Abstract
Shengdong coal was single-step and multi-step pyrolyzed by pyrolysis-gas chromatography/mass spectrometry. In the single-step pyrolysis process, Mono-ring aromatics contents reach a maximum value at 800°C, while phenolic contents are significantly reduced, which might be attributed to the dehydroxylation of phenolics. Naphthalene content also reaches a maximum value at 800°C in the single-step pyrolysis process; however, naphthalene was not detected in the multi-step pyrolysis process. So naphthalene might come from the ring-opening of multi-ring aromatics or the cyclization of mono-ring aromatics in the single-step pyrolysis process. Only phenol and o-cresol were detected, but no p-cresol in the multi-step pyrolysis process; however, p-cresol content reaches a maximum value at 800°C, meanwhile, phenol content was decreased and o-cresol was disappeared in the single-step pyrolysis process, which suggests that p-cresol may be derived from isomerization of o-cresol or alkylation of phenol. It provides an idea for strengthening more basic research related to the rapid pyrolysis of coal.
Graphical abstract
1 Introduction
China is relatively rich in coal resources [1,2]. As one of the eight largest coalfields in the world, Shengdong (SD) coal is easy to mine and it has excellent quality [3]. With the increase in environmental pressures, it is imperative to clean and efficiently use coal [4]. Coal pyrolysis, as the first step in all coal conversion processes, can obtain coal char, coal tar, and fuel gas, which provides an alternative source to the petrochemical industry for the production of liquid fuels and chemicals [5].
Fast pyrolysis is an attractive coal conversion with higher tar yield [6]. Unfortunately, none of fast pyrolysis technologies has been commercialized. It seems necessary to strengthen the basic research related to the rapid pyrolysis of coal [7]. The destructive pyrolysis technique combined with GC/MS is the most effective method to analyze the compositions of organic volatiles during coal pyrolysis and has the advantages of high precision and simplicity, provides not only information about the coal structure but also helps to explain coal pyrolysis process [8].
Pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS) can be used for direct analysis of organic solids without lengthy extractions or derivatizations, saving time and cost [9], which has been widely used in biomass [10], lignin [11], asphaltenes [12], polymer [13], and coal [14,15,16]. The formation of light aromatics during coal pyrolysis was studied by Li et al. [17] and found that the main source of light aromatics was the thermal cracking of coal macromolecular skeleton. Xie et al. [18,19] studied the source of polycyclic aromatic hydrocarbons (PAHs) during coal pyrolysis by Py-GC/MS, and found that the release of PAHs against temperature variation first increased and then decreased. The formation of phenols during coal pyrolysis using in-situ Py-GC/MS were studied by Li et al. [20,21]. The results showed that the main source of phenols came from the thermal decomposition of the oxygen-containing structures in coal. Meanwhile, the emission of thiophenic sulfur compounds during coal pyrolysis were studied by Li et al. [22] and found that the amounts of benzothiophene and dibenzothiophene reached a maximum value at 800°C. Wang et al. [23] studied the release characteristics of N-containing compounds, more than one hundred N-containing compounds have been found. Liu et al. [24] systematically studied the composition of oils produced during pyrolysis at the consecutive temperature ranges. The results showed that mono-ring aromatics were only found in oils at 400–500°C, bicy-ring aromatics were mainly found in oils at 400–500°C.
In summary, pyrolysis characteristics of light aromatics, PAHs, phenols, S-containing and N-containing compounds were investigated. However, there is no report on the comprehensive study of pyrolysis characteristics of many compounds. To our knowledge, most studies used single-step pyrolysis mode of Py-GC/MS, the studies on the multi-step coal pyrolysis of Py-GC/MS were few. Generally, GC/MS is the result of qualitative and semi-quantitative analysis of volatile organic compounds by matching with NIST mass spectrometry library and using area normalization method [25]. The semi-quantitative results represent the relative contents, so maybe the trend of the relative contents is quite different from their actual contents [26 27 28 29]. In literature reports, relative content was replaced by absolute peak area [17] and integrated intensity/mg coal [3,4]. Here, the relative content was first modified by multiplying the pyrolysis rate with the relative content.
In this paper, SD coal was single-step pyrolyzed by in-line Py-GC/MS and the pyrolysis temperatures were 300–1,000°C, with a total of eight temperatures at intervals of 100°C. The pyrolysis rate at each temperature was calculated and used to modify the relative content. A multi-step program was used to investigate the multi-step pyrolysis of SD coal at different temperature ranges, a series of important information about the amounts and species of organic volatiles with temperature was obtained, and the organic volatiles evolution mechanism during coal pyrolysis was analyzed.
2 Experimental
2.1 Coal sample
Shengdong subbituminous coal (SD coal) distributed in the Shanxi province of China was using in this study. The SD coal was pulverized, sieved to pass the 80 mesh standard sieve and dried at 110°C in a vacuum for 4 h before analysis. Proximate and ultimate analyses were determined by Chinese standard methods as described elsewhere [8]. Size distribution was measured according to international standard ISO13320-1 by a Laser particle size analyzer (HYL-2076, China) [30,31]. A summary of the proximate and ultimate analyses as well as the size distribution of SD coal were shown in Table 1.
Conventional analyses and size distribution of SD coal
| Proximate analysis (wt%, ad) | Ultimate analysis (wt%, daf) | Size distribution (μm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | A | V | FC | C | H | N | S | O* | D 10 | D 50 | D 90 | RD |
| 5.85 | 6.10 | 36.87 | 51.18 | 64.49 | 3.68 | 0.90 | 0.23 | 30.70 | 18.81 | 94.86 | 220.73 | 2.13 |
daf: dry and ash-free basis, ad: air-dried basis, *By difference. D 10, D 50, D 90: 10%, 50%, 90% of the particles are smaller than the diameter, respectively, RD: (D 90 − D 10)/D 50.
2.2 Pyrolysis experiments
The destructive pyrolysis technique can provide detailed molecular information because the organic volatiles are related to the original coal structure. Fast pyrolysis of SD coal was performed with directly pyrolysis mode on the CDS 5200 pyrolyzer (CDS Analytical Inc., USA) [4]. In order to detect the chemical composition of complex organic volatiles from SD coal pyrolysis, CDS 5200 pyrolyzer was direct connected to a GC/MS (QP2010 plus, Shimadzu Inc., Japan) and the organic volatiles were identified by the standard mass spectra library used the method of probability-based match algorithm [32]. The detailed parameters have be described in the references [33]. Fast pyrolysis of SD coal was performed by in-line Py-GC/MS (Figure 1).
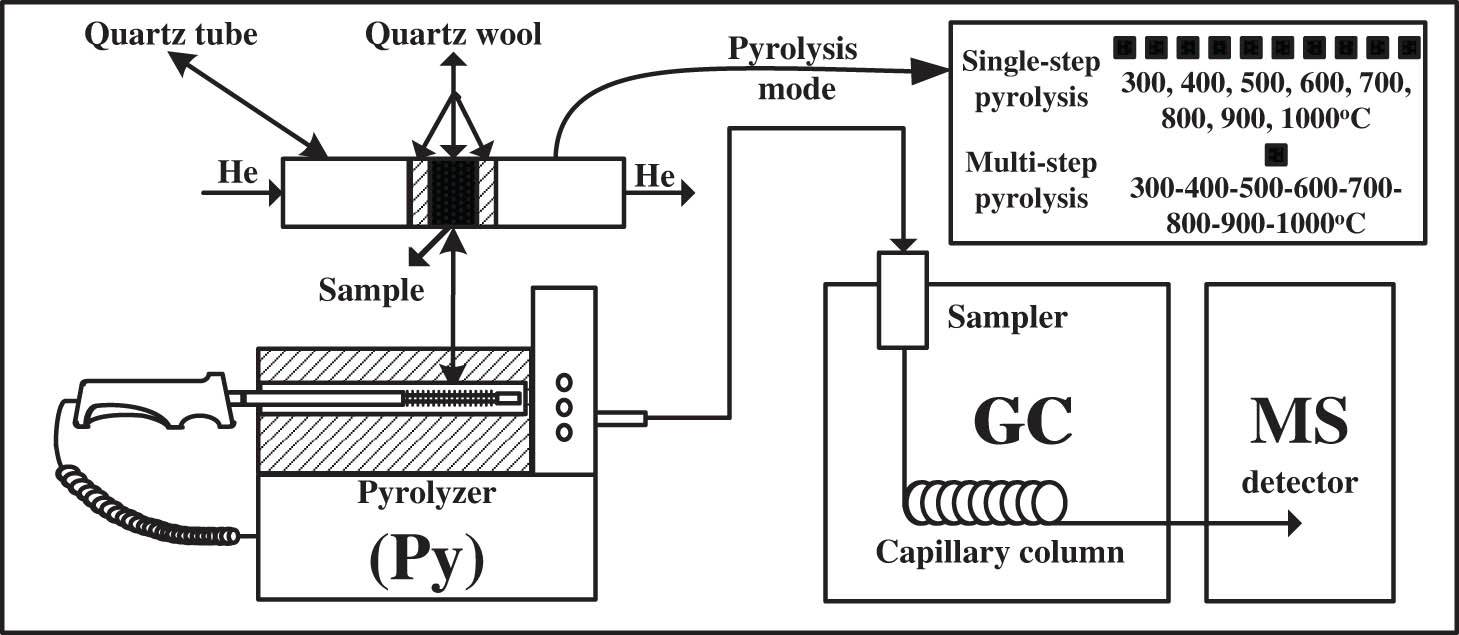
The schematic diagram of Py-GC/MS.
2.2.1 Single-step pyrolysis
In a single-step run, each run corresponds to a sample and a pyrolysis condition. SD coal was single-step pyrolyzed by in-line Py-GC/MS at a heating rate up to 20°C/ms to the final pyrolysis temperature from 300 to 1,000°C at intervals of 100°C and the pyrolysis time at each temperature was 20 s. The organic volatiles produced in sample chamber during coal pyrolysis were transferred to GC by helium purge gas with 40 mL/min. The sample (about 2 mg) was placed at the center of the filament for pyrolysis and held in the place using small plugs of quartz wool.
One hundred thousand molecule-accurate weighing balance (ESJ80-5, China) was used to calculate the pyrolysis rate at different pyrolysis temperatures by equation (1). The corrective content of organic volatiles was obtained by equation (2).
In the equations, R s is the pyrolysis rate in the single-step mode, wt%, W 1, W 2, W 3 and W 4 are the mass of before sample addition, after sample addition, before pyrolysis and after pyrolysis, 0.01 mg, respectively. C c denotes the corrective content of organic volatiles in the single-step pyrolysis, wt%. C r denotes the relative content of organic volatiles, wt%.
2.2.2 Multi-step pyrolysis
A sample was put into the pyrolysis probe and analyzed for many times, which was completed by establishing the sequence method. SD coal was multi-step pyrolyzed (the only difference from single-step pyrolysis is that the sample was not taken out of the pyrolysis tube at different pyrolysis temperatures) by in-line Py-GC/MS at the temperature sequence of 300, 400, 500, 600, 700, 800, 900 and 1,000°C [33]. The pyrolysis rate during sequence pyrolysis temperatures was calculated by equation (3) and the corrective content of organic volatiles in multi-step pyrolysis was obtained by equation (4).
where n is 300, 400, 500, 600, 700, 800 and 900°C, the value of R s can be obtained based on equation (1). R m is the pyrolysis rate during sequence pyrolysis temperatures, wt%.
3 Results and discussion
3.1 Organic volatiles during single-step pyrolysis
When analyzing pyrolytic volatiles by Py-GC/MS, the corrective content was calculated by the pyrolysis rate multiplied by the relative content. Figure 2 shows the pyrolysis rates (R s) of SD coal during the single-step pyrolysis process. The value of R s increased with the increase of pyrolysis temperature. The structure of coal is very complex, which is composed of different covalent bonds with different bond energies. The primary driving force of coal pyrolysis comes from the thermal cleavage of these bonds. Generally, covalent bonds with lower bond energies crack at a lower temperature, while covalent bonds with higher bond energies crack at a higher temperature [34]. The amounts of volatile organic compounds in coal pyrolysis process can be attributed to the devolatilization of fragments produced by covalent bonds breakage. As the pyrolysis temperature increased, the destroyed covalent bonds were increased.
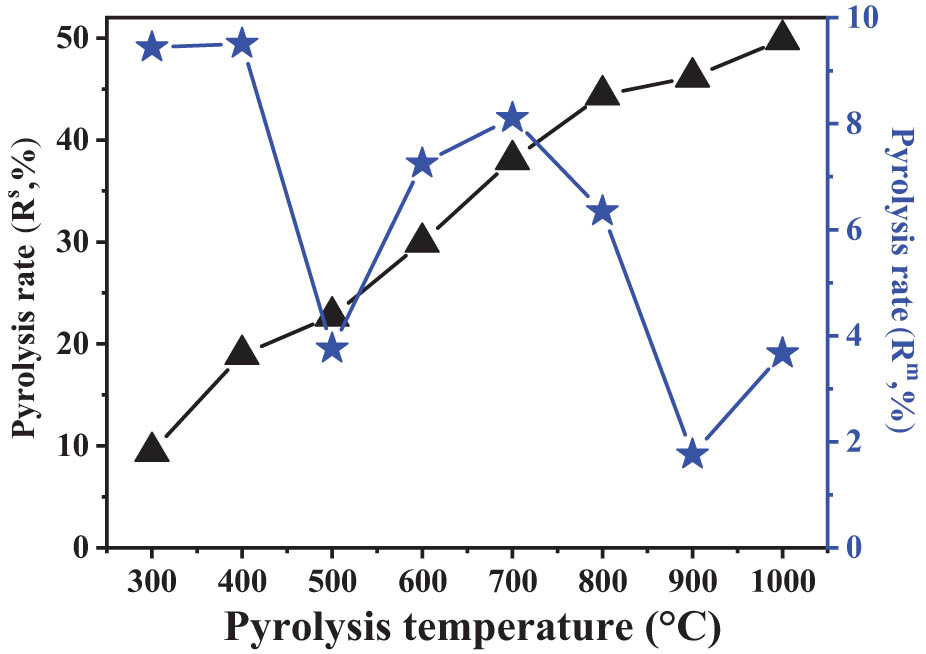
Pyrolysis rates of SD coal during single-step and multi-step pyrolysis process.
The pyrolysis rate (R m) of SD coal during multi-step pyrolysis process (Figure 2) was calculated by subtracting the pyrolysis rate (R s) based on equation (3). The pyrolysis rate of SD coal is higher in the medium-low temperature range. The two temperature regions with higher pyrolysis rates are <400°C and 600–700°C, which can be attributed to the contents of low molecular compounds, carboxyl groups and aromatic branched chains in SD coal [35]. More than 50% of the components in SD coal can’t be thermal fractured, and the emission temperature of organic volatiles during coal pyrolysis are mainly concentrated before 800°C. The pyrolysis rate distribution (R m) of SD coal in different temperature ranges can reflect the distribution of fracture difficulty in the structure.
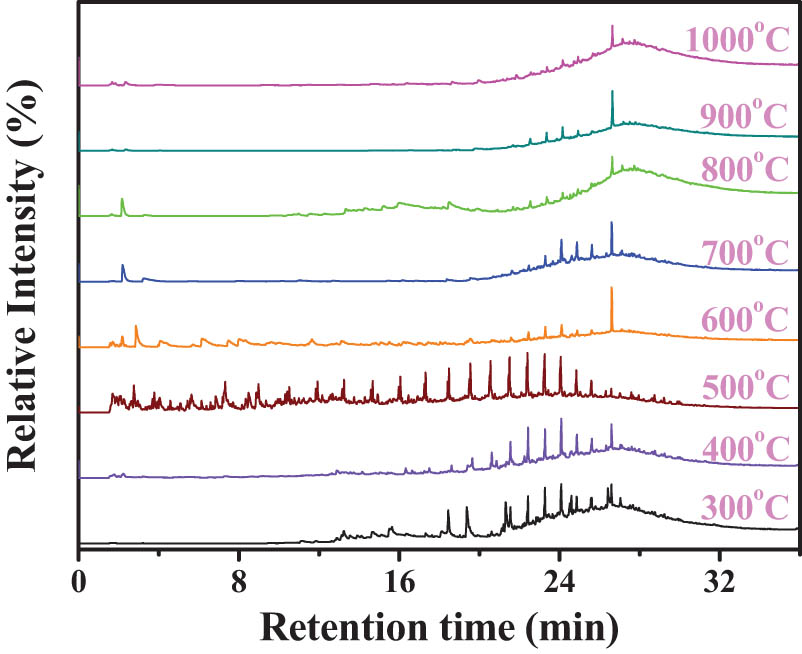
SD coal was single-step pyrolyzed to 300, 400, 500, 600, 700, 800, 900 and 1,000°C by Py-GC/MS in-situ respectively and the individually total ion chromatograms were shown in Figure 3. The organic volatiles were identified by the standard mass spectra library used the method of probability-based match algorithm [32]. Coal pyrolysis products are enormous, for comparison conveniently, all identified components were categorized as aliphatics, aromatics, O-compounds and others [36].
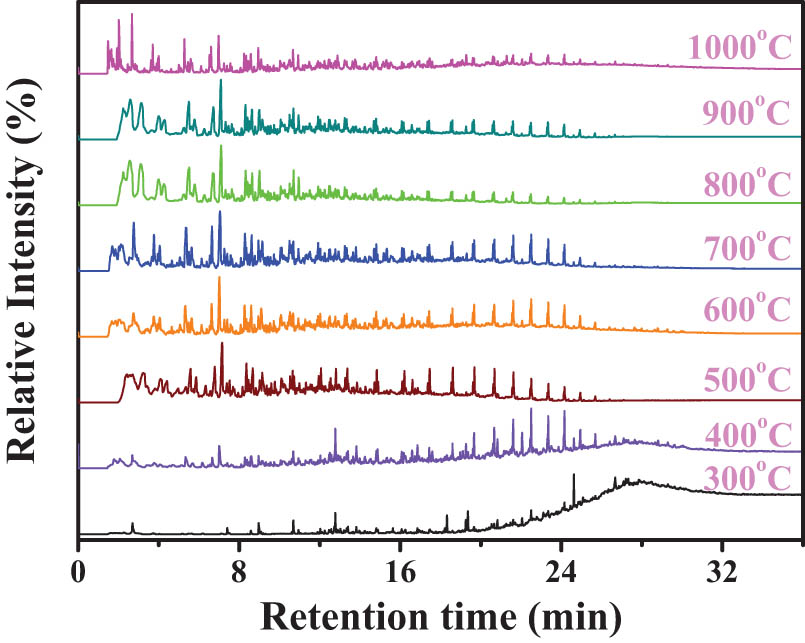
Total ion chromatograms of organic volatiles during single-step pyrolysis.
Figure 4(a) is the relative contents of each component in the single-step pyrolysis process. The total peak number and peak intensity of volatile organic compounds are different at different pyrolysis temperatures. Consequently, the treads of the relative contents of organic volatiles will affect each other, which would be different from the treads of their actual contents [26]. The relative contents of aliphatics and O-compounds (Figure 4(a)) reach a maximum value at 600°C, and the relative content of aromatics reaches a maximum value at 800°C. Without considering other reactions, the higher the pyrolysis temperature, the higher the degree of thermal decomposition in coal. Therefore, the higher the temperature, the higher the contents of organic volatile matter. But as the pyrolysis temperature increasing, the relative contents of organic volatiles change irregularly. After modified by equations (1) and (2), the corrective contents of group compositions during single-step pyrolysis process were obtained (Figure 4(b)). As the pyrolysis temperature increasing, the change regularity of the corrective contents of organic volatiles was obviously enhanced, which can better explain the content changes of organic volatiles during coal pyrolysis at different pyrolysis temperatures.
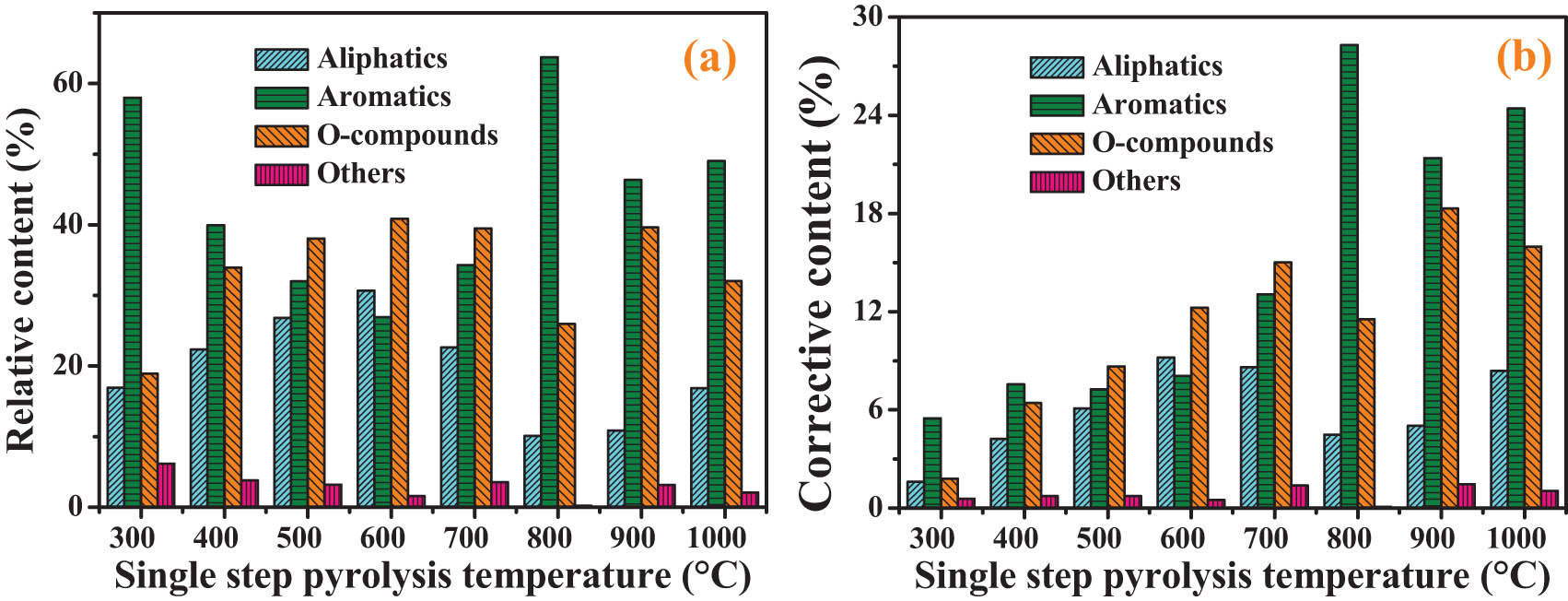
Group compositions of organic volatiles during single-step pyrolysis process (a) relative content, (b) corrective content.
The number of carbons in the chains of aliphatics concentrates on 11 and 32, and the other compounds are mainly N-containing compounds. With the increase of the single-step pyrolysis temperature, the contents of organic volatiles should increase successively. When the single-step pyrolysis temperature is higher than 600°C, the content of aliphatics did not continue to increase. Possible reasons are as follows: at high temperature, the pyrolysis rate of coal increases, releasing more organic volatiles, which leads to the overload of gas chromatography column and cannot effectively separate and detect the products [18]. The released organic volatiles affect each other. For example, when single-step pyrolysis temperature is higher than 600°C, a large amount of aromatics are released, resulting in a high peak strength of aromatics, which may cover the peak strength of aliphatics.
3.1.1 Emission characteristics of aromatics during single-step pyrolysis process
Aromatic hydrocarbons are subdivided into monocyclic, ice ring, and polycyclic structures (Figure 5a). With the pyrolysis temperature increasing from 300 to 1,000°C, the content of mono-ring aromatics first increases and reaches a maximum value (23.3 wt%) at 800°C and then decreases, which gives a single peak trend. This result indicates that with the increase in pyrolysis temperature, more aromatic ring macromolecule structures in SD coal were broken into mono-ring fragments. However, too high temperature may cause the mono-ring fragments to be cracked into aliphatics or condensed into multi-ring aromatics [9], which can be inferred from the increases in the contents of aliphatics and multi-ring aromatics at a high temperature. With the increase in pyrolysis temperature, the content of bicy-ring aromatics increases in general. The change in the content of bicy-ring aromatics is caused by its own ring-opening reaction and the cracking of multi-ring aromatics [19]. The content of multi-ring aromatics disappears at 800°C, which can be attributed to the cracking of multi-ring aromatics, the interaction between organic volatiles or the limitation of the GC/MS column in the detection of multi-ring aromatics [18].
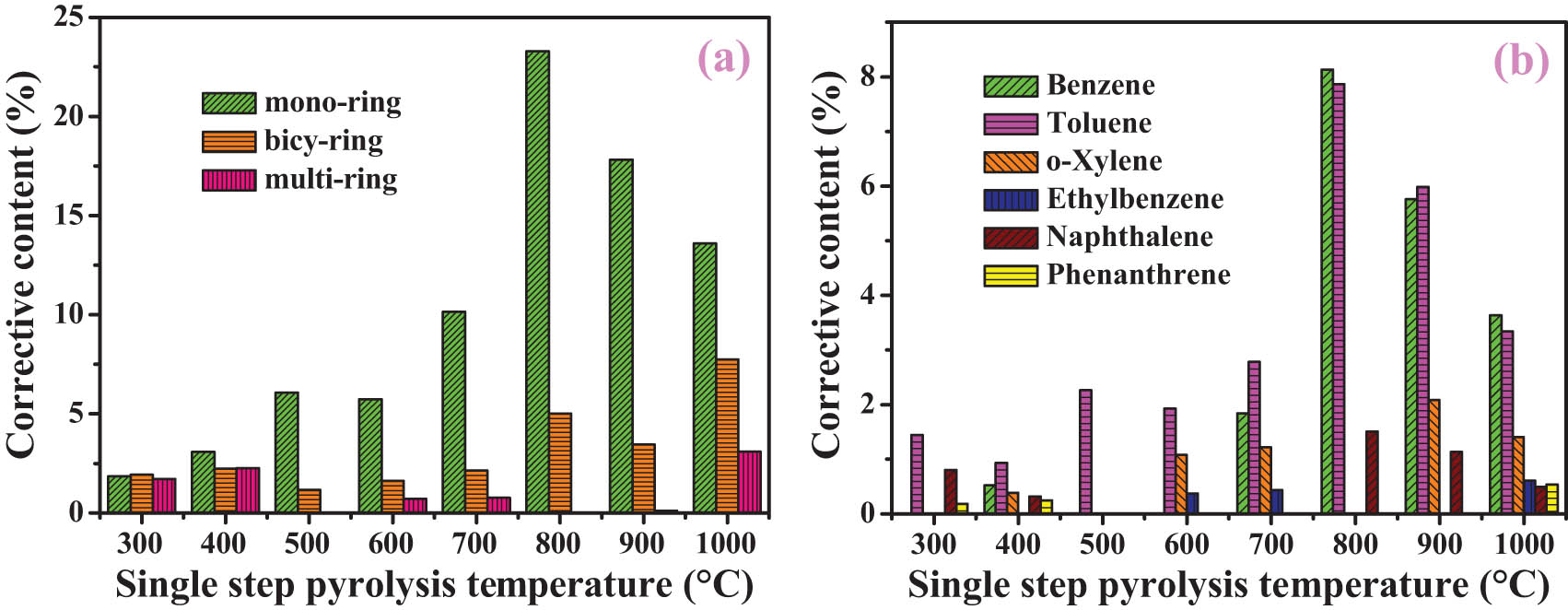
Corrective content of aromatics (a) and the typical compounds (b) during single-step pyrolysis process.
The non-fuel use of coal, for example, the raw materials for obtaining chemicals, is an important utilization method. The content of typical compounds is different at different pyrolysis temperatures, which can provide guidance for the directional transformation of coal [37]. Figure 5b shows the typical aromatics during the single-step pyrolysis process. The contents of benzene, toluene, and naphthalene reach the maximum values in the organic volatiles at 800°C, while o-xylene, ethylbenzene, and phenanthrene disappear. It is speculated that the ring-opening reaction of phenanthrene, the dealkylation, and cyclization of o-xylene and ethylbenzene may be responsible. With the further increase of pyrolysis temperature, the contents of benzene, toluene, and naphthalene decrease, while the content of phenanthrene increases, indicating that aromatic ring condensation is dominant at a high temperature [19].
3.1.2 Emission characteristics of O-compounds during single-step pyrolysis process
O-compounds were subdivided into phenolics, carboxylic acids, and the remaining compounds. Phenolics (Figure 6(a)) are the main body of O-compounds. Phenolics are mainly derived from mono-ring aromatics linked with ether bridges in coal [3]. When the pyrolysis temperature is less than 700°C, the content of phenolics increases with the increase in pyrolysis temperature. At 800°C, together with a decrease in phenolics, a maximum value of mono-ring aromatics has been observed (Figure 5). These trends suggest that one possible pathway for the formation of mono-ring aromatics is the dehydroxylation of phenolics.
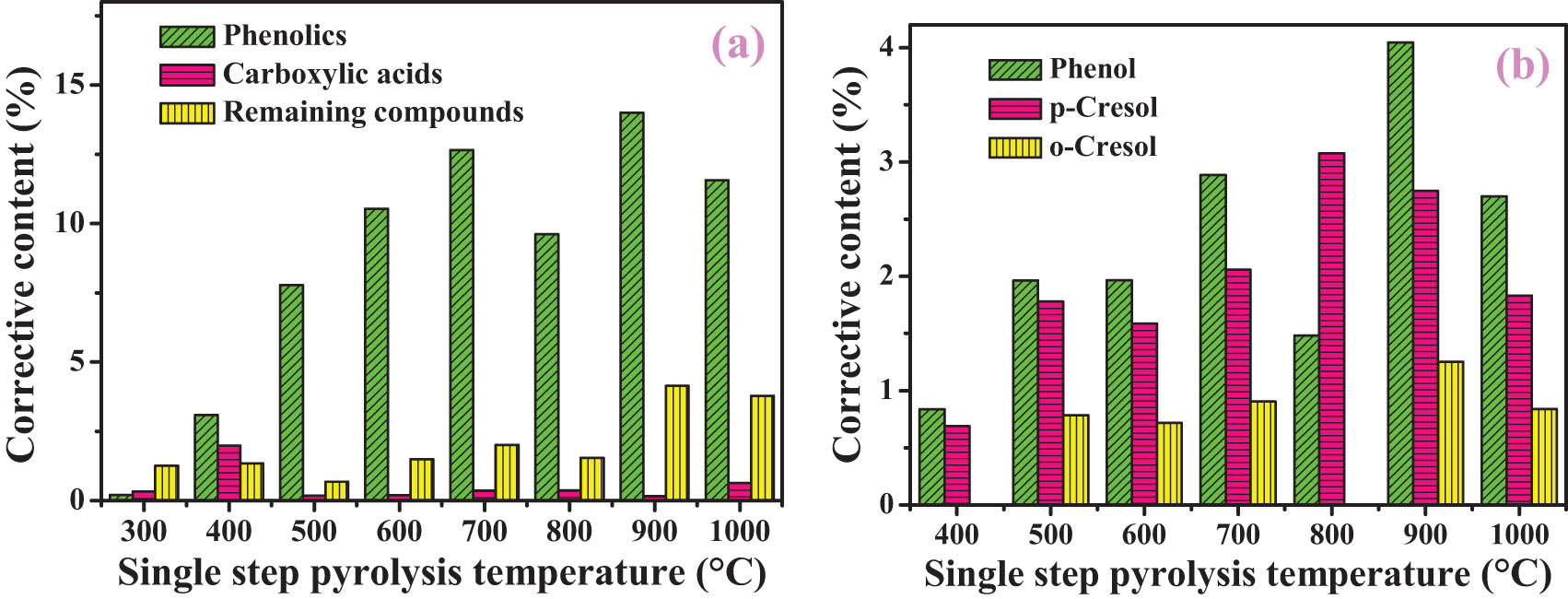
Corrective content of O-compounds (a) and the typical compounds (b) during the single-step pyrolysis process.
At 400℃, the content of released carboxylic acid reached the maximum (1.9 wt%). At a higher pyrolysis temperature, the content of carboxylic acids decreases. The reasons are as follows: on the one hand, the structure of most carboxylic acids in SD coal has broken at a low temperature and its amount will not continue to increase with the increase in pyrolysis temperature, while the contents of other compounds increase with the increase in pyrolysis temperature, which has a great influence on the peak strength of the carboxylic acids [26]. On the other hand, carboxylic acids are decarboxylated or hydrogenated to aromatics or phenolics, which reduce their contents [38].
Figure 6b shows typical phenols in the one-step pyrolysis process. Typical phenols appear when the pyrolysis temperature is higher than 400℃. For example, at 900℃, the content of phenol reaches the maximum (4.1 wt%), that of p-cresol reaches the maximum (3.1 wt%) at 800℃, and that of o-cresol disappears at 800℃. So it can be speculated that o-cresol is easier to be dehydroxylated to form aromatics than phenol and p-cresol.
3.2 Organic volatiles during multi-step pyrolysis
SD coal was multi-step pyrolyzed to 300, 400, 500, 600, 700, 800, 900, and 1,000°C by in-line Py-GC/MS, and the ion chromatograms are shown in Figure 7. Multi-step pyrolysis is a batch of cleavage reactions of a sample, which is used to analyze the difference or difficulty degree of coal pyrolysis at different temperature intervals [2]. For example, different from the single-step pyrolysis at 500°C, the organic volatiles from multi-step pyrolysis at 500°C are actually the release of organic volatiles in the temperature range of 400–500°C.
Total ion chromatograms of organic volatiles during multi-step pyrolysis.
Figure 8(a) shows the relative content of group compositions during the multi-step pyrolysis process. After modified by equations (3) and (4), during the multi-step pyrolysis process, the corrected content of composition was obtained (Figure 8(b)). In different pyrolysis temperature ranges, with the increase in pyrolysis temperature, the composition of pyrolysis volatile matter is irregular. The corrective contents (Figure 8(b)) are close to the real values. Aliphatics and aromatics (Figure 8(b)) were released at each pyrolysis temperature range. The highest content of aliphatics was obtained at 300–400°C and the temperature range of 600–800°C is the main temperature for the release of aromatics, which is in accord with the finding [19]. The highest content of O-compounds was obtained at the pyrolysis temperature range of less than 300°C, and the O-compounds are mainly carboxylic acids. The higher content of O-compounds was obtained at 500–600°C, and the O-compounds are mainly phenolics [36]. The O-compounds disappear when the pyrolysis temperature is higher than 700°C, which indicates that the O-containing structure in SD coal can be completely broken before 700°C. Other compounds are mainly N-containing compounds, and the main pyrolysis temperature is below 300°C. From the main pyrolysis temperature range of different compounds, it can be inferred that the order of the difficulty of compounds escaping from SD coal is carboxylic acids, N-containing compounds < aliphatics < phenolics < aromatics.
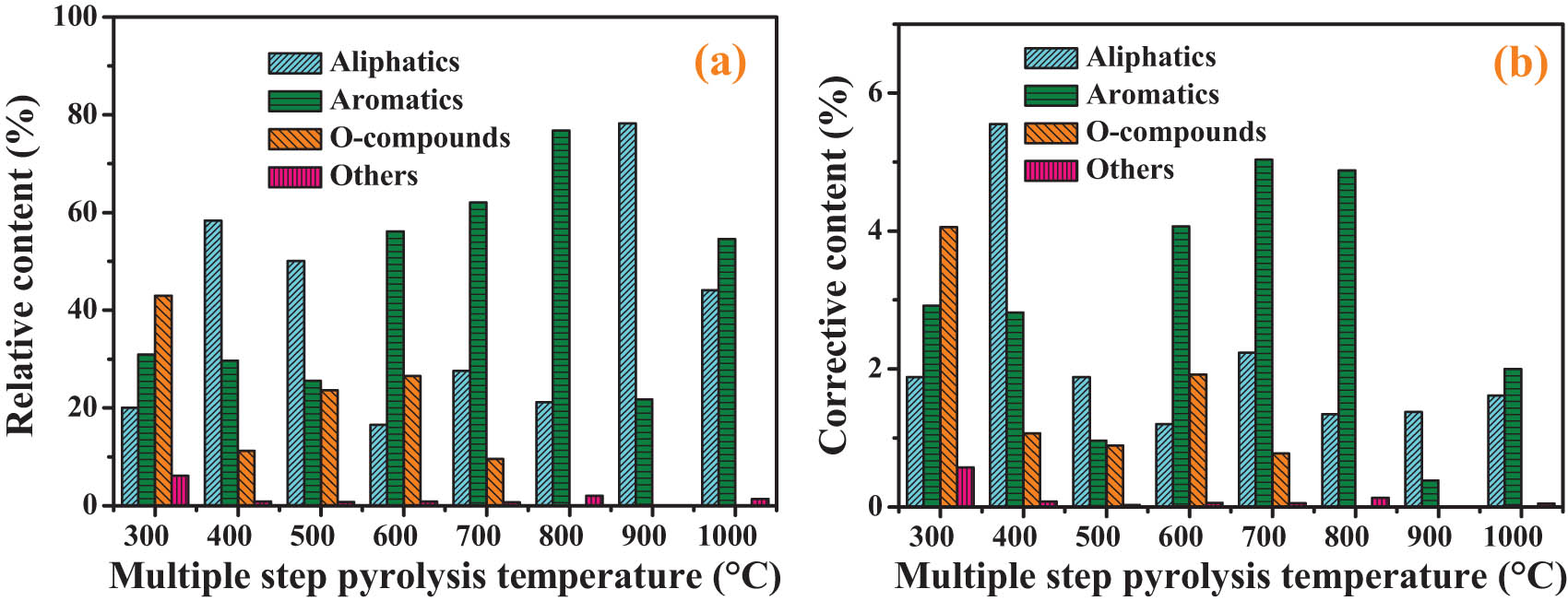
Group compositions of organic volatiles during multi-step pyrolysis process (a) relative content and (b) corrective content.
3.2.1 Emission characteristics of aromatics during multi-step pyrolysis process
Figure 9a shows the correct content of aromatic hydrocarbons during the multi-step pyrolysis. With the pyrolysis temperature increasing from 400 to 800°C during multi-step pyrolysis, the content of mono-ring aromatics increases and reaches a maximum value (3.5 wt%) at 800°C and its main release temperature range is concentrated at 600–800°C, which indicate that pyrolysis at a high temperature is beneficial to the formation of mono-ring aromatics. Bicy-ring aromatics would be cracked into mono-ring aromatics at higher temperatures, so the content of mono-ring aromatics increases and the content of bicy-ring aromatics decreases. When the multi-step pyrolysis temperature is higher than 800°C, the residual semi-coke is polycyclic aromatic hydrocarbons, which makes it difficult to thermally crack [20].
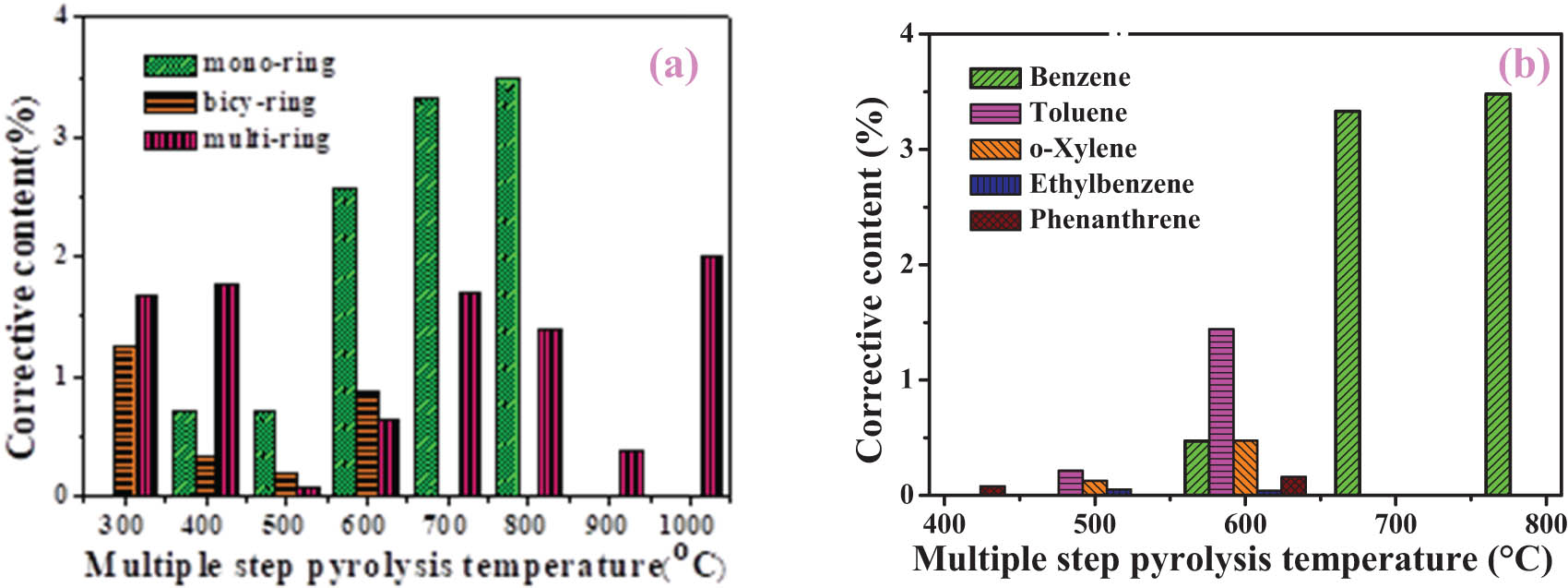
Corrective content of aromatics (a) and the typical compounds (b) during the multi-step pyrolysis process.
Compared with the single-step pyrolysis, multi-step pyrolysis was taken place in the temperature range [37], which is the cutting temperature of organic volatiles in the single-step pyrolysis. The total amount of volatile organic compounds decreased, and the interaction between volatile organic compounds weakened. Figure 9b shows the corrective content of typical aromatic compounds during multi-step pyrolysis process. Benzene is produced when the multi-step pyrolysis temperature is higher than 600°C [39]. The favorable pyrolysis temperature range for toluene and o-xylene is mainly at 500–600°C, while their contents continue to increase at a high temperature during the single-step pyrolysis process (Figure 5b), which are caused by dehydroxylation, alkylation, and ring opening reactions. There is no naphthalene in the volatile products during multi-step pyrolysis, which suggests that naphthalene may mainly derived from the chemical reactions between pyrolysis volatiles during the single-step pyrolysis.
3.2.2 Emission characteristics of O-compounds during multi-step pyrolysis process
Figure 10 shows the corrective content of O-compounds during multi-step pyrolysis process. With the multi-step pyrolysis temperature increasing from 300 to 700°C, the content of phenolics first increases and reaches a maximum value (1.5 wt%) at 600°C and then decreases, which gives a single peak trend, and this result is consistent with the literature [21]. With the increase in multi-step pyrolysis temperature, the content of carboxylic acids decreases gradually and the main release temperature range of aliphatic chain carboxylic acids is concentrated at <300°C. At a higher temperature, carboxylic acids are mainly aromatic chain carboxylic acids. So the cracking order of O-containing bonds in coal is C═O bonds with aliphatic side chains > aromatic C–O bonds > C═O bonds with aromatic side chains [17,34]. Typical O-compounds during multi-step pyrolysis include phenol and o-cresol without p-cresol, which suggests that p-cresol in the single-step pyrolysis volatiles (Figure 6b) may be derived from isomerization of o-cresol or alkylation of phenol. The initial escape temperature ranges of o-cresol and phenol are 400–500 and 500–600°C, respectively, which indicates that oxo-aromatic structure containing substituted alkyl groups is easier to be broken from the SD coal structure.
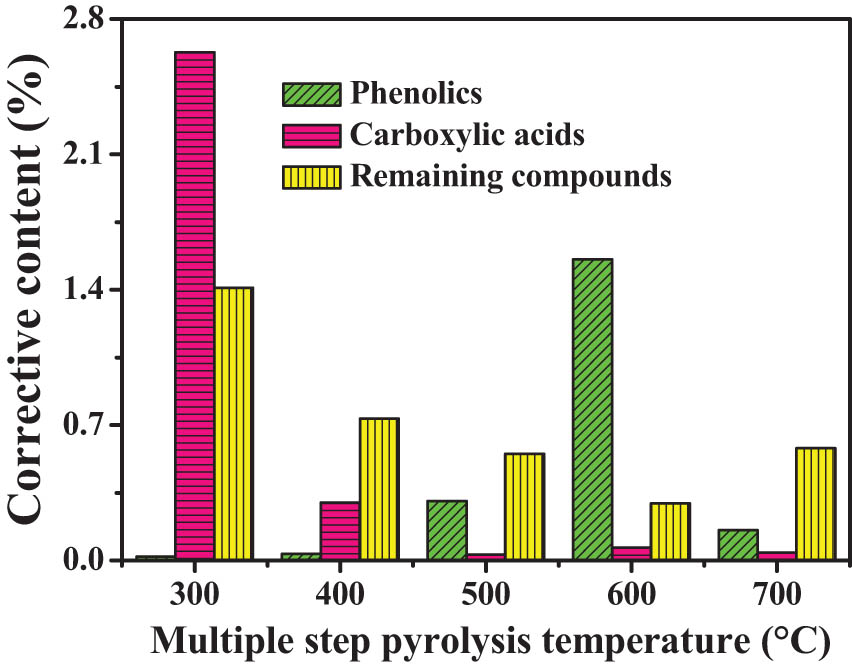
Corrective content of O-compounds during the multi-step pyrolysis process.
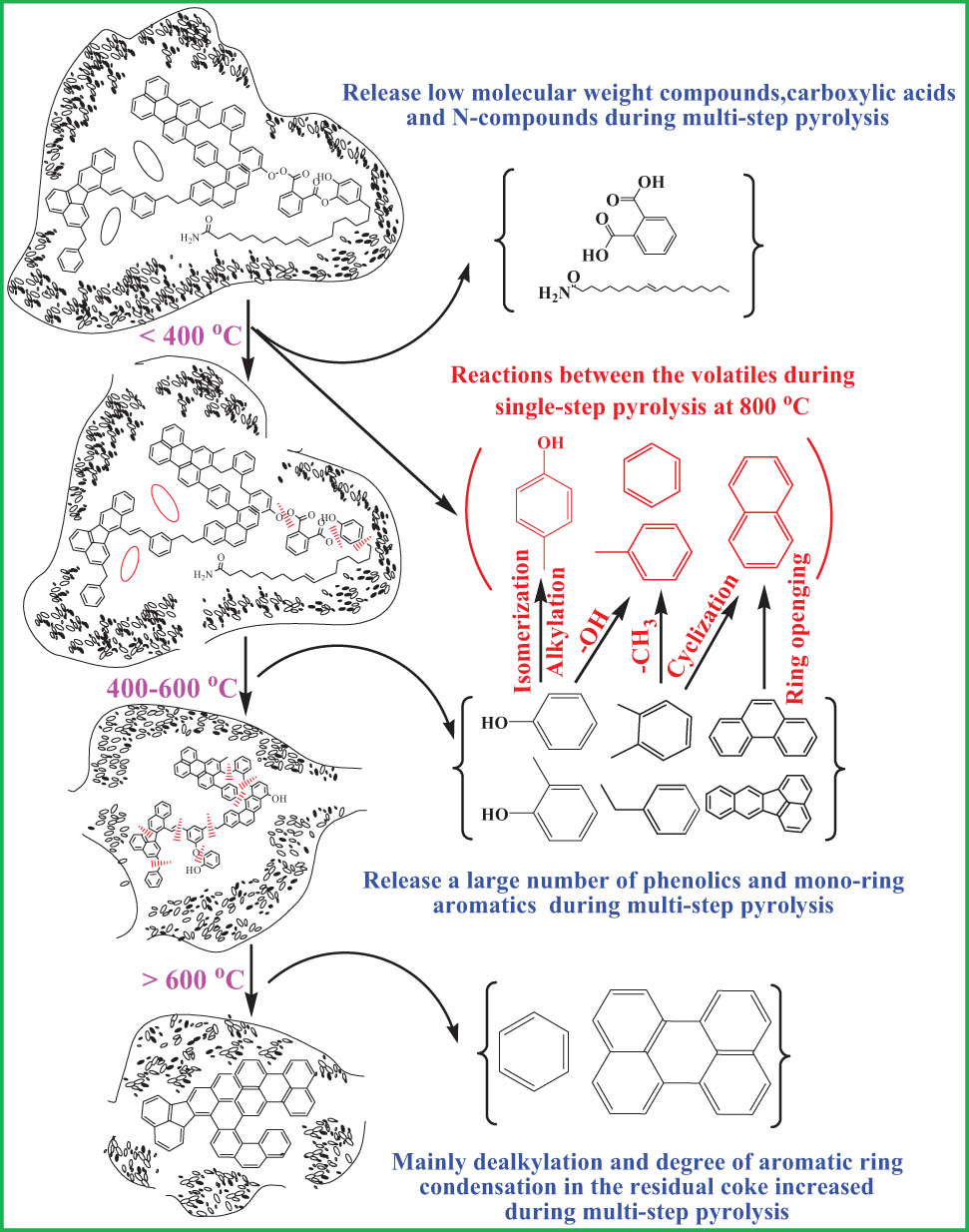
3.3 Pyrolysis process and mutual reactions of organic volatiles
Py-GC/MS can provide molecular information and the pyrolysis volatiles are related to the original macromolecular structure of coal. The multi-step pyrolysis can be used to analyze the pyrolysis volatiles at different temperature intervals. Compared with multi-step pyrolysis, the total amount of volatiles is more and the mutual reactions between organic volatiles become easier. Figure 11 shows the pyrolysis process and mutual reactions of organic volatiles. Compared with coal skeletons, low molecular weight compounds have higher activity in the process of coal pyrolysis [17]. When the multi-step pyrolysis temperature is lower than 400°C, the main released volatiles are the low molecular compounds [40], mainly including polycyclic aromatic hydrocarbons [38], carboxylic acids, and N-containing compounds. Carboxylic acids are alkyl carboxylic acids and aryl carboxylic acids. Alkyl carboxylic acids are concentrated in C14–C18 alkyl acids. Aryl carboxylic acid is mainly phthalic acid. The typical N-containing compound is oleamide. 400–600°C is the main pyrolysis temperature range during the multi-step pyrolysis process, and at this temperature range, large amounts of phenolics and mono-ring aromatics and a small amount of multi-ring aromatics were produced. When the multi-step pyrolysis temperature is higher than 600°C, the degree of aromatic ring condensation in the residual semi-coke increases and multi-ring aromatics are released. Meanwhile, there is a dealkylation reaction and the benzene is released.
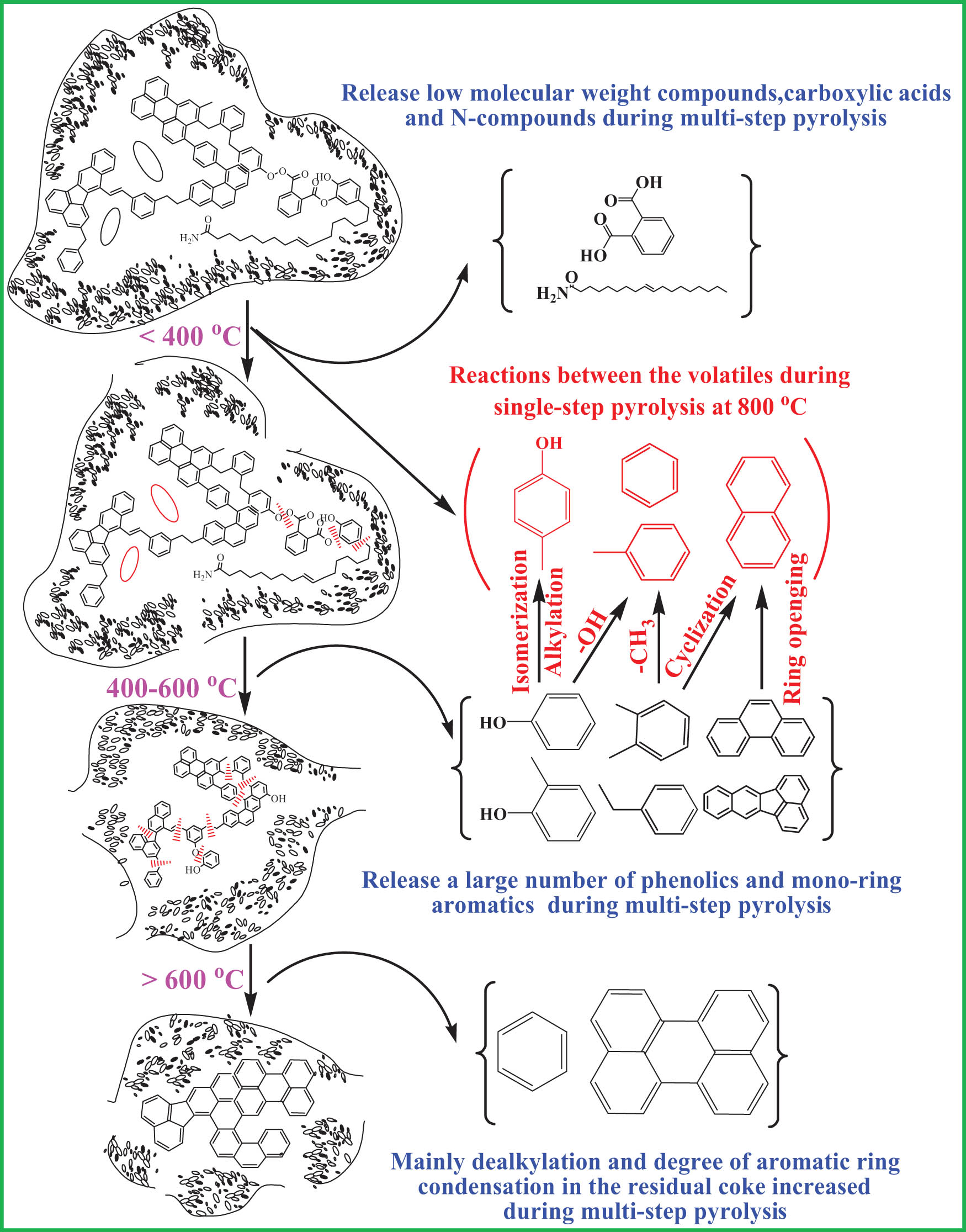
Pyrolysis process and mutual reactions of organic volatiles.
During the multi-step pyrolysis process, only phenol and o-cresol were found in the pyrolysis products, but no p-cresol. However, the content of a single pyrolysis product of p-cresol is obvious, reaching the maximum at 800℃, indicating that p-cresol mainly comes from the interaction of organic volatiles, rather than the direct fracture of SD coal structure. Corresponding to the decrease in the phenol content and the disappearance of o-cresol at 800°C, it is presumed that p-cresol may come from the alkylation of phenol or isomerization of o-cresol [41]. In the multi-step pyrolysis process, toluene is mainly released at 400–600℃, but at higher temperature, its content continues to increase, reaching the maximum at 800℃, and then decreases. So toluene may be produced by dehydroxylation of phenolics and dealkylation of mono-ring aromatics [21]. Benzene is mainly released at 500–800°C during the multi-step pyrolysis process and reaches a maximum value at 800°C and then decreases, which matches well with the previous study [9]. O-xylene disappears at 800°C during multi-step pyrolysis. When the single-step pyrolysis temperature is higher than 800°C, the content of o-xylene and ethylbenzene is obvious, while the content of benzene and toluene decreases. It is indicated that the dealkylation reaction was dominant at 800°C and the alkylation reaction was dominant at >800°C. There is no naphthalene in the multi-step pyrolysis products, and the content of naphthalene reaches a maximum value at 800°C during the single-pyrolysis process. Phenanthrene and pyrene were released below 800°C during the multiple pyrolysis process and disappeared at 800°C. It is speculated that naphthalene may be derived from the ring-opening of polycyclic aromatics and the cyclization of monocyclic aromatics.
4 Conclusion
It is necessary to strengthen basic research on the rapid pyrolysis of coal. In this study, SD coal was single-step and multi-step pyrolyzed by in-line Py-GC/MS at the temperature range of 300–1,000°C. Using the proposed modified method of pyrolysis rate multiplying relative content, the change regularity of the corrective content of pyrolysis products with temperatures during single-step pyrolysis was obviously enhanced. 400–600°C is the main pyrolysis temperature region during the multi-step pyrolysis process. In this temperature range, large amounts of phenolic and mono-ring aromatics, as well as a small amount of multi-ring aromatics, were produced. One possible pathway for the formation of mono-ring aromatics is the dehydroxylation of phenolics. Naphthalene may mainly be derived from the chemical reactions (the ring-opening of multi-ring aromatics and the cyclization of mono-ring aromatics) between pyrolysis volatiles during single-step pyrolysis. p-cresol in the organic volatiles during the single-step pyrolysis process is mainly derived from the mutual reactions of organic volatiles rather than the direct fracture of SD coal structure and it is presumed that p-cresol may come from the alkylation of phenol or isomerization of o-cresol.
-
Funding information: This study was financed by the Department of Education of Gansu Province: Young Doctor Fund Project (2022QB-029), the Fundamental Research Funds for the Central Universities (31920230023, 31920230003, 31920230051, 31920220031), the Scientific Research Project of Introducing Talents of Northwest Minzu University (xbmuyjrc202215, xbmuyjrc202216), the Key Research and Development Project of Gansu Province (21YF5GA061), the National Natural Science Foundation of China (22178289), and the Key Research and Development Program of Shaanxi (2020ZDLGY11-02).
-
Author contributions: Xiaoping Su was responsible for conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, writing original draft, review, and editing; Xiangtong Wang for resources; Ning Li for data curation; Tao Shen for supervision; Ping Zhang for software; Ming Sun for formal analysis; and Xiaoxun Ma for funding acquisition. All authors contributed to the manuscript revision and read and approved the submitted version.
-
Conflict of interest: The authors state no conflict of interest.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Qin ZH. New advances in coal structure mode. Int J Min Sci Technol. 2018;28(4):541–59. 10.1016/j.ijmst.2018.06.010.Suche in Google Scholar
[2] Olivella MA, Palacios JM, Vairavamurthy A, del Rı́o JC, de Las Heras FXC. A study of sulfur functionalities in fossil fuels using destructive-(ASTM and Py-GC-MS) and non-destructive-(SEM-EDX, XANES and XPS) techniques. Fuel. 2002;81(4):405–11. 10.1016/S0016-2361(01)00198-3.Suche in Google Scholar
[3] Lievens C, Ci DH, Bai Y, Ma LG, Zhang R, Chen JY, et al. A study of slow pyrolysis of one low rank coal via pyrolysis-GC/MS. Fuel Process Technol. 2013;116:85–93. 10.1016/j.fuproc.2013.04.026.Suche in Google Scholar
[4] He C, Min XJ, Zheng HA, Fan YJ, Yao QX, Zhang D, et al. Study on the volatiles and kinetic of in-situ catalytic pyrolysis of swelling low rank coal. Energy Fuels. 2017;31(12):13558–71. 10.1021/acs.energyfuels.7b02952.Suche in Google Scholar
[5] Iglesias MJ, Jiménez A, del Río JC, Suárez-Ruiz I. Molecular characterisation of vitrinite in relation to natural hydrogen enrichment and depositional environment. Org Geochem. 2000;31(12):1285–99. 10.1016/S0146-6380(00)00086-3.Suche in Google Scholar
[6] He WJ, Liu ZY, Liu QY, Ci DH, Lievens C, Guo XF. Behaviors of radical fragments in tar generated from pyrolysis of 4 coals. Fuel. 2014;134:375–80. 10.1016/j.fuel.2014.05.064.Suche in Google Scholar
[7] Liu ZY, Guo XJ, Shi L, He WJ, Wu JF, Liu QY, et al. Reaction of volatiles-A crucial step in pyrolysis of coals. Fuel. 2015;154:361–9. 10.1016/j.fuel.2015.04.006.Suche in Google Scholar
[8] Wang SQ, Wang DX, Tang YG, Sun YB, Jiang D, Su T. Study of pyrolysis behavior of hydrogen-rich bark coal by TGA and Py-GC/MS. J Anal Appl Pyrol. 2018;128:136–42. 10.1016/j.jaap.2017.10.016.Suche in Google Scholar
[9] Dong J, Cheng Z, Li F. PAHs emission from the pyrolysis of Western Chinese coal. J Anal Appl Pyrol. 2013;104:502–7. 10.1016/j.jaap.2013.05.020.Suche in Google Scholar
[10] Fahmi R, Bridgwater AV, Darvell LI, Jones JM, Yates N, Thain S, et al. The effect of alkali metals on combustion and pyrolysis of Lolium and Festuca grasses, switchgrass and willow. Fuel. 2007;86(10–11):1560–9. 10.1016/j.fuel.2006.11.030.Suche in Google Scholar
[11] González-Pérez JA, Almendros G, de la Rosa JM, González-Vila FJ. Appraisal of polycyclic aromatic hydrocarbons (PAHs) in environmental matrices by analytical pyrolysis (Py-GC/MS). J Anal Appl Pyrol. 2014;109:1–8. 10.1016/j.jaap.2014.07.005.Suche in Google Scholar
[12] Riley BJ, Lennard C, Fuller S, Spikmans V. Pyrolysis-GC-MS analysis of crude and heavy fuel oil asphaltenes for application in oil fingerprinting. Environ Forensics. 2018;19(1):14–26. 10.1080/15275922.2017.1408163.Suche in Google Scholar
[13] Khabbaz F, Karlsson S, Albertsson AC. PY-GC/MS an effective technique to characterizing of degradation mechanism of poly (L-lactide) in the different environment. J Appl Polym Sci. 2015;78(13):2369–78. 10.1002/1097-4628(20001220)78:13<2369:AID-APP140>3.0.CO;2-N.Suche in Google Scholar
[14] Olivella MA, del Río JC, Palacios J, Vairavamurthy MA, de las Heras FXC. Characterization of humic acid from leonardite coal: an integrated study of PY-GC-MS, XPS and XANES techniques. J Anal Appl Pyrol. 2002;63(1):59–68. 10.1016/S0165-2370(01)00141-3.Suche in Google Scholar
[15] Song JZ, Peng PA. Characterisation of black carbon materials by pyrolysis-gas chromatography-mass spectrometry. J Anal Appl Pyrol. 2010;87(1):129–37. 10.1016/j.jaap.2009.11.003.Suche in Google Scholar
[16] Iglesias MJ, del Rı́o JC, Laggoun-Défarge F, Cuesta MJ, Suárez-Ruiz I. Control of the chemical structure of perhydrous coals; FTIR and Py-GC/MS investigation. J Anal Appl Pyrol. 2002;62(1):1–34. 10.1016/S0165-2370(00)00209-6.Suche in Google Scholar
[17] He YY, Zhao RF, Yan LJ, Bai YH, Li F. The effect of low molecular weight compounds in coal on the formation of light aromatics during coal pyrolysis. J Anal Appl Pyrol. 2017;123:49–55. 10.1016/j.jaap.2016.12.030.Suche in Google Scholar
[18] Dong J, Li F, Xie KC. Study on the source of polycyclic aromatic hydrocarbons (PAHs) during coal pyrolysis by PY-GC-MS. J Hazard Mater. 2012;243:80–5. 10.1016/j.jhazmat.2012.09.073.Suche in Google Scholar
[19] Gao MQ, Wang YL, Dong J, Li F, Xie KC. Release behavior and formation mechanism of polycyclic aromatic hydrocarbons during coal pyrolysis. Chemosphere. 2016;158:1–8. 10.1016/j.chemosphere.2016.05.024.Suche in Google Scholar
[20] Jiao K, Zhao RF, Bai YH, Li GL, Zhang C, Li F. Study on the formation of phenols during coal flash pyrolysis using pyrolysis-GC/MS. Fuel Process Technol. 2014;127:41–6. 10.1016/j.fuproc.2014.06.004.Suche in Google Scholar
[21] Bai YH, Yan LJ, Li GH, Zhao RF, Li F. Effects of demineralization on phenols distribution and formation during coal pyrolysis. Fuel. 2014;134:368–74. 10.1016/j.fuel.2014.05.076.Suche in Google Scholar
[22] Xing MW, Kong J, Dong J, Jiao HL, Li F. Thiophenic sulfur compounds released during coal pyrolysis. Environ Eng Sci. 2013;30(6):273–9. 10.1089/ees.2011.0540.Suche in Google Scholar PubMed PubMed Central
[23] Li Q, Wang ZH, Lin ZM, He Y, Zhang K, Whiddon R, et al. Influences of hydrothermal modification on nitrogen thermal conversion of low-rank coals. Energy Fuels. 2016;30(10):8125–33. 10.1021/acs.energyfuels.6b01255.Suche in Google Scholar
[24] Shi L, Liu QY, Liu ZY, Wu WZ. Oils and phenols-and-water-free tars produced in pyrolysis of 23 Chinese coals in consecutive temperature ranges. Energy Fuels. 2013;27(10):5816–22. 10.1021/ef401215h.Suche in Google Scholar
[25] Lu XQ, Hanna JV, Johnson WD. Source indicators of humic substances: an elemental composition, solid state 13C CP/MAS NMR and Py-GC/MS study. Appl Geochem. 2000;15(7):1019–33. 10.1016/S0883-2927(99)00103-1.Suche in Google Scholar
[26] Zhou P, Xiong SJ, Zhang YX, Jiang H, Chi YC, Li L. Study on the nitrogen transformation during the primary pyrolysis of sewage sludge by Py-GC/MS and Py-FTIR. Int J Hydrog Energy. 2017;42(29):18181–8. 10.1016/j.ijhydene.2017.04.144.Suche in Google Scholar
[27] Song FH, Li TT, Zhang J, Wang XJ, Bai YC, John P, et al. Novel insights into the kinetics, evolved gases, and mechanisms for biomass (sugar cane residue) pyrolysis. Environ Sci Technol. 2019;53(22):13495–505. 10.1021/acs.est.9b04595.Suche in Google Scholar PubMed
[28] Li TT, Song FH, Bai YC, Wu FC, Ruan MQ, Ca YH, et al. Real-Time Emission, Chemical Properties, and Dynamic Evolution Mechanism of Volatile Organic Compounds during Co-Pyrolysis of Rice Straw and Semi-Bituminous Coal. ACS ES&T Eng. 2023;3(5):690–705. 10.1021/acsestengg.2c00391.Suche in Google Scholar
[29] Zhou Y, Li L, Jin LJ, Zhu JL, Li JG, Li Y, et al. Effect of functional groups on volatile evolution in coal pyrolysis process with in-situ pyrolysis photoionization time-of-flight mass spectrometry. Fuel. 2020;260:116322. 10.1016/j.fuel.2019.116322.Suche in Google Scholar
[30] Luo L, Yao W, Liu JX, Zhang H, Ma JF, Jiang XM. The effect of the grinding process on pore structures, functional groups and release characteristic of flash pyrolysis of superfine pulverized coal. Fuel. 2019;235(1):1337–46. 10.1016/j.fuel.2018.08.081.Suche in Google Scholar
[31] Bhanarkar AD, Gavane AG, Tajne DS, Tamhane SM, Nema P. Composition and size distribution of particules emissions from a coal-fired power plant in India. Fuel. 2008;87(10–11):2095–101. 10.1016/j.fuel.2007.11.001.Suche in Google Scholar
[32] Wang GQ, Hou ZY, Sun YA, Zhang RJ, Xie K, Liu RL. Investigation of pyrolysis behavior of carbofuran by pyrolysis-gas chromatography-mass spectrometry. J Hazard Mater. 2006;129(1–3):22–30. 10.1016/j.jhazmat.2005.08.015.Suche in Google Scholar PubMed
[33] Sun M, Zhang D, Yao QX, Liu YQ, Su XP, Jia CQ, et al. Separation and composition analysis of GC/MS analyzable and unanalyzable parts from coal tar. Energy Fuels. 2018;32(7):7404–11. 10.1021/acs.energyfuels.8b01054.Suche in Google Scholar
[34] Shi L, Liu QY, Guo XJ, Wu WZ, Liu ZY. Pyrolysis behavior and bonding information of coal-A TGA study. Fuel Process Technol. 2013;108:125–32. 10.1016/j.fuproc.2012.06.023.Suche in Google Scholar
[35] Zeng C, Wu HW, Hayashi JI, Li CZ. Effects of thermal pretreatment in helium on the pyrolysis behaviour of Loy Yang brown coal. Fuel. 2005;84(12–13):1586–92. 10.1016/j.fuel.2005.02.025.Suche in Google Scholar
[36] Nali M, Corana F, Montanari L, Pellegrini L. A pyrolysis-gas chromatography/mass spectrometry study on coals. J Anal Appl Pyrol. 1994;29(1):15–23. 10.1016/0165-2370(93)00765-F.Suche in Google Scholar
[37] Yan LJ, Bai YH, Kong XJ, Li F. Effects of alkali and alkaline earth metals on the formation of light aromatic hydrocarbons during coal pyrolysis. J Anal Appl Pyrol. 2016;122:169–74. 10.1016/j.jaap.2016.10.001.Suche in Google Scholar
[38] Ribeiro J, Silva T, Filho JGM, Flores D. Polycyclic aromatic hydrocarbons (PAHs) in burning and non-burning coal waste piles. J Hazard Mater. 2012;199–200:105–10. 10.1016/j.jhazmat.2011.10.076.Suche in Google Scholar PubMed
[39] Landete JM, Curiel JA, Rodríguez H, de las Rivas B, Muñoz R. Study of the inhibitory activity of phenolic compounds found in olive products and their degradation by Lactobacillus plantarum strains. Food Chem. 2008;107(1):320–6. 10.1016/j.foodchem.2007.08.043.Suche in Google Scholar
[40] Arenillas A, Pevida C, Rubiera F, Garcı́a R, Pis JJ. Characterisation of model compounds and a synthetic coal by TG/MS/FTIR to represent the pyrolysis behaviour of coal. J Anal Appl Pyrol. 2004;71(2):747–63. 10.1016/j.jaap.2003.10.005.Suche in Google Scholar
[41] Sad ME, Padró CR, Apesteguía CR. Selective synthesis of p-cresol by methylation of phenol. Appl Catal A-Gen. 2008;342(1–2):40–8. 10.1016/j.apcata.2007.12.038.Suche in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Characteristics, source, and health risk assessment of aerosol polyaromatic hydrocarbons in the rural and urban regions of western Saudi Arabia
- Regular Articles
- A network-based correlation research between element electronegativity and node importance
- Pomegranate attenuates kidney injury in cyclosporine-induced nephrotoxicity in rats by suppressing oxidative stress
- Ab initio study of fundamental properties of XInO3 (X = K, Rb, Cs) perovskites
- Responses of feldspathic sandstone and sand-reconstituted soil C and N to freeze–thaw cycles
- Robust fractional control based on high gain observers design (RNFC) for a Spirulina maxima culture interfaced with an advanced oxidation process
- Study on arsenic speciation and redistribution mechanism in Lonicera japonica plants via synchrotron techniques
- Optimization of machining Nilo 36 superalloy parameters in turning operation
- Vacuum impregnation pre-treatment: A novel method for incorporating mono- and divalent cations into potato strips to reduce the acrylamide formation in French fries
- Characterization of effective constituents in Acanthopanax senticosus fruit for blood deficiency syndrome based on the chinmedomics strategy
- Comparative analysis of the metabolites in Pinellia ternata from two producing regions using ultra-high-performance liquid chromatography–electrospray ionization–tandem mass spectrometry
- The assessment of environmental parameter along the desalination plants in the Kingdom of Saudi Arabia
- Effects of harpin and carbendazim on antioxidant accumulation in young jujube leaves
- The effects of in ovo injected with sodium borate on hatching performance and small intestine morphology in broiler chicks
- Optimization of cutting forces and surface roughness via ANOVA and grey relational analysis in machining of In718
- Essential oils of Origanum compactum Benth: Chemical characterization, in vitro, in silico, antioxidant, and antibacterial activities
- Translocation of tungsten(vi) oxide/gadolinium(iii) fluoride in tellurite glasses towards improvement of gamma-ray attenuation features in high-density glass shields
- Mechanical properties, elastic moduli, and gamma ray attenuation competencies of some TeO2–WO3–GdF3 glasses: Tailoring WO3–GdF3 substitution toward optimum behavioral state range
- Comparison between the CIDR or sponge with hormone injection to induce estrus synchronization for twining and sex preselection in Naimi sheep
- Exergetic performance analyses of three different cogeneration plants
- Psoralea corylifolia (babchi) seeds enhance proliferation of normal human cultured melanocytes: GC–MS profiling and biological investigation
- A novel electrochemical micro-titration method for quantitative evaluation of the DPPH free radical scavenging capacity of caffeic acid
- Comparative study between supported bimetallic catalysts for nitrate remediation in water
- Persicaline, an alkaloid from Salvadora persica, inhibits proliferation and induces apoptosis and cell-cycle arrest in MCF-7 cells
- Determination of nicotine content in locally produced smokeless tobacco (Shammah) samples from Jazan region of Saudi Arabia using a convenient HPLC-MS/MS method
- Changes in oxidative stress markers in pediatric burn injury over a 1-week period
- Integrated geophysical techniques applied for petroleum basins structural characterization in the central part of the Western Desert, Egypt
- The impact of chemical modifications on gamma-ray attenuation properties of some WO3-reinforced tellurite glasses
- Microwave and Cs+-assisted chemo selective reaction protocol for synthesizing 2-styryl quinoline biorelevant molecules
- Structural, physical, and radiation absorption properties of a significant nuclear power plant component: A comparison between REX-734 and 316L SS austenitic stainless steels
- Effect of Moringa oleifera on serum YKL-40 level: In vivo rat periodontitis model
- Investigating the impact of CO2 emissions on the COVID-19 pandemic by generalized linear mixed model approach with inverse Gaussian and gamma distributions
- Influence of WO3 content on gamma rays attenuation characteristics of phosphate glasses at low energy range
- Study on CO2 absorption performance of ternary DES formed based on DEA as promoting factor
- Performance analyses of detonation engine cogeneration cycles
- Sterols from Centaurea pumilio L. with cell proliferative activity: In vitro and in silico studies
- Untargeted metabolomics revealing changes in aroma substances in flue-cured tobacco
- Effect of pumpkin enriched with calcium lactate on iron status in an animal model of postmenopausal osteoporosis
- Energy consumption, mechanical and metallographic properties of cryogenically treated tool steels
- Optimization of ultra-high pressure-assisted extraction of total phenols from Eucommia ulmoides leaves by response surface methodology
- Harpin enhances antioxidant nutrient accumulation and decreases enzymatic browning in stored soybean sprouts
- Physicochemical and biological properties of carvacrol
- Radix puerariae in the treatment of diabetic nephropathy: A network pharmacology analysis and experimental validation
- Anti-Alzheimer, antioxidants, glucose-6-phosphate dehydrogenase effects of Taverniera glabra mediated ZnO and Fe2O3 nanoparticles in alloxan-induced diabetic rats
- Experimental study on photocatalytic CO2 reduction performance of ZnS/CdS-TiO2 nanotube array thin films
- Epoxy-reinforced heavy metal oxides for gamma ray shielding purposes
- Black mulberry (Morus nigra L.) fruits: As a medicinal plant rich in human health-promoting compounds
- Promising antioxidant and antimicrobial effects of essential oils extracted from fruits of Juniperus thurifera: In vitro and in silico investigations
- Chloramine-T-induced oxidation of Rizatriptan Benzoate: An integral chemical and spectroscopic study of products, mechanisms and kinetics
- Study on antioxidant and antimicrobial potential of chemically profiled essential oils extracted from Juniperus phoenicea (L.) by use of in vitro and in silico approaches
- Screening and characterization of fungal taxol-producing endophytic fungi for evaluation of antimicrobial and anticancer activities
- Mineral composition, principal polyphenolic components, and evaluation of the anti-inflammatory, analgesic, and antioxidant properties of Cytisus villosus Pourr leaf extracts
- In vitro antiproliferative efficacy of Annona muricata seed and fruit extracts on several cancer cell lines
- An experimental study for chemical characterization of artificial anterior cruciate ligament with coated chitosan as biomaterial
- Prevalence of residual risks of the transfusion-transmitted infections in Riyadh hospitals: A two-year retrospective study
- Computational and experimental investigation of antibacterial and antifungal properties of Nicotiana tabacum extracts
- Reinforcement of cementitious mortars with hemp fibers and shives
- X-ray shielding properties of bismuth-borate glass doped with rare earth ions
- Green supported silver nanoparticles over modified reduced graphene oxide: Investigation of its antioxidant and anti-ovarian cancer effects
- Orthogonal synthesis of a versatile building block for dual functionalization of targeting vectors
- Thymbra spicata leaf extract driven biogenic synthesis of Au/Fe3O4 nanocomposite and its bio-application in the treatment of different types of leukemia
- The role of Ag2O incorporation in nuclear radiation shielding behaviors of the Li2O–Pb3O4–SiO2 glass system: A multi-step characterization study
- A stimuli-responsive in situ spray hydrogel co-loaded with naringenin and gentamicin for chronic wounds
- Assessment of the impact of γ-irradiation on the piperine content and microbial quality of black pepper
- Antioxidant, sensory, and functional properties of low-alcoholic IPA beer with Pinus sylvestris L. shoots addition fermented using unconventional yeast
- Screening and optimization of extracellular pectinase produced by Bacillus thuringiensis SH7
- Determination of polyphenols in Chinese jujube using ultra-performance liquid chromatography–mass spectrometry
- Synergistic effects of harpin and NaCl in determining soybean sprout quality under non-sterile conditions
- Field evaluation of different eco-friendly alternative control methods against Panonychus citri [Acari: Tetranychidae] spider mite and its predators in citrus orchards
- Exploring the antimicrobial potential of biologically synthesized zero valent iron nanoparticles
- NaCl regulates goldfish growth and survival at three food supply levels under hypoxia
- An exploration of the physical, optical, mechanical, and radiation shielding properties of PbO–MgO–ZnO–B2O3 glasses
- A novel statistical modeling of air pollution and the COVID-19 pandemic mortality data by Poisson, geometric, and negative binomial regression models with fixed and random effects
- Treatment activity of the injectable hydrogels loaded with dexamethasone In(iii) complex on glioma by inhibiting the VEGF signaling pathway
- An alternative approach for the excess lifetime cancer risk and prediction of radiological parameters
- Panax ginseng leaf aqueous extract mediated green synthesis of AgNPs under ultrasound condition and investigation of its anti-lung adenocarcinoma effects
- Study of hydrolysis and production of instant ginger (Zingiber officinale) tea
- Novel green synthesis of zinc oxide nanoparticles using Salvia rosmarinus extract for treatment of human lung cancer
- Evaluation of second trimester plasma lipoxin A4, VEGFR-1, IL-6, and TNF-α levels in pregnant women with gestational diabetes mellitus
- Antidiabetic, antioxidant and cytotoxicity activities of ortho- and para-substituted Schiff bases derived from metformin hydrochloride: Validation by molecular docking and in silico ADME studies
- Antioxidant, antidiabetic, antiglaucoma, and anticholinergic effects of Tayfi grape (Vitis vinifera): A phytochemical screening by LC-MS/MS analysis
- Identification of genetic polymorphisms in the stearoyl CoA desaturase gene and its association with milk quality traits in Najdi sheep
- Cold-acclimation effect on cadmium absorption and biosynthesis of polyphenolics, and free proline and photosynthetic pigments in Spirogyra aequinoctialis
- Analysis of secondary metabolites in Xinjiang Morus nigra leaves using different extraction methods with UPLC-Q/TOF-MS/MS technology
- Nanoarchitectonics and performance evaluation of a Fe3O4-stabilized Pickering emulsion-type differential pressure plugging agent
- Investigating pyrolysis characteristics of Shengdong coal through Py-GC/MS
- Extraction, phytochemical characterization, and antifungal activity of Salvia rosmarinus extract
- Introducing a novel and natural antibiotic for the treatment of oral pathogens: Abelmoschus esculentus green-formulated silver nanoparticles
- Optimization of gallic acid-enriched ultrasonic-assisted extraction from mango peels
- Effect of gamma rays irradiation in the structure, optical, and electrical properties of samarium doped bismuth titanate ceramics
- Combinatory in silico investigation for potential inhibitors from Curcuma sahuynhensis Škorničk. & N.S. Lý volatile phytoconstituents against influenza A hemagglutinin, SARS-CoV-2 main protease, and Omicron-variant spike protein
- Physical, mechanical, and gamma ray shielding properties of the Bi2O3–BaO–B2O3–ZnO–As2O3–MgO–Na2O glass system
- Twofold interpenetrated 3D Cd(ii) complex: Crystal structure and luminescent property
- Study on the microstructure and soil quality variation of composite soil with soft rock and sand
- Ancient spring waters still emerging and accessible in the Roman Forum area: Chemical–physical and microbiological characterization
- Extraction and characterization of type I collagen from scales of Mexican Biajaiba fish
- Finding small molecular compounds to decrease trimethylamine oxide levels in atherosclerosis by virtual screening
- Prefatory in silico studies and in vitro insecticidal effect of Nigella sativa (L.) essential oil and its active compound (carvacrol) against the Callosobruchus maculatus adults (Fab), a major pest of chickpea
- Polymerized methyl imidazole silver bromide (CH3C6H5AgBr)6: Synthesis, crystal structures, and catalytic activity
- Using calcined waste fish bones as a green solid catalyst for biodiesel production from date seed oil
- Influence of the addition of WO3 on TeO2–Na2O glass systems in view of the feature of mechanical, optical, and photon attenuation
- Naringin ameliorates 5-fluorouracil elicited neurotoxicity by curtailing oxidative stress and iNOS/NF-ĸB/caspase-3 pathway
- GC-MS profile of extracts of an endophytic fungus Alternaria and evaluation of its anticancer and antibacterial potentialities
- Green synthesis, chemical characterization, and antioxidant and anti-colorectal cancer effects of vanadium nanoparticles
- Determination of caffeine content in coffee drinks prepared in some coffee shops in the local market in Jeddah City, Saudi Arabia
- A new 3D supramolecular Cu(ii) framework: Crystal structure and photocatalytic characteristics
- Bordeaux mixture accelerates ripening, delays senescence, and promotes metabolite accumulation in jujube fruit
- Important application value of injectable hydrogels loaded with omeprazole Schiff base complex in the treatment of pancreatitis
- Color tunable benzothiadiazole-based small molecules for lightening applications
- Investigation of structural, dielectric, impedance, and mechanical properties of hydroxyapatite-modified barium titanate composites for biomedical applications
- Metal gel particles loaded with epidermal cell growth factor promote skin wound repair mechanism by regulating miRNA
- In vitro exploration of Hypsizygus ulmarius (Bull.) mushroom fruiting bodies: Potential antidiabetic and anti-inflammatory agent
- Alteration in the molecular structure of the adenine base exposed to gamma irradiation: An ESR study
- Comprehensive study of optical, thermal, and gamma-ray shielding properties of Bi2O3–ZnO–PbO–B2O3 glasses
- Lewis acids as co-catalysts in Pd-based catalyzed systems of the octene-1 hydroethoxycarbonylation reaction
- Synthesis, Hirshfeld surface analysis, thermal, and selective α-glucosidase inhibitory studies of Schiff base transition metal complexes
- Protective properties of AgNPs green-synthesized by Abelmoschus esculentus on retinal damage on the virtue of its anti-inflammatory and antioxidant effects in diabetic rat
- Effects of green decorated AgNPs on lignin-modified magnetic nanoparticles mediated by Cydonia on cecal ligation and puncture-induced sepsis
- Treatment of gastric cancer by green mediated silver nanoparticles using Pistacia atlantica bark aqueous extract
- Preparation of newly developed porcelain ceramics containing WO3 nanoparticles for radiation shielding applications
- Utilization of computational methods for the identification of new natural inhibitors of human neutrophil elastase in inflammation therapy
- Some anticancer agents as effective glutathione S-transferase (GST) inhibitors
- Clay-based bricks’ rich illite mineral for gamma-ray shielding applications: An experimental evaluation of the effect of pressure rates on gamma-ray attenuation parameters
- Stability kinetics of orevactaene pigments produced by Epicoccum nigrum in solid-state fermentation
- Treatment of denture stomatitis using iron nanoparticles green-synthesized by Silybum marianum extract
- Characterization and antioxidant potential of white mustard (Brassica hirta) leaf extract and stabilization of sunflower oil
- Characteristics of Langmuir monomolecular monolayers formed by the novel oil blends
- Strategies for optimizing the single GdSrFeO4 phase synthesis
- Oleic acid and linoleic acid nanosomes boost immunity and provoke cell death via the upregulation of beta-defensin-4 at genetic and epigenetic levels
- Unraveling the therapeutic potential of Bombax ceiba roots: A comprehensive study of chemical composition, heavy metal content, antibacterial activity, and in silico analysis
- Green synthesis of AgNPs using plant extract and investigation of its anti-human colorectal cancer application
- The adsorption of naproxen on adsorbents obtained from pepper stalk extract by green synthesis
- Treatment of gastric cancer by silver nanoparticles encapsulated by chitosan polymers mediated by Pistacia atlantica extract under ultrasound condition
- In vitro protective and anti-inflammatory effects of Capparis spinosa and its flavonoids profile
- Wear and corrosion behavior of TiC and WC coatings deposited on high-speed steels by electro-spark deposition
- Therapeutic effects of green-formulated gold nanoparticles by Origanum majorana on spinal cord injury in rats
- Melanin antibacterial activity of two new strains, SN1 and SN2, of Exophiala phaeomuriformis against five human pathogens
- Evaluation of the analgesic and anesthetic properties of silver nanoparticles supported over biodegradable acacia gum-modified magnetic nanoparticles
- Review Articles
- Role and mechanism of fruit waste polyphenols in diabetes management
- A comprehensive review of non-alkaloidal metabolites from the subfamily Amaryllidoideae (Amaryllidaceae)
- Discovery of the chemical constituents, structural characteristics, and pharmacological functions of Chinese caterpillar fungus
- Eco-friendly green approach of nickel oxide nanoparticles for biomedical applications
- Advances in the pharmaceutical research of curcumin for oral administration
- Rapid Communication
- Determination of the contents of bioactive compounds in St. John’s wort (Hypericum perforatum): Comparison of commercial and wild samples
- Retraction
- Retraction of “Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: The protective effect on periodontitis via reducing the release of IL-1β and TNF-α”
- Topical Issue on Phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Anti-plasmodial potential of selected medicinal plants and a compound Atropine isolated from Eucalyptus obliqua
- Anthocyanin extract from black rice attenuates chronic inflammation in DSS-induced colitis mouse model by modulating the gut microbiota
- Evaluation of antibiofilm and cytotoxicity effect of Rumex vesicarius methanol extract
- Chemical compositions of Litsea umbellata and inhibition activities
- Green synthesis, characterization of silver nanoparticles using Rhynchosia capitata leaf extract and their biological activities
- GC-MS analysis and antibacterial activities of some plants belonging to the genus Euphorbia on selected bacterial isolates
- The abrogative effect of propolis on acrylamide-induced toxicity in male albino rats: Histological study
- A phytoconstituent 6-aminoflavone ameliorates lipopolysaccharide-induced oxidative stress mediated synapse and memory dysfunction via p-Akt/NF-kB pathway in albino mice
- Anti-diabetic potentials of Sorbaria tomentosa Lindl. Rehder: Phytochemistry (GC-MS analysis), α-amylase, α-glucosidase inhibitory, in vivo hypoglycemic, and biochemical analysis
- Assessment of cytotoxic and apoptotic activities of the Cassia angustifolia aqueous extract against SW480 colon cancer
- Biochemical analysis, antioxidant, and antibacterial efficacy of the bee propolis extract (Hymenoptera: Apis mellifera) against Staphylococcus aureus-induced infection in BALB/c mice: In vitro and in vivo study
- Assessment of essential elements and heavy metals in Saudi Arabian rice samples underwent various processing methods
- Two new compounds from leaves of Capparis dongvanensis (Sy, B. H. Quang & D. V. Hai) and inhibition activities
- Hydroxyquinoline sulfanilamide ameliorates STZ-induced hyperglycemia-mediated amyleoid beta burden and memory impairment in adult mice
- An automated reading of semi-quantitative hemagglutination results in microplates: Micro-assay for plant lectins
- Inductively coupled plasma mass spectrometry assessment of essential and toxic trace elements in traditional spices consumed by the population of the Middle Eastern region in their recipes
- Phytochemical analysis and anticancer activity of the Pithecellobium dulce seed extract in colorectal cancer cells
- Impact of climatic disturbances on the chemical compositions and metabolites of Salvia officinalis
- Physicochemical characterization, antioxidant and antifungal activities of essential oils of Urginea maritima and Allium sativum
- Phytochemical analysis and antifungal efficiency of Origanum majorana extracts against some phytopathogenic fungi causing tomato damping-off diseases
- Special Issue on 4th IC3PE
- Graphene quantum dots: A comprehensive overview
- Studies on the intercalation of calcium–aluminium layered double hydroxide-MCPA and its controlled release mechanism as a potential green herbicide
- Synergetic effect of adsorption and photocatalysis by zinc ferrite-anchored graphitic carbon nitride nanosheet for the removal of ciprofloxacin under visible light irradiation
- Exploring anticancer activity of the Indonesian guava leaf (Psidium guajava L.) fraction on various human cancer cell lines in an in vitro cell-based approach
- The comparison of gold extraction methods from the rock using thiourea and thiosulfate
- Special Issue on Marine environmental sciences and significance of the multidisciplinary approaches
- Sorption of alkylphenols and estrogens on microplastics in marine conditions
- Cytotoxic ketosteroids from the Red Sea soft coral Dendronephthya sp.
- Antibacterial and biofilm prevention metabolites from Acanthophora spicifera
- Characteristics, source, and health risk assessment of aerosol polyaromatic hydrocarbons in the rural and urban regions of western Saudi Arabia
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part II
- Green synthesis, characterization, and evaluation of antibacterial activities of cobalt nanoparticles produced by marine fungal species Periconia prolifica
- Combustion-mediated sol–gel preparation of cobalt-doped ZnO nanohybrids for the degradation of acid red and antibacterial performance
- Perinatal supplementation with selenium nanoparticles modified with ascorbic acid improves hepatotoxicity in rat gestational diabetes
- Evaluation and chemical characterization of bioactive secondary metabolites from endophytic fungi associated with the ethnomedicinal plant Bergenia ciliata
- Enhancing photovoltaic efficiency with SQI-Br and SQI-I sensitizers: A comparative analysis
- Nanostructured p-PbS/p-CuO sulfide/oxide bilayer heterojunction as a promising photoelectrode for hydrogen gas generation
Artikel in diesem Heft
- Characteristics, source, and health risk assessment of aerosol polyaromatic hydrocarbons in the rural and urban regions of western Saudi Arabia
- Regular Articles
- A network-based correlation research between element electronegativity and node importance
- Pomegranate attenuates kidney injury in cyclosporine-induced nephrotoxicity in rats by suppressing oxidative stress
- Ab initio study of fundamental properties of XInO3 (X = K, Rb, Cs) perovskites
- Responses of feldspathic sandstone and sand-reconstituted soil C and N to freeze–thaw cycles
- Robust fractional control based on high gain observers design (RNFC) for a Spirulina maxima culture interfaced with an advanced oxidation process
- Study on arsenic speciation and redistribution mechanism in Lonicera japonica plants via synchrotron techniques
- Optimization of machining Nilo 36 superalloy parameters in turning operation
- Vacuum impregnation pre-treatment: A novel method for incorporating mono- and divalent cations into potato strips to reduce the acrylamide formation in French fries
- Characterization of effective constituents in Acanthopanax senticosus fruit for blood deficiency syndrome based on the chinmedomics strategy
- Comparative analysis of the metabolites in Pinellia ternata from two producing regions using ultra-high-performance liquid chromatography–electrospray ionization–tandem mass spectrometry
- The assessment of environmental parameter along the desalination plants in the Kingdom of Saudi Arabia
- Effects of harpin and carbendazim on antioxidant accumulation in young jujube leaves
- The effects of in ovo injected with sodium borate on hatching performance and small intestine morphology in broiler chicks
- Optimization of cutting forces and surface roughness via ANOVA and grey relational analysis in machining of In718
- Essential oils of Origanum compactum Benth: Chemical characterization, in vitro, in silico, antioxidant, and antibacterial activities
- Translocation of tungsten(vi) oxide/gadolinium(iii) fluoride in tellurite glasses towards improvement of gamma-ray attenuation features in high-density glass shields
- Mechanical properties, elastic moduli, and gamma ray attenuation competencies of some TeO2–WO3–GdF3 glasses: Tailoring WO3–GdF3 substitution toward optimum behavioral state range
- Comparison between the CIDR or sponge with hormone injection to induce estrus synchronization for twining and sex preselection in Naimi sheep
- Exergetic performance analyses of three different cogeneration plants
- Psoralea corylifolia (babchi) seeds enhance proliferation of normal human cultured melanocytes: GC–MS profiling and biological investigation
- A novel electrochemical micro-titration method for quantitative evaluation of the DPPH free radical scavenging capacity of caffeic acid
- Comparative study between supported bimetallic catalysts for nitrate remediation in water
- Persicaline, an alkaloid from Salvadora persica, inhibits proliferation and induces apoptosis and cell-cycle arrest in MCF-7 cells
- Determination of nicotine content in locally produced smokeless tobacco (Shammah) samples from Jazan region of Saudi Arabia using a convenient HPLC-MS/MS method
- Changes in oxidative stress markers in pediatric burn injury over a 1-week period
- Integrated geophysical techniques applied for petroleum basins structural characterization in the central part of the Western Desert, Egypt
- The impact of chemical modifications on gamma-ray attenuation properties of some WO3-reinforced tellurite glasses
- Microwave and Cs+-assisted chemo selective reaction protocol for synthesizing 2-styryl quinoline biorelevant molecules
- Structural, physical, and radiation absorption properties of a significant nuclear power plant component: A comparison between REX-734 and 316L SS austenitic stainless steels
- Effect of Moringa oleifera on serum YKL-40 level: In vivo rat periodontitis model
- Investigating the impact of CO2 emissions on the COVID-19 pandemic by generalized linear mixed model approach with inverse Gaussian and gamma distributions
- Influence of WO3 content on gamma rays attenuation characteristics of phosphate glasses at low energy range
- Study on CO2 absorption performance of ternary DES formed based on DEA as promoting factor
- Performance analyses of detonation engine cogeneration cycles
- Sterols from Centaurea pumilio L. with cell proliferative activity: In vitro and in silico studies
- Untargeted metabolomics revealing changes in aroma substances in flue-cured tobacco
- Effect of pumpkin enriched with calcium lactate on iron status in an animal model of postmenopausal osteoporosis
- Energy consumption, mechanical and metallographic properties of cryogenically treated tool steels
- Optimization of ultra-high pressure-assisted extraction of total phenols from Eucommia ulmoides leaves by response surface methodology
- Harpin enhances antioxidant nutrient accumulation and decreases enzymatic browning in stored soybean sprouts
- Physicochemical and biological properties of carvacrol
- Radix puerariae in the treatment of diabetic nephropathy: A network pharmacology analysis and experimental validation
- Anti-Alzheimer, antioxidants, glucose-6-phosphate dehydrogenase effects of Taverniera glabra mediated ZnO and Fe2O3 nanoparticles in alloxan-induced diabetic rats
- Experimental study on photocatalytic CO2 reduction performance of ZnS/CdS-TiO2 nanotube array thin films
- Epoxy-reinforced heavy metal oxides for gamma ray shielding purposes
- Black mulberry (Morus nigra L.) fruits: As a medicinal plant rich in human health-promoting compounds
- Promising antioxidant and antimicrobial effects of essential oils extracted from fruits of Juniperus thurifera: In vitro and in silico investigations
- Chloramine-T-induced oxidation of Rizatriptan Benzoate: An integral chemical and spectroscopic study of products, mechanisms and kinetics
- Study on antioxidant and antimicrobial potential of chemically profiled essential oils extracted from Juniperus phoenicea (L.) by use of in vitro and in silico approaches
- Screening and characterization of fungal taxol-producing endophytic fungi for evaluation of antimicrobial and anticancer activities
- Mineral composition, principal polyphenolic components, and evaluation of the anti-inflammatory, analgesic, and antioxidant properties of Cytisus villosus Pourr leaf extracts
- In vitro antiproliferative efficacy of Annona muricata seed and fruit extracts on several cancer cell lines
- An experimental study for chemical characterization of artificial anterior cruciate ligament with coated chitosan as biomaterial
- Prevalence of residual risks of the transfusion-transmitted infections in Riyadh hospitals: A two-year retrospective study
- Computational and experimental investigation of antibacterial and antifungal properties of Nicotiana tabacum extracts
- Reinforcement of cementitious mortars with hemp fibers and shives
- X-ray shielding properties of bismuth-borate glass doped with rare earth ions
- Green supported silver nanoparticles over modified reduced graphene oxide: Investigation of its antioxidant and anti-ovarian cancer effects
- Orthogonal synthesis of a versatile building block for dual functionalization of targeting vectors
- Thymbra spicata leaf extract driven biogenic synthesis of Au/Fe3O4 nanocomposite and its bio-application in the treatment of different types of leukemia
- The role of Ag2O incorporation in nuclear radiation shielding behaviors of the Li2O–Pb3O4–SiO2 glass system: A multi-step characterization study
- A stimuli-responsive in situ spray hydrogel co-loaded with naringenin and gentamicin for chronic wounds
- Assessment of the impact of γ-irradiation on the piperine content and microbial quality of black pepper
- Antioxidant, sensory, and functional properties of low-alcoholic IPA beer with Pinus sylvestris L. shoots addition fermented using unconventional yeast
- Screening and optimization of extracellular pectinase produced by Bacillus thuringiensis SH7
- Determination of polyphenols in Chinese jujube using ultra-performance liquid chromatography–mass spectrometry
- Synergistic effects of harpin and NaCl in determining soybean sprout quality under non-sterile conditions
- Field evaluation of different eco-friendly alternative control methods against Panonychus citri [Acari: Tetranychidae] spider mite and its predators in citrus orchards
- Exploring the antimicrobial potential of biologically synthesized zero valent iron nanoparticles
- NaCl regulates goldfish growth and survival at three food supply levels under hypoxia
- An exploration of the physical, optical, mechanical, and radiation shielding properties of PbO–MgO–ZnO–B2O3 glasses
- A novel statistical modeling of air pollution and the COVID-19 pandemic mortality data by Poisson, geometric, and negative binomial regression models with fixed and random effects
- Treatment activity of the injectable hydrogels loaded with dexamethasone In(iii) complex on glioma by inhibiting the VEGF signaling pathway
- An alternative approach for the excess lifetime cancer risk and prediction of radiological parameters
- Panax ginseng leaf aqueous extract mediated green synthesis of AgNPs under ultrasound condition and investigation of its anti-lung adenocarcinoma effects
- Study of hydrolysis and production of instant ginger (Zingiber officinale) tea
- Novel green synthesis of zinc oxide nanoparticles using Salvia rosmarinus extract for treatment of human lung cancer
- Evaluation of second trimester plasma lipoxin A4, VEGFR-1, IL-6, and TNF-α levels in pregnant women with gestational diabetes mellitus
- Antidiabetic, antioxidant and cytotoxicity activities of ortho- and para-substituted Schiff bases derived from metformin hydrochloride: Validation by molecular docking and in silico ADME studies
- Antioxidant, antidiabetic, antiglaucoma, and anticholinergic effects of Tayfi grape (Vitis vinifera): A phytochemical screening by LC-MS/MS analysis
- Identification of genetic polymorphisms in the stearoyl CoA desaturase gene and its association with milk quality traits in Najdi sheep
- Cold-acclimation effect on cadmium absorption and biosynthesis of polyphenolics, and free proline and photosynthetic pigments in Spirogyra aequinoctialis
- Analysis of secondary metabolites in Xinjiang Morus nigra leaves using different extraction methods with UPLC-Q/TOF-MS/MS technology
- Nanoarchitectonics and performance evaluation of a Fe3O4-stabilized Pickering emulsion-type differential pressure plugging agent
- Investigating pyrolysis characteristics of Shengdong coal through Py-GC/MS
- Extraction, phytochemical characterization, and antifungal activity of Salvia rosmarinus extract
- Introducing a novel and natural antibiotic for the treatment of oral pathogens: Abelmoschus esculentus green-formulated silver nanoparticles
- Optimization of gallic acid-enriched ultrasonic-assisted extraction from mango peels
- Effect of gamma rays irradiation in the structure, optical, and electrical properties of samarium doped bismuth titanate ceramics
- Combinatory in silico investigation for potential inhibitors from Curcuma sahuynhensis Škorničk. & N.S. Lý volatile phytoconstituents against influenza A hemagglutinin, SARS-CoV-2 main protease, and Omicron-variant spike protein
- Physical, mechanical, and gamma ray shielding properties of the Bi2O3–BaO–B2O3–ZnO–As2O3–MgO–Na2O glass system
- Twofold interpenetrated 3D Cd(ii) complex: Crystal structure and luminescent property
- Study on the microstructure and soil quality variation of composite soil with soft rock and sand
- Ancient spring waters still emerging and accessible in the Roman Forum area: Chemical–physical and microbiological characterization
- Extraction and characterization of type I collagen from scales of Mexican Biajaiba fish
- Finding small molecular compounds to decrease trimethylamine oxide levels in atherosclerosis by virtual screening
- Prefatory in silico studies and in vitro insecticidal effect of Nigella sativa (L.) essential oil and its active compound (carvacrol) against the Callosobruchus maculatus adults (Fab), a major pest of chickpea
- Polymerized methyl imidazole silver bromide (CH3C6H5AgBr)6: Synthesis, crystal structures, and catalytic activity
- Using calcined waste fish bones as a green solid catalyst for biodiesel production from date seed oil
- Influence of the addition of WO3 on TeO2–Na2O glass systems in view of the feature of mechanical, optical, and photon attenuation
- Naringin ameliorates 5-fluorouracil elicited neurotoxicity by curtailing oxidative stress and iNOS/NF-ĸB/caspase-3 pathway
- GC-MS profile of extracts of an endophytic fungus Alternaria and evaluation of its anticancer and antibacterial potentialities
- Green synthesis, chemical characterization, and antioxidant and anti-colorectal cancer effects of vanadium nanoparticles
- Determination of caffeine content in coffee drinks prepared in some coffee shops in the local market in Jeddah City, Saudi Arabia
- A new 3D supramolecular Cu(ii) framework: Crystal structure and photocatalytic characteristics
- Bordeaux mixture accelerates ripening, delays senescence, and promotes metabolite accumulation in jujube fruit
- Important application value of injectable hydrogels loaded with omeprazole Schiff base complex in the treatment of pancreatitis
- Color tunable benzothiadiazole-based small molecules for lightening applications
- Investigation of structural, dielectric, impedance, and mechanical properties of hydroxyapatite-modified barium titanate composites for biomedical applications
- Metal gel particles loaded with epidermal cell growth factor promote skin wound repair mechanism by regulating miRNA
- In vitro exploration of Hypsizygus ulmarius (Bull.) mushroom fruiting bodies: Potential antidiabetic and anti-inflammatory agent
- Alteration in the molecular structure of the adenine base exposed to gamma irradiation: An ESR study
- Comprehensive study of optical, thermal, and gamma-ray shielding properties of Bi2O3–ZnO–PbO–B2O3 glasses
- Lewis acids as co-catalysts in Pd-based catalyzed systems of the octene-1 hydroethoxycarbonylation reaction
- Synthesis, Hirshfeld surface analysis, thermal, and selective α-glucosidase inhibitory studies of Schiff base transition metal complexes
- Protective properties of AgNPs green-synthesized by Abelmoschus esculentus on retinal damage on the virtue of its anti-inflammatory and antioxidant effects in diabetic rat
- Effects of green decorated AgNPs on lignin-modified magnetic nanoparticles mediated by Cydonia on cecal ligation and puncture-induced sepsis
- Treatment of gastric cancer by green mediated silver nanoparticles using Pistacia atlantica bark aqueous extract
- Preparation of newly developed porcelain ceramics containing WO3 nanoparticles for radiation shielding applications
- Utilization of computational methods for the identification of new natural inhibitors of human neutrophil elastase in inflammation therapy
- Some anticancer agents as effective glutathione S-transferase (GST) inhibitors
- Clay-based bricks’ rich illite mineral for gamma-ray shielding applications: An experimental evaluation of the effect of pressure rates on gamma-ray attenuation parameters
- Stability kinetics of orevactaene pigments produced by Epicoccum nigrum in solid-state fermentation
- Treatment of denture stomatitis using iron nanoparticles green-synthesized by Silybum marianum extract
- Characterization and antioxidant potential of white mustard (Brassica hirta) leaf extract and stabilization of sunflower oil
- Characteristics of Langmuir monomolecular monolayers formed by the novel oil blends
- Strategies for optimizing the single GdSrFeO4 phase synthesis
- Oleic acid and linoleic acid nanosomes boost immunity and provoke cell death via the upregulation of beta-defensin-4 at genetic and epigenetic levels
- Unraveling the therapeutic potential of Bombax ceiba roots: A comprehensive study of chemical composition, heavy metal content, antibacterial activity, and in silico analysis
- Green synthesis of AgNPs using plant extract and investigation of its anti-human colorectal cancer application
- The adsorption of naproxen on adsorbents obtained from pepper stalk extract by green synthesis
- Treatment of gastric cancer by silver nanoparticles encapsulated by chitosan polymers mediated by Pistacia atlantica extract under ultrasound condition
- In vitro protective and anti-inflammatory effects of Capparis spinosa and its flavonoids profile
- Wear and corrosion behavior of TiC and WC coatings deposited on high-speed steels by electro-spark deposition
- Therapeutic effects of green-formulated gold nanoparticles by Origanum majorana on spinal cord injury in rats
- Melanin antibacterial activity of two new strains, SN1 and SN2, of Exophiala phaeomuriformis against five human pathogens
- Evaluation of the analgesic and anesthetic properties of silver nanoparticles supported over biodegradable acacia gum-modified magnetic nanoparticles
- Review Articles
- Role and mechanism of fruit waste polyphenols in diabetes management
- A comprehensive review of non-alkaloidal metabolites from the subfamily Amaryllidoideae (Amaryllidaceae)
- Discovery of the chemical constituents, structural characteristics, and pharmacological functions of Chinese caterpillar fungus
- Eco-friendly green approach of nickel oxide nanoparticles for biomedical applications
- Advances in the pharmaceutical research of curcumin for oral administration
- Rapid Communication
- Determination of the contents of bioactive compounds in St. John’s wort (Hypericum perforatum): Comparison of commercial and wild samples
- Retraction
- Retraction of “Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: The protective effect on periodontitis via reducing the release of IL-1β and TNF-α”
- Topical Issue on Phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Anti-plasmodial potential of selected medicinal plants and a compound Atropine isolated from Eucalyptus obliqua
- Anthocyanin extract from black rice attenuates chronic inflammation in DSS-induced colitis mouse model by modulating the gut microbiota
- Evaluation of antibiofilm and cytotoxicity effect of Rumex vesicarius methanol extract
- Chemical compositions of Litsea umbellata and inhibition activities
- Green synthesis, characterization of silver nanoparticles using Rhynchosia capitata leaf extract and their biological activities
- GC-MS analysis and antibacterial activities of some plants belonging to the genus Euphorbia on selected bacterial isolates
- The abrogative effect of propolis on acrylamide-induced toxicity in male albino rats: Histological study
- A phytoconstituent 6-aminoflavone ameliorates lipopolysaccharide-induced oxidative stress mediated synapse and memory dysfunction via p-Akt/NF-kB pathway in albino mice
- Anti-diabetic potentials of Sorbaria tomentosa Lindl. Rehder: Phytochemistry (GC-MS analysis), α-amylase, α-glucosidase inhibitory, in vivo hypoglycemic, and biochemical analysis
- Assessment of cytotoxic and apoptotic activities of the Cassia angustifolia aqueous extract against SW480 colon cancer
- Biochemical analysis, antioxidant, and antibacterial efficacy of the bee propolis extract (Hymenoptera: Apis mellifera) against Staphylococcus aureus-induced infection in BALB/c mice: In vitro and in vivo study
- Assessment of essential elements and heavy metals in Saudi Arabian rice samples underwent various processing methods
- Two new compounds from leaves of Capparis dongvanensis (Sy, B. H. Quang & D. V. Hai) and inhibition activities
- Hydroxyquinoline sulfanilamide ameliorates STZ-induced hyperglycemia-mediated amyleoid beta burden and memory impairment in adult mice
- An automated reading of semi-quantitative hemagglutination results in microplates: Micro-assay for plant lectins
- Inductively coupled plasma mass spectrometry assessment of essential and toxic trace elements in traditional spices consumed by the population of the Middle Eastern region in their recipes
- Phytochemical analysis and anticancer activity of the Pithecellobium dulce seed extract in colorectal cancer cells
- Impact of climatic disturbances on the chemical compositions and metabolites of Salvia officinalis
- Physicochemical characterization, antioxidant and antifungal activities of essential oils of Urginea maritima and Allium sativum
- Phytochemical analysis and antifungal efficiency of Origanum majorana extracts against some phytopathogenic fungi causing tomato damping-off diseases
- Special Issue on 4th IC3PE
- Graphene quantum dots: A comprehensive overview
- Studies on the intercalation of calcium–aluminium layered double hydroxide-MCPA and its controlled release mechanism as a potential green herbicide
- Synergetic effect of adsorption and photocatalysis by zinc ferrite-anchored graphitic carbon nitride nanosheet for the removal of ciprofloxacin under visible light irradiation
- Exploring anticancer activity of the Indonesian guava leaf (Psidium guajava L.) fraction on various human cancer cell lines in an in vitro cell-based approach
- The comparison of gold extraction methods from the rock using thiourea and thiosulfate
- Special Issue on Marine environmental sciences and significance of the multidisciplinary approaches
- Sorption of alkylphenols and estrogens on microplastics in marine conditions
- Cytotoxic ketosteroids from the Red Sea soft coral Dendronephthya sp.
- Antibacterial and biofilm prevention metabolites from Acanthophora spicifera
- Characteristics, source, and health risk assessment of aerosol polyaromatic hydrocarbons in the rural and urban regions of western Saudi Arabia
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part II
- Green synthesis, characterization, and evaluation of antibacterial activities of cobalt nanoparticles produced by marine fungal species Periconia prolifica
- Combustion-mediated sol–gel preparation of cobalt-doped ZnO nanohybrids for the degradation of acid red and antibacterial performance
- Perinatal supplementation with selenium nanoparticles modified with ascorbic acid improves hepatotoxicity in rat gestational diabetes
- Evaluation and chemical characterization of bioactive secondary metabolites from endophytic fungi associated with the ethnomedicinal plant Bergenia ciliata
- Enhancing photovoltaic efficiency with SQI-Br and SQI-I sensitizers: A comparative analysis
- Nanostructured p-PbS/p-CuO sulfide/oxide bilayer heterojunction as a promising photoelectrode for hydrogen gas generation

