Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
-
Umer Younas
, Ikram Ahmad
, Zohaib Saeed
, Adnan Ashraf
and Sung Jea Park
Abstract
In the last few years, metal nanoparticles (NPs) have become one of the major components in the field of nanotechnology. NPs with fascinating and tunable properties (size and shape) have provided solutions for many problems including water pollution which has now become alarming in the current era. Herein, natural polymer-supported AgSr bimetallic NPs have been synthesized. For this purpose, sodium alginate (Na-Alg) was used as a stabilizer along with sodium borohydride (NaBH4) as a reducing agent. The synthesized Na-Alg-supported AgSr NPs were characterized employing UV–Vis, FTIR, SEM, and XRD techniques. The spectrophotometric analysis confirmed the formation and SEM and XRD confirmed the size of NPs up to 24.18 and 12.95 nm, respectively. These NPs were tested for catalytic degradation potential against malachite green (MG) and methyl orange (MO) dyes in the aqueous medium. The catalytic activity of NPs was evaluated in terms of kinetics and percent removal of the dyes. The results revealed that the MO dye was degraded in 21 min with a removal efficiency of 86.45% and MG dye in 24 min with 91.74%. Catalytic degradation of MO and MG dyes was also monitored in the absence of AgSr NPs which showed no catalytic degradation of dyes even after half an hour. The study has confirmed that biopolymer-supported NPs can be synthesized with suitable morphology for catalytic applications and these NPs can be further used for the removal of dyes from aqueous medium.
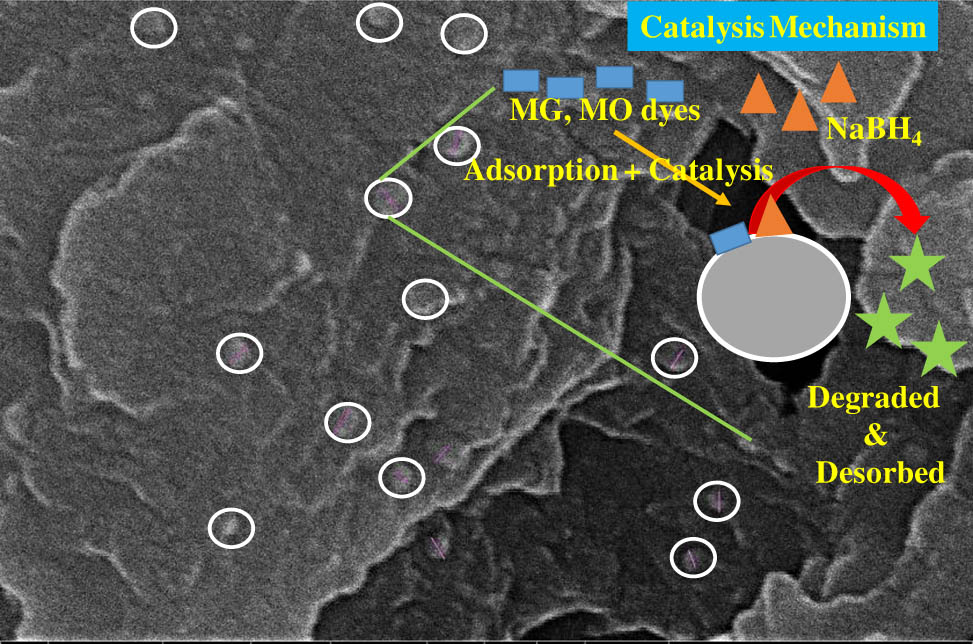
Graphical abstract
1 Introduction
Scientists have focused modifications in different materials in a controlled way to achieve specific properties under the umbrella of nanotechnology. This technology combines the knowledge of different subjects including chemistry [1], physics [2], materials science [3], and biology [4] to design novel materials for multiple applications in different domains of science. Nanomaterials are getting popular as a result of their uses in waste treatment [5] health care [6], cosmetics [7], food [8], biomedical sciences [9], drug delivery [10], and cancer therapy [11]. To meet the demand for nanomaterials, scientists are focusing on discovering new routes toward designing and development of these nanostructures [12]. There are many methods including chemical, sol–gel, green, biological, laser ablation, evaporation/condensation, and co-precipitation that are used for the synthesis of metallic nanoparticles (NPs) [13]. Many advantages have been reported for physical methods i.e. high yield, lesser energy consumption, however, sample impureness, and uneven size distribution can be the disadvantages [14]. The chemical reduction method is the most often used method due to its simplicity and the characteristics of the synthesized NPs [15]. In this method, the reactant’s molar content and feed rate can be changed to generate metal NPs with specified particle sizes, morphologies, and regular particle size distribution. Choosing a suitable reducing agent, stabilizing materials, synthesis conditions, and other parameters can provide NPs with the required characteristics.
The incorporation of metal NPs into different polymers is one of the most common ways to modify their properties. These aspects help in improving biological response, mechanical qualities, drug release, and pollutant removal potential [16,17]. Researchers have also focused on the use of different polymeric materials to modify the morphology, stability, and efficiency of NPs. Owing to biocompatibility and biodegradability, biopolymers are intriguing and worthy of research. The combination of biopolymers with inorganic NPs (metal oxides, oxides, phosphates, silicates, and others) produces biopolymer-based nanocomposites (also known as bio-nanocomposites) with better mechanical and thermal characteristics. These bio-nanocomposites own multi-functional features that make them suitable for a variety of applications including adsorption [13], bioplastic synthesis [18], environmental premeditator [19], chemical sensors [20], electrochemical [21], and packaging materials [22]. Polymeric stabilized nanomaterials synthesized using metals/metal oxide NPs and composites have been reported to exhibit excellent adsorbing characteristics and may also be used as catalysts [23], chemical sensors [24,25], bactericides [26], and biomedical agents (such as tissue engineering, drug delivery, regenerative medicine, and other health care applications) [27].
The combinations of noble metals with other d-block metals enhance their catalytic potential through the synergistic effect. As a result, these metal NPs possess a greater surface-to-volume ratio with enhanced surface area which provides a vast range of active sites for other substrates to adsorb and degrade in the presence of reducing agents [28]. Among these metal NPs, silver and strontium are frequently used due to their unique combination which facilitates each other by enhancing one another’s surface area and thus becomes an excellent catalyst [29,30]. Metal-based nanomaterials have played a major role as nanocatalysts for the degradation of organic–inorganic pollutants from wastewater [31,32,33]. Strontium (Sr) is an alkaline earth metal and its oxide (SrO) serves as a very basic oxide among metal oxides. It is a well-known chemical of industrial significance and is mostly found in clay and used to prepare glass and ceramics. It has a morphology of stacked particles with outstanding thermal stability and good optical qualities [34]. Strontium (Sr) has also been demonstrated to speed up the healing of fractured bones and reduce bone resorption [35], whereas the role of Ag NPs has proven itself in many applications (catalysis, electronics, optics, sensors) and has been used immensely over many decades [36,37].
In many previous studies, authors have reported the successful incorporation of Ag and strontium oxide aluminate NPs into the cotton fabric to achieve luminescence and hydrophobic properties [38]. In another report, AgSr incorporated natural polymer (hydroxyapatite) has been studied for biomedical applications [39]. The silver-strontium titanate NPs and AgSr NPs doped bioactive mesoporous glass have been reported for multiple applications [40,41]. All these previous research on AgSr have shown effective combinations with one another and other materials including natural polymers. In the current study, the synthesis of biopolymer-stabilized AgSr NPs has been synthesized successfully. Stabilization of AgSr NPs was achieved with a novel approach using Na-Alg as a stabilizer and NaBH4 was used as a reducing agent. The catalytic potential of AgSr NPs was tested by carrying out the degradation of the two dyes (malachite green [MG] and methyl orange [MO]). The kinetics and percent removal potential of the synthesized samples were also evaluated.
2 Materials and methods
2.1 Chemicals
The chemicals used for the synthesis and catalytic applications were as follows: silver nitrate (AgNO3; >99%, Sigma Aldrich), strontium nitrate (Sr(NO3)2; >99%, Sigma Aldrich), sodium alginate (Na-Alg; >97%, Fischer), sodium borohydride (NaBH4; >97%, Fischer), sodium hydroxide (NaOH; >97%, Fischer), and hydrochloric acid (HCl; >97%, Fischer). All the sample solutions and reactions were made using deionized water as aqueous media.
2.2 Synthesis of Na-Alg-supported AgSr NPs
The AgSr NPs stabilized by Na-Alg were prepared following an already reported method. For this purpose, a chemical reduction method was adopted to synthesize Na-Alg-stabilized AgSr NPs [42,43]. In a beaker, 0.54 g of Na-Alg powder was taken and dissolved in 250 mL of deionized water with vigorous stirring under continuous N2 purge. A thick layer appeared initially and its stirring was performed for 30 min at 50°C that turned into a smooth colloidal dispersion. Metal precursors (AgNO3 and Sr(NO3)2) solutions were prepared separately in 20 mL of deionized water and added to the Na-Alg mixture followed by adding 0.05 M NaBH4 solution. Then, the pH of the solution was maintained at 11.3 by adding 0.1 M NaOH with continuous stirring and the reaction was carried out for 2 h. The final solution was incubated for 4 h to generate the final dispersion, which was then subjected to centrifuge at 7,000 rpm for 30 min. Then, the liquid was stored in sample tubes and the solid was separated and dried in heat for 5 h over 300°C (Figure 1). Previously, many researchers have successfully synthesized AgSr NPs with suitable polymers following the above methodology [38,44].

Schematic representation of preparation of Na-Alg-supported AgSr NPs.
2.3 Characterization of Na-Alg-supported AgSr NPs
The sample of Na-Alg-supported AgSr NPs were characterized and the UV–Vis spectrum was obtained using Cecil Aquarius 7400s, UK, at the range of 275–800 nm. Fourier-transform infrared (FTIR) analysis was performed on α-II Bruker, UK ranges from 4,000 to 400 cm−1 for solid samples of AgSr stabilized by Na-Alg and Na-Alg itself to evaluate the presence of different functional groups. Similarly, the crystallinity/impurity of the samples was evaluated using X-ray diffraction (XRD) Bruker D2, UK, having CuKα at 1.54 nm λ. Visual confirmation of the morphology of AgSr NPs stabilized by Na-Alg was taken using field emission scanning electron microscopy (FE-SEM) having a resolution range from 200 nm to 10 µm (450 NOVA, LUMS Pakistan).
2.4 Catalytic potential of Na-Alg-supported AgSr NPs
The AgSr NPs were used as catalysts for the degradation of MO and MG dyes. The catalytic degradation of MO dye was carried out after optimizing the conditions. For this purpose, solutions of 0.2 mM MO, 14.5 mg/mL NPs, and 20.1 mM NaBH4 were used. Similarly, for catalytic degradation of MG, 0.32 mM MG, 14.5 mg/mL NPs, and 22.4 mM NaBH4 solutions were prepared. Dye degradation was monitored using UV–Vis spectrophotometer ranges between 250 and 800 nm. The catalytic degradation of the dyes was also monitored in the absence of Na-Alg-supported AgSr NPs at the same optimized conditions. Other than the model reaction, the effect of NPs was also monitored at various concentrations (4.76, 6.72, 8.91, and 10.45 mg/mL) of NPs by keeping the concentration of other parameters constant. The percentage removal of MO and MG dyes was calculated using % removal = (C o – C t )/ C o × 100. Kinetics was observed using ln (C t / C o) = −k app × t, where k aap represents the apparent rate constant. C o and C t are concentrations at zero time and any time interval (t).
3 Results and discussion
3.1 UV–Vis analysis
A sample of Na-Alg-supported AgSr NPs stabilized by Na-Alg was successfully fabricated and UV–visible analysis was performed to confirm the synthesis. UV–Vis spectra revealed the presence of AgSr NPs and Na-Alg in the synthesized colloid dispersions showing maximum absorption at wavelengths between 250 and 800 nm. Figure 2 displays UV–Vis spectra for all the samples and the observed λ max for Ag, Sr, and AgSr NPs were obtained at 400, 530, and 420 nm both absorptions, respectively [42,45] and Na-Alg shows no corresponding higher absorption peak.
The UV–Vis spectra of Ag NPs and Sr NPs were determined before and after association with Na-Alg and results revealed the formation of Ag NPs, Sr NPs, AgSr NPs and their association with Na-Alg in colloidal dispersions [46].

UV–Vis spectra of Ag, Sr, and Na-Alg-supported AgSr NPs.
3.2 FTIR analysis
FTIR spectroscopy was used to confirm the presence and association of functional groups in AgSr NPs stabilized by Na-Alg. The spectrum of Na-Alg, as well as its various peaks, has been presented in Figure 3. The figure shows that the stretching vibrations of the symmetric and asymmetric carboxylate anions exhibit corresponding absorption bands at 1596.88, 1,416, and 1,306 cm−1. The hydroxyl group’s stretching vibrations exhibited a peak at 3,200–3,430 cm−1 [47]. The moderate intensity band at 3248.17 cm−1 recorded for O–H stretching vibrations may be attributed to its involvement towards stabilization of AgSr NPs using Na-Alg when compared to the FTIR spectra of Na-Alg(s) (3332.48 cm−1). The formation of the band was hampered using certain −OH groups from Na-Alg [48]. The slight shift in peak after the incorporation of AgSr NPs can be seen which may be attributed to the successful stabilization of AgSr NPs.

FTIR spectra of (i) Na-Alg and (ii) Na-Alg-supported AgSr NPs.
The AgSr@Na-Alg spectra indicate an additional stronger absorption band at 892.66 cm−1, which corresponds to AgSr absorption, suggesting the synthesis of NPs in addition to the above-mentioned bands of pure Na-Alg. Furthermore, the large absorption bands observed at 1021.14 cm−1 could be explained by stretching of C═O and the hydroxyl group, respectively. The asymmetric and symmetric stretching of the carboxylate group vibrations could be explained by absorption peaks appearing at 1596.48 and 1405.62 cm−1, respectively. Results indicate that functional groups of Na-Alg are involved in synthesis/stabalization of NPs [49,50].
3.3 XRD studies
The crystalline material and amorphous organic biopolymeric phases of the Na-Alg powder sample were detected in this X-ray diffractogram. The increase in the size of the biopolymer-associated crystallites has been reflected by the peak height. The data for the powdered sample of Na-Alg-supported AgSr NPs was recorded over 2θ range of 10–80° (Figure 4). The crystal phase of the NPs and purity were also assessed using powder XRD. Results revealed the presence of a single-phase orthorhombic crystalline structure with a preferential orientation along (212) reflection. At different values of (their maxima centered) 2θ with varying miller indices indicated strong Bragg’s peaks with JCPDS card No. 65-4973. The diffraction peaks in the XRD pattern are narrow and crisp, indicating that the samples acquired so far are good crystalline materials indicating the crystallinity of the AgSr/Na-Alg sample. The results described above are in close comparison with previous reports [39,42,51]. The effect of annealing temperature on peak growth is considerable, indicating increased intensity of (300) peak. The 2θ = 29.97° for carbon and 21.2° can be related to Na-Alg and all other peaks indicate the presence of AgSr NPs. The intensity of the peaks at 31.8°, 35.5°, 38.1°, 40.6°, 42.1°, 46.7°, and 47.8° corresponds to the presence of higher concentration of AgSr metals in the sample. Using the Debye–Scherrer formula D avg = kλ/βcosθ, the crystallite size (D avg) of the AgSr NPs sample calculated is 12.95 nm. The shift in the nucleation process produced by metals’ interaction among themselves and with Na-Alg could be the reason behind the increase in crystallite size.

XRD pattern of Na-Alg-supported AgSr NPs.
3.4 Scanning electron microscopy characterization
The FE-SEM analysis of AgSr NPs stabilized by Na-Alg was performed to evaluate the surface morphology and analysis was done at different resolutions (Figure 5a–d). The visual representation of AgSr NPs at 5 and 10 µm can be seen in Figure 5a and b respectively. The AgSr NPs observed at 10 µm were found to have a pearl-like appearance with good separation and nearly zero agglomeration morphology. This means that AgSr NPs are well dispersed with good crystalline structure (Figure 5a). On the other hand, spherical and agglomeration-free AgSr NPs are embedded successfully on the surface of Na-Alg, which confirms the formation of stabilized NPs and can be seen from two different sites at the nanoscale (Figure 5a and b). The other perspective is that these NPs and Na-Alg also have greater active sites. So, these NPs stabilized by Na-Alg are a complete package for the catalysis process. Furthermore, the particle size distribution was also calculated for AgSr NPs ranging from 18 to 32 nm in terms of histogram using FE-SEM image. These results confirmed that the AgSr NPs were successfully embedded over semi-crystalline Na-Alg particles.

FE-SEM images of Na-Alg-supported AgSr NPs (a) 100 nm, (b) 200 nm, (c) 5 μm, and (d) 10 μm.
3.5 Catalytic potential of Na-Alg-supported AgSr NPs
The catalytic activity of Na-Alg-supported AgSr NPs has been investigated to check catalytic potential toward the degradation of organic pollutants (MO, MG dyes) present in wastewater. These NPs were used as water cleanser catalysts and time-dependent catalytic degradation of MO and MG was performed using AgSr NPs in the presence of NaBH4. A considerable decrease in the absorbance of MO solution was recorded at 476 nm λ max, indicating a decrease in its concentration correspondingly (Figure 6a). The UV–Vis spectra were monitored after 03 min interval and the spectral line became flat after 21 min which shows that total dye molecules have been degraded. The catalytic degradation of azo dyes using AgSr NPs with NaBH4 follows Langmuir Hinshelwood’s mechanism with pseudo-first-order kinetics [52]. Catalyst has shown outstanding performance for the removal of MO dye with 86.45% after 21 min with the apparent rate constant (k aap) value 0.1474 min−1 (Figure 6b and c). The results proved that Na-Alg-supported AgSr NPs can exhibit excellent catalytic ability to degrade organic pollutants in lesser time.

Spectra of catalytic degradation of MO dye (a) using NPs with NaBH4, (b) % removal, (c) kinetic studies, and (d) in the absence of NPs catalyst.
In the reaction mixture, both the ingredients, i.e., NPs and NaBH4 may instigate the process of dye degradation. However, to confirm whether degradation was due to Na-Alg-supported AgSr NPs with NaBH4 or just NaBH4 reducing the MO dye molecule. UV–Vis studies of MO dye degradation were also carried out in the absence of Na-Alg-supported AgSr NPs (Figure 6d). The spectra at 05 min interval show no significant decrease in the absorbance and the same was recorded even after 30 min. This confirms that the degradation was purely done in the presence of the catalyst and NaBH4.
Likewise, the degradation of MG dye was also tested using Na-Alg stabilized AgSr NPs in the presence of NaBH4 (Figure 7a), and the degradation was achieved in 24 min. The catalytic degradation process was completed in 21 min, but the slight decline in UV–Vis spectra shows some leftovers of the substrate were degraded and almost no molecule of MG dye remained in the aqueous medium. The percent removal was remarkable having a value of approximately 92% (Figure 7b) and the kinetics of this model experiment was also evaluated to determine the speed of reaction in terms of rate-constant; the result was according to need, with a value of 0.160 min−1 (Figure 7c). The confirmation of MG degradation was only possible with a catalyst in the presence of NaBH4. The role of the catalyst was confirmed by monitoring the degradation without the catalyst (Figure 7d). In the end, the degradation was not so considerable, and only a slight decrease was observed due to the absence of the catalyst. So, Na-Alg-supported AgSr NPs are important for the degradation of dyes to clean industrial wastewater.

Spectra of catalytic degradation of MG dye (a) using NPs with NaBH4, (b) % removal, (c) kinetic studies, and (d) in the absence of NPs catalyst.
3.6 Factor affecting dye degradation
The Na-Alg-supported AgSr NPs affecting catalytic degradation of MO and MB dyes were carried out in the presence of NaBH4. Different concentrations of the NPs (4.76, 6.72, 8.91, and 10.45 mg/mL) with constant concentrations of 20.1 mM NaBH4 and 0.02 mM MO dye were taken in separate flasks (Figure 8a). The slopes (Figure 8b) exhibited rapid degradation with k app values 0.1207, 0.1303, 0.1474, and 0.161 min−1 at 4.76, 6.72, 8.91, and 10.45 mg/mL, respectively. The apparent rate-constant (k app) was determined (Figure 8c), which shows a sharply inclined pattern corresponding to the fast removal percentage of the dye with increasing catalyst concentration. The percent removal (78.99–89.91%) recorded for different concentrations of catalyst was due to greater surface area availability by increasing catalyst concentration (Figure 8d). Pseudo-first-order kinetics was also observed for the catalytic degradation of MG dye using the same concentration as used in the case of MO dye in the presence of NaBH4 (Figure 9a).

(a) Effect of NPs’ different concentrations on catalytic degradation of MO dye, (b) slopes, (c) apparent rate-constant (k app) vs catalyst (NPs) various concentrations, and (d) % removal of different concentrations of NPs.

(a) Effect of NPs’ different concentrations on catalytic degradation of MG dye, (b) slopes, (c) apparent rate-constant (k app) vs catalyst (NPs) various concentrations, and (d) % removal of different concentrations of NPs.
The slope of kinetics was also determined to find the k app value (Figure 9b) and a sharp decline in slopes with time represents the fast degradation of MG dye triggered by Na-Alg-supported AgSr NPs. The trend of k app w.r.t. time is linear, which explains that the catalytic degradation increases by increasing catalyst concentration (Figure 9c), and the same trend was also reported by our group, in comparison with these results [53]. The results have proven that Na-Alg-supported AgSr NPs exhibited exceptional catalytic activity, as the concentration of MG dye was increased with robust % removal having values from 88 to 93% (Figure 9d). The catalytic potential of AgSr NPs has been compared with previously reported data in Table 1.
Comparison of catalytic potential of Na-Alg-supported AgSr NPs with previous studies
| Nanomaterial | Catalyst quantity | Dyes | Type of reaction | Percent degradation (%) | Degradation time | Ref. |
|---|---|---|---|---|---|---|
| AgSr NPs | 14.5 mg/mL | MO | Catalysis | 86.45 | 21 min | This study |
| MG | 91.74 | 24 min | ||||
| Sr/Ag2O (Sr-doped Ag2O) nanocomposite (NC) | 5% of 0.01 g | MB | Photocatalysis | 96.63 | 60 min | [54] |
| 5% of 0.01 g | MO | 69.23 | 80 min | |||
| 9% of 0.01 g | Rh-B | 96.89 | 70 min | |||
| AgNPs@SrCO3 (AgNPs decorated with Sr-carbonate) NC | 0.1 g | CR | Adsorption | 73.90 | 15 min | [55] |
| Ag-SrTiO3 NC | 4.2% | AO7 | Photocatalysis | 78.10 | 6 h | [56] |
| Ag/AgCl/SrTiO3 nanocubes | 0.050 g | Rh-B | Photocatalysis | 96 | 30 min | [57] |
| MO | 93 | 40 min | ||||
| MB | 96 | 70 min | ||||
| Phenol | 70 | 4 h | ||||
| Bisphenol | 83 | 4 h |
4 Conclusion
The synthesis of Na-Alg stabilized AgSr NPs has been achieved followed by evaluation of their morphological features. According to FE-SEM observations, NPs were obtained orthorhombic crystal phase and the particle size ranged from 18 to 32 nm without significant agglomeration. These spherical-shaped NPs were found to have outstanding catalytic potential for the degradation of MO and MG dyes in aqueous media. The catalytic potential of NPs in the presence of NaBH4 was monitored and the results have proven that NPs own remarkable catalytic properties to remove carcinogenic dyes from wastewater and can be used as a water cleanser tool in the future against different organic pollutants. Researchers have extended their spectrum of studies toward developing novel composite materials and their applications in environmental remediation. In the future, the fabrication of a new combination of biopolymer-supported metal NPs can be achieved followed by utilizing these composites for environmental, biomedical, and energy storage applications.
Acknowledgments
S. J. Park was supported by the Basic Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2018R1A6A1A03025526 and No. 2020R1I1A3063782). This work was also supported by the BK-21 FOUR program through the National Research Foundation of Korea (NRF) under the Ministry of Education. Authors acknowledge the support of ORIC UOL for the completion of research project. The work was supported by Researchers Supporting Project number (RSPD2024R663), King Saud University, Riyadh, Saudi Arabia.
-
Funding information: S. J. Park was supported by the Basic Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2018R1A6A1A03025526 and No. 2020R1I1A3063782). This work was also supported by the BK-21 FOUR program through the National Research Foundation of Korea (NRF) under the Ministry of Education. The work was supported by Researchers Supporting Project number (RSPD2024R663), King Saud University, Riyadh, Saudi Arabia.
-
Author contributions: All authors have accepted responsibility for the entire content of this article and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Soni RA, Rizwan MA, Singh S. Opportunities and potential of green chemistry in nanotechnology. Nanotechnol Environ Eng. 2022;7(3):661–73.10.1007/s41204-022-00233-5Search in Google Scholar
[2] Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F. The history of nanoscience and nanotechnology: from chemical–physical applications to nanomedicine. Molecules. 2019;25(1):112.10.3390/molecules25010112Search in Google Scholar PubMed PubMed Central
[3] Zhu S, Meng H, Gu Z, Zhao Y. Research trend of nanoscience and nanotechnology–A bibliometric analysis of Nano Today. Nano Today. 2021;39:101233.10.1016/j.nantod.2021.101233Search in Google Scholar
[4] Khalid M. Nanotechnology and chemical engineering as a tool to bioprocess microalgae for its applications in therapeutics and bioresource management. Crit Rev Biotechnol. 2020;40(1):46–63.10.1080/07388551.2019.1680599Search in Google Scholar PubMed
[5] Liu W, Huang F, Liao Y, Zhang J, Ren G, Zhuang Z, et al. Treatment of CrVI‐containing Mg(OH)2 nanowaste. Angew Chem. 2008;120(30):5701–4.10.1002/ange.200800172Search in Google Scholar
[6] Lee G, Choi HE, Hong SH, Choi M, Han DW, Lee J, et al. Upconversion nanomaterials and delivery systems for smart photonic medicines and healthcare devices. Adv Drug Delivery Rev. 2022;188:114419.10.1016/j.addr.2022.114419Search in Google Scholar PubMed
[7] Abbasi BH, Fazal H, Ahmad N, Ali M, Giglioli-Guivarch N, Hano C. Nanomaterials for cosmeceuticals: nanomaterials-induced advancement in cosmetics, challenges, and opportunities. Nanocosmetics. Elsevier; 2020. p. 79–108.10.1016/B978-0-12-822286-7.00005-XSearch in Google Scholar
[8] Chaudhary P, Fatima F, Kumar A. Relevance of nanomaterials in food packaging and its advanced future prospects. J Inorg Organomet Polym Mater. 2020;30:5180–92.10.1007/s10904-020-01674-8Search in Google Scholar PubMed PubMed Central
[9] Gautam S, Bhatnagar D, Bansal D, Batra H, Goyal N. Recent advancements in nanomaterials for biomedical implants. Biomed Eng Adv. 2022;3:100029.10.1016/j.bea.2022.100029Search in Google Scholar
[10] Chandrakala V, Aruna V, Angajala G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emerg Mater. 2022;5:1593–615.10.1007/s42247-021-00335-xSearch in Google Scholar PubMed PubMed Central
[11] Li Y, Zheng X, Chu Q. Bio-based nanomaterials for cancer therapy. Nano Today. 2021;38:101134.10.1016/j.nantod.2021.101134Search in Google Scholar
[12] Song Z, Han D, Yang M, Huang J, Shao X, Li H. Formic acid formation via direct hydration reaction (CO + H2O → HCOOH) on magnesia-silver composite. Appl Surf Sci. 2023;607:155067.10.1016/j.apsusc.2022.155067Search in Google Scholar
[13] Saleh TA. Protocols for synthesis of nanomaterials, polymers, and green materials as adsorbents for water treatment technologies. Environ Technol Innov. 2021;24:101821.10.1016/j.eti.2021.101821Search in Google Scholar
[14] Jamkhande PG, Ghule NW, Bamer AH, Kalaskar MG. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J Drug Delivery Sci Technol. 2019;53:101174.10.1016/j.jddst.2019.101174Search in Google Scholar
[15] Saleh TA. Nanomaterials: Classification, properties, and environmental toxicities. Environ Technol Innov. 2020;20:101067.10.1016/j.eti.2020.101067Search in Google Scholar
[16] Xiao Y, Du J. Superparamagnetic nanoparticles for biomedical applications. J Mater Chem B. 2020;8(3):354–67.10.1039/C9TB01955CSearch in Google Scholar
[17] Chen Z, Ma T, Li Z, Zhu W, Li L, et al. Enhanced photocatalytic performance of S-scheme CdMoO4/CdO nanosphere photocatalyst. J Mater Sci & Technol. 2024;179:198–207.10.1016/j.jmst.2023.07.029Search in Google Scholar
[18] Banvillet G, Bugaut M, Doineau E, Taguet A, Le Moigne N, Rojas OJ. Advances in the production of cellulose nanomaterials and their use in engineering (Bio) plastics. Annual Plant: Sources of Fibres, Nanocellulose and Cellulosic Derivatives. Composites Science and Technology. Singapore: Springer; 2023. p. 333–93.10.1007/978-981-99-2473-8_12Search in Google Scholar
[19] Narancic T, Cerrone F, Beagan N, O'Connor KE. Recent advances in bioplastics: application and biodegradation. Polymers. 2020;12(4):920.10.3390/polym12040920Search in Google Scholar PubMed PubMed Central
[20] Debnath B, Gharami S, Bhattacharyya S, Das A, Das A. Paradigm shift in environmental remediation toward sustainable development: biodegradable materials and ICT applications. Biodegradable Materials and Their Applications. Wiley; 2022. p. 565–91.10.1002/9781119905301.ch20Search in Google Scholar
[21] Aslan S, Altuntaş DB. Biocomposites and bioplastics in electrochemical applications. Handbook of Bioplastics and Biocomposites Engineering Applications. Wiley; 2023. p. 491–512.10.1002/9781119160182.ch23Search in Google Scholar
[22] Zhao X, Vodovotz Y. Bioplastics‐based nanocomposites for packaging applications. Handbook of Bioplastics and Biocomposites Engineering Applications. Wiley; 2023. p. 425–44.10.1002/9781119160182.ch19Search in Google Scholar
[23] Wang Z, Zou Y, Li Y, Cheng Y. Metal‐containing polydopamine nanomaterials: catalysis, energy, and theranostics. Small. 2020;16(18):1907042.10.1002/smll.201907042Search in Google Scholar PubMed
[24] Mamun MAA, Yuce MR. Recent progress in nanomaterial enabled chemical sensors for wearable environmental monitoring applications. Adv Funct Mater. 2020;30(51):2005703.10.1002/adfm.202005703Search in Google Scholar
[25] Guo L, Wang H, Wang Y, Liu F, Feng L. Organic polymer nanoparticles with primary ammonium salt as potent antibacterial nanomaterials. ACS Appl Mater Interfaces. 2020;12(19):21254–62.10.1021/acsami.9b19921Search in Google Scholar PubMed
[26] Shahzadi I, Islam M, Saeed H, Haider A, Shahzadi A, Haider J, et al. Formation of biocompatible MgO/cellulose grafted hydrogel for efficient bactericidal and controlled release of doxorubicin. Int J Biol Macromolecules. 2022;220:1277–86.10.1016/j.ijbiomac.2022.08.142Search in Google Scholar PubMed
[27] Pandit S, Gaska K, Mokkapati V, Forsberg S, Svensson M, Kádár R, et al. Antibacterial effect of boron nitride flakes with controlled orientation in polymer composites. RSC Adv. 2019;9(57):33454–9.10.1039/C9RA06773FSearch in Google Scholar
[28] Zhang Y, Zhao M, Huang J, Zhao N, Yu H. Controllable synthesis, photocatalytic property, and mechanism of a novel POM-based direct Z-scheme nano-heterojunction α-Fe2O3/P2Mo18. Molecules. 2023;28(18):6671.10.3390/molecules28186671Search in Google Scholar PubMed PubMed Central
[29] Ramos-Sanchez JE, Camposeco R, Lee SW, Rodríguez-González V. Sustainable synthesis of AgNPs/strontium-titanate-perovskite-like catalysts for the photocatalytic production of hydrogen. Catal Today. 2020;341:112–9.10.1016/j.cattod.2019.08.020Search in Google Scholar
[30] Wang Z, Chen C, Liu H, Hrynshpan D, Savitskaya T, Chen J, et al. Enhanced denitrification performance of Alcaligenes sp. TB by Pd stimulating to produce membrane adaptation mechanism coupled with nanoscale zero-valent iron. Sci Total Environ. 2020;708:135063.10.1016/j.scitotenv.2019.135063Search in Google Scholar PubMed
[31] Jin W, Zhang Y. Sustainable electrochemical extraction of metal resources from waste streams: from removal to recovery. ACS Sustain Chem Eng. 2020;8(12):4693–707.10.1021/acssuschemeng.9b07007Search in Google Scholar
[32] Lin Y, Cao Y, Yao Q, Chai OJH, Xie J. Engineering noble metal nanomaterials for pollutant decomposition. Ind Eng Chem Res. 2020;59(47):20561–81.10.1021/acs.iecr.0c04258Search in Google Scholar
[33] Zheng Y, Liu Y, Guo X, Chen Z, Zhang W, Wang Y, et al. Sulfur-doped g-C3N4/rGO porous nanosheets for highly efficient photocatalytic degradation of refractory contaminants. J Mater Sci Technol. 2020;41:117–26.10.1016/j.jmst.2019.09.018Search in Google Scholar
[34] Iqbal MZ, Alam S, Afzal AM, Iqbal MJ, Yaqoob K, Kamran MA, et al. Binary composites of strontium oxide/polyaniline for high performance supercapattery devices. Solid State Ion. 2020;347:115276.10.1016/j.ssi.2020.115276Search in Google Scholar
[35] Zaoui M, Sellami B, Boufahja F, Faloda F, Nahdi S, Alrezaki A, et al. Effects of ferroelectric oxides of barium strontium titanate (Ba0. 85Sr0. 15TiO3) nanoparticles on Ruditapes decussatus assessed through chemical, physiological, and biochemical methods. Chemosphere. 2021;265:129078.10.1016/j.chemosphere.2020.129078Search in Google Scholar PubMed
[36] Ma X, He S, Qiu B, Luo F, Guo L, Lin Z. Noble metal nanoparticle-based multicolor immunoassays: an approach toward visual quantification of the analytes with the naked eye. ACS Sens. 2019;4(4):782–91.10.1021/acssensors.9b00438Search in Google Scholar PubMed
[37] Mustieles Marin I, Asensio JM, Chaudret B. Bimetallic nanoparticles associating noble metals and first-row transition metals in catalysis. ACS Nano. 2021;15(3):3550–6.10.1021/acsnano.0c09744Search in Google Scholar PubMed
[38] Atta AM. Immobilization of silver and strontium oxide aluminate nanoparticles integrated into plasma‐activated cotton fabric: Luminescence, superhydrophobicity, and antimicrobial activity. Luminescence. 2021;36(4):1078–88.10.1002/bio.4033Search in Google Scholar PubMed
[39] Li Y, Wang W, Han J, Li Z, Wang Q, Lin X, et al. Synthesis of silver-and strontium-substituted hydroxyapatite with combined osteogenic and antibacterial activities. Biol Trace Elem Res. 2022;200:1–12.10.1007/s12011-021-02697-zSearch in Google Scholar PubMed
[40] Bano S, Akhtar M, Yasir M, Salman Maqbool M, Niaz A, Wadood A, et al. Synthesis and characterization of silver–strontium (Ag–Sr)-doped mesoporous bioactive glass nanoparticles. Gels. 2021;7(2):34.10.3390/gels7020034Search in Google Scholar PubMed PubMed Central
[41] Ueno S, Nakashima K, Sakamoto Y, Wada S. Synthesis of silver-strontium titanate hybrid nanoparticles by sol-gel-hydrothermal method. Nanomaterials. 2015;5(2):386–97.10.3390/nano5020386Search in Google Scholar PubMed PubMed Central
[42] Sasikala R, Kandasamy M, Suresh S, Ragavendran V, Sasirekha V, Pearce JM, et al. Enhanced dye-sensitized solar cell performance using strontium titanate perovskite integrated photoanodes modified with plasmonic silver nanoparticles. J Alloy Compd. 2021;889:161693.10.1016/j.jallcom.2021.161693Search in Google Scholar
[43] Ranga N, Poonia E, Jakhar S, Sharma AK, Kumar A, Devi S, et al. Enhanced antimicrobial properties of bioactive glass using strontium and silver oxide nanocomposites. J Asian Ceram Societies. 2019;7(1):75–81.10.1080/21870764.2018.1564477Search in Google Scholar
[44] He Z, Qi S, Zhong X, Ma H, Wang P, Qiu H. Preparation and microwave-absorbing properties of silver-coated strontium ferrite with polyaniline via in situ polymerization. J Alloy Compd. 2015;621:194–200.10.1016/j.jallcom.2014.09.187Search in Google Scholar
[45] Yaseen B, Gangwar C, Kumar I, Sarkar J, Naik RM. Detailed kinetic and mechanistic study for the preparation of silver nanoparticles by a chemical reduction method in the presence of a Neuroleptic Agent (Gabapentin) at an alkaline pH and its characterization. ACS Omega. 2022;7(7):5739–50.10.1021/acsomega.1c05499Search in Google Scholar PubMed PubMed Central
[46] Zahran MK, Ahmed HB, El-Rafie MH. Alginate mediate for synthesis controllable sized AgNPs. Carb Poly. 2014;111:10–7.10.1016/j.carbpol.2014.03.029Search in Google Scholar PubMed
[47] Farea M, Abdelghany A, Oraby AJR. Optical and dielectric characteristics of polyethylene oxide/sodium alginate-modified gold nanocomposites. RSC Adv. 2020;10(62):37621–30.10.1039/D0RA07601ESearch in Google Scholar
[48] Shao Y, Wu C, Wu T, Yuan C, Chen S, Ding T, et al. Green synthesis of sodium alginate-silver nanoparticles and their antibacterial activity. Int J Biol Macromol. 2018;111:1281–92.10.1016/j.ijbiomac.2018.01.012Search in Google Scholar PubMed
[49] Marangoni Júnior L, da Silva RG, Anjos CAR, Vieira RP, Alves RMV. Effect of low concentrations of SiO2 nanoparticles on the physical and chemical properties of sodium alginate-based films. Carbohydr Polym. 2021;269:118286.10.1016/j.carbpol.2021.118286Search in Google Scholar PubMed
[50] Belattmania Z, Bentiss F, Jama C, Barakate M, Katif C, Reani A, Sabour B. Biosynthesis and characterization of silver nanoparticles using sodium alginate from the invasive macroalga Sargassum muticum. Bionanosci. 2018;8:617–23.10.1007/s12668-018-0518-3Search in Google Scholar
[51] Arumugam B, Muthukutty B, Chen SM, Subramanian BT, Biju VMN, Ramaraj SK. Electrochemical reduction of Procardia drug with aid of silver phosphate/strontium phosphate nanoparticles (AgP/SrP NPs) modified glassy carbon electrode. Microchem J. 2020;159:105565.10.1016/j.microc.2020.105565Search in Google Scholar
[52] Razdan N, Bhan A. Catalytic site ensembles: A context to reexamine the Langmuir-Hinshelwood kinetic description. J Catal. 2021;404:726–44.10.1016/j.jcat.2021.09.016Search in Google Scholar
[53] Ali F, Mehmood S, Ashraf A, Saleem A, Younas U, Ahmad A, et al. Ag–Cu embedded SDS nanoparticles for efficient removal of toxic organic dyes from water medium. Ind Eng Chem Res. 2023;62(11):4765–77.10.1021/acs.iecr.2c03460Search in Google Scholar
[54] Kiani FA, Shamraiz U, Badshah A. Enhanced photo catalytic activity of Ag2O nanostructures through strontium doping. Mater Res Express. 2020;7(1):015035.10.1088/2053-1591/ab608cSearch in Google Scholar
[55] Nassar AM, Elseman AM, Alsohaimi IH, Alotaibi NF, Khan A. Diaqua oxalato strontium(ii) complex as a precursor for facile fabrication of Ag-NPs@SrCO3, characterization, optical properties, morphological studies and adsorption efficiency. J Coord Chem. 2019;72(5–7):771–85.10.1080/00958972.2019.1588964Search in Google Scholar
[56] Xian T, Yang H, Di LJ, Dai JF. Enhanced photocatalytic activity of SrTiO3 particles by surface decoration with Ag nanoparticles for dye degradation. Phys Scr. 2015;90(5):055801.10.1088/0031-8949/90/5/055801Search in Google Scholar
[57] Yang SF, Niu CG, Huang DW, Zhang H, Liang C, Zeng GM. SrTiO3 nanocubes decorated with Ag/AgCl nanoparticles as photocatalysts with enhanced visible-light photocatalytic activity towards the degradation of dyes, phenol and bisphenol A. Environ Sci: Nano. 2017;4(3):585–95.10.1039/C6EN00597GSearch in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy

