Abstract
A new process for preparing vanadium by direct reduction of V2O5 from the Mg–V2O5 self-propagating system is proposed in this article. The reaction behavior and path of V2O5 in the magnesiothermic reduction process were investigated using the XRD, SEM-EDS, laser particle size analyzer, and specific surface area analyzer. The experimental results show that the reaction of the V2O5–Mg system is a solid-solid reaction, and the initial reaction temperature is 570°C. Although the formation of MgV2O4 spinel cannot be predicted via the calculation of thermodynamics, the presence of MgV2O4 spinel is of great significance to the V2O5 reduction process. Taking into account the characteristics of the gradual reduction of V2O5 by Mg and the appearance of the MgV2O4 spinel phase, the limiting link of the reaction may be the transition from MgV2O4 to V. A reduction path of V2O5 beyond the thermodynamic prediction is proposed: V2O5 → V3O5 → MgV2O4 → V. The reaction temperature and the phase transformation process can be effectively controlled by adjusting the ratio of reactants and additives, and element V can be obtained by a one-step rapid self-propagating reaction and breaking through the reaction restriction link. In this experiment, the vanadium powder with a porous structure, a specific surface area of 3.44 m2 g−1, and the oxygen content of 4.86 wt% were obtained.
1 Introduction
Vanadium is commonly used as an additive in the iron and steel industry, which can refine the microscopic grains of steel and improve the strength, toughness, and corrosion resistance of steel [1,2,3]. Vanadium is also used as a stable element of the β phase in titanium alloys, which significantly improves the ductility and plasticity of titanium alloys. In addition, vanadium is an important component of superconducting materials, battery materials, phosphors, catalysts, photosensitive materials, and hydrogen storage alloys [4,5,6,7]. Therefore, vanadium has become a strategic material that countries around the world are competing to reserve [8,9,10].
Metal V and Ti–V alloys were obtained by electrolyzing V2O3 in the CaO–CaCl2 molten salt system based on the principle of the “OS” method [11,12,13,14,15]. Wu et al. studied the electrochemical behavior during the electrolysis of V2O3 and achieved satisfactory results in controlling the current efficiency and energy consumption index [16]. Gussone et al. used the LiCl–KCl molten salt system to electrolyze VCl3 and TiCl2 to prepare Ti–V alloy [17]. Weng et al. electrolyzed NaVO3 from the CaCl2–NaCl molten salt to obtain V [18,19]. Cai et al. improved the FFC process, studied the electrochemical behavior of V2O5 in the CaCl2–NaCl electrolyte system, and found that V2O5 was first reduced to V3O5 and finally reduced to V [20,21,22,23]. However, the defect of low current efficiency still remained in this method. High-purity metallic vanadium is currently mainly prepared by reducing vanadium chloride with Mg. This idea is derived from the Kroll method to produce sponge titanium, but the inherent problems of the Kroll method such as high energy consumption and high pollution still need to be overcome [24,25]. Inazu et al. [26] proposed a new idea on the production of V, that is, V2O5 and MgO were sintered to obtain the MgV2O4 precursor, and then the MgV2O4 precursor was reduced by the Mg steam in a microwave field to obtain V. In the decomposition method, VN is first obtained by reducing V2O3/V2O5 with carbon/magnesium in nitrogen, and then VN is decomposed at high temperature to obtain the metal V [27,28,29]. In the silicothermic method, V2O5 is reduced with Si to obtain the primary product, and then high-purity metal V is produced by the deep deoxidation of molten salt electrolysis [30]. Xu used V2O3 as raw material and CaCl2 as a pore-forming agent, and reduced V2O3 with Ca steam under vacuum to obtain metal V[31]. Lee et al. used hydrogen to reduce V2O5 to obtain V2O3 and then reduced V2O3 at 1,073 K for 48 h with magnesium to obtain metal V [32].
Although metallic vanadium was successfully prepared in the above processes, the intermediate such as VCl x , MgV2O4, V2O3, or VN were also produced in the preparation process, and the research on the preparation of metal V using a direct metal thermal reduction is seldom reported. V2O5 is the most stable and common in vanadium-containing compounds, and the preparation process of V2O5 is very mature [33,34]. Therefore, the preparation of the vanadium metal by direct reduction of V2O5 has great application prospects. At present, the preparation of metals and their alloys by direct reduction methods of metal high-valent oxides has gradually matured. For example, Ti, TiAlV, etc. have been successfully prepared by using TiO2 as raw material and using magnesium thermal self-propagation [35,36].
2 Experimental
In this study, the raw materials were chosen with chemical reagents of vanadium pentoxide (V2O5, >99%, Macklin, China), magnesium powder (Mg, >99%, Sinopharm, China), sodium chloride (NaCl, >99%, Sinopharm, China), and hydrochloric acid (HCl, 36%, Sinopharm, China). The ratio of raw materials is listed in Table 1. The raw materials were weighed precisely and mixed thoroughly according to the ingredient ratio shown in Table 1, and then pressed into a cylindrical parison sample. The sample was placed in a self-propagating reactor and performed a self-propagating reaction in a vacuum to obtain a self-propagating reaction product. The reaction product was taken out and pulverized, and then soaked in a dilute hydrochloric acid solution for 3 h. After soaking, the solution was filtered. The filtered product was washed to neutrality, and finally, dried in a vacuum to obtain the reduced product.
The ratio of raw materials
| No. | Molar ratio (V2O5:Mg:NaCl) | Raw materials (g) | ||
|---|---|---|---|---|
| V2O5 | Mg | NaCl | ||
| 1# | 1:1:– | 36.4 | 4.8 | 0 |
| 2# | 1:2:– | 36.4 | 9.6 | 0 |
| 3# | 1:3:– | 36.4 | 14.4 | 0 |
| 4# | 1:4:– | 36.4 | 19.2 | 0 |
| 5# | 1:5:– | 36.4 | 24 | 0 |
| 6# | 1:8:3.5 | 36.4 | 38.4 | 41 |
| 7# | 1:8:– | 36.4 | 38.4 | 0 |
| 8# | 1:10:– | 36.4 | 48 | 0 |
–: not detected.
The product phase was analyzed by X-ray diffraction (XRD, copper target, Bruke, D8, Germany). The micromorphology of the product was characterized by field emission scanning electron microscopy (SEM-EDS, Hitachi, su8010, Japan). The specific surface area of the product was obtained using a specific surface area analyzer (ASAP2020 m, U.S.A.). The oxygen content of the product was detected with an oxygen nitrogen hydrogen analyzer (LECO onh836). The particle size distribution of the product was characterized with a laser particle size analyzer (Mastersizer, 2000, UK).
3 Results and discussion
3.1 Analysis of the reaction kinetics of the V2O5–Mg system
The TG–DTA curve of the V2O5–Mg system is presented in Figure 1(a). A sharp exothermic peak appeared near 570°C on the DTA curve, indicating that the reduction reaction was initiated at low temperatures, and the sharp exothermic peak indicated that the reaction was rapid and violent. Simultaneously, the obvious weight loss occurred near 570°C in the TG curve, which may be caused by splashing during the violent reaction and the volatilization loss of the metal magnesium in a high-temperature environment. The melting point of Mg is 651°C and that of vanadium pentoxide is 690°C, but the actual reaction temperature was around 570°C, which suggested that the self-propagating reaction was a solid-solid reaction. The DTA curve shown in Figure 1(a) was analyzed using the Freeman–Carroll differential method [37]. It can be seen that the reaction order n = 0.14 and the apparent activation energy E = 1816.227 kJ mol−1.
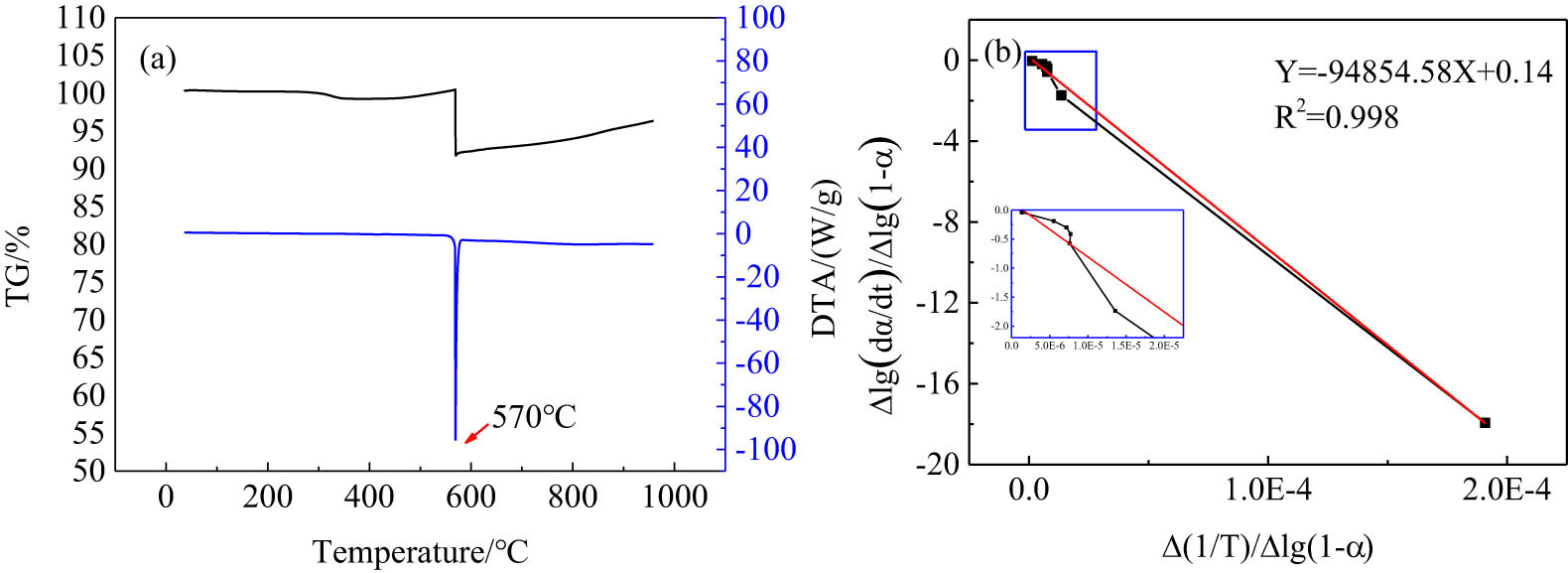
(a) TG–DTA curve of the V2O5–Mg system. (b) The fitting curve of the relationship between Δlg(dα/dt)/Δlg(1 − α) − Δ(1/T)/Δlg(1 − α) when the heating rate is 10°C min−1.
3.2 Evolution of the theoretical equilibrium phase during the reduction reaction
Based on the minimum Gibbs free energy principle, the equilibrium phase evolution law of the Mg–V2O5 system with different molar ratios of Mg/V2O5 at different temperatures was calculated, as shown in Figure 2. When the molar ratio of Mg/V2O5 (replaced by Mg:V2O5) was 1:1 (Figure 2a), the Mg2V2O7 composite oxide phase was formed in the reduction process, and there were also a large number of intermediate oxides and Magne’li phases such as VO2, V2O3, and V3O5 in the system. When the Mg:V2O5 ratio was 2:1 (Figure 2b), in the reduction process, the Mg2V2O7 compound disappeared, the content of V2O4 decreased, and the contents of V2O3 and VO increased, indicating that the degree of reduction of V2O5 increased. When the Mg:V2O5 ratio was 3:1 (Figure 2c), only two phases MgO and VO existed in the reduction products. When the Mg:V2O5 ratio reached 4:1 (Figure 2d), metal V appeared in the reduction products, and a large amount of VO and unreacted Mg also existed. Especially with the increase of the reaction temperature, the content of unreacted Mg and VO increased, even if the Mg:V2O5 ratio exceeded 5:1 to 10:1 (Figure 2d–g); there was still an unreduced VO phase in the products. This change rule of the equilibrium phase with the temperature showed that high temperatures were not conducive to completing the reduction reaction thoroughly, which was consistent with the strong exothermic thermodynamic properties of the self-propagating reaction system; in other words, increasing the amount of the reducing agent cannot promote the complete reduction reaction. It can be theoretically speculated that the composite oxide phase may be formed in the self-propagating rapid reaction process, and then the composite oxide phase was reduced. Therefore, the phase evolution path during the reduction process was V2O5 → Mg2V2O7 → V2O4 → V2O3 → VO → V.
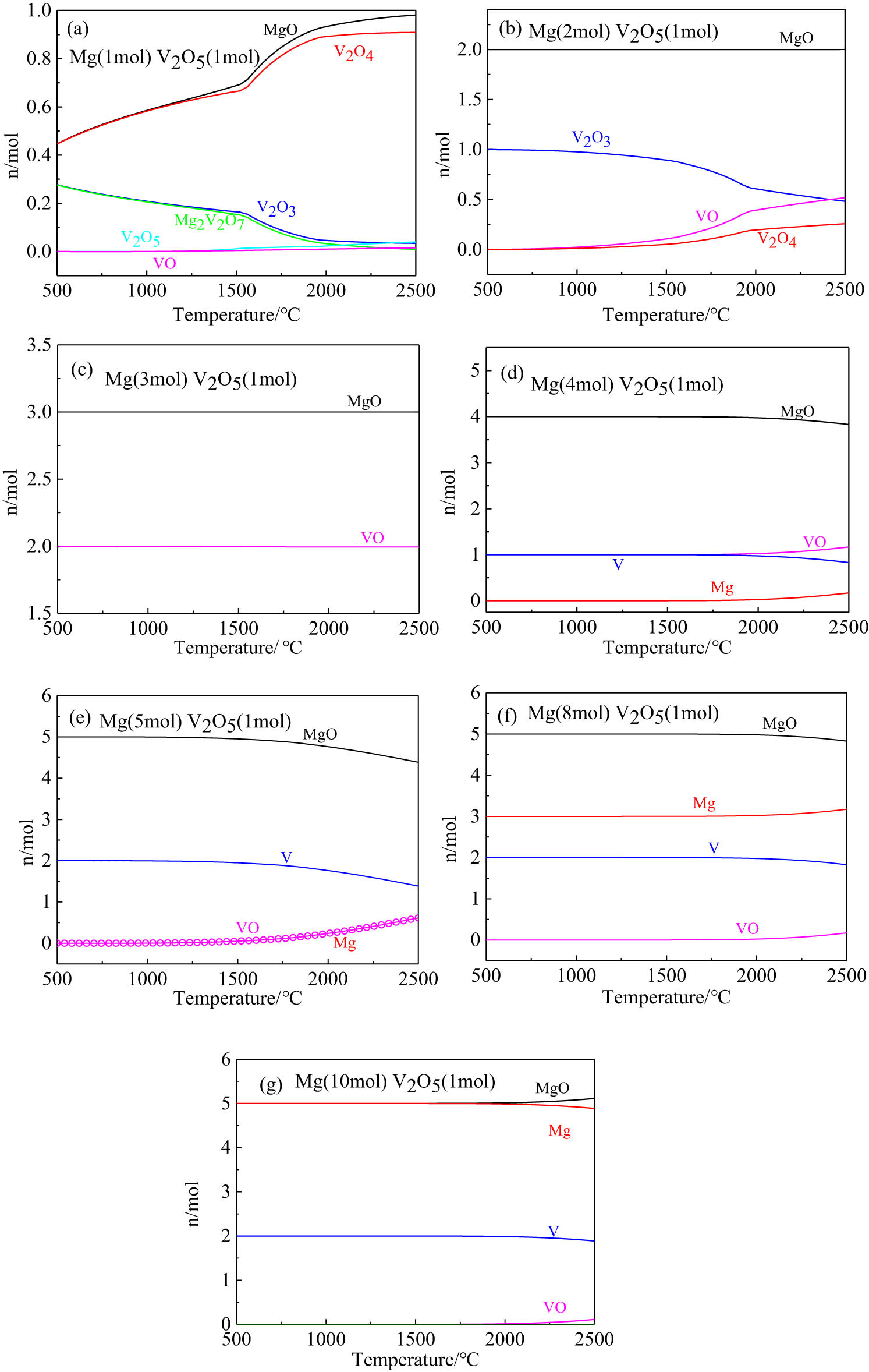
Evolution of equilibrium phases in different systems: (a) Mg:V2O5 = 1:1; (b) Mg:V2O5 = 2:1; (c) Mg:V2O5 = 3:1; (d) Mg:V2O5 = 4:1; (e) Mg:V2O5 = 5:1; (f) Mg:V2O5 = 8:1; and (g) Mg:V2O5 = 10:1.
3.3 Product phase analysis
Figure 3(a) shows the XRD pattern of the self-propagating product before acid leaching. When the Mg:V2O5 ratio was 1:1 (1#), the diffraction peaks of Mg2V2O7, MgO, V3O5, and MgV2O4 phases emerged. When the Mg:V2O5 molar ratios increased to 2, 3, and 4 (2#, 3#, and 4#), the phases of the reaction products were only MgV2O4 and MgO, indicating that the actual self-propagating reaction was in the thermodynamical nonequilibrium and free escape system state, and there was a certain lag in the evolution of phases in the actual reaction process. When the ratio of Mg:V2O5 was 8:1 (7#), the diffraction peak intensity of MgV2O4 decreased, and the diffraction peak of V appeared. After adding NaCl, the diffraction peak of MgV2O4 basically disappeared (6#). When the Mg:V2O5 ratio was 10:1 (8#), the diffraction peak of MgV2O4 also disappeared. The XRD pattern of the pickled product is shown in Figure 3(b). It can be seen that after acid leaching, the by-product phases such as NaCl and MgO in the self-propagating reaction products could be effectively removed, but the by-product phases such as Mg2V2O7 and MgV2O4 could not be removed; when the Mg:V2O5 ratio was 8:1 (6#) and 10:1 (8#), only the elemental V phase existed, indicating that pure metal V could be prepared by adjusting the material ratio of the self-propagating reaction experiment. The XRD analysis results showed that the evolution path of the product phase in the self-propagating reaction of the Mg–V2O5 process was V2O5 → V3O5 → MgV2O4 → V, which was somewhat different from the thermodynamic equilibrium prediction.
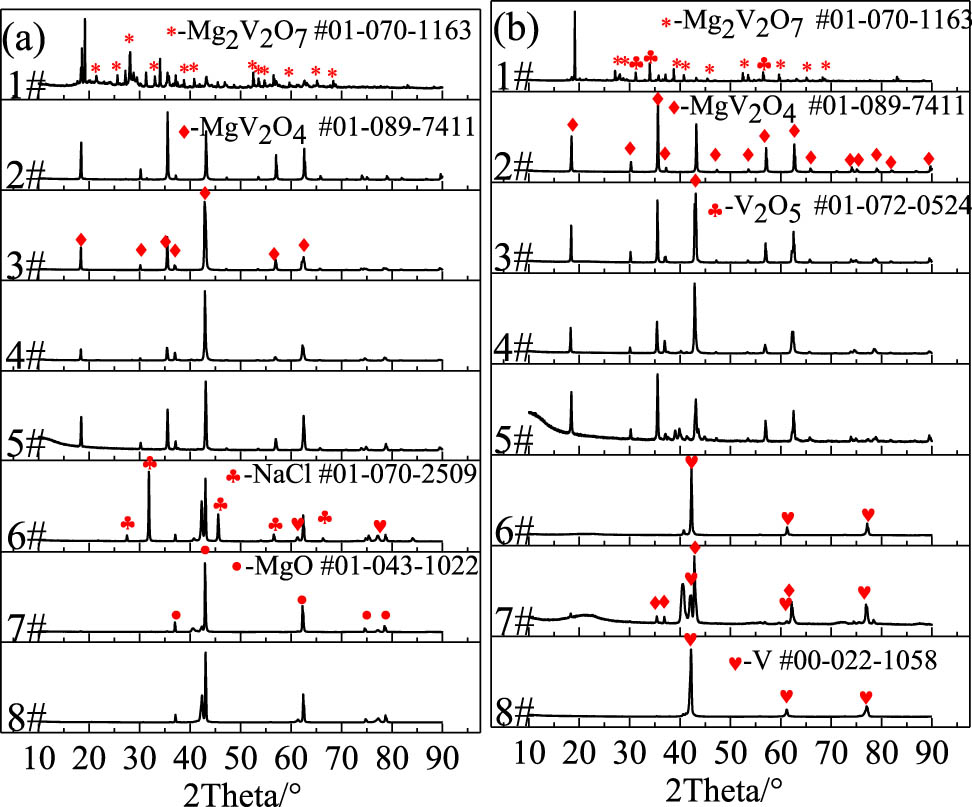
XRD pattern of the products: (a) before acid leaching and (b) after acid leaching.
Table 2 shows the chemical composition analysis results of the products after pickling. As can be seen, when Mg:V2O5 ratios were 8:1 and 10:1 (6# and 8#), the mass fraction of oxygen in the metal vanadium was about 5%. When the Mg:V2O5 molar ratios were 2, 3, and 4 (2#, 3#, and 4#), although the acid leaching product phase was only MgV2O4, the contents of Mg and V in products were significantly different. The three main diffraction peaks corresponding to the XRD pattern in the acid leaching product were analyzed, as shown in Table 3. It can be seen from Table 3 that with the increase of the Mg content in the raw material ratio, the diffraction peak of the products tended to shift to a low angle, the intensity of diffraction peaks near 35 and 18° decreased, and the intensity of diffraction peaks near 43° increased. This may be related to the atomic ratio of Mg and V.
Product composition analyses after acid leaching, mass %
| Sample number | O | Mg | V |
|---|---|---|---|
| 1# | Bal. | 7.36 | 52.73 |
| 2# | Bal. | 15.19 | 44.24 |
| 3# | Bal. | 20.44 | 45.97 |
| 4# | Bal. | 24.82 | 47.11 |
| 5# | Bal. | 25.2 | 50.2 |
| 6# | 5.02 | 0.68 | Bal. |
| 7# | Bal. | 9.52 | 78.59 |
| 8# | 4.86 | 0.46 | Bal. |
Diffraction peak analyses of acid leaching products
| No. 1 | No. 2 | No. 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pos. | FWHM left | Area | Height | Pos. | FWHM left | Area | Height | Pos. | FWHM left | Area | Height | |
| 5# | 42.88 | 0.137 | 2,728 | 14,955 | 35.34 | 0.114 | 685 | 4,509 | 18.3 | 0.114 | 675 | 4,454 |
| 4# | 42.88 | 0.137 | 2,736 | 1,500 | 35.51 | 0.114 | 2,316 | 15,240 | 18.4 | 0.114 | 1,132 | 7,446 |
| 3# | 43.18 | 0.093 | 1,525 | 11,021 | 35.58 | 0.13 | 3,170 | 16,370 | 18.5 | 0.15 | 1,843 | 8,326 |
3.4 Product morphology analysis
From the macro morphology photo of the self-propagating reaction product in Figure 4, it can be seen that when Mg:V2O5 was less than 5:1 (1#, 2#, 3#, 4#, and 5#), the volume of the self-propagating reaction product shrank significantly. When Mg:V2O5 was greater than 8:1 (6#, 7#, and 8#), the volume of the self-propagating reaction product expanded significantly.

The macro morphology of the samples before and after the reaction.
Figures 5 and 6 present the SEM images of products before and after pickling, respectively. Table 4 shows the EDS results of the products in Figures 5 and 6. It can be seen that the morphologies of 2# and 4# products before pickling (Figure 5) and after pickling (Figure 6) were basically the same, and they were both dense blocks, and the EDS results (Table 4) indicated that a large amount of Mg element still remained in the 2# and 4# products after pickling, which was consistent with the XRD pattern analyses in Figure 3. The structure of the 5# product was loose after pickling, which was obviously different from the 2# and 4# products (Figure 6). The surface of the 8# product was dense before pickling, while the surface of the 6# product was attached with small particles (NaCl) before pickling (Figure 5). After pickling, both the 8# and 6# products were elementary V, proved by EDS analysis (Table 4), and exhibited porous morphology, which was obviously different from other products (Figure 6). These holes were divided into two types, one was the closing hole formed by interconnecting adjacent metal vanadium particles and the other was the tunnel-type hole formed by a hole and a skeleton together. In addition, it can be seen from the comparison of the morphology of products after pickling (Figure 6) that there were more sintered necks in the 8# product after pickling, indicating that the reaction temperature of the 8# product was higher than that of the 6# product; as the Mg content in the reaction system increased, the structure of the product gradually changed from dense blocky to porous after pickling. When the molar ratio of Mg and V2O5 was low (such as 2# and 4#), Mg was wrapped by liquid V2O5, solid MgO was formed by combining Mg with O2−, and then the composite MgV2O4 was formed by combining solid MgO with low-valence V oxide. After the reaction, the volume shrank. As the Mg:V2O5 molar ratio increased, the amount of MgO produced after the reaction also gradually increased, and the degree of reduction of V2O5 also gradually increased, but part of the Mg escaped in gaseous form during the reaction. When enough Mg content was involved in the reaction, V2O5 would be wrapped by liquid Mg during the reaction, and then O2− would be continuously transferred from V2O5 to liquid Mg to produce MgO. With the decrease of O atoms in V2O5, the volume of V2O5 continued to shrink, liquid and gaseous Mg would enter the gap of the raw material under the action of the capillary force, and the removal of MgO after pickling would leave a porous morphology (such as 6# and 8#). However, MgO combined with the low-valent vanadium oxide cannot be removed by pickling. Therefore, the 2# and 4# products after pickling were dense and blocky, and the 5# and 7# products showed a “transition from dense blocky to porous” morphology.
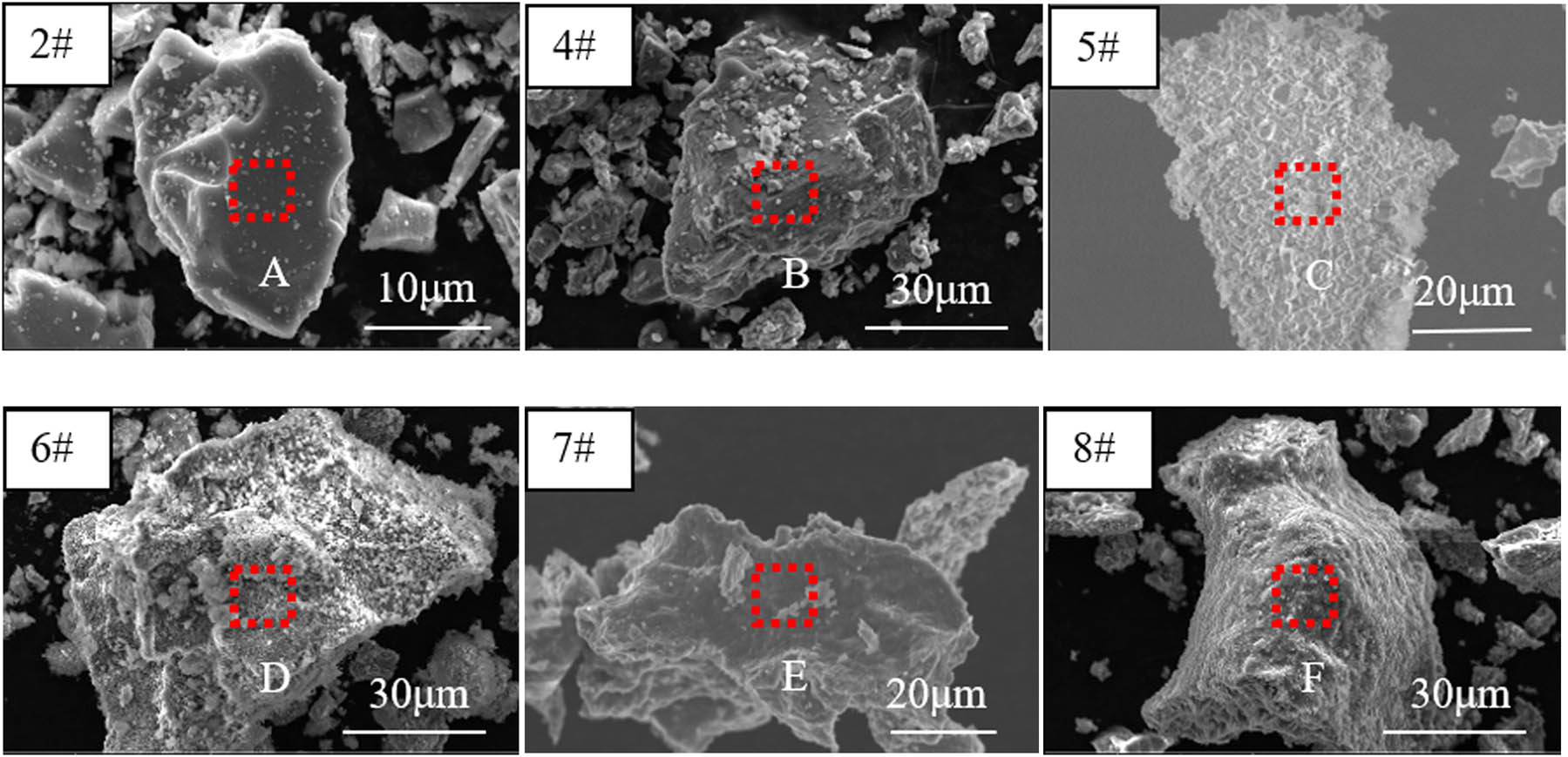
Microstructure of products before pickling.

Microstructure of products after pickling.
EDS analysis results of different regions of the product
| Element (at%) | |||||
|---|---|---|---|---|---|
| V | Mg | O | Na | Cl | |
| A | 28.2 | 27.99 | 43.81 | — | — |
| B | 14.68 | 35.25 | 50.06 | — | — |
| C | 18.22 | 32.40 | 49.37 | ||
| D | 3.04 | 40.51 | 55.48 | 0.49 | 0.46 |
| E | 9.07 | 53.28 | 37.65 | — | — |
| F | 0.78 | 41.07 | 58.15 | — | — |
| G | 41.28 | 19.43 | 39.29 | — | — |
| H | 27.74 | 32.6 | 39.66 | — | — |
| I | 22.57 | 32.43 | 44 | ||
| J | 100 | — | — | — | — |
| K | 39.91 | 17.68 | 39.91 | — | — |
| L | 100 | — | — | — | — |
3.5 Product particle size and specific surface area analysis
Because 1#–5# and 7# products were relatively hard and difficult to break, only the 6# and 8# products were analyzed for the particle size. Figure 7 shows the particle size distribution curves of 6# and 8# products after pickling. Compared with the particle size distribution curve of 8# product after pickling, the particle size distribution curve of 6# product after pickling was obviously shifted to the left, indicating that the particle size of 6# product after pickling was smaller than that of 8# product. Table 5 shows the particle size characteristics of the 6# and 8# products after pickling. The characteristic values of particle size D10, D50, D90, and D [3,4] of the 6# product after pickling were 7.63, 31.69, 73.23, and 36.99 µm, respectively, which were all smaller than the those of 8# product.
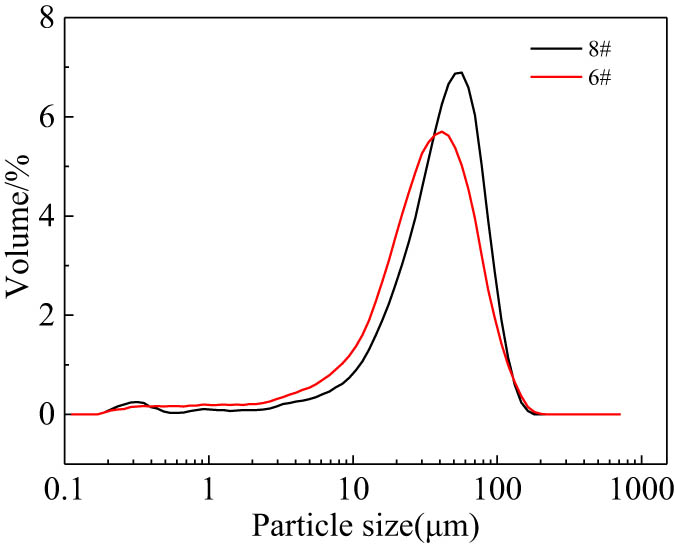
Particle size distribution curve of 6# and 8# samples.
Particle size characteristic values of 6# and 8# samples
| 8# | 6# | |
|---|---|---|
| D10/µm | 12.22 | 7.63 |
| D50/µm | 40.48 | 31.69 |
| D90/µm | 79.21 | 73.23 |
| D[4,3]/µm | 43.61 | 36.99 |
Figure 8 are the adsorption–desorption curves of 6# and 8# products after pickling. According to the IUPAC classification standard, the adsorption–desorption curves conformed to the characteristics of the type II adsorption isotherm. An obvious inflection point occurred in the low-pressure section of the adsorption curve, suggesting that the single-layer adsorption was completed at this time. Then, multilayer adsorption occurred as the partial pressure increased. Obvious hysteresis appeared in the high-pressure section of the desorption curve because of the occurrence of capillary condensation between the particles and in the macropores. The analysis results of the specific surface area, pore volume, and average pore diameter of the 6# and 8# products after pickling are listed in Table 6. It can be seen that the specific surface areas of the 6# and 8# products were 2.01 and 3.44 m2 g−1, respectively. The 8# product had a larger specific surface area and pore volume, which was related to the Mg content in the 8# system and temperature. The higher reaction temperature caused the vaporization of metal Mg, and one part of the gasification Mg escaped from the reaction system, and the other part entered the gap of the raw material to participate in the reduction reaction of V2O5 to form metal V. The reduced metal V was sintered together under the action of high temperatures. Therefore, as more Mg was vaporized, the content of gasification Mg that participated in the reduction reaction of V2O5 increased, which led to an increase in the specific surface area and pore volume of the product after pickling. In the 6# product, NaCl did not participate in the reduction reaction of V2O5 but acted as “pore former,” resulting in larger pore size of 6# product compared with that of the 8# product.
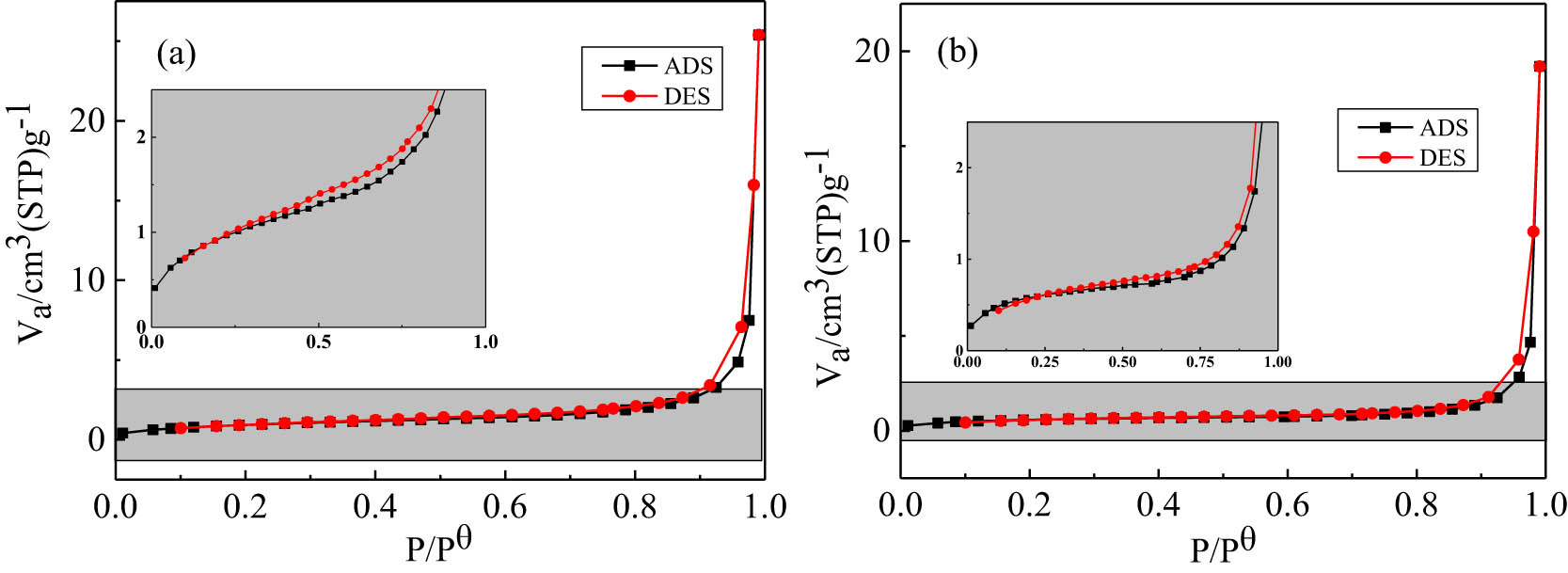
The adsorption–desorption curves of the products after pickling (a) 8# and (b) 6#.
BET analysis test results of products after pickling
| Sample | Specific surface area/m2 g−1 | Pore volume/cm3 g−1 | Average pore size/nm |
|---|---|---|---|
| 8# | 3.44 | 0.039 | 45.718 |
| 6# | 2.01 | 0.030 | 59.056 |
3.6 Reaction mechanism analysis
Figure 9(a) shows the temperature–time curves during the reaction of 6# and 8# samples, and the illustration on the lower right of Figure 9(a) is a schematic diagram of the temperature measurement. It can be seen that the maximum combustion temperature and combustion rate of the 8# reaction system were higher than those of the 6# reaction system. The highest reaction temperature of both 6# and 8# reaction systems was much higher than the boiling point of Mg and slightly higher than the boiling point of NaCl, but lower than the melting point of metal V. Therefore, in the XRD analyses of the products before pickling, the phase diffraction peak of NaCl was present but no phase diffraction peak of Mg. The self-propagating reaction of the V2O5–Mg system relied on the flow of liquid/gas Mg and the transfer of heat. During the reaction, NaCl did not participate in the reaction but only played a role in reducing the adiabatic temperature. Since there was no NaCl in the 8# system and the heat release of the system was larger, the production rate of liquid/gas Mg of 8# system was faster than that of the 6# system, resulting in the higher combustion rate of the 8# reaction system than that of the 6# reaction system. The adiabatic temperature of systems with different Mg and NaCl contents is shown in Figure 9(b). It indicated that NaCl or excessive Mg could reduce the adiabatic temperature of the system as a diluent, and the dilution capacity of NaCl was larger than that of Mg under the same quantity condition. It was assumed that all the V2O5 in the raw materials were converted into the target product V in the process of calculating the adiabatic temperature, that is, no secondary reactions occurred, but only the experimental results of 8# and 6# systems were close to the ideal process in the present research. The adiabatic temperatures of the 8# and 6# systems were 1714.7°C and 1490.6°C, respectively. The highest temperature of 8# and 6# samples during the reaction (Figure 9a) was lower than the adiabatic temperature. This is because the system dissipated heat as it reacted, and there was dissolved oxygen with a mass fraction of 5% in element V obtained by the reduction reaction, which means that the reaction did not proceed completely. The changing trend of the calculated system reaction temperature in Figure 9(b) was basically consistent with the measured temperature in Figure 9(a), suggesting that the addition of NaCl could reduce the adiabatic temperature and the loss of Mg, so as to save the reducing agent. There was no active addition of MgO in this study, and MgV2O4 appeared when the compound of V2O5 and MgO were heated in the air, so the MgV2O4 in this experiment was likely to come from the reduction of V2O5 [26]. The chemical reactions that may occur in this process are shown in formulas (1)–(3).
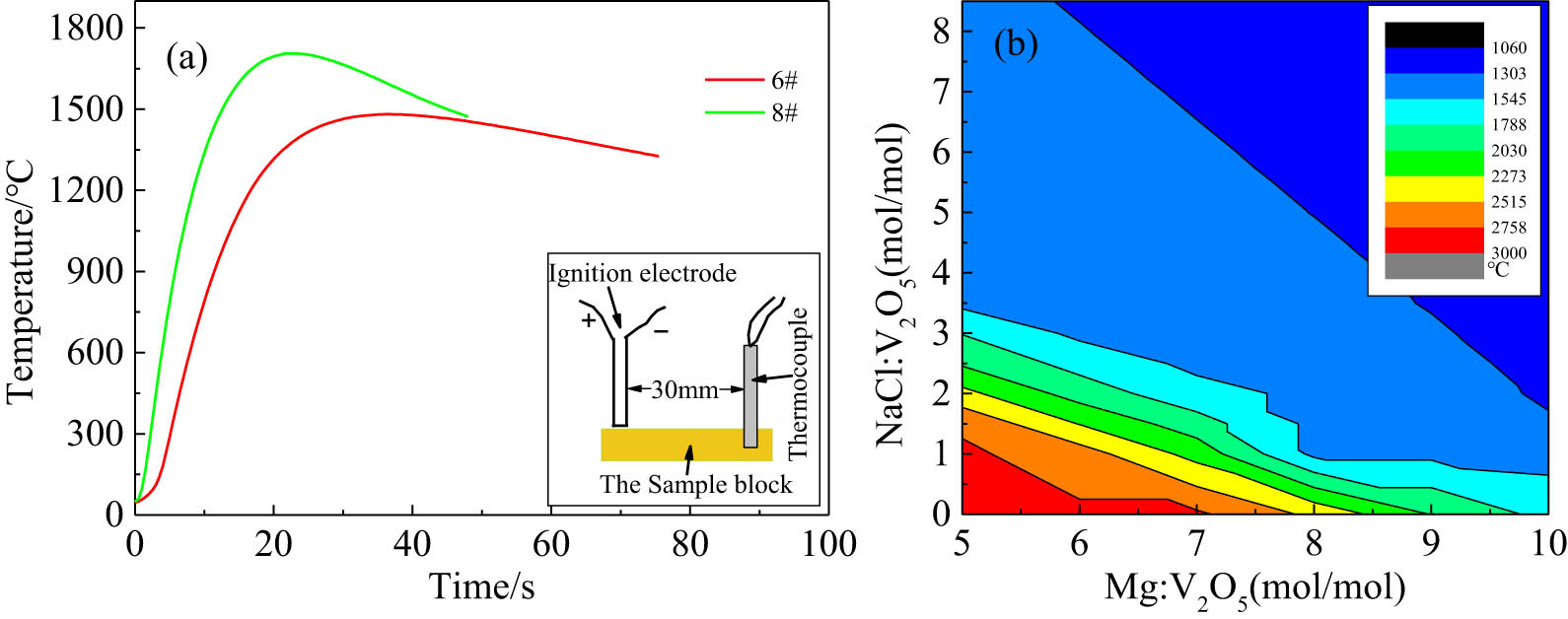
(a) Time–temperature curve of 6# and 8# samples. (b) The adiabatic temperature of different Mg and NaCl content systems.
4 Conclusion
In this article, V2O5, Mg, and NaCl were used as raw materials, and ultrafine metal V powder was successfully prepared by the magnesiothermic self-propagating reaction method. The conclusions are as follows:
The TG–DTA analysis showed that the reaction temperature of the V2O5–Mg system was around 570°C, so the self-propagating reaction of the V2O5–Mg system was a solid–solid reaction. The reaction order n and the apparent activation energy E calculated by the Freeman–Carroll differential method were 0.14 and 1816.227 kJ mol−1, respectively.
The change path of phase during the reaction was V2O5 → V3O5 → MgV2O4 → V. The formation of the complex MgV2O4 was related to the content of Mg involved in the reaction. MgV2O4 was formed in the case of insufficient Mg content, and MgV2O4 cannot be removed by pickling. With the increase of the Mg content involved in the reaction, the morphology of the product after pickling gradually changed from blocky to porous.
The temperature–time curve showed that it was feasible to control the reaction temperature of the system by adding NaCl, and the addition of NaCl could achieve the purpose of reducing the reaction temperature and saving the reducing agent. The finally obtained metal V powder containing oxygen with a mass fraction of 4.86 had a porous network structure with a specific surface area of 3.44 m2 g−1 and an average pore diameter of 45.718 nm.
-
Funding information: The authors acknowledge funding from the National Natural Science Foundation of China (U1908225, U1702253) and Fundamental Research Funds for the Central Universities (N182515007, N170908001, N2025004).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Wang K, Yu HY, He JC, Liu CM. Influence of cooling rate on microstructure evolution due to deformation induced ferrite transformation in vanadium microalloyed steel. J Northeast Univ Nat Sci. 2009;30(12):1739–42.Suche in Google Scholar
[2] Yang CF. Recent development and applications of vanadium microalloying technology. J Iron Steel Res Int. 2020;12:1029–43.Suche in Google Scholar
[3] Zhang Y, Zhang TA, Dreisinger D, Lv GZ, Zhang GQ, Zhang WG, et al. Extraction of vanadium from direct acid leach solution of converter vanadium slag. Can Metall Q. 2017;56(3):281–93.10.1080/00084433.2017.1327501Suche in Google Scholar
[4] Kocyigit N, Gencten M, Sahin M, Sahin Y. A novel vanadium/cobalt redox couple in aqueous acidic solution for redox flow batteries. Int J Energy Res. 2020;44(1):411–24.10.1002/er.4938Suche in Google Scholar
[5] Niitaka S, Ohsumi H, Sugimoto K, Lee S, Oshima Y, Kato K, et al. A-type antiferro-orbital ordering with I4(1)/a symmetry and geometrical frustration in the spinel vanadate MgV2O4. Phys Rev Lett. 2013;111(26):267201.10.1103/PhysRevLett.111.267201Suche in Google Scholar
[6] Mamiya H, Onoda M. Electronic states of vanadium spinels MgV2O4 and ZnV2O4. Solid State Commun. 1995;95(4):217–21.10.1016/0038-1098(95)00253-7Suche in Google Scholar
[7] Tang W, Lan B, Tang C, An Q, Chen L, Zhang W, et al. Urchin-like spinel MgV2O4 as a cathode material for aqueous zinc-ion battery. ACS Sustain Chem Eng. 2020;8(9):3681–8.10.1021/acssuschemeng.9b06613Suche in Google Scholar
[8] Huang W, Shan Y, Xiang S, Yu S, Shen X, Xu L, et al. Kinetic study on the oxidation of elements in hot metal during vanadium-extraction process. Steel Res Int. 2016;87(9):1228–37.10.1002/srin.201500333Suche in Google Scholar
[9] Mu W, Zhang TA, Dou ZH, Zhang T, Dou Z, Lü G, et al. φ-pH diagram of V-Ti-H2O system during pressure acid leaching of converter slag containing vanadium and titanium. T Nneferr Metal Soc. 2011;21(9):2078–86.10.1016/S1003-6326(11)60976-XSuche in Google Scholar
[10] Qu JW, Zhang TA, Niu LP, Lv GZ, Zhang WG, Chen Y. Technology progress in comprehensive utilization of vanadium-chromium reducing slag. Nonferr Metal. 2020;1:79–83.Suche in Google Scholar
[11] Suzuki RO, Ono K, Teranuma K. Calciothermic reduction of titanium oxide and in-situ electrolysis in molten CaCl2. Metall Mater Trans B. 2003;34(3):287–95.10.1007/s11663-003-0074-1Suche in Google Scholar
[12] Ono K, Suzuki RO. A new concept for producing Ti sponge: calciothermic reduction. JOM. 2002;54(2):59–61.10.1007/BF02701078Suche in Google Scholar
[13] Ono K, Okabe TH, Suzuki RO. Design, test and theoretical assessments for reduction of titanium oxide to produce titanium in molten salt. Mater Trans. 2017;58(3):313–8.10.2320/matertrans.MK201604Suche in Google Scholar
[14] Suzuki RO, Ishikawa H, Ishikawa H. Direct reduction of vanadium oxide in molten CaCl2. ECS Trans. 2008;3(35):347–56.10.1149/1.2798678Suche in Google Scholar
[15] Suzuki RO, Tatemoto K, Kitagawa H. Direct synthesis of the hydrogen storage V–Ti alloy powder from the oxides by calcium co-reduction. J Alloy Compd. 2004;385(1–2):173–80.10.1016/j.jallcom.2004.04.137Suche in Google Scholar
[16] Wu T, Ma X, Jin X. Preparation of vanadium powder and vanadium–titanium alloys by the electroreduction of V2O3 and TiO2 powders. J Mater Res. 2016;31(3):405–17.10.1557/jmr.2016.18Suche in Google Scholar
[17] Gussone J, Vijay CRY, Watermeyer P, Vijay CRY, Watermeyer P, Milicevic K, et al. Electrodeposition of titanium–vanadium alloys from chloride-based molten salts: influence of electrolyte chemistry and deposition potential on composition, morphology and microstructure. J Appl Electrochem. 2020;50(3):355–66.10.1007/s10800-019-01385-0Suche in Google Scholar
[18] Weng W, Wang M, Gong X, Wang M, Gong X, Wang Z, et al. One-step electrochemical preparation of metallic vanadium from sodium metavanadate in molten chlorides. Int J Refract Met Hard Mater. 2016;55:47–53.10.1016/j.ijrmhm.2015.11.007Suche in Google Scholar
[19] Weng W, Wang M, Gong X, Wang M, Gong X, Wang Z, et al. Thermodynamic analysis on the direct preparation of metallic vanadium from NaVO3 by molten salt electrolysis. Chin J Chem Eng. 2016;24(5):671–6.10.1016/j.cjche.2016.01.006Suche in Google Scholar
[20] Chen GZ, Fray DJ, Farthing TW. Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride. Chem Inf. 2010;32(2):361–4.10.1038/35030069Suche in Google Scholar PubMed
[21] Chen GZ, Fray DJ. A morphological study of the FFC chromium and titanium powders. Miner Process Extr Metall. 2006;115(1):49–54.10.1179/174328506X91365Suche in Google Scholar
[22] Tang SB, Li Z, Peng H, Xing PF, Luo Z. Preparation and property of TiO2 electrode for production of titanium sponge by FFC process. Dev Appl Mater. 2009;24(04):18–20.Suche in Google Scholar
[23] Cai Z, Zhang ZM, Guo ZC, Zhang Z, Guo Z, Tang H. Direct electrochemical reduction of solid vanadium oxide to metal vanadium at low temperature in molten CaCl2-NaCl. Int J Miner Metall Mater. 2012;19(6):499–505.10.1007/s12613-012-0586-2Suche in Google Scholar
[24] Varhegyi G, Fekete I, Sandor I. Morphological investigation on a vanadium product of the magnesiothermic reduction. Spine. 1971;35(18):E904–7.Suche in Google Scholar
[25] Campbell TT, Schaller JL, Block FE. Preparation of high-purity vanadium by magnesium reduction of vanadium dichloride. Metall Trans. 1973;4(1):237–41.10.1007/BF02649623Suche in Google Scholar
[26] Inazu N, Suzuki E, Fujii S, Suzuki E, Fujii S, Tsubaki S, et al. A facile formation of vanadium(0) by the reduction of vanadium pentoxide pelletized with magnesium oxide enabled by microwave irradiation. Chemistry Select. 2020;5(10):2949–53.10.1002/slct.201904547Suche in Google Scholar
[27] Rui XU, Wu YD, Zhang GH. Preparation of high purity vanadium nitride by magnesiothermic reduction of V2O3 followed by nitriding in N2 atmosphere. Trans Nonferrous Met Soc China. 2019;29(8):1776–83.10.1016/S1003-6326(19)65085-5Suche in Google Scholar
[28] Marukawa Y, Ebihara K. Production of vanadium [P]. JP 60128230. 1985-07-09.Suche in Google Scholar
[29] Tripathy PK, Suri AK. A new process for the preparation of vanadium metal. High Temp Mater Processes. 2002;21(3):127–38.10.1515/HTMP.2002.21.3.127Suche in Google Scholar
[30] Tripathy PK, Juneja JM. Preparation of high purity vanadium metal by silicothermic reduction of oxides followed by electrorefining in a fused salt bath. High Temp Mater Processes. 2004;23(4):237–46.10.1515/HTMP.2004.23.4.237Suche in Google Scholar
[31] Wang F, Xu BQ, Wan HL, Xu B, Wan H, Yang J, et al. Preparation of vanadium powders by calcium vapor reduction of V2O3 under vacuum. Vacuum. 2020;173:109133.10.1016/j.vacuum.2019.109133Suche in Google Scholar
[32] Lee DW, Lee HS, Yun JY, Lee HS, Yun JY, Kim YH, et al. Synthesis of vanadium powder by magnesiothermic reduction. Adv Mater Res. 2014;1025–1026:509–14.10.4028/www.scientific.net/AMR.1025-1026.509Suche in Google Scholar
[33] Fang X, Shen S, Li N, Shen S, Li N, Hou Q. An innovative method to increase vanadium extraction from vanadium slag by increasing the porosity of roasted pellet. Steel Res Int. 2020;91:1900533.10.1002/srin.201900533Suche in Google Scholar
[34] Li J, Zhang YM, Liu T. Assessment method for cleaner production of vanadium extraction from stone coal. Environ Sci Technol. 2013;36(8):200–5.Suche in Google Scholar
[35] Zhang TA, Dou ZH. A novel multi-stage deep thermal reduction technology for titanium and its alloys. Titanium Ind Prog. 2020;37(2):43–8.Suche in Google Scholar
[36] Yan JS, Dou ZH, Zhang TA, Fan SG, Niu LP. A new process of preparing Ti6Al4V powder by a multistage depth reduction process. Rare Metal Mat Eng. 2021;50(9):3094–101.Suche in Google Scholar
[37] Wang X, Liu JM, Yang QS, Liu J, Yang Q, Du J, et al. Decomposition process and kinetics of waste rare earth polishing powder TG–DTA–FTIR studies. J Therm Anal Calorim. 2012;109(1):419–24.10.1007/s10973-011-1500-2Suche in Google Scholar
© 2022 Yan Jisen et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Artikel in diesem Heft
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption

