Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
-
Samar Moustafa
Abstract
In this study, V2O5, 5ZnO/10V2O5, and ZnO, 10ZnO/10V2O5 nanocomposites were synthesized by the sol–gel method. The sol–gel technique is an important process for the fabrication of advanced oxide materials with desirable catalytic, optical, and structural properties. The varieties and flexibilities of sol–gel techniques help in preparing materials with extremely specific properties. For the presented samples, three types of phases were assessed. The average crystalline size of V2O5, 5ZnO/10V2O5 and ZnO, 10ZnO/10V2O5 nanocomposites were found to be 25, 26, 14.5, and 15.5 nm, respectively. SEM images showed three different shapes of semi-tube, semi-spherical, and semi-flower. The pure samples of V2O5 and ZnO showed semi-tube shapes. 5ZnO/10V2O5 shows a spherical shape with average dimeter of 0.6 µm. Strong dependence of the direct optical band gap was observed on different compositions that varied within the range of (2.33–2.73 eV). Conversely, the indirect values varied within the range of 2.119–2.35 eV. On the other hand, 10ZnO/10V2O5 has semi flower shape with different layers. Optical parameters, such as optical band gap, extension coefficient, tails of localized states, and refractive index, were gauged for these nanocomposites. In addition, the mean refractive index of ZnO is lower than that of V2O5, with differences observed between 5ZnO/10V2O5 and 10ZnO/10V2O5 nanocomposites.
1 Introduction
Nanocrystalline metal oxides with uniform shapes and sizes are considered one of the main building blocks of nanotechnology owing to their high surface area, surface chemistry, and intrinsic optical and catalytic characteristics [1,2]. In addition, these metal oxides offer advantageous electronic and optical properties by combining different properties of their constituent materials. The electronic structures, charge transportation characteristics, and light absorption properties are the key aspects of metal oxides that determine their prowess as photocatalysts. A photocatalyst is a material that can absorb light and produce electron/hole pairs (e−/h+), thus enabling chemical transformations of reactants and regenerating its chemical composition after each cycle of such interactions. The main features of a photocatalytic system are wide optical band gap energy (E g), suitable morphology, high surface area, excellent stability, and reusability.
Recently, photocatalysts garnered a lot of attention from scientists because of their appealing applications toward the advantage of humanity. Among these, vanadium-based oxides, especially vanadium pentoxide (V2O5), are broadly considered due to their exceptional properties [3]. Due to its promising and remarkable optoelectronic properties [4], gas sensors, storage systems, laser scanners, thermochromic coatings, solar cells, optical fiber switching devices, batteries, IR detectors, and ultrafast switching applications [5,6,7,8,9,10,11,12,13] are applicable to V2O5. ZnO is an n-type semiconductor material with a wide E g of 3.37 eV at 300 K and a large exciton binding energy of about 60 meV. Research is currently being conducted to investigate its potential use in optoelectronics, sensors, and ferroelectric memory devices [14].
One of the unique inorganic semiconductor materials is metal-doped zinc oxide (Ni, Co). Based on the oxygen vacancies and wurtzite structure, it has a large exciton binding energy of 60 meV at room temperature and a wide direct bandgap of 3.37 eV [15]. Because of its use in optoelectronic devices, ZnO is a significant II–IV n-type direct bandgap semiconductor material that has garnered a lot of attention [16]. It is regarded as a multifunctional semiconductor compound because of its good physical properties [17]. ZnO belongs to the II–VI groups of the periodic table and manifests three crystalline structures: wurtzite, rock salt (NaCl or Rochelle salt), and zinc blend (ZnS). The wurtzite form is the thermodynamically stable phase under ambient conditions. The wurtzite ZnO exhibits intriguing optical, electrical, and optoelectronic properties in both thin-film and nanostructured forms [18]. The properties of ZnO mainly depend on the growth parameters, such as temperature, concentrations of solutions, the stoichiometry of reagents, and pH of the synthesis method [19]. The size, orientation, density, and morphology of ZnO crystal mainly contribute to numerous applications. The physical properties of ZnO are mainly affected by dopant impurities and defects [18]. Defects play an important role in obtaining desirable optoelectronic and electronic properties of semiconductor materials. ZnO can be fabricated in several shapes, such as single crystal, pellet, fine powder, and thick and thin films. Different experimental conditions, such as solution concentration, temperature, and substrate pretreatment, greatly influence the growth and shape of ZnO nanostructure; therefore, a good understanding of the ZnO growth condition is imperative. The applications of ZnO can be found in electronic apparatuses [20], for eco-friendly buffer layer for thin film solar cell applications [21], gas sensors [17], and photocatalysis processes [22]; hence, modifications of its properties are essential for both practical and scientific interests.
Many nanostructured materials have been used as lithium-ion batteries electrode materials in the last 10 years due to significant advancements in materials science and nanotechnology. Because of its low cost, abundance, ease of synthesis, and good safety, layered V2O5 is one of the most appealing potential cathode materials and has been thoroughly studied [23]. Moreover, the cathode material, a crucial component of lithium-ion batteries, has a direct impact on how well these batteries function. However, there is still a need to further improve the energy and power density of electrode materials due to the growing demand for high-power and high-energy devices. V2O5 is thought to be the most promising metal oxide to use as a cathode material for lithium-ion batteries [24]. The kinetic behaviors of photogenerated carriers, which increase light absorption and significantly boost photocatalytic activity, are influenced by the heterostructures of V2O5. Determining the remaining optical and electrical properties of these materials requires an understanding of their structural and topographical characteristics as well as an understanding of the mechanism underlying grain growth [25,26]. Therefore, in this research, we will focus on these electrical and optical properties of ZnO–V2O5 nanocomposites. Therefore, the main purpose of this work is to investigate the structural and optical properties of ZnO–V2O5 nanocomposites.
2 Experimental technique
2.1 Materials
The chemicals used to prepare ZnO–V2O5 nanoparticles were distilled water, zinc acetate Zn(CH3COO)2(H2O)2, and ammonium metavanadate (NH4VO3). Four samples (pure ZnO, pure V2O5, 0.5% ZnO-1.0%V2O5, and 1.0%ZnO-1.0%V2O5 (molar ratio)) were prepared by the sol–gel method.
2.2 Methods
To synthesize ZnO/V2O5 nanocomposites, various routes were proposed, such as the solvothermal process, homogeneous co-precipitation method [27], pulsed-laser ablation [28], electrospinning method [24], spray pyrolysis technique [29], and the sol–gel method [30]. The sol–gel method was used to synthesize metal oxide nanocomposite specimens. The narrow particle size distribution, uniform nanostructure at low temperatures, and high product purity are the main benefits of the sol–gel process. Metal nanooxides are frequently synthesized using this technique [31]. High-purity metal salt powders were used to prepare ZnO–V2O5 nanocomposite specimens. In the experiment, (NH4VO3) was added to 100 mL of distilled water, and the solution was stirred using a magnetic stir bar at 55°C for 30 min (CH3.COO)2Zn·2H2O) was added to the above solution. The stirring of the solution was continued for additional 2 h. The obtained mixture was stirred at room temperature for 24 h utilizing a magnetic stirring hotplate to produce a homogenous solution. Scheme 1 shows the steps of the used chemical method.

The steps of the used chemical method.
Upon the completion of the stirring, the mixture was dried at 120°C for 24 h. The solution was then gradually dried until it became an amorphous solid or a gel. The as-prepared samples were annealed at 500°C for 3 h. The gel was annealed at high temperatures to crystallize the amorphous solid and to remove the residual carbon and organic chemicals. The properties of ZnO/V2O5 were measured to achieve the desired doping concentration.
2.3 Characterizations
Using X-ray Diffraction (XRD) of Cu-Kα radiation of 1.5406 Å, the structural investigation of ZnO–V2O5 nanocomposites was done. Moreover, the optical properties were assessed using an Ultraviolet-Visible (UV-vis) spectroscopy. SEM (JEOL Type T200) was used to examine the morphology of samples at 15 kV. Using ImageJ software, a few hundred of the particles were measured in order to estimate the particle size distribution. Reflectance (R) was in the range of wavelength 190–1,000 nm, gauged using a Shimadzu UV-2101 spectrophotometer.
3 Results and discussion
3.1 Structural investigations
XRD spectra were analyzed for the crystal structures. Figure 1 shows the XRD charts of V2O5, 5ZnO/10V2O5 and ZnO, 10ZnO/10V2O5 nanocomposites. Three types of phases are detected and illustrated in Figure 1. The first phase type indexed well to orthorhombic-structured V2O5 (JCPDS card no: 41-1426). The main reflection peaks at 2θ values, 15.6°, 20.3°, 21.7°, 26.3°, 31.17°, 32.4°, 34.47°, 41.39°, 45.6° and 47.5°, were attributed to the (200), (001), (101), (110), (400), (011), (310), (002), (411) and (600) crystal planes, respectively, of the V2O5. On the other hand, the second phase found was a hexagonal ZnO structure (JCPDS card no: 36-1451).
The peaks at 2θ were 31.86°, 34.5°, 36.27°, 47.59, 56.7, 63.0, 66.46, 58.1, 69.2, 72.5 and 77.1 which were attributed, respectively, to the (100), (002), (101), (102), (110), (103), (200), (112), (201), (004) and (202) of ZnO nanoparticles. These peaks positions error is ±0.2. The most important phase was ZnV2O6. The obtained ZnV2O6 nanostructures exhibited promising hydrogen absorption.
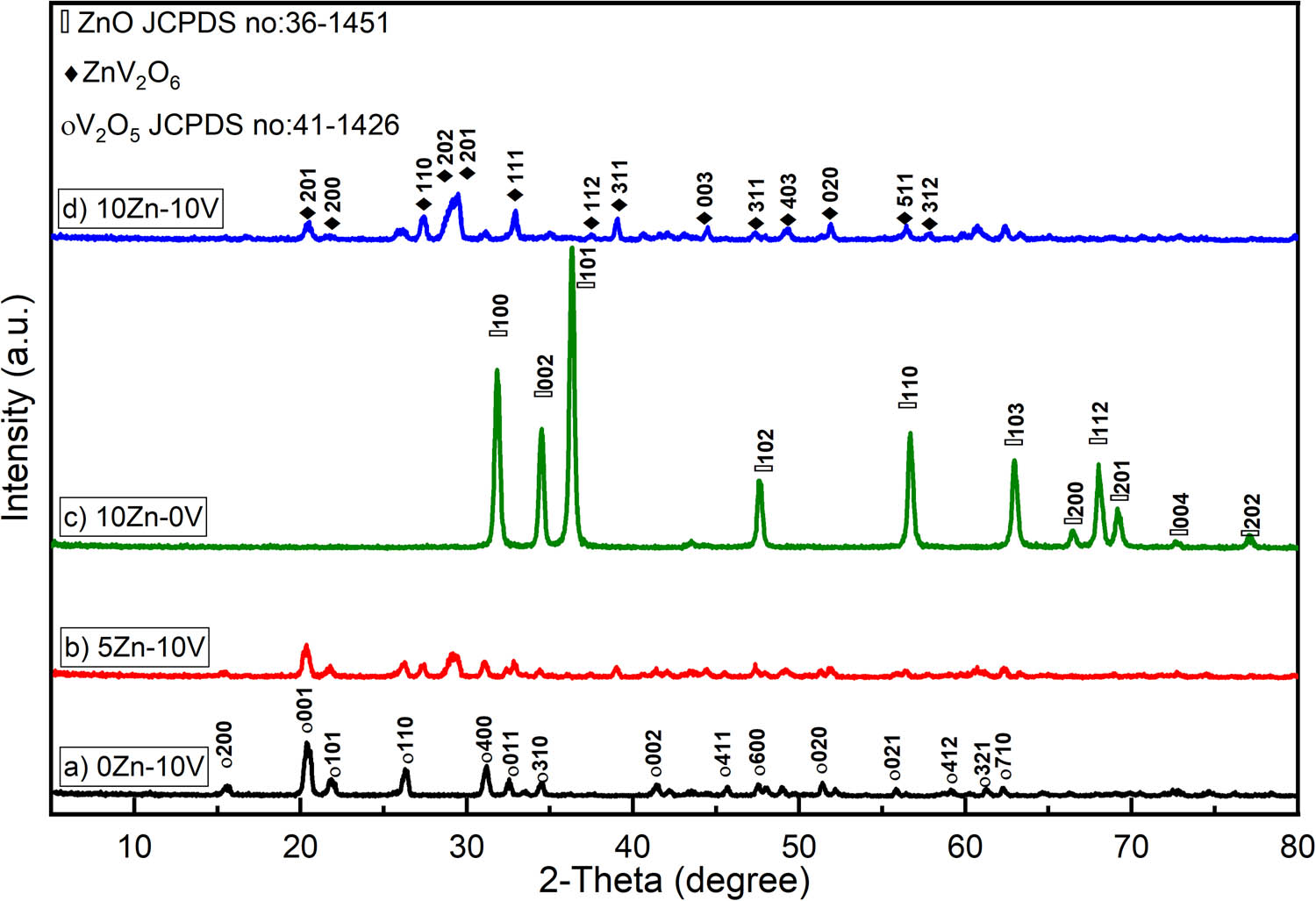
XRD charts of (a) V2O5, (b) 5ZnO/10V2O5, (c) ZnO, and (d) 10ZnO/10V2O5 nanocomposites.
The average crystallite size (D) using Scherrer’s equation, dislocation density (δ), and microstrain (
Structural parameters for V2O5, 5ZnO/10V2O5, ZnO, 10ZnO/10V2O5 nanocomposites
| Nanocomposites | D (nm) |
|
|---|---|---|
| 0ZnO/10V2O5 | 25 | 1.6 |
| 5ZnO/10V2O5 | 26 | 1.5 |
| 10ZnO/0V2O5 | 14.5 | 4.7 |
| 10ZnO/10V2O5 | 15.5 | 4.2 |
The surface morphology of the V2O5, 5ZnO/10V2O5 and ZnO, 10ZnO/10V2O5 nanocomposites is illustrated in Figure 2 in two different magnifications. The image of the V2O5 shows a semi tube shape with length and width of 1.12 and 0.2 µm, respectively. On the other hand, 5ZnO/10V2O5 nanocomposite shows a spherical shape with average dimeter of 0.6 µm. Regarding the ZnO nanocomposite, it shows again semi-tube shape with length and width of 0.44 and 0.12 µm, respectively. Finally, 10ZnO/10V2O5 nanocomposite shows a semi flower shape with different layers with length and width of 1 µm. By measuring several hundred particles from the SEM image, the particle size distribution was computed using ImageJ software. The average size of 5ZnO/10V2O5 is found to be smaller than that of V2O5. The nucleation process may be responsible for the phenomenon’s potential. A faster rate of nucleation leads to a higher concentration of nanoparticles. It is interesting to note that semi-flower 10ZnO/10V2O5 has a smaller average size than ZnO. This indicates that the presence of V2O5 particles within the nanocomposite can inhibit particle growth, leading to a reduction in size [35].
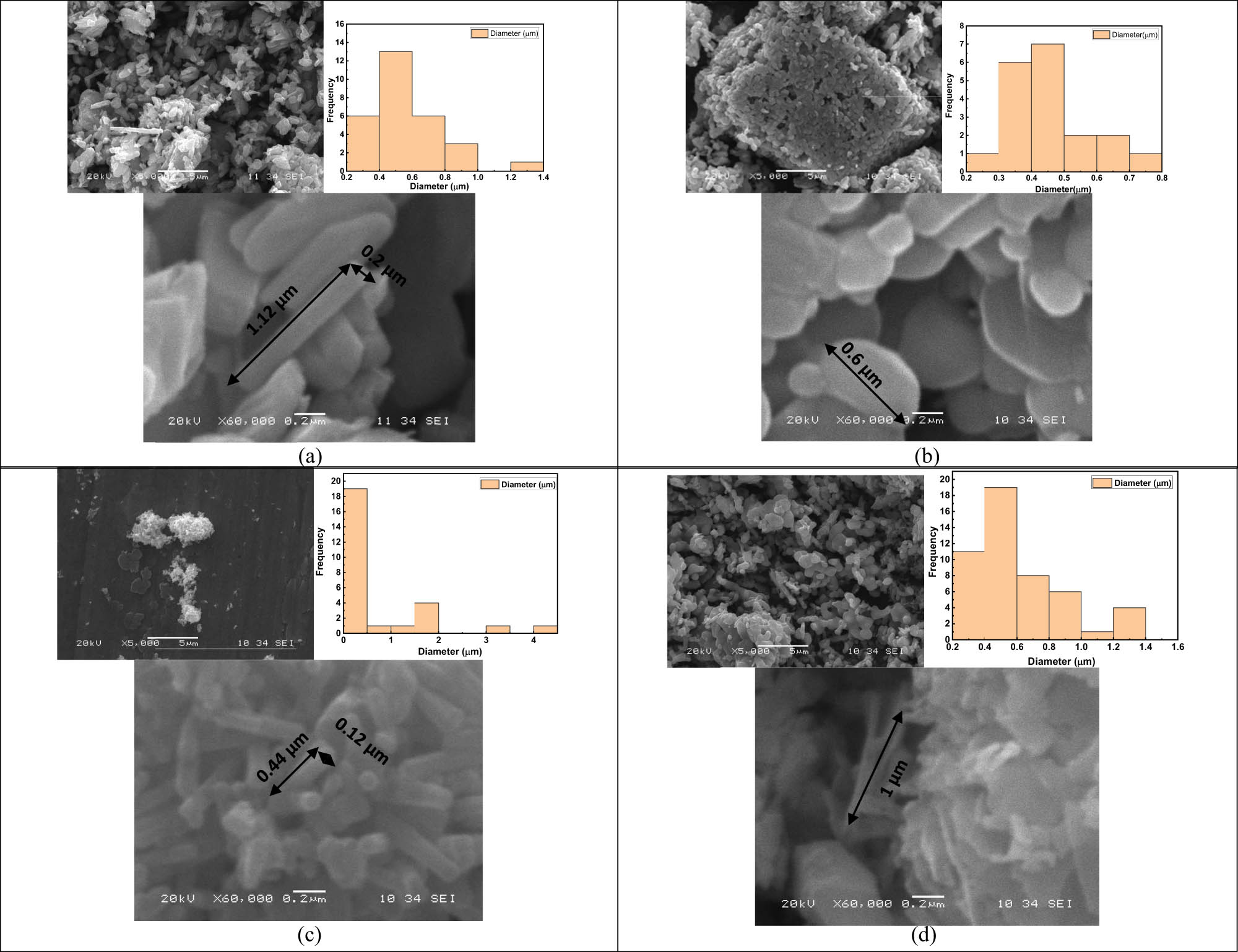
SEM images of (a) V2O5, (b) 5ZnO/10V2O5, (c) ZnO, and (d) 10ZnO/10V2O5 nanocomposites. Insert Figure: another magnifications of the images, nanocomposites size distribution determined with ImageJ software.
To confirm the presence, composition, and homogeneity of all elements in the samples, we have performed the EDX analyses as displayed in Figure 3. Figure 3(a) indicates the presence of V and O in pure V2O5, while Figure 3(b) shows the presence of Zn and O in ZnO, and Figure 3(c) and (d) shows the presence of V, Zn, and O in the final composites.
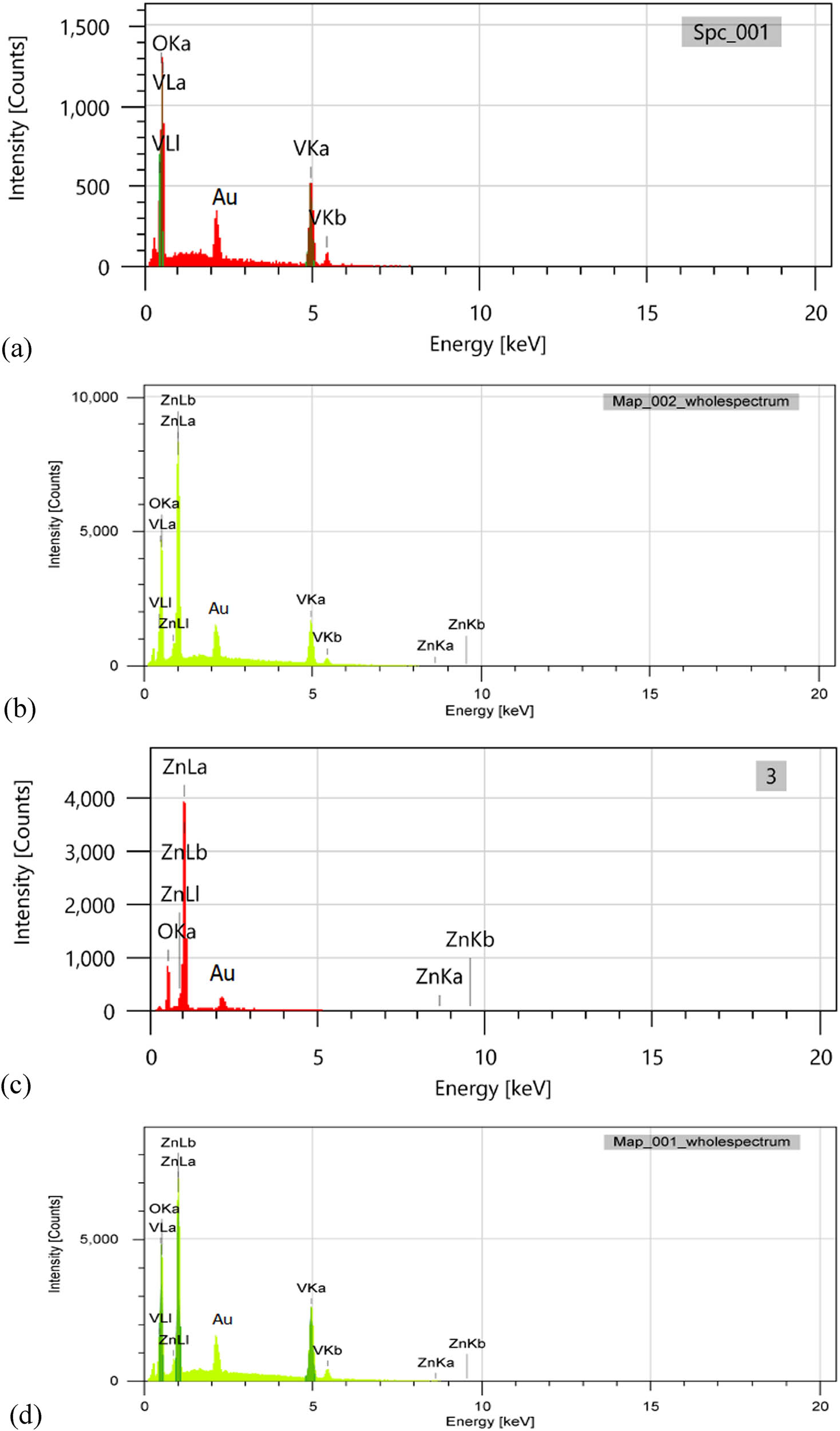
EDX of (a) V2O5, (b) 5ZnO/10V2O5, (c) ZnO, and (d) 10ZnO/10V2O5 nanocomposites (Au peak comes from sputtering system).
3.2 Linear optical investigations
3.2.1 Optical absorption region
The optical properties of V2O5, 5ZnO/10V2O5 and ZnO, 10ZnO/10V2O5 nanocomposites were probed by room-temperature UV-Vis Diffusion Reflectance Spectra (UV-Vis DRS). The diffusion reflectance (R%) spectra of V2O5, 5ZnO/10V2O5, and ZnO, 10ZnO/10V2O5 nanocomposites are shown in Figure 4. It is observed that the prepared ZnO/V2O5 gives a strong visible absorption edge at spectra at around 410 nm. UV-vis measurements were executed in diffusion reflectance mode (R) and converted to the Kubelka-Munk function F(R) to separate the light absorption from scattering. The absorption coefficient α = (R) was found utilizing the Schuster–Kubelka–Munk function expressed as follows [36]:
where
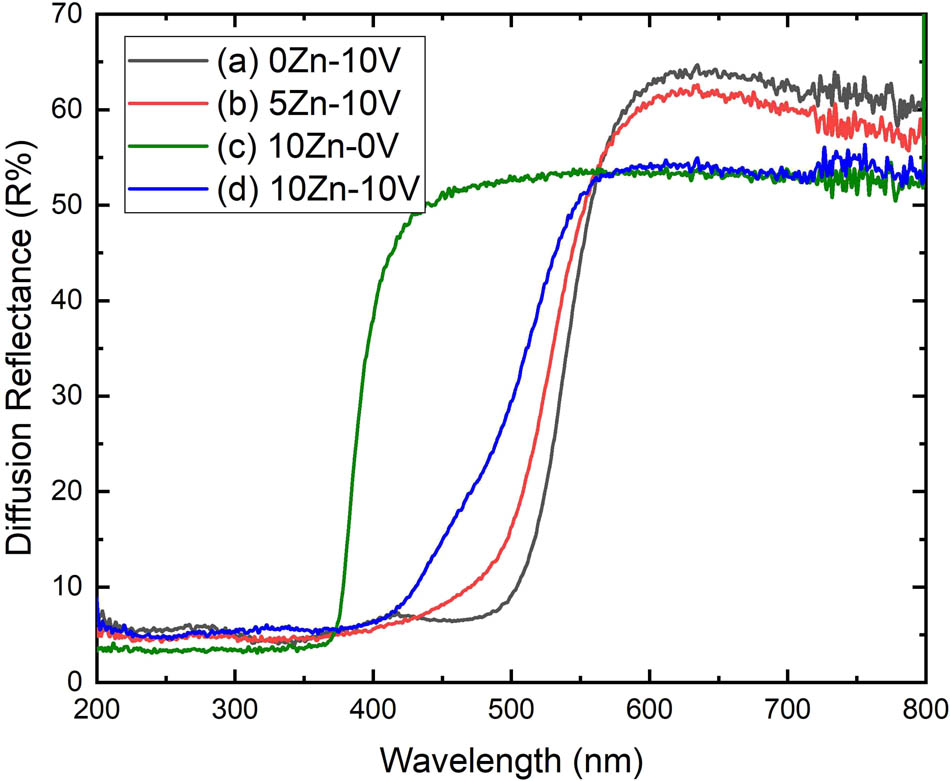
Reflectance charts of (a) V2O5, (b) 5ZnO/10V2O5, (c) ZnO, and (d) 10ZnO/10V2O5 nanocomposites.
The absorption coefficient vs photon energy for V2O5, 5ZnO/10V2O5 and ZnO, 10ZnO/10V2O5 nanocomposites are shown in Figure 5. At E ≥ 3.1 eV, the α is higher vaue, on the constract, the minimum value of α at green area. Moreover, it was noticed that at the lower region of absorption, α value as a function of photon energy obeyed the Urbach relation [37].
where α o is a constant, and the Urbach tail width is represented by E r.
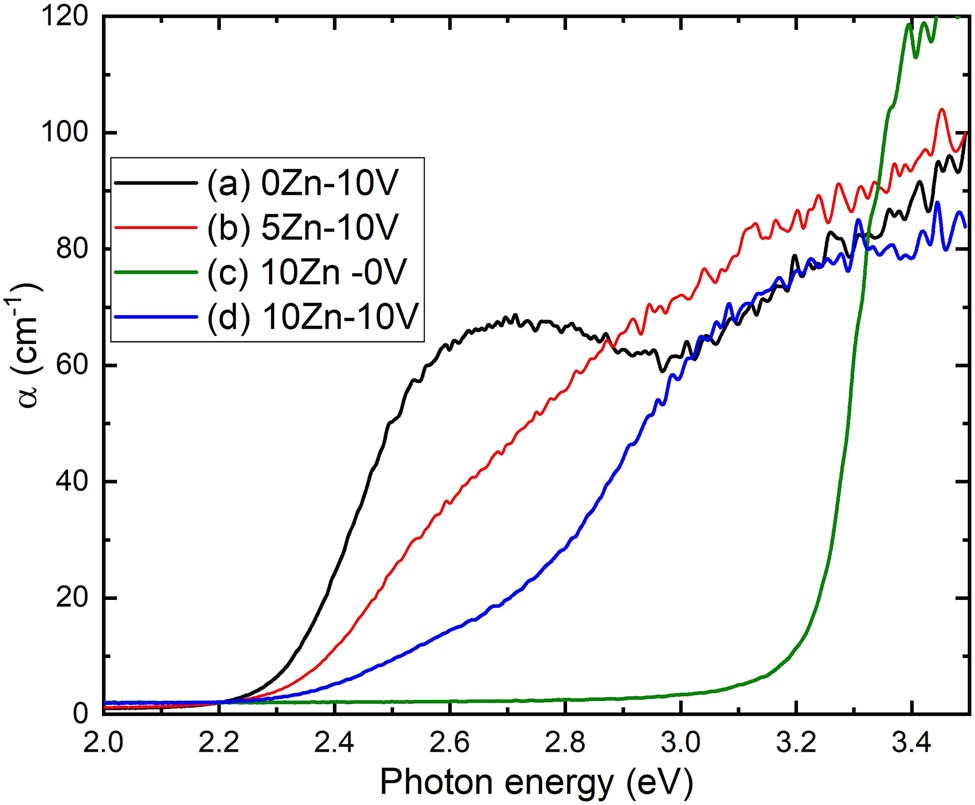
Absorption coefficient vs photon energy of (a) V2O5, (b) 5ZnO/10V2O5, (c) ZnO, and (d) 10ZnO/10V2O5 nanocomposites.
A relation between ln(α) and hυ for the V2O5, 5ZnO/10V2O5 and ZnO, 10ZnO/10V2O5 nanocomposites is presented in Figure 6. The evaluated E r for various samples is computed from the slope in Figure 6 and is listed in Table 2. The change in E r corresponding to the change in ZnO/V2O5 ratio could be related to the change in the disorder degree which changes the band tailing.
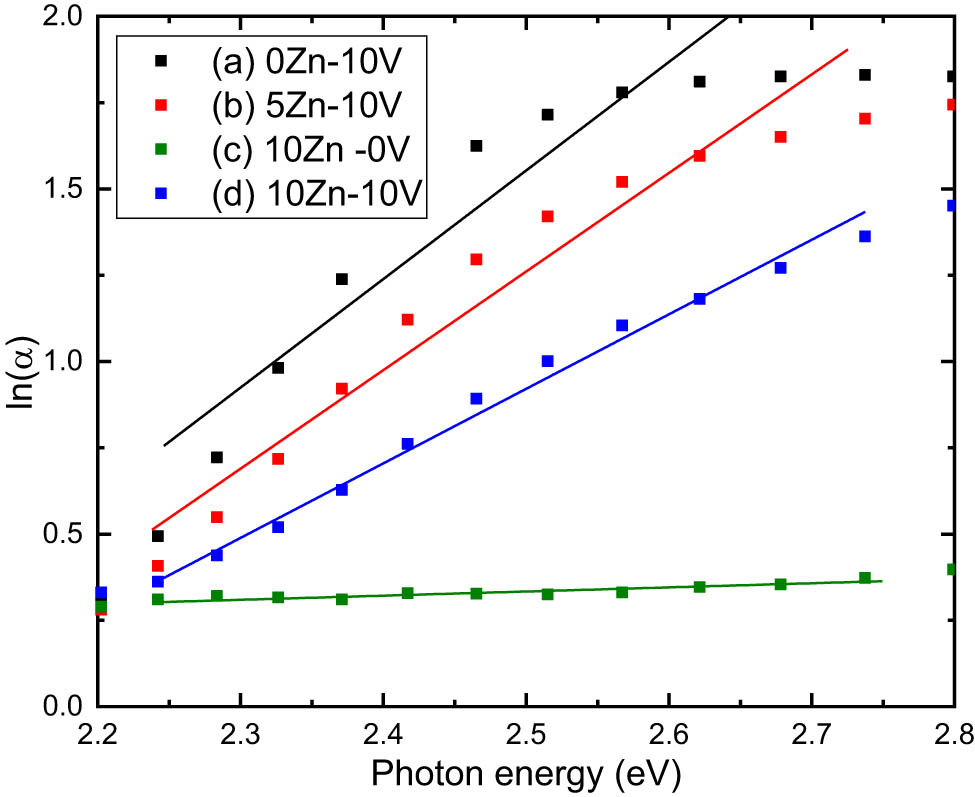
ln(α) vs photon energy of (a) V2O5, (b) 5ZnO/10V2O5, (c) ZnO, and (d) 10ZnO/10V2O5 nanocomposites.
Optical constants for V2O5, 5ZnO/10V2O5, ZnO, 10ZnO/10V2O5 nanocomposites
| Nanocomposites | E r (eV) |
|
|
|---|---|---|---|
| 0ZnO/10V2O5 | 0.32 | 2.33 | 2.19 |
| 5ZnO/10V2O5 | 0.35 | 2.46 | 2.20 |
| 10ZnO/0V2O5 | 0.47 | 3.20 | 3.16 |
| 10ZnO/10V2O5 | 8.33 | 2.73 | 2.35 |
The extinction coefficient (k ext.) for the V2O5, 5ZnO/10V2O5 and ZnO, 10ZnO/10V2O5 nanocomposites is investigated. Hence, k ext. can be computed from [37]:
The plots of k ext vs λ of the incident electromagnetic waves for V2O5, 5ZnO/10V2O5 and ZnO, 10ZnO/10V2O5 nanocomposites are illustrated in Figure 7. It can be seen, k ext., of the V2O5, 5ZnO/10V2O5 and ZnO, 10ZnO/10V2O5 nanocomposites is steadily independent at λ for λ > 550 nm, while it significantly increased to peak values at λ < 550 nm. Besides, the value and peak position of k ext. are affected by the change in concentration of V2O5, 5ZnO/10V2O5 and ZnO, 10ZnO/10V2O5 nanocomposites. These peaks are attributed to the process of particle absorption.
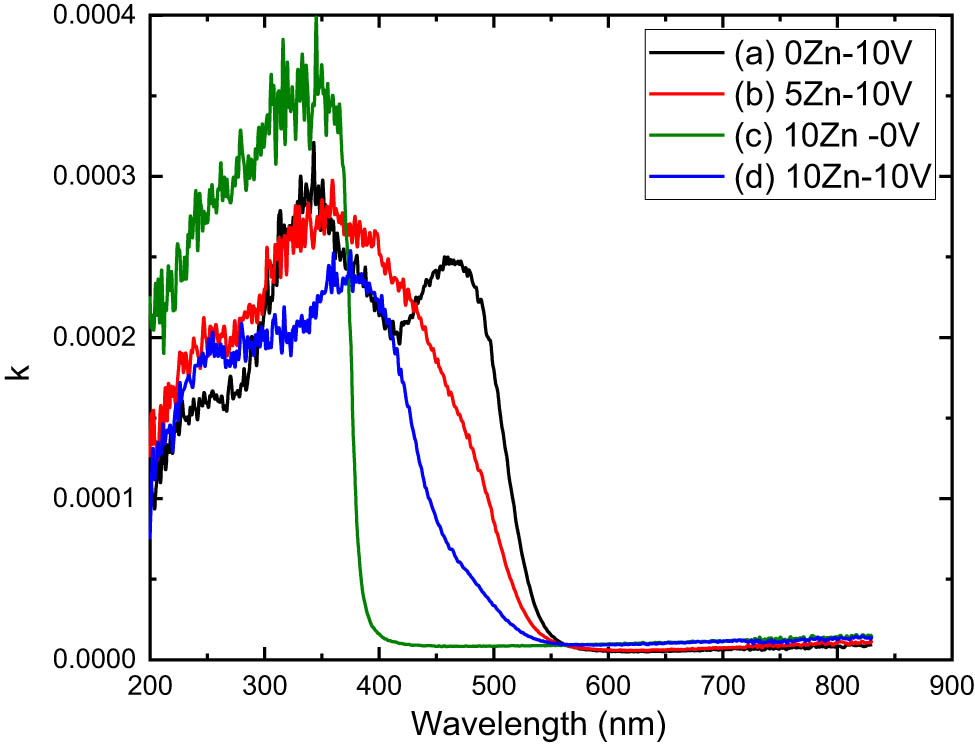
Extinction coefficient vs photon energy of (a) V2O5, (b) 5ZnO/10V2O5, (c) ZnO, and (d) 10ZnO/10V2O5 nanocomposites.
The direct/indirect optical band gaps,
where
Using equation (1), we can compute the following expression [39]:
Figures 8 and 9 show
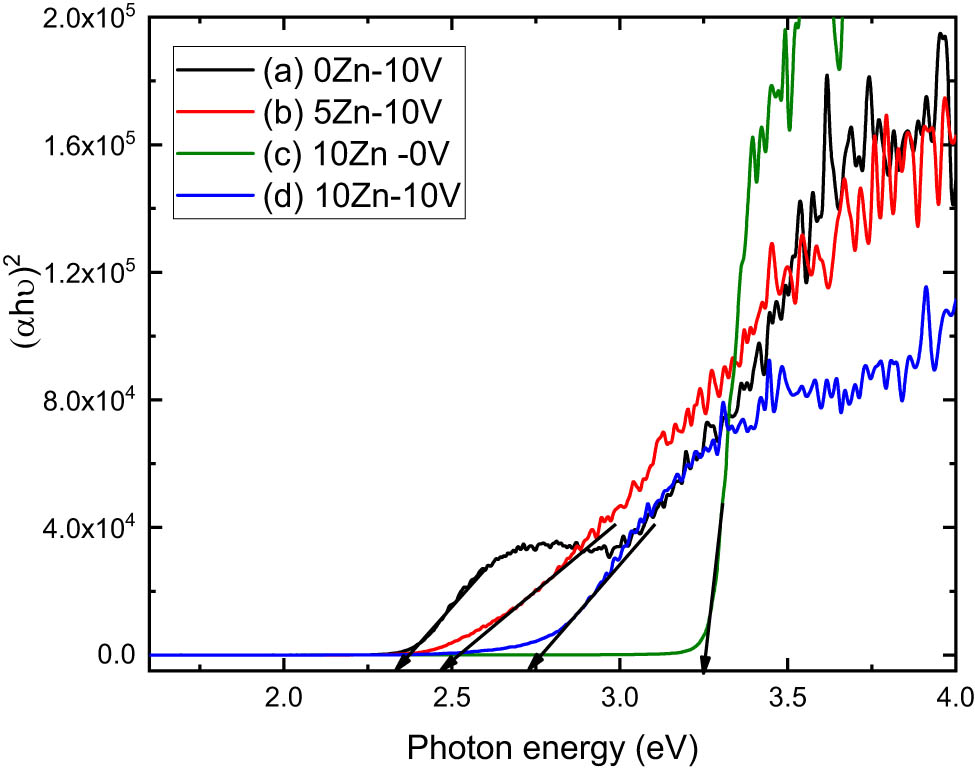
(αhν)2 vs photon energy of (a) V2O5, (b) 5ZnO/10V2O5, (c) ZnO, and (d) 10ZnO/10V2O5 nanocomposites.
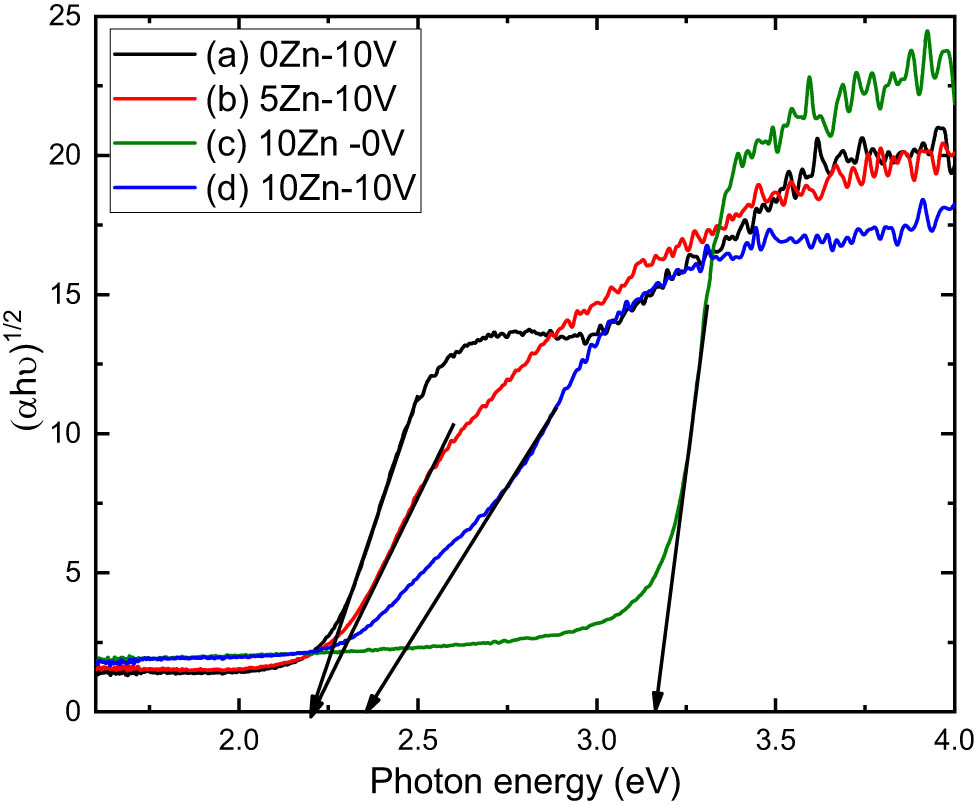
(αhν)1/2 vs photon energy of (a) V2O5, (b) 5ZnO/10V2O5, (c) ZnO, and (d) 10ZnO/10V2O5 nanocomposites.
3.2.2 Relation between the refractive index and the energy gap
The values of static refractive index,
Moss Model corrected by Ravindra [50]
Ravindra and Guptau Model [50]
Reddy and Anjanyuku Model [50,51]
Refractive index obtained from the deduced value of E g by various approaches along with its average value for V2O5, 5ZnO/10V2O5, ZnO, 10ZnO/10V2O5 nanocomposites
| Nanocomposites |
|
n RS | n R | n RA | n Ks |
|
|---|---|---|---|---|---|---|
| 0ZnO/10V2O5 | 2.33 | 2.61 | 2.64 | 2.75 | 2.56 | 2.64 |
| 5ZnO/10V2O5 | 2.46 | 2.57 | 2.56 | 2.69 | 2.52 | 2.59 |
| 10ZnO/0V2O5 | 3.20 | 2.41 | 2.1 | 2.43 | 2.31 | 2.31 |
| 10ZnO/10V2O5 | 2.73 | 2.51 | 2.39 | 2.59 | 2.44 | 2.48 |
The Z-scan technique was used for obtaining the value of the nonlinear refractive index and nonlinear absorption directly. Also, these values of the nonlinear refractive index could be determined from the calculated linear refractive index values which were recorded from reflectance and transmittance. Finally, it is found that the direct and indirect optical band gap of V2O5 is smaller than that of ZnO. On the other hand, the optical band gap of V2O5 increases for 5ZnO/10V2O5 and 10ZnO/10V2O5 nanocomposites.
3.3 Nonlinear optical investigations
The calculated values of the refractive index can be used to obtain other optical parameters, including the dielectric constant (
where A = 1.7 × 10−10 esu. The numerical values of
Nonlinear optical parameters for V2O5, 5ZnO/10V2O5, ZnO, 10ZnO/10V2O5 nanocomposites
| Nanocomposites |
|
|
|
|
|---|---|---|---|---|
| 0ZnO/10V2O5 | 2.64 | 6.97 | 1.38 | 19.76 |
| 5ZnO/10V2O5 | 2.59 | 6.71 | 1.16 | 16.83 |
| 10ZnO/0V2O5 | 2.31 | 5.34 | 0.39 | 6.29 |
| 10ZnO/10V2O5 | 2.48 | 6.15 | 0.77 | 11.65 |
4 Conclusion
ZnO/V2O5 nanocomposites have been synthesized by the sol–gel technique. The samples are in the form of V2O5, 5ZnO/10V2O5 and ZnO, 10ZnO/10V2O5 nanocomposites. The orthorhombic structure of V2O5 and the hexagonal structure of ZnO were confirmed by the XRD analysis. The average crystal size of the nanocomposites of V2O5, 5ZnO/10V2O5, and ZnO, 10ZnO/10V2O5 was determined to be 25, 26, and 14.5 nm, respectively. Semi-flower, semi-sphere, and semi-tube are three different shapes that SEM captures. V2O5 and ZnO exhibit semi-tube forms in their pure samples. The average diameter of 0.6 m was obtained for 5ZnO/10V2O5 which has a sphere-like form. However, 10ZnO/10V2O5 has a semi-flower shape with several layers. Optical diffusion reflectance spectra showed the absorption edge of ZnO/V2O5 nanocomposites in the visible region of the spectra. The direct optical band gap showed a strong dependence on various compositions which changed in the range of 2.33–2.73 eV. On the other hand, the values of the indirect changed in the range of 2.119–2.35 eV. Our investigations have shown that ZnV2O6 nanostructures have the potential to be used as an energy storage material.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number 445-9-750.
-
Funding information: This research work was funded by the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia through the project number 445-9-750.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Suwanboon S. Structural and optical properties of nanocrystalline ZnO powder from sol-gel method. Sci Asia. 2008;34(1):31–4.10.2306/scienceasia1513-1874.2008.34.031Suche in Google Scholar
[2] Athar T. Metal oxide nanopowder. Emerging nanotechnologies for manufacturing. USA: Elsevier; 2015. p. 343–401.10.1016/B978-0-323-28990-0.00014-2Suche in Google Scholar
[3] Beke S. A review of the growth of V2O5 films from 1885 to 2010. Thin Solid Films. 2011;519(6):1761–71.10.1016/j.tsf.2010.11.001Suche in Google Scholar
[4] Jameel MH, Saleem S, Hashim M, Roslan MS, Somaily HH, Hessin MM, et al. A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications. Nanotechnol Rev. 2021;10(1):1484–92.10.1515/ntrev-2021-0100Suche in Google Scholar
[5] Mane AA, Suryawanshi MP, Kim JH, Moholkar AV. Fast response of sprayed vanadium pentoxide (V2O5) nanorods towards nitrogen dioxide (NO2) gas detection. Appl Surf Sci. 2017;403:540–50.10.1016/j.apsusc.2017.01.220Suche in Google Scholar
[6] Lao ZJ, Konstantinov K, Tournaire Y, Ng SH, Wang GX, Liu H-K. Synthesis of vanadium pentoxide powders with enhanced surface-area for electrochemical capacitors. J Power Sources. 2006;162(2):1451–4.10.1016/j.jpowsour.2006.07.060Suche in Google Scholar
[7] Huo F-J, Su J, Sun Y-Q, Yin C-X, Tong H-B, Nie Z-X. A rhodamine-based dual chemosensor for the visual detection of copper and the ratiometric fluorescent detection of vanadium. Dye Pigment. 2010;86(1):50–5.10.1016/j.dyepig.2009.11.007Suche in Google Scholar
[8] Tong Z, Li N, Lv H, Tian Y, Qu H, Zhang X, et al. Annealing synthesis of coralline V2O5 nanorod architecture for multicolor energy-efficient electrochromic device. Sol Energy Mater Sol Cell. 2016;146:135–43.10.1016/j.solmat.2015.11.008Suche in Google Scholar
[9] Shen K, Yang R, Wang D, Jeng M, Chaudhary S, Ho K, et al. Stable CdTe solar cell with V2O5 as a back contact buffer layer. Sol Energy Mater Sol Cell. 2016;144:500–8.10.1016/j.solmat.2015.09.036Suche in Google Scholar
[10] Lee CE, Atkins RA, Gibler WN, Taylor HF. Fiber optic application for thermal switching in vanadium dioxide films. Appl Opt. 1989;28(21):4511–2.10.1364/AO.28.004511Suche in Google Scholar PubMed
[11] Julien C, Haro-Poniatowski E, Camacho-Lopez MA, Escobar-Alarcon L, Jı́menez-Jarquı́n J. Growth of V2O5 thin films by pulsed laser deposition and their applications in lithium microbatteries. Mater Sci Eng B. 1999;65(3):170–6.10.1016/S0921-5107(99)00187-7Suche in Google Scholar
[12] Subrahmanyam A, Reddy YBK, Nagendra CL. Nano-vanadium oxide thin films in mixed phase for microbolometer applications. J Phys D Appl Phys. 2008;41(19):195108.10.1088/0022-3727/41/19/195108Suche in Google Scholar
[13] Gökdemir FP, Özdemir O, Kutlu K. Comparison of structural and electrochemical properties of V2O5 thin films prepared by organic/inorganic precursors. Electrochim Acta. 2014;121:240–4.10.1016/j.electacta.2013.12.164Suche in Google Scholar
[14] Zargar RA, Arora M, Ahmad M, Hafiz AK. Synthesis and characterization of vanadium doped zinc oxide thick film for chemical sensor application. 2015;2015(1):196545.10.1155/2015/196545Suche in Google Scholar
[15] Saleem S, Jameel MH, Akhtar N, Nazir N, Ali A, Zaman A, et al. Modification in structural, optical, morphological, and electrical properties of zinc oxide (ZnO) nanoparticles (NPs) by metal (Ni, Co) dopants for electronic device applications. Arab J Chem. 2022;15(1):103518.10.1016/j.arabjc.2021.103518Suche in Google Scholar
[16] Saleem S, Jameel MH, Rehman A, Tahir MB, Irshad MI, Jiang ZY, et al. Evaluation of structural, morphological, optical, and electrical properties of zinc oxide semiconductor nanoparticles with microwave plasma treatment for electronic device applications. J Mater Res Technol. 2022;19:2126–34.10.1016/j.jmrt.2022.05.190Suche in Google Scholar
[17] Maswanganye MW, Rammutla KE, Mosuang TE, Mwakikunga BW. The effect of Co and In combinational or individual doping on the structural, optical and selective sensing properties of ZnO nanoparticles. Sens Actuators B Chem. 2017;247:228–37.10.1016/j.snb.2017.02.039Suche in Google Scholar
[18] Rao MSR, Okada T. ZnO Nanocrystals and allied materials. Vol. 180, New Delhi: Springer; 2013.10.1007/978-81-322-1160-0Suche in Google Scholar
[19] Shankar S, Saroja M, Venkatachalam M, Muthukumarasamy N, Kumar V. Influence of pH on structural and optical properties of spin coated ZnO thin films. IJIRSET. 2014;2:8990–3.Suche in Google Scholar
[20] Strelchuk VV, et al. Structural and optical properties of ZnO films produced by a nonvacuum chemical technique. Semiconductors. 2014;48:1145–50.10.1134/S106378261409019XSuche in Google Scholar
[21] Wu S, Chen Z, Wang T, Ji X. A facile approach for the fabrication of Au/ZnO-hollow-sphere-monolayer thin films and their photocatalytic properties. Appl Surf Sci. 2017;412:69–76.10.1016/j.apsusc.2017.03.166Suche in Google Scholar
[22] Purohit A, Chander S, Sharma A, Nehra SP, Dhaka MS. Impact of low temperature annealing on structural, optical, electrical and morphological properties of ZnO thin films grown by RF sputtering for photovoltaic applications. Opt Mater (Amst). 2015;49:51–8.10.1016/j.optmat.2015.08.021Suche in Google Scholar
[23] Wang CC. Empirical relation between the linear and the third-order nonlinear optical susceptibilities. Phys Rev B. 1970;2(6):2045.10.1103/PhysRevB.2.2045Suche in Google Scholar
[24] Liu X, Zeng J, Yang H, Zhou K, Pan D. RSC Advances REVIEW V2O5–based nanomaterials: synthesis and their applications. RSC Adv. 2018;8:4014–31. 10.1039/C7RA12523B.Suche in Google Scholar
[25] Chasta G, Suthar D, Thakur A, Kannan MD, Dhaka MS. Comprehensive investigation on influence of copper doping on physical properties of CdSe thin films for solar cell applications. Mater Res Bull. 2022;152:111845.10.1016/j.materresbull.2022.111845Suche in Google Scholar
[26] Kumari S, Suthar D, Kannan MD, Kumari N, Dhaka MS. Understanding the grain growth mechanism in CdS thin films by CdCl2 treatment and thermal annealing evolution. Opt Mater (Amst). 2022;123:111900.10.1016/j.optmat.2021.111900Suche in Google Scholar
[27] Taleb R, Hadeel R, Thamer SM, Abdullah A, Juzsakova T, Al N. Synthesis, characterization of V2O5 nanoparticles and determination of catalase mimetic activity by new colorimetric method. J Therm Anal Calorim. 2021;145(2):297–307. 10.1007/s10973-020-09725-5.Suche in Google Scholar
[28] Boaes Mendonça LT, Bezerra AG, Mendes de Azevedo W. Preparation and characterization of V2O5 and V2O5/PANI nanocomposite by laser ablation technique in liquid. Mater Chem Phys. 2021;273:125084. 10.1016/j.matchemphys.2021.125084.Suche in Google Scholar
[29] Ahmed NM, Sabah ZH. Characterization of V2O5 nanorods grown by spray pyrolysis technique. J Mater Sci Mater Electron. 2016;27(5):4613–21. 10.1007/s10854-016-4338-3.Suche in Google Scholar
[30] Babar BM, Mohite AA, Patil VL, Pawar UT, Kadam LD, Kadam PM, et al. Sol-gel prepared vanadium oxide for photocatalytic degradation of methylene blue dye. Mater Today Proc. 2021;43:2673–7. 10.1016/j.matpr.2020.04.205.Suche in Google Scholar
[31] Bokov D, Turki Jalil A, Chupradit S, Suksatan W, Javed Ansari M, Shewael IH, et al. Nanomaterial by sol-gel method: synthesis and application. 2021;2021(1):5102014.10.1155/2021/5102014Suche in Google Scholar
[32] Rashad M, Darwish AAA. Blue shift of band gap for vanadyl 2,3-naphthalocyanine (VONc) thin films monitored at thermal effect. Mater Res Express. 2018;5(2):026402. 10.1088/2053-1591/aa9ef3.Suche in Google Scholar
[33] Chandekar KV, Alkallas FH, Trabelsi AB, Shkir M, Hakami J, Khan A, et al. Improved linear and nonlinear optical properties of PbS thin films synthesized by spray pyrolysis technique for optoelectronics: An effect of Gd3+ doping concentrations. Phys B Condens Matter. 2022;641:414099.10.1016/j.physb.2022.414099Suche in Google Scholar
[34] Shkir M, Chandekar KV, Khan A, Alshahrani T, El-Toni AM, Sayed MA, et al. Tailoring the structure-morphology-vibrational-optical-dielectric and electrical characteristics of Ce@ NiO NPs produced by facile combustion route for optoelectronics. Mater Sci Semicond Process. 2021;126:105647.10.1016/j.mssp.2020.105647Suche in Google Scholar
[35] Fabrication of ZnO-SiO2 nanocomposite materials prepared by a spray pyrolysis for the photocatalytic activity under UV and Sunlight irradiations fabrication of ZnO-SiO2. In:IOP Conference Series: Materials Science and Engineering; 2020. 10.1088/1757-899X/778/1/012105.Suche in Google Scholar
[36] Faisal M, Ibrahim AA, Harraz FA, Bouzid H, Al-Assiri MS, Ismail AA. SnO2 doped ZnO nanostructures for highly efficient photocatalyst. J Mol Catal A Chem. 2015;397:19–25.10.1016/j.molcata.2014.10.027Suche in Google Scholar
[37] Urbach F. The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys Rev. 1953;92(5):1324.10.1103/PhysRev.92.1324Suche in Google Scholar
[38] Mohamed GA, El-Moiz ABA, Rashad M. Li-doping effects on the electrical properties of ZnO films prepared by the chemical-bath deposition method. Phys B Condens Matter. 2005;370(1–4):158–67.10.1016/j.physb.2005.09.006Suche in Google Scholar
[39] El-Menyawy EM, Elagamey AA, Elgogary SR, El-Enein RANA. Synthesis, crystal structure and thin-film-optical properties of 3-amino-2-(2-nitrophenyl) diazinyl-3-(morpholin-1-yl) acrylonitrile. Spectrochim Acta Part A Mol Biomol Spectrosc. 2013;108:75–81.10.1016/j.saa.2013.02.001Suche in Google Scholar PubMed
[40] Srikant V, Clarke DR. On the optical band gap of zinc oxide. J Appl Phys. 1998;83(10):5447–51.10.1063/1.367375Suche in Google Scholar
[41] Debanath MK, Karmakar S. Study of blueshift of optical band gap in zinc oxide (ZnO) nanoparticles prepared by low-temperature wet chemical method. Mater Lett. 2013;111:116–9.10.1016/j.matlet.2013.08.069Suche in Google Scholar
[42] Sáenz-Trevizo A, Amézaga-Madrid, Pizá-Ruiz, Antúnez-Flores W, Miki-Yoshida M. Optical band gap estimation of ZnO nanorods. Mater Res. 2016;19:33–8.10.1590/1980-5373-mr-2015-0612Suche in Google Scholar
[43] Shaalan NM, Rashad M, Abdel-Rahim MA. CuO nanoparticles synthesized by microwave-assisted method for methane sensing. Opt Quantum Electron. 2016;48(12):2–12. 10.1007/s11082-016-0802-9.Suche in Google Scholar
[44] Scanlon DO, Walsh A, Morgan BJ, Watson GW. An ab initio study of reduction of V2O5 through the formation of oxygen vacancies and Li intercalation. J Phys Chem C. 2008;112(26):9903–11.10.1021/jp711334fSuche in Google Scholar
[45] Kenny N, Kannewurf CR, Whitmore DH. Optical absorption coefficients of vanadium pentoxide single crystals. J Phys Chem Solids. 1966;27(8):1237–46.10.1016/0022-3697(66)90007-2Suche in Google Scholar
[46] Luo Z, Wu Z, Xu X, Du M, Wang T, Jiang Y. Impact of substrate temperature on the microstructure, electrical and optical properties of sputtered nanoparticle V2O5 thin films. Vacuum. 2010;85(2):145–50.10.1016/j.vacuum.2010.05.001Suche in Google Scholar
[47] Ravindra N, Srivastava VK. Variation of refractive index with energy gap in semiconductors. Infrared Phys. 1979;19(5):603–4.10.1016/0020-0891(79)90081-2Suche in Google Scholar
[48] Tripathy SK. Refractive indices of semiconductors from energy gaps. Opt Mater (Amst). 2015;46:240–6.10.1016/j.optmat.2015.04.026Suche in Google Scholar
[49] Moss TS. A relationship between the refractive index and the infra-red threshold of sensitivity for photoconductors. Proc Phys Soc Sect B. 1950;63(3):167.10.1088/0370-1301/63/3/302Suche in Google Scholar
[50] Hervé, Vandamme LKJ. General relation between refractive index and energy gap in semiconductors. Infrared Phys Technol. 1994;35(4):609–15.10.1016/1350-4495(94)90026-4Suche in Google Scholar
[51] Reddy RR, Anjaneyulu S. Analysis of the moss and Ravindra relations. Phys Status Solidi. 1992;174(2):K91–3.10.1002/pssb.2221740238Suche in Google Scholar
[52] V, Gupta, Ravindra NM. Comments on the moss formula. Phys Status Solidi. 1980;100(2):715–9.10.1002/pssb.2221000240Suche in Google Scholar
[53] Kumar V, Singh JK. Model for calculating the refractive index of different materials. Indian J Pure Appl Phys. 2010;48:571–4.Suche in Google Scholar
[54] Ravindra NM, Auluck S, Srivastava VK. On the Penn gap in semiconductors. Phys Status Solidi. 1979;93(2):K155–60.10.1002/pssb.2220930257Suche in Google Scholar
[55] Wemple SH, DiDomenico Jr M. Behavior of the electronic dielectric constant in covalent and ionic materials. Phys Rev B. 1971;3(4):1338.10.1103/PhysRevB.3.1338Suche in Google Scholar
[56] Ticha H, Tichy L. Semiempirical relation between non-linear susceptibility (refractive index), linear refractive index and optical gap and its application to amorphous chalcogenides. J Optoelectron Adv Mater. 2002;4(2):381–6.Suche in Google Scholar
[57] Shkir M, Khan ZR, Khan A, Chandekar KV, Sayed MA, AlFaify S. A comprehensive study on structure, opto-nonlinear and photoluminescence properties of Co3O4 nanostructured thin films: An effect of Gd doping concentrations. Ceram Int. 2022;48(10):14550–9.10.1016/j.ceramint.2022.01.348Suche in Google Scholar
[58] Shkir M, Khan A, Imran M, Khan MA, Zargar RA, Alshahrani T, et al. Spray pyrolysis developed Nd doped Co3O4 nanostructured thin films and their structural, and opto-nonlinear properties for optoelectronics applications. Opt Laser Technol. 2022;150:107959.10.1016/j.optlastec.2022.107959Suche in Google Scholar
[59] Khan ZR, Chandekar KV, Khan A, Akhter N, Sayed MA, Shkir M, et al. An impact of novel Terbium (Tb) doping on key opto-nonlinear optical characteristics of spray pyrolyzed NiO nanostructured films for opto-nonlinear applications. Mater Sci Semicond Process. 2022;138:106260.10.1016/j.mssp.2021.106260Suche in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy
Artikel in diesem Heft
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy

