Abstract
Objective
The differences in the chemical composition of Morus nigra (M. nigra) extracts from four different extraction methods, ultrasound-assisted extraction with pure water (WU), pure water decoction extraction (WD), ultrasonic-assisted extraction with formic acid water (FAU), and pure water heat reflux extraction (WHR), were identified using ultra-high performance liquid chromatography-quadrupole time-of-flight tandem mass spectrometry (UPLC-Q-TOF-MS/MS) technology.
Method
A Waters ACQUITY BEH C18 (1.7 μm, 2.1 mm × 100 mm) was used, with a column temperature of 45°C, mobile phase of methanol and 0.1% formic acid aqueous solution, and gradient elution with a flow rate of 0.4 mL/min. Detection was performed in positive and negative ion modes, and compounds were identified using Progenesis QI software and mass spectrometry data reported according to the literature and laboratory self-built databases of the Mulberry genus. Multivariate statistical techniques, such as principal component analysis and orthogonal partial least squares-discriminant analysis, were applied to differential cluster metabolic profiles and chemical components and to screen the differential chemical components of M. nigra leaves.
Results
There were significant differences in the chemical composition between WD and the other extraction methods of M. nigra leaves. A total of 13 differential metabolites (4 flavonoids, 3 organic acids, 3 phenylpropanoids, 2 alkaloids, and 1 trisaccharide) were identified.
Conclusion
The multivariate statistical analysis and UPLC-Q-TOF-MS/MS method established in this study identified the differential chemical constituents of Xinjiang M. nigra leaves using different extraction methods, which provides a basis for the quality control of M. nigra leaves, and provides basic data for revealing the influence of extraction methods on the synthesis and accumulation of M. nigra leaf metabolites, which has certain reference significance.
Abbreviations
- DNJ
-
1-deoxynojirimycin
- FAU
-
ultrasonic-assisted extraction with formic acid water
- OPLS-DA
-
orthogonal partial least squares-discrimination analysis
- PCA
-
principal component analysis
- UPLC-Q/TOF-MS
-
ultra-high performance liquid chromatography-quadrupole time-of-flight tandem mass spectrometry
- WD
-
pure water decoction extraction
- WHR
-
pure water heat to reflux extraction
- WU
-
ultrasound-assisted extraction with pure water
1 Introduction
Mulberry leaves are dried leaves of Morus alba L. or Morus nigra L. (M. nigra), members of the Moraceae family. Mulberry leaves have a wide range of pharmacological effects, including antibacterial [1], hypolipidemic [2], hypoglycemic [2,3], anti-inflammatory [4], anti-aging [5], and anti-tumor [6] effects. Modern studies have found that mulberry leaves contain flavonoids, steroids, alkaloids, amino acids, organic acids, phenylpropanoids, and other components [7]. As a widely planted mulberry variety in Xinjiang, China, M. nigra is an important supplement and the source of medicinal mulberry leaves.
At present, the research on M. nigra leaves mainly focuses on the pharmacological effects of single chemical components [8] or pharmacodynamic evaluation of crude extracts [4,6,9,10], and there is no in-depth study on the components and contents of mulberry leaf extracts in different extraction solvents. The study of chemical constituents of traditional Chinese medicine is premised on clarifying pharmacological actions, mechanism of actions, and clinical efficacy, and it is of great reference value to analyze and compare the differential chemical constituents of M. nigra leaves using different extraction methods based on plant metabolomic techniques (Table 1).
Metabolomics analysis
| Methods | Advantages | Disadvantages | References |
|---|---|---|---|
| Gas chromatography–mass spectrometry | High resolution, high sensitivity, a large number of reference databases | Pretreatment such as derivatization is required; compounds with poor thermal stability and high molecular weight are not suitable | [11,12,13] |
| Liquid chromatography–mass spectrometry | High resolution, fast analysis speed, and high sensitivity, suitable for the detection of high boiling point, thermally unstable, and high molecular weight compounds | — | [14,15,16] |
| Capillary electrophoresis–mass spectrometry | Analysis of ionic compounds; samples do not need to be derivatized | Separation analysis and micro samples and specific uses; not well-used in plant research | [17,18] |
| Nuclear magnetic resonance | Simple pre-processing; low sample usage; the amount of information that can be measured is large | The detection sensitivity is low and the detection range is narrow, which cannot realize the detection of trace substances | [19,20] |
Extensive untargeted metabolomics analysis based on ultra-high performance liquid chromatography-quadrupole time-of-flight tandem mass spectrometry (UPLC-Q-TOF-MS/MS) is a rapid and reliable method for the detection of metabolites in a variety of plant medicinal herbs [21]. In this study, UPLC-Q-TOF-MS/MS technology was used for non-targeted analysis to more comprehensively evaluate M. nigra leaves using different extraction methods. In addition, statistical analysis was performed to identify and quantify signature compounds and to provide the basis for quality control (QC) of M. nigra leaves.
2 Materials and methods
2.1 Materials and reagents
Sample: M. nigra leaves, collected in 2021 from Xinhe County, Aksu City, Xinjiang, China, were naturally dried and stored in the laboratory for later use.
Reagents: Purified water was prepared by purification with Milli-Q. Methanol, acetonitrile, and formic acid were of MS grade (Thermo Fisher Scientific China Ltd). The purity of the 1-deoxynojirimycin (DNJ) standard (lot number ZZS-20-052-A4, specification 25 mg) and fagomine standard (lot number 21A029-A1, specification 2 mg) was >98%, and the standards were purchased from Shanghai ZZBIO Co., Ltd.
2.2 Instruments and equipment
The instruments and equipment used in this study included ultra-high performance phase quadrupole time-of-flight mass spectrometer (Waters, USA); SW23 water bath (Julabo, Germany); desktop low-temperature high-speed centrifuge (Thermo, USA); ultrapure water system (Millipore, USA); KQ-600E CNC ultrasonic cleaner (Jiangsu Kunshan Ultrasonic Instrument Co., Ltd); analytical balance (Mettler-Toledo Instruments Shanghai Co., Ltd); and a QL-866 rapid mixer (Haimen Kylin-Bell Lab Instruments).
2.3 Sample preparation procedures
2.3.1 Ultrasound-assisted extraction
Extractions with solvents of pure water, 40% ethanol, 70% ethanol, and 95% ethanol were investigated. Briefly, 1 g of M. nigra leaf powder was weighed and added to 20 mL of extract solvent in a 50 mL conical flask with a stopper. The bottle was placed into an ultrasonic cleaner for the extraction process carried out under the following experimental conditions: ultrasonic temperature 25°C, ultrasonic time 30 min, and material ratio 1:20 (w/v, sample powder/purified water). The sample was filtered after extraction, and 1 mL of filtrate was diluted to 10 mL with methanol in a volumetric flask. After shaking, the mixture was centrifuged at 10,000 rpm for 5 min. Each sample was extracted three times in parallel.
2.3.2 Pure water decoction extraction
This referred to the method of Deng et al. [22] with a slight modification. Briefly, 10 g M. nigra leaves were weighed in a round-bottomed flask. Then, 200 mL of purified water was added for a material ratio of 1:20 (w/v, sample/purified water), followed by decoction and extraction in a 95°C water bath for 2 h. Each extract was filtered and fixed to 150 mL, then pipetted into a 0.05 mL to 2 mL volumetric flask, and the volume was fixed with methanol. Each sample was extracted three times in parallel. This method was denoted as WD.
2.3.3 Ultrasonic-assisted extraction with formic acid water
The laboratory established methods were as follows. Briefly, 1 g of M. nigra leaf powder was weighed, 10 mL of 70% methanol and 0.05% formic acid water was added to a 25 mL stoppered Erlenmeyer flask with the powder, and then the bottle was placed into an ultrasonic cleaner for the extraction process. This was carried out under the following experimental conditions: the ultrasonic temperature was 25°C, the ultrasonic processing time was 10 min, and the material ratio was 1:10 (w/v, sample powder/70% methanol + 0.05% formic acid water). Next the extracted sample was moved to a centrifuge tube and centrifuged at 10,000 rpm for 5 min, and 0.2 mL of the supernatant was placed in a 2 mL volumetric flask. The volume was fixed with methanol and mixed well. Each sample was extracted three times in parallel. This method was denoted as FAU.
2.3.4 Heat reflux extraction
According to the existing methods of the laboratory, the extraction of solvent pure water and 90% ethanol was investigated. First, 100 g M. nigra leaves were weighed in a 2,000 mL round-bottomed flask, and 1,000 mL of extract solvent was added for a material ratio of 1:10 (w/v, sample/purified water). Reflux extraction was performed with an electric heating jacket for 2 h, the filtrate was filtered with gauze, and 1,000 mL of purified water was added to a round-bottomed flask containing the filter residue. Reflux extraction was performed for 2 h, during which the filtrate was combined with the first filtrate. The filtrate was concentrated using a pressure reducing device to generate an extract. The extract was heated to remove the remaining solvent to obtain 39 g of water-extracted extract. Then, weigh 0.1 g of extract into a 2 mL volumetric flask, and fix the volume to the scale with 70% methanol, mixed, and centrifuged at 10,000 rpm for 5 min. Then, 0.05 mL of the supernatant was placed in a 2 mL volumetric flask, and the volume was set with methanol and mixed. Each sample was extracted three times in parallel.
2.3.5 QC sample
Briefly, 50 μL of each prepared sample was mixed well to prepare the QC sample. The QC sample was used to balance the system by continuously injecting the sample. Inject one QC sample for every four samples when checking the stability of the instrument [23].
2.3.6 Preparation of standard solution
For the standard solution, 2 mg of the reference substance DNJ was accurately weighed and dissolved with 2 mL of 70% acetonitrile. A standard solution of 1 mg/mL was obtained. The standard solution was diluted with mass spectrometry methanol to obtain a 500 ng/mL standard solution.
Next 0.8 mg of the control buckwheat base was weighed and dissolved in 2 mL of 60% acetonitrile. A standard solution of 0.4 mg/mL was obtained. The standard was then diluted with MS-grade methanol to 500 ng/mL of the standard solution.
2.3.7 Chromatographic conditions
The chromatographic column was a Waters ACQUITY BEH C18 (1.7 μm, 2.1 mm × 100 mm). The mobile phase was methanol (A) and a 0.1% formic acid aqueous solution (B). Gradient elution conditions are shown in Table 2. The flow rate was 0.4 mL/min, the column temperature was 45°C, the pool temperature was 4°C, and the injection volume was 2 μL.
Mobile phase gradient elution conditions for samples
| Time (min) | A (%) | B (%) |
|---|---|---|
| 0 | 5 | 95 |
| 3 | 40 | 60 |
| 10 | 95 | 5 |
| 12 | 95 | 5 |
| 13 | 5 | 95 |
| 15 | 5 | 95 |
2.3.8 Mass spectrometry conditions
The ion source was an electrospray ionization (ESI) source in both positive and negative ion modes with MSE and a source temperature of 120°C. The desolvation gas was nitrogen at 800 L/h and 450°C. The capillary voltage was 3 kV in positive mode and 2.5 kV in negative mode. The cone voltage was 50 V and the molecular weight scan range was 100–1,200 Da. The energy at the high energy scan was 15–40 eV. Accurate mass numbers were corrected with a leucine enkephalin (LE) solution at 200 pg/mL. Other parameters were m/z 556.2771 in positive ion mode, 554.2615 in negative ion mode, and acquisition mode was without correction. The operating software for the UPLC-Q-TOF-MS system was Masslynx4.1.
2.3.9 Data processing and analysis
A MassLynx 4.1 workstation controlled the instrument and acquired MS data. The data were processed with Progenesis QI, and the import data format was profile mass spectrum of the high-resolution mass spectrum. LockMass correction was selected. The positive ion was 556.2766, and the negative ion was 554.2620; the adduct ions in the positive ion mode were [M + H]+, [M + Na]+, and [M + H-H2O]+; and the adduct ions in the negative ion mode were [M-H]−, [M + HCOO]−, and [M-H-H2O]−. When data were aligned, the data file with common features among all data was selected as the reference file. At peak extraction, the chromatographic peaks of the buffer column were not analyzed based on the total ion current profile, and the default parameters were selected for the remaining parameters. When samples were grouped, they were grouped according to their experimental design. For compound identification, the compounds with fragment ions were selected first, the database built for mulberry was searched, and the mass error of parent ions and fragment ions was ≤5 ppm. Mapping software used SIMCA-P14.1 and GraphPad Prism 9.2.0, and data were calculated using Excel spreadsheets.
The data matrices of ESI+ and ESI− modes were synthesized and imported into SIMCA-P14.1 software for statistical analysis. The differences in the chemical composition of M. nigra leaf herbs with different extraction methods were visually expressed by preliminarily observing the aggregation of each sample. Each sample was classified via orthogonal partial least squares-discriminant analysis (OPLS-DA) based on principal component analysis (PCA). Specifically, differential indicator components were screened by combining the score scatter plot (S-plot) and variable importance projection (VIP) values from the OPLS-DA model. The software GraphPad Prism 9.2.0 was used to calculate and compare the contents in different extraction methods of M. nigra leaves.
3 Results and discussion
3.1 Optimization of sample preparation methods
Pure water, 40% ethanol, 70% ethanol, and 95% ethanol were selected for the extraction solvent of the ultrasound-assisted extraction method of M. nigra leaves. In the heating and reflux extraction method of M. nigra leaves, 90% ethanol and pure water were selected for the investigation of the extraction solvent, and the sample treatment method was determined through investigation.
For the ultrasound-assisted extraction method, the total ion chromatograms (TIC) of the four different extraction solvents are as shown in Figure 1. The peak shapes were similar with essentially no difference. Compared with the other extraction solvents, pure water ultrasound-assisted extraction (WU) had the highest response value, and pure water as the extraction solvent was more advantageous.
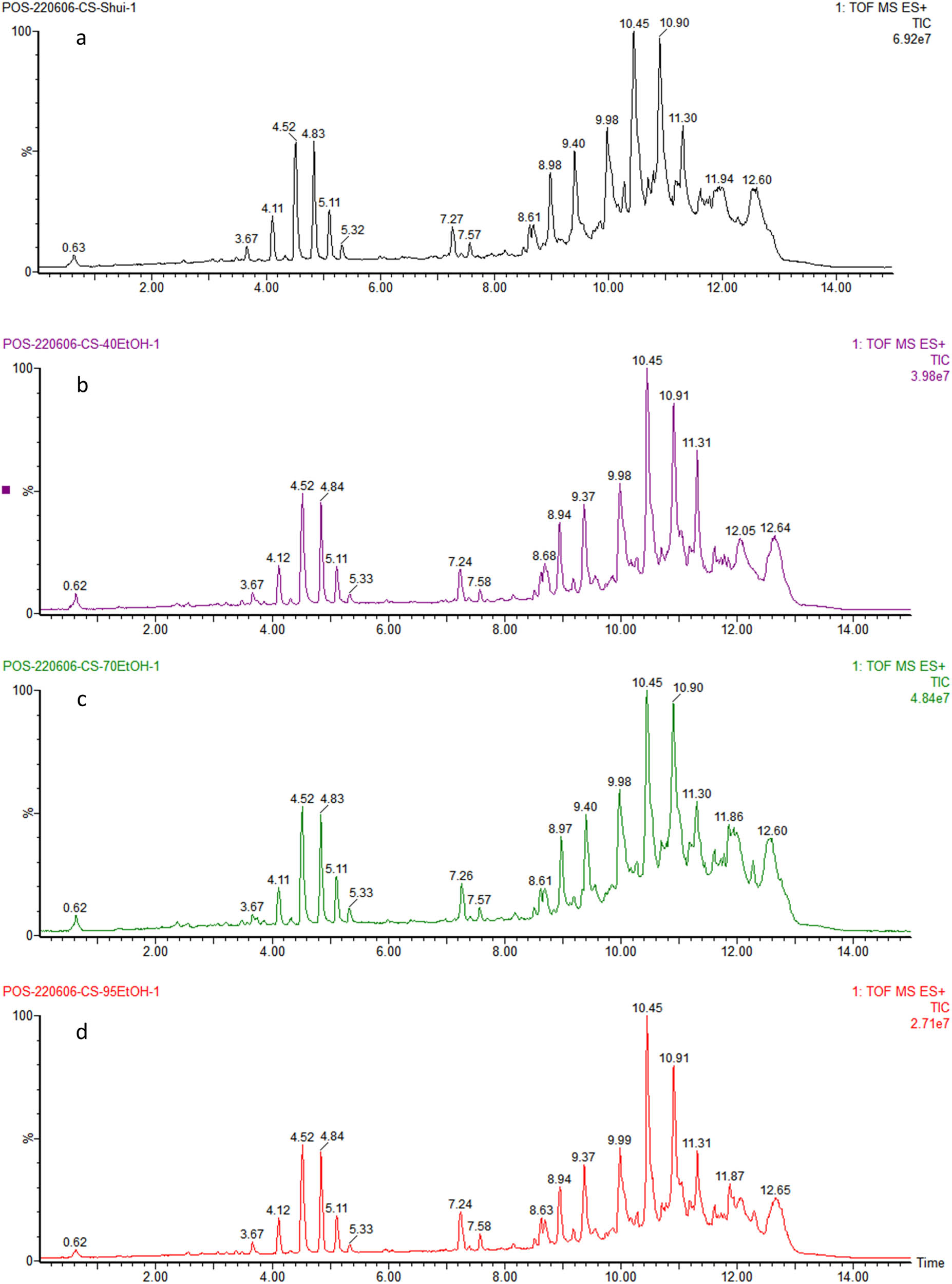
Ultrasound-assisted extraction TICs with different extraction solvents: pure water (a), 40% ethanol (b), 70% ethanol (c), and 95% ethanol (d).
The TIC diagrams of the heat reflux extraction method employing 90% ethanol and water as solvents are compared in Figure 2. The peak shape of the two TIC diagrams was similar, and there is no obvious difference from the response value. The heat reflux extraction method with pure water (WHR) as the extraction solvent was higher and more advantageous.
Heat reflux extraction TICs using different extraction solvents: 90% ethanol (a) and pure water (b).
Pure water was chosen as the extraction solvent for both methods for subsequent experiments.
3.2 QC analysis
Because of the large number of samples analyzed, the TIC of QC samples was visually compared (Figures 3 and 4) to rule out analytical interferences caused by differences in test results from instrument or method instability. The results showed that the response intensities of each peak and retention time were the same, indicating that the instrument was in a stable state and the analytical method repeatability was good.
TIC of M. nigra leaf QC sample in positive ion mode.
TIC of M. nigra leaf QC sample in negative ion mode.
3.3 TIC comparison
UPLC-Q-TOF-MS analysis of M. nigra leaves using different extraction methods showed that the total ion current maps of the samples from the four different extraction methods were generally similar, but there were some differences, indicating that the types and contents of the main components of M. nigra leaves were different to varying degrees (Figures 5 and 6).
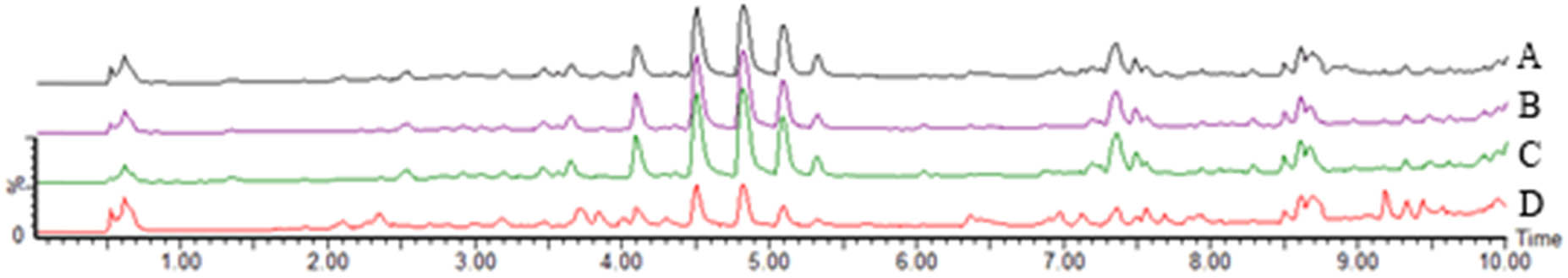
TIC of M. nigra leaves extracted using different methods in positive ion mode. Notes: A: FAU; B: WD; C: WU; and D: WHR.
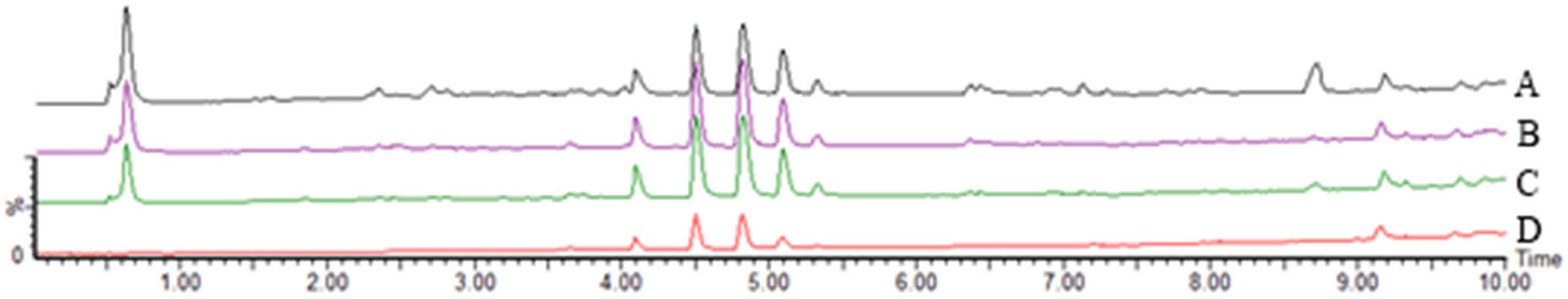
TIC of M. nigra leaves extracted using different methods in negative ion mode. Notes: A: FAU; B: WD; C: WU; and D: WHR.
3.4 Analysis of compounds in different extraction methods of M. nigra leaves
Substance peaks detected in the ESI+ and ESI− patterns were abundant, and 46 and 80 secondary metabolites were identified in the mulberry self-built library based on fragment score screening. Table 3 shows information on the identified compounds.
Compounds identified in M. nigra leaves using four different extraction methods
| No. | Identification | Rt (min) | m/z | Adducts | Formula | Mass error (ppm) | Compound type |
|---|---|---|---|---|---|---|---|
| 1 | Guangsangon I | 0.55 | 625.170 | M + H | C35H28O11 | −0.09 | Flavone |
| 2 | DNJ | 0.56 | 164.092 | M + H | C6H13NO4 | 2.50 | Alkaloid |
| 3 | Fagomine | 0.57 | 148.097 | M + H | C6H13NO3 | 2.50 | Alkaloid |
| 4 | Macrourin B | 0.59 | 523.102 | M + Na | C28H20O9 | 4.14 | Flavonoid analog |
| 5 | Lactic Acid | 0.61 | 135.030 | M + FA-H | C3H6O3 | −2.62 | Organic acid |
| 6 | Galacturonic acid | 0.61 | 193.035 | M-H | C6H10O7 | −0.09 | Organic acid |
| 7 | Melezitose | 0.63 | 503.162 | M-H, M + FA-H | C18H32O16 | −0.38 | Trisaccharide |
| 8 | 2′,3′-Dihydro-4′,6-dihydroxy-2′-(1-hydroxy-1-methylethyl)-3,6′-bibenzofuran | 0.63 | 371.113 | M + FA-H | C19H18O5 | −3.55 | Flavonoid analog |
| 9 | Quinic acid | 0.64 | 191.056 | M-H2O-H, M-H, M + FA-H | C7H12O6 | −2.98 | Organic acid |
| 10 | Maltose | 0.65 | 323.098 | M-H2O-H | C12H22O11 | −2.67 | Disaccharide |
| 11 | Citric acid | 0.7 | 191.019 | M-H2O-H, M-H | C6H8O7 | −3.08 | Organic acid |
| 12 | 3-Epi-fagomine | 1.07 | 170.079 | M + Na | C6H13NO3 | 1.81 | Alkaloid |
| 13 | Protocatechuic acid methyl ester | 1.42 | 149.024 | M-H2O-H | C8H8O4 | −3.89 | Phenol |
| 14 | Cyanidin-3-glucoside | 1.52 | 488.072 | M + K | C21H21O11 + | 1.05 | Flavone |
| 15 | Syringic acid | 1.7 | 197.045 | M-H | C9H10O5 | −4.14 | Organic acid |
| 16 | Vanillic acid | 1.71 | 167.034 | M-H | C8H8O4 | −3.68 | Organic acid |
| 17 | Cryptochlorogenic acid | 1.84 | 353.087 | M-H | C16H18O9 | −2.05 | Phenylpropin |
| 18 | Caffeic acid | 1.84 | 179.034 | M-H2O-H, M-H | C9H8O4 | −2.15 | Phenylpropin |
| 19 | Cycloartomunin | 1.94 | 471.139 | M + Na | C26H24O7 | −4.99 | Flavone |
| 20 | Moracin R | 1.95 | 389.125 | M + FA-H | C19H20O6 | 2.91 | Flavonoid analog |
| 21 | 2′,4′,7-Trihydroxy-8-(2-hydroxyethyl)flavone | 1.97 | 313.072 | M-H | C17H14O6 | 0.54 | Flavone |
| 22 | m-Coumaric acid | 2.03 | 163.039 | M-H | C9H8O3 | −3.96 | Phenylpropin |
| 23 | Oxyresveratrol | 2.07 | 243.065 | M-H | C14H12O4 | −3.71 | Phenylpropin |
| 24 | Dauroside D | 2.07 | 339.071 | M-H | C15H16O9 | −2.89 | Phenylpropin |
| 25 | Plantagoside | 2.13 | 465.103 | M-H | C21H22O12 | −2.60 | Flavone |
| 26 | Cis-Mulberroside A | 2.16 | 567.171 | M-H | C26H32O14 | −2.49 | Phenylpropin |
| 27 | 4-[(2R)-8-(2-Hydroxyethyl)-7-methoxy-3,4-dihydro-2H-chromen-2-yl]-1,3-benzenediol | 2.18 | 355.093 | M + K | C18H20O5 | −2.51 | Flavonoid |
| 28 | Cyanidin-3,5-diglucoside | 2.22 | 609.145 | M-H | C27H30O16 | −1.24 | Flavone |
| 29 | Dihydrooxyresveratrol | 2.3 | 227.072 | M-H2O-H | C14H14O4 | 1.84 | Other |
| 30 | Ferulic acid 4-O-β-d-glucuronide | 2.34 | 351.072 | M-H2O-H, M-H | C16H18O10 | −1.29 | Phenylpropin |
| 31 | 7-Hydroxycoumarin | 2.34 | 161.024 | M-H | C9H6O3 | −2.44 | Phenylpropin |
| 32 | Mongolicin G | 2.37 | 681.270 | M + H | C40H40O10 | 1.38 | Phenylpropin |
| 33 | Neochlorogenic acid | 2.46 | 353.087 | M-H | C16H18O9 | −1.87 | Phenylpropin |
| 34 | Eriodictyol | 2.48 | 287.058 | M-H | C15H12O6 | 4.93 | Flavone |
| 35 | Delphinidin-3,5-diglucoside | 2.51 | 625.141 | M-H | C27H30O17 | −0.71 | Flavone |
| 36 | Morusimic acid D | 2.53 | 312.254 | M + H-H2O | C18H35NO4 | 1.36 | Organic acid |
| 37 | Kaempferol-7-O-β-d-glucopyranoside | 2.54 | 487.065 | M + K | C21H20O11 | 2.57 | Flavone |
| 38 | p-Coumaric acid | 2.54 | 163.039 | M-H | C9H8O3 | −4.32 | Phenylpropin |
| 39 | Luteolin-4′-glucoside | 2.54 | 447.095 | M-H | C21H20O11 | 3.57 | Flavone |
| 40 | Morusignin G | 2.58 | 379.154 | M + H-H2O | C23H24O6 | −0.76 | Flavone |
| 41 | Mesozygin A | 2.66 | 589.152 | M + H-H2O | C35H26O10 | 4.99 | Other |
| 42 | Mulberroside F. 6-glc | 2.66 | 403.103 | M-H | C20H20O9 | −1.11 | Flavonoid analog |
| 43 | Mulberroside F | 2.66 | 565.155 | M-H, M + FA-H | C26H30O14 | −1.81 | Flavonoid analog |
| 44 | Oxyresveratrol 2-O-β-d-glucopyranoside | 2.68 | 405.119 | M-H | C20H22O9 | 0.02 | Phenylpropin |
| 45 | d-Glucopyranose | 2.71 | 161.045 | M-H2O-H | C6H12O6 | −4.49 | Monosaccharide |
| 46 | Mulberrofuran E | 2.79 | 671.205 | M + K | C39H36O8 | 1.34 | Flavonoid analog |
| 47 | 3-(3,4-Dihydroxyphenyl)prop-2-enoic acid | 2.8 | 179.035 | M-H2O-H, M-H | C9H8O4 | 0.41 | Phenylpropin |
| 48 | Spiraeoside | 2.8 | 463.088 | M-H | C21H20O12 | −1.23 | Flavone |
| 49 | 6,7-Dihydroxycoumarin | 2.81 | 177.019 | M-H | C9H6O4 | −3.74 | Phenylpropin |
| 50 | dl-Arginine | 2.82 | 175.119 | M + H | C6H14N4O2 | 2.43 | Amino acid |
| 51 | Morusyunnansin F | 2.98 | 309.149 | M + H-H2O | C20H22O4 | 1.31 | Flavonoid |
| 52 | Wittifuran H | 2.98 | 475.214 | M-H2O-H | C29H34O7 | 2.33 | Flavonoid analog |
| 53 | Cathafuran C | 2.98 | 421.164 | M + FA-H | C24H24O4 | −3.93 | Flavonoid analog |
| 54 | Astragalin | 3.03 | 487.065 | M+K | C21H20O11 | 1.73 | Flavone |
| 55 | Sinapic acid | 3.03 | 223.061 | M-H | C11H12O5 | −3.17 | Phenylpropin |
| 56 | 5-Hydroxy Apiosylskimmin | 3.03 | 453.105 | M-H2O-H | C20H24O13 | 2.46 | Phenylpropin |
| 57 | Isoquercitrin | 3.05 | 463.088 | M-H | C21H20O12 | −0.27 | Flavone |
| 58 | (2S)-8-Hydroxyethyl-7,4′-dimethoxyflavane-2′-O-β-d-glucopyranoside | 3.07 | 537.196 | M + FA-H | C25H32O10 | −2.67 | Flavonoid |
| 59 | (2R,3S)-Guibourtinidol-3-0-α-d-apiofuranosyl-(1→6)-O-β-d-glucopyranoside | 3.09 | 551.178 | M-H | C26H32O13 | 1.69 | Flavonoid |
| 60 | Resveratrol | 3.15 | 229.086 | M + H | C14H12O3 | −0.19 | Phenylpropin |
| 61 | Kaempferol-3-O-β-d-sophoroside | 3.15 | 655.153 | M + FA-H | C27H30O16 | 2.82 | Flavone |
| 62 | Kuwanol D | 3.21 | 409.201 | M + H | C25H28O5 | 1.20 | Phenylpropin |
| 63 | Umbelliferone | 3.24 | 161.024 | M-H | C9H6O3 | −2.83 | Phenylpropin |
| 64 | Austrafuran A | 3.25 | 449.123 | M + H-H2O | C25H22O9 | −0.82 | Flavonoid analog |
| 65 | Wittifuran E | 3.25 | 303.051 | M + FA-H | C14H10O5 | −1.55 | Flavonoid analog |
| 66 | Chlorogenic acid | 3.57 | 353.089 | M-H | C16H18O9 | 2.26 | Phenylpropin |
| 67 | Lariciresinol | 3.6 | 359.150 | M-H | C20H24O6 | −0.92 | Phenylpropin |
| 68 | Rutin | 3.71 | 609.146 | M-H | C27H30O16 | −0.02 | Flavone |
| 69 | Morin | 3.74 | 301.034 | M-H | C15H10O7 | −3.99 | Flavone |
| 70 | Isoquercetin | 3.74 | 463.088 | M-H | C21H20O12 | −0.93 | Flavone |
| 71 | Gossypin | 3.74 | 461.072 | M-H2O-H, M-H | C21H20O13 | −1.37 | Flavone |
| 72 | Norartocarpetin 7-glucoside | 3.75 | 493.098 | M + FA-H | C21H20O11 | −0.84 | Flavone |
| 73 | Pinoresinol | 3.76 | 403.161 | M-H2O-H, M + FA-H | C20H22O6 | −1.62 | Phenylpropin |
| 74 | Moracinflavan B | 3.81 | 313.145 | M + H-H2O | C19H22O5 | 4.98 | Flavone |
| 75 | Quercetin-7-O-β-d-glucopyranoside | 3.84 | 445.077 | M-H2O-H | C21H20O12 | −1.65 | Flavone |
| 76 | Morin-3-O-β-d-glucopyranoside | 3.84 | 341.030 | M-H2O-H | C17H12O9 | −1.39 | Flavone |
| 77 | 2′,4′,5,7-Tetrahydroxy-3-methoxyflavone | 3.84 | 297.040 | M-H2O-H | C16H12O7 | −1.59 | Flavone |
| 78 | Oxyresveratrol 3-glc | 3.86 | 387.110 | M-H2O-H | C20H22O9 | 4.41 | Phenylpropin |
| 79 | 2′,7-Dihydroxy-4′-methoxy-8-prenylflavan 2,7-diglc | 3.94 | 645.255 | M-H2O-H | C33H44O14 | −0.41 | Flavonoid |
| 80 | Syringaresinol | 4 | 417.155 | M-H | C22H26O8 | −1.43 | Phenylpropin |
| 81 | Stearyltriarabinoferulate | 4 | 901.443 | M + FA-H | C43H68O17 | −1.19 | Phenylpropin |
| 82 | 14-Methoxy-dihydromorusin | 4.01 | 435.180 | M + H-H2O | C26H28O7 | −0.58 | Flavone |
| 83 | Morusignin D | 4.01 | 387.109 | M + FA-H | C19H18O6 | 1.24 | Xanthones |
| 84 | Moracinflavan C | 4.13 | 331.154 | M + H | C19H22O5 | −0.85 | Flavone |
| 85 | Kaempferol-3-O-glucoside | 4.13 | 447.093 | M-H | C21H20O11 | −1.25 | Flavone |
| 86 | (E)-4-Isopentenyl-3,5,2′,4′-tetrahydroxystilbene | 4.16 | 357.135 | M + FA-H | C19H20O4 | 1.59 | Phenylpropin |
| 87 | Kuwanon O | 4.18 | 717.228 | M + Na | C40H38O11 | −4.56 | Flavone |
| 88 | (2S)-2′,4′-Dihydroxyl-7-methoxyl-8-butyricflavane | 4.18 | 339.123 | M-H2O-H | C20H22O6 | −2.17 | Flavonoid |
| 89 | Sanggenol G | 4.21 | 529.219 | M + Na | C30H34O7 | −2.25 | Flavone |
| 90 | Kuwanon N | 4.25 | 799.249 | M + K | C45H44O11 | −4.03 | Flavone |
| 91 | 6-Methoxy-5,7,4′-trihydroxyisoflavone | 4.3 | 339.026 | M + K | C16H12O6 | −0.84 | Flavone |
| 92 | Taxifolin | 4.3 | 285.040 | M-H2O-H | C15H12O7 | −1.97 | Flavone |
| 93 | Moracenin C | 4.39 | 799.250 | M + K | C45H44O11 | −2.50 | Flavone |
| 94 | Kuwanon U | 4.39 | 483.204 | M + FA-H | C26H30O6 | 3.99 | Flavone |
| 95 | 7-O-β-d-Glucopyranosid Benzofuran | 4.56 | 339.072 | M-H | C15H16O9 | −0.39 | Phenylpropin |
| 96 | Linoleic acid | 4.68 | 303.230 | M + Na | C18H32O2 | 1.40 | Organic acid |
| 97 | Alboctalol | 4.79 | 471.142 | M + H-H2O | C28H24O8 | −4.42 | Other |
| 98 | Notabilisin B | 4.87 | 547.207 | M + K | C30H36O7 | −3.84 | Flavone |
| 99 | Morusignin A | 4.96 | 329.103 | M + H | C18H16O6 | 3.15 | Xanthones |
| 100 | Sanggenol E | 5.59 | 559.306 | M + H-H2O | C35H44O7 | 1.19 | Flavone |
| 101 | Mulberroside A. 2-glc | 5.62 | 405.118 | M-H | C20H22O9 | −1.99 | Phenylpropin |
| 102 | Wittifuran U | 5.7 | 393.171 | M + H-H2O | C24H26O6 | 3.96 | Flavonoid analog |
| 103 | Yunanensol A | 5.74 | 441.189 | M + H | C25H28O7 | −4.39 | Flavone |
| 104 | 2-(5-Hydroxymethyl-2-formylpyrrol-1-yl)isovaleric acid lactone | 5.97 | 246.052 | M + K | C11H13NO3 | −2.46 | Other |
| 105 | Albafuran C | 6.37 | 563.172 | M + H-H2O, M + K | C34H28O9 | 2.83 | Flavonoid analog |
| 106 | Mornigrol G | 6.37 | 477.131 | M + K | C25H26O7 | −0.24 | Flavone |
| 107 | Moracin C | 6.37 | 309.113 | M-H | C19H18O4 | −0.23 | Flavonoid analog |
| 108 | 2′,4′,7-Trihydroxy-(2S)-flavone | 6.37 | 253.050 | M-H2O-H | C15H12O5 | −0.90 | Flavone |
| 109 | Leachianone G | 6.38 | 355.120 | M-H | C20H20O6 | 4.18 | Flavone |
| 110 | Wittifuran D. | 6.4 | 293.117 | M + H-H2O | C19H18O4 | −1.15 | Flavonoid analog |
| 111 | Sanggenol K | 6.51 | 509.251 | M + H | C30H36O7 | −3.99 | Flavone |
| 112 | 2′,4′,5,7-Tetrahydroxyflavanone | 6.77 | 271.060 | M + H-H2O | C15H12O6 | −0.49 | Flavone |
| 113 | Oleic acid | 7.22 | 265.251 | M + H-H2O | C18H34O2 | −4.81 | Organic acid |
| 114 | Nigrasin J | 7.36 | 451.177 | M + H-H2O | C26H28O8 | 4.56 | Flavone |
| 115 | Wittifuran R | 7.85 | 457.203 | M-H2O-H | C29H32O6 | 1.63 | Flavonoid analog |
| 116 | Mortatarin D | 7.96 | 521.252 | M + FA-H | C30H36O5 | −4.30 | Flavone |
| 117 | Mornigrol E | 8.16 | 437.160 | M-H | C25H26O7 | −2.02 | Flavone |
| 118 | Notabilisin A | 8.19 | 451.175 | M-H | C26H28O7 | −2.66 | Flavone |
| 119 | Chaminic acid | 8.22 | 167.106 | M + H | C10H14O2 | −4.67 | Organic acid |
| 120 | 2′,4′-Dihydroxy-7-methoxy-8-prenylflavan | 8.43 | 339.161 | M-H | C21H24O4 | 1.32 | Flavonoid |
| 121 | Notabilisin C | 8.51 | 507.236 | M + H | C30H34O7 | −4.41 | Flavone |
| 122 | Wittifuran P | 8.69 | 505.225 | M + FA-H | C29H32O5 | 3.10 | Flavonoid analog |
| 123 | Nonadecanoic acid | 9.46 | 343.285 | M + FA-H | C19H38O2 | −3.06 | Organic acid |
| 124 | Wittifuran T | 9.51 | 505.223 | M + FA-H | C29H32O5 | −0.99 | Flavonoid analog |
| 125 | Behenic acid | 9.67 | 323.330 | M + H-H2O | C22H44O2 | −3.21 | Organic acid |
| 126 | Stearic acid | 9.96 | 283.263 | M-H | C18H36O2 | −3.29 | Organic acid |
In both models, mass spectrometry information was identified using software, secondary metabolites of M. nigra leaves were identified through the mulberry plant database, and a total of 126 compounds were identified by referring to the relevant literature. The compounds were assigned to 54 flavonoids, including flavonoids and flavanone, 17 stilbenoids, including benzofuran, 3 alkaloids, 28 phenylpropanoids, 13 organic acids, 1 amino acid, 1 sugar, 2 xanthones, 1 phenol, and 4 other compounds.
The mass spectrometry data detected in positive and negative ion modes were analyzed using SIMCA-P14.1. PCA was used to cluster the data, and the score plot and the degree of convergence of M. nigra leaves with different extraction methods were obtained, as shown in Figure 7. PCA of the four extraction methods showed that the first two principal components explained 78.50% of the original variable information (PC1: 56.30% and PC2: 22.20%). It can be seen from the figure that the PCA model established in this experiment was well clustered. The sample sites of M. nigra leaves with different extraction methods were completely separated, and the samples of medicinal materials of the same extraction method were well gathered in the same area. In addition, as seen in Figure 7, the sample points of WU, FAU, and WHR were close in the first principal component, indicating that the chemical composition structure between the extraction methods was similar. As shown in Figure 7, the sample point aggregation was divided into two areas, which were far apart, indicating that the difference was large.
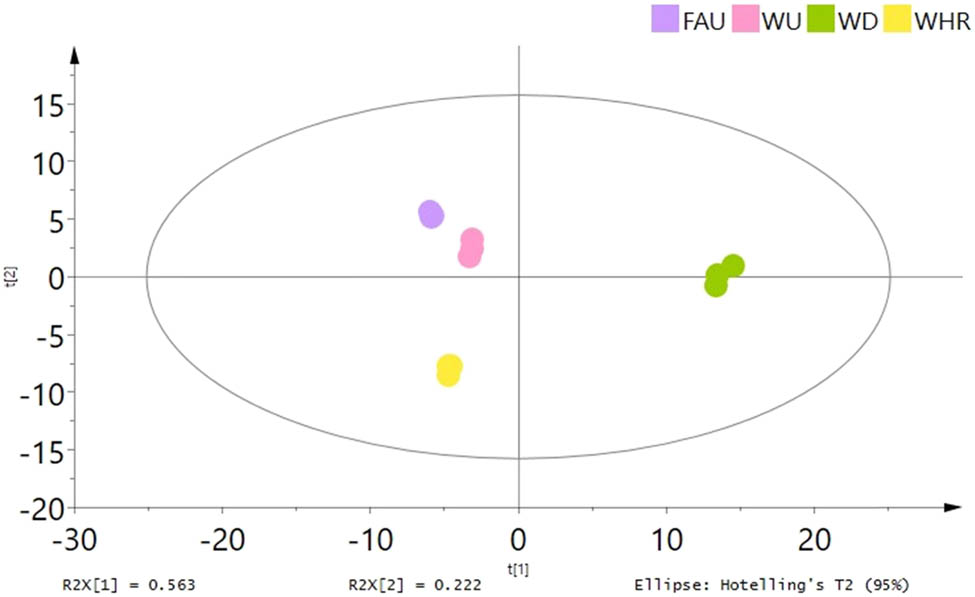
PCA score plots of M. nigra leaf samples using different extraction methods.
3.5 Analysis of differential metabolites using different extraction methods of M. nigra leaves
Due to different extraction methods, the chemical constituents in M. nigra leaf extracts were different, and 126 secondary metabolites identified were stoichiometrically analyzed using the map cloud mapping platform to find the number of differential compounds under the different extraction methods. The results are shown in Figure 8. From the Wayne diagram, it can be seen that there are 55 common components in the four extractions. There were six common components from the other three extraction methods but not the reflux extraction, which showed that the number of metabolites in the reflux extraction was less than that from the other three extraction methods. Among them, there were four signature differential metabolites extracted by formic acid and one signature differential metabolite extracted by water decoction. Some differences in chemical composition were observed among the four groups, and the results were consistent with the PCA results.
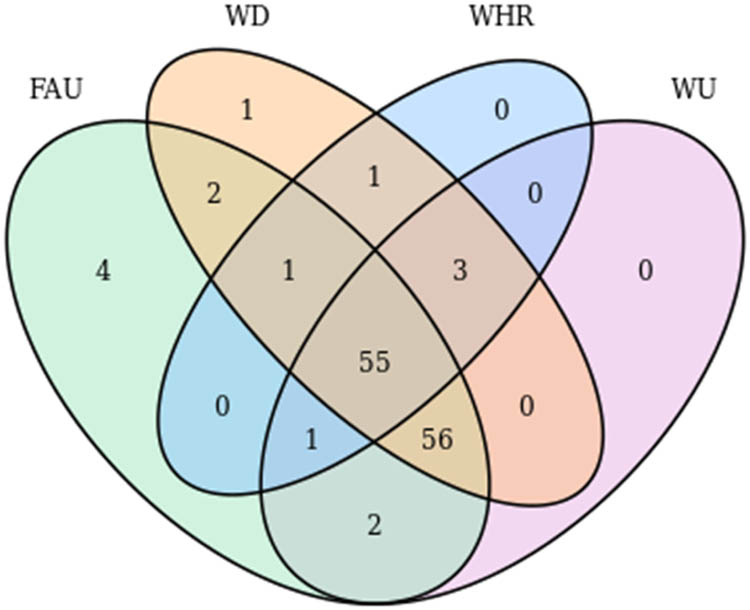
Wayne diagram of metabolites from M. nigra leaf using different extraction methods.
PCA analysis showed that there were some differences in the chemical composition of M. nigra leaves extracted by different extraction methods, and the chemical compositions of M. nigra leaves extracted by UA, FAU, and WHR showed more similarities compared with mulberry leaves extracted by WD. To understand the differences of M. nigra leaf extracts from different extraction methods, according to the PCA model results of the different extraction methods, OPLS-DA modeling was adopted for different groups of M. nigra leaves. The results showed that almost all of the M. nigra leaves extracted by WD and other groups could be divided into two groups, indicating that the compounds in the two groups of M. nigra leaves were significantly different (Figure 8). The model was validated by setting the number of tests to 200. The R 2 and Q 2 points on the left side (Figure 9b) were lower than those on the right side. The regression line of the Q 2 points intersected the longitudinal axis below the origin, and the model validation results (R 2 X = 0.951, R 2 Y = 0.991, Q 2 = 0.988) suggested that no overfitting occurred and the model was reliable (Figure 9). To further elucidate the differential compounds in the M. nigra leaves extracted by WD and other extraction methods, S-plots were plotted according to the established OPLS-DA model, and the compounds were screened according to a VIP value ≥1. According to the m/z value and retention time of differential metabolites, these data were matched with the component identification results or database, and a total of 13 differential components were screened and identified. These included four organic acids, three flavonoids, three phenylpropanoids, two alkaloids, and one trisaccharide. These differential metabolites were quinic acid, DNJ, delphinidin-3,5-diglucoside, melezitose, ferulic acid, 4-O-β-d-glucuronide, isoquercetin, rutin, stearic acid, fagomine, citric acid, neochlorogenic acid, cryptochlorogenic acid, and caffeic acid. Among them, DNJ and fagomine were confirmed by comparison with the control. Tables 3 and 4 shows information on the differential compounds.
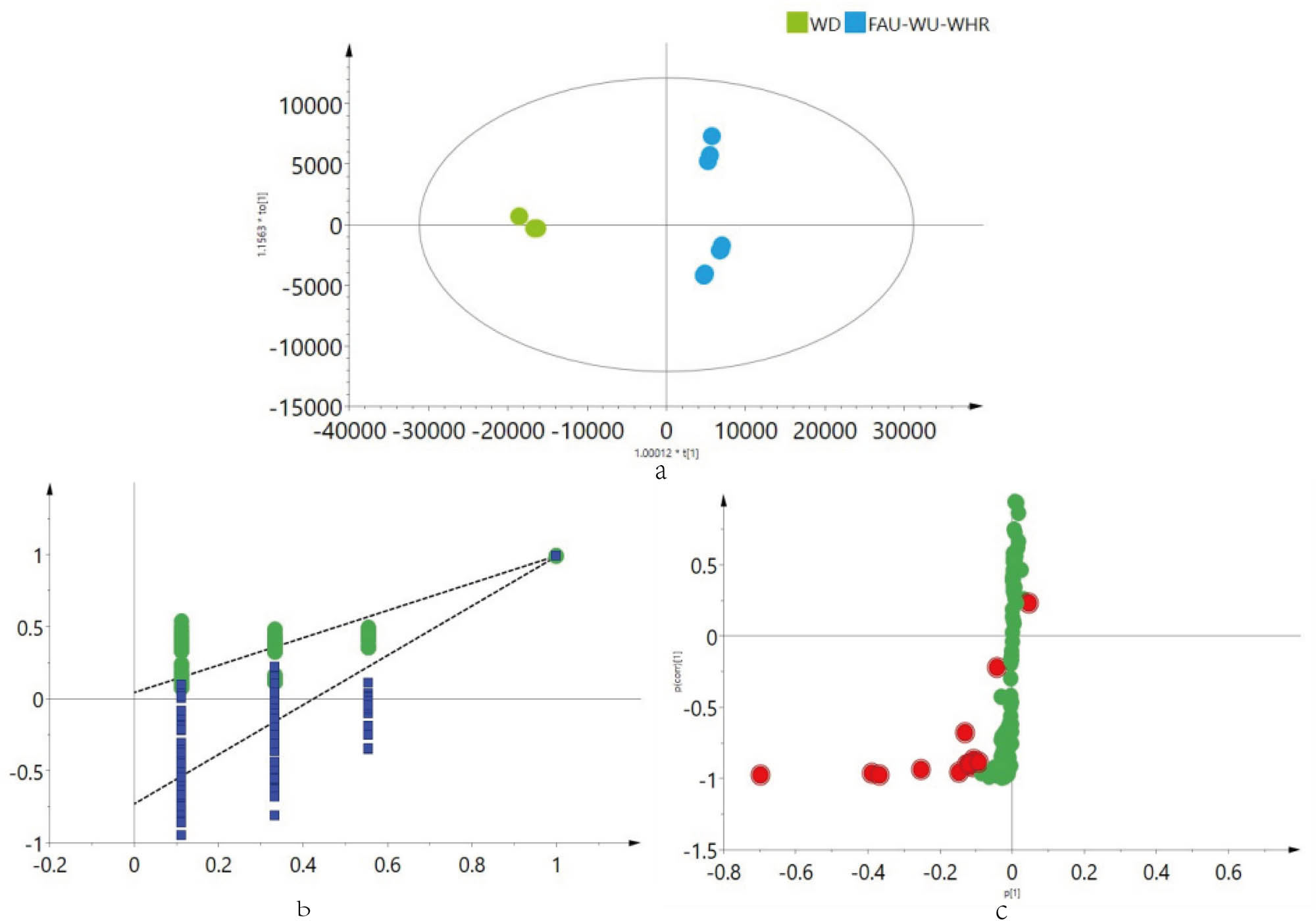
OPLS-DA diagram (a), substitution test diagram (b) of M. nigra leaves using different extraction methods, and S-plot results (c).
Differential metabolites of M. nigra leaves using four different extraction methods
| No. | Identification | VIP | Compound type | MS/MS fragmentation |
|---|---|---|---|---|
| 1 | Quinic Acid | 7.60 | Organic acid | 191.056, 179.056, 175.025, 173.046, 161.046, 149.046, 133.014, 101.024 |
| 2 | DNJ | 4.20 | Alkaloid | 146.147, 128.136, 110.122, 96.106, 82.135, 69.091 |
| 3 | Delphinidin-3,5-diglucoside | 3.99 | Flavone | 625.141, 463.088, 462.080, 461.073 |
| 4 | Melezitose | 2.79 | Trisaccharide | 503.162, 341.109, 323.098, 267.072, 237.062, 191.056, 143.035 |
| 5 | Ferulic acid 4-O-β-d-glucuronide | 1.61 | Phenylpropanoids | 369.083, 353.088, 341.088, 307.082, 287.056, 199.040, 191.020 |
| 6 | Isoquercetin | 1.52 | Flavone | 463.088, 300.028, 283.025, 271.025, 255.030, 254.022,2 43.030 |
| 7 | Rutin | 1.44 | Flavone | 609.146, 463.082, 461.073, 343.046, 300.028, 271.025, 255.030, 243.030, 227.035, 151.004 |
| 8 | Stearic acid | 1.42 | Organic acid | 283.264, 255.233 |
| 9 | Fagomine | 1.37 | Alkaloid | 130.152, 112.139, 94.127, 86.121, 80.109, 68.107 |
| 10 | Citric acid | 1.33 | Organic acid | 175.025, 173.009, 157.014, 147.030, 133.014, 129.019, 115.004, 111.009 |
| 11 | Neochlorogenic acid | 1.22 | Phenylpropanoids | 343.285, 295.228, 269.212, 199.170, 171.103 |
| 12 | Cryptochlorogenic acid | 1.22 | Phenylpropanoids | 353.088, 307.082, 191.056, 179.035, 177.019, 173.046, 135.045, 134.037, 133.030, 127.040 |
| 13 | Caffeic acid | 1.07 | Organic acid | 179.035, 135.045, 134.037, 133.030 |
3.6 Analysis of a differential metabolite cleavage rule under different extraction methods of medicinal M. nigra leaves
3.6.1 Flavonoid assay identification
Flavonoids are a class of characteristic compounds in M. nigra leaves, which have pharmacological effects, such as anti-oxidation and anti-cancer, and improve cardiovascular function [24]. Mass spectrometric fragmentation of flavonoids was investigated using rutin as an example, which formed a [M-H]− quasi-molecular ion peak at m/z 609.1461, and a fragment from lost rutinose at m/z 300.0275 upon collision-induced dissociation. Fragment m/z 300.0275 formed fragment m/z 151.0037 through reverse Diels–Alder (RDA) cleavage [25]. Possible mass spectrometric fragmentation pathways of rutin are shown in Figure 10.
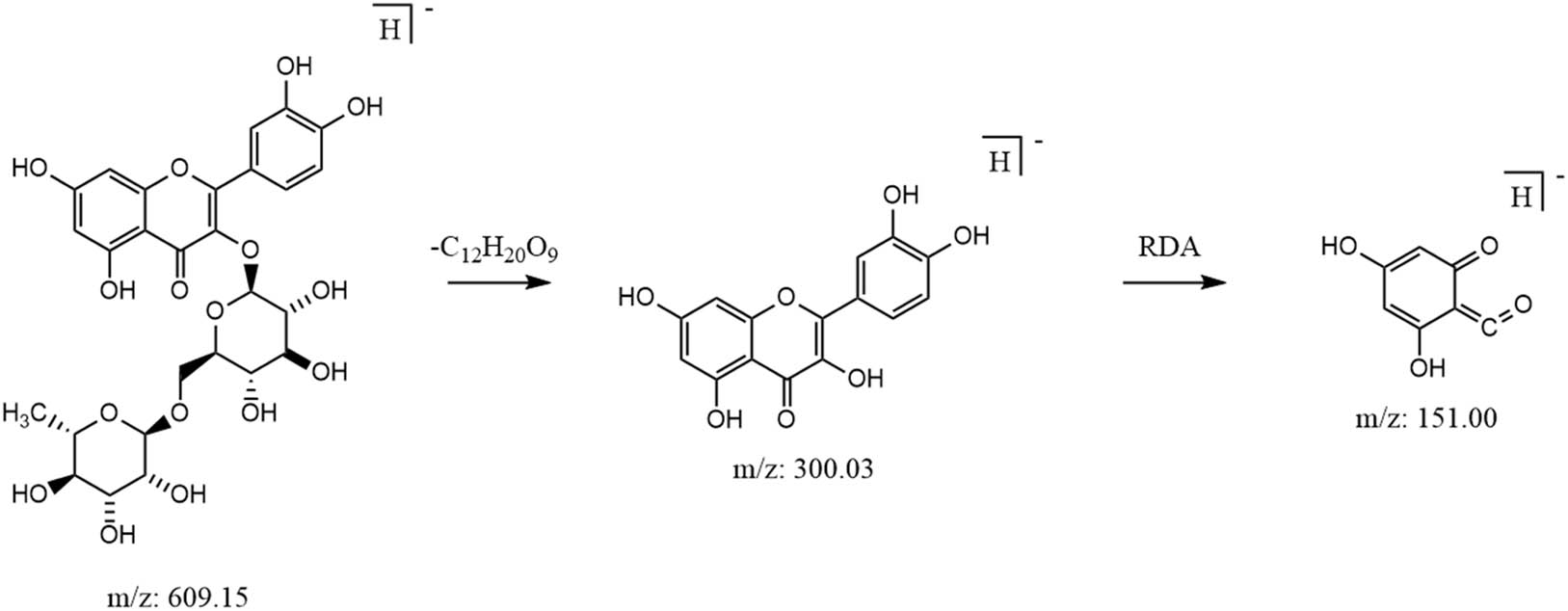
Possible cleavage pathways for rutin.
3.6.2 Organic acids
Organic acid compounds are important compounds in M. nigra leaves, which have the effects of eliminating soreness and freckles and enhancing skin beauty. Using citric acid as an example, possible fragmentation patterns of organic acid compounds were analyzed, as shown in Figure 11. Shown in negative mode [M-H]−, a quasi-molecular ion peak was found at m/z 190, and fragmentation by secondary scanning yielded m/z 147 [M-H-H2O-CO2]− and m/z 111 [M-H-CO2-2H2O]− fragments [26].
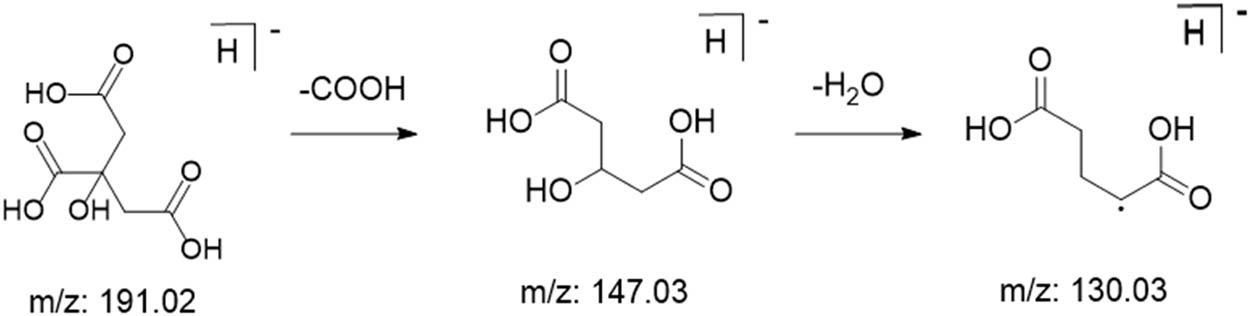
Possible cleavage pathways of citric acid.
3.6.3 Alkaloids
Alkaloids are important components in M. nigra leaves. Modern pharmacological studies have shown that mulberry leaves can inhibit increased blood glucose, with the effect of preventing and treating diabetes. The main active component of lowering blood glucose in mulberry leaves is DNJ-based polyhydroxyalkaloids [27]. Using DNJ as an example to investigate the possible mass spectrometric fragmentation of alkaloids, molecular ion peaks were obtained from DNJ in the positive ion mode at 164.1580 [M + H]+, and two characteristic fragment ions were obtained by energy collision, which were m/z 96.1063 [M + H-(OH)4]+ and m/z 82.1253 [M + H-CH6O4]+. The possible mass spectrometric fragmentation pathway of DNJ is shown in Figure 12 [28].
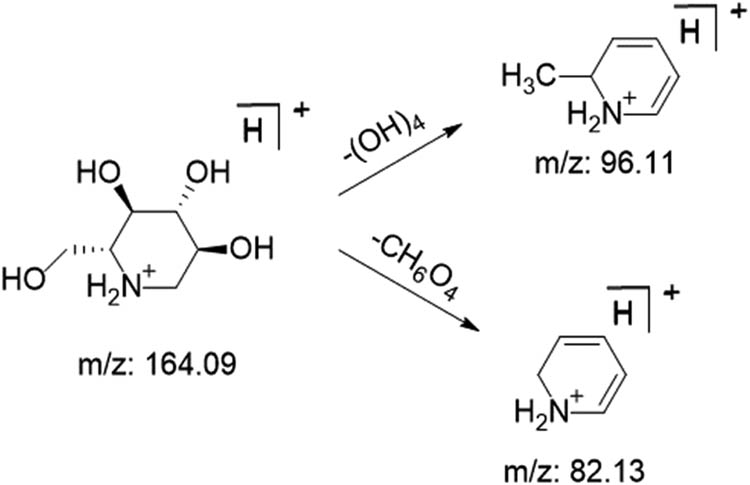
DNJ possible cleavage pathways.
3.6.4 Phenylpropanoids
Phenylpropanoid compounds are important compounds in M. nigra leaves and have antioxidant activity [29]. The possible mass spectrometric fragmentation of phenylpropanoid compounds was analyzed using cryptochlorogenic acid as an example, as shown in Figure 13. The cryptochlorogenic acid parent ion in negative mode was 353.0878 [M-H]−, and a secondary fragment ion m/z 191.0561 was produced by the loss of one molecule of caffeoyl from the parent ion [M-H-caffeoyl]−. Then, m/z 179.0349 was produced by the loss of one molecule of caffeoyl from the parent ion [caffeoyl-H]−. On this basis, a portion of CO2 was removed to produce m/z 135.0444 [caffeoyl-H-CO2]− [30,31].
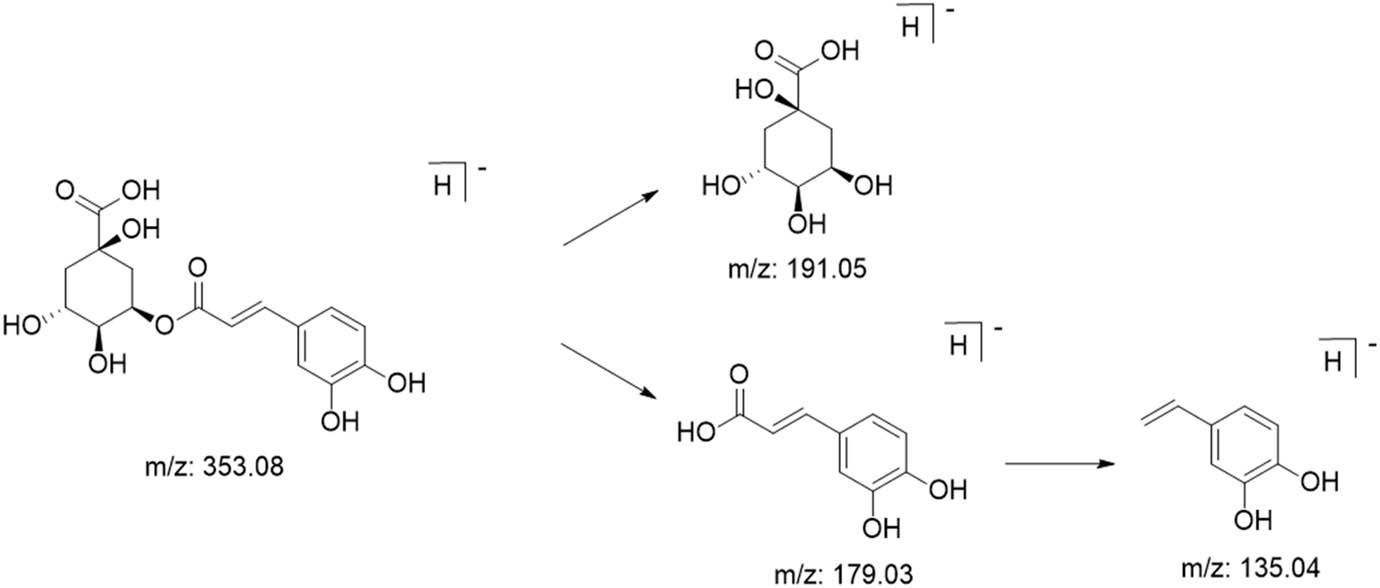
Possible cleavage pathway of cryptochlorogenic acid.
3.6.5 Trisaccharides
Trisaccharides are important active components in M. nigra leaves and can be used to repair islet cells with a significant hypoglycemic effect. Melezitose was used as an example to elaborate on the possible mass spectrometric behavior of trisaccharides. In the negative ion mode, the matrix parent ion was 503.1617 [M-H]−, and the fragment ion m/z 341.1089 was obtained by losing one molecule of glucose (the matrix ion), and the fragment ion m/z 323.0983 was obtained by losing one molecule of water.
3.7 Content analysis of the main compounds in M. nigra leaves using different extraction methods
A total of 13 different chemical components were identified by analysis of M. nigra leaf samples from four different extraction methods, which can serve as specific markers to distinguish different extraction methods. The flavonoid rutin is the main active ingredient against cancer and diabetes [32,33]. The alkaloid DNJ is a natural glycosidase inhibitor with strong postprandial glucose inhibition [34]. The phenylpropanoid compound neochlorogenic acid is an important component in the treatment of inflammation [35]. According to the standard DNJ, the corresponding contents of the differential components were calculated from the corresponding abundance values between the samples of each group under each extraction method. The standard deviations of the mean values of the content of each substance between the samples of M. nigra leaves from different extraction methods were calculated to obtain the content changes in the differential components between the M. nigra leaves from different extraction methods (Figure 14). Rutin and isoquercitrin were obtained at a higher yield by WHR. The remaining 11 differential compounds were extracted by WD, and the content in M. nigra leaves was higher than that of the other extraction methods, indicating that the WD method was more efficient compared with the other three extraction methods. This indicated that the WD extraction method of M. nigra leaves had higher medicinal value. Among them, quinic acid, delphinidin-3,5-diglucoside, ferulic acid 4-O-β-d-glucuronide, neochlorogenic acid, cryptochlorogenic acid, isoquercetin, rutin, and caffeic acid were not found in the reflux extraction method, which is important identification information for distinguishing the extraction of M. nigra leaves using different extraction methods.
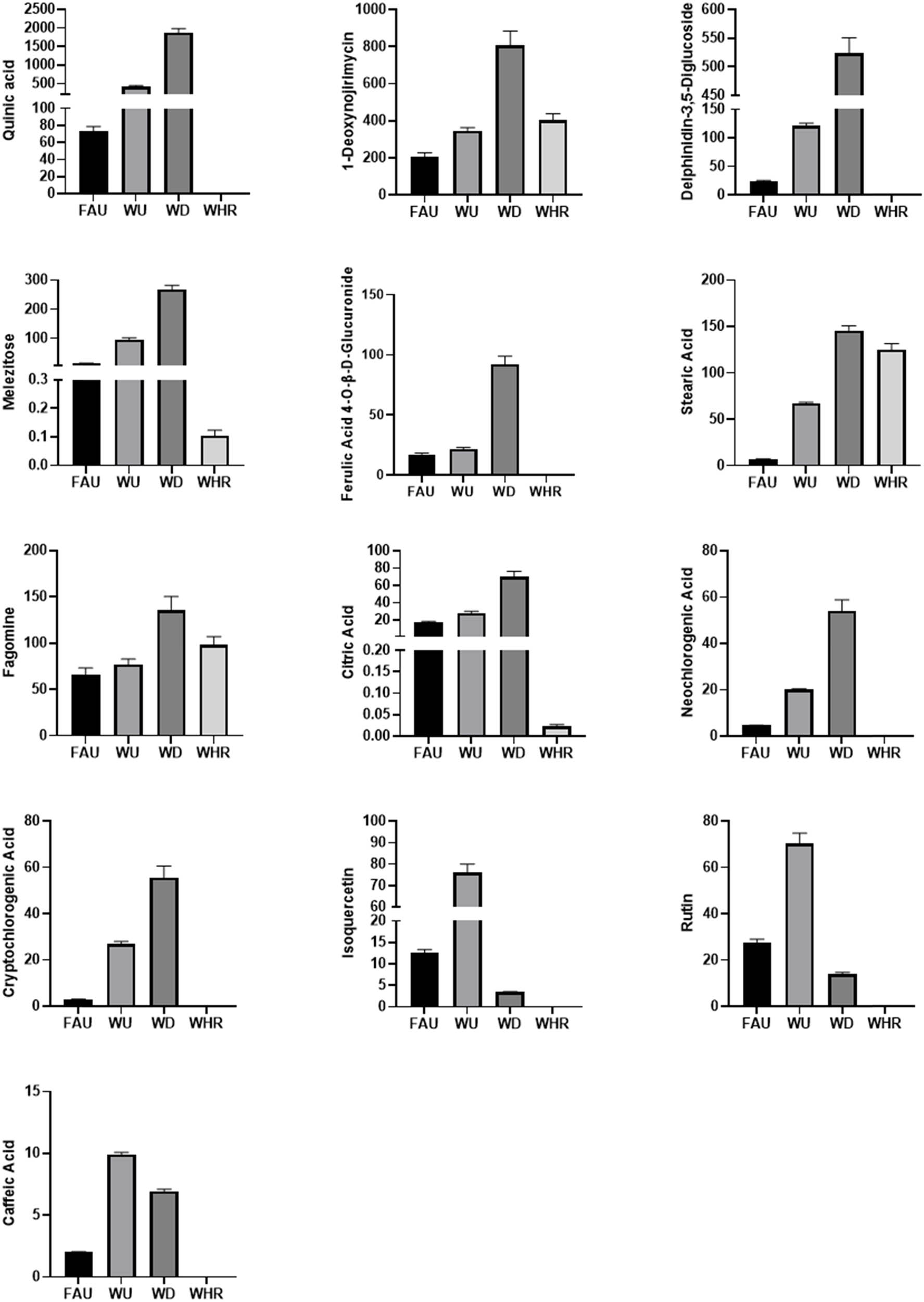
Content of differential chemical constituents in M. nigra leaves extracted using different methods.
4 Conclusion
In this study, UPLC-Q-TOF-MS/MS and multivariate statistical analysis were used to analyze different extraction methods, WU, FAU, WHR, and WD, of M. nigra leaves. A total of 128 chemical constituents were identified and classified. The results showed that WD was different from the other three methods. Compared to other methods evaluated in this study, rutin and isoquercitrin in the differential metabolites were more abundant in WHR. The differential metabolites quinic acid, DNJ, delphinidin-3,5-diglucoside, melezitose, ferulic acid 4-O-β-d-glucuronide, isoquercetin, rutin, stearic acid, fagomine, citric acid, neochlorogenic acid, cryptochlorogenic acid, and caffeic acid were more abundant with WD extractions. This result laid a foundation for the selection of extraction methods for M. nigra leaves in certain pharmacological applications, but further studies are still needed. The results can provide a reference for further optimization of the extraction method of metabolites and provide a basis for the QC of M. nigra leaves.
Acknowledgements
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
-
Funding information: This research was funded by the Application for Open Project of the Key Laboratory of Xinjiang Autonomous Region (Analysis of Genetic Variation in Morus nigra L. and Research on Key Genes of 1-DNJ Alkaloid Synthesis) and Innovation project of germplasm resources of main forest and fruit tree species (lgxy202108).
-
Author contributions: L.Y. and H.Y.W.: conceived and supervised the study; L.Y., W.J.H., and K.Z.: designed the experiments; X.L.G. and S.L.Y.: performed the experiments; and X.L.G.: analyzed the data and wrote the manuscript. All authors reviewed the results and approved the final version of the manuscript.
-
Conflict of interest: Authors state no conflict of interest.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Yiğit D, Yiğit N. Antibacterial activty of black mulberry (morus nigra) fruits and leaves. Erzincan Univ J Sci Technol. 2008;1:39–48.Search in Google Scholar
[2] Yi-heng LIU, Ling Y. Hypoglycemic and hypolipidemic effects of Morus nigra leaves on type 2-diabetic mice. Nat Prod Res Dev. 2015;27:1411.Search in Google Scholar
[3] Oryan S, Eidi M, Yazdi E, Eidi A, Solati J. Hypoglycaemic effect of alcoholic extract of Morus nigra L. leaves in normal and diabetic rats. J Med Plants. 2003;2:27–32.Search in Google Scholar
[4] Padilha MM, Vilela FC, Rocha CQ, Dias MJ, Soncini R, dos Santos MH, et al. Antiinflammatory properties of Morus nigra leaves. Phytother Res. 2010;24:1496–500.10.1002/ptr.3134Search in Google Scholar PubMed
[5] Turgut NH, Mert DG, Kara H, Egilmez HR, Arslanbas E, Tepe B, et al. Effect of black mulberry (Morus nigra) extract treatment on cognitive impairment and oxidative stress status of d-galactose-induced aging mice. Pharm Biol. 2016;54:1052–64.10.3109/13880209.2015.1101476Search in Google Scholar PubMed
[6] Qadir MI, Ali M, Ibrahim Z. Anti-cancer activity of Morus nigra leaves extract. Bangladesh J Pharmacol. 2014;9:496–97.10.3329/bjp.v9i4.19783Search in Google Scholar
[7] Song W, Wang HJ, Bucheli P, Zhang PF, Wei DZ, Lu YH. Phytochemical profiles of different mulberry (Morus sp.) species from China. J Agric Food Chem. 2009;57:9133–40.10.1021/jf9022228Search in Google Scholar PubMed
[8] Li B. Study on the chemical constituents and bioactivities of leaves of Morus nigra L. Xinjiang: Tarim University; 2019.Search in Google Scholar
[9] Zeni A, Moreira TD, Dalmagro AP, Camargo A, Bini LA, Simionatto EL, et al. Evaluation of phenolic compounds and lipid-lowering effect of Morus nigra leaves extract. An Acad Bras Ciênc. 2017;89:2805–15.10.1590/0001-3765201720160660Search in Google Scholar PubMed
[10] Araujo CM, Lúcio Kde P, Silva ME, Isoldi MC, de Souza GH, Brandão GC, et al. Morus nigra leaf extract improves glycemic response and redox profile in the liver of diabetic rats. Food Funct. 2015;6:3490–9.10.1039/C5FO00474HSearch in Google Scholar PubMed
[11] Dobson G, Shepherd T, Verrall SR, Conner S, McNicol JW, Ramsay G, et al. Phytochemical diversity in tubers of potato cultivars and landraces using a GC-MS metabolomics approach. J Agric Food Chem. 2008;56:10280–91.10.1021/jf801370bSearch in Google Scholar PubMed
[12] Styczynski MP, Moxley JF, Tong LV, Walther JL, Jensen KL, Stephanopoulos GN. Systematic identification of conserved metabolites in GC/MS data for metabolomics and biomarker discovery. Anal Chem. 2007;79:966–73.10.1021/ac0614846Search in Google Scholar PubMed
[13] Wiklund S, Johansson E, Sjöström L, Mellerowicz EJ, Edlund U, Shockcor JP, et al. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal Chem. 2008;80:115–22.10.1021/ac0713510Search in Google Scholar PubMed
[14] Yoshida H, Yamazaki J, Ozawa S, Mizukoshi T, Miyano H. Advantage of LC-MS metabolomics methodology targeting hydrophilic compounds in the studies of fermented food samples. J Agric Food Chem. 2009;57:1119–26.10.1021/jf803235mSearch in Google Scholar PubMed
[15] Anacardio R, Mullins FG, Hannam S, Sheikh MS, O'Shea K, Aramini A, et al. Development and validation of an LC–MS/MS method for determination of methanesulfonamide in human urine. J Chromatogr B. 2009;877:2087–92.10.1016/j.jchromb.2009.05.051Search in Google Scholar PubMed
[16] Helander A, Zheng Y. Molecular species of the alcohol biomarker phosphatidylethanol in human blood measured by LC-MS. Clin Chem. 2009;55:1395–405.10.1373/clinchem.2008.120923Search in Google Scholar PubMed
[17] López-Gonzálvez Á, Godzien J, García A, Barbas C. Capillary electrophoresis mass spectrometry as a tool for untargeted metabolomics. In: D’Alessandro A, editor. High-Throughput Metabolomics. Vol. 1978. New York: Springer; 2019. p. 55–77.10.1007/978-1-4939-9236-2_5Search in Google Scholar PubMed
[18] Villate A, San Nicolas M, Gallastegi M, Aulas PA, Olivares M, Usobiaga A, et al. Review: Metabolomics as a prediction tool for plants performance under environmental stress. Plant Sci. 2021;303:110789.10.1016/j.plantsci.2020.110789Search in Google Scholar PubMed
[19] McKay RT. Metabolomics and NMR. In Springer Berlin Heidelberg; 2022.10.1007/164_2022_616Search in Google Scholar PubMed
[20] Reo NV. NMR-based metabolomics. Drug Chem Toxicol. 2002;25:375–82.10.1081/DCT-120014789Search in Google Scholar PubMed
[21] Wang Z-Y, Xiong H, Duan LY, Wang CF, Du YL, Hong X, et al. UPLC-Q-TOF-MS based metabolomics study of hawthorn leaves in different geographical regions. Anal Methods. 2021;13:5458–66.10.1039/D1AY01150BSearch in Google Scholar
[22] Deng W, Sun Z, Luo X, Wu X. Optimization of extraction technology of total alkaloids from mulberry leaves by orthogonal design. Chin Arch Tradit Chin Med. 2012;30:608–9.Search in Google Scholar
[23] Arapitsas P, Ugliano M, Marangon M, Piombino P, Rolle L, Gerbi V, et al. Use of untargeted liquid chromatography–mass spectrometry metabolome to discriminate Italian monovarietal red wines produced in their different terroirs. J Agric Food Chem. 2020;14:13353–66.10.1021/acs.jafc.0c00879Search in Google Scholar PubMed PubMed Central
[24] Luo W-T, Feng JH, Wang LX, He SN, Sun KH, Liu Q, et al. Research progress on flavonoids from mulberry leaves. Chem Bioeng. 2021;38:8–13.Search in Google Scholar
[25] Li Z, Wei Y, Fan Y, Zhu J, Zhao T. Analysis of rutin by electrospray iontrap mass spectrometry. Chin J Anal Lab. 2015;34:186–9.Search in Google Scholar
[26] Qiao X, Wu S, Qi X, Feng J, Xiao X. Identification of chemical constituents in Crataegus pinnatifida var. major by UPLC/ESI-TOF/MS. Drugs Clin. 2014;29:120–4.Search in Google Scholar
[27] Jakobsen P, Lundbeck JM, Kristiansen M, Breinholt J, Demuth H, Pawlas J, et al. Iminosugars: potential inhibitors of liver glycogen phosphorylase. Bioorg Med Chem. 2001;9:733–44.10.1016/S0968-0896(00)00291-1Search in Google Scholar PubMed
[28] Xiong Z-Q, Li Y, Xu Y, Li SY, Cai JY, Lv Q, et al. Study on the difference of principal components different processed sericulture by UPLC-Q/TOF-MS. J Jiangxi Univ Chin Med. 2021;33:89–94.Search in Google Scholar
[29] Zhang W, Han F, He J. HPLC-DAD-ESI-MS/MS analysis and antioxidant activities of nonanthocyanin phenolics in mulberry (Morus alba L.). J Food Sci. 2008;73(6):C512–8.10.1111/j.1750-3841.2008.00854.xSearch in Google Scholar PubMed
[30] Zhao G-L, Miao XL, Song ZH, Gao W, Zhou GR, Li P. Analysis of chemical composition of Kun Yining Granules by UPLC-Q-TOF/MS method. Chin Tradit Pat Med. 2020;42:1074–82.Search in Google Scholar
[31] Lan X-Y, Zhu LB, Huang XZ, Liu DH, Hao QQ, Zhou L, et al. Study on identification and quantitation of main components in Artemisiae Argyi Folium. Chin Tradit Herb Drugs. 2021;52:7630–7.Search in Google Scholar
[32] Saha S, Prajapati DG, Ratrey P, Mishra A. Co-delivery nanosystem of Epigallocatechin Gallate and Rutin for anticancer and antibacterial activities. J Drug Deliv Sci Technol. 2022;70:103191.10.1016/j.jddst.2022.103191Search in Google Scholar
[33] Tian R, Yang W, Xue Q, Gao L, Huo J, Ren D, et al. Rutin ameliorates diabetic neuropathy by lowering plasma glucose and decreasing oxidative stress via Nrf2 signaling pathway in rats. Eur J Pharmacol. 2016;771:84–92.10.1016/j.ejphar.2015.12.021Search in Google Scholar PubMed
[34] Cipolla L, Cardona F, La Ferla B, Fusi P. 1-Deoxynojirimycin (DNJ) and pyrrolizidine derivatives as glycosidase inhibitors. Abstr Pap - Am Chem Soc; 2020.Search in Google Scholar
[35] Yu, M-H, Hung TW, Wang CC, Wu SW, Yang TW, Yang CY, et al. Neochlorogenic acid attenuates hepatic lipid accumulation and inflammation via regulating miR-34a in vitro. Int J Mol Sci. 2021;22:13163.10.3390/ijms222313163Search in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Characteristics, source, and health risk assessment of aerosol polyaromatic hydrocarbons in the rural and urban regions of western Saudi Arabia
- Regular Articles
- A network-based correlation research between element electronegativity and node importance
- Pomegranate attenuates kidney injury in cyclosporine-induced nephrotoxicity in rats by suppressing oxidative stress
- Ab initio study of fundamental properties of XInO3 (X = K, Rb, Cs) perovskites
- Responses of feldspathic sandstone and sand-reconstituted soil C and N to freeze–thaw cycles
- Robust fractional control based on high gain observers design (RNFC) for a Spirulina maxima culture interfaced with an advanced oxidation process
- Study on arsenic speciation and redistribution mechanism in Lonicera japonica plants via synchrotron techniques
- Optimization of machining Nilo 36 superalloy parameters in turning operation
- Vacuum impregnation pre-treatment: A novel method for incorporating mono- and divalent cations into potato strips to reduce the acrylamide formation in French fries
- Characterization of effective constituents in Acanthopanax senticosus fruit for blood deficiency syndrome based on the chinmedomics strategy
- Comparative analysis of the metabolites in Pinellia ternata from two producing regions using ultra-high-performance liquid chromatography–electrospray ionization–tandem mass spectrometry
- The assessment of environmental parameter along the desalination plants in the Kingdom of Saudi Arabia
- Effects of harpin and carbendazim on antioxidant accumulation in young jujube leaves
- The effects of in ovo injected with sodium borate on hatching performance and small intestine morphology in broiler chicks
- Optimization of cutting forces and surface roughness via ANOVA and grey relational analysis in machining of In718
- Essential oils of Origanum compactum Benth: Chemical characterization, in vitro, in silico, antioxidant, and antibacterial activities
- Translocation of tungsten(vi) oxide/gadolinium(iii) fluoride in tellurite glasses towards improvement of gamma-ray attenuation features in high-density glass shields
- Mechanical properties, elastic moduli, and gamma ray attenuation competencies of some TeO2–WO3–GdF3 glasses: Tailoring WO3–GdF3 substitution toward optimum behavioral state range
- Comparison between the CIDR or sponge with hormone injection to induce estrus synchronization for twining and sex preselection in Naimi sheep
- Exergetic performance analyses of three different cogeneration plants
- Psoralea corylifolia (babchi) seeds enhance proliferation of normal human cultured melanocytes: GC–MS profiling and biological investigation
- A novel electrochemical micro-titration method for quantitative evaluation of the DPPH free radical scavenging capacity of caffeic acid
- Comparative study between supported bimetallic catalysts for nitrate remediation in water
- Persicaline, an alkaloid from Salvadora persica, inhibits proliferation and induces apoptosis and cell-cycle arrest in MCF-7 cells
- Determination of nicotine content in locally produced smokeless tobacco (Shammah) samples from Jazan region of Saudi Arabia using a convenient HPLC-MS/MS method
- Changes in oxidative stress markers in pediatric burn injury over a 1-week period
- Integrated geophysical techniques applied for petroleum basins structural characterization in the central part of the Western Desert, Egypt
- The impact of chemical modifications on gamma-ray attenuation properties of some WO3-reinforced tellurite glasses
- Microwave and Cs+-assisted chemo selective reaction protocol for synthesizing 2-styryl quinoline biorelevant molecules
- Structural, physical, and radiation absorption properties of a significant nuclear power plant component: A comparison between REX-734 and 316L SS austenitic stainless steels
- Effect of Moringa oleifera on serum YKL-40 level: In vivo rat periodontitis model
- Investigating the impact of CO2 emissions on the COVID-19 pandemic by generalized linear mixed model approach with inverse Gaussian and gamma distributions
- Influence of WO3 content on gamma rays attenuation characteristics of phosphate glasses at low energy range
- Study on CO2 absorption performance of ternary DES formed based on DEA as promoting factor
- Performance analyses of detonation engine cogeneration cycles
- Sterols from Centaurea pumilio L. with cell proliferative activity: In vitro and in silico studies
- Untargeted metabolomics revealing changes in aroma substances in flue-cured tobacco
- Effect of pumpkin enriched with calcium lactate on iron status in an animal model of postmenopausal osteoporosis
- Energy consumption, mechanical and metallographic properties of cryogenically treated tool steels
- Optimization of ultra-high pressure-assisted extraction of total phenols from Eucommia ulmoides leaves by response surface methodology
- Harpin enhances antioxidant nutrient accumulation and decreases enzymatic browning in stored soybean sprouts
- Physicochemical and biological properties of carvacrol
- Radix puerariae in the treatment of diabetic nephropathy: A network pharmacology analysis and experimental validation
- Anti-Alzheimer, antioxidants, glucose-6-phosphate dehydrogenase effects of Taverniera glabra mediated ZnO and Fe2O3 nanoparticles in alloxan-induced diabetic rats
- Experimental study on photocatalytic CO2 reduction performance of ZnS/CdS-TiO2 nanotube array thin films
- Epoxy-reinforced heavy metal oxides for gamma ray shielding purposes
- Black mulberry (Morus nigra L.) fruits: As a medicinal plant rich in human health-promoting compounds
- Promising antioxidant and antimicrobial effects of essential oils extracted from fruits of Juniperus thurifera: In vitro and in silico investigations
- Chloramine-T-induced oxidation of Rizatriptan Benzoate: An integral chemical and spectroscopic study of products, mechanisms and kinetics
- Study on antioxidant and antimicrobial potential of chemically profiled essential oils extracted from Juniperus phoenicea (L.) by use of in vitro and in silico approaches
- Screening and characterization of fungal taxol-producing endophytic fungi for evaluation of antimicrobial and anticancer activities
- Mineral composition, principal polyphenolic components, and evaluation of the anti-inflammatory, analgesic, and antioxidant properties of Cytisus villosus Pourr leaf extracts
- In vitro antiproliferative efficacy of Annona muricata seed and fruit extracts on several cancer cell lines
- An experimental study for chemical characterization of artificial anterior cruciate ligament with coated chitosan as biomaterial
- Prevalence of residual risks of the transfusion-transmitted infections in Riyadh hospitals: A two-year retrospective study
- Computational and experimental investigation of antibacterial and antifungal properties of Nicotiana tabacum extracts
- Reinforcement of cementitious mortars with hemp fibers and shives
- X-ray shielding properties of bismuth-borate glass doped with rare earth ions
- Green supported silver nanoparticles over modified reduced graphene oxide: Investigation of its antioxidant and anti-ovarian cancer effects
- Orthogonal synthesis of a versatile building block for dual functionalization of targeting vectors
- Thymbra spicata leaf extract driven biogenic synthesis of Au/Fe3O4 nanocomposite and its bio-application in the treatment of different types of leukemia
- The role of Ag2O incorporation in nuclear radiation shielding behaviors of the Li2O–Pb3O4–SiO2 glass system: A multi-step characterization study
- A stimuli-responsive in situ spray hydrogel co-loaded with naringenin and gentamicin for chronic wounds
- Assessment of the impact of γ-irradiation on the piperine content and microbial quality of black pepper
- Antioxidant, sensory, and functional properties of low-alcoholic IPA beer with Pinus sylvestris L. shoots addition fermented using unconventional yeast
- Screening and optimization of extracellular pectinase produced by Bacillus thuringiensis SH7
- Determination of polyphenols in Chinese jujube using ultra-performance liquid chromatography–mass spectrometry
- Synergistic effects of harpin and NaCl in determining soybean sprout quality under non-sterile conditions
- Field evaluation of different eco-friendly alternative control methods against Panonychus citri [Acari: Tetranychidae] spider mite and its predators in citrus orchards
- Exploring the antimicrobial potential of biologically synthesized zero valent iron nanoparticles
- NaCl regulates goldfish growth and survival at three food supply levels under hypoxia
- An exploration of the physical, optical, mechanical, and radiation shielding properties of PbO–MgO–ZnO–B2O3 glasses
- A novel statistical modeling of air pollution and the COVID-19 pandemic mortality data by Poisson, geometric, and negative binomial regression models with fixed and random effects
- Treatment activity of the injectable hydrogels loaded with dexamethasone In(iii) complex on glioma by inhibiting the VEGF signaling pathway
- An alternative approach for the excess lifetime cancer risk and prediction of radiological parameters
- Panax ginseng leaf aqueous extract mediated green synthesis of AgNPs under ultrasound condition and investigation of its anti-lung adenocarcinoma effects
- Study of hydrolysis and production of instant ginger (Zingiber officinale) tea
- Novel green synthesis of zinc oxide nanoparticles using Salvia rosmarinus extract for treatment of human lung cancer
- Evaluation of second trimester plasma lipoxin A4, VEGFR-1, IL-6, and TNF-α levels in pregnant women with gestational diabetes mellitus
- Antidiabetic, antioxidant and cytotoxicity activities of ortho- and para-substituted Schiff bases derived from metformin hydrochloride: Validation by molecular docking and in silico ADME studies
- Antioxidant, antidiabetic, antiglaucoma, and anticholinergic effects of Tayfi grape (Vitis vinifera): A phytochemical screening by LC-MS/MS analysis
- Identification of genetic polymorphisms in the stearoyl CoA desaturase gene and its association with milk quality traits in Najdi sheep
- Cold-acclimation effect on cadmium absorption and biosynthesis of polyphenolics, and free proline and photosynthetic pigments in Spirogyra aequinoctialis
- Analysis of secondary metabolites in Xinjiang Morus nigra leaves using different extraction methods with UPLC-Q/TOF-MS/MS technology
- Nanoarchitectonics and performance evaluation of a Fe3O4-stabilized Pickering emulsion-type differential pressure plugging agent
- Investigating pyrolysis characteristics of Shengdong coal through Py-GC/MS
- Extraction, phytochemical characterization, and antifungal activity of Salvia rosmarinus extract
- Introducing a novel and natural antibiotic for the treatment of oral pathogens: Abelmoschus esculentus green-formulated silver nanoparticles
- Optimization of gallic acid-enriched ultrasonic-assisted extraction from mango peels
- Effect of gamma rays irradiation in the structure, optical, and electrical properties of samarium doped bismuth titanate ceramics
- Combinatory in silico investigation for potential inhibitors from Curcuma sahuynhensis Škorničk. & N.S. Lý volatile phytoconstituents against influenza A hemagglutinin, SARS-CoV-2 main protease, and Omicron-variant spike protein
- Physical, mechanical, and gamma ray shielding properties of the Bi2O3–BaO–B2O3–ZnO–As2O3–MgO–Na2O glass system
- Twofold interpenetrated 3D Cd(ii) complex: Crystal structure and luminescent property
- Study on the microstructure and soil quality variation of composite soil with soft rock and sand
- Ancient spring waters still emerging and accessible in the Roman Forum area: Chemical–physical and microbiological characterization
- Extraction and characterization of type I collagen from scales of Mexican Biajaiba fish
- Finding small molecular compounds to decrease trimethylamine oxide levels in atherosclerosis by virtual screening
- Prefatory in silico studies and in vitro insecticidal effect of Nigella sativa (L.) essential oil and its active compound (carvacrol) against the Callosobruchus maculatus adults (Fab), a major pest of chickpea
- Polymerized methyl imidazole silver bromide (CH3C6H5AgBr)6: Synthesis, crystal structures, and catalytic activity
- Using calcined waste fish bones as a green solid catalyst for biodiesel production from date seed oil
- Influence of the addition of WO3 on TeO2–Na2O glass systems in view of the feature of mechanical, optical, and photon attenuation
- Naringin ameliorates 5-fluorouracil elicited neurotoxicity by curtailing oxidative stress and iNOS/NF-ĸB/caspase-3 pathway
- GC-MS profile of extracts of an endophytic fungus Alternaria and evaluation of its anticancer and antibacterial potentialities
- Green synthesis, chemical characterization, and antioxidant and anti-colorectal cancer effects of vanadium nanoparticles
- Determination of caffeine content in coffee drinks prepared in some coffee shops in the local market in Jeddah City, Saudi Arabia
- A new 3D supramolecular Cu(ii) framework: Crystal structure and photocatalytic characteristics
- Bordeaux mixture accelerates ripening, delays senescence, and promotes metabolite accumulation in jujube fruit
- Important application value of injectable hydrogels loaded with omeprazole Schiff base complex in the treatment of pancreatitis
- Color tunable benzothiadiazole-based small molecules for lightening applications
- Investigation of structural, dielectric, impedance, and mechanical properties of hydroxyapatite-modified barium titanate composites for biomedical applications
- Metal gel particles loaded with epidermal cell growth factor promote skin wound repair mechanism by regulating miRNA
- In vitro exploration of Hypsizygus ulmarius (Bull.) mushroom fruiting bodies: Potential antidiabetic and anti-inflammatory agent
- Alteration in the molecular structure of the adenine base exposed to gamma irradiation: An ESR study
- Comprehensive study of optical, thermal, and gamma-ray shielding properties of Bi2O3–ZnO–PbO–B2O3 glasses
- Lewis acids as co-catalysts in Pd-based catalyzed systems of the octene-1 hydroethoxycarbonylation reaction
- Synthesis, Hirshfeld surface analysis, thermal, and selective α-glucosidase inhibitory studies of Schiff base transition metal complexes
- Protective properties of AgNPs green-synthesized by Abelmoschus esculentus on retinal damage on the virtue of its anti-inflammatory and antioxidant effects in diabetic rat
- Effects of green decorated AgNPs on lignin-modified magnetic nanoparticles mediated by Cydonia on cecal ligation and puncture-induced sepsis
- Treatment of gastric cancer by green mediated silver nanoparticles using Pistacia atlantica bark aqueous extract
- Preparation of newly developed porcelain ceramics containing WO3 nanoparticles for radiation shielding applications
- Utilization of computational methods for the identification of new natural inhibitors of human neutrophil elastase in inflammation therapy
- Some anticancer agents as effective glutathione S-transferase (GST) inhibitors
- Clay-based bricks’ rich illite mineral for gamma-ray shielding applications: An experimental evaluation of the effect of pressure rates on gamma-ray attenuation parameters
- Stability kinetics of orevactaene pigments produced by Epicoccum nigrum in solid-state fermentation
- Treatment of denture stomatitis using iron nanoparticles green-synthesized by Silybum marianum extract
- Characterization and antioxidant potential of white mustard (Brassica hirta) leaf extract and stabilization of sunflower oil
- Characteristics of Langmuir monomolecular monolayers formed by the novel oil blends
- Strategies for optimizing the single GdSrFeO4 phase synthesis
- Oleic acid and linoleic acid nanosomes boost immunity and provoke cell death via the upregulation of beta-defensin-4 at genetic and epigenetic levels
- Unraveling the therapeutic potential of Bombax ceiba roots: A comprehensive study of chemical composition, heavy metal content, antibacterial activity, and in silico analysis
- Green synthesis of AgNPs using plant extract and investigation of its anti-human colorectal cancer application
- The adsorption of naproxen on adsorbents obtained from pepper stalk extract by green synthesis
- Treatment of gastric cancer by silver nanoparticles encapsulated by chitosan polymers mediated by Pistacia atlantica extract under ultrasound condition
- In vitro protective and anti-inflammatory effects of Capparis spinosa and its flavonoids profile
- Wear and corrosion behavior of TiC and WC coatings deposited on high-speed steels by electro-spark deposition
- Therapeutic effects of green-formulated gold nanoparticles by Origanum majorana on spinal cord injury in rats
- Melanin antibacterial activity of two new strains, SN1 and SN2, of Exophiala phaeomuriformis against five human pathogens
- Evaluation of the analgesic and anesthetic properties of silver nanoparticles supported over biodegradable acacia gum-modified magnetic nanoparticles
- Review Articles
- Role and mechanism of fruit waste polyphenols in diabetes management
- A comprehensive review of non-alkaloidal metabolites from the subfamily Amaryllidoideae (Amaryllidaceae)
- Discovery of the chemical constituents, structural characteristics, and pharmacological functions of Chinese caterpillar fungus
- Eco-friendly green approach of nickel oxide nanoparticles for biomedical applications
- Advances in the pharmaceutical research of curcumin for oral administration
- Rapid Communication
- Determination of the contents of bioactive compounds in St. John’s wort (Hypericum perforatum): Comparison of commercial and wild samples
- Retraction
- Retraction of “Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: The protective effect on periodontitis via reducing the release of IL-1β and TNF-α”
- Topical Issue on Phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Anti-plasmodial potential of selected medicinal plants and a compound Atropine isolated from Eucalyptus obliqua
- Anthocyanin extract from black rice attenuates chronic inflammation in DSS-induced colitis mouse model by modulating the gut microbiota
- Evaluation of antibiofilm and cytotoxicity effect of Rumex vesicarius methanol extract
- Chemical compositions of Litsea umbellata and inhibition activities
- Green synthesis, characterization of silver nanoparticles using Rhynchosia capitata leaf extract and their biological activities
- GC-MS analysis and antibacterial activities of some plants belonging to the genus Euphorbia on selected bacterial isolates
- The abrogative effect of propolis on acrylamide-induced toxicity in male albino rats: Histological study
- A phytoconstituent 6-aminoflavone ameliorates lipopolysaccharide-induced oxidative stress mediated synapse and memory dysfunction via p-Akt/NF-kB pathway in albino mice
- Anti-diabetic potentials of Sorbaria tomentosa Lindl. Rehder: Phytochemistry (GC-MS analysis), α-amylase, α-glucosidase inhibitory, in vivo hypoglycemic, and biochemical analysis
- Assessment of cytotoxic and apoptotic activities of the Cassia angustifolia aqueous extract against SW480 colon cancer
- Biochemical analysis, antioxidant, and antibacterial efficacy of the bee propolis extract (Hymenoptera: Apis mellifera) against Staphylococcus aureus-induced infection in BALB/c mice: In vitro and in vivo study
- Assessment of essential elements and heavy metals in Saudi Arabian rice samples underwent various processing methods
- Two new compounds from leaves of Capparis dongvanensis (Sy, B. H. Quang & D. V. Hai) and inhibition activities
- Hydroxyquinoline sulfanilamide ameliorates STZ-induced hyperglycemia-mediated amyleoid beta burden and memory impairment in adult mice
- An automated reading of semi-quantitative hemagglutination results in microplates: Micro-assay for plant lectins
- Inductively coupled plasma mass spectrometry assessment of essential and toxic trace elements in traditional spices consumed by the population of the Middle Eastern region in their recipes
- Phytochemical analysis and anticancer activity of the Pithecellobium dulce seed extract in colorectal cancer cells
- Impact of climatic disturbances on the chemical compositions and metabolites of Salvia officinalis
- Physicochemical characterization, antioxidant and antifungal activities of essential oils of Urginea maritima and Allium sativum
- Phytochemical analysis and antifungal efficiency of Origanum majorana extracts against some phytopathogenic fungi causing tomato damping-off diseases
- Special Issue on 4th IC3PE
- Graphene quantum dots: A comprehensive overview
- Studies on the intercalation of calcium–aluminium layered double hydroxide-MCPA and its controlled release mechanism as a potential green herbicide
- Synergetic effect of adsorption and photocatalysis by zinc ferrite-anchored graphitic carbon nitride nanosheet for the removal of ciprofloxacin under visible light irradiation
- Exploring anticancer activity of the Indonesian guava leaf (Psidium guajava L.) fraction on various human cancer cell lines in an in vitro cell-based approach
- The comparison of gold extraction methods from the rock using thiourea and thiosulfate
- Special Issue on Marine environmental sciences and significance of the multidisciplinary approaches
- Sorption of alkylphenols and estrogens on microplastics in marine conditions
- Cytotoxic ketosteroids from the Red Sea soft coral Dendronephthya sp.
- Antibacterial and biofilm prevention metabolites from Acanthophora spicifera
- Characteristics, source, and health risk assessment of aerosol polyaromatic hydrocarbons in the rural and urban regions of western Saudi Arabia
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part II
- Green synthesis, characterization, and evaluation of antibacterial activities of cobalt nanoparticles produced by marine fungal species Periconia prolifica
- Combustion-mediated sol–gel preparation of cobalt-doped ZnO nanohybrids for the degradation of acid red and antibacterial performance
- Perinatal supplementation with selenium nanoparticles modified with ascorbic acid improves hepatotoxicity in rat gestational diabetes
- Evaluation and chemical characterization of bioactive secondary metabolites from endophytic fungi associated with the ethnomedicinal plant Bergenia ciliata
- Enhancing photovoltaic efficiency with SQI-Br and SQI-I sensitizers: A comparative analysis
- Nanostructured p-PbS/p-CuO sulfide/oxide bilayer heterojunction as a promising photoelectrode for hydrogen gas generation
Articles in the same Issue
- Characteristics, source, and health risk assessment of aerosol polyaromatic hydrocarbons in the rural and urban regions of western Saudi Arabia
- Regular Articles
- A network-based correlation research between element electronegativity and node importance
- Pomegranate attenuates kidney injury in cyclosporine-induced nephrotoxicity in rats by suppressing oxidative stress
- Ab initio study of fundamental properties of XInO3 (X = K, Rb, Cs) perovskites
- Responses of feldspathic sandstone and sand-reconstituted soil C and N to freeze–thaw cycles
- Robust fractional control based on high gain observers design (RNFC) for a Spirulina maxima culture interfaced with an advanced oxidation process
- Study on arsenic speciation and redistribution mechanism in Lonicera japonica plants via synchrotron techniques
- Optimization of machining Nilo 36 superalloy parameters in turning operation
- Vacuum impregnation pre-treatment: A novel method for incorporating mono- and divalent cations into potato strips to reduce the acrylamide formation in French fries
- Characterization of effective constituents in Acanthopanax senticosus fruit for blood deficiency syndrome based on the chinmedomics strategy
- Comparative analysis of the metabolites in Pinellia ternata from two producing regions using ultra-high-performance liquid chromatography–electrospray ionization–tandem mass spectrometry
- The assessment of environmental parameter along the desalination plants in the Kingdom of Saudi Arabia
- Effects of harpin and carbendazim on antioxidant accumulation in young jujube leaves
- The effects of in ovo injected with sodium borate on hatching performance and small intestine morphology in broiler chicks
- Optimization of cutting forces and surface roughness via ANOVA and grey relational analysis in machining of In718
- Essential oils of Origanum compactum Benth: Chemical characterization, in vitro, in silico, antioxidant, and antibacterial activities
- Translocation of tungsten(vi) oxide/gadolinium(iii) fluoride in tellurite glasses towards improvement of gamma-ray attenuation features in high-density glass shields
- Mechanical properties, elastic moduli, and gamma ray attenuation competencies of some TeO2–WO3–GdF3 glasses: Tailoring WO3–GdF3 substitution toward optimum behavioral state range
- Comparison between the CIDR or sponge with hormone injection to induce estrus synchronization for twining and sex preselection in Naimi sheep
- Exergetic performance analyses of three different cogeneration plants
- Psoralea corylifolia (babchi) seeds enhance proliferation of normal human cultured melanocytes: GC–MS profiling and biological investigation
- A novel electrochemical micro-titration method for quantitative evaluation of the DPPH free radical scavenging capacity of caffeic acid
- Comparative study between supported bimetallic catalysts for nitrate remediation in water
- Persicaline, an alkaloid from Salvadora persica, inhibits proliferation and induces apoptosis and cell-cycle arrest in MCF-7 cells
- Determination of nicotine content in locally produced smokeless tobacco (Shammah) samples from Jazan region of Saudi Arabia using a convenient HPLC-MS/MS method
- Changes in oxidative stress markers in pediatric burn injury over a 1-week period
- Integrated geophysical techniques applied for petroleum basins structural characterization in the central part of the Western Desert, Egypt
- The impact of chemical modifications on gamma-ray attenuation properties of some WO3-reinforced tellurite glasses
- Microwave and Cs+-assisted chemo selective reaction protocol for synthesizing 2-styryl quinoline biorelevant molecules
- Structural, physical, and radiation absorption properties of a significant nuclear power plant component: A comparison between REX-734 and 316L SS austenitic stainless steels
- Effect of Moringa oleifera on serum YKL-40 level: In vivo rat periodontitis model
- Investigating the impact of CO2 emissions on the COVID-19 pandemic by generalized linear mixed model approach with inverse Gaussian and gamma distributions
- Influence of WO3 content on gamma rays attenuation characteristics of phosphate glasses at low energy range
- Study on CO2 absorption performance of ternary DES formed based on DEA as promoting factor
- Performance analyses of detonation engine cogeneration cycles
- Sterols from Centaurea pumilio L. with cell proliferative activity: In vitro and in silico studies
- Untargeted metabolomics revealing changes in aroma substances in flue-cured tobacco
- Effect of pumpkin enriched with calcium lactate on iron status in an animal model of postmenopausal osteoporosis
- Energy consumption, mechanical and metallographic properties of cryogenically treated tool steels
- Optimization of ultra-high pressure-assisted extraction of total phenols from Eucommia ulmoides leaves by response surface methodology
- Harpin enhances antioxidant nutrient accumulation and decreases enzymatic browning in stored soybean sprouts
- Physicochemical and biological properties of carvacrol
- Radix puerariae in the treatment of diabetic nephropathy: A network pharmacology analysis and experimental validation
- Anti-Alzheimer, antioxidants, glucose-6-phosphate dehydrogenase effects of Taverniera glabra mediated ZnO and Fe2O3 nanoparticles in alloxan-induced diabetic rats
- Experimental study on photocatalytic CO2 reduction performance of ZnS/CdS-TiO2 nanotube array thin films
- Epoxy-reinforced heavy metal oxides for gamma ray shielding purposes
- Black mulberry (Morus nigra L.) fruits: As a medicinal plant rich in human health-promoting compounds
- Promising antioxidant and antimicrobial effects of essential oils extracted from fruits of Juniperus thurifera: In vitro and in silico investigations
- Chloramine-T-induced oxidation of Rizatriptan Benzoate: An integral chemical and spectroscopic study of products, mechanisms and kinetics
- Study on antioxidant and antimicrobial potential of chemically profiled essential oils extracted from Juniperus phoenicea (L.) by use of in vitro and in silico approaches
- Screening and characterization of fungal taxol-producing endophytic fungi for evaluation of antimicrobial and anticancer activities
- Mineral composition, principal polyphenolic components, and evaluation of the anti-inflammatory, analgesic, and antioxidant properties of Cytisus villosus Pourr leaf extracts
- In vitro antiproliferative efficacy of Annona muricata seed and fruit extracts on several cancer cell lines
- An experimental study for chemical characterization of artificial anterior cruciate ligament with coated chitosan as biomaterial
- Prevalence of residual risks of the transfusion-transmitted infections in Riyadh hospitals: A two-year retrospective study
- Computational and experimental investigation of antibacterial and antifungal properties of Nicotiana tabacum extracts
- Reinforcement of cementitious mortars with hemp fibers and shives
- X-ray shielding properties of bismuth-borate glass doped with rare earth ions
- Green supported silver nanoparticles over modified reduced graphene oxide: Investigation of its antioxidant and anti-ovarian cancer effects
- Orthogonal synthesis of a versatile building block for dual functionalization of targeting vectors
- Thymbra spicata leaf extract driven biogenic synthesis of Au/Fe3O4 nanocomposite and its bio-application in the treatment of different types of leukemia
- The role of Ag2O incorporation in nuclear radiation shielding behaviors of the Li2O–Pb3O4–SiO2 glass system: A multi-step characterization study
- A stimuli-responsive in situ spray hydrogel co-loaded with naringenin and gentamicin for chronic wounds
- Assessment of the impact of γ-irradiation on the piperine content and microbial quality of black pepper
- Antioxidant, sensory, and functional properties of low-alcoholic IPA beer with Pinus sylvestris L. shoots addition fermented using unconventional yeast
- Screening and optimization of extracellular pectinase produced by Bacillus thuringiensis SH7
- Determination of polyphenols in Chinese jujube using ultra-performance liquid chromatography–mass spectrometry
- Synergistic effects of harpin and NaCl in determining soybean sprout quality under non-sterile conditions
- Field evaluation of different eco-friendly alternative control methods against Panonychus citri [Acari: Tetranychidae] spider mite and its predators in citrus orchards
- Exploring the antimicrobial potential of biologically synthesized zero valent iron nanoparticles
- NaCl regulates goldfish growth and survival at three food supply levels under hypoxia
- An exploration of the physical, optical, mechanical, and radiation shielding properties of PbO–MgO–ZnO–B2O3 glasses
- A novel statistical modeling of air pollution and the COVID-19 pandemic mortality data by Poisson, geometric, and negative binomial regression models with fixed and random effects
- Treatment activity of the injectable hydrogels loaded with dexamethasone In(iii) complex on glioma by inhibiting the VEGF signaling pathway
- An alternative approach for the excess lifetime cancer risk and prediction of radiological parameters
- Panax ginseng leaf aqueous extract mediated green synthesis of AgNPs under ultrasound condition and investigation of its anti-lung adenocarcinoma effects
- Study of hydrolysis and production of instant ginger (Zingiber officinale) tea
- Novel green synthesis of zinc oxide nanoparticles using Salvia rosmarinus extract for treatment of human lung cancer
- Evaluation of second trimester plasma lipoxin A4, VEGFR-1, IL-6, and TNF-α levels in pregnant women with gestational diabetes mellitus
- Antidiabetic, antioxidant and cytotoxicity activities of ortho- and para-substituted Schiff bases derived from metformin hydrochloride: Validation by molecular docking and in silico ADME studies
- Antioxidant, antidiabetic, antiglaucoma, and anticholinergic effects of Tayfi grape (Vitis vinifera): A phytochemical screening by LC-MS/MS analysis
- Identification of genetic polymorphisms in the stearoyl CoA desaturase gene and its association with milk quality traits in Najdi sheep
- Cold-acclimation effect on cadmium absorption and biosynthesis of polyphenolics, and free proline and photosynthetic pigments in Spirogyra aequinoctialis
- Analysis of secondary metabolites in Xinjiang Morus nigra leaves using different extraction methods with UPLC-Q/TOF-MS/MS technology
- Nanoarchitectonics and performance evaluation of a Fe3O4-stabilized Pickering emulsion-type differential pressure plugging agent
- Investigating pyrolysis characteristics of Shengdong coal through Py-GC/MS
- Extraction, phytochemical characterization, and antifungal activity of Salvia rosmarinus extract
- Introducing a novel and natural antibiotic for the treatment of oral pathogens: Abelmoschus esculentus green-formulated silver nanoparticles
- Optimization of gallic acid-enriched ultrasonic-assisted extraction from mango peels
- Effect of gamma rays irradiation in the structure, optical, and electrical properties of samarium doped bismuth titanate ceramics
- Combinatory in silico investigation for potential inhibitors from Curcuma sahuynhensis Škorničk. & N.S. Lý volatile phytoconstituents against influenza A hemagglutinin, SARS-CoV-2 main protease, and Omicron-variant spike protein
- Physical, mechanical, and gamma ray shielding properties of the Bi2O3–BaO–B2O3–ZnO–As2O3–MgO–Na2O glass system
- Twofold interpenetrated 3D Cd(ii) complex: Crystal structure and luminescent property
- Study on the microstructure and soil quality variation of composite soil with soft rock and sand
- Ancient spring waters still emerging and accessible in the Roman Forum area: Chemical–physical and microbiological characterization
- Extraction and characterization of type I collagen from scales of Mexican Biajaiba fish
- Finding small molecular compounds to decrease trimethylamine oxide levels in atherosclerosis by virtual screening
- Prefatory in silico studies and in vitro insecticidal effect of Nigella sativa (L.) essential oil and its active compound (carvacrol) against the Callosobruchus maculatus adults (Fab), a major pest of chickpea
- Polymerized methyl imidazole silver bromide (CH3C6H5AgBr)6: Synthesis, crystal structures, and catalytic activity
- Using calcined waste fish bones as a green solid catalyst for biodiesel production from date seed oil
- Influence of the addition of WO3 on TeO2–Na2O glass systems in view of the feature of mechanical, optical, and photon attenuation
- Naringin ameliorates 5-fluorouracil elicited neurotoxicity by curtailing oxidative stress and iNOS/NF-ĸB/caspase-3 pathway
- GC-MS profile of extracts of an endophytic fungus Alternaria and evaluation of its anticancer and antibacterial potentialities
- Green synthesis, chemical characterization, and antioxidant and anti-colorectal cancer effects of vanadium nanoparticles
- Determination of caffeine content in coffee drinks prepared in some coffee shops in the local market in Jeddah City, Saudi Arabia
- A new 3D supramolecular Cu(ii) framework: Crystal structure and photocatalytic characteristics
- Bordeaux mixture accelerates ripening, delays senescence, and promotes metabolite accumulation in jujube fruit
- Important application value of injectable hydrogels loaded with omeprazole Schiff base complex in the treatment of pancreatitis
- Color tunable benzothiadiazole-based small molecules for lightening applications
- Investigation of structural, dielectric, impedance, and mechanical properties of hydroxyapatite-modified barium titanate composites for biomedical applications
- Metal gel particles loaded with epidermal cell growth factor promote skin wound repair mechanism by regulating miRNA
- In vitro exploration of Hypsizygus ulmarius (Bull.) mushroom fruiting bodies: Potential antidiabetic and anti-inflammatory agent
- Alteration in the molecular structure of the adenine base exposed to gamma irradiation: An ESR study
- Comprehensive study of optical, thermal, and gamma-ray shielding properties of Bi2O3–ZnO–PbO–B2O3 glasses
- Lewis acids as co-catalysts in Pd-based catalyzed systems of the octene-1 hydroethoxycarbonylation reaction
- Synthesis, Hirshfeld surface analysis, thermal, and selective α-glucosidase inhibitory studies of Schiff base transition metal complexes
- Protective properties of AgNPs green-synthesized by Abelmoschus esculentus on retinal damage on the virtue of its anti-inflammatory and antioxidant effects in diabetic rat
- Effects of green decorated AgNPs on lignin-modified magnetic nanoparticles mediated by Cydonia on cecal ligation and puncture-induced sepsis
- Treatment of gastric cancer by green mediated silver nanoparticles using Pistacia atlantica bark aqueous extract
- Preparation of newly developed porcelain ceramics containing WO3 nanoparticles for radiation shielding applications
- Utilization of computational methods for the identification of new natural inhibitors of human neutrophil elastase in inflammation therapy
- Some anticancer agents as effective glutathione S-transferase (GST) inhibitors
- Clay-based bricks’ rich illite mineral for gamma-ray shielding applications: An experimental evaluation of the effect of pressure rates on gamma-ray attenuation parameters
- Stability kinetics of orevactaene pigments produced by Epicoccum nigrum in solid-state fermentation
- Treatment of denture stomatitis using iron nanoparticles green-synthesized by Silybum marianum extract
- Characterization and antioxidant potential of white mustard (Brassica hirta) leaf extract and stabilization of sunflower oil
- Characteristics of Langmuir monomolecular monolayers formed by the novel oil blends
- Strategies for optimizing the single GdSrFeO4 phase synthesis
- Oleic acid and linoleic acid nanosomes boost immunity and provoke cell death via the upregulation of beta-defensin-4 at genetic and epigenetic levels
- Unraveling the therapeutic potential of Bombax ceiba roots: A comprehensive study of chemical composition, heavy metal content, antibacterial activity, and in silico analysis
- Green synthesis of AgNPs using plant extract and investigation of its anti-human colorectal cancer application
- The adsorption of naproxen on adsorbents obtained from pepper stalk extract by green synthesis
- Treatment of gastric cancer by silver nanoparticles encapsulated by chitosan polymers mediated by Pistacia atlantica extract under ultrasound condition
- In vitro protective and anti-inflammatory effects of Capparis spinosa and its flavonoids profile
- Wear and corrosion behavior of TiC and WC coatings deposited on high-speed steels by electro-spark deposition
- Therapeutic effects of green-formulated gold nanoparticles by Origanum majorana on spinal cord injury in rats
- Melanin antibacterial activity of two new strains, SN1 and SN2, of Exophiala phaeomuriformis against five human pathogens
- Evaluation of the analgesic and anesthetic properties of silver nanoparticles supported over biodegradable acacia gum-modified magnetic nanoparticles
- Review Articles
- Role and mechanism of fruit waste polyphenols in diabetes management
- A comprehensive review of non-alkaloidal metabolites from the subfamily Amaryllidoideae (Amaryllidaceae)
- Discovery of the chemical constituents, structural characteristics, and pharmacological functions of Chinese caterpillar fungus
- Eco-friendly green approach of nickel oxide nanoparticles for biomedical applications
- Advances in the pharmaceutical research of curcumin for oral administration
- Rapid Communication
- Determination of the contents of bioactive compounds in St. John’s wort (Hypericum perforatum): Comparison of commercial and wild samples
- Retraction
- Retraction of “Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: The protective effect on periodontitis via reducing the release of IL-1β and TNF-α”
- Topical Issue on Phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Anti-plasmodial potential of selected medicinal plants and a compound Atropine isolated from Eucalyptus obliqua
- Anthocyanin extract from black rice attenuates chronic inflammation in DSS-induced colitis mouse model by modulating the gut microbiota
- Evaluation of antibiofilm and cytotoxicity effect of Rumex vesicarius methanol extract
- Chemical compositions of Litsea umbellata and inhibition activities
- Green synthesis, characterization of silver nanoparticles using Rhynchosia capitata leaf extract and their biological activities
- GC-MS analysis and antibacterial activities of some plants belonging to the genus Euphorbia on selected bacterial isolates
- The abrogative effect of propolis on acrylamide-induced toxicity in male albino rats: Histological study
- A phytoconstituent 6-aminoflavone ameliorates lipopolysaccharide-induced oxidative stress mediated synapse and memory dysfunction via p-Akt/NF-kB pathway in albino mice
- Anti-diabetic potentials of Sorbaria tomentosa Lindl. Rehder: Phytochemistry (GC-MS analysis), α-amylase, α-glucosidase inhibitory, in vivo hypoglycemic, and biochemical analysis
- Assessment of cytotoxic and apoptotic activities of the Cassia angustifolia aqueous extract against SW480 colon cancer
- Biochemical analysis, antioxidant, and antibacterial efficacy of the bee propolis extract (Hymenoptera: Apis mellifera) against Staphylococcus aureus-induced infection in BALB/c mice: In vitro and in vivo study
- Assessment of essential elements and heavy metals in Saudi Arabian rice samples underwent various processing methods
- Two new compounds from leaves of Capparis dongvanensis (Sy, B. H. Quang & D. V. Hai) and inhibition activities
- Hydroxyquinoline sulfanilamide ameliorates STZ-induced hyperglycemia-mediated amyleoid beta burden and memory impairment in adult mice
- An automated reading of semi-quantitative hemagglutination results in microplates: Micro-assay for plant lectins
- Inductively coupled plasma mass spectrometry assessment of essential and toxic trace elements in traditional spices consumed by the population of the Middle Eastern region in their recipes
- Phytochemical analysis and anticancer activity of the Pithecellobium dulce seed extract in colorectal cancer cells
- Impact of climatic disturbances on the chemical compositions and metabolites of Salvia officinalis
- Physicochemical characterization, antioxidant and antifungal activities of essential oils of Urginea maritima and Allium sativum
- Phytochemical analysis and antifungal efficiency of Origanum majorana extracts against some phytopathogenic fungi causing tomato damping-off diseases
- Special Issue on 4th IC3PE
- Graphene quantum dots: A comprehensive overview
- Studies on the intercalation of calcium–aluminium layered double hydroxide-MCPA and its controlled release mechanism as a potential green herbicide
- Synergetic effect of adsorption and photocatalysis by zinc ferrite-anchored graphitic carbon nitride nanosheet for the removal of ciprofloxacin under visible light irradiation
- Exploring anticancer activity of the Indonesian guava leaf (Psidium guajava L.) fraction on various human cancer cell lines in an in vitro cell-based approach
- The comparison of gold extraction methods from the rock using thiourea and thiosulfate
- Special Issue on Marine environmental sciences and significance of the multidisciplinary approaches
- Sorption of alkylphenols and estrogens on microplastics in marine conditions
- Cytotoxic ketosteroids from the Red Sea soft coral Dendronephthya sp.
- Antibacterial and biofilm prevention metabolites from Acanthophora spicifera
- Characteristics, source, and health risk assessment of aerosol polyaromatic hydrocarbons in the rural and urban regions of western Saudi Arabia
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part II
- Green synthesis, characterization, and evaluation of antibacterial activities of cobalt nanoparticles produced by marine fungal species Periconia prolifica
- Combustion-mediated sol–gel preparation of cobalt-doped ZnO nanohybrids for the degradation of acid red and antibacterial performance
- Perinatal supplementation with selenium nanoparticles modified with ascorbic acid improves hepatotoxicity in rat gestational diabetes
- Evaluation and chemical characterization of bioactive secondary metabolites from endophytic fungi associated with the ethnomedicinal plant Bergenia ciliata
- Enhancing photovoltaic efficiency with SQI-Br and SQI-I sensitizers: A comparative analysis
- Nanostructured p-PbS/p-CuO sulfide/oxide bilayer heterojunction as a promising photoelectrode for hydrogen gas generation


