Nanoparticles and the treatment of hepatocellular carcinoma
-
Ziyu Zhang
, Yao Xie
und Minghui Li
Abstract
Currently, liver cancer is the leading cause of cancer-related death worldwide, with a low 5-year survival rate, which will further decrease if advanced metastasis is present. Hepatocellular carcinoma (HCC) is the main type. However, due to the lack of specific symptoms in the early stages, it is more difficult to detect HCC, and many patients would have already been diagnosed with advanced liver cancer. At this point, many treatment methods available at early diagnosis would have become ineffective. Therefore, there is an urgent need for more effective treatment methods for HCC. In recent years, nanoparticles have been used in the treatment of HCC due to their good biocompatibility and other advantages. Different types of nanoparticles are modified to play a role in the treatment of HCC, such as regulating tumor microenvironment, enhancing the activity of drug targeting and killing cancer cells, and reducing systemic side effects. It can significantly improve the therapeutic effect of HCC and bring more hope for the treatment of HCC. In this review, several common nanoparticles are introduced, and their characteristics are described in detail. In addition, the construction of a highly efficient drug delivery system by nanoparticles and the combination of nanoparticle-targeted therapy, chemotherapy, and radiotherapy are reviewed.
List of Abbreviations
- HCC
-
hepatocellular carcinoma
- ICC
-
intrahepatic cholangiocarcinoma
- HBV
-
hepatitis B virus
- HCV
-
hepatitis C virus
- AFP
-
alpha fetoprotein
- BCLC
-
Barcelona Clinic Liver Cancer
- CNLC
-
Chinese Liver Cancer Staging
- RFA
-
radiofrequency ablation
- MWA
-
microwave ablation
- LA
-
laser ablation
- IRE
-
irreversible electroporation
- CRA
-
cryoablation
- HIFU
-
high-intensity focused ultrasound
- TACE
-
transcatheter arterial chemoembolization
- ICIs
-
immune checkpoint inhibitors
- ICB
-
immune checkpoint blockade
- PD-1
-
programmed cell death-protein 1
- PD-L1
-
programmed cell death-ligand 1
- CTLA-4
-
cytotoxic T lymphocyte antigen
- TME
-
tumor microenvironment
- TAMs
-
tumor-associated macrophages
- CAFs
-
cancer-associated fibroblasts
- Tregs
-
regulatory T cells
- DDS
-
drug delivery system
- EPR effect
-
enhanced permeability and retention effect
- RES
-
reticuloendothelial system
- LNPs
-
lipid nanoparticles
- PNPs
-
polymer nanoparticles
- SPR
-
surface plasmon resonance
- OS NPs
-
organosilica NPs
- MRI
-
magnetic resonance imaging
- PAI
-
photoacoustic imaging
- SLN
-
solid lipid nanoparticles
- NLC
-
nanostructured lipid carriers
- PEG
-
polyethylene glycol
- LPHNPs
-
lipid–polymer hybrid nanoparticles
- PAMAM
-
polyamidoamine
- RGD
-
arginine-glycine-aspartic acid
- MSNs
-
mesoporous silica nanoparticles
- FDA
-
Food and Drug Administration
- APCs
-
antigen-presenting cells
- IONPs
-
iron oxide nanoparticles
- MNPs
-
magnetic nanoparticles
- MTC
-
magnetic targeting carriers
- SPIONs
-
superparamagnetic iron oxide nanoparticles
- QT
-
quercetin
- QMNPs
-
quercetin-conjugated magnetite nanoparticles
- CRISPR
-
clustered, regularly interspaced short palindromic repeats
- Cas9
-
CRISPR-associated nuclease protein 9
- RNP
-
ribonucleoprotein
- siRNA
-
small interfering RNA
- SNALPs
-
stable nucleic acid lipid particles
- GA
-
glycyrrhetinic acid
- LA
-
lactobionic acid
- CA
-
cationized amylose
- TPE
-
tetraphenylethylene
- FA
-
folic acid
1 Introduction
Liver cancer is the sixth most frequently diagnosed cancer worldwide and is among the top three leading causes of cancer-related death worldwide [1,2]. Each year, up to 854,000 new cases of hepatocellular carcinoma (HCC) are diagnosed, and up to 810,000 deaths occur [3]. As the population ages, the number of HCCs is increasing globally and the mortality rate continues to rise annually [4], with a 5-year relative survival rate of only 21% [5]. Therefore, liver cancer treatment has garnered significant attention. The types of liver cancer include HCC, intrahepatic cholangiocarcinoma (ICC), and mixed HCC. HCC represents the predominant diagnosis and cause of death, accounting for 75–85% of cases [1]. Hepatitis B virus (HBV) and hepatitis C virus (HCV) currently stand as the foremost global risk factors for HCC. Due to the implementation of the hepatitis B vaccination program, the incidence and mortality of HCC caused by hepatitis B have been relatively low in recent years. Other risk factors include alcohol, metabolic syndrome, diabetes, obesity, non-alcoholic fatty liver disease, and aflatoxin-contaminated food [4,6,7]. The main risk factors vary across different regions. For example, HBV and aflatoxin infection are considered the most prominent risk factors for HCC in most high-risk countries in Asia, with the exception of Japan and Egypt, where HCV is the leading cause of HCC, particularly in Japan, where more than 70% of HCC cases are thought to be HCV-related [8]. HCV is the leading cause of HCC in Western Europe, while in Central and Eastern Europe, nearly 50% of the HCC cases are associated with alcohol-related liver disease [9].
Early detection, diagnosis, and treatment are crucial for cancer prevention and treatment. HCC can be detected by serum biomarkers such as AFP (alpha fetoprotein) [10], PIVKA-II combined with AFP [11], abdominal ultrasound, multi-item computed tomography, and other imaging examinations [12]. However, due to its insidious onset without specific symptoms during early stages, most patients are diagnosed at advanced stages. Current treatments for liver cancer comprise surgical resection and liver transplantation, ablation therapy, transarterial chemoembolization, immunotherapy, and others [13]. Surgery serves as a radical treatment for liver cancer [14], but the diagnosis of HCC often presents at an advanced stage, involving peripheral blood vessels or metastasis, rendering surgical resection unsuitable [15]. As the first approved first-line therapy for advanced HCC, Sorafenib, and the emerging first-line therapy Lenvatinib may lead to drug resistance and adverse side effects with prolonged administration [16,17]. A high recurrence rate after surgical resection, limited eligibility for transplantation, drug resistance, and chemotherapy-induced side effects all contribute to poor clinical outcomes in HCC. Therefore, there is an urgent need for novel approaches to treat HCC more effectively.
Nanotechnology has emerged as a promising field. Nanomaterials have received extensive attention due to their low toxicity and biocompatibility, and significant progress in cancer diagnosis and treatment has been made in recent years. Different nanoparticles have specific characteristics, such as the high stability of magnetic nanoparticles (MNPs), high surface area of gold nanoparticles, and drug encapsulation of lipid particles [18], which enable nanoparticles to not only reduce the systemic toxicity caused by chemotherapy in tumor treatment but also effectively improve tumor reactivity [19]. Therefore, nanoparticles can not only build an efficient DDS but also regulate the tumor microenvironment (TME) to improve the therapeutic effect on tumors. In this study, the characteristics of common nanoparticles and the role of nanoparticles in the current common treatment are introduced, and some clinical studies of nanoparticles are also described, hoping to provide a new method and approach for the treatment of HCC by nanoparticles (Figure 1).
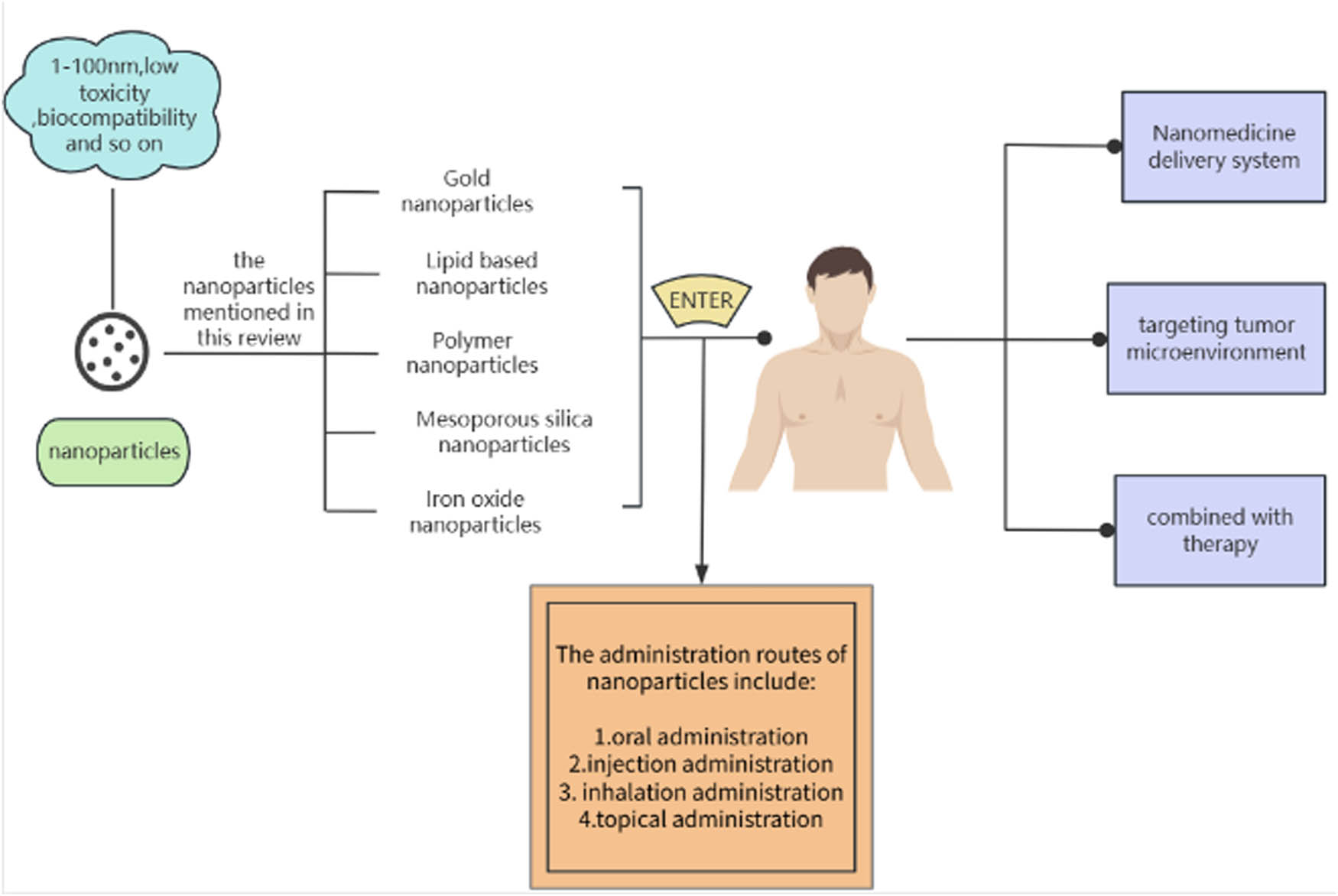
Mind map of the review.
2 Current liver cancer treatments and their limitations
2.1 Surgical resection and liver transplantation
Surgical resection is the main treatment for HCC [3] and also a radical treatment for early HCC. Indications for surgical excision vary by country and equipment. Surgical resection is generally recommended for patients with tumor diameter ≥3 cm and tumor number ≤3 [3]. Hepatectomy is considered for patients with nonmetastatic disease and normal underlying liver function or with compensated cirrhosis and no evidence of portal hypertension [20]. However, surgical removal is not always a cure. The 10-year recurrence-free survival rate after surgery is only about 22–25% [21]. Among them, the 5-year recurrence rate of small liver cancer (<2 cm) without microvascular invasion is 50–60%, of which about 74.6% of the recurrent lesions are located in the liver, and only 15% of the recurrent tumors can be resected [22,23]. In addition, complications such as liver failure may occur after surgery [3]. Studies have shown that the cost, time, and complication rate of ablation are lower than that of surgical resection [24]
In addition, liver transplantation is allowed if the tumor meets the Milan criteria [25], with a single tumor ≤5 cm or 2–3 tumors ≤3 cm, and no large vascular invasion or extrahepatic tumor dissemination on imaging. Liver transplantation is regarded as the first choice for liver cancer treatment because of its good survival rate and recurrence-free survival rate [20]. However, currently, the number of liver donors is outrunning supply [22].
2.2 Ablation therapy
For patients with early liver cancer, there are generally three strategies: surgical resection, ablation therapy, and liver transplantation. Ablation therapy includes radiofrequency ablation (RFA), microwave ablation (MWA), and laser ablation (LA).
2.2.1 RFA
For single HCC ≤ 2 cm without vascular invasion or extrahepatic dissemination, ablation is the preferred initial treatment option, and RFA is the most widely used technique [26]. The primary goal of ablation therapy is complete necrosis of liver cancer cells and establishment of a safety margin > 10 mm at the outer edge of the lesion [27]. Therefore, tumor size can influence the efficacy of ablation. When the tumor exceeds 3 cm, the therapeutic effectiveness of RFA is significantly reduced, characterized by incomplete ablation and high local recurrence rates [27]. Emerging evidence suggests that ablation therapy not only acts as a localized treatment but also exerts immunomodulatory effects. RFA can promote the expression of PD-1/PD-L1, and sunitinib can activate the immune response to inhibit the effect of PD-1/PD-L1 [28], thus supporting the combination of ablation and immunotherapy for liver cancer management [29].
2.2.2 MWA
The first generation of MWA for clinical use was reported in the 1990s [30]. Tumor tissues can be directly destroyed by the thermal damage generated by electromagnetic waves in the MWA [31]. MWA has a high frequency, so it can increase the temperature to enlarge the cauterization range of tumors and shorten the cauterization time [29]. Compared to hepatectomy, MWA is easier to treat lesions located in the center of the liver and can reduce liver damage while maximizing the preservation of normal liver tissue [32]. Compared to RFA, MWA has the advantages of larger heating volume, insensitivity to carbonization, and less susceptibility to the heat sink effect of blood flow [31]. T cells and macrophages are seen to increase in HCC lesions after MWA treatment and tumor-specific immune responses appear or increase [33]. However, clinical data on RFA are limited [34].
2.2.3 Other ablation therapy
At present, when ablation is mentioned clinically, most of them refer to the above two ablation methods. However, in addition to the above two methods, there are other ways of ablation, such as LA, irreversible electroporation (IRE), cryoablation (CRA), and high-intensity focused ultrasound (HIFU). However, other forms of ablation have their limitations. For example, CRA is recommended when HCC is located near other organs or under the diaphragm because RFA is not effective. CRA uses low temperatures to form ice inside the cell and crystals outside the cell, which destroys the cell and causes the cell contents to be released outside the cell, triggering an immune response that kills the tumor cells [35]. However, there are little data about RFA, and RFA can easily lead to complications such as shock. Compared to RFA, LA is more effective for lesions with a diameter of 20 mm or less and has a lower complication rate [36]. Adwan et al. conducted a study comparing the safety and efficacy of MWA and LA, and the results showed that the MWA group had a higher survival rate, indicating that MWA was superior to LA [37]. It was found that the local efficacy of LA was affected by the tumor size and volume [38]. IRE is a non-thermal ablation technology that forms pores on the phospholipid bilayer of the cell membrane through high pulses, leading to cell apoptosis. However, the operation of IRE requires the placement of electrodes close to the critical anatomical structure, which is challenging and carries the risk of primary incomplete ablation [39]. In addition, the IRE may be affected by the presence of pacemakers or surgical clips that ablate the edges, resulting in inaccurate ablation edges [40]. As a non-invasive therapy, HIFU has attracted widespread attention in recent years for its excellent performance in the treatment of small HCC. Compared with RFA, HIFU can not only avoid tumor-targeted puncture but also avoid tumor spreading along the needle path that often occurs in RFA therapy [36]. However, it should not be ignored that HIFU may cause skin burn or pain after treatment and even lead to serious complications such as ablation of the surrounding small intestine, with a high recurrence rate [41,42]. These problems limit the application of these ablation methods.
2.3 Transcatheter arterial chemoembolization (TACE)
According to the widely used Barcelona staging system (BCLC), TACE is recommended as the first-line treatment for advanced HCC [43]. TACE inhibits the growth of tumors by injecting chemotherapy drugs into the hepatic artery [44]. However, due to the heterogeneity of tumors, the clinical response to TACE therapy varies with different individuals [43]. Adverse reactions of post-embolization syndrome, such as abdominal pain, fever, and intestinal obstruction, are common after TACE [45]. Tumor recurrence is common since TACE needs to be repeated many times to control the tumor well [44]. Due to the adverse effects of chemotherapeutic drugs, repeated TACE can lead to deterioration of liver function and miss the opportunity for other treatments [45,46]. Therefore, TACE is often used in combination with other therapies, such as thermal ablation, RFA, sorafenib, and camrelizumab [44,47–50], or used as downstaging therapy to make the patient eligible for liver transplantation [48].
2.4 Systemic anti-tumor therapy
2.4.1 Targeted therapies
Systemic anti-tumor therapy includes molecular targeted therapy, immunotherapy, and chemotherapy [13]. HCC has a poor response to chemotherapy, and the average patient cannot tolerate chemotherapy due to poor liver functions. Some molecules play an important role in the development of cancer, and these can be potential targets for cancer-targeted therapies. Common targeted therapeutic drugs and their targets are shown in Table 1. For HCC, the most important target is the vascular endothelial growth factor and its receptor [58]. Bevacizumab, a humanized monoclonal antibody that binds VEGF-A, has been investigated as a potential treatment for advanced HCC [59]. Molecularly targeted drugs have significantly improved the current survival rate of liver cancer. IMbrave150 is a global open Phase III clinical trial investigating atezolizumab in combination with Bevacizumab for advanced HCC [60]. Compared with first-line drugs, the survival rate in the experimental group was significantly improved, but there was no significant difference in toxicity [60]. However, drug resistance caused by tumor heterogeneity is still the main reason for the failure of current targeted therapy, and the economic cost of treatment is also an unavoidable problem in the current treatment process [61].
Common targeted drugs and their corresponding targets, mechanisms of action, and routes of administration
| Targeted drug | Targets | Mechanism | Route of administration | Ref. |
|---|---|---|---|---|
| Sorafenib | VEGFR, PDGFR, Raf, c-kit, FLT-3 | Inhibits tumor peripheral angiogenesis, tumor cell hypoxic necrosis, and interferes with the signal pathway | Oral administration | [51,52] |
| Lenvatinib | VEGFR, PDGFR, FGFR | Inhibits tumor angiogenesis and hepatoma cell proliferation and interferes with the signal pathway | Oral administration | [52] |
| Apatinib | VEGFR-2, PDGFR c-kit | Inhibits tumor angiogenesis and hepatoma cell proliferation | Oral administration | [52] |
| Bevacizumab | VEGF-A | Inhibits tumor angiogenesis and hepatoma cell proliferation, increased sensitivity to chemotherapy, and improves TME | Intravenous injection | [53] |
| Imatinib | Bcr-Abl fusion protein, kinasec-Met, PDGFR, VEGFR | Inhibits tumor angiogenesis and hepatoma cell proliferation | Oral administration | [54,55] |
| Pembrolizumab | PD-1 receptors | ICIs, restores T cell activity and enhances immune response | Intravenous injection | [53] |
| Rapamycin | mTOR | Inhibitory mTOR activity and regulates cell metabolism and immunity | Oral administration and intravenous injection | [56,57] |
2.4.2 Immune checkpoint inhibitors (ICIs)
In recent years, immunotherapy has been widely studied. ICIs, peptide vaccines, dendritic vaccines, etc., are currently available for HCC immunotherapy [62]. Immune checkpoint blockade (ICB) therapy is a recently developed treatment [63]. ICIs are monoclonal antibodies that can prevent the interaction of immune checkpoint proteins with their ligands, avoiding T cell inactivation thereby restoring and enhancing their immune effects [64,65]. Currently, the main targets of ICIs are programmed cell death protein 1 (PD-1) along with its ligand (PD-L1) and cytotoxic T lymphocyte antigen (CTLA-4) [66]. After the use of PD-1 and PD-L1 inhibitors, PD-L1 on tumor cells can be effectively inhibited from binding to PD-1 on T cells so as to restore the immune function of suppressed T cells, thereby killing tumors. Immunotherapy of PD-1 and PD-L1 is promising, especially for patients with advanced HCC [67]. However, due to the heterogeneity of tumors, ICI monotherapy has limited efficacy and some immune-related adverse reactions [64,68]. Therefore, combining ICIs with anti-angiogenic targeted drug therapy, such as carrelizumab and apatinib, has received wide attention [69,70].
3 Nanoparticles
Nanoparticles refer to particles with diameters in the range of 1–100 nm [71]. The administration routes of nanoparticles include oral, injection, inhalation, and topical administration (Figure 2). Different kinds of nanoparticles have different synthesis methods. Metal nanoparticles can be synthesized by LA, pyrolysis, chemical reduction, radiation-induced synthesis, microwave-assisted synthesis, and other methods. The polymer nanoparticles (PNPs) can be synthesized by Ouzo polymerization, multi-solvent nanoprecipitation, and emulsion polymerization. Methods for the synthesis of lipid-based nanoparticles include emulsion solvent evaporation and the use of magnetic substances [72]. The effect of NPs is not only related to the phase size, charge, and surface area but also to surface hydrophobicity and ligand density shape. Some studies have shown that particles with a diameter of about 50 nm can maintain good intracellular transport and cellular uptake [73]. Larger NPs appear to be safer than smaller nanoparticles due to their small surface area relative to their total mass, as this reduces the chances of the nanoparticles interacting with surrounding biomolecules, thereby reducing possible adverse reactions in the body [74]. Under the enhanced permeability and retention effect (EPR effect), large NPs are well preserved in blood vessels but easily cleared by tumor tissues. Small NPs can penetrate deep into tumor tissue. Therefore, it is necessary to consider the size of the NP design in advance when designing the target NPs [75].
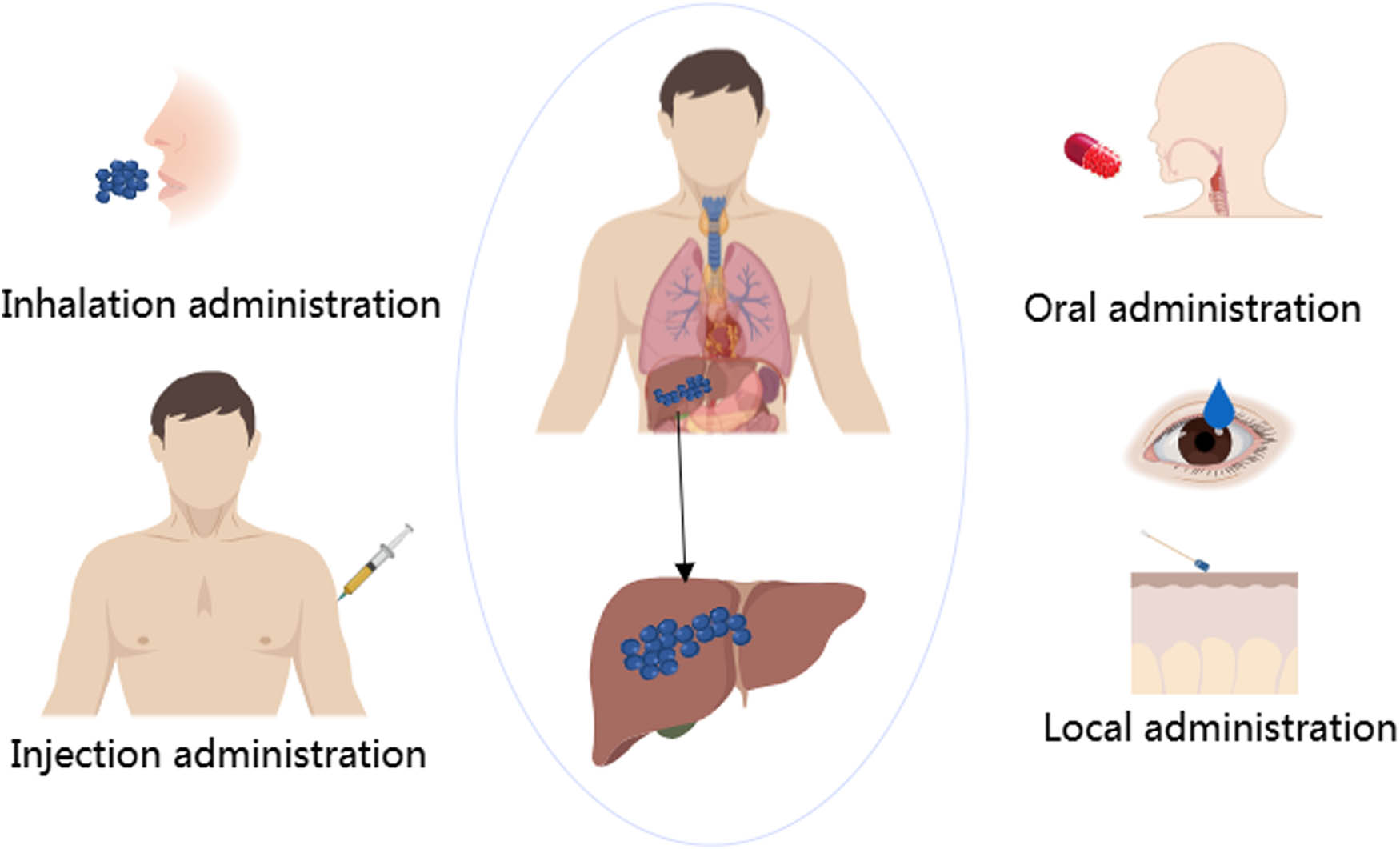
Drug delivery pathway of nanoparticles.
Nanoparticles for DDS include organic and inorganic nanoparticles. The organic nanoparticles include lipid nanoparticles (LNPs) and PNPs, while the inorganic nanoparticles include metal and inorganic nonmetal nanoparticles. Nanoparticles can deliver therapeutic drugs to specific sites, improve the uptake rate of drugs, and enhance the therapeutic effect [76–79]. Several common nanoparticles are introduced in this review.
3.1 Gold nanoparticles
Gold nanoparticles are effective and safe, with tunable size, low osmotic pressure, good biocompatibility, chemical stability, high X-ray absorption coefficient, unique photoelectric properties, and tunable surface plasmon resonance (SPR) [80,81]. Owing to these properties, gold nanoparticles are widely used in medicine [82]. Gold nanoparticles can be used to deliver a variety of substances. Because of its biocompatibility, it can be designed into spherical, oval, rod-shaped, triangular, or flower-shaped shapes according to the requirements [83,84], with safety achieved by changing the size or composition of the gold nanocarrier [85]. Nanoparticles of limited size can easily pass through the body efficiently, and the smallest nanoparticles have lower toxicity and uptake [80]. The uptake of gold nanoparticles is influenced by their size, with smaller nanoparticles exhibiting reduced toxicity and enhanced absorption efficiency [86]. Among them, ultra-small gold nanoparticles can be completely removed by the kidney or liver [87].
Gold nanoparticles are highly efficient due to their inertness, chemical stability, and ability to protect molecules such as cytokines from degradation [85]. They are considered to be suitable drug carriers for tumor therapy due to their small size, strong biocompatibility, and precise targeting ability [88]. Compared with lipid particles, gold nanoparticles have the advantage of absorbing and generating heat by photons [89], which can greatly enhance the effectiveness of photothermal therapy [90]. Small-sized gold nanoparticles can achieve surface functionalization of organosilica nanoparticles (OS NPs), which is conducive to improving the stability and radiation-sensitizing ability of nanoparticles, as well as improving the targeting ability of silica nanoparticles [91]. In addition, gold nanoparticles can produce immune responses with microenvironmental stimuli [92]. The surface particles of gold nanoparticles are rough and have a high surface-to-volume ratio, which is favorable for synthesizing nanomaterials with low toxicity [93]. Therefore, based on the above characteristics, gold nanoparticles are widely used in MRI (magnetic resonance imaging), PAI (photoacoustic imaging), and other tumor diagnostic methods and are widely used as drug carriers in chemotherapy and radiotherapy [94].
3.2 Lipid-based nanoparticles
Lipid-based nanoparticles include liposomes, solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), and other forms. LNPs are one of the most common carriers for delivery systems [95], and most of the nanoparticles currently used for targeted delivery are composed of polymers or lipids [96]. Ideally, the drug carrier should be cleared quickly after drug release [97]. LNPs are mainly composed of physiological lipids and have low toxicity, so lipid carriers are also referred to as “nanosafe” carriers [98]. Lipid-based nanoparticles have good biocompatibility and biodegradability [99]. LNPs are mainly composed of lipid or lipoid materials plus auxiliary cholesterol, lipid, and polyethylene glycol (PEG)-lipid [100], in which the key component, cationic ionizable lipid, can achieve efficient nucleic acid encapsulation, cell delivery, and endosomal release [101]. Liposomes are easily degraded by lipase in the digestive system and recognized by the reticuloendothelial system (RES), resulting in reduced bioavailability [102,103]. Liposome complexes were developed to address this issue, in which polymers such as alginate, chitosan, cyclodextrin, and collagen are used to modify the structure of liposomes [104]. Liposome complexes improve the physicochemical properties of liposomes, have better stability, and protect the activity of delivery molecules [104]. SLN formulations can be integrated as solids or liquids, capable of loading a large amount of drugs, and can adhere to the intestinal wall to increase the bioavailability and solubility of drugs, thereby protecting drugs from degradation in the acidic environment of the stomach [105]. LNPs have now been widely utilized for systemic delivery of RNA therapeutics [100]. LNPs loaded with siRNA enter the cell membrane by forming endosomes and then release siRNA inside the cell to achieve therapeutic effect (Figure 3). Lipid-based nanoparticles are commonly employed in liver cancer treatment due to their many inherent advantages as drug carriers and their preferential accumulation in the liver after systemic administration [106].
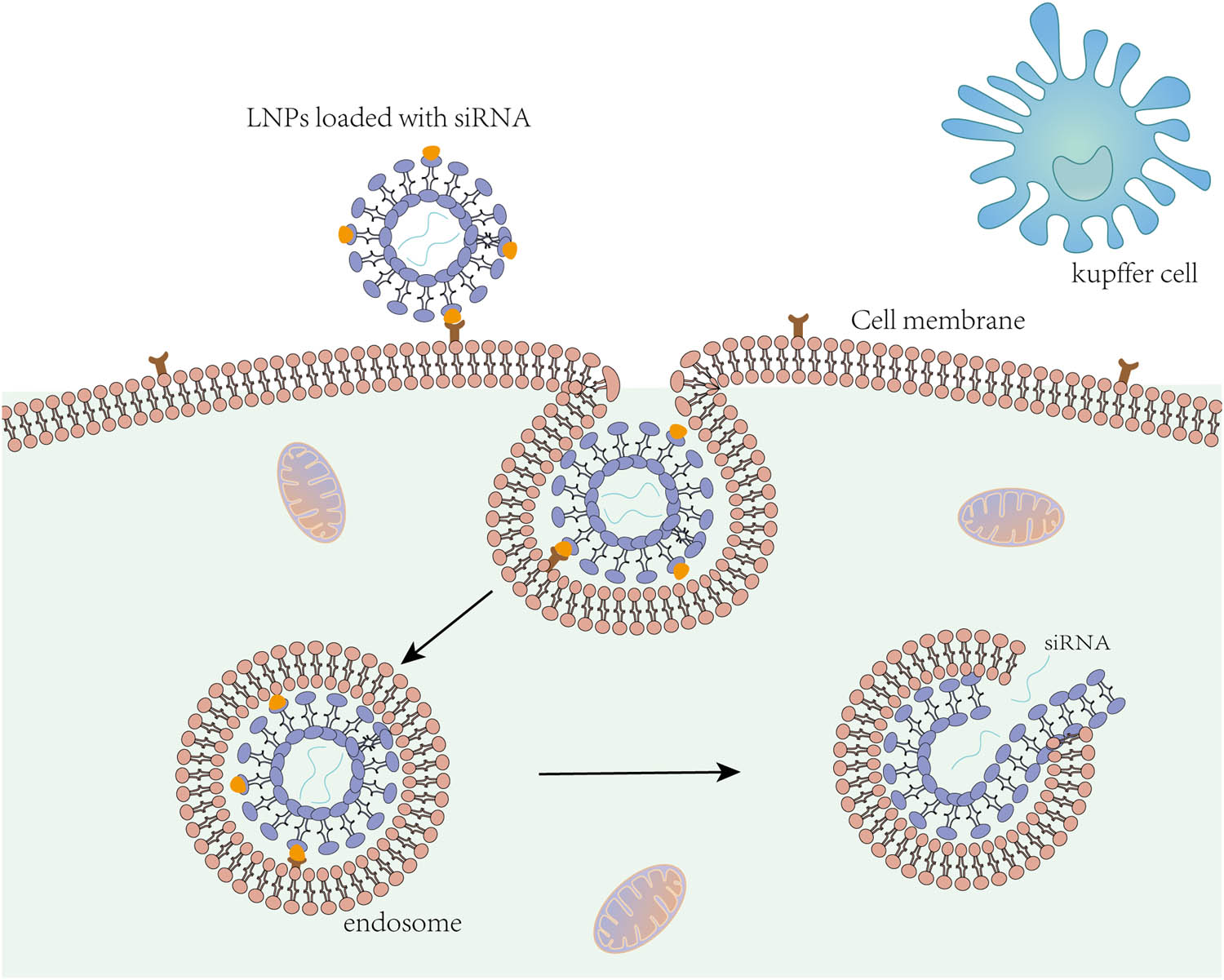
Lipid nanoparticles loaded with siRNA enter liver cells.
3.3 Polymer nanoparticles
Polymer nanoparticles have a variety of structural forms, such as nanocapsules, nanospheres, and micelles [107]. They have high physical and chemical stability, strong modification ability with multiple targeting groups, and a high transfection success rate [108]. Polymer nanoparticles have diverse functions, which can not only target and image cancer cells but also encapsulate drugs in their core, improving the solubility and delivery efficiency of drugs. In addition, appropriate shapes and sizes can be designed to control the release dose of drugs as requested [109,110]. Additionally, control of drug release in the polymer nano-drug delivery system (nano-DDS) can also be achieved by changing the pH, magnetic thermal environment, and other conditions [111,112]. Various forms of polymers, such as poly l-lysine (PLL) [109], cyclodextrin [113], and poly l-arginine (PLA) [114], can be utilized. Lipid–polymer hybrid nanoparticles (LPHNPs) consist of liposomes and polymeric nanoparticles, with a lipid layer enveloping a polymer-formed core. Therefore, LPHNPs possess the advantages of both polymer and LNPs, including high encapsulation efficiency and good stability of the polymer core. The lipid shell enhances stability, facilitates cellular uptake, and promotes better interaction with biomolecules [115,116]. Anbazhagan et al. prepared FA and PTX-supported polyamylamine (PAMAM) dendrimer and conjugated arginine-glycine-asparagine (RGD) to overcome multidrug resistance in oral cancer cells. Moreover, RGD-PAMAM-FP can deliver more PTX in KBCH-R8-5 cells than PAMAM-FP [117].
3.4 Mesoporous silica nanoparticles (MSNs)
MSNs are commonly used in biomedicine. Silica is considered safe by the US Food and Drug Administration (FDA) [118]. When using nanoparticles for disease treatment, it is necessary to consider cellular uptake and intracellular transport, which are closely related to the properties of nanomaterials. Due to their unique properties, MSNs are one of the ideal carriers for loading and delivering various drugs and biomolecules due to their stability, large surface area, pore volume, storage space for loaded drugs, high thermal stability, and good biocompatibility [119,120]. The amount of therapeutic molecules loaded can be varied by changing the size of the MSN pores and channels [121]. At the same time, MSNs have great advantages in biological degradation and excretion [122]. Functionalization or modification of the MSN surface can improve cargo loading and cell uptake [123]. Porous and nonporous silica NPs can be hydrolytically degraded to water-soluble acids and excreted in urine [124]. Compared with traditional drugs, MSNs may immunomodulate HCC TME after being taken up by immune cells [125], allowing antigen-presenting cells (APCs) to recognize tumor vaccines carried by MSNs. Furthermore, miRNAs and growth factors released from MSNs can enrich regulatory T cells (Tregs) through the PI3K/Akt pathway [126,127].
3.5 Iron oxide nanoparticles (IONPs)
MNPs have gained widespread use in recent years. Compared to other inorganic nanoparticles, MNPs have more potential for targeted delivery due to their unique properties. Among these, IONPs are particularly noteworthy due to their good biocompatibility, unique magnetic properties, stable biochemical properties, easy surface functionalization, and shape change. Excessive iron will lead to an increase in active oxygen and cause poisoning [128]. IONPs are typically coated with protective materials such as PEG, polyvinyl alcohol, or chitosan coated with γ-Fe3O2 or Fe3O4 [129]. As Fe3O4 has broad near-infrared absorption and high photothermal conversion efficiency, IONPs are often used for drug delivery by binding specific proteins or utilizing external magnetic fields known as magnetic targeting carriers (MTCs) [130]. IONPs can also increase tumor temperature, thereby enhancing the therapeutic effect of radiotherapy and chemotherapy [131]. Studies have shown that IONPs can inhibit tumor cell growth by transforming macrophages to an M1-like phenotype and increasing reactive oxygen species production (Figure 4) [132]. IONPs can be functionalized with other bioactive substances and then bound to the composite to enter cells [133]. Superparamagnetic iron oxide nanoparticles (SPIONs), formed after surface modification with a silicon dioxide layer, exhibit greater stability, and better biocompatibility while increasing the volume-surface-area ratio and reducing the release of iron.

IONPs increase the production of ROS in macrophages and promote the polarization of M1 tumor-associated macrophages (TAMs) in M2 TAMs.
4 Nanoparticles and liver
4.1 Nanoparticles enter the liver cells
Nanoparticles can be administered orally or intravenously. Studies have shown that after administration, nanoparticles can be distributed in multiple organs, including the heart, lungs, brain, liver, and kidney [134]. Nanoparticles smaller than 6 nm can be excreted into the urine through the kidneys [135]. As a biological filtration system, the liver has the highest accumulation of nanoparticles, especially those larger than 6 nm [134]. After enrichment and accumulation in the liver, nanoparticles are metabolized and excreted from the body, resulting in less therapeutic effect of drugs than expected or even producing hepatotoxicity [136]. Further, the clearance of nanoparticles by the liver is related to the size and surface charge of the nanoparticles. Nanoparticles have to pass through many barriers before reaching the target sites. The first one is the RES, composed of the liver and spleen, which can quickly remove foreign bodies in circulation. Others are crossing the vascular endothelium into the target tissue, passing through the extracellular matrix, cytoplasmic membrane, and intracellular localization [137]. RES is mainly composed of Kupffer cells, which can phagocytize nanoparticles, remove foreign bodies, and block the entry of nanoparticles into liver cells. Therefore, nanoparticles need to avoid Kupffer cells, penetrate the endothelium, and finally, enter the liver cells after passing through the Disse gap [138].
4.2 Interaction between nanoparticles and liver cells
The liver is composed of 60–70% parenchymal cells and non-parenchymal cells; parenchymal cells are hepatocytes, and non-parenchymal cells include hepatic sinusoidal endothelial cells, Kupffer cells, and hepatic stellate cells. Most nanoparticles are removed via the hepatobiliary pathway after absorption by non-parenchymal cells. Monocytes in the blood adhere to the liver tissue and are polarized in Kupffer cells. When the nanoparticles have high cationic and anionic surface charges, they adsorb a large amount of serum proteins to form a “protein crown” and increase the interaction with macrophages [138]. In addition, the larger surface area of the nanoparticles increases their affinity for interacting with neighboring receptors [138]. After being ingested into liver cells, nanoparticles can interact with a variety of enzymes. For example, large SiO2 nanoparticles (160–180 nm) significantly inhibited the catalytic activity of human liver microsomes [134]. When nanoparticles are present in the liver tissue, they stimulate oxidative stress and inflammation. Gaiser et al. found that Ag nanoparticles induced upregulation of mRNA expression of different inflammatory cytokines in liver tissue, especially IL-8, TNF-α, and receptor IL-1RI, suggesting that repeated exposure to Ag nanoparticles induced an increase in serum proinflammatory cytokines [139].
5 Nanoparticles and tumor
5.1 TME
TME is composed of immune cells, non-immune components, and extracellular matrix [140–142], including T cells, B cells, TAMs, extracellular matrix, and cytokines [143]. The common characteristics of TME are hypoxia and acidic pH, which can reduce the sensitivity of tumor cells to anticancer drugs [144,145]. TME has gained extensive attention because immune cells in the TME play an important role in the process of tumorigenesis [146]. Nanoparticles accumulate in the liver because of isolation by macrophages or Kupffer, which is a great challenge to transport nanoparticles to the tumor site for treatment. Studies have shown that Kupffer cells with high expression of M2 marker (CD163) can absorb more nanoparticles than Kupffer cells with low expression of surface CD163 [147]. TME is heterogeneous, and early TME even tends to play an anti-tumor role [148]. Among them, macrophages infiltrating the TME become TAMs [149], which can be divided into M1 and M2 macrophages. Type M1 has pro-inflammatory and anti-tumor properties, whereas type M2 has pro-tumor effects [150]. Hypoxia is a typical feature of TME, which promotes the M2 phenotype by hypoxia [151], interferes with the function of immune cells [146], and supports the growth of tumors. Cancer-associated fibroblasts (CAFs) participate in tumor growth and metastasis, prevent immune cells from entering TME, and play an important role in the occurrence and development of TME [152]. Tregs support tumor cells indirectly and directly by interacting with stromal cells and secreting growth factors [151]. The composition of TME and the regulation of nanoparticles on the components of TME are shown in Figure 5.
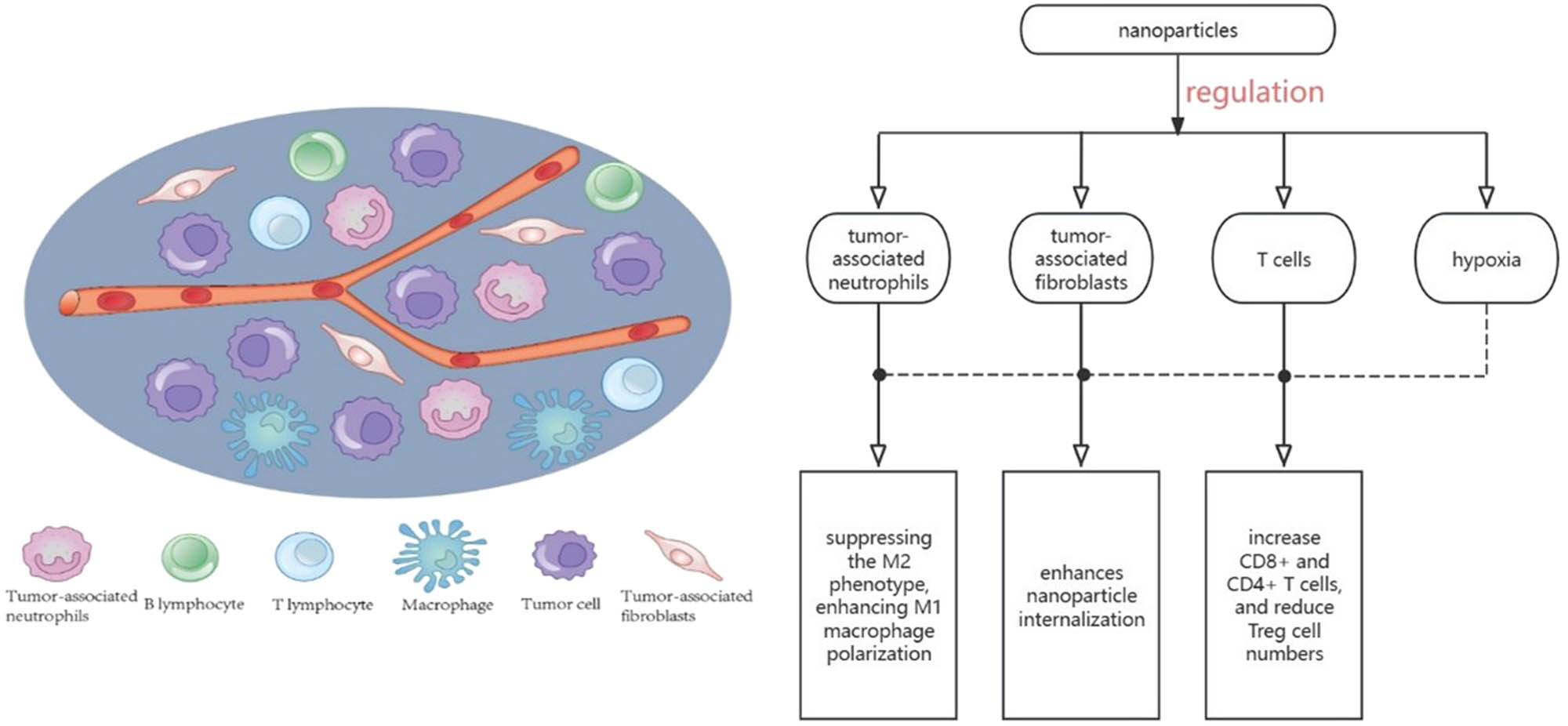
TME is composed of immune cells, non-immune components, and extracellular matrix, including T cells, B cells, TAMs, extracellular matrix, and cytokines. Nanoparticles can play an important role in tumor development by regulating the composition of TME.
5.2 Nanomedicine delivery system
DDS refers to successful targeting and accumulation of therapeutic substances at the desired site to improve drug efficacy and reduce side effects [153]. DDS can be selected to actively or passively target malignant cells or tissues according to the characteristics of nanoparticles [154]. Nanocarriers have EPR effects dependent on passive targeting, which can better promote accumulation in tumor cells [155]. However, during passive targeting, DDS efficiency may be greatly reduced due to the non-specific uptake of nanoparticles by mucosal barriers or particles. Active targeting is the selective recognition of targeted cells by carrying ligands that bind to receptors or carriers on the surface of nanoparticles [96]. When used as DDS, nanoparticles can improve the half-life of drugs and have better solubility and bioavailability, thus better controlling distribution, reducing toxicity of drugs, and improving their efficacy [156,157]. Therefore, the nanocarrier can deliver drugs to targets without damaging the surrounding normal cells [158]. Nanoparticle delivery includes anti-tumor drugs, compounds, and siRNAs [159]. Common nanocarriers and their loaded drugs are shown in Table 2.
Common nanocarriers and their loaded drugs
| Nanocarriers | Drug | Target | Mechanism | Ref. |
|---|---|---|---|---|
| PLA and PLGA NPs | Sorafenib, PTX, 5-FU, genes | HepG2, HCC1, Hep3B | Delivery of drugs and genes | [160–162] |
| PEG NPs | Sorafenib and SAL | HepG2 | Increase the cellular uptake and reduce general cytotoxicity and delivery of drugs | [162–164] |
| Lipid NPs | Sorafenib and doxorubicin | HepG2, Hep3B | Improve efficiency and delivery of drugs | [162,165,166] |
| Silica NPs | Sorafenib, doxorubicin, and genes | HepG2, SMMC-7721 | Delivery of drugs and genes and photothermal therapy | [162,167] |
| Carbon NPs | Sorafenib and doxorubicin | HepG2, SMMC-7721 | Delivery of drugs, enhance drug release, and photothermal therapy | [162,168] |
| Calcium NPs | Sorafenib, doxorubicin, and genes | HepG2, SMMC-7721 | Delivery of drugs, immune adjuvant properties, and reduce the general cytotoxicity | [169–171] |
| Gold NPs | Sorafenib, doxorubicin, 5-FU, and genes | HepG2, Huh-7 | Delivery of drugs, immunotherapy, photothermal therapy, and radiotherapy | [85,89,172,173] |
| Liposomes | Sorafenib, doxorubicin, 5-FU, and genes | HepG2, H22 | Delivery of drugs and genes | [99,174,175] |
| Zinc oxide NPs | Sorafenib, doxorubicin, and genes | HepG2 | Delivery of drugs and genes | [176–178] |
| Albumin NPs | Doxorubicin | HepG2 | Delivery of drugs | [179,180] |
| Chitosan-based NPs | Gemcitabine, 5-FU, doxorubicin, and curcumin | HepG2 | Delivery of drugs | [179,181] |
5.3 Nanoparticles targeting TME
As mentioned above, various composition structures of the tumor site are of great significance for the occurrence and development of tumors and also have an impact on the targeted therapy of tumors. For example, the abnormal vascular structures at the tumor site can sometimes render targeted therapy ineffective, the extracellular matrix can sometimes inhibit the penetration of drugs into the deep tissue of the tumor, and the hypoxic environment of the tumor sometimes leads to decreased responsiveness to anticancer therapy [182]. The regulation of TME can not only reduce the obstacles of nanoparticle delivery but also improve the sensitivity of the tumor to treatment and improve the therapeutic effect of the tumor. Therefore, the role of nanoparticles targeting various components of TME to regulate TME has been widely studied.
Nanoparticles can regulate the polarization of TAMs, inhibit the survival and function of TAMs, and target TAMs localized to TME. The problems of short cycle time, non-specific delivery, and poor drug solubility in traditional TAM-targeted therapy have been overcome [183]. Jiang et al. also designed biomimetic MNPs. Fe3O4-SAS@PLT can induce apoptosis, promote macrophage polarization M1, and disrupt immunosuppression of TME [184]. TAMs not only play a role in polarization but also regulate antigen processing. ZnCDA, for example, is made up of nanopolymer coating cyclic dimeric adenosine monophosphate formation. ZnCDA priority targets TAMs in order to regulate the antigen processing and rendering, thereby generating anti-tumor T cell responses, to a certain extent, solves the problem of lack of targeted delivery of STING pathway [185]. In addition, nanoparticles can be combined with other therapies to relieve the hypoxic environment of TME by producing oxygen in situ or delivering oxygen to TME, thus inhibiting tumor cells [183]. The structure of nanostructured manganese dioxide under acidic pH can be converted into oxygen and Mn2+, and glutathione oxidation for oxidized glutathione, which regulates the TME hypoxia environment to increase the anticancer curative effect [186]. Core–shell nanoparticles are nanoparticles with gold as the core and silver as the shell, which can target CAFs in TME and effectively inhibit the expression of osteopontin in CAFs without affecting the expression of CAF biomarkers, thus impeding the progression of cancer [187]. Abnormal blood vessels in TME limit T cell infiltration, impede drug delivery, block tumors, and hinder therapeutic response. Nanoparticles can simultaneously deliver anti-angiogenic drugs and other anti-tumor drugs, significantly amplifying the effects of both nanoparticles and chemotherapy. Antiangiogenic drugs are able to normalize blood vessels temporarily, and the purpose of promoting vascular normalization is to improve the efficacy of other antitumor treatments [183].
6 Nanoparticles in combination with therapies
6.1 Nanoparticles combined with chemotherapy
Although chemotherapy is widely used for cancer treatment, it is easy to develop drug resistance along with non-specificity, leading to adverse reactions. To address these issues, nanoparticles have been used for drug delivery [188]. Encapsulating chemotherapeutic drugs in nanoparticles not only reduces their toxicity associated with cosolvents but also reduces precipitation and improves drug stability [71]. As an effective DDS, nanoparticles can reshape TME, penetrate barriers of tumor tissue, and target drugs to tumor tissue. They can achieve active and passive targeting while improving the properties of drugs and increasing the concentration of drugs in tumor sites, thereby improving efficacy and reducing side effects [189]. Li et al. established an active target for mesoporous silica DDS nanoparticles by folic acid (FA)-modified pegylated lipid bilayer (FA-LB-MSNs). Bioadhesion between FA and its receptor significantly increased the uptake of FA-LB-MSNs by NB4 cells. Compared with MSN alone, FA-LB-MSNs exhibited sustained drug release, enabling the simultaneous release of PTX and TanIIA from the carrier [190].
6.2 Nanoparticles combined with targeted therapy
Most anticancer drugs exhibit limited accumulation at cancer sites, resulting in systemic toxicity. The nanoparticles have no heterogeneity in the tumor, along with superior encapsulation capabilities and EPR effect, which enables better aggregation within tumor regions. Therefore, utilizing nanoparticles for targeted therapy offers improved therapeutic outcomes while minimizing off-target effects.
6.2.1 Passive targeting
The EPR effect was first proposed by Matsumura and Maeda [191] more than 30 years ago when they found that some macromolecules accumulated preferentially in tumor tissues. Passive targeting of nanoparticles refers to the passive accumulation of nanoparticles in tumor sites [192], which can be achieved by the EPR effect in tumor cells [155]. Passive targeting mainly depends on the physical and chemical properties of the drug and carrier, such as size and surface charge [193]. Small nanoparticles can penetrate tumor tissues more effectively than large nanoparticles through the EPR effect. Although large nanoparticles have better retention in blood vessels, they are quickly eliminated from the tumor tissue after extravasation [194]. Passive targeting enhances the EPR effect by exploiting the incomplete basement membrane of vasculature in the TME, while drug release targeting specific sites can be achieved by utilizing the specific pH and enzyme environment of tumors [193].
6.2.2 Active targeting
However, passive targeting methods suffer from the heterogeneity of the EPR effect, and the EPR effect alone is not sufficient to achieve nanoparticle accumulation. Active targeting can enhance the EPR effect and improve drug delivery efficiency beyond passive targeting. Active targeting can serve as an improved strategy for passive targeting to enhance the stagnation of nanoparticle accumulation at tumor sites [195]. To achieve accurate tumor targeting, active targeting requires affinity recognition, retention, and promotion of target cell uptake by functionalizing the surface of the nanoparticle carrier [193,196]. Active targeting is based on specific intermolecular recognition [162], and the binding of ligands to receptors on the surface of target cells can increase the uptake of nanoparticles by cells and improve therapeutic efficacy [197]. Strategies for active targeting include receptor–antibody, aptamer–ligand, and polysaccharide. Ligands are a variety of biomolecules, such as antibodies, proteins, vitamins, and other small organic molecules, while receptors are surface molecules of tumor cells, molecules secreted by tumor cells, or proteins in organs. The density of ligands on nanoparticles determines their affinity for substrates, with higher densities leading to increased cellular uptake capacity [198]. Active targeting enables more particles to enter tumor tissues by specifically identifying targeted cancer cells while reducing free nanoparticle concentration, thereby enhancing the EPR effect [192].
6.3 Nanoparticles combined with radiotherapy
Radiotherapy relies on high radiation to destroy DNA in tumor cells directly or indirectly. With the development of nanotechnology, heavy metal nanoparticles such as gold and silver nanoparticles have shown good radiosensitization effect and are easy to metabolize and eliminate while effectively absorbing and emitting radiation energy [199,200]. Gold nanoparticles are capable of accelerating DNA fragmentation when exposed to radiation [201]. Additionally, liposomes and polymers have also been used as carriers to deliver radiosensitizers [202]. Tumor recurrence after radiotherapy is common due to radiation resistance of tumor cells caused by tumor hypoxia [203,204]. Due to limited angiogenesis of the tumor, extensive hypoxia occurs in tumor cells, thereby promoting their proliferation [205]. It has been demonstrated that combining gold nanoparticles with radiotherapy can enhance the efficacy of anti-tumor therapy, especially in hypoxic regions [206]. Quercetin (QT) also has radiosensitizing properties and can be conjugated with the radiosensitizer agent iron oxide to form quercetin-conjugated magnetite nanoparticles (QMNPs). It can also be combined with MSNs, which, when used in conjunction with radiotherapy, exhibit potent anti-cancer effects by inducing apoptosis of tumor cells [207,208]. Yang et al. designed a nanoparticle called AU-Pt NPS, composed of the metallic elements Au and Pt. Au-Pt NPs can enhance the killing effect of radiation and inhibit the growth of tumors without obvious damage to non-tumor tissue. Because Au-Pt NPs mimic enzyme activity, they can enhance co-radiosensitivity and catalyze the conversion of H2O2 into O2 [209]. Guan et al. designed a radio-immunostimulant nanomedical drug (IPI549@HMP), which was composed of monodisperse solid silica reacting with potassium permanganate (KMnO4) solution and coated with a uniform layer of MnO2. Then, the resulting sSiO2@MnO2 is etched with Na2CO3 solution to obtain H MnO2. The final IPI549@HMP nanosystem can be obtained by modifying and loading drugs in turn. The results show that IPI549@HMP can be an effective radiosensitizer under anaerobic TME [210]. However, because liver cancer is not sensitive to radiotherapy, radiotherapy is typically used in breast cancer, bone metastasis, etc.
6.4 Nanotherapy combined with gene therapy
6.4.1 CRISPR-Cas9 therapy
Many liver-related diseases are highly correlated with gene mutations, so gene editing is a reasonable approach to treat liver diseases by changing genome sequences. Among these, clustered, regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated nuclease protein 9 (Cas9) have been shown to be a powerful genome editing tool [211]. CRISPR-Cas9 is delivered by viral vectors, non-viral vectors, or physical methods to overcome tissue and cell membrane barriers to be transported to the nucleus [212]. One of the most commonly used is viral vector-mediated gene delivery, which has a very small loading capacity [213] and may cause mutations and immune responses if the viral vector integrates into host cells [214]. A number of nanodelivery systems have been developed to deliver CRISPR-Cas9, such as liposomes, LNPs, polymers, and gold nanoparticles [215]. The delivery complex can select Cas9 ribonucleoprotein (RNP), which avoids the trouble of DNA and RNA during transcription and translation, but the delivery of RNPs is limited by the loading capacity of viral and non-viral vectors [212]. The delivery of RNP in vivo is currently focused on polymeric nanoparticles and liposomes [216]. Tang et al. prepared a hybrid lipid NP (named Gal-LGCP) with a particle size of 106 nm, which has cell targeting, cell membrane penetration, and subcellular targeting, and can be used to deliver CRISPR-Cas9. In in vitro experiments, Gal-LGCP induced about 60% PCSK9 knockouts in Hepa1-6 cells; in vivo, Gal-LGCP effectively targets liver cells. In addition, Gal-LGCP can also knock down PCSK9 in hepatocytes and downregulate LDLC levels [217].
6.4.2 siRNA therapy
Small interfering RNA (siRNA), mRNA, and plasmid RNA have the potential to treat many diseases through gene editing, silencing pathological genes, or expressing therapeutic proteins [218,219]. SiRNA can inhibit oncogene expression by stimulating enzymatic cleavage of target mRNA [220]. Due to the rapid degradation of naked DNA or RNA in body fluids, siRNA is easily degraded during application and has poor cellular uptake. SiRNA is difficult to accumulate in target tissues and penetrate target cells [218,221]. Previous studies have demonstrated that stable nucleic acid-lipid particles (SNALPs) formulated siRNAs of HBV exhibit higher efficacy in the liver [222]. The US FDA has approved the delivery of siRNAs by LNPs to target liver cells for treating transthyretin-induced amyloidosis [223]. Additionally, there are ongoing studies on synthesizing GCGA NPs, a new targeting carrier chitosan nanoparticles modified with glycyrrhetinic acid (GA) and lactobionic acid (LA), which protect siRNA from degradation and target hepatoma cells [224]. Furthermore, by loading siRNA into SP94 peptide-modified nanospheres assembled using cationized amylose (CA), SPIONs, and tetraphenylethylene (TPE), the formation of CSP/TPE@siRNA-SP94 NPs can significantly inhibit Huh-7 cell proliferation [225].
7 Partial clinical studies of nanoparticles
At present, many nanoparticles have entered clinical research. For example, Su prepared NP-SFB nanoparticles loaded with sorafenib in acetone water using the nanoprecipitation method and then modified them with the Traut reagent to obtain thiol-modified antibody (AB-SH). Then, the Ab-SFB-NP nano-DDS was prepared by mixing NP-SFB and AB-SH at room temperature for 2 h [226]. In controlled experiments, sorafenib delivery using the Ab-SFB-NP nanodelivery system has a significantly higher control rate than direct oral administration. The AB-SFB-NP nanodelivery system has high safety, although, in the experiments, diarrhea and other toxic reactions occurred, but after symptomatic treatment, the symptoms were controlled, and no death was observed [226]. He et al. [227] prepared carrier-free indocyanine green nanoparticles (nanoICG) using a green and safe technique without altering the molecular structure of ICG. It has excellent photobleaching resistance, imaging sensitivity, and deposition of iodol in tumors. Therefore, nano-ICG is dispersed into iodide by ultra-stable homogeneous mixed preparation technology (SHIFT and nanoICG) for hepatic artery embolization combined with fluorescence laparoscopic hepatectomy, which is still in the first stage of research.
Zheng et al. added chitosan-SeNPs and lipiodol into the self-made Gel-SOR solution, stirred, and dialyzed for 24 h to obtain Gel-SOR-LUF-SeNPs. It was found that Gel-SOR-LUF-SeNPs not only have high anti-tumor efficacy but also low toxicity towards nude mice. Gel-SOR-LUF-SeNPs reduced the expressions of Ki67 and CD34, thereby inhibiting the proliferation of tumor cells, the formation of tumor blood vessels, and promoting the apoptosis of tumor cells, thereby significantly increasing the concentration of selenium in tumors and significantly inhibiting the growth of HepG2 tumors in nude mouse models [228]. Yang et al. prepared (ICG + S)@mSiO2 by adding ICG and sorafenib to the MSN aqueous solution and used it to simulate mice. Experiments have shown that SiO2 is an ideal drug carrier with good biocompatibility and a high drug loading rate. (ICG + S)@mSiO2 is a multifunctional diagnostic and therapeutic nanosystem with a highly efficient photothermal therapeutic effect, which can enhance immunity and release sorafenib to enhance tumor therapeutic effect [229].
However, not all clinical studies are in good progress. miR-34a is a naturally occurring tumor suppressor that is lost or expressed at reduced levels in a wide range of tumor types. MRX34, developed by Hong et al. [230], is a liposomal formulation of miR-34a with a length of 23-nt encapsulated in liposomal nanoparticles. In the Phase 1 study, one patient with HCC died due to severe immune-mediated toxicity. Therefore, because the overall response rate of 4% did not exceed the risk of serious immune-related adverse reactions, clinical development of MRX34 was discontinued [74].
8 Summary and outlook
As an emerging discipline, nanotechnology has made remarkable advancements in various fields due to its distinctive advantages. Especially in the medical domain, nanoparticles exhibit exceptional biocompatibility, superior body surface area ratio, and stable biochemical properties that offer a wide range of new approaches for more effective HCC treatment. Nanoparticles can construct efficient DDS and synergize with conventional therapies to enhance drug targeting and improve therapeutic outcomes across diverse modalities. Nanoparticles have demonstrated their ability to modulate TME and facilitate increased production of reactive oxygen species to inhibit tumor cell growth.
At present, most nanoparticles used for targeted delivery in clinics are nanoparticles composed of lipids or polymers. LNPs possess low toxicity levels and are considered nano-safe carriers; hence, they represent the most commonly utilized carriers for siRNA delivery while exhibiting liver-targeting capabilities upon systemic administration. Polymer nanoparticles exhibit diverse morphological structures along with a variety of target groups. Furthermore, research on synthesizing LPHNPs has been conducted. These LPHNPs are composed of polymer encapsulated by lipid layers, thereby combining the advantages of both materials, such as high encapsulation efficiency and enhanced cellular uptake capability. Additionally, other types of nanoparticles, such as gold nanoparticles, MSNs, and IONPs, possess their unique advantages. By functionalizing the surfaces of these various nanoparticle types, they can better serve HCC treatment by enhancing the efficacy of conventional therapies, such as photothermal and targeted therapy, while also increasing the load of therapeutic drugs.
Although nanoparticles possess numerous advantages, there are still unsolved issues, such as the drug release kinetics and safety concerns associated with their use. In particular, the safety of heavy metal nanoparticles has hindered their wide clinical application. Consequently, it is imperative to allocate more attention to addressing these challenges and providing viable methodologies and approaches for the treatment of HCC utilizing nanoparticles.
This review first summarizes the limitations of common HCC treatments. Then, the common nanoparticles and their characteristics, as well as the regulation of TME by nanoparticles, are described. Next, we summarize the role of nanoparticles in some common treatments. Finally, we describe several clinical studies that included both success and failure. Our review aims to provide ideas for the use of nanoparticles in the treatment of HCC and bring better therapeutic effects to HCC patients.
-
Funding information: This work was supported by Beijing Hospitals Authority Clinical Medicine Development of special funding support (XMLX 202127); the Capital Health Research and Development of special funding support (2022-1-2172); the National Key Research and Development Program (2022YFC2603500, 2022YFC2603505); Beijing Municipal Health Commission High-Level Public Health Technical Personnel Construction Project (discipline leader-03-26); and the Digestive Medical Coordinated Development Center of Beijing Hospitals Authority (XXZ0302).
-
Author contributions: ML, YX, and WY contributed to the study concept and design. ZZ, WC, XL, WD, TJ, SW, LY, YL, XB, YL, MX, and LZ collected and sorted out the literature. ZZ, WC, XL, TJ, and SW drew pictures. ZZ, WC, XL, DW, TJ, and SW wrote the first draft. ML edited the English version. ML, YX, and WY approved the submitted version after modification. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. 2021;71(3):209–49.10.3322/caac.21660Suche in Google Scholar PubMed
[2] Dopazo C, Søreide K, Rangelova E, Mieog S, Carrion-Alvarez L, Diaz-Nieto R, et al. Hepatocellular carcinoma. Eur J Surg Oncol. 2024 Jan;50(1):107313.10.1016/j.ejso.2023.107313Suche in Google Scholar PubMed
[3] Maki H, Hasegawa K. Advances in the surgical treatment of liver cancer. BST. 2022 Jun;16(3):178–88.10.5582/bst.2022.01245Suche in Google Scholar PubMed
[4] Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021 Jan 21;7(1):6.10.1038/s41572-020-00240-3Suche in Google Scholar PubMed
[5] Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: A Cancer J Clin. 2023;73(1):17–48.10.3322/caac.21763Suche in Google Scholar PubMed
[6] McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatol. 2021 Jan;73(Suppl 1):4–13.10.1002/hep.31288Suche in Google Scholar PubMed PubMed Central
[7] Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. 2018;68(6):394–424.10.3322/caac.21492Suche in Google Scholar PubMed
[8] Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020 Jul;159(1):335–49.e15.10.1053/j.gastro.2020.02.068Suche in Google Scholar PubMed PubMed Central
[9] Singal AG, Kanwal F, Llovet JM. Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy. Nat Rev Clin Oncol. 2023 Dec;20(12):864–84.10.1038/s41571-023-00825-3Suche in Google Scholar PubMed
[10] Piñero F, Dirchwolf M, Pessôa MG. Biomarkers in hepatocellular carcinoma: Diagnosis, prognosis and treatment response assessment. Cells. 2020 Jun;9(6):1370.10.3390/cells9061370Suche in Google Scholar PubMed PubMed Central
[11] Feng H, Li B, Li Z, Wei Q, Ren L. PIVKA-II serves as a potential biomarker that complements AFP for the diagnosis of hepatocellular carcinoma. BMC Cancer. 2021 Apr;21:401.10.1186/s12885-021-08138-3Suche in Google Scholar PubMed PubMed Central
[12] Cunha GM, Sirlin CB, Fowler KJ. Imaging diagnosis of hepatocellular carcinoma: LI-RADS. Chin Clin Oncol. 2021 Feb;10(1):3.10.21037/cco-20-107Suche in Google Scholar PubMed
[13] He Q, Liu K, Wang C, Zhang J. Interpretation on the key points of Guideline for the Diagnose and Treatment of Primary Liver Cancer (2022 Edition). Med J West China. 2023;35(4):474–9.Suche in Google Scholar
[14] Bao X, Chen L, Liu Y, Sheng H, Wang K, Luo Y, et al. Treatment of liver cancer: Role of the traditional mongolian medicine. Evid Based Complement Altern Med. 2022 Feb;2022:6535977.10.1155/2022/6535977Suche in Google Scholar PubMed PubMed Central
[15] Álvarez-Mercado AI, Caballeria-Casals A, Rojano-Alfonso C, Chávez-Reyes J, Micó-Carnero M, Sanchez-Gonzalez A, et al. Insights into growth factors in liver carcinogenesis and regeneration: An ongoing debate on minimizing cancer recurrence after liver resection. Biomedicines. 2021 Sep;9(9):1158.10.3390/biomedicines9091158Suche in Google Scholar PubMed PubMed Central
[16] Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020 Jan;1873(1):188314.10.1016/j.bbcan.2019.188314Suche in Google Scholar PubMed PubMed Central
[17] Zhao Y, Zhang YN, Wang KT, Chen L. Lenvatinib for hepatocellular carcinoma: From preclinical mechanisms to anti-cancer therapy. Biochim Biophys Acta (BBA) – Rev Cancer. 2020 Aug;1874(1):188391.10.1016/j.bbcan.2020.188391Suche in Google Scholar PubMed
[18] Graur F, Puia A, Mois EI, Moldovan S, Pusta A, Cristea C, et al. Nanotechnology in the diagnostic and therapy of hepatocellular carcinoma. Materials (Basel). 2022 May;15(11):3893.10.3390/ma15113893Suche in Google Scholar PubMed PubMed Central
[19] Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F. The history of nanoscience and nanotechnology: From chemical–physical applications to nanomedicine. Molecules. 2019 Dec;25(1):112.10.3390/molecules25010112Suche in Google Scholar PubMed PubMed Central
[20] Orcutt ST, Anaya DA. Liver resection and surgical strategies for management of primary liver cancer. Cancer Control. 2018 Jan;25(1):1073274817744621.10.1177/1073274817744621Suche in Google Scholar PubMed PubMed Central
[21] Vibert E, Schwartz M, Olthoff KM. Advances in resection and transplantation for hepatocellular carcinoma. J Hepatol. 2020 Feb;72(2):262–76.10.1016/j.jhep.2019.11.017Suche in Google Scholar PubMed
[22] Sugawara Y, Hibi T. Surgical treatment of hepatocellular carcinoma. BST. 2021 Jun;15(3):138–41.10.5582/bst.2021.01094Suche in Google Scholar PubMed
[23] Famularo S, Donadon M, Cipriani F, Ardito F, Carissimi F, Perri P, et al. Hepatocellular carcinoma surgical and oncological trends in a national multicentric population: The HERCOLES experience. Updates Surg. 2020 Jun;72(2):399–411.10.1007/s13304-020-00733-6Suche in Google Scholar PubMed
[24] Wang Q, Tang M, Zhang S. Comparison of radiofrequency ablation and surgical resection for hepatocellular carcinoma conforming to the Milan criteria: A meta-analysis. ANZ J Surg. 2021;91(7–8):E432–8.10.1111/ans.16560Suche in Google Scholar PubMed
[25] Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N Engl J Med. 1996 Mar;334(11):693–700.10.1056/NEJM199603143341104Suche in Google Scholar PubMed
[26] Sidali S, Trépo E, Sutter O, Nault JC. New concepts in the treatment of hepatocellular carcinoma. United Eur Gastroenterol J. 2022 Sep;10(7):765–74.10.1002/ueg2.12286Suche in Google Scholar PubMed PubMed Central
[27] Izzo F, Granata V, Grassi R, Fusco R, Palaia R, Delrio P, et al. Radiofrequency ablation and microwave ablation in liver tumors: An update. Oncologist. 2019 Oct;24(10):e990–1005.10.1634/theoncologist.2018-0337Suche in Google Scholar PubMed PubMed Central
[28] Qi X, Yang M, Ma L, Sauer M, Avella D, Kaifi JT, et al. Synergizing sunitinib and radiofrequency ablation to treat hepatocellular cancer by triggering the antitumor immune response. J Immunother Cancer. 2020 Oct;8(2):e001038.10.1136/jitc-2020-001038Suche in Google Scholar PubMed PubMed Central
[29] Wang K, Wang C, Jiang H, Zhang Y, Lin W, Mo J, et al. Combination of ablation and immunotherapy for hepatocellular carcinoma: Where we are and where to go. Front Immunol. 2021 Dec;12:792781.10.3389/fimmu.2021.792781Suche in Google Scholar PubMed PubMed Central
[30] Seki T, Wakabayashi M, Nakagawa T, Itho T, Shiro T, Kunieda K, et al. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer. 1994 Aug;74(3):817–25.10.1002/1097-0142(19940801)74:3<817::AID-CNCR2820740306>3.0.CO;2-8Suche in Google Scholar
[31] Shiina S, Sato K, Tateishi R, Shimizu M, Ohama H, Hatanaka T, et al. Percutaneous ablation for hepatocellular carcinoma: Comparison of various ablation techniques and surgery. Can J Gastroenterol Hepatol. 2018 Jun;2018:4756147.10.1155/2018/4756147Suche in Google Scholar
[32] Zhang M, Ma H, Zhang J, He L, Ye X, Li X. Comparison of microwave ablation and hepatic resection for hepatocellular carcinoma: A meta-analysis. Onco Targets Ther. 2017 Oct;10:4829–39.10.2147/OTT.S141968Suche in Google Scholar PubMed PubMed Central
[33] Leuchte K, Staib E, Thelen M, Gödel P, Lechner A, Zentis P, et al. Microwave ablation enhances tumor-specific immune response in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2021;70(4):893–907.10.1007/s00262-020-02734-1Suche in Google Scholar PubMed PubMed Central
[34] Groeschl RT, Pilgrim CHC, Hanna EM, Simo KA, Swan RZ, Sindram D, et al. Microwave ablation for hepatic malignancies: a multiinstitutional analysis. Ann Surg. 2014 Jun;259(6):1195–200.10.1097/SLA.0000000000000234Suche in Google Scholar PubMed
[35] Chen L, Ren Y, Sun T, Cao Y, Yan L, Zhang W, et al. The efficacy of radiofrequency ablation versus cryoablation in the treatment of single hepatocellular carcinoma: A population‐based study. Cancer Med. 2021 May;10(11):3715–25.10.1002/cam4.3923Suche in Google Scholar PubMed PubMed Central
[36] Zhao Y, Bai J, Wang X, Zhang Y, Yan X, Qi J, et al. Threatment strategies for recurrent hepatocellular carcinoma patients: Ablation and its combination patterns. J Cancer. 2024 Feb;15(8):2193–205.10.7150/jca.93885Suche in Google Scholar PubMed PubMed Central
[37] Adwan H, Vogl TJ, Balaban Ü, Nour-Eldin NEA. Percutaneous thermal ablation therapy of hepatocellular carcinoma (HCC): Microwave ablation (MWA) versus laser-induced thermotherapy (LITT). Diagnostics (Basel). 2022 Feb;12(3):564.10.3390/diagnostics12030564Suche in Google Scholar PubMed PubMed Central
[38] Long H, Zhuang B, Huang G, Li X, Lin M, Long J, et al. Safety and local efficacy of laser ablation for the extrahepatic metastasis of hepatocellular carcinoma: An available treatment strategy. Coatings. 2020 Oct;10(10):951.10.3390/coatings10100951Suche in Google Scholar
[39] Bäumler W, Beyer LP, Lürken L, Wiggermann P, Stroszczynski C, Dollinger M, et al. Detection of incomplete irreversible electroporation (IRE) and microwave ablation (MWA) of hepatocellular carcinoma (HCC) using iodine quantification in dual energy computed tomography (DECT). Diagnostics (Basel). 2022 Apr;12(4):986.10.3390/diagnostics12040986Suche in Google Scholar PubMed PubMed Central
[40] Freeman E, Cheung W, Kavnoudias H, Majeed A, Kemp W, Roberts SK. Irreversible electroporation for hepatocellular carcinoma: Longer-term outcomes at a single centre. Cardiovasc Intervent Radiol. 2021 Feb;44(2):247–53.10.1007/s00270-020-02666-4Suche in Google Scholar PubMed
[41] Cheung TT, Ma KW, She WH. A review on radiofrequency, microwave and high-intensity focused ultrasound ablations for hepatocellular carcinoma with cirrhosis. Hepatobiliary Surg Nutr. 2021 Apr;10(2):193.10.21037/hbsn.2020.03.11Suche in Google Scholar PubMed PubMed Central
[42] Gu L, Shen Z, Ji L, Ng DM, Du N, He N, et al. High-intensity focused ultrasound alone or combined with transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with unsuitable indications for hepatectomy and radiofrequency ablation: A phase II clinical trial. Surg Endosc. 2022 Mar;36(3):1857–67.10.1007/s00464-021-08465-3Suche in Google Scholar PubMed
[43] Kong C, Zhao Z, Chen W, Lv X, Shu G, Ye M, et al. Prediction of tumor response via a pretreatment MRI radiomics-based nomogram in HCC treated with TACE. Eur Radiol. 2021;31(10):7500–11.10.1007/s00330-021-07910-0Suche in Google Scholar PubMed PubMed Central
[44] Liu B, Zhang Y, Chen H, Li W, Tsochatzis E. The combination of transcatheter arterial chemoembolisation (TACE) and thermal ablation versus TACE alone for hepatocellular carcinoma. Cochrane Database Syst Rev. 2022 Jan;2022(1):CD013345.10.1002/14651858.CD013345.pub2Suche in Google Scholar PubMed PubMed Central
[45] Chang Y, Jeong SW, Young Jang J, Jae Kim Y. Recent updates of transarterial chemoembolilzation in hepatocellular carcinoma. Int J Mol Sci. 2020 Oct;21(21):8165.10.3390/ijms21218165Suche in Google Scholar PubMed PubMed Central
[46] Xu Z, Xie H, Zhou L, Chen X, Zheng S. The combination strategy of transarterial chemoembolization and radiofrequency ablation or microwave ablation against hepatocellular carcinoma. Anal Cell Pathol (Amst). 2019 Aug;2019:8619096.10.1155/2019/8619096Suche in Google Scholar PubMed PubMed Central
[47] Zhou H, Song T. Conversion therapy and maintenance therapy for primary hepatocellular carcinoma. BST. 2021 Jun;15(3):155–60.10.5582/bst.2021.01091Suche in Google Scholar PubMed
[48] Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul JL, et al. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018 Jul;69(1):182–236.10.1016/j.jhep.2018.03.019Suche in Google Scholar PubMed
[49] Ju S, Zhou C, Hu J, Wang Y, Wang C, Liu J, et al. Late combination of transarterial chemoembolization with apatinib and camrelizumab for unresectable hepatocellular carcinoma is superior to early combination. BMC Cancer. 2022 Mar;22:335.10.1186/s12885-022-09451-1Suche in Google Scholar PubMed PubMed Central
[50] Zheng L, Fang S, Wu F, Chen W, Chen M, Weng Q, et al. Efficacy and safety of TACE combined with sorafenib plus immune checkpoint inhibitors for the treatment of intermediate and advanced TACE-refractory hepatocellular carcinoma: A retrospective study. Front Mol Biosci. 2021 Jan;7:609322.10.3389/fmolb.2020.609322Suche in Google Scholar PubMed PubMed Central
[51] Tang W, Chen Z, Zhang W, Cheng Y, Zhang B, Wu F, et al. The mechanisms of sorafenib resistance in hepatocellular carcinoma: Theoretical basis and therapeutic aspects. Signal Transduct Target Ther. 2020 Jun;5:87.10.1038/s41392-020-0187-xSuche in Google Scholar PubMed PubMed Central
[52] Zhang L, Feng Q, Wang J, Tan Z, Li Q, Ge M. Molecular basis and targeted therapy in thyroid cancer: Progress and opportunities. Biochim Biophys Acta – Rev Cancer. 2023 Jul;1878(4):188928.10.1016/j.bbcan.2023.188928Suche in Google Scholar PubMed
[53] Ohishi T, Kaneko MK, Yoshida Y, Takashima A, Kato Y, Kawada M. Current targeted therapy for metastatic colorectal cancer. Int J Mol Sci. 2023 Jan;24(2):1702.10.3390/ijms24021702Suche in Google Scholar PubMed PubMed Central
[54] Masucci MT, Motti ML, Minopoli M, Di Carluccio G, Carriero MV. Emerging targeted therapeutic strategies to overcome imatinib resistance of gastrointestinal stromal tumors. Int J Mol Sci. 2023 Mar;24(7):6026.10.3390/ijms24076026Suche in Google Scholar PubMed PubMed Central
[55] Zhong L, Li Y, Xiong L, Wang W, Wu M, Yuan T, et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct Target Ther. 2021 May;6(1):201.10.1038/s41392-021-00572-wSuche in Google Scholar PubMed PubMed Central
[56] Selvarani R, Mohammed S, Richardson A. Effect of rapamycin on aging and age-related diseases—past and future. GeroScience. 2020 Oct;43(3):1135–58.10.1007/s11357-020-00274-1Suche in Google Scholar PubMed PubMed Central
[57] Hou SJ, Zhang SX, Li Y, Xu SY. Rapamycin responds to Alzheimer’s disease: A potential translational therapy. Clin Interv Aging. 2023 Oct;18:1629–39.10.2147/CIA.S429440Suche in Google Scholar PubMed PubMed Central
[58] Liu ZL, Liu JH, Staiculescu D, Chen J. Combination of molecularly targeted therapies and immune checkpoint inhibitors in the new era of unresectable hepatocellular carcinoma treatment. Ther Adv Med Oncol. 2021 May;13:17588359211018026.10.1177/17588359211018026Suche in Google Scholar PubMed PubMed Central
[59] Damaskos C, Garmpis N, Dimitroulis D, Garmpi A, Psilopatis I, Sarantis P, et al. Targeted therapies for hepatocellular carcinoma treatment: A new era ahead—A systematic review. Int J Mol Sci. 2022 Nov;23(22):14117.10.3390/ijms232214117Suche in Google Scholar PubMed PubMed Central
[60] Lee MS, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study. Lancet Oncol. 2020 Jun;21(6):808–20.10.1016/S1470-2045(20)30156-XSuche in Google Scholar PubMed
[61] Huang A, Yang XR, Chung WY, Dennison AR, Zhou J. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther. 2020 Aug;5:146.10.1038/s41392-020-00264-xSuche in Google Scholar PubMed PubMed Central
[62] Mizukoshi E, Kaneko S. Immune cell therapy for hepatocellular carcinoma. J Hematol Oncol. 2019 May;12:52.10.1186/s13045-019-0742-5Suche in Google Scholar PubMed PubMed Central
[63] Meng F, Zhao J, Tan AT, Hu W, Wang SY, Jin J, et al. Immunotherapy of HBV-related advanced hepatocellular carcinoma with short-term HBV-specific TCR expressed T cells: results of dose escalation, phase I trial. Hepatol Int. 2021 Nov;15(6):1402–12.10.1007/s12072-021-10250-2Suche in Google Scholar PubMed PubMed Central
[64] Liu Z, Liu X, Liang J, Liu Y, Hou X, Zhang M, et al. Immunotherapy for hepatocellular carcinoma: Current status and future prospects. Front Immunol. 2021 Oct;12:765101.10.3389/fimmu.2021.765101Suche in Google Scholar PubMed PubMed Central
[65] Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(8):525–43.10.1038/s41575-021-00438-0Suche in Google Scholar PubMed PubMed Central
[66] Zongyi Y, Xiaowu L. Immunotherapy for hepatocellular carcinoma. Cancer Lett. 2020 Feb;470:8–17.10.1016/j.canlet.2019.12.002Suche in Google Scholar PubMed
[67] Shi J, Liu J, Tu X, Li B, Tong Z, Wang T, et al. Single-cell immune signature for detecting early-stage HCC and early assessing anti-PD-1 immunotherapy efficacy. J Immunother Cancer. 2022 Jan;10(1):e003133.10.1136/jitc-2021-003133Suche in Google Scholar PubMed PubMed Central
[68] Shen J, Shen H, Ke L, Chen J, Dang X, Liu B, et al. Knowledge mapping of immunotherapy for hepatocellular carcinoma: A bibliometric study. Front Immunol. 2022 Jan;13:815575.10.3389/fimmu.2022.815575Suche in Google Scholar PubMed PubMed Central
[69] Jácome AA, Castro ACG, Vasconcelos JPS, Silva MHCR, Lessa MAO, Moraes ED, et al. Efficacy and safety associated with immune checkpoint inhibitors in unresectable hepatocellular carcinoma. JAMA Netw Open. 2021 Dec;4(12):e2136128.10.1001/jamanetworkopen.2021.36128Suche in Google Scholar PubMed PubMed Central
[70] Xia Y, Tang W, Qian X, Li X, Cheng F, Wang K, et al. Efficacy and safety of camrelizumab plus apatinib during the perioperative period in resectable hepatocellular carcinoma: A single-arm, open label, phase II clinical trial. J Immunother Cancer. 2022 Apr;10(4):e004656.10.1136/jitc-2022-004656Suche in Google Scholar PubMed PubMed Central
[71] Najahi-Missaoui W, Arnold RD, Cummings BS. Safe nanoparticles: Are we there yet? Int J Mol Sci. 2020 Dec;22(1):385.10.3390/ijms22010385Suche in Google Scholar PubMed PubMed Central
[72] Bommakanti V, Banerjee M, Shah D, Manisha K, Sri K, Banerjee S. An overview of synthesis, characterization, applications and associated adverse effects of bioactive nanoparticles. Environ Res. 2022 Nov;214:113919.10.1016/j.envres.2022.113919Suche in Google Scholar PubMed
[73] Sun Y, Ma W, Yang Y, He M, Li A, Bai L, et al. Cancer nanotechnology: Enhancing tumor cell response to chemotherapy for hepatocellular carcinoma therapy. Asian J Pharm Sci. 2019 Nov 1;14(6):581–94.10.1016/j.ajps.2019.04.005Suche in Google Scholar PubMed PubMed Central
[74] Hu X, Zhu H, He X, Chen J, Xiong L, Shen Y, et al. The application of Nanoparticles in immunotherapy for hepatocellular carcinoma. J Controlled Rel. 2023 Mar;355:85–108.10.1016/j.jconrel.2023.01.051Suche in Google Scholar PubMed
[75] Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–24.10.1038/s41573-020-0090-8Suche in Google Scholar PubMed PubMed Central
[76] Yokoyama R, Ii M, Masuda M, Tabata Y, Hoshiga M, Ishizaka N, et al. Cardiac regeneration by statin-polymer nanoparticle-loaded adipose-derived stem cell therapy in myocardial infarction. Stem Cell Transl Med. 2019 Oct;8(10):1055–67.10.1002/sctm.18-0244Suche in Google Scholar PubMed PubMed Central
[77] Wang W, Liu H, Lu Y, Wang X, Zhang B, Cong S, et al. Controlled-releasing hydrogen sulfide donor based on dual-modal iron oxide nanoparticles protects myocardial tissue from ischemia– reperfusion injury. IJN. 2019 Jan;14:875–88.10.2147/IJN.S186225Suche in Google Scholar PubMed PubMed Central
[78] Banik B, Surnar B, Askins BW, Banerjee M, Dhar S. Dual-targeted synthetic nanoparticles for cardiovascular diseases. ACS Appl Mater Interfaces. 2020 Feb;12(6):6852–62.10.1021/acsami.9b19036Suche in Google Scholar PubMed
[79] Fang J, Islam W, Maeda H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv Drug Delivery Rev. 2020 Jan;157:142–60.10.1016/j.addr.2020.06.005Suche in Google Scholar PubMed
[80] Kim JY, Lee WS, Seo SJ, Jung CW, Kim EH. Effects of gold nanoparticles on normal hepatocytes in radiation therapy. Transl Cancer Res. 2022 Aug;11(8):2572–81.10.21037/tcr-21-1855Suche in Google Scholar PubMed PubMed Central
[81] Zhu J, Liu Z, Pu Y, Xu J, Zhang S, Bao Y. Green synthesized gold nanoparticles from Pseudobulbus Cremastrae seu Pleiones show efficacy against hepatic carcinoma potentially through immunoregulation. Drug Deliv 29(1):1983–93.10.1080/10717544.2022.2092238Suche in Google Scholar PubMed PubMed Central
[82] Leng F, Liu F, Yang Y, Wu Y, Tian W. Strategies on nanodiagnostics and nanotherapies of the three common cancers. Nanomaterials (Basel). 2018 Mar;8(4):202.10.3390/nano8040202Suche in Google Scholar PubMed PubMed Central
[83] Ahn S, Singh P, Jang M, Kim YJ, Castro-Aceituno V, Simu SY, et al. Gold nanoflowers synthesized using Acanthopanacis cortex extract inhibit inflammatory mediators in LPS-induced RAW264.7 macrophages via NF-κB and AP-1 pathways. Colloids Surf B: Biointerfaces. 2018 Feb;162:398–404.10.1016/j.colsurfb.2017.11.037Suche in Google Scholar PubMed
[84] Lu SL, Liu WW, Cheng JCH, Lin LC, Wang CRC, Li PC. Enhanced radiosensitization for cancer treatment with gold nanoparticles through sonoporation. Int J Mol Sci. 2020 Nov;21(21):8370.10.3390/ijms21218370Suche in Google Scholar PubMed PubMed Central
[85] Liu Q, Liu H, Sacco P, Djaker N, Lamy de la Chapelle M, Marsich E, et al. CTL–doxorubicin (DOX)–gold complex nanoparticles (DOX–AuGCs): From synthesis to enhancement of therapeutic effect on liver cancer model. Nanoscale Adv. 2(11):5231–41.10.1039/D0NA00758GSuche in Google Scholar PubMed PubMed Central
[86] Tsai CY, Lu SL, Hu CW, Yeh CS, Lee GB, Lei HY. Size-dependent attenuation of TLR9 signaling by gold nanoparticles in macrophages. J Immunol. 2012 Jan;188(1):68–76.10.4049/jimmunol.1100344Suche in Google Scholar PubMed
[87] Loynachan CN, Soleimany AP, Dudani JS, Lin Y, Najer A, Bekdemir A, et al. Renal clearable catalytic gold nanoclusters for in vivo disease monitoring. Nat Nanotechnol. 2019 Sep;14(9):883–90.10.1038/s41565-019-0527-6Suche in Google Scholar PubMed PubMed Central
[88] Deng R, Shen N, Yang Y, Yu H, Xu S, Yang YW, et al. Targeting epigenetic pathway with gold nanoparticles for acute myeloid leukemia therapy. Biomaterials. 2018 Jun;167:80–90.10.1016/j.biomaterials.2018.03.013Suche in Google Scholar PubMed
[89] Sheth RA, Wen X, Li J, Melancon MP, Ji X, Wang YA, et al. Doxorubicin-loaded hollow gold nanospheres for dual photothermal ablation and chemoembolization therapy. Cancer Nanotechnol. 2020;11(1):6.10.1186/s12645-020-00062-8Suche in Google Scholar PubMed PubMed Central
[90] Kabiri S, Rezaei F. Liver cancer treatment with integration of laser emission and microwave irradiation with the aid of gold nanoparticles. Sci Rep. 2022 Jun;12(1):9271.10.1038/s41598-022-13420-wSuche in Google Scholar PubMed PubMed Central
[91] Mochizuki C, Kayabe Y, Nakamura J, Igase M, Mizuno T, Nakamura M. Surface functionalization of organosilica nanoparticles with Au nanoparticles inhibits cell proliferation and induces cell death in 4T1 mouse mammary tumor cells for DNA and mitochondrial-synergized damage in radiotherapy. Front Chem. 2022 May;10:907642.10.3389/fchem.2022.907642Suche in Google Scholar PubMed PubMed Central
[92] Al-Omar MS, Jabir M, Karsh E, Kadhim R, Sulaiman GM, Taqi ZJ, et al. Gold nanoparticles and graphene oxide flakes enhance cancer cells’ phagocytosis through granzyme-perforin-dependent biomechanism. Nanomaterials (Basel). 2021 May;11(6):1382.10.3390/nano11061382Suche in Google Scholar PubMed PubMed Central
[93] Khan SA, Shahid S, Lee CS. Green synthesis of gold and silver nanoparticles using leaf extract of Clerodendrum inerme; characterization, antimicrobial, and antioxidant activities. Biomolecules. 2020 May;10(6):835.10.3390/biom10060835Suche in Google Scholar PubMed PubMed Central
[94] Fan M, Han Y, Gao S, Yan H, Cao L, Li Z, et al. Ultrasmall gold nanoparticles in cancer diagnosis and therapy. Theranostics. 2020 Mar;10(11):4944–57.10.7150/thno.42471Suche in Google Scholar PubMed PubMed Central
[95] Witzigmann D, Kulkarni JA, Leung J, Chen S, Cullis PR, van der Meel R. Lipid nanoparticle technology for therapeutic gene regulation in the liver. Adv Drug Deliv Rev. 2020;159:344–63.10.1016/j.addr.2020.06.026Suche in Google Scholar PubMed PubMed Central
[96] Yetisgin AA, Cetinel S, Zuvin M, Kosar A, Kutlu O. Therapeutic nanoparticles and their targeted delivery applications. Molecules. 2020 May;25(9):2193.10.3390/molecules25092193Suche in Google Scholar PubMed PubMed Central
[97] Scioli Montoto S, Muraca G, Ruiz ME. Solid lipid nanoparticles for drug delivery: Pharmacological and biopharmaceutical aspects. Front Mol Biosci. 2020 Oct;7:587997.10.3389/fmolb.2020.587997Suche in Google Scholar PubMed PubMed Central
[98] Sheoran S, Arora S, Samsonraj R, Govindaiah P, vuree S. Lipid-based nanoparticles for treatment of cancer. Heliyon. 2022 May;8(5):e09403.10.1016/j.heliyon.2022.e09403Suche in Google Scholar PubMed PubMed Central
[99] Shmeeda H, Amitay Y, Gorin J, Tzemach D, Mak L, Stern ST, et al. Coencapsulation of alendronate and doxorubicin in pegylated liposomes: a novel formulation for chemoimmunotherapy of cancer. J Drug Target. 2016 Nov;24(9):878–89.10.1080/1061186X.2016.1191081Suche in Google Scholar PubMed
[100] Kim M, Jeong M, Hur S, Cho Y, Park J, Jung H, et al. Engineered ionizable lipid nanoparticles for targeted delivery of RNA therapeutics into different types of cells in the liver. Sci Adv. 2021 Feb;7(9):eabf4398.10.1126/sciadv.abf4398Suche in Google Scholar PubMed PubMed Central
[101] Rosenblum D, Gutkin A, Kedmi R, Ramishetti S, Veiga N, Jacobi AM, et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci Adv. 2020 Nov;6(47):eabc9450.10.1126/sciadv.abc9450Suche in Google Scholar PubMed PubMed Central
[102] Caddeo C, Díez-Sales O, Pons R, Carbone C, Ennas G, Puglisi G, et al. Cross-linked chitosan/liposome hybrid system for the intestinal delivery of quercetin. J Colloid Interface Sci. 2016 Jan;461:69–78.10.1016/j.jcis.2015.09.013Suche in Google Scholar PubMed
[103] Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, et al. Sterically stabilized liposomes: Improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci U S A. 1991 Dec;88(24):11460–4.10.1073/pnas.88.24.11460Suche in Google Scholar PubMed PubMed Central
[104] Sriwidodo, Umar AbdK, Wathoni N, Zothantluanga JH, Das S, Luckanagul JA. Liposome-polymer complex for drug delivery system and vaccine stabilization. Heliyon. 2022 Feb;8(2):e08934.10.1016/j.heliyon.2022.e08934Suche in Google Scholar PubMed PubMed Central
[105] Mendoza-Muñoz N, Urbán-Morlán Z, Leyva-Gómez G, de la Luz Zambrano-Zaragoza M, P¨iñón-Segundo E, Quintanar-Guerrero D. Solid lipid nanoparticles: An approach to improve oral drug delivery. J Pharm Pharm Sci. 2021 Oct;24:509–32.10.18433/jpps31788Suche in Google Scholar PubMed
[106] Qiu M, Tang Y, Chen J, Muriph R, Ye Z, Huang C, et al. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2022 Feb;119(8):e2116271119.10.1073/pnas.2116271119Suche in Google Scholar PubMed PubMed Central
[107] Ferrari R, Sponchioni M, Morbidelli M, Moscatelli D. Polymer nanoparticles for the intravenous delivery of anticancer drugs: the checkpoints on the road from the synthesis to clinical translation. Nanoscale. 2018 Dec;10(48):22701–19.10.1039/C8NR05933KSuche in Google Scholar
[108] Prajapati SK, Jain A, Jain A, Jain S. Biodegradable polymers and constructs: A novel approach in drug delivery. Eur Polym J. 2019 Nov;120:109191.10.1016/j.eurpolymj.2019.08.018Suche in Google Scholar
[109] Yang DH, Kim HJ, Park K, Kim JK, Chun HJ. Preparation of poly-l-lysine-based nanoparticles with pH-sensitive release of curcumin for targeted imaging and therapy of liver cancer in vitro and in vivo. Drug Delivery. 2018 Apr;25(1):950–60.10.1080/10717544.2018.1461957Suche in Google Scholar PubMed PubMed Central
[110] Sun Y, Li B, Cao Q, Liu T, Li J. Targeting cancer stem cells with polymer nanoparticles for gastrointestinal cancer treatment. Stem Cell Res Ther. 2022 Oct;13:489.10.1186/s13287-022-03180-9Suche in Google Scholar PubMed PubMed Central
[111] Zahiri M, Babaei M, Abnous K, Taghdisi SM, Ramezani M, Alibolandi M. Hybrid nanoreservoirs based on dextran-capped dendritic mesoporous silica nanoparticles for CD133-targeted drug delivery. J Cell Physiol. 2020 Feb;235(2):1036–50.10.1002/jcp.29019Suche in Google Scholar PubMed
[112] Fernandes S, Fernandez T, Metze S, Balakrishnan PB, Mai BT, Conteh J, et al. Magnetic nanoparticle-based hyperthermia mediates drug delivery and impairs the tumorigenic capacity of quiescent colorectal cancer stem cells. ACS Appl Mater Interfaces. 2021 Apr;13(14):15959–72.10.1021/acsami.0c21349Suche in Google Scholar PubMed PubMed Central
[113] Mendonça MC, Cronin MF, Cryan JF, O’Driscoll CM. Modified cyclodextrin-based nanoparticles mediated delivery of siRNA for huntingtin gene silencing across an in vitro BBB model. Eur J Pharm Biopharm. 2021 Dec;169:309–18.10.1016/j.ejpb.2021.11.003Suche in Google Scholar PubMed
[114] El-Naggar ME, Al-Joufi F, Anwar M, Attia MF, El-Bana MA. Curcumin-loaded PLA-PEG copolymer nanoparticles for treatment of liver inflammation in streptozotocin-induced diabetic rats. Colloids Surf B: Biointerfaces. 2019 May;177:389–98.10.1016/j.colsurfb.2019.02.024Suche in Google Scholar PubMed
[115] Bazsefidpar S, Moyano A, Gutiérrez G, Matos M, Blanco-López MC. Lipid–polymer hybrids encapsulating iron-oxide nanoparticles as a label for lateral flow immunoassays. Biosensors (Basel). 2021 Jul;11(7):218.10.3390/bios11070218Suche in Google Scholar PubMed PubMed Central
[116] Rouco H, García-García P, Évora C, Díaz-Rodríguez P, Delgado A. Screening strategies for surface modification of lipid-polymer hybrid nanoparticles. Int J Pharm. 2022 Aug;624:121973.10.1016/j.ijpharm.2022.121973Suche in Google Scholar PubMed
[117] Anbazhagan R, Muthusamy G, Krishnamoorthi R, Kumaresan S, Rajendra Prasad N, Lai JY, et al. PAMAM G4.5 dendrimers for targeted delivery of ferulic acid and paclitaxel to overcome P-glycoprotein-mediated multidrug resistance. Biotechnol Bioeng. 2021 Mar;118(3):1213–23.10.1002/bit.27645Suche in Google Scholar PubMed
[118] Narayan R, Nayak UY, Raichur AM, Garg S. Mesoporous silica nanoparticles: A comprehensive review on synthesis and recent advances. Pharmaceutics. 2018 Aug;10(3):118.10.3390/pharmaceutics10030118Suche in Google Scholar PubMed PubMed Central
[119] Mekaru H, Lu J, Tamanoi F. Development of mesoporous silica-based nanoparticles with controlled release capability for cancer therapy. Adv Drug Deliv Rev. 2015 Dec;95:40–9.10.1016/j.addr.2015.09.009Suche in Google Scholar PubMed PubMed Central
[120] Argyo C, Weiss V, Bräuchle C, Bein T. Multifunctional mesoporous silica nanoparticles as a universal platform for drug delivery. Chem Mater. 2014 Jan;26(1):435–51.10.1021/cm402592tSuche in Google Scholar
[121] Singh RK, Knowles JC, Kim HW. Advances in nanoparticle development for improved therapeutics delivery: nanoscale topographical aspect. J Tissue Eng. 2019 Sep;10:2041731419877528.10.1177/2041731419877528Suche in Google Scholar PubMed PubMed Central
[122] Li H, Zhu J, Xu YW, Mou FF, Shan XL, Wang QL, et al. Notoginsenoside R1-loaded mesoporous silica nanoparticles targeting the site of injury through inflammatory cells improves heart repair after myocardial infarction. Redox Biol. 2022 Jun;54:102384.10.1016/j.redox.2022.102384Suche in Google Scholar PubMed PubMed Central
[123] Baeza A, Manzano M, Colilla M, Vallet-Regí M. Recent advances in mesoporous silica nanoparticles for antitumor therapy: Our contribution. Biomater Sci. 2016 May;4(5):803–13.10.1039/C6BM00039HSuche in Google Scholar PubMed
[124] Croissant JG, Fatieiev Y, Khashab NM. Degradability and clearance of silicon, organosilica, silsesquioxane, silica mixed oxide, and mesoporous silica nanoparticles. Adv Mater. 2017;29(9):1604634.10.1002/adma.201604634Suche in Google Scholar PubMed
[125] Wu H, Xu XF, Zhu JQ, Wang MD, Li C, Liang L, et al. Mesoporous silica nanoparticles for potential immunotherapy of hepatocellular carcinoma. Front Bioeng Biotechnol. 2021 Oct;9:695635.10.3389/fbioe.2021.695635Suche in Google Scholar PubMed PubMed Central
[126] Liu Z, Chen X, Zhang Z, Zhang X, Saunders L, Zhou Y, et al. Nanofibrous spongy microspheres to distinctly release miRNA and growth factors to enrich regulatory T cells and rescue periodontal bone loss. ACS Nano. 2018 Oct;12(10):9785–99.10.1021/acsnano.7b08976Suche in Google Scholar PubMed PubMed Central
[127] Nguyen TL, Choi Y, Kim J. Mesoporous silica as a versatile platform for cancer immunotherapy. Adv Mater. 2019;31(34):1803953.10.1002/adma.201803953Suche in Google Scholar PubMed
[128] Geppert M, Himly M. Iron oxide nanoparticles in bioimaging – An immune perspective. Front Immunol. 2021 Jun;12:688927.10.3389/fimmu.2021.688927Suche in Google Scholar PubMed PubMed Central
[129] Ohannesian N, De Leo CT, Martirosyan KS. Dextran coated superparamagnetic iron oxide nanoparticles produced by microfluidic process. Mater Today: Proc. 2019 Jan;13:397–403.10.1016/j.matpr.2019.03.172Suche in Google Scholar
[130] Kumar P, Agnihotri S, Roy I. Preparation and characterization of superparamagnetic iron oxide nanoparticles for magnetically guided drug delivery. Int J Nanomed. 2018 Mar;13(T-NANO 2014 Abstracts):43–6.10.2147/IJN.S125002Suche in Google Scholar PubMed PubMed Central
[131] Bardestani A, Ebrahimpour S, Esmaeili A, Esmaeili A. Quercetin attenuates neurotoxicity induced by iron oxide nanoparticles. J Nanobiotechnol. 2021 Oct;19:327.10.1186/s12951-021-01059-0Suche in Google Scholar PubMed PubMed Central
[132] Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016 Nov;11(11):986–94.10.1038/nnano.2016.168Suche in Google Scholar PubMed PubMed Central
[133] Friedrich RP, Janko C, Unterweger H, Lyer S, Alexiou C. SPIONs and magnetic hybrid materials: Synthesis, toxicology and biomedical applications. Phys Sci Rev. 2023;8(8):1435–64.10.1515/psr-2019-0093Suche in Google Scholar
[134] Cornu R, Béduneau A, Martin H. Influence of nanoparticles on liver tissue and hepatic functions: A review. Toxicology. 2020 Jan;430:152344.10.1016/j.tox.2019.152344Suche in Google Scholar PubMed
[135] Ngo W, Ahmed S, Blackadar C, Bussin B, Ji Q, Mladjenovic SM, et al. Why nanoparticles prefer liver macrophage cell uptake in vivo. Adv Drug Delivery Rev. 2022 Jun;185:114238.10.1016/j.addr.2022.114238Suche in Google Scholar PubMed
[136] Xu M, Yang L, Lin Y, Lu Y, Bi X, Jiang T, et al. Emerging nanobiotechnology for precise theranostics of hepatocellular carcinoma. J Nanobiotechnol. 2022 Sep;20(1):427.10.1186/s12951-022-01615-2Suche in Google Scholar PubMed PubMed Central
[137] Barua S, Mitragotri S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today. 2014 Apr;9(2):223–43.10.1016/j.nantod.2014.04.008Suche in Google Scholar PubMed PubMed Central
[138] Zhang YN, Poon W, Tavares AJ, McGilvray ID, Chan WCW. Nanoparticle–liver interactions: Cellular uptake and hepatobiliary elimination. J Controlled Rel. 2016 Oct;240:332–48.10.1016/j.jconrel.2016.01.020Suche in Google Scholar PubMed
[139] Gaiser BK, Hirn S, Kermanizadeh A, Kanase N, Fytianos K, Wenk A, et al. Effects of silver nanoparticles on the liver and hepatocytes in vitro. Toxicol Sci. 2013 Feb;131(2):537–47.10.1093/toxsci/kfs306Suche in Google Scholar PubMed
[140] Ho DWH, Tsui YM, Chan LK, Sze KMF, Zhang X, Cheu JWS, et al. Single-cell RNA sequencing shows the immunosuppressive landscape and tumor heterogeneity of HBV-associated hepatocellular carcinoma. Nat Commun. 2021 Jun;12:3684.10.1038/s41467-021-24010-1Suche in Google Scholar PubMed PubMed Central
[141] Chew V, Lai L, Pan L, Lim CJ, Li J, Ong R, et al. Delineation of an immunosuppressive gradient in hepatocellular carcinoma using high-dimensional proteomic and transcriptomic analyses. Proc Natl Acad Sci U S A. 2017 Jul;114(29):E5900–9.10.1073/pnas.1706559114Suche in Google Scholar PubMed PubMed Central
[142] Sas Z, Cendrowicz E, Weinhäuser I, Rygiel TP. Tumor microenvironment of hepatocellular carcinoma: Challenges and opportunities for new treatment options. Int J Mol Sci. 2022 Mar;23(7):3778.10.3390/ijms23073778Suche in Google Scholar PubMed PubMed Central
[143] Lin A, Zhang J, Luo P. Crosstalk between the MSI status and tumor microenvironment in colorectal cancer. Front Immunol. 2020 Aug;11:2039.10.3389/fimmu.2020.02039Suche in Google Scholar PubMed PubMed Central
[144] Zhao Z, Xu H, Li S, Han Y, Jia J, Han Z, et al. Hypoxic radiosensitizer-lipid coated gold nanoparticles enhance the effects of radiation therapy on tumor growth. J Biomed Nanotechnol. 2019 Sep;15(9):1982–93.10.1166/jbn.2019.2830Suche in Google Scholar PubMed
[145] Wang Y, Shang W, Niu M, Tian J, Xu K. Hypoxia-active nanoparticles used in tumor theranostic. Int J Nanomed. 2019;14:3705.10.2147/IJN.S196959Suche in Google Scholar PubMed PubMed Central
[146] Zhang B, Tang B, Gao J, Li J, Kong L, Qin L. A hypoxia-related signature for clinically predicting diagnosis, prognosis and immune microenvironment of hepatocellular carcinoma patients. J Transl Med. 2020 Sep;18:342.10.1186/s12967-020-02492-9Suche in Google Scholar PubMed PubMed Central
[147] MacParland SA, Tsoi KM, Ouyang B, Ma XZ, Manuel J, Fawaz A, et al. Phenotype determines nanoparticle uptake by human macrophages from liver and blood. ACS Nano. 2017 Mar;11(3):2428–43.10.1021/acsnano.6b06245Suche in Google Scholar PubMed
[148] Jin MZ, Jin WL. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther. 2020 Aug;5:166.10.1038/s41392-020-00280-xSuche in Google Scholar PubMed PubMed Central
[149] Liu Y, Li C, Lu Y, Liu C, Yang W. Tumor microenvironment-mediated immune tolerance in development and treatment of gastric cancer. Front Immunol. 2022 Oct;13:1016817.10.3389/fimmu.2022.1016817Suche in Google Scholar PubMed PubMed Central
[150] Neophytou CM, Panagi M, Stylianopoulos T, Papageorgis P. The role of tumor microenvironment in cancer metastasis: Molecular mechanisms and therapeutic opportunities. Cancers (Basel). 2021 Apr;13(9):2053.10.3390/cancers13092053Suche in Google Scholar PubMed PubMed Central
[151] Anderson NM, Simon MC. Tumor microenvironment. Curr Biol. 2020 Aug;30(16):R921–5.10.1016/j.cub.2020.06.081Suche in Google Scholar PubMed PubMed Central
[152] Kasprzak A. The role of tumor microenvironment cells in colorectal cancer (CRC) cachexia. Int J Mol Sci. 2021 Feb;22(4):1565.10.3390/ijms22041565Suche in Google Scholar PubMed PubMed Central
[153] Cheng X, Xie Q, Sun Y. Advances in nanomaterial-based targeted drug delivery systems. Front Bioeng Biotechnol. 2023 Apr;11:1177151.10.3389/fbioe.2023.1177151Suche in Google Scholar PubMed PubMed Central
[154] Okamatsu A, Motoyama K, Onodera R, Higashi T, Koshigoe T, Shimada Y, et al. Folate-appended β-cyclodextrin as a promising tumor targeting carrier for antitumor drugs in vitro and in vivo. Bioconjug Chem. 2013 Apr;24(4):724–33.10.1021/bc400015rSuche in Google Scholar PubMed
[155] Li F, Chen WL, You BG, Liu Y, Yang SD, Yuan ZQ, et al. Enhanced cellular internalization and on-demand intracellular release of doxorubicin by stepwise pH-/reduction-responsive nanoparticles. ACS Appl Mater Interfaces. 2016 Nov;8(47):32146–58.10.1021/acsami.6b09604Suche in Google Scholar PubMed
[156] Dang Y, Guan J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater Med. 2020;1:10–9.10.1016/j.smaim.2020.04.001Suche in Google Scholar PubMed PubMed Central
[157] Zhou L, Zou M, Xu Y, Lin P, Lei C, Xia X. Nano drug delivery system for tumor immunotherapy: Next-generation therapeutics. Front Oncol. 2022 May;12:864301.10.3389/fonc.2022.864301Suche in Google Scholar PubMed PubMed Central
[158] Huang L, Huang J, Huang J, Xue H, Liang Z, Wu J, et al. Nanomedicine - A promising therapy for hematological malignancies. Biomater Sci. 2020 May;8(9):2376–93.10.1039/D0BM00129ESuche in Google Scholar PubMed
[159] Zhang M, Merlin D. Nanoparticle-based oral drug delivery systems targeting the colon for treatment of ulcerative colitis. Inflamm Bowel Dis. 2018 Jul;24(7):1401–15.10.1093/ibd/izy123Suche in Google Scholar PubMed PubMed Central
[160] Ma X, Cheng Z, Jin Y, Liang X, Yang X, Dai Z, et al. SM5-1-conjugated PLA nanoparticles loaded with 5-fluorouracil for targeted hepatocellular carcinoma imaging and therapy. Biomaterials. 2014 Mar;35(9):2878–89.10.1016/j.biomaterials.2013.12.045Suche in Google Scholar PubMed
[161] Nasab SH, Amani A, Ebrahimi HA, Hamidi AA. Design and preparation of a new multi-targeted drug delivery system using multifunctional nanoparticles for co-delivery of siRNA and paclitaxel. J Pharm Anal. 2021 Apr;11(2):163.10.1016/j.jpha.2020.04.005Suche in Google Scholar PubMed PubMed Central
[162] Kong FH, Ye QF, Miao XY, Liu X, Huang SQ, Xiong L, et al. Current status of sorafenib nanoparticle delivery systems in the treatment of hepatocellular carcinoma. Theranostics. 2021 Mar;11(11):5464–90.10.7150/thno.54822Suche in Google Scholar PubMed PubMed Central
[163] Ebadi M, Rifqi Md Zain A, Tengku Abdul Aziz TH, Mohammadi H, Tee CAT, Rahimi Yusop M. Formulation and characterization of Fe3O4@PEG nanoparticles loaded Sorafenib; molecular studies and evaluation of cytotoxicity in liver cancer cell lines. Polymers (Basel). 2023 Feb;15(4):971.10.3390/polym15040971Suche in Google Scholar PubMed PubMed Central
[164] Wang Z, Zhou Q, Guo Y, Hu H, Zheng Z, Li S, et al. Rapid detection of ractopamine and salbutamol in swine urine by immunochromatography based on selenium nanoparticles. Int J Nanomed. 2021;16:2059.10.2147/IJN.S292648Suche in Google Scholar PubMed PubMed Central
[165] Eljack S, David S, Faggad A, Chourpa I, Allard-Vannier E. Nanoparticles design considerations to co-deliver nucleic acids and anti-cancer drugs for chemoresistance reversal. Int J Pharm X. 2022 Sep;4:100126.10.1016/j.ijpx.2022.100126Suche in Google Scholar PubMed PubMed Central
[166] Younis MA, Khalil IA, Elewa YHA, Kon Y, Harashima H. Ultra-small lipid nanoparticles encapsulating sorafenib and midkine-siRNA selectively-eradicate sorafenib-resistant hepatocellular carcinoma in vivo. J Control Rel. 2021 Mar;331:335–49.10.1016/j.jconrel.2021.01.021Suche in Google Scholar PubMed
[167] Huang Y, Li P, Zhao R, Zhao L, Liu J, Peng S, et al. Silica nanoparticles: Biomedical applications and toxicity. Biomed Pharmacother. 2022 Jul;151:113053.10.1016/j.biopha.2022.113053Suche in Google Scholar PubMed
[168] Ilhan H. Nanoarchitectonics of the effects of curcumin carbon dot-decorated chitosan nanoparticles on proliferation and apoptosis-related gene expressions in HepG2 hepatocellular carcinoma cells. ACS Omega. 2023 Sep;8(37):33554–63.10.1021/acsomega.3c03405Suche in Google Scholar PubMed PubMed Central
[169] Zhao P, Wu S, Cheng Y, You J, Chen Y, Li M, et al. MiR-375 delivered by lipid-coated doxorubicin-calcium carbonate nanoparticles overcomes chemoresistance in hepatocellular carcinoma. Nanomed: Nanotechnol Biol Med. 2017 Nov;13(8):2507–16.10.1016/j.nano.2017.05.010Suche in Google Scholar PubMed
[170] Zhao P, Li M, Wang Y, Chen Y, He C, Zhang X, et al. Enhancing anti-tumor efficiency in hepatocellular carcinoma through the autophagy inhibition by miR-375/sorafenib in lipid-coated calcium carbonate nanoparticles. Acta Biomater. 2018 May 1;72:248–55.10.1016/j.actbio.2018.03.022Suche in Google Scholar PubMed
[171] Huang KW, Lai YT, Chern GJ, Huang SF, Tsai CL, Sung YC, et al. Galactose derivative-modified nanoparticles for efficient siRNA delivery to hepatocellular carcinoma. Biomacromolecules. 2018 Jun;19(6):2330–9.10.1021/acs.biomac.8b00358Suche in Google Scholar PubMed
[172] Cai H, Yang Y, Peng F, Liu Y, Fu X, Ji B. Gold nanoparticles-loaded anti-miR221 enhances antitumor effect of sorafenib in hepatocellular carcinoma cells. Int J Med Sci. 2019;16(12):1541–8.10.7150/ijms.37427Suche in Google Scholar PubMed PubMed Central
[173] Mohd-Zahid MH, Zulkifli SN, Che Abdullah CA, Lim J, Fakurazi S, Wong KK, et al. Gold nanoparticles conjugated with anti-CD133 monoclonal antibody and 5-fluorouracil chemotherapeutic agent as nanocarriers for cancer cell targeting. RSC Adv 11(26):16131–41.10.1039/D1RA01093JSuche in Google Scholar
[174] Lehrich BM, Delgado ER. Lipid nanovesicle platforms for hepatocellular carcinoma precision medicine therapeutics: Progress and perspectives. Organogenesis. 2024;20(1):2313696.10.1080/15476278.2024.2313696Suche in Google Scholar PubMed PubMed Central
[175] Wainberg ZA, Bekaii-Saab T, Boland PM, Dayyani F, Macarulla T, Mody K, et al. First-line liposomal irinotecan with oxaliplatin, 5-fluorouracil and leucovorin (NALIRIFOX) in pancreatic ductal adenocarcinoma: A phase I/II study. Eur J Cancer. 2021 Jul;151:14–24.10.1016/j.ejca.2021.03.028Suche in Google Scholar PubMed
[176] Sayed HM, Said MM, Morcos NYS, El Gawish MA, Ismail AFM. Antitumor and radiosensitizing effects of zinc oxide-caffeic acid nanoparticles against solid ehrlich carcinoma in female mice. Integr Cancer Ther. 2021 Jun;20:15347354211021920.10.1177/15347354211021920Suche in Google Scholar PubMed PubMed Central
[177] Nabil A, Elshemy MM, Asem M, Abdel-Motaal M, Gomaa HF, Zahran F, et al. Zinc oxide nanoparticle synergizes sorafenib anticancer efficacy with minimizing its cytotoxicity. Oxid Med Cell Longev. 2020 May;2020:1362104.10.1155/2020/1362104Suche in Google Scholar PubMed PubMed Central
[178] Li Z, Zhang S, Liu M, Zhong T, Li H, Wang J, et al. Antitumor activity of the zinc oxide nanoparticles coated with low-molecular-weight heparin and doxorubicin complex in vitro and in vivo. Mol Pharm. 2022 Nov;19(11):4179–90.10.1021/acs.molpharmaceut.2c00553Suche in Google Scholar PubMed
[179] Zhang Y, Sun T, Jiang C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm Sin B. 2018 Jan;8(1):34–50.10.1016/j.apsb.2017.11.005Suche in Google Scholar PubMed PubMed Central
[180] Yang Y, Li X, Song J, Li L, Ye Q, Zuo S, et al. Structure-activity relationship of pH-sensitive doxorubicin-fatty acid prodrug albumin nanoparticles. Nano Lett. 2023 Feb;23(4):1530–8.10.1021/acs.nanolett.2c04976Suche in Google Scholar PubMed
[181] Ashrafizadeh M, Hushmandi K, Mirzaei S, Bokaie S, Bigham A, Makvandi P, et al. Chitosan‐based nanoscale systems for doxorubicin delivery: Exploring biomedical application in cancer therapy. Bioeng Transl Med. 2022 Sep;8(1):e10325.10.1002/btm2.10325Suche in Google Scholar PubMed PubMed Central
[182] Sullivan R, Paré GC, Frederiksen LJ, Semenza GL, Graham CH. Hypoxia-induced resistance to anticancer drugs is associated with decreased senescence and requires hypoxia-inducible factor-1 activity. Mol Cancer Ther. 2008 Jul;7(7):1961–73.10.1158/1535-7163.MCT-08-0198Suche in Google Scholar PubMed
[183] Yang M, Li J, Gu P, Fan X. The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment. Bioact Mater. 2020 Dec;6(7):1973–87.10.1016/j.bioactmat.2020.12.010Suche in Google Scholar PubMed PubMed Central
[184] Jiang Q, Wang K, Zhang X, Ouyang B, Liu H, Pang Z, et al. Platelet membrane-camouflaged magnetic nanoparticles for ferroptosis-enhanced cancer immunotherapy. Small. 2020 Jun;16(22):e2001704.10.1002/smll.202001704Suche in Google Scholar PubMed
[185] Yang K, Han W, Jiang X, Piffko A, Bugno J, Han C, et al. Zinc cyclic di-AMP nanoparticles target and suppress tumours via endothelial STING activation and tumour-associated macrophage reinvigoration. Nat Nanotechnol. 2022 Dec;17(12):1322–31.10.1038/s41565-022-01225-xSuche in Google Scholar PubMed
[186] Yang G, Ji J, Liu Z. Multifunctional MnO2 nanoparticles for tumor microenvironment modulation and cancer therapy. WIREs Nanomed Nanobiotechnol. 2021 Nov;13(6):e1720.10.1002/wnan.1720Suche in Google Scholar PubMed
[187] Kovács D, Igaz N, Marton A, Rónavári A, Bélteky P, Bodai L, et al. Core–shell nanoparticles suppress metastasis and modify the tumour-supportive activity of cancer-associated fibroblasts. J Nanobiotechnol. 2020 Jan;18:18.10.1186/s12951-020-0576-xSuche in Google Scholar PubMed PubMed Central
[188] Hong W, Guo F, Yu N, Ying S, Lou B, Wu J, et al. A novel folic acid receptor-targeted drug delivery system based on curcumin-loaded β-cyclodextrin nanoparticles for cancer treatment. Drug Des Dev Ther. 2021 Jun;15:2843–55.10.2147/DDDT.S320119Suche in Google Scholar PubMed PubMed Central
[189] Pelaz B, Alexiou C, Alvarez-Puebla RA, Alves F, Andrews AM, Ashraf S, et al. Diverse applications of nanomedicine. ACS Nano. 2017 Mar;11(3):2313–81.Suche in Google Scholar
[190] Li Z, Zhang Y, Zhu C, Guo T, Xia Q, Hou X, et al. Folic acid modified lipid-bilayer coated mesoporous silica nanoparticles co-loading paclitaxel and tanshinone IIA for the treatment of acute promyelocytic leukemia. Int J Pharm. 2020 Aug;586:119576.10.1016/j.ijpharm.2020.119576Suche in Google Scholar PubMed
[191] Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986 Dec;46(12 Pt 1):6387–92.Suche in Google Scholar
[192] Fu Z, Xiang J. Aptamer-functionalized nanoparticles in targeted delivery and cancer therapy. Int J Mol Sci. 2020 Nov;21(23):9123.10.3390/ijms21239123Suche in Google Scholar PubMed PubMed Central
[193] Guo Y, Wang M, Zou Y, Jin L, Zhao Z, Liu Q, et al. Mechanisms of chemotherapeutic resistance and the application of targeted nanoparticles for enhanced chemotherapy in colorectal cancer. J Nanobiotechnol. 2022 Aug;20:371.10.1186/s12951-022-01586-4Suche in Google Scholar PubMed PubMed Central
[194] Alavi M, Hamidi M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab Pers Ther. 2019;34(1):20180032.10.1515/dmpt-2018-0032Suche in Google Scholar PubMed
[195] Shi Y, van der Meel R, Chen X, Lammers T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics. 2020 Jun;10(17):7921–4.10.7150/thno.49577Suche in Google Scholar PubMed PubMed Central
[196] Navya PN, Kaphle A, Srinivas SP, Bhargava SK, Rotello VM, Daima HK. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019 Jul;6:23.10.1186/s40580-019-0193-2Suche in Google Scholar PubMed PubMed Central
[197] Yoo J, Park C, Yi G, Lee D, Koo H. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers (Basel). 2019 May;11(5):640.10.3390/cancers11050640Suche in Google Scholar PubMed PubMed Central
[198] Montet X, Funovics M, Montet-Abou K, Weissleder R, Josephson L. Multivalent effects of RGD peptides obtained by nanoparticle display. J Med Chem. 2006 Oct;49(20):6087–93.10.1021/jm060515mSuche in Google Scholar PubMed
[199] Choi HS, Liu W, Liu F, Nasr K, Misra P, Bawendi MG, et al. Design considerations for tumour-targeted nanoparticles. Nat Nanotechnol. 2010 Jan;5(1):42–7.10.1038/nnano.2009.314Suche in Google Scholar PubMed PubMed Central
[200] Ma N, Wu FG, Zhang X, Jiang YW, Jia HR, Wang HY, et al. Shape-dependent radiosensitization effect of gold nanostructures in cancer radiotherapy: Comparison of gold nanoparticles, nanospikes, and nanorods. ACS Appl Mater Interfaces. 2017 Apr;9(15):13037–48.10.1021/acsami.7b01112Suche in Google Scholar PubMed
[201] Zhang XD, Wu D, Shen X, Chen J, Sun YM, Liu PX, et al. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials. 2012 Sep;33(27):6408–19.10.1016/j.biomaterials.2012.05.047Suche in Google Scholar PubMed
[202] Jiang X, Zhang B, Zhou Z, Meng L, Sun Z, Xu Y, et al. Enhancement of radiotherapy efficacy by pleiotropic liposomes encapsulated paclitaxel and perfluorotributylamine. Drug Deliv 24(1):1419–28.10.1080/10717544.2017.1378939Suche in Google Scholar PubMed PubMed Central
[203] Lee AWM, Ng WT, Chan JYW, Corry J, Mäkitie A, Mendenhall WM, et al. Management of locally recurrent nasopharyngeal carcinoma. Cancer Treat Rev. 2019 Sep;79:101890.10.1016/j.ctrv.2019.101890Suche in Google Scholar PubMed
[204] Elming PB, Sørensen BS, Oei AL, Franken NAP, Crezee J, Overgaard J, et al. Hyperthermia: The optimal treatment to overcome radiation resistant hypoxia. Cancers (Basel). 2019 Jan;11(1):60.10.3390/cancers11010060Suche in Google Scholar PubMed PubMed Central
[205] Tao J, Yang G, Zhou W, Qiu J, Chen G, Luo W, et al. Targeting hypoxic tumor microenvironment in pancreatic cancer. J Hematol Oncol. 2021 Jan;14(1):14.10.1186/s13045-020-01030-wSuche in Google Scholar PubMed PubMed Central
[206] Kim MS, Lee EJ, Kim JW, Chung US, Koh WG, Keum KC, et al. Gold nanoparticles enhance anti-tumor effect of radiotherapy to hypoxic tumor. Radiat Oncol J. 2016;34(3):230–8.10.3857/roj.2016.01788Suche in Google Scholar PubMed PubMed Central
[207] Huang C, Chen T, Zhu D, Huang Q. Enhanced tumor targeting and radiotherapy by quercetin loaded biomimetic nanoparticles. Front Chem. 2020 Mar;8:225.10.3389/fchem.2020.00225Suche in Google Scholar PubMed PubMed Central
[208] Askar MA, El-Nashar HA, Al-Azzawi MA, Rahman SSA, Elshawi OE. Synergistic effect of quercetin magnetite nanoparticles and targeted radiotherapy in treatment of breast cancer. Breast Cancer (Auckl). 2022 Mar;16:11782234221086728.10.1177/11782234221086728Suche in Google Scholar PubMed PubMed Central
[209] Yang S, Han G, Chen Q, Yu L, Wang P, Zhang Q, et al. Au-Pt nanoparticle formulation as a radiosensitizer for radiotherapy with dual effects. IJN. 2021 Jan;16:239–48.10.2147/IJN.S287523Suche in Google Scholar PubMed PubMed Central
[210] Guan X, Sun L, Shen Y, Jin F, Bo X, Zhu C, et al. Nanoparticle-enhanced radiotherapy synergizes with PD-L1 blockade to limit post-surgical cancer recurrence and metastasis. Nat Commun. 2022 May;13(1):2834.10.1038/s41467-022-30543-wSuche in Google Scholar PubMed PubMed Central
[211] Doudna JA. The promise and challenge of therapeutic genome editing. Nature. 2020 Feb;578(7794):229–36.10.1038/s41586-020-1978-5Suche in Google Scholar PubMed PubMed Central
[212] Wan T, Zhong J, Pan Q, Zhou T, Ping Y, Liu X. Exosome-mediated delivery of Cas9 ribonucleoprotein complexes for tissue-specific gene therapy of liver diseases. Sci Adv. 8(37):eabp9435.10.1126/sciadv.abp9435Suche in Google Scholar PubMed PubMed Central
[213] Chew WL, Tabebordbar M, Cheng JKW, Mali P, Wu EY, Ng AHM, et al. A multi-functional AAV-CRISPR-Cas9 and its host response. Nat Methods. 2016 Oct;13(10):868–74.10.1038/nmeth.3993Suche in Google Scholar PubMed PubMed Central
[214] Duan L, Ouyang K, Xu X, Xu L, Wen C, Zhou X, et al. Nanoparticle delivery of CRISPR/Cas9 for genome editing. Front Genet. 2021;12:673286.10.3389/fgene.2021.673286Suche in Google Scholar PubMed PubMed Central
[215] Chin JS, Chooi WH, Wang H, Ong W, Leong KW, Chew SY. Scaffold-mediated non-viral delivery platform for CRISPR/Cas9-based genome editing. Acta Biomater. 2019 May;90:60–70.10.1016/j.actbio.2019.04.020Suche in Google Scholar PubMed
[216] Gong J, Wang HX, Lao YH, Hu H, Vatan N, Guo J, et al. A versatile nonviral delivery system for multiplex gene-editing in the liver. Adv Mater. 2020 Nov;32(46):e2003537.10.1002/adma.202003537Suche in Google Scholar PubMed PubMed Central
[217] Tang H, Zhao X, Jiang X. Synthetic multi-layer nanoparticles for CRISPR-Cas9 genome editing. Adv Drug Delivery Rev. 2021 Jan;168:55–78.10.1016/j.addr.2020.03.001Suche in Google Scholar PubMed
[218] Cullis PR, Hope MJ. Lipid nanoparticle systems for enabling gene therapies. Mol Ther. 2017 Jul;25(7):1467–75.10.1016/j.ymthe.2017.03.013Suche in Google Scholar PubMed PubMed Central
[219] Zimmermann TS, Lee ACH, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006 May;441(7089):111–4.10.1038/nature04688Suche in Google Scholar PubMed
[220] Zheng QC, Jiang S, Wu YZ, Shang D, Zhang Y, Hu SB, et al. Dual-targeting nanoparticle-mediated gene therapy strategy for hepatocellular carcinoma by delivering small interfering RNA. Front Bioeng Biotechnol. 2020 Jun;8:512.10.3389/fbioe.2020.00512Suche in Google Scholar PubMed PubMed Central
[221] de Wolf HK, Snel CJ, Verbaan FJ, Schiffelers RM, Hennink WE, Storm G. Effect of cationic carriers on the pharmacokinetics and tumor localization of nucleic acids after intravenous administration. Int J Pharm. 2007 Mar;331(2):167–75.10.1016/j.ijpharm.2006.10.029Suche in Google Scholar PubMed
[222] Owens J. Knocked down for the count. Nat Rev Drug Discov. 2005 Sep;4(9):717–7.10.1038/nrd1833Suche in Google Scholar
[223] Paredes KO, Ruiz-Cabello J, Alarcón DI, Filice M. Chapter 14 - The state of the art of investigational and approved nanomedicine products for nucleic acid delivery. In: Nucleic Acid Nanotheranostics. 2019. p. 421–56.10.1016/B978-0-12-814470-1.00015-0Suche in Google Scholar
[224] Li M, Wang Y, Jiang S, Gao Y, Zhang W, Hu S, et al. Biodistribution and biocompatibility of glycyrrhetinic acid and galactose-modified chitosan nanoparticles as a novel targeting vehicle for hepatocellular carcinoma. Nanomedicine (Lond). 2020 Jan;15(2):145–61.10.2217/nnm-2018-0455Suche in Google Scholar PubMed
[225] Zhang H, Deng L, Liu H, Mai S, Cheng Z, Shi G, et al. Enhanced fluorescence/magnetic resonance dual imaging and gene therapy of liver cancer using cationized amylose nanoprobe. Mater Today Bio. 2022 Feb;13:100220.10.1016/j.mtbio.2022.100220Suche in Google Scholar PubMed PubMed Central
[226] Su D. The transcatheter arterial chemoembolization combined with targeted nanoparticle delivering sorafenib system for the treatment of microvascular invasion of hepatocellular carcinoma. Bioengineered. 2021 Dec;12(2):11124–35.10.1080/21655979.2021.2001239Suche in Google Scholar PubMed PubMed Central
[227] He P, Xiong Y, Ye J, Chen B, Cheng H, Liu H, et al. A clinical trial of super-stable homogeneous lipiodol-nanoICG formulation-guided precise fluorescent laparoscopic hepatocellular carcinoma resection. J Nanobiotechnol. 2022 Jun;20(1):250.10.1186/s12951-022-01467-wSuche in Google Scholar PubMed PubMed Central
[228] Zheng L, Li C, Huang X, Lin X, Lin W, Yang F, et al. Thermosensitive hydrogels for sustained-release of sorafenib and selenium nanoparticles for localized synergistic chemoradiotherapy. Biomaterials. 2019 Sep;216:119220.10.1016/j.biomaterials.2019.05.031Suche in Google Scholar PubMed
[229] Yang H, Liu HS, Hou W, Gao JX, Duan Y, Wei D, et al. An NIR-responsive mesoporous silica nanosystem for synergetic photothermal-immunoenhancement therapy of hepatocellular carcinoma. J Mater Chem B. 2020 Jan;8(2):251–9.10.1039/C9TB01891CSuche in Google Scholar PubMed
[230] Hong DS, Kang YK, Borad M, Sachdev J, Ejadi S, Lim HY, et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br J Cancer. 2020 May;122(11):1630–7.10.1038/s41416-020-0802-1Suche in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy
Artikel in diesem Heft
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy

