Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
-
Mohamad Abd Elkodous
, Gomaa A. M. Ali
, Mouna El Abboubi
, Marina R. Abdelnour
Abstract
Agriculture and industrial wastes (AIWs) have attracted much attention because of their huge environmental, economic, and social impacts. AIWs have been considered a crucial link of a closed-loop for the fabrication of nanomaterials and composites wherein they replace traditional resources with sustainable waste in waste management. In this context, the proper disposal of AIWs is required. This review aims to investigate the technical feasibility of using innovative AIW resources and various strategies for the fabrication of nanomaterials for improving energy applications. First, the utilization of AIWs is classified comprehensively. Second, key technologies to produce nanomaterials are summarized. In addition, this review discusses the potential applications of the fabricated nanomaterials in energy storage and energy conversion.
Graphical abstract
Abbreviations
- 3D
-
three-dimensional
- ABS
-
acrylonitrile butadiene styrene
- AC
-
activated carbon
- ACPKS
-
activated carbon palm kernel shell
- AFC
-
alkaline fuel cell
- AIWs
-
agriculture and industrial wastes
- AuNPs
-
gold nanoparticles
- BA
-
bottom ash
- BEW
-
boron enrichment waste
- BFW
-
banana fruit waste
- BNC
-
bacterial nanocellulose
- CNTs
-
carbon nanotubes
- CE
-
counter electrode
- CR
-
congo red
- CFA
-
coal fly ash
- CLR
-
coal liquefaction residue
- CNC
-
cellulose nanocrystal
- CNFs
-
carbon nanofibers
- CNMs
-
carbon nanomaterials
- CQDs
-
carbon quantum dots
- CTP
-
coal tar pitch
- Cu2O
-
copper(i) oxide or cuprous oxide
- CuO
-
cupric oxide
- CVD
-
chemical vapor deposition
- DMFCs
-
direct methanol fuel cells
- DPW
-
date pulp waste
- DSSCs
-
dye-sensitized solar cells
- EY
-
eosin yellow
- E-CQDs
-
carbon quantum dots prepared by electrochemical oxidation
- EOL
-
end-of-life
- EPCB
-
end-of-life flexible printed circuit board
- E-Recycling
-
electronic recycling
- ESM
-
eggshell membrane
- FeNG
-
Fe–N-doped graphene
- FF
-
fill factor
- FA
-
fly ash
- FAO
-
Food and Agriculture Organization
- FCCVD
-
floating catalyst chemical vapor deposition
- F-CQDs
-
carbon quantum dots from fenugreek leaves
- G
-
graphene
- GA
-
gum Arabic
- GO
-
graphene oxide
- GQDs
-
graphene quantum dots
- GR-sludge
-
gold refining sludge
- GC
-
glass carbon
- GCD
-
galvanostatic charge-discharge
- HPC
-
honeycomb-like activated porous carbon
- HSs
-
hierarchical superstructures
- IPCE
-
incident photon-to-current efficiency
- KL
-
kraft lignin
- HDPE
-
high-density polyethylene
- LCAs
-
life cycle assessments
- LiBs
-
lithium-ion batteries
- LTFCs
-
low-temperature fuel cells
- LDPE
-
low-density polyethylene
- LED
-
light-emitting diode
- LLDPE
-
linear low-density polyethylene
- LSV
-
linear sweep voltammetry
- LSW
-
leather solid waste
- MAP
-
microwave-assisted pyrolysis
- MFCQDs
-
magneto fluorescent carbon quantum dots
- M-CQDs
-
carbon quantum dots from Miscanthus
- MP
-
mixed plastic
- MSW
-
municipal solid waste
- MWCNTs
-
multiwalled carbon nanotubes
- MF
-
magnetic field
- MFCs
-
microbial fuel cells
- MO
-
methyl orange
- MSI
-
metal-support interaction
- NDC
-
nitrogen-doped carbon
- NDPG
-
N-doped porous graphene
- NPs
-
nanoparticles
- NRs
-
nanorods
- N-ACs
-
nitrogen-doped activated carbons
- NBC
-
nitrogen-doped carbon
- N-CNFO
-
nitrogen-doped carbon nanofibers with open channels
- N-CQDs
-
nitrogen-doped carbon quantum dots
- NM-CQDs
-
nitrogen-doped microspore carbon quantum dots
- NMs
-
nanomaterials
- N-MWCNTs
-
nitrogen-doped multiwalled carbon nanotubes
- N-ACs
-
nitrogen-doped activated carbons
- NTs
-
nanotubes
- ORR
-
oxygen reduction reaction
- OER
-
oxygen evolution reaction
- OTC
-
oxytetracycline
- PAN
-
polyacrylonitrile
- PCNs
-
porous carbon nanoparticles
- PAC
-
porous activated carbon
- POME
-
palm oil mill effluent
- PA
-
polyamide
- PAC
-
porous activated carbon
- PCBs
-
printed circuit boards
- PE
-
polyethylene
- PP
-
polypropylene
- PS
-
polystyrene
- PET
-
polyethylene terephthalate
- PMMA
-
polymethylmethacrylate
- PVA
-
polyvinyl alcohol
- PVC
-
polyvinyl chloride
- PVP
-
polyvinylpyrrolidone
- PW
-
plastic waste
- PCE
-
power conversion efficiency
- PSCs
-
perovskite solar cells
- QDs
-
quantum dots
- QY
-
quantum yield
- r-PET
-
recycled polyethylene terephthalate
- rGO
-
reduced graphene oxide
- RHE
-
reversible hydrogen electrode
- RhB
-
rhodamine B
- C s
-
specific capacitance
- SG
-
S-doped graphene
- sGQDs
-
self-assembled graphene quantum dots
- SMNPs
-
spherical magnetic nanoparticles
- SWCNTs
-
single-walled carbon nanotubes
- SS
-
spider silk
- SCE
-
saturated calomel electrode
- J SC
-
short circuit current density
- TiRR
-
triiodide reduction reaction
- TAW
-
tricomposite agro waste
- TCs
-
tetracycline
- TEMPO
-
2,2,6,6-tetramethylpiperidine-1-oxyl radical
- TPO
-
tire pyrolysis oil
- TTDDA
-
4,7,10-trioxa-1,13-tridecanediamine
- WEEE
-
waste electrical and electronic equipment
- WEO
-
waste engine oil
- WMPs
-
waste mobile phones
- WPC
-
wood–plastic waste
- WPCBs
-
waste printed circuit boards
- WPT
-
waste palm trunk
- WPVB
-
waste poly vinyl butyral
- WWTPs
-
wastewater treatment plants
- Zn–C
-
zinc–carbon
- Zn–MnO2
-
zinc–manganese oxide
- ZnO NPs
-
zinc oxide nanoparticles
1 Introduction
Because of human population growth, accelerated industrialization, and urbanization, there is a steady rise in generated wastes. According to the World Bank project, by 2050, wastes will significantly exceed population growth by more than double [1,2]. Meanwhile, approximately 2.01 billion tons of municipal solid wastes (MSWs) are being collected globally annually and are anticipated to grow to 3.40 billion tons by 2025 [3]. Furthermore, solid waste management alone produced 1.6 billion tons of CO2 equivalent greenhouse gas emissions in 2016, accounting for approximately 5% of global emissions. Low-income countries spend 20% of their budgets on waste treatment on average, with an upward of 90% of wastes either openly dumped or burned, a key cause of environmental pollution and harmful health impacts [2,4]. This condition has encouraged the creation, adoption, and strengthening of various policy strategies and novel processes to minimize the waste impact on humans and the environment. Thus far, wastes have been used as a utility source to create value-added products.
Recycling waste materials into high-value products is a distinct possibility. However, the global economy remains far from achieving a closed-loop materials cycle because of inefficient or nonexistent recycling practices. For example, only approximately 10% of the 280 million tons of plastics produced each year are recycled [5]. It is worth noting that broadcast recycling is being implemented in different areas for general products (e.g., newspapers, glass bottles, plastic, and cans). However, this situation is less assured with long-lived products, including laptops, fridges, and vehicles, collectively known as e-waste. At the global level, approximately 30–50 million tons of waste from electrical and electronic equipment (WEEE), with an estimated annual growth rate of 5%, accumulates annually [6]. When manufactured in an environmentally sustainable manner, life cycle assessments have shown that nanomaterials’ (NMs) processing requires more energy and natural resources than traditional technologies. Waste materials as feedstock are appealing because they are readily available and inexpensive, whereas NMs synthesis is revolutionary waste management and recycling process [7]. Consequently, sustainable production of waste-derived NMs has become a hot topic of study in recent years. New and more stringent environmental regulations fuel the rising demand for waste recycling.
This has inspired researchers to synthesize new high-value and marketable NM-based products and serve as a strategy to boost industry interest in serious recycling efforts because of potentially attractive economic returns from state subsidies [6]. NMs have physicochemical properties that make them appealing for various applications in healthcare, textiles, food, and electronics. Interestingly, the distinct physicochemical properties of NMs play an important role in their energy applications [8,9].
One of the major problems in the 21st century is energy storage and energy conversion. With the accelerated decline of fossil fuels and worsening air pollution, there is an increased need for energy efficiency and the pursuit of sustainable and clean energy sources. Fuel cells, solar cells, supercapacitors, and lithium-ion batteries (LiBs) are examples of these technologies. All materials used in these devices are considered key to major energy conversion and storage advancements. Consequently, high-performance materials should have unique properties and be rationally engineered for critical energy conversion and storage applications. Various waste residues have been used as initial sources to produce NMs. Researchers have synthesized several NMs, such as metal oxide nanoparticles (NPs), carbon NPs, carbon nanotubes (CNTs), activated carbon (AC), graphene (G), and graphene quantum dots (GQDs), which have recently spurred attention because of their unique properties [10]. The utilization of waste materials is not only a way to find a plentiful and less expensive source for NM production but also a way to use waste materials that seem to be environmentally harmful in general. Future research in this field remains very open to obtaining high-value NMs from less expensive sources and more straightforward methods.
In recent years, waste-derived NMs have become a hot topic of study and research. As depicted in Figure 1, there has been growing evolution in the number of publications on waste-derived NMs from 2013 to early 2021. The academic interest in this field has steadily increased. The scientific output of waste-derived NM-related research has almost doubled over the last 8 years. Carbon nanomaterials (CNMs) and metal oxide NPs are the most investigated; most publications analyze these materials. Interest in CNMs’ research has been increasing steadily and at a speedy rate, from 764 publications in 2012 to 2,853 in 2021, with a 73% increase compared to 73% for metal oxide NPs and 70% for AuNPs. However, the number of studies for the last reported NMs remains low. This deficiency in research productivity could be attributed to several possible factors that are not mutually exclusive, such as the lack of interest in the subject matter among scientists, the small number of scientists in the field, and the lack of funding and governmental support.
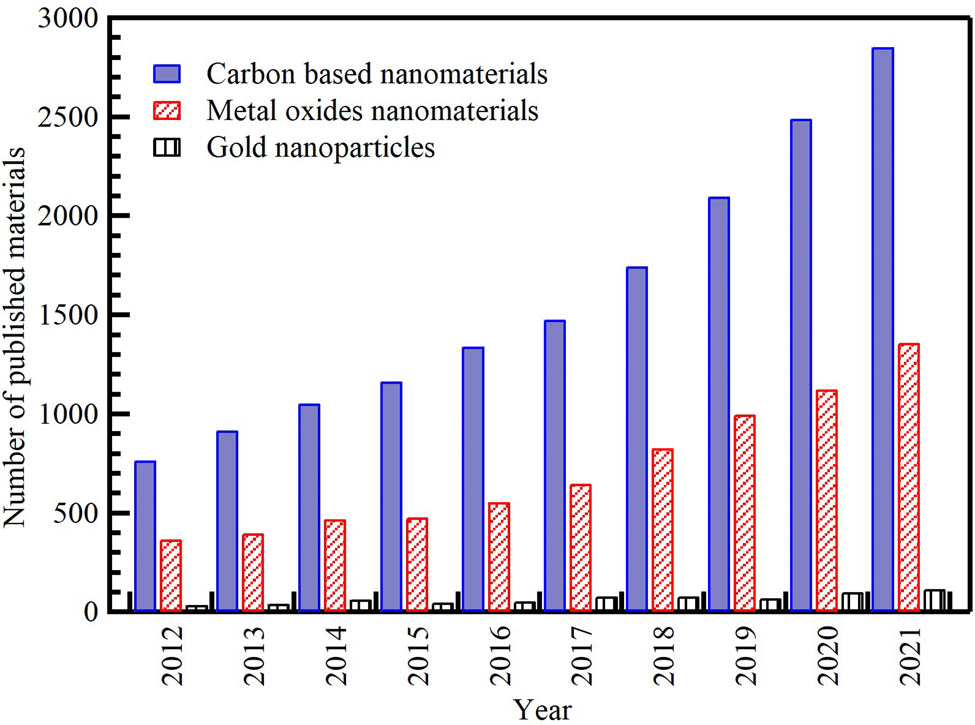
Distribution of the cumulative number of publications on waste-derived NPs per year from the Scopus database (December 2021) according to keywords. Search strategy example: “waste” AND (“carbon nanomaterials” OR “carbon nanofibers” OR “graphene” OR “activated carbon” OR “carbon nanotubes”), metal oxide, gold nanoparticles in the title, abstract, or keywords field.
To meet the demands of modern society and address evolving environmental challenges, new, low-cost, and environmentally sustainable energy conversion and storage solutions must be established. Consequently, science in this area is rapidly progressing. Advanced materials are crucial for the high-efficiency conversion of clean and renewable energy to electrical energy and high energy density electrical storage that can be effectively recycled from waste. The characteristics of materials used in these applications significantly impact their performance. Figure 2 shows a bar chart of publications on waste-derived NMs in storage and conversion energy applications over the past 10 years. Between 2013 and 2021, there has been a large increase in publications for both applications. The number of publications for energy storage applications, such as batteries and supercapacitors, rose from 298 in 2012 to 1,584 in 2021. Whereas the number of publications for energy conversion applications, such as fuel cells, solar cells, green H2 production, and CO2 reduction, steadily rose from 643 in 2012 to 2,080 in 2021.
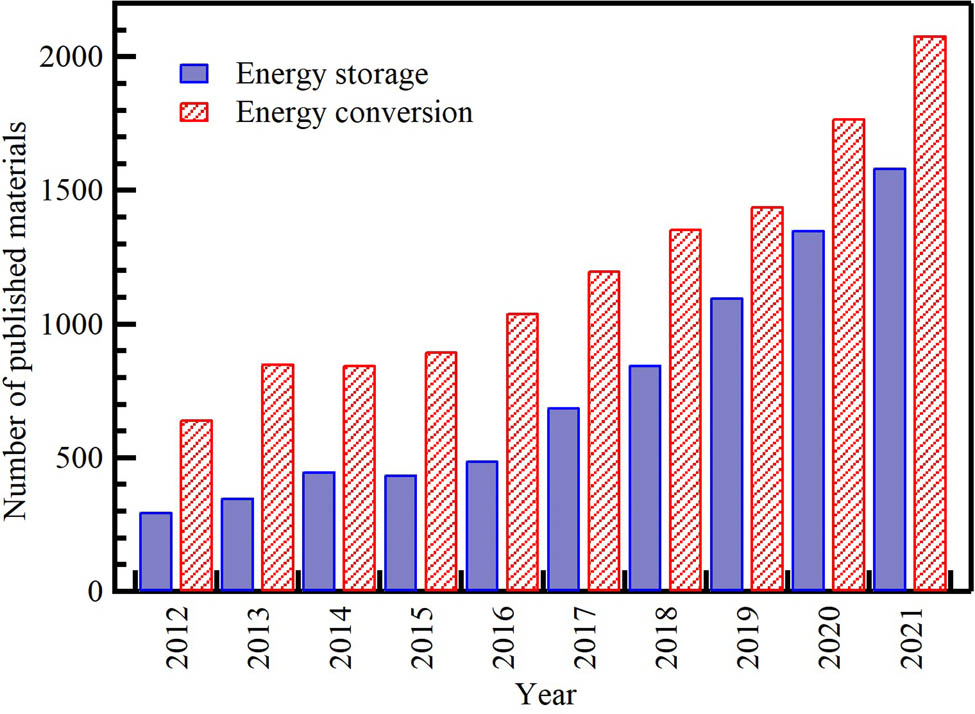
Distribution of the cumulative number of publications on waste-derived NP applications per year from the Scopus database (December 2021) according to keywords. Search strategy example: “waste” AND (“energy storage applications” OR “batteries” OR “supercapacitors”), (“energy conversion applications” OR “fuel cells” OR “solar cells” OR “green H2 production” OR “CO2 reduction”), in the title, abstract, or keywords field.
Generally, there has been an upward trend in waste-derived NMs for energy applications. The scientific community’s interest has focused on energy conversion applications from 2013 to 2016, with 327 publications in 2013 and 108 for energy storage. However, this situation later improved; since 2017, there has been a large spike in articles on energy storage. This substantial rise in the overall number of publications may be due to innovations in waste management, which have occurred in tandem with technological advancements and innovations, especially in electronics. Lithium and Ni–MeH battery systems follow a similar path for the transportation sector and the power grid.
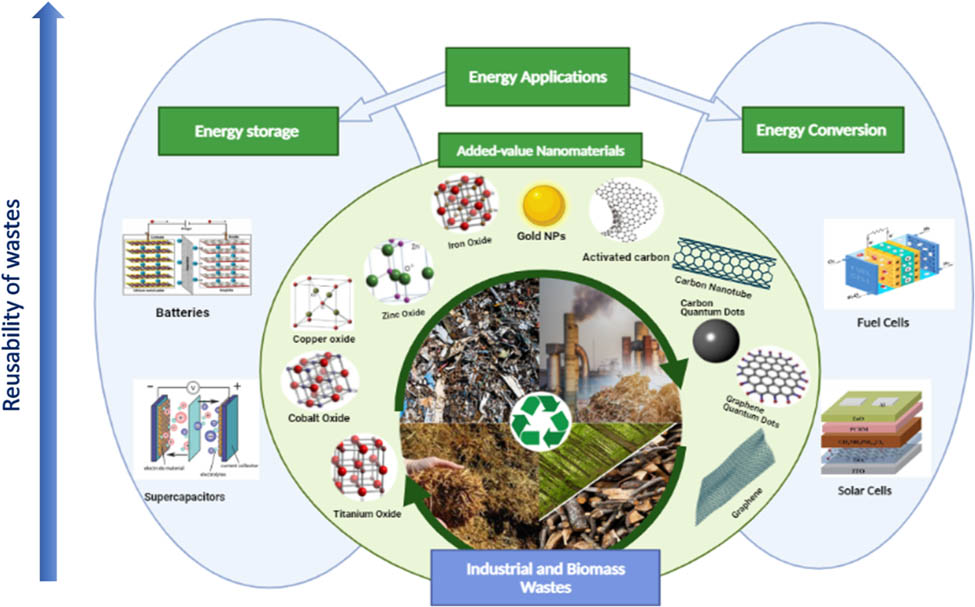
The current review aims to thoroughly evaluate the status, challenges, and future directions for domestic and industrial waste recycling as suitable input to produce NPs as added-value products. Herein the proprieties and the currently available synthesis techniques of NMs (e.g., metal oxide NPs, CNMs, AuNPs, and inorganic QDs) to critically analyze their efficiencies and drawbacks are explored. Various pretreatment methods for wastes are considered. Furthermore, the commercial viability of waste-derived NMs and several proof-of-concept implementations in advanced technologies, especially energy storage and energy conversion applications (e.g., batteries, supercapacitors, fuel cells, and solar cells), are emphasized. Literature classified the recycled NMs for energy storage and conversion into three categories: CNMs, AuNPs, and metal oxide NPs. The primary scheme of this review is summarized graphically in Figure 3. Finally, this study addresses knowledge deficiencies in literature by carefully evaluating this novel and forward-thinking research subject. Overall, results from this literature review indicate a need for continued research that focuses on the critical aspects required for scaling up production and application of waste-derived NMs. By thoroughly evaluating this emerging and forward-looking research issue, our review fills in the information gaps in the literature.
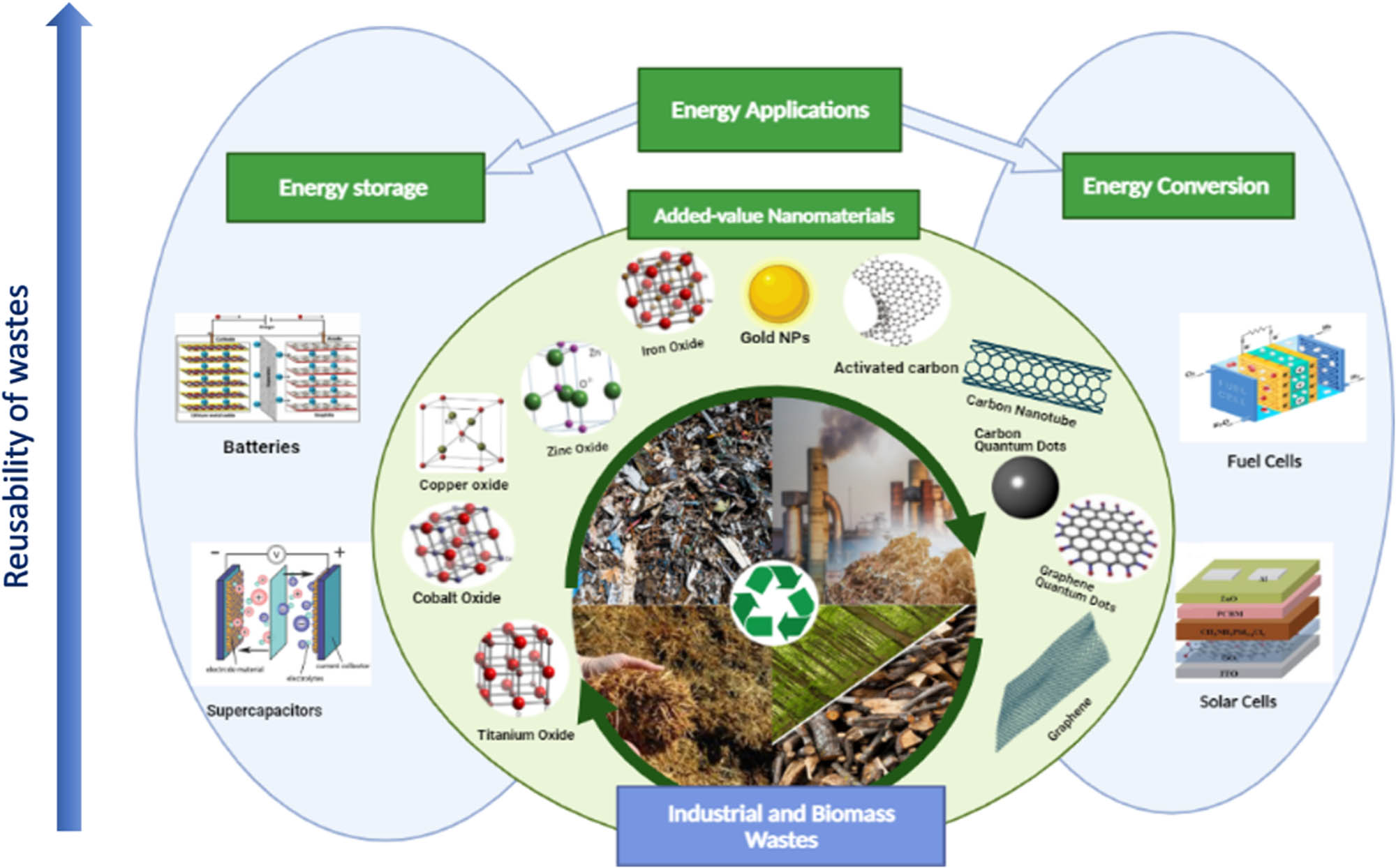
Schematic of recycled NMs from biomass and industrial wastes and their energy applications.
2 Synthesis and properties of nanomaterials recycled from biomass and industrial waste
Nanomaterials are a broad category that includes particulate compounds ranging from 1 to 100 nm with unique physical and chemical characteristics, large specific surface area, and nanoscale size. According to reports, their optical properties are influenced by their size. Their distinctive size, shape, and structure influence reactivity, robustness, and other features. Due to their properties, they are excellent candidates for residential and commercial applications, including catalysis, imaging, medical applications, energy-based research, and environmental applications. NMs might be classified into various groups depending on their physical and chemical properties.
Carbon-based nanomaterials, metal oxide-based nanomaterials, and gold nanoparticles are interesting and have applications in biology, medicine, industry, and energy. It is critical to conduct more research on the progress of recycled NMs derived from biomass and industrial wastes. Diverse sorts of NMs from various sources are summarized in the present review. Up to now, the excellent potential of recycling waste materials into value-added NMs is a hot topic. This section provides a complete, critical, and easily understandable overview of this original trending issue and a correlation between the synthetic procedure and the resulted properties of the fabricated nanomaterials. The first step in fabricating NMs from waste materials is selecting suitable waste detailed in this section. Additionally, several synthetic techniques have been employed to generate the final product of NMs, and the detailed methods for each type of NMs are discussed well in this section.
Waste management is requested to minimize the waste, reduce the environmental impacts, and create a source of added value NMs. Various waste materials have been recycled for the initial feedstock to produce NMs. In the past few years, researchers have synthesized NMs, such as metallic/carbon NPs, CNTs/nanosheets, AC, and nanofibers, from industrial or biomass waste residues. More recently, recycled NMs from cooking oil, biomass, and industrial waste and their subsequent applications have become highly attractive. There are many resources to produce NMs that can be simply categorized into biomass and industrial wastes. Indeed, the proportion of industrial and household wastes varies from country to country because of variations in economic activity [11].
More than 220 billion tons of biomass residues, each year, from which a significant amount of lignin could be extracted. [12]. This includes all biologically generated materials used to characterize all living things on the planet. Energy crops, crops, residues, timber and wood wastes, municipal and animal wastes, aquatic plants, and algae are plentiful biomass resources (Figure 4).
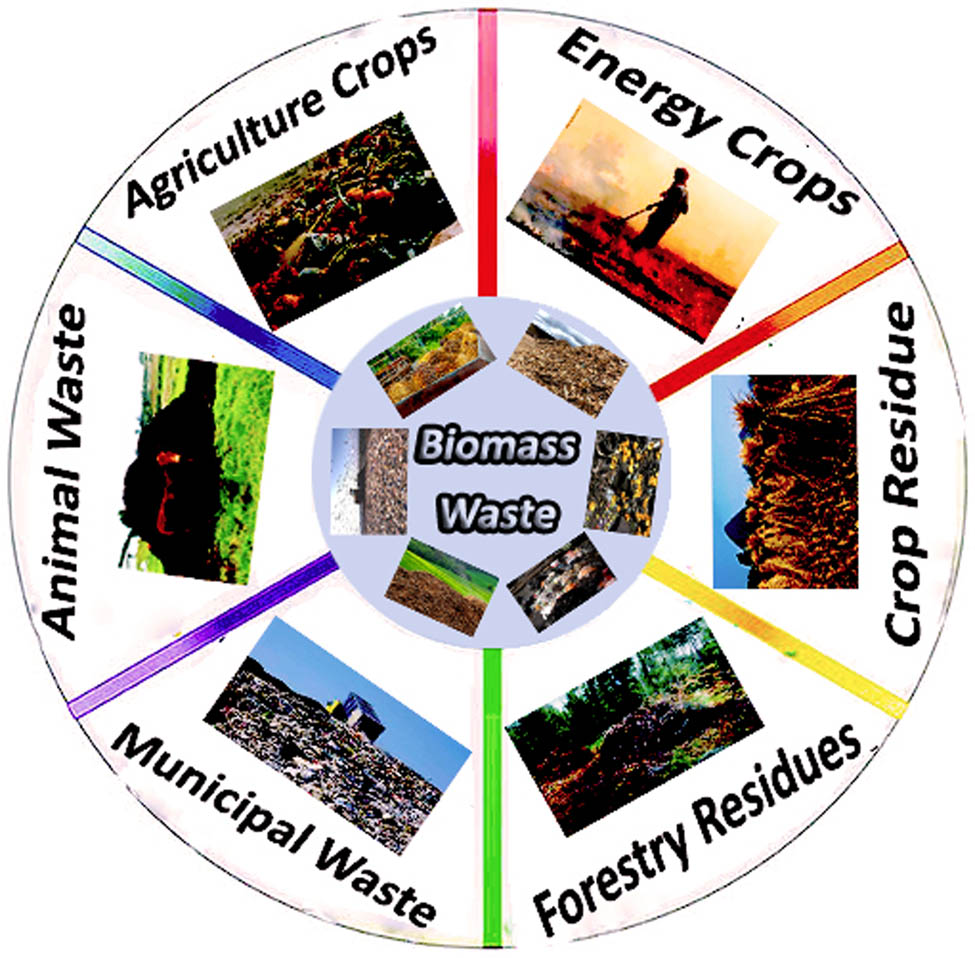
Schematic of biomass resources in nature.
Biomass waste can be converted into NMs with several benefits. A simple pyrolysis process, for example, can turn tea waste into biochar for use as a fluoride adsorbent. Sugarcane and camphor were also used as precursors to produce graphene oxide (GO) and CNTs. Carbon-based structures are the most adaptable materials for current renewable energy and natural science technology [13]. The use of biomass as a raw material in the manufacture of NMs has been praised as a biobased economy and green solution to local and global pollution problems. Agricultural waste biomass, for example, has gained international recognition as a low-cost resource for NM synthesis [14]. Since carbon-based materials can be made from renewable biomass feedstocks, they are considered sustainable.
Nanoelectronics, biomedical research, renewable solar energy, drug-gene delivery, thermal insulation, and other fields have potential applications using metal oxides, nanoporous materials, and NPs. However, scientists must recognize that agricultural waste biomass-assisted synthesis is a less expensive, environmentally sustainable, and renewable technique. Because of their specific properties, the development of noble metal NPs, such as AuNPs, is of great interest. Manipulation of their size and form results in unique properties that could be useful in semiconductors [15].
Generally, industrial waste is classified into two categories: nonhazardous and hazardous. Nonhazardous industrial waste, such as cartons, plastics, metals, and organic waste (Figure 5), is waste from industrial activity that does not threaten public health or the environment. Conversely, hazardous waste, of which only approximately 10% of the 280 million tons of plastic generated each year in the world is recycled, is a byproduct of industrial operation that poses a danger to public health or the environment, such as flammable, corrosive, active, and toxic materials. According to reports, just 3.8% of total industrial waste in Europe (EU-28) was listed as hazardous waste. Waste composition in industrial sectors in Europe (in 2014) and waste from households (MSW) accounted for just 8.3% of total waste, emphasizing the dominance of industrial waste.
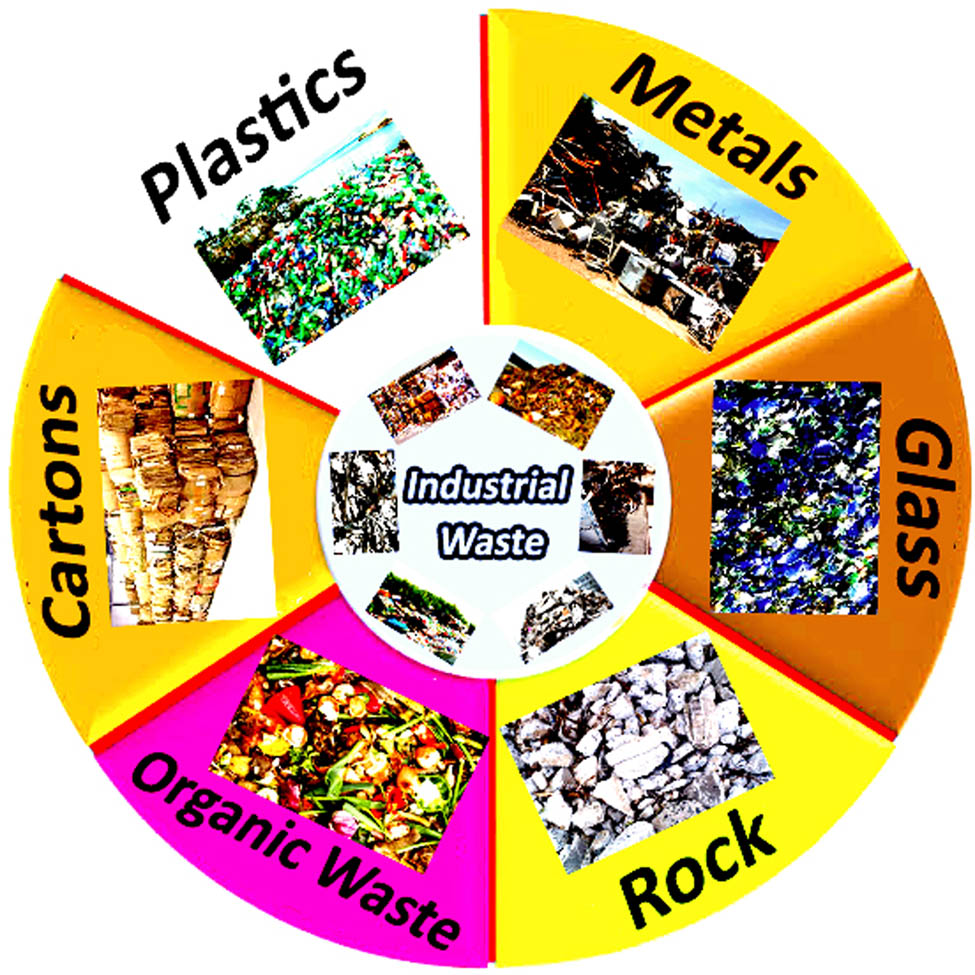
Schematic of industrial waste in nature.
NMs can also use a range of industrial wastes as initial feedstocks, including batteries, polymers, and tires. Consequently, NM processing from various industrial wastes may be a long-term recycling process that protects the environment. China, for example, generates between 50 and 60 million units of waste lead batteries per year. Because of their insolubility, lead pastes primarily contain lead dioxide, lead sulfate, and lead oxide, which are difficult to recycle. Many spent lead batteries are currently being made, and recycling is one of the best ways to address this issue.
In China, Japan, Korea, and the United States, Zn–Mn batteries account for more than 90% of annual portable battery sales. China manufactures over 15 billion Zn–Mn batteries per year. Exposure to high levels of Zn will delay calcium absorption, resulting in lower Ca levels in the body. Consequently, recycling Zn from Zn–Mn battery waste encourages resource sustainability and avoids the introduction of these pollutants into the environment. Zn–Mn battery waste recycling has been extensively studied to produce various NMs, such as Zn NPs, nanofibers, and flaky NMs [16].
In summary, several sources of waste have been utilized as a precursor for the production of NMs, concerning two categories: biomass and industrial wastes. Figure 6 summarizes the classification of the fabricated NMs from sustainable waste.
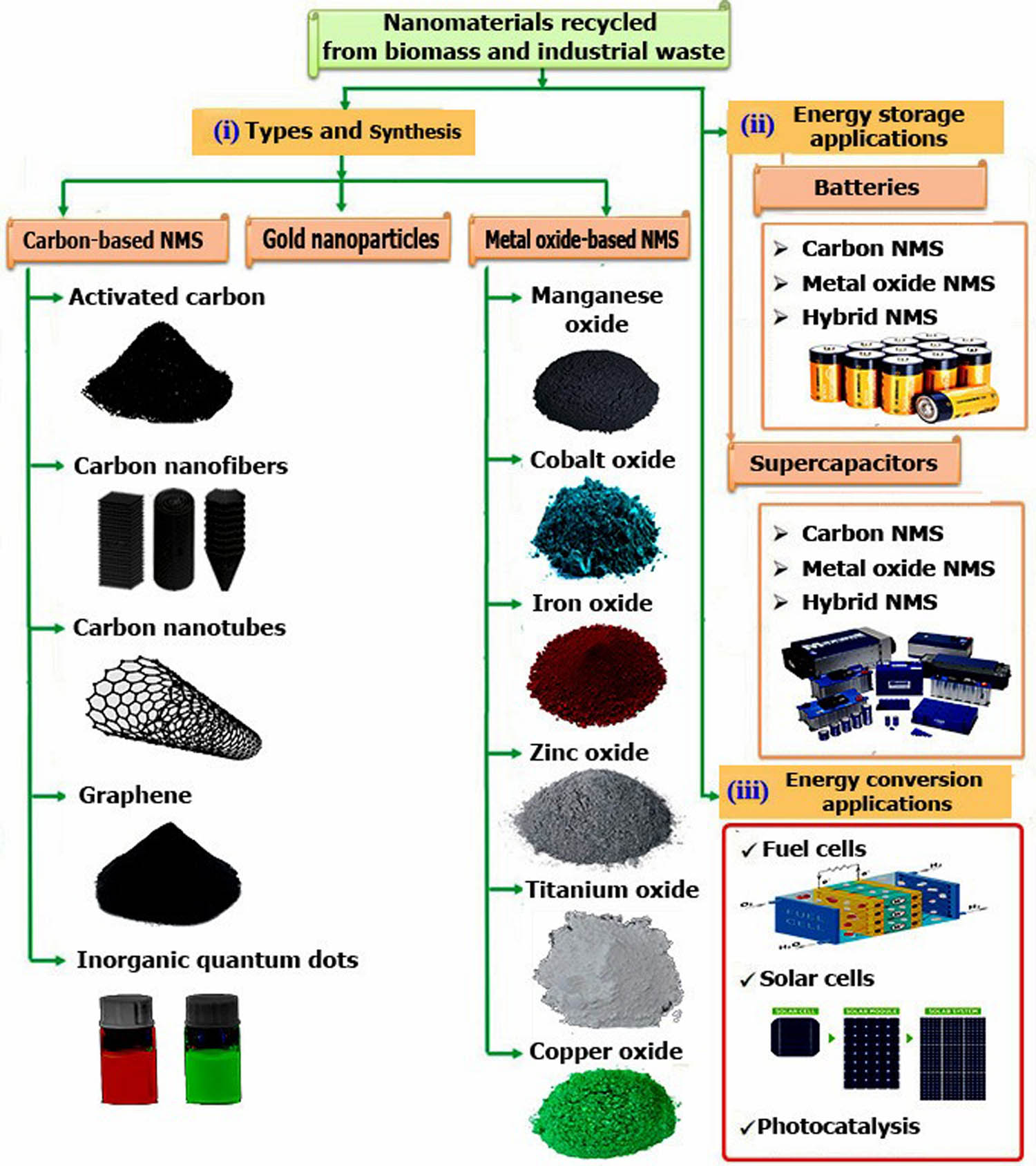
Classification of nanomaterials from wastes.
2.1 CNMs
CNMs fabrication still faces challenges in the exploration of new resources. These challenges are based on improving CNMs’ properties to obtain high-quality and high-purity materials, while meeting the current demands of energy applications [17,18,19,20]. In this respect, a search for new resources is required, increasing different morphology types while considering other factors. Various CNMs fabricated using different resources are discussed in detail with this motivation. The main sources for these CNMs are biomass and industrial waste (Figure 7). CNMs are classified into four categories: AC, carbon nanofibers (CNFs), CNTs, and G.

Types of carbon nanomaterial obtained from recycled waste.
2.1.1 AC
AC is a nongraphitic material and is one of the most famous carbonaceous materials because of its high specific surface area, distinctive pore size distribution, high degree of surface-active sites, and physicochemical stability [21,22,23]. Three recycled source materials that produce AC are biomass, industrial waste, and agro-industrial waste. Numerous biomass wastes have been explored for AC production. The greatest advantage of biomass waste is creating AC via various plant types, cellulose, hemicellulose, and lignin constituents. Hemicellulose in biomass partially hydrolyzes at low temperatures, resulting in the formation of AC out of polymerization [21].
Because of the ban on environmentally harmful palm waste disposal mechanisms, especially in the palm milling industry, challenges of palm waste should be managed by converting it into valuable materials, like AC [23,24,25]. Porous AC (PAC), associated with mesoporous and microporous properties, is also strongly dependent on the utilization of the biomass type and synthesis methodology [26,27,28]. The conversion of fruit peel wastes into PAC is interesting to activate alkalis like H3PO4 [29]. Interestingly, PAC nanosheets are derived from leaves, such as Syzygium oleana leaves, via pretreatment with KOH or NaOH before the pyrolysis process [30,31]. For the development of 3D biomass PAC, three standard biomass waste precursors have been chosen as carbon sources: bagasse, wheat straw, and wood shavings [32]. Mangrove and waste palm trunk (WPT) have been used as low-cost and sustainable precursor materials for the development of AC, which can be used in various applications under different carbonization and activation conditions. The surface area of AC derived from WPT was 1,402 m2 g−1, whereas that derived from mangrove was 2,131 m2 g−1 at the same activation conditions [33].
Coal as waste material has been used for the synthesis of AC. Recent research investigated the possibility of producing alternative and cheap triggering agents to improve the adsorption properties of AC using mine coal [34]. Rubber tire wastes are non-biodegradable and harm climate and land management. It was suggested that this material could be used in a management strategy for diverse applications, like energy and carbon production [35]. In one study, plastic waste (PW) was separated from the dry waste of an institute, which primarily consisted of packaging and laboratory waste. Polypropylene (PP), polyethylene terephthalate (PET), high-density polyethylene (HDPE), and low-density polyethylene (LDPE) are among the thermoplastic polymers used in PW [36]. End-of-life flexible printed circuit boards (PCBs) typically have a polyamide (PA)-based substrate with metal foils, such as copper, interconnecting the circuits.
Several resources are available for this process, including sawdust, wood–plastic, and leather solid wastes (LSWs). Sawdust is ideal for fabricating carbonaceous NMs because the carbon content from sawdust varies from 77.51 to 93.59%, with ash content as low as 0.08%. Agro-industrial wastes are also used as alternatives for the production of AC. Extrusion, hot pressing, and injection are used to create wood–plastic composite materials [37] from plastics like PP, PE, and polystyrene (PS), and biomass fibers like rice husk (RH), corn stalk, and peanut shell. By increasing the carbonization temperature, the surface area of biochar is increased, reaching a maximum value of 518.72 m2 g−1 [38].
Nagaraju et al. [39] successfully used naturally available pine cone flowers, which have abundant carbon contents, as biomass to prepare honeycomb-like porous AC powder with meso/macropore structure and porous 3D properties. Nagaraju used chemical activation with KOH and pyrolysis processes under Ar inert gas atmosphere. The synthesized sample was uniformly coated on fluorine-doped tin oxide (FTO) glass using a smooth brush. Compared with commercially available AC, the obtained results were improved (Figure 8).
![Figure 8
Schematic of (a–d) porous AC fabrication and (e–f) dye-sensitized solar cell preparation process with porous AC-coated FTO glass. Copied with permission from ref. [39]; Elsevier, 2017.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_008.jpg)
Schematic of (a–d) porous AC fabrication and (e–f) dye-sensitized solar cell preparation process with porous AC-coated FTO glass. Copied with permission from ref. [39]; Elsevier, 2017.
2.1.2 CNFs
CNFs resemble CNTs in structure but have more advantageous properties, such as decreased cost and ease of synthesis. The properties of CNFs make them the principal substituting blocks for the synthesis of CNFs at the nanoscale [40,41]. CNFs structures appear hollow, porous, smooth, helical, stacked with a high specific area, with good electrical and thermal conductivity with low weight, and are primarily used in energy and environmental sciences [42].
CNFs biomass waste is considered one of the most valuable CNM sources. It can be extracted from biomass waste sources, like bamboo, palm kernel shell, and pine nut shell-derived char. The process for determining extracted species is pyrolysis, wherein the process temperature is important to release different constituents of the CNMs [43,44].
CNFs are generated from pyrolytic bio-oil and biogas, depending on the heat extraction method [45,46]. The bio-oil microwave method’s production of phenols, methoxy phenols, substituted methoxyphenols, naphthalene, benzene, and alkenes leads to the fabrication of CNFs. Additionally, the biogas microwave-assisted pyrolysis (MAP) process, i.e., CO and CO2, leads to hollow CNFs, like those extracted from pine nut shells and palm kernel shells, at low temperatures via isothermal oxidation [46].
The extraction of cellulose nanofibrils is usually applied for various biomass, such as soft and hard woods, corn husk, and banana peel [47,48]. Waste-tire pyrolysis oil (TPO) and coal fly ash (CFA) are used to synthesize CNFs/CNTs [49]. TPO acts as a carbon precursor and is one of the most important byproducts of waste-tire thermal degradation in an oxygen-free environment [49]. The presence of iron (in the form of oxide) in the CFA is a catalyst that aids in growth [50].
Nitrogen-doped CNFs with open channels can be prepared using a simple electrospinning method with subsequent two-step carbonization using polyacrylonitrile, waste poly(vinyl butyral), and urea [51]. Also, using waste HDPE, self-prepared micron-sized silicon/CNFs/carbon (Si/CNFs/C) composite has been fabricated with pyrolyzed carbon and CNFs [52].
Coal liquefaction residue waste has been used to prepare CNFs films via electrospinning [53]. Also, coal-derived CNFs can be obtained from powder River Basin coal via electrospinning; the raw coal is depolymerized to form coal chars that are then combined with polymeric precursors (polyacrylonitrile [PAN]/polyvinylpyrrolidone [PVP]) to form a homogeneous solution for fabrication [54,55,56].
Zhang et al. [57] synthesized CNFs decorated with a carbonized loofah from loofah sheets using in situ chemical vapor deposition (CVD). The CNFs were used for water purification via solar energy due to the high lighttrapping capability of the CNFs. The loofah sheets were first carbonized at 500°C, washed with alcohol, dried, and then soaked in a dopamine-based solution. The polydopamine-coated carbonized loofah was further carbonized at 800°C and then impregnated with Ni(NO3)2 as a catalyst. Lastly, the sample was placed in a CVD furnace with a gas flow of N2 and ethanol. The CNFs showed a degree of crystallization with a lattice spacing of 0.34 nm and a diameter of 20 nm. X-ray diffraction (XRD) analysis confirmed the crystal structure of the carbonized loofah CNFs.
Cao et al. [58] used alkali lignin as a renewable carbon source, mixing it with PAN in a solution as a carbon precursor for CNFs preparation. It was blended with Sn-based PVP solution and then electro-spun at 23 kV at fixed temperature and humidity. Afterward, the spun nanofibers were preheated before being carbonized at 800°C. The result was porous CNFs with SnO x nanonodules distributed homogenously throughout the composite. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used to characterize the composite morphology (Figure 9), whereas XRD and Brunauer–Emmett–Teller (BET) surface area analyses were utilized to study the crystal and pore structure of the composite, respectively. Additionally, two- and three-symmetric electrode systems were built to test the electrochemical performance of the composite for supercapacitor applications.
![Figure 9
(a) SEM and (b) TEM images of CNFs. Copied with permission from ref. [58]; Elsevier, 2021.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_009.jpg)
(a) SEM and (b) TEM images of CNFs. Copied with permission from ref. [58]; Elsevier, 2021.
2.1.3 CNTs
CNTs have attracted attention because of their superior electrical, thermal, mechanical, and optical properties. Synthesis of CNTs from renewable resources will reduce costs and make the world more sustainable [59]. CNT feedstocks include biopolymers or bioderived chemical compounds, such as essential oils. CVD has completed the fabrication of CNTs from essential oils in the presence of ferrocene as a catalyst. Many precursors act as bio-feedstocks to produce CNTs. Palm oil is not a good carbon precursor for the production of CNTs [60]. However, poor quality CNTs are obtained from sunflower, palm, and sesame oils (I D/I G ratio of 1.00), which indicates that these precursors are not ideal for CNT synthesis.
There are other essential oils available to produce high-quality CNTs. The production of multiwalled carbon nanotubes (MWCNTs) by turpentine oil was discovered by Afre et al. [61]. High-quality MWCNTs were fabricated on quartz rather than silicon via CVD [62]. MWCNTs can easily be created using camphor oil in the presence of ferrocene [63]. According to Awasthi and coworkers, MWCNTs with 20 and 60 nm diameters were fabricated with castor oil [64]. Also, bamboo-shaped N-doped MWCNTs were produced by castor oil in the presence of ammonia [65].
Cobalt catalyzed carbonization of biomass chitosan results in the formation of cobalt/nitrogen-doped CNTs. The presence of cobalt causes a transition from graphene-like carbon nanosheets to tubular graphitic carbon [66]. Biochar is a carbon-rich porous material made from thermochemically treated biomass. Microwave-assisted heating makes CNTs from a mixture of biochar and ferrocene [67]. The coconut shell is an example of a mineral-rich biomass natural resource. By using the mineral content in the source material as catalysts for CNT growth, MWCNTs have been synthesized over coconut shell-derived charcoal pyrolyzed at 900°C [68].
Plastic wastes are considered as a main source of CNMs because of their high amounts of carbon. The most common waste plastics are PE and PP. Plastics are first decomposed in a pyrolysis reactor, and then CNTs are synthesized in a separate reactor using a catalyst. Catalyst growth is a major challenge for increasing CNT amount and quality [69].
High quality and quantity of CNTs can be obtained using 5 wt% of Mn in Fe-based catalyst via catalytic pyrolysis of PP rather than a Fe-based catalyst [69]. Therefore, selecting catalysts, especially bimetallic catalysts, is important for the fabrication of high-quality CNTs. MWCNTs have been produced by single-stage chemical vapor decomposition using PP in the presence of bimetallic Fe–Mn/Al2O3 catalyst [70].
CNTs can be manufactured from PE wastes using heavy metal-support interaction, e.g., La0.8Ni x Fe1−x O3−δ , at high temperatures [71]. Also, CNTs can be manufactured from PE waste at 800°C using a spherical alumina-supported catalyst, the diameter and yield increasing significantly as the Ni content increases [72].
A mixture of PP and LDPE was also used as the main source of CNTs using a two-stage fluidized catalytic bed reactor system with a fraction of 48 wt%. In one study, temperatures of the first- and second-stage reactors were regulated at 600 and 800°C, respectively [73]. MWCNTs can be obtained by using a pure mixture of LDPE, PP, and mixed plastics (MPs) over a Ni-based catalyst at two different temperatures, 500 and 800°C [74].
Coproduction of H2 with high yield and CNTs with high quality can be prepared from the pyrolytic product of waste tires using Ni supported on AC as a catalyst [75]. Single-walled carbon nanotubes (SWCNTs) with very efficient yield have been synthesized using vulcanized scrap rubber via thermal CVD at 850°C on suitable catalytic systems, such as bimetallic oxides of Fe and Ni supported on zeolites [76]. Also, well-defined CNTs can be obtained from scrap rubber by thermal aging the rubber at 90°C for 14 days before CVD using Fe–Ni–Cu/MgO as a catalyst and a growth temperature of 750°C applied for 60 min [77]. The stress buffer layer shell that occurs on the surface of micron-sized silicon waste is a CNT. A high-performance Si/nano-Cu/CNT/C porous structure was obtained from photovoltaic silicon waste by integrating nanocopper-assisted chemical etching with graphite and CNT coating technology [78]. CNTs with a high degree of graphitization can be obtained using a red mud sample by alkali treatment followed by fabrication of bio-composite films of PVA and, finally, the pyrolysis of the composite film at 500°C [79]. The floating catalyst CVD approach offers a continuous single-step process to synthesize aerogel CNTs using waste engine oil as a carbon source in a ferrocene catalyst at a temperature of 1,150°C [80].
Wu et al. [81] have shown that the same process from which hydrogen is produced from the plastic can be utilized to create cost-effective CNTs as a byproduct in NiMnAl-based catalysts. The examination of the resulting mixture showed that the carbon yield depends on the molar ratios of the NiMnAl catalyst used. NiMnAl with a molar ratio of 4:4:4 (Ni:Mn:Al) had a higher carbon yield (57.7 wt%) and a slightly higher CNT yield (91.2 wt%). However, CNTs formed using NiMnAl with a molar ratio of 4:2:4 had better uniformity and higher crystallinity overall. CNTs produced this way were shown to improve the mechanical strength of LPDE significantly. The CNT–LPDE composite had more stiffness than virgin LPDE and improved dynamic load handling abilities. The overall process is also cost-effective, which opens the door to potentially wide industrial applications.
Biomass-based methods for CNT formation have also undergone considerable developments over the last few years. Hidalgo et al. used biochar as a precursor for synthesizing CNTs by microwave irradiation [82]. Their method involves heating a mixture of biochar and ferrocene in a microwave reactor operating at 2.45 GHz, 80°C, 200 W, and 17 psi. Different kinds of biomass from different agricultural wastes were pyrolyzed to form different biochar samples, then used as carbon precursors in the synthesis process (Figure 10). The different biochar samples had diverse surface areas, pore volumes, aromaticity, etc., depending on the agricultural waste used and the pyrolysis conditions, which affected the characteristics of the CNTs produced. Biochar samples obtained from hazelnut hull and wheat straw pyrolyzed at 600°C yielded CNTs with overall higher quality. Biomass gasification also generates a carbon-rich mixture of gases, such as CO, CO2, and CH4 [83].
![Figure 10
SEM images of carbon products from various sources. Copied with permission from ref. [84]; Elsevier, 2018. (a) CNTs from CH4 decomposition process (P
CH4
= 0.2 atm), (b) CNTs from CO decomposition process (P
CO = 0.2 atm), (c) CNTs from CO/CH4 decomposition process (P
CO/P
CH4
= 0.5), and (d) CNTs from CO/CH4 decomposition process (P
CO/P
CH4
= 2).](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_010.jpg)
SEM images of carbon products from various sources. Copied with permission from ref. [84]; Elsevier, 2018. (a) CNTs from CH4 decomposition process (P CH4 = 0.2 atm), (b) CNTs from CO decomposition process (P CO = 0.2 atm), (c) CNTs from CO/CH4 decomposition process (P CO/P CH4 = 0.5), and (d) CNTs from CO/CH4 decomposition process (P CO/P CH4 = 2).
2.1.4 Graphene
Graphene and its derivatives can be synthesized from unused waste material to produce novel materials. There are four main categories of recycled waste material sources to synthesize graphene and its derivatives: biomass, plastic, industrial, and other domestic wastes. In the following sections, each source will be discussed individually.
Graphene and its derivatives can be synthesized using different biomass wastes, such as RH, hemp fiber, sugarcane bagasse, glucose, and chitosan. The merits of using recycled agriculture wastes are their simplicity, environmental friendliness, high crystallinity, and the lack of toxic gas emissions during synthesis. RH contains more than 70% of carbonaceous components besides its silica (15–20%), lignin (25–30%), and cellulose (50%) constituents [85]. For high-purity graphene materials, a pretreatment process with a strong alkali, like KOH or NaOH, is required to eliminate SiO2 impurities [86].
Farmers burn hemp fibers every year; therefore, it is important to decrease this biowaste by benefiting from the availability and eco-friendliness of hemp fibers to produce distinctive materials, like graphene and its derivatives [87]. Hemp has been hydrothermally treated and accompanied by KOH activation to create extremely porous graphene materials [87].
Glucose is considered the only renewable monosaccharide plant-based source of carbon in nature produced simultaneously in plants’ photosynthesis process. Researchers used glucose as a raw material for the processing of graphene and its derivatives because of its uniqueness in nature [13,88].
Sugarcane bagasse is one of the main sources of glucose for the production of graphene and its derivatives [89]. The most common fabrication technique for graphene and its derivatives from sugarcane bagasse is the direct oxidation under a muffled atmosphere with ferrocene as a catalyst. Using a pyrolysis technique combined with a simple treatment, different forms of graphene, such as N-doped graphene, have been manufactured from chitosan [90].
Plastic bags and plastic water bottles (PET) are massively used today. All packaging materials formed of different polymeric composites are collected together as layers to form plastic materials with an inner layer of aluminum [91]. PE has been recycled to prepare promising graphene/mesoporous carbon composites for energy storage devices through the monitoring of graphene oxide (GO) during a lower temperature carbonization process [92]. High-quality graphene flakes have been prepared using PP waste while organically catalyzed with montmorillonite [93]. High-quality single-layered graphene can be made from PS and other carbon-containing wastes. Additionally, multilayer graphene sheets were prepared from waste expanded PS using FeCl3 as a catalyst [94].
High-quality monolayer and multilayer graphene was successfully fabricated using synthetic polymers, such as polymethylmethacrylate (PMMA), polyimide, and waste plastics, as the carbon precursor, whereas hydrogen gas acts as a reducing agent [94]. PMMA film was also used as a starting medium for CVD-grown graphene sheets on sapphire substrates with Cu catalysts [95].
High-quality graphene from PET waste was developed without any catalyst [96]. The treatment of PET wastes involves two main steps before forming carbon-based materials: grinding and crushing. Pyrolysis-generated mixed PWs are a major source of bulk graphene nanosheets [97]. In one study, PE, PP, and PS combined PWs were obtained from a local city council and second-hand markets, cleaned, and dried. The properties of the produced GO sheets varied from the starting raw materials such that the characteristic properties of the GO sheets were nearly identical to those of highly pure graphene [98,99].
Graphene films were developed from pure coal through a graphitization process that has been identified as simple and cost-effective. Since coal is a carbon-rich material for developing CNTs, fullerenes, and amorphous carbon thin films, it is also used to construct graphene, graphene derivatives, and anthracite coal [100,101]. Graphene materials have been recycled from lead and zinc–manganese batteries, besides some types of transportable batteries. This recycling method was carried out in a heating system where NPs of various morphologies were formed, such as hexagonal prisms, fibers, and sheets [16,102].
A significant number of tires, approximately one billion, are produced globally per year. Studies investigating the control of rubber tire disposal as an economically friendly sustainable source for carbonaceous materials composed of 81.2 wt% carbon [103] are in great demand. The high-value carbon graphene NPs resulting from these studies were derived from waste-tire rubber and exhibited good thermal stability and conductivity [104].
Electronic gadgets (phones, laptops, iPads, etc.) have become essential to everyday life. These electronic equipment comprise diverse mixtures of metals, plastics, glass, and even nanomaterials [105]. PCBs are considered the main constituents of such electronics, and they have been considered a good source for graphene synthesis. The recycling process of valuable and high-quality graphene NMs from high pollutant wastes offers a more energy-saving manufacturing process than extraction from ores [106].
Graphene has been prepared using different low-cost carbon-containing materials from daily use, such as cookies, chocolate, leftover bread, salads, grass, cockroaches, and even animal feces [107]. Although different elements, such as O, N, Fe, S, and P, were present in the primary material sources, the graphene was free of those elements. Thus, the synthesis of graphene from such sources is preferred [108].
Several natural waste materials for the synthesis of graphene and GO, such as tea leaves; coconut shells; peanut shells; fruit peels, i.e., orange, banana, and mango peels [109,110,111]; wood; bagasse; leaves; vegetation waste; fruit waste; and powder soot collected from the exhaust of diesel engine and waste newspapers have been used in graphene sheet synthesis [98]. Tea waste contains various components, such as polyphenol, caffeine, amino acids, and tannins, whereas tea leaf constituents include cellulose, hemicelluloses, lignin, tannins, and proteins [112]. Graphitic carbon can be produced because of the presence of these cellulosic materials by treatment at high pyrolysis temperatures [109].
Nut shell waste is globally used in animal feed or construction materials because of its novelty, low cost, and existence as a useful biological resource. Nut shells are of high carbon content and first produce biochar that can be easily converted into graphene [113]. Plenty of application fields encourage recycling such a low-cost graphene resource as it is used as a bio-adsorber or in metal ion removal and wastewater treatment [114].
Fungus can be treated hydrothermally to produce porous graphene materials; for example, the use of a fungus (Auricularia) with a KOH pretreatment and then a carbonization process. The resultant porous graphene material net structure is used in supercapacitor electrode production [115]. The pulping industry is a rich source of alkaline lignin, which has been used to successfully prepare graphene sheets thousands of nanometers in size [116].
Ding et al. [116] prepare 3D graphene from the precursor of black liquor (Figure 11). The black liquor was collected and filtrated to remove impurities. A 15% weight percentage of black liquor was dissolved in deionized water and heated at 180°C in an autoclave. Then, it was cooled at room temperature and washed with deionized water. Finally, graphene was obtained by centrifugation of suspension.
![Figure 11
Three-dimensional graphene schematic. Copied with permission from ref. [116]; Elsevier, 2020.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_011.jpg)
Three-dimensional graphene schematic. Copied with permission from ref. [116]; Elsevier, 2020.
Three-dimensional graphene comprises tightly combined micron-sized graphene sheets, and the graphene sheets have dihedral angles. Both sp2 and sp3 carbon atoms, with a ratio of 8:1, are present. Three-dimensional graphene with porous carbon, which consists of multilayers of graphene, is of considerable interest in many applications because of its distinctive properties, such as self-supporting, high surface area, high mechanical properties, high electrical conductivity, and a high degree of crystallization [117].
Researchers have also used agricultural waste as biowaste precursors to synthesize graphene to reduce toxic chemicals. Agriculture produces millions of tons of RH per year. Therefore, it is of great interest for the large-scale production of graphene. Kumar et al. [118] have synthesized graphene using RH as the precursor via the microwave method with ferrocene as a catalyst. The RH was washed and dried and, after drying, was mechanically ground to powder and mixed with ethanol using ferrocene as a catalyst [119]. Yeleuov et al. [120] synthesized graphene with RH as a precursor using a two-step chemical method. The graphene was then modified with nickel hydroxide through a simple chemical precipitation method. The properties of graphene provided it with high performance in energy storage applications.
2.1.5 Inorganic quantum dots
2.1.5.1 CQDs
CQDs are a fascinating type of carbon NPs with diameters of approximately 10 nm. From a synthesis point of view, CQDs have been produced from several natural carbon sources or waste organic products; the benefits of using green carbon sources are their cost-effectiveness, eco-friendliness, and wide availability in nature [121,122]. Figure 12 shows the types of waste used to produce CQDs, which will be discussed in detail.
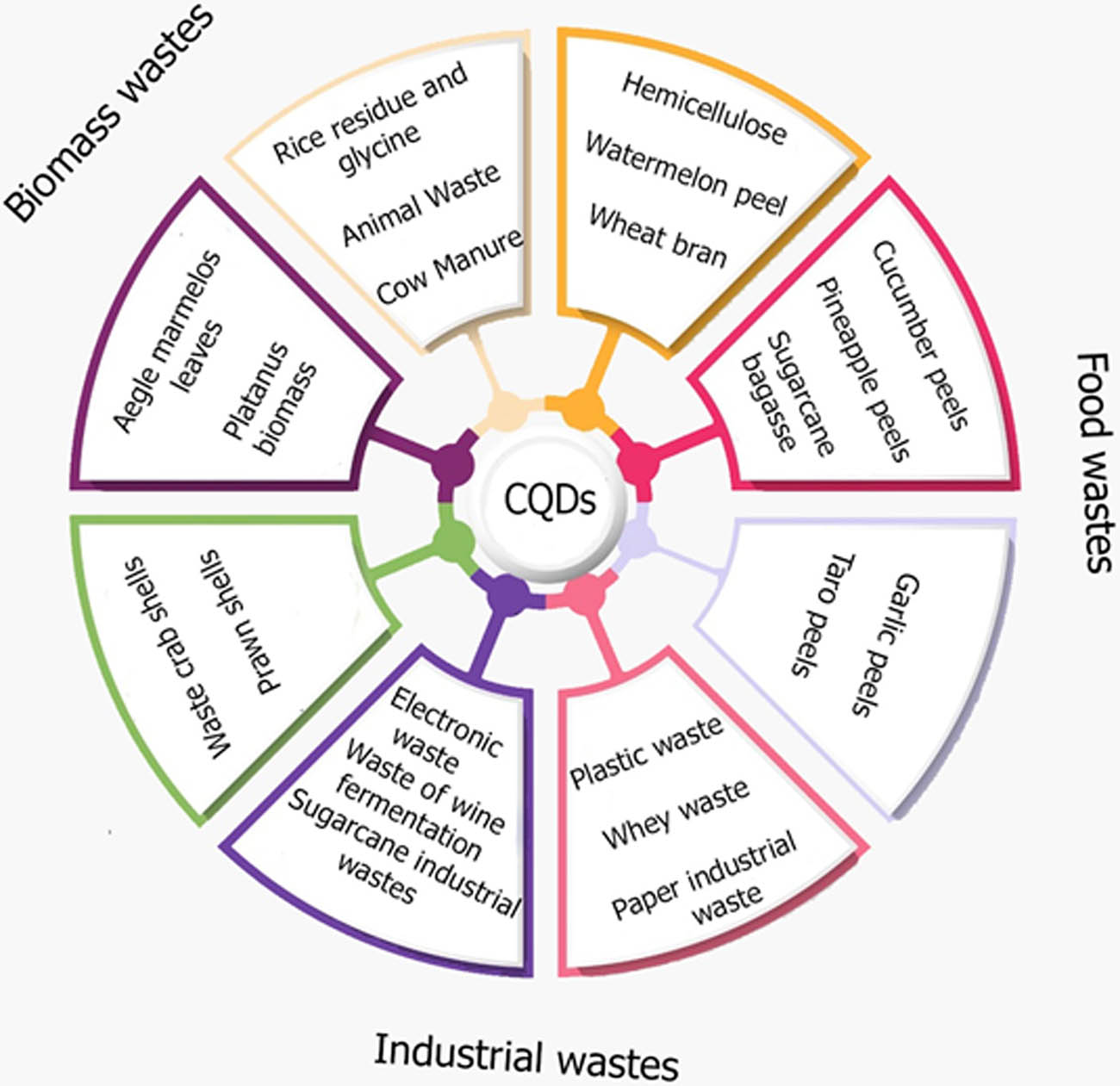
Wastes used as precursors for synthesizing CQDs.
The raw materials needed to produce CQDs from food waste are abundant, such as cucumber peel, pineapple peel, sugarcane bagasse, garlic peel, and taro peel. The type of food waste utilized in the synthesis of CQDs can affect its quality. For example, there were significant variations in essential properties of CQDs obtained from cucumber and pineapple peels using the same synthesis approach [123]. The CQDs obtained from pineapple peel were fully degraded after a few weeks of storage, whereas those from cucumber peel remained stable. Additionally, it was found that CQDs prepared from sugarcane bagasse, garlic peel, and taro peel using the same synthesis process (the ultrasonic-assisted wet-chemical oxidation method) had very different quantum yields (QYs) (4.5, 13.8, and 26.2%, respectively) [124].
From the waste management perspective, researchers have synthesized CQDs from industrial waste to dispose of the waste. There has been a significant advancement in plastic technology in several fields, including electronics, packaging, etc. Because of the negative health and environmental impacts of the inadequate management of PW and the large amounts of waste from plastic industries, it is urgent to convert this waste into CQDs [125,126]. These conversions are obtained from the pyrolysis of wastes of polyolefins. Plastic bottles, cups, and PE bags have been used for CQDs by simple hydrothermal carbonization and have notable QY values of 64, 65, and 62%, respectively, with sizes ranging from 5 to 30 nm [126].
Whey is the liquid remaining after curds have been removed during the cheese-making process [127]. The discharge of watery portions after separating fat and caseins from whole milk creates serious environmental problems. Scientists utilized whey waste, a major dairy, and cheese industry waste product, to resolve these problems into CQDs. Using a facile and environmentally friendly synthetic method and underoptimized synthesis parameters, the CQDs prepared from whey waste exhibited a notable QY (11.4%) and excitation-dependent emission behavior [128].
The paper industrial sector creates large quantities of pulp residual fiber waste that needs further treatment before discharge. In this context, the transformation of such waste into CQD materials has piqued interest in several applications [129]. The formation of tiny CQDs with excellent physicochemical characteristics, such as a QY of 2.7%, particle size of 17.5 nm, and steady-state and lifetime fluorescence, has been achieved by facile microwave-assisted protocol [129].
Additionally, printed office paper has been successfully converted into fluorescent CQDs associated with small particle size, good photostability, high photoluminescence QY (10.8%), and low toxicity [130]. Also, N-doped CQDs from office paper have demonstrated perfect optoelectronic properties, faster response, and better sensitivity in the visible range than undoped CQDs [131]. Interestingly, CQDs fabricated from paper waste from a supermarket showed a significantly higher photoluminescence QY (5.1%) than CQDs prepared from the lignocellulosic residue, with a particle size of approximately 4.8 nm and a high response rate for trinitrotoluene [132].
In recent decades, dumped sugarcane bagasse is soil and earth waste that poses major environmental and health problems, especially in developing countries [133]. Therefore, researchers have utilized industrial sugarcane waste as a carbon precursor to produce highly fluorescent CQDs with an effective QY of 17.98% [134,135].
Discharged batteries, the main component of electronic waste, were used as a precursor for synthesizing uniform spherical CQDs with a particle size of 5 nm and QY of ∼15.3% [136]. White wine lees have also been used as raw material for producing CQDs, exhibiting a QY of 2.53% and a particle size of 10 nm [137].
Biomass is a biodegradable, bio-organic, and abundant element derived from various sources, including agricultural, fishery, animal, and forestry wastes. High-quality and green CQDs have been successfully synthesized using potential valuable byproducts of plant refineries, like hemicellulose and ammonium hydroxide, as solvents. The highest QY of N-doped CQDs produced with these byproducts was up to 16%, higher than undoped CQDs (only 2%) [138]. Watermelon peel is another precursor used to fabricate CQDs and has a particle size of 2.0 nm, strong blue luminescence, satisfactory fluorescence lifetime, and high stability across a wide pH range and at high salt concentrations [139]. Wheat bran has also been used as a precursor for preparing CQDs via hydrothermal treatment. According to fluorescence emission studies, wheat bran CQDs showed the highest fluorescence emission at a wavelength of 500 nm, and the QY of these prepared CQDs was 33.23% [140]. Rice residue and glycine have been used as carbon and nitrogen sources to synthesize N-CQDs using a one-step hydrothermal approach. These N-CQDs showed excellent results as a probe to detect Fe3+ and tetracycline (TC) antibiotics, with a maximum emission at a wavelength of 440 nm and a high QY percentage (23.48%) [141,142]. A single-step hydrothermal carbonization method has been used for the green synthesis of water-soluble monodisperse CQDs with coconut husks as the carbon precursor. Hydroxyl and carboxyl functionals, which play crucial roles in surface passivation and result in more stable NP dispersion, were confirmed by Fourier transform infrared (FTIR) spectroscopy on the surface of the CQDs [143]. Waste tea leaves and peanut shells were also carbon sources used to synthesize CQDs by the one-step hydrothermal method. These CQDs showed high stability and high QY. There was no discernible morphological distinction between tea CQDs and peanut shell CQDs; both had diameters of 7–9 nm, uniform dispersion, and spherical shape, indicating that they were more stable in water [144].
Recently, numerous attempts have been made to upcycle polymeric waste to reduce polymer waste buildup. Chaudhary et al. [145] have reported a sustainable process to convert PW from bottles, cups, and PE bags through facile heating to create FL-CQDs (Figure 13). The XRD of the CQDs showed crystalline characteristics. Also, the CQDs possessed crystallite sizes ranging from 7.5 to 28.3 nm.
![Figure 13
Schematic displaying the production of FL-CQDs from various types of plastic waste. Copied with permission from ref. [145]; Elsevier, 2020.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_013.jpg)
Schematic displaying the production of FL-CQDs from various types of plastic waste. Copied with permission from ref. [145]; Elsevier, 2020.
Biowaste Aegle marmelos leaf powder synthesized CQDs via a hydrothermal carbonization process. The obtained CQDs exhibited a high photoluminescence QY of 22% and, in the presence of a wide range of ionic (KCl) concentrations, revealed their suitability for in vivo and in vitro biosensing applications [146]. Using a pulsed laser ablation method, n-doped microspore CQDs (NM-CQDs) were produced from waste Platanus biomass. The prepared NM-CQDs exhibited a high QY and fluorescence lifetime (32.4% and 6.56 ns, respectively). Furthermore, stable emission behaviors have been obtained for NM-CQDs under various conditions, including varying ionic salt concentrations, temperatures, irradiation times, pH values, and different excitation wavelengths. NM-CQDs are ideal for cellular staining images because of their strong and stable PL emission [147,148]. Additionally, the green synthesis of carbon NPs using a hydrothermal process from cow manure is becoming more prevalent. These CQDs have an approximately spherical morphology and a narrow distribution, with most particle sizes varying between 10 and 15 nm. Photocatalytic activity of the CQDs was observed by the degradation of methylene blue under visible light, which decreased up to 40% after 12 h [149].
Waste crab shells and three different transition metal ions, Gd3+, Mn2+, and Eu3+ were used in a one-pot MAP procedure to fabricate magnetofluorescent CQDs (MFCQDs). Waste crab shells served as carbon sources and ligands to form complexes with transition metal ions. Consequently, MFCQDs exhibit good stability at different ionic strengths and pH values, making them useful for biomedical applications in vivo. Additionally, intense fluorescence, perfect aqueous dispersibility, excellent magnetic resonance response using various transition metal ions, and a fluorescent QY of 19.84, 12.86, and 14.97% were observed for Gd-, Mn-, and Eu-CQDs, respectively [150,151]. Also, prawn shells have been used to synthesize fluorescent carbon dots. Results revealed that these CQDs (with an average diameter of 4 nm) have several desirable characteristics, including high monodispersity, good stability, the ability to remove blue fluorescence under UV light (365 nm), high QY (9%), and excellent water solubility [152].
With a green synthesis route, several synthesis methods have been developed to produce CQDs, including hydrothermal or solvothermal synthesis, microwave-assisted synthesis, laser ablation methods, and chemical oxidation methods. However, toxic chemical reagents or organic solvents are widely used as precursors in these traditional synthesis processes. Hydrothermal or solvothermal methods were used to prepare CQDs with citric acid and l-histidine as precursors; these synthesis techniques require large heat energy of 200°C for 5 h. The fluorescence QY of the obtained CQDs was approximately 22%, with a maximum emission wavelength of 414 nm [153]. Using these methods, CQDs obtained from rice residue and glycine has shown a high QY percentage (23.48%) [141]. Furthermore, highly fluorescent amphiphilic CQDs have been prepared via MAP using citric acid and 4,7,10-trioxa-1,13-tridecanediamine (TTDDA), which served as an A3 and B2 polyamidation-form monomer collection. TTDDA-based CQDs have exhibited a fluorescence QY of 29%, which is higher than CQDs prepared via waste crab shells [154]. Thus, the MAP technique is affected by uncontrollable reaction conditions and high energy demand.
Chemical oxidation, which is one of the most common synthesis methods, typically necessitates using a strong oxidizing agent (strong acid or alkali) during the synthesis process. This poses environmental concerns because it is difficult to eliminate excess oxidizing agents fully. Also, a serious drawback of this approach is the absence of homogeneity in the size distribution of the resulting particles [155].
2.1.5.2 GQDs
GQDs are zero-dimensional (0D) derivatives of graphene [156,157]. They have unique physical and biological properties that are interesting for energy applications. The renewable, available, and inexpensive routes toward GQDs from biomass and industrial wastes are discussed. Figure 14 shows some types of waste used as precursors for producing GQDs. Natural biomass represents cost-effective and renewable resources for the fabrication of GQDs. High-quality GQDs with an average size of 3.9 nm have been obtained using the byproducts of rice milling [158]. RHs undergo pyrolysis to produce RH carbon. Strong acids were used to activate the RH carbon under a hydrothermal treatment at 200°C for 10 h to produce GQDs. The product achieved a yield of 15% and showed intense photoluminescence and high biocompatibility, exhibiting high functionality for biomedical fields [159]. Spent tea represents an inexpensive, renewable, and green biomass waste for GQDs. As a carbon precursor, spent tea has been used to synthesize GQDs with high yields of over 84% via simple microwave treatment [157].
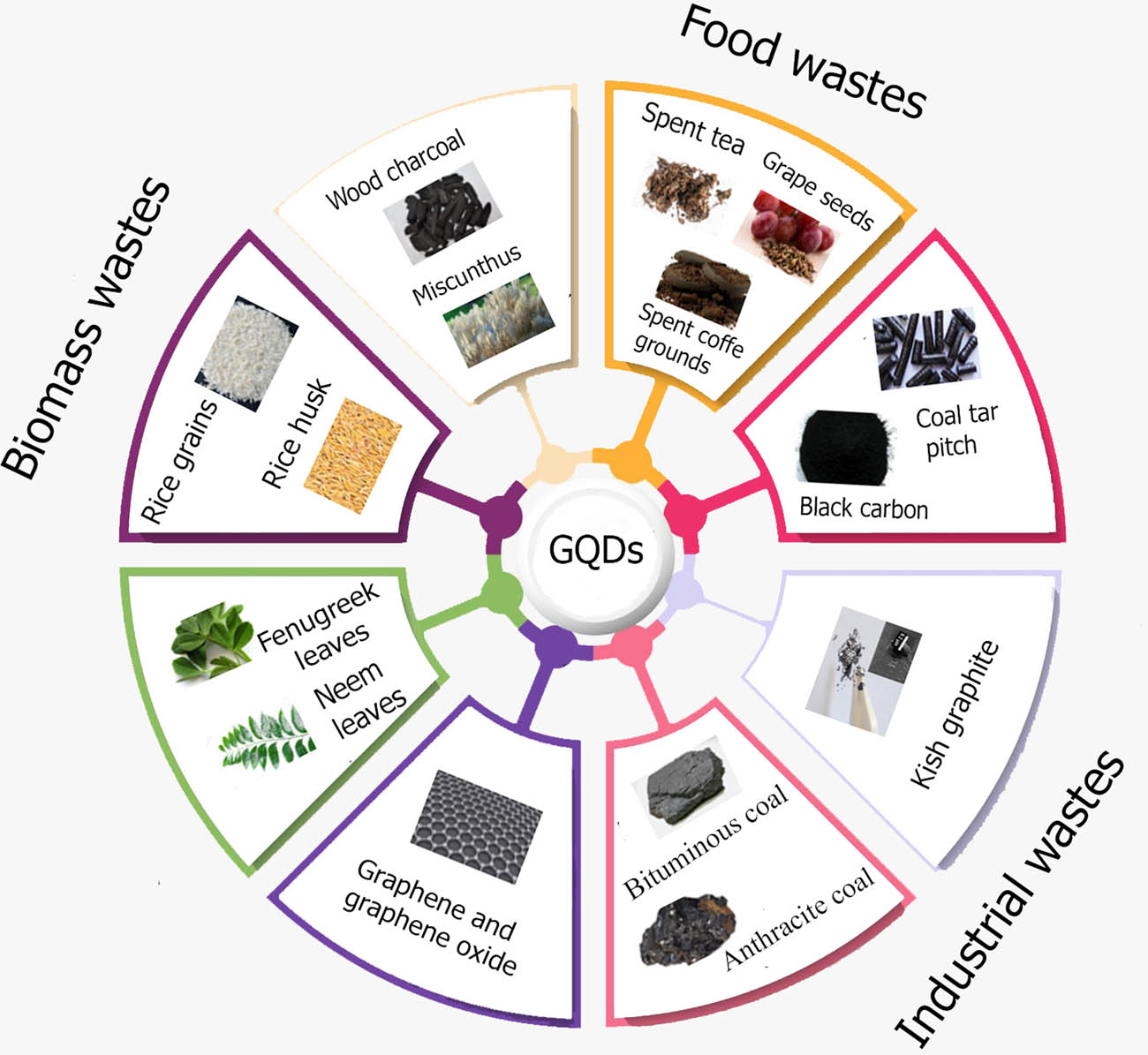
Wastes used as precursors for synthesizing GQDs.
The green synthesis of GQDs by microwave treatment has been confirmed using grape seeds as the carbon source. The GQDs are easily collected and organized in an aqueous medium without any external direction; therefore, they are called “self-assembled” GQDs or (sGQDs). These sGQDs exhibited a QY of 31.79% and a production yield of 53.6%, with sizes in the range of 50–60 nm, which is large compared with other GQDs, i.e., sizes typically 1–8 nm [160].
Plant leaves have been used for the green production of GQDs without oxidation, reduction, passivation agents, or organic solvents [159]. It has been confirmed that biomass pyrolysis treatments can produce a disordered, highly porous form of carbon on the nanometer scale. The thermal treatment of this disordered carbon at 90°C can lead to GQDs at a large scale, which is favorable for inexpensive mercantile production [161]. Neem leaves (Azadirachta indica) have been treated for the fabrication of GO sheets at 300°C for 2 h. After 8 h of treatment, these GO sheets were subsequently broken down into GQDs. The process used green and facile one-pot hydrothermal synthesis method with water as a solvent [162]. The as-produced GQDs were highly photostable and suitable for white light-emitting diodes (LEDs); they had an average size of 5 nm and a high QY of 41.2% [163]. Fenugreek leaves (Trigonella foenum-graecum), a type of green plant, can also be used to produce GQDs. Fenugreek extracts are highly carbonaceous with many hydrocarbons, making them an ideal precursor for GQDs synthesis [163].
Ugly food (any food considered imperfect to sell) is one type of food waste; rice grains are included in this group. According to the FAO of the United Nations, these waste grains contain a significant carbon footprint [164]. Rice grains have been used as a carbon source in a facile and green approach to synthesize monodispersed GQDs with sizes of 2–6.5 nm. The QY of the as-produced GQDs in water depends on their size; it increases from 16% to 24% with a decrease in GQDs’ size (from 6.5 to 2.0 nm) [165].
GQDs have been synthesized by the electrochemical scissoring of wood charcoal, a type of biomass. Electrochemical oxidation has been used to cut charcoal graphene sheets into very small particles called E-GQDs [159]. The size of the product was uniform and ∼5 nm, with optical and structural properties. Wood charcoal is a cheap and widely available source for synthesizing E-GQDs [166].
Miscanthus or silver grass is a genus of African, Eurasian, and Pacific Island plants in the grass family. It is a biorefinery waste comprising sugars and depolymerized lignin. A straightforward, effective, and general strategy has been developed for fabricating GQDs from Miscanthus (M-GQDs). As-produced M-GQDs show many advantages, such as few-layer graphene-like single crystalline structure, sulfur and nitrogen codoping, bright fluorescence, excitation-dependent photoluminescence, and long fluorescence lifetime (11.95 ns). Moreover, M-GQDs exhibit notable fluorescence reduction with good linearity (≤0.995) toward a trace amount of Fe3+. M-GQDs are built from well-dispersed NPs with a uniform size of 4.05 ± 0.61 nm [167].
Green production of highly fluorescent GQDs with a high yield of more than 80 wt% was obtained using coal tar pitch, a thick liquid byproduct from coke and coal gas production. The as-produced GQDs exhibit a tight size distribution of 1.7 ± 0.4 nm and high solubility in aqueous solutions [168]. Additionally, gram-scale GQDs with a yield of 75 wt% and high purity (99.96 wt%) were obtained using Vulcan XC-72 carbon black from battery and fuel cell manufacturing [169]. These GQDs have exhibited multicolor photoluminescence, from green to light red. Bituminous coal is one of the largest types of industrial waste, especially in power plants, and has been used to synthesize GQDs with a uniform size of 2.96 ± 0.96 nm [170]. Anthracite coal is another coal type used to synthesize GQDs, similar to bituminous coal. The average diameter of the GQDs produced from anthracite coal was 29 ± 11 nm [171,172]. GO is a typical starting material for nanosized GQDs when in the presence of other facilitating functional groups [173]. A new path of transforming industrial waste, particularly car bumper waste, into CNMs has also been developed. Reduced GO (rGO) was obtained as a catalyst for the upcycling of waste bumper via an economical thermal decomposition method [174]. GQDs have been synthesized using GO as a precursor through acidic oxidation. The as-produced GQDs exhibited photoluminescence features and the same properties as peroxide catalytic functionality, which can be used to detect H2O2 with a detection limit of 87 nm [175]. GQDs have been obtained from GO by hydrothermal reaction. GQDs with 40 nm uniform lateral dimensions, a yield of 32%, and a QY of 3.6% have been obtained by the hydrothermal cutting method. This simple method shows the notable advantages of a one-step reaction and short time consumption [176]. This method was also used to synthesize GQDs from RH, neem leaf, and fenugreek leaf waste. GQDs were produced with QYs of 41.2, 38.9, and 15% from neem leaves, fenugreek leaves, and RH, respectively. A facile synthesis method of high-quality GQDs from 3D graphene grown by chemical vapor decomposition has been used via a new, highly efficient, and green electrochemical strategy. The as-produced GQDs possess a uniform distribution in diameter (3 nm) and thickness and are mostly single-layered [177]. Also, GQDs have been synthesized using wood charcoal in the same technique. This product is called E-GQDs and is uniform in size (∼5 nm) but is not a big different from this precursor and 3D graphene.
Wang and his team [178] reported a green fractionation bottom-up (two-step) approach to convert lignin into glowing carbon nanocrystal GQDs, as shown in Figure 15. Alkali lignin is fractionated using an acid hydrotrope, which can be readily recovered for sustainability. The GQDs had a nanoscale few-layer structure, were water-soluble, and exhibited long-term photostability, bright fluorescence (ultrahigh UV transmission ≥ 305 nm), outstanding biocompatibility, and ultralow cytotoxicity. With these features, lignin-based GQDs were utilized as probes for detecting hydrogen peroxide in biological systems. The researchers succeeded in detecting low concentrations of hydrogen peroxide (reaching 0.13 nM). Further theoretical validation for GQDs’ ultrasensitivity was confirmed using density functional theory. Synthesis methods of different carbon-based nanomaterials derived from different biomass/waste sources are listed in Table 1.
![Figure 15
Schematic for the green bottom-up synthesis of lignin-based GQDs via a two-step method. Copied with permission from ref. [178]; Royal Society of Chemistry, 2019.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_015.jpg)
Schematic for the green bottom-up synthesis of lignin-based GQDs via a two-step method. Copied with permission from ref. [178]; Royal Society of Chemistry, 2019.
Synthesis of different carbon-based nanomaterials derived from different biomass/waste sources
| Carbon nanomaterials | Biomass source | Synthesis method/technique | Reagent | Conditions | Result/yield morphology and structure | Ref. |
|---|---|---|---|---|---|---|
| Activated carbon | Tamarind fruit shell | Pyrolysis process/CVD technique | KOH/Ar gas | Muffle (800°C for 140 min) and Ar (5°C/min and 100 SCCM) | Activated carbon nanosheets | [179] |
| Coconut shells | Concurrent activation and magnetization processes on the biochars of coconut shells | FeCl3.6H2O/N2 gas | Mixing (2 h), oven (120°C), muffle (700°C), and pH (∼6.5–7.0) | Magnetic activated carbon | [180] | |
| RHs and straw | Pulping processes (pyrolysis)/biological process | Sodium hydroxide/sodium sulfite/sodium sulfite–sodium carbonate blend/NaOH/ammonia thermodesorption/cellulose and peroxidase enzymes | Pulping processes: Na2O (140°C for 2 h), NaOH (60°C for 1 h)Biological process: activation (27°C for 14 days) | Microporous activated carbons | [181] | |
| Carbon nanotubes | Palm kernel shell biomass | Fast microwave pyrolysis | N2 gas | Microwave (200°C), N2 (30 min), and microwave (1,200, 1,300, and 1,400°C for 30 min) | CNTs | [182] |
| Waste polypropylene | Pyrolysis | Waste polypropylene/nickel nitrate | Thermal treatment (H2 at 10 SCCM and Ar at 90 SCCM; ramp temperature [30°C/min], and reaction temperature [600°C, 700°C, and 800°C]), and cooling (RT) | MWCNTs | [183] | |
| Plastic wastes (low-density polyethylene, high-density polyethylene, polypropylene, polyethylene terephthalate, and polystyrene) | Pyrolysis | Ni–Mo/Al2O3 catalyst/H2/N2 gases | Catalytic conditions (600°C for 1 h of H2 [50 SCCM] and N2 [30 SCCM], and then 650°C/N2) | MWCNTs | [184] | |
| CNTs (650°C then RT, N2 at 100 SCCM) | ||||||
| Pomelo peel biomass | Microwave-assisted pyrolysis | Tantalum pentoxide, melamine, trithiocyanuric acid, potassium hydroxide, sulfuric acid, n-Hexane, and surfactant | Pyrolysis (N2 at 400 ml/min and 600°C for 30 min) | Ta-decorated and N, S-doped, CNT enriched mesoporous electrocatalyst | [185] | |
| Liquefied larch sawdust | In situ polymerization, foaming, and carbonization | Formaldehyde solution/sodium carbonate/sulfuric acid/tween 80/n-hexane | Carbonization (N2 at 800°C for 2 h) | MWCNTs/carbon foam nanocomposites | [186] | |
| Carbon nanofiber | Loofah biomass | Calcination/CVD | Dopamine hydrochloride/Tris-Cl solution/N2/Ni (NO3)2 solution/ethanol | Carbonization (N2 at 500°C for 2 h), drying (80°C), soaking (1 day), and further treatment (N2 at 800°C for 2 h) | CNFs | [57] |
| Rubber fruit shell | Hydrothermal process | H2SO4/H3PO4/NaOH | Rinsing (4 h), drying (60°C for 24 h), centrifugation (10,000 rpm for 30 min), stirring (300 rpm and 60°C for 30 h), and drying (90°C) | CNFs | [187] | |
| Sapindus trifoliatus nut shells | High-temperature carbonization followed by physical activation method | N2/Alumina boat/CO2 | Carbonization (N2 at 700°C for 3 h), furnace under N2 flow (80 cm3 min−1) at a heating rate of 5°C min−1 up to 700°C, and then CO2 for 2 h | CNFs | [188] | |
| Pithecellobium Jiringa shell waste | Pyrolysis | KOH/ZnCl2/N2 gas/CO2 gas | Precarbonization (250°C), carbonization (N2 at 600°C), and activation (CO2 for 850°C) | Carbon nanofiber/nanosheet | [189] | |
| Bamboo waste materials | Pyrolysis | KOH/N2 gas/CO2 gas | Carbonization (N2 for 1 h at 600–900°C) and activation (CO2 at 900°C for 2.5 h). | Highly porous activated carbon nanofibers | [190] | |
| Graphene | Pomelo peels | Ultralight microwave absorption | Hydrogen peroxide (H2O2) and acetic acid (HAc) | Autoclave (120°C for 3 h) and pyrolysis (Ar at 800°C for 3 h) | Graphene-like porous carbon nanosheets | [191] |
| Sawdust (from Betula platyphylla) | Shear exfoliation and carbothermal redox process | FeCl3·6H2O and hydrochloric acid | Precarbonization (N2 at 450°C for 2 h), annealing (N2 for 1,000°C for 3 h at 10°C min−1), and centrifugation (3,000 rpm for 20 min) | Graphene sheets | [192] | |
| Orange peel wastes | Ball-milled/the carbonization/activation process | KOH/N2/HCl | Precarbonization (N2 at 400°C for 2 h), stirring (RT for 12 h), and drying (90°C overnight) | Honeycomb-like architecture with a 3D hierarchically ordered pore size distribution | [193] | |
| Biomass film (kraft lignin [KL] and cellulose nanofibers) | Ultrafast laser writing technique | CNFs/KL/NaOH | — | Three-dimensional interconnected porous graphene network with defect-rich boundaries | [194] | |
| Quantum Dots | Spent black tea | Hydrothermal treatment | Sodium hydroxide (NaOH), nitric acid (HNO3 > 67%) and sulfuric acid (H2SO4 > 97%) were purchased from Fisher Scientific, UK. The metal salts include CoCl2, CaCl2, AlCl3, AgNO3, CrCl3, FeCl2, CuCl2, ZnCl2, SrCl2, FeCl3, PbCl2, NiCl2, MoCl2, LiCl, NaCl, MnCl2, and MgCl2 | Autoclave (200°C for 12 h) and dialysis (0.1 µm PVDF for 1 day) | NPs/Nanospheres (NSs)/GQDs | [195] |
| Corn stalk shell | Hydrothermal approach in near-critical water pyrolysis | — | Reaction (270°C and 5 MPa for 10 min), cooling (RT), and dialysis bag (1,000 Da for 24 h) | CQDs | [196] | |
| Lemon peel waste | Hydrothermal process | Waste lemon peels, titanium isopropoxide, methyl, 6-aminohexanoic acid, and sodium hypo chloride | Oven (100°C for 10 h), drying (100°C for 4 h), pH = ∼7, autoclave (200°C for 12 h), centrifugation (10,000 rpm for 30 min), and drying (100°C) | CQDs | [197] |
2.2 Metal oxide-based nanomaterials
Metal oxides have been used in energy storage, energy conversion, and catalysis based on need. This usage only increases based on improvements in metal oxides’ physical and chemical properties and the observation of new sources [198–200]. Figure 16 shows the wastes used to produce the different metal oxide NPs.
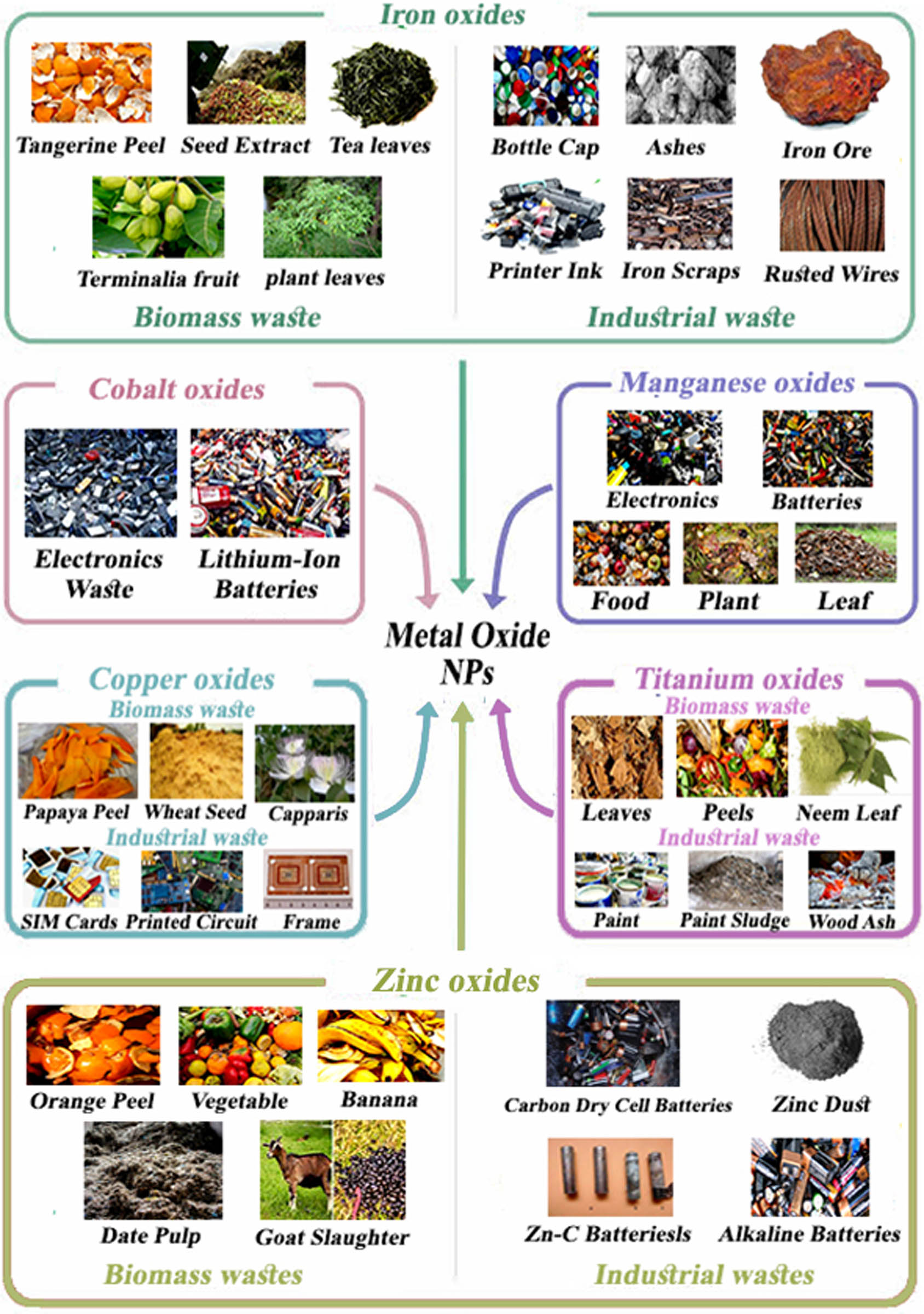
Wastes used as precursors for synthesizing different types of metal oxide nanoparticles.
The chemical treatment of metals, such as Mn and Co, and their oxides for NP fabrication has several advantages, such as high purity, size controllability, and high yield. However, many disadvantages also exist, such as the high cost of precursors and their poor availability. Conversely, extracting these materials from different waste sources has received much attention due to high production, low cost, increased availability, ease of handling, safety, speed, and high production yield. For recycling waste residues, various physical, chemical, and biological techniques have been reported to produce metal oxide NPs [201,202]. However, LiBs sources still produce a large amount of metal oxide. Current issues facing the production of metal oxides are based on green and biosynthesis techniques.
2.2.1 Manganese oxide
More than 60 billion alkaline manganese-based batteries are manufactured annually. The development of technology to recover or isolate Mn from Co, Ni, and Li is critical as Mn concentration of 3 g L−1 greatly reduces the selective separation of Co and Ni [203]. Since industrial LiB wastes typically contain both active materials and impurities, such as Al, Fe, and Cu, few major recycling technologies are available for recovering Mn [204]. Mn can be extracted in different forms, such as powder, metal, metal oxide, and NM, from electronic waste, battery waste, etc., [5,16,204,205]. Additionally, nanoscale MnO2 can be extracted from the waste of portable batteries [206–211], whereas porous flower-like MnO2-NiO has been recovered from spent Zn–Mn batteries [212], and MnCo2O4 and Li x MnO x+1 have been recovered from spent LiBs [213].
Interconnected spheroidal MnO x NPs, in nanorods (NRs) and nanoflowers have been recovered using Urginea sanguinea [214]. A green synthesis method for MnO2 NPs, with sizes of approximately 32 nm, has been achieved using Yucca gloriosa leaf extract and was stabilized using turmeric extract [215]. As a reducing and stabilizing agent, clove (i.e., Syzygium aromaticum extract) was used to prepare MnO NPs [216]. The successful biosynthesis of MnO2 NPs from rhizophytic bacteria Paenibacillus polymyxa strain is achieved. Utilization of KMnO4 as a precursor with an aqueous leaf extract of Kalopanax pictus for the production of MnO2 NPs, with an average particle size of 19.2 nm and a particle diameter ranging from 1 to 60 nm, was also achieved [217]. MnO2 NRs with an average size of 40–50 nm were successfully fabricated using a leaf extract of Phyllanthus amarus [218]. Mn3O4 NPs with an average crystallite size of 44 nm were recovered using manganese sulfate monohydrate as the precursor salt and leaf extract of Malabar nut (Adhatoda vasica Nees/Justicia adhatoda) as the reductant [218]. Moreover, Ananas comosus (L.) peel extract was used to fabricate Mn3O4 nanospheres with an average size of 10–34 nm [219]. Chen et al. also showed recovery in MnO2/Fe(0) composites from Li-ion batteries via ferrous sulfate, lithium manganite, and the hydrothermal technique. XRD peaks confirmed the presence of the β-MnO2 phase (Figure 17) [220]. Min et al. [221] have also reported the recovery of MnO2 from spent LiBs, which can fundamentally be formed from three steps: (1) pretreatment, (2) leaching, and (3) catalyst preparation (Figure 18).
![Figure 17
(a) TEM, (b) SEM, and (c–e) mapping images of MnO2/Fe(0) composites. Copied with permission from ref. [220]; Elsevier, 2021.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_017.jpg)
(a) TEM, (b) SEM, and (c–e) mapping images of MnO2/Fe(0) composites. Copied with permission from ref. [220]; Elsevier, 2021.
![Figure 18
Schematic with photographs of MnO2 fabrication from spent LiBs. Copied with permission from ref. [221]; Elsevier, 2021.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_018.jpg)
Schematic with photographs of MnO2 fabrication from spent LiBs. Copied with permission from ref. [221]; Elsevier, 2021.
2.2.2 Cobalt oxide
Electronic waste (e-waste) generation now exceeds 50 million metric tons per year globally [2]. The bulk of this waste does not get recycled and ends up in landfills in developing countries. Heavy metal residues, common in e-waste landfills, leach into soil and rivers, posing a health risk to residents. Sulfuric and citric acid systems have been used to extract Co from cobalt-bearing waste with a Co leaching efficiency of over 99% [222]. The recovery of 70% of Co from LiBs in cell phones was accomplished by a hydrometallurgical route [223]. Moreover, the extraction of Co and LiCoO2 from LiB wastes, using the green glycine-based method, has been reported [224]. Co3O4 nanocatalysts have been produced from spent LiBs, Ni–Cd batteries, and LCD panels using a hydrometallurgical technique [225]. Co has been recovered from spent LiBs by using supercritical carbon dioxide extraction with sulfuric acid and H2O2 as reagents [226,227]. Interestingly, the electrochemical-hydrothermal process produced cobalt oxide from waste LiCoO2 [228]. The removal of Co from mixed and polymetallic wastes was achieved via biohydrometallurgy [229], and the highest leach yields (53.2% cobalt) have been achieved using biogenic ferric leaching augmented with 100 mM H2SO4. For long-term recovery of Co from spent LiBs, a green process with potential environmental and economic benefits has been suggested [230].
Bioreagents are a feasible and environmentally sustainable alternative to conventional mineral processing that could be improved even further with proper LiB wastes pretreatment [231]. Biohydrometallurgy could be used to remove and recycle metals from mixed and polymetallic wastes. LiB wastes were removed using noncontact bioleaching with biogenic ferric iron and sulfuric acid [229]. The highest leach, providing 53.2% cobalt, was achieved using biogenic ferric leaching augmented with 100 mM H2SO4 [230]. Compared with the chemical leaching process, bacterial communities recovered 100% of Li(i) and 99.3% of Co(ii) after 72 h (91.4 and 94.2%, respectively) [232].
Cobalt ions were collected of waste LiCoO2 cathodes of LiBs waste using a nitric acid leaching solution. For cobalt recovery, solvent extraction and chemical precipitation extracted Co(ii) from a chloride medium [233]. Ni and Co concentrations, conversely, have not exceeded 2.5 and 0.15%, respectively. To recover this material, a leaching phase must first occur. High-pressure acid leaching, which is commonly used, is costly because of its high energy consumption. However, there are now many alternatives to atmospheric leaching [234].
Ni and Co are chemically close, making separation impossible. Solvent extraction is considered the only method for extracting high-rate outputs from a mixture of these elements [235] and is also the only successful method regardless of the metal content in treated solutions [236]. Arsenic is considered the most known contaminant in soil, especially in former mining areas, but if present as arsenide, it is considered a new possibility for exploitable metals. In the presence of citric acid, bleached cobalt produces up to 92% but, with noninoculated and chemical controls, produces only 4 and 10%, respectively. Although the addition of citric acid enhances cobalt liberation, which has appeared during a more stable operation, excluding it has resulted in cobalt liberation ranging from 35 to 82%, based on the ability of the arsenide to deal with [237]. Recently, Dubey et al. [238] used Calotropis procera to assist in synthesizing Co3O4 NPs (Figure 19). XRD data and TEM analysis confirmed that the Co3O4 NPs ranged in size from 3 to 5 nm and were spherical in shape without displaying aggregation.
![Figure 19
Synthesis steps of Co3O4 NPs. Copied with permission from ref. [238]; Elsevier, 2018.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_019.jpg)
Synthesis steps of Co3O4 NPs. Copied with permission from ref. [238]; Elsevier, 2018.
2.2.3 Iron oxide
Recycled resources are one of the main precursors in producing iron oxides [239–241]. Every year, the TiO2 industry produces massive amounts of ferrous sulfate with excess sulfuric acid, stored or disposed as solid waste in landfills. Also, vast amounts of iron ore tailings are collected in the steel industry, in iron ore plants, and from the bottom (BA) and fly (FA) ashes in MSW incineration, which can liberate Fe-bearing minerals during incinerator operation, management, and landfilling [242].
Iron has been recovered from iron ore tailings with a total iron content of nearly 72.36%. This iron was cubic or spheroidal in shape, ranging from 8.3 to 23 nm [243]. The TiO2 industry produces a large amount of ferrous sulfate with excess sulfuric acid, which is harmful to the environment and urgently needs to be recycled. Nanosphere α-Fe2O3 red pigment powders with an average particle size of 45 nm were produced using waste ferrous sulfate as the secondary raw material via Acidithiobacillus ferrooxidans with high-purity Fe2O3 of 98.24 wt% [244–246]. Rusted iron collected from scraps (iron waste) can be used to synthesize an interconnected hematite phase (α-Fe2O3 NPs) [247]. The synthesis of iron oxides, goethite (α-FeO(OH)), magnetite (Fe3O4), and/or maghemite (γ-Fe2O3) from the razor blade and bottle cap wastes has been achieved with iron being the predominant element (at percentages above 70%). Iron oxides in magnetite and impure forms (maghemite, titanomagnetite, titanohematite, and ferrite) can be produced from ash from incinerator operations [242].
Iron nanocomposites (T-Fe3O4) have been synthesized with mesoporous hexagonal nanocrystallinity and with dimensions of less than 100 nm [248]. Green tea leaves (Camellia sinensis) were used to synthesize mesoporous α-Fe2O3 particles at a large scale; the particles were well crystallized, highly pure, and had an average particle size of 60 nm [249]. Iron NPs prepared by this method were used to degrade malachite green. Iron-based NPs can also be sensitized using oolong tea extracts, resulting in spherical particles ranging from 40 to 50 nm [250,251]. Fruit waste materials can produce Fe3O4 mesoporous spherical magnetic NPs with a surface area of 3.517 m2 g−1 and an average pore size relative to single-point adsorption total volume (P/P o) equal to 0.9905 cm3 g−1 [252]. Yadav et al. [253] have reported the synthesis of iron oxide NPs from incense stick waste ash (Figure 20). XRD data confirmed the amorphous character of iron oxide NPs and SEM images verified they possess a polyhedral form. Furthermore, the NPs have an amorphous nature, as presented in the TEM images in Figure 21.
![Figure 20
Synthesis of iron oxide NPs. Copied with permission from ref. [253]; Elsevier, 2020.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_020.jpg)
Synthesis of iron oxide NPs. Copied with permission from ref. [253]; Elsevier, 2020.
![Figure 21
(a) and (b) TEM images of iron oxide NPs, (c) d-spacing, and (d) selected area electron diffraction pattern. Copied with permission from ref. [253]; Elsevier, 2020.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_021.jpg)
(a) and (b) TEM images of iron oxide NPs, (c) d-spacing, and (d) selected area electron diffraction pattern. Copied with permission from ref. [253]; Elsevier, 2020.
2.2.4 Titanium oxide
Despite the high quality of TiO2 fabricated by chemical methods, synthesis from recycled wastes is a promising inexpensive alternative to chemically synthesized TiO2 NPs [254,255]. TiO2 nanocrystals with a size range between 50 and 150 nm can be synthesis-biomediated using tangerine peel waste [256]. Echinacea purpurea extract can be used as a bioreductant to synthesize 120 nm TiO2 NPs [257]. Spherical TiO2 NPs can be synthesized using Murraya koenigii leaf extract without any surfactant, catalyst, or template; these NPs have a particle size of nearly 10 nm [258]. TiO2 NPs with an average size of 19 nm have been prepared using neem leaf extract in a convenient and biodegradable procedure [259].
Alumina-coated TiO2 pigment was produced using a thermal recycling process from a paint waste matrix with a TiO2 content of 30.47 wt% [260]. Furthermore, paint manufacturing industry waste (paint waste sludge) can be recycled into TiO2 powders by sintering the waste containing TiO2 [261]. Boron enrichment waste (BEW) was used to synthesize (TiO2-BEW) photocatalysts, and the TiO2-BEW contained nearly 13.7 wt% TiO2 [262]. Nanocomposite K2Ti6O13/TiO2 has been prepared from water extracted from wood ash using calcination–precipitation method; particle size varied with calcination temperature [263]. Based on oxalic acid leaching, WO3/TiO2 can be produced from the spent V2O5–WO3/TiO2 catalyst, containing approximately 80–90 wt% TiO2 [264].
Zalas et al. proposed a new method for producing renewable energy (via dye-sensitized solar cells, DSSCs) by converting titania-rich waste materials from the paper industry into usable materials. The fabricated DSSCs provided solar electricity but low photon-to-current efficiencies and short circuit photocurrent densities. The fill factor (FF) and open photovoltage in the circuit reached 67% and 719 mV, respectively. A titania paste annealing phase is required to make a compact mesoporous semiconducting layer via the normal working electrode development process. This phase temperature is usually approximately 723–783 K. The obtained semiconducting layer was unstable because of the high cellulose content, in contrast to TiO2 in waste materials. This compelled a calcination step to acquire cellulose-free materials [265].
Qiang et al. [266] synthesized hierarchically porous TiO2 using a simple and reproducible approach combining biofuel materials (Figure 22). Acacia mangium tannin extract (BA tannin) and triblock copolymer pluronic (P123) were used as a dual template, and traditional poisonous and expensive organic alkoxide was substituted with nontoxic and low-cost titanium sulfate (Ti(SO4)2). The biofuel BA tannin processed sulfonic and phenolic hydroxyl groups, effectively coordinating ligands. The results revealed that TiO2 has a multiscale porous structure with macropore and mesopore sizes of 4 nm and 13 nm, respectively. The pore structure could be modified by adjusting the P123 and BA tannin ratios.
![Figure 22
Synthesis steps for porous TiO2. Copied with permission from ref. [266]; Elsevier, 2020.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_022.jpg)
Synthesis steps for porous TiO2. Copied with permission from ref. [266]; Elsevier, 2020.
2.2.5 Zinc oxide
Because of their abundance and low cost, recycled wastes are one of the main sources of producing ZnO NPs [15]. Green synthesis of ZnO NPs is an efficient, environmentally friendly, and simple method that aims to minimize the use of toxic chemicals in NPs. In this regard, high-purity ZnO nanospheres with an average particle size of 21 nm have been fabricated from Tragopogon collinus [267]. ZnO nanopowder acquired from orange fruit peel extract has a fluffy shape and different colors, with small spherical particles (10–20 nm) [268]. ZnO NPs from Kappaphycus alvarezii have exhibited a rod shape with a diameter range of 62–74 nm [269], whereas those from Punica granatum (pomegranate) fruit peel have spherical and hexagonal shapes at different annealing temperatures (600–700°C) [270]. Banana peel extract is an interesting precursor that creates ZnO rod-like or sheet-like structures with diameters ranging from 8 to 10 nm [271]. ZnO NPs from Lippia adoensis leaf extract are pure, hexagonal wurtzite, and thermally stable after 400°C [272,273], whereas those from goat slaughter waste are also pure, hexagonal wurtzite, spherical in shape, and have a size of 3–11 nm [274]. ZnO NPs from Phoenix dactylifera waste (date pulp waste [DPW]) are spherical, pure, crystalline, have hexagonal wurtzite phases, and have a mean diameter of 30 nm, and do not agglomerate [275]. ZnO NPs from leaves of the Ocimum tenuiflorum plant have a hexagonal shape with a diameter range of 11–25 nm and an average particle size of 13.86 nm [276].
Commercially, ZnO was synthesized from top submerged lance furnace flue dust (tire grade, purity > 99.0%) [277]. Synthesized ZnO from end-of-life (EOL) Zn–C batteries is a porous nanosheet with a thickness of up to 100 nm and a hexagonal step. Long-term calcination promotes crystal growth, but agglomeration can occur if the process is prolonged [278]. The purity of ZnO synthesized from spent Zn–C batteries is 99.6%, with impurities like SiO2, and particle size decreases from 50 to 20 nm with a decrease in NaOH concentration. The resultant ZnO particles from this process are hexagonal zincite phases of ZnO [279,280].
ZnO/diatomite hybrid is made of pure Zn, Si, and O with no impurities [11]. CuO/ZnO hybrid nanocatalysts with honeycomb-like structures have been fabricated from solid waste developed in the organosilane industry. CuO and ZnO phases were present in all samples, and there were no other visible crystalline impurities [102]. Pure ZnO particles synthesized from spent Zn–MnO2 alkaline batteries exhibited a size range of 40–50 nm [16]. Rambabu et al. synthesized ZnO NPs from DPW (Phoenix dactylifera) [275]. A high yield of 89.3% ZnO NPs was obtained (Figure 23). UV-vis absorption was carried out, and a red-shifted characteristic peak at 381 nm was obtained compared to the bulk material. This is due to the plasmonic resonance of the NPs. The NPs possessed a spherical appearance with a smooth distribution and no aggregate, as illustrated in Figure 24.
![Figure 23
Synthesis steps of ZnO NPs. Copied with permission from ref. [275]; Elsevier, 2021.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_023.jpg)
Synthesis steps of ZnO NPs. Copied with permission from ref. [275]; Elsevier, 2021.
![Figure 24
SEM (a and b) and TEM (c and d) for ZnO-NPs at different magnifications. Copied with permission from ref. [275]; Elsevier, 2021.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_024.jpg)
SEM (a and b) and TEM (c and d) for ZnO-NPs at different magnifications. Copied with permission from ref. [275]; Elsevier, 2021.
2.2.6 Copper oxide
CuO NPs are synthesized using various physical, chemical, biological, and hybrid approaches. Physical and chemical methods are commonly used to synthesize CuO-NPs [281]. Control of size on the nanoscale, good stability, and high purity have all been demonstrated via biosynthesis of CuO nanostructures [282] using Lantana camara (flower) [283], Capparis spinosa (leaf) [284], [282,285], Allium cepa L. (peel), banana peel [286], Bauhinia tomentosa leaf [287,288], Madhuca longifolia [121,283,284], silver grass leaf extract [285,289–291], and gum arabic (GA) [292]. Conversely, mixed CuO/Cu2O nanospheres can be biosynthesized in the presence of GA [292]. By selecting the appropriate extract, the morphology and size of the synthesized CuO can be monitored. For example, the synthesis of highly pure porous CuO NRs is achieved in the presence of Lantana camara flower extract versus CuO NRs in the absence of the extract [293,294].
Cu and CuO NPs from copper scrap have spherical and twisted sphere morphologies, with average particle sizes of 78 and 67 nm, respectively. Copper scrap processed under N2 gas atmosphere yields single-phase cubic Cu NPs free of impurities and oxide [295]. Additionally, there were no impurity products, such as Cu(OH)2 or Cu2O, indicating that the CuO NPs are in a pure phase. Cauliflower morphology was observed in CuO NPs made from waste SIM cards [296]. The crystallinity of the recycled CuO NSs from Cu2+ waste is low, with wrinkled nanosheet-like aggregate structures and Cu2+ ion recovery performance of 88 and 99% as the pH rises to 11 and 12, respectively [297]. The yield of copper(ii) oxide from lead frame etching waste is 98.0 wt%, with an average particle size of 1.49 mm [15].
Phang et al. [298] managed to produce CuO NPs from papaya peel extract. The characterization studies showed that the CuO NPs were spherical, with sizes ranging from 85 to 140 nm, and the crystalline phase was monoclinic with good purity. Sharma et al. [299] used Ocimum tenuiflorum to synthesize CuO NPs, as illustrated in Figure 25. These CuO NPs possessed different morphologies dependent on copper acetate monohydrate concentrations (Figure 26). Also, unique synthesis methods of different metal oxide-based nanomaterials derived from different waste sources are listed in Table 2.
![Figure 25
Synthesis of CuO NPs. Copied with permission from ref. [299]; Elsevier, 2021.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_025.jpg)
Synthesis of CuO NPs. Copied with permission from ref. [299]; Elsevier, 2021.
![Figure 26
SEM images of CuO NPs created from various copper acetate monohydrate concentrations. Copied with permission from ref. [299]; Elsevier, 2021. (a) [Cu(CH3COO)2·H2O] = 5 mmol kg−1 (b) [Cu(CH3COO)2·H2O] = 10 mmol kg−1 (c) [Cu(CH3COO)2·H2O] = 50 mmol kg−1.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_026.jpg)
SEM images of CuO NPs created from various copper acetate monohydrate concentrations. Copied with permission from ref. [299]; Elsevier, 2021. (a) [Cu(CH3COO)2·H2O] = 5 mmol kg−1 (b) [Cu(CH3COO)2·H2O] = 10 mmol kg−1 (c) [Cu(CH3COO)2·H2O] = 50 mmol kg−1.
Synthesis of different metal oxide-based nanomaterials derived from various waste sources
| Metal oxide nanomaterials | Biomass source | Synthesis method | Reagents | Temperature, time, and atmospheric conditions | Result/yield morphology and structure | Ref. |
|---|---|---|---|---|---|---|
| Manganese oxide | Amorphous MnO2 NPs formed by the reduction of KMnO4 with HCl | Simple wet-chemical technique | KMnO4/HCl/(CH3)2CHOH/DMF/C2H5OH/CH3COCH3/KCl/PVDF | First step; formation of amorphous MnO2: reduction (RT for 20 min.), stirring (RT for 4 h), and centrifugation | Manganese oxide crystalline nanorods | [300] |
| Second step; formation of various nanostructures of crystalline manganese oxides: air-annealing (650°C for 5 h) | ||||||
| Radish | Hydrothermal treatment, carbonization, calcination, impregnation, and annealing | Manganese nitrate solution/anhydrous ethanol/KOH/CO2 | Autoclave (200°C for 6 h), freeze-drying (−50°C for 48 h), activation (CO2), evaporation at 800°C for 1.5 h), evaporation (105°C), and drying (180°C for 2 h in air) | A cross-linked 3D carbon structure with uniform growth of MnO x (3D carbon aerogels incorporated with MnO x NPs) | [301] | |
| Cotton | Hydrothermal treatment and subsequent pyrolysis | KMnO4/C4H5N/KOH/C2H5OH/NMP/PVDF | Autoclave (6 h for 160°C), drying (80°C for 12 h), and annealing (N2 at 600°C for 2 h) | Cotton carbon fiber/MnO/C | [302] | |
| Freshwater red alga (Lemanea manipurensis) | Controllable green synthesis | KMnO4/H2O2 | Stirring (at 28°C for 8 h), pH = 5, air drying, and centrifugation | MnO2 NPs | [303] | |
| Cobalt oxide nanoparticle | Dry anaerobic co-digestion of manure | Coprecipitation method | NaOH/CoCl2·6H2O | Stirring (0.5 days), drying (100°C), calcination (500°C for 2 h), and photoactivation (using halogen lamp for 30 min) | Co3O4 NPs | [304] |
| Jumbo Muscadine (Vitis rotundifolia) | Coprecipitation | Cobalt chloride (CoCl2·6H2O) and sodium hydroxide pellets (NaOH)/ethanol | Crushing (15 min), filtrate storing (4°C), stirring (at 75°C and 500 rpm for 60 min), pH = ∼9, air drying (75°C for 6 h), and calcination (600°C for 4 h) | NPs | [305] | |
| Waste paper biomass | Magnetization | FeCl3·6H2O, FeCl2·4H2O, and NH3·H2O/pure cobalt (II and III) oxide (CoO NPs) | Shaking (200 rpm for 1, 3, 6, 9, 12, 24, 48, and 72 h) and shaking (200 rpm/48 h) at RT | Cobalt oxide NPs | [306] | |
| Spent LiBs | Physical preparation | NaOH/H2C2O4 | Immersion in an electrolyte solution (24 h), drying (90°C for 12 h), separation (250–300°C/30 min), pH (∼4), stirring (30°C for 1 h), drying (120°C for 24 h), calcination (under air at 500°C for 2 h), and ball milling (30 Hz for 16 h) | Cobalt oxide NPs | [307] | |
| Metals oxides loaded with biochar | Thermal treatment is followed by calcination | Anhydrous nickel and cobalt chloride/ammonium hydroxide | — | Biochar supported nickel and cobalt oxide NPs | [308] | |
| Iron oxides nanoparticles | Municipal solid waste | Combustion (incineration) | — | — | — | [309] |
| Green tea extract | Nadagouda et al. (2010) under a slight change that maintains the reaction under an inert nitrogen atmosphere | Fe(NO3)3·9H2O/nitrogen | Vacuum filtration, and cooling at RT and under N2 atmosphere (4°C for 24 h) | — | [310] | |
| Pomegranate (Punica granatum) seeds extract | Green synthesis | PSE fruits/iron chloride | Heating (70°C for 15 min), centrifugation (15,000 rpm for 10 min), and drying (60°C for 3 h) | — | [311] | |
| Carica papaya leaf extract | Green physical method (stirring and centrifugation) | FeCl3·6H2O | RT, pH = ∼11, stirring (30 min), centrifugation (8,000 rpm for 20 min), and drying (80°C for 3 h) | — | [312] | |
| Avicennia marina flower extract | Biosynthesis method | Ferric chloride | Filtration (4°C), centrifugation (10,000 rpm for 30 min), and vacuum hot air oven treatment (125°C and 15 psi for 120 min) | — | [313] | |
| Hylocereus undantus (pitaya or dragon fruit) | Green method | Ferric sulfate and ferrous sulfate/ammonium hydroxide | pH = ∼11, decantation (magnetic field), and drying (60°C) | — | [314] | |
| Titanium dioxide nanoparticles | Sesbania grandiflora | Green synthesis | Titanium dioxide | Incubated (light conditions and RT for 24 h) | TiO2 NPs | [315] |
| Acacia nilotica | Green synthesis route | Silver nitrate, tetrabutyl orthotitanate, and extract of Acacia nilotica | Stirring (RT for 20 min), storing (RT for 1 day), drying (80°C for 1day), and annealing (400°C for 3 h) | Ag/TiO2 spherical NPs | [316] | |
| Artemisia haussknechtii leaf extract | Different green methods | AgNO3, CuSO4, TiO(OH)2, sodium carbonate, gallic acid, NaNO2, AlCl3, NaOH, rutin, HCl, sulfuric acid, sodium phosphate, and ammonium molybdate, gram-negative (E. coli ATCC 25922 and S. marcescens ATTC 13880), and gram-positive (S. aureus ATCC 43300 and S. epidermidis ATCC 12258) | Stirring (3 h), centrifugation (8,000 rpm for 30 min), and storing (4°C) | TiO2 NPs | [317] | |
| Gum Kondagogu | Calcination | Titanium oxysulfate | Stirring (750 rpm at 90–95°C) and calcination (500°C and 4 h) | Anatase TiO2 NPs | [318] | |
| Mentha arvensis leaves extract | Green synthesis | Ethanol, titanium tetra isopropoxide, Mueller hinton agar, and potato dextrose agar | Drying (RT for 7 days) and Soxhlet extraction (50°C for 8 h) | TiO2 NPs | [319] | |
| Zinc oxide nanoparticles | Phoenix dactylifera waste | Biosynthetic approach (valorization) | Zinc nitrate hexahydrate | Ultrasonicated (30 min) and calcination (400°C for 1 h) | ZnO NPs | [275] |
| Allium sativum skin (garlic skin) | Green synthesis | Zinc nitrate, sodium hydroxide, sodium hypochlorite, acetic acid, and sulfuric acid | Stirring (20 h), pH = ∼8, stirring (4 h), centrifugation (7,000 rpm for 15 min, and drying (110°C overnight) | ZnO NPs | [320] | |
| Cornhusk and ZnO powder | Alkali-acid treatments, pyrolysis, and activation | MLG and ZnO powder | Ultrasonicated (1 h), stirring (24 h, RT), and drying (60°C overnight) | Graphene/ZnO nanocomposite | [321] | |
| Musa balbisiana Colla pseudostem biowaste | Green synthesis | Zinc sulfate, NaOH, and bacterial strains, such as Escherichia coli (MTCC-443), Staphylococcus aureus (MTCC-3160), Pseudomonas aeruginosa (MTCC-741), and Bacillus subtilis (MTCC-121) | Stirring (2 h, RT), centrifugation (10,000 rpm for 10 min), and drying (60°C overnight) | ZnO NPs | [322] | |
| Orange waste | Green and chemical sol–gel method | Zinc nitrate and sodium hydroxide | Stirring (2 h), centrifugation (3,000×g for 15 min), and drying (60°C overnight) | ZnO NPs | [323] | |
| Waste pericarp of ananas comosus | Stirring during sonication | Zinc acetate dihydrate and sodium hydroxide | Sonication (40°C for 60 min), pH = ∼9–10, cooling (RT), centrifugation (4,000 rpm), and drying (120°C for 6 h) | ZnO NPs | [324] | |
| Copper oxide nanoparticles | Waste Colocasia esculenta leaves’ extract | Biosynthesis method (extraction followed by centrifugation) | Cu(NO3)2·3H2O | Immersion (70°C and 30 min) and stirring (2 h) | CuO NPs | [325] |
| Waste printed circuit boards (WPCBs) | Dissolution | Dimethylformamide, ammonium chloride, nitric acid, and hydrochloric acid | Leaching (80°C for 30 min), pH = ∼ 6–7, precipitation (60°C), and calcination (500°C for 180 min) | Cu oxide NPs | [326] | |
| Terminalia chebula | Combustion route | Sulfuric acid, methanol, hexane, and cupric nitrate | Stirring (10 min) and calcination (400°C for 4–5 min) | Cu oxide NPs | [327] | |
| Orange peel extract or mint leaves’ extract | Green synthesis technique | Orange peel or mint leaves’ extract and copper sulfate | Stirring (50°C), incubation (RT for 24 h), centrifugation (12,000×g for 15 min), and drying (45°C for 24 h) | Cu oxide NPs | [328] | |
| Recovery from discarded printed circuit boards | Chemical precipitation | Hydrochloric acid, nitric acid, potassium hydroxide, sodium hydroxide, and ethanol | Stirring, drying (80°C for 6 h), and calcination (400°C for 4 h) | Cu oxide NPs | [329] |
2.3 Gold nanoparticles
Over the last decade, the grades of gold ore have reduced, and the extraction cost of gold has constantly increased. The current demand for gold is limited compared to requirements for nanotechnology applications (e.g., jewelry) [330]. The size of the market in 2014 reached more than 1.3 billion USD and is expected to consume over 25% of the market by 2022. This would require more than 20,000 kg of gold to flow into the nanotechnology industry. The increased market growth of AuNPs could prompt increasing product demand, such as for high-efficiency compact storage devices and photovoltaics. AuNPs are also widely used in personal care items because of their skin antiaging properties [331]. Different approaches for fabricating AuNPs have been established, including green (biological), chemical, and physical methods. For chemical pathways in which the synthesis process is initiated, sodium borohydride (NaBH4), hydrazine, and citrates are the most popular [332].
Green synthesis is possible for large-scale production, and it produces extremely stable, well-defined AuNPs with different shapes. Extraordinary outside conditions are avoided with this synthesis method; thus, lower energy consumption is also avoided. Green synthesis’s main issues are the solution medium, reducing agent, and nonhazardous stabilizers [332,333]. Researchers are attracted by its financial significance and by reducing potential ecotoxicity from waste caused by precious metals obtained from secondary resources (for example, industrial effluents, electronic waste, and spent catalysts). Generally, purified metals and alloys supplied by the recycling market also represent a limited proportion of primary production. The approximate recycling rates for the EOL of precious metals are falling in major end-use industries. Gold is just one of those metals with an EOL higher than 50%, providing a valuable assessment of the different types of waste [334]. Waste products for NM production can be divided into industrial and biomass waste categories [335]. Waste products for AuNP production can be divided into the following categories: jewelry polishing wastewater, sewage sludge, electronic waste, gold refining sludge, industrial wastewater, and aqueous laboratory nanowaste, as shown in Figure 27.
Waste sources for gold nanoparticle production.
Industrial waste, such as WEEE, is a desirable source of AuNPs because it is predicted that the Au content of WEEE is 80 times greater than that present in the primary mines of the world. Precious metals are relatively abundant in many electronic waste streams, as shown in Table 3 [336]. In this scope, most active research has focused on metal recycling from waste mobile phones (WMPs) and electronic scraps [337].
Gold content in various types of electronic wastes
| Electronic waste | Gold content (ppm) |
|---|---|
| Information communication technology | 120 |
| Mobile phone scrap | 350 |
| Portable audio scrap | 10 |
| Printed circuit board scrap | 250 |
| Shredded WEEE | 130 |
| TV board scrap | 20 |
Pretreatment of WEEE is achieved in two stages: segmentation and reduction in size. For PCB pretreatment, the PCB must be divided into metallic (ferrous and nonferrous) and nonmetallic (polymer and ceramic) components. A wide range of methods, including mechanical cracking and separation by gravity, electrical conductivity, magnetism, and organic solvent delamination, have been identified for this process [338]. The latest scientific recycling hydrometallurgical direction for copper and gold from cell phone waste circuit boards is a two-step acid leaching process. This process allows for a wide separation between Cu and Au from the other metals present and subsequent Cu and Au solution regeneration by solvent extraction [339]. Another tested adsorbent is persimmon tannin, which could be used with hydrochloric acid solutions to efficiently absorb Au(iii) and Pd(ii) from WPCBs. This bifunctional adsorbent showed a substantial increase in Pd and Au adsorption after modification of ethylenediamine [340].
NMs are released into municipal wastewater treatment systems during the washing process of personal toiletries, cosmetics, and showers. NMs may be sealed into sewerage sludge following collection by wastewater treatment plants. This produces nanosludges that contain abundant NPs, including nanoscale TiO2, Ag, and Au. A study using raw biomass as biosorbent demonstrated very high Au recovery. The refining of Au in a local gold processing factory in India was also used to obtain sludge and jewelry polishing wastewater [341]. AuNPs are produced in many academic and industrial laboratories using chemical techniques, which allow them to be produced with various morphologies and sizes. Testing and error are common approaches to developing these synthesis processes, and AuNP physicochemical properties are tested and functionalized. Attachment of AuNPs to substrates has also been completed using a wide range of methodologies [342]. The resulting production or consumption of AuNPs results from all of these processes and tests [343].
Formal e-recycling systems provide the community with environmental resources. When used suitably, electronic recycling is environmentally friendly because it recovers material for recycling and eliminates waste in waste disposal sites. For the e-recycling industry, formalization is typically desirable. It is important to remember that health is central to creating e-recycling opportunities because of the dangerous chemicals inherent in this waste stream. There are challenges in evaluating and controlling frequently discovered chemical exposures in this field, especially in highly technical and formal e-recycling plants [342,344].
Adsorption is one of the most appropriate and effective technologies to recover Au because of its easy operation, low-cost setup, high efficiency, and minimum secondary waste generation. Economic and environmental features, such as natural abundance, cost-effectiveness, and respect for eco-compliance, should also ideally be considered in adsorption to recover precious metals. In the last two decades, significant efforts have been made to produce new adsorbents with biomass resources, like chitosan, persimmon, eggshell membrane (ESM), cyclodextrin, and cellulose [345]. Most of these studies have used extracts from biomass wastes as reducing and stabilizing agents.
Bioreduction of Au(iii) ions to their metallic form (Au0) was examined in an aqueous extract from waste macadamia nut shell. Advanced characterization showed that the AuNPs were crystalline, between 50 nm and 2 μm in size, and have spherical, triangular, and hexagonal morphologies [346]. AuNPs have also been developed with an Au precursor using palm oil mill effluent (POME) without a surfactant or capping agent. The synthesized AuNPs were mostly spherical, with an average size of 18.75 nm. AuNP morphology and size were controlled by varying the reaction conditions [347]. A one-stage, easy-to-use, and fast green chemical method were developed by using banana fruit waste (BFW) extract to reduce the room temperature of hydrogen tetrachloroaurate(iii) hydrate (HAuCl4·3H2O). This technique illuminated the multifunctionality of BFW extract; it acted as a novel reduction, capping, and stabilization agent for the synthesis of AuNPs while no physical or chemical agents were used [348].
AuNPs have also been synthesized by polyphenolic compounds found in grape seed waste, skin, and stalk. An additional advantage of this method for synthesizing AuNPs with all polyphenol-rich food waste materials is conversion to a high-quality nanoproduct that can be used in medical applications, molecular imaging, and cancer treatment. De-oiled jatropha waste is a highly efficient reducing agent in Au(iii) conversion to Au0. With an average particle size of 14 nm, triangle, hexagonal, and spherical shaped AuNPs were obtained. [344]. ESM and even some active functional groups of ESM fibers may serve as Au(iii) ion reduction agents. The growth of AuNPs in ESM is mainly caused by the adsorption of Au(iii) ions into ESM fiber, which decreases the movement of aldehyde fiber and encourages NP nucleation [349]. Mythili et al. [350] have reported the synthesis of AuNPs using vegetable waste. The AuNPs obtained from vegetable waste possess a particle size in the nano regime (10–70 nm). Also, Usman et al. have reported the synthesis of AuNPs via palm oil leaf extracts [351]. Furthermore, Krishnaswamy et al. [352] have studied the use of grape waste for the synthesis of AuNPs, as presented in Figure 28. Also, Table 4 shows the synthesis of AuNPs derived from different waste sources.
![Figure 28
Schematic for AuNPs synthesis. Copied with permission from ref. [352]; Elsevier, 2014.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_028.jpg)
Schematic for AuNPs synthesis. Copied with permission from ref. [352]; Elsevier, 2014.
Synthesis of gold nanomaterials derived from different biomass/waste sources
| Biomass source | Synthesis method | Reagent | Result/yield morphology and structure | Ref. |
|---|---|---|---|---|
| Waste mobile phone circuit boards | Emulsion liquid membrane method | Nitric acid, sodium hydroxide, hydrochloric acid, and perchloric acid | AuNPs | [353] |
| Action of extremophilic yeasts | Biosynthesis | Yeast biomass, AgNO3, and HAuCl4 | Nanocrystalline gold particles | [354] |
| Banyan leaves | Carbonization followed by hydrothermal method | Luteolin and HAuCl4 | Gold nanoflakes decorated biomass-derived porous carbon | [355] |
| RH biomass | Biosynthesis | Choloauric acid, sodium, HCl, and NaOH | AuNPs | [356] |
| Centaurea behen leaf aqueous extract | Green synthesis | HAuCl4 | AuNPs | [357] |
| Mangifera indica leaf | Biological synthesis | Dried M. indica leaf and HAuCl4·3H2O. | Spherical AuNPs | [358] |
| Garcinia kola pulp extract | Green synthesis | Garcinia kola pulp extract and HAuCl4. | AuNPs | [359] |
3 Energy storage applications
3.1 Supercapacitors
Supercapacitors are energy storage systems that act as alternatives to batteries. They store energy by either non-faradaic or faradaic methods [360,361]. Recently, researchers have been interested in supercapacitors based on faradaic processes because of their fast and reversible multielectron redox. Transition metal oxides are frequently used as redox-active electrode materials [360,362,363]. Additionally, in the non-faradic process, electrolyte ions form a double layer on the electrode material surface. Conversely, to maintain a clean environment, waste precursors could be a potential source for producing low-cost supercapacitor electrode materials [364–369]. As shown in Figure 29, the number of papers published on supercapacitor electrodes fabricated from recycled materials has increased dramatically, with up to 272 studies in 2021, 33 times higher than those reported in 2012.
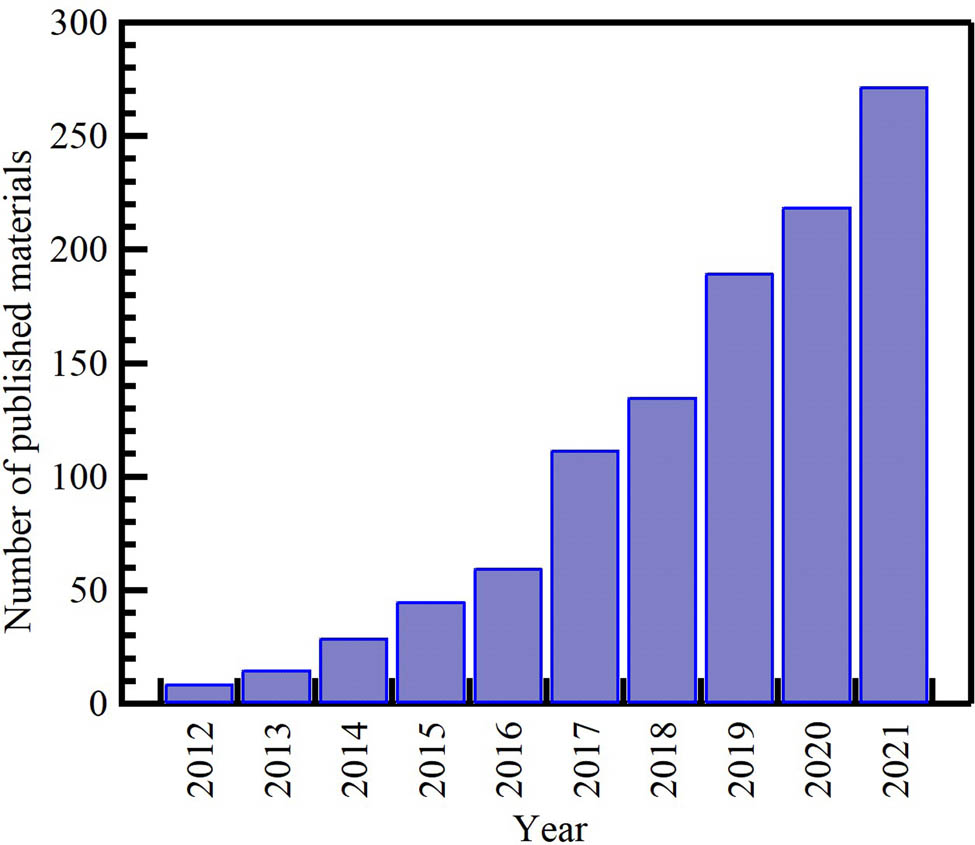
Publication distribution of papers on waste recycling into supercapacitors.
3.1.1 Carbon-based nanomaterials
Carbon-based materials as supercapacitor electrodes are widely studied and used commercially. Applications of carbon-based materials have proven promising for solving current environmental and energy problems. Agriculture waste precursors could be used as target sources for producing carbon-based materials to preserve the environment and maintain low-cost materials. This includes oil palm biomass residues (leaves, fronds, trunks, empty fruit bunches, shells, and fibers), banana fibers, Lablab purpureus, sago bark, Allium cepa peel, RH, waste tires, pineapple leaf fibers, and cellulose [17,19,20,28,370–375]. Several biowaste sources have been used to fabricate CNFs, which act as supercapacitor electrodes. For example, CNFs derived from pineapple leaf fibers exhibited a specific capacitance (C s) of 175 F g−1 [375]. Also, cellulose-derived CNFs (CCNFs) with a diameter of 200 nm resulted in C s of 145 F g−1 [376].
Graphene has recently emerged as a powerful and efficient material for electrodes in supercapacitors because of its excellent characteristics, such as large surface area, carrier mobility, and mechanical power. Graphene is distinguished by its properties, making it an excellent choice for supercapacitor electrodes [377]. Supercapacitors currently rely on unsatisfactory AC, which has less than 120 F g−1 and a low rate for charge and discharge ratio [378]. According to Jinag and Pickering, graphene could be recycled from supercapacitor electrodes and reused [369]. Also, graphite waste could be recycled and turned into graphene; waste graphite is collected from batteries and other electronics [368].
Karakoti and his team produced GNs from PW for supercapacitor applications and reported C s equal to 38.78 F g−1 [379]. Additionally, Sankar et al. used RHs to prepare GNs for supercapacitor electrodes using KOH activation [86]. They recorded a capacitance of 115 F g−1 at a current density of 0.5 mA cm−1 and capacitance retention of 88% after 2,000 cycles, illustrating the excellent stability of GN electrodes [86]. The recycled GNs showed considerable energy and power densities of 36.8 W h kg−1 and 323 W kg−1, respectively, in an aqueous 1 M Na2SO4 electrolyte [86]. Krishna et al. studied the effective preparation of porous GO nanosheets from waste rice, resulting in capacitance of 158.6 F g−1 at 1 A g−1. The quasirectangular form of the capacitance–voltage (CV) curve indicates good capacitance activity and significant charge transfer capability at various scan rates. If the CV curve maintains its quasirectangular form, the electrode has good rate capability.
Using a mix of chemical and physical activation techniques, Yuli et al. used cocoa skin to produce AC, showing C s produced by chemical activation of 90.2 F g−1 [380]. Additionally, corn husk was used as a precursor for AC supercapacitor electrodes with a C s of 127 F g−1 at 1 A g−1 in 6 M KOH with a small energy density (4.4 W h kg−1) [381]. The galvanostatic charge-discharge (GCD) curves for these electrodes had a triangular outline and linear patterns, indicating that the charge storage is an electrical double layer formation and is highly reversible [381]. In 2021, Ramayani et al. reported AC preparation from carrot juice waste via a one-stage integrated pyrolysis approach and KOH chemical activation [382]. The obtained C s, power, and energy were 155 F g−1, 77.57 W kg−1, and 21.52 W h kg−1, respectively, in 1 M H2SO4 [382]. Cui and Liu prepared AC from industrial pyrolytic tires and achieved a C s of 190 F g−1 [383].
CNTs have a novel structure, accessible surface area, a narrow size distribution (in the nanometer range), high stability, and low resistivity, suggesting that they are suitable materials for supercapacitor electrodes. Mishra et al. have synthesized MWCNTs from PP PW and used them for supercapacitor applications [384]. The recycled MWCNTs showed C s of 59 F g−1 in 1 M KOH solution at a scan rate of 5 mV s−1, and it was directly proportional to their surface area. POME was used to produce CNTs [385] by a pyrolysis process at 900°C in a flowing N2 atmosphere. The electrical conductivity of CNTs is 107 S cm−1, making them suitable for use as a supercapacitor electrode. C s obtained for POME recycled CNT electrodes were lower than that for commercial CNTs. Throughout the charging and discharging process, all the cells have similar linear curves of voltage vs time [385]. Sunflower seed hulls and sago were pyrolyzed at 800°C to produce CNTs [386]. The capacitances of CNTs obtained from pyrolyzing natural sunflower seed hulls and sago were 86.9 and 26.7 F g−1, respectively.
CQDs can be generated from denatured (spoiled) milk [387]. The CQDs electrode demonstrated C s of 95 F g−1 over 1,000 cycles with exceptional stability and Coulombic efficiency. The stability of the fabricated CQDs electrode material was tested at a current density of 0.12 A g−1, even after 1,000 cycles, no noticeable change in capacitive behavior was identified, demonstrating excellent long-term cycling stability [387].
Additionally, CQDs were made from durian peel waste using a pyrolysis process [388]. CQDs have also been applied successfully to AC-based electrodes to improve their electrochemical qualities and supercapacitor performance. According to the cyclic voltammogram, the C s of the AC-CQDs composite electrode was 1.4 times higher (approximately 60 F g−1) than that of the pure AC electrode (43 F g−1) [388]. This increase in capacitance is mainly due to the introduction of high surface area CQDs and the pseudocapacitive behaviors provided by CQDs functional groups.
Porous carbon NPs (PCNs) have been prepared from different biowastes, such as sago bark, Allium cepa peel, Lablab purpureus, and oil palm leaves [17–20,374]. The PCNs obtained from these agricultural wastes have been electrochemically studied in detail, either as single electrodes or complete symmetrical devices in 5 M KOH or 1 M Na2SO4. For the full practical supercapacitor fabricated using PCNs obtained from palm oil fronds, the C s values were 309 F g−1 in KOH [374]. The single electrode fabricated from Lablab purpureus showed 300 F g−1 with high stability of 94% up to 52,000 cycles (as shown in Figure 30). Simultaneously, the symmetrical supercapacitor showed C s of 129 F g−1 with a high energy density of 17.9 W h kg−1 under a wider potential window of 1.5 V [19].
![Figure 30
Capacitance retention and Coulombic efficiency of PCNs obtained from Lablab purpureus. Copied with permission from ref. [19]; Royal Society of Chemistry, 2018.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_030.jpg)
Capacitance retention and Coulombic efficiency of PCNs obtained from Lablab purpureus. Copied with permission from ref. [19]; Royal Society of Chemistry, 2018.
3.1.2 Metal oxide-based nanomaterials
Metal oxides are widely used for energy storage due to their excellent morphologies, high surface area, high theoretical specific capacity, and eco-friendliness [209,210,362,363,389]. Metal oxide electrodes could be a single metal oxide, bimetallic oxides, or metal oxide heterostructures. The recycling process of metal oxide from waste is essential to obtain low-cost electrode materials. Different waste sources, such as Zn–C batteries, LiBs, SIM cards, and red mud, have been reported as effective precursors for metal oxide recovery [209,211,296,390–392]. Various metal oxide materials have been recovered from electronic waste and used as supercapacitor electrodes, including MnO2, Co3O4, ZnO, Fe3O4, Fe2O3, and CuO [211,366,391,393]. The supercapacitors fabricated from these recovered materials showed electrochemical behavior close to that found using chemically prepared materials.
Recycling household Zn–C batteries into manganese oxide has been studied. Nanoflower MnO2 was successfully obtained by electrodeposition of Zn–C battery cathode powder [209–211]. A schematic diagram of the recovery of MnO2 nanoflowers from Zn–C battery is presented in Figure 31 [209]. The charge-discharge curve for the MnO2 nanoflower electrode shows linear curves and C s of 294 F g−1 [211]. This high storage efficiency is due to the nanoflower morphology, which is preferable for the diffusion of ions.
![Figure 31
Schematic of the recovery of MnO2 nanoflowers from a Zn–C battery. Copied with permission from ref. [209]; Elsevier, 2017.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_031.jpg)
Schematic of the recovery of MnO2 nanoflowers from a Zn–C battery. Copied with permission from ref. [209]; Elsevier, 2017.
Another method to recover MnO2 from spent batteries is using the sol–gel process with cathode powder leaching solution [365]. The recovered electrode showed 275 F g−1 at 1 A g−1. The electrochemical cycling of Mn3O4, produced from the heat treatment of Zn–C batteries, led to nanoflower MnO2 particles that showed C s of 309 F g−1 at 0.1 A g−1 [209]. Moreover, nanoflower MnO2 showed capacitance retention of 93% after 1,650 cycles; therefore, recycled nanoflower MnO2 is an excellent material for the supercapacitor industry [209].
There are different methods to recover cobalt oxides from spent LiB, and metal oxide electrode capacitance varies according to these methods. The high surface area and microporosity of the as-prepared Co3O4 enhance electron transfer and ion diffusion. There is a connection between the number of cycles and the amount of capacitance retained. The Co3O4 samples maintained approximately 89.1% of their capacitance after 1,000 cycles [366]. The redox mechanism primarily regulates the capacitance action.
Aboelazm et al. recycled LiBs into hierarchical nanostructures of Co3O4 using an electrodeposition technique combined with a magnetic field (MF) [391]. The recovered Co3O4 nanostructures had a charge storage capacity of 1,273 F g−1 at 1 A g−1 (four times higher than electrodeposited Co3O4 produced in the absence of an MF). A stability of 96% after 5,000 cycles also demonstrates the enhanced high cycling stability of the Co3O4 nanostructures. Co3O4 recovery from spent LiBs improved in the experiments, suggesting that it could be used as a supercapacitor electrode material. When calculating the diffusion coefficient, the electroactive surface area is essential. The diffusion value for Co3O4(MF) was much higher than that of Co3O4 as a result of Co3O4(MF) hierarchical nanostructures [391]. Figure 32 shows the stability of a supercapacitor fabricated from Co3O4 recycled from LiBs [391]. Additionally, a facile solvothermal method was used to recover Co3O4 from LiBs [366]. C s and capacitance retention of the recovered Co3O4 were 50.8 F g−1 and 89.1%, respectively, making it a good candidate for supercapacitor devices.
![Figure 32
Capacitance retention (stability) for Co3O4(MF) compared with Co3O4. The Coulombic efficiency and some GCD cycles are shown in the insets. Copied with permission from ref. [391]; American Chemical Society, 2018.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_032.jpg)
Capacitance retention (stability) for Co3O4(MF) compared with Co3O4. The Coulombic efficiency and some GCD cycles are shown in the insets. Copied with permission from ref. [391]; American Chemical Society, 2018.
Copper oxide (CuO) NPs were recycled from SIM card waste through a one-pot green process [296]. The supercapacitive behavior of the obtained CuO NPs was investigated because of their extensive range of technological applications for CuO. GCD revealed typical pseudocapacitive behavior for CuO NPs (as shown in Figure 33a), with 542 F g−1. Additionally, cyclic stability of the CuO electrode material was achieved even after 5,000 GCD cycles, with 95.3% retention (as shown in Figure 33b). Moreover, the CuO electrode has lower resistance, a reasonable rate, and good reversibility. The CuO NP electrodes showed excellent electrochemical characteristics; therefore, they are promising candidates for supercapacitors [296].
![Figure 33
(a) GCD curves and (b) stability of copper oxide recovered from SIM cards. Copied with permission from ref. [296]; Elsevier, 2020.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_033.jpg)
(a) GCD curves and (b) stability of copper oxide recovered from SIM cards. Copied with permission from ref. [296]; Elsevier, 2020.
Spent Zn–C batteries have been used as a precursor to produce ZnO supercapacitor electrodes [394] using in situ production of ZnO NPs in the form of an ultrathin film. The CV curve at 100 mV s−1 and other scan rates investigated the charge transfer through a surface redox reaction (Figure 34). ZnO NPs showed high C s of 547 F g−1 at 5 mV s−1 and a low charge transfer resistance of 6.5 Ω [394]. This excellent electrochemical behavior is due to the small size of the ZnO NPs (45 nm) and their spherical morphology.
![Figure 34
Electrochemical behavior (CV) of ZnO NPs in 0.6 M KOH at (a) 100 mV s−1 and (b) at different scan rates. Copied with permission from ref. [394]; Springer, 2019.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_034.jpg)
Electrochemical behavior (CV) of ZnO NPs in 0.6 M KOH at (a) 100 mV s−1 and (b) at different scan rates. Copied with permission from ref. [394]; Springer, 2019.
Fe2O3 has piqued interest in supercapacitor applications among various iron oxides. It has many crystal structures (α, β, γ, and ε); however, the most thermodynamically stable phase is α-Fe2O3 [395]. To improve its electrochemical properties, Fe2O3 nanomaterials, including NPs [396], NRs [397], and NTs [398], with different morphologies, have been explored. Mill scale is a rich source of iron oxide steel industry waste and can be used as a source of iron oxide recovery [399]. Supercapacitor electrode from iron oxide was fabricated by spray deposition onCu with spider silk (SS) as a current collector. The recovered iron oxides (Fe2O3 and Fe3O4) gave a capacitance of 92 F g−1 in a half-cell design; 25 F g−1 was achieved for the full cell arrangement with a stability of 83% [399]. Compared with commercially available electrodes, the fabricated electrodes significantly differed in prices. The recovered iron oxide-based supercapacitors cost less and are easily fabricated. Comparing mill-scale-based supercapacitors with commercial LiBs and supercapacitors, using their data from other sources, [400] shows that high energy costs are a characteristic of supercapacitors. By contrast, LiBs are characterized by high cost, whereas mill-scale supercapacitors using the data from ref. [399] are broadly competitive with commercial LiBs and supercapacitors. In another work by Fu et al., mill scale was used to produce Fe2O3 and then convert it to hollow porous Fe2O3 microrods [393]. The hollow and porous Fe2O3 microrod-based supercapacitor electrodes showed 346 F g−1 at 2 mV s−1 with 88% capacitance retention over 5,000 cycles. Industrial waste red mud has also been used as a supercapacitor electrode material as it contains a rich mixture of metal oxides. Red mud-based supercapacitor electrodes have C s of 317 F g−1 at a 10 mV s−1 scan rate with retention of approximately 97% after 5,000 cycles with high electrode stability and performance [392].
3.1.3 Hybrid nanomaterials
Carbon materials possess good stability, but they have low capacitance. By contrast, pseudocapacitor materials show high capacitive values but low stability. Therefore, it is necessary to develop a hybrid energy system comprising both materials and investigate their synergetic effect on capacitive storage. To improve the electrochemical efficiency of transition metal oxides, Co3O4 recovered from industrial and domestic wastes has been combined with nanocarbon materials, such as graphene, CNTs, and several mesoporous materials with specific structures. Many hybrid materials have been obtained from waste including carbon/carbon (AC/MWCNTs and CQDs/AC) [388], metal oxide/carbon (MnO2/graphene, CaO/AC, AC/SrFe12O19, and NiCo2O4/AC) [28,210,364], and metal sulfide/carbon (NiS2/graphene) [401].
CaO/palm kernel shells (ACPKS) are composed of calcium oxide, and AC extracted from the chicken eggshell and ACPKS waste, respectively [28,402]. By comparing CaO/ACPKS and ACPKS electrodes, it was found that CaO/ACPKS has a long discharge time, high charge accumulation, and high capacitance (222 F g−1 at 0.025 A g−1) [28]. CaO/ACPKS electrodes have lower resistances (0.44 Ω) than ACPKS (0.86 Ω). This indicates an improvement in conductivity with the addition of CaO to the AC. CaO/ACPKS has high electrochemical stability of 93% and a high energy density of 27.9 W h kg−1 [28]. This excellent electrochemical behavior could be attributed to the synergistic effects of the hybrid structure of CaO and AC and its unique honeycomb morphology, as shown in Figure 35. Another type of hybrid supercapacitor includes AC from crab shells and SrFe12O19 NPs [364]. At 0.5 and 10 A g−1, AC–CS/SrFe12O19 has C s of 742.6 and 401.3 F g−1, respectively, suggesting good rate capability.
![Figure 35
Schematic of the recovery of CaO from eggshells and AC from palm kernel shells. Copied with permission from ref. [28]; Springer Nature, 2018.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_035.jpg)
Schematic of the recovery of CaO from eggshells and AC from palm kernel shells. Copied with permission from ref. [28]; Springer Nature, 2018.
Porous carbon was made from lemon peel waste [403]. A hybrid supercapacitor with NiCo2O4 and nanograss@biowaste-derived porous carbon was fabricated and evaluated [403]. At a current of 1 mA, the fabricated supercapacitor showed a C s of 17.5 F g−1 and an energy density of 6.61 W h kg−1. The presence of regenerative graphene oxide with NiS2 enhances electrochemical efficiency compared with pure NiS2 [401]. Figure 36 and Table 5 summarize the main sources and findings of supercapacitors fabricated from different waste precursors.
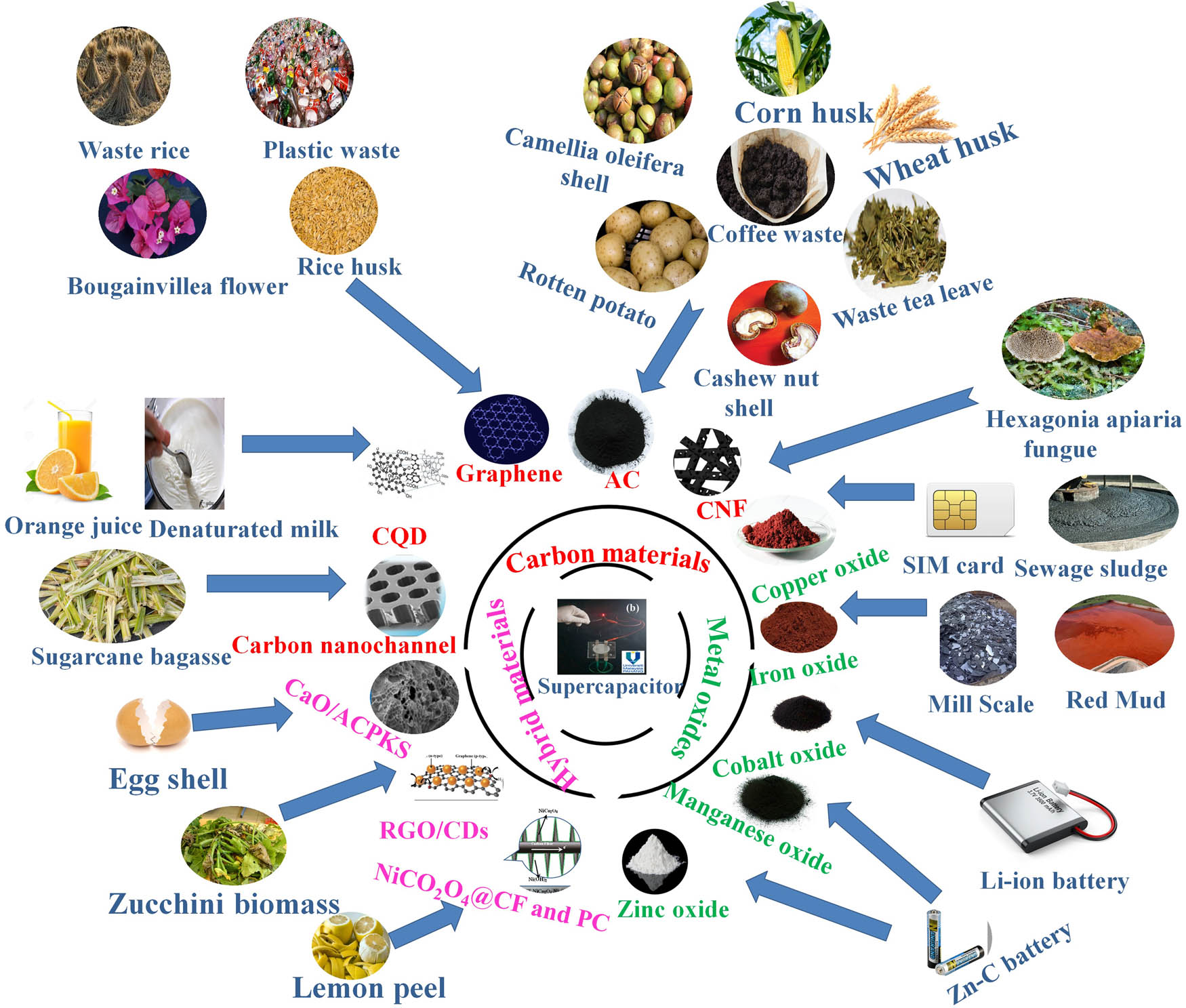
Schematic of the main waste sources used to fabricate supercapacitor electrode materials.
Details regarding supercapacitors fabricated from different waste precursors
| Material type | Waste source | Material | Characteristics | Specific capacitance (F g−1) | Stability (%) | Electrolyte | Ref. |
|---|---|---|---|---|---|---|---|
| Metal oxides | Zn–C batteries | MnO2 | Nanoflower | 208.5 | 88 | 1 M Na2SO4 | [211] |
| Alkaline battery | MnO2 | Spherical cluster shape (15 nm) | 549 | 87.2 | 0.5 M K2SO4 | [404] | |
| Zn–MnO2 battery | MnO2 | Cubic and octahedral particles (10.5 m−2 g−1) | 250 | -- | 0.5 M Na2SO4 | [365] | |
| Zn–C battery | MnO2 | Nanoflower (12 nm and 340 m−2 g−1) | 309 | 93 | 1 M Na2SO4 | [209] | |
| Alkaline battery | MnO2 | Spherical cluster (15 nm) | 549 | 87.2 | 0.5 M K2SO4 | [404] | |
| Zn–MnO2 battery | MnO2 | Well-defined particles (50 nm) | 350 | 0.5 M Na2SO4 | [365] | ||
| LiB | Co3O4 | Hierarchical nanosheets structure (3.2 nm) | 1,273 | 96 | 3 M KOH | [391] | |
| LiB | Co3O4 | Hollow microspheres | 1,256 | 89.1 | 2 M NaOH | [366] | |
| Mill scale | Fe2O3 | Hollow microrods | 346 | 88 | 0.5 M Na2SO3 | [393] | |
| Mill scale | Fe3O4 | Crystalline particles (50 nm to 5 µm) | 92 | 80 | 0.5 M Na2SO3 | [399] | |
| Red mud | Fe2O3 | Spherical NPs (30–50 nm) | 317 | 97 | 6 M KOH | [392] | |
| Zn–C battery | ZnO | Semi-spherical NPs (45 nm) | 547 | — | 0.6 M KOH | [394] | |
| SIM card | CuO | Cauliflower (3.05 nm and 16 m−2 g−1) | 542 | 95.5 | 1 M KOH | [296] | |
| Carbon materials | Sugarcane bagasse | Carbon nanochannel | Open channel morphology (100–150 nm and 1,260 m−2 g−1) | 280 | 72 | 1 M H2SO4 | [405] |
| Corncob | Porous carbon | Unordered porous structure (1,210 m−2 g−1) | 314 | 82 | 6 M KOH | [406] | |
| Allium cepa peel | Oxygen self-doped carbon nanospheres | Spherical shapes (63–66 nm and 2,962 m−2 g−1) | 189.4 | 90 | 3 M KOH | [18] | |
| Rotten potatoes | Activated carbon | Three-dimensional micro\meso\macropore structure (960 m−2 g−1) | 269 | 95 | 6 M KOH | [407] | |
| Loofah sponge | Porous activated carbon | Hierarchical porous structure (2,718 m−2 g−1) | 309.6 | 81.3 | 6 M KOH | [408] | |
| Flowers of Borassus flabellifer | Activated carbon | Sheets-like morphology (930.3 m−2 g−1) | 247 | 94 | 1 M H2SO4 | [409] | |
| Leather waste | Activated carbon | Smooth porous structure (381 m−2 g−1) | 550 | 82 | 6 M KOH | [410] | |
| Hexagonia apiaria fungus | Carbon nanofiber | Fibrous morphology for CNFs (620 nm) | 324 | 70 | 6 M KOH | [411] | |
| Biowaste sago bark | Carbon nanospheres | Porous structure | 180 | 94 | 5 M KOH | [17] | |
| Lablab purpureus | Carbon nanospheres | Uniform and spherical shape | 300 | 94 | 5 M KOH | [19] | |
| Biowaste oil palm leaves | Porous nanocarbons | Spherical shape (20–40 nm) | 368 | 96 | 5 M KOH | [20] | |
| Oil palm fronds | Porous carbon NPs | Uniform particle (35–45 nm) | 309 | 95 | 5 M KOH | [374] | |
| Hybrid materials | Egg shells and palm kernel shells | CaO/ACPKS | Honeycomb morphology (476.7 m−2 g−1) | 222 | 93 | 1 M Na2SO4 | [28] |
| Sewage sludge | Cu2O–Cu cubes | Hierarchical flower-like (285.24 m−2 g−1) | 389.9 | 96 | 6 M KOH | [412] | |
| Zn–C battery | MnO2/graphene | Nanoflowers/nanosheets | 177.6 | 95.2 | 1 M Na2SO4 | [210] | |
| Durian peel | CQDs/AC | Spherical shape (10 nm) | 60 | — | 1 M KOH | [388] | |
| Waste dry battery | NiS2@graphene oxide | Three-dimensional nanoflower structure | 1,932.5 | 83.34 | 6 M KOH | [401] | |
| Crab shell | AC–CS/SrFe12O19 | Multihierarchical porous carbon composites | 742.6 | 94.5 | 6 M KOH | [364] | |
| Lemon peel | NiCo2O4@ porous carbon | Porous carbon (1,012 m−2 g−1) | 735.9 | 92 | PVA/KOH | [403] |
3.2 Batteries
Batteries are electrochemical systems that store electricity and then emit the energy as electrical output during the discharge process. There are two types of cells: primary (nonrechargeable batteries, such as alkaline and dry cell batteries) as well as secondary (rechargeable, which can be repeatedly discharged and recharged, e.g., LiBs and lead-acid) [413]. Besides considerations of NM purity, performance quality, and process scalability, the total cost of extracting NMs from waste sources is crucial in increasing waste use. By minimizing the number of processing steps, costs can be reduced and streamlined. A breakthrough in this field may be an essential step toward the number of recycled materials used in batteries for energy storage [399]. Similar to supercapacitors, batteries suffer from high electrode material production costs. Therefore, recycling waste materials into NMs as battery electrodes is promising. As shown in Figure 37, the number of reports on recycling waste materials into battery electrode materials (or recycling spent batteries into valuable materials) has dramatically increased.
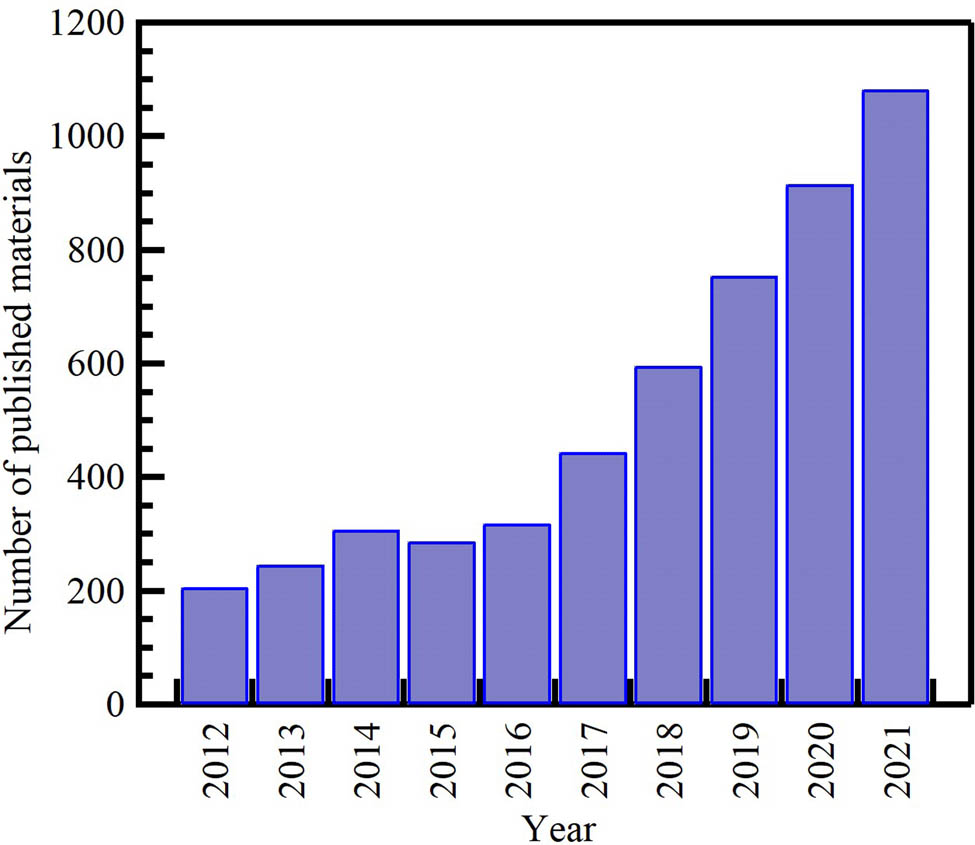
Publication distribution about waste recycled into batteries (or battery recycling).
3.2.1 CNMs
Carbon-based materials have been commonly used as anodes in LiBs. Carbon-based materials, including graphene, AC, CNTs, and CNFs, are promising as advanced electrodes for high-performance batteries. This is because of their high electrical conductivity, structural tunability at the atomic/morphological levels, stability under extreme conditions [414], low cost, variety of forms (composites, fibers, aerogels, sheets, powders, tubes, etc.), electrocatalytically active sites for a wide range of redox reactions, and large specific surface area [415]. Carbon-based materials have been consumed exponentially because they have shown excellent energy storage characteristics. Therefore, they have to be obtained in different ways. Scientists have developed materials with excellent electrochemical properties by converting waste into valuable nanostructured carbon materials. These waste-derived carbon materials have demonstrated promising effects in various applications, particularly energy storage.
CNFs are promising candidates for LiBs due to their large surface-to-volume ratio, fast kinetics, and 3D conductive networks. LiBs can be stored through the following mechanisms: (1) intercalation; (2) vacant space between bundles; (3) adsorption on the outer surface; and (4) defect sites, nanopores, and cavities [416]. CNFs are 1D sp2 hybridized carbon nanostructures with aspect ratios (length/diameter) exceeding 100. CNFs are promising alternatives to commonly used graphite-based anode materials.
Since CNFs are derived from nonrenewable precursors, finding other sustainable resources is necessary to develop high-performance CNFs materials and reduce fossil fuel use.
Tao et al. prepared CNFs from waste biomass (walnut shells) and demonstrated a significant possibility for transforming low-cost biomass into high-value carbon materials for energy storage. The electrochemical performance was investigated and showed that CNFs fabricated at 1,000°C have a high capacity of 271.7 mA h g−1 at 30 mAg−1. The charge–discharge curves of the electrodes are shown in Figure 38 [417].
![Figure 38
CV curves of CNFs at first cycle (a), and CNFs-1000 at first five cycles (b), charge/discharge curves (c). Adapted with permission from Ref. [419], Royal Society of Chemistry, 2018.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_038.jpg)
CV curves of CNFs at first cycle (a), and CNFs-1000 at first five cycles (b), charge/discharge curves (c). Adapted with permission from Ref. [419], Royal Society of Chemistry, 2018.
Graphene has only recently been introduced as a LiBs cathode material, although it is considered a promising material because of its excellent electrochemical performance [418]. Stacked platelet graphene NFs have good ion diffusion because of their lateral dimensions, which allow Li+ to diffuse into the interlayer space much more easily and with reversibility. Therefore, graphene has improved energy storage capacity (461 mA h g−1).
It has recently become highly desirable to synthesize graphene sheets using biomass sources such as butter, honey, foodstuffs, and gelatin. Chen et al. have developed graphene from useless wheat straw by a hydrothermal and graphitization method [419]. The biomass recovered graphene sheets exhibited superior electrochemical properties when used as anode material in LiBs. The results showed excellent rate capability (463.5, 431.4, and 306.8 mAh g−1 at 1, 2, and 5 C, respectively), high capacity (502 mAh g−1 at 0.1 C), and excellent cycling performance (392.8 mAh g−1 at 1 C after 300 cycles), as shown in Figure 39 [419].
![Figure 39
Graphene nanosheet d-spacing and charge capacity relationship. Adapted with permission from ref. [420]; Royal Society of Chemistry, 2011.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_039.jpg)
Graphene nanosheet d-spacing and charge capacity relationship. Adapted with permission from ref. [420]; Royal Society of Chemistry, 2011.
AC has been commonly used in different industries (food, petrochemical, environmental protection, and electric power system) because of its excellent conductivity, high surface area, and large pore volume. AC is expensive and efficient, so low cost and readily available alternatives from recycled material are needed. Recently, much attention has been placed on the use of waste, such as banana peel [370], corn stalk [421], tires [371], and RH [372], to construct AC.
Li et al. prepared mesoporous AC from corn stalk by a simple carbonized-activated process for LiBs anode materials. The mesoporous structure of AC significantly improves its electrochemical performance. After 100 cycles at 0.2 C, the mesoporous AC demonstrated a high reversible capacity of 504 mAh g−1 [421]. Shilpa Kumar and Sharma prepared high surface area carbon material from waste tires [371]. The acid-treated carbon showed excellent electrochemical performance with a high reversible capacity of ∼670 mAh g−1 after 100 cycles [371]. Yu et al. prepared AC derived from RH via carbonization at a low temperature and activation at a high temperature. After 100 cycles at 0.2 C, the capacity remained at 448 mAh g−1 [372]. Fu et al. prepared AC derived from RH for LiBs anode materials. After charging–discharging for 60 cycles, the capacity of LiBs remained at 400 mAh g−1 [422].
CNTs, like many other NMs, are still relatively new in the industrial field. Thus, CNT EOL considerations and the application of recycled waste have still not been widely experimented with or studied [367]. One of the primary considerations is the application of recycled industrial waste in CNTs and their reuse in batteries. As for batteries in general, critical aspects to evaluate or measure performance are capacity, life cycle, charge, discharge rates, and safety. Those are the main properties that should be investigated while studying prospective materials and making recycled CNTs in batteries fabrication worthy. CNTs have proved their applicability in high energy and high-power density LiBs because of their unique electromechanical and chemical properties. One of the critical factors to consider while reusing CNTs in batteries is the energy required to reproduce CNTs from waste compared with the energy needed for virgin material and the energy produced by the recycled CNTs. Recycled SWCNTs have been effectively used for Li+ battery coins for more than one life cycle, with promising results. As presented in Figure 40, recycled CNTs perform better than pure SWCNTs [423].
![Figure 40
(a) CV and (b) charge–discharge curves of an electrode obtained from rice husk. Adapted with permission from ref. [372]; Elsevier, 2018.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_040.jpg)
(a) CV and (b) charge–discharge curves of an electrode obtained from rice husk. Adapted with permission from ref. [372]; Elsevier, 2018.
CNTs have a much lower resistance than AC, which allows them to have a much higher power density. If all interstitial sites (intertube channels, inner-shell van der Waals spaces, and inner cores) are accessible for Li intercalation, CNTs might have a higher Li capacity. The reversible lithium-ion capacities of CNT-based anodes are substantially higher than those of conventional graphite anodes. Furthermore, the open structure and enriched chirality of CNTs help improve power and electrical transport in CNT-based LiBs.
Yeast-fermented wheat dough scaffolds were used to prepare CNTs via a simple, green, and sustainable activation method. The recovered CNTs were used as a sulfur host for a lithium-sulfur battery. The Li–S cell demonstrated excellent cyclic performance with a capacity of 450 mAh g−1 [423]. Figure 40 shows the charge–discharge curves of the electrode at different cycles.
3.2.2 Metal oxide-based nanomaterials
Generally, metal oxides are used in batteries as cathode- or anode-like lithium metal oxide, the cathode material for LiBs [424]. Particularly, nanostructured metal oxides, such as MnO2, Fe3O4, and Co3O4, have the potential as electrodes for energy storage systems.
MnO2 has been used as both cathode and anode for LiBs because of its environmentally friendly characteristics, low cost, and high theoretical capacity (1,230 mAh g−1) [425]. MnO2 is considered an outstanding anode material for LiBs [426] but has disadvantages, such as low specific charge–discharge capacity, poor electrical conductivity, and volume expansion, which inhibit its commercial use [427]. Waste eggshell, considered as a natural hard template with high porosity, can synthesize MnO2 with different morphologies, limit particle size, and improve electrochemical performance [428]. MnO2 produced from manganese sulfate using eggshell as a hard template demonstrates the best electrochemical performance (2156.6/1008.2 mA h g−1 at 100 mA g−1 and average Coulombic efficiency of 97.7%) [429]. LiMn2O4 could be obtained from waste Zn–Mn batteries and delivered a high discharge capacity of 103.6 mAh g−1 [430].
Using a hydrometallurgical process, nanostructured Co3O4 has been successfully recovered from spent LiBs [431]. A GCD between 0.02 and 2 V vs 3 V vs Li/Li+ with a constant current density of 125 mA g−1 evaluated the electrochemical performance of Co3O4 at 760.9 mAh g−1 with a Coulombic efficiency of 99.7% [431]. A waste Zn–C battery was separated to access the powdered materials and used as a precursor to synthesize ZnO NPs [432]. The formation of new composites has also included using ZnO NPs recycled from alkaline batteries. For example, ZnO NPs synthesized from waste Zn–C batteries have effective optical properties, spherical shape, and a 50 nm size [432].
Conversely, an alternate research paper prepared a ZnO sample using a chemical method (the sol–gel synthetic method) to produce ZnO NPs with high electrochemical performance, calculated by GCD cycling (like anodes for LiBs), and a reversible capacity of 1,652 and 318 mAh g−1 at 1st and 100th cycles, respectively. Additionally, the methodology can perhaps carry a reversible capacity of 229 mAh g−1 at a high current density of 1.5 C [433]. Therefore, since the recycled ZnO has similar characteristics, it will be helpful for battery applications.
Interconnected α-Fe2O3 NPs (30–60 nm) used as anode material for LiBs have been prepared by recycling tin ore tailings [434]. The electrochemical behavior of α-Fe2O3 was observed to exhibit the finest performance for LiBs, displaying a capacity of 1,146 mAh g−1 at 0.5 A g−1 after 300 cycles [434]. Additionally, it was hypothesized that an α-Fe2O3 electrode with a smaller surface area might maintain a smaller irreversible capacity loss through the cycles than that of γ-Fe2O3. Simultaneously, the larger average pore size provides an earlier gain for the electrolyte infiltration into the α-Fe2O3 nanoelectrode; rapid infiltration is advantageous for transporting Li+ from the electrode substance to the electrolyte [435]. Fe2O3 is produced by direct chemical processing using an industrial mill and has a theoretical capacity of 1,007 mAh g−1 [393]. It is also nontoxic, low-cost, and environmentally friendly. Chemical treatment has already transformed mill scale, a waste material from the steel industry widely accessible and composed of a mixture of iron oxides, into hollow and porous Fe2O3 microrods [393].
Recently, TiO2 NPs were effectively biosynthesized by applying remnant water, an ideal kitchen waste [436]. The nanosized TiO2 enhances cell performance and cycling stability by increasing the surface area, the electrode interaction area, and consequently shortening the path lengths for Li-ion and electron transport. The electrochemical significance of TiO2 was evaluated in a half-cell configuration, from 1 to 3 V at room temperature via a current density of 33 mA g−1, with a high reversible capacity of 164 mA g−1 following 60 cycles 98% capacity retention was revealed. This technique can be studied further for synthesizing other materials for LiBs.
3.2.3 Hybrid nanomaterials
Recycling spent LiBs make it possible to create LiFePO4/rGO composites from cathode material and spent graphite anode. Nanostructured LiFePO4/rGO batteries have high capacities and high Coulombic efficiencies. Using melamine as a nitrogen source, biowaste bagasse was turned into carbon and nitrogen-doped carbon (NBC) [437]. The electrochemical performance of S@NBC (sulfur and NBC) composite cathodes created by assembling Li–S cells exhibited significantly high performance. S@NBC had the highest output with a reversible capacity of 1,169 mAh g−1 at 0.2 C, and capacitance of 8.98 mAh g−1 was retained after 200 cycles with a capacity retention of 77%. Furthermore, the composite had an outstanding rate potential of 454 mAh g−1 at a 4 C rate [437].
Xu et al. constructed 3D porous carbon@graphene composites from waste cigarette filters. Their results showed a high capacity of 1,010 mAh g−1 at 0.2 C after 200 cycles, with only ∼0.04% capacity loss per cycle [438]. Additionally, Wong et al. were able to generate silicon NPs from RH using a magnesiothemic reduction method; these NPs were used to manufacture Si/graphene, which serves as an anode in LiB. The silica composites displayed C s of 1,000 mAh g−1 at 1,000 mA d−1 [103]. Figure 41 and Table 6 summarize the main sources and findings of batteries fabricated from different waste precursors.
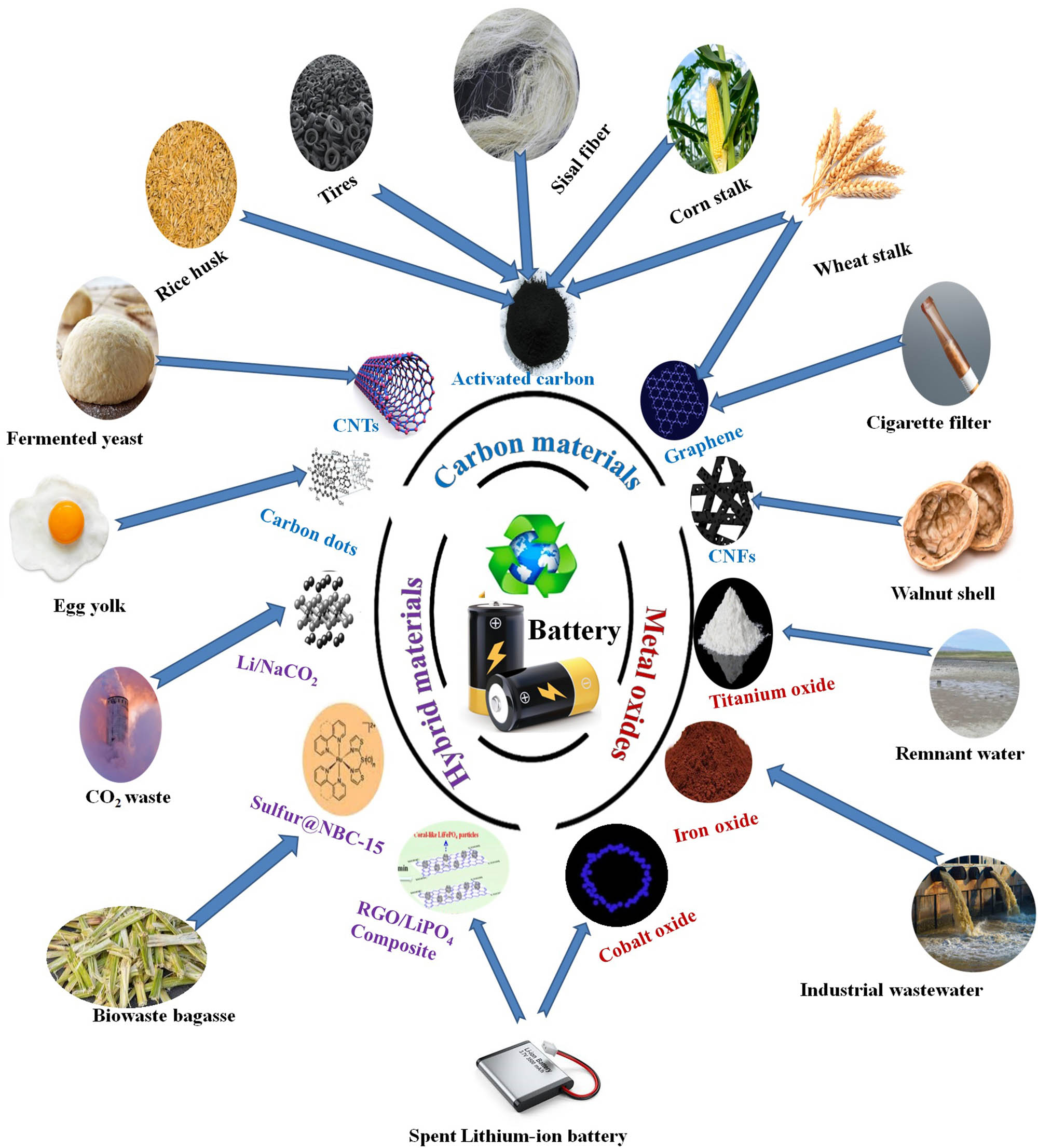
Schematic diagram of the primary waste sources used to fabricate battery electrode materials.
Details regarding batteries fabricated from different waste precursors
| Material type | Waste source | Material | Characteristics | Capacity (m Ah g−1) | Capacity retention (%) | Electrolyte | Ref. |
|---|---|---|---|---|---|---|---|
| Metal oxide | LiBs | Co3O4 | Spherical particles, 80 nm | 760.9 | 99.7 | 1 M LiPF6 | [431] |
| Industrial wastewater | α-Fe2O3 | Nanopowder, 37.4 nm | 760 | 74.1 | 1 M LiPF6 | [435] | |
| Tin ore tailings | α-Fe2O3 | Irregular NPs, 30–60 nm | 1,146 | 64.4 | 1 M LiPF6 | [434] | |
| Mill scale | Fe2O3 | Hollow and porous structure, 74 m2 g−1 | 953 | 71 | 1 M LiPF6 | [393] | |
| Remnant water | TiO2 | Rough and porous morphology, 34.07 m2 g−1 | 1,276 | 98 | 1 M LiTFSI | [439] | |
| Zn–Mn batteries | LiMn2O4 | — | 103.6 | 71.4 | 1 M LiPF6 | [430] | |
| Carbon material | Walnut shells | Carbon nanofibers | Long and smooth surface, 280 nm | 271.7 | 80 | 1 M LiPF6 | [417] |
| Wheat straw | Graphene | Ultrathin nanosheet frameworks | 502 | 92.4 | 1 M LiPF6 | [419] | |
| Banana peel | Activated carbon | Hierarchically porous structure, 194 m2 g−1 | 1,205 | 100 | PVA/KOH | [370] | |
| Corn stalk | Activated carbon | Novel mesoporous AC, 2–10 nm | 504 | 90 | 1 M LiPF6 | [421] | |
| Tires | Activated carbon | 870 m2 g−1 | 880 | 80 | 1 M LiPF6 | [371] | |
| LiB | SWCNTs | High purity SWCNTs | 650 | — | 1 M LiPF6 | [367] | |
| Sisal fibers | Activated carbon | No definite shape | 998 | — | 1 M LiPF6 | [440] | |
| RH | Activated carbon | Hierarchical porous structure, 2,176 m2 g−1 | 448 | 100 | 1 M LiPF6 | [372] | |
| Hybrid material | Spent batteries | LiFePO4/graphene | Graphene nanoribbons covering LiFePO4 | 162.6 | 98 | 1 M LiPF6 | [441] |
| Discarded cigarette filters | Hierarchically porous carbon@graphene | 3D porous carbon@graphene layers, 436.9 m2 g−1 | 1,010 | 91.4 | Li2S6/DOL/DME | [438] |
4 Energy conversion applications
4.1 Fuel cells
Fuel cells have attracted several researchers’ interest due to their promising applications. Fuel cells are electrochemical cells in which the chemical energy of a fuel (often H2) is converted into electricity in the presence of an oxidizing agent (such as O2) through redox reactions [442]. Sir William Grove invented the earliest known fuel cell in 1838 [443]. Fuel cells usually consist of an anode, cathode, and an electrolyte that allows charges (often protons) to move between the anode and cathode [444]. A catalyst (such as Pt) oxidizes the fuel (H2) into protons and electrons at the anode. Through the electrolyte, protons move from the anode to the cathode. Simultaneously, through an external circuit, electrons travel from the anode to the cathode leading to direct current electricity. Another catalyst assists ions, electrons, and oxygen in reacting with each other to form water and other products at the cathode [445]. There are many types of fuel cells, such as proton exchange membrane fuel cells, phosphoric acid fuel cells, solid acid fuel cells, alkaline fuel cells (AFCs), high-temperature fuel cells, and microbial fuel cells (MFCs) [446].
4.1.1 CNMs
Zhou et al. prepared heteroatom-doped porous CNFs via a simple pyrolysis method using natural SS as a precursor for the oxygen reduction reaction (ORR) in MFCs [447]. The prepared CNFs showed good ORR activity in alkaline conditions (0.98 V onset potential versus the reversible hydrogen electrode (RHE) and 0.85 V half-wave potential), which are higher than Pt/C and common metal-free carbon catalysts. The improved catalytic properties were ascribed to the enriched carbon lattice with electronegative N and S atoms and the high surface area due to the porous and nanofibrillar CNFs structure. Additionally, MFCs applying a prepared CNFs cathode exhibited an 1,800 mW m−2 power density, 1.56 times higher than those applying a Pt/C cathode.
Zhou et al. used eggplant-based biomass with plate-like structures and rich porous networks to prepare N-doped porous graphene (NDPG) catalyst for the ORR in low-temperature fuel cells (LTFCs) [448]. Compared with costly commercial Pt/C catalysts, the synthesized NDPG displayed excellent ORR activity and stability. The measured half-wave potential was approximately 10 mV higher than that of Pt/C in alkaline media, and the stability was higher than Pt/C in alkaline and acidic media. The exceptional catalytic efficiency of the synthesized graphene can be ascribed to its mesoporous structure with an ultra-high surface area (1,969 m2 g−1), along with its high electron conductivity and effective nitrogen content.
Zhao et al. produced high yield and quality graphene sheets using disposable paper cups as a carbon source and Fe2+ as a catalyst [449]. Moreover, Pt/graphene and Fe/graphene were obtained from the synthesis. Pt/graphene catalysts showed high ORR activity in fuel cells. Additionally, their electrochemical measurements displayed that prepared Pt/graphene catalyst possessed a better electrochemical efficiency for ORR than Pt/C catalyst.
Ma et al. extracted S-doped graphene (SG) from Li–S batteries and used it as a sustainable and economical metal-free electrocatalyst for the ORR [450]. The prepared SG electrocatalyst, with transferred electron number (n ≈ 3.13), exhibited higher electrocatalytic activity than pristine undoped graphene, higher fuel selectivity, and longer ORR durability. Additionally, SG electrocatalyst had more stable electrocatalytic activity than commercial Pt/C.
Through pyrolysis of economic, biobased corn starch, urea, and FeCl3, Pan et al. synthesized Fe–N-doped graphene (FeNG) electrocatalysts for ORR in fuel cells [451]. Compared with the commercial Pt/C catalyst, the prepared FeNG catalysts showed a high electrocatalytic activity that was approximately 99 mV more positive in KOH, as shown in Figure 42. Additionally, the catalyst was 34 mV more negative in H2SO4. By adjusting the form of the doped species, the ORR of the prepared FeNG catalysts could be controlled at various pH levels. Additionally, ORR potential in basic media may be attributed to the sites of quaternary N and Fe/Fe3C, whereas acidic media ORR performance originated mainly from Fe–N x complexes.
![Figure 42
ORR electrochemical activities of FeNG catalysts in 0.1 M KOH electrolyte: (a) various linear sweep voltammetry (LSV) curves of samples at a 5 mV s−1 scan rate and 1,200 rpm rotation rate; (b) E
1/2 and E
O values obtained from LSV curves in (a); (c) FeNG-1,100 LSV curves at a 5 mV s−1 scan rate and various rotation speeds, and (d) LSV data-based Tafel plots for FeNG catalysts. Copied with permission from ref. [451]; John Wiley and Sons, 2015.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_042.jpg)
ORR electrochemical activities of FeNG catalysts in 0.1 M KOH electrolyte: (a) various linear sweep voltammetry (LSV) curves of samples at a 5 mV s−1 scan rate and 1,200 rpm rotation rate; (b) E 1/2 and E O values obtained from LSV curves in (a); (c) FeNG-1,100 LSV curves at a 5 mV s−1 scan rate and various rotation speeds, and (d) LSV data-based Tafel plots for FeNG catalysts. Copied with permission from ref. [451]; John Wiley and Sons, 2015.
Zhou et al. used a novel, facile, and inexpensive technique to prepare PNDG as a high-performance electrocatalyst (with a very large surface area of 1,152 m2 g−1) for ORR in LTFCs using intrinsically porous biomass (soybean shells) as a carbon and nitrogen source [452]. The prepared NDG catalyst showed an onset and half-wave potential of −0.009 and −0.202 V (vs a saturated calomel electrode), respectively, which are similar to the catalytic behavior of common Pt/C catalyst in alkaline media. Compared with Pt/C, the catalyst performed well in acidic media and exhibited higher ORR stability. Furthermore, the NDG catalyst showed a higher ORR stability and a greater CO and CH3OH tolerance than the Pt/C catalyst in alkaline media.
Liu et al. investigated biomass-derived AC doped with N as a cost-effective catalyst with high-performance ORR performance in an MFC with a maximum output voltage of 544 ± 6 mV and power density of 977 ± 32 mW m−2. The generated electricity from four of the air cathode MFCs connected in series was able to light a LED. Using iron NPs anchored on graphene as a catalyst and urea as a combined source of nitrogen moieties and depolymerization agent, Sridhar and Park presented an easy and efficient synthesis of iron and nitrogen codoped CNTs (Fe@NCNTs-rGO) from PET waste using microwaves for ORR in fuel cells [453]. The prepared Fe@NCNT-rGO electrocatalyst (n ≈ 4) showed a positive E 1/2 of 0.75 V relative to the RHE. Synthesized Fe@NCNT-rGO exhibited unique electrocatalytic ORR behavior with a good onset potential of 0.896 V vs RHE.
Additionally, Fe@NCNT-rGO electrocatalyst possessed a lower current density of 4.8 mA cm−2 concerning commercial Pt/C electrodes (current density of 5.7 mA cm−2). Furthermore, compared with commercially available 20% Pt/C, Fe@NCNT-rGO had outstanding methanol tolerance in direct methanol fuel cells. This can be attributed to embedding catalytically active Fe–N x moieties inside the CNT walls. Consequently, oxidation and consequent fouling of catalyst due to the crossover of methanol was hampered.
Cai et al. synthesized iron and nitrogen codoped CNTs (Fe–N-CNTs) for ORR in fuel cells by catalytic pyrolysis of PP waste plastics over Fe–Al2O3 [454]. The prepared Fe–N-CNT850 catalyst outperformed pristine Fe–CNT in terms of catalytic activity, as shown in Figure 43. Compared with Fe–CNT, the onset and half-wave potential of the Fe–N-CNT850 were 137 and 156 mV, respectively. The limiting current density of Fe–N-CNT850 was also higher than that of Fe–CNT. Compared with commercial Pt/C catalyst, the prepared Fe–N-CNTs showed an onset potential of 0.943 V vs RHE, a half-wave potential of 0.811 V vs RHE, and increased stability anti-poisoning properties. Additionally, the prepared Fe–N-CNTs exhibited greater methanol tolerance than that of 20% Pt/C catalyst, and the current of Fe–N-CNT850 was slightly lowered upon methanol addition, continuing to be stable for about 800 s. This indicated that Fe–N-CNT850 had greater methanol tolerance and poison resistance than the reference Pt/C electrocatalyst.
![Figure 43
(a) LSV curves of O2-saturated solutions for all catalysts at a scan rate of 5 mV s−1 and a rotation speed of 1,600 rpm. (b) Fe–NCNT850 LSV curves at various rotation speeds. (c) Fe–NCNT850 K–L plot at various potentials. (d) Fe–NCNT850 catalyst electron transfer number (n). Copied with permission from ref. [454]; John Wiley and Sons, 2020.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_043.jpg)
(a) LSV curves of O2-saturated solutions for all catalysts at a scan rate of 5 mV s−1 and a rotation speed of 1,600 rpm. (b) Fe–NCNT850 LSV curves at various rotation speeds. (c) Fe–NCNT850 K–L plot at various potentials. (d) Fe–NCNT850 catalyst electron transfer number (n). Copied with permission from ref. [454]; John Wiley and Sons, 2020.
Moo et al. transformed waste plastics (PE, PP, PS, and PET) into CNTs for ORR in fuel cells using sequential pyrolysis and catalytic CVD techniques [74]. At temperatures between 500°C and 800°C, there were only small variations in the performance of CNTs based on the plastic source. ORR onset potentials range from −0.18 to −0.21 V for electrode surfaces modified with CNTs synthesized at 800°C. However, electrode surfaces modified with CNTs synthesized at 500°C showed ORR potentials ranging from −0.11 to −0.14 V. The lowest recorded onset ORR potential of CNTs synthesized from MP at 500°C was −0.11 V.
Veksha et al. synthesized MWCNTs by catalytic CVD using 11.8 and 27.5 wt% PET waste plastics for ORR in fuel cells [455]. MWCNTs from PET-12 and PET-28 had heterogeneous electron transfer concentrations of 0.0042 cm s−1, like those observed in Pt/C and commercial MWCNTs (CCNTs), 0.0043 and 0.0040 cm s−1, respectively. GC and Pt/C had onset potentials of −0.19 and −0.096 V, respectively, whereas CCNTs, MWCNTs from PET-28, and MWCNTs from PET-12 exhibited onset potentials of −0.091, −0.057, and −0.033 V, respectively. These overpotentials are smaller than those recorded for graphene-based materials, like pure graphene (−0.18 V), NDG (−0.16 V), GO (−0.18 V), and electrochemically fabricated rGO (−0.11 V). The lower onset potentials of commercial and synthesized MWCNTs indicated their high electrocatalytic activity and confirmed that these materials would substitute noble metal electrocatalysts and graphene-based materials for ORR applications. Interestingly, compared with CCNTs, MWCNTs from PET-28 and PET-12 displayed higher current densities (0.037 and 0.060 mA cm−2) at peak potentials, suggesting a higher volume of decreased oxygen molecules per unit of time. Table 7 summarizes common waste recycled carbon materials used for fuel cell applications.
Summary of common waste-recycled carbon materials used for fuel cell applications
| Waste source | Material | Transferred electrons (n) | Onset potential/half-wave potential (V) | Electrolyte | Ref. |
|---|---|---|---|---|---|
| Human urine | Urine carbon | 3.7 ± 0.05 | −0.03/−0.13 | 0.1 M KOH | [456] |
| Grass | N-doped carbon nanodots | 3.94 | −0.08/−0.18 | 0.1 M KOH | [457] |
| Pomelo peels | Nitrogen and cobalt-activated carbon | 3.90 | −0.09/−0.18 | 0.1 M KOH | [458] |
| Soymilk | N/Co codoped carbon | 3.80 | −0.005/−0.14 | 0.1 M KOH | [459] |
| Sheep horn | N/S dual-doped 3D porous graphene | 3.52–3.83 | −0.06/−0.186 | 0.1 M KOH | [460] |
| Catkins | Fe/N codoped CNTs@hollow carbon fibers | 3.91 | −0.098/−0.194 | 0.1 M KOH | [461] |
| Chicken feather | N/S codoped biocarbon flocs | 3.98 | 0.03/−0.13 | 0.1 M KOH | [462] |
| Human hair | Heteroatom-doped carbons with valuable N and S | 3.8–3.90 | −0.016/−0.11 | 0.1 M KOH | [463] |
4.1.2 Metals and metal oxide-based nanomaterials
Passaponti et al. recycled chars (the least valuable waste material from waste-tire pyrolysis) to prepare extremely effective ORR catalysts applied in AFCs and metal-air batteries [464]. The chars were derived from MAP and showed −90 mV (RHE) ORR onset potential, and a 4e− mechanism was observed. The obtained activity was attributed to ZnO NPs in the carbon matrix, its porosity, and its large surface area.
4.1.3 Hybrid nanomaterials
Via pyrolysis of a mixture of a naturally abundant source of seaweed biomass (Sargassum tenerrimum) and deep eutectic solvent (a mixture of choline chloride and FeCl3), Mondal et al. synthesized Fe3O4/Fe-doped magnetic GNs that possessed both high electrical conductivity of 2384.6 mS m−1 and a 220 m2 g−1 surface area [465]. Compared with catalysts commonly used in proton exchange fuel cells in alkaline media, the prepared GNs showed higher ORR performance. High cathodic current density, positive onset potential, and less than 5% hydrogen peroxide formation were observed. Additionally, more than 80% activity retention after 30,000 cycles was obtained, making Sargassum tenerrimum-based GNs sustainable substitutes for currently available metal-based ORR catalysts.
Elessawy et al. prepared a nonprecious and highly active fullerene iron oxide composite as a promising ORR and oxygen evolution reaction (OER) catalyst in AFCs via thermal catalytic dissociation of PET bottle waste [466]. The prepared catalyst on glass carbon (fullerene/GC) (with n
4.2 Solar cells
Solar cells convert solar light into electricity through the photovoltaic effect [468]. Electrical solar cells are usually composed of photovoltaic modules known as solar panels. The operation of solar cells requires the integration of three parameters: (1) the absorption of light (solar light or artificial light) to generate excitons or electron-hole pairs, (2) charge–carrier separation, and (3) charge and separated carrier extraction to an external circuit [469]. There are many types of solar cells, such as silicon solar cells, perovskite (PVK) solar cells (PSCs), DSSCs, bifacial solar cells, QD solar cells, and organic polymer solar cells [470]. Solar cells are currently applied in various applications that include transportation, solar farms, space power, and addressing the energy needs of buildings [471].
4.2.1 CNMs
Graphene has received considerable attention due to its versatile applications. Pandey et al. upcycled waste plastics (PP, PE, and PET) into valuable GNs for DSSCs. Bentonite nanoclay was used as a degradation agent for waste plastics via pyrolysis processes in a nitrogen (N2) atmosphere [92]. Recorded results revealed that DSSCs applying GNs part of the photoanode with polymeric electrolyte exhibited a promising FF of approximately 86.4% and an open-circuit voltage (V OC) of 0.77 V, as shown in Figure 44. Interestingly, a short circuit current density (J SC) of 0.302 mA cm−2 was obtained.
![Figure 44
(a) Schematic of a designed DSSC with corresponding (b) J–V curves, (c) EIS curves, and (d) equivalent circuits. Copied with permission from ref. [92]; Nature, 2021.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_044.jpg)
(a) Schematic of a designed DSSC with corresponding (b) J–V curves, (c) EIS curves, and (d) equivalent circuits. Copied with permission from ref. [92]; Nature, 2021.
Wu et al. synthesized green and affordable electrochemically active graphene-encapsulated cobalt NPs embedded in an N, P, and S codoped carbon 3D matrix through direct pyrolysis of kelp biomass [472]. The prepared composite was labeled as Co-KCM-1000 and demonstrated promising activity as a counter electrode (CE) for DSSCs because of its excellent electrocatalytic performance in a triiodide reduction reaction. The exceptional structure of multilayer-graphene-encapsulated cobalt NPs is valuable for the increase in the contact area and the prohibition of dissolution and accumulation of metal NPs. The prepared Co-KCM-1000 and Pt as CEs in DSSCs were compared. The photovoltaic parameters, including open-circuit V OC, J SC, FF, and power conversion efficiency (PCE), are recorded in Table 8. The DSSCs assembled by Co-KCM-1000-CE showed a PCE of 7.48%, and the Pt-CE showed 7.64%.
DSSC photovoltaic parameters for Co-KCM-1000- and Pt-CEs
| CE | V OC (V) | J SC (mA cm−2) | FF | PCE (%) |
|---|---|---|---|---|
| Pt | 0.71 | 14.73 | 0.73 | 7.64 |
| Co-KCM-1000 | 0.75 | 14.71 | 0.68 | 7.48 |
Note: Copied with permission from ref. [472]; American Chemical Society, 2018.
DSSCs are important energy conversion devices as they convert solar energy into electricity. Nagaraju et al. prepared biomass-based 3D-activated porous carbon from fallen pine coneflowers that possessed meso/macropores and led to DSSCs with excellent solar energy conversion characteristics [39]. Their study prepared honeycomb-like activated porous carbon (HPC) via pyrolysis and chemical activation under an inert gas atmosphere. A smooth brush was used to uniformly coat the formed HPC material onto fluorine-doped tin oxide (SnO2) glass for cost-effective DSSCs. Under AM 1.5 G illumination, a high J SC of approximately 13.51 mA cm−2 and a PCE of 4.98% were observed, as shown in Figure 45. The observed activity was ascribed to the prepared HPC sample’s hierarchical porous structure and high specific surface area. Interestingly, the recorded data were better than commercially available AC-based CEs applied in DSSCs (J SC = 12.11 mA cm−2 and PCE = 4.45%).
![Figure 45
(a) Schematic of a DSSC applying HPC as the CE with corresponding (b) J–V curves and (c) incident photon-to-current efficiency (IPCE) spectra (with a TiO2 NP photoanode and reference Pt). Copied with permission from ref. [39]; Elsevier, 2017.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_045.jpg)
(a) Schematic of a DSSC applying HPC as the CE with corresponding (b) J–V curves and (c) incident photon-to-current efficiency (IPCE) spectra (with a TiO2 NP photoanode and reference Pt). Copied with permission from ref. [39]; Elsevier, 2017.
GQDs are carbon NMs with fascinating optical properties suitable for solar cell applications. Teymourinia et al. reported the synthesis of biomass-derived GQDs as downconversion materials for DSSCs using corn powder via the hydrothermal method [473]. The GQDs converted UV light into DSSC-favored 450- and 520-nm light. Their results showed a 21% J SC enhancement in solar cells modified with GQDs concerning reference cells. The recorded enhancement was due to the downconversion effect of GQDs, as shown in Figure 46.
![Figure 46
Schematic of the possible mechanism of DSSC enhancement by GQDs. Copied with permission from ref. [473]; Elsevier, 2018.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_046.jpg)
Schematic of the possible mechanism of DSSC enhancement by GQDs. Copied with permission from ref. [473]; Elsevier, 2018.
4.2.2 Metals and metal oxide-based nanomaterials
Ecofriendly and low-cost green synthesized CuO NPs were prepared using Calotropis gigantea plant leaf extract and were applied as the CE in DSSCs [474]. The prepared sample exhibited crystalline nature and good structural, electrochemical, and photovoltaic properties. The results revealed a relatively high solar to electrical energy conversion efficacy of ∼3.4%, a J SC of ∼8.13 mA, a V OC of ∼0.676 V, and an FF of approximately 0.62 via CE-based CuO NPs in the fabricated DSSC.
ZnO nanostructures possess promising efficiency for photovoltaics. Ansari et al. reported preparing 3D ZnO hierarchical superstructures (HSs) derived from biomass for enhanced photovoltaic performance [475]. They used a facile one-step hydrothermal method for assembling compacted ZnO NRs to build a ZnO HS through the assistance of anionic polygalacturonic acid as a crystal growth modifier. Their results showed that 3D ZnO HSs exhibited an efficiency (η) of approximately 5.37% compared with only 3.48% for ZnO NRs. This substantial enhancement in ZnO HSs was attributed to good charge separation and collection because of the enhanced electron transfer capability of 3D-compacted ZnO HSs, as shown in Figure 47. Additionally, improved light scattering, higher BET surface area for sensitizer loading, and efficient electron injection from the applied dye (D1) were observed.
![Figure 47
(a) J–V for ZnO heterostructure-based photovoltaic devices and corresponding (b) IPCE, (c) Nyquist, and (d) Bode phase plots. Copied with permission from ref. [475]; Elsevier, 2018.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_047.jpg)
(a) J–V for ZnO heterostructure-based photovoltaic devices and corresponding (b) IPCE, (c) Nyquist, and (d) Bode phase plots. Copied with permission from ref. [475]; Elsevier, 2018.
Zhu et al. improved efficiency, reduced cost, and made sustainable and renewable PSCs by recycling FTO/TiO2 substrates from degraded CsPbIBr2 PSCs [476]. They found that some residual CsPbIBr2 decreased the conduction band minimum difference of recycled FTO/TiO2 from 0.51 to 0.36 eV at the CsPbIBr2/TiO2 interface. Work characteristics of the TiO2 layer decreased from 4.13 to 3.89 eV, whereas defects decreased, and halide phase separation was suppressed in the upper CsPbIBr2 film. These characteristics help decrease nonradiative recombination in the subsequent PSCs and expand charge transporter extraction. Accordingly, the average efficiency of the CsPbIBr2 increased, with PSC efficacy enhanced by 20%, from 6.51% ± 0.62% to 8.14% ± 0.63%, with an excellent PSC producing an impressive PCE of 9.12%, as shown in Figure 48. Consequently, recycling FTO/TiO2 substrates was an eco-friendly method to reduce cost and improve the PSC efficiency.
![Figure 48
CsPbIBr2 PSC performance as synthesized from recycled and pristine FTO/TiO2. Copied with permission from ref. [476]; American Chemical Society, 2020.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_048.jpg)
CsPbIBr2 PSC performance as synthesized from recycled and pristine FTO/TiO2. Copied with permission from ref. [476]; American Chemical Society, 2020.
4.2.3 Hybrid nanomaterials
Ahmed et al. reported the green synthesis of GQDs from corn power by a simple eco-friendly method for application in PSCs [477]. They prepared a ZnO/GQDs hybrid photoelectrode to employ as the electron transport layer. Because of the oxygen agent passivation of GQDs on the ZnO surface, electrons were quickly extracted from the PVK film, and electron-hole pair recombination was elongated. Their recorded results indicated that GQDs improved the light-harvesting ability of PVK by 2% and increased its PCE from 10 to 17.67% under AM 1.5 G illumination.
Arshad et al. proposed an efficient strategy for advanced photovoltaics by embedding a mixture of cobalt and manganese oxides in biomass-derived porous carbon (Co/MnO2–PC) prepared from aloe vera peel [478]. They proposed a unique 3D design to improve the active catalytic sites and increase surface areas, allowing multiple and fast electron transfer channels. The proposed hybrid was applied as an accelerant to reduce triiodide in DSSCs, as shown in Figure 49. Promising cell efficiency and attractive electrochemical stability were observed. Compared with a platinum electrode with an efficiency of 6.44%, higher photovoltaic efficiency of 7.01% was recorded in the hybrid. Additionally, the prepared hybrid showed long-term electrochemical stability in I3 −/I− electrolyte; it exhibited 97% retention of its original efficiency after going through many cyclic voltammetry scans. Table 9 lists the recycled nanomaterials used for solar cell applications.
![Figure 49
J–V curves and PCE values of the prepared Co/MnO2–PC hybrid; inset illustrates the concept of multiple electron diffusion routes for I3
− reduction. Copied with permission from ref. [478]; Elsevier, 2020.](/document/doi/10.1515/ntrev-2022-0129/asset/graphic/j_ntrev-2022-0129_fig_049.jpg)
J–V curves and PCE values of the prepared Co/MnO2–PC hybrid; inset illustrates the concept of multiple electron diffusion routes for I3 − reduction. Copied with permission from ref. [478]; Elsevier, 2020.
Summary of recycled nanomaterials used for solar cell applications
| Material type | Waste source | Material | Solar to electrical energy conversion efficiency (%) | Current density (J SC) (mA cm−2) | Open-circuit voltage (V OC) (V) | Ref. |
|---|---|---|---|---|---|---|
| Metal oxide | Tilia tomentosa (Ihlamur) leaves extract | ZnO | 1.97 | 6.26 | 0.65 | [479] |
| Azadirachta indica (Neem) leaves extract | ZnO | 2.1 | 4.5 | 0.73 | [480] | |
| Plectranthus amboinicus leaves extract | TiO2 | 1.35 | 3.2948 | 0.5793 | [481] | |
| Titania-rich paper industry waste | TiO2 | 0.47 | 1.14 | 0.7 | [265] | |
| Calotropis gigantea leaf extract | Co3O4 | 0.66 | 1.79 | 0.646 | [482] | |
| Red dragon fruit peel wastes | TiO2 | 0.029 | 23.46 | 0.47 | [483] | |
| Carbon material | Waste materials (Chitosan-chitin) | CQDs | 0.077 | 0.674 | 0.265 | [484] |
| Invasive plant species of Eichhornia crassipes | Graphitic carbon | 8.52 | 23.49 | 0.672 | [485] | |
| (Different biomass sources) l-arginine | Carbon nanodots | 0.362 | 0.97 | 0.660 | [486] | |
| Pinecone flowers | AC | 4.98 | 13.51 | 0.71 | [39] | |
| Quince leaves | Porous carbon | 5.52 | ∼14.99 | 0.70 | [487] | |
| Biowaste (palm leaf) | Carbon | 1.85 | 11.53 | 0.632 | [488] | |
| Hybrid material | Rice husk (RH) | nano-Si@ACs | 8.01 | 15.50 | 0.76 | [489] |
| Aloe peel waste | ZnWO4-C | 7.61 | 17.02 | 0.67 | [490] | |
| Fruit peel wastes | Se@AC | 5.67 | 13.26 | 0.648 | [491] | |
| Aloe vera-peel | Carbon integrated Co/Mn-oxide | 3.05 ± 0.02 | 10.43 ± 0.07 | 0.66 ± 0.02 | [478] | |
| Biomass- derived carbon (Aloe peel) | MWO–C, ZWO–C, and CWO–C | 7.33 ± 0.02 | 15.52 ± 0.15 | 0.69 ± 0.02 | [492] |
4.3 Green hydrogen production
Modern industrialization and population growth push the world to find new energy substitutes. Recently, renewable energy resources have received significant attention for sustainable development. Among them, hydrogen is considered the future energy because of its relatively higher energy density (energy per unit mass), ease of transportation in either liquid or a gaseous form with cost-effectiveness, and multiple ways of its production. H2 can be produced on a large scale using renewable and nonrenewable resources and processes. Renewable resources, such as water splitting, can be divided into electrolysis, photoelectrochemical water splitting, and photochemical water splitting. Nonrenewable resources can be generated from hydrocarbon fuels through three main processes: steam reforming, partial oxidation, and autothermal reforming [493] (Table 10).
Summary of recycled nanomaterials used for H2 production
| Material type | Waste source | Material | Process | Conditions/light source/catalyst | Photocurrent/produced H2 amount | Ref. |
|---|---|---|---|---|---|---|
| Hybrid material | Sucrose biomass | TiO2/Pt NPs | Photocatalysis | Simulated sunlight | 6 µmol−1 h−1 | [494] |
| Edible mushroom Agaricus bisporus | TiO2/CQDs/Pt | Photocatalysis with triethanolamine (TEOA, sacrificial agent) | Visible light | 1,458 μmol g−1 h−1 | [495] | |
| Organic waste of citrus limetta | Au and Ag/carbon nanolight-decorated TiO2 nanofibers | Photoelectrochemical | Simulated AM 1.5 G solar illumination | Photocurrent density of 18 and 13 mA cm−2 | [496] | |
| Biomass/extracts of Brassica oleracea and Azadirachta indica | MnO2–AC nanoflakes | Photocatalysis in sulfide wastewater | Direct solar irradiation | 395 mL h−1 | [497] | |
| Biomass/Corncob | AC/MgO | Waste plastics catalytic pyrolysis | MgO–AC catalyst | 94.8 vol% | [498] | |
| Hybrid material | Date pulp waste | Fe3O4/date seed AC nanocomposite | Enterobacter aerogenes-mediated dark fermentation | Fe3O4/AC (150 mg/L) | 238.7 mL g−1 | [499] |
| Green tea and coffee | Cuprous oxide-modified titanate nanotube arrays (Cu2O/TNTAs) | Photoelectrochemical | 100 W mercury light with a power of 95.5 mW cm−2 | 2,132 μM cm−2 after 4 h | [500] | |
| PET plastics | N-doped mesoporous carbon-functionalized ZnO (ZnO@NMC) nanocomposite | Electrocatalysis | 0.5 M KOH solution | ∼90 mA cm−2 current density at 50 mV s−1/0.39 V over-potential at 10 mA cm−2 | [501] | |
| Volcanic ash | ZnO/CuO/zeolite composite | Photocatalysis | UVA light | 187 μmol g−1 after 3 h | [502] | |
| Metal oxides | Murraya koenigii leaf extract | γ-Fe2O3 | Clostridium acetobutylicum-mediated glucose fermentation | 175 mg L−1 of Fe2O3 NPs | 2.33 ± 0.09 mol H2 mol−1 glucose | [503] |
| Pomegranate peels | Fe2O3 | Photoelectrochemical | Nanoporous structure | 50 μmol h−1 cm−2 | [504] |
4.4 CO2 reduction
As a result of relying on fossil fuels as the main energy source, tremendous amounts of CO2 are produced and released into the atmosphere. Thus, converting CO2 into useful, energy-rich hydrocarbon fuels is important for solving climate change and global warming challenges [505]. Photocatalytic CO2 conversion requires three pivotal processes: (1) absorption of solar light, (2) separation/migration of generated charges, and (3) CO2 reduction–H2O oxidation [506]. Some recycled materials used for CO2 conversion are listed in Table 11.
Summary of recycled nanomaterials used for CO2 conversion
| Material type | Waste source | Material | Process | Conditions/light source/catalyst | Efficiency | Ref. |
|---|---|---|---|---|---|---|
| Hybrid material | Beet molasses | AC/TiO2 | Adsorption | 1 atm pressure at 40°C | 1.9 mmol g−1 adsorption of CO2 | [507] |
| Volcanic ash | ZnO–CuO–zeolite composite | Photocatalytic CO2 reduction | UVA light | 2,721 μmol g−1 evolution of HCOOH after 3 h | [502] | |
| Metal oxide | Industrial green agent | B/N-doped TiO2 | Photocatalytic CO2 reduction | Simulated sunlight | 3.5 times larger carbon monoxide production concerning pristine TiO2 | [508] |
| Carbon | Vitamin B9 | Porous AC | Photocatalytic CO2 reduction | 0°C/1 bar and 25°C/1 bar | 5.41 and 3.66 mmol g−1 | [509] |
5 Conclusion and future prospects
It is necessary to recycle domestic and industrial wastes and recover value products such as carbon-based NMs, metal/metal oxides, and hybrid NMs. However, due to a lack of cost-effective and technically competent recycling design, research in this field has yet to be turned into applicable technology. This review discussed the recent developments in waste-derived NMs, divided into three categories: carbon-based NMs, metal/metal oxides, and hybrid NMs, due to their cost-effectiveness, technical and economic feasibility, abundance, availability, simplicity, environmental benefits, and eco-friendliness energy applications. Millions of tons of various wastes are produced annually, with just a low percentage recycled globally. Despite their appealing features and wide range of potential applications, the published research has concentrated chiefly on biomass sources for production, with little information on the preparation of NMs from frying oil and other industrial wastes. Overall, the most currently available recycling procedures require considerable development to produce commercial-grade end products. Furthermore, recycled NMs have not appropriately been employed in several essential energy applications, including green hydrogen production and CO2 reduction. More research into the preparation and applications of recycled NMs in numerous industrial, environmental, biomedical, and energy domains are needed to address a large void in the literature. Owing to low cost, technical and economic feasibility, availability, simplicity, environmental benefits, and eco-friendly of the domestic and industrial wastes, it can be utilized as a promising precursor for the large-scale production of nanomaterials and its composites for boosting specific energy applications. Furthermore, while recycling benefits the environment, large-scale companies frequently cannot achieve economic viability without government subsidies due to minimal or no profit. Recent trends, however, appear to be more optimistic, possibly as a result of new business models becoming more adept at producing cash from the wreckage. Recycling has significant economic benefits by providing an alternative approach for materials production instead of mines and limited natural resources. This review provides readers with the technical know-how needed to scale-up the current recycling systems from lab-bench to pilot-plant scale applications.
Acknowledgments
Hesham A. Hamad gratefully thanks the support of the Polish National Agency for Academic Exchange (NAWA) for the post-doctoral fellowship within Ulam program (Grant Agreement No. PPN/ULM/2019/1/00149/U/00001). A. Matsuda and G. Kawamura would like to acknowledge Japan Society for Promotion of Science (JSPS) KAKENHI Grant Nos. 18H03841 and 21K18823.
-
Funding information: The authors state no funding involved.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Elkady M, Shokry H, Hamad H. New activated carbon from mine coal for adsorption of dye in simulated water or multiple heavy metals in real wastewater. Materials. 2020;13:2498.10.3390/ma13112498Suche in Google Scholar PubMed PubMed Central
[2] Kaza S, Yao L, Bhada-Tata P, Van Woerden F. What a waste 2.0: a global snapshot of solid waste management to 2050. USA: World Bank Publications; 2018.10.1596/978-1-4648-1329-0Suche in Google Scholar
[3] Hoornweg D, Bhada-Tata P. What a waste: a global review of solid waste management; Washington, USA; 2012.Suche in Google Scholar
[4] Wilson D, Rodic-Wiersma L, Modak P, Soós R, Rogero A, Velis C, et al. Global Waste Management outlook. Vienna, Austria: United Nations Environment Programme (UNEP) and International Solid Waste Association (ISWA); 2015.Suche in Google Scholar
[5] Dutta T, Kim K-H, Deep A, Szulejko JE, Vellingiri K, Kumar S, et al. Recovery of nanomaterials from battery and electronic wastes: A new paradigm of environmental waste management. Renew Sustain Energy Rev. 2018;82:3694–704.10.1016/j.rser.2017.10.094Suche in Google Scholar
[6] Afroz R, Masud MM, Akhtar R, Duasa JB. Survey and analysis of public knowledge, awareness and willingness to pay in Kuala Lumpur, Malaysia–a case study on household WEEE management. J Clean Prod. 2013;52:185–93.10.1016/j.jclepro.2013.02.004Suche in Google Scholar
[7] Al-Rahbi AS, Williams PT. Production of activated carbons from waste tyres for low temperature NOx control. Waste Manag. 2016;49:188–95.10.1016/j.wasman.2016.01.030Suche in Google Scholar PubMed
[8] Dresselhaus MS, Terrones M. Carbon-based nanomaterials from a historical perspective. Proc IEEE. 2013;101:1522–35.10.1109/JPROC.2013.2261271Suche in Google Scholar
[9] Rahman ST, Rhee KY, Park S-J. Nanostructured multifunctional electrocatalysts for efficient energy conversion systems: Recent perspectives. Nanotechnol Rev. 2021;10:137–57.10.1515/ntrev-2021-0008Suche in Google Scholar
[10] Hamad H, Bailon-Garcia E, Maldonado-Hodar FJ, Perez-Cadenas AF, Carrasco-Marin F, Morales-Torres S. Synthesis of TixOy nanocrystals in mild synthesis conditions for the degradation of pollutants under solar light. Appl Catal B Environ. 2019;241:385–92.10.1016/j.apcatb.2018.09.016Suche in Google Scholar
[11] Duan H, Huang Q, Wang Q, Zhou B, Li J. Hazardous waste generation and management in China: A review. J Hazard Mater. 2008;158:221–7.10.1016/j.jhazmat.2008.01.106Suche in Google Scholar PubMed
[12] Duarah P, Haldar D, Purkait MK. Technological advancement in the synthesis and applications of lignin-based nanoparticles derived from agro-industrial waste residues: A review. Int J Biol Macromol. 2020;163:1828–43.10.1016/j.ijbiomac.2020.09.076Suche in Google Scholar PubMed
[13] Mohamed MA, Elessawy NA, Carrasco-Marín F, Hamad HA. A novel one-pot facile economic approach for the mass synthesis of exfoliated multilayered nitrogen-doped graphene-like nanosheets: new insights into the mechanistic study. Phys Chem Chem Phys. 2019;21:13611–22.10.1039/C9CP01418GSuche in Google Scholar PubMed
[14] Lavorato C, Primo A, Molinari R, Garcia H. N-doped graphene derived from biomass as a visible‐light photocatalyst for hydrogen generation from water/methanol mixtures. Chemistry–A Eur J. 2014;20:187–94.10.1002/chem.201303689Suche in Google Scholar PubMed
[15] Shalaby T, Hamad H, Ibrahim E, Mahmoud O, Al-Oufy A. Electrospun nanofibers hybrid composites membranes for highly efficient antibacterial activity. Ecotoxicol Environ Saf. 2018;162:354–64.10.1016/j.ecoenv.2018.07.016Suche in Google Scholar PubMed
[16] Xiang X, Xia F, Zhan L, Xie B. Preparation of zinc nano structured particles from spent zinc manganese batteries by vacuum separation and inert gas condensation. Sep Purif Technol. 2015;142:227–33.10.1016/j.seppur.2015.01.014Suche in Google Scholar
[17] Hegde G, Abdul Manaf SA, Kumar A, Ali GAM, Chong KF, Ngaini Z, et al. Biowaste sago bark based catalyst free carbon nanospheres: waste to wealth approach. ACS Sustain Chem & Eng. 2015;5:2247–53.10.1021/acssuschemeng.5b00517Suche in Google Scholar
[18] Ali GAM, Supriya S, Chong KF, Shaaban ER, Algarni H, Maiyalagan T, et al. Superior supercapacitance behavior of oxygen self-doped carbon nanospheres: a conversion of Allium cepa peel to energy storage system. Biomass Convers Biorefinery. 2019;3:1311–23. 10.1007/s13399-019-00520.Suche in Google Scholar
[19] Ali GAM, Divyashree A, Supriya S, Chong KF, Ethiraj AS, Reddy MV, et al. Carbon nanospheres derived from Lablab purpureus for high performance supercapacitor electrodes: a green approach. Dalton Trans. 2017:46;14034–44.10.1039/C7DT02392HSuche in Google Scholar
[20] Ali GA, Manaf SA, Divyashree A, Chong KF, Hegde G. Superior supercapacitive performance in porous nanocarbons. J Energy Chem. 2016;25:734–9.10.1016/j.jechem.2016.04.007Suche in Google Scholar
[21] Jain A, Balasubramanian R, Srinivasan MP. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem Eng J. 2016;283:789–805.10.1016/j.cej.2015.08.014Suche in Google Scholar
[22] Habeeb OA, Ramesh K, Ali GAM, Yunus RM. Application of response surface methodology for optimization of palm kernel shell activated carbon preparation factors for removal of H2S from industrial wastewater. J Teknologi. 2017;79:1–10.10.11113/jt.v79.10183Suche in Google Scholar
[23] Habeeb OA, Ramesh K, Ali GAM, Yunus RM. Isothermal modelling based experimental study of dissolved hydrogen sulfide adsorption from waste water using eggshell based activated carbon. Malaysian J Anal Sci. 2017;21:334–45.10.17576/mjas-2017-2102-08Suche in Google Scholar
[24] Ayinla RT, Dennis JO, Zaid HM, Sanusi YK, Usman F, Adebayo LL. A review of technical advances of recent palm bio-waste conversion to activated carbon for energy storage. J Clean Prod. 2019;229:1427–42.10.1016/j.jclepro.2019.04.116Suche in Google Scholar
[25] Habeeb OA, Ramesh K, Ali GAM, Yunus RM. Optimization of activated carbon synthesis using response surface methodology to enhance H2S removal from refinery wastewater. J Chem Eng Ind Biotechnol. 2017;1:1–17.10.15282/jceib.v1i1.3715Suche in Google Scholar
[26] Habeeb OA, Ramesh K, Ali GAM, Yunus RM. Low-cost and eco-friendly activated carbon from modified palm kernel shell for hydrogen sulfide removal from wastewater: adsorption and kinetic studies. Desalination Water Treat. 2017;84:205–14.10.5004/dwt.2017.21196Suche in Google Scholar
[27] Habeeb OA, Ramesh K, Ali GAM, Yunus RM, Olalere OA. Kinetic, isotherm and equilibrium study of adsorption of hydrogen sulfide from wastewater using modified eggshells. IIUM Eng J. 2017;18:13–25.10.31436/iiumej.v18i1.689Suche in Google Scholar
[28] Ali GAM, Habeeb OA, Algarni H, Chong KF. CaO impregnated highly porous honeycomb activated carbon from agriculture waste: symmetrical supercapacitor study. J Mater Sci. 2018;54:683–92.10.1007/s10853-018-2871-6Suche in Google Scholar
[29] Jothi Ramalingam R, Sivachidambaram M, Vijaya JJ, Al-Lohedan HA, Muthumareeswaran MR. Synthesis of porous activated carbon powder formation from fruit peel and cow dung waste for modified electrode fabrication and application. Biomass Bioenergy. 2020;142:105800.10.1016/j.biombioe.2020.105800Suche in Google Scholar
[30] Taer E, Apriwandi A, Taslim R, Agutino A, Yusra DA. Conversion Syzygium oleana leaves biomass waste to porous activated carbon nanosheet for boosting supercapacitor performances. J Mater Res Technol. 2020;9:13332–40.10.1016/j.jmrt.2020.09.049Suche in Google Scholar
[31] Manasa P, Lei ZJ, Ran F. Biomass waste derived low cost activated carbon from carchorus olitorius (Jute Fiber) as sustainable and novel electrode material. J Energy Storage. 2020;30:101494.10.1016/j.est.2020.101494Suche in Google Scholar
[32] Wan H, Hu X. From biomass-derived wastes (bagasse, wheat straw and shavings) to activated carbon with three-dimensional connected architecture and porous structure for Li-ion batteries. Chem Phys. 2019;521:108–14.10.1016/j.chemphys.2019.01.012Suche in Google Scholar
[33] Pal A, Uddin K, Thu K, Saha BB, Kil H-S, Yoon S-H, et al. Thermophysical and adsorption characteristics of waste biomass-derived activated carbons. Encyclopedia of Renewable and Sustainable Materials. 2020;4:617–28. 10.1016/b978-0-12-803581-8.10832-x;617-628. Suche in Google Scholar
[34] Shokry H, Elkady M, Hamad H. Nano activated carbon from industrial mine coal as adsorbents for removal of dye from simulated textile wastewater: operational parameters and mechanism study. J Mater Res Technol. 2019;8:4477–88.10.1016/j.jmrt.2019.07.061Suche in Google Scholar
[35] Saleh TA, Al-Saadi AA. Surface characterization and sorption efficacy of tire-obtained carbon: experimental and semiempirical study of rhodamine B adsorption. Surf Interface Anal. 2015;47:785–92.10.1002/sia.5775Suche in Google Scholar
[36] Patel H, Mangukiya H, Maiti P, Maiti S. Empty cotton boll crop-residue and plastic waste valorization to bio-oil, potassic fertilizer and activated carbon – A bio-refinery model. J Clean Prod. 2021;290:125738.10.1016/j.jclepro.2020.125738Suche in Google Scholar
[37] Oladimeji TE, Odunoye BO, Elehinafe FB, Obanla OR, Odunlami OA. Production of activated carbon from sawdust and its efficiency in the treatment of sewage water. Heliyon. 2021;7:e05960.10.1016/j.heliyon.2021.e05960Suche in Google Scholar
[38] Zhang D, Lin X, Zhang Q, Ren X, Yu W, Cai H. Catalytic pyrolysis of wood-plastic composite waste over activated carbon catalyst for aromatics production: Effect of preparation process of activated carbon. Energy. 2020;212:118983.10.1016/j.energy.2020.118983Suche in Google Scholar
[39] Nagaraju G, Lim JH, Cha SM, Yu JS. Three-dimensional activated porous carbon with meso/macropore structures derived from fallen pine cone flowers: A low-cost counter electrode material in dye-sensitized solar cells. J Alloy Compd. 2017;693:1297–304.10.1016/j.jallcom.2016.10.015Suche in Google Scholar
[40] Wang Z, Wu S, Wang J, Yu A, Wei G. Carbon nanofiber-based functional nanomaterials for sensor applications. Nanomaterials (Basel). 2019;9:1045.10.3390/nano9071045Suche in Google Scholar PubMed PubMed Central
[41] Zhou X, Wang Y, Gong C, Liu B, Wei G. Production, structural design, functional control, and broad applications of carbon nanofiber-based nanomaterials: A comprehensive review. Chem Eng J. 2020;402:126189.10.1016/j.cej.2020.126189Suche in Google Scholar
[42] Zhou X, Liu B, Chen Y, Guo L, Wei G. Carbon nanofiber-based three-dimensional nanomaterials for energy and environmental applications. Mater Adv. 2020;1:2163–81.10.1039/D0MA00492HSuche in Google Scholar
[43] Vallejos ME, Felissia FE, Area MC, Ehman NV, Tarres Q, Mutje P. Nanofibrillated cellulose (CNF) from eucalyptus sawdust as a dry strength agent of unrefined eucalyptus handsheets. Carbohydr Polym. 2016;139:99–105.10.1016/j.carbpol.2015.12.004Suche in Google Scholar PubMed
[44] Wang Z, Shen D, Wu C, Gu S. State-of-the-art on the production and application of carbon nanomaterials from biomass. Green Chem. 2018;20:5031–57.10.1039/C8GC01748DSuche in Google Scholar
[45] Lu H, Zhang L, Liu C, He Z, Zhou X, Ni Y. A novel method to prepare lignocellulose nanofibrils directly from bamboo chips. Cellulose. 2018;25:7043–51.10.1007/s10570-018-2067-xSuche in Google Scholar
[46] Omoriyekomwan JE, Tahmasebi A, Zhang J, Yu J. Formation of hollow carbon nanofibers on bio-char during microwave pyrolysis of palm kernel shell. Energy Convers Manage. 2017;148:583–92.10.1016/j.enconman.2017.06.022Suche in Google Scholar
[47] Abitbol T, Rivkin A, Cao Y, Nevo Y, Abraham E, Ben-Shalom T, et al. Nanocellulose, a tiny fiber with huge applications. Curr Opin Biotechnol. 2016;39:76–88.10.1016/j.copbio.2016.01.002Suche in Google Scholar PubMed
[48] Farooq A, Patoary MK, Zhang M, Mussana H, Li M, Naeem MA, et al. Cellulose from sources to nanocellulose and an overview of synthesis and properties of nanocellulose/zinc oxide nanocomposite materials. Int J Biol Macromol. 2020;154:1050–73.10.1016/j.ijbiomac.2020.03.163Suche in Google Scholar PubMed
[49] Rambau KM, Musyoka NM, Manyala N, Ren J, Langmi HW, Mathe MK. Preparation of carbon nanofibers/tubes using waste tyres pyrolysis oil and coal fly ash derived catalyst. J Environ Sci Health - Part A Toxic/Hazardous Substances Environ Eng. 2018;53:1115–22.10.1080/10934529.2018.1474594Suche in Google Scholar PubMed
[50] Hintsho N, Shaikjee1 A, Masenda1 H, Naidoo D, Billing D, Franklyn P, et al. Direct synthesis of carbon nanofibers from South African coal fly ash. Nanoscale Research Letters. 2014;9:387.10.1186/1556-276X-9-387Suche in Google Scholar PubMed PubMed Central
[51] Park S-W, Kim J-C, Dar MA, Shim H-W, Kim D-W. Superior lithium storage in nitrogen-doped carbon nanofibers with open-channels. Chem Eng J. 2017;315:1–9.10.1016/j.cej.2017.01.005Suche in Google Scholar
[52] Wei T, Zhang Z, Zhu Z, Zhou X, Wang Y, Wang Y, et al. Recycling of waste plastics and scalable preparation of Si/CNF/C composite as anode material for lithium-ion batteries. Ionics. 2019;25:1523–9.10.1007/s11581-019-02892-ySuche in Google Scholar
[53] Li X, Tian X, Yang T, He Y, Liu W, Song Y, et al. Coal liquefaction residues based carbon nanofibers film prepared by electrospinning: an effective approach to coal waste management. ACS Sustain Chem & Eng. 2019;7:5742–50.10.1021/acssuschemeng.8b05210Suche in Google Scholar
[54] Tan S, Kraus TJ, Li-Oakey KD. Understanding the supercapacitor properties of electrospun carbon nanofibers from Powder River Basin coal. Fuel. 2019;245:148–59.10.1016/j.fuel.2019.01.141Suche in Google Scholar
[55] Oliveira AAS, Tristão JC, Ardisson JD, Dias A, Lago RM. Production of nanostructured magnetic composites based on Fe 0 nuclei coated with carbon nanofibers and nanotubes from red mud waste and ethanol. Appl Catal B Environ. 2011;105:163–70.10.1016/j.apcatb.2011.04.007Suche in Google Scholar
[56] Guerreiro GG, Vieira de Andrade F, Roberto, de Freitas M. Carbon nanostructures based-adsorbent obtained from iron ore tailings. Ceram Int. 2020;46:29271–81.10.1016/j.ceramint.2020.08.101Suche in Google Scholar
[57] Zhang C, Yuan B, Liang Y, Yang L, Bai L, Yang H, et al. Carbon nanofibers enhanced solar steam generation device based on loofah biomass for water purification. Mater Chem Phys. 2021;258:123998.10.1016/j.matchemphys.2020.123998Suche in Google Scholar
[58] Cao M, Wang D, Lu J, Cheng W, Han G, Zhou J. Electrospun porous carbon nanofibers@SnOx nanocomposites for high-performance supercapacitors: Microstructures and electrochemical properties. Compos Part A Appl Sci Manuf. 2021;143:106278.10.1016/j.compositesa.2021.106278Suche in Google Scholar
[59] Janas D. From bio to nano: a review of sustainable methods of synthesis of carbon nanotubes. Sustainability. 2020;12:4115.10.3390/su12104115Suche in Google Scholar
[60] Maryam M, Suriani AB, Shamsudin MS, Rusop Mahmood M. Synthesis of carbon nanotubes from palm oil precursor by aerosol-assisted catalytic CVD method. Appl Mech Mater. 2012;229–231:247–51.10.4028/www.scientific.net/AMM.229-231.247Suche in Google Scholar
[61] Afre RA, Soga T, Jimbo T, Kumar M, Ando Y, Sharon M. Growth of vertically aligned carbon nanotubes on silicon and quartz substrate by spray pyrolysis of a natural precursor: Turpentine oil. Chem Phys Lett. 2005;414:6–10.10.1016/j.cplett.2005.08.040Suche in Google Scholar
[62] Afre RA, Soga T, Jimbo T, Kumar M, Ando Y, Sharon M, et al. Carbon nanotubes by spray pyrolysis of turpentine oil at different temperatures and their studies. Microporous Mesoporous Mater. 2006;96:184–90.10.1016/j.micromeso.2006.06.036Suche in Google Scholar
[63] Salifairus MJ, Rusop M. Synthesis of carbon nanotubes by chemical vapour deposition of camphor oil over ferrocene and aluminum isopropoxide catalyst. Adv Mater Res. 2013;667:213–7.10.4028/www.scientific.net/AMR.667.213Suche in Google Scholar
[64] Awasthi K, Kumar R, Raghubanshi H, Awasthi S, Pandey R, Singh D, et al. Synthesis of nano-carbon (nanotubes, nanofibres, graphene) materials. Indian Acad Sci. 2011;34:607.10.1007/s12034-011-0170-9Suche in Google Scholar
[65] Raziah AZ, Junizah AR, Saifuddin N. Synthesis of carbon nanotubes using natural carbon precursor: Castor oil. AIP Conf Proc. 2012;1482:564. 10.1063/1.4757535;564-567. Suche in Google Scholar
[66] Zhang Y, Lu L, Zhang S, Lv Z, Yang D, Liu J, et al. Biomass chitosan derived cobalt/nitrogen doped carbon nanotubes for the electrocatalytic oxygen reduction reaction. J Mater Chem A. 2018;6:5740–5.10.1039/C7TA11258KSuche in Google Scholar
[67] Hildago-Oporto P, Navia R, Hunter R, Coronado G, Gonzalez ME. Synthesis of carbon nanotubes using biochar as precursor material under microwave irradiation. J Environ Manag. 2019;244:83–91.10.1016/j.jenvman.2019.03.082Suche in Google Scholar
[68] Araga R, Sharma CS. One step direct synthesis of multiwalled carbon nanotubes from coconut shell derived charcoal. Mater Lett. 2017;188:205–7.10.1016/j.matlet.2016.11.014Suche in Google Scholar
[69] He S, Xu Y, Zhang Y, Bell S, Wu C. Waste plastics recycling for producing high-value carbon nanotubes: Investigation of the influence of Manganese content in Fe-based catalysts. J Hazard Mater. 2021;402:123726.10.1016/j.jhazmat.2020.123726Suche in Google Scholar
[70] Dlova S, Olaitan Ayeleru O, Victoria Adams F, Mamo MA, Apata, Olubambi P. Development of multi-walled carbon nanotubes obtained from recycled plastics by single stage pyrolysis process. Mater Today Proc. 2021;38:1170–3.10.1016/j.matpr.2020.07.460Suche in Google Scholar
[71] Jia J, Veksha A, Lim TT, Lisak G. Weakening the strong Fe-La interaction in A-site-deficient perovskite via Ni substitution to promote the thermocatalytic synthesis of carbon nanotubes from plastics. J Hazard Mater. 2021;403:123642.10.1016/j.jhazmat.2020.123642Suche in Google Scholar
[72] Liu X, He S, Han Z, Wu C. Investigation of spherical alumina supported catalyst for carbon nanotubes production from waste polyethylene. Process Saf Environ Prot. 2021;146:201–7.10.1016/j.psep.2020.08.027Suche in Google Scholar
[73] Yang R-X, Wu S-L, Chuang K-H, Wey M-Y. Co-production of carbon nanotubes and hydrogen from waste plastic gasification in a two-stage fluidized catalytic bed. Renew Energy. 2020;159:10–22.10.1016/j.renene.2020.05.141Suche in Google Scholar
[74] Moo JGS, Veksha A, Oh W-D, Giannis A, Udayanga WDC, Lin S-X, et al. Plastic derived carbon nanotubes for electrocatalytic oxygen reduction reaction: Effects of plastic feedstock and synthesis temperature. Electrochem Commun. 2019;101:11–8.10.1016/j.elecom.2019.02.014Suche in Google Scholar
[75] Li W, Wei M, Liu Y, Ye Y, Li S, Yuan W, et al. Catalysts evaluation for production of hydrogen gas and carbon nanotubes from the pyrolysis-catalysis of waste tyres. Int J Hydrog Energy. 2019;44:19563–72.10.1016/j.ijhydene.2019.05.204Suche in Google Scholar
[76] Essawy H, Fathy N, Tawfik M, El-Sabbagh S, Ismail N, Youssef H. Fabrication of single-walled carbon nanotubes from vulcanized scrap rubber via thermal chemical vapor deposition. RSC Adv. 2017;7:12938–44.10.1039/C7RA00349HSuche in Google Scholar
[77] Essawy HA, Fathy NA. Enhancing the pyrolysis of scrap rubber for carbon nanotubes/graphene production via chemical vapor deposition. J Macromol Sci Part A. 2018;55:347–54.10.1080/10601325.2018.1434416Suche in Google Scholar
[78] Zhang Z, Xi F, Li S, Wan X, Ma W, Chen X, et al. High-performance Si/nano-Cu/CNTs/C anode derived from photovoltaic silicon waste: A potential photovoltaic-energy storage strategy. Mat Today Energy. 2021;20:100671.10.1016/j.mtener.2021.100671Suche in Google Scholar
[79] Singh R, Volli V, Lohani L, Purkait MK. Synthesis of carbon nanotubes from industrial wastes following alkali activation and film casting method. Waste Biomass Valoriz. 2019;11:4957–66.10.1007/s12649-019-00827-2Suche in Google Scholar
[80] Abdullah HB, Irmawati R, Ismail I, Yusof NA. Utilization of waste engine oil for carbon nanotube aerogel production using floating catalyst chemical vapor deposition. J Clean Prod. 2020;261:121188.10.1016/j.jclepro.2020.121188Suche in Google Scholar
[81] Wu C, Nahil MA, Miskolczi N, Huang J, Williams PT. Production and application of carbon nanotubes, as a co-product of hydrogen from the pyrolysis-catalytic reforming of waste plastic. Process Saf Environ Prot. 2016;103:107–14.10.1016/j.psep.2016.07.001Suche in Google Scholar
[82] Hidalgo P, Navia R, Hunter R, Coronado G, Gonzalez M. Synthesis of carbon nanotubes using biochar as precursor material under microwave irradiation. J Environ Manag. 2019;244:83–91.10.1016/j.jenvman.2019.03.082Suche in Google Scholar PubMed
[83] Al-Rahbi AS, Onwudili JA, Williams PT. Thermal decomposition and gasification of biomass pyrolysis gases using a hot bed of waste derived pyrolysis char. Bioresour Technol. 2016;204:71–9.10.1016/j.biortech.2015.12.016Suche in Google Scholar PubMed
[84] Zhang B, Piao G, Zhang J, Bu C, Xie H, Wu B, et al. Synthesis of carbon nanotubes from conventional biomass-based gasification gas. Fuel Process Technol. 2018;180:105–13.10.1016/j.fuproc.2018.08.016Suche in Google Scholar
[85] Abd Elhafez S, Hamad H, Zaatout A, Malash G. Management of agricultural waste for removal of heavy metals from aqueous solution: adsorption behaviors, adsorption mechanisms, environmental protection, and techno-economic analysis. Environ Sci Pollut Res. 2017;24:1397–1415.10.1007/s11356-016-7891-7Suche in Google Scholar PubMed
[86] Sankar S, Lee H, Jung H, Kim A, Ahmed ATA, Inamdar AI, et al. Ultrathin graphene nanosheets derived from rice husks for sustainable supercapacitor electrodes. N J Chem. 2017;41:13792–97.10.1039/C7NJ03136JSuche in Google Scholar
[87] Wang H, Xu Z, Kohandehghan A, Li Z, Cui K, Tan X, et al. Interconnected carbon nanosheets derived from hemp for ultrafast supercapacitors with high energy. ACS Nano. 2013;7:5131–41.10.1021/nn400731gSuche in Google Scholar PubMed
[88] Mohamed MA, Carrasco‐Marín F, Elessawy NA, Hamad HA. Glucose‐derived n‐doped graphitic carbon: facile one‐pot graphitic structure‐controlled chemical synthesis with comprehensive insight into the controlling mechanisms. ChemistrySelect. 2020;5:14685–702.10.1002/slct.202003014Suche in Google Scholar
[89] Somanathan T, Prasad K, Ostrikov KK, Saravanan A, Krishna VM. Graphene oxide synthesis from agro waste. Nanomaterials. 2015;5:826–34.10.3390/nano5020826Suche in Google Scholar PubMed PubMed Central
[90] Hao P, Zhao Z, Tian J, Li H, Sang Y, Yu G, et al. Hierarchical porous carbon aerogel derived from bagasse for high performance supercapacitor electrode. Nanoscale. 2014;6:12120–9.10.1039/C4NR03574GSuche in Google Scholar PubMed
[91] Banu JR, Sharmila VG, Ushani U, Amudha V, Kumar G. Impervious and influence in the liquid fuel production from municipal plastic waste through thermo-chemical biomass conversion technologies-A review. Sci Total Env. 2020;718:137287.10.1016/j.scitotenv.2020.137287Suche in Google Scholar PubMed
[92] Pandey S, Karakoti M, Surana K, Dhapola PS, SanthiBhushan B, Ganguly S, et al. Graphene nanosheets derived from plastic waste for the application of DSSCs and supercapacitors. Sci Rep. 2021;11:1–17.10.1038/s41598-021-83483-8Suche in Google Scholar PubMed PubMed Central
[93] Marek AA, Zawadiak J, Piotrowski T, Hefczyc B. A new efficient method for the processing of post-consumer polypropylene and other polyolefin wastes into polar waxes. Waste Manag. 2015;46:62–7.10.1016/j.wasman.2015.08.042Suche in Google Scholar PubMed
[94] Gu J, Pang A, Guo X, Li L, Huang D, Li F. Green preparation of high-quality and low-cost graphene from discarded polyethylene plastic bags. Chem Commun. 2021;57:129–32.10.1039/D0CC06999JSuche in Google Scholar PubMed
[95] Ferronato N, Torretta V. Waste mismanagement in developing countries: a review of global issues. Int J Environ Res Public Health. 2019;16:1060.10.3390/ijerph16061060Suche in Google Scholar PubMed PubMed Central
[96] El Essawy NA, Ali SM, Farag HA, Konsowa AH, Elnouby M, Hamad HA. Green synthesis of graphene from recycled PET bottle wastes for use in the adsorption of dyes in aqueous solution. Ecotoxicol Environ Saf. 2017;145:57–68.10.1016/j.ecoenv.2017.07.014Suche in Google Scholar PubMed
[97] Ahmadi S, Afzalzadeh R. Few-layer graphene growth from polystyrene as solid carbon source utilizing simple APCVD method. Phys E. 2016;81:302–7.10.1016/j.physe.2016.01.028Suche in Google Scholar
[98] Akhavan O, Bijanzad K, Mirsepah A. Synthesis of graphene from natural and industrial carbonaceous wastes. RSC Adv. 2014;4:20441–8.10.1039/c4ra01550aSuche in Google Scholar
[99] Siaw W, Tsuji T, Manaf NA, Patah MA, Jan BM. Synthesis of graphene oxide from industrial waste. Proceedings of IOP Conference Series: Materials Science and Engineering. p. 01205010.1088/1757-899X/778/1/012050Suche in Google Scholar
[100] Kang S, Kim KM, Son Y, Mhin S, Ryu JH, Shim KB, et al. Graphene oxide quantum dots derived from coal for bioimaging: facile and green approach. Sci Rep. 2019;9:1–7.10.1038/s41598-018-37479-6Suche in Google Scholar PubMed PubMed Central
[101] Vijapur SH, Wang D, Botte GG. Raw coal derived large area and transparent graphene films. ECS Solid State Lett. 2013;2:M45.10.1149/2.005307sslSuche in Google Scholar
[102] Zhou H, Su M, Lee P-H, Shih K. Synthesis of submicron lead oxide particles from the simulated spent lead paste for battery anodes. J Alloy Compd. 2017;690:101–7.10.1016/j.jallcom.2016.08.094Suche in Google Scholar
[103] Samaddar P, Ok YS, Kim K-H, Kwon EE, Tsang DC. Synthesis of nanomaterials from various wastes and their new age applications. J Clean Prod. 2018;197:1190–209.10.1016/j.jclepro.2018.06.262Suche in Google Scholar
[104] Maroufi S, Mayyas M, Sahajwalla V. Nano-carbons from waste tyre rubber: An insight into structure and morphology. Waste Manag. 2017;69:110–6.10.1016/j.wasman.2017.08.020Suche in Google Scholar PubMed
[105] Abdelbasir SM, El-Sheltawy CT, Abdo DM. Green processes for electronic waste recycling: a review. J Sustain Metall. 2018;4:295–311.10.1007/s40831-018-0175-3Suche in Google Scholar
[106] Abdelbasir SM, Hassan SSM, Kamel AH, El-Nasr RS. Status of electronic waste recycling techniques: a review. Environ Sci Pollut Res Int. 2018;25:16533–47.10.1007/s11356-018-2136-6Suche in Google Scholar PubMed
[107] Ruan G, Sun Z, Peng Z, Tour JM. Growth of graphene from food, insects, and waste. ACS Nano. 2011;5:7601–7.10.1021/nn202625cSuche in Google Scholar PubMed
[108] Panahi-Kalamuei M, Amiri O, Salavati-Niasari M. Green hydrothermal synthesis of high quality single and few layers graphene sheets by bread waste as precursor. J Mater Res Technol. 2020;9:2679–90.10.1016/j.jmrt.2020.01.001Suche in Google Scholar
[109] Faiz MA, Azurahanim CC, Yazid Y, Suriani A, Ain MSN. Preparation and characterization of graphene oxide from tea waste and its photocatalytic application of TiO2/graphene nanocomposite. Mater Res Express. 2020;7:015613.10.1088/2053-1591/ab689dSuche in Google Scholar
[110] Sujiono EH, Zabrian D, Dahlan M, Amin B, Agus J. Graphene oxide based coconut shell waste: synthesis by modified Hummers method and characterization. Heliyon. 2020;6:e04568.10.1016/j.heliyon.2020.e04568Suche in Google Scholar PubMed PubMed Central
[111] Shah J, Lopez-Mercado J, Carreon MG, Lopez-Miranda A, Carreon ML. Plasma synthesis of graphene from mango peel. ACS Omega. 2018;3:455–63.10.1021/acsomega.7b01825Suche in Google Scholar PubMed PubMed Central
[112] Hussain S, Anjali K, Hassan ST, Dwivedi PB. Waste tea as a novel adsorbent: a review. Appl Water Sci. 2018;8:1–16.10.1007/s13201-018-0824-5Suche in Google Scholar
[113] Mgaya J, Shombe GB, Masikane SC, Mlowe S, Mubofu EB, Revaprasadu N. Cashew nut shell: a potential bio-resource for the production of bio-sourced chemicals, materials and fuels. Green Chem. 2019;21:1186–201.10.1039/C8GC02972ESuche in Google Scholar
[114] ALOthman ZA, Naushad M, Ali R. Kinetic, equilibrium isotherm and thermodynamic studies of Cr (VI) adsorption onto low-cost adsorbent developed from peanut shell activated with phosphoric acid. Environ Sci Pollut Res. 2013;20:3351–65.10.1007/s11356-012-1259-4Suche in Google Scholar PubMed
[115] Long C, Chen X, Jiang L, Zhi L, Fan Z. Porous layer-stacking carbon derived from in-built template in biomass for high volumetric performance supercapacitors. Nano Energy. 2015;12:141–51.10.1016/j.nanoen.2014.12.014Suche in Google Scholar
[116] Ding Z, Yuan T, Wen J, Cao X, Sun S, Xiao L-P, et al. Green synthesis of chemical converted graphene sheets derived from pulping black liquor. Carbon. 2020;158:690–7.10.1016/j.carbon.2019.11.041Suche in Google Scholar
[117] Du Q-S, Li D-P, Long S-Y, Tang P-D, Du F-L, Huang H-L, et al. Graphene like porous carbon with wood-ear architecture prepared from specially pretreated lignin precursor. Diam Relat Mater. 2018;90:109–15.10.1016/j.diamond.2018.10.011Suche in Google Scholar
[118] Kumar M, Sachdeva A, Garg RK, Singh S. Synthesis and characterization of graphene prepared from rice husk by a simple microwave process. Proceedings of Nano Hybrids and Composites. 2020;29:74–83.10.4028/www.scientific.net/NHC.29.74Suche in Google Scholar
[119] Yan Y, Nashath FZ, Chen S, Manickam S, Lim SS, Zhao H, et al. Synthesis of graphene: Potential carbon precursors and approaches. Nanotechnol Rev. 2020;9:1284–314.10.1515/ntrev-2020-0100Suche in Google Scholar
[120] Yeleuov M, Seidl C, Temirgaliyeva T, Taurbekov A, Prikhodko N, Lesbayev B, et al. Modified activated graphene-based carbon electrodes from rice husk for supercapacitor applications. Energies. 2020;13:4943.10.3390/en13184943Suche in Google Scholar
[121] Das R, Bandyopadhyay R, Pramanik P. Carbon quantum dots from natural resource: A review. Mater Today Chem. 2018;8:96–109.10.1016/j.mtchem.2018.03.003Suche in Google Scholar
[122] Shi Y, Liu X, Wang M, Huang J, Jiang X, Pang J, et al. Synthesis of N-doped carbon quantum dots from bio-waste lignin for selective irons detection and cellular imaging. Int J Biol Macromol. 2019;128:537–45.10.1016/j.ijbiomac.2019.01.146Suche in Google Scholar PubMed
[123] Himaja A, Karthik PS, Sreedhar B, Singh SP. Synthesis of carbon dots from kitchen waste: conversion of waste to value added product. J Fluorescence. 2014;24:1767–73.10.1007/s10895-014-1465-1Suche in Google Scholar PubMed
[124] Boruah A, Saikia M, Das T, Goswamee RL, Saikia BK. Blue-emitting fluorescent carbon quantum dots from waste biomass sources and their application in fluoride ion detection in water. J Photochem Photobiol B Biol. 2020;209:111940.10.1016/j.jphotobiol.2020.111940Suche in Google Scholar PubMed
[125] Sahajwalla V, Gaikwad V. The present and future of e-waste plastics recycling. Curr OpGreen SustaChem. 2018;13:102–7.10.1016/j.cogsc.2018.06.006Suche in Google Scholar
[126] Kumari A, Kumar A, Sahu SK, Kumar S. Synthesis of green fluorescent carbon quantum dots using waste polyolefins residue for Cu2+ ion sensing and live cell imaging. Sens Actuators B Chem. 2018;254:197–205.10.1016/j.snb.2017.07.075Suche in Google Scholar
[127] Papademas P, Kotsaki P. Technological utilization of whey towards sustainable exploitation. Adv Dairy Res. 2019;7:231.Suche in Google Scholar
[128] Devi P, Kaur G, Thakur A, Kaur N, Grewal A, Kumar P. Waste derivitized blue luminescent carbon quantum dots for selenite sensing in water. Talanta. 2017;170:49–55.10.1016/j.talanta.2017.03.069Suche in Google Scholar PubMed
[129] Rodríguez-Padrón D, Algarra M, Tarelho LA, Frade J, Franco A, de Miguel G, et al. Catalyzed microwave-assisted preparation of carbon quantum dots from lignocellulosic residues. ACS Sustain Chem & Eng. 2018;6:7200–5.10.1021/acssuschemeng.7b03848Suche in Google Scholar
[130] Wei J, Zhang X, Sheng Y, Shen J, Huang P, Guo S, et al. Simple one-step synthesis of water-soluble fluorescent carbon dots from waste paper. N J Chem. 2014;38:906–9.10.1039/c3nj01325aSuche in Google Scholar
[131] Wang R-C, Lu J-T, Lin Y-C. High-performance nitrogen doped carbon quantum dots: facile green synthesis from waste paper and broadband photodetection by coupling with ZnO nanorods. J Alloy Compd. 2020;813:152201.10.1016/j.jallcom.2019.152201Suche in Google Scholar
[132] Devi S, Gupta RK, Paul AK, Tyagi S. Waste carbon paper derivatized Carbon Quantum Dots/(3-Aminopropyl) triethoxysilane based fluorescent probe for trinitrotoluene detection. Mater Res Express. 2018;6:025605.10.1088/2053-1591/aaf03cSuche in Google Scholar
[133] Thambiraj S, Shankaran R. Green synthesis of highly fluorescent carbon quantum dots from sugarcane bagasse pulp. Appl Surf Sci. 2016;390:435–43.10.1016/j.apsusc.2016.08.106Suche in Google Scholar
[134] Pandiyan S, Arumugam L, Srirengan SP, Pitchan R, Sevugan P, Kannan K, et al. Biocompatible carbon quantum dots derived from sugarcane industrial wastes for effective nonlinear optical behavior and antimicrobial activity applications. ACS Omega. 2020;5:30363–72.10.1021/acsomega.0c03290Suche in Google Scholar PubMed PubMed Central
[135] Ma L, Xiang W, Gao H, Wang J, Ni Y, Liang X. Facile synthesis of tunable fluorescent carbon dots and their third-order nonlinear optical properties. Dye Pigment. 2016;128:1–7.10.1016/j.dyepig.2016.01.005Suche in Google Scholar
[136] Devi P, Hipp KN, Thakur A, Lai RY. Waste to wealth translation of e-waste to plasmonic nanostructures for surface-enhanced Raman scattering. Appl Nanosci. 2020;10:1615–23.10.1007/s13204-020-01273-6Suche in Google Scholar
[137] Varisco M, Zufferey D, Ruggi A, Zhang Y, Erni R, Mamula O. Synthesis of hydrophilic and hydrophobic carbon quantum dots from waste of wine fermentation. R Soc Open Sci. 2017;4:170900.10.1098/rsos.170900Suche in Google Scholar PubMed PubMed Central
[138] Liang Z, Zeng L, Cao X, Wang Q, Wang X, Sun R. Sustainable carbon quantum dots from forestry and agricultural biomass with amplified photoluminescence by simple NH4OH passivation. J Mater Chem C. 2014;2:9760–6.10.1039/C4TC01714ESuche in Google Scholar
[139] Zhou J, Sheng Z, Han H, Zou M, Li C. Facile synthesis of fluorescent carbon dots using watermelon peel as a carbon source. Mater Lett. 2012;66:222–4.10.1016/j.matlet.2011.08.081Suche in Google Scholar
[140] John TS, Yadav PK, Kumar D, Singh SK, Hasan SH. Highly fluorescent carbon dots from wheat bran as a novel drug delivery system for bacterial inhibition. Luminescence. 2020;35:913–23.10.1002/bio.3801Suche in Google Scholar PubMed
[141] Qi H, Teng M, Liu M, Liu S, Li J, Yu H, et al. Biomass-derived nitrogen-doped carbon quantum dots: highly selective fluorescent probe for detecting Fe3+ ions and tetracyclines. J Colloid Interface Sci. 2019;539:332–41.10.1016/j.jcis.2018.12.047Suche in Google Scholar PubMed
[142] Cheng C, Shi Y, Li M, Xing M, Wu Q. Carbon quantum dots from carbonized walnut shells: structural evolution, fluorescence characteristics, and intracellular bioimaging. Mater Sci Eng C. 2017;79:473–80.10.1016/j.msec.2017.05.094Suche in Google Scholar PubMed
[143] Chunduri L, Kurdekar A, Patnaik S, Dev BV, Rattan TM, Kamisetti V. Carbon quantum dots from coconut husk: evaluation for antioxidant and cytotoxic activity. Mater Focus. 2016;5:55–61.10.1166/mat.2016.1289Suche in Google Scholar
[144] Zhu J, Zhu F, Yue X, Chen P, Sun Y, Zhang L, et al. Waste utilization of synthetic carbon quantum dots based on tea and peanut shell. J Nanomaterials. 2019;2019:1045.10.1155/2019/7965756Suche in Google Scholar
[145] Chaudhary S, Kumari M, Chauhan P, Ram Chaudhary G. Upcycling of plastic waste into fluorescent carbon dots: An environmentally viable transformation to biocompatible C-dots with potential prospective in analytical applications. Waste Manag. 2021;120:675–86.10.1016/j.wasman.2020.10.038Suche in Google Scholar PubMed
[146] Pramanik A, Biswas S, Kumbhakar P. Solvatochromism in highly luminescent environmental friendly carbon quantum dots for sensing applications: Conversion of bio-waste into bio-asset. Spectrochim Acta – Part A Mol Biomol Spectrosc. 2018;191:498–512.10.1016/j.saa.2017.10.054Suche in Google Scholar PubMed
[147] Ren X, Zhang F, Guo B, Gao N, Zhang X. Synthesis of N-doped micropore carbon quantum dots with high quantum yield and dual-wavelength photoluminescence emission from biomass for cellular imaging. Nanomaterials. 2019;9:495.10.3390/nano9040495Suche in Google Scholar PubMed PubMed Central
[148] D’Angelis do ES, Barbosa C, Corrêa JR, Medeiros GA, Barreto G, Magalhães KG, de Oliveira AL, et al. Carbon dots (C‐dots) from cow manure with impressive subcellular selectivity tuned by simple chemical modification. Chemistry–A Eur J. 2015;21:5055–60.10.1002/chem.201406330Suche in Google Scholar PubMed
[149] Haryadi H, Purnama MRW, Wibowo A. C Dots Derived from Waste of Biomass and Their Photocatalytic Activities. Indonesian J Chem. 2018;18:594–9.10.22146/ijc.26652Suche in Google Scholar
[150] Yao Y-Y, Gedda G, Girma WM, Yen C-L, Ling Y-C, Chang J-Y. Magnetofluorescent carbon dots derived from crab shell for targeted dual-modality bioimaging and drug delivery. ACS Appl Mater Interfaces. 2017;9:13887–99.10.1021/acsami.7b01599Suche in Google Scholar PubMed
[151] Dehvari K, Liu KY, Tseng P-J, Gedda G, Girma WM, Chang J-Y. Sonochemical-assisted green synthesis of nitrogen-doped carbon dots from crab shell as targeted nanoprobes for cell imaging. J Taiwan Inst Chem Eng. 2019;95:495–503.10.1016/j.jtice.2018.08.037Suche in Google Scholar
[152] Gedda G, Lee C-Y, Lin Y-C, Wu H-F. Green synthesis of carbon dots from prawn shells for highly selective and sensitive detection of copper ions. Sens Actuators B Chem. 2016;224:396–403.10.1016/j.snb.2015.09.065Suche in Google Scholar
[153] Han Z, He L, Pan S, Liu H, Hu X. Hydrothermal synthesis of carbon dots and their application for detection of chlorogenic acid. Luminescence. 2020;35:989–97.10.1002/bio.3803Suche in Google Scholar PubMed
[154] Choi Y, Jo S, Chae A, Kim YK, Park JE, Lim D, et al. Simple microwave-assisted synthesis of amphiphilic carbon quantum dots from A3/B2 polyamidation monomer set. ACS Appl Mater Interfaces. 2017;9:27883–93.10.1021/acsami.7b06066Suche in Google Scholar PubMed
[155] Ventrella A, Camisasca A, Fontana A, Giordani S. Synthesis of green fluorescent carbon dots from carbon nano-onions and graphene oxide. RSC Adv. 2020;10:36404–12.10.1039/D0RA06172GSuche in Google Scholar PubMed PubMed Central
[156] Güçlü AD, Potasz P, Korkusinski M, Hawrylak P. Graphene quantum dots. London, UK: Springer; 2014.10.1007/978-3-662-44611-9Suche in Google Scholar
[157] Abbas A, Tabish TA, Bull SJ, Lim TM, Phan AN. High yield synthesis of graphene quantum dots from biomass waste as a highly selective probe for Fe3+ sensing. Sci Rep. 2020;10:1–16.10.1038/s41598-020-78070-2Suche in Google Scholar PubMed PubMed Central
[158] Wang Z, Yu J, Zhang X, Li N, Liu B, Li Y, et al. Large-scale and controllable synthesis of graphene quantum dots from rice husk biomass: a comprehensive utilization strategy. ACS Appl Mater Interfaces. 2016;8:1434–9.10.1021/acsami.5b10660Suche in Google Scholar PubMed
[159] Kumawat MK, Thakur M, Gurung RB, Srivastava R. Graphene quantum dots for cell proliferation, nucleus imaging, and photoluminescent sensing applications. Sci Rep. 2017;7:1–16.10.1038/s41598-017-16025-wSuche in Google Scholar PubMed PubMed Central
[160] Abbas A, Mariana LT, Phan AN. Biomass-waste derived graphene quantum dots and their applications. Carbon. 2018;140:77–99.10.1016/j.carbon.2018.08.016Suche in Google Scholar
[161] Suryawanshi A, Biswal M, Mhamane D, Gokhale R, Patil S, Guin D, et al. Large scale synthesis of graphene quantum dots (GQDs) from waste biomass and their use as an efficient and selective photoluminescence on–off–on probe for Ag+ ions. Nanoscale. 2014;6:11664–70.10.1039/C4NR02494JSuche in Google Scholar PubMed
[162] Chaudhary S, Kumari M, Chauhan P, Chaudhary GR. Upcycling of plastic waste into fluorescent carbon dots: An environmentally viable transformation to biocompatible C-dots with potential prospective in analytical applications. Waste Manag. 2021;120:675–86.10.1016/j.wasman.2020.10.038Suche in Google Scholar PubMed
[163] Roy P, Periasamy AP, Chuang C, Liou Y-R, Chen Y-F, Joly J, et al. Plant leaf-derived graphene quantum dots and applications for white LEDs. N J Chem. 2014;38:4946–51.10.1039/C4NJ01185FSuche in Google Scholar
[164] Scialabba N. Food wastage footprint & climate change. UN: FAO; 2015. p. 15–9.Suche in Google Scholar
[165] Kalita H, Mohapatra J, Pradhan L, Mitra A, Bahadur D, Aslam M. Efficient synthesis of rice based graphene quantum dots and their fluorescent properties. RSC Adv. 2016;6:23518–24.10.1039/C5RA25706ASuche in Google Scholar
[166] Nirala NR, Khandelwal G, Kumar B, Prakash R, Kumar V. One step electro-oxidative preparation of graphene quantum dots from wood charcoal as a peroxidase mimetic. Talanta. 2017;173:36–43.10.1016/j.talanta.2017.05.061Suche in Google Scholar PubMed
[167] Wang R, Jiao L, Zhou X, Guo Z, Bian H, Dai H. Highly fluorescent graphene quantum dots from biorefinery waste for tri-channel sensitive detection of Fe3+ ions. J Hazard Mater. 2021;412:125096.10.1016/j.jhazmat.2021.125096Suche in Google Scholar PubMed
[168] Liu Q, Zhang J, He H, Huang G, Xing B, Jia J, et al. Green preparation of high yield fluorescent graphene quantum dots from coal-tar-pitch by mild oxidation. Nanomaterials. 2018;8:844.10.3390/nano8100844Suche in Google Scholar PubMed PubMed Central
[169] Liu F, Sun Y, Zheng Y, Tang N, Li M, Zhong W, et al. Gram-scale synthesis of high-purity graphene quantum dots with multicolor photoluminescence. RSC Adv. 2015;5:103428–32.10.1039/C5RA19219FSuche in Google Scholar
[170] Dong Y, Chen C, Zheng X, Gao L, Cui Z, Yang H, et al. One-step and high yield simultaneous preparation of single-and multi-layer graphene quantum dots from CX-72 carbon black. J Mater Chem. 2012;22:8764–6.10.1039/c2jm30658aSuche in Google Scholar
[171] Ye R, Xiang C, Lin J, Peng Z, Huang K, Yan Z, et al. Coal as an abundant source of graphene quantum dots. Nat Commun. 2013;4:1–7.10.1038/ncomms3943Suche in Google Scholar PubMed
[172] Kumari TSD. Catalytic graphitization: A bottom-up approach to graphene and quantum dots derived therefrom–A review. Mater Today Proc. 2021;46:3069–74.10.1016/j.matpr.2021.02.499Suche in Google Scholar
[173] Pierrat P, Gaumet J-J. Graphene quantum dots: Emerging organic materials with remarkable and tunable luminescence features. Tetrahedron Lett. 2020;61(49):152554.10.1016/j.tetlet.2020.152554Suche in Google Scholar
[174] Mohamed HH, Alsanea AA. TiO2/carbon dots decorated reduced graphene oxide composites from waste car bumper and TiO2 nanoparticles for photocatalytic applications. Arab J Chem. 2020;13:3082–91.10.1016/j.arabjc.2018.08.016Suche in Google Scholar
[175] Tang D, Liu J, Yan X, Kang L. Graphene oxide derived graphene quantum dots with different photoluminescence properties and peroxidase-like catalytic activity. RSC Adv. 2016;6:50609–17.10.1039/C5RA26279HSuche in Google Scholar
[176] Tian R, Zhong S, Wu J, Jiang W, Wang T. Facile hydrothermal method to prepare graphene quantum dots from graphene oxide with different photoluminescences. RSC Adv. 2016;6:40422–6.10.1039/C6RA00780ESuche in Google Scholar
[177] Ananthanarayanan A, Wang X, Routh P, Sana B, Lim S, Kim DH, et al. Facile synthesis of graphene quantum dots from 3D graphene and their application for Fe3+ sensing. Adv Funct Mater. 2014;24:3021–6.10.1002/adfm.201303441Suche in Google Scholar
[178] Wang R, Xia G, Zhong W, Chen L, Chen L, Wang Y, et al. Direct transformation of lignin into fluorescence-switchable graphene quantum dots and their application in ultrasensitive profiling of a physiological oxidant. Green Chem. 2019;21:3343–52.10.1039/C9GC01012BSuche in Google Scholar
[179] Thirumal V, Dhamodharan K, Yuvakkumar R, Ravi G, Saravanakumar B, Thambidurai M, et al. Cleaner production of tamarind fruit shell into bio-mass derived porous 3D-activated carbon nanosheets by CVD technique for supercapacitor applications. Chemosphere. 2021;282:131033.10.1016/j.chemosphere.2021.131033Suche in Google Scholar PubMed
[180] Cazetta AL, Pezoti O, Bedin KC, Silva TL, Paesano Junior A, Asefa T, et al. Magnetic Activated Carbon Derived from Biomass Waste by Concurrent Synthesis: Efficient Adsorbent for Toxic Dyes. ACS Sustain Chem & Eng. 2016;4:1058–68.10.1021/acssuschemeng.5b01141Suche in Google Scholar
[181] Ho PH, Lofty V, Basta A, Trens P. Designing microporous activated carbons from biomass for carbon dioxide adsorption at ambient temperature. A comparison between bagasse and rice by-products. J Clean Prod. 2021;294:126260.10.1016/j.jclepro.2021.126260Suche in Google Scholar
[182] Esohe Omoriyekomwan J, Tahmasebi A, Zhang J, Yu J. Synthesis of super-long carbon nanotubes from cellulosic biomass under microwave radiation. Nanomaterials. 2022;12:737.10.3390/nano12050737Suche in Google Scholar PubMed PubMed Central
[183] Mishra N, Das G, Ansaldo A, Genovese A, Malerba M, Povia M, et al. Pyrolysis of waste polypropylene for the synthesis of carbon nanotubes. J Anal Appl Pyrolysis. 2012;94:91–8.10.1016/j.jaap.2011.11.012Suche in Google Scholar
[184] Aboul-Enein AA, Awadallah AE, Abdel-Rahman AAH, Haggar AM. Synthesis of multi-walled carbon nanotubes via pyrolysis of plastic waste using a two-stage process. Fullerenes Nanotubes Carbon Nanostruct. 2018;26:443–50.10.1080/1536383X.2018.1447929Suche in Google Scholar
[185] Maliutina K, Huang J, Su T, Yu J, Fan L. Biomass-derived Ta, N, S co-doped CNTs enriched carbon catalyst for efficient electrochemical oxygen reduction. J Alloy Compd. 2021;888:161479.10.1016/j.jallcom.2021.161479Suche in Google Scholar
[186] Zhang Y, Sun J, Tan J, Ma C, Luo S, Li W, et al. Multi-walled carbon nanotubes/carbon foam nanocomposites derived from biomass for CO2 capture and supercapacitor applications. Fuel. 2021;305:121622.10.1016/j.fuel.2021.121622Suche in Google Scholar
[187] Suhdi S, Wang S-C. The production of carbon nanofiber on rubber fruit shell-derived activated carbon by chemical activation and hydrothermal process with low temperature. Nanomaterials. 2021;11:2038.10.3390/nano11082038Suche in Google Scholar PubMed PubMed Central
[188] Vinayagam M, Suresh Babu R, Sivasamy A, de Barros ALF. Biomass-derived porous activated carbon nanofibers from Sapindus trifoliatus nut shells for high-performance symmetric supercapacitor applications. Carbon Lett. 2021;31:1133–43.10.1007/s42823-021-00235-4Suche in Google Scholar
[189] Taer E, Apriwandi A, Agustino A, Dewi MR, Taslim R. Porous hollow biomass-based carbon nanofiber/nanosheet for high-performance supercapacitor. Int J Energy Res. 2022;46:1467–80.10.1002/er.7262Suche in Google Scholar
[190] Farma R, Putri A, Taer E, Awitdrus A, Apriwandi A. Synthesis of highly porous activated carbon nanofibers derived from bamboo waste materials for application in supercapacitor. J Mater Sci Mater Electron. 2021;32:7681–91.10.1007/s10854-021-05486-5Suche in Google Scholar
[191] Zhao H, Cheng Y, Zhang Z, Zhang B, Pei C, Fan F, et al. Biomass-derived graphene-like porous carbon nanosheets towards ultralight microwave absorption and excellent thermal infrared properties. Carbon. 2021;173:501–11.10.1016/j.carbon.2020.11.035Suche in Google Scholar
[192] Yuan S-J, Dong B, Dai X-H. Facile and scalable synthesis of high-quality few-layer graphene from biomass by a universal solvent-free approach. Appl Surf Sci. 2021;562:150203.10.1016/j.apsusc.2021.150203Suche in Google Scholar
[193] Karaman C, Karaman O, Atar N, Yola ML. Sustainable electrode material for high-energy supercapacitor: biomass-derived graphene-like porous carbon with three-dimensional hierarchically ordered ion highways. Phys Chem Chem Phys. 2021;23:12807–21.10.1039/D1CP01726HSuche in Google Scholar PubMed
[194] Mahmood F, Sun Y, Wan C. Biomass-derived porous graphene for electrochemical sensing of dopamine. RSC Adv. 2021;11:15410–15.10.1039/D1RA00735ASuche in Google Scholar PubMed PubMed Central
[195] Abbas A, Abbas S, Tabish TA, Bull SJ, Phan AN, Lim TM. Role of precursor microstructure in the development of graphene quantum dots from biomass. J Environ Chem Eng. 2021;9:106154.10.1016/j.jece.2021.106154Suche in Google Scholar
[196] Li Z, Wang Q, Zhou Z, Zhao S, Zhong S, Xu L, et al. Green synthesis of carbon quantum dots from corn stalk shell by hydrothermal approach in near-critical water and applications in detecting and bioimaging. Microchem J. 2021;166:106250.10.1016/j.microc.2021.106250Suche in Google Scholar
[197] Tyagi A, Tripathi KM, Singh N, Choudhary S, Gupta RK. Green synthesis of carbon quantum dots from lemon peel waste: applications in sensing and photocatalysis. RSC Adv. 2016;6:72423–32.10.1039/C6RA10488FSuche in Google Scholar
[198] Hamad H, Sadik W, Abd El-latif M, Kashyout A, Feteha M. Photocatalytic parameters and kinetic study for degradation of dichlorophenol-indophenol (DCPIP) dye using highly active mesoporous TiO2 nanoparticles. J Environ Sci. 2016;43:26–39.10.1016/j.jes.2015.05.033Suche in Google Scholar PubMed
[199] Shokry H, Elkady MF, Hamad H. Synthesis and characterization of stabilized tetragonal nano Zirconia by precipitation method. Proceedings of Journal of Nano Research. 2019;56:142–51.10.4028/www.scientific.net/JNanoR.56.142Suche in Google Scholar
[200] El-Sayed EM, Hamad HA, Ali RM. Journey from ceramic waste to highly efficient toxic dye adsorption from aqueous solutions via one-pot synthesis of CaSO4 rod-shape with silica. J Mater Res Technol. 2020;9:16051–63.10.1016/j.jmrt.2020.11.037Suche in Google Scholar
[201] Nguyen HL, Gropp C, Yaghi OM. Reticulating 1D ribbons into 2D covalent organic frameworks by imine and imide linkages. J Am Chem Soc. 2020;142:2771–6.10.1021/jacs.9b13971Suche in Google Scholar PubMed
[202] Matheu R, Gutierrez-Puebla E, Monge MÁ, Diercks CS, Kang J, Prévot MS, et al. Three-dimensional phthalocyanine metal-catecholates for high electrochemical carbon dioxide reduction. J Am Chem Soc. 2019;141:17081–5.10.1021/jacs.9b09298Suche in Google Scholar PubMed
[203] Granata G, Pagnanelli F, Moscardini E, Takacova Z, Havlik T, Toro L. Simultaneous recycling of nickel metal hydride, lithium ion and primary lithium batteries: Accomplishment of European Guidelines by optimizing mechanical pre-treatment and solvent extraction operations. J Power Sources. 2012;212:205–11.10.1016/j.jpowsour.2012.04.016Suche in Google Scholar
[204] Peng C, Chang C, Wang Z, Wilson BP, Liu F, Lundström M. Recovery of High-Purity MnO2 from the Acid Leaching Solution of Spent Li-Ion Batteries. JOM. 2020;72:790–9.10.1007/s11837-019-03785-1Suche in Google Scholar
[205] Kim T, Senanayake G, Kang J, Sohn J, Rhee K, Lee S, et al. Reductive acid leaching of spent zinc–carbon batteries and oxidative precipitation of Mn–Zn ferrite nanoparticles. Hydrometallurgy. 2009;96:154–8.10.1016/j.hydromet.2008.10.001Suche in Google Scholar
[206] Mylarappa M, Lakshmi VV, Mahesh KV, Nagaswarupa H, Raghavendra N. A facile hydrothermal recovery of nano sealed MnO2 particle from waste batteries: An advanced material for electrochemical and environmental applications. Proceedings of IOP Conference Series: Materials Science and Engineering. 2016. p. 012178.10.1088/1757-899X/149/1/012178Suche in Google Scholar
[207] Rahmat M, Rehman A, Rahmat S, Bhatti HN, Iqbal M, Khan WS, et al. Laser ablation assisted preparation of MnO2 nanocolloids from waste battery cell powder: evaluation of physico-chemical, electrical and biological properties. J Mol Struct. 2019;1191:284–90.10.1016/j.molstruc.2019.04.094Suche in Google Scholar
[208] Ali GAM, Yusoff MM, Feng CK. Electrochemical properties of electrodeposited MnO2 nanoparticles. Adv Mater Res. 2015;1113:550–3.10.4028/www.scientific.net/AMR.1113.550Suche in Google Scholar
[209] Ali GAM, Yusoff MM, Shaaban ER, Chong KF. High performance MnO2 nanoflower supercapacitor electrode by electrochemical recycling of spent batteries. Ceram Int. 2017;43:8440–8.10.1016/j.ceramint.2017.03.195Suche in Google Scholar
[210] Ali GAM. Recycled MnO2 nanoflowers and graphene nanosheets for low-cost and high performance asymmetric supercapacitor. J Electron Mater. 2020;49:5411–21.10.1007/s11664-020-08268-7Suche in Google Scholar
[211] Ali GAM, Tan LL, Jose R, Yusoff MM, Chong KF. Electrochemical performance studies of MnO2 nanoflowers recovered from spent battery. Mater Res Bull. 2014;60:5–9.10.1016/j.materresbull.2014.08.008Suche in Google Scholar
[212] Zhang N, Guo G, He B, Zhu J, Wu J, Qiu J. Synthesis and research of MnO2-NiO composite as lithium-ion anode Zn-Mn batteries as source. J Alloy Compd. 2020;838:1045.10.1016/j.jallcom.2020.155578Suche in Google Scholar
[213] Natarajan S, Anantharaj S, Tayade RJ, Bajaj HC, Kundu S. Recovered spinel MnCo2O4 from spent lithium-ion batteries for enhanced electrocatalytic oxygen evolution in alkaline medium. Dalton Trans. 2017;46:14382–92.10.1039/C7DT02613GSuche in Google Scholar
[214] Mahlangeni NT, Moodley R. Biosynthesis of manganese oxide nanoparticles using Urginea sanguinea and their effects on cytotoxicity and antioxidant activity. Adv Nat Sci Nanosci Nanotechnol. 2021;12:015015.10.1088/2043-6262/abe8d5Suche in Google Scholar
[215] Souri M, Hoseinpour V, Ghaemi N, Shakeri A. Procedure optimization for green synthesis of manganese dioxide nanoparticles by Yucca gloriosa leaf extract. Int Nano Lett. 2019;9:73–81.10.1007/s40089-018-0257-zSuche in Google Scholar
[216] Kumar V, Singh K, Panwar S, Mehta SK. Green synthesis of manganese oxide nanoparticles for the electrochemical sensing of p-nitrophenol. Int Nano Lett. 2017;7:123–31.10.1007/s40089-017-0205-3Suche in Google Scholar
[217] Moon SA, Salunke BK, Alkotaini B, Sathiyamoorthi E, Kim BS. Biological synthesis of manganese dioxide nanoparticles by Kalopanax pictus plant extract. IET nanobiotechnology. 2015;9:220–5.10.1049/iet-nbt.2014.0051Suche in Google Scholar PubMed
[218] Prasad KS, Patra A. Green synthesis of MnO2 nanorods using Phyllanthus amarus plant extract and their fluorescence studies. Green Process Synth. 2017;6:549–54.10.1515/gps-2016-0166Suche in Google Scholar
[219] Prasad AS. Green synthesis of nanocrystalline manganese (II, III) oxide. Mater Sci Semicond Process. 2017;71:342–7.10.1016/j.mssp.2017.08.020Suche in Google Scholar
[220] Chen X, Deng F, Liu X, Cui K-P, Weerasooriya R. Hydrothermal synthesis of MnO2/Fe(0) composites from Li-ion battery cathodes for destructing sulfadiazine by photo-Fenton process. Sci Total Env. 2021;774:145776.10.1016/j.scitotenv.2021.145776Suche in Google Scholar
[221] Min X, Guo M, Liu L, Li L, Gu J-N, Liang J, et al. Synthesis of MnO2 derived from spent lithium-ion batteries via advanced oxidation and its application in VOCs oxidation. J Hazard Mater. 2021;406:124743.10.1016/j.jhazmat.2020.124743Suche in Google Scholar PubMed
[222] Ma L, Nie Z, Xi X, Han XG. Cobalt recovery from cobalt-bearing waste in sulphuric and citric acid systems. Hydrometallurgy. 2013;136:1–7.10.1016/j.hydromet.2013.01.016Suche in Google Scholar
[223] Jha MK, Kumari A, Jha AK, Kumar V, Hait J, Pandey BD. Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone. Waste Manag. 2013;33:1890–7.10.1016/j.wasman.2013.05.008Suche in Google Scholar PubMed
[224] Chen M, Wang R, Qi Y, Han Y, Wang R, Fu J, et al. Cobalt and lithium leaching from waste lithium ion batteries by glycine. J Power Sources. 2021;482:228942.10.1016/j.jpowsour.2020.228942Suche in Google Scholar
[225] Assefi M, Maroufi S, Yamauchi Y, Sahajwalla V. Core–shell nanocatalysts of Co3O4 and NiO shells from new (discarded) resources: sustainable recovery of cobalt and nickel from spent lithium-ion batteries, Ni–Cd batteries, and LCD panel. ACS Sustain Chem & Eng. 2019;7:19005–14.10.1021/acssuschemeng.9b04618Suche in Google Scholar
[226] Bertuol DA, Machado CM, Silva ML, Calgaro CO, Dotto GL, Tanabe EH. Recovery of cobalt from spent lithium-ion batteries using supercritical carbon dioxide extraction. Waste Manag. 2016;51:245–51.10.1016/j.wasman.2016.03.009Suche in Google Scholar
[227] Li J, Shi P, Wang Z, Chen Y, Chang C-C. A combined recovery process of metals in spent lithium-ion batteries. Chemosphere. 2009;77:1132–6.10.1016/j.chemosphere.2009.08.040Suche in Google Scholar
[228] Myoung J, Jung Y, Lee J, Tak Y. Cobalt oxide preparation from waste LiCoO2 by electrochemical–hydrothermal method. J Power Sources. 2002;112:639–42.10.1016/S0378-7753(02)00459-7Suche in Google Scholar
[229] Boxall NJ, Cheng KY, Bruckard W, Kaksonen AH. Application of indirect non-contact bioleaching for extracting metals from waste lithium-ion batteries. J Hazard Mater. 2018;360:504–11.10.1016/j.jhazmat.2018.08.024Suche in Google Scholar PubMed
[230] Ouyang W, Zhao D, Wang Y, Balu AM, Len C, Luque R. Continuous flow conversion of biomass-derived methyl levulinate into γ-valerolactone using functional metal organic frameworks. ACS Sustain Chem & Eng. 2018;6:6746–52.10.1021/acssuschemeng.8b00549Suche in Google Scholar
[231] Kaksonen AH, Mudunuru BM, Hackl R. The role of microorganisms in gold processing and recovery – A review. Hydrometallurgy. 2014;142:70–83.10.1016/j.hydromet.2013.11.008Suche in Google Scholar
[232] Yu Z, Han H, Feng P, Zhao S, Zhou T, Kakade A, et al. Recent advances in the recovery of metals from waste through biological processes. Bioresour Technol. 2020;297:122416.10.1016/j.biortech.2019.122416Suche in Google Scholar PubMed
[233] Dhiman S, Gupta B. Partition studies on cobalt and recycling of valuable metals from waste Li-ion batteries via solvent extraction and chemical precipitation. J Clean Prod. 2019;225:820–32.10.1016/j.jclepro.2019.04.004Suche in Google Scholar
[234] Janiszewska M, Markiewicz A, Regel-Rosocka M. Hydrometallurgical separation of Co (II) from Ni (II) from model and real waste solutions. J Clean Prod. 2019;228:746–54.10.1016/j.jclepro.2019.04.285Suche in Google Scholar
[235] Kursunoglu S, Ichlas ZT, Kaya M. Solvent extraction process for the recovery of nickel and cobalt from Caldag laterite leach solution: The first bench scale study. Hydrometallurgy. 2017;169:135–41.10.1016/j.hydromet.2017.01.001Suche in Google Scholar
[236] Swain B, Cho S-S, Lee GH, Lee CG, Uhm S. Extraction/separation of cobalt by solvent extraction: a review. Appl Chem Eng. 2015;26:631–9.10.14478/ace.2015.1120Suche in Google Scholar
[237] Giebner F, Kaden L, Wiche O, Tischler J, Schopf S, Schlömann M. Bioleaching of cobalt from an arsenidic ore. Min Eng. 2019;131:73–8.10.1016/j.mineng.2018.10.020Suche in Google Scholar
[238] Dubey S, Kumar J, Kumar A, Sharma YC. Facile and green synthesis of highly dispersed cobalt oxide (Co3O4) nano powder: Characterization and screening of its eco-toxicity. Adv Powder Technol. 2018;29:2583–90.10.1016/j.apt.2018.03.009Suche in Google Scholar
[239] Shokry H, Hamad H. Effect of superparamagnetic nanoparticles on the physicochemical properties of nano hydroxyapatite for groundwater treatment: adsorption mechanism of Fe (II) and Mn (II). RSC Adv. 2016;6:82244–59.10.1039/C6RA14497GSuche in Google Scholar
[240] Ali MAEAA, Hamad H. Synthesis and characterization of highly stable superparamagnetic CoFe2O4 nanoparticles as a catalyst for novel synthesis of thiazolo [4, 5-b] quinolin-9-one derivatives in aqueous medium. J Mol Catal A Chem. 2015;404:148–55.10.1016/j.molcata.2015.04.023Suche in Google Scholar
[241] Ali RM, Elkatory MR, Hamad HA. Highly active and stable magnetically recyclable CuFe2O4 as a heterogenous catalyst for efficient conversion of waste frying oil to biodiesel. Fuel. 2020;268:117297.10.1016/j.fuel.2020.117297Suche in Google Scholar
[242] Funari V, Mantovani L, Vigliotti L, Tribaudino M, Dinelli E, Braga R. Science of the Total Environment Superparamagnetic iron oxides nanoparticles from municipal solid waste incinerators. Sci Total Env. 2018;621:687–96.10.1016/j.scitotenv.2017.11.289Suche in Google Scholar PubMed
[243] Giri SK, Das NN, Pradhan GC. Colloids and Surfaces A: Physicochemical and Engineering Aspects Synthesis and characterization of magnetite nanoparticles using waste iron ore tailings for adsorptive removal of dyes from aqueous solution. Colloids Surf A Physicochem Eng Asp. 2011;389:43–9.10.1016/j.colsurfa.2011.08.052Suche in Google Scholar
[244] Li X, Wang C, Zeng Y, Li P, Xie T, Zhang Y. Bacteria-assisted preparation of nano α-Fe2O3 red pigment powders from waste ferrous sulfate. J Hazard Mater. 2016;317:563–9.10.1016/j.jhazmat.2016.06.021Suche in Google Scholar PubMed
[245] Saini D, Aggarwal R, Anand SR, Satrawala N, Joshi RK, Sonkar SK. Sustainable feasibility of waste printer ink to magnetically separable iron oxide–doped nanocarbons for styrene oxidation. Mater Today Chem. 2020;16:100256.10.1016/j.mtchem.2020.100256Suche in Google Scholar
[246] Biswas A, Patra AK, Sarkar S, Das D, Chattopadhyay D, De S. Synthesis of highly magnetic iron oxide nanomaterials from waste iron by one-step approach. Colloids Surf A. 2020;589:124420–20.10.1016/j.colsurfa.2020.124420Suche in Google Scholar
[247] Mhamane D, Kim H-K, Aravindan V, Roh KC, Srinivasan M, Kim K-B. Rusted iron wire waste into high performance anode (α-Fe2O3) for Li-ion batteries: an efficient waste management approach. Green Chem. 2016;18:1395–404. 10.1039/c5gc01747e;1395-1404. Suche in Google Scholar
[248] Lingamdinne LP, Vemula KR, Chang YY, Yang JK, Karri RR, Koduru JR. Process optimization and modeling of lead removal using iron oxide nanocomposites generated from bio-waste mass. Chemosphere. 2020;243:125257.10.1016/j.chemosphere.2019.125257Suche in Google Scholar PubMed
[249] Ahmmad B, Leonard K, Shariful Islam M, Kurawaki J, Muruganandham M, Ohkubo T, et al. Green synthesis of mesoporous hematite (α-Fe2O3) nanoparticles and their photocatalytic activity. Adv Powder Technol. 2013;24:160–7.10.1016/j.apt.2012.04.005Suche in Google Scholar
[250] Wu Y, Zeng S, Wang F, Megharaj M, Naidu R, Chen Z. Heterogeneous Fenton-like oxidation of malachite green by iron-based nanoparticles synthesized by tea extract as a catalyst. Sep Purif Technol. 2015;154:161–7.10.1016/j.seppur.2015.09.022Suche in Google Scholar
[251] Huang L, Weng X, Chen Z, Megharaj M, Naidu R. Synthesis of iron-based nanoparticles using oolong tea extract for the degradation of malachite green. Spectrochimica Acta - Part A Mol Biomolecular Spectrosc. 2014;117:801–4.10.1016/j.saa.2013.09.054Suche in Google Scholar PubMed
[252] Venkateswarlu S, Natesh Kumar B, Prasad CH, Venkateswarlu P, Jyothi NVV. Bio-inspired green synthesis of Fe3O4 spherical magnetic nanoparticles using Syzygium cumini seed extract. Phys B Condens Matter. 2014;449:67–71.10.1016/j.physb.2014.04.031Suche in Google Scholar
[253] Yadav VK, Yadav KK, Gnanamoorthy G, Choudhary N, Khan SH, Gupta N, et al. A novel synthesis and characterization of polyhedral shaped amorphous iron oxide nanoparticles from incense sticks ash waste. Environ Technol & Innov. 2020;20:101089.10.1016/j.eti.2020.101089Suche in Google Scholar
[254] Hamad H, Abd El-Latif M, Kashyout AE-H, Sadik W, Feteha M. Synthesis and characterization of core–shell–shell magnetic (CoFe2O4–SiO2–TiO2) nanocomposites and TiO2 nanoparticles for the evaluation of photocatalytic activity under UV and visible irradiation. N J Chem. 2015;39:3116–28.10.1039/C4NJ01821DSuche in Google Scholar
[255] Hamad H, Bailón-García E, Morales-Torres S, Carrasco-Marín F, Pérez-Cadenas AF, Maldonado-Hódar FJ. A new platform for facile synthesis of hybrid TiO2 nanostructures by various functionalizations of cellulose to be used in highly-efficient photocatalysis. Mater Lett. 2020;274:128016.10.1016/j.matlet.2020.128016Suche in Google Scholar
[256] Rueda D, Arias V, Zhang Y, Cabot A, Agudelo AC, Cadavid D. Low-cost tangerine peel waste mediated production of Titanium Dioxide Nanocrystals: Synthesis and characterization. Environ Nanotechnol Monit Manag. 2020;13:100285.10.1016/j.enmm.2020.100285Suche in Google Scholar
[257] Dobrucka R. Synthesis of titanium dioxide nanoparticles using Echinacea purpurea herba. Iran J Pharm Res. 2017;16:753–9.Suche in Google Scholar
[258] Shimpi NG, Shirole S, Suryawanshi Y, Mishra S. Optimized Synthesis of nTiO2 using murraya koenigii leaf extract and its application in iPP–EPDM blends. Adv Polym Technol. 2017;36:160–7.10.1002/adv.21595Suche in Google Scholar
[259] El-Remaily MAEAAA, Abu-Dief AM, Elhady O. Green synthesis of TiO2 nanoparticles as an efficient heterogeneous catalyst with high reusability for synthesis of 1,2-dihydroquinoline derivatives. Appl Organomet Chem. 2019;33:1–10.10.1002/aoc.5005Suche in Google Scholar
[260] Karlsson MCF, Abbas Z, Bordes R, Cao Y, Larsson A, Rolland A, et al. Surface properties of recycled titanium oxide recovered from paint waste. Prog Org Coat. 2018;125:279–86.10.1016/j.porgcoat.2018.09.012Suche in Google Scholar
[261] Bilici Z, Bouchareb R, Sacak T, Yatmaz HC, Dizge N. Recycling of TiO2-containing waste and utilization by photocatalytic degradation of a reactive dye solution. Water Sci Technol. 2021;83:1242–9.10.2166/wst.2020.606Suche in Google Scholar PubMed PubMed Central
[262] Yola ML, Eren T, Atar N. A novel efficient photocatalyst based on TiO2 nanoparticles involved boron enrichment waste for photocatalytic degradation of atrazine. Chem Eng J. 2014;250:288–94.10.1016/j.cej.2014.03.116Suche in Google Scholar
[263] Amornpitoksuk P, Suwanboon S, Kaowphong S, Randorn C, Graidist P. Photocatalytic activity of K2Ti6O13/TiO2 nanocomposite prepared using water extract of wood ash from waste for degradation of dye pollutants. J Taiwan Inst Chem Eng. 2020;117:242–51.10.1016/j.jtice.2020.12.009Suche in Google Scholar
[264] Zhang Q, Wu Y, Yuan H. Recycling strategies of spent V2O5-WO3/TiO2 catalyst: A review. Resources Conserv Recycling. 2020;161:155578.10.1016/j.resconrec.2020.104983Suche in Google Scholar
[265] Zalas M, Cynarzewska A. Application of paper industry waste materials containing TiO2 for dye-sensitized solar cells fabrication. Optik. 2018;158:469–76.10.1016/j.ijleo.2017.12.125Suche in Google Scholar
[266] Qiang T, Song Y, Zhu R, Yuan W. Biomass material derived hierarchical porous TiO2: Adjustable pore size for protein adsorption. J Alloy Compd. 2020;829:154512.10.1016/j.jallcom.2020.154512Suche in Google Scholar
[267] Seifipour R, Nozari M, Pishkar L. Preparation of ZnO nanoparticles using Tragopogon Collinus leaf extract and study of its antibacterial effects for therapeutic applications. J Plant Biochem Biotechnol. 2021;30:1–10.10.1007/s13562-020-00638-wSuche in Google Scholar
[268] Thi TUD, Nguyen TT, Thi YD, Thi KHT, Phan BT, Pham KN. Green synthesis of ZnO nanoparticles using orange fruit peel extract for antibacterial activities. RSC Adv. 2020;10:23899–907.10.1039/D0RA04926CSuche in Google Scholar
[269] Pachiyappan J, Gnanasundaram N, Rao GL. Preparation and characterization of ZnO, MgO and ZnO–MgO hybrid nanomaterials using green chemistry approach. Results in. Materials. 2020;7:100104.10.1016/j.rinma.2020.100104Suche in Google Scholar
[270] Ifeanyichukwu UL, Fayemi OE, Ateba CN. Green synthesis of zinc oxide nanoparticles from pomegranate (Punica granatum) extracts and characterization of their antibacterial activity. Molecules. 2020;25:4521.10.3390/molecules25194521Suche in Google Scholar PubMed PubMed Central
[271] Ruangtong J, Jiraroj T, T-Thienprasert NP. Green synthesized ZnO nanosheets from banana peel extract possess anti-bacterial activity and anti-cancer activity. Materials Today. Communications. 2020;24:101224.10.1016/j.mtcomm.2020.101224Suche in Google Scholar
[272] Demissie MG, Sabir FK, Edossa GD, Gonfa BA. Synthesis of zinc oxide nanoparticles using leaf extract of Lippia adoensis (koseret) and evaluation of its antibacterial activity. J Chem. 2020;2020:7459042.10.1155/2020/7459042Suche in Google Scholar
[273] Dobrucka R, Długaszewska J. Biosynthesis and antibacterial activity of ZnO nanoparticles using Trifolium pratense flower extract. Saudi J Biol Sci. 2016;23:517–23.10.1016/j.sjbs.2015.05.016Suche in Google Scholar PubMed PubMed Central
[274] Jha AK, Prasad K. Synthesis of ZnO nanoparticles from goat slaughter waste for environmental protection. Int J Curr Eng Technol. 2016;6:147–51.10.14741/Ijcet/22774106/6.612016.26Suche in Google Scholar
[275] Rambabu K, Bharath G, Banat F, Show PL. Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment. J Hazard Mater. 2021;402:123560.10.1016/j.jhazmat.2020.123560Suche in Google Scholar PubMed
[276] Sagar Raut D, Thorat RT. Green synthesis of zinc oxide (ZnO) nanoparticles using OcimumTenuiflorum leaves. Int J Sci Res. 2015;4:1225–8.Suche in Google Scholar
[277] Abioye AM, Ani FN. Recent development in the production of activated carbon electrodes from agricultural waste biomass for supercapacitors: a review. Renew Sustain Energy Rev. 2015;52:1282–93.10.1016/j.rser.2015.07.129Suche in Google Scholar
[278] Divyashree A, Hegde G. Activated carbon nanospheres derived from bio-waste materials for supercapacitor applications–a review. RSC Adv. 2015;5:88339–52.10.1039/C5RA19392CSuche in Google Scholar
[279] Zhi M, Yang F, Meng F, Li M, Manivannan A, Wu N. Effects of pore structure on performance of an activated-carbon supercapacitor electrode recycled from scrap waste tires. ACS Sustain Chem & Eng. 2014;2:1592–8.10.1021/sc500336hSuche in Google Scholar
[280] Yang Z, Ren J, Zhang Z, Chen X, Guan G, Qiu L, et al. Recent advancement of nanostructured carbon for energy applications. Chem Rev. 2015;115:5159–223.10.1021/cr5006217Suche in Google Scholar PubMed
[281] Sadik WA, El-Demerdash A-GM, Nashed AW, Mostafa AA, Hamad HA. Highly efficient photocatalytic performance of Cu2O@ TiO2 nanocomposite: influence of various inorganic oxidants and inorganic anions. J Mater Res Technol. 2019;8:5405–14.10.1016/j.jmrt.2019.09.007Suche in Google Scholar
[282] Eltarahony M, Abu-Serie M, Hamad H, Zaki S, Abd-El-Haleem D. Unveiling the role of novel biogenic functionalized CuFe hybrid nanocomposites in boosting anticancer, antimicrobial and biosorption activities. Sci Rep. 2021;11:1–22.10.1038/s41598-021-87363-zSuche in Google Scholar PubMed PubMed Central
[283] Chowdhury R, Khan A, Rashid MH. Green synthesis of CuO nanoparticles using Lantana camara flower extract and their potential catalytic activity towards the aza-Michael reaction. RSC Adv. 2020;10:14374–85.10.1039/D0RA01479FSuche in Google Scholar
[284] Samari F, Baluchi L, Salehipoor H, Yousefinejad S. Controllable phyto-synthesis of cupric oxide nanoparticles by aqueous extract of Capparis spinosa (caper) leaves and application in iron sensing. Microchem J. 2019;150:104158.10.1016/j.microc.2019.104158Suche in Google Scholar
[285] Sharma S, Kumar K. Aloe-vera leaf extract as a green agent for the synthesis of CuO nanoparticles inactivating bacterial pathogens and dye. J Dispers Sci Technol. 2020;42(13):1–13.10.1080/01932691.2020.1791719Suche in Google Scholar
[286] Aminuzzaman M, Kei LM, Liang WH. Green synthesis of copper oxide (CuO) nanoparticles using banana peel extract and their photocatalytic activities. AIP Conference Proceedings. 2017;1828:020016.10.1063/1.4979387Suche in Google Scholar
[287] Sharmila G, Pradeep RS, Sandiya K, Santhiya S, Muthukumaran C, Jeyanthi J, et al. Biogenic synthesis of CuO nanoparticles using Bauhinia tomentosa leaves extract: characterization and its antibacterial application. J Mol Struct. 2018;1165:288–92.10.1016/j.molstruc.2018.04.011Suche in Google Scholar
[288] Sackey J, Nwanya A, Bashir A, Matinise N, Ngilirabanga J, Ameh A, et al. Electrochemical properties of Euphorbia pulcherrima mediated copper oxide nanoparticles. Mater Chem Phys. 2020;244:122714.10.1016/j.matchemphys.2020.122714Suche in Google Scholar
[289] Saeed F, Arshad MU, Pasha I, Naz R, Batool R, Khan AA, et al. Nutritional and phyto-therapeutic potential of papaya (Carica papaya Linn.): an overview. Int J Food Prop. 2014;17:1637–53.10.1080/10942912.2012.709210Suche in Google Scholar
[290] Xu T, Bian N, Wen M, Xiao J, Yuan C, Cao A, et al. Characterization of a common wheat (Triticum aestivum L.) high-tillering dwarf mutant. Theor Appl Genet. 2017;130:483–94.10.1007/s00122-016-2828-6Suche in Google Scholar PubMed
[291] Bordbar M, Sharifi-Zarchi Z, Khodadadi B. Green synthesis of copper oxide nanoparticles/clinoptilolite using Rheum palmatum L. root extract: high catalytic activity for reduction of 4-nitro phenol, rhodamine B, and methylene blue. J Sol-Gel Sci Technol. 2017;81:724–33.10.1007/s10971-016-4239-1Suche in Google Scholar
[292] Liu Q-M, Zhou D-B, Yamamoto Y, Ichino R, Okido M. Preparation of Cu nanoparticles with NaBH4 by aqueous reduction method. Trans Nonferrous Met Soc China. 2012;22:117–23.10.1016/S1003-6326(11)61149-7Suche in Google Scholar
[293] Su X, Zhao J, Bala H, Zhu Y, Gao Y, Ma S, et al. Fast synthesis of stable cubic copper nanocages in the aqueous phase. J Phys Chem C. 2007;111:14689–93.10.1021/jp074550wSuche in Google Scholar
[294] Zhou R, Wu X, Hao X, Zhou F, Li H, Rao W. Influences of surfactants on the preparation of copper nanoparticles by electron beam irradiation. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater At. 2008;266:599–603.10.1016/j.nimb.2007.11.040Suche in Google Scholar
[295] Vincent M, Duval RE, Hartemann P, Engels‐Deutsch M. Contact killing and antimicrobial properties of copper. J Appl Microbiology. 2018;124:1032–46.10.1111/jam.13681Suche in Google Scholar PubMed
[296] Rajkumar S, Elanthamilan E, Balaji TE, Sathiyan A, Jafneel NE, Merlin JP. Recovery of copper oxide nanoparticles from waste SIM cards for supercapacitor electrode material. J Alloy Compd. 2020;849:156582.10.1016/j.jallcom.2020.156582Suche in Google Scholar
[297] Fang M, Zheng R, Wu Y, Yue D, Qian X, Zhao Y, et al. CuO nanosheet as a recyclable Fenton-like catalyst prepared from simulated Cu (II) waste effluents by alkaline H2O2 reaction. Environ Sci Nano. 2019;6:105–14.10.1039/C8EN00930ASuche in Google Scholar
[298] Phang Y-K, Aminuzzaman M, Akhtaruzzaman M, Muhammad G, Ogawa S, Watanabe A, et al. Green synthesis and characterization of CuO nanoparticles derived from papaya peel extract for the photocatalytic degradation of palm oil mill effluent (POME). Sustainability. 2021;13:796.10.3390/su13020796Suche in Google Scholar
[299] Sharma S, Kumar K, Thakur N, Chauhan S, Chauhan MS. Eco-friendly Ocimum tenuiflorum green route synthesis of CuO nanoparticles: Characterizations on photocatalytic and antibacterial activities. J Environ Chem Eng. 2021;9:105395.10.1016/j.jece.2021.105395Suche in Google Scholar
[300] Barai HR, Banerjee AN, Hamnabard N, Joo SW. Synthesis of amorphous manganese oxide nanoparticles – to – crystalline nanorods through a simple wet-chemical technique using K+ ions as a ‘growth director’ and their morphology-controlled high performance supercapacitor applications. RSC Adv. 2016;6:78887–908.10.1039/C6RA18811GSuche in Google Scholar
[301] Zhou H, Zhan Y, Guo F, Du S, Tian B, Dong Y, et al. Synthesis of biomass-derived carbon aerogel/MnOx composite as electrode material for high-performance supercapacitors. Electrochim Acta. 2021;390:138817.10.1016/j.electacta.2021.138817Suche in Google Scholar
[302] Zhan D, Yuan X, Xiang C, Lu J, Dai G, Hu R, et al. Facile route to biomass-derived 1D carbon fiber supported high-performance MnO-based nanocomposite anode material. Sustain Mater Technol. 2021;29:e00322.10.1016/j.susmat.2021.e00322Suche in Google Scholar
[303] Borah D, Rout J, Gogoi D, Nath Ghosh N, Bhattacharjee CR. Composition controllable green synthesis of manganese dioxide nanoparticles using an edible freshwater red alga and its photocatalytic activity towards water soluble toxic dyes. Inorg Chem Commun. 2022;138:109312.10.1016/j.inoche.2022.109312Suche in Google Scholar
[304] Samer M, Abdelsalam EM, Mohamed S, Elsayed H, Attia Y. Impact of photoactivated cobalt oxide nanoparticles addition on manure and whey for biogas production through dry anaerobic co-digestion. Environ Dev Sustain. 2021;7:7776–93. 10.1007/s10668-021-01757.Suche in Google Scholar
[305] Samuel MS, Selvarajan E, Mathimani T, Santhanam N, Phuong TN, Brindhadevi K, et al. Green synthesis of cobalt-oxide nanoparticle using jumbo Muscadine (Vitis rotundifolia): Characterization and photo-catalytic activity of acid Blue-74. J Photochem Photobiol B Biol. 2020;211:112011.10.1016/j.jphotobiol.2020.112011Suche in Google Scholar PubMed
[306] Kadam A, Saratale RG, Shinde S, Yang J, Hwang K, Mistry B, et al. Adsorptive remediation of cobalt oxide nanoparticles by magnetized α-cellulose fibers from waste paper biomass. Bioresour Technol. 2019;273:386–93.10.1016/j.biortech.2018.11.041Suche in Google Scholar PubMed
[307] Fuentes CA, Gallegos MV, García JR, Sambeth J, Peluso MA. Catalytic glycolysis of poly(ethylene terephthalate) using zinc and cobalt oxides recycled from spent batteries. Waste Biomass Valoriz. 2020;11:4991–5001.10.1007/s12649-019-00807-6Suche in Google Scholar
[308] El Naggar AMA, Ali MM, Abdel Maksoud SA, Taha MH, Morshedy AS, Elzoghby AA. Waste generated bio-char supported co-nanoparticles of nickel and cobalt oxides for efficient adsorption of uranium and organic pollutants from industrial phosphoric acid. J Radioanal Nucl Chem. 2019;320:741–55.10.1007/s10967-019-06529-2Suche in Google Scholar
[309] Funari V, Mantovani L, Vigliotti L, Tribaudino M, Dinelli E, Braga R. Superparamagnetic iron oxide nanoparticles from municipal solid waste incinerators. Sci Total Env. 2018;621:687–96.10.1016/j.scitotenv.2017.11.289Suche in Google Scholar PubMed
[310] Plachtová P, Medříková Z, Zbořil R, Tuček J, Varma RS, Maršálek B. Iron and iron oxide nanoparticles synthesized with green tea extract: differences in ecotoxicological profile and ability to degrade malachite green. ACS Sustain Chem & Eng. 2018;6:8679–87.10.1021/acssuschemeng.8b00986Suche in Google Scholar PubMed PubMed Central
[311] Bibi I, Nazar N, Ata S, Sultan M, Ali A, Abbas A, et al. Green synthesis of iron oxide nanoparticles using pomegranate seeds extract and photocatalytic activity evaluation for the degradation of textile dye. J Mater Res Technol. 2019;8:6115–24.10.1016/j.jmrt.2019.10.006Suche in Google Scholar
[312] Bhuiyan MSH, Miah MY, Paul SC, Aka TD, Saha O, Rahaman MM, et al. Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon. 2020;6:e04603.10.1016/j.heliyon.2020.e04603Suche in Google Scholar PubMed PubMed Central
[313] Karpagavinayagam P, Vedhi C. Green synthesis of iron oxide nanoparticles using Avicennia marina flower extract. Vacuum. 2019;160:286–92.10.1016/j.vacuum.2018.11.043Suche in Google Scholar
[314] Rizvi M, Bhatia T, Gupta R. Green & sustainable synthetic route of obtaining iron oxide nanoparticles using Hylocereus undatus (pitaya or dragon fruit). Mater Today Proc. 2022;50:1100–6.10.1016/j.matpr.2021.07.469Suche in Google Scholar
[315] Srinivasan M, Venkatesan M, Arumugam V, Natesan G, Saravanan N, Murugesan S, et al. Green synthesis and characterization of titanium dioxide nanoparticles (TiO2 NPs) using Sesbania grandiflora and evaluation of toxicity in zebrafish embryos. Process Biochem. 2019;80:197–202.10.1016/j.procbio.2019.02.010Suche in Google Scholar
[316] Rao TN, Riyazuddin, Babji P, Ahmad N, Khan RA, Hassan I, et al. Green synthesis and structural classification of Acacia nilotica mediated-silver doped titanium oxide (Ag/TiO2) spherical nanoparticles: Assessment of its antimicrobial and anticancer activity. Saudi J Biol Sci. 2019;26:1385–91.10.1016/j.sjbs.2019.09.005Suche in Google Scholar PubMed PubMed Central
[317] Alavi M, Karimi N. Characterization, antibacterial, total antioxidant, scavenging, reducing power and ion chelating activities of green synthesized silver, copper and titanium dioxide nanoparticles using Artemisia haussknechtii leaf extract. Artificial Cells. Nanomedi Biotechnol. 2018;46:2066–81.10.1080/21691401.2017.1408121Suche in Google Scholar PubMed
[318] Saranya KS, Vellora Thekkae Padil V, Senan C, Pilankatta R, Saranya K, George B, et al. Green synthesis of high temperature stable anatase titanium dioxide nanoparticles using gum kondagogu: characterization and solar driven photocatalytic degradation of organic dye. Nanomaterials. 2018;8:1002.10.3390/nano8121002Suche in Google Scholar PubMed PubMed Central
[319] Ahmad W, Jaiswal KK, Soni S. Green synthesis of titanium dioxide (TiO2) nanoparticles by using Mentha arvensis leaves extract and its antimicrobial properties. Inorg Nano-Metal Chem. 2020;50:1032–8.10.1080/24701556.2020.1732419Suche in Google Scholar
[320] Modi S, Fulekar MH. Synthesis and characterization of zinc oxide nanoparticles and zinc oxide/cellulose nanocrystals nanocomposite for photocatalytic degradation of Methylene blue dye under solar light irradiation. Nanotechnol Environ Eng. 2020;5:18.10.1007/s41204-020-00080-2Suche in Google Scholar
[321] Sebuso DP, Kuvarega AT, Lefatshe K, King’ondu CK, Numan N, Maaza M, et al. Corn husk multilayered graphene/ZnO nanocomposite materials with enhanced photocatalytic activity for organic dyes and doxycycline degradation. Mater Res Bull. 2022;151:111800.10.1016/j.materresbull.2022.111800Suche in Google Scholar
[322] Basumatari M, Devi RR, Gupta MK, Gupta SK, Raul PK, Chatterjee S, et al. Musa balbisiana Colla pseudostem biowaste mediated zinc oxide nanoparticles: Their antibiofilm and antibacterial potentiality. Curr Res Green SustaChem. 2021;4:100048.10.1016/j.crgsc.2020.100048Suche in Google Scholar
[323] Menazea AA, Ismail AM, Samy A. Novel green synthesis of zinc oxide nanoparticles using orange waste and its thermal and antibacterial activity. J Inorg Organomet Polym Mater. 2021;31:4250–9.10.1007/s10904-021-02074-2Suche in Google Scholar
[324] Mirgane NA, Shivankar VS, Kotwal SB, Wadhawa GC, Sonawale MC. Waste pericarp of ananas comosus in green synthesis zinc oxide nanoparticles and their application in waste water treatment. Mater Today Proc. 2021;37:886–9.10.1016/j.matpr.2020.06.045Suche in Google Scholar
[325] Barman K, Dutta P, Chowdhury D, Baruah PK. Green biosynthesis of copper oxide nanoparticles using waste colocasia esculenta leaves extract and their application as recyclable catalyst towards the synthesis of 1,2,3-triazoles. BioNanoScience. 2021;11:189–99.10.1007/s12668-021-00826-5Suche in Google Scholar
[326] Panda R, Pant KK, Bhaskar T, Naik SN. Dissolution of brominated epoxy resin for environment friendly recovery of copper as cupric oxide nanoparticles from waste printed circuit boards using ammonium chloride roasting. J Clean Prod. 2021;291:125928.10.1016/j.jclepro.2021.125928Suche in Google Scholar
[327] Yatish KV, Prakash RM, Ningaraju C, Sakar M, GeethaBalakrishna R, Lalithamba HS. Terminalia chebula as a novel green source for the synthesis of copper oxide nanoparticles and as feedstock for biodiesel production and its application on diesel engine. Energy. 2021;215:119165.10.1016/j.energy.2020.119165Suche in Google Scholar
[328] Mahmoud AED, Al-Qahtani KM, Alflaij SO, Al-Qahtani SF, Alsamhan FA. Green copper oxide nanoparticles for lead, nickel, and cadmium removal from contaminated water. Sci Rep. 2021;11:12547.10.1038/s41598-021-91093-7Suche in Google Scholar PubMed PubMed Central
[329] Rajkumar S, Elanthamilan E, Wang S-F, Chryso H, Balan PVD, Merlin JP. One-pot green recovery of copper oxide nanoparticles from discarded printed circuit boards for electrode material in supercapacitor application. Resources Conserv Recycling. 2022;180:106180.10.1016/j.resconrec.2022.106180Suche in Google Scholar
[330] Pati P, McGinnis S, Vikesland PJ. Waste not want not: Life cycle implications of gold recovery and recycling from nanowaste. Environ Science Nano. 2016;3:1133–43.10.1039/C6EN00181ESuche in Google Scholar
[331] a Waste W. An Updated look into the future of solid waste management. World Bank; 2018.Suche in Google Scholar
[332] Lee KX, Shameli K, Yew YP, Teow SY, Jahangirian H, Rafiee-Moghaddam R, et al. Recent developments in the facile bio-synthesis of gold nanoparticles (AuNPs) and their biomedical applications. Int J Nanomed. 2020;15:275–300.10.2147/IJN.S233789Suche in Google Scholar PubMed PubMed Central
[333] Khan AK, Rashid R, Murtaza G, Zahra A. Gold nanoparticles: Synthesis and applications in drug delivery. Tropical J Pharm Res. 2014;13:1169–77.10.4314/tjpr.v13i7.23Suche in Google Scholar
[334] Bisceglie F, Civati D, Bonati B, Faraci FD. Reduction of potassium cyanide usage in a consolidated industrial process for gold recovery from wastes and scraps. J Clean Prod. 2017;142:1810–8.10.1016/j.jclepro.2016.11.103Suche in Google Scholar
[335] Masilela M, Ndlovu S. Extraction of Ag and Au from chloride electronic waste leach solutions using ionic liquids. J Environ Chem Eng. 2019;7:10280.10.1016/j.jece.2018.11.054Suche in Google Scholar
[336] Sun Z, Xiao Y, Agterhuis H, Sietsma J, Yang Y. Recycling of metals from urban mines - A strategic evaluation. J Clean Prod. 2016;112:2977–87.10.1016/j.jclepro.2015.10.116Suche in Google Scholar
[337] Gu F, Summers PA, Hall P. Recovering materials from waste mobile phones: Recent technological developments. 2019;237:117657.10.1016/j.jclepro.2019.117657Suche in Google Scholar
[338] Rao MD, Morrison CA. Challenges and opportunities in the recovery of gold from electronic waste. RSC Adv. 2020;10:4300–9. 10.1039/c9ra07607g;4300-4309. Suche in Google Scholar
[339] Dhanunjaya M, Singh KK, Morrison CA, Love JB. Recycling copper and gold from e-waste by a two-stage leaching and solvent extraction process. Sep Purif Technol. 2021;263:118400.10.1016/j.seppur.2021.118400Suche in Google Scholar
[340] Yi Q, Fan R, Xie F, Min H, Zhang Q, Luo Z. Selective recovery of Au(iii) and Pd(II) from waste PCBs using ethylenediamine modified persimmon tannin adsorbent. Procedia Environ Sci. 2016;31:185–94.10.1016/j.proenv.2016.02.025Suche in Google Scholar
[341] Choudhary BC, Paul D, Borse AU, Garole DJ. Surface functionalized biomass for adsorption and recovery of gold from electronic scrap and refinery wastewater. Sep Purif Technol. 2018;195:260–70.10.1016/j.seppur.2017.12.024Suche in Google Scholar
[342] Anand K, Gengan R, Phulukdaree A, Chuturgoon A. Agroforestry waste Moringa oleifera petals mediated green synthesis of gold nanoparticles and their anti-cancer and catalytic activity. J Ind Eng Chem. 2015;21:1105–11.10.1016/j.jiec.2014.05.021Suche in Google Scholar
[343] Oestreicher V, García CS, Soler Illia GJ, Angelome PC. Gold recycling at laboratory scale: from nanowaste to nanospheres. ChemSusChem. 2019;8(21):4882–8.10.1002/cssc.201901488Suche in Google Scholar PubMed
[344] Kanchi S, Kumar G, Lo A-Y, Tseng C-M, Chen S-K, Lin C-Y, et al. Exploitation of de-oiled Jatropha waste for gold nanoparticles synthesis: a green approach. Arab J Chem. 2018;11:247–55.10.1016/j.arabjc.2014.08.006Suche in Google Scholar
[345] Biswas FB, Rahman IMM, Nakakubo K, Endo M, Nagai K, Mashio AS, et al. Highly selective and straightforward recovery of gold and platinum from acidic waste effluents using cellulose-based bio-adsorbent. J Hazard Mater. 2020;410:124569. 10.1016/j.jhazmat.2020.124569;124569-124569. Suche in Google Scholar
[346] Dang H, Fawcett D, Poinern GEJ. Green synthesis of gold nanoparticles from waste macadamia nut shells and their antimicrobial activity against Escherichia coli and Staphylococcus epidermis. Int J Res Med Sci. 2019;7:1171.10.18203/2320-6012.ijrms20191320Suche in Google Scholar
[347] Gan PP, Ng SH, Huang Y, Li SFY. Green synthesis of gold nanoparticles using palm oil mill effluent (POME): A low-cost and eco-friendly viable approach. Bioresour Technol. 2012;113:132–5.10.1016/j.biortech.2012.01.015Suche in Google Scholar
[348] Deokar GK, Ingale AG. Green synthesis of gold nanoparticles (Elixir of Life) from banana fruit waste extract – an efficient multifunctional agent. RSC Adv. 2016;6:74620–9. 10.1039/c6ra14567a;74620-74629. Suche in Google Scholar
[349] Zheng B, Qian L, Yuan H, Xiao D, Yang X, Paau MC, et al. Preparation of gold nanoparticles on eggshell membrane and their biosensing application. Talanta. 2010;82:177–83.10.1016/j.talanta.2010.04.014Suche in Google Scholar
[350] Mythili R, Selvankumar T, Srinivasan P, Sengottaiyan A, Sabastinraj J, Ameen F, et al. Biogenic synthesis, characterization and antibacterial activity of gold nanoparticles synthesised from vegetable waste. J Mol Liq. 2018;262:318–21.10.1016/j.molliq.2018.04.087Suche in Google Scholar
[351] Usman AI, Aziz AA, Noqta OA. Green sonochemical synthesis of gold nanoparticles using palm oil leaves extracts. Mater Today Proc. 2019;7:803–7.10.1016/j.matpr.2018.12.078Suche in Google Scholar
[352] Krishnaswamy K, Vali H, Orsat V. Value-adding to grape waste: Green synthesis of gold nanoparticles. J Food Eng. 2014;142:210–20.10.1016/j.jfoodeng.2014.06.014Suche in Google Scholar
[353] Zhou W, Liang H, Xu H. Recovery of gold from waste mobile phone circuit boards and synthesis of nanomaterials using emulsion liquid membrane. J Hazard Mater. 2021;411:125011.10.1016/j.jhazmat.2020.125011Suche in Google Scholar PubMed
[354] Mourato A, Gadanho M, Lino AR, Tenreiro R. Biosynthesis of crystalline silver and gold nanoparticles by extremophilic yeasts. Bioinorganic Chem Appl. 2011;2011:546074.10.1155/2011/546074Suche in Google Scholar PubMed PubMed Central
[355] Niu X, Huang Y, Zhang W, Yan L, Wang L, Li Z, et al. Synthesis of gold nanoflakes decorated biomass-derived porous carbon and its application in electrochemical sensing of luteolin. J Electroanal Chem. 2021;880:114832.10.1016/j.jelechem.2020.114832Suche in Google Scholar
[356] Harby AG, El-Borady OM, El-Kemary M. The exploitation of rice husk biomass for the bio-inspired synthesis of gold nanoparticles as a multifunctional material for various biological and photocatalytic applications. Bioprocess Biosyst Eng. 2022;45:61–74.10.1007/s00449-021-02639-ySuche in Google Scholar PubMed
[357] Abdoli M, Arkan E, Shekarbeygi Z, Khaledian S. Green synthesis of gold nanoparticles using Centaurea behen leaf aqueous extract and investigating their antioxidant and cytotoxic effects on acute leukemia cancer cell line (THP-1). Inorg Chem Commun. 2021;129:108649.10.1016/j.inoche.2021.108649Suche in Google Scholar
[358] Philip D. Rapid green synthesis of spherical gold nanoparticles using Mangifera indica leaf. Spectrochim Acta Part A Mol Biomolecular Spectrosc. 2010;77:807–10.10.1016/j.saa.2010.08.008Suche in Google Scholar PubMed
[359] Akintelu SA, Yao B, Folorunso AS. Green synthesis, characterization, and antibacterial investigation of synthesized gold nanoparticles (AuNPs) from Garcinia kola pulp extract. Plasmonics. 2021;16:157–65.10.1007/s11468-020-01274-9Suche in Google Scholar
[360] Yin Y, Xiang C, Chu H, Zhang H, Fen X, Yan E, et al. Facile synthesis of honeycomb-structured Co–W–B composite for high-performance supercapacitors. Appl Surf Sci. 2018;460:25–32.10.1016/j.apsusc.2017.12.013Suche in Google Scholar
[361] Marina PE, Ali GAM, See LM, Teo EYL, Ng E-P, Chong KF. In situ growth of redox-active iron-centered nanoparticles on graphene sheets for specific capacitance enhancement. Arab J Chem. 2016;12:3883–9.10.1016/j.arabjc.2016.02.006Suche in Google Scholar
[362] Thalji MR, Ali GAM, Liu P, Zhong YL, Chong KF. W18O49 nanowires-graphene nanocomposite for asymmetric supercapacitors employing AlCl3 aqueous electrolyte. Chem Eng J. 2021;409:128216.10.1016/j.cej.2020.128216Suche in Google Scholar
[363] Ali GAM, Fouad OA, Makhlouf SA, Yusoff MM, Chong KF. Co3O4/SiO2 nanocomposites for supercapacitor application. J Solid State Electrochem. 2014;18:2505–12.10.1007/s10008-014-2510-3Suche in Google Scholar
[364] Fu M, Chen W, Zhu X, Yang B, Liu Q. Crab shell derived multi-hierarchical carbon materials as a typical recycling of waste for high performance supercapacitors. Carbon. 2019;141:748–57.10.1016/j.carbon.2018.10.034Suche in Google Scholar
[365] Dixini P, Carvalho B, Gonçalves G, Pegoretti V, Freitas M. Sol–gel synthesis of manganese oxide supercapacitor from manganese recycled from spent Zn–MnO2 batteries using organic acid as a leaching agent. Ionics. 2019;25:4381–92.10.1007/s11581-019-02995-6Suche in Google Scholar
[366] Mao Y, Shen X, Wu Z, Zhu L, Liao G. Preparation of Co3O4 hollow microspheres by recycling spent lithium-ion batteries and their application in electrochemical supercapacitors. J Alloy Compd. 2020;816:152604.10.1016/j.jallcom.2019.152604Suche in Google Scholar
[367] Schauerman CM, Ganter MJ, Gaustad G, Babbitt CW, Raffaelle RP, Landi BJ. Recycling single-wall carbon nanotube anodes from lithium ion batteries. J Mater Chem. 2012;22:12008–15.10.1039/c2jm31971cSuche in Google Scholar
[368] Ahmed TM, Rahman MM, Asmatulu E. Recycling of graphite waste into high quality graphene products. Mater Energy Efficiency Sustain TechConnect Briefs. 2018;2:207–9.Suche in Google Scholar
[369] Jiang G, Pickering SJ. Recycling graphene from supercapacitor electrodes as reinforcing filler for epoxy resins. Waste Biomass Valoriz. 2019;10:215–21.10.1007/s12649-017-0039-2Suche in Google Scholar
[370] Zhang Y, Gao Z, Song N, Li X. High-performance supercapacitors and batteries derived from activated banana-peel with porous structures. Electrochim Acta. 2016;222:1257–66.10.1016/j.electacta.2016.11.099Suche in Google Scholar
[371] Shilpa Kumar R, Sharma A. Morphologically tailored activated carbon derived from waste tires as high-performance anode for Li-ion battery. J Appl Electrochem. 2018;48:1–13.10.1007/s10800-017-1129-3Suche in Google Scholar
[372] Yu K, Li J, Qi H, Liang C. High-capacity activated carbon anode material for lithium-ion batteries prepared from rice husk by a facile method. Diam Relat Mater. 2018;86:139–45.10.1016/j.diamond.2018.04.019Suche in Google Scholar
[373] Barhoum A, Shalan AE, El-Hout SI, Ali GAM, Abdelbasir SM, Abu Serea ES, et al. A Broad family of carbon nanomaterials: classification, properties, synthesis, and emerging applications. In: Barhoum A, Bechelany M, Makhlouf A, editors. Handbook of nanofibers. Cham: Springer International Publishing; 2019. p. 1–40. 10.1007/978-3-319-42789-8_59-1pp. Suche in Google Scholar
[374] Ali GAM, Abdul Manaf SA, Kumar A, Chong KF, Hegde G. High performance supercapacitor using catalysis free porous carbon nanoparticles. J Phys D Appl Phys. 2014;47:495307–13.10.1088/0022-3727/47/49/495307Suche in Google Scholar
[375] Taer E, Agustino A, Awitdrus A, Farma R, Taslim R. The synthesis of carbon nanofiber derived from pineapple leaf fibers as a carbon electrode for supercapacitor application. J Electrochem Energy Convers Storage. 2021;18:031004–12.10.1115/1.4048405Suche in Google Scholar
[376] Deng L, Young RJ, Kinloch IA, Abdelkader AM, Holmes SM, De Haro-Del Rio DA, et al. Supercapacitance from cellulose and carbon nanotube nanocomposite fibers. ACS Appl Mater Interfaces. 2013;5:9983–90.10.1021/am403622vSuche in Google Scholar PubMed PubMed Central
[377] Lee SP, Ali GAM, Hegazy HH, Lim HN, Chong KF. Optimizing reduced graphene oxide aerogel for a supercapacitor. Energy & Fuels. 2021;35:4559–69. 10.1021/acs.energyfuels.0c04126.Suche in Google Scholar
[378] Xu Y, Shi G, Duan X. Self-assembled three-dimensional graphene macrostructures: synthesis and applications in supercapacitors. Acc Chem Res. 2015;48:1666–75.10.1021/acs.accounts.5b00117Suche in Google Scholar PubMed
[379] Karakoti M, Pandey S, Jangra R, Dhapola PS, Singh PK, Mahendia S, et al. Waste plastics derived graphene nanosheets for supercapacitor application. Mater Manuf Process. 2021;36:171–7.10.1080/10426914.2020.1832680Suche in Google Scholar
[380] Yuli Y, Eka S, Yazmendra R. Biomass waste of cocoa skin for basic activated carbon as source of eco-friendly energy storage. Proceedings of Journal of Physics: Conference Series; 2021. p. 012020.10.1088/1742-6596/1788/1/012020Suche in Google Scholar
[381] Rani MU, Nanaji K, Rao TN, Deshpande AS. Corn husk derived activated carbon with enhanced electrochemical performance for high-voltage supercapacitors. J Power Sources. 2020;471:228387.10.1016/j.jpowsour.2020.228387Suche in Google Scholar
[382] Ramayani D, Hamzah Y, Taer E, Yanti N, Apriwandi A. Analysis of activated carbon monolith derived from carrot juice waste for supercapacitor electrode application. J Aceh Phys Soc. 2021;10:26–31.10.24815/jacps.v10i2.18392Suche in Google Scholar
[383] Utetiwabo W, Yang L, Tufail MK, Zhou L, Chen R, Lian Y, et al. Electrode materials derived from plastic wastes and other industrial wastes for supercapacitors. Chin Chem Lett. 2020;31:1474–89.10.1016/j.cclet.2020.01.003Suche in Google Scholar
[384] Mishra N, Shinde S, Vishwakarma R, Kadam S, Sharon M, Sharon M. MWCNTs synthesized from waste polypropylene plastics and its application in super-capacitors. USA: AIP Conference Proceedings; 2013. p. 228–36.10.1063/1.4810063Suche in Google Scholar
[385] Widiatmoko P, Nugraha N, Devianto H, Nurdin I, Prakoso T. Upscaled synthesis of carbon nanotube from palm oil mill effluent using pyrolysis for supercapacitor application. Proceedings of IOP Conference Series: Materials Science and Engineering. 2020. p. 012040.10.1088/1757-899X/823/1/012040Suche in Google Scholar
[386] Zhang H, Niu H, Wang Y, Wang C, Bai X, Wang S, et al. A simple method to prepare carbon nanotubes from sunflower seed hulls and sago and their application in supercapacitor. Pigment Resin Technol. 2015;44:7–12.10.1108/PRT-10-2013-0090Suche in Google Scholar
[387] Athika M, Prasath A, Duraisamy E, Devi VS, Sharma AS, Elumalai P. Carbon-quantum dots derived from denatured milk for efficient chromium-ion sensing and supercapacitor applications. Mater Lett. 2019;241:156–9.10.1016/j.matlet.2019.01.064Suche in Google Scholar
[388] Praneerad J, Neungnoraj K, In I, Paoprasert P. Environmentally friendly supercapacitor based on carbon dots from durian peel as an electrode. Key Eng Mater. 2019;803:115–9.10.4028/www.scientific.net/KEM.803.115Suche in Google Scholar
[389] Ali GAM, Yusoff MM, Algarni H, Chong KF. One-step electrosynthesis of MnO2/rGO nanocomposite and its enhanced electrochemical performance. Ceram Int. 2018;44:7799–807.10.1016/j.ceramint.2018.01.212Suche in Google Scholar
[390] Aboelazm EAA, Ali GAM, Chong KF. Cobalt oxide supercapacitor electrode recovered from spent lithium-ion battery. Chem Adv Mater. 2018:3;67–74.Suche in Google Scholar
[391] Aboelazm EAA, Ali GAM, Algarni H, Yin H, Zhong YL, Chong KF. Magnetic electrodeposition of the hierarchical cobalt oxide nanostructure from spent lithium-ion batteries: its application as a supercapacitor electrode. J Phys Chem C. 2018;122:12200–6.10.1021/acs.jpcc.8b03306Suche in Google Scholar
[392] Bhattacharya G, Fishlock SJ, Roy JS, Pritam A, Banerjee D, Deshmukh S, et al. Effective utilization of waste red mud for high performance supercapacitor electrodes. Glob Chall. 2019;3:1800066.10.1002/gch2.201800066Suche in Google Scholar PubMed PubMed Central
[393] Fu C, Mahadevegowda A, Grant PS. Production of hollow and porous Fe2O3 from industrial mill scale and its potential for large-scale electrochemical energy storage applications. J Mater Chem A. 2016;4:2597–604.10.1039/C5TA09141ASuche in Google Scholar
[394] Hassan K, Farzana R, Sahajwalla V. In-situ fabrication of ZnO thin film electrode using spent Zn–C battery and its electrochemical performance for supercapacitance. SN Appl Sci. 2019;1:302.10.1007/s42452-019-0302-1Suche in Google Scholar
[395] Zeng Y, Yu M, Meng Y, Fang P, Lu X, Tong Y. Iron‐based supercapacitor electrodes: advances and challenges. Advanced Energy. Materials. 2016;6:1601053.10.1002/aenm.201601053Suche in Google Scholar
[396] Gund GS, Dubal DP, Chodankar NR, Cho JY, Gomez-Romero P, Park C, et al. Low-cost flexible supercapacitors with high-energy density based on nanostructured MnO2 and Fe2O3 thin films directly fabricated onto stainless steel. Sci Rep. 2015;5:1–13.10.1038/srep12454Suche in Google Scholar PubMed PubMed Central
[397] Xu X, Cao C, Zhu Y. Facile synthesis of single crystalline mesoporous hematite nanorods with enhanced supercapacitive performance. Electrochim Acta. 2015;155:257–62.10.1016/j.electacta.2014.12.105Suche in Google Scholar
[398] Xie K, Li J, Lai Y, Lu W, Liu Y, Zhou L, et al. Highly ordered iron oxide nanotube arrays as electrodes for electrochemical energy storage. Electrochem Commun. 2011;13:657–60.10.1016/j.elecom.2011.03.040Suche in Google Scholar
[399] Fu C, Grant PS. Toward low-cost grid scale energy storage: supercapacitors based on up-cycled industrial mill scale waste. ACS Sustain Chem & Eng. 2015;3:2831–8.10.1021/acssuschemeng.5b00757Suche in Google Scholar
[400] Miller JR, Burke A. Electrochemical capacitors: challenges and opportunities for real-world applications. Electrochem Soc Interface. 2008;17:53.10.1149/2.F08081IFSuche in Google Scholar
[401] Zhang L, Guo H, Xue R, Yue L, Li Q, Liu H, et al. In-situ facile synthesis of flower shaped NiS2@regenerative graphene oxide derived from waste dry battery nano-composites for high-performance supercapacitors. J Energy Storage. 2020;31:101630.10.1016/j.est.2020.101630Suche in Google Scholar
[402] Habeeb OA, Ramesh K, Ali GAM, Yunus RM. Experimental design technique on removal of hydrogen sulfide using CaO-eggshells dispersed onto palm kernel shell activated carbon: Experiment, optimization, equilibrium and kinetic studies. J Wuhan Univ Technology-Mater Sci Ed. 2017;32:305–20.10.1007/s11595-017-1597-7Suche in Google Scholar
[403] Senthilkumar ST, Fu N, Liu Y, Wang Y, Zhou L, Huang H. Flexible fiber hybrid supercapacitor with NiCo2O4 nanograss@carbon fiber and bio-waste derived high surface area porous carbon. Electrochim Acta. 2016;211:411–9.10.1016/j.electacta.2016.06.059Suche in Google Scholar
[404] Edison TNJI, Atchudan R, Karthik N, Xiong D, Lee YR. Direct electro-synthesis of MnO2 nanoparticles over nickel foam from spent alkaline battery cathode and its supercapacitor performance. J Taiwan Inst Chem Eng. 2019;97:414–23.10.1016/j.jtice.2019.01.019Suche in Google Scholar
[405] Wahid M, Puthusseri D, Phase D, Ogale S. Enhanced capacitance retention in a supercapacitor made of carbon from sugarcane bagasse by hydrothermal pretreatment. Energy & Fuels. 2014;28:4233–40.10.1021/ef500342dSuche in Google Scholar
[406] Qu W-H, Xu Y-Y, Lu A-H, Zhang X-Q, Li W-C. Converting biowaste corncob residue into high value added porous carbon for supercapacitor electrodes. Bioresour Technol. 2015;189:285–91.10.1016/j.biortech.2015.04.005Suche in Google Scholar PubMed
[407] Chen X, Wu K, Gao B, Xiao Q, Kong J, Xiong Q, et al. Three-dimensional activated carbon recycled from rotten potatoes for high-performance supercapacitors. Waste Biomass Valoriz. 2016;7:551–7.10.1007/s12649-015-9458-0Suche in Google Scholar
[408] Su X-L, Chen J-R, Zheng G-P, Yang J-H, Guan X-X, Liu P, et al. Three-dimensional porous activated carbon derived from loofah sponge biomass for supercapacitor applications. Appl Surf Sci. 2018;436:327–36.10.1016/j.apsusc.2017.11.249Suche in Google Scholar
[409] Iro ZS, Subramani C, Rajendran J, Sundramoorthy AK. Promising nature-based activated carbon derived from flowers of Borassus flabellifer for supercapacitor applications. Carbon Lett. 2021;31:1145–53.10.1007/s42823-021-00237-2Suche in Google Scholar
[410] El-Hout SI, Attia SY, Mohamed SG, Abdelbasir SM. From waste to value-added products: Evaluation of activated carbon generated from leather waste for supercapacitor applications. J Environ Manag. 2022;304:114222.10.1016/j.jenvman.2021.114222Suche in Google Scholar PubMed
[411] Deng L, Zhong W, Wang J, Zhang P, Fang H, Yao L, et al. The enhancement of electrochemical capacitance of biomass-carbon by pyrolysis of extracted nanofibers. Electrochim Acta. 2017;228:398–406.10.1016/j.electacta.2017.01.099Suche in Google Scholar
[412] Tan Z, Yu F, Liu L, Jia X, Lv Y, Chen L, et al. Cu-doped porous carbon derived from heavy metal-contaminated sewage sludge for high-performance supercapacitor electrode materials. Nanomaterials. 2019;9:892.10.3390/nano9060892Suche in Google Scholar PubMed PubMed Central
[413] Singh R, Singh H, Farina I, Colangelo F, Fraternali F. On the additive manufacturing of an energy storage device from recycled material. Compos Part B Eng. 2019;156:259–65.10.1016/j.compositesb.2018.08.080Suche in Google Scholar
[414] Ma Q, Yu Y, Sindoro M, Fane AG, Wang R, Zhang H. Carbon-based functional materials derived from waste for water remediation and energy storage. Adv Mater. 2017;29:1605361.10.1002/adma.201605361Suche in Google Scholar PubMed
[415] Yao F, Pham DT, Lee YH. Carbon-based materials for lithium-ion batteries, electrochemical capacitors, and their hybrid devices. ChemSusChem. 2015;8:2284–311.10.1002/cssc.201403490Suche in Google Scholar PubMed
[416] Yao F, Cojocaru CS. Carbon-based nanomaterials as an anode for lithium ion battery. Éc Polytech X. Paris: Institute of Technology, 2013 (thesis) https://pastel.archives-ouvertes.fr/pastel-00967913.Suche in Google Scholar
[417] Tao L, Huang Y, Yang X, Zheng Y, Liu C, Di M, et al. Flexible anode materials for lithium-ion batteries derived from waste biomass-based carbon nanofibers: I. Effect of carbonization temperature. RSC Adv. 2018;8:7102–9.10.1039/C7RA13639KSuche in Google Scholar
[418] Kucinskis G, Bajars G, Kleperis J. Graphene in lithium ion battery cathode materials: A review. J Power Sources. 2013;240:66–79.10.1016/j.jpowsour.2013.03.160Suche in Google Scholar
[419] Chen F, Yang J, Bai T, Long B, Zhou X. Facile synthesis of few-layer graphene from biomass waste and its application in lithium ion batteries. J Electroanal Chem. 2016;768:18–26.10.1016/j.jelechem.2016.02.035Suche in Google Scholar
[420] Yoo E, Kim J, Hosono E, Zhou H-S, Kudo T, Honma I. Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano Lett. 2008;8:2277–82.10.1021/nl800957bSuche in Google Scholar PubMed
[421] Li Y, Li C, Qi H, Yu K, Liang C. Mesoporous activated carbon from corn stalk core for lithium ion batteries. Chem Phys. 2018;506:10–6.10.1016/j.chemphys.2018.03.027Suche in Google Scholar
[422] Peng X, Fu J, Zhang C, Tao J, Sun L, Chu PK. Rice husk-derived activated carbon for Li-ion battery anode. Nanosci Nanotechnol Lett. 2014;6:68–71.10.1166/nnl.2014.1714Suche in Google Scholar
[423] Gao Z, Song N, Zhang Y, Schwab Y, He J, Li X. Carbon nanotubes derived from yeast-fermented wheat flour and their energy storage application. ACS Sustain Chem & Eng. 2018;6:11386–96.10.1021/acssuschemeng.8b01292Suche in Google Scholar
[424] Ceder G, Chiang YM, Sadoway DR, Aydinol MK, Jang YI, Huang B. Identification of cathode materials for lithium batteries guided by first-principles calculations. Nature. 1998;392:694–6.10.1038/33647Suche in Google Scholar
[425] Poizot P, Laruelle S, Grugeon S, Dupont L, Tarascon JM. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature. 2000;407:496–9.10.1038/35035045Suche in Google Scholar PubMed
[426] Xia H, Lai M, Lu L. Nanoflaky MnO2/carbon nanotube nanocomposites as anode materials for lithium-ion batteries. J Mater Chem. 2010;20:6896–902.10.1039/c0jm00759eSuche in Google Scholar
[427] Wang M, Yang Q, Zhang T, Zhu B, Li G. Facile synthesis of β-MnO2/polypyrrole nanorods and their enhanced lithium-storage properties. RSC Adv. 2016;6:19952–56.10.1039/C5RA26067ASuche in Google Scholar
[428] Xia H, Wang Y, Lin J, Lu L. Hydrothermal synthesis of MnO2/CNT nanocomposite with a CNT core/porous MnO2 sheath hierarchy architecture for supercapacitors. Nanoscale Res Lett. 2012;7:33.10.1186/1556-276X-7-33Suche in Google Scholar PubMed PubMed Central
[429] Zhang W, Zhang B, Jin H, Li P, Zhang Y, Ma S, et al. Waste eggshell as bio-template to synthesize high capacity δ-MnO2 nanoplatelets anode for lithium ion battery. Ceram Int. 2018;44:20441–8.10.1016/j.ceramint.2018.08.038Suche in Google Scholar
[430] Cao X, Guo G, Liu F, Zhou Y, Zhang S. The properties of LiMn2O4 synthesized by molten salt method using MnO2 as manganese source recycled from spent Zn-Mn batteries. Int J Electrochem Sci. 2014;10:3841–7.10.1016/S1452-3981(23)06584-7Suche in Google Scholar
[431] Hu C, Guo J, Wen J, Peng Y. Preparation and electrochemical performance of nano-Co3O4 anode materials from spent Li-ion batteries for lithium-ion batteries. J Mater Sci & Technol. 2013;29:215–20.10.1016/j.jmst.2013.01.009Suche in Google Scholar
[432] Farzana R, Rajarao R, Behera PR, Hassan K, Sahajwalla V. Zinc oxide nanoparticles from waste Zn-C battery via thermal route: characterization and properties. Nanomaterials. 2018;8:717.10.3390/nano8090717Suche in Google Scholar PubMed PubMed Central
[433] Li H, Wei Y, Zhang Y, Yin F, Zhang C, Wang G, et al. Synthesis and electrochemical investigation of highly dispersed ZnO nanoparticles as anode material for lithium-ion batteries. Ionics. 2016;22:1387–93.10.1007/s11581-016-1661-xSuche in Google Scholar
[434] Yao J, Yang Y, Li Y, Jiang J, Xiao S, Yang J. Interconnected α-Fe2O3 nanoparticles prepared from leaching liquor of tin ore tailings as anode materials for lithium-ion batteries. J Alloy Compd. 2021;855:157288.10.1016/j.jallcom.2020.157288Suche in Google Scholar
[435] Wu N, Zhang X, Ma C, Shi Y-R, Zhou J-M, Wang Z, et al. High performance isomeric Fe2O3 nanospheres anode materials derived from industrial wastewater for lithium ion batteries. Electrochim Acta. 2019;297:1028–34.10.1016/j.electacta.2018.12.059Suche in Google Scholar
[436] Kashale AA, Gattu KP, Ghule K, Ingole VH, Dhanayat S, Sharma R, et al. Biomediated green synthesis of TiO2 nanoparticles for lithium ion battery application. Compos Part B Eng. 2016;99:297–304.10.1016/j.compositesb.2016.06.015Suche in Google Scholar
[437] Babu DB, Ramesha K. Melamine assisted liquid exfoliation approach for the synthesis of nitrogen doped graphene-like carbon nano sheets from bio-waste bagasse material and its application towards high areal density Li-S batteries. Carbon. 2019;144:582–90.10.1016/j.carbon.2018.12.101Suche in Google Scholar
[438] Xu H, Liu Y, Bai Q, Wu R. Discarded cigarette filter-derived hierarchically porous carbon@graphene composites for lithium–sulfur batteries. J Mater Chem A. 2019;7:3558–62.10.1039/C8TA11615FSuche in Google Scholar
[439] Zhang X, Yuan W, Yang Y, Yang S, Wang C, Yuan Y, et al. Green and facile fabrication of porous titanium dioxide as efficient sulfur host for advanced lithium-sulfur batteries: An air oxidation strategy. J Colloid Interface Sci. 2021;583:157–65.10.1016/j.jcis.2020.09.020Suche in Google Scholar
[440] Du R, Tong Z, Wei C, Qin A. Preparation of activated carbons from sisal fibers as anode materials for lithium ion batteries. Int J Electrochem Sci. 2016;11:8418–29.10.20964/2016.10.19Suche in Google Scholar
[441] Song W, Liu J, You L, Wang S, Zhou Q, Gao Y, et al. Re-synthesis of nano-structured LiFePO4/graphene composite derived from spent lithium-ion battery for booming electric vehicle application. J Power Sources. 2019;419:192–202.10.1016/j.jpowsour.2019.02.065Suche in Google Scholar
[442] Anson CW, Stahl SS. Mediated fuel cells: soluble redox mediators and their applications to electrochemical reduction of O2 and oxidation of H2, alcohols, biomass, and complex fuels. Chem Rev. 2020;120:3749–86.10.1021/acs.chemrev.9b00717Suche in Google Scholar
[443] Andújar JM, Segura F. Fuel cells: History and updating. A walk along two centuries. Renew Sustain energy Rev. 2009;13:2309–22.10.1016/j.rser.2009.03.015Suche in Google Scholar
[444] Winter M, Brodd RJ. What are batteries, fuel cells, and supercapacitors? Chem Rev. 2004;104:4245–70.10.1021/cr020730kSuche in Google Scholar
[445] Appleby AJ. Fuel cell handbook; Texas, USA 1988.Suche in Google Scholar
[446] Carrette L, Friedrich KA, Stimming U. Fuel cells: principles, types, fuels, and applications. ChemPhysChem. 2000;1:162–93.10.1002/1439-7641(20001215)1:4<162::AID-CPHC162>3.0.CO;2-ZSuche in Google Scholar
[447] Zhou L, Fu P, Cai X, Zhou S, Yuan Y. Naturally derived carbon nanofibers as sustainable electrocatalysts for microbial energy harvesting: A new application of spider silk. Appl Catal B Environ. 2016;188:31–8.10.1016/j.apcatb.2016.01.063Suche in Google Scholar
[448] Zhou H, Zhang J, Zhu J, Liu Z, Zhang C, Mu S. A self-template and KOH activation co-coupling strategy to synthesize ultrahigh surface area nitrogen-doped porous graphene for oxygen reduction. RSC Adv. 2016;6:73292–300.10.1039/C6RA16703ASuche in Google Scholar
[449] Zhao H, Zhao T. Graphene sheets fabricated from disposable paper cups as a catalyst support material for fuel cells. J Mater Chem A. 2013;1:183–7.10.1039/C2TA00018KSuche in Google Scholar
[450] Ma Z, Dou S, Shen A, Tao L, Dai L, Wang S. Sulfur‐doped graphene derived from cycled lithium–sulfur batteries as a metal‐free electrocatalyst for the oxygen reduction reaction. Angew Chem Int Ed. 2015;54:1888–92.10.1002/anie.201410258Suche in Google Scholar PubMed
[451] Pan F, Zhao Q, Wang J, Zhang J. High-performance Fe–N-doped graphene electrocatalysts with ph-dependent active sites for the oxygen reduction reaction. ChemElectroChem. 2015;2:2032–40.10.1002/celc.201500301Suche in Google Scholar
[452] Zhou H, Zhang J, Amiinu IS, Zhang C, Liu X, Tu W, et al. Transforming waste biomass with an intrinsically porous network structure into porous nitrogen-doped graphene for highly efficient oxygen reduction. Phys Chem Chem Phys. 2016;18:10392–9.10.1039/C6CP00174BSuche in Google Scholar PubMed
[453] Sridhar V, Park H. Transforming waste poly(ethylene terephthalate) into nitrogen doped carbon nanotubes and its utility in oxygen reduction reaction and bisphenol-a removal from contaminated water. Mater (Basel). 2020;13:4144.10.3390/ma13184144Suche in Google Scholar PubMed PubMed Central
[454] Cai N, Xia S, Zhang X, Meng Z, Bartocci P, Fantozzi F, et al. Preparation of iron- and nitrogen-codoped carbon nanotubes from waste plastics pyrolysis for the oxygen reduction reaction. ChemSusChem. 2020;13:938–44.10.1002/cssc.201903293Suche in Google Scholar PubMed
[455] Veksha A, Yin K, Moo JGS, Oh WD, Ahamed A, Chen WQ, et al. Processing of flexible plastic packaging waste into pyrolysis oil and multi-walled carbon nanotubes for electrocatalytic oxygen reduction. J Hazard Mater. 2020;387:121256.10.1016/j.jhazmat.2019.121256Suche in Google Scholar PubMed
[456] Chaudhari NK, Song MY, Yu J-S. Heteroatom-doped highly porous carbon from human urine. Sci Rep. 2014;4:1–10.10.1038/srep05221Suche in Google Scholar PubMed PubMed Central
[457] Zhang H, Chen J, Li Y, Liu P, Wang Y, An T, et al. Nitrogen-doped carbon nanodots@ nanospheres as an efficient electrocatalyst for oxygen reduction reaction. Electrochim Acta. 2015;165:7–13.10.1016/j.electacta.2015.02.240Suche in Google Scholar
[458] Zhang M, Jin X, Wang L, Sun M, Tang Y, Chen Y, et al. Improving biomass-derived carbon by activation with nitrogen and cobalt for supercapacitors and oxygen reduction reaction. Appl Surf Sci. 2017;411:251–60.10.1016/j.apsusc.2017.03.097Suche in Google Scholar
[459] Wang L, Zhang L, Bai L, Han L, Dong S. Nitrogen, cobalt-codoped carbon electrocatalyst for oxygen reduction reaction using soy milk and cobalt salts as precursors. Electrochem Commun. 2013;34:68–72.10.1016/j.elecom.2013.05.019Suche in Google Scholar
[460] Amiinu IS, Zhang J, Kou Z, Liu X, Asare OK, Zhou H, et al. Self-organized 3D porous graphene dual-doped with biomass-sponsored nitrogen and sulfur for oxygen reduction and evolution. ACS Appl Mater Interfaces. 2016;8:29408–18.10.1021/acsami.6b08719Suche in Google Scholar PubMed
[461] Li M, Xiong Y, Liu X, Han C, Zhang Y, Bo X, et al. Iron and nitrogen co-doped carbon nanotube@ hollow carbon fibers derived from plant biomass as efficient catalysts for the oxygen reduction reaction. J Mater Chem A. 2015;3:9658–67.10.1039/C5TA00958HSuche in Google Scholar
[462] Fang Y, Wang H, Yu H, Peng F. From chicken feather to nitrogen and sulfur co-doped large surface bio-carbon flocs: an efficient electrocatalyst for oxygen reduction reaction. Electrochim Acta. 2016;213:273–82.10.1016/j.electacta.2016.07.121Suche in Google Scholar
[463] Chaudhari KN, Song MY, Yu JS. Transforming hair into heteroatom‐doped carbon with high surface area. Small. 2014;10:2625–36.10.1002/smll.201303831Suche in Google Scholar PubMed
[464] Passaponti M, Rosi L, Savastano M, Giurlani W, Miller HA, Lavacchi A, et al. Recycling of waste automobile tires: Transforming char in oxygen reduction reaction catalysts for alkaline fuel cells. J Power Sources. 2019;427:85–90.10.1016/j.jpowsour.2019.04.067Suche in Google Scholar
[465] Mondal D, Sharma M, Wang C-H, Lin Y-C, Huang H-C, Saha A, et al. Deep eutectic solvent promoted one step sustainable conversion of fresh seaweed biomass to functionalized graphene as a potential electrocatalyst. Green Chem. 2016;18:2819–26.10.1039/C5GC03106KSuche in Google Scholar
[466] Elessawy NA, Elnouby M, Gouda M, Mohy Eldin MS, Farag HA, Konsowa AH. Simple self-assembly synthesis for cost-effective alkaline fuel cell bi-functional electrocatalyst synthesized from polyethylene terephthalate waste bottles. J Electron Mater. 2019;49:1009–16.10.1007/s11664-019-07684-8Suche in Google Scholar
[467] Ma M, You S, Gong X, Dai Y, Zou J, Fu H. Silver/iron oxide/graphitic carbon composites as bacteriostatic catalysts for enhancing oxygen reduction in microbial fuel cells. J Power Sources. 2015;283:74–83.10.1016/j.jpowsour.2015.02.100Suche in Google Scholar
[468] Brown AS, Green MA. Impurity photovoltaic effect: Fundamental energy conversion efficiency limits. J Appl Phys. 2002;92:1329–36.10.1063/1.1492016Suche in Google Scholar
[469] Duan C, Cai W, Hsu BB, Zhong C, Zhang K, Liu C, et al. Toward green solvent processable photovoltaic materials for polymer solar cells: the role of highly polar pendant groups in charge carrier transport and photovoltaic behavior. Energy Env Sci. 2013;6:3022–34.10.1039/c3ee41838cSuche in Google Scholar
[470] Bagher AM, Vahid MMA, Mohsen M. Types of solar cells and application. Am J Opt Photonics. 2015;3:94–113.10.11648/j.ajop.20150305.17Suche in Google Scholar
[471] Fraas LM, Partain LD. Solar cells and their applications. Vol. 236, New York, USA: John Wiley & Sons; 2010.10.1002/9780470636886Suche in Google Scholar
[472] Wu D, Zhu C, Shi Y, Jing H, Hu J, Song X, et al. Biomass-derived multilayer-graphene-encapsulated cobalt nanoparticles as efficient electrocatalyst for versatile renewable energy applications. ACS Sustain Chem & Eng. 2018;7:1137–45.10.1021/acssuschemeng.8b04797Suche in Google Scholar
[473] Teymourinia H, Salavati-Niasari M, Amiri O, Farangi M. Facile synthesis of graphene quantum dots from corn powder and their application as down conversion effect in quantum dot-dye-sensitized solar cell. J Mol Liq. 2018;251:267–72.10.1016/j.molliq.2017.12.059Suche in Google Scholar
[474] Sharma JK, Akhtar MS, Ameen S, Srivastava P, Singh G. Green synthesis of CuO nanoparticles with leaf extract of Calotropis gigantea and its dye-sensitized solar cells applications. J Alloy Compd. 2015;632:321–5.10.1016/j.jallcom.2015.01.172Suche in Google Scholar
[475] Ansari MS, Maragani R, Banik A, Misra R, Qureshi M. Enhanced photovoltaic performance using biomass derived nano 3D ZnO hierarchical superstructures and a D−A type CS-Symmetric triphenylamine linked bisthiazole. Electrochim Acta. 2018;259:262–75.10.1016/j.electacta.2017.10.174Suche in Google Scholar
[476] Zhu W, Chai W, Chen D, Xi H, Chen D, Chang J, et al. Recycling of FTO/TiO2 substrates: route toward simultaneously high-performance and cost-efficient carbon-based, all-inorganic CsPbIBr2 solar cells. ACS Appl Mater Interfaces. 2020;12:4549–57.10.1021/acsami.9b21331Suche in Google Scholar PubMed
[477] Ahmed DS, Mohammed MKA, Majeed SM. Green synthesis of eco-friendly graphene quantum dots for highly efficient perovskite solar cells. ACS Appl Energy Mater. 2020;3:10863–71.10.1021/acsaem.0c01896Suche in Google Scholar
[478] Arshad A, Yun S, Si Y, Han F, Zhang Y, Zhang Y, et al. Aloe vera-peel derived porous carbon integrated Co/Mn-oxide based nano-hybrids: An efficient electrocatalyst in advanced photovoltaics. J Power Sources. 2020;451:227731.10.1016/j.jpowsour.2020.227731Suche in Google Scholar
[479] Shashanka R, Esgin H, Yilmaz VM, Caglar Y. Fabrication and characterization of green synthesized ZnO nanoparticle based dye-sensitized solar cells. J Sci Adv Mater Devices. 2020;5:185–91.10.1016/j.jsamd.2020.04.005Suche in Google Scholar
[480] Saravanan P, SenthilKannan K, Divya R, Vimalan M, Tamilselvan S, Sankar D. A perspective approach towards appreciable size and cost-effective solar cell fabrication by synthesizing ZnO nanoparticles from Azadirachta indica leaves extract using domestic microwave oven. J Mater Sci Mater Electron. 2020;31:4301–9.10.1007/s10854-020-02985-9Suche in Google Scholar
[481] Rajendhiran R, Deivasigamani V, Palanisamy J, Pitchaiya S, Eswaramoorthy N, Masan S. Plectranthus amboinicus leaf extract synthesized spherical like-TiO2 photoanode for dye-sensitized solar cell application. Silicon. 2021;13:3329–36.10.1007/s12633-020-00709-6Suche in Google Scholar
[482] Sharma J, Srivastava P, Singh G, Akhtar MS, Ameen S. Green synthesis of Co3O4 nanoparticles and their applications in thermal decomposition of ammonium perchlorate and dye-sensitized solar cells. Mater Sci Eng B. 2015;193:181–8.10.1016/j.mseb.2014.12.012Suche in Google Scholar
[483] Setiawan IN, Giriantari ID, Ariastina W, Swamardika IA Natural dyes extracted from bioactive components of fruit waste for dye-sensitized solar cell. India: International Research Publication House; 2020.Suche in Google Scholar
[484] Briscoe J, Marinovic A, Sevilla M, Dunn S, Titirici M. Biomass‐derived carbon quantum dot sensitizers for solid‐state nanostructured solar cells. Angew Chem Int Ed. 2015;54:4463–8.10.1002/anie.201409290Suche in Google Scholar PubMed
[485] Pitchaiya S, Eswaramoorthy N, Natarajan M, Santhanam A, Asokan V, Madurai Ramakrishnan V, et al. Perovskite Solar cells: A porous graphitic carbon based hole transporter/counter electrode material extracted from an invasive plant species Eichhornia Crassipes. Sci Rep. 2020;10:1–16.10.1038/s41598-020-62900-4Suche in Google Scholar PubMed PubMed Central
[486] Marinovic A, Kiat LS, Dunn S, Titirici MM, Briscoe J. Carbon‐nanodot solar cells from renewable precursors. ChemSusChem. 2017;10:1004–13.10.1002/cssc.201601741Suche in Google Scholar PubMed
[487] Cha SM, Nagaraju G, Sekhar SC, Bharat LK, Yu JS. Fallen leaves derived honeycomb-like porous carbon as a metal-free and low-cost counter electrode for dye-sensitized solar cells with excellent tri-iodide reduction. J Colloid Interface Sci. 2018;513:843–51.10.1016/j.jcis.2017.11.080Suche in Google Scholar PubMed
[488] Xu S, Liu C, Wiezorek J. 20 renewable biowastes derived carbon materials as green counter electrodes for dye-sensitized solar cells. Mater Chem Phys. 2018;204:294–304.10.1016/j.matchemphys.2017.10.056Suche in Google Scholar
[489] Ahmad W, Yang Z, Khan J, Jing W, Jiang F, Chu L, et al. Extraction of nano-silicon with activated carbons simultaneously from rice husk and their synergistic catalytic effect in counter electrodes of dye-sensitized solar cells. Sci Rep. 2016;6:1–11.10.1038/srep39314Suche in Google Scholar PubMed PubMed Central
[490] Nithya P, Roumana C, Velraj G. Biomass-derived carbon (BC) boosted catalytic properties of zinc tungstate (ZnWO4) hybrid photoanodes for accelerating the triiodide reduction in dye-sensitized solar cells. Diam Relat Mater. 2022;108821.10.1016/j.diamond.2022.108821Suche in Google Scholar
[491] Akman E, Karapinar HS. Electrochemically stable, cost-effective and facile produced selenium@ activated carbon composite counter electrodes for dye-sensitized solar cells. Sol Energy. 2022;234:368–76.10.1016/j.solener.2022.02.011Suche in Google Scholar
[492] Li J, Yun S, Han F, Si Y, Arshad A, Zhang Y, et al. Biomass-derived carbon boosted catalytic properties of tungsten-based nanohybrids for accelerating the triiodide reduction in dye-sensitized solar cells. J Colloid Interface Sci. 2020;578:184–94.10.1016/j.jcis.2020.04.089Suche in Google Scholar PubMed
[493] Kalamaras CM, Efstathiou AM. Hydrogen production technologies: current state and future developments. Conf Pap Energy. 2013;2013:690627.10.1155/2013/690627Suche in Google Scholar
[494] Lin X, Wang J. Green synthesis of well dispersed TiO2/Pt nanoparticles photocatalysts and enhanced photocatalytic activity towards hydrogen production. Int J Hydrog Energy. 2019;44:31853–9.10.1016/j.ijhydene.2019.10.062Suche in Google Scholar
[495] Sargin I, Yanalak G, Arslan G, Patir IH. Green synthesized carbon quantum dots as TiO2 sensitizers for photocatalytic hydrogen evolution. Int J Hydrog Energy. 2019;44:21781–9.10.1016/j.ijhydene.2019.06.168Suche in Google Scholar
[496] Thakur A, Kumar P, Bagchi S, Sinha RK, Devi P. Green synthesized plasmonic nanostructure decorated TiO2 nanofibers for photoelectrochemical hydrogen production. Sol Energy. 2019;193:715–23.10.1016/j.solener.2019.10.022Suche in Google Scholar
[497] Sekar S, Lee S, Vijayarengan P, Kalirajan KM, Santhakumar T, Sekar S, et al. Upcycling of wastewater via effective photocatalytic hydrogen production using MnO2 nanoparticles—decorated activated carbon nanoflakes. Nanomaterials. 2020;10:1610.10.3390/nano10081610Suche in Google Scholar PubMed PubMed Central
[498] Huo E, Lei H, Liu C, Zhang Y, Xin L, Zhao Y, et al. Jet fuel and hydrogen produced from waste plastics catalytic pyrolysis with activated carbon and MgO. Sci Total Env. 2020;727:138411.10.1016/j.scitotenv.2020.138411Suche in Google Scholar PubMed
[499] Rambabu K, Bharath G, Banat F, Hai A, Show PL, Phong Nguyen TH. Ferric oxide/date seed activated carbon nanocomposites mediated dark fermentation of date fruit wastes for enriched biohydrogen production. Int J Hydrog Energy. 2021;46:16631–43.10.1016/j.ijhydene.2020.06.108Suche in Google Scholar
[500] Chen C-H, Lin Y-C, Peng Y-P, Lin M-H. Simultaneous hydrogen production and ibuprofen degradation by green synthesized Cu2O/TNTAs photoanode. Chemosphere. 2021;284:131360.10.1016/j.chemosphere.2021.131360Suche in Google Scholar PubMed
[501] Ubaidullah M, Al-Enizi AM, Shaikh S, Ghanem MA, Mane RS. Waste PET plastic derived ZnO@NMC nanocomposite via MOF-5 construction for hydrogen and oxygen evolution reactions. J King Saud Univ - Sci. 2020;32:2397–405.10.1016/j.jksus.2020.03.025Suche in Google Scholar
[502] Luévano-Hipólito E, Torres-Martínez LM, Fernández-Trujillo A. Ternary ZnO/CuO/Zeolite composite obtained from volcanic ash for photocatalytic CO2 reduction and H2O decomposition. J Phys Chem Solids. 2021;151:109917.10.1016/j.jpcs.2020.109917Suche in Google Scholar
[503] Mohanraj S, Kodhaiyolii S, Rengasamy M, Pugalenthi V. Green synthesized iron oxide nanoparticles effect on fermentative hydrogen production by clostridium acetobutylicum. Appl Biochem Biotechnol. 2014;173:318–31.10.1007/s12010-014-0843-0Suche in Google Scholar PubMed
[504] Mohamed F, Rabia M, Shaban M. Synthesis and characterization of biogenic iron oxides of different nanomorphologies from pomegranate peels for efficient solar hydrogen production. J Mater Res Technol. 2020;9:4255–71.10.1016/j.jmrt.2020.02.052Suche in Google Scholar
[505] Sahara G, Ishitani O. Efficient photocatalysts for CO2 reduction. Inorg Chem. 2015;54:5096–104.10.1021/ic502675aSuche in Google Scholar PubMed
[506] Ran J, Jaroniec M, Qiao SZ. Cocatalysts in semiconductor‐based photocatalytic CO2 reduction: achievements, challenges, and opportunities. Adv Mater. 2018;30:1704649.10.1002/adma.201704649Suche in Google Scholar PubMed
[507] Glonek K, Sreńscek-Nazzal J, Narkiewicz U, Morawski A, Wróbel R, Michalkiewicz B. Preparation of activated carbon from beet molasses and TiO2 as the adsorption of CO2. Acta Phys Pol A. 2016;129:158–61.10.12693/APhysPolA.129.158Suche in Google Scholar
[508] Wu D, Guo J, Wang H, Zhang X, Yang Y, Yang C, et al. Green synthesis of boron and nitrogen co-doped TiO2 with rich B-N motifs as Lewis acid-base couples for the effective artificial CO2 photoreduction under simulated sunlight. J Colloid Interface Sci. 2021;585:95–107.10.1016/j.jcis.2020.11.075Suche in Google Scholar PubMed
[509] Wang X, Hui W, Hu A, Li X, Li Y, Wang H. A synthesis of porous activated carbon materials derived from vitamin B9 base for CO2 capture and conversion. Materials Today. Chemistry. 2021;20:100468.10.1016/j.mtchem.2021.100468Suche in Google Scholar
© 2022 Mohamed Abd Elkodous et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption

