Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
-
Bhargav Akkinepally
and Dongwhi Choi
Abstract
Electrode materials comprising SnO2 quantum dots embedded within ZnO hexagonal prisms were successfully synthesized for building cost-effective energy-storage devices. Extensive structural and functional characterizations were performed to assess the electrochemical performance of the electrodes. SEM–EDS results confirm a uniform distribution of SnO2 quantum dots across ZnO. The integration of SnO2 quantum dots with ZnO hexagonal prisms markedly improved the electrochemical behavior. The analysis of electrode functionality conducted in a 3 M KOH electrolyte revealed specific capacitances of 949.26 and 700.68 F g⁻1 for SnO2@ZnO and ZnO electrodes, respectively, under a current density of 2 A g⁻1. After undergoing 5,000 cycles at a current density of 15 A g⁻1, the SnO2@ZnO and ZnO electrodes displayed impressive cycling stability, maintaining specific capacitance retention rates of 89.9 and 92.2%, respectively. Additionally, a symmetric supercapacitor (SSC) device constructed using the SnO2@ZnO electrode showcased exceptional performance, exhibiting a specific capacitance of 83 F g⁻1 at 1.2 A g⁻1. Impressive power and energy densities were achieved by the device, with values reaching 2,808 and 70.2 W kg⁻1, respectively. Notably, the SnO2@ZnO SSC device maintained a capacity preservation of 75% throughout 5,000 galvanostatic charge–discharge sequences. The outcomes highlight the potential of SnO2@ZnO hexagonal prisms as candidates for energy-storage applications, offering scalability and cost-effectiveness. The proposed approach enhances the electrochemical performance while ensuring affordability, facilitating the creation of effective and financially feasible energy storage solutions.
1 Introduction
The increasing need for clean and sustainable energy solutions has accelerated the advancement of effective energy storage devices [1,2,3,4,5,6]. Supercapacitors, also known as electrochemical capacitors, have emerged as potential candidates because of their unique characteristics [7]. They serve as a link between traditional capacitors and batteries, offering superior power density than batteries and remarkable energy density than capacitors [8,9]. This translates to exceptionally fast charge/discharge cycles, making them ideal for applications that require instantaneous power delivery and uptake (such as in electric vehicles), integration of renewable energy sources into the grid, and portable electronics [10]. Despite their immense potential, supercapacitors still face challenges that limit their widespread application, particularly in the identification and development of effective electrode materials [11,12]. Ideally, these materials should possess a synergistic combination of properties, such as high capacitance, excellent rate capability, and extended cyclic stability [13]. A high capacitance corresponds to the capacity to store a significant quantity of electrical energy, directly affecting the energy density of a supercapacitor device [14,15]. To achieve excellent rate capability, the electrode material should allow for rapid charge/discharge cycles, ensuring that the supercapacitor can deliver and absorb energy quickly [16]. This attribute is vital for scenarios necessitating frequent bursts of power, such as the acceleration of electric vehicles. In addition to achieving extended cycling stability, the developed supercapacitors should undergo degradation, resulting in a gradual decline in capacitance over time. Electrodes with exceptional cycling stability can maintain their performance for long durations, thereby extending the lifespan of the device [17]. In this regard, metal oxides [18] have been extensively explored for supercapacitor electrodes (cerium dioxide [19], iron(iii) oxide [20]) because of their abundance, environmental friendliness, and inherent electrochemical properties. SnO2 and ZnO are two prominent materials in this category [21,22,23]. ZnO is acknowledged as a versatile semiconductor with notable electrical conductivity, reaching up to 230 S/cm [24,25]. The ability to adjust the bandgap, morphology, and size of ZnO allows for its extensive utilization across various device technologies [26]. ZnO offers several advantages, including a high theoretical capacitance, good environmental stability, and relatively high conductivity compared to that of other metal oxides [27]. However, ZnO suffers from fast capacitance decay during charge/discharge cycles. This rapid degradation significantly reduces the long-term performance and stability of a supercapacitor [28]. SnO2 boasts several attractive features for supercapacitor applications. It possesses a high theoretical capacitance, indicating its potential to store significant amounts of electrical energy [29,30]. Additionally, SnO2 exhibits pseudocapacitive behavior, a mechanism that enhances the capacitance compared with the pure electrical double-layer capacitance [31]. However, a significant drawback of using SnO2 is its low electrical conductivity, which hinders the efficient transfer of electrons within the electrode, ultimately affecting the rate capability of the supercapacitor [32]. Hence, research endeavors have concentrated on mitigating the drawbacks of individual metal oxides and exploring composite materials that leverage the strength of each component [33]. In this context, the utilization of SnO2-decorated ZnO hexagonal prisms shows promise in advancing the development of high-performance supercapacitor electrodes. However, working on SnO2/ZnO composites for supercapacitor applications may reveal a gap in knowledge regarding the specific design and morphology of the composite material for optimal performance. The use of ZnO hexagonal prisms as a scaffold decorated with SnO2 nanoparticles offers a unique approach with several potential benefits. The hexagonal prismatic structure of ZnO provides a large surface area to accommodate the deposition of SnO2 nanoparticles [34]. This increased surface area can enhance the number of electrochemically active sites available for charge storage, potentially leading to higher capacitance. Further decoration of the ZnO hexagonal prisms with well-dispersed SnO2 quantum dots can improve the overall conductivity of the composite electrode. This addresses the limitations of pristine SnO2 while retaining its high theoretical capacitance. Thus, the combination of SnO2 and ZnO could potentially generate a synergistic effect in which the advantages of each material complement the other. For instance, SnO2 can enhance the capacitance of a composite, whereas ZnO can improve its cycling stability.
This study aims to bridge this knowledge gap by comprehensively investigating SnO2-decorated ZnO hexagonal prisms as supercapacitor electrodes. We explored the synthesis of these composite materials with controlled morphology and tailored SnO2 loading. Extensive testing was conducted to evaluate the electrochemical performance of the electrodes, with specific attention given to capacitance, rate capability, and cycling stability. A detailed analysis was conducted to decipher the structure–property relationships between the morphology, composition, and resulting electrochemical behavior of the composite electrodes by unveiling the performance potential and underlying mechanisms of SnO2-decorated ZnO hexagonal prisms.
2 Materials and methods
2.1 Material synthesis and preparation
2.1.1 SnO2 quantum dots
To prepare SnO2 quantum dots, 2.5 g of SnCl4·5H2O (Daejung Chemicals and Metals Co., Korea) was dissolved in DI water (130 ml) and agitated at 600 rpm for 5 min. Subsequently, 5.3 ml of hydrazine (N2H4 – Sigma-Aldrich, USA) was gradually added dropwise to the solution while maintaining the agitation for 20 more minutes at a temperature of 25°C. The solution was then heated to 105°C (in the absence of agitation) for 19 h. The residue from the mixture was retrieved via centrifugation, followed by thorough washing with water and ethanol, typically lasting 4–5 cycles. Following that, it was placed in an oven at 75°C and dried for 14 h.
2.1.2 SnO2 quantum dots interspersed ZnO hexagonal prisms
To prepare SnO2-decorated ZnO nanostructures, equivalent amounts of commercially obtained ZnO (Sigma-Aldrich, USA) and synthesized SnO2 quantum dots were separately dispersed in 50 ml of water and stirred for 60 min to achieve a homogeneous liquid solution. Subsequently, the SnO2-based solution was added dropwise to the ZnO solution under continuous stirring for 3 h at room temperature (25°C). Following that, the resulting material was subjected to centrifugation and washed with water and ethanol four to six times, followed by drying for 20 h at 80°C in a hot air oven.
2.2 Electrode fabrication
The assembly process of the working electrode entailed the meticulous application of a slurry onto a Ni-foam using the drop-casting technique. Ethanol was added to ensure a homogeneous mixture, which was then uniformly applied to pre-cleaned and pre-weighed Ni-foam substrates (1 × 1 cm2). The slurry was prepared by mixing the active material with carbon spheres, which served as an electrically conductive additive, in a weight ratio of 8:2. The active material was loaded onto each electrode at a mass of approximately 1 mg. Following that, the electrodes were dried overnight at 80°C to eliminate any residual solvent and ensure the formation of well-defined electrode structures.
2.3 Electrochemical performance assessment
In the electrochemical setup, an Ag/AgCl and a Pt wire were utilized as the reference electrode and counter electrode, respectively. An active material (ZnO, SnO2, or SnO2@ZnO) drop-cast onto a Ni-foam substrate served as the working electrode. All electrochemical assessments were carried out in a 3.0 M KOH electrolyte. Several analytical methods were used to explore the electrochemical attributes. Cyclic voltammetry (CV) was employed to examine the redox characteristics of the electrodes. To investigate the charge-transfer resistance and interfacial features, electrochemical impedance spectroscopy (EIS) was used. To assess the energy-storage capabilities of the electrodes, galvanostatic charge/discharge (GCD) analyses were employed. A potentiostat instrument (SP-200 Bio-Logic) was utilized to assess all the electrochemical evaluations.
2.4 Device assembly and testing
To assemble the symmetric supercapacitor (SSC) device, SnO2@ZnO was deposited onto Ni-foam, fulfilling the roles of both positive and negative electrodes. A 3.0 M KOH solution was used as an electrolyte. CV of the device was performed by employing incremental sweep rates from 5 to 200 mV s⁻1 under a potential span of 0–1.3 V. GCD analysis was carried out by employing current densities ranging from 1.2 to 2 A g⁻1. Furthermore, EIS assessments were conducted within a frequency range of 200 kHz to 0.1 Hz using an open circuit voltage. The specific capacitance (C s) was calculated using the following equation:
where I stands for the applied current (A), Δt signifies the discharge time (s), ΔV indicates the voltage window (V), m represents the active material mass (g), and C s denotes the specific capacitance (F g⁻1). The following equations were used to calculate the energy density (E d) and power density (P d) of the system:
3 Results and discussion
3.1 Material characterization
X-ray diffraction (XRD) was utilized to examine the crystalline properties of ZnO, SnO2, and SnO2@ZnO, and the obtained XRD data are presented in Figure 1. All the discernible peaks of ZnO are precisely indexed and aligned with JCPDS card No. 75-1526, confirming its high crystallinity. A prominent high-intensity peak is observed at 36.1°, corresponding to the (101) plane of hexagonal ZnO. The XRD pattern for the SnO2 quantum dots exhibits peaks at 26.2° (110), 33.6° (101), and 51.8° (211), indicating a tetragonal crystal structure (JCPDS card No. 77-0450). The XRD peaks for the SnO2-decorated ZnO nanostructure include one at 36.5°, corresponding to the (101) plane, with a slight increase attributed to SnO2. Importantly, the hexagonal crystal structure of the ZnO nanostructure remains unaffected, indicating that the introduction of SnO2 does not alter the ZnO structure but rather enhances its crystallinity. The X-ray intensity initially exhibits a maximum on the surface of SnO2 compared to that in the ZnO nanostructure because the characteristic peaks of ZnO have higher intensities in the SnO2@ZnO samples. Information on the interplanar distance, particle size, and strain was obtained from the XRD results utilizing Bragg’s law, Scherrer’s formula, and the Williamson–Hall formula, respectively. The interplanar distance d (101) increased in the SnO2@ZnO sample, indicating the introduction of smaller Sn atoms into the lattice. The crystalline sizes for (100), (101), (102), and (110) peaks were increased in SnO2@ZnO when compared to pristine ZnO but remained constant for the (002) peak. This can be due to the interaction between SnO2 and ZnO that may alter the surface energy of the composite material, affecting the kinetics of crystal growth differently for various crystallographic planes. These subtle variations in size can be attributed to differences in the growth mechanisms, preparation temperatures, and particle sizes. The observed distinctions align with prior reports on similarly decorated compounds, confirming the successful decoration of SnO2 on ZnO.

XRD patterns for ZnO hexagonal prisms (ZnO), SnO2 quantum dots (SnO2), and SnO2 quantum dot-decorated ZnO hexagonal prisms (SnO2@ZnO).
Examination of the morphology of the samples was carried out by utilizing field emission scanning electron microscopy (FESEM), as shown in Figure 2(a)–(c). The FESEM images reveal hexagonal prisms with an irregular size distribution for the ZnO nanocrystals. Figure 2(b) shows large grains of SnO2 with irregular shapes, likely resulting from particle accumulation owing to the reaction conditions. Rapid nucleation facilitates the even distribution of SnO2 quantum dots over the ZnO hexagonal nanostructure, as observed in Figure 2(c).
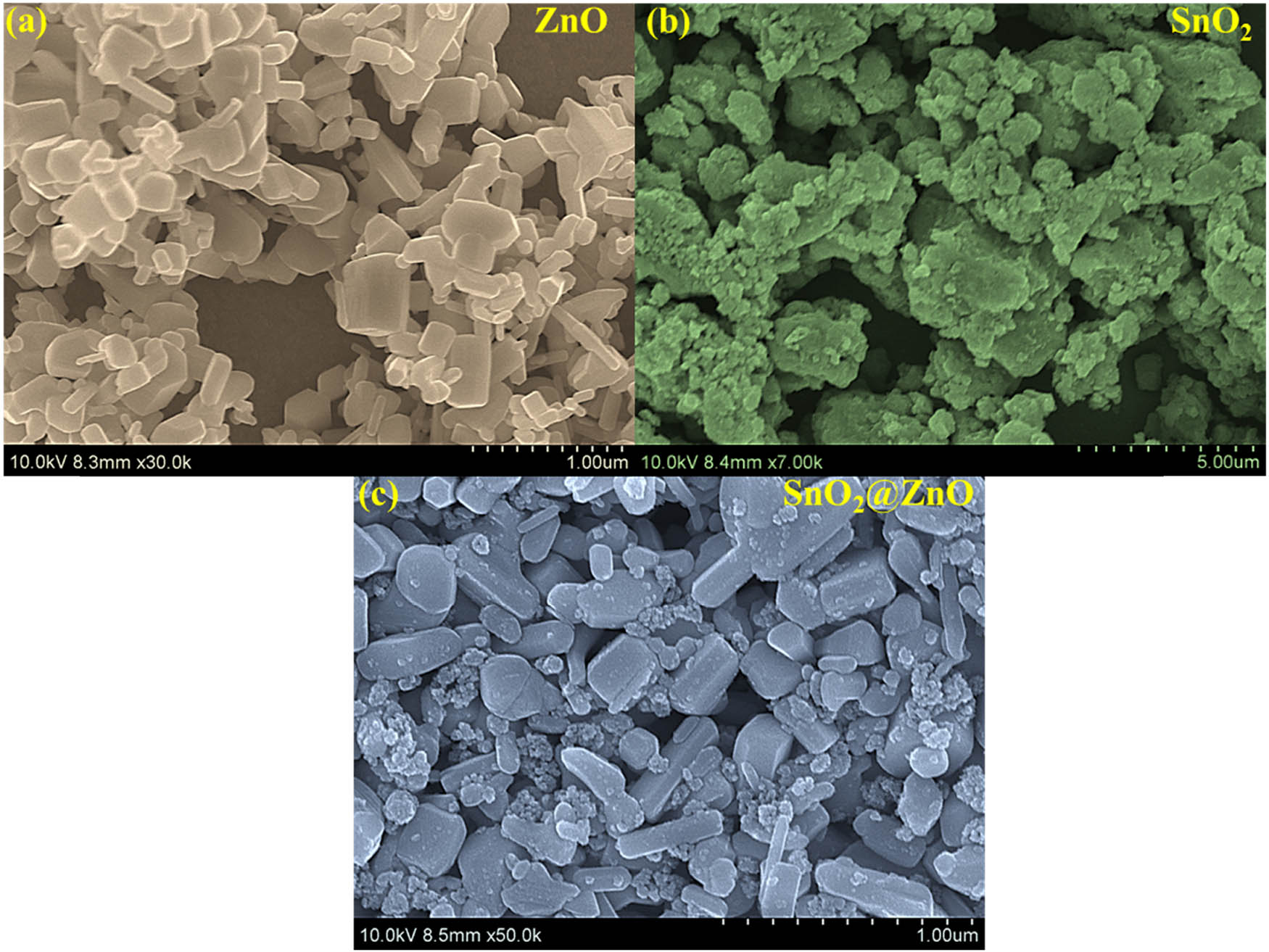
SEM analysis of (a) ZnO hexagonal prisms, (b) SnO2 quantum dots, and (c) SnO2@ZnO hexagonal prisms.
The morphological characteristics of SnO2@ZnO were further investigated by field emission transmission electron microscopy (FETEM). Figure 3(a)–(c) presents FETEM images of the SnO2@ZnO hexagonal prisms. Upon magnification, the FETEM micrographs present well-defined lattice fringes, indicating interplanar spacings of 0.47 nm (ZnO) and 0.33 nm (SnO2) (Figure 3(b) and (c)). The selected area electron diffraction (SAED) pattern (Figure 3(d)) conclusively illustrates the polycrystalline nature of the SnO2@ZnO hexagonal prisms, revealing interconnected nanostructures [35]. The results align with the XRD findings, offering additional confirmation of the effective amalgamation of SnO2 quantum dots and ZnO nanostructures. Additionally, the elemental distribution within the SnO2@ZnO specimen is shown in Figure 3(e)–(h) illustrates the EDS mapping. The homogeneous dispersion of core elements across the sample, revealed by the mapping, suggests a uniform distribution.
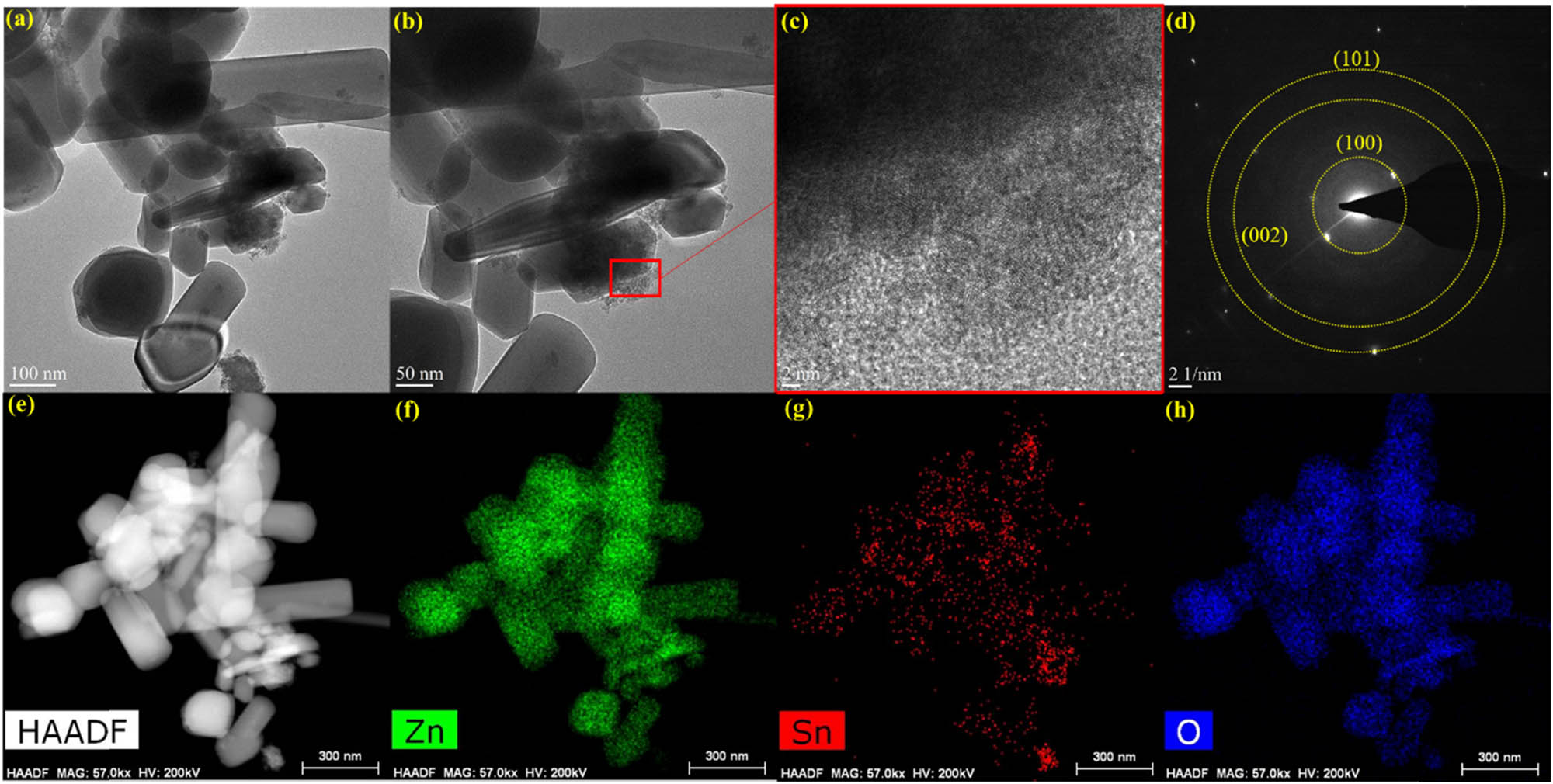
(a)–(c) FETEM images of SnO2@ZnO, (d) SAED patterns of SnO2@ZnO, and (e)–(h) EDS mapping.
Porosity and specific surface area characteristics were also assessed through Brunauer–Emmett–Teller (BET) analysis (Figure 4). Nitrogen adsorption–desorption isotherms of the specimens display a distinct type IV hysteresis, featuring a loop at relative pressures between 0.9 and 1.0, which signifies the existence of mesopores, as depicted in Figure 4(a). This mesoporous configuration aids in minimizing the electron transport distance, thereby optimizing the electron transfer efficiency [36]. In Figure 4(b), the average pore diameters are shown to be 3.6 nm for ZnO and 2.7 nm for SnO2@ZnO, employing the Barrett–Joyner–Halenda (BJH) method. These results indicate the presence of a conventional mesoporous configuration in both samples [37]. The BET surface area measurements (Figure 4(c)) reveal values of 3.91 m2/g for ZnO and 9.39 m2/g for SnO2@ZnO, demonstrating a threefold rise in surface area ascribed to the decoration of SnO2 onto ZnO. This enhancement in surface area is evident in the comparative electrochemical performance analysis of the two electrodes.
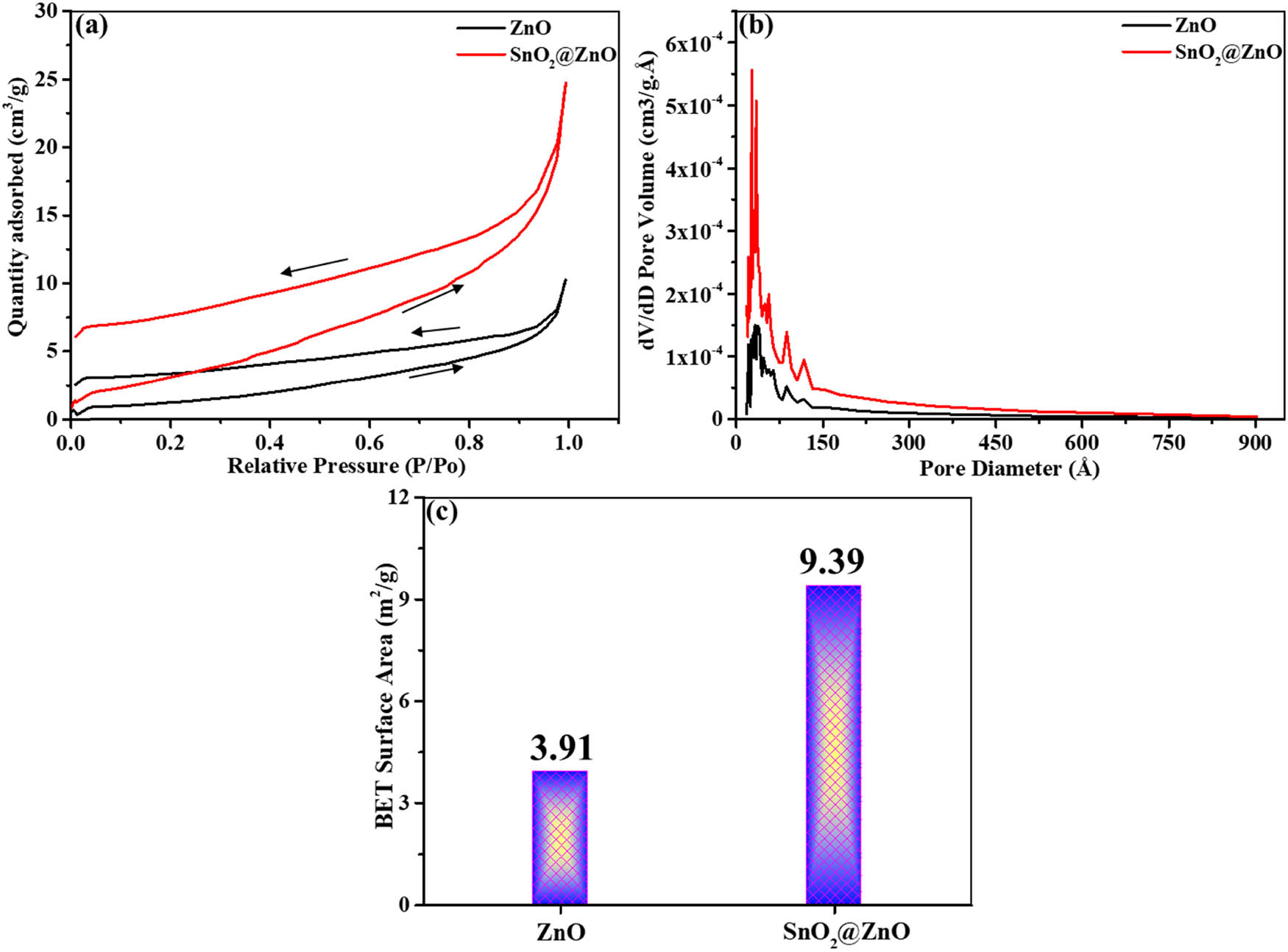
BET analysis. (a) Adsorption–desorption isotherms of ZnO and SnO2@ZnO, (b) BJH pore volume vs pore diameter of ZnO and SnO2@ZnO, and (c) BET surface area of ZnO and SnO2@ZnO.
X-ray photoelectron spectroscopy (XPS) is a suitable technique for examining the chemical structure of samples. In this study, ZnO hexagonal prisms, SnO2 quantum dots, and SnO2@ZnO hexagonal prisms were subjected to XPS analysis, as shown in Figure 5(a). The core element peaks of Zn, Sn, and O are identified at their predicted positions, indicating high quality of the samples. The Zn 2p spectra for pure ZnO and SnO2@ZnO (Figure 5(b) and (c)) reveal two strong peaks at energies of 1021.2 eV and 1044.3, corresponding to the Zn 2p3/2 and Zn 2p1/2 lines, respectively. The difference of 23.1 eV between these energy levels indicates the oxidation state of Zn to be +2 [38]. Similarly, the results in Figure 5(d) and (e) indicate that Sn is in the +4 oxidation state, confirmed by two strong peaks occurring at energies of 486.1 and 494.5 eV, equivalent to Sn 3d5/2 and Sn 3d3/2, respectively. A distinction of 8.4 eV between the major Sn peaks corresponds to the energy variance. No significant changes are observed in these results. Figure 6(a)–(c) illustrates the deconvolution of the O 1s spectra for ZnO and SnO2 quantum dots and that of SnO2@ZnO reveals a distinct peak at 529.4 eV, attributable to lattice oxygen (Ob). An adsorbed oxygen (Oad) peak also appears at 530.9 eV in the samples, indicating the quality of all materials.
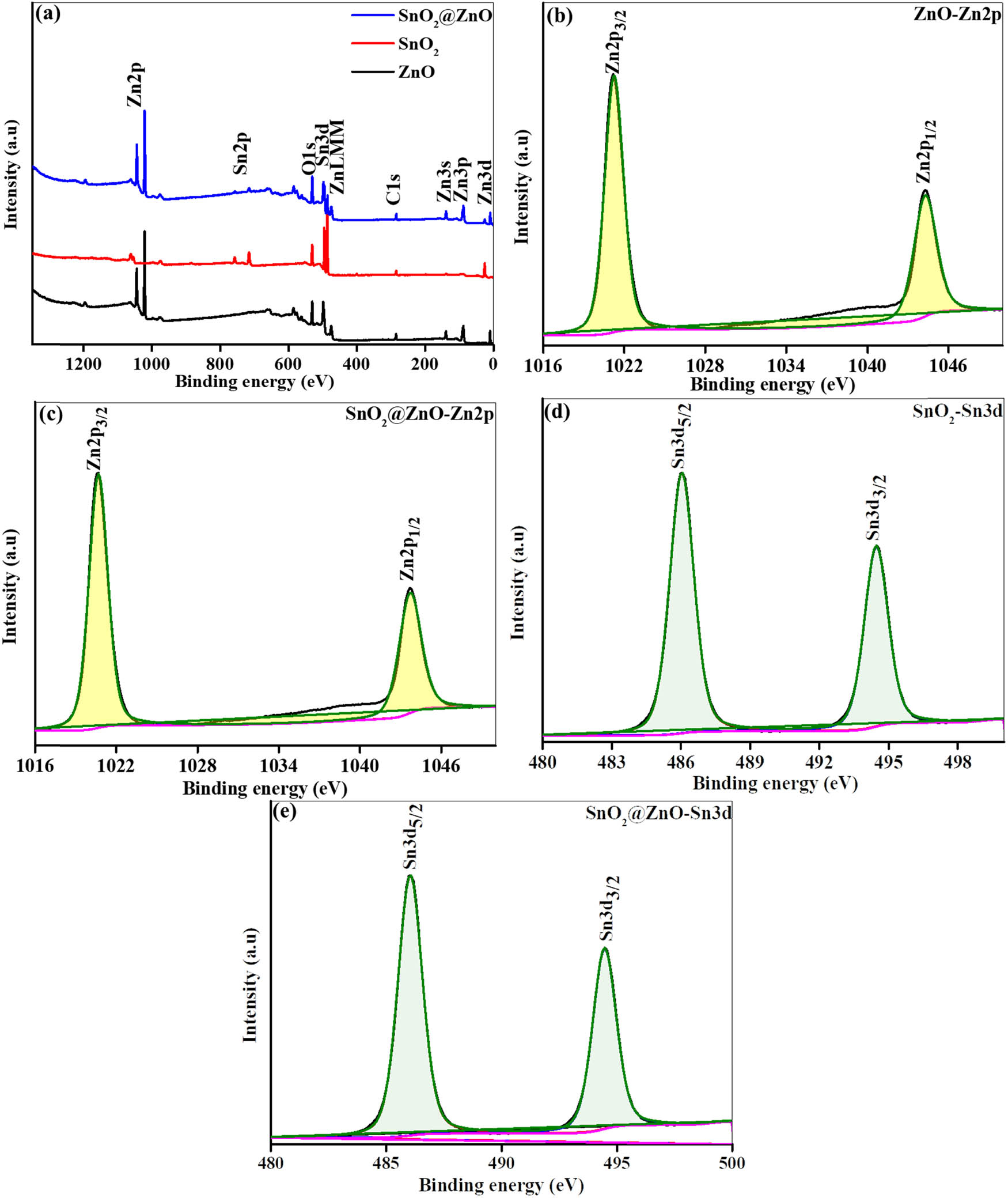
(a) XPS survey spectra of ZnO, SnO2, and SnO2@ZnO, (b) and (c) XPS core level spectra of Zn 2p for (b) ZnO and (c) SnO2@ZnO, and (d) and (e) XPS core level spectra of Sn 3d for (d) SnO2 and (e) SnO2@ZnO.
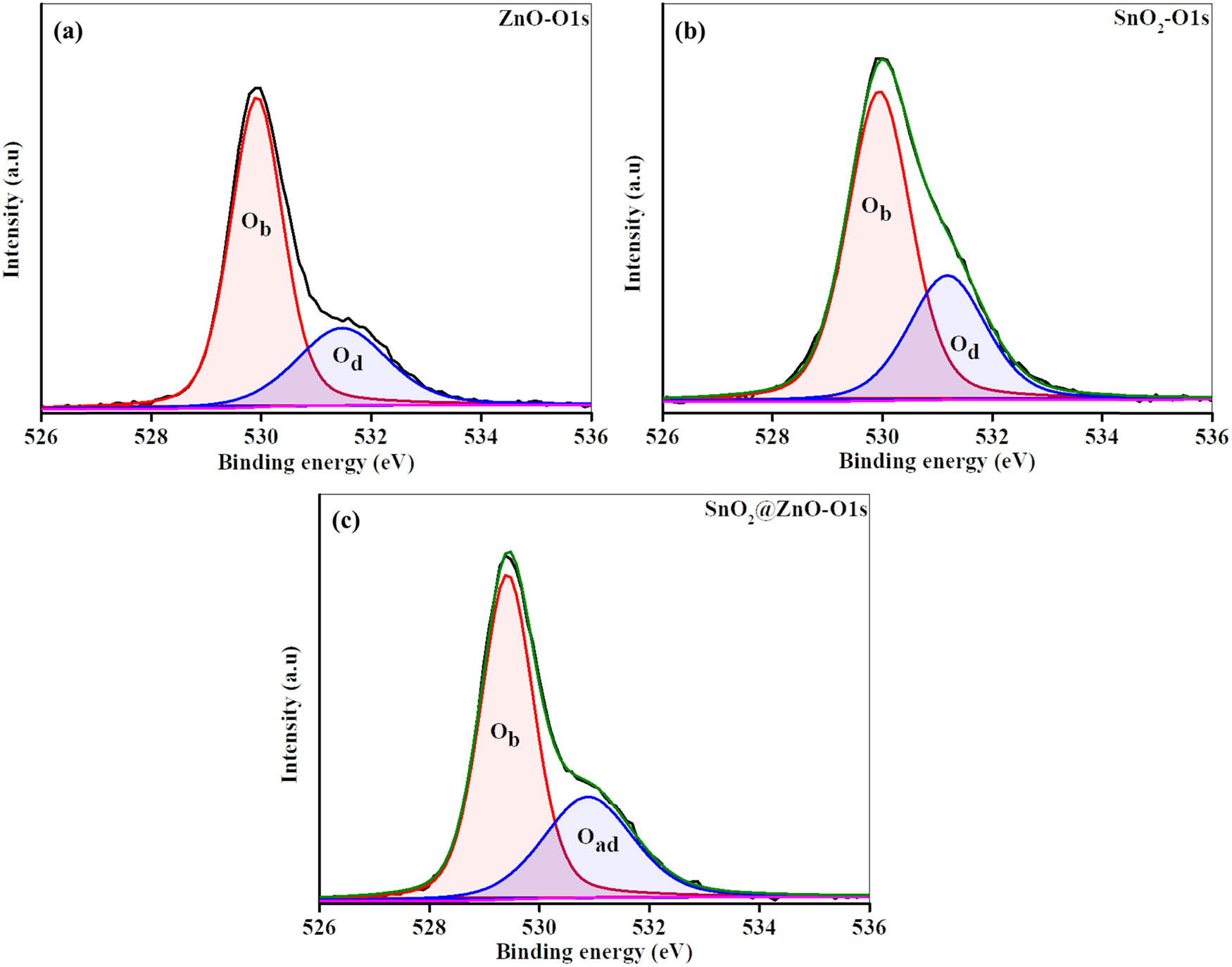
(a)–(c) XPS O 1s spectra for ZnO, SnO2, and SnO2@ZnO, respectively.
3.2 Three-electrode system electrochemical studies
Electrochemical assessments of the ZnO hexagonal prisms and SnO2@ZnO nanostructures were conducted within an aqueous electrolyte of 3.0 M KOH. The CV results (Figure 7) show distorted rectangular shapes, suggesting that the electrodes operate in a manner that is characteristic of electric double-layer capacitors (EDLCs). Additionally, the detection of successive redox peaks in the CV profiles of all electrodes suggests the occurrence of faradaic processes during electrochemical cycling. CV signals for ZnO hexagonal prisms (Figure 7(a)) are presented in the voltage range of 0.1–0.5 V, with varying sweep rates from 5 to 200 mV s⁻1. The oxidative and reductive peak currents of the ZnO hexagonal prisms exhibit a linear relationship (Figure 7(c)), indicating surface-driven electrochemical behavior stemming from a blend of pseudocapacitance and electric double-layer capacitance [39]. The change in peak positions with increasing scan rates reflects the polarization effects occurring in the system [40].
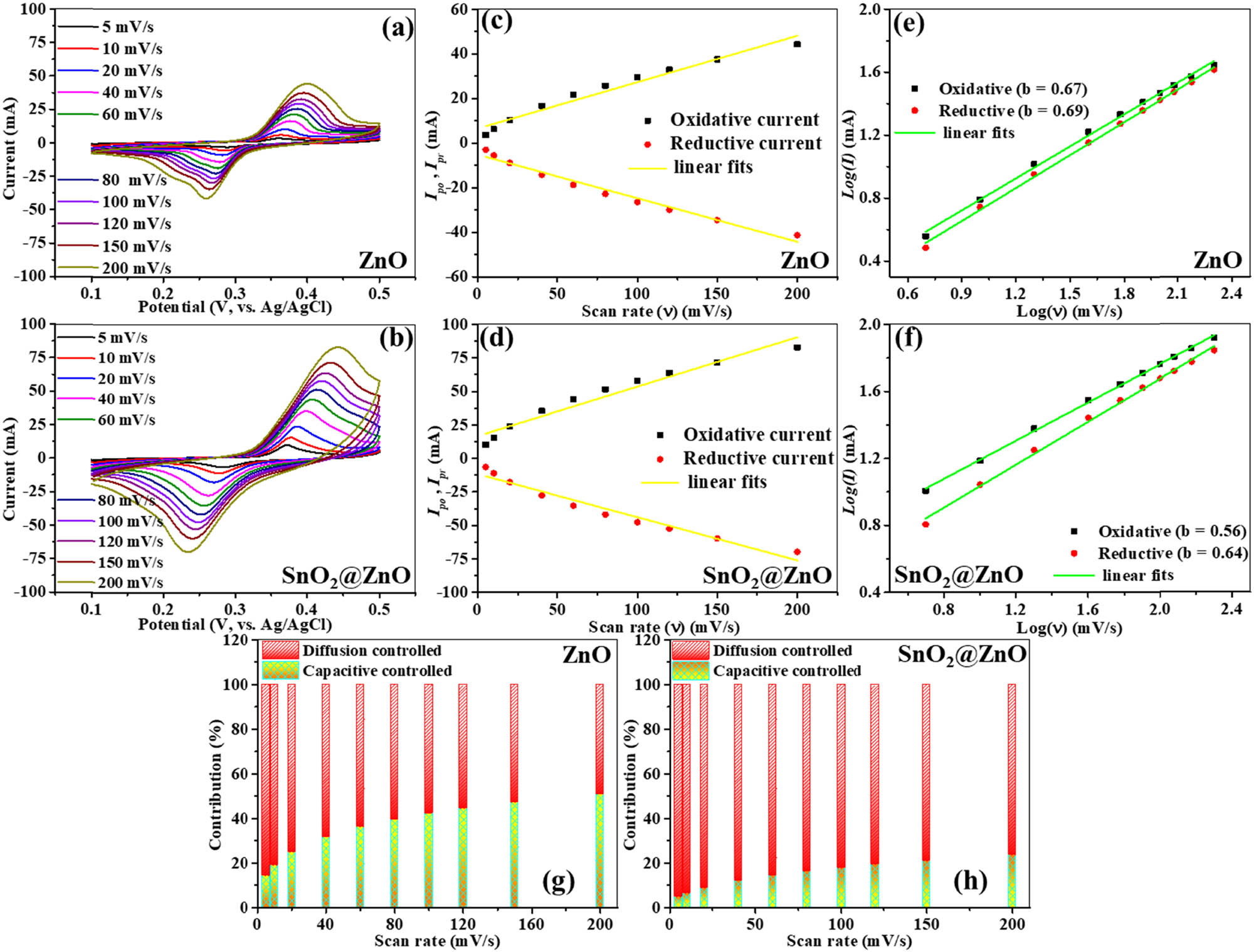
Three-electrode system evaluation. CV curves of (a) ZnO and (b) SnO2@ZnO. Variations of oxidative peak (I po) and reductive peak (I pr) currents versus scan rate of (c) ZnO electrode and (d) SnO2@ZnO electrode. Logarithmic plots of redox peaks for (e) ZnO and (f) SnO2@ZnO. Diffusion- and capacitive-controlled contributions of (g) ZnO and (h) SnO2@ZnO.
Figure 7(b) illustrates the CV profiles of SnO2@ZnO nanostructures at scan rates ranging from 5 to 200 mV s⁻1 within the voltage range 0.1–0.5 V. A comparable trend is observed in the electrochemical behavior of both the SnO2@ZnO samples and the ZnO hexagonal prisms, as they both exhibit a linear association between oxidative and reductive peak currents. The presence of discernible redox peaks implies significant faradaic characteristics inherent in the prepared electrode material [41]. Decoration of SnO2 quantum dots on ZnO hexagonal prism surfaces enhances the efficiency of electron transfer, boosting both anodic and cathodic currents. From the CV profile of SnO2@ZnO (Figure 7(d)), the disparity between the reduction and oxidation currents is highlighted, associated with the boosted oxidation peak current. The observed increment in the anodic peak potential (E pa) is a result of the synergistic impact of SnO2 over ZnO hexagonal prisms, leading to a diminished impedance at the electrode/electrolyte interface. This results in the ZnO electrode displaying a lower anodic peak potential compared to that of the SnO2@ZnO nanostructures. Moreover, while the cathodic peak potential (E pc) for ZnO hexagonal prisms remains lower than that of the SnO2@ZnO samples, broader peak bases are observed, suggesting a more substantial accumulation of electrons. The association between the scan rate (ν) and the recorded current (I) was explored utilizing the power-law model expressed as equation (4), as postulated by Lindström et al. [42].
A linear dependency equation (equation (5)) is employed to fit the experimental data to determine the parameters
The parameter
In this context, ν denotes the scan rate, I represents the peak current, and m 1 and m 2 are variable parameters. Analysis of the current behavior at different potentials under varying sweep rates yielded the gradient and intercept values of m 1 and m 2. Across incremental scan rates from 5 to 200 mV s⁻1, the capacity contributions for the ZnO hexagonal prism electrode (Figure 7(e)) were 14, 19, 25, 32, 36, 40, 42, 44, 47, and 51% of the total current, respectively. These values suggest a combination of both surface-controlled and diffusion-controlled kinetics. At the same scan rates (Figure 7(f)), the electrode composed of SnO2@ZnO nanostructures exhibited capacity contributions of 5, 6, 9, 12, 14, 16, 18, 19, 21, and 23% of the total current, respectively, representing a dominant influence of diffusion-controlled kinetics. Figure S1 represents the capacitive and diffusion contribution of ZnO and SnO2@ZnO samples. This highlights the significant role played by the diffusion-controlled mechanism in determining the charge storage performance of SnO₂@ZnO [46].
The exploration of the charge and discharge behaviors was facilitated by comprehensive GCD measurements, as depicted in Figure 8(a) and (b). Within a spectrum of current densities from 2 to 15 A g⁻1, both the ZnO hexagonal prism and SnO2@ZnO nanostructure electrodes displayed asymmetric behavior in their GCD curves. The non-linear profiles observed for both ZnO hexagonal prisms and SnO2@ZnO nanostructures indicate the presence of faradaic redox dynamics that can correspond to the detected oxidation and reduction behavior in the CV profiles. The ZnO hexagonal prisms demonstrated a specific capacitance of 700.68 F g⁻1, whereas the SnO2@ZnO electrode exhibited an enhanced specific capacitance of 949.26 F g⁻1 at a current density of 2 A g⁻1. As the current density increased to 4, 6, 8, 10, 12, and 15 A g⁻1, the specific capacitance of the ZnO hexagonal prism electrode reduced to 582, 530, 406, 300, 249, and 219 F g⁻1, respectively. On the contrary, for the same current densities, the SnO2@ZnO electrode sustained higher specific capacitances of 749, 630, 562, 517, 487, and 459 F g⁻1 (Figure 8(c)). These findings highlight the superior electrochemical performance of the SnO2@ZnO electrode, offering higher specific capacitances across different current densities compared to the ZnO hexagonal prism electrode. Both electrodes displayed notable cycling stability, with the ZnO hexagonal prism and SnO2@ZnO electrodes retaining 92.5 and 89.9% of their capacity, respectively, after 5,000 cycles of charge and discharge under an elevated current density of 15 A g⁻1 (Figure 8(d)). Alongside remarkable cycling stability, both the ZnO hexagonal prism and SnO2@ZnO electrodes demonstrated consistent Coulombic efficiency.
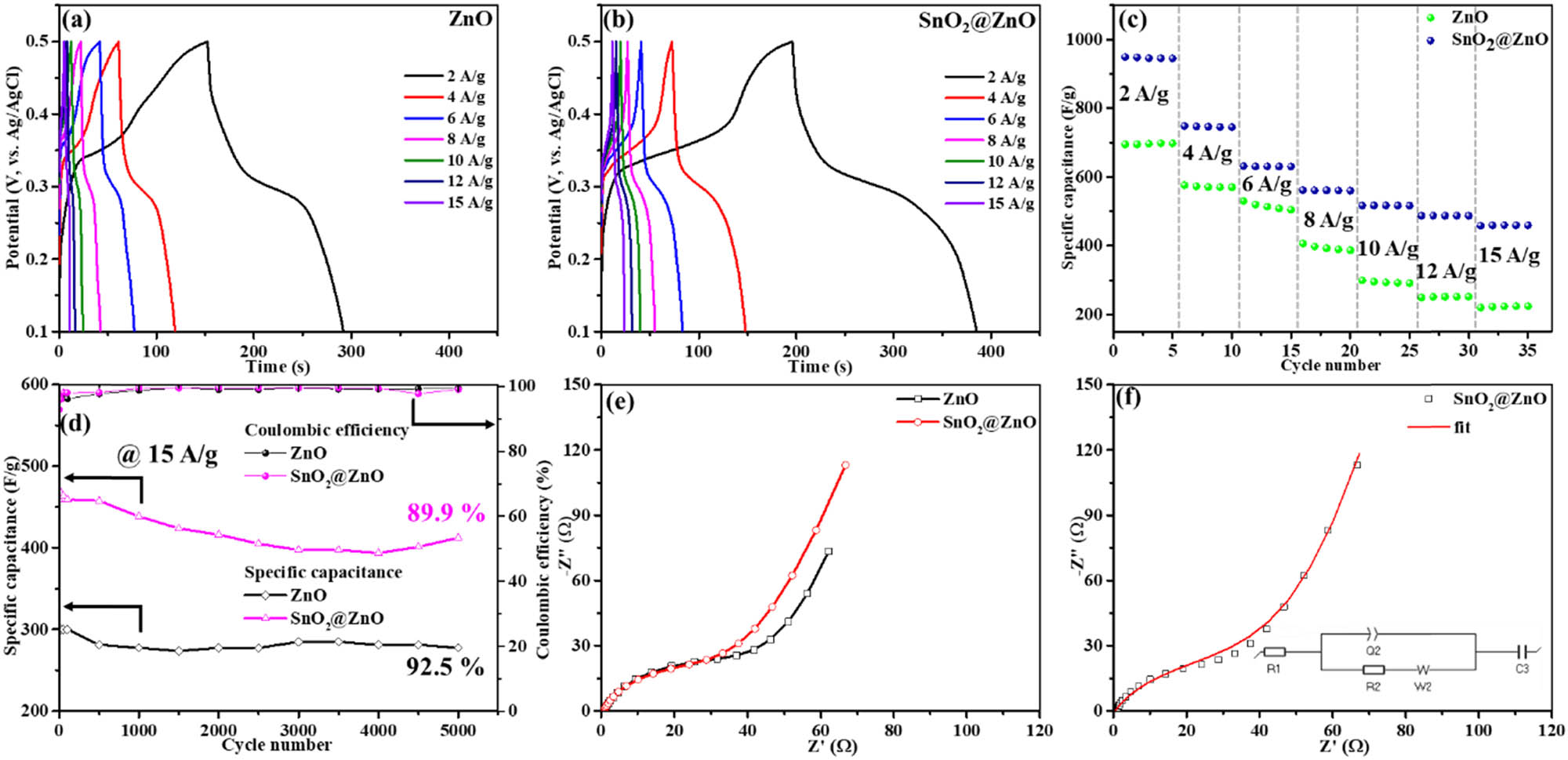
Galvanostatic charge–discharge curves of (a) ZnO and (b) SnO2@ZnO. (c) Specific capacitance vs cycle number at various current densities. (d) Galvanostatic charge–discharge cycling stability of ZnO and SnO2@ZnO at a current density of 15 A/g for 5,000 cycles. (e) EIS spectra of ZnO and SnO2@ZnO. (f) EIS fitted circuit.
The EIS technique was utilized to examine the charge-transfer behavior of ZnO hexagonal prism and SnO2@ZnO electrodes, as depicted in Figure 8(e). Figure 8(f) shows the fitted impedance plot along with the corresponding parameters, and the comprehensive values are listed in Table 1. Consistency in the Nyquist plots for all electrodes is achieved by employing an equivalent circuit model. The determined internal impedance (R1) values for the ZnO hexagonal prism and SnO2@ZnO electrodes are 0.53 and 0.48 Ω, respectively. The resistance arising from the charge transfer process (R2) for the ZnO hexagonal prism electrode was measured at 50 Ω, higher than that of the SnO₂@ZnO electrode (45 Ω). This implies that the decoration of SnO2 over ZnO hexagonal prisms in the SnO2@ZnO electrode promotes charge transport between the prisms and quantum dots, thereby enhancing the overall electrochemical performance [47]. The Q2 value, which is a double layer capacitance, for the ZnO hexagonal prism and SnO2@ZnO electrodes was determined at 3.7 and 2.9 mF s(α−1), respectively. The pseudocapacitance (C3) values of ZnO hexagonal prisms and SnO2@ZnO were determined as 0.05 and 0.1 F, respectively. The improved electrochemical traits detected for the SnO2@ZnO electrode, validated by the EIS investigation, corroborate the conclusions drawn from the GCD and CV assessments, further reinforcing the findings.
The impedance parameters obtained from fitting for ZnO and SnO2@ZnO electrodes tested in a three-electrode configuration
| Electrode | R1 (Ω) | R2 (Ω) | Q2 (mF s(α−1)) | C3 (F) |
|---|---|---|---|---|
| ZnO | 0.53 | 50 | 3.79 | 0.05 |
| SnO2@ZnO | 0.48 | 45 | 2.92 | 0.1 |
3.3 SSC device electrochemical studies
To accurately assess the electrochemical capabilities of the SnO2@ZnO electrode material, a two-electrode device (SSC) was developed employing SnO2-decorated ZnO hexagonal prisms as the positive and negative electrodes. As depicted in Figure 9(a), the CV curves of the fabricated SSC device were recorded at incremental scan rates from 5 to 200 mV s⁻1, encompassing a potential interval of 0–1.3 V. Significantly, the CV curves display clearly defined rectangular shapes with evident reduction and oxidation peaks, suggestive of EDLC behavior. The pseudocapacitive behavior of the SSC can be observed owing to the occurrence of a pair of redox peaks. Specifically, even at elevated sweep rates, the CV plots retain their rectangular shapes, highlighting the exceptional rate capability of the SSC. This preservation of rectangular shapes underscores the ability of the SSC to maintain its electrochemical efficiency under heightened scan rates, highlighting its appropriateness for high-rate applications [48]. The CV for the SnO2@ZnO SSC device exhibited a pair of redox peaks (Figure 9(a)), indicating the battery-type behavior of the material [49]. This behavior can also be seen in the GCD results as well. At various current densities (1.2–2 A g⁻1), the GCD curves of the SnO2@ZnO SSC were recorded (Figure 9(b)). Across the spectrum of current densities, a noticeable extended discharge time is observed, signifying the advantageous electrochemical behavior of the SSC device and emphasizing its remarkable efficacy in energy storage applications [50]. The SnO2@ZnO SSC device achieves a consistent specific capacitance of 83 F g⁻1 (Figure 9(c)) at a current density of 1.2 A g⁻1. With a rise in the current density to 2 A g⁻1, a proportional decline in specific capacitance to 19.2 F g⁻1 is evident. With higher current densities, there is a reduction in specific capacitance due to limitations in charge transfer kinetics and ion diffusion within the electrode material. Such findings underscore the relationship between the applied current density and specific capacitance. To evaluate the enduring capacitance retention of SnO2@ZnO SSC, a rigorous GCD examination was carried out for an extended period of 5,000 cycles, at 2 A g⁻1 current density. The results portrayed in Figure 9(d) reveal a consistent capacitance retention of 75% even after enduring 5,000 GCD cycles, showcasing the SSC device’s satisfactory cyclic stability. The obtained results emphasize the viability of the SnO2@ZnO-based SSC for extended energy-storage applications. Figure 9(e) illustrates a Ragone plot showcasing the correlation between the power and energy densities of the prepared SSC, computed by means of equations (2) and (3). Remarkably, SnO2@ZnO SSC displays an energy density of 70.2 W kg⁻1 at a power density of 2,808 W kg⁻1, exceeding earlier documented benchmarks for energy and power densities [51,52,53,54,55]. A thorough comparison of these metrics with those of alternative supercapacitor systems is presented in Table 2. The EIS spectrum of the assembled SnO₂@ZnO SSC is depicted in Figure 9(f), which shows a small internal resistance of 0.79 Ω, indicating enhanced specific power capability. An enhanced double-layer capacitance in combination with 62 Ω of minimal charge-transfer resistance emphasizes the decent electrochemical performance of the fabricated SnO2@ZnO device. These results emphasize the promise of the SnO2@ZnO SSC device for sophisticated energy-storage applications.
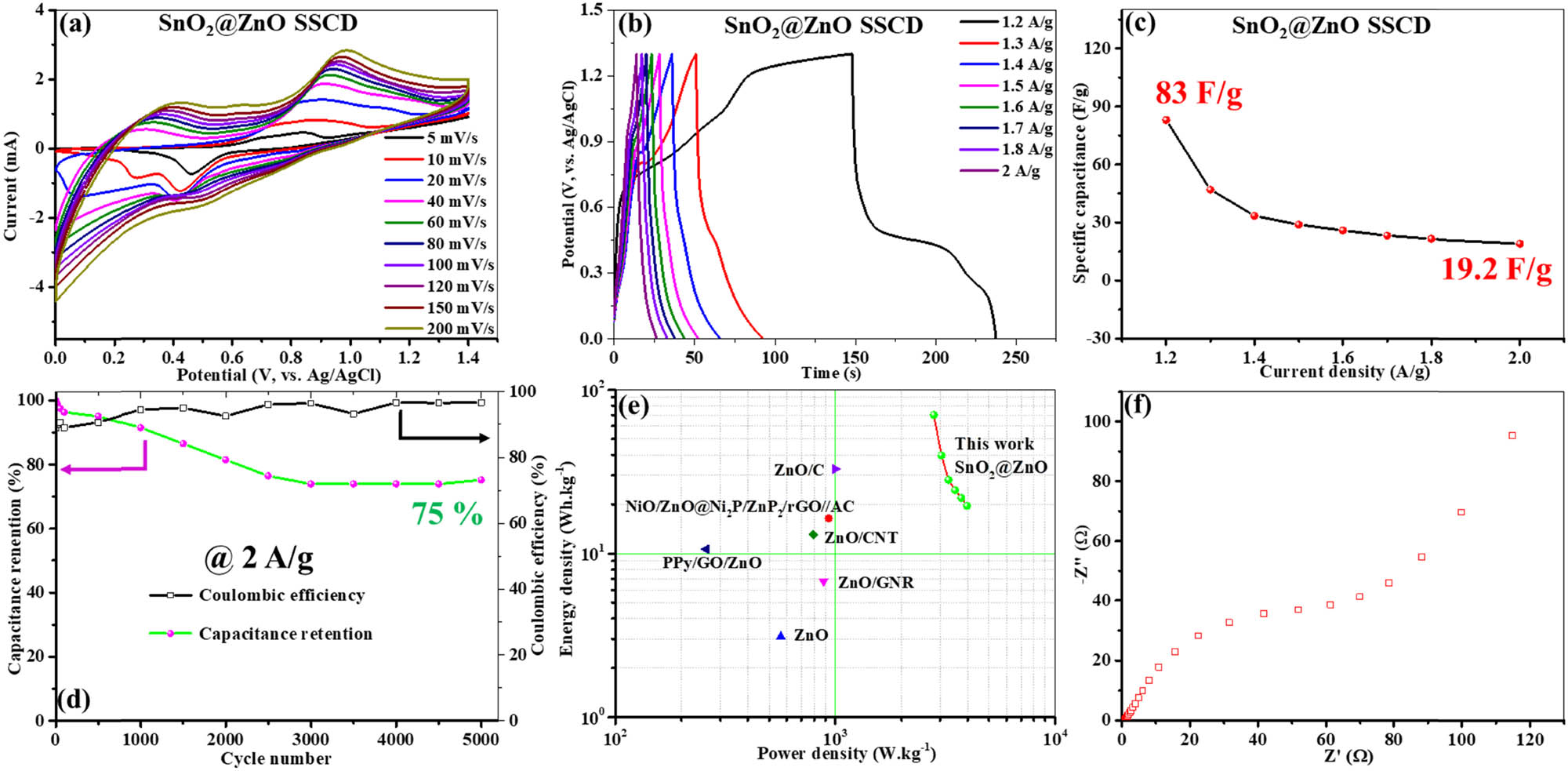
Two-electrode electrochemical evaluation. (a) CV curves, (b) galvanostatic charge–discharge profiles, (c) specific capacitance vs current density, (d) capacitance retention and Coulombic efficiency w.r.t. cycle number, (e) Ragone plot, and (f) EIS spectra after 5,000th GCD cycle.
Comparison of the performance of SnO2@ZnO with various SSCs
| Electrodes | Electrolyte | Specific capacitance (F g−1) | Power density (W kg−1) | Energy density (W kg−1) | Capacitance retention | Ref. |
|---|---|---|---|---|---|---|
| NiO/ZnO@Ni2P/ZnP2/rGO//AC HSC | 6 M KOH | 76 C g−1 | 932.74 | 16.43 | 91% after 10,000 cycles | [51] |
| ZnO SSC | 0.5 M Na2SO4 | NA | 565 | 3.1 | NA | [52] |
| ZnO/GNR SSC | 0.5 M Na2SO4 | NA | 884 | 6.8 | NA | [52] |
| ZNO/CNT SSC | 0.1 M TBAPC/DMF | 48 | 792 | 13.1 | ∼95% after 300 cycles | [53] |
| PPy/GO/ZnO SSC | 1 M Na2SO4 | 94.6 | 258.26 | 10.65 | 73.6% after 1,000 cycles | [54] |
| ZnO/C SSC | 1 M Na2SO4 | 92 | 1,000 | 32.78 | 85% after 250 cycles | [55] |
| SnO 2 @ZnO SSC | 3 M KOH | 83.07 | 2,808 | 70.2 | 75% after 5,000 cycles | This work |
Bold values represents the results of the current work.
Overall, the key findings and advantages observed in the experimental results are as follows:
XRD analysis confirmed the high crystallinity of ZnO and SnO2@ZnO, indicating that the introduction of SnO2 did not alter the hexagonal crystal structure of ZnO but rather enhanced its crystallinity. This structural stability is crucial for maintaining consistent electrochemical performance over multiple cycles.
FESEM and FETEM imaging revealed well-defined hexagonal prisms with uniform SnO2 decoration, suggesting effective integration of SnO2 into ZnO nanostructures. Additionally, BET analysis demonstrated a threefold increase in the surface area for SnO2@ZnO compared to that of pristine ZnO, which is advantageous for facilitating more active sites for charge storage and transfer.
Electrochemical studies, including CV and GCD measurements, showcased superior electrochemical performance of SnO2@ZnO compared to ZnO alone. SnO2@ZnO electrodes exhibited higher specific capacitance across various current densities, indicating their ability to store more charge per unit mass and deliver higher energy densities.
CV profiles of SnO2@ZnO electrodes displayed well-defined rectangular shapes even at elevated scan rates, indicative of excellent rate capability. Moreover, GCD measurements revealed extended discharge times and consistent capacitance retention over 5,000 cycles, highlighting the enhanced cycling stability of SnO2@ZnO electrodes.
Ragone plot analysis demonstrated that the SnO2@ZnO SSC device achieved higher energy and power densities compared to conventional ZnO-based devices, showcasing their potential for high-performance energy storage applications.
4 Conclusions
In summary, this research introduced a novel electrode material for supercapacitors, comprising SnO2 quantum dots integrated into ZnO hexagonal prisms (SnO2@ZnO), marking the first usage of the SnO2@ZnO electrode material in supercapacitor application. A comprehensive examination was undertaken to assess the electrochemical performance of the prepared material, yielding significant results. At a current density of 2 A g⁻1, the ZnO electrode demonstrated a specific capacitance of 700.68 F g⁻1, while the SnO2@ZnO electrode demonstrated a specific capacitance of 949.26 F g⁻1 . Notably, both electrodes, SnO2@ZnO and ZnO, showcased outstanding cycling stability. The SnO2@ZnO electrode retained a specific capacitance of 412.5 F g⁻1, whereas the ZnO electrode maintained a specific capacitance of 277.5 F g⁻1, even after 5,000 cycles at a high current density of 15 A g⁻1. These results underscore the exceptional robustness and durability of the electrode material. To evaluate the real-world applicability of SnO2@ZnO, an SSC device was fabricated with SnO2@ZnO acting as positive and negative electrodes. Remarkably, at a current density of 1.2 A g⁻1, the SnO2@ZnO SSC device showcased a decent specific capacitance, achieving 83.07 F g⁻1. Additionally, remarkable power and energy densities of 2,808 W kg⁻1 and 70.2 W kg⁻1, respectively, were achieved. Furthermore, a significant capacity retention of approximately 75% was exhibited by the SSC device after 5,000 GCD cycles. This underscores the material’s ability to endure prolonged cycling while maintaining a consistent electrochemical performance. The outcomes of this investigation provide significant insight into understanding the synthesis and electrochemical attributes of SnO2@ZnO hexagonal prisms. These findings contribute substantially to the continuous advancement of sophisticated supercapacitors, paving the way for the enhanced optimization and applied integration of SnO2@ZnO hexagonal prisms in energy-storage systems.
Acknowledgments
This work was supported by Researchers Supporting Project number (RSPD2024R765), King Saud University, Riyadh, Saudi Arabia. This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Korea government(MSIT) (RS-2024-00357072).
-
Funding information: This work was supported by Researchers Supporting Project number (RSPD2024R765), King Saud University, Riyadh, Saudi Arabia. This work was also supported by the National Research Foundation (NRF) of Korea funded by the Korean government (RS-2023-00280665).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Vaghela P, Pandey V, Sircar A, Yadav K, Bist N, Kumari R. Energy storage techniques, applications, and recent trends: A sustainable solution for power storage. MRS Energy Sustainability. 2023;10:261–76. 10.1557/s43581-023-00069-9.Search in Google Scholar
[2] Zhang X, Tang Y, Zhang F, Lee C-S, Novel A. Aluminum–graphite dual-ion battery. Adv Energy Mater. 2016;6:1502588. 10.1002/aenm.201502588.Search in Google Scholar
[3] Zhang M, Zhang W, Zhang F, Lee C-S, Tang Y. Anion-hosting cathodes for current and late-stage dual-ion batteries. Sci China Chem. 2024;67:1485–509. 10.1007/s11426-023-1957-3.Search in Google Scholar
[4] Wenkai LI, Ning Z, Zhijie BI, Xiangxin GUO. Na3Zr2Si2PO12 ceramic electrolytes for Na-ion battery: preparation using spray-drying method and its property. J Inorg Mater. 2022;37:189. 10.15541/jim20210486.Search in Google Scholar
[5] Wang X, Xia Y, Huang J, Su Y, Chen Z, Chen L, et al. A facile strategy for large-scale production of 2D nanosheets exfoliated by three-roll milling. J Adv Ceram. 2024;13(1):11–8. 10.26599/JAC.2024.9220831.Search in Google Scholar
[6] Wang X, Luo Z, Huang J, Chen Z, Xiang T, Feng Z, et al. S/N-co-doped graphite nanosheets exfoliated via three-roll milling for high-performance sodium/potassium ion batteries. J Mater Sci Technol. 2023;147:47–55. 10.1016/j.jmst.2022.11.015.Search in Google Scholar
[7] Kumar YA, Alagarasan JK, Ramachandran T, Rezeq M, Bajaber MA, Alalwiat AA, et al. The landscape of energy storage: Insights into carbon electrode materials and future directions. J Energy Storage. 2024;86:111119. 10.1016/j.est.2024.111119.Search in Google Scholar
[8] Dutta A, Mitra S, Basak M, Banerjee T. A comprehensive review on batteries and supercapacitors: Development and challenges since their inception. Energy Storage. 2023;5:e339. 10.1002/est2.339.Search in Google Scholar
[9] Ali S, Ahmad T, Tahir MY, Usman M, Chhattal M, Hussain I, et al. The emergence of density functional theory for supercapacitors: Recent progress and advances. J Energy Storage. 2023;73:109100. 10.1016/j.est.2023.109100.Search in Google Scholar
[10] Kularatna N, Gunawardane K. Energy storage devices for renewable energy-based systems: rechargeable batteries and supercapacitors. Academic Press; 2021. 10.1016/C2019-0-00796-0.Search in Google Scholar
[11] Guo W, Yu C, Li S, Qiu J. Toward commercial-level mass-loading electrodes for supercapacitors: opportunities, challenges and perspectives. Energy Env Sci. 2021;14:576–601. 10.1039/D0EE02649B.Search in Google Scholar
[12] Liu Q, Liu L, Zheng Y, Li M, Ding B, Diao X, et al. On-demand engineerable visible spectrum by fine control of electrochemical reactions. Natl Sci Rev. 2024;11:nwad323. 10.1093/nsr/nwad323.Search in Google Scholar PubMed PubMed Central
[13] Kumar N, Kim S-B, Lee S-Y, Park S-J. Recent advanced supercapacitor: a review of storage mechanisms, electrode materials, modification, and perspectives. Nanomaterials. 2022;12:3708. 10.3390/nano12203708.Search in Google Scholar PubMed PubMed Central
[14] Pathak M, Bhatt D, Bhatt RC, Bohra BS, Tatrari G, Rana S, et al. High energy density supercapacitors: an overview of efficient electrode materials, electrolytes, design, and fabrication. Chem Rec. 2024;24:e202300236. 10.1002/tcr.202300236.Search in Google Scholar PubMed
[15] Yu H, Zhang J, Shaheen I, Ahmad M, Chen X, Akkinepally B, et al. Nature-resembled nanostructures for energy storage/conversion applications. J Ind Eng Chem. 2024;129:53–68. 10.1016/j.jiec.2023.08.041.Search in Google Scholar
[16] Shah SS, Niaz F, Ehsan MA, Das HT, Younas M, Khan AS, et al. Advanced strategies in electrode engineering and nanomaterial modifications for supercapacitor performance enhancement: A comprehensive review. J Energy Storage. 2024;79:110152. 10.1016/j.est.2023.110152.Search in Google Scholar
[17] He J, Cao L, Cui J, Fu G, Jiang R, Xu X, et al. Flexible energy storage devices to power the future. Adv Mater. 2024;36:2306090. 10.1002/adma.202306090.Search in Google Scholar PubMed
[18] Parveen N. Resent development of binder-free electrodes of transition metal oxides and nanohybrids for high performance supercapacitors – A review. Chem Rec. 2024;24:e202300065. 10.1002/tcr.202300065.Search in Google Scholar PubMed
[19] Cao X-M, Chen J-Q, Zhao X-R, Ge H, Liu D, Wu Q, et al. Facile synthesis of bead-chain structured MWCNTs@CeO2 with oxygen vacancies-rich for promoting electrochemical energy storage. Chem Eng J. 2024;479:147663. 10.1016/j.cej.2023.147663.Search in Google Scholar
[20] Akkinepally B, Kumar GD, Reddy IN, Rao HJ, Nagajyothi PC, Alothman AA, et al. Investigation of supercapacitor electrodes based on MIL-101(Fe) metal-organic framework: evaluating electrochemical performance through hydrothermal and microwave-assisted synthesis. Crystals. 2023;13:1547. 10.3390/cryst13111547.Search in Google Scholar
[21] Aizudin M, Fu W, Pottammel RP, Dai Z, Wang H, Rui X, et al. Recent advancements of graphene-based materials for zinc-based batteries: beyond lithium-ion batteries. Small. 2024;20:2305217. 10.1002/smll.202305217.Search in Google Scholar PubMed
[22] Fei T, Ahmad T, Usman M, Ahmad A, Saleem A, Hanif MB, et al. Zn-doped Cr2O3 oxides boosted the electrochemical performance of aqueous hybrid supercapacitor. Electrochim Acta. 2024;476:143673. 10.1016/j.electacta.2023.143673.Search in Google Scholar
[23] Wang M, Jiang C, Zhang S, Song X, Tang Y, Cheng H-M. Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage. Nat Chem. 2018;10:667–72. 10.1038/s41557-018-0045-4.Search in Google Scholar PubMed
[24] Mohapatra D, Parida S, Badrayyana S, Singh BK. High performance flexible asymmetric CNO-ZnO//ZnO supercapacitor with an operating voltage of 1.8 V in aqueous medium. Appl Mater Today. 2017;7:212–21. 10.1016/j.apmt.2017.03.006.Search in Google Scholar
[25] Shi S, Zhuang X, Cheng B, Wang X. Solution blowing of ZnO nanoflake-encapsulated carbon nanofibers as electrodes for supercapacitors. J Mater Chem A. 2013;1:13779–88. 10.1039/C3TA13247A.Search in Google Scholar
[26] Najib S, Bakan F, Abdullayeva N, Bahariqushchi R, Kasap S, Franzò G, et al. Tailoring morphology to control defect structures in ZnO electrodes for high-performance supercapacitor devices. Nanoscale. 2020;12:16162–72. 10.1039/D0NR03921G.Search in Google Scholar
[27] Dalapati GK, Sharma H, Guchhait A, Chakrabarty N, Bamola P, Liu Q, et al. Tin oxide for optoelectronic, photovoltaic and energy storage devices: a review. J Mater Chem A. 2021;9:16621–84. 10.1039/D1TA01291F.Search in Google Scholar
[28] Yang X, Gong M, Liu Z, Huangfu C, Yan Y, Chi C, et al. Multi-dimensional assembly of ZnO nanodots in the reticular carbon nanofibers for high-performance lithium-ion batteries. Carbon. 2024;223:119001. 10.1016/j.carbon.2024.119001.Search in Google Scholar
[29] Gaber A, Attia SY, Salem AMS, Mohamed SG, El-Hout SI. Microwave-assisted fabrication of SnO2 nanostructures as electrode for high-performance pseudocapacitors. J Energy Storage. 2023;59:106358. 10.1016/j.est.2022.106358.Search in Google Scholar
[30] Harisha BS, Akkinepally B, Shim J, Lim J. Hybrid NiO@TiO2 nano-architecture for improved electrochemical performance with simulation corroboration. J Energy Storage. 2024;87:111466. 10.1016/j.est.2024.111466.Search in Google Scholar
[31] Rafique K, Hassan N, Shah MZU, Al-Saeedi SI, Shah A, Shah MSU, et al. Improved performance in asymmetric supercapacitors using SnO2–MoS2 composite microspheres. Surf Interfaces. 2024;44:103650. 10.1016/j.surfin.2023.103650.Search in Google Scholar
[32] Shaheen Shah S, Oladepo S, Ali Ehsan M, Iali W, Alenaizan A, Nahid Siddiqui M, et al. Recent progress in polyaniline and its composites for supercapacitors. Chem Rec. 2024;24:e202300105. 10.1002/tcr.202300105.Search in Google Scholar PubMed
[33] Hosseini SM, Safarifard V. MoS2@MOF composites: Design strategies and photocatalytic applications. Mater Sci Semicond Process. 2024;169:107892. 10.1016/j.mssp.2023.107892.Search in Google Scholar
[34] Sonkar R, Mondal NJ, Boro B, Ghosh MP, Chowdhury D. Cu doped ZnO nanoparticles: Correlations between tuneable optoelectronic, antioxidant and photocatalytic activities. J Phys Chem Solids. 2024;185:111715. 10.1016/j.jpcs.2023.111715.Search in Google Scholar
[35] Mao W, Wang T, Wang H, Zou S, Liu S. Novel Bi2WO6 loaded g-C3N4 composites with enhanced photocatalytic degradation of dye and pharmaceutical wastewater under visible light irradiation. J Mater Sci: Mater Electron. 2018;29:15174–82. 10.1007/s10854-018-9659-y.Search in Google Scholar
[36] Shah MSU, Zuo X, Shah A, Al-Saeedi SI, Shah MZU, Alabbad EA, et al. CoSe nanoparticles supported NiSe2 nanoflowers cathode with improved energy storage performance for advanced hybrid supercapacitors. J Energy Storage. 2023;65:107267. 10.1016/j.est.2023.107267.Search in Google Scholar
[37] Selvaraj Y, Kuzhandaivel H, Nallathambi KS, Elayappan V. Enhanced cyclic stability of cobalt doped Bi25FeO40/BiFeO3 as an electrode material for a super long life symmetric supercapacitor device. Energy Fuels. 2023;37:8624–36. 10.1021/acs.energyfuels.3c00135.Search in Google Scholar
[38] Zhang D. Enhanced photocatalytic activity for titanium dioxide by co-modification with copper and iron. Transit Met Chem. 2010;35:933–8. 10.1007/s11243-010-9414-6.Search in Google Scholar
[39] Goswami S, Dillip GR, Nandy S, Banerjee AN, Pimentel A, Joo SW, et al. Biowaste-derived carbon black applied to polyaniline-based high-performance supercapacitor microelectrodes: Sustainable materials for renewable energy applications. Electrochim Acta. 2019;316:202–18. 10.1016/j.electacta.2019.05.133.Search in Google Scholar
[40] Park HW, Na B-K, Cho BW, Park S-M, Roh KC. Influence of vanadium doping on the electrochemical performance of nickel oxide in supercapacitors. Phys Chem Chem Phys. 2013;15:17626–35. 10.1039/C3CP52498A.Search in Google Scholar PubMed
[41] Roy N, Mangiri R, Phaneendra Reddy G, Manohar A, Chung E, Deva Prasad Raju B, et al. Carbon nanofiber-supported elongated square bipyramid-like MnWO4 composite electrodes for high-performance battery-type supercapacitors: Enhanced electrochemical performance via synergistic effect. J Electroanal Chem. 2023;947:117764. 10.1016/j.jelechem.2023.117764.Search in Google Scholar
[42] Lindström H, Södergren S, Solbrand A, Rensmo H, Hjelm J, Hagfeldt A, et al. Li+ ion insertion in TiO2 (Anatase). 1. Chronoamperometry on CVD films and nanoporous films. J Phys Chem B. 1997;101:7710–6. 10.1021/jp970489r.Search in Google Scholar
[43] Liu T-C, Pell WG, Conway BE, Roberson SL. Behavior of molybdenum nitrides as materials for electrochemical capacitors: comparison with ruthenium oxide. J Electrochem Soc. 1998;145:1882. 10.1149/1.1838571.Search in Google Scholar
[44] Aoki K, Tokuda K, Matsuda H. Theory of linear sweep voltammetry with finite diffusion space: Part II. Totally irreversible and quasi-reversible cases. J Electroanal Chem Interfacial Electrochem. 1984;160:33–45. 10.1016/S0022-0728(84)80113-8.Search in Google Scholar
[45] Su Y, Shang J, Liu X, Li J, Pan Q, Tang Y. Constructing π–π superposition effect of tetralithium naphthalenetetracarboxylate with electron delocalization for robust dual-ion batteries. Angew Chem Int Ed. 2024;63:e202403775. 10.1002/anie.202403775.Search in Google Scholar PubMed
[46] Pallavolu MR, Nallapureddy RR, Goli HR, Banerjee AN, Reddy GR, Joo SW. Bioinspired tailoring of nanoarchitectured nickel sulfide@nickel permeated carbon composite as highly durable and redox chemistry enabled battery-type electrode for hybrid supercapacitors. J Mater Chem A. 2021;9:25208–19. 10.1039/D1TA08122E.Search in Google Scholar
[47] Palani NS, Kavitha NS, Venkatesh KS, Kumar KA, Senthilkumar M, Pandurangan A, et al. The synergistic effect of the RuO2 nanoparticle-decorated V2O5 heterostructure for high-performance asymmetric supercapacitors. N J Chem. 2021;45:14598–607. 10.1039/D1NJ00011J.Search in Google Scholar
[48] Omar FS, Numan A, Duraisamy N, Bashir S, Ramesh K, Ramesh S. Ultrahigh capacitance of amorphous nickel phosphate for asymmetric supercapacitor applications. RSC Adv. 2016;6:76298–306. 10.1039/C6RA15111F.Search in Google Scholar
[49] Hussain I, Mak T, Zhang K. Boron-doped trimetallic Cu-Ni-Co oxide nanoneedles for supercapacitor application. ACS Appl Nano Mater. 2021;4:129–41. 10.1021/acsanm.0c02411.Search in Google Scholar
[50] Ghaly HA, El-Deen AG, Souaya ER, Allam NK. Asymmetric supercapacitors based on 3D graphene-wrapped V2O5 nanospheres and Fe3O4@3D graphene electrodes with high power and energy densities. Electrochim Acta. 2019;310:58–69. 10.1016/j.electacta.2019.04.071.Search in Google Scholar
[51] Mao M, Cui L, Yang K, Xue T, An J, Yao M, et al. Ni2P/ZnP2 nanocrystals/rGO decorated on NiO/ZnO nanoflakes as a high-performance cathode for flexible hybrid supercapacitor. J Energy Storage. 2023;73:109226. 10.1016/j.est.2023.109226.Search in Google Scholar
[52] Sahu V, Goel S, Sharma RK, Singh G. Zinc oxide nanoring embedded lacey graphene nanoribbons in symmetric/asymmetric electrochemical capacitive energy storage. Nanoscale. 2015;7:20642–51. 10.1039/C5NR06083D.Search in Google Scholar
[53] Aravinda LS, Nagaraja KK, Nagaraja HS, Bhat KU, Bhat BR. ZnO/carbon nanotube nanocomposite for high energy density supercapacitors. Electrochim Acta. 2013;95:119–24. 10.1016/j.electacta.2013.02.027.Search in Google Scholar
[54] Chee WK, Lim HN, Harrison I, Chong KF, Zainal Z, Ng CH, et al. Performance of flexible and binderless polypyrrole/graphene oxide/zinc oxide supercapacitor electrode in a symmetrical two-electrode configuration. Electrochim Acta. 2015;157:88–94. 10.1016/j.electacta.2015.01.080.Search in Google Scholar
[55] Sasirekha C, Arumugam S, Muralidharan G. Green synthesis of ZnO/carbon (ZnO/C) as an electrode material for symmetric supercapacitor devices. Appl Surf Sci. 2018;449:521–7. 10.1016/j.apsusc.2018.01.172.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy

