Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
-
Nouf Omar Alafaleq
, Gouse M. Shaik
Abstract
This study synthesized gold nanoparticles (AuNPs) using a facile microwave-assisted chemical route and evaluated them as potential anticancer candidates against breast and colon cancer cell lines. Numerous spectral characterization tools were used to study the optical properties, structure, and morphology of the prepared AuNPs. UV-Vis spectroscopy showed a characteristic peak at 517 nm, which confirms the formation of AuNPs. The crystalline structure of NPs was studied by X-ray diffraction, and the NPs’ shape and size were calculated with Field emission transmission electron microscopy. The synthesized AuNPs were found to be uniform in size in the range of 2–6 nm. A variety of biological tests, including MTT, scratch, real time polymerase chain reaction (RT-PCR), and comet assays were adopted to assess the anticancer potential of these AuNPs in the studied cancer cell models. The findings suggested a cell-dependent cytotoxicity of AuNPs. Different cell viability of 40.3 and 66.4% were obtained for MCF-7 and HCT-116, respectively, at 5 µg/mL of AuNPs. The scratch assay showed AuNPs impede cell migration in a concentration-dependent manner in the MCF-7 cell line. On the other hand, real-time polymerase chain reaction (RT-PCR) of apoptotic (p53, Bax, and caspase-3) and anti-apoptotic (BCl-2) genes revealed upregulation and downregulation of these genes, respectively, probably leading to its cytotoxicity. At 5 µg/mL concentration of AuNPs, reactive oxygen species (ROS) production was found to be increased by 26.4 and 42.7%, respectively, in MCF-7 and HCT-116 cells. Similarly, comet assay demonstrated AuNPs induced DNA damage in the studied cancer cell lines. These findings suggest that the observed anticancer efficacy of AuNPs was driven by ROS generation. The synthesized AuNPs appeared to be a promising therapeutic against cancer cells. However, our in vitro data need to be confirmed and validated in ex vivo and in vivo models so that this NP can be further exploited for human use.
1 Introduction
Cancer is one of the most clinically relevant pathologies driven by highly heterogeneous processes and has remained a major public health concern worldwide [1,2,3]. Among the various types of cancer, breast and colorectal cancer have significant occurrence, expression, and mortality. Breast cancer is the most frequently diagnosed and the leading cause of cancer death in women worldwide, with more than 2 million new cases in 2020 [4,5]. Conversely, colorectal cancer is the third most prevalent cancer in men and the second most common cancer in women worldwide [6,7].
Despite the potential advances in anticancer research, limited progress has been made toward the safe treatment and prognosis of cancer [8]. The escalating complexity of the cancer problem, combined with the inability of traditional chemotherapy to achieve significant reductions in mortality rates, indicates the requirement of noble, harmless, yet concrete chemotherapeutic approaches to impede cancer progression. Nanotechnology can be pivotal in cancer treatment and prevention [9,10]. The nanoparticles (NPs) can be modulated to prolong circulation, improve drug localization and efficacy, and reduce the chances of multi-drug resistance [11,12].
Over the past few years, precision-engineered nanomaterials have risen as a standout among cancer therapeutics with a tremendous anticancer therapeutic perspective [13,14]. They can improve the delivery of therapeutics to the specific sites of the body at particular times and at an effective concentration. Several reports suggested different therapeutic benefits of NP-based treatment that could act as molecular probes, antiangiogenic, antitumor, anti-permeability, and antiproliferative [2,15,16,17].
Among nanotherapeutics, gold nanoparticles (AuNPs) have attracted extensive scientific attention in recent decades because of their convenience of synthesis. AuNPs are increasingly used in medicine because of their excellent biocompatibility due to their chemical and physical stability and ease of functionalization with biologically active molecules [18]. AuNPs can directly conjugate and interact with a wide range of molecules on their surface, including proteins, medicines, antibodies, enzymes, nucleic acids (DNA or RNA), and fluorescent dyes [19]. AuNPs are qualified as efficient nanomedicine with potent anticancer activity due to their unique physicochemical and bio-reactive properties [20]. Distinctive properties of AuNPs, viz., high X-ray absorption coefficient, localized surface plasmon resonance, and radioactivity, enable its prominent clinical applications [21]. AuNPs can serve as molecular sensors, therapeutics, and delivery systems for imaging agents and medications [22]. Functionalized AuNPs have made significant strides as they have the potential for effective cellular uptake. However, other factors, such as the nature of the ligand, molecular weight, and grafting density, also affect the cellular uptake [23]. Along with other factors like size, cell type, tissue distribution, tissue absorption, and penetrating ability, AuNPs-induced cytotoxicity is also influenced by these [24]. Due to the impact of surface energy transfer, AuNPs are a potent quencher for fluorescence donors, making them an ideal building block for fluorescent nanoprobes [22]. Although no pharmaceutical approval of AuNPs has been achieved, several studies suggested their usage in cancer therapy and tumor-targeting [25,26,27].
Our earlier study reported the anti-metastasis potential of biogenic synthesized AgNPs against breast and colorectal cancer cells and suggested it as a promising anticancer therapy [2]. In the current study, the anticancer potential of the synthesized AuNPs was assessed employing a battery of in vitro tests, including cell viability, cell morphology, cell migration, reactive oxygen species (ROS) production, DNA damage, modulation in pro/anti-apoptotic gene expression, and induction of apoptosis.
2 Materials and methods
2.1 Materials
Cell lines (MCF-7 and HCT-116) were obtained from ATCC (Manassas, VA, USA). Chloroauric acid (HAuCl4), citric acid, and cetyltrimethylammonium bromide (CTAB), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT), 2,7 dichlorofluorescin diacetate (DCFH-DA), Dulbecco’s modified Eagle’s medium (DMEM) high glucose, Fetal bovine serum (FBS), and penicillin were obtained from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals used for this study were of the highest purity grade and procured from different commercial sources.
2.2 Preparation of AuNPs
To synthesize AuNPs, a solution containing 15 mL of 10 mM HAuCl4·3H2O, 0.3 g citric acid, and 0.2 g CTAB were mixed under continuous stirring to obtain an aqueous solution. This solution was then heated for 50 s at 100 W in a microwave oven (SAMSUNG; 750 W), resulting in an immediate shift in color from light yellow to bright orange, indicating the formation of gold NPs. The AuNPs were extracted from the solution using the Whatman qualitative filter paper of grade 1.
2.3 Characterization of AuNPs
A UV-Vis Spectrophotometer (Perkin Elmer, USA) with a 1 cm quartz cell was used to characterize the synthesized AuNPs. In a quartz cell, colloidal AuNPs were mixed with 5 mL of distilled water, and the blank was filled with a distilled water solution. The crystalline structure of the synthesized NPs was studied using X-ray diffraction (Philips-PW 1729, Holland) with Cu radiation [30 kV, 40 mA, Kα radiation (1.54430 A°)]. In addition, field emission transmission electron microscopy (FETEM; JEOL, JEM-2100F) at 200 keV was used to examine the NPs’ shape and size. A small drop of NP solution was dropped onto a carbon-coated copper grid, which was then dried in the air before being transferred to the microscope. “Image J” program was used to determine the NP’s average particle size and distribution.
2.4 Cytotoxicity/MTT assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) bromide dye reduction test was used to determine the cytotoxicity of AuNPs, as described previously [2,28]. Briefly, MCF-7 and HCT-116 cells (10,000 cells/well) were seeded on 96-well culture plates and treated with variable concentrations of AuNPs (0.5–100 µg/mL) for 24 h and kept in CO2 incubator. Following the incubation period, 10 µL of MTT (5 mg/mL stock in PBS) was added to each well, and the plates were further incubated at 37°C for 4 h. The formazan product was then dissolved in 100 µL of dimethyl sulfoxide (DMSO) with moderate shaking at 37°C. and the absorbance was recorded at 570 nm on a microplate reader (Chameleon-Multilabel microplate reader, Hidex, Turku, Finland).
2.5 Cell migration (scratch assay)
To assess the anti-metastasis ability of AuNPs, we performed an in vitro scratch assay following the protocol described by Khan et al. [2]. Briefly, MCF-7 cells were seeded in a 24-well cell culture plate and incubated for 24 h. Once monolayers of MCF-7 cells were grown to confluency, a thin scratch was introduced with a sterile tip, and cells were briefly washed with DMEM to decant any detached cells. After the scratch was introduced, cells monolayer was incubated with control and varying concentrations of AuNPs (1–4 µg/mL). Pictures of scratch were taken every 24 h up to 48 h at 10× magnification of Leica DFC 450 microscope equipped with a camera. The pictures were processed using Image J software and aligned with control.
2.6 Comet assay
The assay was carried out according to method adopted and modified by Hassan et al. [29]. The full-grown cells were treated with AuNPs (5 µg/mL) for 3 h in Petri-dishes. The cells were then trypsinized to prepare the cell suspension and homogenized in 1 mL of media. The cells were then centrifuged for 5 min at 500 g. An equal volume of cell suspension (100 μL) was mixed with 1% low melting agarose (100 μL). This solution was placed on conventional melting agarose base-coated slides. For 10 min, the slides were immersed in lysis buffer (0.045 M TBE, pH 8.4, containing 2.5% SDS). After that, the slides were exposed to the same TBE buffer without SDS in a comet assay tank for 10 min at 2 V/cm and 80 mA, respectively. Finally, the cells were stained with ethidium bromide (20 g/mL, Sigma-Aldrich, USA). After proper washing, the slides were covered with coverslips and preserved in a humidified environment. 100 cells from each group were examined using an upright fluorescence microscope (Leica DM2500, Germany) equipped with a digital CCD camera to measure the tail length of nuclear DNA (Andor Zyla 5.5, UK). Komet 5.5 image analysis software was used to measure and picture the tail length (Andor, UK).
2.7 ROS measurement
DCFH-DA was used to detect the intracellular generation of ROS [17,30]. The extremely fluorescent chemical DCF is formed when the DCFH-DA reacts with ROS after passively entering the cell. In 24-well plates, cancer cells (5 × 104 cells/well) were seeded and left to adhere to the flask for 24 h. It was then exposed to various concentrations of AuNPs (1–10 µg/mL) and incubated for an additional 24 h. After exposure, the cells underwent two PBS washes before being incubated for 30 min at 37°C in 1 mL of the working solution of DCFH-DA (100 µM). After 10 min of centrifugation, 200 µL of the cell supernatant was transferred to a 96-well plate. The fluorescence intensity was measured at wavelengths of 485 nm for excitation and 525 nm for emission.
2.8 Gene expression analysis (Real time-PCR)
MCF-7 and HCT-116 cells were grown to confluency in 6-well plates and treated with AuNPs (5 µg/mL) for 24 h. Appropriate control cells were also treated with PBS. These cells were further subjected to RNA extraction using the RNeasy mini kit (Qiagen, Germany). The concentration and purity of total RNA were confirmed by a Nanodrop spectrophotometer (Thermo Scientific, USA). A total of 1 µg of RNA was used to synthesize the cDNA using 100 ng of oligo dTs and 2 units of reverse transcriptase. The following sets of specific primers were employed for amplification of each cDNA: p53 (5′F-CCCAGCCAAAGAAGAAACCA-3′, 5′R-TTCCAAGGCCTCATTCAGCT-3′), caspase3 (5′F-ACATGGCGTGTCATAAAATACC-3′, 5′R-CACAAAGCGACTGGATGAAC-3′), Bax (5′F-TGCTTCAGGGTTTCATCCAG-3′, 5′R-GGCGGCAATCATCCTCTG-3′), Bcl2 (5′F-AGGAAGTGAACATTTCGGTGAC-3′, 5′R-GCTCAGTTCCAGGACCAGGC-3′), and GAPDH (5′F-CCACTCCTCCACCTTTGAC-3′, 5′R-ACCCTGTTGCTGTAGCCA-3′). Real-time quantification was performed in the light of Cycler® 480 instrument with 96-well plate (Roche Diagnostics, Rotkreuz, Switzerland) using Light Cycler® 480 SYBR Green I Master (Roche Diagnostics, Switzerland). The RT-PCR cycle conditions were 10 min of initial denaturation at 95°C, followed by 40 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 20 s, and elongation at 72°C for 20 s. Gene expression levels were normalized to GAPDH gene expression, which was used as an internal housekeeping control.
2.9 Flow cytometry
The induction of apoptosis as a result of AuNPs treatment was measured using a Flow cytometer (BD FACSCalibur®, USA) following the method demonstrated by Hailan et al. [31]. A commercially available FITC-Annexin V apoptosis detection kit supplied by BD Pharmingen™, (CA, USA) was employed. Briefly, the cancer cells (MCF-7 and HCT-116) were grown to confluency and further exposed to the different concentrations of AuNPs (2 and 5 µg/mL) for 24 h. After that, cells were resuspended in 1× binding buffer and treated for 20 min in the dark with annexin V-FITC and propidium iodide (5 µL each). A flow cytometer was used to quickly analyze each sample, and Cell Quest® Pro software was used to analyze the results (BD).
2.10 Statistical analysis
The results of each experiment were provided as the average of three independent replicates ± standard error. Unless otherwise specified in the legends, the student’s t-test was used to analyze the difference between the control and test groups.
3 Results
3.1 Synthesis and characterization of AuNPs
Citric acid was used as a reducing agent and CTAB as a binding agent during microwave irradiation. After 50 s of irradiation, the produced AuNPs were discovered to be stable and to have a size range of 2–6 nm. The UV-Vis absorption spectra of AuNPs showed the surface plasmon resonance peak at ∼517 nm, confirming the AuNP synthesis (Figure 1a). The size of the NPs can be determined with accuracy by the peak’s width. The peak narrows with the decrease in bandwidth and increase in the band strength as the particle size increases [32]. The size, shape, and dielectric constant of the surrounding media all affect the form and location of surface plasmon absorption. The optical absorption spectra of metal NPs are mostly determined by the surface plasmon resonance, which moves to a longer wavelength as particle size increases.
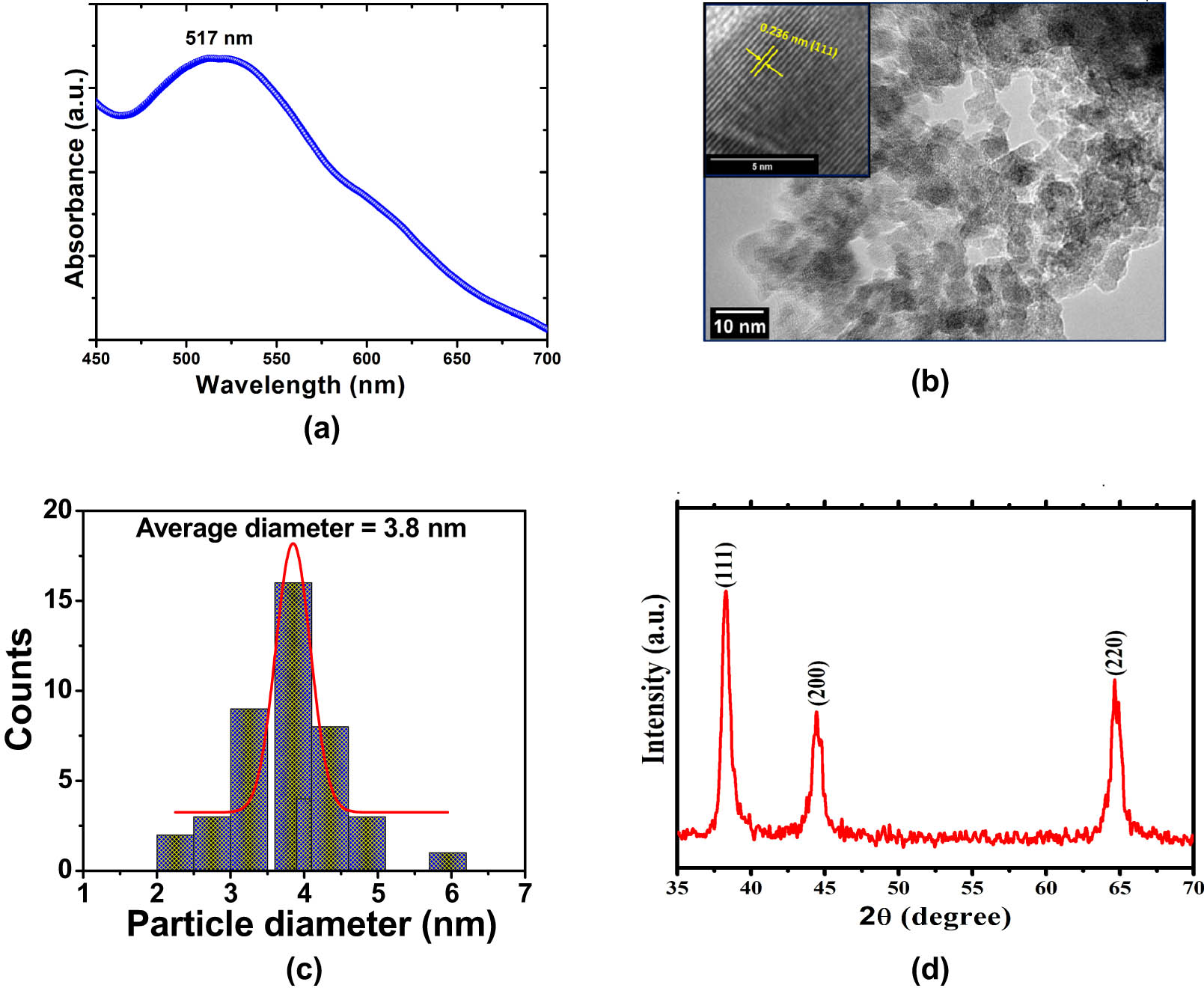
(a) UV-Vis spectrum of AuNPs synthesized at 50 s MWI. (b) TEM image and inset show HRTEM image of AuNPs synthesized at 50 s MWI. (c) Size distribution histogram obtained from TEM images of AuNPs. (d) XRD pattern of AuNPs synthesized at 50 s MWI.
The detailed morphology of AuNPs was investigated by high-resolution transmission electron microscopy (HRTEM) and TEM at room temperature. The TEM images showed stable, well-dispersed, spherical AuNPs with good morphological characteristics (Figure 1b). In addition, TEM measurements also indicated that the obtained material consists of uniform populations of NPs. Particle size was calculated using approximately 50 randomly selected individual NPs from TEM micrographs, and found the particle size was in the 2–6 nm diameter range (Figure 1c). TEM images also showed an essential role played by CTAB in stabilizing the AuNPs. Structural information of AuNPs was further analyzed by HRTEM image. The inset of Figure 1b shows the HRTEM image of a single AuNP, where clear lattice fringes could be easily seen. The distance between two adjacent planes was found to be 0.236 nm, which is linked with (111) plane of Au structure.
XRD pattern analysis was used to assess the crystal structure and phase determinations of the produced NPs. The XRD pattern of AuNPs, displaying the diffraction peaks of AuNPs, is shown in Figure 1d. The (1 1 1), (2 0 0), and (2 2 0) reflections of the face-centered cubic structure of metallic gold, respectively, can be indexed to the peaks located at 38.16, 44.47, and 64.63° [33]. The peak corresponding to (1 1 1) was also found to be more intense than the other peaks, indicating that this orientation is the most common and that the produced AuNPs are naturally crystalline. Within the XRD’s detection range, no additional phase was found. In addition, the average crystal size of AuNPs was calculated using the Debye–Scherrer’s equation by determining the width (111) of the Bragg’s reflection was found to be 5.8 nm.
3.2 Anticancer activity of AuNPs: MTT, scratch, and comet assay
After establishing the purity of the synthesized AuNPs, a battery of in vitro experiments was performed to evaluate the biological efficacy of AuNPs. The synthesized NPs were found to be cytotoxic in a concentration-dependent manner to MCF-7 and HCT-116 cell lines. Different cell viability of 40.3 and 66.4% were obtained for MCF-7 and HCT-116, respectively, at 5 µg/mL of AuNPs (Figure 2a and b). The percentage of cell viability was found to be declined even at a very low concentration (0.5 µg/mL) of AuNPs, suggesting its high toxicity toward cancer cells. On the other hand, the morphology of the cancer cells was also found to be notably distorted in both cell lines with increase in the concentration of AuNPs treatment for 24 h (Figure 3). Scratch assays examined the cancer cell migration and invasion in the presence of AuNPs. The data showed a significant suppression in wound healing and cell migration following the treatment with 2 µg/mL of AuNPs in MCF-7 cells after 48 h of incubation compared to control (Figure 4). Less migration was observed at higher concentrations of AuNPs, which was also found to be variable with time. Because HCT-116 cells develop in bunches, we were unable to measure their migration pattern (data not shown).
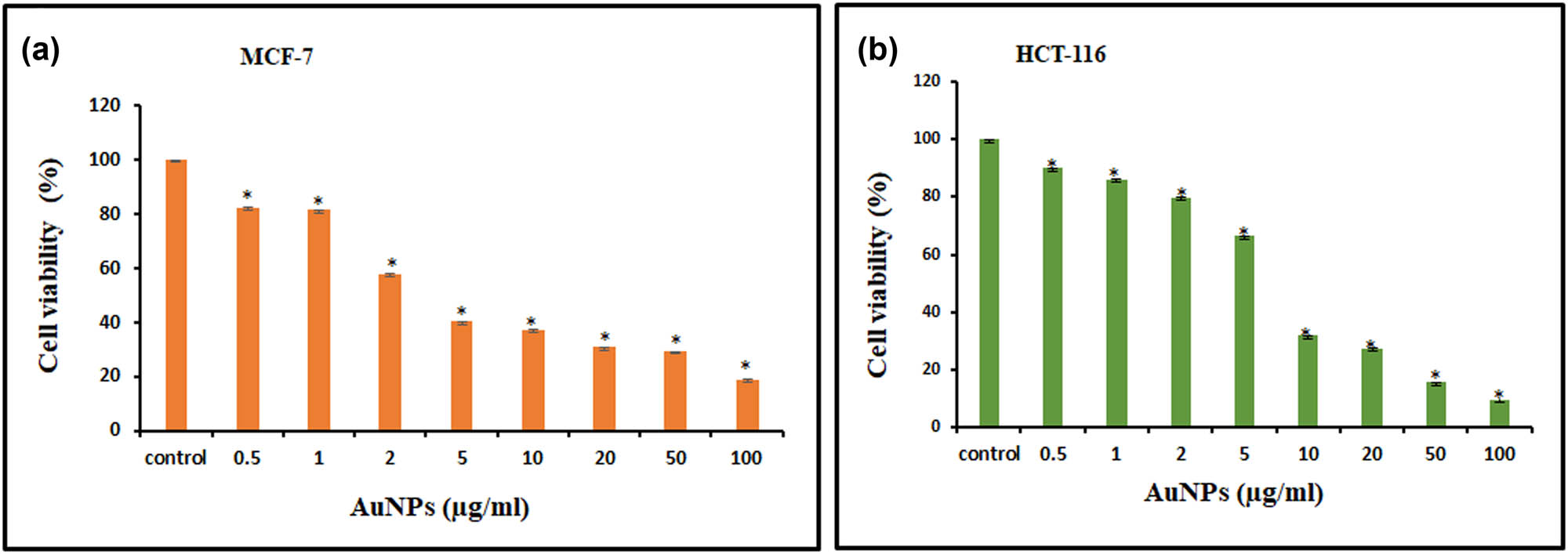
MTT-based cell viability assay. For 24 h, cells were treated with varying concentrations of AuNPs (0.5–100 µg/mL). For both MCF-7 (a) and HCT-116 (b) cell lines, results are reported as column graph with normalized percent cell viability with standard deviation. Data are the mean value ± SD of three independent experiments. Student t-test was performed to determine the significance. P-values < 0.05 (compared to control) were considered a significant criterion and indicated with * mark over the column.
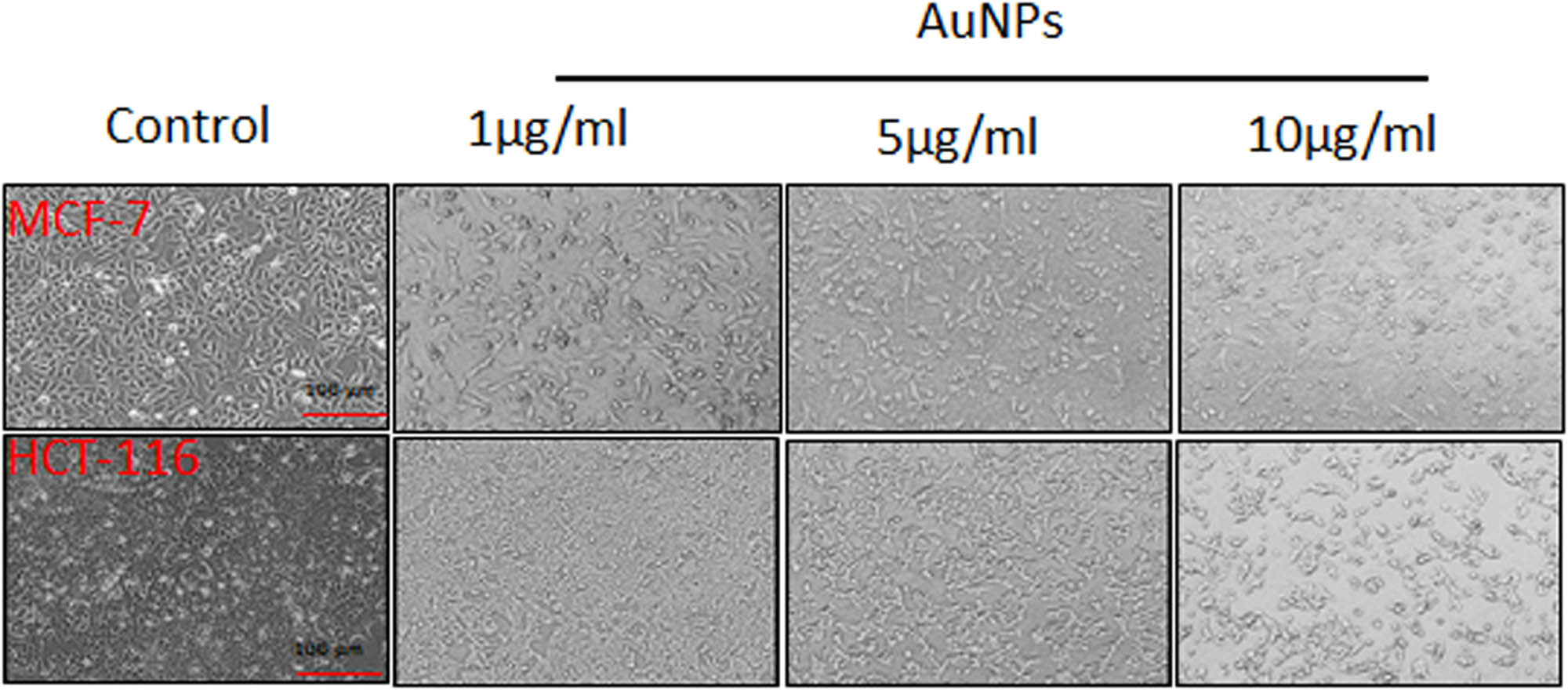
MCF-7 and HCT-116 cells were treated with increasing concentrations of AuNPs for 24 h and showed morphological alterations. The images were captured using a Leica microscope at 10× magnification.
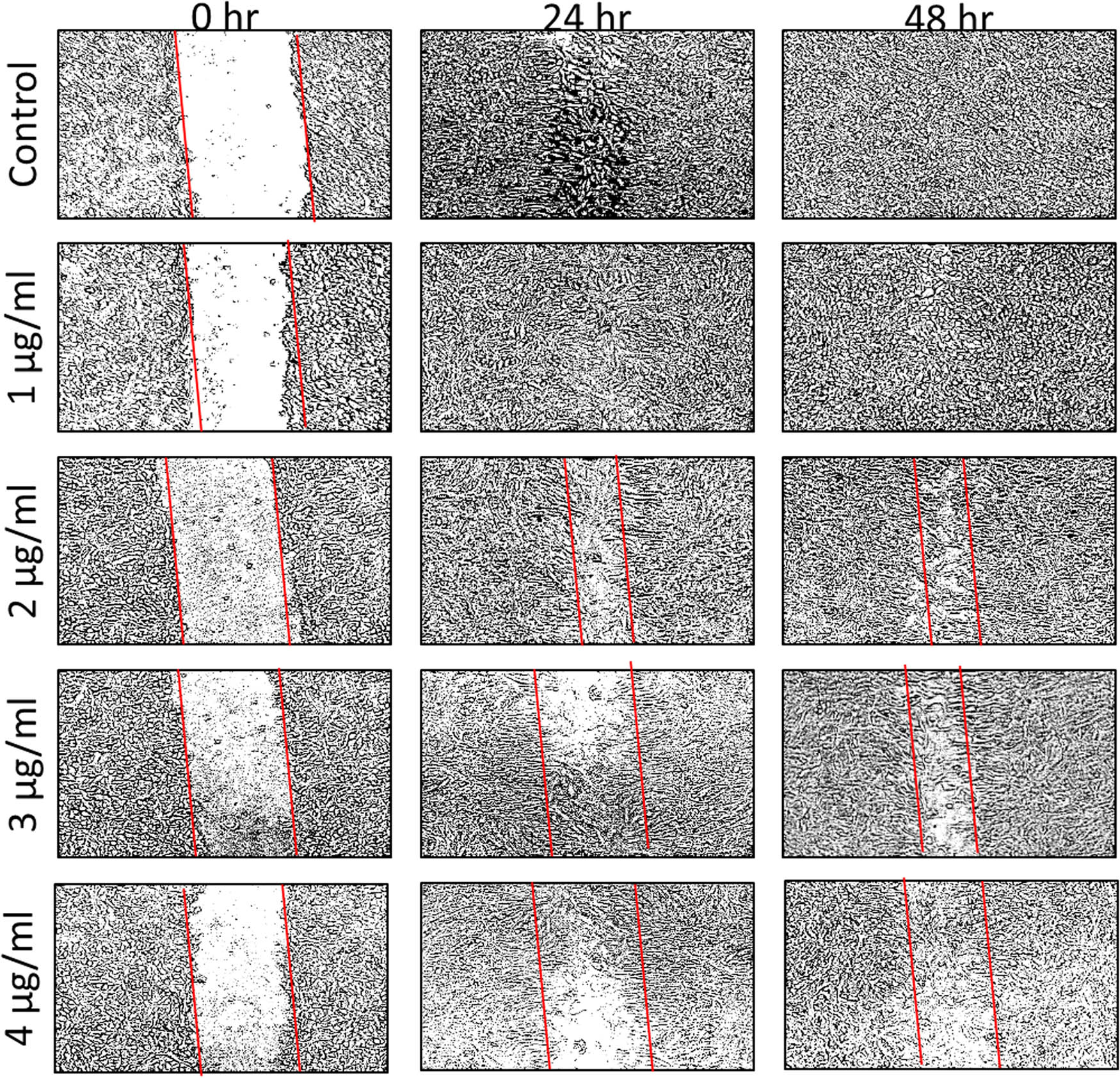
Scratch was applied to a monolayer of cells, which was then treated with 1–4 µg/mL AuNPs at various time intervals (0, 24, and 48 h). The number of cells that migrated to the scratch area, indicating metastatic inhibition, is visually displayed by outlining the scratch with parallel lines and visually exhibiting the number of cells that migrated to the scratch area.
3.3 AuNPs-induced DNA damage in MCF-7 and HCT-116 cells
The present study used a comet assay to evaluate the DNA damage induced by AuNPs, resulting in a combination of single-strand breaks, double-strand breaks, and alkaline-labile sites. In this experiment, negatively charged DNA fragments move towards the positive electrode, whereas the broken DNA fragments move towards the positive electrode by carrying a negative charge. At the same time, the unbroken DNA stays intact without any movement. The untreated control cells showed an intact round-shaped nucleus without a noticeable tail comet-like structure. However, the treatment with AuNPs (5 µg/mL) showed a significant damage to the nuclear DNA of MCF-7 and HCT-116 cells, reflected by a longer tail length (Figure 5). The comet tail was found to be increased by 56% compared to the untreated control in MCF-7 cells. Similarly, the comet tail in the HCT-116 cell was found to be increased by 62% compared to the untreated control.
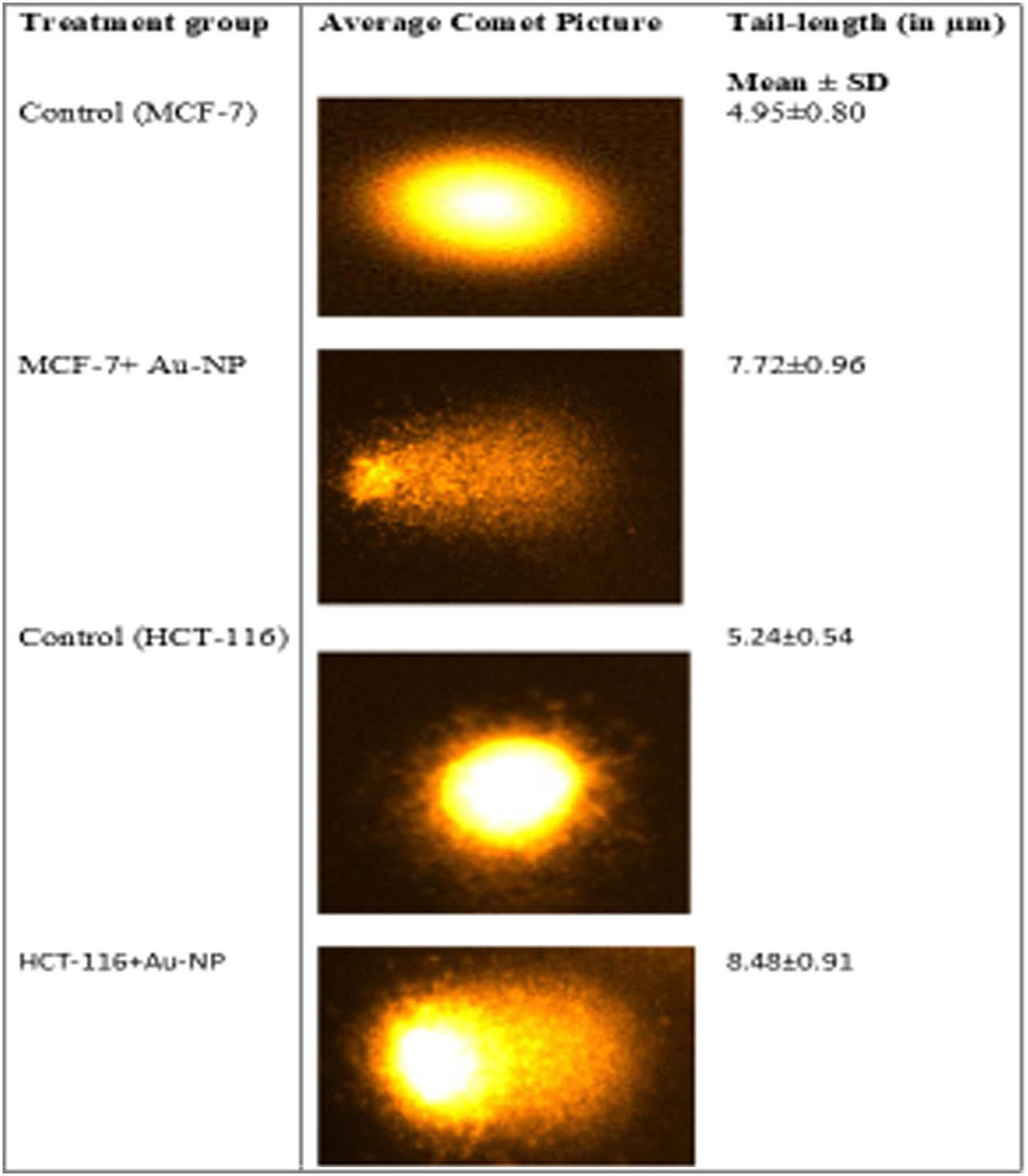
Comet assay was used to evaluate the DNA damage caused by AuNPs (5 µg/mL) in the studied cancer cells. The results showed a significant DNA damage caused by AuNPs treatment, as observed by increase in the tail length of DNA in cancer cells.
3.4 AuNPs-induced intracellular ROS generation in MCF-7 and HCT-116 cell lines
We evaluated the intracellular ROS level by measuring DCF fluorescence. The DCF fluorescence was assessed in AuNPs-treated cancer cells at 1–10 µg/mL doses for 24 h. A dose-dependent increase in ROS production was observed after the treatment with AuNPs in both the studied cell lines. We observed a considerable increase (26.4%) in ROS generation in MCF-7 cells compared to control at 5 µg/mL of AuNPs. Similarly, HCT-116 cells also showed an increase (42.7%) in ROS generation after the treatment with the same concentration of AuNPs (Figure 6). Our results showed significant ROS-producing capabilities of AuNPs in MCF-7 and HCT-116 cell lines (Figure 6).
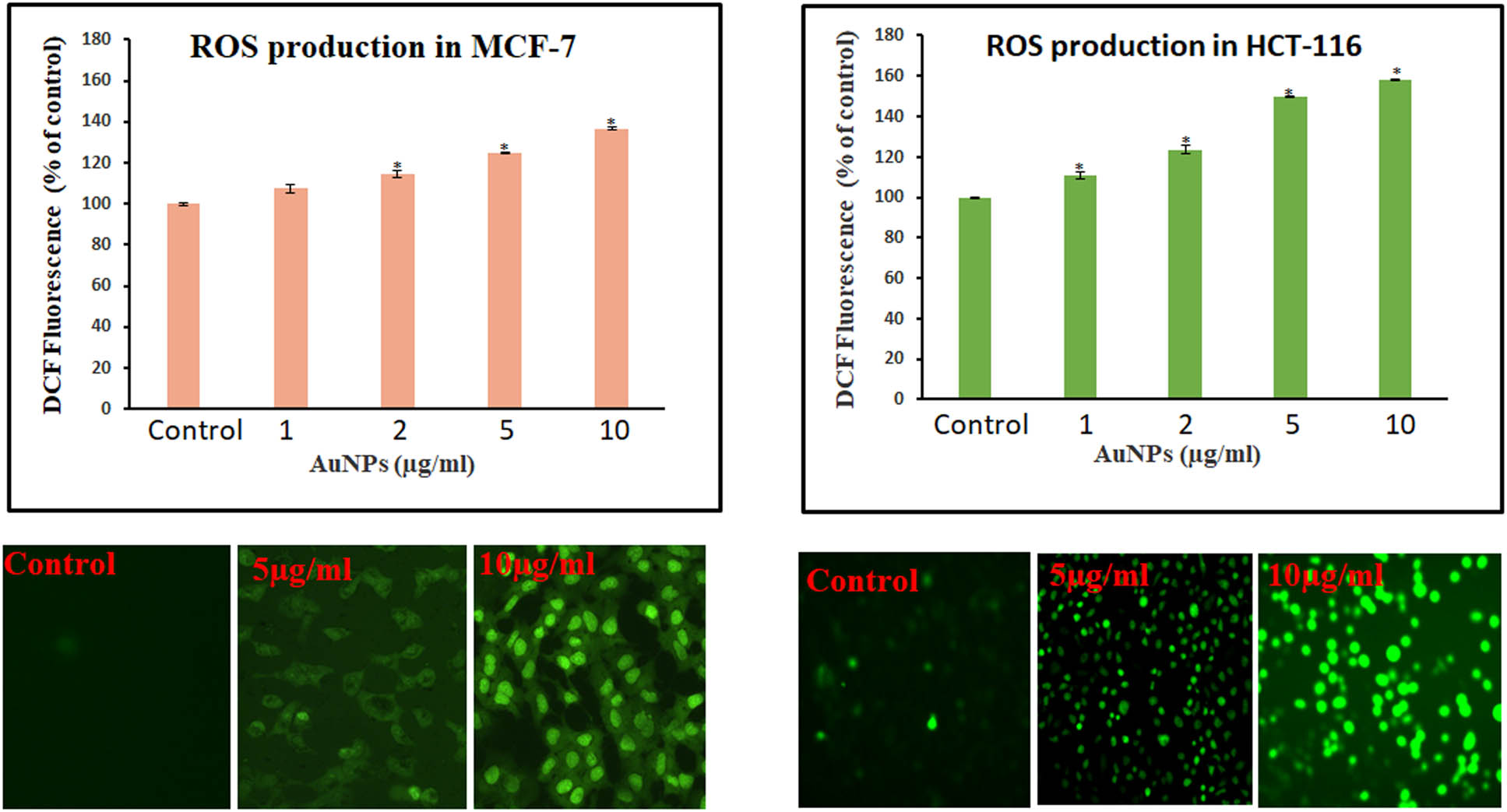
ROS production in MCF-7 and HCT-116 cells treated with AuNPs. A spectrofluorometer was used to detect the relative fluorescence of DCF, with excitation and emission spectra of 485 and 530 nm, respectively. Student t-test was performed to determine the significance. Values <0.05 were considered significant and indicated with * mark above the column. A Leica microscope (20× magnification) was used to take the image of the fluorescing cells.
3.5 Effect of AuNPs on mRNA expression of pro/anti-apoptotic marker genes in MCF-7 and HCT-116 cancer cells
The treatment with 5 µg/mL AuNPs showed increased expression of tumor suppressor gene p53 and elevated expression of pro-apoptotic genes, Bax, and caspase-3. On the other hand, the expression of the anti-apoptotic gene BCL-2 was found to be downregulated in MCF-7 cells (Figure 7a). A similar trend was also recorded in HCT-116 cells at the same concentration of AuNPs (Figure 7b).
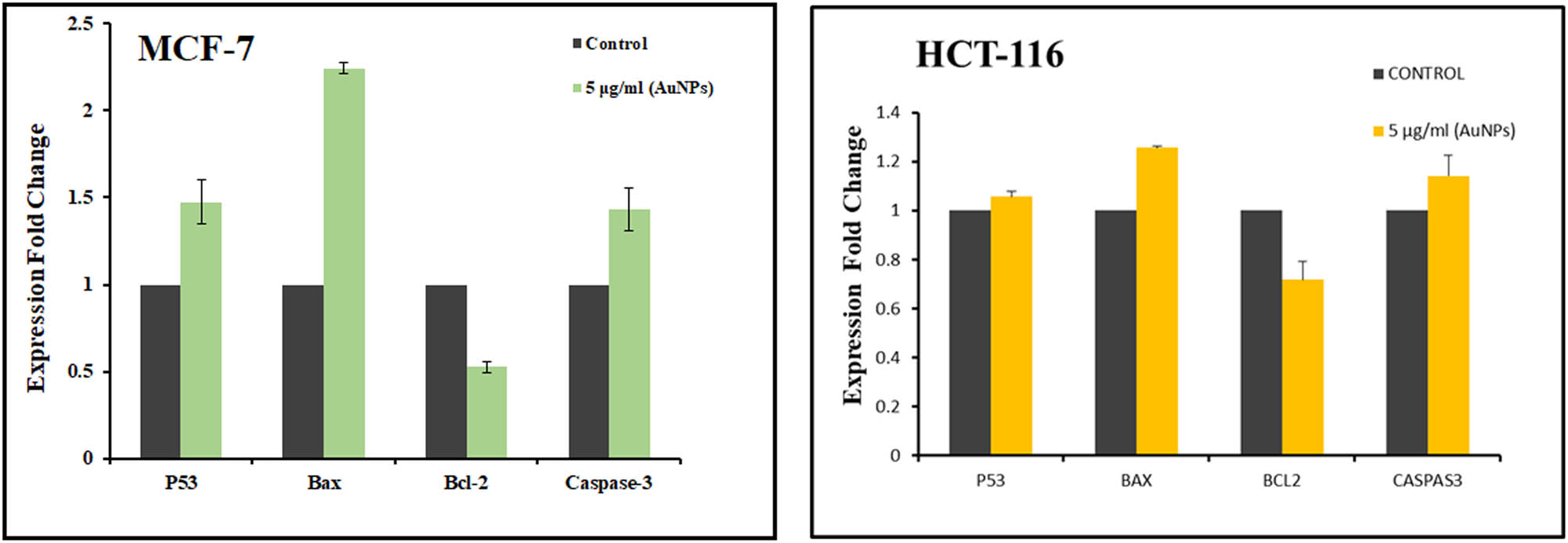
Effect of AuNPs on pro/anti-apoptotic marker mRNA expression levels. Changes in mRNA expression levels caused by nanostructures are expressed as a fold change in relative quantity compared to control cells. Other genes’ cycle threshold values were computed using GAPDH as a control.
3.6 Induction of apoptosis by AuNPs in MCF-7 and HCT-116 cells
The results of the apoptosis quantification in MCF-7 and HCT-116 cells are presented in dot plots (Figures 8a and b; 9a and b). Both the cells experienced a significant increase in apoptosis in a dose-dependent manner. At 2 µg/mL, the rates of early apoptosis were 15.59 and 12.32%, respectively, for MCF-7 and HCT-116 cells. At the same time, the levels of late apoptosis were 10.69 and 13.19% at the same concentration. Similarly, the highest concentration of AuNPs (5 µg/mL) treatment showed 18.78 and 18.92% early apoptotic cells in MCF-7 and HCT-116 cell lines. In addition, the treatment of AuNPs resulted in a slight elevation in necrotic cells in a concentration-independent manner. Conclusively, apoptosis analysis reaffirms the cytotoxicity data of cancer cells towards AuNPs.
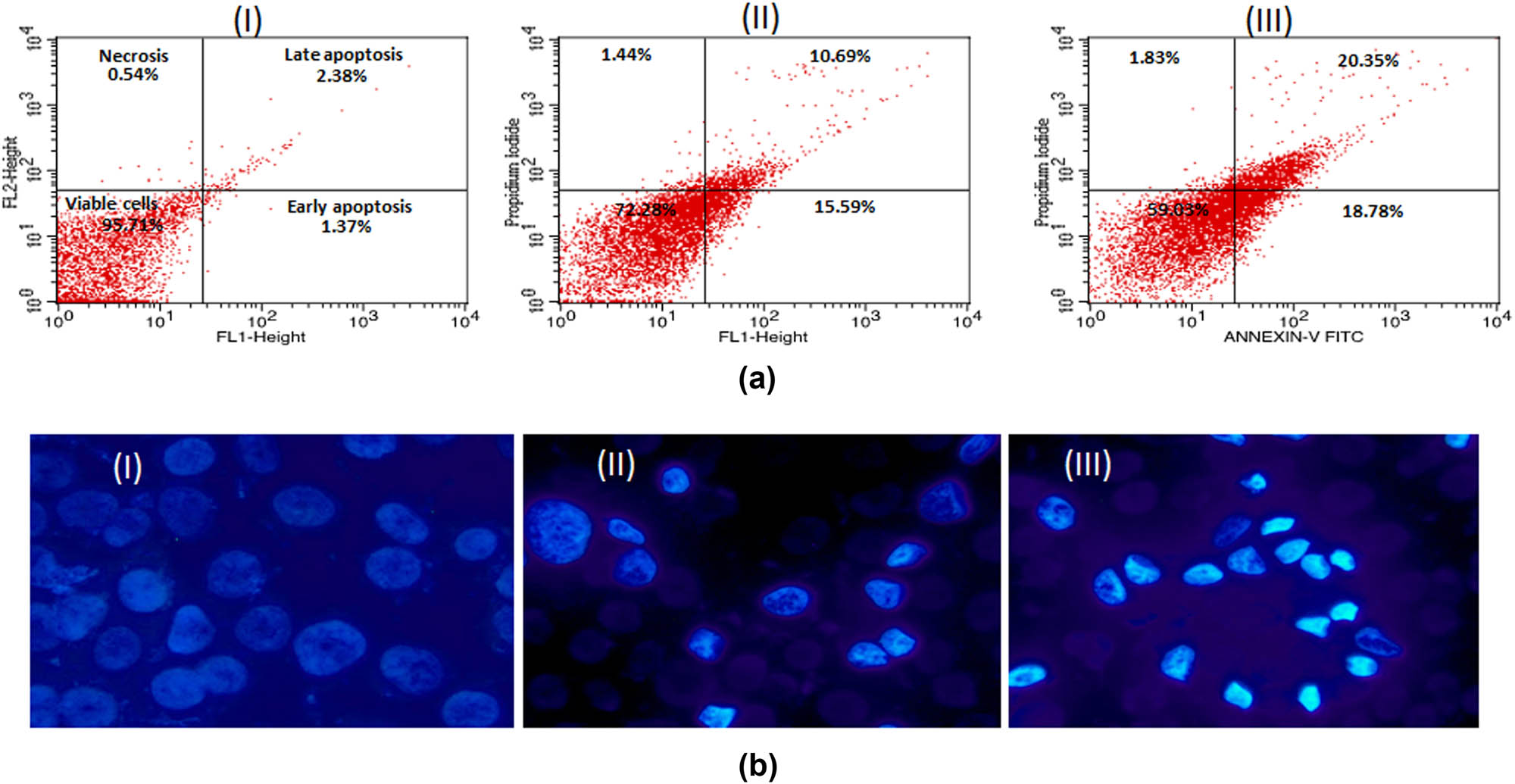
(a) Flow cytometry profile of annexin V-FITC/PI staining of MCF-7 cells showing percentage of viable cells, early apoptosis, late apoptosis, and necrotic cells. (I) Control, (II) 5 μg/mL of AuNPs, (III) 10 μg/mL of AuNPs. (b) Representative images of AuNPs-treated MCF-7 cells captured under fluorescence microscope after staining with nuclear dye Hoechst 33324. (I) Control, (II) 5 μg/mL, (III) 10 μg/mL. Magnification 200×.
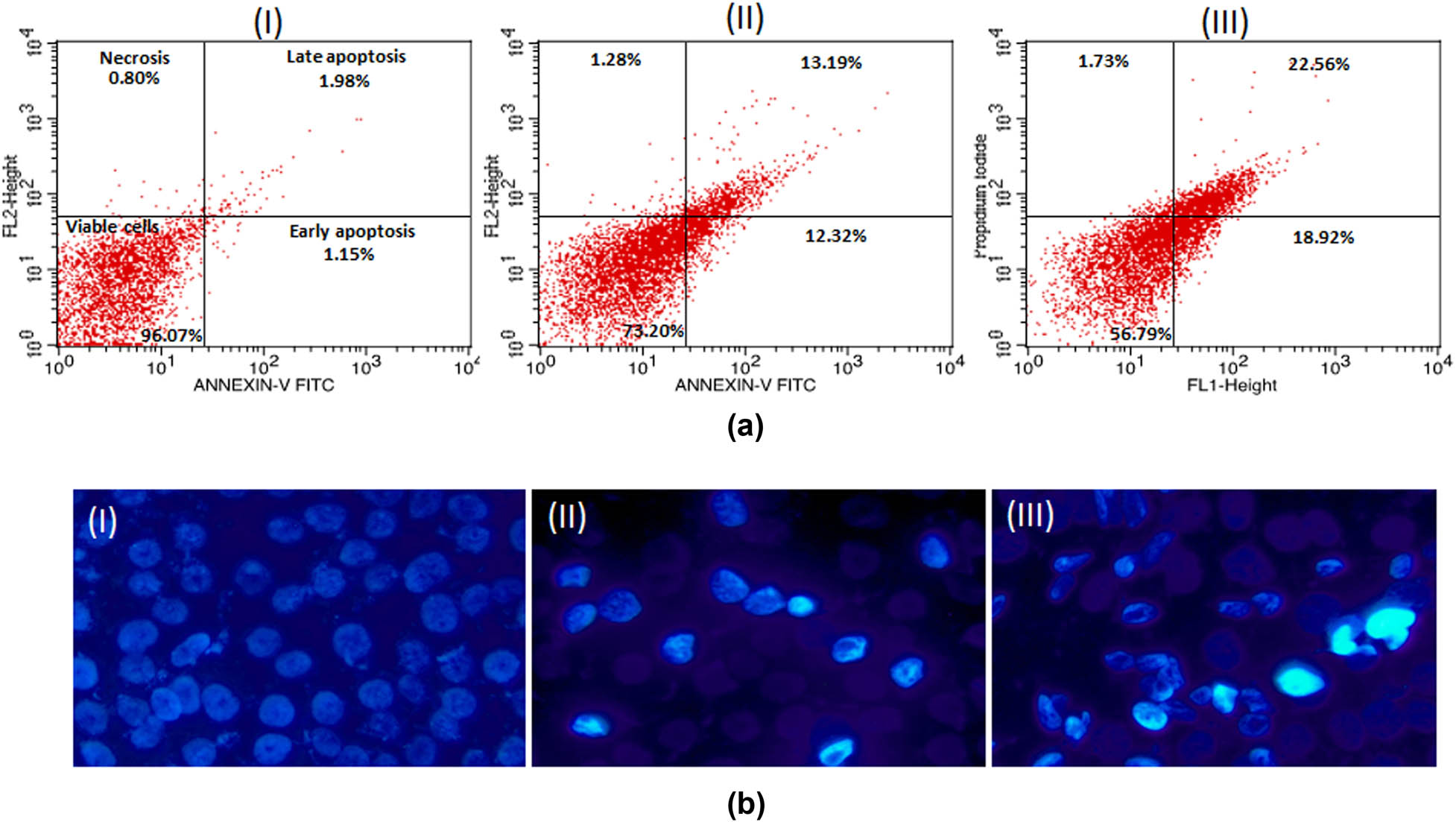
(a) Flow cytometry profile of annexin V-FITC/PI staining of HCT-116 cells showing percentage of viable cells, early apoptosis, late apoptosis and necrotic cells. (I) Control, (II) 5 μg/mL of AuNPs, (III) 10 μg/mL of AuNPs. (b) Representative images of AuNPs-treated HCT-116 cells captured under fluorescence microscope after staining with nuclear dye Hoechst 33324. (I) Control, (II) 5 μg/mL, (III) 10 μg/mL. Magnification 200×.
4 Discussion
The growing body of evidence indicates that NPs are up-and-coming candidates for drug delivery as an adjuvant to existing treatment or a possible therapeutic agent for various disorders, including cancer [34,35,36]. The current study exploited the microwave irradiation technique with citric acid as a reducing agent to synthesize AuNPs, while multi-technique approaches were adopted for the characterization purpose. The synthesized AuNPs were stable and ranged between 2 and 6 nm for 50 s irradiation time. In recent years, the microwave irradiation (MWI) approach has emerged as a new strategy to synthesize smaller size AuNPs rapidly [37,38]. The method allows the regulation of AuNP properties and structure, while avoiding contamination by altering experimental conditions in the presence of a reducing agent [19]. Recently, Adnan et al. [25] reported a microwave-assisted synchronous nanogold synthesis with superior characteristics in terms of high-quality crystal, spherical shape, amorphous, enhanced colloidal stability, and no agglomeration with feasible biocompatibility, biosafety, and anticancer therapeutic value. The specific size and configuration of AuNPs greatly affect the anticancer potential, including cytotoxicity [39]. Despite favorable results with smaller size AuNPs, the very low size could result in rapid excretion through the renal filtration system [40,41]. In one study, Zhang et al. [24] reported that smaller AuNPs (4–5 nm) have a higher cytotoxicity potential compared to larger particles (18–20 nm). Hence, our synthesized AuNPs (2–6 nm) are expected to provide an optimum anticancer therapeutic effect.
The cytotoxicity of AuNPs has been reported in many cancer cell lines, such as Hep2, MDA-MB-231, Caco-2, and MCF-7 cancer cells [27,42,43]. The cytotoxicity of AuNPs is associated with NP size, surface charge, and functional groups [44]. The smaller size of AuNPs results in extensive tissue distribution, deep penetration inside specific tissues, better cellular uptake, and increased toxic effects [45]. Considering the abovementioned facts, we observed significant cytotoxicity of the synthesized AuNPs at very low concentrations in both the cell lines. The smaller size and varied surface characteristics of AuNPs could be the reason behind the observed significant cytotoxicity.
The migration of cells is crucial for the progression of cancer. In this study, we used an in vitro scratch assay since it is a reliable tool for probing cell migration [46]. The potential of AuNPs to interfere with the cytoskeleton of MCF-7 cells may explain the suppression of migration observed in our study. Cell division and migration necessitate cytoskeleton rearrangement and disrupting one or both processes significantly impacts cell proliferation and migration [47]. Our findings are consistent with earlier studies that reported NPs limit cancer cell migration [26,48].
For the quantitative measurement of DNA damage, the comet assay assessing a mixture of single-strand breaks, double-strand breaks, and alkaline-labile sites is frequently performed [49]. Exposure time, particle size, cell types, and NP surface coating significantly impact DNA damage [50]. A recent study reported that a positive surface charge increases the cellular absorption and cytotoxicity of AuNP. Neither functionalization nor size can result in genotoxicity [51]. However, one study reported a dose-dependent increase in DNA damage in HepG2 cells and suggested that the particle size of AuNPs affects their genotoxicity [39]. An earlier study reported that HepG2 cells are more susceptible to DNA damage by AuNPs than peripheral blood mononuclear cells, implying that specific cells are more sensitive to AuNPs cytotoxicity than others [52]. This could be explained because AuNPs affect physiological processes in different cell types through multiple signaling pathways [50]. We also observed genotoxicity/DNA damage in both the studied cancer cell lines due to AuNPs treatment.
A physiological system’s redox state balance is required for proper biochemical functioning, and an imbalance creates oxidative stress, leading to pathogenic processes [53,54]. AuNPs’ response to cellular ROS production is one of the primary factors of NP-induced cytotoxicity. The interaction of AuNPs and mammalian cells might cause oxidative stress by promoting ROS production over the cellular antioxidant defenses [55]. ROS production could also play a significant role in the apoptosis induced by AuNPs treatment [53]. AuNPs have also been shown to stimulate ROS generation in different cancer cell lines [53,54,55]. Our findings revealed a molecular basis of AuNPs-induced ROS production, leading to apoptosis. These findings also suggest that the cell death observed in our study could be due to ROS generation, which may have disturbed the cellular redox balance.
p53 encourages cell arrest in the presence of DNA damage or cellular stress so that the damage can be repaired, or self-mediated apoptosis can take place [17,56]. The activated caspases-3 are capable of autocatalysis, cleaving, and activating other caspase family members, culminating in irreversible apoptosis [57]. Our study also observed DNA fragmentation and increased caspase-3 activity in MCF-7 and HCT-116 cancer cells treated with AuNPs. Apoptosis is crucial for many biological processes and systems, including the immune system, the normal cell cycle, anticancer defenses, embryonic development, morphological changes, and chemically induced cell death [58]. Our findings are in accordance with the previous reports by Kim et al. [59] and Ahmadian et al. [60]. Given the specific apoptotic response in cancer cells, the RT-PCR results also revealed a modulation in expression levels of the studied pro/anti-apoptotic proteins in both cancer cell lines, confirming the anticancer potential of our synthesized AuNPs.
5 Conclusion
In summary, the current study observed the diverse impacts of synthesized AuNPs on human cancer cell (HCT-116 and MCF-7) viability. The considerable difference in AuNP-induced cytotoxicity in cancer cells highlights that it could be a viable alternative to more harmful traditional cancer therapies. The AuNPs trigger apoptosis in cancer cells via the ROS-regulated p53, Bax/Bcl-2, and caspase pathways. However, more in-depth research is needed to understand the exact mechanism resulting from AuNPs’ cancer cell-specific toxicity, which is still mostly unknown.
-
Funding information: The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project no. (IFKSURG-2-1734).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Pradhan RK, Ramakrishna W. Transposons: Unexpected players in cancer. Gene. 2022;808:145975.10.1016/j.gene.2021.145975Suche in Google Scholar PubMed
[2] Khan MS, Alomari A, Tabrez S, Hassan I, Wahab R, Bhat SA, et al. Anticancer potential of biogenic silver nanoparticles: A mechanistic study. Pharmaceutics. 2021;13(5):707.10.3390/pharmaceutics13050707Suche in Google Scholar PubMed PubMed Central
[3] Zughaibi TA, Suhail M, Tarique M, Tabrez S. Targeting PI3K/Akt/mTOR pathway by different flavonoids: A cancer chemopreventive approach. Int J Mol Sci. 2021;22(22):12455.10.3390/ijms222212455Suche in Google Scholar PubMed PubMed Central
[4] Hadhri A, Abidi R, Mahjoub N, Mousli A, Mahjoubi K, Boujelbene N, et al. Metastasis of breast cancer to bladder. Afr J Urol. 2021;27(1):123.10.1186/s12301-021-00224-zSuche in Google Scholar
[5] Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Breast cancer epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancers. 2021;13(17):4287.10.3390/cancers13174287Suche in Google Scholar PubMed PubMed Central
[6] Veettil SK, Wong TY, Loo YS, Playdon MC, Lai NM, Giovannucci EL, et al. Role of diet in colorectal cancer incidence: Umbrella review of meta-analyses of prospective observational studies. JAMA Netw Open. 2021;4(2):e2037341.10.1001/jamanetworkopen.2020.37341Suche in Google Scholar PubMed PubMed Central
[7] Tabrez S, Khan AU, Mirza AA, Suhail M, Jabir NR, Zughaibi TA, et al. Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer. Nanotechnol Rev. 2022;11(1):1322–31.10.1515/ntrev-2022-0081Suche in Google Scholar
[8] Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. 2021;14(1):85.10.1186/s13045-021-01096-0Suche in Google Scholar PubMed PubMed Central
[9] Kemp JA, Kwon YJ. Cancer nanotechnology: current status and perspectives. Nano Convergence. 2021;8(1):34.10.1186/s40580-021-00282-7Suche in Google Scholar PubMed PubMed Central
[10] Alserihi RF, Mohammed MRS, Kaleem M, Khan MI, Sechi M, Sanna V, et al. Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment. Nanotechnol Rev. 2022;11(1):298–311.10.1515/ntrev-2022-0013Suche in Google Scholar
[11] Tabrez S, Jabir NR, Adhami VM, Khan MI, Moulay M, Kamal MA, et al. Nanoencapsulated dietary polyphenols for cancer prevention and treatment: successes and challenges. Nanomed (Lond). 2020;15(11):1147–62.10.2217/nnm-2019-0398Suche in Google Scholar PubMed
[12] Tabrez S, Khan AU, Hoque M, Suhail M, Khan MI, Zughaibi TA. Investigating the anticancer efficacy of biogenic synthesized MgONPs: An in vitro analysis. Front Chem. 2022;10:970193.10.3389/fchem.2022.970193Suche in Google Scholar PubMed PubMed Central
[13] Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–24.10.1038/s41573-020-0090-8Suche in Google Scholar PubMed PubMed Central
[14] Shait Mohammed MR, Ahmad V, Ahmad A, Tabrez S, Choudhry H, Zamzami MA, et al. Prospective of nanoscale metal organic frameworks [NMOFs] for cancer therapy. Semin Cancer Biol. 2021;69:129–39.10.1016/j.semcancer.2019.12.015Suche in Google Scholar PubMed
[15] Gerosa C, Crisponi G, Nurchi VM, Saba L, Cappai R, Cau F, et al. Gold nanoparticles: A new golden era in oncology? Pharmaceuticals. 2020;13(8):192.10.3390/ph13080192Suche in Google Scholar PubMed PubMed Central
[16] Saeed BA, Lim V, Yusof NA, Khor KZ, Rahman HS, Samad NA. Antiangiogenic properties of nanoparticles: a systematic review. Int J Nanomed. 2019;14:5135–46.10.2147/IJN.S199974Suche in Google Scholar PubMed PubMed Central
[17] Tabrez S, Khan AU, Hoque M, Suhail M, Khan MI, Zughaibi TA. Biosynthesis of ZnO N.P.s from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer. Nanotechnol Rev. 2022;11(1):2714–25.10.1515/ntrev-2022-0154Suche in Google Scholar
[18] Elahi N, Kamali M, Baghersad MH. Recent biomedical applications of gold nanoparticles: A review. Talanta. 2018;184:537–56.10.1016/j.talanta.2018.02.088Suche in Google Scholar PubMed
[19] Hu X, Zhang Y, Ding T, Liu J, Zhao H. Multifunctional gold nanoparticles: A novel nanomaterial for various medical applications and biological activities. Front Bioeng Biotechnol. 2020;8:990.10.3389/fbioe.2020.00990Suche in Google Scholar PubMed PubMed Central
[20] Albarwary SA, Kibarer AG, Mustapha MT, Hamdan H, Ozsahin DU. The efficiency of AuNPs in cancer cell targeting compared to other nanomedicine technologies using fuzzy PROMETHEE. J Healthc Eng. 2021;2021:e1566834.10.1155/2021/1566834Suche in Google Scholar PubMed PubMed Central
[21] Bai X, Wang Y, Song Z, Feng Y, Chen Y, Zhang D, et al. The basic properties of gold nanoparticles and their applications in tumor diagnosis and treatment. Int J Mol Sci. 2020;21(7):E2480.10.3390/ijms21072480Suche in Google Scholar PubMed PubMed Central
[22] Siddique S, Chow JCL. Gold nanoparticles for drug delivery and cancer therapy. Appl Sci. 2020;10(11):3824.10.3390/app10113824Suche in Google Scholar
[23] Lu H, Su J, Mamdooh R, Li Y, Stenzel MH. Cellular uptake of gold nanoparticles and their movement in 3D multicellular tumor spheroids: Effect of molecular weight and grafting density of poly(2-hydroxyl ethyl acrylate). Macromol Biosci. 2020;20(1):1900221.10.1002/mabi.201900221Suche in Google Scholar PubMed
[24] Zhang X-D, Wu D, Shen X, Liu P-X, Yang N, Zhao B, et al. Size-dependent in vivo toxicity of PEG-coated gold nanoparticles. Int J Nanomed. 2011;6:2071–81.10.2147/IJN.S21657Suche in Google Scholar PubMed PubMed Central
[25] Adnan M, Oh K-K, Husen A, Wang M-H, Alle M, Cho D-H. Microwave-assisted synchronous nanogold synthesis reinforced by kenaf seed and decoding their biocompatibility and anticancer activity. Pharmaceuticals. 2022;15(2):111.10.3390/ph15020111Suche in Google Scholar PubMed PubMed Central
[26] Ali MRK, Wu Y, Ghosh D, Do BH, Chen K, Dawson MR, et al. Nuclear membrane-targeted gold nanoparticles inhibit cancer cell migration and invasion. ACS Nano. 2017;11(4):3716–26.10.1021/acsnano.6b08345Suche in Google Scholar PubMed PubMed Central
[27] Priya K, Iyer PR. Antiproliferative effects on tumor cells of the synthesized gold nanoparticles against Hep2 liver cancer cell line. Egypt Liver J. 2020;10(1):15.10.1186/s43066-020-0017-4Suche in Google Scholar
[28] Alharthy SA, Tabrez S, Mirza AA, Zughaibi TA, Firoz CK, Dutta M. Sugiol suppresses the proliferation of human U87 glioma cells via induction of apoptosis and cell cycle arrest. Evid Based Complement Altern Med. 2022;2022:7658899.10.1155/2022/7658899Suche in Google Scholar PubMed PubMed Central
[29] Hassan I, Khan AA, Aman S, Qamar W, Ebaid H, Al-Tamimi J, et al. Restrained management of copper level enhances the antineoplastic activity of imatinib in vitro and in vivo. Sci Rep. 2018;8(1):1682.10.1038/s41598-018-19410-1Suche in Google Scholar PubMed PubMed Central
[30] Jabir NR, Khan MS, Alafaleq NO, Naz H, Ahmed BA. Anticancer potential of yohimbine in drug-resistant oral cancer KB-ChR-8-5 cells. Mol Biol Rep. 2022;49:9565–73.10.1007/s11033-022-07847-7Suche in Google Scholar PubMed
[31] Hailan WA, Al-Anazi KM, Farah MA, Ali MA, Al-Kawmani AA, Abou-Tarboush FM. Reactive oxygen species-mediated cytotoxicity in liver carcinoma cells induced by silver nanoparticles biosynthesized using Schinus molle extract. Nanomaterials (Basel, Switz). 2022;12(1):161.10.3390/nano12010161Suche in Google Scholar PubMed PubMed Central
[32] Link S, El-Sayed MA. Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles. J Phys Chem B. 1999;103(21):4212–7.10.1021/jp984796oSuche in Google Scholar
[33] Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res. 2008;10(3):507–17.10.1007/s11051-007-9275-xSuche in Google Scholar
[34] Yetisgin AA, Cetinel S, Zuvin M, Kosar A, Kutlu O. Therapeutic nanoparticles and their targeted delivery applications. Molecules (Basel, Switz). 2020;25(9):E2193.10.3390/molecules25092193Suche in Google Scholar PubMed PubMed Central
[35] Jabir NR, Firoz CK, Bhushan A, Tabrez S, Kamal MA. The use of azoles containing natural products in cancer prevention and treatment: An overview. Anticancer Agents Med Chem. 2018;18(1):6–14.10.2174/1871520616666160520112839Suche in Google Scholar PubMed
[36] Ullah F, Shah KU, Shah SU, Nawaz A, Nawaz T, Khan KA, et al. Synthesis, characterization and in vitro evaluation of chitosan nanoparticles physically admixed with lactose microspheres for pulmonary delivery of montelukast. Polym (Basel). 2022;14(17):3564.10.3390/polym14173564Suche in Google Scholar PubMed PubMed Central
[37] Nguyen VP, Le Trung H, Nguyen TH, Hoang D, Tran TH. Advancement of microwave-assisted biosynthesis for preparing Au nanoparticles using Ganoderma lucidum extract and evaluation of their catalytic reduction of 4-Nitrophenol. ACS Omega. 2021;6(47):32198–207.10.1021/acsomega.1c05033Suche in Google Scholar PubMed PubMed Central
[38] Perveen K, Husain FM, Qais FA, Khan A, Razak S, Afsar T, et al. Microwave-assisted rapid green synthesis of gold nanoparticles using seed extract of Trachyspermum ammi: ROS mediated biofilm inhibition and anticancer activity. Biomolecules. 2021;11(2):197.10.3390/biom11020197Suche in Google Scholar PubMed PubMed Central
[39] Xia Q, Li H, Liu Y, Zhang S, Feng Q, Xiao K. The effect of particle size on the genotoxicity of gold nanoparticles. J Biomed Mater Res A. 2017;105(3):710–9.10.1002/jbm.a.35944Suche in Google Scholar PubMed
[40] Zhang X-D, Wu D, Shen X, Liu P-X, Fan F-Y, Fan S-J. In vivo renal clearance, biodistribution, toxicity of gold nanoclusters. Biomaterials. 2012;33(18):4628–38.10.1016/j.biomaterials.2012.03.020Suche in Google Scholar PubMed
[41] Vines JB, Yoon J-H, Ryu N-E, Lim D-J, Park H. Gold nanoparticles for photothermal cancer therapy. Front Chem. 2019;7:167.10.3389/fchem.2019.00167Suche in Google Scholar
[42] Majoumouo MS, Sharma JR, Sibuyi NRS, Tincho MB, Boyom FF, Meyer M. Synthesis of biogenic gold nanoparticles from Terminalia mantaly extracts and the evaluation of their in vitro cytotoxic effects in cancer cells. Molecules. 2020;25(19):4469.10.3390/molecules25194469Suche in Google Scholar
[43] Jeyarani S, Vinita NM, Puja P, Senthamilselvi S, Devan U, Velangani AJ, et al. Biomimetic gold nanoparticles for its cytotoxicity and biocompatibility evidenced by fluorescence-based assays in cancer (MDA-MB-231) and non-cancerous (HEK-293) cells. J Photochem Photobiol B: Biol. 2020;202:111715.10.1016/j.jphotobiol.2019.111715Suche in Google Scholar
[44] Kus-Liśkiewicz M, Fickers P, Ben Tahar I. Biocompatibility and cytotoxicity of gold nanoparticles: recent advances in methodologies and regulations. Int J Mol Sci. 2021;22(20):10952.10.3390/ijms222010952Suche in Google Scholar
[45] Peng J, Liang X. Progress in research on gold nanoparticles in cancer management. Med (Baltim). 2019;98(18):e15311.10.1097/MD.0000000000015311Suche in Google Scholar
[46] Liang C-C, Park AY, Guan J-L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329–33.10.1038/nprot.2007.30Suche in Google Scholar
[47] Hevia LG, Fanarraga ML. Microtubule cytoskeleton-disrupting activity of MWCNTs: applications in cancer treatment. J Nanobiotechnology. 2020;18(1):181.10.1186/s12951-020-00742-ySuche in Google Scholar
[48] Zughaibi TA, Mirza AA, Suhail M, Jabir NR, Zaidi SK, Wasi S, et al. Evaluation of anticancer potential of biogenic copper oxide nanoparticles (CuO N.P.s) against breast cancer. J Nanomater. 2022;2022:5326355. 10.1155/2022/5326355.Suche in Google Scholar
[49] Bankoglu EE, Schuele C, Stopper H. Cell survival after DNA damage in the comet assay. Arch Toxicol. 2021;95(12):3803–13.10.1007/s00204-021-03164-3Suche in Google Scholar
[50] Wang Y, Zhang H, Shi L, Xu J, Duan G, Yang H. A focus on the genotoxicity of gold nanoparticles. Nanomedicine. 2020;15(4):319–23.10.2217/nnm-2019-0364Suche in Google Scholar
[51] Vales G, Suhonen S, Siivola KM, Savolainen KM, Catalán J, Norppa H. Genotoxicity and cytotoxicity of gold nanoparticles in vitro: role of surface functionalization and particle size. Nanomaterials. 2020;10(2):271.10.3390/nano10020271Suche in Google Scholar PubMed PubMed Central
[52] Paino IMM, Marangoni VS, de Oliveira RD, Antunes LMG, Zucolotto V. Cyto and genotoxicity of gold nanoparticles in human hepatocellular carcinoma and peripheral blood mononuclear cells. Toxicol Lett. 2012;215(2):119–25.10.1016/j.toxlet.2012.09.025Suche in Google Scholar PubMed
[53] Jawaid P, Rehman MU, Zhao Q-L, Misawa M, Ishikawa K, Hori M, et al. Small size gold nanoparticles enhance apoptosis induced by cold atmospheric plasma via depletion of intracellular GSH and modification of oxidative stress. Cell Death Discov. 2020;6(1):1–12.10.1038/s41420-020-00314-xSuche in Google Scholar PubMed PubMed Central
[54] Sen GT, Ozkemahli G, Shahbazi R, Erkekoglu P, Ulubayram K, Kocer-Gumusel B. The effects of polymer coating of gold nanoparticles on oxidative stress and DNA damage. Int J Toxicol. 2020;39(4):328–40.10.1177/1091581820927646Suche in Google Scholar PubMed
[55] Enea M, Pereira E, Peixoto de Almeida M, Araújo AM, Bastos MD, Carmo H. Gold nanoparticles induce oxidative stress and apoptosis in human kidney cells. Nanomaterials. 2020;10(5):995.10.3390/nano10050995Suche in Google Scholar PubMed PubMed Central
[56] Bieging KT, Mello SS, Attardi LD. Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer. 2014;14(5):359–70.10.1038/nrc3711Suche in Google Scholar PubMed PubMed Central
[57] Araya LE, Soni IV, Hardy JA, Julien O. Deorphanizing Caspase-3 and Caspase-9 Substrates In and Out of Apoptosis with Deep Substrate Profiling. ACS Chem Biol. 2021;16(11):2280–96.10.1021/acschembio.1c00456Suche in Google Scholar PubMed PubMed Central
[58] Zhang Y, Li X, Huang Z, Zheng W, Fan C, Chen T. Enhancement of cell permeabilization apoptosis-inducing activity of selenium nanoparticles by ATP surface decoration. Nanomedicine. 2013;9(1):74–84.10.1016/j.nano.2012.04.002Suche in Google Scholar PubMed
[59] Kim S, Choi JE, Choi J, Chung K-H, Park K, Yi J, et al. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol Vitro. 2009;23(6):1076–84.10.1016/j.tiv.2009.06.001Suche in Google Scholar PubMed
[60] Ahmadian E, Dizaj SM, Rahimpour E, Hasanzadeh A, Eftekhari A, Hosain Zadegan H, et al. Effect of silver nanoparticles in the induction of apoptosis on human hepatocellular carcinoma (HepG2) cell line. Mater Sci Eng C Mater Biol Appl. 2018;93:465–71.10.1016/j.msec.2018.08.027Suche in Google Scholar PubMed
© 2022 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption

