Abstract
Most nanoparticles are metabolized and accumulated in the liver; therefore, this review, based on most data collected from PubMed.gov between 2012 and 2023 with the keywords “nanomaterials induced hepatotoxicity,” aims to elucidate the mechanism of nanoparticles leading to liver injury and propose relevant strategies. We discuss the biomedical approaches and strategies for mitigating liver injury, including 1) principle and recommendation of material selection; 2) nanoparticle surface modulation; 3) strategies inspired by virus and other biological phenomenon; and 4) drug and other possible adjunctive strategies. The optimal design of nanomaterials and therapeutic strategies to attenuate hepatotoxicity is critical for the development of nanomedicine.
Graphical abstract
1 Introduction
Nanomaterials have been widely used in the medical field to deliver drugs and improve therapeutic efficacy due to their unique nanoscale size [1]. Nanoparticles can improve vascular permeability and favor the enhanced permeability and retention (EPR) effect, which is very advantageous in the treatment of inflammation or cancer with non-targeted drugs. In addition, bio-inspired nanotechnology, including vesicles, exosomes, or engineered cell membranes, endows the active-targeting capability to the specific lesion [2]. The incorporation of molecular imaging probes could also monitor the delivery and release behavior of nanomaterials using computed tomography (CT), magnetic resonance imaging (MRI), ultrasound (US), fluorescence imaging (FL), photoacoustic imaging (PAI), and some emerging imaging methods. Importantly, some nanomaterials can act as sensitizers to improve efficacy and increase bioavailability, thereby reducing tumor drug resistance. The development of multifunctional nanomaterials also endows nanocarriers with other biological effects, such as light response (photothermal therapy or photodynamic therapy), ultrasonic response (sonodynamic therapy or high-intensity focused ultrasound), magnetic response (magnetic hyperthermia), and other synergistic therapeutic effects.
However, there are still safety concerns, among which are the reactive oxygen species (ROS). Besides the immediate cytotoxicity to damage cell components, ROS also triggers signal pathways to cause necrosis, necroptosis, or apoptosis [3]. Nanomaterial-induced inflammation, both acute and chronic, increases the risk of liver fibrosis and pathological changes [4]. Genotoxicity, including damage to DNA structure, revealed potential carcinogenicity. Some evidence indicates that nanomaterial-induced epigenetic effects include abnormal DNA methylation and histone modifications, which could act as potential biomarkers for predicting the adverse effects of nanomaterials [5].
To address these hidden hazards, sufficient efforts are being made to focus on physical and chemical strategies. Rod-shaped nanoparticles seemed to reduce the uptake by the liver compared to spherical nanoparticles [6]. Various aspect ratios of different shapes have an influence on the retention time in organs [7]. Enhanced elasticity and deformability also help nanoparticles avoid recognition and sequestration by the MPS (mononuclear phagocyte system) [8]. Even the same element, with a different valence, displayed a disparity in hepatotoxicity. Under the same injected dose, 70% MnO nanoparticles can be metabolized by the liver within 48 h [9], while only approximately 50% Mn3O4 is eliminated in 1.5 weeks [10], indicating the metabolic process of nanoparticles in vivo is complex, and its safety requires a comprehensive evaluation.
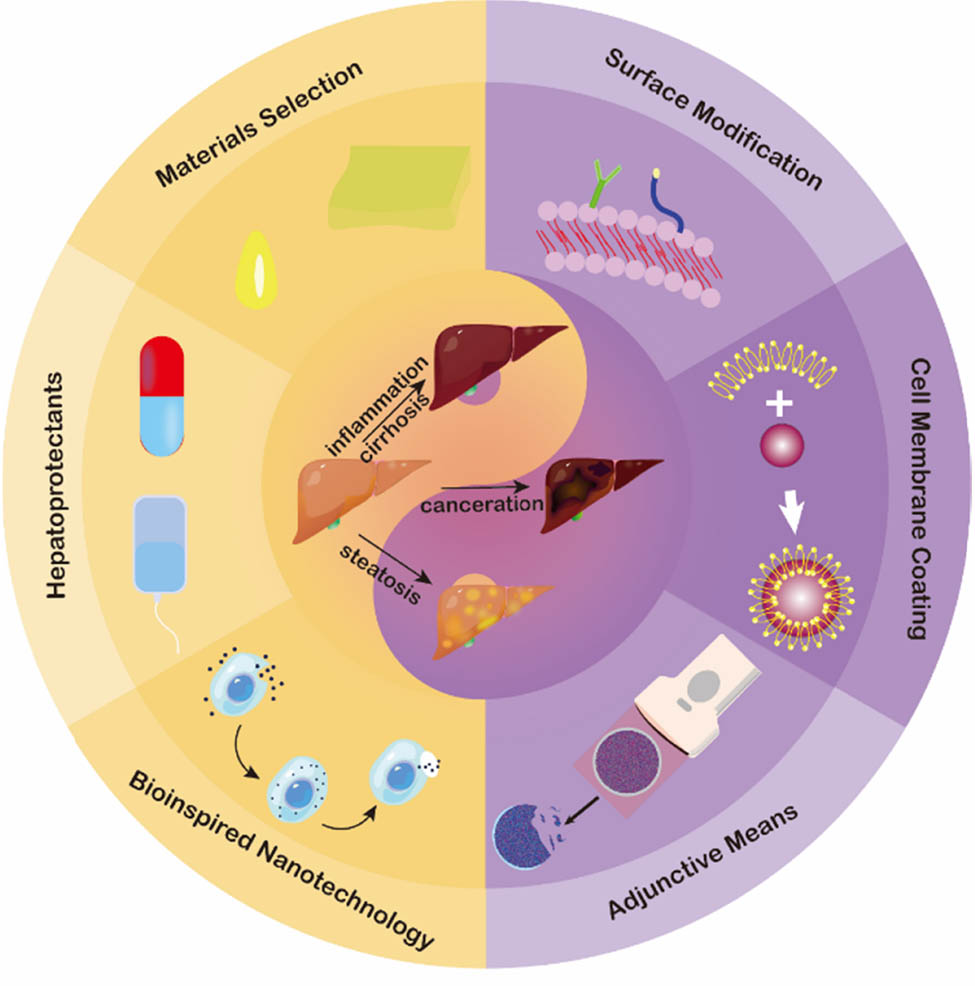
Given that most nanomaterials are sequestered by the liver, we mainly review how to mitigate the damages of nanomaterials to the liver on a biological basis, including materials selection, surface modification of nanomaterials, bio-inspired construction, and some adjunctive or strategies, to provide ideas for the clinical translation of nanomedicine (Scheme 1).

Illustration of strategies for nanomaterial-induced hepatotoxicity.
2 Mechanism of nanomaterial-induced hepatotoxicity
Conventional drug-induced hepatotoxicity is mainly based on metabolism or accumulation of the drug in hepatocytes, with activation of CD4 or cytotoxic CD8+ T-cells [11] leading to antigenic binding, which triggers an immune response, whereas nanomedicine-induced hepatotoxicity is characterized by free radical generation and oxidative stress [12,13]. Therefore, we focus here on ROS and inflammation, with a broader discussion of other related issues.
2.1 ROS and inflammation
Hepatotoxicity is often the most significant safety concern associated with nanomaterial toxicity. The main cause of hepatotoxicity is the production of ROS, such as mono-linear oxygen species, superoxide anion radicals, oxygen radicals, peroxide ions, hydrogen peroxide, and hydroxyl radicals, which enter cells via endocytosis and trigger a series of oxidative stress-related events [14]. In liver LO2 cells, mitochondria-derived ROS were found to activate the NOD-like receptor protein 3 (NLRP3) inflammasome, which promotes caspase-1-dependent thermal apoptosis [15]. ROS-induced mitochondrial swelling, loss of inner membrane and cristae, and increased mRNA levels of caspase-12, a marker of mitochondrial-caspase pathway activation, were found in the livers of normal mice exposed to SiNPs, confirming mitochondrial damage and apoptotic signaling. Upregulation of p53, Bax, and cleaved caspase-3 expression, as well as down-regulation of Bcl-2 and caspase-3 levels, was also detected when human and rat hepatocytes were exposed to SiO2 nanoparticles [16].
The vast majority of nanodrugs are exogenous chemicals that inevitably cause immune recognition and inflammatory response. In HepG2 cells, nanoparticles activate cellular stress response signaling pathways, including mitogen-activated protein kinase (MAPK) and NF-κB (nuclear factor-κ-gene binding) signaling pathways, with significant changes in the phosphorylation levels of stress-activated protein kinase such as ERK1/2, p38, and JNK. The levels of TNF, a pro-inflammatory factor that promotes lymphocyte infiltration and necrosis, and IL-8, a chemokine that recruits and activates neutrophils, were also significantly increased, whereas the expression of A20, an anti-inflammatory gene, was suppressed [17]. Similarly, mice exposed to multi-walled carbon nanotubes exhibited severe inflammatory cell infiltration in the portal vein region, cellular necrosis and localized necrosis, mitochondrial damage, and lysis.
Changes in gene expression involving the antigen processing and presentation pathways, cholesterol biosynthesis, the IL-6 signaling pathway, the cell cycle, and the metabolism of cytochrome P450 to xenobiotics were detected across genomes, with TNF-α and NF-κB signaling pathways showing the most significant changes [18]. Exposure of C57BL/6 mice to peptide-functionalized gold nanorods resulted in a decrease in the activity of the anti-inflammatory genes Arg-1 and IL-4, while at the same time, the levels of TNF-α, a marker of M1 macrophages, were very high, suggesting activation of this cell, and Arg-1 is a marker of M2 macrophages. The time course of the nanoparticle-induced inflammatory response was also investigated. Injected silica nanoparticles activate a variety of inflammatory signaling pathways at different time points, including the G1-S cell cycle, IL-10, IL-6 pathways, phagocytosis-related inflammatory pathways, and Th-17-derived pathways [19]. These results indicate that there are different time windows for specific blocking strategies. However, two questions need to be answered:
Does human nanomaterial-induced hepatotoxicity also share similar physiological courses?
Do different materials or the same material in different doses and states also trigger similar changes in vivo?
Inflammation caused by ROS is difficult to distinguish from other types of inflammation. However, persistent reports of the NF-κB pathway and TNF-α factor may highlight key locations for blocking the inflammatory cascade. NF-κB nuclear factor and erythroid 2-related factor (NRF2) may be crucial to indicate whether the level of ROS is out of control [20]. In addition, inhibition of M1 macrophage activity may be another strategy to reduce nanoparticle-induced hepatotoxicity (Figure 1). Mitochondrial protectants can also be considered in the future design of biomedical nanoparticles.

The genetic and epigenetic pathways and other variations in nanomaterial- induced hepatotoxicity.
2.2 Lipoapoptosis
Liposomes, the most common organic materials used in biological applications, are well-documented to be hepatotoxic upon systemic administration of cationic liposomes, exhibiting significant liver biochemical function abnormalities and histological damage [21]. In Gregory’s report, inflammatory extracellular vesicles (EVs) containing expression of tumor necrosis factor-associated apoptosis-inducing ligand (TRAIL) can be induced by lipid in death receptor 5 (DR5)-dependent manner [22]. DR5 is a promoter that regulates hepatocyte lipoapoptosis and inflammatory signaling [23], and the knockdown of DR5 in vivo could significantly reduce hepatocyte lipotoxicity. Blocking Rho-associated-coiled-containing protein kinase-1 (ROCK1), which promoted the release of EVs, could reduce lipid-induced liver damage [24]. Receptor-interacting protein kinase 1 (RIP1) also plays an important role in the expression of pro-inflammatory factors, such as the typical M1 macrophage markers IL-1β or IL-6, which act as pro-inflammatory factors and contribute to the inflammatory response in hepatocytes. From this perspective, the hepatotoxicity of EV applications deserves more attention. As emerging bio-inspired materials, EVs function as a drug delivery system that can deliver therapeutic drugs or imaging probes to designated disease sites through active targeting. Besides, the nanoscale size of EVs facilitates their passive targeting ability, which improves the potency of drug delivery and promotes diagnostic and therapeutic efficacy. Considering the potential role of lipid-induced EVs in the activation of pro-inflammatory macrophage, an in-depth assessment of the role of EVs in hepatotoxicity, particularly in the regulation of hepatic biochemical factors, is essential for biological applications.
However, though anti-DR5 chimeric antibodies work for tumor suppression [25], there are no reports on blocking DR5 to mitigate nanoparticle lipotoxicity. It remains to be further explored whether any small molecule or monoclonal antibody to DR5 can effectively inhibit hepatocyte lipoapoptosis in vivo (Figure 2).

The mechanism of DR5-introduced lipoapoptosis. Lipid activates DR5, which regulates the expression of TRAIL and RIP1, causing inflammation and activating M1 macrophages. ROCK1 contributes to the release of TRAIL.
2.3 Genotoxicity
The genotoxicity of nanoparticles has also caused great concerns. Researchers have found that titanium oxide nanoparticles deposited in liver DNA not only insert DNA base pairs but also bind to DNA nucleotides and alter the secondary structure of DNA. Furthermore, high doses of nano-anatase TiO2 cause DNA breakage in hepatocytes [26]. Morphological scans show markedly reduced nuclei, nuclear vacuolization, and chromatin margination [27]. In HepG2 cells exposed to TiO2 nanoparticles, DNA repair-related genes (including p53, MDM2, GADD 45α, and p21) were dramatically upregulated, and oxidative damage led to DNA strand breaks [28]. In another example, CuO nanoparticles induced the expression of 8-hydroxy-2′-deoxyguanosine(8-OH-dG), an indicator of oxidative DNA damage in the liver [29]. Nanoparticle-induced genotoxicity has also been reported in silica and gold nanoparticles, where significant DNA damage was detected after intravenous injection, especially in the smaller nanoparticles [19,30]. All of these studies suggest that DNA damage caused by nanomaterials may lead to carcinogenicity and severe cellular degeneration (Figure 3).

The genotoxicity induced by nanomaterials, including DNA cleavage, DNA strands break, DNA secondary structure damage, and DNA repair system damage.
3 Material selection for low hepatotoxicity in biological view
Material strategies to reduce hepatotoxicity are as follows: (1) selecting materials that can be degraded by more pathways in the body; (2) selecting materials that can be rapidly metabolized in liver; (3) targeting the site of the lesions, thus reducing the total amount of drugs; and (4) sustained and stable release of the drug, thus keeping the blood level low.
The longer metabolism time of iron nanoparticles compared to zinc or manganese is due to a later breakdown in the liver, particularly in Kupffer cells [31]. For organic materials, lipid H, lipid M, lipid P, lipid Q, lipid N, and lipid Y showed significantly less accumulation in the liver after intramuscular injection compared to lipid MC3. However, it must be recognized that these molecules, from the degradation of organic materials like hydrogel and lipids, would increase the liver burden [32,33] and aggravate hepatopathy [34].
Gelatin nanoparticles are good colloidal drug carriers for anticancer chemotherapy, which have, e.g., an arginine–glycine–aspartic acid sequence identified by integrin αV (especially αVβ3). Integrin αV is highly expressed in tumor vasculature and endothelial cells lining tumor tissues but less so in normal organs [2]. From this, gelatin-based nanocarriers (GNPs) are also suited for the central nervous system as they may passively target the injured brain tissue or brain tumors upregulated by gelatinases A and B [35]. Organic nanomaterials have advantages such as high loading capacity and low hepatotoxicity, but they also have drawbacks like limited stability. In contrast, the opposite is metal-based nanomaterials. To mitigate hepatotoxicity, metal–organic frameworks (MOFs) have been designed. In 2006, it was discovered that MOFs can hold a high concentration of medication and release them steadily over time. Stability and stiffness from the metals enable a high drug loading, while the safety and porosity of organic components ensure tunable release behaviors [36]. Works including stimuli-responsive MOF framework (e.g., pH/ROS dual-sensitive) and tumor-designed MOFs with decoys have reported better therapeutic efficacy of MOFs with minimal damage to normal liver cells due to improved drug efficiency, controlled drug release, prolonged duration of action, and drug targeting [3,31,35–39] (Table 1).
Comparison of different hypotoxic materials for the nanocarriers and their mechanism to reduce hepatotoxicity
| Nanocarrier | Categories | Mechanism | Subjects/cells | Ref. |
|---|---|---|---|---|
| Zinc oxide-engineered nanoparticles | Inorganic materials | Degraded by MPS in the endolysosomal compartments of phagocytic cells | BALB/c mice and Wistar–Han rats | [9,31,37] |
| MnO | Inorganic materials | Unknown hepatobiliary clearance pathway | Balb/c female mice | [9] |
| Lipid H | Organic material | Degraded by MPS or serum protein | Sprague–Dawley rats | [38] |
| cRGD–chitosan–gold nanoparticles | Organic materials (gelatin and hydrogel) | Targeting delivery | MCF-7 and HUVEC cells | [2] |
| 5-FAM/FA/TP@Fe-MIL-101 | Composite materials (MOF) | Targeting delivery | Balb/c nude mice | [39] |
| γ-CD-MOF | Composite materials | Sustained drug release | L929 cells | [40] |
| PCP-Mn-DTA@GOx@1-MT | Composite materials (MOF) | Targeting delivery | C57BL/6 mice and Balb/c mice | [41] |
4 Engineering strategies for modifying nanoparticles
Surface engineering can be achieved by targeting while preventing non-specific interactions. There are two common ways to alter the surface of nanomaterials: non-covalent conjugation and covalent conjugation, both of which can be used to target lesions and mitigate their hepatotoxicity.
4.1 PEGylation and conditional release modulation
When the nanomedicines enter the body, the surface coating of its nanoparticles with polyethylene glycol (PEG: PEGylation) prevents the nanoparticles from aggregating in the blood circulation and has a phagocytic effect. The MPS in the body has many opsonin-recognizing receptors on the cell surface, which can form a hydration cloud that sterically prevents nanomaterials from binding or interacting with the PEG chains immobilized on the surface of the nanomaterials, preventing the nanomaterials from combining or interacting with other nanomaterials or blood components from a three-dimensional structure [42,43]. The molecular weight (MW) and surface density of PEG are the main parameters affecting interactions and nanodrug metabolism; an MW greater than 2–10 kDa is essential for a low recognition level of MPS, partly due to less protein absorption [44,45].
PEG with MW less than 20 kDa are primarily metabolized in the renal system, whereas those more than 30 kDa are metabolized in the liver [46,47]. When PEGylated gold NPs are administered systemically, the circulation half-life is prolonged with MW increase between 2 and 10 kDa MW [48]. Also, it was shown that liver uptake of 5 kDa PEG was higher compared to 20 kDa PEG-coated NPs. In a subsequent study, NPs coated with 20 kDa PEG decreased hepatic uptake in vivo compared to NPs coated with 5 kDa PEG, resulting in prolonged circulation time [49]. Increased surface PEG density leads to PEG chain overlap and constitutes a maskant, which decreases the uptake of MPS cells by the liver [50,51]. Alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase level, and histopathological evaluation are the main indicators that support the hepatoprotective effect of PEG-coated gold nanomaterials [52]. However, besides the high cost, the shortcomings of conventional PEGylation involve obvious loss of efficacy, as the PEG chains may occupy the active sites of drugs [53,54], and most polyacids are difficult to degrade enzymatically [55].
High lactic acid level leads to paradoxically acidic extracellular and intracellular tumor microenvironments in almost all tumors. In order to coat nanomaterials with pH-responsive layers used to regulate the release of cancer chemotherapeutic drugs, researchers have coated the nanomaterials with poly(β-amino ester)(PBAE), whose tertiary amine residues may be ionized in an acidic environment. In BALB/c nude mice injected with A549 cells, doxorubicin(DOX)-coated PBAE nanomaterials were more efficiently absorbed by cancer tissues despite causing modest levels of hepatic function indicators (ALT and AST) [56].
In addition, the surface of nanomaterials can be modified with ligands (e.g., peptides, nucleic aptamers, polysaccharides, or antibodies) that recognize antigens expressed on the surface of diseased cells to improve the specificity of nanodrug delivery. In SCID Beige mice, gelatin nanoparticle surface-modified with an epidermal growth factor receptor (EGFR)-targeting peptide were almost double as effective as PEG-modified or untreated nanoparticles for tumor targeting without adding any additional hepatotoxicity [57]. High levels of programmed death ligand protein-1(PD-L1) expressed in cancer cells are associated with immune evasion and a dismal prognosis. Hyperbranched, multivalent poly(amidoamine) dendrimers can be used to bind the PD-1/PD-L1 antibody. Nanoparticle–antibody conjugates promoted T-cell antitumor immunity while reducing tumor chemoresistance to DOX compared to PD-L1 human antibody and showed no additional hepatotoxicity [58].
4.2 Bioinspired cell membrane coating nanotechnology
Cell membrane coating nanotechnology further advances surface modification [59,60]. The particle cores of cell membrane-coated nanoparticles are covered by natural or artificial cell membranes and have some of the intrinsic features of source cells (Figure 4) [61].
![Figure 4
A drawing of nanoparticles with cell membrane coatings. Membrane sources for coating nanoparticles. Each cell membrane type may use different features to offer functionality to nanoparticulate cores, the substance of which can vary depending on the application [53]. Reproduced with permission from the study of Fang et al. [61]. Copyright 2018, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.](/document/doi/10.1515/ntrev-2024-0074/asset/graphic/j_ntrev-2024-0074_fig_004.jpg)
A drawing of nanoparticles with cell membrane coatings. Membrane sources for coating nanoparticles. Each cell membrane type may use different features to offer functionality to nanoparticulate cores, the substance of which can vary depending on the application [53]. Reproduced with permission from the study of Fang et al. [61]. Copyright 2018, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Erythrocytes were the first cells of origin to be utilized to encapsulate cell membranes with nanoparticles and are also the most intensively researched cells in this field [62,63]. Erythrocytes are the best source of nanocarriers due to (1) the lack of organelles in this cell, (2) circulation in the blood for up to 4 months, and (3) the detoxification activity of its surface proteins. The liver is the primary organ responsible for the detoxification of hemoglobin and the majority of chemotherapeutic drugs. The toxin affinity of the erythrocyte membrane may be used to prevent the hemolytic impact of hemolysin [64], though mechanisms are not clear. RBC-membrane-coated nanomaterials carrying doxorubicin significantly increased the survival rates of C57BL/6 mice carrying EL4 cells compared to free doxorubicin administration in the same dose, while no significant increase in ALT and AST was detected, and low IL-6 levels indicated no acute liver and systemic responses [65]. Similar findings revealed that RBC membranes could bind DOX, thereby reducing its harmful activity, and their complexes are stable in serum, most likely due to the surface charge of RBC surface proteins of membrane-coated nanoparticles. In contrast to the total retention of PEG-coated nanoparticles of 11% (24 h after injection) and 2% (48 h after injection), the retention of RBC membrane-coated nanoparticles exhibited 29 and 16%, showing that RBC membrane coating was efficient in evading the reticuloendothelial system (RES) [62].
Besides RBC membranes, other sources such as leukocyte membranes, stem cell membranes, endothelial cell membranes, cancer cell membranes, and even hybrid cell membranes have shown promise for liver protection in the way of increasing the targeting of drug delivery; immune modulation or direct binding ability by surface proteins [58,66–68]. However, a certain amount of antigens or even autoantigens may be present in T/B cells and cause serious immune response [69–71].
5 Biological camouflage strategy
5.1 Hijacking cells
Besides MPS, the tumor vasculature and blood–brain barrier (BBB) remain barriers to nanodrug delivery [72]. A strategy to hijack cells in vivo across vessel barriers and to target the inflamed lesion can dramatically alleviate hepatotoxicity because of little liver uptake. Liberal amounts, excellent locomotion ability, and 8 h half-time make neutrophils the most popular platform for the strategy [73–75]. Among PEG, IgG, and anti CD11b antibody-modified nanoparticles, the CD11b Abs decorated nanomaterials have shown the highest uptake specificity of neutrophils. Under the conditions of acute inflammation induced by photodynamic therapy, the accumulation of CD11b nanoparticles was 35 times higher than PEG-modified nanoparticles in lesions [76]. TA99 administration further enhances the therapeutic effect due to antibody-dependent cellular cytotoxicity mechanisms [75], and the pretreatment of TA99 in a mice model of melanoma has the following three merits: (1) promoting neutrophils to infiltrate into tumors; (2) significantly increased uptake radio of the nanoparticles by neutrophil, and thus (3) improved the survival rate (Figure 5) [77].

Diagram of hijacking cell strategy. Neutrophils hijacked by nanoparticles in the bloodstream are attracted by chemokines and then bind to tumor cells with TA99.
5.2 Transient depletion of host Kupffer cells
Kupffer cells in hepatic sinusoids are resident macrophages that play a major role in phagocytosing nanoparticles in the bloodstream, consistent with the role of MPS [78]. A previous study has shown that transient depletion of host Kupffer could alleviate liver injury induced by alcohol in rats [79]. This strategy is equally effective for nanoparticle-induced hepatotoxicity. Substances including gadolinium chloride [80], methyl palmitate [81], dextran sulfate (500 kDa) [82], carrageenan [83], and clodronate liposomes [78] were used for transient depletion of Kupffer cells. In mice models bearing SUIT-2 cells (a human pancreatic adenocarcinoma cell line), injection of clodronate caused almost complete depletion of Kupffer cells on Days 2 and 3, and the amount of Kupffer cells started to recover on Day 5. Clodronate pretreatment reduced the accumulation of doxorubicin from 6.4 to 4.7 μg/g-tissue by Kupffer cell depletion [84]. However, recovery in cell mass does not mean recovery in cell quality. All these studies did not present convincing evidence that regenerated cells have no genetic damage and epigenetic changes to further induce cirrhosis or malignant transformation. Notably, depletion of Kupffer cells may lead to significant accumulation of drugs in other organs (e.g., spleen, lung, heart, or kidney). Whether this strategy poses a risk by disrupting the immune system, especially for those with immunologic deficiency and serious infection, needs to be confirmed.
6 Drugs and other possible strategies
In addition to the development of nanoparticles, combination therapy with other drugs and methods (e.g., ultrasound or photodynamic therapy) may be considered to alleviate hepatotoxicity. Standard methods of clinical treatment of drug-induced liver injury include N-acetylcysteine and corticosteroids [85,86]. However, there are no specific treatments for nanomaterial-induced toxicity.
6.1 Hepatoprotectors
There are a number of hepatoprotective species, including vitamin E (Vit E), α-lipoic acid (ALA), quercetin (Qur), and arginine (Arg), which act as antioxidants mainly by inhibiting the peroxidation of fatty acids and by increasing the levels of cysteine and glutathione in the liver [87–89]. In a study aimed at comparing the hepatoprotective effects of these four antioxidants with those of melanin, melanin was shown to be the most significant in ameliorating gold nanoparticle-induced hepatic dysfunction, as measured by a comprehensive evaluation of all indicators of liver functions in experimental male rats [90]. ALA also downregulated iNOS, which is responsible for nitrosative stress, and reduced the overexpression levels of apoptotic genes C-Jun and C-Myc in the liver [91]. Moreover, ALA reduces CNP accumulation in hepatic tissue by unknown mechanisms [91].
In terms of natural extracts, beetroot juice has also shown powerful liver protective effects and anti-inflammatory and anti-mutagenic properties [92]. The hepatoprotective effect of beetroot juice is supported by the fact that the effects of oxidative stress on silver nanoparticle-induced liver injury in rats can be attenuated by maintaining the activity of antioxidant systems (e.g., SOD and CAT) and by increasing the amount of GSH in the liver, and by the improvement in the expression of p53 and anti-apoptotic Bcl-d2, with little DNA fragmentation [92] supported the hepatoprotective effects of beetroot juice. However, it is unclear what played a major role in this effect. Grape seed proanthocyanidin extract (GSE) also alleviated TiO2 nanoparticle-induced liver injury and oxidative stress in rats. GSE treatment mediated the expressions of TLR-4, NF-κB, NIK, and TNF-α genes and maintained a high level of GSH in the liver.
Despite various advances, it is disputed whether the effects of hepatoprotectors could be repeated on animal models induced by different nanomaterials. Most studies focused on anti-inflammation strategy. Unfortunately, no reports have shown a critical factor in nanoparticle-induced inflammation, probably because there are too many imponderables, for example, sizes, doses, or subjects themselves, to distinguish one significant blocking target. Thus, it is practical to try compound hepatoprotection in clinical study and practice (Table 2).
Comparison of different hepatoprotectors and their mechanism to reduce hepatotoxicity
| Hepatoprotectors | Mechanism | Ref. |
|---|---|---|
| Melanin | Anti-inflammation and activating antioxidant system | [93] |
| α-Lipoic acid (ALA) | Relieving nitrosative and inhabiting apoptosis | [91] |
| Beetroot juice | Anti-inflammation and attenuate ROS, activating the antioxidant system | [92] |
6.2 Ultrasound-assisted strategy
The ultrasound cavitation effects create pores between cells, which means improved vascular permeability and, thus, effective nanodrug transportation and fewer side effects [94]. Ultrasound-induced transient opening of the BBB is of great significance for nanoparticles to reach the central nervous systems and target lesions. Temozolomide (TMZ), a clinical first-line glioma chemotherapy drug, can be loaded in a zirconium-based frame, which collapses under ultrasonic stimulation due to low-frequency oscillations and cavitation effect [95]. Ultrasound can be combined with other strategies to further improve efficacy and alleviate hepatotoxicity. Nanoparticles modified with angiopep-2 peptide, a ligand targeting LRP1 (low-density lipoprotein receptor-related protein 1), which is highly expressed in tumors, achieved glioma-specific delivery in mice, but nanoparticles remain in the liver [96]. Further attempts included a nanodrug for luminescence imaging. Its hollow acoustic-sensitive TiO2 shell produced ROS for controlled drug release. It also encapsulates an immune checkpoint inhibitor (anti‐PD‐1 antibody) for glioma immunosuppression and paclitaxel. In vivo, liver luminescence images show fewer residues in the livers of C57 mice using ultrasound compared to those not using ultrasound [97]. Compared with photodynamic therapy and intervention techniques, ultrasound is non-invasive, focusable, and more penetrating, which also makes it difficult to handle ultrasound-induced bleeding. Whether ultrasound irradiation does not influence molecular structural stability of loading cannot be neglected (Figure 6) [98,99].
![Figure 6
Schematic illustration of hollow TiO2 covered persistent luminescent nanosensitizer for ultrasound amplified chemo/immuno GBM therapy. (a) Composition of ZGO@TiO2@APL. “A” represents anti‐PD‐1 antibody, “L” represents liposome, and “P” represents PTX in the abbreviation “ALP”. (b) BBB penetration process of ZGO@TiO2@APL‐NEs. ZGO@TiO2@APL was loaded by neutrophils to form ZGO@TiO2@APL‐NEs in vitro. The injected ZGO@TiO2@APL‐NEs could be attracted by the inflammation in GBM to traverse the BBB. (c) Ultrasound-triggered drug release from ZGO@TiO2@APL‐NEs for GBM therapy. Activated by ultrasound, ROS was generated from ZGO@TiO2@ALP to break up liposome coverage for PTX, and an anti‐PD‐1 antibody was released to kill the tumor and induce local inflammation, which in turn attracted more ZGO@TiO2@ALP‐NEs to GBM sites for sustained therapy [97]. Reproduced with permission from the study of Li et al. [97]. Copyright 2021, Advanced Science, published by Wiley-VCH GmbH.](/document/doi/10.1515/ntrev-2024-0074/asset/graphic/j_ntrev-2024-0074_fig_006.jpg)
Schematic illustration of hollow TiO2 covered persistent luminescent nanosensitizer for ultrasound amplified chemo/immuno GBM therapy. (a) Composition of ZGO@TiO2@APL. “A” represents anti‐PD‐1 antibody, “L” represents liposome, and “P” represents PTX in the abbreviation “ALP”. (b) BBB penetration process of ZGO@TiO2@APL‐NEs. ZGO@TiO2@APL was loaded by neutrophils to form ZGO@TiO2@APL‐NEs in vitro. The injected ZGO@TiO2@APL‐NEs could be attracted by the inflammation in GBM to traverse the BBB. (c) Ultrasound-triggered drug release from ZGO@TiO2@APL‐NEs for GBM therapy. Activated by ultrasound, ROS was generated from ZGO@TiO2@ALP to break up liposome coverage for PTX, and an anti‐PD‐1 antibody was released to kill the tumor and induce local inflammation, which in turn attracted more ZGO@TiO2@ALP‐NEs to GBM sites for sustained therapy [97]. Reproduced with permission from the study of Li et al. [97]. Copyright 2021, Advanced Science, published by Wiley-VCH GmbH.
7 Conclusions and discussion
In this article, we discuss innovative ways to reduce liver damage caused by nanoparticles. Hydrogel and lipids are recommended for their high degree of biocompatibility. There are also more niche options, such as gelatin-based nanoparticles for brain medication delivery. Surface modification, bioinspired nanotechnology, and techniques like cell hijacking can be used to avoid liver uptake and achieve targeted medication distribution. Transient reduction of Kupffer cells reduces nanoparticle accumulation in the liver despite safety concerns. Hepatoprotective agents have unique pharmacological effects that help to reduce hepatotoxicity. Ultrasound, for example, can be used to aid nanoparticles in infiltrating lesions and to regulate the release of loads contained inside the nanoparticles.
Our knowledge about nanoparticle-induced damage is still limited, and few common features could cover most pathological processes. It seems that a special type of nanoparticle causes similar liver injury. Therefore, toxicological research focusing on FDA-approved carriers or nanoparticles of clinical translational value should be encouraged. Most studies have determined liver health only by testing for AST, ALA, and morphology. To thoroughly examine all potential alterations at the molecular level, it is advisable to provide evidence from protein mass spectrometry, RNA sequence, or gene sequencing. Second, studies on the long-term effects of nanoparticle-induced liver injury are lacking. However, cancer and AIDS treatment require long-term, often lifetime, care. Therefore, it is crucial to study the long-term effects of routinely used nanoparticles on the liver. In order to guide clinical therapy, we need more information to demonstrate a quantifiable link between targeted drug delivery and additional adjunctive techniques (e.g., ultrasonography) and their indirect hepatoprotective effects.
There is a need to investigate some overarching therapy strategies for acute liver damage caused by nanoparticles for usage in clinical settings. The contradiction between the anti-tumor effect of ROS and its toxicity cannot be ignored. In order to maximize the protective effects on the liver, it may be necessary to combine several approaches.
In the future, 3D liver models may be a better option for in vitro assessment of hepatotoxicity [100–102]. In addition, it may be possible to develop small molecules or monoclonal antibodies against receptors such as DR5 or to inactivate Kupffer cells. Efforts to reduce the costs of cell membrane coating nanotechnology and materials are significant for clinical transformation. The development of wearable (ultrasound) devices could also assist in the long-term control of nanodrugs to a great extent [103].
-
Funding information: This work was supported by The Science and Technology Projects of Xiamen Municipal Bureau of Science and Technology (Grant Nos 3502Z20194042 and 2020J02063), Open Research Fund of Key Laboratory of Nanomedical Technology of Fujian Medical University (Grant No. 2022KLNT201), the Science Foundation of Fujian Province (2022J01019), Natural Science Foundation of Guangxi (2023GXNSFAA026023), Central Government Guided Local Science and Technology Development Fund Projects (2023ZYZX1090), National Natural Science Foundation of China (82360360), and National Natural Science Foundation of China (Grant No. 8200011769).
-
Author contributions: T.C.G., M.D.W., Y.L.Z., S.J.M., H.X.L., and Z.X.W. participated in the organization, writing and editing of this manuscript. S.B.L., L.Z., and J.W.H. assisted in the editorial process. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Amreddy N, Babu A, Muralidharan R, Panneerselvam J, Srivastava A, Ahmed R, et al. Recent advances in nanoparticle-based cancer drug and gene delivery. Adv Cancer Res. 2018;137:115–70.10.1016/bs.acr.2017.11.003Search in Google Scholar PubMed PubMed Central
[2] Saber MM, Bahrainian S, Dinarvand R, Atyabi F. Targeted drug delivery of Sunitinib Malate to tumor blood vessels by cRGD-chiotosan-gold nanoparticles. Int J Pharm. 2017;517(1–2):269–78.10.1016/j.ijpharm.2016.12.016Search in Google Scholar PubMed
[3] Mohammadinejad R, Moosavi MA, Tavakol S, Vardar D, Hosseini A, Rahmati M, et al. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy. 2019;15(1):4–33.10.1080/15548627.2018.1509171Search in Google Scholar PubMed PubMed Central
[4] Bartucci R, van der Meer AZ, Boersma YL, Olinga P, Salvati A. Nanoparticle-induced inflammation and fibrosis in ex vivo murine precision-cut liver slices and effects of nanoparticle exposure conditions. Arch Toxicol. 2021;95(4):1267–85.10.1007/s00204-021-02992-7Search in Google Scholar PubMed PubMed Central
[5] Pogribna M, Hammons G. Epigenetic effects of nanomaterials and nanoparticles. J Nanobiotechnol. 2021;19(1):2.10.1186/s12951-020-00740-0Search in Google Scholar PubMed PubMed Central
[6] Decuzzi P, Godin B, Tanaka T, Lee SY, Chiappini C, Liu X, et al. Size and shape effects in the biodistribution of intravascularly injected particles. J Control Rel. 2010;141(3):320–7.10.1016/j.jconrel.2009.10.014Search in Google Scholar PubMed
[7] Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res. 2009;26(1):244–9.10.1007/s11095-008-9626-zSearch in Google Scholar PubMed PubMed Central
[8] Bertrand N, Leroux JC. The journey of a drug-carrier in the body: an anatomo-physiological perspective. J Control Rel. 2012;161(2):152–63.10.1016/j.jconrel.2011.09.098Search in Google Scholar PubMed
[9] Chevallier P, Walter A, Garofalo A, Veksler I, Lagueux J, Bégin-Colin S, et al. Tailored biological retention and efficient clearance of pegylated ultra-small MnO nanoparticles as positive MRI contrast agents for molecular imaging. J Mater Chem B. 2014;2(13):1779–90.10.1039/C3TB21634ASearch in Google Scholar
[10] Xiao J, Tian XM, Yang C, Liu P, Luo NQ, Liang Y, et al. Ultrahigh relaxivity and safe probes of manganese oxide nanoparticles for in vivo imaging. Sci Rep. 2013;3:3424.10.1038/srep03424Search in Google Scholar PubMed PubMed Central
[11] Björnsson HK, Björnsson ES. Drug-induced liver injury: Pathogenesis, epidemiology, clinical features, and practical management. Eur J Intern Med. 2022;97:26–31.10.1016/j.ejim.2021.10.035Search in Google Scholar PubMed
[12] Luo Z, Li Z, Xie Z, Sokolova IM, Song L, Peijnenburg W, et al. Rethinking nano-TiO(2) safety: Overview of toxic effects in humans and aquatic animals. Small. 2020;16(36):e2002019.10.1002/smll.202002019Search in Google Scholar PubMed
[13] Zhang J, Wang F, Yalamarty SSK, Filipczak N, Jin Y, Li X. Nano silver-induced toxicity and associated mechanisms. Int J Nanomed. 2022;17:1851–64.10.2147/IJN.S355131Search in Google Scholar PubMed PubMed Central
[14] Rang FJ, Boonstra J. Causes and consequences of age-related changes in DNA methylation: A role for ROS? Biology (Basel). 2014;3(2):403–25.10.3390/biology3020403Search in Google Scholar PubMed PubMed Central
[15] Zhang X, Luan J, Chen W, Fan J, Nan Y, Wang Y, et al. Mesoporous silica nanoparticles induced hepatotoxicity via NLRP3 inflammasome activation and caspase-1-dependent pyroptosis. Nanoscale. 2018;10(19):9141–52.10.1039/C8NR00554KSearch in Google Scholar
[16] Zuo D, Duan Z, Jia Y, Chu T, He Q, Yuan J, et al. Amphipathic silica nanoparticles induce cytotoxicity through oxidative stress mediated and p53 dependent apoptosis pathway in human liver cell line HL-7702 and rat liver cell line BRL-3A. Colloids Surf B Biointerfaces. 2016;145:232–40.10.1016/j.colsurfb.2016.05.006Search in Google Scholar PubMed
[17] Chen J, Zhang J, Cao J, Xia Z, Gan J. Inflammatory MAPK and NF-κB signaling pathways differentiated hepatitis potential of two agglomerated titanium dioxide particles. J Hazard Mater. 2016;304:370–8.10.1016/j.jhazmat.2015.11.002Search in Google Scholar PubMed
[18] Zhang D, Deng X, Ji Z, Shen X, Dong L, Wu M, et al. Long-term hepatotoxicity of polyethylene-glycol functionalized multi-walled carbon nanotubes in mice. Nanotechnology. 2010;21(17):175101.10.1088/0957-4484/21/17/175101Search in Google Scholar PubMed
[19] Pfuhler S, Downs TR, Allemang AJ, Shan Y, Crosby ME. Weak silica nanomaterial-induced genotoxicity can be explained by indirect DNA damage as shown by the OGG1-modified comet assay and genomic analysis. Mutagenesis. 2017;32(1):5–12.10.1093/mutage/gew064Search in Google Scholar PubMed
[20] Sies H, Mailloux RJ, Jakob U. Fundamentals of redox regulation in biology. Nat Rev Mol Cell Biol. 2024. 10.1038/s41580-024-00730-2.Search in Google Scholar PubMed
[21] Loisel S, Le Gall C, Doucet L, Ferec C, Floch V. Contribution of plasmid DNA to hepatotoxicity after systemic administration of lipoplexes. Hum Gene Ther. 2001;12(6):685–96.10.1089/104303401300057405Search in Google Scholar PubMed
[22] Hirsova P, Ibrahim SH, Krishnan A, Verma VK, Bronk SF, Werneburg NW, et al. Lipid-induced signaling causes release of inflammatory extracellular vesicles from hepatocytes. Gastroenterology. 2016;150(4):956–67.10.1053/j.gastro.2015.12.037Search in Google Scholar PubMed PubMed Central
[23] Park KM, Park JY, Pyo J, Lee SY, Kim HS. Induction of DR5-dependent apoptosis by PGA(2) through ATF4-CHOP pathway. Molecules. 2022;27(12):1–10.10.3390/molecules27123804Search in Google Scholar PubMed PubMed Central
[24] Wang QL, Xing W, Yu C, Gao M, Deng LT. ROCK1 regulates sepsis-induced acute kidney injury via TLR2-mediated endoplasmic reticulum stress/pyroptosis axis. Mol Immunol. 2021;138:99–109.10.1016/j.molimm.2021.07.022Search in Google Scholar PubMed
[25] Qiu Y, Zhang Z, Shi J, Liu S, Liu Y, Zheng D. A novel anti-DR5 chimeric antibody and epirubicin synergistically suppress tumor growth. IUBMB Life. 2012;64(9):757–65.10.1002/iub.1064Search in Google Scholar PubMed
[26] Li N, Ma L, Wang J, Zheng L, Liu J, Duan Y, et al. Interaction between nano-anatase TiO(2) and liver DNA from mice in vivo. Nanoscale Res Lett. 2009;5(1):108–15.10.1007/s11671-009-9451-2Search in Google Scholar PubMed PubMed Central
[27] Cui Y, Liu H, Ze Y, Zengli Z, Hu Y, Cheng Z, et al. Gene expression in liver injury caused by long-term exposure to titanium dioxide nanoparticles in mice. Toxicol Sci. 2012;128(1):171–85.10.1093/toxsci/kfs153Search in Google Scholar PubMed
[28] Petković J, Zegura B, Stevanović M, Drnovšek N, Uskoković D, Novak S, et al. DNA damage and alterations in expression of DNA damage responsive genes induced by TiO2 nanoparticles in human hepatoma HepG2 cells. Nanotoxicology. 2011;5(3):341–53.10.3109/17435390.2010.507316Search in Google Scholar PubMed
[29] Song MF, Li YS, Kasai H, Kawai K. Metal nanoparticle-induced micronuclei and oxidative DNA damage in mice. J Clin Biochem Nutr. 2012;50(3):211–6.10.3164/jcbn.11-70Search in Google Scholar PubMed PubMed Central
[30] Lopez-Chaves C, Soto-Alvaredo J, Montes-Bayon M, Bettmer J, Llopis J, Sanchez-Gonzalez C. Gold nanoparticles: Distribution, bioaccumulation and toxicity. In vitro and in vivo studies. Nanomedicine. 2018;14(1):1–12.10.1016/j.nano.2017.08.011Search in Google Scholar PubMed
[31] Zhang YN, Poon W, Tavares AJ, McGilvray ID, Chan WCW. Nanoparticle-liver interactions: Cellular uptake and hepatobiliary elimination. J Control Rel. 2016;240:332–48.10.1016/j.jconrel.2016.01.020Search in Google Scholar PubMed
[32] Wang K, Zhu K, Zhu Z, Shao F, Qian R, Wang C, et al. Triptolide with hepatotoxicity and nephrotoxicity used in local delivery treatment of myocardial infarction by thermosensitive hydrogel. J Nanobiotechnol. 2023;21(1):227.10.1186/s12951-023-01980-6Search in Google Scholar PubMed PubMed Central
[33] Lee W, Kim HY, Choi YJ, Jung SH, Nam YA, Zhang Y, et al. SNX10-mediated degradation of LAMP2A by NSAIDs inhibits chaperone-mediated autophagy and induces hepatic lipid accumulation. Theranostics. 2022;12(5):2351–69.10.7150/thno.70692Search in Google Scholar PubMed PubMed Central
[34] Abulikemu A, Zhao X, Xu H, Li Y, Ma R, Yao Q, et al. Silica nanoparticles aggravated the metabolic associated fatty liver disease through disturbed amino acid and lipid metabolisms-mediated oxidative stress. Redox Biol. 2023;59:102569.10.1016/j.redox.2022.102569Search in Google Scholar PubMed PubMed Central
[35] Elzoghby AO, Abd-Elwakil MM, Abd-Elsalam K, Elsayed MT, Hashem Y, Mohamed O. Natural polymeric nanoparticles for brain-targeting: Implications on drug and gene delivery. Curr Pharm Des. 2016;22(22):3305–23.10.2174/1381612822666160204120829Search in Google Scholar PubMed
[36] Horcajada P, Serre C, Vallet-Regí M, Sebban M, Taulelle F, Férey G. Metal-organic frameworks as efficient materials for drug delivery. Angew Chem Int Ed Engl. 2006;45(36):5974–8.10.1002/anie.200601878Search in Google Scholar PubMed
[37] Watson CY, Molina RM, Louzada A, Murdaugh KM, Donaghey TC, Brain JD. Effects of zinc oxide nanoparticles on Kupffer cell phagosomal motility, bacterial clearance, and liver function. Int J Nanomed. 2015;10:4173–84.10.2147/IJN.S82807Search in Google Scholar PubMed PubMed Central
[38] Hassett KJ, Benenato KE, Jacquinet E, Lee A, Woods A, Yuzhakov O, et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol Ther Nucleic Acids. 2019;15:1–11.10.1016/j.omtn.2019.01.013Search in Google Scholar PubMed PubMed Central
[39] Cai M, Zeng Y, Liu M, You L, Huang H, Hao Y, et al. Construction of a multifunctional nano-scale metal-organic framework-based drug delivery system for targeted cancer therapy. Pharmaceutics. 2021;13(11):1–14.10.3390/pharmaceutics13111945Search in Google Scholar PubMed PubMed Central
[40] Wei Y, Chen C, Zhai S, Tan M, Zhao J, Zhu X, et al. Enrofloxacin/florfenicol loaded cyclodextrin metal-organic-framework for drug delivery and controlled release. Drug Deliv. 2021;28(1):372–9.10.1080/10717544.2021.1879316Search in Google Scholar PubMed PubMed Central
[41] Dai L, Yao M, Fu Z, Li X, Zheng X, Meng S, et al. Multifunctional metal-organic framework-based nanoreactor for starvation/oxidation improved indoleamine 2,3-dioxygenase-blockade tumor immunotherapy. Nat Commun. 2022;13(1):2688.10.1038/s41467-022-30436-ySearch in Google Scholar PubMed PubMed Central
[42] Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623.10.1146/annurev.immunol.17.1.593Search in Google Scholar PubMed
[43] Yang Q, Lai SK. Anti-PEG immunity: Emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(5):655–77.10.1002/wnan.1339Search in Google Scholar PubMed PubMed Central
[44] Owens DE 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307(1):93–102.10.1016/j.ijpharm.2005.10.010Search in Google Scholar PubMed
[45] He Q, Zhang J, Shi J, Zhu Z, Zhang L, Bu W, et al. The effect of PEGylation of mesoporous silica nanoparticles on nonspecific binding of serum proteins and cellular responses. Biomaterials. 2010;31(6):1085–92.10.1016/j.biomaterials.2009.10.046Search in Google Scholar PubMed
[46] Turecek PL, Bossard MJ, Schoetens F, Ivens IA. PEGylation of biopharmaceuticals: A review of chemistry and nonclinical safety information of approved drugs. J Pharm Sci. 2016;105(2):460–75.10.1016/j.xphs.2015.11.015Search in Google Scholar PubMed
[47] Shi D, Beasock D, Fessler A, Szebeni J, Ljubimova JY, Afonin KA, et al. To PEGylate or not to PEGylate: Immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv Drug Deliv Rev. 2022;180:114079.10.1016/j.addr.2021.114079Search in Google Scholar PubMed PubMed Central
[48] Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WC. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009;9(5):1909–15.10.1021/nl900031ySearch in Google Scholar PubMed
[49] Mosqueira VC, Legrand P, Gref R, Heurtault B, Appel M, Barratt G. Interactions between a macrophage cell line (J774A1) and surface-modified poly (D,L-lactide) nanocapsules bearing poly(ethylene glycol). J Drug Target. 1999;7(1):65–78.10.3109/10611869909085493Search in Google Scholar PubMed
[50] Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine (Lond). 2011;6(4):715–28.10.2217/nnm.11.19Search in Google Scholar PubMed PubMed Central
[51] Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99(Pt A):28–51.10.1016/j.addr.2015.09.012Search in Google Scholar PubMed PubMed Central
[52] Patlolla AK, Kumari SA, Tchounwou PB. A comparison of poly-ethylene-glycol-coated and uncoated gold nanoparticle-mediated hepatotoxicity and oxidative stress in Sprague Dawley rats. Int J Nanomed. 2019;14:639–47.10.2147/IJN.S185574Search in Google Scholar PubMed PubMed Central
[53] Mero A, Ishino T, Chaiken I, Veronese FM, Pasut G. Multivalent and flexible PEG-nitrilotriacetic acid derivatives for non-covalent protein pegylation. Pharm Res. 2011;28(10):2412–21.10.1007/s11095-011-0468-8Search in Google Scholar PubMed
[54] Zaghmi A, Mendez-Villuendas E, Greschner AA, Liu JY, de Haan HW, Gauthier MA. Mechanisms of activity loss for a multi-PEGylated protein by experiment and simulation. Mater Today Chem. 2019;12:121–31.10.1016/j.mtchem.2018.12.007Search in Google Scholar
[55] Andrianov AK. Noncovalent PEGylation of protein and peptide therapeutics. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023;15(5):e1897.10.1002/wnan.1897Search in Google Scholar PubMed
[56] Men W, Zhu P, Dong S, Liu W, Zhou K, Bai Y, et al. Layer-by-layer pH-sensitive nanoparticles for drug delivery and controlled release with improved therapeutic efficacy in vivo. Drug Deliv. 2020;27(1):180–90.10.1080/10717544.2019.1709922Search in Google Scholar PubMed PubMed Central
[57] Xu J, Gattacceca F, Amiji M. Biodistribution and pharmacokinetics of EGFR-targeted thiolated gelatin nanoparticles following systemic administration in pancreatic tumor-bearing mice. Mol Pharm. 2013;10(5):2031–44.10.1021/mp400054eSearch in Google Scholar PubMed PubMed Central
[58] Bu J, Nair A, Iida M, Jeong WJ, Poellmann MJ, Mudd K, et al. An avidity-based PD-L1 antagonist using nanoparticle-antibody conjugates for enhanced immunotherapy. Nano Lett. 2020;20(7):4901–9.10.1021/acs.nanolett.0c00953Search in Google Scholar PubMed PubMed Central
[59] Luk BT, Zhang L. Cell membrane-camouflaged nanoparticles for drug delivery. J Control Rel. 2015;220(Pt B):600–7.10.1016/j.jconrel.2015.07.019Search in Google Scholar PubMed PubMed Central
[60] Wang Y, Zhang P, Wei Y, Shen K, Xiao L, Miron RJ, et al. Cell-membrane-display nanotechnology. Adv Healthc Mater. 2021;10(1):e2001014.10.1002/adhm.202001014Search in Google Scholar PubMed
[61] Fang RH, Kroll AV, Gao W, Zhang L. Cell membrane coating nanotechnology. Adv Mater. 2018;30(23):e1706759.10.1002/adma.201706759Search in Google Scholar PubMed PubMed Central
[62] Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108(27):10980–5.10.1073/pnas.1106634108Search in Google Scholar PubMed PubMed Central
[63] Hang L, Zhang T, Wen H, Li M, Liang L, Tang X, et al. Rational design of non-toxic GOx-based biocatalytic nanoreactor for multimodal synergistic therapy and tumor metastasis suppression. Theranostics. 2021;11(20):10001–11.10.7150/thno.65399Search in Google Scholar PubMed PubMed Central
[64] Hu CM, Fang RH, Copp J, Luk BT, Zhang L. A biomimetic nanosponge that absorbs pore-forming toxins. Nat Nanotechnol. 2013;8(5):336–40.10.1038/nnano.2013.54Search in Google Scholar PubMed PubMed Central
[65] Luk BT, Fang RH, Hu CM, Copp JA, Thamphiwatana S, Dehaini D, et al. Safe and immunocompatible nanocarriers cloaked in RBC membranes for drug delivery to treat solid tumors. Theranostics. 2016;6(7):1004–11.10.7150/thno.14471Search in Google Scholar PubMed PubMed Central
[66] Meng T, Jiang R, Wang S, Li J, Zhang F, Lee JH, et al. Stem cell membrane-coated Au-Ag-PDA nanoparticle-guided photothermal acne therapy. Colloids Surf B Biointerfaces. 2020;192:111145.10.1016/j.colsurfb.2020.111145Search in Google Scholar PubMed
[67] Wang J, Cheng H, Wang Z, Yang E, Guo F, Wang W, et al. Human small intestine cancer cell membrane-camouflaged quercetin-melanin for antibacterial and antitumor activity. J Biomed Mater Res B Appl Biomater. 2021;109(10):1534–51.10.1002/jbm.b.34813Search in Google Scholar PubMed
[68] Zhang Y, Cai K, Li C, Guo Q, Chen Q, He X, et al. Macrophage-membrane-coated nanoparticles for tumor-targeted chemotherapy. Nano Lett. 2018;18(3):1908–15.10.1021/acs.nanolett.7b05263Search in Google Scholar PubMed PubMed Central
[69] Fiorentino DF, Casciola-Rosen L. Autoantibodies and cancer association: The case of systemic sclerosis and dermatomyositis. Clin Rev Allergy Immunol. 2022;63(3):330–41.10.1007/s12016-022-08944-ySearch in Google Scholar PubMed PubMed Central
[70] Wigerblad G, Kaplan MJ. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat Rev Immunol. 2023;23(5):274–88.10.1038/s41577-022-00787-0Search in Google Scholar PubMed PubMed Central
[71] Torres-Aguilar H, Sosa-Luis SA, Ríos-Ríos WJ, Romero-Tlalolini M, Aguilar-Ruiz SR. Silent red blood cell autoantibodies: Are they naturally occurring or an effect of tolerance loss for a subsequent autoimmune process? Autoimmunity. 2020;53(7):367–75.10.1080/08916934.2020.1799989Search in Google Scholar PubMed
[72] Bae YH, Park K. Targeted drug delivery to tumors: Myths, reality and possibility. J Control Rel. 2011;153(3):198–205.10.1016/j.jconrel.2011.06.001Search in Google Scholar PubMed PubMed Central
[73] Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31(8):318–24.10.1016/j.it.2010.05.006Search in Google Scholar PubMed PubMed Central
[74] Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–75.10.1038/nri3399Search in Google Scholar PubMed
[75] Liew PX, Kubes P. The Neutrophil’s role during health and disease. Physiol Rev. 2019;99(2):1223–48.10.1152/physrev.00012.2018Search in Google Scholar PubMed
[76] Chu D, Dong X, Zhao Q, Gu J, Wang Z. Photosensitization priming of tumor microenvironments improves delivery of nanotherapeutics via neutrophil infiltration. Adv Mater. 2017;29(27). 10.1002/adma.201701021.Search in Google Scholar PubMed PubMed Central
[77] Chu D, Zhao Q, Yu J, Zhang F, Zhang H, Wang Z. Nanoparticle targeting of neutrophils for improved cancer immunotherapy. Adv Healthc Mater. 2016;5(9):1088–93.10.1002/adhm.201500998Search in Google Scholar PubMed PubMed Central
[78] Tavares AJ, Poon W, Zhang YN, Dai Q, Besla R, Ding D, et al. Effect of removing Kupffer cells on nanoparticle tumor delivery. Proc Natl Acad Sci U S A. 2017;114(51):E10871–80.10.1073/pnas.1713390114Search in Google Scholar PubMed PubMed Central
[79] Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275(4):G605–11.10.1152/ajpgi.1998.275.4.G605Search in Google Scholar PubMed
[80] Diagaradjane P, Deorukhkar A, Gelovani JG, Maru DM, Krishnan S. Gadolinium chloride augments tumor-specific imaging of targeted quantum dots in vivo. ACS Nano. 2010;4(7):4131–41.10.1021/nn901919wSearch in Google Scholar PubMed PubMed Central
[81] Cai P, Kaphalia BS, Ansari GA. Methyl palmitate: Inhibitor of phagocytosis in primary rat Kupffer cells. Toxicology. 2005;210(2–3):197–204.10.1016/j.tox.2005.02.001Search in Google Scholar PubMed
[82] Gemsa D, Seitz M, Kramer W, Till G, Resch K. The effects of phagocytosis, dextran sulfate, and cell damage on PGE1 sensitivity and PGE1 production of macrophages. J Immunol. 1978;120(4):1187–94.10.4049/jimmunol.120.4.1187Search in Google Scholar
[83] Magaña IB, Yendluri RB, Adhikari P, Goodrich GP, Schwartz JA, Sherer EA, et al. Suppression of the reticuloendothelial system using λ-carrageenan to prolong the circulation of gold nanoparticles. Ther Deliv. 2015;6(7):777–83.10.4155/tde.15.33Search in Google Scholar PubMed
[84] Ohara Y, Oda T, Yamada K, Hashimoto S, Akashi Y, Miyamoto R, et al. Effective delivery of chemotherapeutic nanoparticles by depleting host Kupffer cells. Int J Cancer. 2012;131(10):2402–10.10.1002/ijc.27502Search in Google Scholar PubMed
[85] Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39(36):4073–126.10.1200/JCO.21.01440Search in Google Scholar PubMed
[86] European Association For The Study Of The L. Corrigendum to ‘EASL recommendations on treatment of hepatitis C: Final update of the series(‘) [J Hepatol 73 (2020) 1170-1218]. J Hepatol. 2023;78(2):452.10.1016/j.jhep.2022.10.006Search in Google Scholar PubMed
[87] Vasdev S, Ford CA, Parai S, Longerich L, Gadag V. Dietary alpha-lipoic acid supplementation lowers blood pressure in spontaneously hypertensive rats. J Hypertens. 2000;18(5):567–73.10.1097/00004872-200018050-00009Search in Google Scholar PubMed
[88] Tahan G, Aytac E, Aytekin H, Gunduz F, Dogusoy G, Aydin S, et al. Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid-induced ulcerative colitis in rats. Can J Surg. 2011;54(5):333–8.10.1503/cjs.013610Search in Google Scholar PubMed PubMed Central
[89] Faddah LM, Abdel Baky NA, Al-Rasheed NM, Al-Rasheed NM, Fatani AJ, Atteya M. Role of quercetin and arginine in ameliorating nano zinc oxide-induced nephrotoxicity in rats. BMC Complement Altern Med. 2012;12:60.10.1186/1472-6882-12-60Search in Google Scholar PubMed PubMed Central
[90] Abdelhalim MAK, Moussa SAA, Qaid HA, Al-Ayed MS. Potential effects of different natural antioxidants on inflammatory damage and oxidative-mediated hepatotoxicity induced by gold nanoparticles. Int J Nanomed. 2018;13:7931–8.10.2147/IJN.S171931Search in Google Scholar PubMed PubMed Central
[91] Khalaf AA, Zaki AR, Galal MK, Ogaly HA, Ibrahim MA, Hassan A. The potential protective effect of α-lipoic acid against nanocopper particle-induced hepatotoxicity in male rats. Hum Exp Toxicol. 2017;36(9):881–91.10.1177/0960327116674526Search in Google Scholar PubMed
[92] Albasher G, Albrahim T, Alsultan N, Alfaraj S, Alharthi MS, Kassab RB, et al. Red beetroot extract mitigates chlorpyrifos-induced reprotoxicity associated with oxidative stress, inflammation, and apoptosis in rats. Environ Sci Pollut Res Int. 2020;27(4):3979–91.10.1007/s11356-019-07009-6Search in Google Scholar PubMed
[93] Yuan Y, Wu F, Zhang F, Li X, Wu X, Fu J. Hepatoenteric protective effect of melanin from Inonotus hispidus on acute alcoholic liver injury in mice. Mol Nutr Food Res. 2023;67(14):e2200562.10.1002/mnfr.202200562Search in Google Scholar PubMed
[94] Shang X, Wang P, Liu Y, Zhang Z, Xue Y. Mechanism of low-frequency ultrasound in opening blood-tumor barrier by tight junction. J Mol Neurosci. 2011;43(3):364–9.10.1007/s12031-010-9451-9Search in Google Scholar PubMed
[95] Wan Z, Li C, Gu J, Qian J, Zhu J, Wang J, et al. Accurately controlled delivery of temozolomide by biocompatible UiO-66-NH(2) through ultrasound to enhance the antitumor efficacy and attenuate the toxicity for treatment of malignant glioma. Int J Nanomed. 2021;16:6905–22.10.2147/IJN.S330187Search in Google Scholar PubMed PubMed Central
[96] Qu F, Wang P, Zhang K, Shi Y, Li Y, Li C, et al. Manipulation of mitophagy by “All-in-One” nanosensitizer augments sonodynamic glioma therapy. Autophagy. 2020;16(8):1413–35.10.1080/15548627.2019.1687210Search in Google Scholar PubMed PubMed Central
[97] Li Y, Teng X, Wang Y, Yang C, Yan X, Li J. Neutrophil delivered hollow titania covered persistent luminescent nanosensitizer for ultrosound augmented chemo/immuno glioblastoma therapy. Adv Sci (Weinh). 2021;8(17):e2004381.10.1002/advs.202004381Search in Google Scholar PubMed PubMed Central
[98] Miller DL, Smith NB, Bailey MR, Czarnota GJ, Hynynen K, Makin IR. Overview of therapeutic ultrasound applications and safety considerations. J Ultrasound Med. 2012;31(4):623–34.10.7863/jum.2012.31.4.623Search in Google Scholar PubMed PubMed Central
[99] Basavarajappa L, Rijal G, Hoyt K. Multifocused ultrasound therapy for controlled microvascular permeabilization and improved drug delivery. IEEE Trans Ultrason Ferroelectr Freq Control. 2021;68(4):961–8.10.1109/TUFFC.2020.3026697Search in Google Scholar PubMed PubMed Central
[100] Ma L, Wu Y, Li Y, Aazmi A, Zhou H, Zhang B, et al. Current advances on 3D-bioprinted liver tissue models. Adv Healthc Mater. 2020;9(24):e2001517.10.1002/adhm.202001517Search in Google Scholar PubMed
[101] Ramaiahgari SC, Ferguson SS. Organotypic 3D HepaRG liver model for assessment of drug-induced cholestasis. Methods Mol Biol. 2019;1981:313–23.10.1007/978-1-4939-9420-5_20Search in Google Scholar PubMed
[102] Brooks A, Liang X, Zhang Y, Zhao CX, Roberts MS, Wang H, et al. Liver organoid as a 3D in vitro model for drug validation and toxicity assessment. Pharmacol Res. 2021;169:105608.10.1016/j.phrs.2021.105608Search in Google Scholar PubMed
[103] Wang C, Chen X, Wang L, Makihata M, Liu HC, Zhou T, et al. Bioadhesive ultrasound for long-term continuous imaging of diverse organs. Science. 2022;377(6605):517–23.10.1126/science.abo2542Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy

