Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
-
Seyyed Behnam Abdollahi Boraei
, Ana Ferrández Montero
Abstract
In this study, three-dimensional (3D) printing of 3D scaffolds containing halloysite nanotubes (HNTs) and strontium ranelate (SrR) as a carrier for the promotion of bone regeneration is investigated. SrR acts as an anabolic bone-forming and anti-catabolic agent, while HNTs act as a carrier of SrR. Poly(lactic acid) (PLA) is used as a biodegradable matrix and carrier for HNTs and SrR. The effects of the SrR addition on the morphological, biological, and in vitro release properties of the scaffolds are evaluated. The morphological results show a homogeneous structure with a proper pore size (approximately 400 µm) suitable for osteogenesis. The contact angle is decreased after the addition of SrR to the scaffold to 67.99°, suitable for cell attachment. X-ray diffraction shows that the SrR is homogenously and molecularly distributed in the PLA matrix and reduces the crystallinity in the prepared scaffolds. The in vitro release results demonstrate that the release profile of the SrR is stable, relatively linear, and continuous within 21 days (504 h). A cumulative release of SrR of approximately 49% is obtained after a controlled release for 504 h (21 days) and a low primary burst release (12%). Human adipose stem cells cultured on the 3D-printed scaffolds demonstrate that the SrR can efficiently promote biocompatibility, alkaline phosphatase activity, and alizarin red staining.
1 Introduction
In general, bone is a composite composition that has three main parts: matrix, fiber, and cell. The most important part is the collagen matrix, which bears the tensile mechanical loads, while the mineral phase, which consists of calcium phosphates, bears the compressive mechanical loads [1,2,3,4]. In this regard, the bone tissue experiences numerous defects throughout life, including problems caused by trauma, injuries of various origins, and aging, which are increasingly studied to address the related problems [5,6,7,8,9]. Considering the importance of these problems, bone tissue engineering is a new progressing method considered to mitigate osteogenesis [10,11,12,13]. Scaffolds are the most important part of tissue engineering science. The scaffold is a matrix in three-dimensional (3D) structures, which has essential characteristics, such as biocompatibility and proper mechanical properties, induces cellular activity and protein production, and provides cell attachment, differentiation, and proliferation [14,15,16,17,18,19]. The interconnecting pore is a significant factor for synthetic scaffolds in bone tissue engineering applications [20,21]. The pore sizes must be around 300 µm for good vascularization, cell attachment, and growth guidance in three dimensions [22]. Several methods are used to obtain porous scaffolds, such as solvent casting, foam gel method, freeze-drying, thermally induced phase separation, particle/salt leaching, and chemical/gas foaming [23,24,25,26,27,28,29]. In recent years, additive manufacturing is widely used in bone repair applications.
Composite structures have been synthesized for various applications, which include ceramic, polymer, and metal compounds [30,31,32,33,34,35,36,37]. Polymer–ceramic nanocomposites with drug-loading properties are widely used in tissue engineering and in cases that require appropriate mechanical properties as well as controlled release of drugs [38,39]. Synthetic biopolymers, including poly(lactic acid) (PLA) and polycaprolactone, are useful for numerous applications in the synthesis of nanocomposites [40,41,42,43,44,45,46,47]. A 3D-printed PLA was investigated in recent years. PLA can be 3D printed at low temperatures, while the agent is bound by a binder solution [48,49,50]. In the 3D-printing method, biocompatible polymers can be used, and thus, composite scaffolds can be designed and manufactured [51]. The osteogenesis agent used in the matrix should be elucidated and its local release should be effective and beneficial. Among such agents, strontium ranelate (SrR), which has anabolic and anticatabolic properties, is increasingly used in tissue engineering scaffolds [52]. The addition of SrR increases the adhesion, proliferation, alkaline phosphatase (ALP) activity, mineralization, and angiogenesis of the 3D-printed scaffolds [53,54]. Another important factor of tissue engineering scaffolds containing drugs is the controlled release of the drug for a proper tissue regeneration. Halloysite nanotubes (HNTs) could help control the release of SrR and reduce its initial burst release [55]. HNT is a natural aluminosilicate that attracts considerable attention and has many applications [56,57,58,59]. HNT has unique properties, such as good mechanical and thermal properties, and is a suitable carrier of drugs. Several studies have been carried out on its applications and properties [60,61,62,63,64].
In this study, the 3D-printing technique was employed to obtain PLA/HNT-SrR scaffolds with different compounds (PLA, PLA/HNT, and PLA/HNT-SrR). The scaffold morphologies were evaluated by scanning electron microscopy (SEM). An X-ray diffraction (XRD) analysis was performed to elucidate the crystallographic features of the scaffolds. The drug release behavior of the SrR-loaded scaffolds was investigated. In addition, the cellular behaviors 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), ALP activity, and calcium assay of the 3D scaffolds were revealed.
2 Experimental section
2.1 Preparation and characterization of scaffolds
SrR was purchased from Servier Co. (UK). PLA (PLA 2003D, D-isomer content: 4.25%, Nature-Works LLC, Minnetonka, USA) and HNT (Sigma-Aldrich, St. Louis, USA) were used. Cetyltrimethylammonium bromide (CTAB, 99%) as a surfactant was purchased from Sigma-Aldrich. Fetal bovine serum, high-glucose Dulbecco’s modified Eagle’s medium (HG-DMEM), phosphate-buffered saline (PBS), and penicillin–streptomycin were purchased from GIBCO. Tetrahydrofuran (THF; purity: 99%, Panreac) was provided by Darmstadt Co. (Germany).
Suspensions of HNT- and SrR-modified HNT dispersed by adding 2 wt% CTAB (with respect to the solid content of HNT) in a PLA solution in THF (80 g/L) were prepared and granulated as described elsewhere [55,65]. Granules were crushed into fine powders for the extrusion of colloidal filaments and printing [66]. The 3D scaffold was obtained by a 3D-printing device (Prusa I3 with the Repetier software). 3D scaffolds with different compositions (PLA, PLA/HNT, and PLA/HNT-SrR) were printed (diameter: 1 cm, height: 0.2 cm, infill density: 40%, four layers). The prepared scaffolds were immersed in NaOH (20 wt%) for 5–10 s for a cross-linking treatment and then washed with deionized H2O.
The PLA-based scaffolds were coated with gold (SC7620, QUOROMTECHNOLOGIES-EMITECH, England), and then, the microstructure was observed by SEM (AIS2100, SERON TECHNOLOGY, South Korea) in the secondary-electron mode at 20 kV to evaluate the morphology. Energy-dispersive spectrometry (EDS) was used to confirm the presence of HNTs and SrR in the scaffolds as well as their uniform distributions.
An attenuated total reflectance (ATR) Fourier-transform infrared (FTIR) analysis (Spectrum 100, PerkinElmer Company, UK) was used to investigate the functional groups and structural composition of the scaffolds.
The crystallography structures of the scaffolds were evaluated by XRD (EQUINOX3000, Inel, France) with Cu Kα radiation at a voltage of 40 kV and a scan rate of 2°/min.
The hydrophilicity of the scaffolds was evaluated by a contact angle analysis. Approximately 20 µL of distilled water was dropped on a flat surface part of the scaffold. The contact angle was recorded after 2 s. A drop shape analysis software was applied to determine the baseline and contact angle. The results are presented as mean and standard deviation of five replications for each sample.
The release profile of SrR from the SrR-containing scaffold (0.5 g) was investigated by immersing the scaffold in 5 mL of PBS. It was then incubated in a shaker incubator at 90 rpm. At selected time points, 2 mL of the PBS was picked up and replaced with 2 mL of fresh PBS. The amount of released SrR was evaluated by an ultraviolet (UV)-visible device (NANODROP 2000c, Thermo Scientific Co., USA) at several time points (24, 48, 72, 120, 168, 336, and 504 h) at a wavelength of 318 nm.
2.2 Cellular assay
The prepared scaffolds were punched, placed into tissue culture polystyrene (TCPS), and then sterilized by 70% ethanol for 2 h and 20 min UV irradiation on each side. After immersing the scaffolds in a culture medium overnight, the density of primitive cells was 2 × 105 cells/cm2. They were suspended in 200 μL of HG-DMEM and then seeded on the prepared scaffolds and controls (TCPS). The incubation was carried out for 30 min. Afterward, the basal medium (800 μL) was poured into the wells. All scaffolds were moved to new plates. The osteogenic medium was added and kept for 7 and 14 days. The renovation of the osteogenic medium was carried out every 2 days.
The cytotoxicity and viability of human adipose-derived stem cells (hASCs) on the prepared scaffolds were investigated by an MTT assay. The scaffolds were punched and placed into the plates. The primitive density of hASCs was 2 × 105 cells/cm2. The plates were then placed into the incubator. Fifty microliters of the MTT solution (5 mg/mL) were then poured into the wells. The medium was removed after 2 h from the incubation. Three hundred microliters of dimethylsulfoxide (Merck) were then replaced to dissolute the dark-blue intracellular formazan. This procedure was carried out at the first, fourth, and seventh days of cell seeding. The dye solutions were then moved to six-well plates to observe the optical density of each well at 570 nm by a spectrophotometer (BioTek Instruments, USA).
Radioimmunoprecipitation assay buffer (200 μL) was used to extract the total protein from hASCs cultured on samples and TCPS for ALP activity evaluation at the time points of 7 and 14 days. The lysate was centrifuged (1,200 rpm, 4°C, 5 min) for the sedimentation of cell debris. An ALP assay kit (Parsazmun Co., Tehran, Iran) was used to measure the ALP activity of the collected supernatant.
The cresolphthalein complexone method was used to evaluate the amount of deposited calcium on the samples and TCPS. The homogenization of the hASCs was carried out by HCl (0.6 N, Merck Co.). They were shaken for 4 h at 4°C. In this stage, after the addition of a reagent to the calcium solution, the optical density was measured at 405 nm [38].
The mentioned analyses were carried out three times. Mean ± standard deviation was used to present the final data. The differences between the results were evaluated by a one-way analysis of variance. All results were statistically investigated at a level of p < 0.05.
3 Results and discussion
3.1 Characterization of the 3D-printed scaffolds
A proper scaffold provides specified features, such as a porous structure and interconnected pores for cell adhesion, proliferation, and differentiation, as well as transfer of nutrients to the cells and removal of waste products from cells [38]. Figure 1 shows SEM images of the 3D-printed scaffolds and macroporous structure. The homogeneous porous and completely interconnected matrix were detected in the scaffolds. According to the fixed setup of the printer, all samples have the same pore size (the mean value is 400 µm), which is in good agreement with other studies and in the suitable range for bone regeneration applications [67,68].
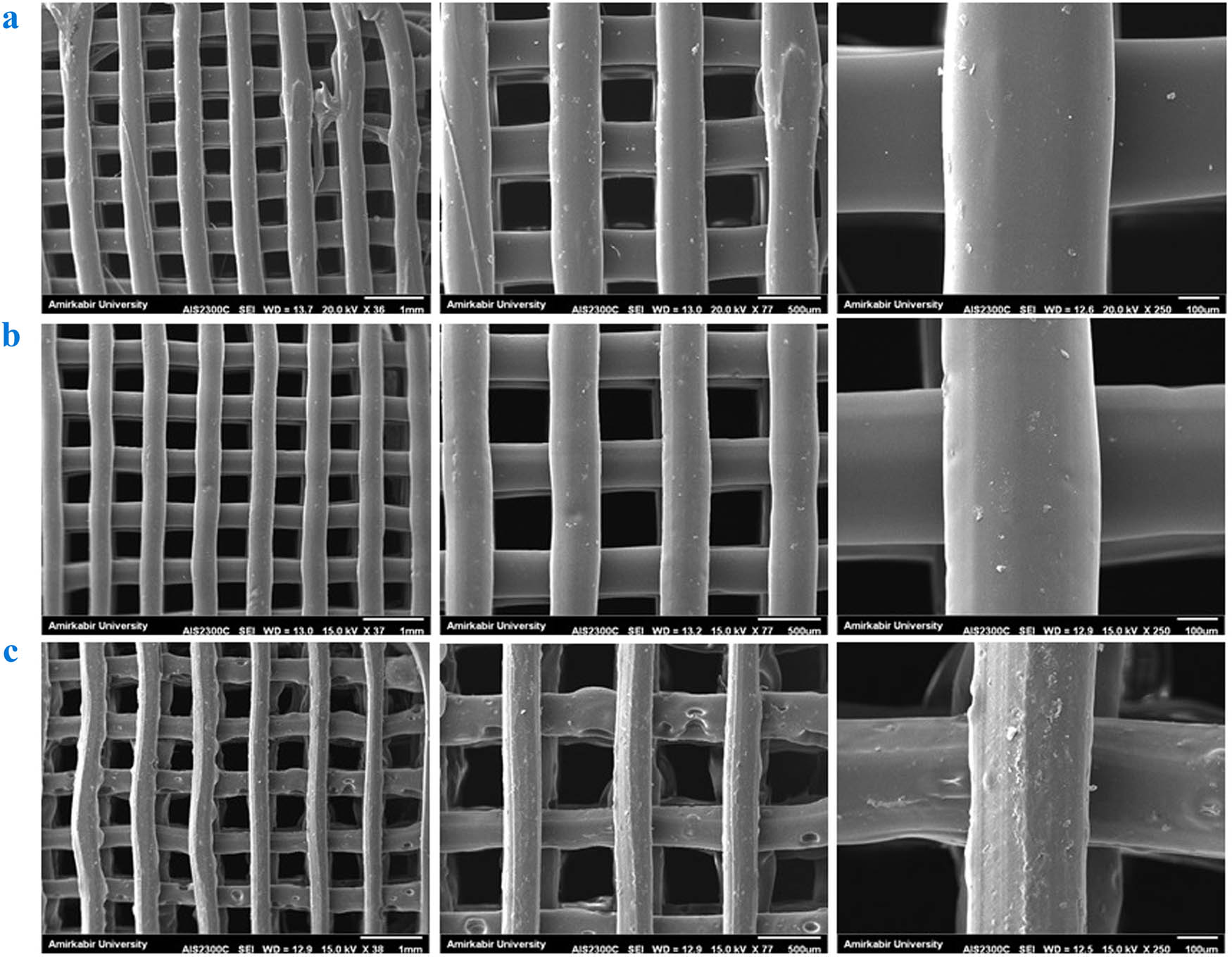
SEM images of (a) PLA, (b) PLA/HNT, and (c) PLA/HNT-SrR scaffolds at various magnifications.
The increase in HNT content reduces the smoothness of the scaffold surface, mainly owing to the tendency of the nanoparticles for agglomeration at high concentrations [69]. HNT is stiffer than molten PLA, and thus, the polymer drags partially with the nozzle movement during the 3D printing [70].
To confirm the presence of HNTs and SrR in the scaffolds, an EDS analysis of Al, Si, and Sr elements was carried out on the scaffolds, as shown in Figure 2. The images show the uniform distribution of HNTs (Al and Si elements) and SrR (Sr element) in the PLA matrix.
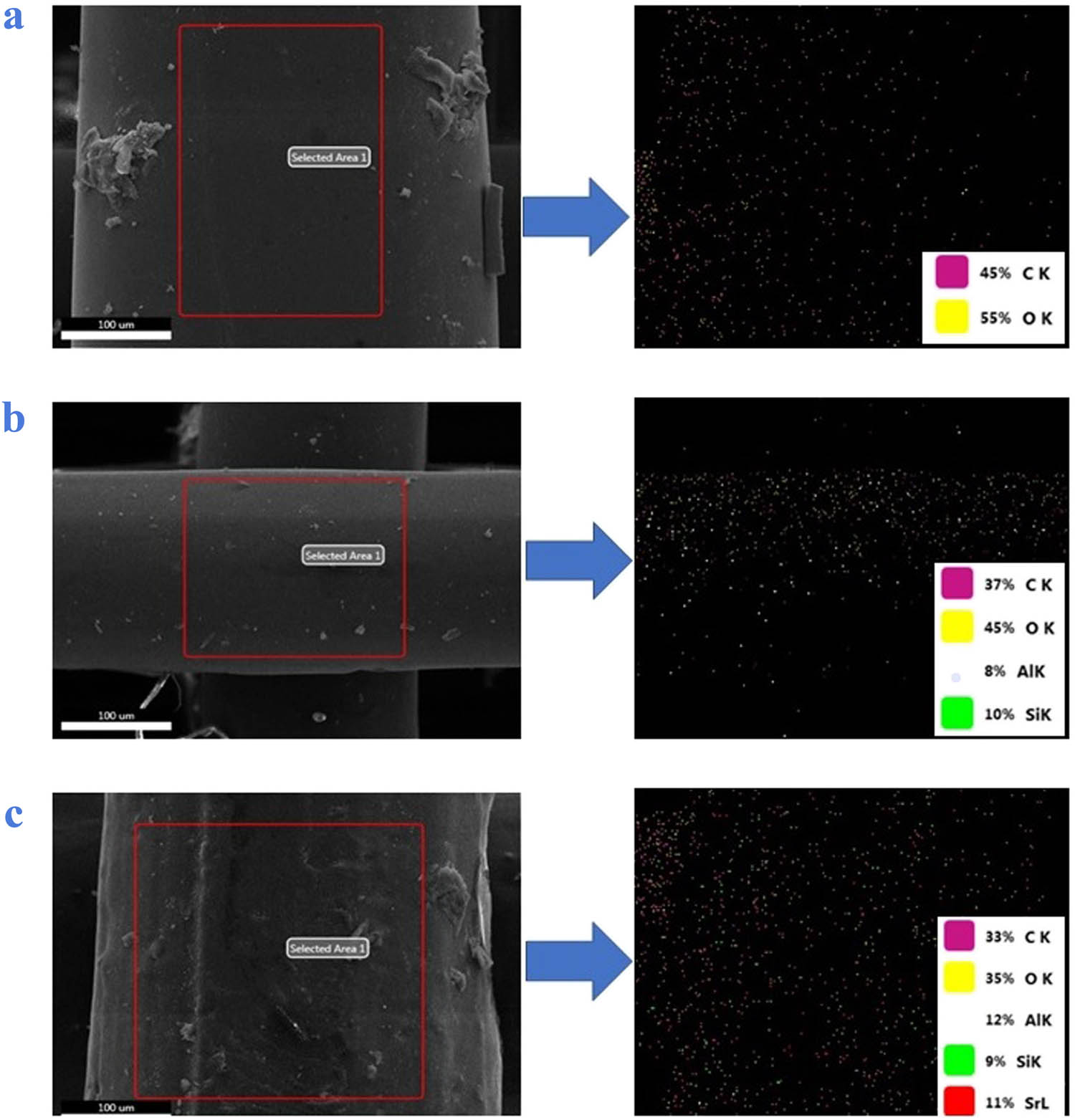
EDS analyses of the (a) PLA, (b) PLA/HNT, and (c) PLA/HNT-SrR scaffolds.
Figure 3 shows the results of the ATR FTIR spectroscopy of the scaffolds. The typical bands of PLA (polyesters) are observed, including those at 2,921 and 2,851 cm−1 attributed to symmetric and asymmetric vibrations of the C–H bond of the CH3 groups, respectively, at 1,741 and 1,179 cm−1 related to C═O and C–O–C stretching, respectively, at 1,450 cm−1 corresponding to C–H stretching vibration in methyl groups, at 1,363 cm−1 assigned to symmetric vibrations of –CH– bending, at 1,076 cm−1 related to carbonyl C═O and –OH groups, and at 867 and 753 cm−1 assigned to the C–C stretching vibration in the structure [71,72]. After the addition of HNTs to the structure, the bands at 2,851 and 2,925 cm−1 attributed to PLA slightly shifted to higher wavenumbers, which indicates hydrogen bonding of the hydroxyl chains of PLA with the siloxane groups of halloysites. The band at 1,023 cm−1 corresponds to the stretching of the Si–O group, which indicates the successful incorporation of the HNT in the structure [73]. After the loading of SrR, bands at 1,260 and 1,355 cm−1 appeared, which are related to the C–N vibration in SrR. This indicates a successful loading of the SrR drug into the scaffold structure.
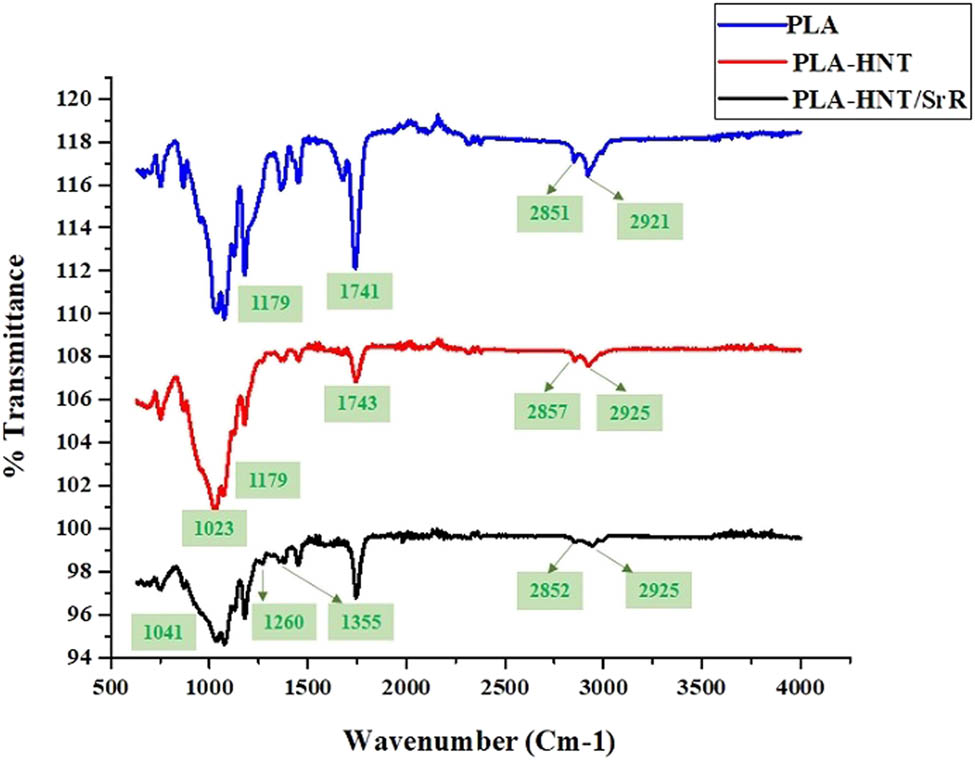
FTIR spectra of the prepared scaffolds.
Figure 4 shows XRD results of the prepared scaffolds. In general, they have two characteristic peaks at 2θ of 17° and 19° [74]. A similar peak is observed in our results (2θ = 16.72°) but is too broad, which indicates that the PLA is amorphous during the scaffold preparation. The HNTs usually have several specific peaks. In this study, owing to the low percentage of HNTs in the structure of the scaffolds, not all of their peaks are clear. Only the characteristic peak at 2θ = 12.05° for the PLA/HNT scaffold, which contains HNTs, is observed. This peak is similar to the major peak of HNTs at 2θ = 11.9° [75]. The peak demonstrates the presence of HNTs in the structure. This peak is in good agreement with the (001) basal plane of HNTs [68]. After the addition of SrR to the structure, no characteristic peaks of SrR were observed owing to two reasons: (1) low percentage of SrR in the scaffold, which makes it undetectable for the device, and (2) with the addition of SrR to the polyesters and polyethers that compose the scaffold matrix, the crystallinity of the SrR is reduced, and thus, it tends to become amorphous [39].
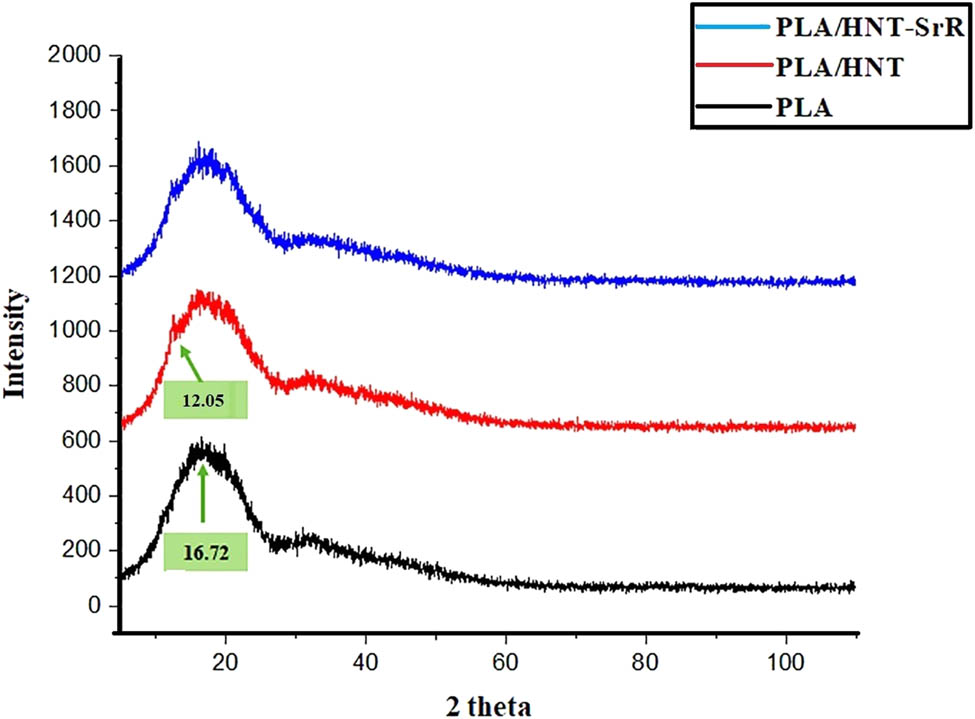
XRD patterns of the PLA, PLA/HNT, and PLA/HNTs-SrR scaffolds.
The contact angle test results of the PLA, PLA/HNT, and PLA/HNT-SrR scaffolds are presented in Figure 5. As expected, the contact angle of the PLA scaffold was the largest, 111.1 ± 13.63°, which is in the range reported for polyesters [76]. Upon the addition of HNTs to the structure, the contact angle and hydrophobicity of the structure are reduced (100.15 ± 11.23°). After the addition of SrR to the structure, which is a completely hydrophilic drug, the hydrophobicity of the structure was reduced and the angle reached 67.99 ± 5.47°. In general, with the increase in the amount of hydrophilic components in the structure (in this case, SrR), a decrease in contact angle is expected. The suitable contact angle range for the hydrophilicity of scaffolds and cell attachment to the scaffold is 40–70°, considered an important parameter [39,77].
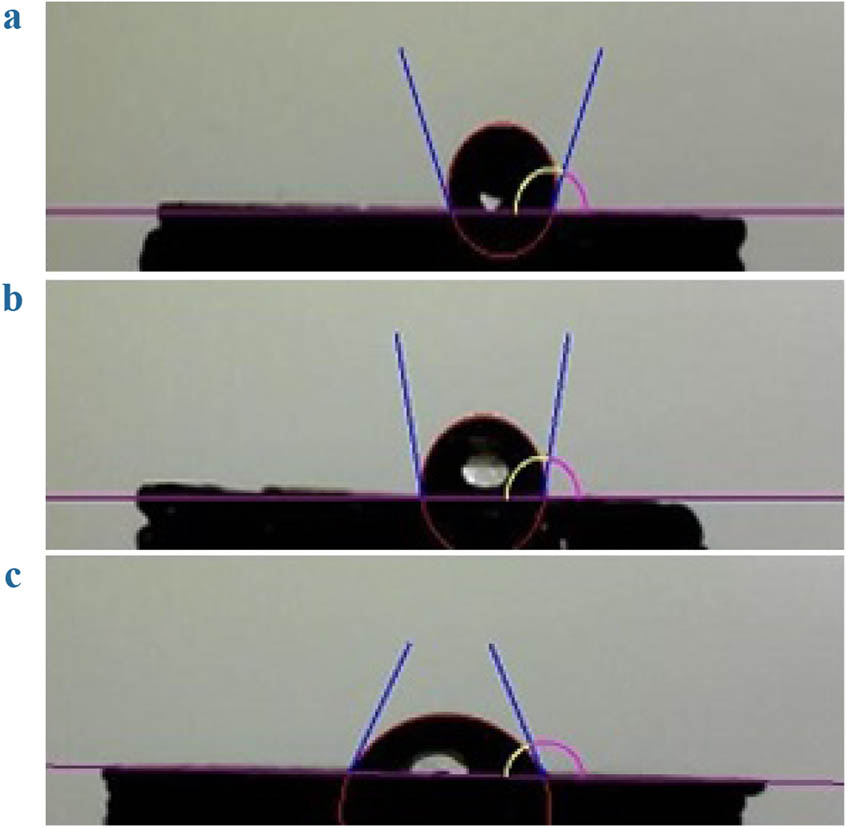
Contact angle results of the (a) PLA, (b) PLA/HNT, and (c) PLA/HNT-SrR scaffolds.
3.2 In vitro SrR release investigations
Figure 6 shows the release profiles of SrR from the scaffolds. The HNT was added as a drug carrier to the scaffold to control the release behavior of the SrR. Twenty-one days (500 h) is a suitable time for osteogenesis differentiation by continuous release of SrR. The porous 3D scaffold exhibited a relatively linear and stable release behavior of SrR. The cumulative release after 500 h was approximately 49%, similar to our previous result [38]. Owing to the formation of SrR bonds on or into the HNT, the SrR release profile exhibits a controlled behavior. As shown in Figure 6, the initial burst release rate of SrR from the scaffold is extremely low, approximately 12%. The initial burst release is very high in many systems, particularly in systems without drug carriers. In general, two mechanisms can be proposed for the release of SrR from HNT-containing nanocomposite scaffolds. The first mechanism is attributed to siloxane groups (Si–O–Si) on the HNT surface and their negative charge. Thus, they can bond the positively charged strontium. The second mechanism is attributed to aluminum hydroxide groups (Al(OH)2) inside the positively charged HNT lumens. Thus, there is a possibility of bond formation between them and the negative terminal of the ranelate [55].
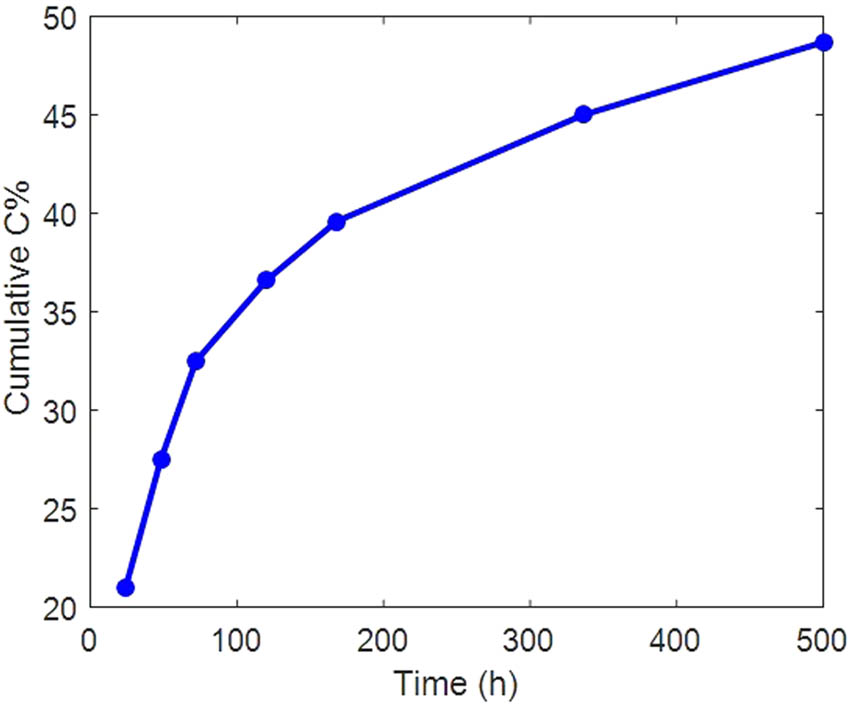
Release behavior of the PLA/HNT-SrR scaffold in PBS.
3.3 Cellular analysis
Biocompatibility and nontoxicity are important factors for a good scaffold. An MTT assay was performed to investigate the cytocompatibility of the prepared samples by culturing hASCs on them (Figure 7). The proliferation of hASCs on the SrR-containing scaffold after 1, 4, and 7 days was better than those for the PLA and PLA/HNT samples. After 5 days, it was better in the case with the SrR scaffold than for the other samples. The extracts acquired from the PLA/HNT and PLA/HNT-SrR scaffolds exhibited superior cell viabilities after 1, 4, and 7 days [78]. Thus, all prepared scaffolds in our experiment exhibited a good cytocompatibility and were suitable for bone tissue engineering. The results of the MTT analysis can be explained using the EDS analysis, where the scaffold containing the drug and element strontium exhibited a higher cell proliferation, as expected.
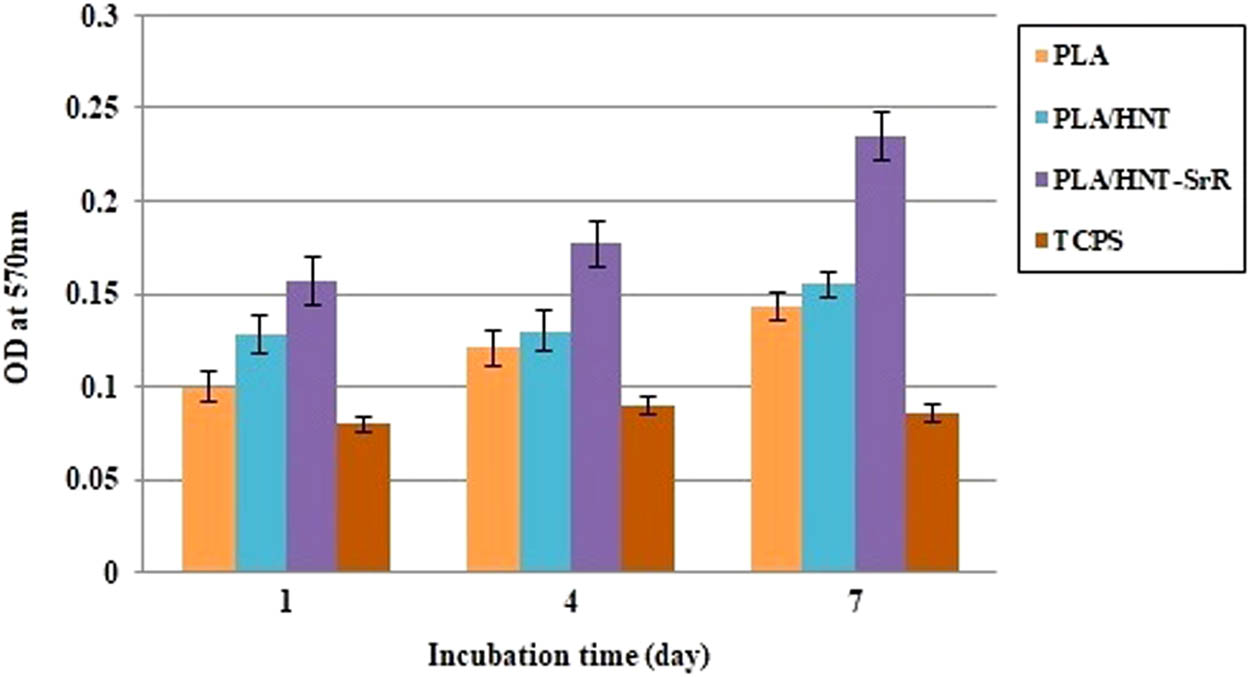
MTT assays of cell proliferation and viability on the scaffolds.
The ALP activity was evaluated to investigate the osteogenic potential of the cells. ALP is an important osteogenic marker expressed in the early stages. The results showed an increase in the ALP activity. Despite the release of SrR, it does not have a significant effect on the hASCs growth in the first week. There is a notable difference between the SrR-containing scaffolds and the rest of scaffolds in the second week [38]. Figure 8 shows a higher ALP activity in the PLA/HNT-SrR scaffold than in the PLA and PLA/HNT scaffolds at day 14 (p < 0.05). These results are consistent with the contact angle analysis, where the scaffold containing SrR had the optimal contact angle and consequently higher hydrophilicity, which, in turn, increased the cell attachment and improved the APL results.
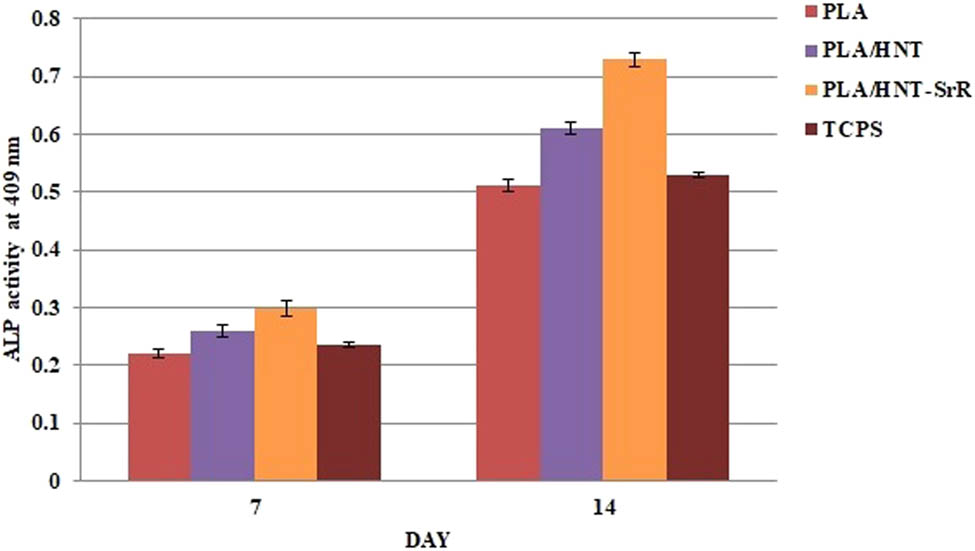
ALP activity of the hASCs on the prepared scaffolds (p < 0.05).
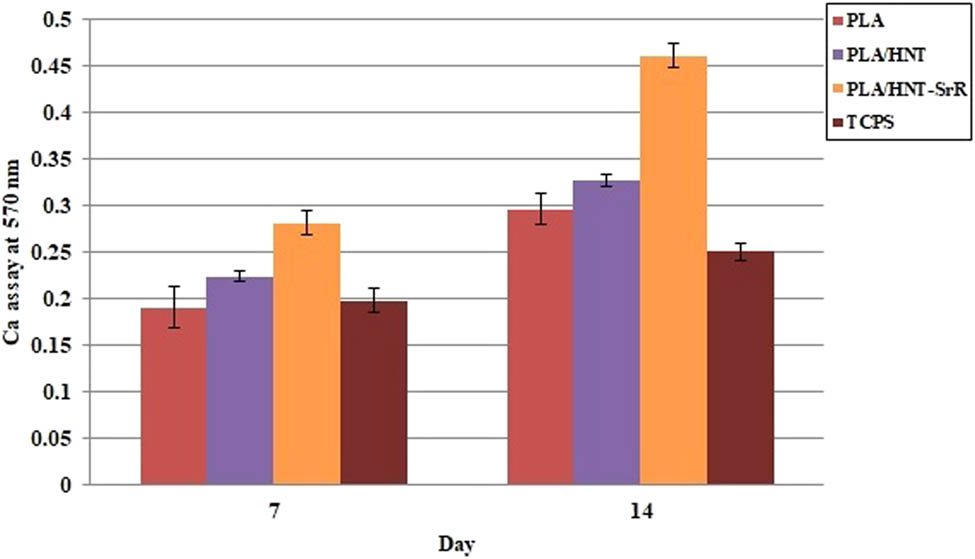
In general, large amounts of calcium deposits are observed in bone tissue. Alizarin red staining (ARS) is one of the approved evaluation methods for calcium deposition and subsequent mineralization of synthesized scaffolds. In this study, such a test was performed after 7 and 14 days. Figure 9 shows an increase in the calcium content after 14 days of culturing, higher than that after 7 days. The scaffold containing SrR exhibited the highest amount on day 7 and the highest increase on day 14. The SrR-containing scaffold had the largest amount of deposits among the prepared scaffolds [79]. As shown in Figure 9, the ARS quantification indicates that the deposition of calcium in the SrR porous scaffold was considerably higher than that in the neat scaffold. Hence, the SrR-containing scaffold accelerates the bone formation-enhanced osteogenic differentiation. Several mechanisms about the osteogenesis ability of SrR have been investigated [80,81,82].
ARS of culture hASCs on the scaffolds at different times.
4 Conclusion
The 3D-printing technology was used to produce proper scaffolds for bone tissue engineering applications. Interconnected porous structures with pore sizes of approximately 400 μm and high porosities were produced. The addition of SrR to the scaffolds remarkably changed the properties of the 3D-printed scaffolds. A cumulative release of SrR of approximately 49% was obtained after the controlled release for 504 h (21 days) and low primary burst release (12%). The sustained release of SrR could be observed for at least 504 h (21 days) owing to the loading of SrR on or into the HNTs. The contact angle largely decreased upon the addition of SrR to the structure to 67.99° in a suitable range for cell attachment. The nontoxicity of HNT and SrR was demonstrated after their addition to the PLA scaffold. The cellular analysis demonstrated that the ALP activity and calcium deposition increased upon the addition of SrR to the scaffold. Thus, the produced scaffolds can provide a good porous 3D structure in bone tissue engineering applications and prompt bone regeneration.
-
Funding information: This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (project number: 2022R1A2C1004437).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Wei S, Ma J-X, Xu L, Gu X-S, Ma X-L. Biodegradable materials for bone defect repair. Mil Med Res. 2020;7(1):1–25.10.1186/s40779-020-00280-6Suche in Google Scholar PubMed PubMed Central
[2] Wang W, Zhang B, Zhao L, Li M, Han Y, Wang L, et al. Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application. Nanotechnol Rev. 2021;10(1):1359–73.10.1515/ntrev-2021-0083Suche in Google Scholar
[3] Wang J, Zhang Y, Yang X, Ma X. Stress effect on 3D culturing of MC3T3-E1 cells on microporous bovine bone slices. Nanotechnol Rev. 2020;9(1):1315–25.10.1515/ntrev-2020-0103Suche in Google Scholar
[4] Xing F, Zhou C, Hui D, Du C, Wu L, Wang L, et al. Hyaluronic acid as a bioactive component for bone tissue regeneration: Fabrication, modification, properties, and biological functions. Nanotechnol Rev. 2020;9(1):1059–79.10.1515/ntrev-2020-0084Suche in Google Scholar
[5] Preethi A, Bellare JR. Tailoring scaffolds for orthopedic application with anti-microbial properties: current scenario and future prospects. Front Mater. 2021;452:1–24.10.3389/fmats.2020.594686Suche in Google Scholar
[6] Abbasi N, Hamlet S, Love RM, Nguyen N-T. Porous scaffolds for bone regeneration. J Sci Adv Mater Devices. 2020;5(1):1–9.10.1016/j.jsamd.2020.01.007Suche in Google Scholar
[7] Cunniffe GM, O’Brien FJ. Collagen scaffolds for orthopedic regenerative medicine. JOM. 2011;63(4):66–73.10.1007/s11837-011-0061-ySuche in Google Scholar
[8] Huo Y, Lu Y, Meng L, Wu J, Gong T, Zou JA, et al. A critical review on the design, manufacturing and assessment of the bone scaffold for large bone defects. Front Bioeng Biotechnol. 2021;946:1–13.10.3389/fbioe.2021.753715Suche in Google Scholar PubMed PubMed Central
[9] Yang YP, Labus KM, Gadomski BC, Bruyas A, Easley J, Nelson B, et al. Osteoinductive 3D printed scaffold healed 5 cm segmental bone defects in the ovine metatarsus. Sci Rep. 2021;11(1):1–12.10.1038/s41598-021-86210-5Suche in Google Scholar PubMed PubMed Central
[10] Koons GL, Diba M, Mikos AG. Materials design for bone-tissue engineering. Nat Rev Mater. 2020;5(8):584–603.10.1038/s41578-020-0204-2Suche in Google Scholar
[11] Qu H, Fu H, Han Z, Sun Y. Biomaterials for bone tissue engineering scaffolds: a review. RSC Adv. 2019;9(45):26252–62.10.1039/C9RA05214CSuche in Google Scholar
[12] Kargozar S, Mozafari M, Hamzehlou S, Brouki Milan P, Kim H-W, Baino F. Bone tissue engineering using human cells: a comprehensive review on recent trends, current prospects, and recommendations. Appl Sci. 2019;9(1):174.10.3390/app9010174Suche in Google Scholar
[13] Perić Kačarević Ž, Rider P, Alkildani S, Retnasingh S, Pejakić M, Schnettler R, et al. An introduction to bone tissue engineering. Int J Artif Organs. 2020;43(2):69–86.10.1177/0391398819876286Suche in Google Scholar PubMed
[14] Hu C, Wu L, Zhou C, Sun H, Gao P, Xu X, et al. Berberine/Ag nanoparticle embedded biomimetic calcium phosphate scaffolds for enhancing antibacterial function. Nanotechnol Rev. 2020;9(1):568–79.10.1515/ntrev-2020-0046Suche in Google Scholar
[15] Radakisnin R, Majid MSA, Jamir MRM, Tahir MFM, Meng CE, Al Alshahrani H. Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique. Nanotechnol Rev. 2021;10(1):1469–83.10.1515/ntrev-2021-0098Suche in Google Scholar
[16] Zhang L, Zheng T, Wu L, Han Q, Chen S, Kong Y, et al. Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth. Nanotechnol Rev. 2021;10(1):50–61.10.1515/ntrev-2021-0004Suche in Google Scholar
[17] Peng Z, Tang P, Zhao L, Wu L, Xu X, Lei H, et al. Advances in biomaterials for adipose tissue reconstruction in plastic surgery. Nanotechnol Rev. 2020;9(1):385–95.10.1515/ntrev-2020-0028Suche in Google Scholar
[18] Ahmed M, Menazea A, Mansour S, Al-Wafi R. Differentiation between cellulose acetate and polyvinyl alcohol nanofibrous scaffolds containing magnetite nanoparticles/graphene oxide via pulsed laser ablation technique for tissue engineering applications. J Mater Res Technol. 2020;9(5):11629–40.10.1016/j.jmrt.2020.08.041Suche in Google Scholar
[19] Mosaddad SA, Yazdanian M, Tebyanian H, Tahmasebi E, Yazdanian A, Seifalian A, et al. Fabrication and properties of developed collagen/strontium-doped Bioglass scaffolds for bone tissue engineering. J Mater Res Technol. 2020;9(6):14799–817.10.1016/j.jmrt.2020.10.065Suche in Google Scholar
[20] Lutzweiler G, Ndreu Halili A, Engin, Vrana N. The overview of porous, bioactive scaffolds as instructive biomaterials for tissue regeneration and their clinical translation. Pharmaceutics. 2020;12(7):602.10.3390/pharmaceutics12070602Suche in Google Scholar PubMed PubMed Central
[21] Arastouei M, Khodaei M, Atyabi SM, Nodoushan MJ. Poly lactic acid-akermanite composite scaffolds prepared by fused filament fabrication for bone tissue engineering. J Mater Res Technol. 2020;9(6):14540–8.10.1016/j.jmrt.2020.10.036Suche in Google Scholar
[22] Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474–91.10.1016/j.biomaterials.2005.02.002Suche in Google Scholar PubMed
[23] Cao H, Kuboyama N. A biodegradable porous composite scaffold of PGA/β-TCP for bone tissue engineering. Bone. 2010;46(2):386–95.10.1016/j.bone.2009.09.031Suche in Google Scholar PubMed
[24] Kucharska M, Butruk B, Walenko K, Brynk T, Ciach T. Fabrication of in-situ foamed chitosan/β-TCP scaffolds for bone tissue engineering application. Mater Lett. 2012;85:124–7.10.1016/j.matlet.2012.07.002Suche in Google Scholar
[25] Sultana N, Wang M. Fabrication of HA/PHBV composite scaffolds through the emulsion freezing/freeze-drying process and characterisation of the scaffolds. J Mater Sci Mater Med. 2008;19(7):2555.10.1007/s10856-007-3214-3Suche in Google Scholar PubMed
[26] Yoshikawa H, Tamai N, Murase T, Myoui A. Interconnected porous hydroxyapatite ceramics for bone tissue engineering. J R Soc Interface. 2009;6(Suppl 3):S341–8.10.1098/rsif.2008.0425.focusSuche in Google Scholar PubMed PubMed Central
[27] Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. The biomaterials: Silver Jubilee Compendium. Elsevier: Amsterdam, Netherlands; 2006. p. 175–89.10.1016/B978-008045154-1/50021-6Suche in Google Scholar
[28] Collins MN, Ren G, Young K, Pina S, Reis RL, Oliveira JM. Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv Funct Mater. 2021;31(21):2010609.10.1002/adfm.202010609Suche in Google Scholar
[29] Hu X, Yang Z, Kang S, Jiang M, Zhou Z, Gou J, et al. Cellulose hydrogel skeleton by extrusion 3D printing of solution. Nanotechnol Rev. 2020;9(1):345–53.10.1515/ntrev-2020-0025Suche in Google Scholar
[30] Zare Y, Rhee KY. Tensile modulus prediction of carbon nanotubes-reinforced nanocomposites by a combined model for dispersion and networking of nanoparticles. J Mater Res Technol. 2019;9:22–32.10.1016/j.jmrt.2019.10.025Suche in Google Scholar
[31] Zare Y, Rhee KY. Analysis of critical interfacial shear strength between polymer matrix and carbon nanotubes and its impact on the tensile strength of nanocomposites. J Mater Res Technol. 2020;9:4123–32.10.1016/j.jmrt.2020.02.039Suche in Google Scholar
[32] Abdollahi Boraei SB, Esmaeili Bidhendib M, Afzali D. Preparation of SiO2/ZrO2 ceramic nanocomposite coating on aluminum alloys as metallic part of the photovoltaic cells and study its corrosion behavior. Environ Energy Economic Res. 2017;1(2):231–8.Suche in Google Scholar
[33] Abdollahi B, Afzali D, Hassani Z. Corrosion inhibition properties of SiO2-ZrO2 nanocomposite coating on carbon steel 178. Anti-Corr Methods Mater. 2018;65:66–72.10.1108/ACMM-12-2015-1618Suche in Google Scholar
[34] Bayat H, Fasihi M, Zare Y, Rhee KY. An experimental study on one-step and two-step foaming of natural rubber/silica nanocomposites. Nanotechnol Rev. 2020;9(1):427–35.10.1515/ntrev-2020-0032Suche in Google Scholar
[35] Naghib SM, Behzad F, Rahmanian M, Zare Y, Rhee KY. A highly sensitive biosensor based on methacrylated graphene oxide-grafted polyaniline for ascorbic acid determination. Nanotechnol Rev. 2020;9(1):760–7.10.1515/ntrev-2020-0061Suche in Google Scholar
[36] Zare Y, Rhee KY. Development of Hashin-Shtrikman model to determine the roles and properties of interphases in clay/CaCO3/PP ternary nanocomposite. Appl Clay Sci. 2017;137:176–82.10.1016/j.clay.2016.12.033Suche in Google Scholar
[37] Zare Y. Modeling approach for tensile strength of interphase layers in polymer nanocomposites. J Colloid Interface Sci. 2016;471:89–93.10.1016/j.jcis.2016.03.029Suche in Google Scholar PubMed
[38] Abdollahi Boraei SB, Nourmohammadi J, Bakhshandeh B, Dehghan MM, Gonzalez Z, Ferrari B. The effect of protelos content on the physicochemical, mechanical and biological properties of gelatin-based scaffolds. J Appl Biotechnol Rep. 2020;7(1):41–7.Suche in Google Scholar
[39] Boraei SBA, Nourmohammadi J, Bakhshandeh B, Dehghan MM, Gholami H, Gonzalez Z, et al. Capability of core-sheath polyvinyl alcohol–polycaprolactone emulsion electrospun nanofibrous scaffolds in releasing strontium ranelate for bone regeneration. Biomed Mater. 2021;16(2):025009.10.1088/1748-605X/abdb07Suche in Google Scholar PubMed
[40] Moradi S, Yeganeh JK. Highly toughened poly (lactic acid)(PLA) prepared through melt blending with ethylene-co-vinyl acetate (EVA) copolymer and simultaneous addition of hydrophilic silica nanoparticles and block copolymer compatibilizer. Polym Test. 2020;91:106735.10.1016/j.polymertesting.2020.106735Suche in Google Scholar
[41] Lohrasbi P, Yeganeh JK. Synergistic toughening of poly (lactic acid)/poly (ethylene vinyl acetate)(PLA/EVA) by dynamic vulcanization and presence of hydrophobic nanoparticles. Polym Adv Technol. 2021;32(11):4326–39.10.1002/pat.5435Suche in Google Scholar
[42] Rashedi S, Afshar S, Rostami A, Ghazalian M, Nazockdast H. Co-electrospun poly (lactic acid)/gelatin nanofibrous scaffold prepared by a new solvent system: morphological, mechanical and in vitro degradability properties. Int J Polym Mater Polym Biomater. 2021;70(8):545–53.10.1080/00914037.2020.1740987Suche in Google Scholar
[43] Ghazalian M, Afshar S, Rostami A, Rashedi S, Bahrami SH. Fabrication and characterization of chitosan-polycaprolactone core-shell nanofibers containing tetracycline hydrochloride. Colloids Surf A Physicochem Eng Asp. 2022;636:128163.10.1016/j.colsurfa.2021.128163Suche in Google Scholar
[44] Tajdari A, Babaei A, Goudarzi A, Partovi R, Rostami A. Hybridization as an efficient strategy for enhancing the performance of polymer nanocomposites. Polym Compos. 2021;42(12):6801–15.10.1002/pc.26341Suche in Google Scholar
[45] Rostami A, Vahdati M, Alimoradi Y, Karimi M, Nazockdast H. Rheology provides insight into flow induced nano-structural breakdown and its recovery effect on crystallization of single and hybrid carbon nanofiller filled poly (lactic acid). Polymer. 2018;134:143–54.10.1016/j.polymer.2017.11.062Suche in Google Scholar
[46] Rostami A, Nazockdast H, Karimi M. Graphene induced microstructural changes of PLA/MWCNT biodegradable nanocomposites: rheological, morphological, thermal and electrical properties. RSC Adv. 2016;6(55):49747–59.10.1039/C6RA08345ESuche in Google Scholar
[47] Zare Y, Garmabi H, Rhee KY. Structural and phase separation characterization of poly (lactic acid)/poly (ethylene oxide)/carbon nanotube nanocomposites by rheological examinations. Compos Part B Eng. 2018;144:1–10.10.1016/j.compositesb.2018.02.024Suche in Google Scholar
[48] Gbureck U, Hölzel T, Klammert U, Würzler K, Müller FA, Barralet JE. Resorbable dicalcium phosphate bone substitutes prepared by 3D powder printing. Adv Funct Mater. 2007;17(18):3940–5.10.1002/adfm.200700019Suche in Google Scholar
[49] Igawa K, Mochizuki M, Sugimori O, Shimizu K, Yamazawa K, Kawaguchi H, et al. Tailor-made tricalcium phosphate bone implant directly fabricated by a three-dimensional ink-jet printer. J Artif Organs. 2006;9(4):234–40.10.1007/s10047-006-0347-ySuche in Google Scholar PubMed
[50] Butscher A, Bohner M, Roth C, Ernstberger A, Heuberger R, Doebelin N, et al. Printability of calcium phosphate powders for three-dimensional printing of tissue engineering scaffolds. Acta Biomater. 2012;8(1):373–85.10.1016/j.actbio.2011.08.027Suche in Google Scholar PubMed
[51] Vorndran E, Klammert U, Ewald A, Barralet JE, Gbureck U. Simultaneous immobilization of bioactives during 3D powder printing of bioceramic drug‐release matrices. Adv Funct Mater. 2010;20(10):1585–91.10.1002/adfm.200901759Suche in Google Scholar
[52] Nair BP, Sindhu M, Nair PD. Polycaprolactone-laponite composite scaffold releasing strontium ranelate for bone tissue engineering applications. Colloids Surf B Biointerf. 2016;143:423–30.10.1016/j.colsurfb.2016.03.033Suche in Google Scholar PubMed
[53] Zhao S, Zhang J, Zhu M, Zhang Y, Liu Z, Tao C, et al. Three-dimensional printed strontium-containing mesoporous bioactive glass scaffolds for repairing rat critical-sized calvarial defects. Acta Biomater. 2015;12:270–80.10.1016/j.actbio.2014.10.015Suche in Google Scholar PubMed
[54] Zhang W, Shi W, Wu S, Kuss M, Jiang X, Untrauer JB, et al. 3D printed composite scaffolds with dual small molecule delivery for mandibular bone regeneration. Biofabrication. 2020;12(3):035020.10.1088/1758-5090/ab906eSuche in Google Scholar PubMed PubMed Central
[55] Boraei SBA, Nourmohammadi J, Mahdavi FS, Yus J, Ferrandez-Montero A, Sanchez-Herencia AJ, et al. Effect of SrR delivery in the biomarkers of bone regeneration during the in vitro degradation of HNT/GN coatings prepared by EPD. Colloids Surf B Biointerf. 2020;190:110944.10.1016/j.colsurfb.2020.110944Suche in Google Scholar PubMed
[56] Zare Y, Rhee KY. The strengthening efficacy of filler/interphase network in polymer halloysite nanotubes system after mechanical percolation. J Mater Res Technol. 2021;15:5343–52.10.1016/j.jmrt.2021.10.116Suche in Google Scholar
[57] Zare Y, Rhee KY, Park S-J. An applicable model for the modulus of polymer halloysite nanotubes samples by the characteristics of halloysite nanotubes, interphase zone and filler/interphase network. Colloids Surf A Physicochem Eng Asp. 2021;628:127330.10.1016/j.colsurfa.2021.127330Suche in Google Scholar
[58] Zhang R, Li Y, He Y, Qin D. Preparation of iodopropynyl butycarbamate loaded halloysite and its anti-mildew activity. J Mater Res Technol. 2020;9(5):10148–56.10.1016/j.jmrt.2020.07.019Suche in Google Scholar
[59] Zhang M, Wang L, Yan H, Lian L, Si J, Long Z, et al. Palladium-halloysite nanocomposites as an efficient heterogeneous catalyst for acetylene hydrochlorination. J Mater Res Technol. 2021;13:2055–65.10.1016/j.jmrt.2021.06.006Suche in Google Scholar
[60] Satish S, Tharmavaram M, Rawtani D. Halloysite nanotubes as a nature’s boon for biomedical applications. Nanobiomedicine. 2019;6:1849543519863625.10.1177/1849543519863625Suche in Google Scholar PubMed PubMed Central
[61] Santos AC, Pereira I, Reis S, Veiga F, Saleh M, Lvov Y. Biomedical potential of clay nanotube formulations and their toxicity assessment. Expert Opin Drug Deliv. 2019;16(11):1169–82.10.1080/17425247.2019.1665020Suche in Google Scholar PubMed
[62] Danyliuk N, Tomaszewska J, Tatarchuk T. Halloysite nanotubes and halloysite-based composites for environmental and biomedical applications. J Mol Liq. 2020;309:113077.10.1016/j.molliq.2020.113077Suche in Google Scholar
[63] Zare Y, Rhee KY. Expansion of Takayanagi model by interphase characteristics and filler size to approximate the tensile modulus of halloysite-nanotube-filled system. J Mater Res Technol. 2021;16:1628–36.10.1016/j.jmrt.2021.12.082Suche in Google Scholar
[64] Zare Y, Rhee KY. A simple model for determining the strength of polymer halloysite nanotube systems. Compos Part B Eng. 2021;227:109411.10.1016/j.compositesb.2021.109411Suche in Google Scholar
[65] Ferrández-Montero A, Eguiluz A, Vazquez E, Guerrero JD, Gonzalez Z, Sanchez-Herencia AJ, et al. Controlled SrR delivery by the incorporation of Mg particles on biodegradable PLA-based composites. Polymers. 2021;13(7):1061.10.3390/polym13071061Suche in Google Scholar PubMed PubMed Central
[66] Ferrández-Montero A, Ferrari-Fernández B, Sánchez Herencia AJ, González Granados Z, González López FJ, Yus J, et al. Method for obtaining a piece by fused filament deposition modelling. Cynical Technologic de Materials; 2019;224:1–9.Suche in Google Scholar
[67] Ghaee A, Nourmohammadi J, Danesh P. Novel chitosan-sulfonated chitosan-polycaprolactone-calcium phosphate nanocomposite scaffold. Carbohydr Polym. 2017;157:695–703.10.1016/j.carbpol.2016.10.023Suche in Google Scholar PubMed
[68] Abdollahi Boraei SB, Nourmohammadi J, Bakhshandeh B, Dehghan MM, Gholami H, Calle Hernández D, et al. Enhanced osteogenesis of gelatin-halloysite nanocomposite scaffold mediated by loading strontium ranelate. Int J Polym Mater Polym Biomater. 2021;70(6):392–402.10.1080/00914037.2020.1725754Suche in Google Scholar
[69] Zare Y, Rhee KY. A simulation work for the influences of aggregation/agglomeration of clay layers on the tensile properties of nanocomposites. JOM. 2019;71:3989–95.10.1007/s11837-019-03768-2Suche in Google Scholar
[70] Alam F, Verma P, Mohammad W, Teo J, Varadarajan K, Kumar S. Architected poly (lactic acid)/poly (ε-caprolactone)/halloysite nanotube composite scaffolds enabled by 3D printing for biomedical applications. J Mater Sci. 2021;56:1–14.10.1007/s10853-021-06145-0Suche in Google Scholar
[71] Redondo FL, Giaroli MC, Ciolino AE, Ninago MD. Preparation of porous poly (lactic acid)/tricalcium phosphate composite scaffolds for tissue engineering. Biointerface Res Appl Chem; 2021;12:5610–24.10.33263/BRIAC124.56105624Suche in Google Scholar
[72] Mofokeng JP, Luyt A, Tábi T, Kovács J. Comparison of injection moulded, natural fibre-reinforced composites with PP and PLA as matrices. J Thermop Compos Mater. 2012;25(8):927–48.10.1177/0892705711423291Suche in Google Scholar
[73] Dong Y, Marshall J, Haroosh HJ, Mohammadzadehmoghadam S, Liu D, Qi X, et al. Polylactic acid (PLA)/halloysite nanotube (HNT) composite mats: Influence of HNT content and modification. Compos Part A Appl Sci Manuf. 2015;76:28–36.10.1016/j.compositesa.2015.05.011Suche in Google Scholar
[74] Hezma A, Abdelrazzak AB, El-Bahy GS. Preparation and spectroscopic investigations of hydroxyapatite-curcumin nanoparticles-loaded polylactic acid for biomedical application. Egypt J Basic Appl Sci. 2019;6(1):1–9.10.1080/2314808X.2019.1586358Suche in Google Scholar
[75] Divi MKP, Rao MAN, Nowshuddin S. Polymorph of strontium ranelate and a process for its preparation. Google Patents; 2011. US20110275834A1.Suche in Google Scholar
[76] Li X, Xie J, Yuan X, Xia Y. Coating electrospun poly (ε-caprolactone) fibers with gelatin and calcium phosphate and their use as biomimetic scaffolds for bone tissue engineering. Langmuir. 2008;24(24):14145–50.10.1021/la802984aSuche in Google Scholar PubMed
[77] Chuah YJ, Koh YT, Lim K, Menon NV, Wu Y, Kang Y. Simple surface engineering of polydimethylsiloxane with polydopamine for stabilized mesenchymal stem cell adhesion and multipotency. Sci Rep. 2015;5(1):1–12.10.1038/srep18162Suche in Google Scholar PubMed PubMed Central
[78] Zhao Y, Guo D, Hou S, Zhong H, Yan J, Zhang C, et al. Porous allograft bone scaffolds: doping with strontium. PLoS One. 2013;8(7):e69339.10.1371/journal.pone.0069339Suche in Google Scholar PubMed PubMed Central
[79] Su W-T, Wu P-S, Huang T-Y. Osteogenic differentiation of stem cells from human exfoliated deciduous teeth on poly (ε-caprolactone) nanofibers containing strontium phosphate. Mater Sci Eng C. 2015;46:427–34.10.1016/j.msec.2014.10.076Suche in Google Scholar PubMed
[80] Pilmane M, Salma-Ancane K, Loca D, Locs J, Berzina-Cimdina L. Strontium and strontium ranelate: historical review of some of their functions. Mater Sci Eng C. 2017;78:1222–30.10.1016/j.msec.2017.05.042Suche in Google Scholar PubMed
[81] Chen Y, Zheng Z, Zhou R, Zhang H, Chen C, Xiong Z, et al. Developing a strontium-releasing graphene oxide-/collagen-based organic–inorganic nanobiocomposite for large bone defect regeneration via MAPK signaling pathway. ACS Appl Mater Interfaces. 2019;11(17):15986–97.10.1021/acsami.8b22606Suche in Google Scholar PubMed
[82] Caverzasio J, Thouverey C. Activation of FGF receptors is a new mechanism by which strontium ranelate induces osteoblastic cell growth. Cell Physiol Biochem. 2011;27(3–4):243–50.10.1159/000327950Suche in Google Scholar PubMed
© 2022 Seyyed Behnam Abdollahi Boraei et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption

