Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
Abstract
Nanomaterials have been widely researched for use in construction materials. Numerous studies demonstrate that nanomaterials in small quantities can significantly improve the macroscopic properties of cement paste, mortar, or concrete through various mechanisms. Nanomaterials retrieved from biomass sources have recently gained particular research interest due to remarkable structural properties and the source material’s abundance and renewability. Cellulose and chitin are the most abundant polysaccharides in nature; thus, they are candidates for nanomaterials extraction as multifunctional additives in cementitious systems. In recent years, cellulose nanomaterials in cementitious composites have been extensively investigated, but chitin nanomaterials and starch derivatives for cement and concrete are still emerging research areas. This review article starts with an overview of polysaccharide nanomaterials’ (PNMs) physicochemical properties as a result of different chemical and mechanical extraction processes. Next a brief overview of cement hydration chemistry and microstructure and the interfacial interactions between the cement and the various surface chemical functionalities of PNMs are discussed. Then, the key mechanisms governing the cement strength enhancement by PNMs, such as bridging, nucleating and filling effect, and internal curing, are described. Finally, the impacts of PNMs on other properties of the cement are discussed.
1 Introduction
Concrete is the most widely used construction material [1]. Increased demand from China, India, the Middle East, Northern Africa, and North America drove the annual global production of cement (the major binder component of concrete) to 4.08 billion metric tons in 2019, with about 86 million tons produced in the U.S. [2]. Concrete is easy to manufacture by proportioning by weight and mechanically mixing the components. Furthermore, it can be made into any desired shape in its fresh state, while some of the cured products gain attractive mechanical properties as early as a few hours [3]. However, CO2 emissions associated with Portland cement production are estimated to be around 5% of the global anthropogenic carbon dioxide [4,5,6,7]. Frequent repairs and infrastructure rebuilding are the main drivers of the high demand for concrete to address cracking. The high susceptibility of current concrete to early cracking is a major concern for the long-term sustainability of infrastructures worldwide.
In the last century, Portland cement has been widely used for different infrastructures, but it results in early cracking [8,9] due to large thermal and drying shrinkage deformations, reduced creep, and increased elastic modulus [8]. Cracks allow water/gas/ions to penetrate the structure faster, leading to steel reinforcement corrosion and subsequent concrete spalling [10]. Therefore, concrete infrastructure requires frequent repairs or replacements, increasing CO2 emissions, and energy usage associated with concrete production. To overcome the burden of recurrent failing infrastructure and reduce concrete-associated CO2 emissions, strategies to enhance the durability of concrete, extend infrastructure life cycle, and delay structural repair and replacement while meeting rapid construction requirements are urgently needed.
In recent years, nanotechnology innovations have enabled improving macroscopic properties of concrete by altering the atomic structure of the calcium–silicate–hydrate (C–S–H) gel [11]. C–S–H is the key cement hydration product (50–70 wt%) and is responsible for the strength development, physical properties, and durability of concrete [12]. However, shrinkage, creep, restructuring, and microcracking can also develop within the C–S–H gel. Therefore, there is a great interest in modifying the structure and properties of C–S–H to achieve desired performance from the concrete composite. Nanomaterials can offer multiple unique advantages such as densification and reinforcement of the C–S–H gel, altering its degree of polymerization (DP) [13] and silica mean chain length of C–S–H [14], and increasing the stiffness of C–S–H [15]. Other effects from nanomaterials on the cement properties have been speculated from the creation of additional nucleation sites to facilitate extra hydration reactions, reduced porosity, and refinement of the cement pore structure [16]. Consequently, improvements in performance, such as subsequent reduction in chloride permeability, have been reported [17,18]. Furthermore, it has been shown that nanomaterials can greatly enhance current ultra-high-performance concrete by reducing cement usage and mitigating their excessive shrinkage and cracking issues [19].
The most researched nanomaterial for cement composites is carbon nanotubes (CNTs), which have demonstrated tremendous abilities in boosting cementitious systems’ mechanical and durability properties. However, wide stream use of CNTs in concrete-based construction is not possible until many challenges associated with CNT manufacturing are overcome to significantly reduce their cost and increase their availability in larger amounts. In addition, due to their inert surface chemistry, CNTs require surface functionalization for good dispersion and interfacial chemical compatibility and reactivity with cement [20,21,22]. Additional treatments and dispersion processes could add to the complexity of concrete production with nanomaterials, concrete cost, and the overall CO2 footprint of concrete production. In order to develop the cost-effective and scalable nanomaterials which can be globally adopted to reduce energy consumption and CO2 emissions of concrete, the nanomaterials should have the following characteristics:
Abundant feedstock resources;
Scalable and low-energy extraction/manufacturing methods,
Convenient dispersion, placement, finishing, and cleanup to maintain construction efficiency and promote implementation by concrete suppliers, builders, and project owners.
Biobased additives from abundant biopolymers in the nanoscale have received significant attention from researchers in recent years. Nanomaterials derived from cellulose (the most abundant biopolymer) have shown great potential for use in the cement industry [23,24,25]. However, less focus has been on chitin as a source for nanomaterials, though chitin is the second most abundant polysaccharide biopolymer [26,27] after cellulose. Other polysaccharides, e.g., starch and its derivatives, are only reported in a few studies [28,29]. Derivatives and nanostructures from these environmentally friendly biopolymers could offer cost-effective and low-energy alternatives to CNTs if sufficient study is carried out for cement and concrete use, providing their manufacturing processes are streamlined. Therefore, this review article aims to turn on the spotlight on these underused biopolymers for nanomaterials extraction for cementitious systems. Furthermore, the effects of polysaccharide nanomaterials (PNMs) on the properties of cement and concrete greatly vary depending on the nature of polysaccharides and the characteristics of PNMs, including surface chemistry, size, morphology, surface charge, etc. The impact of these properties on interfacial interactions with cement and consequential effects on concrete performance has been overlooked in current cement and concrete literature. Therefore, this manuscript aims to give readers an overview of the applications and the ensuing effects and probable reinforcing mechanisms of different types of polysaccharides and their nanomaterials in cement and concrete. This review also aims to draw the attention of researchers to knowledge gaps and areas of future research needed to expand the use of PNMs in cementitious systems.
To do so, this article first presents a comprehensive review of PNMs, including cellulose, chitin, starch (and its derivatives), and their application in cement and concrete. Recent advances in preparation methods and surface modification for making PNMs are reviewed, and their interfacial interactions with cement, and effects on the mechanical properties of cement and concrete are summarized. The mechanisms for nanomaterial-cement interactions and the factors affecting cement and concrete mechanical properties, such as size, morphology, concentration, and dispersion of nanomaterials, are also discussed.
2 Literature review methodology
The literature review methodology used the following search engines; Google scholar, Google patent, SciFinder, Patentsout, and Baidu. The searched keywords included cellulose cement (concrete), cellulose nanocrystal cement, cellulose nanofiber cement (concrete), chitin cement (concrete), chitosan cement (concrete), chitin nanocrystal cement (concrete), chitin nanofiber cement (concrete), starch cement (concrete), polysaccharide cement, among others. The authors reviewed publications and patents reported over the past 30 years.
3 Production methods of PNMs
Emerging biobased PNM additives, particularly cellulose nanomaterials, have been applied in cement and concrete for different end outcomes, e.g., strength enhancement, hydration booster, internal curing, rheological modification, and others. The properties of the PNM-reinforced or modified cement and concrete are usually governed by the characteristics of PNMs, such as their size, morphology, aspect ratio, surface chemistry, charge, etc. PNMs’ characteristics are determined by the nature of the polysaccharides and the extraction methods. Therefore, the following sections provide an overview of different types and production methods of PNMs.
3.1 Sulfated cellulose nanocrystals
Cellulose is a polydisperse linear polymer of poly-β-(1,4)-d-glucose residues and has a flat ribbon-like conformation [30]. The repeating unit (Figure 1a) is comprised of anhydro-glucose rings (C6H10O5) n . Cellulose chains have a degree polymerization of approximately 10,000 glucopyranose units in wood cellulose and 15,000 in native cellulose cotton [31,32]. Nanocellulose can be produced in different ways, leading to different properties and dimensions. Two main forms of cellulose nanomaterials can be obtained: cellulose nanocrystals (CNCs) and cellulose nanofibers (CNFs). Tables 1 and 2 summarize the different methods for producing CNFs or CNCs, such as mechanical fibrillation or sulfuric acid hydrolysis using wood pulp for producing CNFs [33,34,35,36,37]; 2,2,6,6-tetramethylpiperidine-1-oxyl radical ((TEMPO)-mediated oxidation and followed with mild disintegration in water) using wood pulp [38,39,40,41] for producing CNFs; and sulfuric acid hydrolysis with wood pulp, cotton fibers, algae, wood chips, pulp sludge, among other precursors for producing CNCs [42,43,44,45,46].
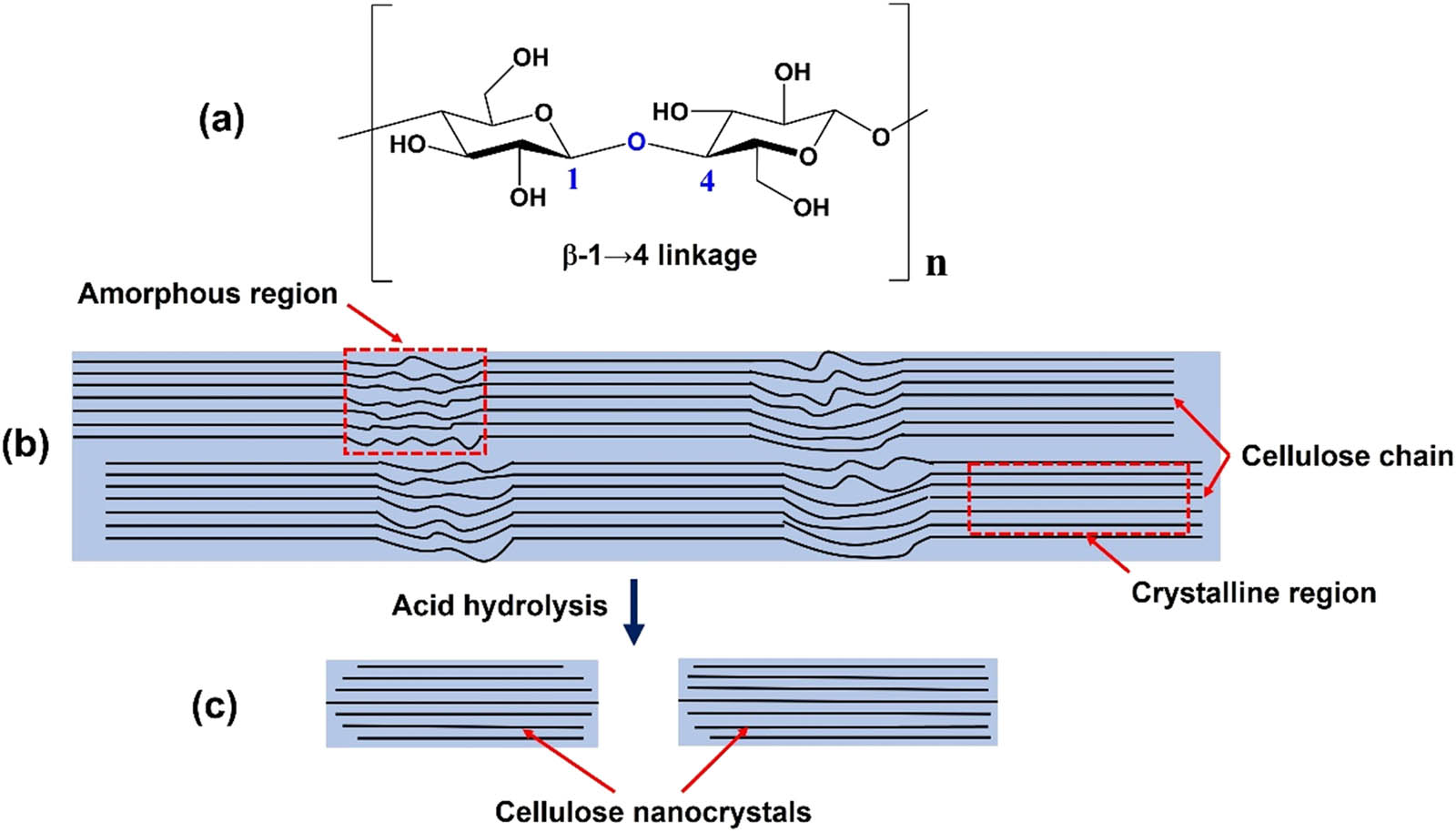
Schematics of (a) repeating unit of cellulose chain with the directionality of the 1 → 4 linkage, (b) idealized cellulose microfibril with one of the suggested configurations of the crystalline and amorphous regions, and (c) cellulose nanocrystals after acid hydrolysis dissolved the disordered regions.
Cellulose nanofibril-reinforced cementitious materials
| PNM type/source/production/size | Nanocellulose dose | Cementitious material | Effects on mechanical properties | Ref. | |
|---|---|---|---|---|---|
| CNF; Source: wood pulp; Production method: mechanical; Diameter: 20–200 nm; Length: 1–2.5
|
0–0.4 wt% of cement | General use limestone cement | ✓ | 106% improvement in fs | [33] |
| ✓ | 184% improvement in energy absorption | ||||
| ✓ | 10% increase in the hardness | ||||
| CNF; Source: wood pulp; Production: TEMPO oxidation; Diameter: 50–90 nm; Length: 400–800 nm | 0–0.4 wt% of cement | Ordinary Portland cement (OPC) (PII 42.5R) | ✓ | 20% increase in Cs | [40] |
| ✓ | 15% increase in fs | ||||
| ✓ | Initial setting time increases from 170 min to 272 min and final setting time increases from 219 min to 310 min | ||||
| CNF; Source: wood pulp; Production: 48% sulfuric acid hydrolysis; Diameter: 20 nm; Length: several microns | 0.04 wt% of cement | Oil well cement (OWC) | ✓ | 23.9% increase in fs | [34] |
| CNF; Source/method: wood pulp with 48% sulfuric acid hydrolysis followed by homogenization; Diameter: 20–30 nm; Length: several hundreds of microns; Aspect ratio: over 1,000 | 0.04 wt% of cement | OWC | ✓ | fs was increased from 11.24 to 13.63 MPa | [35] |
| ✓ | Cs was increased from 39.57 to 39.64 MPa after the addition of 0.04 wt% CNF | ||||
| Nanofibrillated cellulose (NFC); Source: eucalyptus pulp; Production method: bleaching process and deproteination and demineralization; Diameter: 5–10 nm; Length: several microns; Carboxylate content: 0.5 mmol/g fiber | 0.3 wt% of cement | Type I cement 32.5 N EN197-1:2,000 w/c = 0.26 | ✓ | 36% reduction in porosity | [41] |
| ✓ | 43% increase in Cs | ||||
| ✓ | 36% improvement in thermal conductivity | ||||
| ✓ | Both the initial and the final setting times have decreased as NFC contents increase | ||||
| Cellulose filament; Source: wood pulp; Production: mechanical method; Diameter: 10–400 nm; Length: 100–2,000 microns | 0.05, 0.1, 0.15, and 0.2 wt% | Type I cement (ASTM C494 + class F-fly ash | ✓ | Decreased Cs while increased fs | [36] |
| Bleached Chemi-Thermo-Mechanical Pulp (Aspen, grade 325/85/100 H T) was used as the source for the CNF generation by TEMPO method; Diameter:10–20 nm; Length: a few microns | 0–1.18% CNF (kg/m3 paste) | Portland cement type II | ✓ | Addition of CNF increased the yield stress from 11.36 to 22.09 Pa at 0.2% CNF and to 71.06 Pa at 0.8% CNF | [38] |
| ✓ | The viscosity increased only from 0.54 to 0.68 Pa s and to 1.26 Pa s by addition of 0.2 and 0.8% CNF, respectively | ||||
| CNF by InnoTech Alberta; Source: bleached chemo-thermomechanical wood pulp; Production method: TEMPO oxidation followed by Supermass Colloider; Diameter: 5–20 nm; Length: a few microns; Carboxylate content: 0.13 mmol/g fiber | 0–0.185 wt% of cement | Portland cement type GU | ✓ | Significantly alleviated bleeding and shrinkage at early stages of hydration | [39] |
| ✓ | Retarded hydration upon adding CNF | ||||
| ✓ | Reduced ingress of deleterious agents into cement | ||||
| ✓ | Increased degree of hydration (DOH) | ||||
| CNF; Source: wood pulp; Production method: mechanical fibrillation; Diameter: 20–50 nm; Length: typically, less than 0.2 mm; Zeta potential: −34 mV at pH = 7 | 0.015, 0.03, 0.06, 0.09, and 0.15 wt% of cement | Portland cement type I/II | ✓ | 3–31% improvement in 7 days Cs | [37] |
| ✓ | 39–116% improvement in fs | ||||
| ✓ | 49 and 26% reduction in autogenous shrinkage for 0.06 and 0.09 wt% CNF cement, respectively | ||||
| CNF; Source: wood pulp; Production method: mechanical fibrillation; Diameter: 25 and 500 nm; Length: several microns | 0.1–0.8 wt% of cement | OPC | ✓ | Addition of CNF between 0.1 and 0.2 wt% led to a general increase in the modulus of rupture and modulus of elasticity values | [73] |
| CNF; Source: wheat straw; Production method: prepared by HCl, NaOH, peracetic acid pretreatments of wheat straw followed by sonication (30 min) treatment; Diameter: 150–400 nm; Length: several microns | 0.5, 1, 1.5, and 10 wt% of cement mortar | Not provided | ✓ | Improved Cs by 27% at an optimum dosage of 0.5 wt% | [74] |
| CNF; Source: N/A; Production method: N/A. | 0.025, 0.05, 0.1, 0.3, and 0.5% (solids) by weight of cement | OPC type I/II | ✓ | At 90 days, 0.05% CNF in cement paste resulted in 24 and 15% increase in the Cs of cement paste with 0.35 and 0.45 w/c, respectively | [75] |
| ✓ | fs of cement paste increased up to 75 and 55% due to the addition of 0.5% of PCNF and nano silica-CNF, respectively | ||||
| CNF; Production method: mechanically fibrillated cellulose from University of Maine; Diameter: 28.2 ± 20.8 nm; Length: several hundreds of microns | 0.065 wt% by weight of cement | Type I/II OPC | ✓ | Max% increase in 7 days and 28 days Cs 17 and 18%, with 0.065 wt% CNF | [76] |
| ✓ | 0.065 wt% CNF enhanced E by 40% | ||||
| ✓ | Concentrations of CNF resulted in 0 to a maximum of 21 min delay in the initial set time of the control | ||||
| ✓ | 0.075 wt% CNF cement showed a faster initial setting than the control | ||||
| ✓ | Three concentrations of 0.045, 0.05, and 0.055 wt% delayed final setting times by 15, 39, and 56 min | ||||
| ✓ | In the first 90 min period, all CNF-cements showed similar or slightly higher consistency than the control and much higher values than the control + superplasticizer | ||||
| CNF; Production method: N/A; Diameter: 5–20 nm; Length: from 50 to >2,000 nm in length | 0.15 kg/m3 of concrete | Type I cement | ✓ | Slight improvements of Cs and fs were observed for curing times longer than 28 days | [77] |
Cellulose nanocrystal-reinforced cementitious materials
| PNM type/source/production/size | Optimal nanocellulose dose | Cementitious material | Effects on mechanical properties | Ref. | |
|---|---|---|---|---|---|
| CNCs; Source: wood pulp; Production method: sulfuric acid hydrolysis; Width: 3–5 nm; Length: 0.05–0.5
|
0.2 vol% of cement | Type V cement | ✓ | 20–30% improvement in fs | [42] |
| CNCs; Source: wood pulp; Production method: sulfuric acid hydrolysis; Width: 3–5 nm; Length: 0.05–0.5 µm; Zeta potential: –64 mV at pH = 7 | 1.5 vol% of cement | Type V cement | ✓ | 50% improvement in reduced modulus | [43] |
| CNCs; Source: N/A; Production method: Blue Goose Biorefineries Inc.; Width: 90 nm; Length: 500 nm; Zeta potential: –33.4 mV | 0.8 wt% of cement | Type I/II Portland cement | ✓ | Increased fraction of high-density of C–S–H | [78] |
| CNCs Production: Kraft pulp sourced from Alberta’s pulp and paper mill; Width: 5–10 nm; Aspect ratio: 10–60; Zeta potential: N/A | 0–0.44 wt% of OWC | Type G cement | ✓ | Improved Cs and fs by 60% | [45] |
| ✓ | Reduced porosity by 30% | ||||
| CNCs; Source: wood pulp; Production method: mechanical fibrillation; Width: 13–335 nm; Length: 100–400 nm | 0.1–0.8 wt% of cement | OPC | ✓ | Addition of CNC between 0.1 and 0.2 wt% led to a general increase in E and fs | [73] |
| CNCs; Source: wood pulp, cotton fibers, algae, wood chips, and pulp sludge; Production method: sulfuric acid hydrolysis; Width: 3–5 nm; Length: 0.05–0.5
|
0.023–3.311 vol% of cement | Type I/II and type V cement | ✓ | Reduced yield stress by up to 54% at low dosage (<0.2%) | [44] |
| ✓ | Increased yield stress by 10 times at high dosage (1%) | ||||
| CNCs; Source: Oil Palm Empty Fruit Bunch; Production method: 0.2 N HCl at 105°C/15 min, microcrystalline cellulose powder mixed with 64 wt% H2SO4 at 45°C/60 min and constantly stirred to produce CNCs; Zeta potential: –50.4 mV | 0.4 vol% of cement | Portland cement type I | ✓ | Increased Cs by 43–46% | [46] |
| ✓ | Substantial increase in fs | ||||
| Source: any material which comprises a substantial proportion of cellulose; Production method: redox reaction (NaClO with iron sulfate or copper sulfate as a catalyst with buffer) → alkaline extraction → 2nd redox reaction (NaClO with copper sulfate as a catalyst with buffer) → washing and concentrate-CNC; Width: 3–5 nm; Height: 3–20 nm; Length: 50–500 nm | 1–5 g/kg of cement | Cement type GU | ✓ | Marked increase on 3 days, and 30 days, Cs and fs | [79] |
| ✓ | Initial set time: 289 min without CNC and 409 min with 1 g CNC/1 kg cement with superplasticizer and air entrainer | ||||
| Bacterial nanocellulose; Source: derived from cellulose obtained from the aerobic fermentation of bacteria of the genus Gluconacetobacter | 0.05, 0.1, 0.15, and 0.2 wt% of cement | Class G Portland cement | ✓ | Increased Cs at 28 days but decrease Cs at 7 days for 0.15% bacterial nanocellulose-cement samples | [80] |
| CNCs by CelluForce; Production method: sulfuric acid hydrolysis; Width: 6 ± 3 nm; Length: 127 ± 59 nm; Zeta potential: –47.5 ± 2.31 mV at pH = 7.4 | 0.40wt% of cement | Type I/II OPC | ✓ | Max increase in 7 days and 28 days Cs was 9 and 17% at 0.4 wt% CNC, respectively | [76] |
| ✓ | 0.4 wt% CNC increased E by 20% | ||||
| CNCs; Production method: N/A; Width: ∼5 nm; Length: 50–500 nm | 0.15 kg/m3 of concrete | Cement type I | ✓ | Slight improvements in Cs and fs for curing times longer than 28 days | [77] |
CNCs and CNFs, as shown in Figure 1, have distinct physical and chemical properties. For instance, CNCs have a higher degree of crystallinity than CNFs. In addition, CNCs exhibit rod- or whisker-like structures, while CNFs are typically fibrous and include amorphous regions [47].
CNCs are usually isolated from semi-crystalline cellulose fibers using a strong sulfuric acid hydrolysis process in which the amorphous region is digested and the crystalline region is intact. Thus, individual crystallites with a little amorphous phase remaining (CNC has high crystallinity of ∼80%) are released with the assistance of mechanical disintegration processes (e.g., ultrasonication), as represented in Figure 2b and c [48]. Sulfuric acid is one of the widely used inorganic acids for the hydrolysis process because of its low cost and its reactivity with the hydroxyl groups on the surface of crystallites; thus, it can introduce anionic sulfate groups. The resulting cellulose nanocrystals are often referred to as sulfated cellulose nanocrystals. These negatively charged sulfate groups play an important role in stabilizing CNCs in water due to interfibrillar electrostatic repulsion forces [48]. Sulfate cellulose nanocrystals are mainly prepared from wood pulp due to abundant resources and low cost. The resulting cellulose nanocrystals exhibited rod-like or spindle-like shapes and have a diameter of 3–5 nm and a length of 50–500 nm [30,48]. Strong acid hydrolysis treatment results in hydrolysis of the glycosidic bonds of cellulose, especially in the amorphous region, thus reducing the DP. It was reported that the DP of cellulose from bleached wood pulp ranges from 140 to 200 [49]. The number of sulfate groups depends on the hydrolysis time and sulfuric acid concentrations. The sulfate content of the resulting sulfated cellulose nanocrystals was about 0.2–0.3 mmol/g CNCs [50,51,52]. Therefore, there are still a certain number of hydroxyl groups co-existing with sulfate groups on the surface of the sulfated cellulose nanocrystals, as shown in Figure 2(b) and (c).
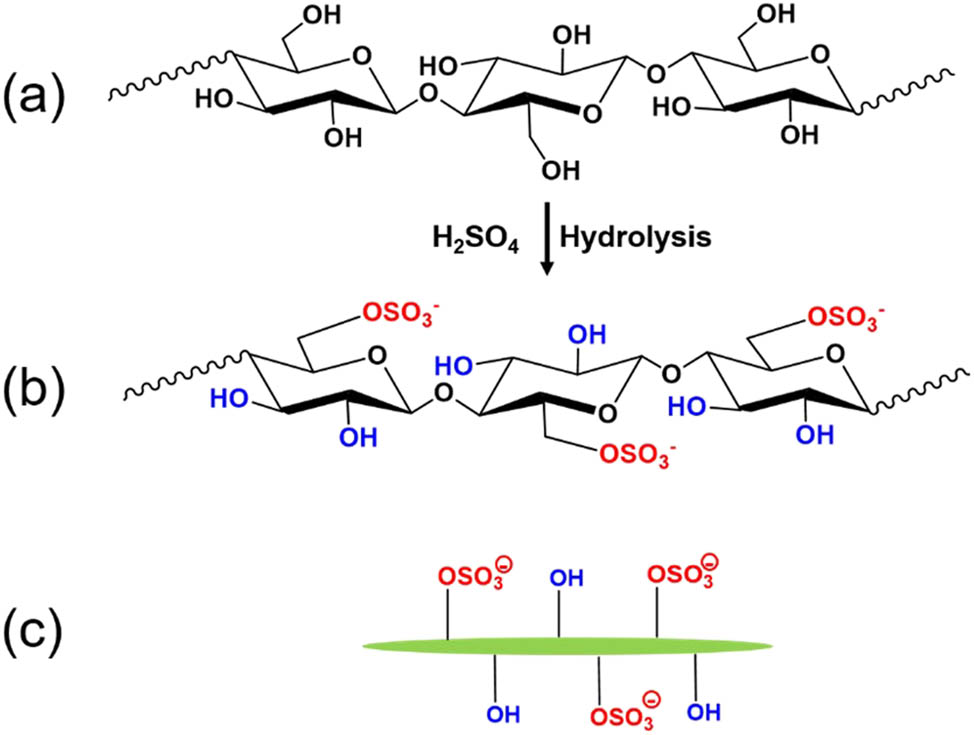
(a) Chemical structure of unmodified cellulose; (b) chemical structure of sulfated cellulose; (c) scheme of rod-shaped sulfated cellulose nanocrystals with hydroxyl and negatively charged sulfate groups on the surface.
3.2 Carboxylated CNFs
The TEMPO-mediated oxidation is regarded as one of the most effective pretreatment processes of cellulose fibers because it can selectively oxidize primary C-6 hydroxyl groups into anionic carboxylate groups (–COO−) under alkaline conditions [53]. This TEMPO-mediated oxidation reaction occurs on the surface of cellulose fibers and in their amorphous regions. As the carboxylate content increases to a certain amount, cellulose begins to disperse in an aqueous solution [53]. The negative charges can induce interfibrillar electrostatic repulsion forces and thereby facilitate nano-fibrillation in the subsequent mechanical separation process resulting in the production of fine and individualized CNFs with a diameter of 3–4 nm, a length of microns, and an aspect ratio greater than 100 [54,55]. The resulting carboxylated CNFs are often referred to as TEMPO-oxidized CNFs (TOCNFs). Compared to the acid-hydrolyzed cellulose nanocrystals, TOCNFs have much higher shear stress and viscosity due to the relatively higher aspect ratio, higher dispersibility, and fewer bundles in water [53]. The carboxylate content on the cellulose surface is highly dependent on the concentration of the oxidant sodium hypochlorite (NaClO). When the NaClO concentration in the TEMPO-mediated oxidation process increases from 2 to 5 mmol/g-pulp, the carboxylate content increases from 1 mmol/g-CNF to about 1.3 mmol/g-CNF. However, the DP decreases from 1,000 to 520 [55]. As mentioned earlier, TEMPO-mediated oxidation only takes place on the surface of CNFs. In the amorphous region of CNFs, most of the hydroxyl groups on the C2 and C3 remain intact. Thus, there is a high density of hydroxyl groups co-existing with carboxylate groups on the surface of TOCNFs, as shown in Figure 3.
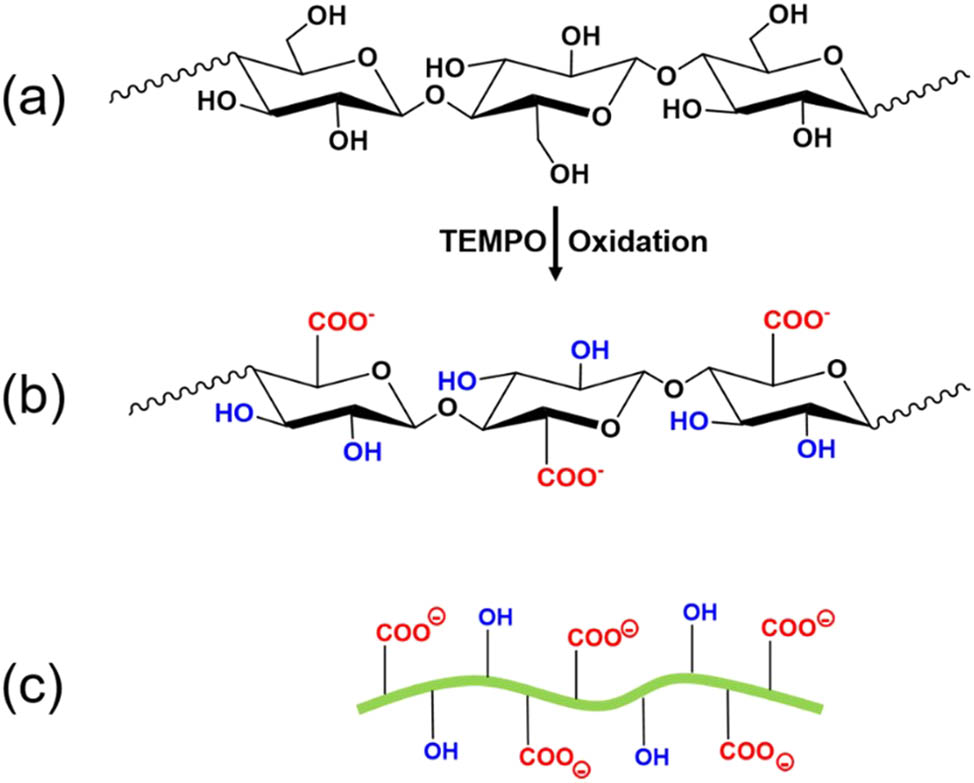
(a) Chemical structure of unmodified cellulose; (b) chemical structure of carboxylated cellulose; (c) scheme of carboxylated CNFs with different groups including hydroxyl and negatively charged carboxylated groups on the surface.
3.3 Mechanically fibrillated CNFs
The mechanical fibrillation method does not involve any chemical treatment; it is less effective in de-fibrillation of cellulose fibers into nanofibers than chemical processes (i.e., strong acid hydrolysis and TEMPO-mediated oxidation). However, it may benefit from lower manufacturing costs and no chemical usage to alleviate the environmental burdens compared to the TEMPO-mediated oxidation method for CNFs and the strong sulfuric acid hydrolysis method for CNCs. A friction grinding process is usually applied for the mechanical pretreatment of cellulose fibers [53]. The grinder often consists of two nonporous ceramic grinding discs with adjustable clearance between the upper and lower discs. While the upper grinding disc is static, the lower one is rotated at high speed. The cellulose fiber slurries are subject to massive compression, shearing, and rolling friction forces when fed into a hopper and dispersed by centrifugal forces into the clearance between the two grinding discs. When the shearing forces are applied to the longitudinal fiber axis of the fibrous materials, cellulose fibers are ground into micro- and nanofibers [53]. Due to the complicated, multilayered structure of plant fibers and interfibrillar hydrogen bonding, the resulting mechanically fibrillated CNFs often include aggregated nanofibers with a wide distribution in width [56]. Thus, mechanically fibrillated CNFs are coarser and larger than TOCNFs [53,56]. Mechanically fibrillated CNFs maintain crystalline and amorphous regions of cellulose chains. CNFs are reported to have a diameter smaller than 100 nm and lengths from 500 nm to several microns [57]. The mechanical fibrillation process by grinding discs will result in a decrease in the DP of the resulting CNFs. The number of the pass of dissolved pulp fibers in the grinder (supermass colloider) increased from 0 to 30. The DP decreased from 750 to about 450 [58]. CNFs produced by the mechanical method show relatively simpler surface chemistry as compared to CNFs obtained by the TEMPO-mediated oxidation method. That means that hydroxyl groups were only functional groups on the cellulose surface, as shown in Figure 4. However, it was reported that a negative charge was sometimes found on the surface of mechanically fibrillated CNFs. This is likely due to the presence of carboxylic groups in the glucuronic acid of hemicellulose left from the pulping process [53,59].
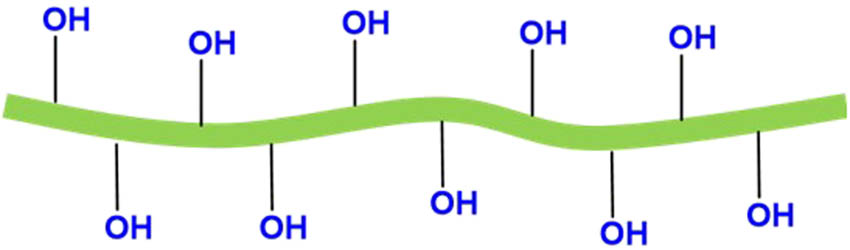
Scheme of mechanically fibrillated CNFs.
3.4 Carboxylated chitin nanofibers and nanocrystals
Chitin is a polymer of poly(b-(1–4)-N-acetyl-d-glucosamine), which is structurally similar to cellulose, but has acetamide groups at the C-2 positions, as shown in Figure 5a [60,61]. The degree of acetylation (DA) of chitin is typically around 0.90 with additional 5–15% amino groups due to deacetylation during extraction [62,63]. Due to chemical structural similarity, the extraction methodologies of nanofibers from cellulose are similar to that from chitin. In our current study, the TEMPO-mediated oxidation process has been applied to chitins. During the TEMPO-mediated oxidation process, the primary C-6 hydroxyl groups on the chitin surface or in amorphous regions can be selectively oxidized to carboxylate groups [64]. Most of the TEMPO-oxidized cellulose nanomaterials derived from bleached wood pulp exhibit a length of several microns and an aspect ratio of greater than 100, although the length and DP decreased with the increasing addition of oxidant NaClO in the TEMPO-mediated oxidation process [55,65]. The amount of oxidant NaClO plays a key role in changing the morphology of the chitin nanofibers derived from shrimp shells. We found that when NaClO increased from 3 to 9.5 mmol NaClO/g-chitin, the chitin nanofiber suspensions changed from opaque to transparent suspensions, as shown in Figure 6. The surface chemistry of carboxylated chitin nanofibers or carboxylated chitin nanocrystals is more complex than that of carboxylated CNFs due to the existence and distribution of the acetamido groups or amine groups at the C-2 position, as shown in Figures 5b and 7b. In our experiments, long and aggregated chitin nanofibers were obtained for a low dosage of NaClO, while short and rod-like chitin nanocrystals were obtained for a high dosage of NaClO (as schematically depicted in Figure 6c).
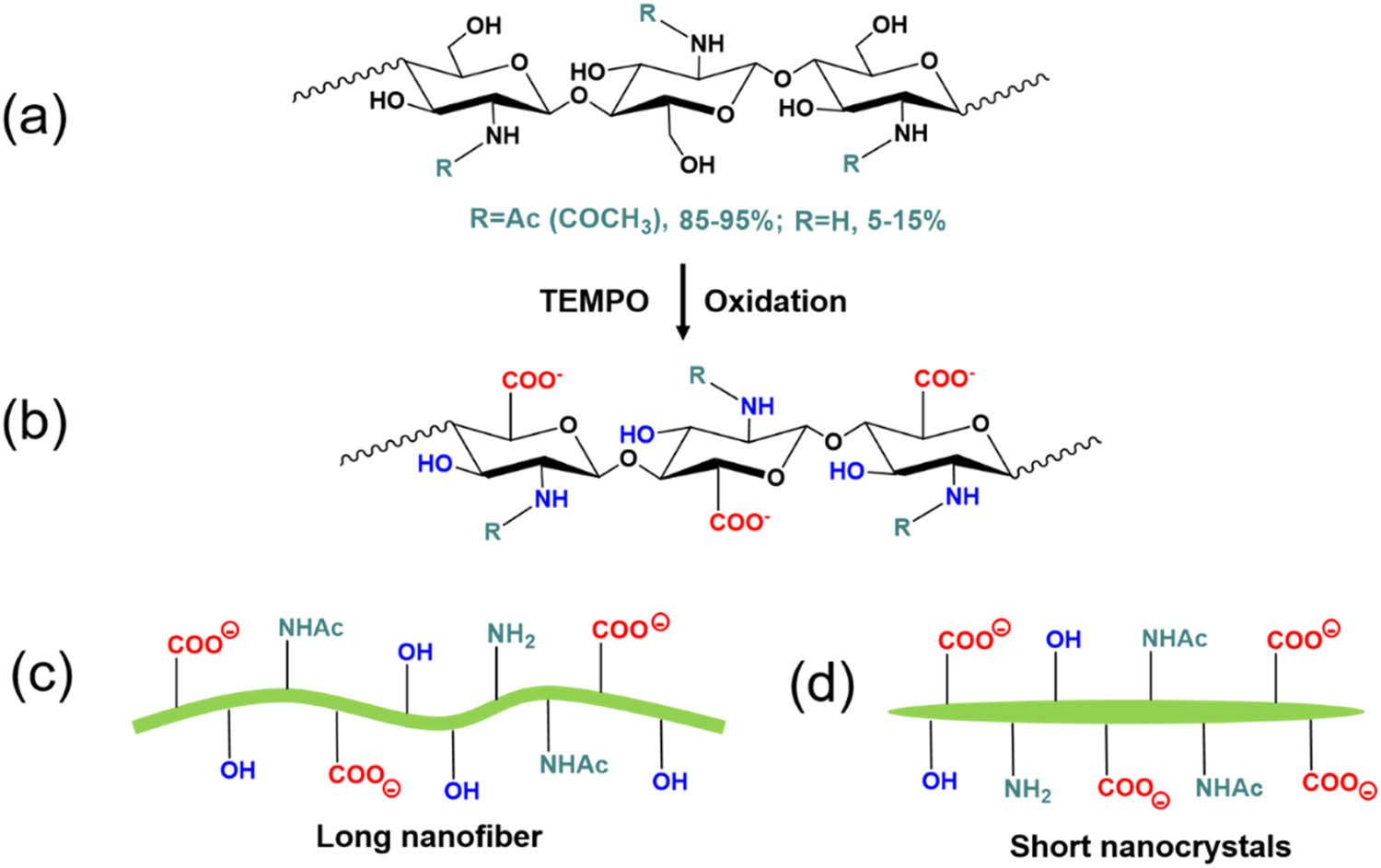
(a) Chemical structure of unmodified chitin; (b) chemical structure of carboxylated chitin; (c) scheme of carboxylated chitin nanofibers with a low degree of oxidation; (d) scheme of carboxylated chitin nanocrystals with a high degree of oxidation.
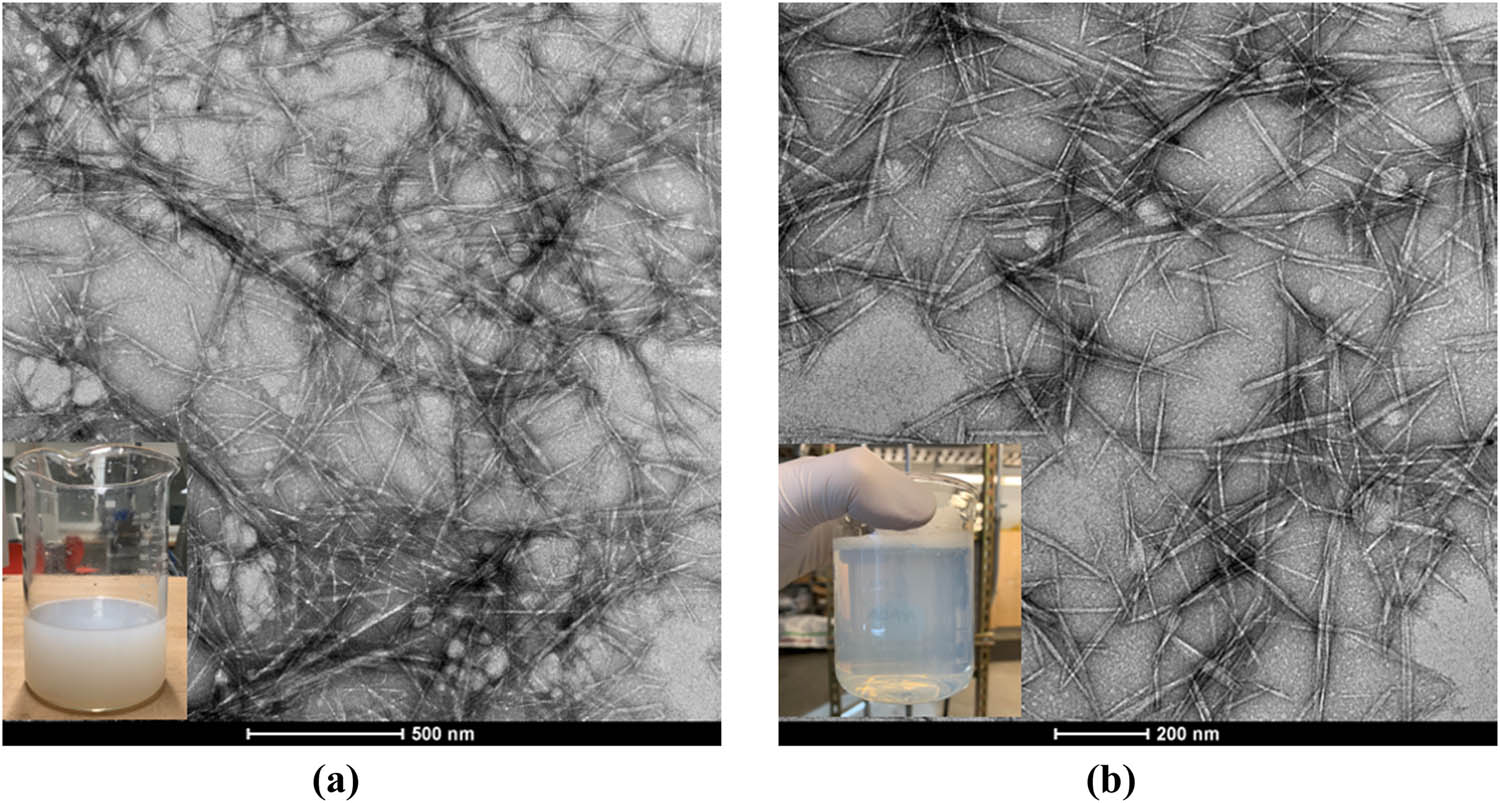
(a) TEMPO-oxidized chitin nanofibers (3 mmol NaClO/g chitin); (b) TEMPO-oxidized chitin nanocrystals (9.5 mmol NaClO/g chitin).
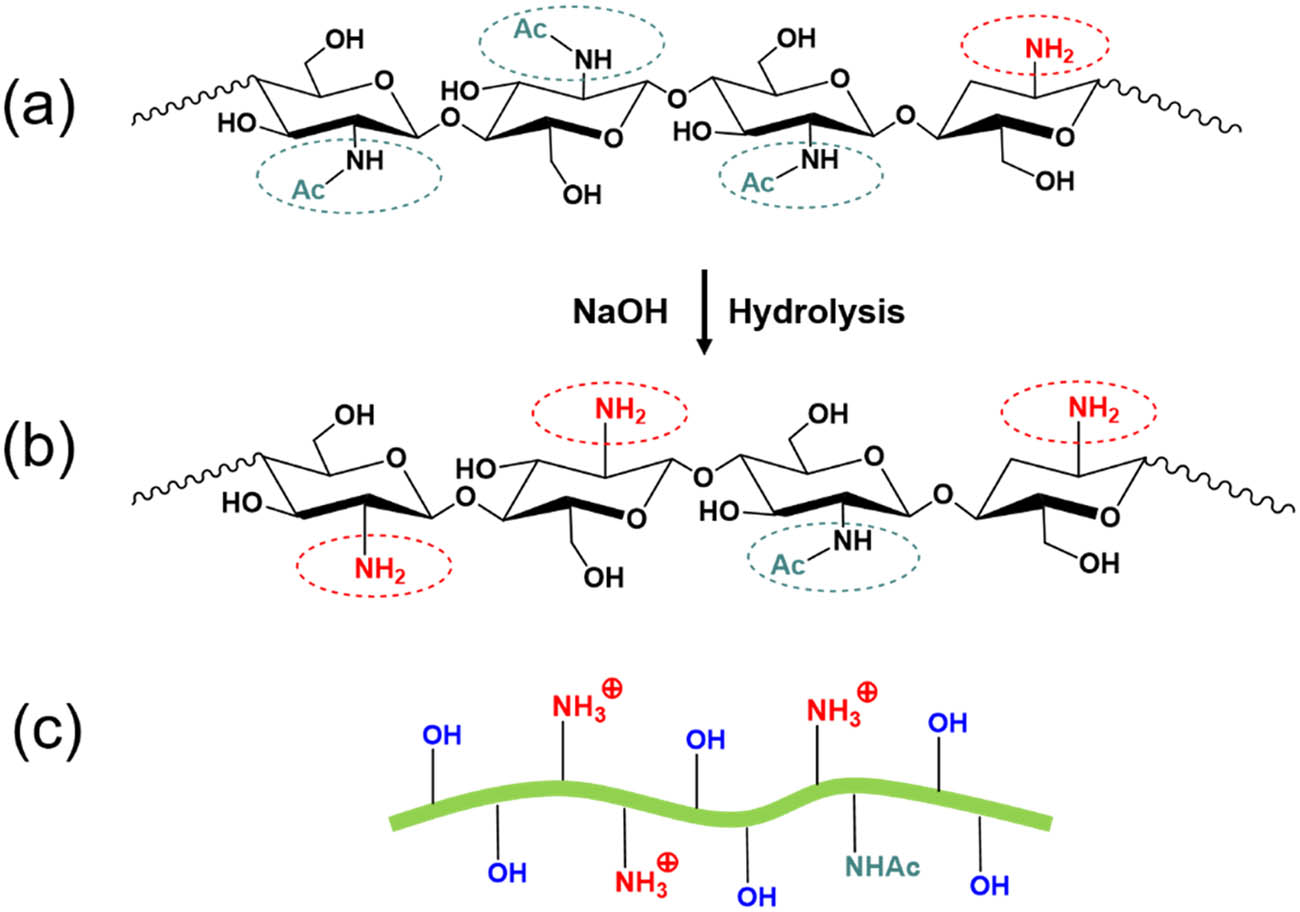
(a) Chemical structure of unmodified chitin; (b) chemical structure of partially deacetylated chitin; (c) scheme of partially deacetylated chitin nanofibers with protonated amino groups at pH 3–4.
3.5 Partially deacetylated chitin nanofibers
Alkali hydrolysis is a conventional method for producing chitin with positively charged surfaces, as displayed in Figure 7. The variables (e.g., reaction temperature, reaction time, alkali concentration, etc.) during a typical process can be tuned to control the degree of deacetylation to prevent severe degradation of the crystalline structure. Fan et al. [66] reported that α-chitin was partially deacetylated with a degree of N-acetylation (DNAc) around 0.74–0.70 in the presence of 33% sodium hydroxide (90°C for 2–4 h). The yields of the products are around 85–90%. There is no change in the crystallinity index and crystal size of the α-chitin, indicating that most of the deacetylation occurred on the α-chitin crystallite surfaces. After partial alkali deacetylation, acetamido groups in chitin molecules can be converted into amino groups, which can be further protonated under mildly acidic conditions [66], as shown in Figure 7. Positively charged surfaces can facilitate the stable dispersion of chitin nanofibers in acidic water by interfibrillar electrostatic repulsion. It should be noted that pH 3–4 of the dispersing medium was required to ensure protonation of the C2–NH2 groups in partially deacetylated chitin [66]. It is not unclear whether these partially deacetylated chitin nanofibers can be used in alkali cement systems since the pH of pore solution for cement is often 12 or higher. In such alkaline conditions, the C2–NH2 groups in the deacetylated chitin nanofibers may not change; however, the surface charge of chitin nanofibers will drop significantly, which ultimately can lead to the agglomeration of the chitin nanofibers.
3.6 Mechanically fibrillated chitin nanofibers
The fully mechanical fibrillation process using grinding discs has been applied to generate nanofibers without changing the chemical structure of chitin. However, the density of hydroxyl groups and amine groups are increased due to the significant increase in the surface areas of chitin nanofibers after ultra-fine grinding. As shown in Figure 8b, the amino groups within mechanically fibrillated chitin nanofibers can be protonated with the addition of acid at a pH of 3–4. This process makes chitin nanofibers positively charged in an acidic aqueous medium. There is no doubt that the positive surface charges of mechanically fibrillated chitin nanofibers are lower than those of partially deacetylated chitin nanofibers because partial deacetylation via NaOH hydrolysis yields more amino groups (–NH2). In our previous research [67], we successfully produced mechanically fibrillated chitin nanofibers with an average diameter of 12 nm using planetary ball milling as pretreatment and further high-pressure homogenization. These mechanically fibrillated chitin nanofibers also exhibited a positive charge in solution, as evidenced by the zeta potential of about 39 mV at a pH of 3.
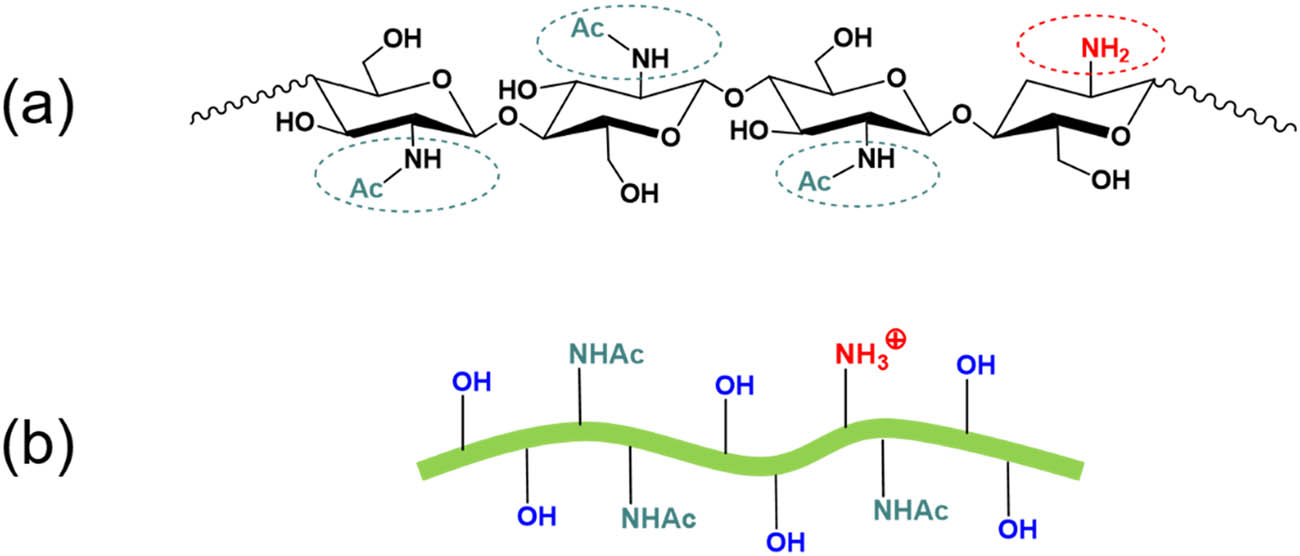
(a) Chemical structure of unmodified chitin; (b) scheme of mechanically fibrillated CNFs with protonated amino groups at pH 3–4.
3.7 Starch and starch derivatives
Amylose and amylopectin are the two main components of starch. Starch has a similar structure to cellulose and chitin. Natural starch consists of 10–30% amylose and 70–90% amylopectin. Amylose is a linear polysaccharide with d-glucose units and the α-1,4-glycosidic linkages. Amylopectin is a branched-chain polysaccharide with glucose units linked primarily by α-1,4-glycosidic bonds or occasionally by α-1,6-glycosidic bonds, which are responsible for the branching. Amylose is similar to cellulose, but glucose units in cellulose are joined by β-1,4-glycosidic linkages, producing a more extended structure than amylose. This high linearity allows multiple hydrogen bonding formations between hydroxyl (–OH) groups which helps dense packing of nanofibers.
There are several publications describing the use of starch in cement [28,68,69,70,71,72]. Dewacker [28] used unmodified starch as a substitute for cellulose to increase water retention in cement and found adding low-cost starch is beneficial for water retention, rheology improvement, and shear bond strength increase. Peschard et al. [69] found the retardation effect was enhanced with the increase in starch-to-cement ratio. This is particularly true for low molecular weight starch. In their study, five admixtures were chosen to add to conventional cement, including cellulose ether (CE), starch ether (SE), native starch (NS), white dextrin (WD), and yellow dextrin (YD). WD and YD are obtained by dextrinization (break down of starch into dextrin’s) of NS by heat and acid treatment. They found that the cement setting was retarded by measuring a reduction in the heat flow of hydration of admixture-modified cement compared to unmodified cement. For cement with YD, the heat of hydration in the first 24 h was reduced from 99 J/g (plain cement) to 9 J/g. Infrared spectroscopy also demonstrated that C–S–H was formed in the first 8 h of hydration for cement modified with CE, while no C–S–H was identified after 8 hs in SE- and YD-modified cement. Vieira et al. [70] investigated the dispersing effect of anionic starch and cellulose on mortar. The starch was processed with a sulfoethylation or a sulfation process. Slump and mortar spread tests showed that sulfoethylation starch (SES) derivatives and starch sulfate (SS) derivatives perform better as dispersants than commercial polycarboxylate (PCE) ethers. The results were explained by the fact that commercial PCE ether has a lower charge density. In addition, due to their high retardation effect, the 24 h compressive strength (Cs) of SES- and SS-modified concrete was only a fraction (0.15 and 0.13 MPa) of that of commercial Glenium 51- and Melment SL-modified cement (26.6 MPa and 27.7 MPa, respectively).
Zhang et al. [71] studied the effect of starch sulfonate as a water-reducing additive. The fluidity of cement paste significantly increases, particularly with low doses of starch sulfonate compared to commercial product naphthalene sulfonated formaldehyde condensates (FDN). According to the authors, this effect is from the thickness of the adsorption layer of the SS (∼5.3 nm) on the cement particle surface being larger than FDN (0.58 nm). Patural et al. [68] studied seven SEs, including four types of hydroxypropyl starch derivatives and two carboxymethyl-hydroxypropyl derivatives, showing an increase in consistency coefficient and a decrease in water retention values. In Tables 1–3, fs stands for the flexural strength, and Cs is the compressive strength. The letter d is the abbreviation of day. E is Young’s modulus.
Chitin, chitosan, starch, and their derivatives reinforced cementitious materials
| PNM type/source/production/size | Dosage of chitin, chitosan, starch and their derivatives | Cementitious material | Effects on mechanical properties | Ref. | |
|---|---|---|---|---|---|
| Hemicellulose, sulfopropylate, starch sulfopropylate, water-soluble starch sulfoethylate | 0.8–1.3 wt% of cement | ASTM specifications C270 and C125 | ✓ | Increased Cs from 2.7 to 6.9 MPa | [81] |
| A mixture of cold-water-soluble (>90 wt%), unmodified starch and a cellulose | 0.25–0.5 wt% of mortar | Portland type 1 cement | ✓ | N/A | [28] |
| New polysaccharide derivative (NPD) which has a cellulose principal chain and two functional groups (ionic and hydrophobic) | 1–4 wt% of cement | Normal Portland cement | ✓ | Shear stress increased from 28 to 174.5 Pa/s | [82] |
| ✓ | Increased the viscosity significantly with a small amount NPD | ||||
| ✓ | Caused less setting retardation than conventional viscosity agents | ||||
| ✓ | Initial setting is 9 h 33 min for 0.03% NPD, while 12 h 21 min for 0.06% hydroxyethyl cellulose | ||||
| Phosphorylated chitin; M w = 2.6–3.9 ×104 Da | 0–14 wt% of cement | Monocalcium phosphate monohydrate and calcium oxide (CaO) or dicalcium phosphate dihydrate and calcium hydroxide [Ca(OH)2] | ✓ | Increased Cs | [83] |
| ✓ | Retarded setting time from 7 up to 60 min | ||||
| NS, WD, and YD; M w = 85,000–25,200,000 Da | 0.5 wt% of cement | White Portland cement (C1), CPA CEM I 52.5, and gray cement (C2), PMES 42.5 | ✓ | Retarding ability is: NS < WD < YD | [69] |
| Sulfoethylation of starch, sulfation of starch, carboxymethylation of limit-dextrin, carboxymethylation of LODP-cellulose, hydroxyethylation, and subsequent carboxymethylation of cellulose; Degree of polymerization (DPw): 56–2,600, which is calculated from the molecular weight M w, of the peracetylated limit-dextrins | 0.9–20 wt% solution | Kiefersfelden 42.5R | ✓ | N/A | [70] |
| Five hydroxypropylmethyl cellulose (HPMC) and four hydroxyethylmethyl cellulose (HEMC); M w = 210 k–1,010 kDa | 0.27 wt% of dry mix | CEM I-52.5R | ✓ | Molecular weight and the hydroxypropyl content seem to have a lower impact on admixed cement hydration process | [84] |
| Chitin and chitosan were used as additives with protein, aliphatic polyester, a poly(lactide), a poly(glycolide), a poly(e-caprolactone), a poly(hydroxy butyrate), a poly(anhydride), an aliphatic polycarbonate, an orthoester, a poly(orthoesters), a poly(amino acid), a poly(ethylene oxide), or a polyphosphazene; Sizes: microcapsules (several microns to tens of microns) | N/A | N/A | ✓ | N/A | [85] |
| SS using corn starch with 83% amylopectin | 0–3% in water | OPC 32.5R | ✓ | Cement paste with SS can reach maximal fluidity at a lower adsorption amount (5 mg/g) | [71] |
| Chitosans with different molecular weights [low molecular weight (LMW), 50–190 kDa; medium molecular weight (MMW), 190–310 kDa; and high molecular weight (HMW), 310–375 kDa, and different deacetylation degrees (92, 80, and 76%, respectively) | 0.1 wt% of dried mortar | OPC (CEM II 32.5N) | ✓ | Increased viscosity in fresh mortars while retaining more water than plain cement | [86] |
| ✓ | HMW chitosan is the polymer with the greatest retarding ability for heavy metals | ||||
| Three panels of HEMC (named as C and TV), two panels of HPMC (named as J and P), two panels of hydroxyethyl cellulose (HEC, named as H and N). M w (HEMC) = 90–410 kDa; M w (HPMC) = 225–910 kDa; M w (HEC) = 40–2,900 kDa | 0.27 wt% of dry mix | Portland cement CEM I 52.5R | ✓ | Decreased yield stress as HEMC molecular weight increased | [68] |
| ✓ | Increased consistency coefficient and water retention with higher HEMC, HEC, and HPMC molecular weight | ||||
| Chitosan powder | N/A | Class A special for oil well cementation | ✓ | Epoxy/chitosan-modified cement slurry for use in environmental-friendly acidizing procedures of oil wells | [88] |
| Two non-ionic chitosan derivatives (hydroxypropyl and hydroxyethyl chitosans) and one ionic derivative (carboxymethylchitosan, CMCH); CMCH with sizes of tens of microns; Negative values of zeta-potential of CMCH | 0–0.5 wt% of cement | OPC (CEM II 32.5N) | ✓ | Non-ionic derivatives had a weak dosage-related influence on the fresh-state properties | [87] |
| ✓ | Ionic CMCH showed a more marked effect: acted as a powerful thickener and reduced the workable life of the fresh mixtures, whereas it caused a delay in the hydration of cement. CMCH reduced the slump by 50% | ||||
| Carboxylated, sulfonated polysaccharide with molecular weight from about 500–1,000,000 Da, and a ratio of carboxylate functionalities to sulfonate functionalities from about 0.1–4 | 0.1–4 wt% of cement | Class H cement | ✓ | N/A | [89] |
| Commercial chitosan powder (84% of deacetylation degree) | A/F-chitosan aqueous suspension (5 wt% of cement) | Class G cement | ✓ | Significantly improved chemical stability of cement slurries in the presence of acidizing fluids | [90] |
| Water-soluble oligochitosan (CO). M w = 30,183 Da | 0.4 wt% of cement | OPC, Shengwei 42.5R and Jidong 42.5 | ✓ | Increased Cs | [91] |
| ✓ | Strong retarding effect on cement hydration | ||||
| ✓ | Max water-reducing ratio was 43% | ||||
| A PCE-based superplasticizer (SP) and three types of polysaccharide-based viscosity modifying agent (VMA) | 0–0.025 wt% of binder (cement + fly ash) | Normal Portland cement (CEM I 42.5N, EN 197) | ✓ | The 7 days C s decreased from 40 MPa to as low as 22 MPa and 28 days C s decreased from 50 MPa to as low as 32 MPa | [92] |
| ✓ | The addition of VMAs reduced the segregation tendency for all concrete mixtures tested | ||||
| Chitosans with different molecular weights (LMW, 8–15 kDa; and HMW, 150–200 kDa) and 90–92% deacetylation degree; commercial products from the Naijin industry (Shanghai, China) | 0.25, 0.5, and 1 wt% of the cement weight | Class G OWC | ✓ | LMW and HMW chitosan reduced fs of cement paste | [93] |
| ✓ | Chitosan has retarding effect. However, after pre-chelating, retarding effect of chitosan will become weaker | ||||
| Chitosan with a size of 74–297 microns | 0.1– 4 wt% of cement | N/A | ✓ | Retarded setting time significantly | [94] |
| Processing chitin with a 40–49% sodium hydroxide aqueous solution under the temperature of 110–140°C for 4–6 h | 0.2–2 wt% of cement | N/A | ✓ | No obvious change in Cs | [95] |
| Modified LMW chitin polymer | 0–0.6% in water | PII 52.5 cement | ✓ | Increased Cs from 20 MPa using 0.6% CG-M-chitin | [96] |
| ✓ | Used as SP, reduced the water significantly with the same slump | ||||
| Modification of chitosan with methacrylic anhydride and crosslinked to ChiMOD hydrogels | 0.5, 1, and 2 wt% of cement | OPC (CEM I 52.5N) | ✓ | 0.5 and 2 wt% of chiMOD hydrogels decreased fs by 9.6 and 16.7%, and Cs decreased by 17 and 22%, respectively | [97] |
| Oxidation (carboxylic acid converted to hydroxyls) via TEMPO of chitin nanocrystal derivatives and hydrocarbon chitin nanocrystal derivatives and combinations thereof. Amine functional groups are substituted by amide groups. Chitin deacetylation followed by amine functionalities and by acylation; Length: 5–50 nm | 1–25 wt% in dry cement | N/A | ✓ | Hydrocarbon chitin nanocrystal derivative serves as a rheology modifier, emulsion stabilizer, and fluid loss additive | [98] |
| Chitosan-g-POEGMA (Chitosan-g-poly[oligo (ethyleneglycol) methyl ether methacrylate]) graft copolymers | N/A | CEM I 42.5R | ✓ | 28 days Cs is determined as 30 MPa for the reference concrete, 35 MPa for sulphonated graft, and 34 MPa for sulfonated chitosan within the reference concrete series | [99] |
| ✓ | Maintained better consistencies than those with chemical admixtures during 45 min (sulfonated graft and chitosan) | ||||
| LMW chitosan polymer | Polylactic acid M w = 6,000–8,000 Da, used as alkali silicic acid inhibitor | N/A | ✓ | Increased Cs and reduced fs | [100] |
| Chitin-acrylic acid copolymerized multifunctional organic anti-dispersant; M w: 2,170,000–2,480,000 Da | 0.5–2.0% in water | N/A | ✓ | Cs decreases but with better anti-dispersion behavior | [101] |
| Commercially available chitosan with a low M w (number-average M w = 529, weight-average M w = 722) | 0–0.9 wt% of the paste | γ-C2S | ✓ | Cs and fs increased by 66% and 299% at 0.6% chitosan content, respectively | [102] |
| Chitin nanofibers and chitin nanocrystals; Source: chitin from shrimp shells; Production method: TEMPO-oxidation and mechanical fibrillation chitin nanofibers width: 16 ± 10 nm, length: 1,068 ± 765 nm | 0.035–0.1 wt% of cement | Type I/II OPC | ✓ | Chitin nanofibers significantly increased the 28 days fs by approximately 40% at optimum concentrations of 0.05 wt% | [103] |
| ✓ | Chitin nanocrystals delayed initial and final set times by up to 56 and 106 min, respectively, which are greater than the delays of 35 and 78 min by chitin nanofibers | ||||
| ✓ | Chitin nanofibers increased the viscosity and yield stress of fresh cement paste, but chitin nanocrystals did not impart a notable effect on rheological properties | ||||
4 Overview of cement hydration and cement hydration products
Cement hydration is a complex reaction process during which multiple phases are formed. PNMs can disperse in the cement slurry system, adsorb on the cement particles and to each other depending on surface charges, create additional nucleation sites for reaction products growth, affect the kinetics of hydration reactions and accelerate or decelerate on various stages of hydration. Therefore, incorporation of PNMs in cementitious systems depends on the composition and nature of the cement and in determining the ultimate performance of the PNM–cementitious material. Thus, it is important to understand the main factors that influence cement hydration kinetics, cementitious systems’ structures, and the properties of cementitious materials. This section provides a brief discussion of cement in terms of composition, cement hydration, pore structure, etc., to help understand the potential interaction of PNMs and cement in the following sections.
4.1 Hydration of clinker
A typical OPC clinker has a composition of 67% CaO, 22% SiO2, 5% A12O3, 3% Fe2O3, and 3% other components, and normally contains 4 major phases, alite (tricalcium silicate: C3S), belite (dicalcium silicate: C2S), aluminate (tricalcium aluminate: C3A), and ferrite (tetracalcium aluminoferrite: C4AF). Several other phases, such as alkalis, sulfates, and calcium oxide, are usually present in trace amounts. The hardening of cement results from reactions between the major clinker phases and water. When the cement is mixed with water, various ions are released such as Ca2+,
Initial hydration (minutes): the dissolution of mainly aluminum-rich clinker phases and precipitation of calcium aluminate sulfate hydrates.
Dormant period (minutes to several hours): hydration rates decrease significantly.
Main hydration (hours to days): acceleration of dissolution of dominated silicate-rich phases and precipitation of calcium silicate hydrates and calcium hydroxide lead to setting and early strength development.
Continuous hydration (days to years): further strength development.
C3S is the primary component of Portland cement, controlling hardening and strength development and the durability of cementitious materials [105]. As a result, C–S–H, which mainly results from C3S hydration, is the primary hydration product (comprising about 50–70 wt% by mass) and binding phases in hydrated Portland cement [106]. The growth and densification may be highly affected by the dissolution and diffusion of C3S [107]. C2S is another component that contributes to the C–S–H hydration products [108].
Despite decades of studies of C–S–H, the relationship between its chemical position and structure remains not fully understood due to its complex structure [109,110]. It is widely reported that C–S–H has layered calcium-silicate crystal structures with linear silicate-“dreierketten” and different amounts of bound water [110]. Once the calcium-to-silicate (C/S) ratio is described correctly, a number of characteristic structural features and physical properties can be correlated through atomistic simulations [109]. Therefore, the C/S ratio plays a critical role in constructing the chemical structure of C–S–H. There are two widely agreed molecular models for C–S–H gel depending on the C/S ratio: (1) 14 Å tobermorite (C/S ratio = 0.83, interlayer distance 14
![Figure 9
Schematic molecular model of 14 Å tobermorite, reprinted with permission from ref. [111]; Copyright 2020, MDPI.](/document/doi/10.1515/ntrev-2022-0149/asset/graphic/j_ntrev-2022-0149_fig_009.jpg)
Schematic molecular model of 14 Å tobermorite, reprinted with permission from ref. [111]; Copyright 2020, MDPI.
4.2 Hydration products and pore structure
Cementitious materials are complex multi-component inorganics with multiscale pore systems. The multiphase microstructure of the hydration products in cement mixtures consists primarily of C–S–H, calcium hydroxide (Ca(OH)2), and a porous phase [78]. The formation of the major hydration product C–S–H often induces a significant volume of internal and nanometer-scale pores, such as small capillary pores and gel pores, as schematically shown in Figure 10 [112]. When dry Portland cement powder mixes with water, hydration reactions are initiated and produce solid hydration products with a greater volume than the initial dry solids. As the hydration continues, the water-filled space is gradually replaced with solids during the reaction. Space not filled by solid hydration products is referred to as the capillary pore space [113]. It has been found that the primary C–S–H gel particles have a lamellar or sheet-like shape with a thickness of 5 nm and up to 60 nm in width, which is confirmed by transmission electron microscopy (TEM) and atomic force microscopy (AFM) [114,115]. The gel pores are specifically associated with the C–S–H gel phase, the primary hydration product of all Portland cement-based cementitious materials [112].
![Figure 10
Schematic of particles of C–S–H, referred to as globules or bricks, reprinted with permission from ref. [112]; Copyright 2018, Japan Concrete Institute. They are about 4 nm across and have a layered structure with interlayer space and small pores imperfectly aligned. Their packing arrangement is such that the pores tend to have specific sizes and overall packing efficiency.](/document/doi/10.1515/ntrev-2022-0149/asset/graphic/j_ntrev-2022-0149_fig_010.jpg)
Schematic of particles of C–S–H, referred to as globules or bricks, reprinted with permission from ref. [112]; Copyright 2018, Japan Concrete Institute. They are about 4 nm across and have a layered structure with interlayer space and small pores imperfectly aligned. Their packing arrangement is such that the pores tend to have specific sizes and overall packing efficiency.
The nanostructure of cement paste, particularly its nanometer-scale pore system, primarily controls important bulk properties, including strength, shrinkage, creep, permeability, and durability [116]. Particularly, the mechanical properties of the cementitious matrices are influenced by the volume, size, and morphology of pores. High-performance cement requires the capillary porosity to be low [112] because connected pores will allow water to enter the matrix to dissolve hydration products, mainly Ca(OH)2, thus leading to poor durability [117].
In general, there are two distinct C–S–H hydration regions depending on the volume fraction of gel pores and different modulus in the matrix: (1) low-density (LD) C–S–H and (2) high-density (HD) C–S–H [117]. The first one has a low packing fraction, and the latter has a high packing fraction of nanoscale solid C–S–H particles, as shown in Figure 11, which can be distinguished by a nanoindentation tool [119]. The densities of different C–S–H phases can be determined by nitrogen penetration tests. HD C–S–H is made up of densely packed particles into which nitrogen cannot penetrate. On the other hand, particles of LD C–S–H are not packed tightly, and nitrogen can penetrate partially into this structure, as shown in Figure 11 [118].
![Figure 11
(a) 2D schematic of LD C–S–H formed by the late-stage and/or by drying and (b) 2D schematic of HD C–S–H, the predominant type of C–S–H that forms during the late-stage at water/cement ratio = 0.4, reprinted with permission from ref. [118]; Copyright 2000, Elsevier Ltd.](/document/doi/10.1515/ntrev-2022-0149/asset/graphic/j_ntrev-2022-0149_fig_011.jpg)
(a) 2D schematic of LD C–S–H formed by the late-stage and/or by drying and (b) 2D schematic of HD C–S–H, the predominant type of C–S–H that forms during the late-stage at water/cement ratio = 0.4, reprinted with permission from ref. [118]; Copyright 2000, Elsevier Ltd.
Four main factors might govern the structural changes in C–S–H hydration products:
dissolution and growth of mineral phases,
diffusion of mobile species in solution,
complexation reactions among species in solution or at solid surfaces, and
nucleation of new phases [112].
5 Surface interactions of PNMs with cement
Tricalcium silicate dissolves rapidly in water to release calcium ions (Ca2+), silicon ions
As discussed previously, the C/S ratio plays a crucial role in the chemical structure of C–S–H, and its phase composition and densification may also be affected by the diffusion of calcium ions. Calcium ions have coordination capacity with oxygen atoms of oxygen-containing functional groups and can be attached to negatively charged polymers via electrostatic interaction, as shown in Figure 12 [120,121]. The chemical reactivity and the electrophilic attack of those functional groups decrease in the following order as phosphate > carboxylate > sulfate > hydroxyl [121]. The binding strength of Ca2+ with different functional groups decreases in the order of phosphate > carboxylate > sulfate (sulfonate) > hydroxyl because the Ca–O distance (bond length) of the complex of the phosphate is the shortest which ranges from 2.28 to 2.31 Å. The second shortest is the complex of the carboxylate, around 2.38 Å, which is shorter than that of the complex of sulfate, about 2.42 Å [121,122].
![Figure 12
The complexes of three monomers (carboxylate, sulfonate, and phosphate) with Ca2+, reprinted with permission from ref. [121]; Copyright 2019, Elsevier Ltd.](/document/doi/10.1515/ntrev-2022-0149/asset/graphic/j_ntrev-2022-0149_fig_012.jpg)
The complexes of three monomers (carboxylate, sulfonate, and phosphate) with Ca2+, reprinted with permission from ref. [121]; Copyright 2019, Elsevier Ltd.
Due to the lack of experimental data and analysis in the literature, it is not well understood about interfacial interaction between PNMs and cement and their influences on the performances of hardened cement composites. The discussion below is mainly based on studies on the interaction between the cement and superplasticizer polymers because superplasticizers contain similar ionic functional groups, particularly sulfate (sulfonate) groups and carboxylate groups similar to those on the surface of polysaccharide nanofibers. It is envisioned that the potential interaction between PNMs and cement may be largely linked to the interfacial interaction of ionic functional groups (anionic sulfate, sulfonate, and carboxylate groups) on the surface of PNMs with the metal cations in the pore solution and cationic compounds.
One of the main functions of superplasticizers is to disperse cement particles. Traditional superplasticizers usually have anionic functional groups, particularly carboxylate and sulfate (sulfonate) groups which can induce the negative charge [123]. Commonly used superplasticizers include sulfonated melamine-formaldehyde condensates, sulfonated naphthalene-formaldehyde condensates, modified lignosulfonates, and PCE derivatives [124,125,126,127], as illustrated in Figure 13.
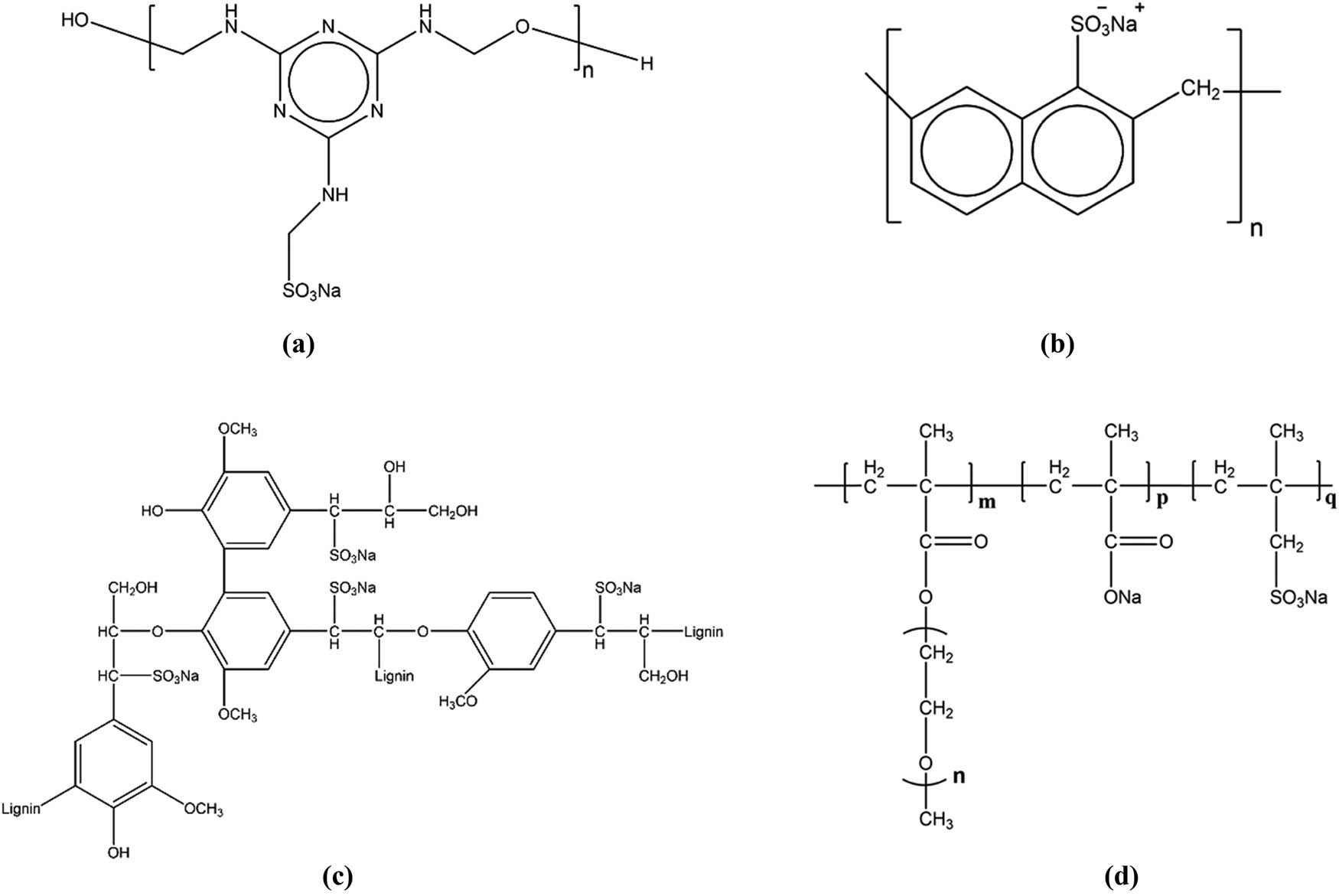
General structure of (a) sulfonated melamine formaldehyde polycondensates; (b) chemical structure of sulfonated naphthalene-formaldehyde condensates; (c) schematic drawing of the main building blocks of the lignosulfonate molecule; and (d) chemical structure of the polycarboxylate copolymer.
It is widely accepted that superplasticizers change the dispersion properties of cement through steric hindrance effect and electrostatic repulsion effect [91,123,128,129]. Figure 14 shows a typical structure of a sulfated chitosan superplasticizer. Lv et al. [130] proposed the adsorption mechanism of sulfated chitosan superplasticizer (SCS) on cement particles. SCS macromolecules adsorb on cement particle surface by the interaction between various functional groups (
![Figure 14
Schematic diagram of the absorption mechanism of SCS on cement particles surface, reprinted with permission from ref. [130]; Copyright 2013, The American Ceramic Society.](/document/doi/10.1515/ntrev-2022-0149/asset/graphic/j_ntrev-2022-0149_fig_014.jpg)
Schematic diagram of the absorption mechanism of SCS on cement particles surface, reprinted with permission from ref. [130]; Copyright 2013, The American Ceramic Society.
As discussed earlier, the TEMPO-mediated oxidation method is one of the most widely used and also simplest methods to produce carboxylated chitin and cellulose nanomaterials with high yield and crystallinity [64]. While there is no information on the chemical interactions between carboxylated chitin and cellulose nanomaterials and cement, we will refer to research on carboxylate-modified CNTs [131].
Within an acidic solution with a pH of ∼2, carboxylate groups are in carboxylic acid form (–COOH). In contrast, within a highly alkaline cement pore solution, carboxylate groups can be fully deprotonated and become negatively charged [125]. In general, the density of negative charges for PCE polymers varies depending on the pH in the pore solution and the molecular structure. The density of negative charges can vary from 20 to 110% when the pH change from 7 to 12.6 [125]. In general, as schematically depicted in Figure 15, there are two forms of coordination bonding between carboxylate groups with Ca2+ [125]. As for a monodentate form, Ca2+ coordinates with only one oxygen atom of the –COO− groups in an end-on configuration. In this way, –COO− is a monodentate ligand for Ca2+. As for a bidentate form, Ca2+ is bound to both oxygen atoms of a –COO− functionality in a side-on configuration. So –COO− is a bidentate ligand for Ca2+.
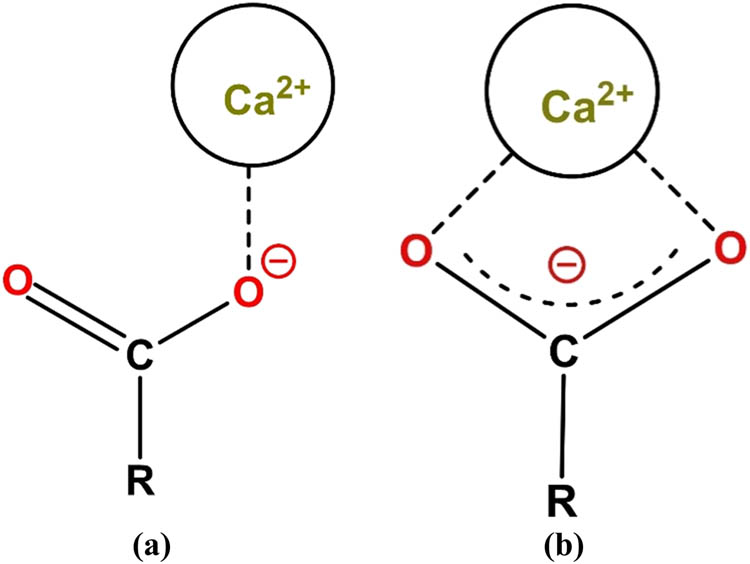
Schematic illustration of two types of coordination between Ca2+ and –COO − groups: –COO − as a (a) monodentate; or (b) bidentate ligand.
The type of coordination between –COO− groups and Ca2+ depend on the steric accessibility of the carboxylate groups. As shown in Figure 16, for PCE molecules with high side chain density, the carboxylate groups will be shielded by the side chains and the bidentate ligand for Ca2+ will be the major coordination type. By contrast, in the PCE molecules containing a high amount of (–COO−), Ca2+ will be coordinated as monodentate [125]. When compared with carboxylate (–COO−) functionality, the individual metal-oxygen interactions with the sulfonate group are weaker when employed with metal cations. Organosulfonates
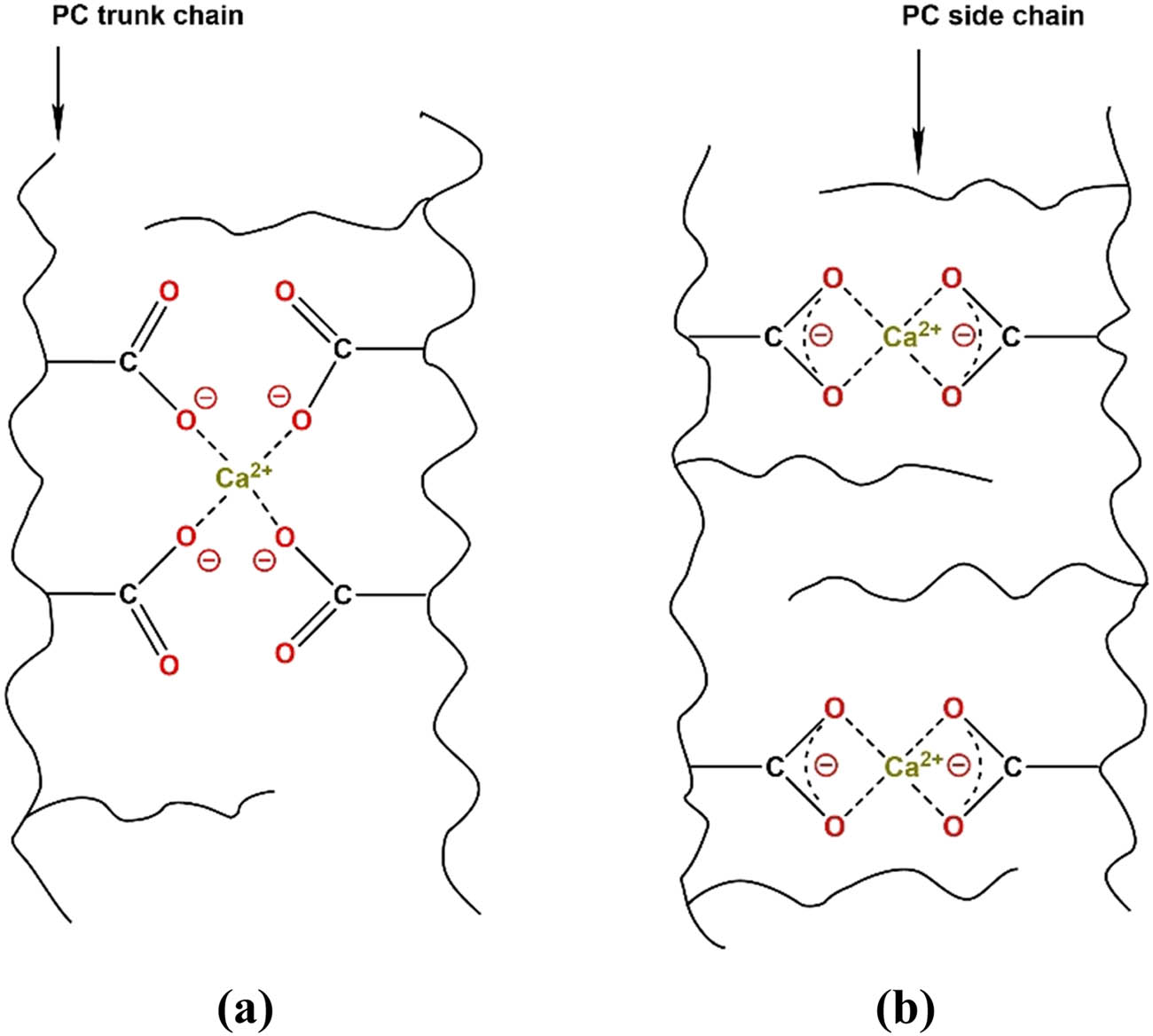
Schematic illustration of (a) monodentate complexation of Ca2+ by two polycarboxylate (PC) trunk chains with low side chain density; and (b) bidentate complexation of Ca2+ by two PC trunk chains with high side chain density.
Chitosan, a major chitin derivative obtained on an industrial scale by strong alkaline deacetylation, has been used as a chelating agent to remove metal ions in solution because metal cations can be chelated with amino groups (–NH2) in near-neutral solutions [134]. The amino groups are strongly reactive with metal ions, which is mainly due to the nitrogen atoms of the amino groups (–NH2) holding free-electron doublets that can react with metal cations [134]. It was reported that amino groups on chitosan have a good affinity for transition metal ions (Zn2+, Cu2+, and Ag+) by chelation mechanisms through the “bridge model” (a transition metal ion is coordinated with four nitrogen atoms of intra- and inter-chitosan chains) or “pendant model” (a transition metal ion is attached to an amino group of the chitosan chain like a pendant). However, the amino groups (–NH2) have a very limited affinity for alkali metals (Na+ and K+) and alkaline-earth metals (Ca2+and Mg2+) [135,136,137,138]. In cement applications, it was known that Ca2+ is one of the major ions in the pore solution. It can be inferred that the binding strength of Ca2+ with the carboxylate groups on TEMPO-oxidized chitin nanofibers (TOChNFs) will be much stronger than that of Ca2+ with the amino groups on partially deacetylated chitin nanofibers or mechanically fibrillated chitin nanofibers. Protonated amino groups
Based on the above discussions, the possible interactions and states of PNMs with cement might exist as follows:
intercalation or the formation of an organo-mineral phase (OMP); the formation of OMP often occurs in the presence of nonionic, anionic, and cationic polymers, which can be formed by coprecipitation, intercalation, or micellization [139];
adsorbed PNMs on the surface of cement particles which help disperse cement agglomerates;
excess PNMs in the suspensions without chemical or mechanical interaction with cement [139].
5.1 Interactions of sulfated
(
–
OSO
3
−
)
CNCs with cement
CNCs obtained from sulfuric acid hydrolysis contain anionic sulfate groups
![Figure 17
The schematic diagram of two different types of CNCs in the fresh cement paste, reprinted with permission from ref. [43]; Copyright 2016, Elsevier.](/document/doi/10.1515/ntrev-2022-0149/asset/graphic/j_ntrev-2022-0149_fig_017.jpg)
The schematic diagram of two different types of CNCs in the fresh cement paste, reprinted with permission from ref. [43]; Copyright 2016, Elsevier.
Aluminate-containing hydrates constitute an essential part (about 25 vol% in pastes) of the hydrate assemblage, which contribute to space-filling (strength development) in the same way as C–S–H and Portlandite. It was reported (as shown in Figure 18) that sulfonated melamine formaldehyde (SMF) polymers are positioned between the (Ca2Al(OH)6)+ main sheets via electrostatic interaction [127]. SMF can be intercalated into a hydrocalcumite host structure yielding stable (layered double hydroxide) LDH compound and the so-called inorganic–organic hybrid materials that were confirmed by XRD analysis, revealing that SMF indeed is located within the host structure [127]. There are no reports in the literature that states negatively charged sulfated cellulose nanocrystals can intercalate into (Ca2Al(OH)6)+ main sheets via electrostatic interaction. However, negatively charged sulfated cellulose nanocrystals may have good interfacial bonding with cationic cement hydrates due to electrostatic interactions.
![Figure 18
Schematic representation of sulfonated melamine formaldehyde intercalation compound, reprinted with permission from ref. [127]; Copyright: 2015, Elsevier.](/document/doi/10.1515/ntrev-2022-0149/asset/graphic/j_ntrev-2022-0149_fig_018.jpg)
Schematic representation of sulfonated melamine formaldehyde intercalation compound, reprinted with permission from ref. [127]; Copyright: 2015, Elsevier.
5.2 Interactions of carboxylated (–COO−) CNFs with cement
Carboxylated CNFs (carboxylate content: 0.5 mmol/g-CNF) obtained by the TEMPO oxidation method have been used in cement applications. The addition of 0.3 wt% carboxylated CNFs improved the compressive strength by 50% [41]. As evidenced in Figure 19, sulfated CNCs were found to retard the hydration process [42], while the carboxylated CNF was found to accelerate the hydration process [41]. The acceleration of cement hydration at an early stage (first 24 h curing) was ascribed to the carboxylated NFC acting as nuclei to promote the formation of the hydration products. X-ray diffraction results revealed an increased volume of C–S–H products and other hydration products such as Portlandite and ettringite. However, Jiao et al. [40] found that the addition of carboxylated CNFs has no clear effect on the cement hydration at an early stage, but it retarded the hydration at a later stage. As a result, the initial and final setting times were extended by about 100 and 90 mins, respectively, with the addition of 0.4 wt% of carboxylated CNFs.
![Figure 19
(a) Heat flow curves of the sulfated CNC-reinforced cement pastes for the first 40 h, reprinted with permission from ref. [42]; Copyright: Elsevier, 2015; (b) setting time of carboxylated NFC-reinforced cement pastes, reprinted with permission from ref. [41]; Copyright 2017, SAGE Publications.](/document/doi/10.1515/ntrev-2022-0149/asset/graphic/j_ntrev-2022-0149_fig_019.jpg)
Opposite cement hydration kinetics after adding carboxylated CNFs were reported in the literature by different researchers; for example, Jiao et al. [40] reported that carboxylated CNFs prolonged the setting time while Mejdoub et al. [41] observed the setting time shortened in the presence of carboxylated CNFs. Furthermore, the overall DOH was increased, and the porosity and pore size was reduced with the addition of carboxylate CNFs [40,41].
As in the previous discussion, the binding capability of carboxylate groups with metal cations, particularly Ca2+, was much stronger than that of sulfate groups. High surface areas and strong binding strength of Ca2+ with carboxylate groups make carboxylated CNFs effective nucleating agents. More hydration products are formed in the open pores which are originally filled with water, resulting in the formation of more homogeneous, dense, and compact microstructure in the presence of carboxylated NFC [40,41,120]. As a result, the interfacial bonding between carboxylated CNFs and cement hydrates can be significantly enhanced to improve the microstructure (reduced pore size and porosity) and mechanical strength of the hardened cement.
The enhanced interfacial bonding combined with high-specific surface areas of carboxylated CNFs can ensure efficient stress transfer and thus prevent the propagation of nano cracks. However, it was reported by Goncalves et al. [39] that the negatively charged ionic groups of CNFs could scavenge some calcium cations in the aqueous phase (as evident from the thermogravimetric analysis), which affect the formation of other hydration products, e.g., ettringite and Ca(OH)2.
As discussed, the presence of carboxylate groups will induce a series of chemical reactions around the interfaces and therefore enhance the reinforcement efficiency. Thus, CNTs have been treated by a mixture solution of sulfuric acid (H2SO4) and nitric acid (HNO3) to introduce carboxylate groups on their surface. The addition of carboxylated CNTs in the cement composites exhibited improved microstructure (finer pore size distribution and reduced porosity) and enhanced mechanical properties [20], as shown in Figure 20. On the other hand, untreated carbon fibers did not improve compressive strength (Figure 20) because the bonding force between the fibers and cement matrix may be insufficient.
![Figure 20
Typical load-displacement curves of cement-CNT composites. PCC: control Portland cement composite; PCCF: Portland cement-untreated carbon fibers composite; PCCT: Portland cement-carboxylated CNT composite, reprinted with permission from ref. [20]; Copyright 2005, Elsevier.](/document/doi/10.1515/ntrev-2022-0149/asset/graphic/j_ntrev-2022-0149_fig_020.jpg)
Typical load-displacement curves of cement-CNT composites. PCC: control Portland cement composite; PCCF: Portland cement-untreated carbon fibers composite; PCCT: Portland cement-carboxylated CNT composite, reprinted with permission from ref. [20]; Copyright 2005, Elsevier.
Based on the discussion above, we can envision the general scheme of the interfacial interaction between the carboxylate groups in CNFs and the C–S–H hydrate or Ca(OH)2, as illustrated in Figure 21. The interaction can lead to a strong covalent bonding in the interface between the nanofibers and matrix in the composites, and therefore increases the load-transfer efficiency from cement matrix to nanofibers, and eventually results in improved mechanical strength [20,140].
There is no direct comparison of the effect of sulfate groups and carboxylate groups of PNMs on the stabilization and growth of C–S–H. However, Wang et al. [141] reported the stabilization effect of SPs with carboxylate groups or sulfate groups on the structure and properties of nano-C–S–H products. The results indicated that PCE superplasticizer is more effective for stabilization of the nano-C–S–H particles than polysulfonate (PSE) superplasticizer results in finer particle forming. This is explained by the stronger bonding between the carboxylate functional groups and C–S–H gels [141]. Moreover, XRD result indicated that PCE or PSE as stabilizers might distort the structure of C–S–H and decrease the crystallinity of C–S–H because the PCE or PSE polymers were inserted into the layer structure of C–S–H by complexation between functional groups and Ca2+, resulting in the interval expansion between the layers [141]. This result is consistent with that reported by Sun et al. [142], who reported that the carboxylate-metal ion groups are arranged vertically in the C–S–H layer, leading to an increase in the interlayer spacing. Sun et al. [142] also pointed out that PCE superplasticizer existed in two different locations in the C–S–H structure: grafted on the surface or partially intercalated in the interlayer regions.
5.3 Interactions and states of mechanically fibrillated CNFs with cement
The surface chemistry on mechanically fibrillated cellulose is much simpler than those obtained by sulfuric acid hydroxide or the TEMPO-mediated oxidation method. The hydroxyl group (–OH) is the major reactive functional group on the surface of mechanical fibrillated CNFs. Carboxylate groups may exist if CNFs are mechanically fibrillated from wood pulp with a small amount of hemicellulose. Thus, some mechanically fibrillated CNFs are negatively charged [53]. Our measurements of zeta potential of mechanically fibrillated CNFs in a simulated pore solution to represent the cement pore solution about 1 h into the hydration gave a value of −51.84 ± 18.74 mV, which is a much higher absolute value than the –9.1 mV value reported for cement particles in pore solution at pH = 12.7, reported by Cao et al. [42]. Mechanically fibrillated CNFs tend to adhere to cement particles rather than agglomeration themselves. Therefore, the affinity strength between the particles have the following order: f (cement–CNF) > f (cement–cement) > f (CNF–CNF). The zeta potential results show that the affinity strength between cement and CNF is stronger than that between cement particles. Thus, mechanically fibrillated CNFs may also have an electrostatic stabilization effect on cement, thus helping improve the dispersion of cement particles. Our results show that CNCs can delay the set time of cement more efficiently than CNFs. Furthermore, the CNCs can delay the final setting time by over an hour compared to unmodified cement. In addition, the longest delay in setting time is reached when the CNC concentration in cement is 0.05 wt% [76].
5.4 Interactions of carboxylated (–COO−) chitin nanofibers and nanocrystals with cement
Chitin has a similar chemical structure to cellulose, as shown in Figures 1 and 3. It can be expected that the surface chemistry of carboxylated chitin is similar to that of carboxylated cellulose. We can envision similar interfacial interactions between carboxylated chitin nanomaterials and cement. As mentioned earlier, controlling the amount of the oxidant NaClO in the TEMPO-mediated oxidation process will result in two distinct forms of carboxylated chitin nanomaterials with different amounts of carboxylate content. A low amount of oxidant NaClO can yield long and more entangled chitin nanofibers with low carboxylate content on the surface, while a high amount of oxidant NaClO can produce short rod-like chitin nanocrystals, as shown in Figure 5.
5.5 Interactions of partially deacetylated chitin nanofibers with cement
The main reactive groups on the surface of partially deacetylated chitin nanofibers are amino groups (–NH2) and hydroxyl groups (–OH). The partially deacetylated chitin nanofibers were stable in the acidic water medium due to the electrostatic repulsion forces induced by the protonation of amino groups. The zeta potential will dramatically drop under neutral or alkaline conditions. In our previous zeta potential measurements, the zeta potential of partially deacetylated chitin nanofibers obtained by 4 h hydrolysis by 33 wt% NaOH decreased from 44 to 16 mV when the pH was increased from 3 to 7. The absolute value of 20 mV is typically assumed to be the minimum value for moderate colloidal stability. Therefore, the partially deacetylated chitin nanofibers will tend to aggregate when increasing the pH to 7, as shown in Figure 22.
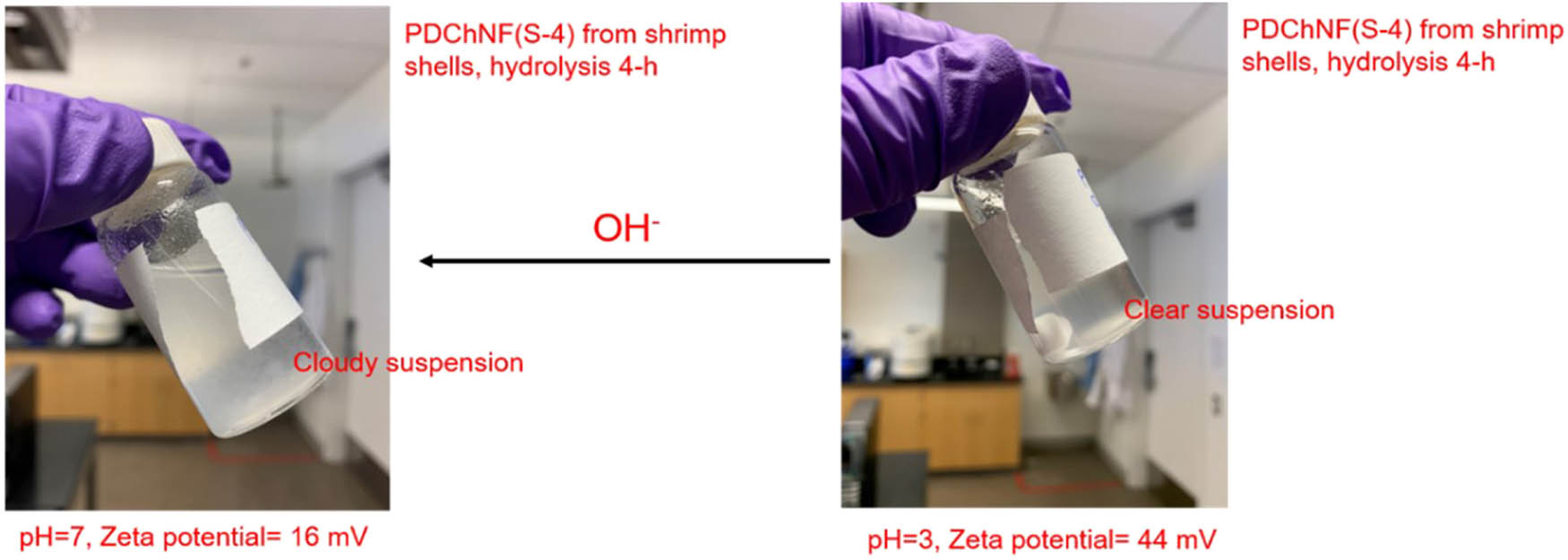
Partially deacetylated chitin nanofibers in acidic or neutral media.
In the earlier discussions regarding the electrostatic stabilization effects of CNFs on the cement particles, the main mechanism might be that the CNFs were preferably attached to the surface of cement particles via electrostatic attraction. Negatively charged CNFs or nanocrystals were absorbed to the cationic surface of cement particles, thus preventing cement aggregations and improving the DOH. Our current research shows that partially deacetylated chitin nanofibers have a positive surface charge under acidic and alkaline conditions.
Hall [98] reported that chitin nanocrystal with amino functional groups (–NH2) or amide groups (–NHR) was ideal to be used as a corrosion inhibitor in the cement because they can form strong films on the surface of metals through non-covalent binding with the metal surfaces. Whether the partially deacetylated chitin nanofibers or mechanically fibrillated chitin nanofibers have a similar corrosion-inhibiting behavior is yet to be proven in future work.
5.6 Interactions of mechanically fibrillated chitin nanofibers with cement
The reactive hydroxyl group (–OH) is the primary functional group on the surface of mechanically fibrillated chitin nanofibers. There are a small number of amino groups on the surface of chitin nanofibers due to a small fraction of deacetylation during the extraction process, as mentioned previously. Suspensions of mechanically fibrillated chitin nanofiber were unstable in the neutral or alkaline condition but did not show any issues dispersing in the alkaline cement pore solution. With their surface charges, we found that chitin nanofibers at a concentration of 0.035 wt% delay the initial and final setting times of cement paste by 35 and 78 min, respectively. Lower or higher concentrations attain shorter setting times. Higher electrostatic repulsion of chitin nanofibers due to high zeta potential values (–28.04 ± 2.6 mV in simulated pore solution) and high density of functional groups might be the contributing factor for the nanochitin function as a set retarder.
Typically, the addition of nanoparticles accelerates cement hydration because small particles might provide more heterogeneous nucleation sites and/or space where hydration products can grow [107,143]. Nucleation sites of nanoparticles will promote a more homogeneous, denser cement microstructure. This is attributed to the filling effect, which can enhance hydration product growth in the pore space and filling voids between the cement (clicker) particles, and hence a reduction in porosity, as shown in Figure 23. PNMs can act as heterogeneous nucleation sites and thereby accelerate the formation of C–S–H nuclei. Peschard et al. also reported that polysaccharides could restrict the C3A hydration rather than promote nucleation [69]. More work is still needed to elucidate the combined effect of the nano-size effect and ionic absorption capability of PNMs on the phase composition chemical and crystalline growth of hydration products.
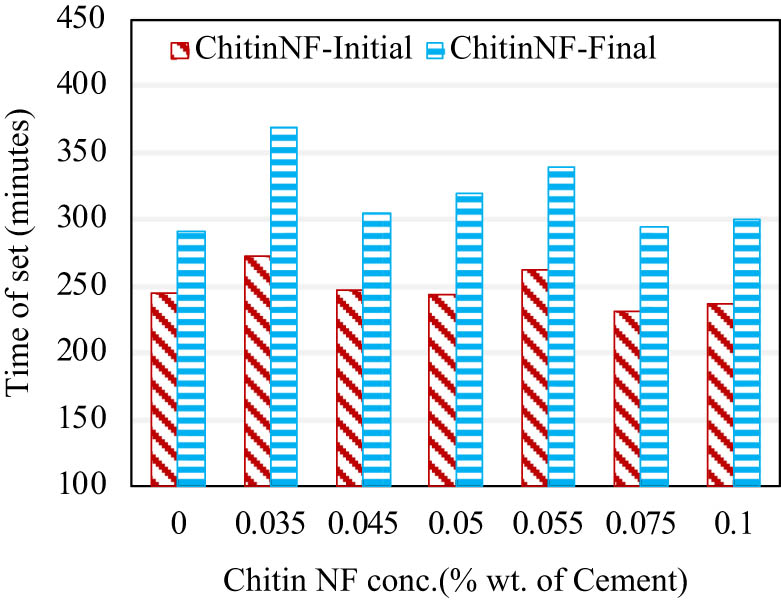
Effect of concentration of chitin nanofibers on the setting time of cement paste.
6 Strength-enhancing mechanisms of PNMs in cement composites
Polysaccharide-based materials could have a significant effect on the mechanical performance of cement composites. Previous studies regarding the effect of PNM type, source material, production method, size, and surface functional groups on the mechanical properties of cement composites, as well as the mechanical properties, were summarized in Tables 1 and 2. Table 3 includes a summary of studies of cement using PNMs and also other derivatives of chitin and chitosan. The effect of PNMs on properties other than strength, such as setting time and rheology, is also listed in these tables wherever available.
A consensus from Tables 1 and 2 is that CNFs and CNCs can increase the compressive and/or flexural strength at different dosages, though contradicting findings were reported in some cases. For example, several studies [34,37,40,144] reported a flexural strength increase from 15 to 72% using CNFs and Jiao et al. and Mejdoub et al. [40,41] reported a 20–43% increase in compressive strength using CNFs. In contrast, Hisseine et al. and Rosato et al. [36,145] reported a reduction in the compressive strength of cement in the presence of CNFs. However, the reason was not explained. For CNCs, Cao, Dousti, and Mazlan et al. [42,45,46] reported increased flexural strength.
Generally, four possible mechanisms are proposed for elucidating the improved mechanical performances of cement by PNMs addition: (1) bridging/reinforcing effect; (2) filling effect, with high modulus PNMs increase the overall modulus of the composite; (3) more nucleation sites due to high surface area; (4) internal curing by releasing adsorbed water to increase the DOH. A detailed discussion of these mechanisms, as seen in the various literature gathered in Tables 1, 3, is provided following the tables. In addition, the effect of properties of PNMs listed in Tables 1–3, i.e., size, aspect ratio, production method, dispersion, concentration, etc., are also discussed following the tables. In Tables 1–3, fs stands for the flexural strength, and Cs is the compressive strength. The letter d is the abbreviation of day. E is Young’s modulus.
6.1 Bridging effect
The bridging effect of nanofibers in the cement composites matrix has been considered as one of the reinforcing mechanisms for the mechanical improvement because the bridging effect in the matrix can provide sufficient load transfer from the matrix to the nanofibers and delay the propagation of nano-cracks and restrain nano-cracks [146]. Much more energy is needed to break the crack bridging by the nanofibers than that for the crack growth of cement matrix, thus leading to improvement in the flexural and compressive strengths of cement [146,147]. The fracture energy was a measure of crack initiation and growth through cementitious materials [148]. With the bridging effect of nanomaterials, more energy is needed to break the crack bridging of nanomaterials. It was reported that the addition of CNFs at a concentration of 0.15% could significantly increase fracture energy of cement by 60%, suggesting that CNFs are effective in preventing crack growth and thus the flexural strength was also increased by 116% [37]. It was also reported that the addition of TOCNFs (carboxylate content: 1.1 mmol/g fiber) could result in a decrease in the average cracking area by about 23% at 0.8 wt% CNF [38]. The bridging effect of cellulose nanomaterials in the polymer matrix has also been considered as a significant factor contributing to the improvement in polymer nanocomposites. The potential scheme of fracture mechanism was also proposed by Xu et al. [149], as shown in Figure 24a. It was reported that at the same nanocellulose concentration, the bridging effect of CNFs in the polyethylene oxide (PEO) matrix was more pronounced than that of CNCs. Thus, CNFs led to higher strength and modulus because of a larger aspect ratio of CNFs and fiber entanglement. To our knowledge, the bridging mechanism of cellulose nanomaterials in the cement matrix is still rarely reported in the literature and has not been fully elucidated [77,150]. Our study recently submitted for peer-review shows that chitin nanofibers and nanocrystals can increase the compressive strength of cement paste by up to 12% and flexural strength by up to 41.4% (Haider et al., [103]).
![Figure 24
Schematic representation of cement hydration and the influence of nucleation seeding with C–S–H. The red arrows display concentration gradients, while the green arrows highlight the different thicknesses of the hydrate formed around the clinker particles, reprinted with permission from ref. [105]; Copyright 2018, Elsevier.](/document/doi/10.1515/ntrev-2022-0149/asset/graphic/j_ntrev-2022-0149_fig_024.jpg)
Schematic representation of cement hydration and the influence of nucleation seeding with C–S–H. The red arrows display concentration gradients, while the green arrows highlight the different thicknesses of the hydrate formed around the clinker particles, reprinted with permission from ref. [105]; Copyright 2018, Elsevier.
As for CNFs-reinforced cementitious materials, the potential scheme of the bridging between CNFs and hydration products is depicted in Figure 25b [34]. Figure 25c shows a typical “pull-out mechanism” of the low weight fraction of CNFs (0.04 wt%) in OWC composite, the authors mainly attributed about 20% increase in the flexural strength to such bridging effect of CNFs in OWC matrix and well dispersion of CNFs at low dosage (0.04 wt%) [34].
![Figure 25
(a) Illustration of fracture mechanisms of polyethylene oxide (PEO)/CNC and PEO/CNF nanocomposites [149]; Copyright 2013, American Chemical Society; (b) scheme of CNF reinforced oil well cement composites; (c) SEM image of CNF reinforced OWC composites with 0.04 wt% CNF loading [34]; Copyright 2016, Nature Portfolio.](/document/doi/10.1515/ntrev-2022-0149/asset/graphic/j_ntrev-2022-0149_fig_025.jpg)
(a) Illustration of fracture mechanisms of polyethylene oxide (PEO)/CNC and PEO/CNF nanocomposites [149]; Copyright 2013, American Chemical Society; (b) scheme of CNF reinforced oil well cement composites; (c) SEM image of CNF reinforced OWC composites with 0.04 wt% CNF loading [34]; Copyright 2016, Nature Portfolio.
6.2 Filling effect and increased hydration for porosity reduction
Research has shown that with the aid of cellulose nanomaterials, the DOH of cement can be enhanced and the porosity can be reduced. Thus, the mechanical performance of cement can be improved. It was reported that the addition of CNCs was beneficial for cement particles reacting more efficiently with water, thus leading to increased DOH and flexural strength [42]. Two mechanisms were proposed to explain CNCs’ contribution to increased DOH. One is the steric stabilization effect of CNCs, by which cement particles are well separated. This mechanism was also supported by the results reported by Flores et al. [78]. The other is a short-circuit-diffusion (SCD) effect suggested by Cao et al. [42] as schematically depicted in Figure 26. One possible scenario is that when CNCs absorb on cement particles and remain in the hydration shells (hydration products), a path will form and help transport water transportation from capillary pores to the inner unhydrated cement cores. This may facilitate a significantly increased fraction of cement reacting with water compared with the cement pastes without CNCs [42,151]. The outcome of increased DOH with adding CNCs is the reduction in total porosity of cement, thus leading to improved mechanical properties [151]. Onuaguluchi et al. [33] also reported that the addition of CNFs could increase the DOH, thus improving strength. Haddad Kolour [37] also proposed a similar “Tunnel” mechanism similar to the SCD mechanism proposed by Cao et al. [42]. According to Haddad Kolour [37], at the hydration acceleration stage, adhered CNFs will be covered by LD C–S–H (outer-products). While at the hydration deceleration stage, when the surface is completely covered by LD C–S–H, HD C–S–H growth starts within the cement particles. The porous space around these adhered CNFs will work as “Tunnels” for transporting water to unhydrated cement grain, which in turn help to produce more HD C–S–H (inner-product) and enhance total hydration reaction. Around 8% hydration improvement [37] was observed in the presence of CNFs. It should be mentioned that though these theories attempt to explain the increase in DOH induced by CNC and CNF additions, they are yet to be experimentally proved at the molecular level and thus remain as working hypotheses.
![Figure 26
A schematics illustration of the proposed hydration products forming around the cement grain from the age of 0–48 h in the (a) plain cement and (b) cement with CNCs on a portion of the cement particle showing short-circuit-diffusion mechanism [42]; Copyright 2015, Elsevier Ltd.](/document/doi/10.1515/ntrev-2022-0149/asset/graphic/j_ntrev-2022-0149_fig_026.jpg)
A schematics illustration of the proposed hydration products forming around the cement grain from the age of 0–48 h in the (a) plain cement and (b) cement with CNCs on a portion of the cement particle showing short-circuit-diffusion mechanism [42]; Copyright 2015, Elsevier Ltd.
6.3 Increased modulus of C–S–H and increased HD C–S–H fraction and influence of nucleation sites
The improved mechanical properties of nanocellulose-reinforced cement may also be attributed to the increased modulus of C–S–H and an increased portion of HD C–S–H. It was reported that the CNC addition could significantly increase the modulus of HD C–S–H [42]. The HD C–S–H fraction was also increased by the CNC addition, as shown in Figure 27 [78]. There are three possible reasons for the increased modulus of HD C–S–H: (1) the incorporation of high-modulus CNC in C–S–H, crystalline cellulose has an elastic modulus in the axial direction of 110–220 GPa [30]; (2) the CNC incorporation can modify the C–S–H structure and chemistry [151]; (3) CNC can act as a nucleating agent to promote the formation of C–S–H crystals. As mentioned earlier, the role of cellulose nanomaterials as a nucleating agent for the crystallization of polymer in polymer composites has been extensively reported and demonstrated in the literature [40,41,120]. As a result, the nucleating agent cellulose nanomaterials can increase the degree of crystallinity of the polymer matrix. The enhanced crystallinity contributed to the improvement in the stiffness of the composite materials, which is difficult to dissociate from the real direct reinforcing effect of the nanoparticles [48,53]. Hydrophilic nature and high specific areas of PNMs may also act as nuclei sites to promote the nucleation of the crystals of C–S–H hydration products, thus leading to the formation of a more compact, denser, and stronger structure than the mixture without nanocellulose [41,78,117]. However, this nucleating effect of cellulose nanomaterials has not been experimentally demonstrated yet. There is also a contradictory view [40] on the role of CNFs as nucleating sites in facilitating the growth of crystalline hydration products. It says that the oxygen atom in the hydroxyl and carboxyl group has unpaired electrons, which can react with calcium ions and form a hydrophilic complex that can adsorb on cement particles. Thus, the number of active sites between cement particles and water decreases, and the distances between cement particles increase. The adsorption and complexation effects reduce the surface activity of the hydration products and retard the production of calcium hydroxide crystals, thus the cement particles with higher addition of CNFs took a longer time to hydrate [40].
![Figure 27
Nanoindentation results showing the probability distribution of Young’s modulus of CNC-reinforced cement corresponding to 28 days of curing, reprinted with permission from ref. [78]; Copyright 2017, MDPI.](/document/doi/10.1515/ntrev-2022-0149/asset/graphic/j_ntrev-2022-0149_fig_027.jpg)
Nanoindentation results showing the probability distribution of Young’s modulus of CNC-reinforced cement corresponding to 28 days of curing, reprinted with permission from ref. [78]; Copyright 2017, MDPI.
6.4 Internal curing agent
Generally, the early-stage autogenous shrinkage of cement occurs within the first 24 h after mixing with water. And the cement matrix is more prone to cracking during the first 12 h of hydration. During this period, the tensile strength of cementitious materials is too low to prevent the crack propagation induced by shrinkage stresses. CNCs can retard the hydration at an early stage because the majority of CNCs (>94%) adhered to the cement particles and reduced the reaction surface area between cement and water [42]. As shown in Figure 19, CNCs decreased the initial hydration rate, but the overall DOH increased with CNCs addition. Figure 19a shows the heat flow curves for the seven CNCs–cement pastes, while Figure 27 shows the cumulative heat flow curves during the first 40 h, from which it can be observed that the heat flow is delayed with increasing CNC concentrations. For instance, the heat flow peak is reached at 12 h for the reference mixture (0%), while the peak is reached at around 17 h for the mixture with 1.5% CNCs. The retardation of the peak heat flow could indicate CNCs adhering to the cement particles and, therefore, blocking the cement particles from reacting with water at an early age. At the early stage, the slowing down of the hydration can help prevent the destabilization of the matrix by preventing fast water loss as cement hydration proceeds. CNFs also exhibited the same retardation effect of cement hydration at an early stage [33]. However, Mejdoub et al. [41] observed that carboxylated CNFs in cement paste accelerate the early hydration. In fact, after 1 day of curing, the DOH increases by 1.6%, 19.3%, 32.6%, 66.0%, 87.3%, and 102% for the mixture containing 0.01, 0.05, 0.1, 0.2, 0.3, and 0.5 wt% CNFs, respectively. The authors ascribed the acceleration of cement hydration at an early stage to the nuclei effect of CNFs.
In addition, CNFs have a high water retention capacity [38]. Haddad Kolour [37] suggested that CNFs can act as a “reservoir” like superabsorbent polymer because CNFs are hydrophilic and can retain some water. This stored water can be released later and work as a supplementary source of water, thus reducing autogenous shrinkage. Adding 0.06 and 0.09% CNFs can reduce autogenous shrinkage up to 49 and 26%, respectively, as shown in Figure 28. Thus, CNFs can work as an internal curing aid to prevent autogenous shrinkage in cementitious materials because water absorbed by CNFs migrates to the cement pastes and provides internal water for unhydrated cement particles, thus offering a decent internal curing environment during the hardening process of cement paste [33,117]. TEMPO-mediated oxidation pretreatment can introduce the hydrophilic carboxylate groups to the surface of CNFs, thus further increasing the water retention capacity. Therefore, CNFs with carboxylate groups resulted in better flow control of the wet cement paste and reduced the crack growth in concrete [37] (Figure 29).
![Figure 28
Cumulative heat flow curves of the cellulose nanocrystal-cement pastes at different CNC concentrations, reprinted with permission from ref. [42]; Copyright 2015, Elsevier. Note: Figure 19a in Section 3.5 shows the heat flow curves.](/document/doi/10.1515/ntrev-2022-0149/asset/graphic/j_ntrev-2022-0149_fig_028.jpg)
Cumulative heat flow curves of the cellulose nanocrystal-cement pastes at different CNC concentrations, reprinted with permission from ref. [42]; Copyright 2015, Elsevier. Note: Figure 19a in Section 3.5 shows the heat flow curves.
![Figure 29
Autogenous shrinkage of cellulose nanofibrils-reinforced cement pastes as a function of time, reprinted with permission from ref. [37]; Copyright 2019, The University of Maine.](/document/doi/10.1515/ntrev-2022-0149/asset/graphic/j_ntrev-2022-0149_fig_029.jpg)
Autogenous shrinkage of cellulose nanofibrils-reinforced cement pastes as a function of time, reprinted with permission from ref. [37]; Copyright 2019, The University of Maine.
7 Impact of other properties of PNMs on cement properties
7.1 Size and morphology
Based on the distinctive size and morphology, PNMs can be categorized into nanofibers and nanocrystals. As shown in Figure 30, nanofibers usually have a complex, highly entangled web-like structure and usually have a large aspect ratio (4–20 nm in width and 500–2,000 nm in length). Nanocrystals often have a needle-like or rod-like structure with a low aspect ratio (less than 10 nm in width, 50–500 nm in length) [30,48]. By controlling the TEMPO-mediated oxidation parameters, i.e., the amount of oxidant NaClO, two different chitin nanomaterials (chitin nanofibers and chitin nanocrystals) were obtained in our current research, as shown in Figure 6.
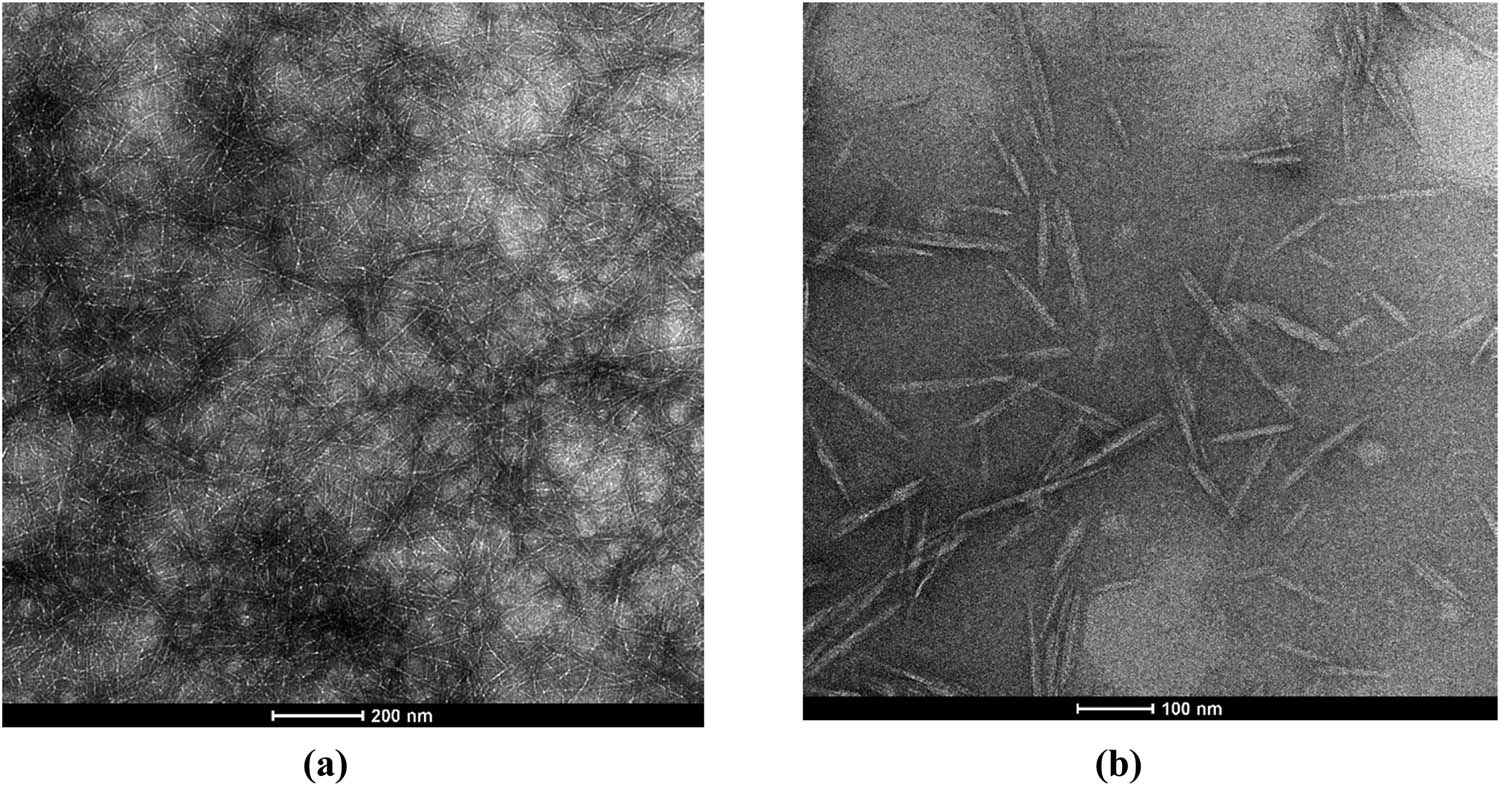
(a) TEMPO-oxidized CNFs and (b) CNCs prepared by acid hydrolysis.
The smaller size of PNMs leads to a higher specific area, thus favoring the effective interaction with the matrix to ensure the load transfer from the matrix to the reinforcement [41,144]. It was reported that the addition of 3.3 wt% NFC in the cement mortar improved the flexural strength and the modulus of elasticity by 26.4 and 41.5%, respectively, in comparison to cement mortar reinforced with the sisal fibers [144]. A high aspect ratio of nanocellulose may be more effective in stabilizing crack tips, thus suppressing cracks [148]. Many claims are based on the fact that long fibers can help delay crack propagation or even lead to crack arrest. While the length of CNCs is much smaller than CNFs, its ability to reinforce the cement needs to be further examined [146]. In addition, systematic studies are required for understanding the effects of nanofibers and nanocrystals on the mechanical performances of cementitious materials.
7.2 Concentration
A high concentration of nanomaterials in the cement paste increases the chances of agglomeration, which can be detrimental to the mechanical properties of cement composite materials. The optimal nanocellulose concentration for improving cement mechanical properties may vary depending on the type of nanocellulose, the sources for nanocellulose production, the way of adding the nanocellulose, the degree of dispersion, the type of cement, water-to-cement ratio, use of water reducer admixtures, etc. Onuaguluchi et al. [33] evaluated cement pastes by incorporating CNFs from bleached pine pulp at levels of 0.05t%, 0.1, 0.2, and 0.4 wt%. The results showed that the pastes reinforced with 0.1% nanofibers showed an increase of 106% in flexural strength and 184% in energy absorption, compared to pastes without nanofibers. High concentration of CNFs (above 0.1 wt%) resulted in a decrease in flexural strength which likely results from CNF agglomerates observed through SEM analysis.
Our results show a 17–18% increase in the compressive strength in the presence of CNFs or CNCs in comparison with the control samples. The maximum strength is reached at a concentration of 0.065 wt% for CNFs and 0.4 wt% for CNCs and after these concentrations, the strength declines. Haddad Kolour et al. [37] reported that adding 0.15% cellulose nanofibrils in the cement pastes could improve flexural strength and fracture energy by 116 and 60%, respectively. Mejdoub et al. [41] used TOCNF and found that maximum compressive strength was achieved in the presence of 0.3 wt% TOCNF. When the concentration of TOCNF is above 0.3 wt%, the strength decreased. It was explained [41] that TOCNF aggregates created weak zones in the form of pores which cause stress concentration in the cementitious matrix and promote premature cracking. The microcracks were found in the presence of 0.5 wt% TOCNF.
7.3 Dispersion
The degree of dispersion of nanomaterials in the matrix is one of the most critical factors governing the reinforcement effects of cement mechanical properties. The agglomerates of PNM in the matrix may act as defects or stress concentrators, which is detrimental to the mechanical properties of the cement. Cao et al. [151] reported that effective dispersion of CNCs to reduce agglomerates could be achieved by tip ultrasonication. As a result, the DOH of the cement could be increased. Flexural strength was also increased by up to 50% for cement paste containing sonicated CNCs compared to the 20–30% increase for the cement pastes containing raw and not sonicated CNCs at 0.2 vol% CNC concentration. Cao et al. [43] also found that CNCs preferred to attach to cement particles without sonication, while they are more randomly dispersed into the matrix after sonication. However, there are no obvious changes in SCD with and without sonification. Haider et al. [76,103] found that ultrasonication seems to have a positive effect on the light transmission of CNC suspensions, as shown in Figure 31. The three figures show that with longer durations of sonication time, suspensions with higher contents of CNCs (0.10 and 0.15 wt%) provide higher transmission of light due to a more uniform dispersion. On the other hand, the mechanically fibrillated CNF suspensions allowed negligible (<5%) light transmission during UV-Vis spectroscopy due to their web-like and entangled morphology. The morphology of the two types of chitin nanomaterials before and after 30 min sonication is shown in representative micrographs in Figure 32. As shown in Figure 32a and b, CNFs displayed an entangled network-like morphology with elongated, interconnected fibrils.
![Figure 31
Effect of sonication on the dispersion of CNCs in DI water based on UV-Vis spectroscopy after (a) 2 min, (b) 10 min, (c) and 30 min sonication, reprinted with permission from ref. [76]; Copyright 2021, Elsevier Ltd.](/document/doi/10.1515/ntrev-2022-0149/asset/graphic/j_ntrev-2022-0149_fig_031.jpg)
Effect of sonication on the dispersion of CNCs in DI water based on UV-Vis spectroscopy after (a) 2 min, (b) 10 min, (c) and 30 min sonication, reprinted with permission from ref. [76]; Copyright 2021, Elsevier Ltd.
![Figure 32
Morphology of CNFs: (a) before sonication and (b) after 30 min sonication; the morphology of CNCs: (c) before sonication and (d) after 30 min sonication, reprinted with permission from ref. [76]; Copyright 2021, Elsevier Ltd.](/document/doi/10.1515/ntrev-2022-0149/asset/graphic/j_ntrev-2022-0149_fig_032.jpg)
Morphology of CNFs: (a) before sonication and (b) after 30 min sonication; the morphology of CNCs: (c) before sonication and (d) after 30 min sonication, reprinted with permission from ref. [76]; Copyright 2021, Elsevier Ltd.
CNCs agglomerates may bring air entrapment, which will cause an increase in the porosity. In addition, CNCs agglomerates absorb water, and unreacted water may form the capillary pores [43]. The pore size distribution study shows that sonication [43] helps reduce the porosity due to less formation of large size capillary pores. This may attribute to the fact that sonification can break the CNC agglomerates and result in better dispersion.
7.4 Surface chemistry
The surface chemistry of nanocellulose is mainly determined by the manufacturing or extraction methods. CNCs are usually isolated from semi-crystalline cellulose fibers using a traditional strong acid hydrolysis process in which the amorphous region is digested. The left intact crystallites are then released with the assistance of mechanical disintegration processes (e.g., ultrasonication) [48]. Sulfuric acid is one of the most used inorganic acids for introducing anionic sulfate half-ester groups on the surface of crystallites through the reaction with the hydroxyl groups. These negatively charged functional groups play important roles in stabilizing CNCs in water [48]. CNFs can be extracted through TEMPO-mediated oxidation pretreatment followed by mechanical disintegration. The TEMPO-mediated oxidation method is regarded as an effective and efficient way to produce CNFs because primary hydroxyl groups are selectively converted into anionic carboxylate groups (–COO−) under alkaline conditions. The negative charges induce interfibrillar electrostatic repulsion forces and thereby facilitate nanofibrillation in the subsequent mechanical separation process [54,55]. The remarkable advantage of sulfuric acid hydrolysis and the TEMPO-mediated oxidation method is that both methods can introduce negatively charged groups on the nanocellulose surface and promote the formation of stable sulfated CNCs and TOCNFs in water due to interfibrillar electrostatic repulsion forces.
Good dispersion of cellulose nanomaterials in water is a prerequisite to ensure their reinforcing effects when applied to cementitious materials. It was reported by Cao et al. [42] that cement particles have a high tendency to aggregate because cement particles have a zeta potential of about −10 mV compared to −64 mV for sulfated CNCs. The addition of negatively charged CNCs helps the dispersion of cement particles and prevents aggregation due to the steric stabilization effect of CNC, thus leading to the increased overall DOH of cement. Negatively charged TOCNFs, TOChNFs, or TEMPO-oxidized chitin nanocrystals (TOChNCs) may have the same electrostatic stabilization effects for cement particles in water, although few reports are found in the literature. As discussed previously, cellulose nanomaterials may work as an internal curing aid to prevent autogenous shrinkage and early crack propagation in cementitious materials with the release of water retained by cellulose nanomaterials [33,117]. Carboxylated CNFs have a higher water retention capacity. When the density of carboxylate groups on the cellulose surface increases from 0.13 to 1.44 mmol/g fiber, the water retention value increases from 3.62 to 13. 4 g/g fiber. Carboxylate-rich CNFs with high water retention value are a better internal curing aid to prevent autogenous shrinkage [38]. The introduction of carboxylate groups (–COO−) can be readily achieved by the TEMPO-mediated oxidation method and the carboxylate content can be conveniently controlled by the oxidation conditions [54,55]. Mejdoub et al. [41] found that TOCNFs could reduce the cement porosity by 36% and increase the compressive strength of cement by 46%. The authors have attributed the improvement to the potential chemical interactions between the carboxylate groups of the TOCNFs and C–S–H hydration products because this reaction can increase a strong interfacial bonding between the reinforcement and the matrix, thus facilitating an effective stress transfer from the matrix to the reinforcement. However, the details of how carboxylate groups on the surface of cellulose nanomaterials react with the components in the cement are not elucidated.
The introduction of carboxylate groups to the surface of CNTs can improve its dispersion in water and enhance the interfacial interaction between carboxylated CNTs and the cement matrix. CNTs can be modified by concentrated sulfuric acid (H2SO4) and nitric acid (HNO3). Nanocomposites with carboxylate-functionalized CNTs [20] clearly showed higher strength than those with pure CNTs. And the treated CNTs were found to be tightly wrapped by C–S–H, indicating interfacial interaction between CNTs and the hydration products (e.g., C–S–H and calcium hydroxide). The strong interfacial interaction between CNTs and the hydration products produces a high bonding strength between the reinforcement and the cement matrix, as schematically depicted in Figure 33 [20]. The interaction leads to a strong covalent force on the interface between the reinforcement and matrix in the composites and therefore increases the load-transfer efficiency from the cement matrix to nanotubes. As a result, the mechanical properties of the composites are improved. Carboxylated CNTs were also found effective in reducing the porosity in the cement composites which is likely due to high strong interfacial bonding between carboxylated CNTs and the cement matrix. As the porosity of the composites is reduced, the strength of composites increases [20].
![Figure 33
Reaction scheme between the carboxylated nanotubes and hydrated production (Ca(OH)2 and C–S–H) of cement, reprinted with permission from ref. [20]; Copyright 2005, Elsevier Ltd.](/document/doi/10.1515/ntrev-2022-0149/asset/graphic/j_ntrev-2022-0149_fig_033.jpg)
Reaction scheme between the carboxylated nanotubes and hydrated production (Ca(OH)2 and C–S–H) of cement, reprinted with permission from ref. [20]; Copyright 2005, Elsevier Ltd.
Calcium ions (Ca2+) can form CaOH+ complexes in an aqueous solution (Wang et al.). In addition, carboxylate groups are functional chelating groups that can absorb metal ions (Palacios et al.). Carboxylate groups on the surface of CNFs thus can chelate with calcium ions and absorb on cement particles via electrostatic attraction, which can act as nucleation sites and form interfacial bonds and produce mechanical interlocking between the reinforcement and the matrix [41,152]. TEMPO-mediated oxidation method has also been used to extract chitin nanomaterials in our study [103]. As shown in Figure 34, negatively charged carboxylate groups can not only induce interfibrillar electrostatic repulsion forces for stable dispersion, but also can improve the interfacial interaction between carboxylated chitin nanomaterials and the cementitious materials. Therefore, carboxylated chitin nanomaterials may be a promising nano-reinforcement alternative to CNTs and cellulose nanomaterials for cementitious materials.
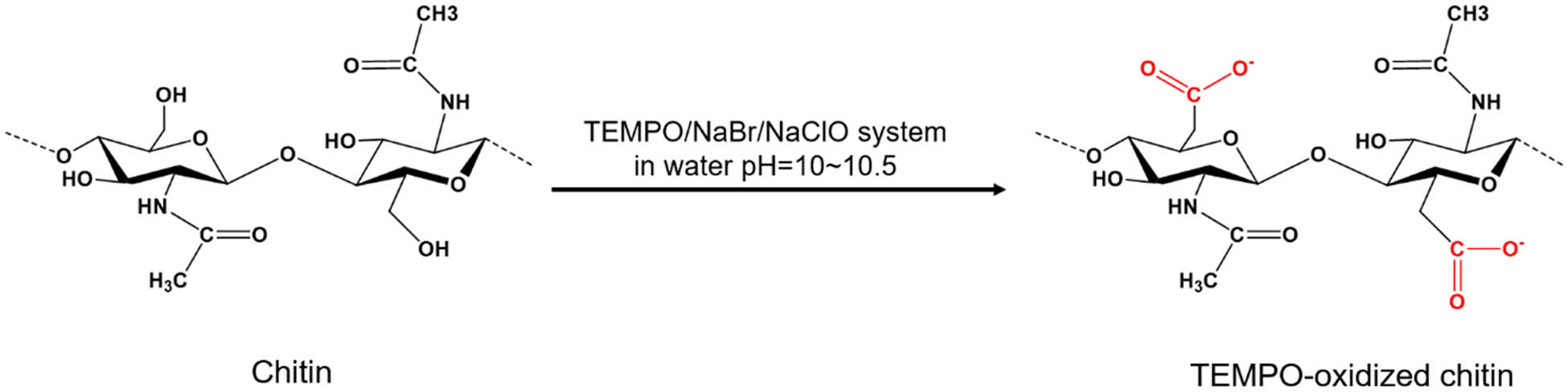
Reaction scheme of chemical pretreatment of chitin to introduce carboxylate groups into the surface of chitin via the TEMPO-mediated oxidation method.
As shown in Tables 1–3, polysaccharides-based materials are widely investigated as cement additives. Polysaccharides and modified polysaccharides (such as starch sulfonate and starch ether) have been used in the cement industry as a water-reducing (dispersing) additive or as a retarder. There are many publications on micron- and nano-cellulose enhanced cement. Results show a general enhancement of compressive strength and tensile strength while reports on rheological properties such as slurry consistency and setting time are limited. Research on chitin nanofibers or chitin nanocrystals enhanced cement is rare. Most of the research on chitin or chitosan is focused on micron size (or larger) chitin, chitosan, or its derivative polymers. Chitin at the micrometer level can be used as a retarder and chitosan and its derivative polymers can be used as additives in cement and concrete, such as superplasticizer, anti-dispersant, retarder, etc. Chitosan derivative polylactic acid can be used as an alkali silicic acid inhibitor for self-packing cement.
8 Conclusion
Many studies focus on the reinforcing mechanisms of carbon nanofibers and CNTs in cement; however, publications on the application of PNMs in cement are far less, and the mechanisms responsible for the cement composite bulk properties are not well understood. This is probably due to the relatively short history of utilizing PNMs as alternative reinforcement materials in cement. This review showed many more studies focusing on cellulose nanocrystals and nanofibers, but only a few studies are available on starch derivatives as reinforcement and other types of modifiers for cement. Furthermore, reports on the use of chitin nanomaterials in cement and concrete are rare. Therefore, the reinforcing mechanisms of these PNMs in cementitious materials are not well understood.
A comprehensive review of the effects of surface chemistry and other properties of PNMs on cement hydration and the performances of the hardened cement composites were summarized based on the published results. Hypothesis on the potential interaction of PNMs with cement was provided in this review based on studies of widely used superplasticizers with sulfate and carboxylate groups. The effect of particle size, morphology, aspect ratio, and functional groups of PNMs on the cement mechanical properties and the potential responsible mechanisms were discussed with the goal of better understanding the following aspects.
The influence of surface chemistry of PNMs on the diffusion of cation compounds and cement hydration.
The diffusion and adsorption behaviors of cations in the cement pore solution in the presence of negatively or positively charged PNMs.
Interfacial interaction between cement and PNMs.
The influence of PNMs on the phase composition and chemical and crystalline structures of hydration products as well as characterization techniques to reliably characterize and evaluate these impacts.
The existence of combined effects of surface chemistry and nano-size/morphology of PNMs on the growth of hydration products and the microstructure of the hardened cement composites.
The relationship between the specific structural arrangements of hydration products in the presence of PNMs and the hydrated cement properties.
Addressing the above research questions will significantly improve our understanding of the effect of PNMs on the properties of nano-reinforced cementitious materials. In addition, it will contribute to the development of new approaches towards advanced, crack-free cement concrete materials.
-
Funding information: This work was funded by the Advanced Research Projects Agency-Energy (ARPA-E) program of the Department of Energy, grant Number DE-AR0001140.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Mehta PK, Monteiro PJ. Concrete microstructure, properties and materials. 4th ed, New York: McGraw-Hill Education; 2014.Suche in Google Scholar
[2] Bernhardt D, James RI. US geological survey, Reston, Virginia: Mineral Commodity Summary. 2020:1–200.Suche in Google Scholar
[3] Hooton RD, Bickley JA. Design for durability: The key to improving concrete sustainability. Constr Build Mater. 2014;67:422–30. 10.1016/j.conbuildmat.2013.12.016.Suche in Google Scholar
[4] Ali MB, Saidur R, Hossain MS. A review on emission analysis in cement industries. Renew Sustain Energy Rev. 2011;15(5):2252–61. 10.1016/j.rser.2011.02.014.Suche in Google Scholar
[5] Hasanbeigi A, Price L, Lin E. Emerging energy-efficiency and CO2 emission-reduction technologies for cement and concrete production: A technical review. Renew Sustain Energy Rev. 2012;16(8):6220–38. 10.1016/j.rser.2012.07.019.Suche in Google Scholar
[6] Rehan R, Nehdi M. Carbon dioxide emissions and climate change: policy implications for the cement industry. Environ Sci Policy. 2005;8(2):105–14. 10.1016/j.envsci.2004.12.006.Suche in Google Scholar
[7] Worrell E, Price L, Martin N, Hendriks C, Meida LO. Carbon dioxide emissions from the global cement industry. Annu Rev Energy Environ. 2001;26(1):303–29. 10.1146/annurev.energy.26.1.303.Suche in Google Scholar
[8] Yang K, Zhong M, Magee B, Yang C, Wang C, Zhu X, et al. Investigation of effects of Portland cement fineness and alkali content on concrete plastic shrinkage cracking. Constr Build Mater. 2017;144:279–90. 10.1016/j.conbuildmat.2017.03.130.Suche in Google Scholar
[9] Ghourchian S, Wyrzykowski M, Baquerizo L, Lura P. Susceptibility of Portland cement and blended cement concretes to plastic shrinkage cracking. Cem Concr Compos. 2018;85:44–55. 10.1016/j.cemconcomp.2017.10.002.Suche in Google Scholar
[10] Pan Z, Sanjayan JG, Kong DLY. Effect of aggregate size on spalling of geopolymer and Portland cement concretes subjected to elevated temperatures. Constr Build Mater. 2012;36:365–72. 10.1016/j.conbuildmat.2012.04.120.Suche in Google Scholar
[11] Singh NB, Kalra M, Saxena SK. Nanoscience of cement and concrete. Mater Today Proc. 2017;4(4, Part E):5478–87. 10.1016/j.matpr.2017.06.003.Suche in Google Scholar
[12] Thomas JJ, Jennings HM, Chen JJ. Influence of nucleation seeding on the hydration mechanisms of tricalcium silicate and cement. J Phys Chem C. 2009;113(11):4327–34. 10.1021/jp809811w.Suche in Google Scholar
[13] Cappelletto E, Borsacchi S, Geppi M, Ridi F, Fratini E, Baglioni P. Comb-shaped polymers as nanostructure modifiers of calcium silicate hydrate: A 29Si solid-state NMR investigation. J Phys Chem C. 2013;117(44):22947–53. 10.1021/jp407740t.Suche in Google Scholar
[14] Beaudoin JJ, Raki L, Alizadeh R. A 29Si MAS NMR study of modified C–S–H nanostructures. Cem Concr Compos. 2009;31(8):585–90. 10.1016/j.cemconcomp.2008.11.004.Suche in Google Scholar
[15] Yakovlev G, Kerienė J, Gailius A, Girnienė I. Cement based foam concrete reinforced by carbon nanotubes. Mater Sci. 2006;12(2):147–51. http://dspace1.vgtu.lt/handle/1/220.Suche in Google Scholar
[16] Parveen S, Rana S, Fangueiro R. A review on nanomaterial dispersion, microstructure, and mechanical properties of carbon nanotube and nanofiber reinforced cementitious composites. J Nanomaterials. 2013;2013(80):80–119. 10.1155/2013/710175.Suche in Google Scholar
[17] Zhang M, Li H. Pore structure and chloride permeability of concrete containing nano-particles for pavement. Constr Build Mater. 2011;25(2):608–16. 10.1016/j.conbuildmat.2010.07.032.Suche in Google Scholar
[18] Papatzani S, Paine K, Calabria-Holley J. A comprehensive review of the models on the nanostructure of calcium silicate hydrates. Constr Build Mater. 2015;74:219–34. 10.1016/j.conbuildmat.2014.10.029.Suche in Google Scholar
[19] Lepech MD, Li VC. Water permeability of cracked cementitious composites. Proceedings of Eleventh International Conference on Fracture. Turin, Italy: Curran Associates, Inc. 2005. p. 20–5.Suche in Google Scholar
[20] Li GY, Wang PM, Zhao X. Mechanical behavior and microstructure of cement composites incorporating surface-treated multi-walled carbon nanotubes. Carbon. 2005;43(6):1239–45. 10.1016/j.carbon.2004.12.017.Suche in Google Scholar
[21] Xie X-L, Mai Y-W, Zhou X-P. Dispersion and alignment of carbon nanotubes in polymer matrix: A review. Mater Sci Engineering R Rep. 2005;49(4):89–112. 10.1016/j.mser.2005.04.002.Suche in Google Scholar
[22] Ma P-C, Siddiqui NA, Marom G, Kim J-K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos Part A Appl Sci Manuf. 2010;41(10):1345–67. 10.1016/j.compositesa.2010.07.003.Suche in Google Scholar
[23] Mohammadkazemi F, Doosthoseini K, Ganjian E, Azin M. Manufacturing of bacterial nano-cellulose reinforced fiber−cement composites. Constr Build Mater. 2015;101:958–64. 10.1016/j.conbuildmat.2015.10.093.Suche in Google Scholar
[24] Hisseine OA, Wilson W, Sorelli L, Tolnai B, Tagnit-Hamou A. Nanocellulose for improved concrete performance: A macro-to-micro investigation for disclosing the effects of cellulose filaments on strength of cement systems. Constr Build Mater. 2019;206:84–96. 10.1016/j.conbuildmat.2019.02.042.Suche in Google Scholar
[25] Akhlaghi MA, Bagherpour R, Kalhori H. Application of bacterial nanocellulose fibers as reinforcement in cement composites. Constr Build Mater. 2020;241:118061. 10.1016/j.conbuildmat.2020.118061.Suche in Google Scholar
[26] El Knidri H, Belaabed R, Addaou A, Laajeb A, Lahsini A. Extraction, chemical modification and characterization of chitin and chitosan. Int J Biol Macromolecules. 2018;120:1181–9. 10.1016/j.ijbiomac.2018.08.139.Suche in Google Scholar PubMed
[27] Rolandi M, Rolandi R. Self-assembled chitin nanofibers and applications. Adv Colloid Interface Sci. 2014;207:216–22. 10.1016/j.cis.2014.01.019.Suche in Google Scholar PubMed
[28] Dewacker DR. Cement mortar systems using blends of polysaccharides and cold-water-soluble, unmodified starches. US5575840A; 1996. https://patents.google.com/patent/US5575840A/en?oq = US5575840A.Suche in Google Scholar
[29] Crépy L, Petit J-Y, Wirquin E, Martin P, Joly N. Synthesis and evaluation of starch-based polymers as potential dispersants in cement pastes and self leveling compounds. Cem Concr Compos. 2014;45:29–38. 10.1016/j.cemconcomp.2013.09.004.Suche in Google Scholar
[30] Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J. Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev. 2011;40(7):3941–94. 10.1039/C0CS00108B.Suche in Google Scholar PubMed
[31] Sjostrom E. Wood chemistry: Fundamentals and applications. Houston: Gulf Professional Publishing; 1993.Suche in Google Scholar
[32] Azizi Samir MAS, Alloin F, Dufresne A. Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromolecules. 2005;6(2):612–26. 10.1021/bm0493685.Suche in Google Scholar PubMed
[33] Onuaguluchi O, Panesar DK, Sain M. Properties of nanofibre reinforced cement composites. Constr Build Mater. 2014;63:119–24. 10.1016/j.conbuildmat.2014.04.072.Suche in Google Scholar
[34] Sun X, Wu Q, Lee S, Qing Y, Wu Y. Cellulose nanofibers as a modifier for rheology, curing and mechanical performance of oil well cement. Sci Rep. 2016;6(1):31654. 10.1038/srep31654.Suche in Google Scholar PubMed PubMed Central
[35] Sun X, Wu Q, Zhang J, Qing Y, Wu Y, Lee S. Rheology, curing temperature and mechanical performance of oil well cement: Combined effect of cellulose nanofibers and graphene nano-platelets. Mater & Des. 2017;114:92–101. 10.1016/j.matdes.2016.10.050.Suche in Google Scholar
[36] Hisseine OA, Basic N, Omran AF, Tagnit-Hamou A. Feasibility of using cellulose filaments as a viscosity modifying agent in self-consolidating concrete. Cem Concr Compos. 2018;94:327–40. 10.1016/j.cemconcomp.2018.09.009.Suche in Google Scholar
[37] Haddad Kolour H. An investigation on the effects of cellulose nanofibrils on the performance of cement based composites. PhD Dissertation, Orono, Maine: The University of Maine; 2019. https://digitalcommons.library.umaine.edu/etd/3128/.Suche in Google Scholar
[38] Bakkari ME, Bindiganavile V, Goncalves J, Boluk Y. Preparation of cellulose nanofibers by TEMPO-oxidation of bleached chemi-thermomechanical pulp for cement applications. Carbohydr Polym. 2019;203:238–45. 10.1016/j.carbpol.2018.09.036.Suche in Google Scholar PubMed
[39] Goncalves J, El-Bakkari M, Boluk Y, Bindiganavile V. Cellulose nanofibres (CNF) for sulphate resistance in cement based systems. Cem Concr Compos. 2019;99:100–11. 10.1016/j.cemconcomp.2019.03.005.Suche in Google Scholar
[40] Jiao L, Su M, Chen L, Wang Y, Zhu H, Dai H. Natural cellulose nanofibers as sustainable enhancers in construction cement. PLoS One. 2016;11(12):e0168422. 10.1371/journal.pone.0168422.Suche in Google Scholar PubMed PubMed Central
[41] Mejdoub R, Hammi H, Suñol JJ, Khitouni M, M’nif A, Boufi S. Nanofibrillated cellulose as nanoreinforcement in Portland cement: Thermal, mechanical and microstructural properties. J Composite Mater. 2017;51(17):2491–503. 10.1177/0021998316672090.Suche in Google Scholar
[42] Cao Y, Zavaterri P, Youngblood J, Moon R, Weiss J. The influence of cellulose nanocrystal additions on the performance of cement paste. Cem Concr Compos. 2015;56:73–83. 10.1016/j.cemconcomp.2014.11.008.Suche in Google Scholar
[43] Cao Y, Tian N, Bahr D, Zavattieri PD, Youngblood J, Moon RJ, et al. The influence of cellulose nanocrystals on the microstructure of cement paste. Cem Concr Compos. 2016;74:164–73. 10.1016/j.cemconcomp.2016.09.008.Suche in Google Scholar
[44] Montes F, Fu T, Youngblood JP, Weiss J. Rheological impact of using cellulose nanocrystals (CNC) in cement pastes. Constr Build Mater. 2020;235:117497. 10.1016/j.conbuildmat.2019.117497.Suche in Google Scholar
[45] Dousti MR, Boluk Y, Bindiganavile V. The effect of cellulose nanocrystal (CNC) particles on the porosity and strength development in oil well cement paste. Constr Build Mater. 2019;205:456–62. 10.1016/j.conbuildmat.2019.01.073.Suche in Google Scholar
[46] Mazlan D, Krishnan S, Din MFM, Tokoro C, Khalid NHA, Ibrahim IS, et al. Effect of cellulose nanocrystals extracted from oil palm empty fruit bunch as green admixture for mortar. Sci Rep. 2020;10:6412. 10.1038/s41598-020-63575-7.Suche in Google Scholar PubMed PubMed Central
[47] Ong KJ, Shatkin JA, Nelson K, Ede JD, Retsina T. Establishing the safety of novel bio-based cellulose nanomaterials for commercialization. NanoImpact. 2017;6:19–29. 10.1016/j.impact.2017.03.002.Suche in Google Scholar
[48] Habibi Y, Lucia LA, Rojas OJ. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem Rev. 2010;110(6):3479–500. 10.1021/cr900339w.Suche in Google Scholar PubMed
[49] Battista OA, Coppick S, Howsmon JA, Morehead FF, Sisson WA. Level-off degree of polymerization. Ind Eng Chem. 1956;48(2):333–5. 10.1021/ie50554a046.Suche in Google Scholar
[50] Takaragi A, Minoda M, Miyamoto T, Liu HQ, Zhang LN. Reaction characteristics of cellulose in the LiCl/1,3‐dimethyl‐2‐imidazolidinone solvent system. Cellulose. 1999;6(2):93–102. 10.1023/A:1009208417954.Suche in Google Scholar
[51] Lin N, Dufresne A. Nanocellulose in biomedicine: Current status and future prospect. Eur Polym J. 2014;59:302–25. 10.1016/j.eurpolymj.2014.07.025.Suche in Google Scholar
[52] Reid MS, Villalobos M, Cranston ED. Benchmarking cellulose nanocrystals: From the laboratory to industrial production. Langmuir. 2017;33(7):1583–98. 10.1021/acs.langmuir.6b03765.Suche in Google Scholar PubMed
[53] Dufresne A. Nanocellulose: from nature to high performance tailored materials. Berlin, Boston: Walter de Gruyter GmbH & Co KG; 2017.10.1515/9783110480412Suche in Google Scholar
[54] Saito T, Isogai A. TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules. 2004;5(5):1983–9. 10.1021/bm0497769.Suche in Google Scholar PubMed
[55] Isogai A, Saito T, Fukuzumi H. TEMPO-oxidized cellulose nanofibers. Nanoscale. 2011;3(1):71–85. 10.1039/C0NR00583E.Suche in Google Scholar
[56] Abe K, Yano H. Comparison of the characteristics of cellulose microfibril aggregates of wood, rice straw and potato tuber. Cellulose. 2009;16(6):1017–23. 10.1007/s10570-009-9334-9.Suche in Google Scholar
[57] de Assis CA, Iglesias MC, Bilodeau M, Johnson D, Phillips R, Peresin MS, et al. Cellulose micro- and nanofibrils (CMNF) manufacturing – financial and risk assessment. Biofuels Bioprod Bioref. 2018;12(2):251–64. 10.1002/bbb.1835.Suche in Google Scholar
[58] Iwamoto S, Nakagaito AN, Yano H. Nano-fibrillation of pulp fibers for the processing of transparent nanocomposites. Appl Phys A. 2007;89(2):461–6. 10.1007/s00339-007-4175-6.Suche in Google Scholar
[59] Chaker A, Alila S, Mutjé P, Vilar MR, Boufi S. Key role of the hemicellulose content and the cell morphology on the nanofibrillation effectiveness of cellulose pulps. Cellulose. 2013;20(6):2863–75. 10.1007/s10570-013-0036-y.Suche in Google Scholar
[60] Kurita K. Chemistry and application of chitin and chitosan. Polym Degrad Stab. 1998;59(1–3):117–20. 10.1016/S0141-3910(97)00160-2.Suche in Google Scholar
[61] Rinaudo M. Chitin and chitosan: Properties and applications. Prog Polym Sci. 2006;31(7):603–32. 10.1016/j.progpolymsci.2006.06.001.Suche in Google Scholar
[62] Dong Y, Xu C, Wang J, Wu Y, Wang M, Ruan Y. Influence of degree of deacetylation on critical concentration of chitosan/dichloroacetic acid liquid-crystalline solution. J Appl Polym Sci. 2002;83(6):1204–8. 10.1002/app.2286.Suche in Google Scholar
[63] Pillai CKS, Paul W, Sharma CP. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog Polym Sci. 2009;34(7):641–78. 10.1016/j.progpolymsci.2009.04.001.Suche in Google Scholar
[64] Fan Y, Saito T, Isogai A. Chitin nanocrystals prepared by TEMPO-mediated oxidation of α-chitin. Biomacromolecules. 2008;9(1):192–8. 10.1021/bm700966g.Suche in Google Scholar PubMed
[65] Shinoda R, Saito T, Okita Y, Isogai A. Relationship between length and degree of polymerization of TEMPO-oxidized cellulose nanofibrils. Biomacromolecules. 2012;13(3):842–9. 10.1021/bm2017542.Suche in Google Scholar PubMed
[66] Fan Y, Saito T, Isogai A. Individual chitin nano-whiskers prepared from partially deacetylated α-chitin by fibril surface cationization. Carbohydr Polym. 2010;79(4):1046–51. 10.1016/j.carbpol.2009.10.044.Suche in Google Scholar
[67] Zhong T, Wolcott MP, Liu H, Glandon N, Wang J. The influence of pre-fibrillation via planetary ball milling on the extraction and properties of chitin nanofibers. Cellulose. 2020;27(11):6205–16. 10.1007/s10570-020-03186-7.Suche in Google Scholar
[68] Patural L, Marchal P, Govin A, Grosseau P, Ruot B, Devès O. Cellulose ethers influence on water retention and consistency in cement-based mortars. Cem Concr Res. 2011;41(1):46–55. 10.1016/j.cemconres.2010.09.004.Suche in Google Scholar
[69] Peschard A, Govin A, Grosseau P, Guilhot B, Guyonnet R. Effect of polysaccharides on the hydration of cement paste at early ages. Cem Concr Res. 2004;34(11):2153–8. 10.1016/j.cemconres.2004.04.001.Suche in Google Scholar
[70] Vieira MC, Klemm D, Einfeldt L, Albrecht G. Dispersing agents for cement based on modified polysaccharides. Cem Concr Res. 2005;35(5):883–90. 10.1016/j.cemconres.2004.09.022.Suche in Google Scholar
[71] Zhang D-F, Ju B-Z, Zhang S-F, He L, Yang J-Z. The study on the dispersing mechanism of starch sulfonate as a water-reducing agent for cement. Carbohydr Polym. 2007;70(4):363–8. 10.1016/j.carbpol.2007.04.024.Suche in Google Scholar
[72] Zhang H, Wang W, Li Q, Tian Q, Li L, Liu J. A starch-based admixture for reduction of hydration heat in cement composites. Constr Build Mater. 2018;173:317–22. 10.1016/j.conbuildmat.2018.03.199.Suche in Google Scholar
[73] Claramunt J, Ventura H, Toledo Filho RD, Ardanuy M. Effect of nanocelluloses on the microstructure and mechanical performance of CAC cementitious matrices. Cem Concr Res. 2019;119:64–76. 10.1016/j.cemconres.2019.02.006.Suche in Google Scholar
[74] Alzoubi HH, Albiss BA. Performance of cementitious composites with nano PCMs and cellulose nano fibers. Constr Build Mater. 2020;236:117483. 10.1016/j.conbuildmat.2019.117483.Suche in Google Scholar
[75] Kamasamudram KS, Ashraf W, Landis EN. Cellulose nanofibrils with and without nanosilica for the performance enhancement of Portland cement systems. Constr Build Mater. 2021;285:121547. 10.1016/j.conbuildmat.2020.121547.Suche in Google Scholar
[76] Nassiri S, Chen Z, Jian G, Zhong T, Haider MM, Li H, et al. Comparison of unique effects of two contrasting types of cellulose nanomaterials on setting time, rheology, and compressive strength of cement paste. Cem Concr Compos. 2021;123:104201. 10.1016/j.cemconcomp.2021.104201.Suche in Google Scholar
[77] Cuenca E, Mezzena A, Ferrara L. Synergy between crystalline admixtures and nano-constituents in enhancing autogenous healing capacity of cementitious composites under cracking and healing cycles in aggressive waters. Constr Build Mater. 2021;266:121447. 10.1016/j.conbuildmat.2020.121447.Suche in Google Scholar
[78] Flores J, Kamali M, Ghahremaninezhad A. An investigation into the properties and microstructure of cement mixtures modified with cellulose nanocrystal. Materials. 2017;10(5):498. 10.3390/ma10050498.Suche in Google Scholar PubMed PubMed Central
[79] Mcalpine S, Nakoneshny J, Kunkel B. Crystalline cellulose reinforced cement. US20200079692A1; 2020. https://patents.google.com/patent/US20200079692A1/en?oq = US20200079692A1.Suche in Google Scholar
[80] Barría JC, Vázquez A, Pereira J-M, Manzanal D. Effect of bacterial nanocellulose on the fresh and hardened states of oil well cement. J Pet Sci Eng. 2021;199:108259. 10.1016/j.petrol.2020.108259.Suche in Google Scholar
[81] Tegiacchi F, Casu B. Alkylsulfonated polysaccharides and mortar and concrete mixes containing them. USH493H; 1988. https://patents.google.com/patent/USH493H/en?oq = USH493H.Suche in Google Scholar
[82] Yamamuro H. Property of new polysaccharide derivative as a viscosity agent for self-compacting concrete. Cachan, France: RILEM Publications; 1999. p. 449–59.Suche in Google Scholar
[83] Wang X, Ma J, Wang Y, He B. Reinforcement of calcium phosphate cements with phosphorylated chitin. Chin J Polym Sci. 2002;20(4):325–32. http://www.cjps.org/article/id/112d256e-9075-421e-bbf3-f9165b56e2b0?pageType = en.Suche in Google Scholar
[84] Pourchez J, Peschard A, Grosseau P, Guyonnet R, Guilhot B, Vallée F. HPMC and HEMC influence on cement hydration. Cem Concr Res. 2006;36(2):288–94. 10.1016/j.cemconres.2005.08.003.Suche in Google Scholar
[85] Roddy CW, Todd BL. Methods of cementing in subterranean formations using crack resistant cement compositions. US7036586B2; 2006. https://patents.google.com/patent/US7036586B2/en?oq = US7036586B2.Suche in Google Scholar
[86] Lasheras-Zubiate M, Navarro-Blasco I, Fernández JM, Alvarez JI. Studies on chitosan as an admixture for cement-based materials: Assessment of its viscosity enhancing effect and complexing ability for heavy metals. J Appl Polym Sci. 2011;120(1):242–52. 10.1002/app.33048.Suche in Google Scholar
[87] Lasheras-Zubiate M, Navarro-Blasco I, Fernández JM, Álvarez JI. Effect of the addition of chitosan ethers on the fresh state properties of cement mortars. Cem Concr Compos. 2012;34(8):964–73. 10.1016/j.cemconcomp.2012.04.010.Suche in Google Scholar
[88] Cestari AR, Vieira EFS, Alves FJ, Silva ECS, Andrade MAS. A novel and efficient epoxy/chitosan cement slurry for use in severe acidic environments of oil wells—Structural characterization and kinetic modeling. J Hazard Mater. 2012;213–214:109–16. 10.1016/j.jhazmat.2012.01.068.Suche in Google Scholar PubMed
[89] Reddy BR, Ghosh A, Fitzgerald R. Polysaccharide based cement additives. US8586508B2; 2013. https://patents.google.com/patent/US8586508B2/en?oq = US8586508B2.Suche in Google Scholar
[90] Vieira EFS, Lima PF, dos Santos IMG, Mangrich AS, de França AA, Le Saoût G, et al. The influence of in situ polymerized epoxidized A/F bisphenol-chitosan on features of oil well cement slurry—Molecular-level analysis and long-term interaction of API fracturing fluid. J Appl Polym Sci. 2014;131(22):41044. 10.1002/app.41044.Suche in Google Scholar
[91] Lv S, Liu J, Zhou Q, Huang L, Sun T. Synthesis of modified chitosan superplasticizer by amidation and sulfonation and its application performance and working mechanism. Ind Eng Chem Res. 2014;53(10):3908–16. 10.1021/ie403786q.Suche in Google Scholar
[92] Isik IE, Ozkul MH. Utilization of polysaccharides as viscosity modifying agent in self-compacting concrete. Constr Build Mater. 2014;72:239–47. 10.1016/j.conbuildmat.2014.09.017.Suche in Google Scholar
[93] Liu H, Bu Y, Sanjayan J, Shen Z. Effects of chitosan treatment on strength and thickening properties of oil well cement. Constr Build Mater. 2015;75:404–14. 10.1016/j.conbuildmat.2014.11.047.Suche in Google Scholar
[94] Liu H, Bu Y, Bu C, Guo S. A kind of pre-chelating chitosan cementing slurry retardant and preparation method thereof. CN103045213B; 2015. https://patents.google.com/patent/CN103045213B/en?oq = CN103045213B.Suche in Google Scholar
[95] Ustinova YV, Nikiforova TP. Cement compositions with the chitosan additive. Procedia Eng. 2016;153:810–5. 10.1016/j.proeng.2016.08.247.Suche in Google Scholar
[96] Zhao H, Chen D, Liao Y, Ouyang F, Du Y, Xu H. A kind of preparation method of the modified chitin bio-based efficient retarding and water reducing agent containing carboxyl. CN107601945A; 2018. https://patents.google.com/patent/CN107601945A/en?oq = CN107601945A.Suche in Google Scholar
[97] Wang J, Mignon A, Trenson G, Van Vlierberghe S, Boon N, De, et al. A chitosan based pH-responsive hydrogel for encapsulation of bacteria for self-sealing concrete. Cem Concr Compos. 2018;93:309–22. 10.1016/j.cemconcomp.2018.08.007.Suche in Google Scholar
[98] Hall LJ. Chitin nanocrystal containing wellbore fluids. US10259981B2; 2019. https://patents.google.com/patent/US10259981B2/en?oq = US10259981B2.Suche in Google Scholar
[99] Arslan H, Aytaç US, Bilir T, Şen Ş. The synthesis of a new chitosan based superplasticizer and investigation of its effects on concrete properties. Constr Build Mater. 2019;204:541–9. 10.1016/j.conbuildmat.2019.01.209.Suche in Google Scholar
[100] Zhao H, Chen D, Liao Y, Xuan W, Ouyang F, Feng J. A kind of high additive of low alkali_silica reaction expansion is discarded to crush glass self-compacting concrete preparation method. CN109534745A; 2019. https://patents.google.com/patent/CN109534745A/en?oq = CN109534745A.Suche in Google Scholar
[101] Zhao H, Chen D, Liao Y, Ouyang F, Xuan W, Xu H. Preparation method of modified natural chitin-acrylic acid copolymerized multifunctional organic anti-dispersant for underwater undispersed concrete. CN110922532A; 2020. https://patents.google.com/patent/CN110922532A/en?oq = CN110922532A.Suche in Google Scholar
[102] Zhao S, Liu Z, Mu Y, Wang F, He Y. Effect of chitosan on the carbonation behavior of γ-C2S. Cem Concr Compos. 2020;111:103637. 10.1016/j.cemconcomp.2020.103637.Suche in Google Scholar
[103] Haider MM, Jian G, Zhong T, Li H, Fernandez CA, Fifield LS, et al. Insights into setting time, rheological and mechanical properties of chitin nanocrystals- and chitin nanofibers-cement paste. Cem Concr Compos. 2022;132:104623–41. 10.1016/j.cemconcomp.2022.104623.Suche in Google Scholar
[104] Zingg A, Winnefeld F, Holzer L, Pakusch J, Becker S, Gauckler L. Adsorption of polyelectrolytes and its influence on the rheology, zeta potential, and microstructure of various cement and hydrate phases. J Colloid Interface Sci. 2008;323(2):301–12. 10.1016/j.jcis.2008.04.052.Suche in Google Scholar PubMed
[105] John E, Matschei T, Stephan D. Nucleation seeding with calcium silicate hydrate – A review. Cem Concr Res. 2018;113:74–85. 10.1016/j.cemconres.2018.07.003.Suche in Google Scholar
[106] Hou D, Zhu Y, Lu Y, Li Z. Mechanical properties of calcium silicate hydrate (C–S–H) at nano-scale: A molecular dynamics study. Mater Chem Phys. 2014;146(3):503–11. 10.1016/j.matchemphys.2014.04.001.Suche in Google Scholar
[107] Joseph S, Bishnoi S, Balen KV, Cizer Ö. Modeling the effect of fineness and filler in early-age hydration of tricalcium silicate. J Am Ceram Soc. 2017;100(3):1178–94. 10.1111/jace.14676.Suche in Google Scholar
[108] Taylor HF. Cement chemistry. Vol. 2. London: Thomas Telford; 1997.10.1680/cc.25929Suche in Google Scholar
[109] Pellenq RJ, Kushima A, Shahsavari R, Van Vliet KJ, Buehler MJ, Yip S, et al. A realistic molecular model of cement hydrates. PNAS. 2009;106(38):16102–7. 10.1073/pnas.0902180106.Suche in Google Scholar
[110] Kunhi Mohamed A, Parker SC, Bowen P, Galmarini S. An atomistic building block description of C–S–H – Towards a realistic C–S–H model. Cem Concr Res. 2018;107:221–35. 10.1016/j.cemconres.2018.01.007.Suche in Google Scholar
[111] Cho BH, Chung W, Nam BH. Molecular dynamics simulation of calcium-silicate-hydrate for nano-engineered cement composites—a review. Nanomaterials. 2020;10(11):2158. 10.3390/nano10112158.Suche in Google Scholar
[112] Jennings HM, Bullard JW, Thomas JJ, Andrade JE, Chen JJ, Scherer GW. Characterization and modeling of pores and surfaces in cement paste. J Adv Concr Technol. 2008;6(1):5–29. 10.3151/jact.6.5.Suche in Google Scholar
[113] Garboczi EJ, Bentz DP. The effect of statistical fluctuation, finite size error, and digital resolution on the phase percolation and transport properties of the NIST cement hydration model. Cem Concr Res. 2001;31(10):1501–14. 10.1016/S0008-8846(01)00593-2.Suche in Google Scholar
[114] Nonat A. The structure and stoichiometry of C–S–H. Cem Concr Res. 2004;34(9):1521–8. 10.1016/j.cemconres.2004.04.035.Suche in Google Scholar
[115] Richardson IG. Tobermorite/jennite- and tobermorite/calcium hydroxide-based models for the structure of C–S–H: applicability to hardened pastes of tricalcium silicate, β-dicalcium silicate, Portland cement, and blends of Portland cement with blast-furnace slag, metakaolin, or silica fume. Cem Concr Res. 2004;34(9):1733–77. 10.1016/j.cemconres.2004.05.034.Suche in Google Scholar
[116] Kendall K, Howard AJ, Birchall JD, Pratt PL, Proctor BA, Jefferis SA, et al. The relation between porosity, microstructure and strength, and the approach to advanced cement-based materials. Philos Trans R Soc Lond. 1983;310(1511):139–53. 10.1098/rsta.1983.0073.Suche in Google Scholar
[117] Balea A, Fuente E, Blanco A, Negro C. Nanocelluloses: Natural-based materials for fiber-reinforced cement composites. A critical review. Polymers. 2019;11(3):518. 10.3390/polym11030518.Suche in Google Scholar
[118] Jennings HM. A model for the microstructure of calcium silicate hydrate in cement paste. Cem Concr Res. 2000;30(1):101–16. 10.1016/S0008-8846(99)00209-4.Suche in Google Scholar
[119] Jennings HM, Thomas JJ, Gevrenov JS, Constantinides G, Ulm F-J. A multi-technique investigation of the nanoporosity of cement paste. Cem Concr Res. 2007;37(3):329–36. 10.1016/j.cemconres.2006.03.021.Suche in Google Scholar
[120] Dalas F, Nonat A, Pourchet S, Mosquet M, Rinaldi D, Sabio S. Tailoring the anionic function and the side chains of comb-like superplasticizers to improve their adsorption. Cem Concr Res. 2015;67:21–30. 10.1016/j.cemconres.2014.07.024.Suche in Google Scholar
[121] Liu J, Yu C, Shu X, Ran Q, Yang Y. Recent advance of chemical admixtures in concrete. Cem Concr Res. 2019;124:105834. 10.1016/j.cemconres.2019.105834.Suche in Google Scholar
[122] Zhao H, Yang Y, Shu X, Wang Y, Wu S, Ran Q, et al. The binding of calcium ion with different groups of superplasticizers studied by three DFT methods, B3LYP, M06-2X and M06. Computational Mater Sci. 2018;152:43–50. 10.1016/j.commatsci.2018.05.034.Suche in Google Scholar
[123] Ilg M, Plank J. Non-adsorbing small molecules as auxiliary dispersants for polycarboxylate superplasticizers. Colloids Surf A Physicochemical Eng Asp. 2020;587:124307. 10.1016/j.colsurfa.2019.124307.Suche in Google Scholar
[124] Lewis JA, Matsuyama H, Kirby G, Morissette S, Young JF. Polyelectrolyte effects on the rheological properties of concentrated cement suspensions. J Am Ceram Soc. 2000;83(8):1905–13. 10.1111/j.1151-2916.2000.tb01489.x.Suche in Google Scholar
[125] Plank J, Sachsenhauser B. Experimental determination of the effective anionic charge density of polycarboxylate superplasticizers in cement pore solution. Cem Concr Res. 2009;39(1):1–5. 10.1016/j.cemconres.2008.09.001.Suche in Google Scholar
[126] Flatt R, Schober I. 7 – Superplasticizers and the rheology of concrete. Understanding the Rheology of Concrete. Cambridge, UK: Woodhead Publishing; 2012. p. 144–208. 10.1533/9780857095282.2.144.Suche in Google Scholar
[127] von Hoessle F, Plank J, Leroux F. Intercalation of sulfonated melamine formaldehyde polycondensates into a hydrocalumite LDH structure. J Phys Chem Solids. 2015;80:112–7. 10.1016/j.jpcs.2015.01.008.Suche in Google Scholar
[128] Yoshioka K, Sakai E, Daimon M, Kitahara A. Role of steric hindrance in the performance of superplasticizers for concrete. J Am Ceram Soc. 1997;80(10):2667–71. 10.1111/j.1151-2916.1997.tb03169.x.Suche in Google Scholar
[129] Lv S, Ju H, Qiu C, Ma Y, Zhou Q. Effects of connection mode between carboxyl groups and main chains on polycarboxylate superplasticizer properties. J Appl Polym Sci. 2013;128(6):3925–32. 10.1002/app.38608.Suche in Google Scholar
[130] Lv S, Cao Q, Zhou Q, Lai S, Gao F. Structure and characterization of sulfated chitosan superplasticizer. J Am Ceram Soc. 2013;96(6):1923–9. 10.1111/jace.12221.Suche in Google Scholar
[131] Goncalves J, Boluk Y, Bindiganavile V. Cellulose nanofibres mitigate chloride ion ingress in cement-based systems. Cem Concr Compos. 2020;114:103780. 10.1016/j.cemconcomp.2020.103780.Suche in Google Scholar
[132] Côté AP, Shimizu GKH. The supramolecular chemistry of the sulfonate group in extended solids. Coord Chem Rev. 2003;245(1–2):49–64. 10.1016/S0010-8545(03)00033-X.Suche in Google Scholar
[133] Zhao H, Yang Y, Wang Y, Shu X, Wu S, Ran Q, et al. Binding of calcium cations with three different types of oxygen-based functional groups of superplasticizers studied by atomistic simulations. J Mol Model. 2018;24(11):321. 10.1007/s00894-018-3853-y.Suche in Google Scholar PubMed
[134] Guibal E. Interactions of metal ions with chitosan-based sorbents: a review. Sep Purif Technol. 2004;38(1):43–74. 10.1016/j.seppur.2003.10.004.Suche in Google Scholar
[135] Ogawa K, Oka K, Yui T. X-ray study of chitosan-transition metal complexes. Chem Mater. 1993;5(5):726–8. 10.1021/cm00029a026.Suche in Google Scholar
[136] Hsien T-Y, Rorrer GL. Effects of acylation and crosslinking on the material properties and cadmium ion adsorption capacity of porous chitosan beads. Sep Sci Technol. 1995;30(12):2455–75. 10.1080/01496399508021395.Suche in Google Scholar
[137] Rhazi M, Desbrières J, Tolaimate A, Rinaudo M, Vottero P, Alagui A, et al. Influence of the nature of the metal ions on the complexation with chitosan.: Application to the treatment of liquid waste. Eur Polym J. 2002;38(8):1523–30. 10.1016/S0014-3057(02)00026-5.Suche in Google Scholar
[138] Vold IMN, Vårum KM, Guibal E, Smidsrød O. Binding of ions to chitosan—selectivity studies. Carbohydr Polym. 2003;54(4):471–7. 10.1016/j.carbpol.2003.07.001.Suche in Google Scholar
[139] Flatt RJ, Houst YF. A simplified view on chemical effects perturbing the action of superplasticizers. Cem Concr Res. 2001;31(8):1169–76. 10.1016/S0008-8846(01)00534-8.Suche in Google Scholar
[140] Gartner E, Maruyama I, Chen J. A new model for the C–S–H phase formed during the hydration of Portland cements. Cem Concr Res. 2017;97:95–106. 10.1016/j.cemconres.2017.03.001.Suche in Google Scholar
[141] Wang F, Kong X, Wang D, Wang Q. The effects of nano-C–S–H with different polymer stabilizers on early cement hydration. J Am Ceram Soc. 2019;102(9):5103–16. 10.1111/jace.16425.Suche in Google Scholar
[142] Sun J, Shi H, Qian B, Xu Z, Li W, Shen X. Effects of synthetic C–S–H/PCE nanocomposites on early cement hydration. Constr Build Mater. 2017;140:282–92. 10.1016/j.conbuildmat.2017.02.075.Suche in Google Scholar
[143] Berodier E, Scrivener K. Understanding the filler effect on the nucleation and growth of C–S–H. J Am Ceram Soc. 2014;97(12):3764–73. 10.1111/jace.13177.Suche in Google Scholar
[144] Ardanuy Raso M, Claramunt Blanes J, Arévalo Peces R, Parés Sabatés F, Aracri E, Vidal Lluciá T. Nanofibrillated cellulose (NFC) as a potential reinforcement for high performance cement mortar composites. BioResources. 2012;7(3):3883–94. https://upcommons.upc.edu/handle/2117/16392.10.15376/biores.7.3.3883-3894Suche in Google Scholar
[145] Rosato L, Stefanidou M, Milazzo G, Fernandez F, Livreri P, Muratore N, et al. Study and evaluation of nano-structured cellulose fibers as additive for restoration of historical mortars and plasters. Mater Today Proc. 2017;4(7, Part 1):6954–65. 10.1016/j.matpr.2017.07.025.Suche in Google Scholar
[146] Liew KM, Kai MF, Zhang LW. Carbon nanotube reinforced cementitious composites: An overview. Compos Part A Appl Sci Manuf. 2016;91:301–23. 10.1016/j.compositesa.2016.10.020.Suche in Google Scholar
[147] da Costa Correia V, Santos SF, Soares Teixeira R, Savastano Junior H. Nanofibrillated cellulose and cellulosic pulp for reinforcement of the extruded cement based materials. Constr Build Mater. 2018;160:376–84. 10.1016/j.conbuildmat.2017.11.066.Suche in Google Scholar
[148] Peters SJ, Rushing TS, Landis EN, Cummins TK. Nanocellulose and microcellulose fibers for concrete. Transportation Res Rec. 2010;2142(1):25–8. 10.3141/2142-04.Suche in Google Scholar
[149] Xu X, Liu F, Jiang L, Zhu JY, Haagenson D, Wiesenborn DP. Cellulose nanocrystals vs. cellulose nanofibrils: A comparative study on their microstructures and effects as polymer reinforcing agents. ACS Appl Mater Interfaces. 2013;5(8):2999–3009. 10.1021/am302624t.Suche in Google Scholar PubMed
[150] Cuenca E, D’Ambrosio L, Lizunov D, Tretjakov A, Volobujeva O, Ferrara L. Mechanical properties and self-healing capacity of ultra high performance fibre reinforced concrete with alumina nano-fibres: Tailoring ultra high durability concrete for aggressive exposure scenarios. Cem Concr Compos. 2021;118:103956. 10.1016/j.cemconcomp.2021.103956.Suche in Google Scholar
[151] Cao Y, Zavattieri P, Youngblood J, Moon R, Weiss J. The relationship between cellulose nanocrystal dispersion and strength. Constr Build Mater. 2016;119:71–9. 10.1016/j.conbuildmat.2016.03.077.Suche in Google Scholar
[152] Khanjani P, Kosonen H, Ristolainen M, Virtanen P, Vuorinen T. Interaction of divalent cations with carboxylate group in TEMPO-oxidized microfibrillated cellulose systems. Cellulose. 2019;26(8):4841–51. 10.1007/s10570-019-02417-w.Suche in Google Scholar
© 2022 Tuhua Zhong et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption


![Figure 21
Reaction scheme between carboxylated CNFs and hydration products, including C–S–H and Ca(OH)2 [20,140].](/document/doi/10.1515/ntrev-2022-0149/asset/graphic/j_ntrev-2022-0149_fig_021.jpg)
