2021 Jul 22;1(1):62–71, https://revistas.anahuac.mx/psrua/article/view/614.10.36105/psrua.2021v1n1.08Search in Google Scholar
[51] Xiao Y, Tang Z, Wang J, Liu C, Kong N, Farokhzad OC, et al. Oral insulin delivery platforms: Strategies to address the biological barriers. Angew Chem Int Ed. 2020;59(45):19787–95.10.1002/anie.202008879Search in Google Scholar PubMed
[52] Liu L, Yao WD, Rao YF, Lu XY, Gao JQ. pH-responsive carriers for oral drug delivery: Challenges and opportunities of current platforms. 2017 Feb;24(1):569–81. 101080/1071754420171279238 Search in Google Scholar
[53] Hu Q, Luo Y. Recent advances of polysaccharide-based nanoparticles for oral insulin delivery. Int J Biol Macromol. 2018;120:775–82.10.1016/j.ijbiomac.2018.08.152Search in Google Scholar PubMed
[54] Zhu Q, Chen Z, Paul PK, Lu Y, Wu W, Qi J. Oral delivery of proteins and peptides: Challenges, status quo and future perspectives. Acta Pharm Sin B. 2021:11:2416–48.10.1016/j.apsb.2021.04.001Search in Google Scholar PubMed PubMed Central
[55] Zhou X, Liu Y, Huang Y, Ma Y, Lv J, Xiao B. Mucus-penetrating polymeric nanoparticles for oral delivery of curcumin to inflamed colon tissue. J Drug Deliv Sci Technol. 2019 Aug;52:157–64.10.1016/j.jddst.2019.04.030Search in Google Scholar
[56] Pelaseyed T, Bergström JH, Gustafsson JK, Ermund A, Birchenough GMH, Schütte A, et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260(1):8–20.10.1111/imr.12182Search in Google Scholar PubMed PubMed Central
[57] Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. 2017 Sep;11(9):821–34.10.1080/17474124.2017.1343143Search in Google Scholar PubMed PubMed Central
[58] Lee SH, Bajracharya R, Min JY, Han JW, Park BJ, Han HK. Strategic approaches for colon targeted drug delivery: An overview of recent advancements. Pharmaceutics. 2020;12(1):68.10.3390/pharmaceutics12010068Search in Google Scholar PubMed PubMed Central
[59] Arévalo-Pérez R, Maderuelo C, Lanao JM. Recent advances in colon drug delivery systems. J Controlled Rel. 2020 Nov;327:703–24.10.1016/j.jconrel.2020.09.026Search in Google Scholar PubMed
[60] Naeem M, Awan UA, Subhan F, Cao J, Hlaing SP, Lee J, et al. Advances in colon-targeted nano-drug delivery systems: Challenges and solutions. Arch Pharm Res. 2020;43(1):153–69.10.1007/s12272-020-01219-0Search in Google Scholar PubMed
[61] Iswandana R, Putri KSS, Putri FA, Gunawan M, Larasati SA. Challenge and development strategy for colon-targeted drug delivery system. Pharm Sci Res. 2022 Apr 30;9(1):3 [cited 2023 Apr 29] https://scholarhub.ui.ac.id/psr/vol9/iss1/3.Search in Google Scholar
[62] Ray S. Advanced colon-specific delivery systems for treating local disorders. Polysaccharide Carriers for. Drug Delivery. 2019 Jan;737–62.10.1016/B978-0-08-102553-6.00025-8Search in Google Scholar
[63] Kumar A, Aggarwal G, HariKumar SL. Colon specific drug delivery by pH sensitive polymers & pulsatile drug delivery system. Indo Glob J Pharm Sci. 2015;5(1):06–11.10.35652/IGJPS.2015.18Search in Google Scholar
[64] Bandi SP, Bhatnagar S, Venuganti VVK. Advanced materials for drug delivery across mucosal barriers. Acta Biomater. 2021 Jan;119:13–29.10.1016/j.actbio.2020.10.031Search in Google Scholar PubMed
[65] Araújo F, Martins C, Azevedo C, Sarmento B. Chemical modification of drug molecules as strategy to reduce interactions with mucus. Adv Drug Deliv Rev. 2018 Jan;124:98–106.10.1016/j.addr.2017.09.020Search in Google Scholar PubMed
[66] Dugad A, Nalawade P, Thakhre R, Kakade S. Colon targeted drug delivery system - A review. J Curr Pharma Res. 2018;9(1):2604–35.10.33786/JCPR.2018.v09i01.007Search in Google Scholar
[67] Iswandana R, Putri KSS, Sandiata CE, Triani S, Sari SP, Djajadisastra J. Formulation of tetrandrine beads using ionic gelation method CA-pectinate coated PH-sensitive polymers as colon-targeted dosage form. Asian J Pharm. Clin Res. 2017;10(10):90–5.10.22159/ajpcr.2017.v10i10.19994Search in Google Scholar
[68] Sankaranarayanan R, Kumar DR, Patel J, Bhat GJ. Do aspirin and flavonoids prevent cancer through a common mechanism involving hydroxybenzoic acids?—The metabolite hypothesis. Molecules. 2020 May 10;25(9):2243 [cited 2023 Jun 11] https://www.mdpi.com/1420-3049/25/9/2243/htm.10.3390/molecules25092243Search in Google Scholar PubMed PubMed Central
[69] Xu Z, Chen L, Duan X, Li X, Ren H. Microparticles based on alginate/chitosan/casein three‐dimensional system for oral insulin delivery. Polym Adv Technol. 2021;32(11):4352–61.10.1002/pat.5437Search in Google Scholar
[70] Liu J, Willför S, Xu C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact Carbohydr Diet Fibre. 2015 Jan;5(1):31–61. https://linkinghub.elsevier.com/retrieve/pii/S2212619814000564.10.1016/j.bcdf.2014.12.001Search in Google Scholar
[71] Krishnamurthy R. Giving rise to life: Transition from prebiotic chemistry to protobiology. Acc Chem Res. 2017 Mar 21;50(3):455–9. https://pubs.acs.org/doi/10.1021/acs.accounts.6b00470.10.1021/acs.accounts.6b00470Search in Google Scholar PubMed
[72] Datta LP, Manchineella S, Govindaraju T. Biomolecules-derived biomaterials. Biomaterials. 2020 Feb;230:119633, https://linkinghub.elsevier.com/retrieve/pii/S014296121930732X.10.1016/j.biomaterials.2019.119633Search in Google Scholar PubMed
[73] Andreani T, Fangueiro JF, Severino P, de Souza AL, Martins-Gomes C, Fernandes PM, et al. The influence of polysaccharide coating on the physicochemical parameters and cytotoxicity of silica nanoparticles for hydrophilic biomolecules delivery. Nanomaterials. 2019 Jul 27;9(8):1081, https://www.mdpi.com/2079-4991/9/8/1081.10.3390/nano9081081Search in Google Scholar PubMed PubMed Central
[74] He S, Liu Z, Xu D. Advance in oral delivery systems for therapeutic protein. J Drug Target. 2019;27(3):283–91.10.1080/1061186X.2018.1486406Search in Google Scholar PubMed
[75] Dos Santos AM, Carvalho SG, Meneguin AB, Sábio RM, Gremião MPD, Chorilli M. Oral delivery of micro/nanoparticulate systems based on natural polysaccharides for intestinal diseases therapy: Challenges, advances and future perspectives. J Controlled Rel. 2021;334:353–66.10.1016/j.jconrel.2021.04.026Search in Google Scholar PubMed
[76] Cao Y, Tan YF, Wong YS, Liew MWJ, Venkatraman S. Recent advances in chitosan-based carriers for gene delivery. Mar Drugs. 2019;17(6):381.10.3390/md17060381Search in Google Scholar PubMed PubMed Central
[77] Jhaveri J, Raichura Z, Khan T, Momin M, Omri A, Stancanelli R, et al. Chitosan nanoparticles-insight into properties, functionalization and applications in drug delivery and theranostics. Molecules. 2021;26:272.10.3390/molecules26020272Search in Google Scholar PubMed PubMed Central
[78] Karava A, Lazaridou M, Nanaki S, Michailidou G, Christodoulou E, Kostoglou M, et al. Chitosan derivatives with mucoadhesive and antimicrobial properties for simultaneous nanoencapsulation and extended ocular release formulations of dexamethasone and chloramphenicol drugs. Pharmaceutics. 2020 Jun;12(6):594.10.3390/pharmaceutics12060594Search in Google Scholar PubMed PubMed Central
[79] Bansal V, Sharma PK, Sharma N, Pal OP, Malviya R. Applications of chitosan and chitosan derivatives in drug delivery. Adv Biol Res (Rennes). 2011;5(1):28–37.Search in Google Scholar
[80] Shariatinia Z. Pharmaceutical applications of chitosan. Adv Colloid Interface Sci. 2019;263:131–94.10.1016/j.cis.2018.11.008Search in Google Scholar PubMed
[81] Parhi R. Drug delivery applications of chitin and chitosan: A review. Environ Chem Lett. 2020 Jan;18(3):577–94.10.1007/s10311-020-00963-5Search in Google Scholar
[82] Yuan B, Jiang X, Chen Y, Guo Q, Wang K, Meng X, et al. Metastatic cancer cell and tissue-specific fluorescence imaging using a new DNA aptamer developed by Cell-SELEX. Talanta. 2017;170:56–62.10.1016/j.talanta.2017.03.094Search in Google Scholar PubMed
[83] Salomon C, Goycoolea FM, Moerschbacher B. Recent trends in the development of chitosan-based drug delivery systems. AAPS PharmSciTech. 2017;18:933–5.10.1208/s12249-017-0764-7Search in Google Scholar PubMed
[84] Ailincai D, Tartau Mititelu L, Marin L. Drug delivery systems based on biocompatible imino-chitosan hydrogels for local anticancer therapy. Drug Deliv. 2018;25(1):1080–90.10.1080/10717544.2018.1466937Search in Google Scholar PubMed PubMed Central
[85] Gonçalves IC, Henriques PC, Seabra CL, Martins MCL. The potential utility of chitosan micro/nanoparticles in the treatment of gastric infection. Expert Rev Anti Infect Ther. 2014;12(8):981–92.10.1586/14787210.2014.930663Search in Google Scholar PubMed
[86] Divya K, Jisha MS. Chitosan nanoparticles preparation and applications. Environ Chem Lett. 2017 Oct 31;16(1):101–12.10.1007/s10311-017-0670-ySearch in Google Scholar
[87] Shah RB, Patel M, Maahs DM, Shah VN. Insulin delivery methods: Past, present and future. Int J Pharm Investig. 2016;6(1):1.10.4103/2230-973X.176456Search in Google Scholar PubMed PubMed Central
[88] Chen MC, Sonaje K, Chen KJ, Sung HW. A review of the prospects for polymeric nanoparticle platforms in oral insulin delivery. Biomaterials. 2011 Dec;32(36):9826–38.10.1016/j.biomaterials.2011.08.087Search in Google Scholar PubMed
[89] Sung HW, Sonaje K, Liao ZX, Hsu LW, Chuang EY. pH-responsive nanoparticles shelled with chitosan for oral delivery of insulin: From mechanism to therapeutic applications. Acc Chem Res. 2012 Apr 17;45(4):619–29. https://pubs.acs.org/doi/10.1021/ar200234q.10.1021/ar200234qSearch in Google Scholar PubMed
[90] M. Ways T, Lau W, Khutoryanskiy V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polym (Basel). 2018 Mar 5;10(3):267. https://www.mdpi.com/2073-4360/10/3/267.Search in Google Scholar
[91] Mura P, Maestrelli F, Cirri M, Mennini N. Multiple Roles of chitosan in mucosal drug delivery: An updated review. Mar Drugs. 2022 May 20;20(5):335. https://www.mdpi.com/1660-3397/20/5/335.10.3390/md20050335Search in Google Scholar PubMed PubMed Central
[92] Lu Z, Chen W, Hamman JH, Ni J, Zhai X. Chitosan-polycarbophil interpolyelectrolyte complex as an excipient for bioadhesive matrix systems to control macromolecular drug delivery. Pharm Dev Technol. 2008 Jan 7;13(1):37–47. http://www.tandfonline.com/doi/full/10.1080/10837450701702636.10.1080/10837450701702636Search in Google Scholar PubMed
[93] Pan Y, Li YJ, Zhao HY, Zheng JM, Xu H, Wei G, et al. Bioadhesive polysaccharide in protein delivery system: chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int J Pharm. 2002 Dec;249(1–2):139–47. https://linkinghub.elsevier.com/retrieve/pii/S0378517302004866.10.1016/S0378-5173(02)00486-6Search in Google Scholar
[94] Tozaki H, Komoike J, Tada C, Maruyama T, Terabe A, Suzuki T, et al. Chitosan capsules for colon-specific drug delivery: improvement of insulin absorption from the rat colon. J Pharm Sci. 1997 Sep;86(9):1016–21. http://linkinghub.elsevier.com/retrieve/pii/S0022354915503729.10.1021/js970018gSearch in Google Scholar PubMed
[95] Krauland AH, Guggi D, Bernkop-Schnürch A. Oral insulin delivery: The potential of thiolated chitosan-insulin tablets on non-diabetic rats. J Controlled Rel. 2004 Mar 24;95(3):547–55.10.1016/j.jconrel.2003.12.017Search in Google Scholar PubMed
[96] Dyer AM, Hinchcliffe M, Watts P, Castile J, Jabbal-Gill I, Nankervis R, et al. Nasal delivery of insulin using novel chitosan based formulations: a comparative study in two animal models between simple chitosan formulations and chitosan nanoparticles. Pharm Res. 2002 Jul;19(7):998–1008. http://www.ncbi.nlm.nih.gov/pubmed/12180553.10.1023/A:1016418523014Search in Google Scholar PubMed
[97] Ways TMM, Lau WM, Khutoryanskiy VV. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polym (Basel). 2018 Mar 5;10(3):267. [cited 2023 Mar 20] /pmc/articles/PMC6414903/.10.3390/polym10030267Search in Google Scholar PubMed PubMed Central
[98] Chuang EY, Lin KJ, Su FY, Chen HL, Maiti B, Ho YC, et al. Calcium depletion-mediated protease inhibition and apical-junctional-complex disassembly via an EGTA-conjugated carrier for oral insulin delivery. J Controlled Rel. 2013 Aug 10;169(3):296–305.10.1016/j.jconrel.2012.11.011Search in Google Scholar PubMed
[99] Sajeesh S, Sharma CP. Mucoadhesive hydrogel microparticles based on poly (methacrylic acid-vinyl pyrrolidone)-chitosan for oral drug delivery. Drug Deliv. 2011;18(4):227–35.10.3109/10717544.2010.528067Search in Google Scholar PubMed
[100] Sonaje K, Chen YJ, Chen HL, Wey SP, Juang JH, Nguyen HN, et al. Enteric-coated capsules filled with freeze-dried chitosan/poly(γ-glutamic acid) nanoparticles for oral insulin delivery. Biomater [Internet]. 2010 Apr;31(12):3384–94, https://linkinghub.elsevier.com/retrieve/pii/S014296121000058X.10.1016/j.biomaterials.2010.01.042Search in Google Scholar PubMed
[101] Khan MZI, Prebeg Ž, Kurjaković N. A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers. J Controlled Rel. 1999 Mar;58(2):215–22, https://linkinghub.elsevier.com/retrieve/pii/S0168365998001515.10.1016/S0168-3659(98)00151-5Search in Google Scholar PubMed
[102] He Z, Santos JL, Tian H, Huang H, Hu Y, Liu L, et al. Scalable fabrication of size-controlled chitosan nanoparticles for oral delivery of insulin. Biomater [Internet]. 2017 Jun;130:28–41, https://linkinghub.elsevier.com/retrieve/pii/S0142961217301709.Search in Google Scholar
[103] Gohil SV, Padmanabhan A, Deschamps J, Nair LS. Chitosan-based scaffolds for growth factor delivery. In: Chitosan based biomaterials. Vol. 2, Sawston, UK: Woodhead Publishing; 2017. p. 175–207. https://linkinghub.elsevier.com/retrieve/pii/B9780081002285000079.10.1016/B978-0-08-100228-5.00007-9Search in Google Scholar
[104] Mao S, Bakowsky U, Jintapattanakit A, Kissel T. Self-assembled polyelectrolyte nanocomplexes between chitosan derivatives and insulin. J Pharm Sci. 2006 May;95(5):1035–48, https://linkinghub.elsevier.com/retrieve/pii/S0022354916320111.10.1002/jps.20520Search in Google Scholar PubMed
[105] Bayat A, Larijani B, Ahmadian S, Junginger HE, Rafiee-Tehrani M. Preparation and characterization of insulin nanoparticles using chitosan and its quaternized derivatives. Nanomedicine. 2008 Jun;4(2):115–20, https://linkinghub.elsevier.com/retrieve/pii/S154996340800004X.10.1016/j.nano.2008.01.003Search in Google Scholar PubMed
[106] Bayat A, Dorkoosh FA, Dehpour AR, Moezi L, Larijani B, Junginger HE, et al. Nanoparticles of quaternized chitosan derivatives as a carrier for colon delivery of insulin: Ex vivo and in vivo studies. Int J Pharm. 2008 May;356(1–2):259–66, https://linkinghub.elsevier.com/retrieve/pii/S037851730701068X.10.1016/j.ijpharm.2007.12.037Search in Google Scholar PubMed
[107] Sadeghi AMM, Dorkoosh FA, Avadi MR, Saadat P, Rafiee-Tehrani M, Junginger HE. Preparation, characterization and antibacterial activities of chitosan, N-trimethyl chitosan (TMC) and N-diethylmethyl chitosan (DEMC) nanoparticles loaded with insulin using both the ionotropic gelation and polyelectrolyte complexation methods. Int J Pharm. 2008 May 1;355(1–2):299–306, https://linkinghub.elsevier.com/retrieve/pii/S037851730700991X.10.1016/j.ijpharm.2007.11.052Search in Google Scholar PubMed
[108] Pawar VK, Meher JG, Singh Y, Chaurasia M, Surendar Reddy B, Chourasia MK. Targeting of gastrointestinal tract for amended delivery of protein/peptide therapeutics: Strategies and industrial perspectives. J Controlled Rel. 2014 Dec;196:168–83, https://linkinghub.elsevier.com/retrieve/pii/S0168365914006750.10.1016/j.jconrel.2014.09.031Search in Google Scholar PubMed
[109] Pai C-M, Min MH, Hwang JS, Cho KM. Nanoparticle compositions of water-soluble drugs for oral administration and preparation methods thereof, US Pat. US20070154559, 2007.Search in Google Scholar
[110] Shukla SK, Mishra AK, Arotiba OA, Mamba BB. Chitosan-based nanomaterials: A state-of-the-art review. Int J Biol Macromol. 2013 Aug;59:46–58.10.1016/j.ijbiomac.2013.04.043Search in Google Scholar PubMed
[111] Mohammed MA, Syeda JTM, Wasan KM, Wasan EK. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics. 2017 Dec;9(4):53.10.3390/pharmaceutics9040053Search in Google Scholar PubMed PubMed Central
[112] Mahdavinia GR, Soleymani M, Sabzi M, Azimi H, Atlasi Z. Novel magnetic polyvinyl alcohol/laponite RD nanocomposite hydrogels for efficient removal of methylene blue. J Env Chem Eng. 2017 Jun;5(3):2617–30.10.1016/j.jece.2017.05.017Search in Google Scholar
[113] Sakloetsakun D, Dünnhaupt S, Barthelmes J, Perera G, Bernkop-Schnürch A. Combining two technologies: Multifunctional polymers and self-nanoemulsifying drug delivery system (SNEDDS) for oral insulin administration. Int J Biol Macromol. 2013;61:363–72.10.1016/j.ijbiomac.2013.08.002Search in Google Scholar PubMed
[114] Sajeesh S, Vauthier C, Gueutin C, Ponchel G, Sharma CP. Thiol functionalized polymethacrylic acid-based hydrogel microparticles for oral insulin delivery. Acta Biomater. 2010;6(8):3072–80.10.1016/j.actbio.2010.02.007Search in Google Scholar PubMed
[115] Bonengel S, Bernkop-Schnürch A. Thiomers — From bench to market. J Controlled Rel. 2014 Dec 10;195:120–9.10.1016/j.jconrel.2014.06.047Search in Google Scholar PubMed
[116] Pathak K, Misra SK, Sehgal A, Singh S, Bungau S, Najda A, et al. Biomedical applications of quaternized chitosan. Polym (Basel). 2021 Aug 1;13(15) [cited 2023 Mar 20] /pmc/articles/PMC8347635/.Search in Google Scholar
[117] Mahjub R, Dorkoosh FA, Amini M, Khoshayand MR, Rafiee-Tehrani M. Preparation, statistical optimization, and in vitro characterization of insulin nanoparticles composed of quaternized aromatic derivatives of chitosan. AAPS PharmSciTech. 2011;12(4):1407–19.10.1208/s12249-011-9716-9Search in Google Scholar PubMed PubMed Central
[118] Marais E, Hamman J, Du Plessis L, Lemmer R, Steenekamp J. Eudragit® L100/N-trimethylchitosan chloride microspheres for oral insulin delivery. Molecules. 2013;18(6):6734–47.10.3390/molecules18066734Search in Google Scholar PubMed PubMed Central
[119] Sonia TA, Rekha MR, Sharma CP. Bioadhesive hydrophobic chitosan microparticles for oral delivery of insulin: In vitro characterization and in vivo uptake studies. J Appl Polym Sci. 2011 Mar 5;119(5):2902–10.10.1002/app.32979Search in Google Scholar
[120] Rekha MR, Sharma CP. Glutamine‐chitosan microparticles as oral insulin delivery matrix: In vitro characterization. J Appl Polym Sci. 2011;122(4):2374–82.10.1002/app.34315Search in Google Scholar
[121] Qu B, Luo Y. Chitosan-based hydrogel beads: Preparations, modifications and applications in food and agriculture sectors – A review. Int J Biol Macromol. 2020 Jun 1;152:437–48.10.1016/j.ijbiomac.2020.02.240Search in Google Scholar PubMed
[122] Cone RA. Barrier properties of mucus. Adv Drug Deliv Rev. 2009 Feb 27;61(2):75–85.10.1016/j.addr.2008.09.008Search in Google Scholar PubMed
[123] Sabra R, Roberts CJ, Billa N. Courier properties of modified citrus pectinate-chitosan nanoparticles in colon delivery of curcumin. Colloid Interface Sci Commun. 2019;32:100192.10.1016/j.colcom.2019.100192Search in Google Scholar
[124] Yuan Y, Xu X, Gong J, Mu R, Li Y, Wu C, et al. Fabrication of chitosan-coated konjac glucomannan/sodium alginate/graphene oxide microspheres with enhanced colon-targeted delivery. Int J Biol Macromol. 2019;131:209–17.10.1016/j.ijbiomac.2019.03.061Search in Google Scholar PubMed
[125] Cao J, Cheng J, Xi S, Qi X, Shen S, Ge Y. Alginate/chitosan microcapsules for in-situ delivery of the protein, interleukin-1 receptor antagonist (IL-1Ra), for the treatment of dextran sulfate sodium (DSS)-induced colitis in a mouse model. Eur J Pharmaceutics Biopharmaceutics. 2019;137:112–21.10.1016/j.ejpb.2019.02.011Search in Google Scholar PubMed
[126] Pilipenko I, Korzhikov-Vlakh V, Sharoyko V, Zhang N, Schäfer-Korting M, Rühl E, et al. pH-sensitive chitosan–heparin nanoparticles for effective delivery of genetic drugs into epithelial cells. Pharmaceutics. 2019 Jul 5;11(7):317, https://www.mdpi.com/1999-4923/11/7/317.10.3390/pharmaceutics11070317Search in Google Scholar PubMed PubMed Central
[127] Barbosa A, Costa Lima S, Reis S. Application of pH-responsive fucoidan/chitosan nanoparticles to improve oral quercetin delivery. Molecules. 2019 Jan 18;24(2):346, http://www.mdpi.com/1420-3049/24/2/346.10.3390/molecules24020346Search in Google Scholar PubMed PubMed Central
[128] Maciel V, Yoshida C, Pereira S, Goycoolea F, Franco T. Electrostatic self-assembled chitosan-pectin nano- and microparticles for insulin delivery. Molecules. 2017 Oct 12;22(10):1707, http://www.mdpi.com/1420-3049/22/10/1707.10.3390/molecules22101707Search in Google Scholar PubMed PubMed Central
[129] Shailender J, Ravi PR, Reddy Sirukuri M, Dalvi A, Keerthi Priya O. Chitosan nanoparticles for the oral delivery of tenofovir disoproxil fumarate: Formulation optimization, characterization and ex vivo and in vivo evaluation for uptake mechanism in rats. Drug Dev Ind Pharm. 2018 Jul 3;44(7):1109–19, https://www.tandfonline.com/doi/full/10.1080/03639045.2018.1438459.10.1080/03639045.2018.1438459Search in Google Scholar PubMed
[130] Sudhakar S, Chandran SV, Selvamurugan N, Nazeer RA. Biodistribution and pharmacokinetics of thiolated chitosan nanoparticles for oral delivery of insulin in vivo. Int J Biol Macromol. 2020 May 1;150:281–8.10.1016/j.ijbiomac.2020.02.079Search in Google Scholar PubMed
[131] He Z, Santos JL, Tian H, Huang H, Hu Y, Liu L, et al. Scalable fabrication of size-controlled chitosan nanoparticles for oral delivery of insulin. Biomaterials. 2017 Jun;130:28–41.10.1016/j.biomaterials.2017.03.028Search in Google Scholar PubMed
[132] Andishmand H, Tabibiazar M, Mohammadifar MA, Hamishehkar H. Pectin-zinc-chitosan-polyethylene glycol colloidal nano-suspension as a food grade carrier for colon targeted delivery of resveratrol. Int J Biol Macromol. 2017 Apr;97:16–22, https://linkinghub.elsevier.com/retrieve/pii/S014181301630798X.10.1016/j.ijbiomac.2016.12.087Search in Google Scholar PubMed
[133] Prabahar K, Udhumansha U, Qushawy M. Optimization of thiolated chitosan nanoparticles for the enhancement of in vivo hypoglycemic efficacy of sitagliptin in streptozotocin-induced diabetic rats. Pharmaceutics. 2020 Mar 26;12(4):300, https://www.mdpi.com/1999-4923/12/4/300.10.3390/pharmaceutics12040300Search in Google Scholar PubMed PubMed Central
[134] Mumuni MA, Kenechukwu FC, Ofokansi KC, Attama AA, Díaz DD. Insulin-loaded mucoadhesive nanoparticles based on mucin-chitosan complexes for oral delivery and diabetes treatment. Carbohydr Polym. 2020 Feb 1;229:115506.10.1016/j.carbpol.2019.115506Search in Google Scholar PubMed
[135] Maity S, Mukhopadhyay P, Kundu PP, Chakraborti AS. Alginate coated chitosan core-shell nanoparticles for efficient oral delivery of naringenin in diabetic animals—An in vitro and in vivo approach. Carbohydr Polym. 2017 Aug;170:124–32, https://linkinghub.elsevier.com/retrieve/pii/S0144861717304654.10.1016/j.carbpol.2017.04.066Search in Google Scholar PubMed
[136] Hirpara MR, Manikkath J, Sivakumar K, Managuli RS, Gourishetti K, Krishnadas N, et al. Long circulating PEGylated-chitosan nanoparticles of rosuvastatin calcium: Development and in vitro and in vivo evaluations. Int J Biol Macromol. 2018 Feb;107:2190–200, https://linkinghub.elsevier.com/retrieve/pii/S0141813017322675.10.1016/j.ijbiomac.2017.10.086Search in Google Scholar PubMed
[137] Mukhopadhyay P, Sarkar K, Chakraborty M, Bhattacharya S, Mishra R, Kundu PP. Oral insulin delivery by self-assembled chitosan nanoparticles: In vitro and in vivo studies in diabetic animal model. Mater Sci Eng: C. 2013 Jan;33(1):376–82, https://linkinghub.elsevier.com/retrieve/pii/S0928493112004316.10.1016/j.msec.2012.09.001Search in Google Scholar PubMed
[138] Thai H, Thuy Nguyen C, Thi Thach L, Thi Tran M, Duc Mai H, Thi Thu Nguyen T, et al. Characterization of chitosan/alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo. Sci Rep. 2020 Jan 22;10(1):909, https://www.nature.com/articles/s41598-020-57666-8.10.1038/s41598-020-57666-8Search in Google Scholar PubMed PubMed Central
[139] Auwal S, Zarei M, Tan C, Basri M, Saari N. Improved in vivo efficacy of anti-hypertensive biopeptides encapsulated in chitosan nanoparticles fabricated by ionotropic gelation on spontaneously hypertensive rats. Nanomaterials. 2017 Dec 2;7(12):421, http://www.mdpi.com/2079-4991/7/12/421.10.3390/nano7120421Search in Google Scholar PubMed PubMed Central
[140] Zhang X, Ma Y, Ma L, Zu M, Song H, Xiao B. Oral administration of chondroitin sulfate-functionalized nanoparticles for colonic macrophage-targeted drug delivery. Carbohydr Polym. 2019 Nov;223:115126, https://linkinghub.elsevier.com/retrieve/pii/S0144861719307933.10.1016/j.carbpol.2019.115126Search in Google Scholar PubMed
[141] Sinha P, Udhumansha U, Rathnam G, Ganesh M, Jang HT. Capecitabine encapsulated chitosan succinate-sodium alginate macromolecular complex beads for colon cancer targeted delivery: in vitro evaluation. Int J Biol Macromol [Internet]. 2018 Oct;117:840–50, https://linkinghub.elsevier.com/retrieve/pii/S0141813018317057.10.1016/j.ijbiomac.2018.05.181Search in Google Scholar PubMed
[142] Castangia I, Nácher A, Caddeo C, Merino V, Díez-Sales O, Catalán-Latorre A, et al. Therapeutic efficacy of quercetin enzyme-responsive nanovesicles for the treatment of experimental colitis in rats. Acta Biomater. 2015 Feb;13:216–27, https://linkinghub.elsevier.com/retrieve/pii/S174270611400511X.10.1016/j.actbio.2014.11.017Search in Google Scholar PubMed
[143] Urimi D, Agrawal AK, Kushwah V, Jain S. Polyglutamic acid functionalization of chitosan nanoparticles enhances the therapeutic efficacy of insulin following oral administration. AAPS PharmSciTech. 2019 Apr 1;20(3):1–14. [cited 2023 Mar 22] https://link.springer.com/article/10.1208/s12249-019-1330-2.10.1208/s12249-019-1330-2Search in Google Scholar PubMed
[144] Melo N, Dias AC, Isidoro L, Duarte R. Bordetella pertussis, an agent not to forget: A case report. Cases J. 2009;2:1–3.10.1186/1757-1626-2-128Search in Google Scholar PubMed PubMed Central
[145] Luque-Alcaraz AG, Lizardi-Mendoza J, Goycoolea FM, Higuera-Ciapara I, Argüelles-Monal W. Preparation of chitosan nanoparticles by nanoprecipitation and their ability as a drug nanocarrier. RSC Adv. 2016;6(64):59250–6.10.1039/C6RA06563ESearch in Google Scholar
[146] Giri TK. Nanoarchitectured polysaccharide-based drug carrier for ocular therapeutics. undefined. 2016 Jul 27;119–41.10.1016/B978-0-323-47347-7.00005-7Search in Google Scholar
[147] Wong CY, Al-Salami H, Dass CR. Formulation and characterisation of insulin-loaded chitosan nanoparticles capable of inducing glucose uptake in skeletal muscle cells in vitro. J Drug Deliv Sci Technol. 2020 Jun 1;57:101738.10.1016/j.jddst.2020.101738Search in Google Scholar
[148] Nagpal K, Singh SK, Mishra DN. Chitosan nanoparticles: A promising system in novel drug delivery. Chem Pharm Bull (Tokyo). 2010 Nov;58(11):1423–30.10.1248/cpb.58.1423Search in Google Scholar PubMed
[149] Jayanudin J, Heriyanto H. A review of encapsulation using emulsion crosslinking method. World Chem Eng J. 2021;5(2):37–43.10.48181/wcej.v5i2.12312Search in Google Scholar
[150] Momoh MA, Franklin KC, Agbo CP, Ugwu CE, Adedokun MO, Anthony OC, et al. Microemulsion-based approach for oral delivery of insulin: formulation design and characterization. Heliyon. 2020 Mar 1;6(3):e03650. [cited 2023 Dec 30] https://pubmed.ncbi.nlm.nih.gov/32258491/.10.1016/j.heliyon.2020.e03650Search in Google Scholar PubMed PubMed Central
[151] Fonte P, Nogueira T, Gehm C, Ferreira D, Sarmento B. Chitosan-coated solid lipid nanoparticles enhance the oral absorption of insulin. Drug Deliv Transl Res. 2011 Aug 29;1(4):299–308. [cited 2023 Jun 11] https://link.springer.com/article/10.1007/s13346-011-0023-5.10.1007/s13346-011-0023-5Search in Google Scholar PubMed
[152] Hoang NH, Thanh TLe, Sangpueak R, Treekoon J, Saengchan C, Thepbandit W, et al. Chitosan nanoparticles-based ionic gelation method: A promising candidate for plant disease management. Polymers. 2022 Feb 9;[cited 2023 Apr 28] 14(4):662. https://www.mdpi.com/2073-4360/14/4/662/htm.10.3390/polym14040662Search in Google Scholar PubMed PubMed Central
[153] Mahdizadeh Barzoki Z, Emam-Djomeh Z, Mortazavian E, Rafiee-Tehrani N, Behmadi H, Rafiee-Tehrani M, et al. Determination of diffusion coefficient for released nanoparticles from developed gelatin/chitosan bilayered buccal films. Int J Biol Macromol. 2018 Jun;112:1005–13. https://linkinghub.elsevier.com/retrieve/pii/S0141813017328131.10.1016/j.ijbiomac.2018.01.215Search in Google Scholar PubMed
[154] El Leithy ES, Abdel-Bar HM, Ali RAM. Folate-chitosan nanoparticles triggered insulin cellular uptake and improved in vivo hypoglycemic activity. Int J Pharm. 2019 Nov;571:118708. https://linkinghub.elsevier.com/retrieve/pii/S0378517319307537.10.1016/j.ijpharm.2019.118708Search in Google Scholar PubMed
[155] Chen T, Li S, Zhu W, Liang Z, Zeng Q. Self-assembly pH-sensitive chitosan/alginate coated polyelectrolyte complexes for oral delivery of insulin. J Microencapsul. 2019 Jan;36(1):96–107.10.1080/02652048.2019.1604846Search in Google Scholar PubMed
[156] Bhattacharyya A, Mukherjee D, Mishra R, Kundu PP. Preparation of polyurethane–alginate/chitosan core shell nanoparticles for the purpose of oral insulin delivery. Eur Polym J. 2017 Jul;92:294–313.10.1016/j.eurpolymj.2017.05.015Search in Google Scholar
[157] Sahoo P, Leong KH, Nyamathulla S, Onuki Y, Takayama K, Chung LY. Optimization of pH-responsive carboxymethylated iota-carrageenan/chitosan nanoparticles for oral insulin delivery using response surface methodology. React Funct Polym. 2017 Oct;119:145–55.10.1016/j.reactfunctpolym.2017.08.014Search in Google Scholar
[158] Tsai LC, Chen CH, Lin CW, Ho YC, Mi FL. Development of mutlifunctional nanoparticles self-assembled from trimethyl chitosan and fucoidan for enhanced oral delivery of insulin. Int J Biol Macromol. 2019 Apr 1;126:141–50.10.1016/j.ijbiomac.2018.12.182Search in Google Scholar PubMed
[159] Al-Remawi M, Elsayed A, Maghrabi I, Hamaidi M, Jaber N. Chitosan/lecithin liposomal nanovesicles as an oral insulin delivery system. Pharm Dev Technol. 2017 Apr;22(3):390–8.10.1080/10837450.2016.1213745Search in Google Scholar PubMed
[160] Erel G, Kotmakçı M, Akbaba H, Sözer Karadağlı S, Kantarcı AG. Nanoencapsulated chitosan nanoparticles in emulsion-based oral delivery system: In vitro and in vivo evaluation of insulin loaded formulation. J Drug Deliv Sci Technol. 2016 Dec;36:161–7.10.1016/j.jddst.2016.10.010Search in Google Scholar
[161] Mukhopadhyay P, Chakraborty S, Bhattacharya S, Mishra R, Kundu PP. PH-sensitive chitosan/alginate core-shell nanoparticles for efficient and safe oral insulin delivery. Int J Biol Macromol. 2015 Jan;72:640–8.10.1016/j.ijbiomac.2014.08.040Search in Google Scholar PubMed
[162] Yang Y, Liu Y, Chen S, Cheong KL, Teng B. Carboxymethyl β-cyclodextrin grafted carboxymethyl chitosan hydrogel-based microparticles for oral insulin delivery. Carbohydr Polym. 2020 Oct 15;246:116617.10.1016/j.carbpol.2020.116617Search in Google Scholar PubMed
[163] Kim JU, Shahbaz HM, Lee H, Kim T, Yang K, Roh YH, et al. Optimization of phytic acid-crosslinked chitosan microspheres for oral insulin delivery using response surface methodology. Int J Pharm. 2020 Oct 15;588:119736.10.1016/j.ijpharm.2020.119736Search in Google Scholar PubMed
[164] Li W, Zhang L, Ge X, Xu B, Zhang W, Qu L, et al. Microfluidic fabrication of microparticles for biomedical applications. Chem Soc Rev. 2018 Aug;47(15):5646–83.10.1039/C7CS00263GSearch in Google Scholar PubMed PubMed Central
[165] Zhang J, Wang Y, Qu Q, Lu T, Li F, Wang J, et al. Preparation of single, heteromorphic microspheres, and their progress for medical applications. Macromol Mater Eng. 2021 Feb;306(2):2000593.10.1002/mame.202000593Search in Google Scholar
[166] Tanhaei A, Mohammadi M, Hamishehkar H, Hamblin MR. Electrospraying as a novel method of particle engineering for drug delivery vehicles. J Controlled Rel. 2021 Feb 10;330:851–65.10.1016/j.jconrel.2020.10.059Search in Google Scholar PubMed
[167] Yurteri CU, Hartman RPA, Marijnissen JCM. Producing pharmaceutical particles via electrospraying with an emphasis on nano and nano structured particles - A review. KONA Powder Part J. 2010;28:91–115.10.14356/kona.2010010Search in Google Scholar
[168] Zhao H, Xu J, Lan W, Wang T, Luo G. Microfluidic production of porous chitosan/silica hybrid microspheres and its Cu (II) adsorption performance. Chem Eng J. 2013;229:82–9.10.1016/j.cej.2013.05.093Search in Google Scholar
[169] White KA, Chalaby R, Olabisi R. Evaluation of microfluidic approaches to encapsulate cells into PEGDA microparticles. Regen Eng Transl Med. 2022 Jun 1;8(2):345–54. [cited 2022 Nov 3] https://link.springer.com/article/10.1007/s40883-021-00232-z.10.1007/s40883-021-00232-zSearch in Google Scholar
[170] Chaurasiya RS, Hebbar HU. Reverse micelles for nanoparticle synthesis and biomolecule separation. In: Ranjan S, Dasgupta N, Lichtfouse E, editors. Nanoscience in food and agriculture 4. Sustainable agriculture reviews. Cham: Springer; 2017. p. 181–211.10.1007/978-3-319-53112-0_5Search in Google Scholar
[171] Garavand F, Cacciotti I, Vahedikia N, Rehman A, Tarhan Ö, Akbari-Alavijeh S, et al. A comprehensive review on the nanocomposites loaded with chitosan nanoparticles for food packaging. Crit Rev Food Sci Nutr. 2020 [cited 2022 Nov 3] 62(5):1383–416. https://www.tandfonline.com/doi/abs/10.1080/10408398.2020.1843133.10.1080/10408398.2020.1843133Search in Google Scholar PubMed
[172] Zhang Y, Wei W, Lv P, Wang L, Ma G. Preparation and evaluation of alginate–chitosan microspheres for oral delivery of insulin. Eur J Pharm Biopharm. 2011 Jan 1;77(1):11–9.10.1016/j.ejpb.2010.09.016Search in Google Scholar PubMed
[173] Wei W, Ma GH, Wang LY, Wu J, Su ZG. Hollow quaternized chitosan microspheres increase the therapeutic effect of orally administered insulin. Acta Biomater. 2010 Jan 1;6(1):205–9.10.1016/j.actbio.2009.06.005Search in Google Scholar PubMed
[174] Shehata TM, Ibrahima MM. BÜCHI nano spray dryer B-90: a promising technology for the production of metformin hydrochloride-loaded alginate–gelatin nanoparticles. Drug Dev Ind Pharm. 2019;45(12):1907–14. 10.1080/03639045.2019.1680992.Search in Google Scholar PubMed
[175] Aranaz I, Paños I, Peniche C, Heras Á, Acosta N. Chitosan spray-dried microparticles for controlled delivery of venlafaxine hydrochloride. Molecules. 2017 Dec;22:1980.10.3390/molecules22111980Search in Google Scholar PubMed PubMed Central
[176] Nasiri Zadeh S, Rajabnezhad S, Zandkarimi M, Dahmardeh S, Mir L, Ali Darbandi M, et al. Mucoadhesive microspheres of chitosan and polyvinyl alcohol as a carrier for intranasal delivery of insulin: in vitro and in vivo studies. MOJ Bioequivalence Bioavailab. 2017 Mar;3(2):00030.10.15406/mojbb.2017.03.00030Search in Google Scholar
[177] Su Z, Wu S, Tao Y, Zhang H. Preparation and characterization of water-soluble chitosan microparticles loaded with insulin using the polyelectrolyte complexation method. J Nanomater. 2011;2011:1–6.10.1155/2011/404523Search in Google Scholar
[178] Shen YB, Du Z, Tang C, Guan YX, Yao SJ. Formulation of insulin-loaded N-trimethyl chitosan microparticles with improved efficacy for inhalation by supercritical fluid assisted atomization. Int J Pharm. 2016 May;505(1–2):223–33.10.1016/j.ijpharm.2016.03.053Search in Google Scholar PubMed
[179] Mumuni MA, Kenechukwu FC, Ernest OC, Oluseun AM, Abdulmumin B, Youngson DC, et al. Surface-modified mucoadhesive microparticles as a controlled release system for oral delivery of insulin. Heliyon. 2019 Sep;5(9):e02366.10.1016/j.heliyon.2019.e02366Search in Google Scholar PubMed PubMed Central
[180] Tian B, Hua S, Tian Y, Liu J. Chemical and physical chitosan hydrogels as prospective carriers for drug delivery: a review. J Mater Chem B. 2020 Nov 18;8(44):10050–64. [cited 2023 Mar 25] https://pubs.rsc.org/en/content/articlehtml/2020/tb/d0tb01869d.10.1039/D0TB01869DSearch in Google Scholar PubMed
[181] Gunasekaran S, Tao W, Chunxiang C. Swelling of pH-sensitive chitosan–poly(vinyl alcohol) hydrogels. J Appl Polym Sci. 2006 Dec 5;102(5):4665–71. [cited 2023 Mar 25] https://onlinelibrary.wiley.com/doi/full/10.1002/app.24825.10.1002/app.24825Search in Google Scholar
[182] Srinatha A, Pandit J, Singh S. Ionic cross-linked chitosan beads for extended release of ciprofloxacin: In vitro characterization. Indian J Pharm Sci. 2008 Jan 1;70(1):16 [cited 2023 Mar 25] /pmc/articles/PMC2852055/.10.4103/0250-474X.40326Search in Google Scholar PubMed PubMed Central
[183] Vakili M, Mojiri A, Zwain HM, Yuan J, Giwa AS, Wang W, et al. Effect of beading parameters on cross-linked chitosan adsorptive properties. React Funct Polym. 2019 Nov 1;144:104354.10.1016/j.reactfunctpolym.2019.104354Search in Google Scholar
[184] Lu Y, Wang Z, Ouyang XK, Ji C, Liu Y, Huang F, et al. Fabrication of cross-linked chitosan beads grafted by polyethylenimine for efficient adsorption of diclofenac sodium from water. Int J Biol Macromol. 2020 Feb 15;145:1180–8.10.1016/j.ijbiomac.2019.10.044Search in Google Scholar PubMed
[185] Kofuji K, Akamine H, Oshirabe H, Maeda Y, Murata Y, Kawashima S. Retention and release behavior of insulin in chitosan gel beads. J Biomater Sci Polym Ed. 2003;14(11):1243–53.10.1163/156856203322553464Search in Google Scholar PubMed
[186] Sacco P, Pedroso-Santana S, Kumar Y, Joly N, Martin P, Bocchetta P. Ionotropic gelation of chitosan flat structures and potential applications. Molecules. 2021;26(3):660.10.3390/molecules26030660Search in Google Scholar PubMed PubMed Central
[187] Barreiro-Iglesias R, Coronilla R, Concheiro A, Alvarez-Lorenzo C. Preparation of chitosan beads by simultaneous cross-linking/ insolubilisation in basic pH: Rheological optimisation and drug loading/release behaviour. Eur J Pharm Sci. 2005 Jan;24(1):77–84.10.1016/j.ejps.2004.09.013Search in Google Scholar PubMed
[188] Maitra J, Shukla VK. Cross-linking in hydrogels - A review. Am J Polym Sci. 2014;4(2):25–31.Search in Google Scholar
[189] Mane S, Ponrathnam S, Chavan N. Effect of chemical cross-linking on properties of polymer microbeads: a review. Can Chem Trans. 2015;3(4):473–85.10.13179/canchemtrans.2015.03.04.0245Search in Google Scholar
[190] Yu S, Xu X, Feng J, Liu M, Hu K. Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int J Pharm. 2019;560:282–93.10.1016/j.ijpharm.2019.02.012Search in Google Scholar PubMed
[191] Frank LA, Onzi GR, Morawski AS, Pohlmann AR, Guterres SS, Contri RV. Chitosan as a coating material for nanoparticles intended for biomedical applications. React Funct Polym. 2020;147:104459.10.1016/j.reactfunctpolym.2019.104459Search in Google Scholar
[192] Kurakula M, Gorityala S, Moharir K. Recent trends in design and evaluation of chitosan-based colon targeted drug delivery systems: Update 2020. J Drug Deliv Sci Technol. 2021 Aug 1;64:102579.10.1016/j.jddst.2021.102579Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy


