Abstact
Copper oxide nanoparticles (CuO NPs) have wide range of application in many fields of industry, agriculture, cosmetics, and health care with probable risk to human health. The present study was conducted to find the chronic toxicity of these nanoparticles in the vital organs. Male healthy Wister Albino rats were subjected on daily bases to 35 intraperitoneal administration of 25 nm CuO NPs (2 mg/kg). All animals were subjected to morphological, histological, histochemical, and ultrastructural examinations. Exposure to CuO NPs induced reduction in body weight gain, urine retention, back arching, and renal calculi formation. The renal tissues demonstrated tubular hydropic degeneration, glomerular hypercellularity, blood vessels congestion and dilatation, renal interstitial oedema, mitochondrial injury, and lysosomal hypertrophy. Conversely, the liver displayed hepatocyte insultation, sinusoidal dilatation, Kupffer cells hyperplasia, inflammatory cell infiltration, hepatocytes mitochondrial cristolysis, mitochondrial swelling, lysosomal hyperplasia, and nuclear alterations. Furthermore, the cardiac tissues demonstrated congestion, cardiocytes disarray, and disorganization. In addition, the neural tissue exhibited Purkinje cells degeneration and cerebral cortex spongiosis. The results suggest that CuO nanomaterials engage with the vital organs’ components, potentially leading to alterations that could affect the organs function. Further research is encouraged for better understanding the mechanisms involved in pathogenesis of CuO NPs.
1 Introduction
Copper oxide nanoparticles (CuO NPs) have been utilized across various sectors, including antimicrobial agents, lubricants, anti-tumor, lubricants for textiles and metallic coatings, osteoporosis treatment, livestock feed additives, scientific and medical imaging, cosmetics, electronics, food industry, and drug delivery [1,2]. These nanomaterials have great catalytic activities and can be applied to biosensors and electrochemical sensors. The investment of CuO NPs and their nanocomposite is increasing with prediction of nano-copper global production to exceed 1,650 tons by 2025 [3].
Nano-copper can enter the body through various exposure routes including inhalation, dermal contact, and ingestion. Some investigations showed that kidney, spleen, and liver are the main target organs for the toxic CuO nanomaterials [4]. Other reports indicated that CuO NPs could decrease the plasma level of catalase, superoxide dismutase, and glutathione but increased malondialdehyde production [5,6]. Furthermore, CuO nanomaterials could cause histological changes such as apoptosis, inflammation, lipid and energy metabolism dysfunction, genotoxicity, oxidative stress, and mitochondrial damage [7–9]. CuO NPs produce reactive oxygen species (ROS) along with antioxidant defense system blockage leading to the oxidative damage and cell death [10]. In addition, CuO NPs can block the cell antioxidant defense [11]. However, the genotoxicity of CuO NPs is dependent upon several factors such as shape, charge, size, dose, and duration of exposure to these nanoparticles [12].
Studies revealed that liver and kidneys are targets for CuO NPs toxicity and subjection to these nanomaterials could cause marked degenerative changes in the renal tissues [3,4]. Moreover, treatment of Hep-2 cell line with copper oxide nanomaterials could induce oxidative damages to these cells and significantly increase of superoxide dismutase activity and level of thiobarbituric acid-reactive substances but reduce catalase activity [13,14]. Few studies explored the potential impacts of CuO NPs on the vital organs. Accordingly, the current investigation was conducted to find out the morphological, histological, histochemical, and ultrastructural alterations in the vital organs that might be induced by chronic exposure to 25 nm nano-CuO.
2 Materials and methods
2.1 Animals and conditions
Eighteen healthy adult male Wistar albino rats (Rattus norvegicus) were procured from Jerash University’s colony. These rats aged 16 weeks and had an average body weight ranging from 200 to 215 g. They were randomly divided into two groups: a control group and a CuO NPs-treated group, each comprising nine rats. Both groups of rats understudy a 7-day acclimatization period and were housed in conventional rat cages (measuring 48.0 × 26.5 × 21.0 cm) under controlled environmental conditions of 24 ± 1°C and 35% humidity, with a 12 h light/12 h dark cycle. The animals had continuous access to standard rodent laboratory diet provided by Jordan Feed Company (Amman, Jordan) and tap water sourced from Jerash University’s animal house.
2.2 CuO NPs
CuO NPs in the form of nanopowder were procured from Sigma (Aldrich, USA) for use in the current study. The manufacturer’s certificate provided the following specifications: a diameter of less than 50 nm, with approximately 82% falling within the range of 20 ± 5 nm and an average size of 25 nm. The nanoparticles also possessed a surface area of 29 m2/g, a boiling point of 2,567°C, and a metal content of 99.99%. The nanoparticle size was verified using transmission electron microscopy (TEM) technique.
2.3 Experimental protocol
A fresh suspension of CuO NPs was subjected to ultrasonication after being diluted in deionized water enriched with 0.1 mM sodium citrate. All NPs solutions were meticulously prepared to facilitate intraperitoneal (IP) administration, which is considered an optimal route for absorption into the circulatory system. These doses were administered in a volume of 0.4 mL. In the experimental group, each rat received 35 IP injections of CuO NPs at a dosage of 2 mg/kg, once per day, with injections given five times per week. The control group rats received a daily dose equivalent in volume to the vehicle solution, consisting of deionized water containing 0.1 mM sodium citrate, warmed to 37°C, using the same injection procedure. The choice of the dose (2 mg/kg) was based on previous in vivo studies investigating the toxicity of CuO NPs [15,16]. Before injection, each animal was securely restrained in a head-down position, with the injection site located in the lower right quadrant of the abdomen to prevent injury to internal organs.
After a 24 h interval following the last injection, the rats were anesthetized via the IP route using a cocktail of ketamine and xylazine at a dosage of 80/8 mg/kg, dissolved in normal saline. They were then dissected to extract vital organs for analysis. Throughout the study, all rats were closely monitored for their responses to CuO NPs exposure, overall health, body weight changes, morbidity, and mortality.
All animals under study were treated and all experimental works were practiced according to the guide of Canadian Council on Animal Care which is followed by Jerash University [17]. In addition, the ethical committee of Jerash University (reference number 3/1/2023/2024) approved the study protocol.
2.3.1 Morphometric observations
All animal understudies were observed for the mortality rate, general wellbeing, behavior patterns, food consumption, water intake, body weight gain, and organs indices. In addition, the treated rats’ body weight, average food consumption, and water intake were monitored weekly during the period of treatment. The chemical content of the demonstrated renal calculi was determined using a Fourier Transform Infrared spectrometer (FTIR; BRUKER Vertex 80, Switzerland) in a (5,000–500) cm−1 wavenumber range.
2.3.2 Histological processing
Tissue samples were collected from the liver, kidney, heart, and brain of all the rats. These samples were initially fixed in a 10% neutral buffered formalin solution. Subsequently, they underwent a dehydration process with a series of gradually increasing concentrations of ethyl alcohol. Following dehydration, the samples were cleared using chloroform, impregnated, embedded, and encased in paraffin wax with a melting point of 58°C. The resulting histological sections, measuring 4–5 µm in thickness, were then subjected to staining with both hematoxylin and eosin (H&E) as well as Mallory trichrome stains [18].
2.3.3 Histochemical processing
Histological sections from all the rats, prepared in parallel with paraffin embedding, were subjected to the histochemical stain: the Periodic acid-Schiff (PAS) reaction, Masson trichrome, and methyl bromophenol blue stain [19].
2.3.4 TEM processing
Tissue blocks from the liver and kidneys of two rats from each group were used to find out the hepatic and renal tissues ultrastructural alterations according to the protocol described by Hayat [20].
2.4 Statistical analysis
Data from the morphometric investigation underwent rigorous statistical analysis, employing student’s t-test. Statistical significance was determined with a threshold of P values <0.05. The analysis was conducted using the Statistical Package for the Social Sciences software (IBM Analytics in the USA).
3 Results
3.1 Body weight gain alteration
After subjection to 35 IP injections of CuO NPs, a change in the body weight gain was demonstrated (Table 1). In comparison with control rats, the treated ones showed nonsignificant body weight gain (P value = 0.068). Control rats had a significant normal weight gain (P value = 0.035) during the treatment period.
Alterations on the average body weight (g) of rats exposed to 35 injections of CuO NPs
| Groups | Starting weight | Weight after 35 injections of CuO NPs | P-value |
|---|---|---|---|
| Control rats | 179 ± 12 | 201 ± 17 | 0.035* |
| CuO NPs-treated rats | 182 ± 15 | 191 ± 8 | 0.068 |
*Indicates statistically significant (P-value ≤ 0.05) under student’s t-test.
3.2 Morphometric alterations
No mortality occurred in CuO NPs-treated rats during the study. However, CuO NPs-treated rats exhibited abdominal ballooning, back arching, nose oozing of pinkish fluid during injection time, testicular enlargement, bloody urination, and urinary bladder congestion. In addition, gross examination with the bare eye and dissecting lenses of the internal organs of each dissected rat under study demonstrated that CuO NPs-treated rats showed lung abscess mainly in the left lung, seminal vesicles enlargement returned to normal after urinary bladder ruptured and urinary bladder ballooning.
Eight out of 18 (44.4%) rats that were exposed to CuO NPs demonstrated renal calculi (Figure 1). The largest calculus had a weight of 1.27 g with the smallest of 1.13 g. The average weight of the control kidney was 1.38 g. FTIR analysis confirmed that the renal calculi contained a mixture of calcium phosphate, uric acid, calcium oxalate monohydrate, and cystine.

Light photographs of renal calculi induced in rats subjected to 35 injections of CuO NPs.
3.3 Histological alterations
The following histological, histochemical, and ultrastructural alterations were detected in all rats exposed to CuO NPs.
3.3.1 Renal histological alterations
Compared with the control kidneys, the renal tissues of the rats that are subjected to 25 nm CuO NPs demonstrated inflammatory cells inflammation, glomerular atrophy, glomerular fragmentation, renal tubules necrosis, renal tubules hydropic degeneration, fibrocytes proliferation, hyaline casts formation, renal oedema, and renal congestion (Figure 2a–j). No indication of glomerular or interstitial fibrosis was detected.
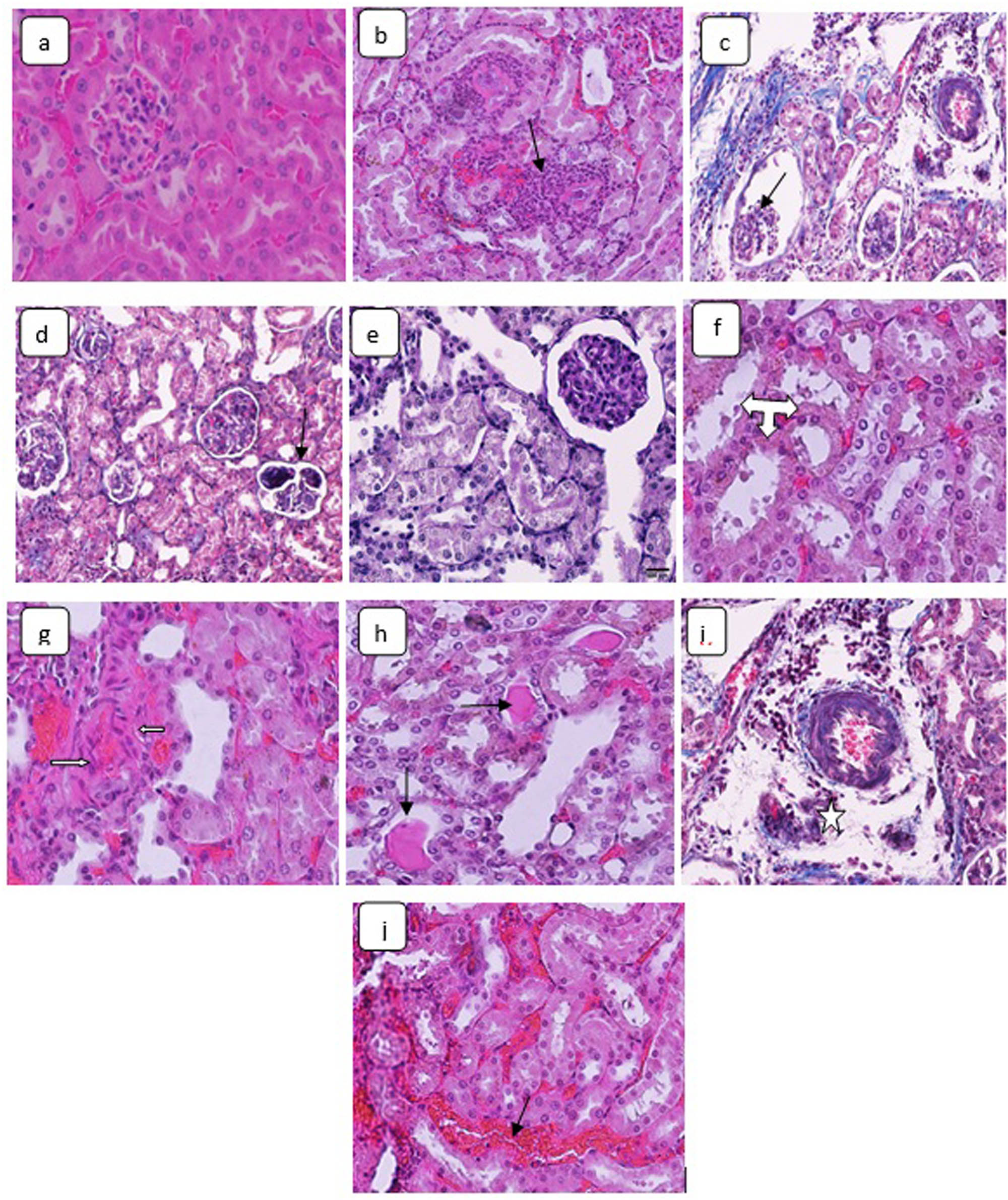
H&E stained sections in the kidneys demonstrating (a) control animal renal tissue, ×160. Kidney sections of CuO NPs-treated rats showing: (b) inflammatory cells inflammation (arrow), ×160; (c) glomerular atrophy (arrow), ×160; (d) glomerular fragmentation (arrow), ×160; (e) tubules necrosis, ×320; (f) tubules hydropic degeneration (triple headed arrow), ×480; (g) fibrocytes proliferation (arrows), ×160; (h) hyaline casts precipitation (arrows), ×160; (i) interstitial edema (star), ×160; and (j) renal congestion (arrow), ×160.
3.3.2 Renal cells ultrastructural changes
The renal cells of animals subjected to CuO NPs demonstrated glomerular alterations and mitochondrial cristolysis and swelling, cytoplasmic vacuolization, lysosomal activity, apoptosis, dilatation of the endoplasmic reticulum, nuclear deformity, chromatin dissolution, and thickening of the basement membrane (Figure 3a and b).

TEM micrographs of renal cells from (a) control rat showing normal euchromatin, nuclear envelop consistency (arrow) with prominent normal mitochondria (cloud, ×8,000). (b) CuO NPs-treated rat showing mitochondrial crystolysis and swelling (arrow-left right), chromatin dissolution (star) basement membrane thickening (arrow), and lysosomal hypertrophy, ×8,000.
3.3.3 Hepatic histological alterations
Chronic subjection to CuO NPs caused the following histological hepatic alterations: hepatocytes hydropic degeneration, sinusoidal dilatation, Kupffer cells hyperplasia, and inflammatory cells infiltration (Figure 4a–e). No hepatic fibrosis or cirrhosis were detected in the liver of all CuO NPs-treated rats.
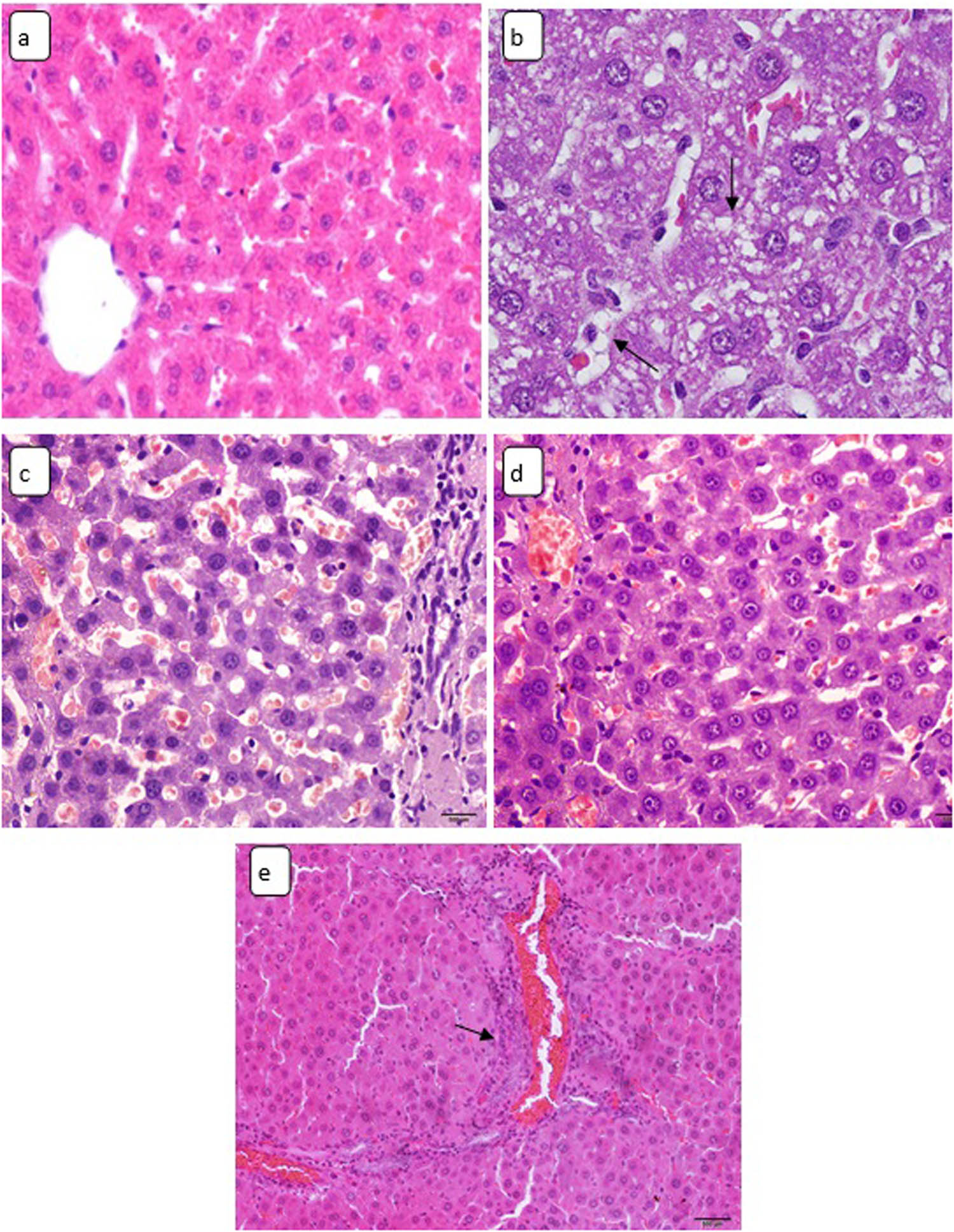
Light micrographs of hepatic tissue stained by H&E stain showing (a) control rat liver, ×180. Sections in the hepatic tissue of CuO NPs-treated animals demonstrating: (b) hydropic degeneration (arrows), ×400; (c) sinusoidal dilatation, ×180; (d) Kupffer cells enlargement, ×180; and (e) inflammatory cells infiltration (arrow), ×128.
3.3.4 Hepatocytes ultrastructural alterations
The hepatic cells of nano-copper-treated rats demonstrated mitochondrial crystolysis, swelling and matrix lysis, lysosomal hyperplasia, fat droplets precipitation, phagocytized bodies, and nuclear envelope irregularity (Figure 5a and b).

TEM micrographs of hepatic cells from (a) control rat showing normal euchromatin with numerous mitochondria. Note that the nuclear envelope is consistent (white arrow), ×5,000. (b) CuO NPs-treated rat demonstrating mitochondrial (m) swelling (stars), crystolysis and matrix lysis, fat droplets (cloud), and nuclear envelope irregularity (white arrow: up), ×20,000.
3.3.5 Hepatocytes glycogen depletion
By investing PAS stain, the hepatocytes of CuO NPs-treated rats showed depletion of glycogen mainly in the degenerative hepatic cells (Figure 6a and b).

Light micrographs of sections stained with PAS reaction in the hepatic tissue showing: (a) control rat hepatic cell with normal glycogen content, ×128 and (b) CuO NPs-treated rats showing partial glycogen depletion, ×128.
3.3.6 Cardiac histological alterations
The cardiac tissue of the CuO NPs-treated rats that were subjected to 35 IP injections showed cardiac congestion, cardiocytes disarray and disorganization, and occasional hydropic degeneration (Figure 7a–d). No cardiac fibrosis was observed in the heart of all rats under study.

Light micrographs of H&E-stained demonstrating: (a) control rat cardiac tissues, ×320; (b) cardiac congestion (star), CuO NPs-treated rats, ×180; (c) cardiocytes disarray and fibers disorganization, CuO NPs-treated rats, ×180; and (d) occasional hydropic degeneration (arrow), ×320.
3.3.7 Neural histological alterations
In comparison with the neural tissue of the control brains, the brain of CuO NPs-treated rats demonstrated necrotic Purkinje cells, occasional cerebral cortex spongiosis characterized by neural loss, and swelling (Figure 8a–c).

Light micrograph sections in the brain of (a) control rat cerebral cortex with normal pyramidal cells (arrows), normal glial cells (triple heads arrow), and normal neuropil (stars), H&E stain ×180. Brain sections of CuO NPs-treated rats showing: (b) necrotic Purkinje cells with normal granular cell layer (arrows), H&E stain ×320 and (c) neural spongiosis (arrow), methyl bromophenol blue stain, ×400.
4 Discussion
The results of the present study indicate that exposure to CuO nanomaterials could cause body weight gain reduction. This might be related to the impacts of nano-copper on the oxidative metabolic enzymes [21]. Moreover, back arching among CuO NPs-treated rats may indicate signs of pain associated with contraction of the abdominal muscles and abdominal pressing [22–24]. The seen urine retention might be associated to urethra blockage by precipitate from the formed calculi or from sloughing the renal epithelial lining. Furthermore, the FTIR analysis indicated that the induced calculi were devoid of copper compounds suggesting that the calculi formation resulted from disturbance on the body metabolism. Several reports demonstrated that the formation of renal calculi involves multiple metabolism-related factors [25]. Literature is devoid of any indications for renal calculi formation due to any kind of nanoparticles or nanostructures.
It is revealed that chronic exposure to CuO nanomaterials induced degenerative alterations in the renal cells, glomeruli, and renal interstitial tissues of the rats. This might be resulted from the interaction of CuO NPs with renal cell proteins forming corona [26]. In addition, the exhibited renal cells hydropic degeneration might be resulted from intracellular and interstitial fluid imbalance resulted from subjection to CuO NPs [27]. Moreover, the renal tissues showed degenerative changes mainly in the proximal convoluted tubules. This injury may indicate the greater viability of CuO NPs in the proximal tubules which are the primary sites of filtrate reabsorption. Furthermore, CuO NPs induced damage in the glomeruli and hyaline casts formation that may affect ions pump across the renal tubules [28].
Nano-copper induced liver injury indicating hepatic tissue susceptible to CuO NPs toxicity affecting liver function as site of detoxification [29]. The induced Kupffer cells hyperplasia may indicate that these nanomaterials could activate phagocytosis as a defense tool for detoxification [30]. Moreover, the sinusoidal dilatation may indicate sinusoids endothelia injury. In addition, the hepatocytes demonstrated partial glycogen depletion indicating an impact of CuO NPs on glycogenesis and glycolysis.
The renal and hepatic cells of animals subjected to CuO nanomaterials exposure show inflammatory cells. This injury may suggest the ability of these nanomaterials to interact with the hepatic and renal tissue components. Nanoparticles including CuO ones have almost similar measurements of the cellular macromolecules with possible interaction with these structures generating free radicals [31,32].
The induced cardiac alterations may reveal that cardic muscle fibers are affected by subjection to CuO NPs. The induced cardiocytes disoragnization may reveal cardiac degeneration, indicating cardiomyopathy [33,34]. Furthermore, the induced cardiocytes disarray might be associated with heart rate reduction [35].
Purkinje cells are specific to cerebellar cortex, play an essential role in movement coordination, cognition, and emotion [36]. Subjection to CuO NPs induced Purkinje cells degeneration indicating neurodegenerative cerebellar disorder [37]. Moreover, the seen spongiform degeneration in the cerebral cortex indicates brain atrophy associated with dementia resulted from loss of connection between neurons [38]. These alterations suggest an effect to CuO nanomaterials on the structure and function of the brain.
Collectively, CuO nanomaterials are found to be toxic and can cause morphological, histochemical, and histological changes in the ultrastructure of the vital organs. These results are congruent to some previous studies [39,40]. The induced injury by nano-copper might be associated to the ability of these nanomaterials to have long circulating residue with the bioavailability of the reactive surface area of these nanoparticles and high dissolution leading for intracellular ROS generation [41,42].
5 Conclusion
CuO nanomaterials could induce hepatic, renal, cardiac, and neural changes that may alter the structure and physiology of these vital organs. These alterations might be resulted from the induced oxidative stress and variable toxicokinetics by CuO NPs, causing injury to the vital organ tissues. It is suggested that there is a need to put more efforts to find out the pathogenesis and toxicity of the nano-copper materials on human and the ecosystems.
Acknowledgments
The authors are grateful to Jerash University and Isra University for putting lab facilities under their disposal.
-
Funding information: The present project was supported by researchers supporting project number (RSPD2024R900) King Saud University, Riyadh, Saudi Arabia and by the Deanship of Scientific Research at King Khalid University, Abha, KSA through the research project (Grant number R.G.P.2/8/45).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Bo S, Durazzo M, Gambino R, Berutti C, Milanesio N, Caropreso A, et al. Associations of dietary and serum copper with inflammation, oxidative stress, and metabolic variables in adults. J Nutr. 2008;138(2):305–10. 10.1093/jn/138.2.305.Search in Google Scholar PubMed
[2] Chakraborty N, Banerjee J, Chakraborty P, Banerjee A, Chanda S, Ray K, et al. Green synthesis of copper/copper oxide nanoparticles and their applications: a review. Green Chem Lett Rev. 2022;185–213. org/10.1080/17518253.2022.2025916.Search in Google Scholar
[3] Privalova LI, Katsnelson BA, Loginova NV, Gurvich VB, Shur VY, Valamina IE, et al. Subchronic toxicity of copper oxide nanoparticles and its attenuation with the help of a combination of bioprotectors. Int J Mol Sci. 2014;15(7):12379–406. 10.3390/ijms150712379.Search in Google Scholar PubMed PubMed Central
[4] Lee IC, Ko JW, Park SH, Shin NR, Shin IS, Moon C, et al. Comparative toxicity and biodistribution assessments in rats following subchronic oral exposure to copper nanoparticles and microparticles. Part Fibre Toxicol. 2016;13(1):56. 10.1186/s12989-016-0169-x.Search in Google Scholar PubMed PubMed Central
[5] Anreddy RN. Copper oxide nanoparticles induce oxidative stress and liver toxicity in rats following oral exposure. Toxicol Rep. 2018;5:903–4. 10.1016/j.toxrep.2018.08.022.Search in Google Scholar PubMed PubMed Central
[6] Liu H, Lai W, Liu X, Yang H, Fang Y, Tian L, et al. Exposure to copper oxide nanoparticles triggers oxidative stress and endoplasmic reticulum (ER)-stress induced toxicology and apoptosis in male rat liver and BRL-3A cell. J Hazard Mater. 2021;401(5):123349. 10.1016/j.jhazmat.2020.123349.Search in Google Scholar PubMed
[7] Lei R, Yang B, Wu C, Liao M, Ding R, Wang Q. Mitochondrial dysfunction and oxidative damage in the liver and kidney of rats following exposure to copper nanoparticles for five consecutive days. Toxicol Res. 2015;2:351–64. 10.1039/c4tx00156g.Search in Google Scholar
[8] Sarkar A, Sarkar D, Poddar K. Nanotoxicity: sources and effects on environment. In: Prasad R, editor. Microbial nanobionics. Cham: Springer; 2019. p. 169–79.10.1007/978-3-030-16534-5_9Search in Google Scholar
[9] Liao M, Liu H. Gene expression profiling of nephrotoxicity from copper nanoparticles in rats after repeated oral administration. Environ Toxicol Pharmacol. 2012;34(1):67–80. 10.1016/j.etap.2011.05.014.Search in Google Scholar PubMed
[10] Arafa AF, Ghanem HZ, Soliman MS, Emad EM. Modulation effects of quercetin against copper oxide nanoparticles-induced liver toxicity in rats. Egypt Pharm J. 2017;16(2):78. 10.4103/epj.epj_15-17.Search in Google Scholar
[11] Sandhu NS, Chopra D, Kaur S. Amelioration of paracetamol induced hepatotoxicity by a protein isolated from the leaves of the herb Cajanus acutifolius Linn. Int J Pharm Sci. 2010;2(3):75–80.Search in Google Scholar
[12] Choi SJ, Oh JM, Choy JH. Toxicological effects of inorganic nanoparticles on human lung cancer A549 cells. J Inorg Bioch. 2009;103(3):463–71. 10.1016/j.jinorgbio.2008.12.017.Search in Google Scholar PubMed
[13] Park S, Lee YK, Jung M, Kim KH, Chung N, Ahn EK, et al. Cellular toxicity of various inhalable metal nanoparticles on human alveolar epithelial cells. Inhal Toxicol. 2007;19(Suppl 1):59–65. 10.1080/08958370701493282.Search in Google Scholar PubMed
[14] Fatahian-Dehkordi RA, Reaisi M, Heidarnejad MS, Mohebbi A. Serum biochemical status and morphological changes in mice ovary associated with copper oxide nanoparticles after thiamine therapy. J Herbmed Pharmacol. 2017;6:doaj.org/article/578d2def8bb94d52ab4a720b27b5defe.Search in Google Scholar
[15] Nair AB, Jacob SA. Simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. 10.4103/0976-0105.177703.Search in Google Scholar PubMed PubMed Central
[16] Naz S, Gul A, Zia M. Toxicity of copper oxide nanoparticles: a review study. IET Nanobiotechnol. 2020;14(1):1–13. 10.1049/iet-nbt.2019.0176.Search in Google Scholar PubMed PubMed Central
[17] Canadian Council on Animal Care (CCAC). CCAC guidelines: Mice. Ontario: CCPA; 2019.Search in Google Scholar
[18] Bancroft JD, Gamble M. Theory and practice of histological techniques. 6th edn. London: Churchill-Livingstone; 2008.Search in Google Scholar
[19] Jarrar B, Taib N. Histocytotechnology. 3rd edn. Riyadh: King Saud University Press; 2018.Search in Google Scholar
[20] Hayat M. 2000. Principles and techniques of electron microscopy, biological applications. 4th edn. London: Cambridge University Press; 2018. p. 371–4.Search in Google Scholar
[21] Aniagu SO, Nwinyi FC, Akumka DD, Ajoku GA, Dzarma S, Izebe KS, et al. Toxicity studies in rats fed nature cure bitters. Afr J Biotechnol. 2005;4(1):72–8, http://www.academicjournals.org/AJB.Search in Google Scholar
[22] Gondim FA, Rodrigues CL, Lopes AC Jr, Leal PR, Camurça FL, Freire CC, et al. Effect of preinjury large bowel emptying on the inhibition of upper gastrointestinal motility after spinal cord injury in rats. Dig Dis Sci. 2003;48:1713–8. 10.1023/A:1025482609323.Search in Google Scholar PubMed
[23] Katos AM, Conti ML, Moran TS, Gordon RK, Doctor BP, Sciuto AM, et al. Abdominal bloating and irritable bowel syndrome like symptoms following microinstillation inhalation exposure to chemical warfare nerve agent VX in guinea pigs. Toxicol Ind Health. 2007;4:231–40. 10.1177/0748233707081720.Search in Google Scholar PubMed
[24] Turner PV, Pang DS, Lofgren JL. A review of pain assessment methods in laboratory rodents. Comp Med. 2019;69(6):451–67. 10.30802/AALAS-CM-19-000042.Search in Google Scholar PubMed PubMed Central
[25] Xu Z, Yao X, Duan C, Liu H, Xu H. Metabolic changes in kidney stone disease. Front Immunol. 2023;14:14. 10.3389/fimmu.2023.1142207.Search in Google Scholar PubMed PubMed Central
[26] Durán N, Silveira CP, Durán M, Martinez DS. Silver nanoparticle protein corona and toxicity: a mini-review. J Nanobiotechnol. 2015;13:55. 10.1186/s12951-015-0114-4.Search in Google Scholar PubMed PubMed Central
[27] Schrand AM, Rahman MF, Hussain SM, Schlager JJ, Smith DA, Syed AF. Metal based nanoparticles and their toxicity assessment. Nanomed Nanobiotechnol. 2010;2:544–68. 10.1002/wnan.103.Search in Google Scholar PubMed
[28] Jarrar BM. Histological and histochemical alterations in the kidney induced by lead. Ann Saudi Med. 2003;23(1–2):10–5. 10.5144/0256-4947.2003.10.Search in Google Scholar PubMed
[29] Singh A, Bhat T, Sharma T. Clinical biochemistry and hepatotoxicity. J Clin Toxicol. 2011;4:11–8. 10.4172/2161-0495.S4-001.Search in Google Scholar
[30] Kegel V, Pfeiffer E, Burkhardt B, Liu JL, Zeilinger K, Nüssler AK, et al. Subtoxic concentrations of hepatotoxic drugs lead to Kupffer cell activation in a human in vitro liver model: an approach to study DILI. Mediat Inflamm. 2015;2015:640631. 10.1155/2015/640631.Search in Google Scholar PubMed PubMed Central
[31] Johar D, Roth JC, Bay GH, Walker JN, Kroczak TJ, Los M. Inflammatory response, reactive oxygen species, programmed (necrotic-like and apoptotic) cell death and cancer. Rocz Akad Med Bialymst. 2004;49:31–9.Search in Google Scholar
[32] Turtle GR. Size and surface area dependent toxicity of silver nanoparticles in zebrafish embryo (Danio rerio). Master Thesis in toxicology submitted to Oregon state University. USA: 2012.Search in Google Scholar
[33] Zhao H, Wald J, Palmer M, Han Y. Hydroxychloroquine-induced cardiomyopathy and heart failure in twins. J Thorac Dis. 2018;10:E70–3. 10.21037/jtd.2017.12.66.Search in Google Scholar PubMed PubMed Central
[34] Al-Doaiss A, Jarrar B, Shati A, Al-Kahtani M, Alfaifi M. Cardiac and testicular alterations induced by acute exposure to titanium dioxide nanoparticles: histopathological study. IET Nanobiotechnol. 2021;15:58–67. 10.1049/nbt2.12000.Search in Google Scholar PubMed PubMed Central
[35] Capel RA, Herring N, Kalla M, Yavari A, Mirams GR, Douglas G, et al. Hydroxychloroquine reduces heart rate by modulating the hyperpolarization-activated current If: novel electrophysiological insights and therapeutic potential. Heart Rhythm. 2015;12:2186–94. 10.1016/j.hrthm.2015.05.027.Search in Google Scholar PubMed PubMed Central
[36] Paul MS, Limaiem F. Histology, Purkinje cells. Treasure Island: StatPearls Publishing; 2023.Search in Google Scholar
[37] Erekat NS. Programmed cell death in cerebellar Purkinje neurons. J Integr Neurosci. 2022;28(211):30. 10.31083/j.jin2101030.Search in Google Scholar PubMed
[38] Kashiwazaki H, Nomura R, Matsuyama S, Taguchi F, Watanabe R. Spongiform degeneration induced by neuropathogenic murine coronavirus infection. Pathol Int. 2011;61(4):184–91. 10.1111/j.1440-1827.2010.02639.x.Search in Google Scholar PubMed PubMed Central
[39] Al-Ruwaili M, Jarrar B, Jarrar Q, Al-Doaiss A, Alshehri M, Melhem W. Renal ultrastructural damage induced by chronic exposure to copper oxide nanomaterials: electron microscopy study. Toxicol Ind Health. 2022;38(2):80–91. 10.1177/07482337211062674.Search in Google Scholar PubMed
[40] Jarrar Q, Almansour M, Jarrar B, Al-Doaiss A, Shati A. Hepatic ultrastructural alterations induced by the toxicity of copper oxide nanoparticles: in vivo electron microscopy study. Toxicol Ind Health. 2023;39(11):1–18. 10.1177/07482337231205921.Search in Google Scholar PubMed
[41] Johnston HJ, Hutchison G, Christensen FM, Peters S, Hankin S, Stone V. A review of the in vivo and in vitro toxicity of silver and gold particulates: particle attributes and biological mechanisms responsible for the observed toxicity. Crit Rev Toxicol. 2010;40:328–46. 10.3109/10408440903453074.Search in Google Scholar PubMed
[42] Ma R, Levard C, Marinakos SM, Cheng Y, Liu J, Michel FM, et al. Size-controlled dissolution of organic-coated silver nanoparticles. Environ Sci Technol. 2012;46:752–9. 10.1021/es201686j.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy


