Abstract
The ability to control the function and structure of some promising nanosystems using an external stimulus is attractive research to develop programmable and reconfigurable intelligent nanomaterials. The focal point of this review is the silicon-based nanoporous materials, and particularly the mesoporous silica materials (MSMs) class (pore size: 2–50 nm), due to their important intrinsic properties, such as high surface area, highly ordered nanostructure, narrow pore size distribution, various dimensions (one-dimensional, two-dimensional, and three-dimensional), and easily functionable. One of the most essential organic components that can be incorporated in MSMs is organic photochromic molecules (OPMs), such as azobenzene, stilbene, dithienylethenes, and spiropyrans. OPMs can be incorporated into MSMs, to form photochromic mesoporous organosilica materials (PMOMs), in two different ways: physical (non-covalent immobilization) or chemical (covalent immobilization) binding. PMOMs are considered smart nanomaterials because they have the ability to undergo reversible changes in the solid state when exposed to an external electromagnetic radiation. PMOMs have been the subject of many research studies during the last decade due to their potential applications, especially as chemosensors. This review discusses the main families of OPMs, their incorporation into MSMs using different methods, and the applications of some PMOMs as chemosensors.
1 Introduction
1.1 Brief classification of nanomaterials
For the past two decades, scientists’ efforts have been focused on the development and design of materials at the nanoscale due to their specific properties and important applications in a variety of fields [1]. Nanoscience and nanotechnology are two research areas of materials science that focus on the development of materials at the nanometer scale (nanomaterials) (Figure 1). Nanotechnology allowed the utilization of nanomaterials in many fields including drug delivery, electronics, medicine, biotechnology, energy storage, catalysis, wastewater treatment, and sensing [2].
The relationship between nanomaterials, nanoscience, and nanotechnology terms.
Nanomaterials, as defined by the International Union of Pure and Applied Chemistry, are materials possessing a minimum of one external dimension measuring from 1 to 100 nm [3]. Nanomaterials can be classified into two major categories: nanoparticles and nanoporous materials (NPMs). Nanoparticles have a diameter of particles between 1 and 100 nm. While NPMs contain pore diameter between 1 and 100 nm [4]. Various parameters can affect the physical and chemical properties of nanomaterials, such as shape, size, and distribution of nanoparticles and nanopores, in addition to the specific surface area, surface morphology, crystallinity, structure, composition, tension, and hydrophilicity/lipophilicity [5]. Nanomaterial particles can be of various shapes such as quantum dots, fullerene, nanoballs, nanosphere, nanotubes, nanofibers, and nanowires [6].
According to Siegel [1], nanostructured materials are divided into four categories based on their dimensions (Figure 2): zero-dimensional (0D); one-dimensional (1D); two-dimensional (2D); and three-dimensional (3D) nanostructures. 0D nanostructure materials mean all dimensions of the material particles are estimated within the nanoscale. This class includes quantum dots and noble metal nanoparticles. 1D nanostructures have one dimension measured within the nanoscale, such as nanorods, nanowires, and nanotubes. 2D nanostructures have two dimensions measured within the nanoscale, this is including nanocoatings, nanolayers, and nanofilms. 3D nanomaterials are not constrained to the nanoscale in any dimension, such as multi nanolayers, nanocore shells, bundles of nanowires, and bundles of nanotubes [2]. Another classification of nanomaterials has been also used by the researchers based on the nature of the material. Accordingly, these materials were divided into carbon-, inorganic-, and organic-based nanomaterials [7].
![Figure 2
Classification of nanomaterials according to Siegel [1] with example(s) of each category.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_002.jpg)
Classification of nanomaterials according to Siegel [1] with example(s) of each category.
One of the most preeminent categories of nanomaterials is NPMs. NPMs are used in many applications, such as catalysis, adsorption, sensing, drug delivery, water treatment, gas purification, and gas storage [8]. The first discovery of NPMs was reported by Kresge et al. and Mobil company in 1992 [9]. NPMs consist of organic, inorganic, or hybrid frameworks containing voids and cavities (pores) with diameter at the nanoscale (1–100 nm). These materials can have interesting features such as narrow pore size distribution, large specific surface area, well-defined ordered morphology, and tunable porosity [10]. NPMs are divided into three main categories: (1) microporous materials: having pores diameter less than 2 nm; (2) mesoporous materials: with pores diameter between 2 and 50 nm; and (3) macroporous materials: having pores size larger than 50 nm. NPMs include silica-, transition metal oxide-, alumina-, and carbon-based NPMs, such as mesoporous silicas, nanoporous alumina and titania, metal-organic frameworks, activated carbons, and zeolites [11,12].
NPMs can be also divided into two main categories: silicon-based mesoporous materials (pure and modified silicates) and non-siliceous mesoporous materials. Pure silicate materials such as Santa Barbara Amorphous (SBA), Mobil Crystalline Materials (MCM), and Hollow Mesoporous Silica (HMSs). Non-siliceous mesoporous materials category includes metal oxides (e.g., titania, zirconia), and non-metallic oxides (e.g., sulfate, phosphate, and mesoporous carbon). The focal point of this work is the silicon-based mesoporous materials and in particular the mesoporous silica materials (MSMs) class. Originally, MSMs were made from pure silica. This class includes Mobil Composition of Matter No. 41 (MCM-41), MCM-48, MCM-50, SBA-15, SBA-16, and FDU (Fudan University Shanghai Materials). MSMs have important properties such as uniform shapes, high surface areas, tailorable pore size, well-defined pore sizes, narrow pore size distributions, low density, well-defined ordered morphology, and tunable porosity [10,13]. Here is a brief description of the preparation process of some famous MSMs.
1.2 Synthesis of MCM-41 and SBA-15
The exploration of MSMs expanded new pathways for researchers interested in porous materials and particles morphology. The most common MSMs are MCM-41 and SBA-15. These materials have a variety of distinct and advantageous properties, including high thermal and chemical stability. These MSMs were prepared via a new synthetic strategy based on the utilization of soft organic templates as described in Scheme 1 [14]. In this method, the template is covered by a silica film formed through sol–gel reaction of silica source (e.g., tetraethyl orthosilicate (TEOS)) in basic or acidic aqueous solution. Then, the template can be removed either by solvent extraction (HCl/EtOH) or a calcination process to obtain the final MSMs [15].
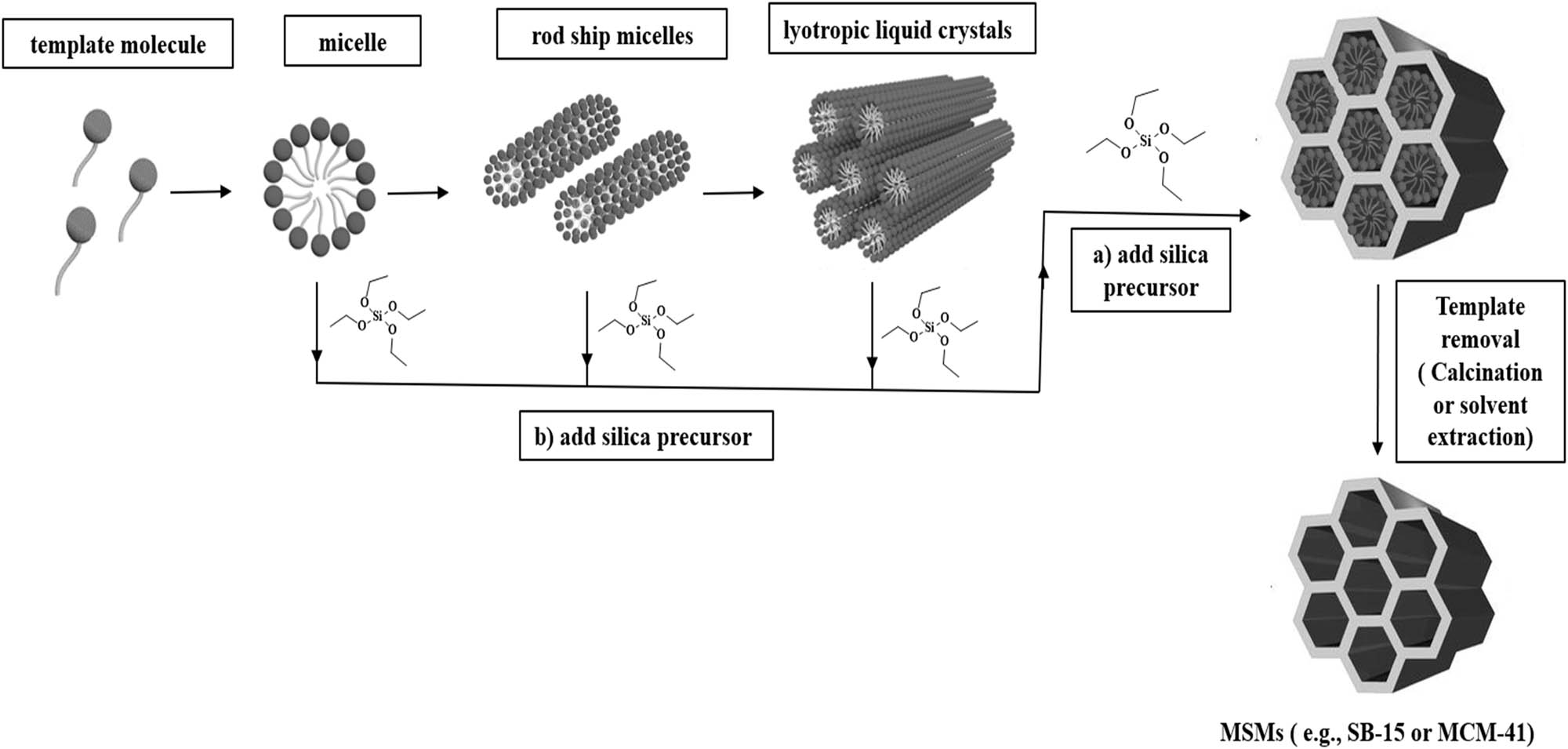
The general synthetic pathway of MCM-41 and SBA-15.
The MCM-41 is a mesoporous material with a hierarchical structure discovered by Mobil Oil Corporation researchers in 1992. It has a regular hexagonal pore structure, with 1D pore system, adjustable pore diameter, narrow pore distribution, large surface area (900–1,100 nm2/g), large pore volume (0.8–1.1 cm3/g), mesopores that range from 2 to 4 nm, and pore wall thickness from 0.8 up to 2.2 nm [16,17]. Mostly, the preparation of MCM-41 involves cetyltrimethylammonium bromide (CTAB) as a surfactant that builds up micelles in basic aqueous solution, and contributes to the formation of the mesoporous framework of MCM-41 (Scheme 1) [18]. The surfactant micelles are transformed into rod ship micelles, which are aligned into hexagonal lyotropic liquid crystals. These liquid crystals are covered by silica film formed by the hydrolysis and condensation of TEOS. After template (CTAB) removal by calcination or solvent extraction, MCM-41 material can be obtained. SBA-15 was prepared for the first time by Stucky and his coworkers in 1998. An amphiphilic triblock copolymer of polypropylene oxide and polyethylene oxide namely Pluronic 123 (P123) was used as a structure-directing agent in acidic aqueous solution following a similar mechanism described for MCM-41[19]. The SBA-15 is distinguished by a highly ordered mesoporous structure, with larger pore size (4–12 nm), pore volume (0.5–1.0 cm3/g), 2D hexagonal structure, thick pore walls (3.1–6.4 nm), straight cylindrical pores, high surface area (450–850 nm2/g), and adjustable pore size. Both MCM-41 and SBA-15 have higher hydrothermal stability and the ability of outstanding modification and functionalization into their surface or even in framework.
1.3 Examples of organic molecules incorporated in MSMs
Later, in 2003, researchers started incorporating organic functional groups into pure MSMs using different methods [20]. The incorporation of organic moieties into silica combines the advantages of both inorganic and organic components and overcomes their drawbacks. Functionalized MSMs have particular properties that make them one of the most important candidates in a variety of applications such as modern nanomedicine, nanocatalysis [21], removal of heavy metals and dyes [22], water purification [23], biotechnology [24], gas separation [25], gas purification [26], and gas storage [27]. Various organic moieties have been incorporated into MSMs to prepare mesoporous organosilica materials, such as pyrene [28], pyrazole derivative [29], azobenzene (AZB) [30,31], 8-hydroxyquinoline [32], phenolic compounds [33], isatin derivative [34], 1,2-bis(2-formylphenoxy)ethane [35], and pyronine derivative moieties [36], and 1‑methyl-2-azaadamanane N‑oxyl moieties [37] (Figure 3).
![Figure 3
Examples of organic moieties incorporated into MSMs. (a) Adapted from ref. [28] with permission. Copyright 2022 American Chemical Society. (b) Adapted from ref. [29] with permission. Copyright 2022 VSP. (c) Adapted from ref. [30] with permission. Copyright 2018 Elsevier. (d) Adapted from ref. [31] with permission. Copyright 2017 Elsevier. (e) Adapted from ref. [32] with permission. Copyright 2024 Iranian Chemical Society. (f) Adapted from ref. [34] with permission. Copyright 2022 Elsevier. (g) Adapted from ref. [36] with permission. Copyright 2020 Elsevier.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_003.jpg)
Examples of organic moieties incorporated into MSMs. (a) Adapted from ref. [28] with permission. Copyright 2022 American Chemical Society. (b) Adapted from ref. [29] with permission. Copyright 2022 VSP. (c) Adapted from ref. [30] with permission. Copyright 2018 Elsevier. (d) Adapted from ref. [31] with permission. Copyright 2017 Elsevier. (e) Adapted from ref. [32] with permission. Copyright 2024 Iranian Chemical Society. (f) Adapted from ref. [34] with permission. Copyright 2022 Elsevier. (g) Adapted from ref. [36] with permission. Copyright 2020 Elsevier.
Recently, Wang et al. grafted pyrene moieties into mesoporous silica surface to obtain pyrene-functionalized mesoporous silica nanoparticles that were used for selective detection of Hg2+ ions (Figure 4) [28]. In addition, Feitosa et al. grafted 3-aminopropyltriethoxysilane (APTS) on the SBA-15 surface for CO2 capture [38]. Zhang et al. also designed a new type of fluorescent chemosensor by incorporating a bis-salicylaldehyde onto the surface of SBA-15 mesoporous silica (MS@NPS) for selective detection of Cu2+ ions in water (Figure 5) [39].
![Figure 4
The mechanism of pyrene-functionalized mesoporous silica nanoparticles to capture Hg2+ ions in an aqueous solution. Adapted from ref. [28] with permission. Copyright 2022 Elsevier.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_004.jpg)
The mechanism of pyrene-functionalized mesoporous silica nanoparticles to capture Hg2+ ions in an aqueous solution. Adapted from ref. [28] with permission. Copyright 2022 Elsevier.
![Figure 5
The mechanism of MS@NPS to capture Cu2+ ions in the water. Adapted from ref. [39] with permission. Copyright 2022 Royal Society of Chemistry.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_005.jpg)
The mechanism of MS@NPS to capture Cu2+ ions in the water. Adapted from ref. [39] with permission. Copyright 2022 Royal Society of Chemistry.
2 Organic photochromic molecules (OPMs): main families
One of the most important organic moieties that can be incorporated in MSMs is OPMs. OPMs are molecules that have the ability to transform between two or more different states upon the absorption of an electromagnetic radiation [40]. Figure 6 presents the main families of OPMs, including diarylethene (DAE), spiropyran (SP)/merocyanine (MC), AZB, and stilbene.
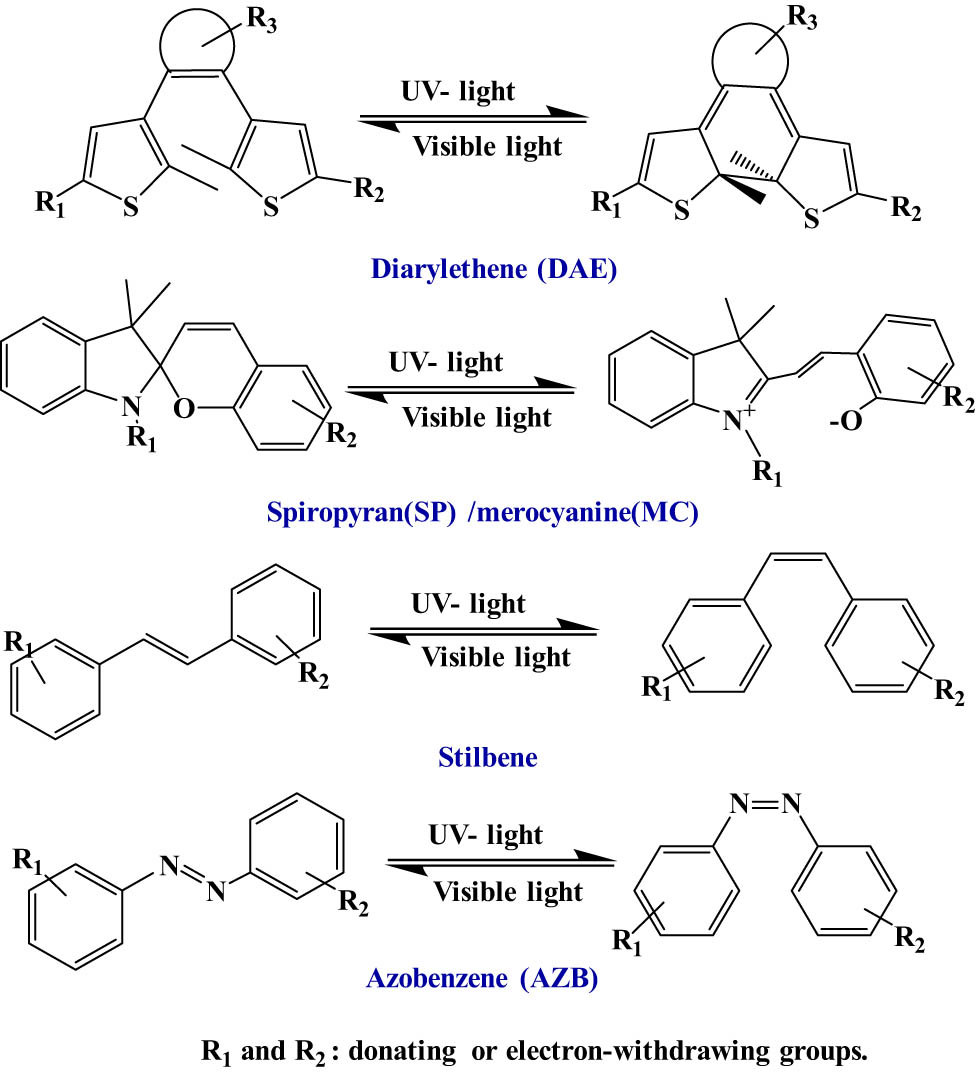
Examples of OPMs.
The incorporation of OPMs into MSMs can afford a new class of mesoporous materials called photochromic mesoporous organosilica materials (PMOMs). PMOMs have been the subject of many research studies during the last decade due to their potential applications, especially in the design and development of optical data storage devices [41], optical switches [42], drug delivery [43], selective separation of dye molecules [44], capture and release of different gases [45], sensing [46], and nanomedicine [47]. PMOMs are considered smart nanomaterials because they have the capacity to undergo reversible changes in the solid state when exposed to an external electromagnetic radiation [48]. Figure 7 presents the position of PMOMs (highlighted) in nanomaterials classification.
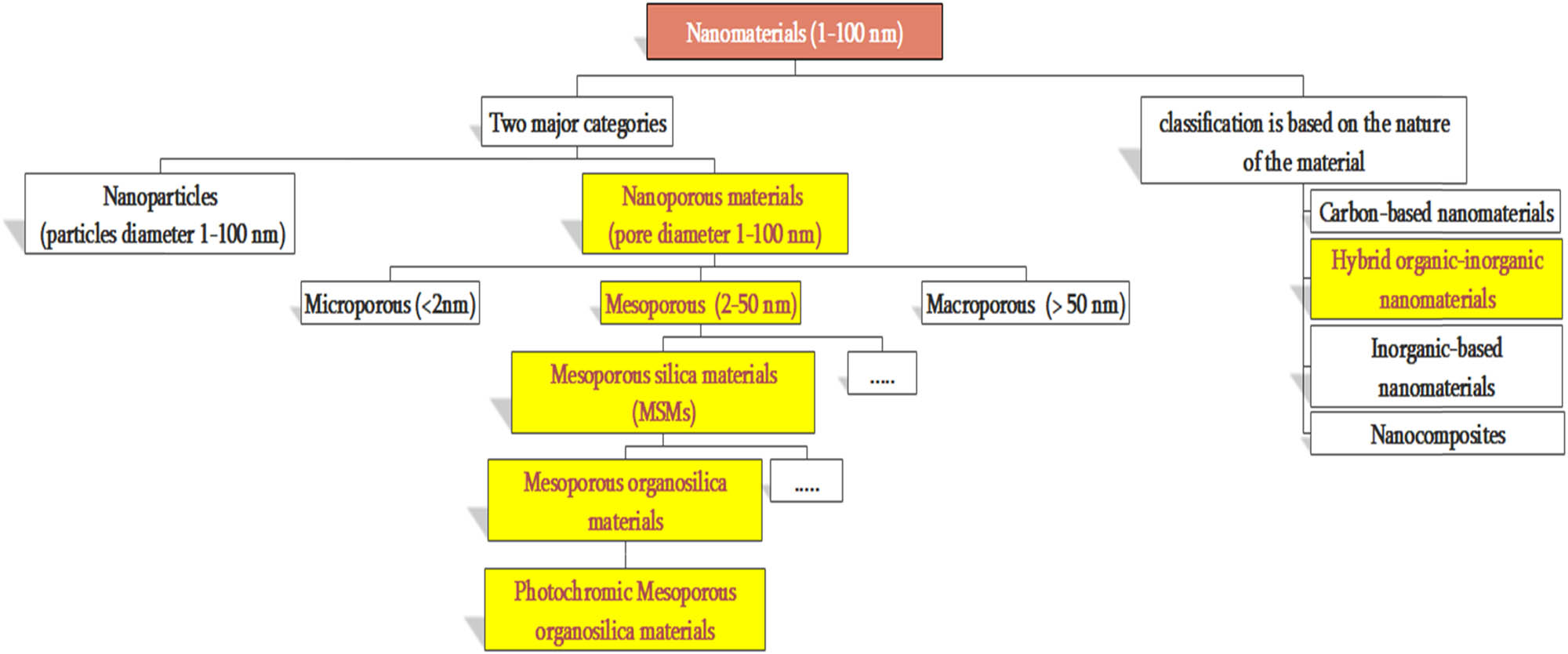
The position of PMOMs in the classification of nanomaterials.
However, only few OPMs have been incorporated in MSMs until now, such as AZB [30,49], SP [50], pyromellitimide [51], spirooxazines (SPZs) [52], and 2-hydroxychalcones [53]. In this review, we will discuss the main families of OPMs, their incorporation into MSMs to prepare PMOMs, and the applications of some PMOMs reported in the literature.
The photochromism phenomenon can be defined as the reversible transformation of some organic, and biological molecules between two forms or more upon the absorption of an electromagnetic radiation. This radiation can be present either in near-IR, UV, or visible spectrum. These two different transformation forms have different absorption spectra and different physicochemical properties such as the geometrical structure, dielectric constant, and oxidation/reduction potential [54]. The features of photochromism can be obtained through the control of parameters such as solubility, stereochemistry, reactivity, phase, interaction, or complexation between molecules or ions [55]. The common model of photochromic system can be described as follows (Figure 8).
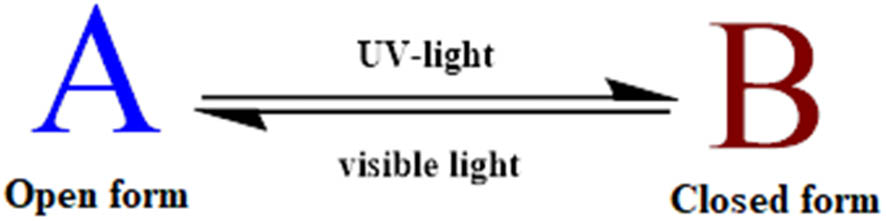
The common model of the photochromic systems.
The most common OPM systems are based on unimolecular reactions. OPM can exhibit positive (direct, A to B) photochromism and negative (reverse, B to A) photochromism. Direct/positive photochromism occurs when the OPM structure changes from a colorless (A-form) to a colored (B-form) situation upon UV irradiation. Negative photochromism known as thermal coloration and photo-induced discoloration is the inverse of normal photochromism. This converts a colored stable situation of OPM (B-form) to a colorless situation (A-form) by irradiation or heat [56,57]. Negative photochromism is especially useful in optical recording materials [58]. Generally, there are two systems that describe the photochromism behavior of the back reaction B to A: P-type and T-type systems. Each P-type and T-type system has a forward reaction A to B that occurs by irradiation with specific wavelength ranges of light. The difference between the two cases is that the P-type system denotes when the back reaction happens through photon absorption (photochemical process). The back reaction can be considered a T-type system when it occurs through a thermal process (heat). The back reaction of the T-type system reverts to its original state in the absence of light. Ordinarily, P-type systems are usually used as dyes because exchanges between A → B and B → A continue indefinitely. Many OPMs of P- and T-type are reported in the literature, such as SPs/SPZs, stilbenes, DAEs, fulgides (FLGs), and AZBs. These OPMs are incorporated in different materials and used for various applications such as cosmetics, clothes, and glass lenses [59]. The suitable photochromic system should be characterized by a good thermal stability, more fatigue resistance, a high quantum yield of photoreactions, perfect solubility in a polymer matrix, reactivity in solid state, and high speed of photoreactions. These OPMs can be classified into different families according to the nature of the chemical transformation during their photoisomerization process [54]. OPMs are used in many applications because the phototransformation causes the rearrangement of electrons and chemical bonds and alters the geometrical structures of OPMs [60].
2.1 Trans/cis photoisomerization
The most studied organic photochromic systems are those that undergo reversible trans/cis isomerization. The trans/cis photoisomerization is a main photoinduced process in AZB and SP systems, but in FLG or DAE systems, trans/cis photoisomerization can occur only as an alternate photoinduced process. Therefore, AZBs and its derivatives represent the main family of photochromic molecules performing trans/cis isomerization [61].
2.1.1 AZB and its derivatives
AZBs undergo trans/cis isomerization between two phenyl rings through an N═N double bond (Figure 9). These are known as “diazenes.” AZB usually converts from yellow to orange-red under UV light. The main application of AZB compounds is their utilization as dyes, but due to their efficiency of trans/cis isomerization in the presence of suitable radiation, these molecules are good candidates to operate as molecular switches [62]. In addition, they are also used in a variety of applications such as optoelectronic devices, sensors, and catalysts [63]. Upon UV light between 300 and 400 nm, AZB moiety transforms from trans to cis, and the trans form can be easily recovered either in visible light (>400 nm) or by heat [64]. A major disadvantage of AZBs is the quick thermal back reaction of the cis isomer to the more stable trans isomer. The back thermal isomerization of AZBs happens in the dark and is more effective in the liquid phase due to the thermodynamic stability of the trans isomer. The temperature of the AZB thermal isomerization reaction depends on the solvent used, for example, AZB in chloroform occurs from 23 to 60°C [65].
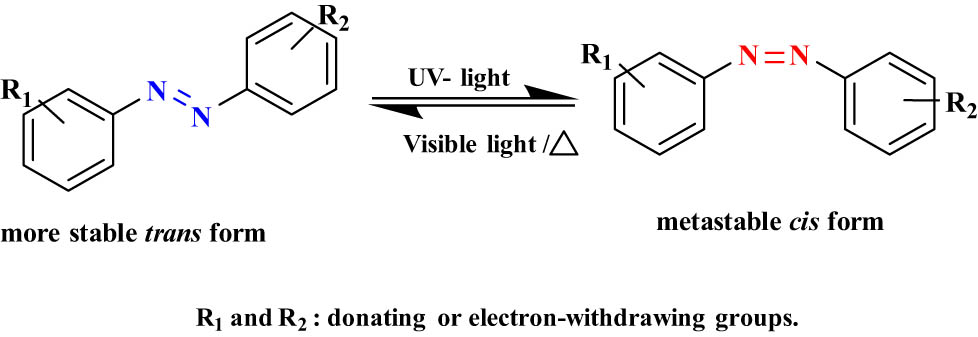
The typical trans/cis photoisomerization of AZBs.
For example, Zhang and his team synthesized a chemosensor containing AZB moiety with good solubility in water. This chemosensor showed a good detection of Hg2+ in wastewater (Figure 10(1)) [66]. In another work, Manubhai and coworkers reported another chemosensor containing two dyes namely AZB and rhodamine for the detection of Al3+ in aqueous ethanol (Figure 10(2)) [67]. The binding of Al3+ causes a considerable change in both colorimetric and fluorometric responses. This chemosensor changes color from colorless to pink after the complexation of Al3+. It was found that this chemosensor has high selectivity and reversibility to Al3+ making it a viable probe for Al3+ detection with naked eyes.
AZB derivatives were also used in catalysis. Arif et al. immobilized an AZB derivative into a bimetallic phosphine–gold complex (Figure 11) [68]. The obtained photochromic organometallic complex was used as a catalyst for an intramolecular hydroamination reaction. It was found that the irradiation of the reaction mixture upon UV light (320 nm) for 10 min transforms the AZB moieties to cis form, leading to the acceleration of the reaction rate.
![Figure 11
Structure and design of a bimetallic AZB-gold-based photochromic catalyst. Adapted from ref. [68] with permission. Copyright 2018 Royal Society of Chemistry.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_011.jpg)
Structure and design of a bimetallic AZB-gold-based photochromic catalyst. Adapted from ref. [68] with permission. Copyright 2018 Royal Society of Chemistry.
2.1.2 Trans-Stilbene
Stilbene is a simple type of DAEs. Under the UV light between 340 and 400 nm, the stilbene undergoes trans to cis isomerization, and the back reaction from cis to trans can occur easily under visible light or heat (T-type) (Figure 12) [69]. Trans-Stilbene has been used in a variety of applications, especially in large-area displays and lighting devices (photoreceptor and photoconductors) [70].
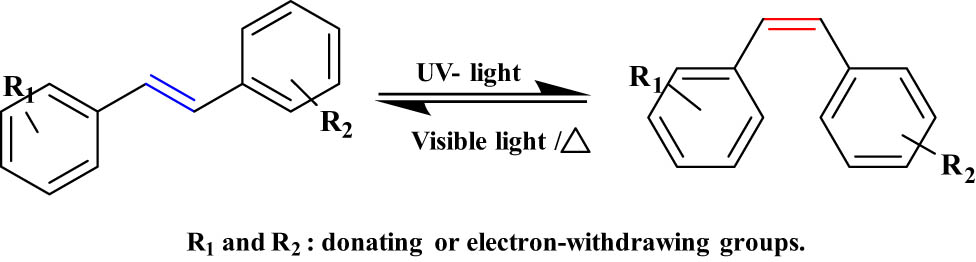
Typical trans/cis photoisomerization of trans-stilbene.
2.2 Electrocyclization reaction
Many OPMs undergo electrocyclic reactions under electromagnetic radiation such as FLGs, DAEs, SPs, and SPZs. Electrocyclic reactions are the concerted cyclization of terminal π-bond in an open conjugated system to create σ-bond by rearrangements of the π-electrons. The back reaction is known as an electrocyclic ring opening.
2.2.1 DAE and its derivatives
DAEs are the most popular class of photochromic families which undergo a reversible electrocyclization reaction upon electromagnetic radiation. DAEs are the most investigated photochromic compounds due to their excellent fatigue resistance and highly bistable nature. Mostly, the “aryl” part is based on heterocycles. DAE structure is composite from an extended π-system of cyclohexadiene and considered P-type systems. Usually, DAEs undergo an electrocyclization reaction from a colorless-open form to a colored-closed form. The most common molecules investigated in this class are DAEs and their derivatives (Figure 13). DAEs usually absorb electro-radiation light to produce two absorption bands between 234–373 and 353–575 nm [60]. DAEs are more used in the optical data storage field because they are thermally stable, with short response time and high quantum yield. In addition, both isomeric forms of DAEs are stable which allow the write–erase process with many cycles without losing the photochromic performance [71]. DAEs also have been used in many applications such as molecular calculators [72], photo-switchable fluorescence [73], and chemosensors [74]. Also, the cis isomer of stilbene can undergo two kinds of isomerization reactions: electrocyclization reaction and the cis–trans photoisomerization (Figure 14) [54]. Cis-stilbene absorbs light to produce dihydrophenanthrene that oxidizes irreversibly by the elimination of hydrogen to produce phenanthrene [69]. Cis-stilbene has applications in many fields such as supramolecular chemistry, catalysis, and biology [75].
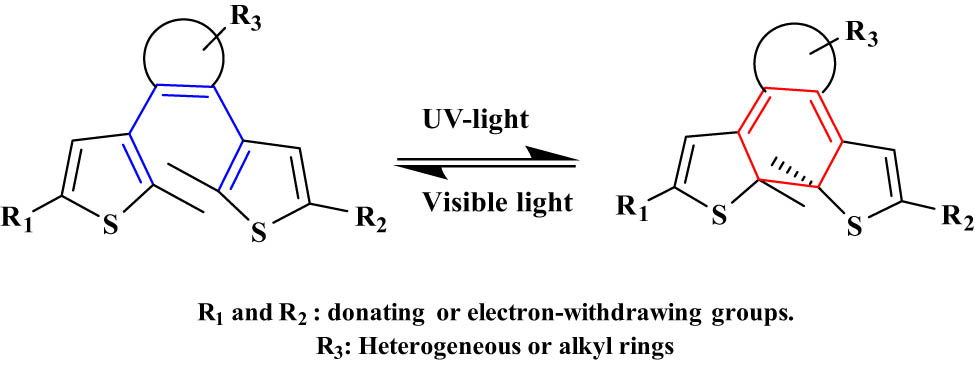
Typical electrocyclization reaction of DAEs.
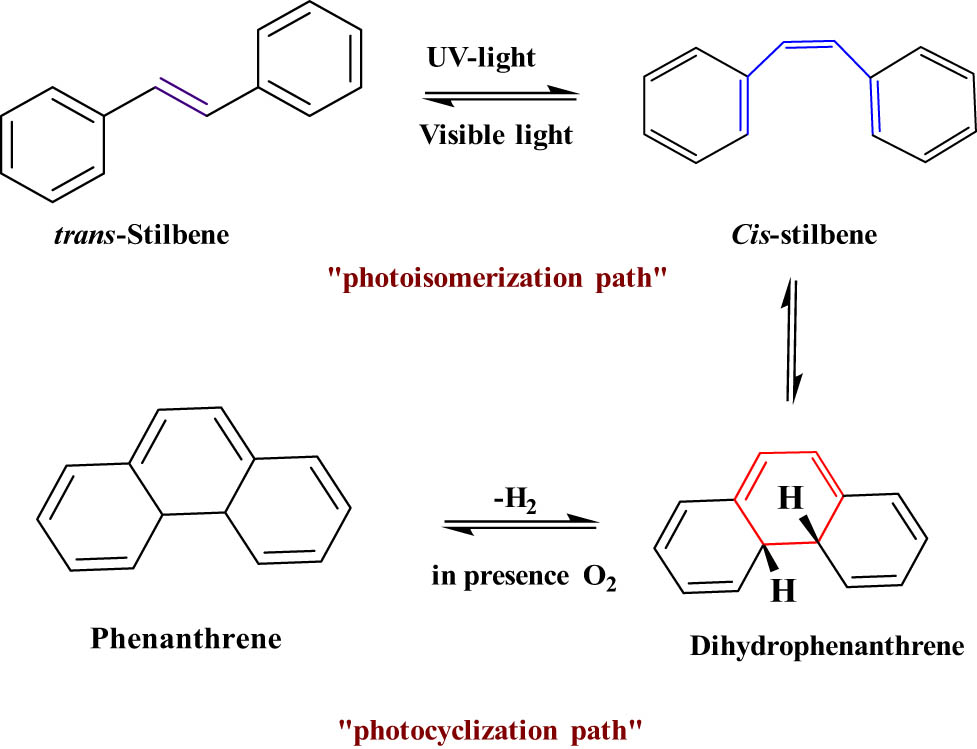
Typical electrocyclization reaction cis-stilbene.
2.2.2 SPs and SPZs
The photochemical behavior of SPs and SPZs is based on the photochemical 6π-electron-ring-closing reactions (Figure 15). These photochromic compounds are considered T-type systems. In addition, they can undergo reversed isomerization by heat or exposure to visible light. The most popular type of SPs contains benzopyran and indoline moieties [76]. SP and SPZs absorb UV light (between 250 and 380 nm) and the carbon–oxygen bond of the pyran ring splits and undergoes a ring-opening reaction to form more conjugated MC (Figure 15). The closed form of SPs is more stable than the MC form which is metastable [77]. The thermal isomerization reaction of SPs occurred at 0–60°C [78]. SPs are utilized in many industrial applications such as drug release [79], smart textiles [80], and optical memory [81].
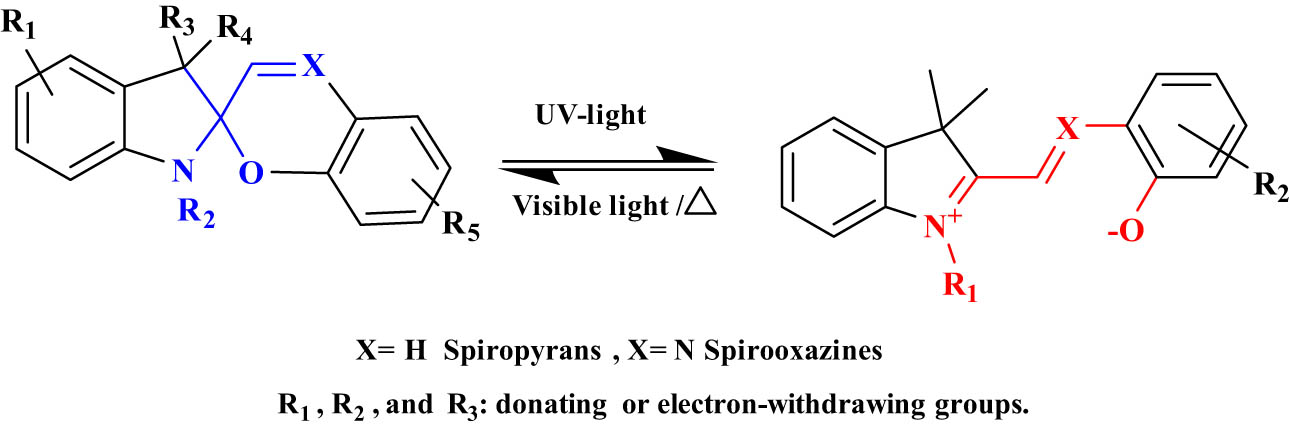
Photoisomerization of SPs/SPZs between the closed and open MC forms.
2.2.3 Chromenes
Chromenes contain a benzene ring with a heterocyclic pyran ring (Figure 16). They undergo photochromic ring-opening reactions type cyclohexadiene 1,3-hexatriene-1,3,5 under UV light between 315 and 380 nm [82]. Chromenes are considered T-type systems because they undergo back reaction (ring closing) under heat or visible light. Compared with SP-based photochromic molecules, chromones have a slow back reaction. Therefore, chromones can be used in photochromic ophthalmic lenses [83].
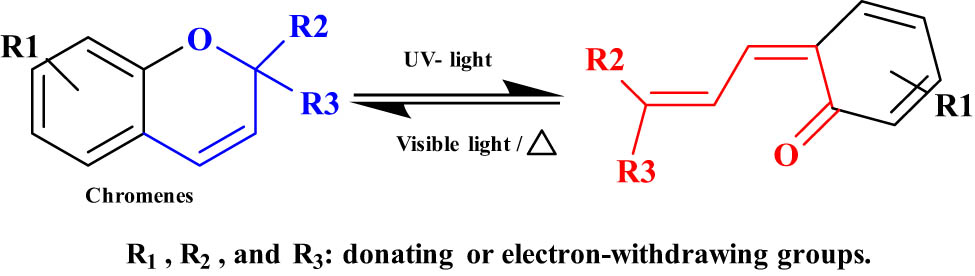
Photoisomerization of chromenes.
Chromones are also used as chemosensors. For example, Ziarani et al. reported a chromone-based chemosensor that was prepared by functionalized mesoporous silica SBA-15 with 4-hydroxy-2-oxo-2H-chromene-3-carbaldehyde (SBA-Pr-NHC) [84]. The sensing property of the obtained material was investigated using photoluminescence spectroscopy. The obtained SBA-Pr-NHC showed a remarkable selectivity toward Ag+ ions in aqueous solutions. After the complexation reaction, the fluorescence emission was significantly increased (Figure 17).
![Figure 17
Detection of Ag+ ions by SBA-Pr-NHC chemosensor through increasing fluorescence emission. Adapted from ref. [84] with permission. Copyright 2022 Elsevier.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_017.jpg)
Detection of Ag+ ions by SBA-Pr-NHC chemosensor through increasing fluorescence emission. Adapted from ref. [84] with permission. Copyright 2022 Elsevier.
2.2.4 FLGs and fulgimides
The nomenclature of “Fulgide” comes from the Latin “Fulgere” which means “shine.” FLG has a bis-methylene-succinic anhydride and an aromatic group as a substituent, but fulgimides contain a succinimide that replaces succinic anhydride (Figure 18) [85]. To obtain photochromic features, FLGs should have exo-methylene carbon substituted with an aryl group. The photochromism in FLGs depends on a cyclization reaction. Originally, FLGs are known as T-type photochromic compounds because they undergo thermal back-reaction. However, when substituted with heterocycles as an aryl group within the structure they become P-type photochromic compounds. FLGs have both cis and trans configurations in the open forms. The main difference between FLGs and SPs is that the open forms of FLGs are colorless, and the closed forms are colored [86]. FLGs have distinctive features such as high thermal and chemical stability and high fatigue resistance. These features lead to the utilization of FLGs in some special applications such as dynamic chemosensing, optical storage devices [81], light-activated tools [87], and molecular switches [88]. However, the utilization of FLGs is relatively limited by their difficult synthesis.
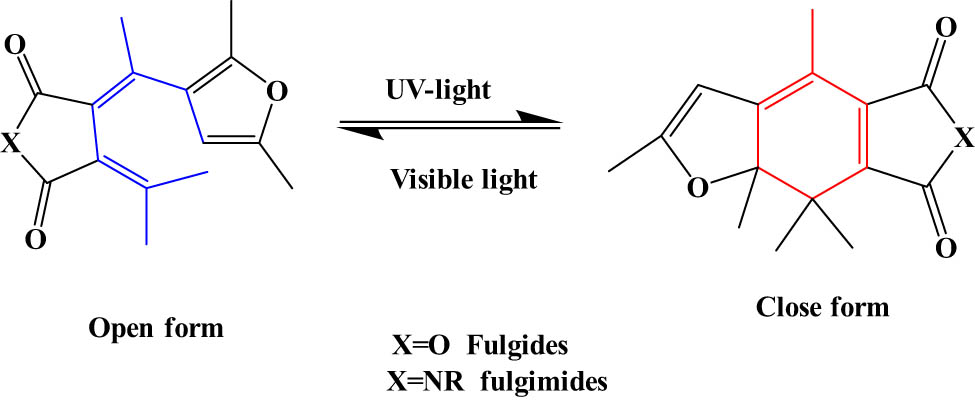
Structures and typical electrocyclization reaction of FLGs.
2.3 Proton transfer
The photochromism assisted by proton transfer reactions is exhibited in a variety of organic natural systems. The most investigated photochromic compounds involving a proton transfer are salicylideneaniline and their derivatives. However, photochromism of this class occurs in a variety of media such as solution, inclusions, solid-state, and encapsulation. Upon UV–Vis irradiation (from 365 to 488 nm), salicylideneaniline derivatives transform from the enol form, by an intramolecular proton transfer, to the trans-keto form (Figure 19). It was found that this process takes a few picoseconds in the solution and a few hundred picoseconds in the solid state [54].
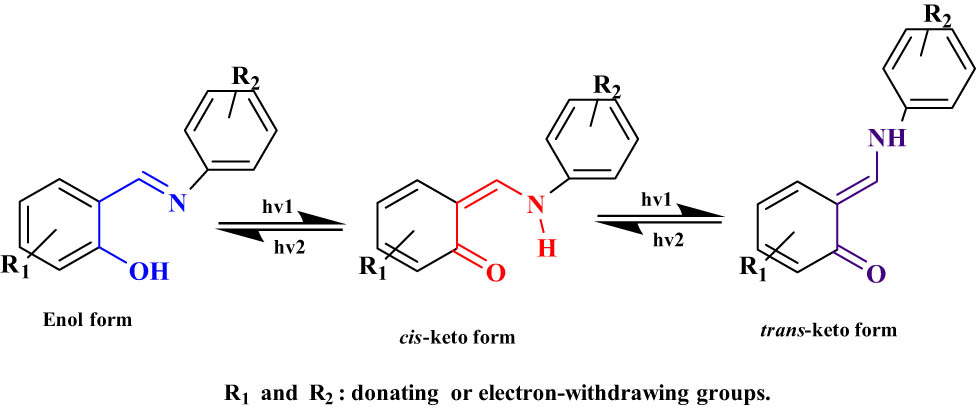
Proton transfer-assisted photochromism salicylideneaniline and their derivatives.
2.4 Homolytic cleavage
In this type of photochromic isomerization of FLGs is assisted by a bond cleavage while the FLG molecule splits into two or more molecules. This group contains hexaarylbiimidazole (HABI) (Figure 20). The C–N bond between the two imidazole rings in HABI undergoes homolytic cleavage upon exposure to UV light irradiation. The two radicals are produced: triphenylimidazolyl radical (TPIR) and triphenylimidazolyldimer (TPID) pair. TPIR absorbs visible light to produce a broad absorption band, but TPID only absorbs UV light and hence is colorless. Although UV irradiation leads to C–N cleavage in less than 100 fs. The recombination at ambient temperature can take up to a few minutes. This class of photochromism can be considered T-type [89,90]. HABI was used in many applications, such as photo initiators, panting, and electronic devices [91].
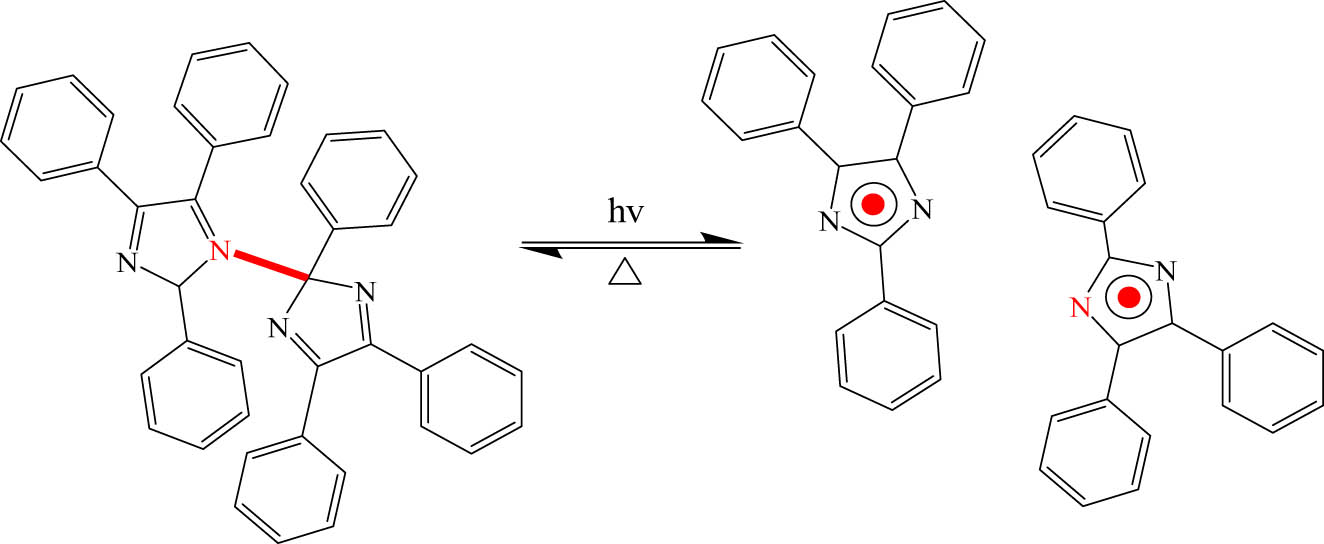
Photochromism of HABI by homolytic cleavage.
2.5 Alternative families
From previous studies, photochromic systems based on dihydroazulene, [92] phenoxynaphthacene-quinone cores, [93] indoline [94], dihydropyrene (DHP) [95], naphthopyran (NP) [96], and biindenylidenedione [97] undergo also ring-opening and ring-closing interconversion reaction under UV light (Figure 21). These families are known as alternative families of OPMs and are used in many important applications such as solar energy storage [98], molecular electronics [99], colorimetric sensing for ions [100], and photoswitches [101].
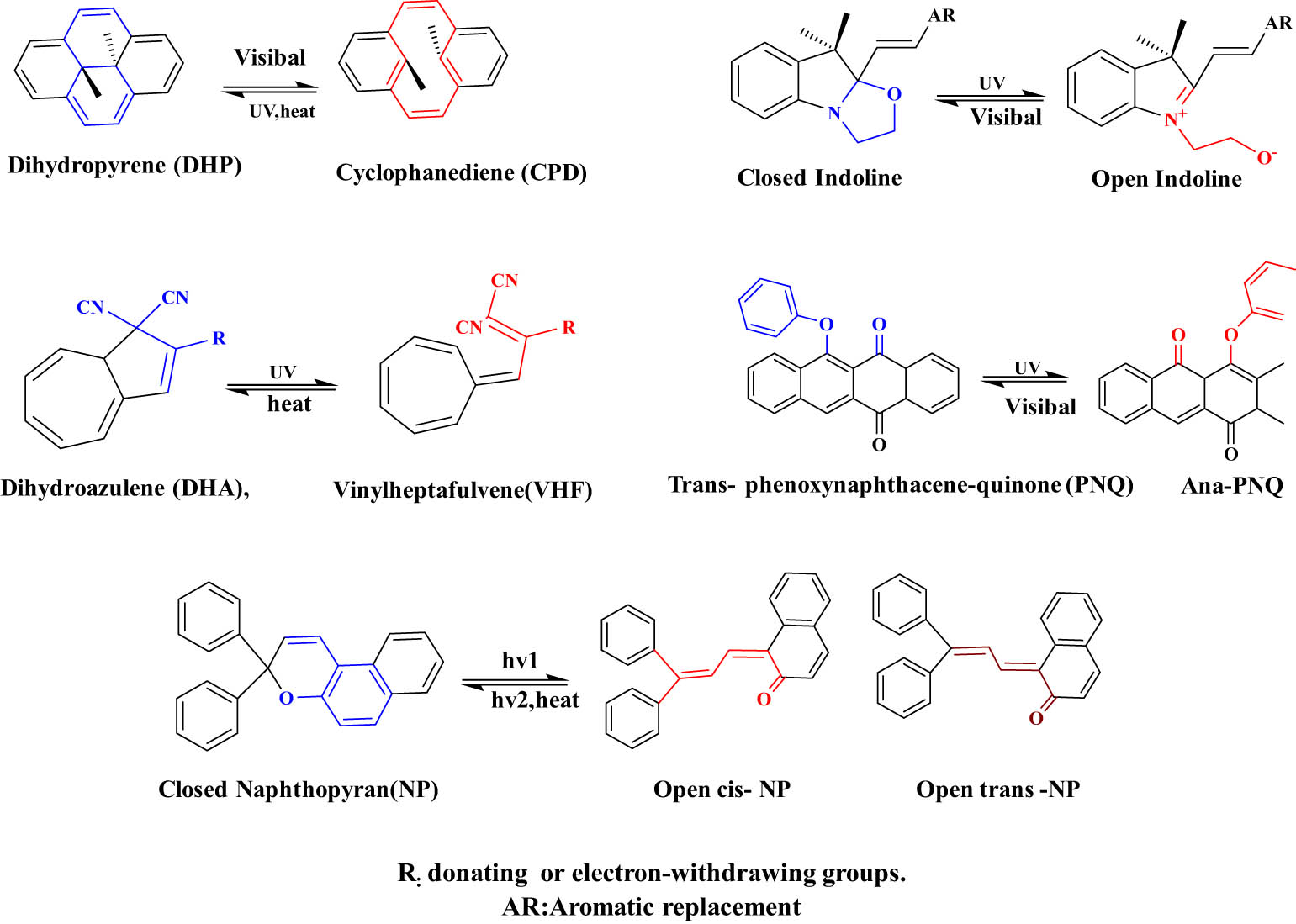
Chemical structures and photoisomerization of alternative families.
3 Incorporation of OPMs into MSMs: synthesis and applications
MSMs are NPMs that have periodic arrays of cavities and channels possessing pore diameters from 2 to 50 nm. These materials are an outgrowth of the initial work of Mobil’s efforts when it revealed the M41S family, which uses long-chain cationic surfactants as templates during the hydrothermal sol–gel process [102]. Combining organic and inorganic components at the nanometric level improves the production of novel multifunctional materials. OPMs can be introduced into silica matrices to produce photochromic MSMs (PMSMs). However, only few OPMs have been incorporated in MSMs until now, such as the AZB [30], SP [103], and stilbene [104]. The design of these OPM systems is critical so that charges can be transferred reversibly between two or more different states (e.g., cis/trans, open/close) in a solid state upon a photoirradiation [105]. The synthesis of OPMs is usually challenging. They cannot be prepared using standard methods such as vacuum evaporation or sputtering because most photochromic moieties decompose when heated at high temperatures [106]. The incorporation of OPMs into MSMs with specific pore diameters is a versatile idea for developing materials with novel features and intriguing applications such as optical data storage devices [41], optical switches [42], support for drug release [43], capture and release of different gases [45], sensors [46], and nanomedicine [47]. Moreover, OPMs can be incorporated into the MSMs matrix in two different ways: physical binding (non-covalent interactions) or chemical binding (covalent interactions) as seen in (Figure 22).

Incorporation of OPMs into MSMs by chemical and physical pathways.
3.1 Physical methods
The physical adsorption of OPMs into MSMs (OPMs@MSMs) is a straightforward pathway that involves unshared electron bonds (non-covalent) such as Van der Waals forces (e.g., dipole–dipole, π−π stacking, and London dispersion forces), hydrogen bonds (HBs), and ionic bonds [107]. The physical incorporation of organic molecules into MSM pores was the basis for the first functional mesostructured materials [108]. Generally, the adsorption of any organic molecules inside a solid can be affected by different parameters such as the ionic strength of the solvent, organic molecules loading, pH, and temperature. In general, low loading is preferred to avoid the leaching of OPMs from the solid, or/and their aggregation inside of the pores. The adsorption process can also be affected by the physical and chemical properties of the MSM surface. In case of the immobilization of OPMs into MSMs via HBs, OPMs must have functional groups, or atoms possess available lone pairs able to form HBs (e.g., OH, O, N). For example, Schomburg et al. impregnated the photochromic dye 2H-spiro [[6] nitrochromene-26] nitrochromene-methylindoline] (NBIPS) into Si-MCM-41 via HBs between the silanol groups in the surface of MCM-41 and the NH groups of NBIPS (Figure 23) [109].
![Figure 23
Incorporation of 2H-spiro[[6] nitrochromene-2,2′-[1,3,3] trimethylindoline] dye into Si-MCM-41 pores through HBs. Adapted from ref. [109] with permission. Copyright 2001 Royal Society of Chemistry.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_023.jpg)
Incorporation of 2H-spiro[[6] nitrochromene-2,2′-[1,3,3] trimethylindoline] dye into Si-MCM-41 pores through HBs. Adapted from ref. [109] with permission. Copyright 2001 Royal Society of Chemistry.
The OPM load in MSMs depends on the type of interactions between OPMs and MSMs surface (non-covalent interactions). The adsorbed OPMs can exist in different forms: isolated molecules, monolayers, or clusters [110].
OPMs can be found in the external or internal surface of MSMs, near the pores entrance, or inside of the pores. The spatial distribution of the OPMs (including position, density, and direction) influences their stability and can reduce the interactions between them in the MSM matrix. Physical adsorption relies on the ability to reach adsorption sites in MSMs, the density and distribution of the adsorption sites, and the confinement possibility of OPMs inside MSM pores. The accessibility of the adsorption sites is determined by the MSM pore size and shape. The greater the pore size, the better the possibility of reaching the adsorption sites. However, increasing pores size can lead to more leaching of OPMs from MSMs. Therefore, in the physical adsorption of OPMs into MSMs, it is vital to enhance the adsorption site accessibility while maintaining the confinement possibility of OPMs. Finding a balance between adsorption site accessibility and confinement is a critical point in the physical adsorption. The inclusion of OPMs inside the MSM pores network can be facilitated by a high accessibility, but the lack of the confinement can lead to a high leaching rate of OPMs. However, low accessibility can also result in increased leaching rates because OPMs are preferentially adsorbed outside the pores [111]. The classical physical adsorption methods have basic problems of leaching, agglomeration, migration, and sintering of organic molecules inside the MSMs [112]. To enhance the adsorption of organic molecules on MSM surface and reduce these drawbacks, the electrostatic interactions between the organic molecules and the silica surface have to be improved, with accessible adsorption sites and adequate pore size [113]. To avoid these disadvantages of the physical adsorption, and to increase the electrostatic interactions between the organic molecules and the silica surface, Dunn et al. suggested the incorporation of the organic molecules during the preparation process of the silica (sol–gel process) [114]. This method allowed the control of MSM porosity, improved the electrostatic interactions, and prevented the migration and aggregation of the organic molecules by their capsulation in closed pores. Various methods have been reported in the literature to incorporate organic molecules into MSMs via physical adsorption [115,116,117,118,119]. Here we will discuss the most used methods to incorporate OPMs into MSMs, such as the incipient wetness impregnation (IWI), photo-induced adsorption, and the encapsulation method.
3.2 Impregnation method
The impregnation method has the general concept that the organic precursor(s) dissolves in a suitable solvent and, then, the MSMs immerse in the obtained solution to allow the precursor molecules to penetrate inside the MSM pores. After removing the solvent by drying OPMs@MSMs is obtained (Scheme 2). The distribution of the OPM molecules into MSMs depends on the balance between the adsorption and diffusion processes. The physical and chemical properties of the obtained materials can be influenced by different parameters, such as process time, OPM load, solvent, pH, and temperature [120,121].
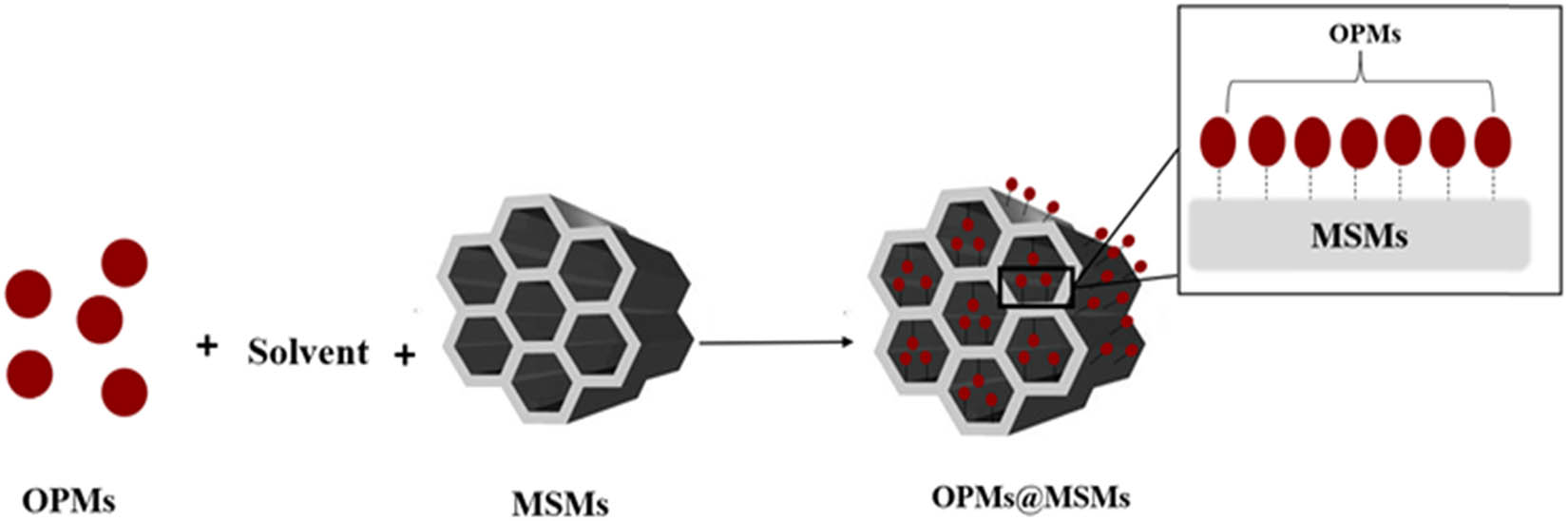
The general concept of the impregnation of OPMs inside MSMs.
If OPMs are dissolved into a volume of solvent equal to the silica total pore volume, a thick-paste-like material will be obtained, this point is named as “incipient wetness point.” This method is called IWI [122,123,124]. After the evaporation of the solvent, washing and drying the OPMs@MSMs can be obtained. Schomburg and his team incorporated SP dye into MCM-41 using the IWI method (Figure 24) [104].

Incorporation of SP into MCM-41 via IWI method.
The SP dye was loaded into MCM-41 with 1 × 10−5 and 5 × 10−5 mol/g. The absorption spectra of SP in MCM-41 at high loading were displayed at 350 and 500 nm and at low loading were observed at 300 and 400 nm. High quantum yields[1] for photochromism (75%) show that chromophores of SP in these materials are readily accessible to the photons from laser pulses that are employed for photoinduced switching between various configurational isomers. The aim of this work was the investigation of the host–guest interaction effects on cis/trans isomer status, reversibility, and stability.
However, the obtained materials were not evaluated in any application.
In another work, Casades et al. reported the incorporation of SP (BIPS) and 6-nitrospiropyran (6-NO2-BIPS) into MCM-41, Al-MCM-41, and silylated MCM-41 using the impregnation method [121]. The objective of this study was the investigation of the effect of the silylation and chemical composition of MCM-41 on the photochromic behavior of the adsorbed SP and 6-nitro-SP. The UV–Vis diffuse reflectance spectra of BIPS@Silica and 6-NO2-BIPS@Silica (Silica: MCM-41, Al-MCM-41, and MCM-41Sil) recorded after 24 h are presented in Figure 25. This study confirmed that the presence of aluminum in MCM-41 has a very minor impact on the distribution of SP and the O-protonated MC form in MCM-41. After the incorporation the SP performs ring opening to form either O-protonated form or zwitterionic MC form (Scheme 3). It was also found that the O-protonated MC was predominantly formed in MCM-41 and Al-MCM-41. Unlikely, after the silylation of MCM-41 by grafting trimethylsilyl groups {Si(CH3)3} using hexamethyldisilazane {(CH3)3Si-NH-Si(CH3)3}, the obtained silylated MCM-41 allowed the presence of a mixture of zwitterionic MC and SP. This is probably due to the significant decrease of the hydrophilicity and acidity after the modification.
![Figure 25
UV–Vis diffuse reflectance spectra recorded after 24 h of the adsorption of BIPS (1) on (a) MCM-41; (b) Al/MCM-41; and (c) MCM-41Sil. (2) 6-NO2-BIPS on (a) MCM-41; (b) Al/MCM-41; and (c) MCM-41Sil. Adapted from ref. [121] with permission. Copyright 2002 Royal Society of Chemistry.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_025.jpg)
UV–Vis diffuse reflectance spectra recorded after 24 h of the adsorption of BIPS (1) on (a) MCM-41; (b) Al/MCM-41; and (c) MCM-41Sil. (2) 6-NO2-BIPS on (a) MCM-41; (b) Al/MCM-41; and (c) MCM-41Sil. Adapted from ref. [121] with permission. Copyright 2002 Royal Society of Chemistry.
![Scheme 3
Processes observed upon the physical adsorption of SP in MCM-41 aluminosilicates. Adapted from ref. [121] with permission. Copyright 2002 Royal Society of Chemistry.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_039.jpg)
Processes observed upon the physical adsorption of SP in MCM-41 aluminosilicates. Adapted from ref. [121] with permission. Copyright 2002 Royal Society of Chemistry.
The photochromic behavior of the obtained photochromic SPs@MSMs materials was investigated using two irradiation techniques namely steady-state irradiation (SSI) and laser flash photolysis (LFP). After SSI (λ > 450 nm) of the prepared materials, the intensity of the absorption bands corresponding to the protonated and unprotonated MC form was decreased. This photochromic behavior is called reverse photochromism. However, when LFP was used as an irradiation method at 308 nm to irradiate SPs@MCM-41, the residual SP was transformed to the corresponding zwitterionic form. Here, the photochromic behavior is named normal or direct photochromism. Also, Barachevsky and his team studied the kinetic and spectral manifestations of photochromism of different photochromic compounds, such as DAEs, chromenes, SPs, and SPZs, with and without silica nanoparticles, using water-ethanol solutions [125]. Comparing the photochromic properties of these OPMs in silica with those without silica, it was found that only compounds 2 and 3 had similar photochromic behavior in both states. Based on this finding, authors claimed that these two OPMs were adsorbed physically into silica surface. However, the absorption bands of compounds 1 and 4–6 were shifted in silica nanoparticles (Figure 26). According to the author’s explanation, this is probably due to chemical interactions (e.g., protonation) between these OPMs and the silica surface.
![Figure 26
Structure of SPs (a), SPZs (b), chromenes (c), and DTEs (d–f) derivatives incorporated into SiO2 nanoparticles via physical binding. Adapted from ref. [125] with permission. Copyright 2018 Springer.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_026.jpg)
Structure of SPs (a), SPZs (b), chromenes (c), and DTEs (d–f) derivatives incorporated into SiO2 nanoparticles via physical binding. Adapted from ref. [125] with permission. Copyright 2018 Springer.
3.3 In situ synthesis
SP was also incorporated in three types of zeolites (DAY, NaY, and HY) by Schomburg et al. [109] using in situ synthesis technique (Scheme 4). In this method, SP@zeolites were prepared in two steps. In the first step, 1,3,3-trimethyl-2-methyleneindoline was inserted into zeolite (DAY, NaY, and HY), followed by the addition of 2-hydroxy-5-nitrobenzaldehyde in the second step. After filtration and extraction by Soxhlet using ethanol as a solvent for 70 h, the desired material was obtained. The luminescence spectra of the colored SP isomers in the prepared materials revealed non-aggregation of the MC forms. Moreover, the SP species exhibited stronger retardation of the thermal relaxation rate (from cis-MC form to the trans-MC form) into faujasite hosts (HY, DAY) than into MCM-41. This is probably due to the imposed spatial limits in zeolites which can increase the rotational barriers.
![Scheme 4
In situ synthesis of physically attached SP into three types of zeolites (DAY, NaY, and HY). Adapted from ref. [109] with permission. Copyright 2001 Royal Society of Chemistry.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_040.jpg)
In situ synthesis of physically attached SP into three types of zeolites (DAY, NaY, and HY). Adapted from ref. [109] with permission. Copyright 2001 Royal Society of Chemistry.
3.4 Photo-induced adsorption
The photo-induced adsorption of OPMs is a method reported by the Ogawa research group [110,115,118,126], which combines photochemical reaction and physical adsorption. This method consists of dissolving SP in a suitable organic solvent (e.g., toluene) with suspended MSMs, followed by photoirradiation or heat to transform SP, which has weak interactions with MSMs, to MC that absorbs well into MSMs. After exposing the mixture to visible light MC can be transformed back to SP and desorbs from MSMs. The photo-induced adsorption of SP into MSMs was monitored by UV–Vis spectra as well as by naked eye observation. The photo-induced adsorption technique of SP/MC can have some potential applications such as the control release of drugs (drug delivery). Generally, photo-induced adsorption methods have many advantages such as controllable kinetic selectivity and stability of the photoreaction. In addition, this method takes advantage of the properties of some OPMs that change their geometry and polarity upon photoirradiation.
Ogawa and Okabe studied the adsorption of SP into SBA-15 spherical particles [118]. It was found that the photochemically formed MC in SBA-15 was very stable at room temperature, which suggests a possible utilization for optical recording. In fact, the silanol groups in SBA-15 pores surface were suggested to stabilize MC molecules through the formation of HBs. The authors concluded that the adsorption yield of MC into SBA-15 can be affected by the relatively large pore size and SBA-15 surface modification.
In another work, Ogawa et al. investigated the effect of MSM pore size on the acceleration of photochromism and negative photochromism of SP/MC using the photo-induced adsorption method in toluene (Figure 27) [116]. Two types of MSMs with different pore sizes were used, MCM-41 (2.2 nm) and SBA-15 with two different pore sizes (5.5 and 9.4 nm).
![Figure 27
SP photochromism in toluene solution (a) and mesoporous silica (b). Adapted from ref. [116] with permission. Copyright 2019 Royal Society of Chemistry.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_027.jpg)
SP photochromism in toluene solution (a) and mesoporous silica (b). Adapted from ref. [116] with permission. Copyright 2019 Royal Society of Chemistry.
The photoinduced absorption method allowed high loading of MC into MSMs. The kinetic study revealed that the thermal coloration and photoinduced adsorption processes of MC into different types of silica are affected by the silica pore size. Therefore, the negative photochromism, thermal coloration, and photoinduced absorption processes can occur faster in mesoporous silicas with larger pore size such as SBA-15 (9.4 nm). The photoinduced adsorption process was considered a pseudo-second-order reaction. The constant rate of adsorption into SBA-15 {9.4 nm – k = 9.55 × 10−3 g (mg min)−1} was larger than MCM-41 {2.3 nm – k = 8.91 × 10−3 g (mg min)−1} due to the MC molecules’ effective entry into the pores of SBA-15 with larger pores. The SBA-15 (t 1/2: 0.37–1.3 h) showed a shorter thermal coloration lifetime than MCM-41 (t 1/2 = 0.69–2.6 h) during negative photochromism. Therefore, the pore size had an impact on the response rates of photochromism through the diffusion of the photochromic molecules rather than being constrained by molecular conformation. Accordingly, this strategy can utilize other photochromic molecules to create solid-state photochromic hybrids. Also, these materials can be used in smart windows and optical recording applications.
Ogawa and Yamaguchi also investigated the effect of hydrophobicity/hydrophilicity of MCM-41 on the photochromic properties of SP/MC [115]. In this work, the surface of MCM-41 particles was functionalized by phenyl groups using phenyltriethoxysilane to obtain a dual domain of mesoporous silica particle (hydrophobic particle surface and hydrophilic mesopore). Thus, Ph-MCM-41 worked as the platform of the SP/MC photochromic reaction.
The decolorization efficiency (negative photochromism) at the photostationary states was improved from 92% in MCM-41 to 95% in Ph-MCM-41. In addition, the thermal colorization of MC@Ph-MCM-41 was accelerated about 1.6 times compared to MC@MCM-41. The authors explain these results by the existence of hydrophobic interactions between SP molecules and phenyl moiety on the Ph-MCM-41 particle surface.
Also, the Ogawa team prepared a hybrid nanoporous-silica-composed dendritic fibrous silica (HNS-MC) through the adsorption of MC into nanoporous silica during UV irradiation [126]. HNS-MC demonstrated a remarkable negative photochromic activity (transition from red-MC to colorless-SP) in the solid state when exposed to visible light. HNS-MC was mixed with an organophilic clay to improve the conversion efficiency. The mixed solid system appeared more efficient in thermal coloration/photodecoloration cycles. Organophilic clay played an important role to accommodate the photogenerated SP. The smooth diffusion of SP molecules between silica particles permitted a negative photochromic reaction in the solid state within a few minutes. In another work, Ogawa and coworkers investigated the photoinduced adsorption of (1-(2-hydroxyethyl)-3,3-dimethylindolino-6′nitrobenzopyrylo-spiran) in a dendritic fibrous nanosilica (DFNS-SP) in toluene solution [127]. In this work, a suspension of mesoporous silica DFNS and SP in toluene was exposed to UV light with and without stirring. Upon UV irradiation, the colorless suspension changed to blue without stirring, while was turned to red under stirring (Figure 28). The blue color was attributed to the photogenerated MC in a nonpolar media (in toluene), while the red color was explained by the protic environment on DFNS. The DFNS with the adsorbed photogenerated MC was easily separable and stable.
![Figure 28
Optical images of the SP in toluene suspension with DFNS (a) after UV irradiation without stirring, (b) before UV irradiation, (c) after UV irradiation with stirring, and (d) after 1-minute sedimentation. Adapted from ref. [127] with permission. Copyright 2017 American Chemical Society.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_028.jpg)
Optical images of the SP in toluene suspension with DFNS (a) after UV irradiation without stirring, (b) before UV irradiation, (c) after UV irradiation with stirring, and (d) after 1-minute sedimentation. Adapted from ref. [127] with permission. Copyright 2017 American Chemical Society.
3.5 Encapsulation method
Encapsulation technique is one of the incorporation methods used to immobilize OPMs into MSMs. This method consists of creating silica walls around OPMs or confining them within closed pores for different applications [128]. Thus far, OPM encapsulation in transparent materials was used to control the photoreaction and to build innovative photo-responsive supramolecular systems [129]. The encapsulation method is usually carried out according to the modified Stöber method [130], which includes the hydrolysis and condensation polymerization of TEOS, either in the presence or absence of a structure directing agent (Scheme 5). Generally, the primary goal of the encapsulation method is to protect the active material from the external environment, such as pH, temperature, moisture, and oxidation. The encapsulation of OPMs into MSMs can also prevent the leaching of OPM molecules from the silica. Different encapsulation conditions can produce different capsules which can be categorized according to their sizes (1–1,000 μm in diameter) and shapes (e.g., tubes and spheres). The encapsulation process has two primary steps: (1) emulsion formation/active component suspension and (2) shell formation [119]. The emulsion step is essential as it determines the size of the capsules. The emulsion step can be affected by the operating conditions (e.g., mass ratio of different phases, viscosity, time, and agitation rate). OPM molecules can be encapsulated into MSMs to create a composite mesoporous material with different photochromic properties [131]. Many studies reported the encapsulation of organic molecules into MSMs, but only few publications reported the encapsulation of OPMs into MSMs.
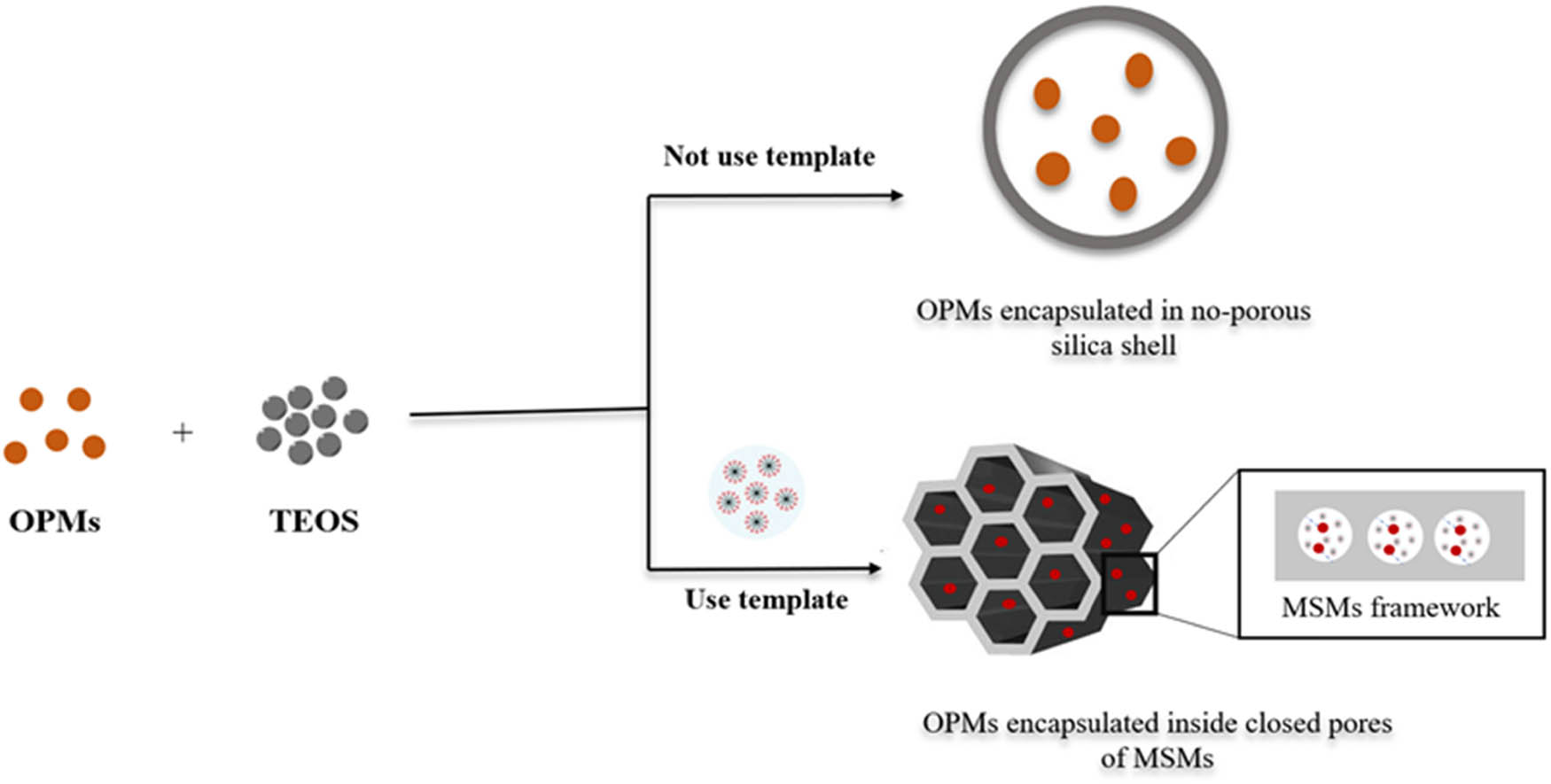
Incorporation of OPMs into MSMs via the encapsulation method.
However, the encapsulation of OPMs into MSMs can limit the applications of the obtained OMPs@MSM materials. Since direct contact between the encapsulated OPMs and any other guest is not possible, this material can be used only as a light or temperature sensor (thermochromism). For example, using these kinds of materials as metallic ion chemosensors is not possible.
Wirnsberger et al. incorporated SP and SO dyes into as made (AM) SBA-15 during the preparation process of SBA-15, using a template-assisted encapsulation method (Scheme 6) [117]. (SP or SO)@AM-SBA-15 materials were prepared by the addition of the dyes (SP and SO) after the hydrolysis of TEOS and the formation of AM-SBA-15 (SBA-15/template). After refluxing a composition of P123 (template), ethanol, H2O, 2 M HCl, and TEOS for 1 h, and cooling the solutions to room temperature, either SP nor SO dye was added to the mixture, and the solution was stirred until complete dissolution of the dye. The obtained solutions were used to prepare thick mesostructured (SP or SO)@AM-SBA-15 films using a deep-coating apparatus. SP and SO were attached to the hydrophobic sites of P123 into AM-SBA-15.
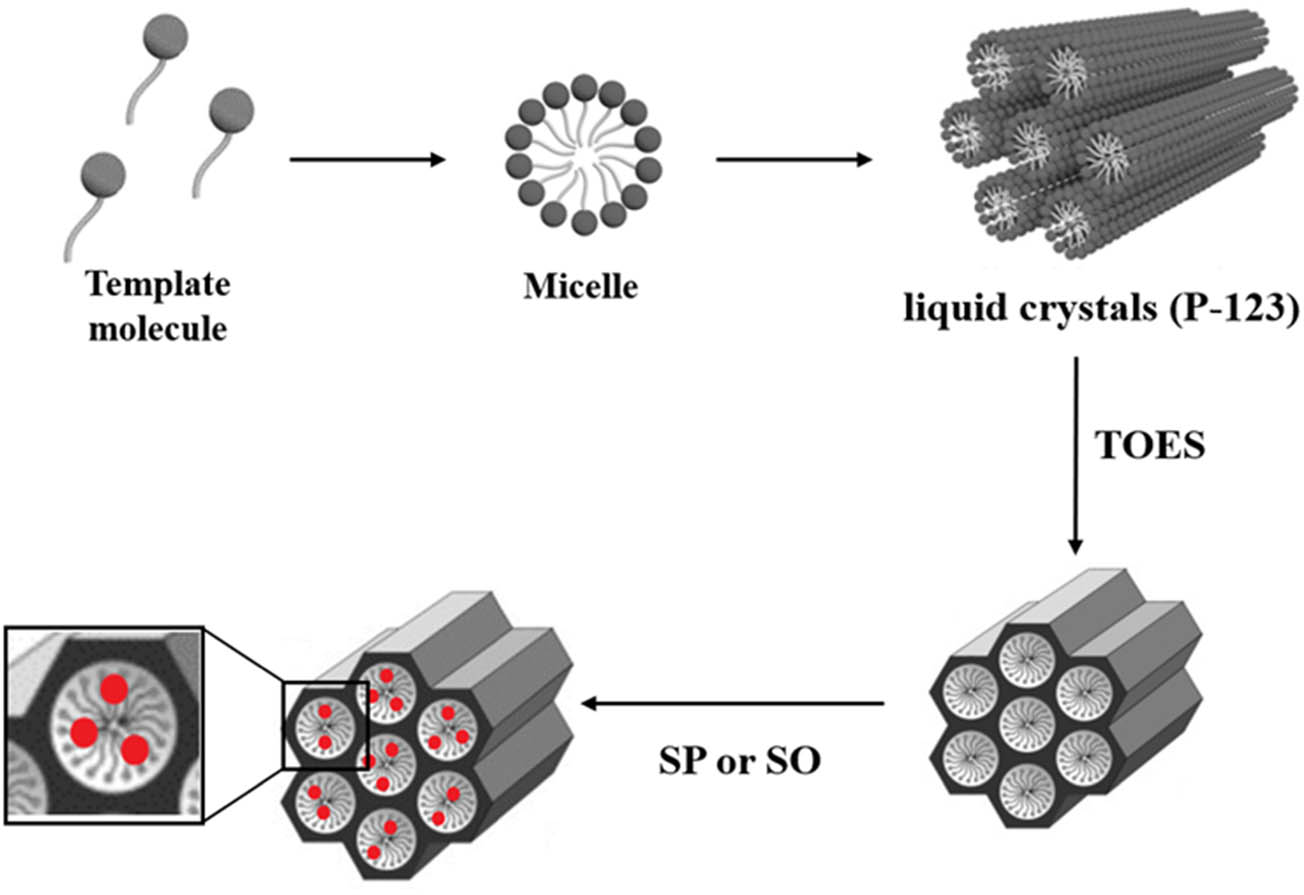
Schematic explanation for the encapsulation of SP and SO into SBA-15/P123.
SP and SO dye molecules in both films were easily switched between open and closed states upon UV–Vis irradiation. Both investigated dyes showed direct photochromism. SP and SO dyes were colored upon UV irradiation and thermally bleached back to their colorless closed forms. The response times of SO@AM-SBA-15 were very fast, with long-term stability and no obvious direct/reverse photochromism competition with time (Figure 29). These kinds of PMSM materials are promising candidates for many applications such as light modulators and optical shutters [132]. These films can be also used for the organic/inorganic nano-separation.
![Figure 29
Time-dependent photocoloration and bleaching of SO@AM-SBA-15 films showing low photodecomposition with time. Adapted from ref. [132] with permission. Copyright 2022 American Chemical Society.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_029.jpg)
Time-dependent photocoloration and bleaching of SO@AM-SBA-15 films showing low photodecomposition with time. Adapted from ref. [132] with permission. Copyright 2022 American Chemical Society.
In another study, Iqbal and coworkers recently reported the encapsulation of twelve new SP derivatives into mesosphere silica [119]. The synthesized SPs were dissolved in ethanol–water mixture, and then, TEOS and ammonium hydroxide were added. After hydrolysis and condensation of TEOS, the obtained solid was filtered, washed with ethanol to remove TEOS residue, and then dried in a vacuum (Scheme 7). However, the material characterization was not reliable. Based on XRD, SEM, and FTIR results, the authors claimed that the SP was fully incorporated within silica mesospheres, but all XRD patterns were similar to those of pure silica, and no peaks can be attributed to SP were observed. In addition, the thermochromic properties of the obtained material SP@Silica were not investigated. Only the UV–Vis absorption spectra of one of the SP derivatives (SP-8) in the temperature range of 0–50°C were reported.
![Scheme 7
Synthetic route of the silica encapsulated SP. Adapted from ref. [119] with permission. Copyright 2022 Royal Society of Chemistry.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_043.jpg)
Synthetic route of the silica encapsulated SP. Adapted from ref. [119] with permission. Copyright 2022 Royal Society of Chemistry.
3.6 Chemical methods
The incorporation of OPMs by chemical binding (shared electrons) into MSMs to prepare PMSMs can be performed via four principal approaches: grafting, co-condensation (co-hydrolysis), periodic mesoporous organosilicas (PMOs), and coordination bonds (ligand–metal bond). These approaches can be divided into two different categories: (1) the incorporation of OPMs into surface of MSMs which can be performed via either grafting, coordination bond, or co-condensation between a silica source (i.e., TEOS) and mono-silylated OPM {(EtO)3Si-OPM} and (2) the introduction of OPMs into MSMs framework using co-condensation of bis- or multi-silylated OPMs {[(EtO)3Si] n OPM, n ≥ 2} with a silica source (i.e., TEOS), or by condensation of 100% of bis- or multi-silylated OPMs without any silica source, this last method called preparation of PMOs.
3.6.1 Chemical incorporation of OPMs into MSM surface
3.6.1.1 Grafting
The grafting method has been used to immobilize many organic and organometallic molecules, including OPMs, into different MSMs. In this method, the OPMs can be grafted into MSM surface via the creation of covalent bonds between OPMs and MSMs. These covalent bonds can be created by two different approaches:
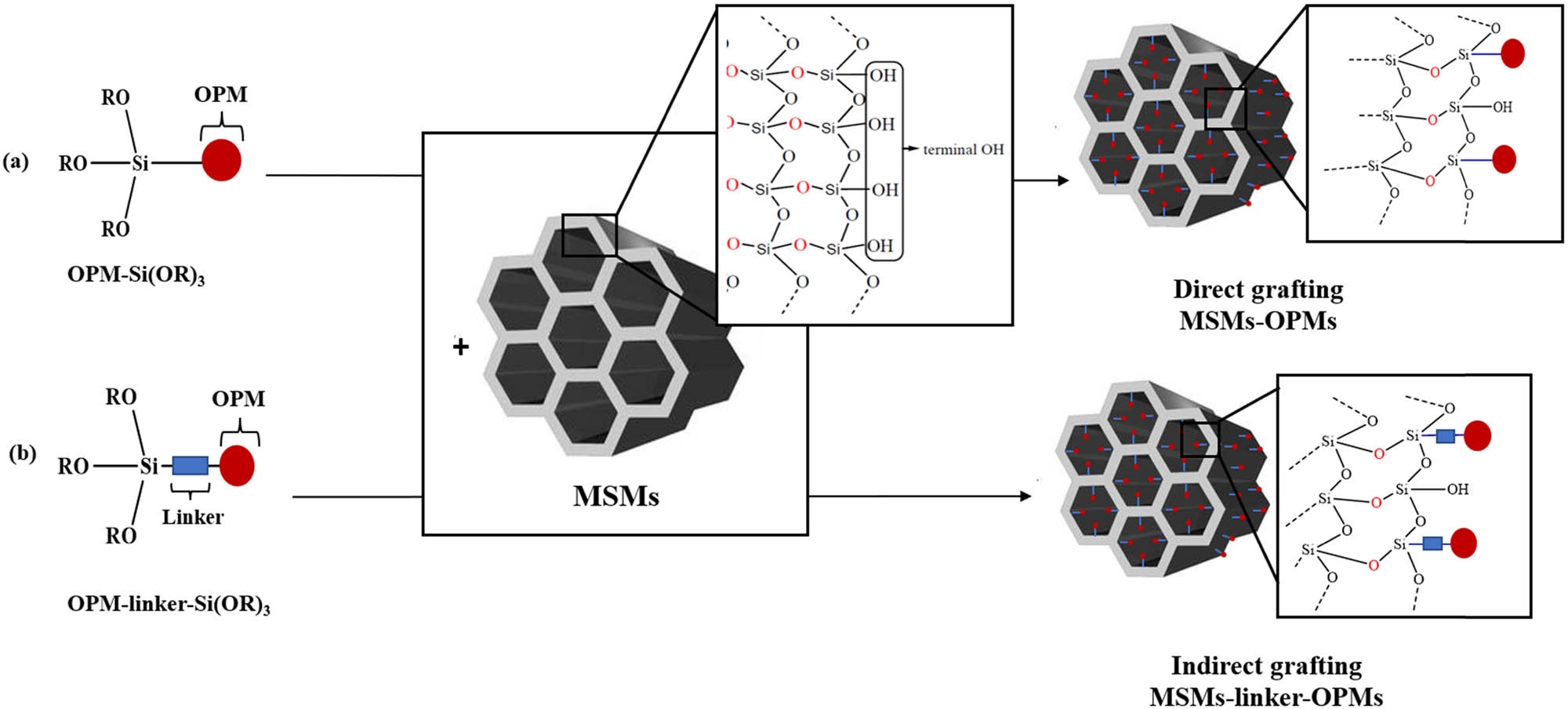
Incorporation of OPMs into MSMs by grafting. Direct grafting (a) and indirect grafting (b).
A. Direct grafting: Functionalization of OPMs by – Si (OR)3 (R: Et or Me) which will react directly with MSMs silanol groups (Si–OH) to create Si–C covalent bonds (bridges) between OPM and silica surface (Scheme 8a).
B. Indirect grafting: Creation of covalent bonds between OPMs and MSMs surface which are performed through an organic linker between OPMs and MSMs surface (Scheme 8b).
To the best of our knowledge, the introduction of OPMs into MSMs using direct grafting has not been reported until now. However, indirect grafting has been used to incorporate various organic molecules into the MSM surface [133,134,135]. Mitran and his team reported the synthesis of a new light-responsive material by functionalizing the surface of AlMCM-41 by AZB groups (Scheme 9) [43]. The AZB moieties were functionalized by the triethoxysilane group using the N-ethylamide group as a linker. Then, the obtained precursor was grafted into the AlMCM-41 surface. The obtained photochromic material AZB-ALMCM-41 was evaluated for irinotecan drug delivery. The initial release rate was increased to 15-fold when the material was exposed to UV light. This was attributed to an increase in the drug diffusion caused by AZB molecular motion (trans to cis) and hydrophilic–hydrophobic surface switching.
![Scheme 9
AZB functionalized AlMCM-41 using indirect grafting (linker). Adapted from ref. [43] with permission. Copyright 2014 De Gruyter.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_045.jpg)
AZB functionalized AlMCM-41 using indirect grafting (linker). Adapted from ref. [43] with permission. Copyright 2014 De Gruyter.
In another work, Burns and his team prepared acidichromic silyl SP 6-(vinyltriethoxysilyl) SP and grafted it into the surface of mesoporous silica (SBA-15) using an indirect grafting method [136]. The SP moieties were incorporated into the silica surface by open form (MC form) via covalent bonds. The obtained PMMs were evaluated as a metal ion chemosensor. The preliminary analysis of the metal ion sorption enabled the selection of monovalent, divalent, and trivalent metal ions compared to an unfunctionalized
C. Two-step indirect grafting: The immobilization of OPMs into MSM surface can be also performed using a two-step indirect grafting process (Scheme 10). The first step consists of functionalizing the silica surface by a functional group 1 (FG1) via grafting FG1-Si(OR)3 {i.e., (3-Aminopropyl)triethoxysilane (APTES), (3-iodopropyl)triethoxysilane (IPTES)} to produce FG1-MSMs. In the second step, the functionalized OPMs by a second functional group (FG2-OPMs) will be added to the prepared FG1-MSMs. After the reaction between FG1 and FG2, the OPMs will be immobilized on the MSM surface. In this case, the linker is formed by FG1–FG2.
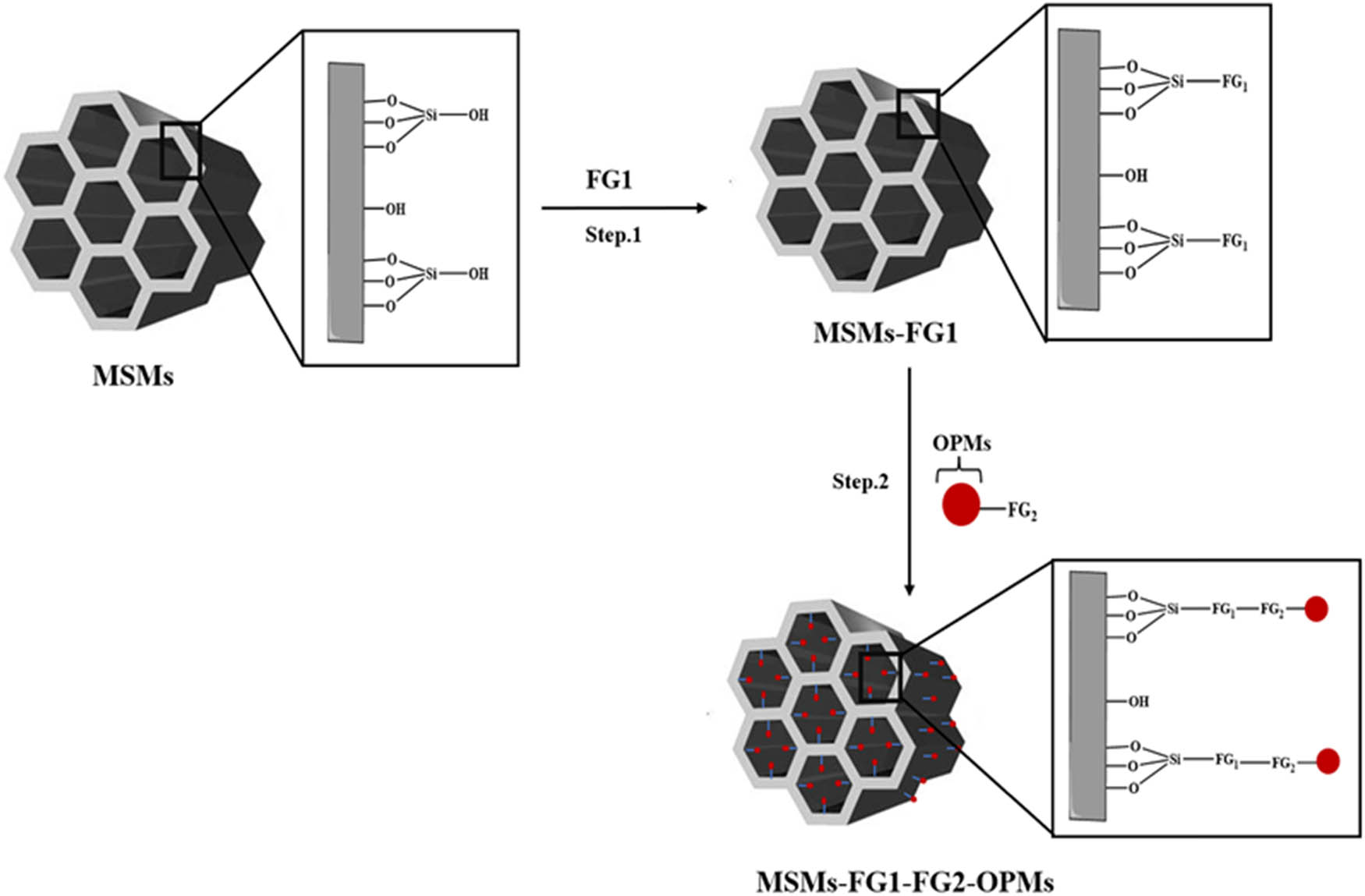
Incorporation of OPMs into MSMs via two-step indirect grafting.
One example of this type of grafting is the immobilization of AZB into amino-functionalized MCM-41 which was reported by Rohlfing and his coworkers (Scheme 11) [137]. The surface of MCM-41 was first functionalized by APTES, then the carbonyl group in 4′-dimethylaminoazobenzene-4-carbonic acid was attacked by NH2 to create amide group and afford the corresponding photochromic material AZB-APETS-MCM-41.
![Scheme 11
Immobilization of the AZB dye into MCM-41 surface by two-step indirect grafting. Adapted from ref. [137] with permission. Copyright 2000 Elsevier.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_047.jpg)
Immobilization of the AZB dye into MCM-41 surface by two-step indirect grafting. Adapted from ref. [137] with permission. Copyright 2000 Elsevier.
The obtained results revealed that the anchoring of AZB has no effect on the order of MCM-41 nanostructure, except for the slight decrease in the pore size. UV–Vis analysis showed a blue shift of the main absorption band of the AZB dye in the solid state (441 nm) compared to the liquid state (468 nm) state. This result was explained by the increased basicity of the chemical environment in MCM-41 pores due to the existence of silanols and non-reacted amines groups.
In another example, Timm et al. reported the grafting of AZB dye into benzene PMO (Bz-PMO), with high AZB density in the pores, using a two-step indirect grafting approach (Scheme 12) [138]. Bz-PMO was first functionalized by APTES, and then, 4-phenylazobenzoyl chloride was added to form an amide group. It has been noted that the creation of amide groups is one of the simplest and most widely used methods to immobilize organic molecules in MSMs, including OPMs and organometallic complexes, into a silica surface.
![Scheme 12
AZB functionalized Bz-PMO material using two-step indirect grafting. Adapted from ref. [138] with permission. Copyright 2016 Elsevier.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_048.jpg)
AZB functionalized Bz-PMO material using two-step indirect grafting. Adapted from ref. [138] with permission. Copyright 2016 Elsevier.
The obtained PMSM material showed a complete reversible cis/trans-isomerization upon light irradiation. The isomerization reaction was not affected by the small distance between the switching units. It was reported also that the half lifetime of cis isomer was very high (87 h) by thermal isomerization. This stability of the cis isomer was explained by the π-electron rich environment of the Bz-PMO.
Flavylium was also incorporated into SBA-15 and MCM-41 using two-step indirect grafting. Gago et al. reported the synthesis of photoisomerizable 2-hydroxychalcones functionalized MCM-41 and SAB-15 using a two-step indirect grafting approach (Scheme 13) [53]. The new hybrid materials exhibited pH-dependent reflectance spectra like those observed in solutions at higher pH ranges (Figure 30). The grafted flavylium exhibited good stability at high pH. Also, the irradiation of these materials equilibrates at adequate pH values, where the photoisomerizable trans-chalcones predominate and the flavylium cations return to their original compositions in the dark (Scheme 14).
![Scheme 13
Incorporation of flavylium into SBA-15 and MCM-41 via two-step indirect grafting. Adapted from ref. [53] with permission. Copyright 2010 Royal Society of Chemistry.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_049.jpg)
Incorporation of flavylium into SBA-15 and MCM-41 via two-step indirect grafting. Adapted from ref. [53] with permission. Copyright 2010 Royal Society of Chemistry.
![Figure 30
The diffuse reflectance UV/Vis absorption spectra of the MCM-41-Fl-OH (a) in the range 2.6 < pH < 8.3 at 447 nm (●) and 485 nm (○); and SBA-15-Fl-OCH3 (b) in the range 2.5 < pH < 7.2 at 447 nm (●) and 380 nm (○). Adapted from ref. [53] with permission. Copyright 2010 Royal Society of Chemistry.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_030.jpg)
The diffuse reflectance UV/Vis absorption spectra of the MCM-41-Fl-OH (a) in the range 2.6 < pH < 8.3 at 447 nm (●) and 485 nm (○); and SBA-15-Fl-OCH3 (b) in the range 2.5 < pH < 7.2 at 447 nm (●) and 380 nm (○). Adapted from ref. [53] with permission. Copyright 2010 Royal Society of Chemistry.
![Scheme 14
The photoisomerization of the flavylium with pH and light. Adapted from ref. [53] with permission. Copyright 2010 Royal Society of Chemistry.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_050.jpg)
The photoisomerization of the flavylium with pH and light. Adapted from ref. [53] with permission. Copyright 2010 Royal Society of Chemistry.
In another study, Zhang and his team investigated the fluorescent switching of NP with carbazole and pyrene dyad immobilized on SBA-15 [139]. The first material was prepared by grafting a naphthopyran-bridge-carbazole dyad (CzNP) into SBA-15-NH2 (SBA-15-APTES) following a two-step indirect grafting method (Scheme 15). The second material was double fluorescent photochromic material which was prepared by reacting 1-pyrenecardboxaldehyde (PY-CHO): naphthopyrancarbazole (CzNP)¼1:1 (molar ratio) with SBA-15-NH2 (Scheme 16).
![Scheme 15
The fluorescence switching behavior of synthesized CzNP-SBA-15-NH2. Adapted from ref. [139] with permission. Copyright 2014 Elsevier.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_051.jpg)
The fluorescence switching behavior of synthesized CzNP-SBA-15-NH2. Adapted from ref. [139] with permission. Copyright 2014 Elsevier.
![Scheme 16
The fluorescence switching behavior of synthesized CzNP-SBA-15-NH2 and PY-CzNP-SBA-15-NH2. Adapted from ref. [139] with permission. Copyright 2014 Elsevier.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_052.jpg)
The fluorescence switching behavior of synthesized CzNP-SBA-15-NH2 and PY-CzNP-SBA-15-NH2. Adapted from ref. [139] with permission. Copyright 2014 Elsevier.
In both systems, the NP moieties displayed superior photochromic performance. The results showed better and modulated fluorescent emission between “on” and “off” due to photoinduced energy transfer from pyrene excimers to the opened-form NP, and photoinduced electron transfer between carbazoles and the opened-form NP moiety in high-degree. Furthermore, both fluorescence photoswitching solutions (v/v, 1:1) demonstrated superior fatigue resistance. This material can have a potential application in optical memory.
3.6.1.2 Co-condensation of mono-silylated OPMs
The incorporation of OPMs into MSMs surface can be also performed using co-condensation of mono-silylated OPMs [(OR)3Si-OPMs, R: Me or Et] with a silica source such as TEOS in the presence of a structure-directing agent (template), in acidic or basic conditions (Scheme 17). The co-condensation approach is a simultaneous reaction referred to as the one-pot route or direct synthesis way. In the obtained material OPMs@MSMs, the OPM moieties will be attached to the silica surface by covalent bonds. In (OR)3Si–OPMs precursor, Si(OR)3 can be either attached directly to OPMs through C–Si bond [30] or via a linker [53,103]. Usually, the utilization of a linker is the easiest pathway to connect OPMs with the Si(OR)3 group {(RO)3Si-linker-OPMs}. However, the preparation and purification of (OR)3Si–OPMs precursors without linker is usually a difficult process, and Si–C bonds can be very sensitive to the temperature [140]. Moreover, the order of the nanostructure, morphology, and the porosity of the obtained material will be affected by OPM/TEOS molar ratio. To preserve the mesoporosity and the high order of the MSMs, the molar ratio OPM/TEOS has to be ≤0.2 (15/75).
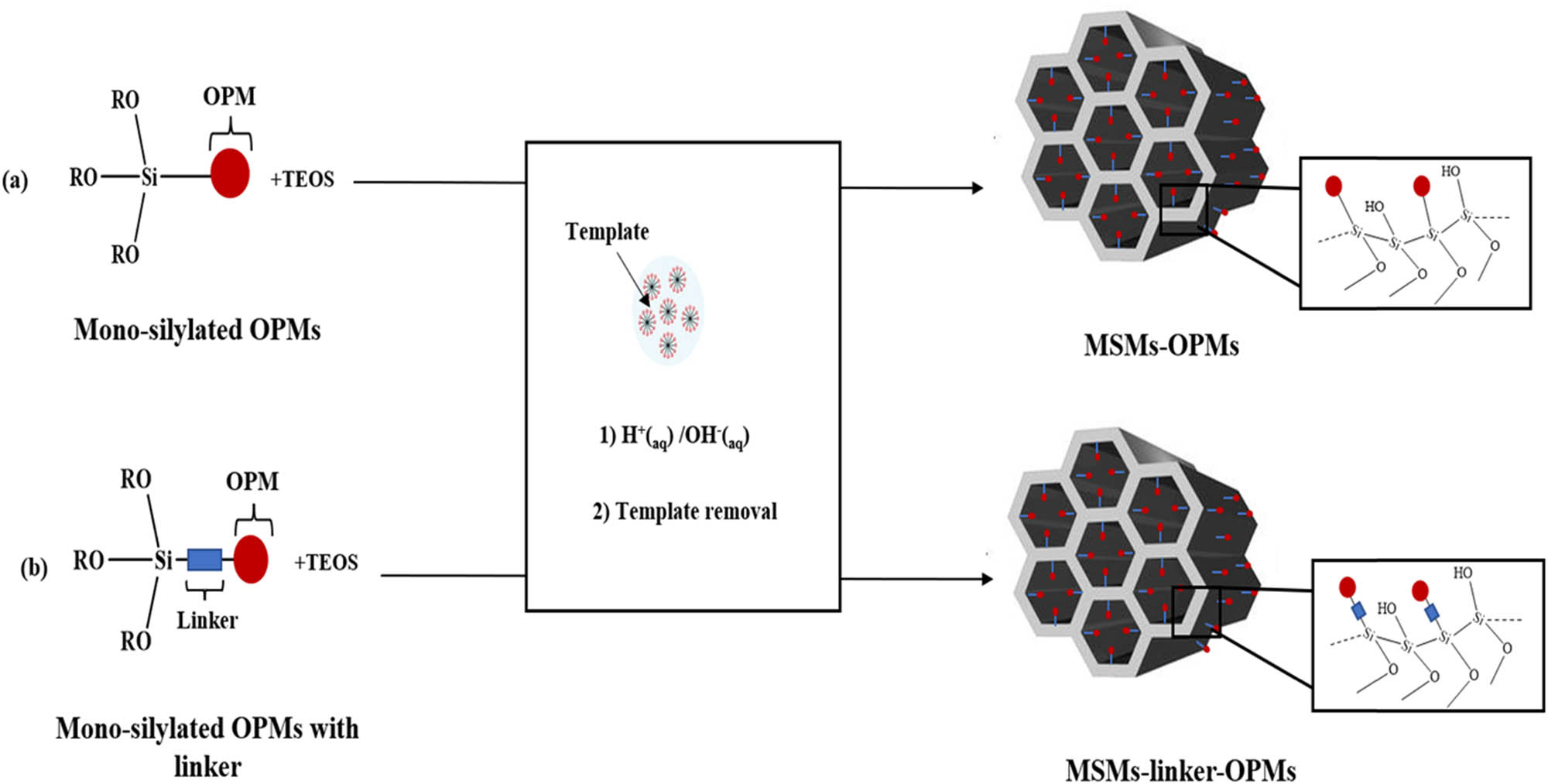
Immobilization of OPMs into MSMs surface using co-condensation method of mono-silylated OPMs with a silica source. (a) Without linker and (b) with linker.
Abboud et al. reported the introduction of ferrocene-AZB (Fc-AZB) moieties in MCM-41 surface via the co-condensation process (Scheme 18) [30]. The synthesis of the mono-silylated 3-ferrocenylazobenzene precursor without using any linker between the silicon atom and phenyl ring was possible by using the lithium-iodine exchange reaction. The obtained mono-silylated ferrocene-azobenzene (TEFA) precursor was co-condensed with TEOS in the presence of CTAB as a template (surfactant) in a basic condition to produce fc-AZB functionalized MCM-41 (Fc-Az-M41). Upon alternate UV–visible irradiations, the AZB moieties on Fc-AZB-M41 exhibited cis/trans isomerization (Figure 31). These new PMSMs can be used as a photochromic heterogeneous catalyst.
![Scheme 18
Preparation of Fc-AZB-MCM-41 photochromic material from the mono-silylated 3-ferrocenylazobenzene precursor by co-condensation reaction with TEOS. Adapted from ref. [30] with permission. Copyright 2018 Elsevier.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_054.jpg)
Preparation of Fc-AZB-MCM-41 photochromic material from the mono-silylated 3-ferrocenylazobenzene precursor by co-condensation reaction with TEOS. Adapted from ref. [30] with permission. Copyright 2018 Elsevier.
![Figure 31
Diffuse reflectance UV–Vis absorption spectra of Fc-AZB-M41 before and after UV irradiation. Adapted from ref. [30] with permission. Copyright 2018 Elsevier.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_031.jpg)
Diffuse reflectance UV–Vis absorption spectra of Fc-AZB-M41 before and after UV irradiation. Adapted from ref. [30] with permission. Copyright 2018 Elsevier.
Chen et al. reported the incorporation of AZB into HMS nanocontainers (HMSs) with linker by co-condensation of a mono-silylated AZB (AZB-ICPES) and TEOS in the presence of CTAB (Scheme 19) and SiO2 NPs as shell [141]. The obtained material was in the form of core/shell hollow (Figure 32). The obtained PMSMs were evaluated as new self-healing anticorrosion and light-responsive coating (Figure 33). The cavity of the nanocontainers in the obtained material can encapsulate active molecules such as benzotriazole in visible light and release them in ultraviolet light. The obtained results showed that the release of benzotriazole from the hollow nanocontainers can be performed with high controllability, which will allow an effective process to avoid excessive release of the benzotriazole after the corrosion healing. UV irradiation at 365 nm converts AZB group to the cis form and leads to pore opening. The cis isomer transforms into the trans under visible-light irradiation (450 nm) and causes pore closing.
![Scheme 19
Synthesis of HMS nanocontainers SiO2@CTAB/AZB-SiO2 by co-condensation method. Adapted from ref. [141] with permission. Copyright 2015 Royal Society of Chemistry.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_055.jpg)
Synthesis of HMS nanocontainers SiO2@CTAB/AZB-SiO2 by co-condensation method. Adapted from ref. [141] with permission. Copyright 2015 Royal Society of Chemistry.
![Figure 32
SEM (a) and TEM (b) image of AZB-HMSs. Adapted from ref. [141] with permission. Copyright 2015 Royal Society of Chemistry.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_032.jpg)
SEM (a) and TEM (b) image of AZB-HMSs. Adapted from ref. [141] with permission. Copyright 2015 Royal Society of Chemistry.
![Figure 33
Illustration of a reversible release system of benzotriazole molecules based on trans-cis photoisomerization of AZB molecules grafted into HMSs. Adapted from ref. [141] with permission. Copyright 2015 Royal Society of Chemistry.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_033.jpg)
Illustration of a reversible release system of benzotriazole molecules based on trans-cis photoisomerization of AZB molecules grafted into HMSs. Adapted from ref. [141] with permission. Copyright 2015 Royal Society of Chemistry.
In another example, Guo and coworkers prepared two different alkoxysilyl-functionalized AZB precursors with linkers, namely 4-[3-(triethoxysilyl)propoxy] AZB (P1) and 4-[3-(diethoxymethylsilyl)propoxy] AZB (P2) and used them to prepare silica thin films via co-condensation process, without template (self-assembly) (Scheme 20) [142]. In this study, two types of AZB–siloxane ordered hybrid films were prepared, without TEOS (films H1 and H2), and with 80% of TEOS (films H1′ and H2). The arrangement of the azo groups between the siloxane’s layers and the photoresponsive behavior were affected by the number of the alkoxy groups and the co-condensation with TEOS. Different degrees of trans/cis photoisomerization of the AZB moieties, as well as change in lamellar periodicity, were observed under UV/Vis irradiation (Figure 34). These kinds of photochromic materials can be used as smart sensors and adsorbents.
![Scheme 20
Structures of diethoxysilyl- and triethoxysilyl-AZB precursors (P1 and P2) and preparation of four types of lamellar, AZB–siloxane hybrid films by co-condensation method with and without TEOS. Adapted from ref. [142] with permission. Copyright 2013 Royal Society of Chemistry.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_056.jpg)
Structures of diethoxysilyl- and triethoxysilyl-AZB precursors (P1 and P2) and preparation of four types of lamellar, AZB–siloxane hybrid films by co-condensation method with and without TEOS. Adapted from ref. [142] with permission. Copyright 2013 Royal Society of Chemistry.
![Figure 34
UV–Vis absorption spectra of H1 (left) and H2 (right) films: (a) (red) before irradiation, (b) (blue) after UV irradiation for 5 min, and (c) (green) after subsequent visible light irradiation for 5 min. Adapted from ref. [142] with permission. Copyright 2013 Royal Society of Chemistry.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_034.jpg)
UV–Vis absorption spectra of H1 (left) and H2 (right) films: (a) (red) before irradiation, (b) (blue) after UV irradiation for 5 min, and (c) (green) after subsequent visible light irradiation for 5 min. Adapted from ref. [142] with permission. Copyright 2013 Royal Society of Chemistry.
SP was also incorporated in MSMs with linker using co-condensation methods. Allouche et al. [103] prepared photochromic hybrid SP-silica nanoparticles with a core–shell structure via a two-step sol–gel procedure, using TEOS and methyltriethoxysilane (MTEOS) as silica source. The core was made by silica nanoparticles which were prepared via Ströber method using TEOS as the silica source in an alkaline medium. The previously prepared core NPs were surrounded by a porous shell using a “one pot” co-condensation of a mono-silylated SP (Scheme 21) with different TEOS/MTEOS molar ratios (100/0, 50/50, and 0/100) in the presence of CTAB as structure directing agent. The mono-silylated SP was prepared by the grafting method and confined inside the nanoporous shell to produce the photoresponsive nanomaterials with a tunable photochromic response. The results suggested that the TEOS/MTEOS ratio affected both chemical organizations and nanostructures of the obtained photochromic materials.
![Scheme 21
Preparation of the mono-silylated hydroxyethyl-SP. Adapted from ref. [103] with permission. Copyright 2010 Royal Society of Chemistry.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_057.jpg)
Preparation of the mono-silylated hydroxyethyl-SP. Adapted from ref. [103] with permission. Copyright 2010 Royal Society of Chemistry.
The optical analysis revealed a high sensitivity of the SP photochromic behavior to the polarity of the matrix. Meanwhile, reverse photochromism was favorable in the polar chemical environment; hence, direct photochromism was observed in a polar material.
In another example, Burns et al. reported the incorporation of SP derivatives in SBA-15 with al inker using the co-condensation technique [136]. In this work, the acidichromic silyl SP 6-(vinyltriethoxysilyl) SP was co-condensed with TEOS in acidic medium in the presence of Pluronic 123 (P123) to prepare SP-SBA-15. As observed in the material prepared by the grafting method, MC form was the most favored in the MSM matrix. Compared to the grafting method, the co-condensation process provided higher SP loading with two different states of attachment, protonated and deprotonated MC. In addition, the color of SP-SBA-15 prepared via post-modification (grafting) procedure was violet, while the materials synthesized by co-condensation were brown. Moreover, the photochromic behavior of SP-SBA-15 prepared via grafting and co-condensation was different. This was attributed to the protonation of MC incorporated in SBA-15 by co-condensation in a very acidic medium compared to that prepared by the grafting method in less acidic conditions. These SP/MC-SBA-15 photochromic materials were evaluated in selective sorption of monovalent, divalent, and trivalent metal ions. However, the obtained results indicated a modest improvement in cation exchange characteristics compared to non-functionalized mesoporous silica.
3.6.1.3 Co-condensation/reaction
Also, Krohm et al. incorporated SP and SO in a mesoporous silica film through co-condensation followed by surface-initiated ring-opening metathesis polymerization (SI-ROMP) (Scheme 22) [52]. In the first step, the mesoporous allyl-silica was prepared by co-condensation reaction between allyl-triethoxysilane and TEOS in the presence of Pluronic F127 as a structure-directing agent in an acidic medium. In the second step, SP- and SPO-norbornene monomers, SP-Nb and SPO-Nb, respectively, were added to the mesoporous allyl-silica films to form the corresponding photochromic homopolymers in mesoporous silica thin films after ring opening metathesis polymerization (ROMP). According to UV/Vis and NMR spectroscopic results, SP- and SPO-homopolymers exhibited very different switching kinetics and photochromic behaviors than in the liquid phase. SP and SPO located inside the mesoporous matrix exhibited faster photoresponse upon irradiation compared to those in the external surface or in solution. This was attributed to the effect of the spatial confinement and the lower molecular weight of the homopolymers inside the pores. These photochromic materials are considered useful for subsequent photochromic control of ion transport through mesopores.
![Scheme 22
Synthetic strategy of the photochromic SP and SO homopolymers in mesoporous thin films by SI-ROMP. Adapted from ref. [52] with permission. Copyright 2016 Royal Society of Chemistry.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_058.jpg)
Synthetic strategy of the photochromic SP and SO homopolymers in mesoporous thin films by SI-ROMP. Adapted from ref. [52] with permission. Copyright 2016 Royal Society of Chemistry.
3.6.2 Chemical immobilization of OPMs into MSM framework
The incorporation of OPMs into MSMs framework can be performed using two main methods, namely co-condensation of bis- or multi-silylated OPMs (≤15%) with silica source (TEOS or TMOS) (≥75%), and PMOs method using 100 % of bis- or multi-silylated OPMs, without silica source. In the obtained material the OPM moieties will be a part of the silica framework (wall). The incorporation of OPMs into MSM wall will affect the texture properties, rigidity, and nanostructure order of MSMs. Furthermore, compared to OPMs immobilized on the surface of MSMs, OMPs as a part of the MSM framework will have less flexibility to perform the photochromic isomerization, especially cis/trans isomerization.
3.6.2.1 Co-condensation of bi- or multi-silylated OPMs
The incorporation of OPMs into the MSM framework using co-condensation method requires the utilization of bi- or multi-silylated OPMs, template, and silica source (TEOS or TMOS) with a small [(OR)3Si] n(n ≥ 2) OPMs/TEOS molar ratio (≤0.2) to preserve the mesoporous silica framework, in acidic or basic conditions (Scheme 23). Here also OPMs can be attached directly to Si(OR)3 groups or via a linker.
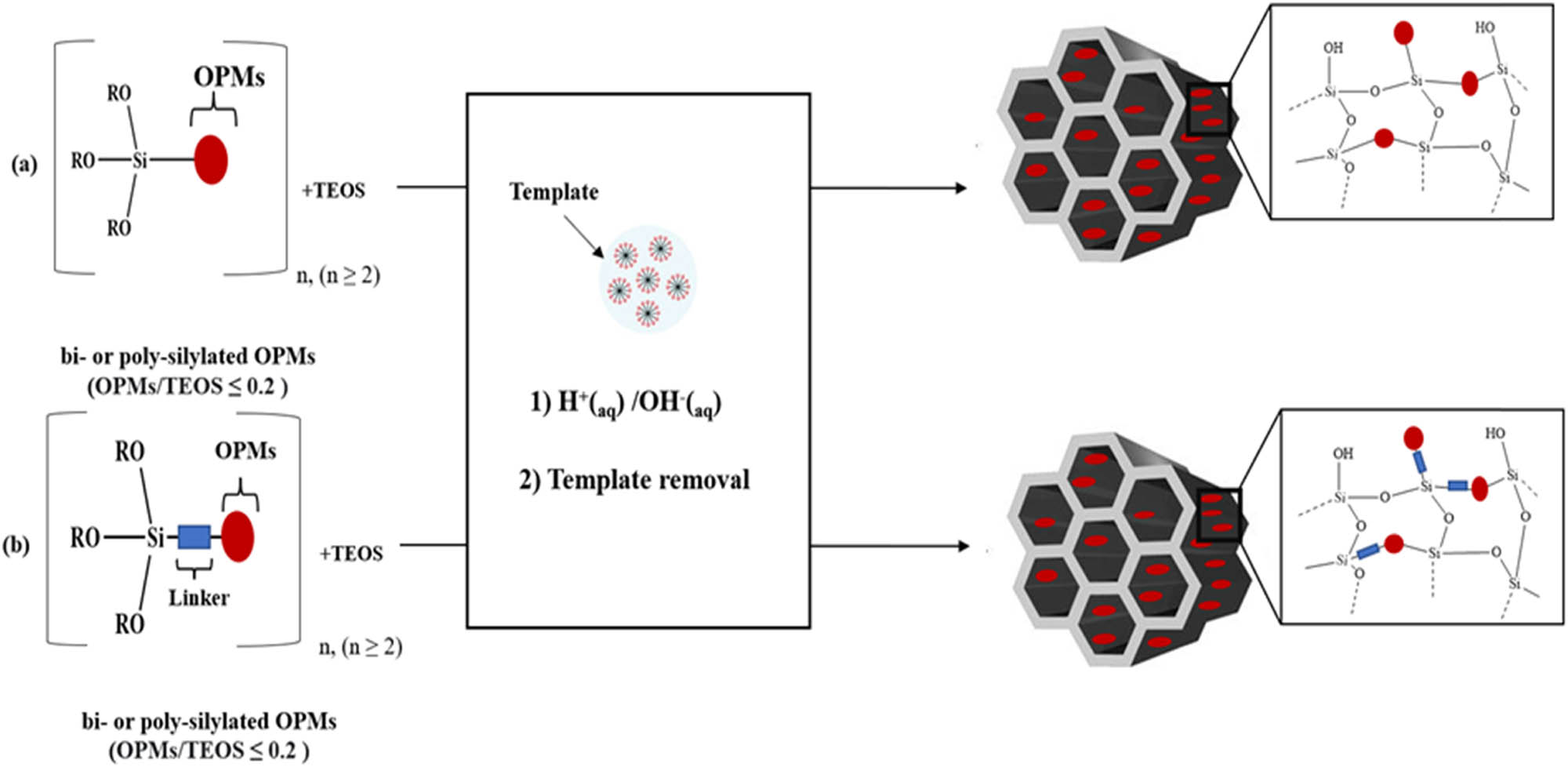
Incorporation of OPMs into MSMs framework using co-condensation method, (a) without linker and (b) with linker.
The utilization of OPMs {Si(OR)3} n(n ≥ 2) without a linker between OPMs and Si atoms is suitable. Because the existence of a linker can significantly affect the order, rigidity, and porosity of the obtained material OPMs–MSMs. However, only few examples reported the incorporation of OPMs into MSMs framework by co-condensation. For instance, Besson and his team reported the preparation of AZB-SBA-15 photochromic material containing bridged AZB groups as OPMs, with the propoxy group as a linker (Scheme 24) [143]. This nanostructured material was obtained by co-hydrolysis of TEOS and (5.24% molar) 4,4′-[(triisopropoxysilyl)propyloxy] AZB in the presence of P123 as a template in acidic condition.
Preparation of SBA-15 containing photochromic AZB into framework by co-condensation method.
3.6.2.2 PMOs
Photochromic PMOs (PPMOs) can be prepared by condensation of 100% of bi- or multi-silylated OPMs {(OR)3Si] n(n ≥ 2) OPMs} without silica source, in the presence of a suitable template (Scheme 25). This method is also suitable to use [(OR)3Si] n(n ≥ 2) OPM precursor without a linker between OPMs and silicon atoms. The existence of any linker will significantly affect the order, rigidity, and texture properties of the obtained PPMOs. Compared to the co-condensation method, the obtained material using the PMO technique will contain a higher ratio of OPMs:Si [144].
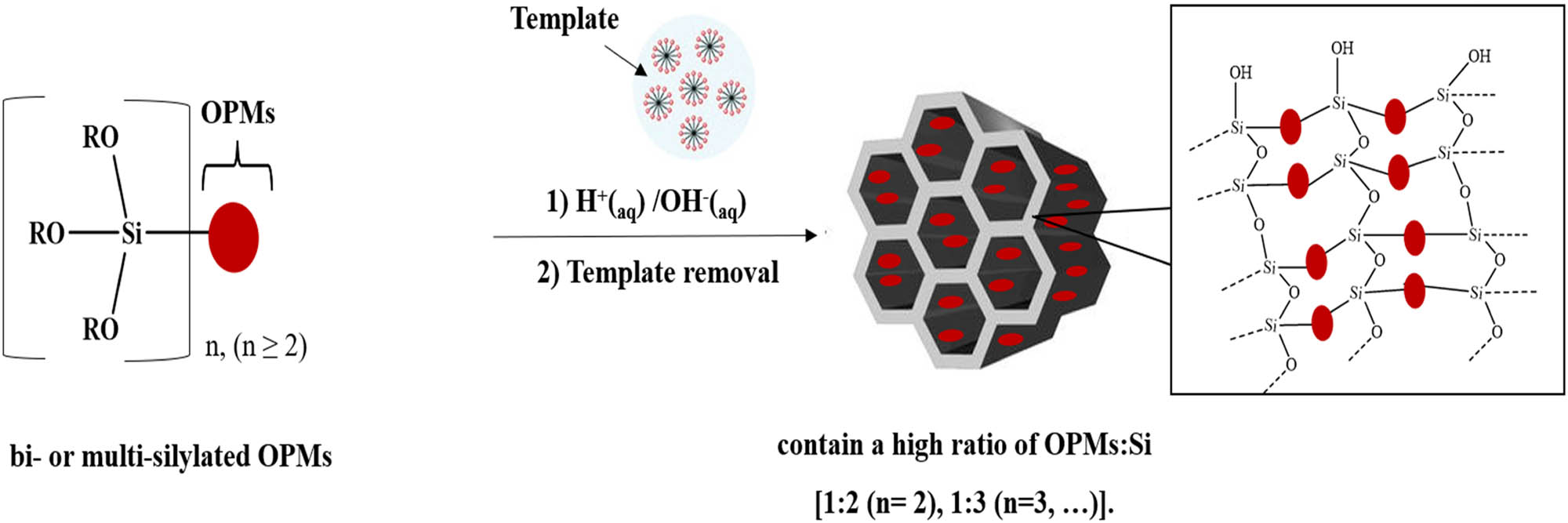
The synthesis of OPMs-MSMs via the POM method using bis or multi-silylated OPMs.
The texture properties (surface area, pore size, and pore volume), nanostructure order, material rigidity, and pores shape (e.g., 2D hexagonal, 3D hexagonal, and cubic) of the obtained PMSMs materials using PMOs or co-condensation methods of bi- or multi-silylated OPMs can be affected by the molar ratios of the initial synthesis mixture (OPMs precursor:silica source:solvent:water:base/acid, etc.), nature of the template, precursor, reaction time, and the temperature. Additionally, the interaction between the silylated precursors and template in the absence of silica source is critical to the synthesis of PPMOs [144]. If the interaction between the precursor and template is weak, it is particularly challenging to synthesize PMOs from 100% bridged organosilane precursors with bulky organic moieties. Functionalizing the organic precursor with more silyl groups, the utilization of small organic precursors, and the addition of TEOS are efficient ways to improve these interactions, especially for the co-condensation method. The stability of the silicon–carbon bond of the organosilane precursors depends on the reaction condition (e.g., temperature and pH), chemical and thermal stability of the organic moiety, and linker between silicon atom and OPMs. Usually, PPMOs are more stable in the presence of an organic linker, but less rigid and ordered [145].
Abboud and Sayari reported the synthesis and characterization of a new family of three AZB-bridged PPMO isomers (Figure 35) [31]. These new PPMOs were prepared from three pure para-bis-silylated AZB precursors, without any linker between the phenyl ring of the AZB and the silicon atom, as communally used in the preparation of AZB bridged MSMs. Lithium-Iodine exchange reaction was used to attach Si(OEt)3 groups directly to AZB moieties. The AZB moieties of two PMOs were tetra-ortho-substituted by methyl and isopropyl groups. These three bis-silylated precursors were subjected to the condensation reaction in the presence of CTAB as a template in basic conditions following MCM-41 preparation process without any other silica source. UV–Vis spectroscopy analysis showed that the prepared PPMOs are formed by a rigid combination of cis- and trans-AZB isomers, which did not exhibit a regular photochemical cis/trans isomerization upon alternating UV–Vis radiation (Figure 36). However, as reported for solid material bearing AZB moieties [146], these materials can exhibit a dynamic and local bending around N═N azo which could be useful for some important applications such as light-controllable gas adsorption and release.
![Figure 35
Synthesis of AZB-bridged PMO (AZB-PMOs). Adapted from ref. [31] with permission. Copyright 2018 Elsevier.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_035.jpg)
Synthesis of AZB-bridged PMO (AZB-PMOs). Adapted from ref. [31] with permission. Copyright 2018 Elsevier.
![Figure 36
Diffuse reflectance UV–Vis spectra of AZB-PMOs. Adapted from ref. [31] with permission. Copyright 2018 Elsevier.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_036.jpg)
Diffuse reflectance UV–Vis spectra of AZB-PMOs. Adapted from ref. [31] with permission. Copyright 2018 Elsevier.
4 Conclusion
This review discussed the synthesis of PMOMs and their applications as chemosensors. PMOMs can be prepared by incorporating OPMs and MSMs. However, only few examples of OPMs have been incorporated in MSMs, such as AZB, SP, and SPZ derivatives. PMOMs have been the subject of many research investigations during the last decade due to their potential applications, especially in the design and development of efficient chemosensors for metallic ion detection. However, the synthesis of OPMs and their immobilization in MSMs is usually a challenging process. OPMs can be incorporated in MSMs by physical binding (non-covalent bonds) or chemical binding (covalent bonds). The physical adsorption of OPMs inside MSMs (OPMs@MSMs) is a non-covalent immobilization. Physical adsorption involves unshared electron bonds between OPMs and the surface of MSMs. These bonds can be Van der Waals forces, or HBs, or ionic bonds. Physical adsorption methods include the impregnation, photo-induced adsorption, and encapsulation methods. The main problem of the physical adsorption is the leaching of OPMs from the MSMs and limited loading. To improve OPM adsorption into MSM surfaces and mitigate these shortcomings, the electrostatic interactions between organic molecules and the silica surface must be enhanced, with accessible adsorption sites, and sufficient and adequate pore size. The incorporation of OPMs via covalent bonding (shared electrons) is the best alternative. However, the creation of chemical bonds between OPMs and MSMs is usually a complicated process. The chemical immobilization of OPMs into MSMs to prepare PMSMs can be divided into two main categories: incorporation into MSMs surface or inside MSMs framework (silica wall). The functionalization of the MSMs surfaces by OPMs via covalent bonds can be performed through grafting or co-condensation of mono-silylated OPMs. While OPMs can be incorporated into MSMs framework either by co-condensation of bis- or multi-silylated OPMs (≤15%) with a silica source (e.g., TEOS), or PMOs method using 100% of bis- or multi-silylated OPMs without any silica source. The inclusion of OPMs into the wall of MSMs will significantly affect the textural characteristics, and nanostructure order of the obtained PMSMs. Furthermore, OPMs will have less flexibility to execute photochromic isomerization in solid state compared to OPMs into MSMs surface, especially for cis/trans transformations.
-
Funding information: The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a large group Research Project under grant number RGP2/390/44.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Siegel RW. Nanostructured materials-mind over matter. Nanostruct Mater. 1993;3(1–6):1–18.10.1016/0965-9773(93)90058-JSearch in Google Scholar
[2] Kolahalam LA, Viswanath IK, Diwakar BS, Govindh B, Reddy V, Murthy Y. Review on nanomaterials: Synthesis and applications. Mater Today: Proc. 2019;18:2182–90.10.1016/j.matpr.2019.07.371Search in Google Scholar
[3] McNaught AD, Wilkinson A. Compendium of chemical terminology: IUPAC recommendations. 2nd edn. Oxford: Blackwell Science Publications; 1997.Search in Google Scholar
[4] ISO T. 27687. Nanotechnologies–terminology and definitions for nano-objects–nanoparticle, nanofibre and nanoplate; 2008.Search in Google Scholar
[5] Patil SP, Burungale VV. Physical and chemical properties of nanomaterials. Nanomedicines for Breast Cancer Theranostics. Amsterdam, Netherlands: Elsevier; 2020. p. 17–31.10.1016/B978-0-12-820016-2.00002-1Search in Google Scholar
[6] Glezer A. Structural classification of nanomaterials. Russian Metall (Metally). 2011;2011(4):263–9.10.1134/S0036029511040057Search in Google Scholar
[7] Singh V, Yadav P, Mishra V. Recent advances on classification, properties, synthesis, and characterization of nanomaterials. In: Green Synthesis of Nanomaterials for Bioenergy Applications. Hoboken, New Jersey, U.S.: John Wiley & Sons Ltd.; 2020. p. 83–97.10.1002/9781119576785.ch3Search in Google Scholar
[8] Sneddon G, Greenaway A, Yiu HH. The potential applications of nanoporous materials for the adsorption, separation, and catalytic conversion of carbon dioxide. Adv Energy Mater. 2014;4(10):1301873.10.1002/aenm.201301873Search in Google Scholar
[9] Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC. Composition of synthetic porous crystalline material, its synthesis, Utility Patent., US5102643A; 1992.Search in Google Scholar
[10] Yang B, Chen Y, Shi J. Mesoporous silica/organosilica nanoparticles: Synthesis, biological effect and biomedical application. Mater Sci Eng: R: Rep. 2019;137:66–105.10.1016/j.mser.2019.01.001Search in Google Scholar
[11] Mishra R, Militky J, Venkataraman M. Nanoporous materials. The Textile Institute Book Series. Amsterdam, Netherlands: Elsevier; 2019. p. 311–5310.1016/B978-0-08-102609-0.00007-9Search in Google Scholar
[12] Polarz S, Smarsly B. Nanoporous materials. J Nanosci Nanotechnol. 2002;2(6):581–612.10.1166/jnn.2002.151Search in Google Scholar
[13] Wang X, Zhang Y, Luo W, Elzatahry AA, Cheng X, Alghamdi A, et al. Synthesis of ordered mesoporous silica with tunable morphologies and pore sizes via a nonpolar solvent-assisted stober method. Chem Mater. 2016;28(7):2356–62.10.1021/acs.chemmater.6b00499Search in Google Scholar
[14] Hoffmann F, Cornelius M, Morell J, Fröba M. Silica‐based mesoporous organic–inorganic hybrid materials. Angew Chem Int Ed. 2006;45(20):3216–51.10.1002/anie.200503075Search in Google Scholar PubMed
[15] Seshadri S. Synthesis and characterization of ordered mesoporous silica with controlled macroscopic morphology for membrane applications. PhD Thesis. Tempe, AZ, USA: Arizona State University; 2011.Search in Google Scholar
[16] Reichinger M. Poröse Silicate mit hierarchischer Porenstruktur: Synthese von mikro-/mesoporösen MCM-41 und MCM-48 Materialien aus zeolithischen Baueinheiten des MFI-Gerüststrukturtyps. Bochum, Univ., Diss. 2007; 2007.Search in Google Scholar
[17] Williams CD, Travis KP, Burton NA, Harding JH. A new method for the generation of realistic atomistic models of siliceous MCM-41. Microporous Mesoporous Mater. 2016;228:215–23.10.1016/j.micromeso.2016.03.034Search in Google Scholar
[18] Sarı Yılmaz M, Dere Özdemir Ö, Pişkin S. Synthesis and characterization of MCM-41 with different methods and adsorption of Sr2 + on MCM-41. Res Chem Intermed. 2015;41(1):199–211.10.1007/s11164-013-1182-4Search in Google Scholar
[19] Zhao D, Feng J, Huo Q, Melosh N, Fredrickson GH, Chmelka BF, et al. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science. 1998;279(5350):548–52.10.1126/science.279.5350.548Search in Google Scholar PubMed
[20] Che S, Garcia-Bennett AE, Yokoi T, Sakamoto K, Kunieda H, Terasaki O, et al. A novel anionic surfactant templating route for synthesizing mesoporous silica with unique structure. Nat Mater. 2003;2(12):801–5.10.1038/nmat1022Search in Google Scholar PubMed
[21] Varshney S, Nigam A, Pawar SJ, Mishra N. An overview on biomedical applications of versatile silica nanoparticles, synthesized via several chemical and biological routes: A review. Phosphorus, Sulfur, Silicon Relat Elem. 2022;197(2):72–88.10.1080/10426507.2021.2017434Search in Google Scholar
[22] Da’na E. Adsorption of heavy metals on functionalized-mesoporous silica: A review. Microporous Mesoporous Mater. 2017;247:145–57.10.1016/j.micromeso.2017.03.050Search in Google Scholar
[23] Nasreen S, Urooj A, Rafique U, Ehrman S. Functionalized mesoporous silica: absorbents for water purification. Desalination Water Treat. 2016;57(60):29352–62.10.1080/19443994.2016.1185744Search in Google Scholar
[24] Huang P, Chen Y, Lin H, Yu L, Zhang L, Wang L, et al. Molecularly organic/inorganic hybrid hollow mesoporous organosilica nanocapsules with tumor-specific biodegradability and enhanced chemotherapeutic functionality. Biomaterials. 2017;125:23–37.10.1016/j.biomaterials.2017.02.018Search in Google Scholar PubMed
[25] Shan W, Zhang Y, Shu Y, Zhang D, Xing C, Xiong Y. Enhanced adsorption and separation of Re (VII) using organic-inorganic hybrid silica adsorbent. Microporous Mesoporous Mater. 2021;317:110996.10.1016/j.micromeso.2021.110996Search in Google Scholar
[26] Shingdilwar S, Dolui S, Banerjee S. Facile Fabrication of Functional Mesoporous Polymer Nanospheres for CO2 Capture. Ind Eng Chem Res. 2022;61(2):1140–7.10.1021/acs.iecr.1c04580Search in Google Scholar
[27] Putz A-M, Policicchio A, Stelitano S, Sfîrloagă P, Ianăşi C, Agostino RG, et al. Tailoring mesoporous silica by functionalization for gases (H2, CH4, CO2) storage applications. Fullerenes Nanotubes Carbon Nanostruct. 2018;26(12):810–9.10.1080/1536383X.2018.1502177Search in Google Scholar
[28] Wang Z, Gao Z, Qiao M, Peng J, Ding L. Pyrene-functionalized mesoporous silica as a fluorescent nanosensor for selective detection of Hg2 + in aqueous solution. Colloids Surf A: Physicochem Eng Asp. 2022;637:128269.10.1016/j.colsurfa.2022.128269Search in Google Scholar
[29] Jamasbi N, Mohammadi Ziarani G, Mohajer F, Badiei A. Highly silver-selective fluorescent sensor based on functionalized nanoporous silica (SBA-Pr-NMP) in aqueous media. Res Chem Intermed. 2022;48(9):3957–69.10.1007/s11164-022-04773-ySearch in Google Scholar
[30] Abboud M, Bel-Hadj-Tahar R, Fakhri N, Sayari A. Synthesis of ferrocenylazobenzene-functionalized MCM-41 via direct co-condensation method. Microporous Mesoporous Mater. 2018;265:179–84.10.1016/j.micromeso.2018.02.013Search in Google Scholar
[31] Abboud M, Sayari A. Novel family of periodic mesoporous organosilicas containing azobenzene within the pore walls. Microporous Mesoporous Mater. 2017;249:157–64.10.1016/j.micromeso.2017.04.061Search in Google Scholar
[32] Lashgari N, Badiei A, Mohammadi Ziarani G. Modification of mesoporous silica SBA-15 with different organic molecules to gain chemical sensors: a review. Nanochem Res. 2016;1(1):127–41.Search in Google Scholar
[33] Mohajer F, Ziarani GM, Badiei A, Ghasemi JB, Varma RS, Karimi-Maleh H. Pomegranate Punica granatum peel waste as a naked-eye natural colorimetric sensor for the detection and determination of Fe + 3 and I− ions in water. Chemosphere. 2022;294:133759.10.1016/j.chemosphere.2022.133759Search in Google Scholar PubMed
[34] Ziarani GM, Roshankar S, Mohajer F, Badiei A, Sillanpää M. The synthesis of SBA-Pr-N-Is-Bu-SO3H as a new Hg2 + fluorescent sensor. Inorg Chem Commun. 2022;146:110100.10.1016/j.inoche.2022.110100Search in Google Scholar
[35] Ziarani GM, Ashtiani ST, Mohajer F, Badiei A, Varma R. Architecture of a functionalized SBA-15 (SBA-Pr-N-BFE) chemosensor for the detection of Fe3 + ions in aqueous media. Inorg Chem Commun. 2023;150:110483.10.1016/j.inoche.2023.110483Search in Google Scholar
[36] Gao Z, Qiao M, Tan M, Peng H, Ding L. Surface functionalization of mesoporous silica nanoparticles with pyronine derivative for selective detection of hydrogen sulfide in aqueous solution. Colloids Surf A: Physicochem Eng Asp. 2020;586:124194.10.1016/j.colsurfa.2019.124194Search in Google Scholar
[37] Tian Y, Guo X, Li M, Li C, Hu X, Jin L, et al. SBA-15 supported 1-methyl-2-azaadamanane n-oxyl (1-Me-AZADO) as recyclable catalyst for oxidation of alcohol. Org Lett. 2021;23(10):3928–32.10.1021/acs.orglett.1c01058Search in Google Scholar PubMed
[38] Feitosa LF, Pozes BB, Silva AS, Castro LF, Júnior LSC, Quitete CB, et al. Surface molecular design of organic–inorganic mesoporous hybrid materials for CO2 capture. J Environ Chem Eng. 2021;9(1):104951.10.1016/j.jece.2020.104951Search in Google Scholar
[39] Zhang Y, Zhu T, Wang H, Zheng L, Chen M, Wang W. Preparation of a bis-Schiff base immobilized mesoporous SBA-15 nanosensor for the fluorogenic sensing and adsorption of Cu 2+. Dalton Trans. 2022;51(18):7210–22.10.1039/D2DT00933ASearch in Google Scholar
[40] Crano JC, Guglielmetti RJ. Organic Photochromic and Thermochromic Compounds. physicochemical studies, biological applications, and thermochromism. New York: Springer New York, NY; Vol. 2, 1999;.Search in Google Scholar
[41] Sanchez C, Lebeau B, Chaput F, Boilot JP. Optical properties of functional hybrid organic–inorganic nanocomposites. Adv Mater. 2003;15(23):1969–94 .10.1002/adma.200300389Search in Google Scholar
[42] Wang M-S, Xu G, Zhang Z-J, Guo G-C. Inorganic–organic hybrid photochromic materials. Chem Commun. 2010;46(3):361–76.10.1039/B917890BSearch in Google Scholar
[43] Mitran R-A, Berger D, Băjenaru L, Năstase S, Andronescu C, Matei C. Azobenzene functionalized mesoporous AlMCM-41-type support for drug release applications. Cent Eur J Chem. 2014;12(7):788–95.10.2478/s11532-014-0534-2Search in Google Scholar
[44] Timm J, Stoltenberg C, Senker J, Bensch W. Azobenzene‐Functionalized SBA‐15 Material for Application in Selective Separation. Z Anorg Allg Chem. 2014;640(3–4):595–603.10.1002/zaac.201300477Search in Google Scholar
[45] Tan P, Jiang Y, Wu Q, Gu C, Qi S, Zhang Q, et al. Light-responsive adsorbents with tunable adsorbent-adsorbate interactions for selective CO2 capture. Chin J Chem Eng. 2022;42(2):104–11.10.1016/j.cjche.2021.07.010Search in Google Scholar
[46] Park SS, Kong J, Selvaraj M, Ha C-S. Functionalized Mesoporous Silica for Highly Selective Sensing of Iron Ion in Water. J Nanosci Nanotechnol. 2021;21(8):4406–11.10.1166/jnn.2021.19425Search in Google Scholar PubMed
[47] Rastegari E, Hsiao Y-J, Lai W-Y, Lai Y-H, Yang T-C, Chen S-J, et al. An update on mesoporous silica nanoparticle applications in nanomedicine. Pharmaceutics. 2021;13(7):1067.10.3390/pharmaceutics13071067Search in Google Scholar PubMed PubMed Central
[48] Pardo R, Zayat M, Levy D. Photochromic organic–inorganic hybrid materials. Chem Soc Rev. 2011;40(2):672–87.10.1039/c0cs00065eSearch in Google Scholar PubMed
[49] Guo S, Okubo T, Kuroda K, Shimojima A. A photoresponsive azobenzene-bridged cubic silsesquioxane network. J Sol–Gel Sci Technol. 2016;79(2):262–9.10.1007/s10971-016-4074-4Search in Google Scholar
[50] Li L, Wang C, Xi P, Shu D, Liu R, Wang X, et al. Synthesis and properties of multi-layer core-shell Tb (BAO) 3 (NO3) 2@ SiO2@(PSPEA-PMMA) microsphere with photoluminescence and photochromic functions. Dye Pigment. 2021;195:109654.10.1016/j.dyepig.2021.109654Search in Google Scholar
[51] dos Santos Andrade L, Otubo L, Castanheira B, Brochsztain S. Novel periodic mesoporous organosilicas containing pyromellitimides and their application for the photodegradation of asphaltenes. Microporous Mesoporous Mater. 2021;312:110740.10.1016/j.micromeso.2020.110740Search in Google Scholar
[52] Krohm F, Kind J, Savka R, Janßen MA, Herold D, Plenio H, et al. Photochromic spiropyran-and spirooxazine-homopolymers in mesoporous thin films by surface initiated ROMP. J Mater Chem C. 2016;4(18):4067–76.10.1039/C5TC04054JSearch in Google Scholar
[53] Gago S, Fonseca IM, Parola AJ. Hybrid mesoporous silica grafted with photoisomerizable 2-hydroxychalcones. Microporous mesoporous Mater. 2013;180:40–7.10.1016/j.micromeso.2013.06.001Search in Google Scholar
[54] Zhang J, Tian H. Surface and interfacial photoswitches. In: Photochromic Materials: Preparation, Properties and Applications. Hoboken, New Jersey, U.S.: John Wiley & Sons Ltd.; 2016; p. 195–242.10.1002/9783527683734.ch6Search in Google Scholar
[55] Kawai T, Kunitake T, Irie M. Novel photochromic conducting polymer having diarylethene derivative in the main chain. Chem Lett. 1999;28(9):905–6.10.1246/cl.1999.905Search in Google Scholar
[56] Aiken S, Edgar RJ, Gabbutt CD, Heron BM, Hobson PA. Negatively photochromic organic compounds: Exploring the dark side. Dye Pigment. 2018;149:92–121.10.1016/j.dyepig.2017.09.057Search in Google Scholar
[57] Barachevsky V. Negative photochromism in organic systems. Rev J Chem. 2017;7(3):334–71.10.1134/S2079978017030013Search in Google Scholar
[58] Burova A, Venidiktova O, Savelyev M, Barachevsky V, Bukreeva T, Borodina T. Encapsulation of photochromic compounds possessing positive and negative photochromism. Mater Lett. 2021;303:130558.10.1016/j.matlet.2021.130558Search in Google Scholar
[59] Żmija J, Małachowski M. New organic photochromic materials and selected applications. J Achiev Mater Manuf Eng. 2010;41:48–56.Search in Google Scholar
[60] Irie M, Fukaminato T, Matsuda K, Kobatake S. Photochromism of diarylethene molecules and crystals: memories, switches, and actuators. Chem Rev. 2014;114(24):12174–277.10.1021/cr500249pSearch in Google Scholar PubMed
[61] Ivashenko O, van Herpt JT, Feringa BL, Rudolf P, Browne WR. UV/vis and NIR light-responsive spiropyran self-assembled monolayers. Langmuir. 2013;29(13):4290–7.10.1021/la400192cSearch in Google Scholar PubMed
[62] Muzdalo A, Saalfrank P, Vreede J, Santer M. Cis-to-trans isomerization of azobenzene derivatives studied with transition path sampling and quantum mechanical/molecular mechanical molecular dynamics. J Chem Theory Comput. 2018;14(4):2042–51.10.1021/acs.jctc.7b01120Search in Google Scholar PubMed
[63] Homocianu M, Fifere N, Airinei A. Azobenzene: Research progress and its reflections in applications. In: Watson LE, editor. Azobenzene: Aspects, Applications and Research. Hauppauge, New York: Nova Science Publishers; 2017.Search in Google Scholar
[64] Purkait MK, Sinha MK, Mondal P, Singh R. Photoresponsive membranes. Interface Science and Technology. Amsterdam, Netherlands: Elsevier; 2018. p. 115–44.10.1016/B978-0-12-813961-5.00004-8Search in Google Scholar
[65] Miniewicz A, Orlikowska H, Sobolewska A, Bartkiewicz S. Kinetics of thermal cis–trans isomerization in a phototropic azobenzene-based single-component liquid crystal in its nematic and isotropic phases. Phys Chem Chem Phys. 2018;20(4):2904–13.10.1039/C7CP06820DSearch in Google Scholar
[66] Zhang L, Tang Z, Hou L, Qu Y, Deng Y, Zhang C, et al. Selective mercury (II) detection in aqueous solutions upon the absorption changes corresponding to the transition moments polarized along the short axis of an azobenzene chemosensor. Analyst. 2020;145(5):1641–5.10.1039/C9AN02286DSearch in Google Scholar PubMed
[67] Mabhai S, Dolai M, Dey S, Dhara A, Das B, Jana A. A novel chemosensor based on rhodamine and azobenzene moieties for selective detection of Al 3 + ions. N J Chem. 2018;42(12):10191–201.10.1039/C8NJ00436FSearch in Google Scholar
[68] Arif T, Cazorla C, Bogliotti N, Saleh N, Blanchard F, Gandon V, et al. Bimetallic gold (i) complexes of photoswitchable phosphines: synthesis and uses in cooperative catalysis. Catal Sci Technol. 2018;8(3):710–5.10.1039/C7CY01614JSearch in Google Scholar
[69] Meier H. The photochemistry of stilbenoid compounds and their role in materials technology. Angew Chem Int Ed Engl. 1992;31(11):1399–420.10.1002/anie.199213993Search in Google Scholar
[70] Likhtenshtein GI. Stilbenes synthesis and applications. In: Kirk-Othmer Encyclopedia of Chemical Technology Article: Stilbenes Synthesis and Applications. Hoboken, New Jersey, U.S.: John Wiley & Sons Ltd.; 2000. p. 1–24.10.1002/0471238961.stillkh.a01Search in Google Scholar
[71] Chan JC-H, Lam WH, Yam VW-W. A highly efficient silole-containing dithienylethene with excellent thermal stability and fatigue resistance: a promising candidate for optical memory storage materials. J Am Chem Soc. 2014;136(49):16994–7.10.1021/ja5101855Search in Google Scholar PubMed
[72] Chatir E, Khettabi A, Lafolet F, Jouvenot D, Royal G, Saint-Aman E, et al. Photochromic metallopolymer based on dithienylethene as a molecular calculator. Chem Mater. 2022;34(13):5912–8.10.1021/acs.chemmater.2c00819Search in Google Scholar
[73] Wang C, Zhang Y-M, Li H, Zhang J, Zhou Y, Liu G, et al. Synergistic activation of photoswitchable supramolecular assembly based on sulfonated crown ether and dithienylethene derivative. Chin Chem Lett. 2022;33(5):2447–50.10.1016/j.cclet.2021.09.106Search in Google Scholar
[74] Jia Y, Lu M, Cui S, Pu S. A new diarylethene based chemosensor for colorimetric recognition of arginine and fluorescent detection of Cu2+. J Photochem Photobiol A: Chem. 2022;423:113592.10.1016/j.jphotochem.2021.113592Search in Google Scholar
[75] Villarón D, Wezenberg SJ. Stiff‐stilbene photoswitches: from fundamental studies to emergent applications. Angew Chem. 2020;132(32):13292–302.10.1002/ange.202001031Search in Google Scholar
[76] Durr H, Bouas-Laurent H. Photochromism: molecules and systems. Amsterdam, Netherlands: Elsevier; 2003.Search in Google Scholar
[77] Moniruzzaman M, Sabey C, Fernando G. Photoresponsive polymers: an investigation of their photoinduced temperature changes during photoviscosity measurements. Polymer. 2007;48(1):255–63.10.1016/j.polymer.2006.08.066Search in Google Scholar
[78] Shiraishi Y, Itoh M, Hirai T. Thermal isomerization of spiropyran to merocyanine in aqueous media and its application to colorimetric temperature indication. Phys Chem Chem Phys. 2010;12(41):13737–45.10.1039/c0cp00140fSearch in Google Scholar PubMed
[79] Yuan W, Gao X, Pei E, Li Z. Light-and pH-dually responsive dendrimer-star copolymer containing spiropyran groups: Synthesis, self-assembly and controlled drug release. Polym Chem. 2018;9(26):3651–61.10.1039/C8PY00721GSearch in Google Scholar
[80] Gupta VK. Photochromic dyes for smart textiles. Dye Pigments-Novel Appl Waste Treat. London, UK: IntechOpen; 2021.Search in Google Scholar
[81] Jiao Y, Yang R, Luo Y, Liu L, Xu B, Tian W. Fulgide derivative-based solid-state reversible fluorescent switches for advanced optical memory. CCS Chem. 2022;4(1):132–40.10.31635/ccschem.021.202000673Search in Google Scholar
[82] Coelho PJ, Carvalho LM, Rodrigues S, Oliveira-Campos A, Dubest R, Aubard J, et al. Synthesis and photochromic behaviour of novel 2H-chromenes derived from fluorenone. Tetrahedron. 2002;58(5):925–31.10.1016/S0040-4020(01)01187-5Search in Google Scholar
[83] Barachevsky V. Photochromic organo-silica nanoparticles. Russian J Gen Chem. 2021;91(9):1875–88.10.1134/S1070363221090346Search in Google Scholar
[84] Ziarani GM, Moradi R, Mohajer F, Badiei A. Synthesis of SBA-Pr-NHC as a selective fluorescent sensor for the detection of Ag + ion in aqueous media. Spectrochim Acta Part A. 2022;267:120580.10.1016/j.saa.2021.120580Search in Google Scholar PubMed
[85] Stobbe H. The colour of the ‘fulgenic acid’and ‘fulgide’. Ber Dtsch Chem Ges. 1905;38:3673–82.10.1002/cber.190503803214Search in Google Scholar
[86] Nakatani K, Piard J, Yu P, Métivier R. Introduction: organic photochromic molecules. In: Photochromic Materials: Preparation, Properties and Applications. Hoboken, New Jersey, U.S.: John Wiley & Sons Ltd.; 2016. p. 1–45.10.1002/9783527683734.ch1Search in Google Scholar
[87] Lachmann D, Lahmy R, König B. Fulgimides as Light‐Activated Tools in Biological Investigations. Eur J Org Chem. 2019;2019(31–32):5018–24.10.1002/ejoc.201900219Search in Google Scholar
[88] Volarić J, Szymanski W, Simeth NA, Feringa BL. Molecular photoswitches in aqueous environments. Chem Soc Rev. 2021;50:12377–449.10.1039/D0CS00547ASearch in Google Scholar
[89] Satoh Y, Ishibashi Y, Ito S, Nagasawa Y, Miyasaka H, Chosrowjan H, et al. Ultrafast laser photolysis study on photodissociation dynamics of a hexaarylbiimidazole derivative. Chem Phys Lett. 2007;448(4–6):228–31.10.1016/j.cplett.2007.09.081Search in Google Scholar
[90] Delbaere S, Orio M, Berthet J, Sliwa M, Hatano S, Abe J. Insights into the recombination of radical pairs in hexaarylbiimidazoles. Chem Commun. 2013;49(52):5841–3.10.1039/c3cc43037eSearch in Google Scholar PubMed
[91] Dessauer R. Photochemistry, history and commercial applications of hexaarylbiimidazoles: all about HABIs. Amsterdam, Netherlands: Elsevier; 2006.10.1016/B978-044452770-7/50007-8Search in Google Scholar
[92] Broman SL, Nielsen MB. Dihydroazulene: from controlling photochromism to molecular electronics devices. Phys Chem Chem Phys. 2014;16(39):21172–82.10.1039/C4CP02442GSearch in Google Scholar
[93] Buchholtz F, Zelichenok A, Krongauz V. Synthesis of new photochromic polymers based on phenoxynaphthacenequinone. Macromolecules. 1993;26(5):906–10.10.1021/ma00057a004Search in Google Scholar
[94] Mançois F, Sanguinet L, Pozzo J-L, Guillaume M, Champagne B, Rodriguez V, et al. Acido-triggered nonlinear optical switches: Benzazolo-oxazolidines. J Phys Chem B. 2007;111(33):9795–802.10.1021/jp073386+Search in Google Scholar
[95] Mitchell RH. The metacyclophanediene‐dihydropyrene photochromic π switch. Eur J Org Chem. 1999;1999(11):2695–703.10.1002/(SICI)1099-0690(199911)1999:11<2695::AID-EJOC2695>3.3.CO;2-KSearch in Google Scholar
[96] Delbaere S, Luccioni-Houze B, Bochu C, Teral Y, Campredon M, Vermeersch G. Kinetic and structural studies of the photochromic process of 3 H-naphthopyrans by UV and NMR spectroscopy. J Chem Soc Perkin Trans 2. 1998;5:1153–8.10.1039/a800906fSearch in Google Scholar
[97] Han J, Li Y-X, Pang M-L, Cheng K-G, Wang Y-M, Ma Y-X, et al. A novel photochromic liquid crystal system based on biindenylidenedione derivatives. N J Chem. 2007;31(4):543–8.10.1039/b613549hSearch in Google Scholar
[98] Wang Z, Udmark J, Börjesson K, Rodrigues R, Roffey A, Abrahamsson M, et al. Evaluating dihydroazulene/vinylheptafulvene photoswitches for solar energy storage applications. ChemSusChem. 2017;10(15):3049–55.10.1002/cssc.201700679Search in Google Scholar
[99] Cacciarini M, Nielsen MB. Dihydroazulene/Vinylheptafulvene (DHA/VHF) and molecular electronics. Mol Photoswitches: Chem, Prop, Appl. 2022;1:379–400.10.1002/9783527827626.ch17Search in Google Scholar
[100] Zhang H, Zhong T, Jiang N, Zhang Z, Gong X, Wang G. Study on the photochromism, photochromic fluorescence switch, fluorescent and colorimetric sensing for Cu2 + of naphthopyran-diaminomaleonitrile dyad and recognition Cu2 + in living cells. Spectrochim Acta Part A. 2020;233:118191.10.1016/j.saa.2020.118191Search in Google Scholar
[101] Lvov AG, Bredihhin A. Azulene as an ingredient for visible-light-and stimuli-responsive photoswitches. Org Biomol Chem. 2021;19(20):4460–8.10.1039/D1OB00422KSearch in Google Scholar
[102] Beck JS, Vartuli JC, Roth WJ, Leonowicz ME, Kresge C, Schmitt K, et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J Am Chem Soc. 1992;114(27):10834–43.10.1021/ja00053a020Search in Google Scholar
[103] Allouche J, Le Beulze A, Dupin J-C, Ledeuil J-B, Blanc S, Gonbeau D. Hybrid spiropyran–silica nanoparticles with a core-shell structure: sol–gel synthesis and photochromic properties. J Mater Chem. 2010;20(42):9370–8.10.1039/c0jm01780aSearch in Google Scholar
[104] Dickson SE, Crudden CM. Transformable periodic mesoporous organosilica materials. Chem Commun. 2010;46(12):2100–2.10.1039/b924570gSearch in Google Scholar
[105] Bousseksou A, Molnár G, Demont P, Menegotto J. Observation of a thermal hysteresis loop in the dielectric constant of spin crossover complexes: towards molecular memory devices. J Mater Chem. 2003;13(9):2069–71.10.1039/B306638JSearch in Google Scholar
[106] He T, Yao J. Photochromism in composite and hybrid materials based on transition-metal oxides and polyoxometalates. Prog Mater Sci. 2006;51(6):810–79.10.1016/j.pmatsci.2005.12.001Search in Google Scholar
[107] Koumpouras K, Larsson JA. Distinguishing between chemical bonding and physical binding using electron localization function (ELF). J Phys: Condens Matter. 2020;32(31):315502.10.1088/1361-648X/ab7fd8Search in Google Scholar
[108] MacLachlan MJ, Aroca P, Coombs N, Manners I, Ozin GA. Ring‐opening polymerization of a [1] silaferrocenophane within the channels of mesoporous silica: poly (ferrocenylsilane)‐MCM‐41 precursors to magnetic iron nanostructures. Adv Mater. 1998;10(2):144–9.10.1002/(SICI)1521-4095(199801)10:2<144::AID-ADMA144>3.3.CO;2-DSearch in Google Scholar
[109] Schomburg C, Wark M, Rohlfing Y, Schulz-Ekloff G, Wöhrle D. Photochromism of spiropyran in molecular sieve voids: effects of host–guest interaction on isomer status, switching stability and reversibility. J Mater Chem. 2001;11(8):2014–21.10.1039/b101516hSearch in Google Scholar
[110] Yoshida A, Kakegawa N, Ogawa M. Adsorption of a cationic porphyrin onto mesoporous silicas. Res Chem Intermed. 2003;29(7):721–31.10.1163/156856703322601735Search in Google Scholar
[111] Ritter H, Ramm JH, Brühwiler D. Influence of the structural properties of mesoporous silica on the adsorption of guest molecules. Materials. 2010;3(8):4500–9.10.3390/ma3084500Search in Google Scholar
[112] Mittal V, Herle V. Physical adsorption of organic molecules on the surface of layered silicate clay platelets: A thermogravimetric study. J Colloid Interface Sci. 2008;327(2):295–301.10.1016/j.jcis.2008.08.036Search in Google Scholar PubMed
[113] Gustafsson H. Enzyme immobilization in mesoporous silica: Chalmers Tekniska Hogskola. Thesis for the degree of doctor of philosophy, Chalmers University of Technology, Göteborg, Sweden; 2013.Search in Google Scholar
[114] Dunn B, Zink JI. Optical properties of sol–gel glasses doped with organic molecules. J Mater Chem. 1991;1(6):903–13.10.1039/JM9910100903Search in Google Scholar
[115] Yamaguchi T, Ogawa M. Hydrophilic internal pore and hydrophobic particle surface of organically modified mesoporous silica particles to host photochromic molecules. Chem Lett. 2019;48(2):170–2.10.1246/cl.180908Search in Google Scholar
[116] Yamaguchi T, Leelaphattharaphan NN, Shin H, Ogawa M. Acceleration of photochromism and negative photochromism by the interactions with mesoporous silicas. Photochem Photobiol Sci. 2019;18(7):1742–9.10.1039/c9pp00081jSearch in Google Scholar PubMed
[117] Wirnsberger G, Scott B, Chmelka B, Stucky G. Fast response photochromic mesostructures. Adv Mater. 2000;12(19):1450–4.10.1002/1521-4095(200010)12:19<1450::AID-ADMA1450>3.3.CO;2-WSearch in Google Scholar
[118] Okabe Y, Ogawa M. Photoinduced adsorption of spiropyran into mesoporous silicas as photomerocyanine. RSC Adv. 2015;5(123):101789–93.10.1039/C5RA18252BSearch in Google Scholar
[119] Iqbal A, Iqbal G, Umar MN, Ur Rashid H, Khan SW. Synthesis of novel silica encapsulated spiropyran-based thermochromic materials. R Soc Open Sci. 2022;9(3):211385.10.1098/rsos.211385Search in Google Scholar
[120] Marceau E, Carrier X, Che M. Impregnation and drying. Synth solid Catalysts. 2009;59–82.10.1002/9783527626854.ch4Search in Google Scholar
[121] Casades I, Álvaro M, García H, Narayana Pillai M. Modified mesoporous MCM-41 as hosts for photochromic spirobenzopyrans. Photochem Photobiol Sci. 2002;1(3):219–23.10.1039/b110936gSearch in Google Scholar
[122] Frey AS, Hinrichsen O. Comparison of differently synthesized Ni (Al) MCM-48 catalysts in the ethene to propene reaction. Microporous mesoporous Mater. 2012;164:164–71.10.1016/j.micromeso.2012.07.015Search in Google Scholar
[123] He S, Zhang L, He S, Mo L, Zheng X, Wang H, et al. Ni/SiO2 catalyst prepared with nickel nitrate precursor for combination of CO2 reforming and partial oxidation of methane: characterization and deactivation mechanism investigation. J Nanomaterials. 2015;2015:659402.10.1155/2015/659402Search in Google Scholar
[124] Nakagawa Y, Nakazawa H, Watanabe H, Tomishige K. Total hydrogenation of furfural over a silica‐supported nickel catalyst prepared by the reduction of a nickel nitrate precursor. ChemCatChem. 2012;4(11):1791–7.10.1002/cctc.201200218Search in Google Scholar
[125] Barachevsky V, Kobeleva O, Gorelik A, Krayushkin M. Spectral manifestations of the interaction of silicon dioxide nanoparticles with molecules of photochromic compounds. Opt Spectrosc. 2018;125(3):362–7.10.1134/S0030400X18090047Search in Google Scholar
[126] Yamaguchi T, Maity A, Polshettiwar V, Ogawa M. Negative photochromism based on molecular diffusion between hydrophilic and hydrophobic particles in the solid state. Inorg Chem. 2018;57(7):3671–4.10.1021/acs.inorgchem.7b03132Search in Google Scholar PubMed
[127] Yamaguchi T, Maity A, Polshettiwar V, Ogawa M. Photochromism of a spiropyran in the presence of a dendritic fibrous nanosilica; simultaneous photochemical reaction and adsorption. J Phys Chem A. 2017;121(42):8080–5.10.1021/acs.jpca.7b08466Search in Google Scholar PubMed
[128] Gu G, Ong P, Li Q. Photoluminescence of coumarin 540 dye confined in mesoporous silica. J Phys D: Appl Phys. 1999;32(17):2287.10.1088/0022-3727/32/17/320Search in Google Scholar
[129] Ogawa M, Kuroda K, Mori JI. Preparation of aluminum-containing mesoporous silica films. Langmuir. 2002;18(3):744–9.10.1021/la011429mSearch in Google Scholar
[130] Masse S, Laurent G, Chuburu F, Cadiou C, Déchamps I, Coradin T. Modification of the Stöber process by a polyazamacrocycle leading to unusual core− shell silica nanoparticles. Langmuir. 2008;24(8):4026–31.10.1021/la703828vSearch in Google Scholar PubMed
[131] Wirnsberger G, Stucky GD. Ordered mesostructured materials with optical functionality. ChemPhysChem. 2000;1(2):90–2.10.1002/1439-7641(20000915)1:2<90::AID-CPHC90>3.3.CO;2-1Search in Google Scholar
[132] Levy D. Photochromic sol−gel materials. Chem Mater. 1997;9(12):2666–70.10.1021/cm970355qSearch in Google Scholar
[133] Ang CW, Tan L, Qu Z, West NP, Cooper MA, Popat A, et al. Mesoporous silica nanoparticles improve oral delivery of antitubercular bicyclic nitroimidazoles. ACS Biomater Sci Eng. 2022;8(10):4196–206.10.1021/acsbiomaterials.1c00807Search in Google Scholar PubMed PubMed Central
[134] Ebrahimi-Gatkash M, Younesi H, Shahbazi A, Heidari A. Amino-functionalized mesoporous MCM-41 silica as an efficient adsorbent for water treatment: batch and fixed-bed column adsorption of the nitrate anion. Appl Water Sci. 2017;7(4):1887–901.10.1007/s13201-015-0364-1Search in Google Scholar
[135] Zhao H, Han H. Synthesis and characterization of functionalized SBA-15 silica through template removal. J Solid State Chem. 2020;282:121074.10.1016/j.jssc.2019.121074Search in Google Scholar
[136] Burns CT, Choi SY, Dietz ML, Firestone MA. Acidichromic spiropyran-functionalized mesoporous silica: Towards stimuli-responsive metal ion separations media. Sep Sci Technol. 2008;43(9–10):2503–19.10.1080/01496390802122311Search in Google Scholar
[137] Rohlfing Y, Wöhrle D, Wark M, Schulz-Ekloff G, Rathousky J, Zukal A. Covalent attachment of dye molecules to the inner surface of MCM-41. Stud Surf Sci Catal. 2000;129:295–302.10.1016/S0167-2991(00)80226-8Search in Google Scholar
[138] Timm J, Schuermann U, Kienle L, Bensch W. High azobenzene functionalization enhances stability of the cis isomer: Periodic mesoporous organosilica network on the way to new light triggered applicable materials. Microporous Mesoporous Mater. 2016;228:30–6.10.1016/j.micromeso.2016.03.027Search in Google Scholar
[139] Zhang Y, Wang G, Zhang J. Study on fluorescent switching of naphthopyran with carbazole and pyrene dyad immobilized on SBA-15. Tetrahedron. 2014;70(35):5966–73.10.1016/j.tet.2014.05.097Search in Google Scholar
[140] Abboud M. Synthesis and characterization of 5, 5′-bis-silylated dithienylethene as a new building block of novel photochromic periodic mesoporous organosilicas. Res Chem Intermed. 2020;46(4):2195–204.10.1007/s11164-020-04086-ySearch in Google Scholar
[141] Chen T, Chen R, Jin Z, Liu J. Engineering hollow mesoporous silica nanocontainers with molecular switches for continuous self-healing anticorrosion coating. J Mater Chem A. 2015;3(18):9510–6.10.1039/C5TA01188DSearch in Google Scholar
[142] Guo S, Sugawara-Narutaki A, Okubo T, Shimojima A. Synthesis of ordered photoresponsive azobenzene–siloxane hybrids by self-assembly. J Mater Chem C. 2013;1(42):6989–95.10.1039/c3tc30587bSearch in Google Scholar
[143] Besson E, Mehdi A, Lerner DA, Reyé C, Corriu RJ. Photoresponsive ordered hybrid materials containing a bridged azobenzene group. J Mater Chem. 2005;15(7):803–9.10.1039/B416262ESearch in Google Scholar
[144] Mandal M, Kruk M. Versatile approach to synthesis of 2-D hexagonal ultra-large-pore periodic mesoporous organosilicas. J Mater Chem. 2010;20(35):7506–16.10.1039/c0jm01170cSearch in Google Scholar
[145] Goto Y, Inagaki S. Synthesis of large-pore phenylene-bridged mesoporous organosilica using triblock copolymer surfactant. Chem Commun. 2002;20:2410–1.10.1039/B207825BSearch in Google Scholar
[146] Gibard C, Gaulier C, Nauton L, Théry V, El-Ghozzi M, Avignant D, et al. Access to functionalised silver (I) and gold (I) N-heterocyclic carbenes by [2 + 3] dipolar cycloadditions. Dalton Trans. 2012;41(22):6803–12.10.1039/c2dt30249gSearch in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy


![Figure 10
Structure of AZB-based chemosensors for the detection of some metallic cations in aqueous solutions. (1) Adapted from ref. [66] with permission. Copyright 2020 Royal Society of Chemistry. (2) Adapted from ref. [67] with permission. Copyright 2018 Royal Society of Chemistry.](/document/doi/10.1515/ntrev-2024-0032/asset/graphic/j_ntrev-2024-0032_fig_010.jpg)
