Abstract
This study provides insights into the effects of Bi2O3 on the physical, mechanical, and gamma ray shielding properties of Bi2O3–BaO–B2O3–ZnO–As2O3–MgO–Na2O glasses. The higher Bi2O3 concentrations result in increased density and molecular weight of the glasses. The molar volume also increases with higher Bi2O3 percentages, accompanied by a decrease in the average distance between boron atoms and a reduction in polaron radius and inter-nuclear distance. Electronegativity decreases and electronic polarizability increases with increasing Bi2O3 concentration, indicating higher electron-donating capacity and greater susceptibility to external electric field distortion. The elastic moduli exhibit a downward trend with increasing Bi2O3 concentration, indicating a decreased degree of elastic behaviour. The decrease in cross-linking is further supported by the reduction in Poisson’s ratio. The decrease in values of the hardness also indicates a decline in the stiffness and connectivity of the glass network. The linear attenuation coefficients (LACs) of three different glasses were obtained using Phy-X software in 0.015–15 MeV energy range. Also, the effective atomic numbers are calculated for the selected glasses. The LAC has the highest values for Bi21, indicating that the addition of Bi2O3 causes an improvement in the LAC.
1 Introduction
A key component of modern science that has improved the quality and durability of life on earth is the use of radiation with sufficiently high energy [1,2]. Radiation is used in various domains such as healthcare, manufacturing, energy production, nuclear research, material analysis, and food preservation, showcasing its beneficial impact on both humanity and the environment [3,4,5]. Despite the inherent risks associated with exposure to any artificial radiation, these practical boundaries are continually expanding. This is due to the fact that such hazards have been effectively reduced to the absolute smallest amount, and in certain instances eliminated, as a result of the availability of sufficient radiation control techniques [6,7,8,9]. The damage caused by ionizing radiation in the bodies of living organisms is a complex interaction. One of the essential processes driving these damages is the formation of radicals during exposure to gamma radiation and X-ray. The assessment of radiation interaction factors plays a crucial role in selecting suitable materials for research and development in radiation shielding. These factors provide valuable insights into the absorption of radiation and its energy by different materials [10,11]. Consequently, the analysis of interaction factors enables the evaluation of a material’s potential for providing effective radiation shielding in nuclear facilities [12]. Extensive investigations have been conducted on a diverse range of functional materials, including alloys, steel, concrete, ceramics, polymers, and glasses, to explore their radiation interaction characteristics and their potential for radiation shielding [13,14,15,16]. The effectiveness of several of these shielding materials has been demonstrated to be comparable to, and in some cases even superior to, that of more conventional shielding materials. Over the past few years, there has been a growing demand for novel conventional shielding materials due to various factors. These factors include the instability and lack of uniformity in chemical composition, the existence of cracks, limited transparency, high density, and environmental concerns associated with conventional shielding materials [17,18].
Currently, a wide range of oxide glasses with diverse structural, optical, thermal, mechanical, and chemical properties have been extensively studied for their effectiveness in radiation shielding against various types of radiations. This increased interest can be attributed to their relatively superior chemical and mechanical stability when compared to other materials [19,20]. Mandal et al. [21] studied the lead-free multi-component non-toxic Bi2O3–BaO–B2O3–ZnO–As2O3–MgO–Na2O glass system for their thermal behaviour using dilatometric test, structural and microstructural properties using the X-ray diffraction, Raman and scanning electron microscope, and optical and photoluminescence studies in detail. But as far as the physical and mechanical characteristics are considered, only the variation of density with mol% of Bi2O3 is discussed so far. As the selected glasses contain the sufficient amount of Bi2O3 and possess fairly good density values, their gamma ray shielding behaviour needs to be studied for the application of the glasses in the field of radiation shielding. So in this proposed work, the physical, mechanical, and gamma ray shielding behaviour of the selected glasses have been studied.
2 Materials and methods
2.1 Details of the samples
The composition and density of the Bi2O3–BaO–B2O3–ZnO–As2O3–MgO–Na2O glass system have been obtained from the study of Mandal et al. [21]. Mandal et al. [21] measured the density (ρ) of the glasses using the helium gas pycnometer and Archimedes principle. In this study, the density values measured using the Archimedes principle are used. The sample codes depending on the amount of Bi2O3 present in the samples and their density values are as follows:
Bi15: 15Bi2O3–8BaO–41B2O3–10ZnO–4As2O3–12MgO–10Na2O (density = 4.59 g/cm3).
Bi18: 18Bi2O3–8BaO–38B2O3–10ZnO–4As2O3–12MgO–10Na2O (density = 4.77 g/cm3).
Bi21: 21Bi2O3–8BaO–35B2O3–10ZnO–4As2O3–12MgO–10Na2O (density = 5.07 g/cm3).
2.2 Physical properties
The molar volume (V m) was calculated from its molar mass (M) using the following formula [22]:
The average boron–boron separation (
where
If x B is the B2O3’s mole fraction [22], then
The dopant concentration (N), inter-nuclear distance (r i), polaron radius (r p), and field strength (F) are calculated as [22,23]:
where “z” represents the oxidation state of the dopant ions.
The formula for determining the oxygen molar volume (V o) of the glasses is as follows [23]:
where n is the number of oxygen atoms in one formula unit.
The equation for calculating oxygen packing density (OPD) is as follows [23]:
The equation for calculating optical basicity (Ʌth) is as follows [23]:
where Ʌ i and x i are the optical basicities and the mole fractions of each oxide (Ʌ1(Bi2O3) = 1.19, Ʌ2(BaO) = 1.15, Ʌ3(ZnO) = 1.03, Ʌ4(B2O3) = 0.43, Ʌ5(As2O3) = 1.02, Ʌ6(MgO) = 0.78, Ʌ7(Na2O) = 1.15).
The equation for calculating average electronegativity (χ av) is as follows [24]:
The equation for calculating electronic polarizability (
2.3 Mechanical properties
The equation for calculating average cross-link density (
where n f is the coordination number of the cations present in the sample.
The equation for calculating number of bonds per unit volume of the glasses (n b) is as follows [28]:
The mechanical characteristics of glasses, according to Makishima–Mackenzie’s theory [29,30] are as follows:
The equation for calculating atomic packing density (V t) is as follows [29,30]:
The equation for calculating interatomic bonding energy (G t) is as follows [29,30]:
where V i and G i represent the atomic packing densities and interatomic bond energies of the constituent atoms in glasses, respectively [31].
The equation for calculating Young’s modulus (E) is as follows:
The equation for calculating bulk modulus (B) is as follows:
The equation for calculating shear modulus (G) is as follows:
The equation for calculating longitudinal modulus (L) is as follows:
The equation for calculating Poisson’s ratio is as follows:
The equation for calculating fractal bond connectivity (d) is as follows:
The equation for calculating Hardness (H) is as follows:
2.4 Gamma ray shielding properties
The radiation shielding parameters for the selected glasses were determined in the range of 0.015–15 MeV. The linear attenuation coefficient (LAC) is a basic parameter that can be used to understand the glass’s ability to attenuate the incoming radiation. Also, it is a useful parameter since the other parameters are calculated from the LAC such as the half value layer (HVL) and mean free path (MFP). Any material’s HVL value refers to its thickness, which reduces 50% of the radiation that enters it. It is related to the LAC by the following equation:
The MFP stands for the average distance that a travelling photon travels between successive encounters. MFP is a crucial component of offering greater protection quality. It is given by:
The tenth-value layer (TVL) is the average quantity of material thickness, measured in cm, which must be present for the radiation to be reduced to one tenth of its initial intensity (a reduction of 90%), which is calculated by:
3 Results and discussion
3.1 Physical properties
Table 1 presents the physical properties of the glasses. The higher concentrations of Bi2O3 (15–21 mol%) resulted in increased density and molecular weight of the glasses. The glass network undergoes a substitution of less dense B2O3 with a denser Bi2O3 form. The greater density observed with increased Bi2O3 concentration (as shown in Figure 1a) can be attributed to the higher atomic mass of Bi2O3 in comparison to B2O3. Figure 1a illustrates that the molar volume of the glasses increases from 30.02 to 31.87 cm3 with an increase in the percentage of Bi2O3 in the glasses. It is due to the fact that incorporation of Bi2O3 results in the increase in molar mass of the sample by higher fraction as compared to increase in its density due to larger size of Bi [32,33,34]. The average distance between boron atoms (d B–B) decreases from 3.48 to 3.44 Å as the concentration of Bi2O3 increases. This is likely due to the increase in ion concentration from 3.01 × 1021 to 3.97 × 1021 ions/cm3 with the increase in Bi2O3 concentration. Additionally, the polaron radius and inter-nuclear distance exhibit a decrease from 2.79 to 2.54 Å and from 6.93 to 6.32 Å, respectively. These changes suggest that the glass network is becoming denser. The excessive presence of Bi2O3 enhances the bonding between Bi ions and oxygen, leading to a rise in the field strength around the Bi ion from 2.57 × 1015 to 3.09 × 1015 cm2.
Physical parameters
| Properties | Physical parameters | ||
|---|---|---|---|
| Bi15 | Bi18 | Bi21 | |
| M (g) | 137.79 | 149.68 | 161.57 |
| V m (cm3) | 30.02 | 31.38 | 31.87 |
| N (×1021 ions cm‒3) | 3.01 | 3.45 | 3.97 |
| V m b (cm3) | 25.44 | 25.31 | 24.51 |
| 〈d B–B〉 (Å) | 3.48 | 3.47 | 3.44 |
| r p (Å) | 2.79 | 2.67 | 2.54 |
| r i (Å) | 6.93 | 6.61 | 6.32 |
| F × 1015 (cm2) | 2.57 | 2.82 | 3.09 |
| OPD | 73.29 | 70.11 | 69.03 |
| V o (cm3 mol‒1) | 13.65 | 14.26 | 14.49 |
| Ʌth | 0.80 | 0.82 | 0.84 |
| χ av | 2.29 | 2.26 | 2.24 |
|
|
1.92 | 1.97 | 2.02 |
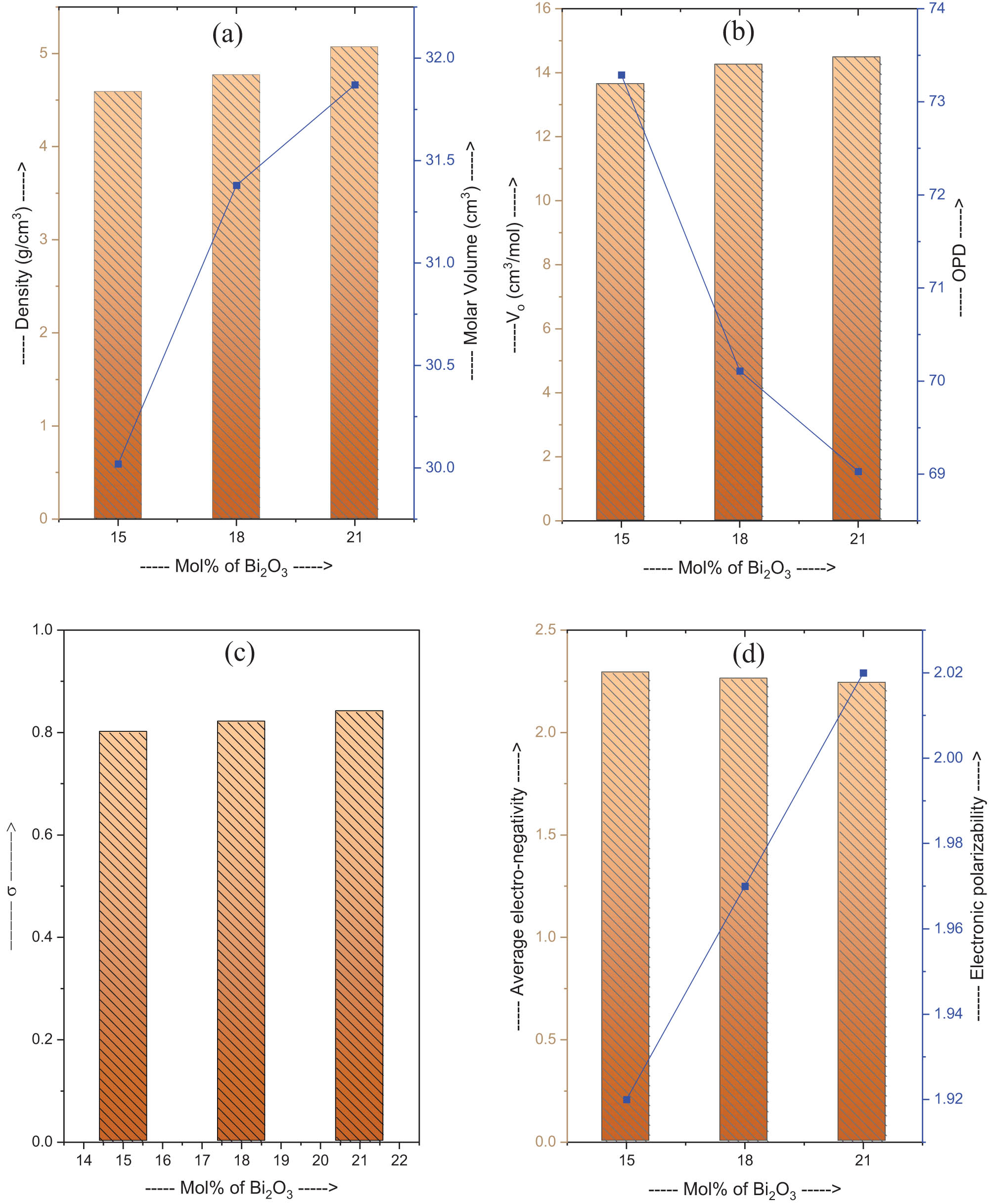
Variation of physical properties. (a) density and molar volume, (b) V o and OPD, (c) σ and (d) average electro-negativity and electronic polarizability with mol% of Bi2O3.
Incorporating Bi2O3 alters the V o and OPD values. The study reveals a positive correlation between the amount of Bi2O3 in the glass network and the V o, which increases from 13.65 to 14.49 cm3/mol. The OPD declines from 73.29 to 69.03. The observed phenomenon indicates an increase in the V o within the glass network, attributed to the greater space occupied by oxygen atoms. Additionally, the observed decrease in OPD is attributed to a less dense packing of oxygen atoms. Figure 1b illustrates the changes in the said parameters as the mol% of Bi2O3 in the glass composition varies. The inverse relationship between these parameters can be attributed to the impact of glass density and molar volume. The study suggests that Bi2O3 impacts the oxygen atom arrangement, leading to alterations in the V o and OPD [32,33,34].
Optical basicity is a property of glass that is determined by the electron accepting or donating ability of its constituent ions. It is closely linked to the glass’s physical and chemical characteristics. The findings indicate that the optical basicity of the glass increased from 0.80 to 0.84 as a consequence of increased Bi2O3 content as shown in Figure 1c. The inclusion of Bi2O3 results in increased negative charges on the oxygen atoms within the glass network. This trend is due to the amphoteric properties of Bi2O3, enabling it to function as both an acid and a base. The optical basicity of glass can be enhanced by substituting acidic oxide (B2O3) within the glass network. This behaviour underscores the superior capacity of oxide ions to convey electrons to adjacent cations.
The relationship between electronegativity and electronic polarizability in glasses as a function of the mol% of Bi2O3 is examined, as illustrated in Figure1d. Lower electronegativity indicates higher electron-donating capacity. The rise in mol% of Bi2O3 in the glasses results in a reduction of electronegativity from 2.29 to 2.24. The results indicate an increase in electron-donating properties of the samples with increasing Bi2O3 concentration. Electronic polarizability measures the susceptibility of an atom or molecule’s electron cloud to distortion by an external electric field. The electronic polarizability of the glasses increases from 1.92 to 2.02 as the mol% of Bi2O3 is increased [32].
3.2 Mechanical properties
The value of
The V t and G t values may be used in conjunction with the Makishima–Mackenzie’s model to derive additional elastic parameters. The elastic parameters that were determined as a consequence are listed in Table 2. The fluctuations of these parameters with the concentration of Bi2O3 are then analysed, which reveals a downward trend in the values of E, B, G, and L. To be more specific, E drops from a value of 76.843 to a value of 70.329, B drops from a value of 52.875 to a value of 46.423, G drops from a value of 32.588 to a value of 30.088, and L drops from a value of 96.327 to a value of 86.541 [33]. Figure 2 illustrates how those values changed in relation to the amount of Bi2O3 in the sample. These lowering values imply that the samples under investigation exhibit a decreased degree of elastic behaviour. As the concentration of Bi2O3 increases, it leads to an expansion of the network. This expansion, in turn, contributes to the decreasing nature of the elastic behaviour observed in these samples. This tendency is supported even further by the drop in the values of the σ variable. The decrease in σ with increasing Bi2O3 concentration, which ranges from 0.259 to 0.248, verifies the decrease in the amount of cross linking in the network. The range of values for d, which is from 2.465 to 2.593, is indicative of a layered structure in 2D. The decline in the stiffness and connectivity of the network is reflected in the values of H, which ranges from 4.914 to 4.727 before showing a downward trend [33].
Mechanical properties
| Sample | Mechanical properties | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n b (×1022 cm−3) |
|
V t (cm3 mol−1) | G t (kcal cm−3) | E (GPa) | B (GPa) | G (GPa) | L (GPa) | σ | d | H (GPa) | |
| Bi15 | 10.23 × 1022 | 3.675 | 0.575 | 15.979 | 76.843 | 52.875 | 32.588 | 96.327 | 0.259 | 2.465 | 4.914 |
| Bi18 | 9.904 × 1022 | 3.750 | 0.555 | 15.612 | 72.481 | 48.149 | 30.967 | 89.438 | 0.250 | 2.573 | 4.834 |
| Bi21 | 9.866 × 1022 | 3.825 | 0.552 | 12.245 | 70.329 | 46.423 | 30.088 | 86.541 | 0.248 | 2.593 | 4.727 |
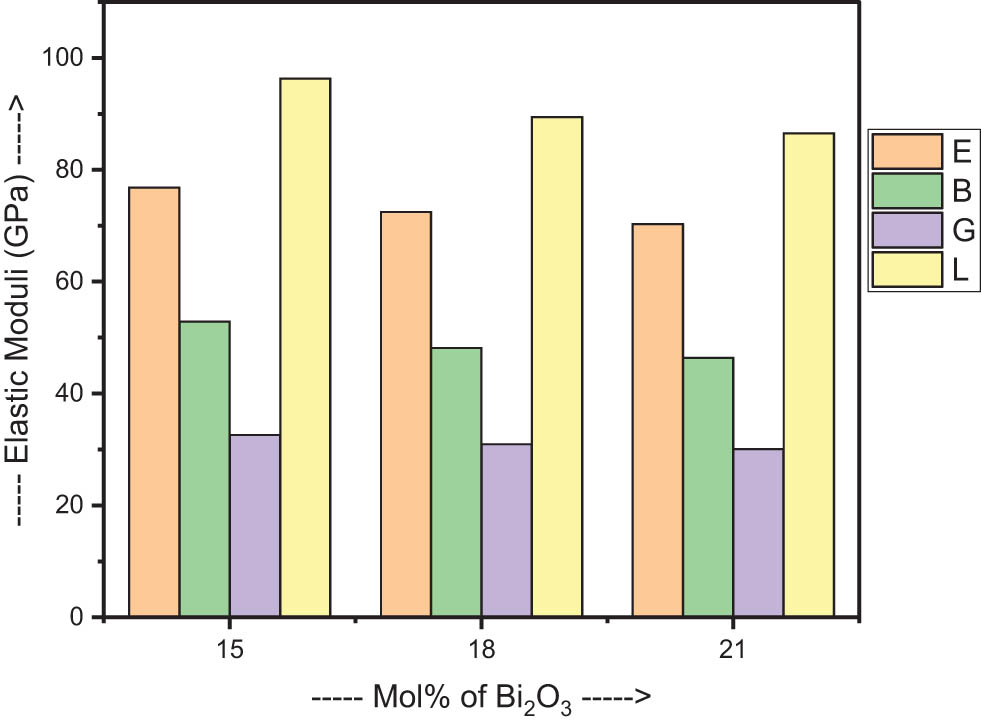
Mechanical properties of the samples.
3.3 Gamma ray shielding properties
Using the Phy-X software, the LACs of three distinct glass types were determined within the energy range of 0.015–15 MeV [35]. Figure 3 illustrates the LAC values for the investigated Bi15, Bi18, and Bi21 glasses. Notably, as the photon energy increases up to 0.2 MeV, the LAC values for all the selected glasses exhibit a significant decline. This can be attributed to the influence of the atomic number (Z) in the Z4–5 form, which affects the cross-section of the photoelectric absorption effect, the primary interaction mechanism at lower energies. Hence, a substantial portion of photon interactions with the glasses occurs within the low-energy region of the electromagnetic spectrum. Beyond 0.2 MeV, where Compton scattering predominates and its cross-section is linearly dependent on the atomic number, the LAC values remain relatively small and consistent across all the samples. However, at higher energies, the dominant interaction process is pair production, which is connected to Z 2. Consequently, a slight improvement in the LAC values is observed in the latter energy range. The LAC has the highest values for Bi21, as can be observed in Figure 3, indicating that the addition of Bi2O3 causes an improvement in the LAC.
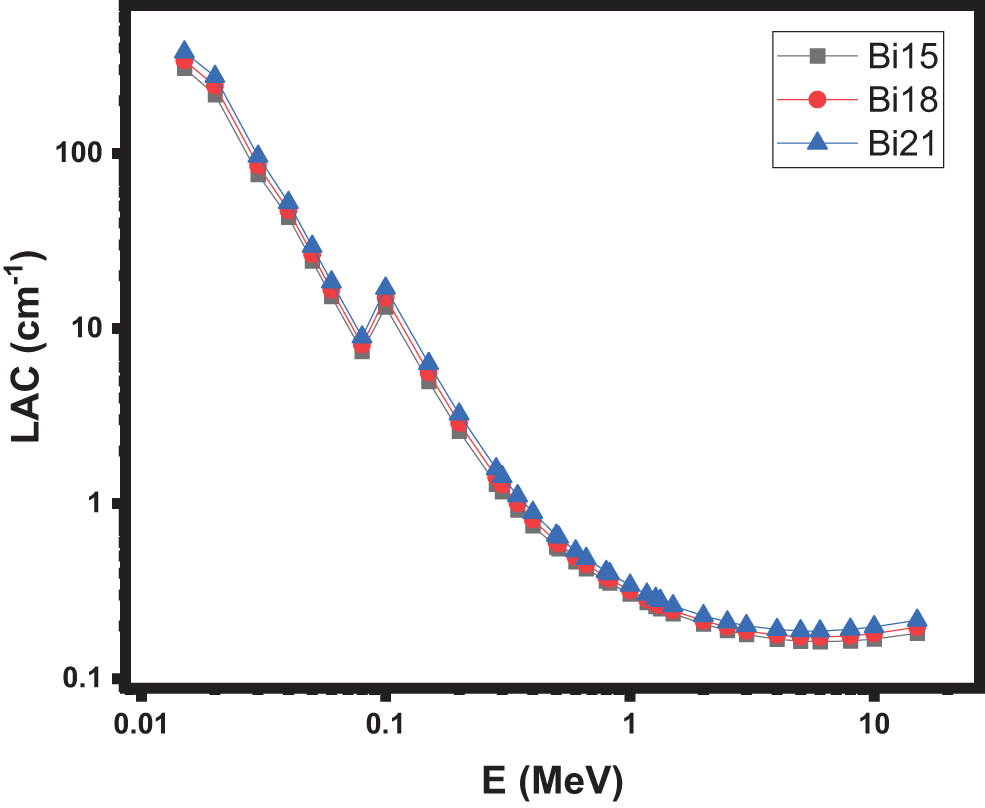
The LAC of the samples.
Figures 4 and 5 illustrate the graphical representation of how the HVL and TVL values vary with photon energy for different glass types. In contrast to the LAC trend shown in the previous figure, the HVL and TVL values increase with increasing energy levels. It is worth noting that both parameters exhibit an inverse relationship with the density of the samples. This can be observed in Figures 4 and 5, where the Bi21 glasses, which have the highest densities, exhibit lower HVL and TVL values compared to the other glasses. These findings align with previous research conducted on various glass systems [13].
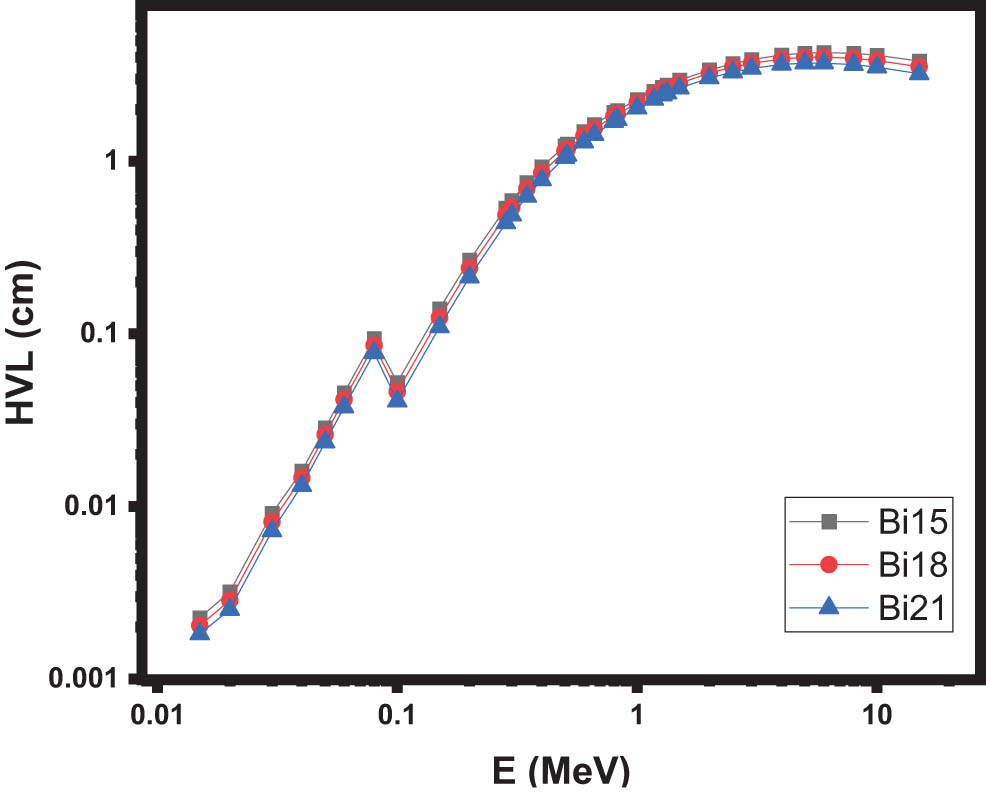
The HVL of the samples.
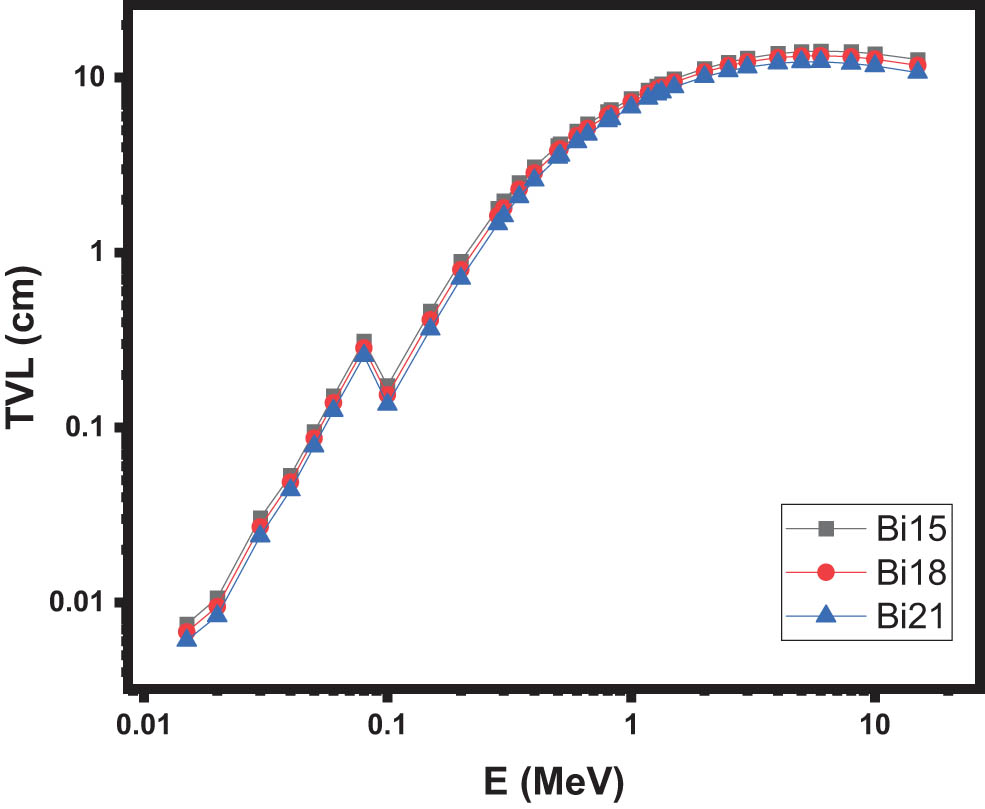
The TVL of the samples.
Additionally, Figure 6 presents the MFP [36] values for the three selected glasses within the energy range of 0.015–15 MeV. The MFP values for Bi15, Bi18, and Bi21 glasses range from 0.0032 to 5.508 cm, 0.0029 to 5.096 cm, and 0.0026 to 4.642 cm, respectively. Similar to the HVL, the Bi21 glass exhibits the lowest MFP value. In other words, the addition of Bi2O3 causes a reduction in the MFP for these glasses.
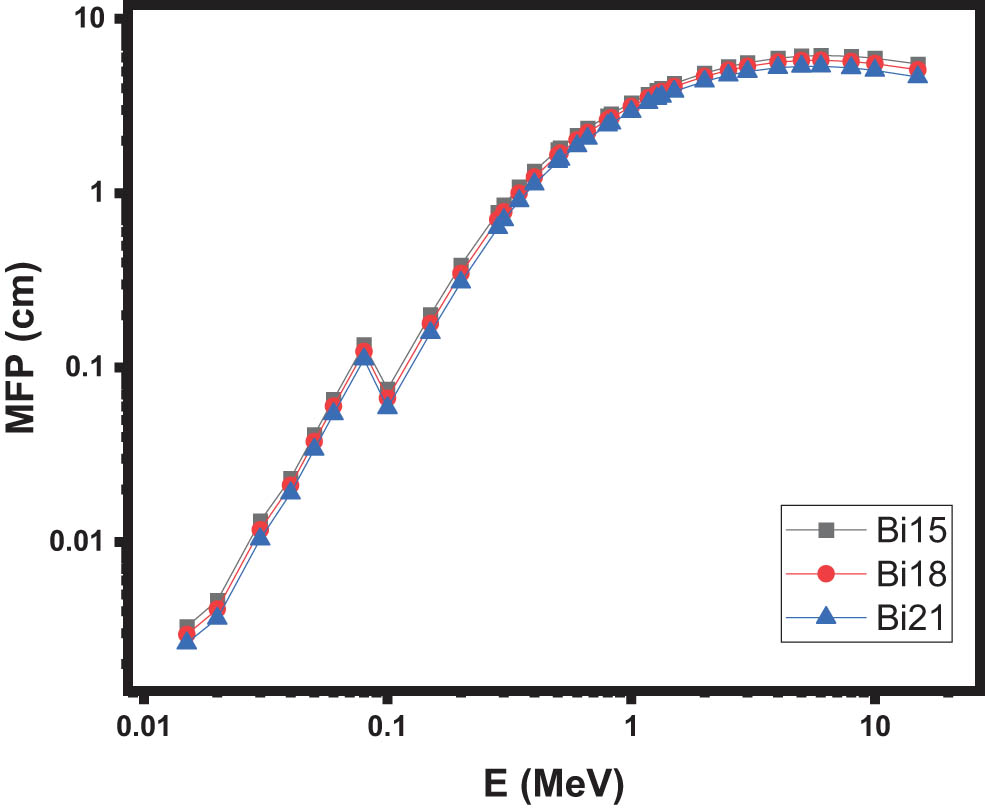
The MFP of the samples.
Additionally, the fluctuations in Z eff values versus energy for the current glasses are presented in Figure 7. Since Z eff and MAC are related for the glass being examined, it is shown that the photoelectric effect causes the Z eff values to first fall rapidly. The smallest value of Z eff was recorded at the range of intermediate energies, a region where Compton scattering influences almost all photon interactions. Bi21 glass was found to have the highest Z eff values of the glasses that were examined, with values ranging from 66.15 to 19.88. Because this sample has an elevated amount of Bi compared to the other glasses, it has comparatively high Z eff values. To put it simply, the variation in Z eff can be categorized into three specific energy ranges, namely low, moderate, and high. Each of these energy intervals arises from a distinct interaction with photons. In regions of low energy, the photoelectric absorption process predominates as the primary mode of photon interaction. Due to this Z eff is high in this energy range. At intermediate energies, Compton scattering predominates, and as a result, it is observed that Z eff variation is essentially constant. Pair production is the dominant process in the high energy (from 3 to 15 MeV) region. In light of this, all changes are defined by the Z dependence of total atomic cross sections.
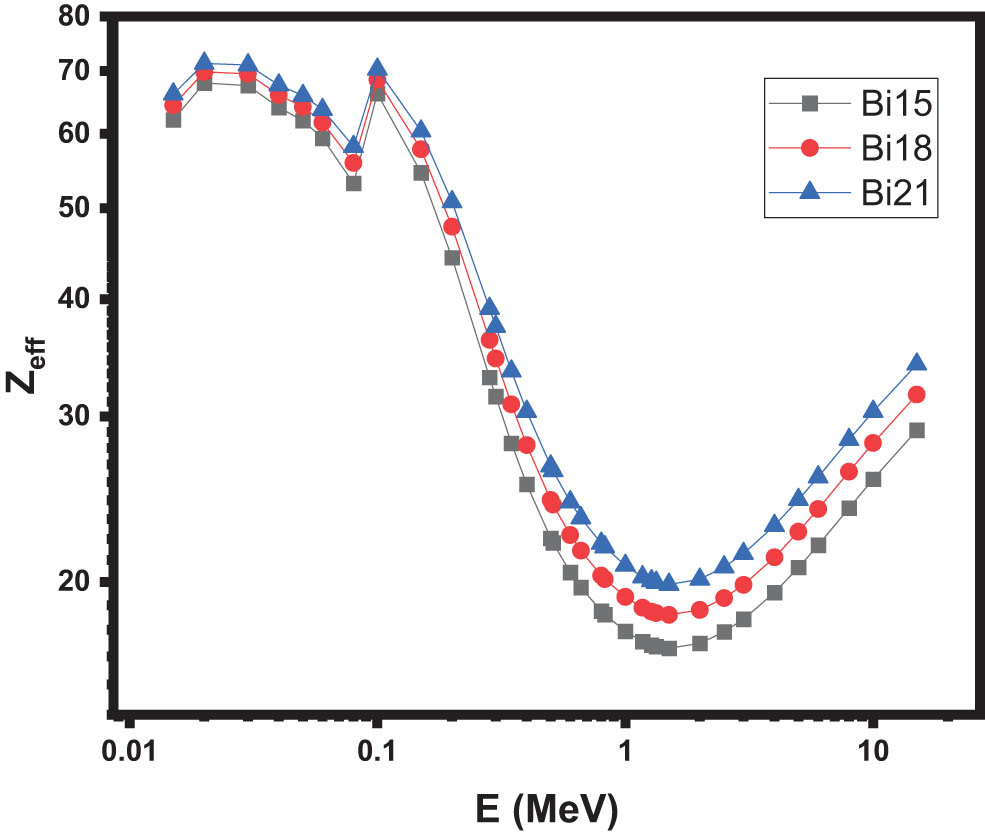
The effective atomic number of the samples.
4 Conclusion
The study examined the effects of incorporating Bi2O3 into glass matrices and its influence on various physical, mechanical, and gamma ray shielding properties. The analysis revealed that higher concentrations of Bi2O3 resulted in increased ρ and M of the glasses. V
m of the glasses also increased with the percentage of Bi2O3, indicating expansion within the network. The incorporation of Bi2O3 caused a decrease in
-
Funding information: The authors express their gratitude to the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R57), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
-
Author contributions: D.A.A.: funding acquisition, methodology, software, validation, writing – review and editing. A.H.A.: project administration, funding acquisition. M.I.A.: conceptualization, data curation, supervision, original draft preparation. A.K.: investigation, formal analysis, writing – review and editing, original draft preparation. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: The authors declare no conflict of interest.
-
Data availability statement: The data presented in this study are available on request from the corresponding author.
-
Ethical approval: The conducted research is not related to either human or animal use.
References
[1] Korkut T, Umaç ZI, Aygün B, Karabulut A, Yapıcı S, Sahin R. Neutron equivalent dose rate measurements of gypsum-waste tire rubber layered structures. Int J Polym Anal Charact. 2013;18:423–9.10.1080/1023666X.2013.814025Search in Google Scholar
[2] Prasad R, Pai AR, Oyadiji SO, Thomas S, Parashar SK. , Utilization of hazardous red mud in silicone rubber/MWCNT nanocomposites for high performance electromagnetic interference shielding. J Clean Prod. 2022;377:134290.10.1016/j.jclepro.2022.134290Search in Google Scholar
[3] Aşkın A, Sayyed MI, Sharma A, Dal M, El-Mallawany R, Kaçal MR. Investigation of the gamma ray shielding parameters of (100-x) [0.5Li2O–0.1B2O3–0.4P2O5]-xTeO2 glasses using Geant4 and FLUKA codes. J Non-Cryst Solids. 2019;521:119489.10.1016/j.jnoncrysol.2019.119489Search in Google Scholar
[4] Tekin HO, Syyed MI, Altunsoy EE, Manici. T. Shielding properties and effects of WO3 and PbO on mass attenuation coefficients by using MCNPX code. Dig J Nanomater Biostruct. 2017;12:861–7.Search in Google Scholar
[5] Dong M, Zhou S, Xue X, Sayyed MI, Tishkevich D, Trukhanov A, et al. Study of comprehensive shielding behaviors of chamber site deposit for neutron and gamma ray. Prog Nucl Energy. 2022;146:104155.10.1016/j.pnucene.2022.104155Search in Google Scholar
[6] Dong M, Xue X, Yang H, Li Z. Highly cost-effective shielding composite made from vanadium slag and boron-rich slag and its properties. Radiat Phys Chem. 2017;141:239–44.10.1016/j.radphyschem.2017.07.023Search in Google Scholar
[7] Dong M, Xue X, Yang H, Liu D, Wang C, Li Z. A novel comprehensive utilization of vanadium slag: As gamma ray shielding material. J Hazard Mater. 2016;318:751–7.10.1016/j.jhazmat.2016.06.012Search in Google Scholar PubMed
[8] Mhareb MH, Slimani Y, Alajerami YS, Sayyed MI, Lacomme E, Almessiere MA. Structural and radiation shielding properties of BaTiO3 ceramic with different concentrations of Bismuth and Ytterbium. Ceram Int. 2020;46:28877–86.10.1016/j.ceramint.2020.08.055Search in Google Scholar
[9] Al-Buriahi MS, Rashad M, Alalawi A, Sayyed MI. Effect of Bi2O3 on mechanical features and radiation shielding properties of boro-tellurite glass system. Ceram Int. 2020;46:16452–8.10.1016/j.ceramint.2020.03.208Search in Google Scholar
[10] Kamislioglu M. Research on the effects of bismuth borate glass system on nuclear radiation shielding parameters. Result Phys. 2021;22:103844.10.1016/j.rinp.2021.103844Search in Google Scholar
[11] Sayyed MI, Kaky KM, Şakar E, Akbaba U, Taki MM, Agar O. Gamma radiation shielding investigations for selected germanate glasses. J Non-Cryst Solids. 2019;512:33–40.10.1016/j.jnoncrysol.2019.02.014Search in Google Scholar
[12] Chaiphaksa W, Borisut P, Chanthima N, Kaewkhao J, Sanwaranatee NW. Mathematical calculation of gamma rays interaction in bismuth gadolinium silicate glass using WinXCom program. Mater Today Proc. 2022;65:2412–5.10.1016/j.matpr.2022.05.529Search in Google Scholar
[13] Bagheri R, Moghaddam AK, Yousefnia H. Gamma Ray shielding study of barium-bismuth-borosilicate glasses as transparent shielding materials using MCNP-4C code, XCOM program, and available experimental data. Nucl Eng Technol. 2017;49:216–23.10.1016/j.net.2016.08.013Search in Google Scholar
[14] Geidam IG, Matori KA, Halimah MK, Chan KT, Muhammad FD, Ishak M, et al. Oxide ion polarizabilities and gamma radiation shielding features of TeO2–B2O3–SiO2 glasses containing Bi2O3 using Phy-X/PSD software. Mater Today Commun. 2022;31:103472.10.1016/j.mtcomm.2022.103472Search in Google Scholar
[15] Aktas B, Acikgoz A, Yilmaz D, Yalcin S, Dogru K, Yorulmaz N. The role of TeO2 insertion on the radiation shielding, structural and physical properties of borosilicate glasses. J Nucl Mater. 2022;563:153619.10.1016/j.jnucmat.2022.153619Search in Google Scholar
[16] Fidan M, Acikgoz A, Demircan G, Yilmaz D, Aktas B. , Optical, structural, physical, and nuclear shielding properties, and albedo parameters of TeO2–BaO–B2O3–PbO–V2O5 glasses. J Phys Chem Solids. 2022;163:110543.10.1016/j.jpcs.2021.110543Search in Google Scholar
[17] Sayyed MI, Lakshminarayana G. Structural, thermal, optical features and shielding parameters investigations of optical glasses for gamma radiation shielding and defence applications. J Non-Cryst Solids. 2018;487:53–9.10.1016/j.jnoncrysol.2018.02.014Search in Google Scholar
[18] Sharma A, Sayyed MI, Agar O, Tekin HO. Simulation of shielding parameters for TeO2-WO3-GeO2 glasses using FLUKA code. Results Phys. 2019;13:102199.10.1016/j.rinp.2019.102199Search in Google Scholar
[19] Sayyed MI, Mhareb MHA, Alajerami YSM, Mahmoud KA, Imheidat MA, Alshahri F, et al. Optical and radiation shielding features for a new series of borate glass samples. Optik. 2021;239:166790.10.1016/j.ijleo.2021.166790Search in Google Scholar
[20] Issa SA, Kumar A, Sayyed MI, Dong MG, Elmahroug Y. Mechanical and gamma-ray shielding properties of TeO2-ZnO-NiO glasses. Mater Chem Phys. 2018;212:12–20.10.1016/j.matchemphys.2018.01.058Search in Google Scholar
[21] Mandal S, Manna S, Biswas K, Nag S, Ambade B. Effect of gamma ray irradiation on optical and luminescence properties of CeO2 doped bismuth glass. Ceram Int. 2023;49:23878–86.10.1016/j.ceramint.2023.04.231Search in Google Scholar
[22] Bhatia V, Kumar D, Kumar A, Mehta V, Chopra S, Vij A, et al. Mixed transition and rare earth ion doped borate glass: structural, optical and thermoluminescence study. J Mater Sci Mater Electron. 2019;30:677–86.10.1007/s10854-018-0336-ySearch in Google Scholar
[23] Kaur A, Khan S, Kumar D, Bhatia V, Rao SM, Kaur N, et al. Effect of MnO on structural, optical and thermoluminescence properties of lithium borosilicate glasses. J Lumin. 2020;219:116872.10.1016/j.jlumin.2019.116872Search in Google Scholar
[24] Morshidy HY, Sadeq MS. Influence of cobalt ions on the structure, phonon emission, phonon absorption and ligand field of some sodium borate glasses. J Non-Cryst Solids. 2019;525:119666–71.10.1016/j.jnoncrysol.2019.119666Search in Google Scholar
[25] Morshidy HY, Sadeq MS, Raouf Mohamed A, El-Okr MM. The role of CuCl2 in tuning the physical, structural and optical properties of some Al2O3–B2O3 glasses. J Non-Cryst Solids. 2020;528:119749–54.10.1016/j.jnoncrysol.2019.119749Search in Google Scholar
[26] Mansour SF, Wageh S, Alotaibi MF, Abdo MA, Sadeq MS. Impact of bismuth oxide on the structure, optical features and ligand field parameters of borosilicate glasses doped with nickel oxide. Ceram Int. 2021;47:21443–9.10.1016/j.ceramint.2021.04.154Search in Google Scholar
[27] Thakur S, Thakur V, Kaur A, Singh L. Structural, optical and thermal properties of nickel doped bismuth borate glasses. J Non-Cryst Solids. 2019;512:60–71.10.1016/j.jnoncrysol.2019.02.012Search in Google Scholar
[28] Almuqrin AH, Al-Ghamdi H, Aloraini DA, Sayyed MI, Kumar A. Assessment of mechanical and gamma ray shielding capability of highly dense PbO-TeO2-CdO glasses. Optik. 2023;273:170429.10.1016/j.ijleo.2022.170429Search in Google Scholar
[29] Makishima A, Mackenzie JD. Direct calculations of Young modulus of glass. J Non-Cryst Solids. 1973;12:35–45.10.1016/0022-3093(73)90053-7Search in Google Scholar
[30] Makishima A, Mackenzie JD. Calculation of Bulk modulus, shear modulus and Poisson’s ratio of glass. J Non-Cryst Solids. 1975;17:147–57.10.1016/0022-3093(75)90047-2Search in Google Scholar
[31] Inaba S, Oda S, Morinaga K. Heat capacity of oxide glasses at high temperature region. J Non-Cryst Solids. 2003;325:258–66.10.1016/S0022-3093(03)00315-6Search in Google Scholar
[32] Saritha D, Salagram M, Bhikshamaiah G. Physical and optical properties of Bi2O3-B2O3 glasses. IOP Conf Ser Mater Sci Eng. 2009;2:01205710.1088/1757-899X/2/1/012057Search in Google Scholar
[33] Naseer KA, Marimuthu K, Mahmoud KA, Sayyed MI. Impact of Bi2O3 modifier concentration on barium–zincborate glasses: physical, structural, elastic, and radiation-shielding properties. Eur Phys J Plus. 2021;136:116.10.1140/epjp/s13360-020-01056-6Search in Google Scholar PubMed PubMed Central
[34] Alajerami YS, Drabold DA, Thapa R, Sayyed MI, Mhareb MH. Physical, structural, optical and gamma-ray shielding properties of Na2O-CdO-Bi2O3-B2O3 glasses. Int J Appl Glas Sci. 2021;12:259–73.10.1111/ijag.15859Search in Google Scholar
[35] Şakar E, Özpolat ÖF, Alım B, Sayyed MI, Kurudirek M. Phy-X/PSD: Development of a user friendly online software for calculation of parameters relevant to radiation shielding and dosimetry. Radiat Phys Chem. 2020;166:108496.10.1016/j.radphyschem.2019.108496Search in Google Scholar
[36] Rammah YS, Abouhaswa AS, Sayyed MI, Tekin HO, El-Mallawany R. Structural, UV and shielding properties of ZBPC glasses. J Non-Cryst Solids. 2019;509:99–105.10.1016/j.jnoncrysol.2018.12.013Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Characteristics, source, and health risk assessment of aerosol polyaromatic hydrocarbons in the rural and urban regions of western Saudi Arabia
- Regular Articles
- A network-based correlation research between element electronegativity and node importance
- Pomegranate attenuates kidney injury in cyclosporine-induced nephrotoxicity in rats by suppressing oxidative stress
- Ab initio study of fundamental properties of XInO3 (X = K, Rb, Cs) perovskites
- Responses of feldspathic sandstone and sand-reconstituted soil C and N to freeze–thaw cycles
- Robust fractional control based on high gain observers design (RNFC) for a Spirulina maxima culture interfaced with an advanced oxidation process
- Study on arsenic speciation and redistribution mechanism in Lonicera japonica plants via synchrotron techniques
- Optimization of machining Nilo 36 superalloy parameters in turning operation
- Vacuum impregnation pre-treatment: A novel method for incorporating mono- and divalent cations into potato strips to reduce the acrylamide formation in French fries
- Characterization of effective constituents in Acanthopanax senticosus fruit for blood deficiency syndrome based on the chinmedomics strategy
- Comparative analysis of the metabolites in Pinellia ternata from two producing regions using ultra-high-performance liquid chromatography–electrospray ionization–tandem mass spectrometry
- The assessment of environmental parameter along the desalination plants in the Kingdom of Saudi Arabia
- Effects of harpin and carbendazim on antioxidant accumulation in young jujube leaves
- The effects of in ovo injected with sodium borate on hatching performance and small intestine morphology in broiler chicks
- Optimization of cutting forces and surface roughness via ANOVA and grey relational analysis in machining of In718
- Essential oils of Origanum compactum Benth: Chemical characterization, in vitro, in silico, antioxidant, and antibacterial activities
- Translocation of tungsten(vi) oxide/gadolinium(iii) fluoride in tellurite glasses towards improvement of gamma-ray attenuation features in high-density glass shields
- Mechanical properties, elastic moduli, and gamma ray attenuation competencies of some TeO2–WO3–GdF3 glasses: Tailoring WO3–GdF3 substitution toward optimum behavioral state range
- Comparison between the CIDR or sponge with hormone injection to induce estrus synchronization for twining and sex preselection in Naimi sheep
- Exergetic performance analyses of three different cogeneration plants
- Psoralea corylifolia (babchi) seeds enhance proliferation of normal human cultured melanocytes: GC–MS profiling and biological investigation
- A novel electrochemical micro-titration method for quantitative evaluation of the DPPH free radical scavenging capacity of caffeic acid
- Comparative study between supported bimetallic catalysts for nitrate remediation in water
- Persicaline, an alkaloid from Salvadora persica, inhibits proliferation and induces apoptosis and cell-cycle arrest in MCF-7 cells
- Determination of nicotine content in locally produced smokeless tobacco (Shammah) samples from Jazan region of Saudi Arabia using a convenient HPLC-MS/MS method
- Changes in oxidative stress markers in pediatric burn injury over a 1-week period
- Integrated geophysical techniques applied for petroleum basins structural characterization in the central part of the Western Desert, Egypt
- The impact of chemical modifications on gamma-ray attenuation properties of some WO3-reinforced tellurite glasses
- Microwave and Cs+-assisted chemo selective reaction protocol for synthesizing 2-styryl quinoline biorelevant molecules
- Structural, physical, and radiation absorption properties of a significant nuclear power plant component: A comparison between REX-734 and 316L SS austenitic stainless steels
- Effect of Moringa oleifera on serum YKL-40 level: In vivo rat periodontitis model
- Investigating the impact of CO2 emissions on the COVID-19 pandemic by generalized linear mixed model approach with inverse Gaussian and gamma distributions
- Influence of WO3 content on gamma rays attenuation characteristics of phosphate glasses at low energy range
- Study on CO2 absorption performance of ternary DES formed based on DEA as promoting factor
- Performance analyses of detonation engine cogeneration cycles
- Sterols from Centaurea pumilio L. with cell proliferative activity: In vitro and in silico studies
- Untargeted metabolomics revealing changes in aroma substances in flue-cured tobacco
- Effect of pumpkin enriched with calcium lactate on iron status in an animal model of postmenopausal osteoporosis
- Energy consumption, mechanical and metallographic properties of cryogenically treated tool steels
- Optimization of ultra-high pressure-assisted extraction of total phenols from Eucommia ulmoides leaves by response surface methodology
- Harpin enhances antioxidant nutrient accumulation and decreases enzymatic browning in stored soybean sprouts
- Physicochemical and biological properties of carvacrol
- Radix puerariae in the treatment of diabetic nephropathy: A network pharmacology analysis and experimental validation
- Anti-Alzheimer, antioxidants, glucose-6-phosphate dehydrogenase effects of Taverniera glabra mediated ZnO and Fe2O3 nanoparticles in alloxan-induced diabetic rats
- Experimental study on photocatalytic CO2 reduction performance of ZnS/CdS-TiO2 nanotube array thin films
- Epoxy-reinforced heavy metal oxides for gamma ray shielding purposes
- Black mulberry (Morus nigra L.) fruits: As a medicinal plant rich in human health-promoting compounds
- Promising antioxidant and antimicrobial effects of essential oils extracted from fruits of Juniperus thurifera: In vitro and in silico investigations
- Chloramine-T-induced oxidation of Rizatriptan Benzoate: An integral chemical and spectroscopic study of products, mechanisms and kinetics
- Study on antioxidant and antimicrobial potential of chemically profiled essential oils extracted from Juniperus phoenicea (L.) by use of in vitro and in silico approaches
- Screening and characterization of fungal taxol-producing endophytic fungi for evaluation of antimicrobial and anticancer activities
- Mineral composition, principal polyphenolic components, and evaluation of the anti-inflammatory, analgesic, and antioxidant properties of Cytisus villosus Pourr leaf extracts
- In vitro antiproliferative efficacy of Annona muricata seed and fruit extracts on several cancer cell lines
- An experimental study for chemical characterization of artificial anterior cruciate ligament with coated chitosan as biomaterial
- Prevalence of residual risks of the transfusion-transmitted infections in Riyadh hospitals: A two-year retrospective study
- Computational and experimental investigation of antibacterial and antifungal properties of Nicotiana tabacum extracts
- Reinforcement of cementitious mortars with hemp fibers and shives
- X-ray shielding properties of bismuth-borate glass doped with rare earth ions
- Green supported silver nanoparticles over modified reduced graphene oxide: Investigation of its antioxidant and anti-ovarian cancer effects
- Orthogonal synthesis of a versatile building block for dual functionalization of targeting vectors
- Thymbra spicata leaf extract driven biogenic synthesis of Au/Fe3O4 nanocomposite and its bio-application in the treatment of different types of leukemia
- The role of Ag2O incorporation in nuclear radiation shielding behaviors of the Li2O–Pb3O4–SiO2 glass system: A multi-step characterization study
- A stimuli-responsive in situ spray hydrogel co-loaded with naringenin and gentamicin for chronic wounds
- Assessment of the impact of γ-irradiation on the piperine content and microbial quality of black pepper
- Antioxidant, sensory, and functional properties of low-alcoholic IPA beer with Pinus sylvestris L. shoots addition fermented using unconventional yeast
- Screening and optimization of extracellular pectinase produced by Bacillus thuringiensis SH7
- Determination of polyphenols in Chinese jujube using ultra-performance liquid chromatography–mass spectrometry
- Synergistic effects of harpin and NaCl in determining soybean sprout quality under non-sterile conditions
- Field evaluation of different eco-friendly alternative control methods against Panonychus citri [Acari: Tetranychidae] spider mite and its predators in citrus orchards
- Exploring the antimicrobial potential of biologically synthesized zero valent iron nanoparticles
- NaCl regulates goldfish growth and survival at three food supply levels under hypoxia
- An exploration of the physical, optical, mechanical, and radiation shielding properties of PbO–MgO–ZnO–B2O3 glasses
- A novel statistical modeling of air pollution and the COVID-19 pandemic mortality data by Poisson, geometric, and negative binomial regression models with fixed and random effects
- Treatment activity of the injectable hydrogels loaded with dexamethasone In(iii) complex on glioma by inhibiting the VEGF signaling pathway
- An alternative approach for the excess lifetime cancer risk and prediction of radiological parameters
- Panax ginseng leaf aqueous extract mediated green synthesis of AgNPs under ultrasound condition and investigation of its anti-lung adenocarcinoma effects
- Study of hydrolysis and production of instant ginger (Zingiber officinale) tea
- Novel green synthesis of zinc oxide nanoparticles using Salvia rosmarinus extract for treatment of human lung cancer
- Evaluation of second trimester plasma lipoxin A4, VEGFR-1, IL-6, and TNF-α levels in pregnant women with gestational diabetes mellitus
- Antidiabetic, antioxidant and cytotoxicity activities of ortho- and para-substituted Schiff bases derived from metformin hydrochloride: Validation by molecular docking and in silico ADME studies
- Antioxidant, antidiabetic, antiglaucoma, and anticholinergic effects of Tayfi grape (Vitis vinifera): A phytochemical screening by LC-MS/MS analysis
- Identification of genetic polymorphisms in the stearoyl CoA desaturase gene and its association with milk quality traits in Najdi sheep
- Cold-acclimation effect on cadmium absorption and biosynthesis of polyphenolics, and free proline and photosynthetic pigments in Spirogyra aequinoctialis
- Analysis of secondary metabolites in Xinjiang Morus nigra leaves using different extraction methods with UPLC-Q/TOF-MS/MS technology
- Nanoarchitectonics and performance evaluation of a Fe3O4-stabilized Pickering emulsion-type differential pressure plugging agent
- Investigating pyrolysis characteristics of Shengdong coal through Py-GC/MS
- Extraction, phytochemical characterization, and antifungal activity of Salvia rosmarinus extract
- Introducing a novel and natural antibiotic for the treatment of oral pathogens: Abelmoschus esculentus green-formulated silver nanoparticles
- Optimization of gallic acid-enriched ultrasonic-assisted extraction from mango peels
- Effect of gamma rays irradiation in the structure, optical, and electrical properties of samarium doped bismuth titanate ceramics
- Combinatory in silico investigation for potential inhibitors from Curcuma sahuynhensis Škorničk. & N.S. Lý volatile phytoconstituents against influenza A hemagglutinin, SARS-CoV-2 main protease, and Omicron-variant spike protein
- Physical, mechanical, and gamma ray shielding properties of the Bi2O3–BaO–B2O3–ZnO–As2O3–MgO–Na2O glass system
- Twofold interpenetrated 3D Cd(ii) complex: Crystal structure and luminescent property
- Study on the microstructure and soil quality variation of composite soil with soft rock and sand
- Ancient spring waters still emerging and accessible in the Roman Forum area: Chemical–physical and microbiological characterization
- Extraction and characterization of type I collagen from scales of Mexican Biajaiba fish
- Finding small molecular compounds to decrease trimethylamine oxide levels in atherosclerosis by virtual screening
- Prefatory in silico studies and in vitro insecticidal effect of Nigella sativa (L.) essential oil and its active compound (carvacrol) against the Callosobruchus maculatus adults (Fab), a major pest of chickpea
- Polymerized methyl imidazole silver bromide (CH3C6H5AgBr)6: Synthesis, crystal structures, and catalytic activity
- Using calcined waste fish bones as a green solid catalyst for biodiesel production from date seed oil
- Influence of the addition of WO3 on TeO2–Na2O glass systems in view of the feature of mechanical, optical, and photon attenuation
- Naringin ameliorates 5-fluorouracil elicited neurotoxicity by curtailing oxidative stress and iNOS/NF-ĸB/caspase-3 pathway
- GC-MS profile of extracts of an endophytic fungus Alternaria and evaluation of its anticancer and antibacterial potentialities
- Green synthesis, chemical characterization, and antioxidant and anti-colorectal cancer effects of vanadium nanoparticles
- Determination of caffeine content in coffee drinks prepared in some coffee shops in the local market in Jeddah City, Saudi Arabia
- A new 3D supramolecular Cu(ii) framework: Crystal structure and photocatalytic characteristics
- Bordeaux mixture accelerates ripening, delays senescence, and promotes metabolite accumulation in jujube fruit
- Important application value of injectable hydrogels loaded with omeprazole Schiff base complex in the treatment of pancreatitis
- Color tunable benzothiadiazole-based small molecules for lightening applications
- Investigation of structural, dielectric, impedance, and mechanical properties of hydroxyapatite-modified barium titanate composites for biomedical applications
- Metal gel particles loaded with epidermal cell growth factor promote skin wound repair mechanism by regulating miRNA
- In vitro exploration of Hypsizygus ulmarius (Bull.) mushroom fruiting bodies: Potential antidiabetic and anti-inflammatory agent
- Alteration in the molecular structure of the adenine base exposed to gamma irradiation: An ESR study
- Comprehensive study of optical, thermal, and gamma-ray shielding properties of Bi2O3–ZnO–PbO–B2O3 glasses
- Lewis acids as co-catalysts in Pd-based catalyzed systems of the octene-1 hydroethoxycarbonylation reaction
- Synthesis, Hirshfeld surface analysis, thermal, and selective α-glucosidase inhibitory studies of Schiff base transition metal complexes
- Protective properties of AgNPs green-synthesized by Abelmoschus esculentus on retinal damage on the virtue of its anti-inflammatory and antioxidant effects in diabetic rat
- Effects of green decorated AgNPs on lignin-modified magnetic nanoparticles mediated by Cydonia on cecal ligation and puncture-induced sepsis
- Treatment of gastric cancer by green mediated silver nanoparticles using Pistacia atlantica bark aqueous extract
- Preparation of newly developed porcelain ceramics containing WO3 nanoparticles for radiation shielding applications
- Utilization of computational methods for the identification of new natural inhibitors of human neutrophil elastase in inflammation therapy
- Some anticancer agents as effective glutathione S-transferase (GST) inhibitors
- Clay-based bricks’ rich illite mineral for gamma-ray shielding applications: An experimental evaluation of the effect of pressure rates on gamma-ray attenuation parameters
- Stability kinetics of orevactaene pigments produced by Epicoccum nigrum in solid-state fermentation
- Treatment of denture stomatitis using iron nanoparticles green-synthesized by Silybum marianum extract
- Characterization and antioxidant potential of white mustard (Brassica hirta) leaf extract and stabilization of sunflower oil
- Characteristics of Langmuir monomolecular monolayers formed by the novel oil blends
- Strategies for optimizing the single GdSrFeO4 phase synthesis
- Oleic acid and linoleic acid nanosomes boost immunity and provoke cell death via the upregulation of beta-defensin-4 at genetic and epigenetic levels
- Unraveling the therapeutic potential of Bombax ceiba roots: A comprehensive study of chemical composition, heavy metal content, antibacterial activity, and in silico analysis
- Green synthesis of AgNPs using plant extract and investigation of its anti-human colorectal cancer application
- The adsorption of naproxen on adsorbents obtained from pepper stalk extract by green synthesis
- Treatment of gastric cancer by silver nanoparticles encapsulated by chitosan polymers mediated by Pistacia atlantica extract under ultrasound condition
- In vitro protective and anti-inflammatory effects of Capparis spinosa and its flavonoids profile
- Wear and corrosion behavior of TiC and WC coatings deposited on high-speed steels by electro-spark deposition
- Therapeutic effects of green-formulated gold nanoparticles by Origanum majorana on spinal cord injury in rats
- Melanin antibacterial activity of two new strains, SN1 and SN2, of Exophiala phaeomuriformis against five human pathogens
- Evaluation of the analgesic and anesthetic properties of silver nanoparticles supported over biodegradable acacia gum-modified magnetic nanoparticles
- Review Articles
- Role and mechanism of fruit waste polyphenols in diabetes management
- A comprehensive review of non-alkaloidal metabolites from the subfamily Amaryllidoideae (Amaryllidaceae)
- Discovery of the chemical constituents, structural characteristics, and pharmacological functions of Chinese caterpillar fungus
- Eco-friendly green approach of nickel oxide nanoparticles for biomedical applications
- Advances in the pharmaceutical research of curcumin for oral administration
- Rapid Communication
- Determination of the contents of bioactive compounds in St. John’s wort (Hypericum perforatum): Comparison of commercial and wild samples
- Retraction
- Retraction of “Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: The protective effect on periodontitis via reducing the release of IL-1β and TNF-α”
- Topical Issue on Phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Anti-plasmodial potential of selected medicinal plants and a compound Atropine isolated from Eucalyptus obliqua
- Anthocyanin extract from black rice attenuates chronic inflammation in DSS-induced colitis mouse model by modulating the gut microbiota
- Evaluation of antibiofilm and cytotoxicity effect of Rumex vesicarius methanol extract
- Chemical compositions of Litsea umbellata and inhibition activities
- Green synthesis, characterization of silver nanoparticles using Rhynchosia capitata leaf extract and their biological activities
- GC-MS analysis and antibacterial activities of some plants belonging to the genus Euphorbia on selected bacterial isolates
- The abrogative effect of propolis on acrylamide-induced toxicity in male albino rats: Histological study
- A phytoconstituent 6-aminoflavone ameliorates lipopolysaccharide-induced oxidative stress mediated synapse and memory dysfunction via p-Akt/NF-kB pathway in albino mice
- Anti-diabetic potentials of Sorbaria tomentosa Lindl. Rehder: Phytochemistry (GC-MS analysis), α-amylase, α-glucosidase inhibitory, in vivo hypoglycemic, and biochemical analysis
- Assessment of cytotoxic and apoptotic activities of the Cassia angustifolia aqueous extract against SW480 colon cancer
- Biochemical analysis, antioxidant, and antibacterial efficacy of the bee propolis extract (Hymenoptera: Apis mellifera) against Staphylococcus aureus-induced infection in BALB/c mice: In vitro and in vivo study
- Assessment of essential elements and heavy metals in Saudi Arabian rice samples underwent various processing methods
- Two new compounds from leaves of Capparis dongvanensis (Sy, B. H. Quang & D. V. Hai) and inhibition activities
- Hydroxyquinoline sulfanilamide ameliorates STZ-induced hyperglycemia-mediated amyleoid beta burden and memory impairment in adult mice
- An automated reading of semi-quantitative hemagglutination results in microplates: Micro-assay for plant lectins
- Inductively coupled plasma mass spectrometry assessment of essential and toxic trace elements in traditional spices consumed by the population of the Middle Eastern region in their recipes
- Phytochemical analysis and anticancer activity of the Pithecellobium dulce seed extract in colorectal cancer cells
- Impact of climatic disturbances on the chemical compositions and metabolites of Salvia officinalis
- Physicochemical characterization, antioxidant and antifungal activities of essential oils of Urginea maritima and Allium sativum
- Phytochemical analysis and antifungal efficiency of Origanum majorana extracts against some phytopathogenic fungi causing tomato damping-off diseases
- Special Issue on 4th IC3PE
- Graphene quantum dots: A comprehensive overview
- Studies on the intercalation of calcium–aluminium layered double hydroxide-MCPA and its controlled release mechanism as a potential green herbicide
- Synergetic effect of adsorption and photocatalysis by zinc ferrite-anchored graphitic carbon nitride nanosheet for the removal of ciprofloxacin under visible light irradiation
- Exploring anticancer activity of the Indonesian guava leaf (Psidium guajava L.) fraction on various human cancer cell lines in an in vitro cell-based approach
- The comparison of gold extraction methods from the rock using thiourea and thiosulfate
- Special Issue on Marine environmental sciences and significance of the multidisciplinary approaches
- Sorption of alkylphenols and estrogens on microplastics in marine conditions
- Cytotoxic ketosteroids from the Red Sea soft coral Dendronephthya sp.
- Antibacterial and biofilm prevention metabolites from Acanthophora spicifera
- Characteristics, source, and health risk assessment of aerosol polyaromatic hydrocarbons in the rural and urban regions of western Saudi Arabia
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part II
- Green synthesis, characterization, and evaluation of antibacterial activities of cobalt nanoparticles produced by marine fungal species Periconia prolifica
- Combustion-mediated sol–gel preparation of cobalt-doped ZnO nanohybrids for the degradation of acid red and antibacterial performance
- Perinatal supplementation with selenium nanoparticles modified with ascorbic acid improves hepatotoxicity in rat gestational diabetes
- Evaluation and chemical characterization of bioactive secondary metabolites from endophytic fungi associated with the ethnomedicinal plant Bergenia ciliata
- Enhancing photovoltaic efficiency with SQI-Br and SQI-I sensitizers: A comparative analysis
- Nanostructured p-PbS/p-CuO sulfide/oxide bilayer heterojunction as a promising photoelectrode for hydrogen gas generation
Articles in the same Issue
- Characteristics, source, and health risk assessment of aerosol polyaromatic hydrocarbons in the rural and urban regions of western Saudi Arabia
- Regular Articles
- A network-based correlation research between element electronegativity and node importance
- Pomegranate attenuates kidney injury in cyclosporine-induced nephrotoxicity in rats by suppressing oxidative stress
- Ab initio study of fundamental properties of XInO3 (X = K, Rb, Cs) perovskites
- Responses of feldspathic sandstone and sand-reconstituted soil C and N to freeze–thaw cycles
- Robust fractional control based on high gain observers design (RNFC) for a Spirulina maxima culture interfaced with an advanced oxidation process
- Study on arsenic speciation and redistribution mechanism in Lonicera japonica plants via synchrotron techniques
- Optimization of machining Nilo 36 superalloy parameters in turning operation
- Vacuum impregnation pre-treatment: A novel method for incorporating mono- and divalent cations into potato strips to reduce the acrylamide formation in French fries
- Characterization of effective constituents in Acanthopanax senticosus fruit for blood deficiency syndrome based on the chinmedomics strategy
- Comparative analysis of the metabolites in Pinellia ternata from two producing regions using ultra-high-performance liquid chromatography–electrospray ionization–tandem mass spectrometry
- The assessment of environmental parameter along the desalination plants in the Kingdom of Saudi Arabia
- Effects of harpin and carbendazim on antioxidant accumulation in young jujube leaves
- The effects of in ovo injected with sodium borate on hatching performance and small intestine morphology in broiler chicks
- Optimization of cutting forces and surface roughness via ANOVA and grey relational analysis in machining of In718
- Essential oils of Origanum compactum Benth: Chemical characterization, in vitro, in silico, antioxidant, and antibacterial activities
- Translocation of tungsten(vi) oxide/gadolinium(iii) fluoride in tellurite glasses towards improvement of gamma-ray attenuation features in high-density glass shields
- Mechanical properties, elastic moduli, and gamma ray attenuation competencies of some TeO2–WO3–GdF3 glasses: Tailoring WO3–GdF3 substitution toward optimum behavioral state range
- Comparison between the CIDR or sponge with hormone injection to induce estrus synchronization for twining and sex preselection in Naimi sheep
- Exergetic performance analyses of three different cogeneration plants
- Psoralea corylifolia (babchi) seeds enhance proliferation of normal human cultured melanocytes: GC–MS profiling and biological investigation
- A novel electrochemical micro-titration method for quantitative evaluation of the DPPH free radical scavenging capacity of caffeic acid
- Comparative study between supported bimetallic catalysts for nitrate remediation in water
- Persicaline, an alkaloid from Salvadora persica, inhibits proliferation and induces apoptosis and cell-cycle arrest in MCF-7 cells
- Determination of nicotine content in locally produced smokeless tobacco (Shammah) samples from Jazan region of Saudi Arabia using a convenient HPLC-MS/MS method
- Changes in oxidative stress markers in pediatric burn injury over a 1-week period
- Integrated geophysical techniques applied for petroleum basins structural characterization in the central part of the Western Desert, Egypt
- The impact of chemical modifications on gamma-ray attenuation properties of some WO3-reinforced tellurite glasses
- Microwave and Cs+-assisted chemo selective reaction protocol for synthesizing 2-styryl quinoline biorelevant molecules
- Structural, physical, and radiation absorption properties of a significant nuclear power plant component: A comparison between REX-734 and 316L SS austenitic stainless steels
- Effect of Moringa oleifera on serum YKL-40 level: In vivo rat periodontitis model
- Investigating the impact of CO2 emissions on the COVID-19 pandemic by generalized linear mixed model approach with inverse Gaussian and gamma distributions
- Influence of WO3 content on gamma rays attenuation characteristics of phosphate glasses at low energy range
- Study on CO2 absorption performance of ternary DES formed based on DEA as promoting factor
- Performance analyses of detonation engine cogeneration cycles
- Sterols from Centaurea pumilio L. with cell proliferative activity: In vitro and in silico studies
- Untargeted metabolomics revealing changes in aroma substances in flue-cured tobacco
- Effect of pumpkin enriched with calcium lactate on iron status in an animal model of postmenopausal osteoporosis
- Energy consumption, mechanical and metallographic properties of cryogenically treated tool steels
- Optimization of ultra-high pressure-assisted extraction of total phenols from Eucommia ulmoides leaves by response surface methodology
- Harpin enhances antioxidant nutrient accumulation and decreases enzymatic browning in stored soybean sprouts
- Physicochemical and biological properties of carvacrol
- Radix puerariae in the treatment of diabetic nephropathy: A network pharmacology analysis and experimental validation
- Anti-Alzheimer, antioxidants, glucose-6-phosphate dehydrogenase effects of Taverniera glabra mediated ZnO and Fe2O3 nanoparticles in alloxan-induced diabetic rats
- Experimental study on photocatalytic CO2 reduction performance of ZnS/CdS-TiO2 nanotube array thin films
- Epoxy-reinforced heavy metal oxides for gamma ray shielding purposes
- Black mulberry (Morus nigra L.) fruits: As a medicinal plant rich in human health-promoting compounds
- Promising antioxidant and antimicrobial effects of essential oils extracted from fruits of Juniperus thurifera: In vitro and in silico investigations
- Chloramine-T-induced oxidation of Rizatriptan Benzoate: An integral chemical and spectroscopic study of products, mechanisms and kinetics
- Study on antioxidant and antimicrobial potential of chemically profiled essential oils extracted from Juniperus phoenicea (L.) by use of in vitro and in silico approaches
- Screening and characterization of fungal taxol-producing endophytic fungi for evaluation of antimicrobial and anticancer activities
- Mineral composition, principal polyphenolic components, and evaluation of the anti-inflammatory, analgesic, and antioxidant properties of Cytisus villosus Pourr leaf extracts
- In vitro antiproliferative efficacy of Annona muricata seed and fruit extracts on several cancer cell lines
- An experimental study for chemical characterization of artificial anterior cruciate ligament with coated chitosan as biomaterial
- Prevalence of residual risks of the transfusion-transmitted infections in Riyadh hospitals: A two-year retrospective study
- Computational and experimental investigation of antibacterial and antifungal properties of Nicotiana tabacum extracts
- Reinforcement of cementitious mortars with hemp fibers and shives
- X-ray shielding properties of bismuth-borate glass doped with rare earth ions
- Green supported silver nanoparticles over modified reduced graphene oxide: Investigation of its antioxidant and anti-ovarian cancer effects
- Orthogonal synthesis of a versatile building block for dual functionalization of targeting vectors
- Thymbra spicata leaf extract driven biogenic synthesis of Au/Fe3O4 nanocomposite and its bio-application in the treatment of different types of leukemia
- The role of Ag2O incorporation in nuclear radiation shielding behaviors of the Li2O–Pb3O4–SiO2 glass system: A multi-step characterization study
- A stimuli-responsive in situ spray hydrogel co-loaded with naringenin and gentamicin for chronic wounds
- Assessment of the impact of γ-irradiation on the piperine content and microbial quality of black pepper
- Antioxidant, sensory, and functional properties of low-alcoholic IPA beer with Pinus sylvestris L. shoots addition fermented using unconventional yeast
- Screening and optimization of extracellular pectinase produced by Bacillus thuringiensis SH7
- Determination of polyphenols in Chinese jujube using ultra-performance liquid chromatography–mass spectrometry
- Synergistic effects of harpin and NaCl in determining soybean sprout quality under non-sterile conditions
- Field evaluation of different eco-friendly alternative control methods against Panonychus citri [Acari: Tetranychidae] spider mite and its predators in citrus orchards
- Exploring the antimicrobial potential of biologically synthesized zero valent iron nanoparticles
- NaCl regulates goldfish growth and survival at three food supply levels under hypoxia
- An exploration of the physical, optical, mechanical, and radiation shielding properties of PbO–MgO–ZnO–B2O3 glasses
- A novel statistical modeling of air pollution and the COVID-19 pandemic mortality data by Poisson, geometric, and negative binomial regression models with fixed and random effects
- Treatment activity of the injectable hydrogels loaded with dexamethasone In(iii) complex on glioma by inhibiting the VEGF signaling pathway
- An alternative approach for the excess lifetime cancer risk and prediction of radiological parameters
- Panax ginseng leaf aqueous extract mediated green synthesis of AgNPs under ultrasound condition and investigation of its anti-lung adenocarcinoma effects
- Study of hydrolysis and production of instant ginger (Zingiber officinale) tea
- Novel green synthesis of zinc oxide nanoparticles using Salvia rosmarinus extract for treatment of human lung cancer
- Evaluation of second trimester plasma lipoxin A4, VEGFR-1, IL-6, and TNF-α levels in pregnant women with gestational diabetes mellitus
- Antidiabetic, antioxidant and cytotoxicity activities of ortho- and para-substituted Schiff bases derived from metformin hydrochloride: Validation by molecular docking and in silico ADME studies
- Antioxidant, antidiabetic, antiglaucoma, and anticholinergic effects of Tayfi grape (Vitis vinifera): A phytochemical screening by LC-MS/MS analysis
- Identification of genetic polymorphisms in the stearoyl CoA desaturase gene and its association with milk quality traits in Najdi sheep
- Cold-acclimation effect on cadmium absorption and biosynthesis of polyphenolics, and free proline and photosynthetic pigments in Spirogyra aequinoctialis
- Analysis of secondary metabolites in Xinjiang Morus nigra leaves using different extraction methods with UPLC-Q/TOF-MS/MS technology
- Nanoarchitectonics and performance evaluation of a Fe3O4-stabilized Pickering emulsion-type differential pressure plugging agent
- Investigating pyrolysis characteristics of Shengdong coal through Py-GC/MS
- Extraction, phytochemical characterization, and antifungal activity of Salvia rosmarinus extract
- Introducing a novel and natural antibiotic for the treatment of oral pathogens: Abelmoschus esculentus green-formulated silver nanoparticles
- Optimization of gallic acid-enriched ultrasonic-assisted extraction from mango peels
- Effect of gamma rays irradiation in the structure, optical, and electrical properties of samarium doped bismuth titanate ceramics
- Combinatory in silico investigation for potential inhibitors from Curcuma sahuynhensis Škorničk. & N.S. Lý volatile phytoconstituents against influenza A hemagglutinin, SARS-CoV-2 main protease, and Omicron-variant spike protein
- Physical, mechanical, and gamma ray shielding properties of the Bi2O3–BaO–B2O3–ZnO–As2O3–MgO–Na2O glass system
- Twofold interpenetrated 3D Cd(ii) complex: Crystal structure and luminescent property
- Study on the microstructure and soil quality variation of composite soil with soft rock and sand
- Ancient spring waters still emerging and accessible in the Roman Forum area: Chemical–physical and microbiological characterization
- Extraction and characterization of type I collagen from scales of Mexican Biajaiba fish
- Finding small molecular compounds to decrease trimethylamine oxide levels in atherosclerosis by virtual screening
- Prefatory in silico studies and in vitro insecticidal effect of Nigella sativa (L.) essential oil and its active compound (carvacrol) against the Callosobruchus maculatus adults (Fab), a major pest of chickpea
- Polymerized methyl imidazole silver bromide (CH3C6H5AgBr)6: Synthesis, crystal structures, and catalytic activity
- Using calcined waste fish bones as a green solid catalyst for biodiesel production from date seed oil
- Influence of the addition of WO3 on TeO2–Na2O glass systems in view of the feature of mechanical, optical, and photon attenuation
- Naringin ameliorates 5-fluorouracil elicited neurotoxicity by curtailing oxidative stress and iNOS/NF-ĸB/caspase-3 pathway
- GC-MS profile of extracts of an endophytic fungus Alternaria and evaluation of its anticancer and antibacterial potentialities
- Green synthesis, chemical characterization, and antioxidant and anti-colorectal cancer effects of vanadium nanoparticles
- Determination of caffeine content in coffee drinks prepared in some coffee shops in the local market in Jeddah City, Saudi Arabia
- A new 3D supramolecular Cu(ii) framework: Crystal structure and photocatalytic characteristics
- Bordeaux mixture accelerates ripening, delays senescence, and promotes metabolite accumulation in jujube fruit
- Important application value of injectable hydrogels loaded with omeprazole Schiff base complex in the treatment of pancreatitis
- Color tunable benzothiadiazole-based small molecules for lightening applications
- Investigation of structural, dielectric, impedance, and mechanical properties of hydroxyapatite-modified barium titanate composites for biomedical applications
- Metal gel particles loaded with epidermal cell growth factor promote skin wound repair mechanism by regulating miRNA
- In vitro exploration of Hypsizygus ulmarius (Bull.) mushroom fruiting bodies: Potential antidiabetic and anti-inflammatory agent
- Alteration in the molecular structure of the adenine base exposed to gamma irradiation: An ESR study
- Comprehensive study of optical, thermal, and gamma-ray shielding properties of Bi2O3–ZnO–PbO–B2O3 glasses
- Lewis acids as co-catalysts in Pd-based catalyzed systems of the octene-1 hydroethoxycarbonylation reaction
- Synthesis, Hirshfeld surface analysis, thermal, and selective α-glucosidase inhibitory studies of Schiff base transition metal complexes
- Protective properties of AgNPs green-synthesized by Abelmoschus esculentus on retinal damage on the virtue of its anti-inflammatory and antioxidant effects in diabetic rat
- Effects of green decorated AgNPs on lignin-modified magnetic nanoparticles mediated by Cydonia on cecal ligation and puncture-induced sepsis
- Treatment of gastric cancer by green mediated silver nanoparticles using Pistacia atlantica bark aqueous extract
- Preparation of newly developed porcelain ceramics containing WO3 nanoparticles for radiation shielding applications
- Utilization of computational methods for the identification of new natural inhibitors of human neutrophil elastase in inflammation therapy
- Some anticancer agents as effective glutathione S-transferase (GST) inhibitors
- Clay-based bricks’ rich illite mineral for gamma-ray shielding applications: An experimental evaluation of the effect of pressure rates on gamma-ray attenuation parameters
- Stability kinetics of orevactaene pigments produced by Epicoccum nigrum in solid-state fermentation
- Treatment of denture stomatitis using iron nanoparticles green-synthesized by Silybum marianum extract
- Characterization and antioxidant potential of white mustard (Brassica hirta) leaf extract and stabilization of sunflower oil
- Characteristics of Langmuir monomolecular monolayers formed by the novel oil blends
- Strategies for optimizing the single GdSrFeO4 phase synthesis
- Oleic acid and linoleic acid nanosomes boost immunity and provoke cell death via the upregulation of beta-defensin-4 at genetic and epigenetic levels
- Unraveling the therapeutic potential of Bombax ceiba roots: A comprehensive study of chemical composition, heavy metal content, antibacterial activity, and in silico analysis
- Green synthesis of AgNPs using plant extract and investigation of its anti-human colorectal cancer application
- The adsorption of naproxen on adsorbents obtained from pepper stalk extract by green synthesis
- Treatment of gastric cancer by silver nanoparticles encapsulated by chitosan polymers mediated by Pistacia atlantica extract under ultrasound condition
- In vitro protective and anti-inflammatory effects of Capparis spinosa and its flavonoids profile
- Wear and corrosion behavior of TiC and WC coatings deposited on high-speed steels by electro-spark deposition
- Therapeutic effects of green-formulated gold nanoparticles by Origanum majorana on spinal cord injury in rats
- Melanin antibacterial activity of two new strains, SN1 and SN2, of Exophiala phaeomuriformis against five human pathogens
- Evaluation of the analgesic and anesthetic properties of silver nanoparticles supported over biodegradable acacia gum-modified magnetic nanoparticles
- Review Articles
- Role and mechanism of fruit waste polyphenols in diabetes management
- A comprehensive review of non-alkaloidal metabolites from the subfamily Amaryllidoideae (Amaryllidaceae)
- Discovery of the chemical constituents, structural characteristics, and pharmacological functions of Chinese caterpillar fungus
- Eco-friendly green approach of nickel oxide nanoparticles for biomedical applications
- Advances in the pharmaceutical research of curcumin for oral administration
- Rapid Communication
- Determination of the contents of bioactive compounds in St. John’s wort (Hypericum perforatum): Comparison of commercial and wild samples
- Retraction
- Retraction of “Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: The protective effect on periodontitis via reducing the release of IL-1β and TNF-α”
- Topical Issue on Phytochemicals, biological and toxicological analysis of aromatic medicinal plants
- Anti-plasmodial potential of selected medicinal plants and a compound Atropine isolated from Eucalyptus obliqua
- Anthocyanin extract from black rice attenuates chronic inflammation in DSS-induced colitis mouse model by modulating the gut microbiota
- Evaluation of antibiofilm and cytotoxicity effect of Rumex vesicarius methanol extract
- Chemical compositions of Litsea umbellata and inhibition activities
- Green synthesis, characterization of silver nanoparticles using Rhynchosia capitata leaf extract and their biological activities
- GC-MS analysis and antibacterial activities of some plants belonging to the genus Euphorbia on selected bacterial isolates
- The abrogative effect of propolis on acrylamide-induced toxicity in male albino rats: Histological study
- A phytoconstituent 6-aminoflavone ameliorates lipopolysaccharide-induced oxidative stress mediated synapse and memory dysfunction via p-Akt/NF-kB pathway in albino mice
- Anti-diabetic potentials of Sorbaria tomentosa Lindl. Rehder: Phytochemistry (GC-MS analysis), α-amylase, α-glucosidase inhibitory, in vivo hypoglycemic, and biochemical analysis
- Assessment of cytotoxic and apoptotic activities of the Cassia angustifolia aqueous extract against SW480 colon cancer
- Biochemical analysis, antioxidant, and antibacterial efficacy of the bee propolis extract (Hymenoptera: Apis mellifera) against Staphylococcus aureus-induced infection in BALB/c mice: In vitro and in vivo study
- Assessment of essential elements and heavy metals in Saudi Arabian rice samples underwent various processing methods
- Two new compounds from leaves of Capparis dongvanensis (Sy, B. H. Quang & D. V. Hai) and inhibition activities
- Hydroxyquinoline sulfanilamide ameliorates STZ-induced hyperglycemia-mediated amyleoid beta burden and memory impairment in adult mice
- An automated reading of semi-quantitative hemagglutination results in microplates: Micro-assay for plant lectins
- Inductively coupled plasma mass spectrometry assessment of essential and toxic trace elements in traditional spices consumed by the population of the Middle Eastern region in their recipes
- Phytochemical analysis and anticancer activity of the Pithecellobium dulce seed extract in colorectal cancer cells
- Impact of climatic disturbances on the chemical compositions and metabolites of Salvia officinalis
- Physicochemical characterization, antioxidant and antifungal activities of essential oils of Urginea maritima and Allium sativum
- Phytochemical analysis and antifungal efficiency of Origanum majorana extracts against some phytopathogenic fungi causing tomato damping-off diseases
- Special Issue on 4th IC3PE
- Graphene quantum dots: A comprehensive overview
- Studies on the intercalation of calcium–aluminium layered double hydroxide-MCPA and its controlled release mechanism as a potential green herbicide
- Synergetic effect of adsorption and photocatalysis by zinc ferrite-anchored graphitic carbon nitride nanosheet for the removal of ciprofloxacin under visible light irradiation
- Exploring anticancer activity of the Indonesian guava leaf (Psidium guajava L.) fraction on various human cancer cell lines in an in vitro cell-based approach
- The comparison of gold extraction methods from the rock using thiourea and thiosulfate
- Special Issue on Marine environmental sciences and significance of the multidisciplinary approaches
- Sorption of alkylphenols and estrogens on microplastics in marine conditions
- Cytotoxic ketosteroids from the Red Sea soft coral Dendronephthya sp.
- Antibacterial and biofilm prevention metabolites from Acanthophora spicifera
- Characteristics, source, and health risk assessment of aerosol polyaromatic hydrocarbons in the rural and urban regions of western Saudi Arabia
- Special Issue on Advanced Nanomaterials for Energy, Environmental and Biological Applications - Part II
- Green synthesis, characterization, and evaluation of antibacterial activities of cobalt nanoparticles produced by marine fungal species Periconia prolifica
- Combustion-mediated sol–gel preparation of cobalt-doped ZnO nanohybrids for the degradation of acid red and antibacterial performance
- Perinatal supplementation with selenium nanoparticles modified with ascorbic acid improves hepatotoxicity in rat gestational diabetes
- Evaluation and chemical characterization of bioactive secondary metabolites from endophytic fungi associated with the ethnomedicinal plant Bergenia ciliata
- Enhancing photovoltaic efficiency with SQI-Br and SQI-I sensitizers: A comparative analysis
- Nanostructured p-PbS/p-CuO sulfide/oxide bilayer heterojunction as a promising photoelectrode for hydrogen gas generation


