Abstract
To date, the encapsulation of therapeutic enzymes in a protective matrix is an optimized strategy for the maintenance of their stability, facilitating their clinical application. However, the stability and activity of therapeutic enzymes are often in tension with each other. A rigid protective matrix may effectively maintain the stability of therapeutic enzymes, but it can reduce the diffusion of substrates toward the therapeutic enzyme active site, dramatically affecting their catalytic efficiency. Here, we exploited a kind of nanogels by in situ polymerization on the arginine deiminase (ADI) surface with 3-acrylamido-phenylboronic acid (APBA) monomer. These nanogels efficiently improved the thermal stability (25–75℃), the pH stability (pH 1–13), and protease (trypsin) stability of ADI due to the strong rigidity of the surface poly(APBA) shell. And even after 60 days of storage, ∼60% of the activity of ADI encapsulated by nanogels remained. Furthermore, ADI encapsulated by nanogels could efficiently degrade arginine to increase the ratio of citrulline to arginine in mice plasma. That is because autologous glucose binds with APBA leading to the hydrophilicity increase of nanogels, and then, the arginine molecules can readily diffuse toward the encapsulated ADI. This nanogel platform eases the tension between the stability and activity of therapeutic enzymes. The resulting nanogels can efficiently maintain the in vitro stability and the in vivo activity of therapeutic enzymes, facilitating the exploitation of new therapeutic enzyme formulations, which can be transported and stored in vitro for a long time and be applied effectively in vivo.
1 Introduction
Therapeutic enzymes have been applied to clinical practice for at least 60 years [1]. As an example, De Duve [2] has first reported that a therapeutic enzyme can be a part of replacement therapies for genetic deficiencies. To date, they have been used to treat various diseases, such as COVID‐19 and cancers [3,4,5,6]. Compared with other kinds of drugs, such as nucleic acid drugs [7], therapeutic enzymes can specially bind and efficiently act on target molecules. Furthermore, the target molecules of therapeutic enzymes are often multiple, endowing them with a broad spectrum of therapeutic ability [8]. Therapeutic enzymes have been used to treat various disorders that small molecules cannot treat. As an example, arginine deiminase (ADI) is a kind of therapeutic enzyme used for arginine (Arg) deprivation that can efficiently suppress argininosuccinate synthetase (ASS1)-deficient tumors. Under normal circumstances, Arg is a kind of nonessential amino acid since normal cells can convert citrulline (Cit) to Arg via the catalysis of ASS1, while ASS1-deficient tumor cells must rely on exogenous Arg to meet the needs of growth and proliferation. Therefore, ASS1-deficient tumors can be efficiently suppressed by ADI-based degradation of the Arg in the blood. However, therapeutic enzymes are proteins with complex and fragile three-dimensional structures, which are easily destroyed by high temperature, pH, organic solvents, or proteases [9,10,11]. The low stability of therapeutic enzymes would limit their in vitro transportation and storage and reduce their in vivo catalytic efficiency [12,13]. The enhancement of therapeutic enzyme stability, therefore, is extremely necessary to realize their efficient clinical application.
To improve the stability of therapeutic enzymes under harsh conditions, numerous strategies have been applied. Thereinto, the conjugation of polyethylene glycol (PEG) to therapeutic enzymes (PEGylation) is one of the most successful strategies to maintain their stability [14]. However, the reduced bioactivity of therapeutic enzymes caused by the random chemical coupling greatly limited their practical application [15]. Besides, immobilization of therapeutic enzymes on scaffolds, which can achieve stabilization effects to maintain the three-dimensional structure of therapeutic enzymes, is used to improve their stability [16]. However, therapeutic enzymes immobilized on macroscaffolds can only be used locally, limiting their application scope [17]. Microscaffolds, such as organic/inorganic nanoparticles, can be applied for the immobilization of therapeutic enzymes realizing their systemic and local application, but the structural stability and catalytic activity of therapeutic enzymes are strongly affected by the physicochemical properties of biointerfaces, such as porosity, surface curvature, and their heterogeneity [18,19,20,21,22]. Immobilization of therapeutic enzymes often results in the lower catalytic activity because the structure of the therapeutic enzyme is easily destroyed [22]. Encapsulation of therapeutic enzymes in a protective matrix, by comparison, is an optimized method to maintain their stability. For example, Zhang et al. have reported a therapeutic enzyme encapsulation technology that is based on a zwitterionic polymer network, efficiently enhancing their stability, improving their pharmacokinetics, and mitigating the immune response [23]. Taking advantage of the rigidity of the protective matrix to maintain the three-dimensional conformation of therapeutic enzymes is the fundamental mechanism of encapsulation methods [24]. Theoretically, the more rigid the protective matrix is, the stronger the stability of therapeutic enzymes is. However, their activity and stability are commonly in tension with each other. A rigid matrix layer surrounding the therapeutic enzymes may effectively maintain their structure, but it can reduce the diffusion of substrates, dramatically affecting their catalytic efficiency [25,26]. Therefore, how to address the tension between the stability and activity of therapeutic enzymes is a huge challenge for therapeutic enzyme encapsulation technologies.
As shown in Scheme 1, we used ADI as a model therapeutic enzyme, and the in situ polymerization on ADI surface with 3-acrylamido-phenylboronic acid (APBA), N-(3-aminopropyl)methacrylamide (APM), and N,N′-methylene-bis(acrylamide) (BIS) was adopted to exploit a kind of nanogel (denoted as P-n(ADI)). To further endow P-n(ADI) with the high in vivo application capacity, it was combined with PEG to form PEGylated P-n(ADI) (denoted as PP-n(ADI)). These nanogels showed strong rigidity in vitro because of the hydrophobicity of poly(APBA) [27]. The rigid polymeric shell could efficiently restrain conformational changes in ADI, dramatically maintaining the in vitro stability of ADI. Once inside the body, APBA molecules located at the surface of PP-n(ADI) could bind with autologous glucose increasing the hydrophilicity and flexibility of the poly(APBA) shell [28]. PP-n(ADI) bound with glucose was denoted as PP-n(ADI)/Glucose. The concentration of glucose was 5 mM similar to the physiological level of glucose unless otherwise mentioned. Then, the Arg molecules could readily diffuse toward the encapsulated ADI, efficiently maintaining the catalytic activity of ADI. Furthermore, the flexible poly(APBA) shell can still protect ADI from degradation and improve its in vivo stability. This kind of nanogel could facilitate the exploitation of new therapeutic enzyme formulations, which can be transported and stored in vitro for a long time and be applied effectively in vivo.

The mechanism for the preparation and action of nanogels. (a) APBA, APM, and BIS were added to ADI solution and they could enrich around ADI molecules because of the hydrogen bond and hydrophobic interaction between them. Then, P-n(ADI) was formed by initiating the free radical polymerization of monomers and crosslinkers. mPEG-ALD was linked to P-n(ADI) to form PP-n(ADI). (b) The polymeric shell was hydrophobic in vitro, efficiently maintaining the stability of ADI. Glucose bound with APBA molecules can locate at the polymeric shell leading to the loosening of nanogels and the restoration of the catalytic activity of ADI. In addition, the loosened polymeric shell still had protective effects.
2 Experimental section
2.1 Materials
Rough extracted ADI was purchased from Baiaolaibo Technology Co., Ltd (Beijing, China). Methoxy PEG propionaldehyde (mPEG-ALD, MW 5 kDa) was provided by Jenkem Technology Co., Ltd (Beijing, China). Bicinchoninic acid (BCA) protein assay kit, Sephadex G-100, and Cell Counting Kit-8 (CCK-8) were supplied by Solarbio (Beijing, China). Amino acid assay kits were bought from BioVision Incorporated. Fluorescein isothiocyanate (FITC) isomer was purchased from Yuanye Biological Technology Co., Ltd (Shanghai, China). Annexin V-FITC/PI cell apoptosis assay kit was bought from Meilunbio Co., Ltd (Dalian, China). Other unindicated chemical reagents were purchased from Sigma.
2.2 The PP-n(ADI) synthesis and its characteristics
PP-n(ADI) was synthesized according to the previous method with slight modification [29]. Crude enzyme extract was filtered by Sephadex G-100 to obtain pure ADI, which was further quantified by a BCA assay kit. To facilitate the encapsulation, ADI (10 mg/mL) was first mixed with N-hydroxysuccinimide ester (the molar ratio was 1:20) at 4℃ for 24 h, and the acrylated ADI was purified by dialyzing against phosphate-buffered saline (PBS). The acrylated ADI (1 mg/mL) was mixed with APM, APBA, and BIS (the molar ratio was 1:300:3,000:300). The polymerization was initiated by taking advantage of ammonium persulfate as the initiator and N,N,N′,N′-tetramethylethylenediamine as the catalyst, and it was carried out under the protection of nitrogen at 4°C for 2 h. The resulting P-n(ADI) was purified by dialyzing against PBS and using a hydrophobic interaction column (Phenyl-Sepharose CL-4B). The ADI loading capacity was determined by a BCA assay kit. To further prepare PP-n(ADI), mPEG-ALD was mixed with P-n(ADI) in the alkaline buffer overnight. The molar ratio of mPEG-ALD to P-n(ADI) (by ADI mass) was 20. The uncoupled PEG was removed by dialyzing against PBS. Bovine serum albumin (BSA) could be used as an ADI substitute, and PP-n(BSA) could be prepared as the above-mentioned method.
The size distribution and zeta potential of samples were measured by the dynamic light scattering (DLS) technique. One milligram per milliliter of samples were measured by DLS, and it was repeated three times. A transmission electron microscope (TEM) was applied to assess the morphologies of samples (0.5 mg/mL) that were prestained with phosphotungstic acid (3%). Native ADI, P-n(ADI), PP-n(ADI), and PP-n(ADI)/glucose (1 mg/mL, by ADI mass) were labeled with FITC and analyzed via agarose gel electrophoresis. The molar ratio of FITC to samples was 5. Gel running electrophoresis was conducted for 30 min on agarose gel (1%). One milligram per milliliter of samples were lyophilized, and they were measured by the Fourier transform infrared spectroscopy (FT-IR) analysis. The UV–visible absorption of sample solutions (0.5 mg/mL) was measured using a spectrophotometer.
To test the surface hydrophobicity of samples, the static water contact angles of samples were measured. Briefly, 1 mg/mL of samples were added onto the surface of a silicon slice, and N2 was used to dry the surface solution forming a film. Then, we added a drop of water onto the film and recorded the static water contact angle.
2.3 The test of samples’ specific activities
The specific activities of samples were measured by an ELISA kit. About 0.1 mg/mL of samples were coincubated with Arg (50 mM) at 37℃, and the process lasted 10 min. Then, an Arg assay kit was applied to quantify the residual Arg, and the enzymatic activity was determined according to the definition. The specific activity was defined as the formula: specific activity (IU/mg) = Enzyme activity/Enzyme concentration.
Then, the specific activities of native ADI and PP-n(ADI), which were preincubated with the same volume of fresh mice serum at 37°C, were tested every 10 days, and it was continued for 60 days. Furthermore, to verify the protective effects of nanogels on therapeutic enzymes, native ADI and PP-n(ADI) were incubated with or without protease (trypsin, 2.0 μM) at 37°C for a day, and their enzymatic activities were also tested. In addition, the specific activities of native ADI and PP-n(ADI), which were stored at different temperatures or in solutions with different pH values, were also tested.
2.4 The glucose responsiveness of samples
To test the glucose-responsive activity of samples, a certain amount of native ADI and PP-n(ADI) was incubated with Arg solution (100 mM) at 37℃, and the concentrations of residual Arg were measured by an Arg assay kit every 1 min. The process lasted for 10 min. Furthermore, the enzyme activities of PP-n(ADI) that was incubated with glucose solution (1, 6, and 10 mM) and an equal volume of serum were also tested according to the above-mentioned methods.
2.5 The cytotoxicity of nanogels
Human umbilical vein endothelial cells (HUVECs) were used to test the cytotoxicity by a CCK-8. Cells were inoculated into a 96-well plate and cultured in a cell incubator for 24 h. Then, fresh medium that contained different concentrations of PP-n(BSA) was employed to replace the culture medium, and untreated cells were used as the control. Each group had six replicates. After 48 h of culture, we added CCK-8 solution to each well, and the absorbance at 450 nm of each well was measured. The cell viability (%) = [absorbance (test well)450 nm − absorbance (blank well)450 nm]/[absorbance (control well)450 nm − absorbance (blank well)450 nm] × 100 [30].
2.6 Cell viability assay
Michigan Cancer Foundation-7 (MCF-7) cells were used as target cells to evaluate the cell suppression effects of samples by using a CCK-8. MCF-7 cells (5,000 cells per well) were inoculated into a 96-well plate and cultured in a cell incubator for 24 h. Then, the medium was replaced with a fresh one that contained different concentrations of samples. At 48 h, we added CCK-8 solution into each well, and the cell viability was assessed. Cells without the treatment of samples were used as the control, and the cell viability (%) = [absorbance (test well)450 nm − absorbance (blank well)450 nm]/[absorbance (control well)450 nm − absorbance (blank well)450 nm] × 100 [30]. Furthermore, the apoptosis of MCF-7 cells induced by samples was measured by using AnnexinV-FITC/PI double staining.
2.7 The intracellular activity of PP-n(ADI)
The ratio of Cit to Arg in cells that were treated with samples was tested. Briefly, MCF-7 cells were inoculated into a six-well plate and cultured in a cell incubator for 24 h. Then, different concentrations of samples were used to treat cells. The treated cells were washed and detached with trypsin after 24 h. We further added distilled water to disperse the cells, and the cells were vortexed and centrifuged for 30 min at 12,000 rpm. Finally, we collected the resulting supernatant and used the corresponding assay kits to quantify the Arg and Cit in cells.
2.8 The blood circulation of samples
Similar to what we did before [31], we randomly divided healthy Kunming mice into two groups, and each group had six mice. FITC-labeled native ADI and PP-n(ADI) (∼1 mg by ADI mass) were intravenously single-dose injected into the corresponding mice. After the injection, we collected the mice blood at selected time points and obtained mice plasma by gentle centrifugation. Finally, a microplate reader was used to measure the fluorescence intensity of the dilute plasma.
2.9 The in vivo activity of PP-n(ADI)
We randomly divided healthy Kunming mice into two groups, and each group had six mice. Samples (∼1 mg by ADI mass) were intravenously injected into the corresponding mice once. After the injection, we cut the mice tail to collect mice blood at certain time points and centrifuged the blood to obtain mice plasma. The Cit and Arg in fresh mice plasma were quantified by the assay kits.
2.10 The biological safety of samples
We randomly divided healthy Kunming mice into two groups, and each group had six mice. According to our previous study, samples (∼1 mg by ADI mass) were intravenously injected into the corresponding mice once [32]. One week after injection, we euthanized the mice and obtained their main organs, which were further stored overnight in paraformaldehyde solution (4%). Then, organs were carefully washed twice with buffer solution and embedded in paraffin. Finally, we section the embedded organs and stained the tissue sections with hematoxylin–eosin (H&E), and the resulting H&E staining tissue sections were observed under a microscope.
2.11 Statistical analysis
Statistical comparisons were achieved using GraphPad Prism 7.04 software. Results are presented as mean ± SEM.
3 Results and discussion
The morphology and diameter of prepared nanogels were characterized as shown in Figure 1. The diameter of P-n(ADI) (16.58 ± 5.18 nm) was significantly larger than that of native ADI (6.04 ± 1.34 nm), implying the formation of poly(APBA) shell on the ADI surface, but it should be noted that not all ADI was encapsulated into P-n(ADI), and the loading efficiency was 87.67 ± 5.29%. The coupling of PEG to P-n(ADI) further increased in size to 22.58 ± 7.68 nm. Furthermore, the diameter of PP-n(ADI)/glucose was 23.20 ± 6.70 nm, which was nearly not different from that of PP-n(ADI). Theoretically, the formation of polymeric shells on ADI and the further coupling of PEG could gradually increase the size of nanoparticles. Therefore, the size changes implied that the preparation process met our design expectations. PP-n(ADI) potentially improved the in vivo application efficiency of ADI because nanogels avoided the removal of ADI by the kidney, which often metabolically cleared drugs smaller than 10 nm [33]. The representative TEM image of samples (the inset picture in Figure 1) presented that they were both spherical shapes and had homogeneous dispersion implying the protection of nanogels to ADI. It had been proved that nanomedicines possessed optimal in vivo performances including long blood circulation time, low immunogenicity, and excellent targeting ability should be with the appropriate size (10–100 nm) and regular structure [34]. Therefore, the above results demonstrated that nanogels could potentially be used as excellent delivery vectors for ADI.
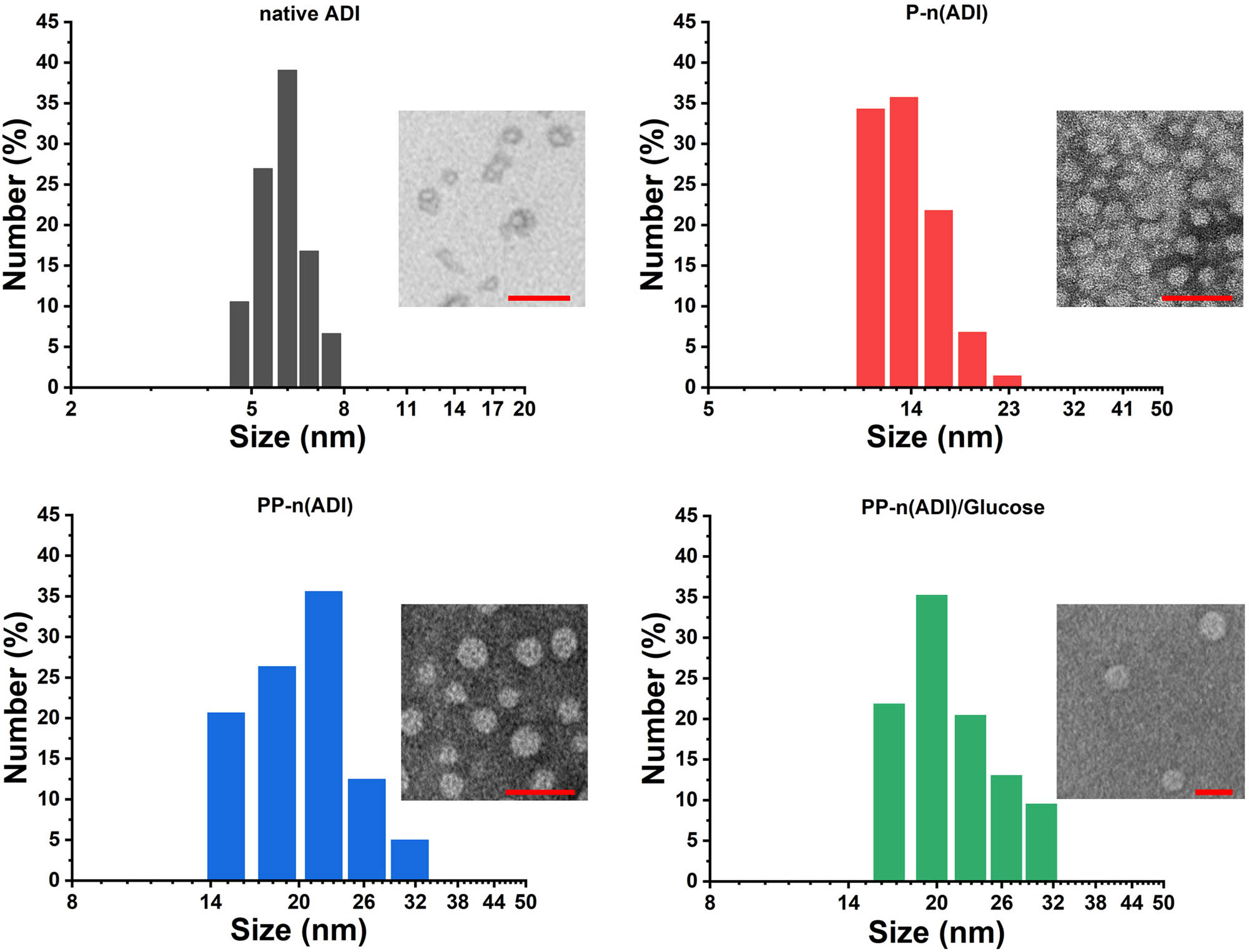
The size distribution of samples. The inset pictures were the corresponding TEM images. The scale bar was 50 nm.
As shown in the agarose gel electrophoresis analysis (Figure 2a), FITC-labeled native ADI moved toward positive electrodes because of its electronegativity (Figure 2b). After encapsulation, the zeta potentials of P-n(ADI) and PP-n(ADI) became positive, and therefore, they both moved toward negative electrodes. The high zeta potential of polymeric nanoparticles could result in their rapid in vivo clearance. For example, it had been reported that micelles (25 ± 6 nm) with a zeta potential of 37.0 ± 2.9 mV were mostly accumulated in liver tissues [35]. On the contrary, the accumulation of micelles (27 ± 6 nm) with a zeta potential of 3.6 ± 0.8 mV in liver tissues was much lower. Therefore, although the surface potential of PP-n(ADI) was slightly positive (2.3 ± 1.1 mV), it would not result in the nonspecific clearance of PP-n(ADI) in animals. The surface components and structure of samples were further characterized. For P-n(ADI), the appearance of new peaks (1426.74, 1335.10, and 709.85 cm−1) in the FT-IR spectrum was attributed to the phenylboronic acid (Figure 2c) [36], indicating the successful formation of poly(APBA) shell on the surface of ADI. New peaks at 2881.62, 1103.03, 959.46, and 846.36 cm−1 were shown in the spectrum of PP-n(ADI), indicating the successful coupling of PEG to P-n(ADI) [37]. Glucose can be bound to PP-n(ADI) since the spectrum exhibited the typical peaks of glucose at 1427.63 cm−1. The binding of glucose to PP-n(ADI) could potentially prolong its circulation time since the autologous glucose was not perceived as a foreign material by the immune system. Furthermore, the autologous glucose may endow PP-n(ADI) with the ability to target lesions due to the associated diseases consuming a lot of glucose. As an example, glucose could be conjugated to nanomedicines realizing the specific targeting and subsequent treatment of cancers [38]. UV-visible spectrum further proved that the polymeric shells could efficiently shield ADI. Native ADI as a kind of protein showed the characteristic peak at 280 nm. On the contrary, the formation of a polymeric shell on ADI shielded this characteristic peak (Figure 2d).
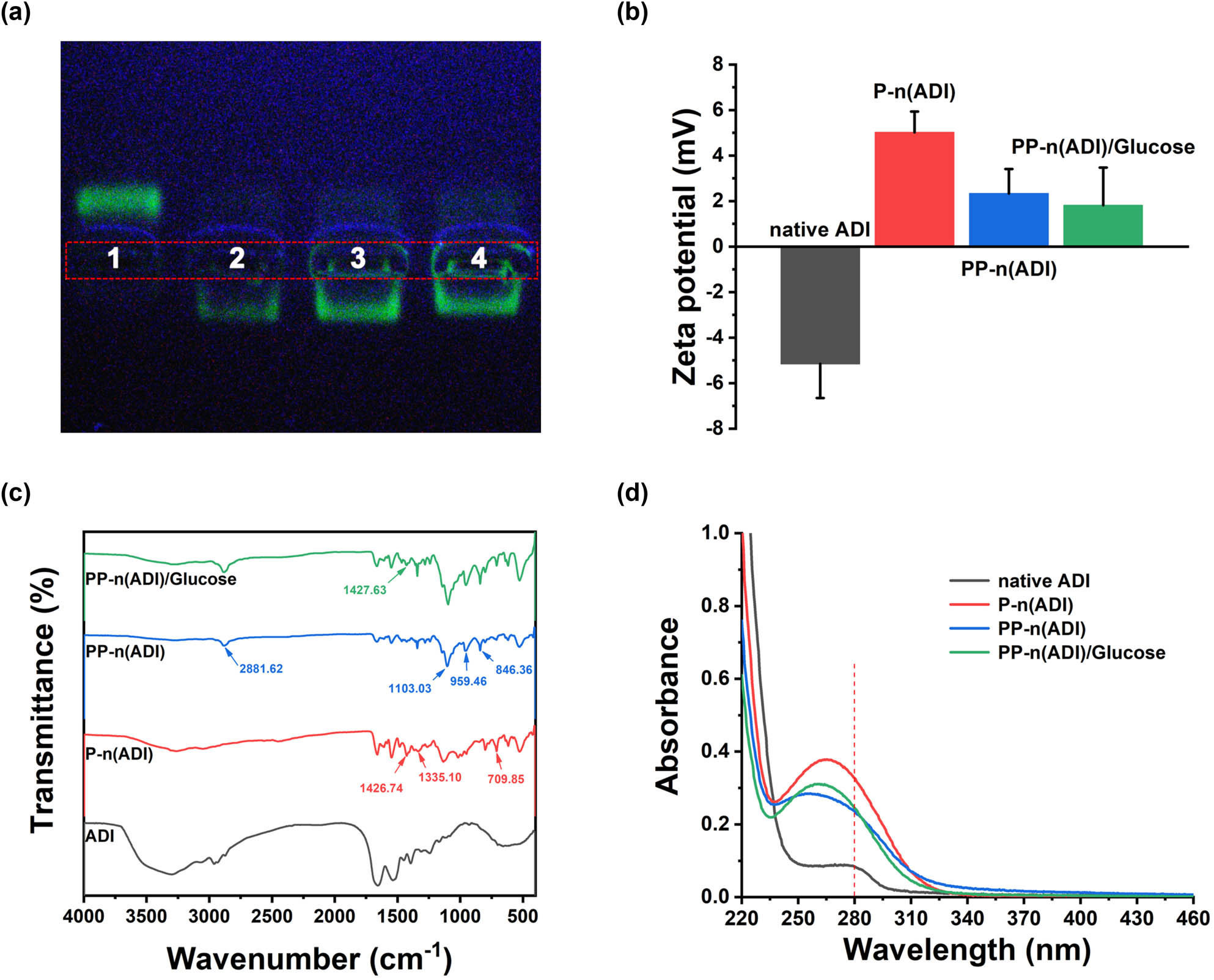
(a) Native ADI (1), P-n(ADI) (2), PP-n(ADI) (3), and PP-n(ADI)/Glucose (4) were analyzed by agarose gel electrophoresis. (b) Zeta potential of corresponding samples. (c) FT-IR spectra of corresponding samples. (d) The UV-visible spectrum of corresponding samples.
The water contact angle of the films that were respectively formed by native ADI, P-n(ADI), PP-n(ADI), and PP-n(ADI)/Glucose was measured. As shown in Figure 3, the water contact angle of the film formed by P-n(ADI) was 40.0 ± 3.1°, while that of the film formed by native ADI was 35.5 ± 2.4°, indicating that the poly(APBA) shell increased the hydrophobicity of the system. At physiological pH, the uncharged phenylboronic acid was hydrophobic because of the existence of the benzene ring, resulting in the hydrophobicity of the polymer layer on the surface of ADI. When phenylboronic acid could react with glucose to form hydrophilic phenylborates, the hydrophobic polymer layer turned hydrophilic. Furthermore, the coupling of PEG and the binding of glucose improved the hydrophilicity of the system. The hydrophilicity of PP-n(ADI)/Glucose could reduce the protein adsorption and the capture of immune cells, potentially prolonging its circulation time [39]. Taken together, these results indicated that the developed nanogels could successfully encapsulate ADI, and the resulting PP-n(ADI) showed excellent physicochemical properties, facilitating its in vivo application.
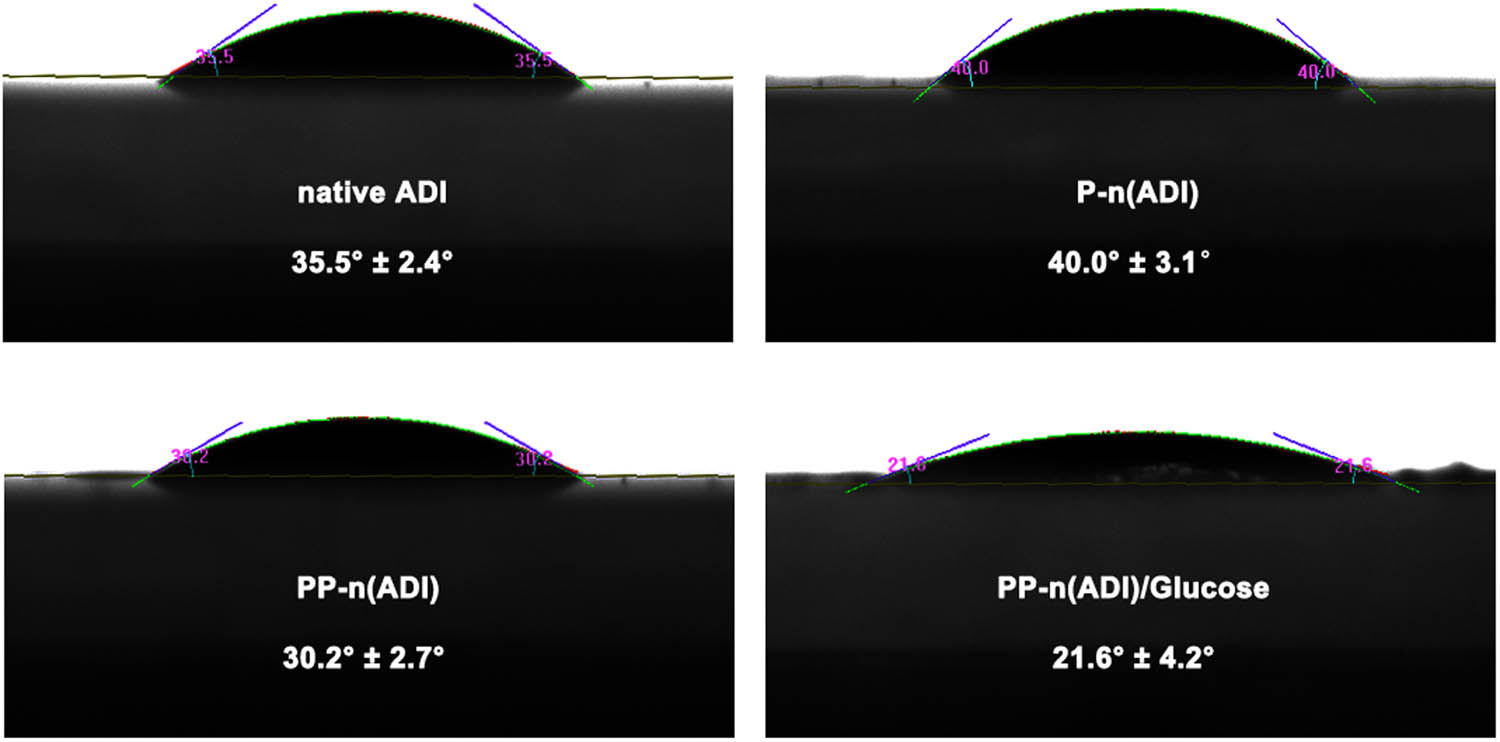
The films were respectively formed by corresponding samples, and their water contact angle was measured.
To investigate the effect of the preparation process on therapeutic enzymes, the activity maintenance of ADI was tested. The specific activity of PP-n(ADI)/Glucose was similar to native ADI’s specific activity (Figure 4a), demonstrating that the preparation process would not result in the inactivation of ADI. Therefore, taking advantage of physical interaction to encapsulate therapeutic enzymes was a more suitable method compared with chemical PEGylation and immobilization, which could result in the inactivation of therapeutic enzymes. To further demonstrate the glucose responsiveness of PP-n(ADI), the degradation efficiency of Arg was tested. As shown in Figure 4b, PP-n(ADI) had a low degradation efficiency of Arg, but its efficiency was rapidly improved after incubation with glucose or serum. Importantly, the activity of serum-incubated PP-n(ADI) was similar to that of native ADI, indicating the efficient in vivo catalytic activity of PP-n(ADI). These results demonstrated that the encapsulation of therapeutic enzymes in nanogels did not cause damage to them.

(a) The measurement of the specific activities of samples. The significance level was ns p > 0.05. (b) Arg was incubated with different samples and its concentration change in 10 min was measured.
In addition, the results indicated that the binding of glucose to nanogels restored the high-efficiency in vivo activity of ADI. That is because the binding of glucose could reduce the rigidity of the nanogel shell to endow the internal ADI with more flexibility [28], efficiently increasing the fitness of the ADI active site to Arg. And Arg could readily diffuse through the flexible poly(APBA)/glucose shell, maintaining the degradation efficiency of ADI.
We further tested the influence of nanogels on the stability of ADI. Samples were stored at different temperatures for 30 min. As shown in Figure 5a, nanogels could effectively maintain the thermostability of ADI, while the activity of native ADI was rapidly decreased when the temperature was adjusted to higher than 55°C. Besides, the activities of PP-n(ADI) stored in solutions with different pH values for 30 min were similar, further proving the protective effects of nanogels on therapeutic enzymes (Figure 5b). When samples were pre-incubated with the same volume of fresh mice serum and stored at 37℃, native ADI rapidly lost its activity as the increase of storage time, and it was only ∼10% of its initial activity after 30 days of storage (Figure 5c). After 60 days, in contrast, the activity of PP-n(ADI) was still ∼60% of its initial activity, implying the protective effects of nanogels on therapeutic enzymes. In addition, the activity maintenance of native ADI and PP-n(ADI), which were both incubated with trypsin, was tested (Figure 5d). The proteases could degrade native ADI and result in its activity loss, while nanogels could efficiently protect ADI from the degradation of proteases. The specific activity of PP-n(ADI) incubated with trypsin was similar to that of native ADI, demonstrating the protective effects of nanogels. Furthermore, to prove whether the nanogel could still resist the attack of proteases when glucose was bound with the nanogel, we first pretreated PP-n(ADI) with glucose and then treated it with trypsin. The activity of the corresponding PP-n(ADI) was still similar to that of native ADI. These results indicated that the rigid nanogels could maintain the in vitro stability of ADI by restraining conformational changes in ADI. The stability enhancement of therapeutic enzymes can facilitate their transportation and storage dramatically increasing the possibility of clinical translation.

(a) The measurement of the specific activities of samples that were stored at different temperatures for 30 min. (b) The measurement of the specific activities of samples that were stored in solutions with different pH values for 30 min. (c) Samples were pre-incubated with the same volume of fresh mice serum and stored at 37℃ for 60 days, and their activity was measured every 10 days. (d) Before and after incubation with trypsin, the specific activity of samples was measured. 1: native ADI; 2: PP-n(ADI); 3: native ADI treated with trypsin; 4: PP-n(ADI) treated with trypsin; 5: PP-n(ADI) first treated with glucose and then treated with trypsin. The significance levels were ****p < 0.0001 and ns p > 0.05.
Figure 6a shows the result of circular dichroism (CD) of the samples. The secondary structure of native ADI treated with trypsin was destroyed. On the contrary, the secondary structure of ADI could be well stabilized by nanogels. These results indicated that nanogels fixed the structure of therapeutic enzymes leading to the maintenance of their activity. To evaluate the cytotoxic effects of nanogels, BSA was selected as the model protein to prepare PP-n(BSA). That is because BSA had no function and the results would only show the effect of nanogels on cells, avoiding interference from the internal therapeutic enzymes. HUVECs incubated with 640 μg/mL of PP-n(BSA) still maintained as high as 90% viability (Figure 6b), which demonstrated that nanogels as a drug delivery vehicle had suitable cytocompatibility.

(a) CD analysis of native ADI, native ADI treated with trypsin, and PP-n(ADI) treated with trypsin. (b) Cytotoxicity of different concentrations of PP-n(BSA) in HUVECs.
MCF-7 cell is a kind of ASS1-deficient tumor cell, and it can be killed by ADI-based Arg deprivation. Here, MCF-7 cells were treated with samples and their cell viabilities were also tested. As shown in Figure 7a, the cell viability was rapidly decreased with the concentration increase of PP-n(ADI). The viability of the PP-n(ADI) (100 ng/mL)-treated MCF-7 cells was ∼20% having no difference from that treated with the same concentration of native ADI. It was found that the change of the intracellular amino acid ratio led to cytotoxicity. The MCF-7 cells’ intracellular ratio of Cit to Arg was increased with the increase in samples’ concentration (Figure 7b). The apoptosis of MCF-7 cells was also measured by AnnexinV-FITC/PI double staining (Figure 7c). Samples could induce the apoptosis of MCF-7 cells, and PP-n(ADI) was potentially more effective because nanogels would alter the cellular interaction of ADI [29]. In conclusion, nanogels had low cytotoxicity and could efficiently maintain the function of therapeutic enzymes.

(a) The cell viability of MCF-7 cells that were treated with various concentrations of samples. (b) MCF-7 cells were incubated with various concentrations of samples, and the ratio of Cit to Arg in them was tested. (c) Fluorescence microscopy images showing the apoptosis of MCF-7 cells treated with native ADI and PP-n(ADI) (100 ng/mL) by AnnexinV-FITC/PI double staining. The scale bar is 100 μm.
As depicted in Figure 8a, the blood circulation half-life of PP-n(ADI) was ∼12 h, which was much longer than that of native ADI (∼2 h). The nanogels significantly prolonged the circulation time of therapeutic enzymes mainly owing to the shielding protection of PEG. Besides, the binding of autologous glucose to the surface of nanogels could avoid the capture of the reticuloendothelial system [40]. The initial ratio of Cit to Arg in mice plasma was 0.56 ± 0.06, and once we intravenously injected the samples, its value was immediately increased to a few hundred (Figure 8b). However, the ratio of Cit to Arg in mice plasma was rapidly decreased in the native ADI group, and it returned to normal after 96 h. In contrast, PP-n(ADI) had a relatively long blood circulation time, and it could realize the sustained degradation of Arg in mice plasma. Even after a week, in the PP-n(ADI) group, the ratio of Cit to Arg in mice plasma was still more than 10. We have given one injection of samples into mice, and their main organs were obtained. We stained histological sections with H&E, and the results showed that samples did not have obvious damage to animals (Figure 8c), indicating the excellent biocompatibility of nanogels. This may be because nanogels did not cause excessive oxidative stress [41]. In addition, Arg deprivation would not damage normal cells because they can convert Cit to Arg via the catalysis of ASS1, indicating the biosafety of ADI based. From the in vivo performance of PP-n(ADI), it could be concluded that the encapsulated therapeutic enzymes still possessed efficient in vivo activity, and nanogels did not further increase side effects. Therefore, this kind of nanogel was an excellent delivery platform for therapeutic enzymes, and they could serve as novel therapeutic enzyme formulations for various disease treatments, including tumor therapy, antibacterial treatment, and anti-inflammatory therapy [7,42,43].

(a) The blood circulation time of samples. FITC was used to label ADI. (b) Samples (∼1 mg) were intravenously injected into the mice once, and the ratio of Cit to Arg in corresponding mice plasma was measured. (c) Images of H&E staining of histological sections of organs.
To further study the biocompatibility of PP-n(ADI) in vivo, hemolytic activity tests were performed to evaluate blood compatibility. No hemolysis was observed in the groups of PP-n(ADI) (Figure 9), which demonstrates that nanogels were biocompatible as a drug delivery vehicle.

Hemolytic activities of PP-n(ADI) and comparison with saline (negative control) and distilled water (positive control).
4 Conclusions
Glucose-responsive nanogels were synthesized by forming a polymeric shell containing phenylboronic acid groups on a therapeutic enzyme surface. These nanogels could efficiently restrain conformational changes in therapeutic enzymes, dramatically maintaining their in vitro stability. When applied in vivo, furthermore, phenylboronic acid molecules located at the surface of nanogels could bind with autogenous glucose, leading to the hydrophilicity and flexibility increase in the polymeric shell. Then, substrate molecules are easily diffused through the flexible polymeric shell, effectively maintaining their in vivo catalytic activity. Moreover, the flexible polymeric shell can still protect ADI from degradation and improve its in vivo stability. This kind of nanogel can significantly improve the in vitro stability of therapeutic enzymes and maintain their in vivo activity by the flexible change of the nanogel rigidity, facilitating the exploitation of new therapeutic enzyme formulations. In the present study, the researchers mainly focused on the in vivo efficiency of the drug. We believe that the efficiency of the drug from in vitro preparation and storage to clinical in vivo application should be considered comprehensively. And we hope our results can inspire others to design more efficient pharmaceutical preparations.
-
Funding information: This work was supported by the Nature Science Foundation of Shandong Province (No. ZR2019BC020) and the National Nature Science Foundation of China (No. 52103170).
-
Author contributions: All authors have accepted responsibility for the entire content of this article and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Vellard M. The enzyme as drug: application of enzymes as pharmaceuticals. Curr Opin Biotech. 2003;14:444–50.10.1016/S0958-1669(03)00092-2Suche in Google Scholar
[2] De Duve C. The significance of lysosomes in pathology and medicine. Proc Inst Med Chic. 1966;26:73–6.Suche in Google Scholar
[3] Wu Q, He Z, Wang X, Zhang Q, Wei Q, Ma S, et al. Cascade enzymes within self-assembled hybrid nanogel mimicked neutrophil lysosomes for singlet oxygen elevated cancer therapy. Nat Commun. 2019;10:1–14.10.1038/s41467-018-08234-2Suche in Google Scholar PubMed PubMed Central
[4] Qin M, Cao Z, Wen J, Yu Q, Liu C, Wang F, et al. An antioxidant enzyme therapeutic for COVID‐19. Adv Mater. 2020;32:2004901.10.1002/adma.202004901Suche in Google Scholar PubMed
[5] Meng Y, Sohar I, Sleat DE, Richardson JR, Reuhl KR, Jenkins RB, et al. Effective intravenous therapy for neurodegenerative disease with a therapeutic enzyme and a peptide that mediates delivery to the brain. Mol Ther. 2014;22:547–53.10.1038/mt.2013.267Suche in Google Scholar PubMed PubMed Central
[6] Puzzo F, Colella P, Biferi MG, Bali D, Paulk NK, Vidal P, et al. Rescue of Pompe disease in mice by AAV-mediated liver delivery of secretable acid α-glucosidase. Sci Transl Med. 2017;9(418):eaam6375.10.1126/scitranslmed.aam6375Suche in Google Scholar PubMed PubMed Central
[7] Li X, Yang Y, Wang Z, Ju H, Fu X, Zou L, et al. Multistage-responsive nanocomplexes attenuate ulcerative colitis by improving the accumulation and distribution of oral nucleic acid drugs in the colon. ACS Appl Mater Inter. 2022;14:2058–70.10.1021/acsami.1c21595Suche in Google Scholar PubMed
[8] Dean SN, Turner KB, Medintz IL, Walper SA. Targeting and delivery of therapeutic enzymes. Ther Deliv. 2017;8:577–95.10.4155/tde-2017-0020Suche in Google Scholar PubMed
[9] Vinogradov VV, Avnir D. Exceptional thermal stability of therapeutical enzymes entrapped in alumina sol-gel matrices. J Mater Chem B. 2014;2:2868–73.10.1039/c3tb21643hSuche in Google Scholar PubMed
[10] Fuhrmann G, Leroux JC. Improving the stability and activity of oral therapeutic enzymes-Recent advances and perspectives. Pharm Res. 2014;31:1099–105.10.1007/s11095-013-1233-ySuche in Google Scholar PubMed
[11] Qi H, Yang L, Shan P, Zhu S, Ding H, Xue S, et al. The stability maintenance of protein drugs in organic coatings based on nanogels. Pharmaceutics. 2020;12:115.10.3390/pharmaceutics12020115Suche in Google Scholar PubMed PubMed Central
[12] Karamitros CS, Yashchenok AM, Möhwald H, Skirtach AG, Konrad M. Preserving catalytic activity and enhancing biochemical stability of the therapeutic enzyme asparaginase by biocompatible multilayered polyelectrolyte microcapsules. Biomacromolecules. 2013;14:4398–406.10.1021/bm401341kSuche in Google Scholar PubMed
[13] Tadepalli S, Yim J, Cao S, Wang Z, Naik RR, Singamaneni S. Metal-organic framework encapsulation for the preservation and photothermal enhancement of enzyme activity. Small. 2018;14:1702382.10.1002/smll.201702382Suche in Google Scholar PubMed
[14] Yari M, Ghoshoon B, Vakili M, Ghasemi B, Y. Therapeutic enzymes: applications and approaches to pharmacological improvement. Curr Pharm Biotechno. 2017;18:531–40.10.2174/1389201018666170808150742Suche in Google Scholar PubMed
[15] Veronese FM, Mero A. The impact of PEGylation on biological therapies. BioDrugs. 2008;22:315–29.10.2165/00063030-200822050-00004Suche in Google Scholar PubMed
[16] Cowan DA, Fernandez-Lafuente R. Enhancing the functional properties of thermophilic enzymes by chemical modification and immobilization. Enzyme Microb Tech. 2011;49:326–46.10.1016/j.enzmictec.2011.06.023Suche in Google Scholar PubMed
[17] Rodriguez-Abetxuko A, Sánchez-deAlcázar D, Muñumer P, Beloqui A. Tunable polymeric scaffolds for enzyme immobilization. Front Bioeng Biotechnol. 2020;8:830.10.3389/fbioe.2020.00830Suche in Google Scholar PubMed PubMed Central
[18] Bomboi F, Tardani F, Gazzoli D, Bonincontro A, La Mesa C. Lysozyme binds onto functionalized carbon nanotubes. Colloid Surf B. 2013;108:16–22.10.1016/j.colsurfb.2013.02.034Suche in Google Scholar PubMed
[19] Chakraborty S, Joshi P, Shanker V, Ansari Z, Singh SP, Chakrabarti P. Contrasting effect of gold nanoparticles and nanorods with different surface modifications on the structure and activity of bovine serum albumin. Langmuir. 2011;27:7722–31.10.1021/la200787tSuche in Google Scholar PubMed
[20] Cukalevski R, Lundqvist M, Oslakovic C, Dahlbäck Br, Linse S, Cedervall T. Structural changes in apolipoproteins bound to nanoparticles. Langmuir. 2011;27:14360–9.10.1021/la203290aSuche in Google Scholar PubMed
[21] Huang R, Carney RP, Stellacci F, Lau BL. Protein-nanoparticle interactions: the effects of surface compositional and structural heterogeneity are scale dependent. Nanoscale. 2013;5:6928–35.10.1039/c3nr02117cSuche in Google Scholar PubMed
[22] Kao KC, Lin TS, Mou CY. Enhanced activity and stability of lysozyme by immobilization in the matching nanochannels of mesoporous silica nanoparticles. J Phys Chem C. 2014;118:6734–43.10.1021/jp4112684Suche in Google Scholar
[23] Zhang P, Sun F, Tsao C, Liu S, Jain P, Sinclair A, et al. Zwitterionic gel encapsulation promotes protein stability, enhances pharmacokinetics, and reduces immunogenicity. P Natl Acad SCI USA. 2015;112:12046–51.10.1073/pnas.1512465112Suche in Google Scholar PubMed PubMed Central
[24] Kim SH, Kim KR, Ahn DR, Lee JE, Yang EG, Kim SY. Reversible regulation of enzyme activity by pH-responsive encapsulation in DNA nanocages. ACS Nano. 2017;11:9352–9.10.1021/acsnano.7b04766Suche in Google Scholar PubMed
[25] Chapman R, Stenzel MH. All wrapped up: Stabilization of enzymes within single enzyme nanoparticles. J Am Chem Soc. 2019;141:2754–69.10.1021/jacs.8b10338Suche in Google Scholar PubMed
[26] Johnson KA. Role of induced fit in enzyme specificity: a molecular forward/reverse switch. J Biol Chem. 2008;283:26297–301.10.1074/jbc.R800034200Suche in Google Scholar PubMed PubMed Central
[27] Chen C, Gu Y, Deng L, Han S, Sun X, Chen Y, et al. Tuning gelation kinetics and mechanical rigidity of β-hairpin peptide hydrogels via hydrophobic amino acid substitutions. ACS Appl Mater Inter. 2014;6:14360–8.10.1021/am5036303Suche in Google Scholar PubMed
[28] Wang Q, Wang H, Chen Q, Guan Y, Zhang Y. Glucose-triggered micellization of Poly (ethylene glycol)-b-poly (N-isopropylacrylamide-co-2-(acrylamido) phenylboronic acid) block copolymer. ACS Appl Polym Mater. 2020;2:3966–76.10.1021/acsapm.0c00635Suche in Google Scholar
[29] Qi H, Wang Y, Yuan X, Li P, Yang L. Selective extracellular arginine deprivation by a single injection of cellular non-uptake arginine deiminase nanocapsules for sustained tumor inhibition. Nanoscale. 2020;12:24030–43.10.1039/D0NR06823CSuche in Google Scholar PubMed
[30] Yang L, Han D, Zhan Q, Li X, Shan P, Hu Y, et al. Blood TfR + exosomes separated by a pH-responsive method deliver chemotherapeutics for tumor therapy. Theranostics. 2019;9:7680.10.7150/thno.37220Suche in Google Scholar PubMed PubMed Central
[31] Qi H, Yang L, Li X, Sun X, Zhao J, Hou X, et al. Systemic administration of enzyme-responsive growth factor nanocapsules for promoting bone repair. Biomater Sci. 2019;7:1675–85.10.1039/C8BM01632ASuche in Google Scholar
[32] Qi H, Liu C, Long L, Ren Y, Zhang S, Chang X, et al. Blood exosomes endowed with magnetic and targeting properties for cancer therapy. ACS Nano. 2016;10:3323–33.10.1021/acsnano.5b06939Suche in Google Scholar PubMed
[33] Tang L, Yang X, Yin Q, Cai K, Wang H, Chaudhury I, et al. Investigating the optimal size of anticancer nanomedicine. P Natl Acad SCI USA. 2014;111:15344–9.10.1073/pnas.1411499111Suche in Google Scholar PubMed PubMed Central
[34] Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941.10.1201/9780429027819-9Suche in Google Scholar
[35] Xiao K, Li Y, Luo J, Lee JS, Xiao W, Gonik AM, et al. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials. 2011;32:3435–46.10.1016/j.biomaterials.2011.01.021Suche in Google Scholar PubMed PubMed Central
[36] Basiruddin S, Swain SK. Phenylboronic acid functionalized reduced graphene oxide based fluorescence nano sensor for glucose sensing. Mat Sci Eng C. 2016;58:103–9.10.1016/j.msec.2015.07.068Suche in Google Scholar PubMed
[37] Elmarzugi NA, Adali T, Bentaleb AM, Keleb EI, Mohamed AT, Hamza AM. Spectroscopic characterization of PEG-DNA biocomplexes by FTIR. J Appl Pharm Sci. 2014;4:6.Suche in Google Scholar
[38] Calvaresi EC, Hergenrother PJ. Glucose conjugation for the specific targeting and treatment of cancer. Chem Sci. 2013;4:2319–33.10.1039/c3sc22205eSuche in Google Scholar
[39] Simon J, Wolf T, Klein K, Landfester K, Wurm FR, Mailänder V. Hydrophilicity regulates the stealth properties of polyphosphoester‐coated nanocarriers. Angew Chem Int Ed. 2018;57:5548–53.10.1002/anie.201800272Suche in Google Scholar PubMed
[40] Cvjetinović Đ, Prijović Ž, Janković D, Radović M, Mirković M, Milanović Z, et al. Bioevaluation of glucose-modified liposomes as a potential drug delivery system for cancer treatment using 177-Lu radiotracking. J Control Rel. 2021;332:301–11.10.1016/j.jconrel.2021.03.006Suche in Google Scholar PubMed
[41] Qi H, Wang Y, Fa S, Yuan C, Yang L. Extracellular vesicles as natural delivery carriers regulate oxidative stress under pathological conditions. Front Bioeng Biotechnol. 2021;810.10.3389/fbioe.2021.752019Suche in Google Scholar PubMed PubMed Central
[42] Qi H, Shan P, Wang Y, Li P, Wang K, Yang L. Nanomedicines for the efficient treatment of intracellular bacteria: the “ART” principle. Front Chem. 2021;924.10.3389/fchem.2021.775682Suche in Google Scholar PubMed PubMed Central
[43] Shan P, Yang F, Qi H, Hu Y, Zhu S, Sun Z. Alteration of MDM2 by the small molecule YF438 exerts antitumor effects in triple-negative breast cancer. Cancer Res. 2021;81:4027–40.10.1158/0008-5472.CAN-20-0922Suche in Google Scholar PubMed
© 2022 Hongzhao Qi et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption

