Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
-
Poonam Dwivedi
, Indu Jatrana
Abstract
This study presents an innovative, environmentally friendly method for biosynthesizing copper oxide–silica (Cu2O/SiO2) nanocomposites (CSNCs) utilizing an aqueous leaf extract of Callistemon viminalis (C. viminalis). The goal of this work is to fabricate CSNCs using a less hazardous and sustainable synthesis approach. Copper acetate and sodium metasilicate were used as precursors, whereas the C. viminalis green leaf extract was used as the reducing and stabilizing agent. Analysis of the plant extract using Fourier transform infrared spectroscopy indicated the presence of polyphenolic compounds, primarily phenolic acids, which functioned as both reducing and stabilizing agents in the synthesis of CSNCs. A combination of energy dispersive X-ray spectroscopy and scanning electron microscopy was used to study the formation of spherical copper–silica hybrid nanostructures. Powder X-ray diffraction analysis revealed the successful integration of silica with copper(i) oxide (Cu2O) through the presence of distinct Cu2O peaks and a broad amorphous SiO2 peak at 2θ = 22.77°. The thermal stability of the nanocomposites (NCs) was assessed using thermogravimetric analysis and differential thermal analysis under a nitrogen atmosphere. The biogenic NCs also successfully inhibited pathogenic strains of Staphylococcus aureus (S. aureus) and Candida albicans (C. albicans); however, S. aureus was found to be more susceptible to the biocidal activity of the NCs than P. aeruginosa. These findings suggest that this simple, cost-effective, and eco-friendly method for producing biologically active hybrid nanomaterials holds significant promise for future applications in both biological and materials sciences.
1 Introduction
Biogenic nanoparticles and composites are synthesized from several natural sources, including different parts of plants, fungi, and bacteria [1]. This synthesis process is more ecologically friendly compared to standard procedures that involve the use of multiple harmful chemicals. This environmentally friendly approach reduces the dependence on dangerous chemicals and minimizes waste [2,3,4]. These nanomaterials frequently demonstrate distinctive and enhanced characteristics as a result of their biological source. Biofabricated nanoparticles exhibit improved antibacterial, catalytic, and optical characteristics, which are advantageous for diverse applications. The as-prepared nanoparticles and composites exhibit enhanced biocompatibility and reduced toxicity, rendering them well suitable for medical and pharmaceutical purposes such as medication delivery, imaging, and tissue engineering [5]. Utilizing biological organisms for nanoparticle synthesis can offer a more economical alternative to chemical synthesis techniques. This is especially crucial for expanding manufacturing and increasing the availability of sophisticated materials [6]. The distinctive characteristics of biogenic nanoparticles and composites create opportunities for advancement in areas such as environmental remediation, agriculture (e.g., as bio-fertilizers or pesticides), and energy storage (e.g., in batteries and supercapacitors). Biogenic nanomaterials are advantageous for sustainable and eco-friendly technology improvements due to their production procedures, which frequently utilize renewable resources and result in less pollution [7,8,9].
The interactions between atoms and their thorough modeling are examined by nanoscale science [10]. A relatively tiny quantity of nanofiller loading improved the thermal and mechanical properties of Nylon-6 nanocomposite (NC), according to the research done by Toyota Central Research Laboratory in Japan in the 1990s [11]. As a result, according to Kanartzidis [12], the qualities of NC materials depend not only on the properties of their parents (nanofiller and nylon) but also on their shape and interfacial properties. In comparison to larger-dimensional materials of the same composition, nanostructured materials have different properties. Nanoscale materials have a high surface area for a given volume [13,14], valuable interactions between chemical and physical properties governed by surface and surface properties [10], and different properties. Significant variations in composite properties can be accomplished depending on the components utilized (layered silicates or nanofibers, cation exchange capacity, and polymer matrix), as well as on the technique of synthesis [15,16,17]. It was eventually discovered that NC research had become well known all over the world, particularly in areas like electronics and computing, data storage, communication, aerospace and sporting goods, health and medicine, energy, environment, transportation, and applications for national defense [10,15,18,19,20,21,22,23,24,25].
The synthesis of functional nanomaterials from natural resources has attracted much attention due to its potential for sustainable and environmentally friendly applications. For example, a study demonstrated the successful fabrication of floral-shaped Cu2O microbeads using the savoy cabbage extract. This approach highlights the potential of plant extracts as green precursors for catalytic materials. The synthesized Cu2O microbeads were effectively used to produce 1,4-disubstituted 1,2,3-triazoles, demonstrating their usefulness in organic synthesis and pharmaceutical applications [26]. Similarly, peanut shells have been used as a source for the synthesis of microcrystalline cellulose and nanocrystalline cellulose, highlighting the possibility of using agricultural waste to produce valuable nanocellulose materials [27]. These studies, as well as the present study, highlight the potential of using natural resources to sustainably synthesize functional nanomaterials.
Due to their potential uses in numerous technical fields, metal and metal oxide nanoparticles, in particular copper and its oxides, have received a lot of attention in recent years. Copper oxide nanoparticles (Cu2O NPs) are non-toxic, inexpensive, widely accessible, and often used in a variety of fields [28,29,30,31]. The Environmental Protection Agency of the United States recognized Cu NPs as an antibacterial substance [32], and they have since been used in several biomedical applications, including the treatment of cancer [28], bacterial infections [33], and other conditions [34].
Thermally stable nanoparticles include silicon dioxide nanoparticles (SiO2 NPs), which have been used as support materials for creating numerous stable and reusable catalysts because of their great stability [35]. In addition, SiO2 NPs have been discovered to have uses in various industries, including ceramics, drug delivery, and diagnostic equipment [36].
Scientifically, it has been reported in the literature that binary metal oxide NCs exhibit enhanced stability, selectivity, and catalytic activity when compared to single metal oxide nanoparticles [37,38]. These NCs also exhibit unique geometrical structures that result in increased functionality [39,40]. Due to their special surface properties, bimetallic NCs have been utilized in a wide range of applications, including catalysis [41], electrical devices [42], biomedical applications [43,44], environmental technology [45,46], and antibacterial activities [47,48]. Various techniques such as sol–gel, micro-emulsion, chemical precipitation, and hydrothermal methods have been employed in the past to synthesize NCs for industrial applications in catalysis and medicine. However, many of these methods do not meet the criteria of green chemical synthesis as they often involve the use of expensive and toxic chemicals, harsh reaction conditions, and the generation of hazardous by-products [49,50]. In recent years, there has been growing interest in eco-friendly biogenic synthesis of NCs using renewable bioproducts such as biomass, bacteria, fungi, and plant parts, which have been extensively reported in the literature [30,43,51,52,53,54,55,56]. These biogenically synthesized NCs have shown promising potential for various sectors, and they offer a more sustainable and environmentally friendly approach to NC fabrication. Using plant extracts for synthesizing nanoparticles offers several advantages, including its environmentally friendly and sustainable nature, as it eliminates the need for harmful chemicals by utilizing natural plant extracts as reducing and stabilizing agents. Additionally, NPs synthesized using plant extracts are often biocompatible, making them suitable for a wide range of biomedical applications, such as drug delivery, tissue engineering, and diagnostics. Furthermore, these NPs typically exhibit low toxicity, minimizing the risk of adverse effects [57]. However, there are also some disadvantages to plant extract-based synthesis. One limitation is that it may result in less precise control over the size, shape, and surface properties of nanoparticles compared to other methods, leading to variability in their properties. Additionally, the composition and potency of plant extracts can vary depending on various factors such as season, geographical location, and plant species, leading to batch-to-batch variability in NP synthesis. A literature survey revealed that the Cu2O/SiO2 NC (CSNC) had been synthesized by various methods such as sol–gel, hydrothermal, impregnation, and so on and had found their applications in various fields [55,56,57,58,59,60]. It was observed that there are few reports on the green synthesis of CSNCs, especially using plant materials. Thus, the biogenic synthesis of CSNC from Callistemon viminalis leaves, in this manner, is a novel, clean, green, environmentally friendly approach that can be applied for various applications. To the best of our knowledge, the synthesis of CSNC from C. viminalis leaves has not been reported to date. Therefore, in this article, the green synthesis of CSNCs using the C. viminalis green leaf extract has been described, and the synthesized sample was confirmed by physicochemical characterization methods such as UV-vis spectroscopy, Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-ray (EDX) spectroscopy, and transmission electron microscopy (TEM). Finally, the synthesized NC was successfully used in an antimicrobial study against the following pathogenic strains: Staphylococcus aureus, Pseudomonas aeruginosa, and Candida albicans.
2 Materials and methods
2.1 Chemicals
All chemicals and solvents used in this study were purchased from Merck India Ltd. and used as received. UV-visible absorption spectra were collected on a Shimadzu UV-1800 spectrophotometer (Japan) with a spectral resolution of 1 nm in the wavelength range of 200 to 800 nm. The size and shape of the CSNCs were characterized using TEM on a TECNAI G-20 instrument and SEM on a Nova Nano FE-SEM 450 FEI microscope. FTIR spectra of both the C. viminalis extract and Cu2O/SiO2 with KBr pellet preparation were obtained in the range of 4,000–400 cm⁻¹ using a PerkinElmer Spectrum 2000 FTIR spectrometer. The presence of copper, silicon, and oxygen in the sample was confirmed through EDX spectroscopy analysis.
2.2 Preparation of extract
The genus Callistemon contains almost 34 species worldwide, with ten of these found in India. Among them, C. viminalis exhibits a wide distribution across tropical Asia, Australia, Sri Lanka, South America, and India [61,62,63]. C. viminalis, commonly known as weeping bottlebrush, is a member of the Myrtaceae plant family. Used in traditional medicine practices, this plant has been used to treat a variety of ailments, including stomach ailments, skin infections, and respiratory problems [64]. C. viminalis (weeping bottle brush) was washed, dried, and finely powdered. Approximately 200 mL of double distilled water was added to a 250 mL round bottom flask containing the powder. The mixture was boiled and kept at this temperature for 30 min, then allowed to cool down to the ambient temperature. Next, the solution was filtered using Whatman filter paper to purify the extract. The filtered liquid was then refrigerated for subsequent NC synthesis.
2.3 Biosynthesis of CSNC
The water extract made from shade-dried green leaves of C. viminalis was employed in the synthesis of CSNC as a bio-reducing and stabilizing agent. At the start of the experiment, C. viminalis extract from green leaves was made in the same way as in the previous study [65]. An amount of 2.0 g of copper acetate monohydrate, diluted in 30 mL of deionized water (DIW), was combined with 1.2 g of sodium metasilicate solution, also made in 30 mL of DIW, to create the CSNC. The resulting mixed solution was heated to 70°C while being swirled, and 30 mL of the leaf extract produced was slowly added to the flask while stirring. After 30 min, 0.1 M NaOH solution was added to the flask to make it alkaline (pH = 12).
After 2 h of nonstop stirring and heating, a blackish-brown suspension emerged, indicating the creation of the CSNC. After that, the suspension was centrifuged at 8,000 rpm and cooled to ambient temperature. The biosynthesized CSNC was then subjected to a drying process in a hot air oven at 100°C after being rinsed five times with ethanol. After calcining at 350°C, the dried NC was kept for later studies.
2.4 Antimicrobial screening assay
The antimicrobial efficacy of CSNCs was evaluated against a panel of microorganisms, including the Gram-positive bacterium S. aureus, the Gram-negative bacterium P. aeruginosa, and the fungus C. albicans. The antimicrobial tests were conducted using two different concentrations of the biogenically synthesized bimetallic NCs. In the procedure [65,66,67], bacterial strains were cultured on agar plates at 37°C for 24 h, while fungal strains were incubated at 28°C and 150 rpm for 48 h. Inoculum concentrations were standardized using McFarland turbidity standards. Sabouraud’s dextrose agar plates were prepared for microbial growth. S. aureus, P. aeruginosa, and C. albicans were separately inoculated onto the agar surface. Filter paper discs impregnated with different concentrations of Cu2O/SiO2 (50 and 100 μg/mL) were placed on the inoculated plates. Streptomycin and itraconazole discs served as positive controls for bacteria and fungi, respectively, while DMSO-soaked discs acted as negative controls. Plates were incubated at 37°C for 24 h (bacteria) or at 28°C for 48 h (fungi). Antimicrobial activity was determined by measuring the inhibition zone diameter around the discs with a precision of ±0.5 mm. All experiments were conducted in triplicate, and the data presented in Tables 1 and 2 represent the average of these replicates.
Outcomes of antibacterial activity of biogenic CSNCs against S. aureus and P. aeruginosa
| Nanomaterials | Zone of inhibition (mm) | |||||
|---|---|---|---|---|---|---|
| S. aureus | P. aeruginosa | |||||
| PC | 1× (100 µg/mL) | 0.5× (50 µg/mL) | PC | 1× (100 µg/mL) | 0.5× (50 µg/mL) | |
| Cu2O/SiO2 | 4.5 | 1.62 | 1.37 | 4 | NZ | NZ |
PC = positive control, streptomycin; NZ = no zone.
Outcomes of antifungal activity of biogenic CSNCs against C. albicans
| Nanomaterials | Zone of inhibition (mm) C. albicans | ||
|---|---|---|---|
| PC | 1× (100 µg/mL) | 0.5× (50 µg/mL) | |
| Cu2O/SiO2 | 30 | 13 | 12 |
PC = positive control, itraconazole.
3 Results and discussion
3.1 NC characterization
To comprehend the role of biomolecules and biomaterials in the creation of the CSNC material, FTIR spectral analysis was used. Using an X-ray diffractometer operating at 40 kV and 30 mA with Cu/K radiation and 2θ varying from 10° to 80°, the crystal phase of the produced materials was identified. SEM and TEM were used to examine the surface morphology of the CSNC. The composition of the components in the NC was investigated using EDX analysis. A UV-visible spectrophotometer was used to examine the UV-visible absorption spectra of Cu2O/SiO2 in their as-prepared states.
3.2 FT-IR spectroscopy
The FT-IR spectrum of the CSNC produced utilizing the C. viminalis green leaf extract via a green method is shown in Figure 1. The stretching vibration of the adsorbed water (H2O) molecules and the surface hydroxyl groups connected to the phenolic compounds may be the cause of the absorption peaks at 3441.4 cm–1. The small peaks in the 2,900–2,800 cm–1 range can be connected to the C–H stretching vibrations of the extract biomolecules used to create the sample. H–O–H bending vibrations cause absorption peaks to appear at 1631.7 cm–1 [17,67]. The typical asymmetric and symmetric stretching vibrations of the Si–O–Si bond of SiO2 are attributed to the IR peaks that occurred at 1,100 and 800.4 cm–1, respectively [68,69]. The FTIR signal at 626.98 cm–1 corresponds to Cu(i)–O stretching, which infers the presence of the copper atom in the produced Cu2O/SiO2 sample [70]. Furthermore, this spectrum is compared with the FTIR spectra of Cu2O NPs and C. viminalis leaf extract (Figure 1a and b), which were previously reported by our group [71]. It can be seen from the spectra that the Cu(i)–O peak at 627.58 cm–1 in the spectrum of Cu2O NP is sharp and intense, as compared to the peak at 626.98 cm–1 in the spectrum of CSNC. Also, two IR peaks at 1,100 and 800.4 cm–1 assigned to the Si–O–Si bond of SiO2 are absent in the spectrum of Cu2O NPs. These observations of FTIR predict the formation of CSNC in which Cu2O is encapsulated by silica (SiO2). The spectrum of C. viminalis leaf extract also shows absorption peaks for O–H, C–H, aryl ketone, aromatic C═C, C–OH, and C–O functional groups. These peaks suggest that biomolecules with phenolic and alcoholic groups are present. The spectra reveal that some peaks in the CSNC spectrum either disappeared or have low intensities, confirming the involvement of biomolecules in the formation of CSNCs. A survey of FTIR studies revealed that betulinic acid, present in the extract, likely played a key role in the production of CSNCs.

FTIR spectra of (a) biosynthesized C. viminalis leaf extract, (b) Cu2O NPs, and (c) CSNC.
3.3 Plausible mechanism
An explanation for the Cu2O/SiO2 production from the extract of C. viminalis leaves can be put forward based on the FTIR results. In essence, betulinic acid in the leaf extract is oxidized by a free radical mechanism, converting betulinic acid to its dihydro form (Figure 2). Dihydrobetulinic acid, in its anionic form, can donate electrons to metal ions such as Cu(i) and the silicate ions present in the solution, leading to the formation of complexes with dihydrobetulinic acid. The biomolecules present in the mixture act as a protective layer, preventing the aggregation of nanoparticles. Upon heating, this complex undergoes a transformation, resulting in the formation of metal oxide nanoparticles [72].

A plausible mechanism for the biogenic synthesis of CSNC.
3.4 Powder X-ray diffraction (PXRD) spectroscopy
The phase composition of the synthesized CSNC was determined using PXRD. The resulting diffraction profile is presented in Figure 3. The diffraction pattern revealed characteristic peaks at 2θ values of 29.68°, 36.78°, 42.67°, 61.79°, and 73.84°, which were indexed to the respective (1 1 0), (1 1 1), (2 0 0), (2 2 0), and (3 1 1) crystallographic planes of Cu2O (JCPDS Card No. 05-0667) [73]. A broad peak at 22.77° was observed and assigned to amorphous SiO2 (JCPDS Card No. 27-1402) [74,75]. These findings are consistent with those obtained from the chemical synthesis of CSNC via an in situ deposition approach [76,77] and the chemical precipitation method [18,78]. Furthermore, as previously mentioned, the intensity and sharpness of peaks in CSNC decreased compared to bare Cu2O (Figure 3(b)), indicating the presence of amorphous SiO2 in conjunction with Cu2O particles, resulting in a reduction in the crystallinity of CSNC. Additionally, CSNC has expanded XRD peaks, which show that the particles are smaller than bare Cu2O [63]. The literature makes it clear that peak broadening caused surface stresses and a decrease in the grain size [79,80]. Another illustration of how SiO2 can be incorporated into Cu2O structures to produce a hybrid structure is provided by this study. Additionally, according to the Debye–Scherrer formula, the CSNC crystallite size at maximum intensity (111 planes) was predicted to be 21.51 nm [55] and that of bare Cu2O was 60.81 nm [77], which was in agreement with the TEM findings.

PXRD spectra of synthesized C. viminalis: (a) Cu2O NPs and (b) CSNCs.
3.5 SEM
SEM was used to investigate the morphology of the produced nanomaterial at various magnifications. Figure 4(a) and (b) displays Cu2O/SiO2 FESEM images, revealing irregular spherical shapes in the particles resulting from the green process. Some nanospheres with diameters of 20–40 nm self-assemble [65], as seen in image. This may be due to the bimolecular protection on the surface of the NC.

SEM images (a and b) and TEM images (c and d) of CSNC fabricated from C. viminalis at different scale bars.
3.6 TEM
In Figure 4(c) and (d), TEM images of Cu2O/SiO2 are displayed. TEM micrographs of the CSNC revealed a shadow-like appearance surrounding spherical dark spots, indicating the presence of SiO2 around Cu2O structures [76,77]. TEM photographs also show that the particles had an atypical spherical form and had nanoscale dimensions ranging from 20 to 40 nm. The green leaf extract of C. viminalis successfully fabricated the nanosized Cu2O/SiO2 composite, as demonstrated by the results of EDX, SEM, and TEM.
3.7 EDX analysis
EDX spectroscopy was used to detect the presence of copper and silicon in the biosynthesized CSNC (Figure 5). Both the copper and silicon emission peaks in the biosynthesized Cu2O/SiO2 EDX analysis revealed distinct spectral signatures corresponding to Cu and Si, constituting 28.16 and 13.65% of the total mass, respectively. The presence of characteristic emission peaks for copper, silicon, and oxygen within the EDX spectra confirmed the elemental composition of the greenly synthesized CSNC material. Additionally, the carbon peak that appears in the EDX spectrum of the NC may be caused by the carbon atoms of biomolecules that have been cap-tied to the surface of the nanomaterial at this point [76].
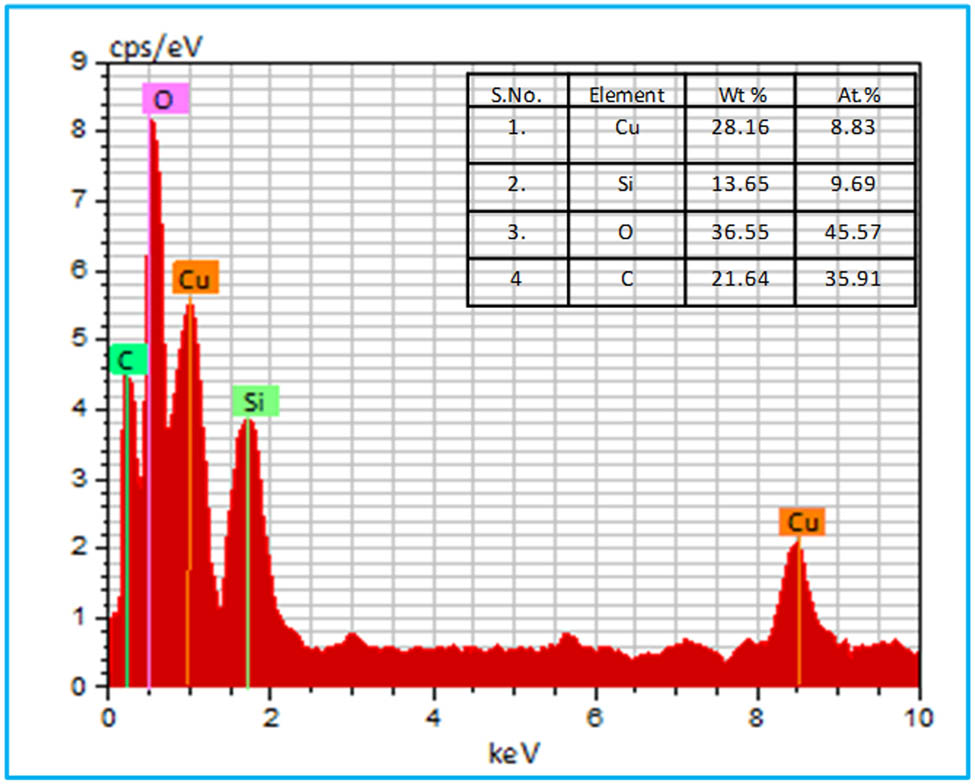
EDX analysis of the biosynthesized CSNC.
3.8 Thermal analysis
Thermogravimetric analysis (TGA), differential thermal analysis (DTA), and derivative thermogravimetry (DTG) were conducted in parallel to evaluate the thermal characteristics of the CSNC. The TGA/DTG graphs in Figure 6 showed that the sample initially experienced a weight loss of 4.68% up to 100°C, which could be attributed to the evaporation of water molecules from the surface of the NC. In the second step, a weight loss of 9.64% was observed between 200 and 350°C, indicating the removal of biomolecules that were cap-bound to the surface of the NC. Further analysis revealed that the biosynthesized CSNC remained thermally stable beyond 400°C, as no significant weight loss was observed between 400 and 1,000°C. Moreover, the DTA curve in Figure 6 showed heat variations associated with the released water and bioactive compounds from the biologically synthesized copper silica NC.

TGA, DTG, and DTA thermograms of the biogenic CSNC.
3.9 Antimicrobial activity
Antimicrobial assays were conducted to determine the inhibitory capacity of CSNCs against specific microorganisms, including pathogenic bacteria and fungi, using the disk-diffusion method. The diameter of the inhibition zone was measured as part of the analysis to determine the degree of drug sensitivity. Gram-positive and -negative bacteria and fungi were tested for their resistance to biogenic NCs. After incubation, inhibition zones were examined around the disc and measured in millimeters with a transparent ruler. A lack of zone inhibition was interpreted as inactivity. The CSNCs did not affect Gram-negative bacteria but had a significant inhibitory effect on Gram-positive bacterial and fungal strains such as S. aureus and C. albicans (Figure 7, Tables 1 and 2). The antimicrobial activity of the composite presented in this study was compared to that of a composite previously reported in the literature (Table 3). It was found that the CSNC displayed intermediate-level activity against the tested strain. The biosynthesized composite may exhibit good activity against other pathogenic strains.

Antimicrobial activity using filter papers disc with different concentrations of CSNC against S. aureus, P. aeruginosa, and C. albicans, showing concentrations, positive control, negative control, and inhibition zones in mm.
Comparison of antimicrobial activity of different composites
| Name of nanomaterials | Antimicrobial activity | Inhibition zone | Ref. |
|---|---|---|---|
| CdO–MgO | The antimicrobial activity was evaluated against a panel of bacteria, including Gram-positive strains (M. luteus and R. rhodochrous) and Gram-negative strains (Vibrio cholerae, Klebsiella pneumoniae, A. hydrophila, and Escherichia coli) | Zone of inhibition diameters of 24 mm (M1) and 25 mm (M2) indicate strong antibacterial efficacy against the Gram-negative bacterium | [94] |
| Chitosan Schiff base and ZnO composite | The antimicrobial properties were assessed against a panel of microorganisms, including two Gram-negative bacteria (E. coli and P. aeruginosa), two Gram-positive bacteria (S. aureus and B. cereus), and a yeast strain (C. albicans) | 47.12, 91.9, and 96.56% | [95] |
| Carbon dots modified polyurethane NC | Antibacterial agent | [96] | |
| Carbon quantum dots modified Ag2S/CS NC | The antibacterial efficacy was evaluated using S. aureus (Gram-positive), E. coli (Gram-negative), and methicillin-resistant S. aureus as model microorganisms | [97] | |
| Ag@biochar | Four common microorganisms, including C. albicans, Bacillus subtilis, P. aeruginosa, and K. pneumoniae | 19, 18, 22, and 16 mm | [98] |
| Cu/Zn galvanic couple composite | Antibacterial activity toward S. aureus | Cu (Φ50.8 × 5 mm, 99.99%) and Zn (Φ50.8 × 5 mm, 99.99%) | [99] |
| Composite copper oxide nanoparticles made of chitosan and cellulose | The substance demonstrated antimicrobial properties against a range of bacteria and fungi, including drug-resistant strains such as methicillin-resistant S. aureus, vancomycin-resistant Enterococcus, and highly resistant forms of E. coli, Streptococcus agalactiae, P. aeruginosa, Stenotrophomonas, and C. albicans | 35 nmol/mg or lower bactericidal activity | [100] |
| Composite Ag–Cu–Au and Ag–Cu | The efficacy was assessed against four bacterial species commonly associated with infections: Streptococcus pneumoniae and P. aeruginosa, which are airborne, and E. coli and S. aureus | MBC values of 62.5 mM for both the S. aureus and P. aeruginosa species, and 31.15 and 15.6 mM for E. coli and S. pneumoniae | [101] |
| Fe3O4/Cu | Gram-positive and Gram-negative bacteria, including S. aureus, B. subtilis, and E.coli | MBC MIC MBC MIC MBC MIC 1.25 0.01 1.00 0.05 1.15 0.02 | [102] |
Based on the results of the experiments, the NC with a concentration of 100 μg/mL had better activity against S. aureus than the concentration of 50 μg/mL, whereas Cu2O/SiO2 with various concentrations did not affect P. aeruginosa. The antibacterial activity of NCs against S. aureus and P. aeruginosa was compared to the reference drug, but the results were not promising; however, the antifungal activity of NCs at different concentrations was better but less than that of the reference drug itraconazole (PC = positive control), as shown in Figure 6 and Tables 1 and 2. It has been demonstrated that the antimicrobial activity of the as-prepared composite increased with concentration. The experimental study also showed that the synthesized composite has a higher fungi potential. The difference in inhibition activity is due to structural differences in the cell walls of bacterial and fungal strains.
The biological activity of nanoparticles, including biosynthesized CSNCs, is significantly affected by various factors, as shown in Figure 8a. These have been identified to evaluate the reactive oxygen species (ROS) and their causes and toxicity of the nanomaterials [81]. The small size of the NCs reported in this paper, ranging from 20 to 40 nm, likely contributes to their enhanced antimicrobial efficacy due to increased surface area and the potential for greater interaction with microbial cells. The observed spherical morphology might facilitate cellular uptake and interaction. The morphology of nanoparticles significantly affects cellular uptake, biodistribution, and subsequent biological interactions. Studies have consistently shown that changes in particle shape (e.g., spherical- versus rod-shaped structures) affect the cellular internalization pathway and rate. This, in turn, affects pharmacokinetic characteristics, including the circulation time and tissue distribution [82,83]. In this study, the biosynthesized Cu2O/SiO2 nanoparticles exhibited spherical morphology, which contributed to their antimicrobial efficacy against S. aureus, P. aeruginosa, and C. albicans. However, studies have shown that different shapes (e.g., rod-shaped and disc-shaped nanomaterials) have different biological responses and cellular uptake patterns [84]. The surface charge of nanomaterials has a significant impact on their behavior in biological systems. Positively charged nanoparticles exhibit stronger interactions with negatively charged cell membranes, resulting in not only increased cellular uptake but also potential cytotoxicity. In contrast, negatively or neutrally charged nanoparticles generally have lower cellular uptake and toxicity. Electrostatic interactions between positively charged nanoparticles and negatively charged bacterial cell membranes facilitate their attachment and subsequent penetration. This interaction can disrupt bacterial membrane integrity, leading to increased intracellular nanoparticle accumulation. As a result, these nanoparticles can cause oxidative stress by making ROS, which damage parts of cells and eventually kill bacteria cells or stop their growth [85,86] (Figure 8b). Surface chemistry with capping agents plays an important role in stabilizing nanomaterials and preventing agglomeration. It is likely that the green synthesis of the CSNC using the plant extract led to the presence of biomolecules, such as those with phenolic and alcoholic functional groups that acted as caps. These molecules interact with nanoparticle surfaces through various mechanisms, such as coordination, electrostatic interactions, and hydrogen bonding. The presence of these capping agents is crucial for stabilizing the nanoparticles, preventing agglomeration, and influencing their overall physicochemical properties. Furthermore, these biomolecules can enhance the NC’s biocompatibility by providing a hydrophilic interface [87].

Mechanisms of NP activity. (a) A schematic overview of how NP properties contribute to the generation of ROS, ultimately causing bacterial cell death and inhibiting biofilm formation, as observed in antifungal studies. (b) A diagrammatic representation of the diverse toxic effects from nanomaterials.
Given the diverse characteristics of nanomaterials, the antimicrobial capabilities of nanoparticles and their associated metal ions have been the subject of considerable research in recent times [88,89]. Studies have indicated that the pathogenic effects of metal and metal oxide nanomaterials on bacteria are mostly dependent on metal ions [90,91,92]. These NPs can be oxidized in biological media, releasing metal ions that generate ROS or create oxidative stress [93]. This oxidative stress leads to biocidal effects, which can include ribosome destabilization, disruption of nucleoid proteins, prevention of fungal biofilm formation in antifungal mechanisms, cell wall damage, disruption of electron transport chains, and ultimately cell death, as shown in Figure 8b. Although the precise mechanism of antibacterial action necessitates additional research, it is likely that the discharge of copper ions from the NC is pivotal in disturbing the integrity of microbial cells. Compared to previous studies on copper oxide nanoparticles and other nanomaterials, our biogenic CSNC demonstrates promising antimicrobial activity against S. aureus, P. aeruginosa, and C. albicans (Table 3). However, a more comprehensive understanding of the factors influencing its biocidal action requires further characterization and mechanistic studies.
4 Conclusions
This study successfully demonstrated the eco-friendly synthesis of CSNCs using the C. viminalis leaf extract, providing a clean and sustainable alternative to conventional methods. FTIR analysis confirmed that biomolecules containing phenolic and alcohol groups present in the leaf extract were involved in the formation of reduced and stabilized NCs. The structure and size of the NCs were characterized using XRD, SEM + EDX, and TEM, confirming the successful synthesis of CSNCs with spherical sizes ranging from 20 to 40 nm. Furthermore, the biocidal activity of the synthesized NCs against bacterial strains such as S. aureus and P. aeruginosa, as well as the fungal strain such as C. albicans was observed, with varying degrees of inhibition. The synthesized NCs exhibited moderate antibacterial activity against S. aureus, with a larger inhibition zone observed at higher concentrations. However, no significant antibacterial effect was observed against P. aeruginosa. On the other hand, the NCs displayed antifungal properties against C. albicans, although the observed inhibition zones were smaller compared to the positive control, Itraconazole (inhibition zone = 30 mm). As a result, this research offers a good foundation for future studies that aim to synthesize copper-based NCs in an economical, environmentally friendly, and biologically friendly manner for use in biological and materials sciences. Future research can further explore the underlying mechanisms of antimicrobial action and optimize the synthesis process for large-scale applications in various fields, including biomedicine and materials science.
-
Funding information: This work was funded by Researchers Supporting Project number (RSPD2024R1106), King Saud University, Riyadh, Saudi Arabia.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Gupta AD, Rajput D, Bionanofabrication A. Green approach towards nanoparticle synthesis using plants and microbes. In Nanochemistry. Boca Raton: CRC Press; 2023. p. 88–113.10.1201/9781003081944-6Search in Google Scholar
[2] Sadhu A, Bhattacharjee D, Sadhu S. Biogenic nanoparticles synthesis, extraction, and purification from agro-wastes. In Agro-waste to microbe assisted value added product: challenges and future prospects: recent developments in agro-waste valorization research. Cham: Springer; 2024. p. 381–404.10.1007/978-3-031-58025-3_17Search in Google Scholar
[3] Agrawal S, Sen SK, Bhui AK, Singh R. Biological synthesis of nanoparticles: a value of ethnomedicine. Nanotechnology and in silico tools. Netherlands: Elsevier; 2024. p. 15–29.10.1016/B978-0-443-15457-7.00017-4Search in Google Scholar
[4] Singhal A, Baweja P, Gupta S, Chopra H, Gandhi PB. Biogenesis of nanoparticles using microorganisms: A review. Plant Sci Today. 2023;10:97–104.10.14719/pst.2373Search in Google Scholar
[5] Ahmad F, Salem-Bekhit MM, Khan F, Alshehri S, Khan A, Ghoneim MM, et al. Unique properties of surface-functionalized nanoparticles for bio-application: functionalization mechanisms and importance in application. Nanomaterials. 2022;12:1333.10.3390/nano12081333Search in Google Scholar PubMed PubMed Central
[6] Singh H, Desimone MF, Pandya S, Jasani S, George N, Adnan M, et al. Revisiting the green synthesis of nanoparticles: uncovering influences of plant extracts as reducing agents for enhanced synthesis efficiency and its biomedical applications. Int J Nanomed. 2023;18:4727–50.10.2147/IJN.S419369Search in Google Scholar PubMed PubMed Central
[7] Osman AI, Zhang Y, Farghali M, Rashwan AK, Eltaweil AS, Abd El-Monaem EM, et al. Synthesis of green nanoparticles for energy, biomedical, environmental, agricultural, and food applications: A review. Environ Chem Lett. 2024;22:841–7.10.1007/s10311-023-01682-3Search in Google Scholar
[8] Wahab A, Muhammad M, Ullah S, Abdi G, Shah GM, Zaman W, et al. Agriculture and environmental management through nanotechnology: Eco-friendly nanomaterial synthesis for soil-plant systems, food safety, and sustainability. Sci Total Environ. 2024;926:171862.10.1016/j.scitotenv.2024.171862Search in Google Scholar PubMed
[9] Su C, Chen A, Liang W, Xie W, Xu X, Zhan X, et al. Copper-based nanomaterials: Opportunities for sustainable agriculture. Sci Total Environ. 2024;926:171948.10.1016/j.scitotenv.2024.171948Search in Google Scholar PubMed
[10] Hussain F, Hojjati M, Okamoto M, Gorga RE. Polymer-matrix nanocomposites, processing, manufacturing, and application: an overview. J Compos Mater. 2006;40:1511–75.10.1177/0021998306067321Search in Google Scholar
[11] Usuki A, Kawasumi M, Kojima Y, Okada A, Kurauchi T, Kamigaito O. Swelling behavior of montmorillonite cation exchanged for ω-amino acids by ∊ -caprolactam. J Mater Res. 1993;8:1174–8.10.1557/JMR.1993.1174Search in Google Scholar
[12] Oriakhi C. Nano sandwiches. Chem Br. 1998;34:59–62.Search in Google Scholar
[13] Luo J-J, Daniel IM. Characterization and modeling of mechanical behavior of polymer/clay nanocomposites. Compos Sci Technol. 2003;63:1607–16.10.1016/S0266-3538(03)00060-5Search in Google Scholar
[14] Ocsoy I, Gulbakan B, Chen T, Zhu G, Chen Z, Sari MM, et al. DNA‐guided metal‐nanoparticle formation on graphene oxide surface. Adv Mater. 2013;25:2319–25.10.1002/adma.201204944Search in Google Scholar PubMed PubMed Central
[15] Wu P, Zhao T, Tian Y, Wu L, Hou X. Protein‐directed synthesis of Mn‐doped ZnS quantum dots: a dual‐channel biosensor for two proteins. Chem – A Eur J. 2013;19:7473–9.10.1002/chem.201204035Search in Google Scholar PubMed
[16] Widakdo J, Chen T-M, Lin M-C, Wu J-H, Lin T-L, Yu P-J, et al. Evaluation of the antibacterial activity of eco-friendly hybrid composites on the base of oyster shell powder modified by metal ions and LLDPE. Polymers. 2022;14:3001.10.3390/polym14153001Search in Google Scholar PubMed PubMed Central
[17] Gharbi AH, Hemmami H, Laouini SE, Bouafia A, Ben Amor I, Zeghoud S, et al. Novel CuO–SiO2 nanocomposites: synthesis, kinetics, recyclability, high stability and photocatalytic efficiency for Rose Bengal dye removal. Transit Met Chem. 2024;49:1–19.10.1007/s11243-024-00574-xSearch in Google Scholar
[18] Park CI, Park OO, Lim JG, Kim HJ. The fabrication of syndiotactic polystyrene/organophilic clay nanocomposites and their properties. Polymer. 2001;42:7465–75.10.1016/S0032-3861(01)00213-0Search in Google Scholar
[19] Shen G, Chen P-C, Ryu K, Zhou C. Devices and chemical sensing applications of metal oxide nanowires. J Mater Chem. 2009;19:828–39.10.1039/B816543BSearch in Google Scholar
[20] Cheng J, Zhang X, Tao X, Lu H, Luo Z, Liu F. Fine-tuning the synthesis of ZnO nanostructures by an alcohol thermal process. J Phys Chem B. 2006;110:10348–53.10.1021/jp060133sSearch in Google Scholar PubMed
[21] Valladares LDLS, Salinas DH, Dominguez AB, Najarro DA, Khondaker S, Mitrelias T, et al. Crystallization and electrical resistivity of Cu2O and CuO obtained by thermal oxidation of Cu thin films on SiO2/Si substrates. Thin Solid Films. 2012;520:6368–74.10.1016/j.tsf.2012.06.043Search in Google Scholar
[22] Lin C-C, Lee W-S, Sun C-C, Whu W-H. A varistor–polymer composite with nonlinear electrical-thermal switching properties. Ceram Int. 2008;34:131–6.10.1016/j.ceramint.2006.09.018Search in Google Scholar
[23] He Q, Liu J, Tian Y, Wu Y, Magesa F, Deng P, et al. Facile preparation of Cu2O nanoparticles and reduced graphene oxide nanocomposite for electrochemical sensing of rhodamine b. Nanomaterials. 2019;9:958.10.3390/nano9070958Search in Google Scholar PubMed PubMed Central
[24] Coeffard V, Aylward M, Guiry PJ. Cover picture: First regio‐and enantioselective chromium‐catalyzed homoallenylation of Aldehydes (Angew. Chem. Int. Ed. 48/2009). Angew Chem Int Ed. 2009;48:9001.10.1002/anie.200905607Search in Google Scholar
[25] Hung AM, Micheel CM, Bozano LD, Osterbur LW, Wallraff GM, Cha JN. Large-area spatially ordered arrays of gold nanoparticles directed by lithographically confined DNA origami. Nat Nanotechnol. 2010;5:121–6.10.1038/nnano.2009.450Search in Google Scholar PubMed
[26] Abdelbaki H, Djemoui A, Souli L, Souadia A, Ouahrani MR, Djemoui B, et al. Plant mediated synthesis of flower-like Cu2O microbeads from artimisia campestris L. Extract for the catalyzed synthesis of 1, 4-disubstituted 1, 2, 3-triazole derivatives. Front Chem. 2024;11:1342988.10.3389/fchem.2023.1342988Search in Google Scholar PubMed PubMed Central
[27] Terea H, Selloum D, Rebiai A, Atia D, Kouadri I, Seghir BB, et al. Characterization, biological, and antimicrobial properties of nanocellulose isolated from peanut shells (Arachis hypogaea L.). Biomass Convers Biorefin. 2023;1–11. 10.1007/s13399-023-04792-8.Search in Google Scholar
[28] Qian Y, Ye F, Xu J, Le Z-G. Synthesis of cuprous oxide (Cu2O) nanoparticles/graphene composite with an excellent electrocatalytic activity towards glucose. Int J Electrochem Sci. 2012;7:10063–73.10.1016/S1452-3981(23)16259-6Search in Google Scholar
[29] Zhang H, Zhu Q, Zhang Y, Wang Y, Zhao L, Yu B. One‐pot synthesis and hierarchical assembly of hollow Cu2O microspheres with nanocrystals‐composed porous multishell and their gas‐sensing properties. Adv Funct Mater. 2007;17:2766–71.10.1002/adfm.200601146Search in Google Scholar
[30] Ocsoy I, Tasdemir D, Mazicioglu S, Celik C, Katı A, Ulgen F. Biomolecules incorporated metallic nanoparticles synthesis and their biomedical applications. Mater Lett. 2018;212:45–50.10.1016/j.matlet.2017.10.068Search in Google Scholar
[31] Kim YH, Lee DK, Cha HG, Kim CW, Kang YC, Kang YS. Preparation and characterization of the antibacterial Cu nanoparticle formed on the surface of SiO2 nanoparticles. J Phys Chem B. 2006;110:24923–8.10.1021/jp0656779Search in Google Scholar PubMed
[32] Meghana S, Kabra P, Chakraborty S, Padmavathy N. Understanding the pathway of antibacterial activity of copper oxide nanoparticles. RSC Adv. 2015;5:12293–9.10.1039/C4RA12163ESearch in Google Scholar
[33] Domek MJ, LeChevallier MW, Cameron SC, McFETERS GA. Evidence for the role of copper in the injury process of coliform bacteria in drinking water. Appl Environ Microbiol. 1984;48:289–93.10.1128/aem.48.2.289-293.1984Search in Google Scholar PubMed PubMed Central
[34] Gao F, Pang H, Xu S, Lu Q. Copper-based nanostructures: promising antibacterial agents and photocatalysts. Chem Commun. 2009;3571–3.10.1039/b904801dSearch in Google Scholar PubMed
[35] Wang J, Shah ZH, Zhang S, Lu R. Silica-based nanocomposites via reverse microemulsions: classifications, preparations, and applications. Nanoscale. 2014;6:4418–37.10.1039/c3nr06025jSearch in Google Scholar PubMed
[36] Jadhav SA. Incredible pace of research on mesoporous silica nanoparticles. Inorg Chem Front. 2014;1:735–9.10.1039/C4QI00118DSearch in Google Scholar
[37] Lim B, Jiang M, Camargo PH, Cho EC, Tao J, Lu X, et al. Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction. Science. 2009;324:1302–5.10.1126/science.1170377Search in Google Scholar PubMed
[38] Omori T, Ando K, Okano M, Xu X, Tanaka Y, Ohnuma I, et al. Superelastic effect in polycrystalline ferrous alloys. Science. 2011;333:68–71.10.1126/science.1202232Search in Google Scholar PubMed
[39] Rodriguez JA, Goodman DW. The nature of the metal-metal bond in bimetallic surfaces. Science. 1992;257:897–903.10.1126/science.257.5072.897Search in Google Scholar PubMed
[40] Tanori J, Duxin N, Petit C, Lisiecki I, Veillet P, Pileni M. Synthesis of nanosize metallic and alloyed particles in ordered phases. Colloid Polym Sci. 1995;273:886–92.10.1007/BF00657639Search in Google Scholar
[41] Ghosh SK, Mandal M, Kundu S, Nath S, Pal T. Bimetallic Pt–Ni nanoparticles can catalyze reduction of aromatic nitro compounds by sodium borohydride in aqueous solution. Appl Catal A: Gen. 2004;268:61–6.10.1016/j.apcata.2004.03.017Search in Google Scholar
[42] Elmes RB, Orange KN, Cloonan SM, Williams DC, Gunnlaugsson T. Luminescent ruthenium (II) polypyridyl functionalized gold nanoparticles; their DNA binding abilities and application as cellular imaging agents. J Am Chem Soc. 2011;133:15862–65.10.1021/ja2061159Search in Google Scholar PubMed
[43] Mittal AK, Kumar S, Banerjee UC. Quercetin and gallic acid mediated synthesis of bimetallic (silver and selenium) nanoparticles and their antitumor and antimicrobial potential. J Colloid Interface Sci. 2014;431:194–9.10.1016/j.jcis.2014.06.030Search in Google Scholar PubMed
[44] Vaidyanathan R, Kalishwaralal K, Gopalram S, Gurunathan S. RETRACTED: Nanosilver—The burgeoning therapeutic molecule and its green synthesis. Biotechnol Adv. 2009;27:924–37.10.1016/j.biotechadv.2009.08.001Search in Google Scholar PubMed
[45] Muradova G, Gadjieva S, Di Palma L, Vilardi G. Nitrates removal by bimetallic nanoparticles in water. Chem Eng Trans. 2016;47:205–10.Search in Google Scholar
[46] Saravanan R, Gupta V, Narayanan V, Stephen A. Visible light degradation of textile effluent using novel catalyst ZnO/γ-Mn2O3. J Taiwan Inst Chem Eng. 2014;45:1910–7.10.1016/j.jtice.2013.12.021Search in Google Scholar
[47] Nazeruddin G, Prasad R, Shaikh Y, Shaikh A. Synergetic effect of Ag-Cu bimetallic nanoparticles on antimicrobial activity. Der Pharmacia Lett. 2014;3:129–36.Search in Google Scholar
[48] Sharma N, Kumar J, Thakur S, Sharma S, Shrivastava V. Antibacterial study of silver doped zinc oxide nanoparticles against Staphylococcus aureus and Bacillus subtilis. Drug Invent Today. 2013;5:50–4.10.1016/j.dit.2013.03.007Search in Google Scholar
[49] Nasrollahzadeh M, Sajadi SM. Preparation of Pd/Fe3O4 nanoparticles by use of Euphorbia stracheyi Boiss root extract: a magnetically recoverable catalyst for one-pot reductive amination of aldehydes at room temperature. J Colloid Interface Sci. 2016;464:147–52.10.1016/j.jcis.2015.11.020Search in Google Scholar PubMed
[50] Lim CW, Lee IS. Magnetically recyclable nanocatalyst systems for the organic reactions. Nano Today. 2010;5:412–34.10.1016/j.nantod.2010.08.008Search in Google Scholar
[51] Akhtar MS, Panwar J, Yun Y-S. Biogenic synthesis of metallic nanoparticles by plant extracts. ACS Sustain Chem & Eng. 2013;1:591–602.10.1021/sc300118uSearch in Google Scholar
[52] Strayer A, Ocsoy I, Tan W, Jones J, Paret M. Low concentrations of a silver-based nanocomposite to manage bacterial spot of tomato in the greenhouse. Plant Dis. 2016;100:1460–5.10.1094/PDIS-05-15-0580-RESearch in Google Scholar PubMed
[53] Shi H, Chen X, Li L, Tan L, Ren X, Ren J, et al. One-pot and one-step synthesis of bioactive urease/ZnFe 2 O 4 nanocomposites and their application in detection of urea. Dalton Trans. 2014;43:9016–21.10.1039/C4DT00825ASearch in Google Scholar
[54] Some S, Bulut O, Biswas K, Kumar A, Roy A, Sen IK, et al. Effect of feed supplementation with biosynthesized silver nanoparticles using leaf extract of Morus indica L. V1 on Bombyx mori L.(Lepidoptera: Bombycidae). Sci Rep. 2019;9:14839.10.1038/s41598-019-50906-6Search in Google Scholar PubMed PubMed Central
[55] Demirbas A, Welt BA, Ocsoy I. Biosynthesis of red cabbage extract directed Ag NPs and their effect on the loss of antioxidant activity. Mater Lett. 2016;179:20–3.10.1016/j.matlet.2016.05.056Search in Google Scholar
[56] Danovaro R, Gambi C. 40 years of chemistry and ecology. Chem Ecol. 2022;38:897–9.10.1080/02757540.2022.2122473Search in Google Scholar
[57] Wu M, He S, Ha E, Hu J, Ruan S. A facile synthesis of PEGylated Cu2O@ SiO2/MnO2 nanocomposite as efficient photo− Fenton− like catalysts for methylene blue treatment. Front Bioeng Biotechnol. 2022;10:1023090.10.3389/fbioe.2022.1023090Search in Google Scholar PubMed PubMed Central
[58] Fan G, Bao M, Wang B, Wu S, Luo L, Li B, et al. Inhibitory effects of Cu2O/SiO2 on the growth of Microcystis aeruginosa and its mechanism. Nanomaterials. 2019;9:1669.10.3390/nano9121669Search in Google Scholar PubMed PubMed Central
[59] Kim S, Kang SW, Kim A, Yusuf M, Park JC, Park KH. A highly efficient nano-sized Cu 2 O/SiO 2 egg-shell catalyst for C–C coupling reactions. RSC Adv. 2018;8:6200–5.10.1039/C7RA13490HSearch in Google Scholar
[60] Zhang X, Zhang D, Ni X, Zheng H. Synthesis and optical properties of Cu2O/SiO2 composite films via gamma-irradiation route. Mater Lett. 2007;61:248–50.10.1016/j.matlet.2006.04.047Search in Google Scholar
[61] Srivastava S, Ahmad A, Syamsunder K, Aggarwal K, Khanuja S. Essential oil composition of Callistemon viminalis leaves from India. Flavour Fragr J. 2003;18:361–3.10.1002/ffj.1143Search in Google Scholar
[62] Oyedeji OO, Lawal OA, Shode FO, Oyedeji AO. Chemical composition and antibacterial activity of the essential oils of Callistemon citrinus and Callistemon viminalis from South Africa. Molecules. 2009;14:1990–8.10.3390/molecules14061990Search in Google Scholar PubMed PubMed Central
[63] Abdelhady MI, Motaal AA, Beerhues L. Total phenolic content and antioxidant activity of standardized extracts from leaves and cell cultures of three Callistemon species. Am J Plant Sci. 2011;2:847.10.4236/ajps.2011.26100Search in Google Scholar
[64] Ahmad K, Athar F. Phytochemistry and pharmacology of Callistemon viminalis (Myrtaceae): A review. Nat Prod J. 2017;7:166–75.10.2174/2210315507666161216100323Search in Google Scholar
[65] Jatrana I, Satiya H, Dwivedi P. Novel green synthesis of ZnO/SiO2 nanocomposite: Characterization and biocidal activity. Mater Today: Proc. 2022;79:148–54.10.1016/j.matpr.2022.11.238Search in Google Scholar
[66] Dwivedi P, Satiya H, Sharma SK. Novel green approach for the synthesis of Co3O4/ZnO nanocomposite, characterization and antimicrobial activity. Mater Today: Proc. 2023;79:80–6.10.1016/j.matpr.2022.09.229Search in Google Scholar
[67] Dwivedi P, Jatrana I, Khan AU, Khan AA, Satiya H, Khan M, et al. Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: a comparative study. Nanotechnol Rev. 2021;10:1912–25.10.1515/ntrev-2021-0117Search in Google Scholar
[68] Pal D, Chattopadhyay S. Surface and interface effects: properties of nanostructured ZnO. In Nanostructured Zinc Oxide. Amsterdam, Netherlands: Elsevier; 2021. p. 253–87.10.1016/B978-0-12-818900-9.00009-7Search in Google Scholar
[69] Cheng L, Liu W, Zhang Z, Zhou Y, Li S. Enhanced breakdown strength and restrained dielectric loss of polypropylene/maleic anhydride grafted polypropylene/core‐shell ZrO2@ SiO2 nanocomposites. Polym Compos. 2022;43:2175–83.10.1002/pc.26530Search in Google Scholar
[70] Zhang P, Wang T, Zeng H. Design of Cu-Cu2O/g-C3N4 nanocomponent photocatalysts for hydrogen evolution under visible light irradiation using water-soluble Erythrosin B dye sensitization. Appl Surf Sci. 2017;391:404–14.10.1016/j.apsusc.2016.05.162Search in Google Scholar
[71] Dwivedi Poonam JI, Satiya Honey LRS. Understanding the synergistic effect of bimetallic Cu2O-ZnO nanocomposites for the antimicrobial activity and facile synthesis of phenyl xanthenedione: A comparative study. Res J Chem Environ. 2022;26:e50–60.10.25303/2604rjce5060Search in Google Scholar
[72] Bibi I, Nazar N, Iqbal M, Kamal S, Nawaz H, Nouren S, et al. Green and eco-friendly synthesis of cobalt-oxide nanoparticle: Characterization and photo-catalytic activity. Adv Powder Technol. 2017;28:2035–43.10.1016/j.apt.2017.05.008Search in Google Scholar
[73] Tenkyong T, Bachan N, Raja J, Kumar PN, Shyla JM. Investigation of sol-gel processed CuO/SiO nanocomposite as a potential photoanode material. Mater Sci-Poland. 2015;33:826–34.10.1515/msp-2015-0097Search in Google Scholar
[74] Thostenson ET, Li C, Chou T-W. Nanocomposites in context. Compos Sci Technol. 2005;65:491–516.10.1016/j.compscitech.2004.11.003Search in Google Scholar
[75] Dubey R, Rajesh Y, More M. Synthesis and characterization of SiO2 nanoparticles via sol-gel method for industrial applications. Mater Today: Proc. 2015;2:3575–9.10.1016/j.matpr.2015.07.098Search in Google Scholar
[76] Schmidt D, Shah D, Giannelis EP. New advances in polymer/layered silicate nanocomposites. Curr OpSolid State Mater Sci. 2002;6:205–12.10.1016/S1359-0286(02)00049-9Search in Google Scholar
[77] Su X, Zhao J, Zhao X, Guo Y, Zhu Y, Wang Z. A facile synthesis of Cu2O/SiO2 and Cu/SiO2 core–shell octahedral nanocomposites. Nanotechnology. 2008;19:365610.10.1088/0957-4484/19/36/365610Search in Google Scholar PubMed
[78] Prajapati JP, Das D, Katlakunta S, Maramu N, Ranjan V, Mallick S. Synthesis and characterization of ultrasmall Cu2O nanoparticles on silica nanoparticles surface. Inorg Chim Acta. 2021;515:120069.10.1016/j.ica.2020.120069Search in Google Scholar
[79] Wang Y, Zhang J, Zhao Y. Strength weakening by nanocrystals in ceramic materials. Nano Lett. 2007;7:3196–9.10.1021/nl0718723Search in Google Scholar PubMed
[80] Zhao Y, Zhang J. Microstrain and grain-size analysis from diffraction peak width and graphical derivation of high-pressure thermomechanics. J Appl Crystallogr. 2008;41:1095–1108.10.1107/S0021889808031762Search in Google Scholar
[81] Misra SK, Dybowska A, Berhanu D, Luoma SN, Valsami-Jones E. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci Total Environ. 2012;438:225–32.10.1016/j.scitotenv.2012.08.066Search in Google Scholar PubMed
[82] Hoshyar N, Gray S, Han H, Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine. 2016;11:673–92.10.2217/nnm.16.5Search in Google Scholar PubMed PubMed Central
[83] Menichetti A, Mavridi-Printezi A, Mordini D, Montalti M. Effect of size, shape and surface functionalization on the antibacterial activity of silver nanoparticles. J Funct Biomater. 2023;14:244.10.3390/jfb14050244Search in Google Scholar PubMed PubMed Central
[84] Augustine R, Hasan A, Primavera R, Wilson RJ, Thakor AS, Kevadiya BD. Cellular uptake and retention of nanoparticles: Insights on particle properties and interaction with cellular components. Mater Today Commun. 2020;25:101692.10.1016/j.mtcomm.2020.101692Search in Google Scholar
[85] Ivask A, Suarez E, Patel T, Boren D, Ji Z, Holden P, et al. Genome-wide bacterial toxicity screening uncovers the mechanisms of toxicity of a cationic polystyrene nanomaterial. Environ Sci & Technol. 2012;46:2398–405.10.1021/es203087mSearch in Google Scholar PubMed
[86] Alam M. Analyses of biosynthesized silver nanoparticles produced from strawberry fruit pomace extracts in terms of biocompatibility, cytotoxicity, antioxidant ability, photodegradation, and in-silico studies. J King Saud Univ-Sci. 2022;34:102327.10.1016/j.jksus.2022.102327Search in Google Scholar
[87] Yougbaré S, Mutalik C, Okoro G, Lin I-H, Krisnawati DI, Jazidie A, et al. Emerging trends in nanomaterials for antibacterial applications. Int J Nanomed. 2021;2021:5831–67.10.2147/IJN.S328767Search in Google Scholar PubMed PubMed Central
[88] Rahman S, Sadaf S, Hoque ME, Mishra A, Mubarak NM, Malafaia G, et al. Unleashing the promise of emerging nanomaterials as a sustainable platform to mitigate antimicrobial resistance. RSC Adv. 2024;14:13862–99.10.1039/D3RA05816FSearch in Google Scholar
[89] Zhang W, Taheri-Ledari R, Ganjali F, Mirmohammadi SS, Qazi FS, Saeidirad M, et al. Effects of morphology and size of nanoscale drug carriers on cellular uptake and internalization process: a review. RSC Adv. 2023;13:80–114.10.1039/D2RA06888ESearch in Google Scholar PubMed PubMed Central
[90] Jakimska A, Konieczka P, Skóra K, Namieśnik J. Bioaccumulation of metals in tissues of marine animals, Part II: metal concentrations in animal tissues. Pol J Environ Stud. 2011;20:1127–46.Search in Google Scholar
[91] Faisal M, Saquib Q, Alatar AA, Al-Khedhairy AA, Hegazy AK, Musarrat J. Phytotoxic hazards of NiO-nanoparticles in tomato: a study on mechanism of cell death. J Hazard Mater. 2013;250:318–32.10.1016/j.jhazmat.2013.01.063Search in Google Scholar PubMed
[92] Thakur S, Neogi S. Effect of doped ZnO nanoparticles on bacterial cell morphology and biochemical composition. Appl Nanosci. 2021;11:159–71.10.1007/s13204-020-01592-8Search in Google Scholar
[93] Fu PP, Xia Q, Hwang H-M, Ray PC, Yu H. Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal. 2014;22:64–75.10.1016/j.jfda.2014.01.005Search in Google Scholar PubMed PubMed Central
[94] Karthik K, Dhanuskodi S, Gobinath C, Prabukumar S, Sivaramakrishnan S. Ultrasonic-assisted CdO–MgO nanocomposite for multifunctional applications. Mater Technol. 2019;34:403–14.10.1080/10667857.2019.1574963Search in Google Scholar
[95] Gaafar MM, Eltaweel FM, Fouda HA, Abdelaal MY. Synthesis of novel chitosan Schiff base and its ZnO nanocomposite for removal of synthetic dye, antimicrobial, and cytotoxicity activity. J Bioact Compat Polym. 2022;37:359–80.10.1177/08839115221119212Search in Google Scholar
[96] Kovacova M, Markovic ZM, Humpolicek P, Micusik M, Svajdlenkova H, Kleinova A, et al. Carbon quantum dots modified polyurethane nanocomposite as effective photocatalytic and antibacterial agents. ACS Biomater Sci Eng. 2018;4:3983–93.10.1021/acsbiomaterials.8b00582Search in Google Scholar PubMed
[97] Gao X, Li H, Niu X, Zhang D, Wang Y, Fan H, et al. Carbon quantum dots modified Ag2S/CS nanocomposite as effective antibacterial agents. J Inorg Biochem. 2021;220:111456.10.1016/j.jinorgbio.2021.111456Search in Google Scholar PubMed
[98] Eltaweil AS, Abdelfatah AM, Hosny M, Fawzy M. Novel biogenic synthesis of a Ag@ Biochar nanocomposite as an antimicrobial agent and photocatalyst for methylene blue degradation. ACS Omega. 2022;7:8046–59.10.1021/acsomega.1c07209Search in Google Scholar PubMed PubMed Central
[99] Dong H, Zhang S, Yang L, Wang N, Chen S, Ma J, et al. Cu/Zn galvanic couples composite antibacterial dressings prepared by template-assisted magnetron sputtering. Compos Part B: Eng. 2021;224:109240.10.1016/j.compositesb.2021.109240Search in Google Scholar
[100] Tran CD, Makuvaza J, Munson E, Bennett B. Biocompatible copper oxide nanoparticle composites from cellulose and chitosan: facile synthesis, unique structure, and antimicrobial activity. ACS Appl Mater Interfaces. 2017;9:42503–15.10.1021/acsami.7b11969Search in Google Scholar PubMed
[101] Medina J, Garcia-Perez VI, Zanella R. Metallic composites based on Ag, Cu, Au and Ag-Cu nanoparticles with distinctive bactericidal effect on varied species. Mater Today Commun. 2021;26:102182.10.1016/j.mtcomm.2021.102182Search in Google Scholar
[102] Heydari R, Koudehi MF, Pourmortazavi SM. Antibacterial activity of Fe3O4/Cu nanocomposite: green synthesis using Carum carvi L. seeds aqueous extract. ChemistrySelect. 2019;4:531–5.10.1002/slct.201803431Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy

