Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
-
Kanchan Yadav
, Sanjeev Kumar Yadav
Abstract
Ultraviolet rays – B (UVB) can be efficiently absorbed by the cellular molecules of skin inducing damage within skin cells and a major cause of melanoma cancer. In recent years, several studies have reported the adverse effects of traditionally used organic and inorganic material-based sunscreens and UVB blockers. In this study, bovine serum albumin (BSA) has been used as a precursor to synthesize temperature- and pressure-dependent phase transition from sol (globular aggregates) – gel (hydrogels) – sol (carbon quantum dots) using a single-step hydrothermal method with an objective to develop an efficient and effective UVB blocker. The synthesized hydrogels exhibit UV – attenuation, self-fluorescence, and high biocompatibility properties that make them a suitable candidate for UV-blocker or sunscreen material. The biological efficacy of the hydrogels was studied through cyto-toxicity studies. Also, UVB blocking efficiency of developed hydrogel in primary mice skin cell culture as well as in vivo in mice model was studied. In vivo study on mice further demonstrated prominent thickening of stratum corneum and epidermis with perivascular edema in the dermis after 5 days of UVB exposure. Hence, this suggesting that hydrogel could be a potential candidate for protecting the skin from UVB exposure and reducing the threat of skin cancer.
Graphical abstract
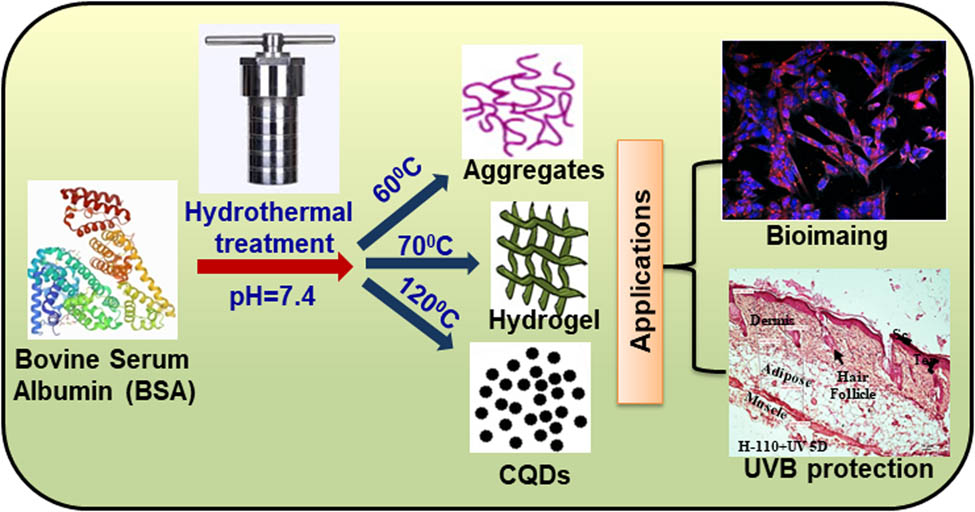
1 Introduction
Skin, the largest organ in the body, has a surface area of about 1.8 m2 and occupies 16% of the total body mass of an adult [1]. The primary and most important function of the skin in mammals is to provide a protective barrier against various infections caused by fungi, bacteria, UV radiation, and other environmental factors [2]. One of the major reasons for skin cancer is exposure to UV radiation (UV) which is classified as UVA (320–420 nm), UVB (290–320 nm), and UVC (200–290 nm). Almost all three, UVC and a part of UVB wavelength get absorbed by the ozone layer and limit their reach upto the earth’s surface [3,4]. UVA has a minor effect on the skin; however, UVB is the primary threat to human health and is responsible for skin cancer, photoaging, immune suppression, and sunburns [5,6].
Cancer is the most challenging disease to treat and the second most cause of mortality in the world. In recent years, there is a tremendous increase in skin cancer worldwide [7,8]. The major types of skin cancers are basal cell carcinoma, squamous cell carcinoma, Markel cell cancer, and melanoma in which melanoma is most dangerous because of its ability to spread faster to other organs if it is not diagnosed at an early stage and even becomes deadly [9,10]. Therefore, treating melanoma skin cancer is a big challenge, and emerging functional materials give a possible route to treat melanoma by enhancing drug uptake in cancer cells, minimizing toxicity, increasing the circulation time in tumor tissue, etc.
Avoiding excessive solar exposure and using sunscreens composed of nanoparticles such as zinc oxide NPs, are recommended to prevent exposure to UV radiation [11,12,13]. Currently, the most commonly used sunscreens are based on the nano-sized TiO2 and ZnO nanoparticles [14,15]. Skin exposure to the nano-sized particles containing sunscreens leads to the incorporation of inorganic material in the stratum corneum which can alter the attenuation properties due to particle-particle, particle-skin, and skin-particle-light physiochemical interactions [16,17]. Photocatalytic effects, nanoparticle stabilization after chronic exposure, and free radical production are some other major concerns to using these materials as sun blockers. The current scenario demands a new alternative material for skincare treatments, which can be more efficient, biocompatible, minimizing the side effects, and reducing the treatment cost significantly for the patients [18,19,20,21]. In this regard, functional biomaterials could be a good alternative to the nano-sized inorganic nanoparticle to prevent sunburns and skin cancer.
Proteins are biomolecules in nature; thus, they are safe and biocompatible. In recent studies, proteins have been extensively utilized by Materials scientists to design novel materials with unique functionality that further improves their practical applicability [22,23,24,25]. The complex structure of proteins is maintained due to the various interactions such as electrostatic, van der Waals, hydrophobic, and cation-pi. This gives tremendous opportunity to tune them into self-assembled hierarchal structures. Another fascinating aspect of self-assembled protein materials is the formation of hydrogel, which have multifunctional properties [26,27,28,29]. Protein-based hydrogels are a promising material for various biomedical applications because of their hydrophilic networks, controllable self-assembly, and biocompatibility. Bovine serum albumin (BSA) is a cost-effective, easily, and widely available protein. Its primary structure contains a single polypeptide chain of 583 amino acids with a molar mass of 66.4 kDa. BSA is a water-soluble protein with 76% sequence homology and identical pH-dependent conformation with human serum albumin [30,31]. Studies show that BSA has been conjugated with many organic and inorganic compounds for the synthesis of bio-nanoconjugates, monolayers, and nanoparticles, and holds promising applications in the biomedical field [32,33,34,35,36].
Thus, BSA has been used as a precursor to synthesize different self-assembled morphologies using a single-step hydrothermal thermal method with an objective to develop an efficient and effective UV-B blocker. By varying the hydrothermal temperature from 25 to 120°C, different phases of BSA protein have been synthesized. First, sol-to-gel transition occurs at 70°C, where BSA protein transforms into a self-assembled hydrogel phase, further heating of BSA protein at 120°C leads to the second-phase transition from gel to carbon quantum dots. Developed phases were thoroughly characterized using various physical and optical techniques such as SEM, TEM, FTIR, and SAXS, for studying their efficient applicability in the biomedical field. Biological efficacy was studied through cytotoxicity studies, and UV-B blocking efficiency of developed hydrogel was studied in primary mice skin cell culture as well as in vivo in mice model.
2 Material and methods
2.1 Chemicals and materials
BSA (lyophilized powder, >96% (agarose gel electrophoresis), M W = 66,430 Da), rutile nanosized TiO2, sodium chloride, hydrochloric acid, disodium hydrogen phosphate, and sodium dihydrogen phosphate were purchased from Sigma-Aldrich and used without further purification. Deionized water (12M-cm) from the Millipore water purification system was used for all aqueous solution preparations.
2.2 Synthesis of temperature-dependent phases of BSA
All the temperature-dependent phases of BSA were synthesized using the single-pot hydrothermal method. The homogenous solutions of concentration 40 mg/mL of lyophilized BSA powder were prepared using Sorensen’s phosphate buffer (pH-7.4) with a sodium salt concentration of 150 mM. The prepared solutions were transferred to a 25 mL Teflon cylinder with a steel autoclave and heated for the hydrothermal reaction at temperature ranges from 25 to 120°C for 15 h. During heating at different temperatures, different phases of BSA were resulted. The resultant samples were named according to their heating temperature, for example, H-100 suggests that BSA solution has been heated at 100°C for 15 h using the hydrothermal method.
2.3 Characterization techniques
A different phase of BSA has been characterized using suitable physical techniques. UV-Vis measurements have been performed using JASCO V770 UV-Vis spectrophotometer and Eppendorf Biospectrometer for solid and liquid samples, respectively. Photoluminescence (PL) properties have been studied using Fluorolog (Horiba). Fourier transform infrared (FT-IR) spectroscopy was performed using the Thermo Scientific Nicolet iD7 spectrometer over the wavenumber 500–4,000 cm−1 region with a resolution of 4 cm−1. Field emission scanning electron microscope (FESEM) images were taken using a FEI Nova NanoSEM 450 FESEM. A transmission electron microscopy (TEM) image was obtained using the TECNAI-T-20 (FEI instrument) at 200 kV accelerating voltage. LV100ND fluorescence microscope and Carl Zeiss 780 LSM laser scanning confocal microscopy system (Germany) were used for fluorescence microscopy. Rheological characterization of BSA hydrogels was performed using the Anton Paar Physica MCR301 rheometer. The BSA hydrogels were prepared in a hydrothermal autoclave and transferred to the rheological geometry of 8 mm parallel plates having a gap of 1 mm at room temperature for further studies. The measurement for storage and loss modulus as a function of strain and frequency was also performed for all hydrogels (H-70 to H-110) between the frequency range of 0.1 and 10 rad s−1 with a constant strain of 0.5% at 25°C. Xenocs SAS, model Xeuses 2.0, with a dual-source module X-ray source (Q range varies from 0.005 to 1.2 Angstrom−1) was used for the small-angle X-ray scattering (SAXS) measurement.
2.4 In vitro studies
2.4.1 Cell culture
For primary skin cell culture, skin tissue was collected from the hairless skin of Swiss albino mice, and the isolation of primary epidermal cells, endothelial cells, dermal cells, and fibroblast cells was performed by following the protocol given by Pauline Henrot et al. [37]. For experimental purposes, epidermal cells were used.
2.4.2 Cell viability
The biocompatibilities of all samples, i.e., H-25, H-60, H-70, H-80, H-90, H-100, H-110, H-120, and H-200, were determined by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay where the primary mice skin cells were plated at 104 cells/well in 96-well plates (Genetix) 24 h prior to the experiments. After completion of 24 h, the cells were treated with H-25, H-60, H-70, H-80, H-90, H-100, H-110, H-120, and H-200 with the same dose (20 mg/mL, i.e., 1:1 sample: complete media). In control (with cells) and negative control or blank (without cells) groups, the complete media were added. After 24 h of treatment, the cells were processed for the MTT assay following the laboratory standard process [38], and the optical density was measured in triplicate at 595 nm in a microplate reader (Biorad).
2.4.3 Cellular uptake
For the cellular uptake study of each sample, primary mice skin cells were seeded at 6,000 cells/well upon the round coverslips placed inside the 24 well plates 24 h prior to treatments. After 24 h, the cells were treated with the samples (H-25, H-60, H-70, H-80, H-90, H-100, H-110, H-120, and H-200) at the concentration of 20 mg/mL for the next 24 h similarly to MTT assay. After completion of 24 h, treated cells were fixed, permeabilized, and stained with DAPI according to the laboratory standard procedure [39], and finally, images were captured by a Carl Zeiss 780 LSM laser scanning confocal microscope (Germany).
2.4.4 UVB treatment study
An UVB lamp (G25T8E, Sankyo Denki) with peak emission at 306 nm was used for the generation of UVB radiation. For the UVB treatment study, the whole experiment was divided into two primary groups, i.e., UVB treated and UVB + H-110 treated. Each group was further subdivided into three subgroups according to UVB exposures, i.e., 5, 10, and 15 min according to ref. [17]. For each subgroup, primary mice skin cells were seeded at 6,000 cells/well on coverslip (3 wells) and 12,000 cells/well in other 3 wells of 6 well plates [thus 3 separate 6-well plates for 5′, 10′, and 15′ UVB treatment] for 24 h for acclimatization. After the completion of 24 h, every six-well plates was placed under UVB light of the self-made UVB chamber (Figure S1) for respective 5′, 10′, and 15′ UVB treatment. On the same day, the cells of the UVB + H-110 group were first layered and incubated with H-110 for 1 h inside the CO2 incubator. After 1 h, the cells were treated with UVB as described earlier. After all treatments, the cells seeded on the coverslips were fixed permeabilized and stained with DAPI, and the images were captured by a Carl Zeiss 780 LSM laser scanning confocal microscope (Germany) for cytological study. Other treated cells were processed for survival analysis through MTT assay.
2.5 In vivo mice model study
Two weeks prior to experimentations, all-female Swiss albino mice (N = 30) were maintained at 12 h light: 12 h dark cycle with 25 ± 2°C temperature at the Department of Zoology, Banaras Hindu University, Varanasi, Uttar Pradesh, India. All maintenance procedures along with experimentations were performed following the revised Animals Act Government of India, 2007, approved rules [Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Registration No. 1802/GO/Re/S/15/CPCSEA].
After acclimatization, animals were divided into six following groups with five animals in each group:
Control (n = 5) – without any treatment,
H-110 treated group (n = 5) – where a single layer of H-110 at the backside was applied to each animal,
3 days UVB-treated group (UV 3D, n = 5), treated with UVB for 20 min for 3 days,
5 days UVB-treated group (UV 5D, n = 5), treated with UVB for 20 min for 5 days,
3 days H-110 + UVB-treated group (H-110 + UV 3D, n = 5), where a single layer of H-110 at the backside was first applied and then the UVB treatment was given for 20 min for 3 days,
5 days H-110 + UVB-treated group (H-110 + UV 5D, n = 5), where a single layer of H-110 at the backside was first applied and then UVB treatment was given for 20 min for 5 days.
During treatment, mild ether anesthesia was continually given to the animals. After completion of the experimental periods, part of the treated skin was collected from each animal for further histoarchitectural analysis. No animals were sacrificed during this experimentation; as after skin collection, animals were given proper medical care to heal the wounds.
2.5.1 Morphometric analysis
For histological analysis, skin were immediately removed from each group of animals and fixed in 10% formalin, followed by dehydration, clearing with xylene, and finally processed in wax and sectioned (7 microns) for hematoxylin and eosin staining (HE staining) [39]. Histoarchitectures were observed under a research microscope (Nikon E 200, Japan) in randomly selected sections.
3 Results and discussion
3.1 Sol–gel–sol transformations of BSA hydrogel
BSA gels were prepared at different hydrothermal temperatures varying from 25 to 120°C with the 40 mg/mL protein concentration without using any cross-linker or chemical agent. At 25°C, the BSA solution does not go for the gelation process even after 15 h of heating, and the resultant has the same sol phase as the BSA initial solution. Further heating at 60°C, the turbidity of the solution is increased; however, no gelation occurs. One of the plausible reasons is that below the melting temperature (63°C), protein starts to unfold and thermal energy and electrostatic forces between the amino acid start to encounter each other during the hydrothermal process [40]. The melting temperature of the BSA is above 63°C, so the BSA solution was heated at 70°C hydrothermally for 15 h. Heating protein solution at a temperature of 70°C changed the turbid sol phase into a very fine gel, and this gel phase remains till the temperature of 100°C. Further heating of the protein solution above 110°C leads to the formation of new phases, and the phase separation between sol and gel was observed. The major portion of the protein solution changes into gel but the color of the gel becomes brownish and the remaining liquid was white. When the protein solution was heated to 120°C, the whole protein solution was converted into a sol phase and shows the physical and optical properties of carbon quantum dots. All the phases of the BSA are shown in Figure 1.
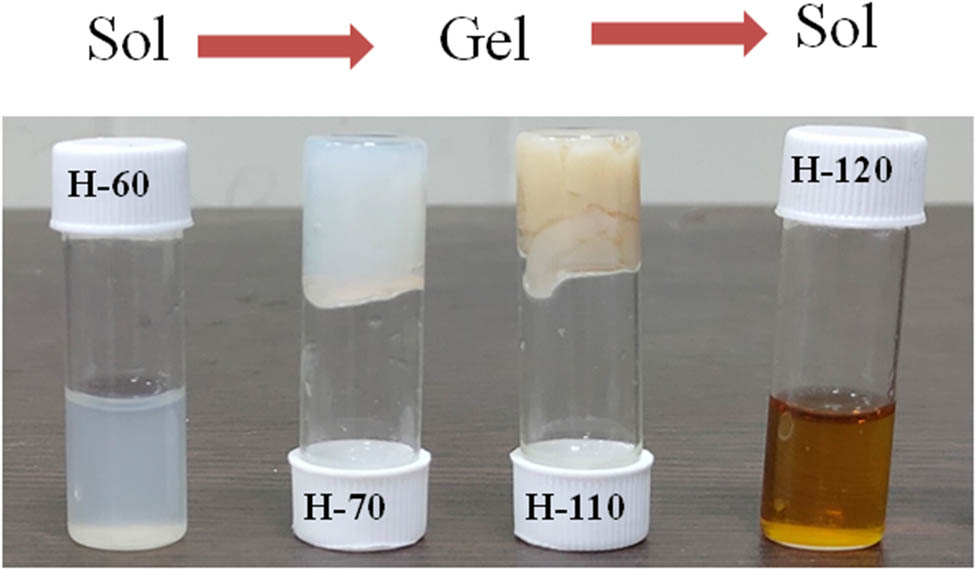
Different phases of developed BSA hydrogel at different temperatures.
The sol–gel–sol phase transition as a function of temperature was tested using the tube inversion method. Thermally elevated phases of BSA show sol-to-gel and gel-to-sol transformation at 70 and 120°C, respectively. The melting temperature of BSA is 63°C, and studies show that BSA has different oligomeric states above and below its melting temperature [41]. The heating of proteins above melting temperature results in weakening the hydrogen bonds and strengthening the hydrophobic interaction which alters the diffusion, accessibility, and exposure of polypeptide chains segment and dilutes the ligand binding properties [42]. Interactions between electrostatic and hydrophobic interaction result in connecting the protein molecules and led to form a gel-like structure. The aggregation of BSA can be elevated with temperature, pH, ionic strength, and other external stimuli. These factors play a significant role in the transition temperature of the various phases. As we heat the protein solution at a higher temperature, the strength of the gel decreases, which was further supported by rheological studies and a tube inversion test. At high temperatures, interactions present between the gels start to break, and gel-to-sol phase transition happens. Physical and optical characterizations of the sol phase confirm the nanostructure of the carbon quantum dots that are homogeneously dispersed in the medium [42,38]. Thus, different phases of BSA hydrogels have been synthesized using a single hydrothermal technique just by varying temperatures without the addition of a cross-linker molecule.
3.2 Optical characterization of various phases of BSA hydrogels
It was observed that all the BSA hydrogels synthesized at various temperatures show different colors in appearance. As the heating temperature increases, there is a change in color. It becomes a transparent solution to translucent white in the gel phase and dense yellow in the CQDs phase. UV-Vis spectroscopy was used to analyze the optical changes shown in Figure 2(a). UV-Vis spectra of H-25 (hydrogel synthesized at 25°C) show the typical characteristic peak of BSA observed at 278 nm. As there is an increase in the temperature, protein starts to aggregate and forms a gel. H-70 (hydrogel synthesized at 70°C) and H-110 (hydrogel synthesized at 110°C) also show the BSA peak but absorb in a broader range from UV to visible, which make them a suitable candidate for skin protection from UVB (Figure S2(a)). H-110 shows a new peak at 340 nm, which reveals the possibility that there might be a generation of new fluorescent compounds during the gelation process. H-120 shows the characteristic peak of CQDs at 275 nm which is due to the π–π* electronic transition of C═C on the CQDs surface. The board absorbance shows the presence of a large number of functional moieties present on the surface. Figure 2(b) shows the auto-fluorescence property of various phases of BSA. It is well established that BSA has blue auto-fluorescence because of the aromatic amino acids such as tyrosine, tryptophan, and phenylalanine [43]. The emission spectra of H-25 and H-60 show intrinsic fluorescence such as BSA solution centered at 340–350 nm with the excitation wavelength of 280 nm. The excitation spectrum is also centered at 270–290 nm and does not show long-range excitation. Moreover, the gel phases of the BSA (H-70 to H-110) exhibit a strong fluorescence at 450 nm when excited with 365 nm. The PL spectrum of the gels (Figure S2(b)) shows a redshift in excitation and emission compared to the sol phase. The emission spectrum ranges from 400 to 550 nm for emission with the broad excitation wavelength ranging from 325 to 375 nm. This phenomenon can be explained by the fact that the heating of the protein reduces the steric hindrance of peptide chains and exposed the aromatic amino acids that can favor the energy transfer among the C═C and C═O bonds that existed in the protein as well as the proximity of amine groups. Another reason could be the formation of new fluorophores that have been promoted by the temperature-induced gelation process. The intensity of the fluorescence also increases with the hydrothermal temperature but does not show a strong redshift. Due to the presence of a larger number of amino acids and the non-availability of information regarding their interactions, it is difficult to probe the accurate reason for the red-shifted and high auto-fluorescence intensity in hydrogels. The CQDs phase of the BSA shows a blue shift in excitation and emission wavelength compared to the gel phase which can be tuned by concentration and excitation wavelength. Wavelength-dependent emission in CQDs can be due to the non-uniform size distribution in dispersive medium and various surface functional groups such as carboxyl, hydroxyl, and other oxygen species.
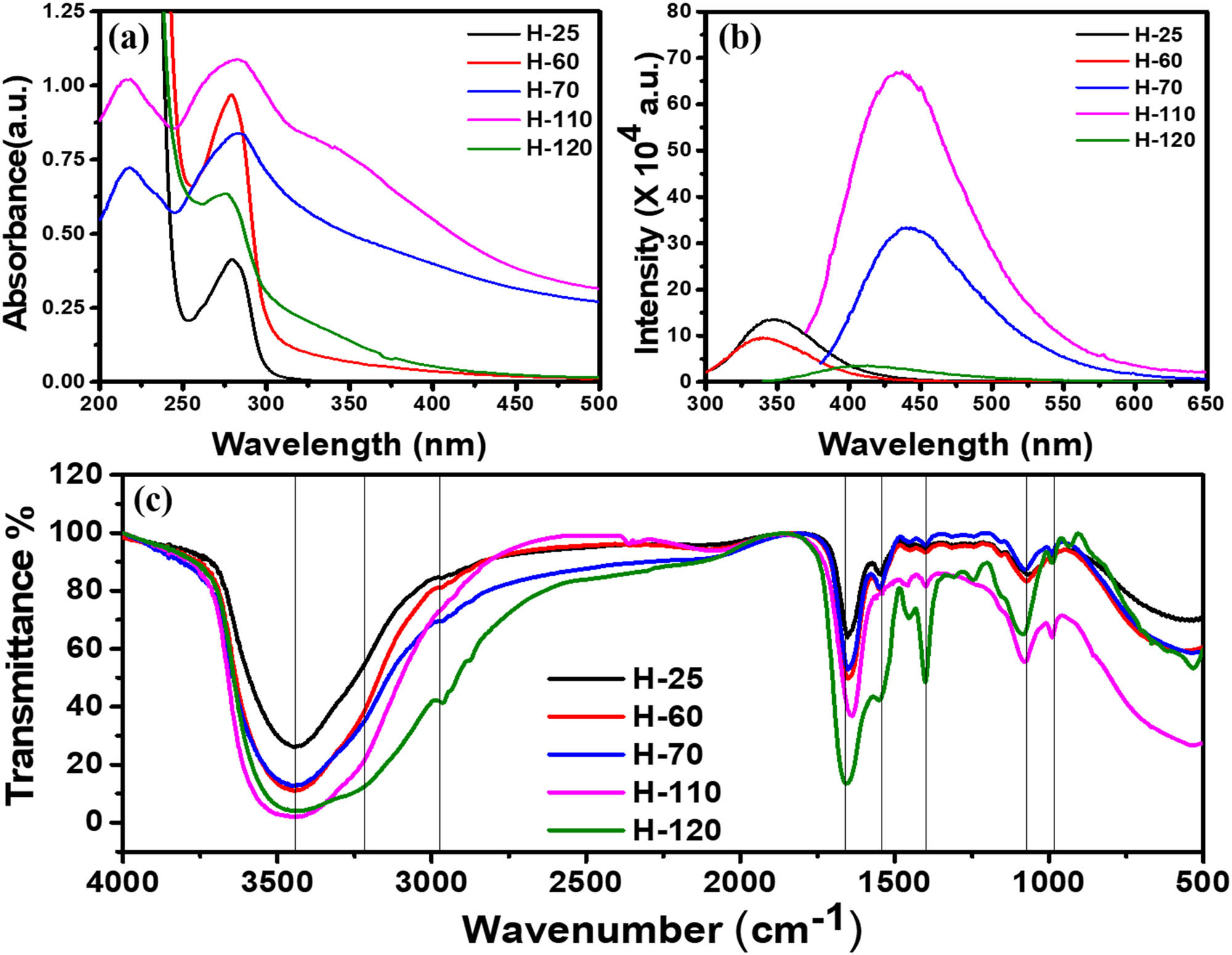
(a) UV-Vis spectrum of different phases of BSA protein; (b) PL spectrum of different phases of protein at their maximum excitation wavelength; (c) FTIR spectrum of different phases.
To get more insight into the involvement of various functional groups to form different phases of BSA at various temperatures, FTIR analysis has been performed. Full scan FITR spectra (4,000–500 cm−1) of all phases of BSA are shown in Figure 2(c). Intensity variation and small spectral shifts have been observed during the gelation process, and some new peaks have been observed when the gel phase is converted to CQDs. The broad peak at 3,500 cm−1 corresponds to the amide II bond due to the N–H stretching present in all the phases. From the sol-to-gel transition, only the peak becomes broader, but during the gel to CQDs transition, new peak occurs at 3,252 cm−1 corresponding to the N–H bond of the amine (–NH2) group. Amide band (1,400–1,700 cm−1) exists during both phase transitions and supports the fact that the protein main chain remains intact after heating at high temperature [44]. Peaks due to amide I bond C═O stretch at 1,650 cm−1 become strong with gel formation but amide II bond (C–N stretch coupled with N–H bending) at 1,580 cm−1 disappears in gel; however, it becomes stronger in the CQD phase. The decrease of amide II at approximately 1,580 cm−1 and increase of Amide II at approximately 1,450 cm−1 are related, which shows the conformational changes at a tertiary structural level. CQDs are nano-dimensional structural with an exposed functional group on the surface. The very strong peak around 1,400 cm−1 in the CQD phase corresponds to the symmetric stretching vibration of COO–, which disappear in gel form due to the CO–NH bond formation and also the peak at 1,093 cm−1 due to C–O vibration becomes prominent in the CQD phase. The limitation of the FTIR instrument investigation of new chemical bond formation overrules and limits the accurate analysis of the sol-to-gel transition.
SAXS has been performed on the samples prepared namely H-25, H-60, H-70, H-90, H-110, and H-120. No gel formation was observed in the samples prepared at the temperatures 25 and 60°C, and they remained in the solution state. On the other hand, gel formation was observed in the samples, which was prepared above the transition temperature. Raw data for the samples are shown in Figure 3(a). Sample H-25 shows a characteristic curve for a sphere. BSA in the solution state was found to form a globular structure, and the radius of the globule has been calculated by fitting the spherical model. The calculated radius of the BSA is found to be 3.198 nm. The fitted data are shown in Figure 3(b).
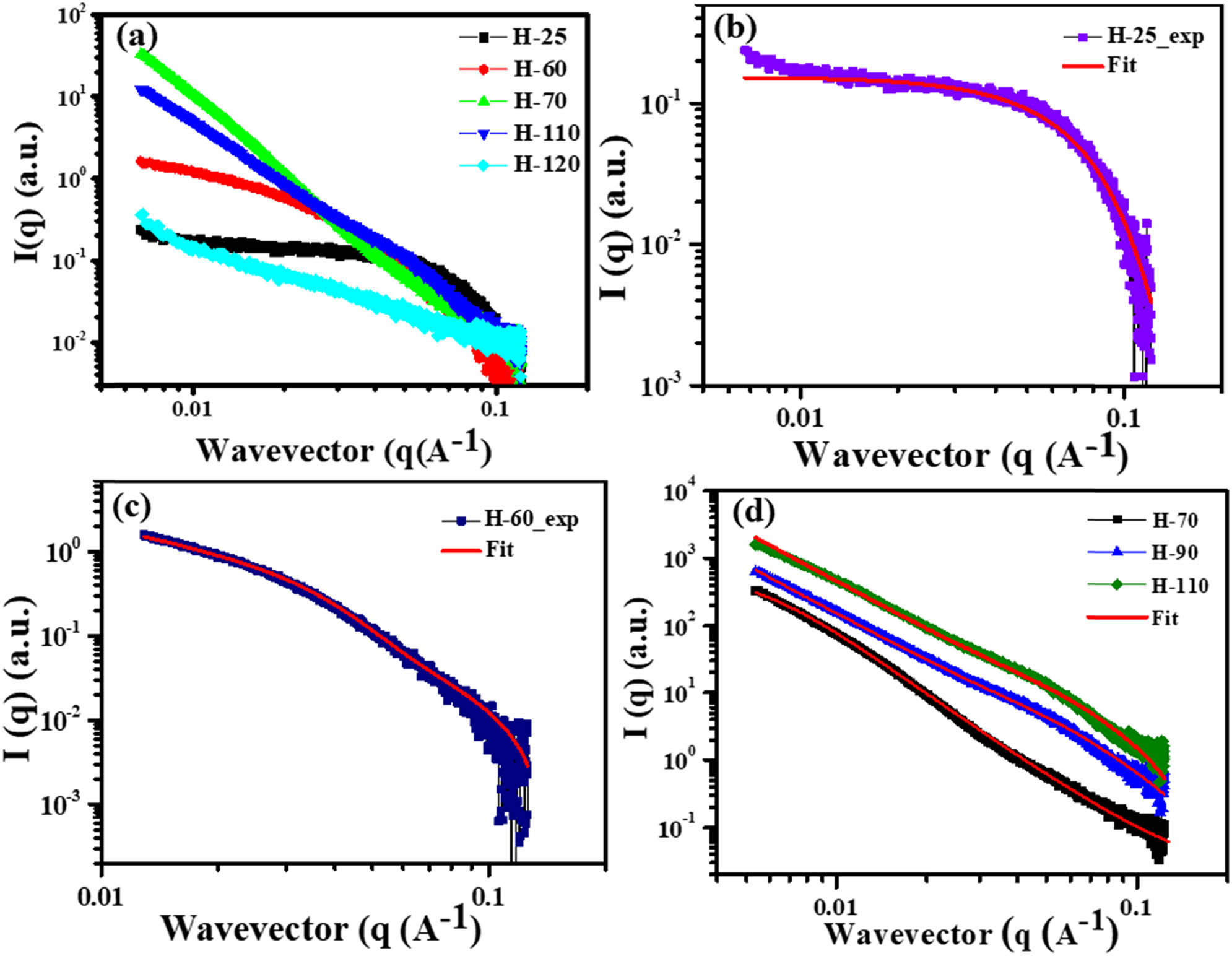
(a) Small-angle X-ray diffraction analysis of all phases of BSA protein; (b) shows that fitting of the SAXS of the H-25 samples using the spherical model; (c) H-60 is prepared near the transition temperature, SAXS for H-60 is fitted using the combination of Lorentz and Guinier equation; (d) shows the fitting of H-70, H-90 and H-110 using the Lorentz and porod fitting.
For the sample H-60, it is near the phase-transition temperature. It was observed that it was neither showing the characteristic curve for a sphere nor a gel. As the samples are prepared near the phase transition temperature, it can be assumed that SAXS data will be a combination of both the solution state sample and hydrogels. The mathematical equation used to fit the curve is given by equation (1) [45]:
where I(0) is the scaling factor, C is the Lorentz scale, G is the Guinier scale,
The fitted curve for the H-60 is shown in Figure 3(c). Fitted parameters for the H-60 are listed in Table S1. The value of D indicates the solvent condition. D value greater than 2 indicates a bad solvent condition; D value less than 2 indicates that the BSA is in good solvent condition and equal to 2 indicates that BSA is in the theta solvent condition. For the H-60 sample, the value of D is 1.75 which is close to the value of 5/3 indicating that the BSA below the transition temperature is in good solvent condition.
where A is the porod constant, C is the Lorentz constant, n is the porod factor, m is the Lorentz factor, and
The fitting parameters are shown in Table S2. n is the factor that corresponds to t homogeneity of the system. n value above 2 indicates that formed gels are homogeneous. As the temperature is increased, the value of n also increases indicating that with an increase in temperature, BSA molecules unfold and form a homogeneous structure. For H-70, as the temperature is closer to the transition temperature, n is a little less than 2 which indicates that the gel structure is not fully homogeneous and the interaction between the molecules to form the crosslinks is not strong enough. For samples H-90 and H-110, the n value is above 2, and it indicates that the samples are homogeneous, and the bonds are strong enough to form the crosslinks to form stable hydrogels.
Lorentz part of the curve has two parameters m and
To investigate the surface morphology and mechanical strength of the temperature elevated self-assembled hydrogels, SEM and rheology studies were employed. The SEM images of lyophilized powder of BSA hydrogels (H-70, H-90, H-110) are shown in Figure 4(a)–(c). All hydrogel phases exhibit a three-dimensional highly porous honeycomb-like structure with irregularities in shape and size. During the lyophilization process, water gets evaporated and lefts the voids between the interconnected areas [49]. As there is an increase in the hydrothermal temperature, the pore size of the hydrogels gets smaller. Relative decreases in pore size of hydrogels are shown in Figure 4(d).
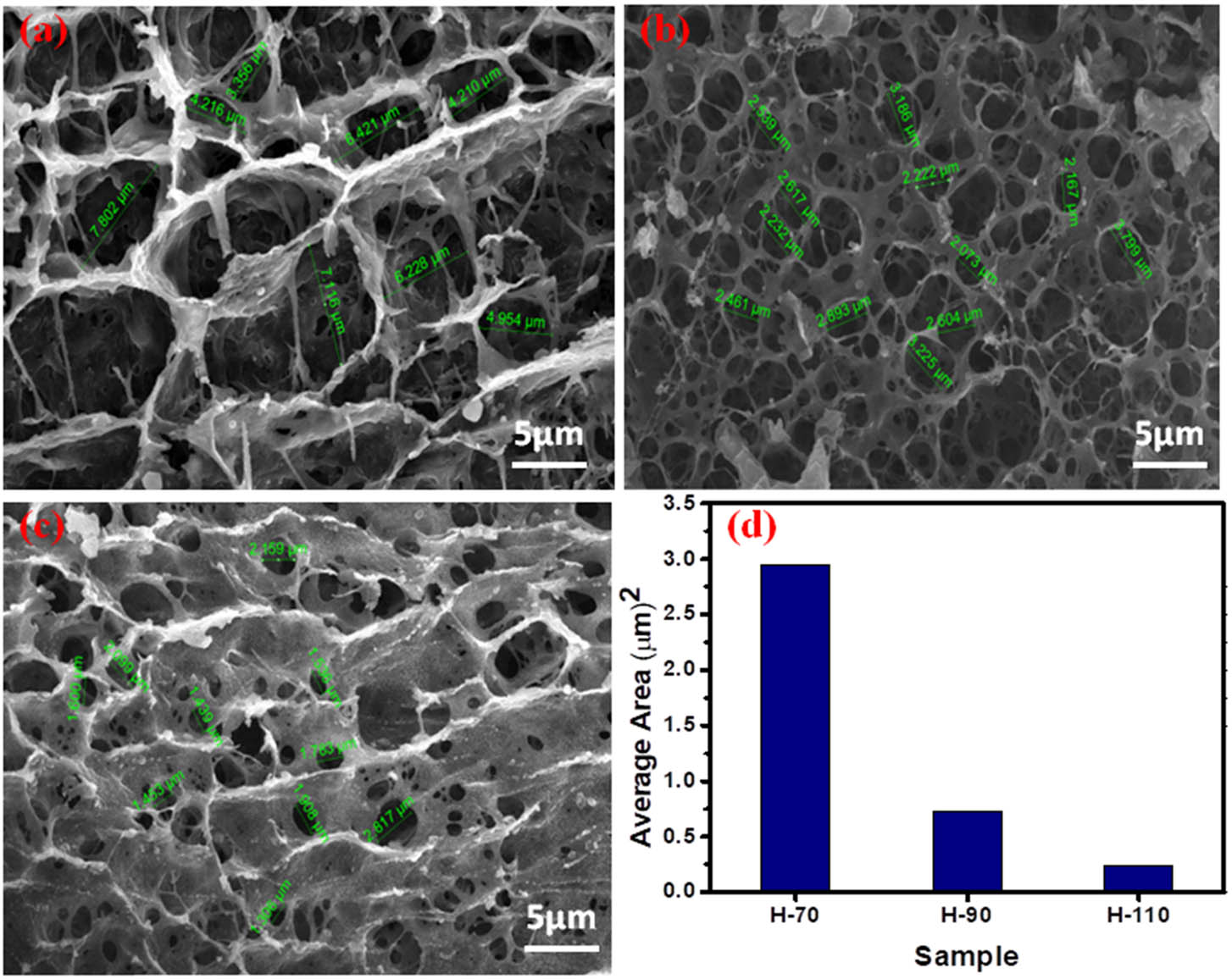
SEM images of hydrogel: (a) H-70; (b) H-90; (c) H-110; and (d) histogram of the area of voids.
3.3 Rheological analysis
Rheological studies have been performed to investigate the viscoelastic behavior of the hydrogels. Strain sweep test and frequency sweep test were performed to get insight into change in storage and loss modulus of gels as a function of strain and frequency. To evaluate the limits of the viscoelastic region, strain sweep tests were carried out on all the hydrothermally synthesized gel phases of BSA. In the linear viscoelastic region, the storage modulus G′ and viscous modulus G′′ are independent of strain and frequency [50]. Under higher strain conditions, the stress response of the gels is no longer sinusoidal, and similarly, higher harmonic contributions lead to a nonlinear stress–strain relationship. In the case of the strain sweep test, G′ and G′′ were plotted as a function of strain (%). As we increase the hydrothermal temperature from 70 to 90°C, both G′ and G′′ increase linearly as shown in Figure 5(a). However, at 100°C, the G′ and G′′ start to decrease, which suggests that due to the hydrothermal process, the lower-order structure starts to form that alters the viscoelastic property of hydrogel. Also, for the H-110 phase, we are getting similar viscoelastic trends as H-70. Additionally, in the case of the frequency sweep test, both G′ and G′′ moduli also increase as we increase the temperature from 70 to 110°C (Figure 5(b)). The higher values of G′ over G′′ indicate that the elastic component is predominant on the viscous one in a swiped frequency range.
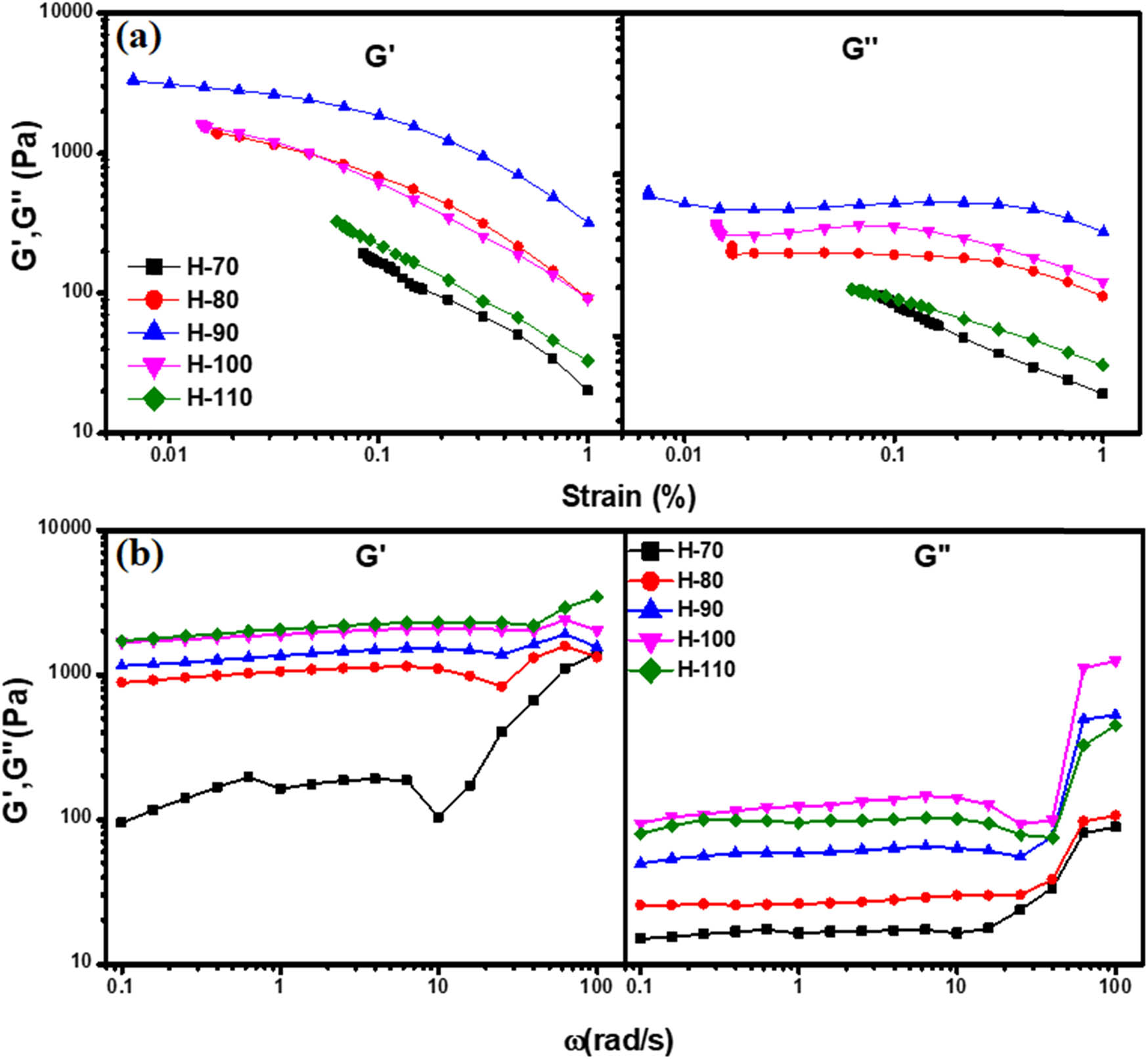
(a) Strain sweep test: G′ and G′′ modulus as a function of strain and (b) frequency sweep test: G′ and G′′ modulus as a function of frequency.
3.4 Biological studies
3.4.1 Cell toxicity and cellular uptake analysis
BSA has been extensively used in biomedical applications due to its higher bioavailability, biocompatibility, and biodegradability properties [51]. Herein, our objective was to study the cytotoxicity aspect of synthesized BSA hydrogel using BSA at various temperatures. In vitro, the biocompatibility of all hydrogel samples, i.e., H-25, H-60, H-70, H-80, H-90, H-100, H-110, H-120, and H-200. is studied and shown in Figure 6. The MTT assay results demonstrated that all samples showed the % cell survival between 93.6 and 99.16%, which suggests its excellent biocompatibility. Among all of them, H-110 showed the highest % cell survival of 99.16%.
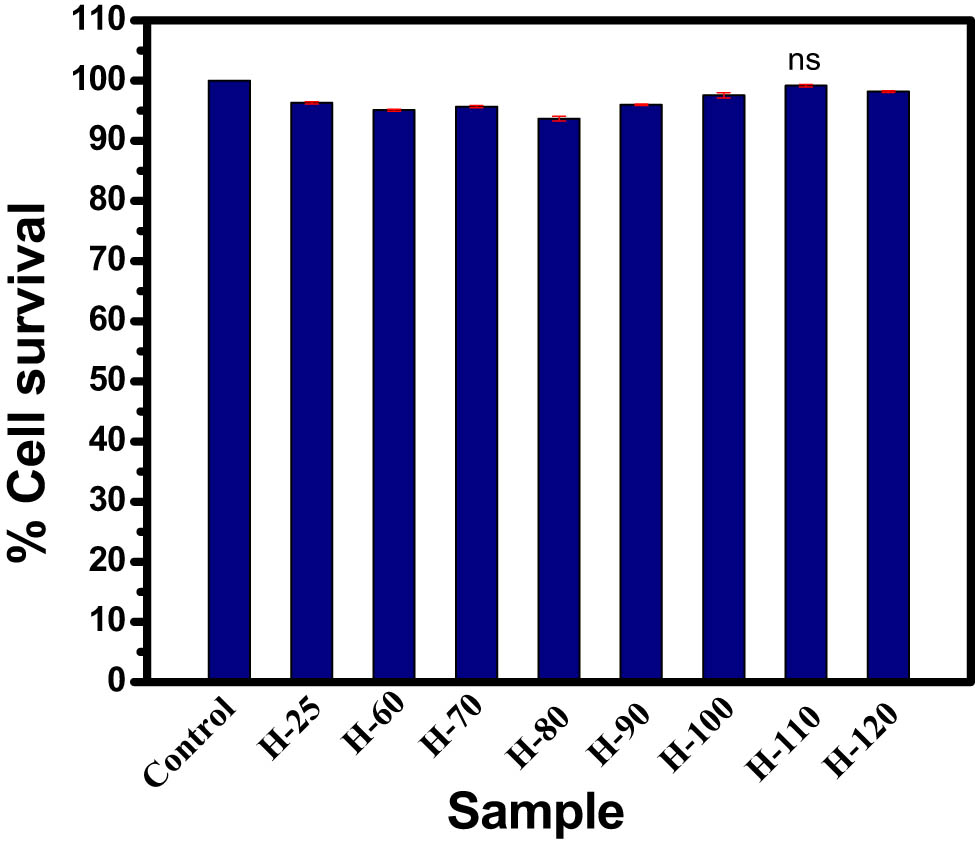
Cell viability study of hydrogel samples for 24 h. Vertical bars representing mean ± SEM. Significance was tested using one-way ANOVA followed by Tukey post-hoc test.
Cellular uptake studies further confirm its good bioimaging green autofluorescence capacity in all hydrogel samples. Interestingly, H-110 has shown maximum cellular penetration (Figure 7) with a higher cytoplasmic and nuclear distribution. Hence, we have applied H-110 for further experimental studies.
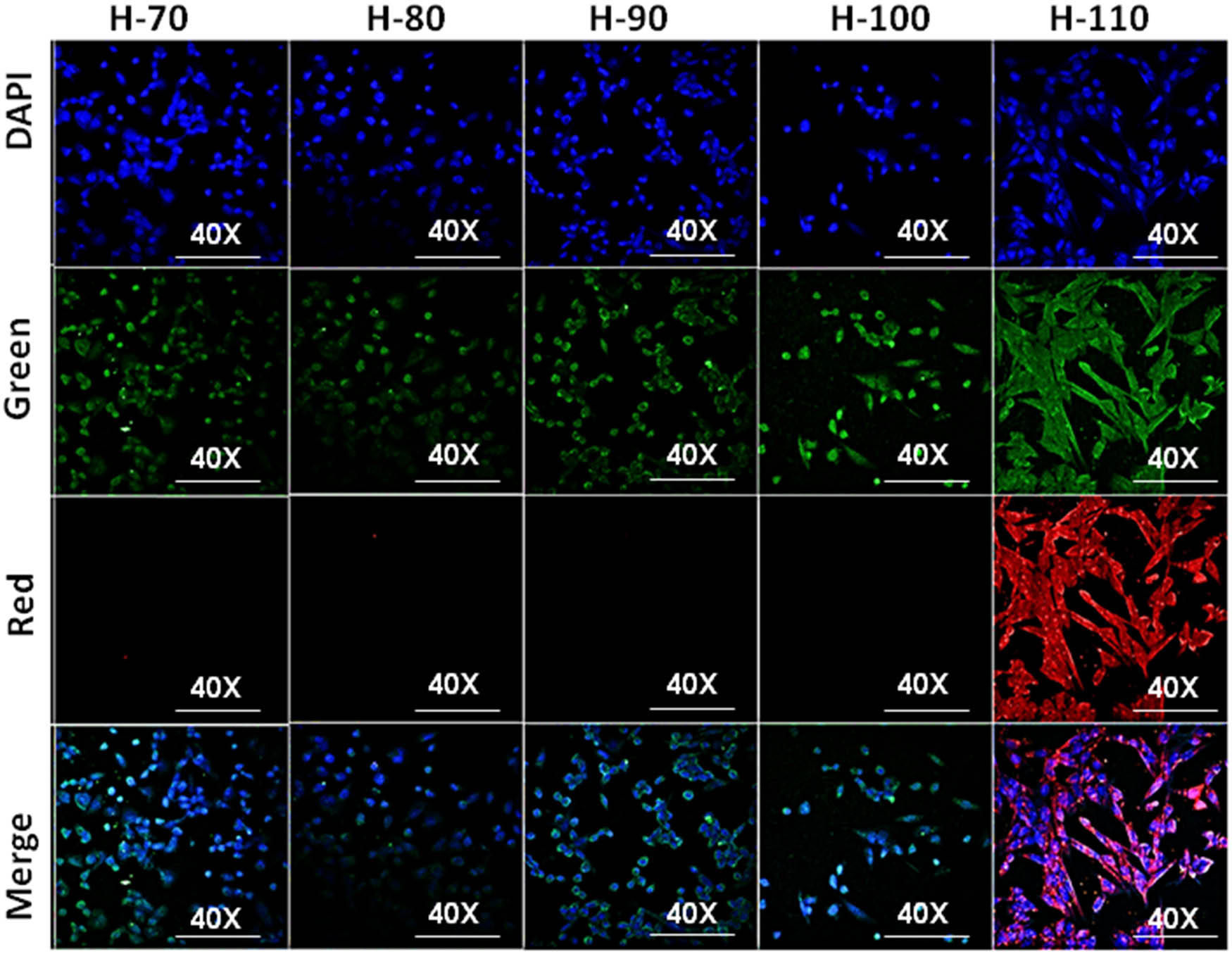
Confocal imaging of cellular uptake of H-60, H-70, H-80, H-90, H-100, and H-110.
3.4.2 Studying the protective role of H-110 on UVB induced cytological damaged primary skin cells
UVB attenuation activity of all the samples is tested and shown in Figure 8(a). All the gel phases have shown a significant reduction in UVB intensity. However, maximum attenuation is shown by H-110 with a density of 5 mg/cm2. The maximum UVB irradiance emitted by the UVB rod was 790 µW/cm2 at 11 cm away from the rod where all the UV attenuation studies were performed. The maximum attenuation is observed by H-110 hydrogel with a 36.7% decrease. Our MTT data have demonstrated aggressive normal skin cell death except UVB treated cells after all three-time points, i.e., 5, 10, and 15 min (P < 0.000, one-way ANOVA, followed by Tukey post-hoc test, Figure 8(b)). Minimum % cell survival was observed after 15 min UVB treatment (29.39 %) in comparison to control skin cells (P < 0.000, one-way ANOVA, followed by Tukey post-hoc test). No significant cell death was observed after 5 min UVB exposure in H-110 applied skin cells in comparison to control skin cells (P = 0.997, one-way ANOVA, followed by Tukey Posthoc test). After 20 and 15 min of UVB exposure, the H-110 applied cells showed 84.06 and 77.76% cell survival. Interestingly, the H-110 layer on skin cells significantly increased skin cell survival at 47.33, 39.09, and 50.36% after respective 5, 10, and 15 min UVB exposure (P < 0.000, one-way ANOVA, followed by Tukey Posthoc test) [52].
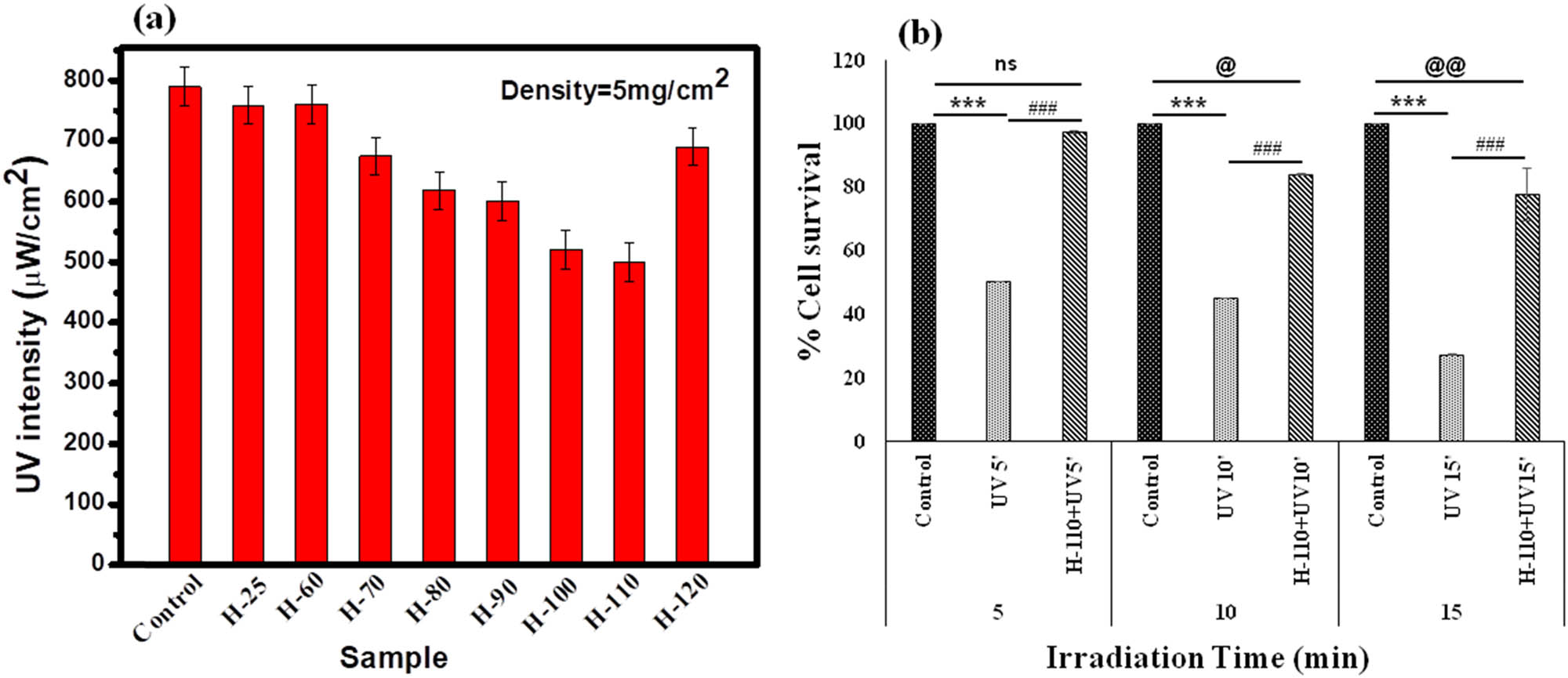
(a) UVB attenuation using different phases of BSA hydrogels, (b) protective role of H-110 from UVB-induced cytological damage primary skin cell culture by % cell viability assay, time-dependent experimental settings (5–15 min). Vertical bars representing mean ± SEM. Significance was tested using one-way ANOVA followed by Tukey Post-hoc test. The symbol * shows the significance level between control and only UVB, # shows the significance level between only UVB and H-110 + UVB, and @ shows the significance level between only control and H-110 + UVB. Data were considered statistically significant as ***/###/@@@ for P < 0.0001, **/##/@@ for P < 0.01, and */#/@ for P < 0.05.
It was observed that the confocal imaging data (Figure 9) are also in the accordance with the results of the MTT assay with the protective role of H-110 after UVB treatment. In our study, with an increase of UVB exposure time to cells from 5 to 15 min, the intensity of emitted red fluorescence decreases. This might be due to the disruption of cellular components with increased time of UVB exposure as the morphology of cells was also changed toward dead cells at 15 min UVB exposed group. Previous studies have reported that the cell partially absorbs the smaller UV wavelength and emit a longer wavelength [52,53]. However, in the case of the H-110 applied group of cells, there was no significant morphological changes were observed after UVB exposure. Hence, this clearly shows that H-110 has the potential to protect the skin cells from UVB exposure.
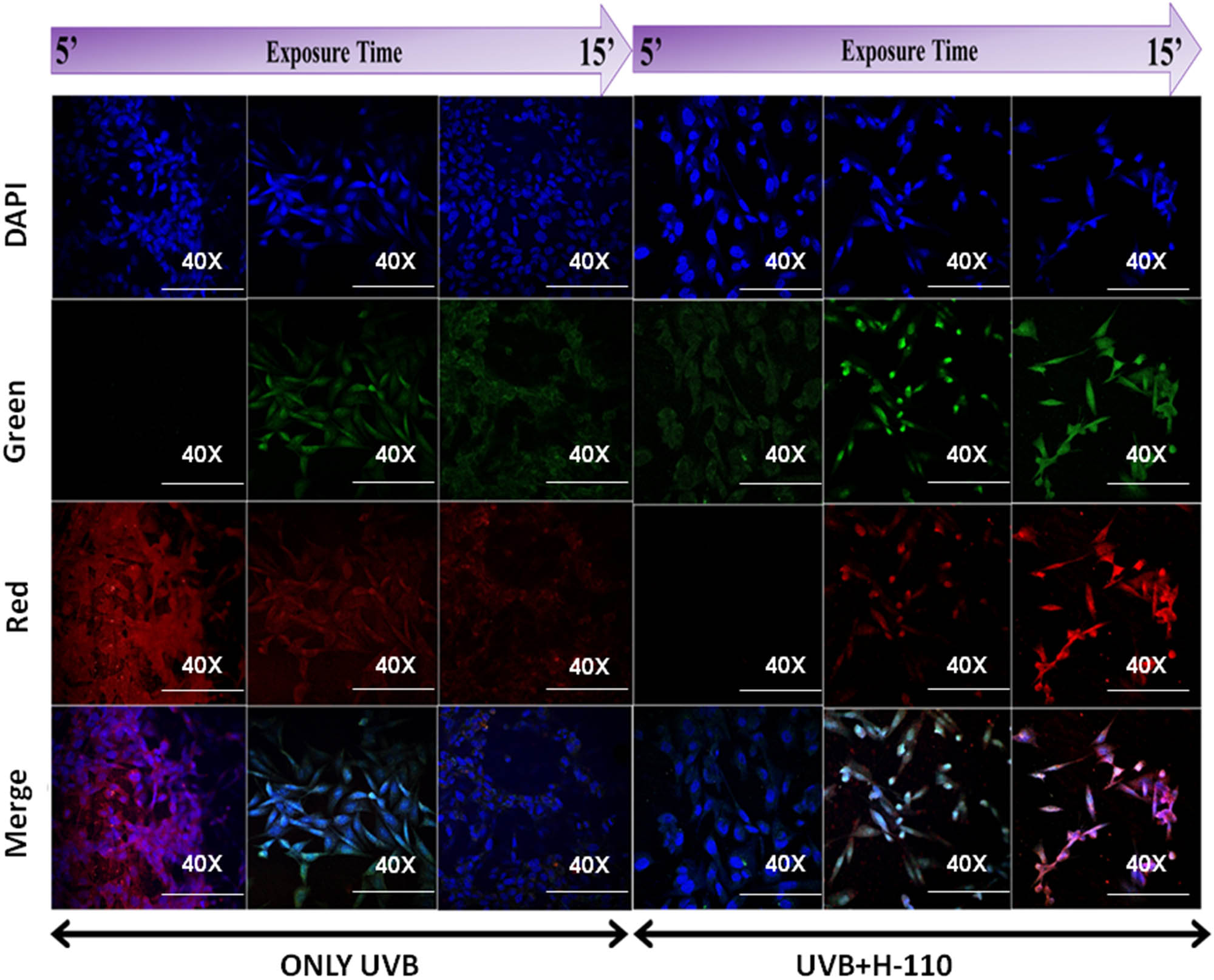
Protective role of H-110 from UVB-induced cytological damage primary skin cell culture by confocal bioimaging, time-dependent experimental settings (5–15 min).
3.4.3 In vivo protective role of H-110 on UVB-induced skin damage in mice
Our histological results with 3 days of UVB exposure (Figure 10(c)) showed no significant change, whereas 5 days of UVB exposure (Figure 10(d)) produced pathomorphological changes in the skin of mice, i.e., significant thickening of stratum corneum (SC) and epidermis (Tep, P < 0.000), and perivascular edema (Ed) in the dermis was prominent. At H-110, the monolayer applied group showed no significant morphometric changes after 3 days of UVB exposure (Figure 10(e)). Interestingly, 5 days of UVB exposure at H-110 monolayer (Figure 10(f)) applied group showed significant epidermal thickening as compared with the control tissues (P < 0.007); however, when the epidermal thickness of H110 + UV5D skin tissues was compared with the epidermal thickness of the UV5D skin tissue, the epiderma was significantly thinner in the H-110 + UV5D skin tissue (P < 0.000, Figure 10(g)). Hence, our mice model study also supports our finding that the topical administration of H-110 is potentially effective in protecting the skin from UVB-induced skin damage.
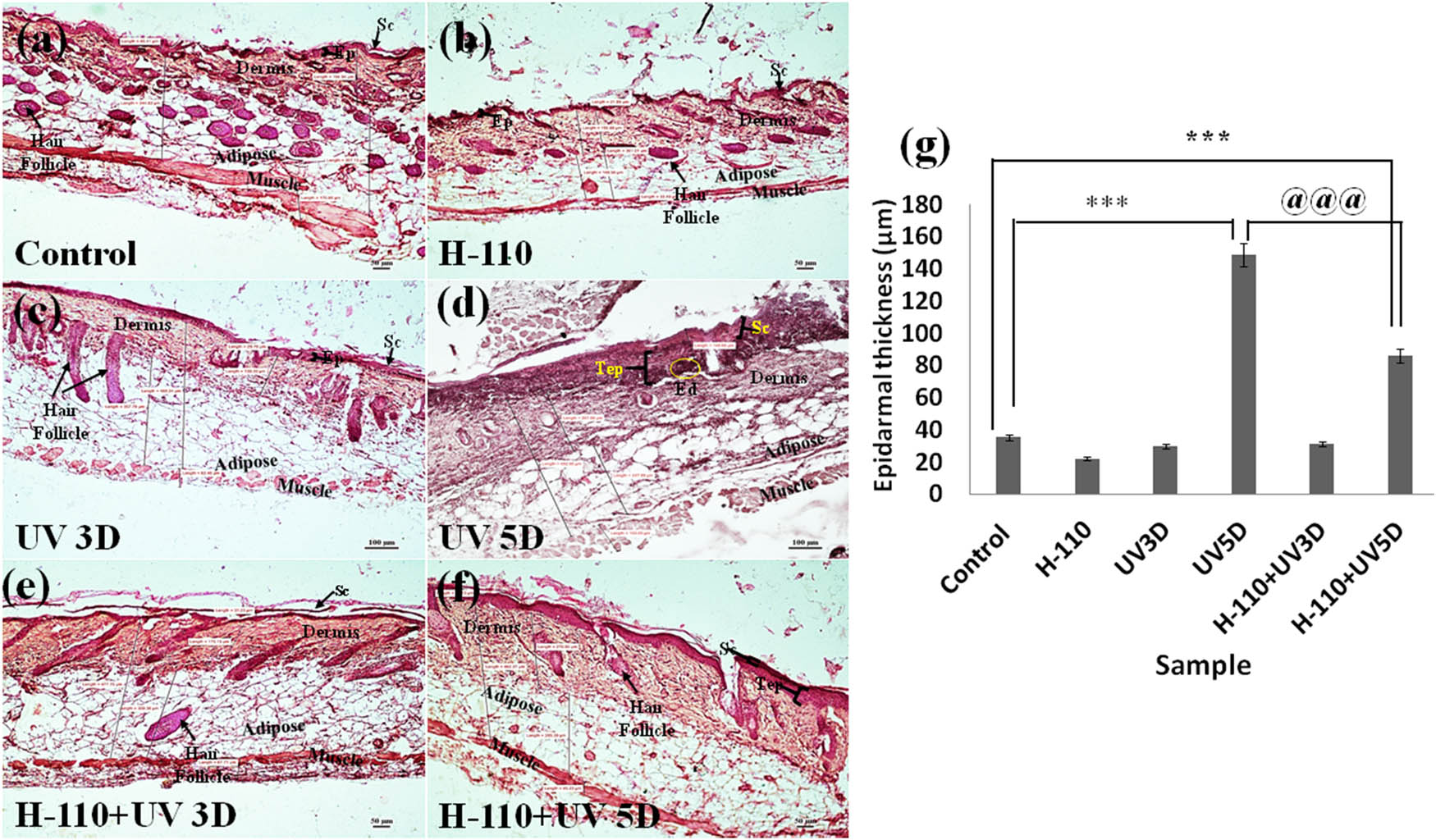
Transverse section showing histoarchitecture of skin tissue of M. musculus: (a) control, (b) H-110 treated, (c) UVB treated for 3 days, (d) UVB treated for 5 days, (e) H-110 + UV treated for 3 days, and (f) H-110 + UV treated for 5 days; Ep represents epidermis, Sc represents stratum corneum, Tep represents thickening epidermis, and Ed represents edema. (g) The vertical bar represents the epidermal thickness of different treatment groups (mean ± SEM). Significance was tested using one-way ANOVA followed by Tukey post-hoc test. The symbol * shows the significance level between control and only UVB 5 days, # shows the significance level between control and H-110 + UVB 5 days, and @ shows the significance level between only UVB 5 days and H-110 + UVB 5 days. Data were considered statistically significant as ***/###/@@@ for P < 0.0001.
4 Conclusion
Using a simple and efficient hydrothermal method, a wide range of phases varying from sol–gel–sol of BSA hydrogel were synthesized. This is the first-of-this-kind of study wherein we observed that even without using cross-linkers, we can synthesize different phases of protein by varying the hydrothermal temperature. Extensive physico-chemical and optical characterization data of synthesized hydrogels has shown its potential application as a UVB blocker for skin protection. To ensure, in vitro cytotoxicity and UV protection ability of hydrogels were tested using primary skin cells. Among all, the H-110 sample has shown extraordinary applicability. Thus, in vivo protective role of H-110 on UVB-induced skin damage in the mice model was studied, and the results have confirmed that the topical administration of H-110 is potentially effective in protecting the skin from UVB-induced skin damages. The present study ensures that using BSA protein as a precursor, it is possible to prepare hydrogels with various functionalities, no cytotoxicity and it holds a prominent role in the biomedical field for the protection of skin and in the cure of skin cancer.
-
Funding information: The authors would like to thank the Department of Science and Technology (SERB), India-CRG/2019/000903 (Core research Grant) & SB/S2/RJN-140/2014 (Ramanujan Fellowship Award) for the financial support of this study.
-
Author contributions: Kanchan Yadav: Conception or design of the work, data collection, data analysis, and interpretation, drafting the article; Megha Das: data collection, data analysis, and interpretation, drafting the manuscript; Nitesh Kumar Mishra: data collection; Anuj Chhabra: data collection, data analysis, and interpretation; Archana Mishra: drafting the manuscript, data analysis and interpretation; Sunita Srivastava: data analysis and interpretation; Poonam Sharma: resource, editing of the manuscript; Sanjeev Kumar Yadav: data analysis and interpretation; Avanish Singh Parmar: conception or design of the work, drafting of the manuscript, funding, and resources. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Ethical approval: All maintenance procedures along with experimentations were performed following the revised Animals Act Government of India, 2007 approved rules [Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Registration No.-1802/GO/Re/S/15/CPCSEA].
References
[1] D’Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14(6):12222–48. 10.3390/ijms140612222.Suche in Google Scholar PubMed PubMed Central
[2] Sanford JA, Gallo RL. Functions of the skin microbiota in health and disease. Semin Immunol. 2013;25(5):370. 10.1016/J.SMIM.2013.09.005.Suche in Google Scholar PubMed PubMed Central
[3] Med CC. Ultraviolet radiation exposure and its impact on skin cancer risk. 2015;40(4):1291–6. 10.1097/CCM.0b013e31823da96d.Hydrogen.Suche in Google Scholar
[4] Teresa M, Petersen S, Prakash G. UV light effects on proteins: from photochemistry to nanomedicine. Mol Photochem – Var Asp. 2012;37947:1–36. 10.5772/37947.Suche in Google Scholar
[5] Harrison SC, Bergfeld WF. Ultraviolet light and skin cancer in athletes. Sports Health. 2009;1(4):335–40. 10.1177/1941738109338923.Suche in Google Scholar PubMed PubMed Central
[6] Craig S, Earnshaw CH, Virós A. Ultraviolet light and melanoma. J Pathol. 2018;244(5):578–85. 10.1002/path.5039.Suche in Google Scholar PubMed
[7] Fontanillas P, Alipanahi B, Furlotte NA, Johnson M, Wilson CH, Pitts SJ, et al. Disease risk scores for skin cancers. Nat Commun. 2021;12(1):1–13. 10.1038/s41467-020-20246-5.Suche in Google Scholar PubMed PubMed Central
[8] Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. 10.3322/CAAC.21654.Suche in Google Scholar
[9] Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med. 2018;379(4):363–74. 10.1056/NEJMRA1708701/SUPPL_FILE/NEJMRA1708701_DISCLOSURES.PDF.Suche in Google Scholar
[10] Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(s61):1–6. 10.1046/J.1365-2133.146.S61.2.X.Suche in Google Scholar PubMed
[11] Xu L, Wu D, Zhou B, Xu Y, Wang W, Yu D, et al. Microencapsulated sunblock nanoparticles based on zeolitic imidazole frameworks for safe and effective UV protection. RSC Adv. 2018;8(22):12315–21. 10.1039/c8ra00632f.Suche in Google Scholar PubMed PubMed Central
[12] Pristovnik M, Maver U, Orthaber K, Skok K, Perić B. Skin cancer and its treatment: novel treatment approaches with emphasis on nanotechnology. J Nanomater. 2017;2017:1–20. 10.1155/2017/2606271.Suche in Google Scholar
[13] Jose J, Netto G. Role of solid lipid nanoparticles as photoprotective agents in cosmetics. J Cosmet Dermatol. 2019;18(1):315–21. 10.1111/jocd.12504.Suche in Google Scholar PubMed
[14] Lu PJ, Fang SW, Cheng WL, Huang SC, Huang MC, Cheng HF. Characterization of titanium dioxide and zinc oxide nanoparticles in sunscreen powder by comparing different measurement methods. J Food Drug Anal. 2018;26(3):1192–200. 10.1016/J.JFDA.2018.01.010.Suche in Google Scholar PubMed PubMed Central
[15] Singh P, Nanda A. Enhanced sun protection of nano-sized metal oxide particles over conventional metal oxide particles: an in vitro comparative study. Int J Cosmet Sci. 2014;36(3):273–83. 10.1111/ICS.12124.Suche in Google Scholar PubMed
[16] Vitiello G, Zanfardino A, Tammaro O, Di Napoli M, Caso MF, Pezzella A, et al. Bioinspired hybrid eumelanin-TiO2 antimicrobial nanostructures: the key role of organo-inorganic frameworks in tuning eumelanin’s biocide action mechanism through membrane interaction. RSC Adv. 2018;8(50):28275–83. 10.1039/c8ra04315a.Suche in Google Scholar PubMed PubMed Central
[17] Wu MS, Sun DS, Lin YC, Cheng CL, Hung SC, Chen PK, et al. Nanodiamonds protect skin from ultraviolet B-induced damage in mice. J Nanobiotechnol. 2015;13(1):1–12. 10.1186/s12951-015-0094-4.Suche in Google Scholar PubMed PubMed Central
[18] Vishnubhakthula S, Elupula R, Durán-Lara EF. Recent advances in hydrogel-based drug delivery for melanoma cancer therapy: a mini review. J Drug Deliv. 2017;2017:1–9. 10.1155/2017/7275985.Suche in Google Scholar PubMed PubMed Central
[19] Rigon RB, Oyafuso MH, Fujimura AT, Gonçalez ML, Prado AH, Gremião MP, et al. Nanotechnology-based drug delivery systems for melanoma antitumoral therapy: a review. Biomed Res Int. 2015;2015:16–20. 10.1155/2015/841817.Suche in Google Scholar PubMed PubMed Central
[20] Tang JQ, Hou XY, Yang CS, Li YX, Xin Y, Guo WW, et al. Recent developments in nanomedicine for melanoma treatment. Int J Cancer. 2017;141(4):646–53. 10.1002/ijc.30708.Suche in Google Scholar PubMed
[21] Vyas A, Das SK, Singh D, Sonker A, Gidwani B, Jain V, et al. Recent nanoparticuulate approches of drug delivery for skin cancer. Trends Appl Sci Res. 2012;7:620–35.10.3923/tasr.2012.620.635Suche in Google Scholar
[22] Chang Z, Zhang S, Li F, Wang Z, Li J, Xia C, et al. Self-healable and biodegradable soy protein-based protective functional film with low cytotoxicity and high mechanical strength. Chem Eng J. 2021;404:126505. 10.1016/J.CEJ.2020.126505.Suche in Google Scholar
[23] Kumar M, Sanford KJ, Cuevas WP, Du M, Collier KD, Chow N. Designer protein-based performance materials. Biomacromolecules. 2006;7(9):2543–51. 10.1021/BM060464A.Suche in Google Scholar
[24] Debele TA, Su WP. Polysaccharide and protein-based functional wound dressing materials and applications. 2020;71:87–108. 10.1080/00914037.2020.1809403.Suche in Google Scholar
[25] Zhang D, Wang Y. Functional protein-based bioinspired nanomaterials: from coupled proteins, synthetic approaches, nanostructures to applications. Int J Mol Sci. 2019;20(12):3054. 10.3390/IJMS20123054.Suche in Google Scholar
[26] Mahler A, Reches M, Rechter M, Cohen S, Gazit E. Rigid, Self-assembled hydrogel composed of a modified aromatic dipeptide. Adv Mater. 2006;18(11):1365–70. 10.1002/ADMA.200501765.Suche in Google Scholar
[27] Nagarajan S, Radhakrishnan S, Kalkura S, Balme S, Miele P, Bechelany M. Overview of protein-based biopolymers for biomedical application. Macromol Chem Phys. 2019;220(14):1900126. 10.1002/MACP.201900126.Suche in Google Scholar
[28] Xing Y, Cheng E, Yang Y, Chen P, Zhang T, Sun Y, et al. Self-assembled dna hydrogels with designable thermal and enzymatic responsiveness. Adv Mater. 2011;23(9):1117–21. 10.1002/adma.201003343.Suche in Google Scholar PubMed
[29] Arabi SH, Aghelnejad B, Schwieger C, Meister A, Kerth A, Hinderberger D. Biomaterials science rsc.li/biomaterials-science serum albumin hydrogels in broad pH and temperature ranges: characterization of their self-assembled structures and nanoscopic and macroscopic properties. Biomater Sci. 2018;6:478. 10.1039/c7bm00820a.Suche in Google Scholar PubMed
[30] Su TJ, Lu JR, Thomas RK, Cui ZF, Penfold J. The conformational structure of bovine serum albumin layers adsorbed at the silica−water interface. J Phys Chem B. 1998;102(41):8100–8. 10.1021/JP981239T.Suche in Google Scholar
[31] Jayabharathi J, Thanikachalam V, Venkatesh Perumal M. Mechanistic investigation on binding interaction of bioactive imidazole with protein bovine serum albumin—a biophysical study. Spectrochim Acta Part A Mol Biomol Spectrosc. 2011;79(3):502–7. 10.1016/J.SAA.2011.03.020.Suche in Google Scholar PubMed
[32] Wu X, Lv L, Han X, Li C. Bovine serum albumin fibrous biofilm template synthesis of metallic nanomeshes for surface-enhanced Raman scattering and electrocatalytic detection. Mater Des. 2020;192:108777. 10.1016/J.MATDES.2020.108777.Suche in Google Scholar
[33] Yuan H, Zheng X, Liu W, Zhang H, Shao J, Yao J, et al. A novel bovine serum albumin and sodium alginate hydrogel scaffold doped with hydroxyapatite nanowires for cartilage defects repair. Colloids Surfaces B Biointerfaces. 2020;192:111041. 10.1016/J.COLSURFB.2020.111041.Suche in Google Scholar
[34] Zewde B, Atoyebi O, Gugssa A, Gaskell KJ, Raghavan D. An investigation of the interaction between bovine serum albumin-conjugated silver nanoparticles and the hydrogel in hydrogel nanocomposites. ACS Omega. 2021;6(17):11614–27. 10.1021/ACSOMEGA.1C00834/SUPPL_FILE/AO1C00834_SI_001.PDF.Suche in Google Scholar
[35] Upadhyay A, Narula A, Rao CP. Copper-based metallogel of bovine serum albumin and its derived hybrid biomaterials as aerogel and sheet: comparative study of the adsorption and reduction of dyes and nitroaromatics. ACS Appl Bio Mater. 2020;3(12):8619–26. 10.1021/ACSABM.0C01028/SUPPL_FILE/MT0C01028_SI_001.PDF.Suche in Google Scholar
[36] Phan VHG, Le TMD, Janarthanan G, Ngo PKT, Lee DS, Thambi T. Development of bioresorbable smart injectable hydrogels based on thermo-responsive copolymer integrated bovine serum albumin bioconjugates for accelerated healing of excisional wounds. J Ind Eng Chem. 2021;96:345–55. 10.1016/J.JIEC.2021.01.041.Suche in Google Scholar
[37] Henrot P, Laurent P, Levionnois E, Leleu D, Pain C, Truchetet ME, et al. A method for isolating and culturing skin cells: application to endothelial cells, fibroblasts, keratinocytes, and melanocytes from punch biopsies in systemic sclerosis skin. Front Immunol. 2020;11:2408. 10.3389/FIMMU.2020.566607.Suche in Google Scholar PubMed PubMed Central
[38] Yadav K, Das M, Hassan N, Mishra A, Lahiri J, Dubey A, et al. Synthesis and characterization of novel protein nanodots as drug delivery carriers with an enhanced biological efficacy of melatonin in breast cancer cells. RSC Adv. 2021;11(16):9076–85. 10.1039/D0RA08959A.Suche in Google Scholar PubMed PubMed Central
[39] Shukla D, Das M, Kasade D, Pandey M, Dubey AK, Yadav SK, et al. Sandalwood-derived carbon quantum dots as bioimaging tools to investigate the toxicological effects of malachite green in model organisms. Chemosphere. 2020;248:125998. 10.1016/j.chemosphere.2020.125998.Suche in Google Scholar PubMed
[40] Jiang B, Jain A, Lu Y, Hoag SW. Probing thermal stability of proteins with temperature scanning viscometer. Mol Pharm. 2019;16(8):3687–93. 10.1021/ACS.MOLPHARMACEUT.9B00598.Suche in Google Scholar PubMed
[41] Michnik A, Michalik K, Drzazga Z. Stability of bovine serum albumin at different pH. J Therm Anal Calorim. 2005;80(2):399–406. 10.1007/S10973-005-0667-9.Suche in Google Scholar
[42] Molodenskiy D, Shirshin E, Tikhonova T, Gruzinov A, Peters G, Spinozzi F. Thermally induced conformational changes and protein–protein interactions of bovine serum albumin in aqueous solution under different pH and ionic strengths as revealed by SAXS measurements. Phys Chem Chem Phys. 2017;19(26):17143–55. 10.1039/C6CP08809K.Suche in Google Scholar
[43] Ma X, Sun X, Hargrove D, Chen J, Song D, Dong Q, et al. A biocompatible and biodegradable protein hydrogel with green and red autofluorescence: preparation, characterization and in vivo biodegradation tracking and modeling. Sci Rep. 2016;6(1):1–12. 10.1038/srep19370.Suche in Google Scholar PubMed PubMed Central
[44] Arabi SH, Aghelnejad B, Schwieger C, Meister A, Kerth A, Hinderberger D. Serum albumin hydrogels in broad pH and temperature ranges: characterization of their self-assembled structures and nanoscopic and macroscopic properties. Biomater Sci. 2018;6(3):478–92. 10.1039/C7BM00820A.Suche in Google Scholar
[45] Shibayama M, Tanaka T, Han CC. Small angle neutron scattering study on poly(N‐isopropyl acrylamide) gels near their volume‐phase transition temperature. J Chem Phys. 1998;97(9):6829. 10.1063/1.463636.Suche in Google Scholar
[46] Hule RA, Nagarkar RP, Altunbas A, Ramay HR, Branco MC, Schneider JP, et al. Correlations between structure, material properties and bioproperties in self-assembled β-hairpin peptide hydrogels. Faraday Discuss. 2008;139:251. 10.1039/B717616C.Suche in Google Scholar
[47] Surita S, Lackey MA, Griffin DM, Kishore S, Tew GN, Bhatia SR. SANS study of highly resilient poly(ethylene glycol) hydrogels. Soft Matter. 2014;10(12):1905–16. 10.1039/C3SM52395K.Suche in Google Scholar PubMed PubMed Central
[48] Amdursky N, Mazo MM, Thomas MR, Humphrey EJ, Puetzer JL, St-Pierre JP, et al. Elastic serum-albumin based hydrogels: mechanism of formation and application in cardiac tissue engineering. J Mater Chem B. 2018;6(35):5604–12. 10.1039/C8TB01014E.Suche in Google Scholar PubMed PubMed Central
[49] Bodenberger N, Kubiczek D, Abrosimova I, Scharm A, Kipper F, Walther P, et al. Evaluation of methods for pore generation and their influence on physio-chemical properties of a protein based hydrogel. Biotechnol Rep. 2016;12:6–12. 10.1016/J.BTRE.2016.09.001.Suche in Google Scholar
[50] De Maria S, Ferrari G, Maresca P. Rheological characterization bovine serum albumin gels induced by high hydrostatic pressure. Food Nutr Sci. 2015;6(9):770–9. 10.4236/FNS.2015.69080.Suche in Google Scholar
[51] Chen J, Ma X, Dong Q, Song D, Hargrove D, Vora SR, et al. Self-healing of thermally-induced, biocompatible and biodegradable protein hydrogel. RSC Adv. 2016;6(61):56183–92. 10.1039/C6RA11239K.Suche in Google Scholar
[52] Allen M. Post hoc tests: tukey honestly significant difference test. SAGE Encycl Commun Res Methods. 2017;4:1–4. 10.4135/9781483381411.N452.Suche in Google Scholar
[53] Notara M, Behboudifard S, Kluth MA, Maßlo C, Ganss C, Frank MH, et al. UV light-blocking contact lenses protect against short-term UVB-induced limbal stem cell niche damage and inflammation. Sci Rep. 2018;8(1):1–12. 10.1038/S41598-018-30021-8.Suche in Google Scholar PubMed PubMed Central
© 2022 Kanchan Yadav et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption

