Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
-
Mehwish Amanat
, Tauheeda Riaz
Abstract
Antibiotics are resistant compounds that become emerging contaminants that cause hazards to human health and the ecological environment due to their wide production and consumption. The present research reveals the remediation of amoxicillin (AMX) antibiotic by catalytic degradation using fabricated zinc oxide (ZnO) and zinc oxide-reduced graphene oxide (ZnO-rGO) catalysts. The characterization of the catalyst was carried out via UV–Vis spectroscopy, Fourier transform infrared spectroscopy, X-ray diffraction, energy dispersive X-ray spectroscopy, and scanning electron microscopy to evaluate the morphology and composition of synthesised catalyst. The catalytic ability of ZnO-rGO and ZnO was investigated by analysing the degradation of AMX. The ZnO-rGO nanocomposites (NCs) showed improved catalytic performance towards AMX degradation (96%) than pure ZnO nanoparticles (85%), which may be attributed to the incorporation of rGO, which enhanced the adsorption rate and changed the electron–hole recombination rate. The antioxidant potential of synthesised nanomaterials was also analysed by three different methods. The adsorption behaviour was explained through the Langmuir and Freundlich models, and the results revealed that AMX adsorption followed the Freundlich model more closely for both catalysts. The adsorption of AMX was also studied thermodynamically at different temperatures. The negative Gibbs energy change, positive enthalpy change, and entropy change showed the reaction’s spontaneity and endothermic nature. Finally, it can be assumed that the ZnO-rGO NCs could be an effective semiconductor for the degradation of AMX from wastewater.
1 Introduction
The entry of different emerging pollutants into water bodies has now become a major environmental threat. In recent years, pharmaceutical products specifically antibiotics have been identified as the most common contaminants found in various water sources, including drinking water, groundwater, and surface water. Various sources such as domestic sewage, human and animal excretion, pharmaceutical manufacturing plants, hospital effluents, and agriculture release pharmaceutical compounds into the water [1]. The antibiotics that enter water bodies and the soil ecosystem cause endocrine disruption and the emergence of antibiotic resistance in microorganisms [2]. In microorganisms, resistance to β-lactams is caused by chromosomal mutations in cell-wall-synthesizing enzymes known as penicillin-binding proteins (PBPs). Such mutations in PBPs are caused by a continuous mutation process that causes resistance [3]. So, it is important to remove and degrade antibiotics from aqueous media to protect the ecosystem.
Amoxicillin (AMX) is a semisynthetic beta-lactam antibiotic that belongs to the penicillin family and destroys bacterial cell walls. It is used to cure human as well as animal infectious diseases caused by susceptible microorganisms. It is the most widely used antibiotic in the penicillin family because of its ability to be absorbed orally. However, unfortunately, AMX is hardly degradable [4].
In the literature, various treatment techniques have been utilized to eliminate antibiotics from an aqueous medium, such as biodegradation [5], flocculation [6], membrane filtration [7], adsorption [8], and various advanced oxidation processes such as ozonation [9] and semiconductor photocatalysis [10]. Among all these approaches, the adsorption process is one of the most potent techniques used to remove organic contaminants from aqueous media owing to its high removal capacity, simplicity, and low operation cost [11].
Different adsorbent materials, i.e., reduced graphene oxide (rGO), carbon nanotubes, activated carbon, magnetic graphene oxide, metal/metal oxide nanoparticles (NPs), and graphene-based nanocomposites (NCs), have been used for antibiotics removal methods [8].
Zinc oxide (ZnO) NPs were chosen as they possess remarkable properties such as a large specific surface area, nano size, broad bandgap (3.37 eV), chemical and physical inertness, non-toxicity, high adsorption capacity, easy to synthesize, biocompatibility, excellent electronic, catalytic, and optical properties [12]. Various approaches have been applied to increase the effectiveness of ZnO NPs. One of the most favourable strategies is the coupling of ZnO NPs with graphene and its derivatives to enhance catalytic performance by enlarging surface area and suppressing electron–hole recombination rates in ZnO [13]. Many antibiotics such as beta-lactams have aromatic rings in their structure, which make the graphene-based material an excellent adsorbent for the removal of antibiotics via π–π interactions [14].
Green synthesis provides significant advantages over traditional approaches and a wide range of biological applications. It avoids the use of toxic reagents and the production of undesirable by-products by offering sustainable synthesis methods. The use of plant leaf extract in the synthesis of metal NPs is one of the most compatible, safe, and environmentally friendly methods. Plant extracts contain a variety of bioactive compounds, including alkaloids, flavonoids, and phenols, which help to stabilise and control the size of NPs [15].
Phyto-mediated synthesised ZnO NPs exhibited many biomedical applications such as antibacterial, anti-inflammatory, and antioxidant. ZnO NPs have received a lot of interest due to their antioxidant properties, which are significant in resisting oxidative stress in biological systems. ZnO NPs have the potential to scavenge reactive free radicals produced by oxidative stress, which cause a variety of diseases such as cancer and neurological diseases. As antioxidants, ZnO NPs neutralise free radicals and minimise oxidative damage. ZnO NPs as antioxidants neutralize free radicals and reduce oxidative damage [16].
The present work reports the green synthesis of ZnO NPs and ZnO-rGO NCs by using an aqueous leaf extract of the Litchi chinensis plant as a reducing and capping agent for the first time. The properties of prepared nanomaterials were analysed by different characterization techniques, and the efficiency of synthesised nanomaterials in AMX degradation was also assessed.
2 Materials and methods
2.1 Chemicals
Leaves of L. chinensis were taken from the local area of Sialkot. The analytical grade chemical reagents used in this study were graphite powder, Zn(NO3)2⸱6H2O, KMnO4, H2O2, H2SO4, DPPH radical (1,1-diphenyl-2-picrylhydrazyl), methanol, Folin–Ciocalteu reagent, Na2CO3, ammonium hexamolybdate, Na3PO4, NaOH, and HCl.
2.2 Preparation of plant leaf extract
Plant leaves were thoroughly washed with distilled H2O, dried under shade, and crushed. For preparing aqueous plant extract, 2 g of powdered leaves were added to 100 mL of distilled water and heated at 80°C for 60 min. The extract was filtered and then placed at 5°C for further use [17].
2.3 Preparation of ZnO NPs
A solution of 2.97 g (1 M) of Zn(NO3)2⸱6H2O in 10 mL of DW was prepared. Afterwards, 10 mL of plant extract was mixed with a salt solution in a 1:1 ratio and stirred for 60 min at 60°C using a magnetic stirrer. The transformation of colour from yellow to brown indicates the formation of NPs. After centrifugation, NPs were washed with distilled H2O to obtain pure ZnO NPs [18].
2.4 Preparation of graphene oxide and ZnO-rGO NC
Graphene oxide was prepared via the modified Hummers method [34]. To synthesize ZnO-rGO NCs, 0.1 g graphene oxide was transferred into distilled H2O (20 mL) and sonicated for 60 min to get a homogeneous mixture. Then, 20 mL solution of 2.97 g Zn(NO3)2⸱6H2O was mixed with rGO dispersion and stirred for 60 min. Then, 40 mL of plant extract was slowly poured into the resulting mixture and then centrifuged. The solid residues were dried overnight at 60°C to get ZnO-rGO NCs [17].
2.5 Determination of point zero charge (pHpzc)
To determine the pHpzc of both synthesised ZnO NPs and ZnO-rGO, 40 mL of 0.1 M NaCl solution was taken in different beakers, and the initial pH (pHi) from 2 to 12 was adjusted using 0.1 M HCl and NaOH solutions. After maintaining pHi, the desired amount of synthesised nanomaterials was added to all beakers and stirred at the speed of 150 rpm on an orbital shaker at room temperature for 24 h. After that, the final pH was measured. The pHpzc was calculated by plotting the graph between pHi and ∆pH [19].
2.6 Characterization
The formation of prepared nanomaterials was confirmed using a UV–Vis spectrophotometer (Specord 210 plus, Analytic Jena AG, Germany), Fourier transform infrared (FTIR), scanning electron microscopy (SEM), energy-dispersive X-ray (EDX) spectroscopy (JEOL JAPAN), and X-ray diffraction (XRD, X-PERT PANalytical diffractometer). High-performance liquid chromatography (HPLC) analysis was performed to analyse the degradation of AMX with Waters Alliance™ e2695 XE HPLC instrument.
2.7 Antioxidant potential
Antioxidant potential of green synthesised nanomaterials was determined by three different methods.
2.7.1 DPPH free radical scavenging activity
DPPH radical scavenging capacity of prepared ZnO NPs and ZnO-rGO was determined by the standard method [20]. To determine free radical scavenging ability, different concentrations (500, 1,000, and 1,500 μg/mL) of synthesised nanomaterials were taken. Then, 3 mL of DPPH solution, which was prepared by adding 4 mg of DPPH in 100 mL of methanol, was added and shaken vigorously. The mixture was kept for 30 min at room temperature in the dark and centrifuged for 10 min at 3,000 rpm. The colour of the solution changed from violet to yellow, indicating that the DPPH radical was reduced. The absorbance of the supernatant was recorded using a spectrophotometer at 517 nm. The DPPH radical scavenging capacity of synthesised nanomaterials was calculated using this formula:
2.7.2 Total phenolic contents (TPCs)
The TPCs were measured by the method of Makkar et al. using the Folin–Ciocalteu reagent [21]. The TPC was determined by adding 0.5 mg/mL of prepared nanomaterials in 0.1 mL Folin–Ciocalteu reagent (2 N). Then, 2.8 mL of 10% Na2CO3 was added to the mixture and shaken vigorously. The solution was stored at room temperature for 40 min, and then absorbance was recorded at 765 nm. TPC was calculated as mg of gallic acid equivalents per gram of sample by extrapolation of different concentrations of gallic acid.
2.7.3 Total antioxidant activity
The total antioxidant capacity was measured by the phosphomolybdate method [22]. About 0.5 mg/mL of synthesised nanomaterials were mixed with 4 mL of reagent solution (4 mM of ammonium molybdate, 28 mM of sodium phosphate, and 0.6 mM of sulphuric acid). The mixture was taken in test tubes, which were then sealed and heated in the water bath at 95°C for 90 min, and then the solution was cooled. After cooling, the absorbance of the solution was checked at 695 nm. The typical blank solution consisted of 4 mL of reagent solution and was heated under the same conditions as the sample solution.
2.8 Adsorption studies
The batch experiment was performed to compare AMX removal by ZnO and ZnO-rGO with 5 mg/L of AMX solution. The appropriate amount of catalyst was mixed into 25 mL of AMX solution, placed in a shaker for 60 min, and then centrifuged. The absorbance value of the supernatant after centrifugation was determined, and the equilibrium concentration C e (mg/L) was converted from the standard concentration curve of AMX. The adsorption capacity is measured using the following formula [23]:
where initial and equilibrium concentrations of AMX are C 0 and C e (mg/L), respectively, V is the volume of the AMX solution (L), and M (g) is the adsorbent quantity.
The AMX degradation efficiency is calculated by using the following equation [24]:
3 Results and discussion
3.1 Proposed mechanism for the formation of ZnO NPs
In the green preparation of ZnO NPs, potential phytochemical agents present in the leaves of L. chinensis plant, such as polyphenols (epicatechin), reduced Zn2+ to ZnO or aggregates of ZnO NPs, as shown in Figure 1a; first, zinc nitrate hexahydrate was ionized in aqueous media to give Zn2+ ions which reacted with epicatechin and formed Zn-epicatechin. It oxidized to quinone and produced electrons and hydrogens, which reduced Zn2+ ions into ZnO NPs [25].

(a) Proposed mechanism for synthesis of ZnO NPs; (b) proposed mechanism for the reduction of GO to rGO; (c) UV–Vis spectra of plant extract, ZnO, GO, and ZnO-rGO; and (d) FTIR spectra of plant extract, ZnO, GO, and ZnO-rGO.
3.2 Proposed mechanism for reduction of GO to rGO by plant extract
The reducing agents (polyphenols) in the leaf extract of the L. chinensis plant reacted with graphene oxide, as depicted in Figure 1b. The epoxide group of GO came into contact with the alcohol group. The epoxide and phenolic groups of GO generated an intermediate due to the nucleophilic attack of the –OH groups, and the water was removed. After reduction, rGO was produced [26].
3.3 Characterization of ZnO NCs
The absorption spectra of plant extract, ZnO NPs, GO, and ZnO-rGO NCs are shown in Figure 1c. The spectrum of ZnO NPs displayed a single broad and strong peak at 250 nm because of surface plasmon resonance. GO absorption peaks in aqueous suspension were detected at 234 nm and a weak peak at 305 nm. The peak at 234 nm was caused by π → π* transition of aromatic –C═C bonds, and the small peak observed at 305 nm was caused by n→π* transition of –C═O bonds of carboxyl and carbonyl groups. The ZnO-rGO absorption spectrum showed a broad peak in the 250–300 nm range. Compared to the GO absorption spectrum, the peak at 234 nm was shifted to 274 nm after reduction, and a weak peak at 305 nm vanished completely due to carboxylic acid group reduction with the regeneration of graphitic nature in ZnO-rGO NCs [27,28]. As a result of the above-mentioned observations, the position of the NC absorption peaks was very close to individual peaks, indicating a strong reaction between ZnO NPs and rGO in ZnO-rGO NCs.
The FTIR spectra of plant leaf extract, ZnO, GO, and ZnO-rGO are presented in Figure 1d. The FTIR spectrum of L. chinensis leaf extract showed a broad peak at 3,229 cm−1 assigned to vibrational stretching of –OH groups that arise due to the presence of phenols, carbohydrates, and alcohols. The peaks at 2,918 and 2,850 cm−1 regions correspond to C–H stretching of CH3 and CH2 functional groups of alkanes, and a band at 1,730 cm−1 was ascribed to −C═O stretching vibration of aldehyde, ketone, carboxylic acid, and ester. The vibrational peaks at 1,615, 1,515, and 1,454 cm−1 were also noted, which were ascribed to the bending of N–H bonds originating from amines or amides and aromatic C═C stretching. The peaks at 1,371 and 1,238 cm−1 originated from the bending of the –OH group of phenol and C–O or C–N stretching, respectively, while the corresponding peaks at 1,032 and 716 cm−1 were attributed to the C–O stretch of esters or ethers and C–X stretching [25].
When the spectra of ZnO NPs were compared with the spectra of L. chinensis leaf extract, shifting of peaks was observed in spectra, indicating the reduction of molecules. As a result, secondary metabolites, such as polyphenol functional groups, covered the ZnO NPs. From FTIR analysis, it was confirmed that bioactive compounds present in plant leaves performed dual functions for the synthesis and stabilization of ZnO NPs [29].
FTIR spectra of graphene oxide showed various characteristic absorption peaks (Figure 1d). A broad absorption peak that appeared at 3,348 cm−1 indicated –OH groups stretching vibrations present on graphene sheets and water absorbed on the surface of GO. The typical absorption peak at 2,934 cm−1 was assigned to CH2 stretching, and the peak at 1,714 cm−1 represented the −C═O stretching of carboxyl groups. Absorption peaks at 2,088 and 1,642 cm−1 were attributed to –N═C═O and –C═C stretch. Peaks observed at 1,338, 1,050, and 878 cm−1 were ascribed to stretching peaks of C–O epoxy and alkoxy groups and bending vibration ═C–H. The presence of functional groups containing oxygen proved that the graphite was oxidized to GO, and the occurrence of hydroxyl groups allowed GO to quickly establish hydrogen bonds with H2O, giving it a hydrophilic appearance [30,31].
The FTIR spectrum of ZnO-rGO NCs displayed various representative absorption bands. Compared with GO spectra, the intensity of the –OH peak at 3,239 cm−1 in ZnO-rGO NCs reduced dramatically due to the loss of –OH groups, which might be due to the bonding of –OH groups with ZnO NPs, while the absorption peak at 1,714 cm−1 for C═O was absent in NCs compared to GO, confirming the GO reduction. The stretching peak at 1,151 cm−1 represented a more powerful Zn–O–C combination. It is worth noting that after reduction, peaks of GO either vanished or appeared with considerably reduced intensities. These results are consistent with the literature [32].
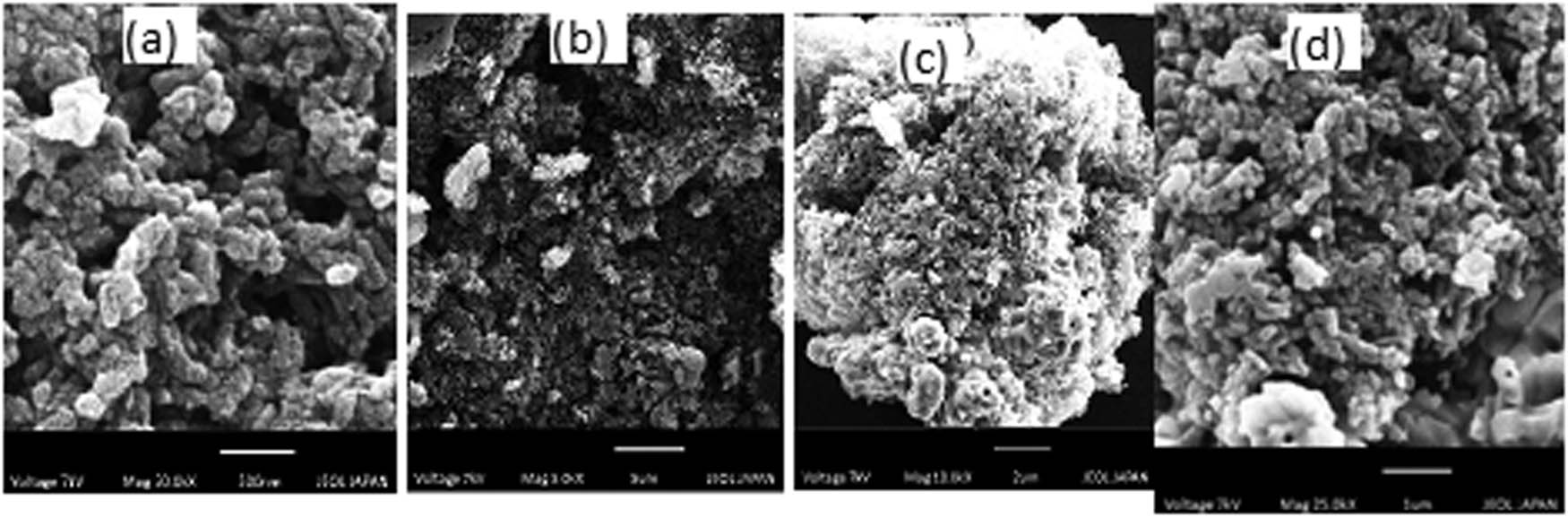
SEM analysis was conducted to analyse the morphology of nanomaterials. The SEM images of ZnO NPs have distinct spherical and hexagonal morphologies (Figure 2). The ZnO NPs that were synthesised were random [33]. The ZnO-rGO NCs were made up of clumps of ZnO nanostructures that were well anchored on the rGO sheet (Figure 3).
SEM images of ZnO NPs: (a) 500 nm, (b) 5 µm, (c) 2 µm, and (d) 1 µm.

SEM images of ZnO-rGO NCs: (a) 500 nm, (b) 5 µm, (c) 2 µm, and (d) 1 µm.
The EDX analysis was carried out to evaluate the chemical composition of prepared samples. The EDX spectra in Figure 4a and b showed the elemental composition of ZnO NPs and ZnO-rGO NCs. It revealed that all the predictable elements were available in the prepared nanomaterials. It also showed the high-purity composition of the samples by detecting only the peaks of elements in NCs. The peaks shown in the ZnO-rGO spectrum were attributed to zinc, oxygen, and carbon constituents. No other peaks of impurities were detected in the spectra [34].
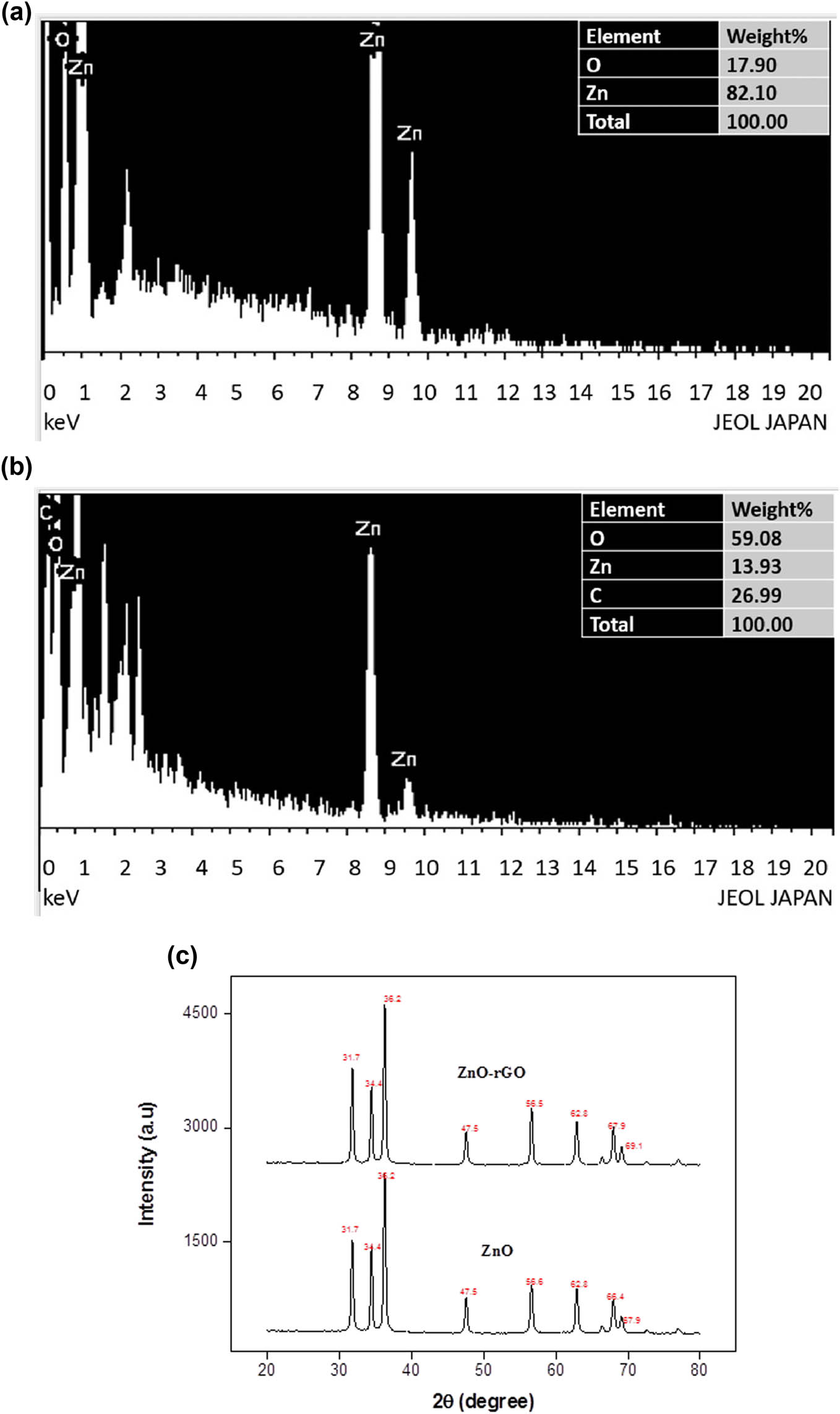
(a) EDX spectrum of ZnO NPs, (b) EDX spectrum of ZnO-rGO NCs, and (c) XRD spectra of ZnO and ZnO-rGO.
XRD patterns of ZnO NPs and ZnO-rGO are shown in Figure 4c. The strong prominent peaks on 2θ values of 31.7°, 34.4°, 36.2°, 47.5°, 56.6°, 62.8°, 66.4°, and 67.9°, for ZnO NPs, and 31.7°, 34.4°, 36.2°, 47.5°, 56.6°, 62.8°, 67.9°, 69.1°, 72.6°, and 76.9°, for NCs were observed. XRD spectra of ZnO NPs exhibited a series of diffraction peaks that perfectly indexed to highly crystalline, spherical, and hexagonal wurtzite structural patterns of ZnO NPs from JCPDS card No. 46-145. When compared to ZnO NPs, XRD spectra of prepared ZnO-rGO NCs exhibited no significant difference in peak shapes and locations, showing that the addition of rGO had no effect on the orientations of crystals of ZnO NPs. There were no peaks identified that could be attributed to foreign impurities. An average crystallite size of the ZnO NPs and ZnO-rGO NCs were 23.4 and 18.7 nm, which were calculated by the Debye–Scherrer equation [35]:
3.4 Adsorption studies
3.4.1 Adsorbent dosage effect
Adsorbent dosage effects of ZnO NPs and ZnO-rGO NCs on degradation efficiency of AMX were studied in the range of 0.5–10 mg by keeping AMX solution concentration constant (5 mg/L, V = 25 mL) for 1. The degradation efficiency decreased by increasing the dosage, as shown in Figure 5(a). This might be attributed to decreased light penetration, increased light scattering, and aggregation of ZnO-rGO NCs in aqueous solution at high adsorbent amounts, which greatly reduced the surface area for adsorption. In the case of ZnO NPs, initially, the degradation efficiency increased by increasing the catalyst amount owing to more binding sites and then declined due to the agglomeration of particles [36]. The result indicated that the degradation efficiency of ZnO-rGO NCs (96% at 0.5 mg) was maximum as compared to ZnO NPs (88% at 5 mg).

(a) Effect of adsorbent amount on AMX degradation efficiency, (b) influence of initial AMX concentration on its degradation efficiency, (c) pH effect on the AMX degradation efficiency, (d) effect of temperature on degradation efficiency of AMX, and (e) determination of pHpcz of ZnO and ZnO-rGO.
3.4.2 Effect of initial AMX concentration
The influence of AMX initial concentration was studied by adjusting AMX solution concentration from 5 to 25 mg/L (V = 25 mL) and keeping the amounts of both catalysts constant (ZnO = 5 mg, ZnO-rGO = 0.5 mg) for a contact time of 60 min. The results are presented in Figure 5(b), which revealed that at a lower concentration (5 mg/L), maximum degradation of AMX occurred as compared to higher concentrations because active sites present on catalysts were sufficient, whereas, at a higher concentration, degradation of AMX dropped because more and more molecules of AMX adsorbed on the catalyst's surface and fully covered the surface of active sites and also reduced the generation of active oxidizing •OH radicals which decreased the degradation of AMX. The saturated active sites of catalysts showed repulsion for incoming AMX molecules and reduced adsorption [37]. The result showed that ZnO-rGO has greater degradation efficiency (92%) compared to ZnO NPs (83%) at the same AMX concentration and reaction time.
3.4.3 Effect of pH
To find out the effect of pH on AMX degradation, the pH was adjusted from 2 to 10 by keeping the concentration of AMX solution and catalysts constant during the entire experiment. The pH of the original AMX solution was 6. The result is presented in Figure 5(c), which depicts that the degradation of AMX increased by increasing the pH of the solution. The highest degradation of AMX occurred in an alkaline medium could be due to two facts. First, the presence of a large number of OH− ions on the surface and in an aqueous solution promoted ˙OH radical formation that has been regarded as potent oxidizing agents in catalytic processes that initiate degradation of adsorbed AMX molecules. Second, the reason was hydrolysis of AMX attributed to β-lactam ring’s instability at high pH [38].
3.4.4 Temperature effect
The temperature effect on AMX degradation efficiency was evaluated at four temperatures (308, 318, 328, and 338 K). The percentage degradation increased by increasing the temperature as presented in Figure 5(d). This might be attributed to the presence of more adsorbent sites because of the activation of adsorbent surfaces at high temperatures. The rate of diffusion of AMX molecules from aqueous solution onto the adsorbent surface enhanced as the temperature elevated. The removal of AMX was facilitated at higher temperatures due to the breakdown of the C–N bond of the β-lactam ring [39]. The result illustrated that AMX adsorption on the adsorbent's surface was endothermic.
3.4.5 pHpzc of ZnO and ZnO-rGO
The pHpzc values calculated for synthesised nanomaterials were 5.2 (ZnO Nps) and 5.4 (ZnO-rGO), as shown in Figure 5(e). The pHpzc is the value of pH at which the adsorbent surface is neutral. When the solution’s pH is less than pHpzc, the adsorbent surface has a positive charge and vice versa. In this case, the pH of the solution is 6, which is higher than pHpzc. As a result, the synthesised adsorbents are negatively charged [40].
3.5 Antioxidant potential
3.5.1 DPPH free radical scavenging activity
The free radical scavenging activity of nanomaterials was evaluated by DPPH (1,1-diphenyl-2-picrylhydrazyl), a stable free radical organic molecule. The DPPH scavenging method based on DPPH molecule reduction. The antioxidant activity of synthesised nanomaterials is owing to their reducing power. Nanomaterials neutralized DPPH free radicals and formed a yellow-coloured stable non-radical molecule. The disappearance of the purple colour indicated the scavenging potential of nanomaterials [41]. The results presented in Figure 6 showed that with the increase in the amount of prepared nanomaterials, the ability to scavenge DPPH free radicals also increased. The scavenging ability of ZnO-rGO (93%) was greater than that of ZnO NPs (88%). It was observed that ZnO-rGO composites were effective in inhibiting DPPH, which was primarily caused by electron charge transfer.
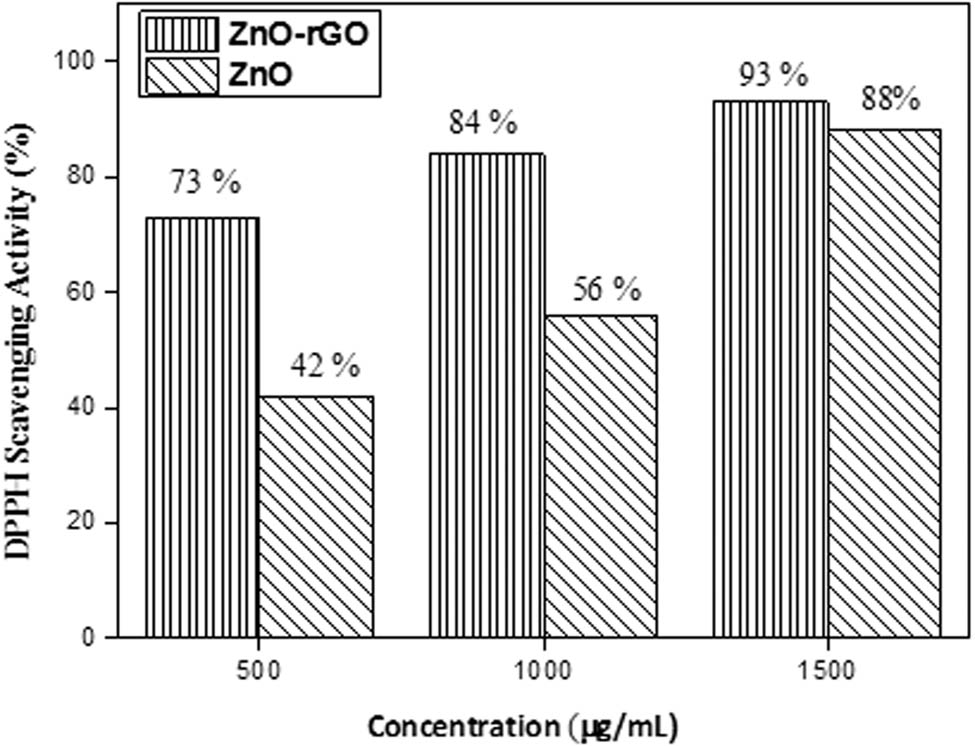
% Scavenging of DPPH radical by ZnO NPs and ZnO-rGO NCs.
3.5.2 TPCs
Using Folin–Ciocalteu reagents, the TPCs of ZnO and ZnO-rGO synthesised by the L. chinensis plant were determined spectrophotometrically. This method relies on the formation of the blue complex as a result of the interaction of phenolic compounds with the Folin–Ciocalteu reagent [42]. The calculated TPC of nanomaterials was measured as given in Table 1. The result implied that ZnO-rGO showed a high TPC, i.e., 71.6 ± 0.02 mg/g GAE compared to ZnO 45.7 ± 0.04 mg/g GAE.
Results of total phenolic content and total antioxidant activity
| Sample | TPC (GAE mg/g of sample) | Total antioxidant activity |
|---|---|---|
| ZnO NPs | 45.7 ± 0.04 | 0.510 ± 0.01 |
| ZnO-rGO NCs | 71.6 ± 0.02 | 0.644 ± 0.04 |
| Blank | 11.2 ± 0.03 | — |
| BHT | 0.813 ± 0.05 |
3.5.3 Total antioxidant activity
The total antioxidant capacity of synthesised nanomaterials was assessed spectrophotometrically using the phosphomolybdenum method. This method depends on the reduction of Mo(vi) to Mo(v) by antioxidants contained in the sample material at an acidic pH, which results in the preparation of the green-coloured phosphate–molybdenum complex, commonly used to identify the antioxidants such as phenolic, carotenoids, and tocopherols [41]. The results of total antioxidant activity are presented in Table 1, which indicates that the antioxidant activity of prepared ZnO-rGO (0.644 ± 0.04) was better than that of ZnO NPs (0.510 ± 0.01).
3.6 Adsorption isotherm
The adsorption isotherm is the most essential parameter that summarises how adsorbate molecules interact with the adsorbent. This study used the two most common isotherm models, Langmuir and Freundlich. A linear form of Langmuir and Freundlich isotherm models is presented by the following equations [43]:
where q e is the amount of AMX adsorbed at equilibrium, C e is the equilibrium concentration of AMX in solution, q m is maximum adsorption capacity, and K L is the Langmuir constant. The values of q m and K L were obtained from the plot C e/q e vs C e, and the values of K f and 1/n were derived from the plot of log q e vs log C e.
The isotherm parameters are shown in Table 2, indicating the value of R 2 (correlation coefficient) for both models was >0.9, but AMX adsorption on ZnO and ZnO-rGO was better explained by the Freundlich model because the values of R 2 for Freundlich were greater than R 2 for the Langmuir model. A similar result was reported in the literature [44]. The 1/n value described the favourability if its value was less than 1, it indicated that adsorption was favourable [45].
Langmuir and Freundlich isotherm parameters
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| Adsorbent | q m (mg/g) | K L (L/mg) | R 2 | K f (mg/g) | 1/n | R 2 |
| ZnO | 109.7 | 0.25 | 0.988 | 23.7 | 0.554 | 0.991 |
| ZnO-rGO | 909.0 | 0.53 | 0.928 | 328.3 | 0.395 | 0.981 |
3.7 Thermodynamics studies
Thermodynamic parameters for the adsorption of AMX on ZnO NPs and ZnO-rGO NCs were examined at four different temperatures (308, 318, 328, and 338 K). It was demonstrated that higher temperatures enhanced AMX adsorption on both adsorbents. Thermodynamic parameters including ∆G 0, ∆H 0, and ∆S 0 are calculated using the following equations:
where K d depicts the distribution coefficient, R is the general gas constant (8.314 J/mol K), and T is the temperature (K).
Thermodynamic parameters are presented in Table 3. At all temperatures, values of ∆G 0 were negative, indicating that AMX adsorption on both adsorbents was feasible and a spontaneous process. Moreover, a decrease in negative values of ∆G 0, as temperature increased, revealed that the adsorption of AMX on ZnO and ZnO-rGO becomes more favourable at higher temperatures. The positive value of enthalpy also implied that the process was endothermic. Furthermore, the positive value of ∆S 0 implies that randomness was increasing throughout the adsorption process [46].
Effect of temperature on AMX adsorption and thermodynamics parameters
| Adsorbent | T (K) | q m (mg/g) | Thermodynamics parameters | ||
|---|---|---|---|---|---|
| ∆G (kJ mol−1) | ∆H (kJ mol−1) | ∆S (J mol−1K−1) | |||
| ZnO | 308 | 21.75 | −8.98 | ||
| 318 | 22.25 | −9.78 | 19.67 | 92.86 | |
| 328 | 22.75 | −10.69 | |||
| 338 | 23.25 | −11.79 | |||
| ZnO-rGO | 308 | 225 | −15.6 | ||
| 318 | 232.5 | −17.18 | 46.77 | 201.66 | |
| 328 | 237.5 | −18.69 | |||
| 338 | 245 | −21.92 | |||
3.8 Degradation mechanism of AMX
The degradation experiment was carried out at room temperature (35°C). When AMX solution and catalyst (ZnO-rGO) were subjected to diffuse light, a hole (h+) was created in VB of ZnO. These electrons were received at the surface of rGO, which was a good electron acceptor, and reacted with O2 available in an aqueous solution, forming superoxide radical (˙O2 −). On the other hand, hole (h+) generated in VB captured H2O or OH− ions and produced ˙OH radicals, which acted as oxidizing agents, converting AMX into degraded products. The degradation mechanism relies on the electron–hole pair recombination rate. The recombination rate was quite fast in pure ZnO, which eventually delayed the degradation rate. However, in ZnO-rGO, excited electrons were received at the surface of rGO, which increased the time duration of the electron pair recombination period and accelerated the degradation process. First, AMX gets adsorbed on the adsorbent material by π–π interaction between aromatic rings of rGO and AMX, and then its degradation takes place. The mechanism of AMX degradation is presented in the following steps [35]:
3.9 HPLC analysis
The degradation of AMX was confirmed by HPLC analysis. A well-defined chromatogram of standard AMX, as shown in Figure 7(a), is obtained at a retention time of 2.70 min with a 1 mL/min flow rate of mobile phase (methanol:DI water) at 228 nm wavelength. No peak was observed at a retention time of 2.70 min in chromatograms of AMX degraded by ZnO-rGO and ZnO in Figure 7(b) and (c), which confirmed the degradation of AMX into its by-products. The degraded products reported in the literature are AMX penicilloic acid, AMX penicilloic acid, AMX diketopiperazine, and phenol hydroxypyrazine [47].
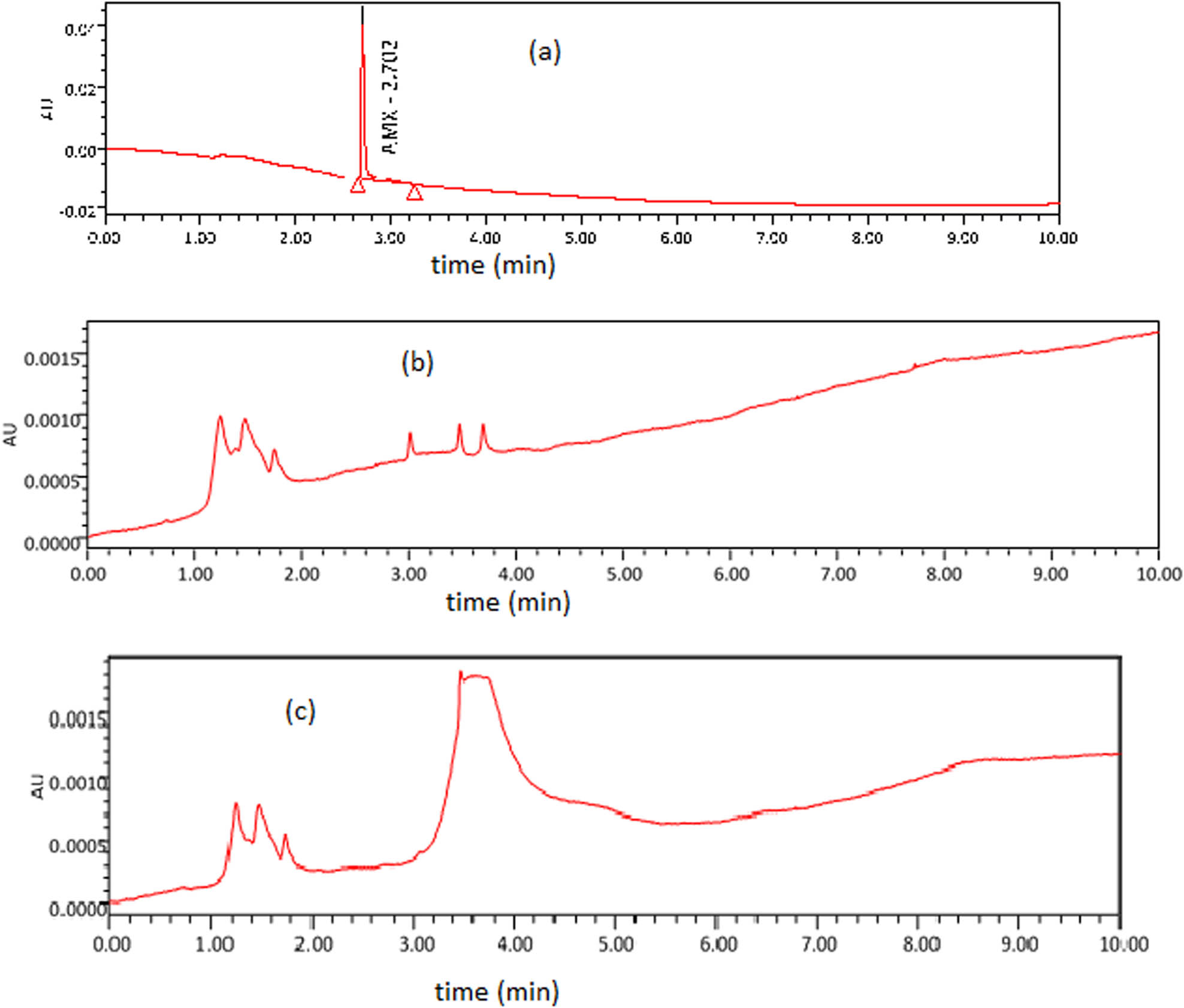
(a) HPLC chromatogram of AMX, (b) HPLC chromatogram of AMX degraded products after reaction with ZnO-rGO, and (c) HPLC chromatogram of AMX degraded products after reaction with ZnO.
3.10 Comparison with literature
AMX degradation efficiency of ZnO and ZnO-rGO has been compared with other adsorbing materials, as shown in Table 4.
Comparison of AMX degradation efficiency of catalysts with other adsorbing material
| Adsorbent material | Synthesis method | Light source | Concentration of AMX (mg/L) | Adsorbent dosage (mg) | Contact time (min) | Removal efficiency (%) | References |
|---|---|---|---|---|---|---|---|
| ZnO NPs | UV light | 15 | 2 g/L | 15 | 48.6 | [48] | |
| ZnO NPs | Precipitation method | Ultrasound | 6.25 | 0.1 g | 60 | 99 | [49] |
| ZnO NPs | UV light | 104 | 0.5 g/L | 180 | 100 | [50] | |
| ZnO NPs | Ultrasonic waves | 150 | 0.05 g/L | 60 | 92.47 | [51] | |
| ZnO–TiO2 nanocomposite | Ball milling | visible light | 100 | 0.1 g/L | 70 | 80 | [4] |
| GO/TiO2 | UV light | 50 | 0.4 g/L | 60 | 99.84 | [46] | |
| NiO | Sol–gel process | UV light | 25 | 0.2 g/L | 120 | 96 | [45] |
| TiO2 | UV light | 30 | 450 mg/L | 270 | 80 | [52] | |
| Iron oxide nanoparticles (gINPs) | Green synthesis (Ceratonia siliqua) | Closed system | 5 | 0.04 g/L | 200 | 99 | [39] |
| Mn-doped Cu2O NPs | Green synthesis (Aloe vera) | Sunlight | 15 | 1 g/L | 180 | 92 | [38] |
| Ag/ZnO NPs | UV light | 5 | 0.15 g/L | 120 | 93.7 | [53] | |
| ZnO NPs | Green Synthesis (L. chinensis) | 5 | 5 mg | 60 | 85 | Present work | |
| ZnO-rGO NCs | Green Synthesis (L. chinensis) | 5 | 0.5 mg | 60 | 96 | Present work |
3.11 Recyclability of catalysts
Recyclability is a crucial feature of heterogeneous catalysts for their practical applications. The reusability of ZnO NPs and ZnO-rGO was investigated under optimum conditions for four consecutive cycles. The catalysts were collected after each run from the reaction mixture by centrifugation, washed with distilled water, and dried in an oven at 60°C. It was found that the degradation of AMX by ZnO-rGO is slightly reduced from 96 to 93% after four cycles, while in the case of ZnO NPs, AMX degradation decreased from 85 to 80%, as shown in Figure 8. Figure 8 shows the recyclability of ZnO NPs and ZnO-rGO for the degradation of AMX. A small loss in the catalytic efficiency confirmed that both catalysts have good stability [49].
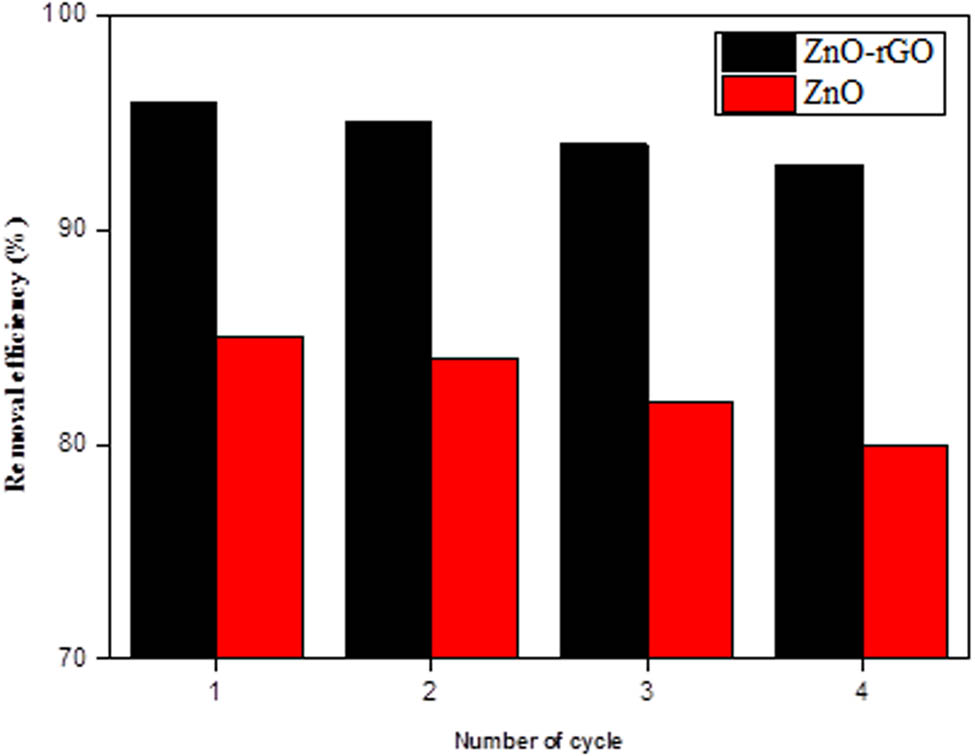
Recyclability of catalysts.
4 Conclusion
ZnO NPs and ZnO-rGO NCs were prepared using the leaf extract of the L. chinensis plant and applied in AMX degradation. The spherical and hexagonal morphologies of prepared nanomaterials were confirmed by SEM analysis. The particle sizes of ZnO and ZnO-rGO were 23.4 and 18.7 nm, respectively, as calculated by XRD measurement. The influence of parameters such as the dose of catalyst, AMX concentration, pH, and temperature on the degradation of AMX was also examined. AMX showed maximum degradation under optimum conditions, i.e., 5 mg/L of solution concentration, 65°C temperature, adsorbent dosages of 5 mg (ZnO) and 0.5 mg (ZnO-rGO), and pH values of 8 (ZnO) and 10 (ZnO-rGO). The catalytic activity of ZnO-rGO NCs showed higher potentiality towards AMX degradation (96%) as compared to ZnO NPs (85%). The antioxidant potential of synthesised nanomaterials was also evaluated, and the result showed that NCs exhibited a higher antioxidant potential than ZnO NPs. The isotherm and thermodynamics analysis revealed that AMX adsorption followed the Freundlich model more closely. Also, it was an endothermic and spontaneous process. So, this research demonstrated that the prepared NC was potent, highly efficient, and suitable adsorbents for the removal and degradation of AMX. It was also confirmed by HPLC.
-
Funding information: S. J. Park was supported by the Basic Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2018R1A6A1A03025526 and No. 2020R1I1A3063782). This work was also supported by the BK-21 FOUR program through the National Research Foundation of Korea (NRF) under the Ministry of Education. The authors thank the Cooperative Equipment Center at KOREATECH for assistance with SEM analysis. This work was funded by the Researchers Supporting Project Number (Project No. RSPD2023R672), King Saud University, Riyadh, Saudi Arabia.
-
Author contribution: M.A.: writing original manuscript; T.S., S.J.P.: supervision; T.R.: validation; M.Z.: data curation, methodology; F.N.: resources; A.M.T.: software; M.R.K., D.W.: revising manuscript. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All data generated or analysed during this study are included in this published article.
References
[1] Sharma M, Rajput D, Kumar V, Jatain I, Aminabhavi TM, Mohanakrishna G, et al. Photocatalytic degradation of four emerging antibiotic contaminants and toxicity assessment in wastewater: A comprehensive study. Environ Res. 2023;231:116132. 10.1016/j.envres.2023.116132.Search in Google Scholar PubMed
[2] Nassri I, Souabi S. Occurrence, pollution sources, and mitigation prospects of Antibiotics, anti-inflammatories, and endocrine disruptors in the aquatic environment. Environ Nanotechnol Monit Manag. 2023;20:100878. 10.1016/j.enmm.2023.100878.Search in Google Scholar
[3] Jian Z, Zeng L, Xu T, Sun S, Yan S, Yang L, et al. Antibiotic resistance genes in bacteria: Occurrence, spread, and control. J Basic Microbiol. 2021;61(12):1049–70. 10.1002/jobm.202100201.Search in Google Scholar PubMed
[4] Thi TDN, Nguyen LH, Nguyen XH, Phung HV, Vinh THT, Van Viet P, et al. Enhanced heterogeneous photocatalytic perozone degradation of amoxicillin by ZnO modified TiO2 nanocomposites under visible light irradiation. Mater Sci Semicond Proc. 2022;142:106456. 10.1016/j.mssp.2022.106456.Search in Google Scholar
[5] Cetecioglu Z, Atasoy M. Biodegradation and inhibitory effects of antibiotics on biological wastewater treatment systems. Toxicity and Biodegradation Testing. New York, NY: Humana Press; 2018. p. 29–55.10.1007/978-1-4939-7425-2_2Search in Google Scholar
[6] Tang X, Fan W, Zhang S, Yan B, Zheng H. The improvement of levofloxacin and tetracycline removal from simulated water by thermosensitive flocculant: Mechanisms and simulation. Sep Purif Technol. 2023;309:123027. 10.1016/j.seppur.2022.123027.Search in Google Scholar
[7] Guo Y. Removal ability of antibiotic resistant bacteria (Arb) and antibiotic resistance genes (Args) by membrane filtration process. IOP Conference Series: Earth Environ Sci. Vol. 801. IOP Publishing; 2021. p. 012004. 10.1088/1755-1315/801/1/012004.Search in Google Scholar
[8] Subaihi A, Shahat A. Synthesis and characterization of super high surface area silica-based nanoparticles for adsorption and removal of toxic pharmaceuticals from aqueous solution. Jo Mol Liq. 2023;378:121615. 10.1016/j.molliq.2023.121615.Search in Google Scholar
[9] Zhang D, Liu Y, Song Y, Sun X, Liu W, Duan J, et al. Synergistic effect of Fe and Ce on Fe doped CeO2 for catalytic ozonation of amoxicillin: Efficiency evaluation and mechanism study. Sep Purif Technol. 2023;31:123430. 10.1016/j.seppur.2023.123430.Search in Google Scholar
[10] Al-Musawi TJ, Yilmaz M, Ramírez-Coronel AA, Al-Awsi GRL, Alwaily ER, Asghari A, et al. Degradation of amoxicillin under a UV or visible light photocatalytic treatment process using Fe2O3/bentonite/TiO2: Performance, kinetic, degradation pathway, energy consumption, and toxicology studies. Optik. 2023;272:170230. 10.1016/j.ijleo.2022.170230.Search in Google Scholar
[11] Laksaci H, Belhamdi B, Khelifi O, Khelifi A, Trari M. Elimination of amoxicillin by adsorption on coffee waste based activated carbon. J Mol Struct. 2023;274:134500. 10.1016/j.molstruc.2022.134500.Search in Google Scholar
[12] Lonkar SP, Pillai V, Abdala A. Solvent-free synthesis of ZnO-graphene nanocomposite with superior photocatalytic activity. Appl Surf Sci. 2019;465:1107–13. 10.1016/j.apsusc.2018.09.264.Search in Google Scholar
[13] Yaqoob AA, Mohd Noor NHB, Serrà A, Mohamad Ibrahim MN. Advances and challenges in developing efficient graphene oxide-based ZnO photocatalysts for dye photo-oxidation. Nanomater. 2020;10:932. 10.3390/nano10050932.Search in Google Scholar PubMed PubMed Central
[14] Zhou Y, Cao S, Xi C, Li X, Zhang L, Wang G, et al. A novel Fe3O4/graphene oxide/citrus peel-derived bio-char based nanocomposite with enhanced adsorption affinity and sensitivity of ciprofloxacin and sparfloxacin. Bioresour Technol. 2019;292:121951. 10.1016/j.biortech.2019.121951.Search in Google Scholar PubMed
[15] Ramesh AM, Pal K, Kodandaram A, Manjula BL, Ravishankar DK, Gowtham HG, et al. Antioxidant and photocatalytic properties of zinc oxide nanoparticles phyto-fabricated using the aqueous leaf extract of Sida acuta. Green Proc Synth. 2022;11(1):857–67. 10.1515/gps-2022-0075.Search in Google Scholar
[16] Hamed R, Obeid RZ, Abu-Huwaij R. Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications. Nanotechnol Rev. 2023;12(1):20230112. 10.1515/ntrev-2023-0112.Search in Google Scholar
[17] Sadhukhan S, Bhattacharyya A, Rana D, Ghosh TK, Orasugh JT, Khatua S, et al. Synthesis of RGO/NiO nanocomposites adopting a green approach and its photocatalytic and antibacterial properties. Mater Chem Phys. 2020;247:122906. 10.1016/j.matchemphys.2020.122906.Search in Google Scholar
[18] Rad SS, Sani AM, Mohseni S. Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Mentha pulegium (L.). Microb Pathog. 2019;131:239–45. 10.1016/j.micpath.2019.04.022.Search in Google Scholar PubMed
[19] Kanwal A, Shahzadi T, Riaz T, Zaib M, Khan S, Habila MA, et al. Photocatalytic degradation studies of organic dyes over novel Cu/Ni loaded reduced graphene oxide hybrid nanocomposite: adsorption, kinetics and thermodynamic studies. Mol. 2023;28(18):6474. 10.3390/molecules28186474.Search in Google Scholar PubMed PubMed Central
[20] Lee KG, Shibamoto T. Antioxidant property of aroma extract isolated from clove buds Syzygium aromaticum (L.) [Merr. et Perry]. Food Chem. 2001;74:443–8. 10.1016/s0308-8146(01)00161-3.Search in Google Scholar
[21] Makkar HPS, Blümmel M, Borowy NK, Becker K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J Sci Food Agric. 1993;61:161–5. 10.1002/jsfa.2740610205.Search in Google Scholar
[22] Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–41. 10.1006/abio.1999.4019.Search in Google Scholar PubMed
[23] Balarak D, Mahvi AH, Shim MJ, Lee SM. Adsorption of ciprofloxacin from aqueous solution onto synthesized NiO: isotherm, kinetic and thermodynamic studies. Desalin Water Treat. 2021;212:390–400. 10.5004/dwt.2021.26603.Search in Google Scholar
[24] Shahzadi T, Anwaar A, Riaz T, Zaib M. Sulfate and phosphate ions removal using novel nano-adsorbents: modeling and optimization, kinetics, isotherm and thermodynamic studies. Int J Phytoremediat. 2022;24:1518–32. 10.1080/15226514.2022.2040421.Search in Google Scholar PubMed
[25] Kaur R, Avti P, Kumar V, Kumar R. Effect of various synthesis parameters on the stability of size controlled green synthesis of silver nanoparticles. Nano Express. 2021;2:020005. 10.1088/2632-959x/abf42a.Search in Google Scholar
[26] Agarwal V, Zetterlund PB. Strategies for reduction of graphene oxide–A comprehensive review. Chem Eng J. 2021;405:127018. 10.1016/j.cej.2020.127018.Search in Google Scholar
[27] Ahmadi R, Nafchi RF, Sangpour P, Bagheri M, Badiei E. A comparative study: Green synthesis and evaluation of ZnO-GO and ZnO-RGO nanocomposites for antibacterial applications. Mater Sci Eng. 2023;294:116555. 10.2139/ssrn.4270332.Search in Google Scholar
[28] Kumar S, Ojha AK, Walkenfort B. Cadmium oxide nanoparticles grown in situ on reduced graphene oxide for enhanced photocatalytic degradation of methylene blue dye under ultraviolet irradiation. J Photochem Photobiol B: Biol. 2016;159:111–9. 10.1016/j.jphotobiol.2016.03.025.Search in Google Scholar PubMed
[29] Sai Saraswathi V, Tatsugi J, Shin PK, Santhakumar K. Facile biosynthesis, characterization, and solar assisted photocatalytic effect of ZnO nanoparticles mediated by leaves of L. speciosa. J Photochem Photobiol B: Biol. 2017;167:89–98. 10.1016/j.jphotobiol.2016.12.032.Search in Google Scholar PubMed
[30] Murali A, Sarswat PK, Free ML. Minimizing electron-hole pair recombination through band-gap engineering in novel ZnO-CeO2-rGO ternary nanocomposite for photoelectrochemical and photocatalytic applications. Env Sci Pollut Res. 2020;27:25042–56. 10.1007/s11356-020-08990-z.Search in Google Scholar PubMed
[31] Hidayah NMS, Liu WW, Lai CW, Noriman NZ, Khe CS, Hashim U et al. Comparison on graphite, graphene oxide and reduced graphene oxide: Synthesis and characterization. AIP Conference Proceedings. AIP Publishing LLC; 2017. p. 150002.10.1063/1.5005764Search in Google Scholar
[32] Boukhvalov DW, Katsnelson MI. Tuning the gap in bilayer graphene using chemical functionalization: Density functional calculations. Phys Rev B. 2008;78(8):085413. 10.1103/physrevb.78.085413.Search in Google Scholar
[33] Rajiv P, Rajeshwari S, Venckates R. Bio-Fabrication of zinc oxide nanoparticles using leaf extract of Parthenium hysterophorus L. and its size-dependent antifungal activity against plant fungal pathogens. Spectrochim Acta Mol Biomol Spectrosc. 2013;112:384–7. 10.1016/j.saa.2013.04.072.Search in Google Scholar PubMed
[34] Jain B, Hashmi A, Sanwaria S, Singh AK, Susan MABH, Singh A. Zinc oxide nanoparticle incorporated on graphene oxide: an efficient and stable photocatalyst for water treatment through the Fenton process. Adv Compos Hybrid Mater. 2020;3:231–42. 10.1007/s42114-020-00153-5.Search in Google Scholar
[35] Gang R, Xu L, Xia Y, Zhang L, Wang S, Li R. Facile one-step production of 2D/2D ZnO/rGO nanocomposites under microwave irradiation for photocatalytic removal of tetracycline. ACS Omega. 2021;6(5):3831–9. 10.1021/acsomega.0c05559.Search in Google Scholar PubMed PubMed Central
[36] Wu S, Zhao X, Li Y, Du Q, Sun J, Wang Y, et al. Adsorption properties of doxorubicin hydrochloride onto graphene oxide: equilibrium, kinetic and thermodynamic studies. Mater. 2013;6:2026–42. 10.3390/ma6052026.Search in Google Scholar PubMed PubMed Central
[37] Zhao D, Sheng G, Chen C, Wang X. Enhanced photocatalytic degradation of methylene blue under visible irradiation on graphene@TiO2 dyade structure. Appl Catal B: Environ. 2012;111–112:303–8. 10.1016/j.apcatb.2011.10.012.Search in Google Scholar
[38] Gaim YT, Yimanuh SM, Kidanu ZG. Enhanced photocatalytic degradation of amoxicillin with MN-doped cu2o under sunlight irradiation. J Compos Sci. 2022;6(10):317. 10.3390/jcs6100317.Search in Google Scholar
[39] Aksu Demirezen D, Yıldız YE, Demirezen Yılmaz D. Amoxicillin degradation using green synthesized iron oxide nanoparticles: Kinetics and mechanism analysis. Env Nanotechnol Monit Manag. 2019;11:100219. 10.1016/j.enmm.2019.100219.Search in Google Scholar
[40] Rodrigues DLC, Machado FM, Osório AG, de Azevedo CF, Lima EC, da Silva RS, et al. Adsorption of amoxicillin onto high surface area–activated carbons based on olive biomass: kinetic and equilibrium studies. Env Sci Pollut Res. 2020;27:41394–404. 10.1007/s11356-020-09583-6.Search in Google Scholar PubMed
[41] Shahzadi T, Rehman S, Riaz T, Zaib M. Eco-friendly synthesis of ZnO nanoparticles using Cannabis sativa and assessment of its activities as efficient dyes removal and antioxidant agent. Int J Env Anal Chem. 2022;102(16):4738–56. 10.1080/03067319.2020.1789610.Search in Google Scholar
[42] Masfria S, Dalimunthe Rasayan A. Determination of total phenolic content, total flavonoid content, and antimutagenic activity of ethanol extract nanoparticles of Rhaphidophora Pinnata (L.F) Schott leaves. Rasayan J Chem. 2018;11:505–10. 10.31788/rjc.2018.1122068.Search in Google Scholar
[43] Balarak D, Mostafapour FK, Joghtaei A. Thermodynamic analysis for adsorption of amoxicillin onto magnetic carbon nanotubes. Br J Pharma Res. 2017;16:1–11. 10.9734/bjpr/2017/33212.Search in Google Scholar
[44] Duman O, Ayranci E. Adsorption characteristics of benzaldehyde, sulphanilic acid, and p‐phenolsulfonate from water, acid, or base solutions onto activated carbon cloth. Sep Sci Technol. 2006;41:3673–92. 10.1080/01496390600915072.Search in Google Scholar
[45] Balarak D, Mostafapour FK. Photocatalytic degradation of amoxicillin using UV/synthesized NiO from pharmaceutical wastewater. Indones J Chem. 2019;19:211. 10.22146/ijc.33837.Search in Google Scholar
[46] Balarak D, Mengelizadeh N, Rajiv P, Chandrika K. Photocatalytic degradation of amoxicillin from aqueous solutions by titanium dioxide nanoparticles loaded on graphene oxide. Env Sci Pollut Res. 2021;28(36):49743–54. 10.1007/s11356-021-13525-1.Search in Google Scholar PubMed
[47] Gozlan I, Rotstein A, Avisar D. Amoxicillin-degradation products formed under controlled environmental conditions: Identification and determination in the aquatic environment. Chemosphere. 2013;91:985–92. 10.1016/j.chemosphere.2013.01.095.Search in Google Scholar PubMed
[48] Fazilati M, Hassani A, Torabian A. Photocatalytic degradation of amoxicillin and cephalexin from aqueous solution by Zno And Tio2. In International Congress on Engineering Science and Sustainable Urban Development Denmark; 2018. p. 1–10.Search in Google Scholar
[49] Ayanda OS, Aremu OH, Akintayo CO, Sodeinde KO, Igboama WN, Oseghe EO, et al. Sonocatalytic degradation of amoxicillin from aquaculture effluent by zinc oxide nanoparticles. Env Nanotechnol Monit Manag. 2021;16:100513. 10.1016/j.enmm.2021.100513.Search in Google Scholar
[50] Elmolla ES, Chaudhuri M. Degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution by the UV/ZnO photocatalytic process. J Hazard Mater. 2010;173(1–3):445–9. 10.1016/j.jhazmat.2009.08.104.Search in Google Scholar PubMed
[51] Rahdar S, Ahmadi S. The removal of amoxicillin with Zno nanoparticles in combination with US-H2O2 advanced oxidation processes from aqueous solutions. Iran J Health Sci. 2019;7(1):36–45. 10.18502/jhs.v7i1.1023.Search in Google Scholar
[52] Verma M, Haritash A. Photocatalytic degradation of amoxicillin in pharmaceutical wastewater: A potential tool to manage residual antibiotics. Env Technol Innov. 2020;20:101072. 10.1016/j.eti.2020.101072.Search in Google Scholar
[53] Rezaei MR, Sayadi MH, Ravankhah N. Photocatalytic degradation of amoxicillin and levofloxacin from aqueous solutions using Ag/ZnO. J Nat Environ. 2021;74(2):331–44. 10.22059/JNE.2021.318062.2160.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy


