Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
-
Mageswari Manimaran
, Mohamad Haafiz Mohamad Kassim
and Victor Feizal Knight
Abstract
In the last century, global awareness of the environmental repercussions associated with petroleum-based polymer composites has surged. This realization urged extensive scientific research directed towards plant-based biomass, particularly nanocellulose, as a reinforcing element in polymer matrices. Global market value of nanocellulose is expected to increase considerably by 2025, to a forecast USD 783 million. Despite nanocellulose’s performance benefits, its poor compatibility with hydrophobic polymer matrices poses challenges, limiting thermal stability and impeding widespread commercialization at higher processing temperatures. To overcome these issues, chemical modification or functionalization emerges as a promising solution to enhance nanocellulose-based polymer nanocomposites’ thermal stability. The abundance of hydroxyl groups on nanocellulose enables specific chemical modifications, such as grafting functional molecules or forming covalent/ionic bonds with the polymer matrix. The aim of this study is to validate that integrating chemically functionalized nanocellulose into various polymer matrices, including thermoset, thermoplastic, and bio-polymer, enhances the thermal stability of resulting polymer nanocomposites, supported by thermogravimetric analysis (TGA). The study also explores six additional factors influencing TGA in nanocomposites, providing a comprehensive understanding of elements impacting the thermal properties of these materials.
1 Introduction
Polymer nanocomposites, consisting of a polymer matrix reinforced with nanosized particles, have garnered considerable attention from researchers due to their potential to yield high-performance materials, even with minimal addition of nanosized particles (less than 5% by weight) [1]. Various types of nanofillers, such as chitosan nanoparticles, nanoclay, nanosilica, and other inorganic or organic nanomaterials, are commonly incorporated to address certain drawbacks of polymer composites [2]. However, with the increasing demand for non-toxic reinforcement materials, there has been a surge in research papers focusing on plant-based nanocellulose as a reinforcing filler to enhance polymer properties [3,4,5]. This trend is gaining popularity among researchers for developing innovative polymers and materials across diverse applications, including nanocomposites, automotive, construction, paper and pulp, electronic sensors, and packaging industries [6].
Researchers are gravitating towards nanoscale cellulose materials over microscale cellulose due to drawbacks like high moisture absorption and poor compatibility. Nanoscale cellulose exhibits unique performance characteristics, including excellent mechanical properties, a high elasticity modulus (up to 150 GPa), tensile strength of nearly 10 GPa, low density (1.6 g/cm3), a low coefficient of thermal expansion (0.01 ppm/K), biodegradability, a large surface area, and an impressive strength-to-weight ratio that is eight times greater than stainless steel [6,7]. Numerous studies have highlighted a significant enhancement in nanocomposites through the additional reinforcement of nanoscale particles. Unlike commonly used carbon nanotubes or graphene, nanocellulose is a natural material that is widely available, with over 1 trillion tonnes of biomass produced annually, making it cost-effective and accessible [8]. The unique properties of nanocellulose, such as high surface area, distinctive morphology, high crystallinity index, sustainability, and biodegradability, contribute to minimal waste generation. Several works have explored hierarchical composites with nanocellulose reinforcements [8].
Nanocellulose emerges as an excellent reinforcing filler for both natural and synthetic polymer matrices due to its cost-effective and environmentally friendly processing, a feature synthetic fillers often lack [9]. This statement was proven by Geng et al. [10], who demonstrated that polymer-based nanocomposites can be effectively reinforced with ultra-low weight fractions (0.1 wt%) of functionalized nanocellulose, and a low-cost drawing process facilitates their application in large-scale scenarios. Regrettably, the low thermal stability of nanocellulose imposes restrictions on its processing at elevated temperatures, given its decomposition temperature of approximately 200–300°C [11]. Consequently, the processing temperature for nanocellulose composites must be maintained around 200°C to prevent nanocellulose degradation. Furthermore, inadequate distribution of nanocellulose within the polymer nanocomposite and poor compatibility between the polymer matrix and cellulose can lead to agglomeration, thereby diminishing the thermal stability of the nanocellulose-reinforced composite [9]. Aggregates introduce stress concentrators, acting as defects that impede stress transmission from nanocellulose to the polymer matrix [12]. Achieving a desired property, such as high thermal stability in a nanocomposite, necessitates the homogeneous dispersion of the filler in the polymer matrix [13]. A biocompatible composite can be attained through proper blending of high-quality polymer and nanocellulose from plant fibers, facilitating effective load transfer between the polymer and fiber and improving composite characteristics, especially thermal stability, even at low filler content [14]. Besides, factors influencing composite thermal stability include the source of nanocellulose, type of polymer matrices, processing techniques, drying process, fiber shape, orientation, dispersion, and volume percentage [14,15].
To address these challenges, various methods have been developed, with chemical modification or functionalization of nanocellulose proving to be an effective approach. Techniques like grafting, acetylation, esterification, etherification, or the introduction of reactants such as isocyanates (e.g., sulfates, amines, hemiacetals, and silanes) are employed to tailor the surface chemistry of nanocellulose, thereby enhancing both miscibility and interfacial compatibility with the polymer matrix while improving composite thermal stability [16]. These functionalized materials, with their unique properties, hold significant value and offer diverse applications, particularly in engineering and technology [17]. Additionally, several thermal analysis methods are available to measure changes in the physical or reactive properties of nanocellulose concerning temperature and time. Thermogravimetric analysis (TGA), which assesses thermal stability by tracking weight changes as a function of temperature, time, and atmospheric conditions, is the primary focus of this review. The characteristics derived from TGA can be utilized during processing to ascertain the degradation of processed substances.
In recent times, an increase in the number of research papers have shifted their focus towards investigating the thermal stability of nanocellulose-reinforced polymer nanocomposites [18]. The uniqueness of this study lies in its structure, aiming to provide an overarching view of plant-based nanocellulose. It begins with discussions on the various types of nanocellulose functionalization. Subsequent sections delve into the factors influencing the TG behavior of nanocomposites, including the impact of sulfate content, nanocellulose type, intermolecular bonding, nanocellulose filler loading, drying processes, fabrication methods, and crystallinity. The study further offers supporting information on the thermal stability of functionalized nanocellulose-based polymer nanocomposites, drawing on the latest research papers from the past 6 years. A comprehensive understanding of the thermal behavior of nanocellulose is crucial for the development of high-thermal-stability nanocellulose composites, especially for applications in high-temperature production processes.
2 Overview on plant-based cellulose
Plant fibers can be categorized into woody and non-woody plants. Non-woody plant fibers, derived from sources such as industrial and agricultural waste, as well as forestry byproducts, are abundant in our environment. These resources are not only cost-effective but are also underutilized when compared to the more limited availability of woody biomasses. Typically, non-woody plants, including agricultural waste, find practical applications such as serving as organic fertilizer, contributing to energy production through combustion, being utilized for gully filling to manage erosion, or serving as animal feed, while excess is often incinerated as waste [19]. However, transforming various types of waste into nanocellulose presents a significant environmental advantage.
In recent years, cellulose nanoparticles derived from lignocellulosic agricultural waste, such as oil palm empty fruit bunch, rice husk, sugarcane bagasse, and sawdust, or various parts of plants, including seeds, fruits, bast, leaves, stalks, or grass, have found applications in a diverse range of industrial uses [20]. Cellulose, lignin, and hemicellulose stand as the three primary biopolymers in the plant cell wall, featuring distinct intra and inter-polymer linkages, as outlined in Table 1 [21]. Additionally, the presence of intra and intermolecular hydrogen bonds contributes to the stability of cellulose, imparting high stiffness and strength to cellulose fibers [22].
Intra and inter polymer linkages between the primary biopolymers
| Polymer linkage | Type of bond | Component |
|---|---|---|
| Intra-polymer linkages | Ether bond | Lignin, hemicellulose |
| Carbon to carbon | Lignin | |
| Hydrogen bond | Cellulose | |
| Ester bond | Hemicellulose | |
| Inter-polymer linkages | Ester bond | Hemicellulose-lignin |
| Ether bond | Cellulose-lignin, hemicellulose-lignin | |
| Hydrogen bond | Cellulose-hemicellulose, hemicellulose-lignin, cellulose-lignin |
Cellulose serves as the principal constituent in plant fibers, constituting a homopolysaccharide comprised of β-linked d-glucose units connected by β 1-4 glycosidic bonds. Each glucose unit contains three −OH groups, with a primary alcohol at position 6C–OH reacting ten times faster than secondary alcohols at positions 2C–OH and 3C–OH. The formation of robust inter and intra-hydrogen bonds occurs between end-to-end glucose units, either on the same chain or different chains, as illustrated in Figure 1. This results in the creation of densely packed crystalline cellulose, which imparts mechanical strength, resistance to organic cellulose chains, and insolubility in water.
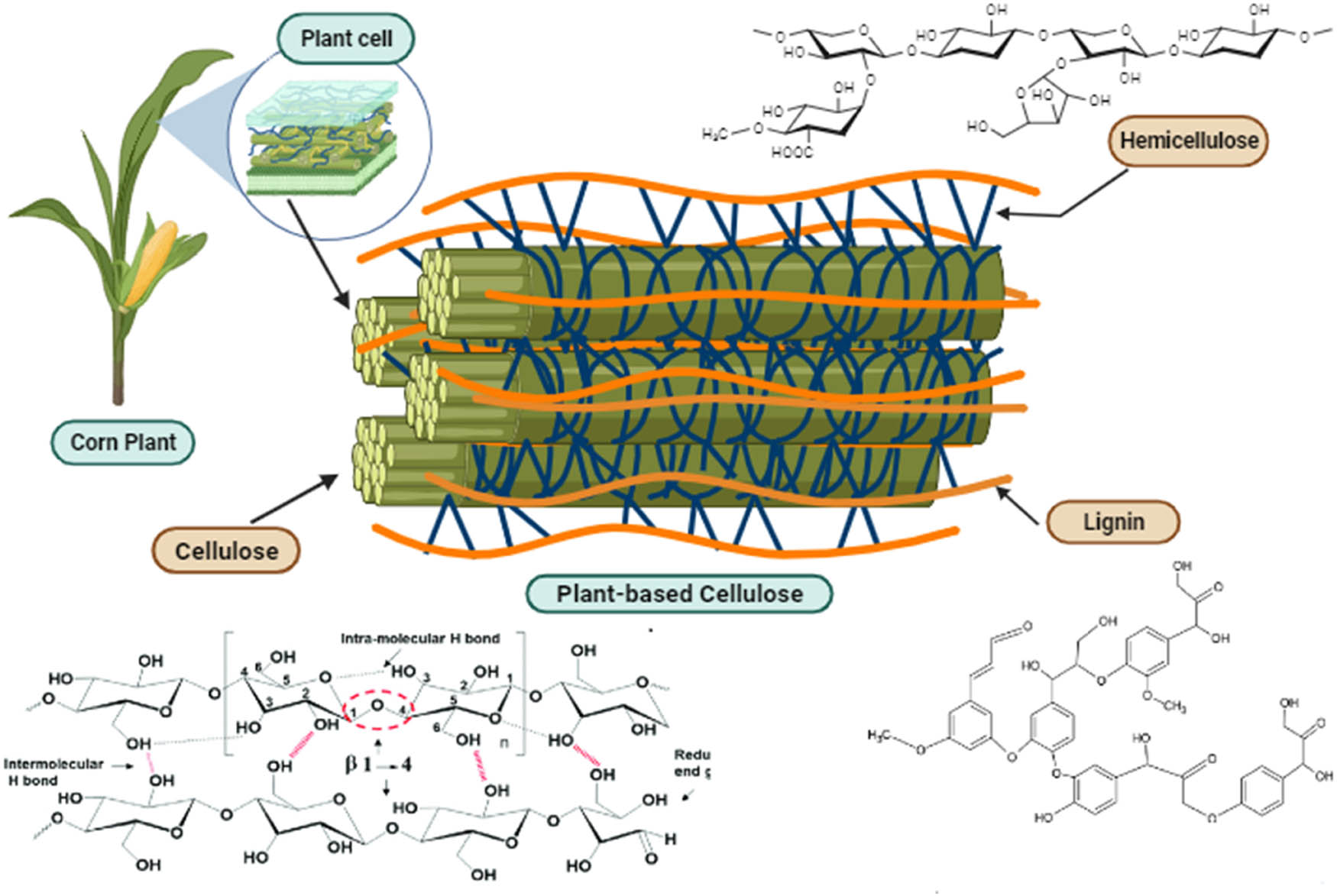
The main component of plant-based cellulose.
Conversely, hemicellulose exhibits an amorphous, randomly assembled, and structurally complex nature [23]. It contributes to plant rigidity by forming links between the hydrophobic lignin and the hydrophilic cellulose. Additionally, lignin, described as a complex phenolic polymer, acts as a protective barrier in plant fibers. It surrounds and safeguards cellulose and hemicellulose, playing a crucial role in providing structural support.
2.1 Isolation of plant-based nanocellulose
Before nanocellulose functionalization, it is essential to isolate lignin and hemicellulose, the non-cellulosic components mentioned earlier. This isolation is achieved through pretreatment, separating the crystalline cellulose from plant fibers using preferred methods such as chemical or mechanical treatments, as illustrated in Figure 2. This process alters internal structures, leading to size reduction, structural modifications, and increased crystallinity in the exposed cellulose sources. The efficient removal of lignin and hemicellulose results in the isolation of nanocellulose with generally uniform sizes, ultimately yielding homogeneous characteristics and reducing its hydrophilicity while significantly enhancing surface roughness [17]. Furthermore, pretreatment can impact the thermal stability, mechanical strength, and crystallinity ratio of the material. Studies have demonstrated that the removal of hemicellulose and lignin during bleaching and delignification pretreatment processes contributes to an increased thermal stability of nanocellulose [24]. Therefore, pretreatment is a crucial consideration when characterizing the thermal stability properties of nanocellulose.
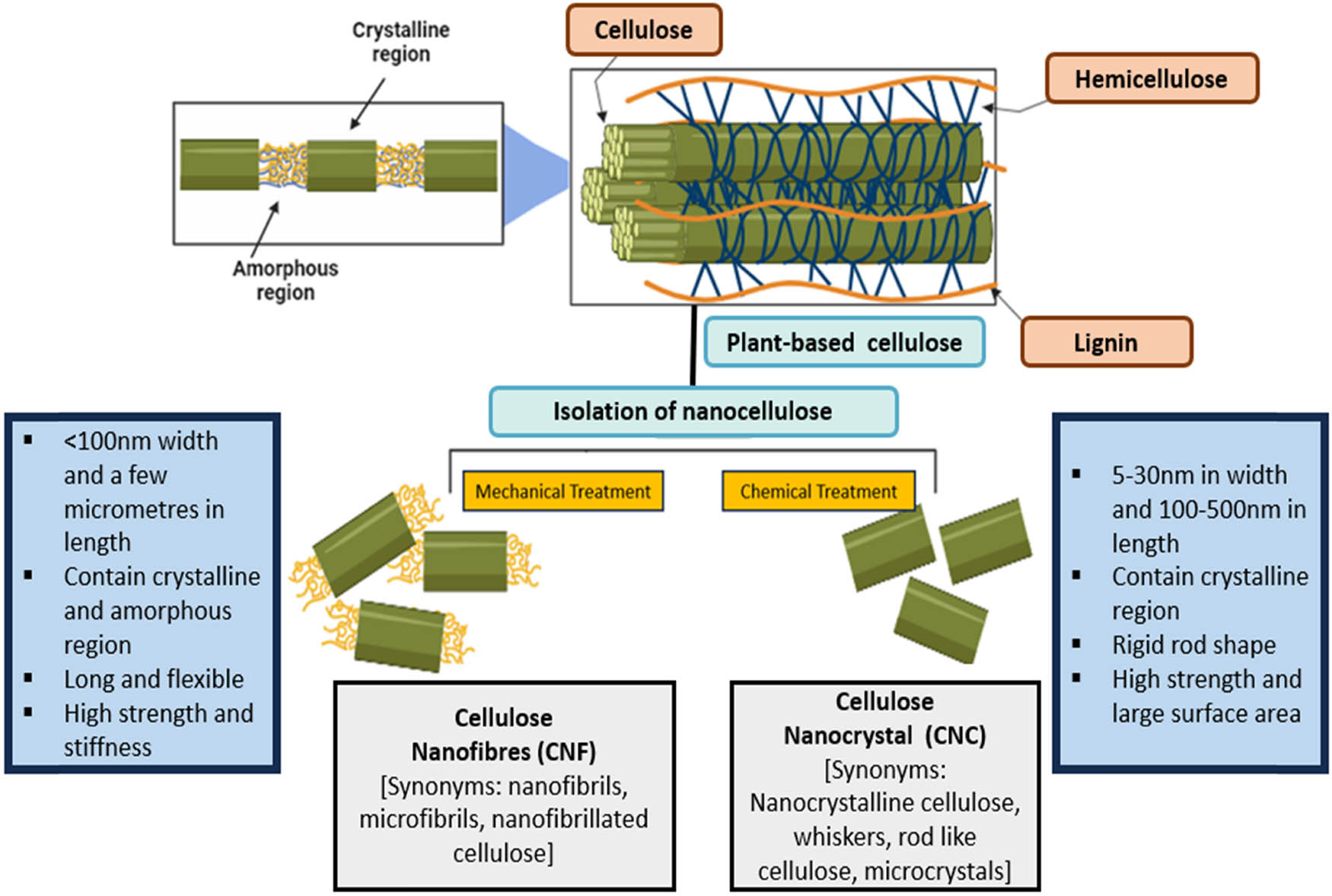
The isolation of nanocellulose through two distinct treatments leads to the synthesis of varying properties in nanocellulose.
The isolation of nanocellulose involves a two-stage process: (i) cleaning of plant fibers, extraction removal, and alkaline treatment with bleaching; and (ii) nanocellulose production involving depolymerization and mechanical isolation for preparing nanocellulose. Prior to alkaline treatment, it is crucial to clean plant-based fibers, removing oil and dust, as impurities on the fiber surface can affect the surface charge during acid hydrolysis [25]. The alkaline treatment permeates an alkaline solution into the crystalline structure, disrupting both inter and intra-molecular hydrogen bonds in cellulose and the adjacent crystalline region [14]. Alkalization precedes the bleaching process because it has been demonstrated that combining alkalization with bleaching enhances product properties, providing increased surface access for greater interaction and yielding more nanosized nanocellulose [26]. Alkalization can be achieved using potassium hydroxide (KOH) or sodium hydroxide (NaOH) at concentrations ranging from 4–20 wt% and temperatures around 70–160°C [20]. Moreover, the bleaching process involves the complete removal of lignin, also known as the delignification process. Sodium chlorite, with acetate buffer as a proton donor, is commonly used for bleaching to oxidize chromophoric groups in lignin and eliminate by-products such as oxidation [24]. Alternatively, hydrogen peroxide (H₂O₂) with NaOH at pH 11.5 is employed. The presence of lignin imparts a brown color to cellulose, which turns white after the bleaching process, signifying the removal of lignin. To ensure the maximum removal of lignin and hemicellulose, these pre-treatment procedures can be repeated multiple times.
The second stage involves depolymerization, where the glycosidic bonds in the amorphous region break through various chemical and/or mechanical treatments to synthesize nanocellulose. Acid hydrolysis is the most commonly employed procedure to obtain pure cellulose material [25]. Universally used reagents for acid hydrolysis include hydrochloric acid (HCl), sulfuric acid (H2SO4), formic acid, phosphoric acid, and nitric acid. Among these acids, H2SO4 is the preferred choice due to its ability to form a stable colloid in water. This stability arises from the half sulfate ester group (OSO3–) on the nanocellulose surface, created through interaction between H2SO4 and –OH groups on the nanocellulose. This interaction induces repulsion forces between negatively charged nanocellulose particles, ensuring colloidal stability and dispersion in water compared to other organic or mineral acids with fewer charges on the nanocellulose surface [27].
During acid hydrolysis, the sulfate ester group (
In addition to acid hydrolysis, mechanical treatments can also be employed for the isolation of nanocellulose, including techniques such as ultra-sonication, ball milling, or high-pressure homogenization. Mechanical methods aim to fibrillate bulk nanocellulose, emphasizing the generation of high uniformity rather than the elimination of amorphous portions, as seen in chemical treatments. High-pressure homogenization and micro-fluidization are the most commonly utilized mechanical treatments, operating at different pressure levels ranging from 50 to 2,000 MPa and up to 276 MPa, respectively [29]. Both methods involve passing nanocellulose slurries through narrow channels, resulting in collisions and shear forces between cellulose fibers at the molecular level.
Ultrasonication, another widely employed mechanical treatment, utilizes hydrodynamic forces generated by ultrasound. Ultrasonication has been shown to significantly increase cellulose sample yield, reaching up to 85.38%, compared to a yield of 48.16% without ultrasonication [6]. Additionally, the sonication time can influence the thermal stability of nanocomposites, as supported by Syafri et al. [30], who demonstrated that longer sonication vibration times (e.g., 1 h) enhance thermal stability and reduce moisture absorption. This improvement is attributed to the kinetic energy of the ultrasonic bath, facilitating even dispersion of fibers in the matrix, resulting in a compact structure, and enhancing interfacial bonding between the fibers and the matrix.
3 Functionalization of nanocellulose
One of the primary factors contributing to the low thermal stability of nanocomposites is the inadequate compatibility between nanocellulose and the polymer matrix [31]. To enhance the compatibility of nanocellulose with a hydrophobic polymer matrix, various surface modification approaches can be employed, encompassing chemical, physical, or enzymatic methods [6]. However, this study primarily concentrates on chemical modifications applied to nanocellulose (Figure 3).
![Figure 3
Illustrates three methods for nanocellulose modification: chemical, physical, and enzymatic approaches. Reproduced from Ref. [9].](/document/doi/10.1515/ntrev-2024-0005/asset/graphic/j_ntrev-2024-0005_fig_003.jpg)
Illustrates three methods for nanocellulose modification: chemical, physical, and enzymatic approaches. Reproduced from Ref. [9].
Chemical modifications of nanocellulose include oxidation, esterification, etherification, silane grafting, acetylation, carboxymethylation, and alkylation, which are key techniques for chemical modification without the use of coupling agents or surfactants [32]. Esterification surface modification occurs between the −OH group of nanocellulose and acids or anhydrides, such as succinic anhydride, forming esters and water. Various esterification types are available, including sulfonation and phosphorylation, occurring during nanocellulose separation based on the corresponding hydrolysis conditions. Acetylated surfaces can be achieved through Fischer–Speier esterification with acetic acid. Sulfonated surfaces are obtained using H2SO4, resulting in a negatively charged surface conducive to nanocellulose dispersion, forming a stable suspension [33]. Additionally, silane treatment is another chemical modification aimed at enhancing nanocellulose dispersion within the polymer matrix to ensure compatibility and distribution by converting the hydrophilic nanocellulose into a hydrophobic form. In the silylation process, the silyl group attaches to the −OH group of nanocellulose. Samarasekara et al. demonstrated that silylated nanocellulose modified using Si-69 (bis[3-(triethoxysilyl)propyl] tetrasulfide) exhibits the highest thermal stability compared to unmodified nanocellulose [34].
Moving on, one prevalent method in the chemical modification of nanocellulose involves oxidation via 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-assisted oxidation, recognized as a green and highly effective approach. This method introduces carboxylate groups (−COOH) on the nanocellulose surface, enhancing reactivity for subsequent grafting or polymer adsorption. It also enhances the stability of TEMPO-oxidized nanocellulose in water, resulting in well-dispersed nanosized particles. The process selectively converts −OH groups at 6C to −COOH, using catalytic amounts of TEMPO with NaBr at a pH around 10–11 in an aqueous NaClO solution containing cellulose, yielding high efficiency of up to 80%, albeit with a longer oxidation time [35].
Essentially, this method serves as a cost-effective chemical pre-treatment step before mechanical processing. The introduction of negatively charged groups weakens the rigidity of the cellulose structure, breaking strong intra-fiber H-bond networks through inter-fibrillar repulsive forces produced by these negatively charged groups. This facilitates nanoscale fibrillation of cellulosic fibers using various mechanical shearing processes [35]. Carboxymethyl nanocellulose is obtained through etherification, where alkaline cellulose, following alkaline treatment, undergoes etherification. The carboxymethyl group combines with the activated −OH group to form carboxymethyl cellulose, rendering it highly charged. Acetylation of nanocellulose is also an effective functionalization to prevent self-aggregation and enhance interaction with non-polar solvents [36]. Acetylation serves as a valuable chemical modification step in nanocomposites of nanocellulose with poly lactic acid (PLA), exhibiting remarkable enhancement in nanocellulose dispersion in non-aqueous media [35].
Furthermore, a common covalent bond modification involves the direct attachment of polymers to nanocellulose through “graft-to,” “graft-from,” and “grafting-completed/through” methods, as illustrated in Figure 4. In “graft-to,” a pre-formed polymer attaches to the nanocellulose surface, while in “grafting from,” polymerization initiates from the nanocellulose surface, enhancing the mechanical properties of the final composite [37]. “Grafting to” directly grafts organic molecules through reactions with available −OH groups on the nanoparticle surface. The drawback is the low yield due to steric hindrance by large grafting molecules. However, it allows for easy characterization of the pre-formed polymer, making it preferable when precise product control is necessary [38]. Successful colloidal bonding in “grafting to” requires efficient coupling chemistry; otherwise, the graft density will be low [39]. Examples of common “grafting to” reactions for preparing polymer-grafted nanocellulose are shown in Figure 5. These include (a) carbodiimide coupling to attach amine-terminated poly(ethylene glycol) (PEG-NH2) to carboxylated nanocellulose, (b) epoxy ring opening in basic conditions to attach epoxy-terminated poly(ethylene oxide) to desulfated nanocellulose, and (c) isocyanate-mediated “grafting to” to attach poly(caprolactone-block-L-lactide) (P(CL-b-LLA)) to unmodified nanocellulose.
![Figure 4
The “grafting to,” “grafting from,” and “grafting through” approaches for covalently immobilizing various moieties onto surfaces. Polymer chains are depicted with thick lines, circles represent reactive groups that can be either immobilized onto the substrate or used as a point for chain growth (in the case of grafting through), and squares represent the monomeric units being attached to the growing chain. Reproduced from Ref. [38].](/document/doi/10.1515/ntrev-2024-0005/asset/graphic/j_ntrev-2024-0005_fig_004.jpg)
The “grafting to,” “grafting from,” and “grafting through” approaches for covalently immobilizing various moieties onto surfaces. Polymer chains are depicted with thick lines, circles represent reactive groups that can be either immobilized onto the substrate or used as a point for chain growth (in the case of grafting through), and squares represent the monomeric units being attached to the growing chain. Reproduced from Ref. [38].
![Figure 5
The example of "grafting to" grafting nanocellulose: (a) carbodiimide coupling to attach amine-terminated poly(ethylene glycol) (PEG-NH2) to carboxylated nanocellulose, (b) epoxy ring opening in basic conditions to attach epoxy-terminated poly(ethylene oxide) to desulfated nanocellulose, and (c) isocyanate-mediated. Reproduced from Ref. [37].](/document/doi/10.1515/ntrev-2024-0005/asset/graphic/j_ntrev-2024-0005_fig_005.jpg)
The example of "grafting to" grafting nanocellulose: (a) carbodiimide coupling to attach amine-terminated poly(ethylene glycol) (PEG-NH2) to carboxylated nanocellulose, (b) epoxy ring opening in basic conditions to attach epoxy-terminated poly(ethylene oxide) to desulfated nanocellulose, and (c) isocyanate-mediated. Reproduced from Ref. [37].
As a consequence, the “grafting-from” methodology is generally preferred over “grafting to” because it cannot achieve very high graft densities. The reactions are conducted in aqueous environments where nanocellulose remains colloidally stable, eliminating the need to attach initiators. This makes it a one-step and potentially more industrially feasible process [37]. Various coupling agents and polymer surface modifications can be employed with the “graft from” strategy, involving radical polymerization, ring-opening polymerization (ROP), atom transfer radical polymerization (ATRP), and single-electron transfer living radical polymerization [40]. The “grafting through” approach is considered an intermediate technique between the “grafting to” and “grafting from” procedures. Table 2 provides details on the types of chemical modifications and their specific benefits [17].
Some type of chemical surface modification of nanocellulose and its specific improvement
| Type of functionalization | Remarks |
|---|---|
| Alkalization | Greater thermal stability, reduce moisture absorption of treated nanocellulose |
| Silylation | Enhance the nanocellulose/polymer interface, impart additional reinforcing properties, and improve the physicochemical characteristics as well as the tensile strength of the composite |
| Grafting | Reduce water absorption rates, enhance mechanical strength, and improve the miscibility of surface-grafted layers on the nanocellulose matrix. Acrylonitrile grafting contributes to improved thermal stability |
| Etherification | Cationizing the nanocellulose surface reduces water absorption, enhances resistance to fungus attacks, and provides dimensional stability |
| Sulfonation | A lower density of surface sulfate groups results in superior thermal stability compared to those achieved by hydrolyzing with pure H2SO4 |
Numerous studies have investigated chemically modified nanocellulose, as depicted in Figure 6. This trend suggests a focused effort by researchers to enhance the versatility of nanocellulose and address certain limitations, particularly its low thermal stability. With exceptional characteristics such as a remarkably small particle size, high specific surface area, and modifiability, nanocellulose stands out as an excellent filler material.
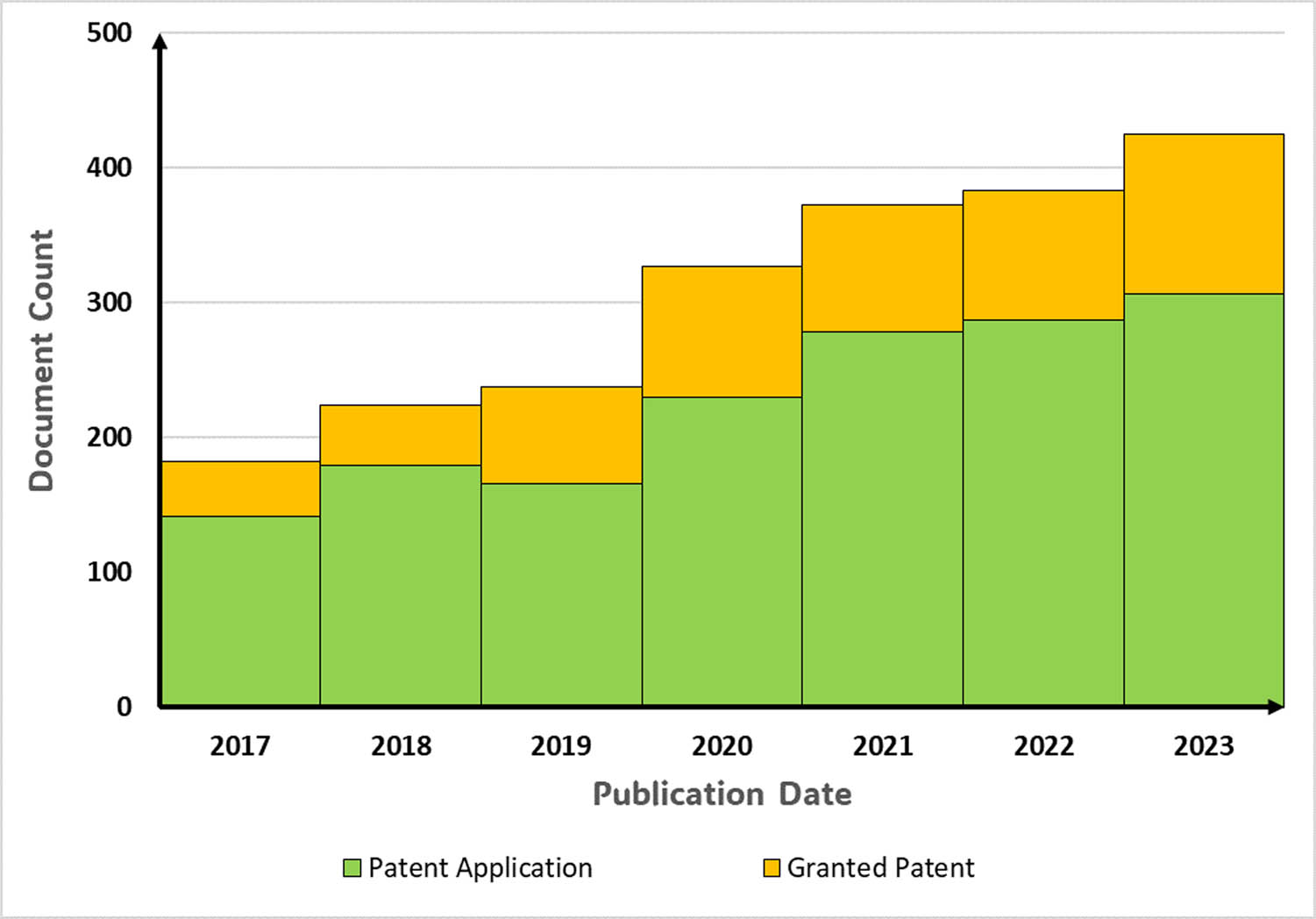
Shows the simplified patent search done using Lens.org with the keywords “functionalized nanocellulose” and “modified nanocellulose.”
4 TGA
One valuable technique for assessing the thermal stability of nanocellulose-reinforced polymer nanocomposites is TGA. TGA detects thermal stability by monitoring weight changes as a function of temperature at a constant rate. It provides both quantitative and qualitative insights into physical changes, such as sample loss or gain, in response to controlled atmospheres (inert or oxygen-rich), measuring the weight change over time. The primary information gleaned from the TGA curve is the sample’s thermal stability, indicating its ability to maintain characteristics almost unaffected when exposed to heat. This knowledge is crucial for engineers in various applications to prevent sample breakdown [41]. For instance, nanocellulose has a decomposition temperature ranging from 200 to 300°C; thus, controlling the processing temperature at 200°C is essential to prevent nanocellulose degradation during processing [9]. Additionally, the TGA curve offers insights into the sample’s composition, showcasing weight loss under heat. This information is significant for chemists to determine the sample’s composition, aiding in understanding the reaction steps involved in the decomposition process [42]. It also enables the identification of unknown compounds in the sample or the evaluation of the amount or percentage of a specific compound within a mixture. Plotting the derivative of mass change with temperature helps identify points where different mass changes occur, particularly useful for multiple decomposition reactions. Some TGA curves of nanocellulose exhibit a single-stage decomposition, indicating high cellulose component purity, while others may show two-stage decomposition [43].
Figure 7 depicts a single-stage TGA curve, providing insight into the thermal stability of the sample through the initial decomposition temperature (T onset)/(T i) and final decomposition temperature (T end)/(T f). The plateau or linear line before (T i) and after (T f) indicates that there is no change in weight occurring in the sample. The ability of the sample to retain its properties nearly unchanged upon heating is referred to as the “thermal stability” of the sample. As the temperature rises, the sample begins to lose weight or degrade at the temperature (T i), and the temperature at which decomposition ends is denoted as (T f). The difference in temperature represents the temperature range over which the present sample thermally degrades. The thermal degradation of cellulose typically involves three processes: dehydration (removal of water molecules), depolymerization (breakage of glycosidic linkages among glucose units), and decomposition (degradation of glycosyl units), ultimately resulting in the formation of charred residue [44].
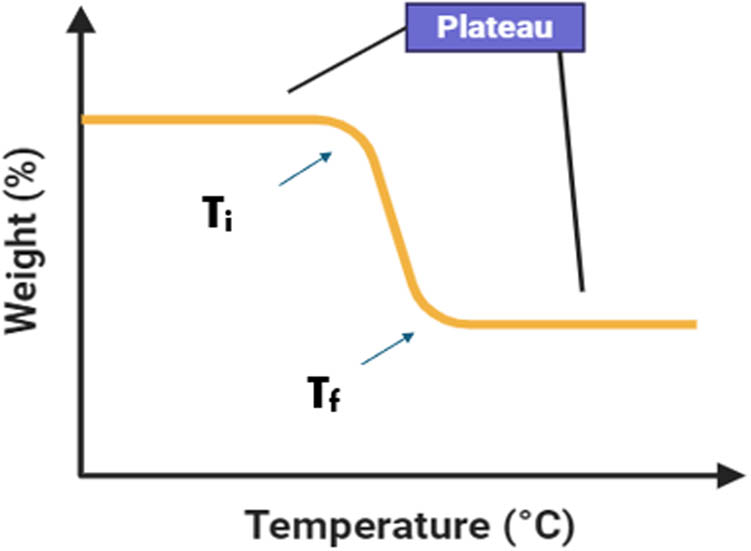
The basic thermogravimetry curve with initial decomposition temperature (T i) and final decomposition temperature (T f).
The initial degradation of nanocellulose occurs around 50–100°C, representing the temperature at which the first weight loss occurs in the TGA curve. This corresponds to the elimination of low molecular weight compounds or loosely bound volatile matter, such as moisture from the composite. It is well recognized that absorbed water in nanocomposites can significantly impact mechanical properties and thermal stability [45]. The second degradation involves a large weight loss rate, indicating the decomposition temperature. The thermal degradation of lignin and hemicellulose occurs between 300 and 450°C and 200 and 300°C, respectively. The decomposition of hemicelluloses results in a substantial weight loss between 200 and 350°C, whereas the thermal decomposition of nanocellulose occurs between 230 and 350°C due to the presence of hemicellulose and lignin [46]. In the third step of degradation, the mass loss is attributed to the degradation of charred residue into gaseous products. The remaining weight on the TGA curve represents the charred residue or ash. The high flame-retardant tendency contributes to char formation, protecting composite materials from heat sources, resulting in lower mass and increased thermal stability. This flame retardation potential is often observed in nanocomposites reinforced by acid-hydrolysis-produced nanocellulose containing sulfated groups, which are inherently flame-resistant. It is essential to note that the production of sulfate groups during H2SO4 hydrolysis can continuously increase with an extended hydrolysis time [45] (Figure 8).
![Figure 8
The degradation step of plant-based nanocellulose reinforced polymer composite. Reproduced from ref. [46].](/document/doi/10.1515/ntrev-2024-0005/asset/graphic/j_ntrev-2024-0005_fig_008.jpg)
The degradation step of plant-based nanocellulose reinforced polymer composite. Reproduced from ref. [46].
Moreover, there is another graph related to TGA known as the derivative thermogravimetric (DTG) curve, which is used to indicate the weight change with respect to temperature and identifies the highest rates of weight change or optimum degradation temperature [47]. Figure 9 presents a combined TGA and DTG analysis. The two peaks in the DTG curve indicate the two maximum decomposition temperatures of cellulose and lignin at 325 and 405°C, respectively. Additionally, the linear line (red line) indicates the thermal stability of cellulose in the range of 154–230°C, with cellulose decomposition starting at 230°C and ending at 375°C. The third stage indicates the decomposition of lignin starting at 375°C and ending at 490°C. Above 490°C, the weight no longer varies, indicating complete calcination of cellulose (fourth stage). As plants require inorganic compounds as nutrients during their growth, the generation of ash occurs, contributing to the observed weight at higher temperatures [48].
![Figure 9
The combination curve of TG (red curve) and DTG (black curve) of cellulose bark of Cola lepidota. Reproduced form ref. [49].](/document/doi/10.1515/ntrev-2024-0005/asset/graphic/j_ntrev-2024-0005_fig_009.jpg)
The combination curve of TG (red curve) and DTG (black curve) of cellulose bark of Cola lepidota. Reproduced form ref. [49].
5 Factors affecting TG characteristics of nanocomposite
Several factors, including sulfate content, type of nanocellulose, intermolecular bonding, nanocellulose filler loading in the polymer matrix, drying process, fabrication method, and crystallinity, can influence the TG characteristics of nanocomposites. These factors are explored in detail in this section.
5.1 Influence of sulfate content on thermal stability
The choice of acid for nanocellulose isolation significantly impacts its thermal stability. The onset degradation temperature of nanocellulose isolated from H2SO4 is approximately 200–240°C, while that of nanocellulose isolated using phosphoric acid or formic acid is higher, around 260–310°C and 300–325°C, respectively. Nanocellulose isolated using HCl shows thermal degradation around 244°C. Comparatively, nanocellulose isolated with H2SO4 exhibits the lowest onset degradation temperature [50]. However, the thermal stability of nanocellulose after acid hydrolysis, especially with H2SO4, remains a challenge for researchers due to the resulting
The increased sulfation area and flame-retardant activity of the
![Figure 10
TGA results of nanocellulose with various concentrations of H2SO4: microcrystalline cellulose (MCC), 40NCC (nanocellulose with 40%), 58NCC (nanocellulose with 58%), 64NCC (nanocellulose with 64%), and 78NCC (nanocellulose with 78%). Reproduced from ref. [28].](/document/doi/10.1515/ntrev-2024-0005/asset/graphic/j_ntrev-2024-0005_fig_010.jpg)
TGA results of nanocellulose with various concentrations of H2SO4: microcrystalline cellulose (MCC), 40NCC (nanocellulose with 40%), 58NCC (nanocellulose with 58%), 64NCC (nanocellulose with 64%), and 78NCC (nanocellulose with 78%). Reproduced from ref. [28].
However, there is a suggested method for improving the thermal stability of nanocellulose, which involves desulfation and neutralization through post-alkaline treatment [50]. It has been demonstrated that desulfated nanocellulose exhibits a higher decomposition temperature compared to sulfated nanocellulose. Figure 11 illustrates the morphology of sulfated and desulfated nanocellulose [52]. While nanocellulose typically decomposes at temperatures around 200–240°C, physical or chemical modifications, such as desulfation of cellulose nanocrystals (CNC) obtained through sulfuric acid hydrolysis, can enhance thermal degradation at 260°C [53].
![Figure 11
SEM image depicting the desulfation process of nanocellulose. Reproduced from ref. [52].](/document/doi/10.1515/ntrev-2024-0005/asset/graphic/j_ntrev-2024-0005_fig_011.jpg)
SEM image depicting the desulfation process of nanocellulose. Reproduced from ref. [52].
The desulfation process can also be accomplished through post-alkaline treatment with a robust base like sodium hydroxide (NaOH), leading to enhanced thermal properties by elevating the melting temperature. As depicted in Figure 12, sodium is employed to neutralize the sulfonic acid hydrogen, resulting in water molecule absorption (hydration). The hydrated ions create a double ionic layer that repels the corresponding double ionic layer of the next particle, providing electrostatic stabilization against long-range attractive forces [54].
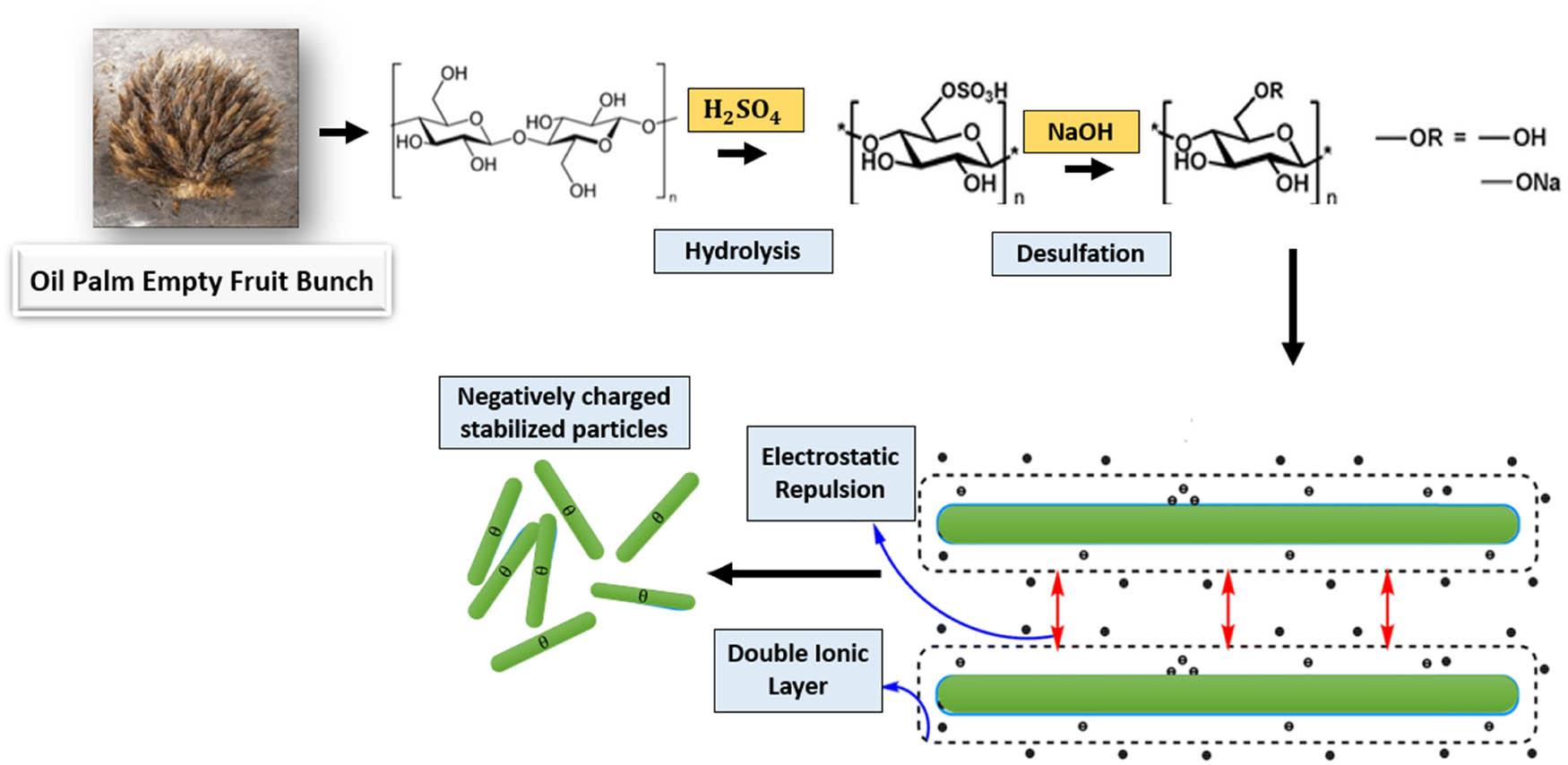
The effect of neutralization of sulfonated nanocellulose using NaOH.
Another approach to enhance thermal stability involves reducing the presence of acid in nanocellulose by employing a washing method, which includes a repeated sequence of centrifugation and filtration. However, this method results in excessive water usage [17]. Recent research has demonstrated that the thermal stability of sulfate group-containing nanocellulose can be increased using N,N-dialkylazetidinium salts or solvolytic desulfation. The latter process involves heating nanocellulose pyridinium salt in dimethyl sulfoxide (DMSO) in the presence of methanol or water [52], as illustrated in Figure 13. The reaction between azetidinium ions and nanocellulose significantly enhances its thermal stability by over 100°C, as indicated by TGA measurements.
![Figure 13
The reaction of N,N-dialkylazetidinium salts with sulfonated nanocellulose. Reproduced from ref. [55].](/document/doi/10.1515/ntrev-2024-0005/asset/graphic/j_ntrev-2024-0005_fig_013.jpg)
The reaction of N,N-dialkylazetidinium salts with sulfonated nanocellulose. Reproduced from ref. [55].
Moreover, Figure 14 shows the thermal stability of neutralized H2SO4 cellulose nanofibers (CNF) neutralized by aqueous Na2CO3 and dialyzed H2SO4 CNFs. It shows that the neutralized cellulose show high thermal stability (thermal degradation temperature: 285°C) compared to dialyzed CNFs (thermal degradation temperature: 225°C) [56] which is present on the CNFs due to the sulfate group.
![Figure 14
The thermal stability of H2SO4 dialyzed and neutralized CNF. Reproduced from ref. [56].](/document/doi/10.1515/ntrev-2024-0005/asset/graphic/j_ntrev-2024-0005_fig_014.jpg)
The thermal stability of H2SO4 dialyzed and neutralized CNF. Reproduced from ref. [56].
5.2 Influence of nanocellulose type on thermal stability
The isolation of nanocellulose from cellulose can be achieved through either mechanical treatment (milling, high-pressure homogenization, and ultrasonication) or chemical treatment (such as acid hydrolysis), resulting in two types of nanocellulose known as CNF and CNC. Figure 15 illustrates that CNC particles exhibit a short rod-like shape and possess smaller particle dimensions when compared to CNF. Although CNF and CNC share the same chemical composition, they differ in terms of surface morphology, particle size, crystallinity, and other attributes depending on the precursor and the type of synthesis process [57]. Different treatments lead to variations in the length of nanocellulose.
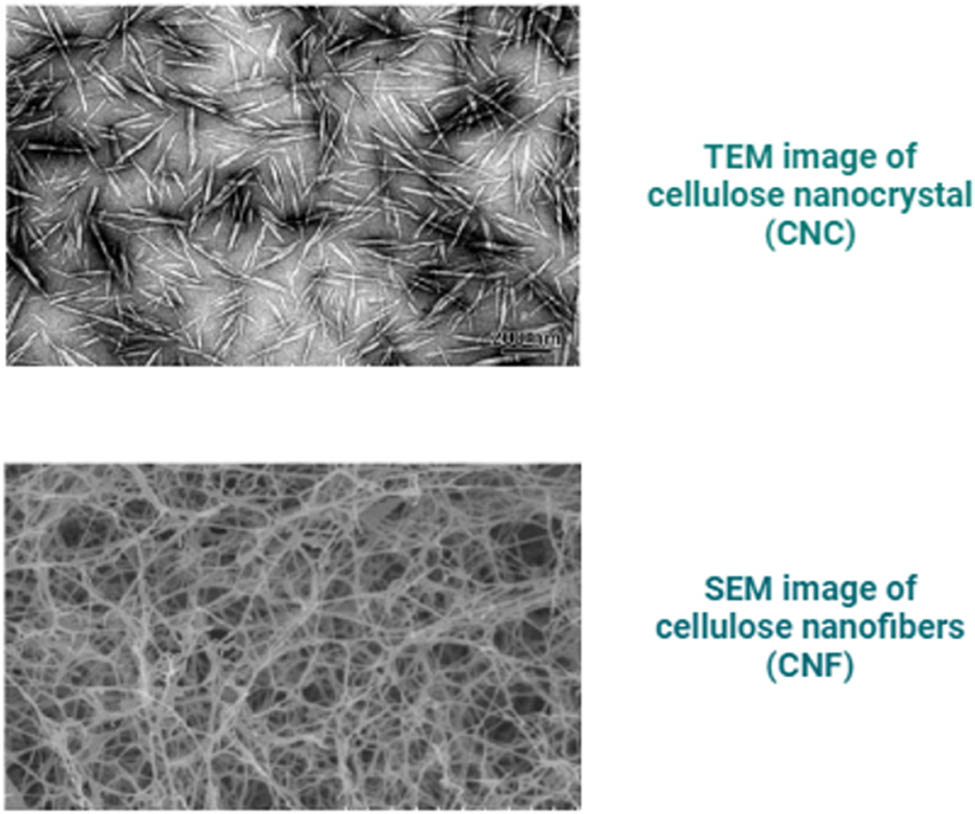
Shows the difference between the morphologies of CNC and CNF.
The thermal degradation of cellulose initiates in the amorphous regions and progresses to the more crystalline domains [58]. Additionally, an amorphous structure is less resistant to heat and high temperatures than a compact and ordered crystalline structure. A study on the thermal stability of nanocellulose conducted by Yildirim and Shaler [59] concluded that CNC demonstrated greater mechanical performance in both ways, possibly due to their higher crystalline structure where molecules are well-organized and capable of smoothly transmitting applied stresses compared to CNF. However, the low thermal stability of CNC can be attributed to their high crystalline structure. CNC, introduced to a greater number of sulfur groups throughout the manufacturing process (based on common chemical treatment), form a route with strong heat transfer potential. This pathway reduces the thermal degradation temperatures of CNC, exposing high surface areas of CNC to the heat source, thereby lowering thermal stability. In some cases, CNC is more commonly used than CNF due to its relatively small aspect ratio and ease of dispersibility. However, Pickering stabilization using CNF has been identified as a promising approach [60]. The incorporation of different types of nanocellulose in different polymers produces different thermal stability composites as shown in Table 3 [5].
Effect of different types of nanocellulose on the thermal stability of nanocellulose composite
| Type of nanocellulose | Type of matrix (°C) | T onset of neat matrix (°C) | T max of neat matrix (°C) | T onset of nanocomposite (°C) | T max of nanocomposite (°C) |
|---|---|---|---|---|---|
| Nanocrystalline cellulose decomposition temperature (257–333°C) | Polyvinyl alcohol (PVA) | 253.5 | 272.5 | 235–271.5 | 273.0–339.0 |
| PLA | 270 | 332 | — | 313–329 | |
| PVA and chitosan | 194 | — | 240–253 | — | |
| Nanofibrillated cellulose decomposition temperature (190–225°C) | Epoxy | 170.1 | 362.1 | 161.5–169.5 | 347.4–350.7 |
| Starch and chitosan | 235.0 | 304–313 | 216–224 | 302–311 | |
| Poly(methyl methacrylate) | 331.3 | 371.4 | 332.3–342.5 | 370.2–376.2 | |
| PLA | 270 | 320 | 282–2,980 | 325–337 |
5.3 Intermolecular bonding between the nanocellulose and polymer matrix
The thermal stability of composite materials is influenced by various factors, with molecular interactions among different macromolecules being a significant contributor, as depicted in Figure 16. The intermolecular bonding between nanocellulose and the polymer matrix is crucial for enhancing thermal stability. Stronger intermolecular bonds contribute to superior thermal stability, requiring substantial energy to break down nanocellulose macromolecules. However, challenges arise from the hydrophobic nature of the polymer matrix and the hydrophilic nature of nanocellulose, leading to agglomeration and suboptimal interfacial adhesion.
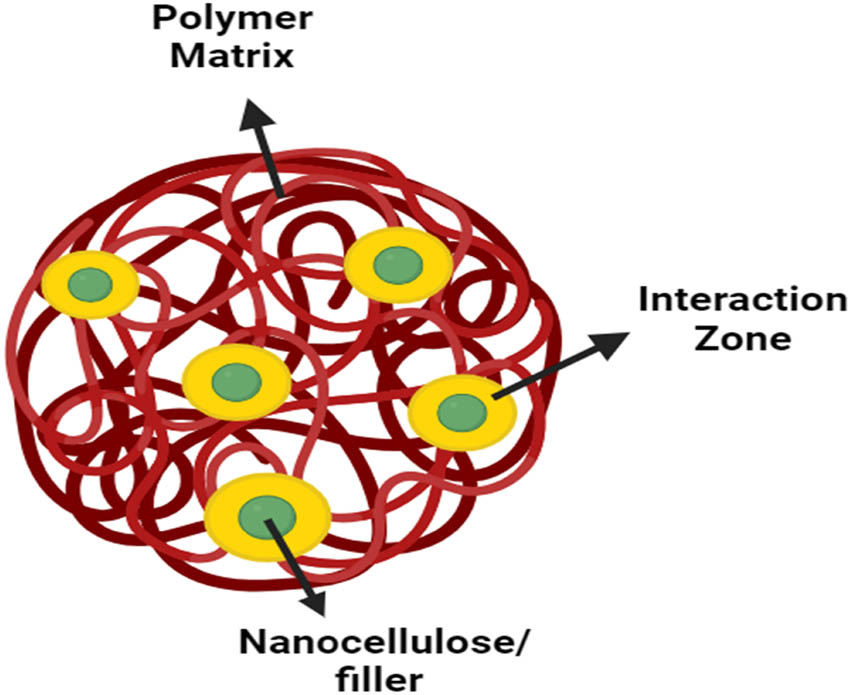
The interaction zone between the polymer matrix and the nanoparticles.
Some researchers address these challenges by employing a combination of chemical and mechanical treatments to produce high-quality products. For example, Yudhanto and his team successfully synthesized a bioplastic film by incorporating 6% CNCs treated with 46 wt% H2SO4 in a PVA matrix. This involved ultrasonication for 15 min at 60°C, resulting in particles with dimensions of 25 nm in diameter and 310 nm in length, and an aspect ratio of 12.4. The intermolecular bonding of the hydroxyl functional group between NCC and PVA raised the maximum temperature (T max) to 300°C, compared to pure PVA with T max at 275°C, as determined by TGA [61].
Two primary methods are employed to create nanocellulose composites: the solution casting technique and the melt blending technique. The solution casting method is generally preferred due to its ability to achieve a more uniform distribution of nanocellulose within the polymer matrix, resulting in a highly crystalline composite structure that significantly impacts thermal stability [62]. During sample preparation, the solution casting method allows particles to self-organize into networks as the solvent forces nanocellulose and the matrix to disperse. If there is low compatibility, self-assembly during solvent evaporation is more likely than binding with the matrix. Solvent selection is critical during solution casting, and melt processing is applicable when the polymer is solvent-soluble while the nanofillers are swellable. Inadequate solvent selection may lead to expensive recovery costs and environmental contamination.
According to research, nanocellulose can be dissolved in N,N-dimethylformamide (DMF) with stability comparable to aqueous solutions, making DMF a common additive to water as a dispersion [63]. Other solvents such as chloroform, toluene, and water can also be used to swell the nanofillers [64]. While wet blending is considered a greener method as it avoids solvent use, it is often incompatible between cellulose and popular polymeric matrices. Therefore, solution casting is generally preferred. Achieving effective dispersibility of nanocellulose within the polymer matrix can be accomplished through various approaches, with the utilization of the solution casting method being one effective strategy [65].
These interactions play a pivotal role in achieving the dispersibility of nanocellulose within the polymer matrix, consequently influencing the ultimate properties of the nanocomposite. The establishment of strong intermolecular bonds between nanocellulose and the matrix can occur through either physical or chemical interactions. These robust and rigid bonds elevate the thermal energy required for chain cleavage at critical decomposition stages. Another approach to enhance compatibility between nanocellulose and the polymer matrix involves chemical modification of nanocellulose. Surface modification leads to a homogeneous dispersion, contributing to increased thermal stability through improved interfacial adhesion between nanocellulose and the polymer matrix [2]. In addition to chemical modification, some researchers employ surfactants, chemical grafting reactions, and compatibilizers to enhance the dispersion of nanocellulose in the polymer matrix and improve composite thermal stability [65]. In a specific study, rod-like CNC were extracted through acid hydrolysis using parameters of 62 wt% H2SO4 at 45°C for 45 min, with an acid-to-fiber ratio of 1:20. Surprisingly, the resulting CNC exhibited a high thermal degradation temperature of 249.5°C compared to untreated nanocellulose. This improvement is attributed to the stronger hydrogen bonding in nanocrystals and crystalline domains formed after acid hydrolysis, removing amorphous regions from cellulose and leaving a predominantly crystalline structure [66, 67]. In essence, the acid hydrolysis pretreatment yields nanoscale cellulose fillers, fostering good compatibility between the PLA matrix and cellulose nanofillers [68]. Furthermore, the high thermal stability is also attributed to the termination of hydrolysis by diluting the solution 20 times with deionized water at 4°C, followed by centrifugation for 20 min at 8,000 rpm to remove residual sulfuric acid. This process reduces the sulfate content, contributing to the increased thermal stability of the CNC derived from date palm waste [67].
5.4 Effect of nanocellulose filler loading in polymer matrix on thermal stability
The quantity of filler loading in the polymer matrix plays a crucial role in determining the final properties of the composite. Numerous studies have theoretically demonstrated that an increase in filler loading within the polymer matrix can enhance the thermal stability of the composite by decelerating the rate of thermal degradation, as depicted in Figure 17 [69].
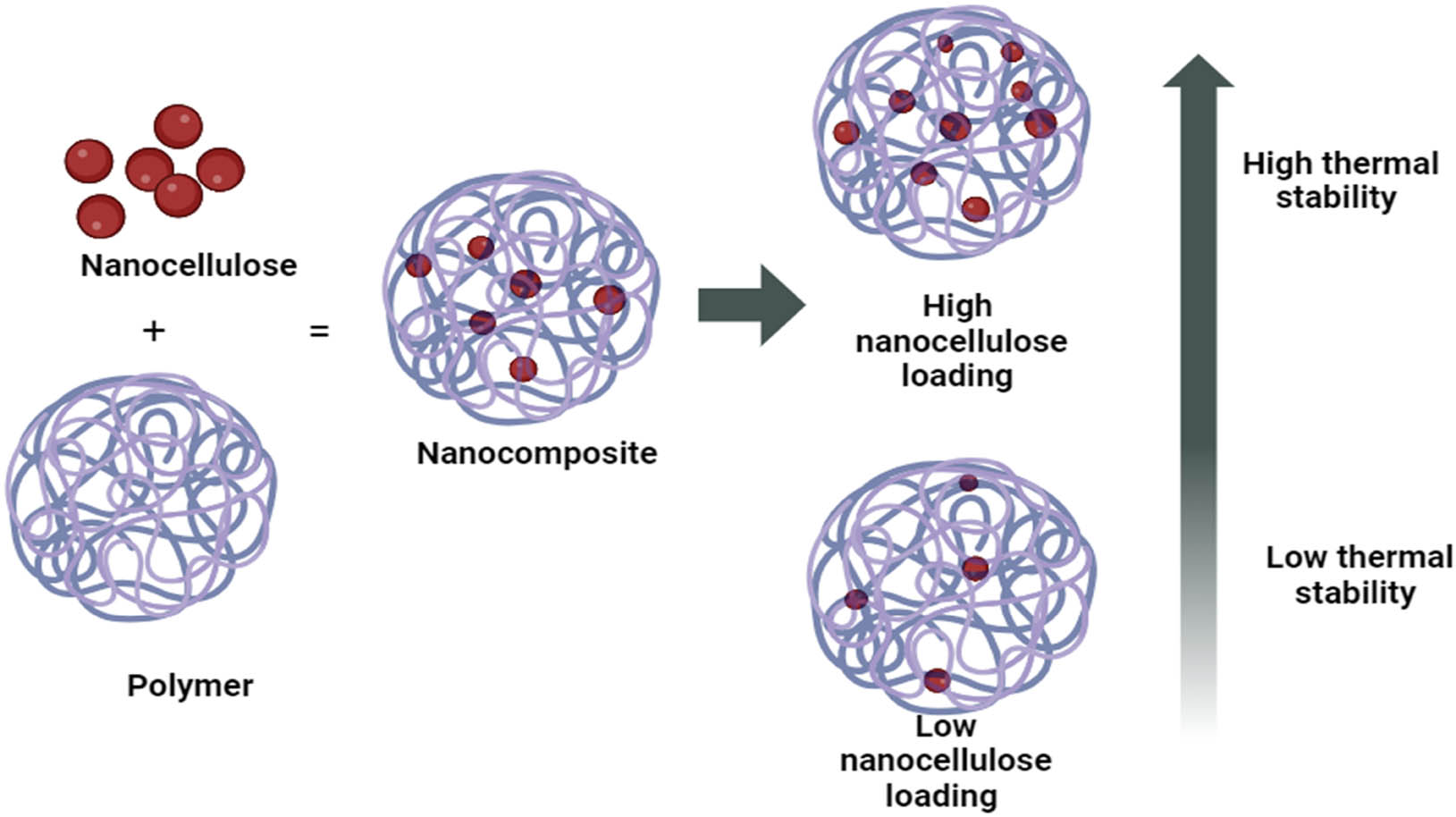
The effect of filler loading in the polymer matrix.
Practically, when nanocellulose loses its high aspect ratio due to a strong tendency to agglomerate, the reinforcing effect may be hindered and even degraded, especially at higher cellulose loading levels [45]. Optimal nanocellulose loading is crucial, reaching the percolation threshold or the saturation point of homogeneous dispersion, to achieve optimum properties. Percolation networks play a vital role in composite materials, as exceeding the percolation threshold dramatically improves material properties, possibly introducing properties that were previously nonexistent. To establish an effective percolation network, close monitoring of nanoparticle density and dispersion in the matrix is essential [22].
Moreover, DMF stands out as a versatile solvent, compatible with water and most organic solvents (excluding halogenated hydrocarbons). It exhibits chemical stability, serves as an effective solvent for a wide range of organic and inorganic compounds, and is excellent for solution casting and film formation. In a study, researchers examined the effects of 0.1 and 0.5 wt% cellulose nanowhiskers, isolated through sulfuric acid treatment and ultrasonication, on reinforcing PLA polymer. Surprisingly, an increase in cellulose nanowhiskers loading led to a decrease in thermal stability. PLA reinforced with 0.1 and 0.5 wt% cellulose nanowhiskers began decomposing at 277 and 259°C, respectively. Additionally, the activation energy of thermal decomposition decreased with the increase in the cellulose nanowhiskers content, indicating insufficient thermal insulation in the PLA matrix [68]. The sulfuric acid treatment further contributed to the significant decrease in thermal stability. However, higher cellulose nanowhiskers loading showed an increase in tensile strength and tensile modulus, attributed to the high crystalline properties of cellulose nanowhiskers promoting favorable behavior in the PLA matrix.
In another study, the effect of Washingtonia fiber loading (10, 20, and 30 wt%) with high-density polyethylene (HDPE) on composite thermal stability was investigated. Results indicated that a 20% fiber loading with HDPE demonstrated superior thermal stability, suggesting a Washingtonia fiber saturation ratio of around 20% by mass, beyond which the thermal stability of the composite declined [70].
5.5 Influence of drying process and fabrication method on thermal stability
A crucial final step in preparing nanofillers involves drying nanocellulose aqueous suspensions. This step is indispensable as the demand for nanocellulose in a dried-solid state is paramount to meet the requirements of cost-effective, large-scale industrial production of nanocomposites. This is facilitated by the ease of processing using melt compounding [45]. An improper drying process can significantly impact the properties of the end product, especially its thermal characteristics [71]. Notably, research has indicated that spray drying of nanocellulose enhances thermal stability and the crystallinity index [17]. Typically, during the cellulose drying process, molecular interactions within the nanocellulose intensify due to the forces generated by water removal and elevated temperatures [69].
Simultaneously, nanocellulose is often supplied as an aqueous dispersion to prevent aggregation, as it is most favorable to process nanocellulose while still in a wet state with the polymer matrix. Lyophilization, a frequently employed technique, involves replacing the water in the aqueous dispersion with tert-butanol and then lyophilizing to prevent agglomeration [60]. However, in some instances, concerns about aggregation persist even when nanocellulose is freeze-dried.
Moreover, the selection of the optimal processing technique stands as a pivotal phase in composite manufacturing. While solvent casting and melt processing are widely utilized for laboratory-scale preparation of polymer nanocomposites, they lack significant industrial appeal compared to melt extrusion processes. In melt extrusion, the reinforcement is typically added in solid form to a polymer melt, catering to industrial-scale production [72]. Achieving good compatibilization is a crucial consideration when synthesizing a desired polymer nanocomposite to ensure improved performance compared to the pristine polymer. The chosen fabrication method also influences the thermal stability of the composite. Melt blending, widely employed in various industries, is the predominant technique for producing polymer nanocomposites with diverse polymer matrices. This method entails the direct dispersion of nanocellulose in molten polymer, thorough mixing in a molten state, and the application of high shear stress, which aids in reducing the size of agglomerations, as illustrated in Figure 18 [73].
![Figure 18
The effect of shearing on the dispersion of the nanocellulose in polymer by melt blending. Reproduced from Ref. [73].](/document/doi/10.1515/ntrev-2024-0005/asset/graphic/j_ntrev-2024-0005_fig_018.jpg)
The effect of shearing on the dispersion of the nanocellulose in polymer by melt blending. Reproduced from Ref. [73].
Referring to Figure 18, the initial step involves the breakdown of large agglomerates, which then disperse into smaller units scattered throughout the polymer matrix. The application of stronger shearing forces, resulting from the transfer of strain from the polymer to these newly formed agglomerates, further breaks them down into individual particles. The success of this process largely depends on factors such as time and the chemical affinity between the polymer and the nanoparticle surface. Melt blending is typically executed using single and twin extruders or through injection molding. This technique offers several advantages, including achieving well-dispersed nanocellulose within the polymer matrix, enhancing thermal stability, improving mechanical properties, cost-effectiveness, and, notably, eco-friendliness as it avoids the use of solvents [73]. However, it is worth noting that high temperatures in melt blending can occasionally have adverse effects on the modified surface of the nanofillers, necessitating the use of optimized performance techniques. Moreover, managing the interactions between the polymer and nanoparticles, alongside controlling processing parameters like temperature and dwell time, can pose significant challenges.
Melt processing is the preferred and widely adopted method, especially for polymers that require melting and molding temperatures in the range of 170–190°C. However, it is important to consider that the degradation temperature of nanocellulose is around 200°C, beyond which chemical dehydration occurs due to the loss of physically and chemically bonded water. This process can result in yellowing and further darkening of the cellulose fibers, leading to increased brittleness. While melt processing offers high dispersion levels, the only drawback is the requirement for toxic and hazardous solvents to dissolve the polymer matrix, as in the case of PLA. Studies have demonstrated that the use of acetylated cellulose as a reinforcement in PLA through melt extrusion leads to significant enhancements in the mechanical performance of the nanocomposite and PLA crystallinity [74]. This may be attributed to less structural damage and the preservation of CNCs orientation in the matrix during solution casting, consequently maintaining the maximum reinforcing effect for the composites [75]. Melt mixing is usually applied for non-polar polymer matrix; thus, surface modification of nanocellulose needs to be conducted to obtain uniform dispersion of nanocellulose in the matrix.
5.6 Effect of crystallinity on thermal stability
Crystallization refers to the ability of polymer chains, in a mobile state, to form a crystalline packing structure that is ordered and compact [45]. Cellulose acts as a nucleating agent in composites, enhancing their crystallization rate and overall crystallinity, thus improving their thermal properties [65]. Higher crystallinity imparts a more ordered and densely packed structure to cellulose chains, making them more resistant to degradation at elevated temperatures. Conversely, a predominantly amorphous cellulose structure renders the chains more accessible and less stable during cellulose pyrolysis. The crystalline content is a crucial parameter, influencing mechanical properties and degradation rates. Additionally, higher crystallinity is typically associated with greater thermal stability, a favorable property during thermal processing [76].
The nucleation action of nanocellulose, particularly highly crystalline nanocellulose, contributes to the formation of a stiff and rigid crystalline structure in composites, making it less sensitive to thermal attack. The addition of cellulose positively impacts the thermal properties of the composite. The degree of crystallization is interrelated to the thermal stability of nanocellulose-reinforced polymer nanocomposites. Nucleating agents, such as talc, carbon nanotubes, graphene nanosheets, nanoclay, zinc phenyl phosphonate, poly(d-lactic acid), polyoxymethylene, and more, are factors that can affect the rate and behavior of crystallization in a composite [77].
The addition of nucleating agents accelerates the crystallization of polymers like PLA. For instance, introducing 0.2% wt./wt. of the heterogeneous nucleating agent TMC-328 in PLA improves heat resistance [78]. Nanocellulose treated with acid hydrolysis (H2SO4) exhibits the highest crystallinity index (83.51%) and good thermal stability with a low aspect ratio. In contrast, nanocellulose treated with TEMPO shows a crystallinity index of 72.33%, resulting in a sharp reduction in thermal stability but exhibiting good dispersion stability due to surface functional groups [79]. The reduced thermal stability of TEMPO-treated nanocellulose is attributed to the introduction of sodium carboxylate groups on the fiber surface during the TEMPO oxidation process. This leads to a decrease in crystalline cellulose chains, represented by unstable sodium anhydroglucuronate units in the TEMPO nanocellulose. However, the compact structure of acid hydrolyzed nanocellulose, as evidenced by a higher crystallinity index, limits the grafting of sulfate groups onto its surface. As a result, the decrease in degradation temperature for acid hydrolyzed nanocellulose is not pronounced [79] (Figure 19).
The six factors influencing the TG characteristics of nanocomposites.
6 TGA characteristics functionalized nanocellulose reinforced polymer nanocomposites
6.1 Functionalized nanocellulose reinforced with thermoset nanocomposites
Epoxy is a widely used polymer known for its applications, yet it possesses drawbacks such as high brittleness and low impact resistance due to its highly cross-linked structure [80]. To address these limitations, nanocellulose (Figure 20(a)) is introduced as a reinforcement agent in epoxy composites. Ureido-pyrimidinone (UPy), recognized as a supramolecular polymer, is incorporated into nanocellulose to enhance the oriented and stable dispersion of nanocellulose, owing to the strong bonding ability of UPy [81].
![Figure 20
The SEM image of (a) NCC and (b) NCC-UPy. Reproduced from Ref. [82].](/document/doi/10.1515/ntrev-2024-0005/asset/graphic/j_ntrev-2024-0005_fig_020.jpg)
The SEM image of (a) NCC and (b) NCC-UPy. Reproduced from Ref. [82].
Wang et al. successfully synthesized UPy-modified nanocellulose (NCC-UPy), which exhibited a tendency to bundle into long rod-like nanocrystals and predominantly aligned in the form of aggregates, as depicted in Figure 20(b). This alignment is attributed to the highly oriented multiple hydrogen bonds formed through the UPy groups of NCC-UPy, in contrast to unmodified nanocellulose shown in Figure 20(a), which presents rod-like shapes and a propensity for self-aggregation due to the abundance of −OH groups [82]. The onset decomposition temperature and maximum decomposition temperature of the modified nanocellulose were recorded at 326.6 and 350.2°C, respectively. NCC-UPy demonstrated an oriented ordered arrangement with superior dispersibility, good thermostability, and high crystallinity compared to unmodified nanocellulose. These attributes are advantageous for its use in reinforcing epoxy resin [82]. This improvement is mainly attributed to the formation of electrostatic repulsion between NCC-UPy nanoparticles, weakening hydrogen bonding and consequently enhancing the dispersion and interfacial compatibility of UPy-modified nanocellulose without aggregation.
While this study lacks information on the thermal stability of the nanocomposite, the positive impact demonstrated by the modified nanocellulose, indicating an improvement in thermal stability, suggests that the resulting supramolecular nanocomposite is expected to exhibit high thermal stability after reinforcement with epoxy resin.
Dharmalingam et al. [83] observed a reduction in the thermal stability of luffa fiber due to the hydrophilic nature of nanocellulose, leading to high water uptake and poor distribution. To address this, they proposed a solution involving the modification of nanocellulose using 3-Aminopropyl trimethoxysilane ((OCH3)3Si(CH2)3NH2). This functional group plays a crucial role in chemical modification, incorporating (a) methoxy (OCH3) to decrease the number of hydrogen groups in the fiber, (b) Si groups to hinder water uptake from atmospheric moisture, and (c) amine (NH2) for forming chemical bonds with epoxy or as a hardener [84]. When these functionalized luffa fibers are employed in the fabrication of epoxy composites, the epoxy groups condense with these aminated luffa fibers, similar to the condensation of epoxy groups with the aminated hardener molecule.
The TGA curve demonstrates a single major transition, indicating a robust interlocking between the amine-functionalized fiber and the epoxy matrix [85]. Additionally, there is a reduction in water uptake, leading to an increase in degradation temperature with filler loading ranging from 4 to 6%, compared to an unmodified nanocellulose-reinforced epoxy matrix.
Furthermore, in a recent study, Wang et al. [86] demonstrated that nanocellulose grafted copolymer CNC-g-PtBA, synthesized via ATRP in the presence of a macromolecular initiator blended with acrylic-based polymer resin, enhanced the compatibility and interaction between nanocellulose and the polymer. This grafting approach via ATRP resulted in a significant improvement in thermal stability. Moreover, Abraham et al. [87] provided a comprehensive review indicating that acetylated CNC exhibits stronger thermal stability (T onset at 298°C) compared to unmodified nanocellulose (T onset at 255°C). This enhanced thermal stability is attributed to the crystalline hydrophobic behavior and the presence of acetate esters, which enhance thermal resistance. Consequently, acetylated CNC can serve as an effective reinforcing agent for hydrophobic polymer matrices, such as epoxy. The mechanism of acetylated nanocellulose begins with nucleophilic attack during acetylation on the acyl carbon of the anhydride molecule by a lone pair of the alcoholic hydroxyl group. Subsequent loss of acetic acid occurs, generating the ester. A catalyst, such as iodine, serves as a Lewis acid catalyst, activating one of the carbonyl carbons of Ac2O. This, in turn, protonates the nearby hydroxyls of cellulose (polyalcohol), eventually leading to the esterification of CNC [87].
Additionally, Girouard et al. [88] undertook nanocellulose modification through a distinct functionalization approach involving the introduction of urethane linkages between polyurethane (PU) and CNC. This modification utilized isophorone diisocyanate (IPDI) monomer, possessing both primary and secondary isocyanate groups (–NCO) with varying reactivity toward the surface –OH groups of CNC. The reaction, facilitated by dibutyl tin dilaurate as a catalyst, involved the secondary –NCO group reacting with the primary −OH group of CNC. Meanwhile, the unreacted primary –NCO units on the modified CNC participated in polymerization in the presence of polyether-based triols to generate PU. The resulting PU/CNC nanocomposite exhibited enhanced thermal degradation, commencing at 300°C, marking a 35°C increase compared to unmodified nanocellulose, as depicted in Figure 21. Notably, it was emphasized that the CNC’s degradation is influenced by the drying method. Following the functionalization of CNC with IPDI, the particles were washed with toluene to remove excess reactants and then dried in a vacuum oven at 80°C. This drying process ensured the absence of moisture for the modified particles, contributing to their enhanced thermal stability [88]. Furthermore, the inclusion of 1 wt% esterified CNC with unsaturated fatty acid–modified nanocellulose demonstrated potential improvement in the thermal stability of unsaturated polyester/m-NC nanocomposites (UPe/m-NC), obtained from waste poly(ethylene terephthalate) (PET) mixed with a reactive vinyl monomer [89]. The notable enhancement can be attributed to the high compatibility of the modified nanocellulose with unsaturated polyester, even at low filler loading (Tables 4–6).
![Figure 21
The (a) TGA curve and (b) DTG curve depict the thermal properties of unmodified CNC (um-CNC) and modified CNC (m-CNC). Reproduced from ref. [88].](/document/doi/10.1515/ntrev-2024-0005/asset/graphic/j_ntrev-2024-0005_fig_021.jpg)
The (a) TGA curve and (b) DTG curve depict the thermal properties of unmodified CNC (um-CNC) and modified CNC (m-CNC). Reproduced from ref. [88].
Summary of the functionalized nanocellulose reinforced with thermosetting polymer nanocomposites
| Origin of nanocellulose | Type of thermoplastic polymer | Type of functionalization | Remarks | Thermal stability of nanocomposite | Ref. |
|---|---|---|---|---|---|
| −(CNC) | Epoxy | UPy-modified nanocellulose | Enhanced crystallinity of NCC-UPy and strong interfacial bonding force between NCC-UPy | Thermal stability of modified nanocellulose increased | [82] |
| Luffa fiber | Epoxy | Amine functionalization | Strong interlocking between the amine-functionalized fiber and epoxy matrix and lesser water uptake | Improvement in thermal stability | [83] |
| Cotton pulp (CNC) | Acrylic-based polymer | Grafted copolymer CNC -g-PtBA | Increased the compatibility and interaction between the nanocellulose and polymer | Thermal stability increased | [86] |
| Cellulosic waste materials (CNC) | Epoxy | Acetylated CNC | Crystalline hydrophobic behavior and the acetate esters enhanced thermal burning | Acetylated CNC has stronger thermal stability (decomposition onset at 298°C) | [87] |
| Southern yellow pine (CNC) | PU | Urethane linkage-CNC | Drying process after the modification play a role | Thermal degradation begins at 300°C, a 35°C increase compared to the unmodified nanocellulose | [88] |
| Cotton (CNC) | Unsaturated polyester | Esterification of CNC | High compatibility of modified nanocellulose | Thermal stability increased | [89] |
Summary of the functionalized nanocellulose reinforced with thermoplastics polymer nanocomposites
| Origin of nanocellulose | Type of thermoplastic polymer | Type of functionalized nanocellulose | Remarks | Thermal stability of nanocomposite | Ref. |
|---|---|---|---|---|---|
| Never-dried bleached hardwood kraft pulp (CNF) | LLDPE | Silanization | Hydrophobic properties | Thermal stability enhanced to 263.4°C after modification with Cef–PE | [90] |
| CNF | PP | Silanization | Hydrophobic properties by silyl group to the −OH group in the cellulose | Better thermal stability | [34] |
| Sugarcane bagasse | PP | Acetylation | Substituted by acetyl groups | Higher thermal stability than the composites reinforced with untreated fibers at temperature 400°C | [48] |
| Short sisal fibers | PS | Acetylation | Improvement in the fiber matrix adhesion by additional intermolecular bonding between the fiber and matrix | High degradation temperature (329°C) | [92] |
| CNC | PEO | Silane treated | Effect of the three-dimensional polysiloxane network grafted onto the surface of the modified CNC | Increased the thermal stability | [93] |
| Pandanus stem | Poly(ethylene-co-acrylic acid) | Diamine functionalized nanocellulose | Fire retardant effect of chlorine | Functionalized nanocellulose degradation start at 286°C | [94] |
| CNC | LLDPE | Modified nanocellulose with AzOH-DEA salt | Azetidinium hinder the acid-catalyzed dehydration process | Thermal stability of the CNC by more than 100°C | [55] |
| Bamboo cellulose | Modified PP | (TEMPO)-oxidized bamboo cellulose fiber | Strong interfacial adhesion between the modified PP and nanocellulose | Thermal stability increased from 265.4 to 284.9°C, respectively | [95] |
| CNC | PVA | Polyacrylamide grafted cellulose nanocrystals | Improve the interfacial interaction | Thermal stability increased | [96] |
Summary of the functionalized nanocellulose reinforced with bio-plastic nanocomposites
| Origin of nanocellulose | Type of thermoplastic polymer | Type of functionalization | Remarks | Thermal stability of nanocomposite | Ref. |
|---|---|---|---|---|---|
| Cotton pulp | PLA | HAP-modified nanocellulose | Strong hydrogen bonding interaction at the interface, leading to good dispersion in the PLA matrix | Higher thermal stability ( 346.6°C) than pure PLA (340.1°C) | [100] |
| CNF | PLA | Acetylated nanocellulose | Improve the dispersion and compatibility of nanocellulose in non-polar PLA | Improved thermal stability | [101] |
| Kenaf bast CNF | PLA | Silane modification | Surface hydrophobicity | Strongest thermal resistance at around 450°C | [103] |
| Cotton | PLA | CNC grafted APS | Compatibility between the polymeric matrix and modified CNC | Improved thermal stability | [104] |
| Eucalyptus wood | PCL | CNC (PCL-g-CNC) | Good compatibility between the matrix and the filler occurred. The | Good thermal stability of modified nanocellulose | [105] |
| CNC | PHBV | CNC (PCL-g-CNC) | Formation of hydrogen bonding interaction | Maximum decomposition temperature at 294.1°C. | [106] |
| Cotton fiber | PHBH | Acetylated CNC | Acetylated and MMT had a best compatibility with PHBH | Increased thermal stability up to 49°C | [108] |
| MCC by sulfuric acid hydrolysis | PBAT | Acetylated CNC | Uniform dispersion and a stronger interfacial adhesion between the matrix and nanocellulose | Degradation temperature of composite with 0.5% modified nanocellulose is 413° C | [109] |
| MCC by sulfuric acid hydrolysis | PBAT | Grafted with PBG | Surface hydrophobicity | Increased in their thermal modification by 20°C | [72] |
| MCC by sulfuric acid hydrolysis | PBAT | Grafted with octadecyl isocyanate reinforced PBAT. | Avoid agglomeration of modified CNC during noncomposites manufacturing | Improve thermal stability | [76] |
6.2 Functionalized nanocellulose reinforced with thermoplastics nanocomposites
There is substantial evidence that CNF synthesized through TEMPO-mediated oxidation, with sodium carboxylate groups on the cellulose, effectively enhance CNF dispersion. However, simultaneous decarboxylation of anhydroglucuronate units poses challenges to thermal stability and other issues [90]. To address this concern, nanofiber cellulose by TEMPO was methylated with trimethylsilyl diazomethane (TMSCHN2), resulting in an increased thermal stability from 222 to 249°C. Alternatively, ion-exchange treatments of the sodium carboxylate groups present in the original total organic carbon were employed [91].
Another strategy to enhance thermal stability involves the use of chain-end-functionalized polyethylenes (Cef–PEs). These polyethylenes can transfer stress across interfaces, thereby improving the performance of nanocomposites [90]. Furthermore, CNF by TEMPO-mediated oxidation, modified with Cef–PEs through alkoxysilane silanization (−Si(OCH3)3), efficiently reacts with −OH groups on nanofiber cellulose, enhancing its thermal stability. The decomposition temperature of unmodified nanocellulose was 326.7°C, whereas after modification, it increased to 350.9°C [90]. The aforementioned modified nanocellulose was incorporated with non-polar linear low-density polyethylene (LLDPE) polymer to synthesize a nanocomposite. This modification rendered the surface properties of the nanocellulose hydrophobic, thereby increasing the thermal stability of the resulting nanocomposite.
Additionally, polypropylene (PP) is a widely used hydrophobic thermoplastic. A PP nanocomposite reinforced with 5% loading of silane surface-modified CNFs demonstrates improved thermal stability [34]. Moreover, cellulose and cellulignin (fiber without hemicellulose), obtained from sugarcane bagasse and chemically modified through acetylation, were used to reinforce PP composites for the study of thermal stability properties [48]. The decrease in the −OH band in Fourier-transform infrared spectroscopy (FTIR) confirms the substitution of −OH groups on nanocellulose by acetyl groups, resulting in fewer −OH groups available for hydrogen bonding. Overall, the composites reinforced with treated fibers exhibited higher thermal stability compared to those reinforced with untreated fibers at a temperature of 400°C [48]. Furthermore, the thermal stability of acetylated short sisal fibers reinforced with polystyrene (PS) composites was investigated. The composites demonstrated higher thermal stability than both sisal fiber and the PS matrix. PS/treated sisal composites exhibited greater thermal stability than unreinforced PS and sisal fiber. The major degradation of the composite initiated at a higher temperature (329°C), and decomposition was nearly complete at 447°C. These improvements were attributed to enhanced fiber-matrix adhesion through additional intermolecular bonding between the fiber and matrix [92].
Moreover, Chanda et al. [93]. employed a novel, safe, effective, and eco-friendly modification to functionalize the −OH groups of CNC through silane treatment. These modified CNCs were utilized as reinforcements in the preparation of poly(ethylene oxide) (PEO)/CNC nanocomposites using the solvent casting method. The results indicated that the composites incorporating silane-treated CNCs exhibited an enhancement in the dispersion behavior of nanoparticles within the matrix. The inclusion of silane-treated CNCs led to increased thermal stability of the composite films. This heightened thermal stability in the nanocomposites can be ascribed to the shielding effect of the three-dimensional polysiloxane network grafted onto the surface of the modified CNC, thus enhancing compatibility between the filler and matrix while reducing the polar characteristics of fillers [93].
Furthermore, Chenampulli et al. [94] synthesized a novel ethylene diamine-functionalized nanocellulose/poly(ethylene-co-acrylic acid) composite, claiming its suitability for biomedical applications and cation removal from wastewater streams containing heavy metal ions. The first step in functionalizing nanocellulose involves nucleophilic substitution of the 6C–OH group with a chlorine atom (a good nucleophile) using thionyl chloride as the chlorinating agent. The second step is the replacement of the chlorine pendent group by ethylene diamine, resulting in aminodeoxy cellulose nanoparticles. The incorporation of an ethylene diamine moiety into cellulose is expected to reduce interparticle attractions, and the −NH2 group is anticipated to interact with the −COOH group of poly(ethylene-co-acrylic acid). According to TGA analysis, the thermal stability of aminodeoxy cellulose nanoparticles improved, as the cellulose main chain degradation began at 287°C, possibly due to the fire retardant effect of chlorine [94].
A novel chemical conversion of sulfate groups on nanocellulose using the azetidinium ion has been introduced to enhance the thermal stability of nanocellulose composites [55]. Two types of azetidinium salts were employed: (a) 1,10-diethyl-3-methoxyazetidinium chloride (AzOMeDEA salt) and (b) 1,10-dihexyl-3-methoxyazetidinium chloride (AzOMe-DHA salt). Surprisingly, the sulfate concentration remained relatively unchanged for samples modified with AzOH-DHA. In contrast, the sulfate content decreased for nanocellulose modified with AzOH-DEA. Consequently, AzOH-DEA-modified nanocellulose was coated with LLDPE to investigate the thermal properties of the resulting nanocomposite. TGA results indicated that the reaction between azetidinium ions and CNC increased the thermal stability of CNCs by more than 100°C. This enhancement was attributed to the removal of the acidic hydrogen associated with the sulfate group. The hydrogen removal occurred through grafting with azetidinium substituents, hindering the acid-catalyzed dehydration process. Methylated groups also prevented intramolecular cyclization, while non-methylated (AzOH-DEA) groups caused desulfation of CNCs. This modification is suitable for heat-processing techniques, such as extrusion, on an industrial scale [55].
Additionally, Wang et al. [95] presented a dual modification approach to enhance the interfacial adhesion between PP and cellulose fibers. Maleic anhydride (MAH) was mechanochemically grafted onto PP to interact with the reactive hydroxyl groups on the surface of 2,2,6,6-tetramethylpiperidine-1-oxy radical (TEMPO)-oxidized bamboo cellulose fiber (TBCF), as depicted in Figure 22. Unmodified nanocellulose reinforced with modified PP and modified cellulose with modified PP exhibited onset decomposition temperatures of 265.4 and 284.9°C, respectively. This difference can be attributed to the released reactive −OH groups on the fiber surface generated by TEMPO-mediated oxidation processing, effectively bonding with MAH-modified PP and creating a strong interfacial adhesion between the modified PP and nanocellulose. Interestingly, the 5 wt% modified PP was tightly attached to TBCF and could not be removed even after 120 h of Soxhlet extraction with dimethylbenzene [95].
![Figure 22
The nanocomposite incorporates a combination of modified PP and TBCF. Reproduced from Ref. [95].](/document/doi/10.1515/ntrev-2024-0005/asset/graphic/j_ntrev-2024-0005_fig_022.jpg)
The nanocomposite incorporates a combination of modified PP and TBCF. Reproduced from Ref. [95].
PVA is a water-soluble polymer known for its non-toxicity, good transparency, and high oxygen barrier properties. However, its limited mechanical strength and stiffness hinder its applications requiring high mechanical performance. To address this limitation, polyacrylamide grafted CNC (CNC-g-PAM) were introduced as reinforcing agents for PVA using a solution casting method to create a nanocomposite film. The incorporation of grafted CNC in the PVA matrix aims to prevent nanocellulose agglomeration and enhance interfacial interactions through hydrogen bonding between nanocellulose and the polymer [96]. The presence of polyacrylamide grafted onto CNC in the nanocomposite film is confirmed by the absorption band at 1,661 cm-1, representing the amide group in CNC-g-PAM.
The TGA of the resulting nanocomposite reveals three stages of weight loss. The first stage, occurring between 80 and 180°C, is attributed to the evaporation of moisture in the films. The second and third stages, observed at 210–380°C and 380–520°C, respectively, represent the thermal degradation of the film. The overall thermal stability of the film shows a slightly higher temperature after incorporating polyacrylamide grafted CNC into PVA compared to pristine PVA film. The initial degradation temperature and maximum degradation temperature of PVA-2.5%, PVA-5%, PVA-7.5%, and PVA-10% have shifted to slightly higher temperatures compared to the pristine PVA film. This indicates only a marginal improvement in thermal stability after the inclusion of CNC-g-PAM into PVA [96].
6.3 Functionalized nanocellulose reinforced with bio-polymer nanocomposites
Biobased and biodegradable polymers, such as starch, PLA, and polycaprolactone (PCL), are emerging alternatives for synthetic polymers like PP, acrylonitrile butadiene styrene, and PS [97]. Chakrabarty [60] has discussed recent advances in biopolymer reinforcement using functionalized nanocellulose.
PLA is a biodegradable thermoplastic polyester synthesized through the polymerization of lactic acid monomers, primarily derived from natural sources via ROP [98]. Rigotti et al. [99] prepared a biopolymer nanocomposite, PLA reinforced with nanocellulose functionalized with a hydrophobic lauryl chain through esterification reaction. In another approach, Lu et al. [100] modified nanocellulose using hydroxyapatite (HAP) to reinforce PLA (CNC-HAP). TGA results indicate that the addition of inorganic HAP significantly enhanced the thermal stability of CNC-HAP in PLA, reaching 346.6°C, especially for the sample (CNC/HAP) at a ratio of 30/70, referred to as PLA/A3, as shown in Figure 23. The study demonstrated that incorporating modified nanocellulose NCC/HAP in PLA significantly improved the interface compatibility with the PLA matrix due to strong hydrogen bonding interactions at the interface, leading to good dispersion in the PLA matrix [100].
![Figure 23
The thermal stability of different ratios of NCC to HAP was 50/50 (A1), 40/60 (A2), 30/70 (A3), 20/80 (A4), and 10/90 (A5) in PLA matrix. Reproduced from Ref. [100].](/document/doi/10.1515/ntrev-2024-0005/asset/graphic/j_ntrev-2024-0005_fig_023.jpg)
The thermal stability of different ratios of NCC to HAP was 50/50 (A1), 40/60 (A2), 30/70 (A3), 20/80 (A4), and 10/90 (A5) in PLA matrix. Reproduced from Ref. [100].
Additionally, Bulota et al. emphasized that incorporating acetylated nanocellulose into PLA can enhance the end properties by esterifying the primary −OH groups of nanocellulose, reducing polarity, and improving the dispersion and compatibility of nanocellulose in the non-polar PLA matrix. The effectiveness of this enhancement depends on the degree of substitution (DS). For instance, DS with 0.43 showed no visible aggregation compared to DS with 0.24, indicating that DS 0.24 is insufficient to prevent severe aggregation due to hydrogen bond formation [101]. Another green strategy was proposed by Ren et al. [102], who added acetylated surface-modified CNF into the PLA matrix using melt-compounding technology.
Moreover, Oyekanmi et al. [103] developed a novel method to synthesize macroalgae film composites reinforced with silane-modified CNF, making them potentially suitable materials for packaging applications. This study assessed the effect of silane treatment modification on the composite properties with different concentrations of CNF loading (1, 2, 3, 4, and 5%) in the biopolymer film. The results showed that silane-modified nanocellulose exhibited the strongest thermal resistance during its peak decomposition, occurring around 450°C. This was attributed to the deterioration of the matrix and the dehydroxylation of the silanol groups (Si–O–Si) on the modified film, promoting surface hydrophobicity. Moreover, the more homogeneous distribution of CNF enhanced the interfacial interaction with macroalgae for both the modified and unmodified film. Consequently, as the temperature rose, the polysaccharide macroalgae chains in the matrix were unable to move, thus increasing thermal stability. The effect of the reinforcements of the CNF loadings indicated improved thermal properties for both modified and unmodified films, with optimum fiber loading at 4%.
Furthermore, another study demonstrated that PEG-grafted CNF exhibited much better dispersion in a PLA matrix compared with unmodified nanocellulose through the covalent grafting process [10]. Yin et al. [104] successfully synthesized CNC grafted aminopropyltriethoxy silane (APS) with PEG epoxide (CNCs-APS-PEG). CNCs modified with APS and PEG were utilized as reinforcement for PLA composites through hot-pressing. PEG served as an effective plasticizer and compatibilizer to increase the dispersion of functionalized CNCs in PLA composites. Electron microscope images showed that the dispersion of CNCs in the polymer matrix improved due to changes in surface characteristics. TGA indicated that the initial decomposition temperature of the modified CNCs increased by approximately 150°C compared to pure CNCs.
PCL, which is gaining growing interest as a biomaterial for surgical applications, was incorporated with PCL-grafted CNC (PCL-g-CNC) to enhance the mechanical properties of PCL-based scaffolds and improve compatibility with the polymeric matrix. PCL chains were grafted onto the surfaces of CNC through ROP, obtained from eucalyptus wood by acid hydrolysis, to enhance their compatibility with the continuous PCL phase [105]. Subsequently, nanocomposites of PCL-g-CNC demonstrated improvements in TGA analysis. The degradation temperature and maximum degradation temperature for CNC were 260 and 314°C, respectively. On the other hand, the onset degradation temperature and maximum degradation temperature for PCL-g-CNC were approximately 295 and 382°C, indicating good thermal stability of the modified nanocellulose [105]. The author also investigated the effect of different PCL-g-CNC loadings (1, 3, and 5 wt%) in PCL. These results clearly indicated a decrease in the thermal stability of the PCL mats with an increasing content of PCL-g-CNC in the biocomposites, attributed to the lower thermal stability of PCL-g-CNC.
Moreover, another biodegradable polymeric matrix, poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), known for its poor thermal properties with a maximum decomposition temperature of 245.8°C, exhibited improved thermal stability after being grafted with functionalized CNC, reaching a maximum decomposition temperature at 294.1°C [106].
The enhancement in thermal stability is attributed to the formation of hydrogen bonding interactions between the ester carbonyl group of PHBV chains and the −OH group of functionalized CNC. This interaction reduces the electron-donating effect of substituent groups on carbon atoms at the α-position and the negative inductive effect of neighboring methylene groups at the β-position to the ester oxygen of the PHBV matrix [106]. Consequently, hydrogen bonding interactions can inhibit the formation of six-membered ring ester during the degradation process of PHBV, thus improving the thermal stability of the nanocomposite.
Polyhydroxyalkanoates are considered more promising bioplastics due to their biodegradable and biocompatible properties similar to polypropylene. Additionally, montmorillonite (MMT) is a low-cost clay that readily yields hydrophilic and environmentally friendly nanoparticles [107]. Xu et al. [108] have conclusively demonstrated that the incorporation of acetylated CNC in poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBH) matrix through solution-casting using MMT can lead to the development of sustainable nanocomposites for food packaging applications. The composite exhibited an increase in thermal stability by up to 49°C compared to unmodified nanocellulose. The primary reason for this improvement lies in the thermal barrier effect of the modified nanocellulose, where the carbon and gas generated by the degradation of acetylated CNC may delay the thermal decomposition of PHBH. Additionally, the study reported that acetylated CNC and MMT demonstrated the best compatibility with PHBH [108].
Furthermore, synthetic thermoplastic polybutylene adipate-co-terephthalate (PBAT) is a fully biodegradable polyester synthesized through the polycondensation of 1,4-butanediol with both adipic and terephthalic acid [98]. Despite having good processing properties and flexible performance, PBAT exhibits low mechanical properties, leading to its reinforcement with nanocellulose. In a specific study, nanocellulose was chemically modified using acetic anhydride to create acetylated nanocellulose, which was then used to reinforce PBAT, aiming to enhance its properties through the melt-blending technique. The resulting nanocomposite demonstrated improved thermal stability attributed to the uniform dispersion and stronger interfacial adhesion between the matrix and nanocellulose [109]. The composite with 0.5% modified nanocellulose exhibited a maximum degradation temperature of 413°C, outperforming the counterpart with unmodified nanocellulose in combination with PBAT.
A similar processing method involving the polymer matrix PBAT was employed by Morelli et al. [72], although with a different type of functionalized nanocellulose synthesized before its incorporation into PBAT. The modification of the CNC was achieved using low molecular weight poly(butylene glutarate) (PBG) through an “in situ” polymerization procedure, as illustrated in Figure 24. The grafting treatment reduced the CNC’s hydrophilic character and elevated the onset temperature of their thermal degradation by approximately 20°C (from 198 to 226°C) compared to the reference CNC sample containing sulfate groups. The sulfate groups tend to catalyze the thermal degradation of CNC and remain as residue after TGA. However, after the grafting process, these sulfate groups were removed, resulting in increased thermal stability.
![Figure 24
The two steps in situ polymerization reaction to synthesis nanocellulose grafted PBG. Reproduced from Ref. [72].](/document/doi/10.1515/ntrev-2024-0005/asset/graphic/j_ntrev-2024-0005_fig_024.jpg)
The two steps in situ polymerization reaction to synthesis nanocellulose grafted PBG. Reproduced from Ref. [72].
Furthermore, using a similar polymer matrix (PBAT) and grafting method, Pinheiro et al. [76] synthesized CNC modified by octadecyl isocyanate to produce a composite with a PBAT matrix. According to TGA, the degradation temperature of unmodified CNC and modified CNC was 233 and 285°C, respectively, due to the removal of sulfate groups after grafting. The crystallinity of the CNC can also influence the thermal stability of the composite.
7 Challenges and future directions
The escalating concern for sustainability is prompted by the environmental repercussions of non-renewable petroleum-based products, particularly plastics. These products, cherished for their utility, affordability, and widespread availability, contribute significantly to the issue, with single-use plastics like PET being a major component. The persistence of plastic waste is exacerbated by the lack of economically viable and energy-efficient recycling methods, compounded by the virtually eternal shelf life of most plastics. If present rates of manufacturing and recycling persist, estimates suggest that by 2050, global plastic accumulation could reach nearly 12,000 metric tonnes [110].
Concurrently, the alarming levels of plastic pollution, especially in marine ecosystems, pose a serious threat to aquatic flora and fauna, projecting that by 2050, the oceans may contain more plastic than fish. While it seems implausible to envision a world without plastics, environmental scientists are actively exploring innovative bio-based plastics that are recyclable, compostable, or biodegradable, offering both environmental sustainability and efficient plastic waste processing. In this pursuit, materials like nanocellulose serve as reinforcement in polymer matrices.
Polymer nanocomposites present exciting opportunities for novel functionalities beyond those offered by traditional materials. However, Garavand [2] has provided a comprehensive explanation of the safety considerations of nanocellulose, both before and after incorporation into biopolymeric matrices. Various studies have suggested that high aspect ratio nanoparticles may pose carcinogenic risks, potentially leading to lung cancer and mesothelioma. This is attributed to the ability of very small particles to penetrate biological barriers more effectively, resulting in increased accumulation in cellular compartments. Recent research on rats indicates that CNFs may induce respiratory issues by causing lung inflammation and fibrosis. Due to their minute size, nanoparticles may accumulate in specific regions of the brain when introduced into a biological environment, such as the liver or brain.
It is crucial to note that while many studies have reported similar procedures for obtaining nanocellulose, variations in results are common, necessitating adaptations to align with reported outcomes. For young scientists, this presents challenges in selecting a reliable method and identifying optimal conditions for their specific plant source or plant part. Achieving nanocomposites with exceptional properties demands precise control of these parameters to enhance the interaction between the filler and matrix. Driven by the demand for products derived from sustainable, renewable, and biodegradable resources with minimal environmental risks to human and animal health and safety, cellulose has emerged as one of the most abundant natural biomaterials with promising potential.
8 Conclusion and future perspective
In conclusion, the growing ecological concerns have fueled a keen interest in utilizing lignocellulosic biomass as nanocellulose, emerging as a promising nanofiller due to its unique properties. However, nanocellulose is prone to self-aggregation in the matrix due to abundant −OH groups, hindering interfacial compatibility and causing low thermal stability. This limitation affects the enhancement performance of composite materials during processing or at high temperatures. Therefore, this review includes a detailed discussion on chemical modification and various techniques for functionalizing the cellulose surface before its incorporation into the polymer matrix.
To mitigate self-aggregation, chemical modification of nanocellulose is crucial, introducing functional groups that reduce agglomeration and enhance interfacial compatibility with the matrix. Incompatibility with a polar polymer has historically hindered the efficient addition of nanoscale cellulose to such matrices. Thus, surface characteristics of polar polymers or cellulose must be modified. The findings clearly indicate that surface modification of nanocellulose before reinforcing the polymer matrix plays a pivotal role in increasing thermal stability. First, it reduces nanocellulose hydrophilicity, impacting its dispersion and integration within the matrix. Second, it promotes a robust interfacial bond between cellulose and the matrix through the formation of strong hydrogen bonds, thereby improving the thermal properties of the nanocomposite. The addition of nanocellulose also enhances the barrier properties of the nanocomposite. When well-dispersed in the polymer matrix, it forms strong interfacial interactions, reducing chain segmental mobility and decreasing penetrant diffusivity. For polar polymer matrices, uniform dispersion is easily achieved due to the shared hydrophilic nature of both materials. However, with non-polar polymer matrices, nanocellulose surface modification is essential for achieving uniform dispersion, often requiring surfactants or other chemicals.
Various factors impacting the thermal performance of composites, such as sulfate content, nanocellulose type, intermolecular bonding, filler loading, drying processes, fabrication methods, and crystallinity, highlight the importance of surface modification for enhanced dispersion and chemical interaction with diverse polymer matrices. This comprehensive understanding underscores the development of biodegradable nanocomposites with improved thermal stability.
Acknowledgments
Special thanks to Ministry of Higher Education (MOHE) for funding under the Fundamental Research Grant Scheme (FRGS) (FRGS/1/2023/STG05/USM/02/3). The authors would like express their gratitude for the financial support received from the Universiti Pertahanan Nasional Malaysia.
-
Funding information: Fundamental Research Grant Scheme (FRGS) (FRGS/1/2023/STG05/USM/02/3).
-
Author contributions: Conceptualization, writing-original draft preparation, review and editing: Mageswari Manimaran, Mohd Nurazzi Norizan, and Mohd Nor Faiz Norrrahim; Conceptualization: Mohamad Haafiz Mohamad Kassim, Mohd Ridhwan Adam, and Victor Feizal Knight. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Al-Haik MY, Aldajah S, Siddique W, Kabir MM, Haik Y. Mechanical and thermal characterization of polypropylene-reinforced nanocrystalline cellulose nanocomposites. J Thermoplast Compos Mater. 2022;35(5):680–91.10.1177/0892705720925125Search in Google Scholar
[2] Garavand F, Nooshkam M, Khodaei D, Yousefi S, Cacciotti I, Ghasemlou M. Recent advances in qualitative and quantitative characterization of nanocellulose-reinforced nanocomposites: A review. Adv Colloid Interface Sci. 2023;318(August):102961. 10.1016/j.cis.2023.102961.Search in Google Scholar PubMed
[3] Rosli N, Ahmad I. Application of cellulose nanocrystals (CNC) as reinforcing materials in bio-nanocomposites. J Polym Sci Technol. 2019;4(1):22–32.Search in Google Scholar
[4] Ilyas RA, Sapuan SM, Ishak MR, Zainudin ES. Nanocellulose reinforced starch polymer composites: a review of preparation, properties and application. In Proceeding: 5th international conference on applied sciences and engineering (ICASEA, 2018). Capthorne Hotel, Cameron Highlands, Malaysia: Global Academic Excellence (M) SDN BHD; 2018. p. 325–41.Search in Google Scholar
[5] Kargarzadeh H, Mariano M, Huang J, Lin N, Ahmad I, Dufresne A, et al. Recent developments on nanocellulose reinforced polymer nanocomposites: A review. Polymer. 2017;132:368–93. 10.1016/j.polymer.2017.09.043.Search in Google Scholar
[6] Tahir D, Karim MRA, Hu H, Naseem S, Rehan M, Ahmad M, et al. Sources, chemical functionalization, and commercial applications of nanocellulose and nanocellulose-based composites: A review. Polymers (Basel). 2022;14:21.10.3390/polym14214468Search in Google Scholar PubMed PubMed Central
[7] Aigaje E, Riofrio A. Processing, properties, modifications, and environmental impact of nanocellulose/biopolymer composites: a review. Polymers. 2023;15(5):1219.Search in Google Scholar
[8] Mishnaevsky L, Mikkelsen LP, Gaduan AN, Lee KY, Madsen B. Nanocellulose reinforced polymer composites: Computational analysis of structure-mechanical properties relationships. Compos Struct . 2019;224:111024. 10.1016/j.compstruct.2019.111024.Search in Google Scholar
[9] Trache D, Tarchoun AF, Derradji M, Hamidon TS, Masruchin N, Brosse N, et al. Nanocellulose: from fundamentals to advanced applications. Front Chem. 2020;8:392.10.3389/fchem.2020.00392Search in Google Scholar PubMed PubMed Central
[10] Geng S, Yao K, Zhou Q, Oksman K. High-strength, high-toughness aligned polymer-based nanocomposite reinforced with ultralow weight fraction of functionalized nanocellulose. Biomacromolecules. 2018;19(10):4075–83.10.1021/acs.biomac.8b01086Search in Google Scholar PubMed
[11] Barkoula NM, Alcock B, Cabrera NO, Peijs T. Effect of nanocrystalline cellulose on morphological, thermal, and mechanical properties of nylon 6 composites. Polym Polym Compos. 2008;16(2):101–13.10.1177/096739110801600203Search in Google Scholar
[12] Dhuiège B, Pecastaings G, Sèbe G. Sustainable approach for the direct functionalization of cellulose nanocrystals dispersed in water by transesterification of vinyl acetate. ACS Sustain Chem Eng. 2019;7(1):187–96.10.1021/acssuschemeng.8b02833Search in Google Scholar
[13] Savadekar NR, Mhaske ST. Synthesis of nano cellulose fibers and effect on thermoplastics starch based films. Carbohydr Polym. 2012;89(1):146–51. 10.1016/j.carbpol.2012.02.063.Search in Google Scholar PubMed
[14] Poulose A, Parameswaranpillai J, George JJ, Gopi JA, Krishnasamy S, Dominic CDM, et al. Nanocellulose: A fundamental material for science and technology applications. Molecules. 2022;27(22):1–27.10.3390/molecules27228032Search in Google Scholar PubMed PubMed Central
[15] Aigaje E, Riofrio A, Baykara H. Processing, properties, modifications, and environmental impact of nanocellulose/biopolymer composites: a review. Polymers. 2023;15(5):1219.10.3390/polym15051219Search in Google Scholar PubMed PubMed Central
[16] Rol F, Belgacem MN, Gandini A, Bras J. Recent advances in surface-modified cellulose nanofibrils. Prog Polym Sci. 2019;88:241–64. 10.1016/j.progpolymsci.2018.09.002.Search in Google Scholar
[17] Norizan MN, Shazleen SS, Alias AH, Sabaruddin FA, Asyraf MRM, Zainudin ES, et al. Nanocellulose-based nanocomposites for sustainable applications: A review. Nanomaterials. 2022;12(19):1–51.10.3390/nano12193483Search in Google Scholar PubMed PubMed Central
[18] Chaturvedi S, Kataria A, Chaudhary V, Verma A, Jain N, Sanjay MR, et al. Bionanocomposites reinforced with cellulose fibers and agro-industrial wastes. In Cellulose Fibre Reinforced Composites. UK: Woodhead Publishing Limited; 2023. p. 317–42.10.1016/B978-0-323-90125-3.00017-3Search in Google Scholar
[19] Wang J, Han X, Zhang C, Liu K, Duan G. Source of nanocellulose and its application in nanocomposite packaging material: A review. Nanomaterials. 2022;12:18.10.3390/nano12183158Search in Google Scholar PubMed PubMed Central
[20] Owonubi SJ, Agwuncha SC, Malima NM, Shombe GB, Makhatha EM, Revaprasadu N. Non-woody biomass as sources of nanocellulose particles: a review of extraction procedures. Front Energy Res. 2021;9:608825.10.3389/fenrg.2021.608825Search in Google Scholar
[21] Afrin S, Karim Z. Isolation and Surface modification of nanocellulose: necessity of enzymes over chemicals. ChemBioEng Rev. 2017;4(5):289–303.10.1002/cben.201600001Search in Google Scholar
[22] Gao T, Liu Y, Liu R, Zhuang W. Research progress and development of near-infrared phosphors. Materials (Basel). 2023;16(8):1–19.10.3390/ma16083145Search in Google Scholar PubMed PubMed Central
[23] Kataria A, Chaturvedi S, Chaudhary V, Verma A, Jain N, Sanjay MR, et al. Cellulose fiber-reinforced composites – History of evolution, chemistry, and structure. In Cellulose fibre reinforced composites. UK: Woodhead Publishing; 2023. p. 1–22.10.1016/B978-0-323-90125-3.00012-4Search in Google Scholar
[24] Van-Pham DT, Pham TYN, Tran MC, Nguyen CN, Tran-Cong-Miyata Q. Extraction of thermally stable cellulose nanocrystals in short processing time from waste newspaper by conventional acid hydrolysis. Mater Res Express. 2020;7(6):65004. 10.1088/2053-1591/ab9668.Search in Google Scholar
[25] Wu Y. Nanocellulose extraction and surface modification toward active packaging applications. Doctoral dissertation. Columbia: University of Missouri; 2017. p. 1–14.Search in Google Scholar
[26] Abd El-Sayed ES, El-Sakhawy M, El-Sakhawy MAM. Non-wood fibers as raw material for pulp and paper industry. Nord Pulp Pap Res J. 2020;35(2):215–30.10.1515/npprj-2019-0064Search in Google Scholar
[27] Le Gars M. Surface modifications of cellulose nanocrystals for biobased food packaging applications. Doctoral dissertation. Université Grenoble Alpes; 2021.Search in Google Scholar
[28] Rashid ESA, Gul A, Yehya WAH, Julkapli NM. Physico-chemical characteristics of nanocellulose at the variation of catalytic hydrolysis process. Heliyon. 2021;7(6):e07267.10.1016/j.heliyon.2021.e07267Search in Google Scholar PubMed PubMed Central
[29] Low DYS, Supramaniam J, Soottitantawat A, Charinpanitkul T, Tanthapanichakoon W, Tan KW, et al. Recent developments in nanocellulose-reinforced rubber matrix composites: A review. Polymers (Basel). 2021;13(4):1–35.10.3390/polym13040550Search in Google Scholar PubMed PubMed Central
[30] Syafri E, Yulianti E, Asrofi M, Abral H, Sapuan SM, Ilyas RA, et al. Effect of sonication time on the thermal stability, moisture absorption, and biodegradation of water hyacinth (Eichhornia crassipes) nanocellulose-filled bengkuang (Pachyrhizus erosus) starch biocomposites. J Mater Res Technol. 2019;8(6):6223–31.10.1016/j.jmrt.2019.10.016Search in Google Scholar
[31] Kargarzadeh H, Huang J, Lin N, Ahmad I, Mariano M, Dufresne A, et al. Recent developments in nanocellulose-based biodegradable polymers, thermoplastic polymers, and porous nanocomposites. Prog Polym Sci. 2018;87:197–227. 10.1016/j.progpolymsci.2018.07.008.Search in Google Scholar
[32] Boujemaoui A. Surface modification of nanocellulose towards composite applications. Doctoral dissertation. KTH Royal Institute of Technology; 2016.Search in Google Scholar
[33] Thakur V, Guleria A, Kumar S, Sharma S, Singh K. Recent advances in nanocellulose processing, functionalization and applications: A review. Mater Adv. 2021;2(6):1872–95.10.1039/D1MA00049GSearch in Google Scholar
[34] Samarasekara AMPB, Kahavita KDHN, Amarasinghe DAS, Karunanayake L. Fabrication and characterization of nanofibrillated cellulose (NFC) reinforced polymer composite. MERCon 2018 – 4th Int Multidiscip Moratuwa Eng Res Conf; 2018. p. 449–54.10.1109/MERCon.2018.8421934Search in Google Scholar
[35] Yadav C, Saini A, Zhang W, You X, Chauhan I, Mohanty P, et al. Plant-based nanocellulose: A review of routine and recent preparation methods with current progress in its applications as rheology modifier and 3D bioprinting. Int J Biol Macromol. 2021;166:1586–616. 10.1016/j.ijbiomac.2020.11.038.Search in Google Scholar PubMed
[36] Wohlert J, Chen P, Berglund LA, Lo Re G. Acetylation of nanocellulose: miscibility and reinforcement mechanisms in polymer nanocomposites. ACS Nano. 2023;18(3):1882–91.10.1021/acsnano.3c04872Search in Google Scholar PubMed PubMed Central
[37] Kedzior SA, Zoppe JO, Berry RM, Cranston ED. Recent advances and an industrial perspective of cellulose nanocrystal functionalization through polymer grafting. Curr Opin Solid State Mater Sci. 2019;23(2):74–91.10.1016/j.cossms.2018.11.005Search in Google Scholar
[38] Lizundia E, Meaurio E, Vilas JL. Grafting of cellulose nanocrystals. In: Multifunctional polymeric nanocomposites based on cellulosic reinforcements. Vol. 61. New York, USA: William Andrew Publishing; Elsevier Inc; 2016. p. 61–113.10.1016/B978-0-323-44248-0.00003-1Search in Google Scholar
[39] Koschevic MT, dos Santos M, de Faria RC, Fakhouri FM, Martelli SM. Cellulose nanocrystals functionalization by grafting. Biopolymer grafting: Synthesis and properties. UK: Elsevier; 2018. p. 409–39. 10.1016/B978-0-323-48104-5.00009-3.Search in Google Scholar
[40] Mokhena TC, Sefadi JS, Sadiku ER, John MJ, Mochane MJ, Mtibe A. Thermoplastic processing of PLA/cellulose nanomaterials composites. Polym (Basel). 2018;10(12):1363.10.3390/polym10121363Search in Google Scholar PubMed PubMed Central
[41] Földvári M. Handbook of thermogravimetric system of minerals and its use in geological practice. Budapest: Geological Institute of Hungary; 2011.Search in Google Scholar
[42] Loganathan S, Valapa RB, Mishra RK, Pugazhenthi G, Thomas S. Thermogravimetric analysis for characterization of nanomaterials. In Thermal and rheological measurement techniques for nanomaterials characterization. Vol. 3. UK: Elsevier; 2017. p. 67–108.10.1016/B978-0-323-46139-9.00004-9Search in Google Scholar
[43] Kian LK, Jawaid M. Thermal properties of nanocrystalline cellulose and cellulose nanowhisker. Int J Innov Technol Explor Eng. 2019;9(1):5430–4.10.35940/ijitee.A8103.119119Search in Google Scholar
[44] Ullah MW, Manan S, Ul-Islam M, Revin VV, Thomas S, Yang G. Introduction to nanocellulose. In Nanocellulose: Synthesis, structure, properties and applications. Singapore: World Scientific; 2021;1–50.10.1142/9781786349477_0001Search in Google Scholar
[45] Robert B, Brown EB. A review nanocellulose polymer composite characteristics and challenges. 2004;1:1–14.Search in Google Scholar
[46] Mohammed M, Jawad AJ, Afar M, Mohammed AM, Oleiwi JK, Adam T, Osman AF, et al. Challenges and advancement in water absorption of natural fiber-reinforced polymer composites. Polym Test. 2023;124(June):108083. 10.1016/j.polymertesting.2023.108083.Search in Google Scholar
[47] Satha H, Kouadri I, Benachour D. Thermal, structural and morphological studies of cellulose and cellulose nanofibers extracted from bitter watermelon of the cucurbitaceae family. J Polym Environ. 2020;28(7):1914–20. 10.1007/s10924-020-01735-6.Search in Google Scholar
[48] Luz SM, Del Tio J, Rocha GJM, Gonçalves AR, Del’Arco AP. Cellulose and cellulignin from sugarcane bagasse reinforced polypropylene composites: Effect of acetylation on mechanical and thermal properties. Compos Part A Appl Sci Manuf. 2008;39(9):1362–9.10.1016/j.compositesa.2008.04.014Search in Google Scholar
[49] Legrand NBR, Lucien M, Pierre O, Fabien BE, Marcel NP, Jean AA. Physico-chemical and thermal characterization of a lignocellulosic fiber, extracted from the bast of cola lepidota stem. J Min Mater Charact Eng. 2020;08(05):377–92.10.4236/jmmce.2020.85024Search in Google Scholar
[50] Sapuan SM, Norrrahim MNF, Ilyas RA, Soutis C. Industrial applications of nanocellulose and its nanocomposites. Sawston, UK: Woodhead Publishing; 2022. p. 327–33.Search in Google Scholar
[51] Sabaruddin FA, Tahir PM, Sapuan SM, Ilyas RA, Lee SH, Abdan K, et al. The effects of unbleached and bleached nanocellulose on the thermal and flammability of polypropylene-reinforced kenaf core hybrid polymer bionanocomposites. Polymers (Basel). 2021;13(1):1–19.10.3390/polym13010116Search in Google Scholar PubMed PubMed Central
[52] Jiang F, Esker AR, Roman M. Acid-catalyzed and solvolytic desulfation of H2SO4 -hydrolyzed cellulose nanocrystals. 2010;26(5):17919–25.10.1021/la1028405Search in Google Scholar PubMed
[53] Kaur P, Sharma N, Munagala M, Rajkhowa R, Aallardyce B, Shastri Y, et al. Nanocellulose: resources, physio-chemical properties, current uses and future applications. Front Nanotechnol. 2021;3(November):1–17.10.3389/fnano.2021.747329Search in Google Scholar
[54] Eslami H, Tzoganakis C, Mekonnen TH. Surface graft polymerization of lactic acid from the surface of cellulose nanocrystals and applications in chloroprene rubber film composites. Cellulose. 2020;27(9):5267–84. 10.1007/s10570-020-03167-w.Search in Google Scholar
[55] Börjesson M, Sahlin K, Bernin D, Westman G. Increased thermal stability of nanocellulose composites by functionalization of the sulfate groups on cellulose nanocrystals with azetidinium ions. J Appl Polym Sci. 2018;135(10):1–10.10.1002/app.45963Search in Google Scholar
[56] Soni B, Hassan EB, Mahmoud B. Chemical isolation and characterization of different cellulose nanofibers from cotton stalks. Carbohydr Polym. 2015;134:581–9. 10.1016/j.carbpol.2015.08.031.Search in Google Scholar PubMed
[57] Kurniawan TW, Sulistyarti H, Rumhayati B, Sabarudin A. Cellulose nanocrystals (CNCs) and cellulose nanofibers (CNFs) as adsorbents of heavy metal ions. J Chem. 2023;2023:1–36.10.1155/2023/5037027Search in Google Scholar
[58] Santmartí A, Lee K-Y. Crystallinity and thermal stability of nanocellulose. In Nanocellulose and sustainability. CRC Press; 2018, p. 67–86.10.1201/9781351262927-5Search in Google Scholar
[59] Yildirim N, Shaler S. A study on thermal and nanomechanical performance of cellulose nanomaterials (CNs). Mater (Basel). 2017;10(7):718.10.3390/ma10070718Search in Google Scholar PubMed PubMed Central
[60] Chakrabarty A, Teramoto Y. Recent advances in nanocellulose composites with polymers: A guide for choosing partners and how to incorporate them. Polymers. 2018;10(5):517.10.3390/polym10050517Search in Google Scholar PubMed PubMed Central
[61] Yudhanto F, Yudha V, Rochardjo HSB, Budiyantoro C, Khan A, Asiri AM, et al. Physical, mechanical, and thermal properties of polyvinyl alcohol/nanocrystalline cellulose bioplastic film. Int J Eng Trans C Asp. 2024;37(1):94–103.10.5829/IJE.2024.37.01A.09Search in Google Scholar
[62] Arjmandi R, Hassan A, Mohamad Haafiz MK, Zakaria Z. Partial replacement effect of montmorillonite with cellulose nanowhiskers on polylactic acid nanocomposites. Int J Biol Macromol. 2015;81:91–9. 10.1016/j.ijbiomac.2015.07.062.Search in Google Scholar PubMed
[63] Azizi Samir MAS, Alloin F, Sanchez JY, El Kissi N, Dufresne A. Preparation of cellulose whiskers reinforced nanocomposites from an organic medium suspension. Macromolecules. 2004;37(4):1386–93.10.1021/ma030532aSearch in Google Scholar
[64] Khan I, Khan I, Saeed K, Ali N, Zada N, Khan A, et al. Polymer nanocomposites: an overview. In Smart Polymer Nanocomposites. Elsevier; 2023. p. 167–84.10.1016/B978-0-323-91611-0.00017-7Search in Google Scholar
[65] Nurazzi NM, Abdullah N, Norrrahim MNF, Kamarudin SH, Ahmad S, Shazleen SS, et al. Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) of PLA/cellulose composites. In Polylactic acid-based nanocellulose and cellulose composites. CRC Press; 2022, p. 145–64.10.1201/9781003160458-7Search in Google Scholar
[66] Windiastuti E, Bindar Y, Hasanudin U. Identification of potential application of oil palm empty fruit bunches (EFB): a review. IOP Conf Ser Earth Environ Sci. 2022;1063(1):012024.10.1088/1755-1315/1063/1/012024Search in Google Scholar
[67] Raza M, Abu-Jdayil B, Banat F, Al-Marzouqi AH. Isolation and characterization of cellulose nanocrystals from date palm waste. ACS Omega. 2022;7(29):25366–79.10.1021/acsomega.2c02333Search in Google Scholar PubMed PubMed Central
[68] Lee JH, Park SH, Kim SH. Preparation of cellulose nanowhiskers and their reinforcing effect in polylactide. Macromol Res. 2013;21(11):1218–25.10.1007/s13233-013-1160-0Search in Google Scholar
[69] Khoo RZ, Ismail H, Chow WS. Thermal and morphological properties of poly (lactic acid)/nanocellulose nanocomposites. Procedia Chem. 2016;19:788–94. 10.1016/j.proche.2016.03.086.Search in Google Scholar
[70] Bahlouli S, Belaadi A, Makhlouf A, Alshahrani H, Khan MKA, Jawaid M. Effect of fiber loading on thermal properties of cellulosic washingtonia reinforced HDPE biocomposites. Polymers. 2023;15(13):2910.10.3390/polym15132910Search in Google Scholar PubMed PubMed Central
[71] Bharimalla AK, Deshmukh SP, Vigneshwaran N, Patil PG, Prasad V. Nanocellulose-polymer composites for applications in food packaging: current status, future prospects and challenges. Polym – Plast Technol Eng. 2017;56(8):805–23. 10.1080/03602559.2016.1233281.Search in Google Scholar
[72] Morelli CL, Belgacem MN, Branciforti MC, Salon M, Bras J, Bretas RES. Nanocomposites of PBAT and cellulose nanocrystals modified by in situ polymerization and melt extrusion. Polym Eng Sci. 2016;56(12):1339–48.10.1002/pen.24367Search in Google Scholar
[73] de Oliveira AD, Beatrice CA. Polymer nanocomposites with different types of nanofiller. Nanocompos – Recent Evol. 2018;103–4.Search in Google Scholar
[74] Scaffaro R, Botta L, Lopresti F, Maio A, Sutera F. Polysaccharide nanocrystals as fillers for PLA based nanocomposites. Cellulose. 2017;24(2):447–78.10.1007/s10570-016-1143-3Search in Google Scholar
[75] Nagalakshmaiah M. Melt processing of cellulose nanocrystals: thermal, mechanical and rheological properties of polymer nanocomposites. Doctoral dissertation. Université Grenoble Alpes; 2017. https://tel.archives-ouvertes.fr/tel-01533422.Search in Google Scholar
[76] Pinheiro IF, Ferreira FV, Souza DHS, Gouveia RF, Lona LMF, Morales AR, et al. Mechanical, rheological and degradation properties of PBAT nanocomposites reinforced by functionalized cellulose nanocrystals. Eur Polym J. 2017;97:356–65. 10.1016/j.eurpolymj.2017.10.026.Search in Google Scholar
[77] Xu T, Zhang A, Zhao Y, Han Z, Xue L. Crystallization kinetics and morphology of biodegradable poly(lactic acid) with a hydrazide nucleating agent. Polym Test. 2015;45:101–6. 10.1016/j.polymertesting.2015.05.009.Search in Google Scholar
[78] Wang L, Wang YN, Huang ZG, Weng YX. Heat resistance, crystallization behavior, and mechanical properties of polylactide/nucleating agent composites. Mater Des. 2015;66:7–15. 10.1016/j.matdes.2014.10.011.Search in Google Scholar
[79] Yang X, Han F, Xu C, Jiang S, Huang L, Liu L, et al. Effects of preparation methods on the morphology and properties of nanocellulose (NC) extracted from corn husk. Ind Crop Prod. 2017;109(July):241–7. 10.1016/j.indcrop.2017.08.032.Search in Google Scholar
[80] Rastogi S, Verma A. VKS. Experimental response of nonwoven waste cellulose fabric-reinforced epoxy composites for high toughness and coating applications. Mater Perform Charact. 2020;9:151–72.10.1520/MPC20190251Search in Google Scholar
[81] Chen Y, Meng Y, Luo Y, Wang Y. Poly(dl-Lactic Acid)-based linear polyurethanes capable of forming physical crosslinking via UPy quadruple hydrogen bonding assembly. Macromol Mater Eng. 2020;305(7):1–10.10.1002/mame.202000042Search in Google Scholar
[82] Wang H, Wu J, Huang B, Lu QL. One-pot synthesis of UPy-functionalized nanocellulose under mechanochemical synergy for high-performance epoxy nanocomposites. Polymers (Basel). 2022;14(12):2428.10.3390/polym14122428Search in Google Scholar PubMed PubMed Central
[83] Dharmalingam S, Meenakshisundaram O, Elumalai V, Boopathy RS. An investigation on the interfacial adhesion between amine functionalized luffa fiber and epoxy resin and its effect on thermal and mechanical properties of their composites. J Nat Fibers. 2021;18(12):2254–69. 10.1080/15440478.2020.1726238.Search in Google Scholar
[84] Arkles B. Silane coupling agents: connecting across boundaries (Version 3.0). J Organomet Chem. 2014;3(1):1–76. www.gelest.com.Search in Google Scholar
[85] Gupta MK, Srivastava RK. Mechanical, thermal and water absorption properties of hybrid sisal/jute fiber reinforced polymer composite. Indian J Eng Mater Sci. 2016;23(4):231–8.Search in Google Scholar
[86] Wang Q, Yang Z, Feng X, Liu X. Modification of nanocellulose via atom transfer radical polymerization and its reinforcing effect in waterborne UV-curable resin. Int J Biol Macromol . 2023;253(P2):126743. 10.1016/j.ijbiomac.2023.126743.Search in Google Scholar PubMed
[87] Abraham E, Kam D, Nevo Y, Slattegard R, Rivkin A, Lapidot S, et al. Highly modified cellulose nanocrystals and formation of epoxy-nanocrystalline cellulose (CNC) nanocomposites. ACS Appl Mater Interfaces. 2016;8(41):28086–95.10.1021/acsami.6b09852Search in Google Scholar PubMed
[88] Girouard NM, Xu S, Schueneman GT, Shofner ML, Meredith JC. “site-selective modification of cellulose nanocrystals with isophorone diisocyanate and formation of polyurethane-CNC composites. ACS Appl Mater Interfaces. 2016;8(2):1458–67. 10.1021/acsami.5b10723.Search in Google Scholar PubMed
[89] Girouard NM, Xu S, Schueneman GT, Shofner ML, Meredith JC. Site-Selective modification of cellulose nanocrystals with isophorone diisocyanate and formation of polyurethane-CNC composites. ACS Appl Mater Interfaces. 2016;8(2):1458–67.10.1021/acsami.5b10723Search in Google Scholar
[90] Dang X, Cao X, Ke L, Ma Y, An J, Wang F. Combination of cellulose nanofibers and chain-end-functionalized polyethylene and their applications in nanocomposites. J Appl Polym Sci. 2017;134(42):1–11.10.1002/app.45387Search in Google Scholar
[91] Fukuzumi H, Saito T, Okita Y, Isogai A. Thermal stabilization of TEMPO-oxidized cellulose. Polym Degrad Stab. 2010;95(9):1502–8. 10.1016/j.polymdegradstab.2010.06.015.Search in Google Scholar
[92] Manikandan Nair KC, Thomas S, Groeninckx G. Thermal and dynamic mechanical analysis of polystyrene composites reinforced with short sisal fibres. Compos Sci Technol. 2001;61(16):2519–29.10.1016/S0266-3538(01)00170-1Search in Google Scholar
[93] Chanda S, Bajwa DS, Holt GA, Stark N, Bajwa SG, Quadir M. Silane compatibilzation to improve the dispersion, thermal and mechancial properties of cellulose nanocrystals in poly (ethylene oxide). Nanocomposites. 2021;7(1):87–96.10.1080/20550324.2021.1942641Search in Google Scholar
[94] Chenampulli S, Unnikrishnan G, Thomas S, Narine SS. Novel ethylene diamine functionalised nanocellulose/poly(ethylene-co-acrylic acid) composites for biomedical applications. Cellulose. 2019;26(3):1795–809. 10.1007/s10570-018-02227-6.Search in Google Scholar
[95] Wang S, Lin Y, Zhang X, Lu C. Towards mechanically robust cellulose fiber-reinforced polypropylene composites with strong interfacial interaction through dual modification. RSC Adv. 2015;5(63):50660–7.10.1039/C5RA01792KSearch in Google Scholar
[96] Li B, Wu C, Zhang Y, Cao X, Luo Z. Microstructure and thermal and tensile properties of Poly(vinyl alcohol) nanocomposite films reinforced by polyacrylamide grafted cellulose nanocrystals. J Macromol Sci Part B Phys. 2020;59(4):223–34. 10.1080/00222348.2019.1710364.Search in Google Scholar
[97] Arpitha GR, Verma A, MR S, Gorbatyuk S, Khan A, Sobahi TR, et al. Bio-composite film from corn starch based vetiver cellulose. J Nat Fibers. 2022;19(16):14634–44.10.1080/15440478.2022.2068174Search in Google Scholar
[98] Ferreira FV, Pinheiro IF, Gouveia RF, Thim GP, Lona LMF. Functionalized cellulose nanocrystals as reinforcement in biodegradable polymer nanocomposites. Polym Compos. 2018;39:E9–29.10.1002/pc.24583Search in Google Scholar
[99] Rigotti D, Checchetto R, Tarter S, Caretti D, Rizzuto M, Fambri L, et al. Polylactic acid-lauryl functionalized nanocellulose nanocomposites: Microstructural, thermo-mechanical and gas transport properties. Express Polym Lett. 2019;13(10):858–76.10.3144/expresspolymlett.2019.75Search in Google Scholar
[100] Lu J, Sun C, Yang K, Wang K, Jiang Y, Tusiime R, et al. Properties of polylactic acid reinforced by hydroxyapatite modified nanocellulose. Polymers (Basel). 2019;11(6):1–13.10.3390/polym11061009Search in Google Scholar PubMed PubMed Central
[101] Yang Z, Peng H, Wang W, Liu T. Acetylated microfibrillated cellulose as a toughening agent in poly(lactic acid). J Appl Polym Sci. 2010;116(5):2658–67.10.1002/app.31787Search in Google Scholar
[102] Ren Q, Wu M, Wang L, Zheng W, Hikima Y, Semba T, et al. Cellulose nanofiber reinforced poly (lactic acid) with enhanced rheology, crystallization and foaming ability. Carbohydr Polym. 2022;286:1–38.10.1016/j.carbpol.2022.119320Search in Google Scholar PubMed
[103] Oyekanmi AA, Saharudin NI, Hazwan CM, Abdul Khalil HPS, Olaiya NG, Abdullah CK, et al. Improved hydrophobicity of macroalgae biopolymer film incorporated with kenaf derived CNF using silane coupling agent. Molecules. 2021;26(8):2254.10.3390/molecules26082254Search in Google Scholar PubMed PubMed Central
[104] Yin Y, Ma J, Tian X, Jiang X, Wang H, Gao W. Cellulose nanocrystals functionalized with amino-silane and epoxy-poly(ethylene glycol) for reinforcement and flexibilization of poly(lactic acid): material preparation and compatibility mechanism. Cellulose. 2018;25(11):6447–63. 10.1007/s10570-018-2033-7.Search in Google Scholar
[105] Bellani CF, Pollet E, Hebraud A, Pereira FV, Schlatter G, Avérous L, et al. Morphological, thermal, and mechanical properties of poly(ε-caprolactone)/poly(ε-caprolactone)-grafted-cellulose nanocrystals mats produced by electrospinning. J Appl Polym Sci. 2016;133:21.10.1002/app.43445Search in Google Scholar
[106] Yu HY, Qin ZY, Yan CF, Yao JM. Green nanocomposites based on functionalized cellulose nanocrystals: A study on the relationship between interfacial interaction and property enhancement. ACS Sustain Chem Eng. 2014;2(4):875–86.10.1021/sc400499gSearch in Google Scholar
[107] Hasnain MS, Nayak AK. Alginate-inorganic composite particles as sustained drug delivery matrices. In Applications of nanocomposite materials in drug delivery. Woodhead publishing; 2018. p. 39–74. 10.1016/B978-0-12-813741-3.00003-0.Search in Google Scholar
[108] Xu X, Ma X, Li D, Dong J. Toward sustainable biocomposites based on MMT and PHBH reinforced with acetylated cellulose nanocrystals. Cellulose. 2021;28(5):2981–93. 10.1007/s10570-021-03735-8.Search in Google Scholar
[109] Zhang X, Ma P, Zhang Y. Structure and properties of surface-acetylated cellulose nanocrystal/poly(butylene adipate-co-terephthalate) composites. Polym Bull. 2016;73(7):2073–85.10.1007/s00289-015-1594-ySearch in Google Scholar
[110] Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017;3(7):e1700782. 10.1126/sciadv.1700782.Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy

