Abstract
Inflammatory bowel disease (IBD) encompasses a group of chronic inflammatory disorders that affect the gastrointestinal tract, with Crohn’s disease and ulcerative colitis being the primary subtypes. Diagnosis and treatment of IBD are challenging due to their unknown etiology and complex pathology. Smart bionanomaterials, which are biocompatible nanometer-sized materials that respond to external stimuli, can be used in the treatment and diagnosis of diseases. In the context of IBD, these materials can deliver drugs, primarily aminosalicylates, and corticosteroids, as well as live probiotics to the inflamed parts of the intestine, with a specific focus on the colon. The controlled release of drugs can be triggered by the conditions present in the IBD-affected intestine, such as inflammation, anaerobic environment, neutral pH, and gut microbiota. This article provides an overview of the use of smart bionanomaterials, including hydrogels, nanoparticles, nanofibers, and hybrid systems. It discusses their manufacturing process and their ability to deliver active ingredients in response to various stimuli, such as pH, temperature, reactive oxygen species, magnetic field, and biomolecules, for the treatment of IBD. We also describe the use of smart probiotics, which have been genetically engineered to recognize specific stimuli and synthesize recombinant proteins for the treatment of IBD. The qualitative or quantitative response to inflammatory stimuli can be exploited in diagnostic applications, with some examples already developed. Smart bionanomaterials offer several advantages, such as encapsulation, targeted delivery, responsiveness to stimuli, and controlled release. These features make them a valuable adjunct tool in the diagnosis and treatment of IBD.
Abbreviations
- 5-ASA
-
5-aminosalicylic acid
- CD
-
Crohn’s disease
- DSS
-
dextran sulfate sodium
- EcN
-
Escherichia coli Nissle 1917
- FOS
-
fructooligosaccharides
- GM-CSF
-
granulocyte-macrophage colony-stimulating factor
- HA
-
hyaluronic acid
- IBD
-
inflammatory bowel disease
- IFNL1
-
interferon lambda 1
- IL
-
interleukin
- KGF
-
keratinocyte growth factor
- MC3, DLin-MC3-DMA; DMG-PEG
-
1,2-dimyristoyl-sn-glycero-3-methoxy(polyethylene glycol); SPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)].
- mRNA
-
messenger RNA
- NO
-
nitric oxide
- PBS
-
phosphate-buffered saline
- PEG
-
polyethylene glycol
- PLA
-
polylactic acid
- PLGA
-
polylactide-co-glycolic acid
- PVA
-
polyvinyl alcohol
- ROS
-
reactive oxygen species
- SCFA
-
short-chain fatty acid
- siRNA
-
small-interfering RNA
- SOD
-
superoxide dismutase
- TGF-β
-
transforming growth factor-β
- TNBS
-
2,4,6-trinitrobenzenesulfonic acid
- TNF-α
-
tumor necrosis factor alpha
- UC
-
ulcerative colitis
1 Introduction
Smart materials are materials that can respond to external stimuli, such as chemicals, light, pH, temperature, or magnetic field, and change their shape, form, and color [1]. They can be designed from various polymers, gels, metals, fibers, minerals, alloys, and composites and can be applied in various fields of science and technology [2,3]. Smart materials have become increasingly popular in recent decades and are of particular interest in medical applications. For example, a smart wireless theranostic contact lens was developed using smart materials to monitor and control intraocular pressure in glaucoma [4]. Conductive hydrogel cardiac patches containing smart materials have shown promising results in the treatment of myocardial infarction [5]. Hydrogels based on hyaluronic acid (HA) show suitable biocompatibility and have great potential for bioprinting of skin, cartilage, nerve tissue, and bone [6]. Some of these materials are called bionanomaterials because of their biocompatibility (as defined by regulatory agencies such as the European Medicines Agency and United States Food and Drug Administration) and nanoscale dimensions. They are synthetic or natural substances that can interact with the human body at different levels in harmless or beneficial manner [7,8]. Bionanomaterials can be used to develop drug delivery systems, especially for oral administration. These materials contribute to the efficacy of delivery systems by encapsulating different active ingredients with therapeutic effects.
Inflammatory bowel disease (IBD) is a chronic inflammatory disease of the gastrointestinal tract. The exact etiology of IBD remains unknown; however, it is characterized by an abnormal immune response and alterations in the gut microbiota and is associated with genetic susceptibility and environmental triggers [9]. The Global Burden of Diseases, Injuries, and Risk Factors Study analyzed data on a variety of diseases, including IBD, from 195 countries and territories between 1990 and 2019. Globally, the prevalence of IBD increased from 3.32 million in 1990 to 4.90 million in 2019, marking a 47% increase. Additionally, in the same time period, the number of deaths attributed to IBD increased by 69%, years lived with disability increased by 48% and years of life lost increased by 18% [10].
Ulcerative colitis (UC) and Crohn’s disease (CD) are two major subtypes of IBD. They are characterized by specific clinical symptoms, such as diarrhea, abdominal pain, rectal bleeding, and weight loss [11]. In up to 38% of patients, extra-intestinal manifestations (ophthalmological, rheumatological, cutaneous, and hepatobiliary) also occur [12]. CD can occur anywhere within the gastrointestinal tract. It can affect all layers of the intestine and is characterized by patchy inflammation, granulomas, fistulas, and deep penetrating ulcers. Conversely, UC is limited to colon and rectum and forms continuous homogeneous lesions, with inflammation limited to the mucosa [13].
Diagnosis of IBD and its subtype requires examination of the clinical symptoms and the use of medical procedures such as endoscopy, colonoscopy, upper gastrointestinal radiography, and biopsy [11]. Differential diagnosis of CD and UC may be challenging; however, it can be assisted by the use of molecular markers. These include serological markers (perinuclear antineutrophil cytoplasmic antibody, anti-Saccharomyces cerevisiae antibody, anti-glycan antibodies), histological markers (specific mRNAs), and fecal markers (fecal calprotectin and fecal lactoferrin). However, they are not consistently present in IBD patients and can also be detected in healthy individuals, limiting their clinical utility in diagnosis [14].
Treatment strategies typically involve pharmacological intervention to control inflammation and, in severe cases, surgical intervention. Among small molecules, aminosalicylates, corticosteroids, and immunomodulators (thiopurines, methotrexate, calcineurin inhibitors, and Janus kinase inhibitors) are used [15]. Biological drugs include antibodies infliximab, golimumab, adalimumab, and certolizumab pegol against tumor necrosis factor alpha (TNF-α), vedolizumab against integrin α4β7, and ustekinumab against interleukin-12/23 [15,16]. On the other hand, probiotics are live microorganisms that, when administered in adequate amounts, confer health benefits to the host. Modulation of the indigenous gut microbiota through the administration of probiotics and prebiotics is another promising strategy for IBD treatment [17,18].
Materials used for diagnosis and treatment of IBD have been reviewed previously [19,20]. To complement these studies, the present review focuses on stimuli-responsive biocompatible materials of nanometer size and presents an exhaustive overview of developed prototypes, their composition, active ingredients, and manufacturing procedure. The review describes the use of hydrogels, nanoparticles, nanofibers, and hybrid systems for the delivery of active ingredients to the intestine, with a focus on the colon. We include the description of their properties, advantages, and limitations, as well as description of their manufacturing. We also describe the use of genetically engineered probiotics as live biomaterials and their application in the diagnosis and treatment of IBD (Figure 1).

Schematic overview of smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease, stimuli to which they respond, and active ingredients they deliver. The image was created with Biorender.com.
2 IBD is associated with the immune system and gut microbiota
Pathogenesis of IBD is associated with the impaired immune response, in which both innate and adaptive immunities are dysregulated. As for the former, neutrophils and macrophages induce tissue damage by producing proinflammatory cytokines [21], and as for the latter, CD4+ T helper cells, such as Th1, Th2, Th9, Th17, and Th22, are activated, and considered to be one of the main triggers of the disease [22]. On the other hand, the relationship between gut immunity and gut microbiota is also an important factor in the progression of IBD. Distortion of the gut mucus layer results in translocation of bacteria causing defects in the host immune response and leading to inflammation [23]. Moreover, changes in gut microbiota composition (dysbiosis) have been observed in IBD patients and its modification has shown positive results in alleviating IBD [17]. Gut microbiota consists of more than 1014 microorganisms that have coevolved with the host and are of critical importance to the overall health [24]. Its composition depends on many factors, such as geographic origin, diet, environment, age, and antibiotic use [25,26,27,28]. The functions of the gut microbiota are related to nutrient and drug metabolism [29], immunomodulation [30], protection against pathogens [31], and maintenance of normal intestinal integrity [32].
Bacteria from the phyla Firmicutes and Bacteroidetes are the most abundant, accounting for 90% of the gut microbiota [33]. Gut microbiota ferments nondigestible carbohydrates and produces various metabolites. Butyrate is a short-chain fatty acid (SCFA) which is produced mainly by the Firmicutes phylum and plays an essential role in gut immunomodulation [34]. The Firmicutes/Bacteroidetes ratio plays a vital role in many diseases including IBD, and a decrease in the ratio is positively associated with IBD [17]. Some studies have also reported an association between a decreased Firmicutes/Proteobacteria ratio and IBD [35]. Several studies have found decreased levels of Faecalibacterium prausnitzii [35], Eubacterium rectale, Roseburia intestinalis, and Bifidobacterium longum in IBD patients, whereas the relative abundance of Bacteroides fragilis was increased [36]. Bacterial species from the genera Bifidobacterium, Lactobacillus, and Faecalibacterium have demonstrated protective properties for the host mucosa by stimulating the production of anti-inflammatory cytokines [37].
A combination of bacterial dysbiosis, destruction of the mucus layer, malfunction of the immune system, and genetic predisposition can lead certain bacterial species to trigger an inflammatory response, which is mediated by the production of inflammatory cytokines such as TNF-α. TNF-α plays a pivotal role in numerous inflammatory disorders, and its inhibitors are effective in treating patients with IBD [38,39]. Proinflammatory cytokines, including interleukin (IL)-1β, IL-6, IL-17, and IL-23, are also involved in the pathogenesis of IBD [40,41]. In contrast, immunosuppressive cytokines such as IL-10, IL-4, IL-12, IL-13, and transforming growth factor-β (TGF-β) downregulate IBD progression and are beneficial for gut immunological homeostasis [42]. Some gut bacteria stimulate IL-10-producing B and regulatory T cells in the colon, which downregulate mucosal inflammation in mice [43]. Metabolites of the gut microbiota, especially SCFAs, promote the production of IL-10 and IL-22, which maintains intestinal homeostasis and suppresses the progression of colitis [44,45]. Gut dysbiosis is also linked with the increased production of reactive oxygen species (ROS), which is observed in patients with IBD. Gut bacteria can generate different ROS, leading to DNA damage and progressive inflammation [46]. Overproduction of nitric oxide (NO) is also typical for IBD and can alter gut homeostasis [47]. Balancing the disrupted gut microbiota with probiotics and prebiotics can stop the progression of IBD and restore immune homeostasis in the gut [48,49].
3 Smart bionanomaterials in the treatment of IBD
Hallmarks of pathophysiological changes in the gut affected by IBD (inflammatory molecules, ROS, and altered microbiota) and conditions specific for the colon (anaerobiosis and higher pH) provide stimuli for smart bionanomaterials and can trigger the controlled release of active ingredients or probiotics. Smart bionanomaterials, such as hydrogels, nanoparticles, nanofibers, and hybrid bio-nano delivery systems, are used to encapsulate active ingredients or live cells and enable targeted delivery to the colon. Conversely, smart probiotics are live biomaterials that can be genetically programmed to sense and respond to external stimuli.
3.1 Smart hydrogels
3.1.1 Definition and properties
Hydrogels are three-dimensional networks of hydrophilic polymers that can swell in water and retain a large amount of water while maintaining the structure due to chemical or physical cross-linking of individual polymer chains [50]. Due to their high water absorption capacity, porosity, flexibility, soft structure, controlled release, and similarity to living tissues, hydrogels are suitable for various biomedical applications such as drug delivery, tissue engineering, and wound dressing. These properties can be adjusted by changing the polymer type, its concentration, and reaction conditions [51,52]. The reported disadvantages of hydrogels are their inhomogeneous mechanical strength, difficulty in shaping them in predesigned geometries, and difficulty in loading hydrophobic drugs [51,53].
3.1.2 Active ingredients and their release
Active ingredients encapsulated in smart hydrogels include anti-inflammatory compounds such as 5-aminosalicylic acid (5-ASA), HA, and dexamethasone. Additionally, probiotic bacteria, such as Lactobacillus reuteri and Lactobacillus acidophilus, have been included in the smart hydrogel formulation against IBD (Table 1). Smart hydrogels involved in the treatment and diagnosis of IBD respond to external stimuli such as pH, temperature, enzymes, and ROS.
Examples of smart hydrogels, their response inducing stimuli, and applications
| Hydrogel | Response inducing stimuli | Active ingredient/application | Reference |
|---|---|---|---|
| Methacrylated (1,3)-(1,6) β-glucan and poly (N-isopropyl acrylamide) | Temperature | 5-ASA | [54] |
| Polyamidoamine and chitosan | pH | 5-ASA | [55] |
| Chitosan-dextran sulfate | pH | 5-ASA | [56] |
| Thiolated-HA and sodium alginate | pH | HA | [57] |
| Ascorbyl palmitate | Enzyme | Dexamethasone | [59] |
| Methacrylated HA | ROS | L. reuteri | [60] |
| Poly-γ-glutamic acid | NO | L. acidophilus | [61] |
HA (hyaluronic acid), 5-ASA (5-aminosalicylic acid), ROS (reactive oxygen species), NO (nitric oxide)
Temperature changes can affect the volume of hydrogels prepared from thermally sensitive polymers, thereby controlling drug release and bioavailability. Different formulations of hydrogels have been composed of (1,3)-(1,6) β-glucan and poly (N-isopropylacrylamide) and loaded with the anti-inflammatory drug 5-ASA. Increasing β-glucan content resulted in a higher swelling ratio and drug-loading capacity. The optimized composition released almost 90% of the drug within 1 h at 37 °C [54]. Similarly, 5-ASA was loaded into a pH-sensitive hydrogel composed of poly(amidoamine) and chitosan. The hydrogel protected the drug in the acidic environment of the stomach and enabled its release at colon pH. The hydrogel delivered the drug in its ionized state, which increased its solubility and allowed the drug to be localized at the site of inflammation [55]. The targeted delivery of 5-ASA was proposed by Saboktakin et al. who encapsulated the drug in a chitosan-dextran sulfate hydrogel [56]. A colon-targeted, pH-sensitive core–shell hydrogel with a core of thiolated-HA hydrogel and shell of alginate hydrogel was developed. HA has anti-inflammatory properties but is susceptible to degradation in the stomach. The use of calcium alginate hydrogel as a shell-protected HA resulted in anti-inflammatory effects and alterations in gut microbiota in mice with colitis. The alginate shell remained stable in artificial gastric fluids (pH 1) but slowly lost its thickness as the pH increased [57].
Enzymes, such as esterases and matrix metalloproteinases, are overexpressed in the colonic lumen and tissue of IBD patients and can be used as biomarkers [58]. The development of an enzyme-responsive hydrogel that can be degraded by IBD-specific enzymes will enable targeted delivery. For example, encapsulation of the corticosteroid drug dexamethasone in hydrogel microfibers generated from ascorbyl palmitate resulted in preferential adhesion to inflamed tissue in vitro and a significant reduction in inflammation in mice with colitis, observed as reduction of the colon weight, myeloperoxidase activity, and TNF-α expression. The dexamethasone-loaded hydrogel was stable for 16 days in phosphate-buffered saline (PBS) at 37°C, and the addition of esterase resulted in a rapid and dose-dependent release of the drug [59].
Smart hydrogels can also be used for the delivery of probiotics. The ROS-responsive hydrogel loaded with L. reuteri was designed for targeted delivery to the inflamed colon. Methacrylated HA crosslinked with thiolated thioketal allowed up to 95% encapsulation and 90% survival rate of the probiotic. Thioketal linkages can be cleaved in the presence of excessive ROS, resulting in the degradation of the hydrogel and the release of the probiotic. Approximately 90% of L. reuteri was released after incubation with 10 mM H2O2 for 60 min. Moreover, the hydrogel scavenged excessive ROS, protected HT-29 cells, and improved the survival rate of mice with colitis [60]. NO can be used as an external stimulus for the local delivery of probiotics to the colon. NO responsive hydrogel microcapsules loaded with probiotic L. acidophilus were fabricated with poly-γ-glutamic acid. Hydrogels treated with nitroferricyanide(iii) dihydrate released 67.9% of the probiotic, whereas only 4.6% of the probiotic was released without nitroferricyanide(iii) dihydrate. In vivo studies in a mouse model of colitis confirmed immunomodulatory effects by improving the disease activity index. In addition, the system improved the microbiota balance by increasing the proportion of beneficial bacterial genera such as Turicibacter, Acidaminococcaceae, Negativicutes, and Phascolarctobacterium [61].
3.1.3 Composition and manufacturing
Hydrogels are synthesized from natural or synthetic polymers [62,63]. Most smart hydrogels for the treatment of IBD are synthesized from natural polymers such as ascorbyl palmitate, chitosan, HA, poly-γ-glutamic acid, and β-glucan, while poly (N-isopropyl acrylamide) is an example of synthetic polymer. Natural polymers have better biocompatibility and biodegradability but lower stability and mechanical strength compared to synthetic polymers [52].
Crosslinking of the polymer chains can be physical, chemical, or a combination of both (Figure 2). The structure of the hydrogel is influenced by various factors, including their composition, crosslinking, and encapsulated ingredient. The chitosan-dextran sulfate hydrogel has a solid and consistent structure, and the incorporation of 5-ASA resulted in a smooth surface and compact structure [56]. Conversely, HA hydrogels are characterized by a porous structure, and encapsulation of L. reuteri did not have a significant influence on the hydrogel structure [60].
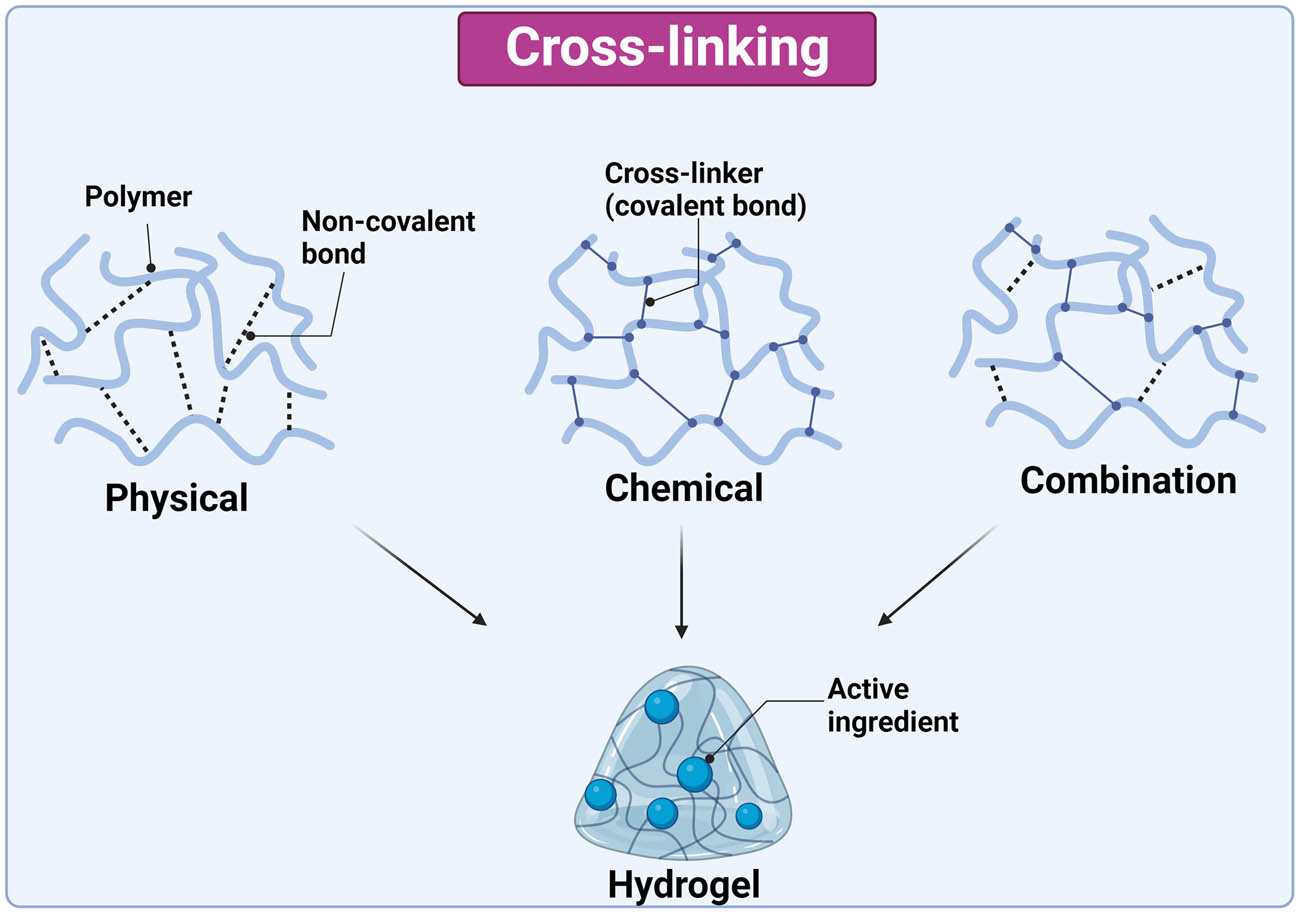
Synthesis methods of hydrogels, their interaction, and encapsulation of active ingredient. The image was created with Biorender.com.
Physically crosslinked hydrogels are prepared by intermolecular reversible interactions such as hydrophobic/hydrophilic interactions, hydrogen, and ionic bonds [64]. These types of hydrogels are simpler because gelation does not require crosslinking molecules, but they lack mechanical strength and stability. In regard to smart physically crosslinked hydrogels in IBD, ascorbyl palmitate has been frequently used as a polymer due to its self-assembly into a gel. Ascorbyl palmitate hydrogel is prepared by dissolving ascorbyl palmitate in heated dimethyl sulfoxide and water, followed by cooling [59].
Chemically crosslinked hydrogels have stronger linkages between polymer chains due to covalent bonds between the polymer and the crosslinker [65]. Crosslinker is a bi-reactive molecule that forms bridges between the chains of polymers, such as chitosan, HA, poly-γ-glutamic acid, β-glucan, and poly (N-isopropyl acrylamide). Chitosan is a natural polymer that can self-crosslink; alternatively, crosslinking can be assisted by adding glutaraldehyde. Carbonyl groups of glutaraldehyde covalently bind with the amino groups of chitosan or other polymers making gel more rigid and stable [66]. Glutaraldehyde-saturated toluene was used to crosslink chitosan with dextran sulfate [56] and polyamidoamine [55] to form a smart hydrogel for colon delivery of 5-ASA.
Likewise, HA and alginate are natural polymers that are used to synthesize smart hydrogels for colon targeting. An aqueous solution of thiolated HA and sodium alginate was injected through a gas shearing device where homogeneous droplets were generated. The addition of silver nitrate and calcium nitrate provided Ag+ and Ca2+ for crosslinking of HA and sodium alginate, respectively [57]. Another crosslinker for the formation of smart HA hydrogel is thioketal, which is sensitive to ROS. Methacrylated HA was mixed with a probiotic suspension, followed by the addition of thioketal dissolved in PBS. The mixture was incubated at 37°C for gelation [60]. Methacrylated β-glucan was used as a linker for poly (N-isopropyl acrylamide) to synthesize a temperature-responsive hydrogel. The hydrogel was synthesized at room temperature with redox polymerization using tetramethylethylenediamine and potassium persulfate as redox pair [54].
Microfluidic technology is an advanced tool for smart hydrogel preparation, as it enables precise particle manipulation and mixing of liquids [67]. Droplet microfluidic technology was used for the synthesis and encapsulation of probiotics in poly-γ-glutamic acid hydrogel, using N,N-(2-amino-1,4-phenylene) diacryl amide as a linker. A microfluidic chip was designed to merge three channels into a single channel, thereby allowing uniform distribution of probiotics and polymer. Droplets of the mixture of probiotics and polymer were obtained under shear stress with perfluorinated oil. The droplets were solidified by irradiation with visible light, resulting in smart hydrogels uniformly loaded with probiotic bacteria [61].
3.2 Smart nanoparticles
3.2.1 Definition and properties
Nanoparticles are a broad class of materials with dimensions below 100 nm. Based on their physical and chemical characteristics, nanoparticles can be classified into carbon, metal, ceramic, semiconductor, polymeric, and lipid nanoparticles [68,69]. Smart nanoparticles for IBD are mainly polymeric. They respond to different physical, chemical, and biological stimuli, such as ROS, pH, ligands, magnetic field, and gut microbiota, and thus provide controlled release. They can protect the drug by encapsulation, reduce adverse effects, and target specific cells [70]. They are also easy to prepare and have good mucoadhesive and biocompatible properties. On the other hand, their use is limited by chemical stability and particle aggregation [71,72]. Compared to polymeric nanoparticles, lipid nanoparticles are more affordable, can be easily scaled up, are biodegradable, and have excellent biocompatibility. However, they are limited by their lower loading capacity due to the lower solubility of the drugs [73]. Inorganic nanoparticles are characterized by good optical and magnetic properties, making them suitable for targeting IBD. However, their main limitations are long-term toxicity and burst drug release. These can be partially overcome by physical or chemical modification of the outer surface [71].
3.2.2 Active ingredients and their release
Smart nanoparticles deliver anti-inflammatory agents to the colon or regulate microbiota homeostasis. Examples of smart nanoparticles are summarized in Table 2. The majority of the smart nanoparticles effectively localize in the inflamed area of the intestines and act by inhibiting the proinflammatory cytokines in vitro and in vivo. Nanoparticles from polyphenol tannic acid and polyethylene glycol (PEG) were used to encapsulate infliximab and protect it from degradation in the digestive tract. The hydroxyl groups of tannic acid are easily oxidized by ROS in the inflamed colon, leading to the cleavage of the hydrogen bond between tannic acid and PEG and the release of the antibody at the site of increased oxidative stress. Oral administration of antibody-loaded nanoparticles resulted in the accumulation of infliximab in the inflamed area and reduced the levels of the proinflammatory cytokines TNF-α, IL-1β, and IL-6 [74]. Another approach to lower the levels of proinflammatory cytokines is the oral administration of small interfering RNA (siRNA) against TNF-α encapsulated in thioketal nanoparticles. The nanoparticles were prepared from poly-(1,4-phenylene acetone dimethylene thioketal), which degrades in the presence of ROS. Oral administration of nanoparticles improved the clinical symptoms of induced colitis in mice by improving the intestinal epithelium, increasing weight, and reducing neutrophil levels [75]. Redox-dependent targeting of IBD by nanoparticles has also been studied for HA [76], tempol [77], budesonide [78], and magnolol [79].
Examples of smart nanoparticles, their response inducing stimuli, and applications
| Nanoparticles | Response inducing stimuli | Active ingredient/application | Reference |
|---|---|---|---|
| Tannic acid and PEG | ROS | Infliximab | [74] |
| Poly-(1,4-phenyleneacetone dimethylene thioketal) | ROS | TNF-α siRNA | [75] |
| HA-bilirubin | ROS | HA | [76] |
| β-Cyclodextrin-derived material | ROS | Tempol | [77] |
| Amphiphilic carboxymethyl inulin with 4-aminothiophenol | pH/ROS | Budesonide | [78] |
| Butyrate-rich polymer | pH/ROS | Magnolol | [79] |
| PLGA and Eudragit S100 | pH | Curcumin | [80] |
| PLGA and Eudragit S100 | pH | Budesonide | [81] |
| PLGA and Eudragit FS30D | pH | Cyclosporine A | [82] |
| Mesoporous Silica and Eudragit S100 | pH | Budesonide and prednisolone | [83] |
| PLGA conjugated with folic acid | Ligand | Resveratrol | [84] |
| MC3, DSPC, cholesterol, DMG-PEG, and DSPE-PEG | Ligand | TNF-α siRNA | [85] |
| MC3, DSPC, cholesterol, DMG-PEG, and DSPE-PEG | Ligand | IL-10-modified mRNA | [86] |
| Iron oxide | Magnetic field | E. coli | [87] |
| Carbon nanotubes and graphene oxide flakes | Gut microbiota | Microbiota homeostasis | [88] |
| Fullerenol | Gut microbiota | Microbiota homeostasis | [89] |
| Selenium nanoparticles | Gut microbiota | Microbiota homeostasis | [90] |
| Curcumin nanoparticles | Gut microbiota | Microbiota homeostasis | [91] |
PEG (polyethylene glycol), HA (hyaluronic acid), PLGA (polylactide-co-glycolic acid), ROS (reactive oxygen species), TNF-α (tumour necrosis factor-α), siRNA (small interfering RNA), IL-10 (interleukin-10), messenger RNA (mRNA), MC3 (DLin-MC3-DMA), DMG-PEG (1,2-dimyristoyl-sn-glycero-3-methoxy(polyethylene glycol)), SPE-PEG (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)])
Several pH-sensitive nanoparticles have been developed for the local treatment of IBD. Curcumin was encapsulated in polylactide-co-glycolic acid (PLGA) and polymethacrylate polymer nanoparticles. The release of the drug was pH dependent, with acidic pH (1.2 and 4.5) limiting the release rate to <15%, and pH 7.2 triggering a rapid release. The nanoparticles enabled better permeation through Caco-2 cell monolayers and significantly reduced TNF-α secretion in murine models [80]. PLGA and Eudragit S100 nanoparticles were used for the encapsulation of budesonide. The release of the drug was pH dependent, with a rapid release in a simulated colon environment (pH 7.4). The nanoparticles showed significant therapeutic effects in a rat colitis model [81]. Similarly, PLGA and Eudragit FS30D were used for the synthesis of nanoparticles loaded with the immunosuppressive drug cyclosporine A, which enabled pH-dependent release and improved the health status of mice with colitis [82]. Eudragit S100 was also used to coat inorganic mesoporous silica nanoparticles, resulting in pH-sensitive nanoparticles suitable for colonic delivery of prednisolone and budesonide. Upon encountering the pH above 7, high percentage of prednisolone and budesonide (71 and 60%, respectively) were released [83].
The upregulation of proinflammatory proteins in IBD provides molecular stimuli for the development of proinflammatory protein-sensitive nanoparticles. For example, folate receptors are proinflammatory proteins that are overexpressed during inflammation. PLGA nanoparticles conjugated with folic acid and loaded with resveratrol demonstrated targeted anti-inflammatory properties by reducing neutrophil and lymphocyte accumulation in rats as a result of binding of conjugated nanoparticles to cells that overexpressed folate receptor [84]. Lipid nanoparticles loaded with TNF-α siRNA and coated on the surface with rat IgG2a antibody against Ly6C showed 94.2% knockdown in vitro and reduced the TNF-α protein levels in the colon of mice by approximately threefold [85]. In a similar fashion, IL-10-modified messenger RNA (mRNA) was encapsulated in lipid nanoparticles and coated with a Ly6C antibody. In mouse models of induced colitis, the nanoparticles recognized Ly6C receptors on inflammatory leukocytes and delivered the modified mRNA encoding IL-10, resulting in increased level of IL-10 and decreased levels of TNF-α and IL-6 in mice models of colitis [86].
Targeted delivery of oral probiotics with nanoparticles was achieved by labeling Escherichia coli with streptavidin-coated superparamagnetic iron oxide nanoparticles, allowing colonization of specific regions of the gut by applying an external magnetic field. Under the influence of magnetic arrays worn by mice, labeled E. coli were localized and retained in distinct regions of the gastrointestinal tract. The application of magnetic field enabled localization and retention of 17.0 ± 1.5% of bacteria in the small intestine 9 h post-gavage, whereas only 0.6 ± 0.5% of bacteria were detected in the small intestine without a magnetic field [87]. Conversely, certain nanoparticles can be degraded by the gut microbiota and serve as nutrients. These nanoparticles can alter the composition of the microbiota and significantly increase the levels of butyrate-producing bacteria, which can downregulate the production of proinflammatory cytokines. Oral administration of small carbon nanotubes and graphene oxide flakes to mice for 28 days resulted in gut microbiota alterations, with increased levels of butyrate-producing bacteria and increased levels of intestinal butyrate [88]. Similar results were obtained with fullerenol nanoparticles administered orally to mice for 4 weeks. These nanoparticles were also degraded by the microbiota, and the number of butyrate-producing bacteria increased after 7–28 days of treatment [89]. Selenium nanoparticles at specific doses also increased the levels of butyrate-producing bacteria from the genera Lactobacillus and Faecalibacterium in chickens and served as an effective selenium supplement [90]. Similarly, curcumin nanoparticles increased the abundance of butyrate-producing bacteria from Clostridium cluster IV, Clostridium subcluster XIVa, and Clostridium cluster XI, possibly by improving curcumin absorption and by inducing mucosal immune cells with regulatory properties [91].
3.2.3 Composition and manufacturing
Smart nanoparticles for the treatment and diagnosis of IBD are most commonly prepared from polymers and lipids using dialysis or emulsification (Figure 3).
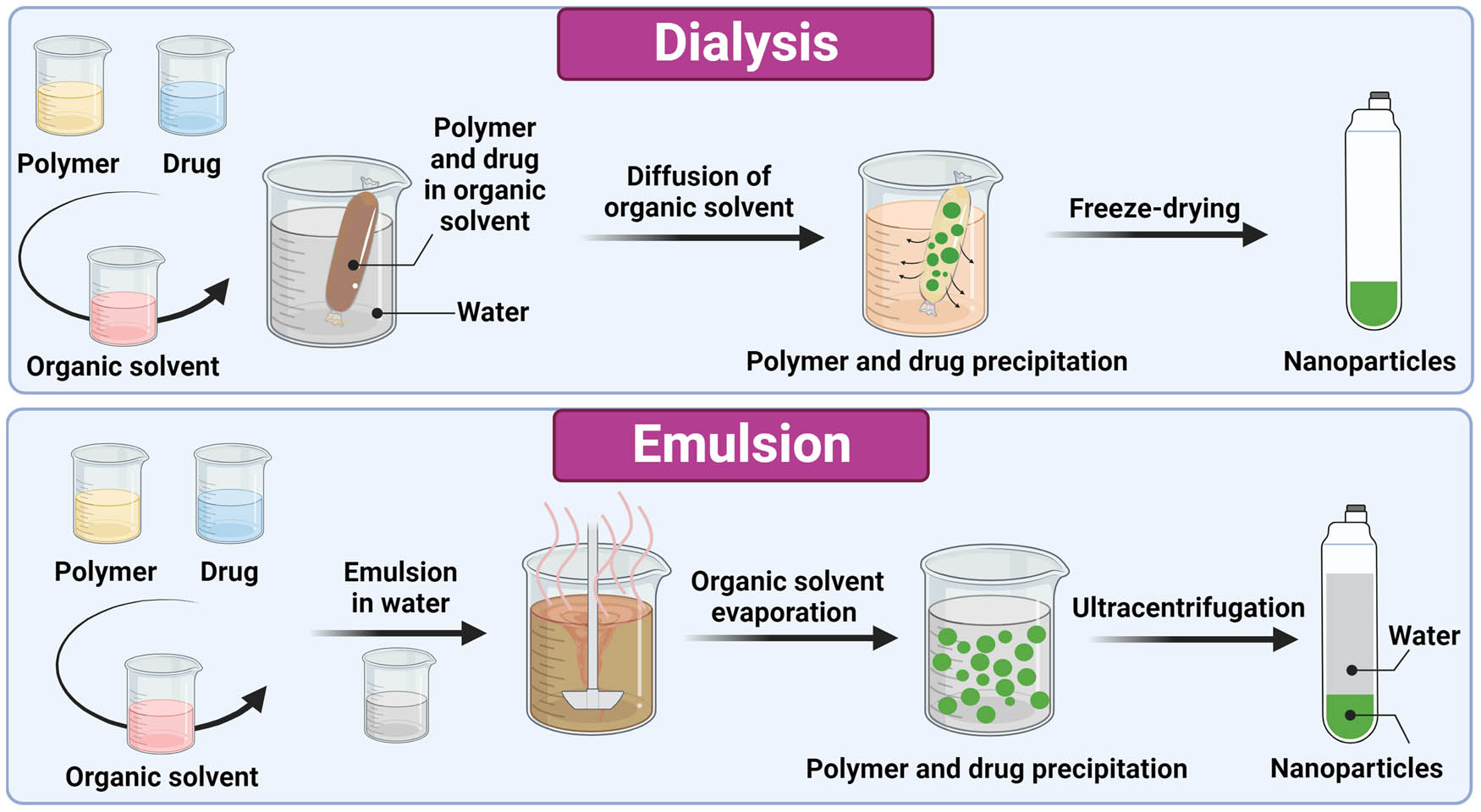
Schematic representation of the preparation process of nanoparticles with dialysis and emulsion methods (solvent evaporation and single emulsion). The image was created with Biorender.com.
When using dialysis, the active ingredient and polymer are dissolved in water-miscible organic solvents and placed in a dialysis tube. The organic solvent diffuses out of the dialysis tube, allowing aggregation of the polymer due to the increase in concentration and decrease in solubility. The suspension is freeze dried, resulting in the formation of nanoparticles [92]. Dialysis against water is used to prepare PEG- [74], HA- [76], β-cyclodextrin- [77], and butyrate-based polymeric [79] smart nanoparticles loaded with different anti-inflammatory agents. Dialysis with microfluidic technology was used to synthesize smart lipid nanoparticles for delivering siRNA [85] and modified mRNA [86]. A mixture of lipids (MC3, DSPC, cholesterol, DMG-PEG, and DSPE-PEG) in ethanol was mixed with siRNA or modified mRNA in acetate buffer. The mixture was injected into a microfluidic mixing device and dialyzed against PBS. The nanoparticles were then coated with recombinant scFv onto which rat IgG2a antibody was attached via the Fc domain [85,86].
When using emulsification, the drug and polymer are dissolved in a volatile organic solvent that forms the oil phase, followed by the addition of water with a stabilizer to form oil in water emulsion [93]. The solvent is then evaporated using high temperature, vacuum, or continuous stirring, resulting in precipitation of the polymer and formation of nanoparticles. The size of the nanoparticles can be controlled by varying the parameters of evaporation [94]. The nanoparticles are collected with ultracentrifugation and can be lyophilized to enhance the shelf life [93]. This method was used to synthesize thioketal nanoparticles composed of poly-(1,4-phenyleneacetone dimethylene thioketal) [75], pH-sensitive PLGA with Eudragit FS30D [82], and PLGA with Eudragit S100 [80,81], as well as ligand-sensitive PLGA [84] nanoparticles.
Other chemical reactions such as alkaline reaction [89] and chemical reduction [90] are also used in the synthesis of smart inorganic nanoparticles. Chemical synthesis was used to manufacture mesoporous silica nanoparticles by mixing hexadecyltrimethylammonium bromide, NaOH, and tetraethylorthosilicate in deionized water for 2 h. The suspension was vacuum-filtered and the particles were dried overnight. Then, the particles were mixed with Eudragit S100 and prednisolone or budesonide in methanol which was then evaporated [83].
3.3 Smart nanofibers
3.3.1 Definition and properties
Smart nanofibers are polymeric fibrous materials with nanometer diameters, high surface-to-volume ratios, low weight, and high, controllable porosity. They are produced by electrospinning [95]. Smart nanofibers can deliver small molecular drugs [96,97,98], as well as larger biological drugs, such as proteins, nucleic acids, microorganisms, and even stem cells [98,99,100,101,102]. Like other smart bionanomaterials, smart nanofibers can respond to external stimuli and enable targeted and controlled drug delivery. The disadvantages of electrospinning include extended manufacturing time and low production efficiency, but these can be circumvented by using multiple jets and nozzle-less electrospinning [103]. Another drawback is a high voltage, which can decrease the stability of biological drugs, such as proteins, nucleic acids, and cells. However, the addition of lyoprotectants can alleviate this challenge, allowing the ingredient to be preserved for a longer period of time [98,99,101].
3.3.2 Active ingredients and their release
In IBD, smart nanofibers were developed for the delivery of anti-inflammatory drugs such as prednisolone, diclofenac sodium, and budesonide, as well as probiotic bacteria such as Lactobacillus plantarum and Lactobacillus rhamnosus (Table 3).
Examples of smart nanofibers, their response inducing stimuli, and applications
| Nanofibers | Response inducing stimuli | Active ingredient/application | Reference |
|---|---|---|---|
| Chitin and sulfobutyl ether β-cyclodextrin gel | pH | Prednisolone | [104] |
| Polyvinylpyrrolidone and shellac | pH | Diclofenac sodium | [105] |
| Ethylcellulose and Eudragit S100 | pH | Budesonide | [106] |
| PVA and sodium alginate | pH | L. plantarum | [107] |
| PLA and FOS | pH | L. plantarum | [108] |
| Eudragit S100 and PVA/pectin | pH | L. rhamnosus | [109] |
| Pullulan and PLGA | pH | L. rhamnosus | [110] |
PVA (polyvinyl alcohol), PLA (polylactic acid), FOS (fructooligosaccharides), PLGA (polylactide-co-glycolic acid)
Chitin nanofibers, surface deacetylated, and reinforced with a sulfobutyl ether β-cyclodextrin gel, were developed for the controlled release of poorly water-soluble prednisolone. The full content of the drug was released slowly over 12 h irrespective of the pH (1.2 or 6.8), enabling colon delivery. The in vivo use of nanofibers in mice showed the anti-inflammatory activity of the material by preserving the length of the colon, reducing symptoms of colitis and histopathological changes in the colon tissue [104]. Polyvinylpyrrolidone nanofibers coated with shellac were produced with coaxial electrospinning. Shellac is not soluble at acidic pH but dissolves at neutral pH. These properties enable the targeted release of diclofenac sodium with a release rate of only 7.1% at pH 2 and rapid release at pH 7.4, making this a promising formulation against IBD [105]. Coaxial electrospinning was also used to encapsulate budesonide in ethyl cellulose nanofibers coated with Eudragit S100. The drug showed a pH-dependent release profile, with the highest drug release at pH 7.4, corresponding to colonic conditions. When budesonide was encapsulated in core–shell nanofibers, the mean peak concentration in the colon of rats was approximately 2.6 times higher than when the drug was encapsulated in single-layer fibers [106].
Sodium alginate is a pH-responsive polysaccharide that can be used to synthesize pH-responsive nanofibers. Alginate was used as a shell for polyvinyl alcohol (PVA) nanofibers loaded with the probiotic bacterium L. plantarum. Encapsulated probiotics in the double-layered nanofibers retained their viability after exposure to acidic conditions compared to the nonencapsulated bacteria. Probiotic bacterial proliferation was observed at higher pH, suggesting that this system could be used to deliver probiotics to the colon [107]. Similarly, L. plantarum was encapsulated in coaxial electrospun nanofibers with polylactic acid (PLA) and fructooligosaccharides (FOS) as the shell material. The survival rate of probiotics at simulated intestinal pH was 72%. PLA is resistant to acid, which allows for the protection of probiotics and targeted release in the colon. FOS remained in the culture media and may have further promoted bacterial growth by acting as a prebiotic [108]. L. rhamnosus was encapsulated in Eudragit S100 and PVA/pectin nanofibers using coaxial electrospinning. Encapsulation of bacteria resulted in 90.07 and 91.96% survivability in simulated gastric and intestinal fluids, respectively [109]. Similarly to coaxial electrospinning, multilayer nanofibers composed of different layers that were spun sequentially showed promising results as probiotic delivery system. L. rhamnosus encapsulated in pullulan nanofibers sandwiched between two PLGA mats provided better retention of viability compared to spray-dried bacteria and bacteria encapsulated in single-layer nanofibers. In addition, sandwiched bacteria demonstrated better tolerance to low gastric pH, while enabling effective colonization of the jejunum and cecum of mice three days after administration [110].
3.3.3 Composition and manufacturing
Smart nanofibers are produced by conventional or coaxial electrospinning (Figure 4). Conventional electrospinning is a method that applies high electric voltage to a droplet of a polymer solution such as polyethylene oxide, PLA, and polystyrene [111]. The voltage overcomes the surface tension of the polymer droplet, resulting in a “whipping” motion, solvent evaporation, and nanofiber formation [95]. The final product is a solid thin, soft, and flexible film. Unlike the other delivery systems, encapsulation of active ingredients, especially bacteria, has a significant influence on the morphology of the nanofibers. Upgraded method of electrospinning is coaxial electrospinning, in which two components (core and shell) are electrospun simultaneously by using an additional syringe pump and a coaxial nozzle. A core polymer flows through the inner nozzle, while a shell polymer flows through the outer nozzle. Similar to the conventional method, a high electrostatic field is applied, resulting in the formation of two-layered nanofibers [112,113].
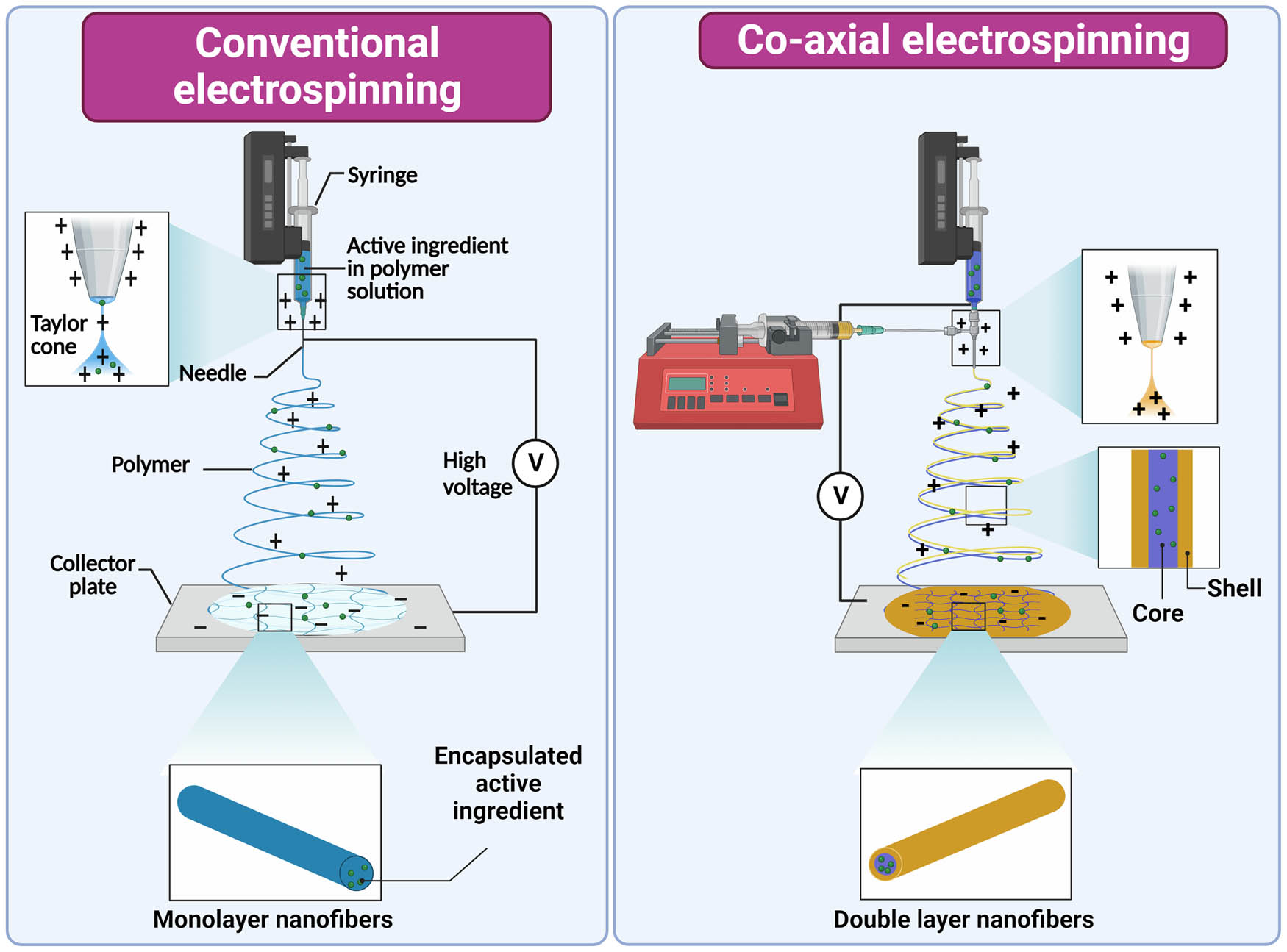
Illustration of conventional and coaxial electrospinning and synthesis of mono and double-layered nanofibers. The image was created with Biorender.com.
Coaxial electrospinning was used to prepare smart nanofibers loaded with diclofenac sodium. A mixed solution of 35% (w/v) polyvinylpyrrolidone and 5% (w/v) diclofenac sodium was used as the core fluid, while 10% (w/v) of shellac solution was used as the sheath fluid [105]. In a similar fashion, budesonide was encapsulated in ethyl cellulose nanofibers as the core, while Eudragit S100 was used for the shell layer [106]. Eudragit S100 was also used as a shell layer to protect the viability of the probiotic bacterium L. rhamnosus. Bacterial suspension was added to a 9:1 mixture of 12% (w/v) PVA and 11% (w/v) pectin as the core layer [109]. Another probiotic encapsulated in core–shell smart nanofibers was L. plantarum. Bacterial suspension (1010 CFU/mL) was mixed with 6% (w/v) PVA as core solution and an 8:2 mixture of 8% (w/v) PVA and 2% sodium alginate as shell solution [107]. L. plantarum was also encapsulated in another smart core and shell nanofiber formulation, using 8% (w/v) PLA and 2.5% (w/v) FOS as shell solution and 1% (v/v) L. plantarum in MRS growth medium as core solution [108].
3.4 Smart hybrid bionanomaterials
3.4.1 Definition and properties
Smart hybrid bionanomaterials combine two or more bionanomaterials to provide better efficacy and fewer side effects of delivered drugs [114]. Assembly of two or more bionanomaterials can improve their characteristics in delivering active ingredients to the inflamed intestine, thereby improving the treatment and/or diagnosis of IBD. Most smart hybrid bionanomaterials combine nanoparticles and hydrogels. They respond to external stimuli such as biomolecules, pH, ROS, and magnetic field.
3.4.2 Active ingredients and their release
Smart hybrid systems can deliver active ingredients into the colon or sample the microbiota to aid in the diagnosis of IBD (Table 4).
Examples of smart hybrid delivery systems, their response inducing stimuli, and applications
| Hybrid delivery system | Response inducing stimuli | Active ingredient/application | Reference |
|---|---|---|---|
| PEG nanoparticles and chitosan/alginate hydrogel | Biomolecule | CD98 siRNA | [115] |
| Polycationic 2-(diethylamino)ethyl methacrylate nanogels and poly(methacrlic acid-co-N-vinyl-2-pyrrolidone) hydrogel | pH/enzyme | TNF-α siRNA | [116] |
| HA-embedded PLGA nanoparticles and chitosan/alginate hydrogel | pH/biomolecule | KPV | [117] |
| HA-coated selenium nanoparticles and calcium alginate hydrogel | pH | Selenoprotein | [118] |
| Medium chain triglyceride and beeswax nano-emulsion coated with chitosan and octenyl succinic anhydride-modified resistant starch | pH | 6-gingerol | [119] |
| Copper nanoneedles/inulin hydrogel | pH | Olsalazine | [120] |
| Sodium alginate and PVA hydrogel microspheres | ROS | L. plantarum | [121] |
| HA and polypropylene sulfide nanoparticles conjugated to EcN surface and encapsulated in poly-norepinephrine layer | ROS | EcN | [122] |
PEG (polyethylene glycol), HA (hyaluronic acid), PLGA (polylactide-co-glycolic acid), PVA (polyvinyl alcohol), TNF-α (tumour necrosis factor-α), siRNA (small interfering RNA), KPV (lysine-proline-valine), EcN (E. coli Nissle 1917), ROS (reactive oxygen species)
Cluster of differentiation 98 (CD98) is a glycoprotein antigen overexpressed on the surface of colonic epithelial cells and macrophages in IBD. A hybrid oral delivery system with nanoparticles in hydrogel was designed to target CD98. PEG-urocanic acid-modified chitosan nanoparticles coated with single-chain CD98 antibodies on their surface and loaded with CD98 siRNA were encapsulated in a chitosan/alginate hydrogel. The modified nanoparticles interacted with CD98 and reduced its levels in vitro and in mice with colitis; moreover, they also reduced the expression of TNF-α, IL-6, and IL-12 [115]. Targeted delivery of siRNA against TNF-α was achieved by encapsulation in a hybrid material that was responsive to changes in pH and intestinal enzymes. siRNA was encapsulated in polycationic 2-(diethylamino)ethyl methacrylate nanogels, which were further encapsulated in an enzymatically degradable poly(methacrylic acid-co-N-vinyl-2-pyrrolidone) hydrogel. At intestinal pH, the hydrogel swelled and enabled the infusion of intestinal fluid and degradation of the gel by intestinal enzymes such as trypsin. The released nanogels loaded with siRNA TNF-α were absorbed by RAW 264.7 macrophages and downregulated the secretion of TNF-α [116].
Naturally occurring tripeptide KPV can alleviate colon inflammation. Its encapsulation in HA-embedded PLGA nanoparticles resulted in an immunosuppressive response following administration to mice with colitis. Moreover, when these nanoparticles were encapsulated in a chitosan/alginate hydrogel, they prevented mucosal damage and downregulated TNF-α even more effectively. The hydrogel degrades at colonic pH, suggesting that the system acts in the colonic lumen and not in other sections of the gastrointestinal tract. In addition, HA can bind to the CD44 receptor, which is abundant on the surface of epithelial cells and macrophages in IBD-affected colon tissues [117].
Selenoprotein, the main in vivo form of selenium, plays a critical role in inflammation and immune cell activation. To increase the in situ production of selenoprotein, selenium nanoparticles were modified with HA and encapsulated in a protective shell of a calcium alginate hydrogel. The hybrid system protected mice with colitis by decreasing epithelial damage and downregulating the expression of proinflammatory cytokines TNF-α, IL-6, and IL-1β. The hybrid system of nanoparticles and hydrogels resulted in a greater downregulation of cytokines than the conventional system using only nanoparticles. The authors also observed that the system increased the relative abundance of Verrucomicrobiota, Firmicutes, and Bacteroides [118].
Anti-inflammatory 6-gingerol is one of the most bioactive compounds in ginger. Its encapsulation in medium-chain triglycerides and beeswax nanoemulsions coated with chitosan and octenyl succinic anhydride-modified resistant starch resulted in pH-responsive microcapsules that mitigated colitis symptoms in mice. In vitro and in vivo studies have confirmed the colon targeting and anti-inflammatory effects of the hybrid material by regulating antioxidant/anti-inflammatory pathways and increasing the Firmicutes/Bacteroidetes ratio [119].
Olsalazine is a prodrug that is not absorbed in the intestines but breaks down into two 5-ASA molecules by the colon bacterial azoreductases. To improve the colon retention, hybrid delivery system composed of olsalazine-containing copper nanoneedles and inulin hydrogel was designed. The hybrid system demonstrated pH-responsive behavior, facilitating targeted release at the colonic pH of 7.4, and enhanced bio-adhesion due to its macroporous structure. In the in vivo studies on mice, reduced proinflammatory cytokines, neutrophil infiltration, and epithelial damage in the colon were observed. In addition, the prebiotic properties of inulin within the hybrid system improved the intestinal microbiota composition by decreasing the abundance of harmful microorganisms (Proteobacteria, Romboutsia, and Enterococcus) and increasing the abundance of butyrate-producing bacteria, particularly Lachnospiraceae [120].
Hydrogel microspheres were produced from sodium alginate and PVA, and loaded with L. plantarum. To decrease the pore size and enable controlled release, sodium alginate, and PVA were crosslinked with ROS-sensitive 1,4-phenylenediboronic acid. The in vitro release rate of L. plantarum depended on the H2O2 concentration. In the presence of 0.1 mM H2O2, the release rate was over 73%, whereas in the presence of 0.05 and 0 mM H2O2 the release rate decreased to 56 and 37%, respectively. Regarding safety, hydrogel microspheres showed no toxicity in MRC-5 and L-929 cell models [121]. Probiotic was also delivered in a hybrid delivery system composed of ROS-reactive nanoparticles conjugated to the surface of EcN and encapsulated in a polynorepinephrine layer. Conjugated nanoparticles were fabricated from HA and polypropylene sulfide, allowing them to scavenge ROS. Encapsulation of EcN in the polynorepinephrine layer not only protected the probiotic from the external environment but also enabled better adhesion to the gut mucosa which extended the retention time in the gut and enabled better histopathological score. The hybrid system was effective in the prevention and treatment of colitis, as well as in balancing the disturbed gut microbiota in mice by increasing the abundance of beneficial bacteria (Muribaculaceae, Prevotellaceae_UCG-001) and decreasing the abundance of harmful bacteria (Desulfovibrionaceae) [122].
3.4.3 Composition and manufacturing
The most common smart hybrid bionanomaterials for treatment of IBD are combinations of hydrogels and nanoparticles. Poly(propylene sulfide) and HA were assembled into nanoparticles by linking them with ROS linker ethylenediamine. The mixture was dialyzed against water/methanol and lyophilized. The nanoparticles were conjugated to the surface of E. coli Nissle 1917 (EcN) and encapsulated in polynorepinephrine by mixing the bacteria and polymer [122].
The nanoemulsion method was used to synthesize nanoparticles loaded with 6-gingerol. The lipid phase consisted of medium-chain triglyceride, beeswax, and 6-gingerol, while the aqueous phase consisted of 3% (w/v) Tween 80 in water. The two phases were mixed, homogenized, sonicated, and added dropwise to a chitosan solution and then to octenyl succinic anhydride-modified resistant starch solution, which was in the end spray dried [119]. The double emulsion solvent evaporation method was used to synthesize PLGA nanoparticles loaded with the tripeptide KPV. The tripeptide (water phase) was mixed with PLGA (oil phase) forming the first emulsion. The emulsion was then sonicated and mixed with the second aqueous phase composed of PVA and chitosan which further stabilized the nanoparticles. In addition, the chitosan allowed the conjugation of HA through the formation of amide bonds. Finally, the nanoparticles were encapsulated in an alginate and chitosan hydrogel allowing delivery to the colonic lumen. The hybrid system exhibited a larger size than that of the individual nanoparticles [117].
Polycationic 2-(diethylamino)ethyl methacrylate gels were chemically crosslinked with tetraethylene glycol dimethacrylate. The gels were then mixed with TNF-α siRNA in PBS (pH 5.5), forming an electrostatic complex. After encapsulation, the gel was mixed with a poly(methacrylic acid-co-N-vinyl-2-pyrrolidone) hydrogel crosslinked with GRRRGK peptide and further lyophilized and milled [116]. A similar method was used for encapsulation of probiotic bacteria. Sodium alginate and PVA solutions were mixed with L. plantarum suspension and ultrasonicated to achieve a uniform distribution of bacteria. The mixture was then added dropwise into CaCl2 solution (100 mM), allowing the Ca2+ ions to crosslink the polymers. The hydrogel was then immersed in a gelatin solution (3% (w/w)) and stirred, forming the hybrid material [121].
Synthesis of copper/olsalazine nanoneedles was done by the addition of CuCl2 solution into olsalazine sodium, followed by heating the mixture at different temperatures. The precipitates were then centrifuged and washed with water. The resulting suspension was mixed with inulin, heated at 70°C, and subsequently left to cool at room temperature overnight [120].
3.5 Smart probiotics
3.5.1 Definition and properties
Unlike inanimate smart bionanomaterials, smart probiotics are live organisms, i.e., genetically engineered safe bacteria. Analogous to smart bionanomaterials, they can respond to external stimuli and deliver active ingredients with diagnostic and therapeutic potential in IBD. This is usually achieved by the inducible expression of the recombinant proteins with anti-inflammatory activities.
3.5.2 Active ingredients and their release
Probiotics can modify the gut microbiota and interact with the immune system at various levels. Their immunomodulatory properties have been associated with the alleviation of IBD symptoms and regulation of gut microbial homeostasis [17,123,124]. These effects can be further upgraded by the delivery of diagnostic and therapeutic recombinant proteins (Table 5).
Examples of smart probiotics, their responsive stimuli, and applications
| Probiotic strain | Responsive stimuli | Active ingredient/application | References |
|---|---|---|---|
| Lc. lactis | Nisin | TNF-α affibody | [126,127] |
| Lc. lactis | Nisin | TNF-α, IL-17, and IL-23 binders | [128] |
| Lc. lactis | Nisin | IL-6 affibody (ZIL) | [129] |
| Lc. lactis | Nisin | IL-23 binders | [130] |
| Lc. lactis and L. plantarum | Nisin | SOD | [131] |
| EcN | Isopropyl-β-d-thiogalactoside | SOD and catalase | [132] |
| EcN | Low oxygen | ®-3-Hydroxybutyrate | [133] |
| Lc. lactis | Heat shock, UV radiation and pH EcN | IL-10 | [134] |
| EcN | NO | GM-CSF | [135] |
| EcN | NO | IFNL1 | [136] |
| B. subtilis | Conditions in colon | IL-1 receptor antagonist | [137] |
| Bc. Ovatus | Xylan | TGF-β | [138] |
| Bc. Ovatus | Xylan | KGF-2 | [139] |
TNF-α (tumor necrosis factor-α), IL (interleukin) SOD (superoxide dismutase) EcN (E. coli Nissle 1917), NO (nitric oxide), GM-CSF (Granulocyte-macrophage colony-stimulating factor), IFNL1 (interferon lambda 1), TGF-β (transforming growth factor-β), KGF-2 (keratinocyte growth factor-2)
Bacteriocin nisin is a food preservative and component of the nisin inducible expression system [125]. The addition of nisin to nisin-inducible species triggers the synthesis of recombinant therapeutic proteins. Nisin-responsive smart probiotics have been developed to exert their anti-inflammatory effects by neutralizing proinflammatory cytokines, producing anti-inflammatory substances, or decreasing oxidative stress. Specific TNF-α-binding affibodies were successfully displayed on the surface of the nisin-sensitive Lactococcus lactis [126,127,128], and engineered bacteria were tested in vitro, ex vivo, and in vivo. Binding of IL-6 by engineered, nisin-responsive Lc. lactis was successfully performed in vitro, and the bacteria neutralized up to 94% of the cytokines in a cell model [129]. IL-23-binding proteins secreted by the recombinant Lc. lactis following nisin induction were successful IL-23 blockers [130]. Superoxide dismutase (SOD) is an enzyme that converts ROS into molecular oxygen and hydrogen peroxide, thus providing a protective function. SOD administration can neutralize ROS and prevent disease progression. Two recombinant lactic acid bacteria (Lc. lactis and L. plantarum) expressing SOD following nisin induction reduced the number of ulcerations and the macroscopic damage score in experimental rats with colitis. In contrast, colonic infusion of bovine SOD resulted in poorer therapeutic outcomes, which confirms the added value of smart probiotics [131].
In addition to nisin, other substances have been tested for the controlled induction of recombinant protein synthesis for the treatment of IBD. EcN was engineered to express SOD and catalase, following induction with isopropyl-β-d-thiogalactoside. The strain was coated in chitosan and sodium alginate biofilm and showed a high superoxide scavenging rate (94%). The strain also improved the IBD symptoms in mice and increased the abundance of microbes that are important for maintaining homeostasis in the intestine, namely Lachnospiraceae_NK4A136 and Odoribacter [132]. EcN was also engineered to synthesize ketone body (R)-3-hydroxybutyrate. The biosynthetic genes were inserted into the genome of EcN under the control of low oxygen inducible promoters, which are suitable for anaerobic conditions in the gut. Engineered EcN produced 0.6 g/L (R)-3-hydroxybutyrate, colonized the gut of mice, and promoted the growth of Akkermansia spp. to over 31% of the microbiota, while with the nonengineered EcN, the proportion of Akkermansia spp. was only 2%. The final outcome of the study of engineered EcN in this mouse model was an improvement in IBD symptoms [133].
Expression of the anti-inflammatory cytokine IL-10 in Lc. lactis under the control of the groESL operon promoter was induced by stresses such as heat shock, UV radiation, and changes in pH [134]. EcN was engineered to express granulocyte macrophage-colony stimulating factor (GM‐CSF) under the control of the NO-responsive promoter hmp. The engineered strain exhibited anti-inflammatory properties in an in vitro CD model [135]. The NO-responsive promoter pNorV, the YebF secretion sequence, and the anti-inflammatory interferon lambda 1 (IFNL1) gene were inserted into the genome of EcN. When exposed to NO, the engineered EcN demonstrated anti-inflammatory and protective properties in vitro. It reduced the concentration of the proinflammatory cytokines such as IL-4, IL-5, IL-13, and IL-33, improved the tight junctions, and decreased the transcription level of inducible NO synthase by 84.1% in the inflamed primary intestinal epithelial cells [136]. Bacillus subtilis was engineered to synthesize IL-1 receptor antagonists and autolyze upon sensing unfavorable conditions in the colon of mice. IL-1 receptor antagonist was detected both in the intestinal washings and in the serum after intracolonic administration of the strain. Furthermore, the anti-inflammatory properties of engineered B. subtilis were demonstrated by improving the survival rate of mice after the administration of a lethal dose of bacterial lipopolysaccharide [137]. Bacteroides ovatus was engineered to produce TGF-β. The gene was cloned downstream of the xylanase promoter that was activated in the presence of xylan. Engineered bacteria, together with xylan, were administered to mice, resulting in improved stool consistency and reduced rectal bleeding. Inflammation was reduced by 38% compared with that in untreated mice [138]. Similarly, the xylan sensitive Bc. ovatus was engineered to produce keratinocyte growth factor-2 (KGF-2), a molecule beneficial for reducing IBD symptoms. Administration of engineered bacteria and xylan to mice with colitis resulted in a significant reduction in inflammation and healing of the epithelium [139]. Another advantage of the xylan-dependent system is the potential prebiotic activity of xylan. This approach consists of the simultaneous administration of probiotics and prebiotics with potential synergistic activity.
3.5.3 Engineering of smart probiotics
A variety of gene editing techniques are implemented in the engineering of smart probiotics [140]. Smart probiotics for the treatment and diagnosis of IBD are prepared using recombinant DNA technology in which a heterologous DNA fragment is inserted into a plasmid vector. This is then transformed into bacteria using an appropriate technique, such as electroporation (Figure 5) [141]. Engineered plasmids often contain genes that enable retention of the plasmid and selection of transformants (e.g., antibiotic resistance genes) [141]. Plasmid-introduced recombinant genes can be inserted into the bacterial chromosome or expressed from the plasmid, whereby the expression is driven by constitutive or inducible promoters that are triggered by external stimuli [142]. Chromosomal integration of DNA allows for better genetic regulation, gene conservation, stability, and lower metabolic burden [143].
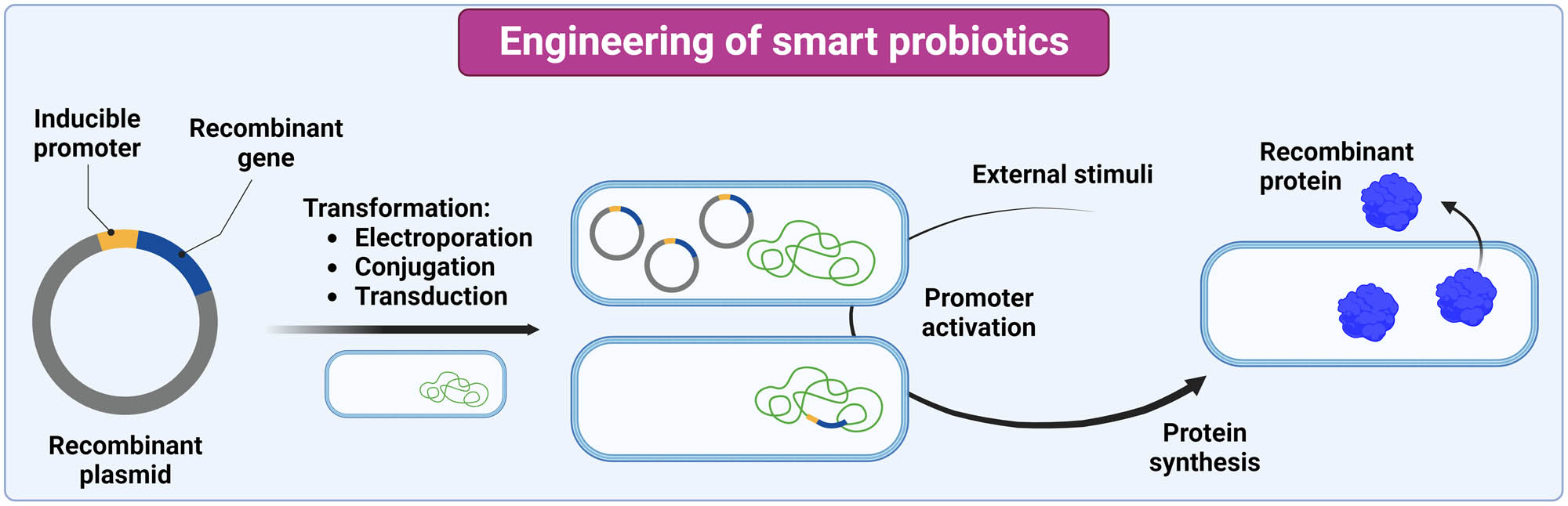
Engineering of smart probiotics and synthesis of recombinant proteins. The image was created with Biorender.com.
The development of smart probiotics for the treatment of IBD has focused on the food-grade bacteria Lc. lactis and EcN, which are amenable to the genetic manipulation. Other species from the genera Lactobacillus (old nomenclature), Bifidobacterium, and Bacteroides have also been engineered for the treatment of IBD. Lc. lactis have been engineered with plasmids containing nisin and groESL inducible promoters and transformed with electroporation [126,127,128,129,130,131,134]. Standard cloning methods have also been used to engineer EcN [132,133,135]. To transform EcN and Bacillus subtilis, electroporation and simpler heat shock method were used [137]. Alternative modes of transformation are conjugation and transduction. In conjugation, bacteria share plasmid DNA through conjugative pili [144], whereas in transduction the DNA sharing is mediated by a bacteriophage [145]. Bacteroides ovatus was engineered by conjugation to produce TGF-β [138] and KGF-2 [139]. Conjugation with phage λ Red recombinase was used to insert pNorV-YebF-IFNL1 into the EcN genome [136].
4 Smart bionanomaterials in diagnosis of IBD
The majority of smart bionanomaterials associated with IBD predominantly focus on therapeutic applications. However, several smart materials are also fabricated for diagnostic strategies. Smart bionanomaterials for diagnosis of IBD are mainly nanoparticles, hybrid delivery systems, and probiotics (Table 6).
Examples of smart bionanomaterials for diagnosis of IBD
| Smart bionanomaterial | Response inducing stimuli | Active ingredient/application | Reference |
|---|---|---|---|
| Poly-(1,4-phenyleneacetone dimethylene thioketal) nanoparticles | ROS | Platinum nanoclusters | [146] |
| PLA nanoparticles | Ligand | Quantum dots | [148] |
| Mesostructured silica nanoparticles | Ligand | Rhodamine B | [149] |
| Mesostructured silica nanoparticles | Ligand | Fluorescein | [149] |
| Chitosan covered with HA and Eudragit S100 | pH/biomolecule | TotalROX | [150] |
| PEG diacrylate and magnetic nanoparticles | Magnetic field | Microbiota sampling | [151] |
| E. coli | Tetrathionate | β-Galactosidase | [152] |
| EcN | Tetrathionate and thiosulfate | Superfolder GFP | [153] |
PLA (polylactic acid), PEG (polyethylene glycol), EcN (E. coli Nissle 1917), GFP (green fluorescent protein)
Poly-(1,4-phenylene acetone dimethylene thioketal) was used as ROS-responsive polymer to fabricate smart nanoparticles. The nanoparticles were synthesized with the emulsification method and were loaded with platinum nanoclusters which were released following an encounter with ROS within the inflamed intestines. The released platinum nanoclusters were filtered through the kidneys in the urine. The detection of nanoclusters in the urine served as an indicator of the presence of ROS in the intestine [146]. Quantum dots, semiconductor nanocrystals that exhibit high photoluminescent emission, have high potential in bio-imaging and sensing [147]. Quantum dots encapsulated in PLA nanoparticles prepared with the emulsification method were coated with the Fab region of the CD98 antibody. Coating the nanoparticles increased their cellular uptake and demonstrated increased accumulation in colitis tissue [148]. Nanoparticle coating was also used to fabricate metallopeptidase 9- and TNF-α-sensing silica nanoparticles loaded with fluorescent dyes rhodamine b and fluorescein, respectively. The nanoparticles released the dyes in the presence of the ligands and enabled IBD detection in IL10–/– mouse model. The limits of detection were 1.1 μg/mL for metallopeptidase 9 and 1 μg/mL for TNF-α [149]. Chemical synthesis was also used to manufacture smart nanoparticles. The mesostructured silica for the detection of metallopeptidase 9 was modified with 6-azido-N-(3-(triethoxysilyl) propyl) hexanamide and suspended in dry toluene. The mixture was centrifuged, washed, dried, and suspended in rhodamine b dye. The nanoparticles were coated with three repeats of the peptide sequence PLGMWSR which is a specific substrate for metallopeptidase 9. The peptide, together with copper sulfate, sodium ascorbate, and t-butanol, was mixed with mesoporous silica, and a microwave reaction was performed at 90°C. Nanoparticles for the detection of TNF-α were suspended in a fluorescein solution, allowing the dye to diffuse into the particles. The surface of the nanoparticles was modified with (3-aminopropyl) triethoxysilane and positively charged with PBS buffer, allowing the negatively charged anti-TNF-α antibodies to attach to the surface [149].
TotalROX is a ROS-responsive fluorescent probe that detects free radicals and emits fluorescent light. However, its poor water solubility and poor targeting of the active site limit its use in IBD diagnostics. To improve these properties, totalROX was encapsulated in chitosan, which was coated with two protective layers of HA and Eudragit S100. Chitosan was crosslinked with tripolyphosphate. HA and Eudragit S100 were then added to the solution dropwise, allowing for their adsorption to the surface of the chitosan hydrogel and forming a protective layer around the gel. Eudragit S100 dissolves at colonic pH and HA binds to the CD44 receptors in the colon. The in vitro tests revealed rapid dissolution of the hybrid system at pH 7.4, in contrast to pH 1.2 and pH 6.8. The hybrid system allowed targeted delivery of totalROX into the colon of IBD mice and the fluorescent signal was detected ex vivo 5–9 days after administration [150]. The diagnosis of gut dysbiosis can provide information on many diseases, including IBD. Sampling the gut microbiota at specific sites in the intestine is a good, but practically challenging method to determine the microbiota status. The PEG diacrylate smart hydrogel with magnetic nanoparticles and two initiators (ascorbic acid and FeCl3) in its core was covered with two protective layers composed of cellulose stearoyl esters and myristic acid. The magnetic particles enable the hydrogel to be moved to a specific location using an external magnetic field. The outer layer provides protection from conditions in the gastrointestinal tract, as cellulose is not digested and myristic acid has a high melting point and is not ionized at low pH. The addition of a magnetic field leads to an increase in temperature, melting of the myristic acid, and trapping of the microbiota in the hydrogel. The smart hydrogel collected microorganisms in vitro and ex vivo in the small and large intestines of pigs [151]. The hybrid system was fabricated with coprecipitation in which FeCl3·6 H2O and FeSO4·7 H2O were dissolved in water and mixed. Ammonia solution and oleic acid were added to the mixture and the nanoparticles were formed. The magnetic nanoparticles were mixed with polyethylene glycol diacrylate, ascorbic acid, and FeCl3 and vacuum dried. After drying, cellulose stearoyl ester was added, forming a protective layer, and dried further. In the end, myristic acid was added, forming another protective layer, and the hybrid smart material was dried in vacuum [151].
Smart probiotics can also be used to diagnose IBD by recognizing specific stimuli. Tetrathionate is a transient product of ROS during inflammation. It was detected by a genetically engineered murine E. coli strain that in response to tetrathionate in the environment initiated the synthesis of β-galactosidase, which converted 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside into a blue metabolite. The strain was engineered by transduction and tetrathionate responsive trigger and β-galactosidase were inserted into the chromosome of E. coli by P1vir bacteriophage [152]. Tetrathionate and thiosulfate-sensitive EcN strains were engineered by standard cloning methods to detect colon inflammation in mice after oral administration. Both biosensor strains were linked to a fluorescent reporter (superfolder GFP) that was expressed upon exposure to an inflamed gut [153].
5 Conclusion and future prospects
Smart materials are designed to sense and respond to changes in environmental conditions. They can adapt their properties, such as shape, color, and solubility, in response to external stimuli like pH or temperature. This adaptability makes smart materials valuable in the biomedical field, where they can be used for treating or diagnosing a variety of diseases. IBD is associated with alterations in the immune and gut microbiota homeostasis. This disruption leads to an increase in proinflammatory molecules and harmful bacteria. Apart from the need for targeting diseased tissue in IBD, the delivery to the intestines is challenging by itself due to physiological and anatomical factors, such as low gastric pH, the presence of enzymes and bile acids, and the presence of a large number of microorganisms. The utilization of smart bionanomaterials has yielded promising results in this respect by protecting the active ingredient, making them useful tools in the treatment of IBD.
Proof-of-principle applications that are described in this review mostly do not differentiate between the two specific subtypes of IBD. The majority of them were tested in mice models of dextran sulfate sodium (DSS)- or trinitrobenzene sulfonic acid (TNBS)-induced colitis, which are the most prevalent models for studying IBD due to their simplicity, reproducibility, and controllability; however, they poorly recapitulate differences in the pathology of CD and UC [154]. Therefore, further studies will have to focus on testing the suitability of smart bionanomaterials for specific subtypes of IBD.
Smart bionanomaterials for the treatment and diagnosis of IBD are mainly synthesized by crosslinking (hydrogels), electrospinning (nanofibers), dialysis and emulsification (nanoparticles), and molecular cloning (smart probiotics). The materials generally respond to the pH, temperature, ROS, specific biomolecules, and magnetic field. The inflammatory microenvironment in IBD is also exploited to trigger the release of drugs from smart bionanomaterials in the inflamed intestine. Molecules that are characteristic and more abundant in IBD, such as enzymes esterases and matrix metalloproteinases, receptors CD98, Ly6C, CD44, receptors for TNF-α and folate receptors, were used as stimuli for the release of active agents from smart bionanomaterials.
Smart bionanomaterials for use in IBD have both advantages and disadvantages. They depend on the type of the material used and need to be considered before utilization. They are summarized in Table 7, together with their methods of preparation.
Methods of preparation, advantages, and disadvantages of smart bionanomaterials in the diagnosis and treatment of IBD
| Smart bionanomaterial | Method of preparation | Advantages | Disadvantages |
|---|---|---|---|
| Hydrogels | Crosslinking | High water absorption capacity, porosity, flexibility, soft structure, controlled release and similarity to living tissues | Inhomogeneous mechanical strength, difficulty to shape them in predesigned geometries and difficulty in loading hydrophobic drugs |
| Nanoparticles | Dialysis and emulsification | Protection of the drug, reduction of adverse effects, targeting option and straightforward preparation | Chemical stability and particle aggregation |
| Nanofibers | Electrospinning | High surface to volume ratio, high porosity and protective properties | Low product yield and sensitivity to the manufacturing environment (voltage, temperature and humidity) |
| Hybrid delivery systems | Dialysis, emulsification, crosslinking and coprecipitation | Better efficacy and fewer adverse effects | Complicated and expensive process |
| Probiotics | Molecular cloning | Flexibility and programmability, beneficial effect on gut microbiota | Sensitivity to gastric acid and intestinal conditions |
Among the substances for the fabrication of smart materials for use in IBD, different forms of HA are the most frequently applied. In addition to HA, sodium alginate and Eudragit are also used as shells to protect the drug. The most commonly used active ingredients encapsulated in smart biomaterials are 5-ASA and corticosteroids among small molecules, and bacteria of the genera Lactobacillus and Bifidobacterium among probiotics. The latter can exert beneficial effects by repopulating the gut and restoring homeostasis.
Unlike most smart materials that use the bottom-up approach to combine materials to gain functionality, smart probiotics use a top-down approach by rationally exploiting parts of the cellular machinery to produce recombinant proteins in response to specific stimuli. Lc. lactis and EcN are the most widely used bacteria for genetic modification, with the goal of expressing various therapeutic proteins. Lc. lactis has been engineered to neutralize proinflammatory cytokines (TNF-α, IL-6, IL-17, and IL-23) or express anti-inflammatory molecules (IL-10, SOD, catalase, TGF-β, KGF-2, and butyrate).
Apart from the treatment of IBD, smart bionanomaterials can also be developed for the diagnosis of IBD. These applications are mainly associated with colorimetric and fluorescent changes in response to inflammation, or with microbiota sampling from specific regions of the gut. Currently, the number of developed applications is limited and relies on nanoparticles, hybrid systems, and probiotics; further studies are therefore needed to fully exploit the potential of smart bionanomaterials for IBD diagnostics. The future of smart materials in the diagnosis of IBD is indicated by micro- or millimeter devices, such as smart 3D-printed capsules which are designed for sampling microbiota and gut metabolites in specific regions of the colon that are difficult to access [155,156,157].
Similarly, the use of smart bionanomaterials in IBD therapy can also take inspiration from micro- and millimeter devices that can perform multiple functions. In this context, combining materials with therapeutic and diagnostic properties in a theranostic material seems particularly promising [158]. With that in mind, a combination of multiple materials, including live organisms, in advanced hybrid materials seems the most promising option. Apart from that, the applications currently focus mostly on delivery into the colon. Additional focus on the small intestine is therefore crucial, particularly for the CD, where the use of complex, larger-scale devices was also previously reported [159,160]. Achieving similar tasks with bionanomaterials would simplify and expedite their introduction into human clinical trials, which are thus far lacking.
To summarize, in this article, we review the literature on smart bionanomaterials for the treatment and diagnosis of IBD. We complement previous publications by focusing on the “smart” aspect of the materials, namely their ability to respond to external stimuli. We describe the techniques and methods for their synthesis, as well as their properties. Smart bionanomaterials are hydrogels, nanoparticles, nanofibers, and hybrid systems that encapsulate specific active ingredients. They protect them during passage through other parts of the gastrointestinal tract and release them on request. We also explain the use of smart probiotics created by genetic engineering which exploit the advantages of living cells to respond to environmental stimuli and synthesize recombinant proteins that target specific proinflammatory pathways in situ in the intestine. Smart bionanomaterials are therefore useful for encapsulating and delivering active ingredients to different parts of the intestine, thereby allowing targeted treatment and diagnosis of IBD.
-
Funding information: This work was funded by the Slovenian Research and Innovation Agency (grants J7-4418, J7-4420, and P4-0127).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Bahl S, Nagar H, Singh I, Sehgal S. Smart materials types, properties and applications: A review. Mater Today-Proc. 2020;28:1302–6. 10.1016/j.matpr.2020.04.505.Search in Google Scholar
[2] Eken GA, Acar MA. PVDF-based shape memory materials. In: Ameduri B, Fomin S, editors. Opportunities for fluoropolymers synthesis, characterization, processing, simulation and recycling. Amsterdam, Netherlands: Elsevier; 2020. p. 247–74. 10.1016/B978-0-12-821966-9.00009-2.Search in Google Scholar
[3] Marin E, Boschetto F, Pezzotti G. Biomaterials and biocompatibility: An historical overview. J Biomed Mater Res A. 2020;108(8):1617–33. 10.1002/jbm.a.36930.Search in Google Scholar PubMed
[4] Kim TY, Mok JW, Hong SH, Jeong SH, Choi H, Shin S, et al. Wireless theranostic smart contact lens for monitoring and control of intraocular pressure in glaucoma. Nat Commun. 2022;13(1):6801. 10.1038/S41467-022-34597-8.Search in Google Scholar
[5] He YT, Li Q, Chen P, Duan QX, Zhan JMA, Cai XH, et al. A smart adhesive Janus hydrogel for non-invasive cardiac repair and tissue adhesion prevention. Nat Commun. 2022;13(1):7666. 10.1038/S41467-022-35437-5.Search in Google Scholar PubMed PubMed Central
[6] Ding YW, Zhang XW, Mi CH, Qi XY, Zhou J, Wei DX. Recent advances in hyaluronic acid-based hydrogels for 3D bioprinting in tissue engineering applications. Smart Mater. 2023;4:59–68. 10.1016/j.smaim.2022.07.003.Search in Google Scholar
[7] Ng IC, Pawijit P, Tan J, Yu H. Anatomy and physiology for biomaterials research and development. In: Narayan R, editor. Encyclopedia of biomedical engineering. Amsterdam, Netherlands: Elsevier; 2019. p. 225–36.10.1016/B978-0-12-801238-3.99876-3Search in Google Scholar
[8] Armarego WLF. Nanomaterials. In: Armarego, editor. Purification of laboratory chemicals. 9th edn. Amsterdam, Netherlands: Elsevier; 2022. p. 586–630. 10.1016/B978-0-323-90968-6.50005-9.Search in Google Scholar
[9] Fakhoury M, Negrulj R, Mooranian A, Al-Salami H. Inflammatory bowel disease: clinical aspects and treatments. J Med Life. 2019;12(2):113–22. 10.2147/JIR.S65979.Search in Google Scholar PubMed PubMed Central
[10] Wang R, Li Z, Liu S, Zhang D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: A systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open. 2023;13:e065186. 10.1136/bmjopen-2022-065186.Search in Google Scholar PubMed PubMed Central
[11] Seyedian SS, Nokhostin F, Malamir MD. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. JML. 2019;12(2):113–22. 10.25122/jml-2018-0075.Search in Google Scholar PubMed PubMed Central
[12] Zhao M, Gonczi L, Lakatos PL, Burisch J. The burden of inflammatory bowel disease in Europe in 2020. J Crohns Colitis. 2021;15:1573–87. 10.1093/ecco-jcc/jjab029.Search in Google Scholar PubMed
[13] Liu TC, Stappenbeck TS. Genetics and pathogenesis of inflammatory bowel disease. Annu Rev Pathol. 2016;11:127–48. 10.1146/annurev-pathol-012615-044152.Search in Google Scholar PubMed PubMed Central
[14] Zhang H, Zeng Z, Mukherjee A, Shen B. Molecular diagnosis and classification of inflammatory bowel disease. Expert Rev Mol Diagn. 2018;18:867–86. 10.1080/14737159.2018.1516549.Search in Google Scholar PubMed
[15] Cai ZB, Wang S, Li JN. Treatment of inflammatory bowel disease: A comprehensive review. Front Med-Lausanne. 2021;8:765474. 10.3389/Fmed.2021.765474.Search in Google Scholar PubMed PubMed Central
[16] Moreno OL, Fernández-Tomé S, Abalo R. Biological treatments in inflammatory bowel disease: a complex mix of mechanisms and actions. Biologics. 2021;1(2):189–210. 10.3390/biologics1020012.Search in Google Scholar
[17] Stojanov S, Berlec A, Strukelj B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020;8(11):1715. 10.3390/microorganisms8111715.Search in Google Scholar PubMed PubMed Central
[18] Selvamani S, Mehta V, Ali El Enshasy H, Thevarajoo S, El Adawi H, Zeini I, et al. Efficacy of probiotics-based interventions as therapy for inflammatory bowel disease: A recent update. Saudi J Biol Sci. 2022;29(5):3546–67. 10.1016/j.sjbs.2022.02.044.Search in Google Scholar PubMed PubMed Central
[19] Fu WY, Xu LL, Chen ZT, Kan LL, Ma Y, Qian HS, et al. Recent advances on emerging nanomaterials for diagnosis and treatment of inflammatory bowel disease. J Control Rel. 2023;363:149–79. 10.1016/j.jconrel.2023.09.033.Search in Google Scholar PubMed
[20] Wang CPJ, Byun MJ, Kim SN, Park W, Park HH, Kim TH, et al. Biomaterials as therapeutic drug carriers for inflammatory bowel disease treatment. J Control Rel. 2022;345:1–19.10.1016/j.jconrel.2022.02.028Search in Google Scholar PubMed
[21] Saez A, Herrero-Fernandez B, Gomez-Bris R, Sanchez-Martinez H, Gonzalez-Granado JM. Pathophysiology of inflammatory bowel disease: Innate immune system. Int J Mol Sci. 2023;24(2):1526. 10.3390/ijms24021526.Search in Google Scholar PubMed PubMed Central
[22] Imam T, Park S, Kaplan MH, Olson MR. Effector T helper cell subsets in inflammatory bowel diseases. Front Immunol. 2018;9:1212. 10.3389/fimmu.2018.01212.Search in Google Scholar PubMed PubMed Central
[23] Kang Y, Park H, Choe BH, Kang B. The role and function of mucins and its relationship to inflammatory bowel disease. Front Med (Lausanne). 2022;9:848344. 10.3389/fmed.2022.848344.Search in Google Scholar PubMed PubMed Central
[24] Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823–36. 10.1042/BCJ20160510.Search in Google Scholar PubMed PubMed Central
[25] Beam A, Clinger E, Hao L. Effect of diet and dietary components on the composition of the gut microbiota. Nutrients. 2021;13(8):2795. 10.3390/nu13082795.Search in Google Scholar PubMed PubMed Central
[26] Syromyatnikov M, Nesterova E, Gladkikh M, Smirnova Y, Gryaznova M, Popov V. Characteristics of the gut bacterial composition in people of different nationalities and religions. Microorganisms. 2022;10(9):1866. 10.3390/microorganisms10091866.Search in Google Scholar PubMed PubMed Central
[27] Dedrick S, Sundaresh B, Huang Q, Brady C, Yoo T, Cronin C, et al. The role of gut microbiota and environmental factors in type 1 diabetes pathogenesis. Front Endocrinol. 2020;11:78. 10.3389/fendo.2020.00078.Search in Google Scholar PubMed PubMed Central
[28] Perez-Cobas AE, Artacho A, Knecht H, Ferrus ML, Friedrichs A, Ott SJ, et al. Differential effects of antibiotic therapy on the structure and function of human gut microbiota. PLoS One. 2013;8(11):e80201. 10.1371/journal.pone.0080201.Search in Google Scholar PubMed PubMed Central
[29] Stojanov S, Kreft S. Gut microbiota and the metabolism of phytoestrogens. Rev Bras Farmacogn. 2020;30(2):145–54. 10.1007/s43450-020-00049-x.Search in Google Scholar
[30] AL-Ishaq RK, Koklesova L, Kubatka P, Busselberg D. Immunomodulation by gut mcrobiome on gastrointestinal cancers: focusing on colorectal cancer. Cancers. 2022;14(9):2140. 10.3390/Cancers14092140.Search in Google Scholar PubMed PubMed Central
[31] Pickard JM, Zeng MY, Caruso R, Núñez G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 2017;279(1):70–89. 10.1111/imr.12567.Search in Google Scholar PubMed PubMed Central
[32] Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Reddy DN. Role of the normal gut microbiota. World J Gastroenterol. 2015;21(29):8787–803. 10.3748/wjg.v21.i29.8787.Search in Google Scholar PubMed PubMed Central
[33] Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14. 10.3390/microorganisms7010014.Search in Google Scholar PubMed PubMed Central
[34] Gasaly N, Hermoso MA, Gotteland M. Butyrate and the fine-tuning of colonic homeostasis: Implication for inflammatory bowel diseases. Int J Mol Sci. 2021;22(6):3061. 10.3390/Ijms22063061.Search in Google Scholar PubMed PubMed Central
[35] Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol. 2015;37(1):47–55. 10.1007/s00281-014-0454-4.Search in Google Scholar PubMed PubMed Central
[36] Vila AV, Imhann F, Collij V, Jankipersadsing SA, Gurry T, Mujagic Z, et al. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci Transl Med. 2018;10(472):eaap8914. 10.1126/scitranslmed.aap8914.Search in Google Scholar PubMed
[37] Zuo T, Ng SC. The gut microbiota in the pathogenesis and therapeutics of inflammatory bowel disease. Front Microbiol. 2018;9:2247. 10.3389/fmicb.2018.02247.Search in Google Scholar PubMed PubMed Central
[38] Gareb B, Otten AT, Frijlink HW, Dijkstra G, Kosterink JGW. Review: Local tumor necrosis factor-alpha inhibition in inflammatory bowel disease. Pharmaceutics. 2020;12(6):539. 10.3390/pharmaceutics12060539.Search in Google Scholar PubMed PubMed Central
[39] Ruder B, Atreya R, Becker C. Tumour necrosis factor alpha in intestinal homeostasis and gut related diseases. Int J Mol Sci. 2019;20(8):1887. 10.3390/ijms20081887.Search in Google Scholar PubMed PubMed Central
[40] McLean MH, Neurath MF, Durum SK. Targeting interleukins for the treatment of inflammatory bowel disease-what lies beyond anti-TNF therapy? Inflamm Bowel Dis. 2014;20(2):389–97. 10.1097/01.MIB.0000437616.37000.41.Search in Google Scholar PubMed PubMed Central
[41] Schmitt H, Neurath MF, Atreya R. Role of the IL23/IL17 pathway in Crohn’s disease. Front Immunol. 2021;12:622934. 10.3389/fimmu.2021.622934.Search in Google Scholar PubMed PubMed Central
[42] Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14(27):4280–8. 10.3748/wjg.14.4280.Search in Google Scholar PubMed PubMed Central
[43] Mishima Y, Oka A, Liu B, Herzog JW, Eun CS, Fan TJ, et al. Microbiota maintain colonic homeostasis by activating TLR2/MyD88/PI3K signaling in IL-10-producing regulatory B cells. J Clin Invest. 2019;129(9):3702–16. 10.1172/JCI93820.Search in Google Scholar PubMed PubMed Central
[44] Sun M, Wu W, Chen L, Yang W, Huang X, Ma C, et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun. 2018;9(1):3555. 10.1038/s41467-018-05901-2.Search in Google Scholar PubMed PubMed Central
[45] Yang W, Yu T, Huang X, Bilotta AJ, Xu L, Lu Y, et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat Commun. 2020;11(1):4457. 10.1038/s41467-020-18262-6.Search in Google Scholar PubMed PubMed Central
[46] Tian T, Wang Z, Zhang J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid Med Cell Longev. 2017;2017:4535194. 10.1155/2017/4535194.Search in Google Scholar PubMed PubMed Central
[47] Leclerc M, Bedu-Ferrari C, Etienne-Mesmin L, Mariadassou M, Lebreuilly L, Tran SL, et al. Nitric oxide impacts human gut microbiota diversity and functionalities. mSystems. 2021;6(5):e0055821. 10.1128/mSystems.00558-21.Search in Google Scholar PubMed PubMed Central
[48] Damaskos D, Kolios G. Probiotics and prebiotics in inflammatory bowel disease: Microflora ‘on the scope’. Br J Clin Pharmacol. 2008;65(4):453–67. 10.1111/j.1365-2125.2008.03096.x.Search in Google Scholar PubMed PubMed Central
[49] Saez-Lara MJ, Gomez-Llorente C, Plaza-Diaz J, Gil A. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: a systematic review of randomized human clinical trials. BioMed Res Int. 2015;2015:505878. 10.1155/2015/505878.Search in Google Scholar PubMed PubMed Central
[50] Bahram M, Mohseni N, Moghtader M. An introduction to hydrogels and some recent applications. In: Majee SB, editor. Emerging concepts in analysis and applications of hydrogels. London, UK: IntechOpen; 2016. 10.5772/64301.Search in Google Scholar
[51] Aswathy SH, Narendrakumar U, Manjubala I. Commercial hydrogels for biomedical applications. Heliyon. 2020;6(4):e03719. 10.1016/j.heliyon.2020.e03719.Search in Google Scholar PubMed PubMed Central
[52] Ho TC, Chang CC, Chan HP, Chung TW, Shu CW, Chuang KP, et al. Properties and applications in miomedicine. Molecules. 2022;27(9):2902. 10.3390/Molecules27092902.Search in Google Scholar
[53] Billiet T, Vandenhaute M, Schelfhout J, Van Vlierberghe S, Dubruel P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials. 2012;33(26):6020–41. 10.1016/j.biomaterials.2012.04.050.Search in Google Scholar PubMed
[54] Eyigor A, Bahadori F, Yenigun VB, Eroglu MS. Beta-glucan based temperature responsive hydrogels for 5-ASA delivery. Carbohydr Polym. 2018;201:454–63. 10.1016/j.carbpol.2018.08.053.Search in Google Scholar PubMed
[55] Saboktakin MR, Tabatabaie RM, Maharramov A, Ramazanov MA. Synthesis and characterization of chitosan hydrogels containing 5-aminosalicylic acid nanopendents for colon: specific drug delivery. J Pharm Sci. 2010;99(12):4955–61. 10.1002/jps.22218.Search in Google Scholar PubMed
[56] Saboktakin MR, Tabatabaie R, Maharramov A, Ramazanov MA. Synthesis and characterization of superparamagnetic chitosan-dextran sulfate hydrogels as nano carriers for colon-specific drug delivery. Carbohydr Polym. 2010;81(2):372–6. 10.1016/j.carbpol.2010.02.034.Search in Google Scholar
[57] Liu H, Cai ZW, Wang F, Hong LW, Deng LF, Zhong J, et al. Colon-targeted adhesive hydrogel microsphere for regulation of gut immunity and flora. Adv Sci. 2021;8(18):e2101619. 10.1002/Advs.202101619.Search in Google Scholar PubMed PubMed Central
[58] Maronek M, Marafini I, Gardlik R, Link R, Troncone E, Monteleone G. Metalloproteinases in inflammatory bowel diseases. J Inflamm Res. 2021;14:1029–41. 10.2147/JIR.S288280.Search in Google Scholar PubMed PubMed Central
[59] Zhang SF, Ermann J, Succi MD, Zhou A, Hamilton MJ, Cao BN, et al. An inflammation-targeting hydrogel for local drug delivery in inflammatory bowel disease. Sci Transl Med. 2015;7(300):300ra128. 10.1126/scitranslmed.aaa5657.Search in Google Scholar PubMed PubMed Central
[60] Huang LJ, Wang JJ, Kong LL, Wang X, Li QL, Zhang LJ, et al. ROS-responsive hyaluronic acid hydrogel for targeted delivery of probiotics to relieve colitis. Int J Biol Macromol. 2022;222:1476–86. 10.1016/j.ijbiomac.2022.09.247.Search in Google Scholar PubMed
[61] Wang R, Gou K, Zhang W, He Y, Yang K, Chen Q, et al. Poly-γ-glutamic acid microgel-encapsulated probiotics with gastric acid resistance and smart inflammatory factor targeted delivery performance to smeliorate volitis. Adv Funct Mater. 2022;32(26):2113034. 10.1002/adfm.202113034.Search in Google Scholar
[62] Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1(12):16071. 10.1038/natrevmats.2016.71.Search in Google Scholar PubMed PubMed Central
[63] Xu F, Dawson C, Lamb M, Mueller E, Stefanek E, Akbari M, et al. Hydrogels for tissue engineering: Addressing key design needs toward clinical translation. Front Bioeng Biotechnol. 2022;10:849831. 10.3389/fbioe.2022.849831.Search in Google Scholar PubMed PubMed Central
[64] Hu W, Wang Z, Xiao Y, Zhang S, Wang J. Advances in crosslinking strategies of biomedical hydrogels. Biomater Sci. 2019;7(3):843–55. 10.1039/c8bm01246f.Search in Google Scholar PubMed
[65] Parhi R. Cross-linked hydrogel for pharmaceutical applications: A review. Adv Pharm Bull. 2017;7(4):515–30. 10.15171/apb.2017.064.Search in Google Scholar PubMed PubMed Central
[66] Arianita AC, Amalia B, Pudjiastuti W, Melanie S, Fauzia V, Imawan C. Effect of glutaraldehyde to the mechanical properties ofchitosan/nanocellulose. J Phys Conf Ser. 2019;1317:012045. 10.1088/1742-6596/1317/1/012045.Search in Google Scholar
[67] Gharib G, Butun I, Muganli Z, Kozalak G, Namli I, Sarraf SS, et al. Biomedical applications of microfluidic devices: A review. Biosensors. 2022;12(11):1023. 10.3390/bios12111023.Search in Google Scholar PubMed PubMed Central
[68] Khan I, Saeed K, Khan I. Nanoparticles: Properties, applications and toxicities. Arab J Chem. 2019;12(7):908–31. 10.1016/j.arabjc.2017.05.011.Search in Google Scholar
[69] Joudeh N, Linke D. Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. J Nanobiotechnol. 2022;20(1):262. 10.1186/s12951-022-01477-8.Search in Google Scholar PubMed PubMed Central
[70] Yusuf A, Almotairy ARZ, Henidi H, Alshehri OY, Aldughaim MS. Nanoparticles as drug delivery systems: A review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers. 2023;15(7):1596. 10.3390/Polym15071596.Search in Google Scholar
[71] Chenthamara D, Subramaniam S, Ramakrishnan SG, Krishnaswamy S, Essa MM, Lin FH, et al. Therapeutic efficacy of nanoparticles and routes of administration. Biomater Res. 2019;23(1). 10.1186/s40824-019-0166-x.Search in Google Scholar PubMed PubMed Central
[72] Zielinska A, Carreiró F, Oliveira AM, Neves A, Pires B, Venkatesh DN, et al. Polymeric nanoparticles: Production, characterization, toxicology and ecotoxicology. Molecules. 2020;25(16):3731. 10.3390/Molecules25163731.Search in Google Scholar PubMed PubMed Central
[73] Mukherjee S, Ray S, Thakur RS. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J Pharm Sci. 2009;71(4):349–58. 10.4103/0250-474X.57282.Search in Google Scholar PubMed PubMed Central
[74] Wang X, Yan J, Wang L, Pan D, Xu Y, Wang F, et al. Oral delivery of anti-TNF antibody shielded by natural polyphenol-mediated supramolecular assembly for inflammatory bowel disease therapy. Theranostics. 2020;10(23):10808–22. 10.7150/thno.47601.Search in Google Scholar PubMed PubMed Central
[75] Wilson DS, Dalmasso G, Wang L, Sitaraman SV, Merlin D, Murthy N. Orally delivered thioketal nanoparticles loaded with TNF-alpha-siRNA target inflammation and inhibit gene expression in the intestines. Nat Mater. 2010;9(11):923–8. 10.1038/nmat2859.Search in Google Scholar PubMed PubMed Central
[76] Lee Y, Sugihara K, Gillilland MG III, Jon S, Kamada N, Moon JJ. Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat Mater. 2020;19(1):118–26. 10.1038/s41563-019-0462-9.Search in Google Scholar PubMed PubMed Central
[77] Zhang Q, Tao H, Lin Y, Hu Y, An H, Zhang D, et al. A superoxide dismutase/catalase mimetic nanomedicine for targeted therapy of inflammatory bowel disease. Biomaterials. 2016;105:206–21. 10.1016/j.biomaterials.2016.08.010.Search in Google Scholar PubMed
[78] Sun Q, Luan L, Arif M, Li J, Dong QJ, Gao Y, et al. Redox-sensitive nanoparticles based on 4-aminothiophenol-carboxymethyl inulin conjugate for budesonide delivery in inflammatory bowel diseases. Carbohydr Polym. 2018;189:352–9. 10.1016/j.carbpol.2017.12.021.Search in Google Scholar PubMed
[79] Fan X, Zhang ZZ, Gao WX, Pan QQ, Luo K, He B, et al. An engineered butyrate-derived polymer nanoplatform as a mucosa-healing enhancer potentiates the therapeutic effect of magnolol in inflammatory bowel disease. ACS Nano. 2023;18:229–44. 10.1021/acsnano.3c05732.Search in Google Scholar PubMed
[80] Beloqui A, Coco R, Memvanga PB, Ucakar B, des Rieux A, Preat V. pH-sensitive nanoparticles for colonic delivery of curcumin in inflammatory bowel disease. Int J Pharm. 2014;473(1–2):203–12. 10.1016/j.ijpharm.2014.07.009.Search in Google Scholar PubMed
[81] Zhou H, Qian H. Preparation and characterization of pH-sensitive nanoparticles of budesonide for the treatment of ulcerative colitis. Drug Des Dev Ther. 2018;12:2601–9. 10.2147/DDDT.S170676.Search in Google Scholar PubMed PubMed Central
[82] Naeem M, Bae J, Oshi MA, Kim MS, Moon HR, Lee BL, et al. Colon-targeted delivery of cyclosporine A using dual-functional Eudragit ® FS30D/PLGA nanoparticles ameliorates murine experimental colitis. Int J Nanomed. 2018;13:1225–40. 10.2147/IJN.S157566.Search in Google Scholar PubMed PubMed Central
[83] Qu Z, Wong KY, Moniruzzaman M, Begun J, Santos HA, Hasnain SZ, et al. One-pot synthesis of pH-responsive eudragit-mesoporous silica nanocomposites enable colonic delivery of glucocorticoids for the treatment of inflammatory bowel disease. Adv Ther-Germany. 2021;4:2000165. 10.1002/adtp.202000165.Search in Google Scholar
[84] Naserifar M, Hosseinzadeh H, Abnous K, Mohammadi M, Taghdisi SM, Ramezani M, et al. Oral delivery of folate-targeted resveratrol-loaded nanoparticles for inflammatory bowel disease therapy in rats. Life Sci. 2020;262:118555. 10.1016/j.lfs.2020.118555.Search in Google Scholar PubMed
[85] Kedmi R, Veiga N, Ramishetti S, Goldsmith M, Rosenblum D, Dammes N, et al. A modular platform for targeted RNAi therapeutics. Nat Nanotechnol. 2018;13(3):214–9. 10.1038/s41565-017-0043-5.Search in Google Scholar PubMed
[86] Veiga N, Goldsmith M, Granot Y, Rosenblum D, Dammes N, Kedmi R, et al. Cell specific delivery of modified mRNA expressing therapeutic proteins to leukocytes. Nat Commun. 2018;9(1):4493. 10.1038/s41467-018-06936-1.Search in Google Scholar PubMed PubMed Central
[87] Buss MT, Ramesh P, English MA, Lee-Gosselin A, Shapiro MG. Spatial control of probiotic bacteria in the gastrointestinal tract assisted by magnetic particles. Adv Mater. 2021;33(17):e2007473. 10.1002/Adma.202007473.Search in Google Scholar PubMed
[88] Cui XJ, Wang XY, Chang XL, Bao L, Wu JG, Tan ZQ, et al. A new capacity of gut microbiota: Fermentation of engineered inorganic carbon nanomaterials into endogenous organic metabolites. Proc Natl Acad Sci USA. 2023;120(20):e2218739120. 10.1073/pnas.2218739120.Search in Google Scholar PubMed PubMed Central
[89] Li J, Lei RH, Li X, Xiong FX, Zhang QY, Zhou Y, et al. The antihyperlipidemic effects of fullerenol nanoparticles via adjusting the gut microbiota in vivo. Part Fibre Toxicol. 2018;15(1):5. 10.1186/S12989-018-0241-9.Search in Google Scholar
[90] Gangadoo S, Dinev I, Chapman J, Hughes RJ, Van TTH, Moore RJ, et al. Selenium nanoparticles in poultry feed modify gut microbiota and increase abundance of Faecalibacterium prausnitzii. Appl Microbiol Biot. 2018;102(3):1455–66. 10.1007/s00253-017-8688-4.Search in Google Scholar PubMed
[91] Ohno M, Nishida A, Sugitani Y, Nishino K, Inatomi O, Sugimoto M, et al. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS One. 2017;12(10):e0185999. 10.1371/journal.pone.0185999.Search in Google Scholar PubMed PubMed Central
[92] Krishnamoorthy K, Mahalingam M. Selection of a suitable method for the preparation of polymeric nanoparticles: multi-criteria decision making approach. Adv Pharm Bull. 2015;5(1):57–67. 10.5681/apb.2015.008.Search in Google Scholar PubMed PubMed Central
[93] Nava-Arzaluz MG, Pinon-Segundo E, Ganem-Rondero A, Lechuga-Ballesteros D. Single emulsion-solvent evaporation technique and modifications for the preparation of pharmaceutical polymeric nanoparticles. Recent Pat Drug Deliv Formul. 2012;6(3):209–23. 10.2174/187221112802652633.Search in Google Scholar PubMed
[94] Gundloori RVN, Singam A, Killi N. Nanobased intravenous and transdermal drug delivery systems. In: Mohapatra SS, Ranjan S, Dasgupta N, Mishra RK, Thomas S, editors. Applications of targeted nano drugs and delivery systems. Amsterdam, Netherlands: Elsevier; 2019. p. 551–94. 10.1016/B978-0-12-814029-1.00019-3.Search in Google Scholar
[95] Xue J, Xie J, Liu W, Xia Y. Electrospun nanofibers: New concepts, materials, and applications. Acc Chem Res. 2017;50(8):1976–87. 10.1021/acs.accounts.7b00218.Search in Google Scholar PubMed PubMed Central
[96] Torres-Martinez EJ, Bravo CJM, Medina AS, González GLP, Gómez LJV. A summary of electrospun nanofibers as drug delivery system: Drugs loaded and biopolymers used as matrices. Curr Drug Deliv. 2018;15(10):1360–74. 10.2174/1567201815666180723114326.Search in Google Scholar PubMed PubMed Central
[97] Duan X, Chen HL, Guo C. Polymeric nanofibers for drug delivery applications: A recent review. J Mater Sci Mater Med. 2022;33(12):78. 10.1007/s10856-022-06700-4.Search in Google Scholar PubMed PubMed Central
[98] Stojanov S, Berlec A. Electrospun nanofibers as carriers of microorganisms, stem cells, proteins, and nucleic acids in therapeutic and other applications. Front Bioeng Biotechnol. 2020;8:130. 10.3389/fbioe.2020.00130.Search in Google Scholar PubMed PubMed Central
[99] Škrlec K, Zupančič Š, Mihevc SP, Kocbek P, Kristl J, Berlec A. Development of electrospun nanofibers that enable high loading and long-term viability of probiotics. Eur J Pharm Biopharm. 2019;136:108–19. 10.1016/j.ejpb.2019.01.013.Search in Google Scholar PubMed
[100] Stojanov S, Plavec TV, Kristl J, Zupančič Š, Berlec A. Engineering of vaginal lactobacilli to express fluorescent proteins enables the analysis of their mixture in nanofibers. Int J Mol Sci. 2021;22(24):13631. 10.3390/ijms222413631.Search in Google Scholar PubMed PubMed Central
[101] Stojanov S, Kristl J, Zupančič Š, Berlec A. Influence of excipient composition on survival of vaginal lactobacilli in electrospun nanofibers. Pharmaceutics. 2022;14(6):1155. 10.3390/pharmaceutics14061155.Search in Google Scholar PubMed PubMed Central
[102] Zupančič Š, Škrlec K, Kocbek P, Kristl J, Berlec A. Effects of electrospinning on the viability of ten species of lactic acid bacteria in poly(ethylene oxide) nanofibers. Pharmaceutics. 2019;11(9):483. 10.3390/pharmaceutics11090483.Search in Google Scholar PubMed PubMed Central
[103] Xu HZ, Yagi S, Ashour S, Du L, Hoque ME, Tan L. A review on current nanofiber technologies: Electrospinning, centrifugal spinning, and electro-centrifugal spinning. Macromol Mater Eng. 2023;308(3):2200502. 10.1002/mame.202200502.Search in Google Scholar
[104] Tabuchi R, Anraku M, Iohara D, Ishiguro T, Ifuku S, Nagae T, et al. Surface-deacetylated chitin nanofibers reinforced with a sulfobutyl ether beta-cyclodextrin gel loaded with prednisolone as potential therapy for inflammatory bowel disease. Carbohydr Polym. 2017;174:1087–94. 10.1016/j.carbpol.2017.07.028.Search in Google Scholar PubMed
[105] Yang YY, Liu ZP, Yu DG, Wang K, Liu P, Chen X. Colon-specific pulsatile drug release provided by electrospun shellac nanocoating on hydrophilic amorphous composites. Int J Nanomed. 2018;13:2395–404. 10.2147/IJN.S154849.Search in Google Scholar PubMed PubMed Central
[106] Xu Q, Zhang N, Qin W, Liu J, Jia Z, Liu H. Preparation, in vitro and in vivo evaluation of budesonide loaded core/shell nanofibers as oral colonic drug delivery system. J Nanosci Nanotechnol. 2013;13(1):149–56. 10.1166/jnn.2013.6920.Search in Google Scholar PubMed
[107] Feng K, Huang RM, Wu RQ, Wei YS, Zong MH, Linhardt RJ, et al. A novel route for double-layered encapsulation of probiotics with improved viability under adverse conditions. Food Chem. 2020;310:125977. 10.1016/j.foodchem.2019.125977.Search in Google Scholar PubMed
[108] Yu H, Liu W, Li D, Liu C, Feng Z, Jiang B. Targeting delivery system for Lactobacillus plantarum based on functionalized electrospun nanofibers. Polymers. 2020;12(7):1565. 10.3390/polym12071565.Search in Google Scholar PubMed PubMed Central
[109] Xu C, Ma J, Liu Z, Wang W, Liu X, Qian S, et al. Preparation of shell-core fiber-encapsulated Lactobacillus rhamnosus 1.0320 using coaxial electrospinning. Food Chem. 2023;402:134253. 10.1016/j.foodchem.2022.134253.Search in Google Scholar PubMed
[110] Ajalloueian F, Guera PR, Bahl MI, Torp AM, Hwu ET, Licht TR, et al. Multi-layer PLGA-pullulan-PLGA electrospun nanofibers for probiotic delivery. Food Hydrocoll. 2022;123:107112. 10.1016/j.foodhyd.2021.107112.Search in Google Scholar
[111] Al-Abduljabbar A, Farooq I. Electrospun polymer nanofibers: Processing, properties, and applications. Polymers. 2023;15:65. 10.3390/polym15010065.Search in Google Scholar PubMed PubMed Central
[112] Qin X. Coaxial electrospinning of nanofibers. Woodhead Publ Ser Te. 2017;186:41–71. 10.1016/B978-0-08-100907-9.00003-9.Search in Google Scholar
[113] Han D, Steckl AJ. Coaxial electrospinning formation of complex polymer fibers and their applications. Chempluschem. 2019;84(10):1453–97. 10.1002/cplu.201900281.Search in Google Scholar PubMed
[114] Rout SR, Gowtham K, Sheikh A, Parvez S, Dandela R, Kesharwani P. Recent advances and future prospective of hybrid drug delivery systems. In: Kesharwani P, Jain NK, editors. Hybrid nanomaterials for drug delivery. Amsterdam, Netherlands: Elsevier; 2022. p. 357–74. 10.1016/B978-0-323-85754-3.00006-X.Search in Google Scholar
[115] Xiao B, Laroui H, Viennois E, Ayyadurai S, Charania MA, Zhang YC, et al. Nanoparticles with surface antibody against CD98 and carrying CD98 small interfering RNA reduce colitis in mice. Gastroenterology. 2014;146(5):1289–300. 10.1053/j.gastro.2014.01.056.Search in Google Scholar PubMed PubMed Central
[116] Knipe JM, Strong LE, Peppas NA. Enzyme- and pH-responsive microencapsulated nanogels for oral delivery of siRNA to induce TNF-α knockdown in the intestine. Biomacromolecules. 2016;17(3):788–97. 10.1021/acs.biomac.5b01518.Search in Google Scholar PubMed
[117] Xiao B, Xu ZG, Viennois E, Zhang YC, Zhang Z, Zhang MZ, et al. Orally targeted delivery of tripeptide KPV via hyaluronic acid-functionalized nanoparticles efficiently alleviates ulcerative colitis. Mol Ther. 2017;25(7):1628–40. 10.1016/j.ymthe.2016.11.020.Search in Google Scholar PubMed PubMed Central
[118] Ouyang J, Deng B, Zou BH, Li YJ, Bu QY, Tian Y, et al. Oral hydrogel microbeads-mediated in situ synthesis of selenoproteins for regulating intestinal immunity and microbiota. J Am Chem Soc. 2023;145(22):12193–205. 10.1021/jacs.3c02179.Search in Google Scholar PubMed
[119] Tian WN, Wang HA, Zhu Y, Wang Q, Song MY, Cao Y, et al. Intervention effects of delivery vehicles on the therapeutic efficacy of 6-gingerol on colitis. J Control Rel. 2022;349:51–66. 10.1016/j.jconrel.2022.06.058.Search in Google Scholar PubMed
[120] Zhang ZZ, Pan Y, Guo ZY, Fan X, Pan QQ, Gao WX, et al. An olsalazine nanoneedle-embedded inulin hydrogel reshapes intestinal homeostasis in inflammatory bowel disease. Bioact Mater. 2024;33:71–84. 10.1016/j.bioactmat.2023.10.028.Search in Google Scholar PubMed PubMed Central
[121] Zhu Y, Wang QH, Chen Y, Xie YT, Han GH, Liu S, et al. Living probiotics-loaded hydrogel microspheres with gastric acid resistance and ROS triggered release for potential therapy of inflammatory bowel disease. ACS Appl Polym Mater. 2023;5(1):957–67. 10.1021/acsapm.2c01893957.Search in Google Scholar
[122] Liu J, Wang YX, Heelan WJ, Chen Y, Li ZT, Hu QY. Mucoadhesive probiotic backpacks with ROS nanoscavengers enhance the bacteriotherapy for inflammatory bowel diseases. Sci Adv. 2022;8(45):eabp8798. 10.1126/sciadv.abp8798.Search in Google Scholar PubMed PubMed Central
[123] Abraham BP, Quigley EMM. Probiotics in inflammatory bowel disease. Gastroenterol Clin North Am. 2017;46(4):769–82. 10.1016/j.gtc.2017.08.003.Search in Google Scholar PubMed
[124] Jakubczyk D, Leszczynska K, Gorska S. The effectiveness of probiotics in the treatment of inflammatory bowel disease (IBD)-a critical review. Nutrients. 2020;12(7):1973. 10.3390/nu12071973.Search in Google Scholar PubMed PubMed Central
[125] Mierau I, Kleerebezem M. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl Microbiol Biotechnol. 2005;68(6):705–17. 10.1007/s00253-005-0107-6.Search in Google Scholar PubMed
[126] Ravnikar M, Štrukelj B, Obermajer N, Lunder M, Berlec A. Engineered lactic acid bacterium Lactococcus lactis capable of binding antibodies and tumor necrosis factor alpha. AEM. 2010;76(20):6928–32. 10.1128/AEM.00190-10.Search in Google Scholar PubMed PubMed Central
[127] Simčič S, Berlec A, Stopinšek S, Štrukelj B, Orel R. Engineered and wild-type L. lactis promote anti-inflammatory cytokine signalling in inflammatory bowel disease patient’s mucosa. World J Microbiol Biotechnol. 2019;35(3):45. 10.1007/s11274-019-2615-z.Search in Google Scholar PubMed
[128] Kosler S, Štrukelj B, Berlec A. Lactic acid bacteria with concomitant IL-17, IL-23 and TNFalpha- binding ability for the treatment of inflammatory bowel disease. Curr Pharm Biotechnol. 2017;18(4):318–26. 10.2174/1389201018666170210152218.Search in Google Scholar PubMed
[129] Zahirović A, Berlec A. Targeting IL-6 by engineered Lactococcus lactis via surface-displayed affibody. Microb Cell Fact. 2022;21(1):143. 10.1186/s12934-022-01873-7.Search in Google Scholar PubMed PubMed Central
[130] Plavec TV, Kuchař M, Benko A, Liškova V, Černy J, Berlec A, et al. Engineered Lactococcus lactis Secreting IL-23 receptor-targeted REX protein blockers for modulation of IL-23/Th17-mediated inflammation. Microorganisms. 2019;7(5):152. 10.3390/microorganisms7050152.Search in Google Scholar PubMed PubMed Central
[131] Han W, Mercenier A, Ait-Belgnaoui A, Pavan S, Lamine F, van Swam II, et al. Improvement of an experimental colitis in rats by lactic acid bacteria producing superoxide dismutase. Inflamm Bowel Dis. 2006;12(11):1044–52. 10.1097/01.mib.0000235101.09231.9e.Search in Google Scholar PubMed
[132] Zhou J, Li M, Chen Q, Li X, Chen L, Dong Z, et al. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat Commun. 2022;13(1):3432. 10.1038/s41467-022-31171-0.Search in Google Scholar PubMed PubMed Central
[133] Yan X, Liu XY, Zhang D, Zhang YD, Li ZH, Liu X, et al. Construction of a sustainable 3-hydroxybutyrate-producing probiotic Escherichia coli for treatment of colitis. Cell Mol Immunol. 2021;18(10):2344–57. 10.1038/s41423-021-00760-2.Search in Google Scholar PubMed PubMed Central
[134] Benbouziane B, Ribelles P, Aubry C, Martin R, Kharrat P, Riazi A, et al. Development of a Stress-Inducible Controlled Expression (SICE) system in Lactococcus lactis for the production and delivery of therapeutic molecules at mucosal surfaces. J Biotech. 2013;168(2):120–9. 10.1016/j.jbiotec.2013.04.019.Search in Google Scholar PubMed
[135] McKay R, Ghodasra M, Schardt J, Quan D, Pottash AE, Shang W, et al. A platform of genetically engineered bacteria as vehicles for localized delivery of therapeutics: Toward applications for Crohn’s disease. Bioeng Transl Med. 2018;3(3):209–21. 10.1002/btm2.10113.Search in Google Scholar PubMed PubMed Central
[136] Chua KJ, Ling H, Hwang IY, Lee HL, March JC, Lee YS, et al. An engineered probiotic produces a type III interferon IFNL1 and reduces inflammations in in vitro inflammatory bowel disease models. ACS Biomater Sci Eng. 2023;9(9):5123–35. 10.1021/acsbiomaterials.2c00202.Search in Google Scholar PubMed PubMed Central
[137] Porzio S, Bossu P, Ruggiero P, Boraschi D, Tagliabue A. Mucosal delivery of anti-inflammatory IL-1Ra by sporulating recombinant bacteria. BMC Biotechnol. 2004;4:27. 10.1186/1472-6750-4-27.Search in Google Scholar PubMed PubMed Central
[138] Hamady ZZ, Scott N, Farrar MD, Wadhwa M, Dilger P, Whitehead TR, et al. Treatment of colitis with a commensal gut bacterium engineered to secrete human TGF-beta1 under the control of dietary xylan 1. Inflamm Bowel Dis. 2011;17(9):1925–35. 10.1002/ibd.21565.Search in Google Scholar PubMed
[139] Hamady ZZ, Scott N, Farrar MD, Lodge JP, Holland KT, Whitehead T, et al. Xylan-regulated delivery of human keratinocyte growth factor-2 to the inflamed colon by the human anaerobic commensal bacterium Bacteroides ovatus. Gut. 2010;59(4):461–9. 10.1136/gut.2008.176131.Search in Google Scholar PubMed
[140] Ma J, Lyu Y, Liu X, Jia X, Cui F, Wu X, et al. Engineered probiotics. Microb Cell Fact. 2022;21(1):72. 10.1186/s12934-022-01799-0.Search in Google Scholar PubMed PubMed Central
[141] Tüzmen S, Baskin Y, Nursal AF, Eraslan S, Esemen Y, Çalibasi G, et al. Techniques for nucleic acid engineering: The foundation of gene manipulation. In: Barh D, Azevedo V, editors. Omics technologies and bio-engineering: Towards improving quality of life. Vol. 1, Amsterdam, Netherlands: Elsevier; 2018. p. 247–315. 10.1016/B978-0-12-804659-3.00014-2.Search in Google Scholar
[142] Yarmush ML, Golberg A, Sersa G, Kotnik T, Miklavcic D. Electroporation-based technologies for medicine: principles, spplications, and vhallenges. Annu Rev Biomed Eng. 2014;16:295–320. 10.1146/annurev-bioeng-071813-104622.Search in Google Scholar PubMed
[143] Qi H, Yu L, Li YZ, Cai M, He JZ, Liu JY, et al. Developing multi-copy chromosomal integration strategies for heterologous biosynthesis of caffeic acid in Saccharomyces cerevisiae. Front Microbiol. 2022;13:851706. 10.3389/Fmicb.2022.851706.Search in Google Scholar
[144] Virolle C, Goldlust K, Djermoun S, Bigot S, Lesterlin C. Plasmid transfer by conjugation in gram-negative bacteria: From the cellular to the community level. Genes. 2020;11(11):1239. 10.3390/genes11111239.Search in Google Scholar PubMed PubMed Central
[145] Goh S. Phage transduction. In: Roberts AP, Mullany P, editors. Clostridium difficile: Methods and protocols. 2nd edn. New York, USA: Springer; 2016. p. 177–85. 10.1007/978-1-4939-6361-4_13.Search in Google Scholar PubMed
[146] Zhou DT, Yin Y, Zhu ZX, Gao YF, Yang JJ, Pan YC, et al. Orally administered platinum nanomarkers for urinary monitoring of inflammatory bowel disease. ACS Nano. 2022;16:18503–14. 10.1021/acsnano.2c06705.Search in Google Scholar PubMed
[147] Shishodia S, Chouchene B, Gries T, Schneider R. Selected I-III-VI(2) Semiconductors: synthesis, properties and applications in photovoltaic cells. Nanomaterials. 2023;13:2889. 10.3390/nano13212889.Search in Google Scholar PubMed PubMed Central
[148] Xiao B, Yang Y, Viennois E, Zhang YC, Ayyadurai S, Baker MT, et al. Glycoprotein CD98 as a receptor for colitis-targeted delivery of nanoparticles. J Mater Chem B. 2014;2(11):1499–508. 10.1039/c3tb21564d.Search in Google Scholar PubMed PubMed Central
[149] Liu D, Viennois E, Fang J, Merlin D, Iyer SS. Toward point-of-care diagnostics to monitor MMP-9 and TNF-alpha levels in inflammatory bowel disease. ACS Omega. 2021;6(10):6582–7. 10.1021/acsomega.0c05115.Search in Google Scholar PubMed PubMed Central
[150] Wang Y, Wang X, Lv Y, Guo Y, He M, Lan M, et al. A ROS-responsive fluorescent probe detecting experimental colitis by functional polymeric nanoparticles. Int J Pharm. 2021;609:121125. 10.1016/j.ijpharm.2021.121125.Search in Google Scholar PubMed
[151] Yoon D, Park S, Park S. Smart hydrogel structure for microbiome sampling in gastrointestinal tract. Sens Actuat B-Chem. 2023;389:133910. 10.1016/J.Snb.2023.133910.Search in Google Scholar
[152] Riglar DT, Giessen TW, Baym M, Kerns SJ, Niederhuber MJ, Bronson RT, et al. Engineered bacteria can function in the mammalian gut long-term as live diagnostics of inflammation. Nat Biotechnol. 2017;35(7):653–8. 10.1038/nbt.3879.Search in Google Scholar PubMed PubMed Central
[153] Daeffler KN, Galley JD, Sheth RU, Ortiz-Velez LC, Bibb CO, Shroyer NF, et al. Engineering bacterial thiosulfate and tetrathionate sensors for detecting gut inflammation. Mol Syst Biol. 2017;13(4):923. 10.15252/msb.20167416.Search in Google Scholar PubMed PubMed Central
[154] Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:15 25 1–4. 10.1002/0471142735.im1525s104.Search in Google Scholar PubMed PubMed Central
[155] Nejati S, Wang J, Sedaghat S, Balog NK, Long AM, Rivera UH, et al. Smart capsule for targeted proximal colon microbiome sampling. Acta Biomater. 2022;154:83–96. 10.1016/j.actbio.2022.09.050.Search in Google Scholar PubMed PubMed Central
[156] Waimin JF, Nejati S, Jiang H, Qiu J, Wang J, Verma MS, et al. Smart capsule for non-invasive sampling and studying of the gastrointestinal microbiome. RSC Adv. 2020;10(28):16313–22. 10.1039/c9ra10986b.Search in Google Scholar PubMed PubMed Central
[157] De la Paz E, Maganti NH, Trifonov A, Jeerapan I, Mahato K, Yin L, et al. A self-powered ingestible wireless biosensing system for real-time in situ monitoring of gastrointestinal tract metabolites. Nat Commun. 2022;13(1):7405. 10.1038/s41467-022-35074-y.Search in Google Scholar PubMed PubMed Central
[158] Zhu W, Wei ZQ, Han C, Weng XS. Nanomaterials as promising theranostic tools in nanomedicine and their applications in clinical disease diagnosis and treatment. Nanomaterials. 2021;11:3346. 10.3390/nano11123346.Search in Google Scholar PubMed PubMed Central
[159] Nejati S, Wang J, Heredia-Rivera U, Sedaghat S, Woodhouse I, Johnson JS, et al. Small intestinal sampling capsule for inflammatory bowel disease type detection and management. Lab Chip. 2021;22:57–70. 10.1039/d1lc00451d.Search in Google Scholar PubMed
[160] Nejad HR, Oliveira BCM, Sadeqi A, Dehkharghani A, Kondova I, Langermans JAM, et al. Ingestible osmotic pill for in vivo sampling of gut microbiomes. Adv Intell Syst-Ger. 2019;1:1900053. 10.1002/aisy.201900053.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy


