Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
-
Faten Ermala Che Othman
, Nik Abdul Hadi Md Nordin
Abstract
This study presents the fabrication of polyethyleneimine (PEI)–graphene-derived rice husk char (GRHC)/activated carbon nanofiber (ACNF) composites via electrospinning and physical activation processes and its adsorption performance toward CO2. This study was performed by varying several parameters, including the loading of graphene, impregnated and nonimpregnated with amine, and tested on different adsorption pressures and temperatures. The resultant ACNF composite with 1% of GRHC shows smaller average fiber diameter (238 ± 79.97 nm) with specific surface area (S BET) of 597 m2/g, and V micro of 0.2606 cm3/g, superior to pristine ACNFs (202 m2/g and 0.0976 cm3/g, respectively). ACNF/GRHC0.01 exhibited CO2 uptakes of 142 cm3/g at atmospheric pressure and 25°C, significantly higher than that of pristine ACNF’s 69 cm3/g. The GRHC/ACNF0.01 was then impregnated with PEI and further achieved impressive increment in CO2 uptake to 191 cm3/g. Notably, the adsorption performance of CO2 is directly proportional to the pressure increment; however, it is inversely proportional with the increased temperature. Interestingly, both amine-impregnated and nonimpregnated GRHC/ACNFs fitted the pseudo first-order kinetic model (physisorption) at 1 bar; however, best fitted the pseudo second-order kinetic model (chemisorption) at 15 bar. Both GRHC/ACNF and PEI-GRHC/ACNF samples obeyed the Langmuir adsorption isotherm model, which indicates monolayer adsorption. At the end of this study, PEI-GRHC/ACNFs with excellent CO2 adsorption performance were successfully fabricated.
1 Introduction
Current dependency on fossil fuel accounts for about 85% of the total global energy production, which contributes to about 40% of the global CO2 emission. The global monitoring laboratory at Mauna Lao observed an estimated CO2 weekly average atmospheric concentration of 413.84 ppm during November 2020, 0.78% higher than last year’s value and 6.25% higher than the weekly value from 10 years ago [1]. The increased level of atmospheric CO2 is worrying as it is the major component of the greenhouse gases (GHG) that are a major concern regarding climate change. It is estimated that the global economic damage cause by climate change (including costs associated with climate change-induced market and nonmarket impacts, effects of sea level rise, and consequences associated with large-scale discontinuities) are projected to be $54 and $69 trillion, respectively, relative to 1961–1990 [2].
Therefore, considerable effort has been made by the international community toward reducing the use of these hazardous materials as well as to combat global warming. The most recent realization of the effort was the 21st Conference of Parties (COP21) that was held in Paris, France, in 2015 to limit global temperature rise well below 2℃ by the end of this century. In light of the current alarming rate of anthropogenic CO2 emission, carbon capture and storage (CCS) technologies receive overwhelming attention to combat this issue. These technologies currently serve as reliable mitigation approaches for climate change as well as the key to controlling the global CO2 emission by controlling the emission of gases from their point source. Through sustainable development along with CCS technology, it is projected that the level of anthropogenic CO2 emission would be reduced up to 22% of total CO2 emissions in 2035 [3]. Currently, there are several techniques for CO2 capture that have been utilized including adsorption, absorption, membrane separation, and cryogenic distillation. Interestingly, adsorption showed great potential due to its economic and easy handling during adsorbent preparation and effective energy consumption for regeneration [4].
A wide variety of adsorbents have been studied over the past decades, such as zeolites [5], silica [6], carbon-based materials [7], and metal organic frameworks (MOFs) [8]. Interestingly, carbon-based adsorbents have aroused intense interest among researchers owing to their tunable pore structure and adjustable surface properties. One example of a newer class of carbon-based sorbents is activated carbon nanofibers (ACNFs), which possess fibrous structure with a large number of active sites and direct accessible micropores for adsorption [9]. Pristine ACNFs have been found to possess moderate specific surface area (S BET) and low carbon yield after the activation process due to high decomposition of polyacrylonitrile (PAN) as the polymer precursor at a high activation temperature. Even though the S BET achieved by ACNFs is moderate, the adsorption capacity of ACNFs is comparable to that of commercial carbon [9]. This is due to the wide range of porosity of the ACNFs with abundant micropores in their structures. The findings from our recent studies [10] have proved that impregnation of nanofillers or additives can improve the physicochemical properties of the adsorbent, especially S BET and porous structure, consequently the adsorption properties.
At present, a number of additive materials are being used, including metal and metal oxides, graphene and graphene oxides (GO), and amine moieties. For instance, ACNFs composited with magnesium oxide (MgO) and showing enhanced porous structure (S BET up to 1,900 m2/g and 0.7336 cm3/g) have been successfully synthesized by Othman et al. [9]. This approach could be potentially useful in improving their CO2 adsorption capacity through physisorption. In addition, introducing amine moieties to the adsorbent was also reported to improve CO2 adsorption capacity via chemisorption. Study by Rouzitalab et al. [11] demonstrated that N-doped adsorbent enhanced the polarity of the adsorbent surface and significantly improved CO2 capacity with the adsorption capacity of 7.42 mmol/g at 1 bar. However, regarding the adsorption-based CO2 separation process, it is well known that, besides the adsorption step, the setup of a reliable and economic sorbent regeneration strategy represents one of the crucial issues to be dealt with. This is because the regeneration mode greatly affects the selection of the most proper reactor configuration, which should be efficient and cost-effective. Currently available regeneration of the sorbent include temperature swing adsorption (TSA), pressure swing adsorption (PSA), vacuum swing adsorption (VSA), and possibly a combination of two modes, for example, hybrid regeneration mode such as VTSA or PTSA [12]. In our previous work, low-cost graphene-derived rice husk char (GRHC) was successfully synthesized and incorporated into the ACNFs [13] and known as GRHC/ACNFs. The GRHC/ACNF composites have shown improvement in S BET and micropore volumes as well as better adsorption capacity. Over these past decades, the carbon-based adsorbents have been further introduced into functional groups that provide basicity to the surface of the GRHC/ACNFs for capturing CO2. This is because the adsorption performance of CO2 on the adsorbents is limited due to the low affinity of CO2 molecules or low moisture toleration as well as limited adsorption at higher temperature (higher than 30°C). To the best of our knowledge, there are no studies available to deliberate on the implementation of polyethyleneimine (PEI) for amine functionalization of the existing GRHC/ACNF adsorbent at the moment. The challenge of amine impregnation is that the amount of amine attached on the surface is limited by the surface area. Therefore, by improving the surface area of the host materials prior to impregnation could potentially elevate their capacity. Thus, this study represents the very first one that extensively discusses the preparation of GRHC/ACNF composites with enhanced properties especially in terms of porosity and impregnation with PEI for excellent postcombustion CO2 capture.
2 Methods
2.1 Synthesis of graphene-derived rice husk char (GRHC)
GRHC is a graphene-based material that is made from rice husks through a chemical activation method. Rice husks were procured from a local paddy mill in Kedah, Malaysia. They were chemically treated with potassium hydroxide (KOH; ≥85% pellets) under pure air flow at 200°C to produce rice husk char (RHC). The RHC were ground to form a powder. GRHC were formed by a simple chemical activation method. Details on the synthesis method to convert rice husks into GRHC has been explained in our previous study [14].
2.2 Fabrication of ACNF composites
PAN (M w 150,000 Da), N,N-dimethylformamide (DMF), and PEI were purchased from Sigma Aldrich and used as received. PAN and DMF were used to make the polymer dope solution and PEI was used for amine functionalization of the ACNF. Nitrogen gas (99.99%), CO2 (99.99%), and purified air (99.99%) were acquired from Alpha Gas Solution Sdn Bhd. For the polymer dope preparation, 8 wt% (4 g) of PAN and 92 wt% (46 g) of DMF were used for the total weight of 50 g of solution. Prior to electrospinning, 1 wt% of GRHC relative to PAN was stirred in DMF for a minimum 6 h to form a homogeneous solution and then PAN was added. The dope solution was left stirring for 24 h at room temperature until homogeneity was achieved. The same procedure was repeated with varied GRHC loadings (2.5, 5, 10 g). The composition of all denoted samples are listed in Table 1 with ACNF acting as the control for this study.
Dope formulation of ACNFs with different graphene loadings of 50 mL solution
| Sample name | PAN to graphene ratio | PAN wt (g) | Graphene wt (g) |
|---|---|---|---|
| ACNF | 1:0 | 4 | 0 |
| GRHC/ACNF0.01 | 1:0.01 | 3.96 | 0.04 |
| GRHC/ACNF0.025 | 1:0.025 | 3.90 | 0.1 |
| GRHC/ACNF0.5 | 1:0.05 | 3.80 | 0.2 |
| GRHC/ACNF0.1 | 1:0.1 | 3.60 | 0.4 |
The dope solution was then loaded into the electrospinning machine to fabricate the nanofiber. The optimum electrospinning parameters were carried out based on our previous work [15]. The syringe pump was set at 1.0 mL/h, voltage supply at 12 kV, and the needle–collector gap at 15 cm while the environment of the electrospinning chamber was controlled at 30°C temperature and 50% relative humidity. Pyrolysis in a horizontal furnace (model Carbolite 12/65/550) that included three stages, namely stabilization, carbonization, and activation, was carried out next. In the stabilization stage, the NFs were heated up to 275°C under O2 flow with a heating rate of 2°C/min. Then, carbonization of the stabilized NFs under N2 atmosphere was carried out with heating up to 600°C (heating rate of 5°C/min) followed by the activation of the carbonized NFs to 800°C (heating rate of 5°C/min), respectively. The gas flow rate and dwelling time used throughout the process were 0.2 L/min and 30 min, respectively.
2.3 Impregnation of PEI on GRHC/ACNF composites
Amine impregnation was adapted from the study by Khalil and coworkers with some modifications. First, 2 g of ACNFs was mixed with 2 g of PEI and 10 g of DI water. The mixture was magnetically stirred at room temperature for 3 h to make sure the PEI were fully attached on the surface of ACNF samples. Then, the mixture was put into a vacuum oven at 100°C for 5 h for complete drying. A similar procedure was also performed on GRHC/ACNFs. The dried PEI-impregnated samples were further investigated, characterized, and tested for CO2 adsorption performance.
2.4 Characterization
Before the N2 adsorption/desorption test, the VacPrep Degasser (VacPrep 061, Micromeritics Instrument Corporation) was used to degas the samples in a processor under vacuum (1 × 10−1 kPa) at 150°C, minimum for 12 h. Then, the textural properties of the porosity of the samples were examined using a porosity analyzer (MicrotracBEL Belsorp-Max) with N2 (99.9999% purity) at −196°C. The Brunauer–Emmett–Teller (BET) method has been used in determining the specific surface area (S BET), total pore volume (TPV), and mean pore diameter of samples. Meanwhile, the t-plot and Barrett–Joyner–Halenda (BJH) methods were used to determine the micropore volume (V micro) of samples. The mesopore volumes (V meso) were evaluated by subtracting the V micro from TPV. In order to study the crystallinity of the materials and their phase structure, X-ray diffractometer (XRD, Rigaku SmartLab) using Cu Kα (λ = 1.54184 Å) at a scanning rate of 1.5°/min was utilized. A Raman spectrophotometer (RMP-510S, Jasco USA) with 514 nm wavelength Ar-ion laser excitation and 20 mW laser power was applied to describe the degree of graphitization and structural transformation of the samples. The Raman spectrum peaks were recorded within the range of 1,000–3,000 cm−1 laser excitation wavelength.
The morphology, structure, and elemental properties and mapping of the samples were conducted with a high-resolution transmission electron microscope (HR-TEM 120 kV; Hitachi HT7700), field-emission scanning electron microscope (FE-SEM), and electron dispersive X-ray (EDX) (Hitachi SU8020; Hitachi Co. Ltd Japan). Prior to characterization, all samples were placed on a copper grid with a carbon film (after ion bombardment) and were left to dry at an ambient atmosphere. Fourier-transform infrared (FTIR, Thermo Scientific/Nicolet iS10) was employed in determining the chemical functionalities in the samples by setting the scanning range of 4,000–1,000 cm−1. For determining the thermal properties of the samples, such as the percentage of carbon yield and weight loss, thermal gravimetric analysis (TGA) (TA Instruments Q600 Simultaneous DSC/TG) was carried out in the range of 25–800°C with a heating rate of 10°C/min under N2 flow.
2.5 CO2 adsorption performance
CO2 sorption was assessed through a volumetric gas adsorption system (Micromeritics TriStar II). Prior to adsorption, all moisture in the samples was firstly removed by degassing the samples for 12 h at 150°C. Each sample with a weight of ±0.1 g was filled into the adsorption column and constant flow of CO2 (0.015 L/min) was used for the adsorption study. Consequently, samples with the best adsorption performance were subjected to the adsorption test at different temperatures (0, 25, and 50°C) to study their adsorption properties at low to moderate temperatures. As both PEI-impregnated and nonimpregnated GRHC/ACNFs exhibited better adsorption performance over pristine ACNFs, these two samples were used for further assessments including the kinetic and equilibrium characteristics as well as stability studies.
2.6 Kinetics and adsorption equilibrium studies
In order to examine the experimental data of CO2 adsorption on the ACNF samples at different times, Lagergren’s pseudo first-order and pseudo second-order of kinetic models have been employed. According to pseudo first-order characteristics, the adsorption rate is proportional to the number of free adsorption sites on the surface of the adsorbent, and it was calculated based on Equation (1):
where q e is the weight-specific adsorbed amount of the adsorbate at the end of equilibrium (mmol/g), q t is the weight-specific adsorbed amount of the adsorbate at time t (s) during the adsorption process (mmol/g), and k 1 is the rate constant of adsorption of the pseudo first-order model.
Thus, it can be assumed that CO2 adsorption conforms to the pseudo first-order model if the representation of
However, according to the pseudo second-order model, the adsorption rate is proportional to the square of vacant adsorption sites as shown by equation (2):
where
Similar to kinetic study, only GRHC/ACNFs (PEI-impregnated and nonimpregnated) were used for adsorption equilibrium study. The CO2 adsorption isotherms show the relationship between the CO2 adsorbed amount at equilibrium at different pressures (1 and 15 bar). The data obtained were adjusted to the Langmuir and Freundlich models to determine the stages and mechanism of the adsorption of CO2.
2.7 Regeneration and stability
One of the crucial parameters that need to be considered for the practical application of any adsorbent in the adsorption process is the long-term cycling stability. Therefore, we carried out the desorption of the cyclic CO2 after each cycle of adsorption to determine the stability of GRHC/ACNFs. In this study, the cycles were repeated five times at the same adsorption conditions in order to evaluate the regeneration of the GRHC/ACNFs in the early stage. This result will give a better understanding of the adsorbent stability for further use in the following cycles. The cycles were conducted under conditions similar to real-life situations as this temperature is where the CO2 is stripped out from the flue gas pipe. The regeneration of both exhausted GRHC/ACNFs and PEI-impregnated GRHC/ACNFs was conducted by sweeping the adsorbents with 0.1 L/min upward pure N2 at 100°C for 5 h. The regenerated adsorbents were reused in an experiment to adsorb CO2 [18].
3 Results and discussion
3.1 Activated carbon nanofibers composited GRHC (GRHC/ACNF) composites
The N2 isotherm of all prepared samples are shown in Figure 1, and their microstructure and textural properties are recorded in Table 2. All prepared NF-based materials, including with GRHC shows no microporous structure, (V micro = 0 cm3/g). After activation, the textural properties of the ACNFs shows the development of meso- and micropores and increment in the surface area. Type I(b) isotherms derived from the N2 adsorption/desorption in Figure 1 explains the microporous attributes in all ACNF samples. [19,20]. Typical adsorption response of microporous materials can be seen from the stagnant plateau isotherm of all the ACNFs at p/p 0 = 0.05–0.95; however, above 0.95 the adsorption curve continually increases. Additionally, as Qi et al. [19] suggested, H3-type of hysteresis loop was observed from the N2 adsorption/desorption isotherms (p/p 0 = 0.4–1.0), which was due to the capillary condensation in the mesoporous pores [21,22]. It is confirmed that all the activated samples were mainly microporous with a high proportion of V micro in relation to the TPV as shown in Table 2. Along with it, the values of V meso indicating the proportion of mesoporous structure are also tabulated.
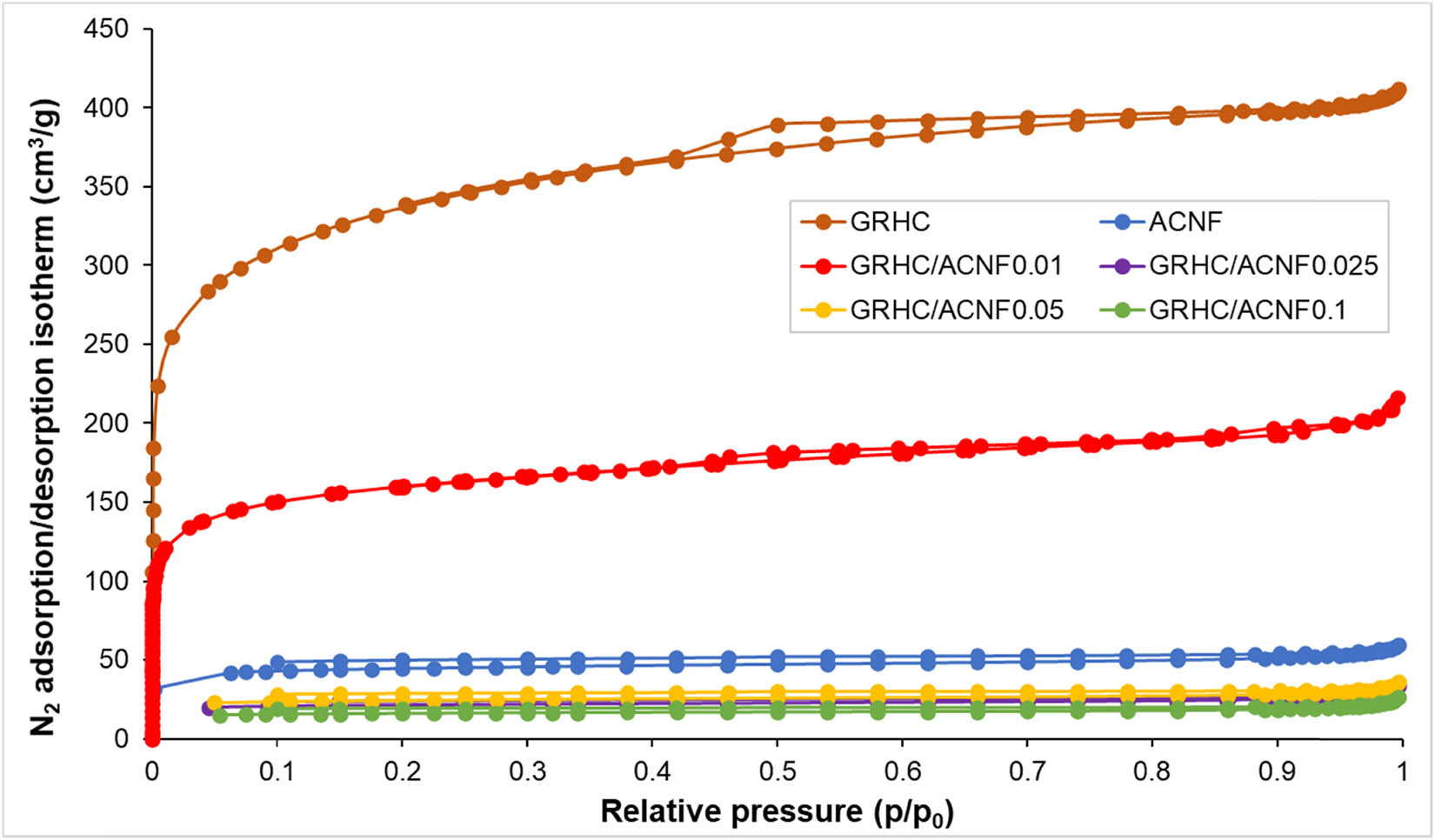
N2 adsorption/desorption isotherms of ACNFs synthesized at different loadings of GRHC after activation.
Porous structure characteristics of pristine and composite ACNFs prior and after activation
| Samples | S BET (m2/g) | TPV (cm3/g) | V micro (cm3/g) | V meso (cm3/g) | DPAve (nm) | |
|---|---|---|---|---|---|---|
| Prior activation | NF | 17.17 | 0.1364 | 0 | 0 | 31.7692 |
| GRHC/NF0.01 | 17.80 | 0.1423 | 0 | 0 | 31.9677 | |
| GRHC/NF0.025 | 18.24 | 0.0053 | 0 | 0 | 21.0940 | |
| GRHC/NF0.05 | 57.71 | 0.4284 | 0 | 0 | 29.6902 | |
| GRHC/NF0.1 | 15.80 | 0.0796 | 0 | 0 | 20.1624 | |
| After activation | ACNF | 138.67 | 0.0930 | 0.0548 | 0.0382 | 2.8424 |
| GRHC/ACNF0.01 | 597.27 | 0.3218 | 0.2606 | 0.0612 | 2.174 | |
| GRHC/ACNF0.025 | 66.41 | 0.0523 | 0.0267 | 0.0256 | 3.3385 | |
| GRHC/ACNF0.05 | 78.26 | 0.0562 | 0.0309 | 0.0253 | 2.8728 | |
| GRHC/ACNF0.1 | 51.65 | 0.0412 | 0.0205 | 0.0207 | 3.1897 |
S BET = specific surface area; TPV = total pore volume; V micro = micropore volume; V meso = mesopores volume; DPAve = Average pore diameter.
At an activation temperature of 800°C, it can be significantly observed that S BET increases in all ACNF samples as shown in Table 2, which represents the formation of mesoporous/microporous structures. This could possibly be due to the catalytic effects of GRHC that takes place at higher temperatures [23]. For example, the S BET of ACNF drastically improved from 17 m2/g to 139 m2/g after the activation. Prior to activation, it can be seen that the S BET is very low in all samples, and it is considered that there is no significant catalytic ability at low temperatures. The S BET considerably increases after activation indicating that GRHC has good catalytic ability for creating porosity at higher temperature. Interestingly, the highest S BET of 597 m2/g was measured for GRHC/ACNF0.1 while further increasing GRHC loading diminished its textural properties. As shown in Table 2, ACNFs with the highest loading of GRHC show the lowest S BET and V micro. Importantly, the adsorbents with high S BET and V micro are preferable as these are important factors for the adsorption of gas molecules [24]. The V meso increases and V micro decreases with the increasing loading of GRHC. This increment of GRHC loading leading to poor textural properties of the ACNF composite could possibly be due to the effects of high conductivity of GRHC that could interfere with the jet ejection during electrospinning for producing nanofibers with larger diameter and formation of beads that results in pore blockage (see Supplementary Materials, Figure S1).
The XRD spectra of all samples in this study are shown in Figure 2. For GRHC, a sharp peak at 28.6° representing the (002) lattice plane and a broad peak at range 17°–31° representing hexagonal graphite were observed. All ACNFs shows two diffraction peaks ranging between 17°–31° and 41°–43°, representing hexagonal graphite and rhombohedral graphite, respectively [25], with variation only in their intensities. Peaks appear around 17°–31° corresponding to the (002) plane of the carbon skeleton [25]. This represents the formation of ladder structures within the ACNF and the composites after the cyclization of PAN activation. Moreover, XRD analysis reveals their crystallographic (100) and amorphous (110) planes [26]. Apparently, the presence of the low and broad peaks in the range of 41°–43° indicates that samples have amorphous structure and possess very low graphitization degree. This finding corresponds well to the one obtained by Raman spectroscopy. All amorphous ACNFs obtained in this study could possibly be due to the destruction of the in-plane aromatic lattices [27], leaving a large number of defect structures in the ACNFs [28,29]. Remarkably, there is no appearance of the GRHC peak in all ACNF composites and this is believed to be due to the very small amount of GRHC used in all composite samples, which is below 10% (relative to PAN wt) that contributed to very weak peak or insignificant peak in the amorphous structure of the carbon materials. In 2012, Guo et al. [30] found that the carbon materials with amorphous and defective structures were considered to have greater ability in modifying the textural characteristics of the ACNFs, which was also observed in this study (Figure 1 and Table 2).
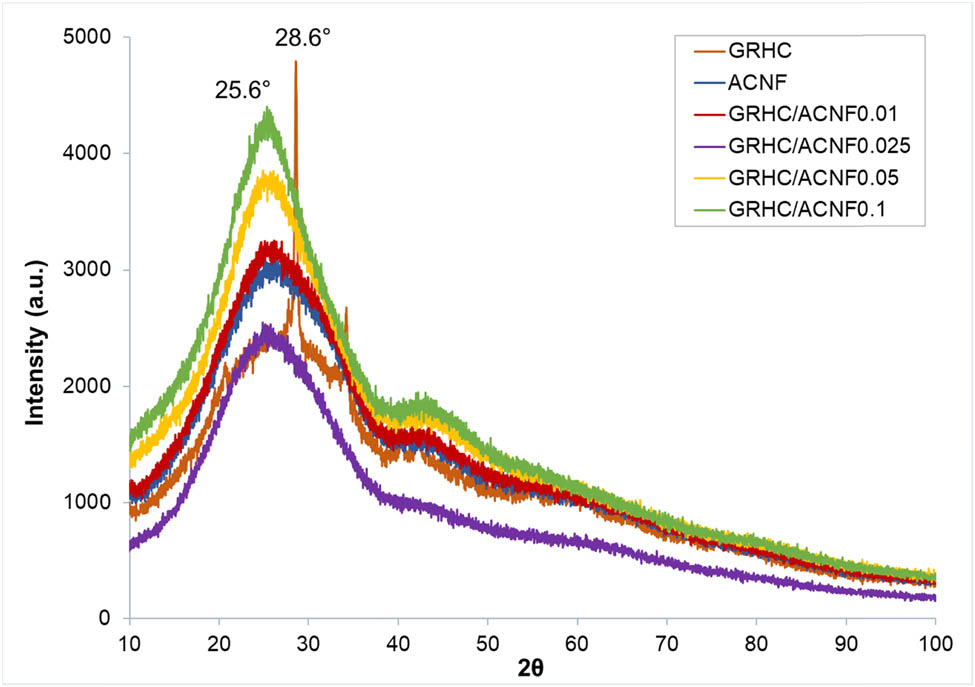
XRD spectra of GRHC, pristine, and composite ACNFs with different loadings of GRHC.
Raman spectroscopy was used in this study to identify the structural features and degree of graphitization of the GRHC and pristine ACNFs, as well as their composites, as shown in Figure 3. All activated samples exhibited two distinguishing peaks, which are at 1,368 cm−1 and 1,612 cm−1 but with different intensities. These two peaks indicated the appearance of the D-band and G-band of graphitic materials in the samples, respectively. In conjunction, the D-band spectra of all samples stipulated the existence of defective and amorphous carbon structure whereas the presence of the G-band signified the orderly structure of the graphene with sp 2 hybridization of the carbon atom. The intensity is relatively high at a low loading of GRHC (1 wt%), and further increasing the loading diminishes their intensity.
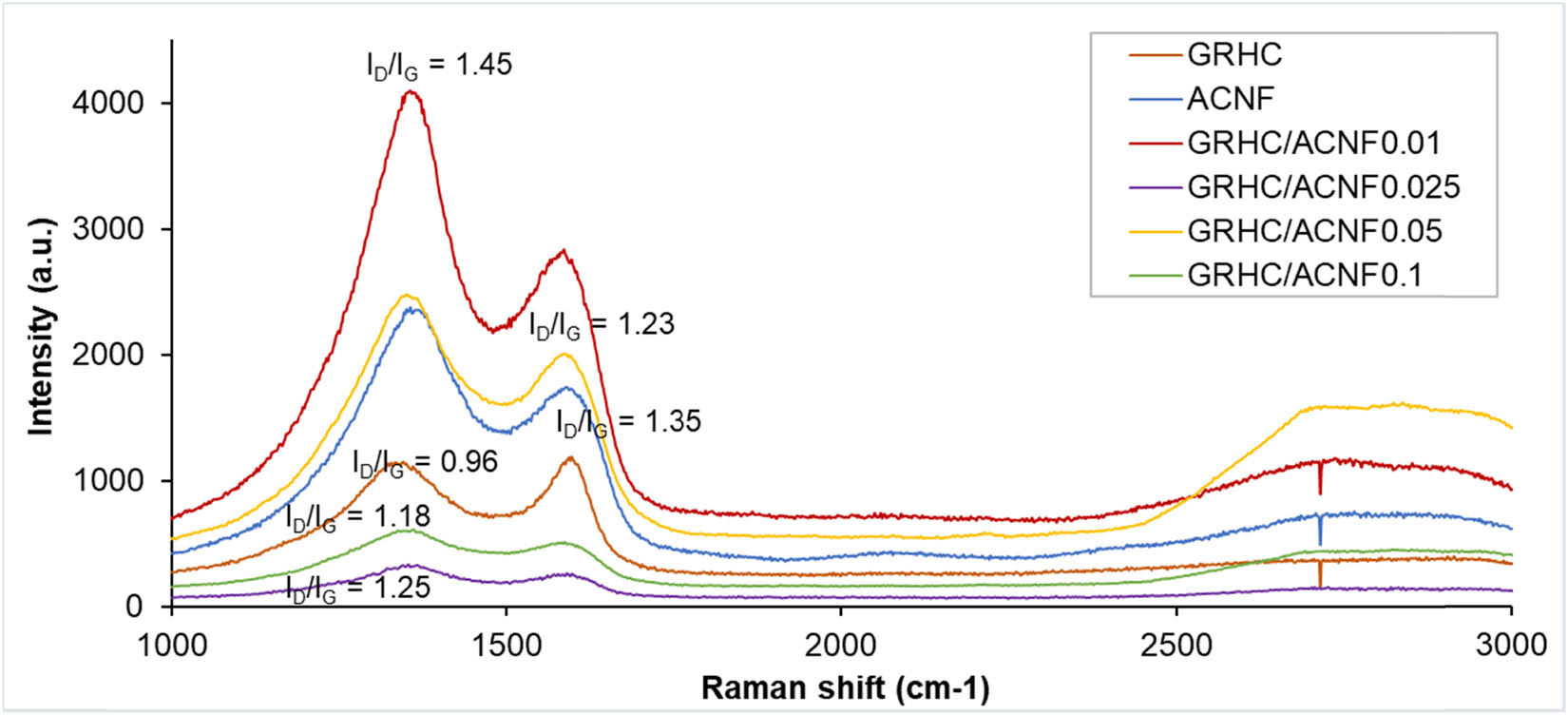
Raman spectra of GRHC, pristine, and composite ACNFs with different loadings of GRHC.
As mentioned in previous literature, the formation of the graphene material can be confirmed from the appearance of the 2D-band spectrum around 2,700 cm−1 of the Raman shift [31]. Broad and weak 2D-bands around 2,700–2,800 cm−1 can be observed in all ACNF samples in Figure 3. The very low intensity of the 2D-band in ACNF composites is believed to be due to too small amount of GRHC composited into the structures. It is worth noting that all ACNF-based samples exhibited defect structures and highly amorphous nature as indicated by the intensity of the D-band being higher than the G-band, concurring with the XRD result analysis. In contrast, the G-band is higher than the D-band in the GRHC sample proving its higher crystallinity as a graphene-based material.
Pristine GRHC exhibits the smallest intensity ratio R(I D/I G) of 0.96 for D-band to G-band among all materials under investigation due to the ordered carbon structures as well as the high degree of graphitization [32,33] whereas ACNF exhibits R(I D/I G) of 1.35 indicating a highly disordered structure. Incorporating GRHC into the ACNF shows the composite structure becoming more ordered as implied by the decrease in R(I D/I G) with increasing GRHC, with exception of GRHC/ACNF0.01. It shows that R(I D/I G) of GRHC/ACNF0.01 is higher than that of pristine ACNF and this is corresponding well with the BET and XRD results discussed earlier, indicating this sample possesses the most disordered structures. As more GRHC was incorporated into ACNFs, the composites became more ordered and this could be explained by the addition of the highly ordered structure of pristine GRHC that could affect the structure of the composites.
The graphitic structure of the GRHC/ACNF composites is expected to diminish with the increment of GRHC loading. Regardless of its lowest R(I D/I G) in comparison with other ACNF composites, the carbon material with this value is believed to possess disordered and defective structures. This is validated by Gayathri et al. [31], who found that the graphene synthesized in their research still possessed defect structure even at a significantly low value of R(I D/I G) of ∼0.2–0.4. Remarkably, in this study, their defective structures are more preferable for better gas adsorption due to porosity enhancement caused by the defects. This means the sample with higher R(I D/I G), such as GRHC/ACNF0.01, will show better performance toward CO2 adsorption.
Figure 4 shows the morphological structure of the ACNF at different magnifications and loadings and summarized in Table 3. The development of the porous structure after the activation of the NFs was proved with the appearance of pores on the ACNF structure as shown in Figure 4(e). Among all samples, GRHC/ACNF composited with 1% of GRHC exhibited the smallest fiber diameter of 238 ± 79.97 nm as shown in Table 3. Bead formation was observed in ACNF composites likely due to conductive properties provided by the GRHC and the viscosity of the solution [34]. This would affect the final diameters of the fibers due to the electrostatic repulsion within the jet spray from the tip of the needle to the collector surface throughout the electrospinning process. Huang et al. [35] reported that high conductivity led to the production of electrospun fibers with smaller diameter due to the increment of the surface tension of the solution, which affects the jet ejection during electrospinning. Thus, it can be hypothesized that increasing GHRC loading would result in a smaller diameter. However, interestingly in this study, only GRHC/ACNF0.01 resulted in the smallest diameter whereas increasing GHRC loading further increases the fiber diameter (albeit smaller than ACNF). The results show correlation with the S BET obtained (Table 2) where smaller diameter provides higher S BET and vice versa. From the results obtained, only GRHC/ACNF0.01 has been used for further impregnation study and pristine ACNF has been used for comparison.
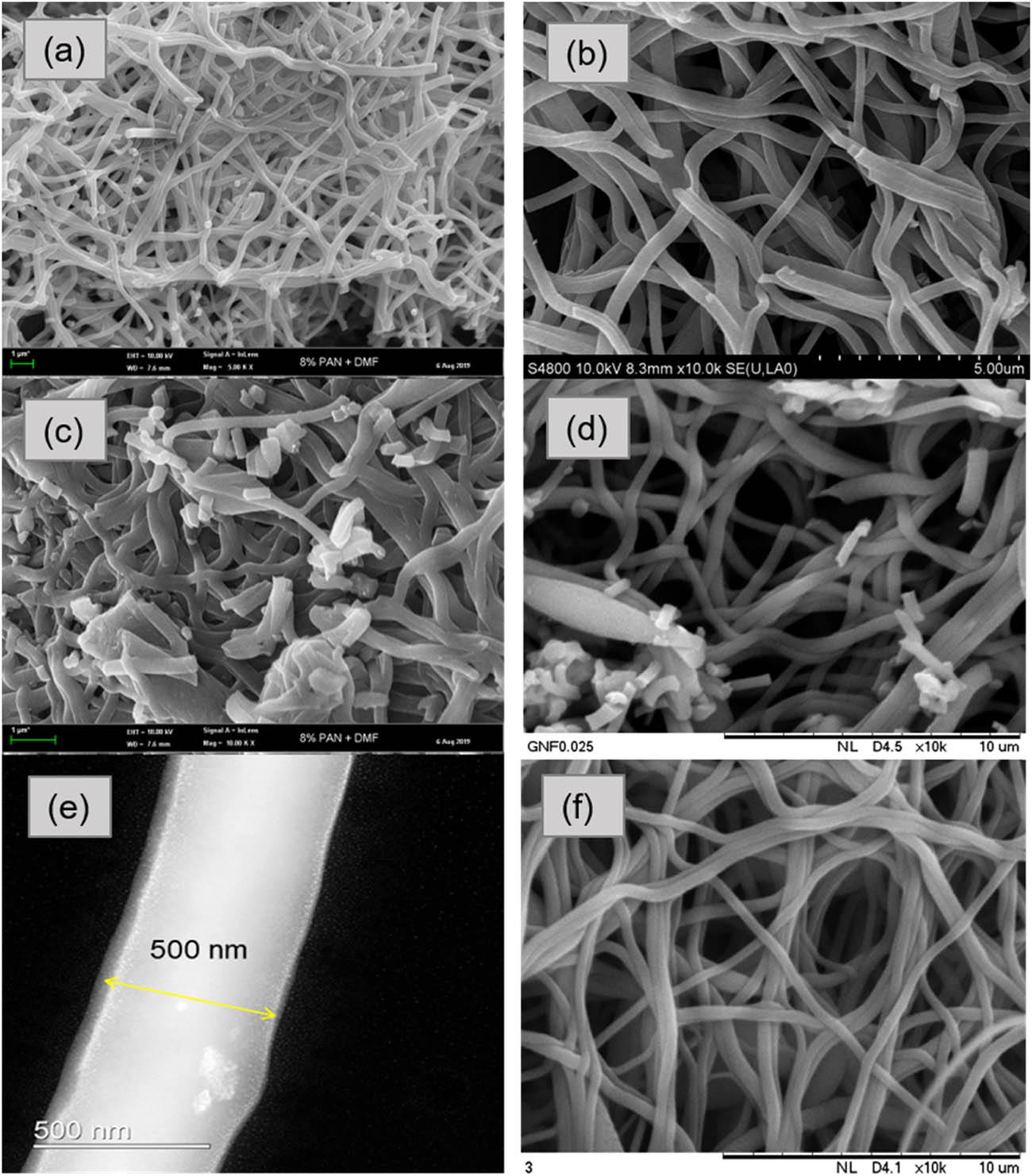
FE-SEM images of pristine ACNFs at different magnifications: (a) 5,000×; (c) 10,000×; (e) 100,000×; and with different loadings of GRHC at 10,000× (b) GRHC/ACNF0.01; (d) GRHC/ACNF0.05; (f) GRHC/ACNF0.1.
Diameter of the pristine and composite ACNFs at different loadings of GRHC
| Sample | Average diameter (nm) | Standard deviation (nm) |
|---|---|---|
| ACNF | 460 | ±57.05 |
| GRHC/ACNF0.01 | 238 | ±79.97 |
| GRHC/ACNF0.025 | 296 | ±40.58 |
| GRHC/ACNF0.05 | 340 | ±57.05 |
| GRHC/ACNF0.1 | 384 | ±52.87 |
Moreover, another major point to be addressed in this study is the particle size of the GRHC/ACNF0.01 in powdered form. From the results of this current study (see Supplementary Materials, Figure S2), the average particle size of GRHC/ACNF obtained ranged from 22.2 to 35.4 µm. Compared with other sorbents, this range of particle size is a good indicator of the suitability of these samples as good sorbents. It is believed that the size of the particles can greatly affect the adsorption properties of the sorbents. This was supported by a study conducted by Li et al. [36] as they found that particle size of 52.5 µm or smaller resulted in higher absorption of CO2 as compared with a particle size of 77.5 and 100.0 µm. Even though this sorbent has been ground into powdered form, however, under high magnification image, the thread-like structure of the nanofibers remains as shown in Figure 4.
In terms of mechanical strength, this proposed GRHC/ACNF is believed to possess good mechanical strength, including high tensile strength, which could be due to the highly oriented fibers during the electrospinning process that affect the mechanical strength of the nanofibers. Due to limitations of time limitation and availability of instruments, the mechanical test on the GRHC/ACNF could not be conducted. However, superiority of the mechanical properties possessed by GRHC/ACNF can be seen from similar materials such carbon nanofibers studied in several previous work reviewed by Yadav et al. [37]. For example, Pu et al. [38] have successfully fabricated electrospun carbon nanofibers with excellent mechanical properties, such as high tensile strength up to 20.7 MPa. According to their studies, the stretching effect of the jet during electrospinning is advantageous to the arrangement of carbon nanofibers along the fiber axis, which significantly enhances the tensile strength and modulus of the fibers [38].
3.2 PEI-impregnated ACNFs and GRHC/ACNFs
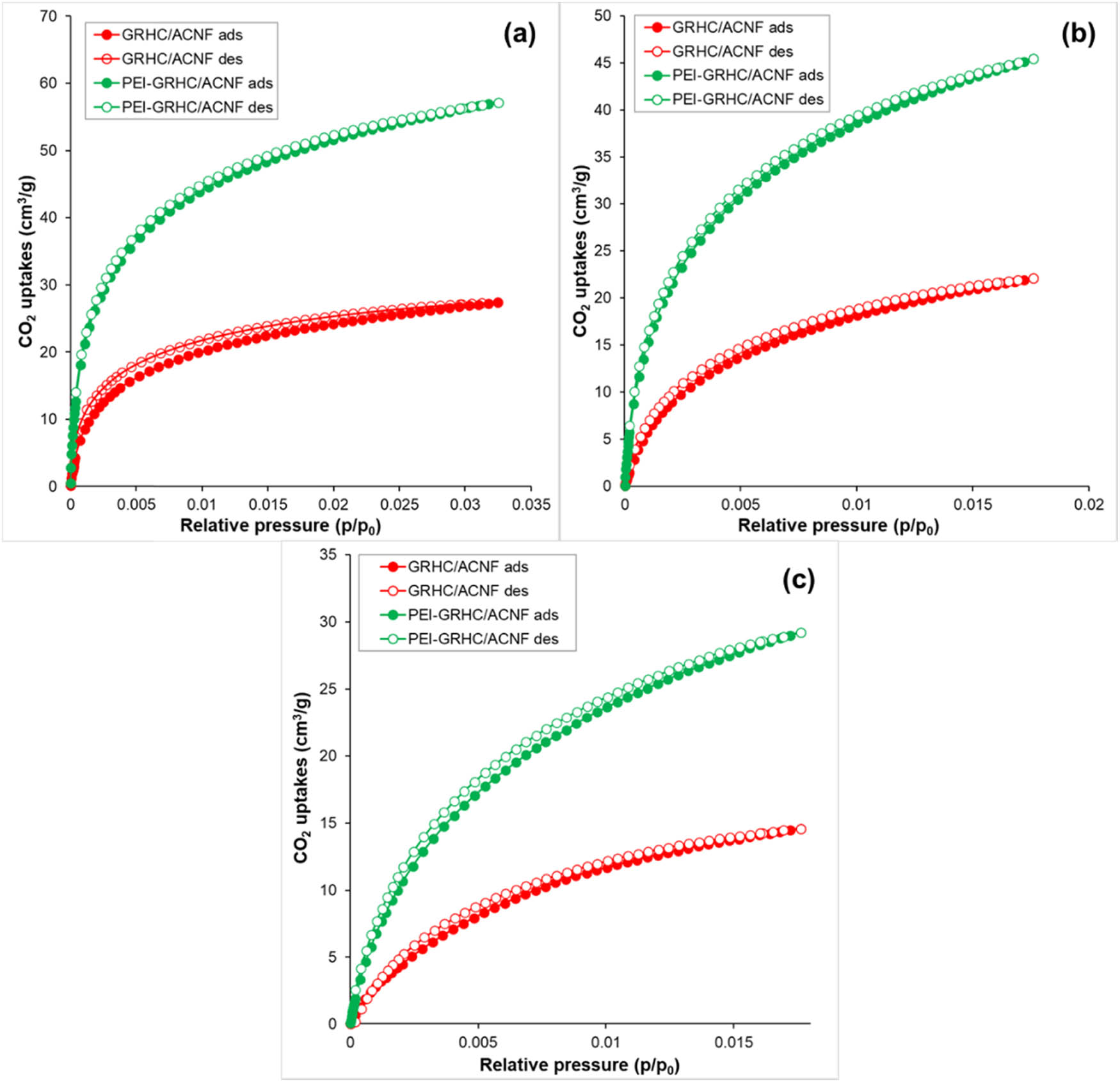
Figure 5 shows the N2 adsorption/desorption isotherms of PEI-impregnated ACNFs and GRHC/ACNF0.01. Both impregnated samples exhibit similar type of isotherm, which is type II indicative of nonporous or macroporous structures [39]. The isotherm type changes from type I(b) prior to activation into type II after activation. This type of isotherm explained the formation of a monolayer at relatively low pressure, and then multilayer formation at higher relative pressure [40]. In contrast with the type I isotherm, the long plateau is absent in type II isotherm as the adsorption uptake continuously increases with the relative pressure without significant halt and it even almost reaches unity, indicating the multilayer adsorption.
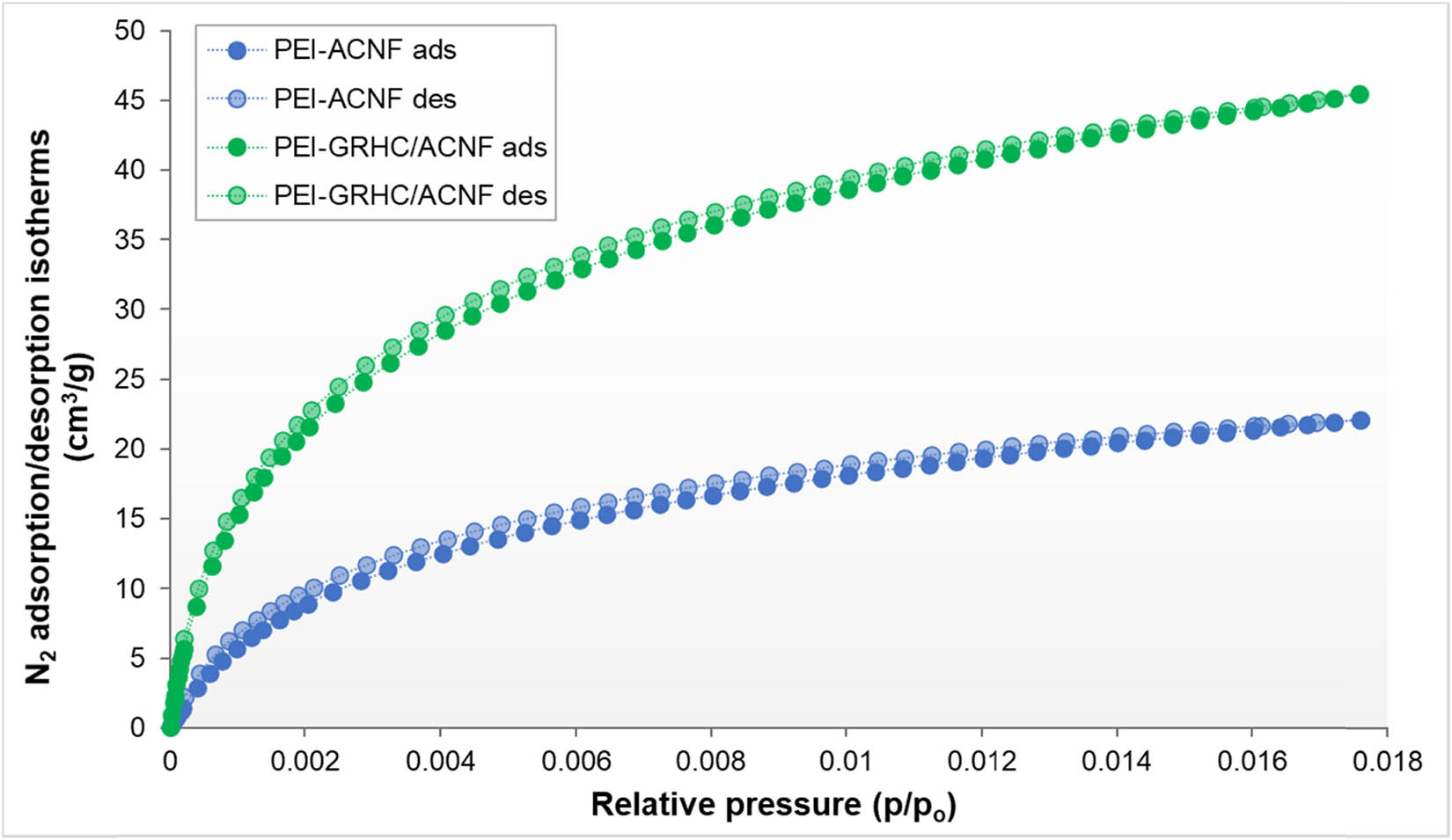
N2 adsorption/desorption isotherms of PEI-impregnated ACNFs and PEI-impregnated GRHC/ACNFs.
Table 4 summarizes the corresponding textural properties of both impregnated samples. As compared with nonimpregnated samples, both PEI-impregnated samples have shown significant reduction in S BET and TPV, with the disappearance of V micro in the structure. This reduction of S BET confirms that PEI was successfully impregnated onto the porous structures of the ACNF samples, and all of its porosity structure was filled with PEI. Rouzitalab and coworkers have reported that adsorbent materials that are high in TPV were easily accessible by the N-functional groups, which results in blocking the pores of the ACNFs, and were reflected by the decrement of the S BET. In this case, the physisorption that mainly depends on S BET, which predominantly lay in its pores, would be mostly excluded and chemisorption takes place.
Textural characteristics of PEI-impregnated ACNFs and GRHC/ACNFs
| Samples | S BET (m2/g) | TPV (cm3/g) | V micro (cm3/g) | DPave (nm) |
|---|---|---|---|---|
| PEI-ACNF | 2.5162 | 0.0052 | 0 | 8.3243 |
| PEI-GRHC/ACNF | 1.9032 | 0.0043 | 0 | 9.1175 |
The thermal stability of the impregnated ACNFs was evaluated using TGA analysis in the temperature range of 25–700°C (Figure 6). All impregnated ACNFs exhibit thermal decomposition at a higher onset temperature of ∼400°C. This result suggests PEI-impregnated ACNFs have better thermal stability as compared with nonimpregnated samples. From the thermogram, the weight percentage of the ACNFs improved from 24.2% to 36.2% as compared to PEI-ACNF. Meanwhile for PEI-impregnated GRHC/ACNFs, the final weight obtained is much higher than the nonimpregnated one, which is up to 68.2%. This is could possibly be due to the low volatility and good stability properties of PEI [41]. This result is expected to contribute to GRHC/ACNF stability and cyclability during the real-life flue gas separation in postcombustion CO2 capture.
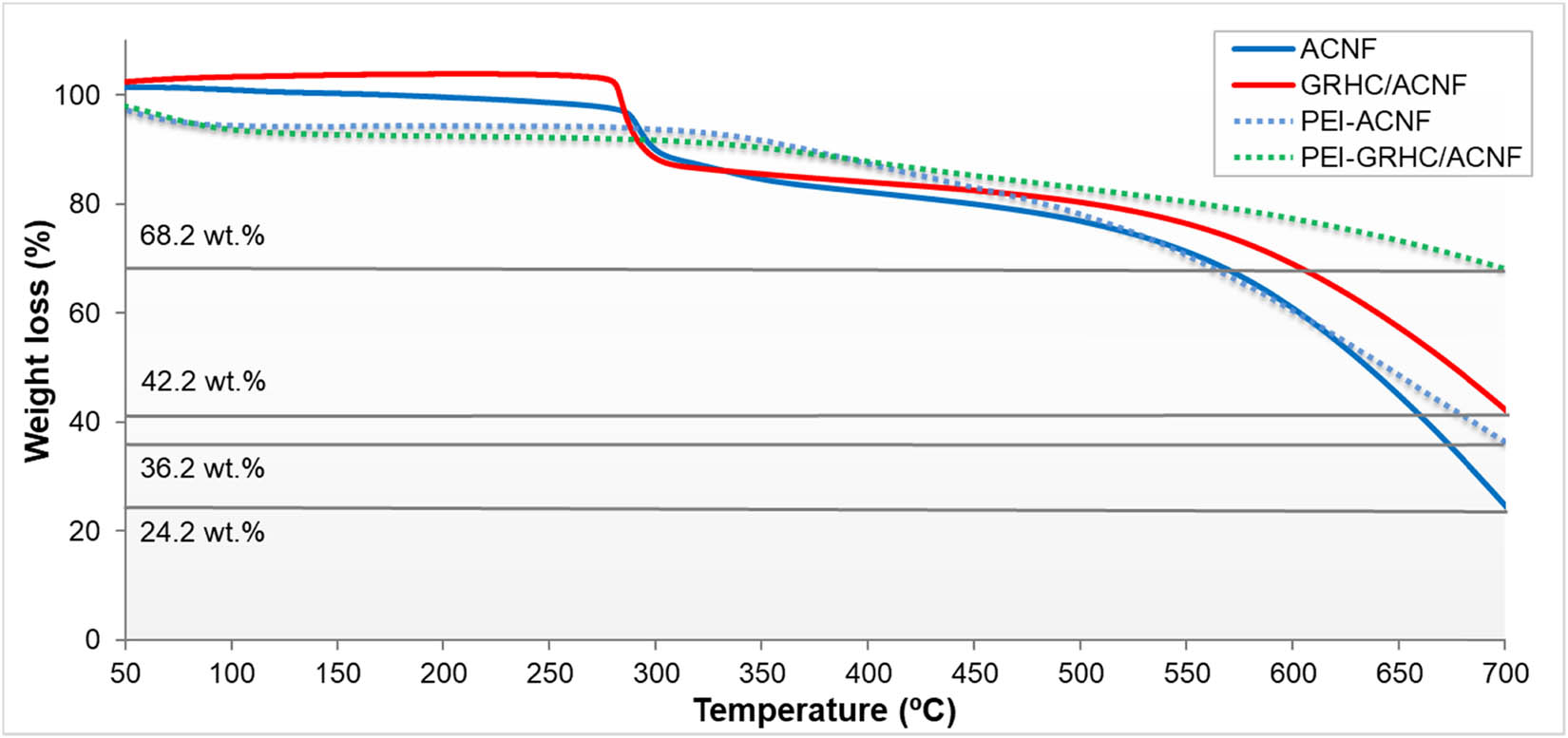
TGA thermogram of PEI-impregnated and nonimpregnated ACNFs and GRHC/ACNFs.
Figure 7 exhibits the FTIR spectra of the nonimpregnated and PEI-impregnated ACNFs and GRHC/ACNFs, respectively. Compared with nonimpregnated ACNFs. A broad band at 2,980 cm−1 was observed, which indicates the existence of –CH2 symmetric stretching modes of the PEI chain in the PEI-impregnated samples. The band at 1,557 cm−1 also can be observed in all impregnated samples associated with the asymmetric bending of the primary amines (–NH2). The appearance of this peak further supports successful impregnation of PEI [42,43].
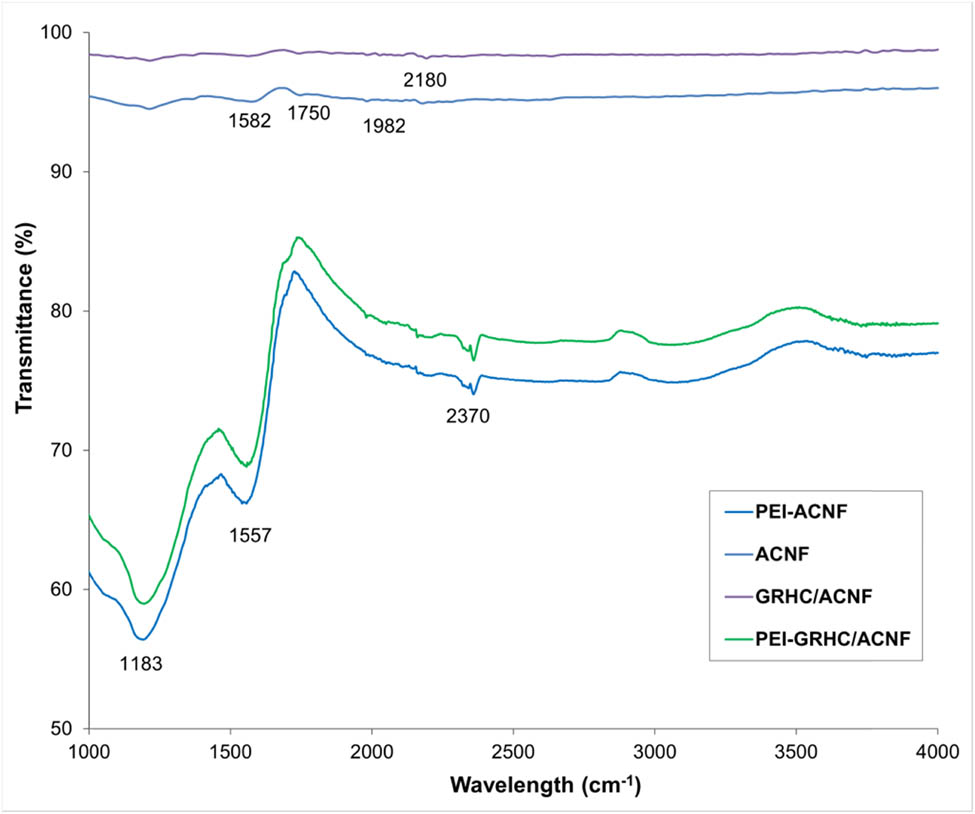
FTIR spectrum of PEI-impregnated and nonimpregnated ACNFs and GRHC/ACNFs.
The SEM, TEM images, and EDX mapping of PEI-impregnated GRHC/ACNFs is presented in Figure 8(a)–(d). As shown in Figure 8(a), it can be seen that the morphology of PEI-GRHC/ACNF was agglomerated with appearance of large beads, which is believed happened during the impregnation process. The presence of black spots within the fibers in Figure 8(b) indicates the GRHC presence onto the structures. Furthermore, the presence of a small amount of silica (0.02 ± 0.02 wt%) in the nanowires was also observed [44] in Figure 8(c). This also can be seen with the appearance of silica in the resultant GRHC/ACNFs through EDX mapping (Supplementary Materials, Figure S3), which indicating the high content of silica in untreated (raw) rice husks (prior conversion into GRHC). Through EDX analysis, Table 5 confirms the existence of a high concentration of N atoms on the impregnated samples (35.21 ± 6.81 wt%). The other major elemental properties such as carbon (C) and oxygen (O) were also detected at 51.45 ± 0.74 wt% and 13.32 ± 1.71 wt%, respectively. As expected, C covers the highest percentage among other elements as ACNFs are carbon-based materials.
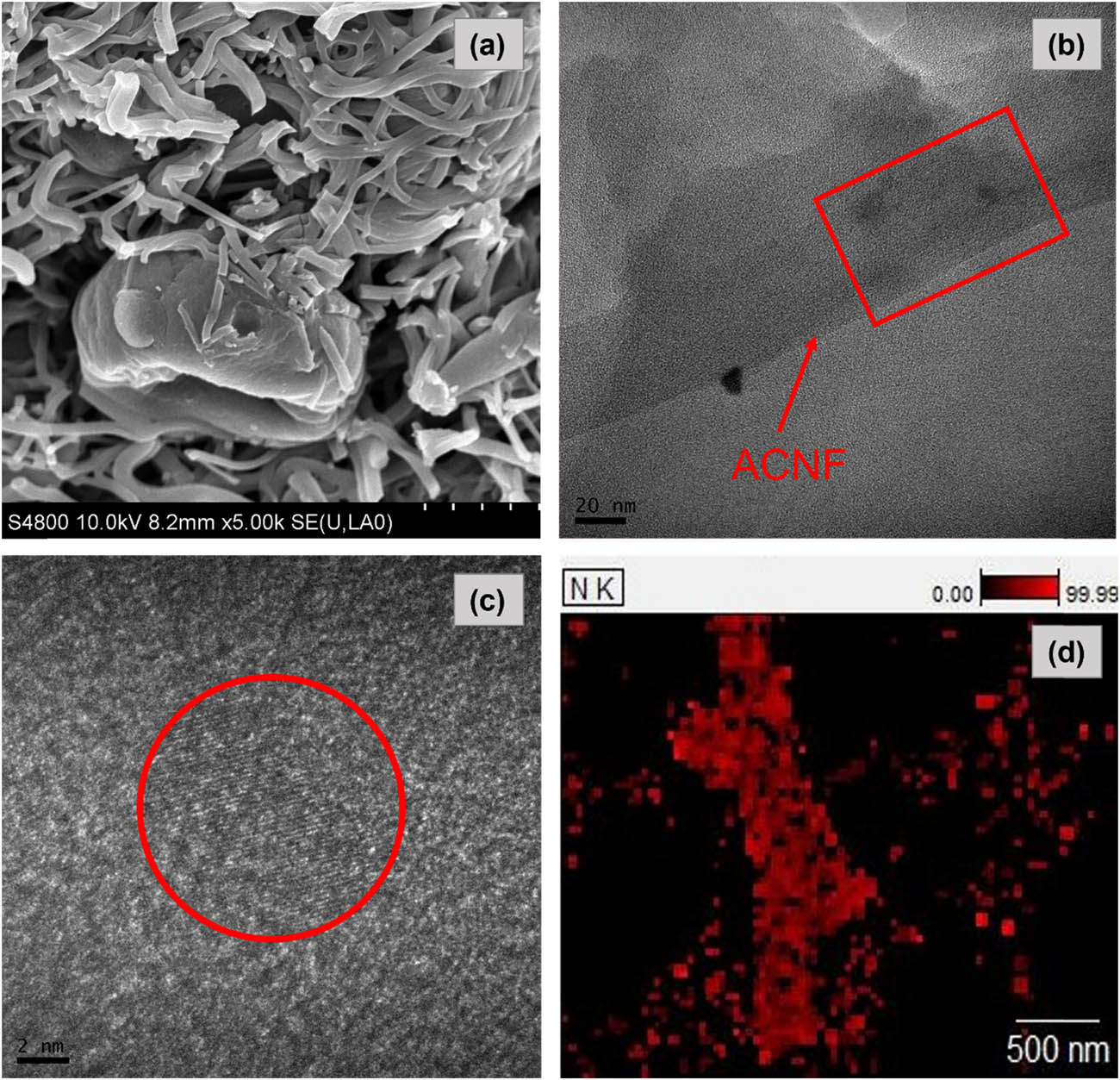
(a) SEM image at magnification of 5,000×; (b and c) TEM images at different magnifications; and (d) EDX mapping of PEI-impregnated GRHC/ACNFs.
Elemental properties of PEI-impregnated GRHC/ACNFs
| Element | Weight (%) | Wt% error | Atom% | Atom% error |
|---|---|---|---|---|
| C | 51.45 | ±0.74 | 56.13 | ±0.80 |
| N | 35.21 | ±6.81 | 32.95 | ±6.39 |
| O | 13.32 | ±1.71 | 10.91 | ±1.42 |
| Si | 0.02 | ±0.02 | 0.01 | ±0.01 |
| Total | 100.00 | 100.00 |
3.3 Adsorption performance and characteristics toward CO2
GRHC/ACNFs showing better CO2 adsorption than the pristine ACNFs (Figure 9) at pressure 1–15 bar indicate that the presence of graphene improves its affinity toward CO2 as it contributes to the enhancement of the textural properties of the adsorbents [45]. These results are in agreement with the BET results where higher S BET and V micro [46] provide more surface for gas to be adsorbed. Compared with other previous works, both ACNFs possess higher CO2 adsorption compared with other carbon-based materials as summarized in Table 6, even though with lower S BET values. It is believed that the high adsorption value is not totally S BET dependent, yet it also could be contributed by the available open micropores on the adsorbents. For example, most of the CNF-based adsorbents in Table 6 showed higher S BET as compared with the samples in this study, however, with lower V micro values and limited CO2 adsorption capacities. This means V micro is a very important characteristic for adsorbents to adsorb higher gas. From this, it can be seen that the resultant ACNFs can be good candidates for gas adsorbents, especially in flue gases, in order to reduce the CO2 emission into the atmosphere, consequently combating the global warming problem.
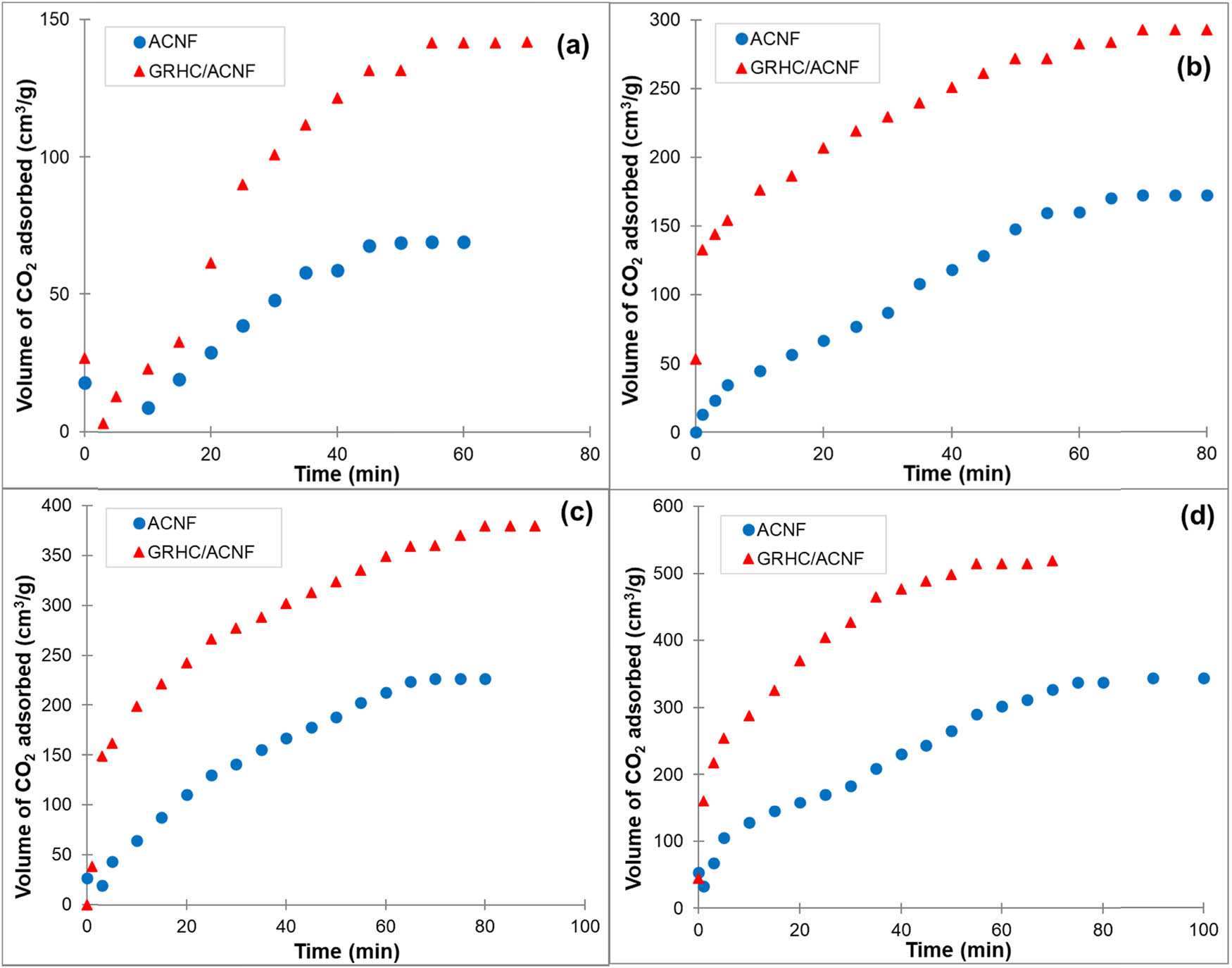
CO2 adsorption of ACNFs and GRHC/ACNFs at different adsorption pressures: (a) 1 bar; (b) 5 bar; (c) 10 bar; (d) 15 bar at 25°C.
Comparison of CO2 adsorption capacity on various types of carbon fibers and their composite-based adsorbents
| Adsorbent | S BET (m2/g) | V micro (cm3/g) | Vol. of CO2 adsorbed (cm3/g) | Temp; pressure | Ref. |
|---|---|---|---|---|---|
| ACNF | 139 | 0.06 | 69 | 25°C; 1 bar | This work |
| GRHC/ACNF | 597 | 0.32 | 142 | 25°C; 1 bar | This work |
| CNF-SnO2 | 434 | 0.20 | 58 | 25°C; 1 atm | [34] |
| CNF-MIL-53 | 140 | — | 30 | 25°C; 1 bar | [47] |
| PAN-CMFs | 966 | — | 64 | 25°C; 1 bar | [48] |
| Graphite NFs | 567 | 0.27 | 59 | 25°C; 1 atm | [49] |
| Graphite NFs | 966 | 0.25 | 71 | 25°C; 1 atm | [50] |
| NiCo2O4/CNTs CNFs | 40 | — | 34 | 25°C; 1 bar | [51] |
| Phenolic resin-based carbon ultrafine fibers | 650 | — | 65 | 25°C; 1 bar | [52] |
Various sorbent materials have been widely used in current adsorption technology and further assessment on their performance needs to be clarified. Table 7 shows the comparison of the sorbents’ properties and their adsorption capacities to further assess the potential of currently synthesized GRHC/ACNFs compared with other available sorbent types, such as conventional activated carbon, zeolites, carbon nanotubes (CNTs), MOFs, graphene, and so on. Among the available adsorbents, carbon-based adsorbents have shown promising adsorption performance as excellent gas adsorbents due to their large surface area and availability (can be produced from wide range of raw materials). In the past few decades, activated carbon is the commonly used adsorbent in various applications. The wide utilization of activated carbon in adsorption industries is advantageous due to their wide distribution of pore structures and surface chemistry for gas adsorption. Most importantly, activated carbon is relatively low in cost, has high S BET, widely available, has an easily modifiable pore structure, , susceptible to surface functionalization, and can be regenerated [53,54].
Comparison of various types of available sorbent materials
| Adsorbent | S BET (m2/g) | Pore volume (cm3/g) | Temperature; pressure (°C; bar) | Volume adsorbed (cm3/g) | Ref. |
|---|---|---|---|---|---|
| GRHC/ACNF | 597 | 0.32 | 25°C; 1 bar | 142.0 | This work |
| MCM-41 | 1211 | 1.3 | —; 1 | 99.9 | [56,57] |
| Mesoporous alumina | 812 | 0.83 | —; 1 | 115.4 | [58] |
| Activated carbon | 2187 | 0.82 | 25; 8 | 24.4 | [59] |
| 13X-zeolites | 710 | — | 120; 20 | 15.5 | [60] |
| Microporous polymers | 1237 | — | 25; 1 | 48.8 | [61] |
| Porous clay heterostructures | 640 | 0.828 | 25; 1 | 14.2 | [62] |
| Graphene | 443 | — | 25; 11 | 47.9 | [29] |
| Carbon nanotubes | 60 | 0.4 | 25; 1.1 | 155.3 | [63] |
| Coconut shell-based AC | 378 | — | 35; 1 | 13.8 | [64] |
| Pitch-based AC | 1053 | — | 28; 20% | 42.6 | [65] |
| Activated carbon fibers (ACF) | 1256 | 0.636 | 30; 0.05% | 0.08 | [66] |
| Carbon molecular sieve | 857 | 0.501 | 30; 0.05% | 0.36 | [66] |
| ACFs (physical activation) | 1,240 | 0.61 | 30; 1.1 | 146.1 | [67] |
| ACFs (chemical activation) | 2,180 | 1.003 | 30; 1 | 126.1 | [68] |
| Graphene sheets | 484 | 0.682 | 0; 1 | 64.2 | [69] |
| Rice-husk char | 1,041 | 0.53 | 0; 1 | 125.0 | [70] |
| Graphite nanofibers | 567 | 0.708 | 25; 1.01 | 30.0 | [49] |
Recently, activated carbon has been obtained in different structures and forms, including powder, granular, tubular, and fibers. It was found that activated carbon in fiber form has shown significant structure improvement, especially in terms of surface area, micropores, active site availability, and gas adsorption capacity as compared with the traditional granular form [55]. The presence of fibril structure in ACNFs has ensured much higher adsorption capacity, which is due to the direct diffusion of gas adsorbates into the available micropores inside and on the surface of the adsorbents. This means there is no resistance for adsorbates to reach the adsorption sites as there are no macropore or mesopore network. Moreover, this fiber form can be prepared from a wide range of precursors such as coal, polymers, petroleum pitch, rayon, and viscose.
As listed in Table 7, the adsorbed volume of CO2 by the ACF and GRHC/ACNF are the highest, which are 6.4 and 6.58 mmol/g, respectively compared with other adsorbents. Even though, the CO2 adsorbed volume is comparable for both samples, the S BET value is significantly different, which is 1240 m2/g for ACF and 597 m2/g for GRHC/ACNF. From this finding, it can be concluded that even at low S BET value, the adsorption performance of the as-proposed adsorbent, GRHC/ACNF, is better than that of the other mentioned adsorbents. It is believed that tuning into higher S BET value can potentially turn this GRHC/ACNF into an excellent CO2 adsorbent. Besides S BET value, another important factor that affects the adsorption performance is the compatibility of the adsorbents for real-life CO2 capture applications. As mentioned in the TGA results above, GRHC/ACNF with good thermal stability (up to 700°C) can withstand the temperature during postcombustion CO2 capture ranging from 40°C to 80°C, which makes this proposed GRHC/ACNF as one of the excellent CO2 adsorbents.
The CO2 adsorption performance of PEI-impregnated GRHC/ACNFs is presented in Table 8. The CO2 adsorption capacity was higher at 107 cm3/g for PEI-ACNFs (55% enhancement) and 191 cm3/g for PEI-GRHC/ACNFs (34% enhancement) as compared with their nonimpregnated samples, which were 69 cm3/g and 142 cm3/g for ACNFs and GRHC/ACNFs, respectively. This has proved the involvement of chemical interaction between the basic N-functionalities in PEI and acidic CO2 molecules. From this finding, it is believed that the high adsorption capacity in PEI-impregnated samples could be contributed by the involvement of both physical and chemical interactions between the adsorbent and adsorbates.
CO2 uptakes at different pressures at ambient temperatures
| Sample | CO2 uptakes (cm3/g) | ||||
|---|---|---|---|---|---|
| PEI-1 bar | 1 bar | 5 bar | 10 bar | 15 bar | |
| ACNF | 107 | 69 | 173 | 242 | 293 |
| GRHC/ACNF | 191 | 142 | 342 | 413 | 522 |
In terms of adsorption in more practical dynamic conditions in multicomponent mixtures such as flue gases, this newly produced both impregnated and nonimpregnated GRHC/ACNFs are believed to have good gas separation properties. This statement was made by considering and comparing the N2 adsorption obtained from BET results and the CO2 adsorption capacity. From BET results, the PEI-impregnated sample shows very low N2 adsorption capacity of 2.67 cm3/g whereas the nonimpregnated sample possesses higher adsorption capacity of 33.7 cm3/g. The low N2 adsorption on PEI-GRHC/ACNFs indicates the blockage of the available porous structure (very low S BET as previously discussed) by PEI for N2 molecules to be adsorbed onto the structure. As N2 is an inert gas, it does not interact with N-functionalities on the surface of PEI-GRHC/ACNFs. In contrast, the nonimpregnated sample exhibits higher N2 adsorption due to the numerous available porous structures, which contributes to physisorption. According to both N2 and CO2 adsorption capacity, it is believed that the resultant GRHC/ACNFs more selective toward CO2. From this finding, GRHC/ACNFs can potentially act as a good adsorbent for flue gas separation in postcombustion CO2 capture.
As previously discussed, the GRHC/ACNFs possessed the highest S BET and TPV prior to PEI impregnation but the lowest after impregnation. It can be attributed to the presence of amine in the GRHC/ACNFs. This phenomenon explains the high CO2 adsorption capacity in PEI-GRHC/ACNFs even though they possessed smallest values of S BET and TPV as compared with GRHC/ACNFs, which is highly believed to be influential in both physisorption and chemisorption. In conclusion, it can be said that high CO2 adsorption capacity in PEI-GRHC/ACNFs is attributed to a synergic effect of amino groups, thus multiplying the CO2 uptakes through chemisorption [71]. With low value of S BET (presence of very low V meso or macropores), it can be said that PEI does not totally block the surface of the GRHC/ACNFs leaving spaces for physisorption to take place. Both GRHC/ACNFs and PEI- GRHC/ACNFs show the highest CO2 uptakes as compared with other samples as shown in Table 8. For further adsorption studies, both samples have been used and were denoted as PEI-GRHC/ACNFs for PEI-impregnated samples and GRHC/ACNFs for nonimpregnated ones.
Figure 10 shows the CO2 adsorption isotherm of the GRHC/ACNFs and PEI-GRHC/ACNFs at atmospheric pressure and temperatures of 0°C, 25°C, and 50°C. Figure 10 reveals that the desorption of both impregnated and nonimpregnated samples at different temperatures shows no hysteresis. This means that both samples have very good adsorption/desorption capacity. It was also observed that CO2 adsorption capacity reduces with increasing temperature for both samples. The adsorbed amount of CO2 in PEI-GRHC/ACNFs and GRHC/ACNFs decreased from 522 to 142 cm3/g and 293 to 69 cm3/g, respectively. At higher temperature, the energized gas molecules were rapidly occupied on the GRHC/ACNFs surface, therefore, resulting in fewer CO2 molecules captured [72]. Moreover, low equilibrium of CO2 at higher temperature was also due to the exothermic nature of the adsorption [73].
CO2 adsorption/desorption on PEI-impregnated and nonimpregnated GRHC/ACNFs at different temperatures (a) 0°C; (b) 25°C; and (c) 50°C under atmospheric pressure.
Table 9 summarizes the kinetic studies of the PEI-GRHC/ACNFs and GRHC/ACNFs in order to determine the most fitted kinetics adsorption for the adsorption of CO2. Based on the R 2 values, both GRHC/ACNFs samples are best fitted to the pseudo first-order kinetic model at low adsorption pressure of 1 bar by displaying greater R 2 value of 0.891 and 0.899. Meanwhile, the significantly low values of R 2 found in both samples in pseudo second-order kinetic model. This means that the CO2 adsorption process of GRHC/ACNFs involved a physisorption phenomenon at low pressure as per our expectation, which means the physisorption is more related to the adsorption toward the surface with higher S BET, TPV, and V micro by weak dipole van der Waals interactions. These results are also in agreement with the results of S BET, N2, and CO2 adsorption previously discussed. Remarkably, even though the nonimpregnated GRHC/ACNFs show the results as expected, however, the PEI-impregnated GRHC/ACNFs show result beyond our expectations as they also obeyed the pseudo first-order kinetic model. This is believed to be because of the small amount of amino groups presence in the impregnated samples (due to the low ratio of PEI:GRHC/ACNF:DI water; 1:1:5 used) that led to weaker interaction between PEI and CO2 molecules resulting to minimum effects on the chemical interaction while involving physical interaction simultaneously [74]. In low pressure, physical interaction exhibits more dominant effect on the adsorption process. This is corresponding with previous study conducted by Liu et al. as they found that PEI-impregnated porous polymer-based adsorbents suggest both physical adsorption and chemical adsorption toward CO2 [75].
The fitted parameters of pseudo first order and pseudo second order of PEI-GRHC/ACNFs and GRHC/ACNFs at 1 and 15 bar
| Sample | Pressure (bar) | q e , exp (cm3/g) | Pseudo first order | Pseudo second order | ||||
|---|---|---|---|---|---|---|---|---|
| k 1 | q e ,cal | R 2 | k 2 | q e ,cal | R 2 | |||
| GRHC/ACNF | 1 | 142 | −0.0414 | 3.05 | 0.899 | 0.0061 | 1.95 | 0.038 |
| PEI-GRHC/ACNF | 191 | −0.0523 | 3.87 | 0.891 | 0.0305 | 11.82 | 0.257 | |
| GRHC/ACNF | 15 | 522 | −0.0343 | 4.13 | 0.915 | 0.1586 | 1.38 | 0.986 |
| PEI-GRHC/ACNF | 606 | −0.0528 | 4.51 | 0.798 | 0.3865 | 2.71 | 0.996 | |
Interestingly, at higher adsorption pressure of 15 bar, both samples can be ascribed by pseudo first-order and pseudo second-order kinetic models as the R 2 values obtained were high in both models. The R 2 values of pseudo first order of PEI-GRHC/ACNFs and GRHC/ACNFs are 0.7978 and 0.9154, respectively. Meanwhile, the R 2 values of pseudo second order of PEI-impregnated GRHC/ACNFs and nonimpregnated GRHC/ACNFs are 0.996 and 0.986, respectively. These findings indicate that the adsorption properties of both GRHC/ACNFs involve both chemical and physical reactions between the free active sites and open pores available on the GRHC/ACNFs and the CO2 molecules. Moreover, this also confirms the main idea of PEI impregnation is to improve the CO2 adsorption by introducing the basic N-functional groups onto the GRHC/ACNFs for better binding with acidic CO2 molecules through chemisorption without cancelling the physisorption properties. To conclude, both kinetic models used in this study are suitable for the present data obtained for the adsorption of CO2 at 15 bar and 25°C; however, it fits the best with pseudo second order and followed by pseudo first order [76].
Table 10 summarizes the linearized plot of the experimental data correlated to the Langmuir, Freundlich, and BET models to determine the homogeneity and heterogeneity energy distribution on the surface of the adsorbents. The R 2 values show that the resultant GRHC/ACNFs best fitted in Langmuir model and followed by BET. This implies that the adsorption of CO2 involves monolayer phenomenon with homogeneous distribution on adsorbent active sites as previously discussed by Kim et al. [24]. In Langmuir isotherm and BET model, the dynamic equilibrium can be obtained by balancing the relative rates of adsorption and desorption based on the GRHC/ACNFs surface coverage [77].
The fitted parameters of Langmuir, Freundlich, and BET isotherm models of PEI-GRHC/ACNFs and GRHC/ACNFs at 1 bar
| Sample | Freundlich | Langmuir | BET | ||||
|---|---|---|---|---|---|---|---|
| n (mmol/g) | K F | R 2 | q max (mmol/g) | K L (bar−1) | R 2 | R 2 | |
| GRHC/ACNF | 0.785 | 2.434 | 0.841 | 8.688 | 39.179 | 0.999 | 0.987 |
| PEI-GRHC/ACNF | 0.761 | 2.339 | 0.810 | 6.379 | 27.073 | 0.998 | 0.972 |
It is believed that the adsorption process on the resultant GRHC/ACNFs does not only involve the formation of monolayer but also the formation of multilayers simultaneously [78]. This result was also supported by N2 adsorption/desorption mentioned previously, in which the adsorption is monolayer at lower pressure but forming multilayers at higher pressures. This multilayer formation is best described by the Freundlich model. This model explains the adsorption process on surface adsorption sites which are energetically homogeneous.
Apart from a high CO2 uptakes and fast adsorption/desorption kinetics, a good adsorbent should be stable and regenerated under mild conditions, in order to avoid high costs of the energy penalty. In this study, the cyclic behavior and stability of both impregnated and nonimpregnated GRHC/ACNFs were investigated via five consecutive CO2 adsorption/desorption cycles for 14 h at 50°C (Figure 11). The selection of this adsorption temperature is to replicate the real condition of postcombustion CO2 capture that is commonly used for adsorbent materials. As shown, there is a slight loss in adsorption capacity after each cycle. After five cycles, the stability of the impregnated GRHC/ACNFs shows almost similar drop percentage of 23% (from 32.2 to 24.6 cm3/g) as compared with nonimpregnated GRHC/ACNFs of 22% (15.1–11.8 cm3/g). This is could possibly be due to imperfect regeneration of the adsorbents due to the high affinity of PEI toward CO2 and the current desorption method is insufficient to evacuate all CO2 from the adsorbents. Moreover, PEI-modified ACNFs are dominated by chemisorption, and hence require higher energy (i.e. temperature) to evacuate CO2. It has been found that this dropping percentage is considered acceptable for amine-impregnated samples after five cycles, as the capacity of the carbon capture under the same conditions on tetraethylenepentamine (TEPA) impregnated electrospun carbon nanofibers by a study conducted by Wang et al. [79] reported the drop is more than 11%. Rather than its higher drop percentage as compared with previously reported studies, the GRHC/ACNFs possessed comparable or superior adsorption capacity over other samples which is up to 27.1 cm3/g at 50°C. Even though the GRHC/ACNFs obtained in this study do not show similar adsorption capacity compared with the first cycle, however it become more stable from the fourth cycle to fifth cycle, which shows that the percentage drop is very low. It has been found that the resultant GRHC/ACNFs have good recyclability as well as mild regeneration conditions, which is believed to have high potential for practical applications in energy-efficient carbon capture processes.
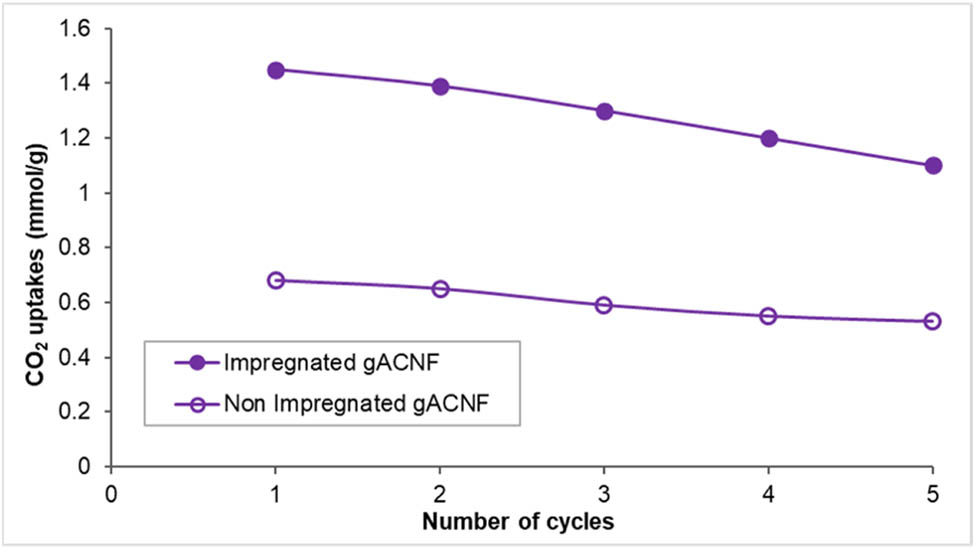
Cyclic ability of the CO2 adsorption on PEI-impregnated and nonimpregnated GRHC/ACNFs at 50°C and 1 bar.
4 Conclusion
In conclusion, it can be said that the physicochemical properties of the pristine ACNFs and their adsorption performances can be improved by suitable loading of additives or nanofillers. In this study, it has been found that graphene-based materials such as GRHC could greatly enhance the properties of the pristine ACNFs, especially in terms of their porosity and textural properties that were the major contributing factors for their excellent CO2 adsorption capacity. GRHC, which shows high S BET as well as good electrical conductivity, produced in this study has been used as additive to improve the solution conductivity during the electrospinning process, which helps in producing fiber with smaller diameter. This is because the adsorption capacity is highly dependent upon the S BET, TPV, and V micro of the adsorbents; the higher the S BET and V micro, the higher the adsorption capacity. Compared with pristine ACNFs, GRHC/ACNFs exhibited better porous properties and consequently, exhibited the highest adsorption capacity toward CO2—up to 142.1 cm3/g. Meanwhile, the adsorption capacity of the samples was enhanced by impregnating amine-based chemicals, such as PEI, onto the ACNFs and GRHC/ACNFs. The presence of amine groups in PEI-impregnated samples enhanced the chemical interaction between the basic properties of amines and acidic properties of CO2 adsorbates. The adsorption capacity in PEI-GRHC/ACNFs composites was found to be the highest with the value of 190.9 cm3/g under atmospheric pressure and 25°C. This is could possibly be due to the interaction of both physical and chemical adsorption that simultaneously occurred and resulted in high CO2 adsorption.
Acknowledgements
The authors would like to acknowledge the financial support from the Malaysian Ministry Education and Universiti Teknologi Malaysia under the UTM Prototype Research grant (UTMPR) (Q.J130000.2851.00L41); collaborative research grant (CRG) (Q.J130000.2451.087G72) and (Q.J130000.2451.08G26); UTM-TDR grant scheme (Q.J130000.3551.06G07); HICOE research grant (R.J090301.7851.4J428), and Professional Development Research University grant (Q.J130000.21A2.05E42). The work was carried out through the financial support of the Ministry of Education, Youth and Sports of the Czech Republic and the European Union (European Structural and Investment Funds – Operational Programme Research, Development and Education) under the project “Modular platform for autonomous chassis of specialized electric vehicles for freight and equipment transportation,” Reg. No. CZ.02.1.01/0.0/0.0/16_025/0007293. The authors would also like to acknowledge the technical and management support from the Research Management Centre, Universiti Teknologi Malaysia (RMC, UTM). One of the authors, Othman, F.E.C. would like to acknowledge the Zamalah scholarship received from UTM, NIMS Internship Scholarship 2018 awarded by the National Institute for Materials Science (NIMS), Japan, and the Mitacs-Globalink Research Award 2020 awarded by Mitacs and Ecole Polytechnique de Montreal, Canada.
-
Funding information: Malaysian Ministry Education and Universiti Teknologi Malaysia under the UTM Prototype Research grant (UTMPR) (Q.J130000.2851.00L41); collaborative research grant (CRG) (Q.J130000.2451.087G72) and (Q.J130000.2451.08G26); UTM-TDR grant scheme (Q.J130000.3551.06G07); HICOE research grant (R.J090301.7851.4J428) and Professional Development Research University grant (Q.J130000.21A2.05E42). The work was carried out through the financial support of the Ministry of Education, Youth and Sports of the Czech Republic and the European Union (European Structural and Investment Funds – Operational Programme Research, Development and Education), Reg. No. CZ.02.1.01/0.0/0.0/16_025/0007293.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Mondal MK, Balsora HK, Varshney P. Progress and trends in CO2 capture/separation technologies: a review. Energy. 2012;46(1):431–41.10.1016/j.energy.2012.08.006Search in Google Scholar
[2] Mauna Lao Lab. Global Monitoring Laboratory – Carbon Cycle Greenhouse Gases. US Department of Commerce, NOAA, Global Monitoring Laboratory Accessed on November 6, 2020 from https://www.esrl.noaa.gov/gmd/ccgg/trends/.Search in Google Scholar
[3] Global Greenhouse Gas Emissions by United States Environmental Protection Agency (EPA). Accessed on November 06, 2020 from https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data.Search in Google Scholar
[4] Ammendola P, Raganati F, Chirone R, Miccio F. Fixed bed adsorption as affected by thermodynamics and kinetics: yellow tuff for CO2 capture. Powder Technol. 2020;373:446–58.10.1016/j.powtec.2020.06.075Search in Google Scholar
[5] Megías-Sayago C, Bingre R, Huang L, Lutzweiler G, Wang Q, Louis B. CO2 adsorption capacities in zeolites and layered double hydroxide materials. Front Chem. 2019;7(51):1–10.10.3389/fchem.2019.00551Search in Google Scholar
[6] Wang K, Shang H, Li L, Yan X. Efficient CO2 capture on low-cost silica gel modified by polyethyleneimine. J Nat Gas Chem. 2012;21(3):319–23.10.1016/S1003-9953(11)60371-XSearch in Google Scholar
[7] Creamer AE, Gao B. Carbon-based adsorbents for postcombustion CO2 capture: a critical review. Environ Sci Technol. 2016;50(14):7276–89.10.1021/acs.est.6b00627Search in Google Scholar PubMed
[8] Hu Z, Wang Y, Shah BB, Zhao D. CO2 capture in metal-organic framework adsorbents: an engineering perspective. Adv Sustain Syst. 2018;3(1):1800080.10.1002/adsu.201800080Search in Google Scholar
[9] Othman FEC, Yusof N, Hasbullah H, Jaafar J, Ismail AF, Abdullah N, et al. Polyacrylnitrile/magnesium oxide-based activated carbon nanofibers with well-developed microporous structure and their adsorption performance for methane. J Ind Eng Chem. 2017;51:281–7.10.1016/j.jiec.2017.03.014Search in Google Scholar
[10] Othman FEC, Yusof N, González-Benito J, Fan X, Ismail AF. Electrospun composites made of reduced graphene oxide and polyacrylonitrile-based activated carbon nanofibers (rGO/ACNF) for enhanced CO2 adsorption. Polymers. 2020;12(9):1–19.10.3390/polym12092117Search in Google Scholar PubMed PubMed Central
[11] Rouzitalab Z, Maklavany DM, Rashidi A. Synthesis of N-doped nanoporous carbon from walnut shell for enhancing CO2 adsorption capacity and separation. J Environ Chem Eng. 2018;6:6653–63.10.1016/j.jece.2018.10.035Search in Google Scholar
[12] Raganati F, Miccio F, Ammendola P. Adsorption of carbon dioxide for post-combustion capture: a review. Energy Fuels. 2021;35:12845–68.10.1021/acs.energyfuels.1c01618Search in Google Scholar
[13] Othman FEC, Yusof N, Ismail AF, Jaafar J, Salleh WNW, Aziz F. Preparation and characterization of polyacrylonitrile-based activated carbon nanofibers/graphene (gACNFs) composite synthesized by electrospinning. AIP Adv. 2020;10:055117.10.1063/5.0008012Search in Google Scholar
[14] Othman FEC, Ismail MS, Yusof N, Samitsu S, Yusop MZ, Ariffin NFT, et al. Methane adsorption by porous graphene-derived from rice husks ashes under various stabilization temperatures. Carbon Lett. 2020;30(5):535–43.10.1007/s42823-020-00123-3Search in Google Scholar
[15] Othman FEC, Yusof N, Ismail AF. Activated carbon nanofibers/graphene nanocomposites and their adsorption performance towards carbon dioxide. Chem Eng Tech. 2020;43(10):2023–30.10.1002/ceat.201900480Search in Google Scholar
[16] Romano MC, Anantharaman R, Arasto A, Ozcan DC, Ahn H, Dijkstra J, et al. Application of advanced technologies for CO2 capture from industrial sources. Energy Procedia. 2013;37:7176–85.10.1016/j.egypro.2013.06.655Search in Google Scholar
[17] Romero JRG, Moreno-Piraján JC, Giraldo L. Kinetic and equilibrium study of the adsorption of CO2 in ultramicropores of resorcinol-formaldehyde aerogels obtained in acidic and basic medium. J Carbon Res C. 2018;4:52.10.3390/c4040052Search in Google Scholar
[18] Khalil SH, Aroua MK, Daud WMAW. Study on the improvement of the capacity of amine-impregnated commercial activated carbon bed for CO2 adsorbing. Chem Eng J. 2012;183:15–20.10.1016/j.cej.2011.12.011Search in Google Scholar
[19] Qi L, Tang X, Wang Z, Peng X. Pore characterization of different types of coal and gas outburst disaster sites using low temperature nitrogen adsorption approach. Int J Min Sci Technol. 2017;37:371–7.10.1016/j.ijmst.2017.01.005Search in Google Scholar
[20] Lee SY, Park SJ. Determination of the optimal pore size for improved CO2 adsorption in activated carbon fibers. J Colloid Interface Sci. 2013;389:230–5.10.1016/j.jcis.2012.09.018Search in Google Scholar PubMed
[21] Sing KS, Williams RT. Physisorption hysteresis loops and the characterization of nanoporous materials. Adsorpt Sci Technol. 2004;22:773–82.10.1260/0263617053499032Search in Google Scholar
[22] Liu H, Ding W, Lei S, Tian X, Zhou F. Selective adsorption of CH4/N2 on Ni-based MOF/SBA-15 composite materials. J Nanomater. 2019;9(149):1–14.10.3390/nano9020149Search in Google Scholar PubMed PubMed Central
[23] Nasrollahzadeh M, Babaei F, Fahkri P, Jaleh B. Synthesis, characterization, structural, optical properties and catalytic activity of reduced graphene oxide/copper nanocomposites. RSC Adv. 2015;5:10782–89.10.1039/C4RA12552ESearch in Google Scholar
[24] Kim DW, Jung DW, Adelodun AA, Jo YM. Evaluation of CO2 adsorption capacity of electrospun carbon fibers with thermal and chemical activation. J Appl Polym Sci. 2017;134:45534.10.1002/app.45534Search in Google Scholar
[25] Lee HM, Kang HR, An KH, Kim HG, Kim BJ. Comparative studies of porous carbon nanofibers by various activation methods. Carbon Lett. 2012;14(3):180–5.10.5714/CL.2013.14.3.180Search in Google Scholar
[26] Manoj B, Kunjomana AG. Study of stacking structure of amorphous carbon by X-ray diffraction technique. Int J Electrochem Sci. 2012;3127–34.10.1016/S1452-3981(23)13940-XSearch in Google Scholar
[27] Ouassim B, Fouad G, Arunabh G, Ouafae A, Tarik C. Excellent CO2 capture by ultra-high microporous activated carbon made out from Natural coal. Chem Eng Technol. 2020;44(1):148–55.10.1002/ceat.202000138Search in Google Scholar
[28] Huang G, Liu Y, Wu X, Cai J. activated carbons prepared by the KOH activation of a hydrochar from garlic peel and their CO2 adsorption performance. New Carbon Mater. 2019;34:247–57.10.1016/S1872-5805(19)60014-4Search in Google Scholar
[29] Mishra AK, Ramaprabhu S. Carbon dioxide adsorption in graphene sheets. AIP Adv. 2011;1:032152.10.1063/1.3638178Search in Google Scholar
[30] Guo B, Chang L, Xie K. Adsorption of carbon dioxide on activated carbon. J Natur Gas Chem. 2006;25:223–38.10.1016/S1003-9953(06)60030-3Search in Google Scholar
[31] Gayathri S, Jayabal P, Kottaisamy M, Ramakrishnan V. Synthesis of few layers graphene by direct exfoliation of graphite and a Raman spectroscopic study. AIP Adv. 2014;4:027116.10.1063/1.4866595Search in Google Scholar
[32] Pei S, Cheng HM. The reduction of graphene oxide. Carbon. 2012;50:3210–28.10.1016/j.carbon.2011.11.010Search in Google Scholar
[33] Hong SM, Kim SH, Jeon BG, Jo SM, Lee KB. Development of porous carbon nanofibers from electrospun polyvinylidene fluoride for CO2 capture. RSC Adv. 2014;4:58956–63.10.1039/C4RA11290CSearch in Google Scholar
[34] Ali N, Babar AA, Zhang Y, Iqbal N, Wang X, Yu J, et al. Porous, flexible, and core-shell structured carbon nanofibers hybridized by tin oxide nanoparticles for efficient carbon dioxide capture. J Colloid Interface Sci. 2020;560:379–87.10.1016/j.jcis.2019.10.034Search in Google Scholar PubMed
[35] Huang ZM, Zhang YZ, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol. 2003;63:2223–53.10.1016/S0266-3538(03)00178-7Search in Google Scholar
[36] Li JH, Zhang GH, Wang Z. CO2 absorption of powdered Ba2Fe2O5 with different particle size. High Temp Mater Process. 2018;37(9–10):1001–6.10.1515/htmp-2017-0100Search in Google Scholar
[37] Yadav D, Amini F, Ehrmann A. Recent advances in carbon nanofibers and their applications – a review. Eur Polym J. 2020;138:109963.10.1016/j.eurpolymj.2020.109963Search in Google Scholar
[38] Pu X, Yang X, Zhang Y, Li L, Xie Y, He B, et al. Fabrication and characterization of highly oriented composite nanofibers with excellent mechanical strength and thermal stability. Macromol Mater Eng. 2020;305:1900691.10.1002/mame.201900691Search in Google Scholar
[39] Cychosz KA, Guillet-Nicolas R, Garcia-Martinez J, Thommes M. Recent advances in the textural characterization of hierarchically structured nanoporous materials. Chem Soc Rev. 2017;46(2):389–414.10.1039/C6CS00391ESearch in Google Scholar
[40] Cychosz KA, Thommes M. Progress in the physisorption characterization of nanoporous gas storage materials. Eng. 2018;4:559–66.10.1016/j.eng.2018.06.001Search in Google Scholar
[41] Martin CF, Sweatman MB, Brandani S, Fan X. Wet impregnation of a commercial low-cost silica using DETA for a fast post-combustion CO2 capture process. Appl Energy. 2016;183:1705–21.10.1016/j.apenergy.2016.09.081Search in Google Scholar
[42] Bai G, Han Y, Du P, Fei Z, Chen X, Zhang Z, et al. Polyethyleneimine (PEI)-impregnated resin adsorbent with high efficiency and capacity for CO2 capture from flue gas. New J Chem. 2019;43(46):18345–54.10.1039/C9NJ03822ASearch in Google Scholar
[43] Fu X. Polyethyleneimine-silica mesoporous hybrid: a novel adsorbent for CO2 capture. Adv Compos Lett. 2012;21(2):37–43.10.1177/096369351202100201Search in Google Scholar
[44] Wang X, Li G, Seo MH, Lui G, Han FM, Feng K, et al. Carbon-coated silicon nanowires on carbon fabric as self-supported electrodes for flexible lithium-ion batteries. ACS Appl Mater Interfaces. 2017;9:9551–8.10.1021/acsami.6b12080Search in Google Scholar PubMed
[45] Zeeshan M, Yalcin K, Oztuna FES, Unal U, Keskin S, Uzun A. A new class of porous materials for efficient CO2 separation: ionic liquid/graphene aerogel composites. Carbon. 2021;171:79–87.10.1016/j.carbon.2020.08.079Search in Google Scholar
[46] Lee SY, Park SJ. Preparation and characterization of ordered porous carbons for increasing hydrogen storage behaviors. J Solid State Chem. 2011;184(10):2655–60.10.1016/j.jssc.2011.07.034Search in Google Scholar
[47] Ullah S, Shariff AM, Bustam MA, Elkhalifah AEI, Murshid G, Riaz N, et al. Modified MIL-53 with multi-wall carbon nanotubes and nanofibers on CO2 adsorption. Appl Mech Mater. 2014;625:870–3.10.4028/www.scientific.net/AMM.625.870Search in Google Scholar
[48] Ojeda-López R, Esparza-Schulz JM, Perez-Hermosillo IJ, Hernandez-Gordillo A, Dominguez-Ortiz A. Improve in CO2 and CH4 adsorption capacity on carbon microfibers synthesized by electrospinning of PAN. Fibers. 2019;7:81.10.3390/fib7100081Search in Google Scholar
[49] Meng LY, Park SJ. Effect of heat treatment on CO2 adsorption of KOH-activated graphite nanofibers. J Colloid Interface Sci. 2010;352:498–503.10.1016/j.jcis.2010.08.048Search in Google Scholar
[50] Yuan H, Meng LY, Park SJ. KOH-activated graphite nanofibers as CO2 adsorbents. Carbon Lett. 2016;19:99–103.10.5714/CL.2016.19.099Search in Google Scholar
[51] Iqbal N, Wang X, Babar AA, Yu J, Din B. Highly flexible NiCo2O4/CNTs doped carbon nanofibers for CO2 adsorption and supercapacitor electrodes. J Colloid Interface Sci. 2016;476:87–93.10.1016/j.jcis.2016.05.010Search in Google Scholar
[52] Nan D, Liu J, Ma W. Electrospun phenolic resin-based carbon ultrafine fibers with abundant ultra-small micropores for CO2 adsorption. Chem Eng J. 2015;276:44–50.10.1016/j.cej.2015.04.081Search in Google Scholar
[53] Aksoylu AE, Madalena M, Freitas A, Pereira MFR, Figueiredo JL. The effects of different activated carbon supports and support modifications on the properties of Pt/AC catalysts. Carbon. 2010;39:175–85.10.1016/S0008-6223(00)00102-0Search in Google Scholar
[54] Gomes HT, Machado BF, Ribeiro A, Moreira I, Rosario M, Silva AMT, et al. Catalytic properties of carbon materials for wet oxidation of aniline. J Hazard Mat. 2008;159:420–6.10.1016/j.jhazmat.2008.02.070Search in Google Scholar PubMed
[55] Diez N, Alvarez P, Granda M, Blanco C, Santamaria R, Menendez R. A novel approach for the production of chemical activated carbon fibers. Chem Eng J. 2015;114:2243–51.Search in Google Scholar
[56] Titinchi SJJ, Piet M, Abbo HS, Bolland O, Schwieger W. Chemically modified solid adsorbents for CO2 capture. Energy Procedia. 2014;63:8153–60.10.1016/j.egypro.2015.12.337Search in Google Scholar
[57] Kishor R, Ghoshal AK. Amine-modified mesoporous silica for CO2 adsorption: the role of structural parameters. Ind Eng Chem Res. 2017;56:6078–87.10.1021/acs.iecr.7b00890Search in Google Scholar
[58] Chen C, Ahn W-S. ‘CO2 capture using mesoporous alumina prepared by a sol-gel process’. Chem Eng J. 2011;166(2):646–51.10.1016/j.cej.2010.11.038Search in Google Scholar
[59] Pellerano M, Pre P, Kacem M, Delebarre A. CO2 capture by adsorption on activated carbons using pressure modulation. Energy Procedia. 2009;1:647–53.10.1016/j.egypro.2009.01.085Search in Google Scholar
[60] Siriwardane RV, Shen MS, Fisher EP. Adsorption of CO2 on zeolites at moderate temperatures. Energy Fuels. 2005;19(3):1153–9.10.1021/ef040059hSearch in Google Scholar
[61] Dawson R, Stockel E, Holst JR, Adams DJ, Cooper AI. Microporous organic polymers for carbon dioxide capture. Energy Environ Sci. 2011;4:4239–45.10.1039/c1ee01971fSearch in Google Scholar
[62] Vilarrasa-Garcia E, Cecilia JA, Azevedo DCS, Cavalcante Jr CL, Rodriguez-Castellon E. Evaluation of porous clay heterostructures modified with amine species as adsorbent for the CO2 capture. Micropor Mesopor Mat. 2017;249:25–33.10.1016/j.micromeso.2017.04.049Search in Google Scholar
[63] Ngoy JM, Wagner N, Riboldi L, Bolland O. A CO2 capture technology using multiwalled carbon nanotubes with polyaspartamide surfactant. Energy Procedia. 2014;63:2230–48.10.1016/j.egypro.2014.11.242Search in Google Scholar
[64] Tan YL, Islam MA, Asif M, Hameed BH. Adsorption of carbon dioxide by sodium hydroxide-modified granular coconut shell activated carbon in a fixed bed. Energy. 2014;77:926–31.10.1016/j.energy.2014.09.079Search in Google Scholar
[65] Shen C, Grande CA, Li P, Yu J, Rodrigues AE. Adsorption equilibria and kinetics of CO2 and N2 on activated carbon beads. Chem Eng J. 2010;160:398.10.1016/j.cej.2009.12.005Search in Google Scholar
[66] Bikshapathi M, Sharma A, Sharma A, Verma N. Preparation of carbon molecular sieves from carbon micro and nanofibers for sequestration of CO2. Chem Eng Res Des. 2011;89:1737–46.10.1016/j.cherd.2010.09.009Search in Google Scholar
[67] Choma J, Osuchowski L, Marszewski M, Dziura A, Jaroneic M. Developing microporosity in Kevlar1-derived carbon fibers by CO2 activation for CO2 adsorption. J CO2 Util. 2016;16:17–22.10.1016/j.jcou.2016.05.004Search in Google Scholar
[68] Lee SY, Park SJ. Preparation and characterization of orderous porous carbons for increasing hydrogen storage behaviors. J Solid State Chem. 2011;184(10):2655–60.10.1016/j.jssc.2011.07.034Search in Google Scholar
[69] Chowdhury S, Parshetti GK, Balasubramaniam R. Post-combustion CO2 capture using mesoporous TiO2/graphene oxide nanocomposites. Chem Eng J. 2015;263:374–84.10.1016/j.cej.2014.11.037Search in Google Scholar
[70] Li D, Ma T, Zhang R, Tian Y, Qiao Y. Preparation of porous carbons with high low-pressure CO2 uptake by KOH activation of rice husk char. Fuels. 2015;139:68–70.10.1016/j.fuel.2014.08.027Search in Google Scholar
[71] Sanz R, Calleja G, Arencibia A, Sanz ES. Development of high efficiency adsorbents for CO2 capture based on a double-functionalization method of grafting and impregnation. J Phys Chem A. 2012;1:1956–62.10.1039/c2ta01343fSearch in Google Scholar
[72] Builes S, Sandler SI, Xiong R. Isosteric heats of gas and liquid adsorption. Langmuir. 2013;29:10416–22.10.1021/la401035pSearch in Google Scholar PubMed
[73] Singh VK, Kumar EA. Measurement and analysis of adsorption isotherms of CO2 on activated carbon. Appl Therm Eng. 2016;97:77–86.10.1016/j.applthermaleng.2015.10.052Search in Google Scholar
[74] Kong X, Li S, Stromme M, Xu C. Synthesis of porous organic polymers with tunable amine loadings for CO2 capture: balanced physisorption and chemisorption. Nanomater. 2019;9(7):1–11.10.3390/nano9071020Search in Google Scholar PubMed PubMed Central
[75] Liu F, Chen S, Gao Y. Synthesis of porous polymer based solid amine adsorbent: effect of pore size and amine loading on CO2 adsorption. J Colloid Interface Sci. 2017;506:236–44.10.1016/j.jcis.2017.07.049Search in Google Scholar PubMed
[76] Guo B, Wang Y, Shen X, Qiao X, Jia L, Xiang J, et al. Study on CO2 capture characteristics and kinetics of modified potassium-based adsorbents. Mater. 2020;13(4):877.10.3390/ma13040877Search in Google Scholar PubMed PubMed Central
[77] Ayawei N, Ebelegi AN, Wankasi D. Modelling and interpretation of adsorption isotherm. J Chem. 2017;1–11.10.1155/2017/3039817Search in Google Scholar
[78] Hu Y, Zhu Y, Zhang Y, Lin T, Zeng G, Zhang S, et al. An efficient adsorbent: simultaneous activated and magnetic ZnO doped biochar derived from camphor leaves for ciprofloxacin adsorption. Bioresour Technol. 2019;288:121511.10.1016/j.biortech.2019.121511Search in Google Scholar PubMed
[79] Wang J, Adelodun AA, Oh JM, Jo YM. TEPA impregnation of electrospun carbon nanofibers for enhanced low-level CO2 adsorption. Nano Converg. 2020;7(7):1–11.10.1186/s40580-020-0217-ySearch in Google Scholar PubMed PubMed Central
© 2022 Faten Ermala Che Othman et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption

