Abstract
The association between electrospinning and polysaccharides corresponds to an important area under exploration, to meet the demands of biotechnological industries claiming for polymers with novel functional properties. Among the group of polysaccharides that attract attention for the manufacture of spun fibers; those from the marine origin that exhibit a remarkable potential, due to the possibilities to act as sulfated glycosaminoglycan mimics; as well as, exhibit an easily modifiable chemical structure that allow the production of derivatives suitable for biotechnological applications. Although electrospinning is a seemingly simple method, its applicability is not an easy task. The problem linked to the spinning of pure biomacromolecules has been generally evaluated embracing polymers from different origins. In this review, the parameters affecting the electrospinning of different marine polysaccharides in their pure form will be considered. The chemical features of these polysaccharides as well as the rheological aspects of their solutions will be in depth analyzed, emphasizing the difficulties associated with the use of water as the working solvent. Strategies used to produce spun fibers from other polymers will be also analyzed in this review, proposing them as an alternative to be studied when the production of spun fibers of marine polysaccharides is envisaged.
1 Introduction
Electrospinning is an electrostatic fiber fabrication technique that allows for the production of submicron fibrillar structures. These structures are referred to as nanofibers when they have diameters of less than 500 nm [1] and are characterized by their large surface-to-mass ratio, high porosity, and mechanical performance; these properties have caught the interest of the scientific community [2,3,4,5].
The large surface-to-mass ratio of nanofibers has encouraged the use of these nanostructures as biotechnological products in a wide range of applications such as tissue engineering, cosmetics, textiles, water filtration, water remediation, sensor fabrication, energy generation, protective clothing, and the production of ultralight materials among others [2,3,6,7,8,9,10].
Despite the availability of various nanofiber fabrication techniques, electrospinning is widely used due to its simplicity, relatively high rate of production, and its versatility, which allows it to spin heterogeneous groups of polymers [3].
Electrospinning has been effectively used to fabricate nanofibers from synthetic and bio-based polymers, but a significant amount of effort has been put into replacing synthetic polymers with biodegradable alternatives due to the serious concerns associated with the former, such as their toxicity, environmental risks, occupational hazards, and the overexploitation of petroleum [11,12,13].
Of the candidates touted to replace synthetic polymers, polysaccharides appear to be an interesting option due to their multiple advantages – it is inexpensive, is abundant, is biocompatible, is biodegradable, is sourced from renewable sources [9,14,15,16,17,18], and has a high production rate when cultivated [19].
The association between electrospinning and polysaccharides is an important area that is currently under exploration [9,13,20,21,22,23,24,25], to meet the demands of biotechnological industries that require polymers that have novel functional properties, are suitable for biological assays, and have environmental applications [9,26,27,28].
Of the groups of polysaccharides, those of marine origin exhibit a remarkable biotechnological potential and have attracted a significant amount of attention regarding the manufacture of spun fibers [11,29]. This group includes chitin, the second most abundant, renewable, naturally occurring polysaccharide [30], and sulfated polysaccharides, which are striking members of these polysaccharide group due to the resemblance of their chemical structures to the glycosaminoglycans from animal cells, which fulfill fundamental roles in cellular processes [27,31,32,33]. This similarity gives these polysaccharides the potential to mimic sulfated glycosaminoglycan [34]. In addition, sulfated polysaccharides have potential applications in other fields (e.g., food science, environmental, cosmetic, and pharmacology), because of their chemical structure decorated with modifiable groups; this allows for the production of polymer derivatives that are suitable for a variety of envisaged applications, such as the removal of metal ions and dyes [9,28], functional additives [14], functional packages [35,36], antitumor activity, drugs and gene delivery, tissue engineering, the encapsulation of active ingredients, antimicrobial activity, and anticoagulant and antihyperlipidemic activities between others [17,26,27,29,36,37,38].
Marine polysaccharides can be extracted from algae, crustaceans, and microorganisms. The physicochemical and biochemical properties of these polysaccharides are dependent on their chemical structures and macromolecular aspects, which are related to the organisms from which they were extracted [17,36]. Chitin is the main component of arthropod exoskeletons and can be found in their respiratory, excretory, and digestive systems, as well as, in the epidermis and eyes of arthropods and cephalopods. Importantly, the chemical structure of chitin somewhat resembles mucopolysaccharides such as hyaluronic acids (specifically the presence of (1 → 4)-linked N-acetyl β-glucosamine units) [30].
Seaweed sulfated polysaccharides refer to the extracellular polysaccharides extracted from brown, red, and green algae; these have been described as bioactive compounds with outstanding properties that can be potentially used in biomedical, food science and technology, environmental, cosmetic, and pharmacological applications [14,17,27,29,37]. The increased interest in seaweed sulfated polysaccharides is due to their complex and diverse chemistry that resembles the structure of glycosaminoglycans as well as the fact that algae are a renewable and underexploited resource.
The microbial marine polysaccharides are an important option from a biotechnological perspective. These polysaccharides can be isolated from bacteria, fungi, and microalgae [26,27,29,39], and they are more interesting due to the advantages associated with their microbial cultures such as their high growth rates, reproducibility (constant physicochemical properties) due to constant monitoring, and the ease at which the bioprocess conditions can be modified [40].
Microbial marine polysaccharides are mainly secretory and can be found as important components of the bacterial capsule, as polysaccharides released to the environment [29,39,40,41], or as part of the extracellular matrix [42]. Polysaccharides that are secreted by the cells are known as exopolysaccharides (EPS) and are large polymers composed of monosaccharide residues linked by glycosidic bonds, and these compounds have high molar masses between 100 and 2,000 kg mol−1 [41]. In addition, there has been a growing interest in marine microorganisms found in extreme marine environments (extremophiles) because of the unique properties exhibited by many of their EPS. Interestingly, many of these extremophilic microorganisms can produce polysaccharides under laboratory conditions [41,43].
Marine polysaccharides possess important properties that are derived from their structure and physicochemical characteristics, and these properties have allowed them to become an emerging and versatile class of biomimetic nanostructures. The variable and complex structures of these polymers (mainly due to their charged groups) have also been associated with the considerable difficulty of producing functional spun fibers from pure polysaccharides.
Marine polysaccharides behave like polyelectrolytes due to the presence of ionic groups, which have different effects on their solubility. Thus, the solvent used to prepare these polymer solutions has a significant influence on their spinnability due to the intermolecular interactions in the polymer–solvent system [3]. This review will consider the variables that can affect the electrospinning process, such as the solution parameters and processing parameters, and will also focus on the problems associated with the electrospinning of pure marine polysaccharides.
2 Challenges during electrospinning
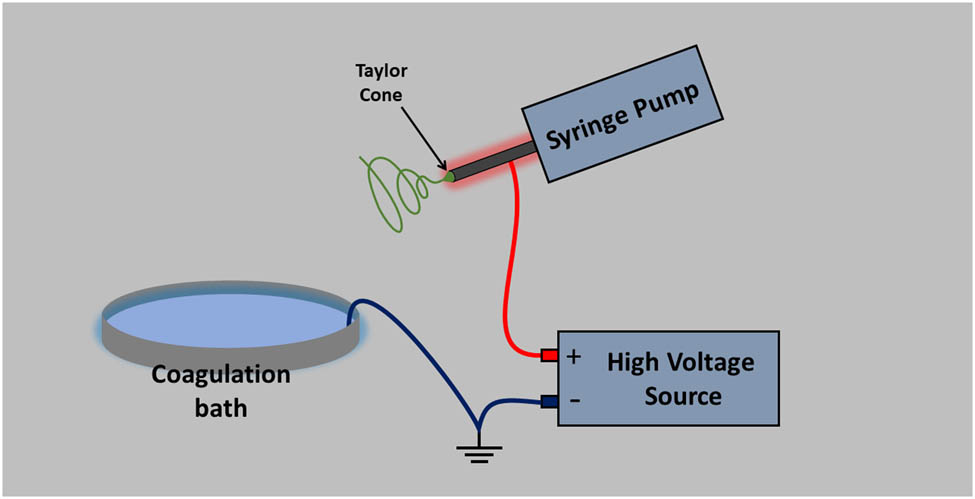
Electrospinning involves the application of a high-voltage electrostatic field to a polymer solution droplet, which charges its surface, causing the ejection of a solution jet through a spinneret (Figure 1). The strong electric field (in the kV range) increases mutual charge repulsion on the surface of the droplet, allowing it to obtain a surface charge that changes the shape of the droplet from spherical to conical. The conducting liquid will exist in the form of a cone (Taylor cone) from which the liquid jet is ejected and accelerated towards a grounded collector. The jet initially extends in a straight line, and then rapid whipping occurs as a result of bending instabilities, leading to the solvent evaporation. This causes the jet to be stretched to finer diameters before it solidifies and is deposited, leading to the formation of a fine fiber structure [1,3,44,45].
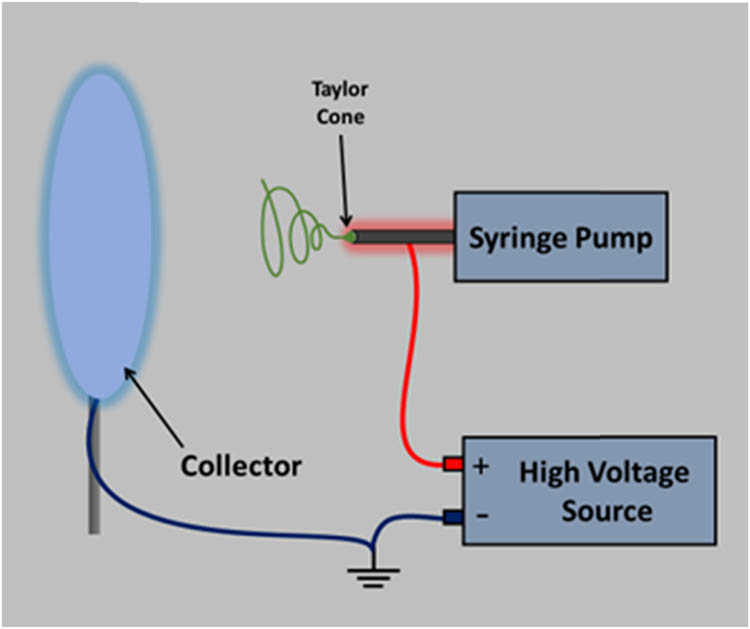
Schematic illustration of electrospinning setup.
Although electrospinning appears to be a simple method, it is not easily applicable as multiple factors must be adjusted during the process before suitable solution properties and adequate instrumental processing conditions can be found [1,3,25,44].
This is especially true in the field of polysaccharides, and the solution properties and processing conditions enormously differ from one polysaccharide to another, making it difficult to tune the electrospinning parameters and produce stable fibers with desirable morphologies and characteristics [24,25,46].
To illustrate the complexity associated with the electrospinning of polysaccharides, the parameters and conditions that influence the electrospinning process are described in detail as follows:
Ecophysiology conditions: Polysaccharides extracted from seaweeds exhibit an inherent variability due to the effects of factors such as seasonality, the period of collection of the algae species, and growth conditions [47,48,49,50,51]. Each of these aspects has an important effect on the structural character of polysaccharides, which in turn also affect the physicochemical properties of these biopolymers.
Chemical structure of polysaccharides: These are the features that define the physicochemical properties of these polymers such as the composition and sequencing of the monosaccharides, the presence of charged groups (including their type, number, and location), the molecular architecture (whether the molecule is branching or linear, as well as its chain length), the glycosidic linkage patterns, the molar mass of the polymer, the distribution of this molar mass, the stiffness associated with its native state, and conformation. Several of these properties have been explored in the literature [29,49,52,53,54,55,56,57,58,59,60,61] and are related to the rheological behavior of polysaccharides.
Processing parameters during the polymer extraction: Several authors have shown that the method by which the polysaccharides are extracted can modify the original chemical structure of polysaccharides and consequently affect their physicochemical behavior [30,49,70,71,62,63,64,65,66,67,68,69]. In addition, the presence of extraction residues has been referred to as an important factor that can affect the homogeneity of the fibers [22].
Solvent characteristics: This refers to the chemical features of the solvent, which affect key solution parameters that influence the outcome of the electrospinning process. Relevant characteristics include the solvent’s volatility, vapor pressure, boiling point, viscosity, surface tension, and conductivity, as well as its toxicity which is an important consideration for its suitability and the potential applications of the electrospun mat [3,4,10,13,72,73].
Solution parameters and properties: This refers to the characteristics that determine the success of the electrospinning process. These properties are intimately linked to the chemical structure of the solvent and polysaccharides, such as polymer concentration [55,74], pH [56,58,75], surface tension, conductivity, viscosity [4], viscoelasticity [76], and volatility [72].
In addition to these processing parameters, the success of electrospinning is highly dependent on the solution parameters, which are intimately linked to the polysaccharide chemical structure and the solvent employed. The main factors that affect the characteristics of marine polysaccharides in aqueous media will be further discussed in this review, with the aim of describing the principal concerns associated with the spinning of pure marine polysaccharides.
3 Chemical structure of polysaccharides
According to the IUPAC-IUBMB Joint Commission on Biochemical Nomenclature, polysaccharides are defined as macromolecules that consist of a large number of monosaccharides joined to each other by glycosidic linkages [77]. These monosaccharides are classified as single units of aldoses or ketoses depending on whether aldehyde or ketone groups are present, respectively. Monosaccharides are typically composed of five (pentoses) or six (hexoses) carbons and can exhibit two types of ring closure (pyranoses or furanoses) and anomericity (α or β) [77].
There is a wide variation in functional groups that can appear in the monosaccharide structure, and this includes the replacement of the hydrogen atom of a hydroxyl group with another element (e.g., sulfate, phosphate) or the replacement of the entire hydroxyl group, such in deoxy sugars (e.g., rhamnose and glucosamine) [77]. Furthermore, monosaccharides can be acidic when they contain a carboxyl group, as in the case of uronic acids (e.g., glucuronic acid). The diversity of monosaccharide structures, their distinct molecular architectures (linear or branching), and the bond configurations of the polymer backbone can easily explain the heterogeneity and complexity found in polysaccharides.
Marine polysaccharides are a good example to illustrate the variability of these macromolecules as they exhibit a diverse set of chemical structures, which are associated with very different sources from which they are extracted (e.g., crustaceans, algae, and microorganisms) [11,14,27,29,36]. Chitin, chitosan, alginate, ulvan, and mauran are all marine polysaccharides that are extracted from different sources, and each of these exhibits very different chemical behaviors. These polysaccharides form the framework of this review and will be used to explain the drawbacks associated with the electrospinning of pure polysaccharides with respect to their diverse structural features.
Chitin is a polysaccharide formed by 2-(acetylamino)-2-deoxy-β-glucose (acetylated glucosamine) units linked by 1 → 4-glycosidic bonds [57]. This biopolymer exhibits three different polymorphisms (α, β, and γ sheets) that are generated by the polymerization of microfibrils with different orientation patterns (Figure 2), and its structure is very similar to that exhibited by the hyaluronic acid and glycosaminoglycan [30].
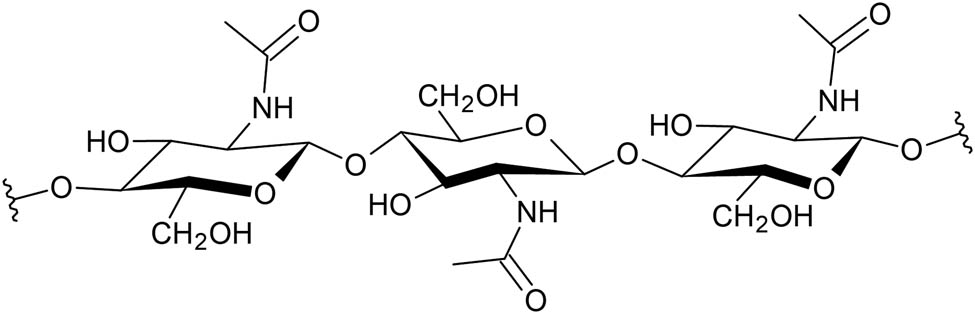
Structure of chitin.
Chitosan is the deacetylated form of chitin and can exhibit varying degrees of deacetylation (typically about 60%) [58,78] (Figure 3). It is produced industrially from different sources of chitin, but it can also be found naturally in some fungi [30] and the shells of crustaceans [79].
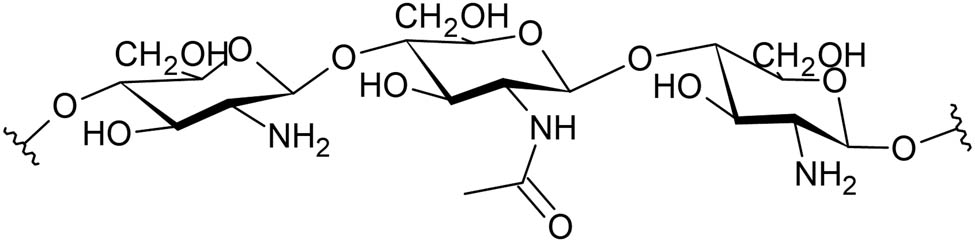
Structure of chitosan.
Alginate is a linear anionic polysaccharides extracted from brown seaweeds and can also be synthesized from Azetobacter and Pseudomonas spp. [48]. It is composed of (1,4)-linked β-d-mannuronic and α-l-guluronic acids that can appear in different proportions and sequences [48,53,54,75] (Figure 4). It possesses an abundance of free hydroxyl groups [52], from which the H can be easily replaced through chemical reactions such as sulfation [20,34], generating interesting derivatives that resemble the structure of glycosaminoglycans. Alginate has emerged as mimic candidates on the basis of the relationship found between alginate sulfate and cellular processes [34]. In addition, alginates are widely used as food gelling, thickening, and stabilizing agents [75].
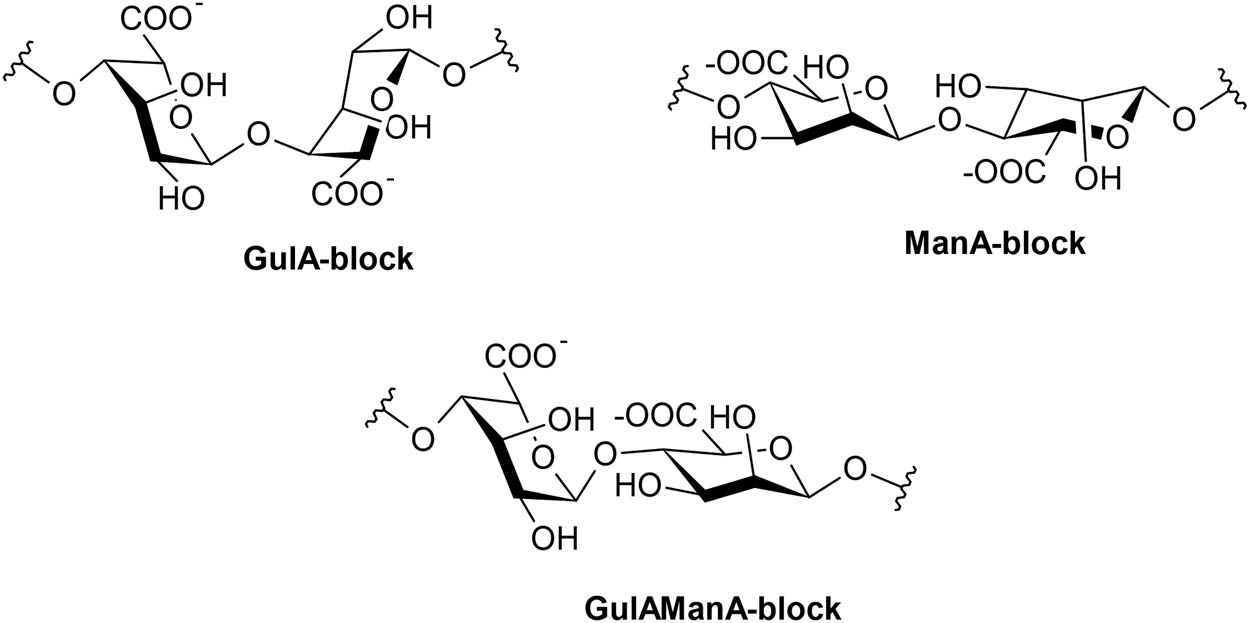
Chemical structure of sodium alginate. GulA, α-l-guluronic acid; ManA, β-d-mannuronic acid.
Ulvan is a lightly branched polysaccharide with broadly distributed charge densities and molar masses. It is extracted from green seaweeds, and its backbone is primarily composed of α- and β-(1,4)-linked monosaccharides (rhamnose, xylose, glucuronic acid, and iduronic acid) [12,37,49,50,69,80,81,82]. Minor proportions of galactose and/or glucose [37,47,69,80,81,82,83] as well as mannose have been also reported in ulvan [69,83].
Glucuronic acid and rhamnose units occur primarily in the form of aldobiuronic acids and make up the main repeating disaccharide units in the backbone structure of ulvan. These repeating units are characterized by the abundance of two types of negatively charged groups: sulfate esters and carboxylate groups [12,50,75], which give this polysaccharide its polyanionic character (Figure 5).
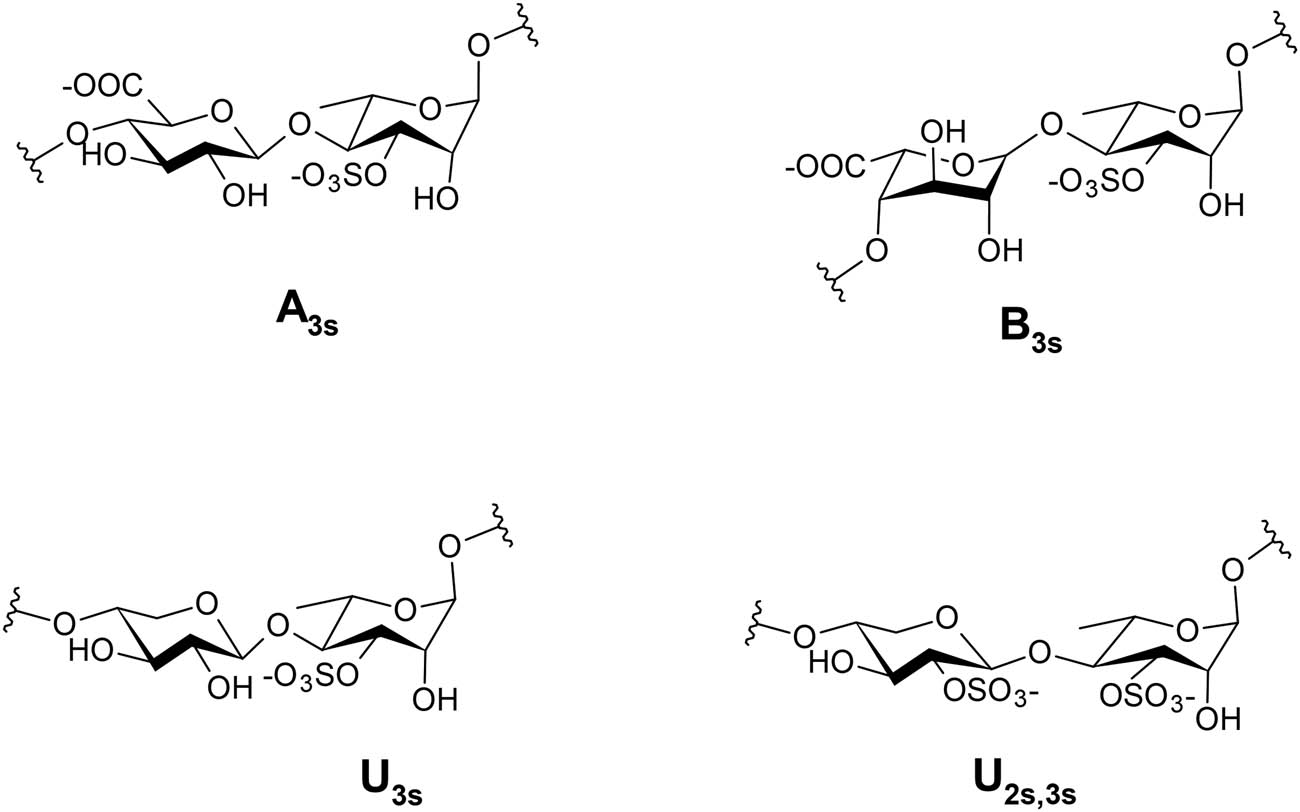
Structure and nomenclature of the main repeating disaccharides: ulvanobiuronic acids (A3S and B3S) and ulvanobioses (U3S and U2S,3S) acids.
Mauran is a sulfated EPS produced by the halophilic bacteria Halomonas maura. Mauran refers to highly anionic polysaccharide composed of sugars such as mannose, galactose, glucose, and glucuronic acid. It is characterized by its high uronic acid content and the presence of phosphate and sulfate groups. This polysaccharide can resist various stress conditions as well as the binding of heavy metals, making it an ideal candidate for industrial and biomedical applications [26,29].
As mentioned earlier, these marine polysaccharides exhibit a wide diversity of chemical structures, having in common an ionic character due to the presence of different charged groups in their backbones. The presence of the charged groups in these biopolymers favors the development of electrostatic interactions that will control their conformations in solution, which consequently influences the behavior of these polysaccharides in aqueous media and other solvents.
4 Extraction
The extraction process and processing conditions may strongly influence the chemical structure (i.e., molar mass distribution and chain length) of the polysaccharides and therefore their potential applications. As a result, the research community is continuously evaluating different extraction methodologies to improve the efficiency of the process while considering its potential applications.
The extraction of chitin from crustacean shell waste involves deproteinization, demineralization, and decolorization [30]. Deproteinization involves the use of dilute sodium hydroxide solution at elevated temperatures. As referred by Yeul and Rayalu [30], severe conditions during the deproteinization treatment could cause structural changes in the polysaccharide because of depolymerization and deacetylation.
After the deproteinization step, the demineralization is achieved through the use of HCl at ambient temperature, followed by the decolorization, which involves the use of acetone and sodium hypochlorite to remove carotenoids and bleaching the sample, respectively. Yeul and Rayalu [30] suggested that the differences in the strength of chitin–pigment bonds between crustacean species could generate variations in the conditions of the decoloration process, and the stronger the bonds, the harsher the process.
To convert chitin into chitosan, some acetyl groups must be removed by deacetylation. This process is usually performed by using concentrated hydroxide solution at temperatures equal to or higher than 100°C. Critical factors that affect this process include temperature, time, the concentration of the alkali, the processes that were used to isolate the chitin, the density of the chitin, and the particle size. Yeul and Rayalu [30] noted that higher temperature tend to increase the degree of deacetylation and also reduce the molecular size.
The extraction of polysaccharides from plant sources requires an alternative sequence of events that includes solvent infiltration, polysaccharide dissolution, and diffusion [66]. Hence, different methodologies have been proposed to improve the yield of the process while not altering the chemical structure and rheological characteristics of the extracted polysaccharides.
The methodologies applied in the extraction of polysaccharides from terrestrial plants and seaweeds include hot water extraction, alkaline extraction, acidic hot water extraction, enzyme-assisted hot water extraction, ultrasonic-assisted hot water extraction, microwave-assisted hot water extraction, and freeze–thaw cold pressing. These methodologies employ high temperatures, chemical solvents, biocatalysts, sound waves, and electromagnetic radiation as strategies to increase the solubility of the polysaccharide chains [62]. Macroalgae have received special attention for several years due to the unique properties of their sulfated polysaccharides, which can be used in industrial applications. In contrast to terrestrial plants, algal cell walls do not contain lignin. Thus, extraction can be performed under milder conditions [64].
Pretreatment and extraction processes are not usually performed separately during the isolation of the macroalgal polysaccharides [84]. Pretreatment processes primarily focus on the swelling of macroalgal fibers by increasing their pore sizes and by reducing sizes of the biomaterial via mechanical, thermal, chemical, and biological processes [84]. Novel technologies such as ultrasound, microwave, and pulsed electric fields have been used to improve extraction efficiency. As mentioned by Ummat et al. [70], the stages at which these technologies are applied during the extraction will have significant effects on the extraction time, yield, energy consumption, and bioactivity of the extracted molecules.
Sulfated polysaccharides are usually extracted with water, acidic solvent, or alkaline solvent followed by precipitation with alcohol or quaternary salt [63]. Acidic or alkaline conditions are often used to facilitate extraction as the hydrogen ion and hydroxyl ions interfere with the hydrogen linkages between polysaccharides [84].
The most commonly used strategy in the extraction of algal polysaccharides is hot water extraction, which disrupts the cell walls and facilitates mass transfer [63,71]. Another advantage of this strategy is the prevention of the autolysis of the polysaccharide at low temperatures over a long time due to the action of enzymes that are naturally present in the raw material. However, hot water extraction is known to be time consuming and energy intensive [62]; in addition, there is an inverse correlation between extraction yield and polymer stability when the process is conducted above a threshold temperature [62,64,65].
Acidic extraction at high temperature involves the use of dilute acetic or hydrochloric acid, which is associated with the degradation of the polysaccharide and affects its degree of polymerization [85] and degree of sulfation [69]. This methodology is associated with the hydrolysis of the linkages of sulfated and nonsulfated polysaccharides. Consequently, long acid extraction times at high temperatures lead to low sulfate contents, low molar masses, and modified sugar structures [63]. As mentioned by Kidgell et al. [49], extraction parameters such as the temperature, pH, and duration of the process influence the degree of polymerization in polysaccharides such as ulvans.
For some polysaccharides, alkaline extraction is used as the primary step of the purification process. For example, the extraction protocol of alginate includes acidification, alkaline extraction, solid/liquid separation, precipitation, and drying. Acidification is used to convert the soluble alginate salts into alginic acid. The insoluble alginic acid is then soaked in a sodium carbonate solution and is converted to soluble sodium alginate (SA), which is then passed into the aqueous phase. Following this, sulfuric acid or calcium chloride is added to precipitate the alginates as alginic acid or calcium alginate, respectively. Finally, the alginate salts are produced by reacting alginic acid with the appropriate base [52].
As mentioned by Jönsson et al. [84], both acid and alkaline extraction had several disadvantages, such as long extraction times, high costs, health hazards, and the degradation of the polysaccharide to compounds of smaller molecular size. Jönsson et al. [84] compared these with other extraction methods such as ultrasound and microwave-assisted extraction, which only require short extraction times and small amounts of solvents but can induce polymer degradation. Enzyme-assisted extraction is another method in which it is possible to isolate sensitive bioactive compounds; however, the target compounds can be degraded by nonspecific enzymes. Finally, the authors described supercritical fluid extraction, a process that does not degrade bioactive compounds but requires high pressures, which has a negative effect on the product and results in increased costs.
Regardless of the methodology, the quantity and quality of the extracted polysaccharide are determined by various factors involved during the process, such as the properties and pretreatment of the biomass, the particle size, the extractant selected, the extractant-to-biomass ratio, the pH, the extraction temperature, and the duration of the extraction. The combined effect of some of these parameters has been discussed by various authors [49,61,63,64,70,71,86].
Variations in the molar mass of polysaccharides have been associated with their origin, cultivation [12,51,71], mode of extraction [12,49,63,64,65,71], solvent pretreatment, and size of the algae powder (during the extraction), which can also affect its polydispersity [61].
Thus, the combined effects of the above mentioned factors will define the extent to which it will be possible the compliance of the criteria pointed by Kidgell et al. [49] to define the proper extraction process: high yield, high selectivity, and low degradation of the polysaccharide. The stages during the methodologies can affect the molar mass and the composition of the monosaccharides, which have an important effect on the electrospinning results (this will be discussed later). In addition, the preservation of the original structure of polysaccharides must be considered and evaluated, especially in terms of their functionality and potential applications.
5 Choice of the solvent in the electrospinning of polysaccharides
The selection of an appropriate solvent during the formulation of spinnable mixtures is an important prerequisite for the success of the electrospinning. It is important to remember that the dissolution of the polymer is the first step in the process, in which the chemical features of the solvent will play a critical role [87].
It is important to note that one of the main drawbacks of working with polysaccharides is their insolubility in most solvents. Indeed, there are only a few solvents (or solvent mixture) that can dissolve polysaccharides [13,23,57,88,89,90,91,92].
The formulation of suitable polysaccharide solutions has been tested with a large number of solvents such as water, strong acids, acetic acid, formic acid, dimethyl formamide, dimethyl sulfoxide, halogenated compounds, basic systems, and ionic liquids (ILs) among others, with the aim of formulating suitable polysaccharide solutions [9,13,23,67,79,89,91,92,93,94]. Several of these studies have emphasized the insolubility of polysaccharides in water or organic solvents, highlighting the fact that converting some polysaccharides into functional forms is never straightforward [87,88,92].
In addition to the difficulties associated with finding a solvent that can guarantee the polysaccharide dissolution, there is another important criterion to be considered when the formulation of spinnable mixtures is envisaging, i.e., the influence of the solvent over critical spinning parameters such as viscosity, surface tension, electrical conductivity, dielectric constant, and volatility of the mixture [3,13,44,87,93,95,96].
Furthermore, the biocompatibility and eco-friendly characteristics of the solvent must also be considered [13,97,98]. Many of the organic solvents that are used to dissolve polysaccharides are toxic, limiting their use in bioactivity assays and environmental projects [57,72,88]. When considering all of these restrictions, water remains a solvent that is used in many functions; it is safe, is eco-friendly, and is the medium in which many biochemical reactions take place. From a chemical perspective, water is a highly versatile solvent whose solubility can be adjusted by varying its pH, temperature, or adding surfactants. Its production, transportation, and disposal are cheap [2,55,97].
Despite the aforementioned advantages, there are several disadvantages to choosing water as the solvent for electrospinning mixtures, such as (1) the insolubility of some polysaccharides in the water [57], (2) high viscosity and tendency of water toward gelling behavior [75]; and (3) the low stability of the spun mats in aqueous media (as a post spinning process concern) [2,23,54,99].
From a chemical point of view, water molecules are composed of two hydrogen atoms covalently bonded to an oxygen atom in a triangular arrangement. This atomic disposition results in the creation of dipoles that attract the neighboring water molecules by their opposite ends, producing a three-dimensional tetrahedral network in which each oxygen atom is strongly hydrogen bonded to four other oxygen atoms [87,100]. Thus, water is a protic solvent characterized by its strong hydrogen bond donor capability, which allows it to interact with solutes that have hydrogen bond acceptors [87]. Because of its dipole characteristics, water is a good solvent for ionic compounds in which the surface ions of the crystalline lattice attract the oppositely charged poles of the water molecules [100].
The primary characteristic that determines whether a polysaccharide can dissolve in water is the presence of charged groups. These polysaccharides behave like polyelectrolytes because of the effect of various ionic groups, such as carboxyl (e.g., alginates) [52] and/or sulfate groups (e.g., carrageenan, ulvan, fucoidans, and mauran) [27,28,55], which can increase the molecular water affinity of the polymer through the prevention of polysaccharidal interchain association via electrostatic repulsions [55].
The mere presence of charged groups in the structure of polysaccharides does not guarantee that it is soluble in water. Indeed, several marine polysaccharides that contain N-acetyl groups (e.g., chitin) or amino groups (e.g., chitosan) are not water soluble [57]. This is due to the effect of additional structural features and properties of the polysaccharides, which can modify their behavior and thus their solubility property such as the number of ionic charges along a chain, the distance between ionic sites, the degree of polymerization, and their conformation [55,56,101]. In fact, as Dedic et al. [96] found that the valence, polarizability, and the size of the ion will exert a strong influence on the structure of the water molecules in its vicinity, which affects the solubility.
Understanding the different effects of functional groups in a polysaccharide on its solubility can be accomplished by first considering the effects of their structural OH groups. It is important to note that while OH groups contribute (via molecule–solvent interactions) to the dissolution of the polysaccharide in water, it is also true that the interactions between polysaccharide chains via hydrogen bonds can also prevent the polymer from being dissolved in aqueous solutions and other solvents [55,88].
Chitin can be used to illustrate the relevance of the previous statement. Chitin is characterized by its acetyl and amino groups; however, it is widely known for its insolubility in water and conventional solvents and its rigid crystalline structure [57,102].
The structure of chitin is composed of a strong network of intermolecular and intramolecular hydrogen bonds. The first type of bond is formed (1) between N-acetyl groups and (2) between N-acetyl and hydroxymethyl groups (not detected in β chitin), while the second type is formed between the consecutive glucosamine units that maintain the length of the chain repeating distance, which keeps the 2-fold helical conformation of the rigid structure of chitin in a solid state [57,58].
Simulations of molecular dynamics based on the distributions and helical conformation of chitin performed by Franca et al. [58] show that chitin in the aqueous solution tends to maintain a 2-fold helical conformation, which is stabilized by intramolecular HO3(n)···O5(n+1) hydrogen bonds between neighboring monosaccharides. Furthermore, the authors noted that there was an inverse relation between the stability (lifetime) of the HO3(n)···O5(n+1) hydrogen bonds and the solubility of chitin and reported high stability of the HO3(n)···O5(n+1) hydrogen bonds respect to other interactions, thus concluding that chitin in the aqueous solution exhibit similar rigidity to that in its crystalline state.
Due to the rigid structure of chitin, only harsh solvents (strong acids, halogenated compounds, and basic systems) with the capacity to break hydrogen bonds and high ionic strength can solubilize it [57,90]. Unfortunately, the high cost and toxicity associated with some of these solvents make the process unsuitable for most applications.
Alternatively, pure chitin with high molar masses can be electrospun into fibrous mats through the use ILs as solvents [13,89,92], which possess physicochemical characteristics that can be quite different from those of conventional volatile organic compounds (VOCs), as they are nonvolatile, much more viscous, highly conductive, and capable of dissolving biopolymers that are not soluble in VOCs [13]. However, the dissolution of chitin in ILs is not a straightforward task. As discussed by Shamshina [103], the different chitin qualities of chitin, such as its polymorphic forms, molecular mass, and degree of acetylation (DA), as well as chemical aspects of the ILs (i.e., anions or type of the cationic ring) and dissolution conditions, can significantly affect the results.
As the IL stream cannot be evaporated in-flight, it must be collected in a coagulation bath (Figure 6), using solvents such as water and ethanol [13,89,92]; this is acceptable due to the insolubility of chitin in water. The fibers are formed while the IL is solubilized. It is important to note that the high viscosities of the chitin−IL solutions only allow for electrospun solutions with low polymer concentrations (˂1–2.5 wt%) [13,89,92], adding the difficulty to control the process due to the lack of IL volatility [89].
Schematic representation of electrospinning from IL solutions.
Barber et al. [89] evaluated the ability of 1,3-diethylimidazolium acetate [C2C2Im][OAc] and 1-ethyl-3-methylimidazolium acetate [C2C1Im][OAc] to produce fibers from different chitin sources. The authors found that the latter was a more appropriate ILs solvent after considering the final viscosity of the solution, the undissolved residue, and the chance of any degradation that would affect the results (Table 1, entry 1). The authors reported the production of novel high molar mass chitin nanofibers directly from shrimp shells in a one-pot system. Zavgorodnya et al. [13] also produced chitin fibers using the same ILs selected by Barber et al.[89], reporting differences in the results due to the source of the chitin and preprocessing effects (Table 1, entry 2). Recently, Gorzędowski et al. [92] tested five different chitin sources (of which four were of marine origin) and six ILs: 1-ethyl-3-methylimidazolium acetate (EMIM OAc), 1-butyl-3-methylimidazolium chloride (BMIM Cl), 1-butyl-3-methylimidazolium bromide (BMIM Br), 1-ethyl-3-methylimidazolium chloride (EMIM Cl), and 1-allyl-3-methylimidazolium chloride (AMIM Cl). The effective production of chitin fibers production was only possible from the chitin source that exhibited the highest degree of N-acetylation and a lower degree of crystallinity. After being regenerated from BMIM OAc, a solvent-free product was obtained after the fibers were repeatedly washed in water (Table 1, entry 3; Figure 7).
Nanofibers produced from marine polysaccharides: composition, processing parameters, and main characteristics
| No. | Polysaccharide | Co-polymer | Solvent | Additive | Polysaccharide concentration: polysaccharide/co-polymer (ratio) | Electric field (kV/cm) | Flow rate (mL/h) | Spun mat characteristics | Postspinning treatment | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PG-chitin (low M) | — | [C2C2Im] [OAc] [C2C1Im] [OAc] | — | 1.50% | 2.21.5–2.2 | 0.05 and g(a), 3 (or) 30-g/3 | Electrospraying/electrospinning of PG-chitin in [C2C2Im][OAc] showed non-consistent fiber formation or fibers with deformed surface. Smooth continuous fibers were produced from shrimp chitin (0.45%) in [C2C1Im][OAc]. [C2C1Im][OAc] has the ability to extract high M chitin from shrimp shells (different from [C2C2Im][OAc]). High M chitin extracted provided optimal viscosity, concentration, and entanglement density for ESP. | — | [89] |
| Source: commercial | 0.45–0.67% | |||||||||
| Chitin (high M) | ||||||||||
| Source: shrimp shells | ||||||||||
| 2 | PG-chitin | _ | [C2mim] [OAc] | _ | 0.2–1.0% | 2.6–2.8 | p/g(b) | Different spinning results were related to the chitin source: droplets and short NF were observed from PG-chitin solutions, while efficient NF formation was showed for P-chitin and R-chitin at ≥ 0.3%. Divergent ESP behaviors were observed at higher P-chitin and R-chitin concentrations (0.75–1.0%): NF, gel particles (P-chitin) or gelation (R-chitin). It was suggested that these divergences could be related to M. Chitin concentration, M and viscosity were key in the EPS, being less dependent on conductivity and surface tension. The diameter of NF increased with the external pressure applied. | IL was removed by keeping the mats in pure DW, which was changed five times. The electrospun mats were air-dried on a porous Teflon-coated mesh. | [13] |
| Source: commercial (extracted from shrimp shells) | ||||||||||
| P-chitin | ||||||||||
| Source: shrimp shells (thermally preprocessed) | ||||||||||
| R-chitin: shrimp shells (unprocessed frozen) | ||||||||||
| 3 | Chitin1 (M: 177 Kda, DA: 80.1%) | _ | EMIM OAc | _ | 2.5% Chitin3 | N.R. | N.R. | Effective dissolution occurred only for Chitin3/BMIM OAc (high viscosity solution at >2%). Chitin3 exhibited the lowest M and highest DA. Chitin3 (regenerated from IL) in the form of microfibers (dd: 0.3–70 µm), exhibited higher DA (5.3%), lower grade of crystallinity and lower M (55 kDa). Chitin fibers were generated through direct-solution blow spinning. EtOH solution (5%) was used as coagulation bath. | The chitin nonwoven was washed three times in DW and dried in an oven (2 h at 100 °C) | [92] |
| Chitin2 (M: 237 Kda, DA: 78.3%) | BMIM OAc | |||||||||
| Chitin3 (M: 61 Kda, DA: 86.4%) | BMIM Cl | |||||||||
| Chitin4 (M: 335 Kda, DA: 82.3%) | BMIM Br | |||||||||
| Chitin1, Chitin2 | EMIM Cl | |||||||||
| Source: commercial | AMIM Cl | |||||||||
| Chitin3, Chitin4 | ||||||||||
| Source: shrimp shells (Pandalus borealis) | ||||||||||
| Chitin1, Chitin3: powder | ||||||||||
| Chitin2, Chitin4: flakes | ||||||||||
| 4 | Chitosan (M: 30 kDa, DD: 56%), Chitosan (M:106 kDa, DD: 54%), Chitosan (M: 398 kDa, DD: 65%) | _ | Aq.AcOH: (1, 10, 30, 50, 70, and 90%) | _ | _ | 4 | 1.2 | AcOH solutions (1%) were not electrospinnable irrespective of chitosan concentration. AcOH (90%) was the most suitable solution. ESP of chitosan (9.5–10.5%, 30 kDa)–AcOH (90%) produced large size beads, several droplets, and fragile fibers. ESP of chitosan (2.5–3%, 398 kDa)–AcOH (90%) produced very fine fibers (d: 60 nm) with rougher surfaces. ESP of chitosan (106 kDa)–AcOH (90%) produced continuous and uniform fibers only at 7–7.5% chitosan. AcOH concentration was the most important parameter in the ESP, influencing surface tension and charge density of the jet. | _ | [93] |
| Source: commercial powder | ||||||||||
| 5 | Chitosan powders (M: 300 kDa, DD: 90%) | _ | AcOH (90%) | _ | 5% | 3.4 | N.R. | NF d: 75 nm were produced from Chitosan (5%)–AcOH (90%) and collected on PET scrim to form composite NF membranes. | The composite membranes were cross-linked with glutaraldehyde vapor at room temperature for 12 h. NF membranes were tested for Cr(IV) ions adsorption, showing to be effective in this process. | [94] |
| Source: commercial powder | ||||||||||
| 6 | Chitosan 10 (M: 2.1 × 105, DD: 78%) | PVA (8.8 × 104), degree of polymerization ≈ 2,000 | FA | _ | 9% PVA in DW | 1 | N.R. | 100/0 Chitosan/PVA was not associated with jet formation (even at >25 kV). The ESP of 90/10 Chitosan/PVA formed beads, while thin fibers and beads were observed from 70/30 Chitosan/PVA. Homogenous fibers with d: 120 nm and dd: 83–170 nm were produced from 50/50 Chitosan/PVA. Homogenous fibers with d: 170nm; dd: 110–220 nm were produced from 30/70 Chitosan/PVA. | _ | [72] |
| Source: commercial | 7% Chitosan 10 in FA: 100/0 Chitosan 10/PVA | |||||||||
| 90/10 Chitosan 10/PVA | ||||||||||
| 70/30 Chitosan 10/PVA | ||||||||||
| 50/50 Chitosan 10/PVA | ||||||||||
| 30/70 Chitosan 10/PVA | ||||||||||
| Chitosan 10 (M: 2.1 × 105, DD: 78%) | _ | TFA and DMC-TFA | — | 5–8% Chitosan | 1 | N.R. | TFA as solvent let the formation of fibers. The NF morphology depended on polysaccharide concentration: Chitosan-10 (5–6%): beads and fibers coexisted on the collector. Chitosan-10 (7%): NF (d: 490 nm and dd: 330–610 nm ) predominated and the bead fraction decreased. Chitosan-10 (8%): An almost homogenous network of NF (d: 490 nm and dd: 390–610 nm) was produced. The formation of TFA amino groups of chitosan salts and the high volatility of TFA were proposed as possible reasons for a successful ESP. The TFA/DMC mixture let the production of more homogeneous NF: d: 380 nm and dd: 200–660 nm (8% Chitosan-10/TFA:DMC 80:20), d: 390 nm and dd: 230–650 nm (8% Chitosan-10/TFA: DMC 90:10), d: 330 nm and dd: 210–650 nm(8% Chitosan-10/TFA:DMC 70:30). At TFA: DMC 70:30, the chitosan partly separated from the solution. | _ | ||
| Source: commercial | ||||||||||
| Chitosan 100 (M: 1.3 × 106, DD 77%)Source: commercial | PVA (8.8 × 104), degree of polymerization ≈ 2000 | FA or AcOH (0.2M) | — | 2% Chitosan 100 in FA, (or 0.2M AcOH) /PVA (9%) | 1 | N.R. | NF with d: 170 nm and dd: 120–220 nm were produced. | _ | ||
| 50/50 Chitosan/PVA | ||||||||||
| 7 | β-chitinSource: cuttlefish bone from a fishery market | PEO (M: 900 kDa) | FA | _ | β-chitin solution and 2% β-chitin in FA and PEO: 2/0.1 β-chitin/PEO |
2.1 | 0.8–1.2 | ESP of β-chitin solution produced relatively spherical beads and not fibrous structure. The electrospinnability of β-chitin/PEO increased from 2/0.1 to 2/1. PEO reduced the surface tension of β-chitin solution and increased the chain entanglement. NF produced from the β-chitin/PEO blend (2/1) exhibited d: 417 nm that decreased to 297 nm after soaking in water. | Pure β-chitin nano- fibers were obtained by soaking the β-chitin/PEO fibers in water to eliminate the water-soluble PEO. β-chitin/PEO nanofibers were evaluated for biocompatibility and wound healing effects. The wound healing ability of β-chitin nanofibers was better than that of β- chitin/PEO | [67] |
| 2/0.5 β-chitin/PEO 2/1 β-chitin/PEO | ||||||||||
| 8 | Sodium alginate (SA)m (MV, M: 490 ± 60 kDa, ManA/GulA: 1.5) | PEO (M: 900 kDa) | DIW | TritonTM X-100 | 4% (SA/PEO) solutions mixed according: 70/30 SA/PEO | 0.73 | 0.5 | Strongly different spun membranes were produced depending on the SA used and characterized after post-spinning treatment: A collapsed membrane with low global porosity (d: 150 ± 30 nm) was obtained from the (SA)m mixture. A mat of thin fibers and highly porous structure and d: 100 ± 30 nm was produced by using the mixture containing (SA)g. The ManA/GulA ratio and the length of the chain influenced the behavior of SA in salt-free solutions. SA and PEO strongly interact with a marked effect for ManA-rich samples. The mat with best properties was obtained from (SA)g. | Crosslinking solution (EtOH/H2O 40/60 containing 3% of CaCl2 for 30 min). The thermo-gravimetric analysis showed that the mat was composed of only SA. The authors proposed that PEO was probably totally dissolved during the electrospinning process | [53] |
| Sodium alginate (SA)l (LV, M: 145 ± 2 kDa, ManA/GulA: 1.5) | ||||||||||
| Sodium alginate (SA)g (MV, M: 360 ± 60 kDa, ManA/GulA: 0.5) | ||||||||||
| Source: commercial | ||||||||||
| 9 | Sodium alginate (SA) (LV, GulA/ManA: 6.6) Source: commercial |
PVA (M: 146–186 kDa), 99% hydrolysis | DIW | Triton TM X-100 | SA was add to PVA (4%) to produce 5.68–7.15% aq. SA/PVA at mixtures ratios: 3/7 SA/PVA | 0.75–1 | 0.5 | Uniform fibers without beads were produced from 3/7 SA/PVA mixture. A higher SA ratio (3.55/6.45 SA/PVA) produced few beads along the fibers. Fiber sizes and increasing numbers of micrometer sized beads were observed as SA/PVA increased to 4/6 and 5/5. The ESP of 6/4 SA/PVA produced irregular shaped fibers and few ca. 15 µm size beads between fibers. | Crosslinking (5% CaCl2 in 75% EtOH for 30 min). The hybrid fibers were rendered water insoluble. | [54] |
| 3.55/6.45 SA/PVA | ||||||||||
| 4/6 SA/PVA | ||||||||||
| 5/5 SA/PVA | ||||||||||
| 6/4 SA/PVA | ||||||||||
| Sodium alginate (SA) (MV, GulA/ManA: 3.5) Source: commercial |
SA was add to PVA (4%) to produce 5% aq. SA/PVA at mixtures ratios: 2/8 SA/PVA 3/7 SA/PVA | Finer and uniform NF (dd: 153–260 nm) and no beads were produced from 2/8 SA/PVA. The ESP of SA/PVA (3.33/6.67) showed fibers characterized for some spindle-shaped and round beads. The ESP result was significantly improvement from SA/PVA (3/7), producing cylindrical fibers and fewer beads. ESP of mixtures at higher SA/PVA (4/6) produced only droplets. SA–PVA and PVA–PVA intermolecular hydrogen bonds could balance SA–SA repulsion, providing necessary chain entanglement to support electrospinning. | ||||||||
| 3.33/6.67 SA/PVA | ||||||||||
| 4/6 SA/PVA | ||||||||||
| 10 | Ulvan (U) (M: 30,58 to 59,95 KDa) Source: Ulva rigida |
PVA (M: 60 kDa) (M: 31–50 kDa) 98–99% hydrolysis | DIW | 15 mM H3BO3; 7mM CaCl2 in a ratio 60/40 (v/v) | U (2.34%) and PVA (12%) at mixture ratios: 50/50 U/PVA | 2.3–2.5 | 0.04–0.10 | The spun mats exhibited different characteristics depending on the ulvan/PVA blend ratio: Uniform fibers (d: 105 ± 4 nm) were produced from U/PVA (50/50). Fibers with some thicker sections and d: 84 ± 4 nm were produced from U/PVA (70/30). Fibers (d: 60 ± 5 nm) and beads (1–2 µm) were produced from U/PVA (85/15). The fiber diameters decrease when increasing ulvan concentration. | _ | [23] |
| 70/30 U/PVA | ||||||||||
| 85/15 U/PVA | ||||||||||
| Ulvan (U) (M: 30,58 to 59,95 kDa) Source: U. rigida | PEO (M: 900 kDa) | aq. AcOH (0.5M) | _ | U (2.34%) and PEO (3%) at mixture ratios: 60/40 U/PEO | 2.3–2.5 | 0.04–0.10 | Bead defect NFs were produced | _ | ||
| 80/20 U/PEO | ||||||||||
| 11 | Ulvan (U) (M: 1,800 kDa) Source: U. rigida |
PCL (M: 80 kDa) | DCM/DMF (80/20, v/v) | _ | U and PCL (8%) at mixture ratios: 1/8 U/PCL | 1.1 | 0.5 | NF generated from ulvan/PCL blends with 1/8, 3/8, and 3/2ratios exhibited d: 994± 5 nm, d: 499 ± 5 nm, and d: 300 ± 5 nm, respectively. In the blend with 3/2 ratio, both fibers and beads (approximately 1 µm) coexisted. The increase of the ulvan content in the polymer blends produced a decrease in the diameter of the fibers. | _ | [21] |
| 3/8 U/PCL | ||||||||||
| 3/2 U/PCL | ||||||||||
| Ulvan (U) (M: 1,800 kDa) Source: U. rigida |
PEO (M: 900 kDa) | DIW | _ | U (2%) and PEO (4%) at mixture ratios: 1/1 U/PEO | 0.9 | 0.1 | Spindle-like NF were obtained when the ulvan/PEO ratio was 1/1, whereas beaded fibers with smaller diameter were produced from the 2/1 ratio. NF from 1/1 (U/PEO) and 2/1 (U/PEO) exhibited d: 935 ± 5 nm and d: 505 ± 5 nm, respectively. The electrospinnability of the solutions was reduced when the ulvan content was increased. | _ | ||
| 2/1 U/PEO | ||||||||||
| 12 | Ulvan (Up) (M: 436.3 kDa) | PVA (M: 125 kDa) | DIW | _ | U (2.4%) and PVA(13%): 1/2 U/PVA | 1.8 | N.R. | NF from UN resulted in a decrease in bead-free samples or developed beads when compared with NF from Up (a possible effect of the lower M of pretreated samples was suggested). Smooth and defect-free NF from Up and UN exhibited d: 77.13 ± 15 nm and d: 70.15 ± 15.2 nm, respectively. | _ | [61] |
| Ulvan (UN) (M: 455.56 kDa) | 98% hydrolysis | |||||||||
| Source: U. fasciata | ||||||||||
| Ulvan (Upnano) (M: 388.21 kDa) | PVA (M:125 kDa) | DIW | _ | U (2.4%) and PVA(13%): 1/2 U/PVA | 1.8 | N.R. | No beads were found in NF from Upnano and Unnano. The effect of OSPS was not pronounced. Smooth and defect-free NF from Upnano and UN exhibited d: 80.24 ± 23.8 nm and d: 84.75 ± 14.4 nm, respectively. | _ | ||
| Ulvan (Unnano) (M: 336.95 kDa) | 98% hydrolysis | |||||||||
| Source: Ulva fasciata | ||||||||||
| Ulvan (Up) (M: 436.3 kDa) | PVA (M:125 kDa) | DIW | 15mM H3BO3; 7mM CaCl2 in a ratio (60/40v/v) | U (2.4%) , PVA(13%) or PVA(11%): 1/1 U/PVA U (2.4%), PVA(13%): 2/1 U/PVA 1/2 U/PVA | 1.8 | N.R. | NF from 1/1 Ulvan/PVA (11 or 13%) solutions were distorted. The distortion was more pronounced with PVA (11%). PVA (13%) was selected as carrier. ESP of 2/1 Ulvan/PVA mixtures results in further distraction of NF morphology (almost creating non-uniform nano-bead fibers). Handle and smooth NF were produced from ulvan/PVA (1/2), d: 80.07 nm and few beads. | _ | ||
| Source: U. fasciata | 98% hydrolysis | |||||||||
| 13 | Mauran (MR) | PVA (M: 31,000–50,000) | DW | _ | MR/PVA mixture (6, 15, 20 and 30 mg/2 mL) of MR concentrations in 12 % PVA aq. Solution | 2 | 1.5 | NF fabricated using 6 mg (MR) exhibited uniform diameter with various interconnected pores (d: 110 nm). Increasing MR from 6 to 30 mg showed smooth and uniform NF (d: 130 nm).The size of the NF (dd: 70–160 nm) were a function of MR concentration. Large population of smaller NF and uniform morphology was retained on varying MR concentrations. | MR/PVA NF were cross-linked using 50% glutaraldehyde and 200 µL TFA vapors to reduce the water solubility. MR/PVA NF showed to be a good biocompatible material for the migration, proliferation and differentiation of mesenchymal stem cells and fibroblast cell derived from mouse. | [26] |
| Source: Halomonas maura | 87–89% hydrolysis |
PG-chitin: practical grade-chitin; M: molar mass; [C2C2Im][OAc]: 1,3-diethylimidazolium acetate; [C2C1Im][OAc]: 1-ethyl-3 methylimidazolium acetate; ESP: electrospinning; NF: nanofibers; DW: distilled water; DIW: deionized water; IL: ionic liquid; [C2mim][OAc]: 1-ethyl-3-methylimidazolium acetate; EMIM OAc: 1-ethyl-3-methylimidazolium acetate; BMIM OAc: 1-butyl-3-methylimidazolium acetate; BMIM Cl: 1-butyl-3-methylimidazolium chloride; BMIM Br: 1-butyl-3-methylimidazolium bromide; EMIM Cl: 1-ethyl-3-methylimidazolium chloride; AMIM Cl: 1-allyl-3-methylimidazolium chloride; N.R: not reported; (a) g: gravity; (b) p/g: solution flow was controlled by gravity or external pressure; PET: polyester; AcOH: acetic acid; FA: neatformic acid; DD: degree of deacetylation; DA: degree of N-acetylation; TFA: trifluoroacetic acid; DCM: dichloromethane; aq: aqueous; d: mean diameter; dd: diameter distribution; PVA: polyvinyl alcohol; PCL: polycaprolactone; PEO: poly(ethylene oxide); DMF: N,N-dimethylformamide; ManA: mannuronic acid; GulA: guluronic acid; LV: low viscosity; MV: medium viscosity; EtOH: ethanol; (UP): ulvan from micro-powdered algae with organic solvent pretreatment; OSPS: organic solvent pretreatment stage; (UN): ulvan extracted from micro-powdered without OSPS; (Upnano): ulvan obtained from nano-powdered algae with OSPS; (Unnano): ulvan obtained from nano-powdered algae without OSPS.
![Figure 7
Microscopic images of chitin fibers (powder of chitin extracted from shrimp shells (Pandalus borealis)) in 1-butyl-3-methylimidazolium acetate BMIM OAc, produced by the solution blow spinning method (a and b) mag: 5×, fiber diameter: 0.3–30 µm; (b) mag: 5×, fiber diameter: 0.3–30 µm. Image reprinted with permission from [92] Łukasiewicz Research Network-Institute of Biopolymers and Chemical Fibres, in accordance with the BOAI.](/document/doi/10.1515/ntrev-2022-0491/asset/graphic/j_ntrev-2022-0491_fig_007.jpg)
Microscopic images of chitin fibers (powder of chitin extracted from shrimp shells (Pandalus borealis)) in 1-butyl-3-methylimidazolium acetate BMIM OAc, produced by the solution blow spinning method (a and b) mag: 5×, fiber diameter: 0.3–30 µm; (b) mag: 5×, fiber diameter: 0.3–30 µm. Image reprinted with permission from [92] Łukasiewicz Research Network-Institute of Biopolymers and Chemical Fibres, in accordance with the BOAI.
The solubility of chitin can be improved by converting it into chitosan, whose solubility depends on the ability of the solvent to protonate this derivative [57]. Consequently, the solubility properties of chitosan are dependent on the pH and ionic strength of the solutions due to the presence of deacetylated amino groups that are able to gain protons [73].
The effect of the protonated amino group (NH3 +) on the solubility of chitosan has been described by Franca et al. [58]. They found that these protonated groups could increase the water exchange in the region surrounding the O3 atoms, destabilizing the HO3(n)···O5(n+1) hydrogen bonds, and thus, the 2-fold helical conformation, improving the solubility of the polymer (Figure 8). For this reason, chitosan is readily soluble in dilute acidic media below its pK a (pH = 6.5), such as dilute hydrochloric acid, acetic acid, formic acid, lactic acid, and succinic acid among others [57,72,94]. Franca et al. [58] reported a significant difference between chitin and chitosan in the number of water molecules that were hydrogen bonded to O3, as well as in the lifetime of the HO3(n)···O5(n+1) interaction as a function of pH. They explained that this was a consequence of micro-solvation and demonstrated that this was the case by calculating the orientation of all the water molecules within a 5-Å radius of all O3 atoms (Figure 8).
![Figure 8
Solvent (water) orientation around the O3 oxygen atom of chitin and chitosan chains. Property is shown as the average orientation (cos θ) over the entire simulation for a 5-Å radius around the O3 atom. θ is defined by the angle formed between the vector formed by O3 and the water oxygen atoms and the water dipole vector (as illustrated by the black arrows). Hydrogen bonds are represented by dashed blue lines. Water molecules are displayed in a ball-and-stick model and the sugar units of chitin are in sticks (atoms are color coded as follows: cyan, carbon; blue, nitrogen; red, oxygen; white, hydrogen). Image reprinted with permission from [58]. Copyright (2008) American Chemical Society.](/document/doi/10.1515/ntrev-2022-0491/asset/graphic/j_ntrev-2022-0491_fig_008.jpg)
Solvent (water) orientation around the O3 oxygen atom of chitin and chitosan chains. Property is shown as the average orientation (cos θ) over the entire simulation for a 5-Å radius around the O3 atom. θ is defined by the angle formed between the vector formed by O3 and the water oxygen atoms and the water dipole vector (as illustrated by the black arrows). Hydrogen bonds are represented by dashed blue lines. Water molecules are displayed in a ball-and-stick model and the sugar units of chitin are in sticks (atoms are color coded as follows: cyan, carbon; blue, nitrogen; red, oxygen; white, hydrogen). Image reprinted with permission from [58]. Copyright (2008) American Chemical Society.
The drawbacks associated with the solubility of chitosan have been overcome by employing dilute acid solutions as the solvent media. However, the repulsive force generated by the cationic groups along the chains increases the viscosity of the chitosan solution, which prevents jet formation [9]. To illustrate this statement, it is worthy to refer to the research of Ohkawa et al. [72] in which despite the authors successfully dissolved chitosan (3–9 wt%) in different acid solvents (dilute hydrochloric acid, acetic acid, neat formic acid, and dichloroacetic acid) as well as in mixtures containing methanol, ethanol, 1,4-dioxane, dichloromethane, N,N-dimethylformamide, or dimethyl sulfoxide, their attempts at producing visible spun jets from these solutions were unsuccessful.
The production of spun fibers from pure native chitosan is possible with the use of concentrated acids [93,94] (Table 1, entries 4 and 5) or strong acids as trifluoroacetic acid (TFA) solutions or TFA-dichloromethane (MC) solutions [72] (Table 1, entry 6). Ohkawa et al. [72] explained the success of TFA as the working solvent as a consequence of the formation of TFA-amino salts, which were able to disrupt the interaction between chitosan molecules. However, trifluoracetic acid is a volatile, toxic, and expensive solvent.
Homayoni et al. [104] avoided the use of strong acids as the working solvent during the electrospinning of pure polysaccharide by using the alkali treatment to hydrolyze the polysaccharide, allowing them to dissolve in aqueous acetic acid (70%). The authors were able to formulate a spinnable solution that generated nanofibers with moisture uptake properties while avoiding toxic solvents.
Another strategy involves chemically modifying chitin and chitosan to improve their solubility. The introduction of chemical groups such as alkyl or carboxymethyl groups into the structure of these polysaccharides resulted in an increase in their solubility at neutral and alkaline pH values while maintaining their characteristics [29,91].
To address the issues associated with the spinning of pure polyelectrolyte polysaccharides, the use of synthetic polymers such as polyvinyl alcohol (PVA), poly(ethylene oxide) (PEO), and polycaprolactone (PCL) has been a strategy used to formulate the mixtures. This results in the creation of new molecular interactions that reduce the repulsive effects between the polysaccharide chains and improves their flexibility. Hence, the addition of synthetic polymers has made it possible to formulate polysaccharide-based spinnable systems [4,10,20,21,23,53,54,61,67,79].
Jung et al. [67] reported unsuccessful attempts to electrospun β-chitin in a solution of formic acid. As an alternative, the authors added PEO during the formulation of the β-chitin mixture, which reduced the surface tension and increased the incidence of the chain entanglements, enhancing the electrospinnability of β-chitin. Following this, Jung et al. [67] produced pure β-chitin nanofibers by soaking the β-chitin/PEO fibers in water to remove the water-soluble PEO polymer (Table 1, entry 7). The authors reported a decrease in the diameter of the fibers (from 417 nm to 297 nm) as well as elongation at break in parallel with an increase in the tensile strength. In addition, the authors showed that pure β-chitin nanofibers exhibited a great potential as a material for use in wound healing processes.
Currently, several studies have reported on the production of spun fibers from binary systems using synthetic polymers. However, considering that the focus of this review is on the electrospinning of pure marine polysaccharides, only some works associated with nanofibers derived from binary systems will be included, aiming to remark on some aspects of our discussion. Specifically, some strategies can be used to produce pure marine polysaccharide nanofibers (even if they cannot produce spinnerets in their pure form) as well as present an overview of the electrospinning of marine polysaccharides that are barely explored in this field (such as ulvan and mauran).
The strategy employed by Jung et al. [67] to obtain pure β-chitin nanofibers cannot be used for fibers that are fabricated from water-soluble polysaccharides, such as ulvan, alginate, and mauran. Specifically, fibers fabricated from water-soluble polymers require additional treatment to prevent them from disintegrating upon contact with water, while also guaranteeing their functionality in different applications.
Chemical and physical crosslinking methods have been used to limit the disintegration of polysaccharide-based spun mat upon contacting with water [4,10,23,26,54,94,99,105]. Once again, there are challenges associated with the crosslinking processes, such as the inherent toxicity of common crosslinker agents (e.g., glutaraldehyde, formaldehyde, and epoxy compounds). Consequently, the search for alternative crosslinkers represents an important field to be explored. An example of an alternative agent is the naturally occurring and nontoxic genipin that has been effectively used to enhance some functional aspects of spun polysaccharide mats [105,106]. However, it is not suitable for polysaccharides that lack the functional groups to interact with genipin. Alternatively, physical crosslinking avoids the use of crosslinking agents altogether [54,99].
Dodero et al. [53] and Shen and Hsieh [54] used physical crosslinking to stabilize hybrid fibers, producing SA-based nanofibrous membranes from solutions blended with PVA and PEO, while using EtOH/CaCl2 as a crosslinker agent (Table 1, entries 8 and 9). In addition to the difficulty associated with the low stability of the spun mats in aqueous media, the authors also described aspects associated with the chemical composition of SA, which, in turn, determined its rheological behavior; these were linked to the difficulties associated with spinning the pure form of this polysaccharide and will be discussed later in this review.
In contrast to chitin and chitosan, the other marine polysaccharides discussed in this review, such as alginate, ulvan, and mauran, are water-soluble polysaccharides, whose solubility depends on their composition, molar mass, and the ionic strength of the solution as well as the presence of the charged groups.
SA is a water-soluble polymer that forms rigid, extended chains in aqueous solutions, which hinders the electrospinning process [107]. Nie et al. [107] reported that the inclusion of a polyol(glycerol) as a cosolvent improved the flexibility of the chains through the disruption of hydrogen bonding, leading to the production of fibers (Figure 9), which were collected in a coagulating bath containing ethanol and CaCl2 solution. Nie et al. [107] concluded that enhanced chain entanglement (or both the viscosity and elasticity) as well as decreased surface tension and conductivity were key factors in the success of the process.
![Figure 9
Scanning electron microscopic images of SA fibers electrospun from SA solution with different concentrations: (a) 1.6, (b) 2.0, and (c) 2.4 w/v%. The volume ratio of glycerol to water was constant (2/1). Image reprinted with permission from [107]. Copyright: 2008 American Chemical Society.](/document/doi/10.1515/ntrev-2022-0491/asset/graphic/j_ntrev-2022-0491_fig_009.jpg)
Scanning electron microscopic images of SA fibers electrospun from SA solution with different concentrations: (a) 1.6, (b) 2.0, and (c) 2.4 w/v%. The volume ratio of glycerol to water was constant (2/1). Image reprinted with permission from [107]. Copyright: 2008 American Chemical Society.
Ulvan is a densely charged polysaccharide, which increases its solubility in water; however, it also possesses a high number of hydrophobic rhamnose methyl groups, giving it an amphiphilic character [82,108]. The amphiphilic character of ulvan was invoked by Robic et al. [50] to justify the bead-like structures that were observed at the structural level in aqueous solutions; they suggested that the bead-like structures were the consequence of aggregation at low ionic strength. The authors also note that the Mark–Houwink–Sakurada (MHS) relationship between molar mass (M) and intrinsic viscosity [η] of the ulvan fraction analyzed by them was consistent with the formation of compact spheres.
Recently, a native ulvan fraction was analyzed by de Carvalho et al. [109] to determine its: intrinsic viscosity ([η]), radius of gyration (R g), persistence length (L p), and the Mark–Houwink–Sakurada exponent α. On the basis of their results, de Carvalho et al. [109] suggested that the native ulvan fraction would have a spherical like-shape conformation, which was consistent with the observations of Robic et al. [82], who described ulvan in aqueous solutions as being aggregates of spherical-shaped structures of varying diameters that were partially linked by strands and filament-like material. Robic et al. [82] noted that this particular behavior of ulvan is derived from the classical rod-shaped or fiber-shaped structures of gel-forming polysaccharides, evidencing that water is a poor solvent for ulvan, explaining the low intrinsic viscosity of ulvan in aqueous saline solutions.
Despite being water-soluble polysaccharides, the electrospinning of aqueous solutions of alginate and mauran is challenging because other equally important conditions must be met to guarantee the success of the electrospinning process. This includes important rheological aspects that must be considered during the electrospinning of pure marine polysaccharides; this will be discussed in the next section.
6 Viscoelastic deformations, chain entanglements, and electrospinning
During the electrospinning of a polymer mixture, it is possible to observe two kinds of behaviors: spraying and spinning (Figure 10).
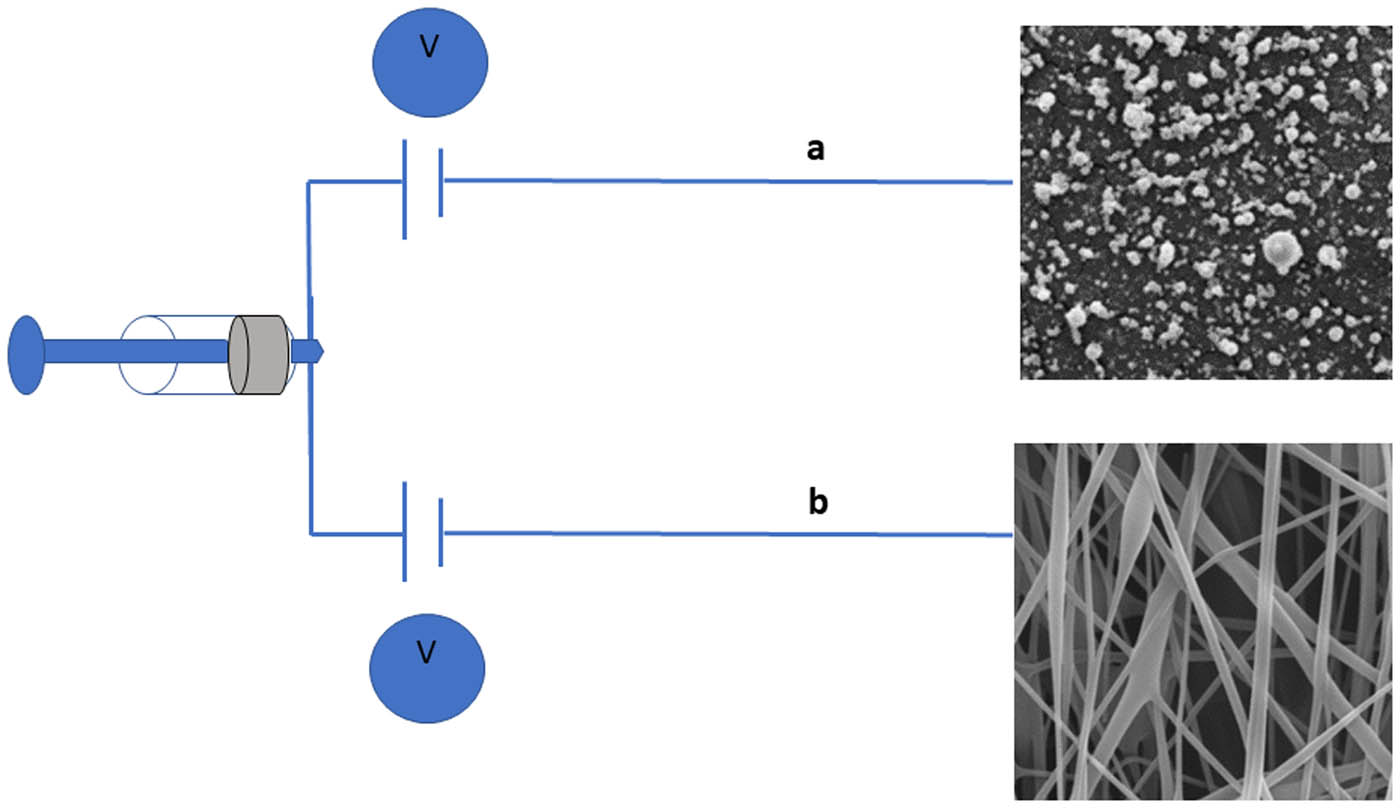
Schematic illustration of electro spraying (a) and electrospinning (b). Source: Author.
Despite the similarity between spraying and spinning in terms of using high voltage to eject liquid jets, they have remarkably different results. Spraying results in the formation of droplets or microbeads, while spinning results in the production of fibers [1,44,74,110].
The transition between spraying and spinning is influenced by at least three parameters: chain entanglements, extensional viscosity [1,60,110], and relaxation time [74,76], which in turn depend on other solution parameters such as the concentration and molar mass of the polymer, the distribution of the molar mass, the viscosity, conductivity, surface tension of the solution, the diffusivity of the solvent, and mass transfer coefficient to the gas phase [44,60,74].
Chain entanglements refer to a special type of intermolecular interactions that occur between polymer chains as they slide past each other, constraining the motion of the chain and impacting the properties of the solution (i.e., viscosity) [74,110,111].
The average number of entanglements in which the polymer chains participate is influenced by several parameters such as the concentration of the polymer, the length of the polymer chain, the molar mass distribution, and the morphology of the polysaccharides [25,60,74,89,110,111].
To begin analyzing the aforementioned parameters, it is important to understand the four concentration regimes of polymer solutions: dilute, semi-dilute unentangled, semi-dilute entangled, and concentrated regimes [53,112].
Dilute regimes refer to solutions that are characterized by very low polymer concentrations, in which polymer chains appear: separated, disengaged, and showing a similar behavior to individual units, characterized by chains that do not overlap [25,53,113,114].
In solutions with a dilute regime, the liquid jet breaks into droplets because of the Rayleigh instability induced by the surface tension [53,76,113,114], for which, a spraying behavior could be expected for polysaccharide solutions in a dilute regime.
Semi-dilute unentangled regime occurs at a critical overlap concentration (c*), which is large enough to promote chain overlapping but not entanglements. However, chain entanglements are the direct consequence of overlapping chains [74].
Finally, the semi-dilute entangled and concentrated regimes are distinguished by the critical entanglement concentration (Ce), which marks the transition from the onset of entanglements (in which a continuous network is formed) to the appearance of a significant number of entanglements [53,76,112,114,115].
The previous classification perfectly describes neutral polysaccharides. However, polymers that behave like polyelectrolytes (such as alginate) exhibit properties that are significantly different in semi-dilute regimes solutions, highlighting an unusual scaling relationship of the specific viscosity upon the concentration of the polymer [53,56,96].
Traditionally, the anomalous viscosity of polyelectrolytes has been ascribed to the effect of intermolecular forces as the concentration decreases [116], which is consistent for small, spherical polyelectrolytes. As referred by Ise and Sogami [117], correlations that influence the viscosity of polyelectrolytes solutions include the interactions between ionized groups, the interactions between ionized groups and nearby counterions associated in the macroion domain, and interactions between macroions [117].
Ise and Sogami [117] also noted that the primary interaction in electrolyte solutions has a long-range electrostatic origin, which persists at low concentrations (this is different for low molar mass electrolytes). Recently, Dedic et al. [96] elaborated upon the effect of the long-range interactions between polyelectrolytes and the H-bond network in water and pointed out that these long-range interactions drive the structural rearrangement of the H-bond of water, producing changes in the water–water orientational correlations and causing significant changes to the reduced specific viscosity. In addition, as suggested by Dedic et al. [96], the type of H-bond breaking (rotation or stretching) could also affect the viscous flow.
Antonietti et al. [116] described the polyelectrolyte effect as a strong increase in the reduced viscosity when there is a decrease in the concentration, and they pointed out that the presence of charges alters the single particle hydrodynamics and highlighted the fact that the cooperative coupling of all particles would have a remarkable influence on the reduced viscosity.
The reduced viscosity strongly increases with molar mass for linear polyelectrolytes, while branched polyelectrolytes exhibit a reduced viscosity that is independent of molar mass [116]. McKee et al. [112], who worked with linear and different branching grade polyesters, reported that there was a weaker correlation between specific viscosity and polymer concentration than theoretically predicted in branched polymers in semi-diluted entangled regimes, showing a larger Ce compared to linear polymers with equivalent molar mass. The authors explained that the higher segment density of the branched polymers resulted in a smaller hydrodynamic volume compared to the hydrodynamic volume of linear polymers of equivalent molar mass.
According to Antonietti et al. [116], the reduced viscosity of solutions of branched polyelectrolytes produced below the critical overlap concentration exhibits a significantly weak molar mass dependence because the intrinsic viscosity of branching polyelectrolytes is directly related to the effective charge number per particle. Thus, the weight dependence of the intrinsic viscosity of branched polyelectrolytes is low, and the influence of cooperative electrostatic coupling is remarkable [116].
In contrast to branched polyelectrolytes, the reduced viscosity of linear polyelectrolytes strongly increases as the molar mass increases [116]. There are remarkable differences between polyelectrolytes and neutral polymers in the semi-dilute regimes, indicating the presence of a different scaling relationship between specific viscosity and the concentration of the polymer. In contrast, no differences are observed between neutral polymers and polyelectrolytes in concentrated regimes [53].
It is worth referring to the research by Dodero et al. [53] in which the authors worked with SA (a linear polysaccharide), with different molar masses (145 ± 2, 360 ± 60 and 490 ± 60 kg mol−1), high polydispersity indices, different monosaccharide compositions: α-l-guluronic acid (GulA-blocks) to β-d-mannuronic acid (ManA-blocks) ratios.
Dodero et al. [53] reported that the SA sample with a molar mass of 360 kg mol−1 and with a higher number of GulA-blocks exhibited polyelectrolyte behavior with rigid and stiff chains in a rod-like conformation, while the SA sample with a molar mass of 145 kg mol−1 with a higher number of ManA-blocks exhibited neutral polymer behavior with flexible chains in random coil-like conformation. An intermediate behavior between neutral and polyelectrolytic was also reported for the 490 kg mol−1 sample with a higher number of ManA-blocks.
Considering the results of the viscosity and the values of c*, ce, and c** determined by Dodero et al. [53] for the three SA solutions, the authors concluded that molar mass strongly influences the critical concentrations; the longer the chains, the lower the values of c*, ce, and c**. The authors proposed that longer chains could more easily overlap and entangle each other at lower concentrations. The entanglement behavior of SA is reflected in the viscosity of the different solutions; a low-value viscosity (0.1 Pa s) was exhibited in the SA with the lowest M, while a high viscosity (117.5 Pa s) was exhibited by the SA with the highest M and more rigid chains. The results of Dodero et al. [53] were perfectly consistent with the direct relationship established by Antonietti et al. [116] between molar mass and viscosity for linear polysaccharides.
Despite the significant differences in the viscosity and c*, ce, and c** exhibited by the pure SA solutions in the study conducted by Dodero et al. [53], none of these solutions were suitable for electrospinning. Dodero et al. [53] discussed some of the reasons why the spinning of pure SA solutions was impossible.
First, the shear-thinning behavior of the SA solutions was evaluated by Dodero et al. [53], reporting shear-thinning for all of the SA solutions performed by them, as well as, thixotropic behavior for those solutions performed from higher SA molar mass.
Shear-thinning behavior is one of the factors that led to envisage the feasibility of producing fibers based on the ability of the polymer to form entanglements [25]. Stijnman et al. [25] found that polysaccharide solutions that exhibit shear-thinning behavior tend to have few entanglements and have a decreased extensional viscosity at higher shear rates, which negatively affects the spinning process.
In general, solutions subjected to electrospinning are exposed to high shear rates in the Taylor cone during the process. Subsequently, a certain degree of elongation strain hardening would be necessary to stretch and orient the dissolved polymer chains, generating the effective fiber formation to prevent the liquid jet breaks (into the corresponding electrospray) under the effect of the high shear rate generated by the electric field [25]. Thus, the shear-thinning behavior reported by Dodero et al. [53] in SA solutions would explain the unsuitability of these solutions in an electrospinning process.
Dodero et al. [53] found that ManA-rich SA (particularly when they had low molar masses) exhibited shear-thinning behavior at low-shear rates. It was explained by the authors as a consequence of its extremely short polymer chains, which hindered the entanglement process and prevented jet formation. In contrast, GulA-rich SA exhibited strong electrostatic repulsions that produced semi-rigid chains, constraining the motion of the polysaccharide under the electric field, which is also associated with the high shear rate values that induce orientation of the chain [53].
However, Stijnman et al. [25] who worked with different polysaccharides observed that a strong shear thinning was not always observed at low shear rates in nonjet polysaccharide solutions (e.g., alginate). They suggested that other factors aside from entanglements would affect the electrospinning, highlighting the homogeneity of the solution on a molecular scale as a very important factor.
Yu et al. [76] concluded that entanglements represent a sufficient but not necessary condition to achieve the elastic response required for a successful electrospinning. In fact, Yu et al. [76] noted that an elastic response could also be achieved at low polymer concentrations (below c*), if the relaxation time of the fluid is longer than the time of extensional deformation (which is typical of Borger fluids). Borger fluids exhibit Newtonian behavior under shear and also exhibit an elasticity that is completely extensional.
Palangetic et al. [60] suggested the finite extensibility limit of the polymer chain, and the maximum bounded extensional viscosity are the parameters that define the physics that dominate the electrospinning process.
By considering the relationship between the next parameters: the critical overlap concentration of a polymer, its molar mass, and the quality of the solvent; Palangetic et al. [60] explored the correlation between the minimum polymer concentration and molar mass during the electrospinning. Furthermore, the authors explored the effect of polydispersity on the critical polymer concentration required to produce fibers as well as its effect on the morphology of the fibers.
Palangetic et al. [60] found that systems performed from low molar mass polymer fit the classic scaling law based on the overlap and entanglement concentration criteria, in which the onset of fiber formation occurs above the entanglement concentration. Palangetic et al. [60] stated that the weight of average molar mass frequently gets the spinning properties in these systems giving the contribution of each species to the total zero-shear viscosity.
In contrast to the low molar mass polymer systems, Palangetic et al. [60] stated that high molar mass polymers were not well described by the scale based on zero-shear viscosity (related to the molar mass of the polymers). As an alternative, Palangetic et al. [60] proposed that the finite extensibility limit of the extensional viscosity, rather than the zero-shear viscosity, would be the factor that controls the spinning process in systems involving high molar mass polymers.
Palangetic et al. [60] proposed a power scaling, in which the minimum viscoelastic stress for spinning would be proportional to the steady-state extensional viscosity of flexible polymer solutions at high strain rates and hence to the finite extensibility of the polymer chains. The power scaling law introduced by Palangetic et al. [60] linked the minimum spinning concentration of a polymer to its molar mass and the quality of the solvent. Palangetic et al. [60] noted that the power scaling law proposed by them becomes dominant at a very high molar mass of the polymers.
In the context of the molar mass distribution of the polymers, Palangetic et al. [60] introduced the concept of the extensibility average molar mass, suggesting that polydisperse solutions undergo an extension-dominated spinning process. The authors pointed out that highly polydisperse systems effectively lower the minimum concentration required for an effective fiber spinning in which the spinnability would be primarily driven by the extensibility of the highest molar mass fractions.
From the results of Dodero et al. [53] who worked with strongly polydisperse SA systems, it is possible to infer that a high polydispersity index is not enough to guarantee the success of the electrospinning of pure polyelectrolyte polysaccharides. This, combined with the work conducted by Palangetic et al. [60], suggested that the proportion of high molar mass chains present during the formulation of polyelectrolyte polysaccharides systems strongly influences the success of the electrospinning. In addition, the monosaccharide composition, which defines the conformation of the polymers in a particular solvent, will be also among the relevant parameters to define the success of the electrospinning process.
The previous statement is supported by the work conducted by Kong et al. [118] who evaluated the dependence of the rheological properties with the adjustment of the molar mass distribution of alginate (maintaining a critical length of GulA blocks to allow ionic cross-linking). The authors generated low molar mass alginate from high molar mass alginate while maintaining a GulA block consistent with that observed in high molar mass alginate. The authors accomplished this by irradiating high molar mass alginate and obtaining molecules of lower molar mass with negligible changes to the guluronic/mannuronic ratio. The authors then evaluated the rheological behavior of the bimodal molar mass solutions and concluded that polymer solutions with a bimodal molar mass distribution decoupled the dependence of the viscosity of pre-gelled solutions. The authors highlighted that low molar mass alginate had a negligible contribution to the viscosity (which they justified in the context of relaxation time); in contrast, the more flexible the high molar mass alginate, easy the formation of physical entanglements, which are related to the increase in the relaxation time. The authors stated that the concentration of high molar mass alginate is the main factor that influences the viscosity of the alginate solutions.
As previously mentioned in this review, Homayoni et al. [104] successfully electrospun pure chitosan after hydrolyzing the polysaccharide by alkali treatment. The authors found that short alkali treatment times (up to 5 h) did not have a considerable effect on fiber’s formation. However, increasing the alkali treatment time to 48 h let resulted in the production of high-quality nanofibers. Homayoni et al. [104] reported 2.94 × 105 g mol−1 as the proper molar mass of hydrolyzed chitosan, which permitted to produce spinnable solutions in 70, 80, and 90% acetic acid solvents after 48 h of depolymerization.
Despite the decrease in the molar mass reported by Homayoni et al. [104], the results of this work led to perceive that the molar mass of the hydrolyzed chitosan is still high. This suggests that there could be a threshold for the fraction of high molar mass chains fraction, which would drive the extensional viscosity of each polymer.
In addition, Shenoy et al. [74] highlighted the importance of the relaxation time (disentanglement time) during the electrospinning process. Referring to it, the authors explained that short chains (low molar mass polymers) require less time than long chains (higher molar mass polymers) to disentangle. Consequently, a considerable entanglement loss can be generated under the elongational flow during the electrospinning of low molar mass polymers.
Referring to the elongation rate and disentanglement time, Akkoyun et al. [115] pointed out that polymer solutions with lower initial concentration exhibit a higher elongation rate, which was explained by the authors as the consequence of the more rapid disentanglement of the polymer chains.
Thus far, a large part of this discussion has focused on polysaccharide solutions that are characterized by an increase in the viscosity at low polysaccharide concentrations. In the following sections, we will explore the behavior of ulvan, a polysaccharide of increasing interest that exhibit a different behavior.
In contrast to phyco-colloids such as alginate, aqueous ulvan solutions are characterized by their low viscosities [12,82]. This occurs because although ulvan is usually referred to as a water-soluble polysaccharide, it appears as small, spherically shaped particles that form aggregates in ionized water (pH = 5) [82].
The rheological behavior of aqueous ulvan systems represents one of its limitations concerning the fabrication of spun fibers [23,108]. These systems could resemble dilute regimen solutions, in which separated or partially aggregated particles exhibit spraying behavior during the electrospinning. In the pioneering work conducted by Toskas et al. [23], the authors reported unsuccessful attempts to produce electrospun pure ulvan-rich extracts even after using different polysaccharide concentrations and different solvent systems.
Considering their aforementioned properties, it is possible to consider ulvan systems as colloidal dispersions that are capable of forming micelles. Xue et al. [1] noted that a colloidal system could be adapted for electrospinning when sufficient entanglements are formed. However, the authors also highlighted the difficulty of fabricating uniform fibers when the nanoscale components tend to aggregate. Considering this fact, the destabilization of the ulvan colloidal system could be an interesting option that would promote stabilization and molecular interactions that would favor the formation of a spinnable system.
Toskas et al. [23] used a solvent composed of boric acid and calcium as well as PVA as co-polymers to increase intermolecular interactions and formulate a system with the appropriate viscosity. The authors reported that this mixture allowed for the production of spun fibers, suggesting that the interpenetration of ulvan aggregates by the PVA molecules caused the system to stabilize. In contrast, the authors also reported unsuccessful attempts to produce electrospun fibrous mats from an aqueous ulvan/PEO mixture. Both Toskas et al. [23] and Kikionis et al. [21] discussed the influence of the solvent on the electrospinning of ulvan-based blends when PEO was used as a copolymer (Table 1, entries 10 and 11). Recently, PVA has been used in co-blended ulvan systems with water as the working solvent [61], producing an aqueous system in an environmentally friendly process. This study also evaluated the effects of pretreatment of the biomass and the particle size of the biomass (Table 1, entry 12).
Kuchibhatla et al. [119] referred that the stabilization of colloids using an organic polymer is based on trapping nanoparticles with an organic polymer, which does not interfere with the chemical characteristics of the nanoparticles. It is possible that ulvan could be trapped by PVA when both are mixed in an aqueous system, especially if the aqueous ulvan system is considered as a dispersion of nanoparticles. In this context, it will be important to consider that referred by Kuchibhatla et al. [119] in relation to that the appropriate selection of the organic polymer, which will depend on the functional groups, molar mass, and the chemical reactivity of this polymer.
Finally, mauran is a highly water-soluble polysaccharide that is known to exhibit pseudoplastic and thixotropic behavior [26]. The viscosity of mauran is highly influenced by the concentration of the salt in the growth medium as well as the incubation time [28]. In addition, the viscosity of mauran solution is stable in the presence of high salt concentrations, sugars, detergents, and extreme pH values [26,28]; however, its viscosity has been shown to decrease at high-temperature treatments [28]. The viscoelastic behavior of mauran was also evaluated by Arias et al. [28] who reported on its liquid behavior at low frequencies. Raveendra et al. [26] evaluated the rheological behavior of PVA, mauran, and mauran/PVA solutions produced with different concentrations of mauran. The authors noted the good compatibility between mauran and PVA and found that the viscosity of the mauran/PVA solutions increased as the concentration of mauran increased.
7 Processing parameters during electrospinning
Processing parameters play an important role in determining the desired quality of the electrospun fiber produced. These parameters correspond to the following factors:
Applied voltage (potential difference): This determines the degree of electrostatic interaction required to distort the solution, resulting in the formation of the Taylor cone and the ejection of the solution from the needle [4]. This parameter affects the speed of the jet, which consequently determines the diameter and morphology of the fibers. In general, the higher the voltage, the thinner the diameter of the resultant fibers [4,45]. However, very high voltages can be discharged directly onto the plates, resulting in the breakdown of the electric field and the termination of the process [4]. Effective operating voltages are usually between 6 and 20 kV [4], but the optimal voltage is mainly dependent on the surface tension and viscosity of the solutions [44].
Flow rate: This refers to the rate at which the polymer solution is pumped into the spinneret to feed the spinning cone jet. This depends on three factors: 1) the inner size of the spinneret orifice, 2) the applied voltage, and 3) the flow rate at which the solution is fed into the needle [44]. The flow rate determines the morphology of the electrospun nanofibers, influencing the diameter and the uniformity of the fibers; it should be tuned by considering the time required for the solvent to evaporate [3,44,45,120]. The flow rates must be low enough to provide enough time for the solvent to evaporate because high flow rates will result in beaded fibers if there is insufficient time for the solvent to evaporate before it reaches the collector [3]. The ideal flow rate should balance the ejection and replacement rates of the polymeric solution, leading to a stable jet cone. There is a critical flow rate for each polymeric solution [120].
Spinneret to collector distance: The distance between the tip and the collector is known to control the diameter and morphology of the fibers. Longer distances produce narrower fibers due to the longer period of elongation. In addition, shorter distances could result in the incomplete solvent evaporation due to the reduction in the flight time [3,44].
Homayoni et al. [104] also evaluated the effect of processing parameters on the characteristics of the electrospun chitosan fibers and found that increasing the gap distance and the voltage decreased and refined the diameters of the nanofibers while also improving their quality.
Toskas et al. [23] evaluated two critical distances for ulvan/PVA nanofibers that were electrospun under the same conditions. The authors reported that the ulvan/PVA solutions produced nanofibers of 150 and 105 nm when the tip-to-collector distance was 7 and 12 cm, respectively.
The results reported by Homayoni et al. [104] and Toskas et al. [23] are in agreement with which has been previously mentioned by Williams et al. [44], i.e., the diameter of the fibers decreases as the distance between the needle and the collector increases.
8 Conclusion
Pure chitin, chitosan, alginate, ulvan, and mauran are either difficult or impossible to electrospun into fibrous due to aspects such as poor solubility in conventional solvents, polysaccharide configuration, and rheological behavior.
Polydispersity and the fraction of high molar mass of polymers have been shown to have an important effect on the effective polysaccharide concentration, the occurrence of physical entanglements, relaxation time, and extensional viscosity [118], which consequently has a strong effect on the results of the electrospinning process. The power scaling law proposed by Palangetic et al. [60] links the minimum spinning concentration of a polymer with its molar mass and the quality of the solvent; suggesting that the spinnability of a polymer is determined by the extensibility of its highest molar mass fractions.
Studies aimed at evaluating the effect of the fraction of the highest molar mass molecules and dispersity in the spinning of pure marine polysaccharides are an interesting field of research. There must be a collaborative effort to consider, modify, and standardize parameters such as the source of the polysaccharides, the ecophysiological conditions, the collection period, the culture conditions, and the extraction methods, among others. The main objective of such studies would be to determine the threshold for the high molar mass molecules with the aim of improving the extensional viscosity and relaxation time of specific marine polysaccharide.
Acknowledgments
L.E.M.A. thanks PAEC (Programa de Alianzas para la Educación y la Capacitación), OEA/GCUB (Organización de los Estados Americanos/Grupo Coimbra de Universidades Brasileiras) and CAPES by a doctoral scholarship and also thanks a doctoral leave by Fac. Ciencias, Universidad Central de Venezuela (UCV).
-
Funding information: C.K.S., R.A.F., M.R.D., and M.D.N. are Research Members of the National Research Council of Brazil (CNPq). This work was supported by CNPq (Universal Call: 462414/2014-0 and PQ-CNPq: 306568/2018-7) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) - Finance Code 001 and PrInt/CAPES.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Xue J, Wu T, Dai Y, Xia Y. Electrospinning and electrospun nanofibers: methods, materials, and applications. Chem Rev. 2019;119(8):5298–415.10.1021/acs.chemrev.8b00593Search in Google Scholar PubMed PubMed Central
[2] Greiner A, Wendorff JH. Electrospinning: a fascinating method for the preparation of ultrathin fibers. Angew Chem - Int Ed. 2007;46(30):5670–703.10.1002/anie.200604646Search in Google Scholar PubMed
[3] Bhardwaj N, Kundu SC. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv. 2010;28(3):325–47.10.1016/j.biotechadv.2010.01.004Search in Google Scholar PubMed
[4] Kriegel C, Arrechi A, Kit K, Mcclements DJ, Weiss J. Fabrication, functionalization, and application of electrospun biopolymer nanofibers. Crit Rev. Food Sci. 2008;48(8):775–97.10.1080/10408390802241325Search in Google Scholar
[5] Ramakrishna S, Fujihara K, Teo WE, Yong T, Ma Z, Ramaseshan R. Electrospun nanofibers: solving global issues. Mater Today. 2006;9(3):40–50.10.1016/S1369-7021(06)71389-XSearch in Google Scholar
[6] Persano L, Camposeo A, Tekmen C, Pisignano D. Industrial upscaling of electrospinning and applications of polymer nanofibers: a review. Macromol Mater Eng. 2013;298(5):504–20.10.1002/mame.201200290Search in Google Scholar
[7] Teo WE, Ramakrishna S. A review on electrospinning design and nanofibre assemblies. Nanotechnology. 2006;17(14):R89–106.10.1088/0957-4484/17/14/R01Search in Google Scholar PubMed
[8] Barnes C, Sell S, Boland E, Simpson D, Bowlin G. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv Drug Deliv Rev. 2007;59(14):1413–33.10.1016/j.addr.2007.04.022Search in Google Scholar PubMed
[9] Phan DN, Khan MQ, Nguyen NT, Phan TT, Ullah A, Khatri M, et al. A review on the fabrication of several carbohydrate polymers into nanofibrous structures using electrospinning for removal of metal ions and dyes. Carbohyd Polym. 2021;252:1–15.10.1016/j.carbpol.2020.117175Search in Google Scholar PubMed
[10] Akshay Kumar KP, Zare EN, Torres-Mendieta R, Wacławek S, Makvandi P, Černík M, et al. Electrospun fibers based on botanical, seaweed, microbial, and animal sourced biomacromolecules and their multidimensional applications. Int J Biol Macromol. 2021;171:130–49.10.1016/j.ijbiomac.2020.12.205Search in Google Scholar PubMed
[11] Majee SB, Avlani D, Biswas GR. Pharmacological, pharmaceutical, cosmetic and diagnostic applications of sulfated polysaccharides from marine algae and bacteria. Afr J Pharm Pharmacol. 2017;11(5):68–77.10.5897/AJPP2016.4695Search in Google Scholar
[12] Lahaye M, Robic A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules. 2007;8(6):1765–74.10.1021/bm061185qSearch in Google Scholar PubMed
[13] Zavgorodnya O, Shamshina JL, Bonner JR, Rogers RD. Electrospinning biopolymers from ionic liquids requires control of different solution properties than volatile organic solvents. ACS Sustain. Chem Eng. 2017;5(6):5512–9.10.1021/acssuschemeng.7b00863Search in Google Scholar
[14] Cunha L, Grenha A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar Drugs. 2016;14(3):1–41.10.3390/md14030042Search in Google Scholar PubMed PubMed Central
[15] Krajewska B. Membrane-based processes performed with use of chitin/chitosan materials. Sep Purif Technol. 2005;41(3):305–12.10.1016/j.seppur.2004.03.019Search in Google Scholar
[16] Morelli A, Chiellini F. Ulvan as a new type of biomaterial from renewable resources: functionalization and hydrogel preparation. Macromol Chem Physic. 2010;211(7):821–32.10.1002/macp.200900562Search in Google Scholar
[17] De Jesus Raposo MF, De Morais AM, Costa, de Morais RM. Marine polysaccharides from algae with potential biomedical applications. Mar Drugs. 2015;13(5):2967–3028.10.3390/md13052967Search in Google Scholar PubMed PubMed Central
[18] Alves A, Sousa R, Reis R. A practical perspective on ulvan extracted from green algae. J Appl Phycol. 2013;25:407–24.10.1007/s10811-012-9875-4Search in Google Scholar
[19] Mata L, Magnusson M, Paul NA, de Nys R. The intensive land-based production of the green seaweeds Derbesia tenuissima and Ulva ohnoi: biomass and bioproducts. J Appl Phycol. 2016;28:365–75.10.1007/s10811-015-0561-1Search in Google Scholar
[20] Daemi H, Mashayekhi M, Modaress MP. Facile fabrication of sulfated alginate electrospun nanofibers. Carbohyd Polym. 2018;198:481–5.10.1016/j.carbpol.2018.06.105Search in Google Scholar PubMed
[21] Kikionis S, Ioannou E, Toskas G, Roussis V. Electrospun biocomposite nanofibers of ulvan/PCL and ulvan/PEO. J Appl Polym Sci. 2015;132:1–5.10.1002/app.42153Search in Google Scholar
[22] Lubambo AF, De Freitas RA, Sierakowski MR, Lucyszyn N, Sassaki GL, Serafim BM, et al. Electrospinning of commercial guar-gum: Effects of purification and filtration. Carbohyd Polym. 2013;93(2):484–91.10.1016/j.carbpol.2013.01.031Search in Google Scholar PubMed
[23] Toskas G, Hund R-D, Laourine E, Cherif C, Smyrniotopoulos V, Roussis V. Nanofibers based on polysaccharides from the green seaweed Ulva rigida. Carbohyd Polym. 2011;84(3):1093–102.10.1016/j.carbpol.2010.12.075Search in Google Scholar
[24] Mendes AC, Stephansen K, Chronakis IS. Electrospinning of food proteins and polysaccharides. Food Hydrocoll. 2017;68(30th anniversary special issue):53–68.10.1016/j.foodhyd.2016.10.022Search in Google Scholar
[25] Stijnman AC, Bodnar I, Tromp RH. Electrospinning of food-grade polysaccharides. Food Hydrocoll. 2011;25(5):1393–8.10.1016/j.foodhyd.2011.01.005Search in Google Scholar
[26] Raveendran S, Dhandayuthapani B, Nagaoka Y, Yoshida Y, Maekawa T, Kumar DS. Biocompatible nanofibers based on extremophilic bacterial polysaccharide, mauran from Halomonas maura. Carbohyd Polym. 2013;92(2):1225–33.10.1016/j.carbpol.2012.10.033Search in Google Scholar PubMed
[27] Raveendran S, Yoshida Y, Maekawa T, Kumar DS. Pharmaceutically versatile sulfated polysaccharide based bionano platforms. Nanomed-Nanotechnol. 2013;9(5):605–26.10.1016/j.nano.2012.12.006Search in Google Scholar PubMed
[28] Arias S, del Moral A, Ferrer M, Tallon R, Quesada E, Béjar V. Mauran, an exopolysaccharide produced by the halophilic bacterium Halomonas maura, with a novel composition and interesting properties for biotechnology. Extremophiles. 2003;7:319–26.10.1007/s00792-003-0325-8Search in Google Scholar PubMed
[29] Manivasagan P, Oh J. Marine polysaccharide-based nanomaterials as a novel source of nanobiotechnological applications. Int J Biol Macromol. 2016;82:315–27.10.1016/j.ijbiomac.2015.10.081Search in Google Scholar PubMed
[30] Yeul VS, Rayalu SS. Unprecedented chitin and chitosan: A chemical overview. J Polym Env. 2013;21:606–14.10.1007/s10924-012-0458-xSearch in Google Scholar
[31] Huang ML, Smith RAA, Trieger GW, Godula K. Glycocalyx remodeling with proteoglycan mimetics promotes neural specification in embryonic stem cells. J Am Chem Soc. 2014;136(30):10565–8.10.1021/ja505012aSearch in Google Scholar PubMed PubMed Central
[32] Du J, Yarema KJ. Carbohydrate engineered cells for regenerative medicine. Adv Drug Deliv Rev. 2010;62(7–8):671–82.10.1016/j.addr.2010.01.003Search in Google Scholar PubMed PubMed Central
[33] Smith R, Meade K, Pickford C, Holley R, Merry C. Glycosaminoglycans as regulators of stem cell differentiation. Biochem Soc T. 2011;39(1):383–7.10.1042/BST0390383Search in Google Scholar PubMed
[34] Mhanna R, Becher J, Schnabelrauch M, Reis RL, Pashkuleva I. Sulfated alginate as a mimic of sulfated glycosaminoglycans: binding of growth factors and effect on stem cell behavior. Adv Biosyst. 2017;1:1–7.10.1002/adbi.201700043Search in Google Scholar PubMed
[35] Amjadi S, Almasi H, Ghorbani M, Ramazani S. Reinforced ZnONPs/rosemary essential oil-incorporated zein electrospun nanofibers by κ-carrageenan. Carbohyd Polym. 2020;232:1–10.10.1016/j.carbpol.2019.115800Search in Google Scholar PubMed
[36] Wan M, Qin W, Lei C, Li Q, Meng M, Fang M, et al. Biomaterials from the sea: future building blocks for biomedical applications. Bioact Mater. 2021;6(12):4255–85.10.1016/j.bioactmat.2021.04.028Search in Google Scholar PubMed PubMed Central
[37] de Carvalho MM, de Freitas RA, Ducatti DRB, Ferreira LG, Gonçalves AG, Colodi FG, et al. Modification of ulvans via periodate-chlorite oxidation: chemical characterization and anticoagulant activity. Carbohyd Polym. 2018;197:631–40.10.1016/j.carbpol.2018.06.041Search in Google Scholar PubMed
[38] Sari-Chmayssem N, Taha S, Mawlawi H, Guégan JP, Jeftić J, Benvegnu T. Extracted ulvans from green algae Ulva linza of Lebanese origin and amphiphilic derivatives: evaluation of their physico-chemical and rheological properties. J Appl Phycol. 2019;31(3):1931–46.10.1007/s10811-018-1668-ySearch in Google Scholar
[39] Singha T. Microbial extracellular polymeric substances: production, isolation and applications. IOSR J Pharm. 2012;2(2):276–81.10.9790/3013-0220276281Search in Google Scholar
[40] Casillo A, Lanzetta R, Parrilli M, Corsaro MM. Exopolysaccharides from marine and marine extremophilic bacteria: structures, properties, ecological roles and applications. Mar Drugs. 2018;16(2):1–34.10.3390/md16020069Search in Google Scholar PubMed PubMed Central
[41] López-ortega MA, Chavarría-hernández N, López-cuellar MR, Rodríguez-hernández AI. A review of extracellular polysaccharides from extreme niches: An emerging natural source for the biotechnology. Int J Biol Macromol. 2021;177:559–77.10.1016/j.ijbiomac.2021.02.101Search in Google Scholar PubMed
[42] Faria-Oliveira F, Carvalho J, Belmiro CLR, Ramalho G, Pavão M, Lucas C, et al. Elemental biochemical analysis of the polysaccharides in the extracellular matrix of the yeast Saccharomyces cerevisiae. J Basic Microbiology. 2015;55(6):685–94.10.1002/jobm.201400781Search in Google Scholar PubMed
[43] Laurienzo P. Marine Polysaccharides in Pharmaceutical Applications: An Overview. Mar Drugs. 2010;8:2435–65.10.3390/md8092435Search in Google Scholar PubMed PubMed Central
[44] Williams G, Raimi-Abraham B, Luo C. Electrospinning fundamentals. Nanofibers in Drug Delivery. London: UCL Press and JSTOR; 2018. p. 24–59.10.2307/j.ctv550dd1.6Search in Google Scholar
[45] Jain R, Shetty S, Yadav KS. Unfolding the electrospinning potential of biopolymers for preparation of nanofibers. J Drug Deliv Sci Tec. 2020;57:1–15.10.1016/j.jddst.2020.101604Search in Google Scholar
[46] Rošic R, Pelipenko J, Kocbek P, Baumgartner S, Bešter-Rogač M, Kristl J. The role of rheology of polymer solutions in predicting nanofiber formation by electrospinning. Eur Polym J. 2012;48(8):1374–84.10.1016/j.eurpolymj.2012.05.001Search in Google Scholar
[47] Robic A, Sassi JF, Dion P, Lerat Y, Lahaye M. Seasonal variability of physicochemical and rheological properties of ulvan in two Ulva species (chlorophyta) from the Brittany coast. J Phycol. 2009;45(4):962–73.10.1111/j.1529-8817.2009.00699.xSearch in Google Scholar PubMed
[48] Yong K, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1):106–26.10.1016/j.progpolymsci.2011.06.003Search in Google Scholar PubMed PubMed Central
[49] Kidgell JT, Magnusson M, de Nys R, Glasson CRK. Ulvan: a systematic review of extraction, composition and function. Algal Res. 2019;39:1–20.10.1016/j.algal.2019.101422Search in Google Scholar
[50] Robic A, Rondeau-Mouro C, Sassi JF, Lerat Y, Lahaye M. Structure and interactions of ulvan in the cell wall of the marine green algae Ulva rotundata (Ulvales, Chlorophyceae). Carbohyd Polym. 2009;77(2):206–16.10.1016/j.carbpol.2008.12.023Search in Google Scholar
[51] Kidgell JT, Carnachan SM, Magnusson M, Lawton RJ, Sims IM, Hinkley SFR, et al. Are all ulvans equal? A comparative assessment of the chemical and gelling properties of ulvan from blade and filamentous Ulva. Carbohyd Polym. 2021;264:1–12.10.1016/j.carbpol.2021.118010Search in Google Scholar PubMed
[52] Masuelli MA, Illanes CO. Review of the characterization of sodium alginate by intrinsic viscosity measurements. Comparative analysis between conventional and single point methods. Int J Biomaterals Sci Eng. 2014;1:1–12.Search in Google Scholar
[53] Dodero A, Vicini S, Alloisio M, Castellano M. Sodium alginate solutions: correlation between rheological properties and spinnability. J Mater Sci. 2019;54:8034–46.10.1007/s10853-019-03446-3Search in Google Scholar
[54] Shen W, Hsieh Y. Biocompatible sodium alginate fibers by aqueous processing and physical crosslinking. Carbohyd Polym. 2014;102:893–900.10.1016/j.carbpol.2013.10.066Search in Google Scholar PubMed
[55] Guo MQ, Hu X, Wang C, Ai L. Polysaccharides: structure and solubility. Zhenbo Xu, editor. Solubility of Polysaccharides. London: IntechOpen; 2017. p. 7–21.10.5772/intechopen.71570Search in Google Scholar
[56] Rinaudo M. Polyelectrolyte properties of a plant and animal polysaccharide. Struct Chem. 2009;20(2):277–89.10.1007/s11224-009-9426-zSearch in Google Scholar
[57] Roy J, Salaün F, Giraud S, Ferri A, Chen G, Guan J. Solubility of chitin: solvents, solution behaviors and their related mechanisms. In: Zhenbo Xu, editor. Solubility of Polysaccharides. London: InTechOpen; 2017. p. 109–27.10.5772/intechopen.71385Search in Google Scholar
[58] Franca EF, Lins RD, Freitas LCG, Straatsma TP. Characterization of chitin and chitosan molecular structure in aqueous solution. J Chem Theory Comput. 2008;4(12):2141–9.10.1021/ct8002964Search in Google Scholar PubMed
[59] Pawlak A. The Entanglements of Macromolecules and Their Influence on the Properties of Polymers. Macromol Chem Physic. 2019;220(10):1–25.10.1002/macp.201900043Search in Google Scholar
[60] Palangetic L, Reddy NK, Srinivasan S, Cohen RE, McKinley GH, Clasen C. Dispersity and spinnability: why highly polydisperse polymer solutions are desirable for electrospinning. Polym (Guildf). 2014;55(19):4920–31.10.1016/j.polymer.2014.07.047Search in Google Scholar
[61] Madany MA, Abdel-Kareem MS, Al-Oufy AK, Haroun M, Sheweita SA. The biopolymer ulvan from Ulva fasciata: extraction towards nanofibers fabrication. Int J Biol Macromol. 2021;177:401–12.10.1016/j.ijbiomac.2021.02.047Search in Google Scholar PubMed
[62] Yi Y, Xu W, Wang H-X, Huang F, Wang L-M. Natural polysaccharides experience physiochemical and functional changes during preparation: a review. Carbohyd Polym. 2020;234(68):1–18.10.1016/j.carbpol.2020.115896Search in Google Scholar PubMed
[63] Peasura N, Laohakunjit N, Kerdchoechuen O, Wanlapa S. Characteristics and antioxidant of Ulva intestinalis sulphated polysaccharides extracted with different solvents. Int J Biol Macromol. 2015;81:912–9.10.1016/j.ijbiomac.2015.09.030Search in Google Scholar PubMed
[64] Pezoa-conte R, Leyton A, Baccini A, Ravanal M, Mäki-Arvela P, Grénman H, et al. Aqueous Extraction of the Sulfated Polysaccharide Ulvan from the Green Alga Ulva rigida Kinetics and Modeling. Bioenerg Res. 2017;10:915–28.10.1007/s12155-017-9853-4Search in Google Scholar
[65] Yamamoto M. Physicochemical studies on sulfated polysaccharides extracted from seaweeds at various temperatures. AgricBiolChem. 1980;44(3):589–93.10.1080/00021369.1980.10863990Search in Google Scholar
[66] He L, Yan X, Liang J, Li S, He H, Xiong Q, et al. Comparison of different extraction methods for polysaccharides from Dendrobium officinale stem. Carbohyd Polym. 2018;198:101–8.10.1016/j.carbpol.2018.06.073Search in Google Scholar PubMed
[67] Jung HS, Kim MH, Shin JY, Park SR, Jung JY, Park WH. Electrospinning and wound healing activity of β-chitin extracted from cuttlefish bone. Carbohyd Polym. 2018;193:205–11.10.1016/j.carbpol.2018.03.100Search in Google Scholar PubMed
[68] Yaich H, Amira A, Abbes F, Bouaziz M, Besbes S, Richel A, et al. Effect of extraction procedures on structural, thermal and antioxidant properties of ulvan from Ulva lactuca collected in Monastir coast. Int J Biol Macromol. 2017;105:1430–9.10.1016/j.ijbiomac.2017.07.141Search in Google Scholar PubMed
[69] Wahlström N, Nylander F, Malmhäll-Bah E, Sjövold K, Edlund U, Westman G, et al. Composition and structure of cell wall ulvans recovered from Ulva spp. along the Swedish west coast. Carbohyd Polym. 2020;233:1–9.10.1016/j.carbpol.2020.115852Search in Google Scholar PubMed
[70] Ummat V, Sivagnanam SP, Rajauria G, O’Donnell C, Tiwari BK. Advances in pre-treatment techniques and green extraction technologies for bioactives from seaweeds. Trends Food Sci Tech. 2021;110:90–106.10.1016/j.tifs.2021.01.018Search in Google Scholar
[71] Mohammed A, Rivers A, Stuckey DC, Ward K. Alginate extraction from Sargassum seaweed in the Caribbean region: optimization using response surface methodology. Carbohyd Polym. 2020;245:1–8.10.1016/j.carbpol.2020.116419Search in Google Scholar PubMed
[72] Ohkawa K, Cha D, Kim H, Nishida A, Yamamoto H. Electrospinning of chitosan. Macromol Rapid Comm. 2004;25(18):1600–5.10.1002/marc.200400253Search in Google Scholar
[73] De Vrieze S, Westbroek P, Van Camp T, Van Langenhove L. Electrospinning of chitosan nanofibrous structures: feasibility study. J Mater Sci. 2007;42:8029–34.10.1007/s10853-006-1485-6Search in Google Scholar
[74] Shenoy S, Bates W, Frisch H, Wnek G. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: good solvent, non-specific. polymer-polymer Interact limit Polym (Guildf). 2005;46(10):3372–84.10.1016/j.polymer.2005.03.011Search in Google Scholar
[75] Lahaye M. Chemistry and physico-chemistry of phycocolloids. Cah de Biologie Mar. 2001;42(1):137–57.Search in Google Scholar
[76] Yu JH, Fridrikh SV, Rutledge GC. The role of elasticity in the formation of electrospun fibers. Polym (Guildf). 2006;47(13):4789–97.10.1016/j.polymer.2006.04.050Search in Google Scholar
[77] IUPAC-IUB Joint Commission on Biochemical Nomenclature. Nomenclature of carbohydrates (Recommendations 1996). Pure & Appl Chem. 1996;68(10):1919–2008.10.1351/pac199668101919Search in Google Scholar
[78] Elieh-Ali-Komi D, Hamblin MR. Chitin and chitosan: production and application of versatile biomedical nanomaterials. Int J Adv Res. 2016;4(3):411–27.Search in Google Scholar
[79] Wani S, Sofi HS, Majeed S, Sheikh FA. Recent trends in chitosan nanofibers: from tissue-engineering to environmental importance: A review. Mater Sci Res India. 2017;14(2):89–99.10.13005/msri/140202Search in Google Scholar
[80] Ray B, Lahaye M. Cell-wall polysaccharides from the marine green alga Ulva “rigida” (Ulvales, Chlorophyta). Chemical structure of ulvan. Carbohyd Res. 1995;274(C):313–8.10.1016/0008-6215(95)00059-3Search in Google Scholar
[81] Quemener B, Lahaye M, Bobin-Dubigeon C. Sugar determination in ulvans by a chemical-enzymatic method coupled to high performance anion exchange chromatography. J Appl Phycol. 1997;9:179–88.10.1023/A:1007971023478Search in Google Scholar
[82] Robic A, Gaillard C, Sassi J, Leral Y, Lahaye M. Ultrastructure of Ulvan: a polysaccharide from green seaweeds. Biopolymers. 2009;91(8):652–64.10.1002/bip.21195Search in Google Scholar PubMed
[83] Shao P, Qin M, Han L, Sun P. Rheology and characteristics of sulfated polysaccharides from chlorophytan seaweeds Ulva fasciata. Carbohyd Polym. 2014;113:365–72.10.1016/j.carbpol.2014.07.008Search in Google Scholar PubMed
[84] Jönsson M, Allahgholi L, Sardari RRR, Hreggviðsson GO, Karlsson EN. Extraction and modification of macroalgal polysaccharides for current and next-generation applications. Molecules. 2020;25:1–29.10.3390/molecules25040930Search in Google Scholar PubMed PubMed Central
[85] Glasson C, Sims I, Carnachan S, Nys R, Magnusson M. A cascading biorefinery process targeting sulfated polysaccharides (ulvan) from Ulva ohnoi. Algal Res. 2017;27(March):383–91.10.1016/j.algal.2017.07.001Search in Google Scholar
[86] Cindana Mo’oFR, Wilar G, Devkota HP, Wathoni N. Ulvan, a polysaccharide from macroalga Ulva sp: a review of chemistry, biological activities and potential for food and biomedical applications. Appl Sci. 2020;10(16):1–21.10.3390/app10165488Search in Google Scholar
[87] Antoniou E, Buitrago CF, Tsianou M, Alexandridis P. Solvent effects on polysaccharide conformation. Carbohyd Polym. 2010;79(2):380–90.10.1016/j.carbpol.2009.08.019Search in Google Scholar
[88] Jayawardena B, Pandithavidana DR, Sameera W. Polysaccharides in Solution: Experimental and Computational Studies. In: Zhenbo Xu, editor. Solubility of Polysaccharides. London: InTechOpen; 2017. p. 1–62.10.5772/intechopen.69863Search in Google Scholar
[89] Barber PS, Griggs CS, Bonner JR, Rogers RD. Electrospinning of chitin nanofibers directly from an ionic liquid extract of shrimp shells. Green Chem. 2013;15(3):601–7.10.1039/c2gc36582kSearch in Google Scholar
[90] Min BM, Lee SW, Lim JN, You Y, Lee TS, Kang PH, et al. Chitin and chitosan nanofibers: electrospinning of chitin and deacetylation of chitin nanofibers. Polym (Guildf). 2004;45(21):7137–42.10.1016/j.polymer.2004.08.048Search in Google Scholar
[91] Narayanan D, Jayakumar R, Chennazhi KP. Versatile carboxymethyl chitin and chitosan nanomaterials: a review. Wires Nanomed Nanotechnol. 2014;6:574–98.10.1002/wnan.1301Search in Google Scholar PubMed
[92] Gorzędowski J, Smiglak M, Struszczyk M, Puchalski M, Krucińska I. Chitin fibre formation by the solution blow spinning method, using 1-butyl- 3 methylimidazolium acetate ionic liquid as a solvent. Fibres Text East Eur. 2020;28(4):42–8.Search in Google Scholar
[93] Geng X, Kwon O-H, Jang J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials. 2005;26:5427–32.10.1016/j.biomaterials.2005.01.066Search in Google Scholar PubMed
[94] Li L, Li Y, Yang C. Chemical filtration of Cr ( VI) with electrospun chitosan nanofiber membranes. Carbohyd Polym. 2016;140:299–307.10.1016/j.carbpol.2015.12.067Search in Google Scholar PubMed
[95] Fong H, Chun I, Reneker DH. Beaded nanofibers formed during electrospinning. Polym (Guildf). 1999;40(16):4585–92.10.1016/S0032-3861(99)00068-3Search in Google Scholar
[96] Dedic J, Okur HI, Roke S. Polyelectrolytes induce water-water correlations that result in dramatic viscosity changes and nuclear quantum effects. Sci Adv. 2019;5:1–8.10.1126/sciadv.aay1443Search in Google Scholar PubMed PubMed Central
[97] Castro-Puyana M, Marina M, Plaza M. Water as green extraction solvent: principles and reasons for its use. Current Opinion in Green and Sustainable. Chemistry. 2017;5:31–6.10.1016/j.cogsc.2017.03.009Search in Google Scholar
[98] Agarwal S, Greiner A. On the way to clean and safe electrospinning – green electrospinning: emulsion and suspension electrospinning. Polym Adv Technol. 2011;22:372–8.10.1002/pat.1883Search in Google Scholar
[99] Patil SV. Crosslinking of polysaccharides: methods and applications. Pharm Rev. 2008;6(2):1–8.Search in Google Scholar
[100] Tölgyessy J. The chemistry of water. Studies in Environmental Science. Elsevier B.V; 1993. p. 14–32510.1016/S0166-1116(08)70067-0Search in Google Scholar
[101] Diener M, Adamcik J, Sánchez-Ferrer A, Jaedig F, Schefer L, Mezzenga R. Primary, secondary, tertiary and quaternary structure levels in linear polysaccharides: from random coil, to single helix to supramolecular assembly. Biomacromolecules. 2019;20(4):1731–9.10.1021/acs.biomac.9b00087Search in Google Scholar PubMed
[102] Sugimoto M, Morimoto M, Sashiwa H, Saimoto H, Shigemasa Y. Preparation and characterization of water-soluble chitin and chitosan derivatives. Carbohyd Polym. 1998;36(1):49–59.10.1016/S0144-8617(97)00235-XSearch in Google Scholar
[103] Shamshina JL. Chitin in ionic liquids: historical insights into the polymer’s dissolution and isolation. A review. Green Chem. 2019;21:3974–93.10.1039/C9GC01830ASearch in Google Scholar
[104] Homayoni H, Hosseini Ravandi S, Valizadeh M. Electrospinning of chitosan nanofibers: processing optimization. Carbohyd Polym. 2009;77(3):656–61.10.1016/j.carbpol.2009.02.008Search in Google Scholar
[105] Hillberg AL, Holmes CA, Tabrizian M. Biomaterials effect of genipin cross-linking on the cellular adhesion properties of layer-by-layer assembled polyelectrolyte films. Biomaterials. 2009;30(27):4463–70.10.1016/j.biomaterials.2009.05.026Search in Google Scholar PubMed
[106] Miras J, Liu C, Blomberg E, Thormann E, Vílchez S, Esquena J. Physicochemical and engineering aspects pH-responsive chitosan nanofilms crosslinked with genipin. Colloids Surf A: Physicochemical Eng Asp. 2021;616:1–10.10.1016/j.colsurfa.2021.126229Search in Google Scholar
[107] Nie H, He A, Zheng J, Xu S, Li J, Han CC. Effects of chain conformation and entanglement on the electrospinning of pure alginate. Biomacromolecules. 2008;9(5):1362–5.10.1021/bm701349jSearch in Google Scholar PubMed
[108] Tziveleka LA, Ioannou E, Roussis V. Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials: a review. Carbohyd Polym. 2019;218:355–70.10.1016/j.carbpol.2019.04.074Search in Google Scholar PubMed
[109] de Carvalho MM, Noseda MD, Dallagnol JCC, Ferreira LG, Ducatti DRB, Gonçalves AG, et al. Conformational analysis of ulvans from Ulva fasciata and their anticoagulant polycarboxylic derivatives. Int J Biol Macromol. 2020;162:599–608.10.1016/j.ijbiomac.2020.06.146Search in Google Scholar PubMed
[110] Bodnár E, Grifoll J, Rosell-Llompart J. Polymer solution electrospraying: a tool for engineering particles and films with controlled morphology. J Aerosol Sci. 2018;125:93–118.10.1016/j.jaerosci.2018.04.012Search in Google Scholar
[111] Graessley WW. Entanglement concept in polymer rheology. In: Springer, editor. The Entanglement Concept in Polymer Rheology. 1st ed. Berlin Heidelberg: Springer-Verlag; 1974. p. 3–164.10.1007/BFb0031037Search in Google Scholar
[112] McKee MG, Wilkes GL, Colby RH, Long TE. Correlations of Solution Rheology with Electrospun Fiber Formation of Linear and Branched Polyesters. Macromolecules. 2004;37(5):1760–7.10.1021/ma035689hSearch in Google Scholar
[113] Porter RS, Johnson JF. The entanglement concept in polymer systems. Chem Rev. 1966;66(1):1–27.10.1021/cr60239a001Search in Google Scholar
[114] Gupta P, Elkins C, Long T, Wilkes G. Electrospinning of linear homopolymers of poly(methyl methacrylate): exploring relationships between fiber formation, viscosity, molecular weight and concentration in a good solvent. Polym (Guildf). 2005;46(13):4799–810.10.1016/j.polymer.2005.04.021Search in Google Scholar
[115] Akkoyun S, Öktem N. Effect of viscoelasticity in polymer nanofiber electrospinning: Simulation using FENE-CR model. Eng Sci Technology, an Int J. 2021;24(3):620–30.10.1016/j.jestch.2020.12.017Search in Google Scholar
[116] Antonietti M, Briel A, Stephan F. Quantitative description of the intrinsic viscosity of branched polyelectrolytes. Macromolecules. 1997;30(9):2700–4.10.1021/ma9617025Search in Google Scholar
[117] Ise N, Sogami IS. Structure formation in solution. Ionic polymers and colloidal particles. In: Springer, editor. Structure Formation in Solution: Ionic Polymers and Colloidal Particles. 1st edn. Berlin Heidelberg: Springer-Verlag; 2005. 1–350.Search in Google Scholar
[118] Kong H-J, Lee KY, Mooney D. Decoupling the dependence of rheological/mechanical properties of hydrogels from solids concentration. Polym (Guildf). 2002;43:6239–46.10.1016/S0032-3861(02)00559-1Search in Google Scholar
[119] Kuchibhatla SV, Karakoti AS, Seal S. Colloidal stability by surface modification. J Min Met Mater Soc. 2005;57:52–6.10.1007/s11837-005-0183-1Search in Google Scholar
[120] Haider A, Haider S, Kang I. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab J Chem. 2018;11(8):1165–88.10.1016/j.arabjc.2015.11.015Search in Google Scholar
© 2022 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption

