Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
-
Djaber Aouf
, Fares Fenniche
, Ghassan M. Sulaiman
, Sofiane Khane
Abstract
The current study proposed a novel simple and environmentally friendly approach for producing silver nanoparticles (AgNPs) using an extract of Moringa oleifera leaves (MOL) and optimizing the different experimental factors required for the formation and stability of AgNPs. The formation of nanoparticles was confirmed by a color change from yellow to reddish-brown with a surface plasmon resonance band at 412 nm. The morphology, size, and elemental composition of AgNPs were investigated by zeta potential dynamic light scattering, field emission scanning electron microscopy, energy dispersive X-ray, X-ray diffraction, and transmission electron microscopy analysis, which showed crystalline and spherical AgNPs. The identification of functional groups was supported by Fourier-transform infrared spectroscopy. The photocatalytic activities of AgNPs were assessed in the degradation of organic Malachite green (MG) dye in the aqueous solution. Two kinetic adsorption models, the pseudo-first-order model and the pseudo-second-order model, and three isotherm models, the Langmuir, Freundlich, and Temkin, were used to mathematically characterize the MG degradation process. The pseudo-second-order kinetics and the Freundlich isotherm model were found to be in good agreement with the experimental data. As a result of their synergistic interaction with the MOL extract solution, the photocatalytic activity of AgNPs increases and they can successfully adapt to the photodegradation of organic dyes in industrial effluents.
1 Introduction
Due to their longevity, resilience, and potentially devastating effects on human health, organic contaminants are mostly partially responsible for the significant increase in water contamination. Most dyes used in the industry are released straight into water bodies, where they produce serious problems that contribute to pollution, eutrophication, disruption, and restriction of biological processes of aquatic life [1]. These problems prompted researchers to develop novel approaches that fulfill some criteria, such as low toxicity, environmental-friendliness, and cost-effectiveness [2,3]. Organic substances have been removed using a variety of methods. For instance, advanced oxidation procedures are efficient at removing organic pollutants. Photocatalysis’s high efficiency, low cost, and environmentally friendly approach make it a popular choice for the breakdown of organic contaminants [4,5]. Modest technical advancements have been made in developing photocatalytic technology for industrial applications in pollutant treatment. One of the technical challenges hindering the widespread application of photocatalytic technology is the presence of several system factors that require rapid testing [6]. During photocatalysis, certain factors can influence the reaction environment, and thus, the efficiency of the photocatalytic activity needs to be controlled. This includes pH, initial concentration, agitation rate, reaction kinetics, light yield, and the distance between light sources and the medium [7,8]. In recent years, biogenic nanoparticles have been utilized effectively to purge water from a wide range of organic pigments and bacterial infections [9,10]. Metal nanoparticles are of enormous significance for their potential application in catalytic, sensing, optical, and antibacterial materials due to their size- and shape-related characteristics [11,12]. The organic dyes can be photocatalytically degraded using a variety of metal nanoparticles. Because of their cost-effectiveness, appropriate band location, and outstanding plasmonic characteristics, silver nanoparticles (AgNPs) are effective catalysts compared to other metal nanoparticles [13]. AgNPs are among the metal nanoparticles with the highest demonstrated actions in several areas, including photocatalysis, antioxidants, and antimicrobials [14,15]. In this sense, nanotechnology utilizes green synthesis techniques to create nanoparticles using environmentally friendly molecules [16]. One of these techniques involves the use of natural medicinal plants to produce metallic nanoparticles since they are rich in proteins, carbohydrates, phenol, vitamins, kaempferol, potassium, calcium, and amino acids [17]. Because of their high concentrations of antioxidants such as isoflavones, metabolites, flavones, and phenolic chemicals, these plants are ideal for producing AgNPs from their metal precursors [18]. Moringa is a moderate-sized tree belonging to the family Moringaceae and the order Capparales [19]. It comprises 13 species and are known by various common names depending on the region it grow in. The Moringa oleifera (M. oleifera) plant has been used for biosynthesizing nanoparticles [20,21]. It has been discovered that the leaves of the Moringa tree possess flavonoids such as myricetin, quercetin, kaempferol, isorhamnetin, or rutin, in addition to phenolic acids. Green foliage serves as an excellent reservoir of carotenoids, including lutein, β-carotene, and zeaxanthin. Thus, it is a wise decision to utilize different capping compositions for the in situ synthesis of AgNPs to assess the impact of various surface-bound biomolecules on the size, shape, and antibacterial properties of the nanoparticles [22]. Additionally, Moringa possesses an extensive range of medicinal uses, including antibacterial, antifungal, antiparasitic, antiviral, wound-healing, antimicrobial, antioxidant, and proliferative properties [23]. Therefore, the current study investigates the bio-synthesis of AgNPs using the Moringa oleifera leaves (MOL) aqueous extract. The biosynthesized nanoparticles were characterized using UV-Vis spectroscopy, X-ray diffraction (XRD), field emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), and dynamic light scattering (DLS)-zeta potential (ZP) analysis, and further evaluated for their photocatalytic activities to remove Malachite green (MG) dye and the deferring effect of factors such as pH, temperature, and dye concentration. To the best of our knowledge, there is no existing literature that reports on the use of green synthesis to create AgNPs utilizing MOL extract, and its subsequent use as a photocatalyst for the efficient breakdown of MG dye under UV light.
2 Materials and methods
2.1 Chemicals
The MG dye and silver nitrate (AgNO3, 99.99%) were acquired from Sigma Aldrich (Germany). Double-distilled water was supplied by the University of Ghardaia, Algeria.
2.2 Selection of M. oleifera
Fresh leaves of M. oleifera were collected in March 2021 from the same tree of AOUF grove in Oasis Ghardaïa, which is a region from the north of the Algerian Sahara. About 100 g of M. oleifera leaves were collected by hand, rinsed multiple times with tap water, and then cleaned again with distilled water to eliminate dust and all possible impurities before being dried for 2 weeks at room temperature (32°C). The obtained dried plant leaves (35 g) were powdered as used for the extraction procedure.
2.3 Aqueous extract of MOL
About 20 g of the M. oleifera dried leaf powder was refluxed in 200 ml of demineralized water at 65°C with stirring for 10 min to obtain an aqueous extract of MOL. The obtained extract was clear, pale yellow in color. The extract was allowed to cool at ambient room temperature and filtered with a qualitative filter paper Ø.47 mm, Grade no 1 Whatman to remove any suspended particles. The first part of the MOL extract was dried at 40°C for 48 h in a vacuum oven to obtain the extract powder, and the other part was refrigerated between 4 and 8°C [20].
2.4 Green synthesis of AgNPs
The green synthesis AgNPs was performed according to a previously published work [24] with some modifications. About 10 mL of the MOL extract was mixed with 90 mL of an aqueous solution of AgNO3. The mixture was stirred at 250 rpm for 20 min at a constant mixing temperature of 60°C, which resulted in a color change from yellow to brown due to the formation of AgNPs. A dark brown precipitate was formed after centrifuging the suspension several times at 20,000 rpm for 15 min. Then, this precipitate was washed three times with sterile water and once with methanol to obtain AgNPs; the powder residue was dried in an oven. To optimize the production of AgNPs with the MOL extract, a series of experiments were conducted, considering various experimental parameters such as the concentration of the leaf extract, the contact time, and the concentration of AgNO3. UV-visible spectroscopy was performed to examine the controlled conditions and study the proportions and form of AgNPs.
2.5 Spectroscopy characterization of MOL-AgNPs
The optical characterization of MOL-AgNPs was performed using a UV-visible spectrophotometer (UviLine 9400C) with 1 nm resolution in the wavelength range from 300 to 800 nm. The chemical bonding and the surface coating of dried MOL were verified by Fourier transform infrared spectroscopy (FT-IR; Agilent Cary 640 spectrophotometer, 400–4,000 cm−1, Germany). A Nano Zeta sizer instrument (ZS; Malvern, Instruments Inc., Germany) was used to determine the particle size of AgNPs in MOL and DLS of AgNPs. The crystalline nature of MOL-AgNPs was established using a PW 1710 diffractometer (Philips Diffractometer) set to 40 kV and 40 mA with Cu Ka radiation (wavelength = 1.5406), a 2 h range of 10–60, a step size of 0.03, and a time/step of 0.3 s. A field emission scanning electron microscope with MIRA 3 TESCAN (Brno, Czech Republic), equipped with an energy dispersive X-ray spectrometer (EDX), operated at 20 kV acceleration voltage with a magnification of Images 30000 was used to record the morphology and form of AgNPs. A transmission electron microscope – Zeiss model (Jena, Germany) was also used to visualize the Ag nanoparticles.
3 Results and discussion
In this work, MOL-AgNPs were synthesized using the aqueous solution of M. oleifera leaves, which contain bioactive compounds. Various spectroscopic analyses were employed to track the progress of the process. The parameters (time, pH, temperature, concentration of AgNO3, and plant extract) were optimized for the rapid and maximum synthesis of AgNPs. The pH was maintained at 1, 3, 5, 7, 9, and 11 and optimized with 0.1 N (HCl) and 0.1 N (NaOH). The reaction was monitored from 0 to 60 min for optimal production of AgNPs. The synthesis of AgNPs was monitored at various incubation temperatures (25, 50, 75, and 100°C). The concentration of AgNO3 was optimized at different concentrations (0.50, 1.0, 1.5, and 2.0 mM). Similarly, the concentration ratio of the plant extract and AgNO3 was optimized by increasing the concentration of the plant extract in a 1 mM AgNO3 solution. The absorbance of nanoparticles was observed within the range of 300–800 nm by a UV–visible spectrophotometer. The parameters time, pH, temperature, concentration of AgNO3, and plant extract were optimized for the rapid and maximum synthesis of AgNPs.
3.1 UV–Visible spectral analysis
Figure 1a shows the follow-up of the bioreduction of Ag+ ions with the MOL extract by UV-vis spectroscopy after different times. The UV-vis spectra of the synthesized nanoparticles showed a single characteristic UV absorption peak at 412 nm. The surface plasmon resonsnce (SPR) is confirmed he formation of AgNPs. The SPR of AgNPs is responsible for the observed shift from a yellow to a reddish-brown hue in the aqueous solution because the free electrons in the metal are excited during the synthesis of AgNPs [25]. When Ag+ ions are exposed to the bioactive components of the plant extract, they are reduced to Ag and then to AgNPs [26]. An increase in the spectrum intensity over time may be attributable to the formation of more AgNPs as a result of a decrease in the concentration of reduced silver ions in the aqueous medium. These results were completely consistent with the most recent published results by Khane et al. [24].
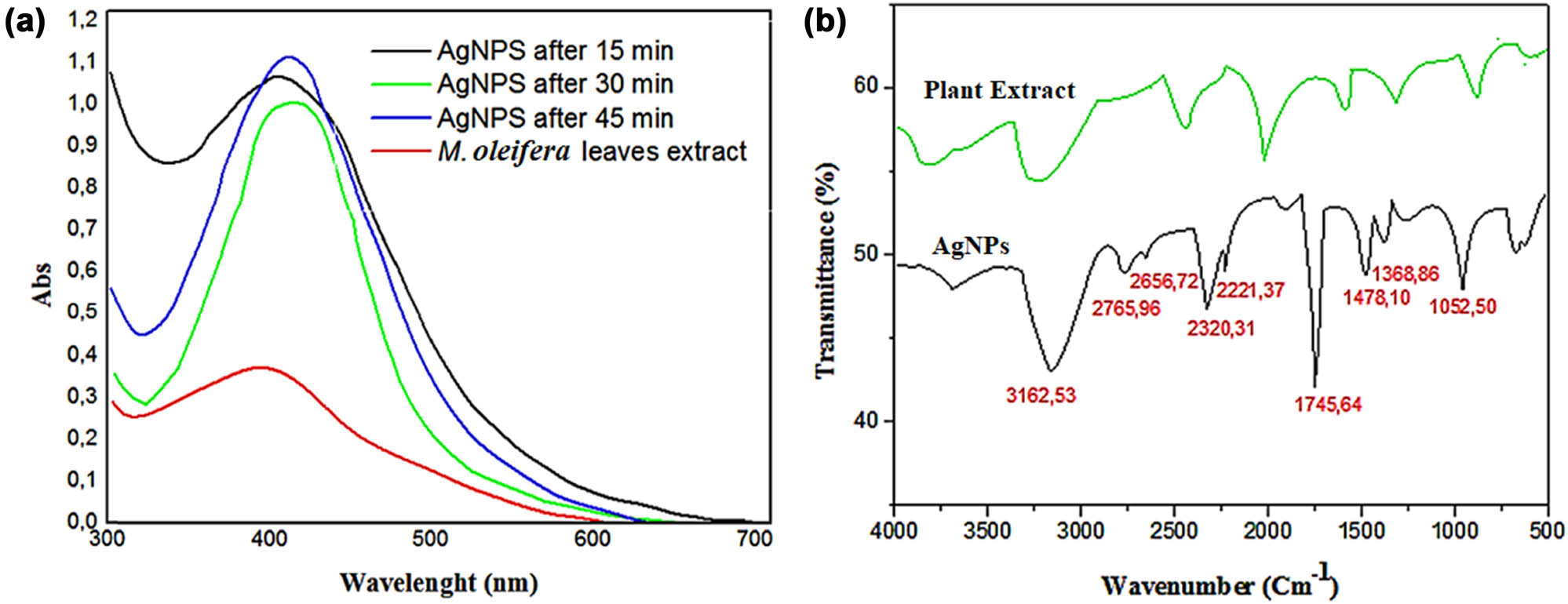
(a) UV-Vis spectra showing the absorbance peak of green synthesized AgNPs using M. oleifera leaf extract after 15, 30, and 45 min of preparation. (b) FT-IR spectra of the plant extract (green line) and synthesized AgNPs (red line). FT-IR: Fourier transform infrared spectroscopy; AgNPs: silver nanoparticles; M. oleifera: Moringa oleifera.
3.2 Fourier transform infrared spectroscopy
Figure 1b shows the FT-IR spectra of dried MOL-AgNPs. The spectra provide information about biofunctional groups contributing to the binding and bioreduction of silver ions (Ag+). The FT-IR spectra of the leaf extract show several absorption peaks, which reveal the existence of capping agents (Figure 1). The band at 1044.18 cm−1 was attributed to the –C–O groups of the polyalcohol, the peaks at 1,157, 1478.10, 2320.31 and 1745.64 cm−1 corresponded to N–O stretching, the N–H bond to the primary amines, the C–H stretching to methylene groups, and carbonyl stretching to the –C═O.
The strong bands at 2675.96 and 2656.72 cm−1 were assigned to the presence of the C–H stretching vibration of aromatic compounds [27], and the strong band at 3162.53 cm−1 corresponds to the stretching vibration of the hydroxyl functional groups (–OH) in alcohols, phenolic compounds, and carboxylic acid [28]. The peak at 1,634 cm−1 is due to the amide function from proteins, whereas the band at 1052.50 cm−1 is due to the C–N stretch vibrations of amines [29]. The FT-IR spectra corresponding to the MOL-AgNPs (Figure 1b) revealed a significant change in peaks, and these peaks entirely disappeared or had significantly diminished intensities. Some strong peaks became weak in nanoparticles, which indicates that the bioactive molecules of the extract compounds are involved in reducing silver ions. Owing to their oxidation-reduction potential [30], functional groups like polyphenols and their derivatives are involved in the reduction of Ag+ into Ag0 and the stabilization of AgNPs [31]. The studies suggest that the functional groups in the plant metabolites present in the MOL extract formed a solid coating on the nanoparticles [32].
3.3 X-ray diffractogram analysis
Table 1 presents the XRD analysis data and further shape description by Bragg’s equation (2dsin θ = nλ) and Scherrer’s equation D = Kλ/βcos θ, confirming the crystalline nature of AgNPs synthesized using the aqueous extract of MOL (Figure 2). The diffraction peaks at 2θ of 38.0615, 44.5231, 63.6769, and 63.6769 correspond to the (111), (200), (220), and (311) facets, respectively, and these findings corroborate the crystalline nature and face-centered cubic structure of the AgNPs generated by the reduction of Ag+ ions by the MOL extract, which is in good agreement with the powder data of Joint Committee on Powder Diffraction Standards file no. 84-0713. Some peaks were also seen, which may have been caused by silver oxide or bio-organic phases crystallizing on the nanoparticles’ surfaces. AgNPs produced from the MOL extract [33] and M. oleifera flower extract [34] were reported to have a similar spectrum. By applying the Debye–Scherrer formula to the full width at half-maximum (FWHM) of peaks, we determined that the average crystallite size of MOL-AgNPs is 25.26 nm.
Data from the X-ray diffractogram analysis and additional shape description of MOL-AgNPs using Bragg’s equation (2dsin θ = nλ) and Scherrer’s equation D = Kλ/β cos θ
| Peak number | Scherrer’s equation | Bragg’s equation | |||
|---|---|---|---|---|---|
| Peak position 2θ (°) | FWHM β (°) | D (nm) | Average (nm) | D-space | |
| 1 | 38.0615 | 0.44583 | 19.69 | 25.26 | 2.36 |
| 2 | 44.5231 | 0.61835 | 14.50 | 2.03 | |
| 3 | 63.6769 | 0.62052 | 15.74 | 1.46 | |
| 4 | 77.2154 | 0.20771 | 51.12 | 1.23 | |

XRD diffraction patterns of the synthesized MOL-AgNPs confirm the crystalline nature of AgNPs and show various Bragg’s reflection peaks. 2θ: 2 theta angle ranging from 20 to 90. XRD: X-ray diffractogram; MOL-AgNPs: Moringa oleifera silver nanoparticles.
3.4 ZP and DLS characterization
The Zetasizer Nano-ZS can measure both the particle size and the ZP of nanoparticles. Static light scattering is used to determine the thickness of the compounds enveloping the particles and the average particle size distribution in the solution of AgNPs [35]. Figure 3a and Table 2 reveal that the AgNPs made using the MOL extract are of good quality because the polydispersity index (PDI) value was found to be 0.331, which is less than 0.7. This indicates that the nanoparticles have well-defined size and high monodispersity [36]. The mean size of the AgNP dispersion was found to be 25.95 nm. By using ZP estimation, we demonstrated that the AgNPs synthesized using the MOL extract are negatively charged (−16.6 mV). Repulsion acts as an electrostatic stabilizer, with the negative charge perhaps coming from the adsorption of free nitrate ions in the mixture [37].
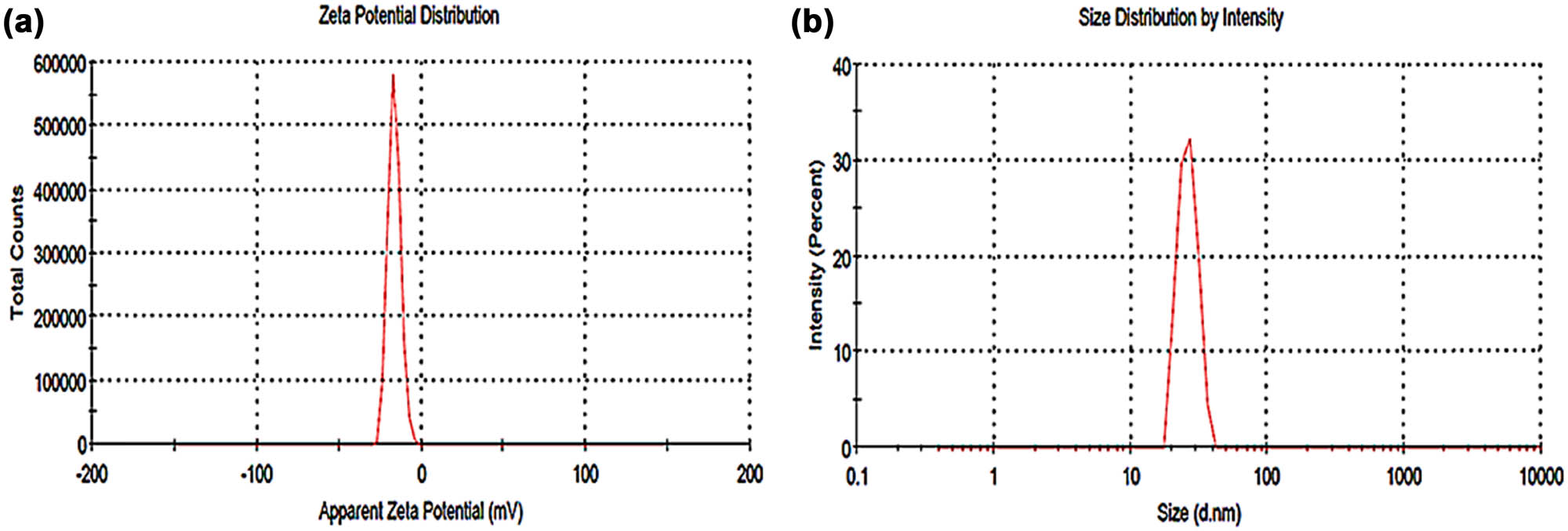
(a) ZP at −16.6 mV and (b) DLS analysis showed a mean size of 25.95 nm of MOL-AgNPs measured using Zetasizer® software. MOL-AgNPs: Moringa oleifera silver nanoparticles.
Conductivity, surface ZP, particle size distribution, and PDI of MOL-AgNPs at 25°C
| T (°C) | Conductivity (mS/cm) | ZP (mV) | z-average size d (nm) | PDI |
|---|---|---|---|---|
| 25 | 0.394 | −16.6 | 25.95 | 0.331 |
T: Temperature.
3.5 FE-SEM-EDX and TEM analysis
The morphological shape and surface of the biosynthesized MOL-AgNPs using FE-SEM micrographs are shown clearly in Figure 4a. The detailed structure and characterization demonstrate that the AgNPs synthesized are spherical and very crystalline in structure, with sizes ranging between 7 and 50 nm, which are confirmed by TEM analysis and DLS results. The TEM analysis of biosynthesized AgNPs confirmed their form and size distributions. The TEM images (Figure 4b) revealed both the individual silver particles and the polydispersity of the nanoparticles. The diameters of the produced nanomaterial ranged from 6.82 to 55.28 nm. MOL-AgNPs of different sizes were produced since the amount of reducing agents in the MOL extract decreased throughout the synthesis. Even inside the aggregates, we found little evidence of direct contact between the nanoparticles, suggesting that MOL-AgNPs were protected by a thin layer of functional material derived from organic chemicals [38]. This result was different from the XRD result because XRD provides an approximation of the NPs’ actual size, but FE-SEM and TEM analyses are superior for visualizing particle size. The observation of larger nanoparticles may be attributed to AgNP’s proclivity to agglomerate due to their high surface energy and ultra-fine nanoparticle high surface tension. Due to this phenomenon, most of the nanoparticles in this micrograph have agglomerated. AgNPs derived from a variety of biological sources are of different sizes, with a spherical shape in the majority of these investigations [39]. The elemental make-up of green synthesized AgNPs from MOL is introduced mostly through the EDX patterns. In Figure 4c, a significant signal for Ag properties (lines L and K) was observed around 3 keV and between 2 and 4 keV, with high atomic percent values as shown in Table 3, confirming the production of AgNPs produced using the MOL aqueous extract. EDX analysis showed that there were a few faint signals of carbon, oxygen, sodium, and chlorine in addition to silver, which indicated that the nanoparticles contained plant-bioactive compounds attached to the surface of AgNPs. There is no ionic silver peak in the EDX spectrum, which proves that Ag+ is bio-reduced to stable Ag0 with a good yield. Based on the EDX patterns that were seen, the biosynthesized AgNPs are very pure and crystalline because polyphenols in the water extract of MOL completely bio-reduce the silver ions [40]. Previous reports [41] shared comparable findings and have suggested that capping structures in biomolecules are responsible [42].
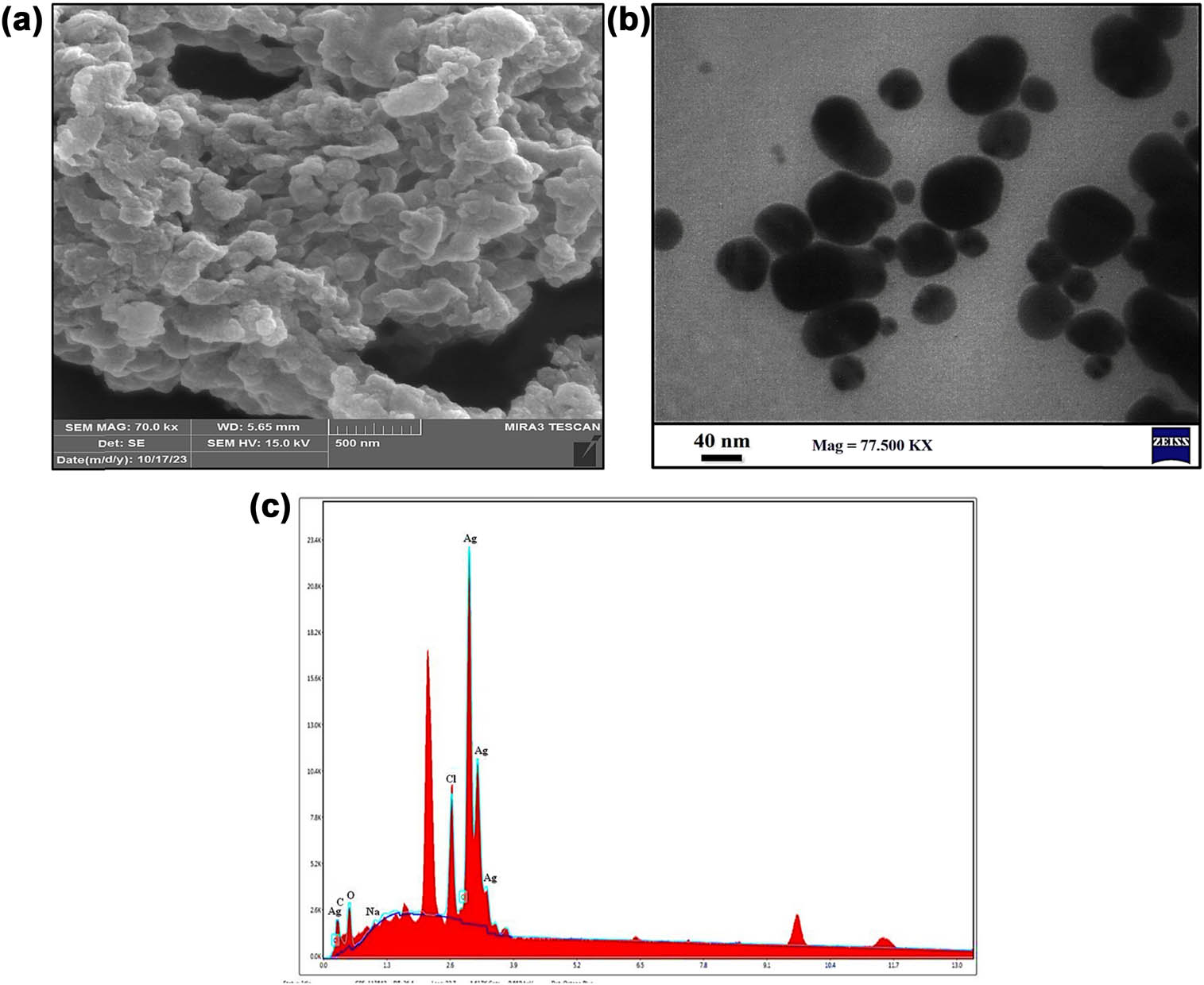
(a) FE-SEM micrographs; scale bar, 500 nm. (b) TEM micrographs; scale bar, 40 nm. (c) EDX spectrum showing the elemental composition of green synthesized MOL-AgNPs. FE-SEM: field emission scanning electron microscopy, TEM: transmission scanning electron microscopy; MOL-AgNPs: Moringa oleifera-silver nanoparticles.
Elemental composition of biosynthesized MOL-AgNPs, as revealed by EDX
| Element | Weight % | Atomic % | Net Int. |
|---|---|---|---|
| C | 5.75 | 18.46 | 347.8 |
| O | 19.65 | 47.31 | 789.12 |
| Ag | 65.2 | 23.29 | 10725.84 |
| Na | 1.21 | 2.03 | 126.53 |
| Cl | 8.19 | 8.9 | 2975.52 |
C: carbon; O: oxygen; Ag: silver; Na: sodium; Cl: chlorine.
3.6 Photocatalytic degradation
AgNPs can be used as a photocatalyst to remove organic pollutants and act as a clean energy source. Photocatalytic activity has garnered more attention due to its simplicity, high efficiency, cost-effectiveness, non-toxicity, good permeability, and reduced secondary pollution. In this study, we have selected MG dyes (4-[(4-dimethyl-aminophenyl)-phenyl-methyl]-N,N-dimethyl-aniline, C23H25ClN2) as pollutants due to their significant characteristics and multiple uses [43]. The triphenylmethane dye, which is a carcinogenic organic molecule, is still illegally used for various purposes, including in aquaculture as a fungicide on larvae and juvenile fish, as a parasiticide in food, textile, and other industries [44].
3.6.1 Photocatalytic degradation process of MG
The MOL-AgNPs were tested for their photocatalytic degradation of MG as a model pollutant utilizing a batch technique and UV irradiation. This dye was degraded when 30 mg of MOL-AgNPs were added to 50 mL of MG with an initial concentration of 2.1 × 105 mol L−1 under optimal conditions of pH = 6.8, duration of 60 min, T = 20°C, and stirring. After mixing thoroughly, the beakers are placed in a dark chamber equipped with two UV lamps (Vilber Company, Germany, 2012) emitting radiation at a wavelength of 360 nm and a radiation intensity of 1 mW/cm2 (Figure 5). The control sample was made without the use of radiation. The absorbance of the MG dye solution was checked using a UV/Visible spectrophotometer at λ max = 625 nm, and the efficiency of decolorization (%) was calculated using the following equation:
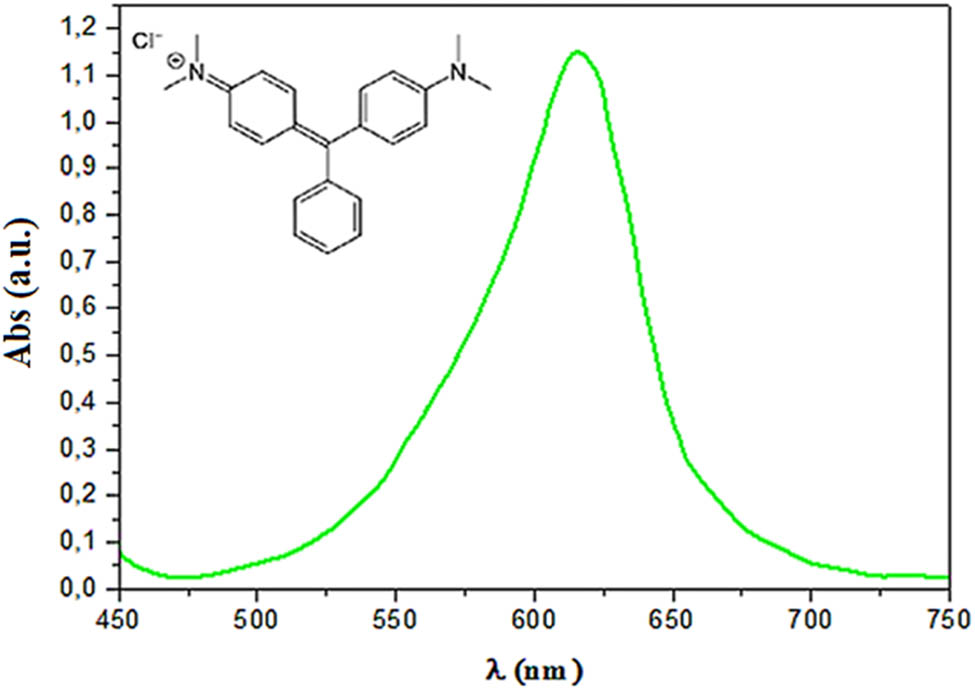
Maximum wavelength of the MG dye. The absorbance of MG was measured using a UV-Vis spectrophotometer at λ max = 625 nm. Abs: absorbance; MG: Malachite green.
C 0 is the concentration of MG at the equilibrium dark adsorption, and C t is the concentration following treatment with MOL-AgNPs. Origin pro 9.0 data analysis package was used to plot all the graphs and data evaluation.
3.6.2 Factors influencing the photocatalytic degradation of MG
Several studies have highlighted the significance of operational parameters, as the oxidation rate and efficiency of the photocatalytic system are strongly dependent on several operational parameters that govern the photodegradation of organic molecules [45].
3.6.2.1 Effect of solution pH
Because protons affect the degree of protonated dispersion of adsorbents in aqueous solution, the pH of the solution has a profound effect on the photocatalytic degradation of MG from aqueous solutions by adsorption. This, in turn, affects the surface charge of MOL-AgNPs in anionic dye solution, which in turn affects the nature of the ionic species of MOL-AgNPs. Generally, most of the oxidation cycle involves maintaining an acidic environment and a low pH [46]. By adding different amounts of hydrochloric acid solution, H2SO4 (0.1 M) or sodium hydroxide NaOH solution (0.1 M) to the solution, the pH was adjusted from 3.6 to 12 and fixing the other parameters with 2.1 × 105 mol/L of MG concentration at 60 min under UV irradiation at 20°C. Figure 6(a) shows the outcomes of MG’s photodegradation using MOL-AgNPs and illustrates that the percent degradation of MG was found to be 78.2, 86, and 98.4% at pH 12, 7.2, and 3.6, respectively. Then, the degradation of MG is strongly influenced by the pH of the dye solution. The degradation efficiency decreased from 76.2 to 98.4% as a result of the decrease in pH from 12 to 3.6, respectively. In acidic solutions, the interaction between the positively charged MOL-AgNPs and the negatively charged dye (pH < pHpzc) increases, leading to a greater rate of photocatalytic degradation of the anionically configured MG dye. In addition, AgNPs’ surface deposition of metal hydroxide can impede the production of reactive oxygen species and restrict MG elimination [47].
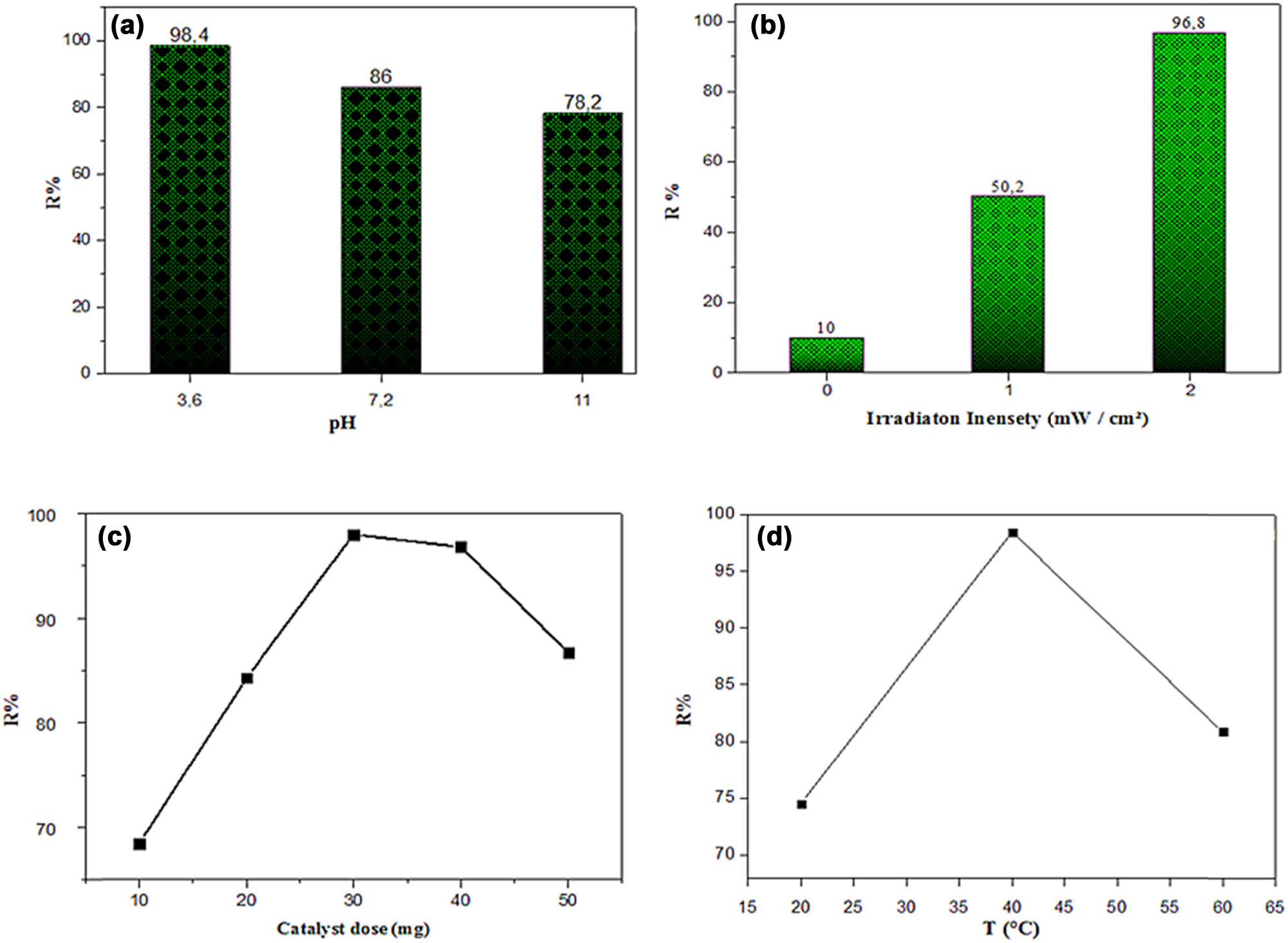
Effect of (a) pH, (b) irradiation intensity, (c) catalyst dose, and (d) the temperature of MOL-AgNPs on the kinetics of MG dye photodegradation using MOL-AgNPs. MOL-AgNPs: Moringa oleifera-silver nanoparticles.
3.6.2.2 Effect of irradiation intensity
The effects of UV irradiation on the photocatalytic of MG with MOL-AgNPs in an aqueous solution are tested using one and two UV lamps (Vilber Company. GERMANY). The UV wavelengths were 365 nm, with an irradiation intensity of 1 mW/cm², fixing all experimental parameters using 30 mg of MOL-AgNPs and under optimal conditions of pH = 6.8 and initial concentration of MG = 2.10 × 10−5 mol/L and at the same reaction time of 60 min. We can observe from the results shown in Figure 6(b) that the photodegradation was increased with an increase in the irradiation intensity of UV light intensity, which increased the rate of reaction because the number of photons striking per unit area also increased [48].
3.6.2.3 Effect of the MOL-AgNP amount
The catalyst dose also affects the photocatalytic process of the dye. To determine the effect of the amount of MOL-AgNPs on the reaction rate, the experiment of photodegradation of MG was carried out using 10–50 mg of MOL-AgNPs and under optimal conditions of pH = 6.8 and initial concentration of MG = 2.1 × 10−5 mol/L and at the same reaction time of 60 min. As shown in Figure 6(c), the degradation rate increased with an increase in several substrates from 10 to 30 mg with a maximum degradation of 98%, which is obviously due to a large surface area for photodegradation, attributed to particular interactions of dye molecules on the active sites. Therefore, the increase in the exposed surface area of photocatalysts with active sites is because the amount of MOL-AgNPs speeds up the reaction of photodegradation of dye [49]. However, as the catalyst dose was increased from 30 to 50 mg, the effectiveness of photodegradation dropped. The slower reaction rate at higher catalyst concentrations may be explained by the fact that activated molecules collided with ground-state catalysts, rendering them inactive due to blockage of multiple active sites [50].
3.6.2.4 Effect of temperature
To study the effect of temperature on the photodegradation of MG in a solution of the MOL-AgNPs, the experiment was performed using one MOL-AgNPs in 50 mL of the MG solution (2.1 × 10−5 mol/L) at different temperatures of 20, 40, and 60°C (Figure 6(d)) for 60 min and pH = 6.8. The percentage of MG degradation was found to increase from 74.52 to 98.46% as the temperature was raised from 20 to 40°C because more MG molecules were being transported from a liquid medium to a nanoadsorbent surface [51]. However, the degradation of Mg reduced to 80% at 60°C, which was due to evaporation of the solution and a decrease in adsorptive pressures between the active sites of the adsorbent and the adsorbate. Dye degradation was shown to be most efficient at 35°C, lending further credence to the endothermic nature of UV light-mediated photocatalytic degradation of MG using MOL-AgNPs. This result agrees with the results reported by Batool et al. [52].
3.6.2.5 Effect of contact time
In order to keep track of the contact time and establish equilibrium, researchers examined the photodegradation of MG using 30 mg over a time range (0 min to 60 min) while holding all other experimental variables constant. As shown in Figure 7(a and b), the photocatalytic effect of MG with MOL-AgNPs was shown to be a highly quick process when exposed to sunlight in first 25 min, which can be attributed to the availability of binding sites on the surface of MOL-AgNPs. This was reflected in a gradual weakening of the dark green hue of the solution as time progressed. However, after 25 min, the rate of photodegradation began to drop and it achieved equilibrium after 45 min due the saturation of the active sites with a maximum amount of dye removal found to be 74.19, 82.65, 97.14, and 98.01% for 5.10 × 10−5, 3.40 × 10−5, 2.10 × 10−5 and 0.86 × 10−5 mol L−1 at 60 min, respectively. Similar findings were reported by Batool et al. [52].
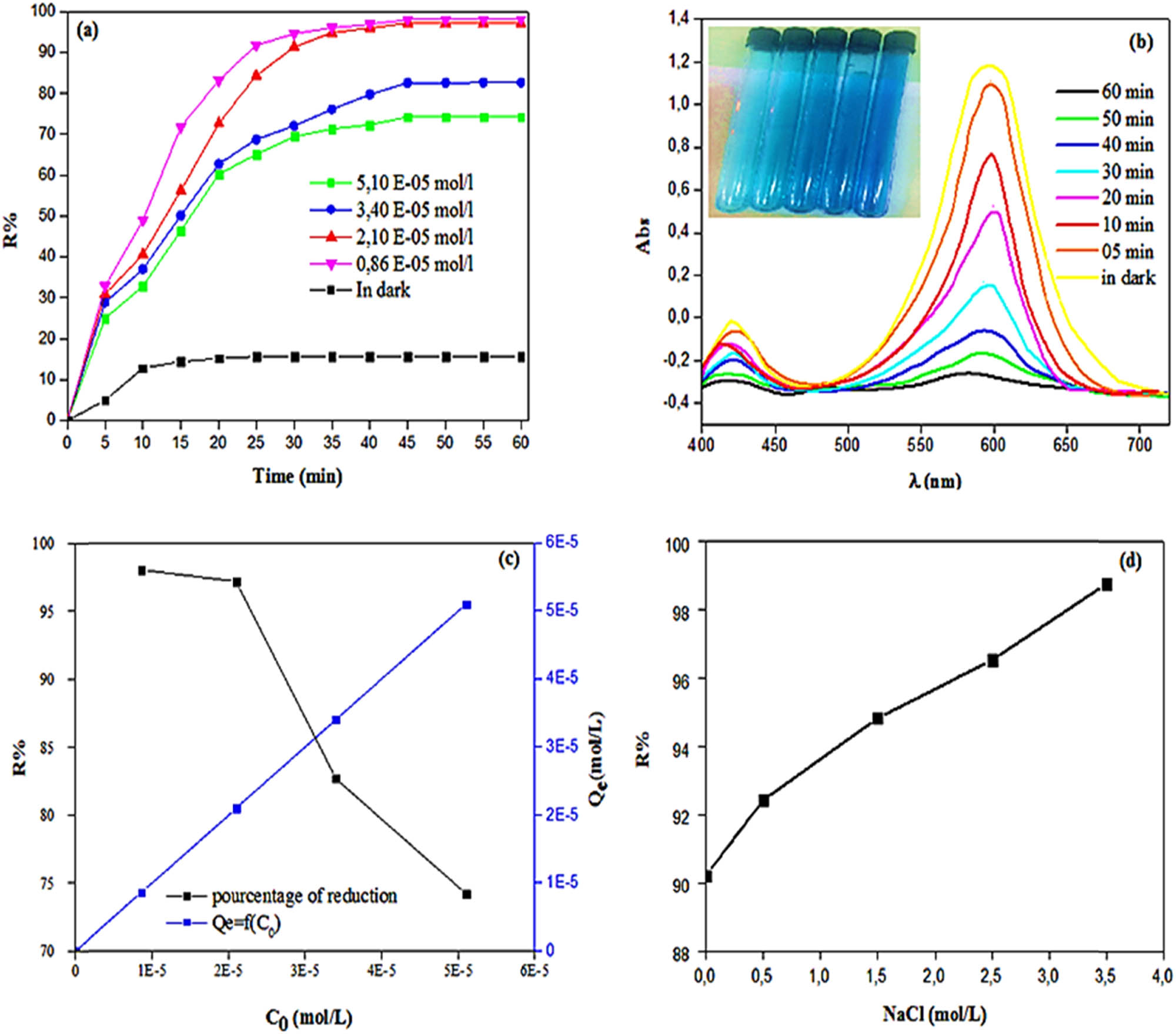
Effect of (a and b) contact time, (c) initial dye concentration, and (d) salinity on the kinetics of MG dye photodegradation using MOL-AgNPs. MOL-AgNPs: Moringa oleifera-silver nanoparticles.
3.6.2.6 Effect of the initial concentration of MG dye
By gradually raising the initial dye concentration from 0.8 × 10−5 to 5.1 × 10−5 mol L−1 and treating the mixture with MOL-AgNPs for 60 min while subjected to UV irradiation, we were able to investigate the effect of the MG concentration on the speed of the photocatalytic degradation reaction. The degradation efficiencies after 60 min of irradiation were 74.19%, 82.65%, 97.14%, and 98.01% for a dye solution of 0.86 × 10−5, 2.10 × 10−5, 3.40 × 10−5, and 5.10 × 10−5 mol L−1, respectively. From Figure 7(c), the degradation efficiency decreased with increasing initial dye concentration. The high dye concentration would have acted as a filter for the incident light, which ultimately decreased the degradation efficiency [53].
3.6.2.7 Effect of salinity
Salinity is also a very significant factor that interferes with the photodegradation efficiency. Treatment procedures like adsorption, photocatalytic degradation, and biological treatment are typically less effective in the presence of auxiliary components like salts, acids, and alkalis in textile dye effluents. The effect of salinity was studied at different concentrations of NaCl from 0.5 to 3.5 g/L, fixing all experimental parameters. The results in Figure 7(d) revealed an increase in photocatalysis of MG from 90.20 to 98.76% as the NaCl concentration is increased from 0.5 to 3.5 g/L. Competitive adsorption is a common mechanism proposed to account for NaCl’s inhibitory impact [54]. In order to neutralize hydroxyl free radicals, chloride ions undergo the following chemical reaction:
The excess chloride competes with the photocatalyst for the occupancy of active sites on its surface, as the Cl˙ radical is less reactive than the OH˙ radical. The photocatalytic efficacy for MG removal was drastically reduced due to an increase in Cl− ions [55]. One possible explanation for the drop is as follows. Some trapping effects were observed at greater NaCl concentrations due to Cl− ions competing with the oxidizing radicals or active sites of the catalyst. Adsorption of Cl− ions reached equilibrium as NaCl concentration increased; as a result, surplus of free Cl− ions enhanced charge transfer in the photocatalytic reactor, leading to greater electron capture created by exposure to ultraviolet light.
3.7 Kinetic study of MG dye photodegradation using MOL-AgNPs
Modeling kinetics involved incubating MOL-AgNPs for 60 min at room temperature with 50 mL of a dye solution at an initial pH of 6.8. The pseudo-first-order and pseudo-second-order models were used to analyze these kinetics using kinetic rate equations [56].
Pseudo-first-order kinetic model:
where Q e and Q t are the adsorption capacities at equilibrium and at different times (mg/L) and k 1 (min−1) is the rate constant of the pseudo-first-order model.
Pseudo-second-order kinetic model:
where Q e and Q t are the adsorption capacities at equilibrium and at different times (mg/L) and k 2 (g mg−1 min−1) is the rate constant of the pseudo-second-order model.
Linear fits to experimental data for pseudo-first-order and pseudo-second-order models are shown in Figure 8, and their results are summarized in Table 4. According to the results, an excellent linear relationship between the experimental data and the pseudo-second-order kinetic model was found, with correlation coefficient values between 0.992 and 0.999. Table 3 displays good agreement between the theoretical values of photodegradation (Q e cal) and experimental values (Q e exp) compared with pseudo-second-order. The calculated capacities of photodegradation (Q e cal) did not correspond with the experimental Q e values, verifying the applicability of the first pseudo-order model, while the produced graph of the pseudo-second-order kinetic model is linear but the intercept is far from zero.
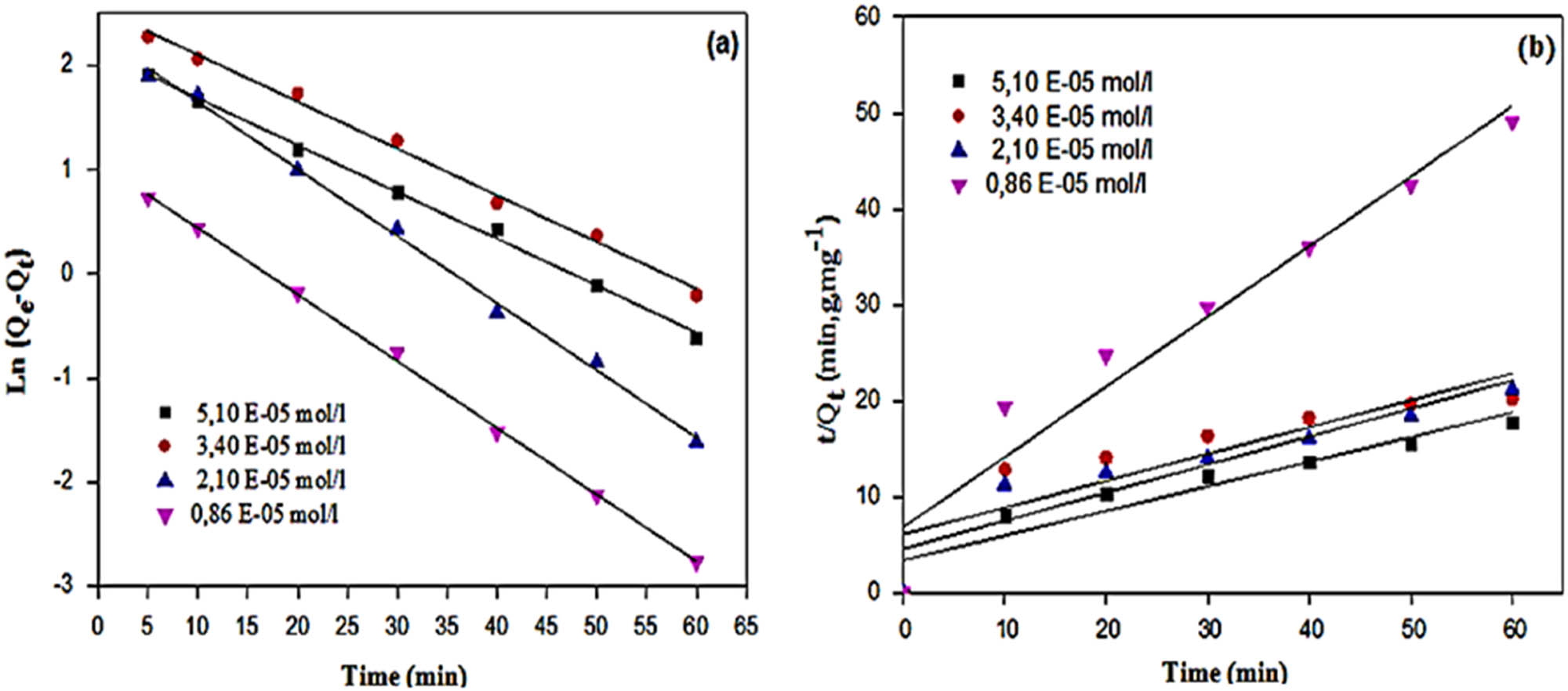
(a) Pseudo-first-order and (b) pseudo-second-order kinetics plots of MG dye photodegradation using MOL-AgNPs. MOL-AgNPs: Moringa oleifera silver nanoparticles.
Kinetic parameters of MG dye photodegradation using MOL-AgNPs at different initial concentrations of the dye
| Kinetic models and their parameters | Initial concentrations of MG (C 0, mg/L) | |||
|---|---|---|---|---|
| 3.14 | 7.66 | 12.4 | 18.6 | |
| Experimental | ||||
| Q e exp (mg/g) | 2.89 | 6.68 | 9.01 | 8.23 |
| Pseudo-first-order | ||||
| Q e cal (mg/g) | 2.89 | 6.12 | 10.36 | 8.48 |
| K 1 (min−1) | 0.03169 | 0.03269 | 0.02341 | 0.02257 |
| R 2 | 0.9991 | 0.99747 | 0.99691 | 0.99786 |
| Pseudo-second-order | ||||
| Q e cal (mg/g) | 3.25224405 | 8.5302397 | 8.14863103 | 9.20386562 |
| K 2 (g mg−1 min−1) | 0.01595145 | 0.00278187 | 0.0028112 | 0.00385661 |
| h (mg g−1 min−1) | 0.16871999 | 0.20242259 | 0.18666398 | 0.3266981 |
| R 2 | 0.96676 | 0.86324 | 0.84056 | 0.93478 |
MG: Malachite green.
3.8 Isotherm models of MG dye photodegradation using MOL-AgNPs
Three isotherm models, the Langmuir, Freundlich, and Temkin, were used to fit the photodegradation process in the presence of MOL-AgNPs, with initial MG concentrations ranging from 0.86 × 105 to 5.10 × 105 mol L−1, after 60 min at room temperature (20°C) with an initial pH of 6.8 (Table 5).
Equations and constant parameters for the isotherm models
| Isotherm mode | Linear plot | Abbreviation |
|---|---|---|
| Langmuir |
|
K L: constant of Langmuir model (L/mg) |
| q e: adsorption capacity (mg/g) | ||
|
|
C e: dye concentration at equilibrium (mg/L) | |
| q max: maximum capacity (mg/g) | ||
| Timken |
q
e = ƒ(
|
b T: Temkin constant related to the heat of adsorption (J mol−1) |
| K T: Temkin isotherm constant (L g−1) | ||
|
|
Ce: dye concentration at equilibrium (mg/L) | |
| R: gas constant (J mol⁻¹ K⁻¹) | ||
| T: temperature (K) | ||
| Freundlich | log
|
K F: constant of the Freundlich model and is related to the surface heterogeneity (L mg−1) |
|
|
n: the degree of linearity |
The results are presented in Figure 9 and listed in Table 6. The Langmuir isotherm assumes that adsorption occurs on a monolayer surface and the number of adsorption sites is limited [57]. In adsorption via the Freundlich mechanism, it is assumed in the isotherm that adsorption on a heterogeneous surface occurs in a multilayer fashion. Because of the effects of the biosorbent-adsorbate interaction, the Temkin isotherm model holds true, and the adsorption energy of all molecules reduces linearly with increasing surface coverage [58]. When comparing the results of fitting experimental data, summarized in the supplementary Table 4, we foud that the Freundlich model provides the best fit, revealing that the organic dyes were catalytically degraded by reactants and NPs on their surfaces, and that the multilayer covering of MG on MOL-AgNPs was viable [18]. This is in contrast to the results obtained using the Langmuir and Temkin models, for which the correlation coefficient R 2 is lower. In addition, we observe that when n is between 2 and 10, the Freundlich constant is smaller than 1, indicating a good and favorable adsorption situation.
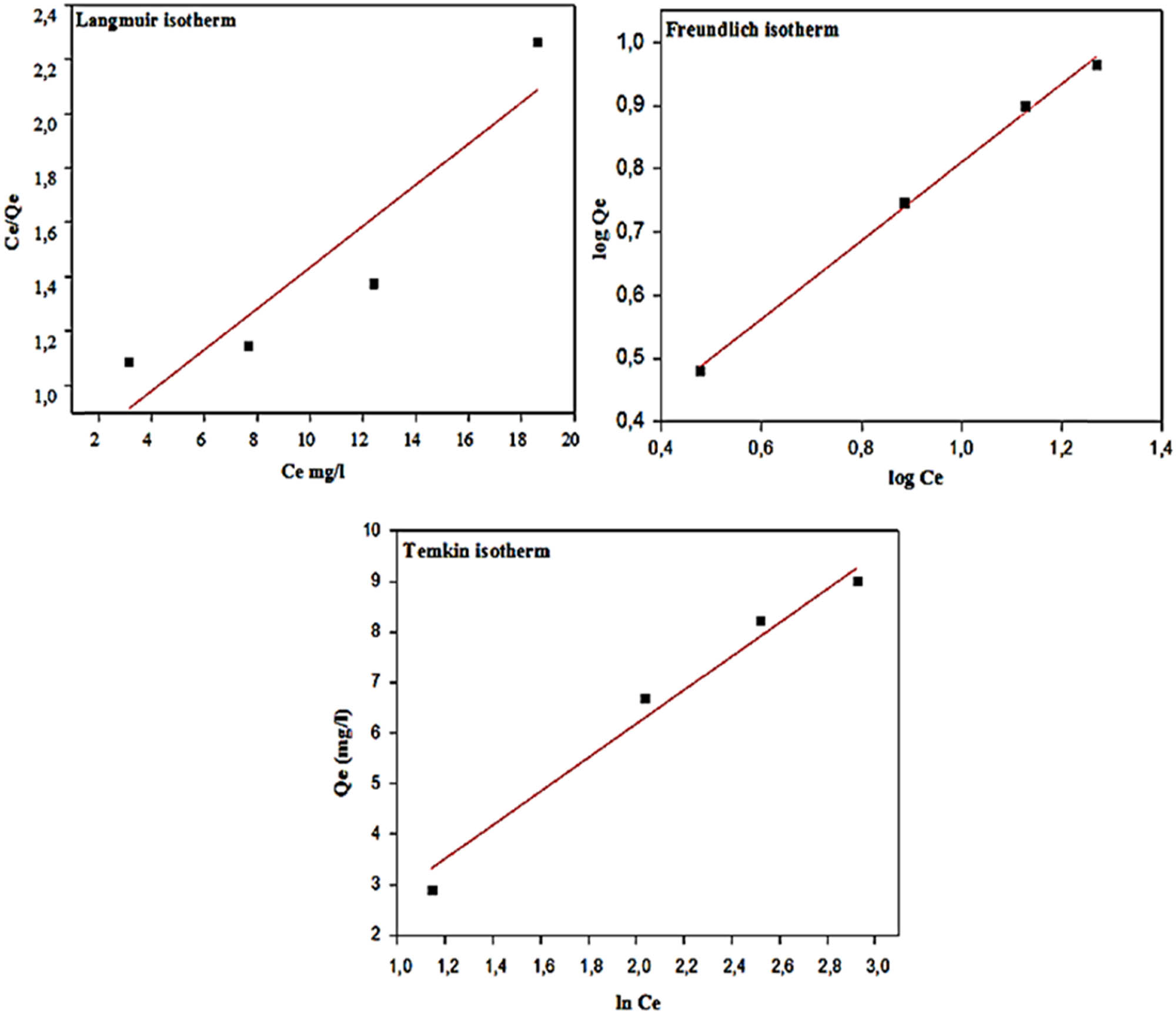
The linear plot of the Langmuir, Freundlich, and Temkin isotherms for the photodegradation of MG with MOL-AgNPs. MOL-AgNPs: Moringa oleifera-silver nanoparticles.
Parameter values of Langmuir, Freundlich, and Temkin isotherms of MG dye photodegradation using MOL-AgNPs (T = 293.15 K)
| Parameter values | ||||
|---|---|---|---|---|
| Langmuir | q max (mg/g) | K L (L/mg) | R 2 | |
| 1.48066 | 28.905 | 0.779 | ||
| Frendlich | K F (mg/g) | 1/n | n | R 2 |
| 1.8581 | 0.19088 | 5.2388 | 0.995 | |
| Temkin | b T (J/mol) | K T (L/g) | R 2 | |
| 4965.808 | 0.998 | 0.82 | ||
MG degradation of the biosynthesized AgNPs is compared to that of reported materials in Table 4. When exposed to UV-light irradiation, the biosynthesized AgNPs displayed higher photocatalytic efficiency than some other catalysts. This finding may be explained by the difference in phytochemical molecules present in the M. oleifera leaf extract and on the nanoparticle surface, which not only prevent the aggregation of nanoparticles during MG degradation but also enhance UV light absorption and inhibit the recombination of photogenerated electrons and holes. Therefore, biosynthesized MOL-AgNPs were more efficient at degrading MG via photocatalysis (Table 7).
Comparison of maximum photocatalytic degradation of MG dye using the biosynthesized AgNPs
| Biosynthesized AgNPs | Light source | Dose of catalyst (mg) | Concentration of MG | Degradation time | Degradation (%) | Ref. |
|---|---|---|---|---|---|---|
| Moringa oleifera leaf extract | UV lamp | 30 | 2.1 10−5 mol/L | 60 min | 98 | Current study |
| Kinnow mandarin peel extract | Sunlight | 20 | 100 mg/L | 120 h | Maximum | [59] |
| Gooseberry extract | Visible light | 100 | 8 10−6 mol/L | 100 min | 69.23 | [60] |
| Alchornea laxiflora leaf extract | Sunlight | — | — | 60 min | 94 | [61] |
| Angelica gigas stem extract | UV light | 5 mg | 10 mg/L | 4 h | 64 | [62] |
| Rhododendron campanulatum tree flowers extract | Solar irradiation | 10 mg | — | 90 min | 47.22 | [63] |
| Tridax procumbens | — | — | — | 180 min | 27.45 | [64] |
3.9 Reusability of the biosynthesized MOL-AgNPs
Under UV light, the biosynthesized MOL-AgNPs were examined for their potential reusability as a photocatalyst for the breakdown of the MG dye. To determine the degradation efficiency, the AgNPs catalyst was washed extensively with a mixture of double-distilled water and ethanol after each reaction cycle to remove any remaining dye molecules from the catalyst surface. Centrifugation was used to recover the catalyst from the reaction mixture. Under the same experimental conditions, the recovered MOL-AgNPs were employed five times for photodegradation tests, including the degradation of MG dye. Taking absorbance readings from small portions of the reaction solution at predetermined intervals allowed us to track the reaction’s development. As shown in Figure 10, the percentage of dye degradation remains consistent across multiple cycles. This indicates that the MOL-AgNPs retain their impressive catalytic efficiency and demonstrate excellent photocatalytic activity up to the third cycle. However, following the third cycle, there was a small decline in activity when the nanocatalyst was reused, which is explained by the deactivation of the reaction sites on the catalyst surface. The findings indicate that the AgNPs produced by phytosynthetic reactions had good stability and showed promise as a catalyst that could effectively reduce degradation rates by more than 50% for over five repetitions. The same results were found, according to David and Moldovan [65].
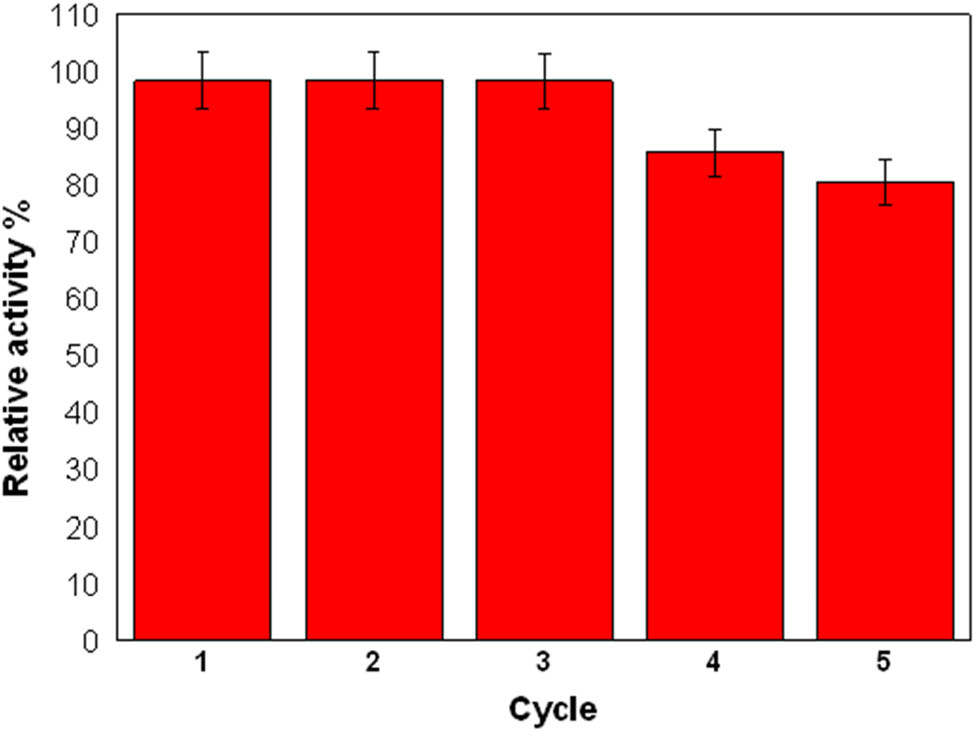
The relative activity (%) for the photodegradation of MG with MOL-AgNPs performed for five cycles. MOL-AgNPs: Moringa oleifera silver nanoparticles.
3.10 Mechanism of photodegradation of MG using MOL-AgNPs
Due to their high pore density and extensive surface area, AgNPs are a potent catalyst for the photodegradation of MG dye under UV light irradiation [66]. As shown in Figure 10, in the process of photocatalysis, electrons in the photocatalyst MOL-AgNPs are excited from the valence band to the conduction band, resulting in the creation of electron–hole pairs [67]. Electrons interact with molecular oxygen in water and produce free radicals when they collectively oscillate from the outermost band to a higher energy state as a result of the SPR phenomenon. Then, these electrons transfer the oxygen molecules and hydroxyl ions into oxygen radicals (O2.) and hydroxyl radicals (HO.), respectively. These radicals oxidize MG dye and electron excitation creates positive holes in the d orbital of nanoparticles [68], which then receive an electron from the adsorbed dye, thus reducing the dye. Furthermore, other free radicals created from the oxidation of H+ ions made by the breaking of water molecules then proceed to oxidize the dye. The dye is completely broken down by the hydroxyl radical into harmless byproducts (CO2, H2O, etc.) [69] (Figure 11).
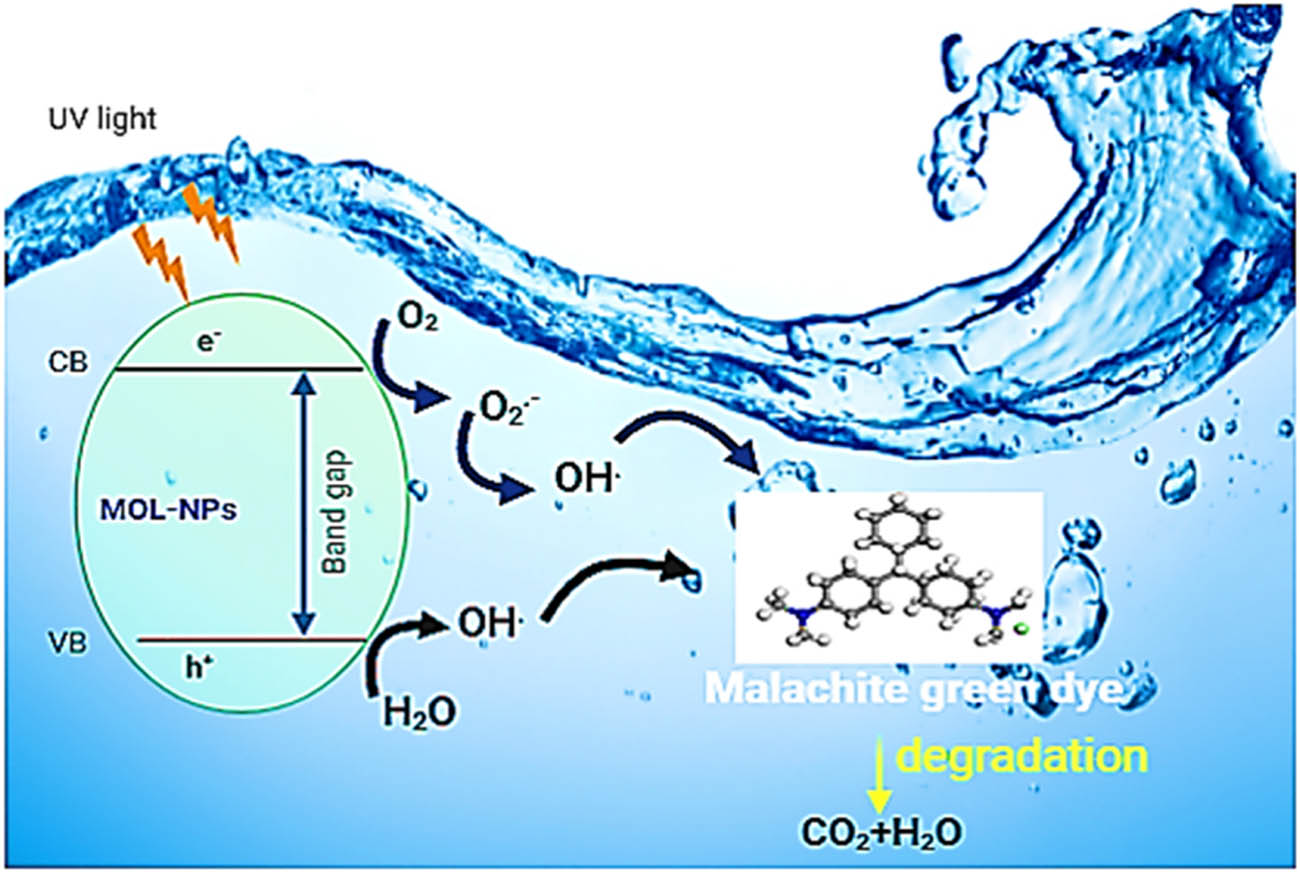
Mechanism of MG dye photodegradation under UV light irradiation using MOL-AgNPs. MOL-AgNPs: Moringa oleifera silver nanoparticles.
4 Conclusions
Ag NPs were successfully synthesized utilizing the M. oleifera plant extract by the green method, which is a very simple, rapid, and eco-friendly approach due to the involvement of active compounds found in plants without the use of toxic chemicals. The crystalline nature and the nanoscale size were determined using XRD and ZP, the presence of elemental Ag was determined using EDX, and the shape of the AgNPs was evaluated using FE-SEM and TEM. MOL-AgNPs exhibited strong photocatalytic activity for degrading MB dye. In particular, the electron-hole pairs formed on the nanoparticle surface highlighted a basic mechanism of photocatalytic activity. Under UV light irradiation for 60 min, biosynthesized AgNPs showed excellent photocatalytic ability with a 98% degradation yield. The degradation of MG by UV light revealed that AgNPs mediated by the M. oleifera leaf extract can have high photocatalytic activity.
Acknowledgments
The authors are thankful to the Deanship of Scientific Research at the University of Bisha for supporting this work through the Fast-Track Research Support Program.
-
Funding information: The authors state no funding involved.
-
Author contributions: Conceptualization; Djaber Aouf, Yasmina Khane, and Fares Fenniche; formal analysis, Sofiane Khan and Nadir Dizge; investigation and data curation, Salim Albukhaty, Hamdoon A. Mohammed, and Ghassan M. Sulaiman; validation, Abdallah Henni; visualization, Abdelhalim Zoukel; original draft preparation Djaber Aouf, Yasmina Khane, Fares Fenniche, and Nadir Dizge; writing – review and editing, Nadir Dizge, Hamdoon A. Mohammed, and Mosleh M. Abomughaid.; supervision, Salim Albukhaty, and Yasmina khane; project administration, Ghassan M. Sulaiman. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Al-Tohamy R, Ali SS, Li F, Okasha KM, Mahmoud YA-G, Elsamahy T, et al. A critical review on the treatment of dye-containing wastewater: ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol Env Saf. 2022;231:113160.10.1016/j.ecoenv.2021.113160Search in Google Scholar PubMed
[2] Crini G. Non-conventional low-cost adsorbents for dye removal: a review. Bioresour Technol. 2006;97:1061–85.10.1016/j.biortech.2005.05.001Search in Google Scholar PubMed
[3] Riva L, Pastori N, Panozzo A, Antonelli M, Punta C. Nanostructured cellulose-based sorbent materials for water decontamination from organic dyes. Nanomaterials. 2020;10:1570.10.3390/nano10081570Search in Google Scholar PubMed PubMed Central
[4] Sree GS, Botsa SM, Reddy BJM, Ranjitha KVB. Enhanced UV–visible triggered photocatalytic degradation of brilliant green by reduced graphene oxide based NiO and CuO ternary nanocomposite and their antimicrobial activity. Arab J Chem. 2020;13:5137–50.10.1016/j.arabjc.2020.02.012Search in Google Scholar
[5] Singh J, Dhaliwal AS. Electrochemical and photocatalytic degradation of methylene blue by using rGO/AgNWs nanocomposite synthesized by electroplating on stainless steel. J Phys Chem Solids. 2022;160:110358. 10.1016/j.jpcs.2021.110358.Search in Google Scholar
[6] Xie A, Jin M, Zhu J, Zhou Q, Fu L, Wu W. Photocatalytic technologies for transformation and degradation of microplastics in the environment: Current achievements and future prospects. Catalysts. 2023;13:846.10.3390/catal13050846Search in Google Scholar
[7] Fakhri A, Naji M. Degradation photocatalysis of tetrodotoxin as a poison by gold doped PdO nanoparticles supported on reduced graphene oxide nanocomposites and evaluation of its antibacterial activity. J Photochem Photobiol B Biol. 2017;167:58–63.10.1016/j.jphotobiol.2016.12.027Search in Google Scholar PubMed
[8] Zhang D, Zeng F. Visible light-activated cadmium-doped ZnO nanostructured photocatalyst for the treatment of methylene blue dye. J Mater Sci. 2012;47:2155–61.10.1007/s10853-011-6016-4Search in Google Scholar
[9] Veisi H, Azizi S, Mohammadi P. Green synthesis of the silver nanoparticles mediated by thymbra spicata extract and its application as a heterogeneous and recyclable nanocatalyst for catalytic reduction of a variety of dyes in water. J Clean Prod. 2018;170:1536–43.10.1016/j.jclepro.2017.09.265Search in Google Scholar
[10] Alyamani AA, Albukhaty S, Aloufi S, AlMalki FA, Al-Karagoly H, Sulaiman GM. Green fabrication of zinc oxide nanoparticles using phlomis leaf extract: characterization and in vitro evaluation of cytotoxicity and antibacterial properties. Molecules. 2021;26:6140.10.3390/molecules26206140Search in Google Scholar PubMed PubMed Central
[11] Mohammed HA, Amin MA, Zayed G, Hassan Y, El-Mokhtar M, Saddik MS. In vitro and in vivo synergistic wound healing and anti-methicillin-resistant staphylococcus aureus (MRSA) evaluation of liquorice-decorated silver nanoparticles. J Antibiot (Tokyo). 2023;76:291–300.10.1038/s41429-023-00603-4Search in Google Scholar PubMed
[12] Safat S, Buazar F, Albukhaty S, Matroodi S. Enhanced sunlight photocatalytic activity and biosafety of marine-driven synthesized cerium oxide nanoparticles. Sci Rep. 2021;11:14734.10.1038/s41598-021-94327-wSearch in Google Scholar PubMed PubMed Central
[13] Singh J, Dhaliwal AS. Plasmon-induced photocatalytic degradation of methylene blue dye using biosynthesized silver nanoparticles as photocatalyst. Environ Technol. 2020;41(12):1520–34. 10.1080/09593330.2018.1540663.Search in Google Scholar PubMed
[14] Alzubaidi AK, Al-Kaabi WJ, Ali AA, Albukhaty S, Al-Karagoly H, Sulaiman GM, et al. Green synthesis and characterization of silver nanoparticles using flaxseed extract and evaluation of their antibacterial and antioxidant activities. Appl Sci. 2023;13:2182.10.3390/app13042182Search in Google Scholar
[15] Messaoudi O, Benamar I, Azizi A, Albukhaty S, Khane Y, Sulaiman GM, et al. Characterization of Silver Carbonate Nanoparticles Biosynthesized Using Marine Actinobacteria and Exploring of Their Antimicrobial and Antibiofilm Activity. Mar Drugs. 2023;21:536. 10.3390/md21100536.Search in Google Scholar PubMed PubMed Central
[16] Raj S, Trivedi R, Soni V. Biogenic synthesis of silver nanoparticles, characterization and their applications – A review. Surfaces. 2022;5:67–90. 10.3390/surfaces5010003.Search in Google Scholar
[17] Hussein NN, Al-Azawi K, Sulaiman GM, Albukhaty S, Al-Majeed RM, Jabir M, et al. Silver-cored Ziziphus spina-christi extract-loaded antimicrobial nanosuspension: overcoming multidrug resistance. Nanomedicine. 2023;18:1839–54.10.2217/nnm-2023-0185Search in Google Scholar PubMed
[18] Ashij MA, Al-Shmgani HS, Sulaiman GM, Mohammed HA, Abdalrazaq EA, Albukhaty S. Investigation of antibacterial activity and wound healing promotion properties induced by Bromelain-Loaded silver nanoparticles. Plasmonics. 2023;23:1–4.10.1007/s11468-023-02127-xSearch in Google Scholar
[19] Boopathi NM, Abubakar BY. Botanical descriptions of Moringa spp. Moringa Genome. 2021;11–20.10.1007/978-3-030-80956-0_2Search in Google Scholar
[20] Mohammed ABA, Mohamed A, El-Naggar NE-A, Mahrous H, Nasr GM, Abdella A, et al. Antioxidant and antibacterial activities of silver nanoparticles biosynthesized by moringa oleifera through response surface methodology. J Nanomater. 2022;2022:1–15.10.1155/2022/9984308Search in Google Scholar
[21] Shalaby EA, Shanab SMM, El-Raheem WMA, Hanafy EA. Biological activities and antioxidant potential of different biosynthesized nanoparticles of Moringa Oleifera. Sci Rep. 2022;12:18400.10.1038/s41598-022-23164-2Search in Google Scholar PubMed PubMed Central
[22] Nizioł-Łukaszewska Z, Furman-Toczek D, Bujak T, Wasilewski T, Hordyjewicz-Baran Z. Moringa oleifera L. extracts as bioactive ingredients that increase safety of body wash cosmetics. Dermatol Res Pract. 2020;2020:8197902. 10.1155/2020/8197902.Search in Google Scholar PubMed PubMed Central
[23] Patil SV, Mohite BV, Marathe KR, Salunkhe NS, Marathe V, Patil VS. Moringa tree, gift of nature: a review on nutritional and industrial potential. Curr Pharmacol Rep. 2022;8:262–80.10.1007/s40495-022-00288-7Search in Google Scholar PubMed PubMed Central
[24] Khane Y, Benouis K, Albukhaty S, Sulaiman GM, Abomughaid MM, Al Ali A, et al. Green synthesis of silver nanoparticles using aqueous citrus limon zest extract: characterization and evaluation of their antioxidant and antimicrobial properties. Nanomaterials. 2022;12:2013.10.3390/nano12122013Search in Google Scholar PubMed PubMed Central
[25] Adhikari A, Lamichhane L, Adhikari A, Gyawali G, Acharya D, Baral ER, et al. Green synthesis of silver nanoparticles using artemisia vulgaris extract and its application toward catalytic and metal-sensing activity. Inorganics. 2022;10:113.10.3390/inorganics10080113Search in Google Scholar
[26] Dubey SP, Lahtinen M, Sillanpää M. Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa. Colloids Surf A Physicochem Eng Asp. 2010;364:34–41.10.1016/j.colsurfa.2010.04.023Search in Google Scholar
[27] Gautam KP, Acharya D, Bhatta I, Subedi V, Das M, Neupane S, et al. Nickel oxide-incorporated polyaniline nanocomposites as an efficient electrode material for supercapacitor application. Inorganics. 2022;10:86.10.3390/inorganics10060086Search in Google Scholar
[28] Irfan MI, Amjad F, Abbas A, Rehman MFU, Kanwal F, Saeed M, et al. Novel carboxylic acid-capped silver nanoparticles as antimicrobial and colorimetric sensing agents. Molecules. 2022;27:3363.10.3390/molecules27113363Search in Google Scholar PubMed PubMed Central
[29] Prakash P, Gnanaprakasam P, Emmanuel R, Arokiyaraj S, Saravanan M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf B Biointerfaces. 2013;108:255–9.10.1016/j.colsurfb.2013.03.017Search in Google Scholar PubMed
[30] Shaikh WA, Chakraborty S, Owens G, Islam RU. A review of the phytochemical mediated synthesis of AgNP (Silver Nanoparticle): The wonder particle of the past decade. Appl Nanosci. 2021;11:2625–60.10.1007/s13204-021-02135-5Search in Google Scholar PubMed PubMed Central
[31] Litvin VA, Minaev BF. Spectroscopy study of silver nanoparticles fabrication using synthetic humic substances and their antimicrobial activity. Spectrochim Acta Part A Mol Biomol Spectrosc. 2013;108:115–22.10.1016/j.saa.2013.01.049Search in Google Scholar PubMed
[32] Anand K, Tiloke C, Phulukdaree A, Ranjan B, Chuturgoon A, Singh S, et al. Biosynthesis of palladium nanoparticles by using Moringa oleifera flower extract and their catalytic and biological properties. J Photochem Photobiol B Biol. 2016;165:87–95.10.1016/j.jphotobiol.2016.09.039Search in Google Scholar PubMed
[33] Sathyavathi R, Krishna M, Rao DN. Biosynthesis of silver nanoparticles using Moringa oleifera leaf extract and its application to optical limiting. J Nanosci Nanotechnol. 2011;11:2031–5.10.1166/jnn.2011.3581Search in Google Scholar PubMed
[34] Bindhu MR, Umadevi M, Esmail GA, Al-Dhabi NA, Arasu MV. Green synthesis and characterization of silver nanoparticles from Moringa oleifera flower and assessment of antimicrobial and sensing properties. J Photochem Photobiol B Biol. 2020;205:111836.10.1016/j.jphotobiol.2020.111836Search in Google Scholar PubMed
[35] Sujitha MV, Kannan S. Green synthesis of gold nanoparticles using Citrus fruits (Citrus Limon, Citrus Reticulata and Citrus Sinensis) aqueous extract and its characterization. Spectrochim Acta Part A Mol Biomol Spectrosc. 2013;102:15–23.10.1016/j.saa.2012.09.042Search in Google Scholar PubMed
[36] Prema P, Veeramanikandan V, Rameshkumar K, Gatasheh MK, Hatamleh AA, Balasubramani R, et al. Statistical optimization of silver nanoparticle synthesis by green tea extract and its efficacy on colorimetric detection of mercury from industrial waste water. Env Res. 2022;204:111915.10.1016/j.envres.2021.111915Search in Google Scholar PubMed
[37] Kumar CG, Mamidyala SK. Extracellular synthesis of silver nanoparticles using culture supernatant of Pseudomonas aeruginosa. Colloids Surf B Biointerfaces. 2011;84:462–6.10.1016/j.colsurfb.2011.01.042Search in Google Scholar PubMed
[38] Jalilian F, Chahardoli A, Sadrjavadi K, Fattahi A, Shokoohinia Y. Green synthesized silver nanoparticle from allium ampeloprasum aqueous extract: characterization, antioxidant activities, antibacterial and cytotoxicity effects. Adv Powder Technol. 2020;31:1323–32.10.1016/j.apt.2020.01.011Search in Google Scholar
[39] Venkatadri B, Shanparvish E, Rameshkumar MR, Arasu MV, Al-Dhabi NA, Ponnusamy VK, et al. Green synthesis of silver nanoparticles using aqueous rhizome extract of Zingiber officinale and Curcuma longa: In-vitro anti-cancer potential on human colon carcinoma HT-29 cells. Saudi J Biol Sci. 2020;27:2980–6.10.1016/j.sjbs.2020.09.021Search in Google Scholar PubMed PubMed Central
[40] Githala CK, Raj S, Dhaka A, Mali SC, Trivedi R. Phyto-fabrication of silver nanoparticles and their catalytic dye degradation and antifungal efficacy. Front Chem. 2022;10:994721.10.3389/fchem.2022.994721Search in Google Scholar PubMed PubMed Central
[41] Baran MF, Keskin C, Baran A, Hatipoğlu A, Yildiztekin M, Küçükaydin S, et al. Green synthesis of silver nanoparticles from Allium Cepa L. Peel extract, their antioxidant, antipathogenic, and anticholinesterase activity. Molecules. 2023;28:2310.10.3390/molecules28052310Search in Google Scholar PubMed PubMed Central
[42] Guilger-Casagrande M, Germano-Costa T, Bilesky-José N, Pasquoto-Stigliani T, Carvalho L, Fraceto LF, et al. Influence of the capping of biogenic silver nanoparticles on their toxicity and mechanism of action towards sclerotinia sclerotiorum. J Nanobiotechnol. 2021;19:1–18.10.1186/s12951-021-00797-5Search in Google Scholar PubMed PubMed Central
[43] Rani B, Kumar V, Singh J, Bisht S, Teotia P, Sharma S, et al. Bioremediation of dyes by fungi isolated from contaminated dye effluent sites for bio-usability. Braz J Microbiol. 2014;45:1055–63.10.1590/S1517-83822014000300039Search in Google Scholar PubMed PubMed Central
[44] Abutalib MM, Alghamdi HM, Rajeh A, Nur O, Hezmad AM, Mannaa MA. Preparation of RGO/FeMoO4 as high-performance photocatalyst for degradation of malachite green, phenol and H2 evolution under natural sunlight. Int J Hydrog Energy. 2022;47:32955–68.10.1016/j.ijhydene.2022.07.189Search in Google Scholar
[45] Nouasria FZ, Selloum D, Henni A, Tingry S, Hrbac J. Improvement of the photocatalytic performance of ZnO thin films in the UV and sunlight range by Cu doping and additional coupling with Cu2O. Ceram Int. 2022;48:13283–94.10.1016/j.ceramint.2022.01.207Search in Google Scholar
[46] Liu W, Zhang H, Cao B, Lin K, Gan J. Oxidative removal of bisphenol a using zero valent aluminum–acid system. Water Res. 2011;45:1872–8.10.1016/j.watres.2010.12.004Search in Google Scholar PubMed
[47] Cheng Z, Fu F, Pang Y, Tang B, Lu J. Removal of phenol by acid-washed zero-valent aluminium in the presence of H2O2. Chem Eng J. 2015;260:284–90.10.1016/j.cej.2014.09.012Search in Google Scholar
[48] Chanderia K, Kumar S, Sharma J, Ameta R, Punjabi PB. Degradation of sunset yellow FCF using copper loaded bentonite and H2O2 as photo-fenton like reagent. Arab J Chem. 2017;10:S205–11.10.1016/j.arabjc.2012.07.023Search in Google Scholar
[49] Khan I, Saeed K, Zekker I, Zhang B, Hendi AH, Ahmad A, et al. Review on methylene blue: its properties, uses, toxicity and photodegradation. Water. 2022;14:242.10.3390/w14020242Search in Google Scholar
[50] Orian L, Mauri P, Roveri A, Toppo S, Benazzi L, Bosello-Travain V, et al. Selenocysteine oxidation in glutathione peroxidase catalysis: an MS-supported quantum mechanics study. Free Radic Biol Med. 2015;87:1–14.10.1016/j.freeradbiomed.2015.06.011Search in Google Scholar PubMed
[51] Amdeha E. Biochar-based nanocomposites for industrial wastewater treatment via adsorption and photocatalytic degradation and the parameters affecting these processes. Biomass Convers Biorefin. 2023;1–26.10.1007/s13399-023-04512-2Search in Google Scholar
[52] Batool M, Daoush WM, Hussain MK. Dye sequestration using biosynthesized silver nanoparticles adsorbent in aqueous solutions. Crystals. 2022;12:662.10.3390/cryst12050662Search in Google Scholar
[53] Tolia J, Chakraborty M, Murthy Z. Photocatalytic degradation of malachite green dye using doped and undoped ZnS nanoparticles. Pol J Chem Technol. 2012;14:16–21.10.2478/v10026-012-0065-6Search in Google Scholar
[54] Wiszniowski J, Robert D, Surmacz-Gorska J, Miksch K, Weber J-V. Photocatalytic mineralization of humic acids with TiO2: Effect of PH, sulfate and chloride anions. Int J Photoenergy. 2003;5:69–74.10.1155/S1110662X03000151Search in Google Scholar
[55] Trabelsi H, Atheba P, Gbassi GK, Ksibi M, Drogui P. Sunlight-activated photocatalysis of malachite green using a TiO2/cellulosic fiber. Int J Hazard Mater. 2012;1:6–10.Search in Google Scholar
[56] Rudzinski W, Plazinski W. Kinetics of solute adsorption at solid/solution interfaces: A theoretical development of the empirical pseudo-first and pseudo-second order kinetic rate equations, based on applying the statistical rate theory of interfacial transport. J Phys Chem B. 2006;110:16514–25.10.1021/jp061779nSearch in Google Scholar PubMed
[57] Blackburn RS, Harvey A, Kettle LL, Payne JD, Russell SJ. Sorption of poly (Hexamethylenebiguanide) on cellulose: mechanism of binding and molecular recognition. Langmuir. 2006;22:5636–44.10.1021/la053002bSearch in Google Scholar PubMed
[58] Shahbeig H, Bagheri N, Ghorbanian SA, Hallajisani A, Poorkarimi S. A new adsorption isotherm model of aqueous solutions on granular activated carbon. World J Model Simul. 2013;9:243–54.Search in Google Scholar
[59] Jaast S, Grewal A. Green synthesis of silver nanoparticles, characterization and evaluation of their photocatalytic dye degradation activity. Curr Res Green Sustain Chem. 2021;4:100195.10.1016/j.crgsc.2021.100195Search in Google Scholar
[60] Lakshmi SP, Dhanya S, Sheeba D. Photcatalytic degradation of malachite green using silver nanoparticles synthesised from gooseberry extract. J Chem Mater Res. 2016;5:68–73.10.1016/j.optmat.2017.08.026Search in Google Scholar
[61] Ekennia AC, Uduagwu DN, Nwaji NN, Nwosa CC, Olowu OJ, Nwanji OL, et al. Facile green synthesis and biological evaluation of biogenic silver nanoparticles using aqueous extract of Alchornea laxiflora leaf. Inorg Nano-Metal Chem. 2022;52:979–90.10.1080/24701556.2021.2025398Search in Google Scholar
[62] Chokkalingam M, Rupa EJ, Huo Y, Mathiyalagan R, Anandapadmanaban G, Ahn JC, et al. Photocatalytic degradation of industrial dyes using Ag and Au nanoparticles synthesized from Angelica gigas ribbed stem extracts. Opt (Stuttg). 2019;185:1213–9.10.1016/j.ijleo.2019.04.065Search in Google Scholar
[63] Sati S, Bartwal D, Ringwal S, Kour G, Rawat R, Nautiyal S. Investigation of catalytic property of plant mediated silver nanoparticles as degradation of toxic dyes in water. J Mt Res. 2021;16:13–9. 10.51220/jmr.v16i2.3.Search in Google Scholar
[64] Bhati–Kushwaha H, Malik CP. Biosynthesis of silver nanoparticles using fresh extracts of Tridax Procumbens Linn; 2014.Search in Google Scholar
[65] David L, Moldovan B. Green synthesis of biogenic silver nanoparticles for efficient catalytic removal of harmful organic dyes. Nanomaterials. 2020;10:202. 10.3390/nano10020202.Search in Google Scholar PubMed PubMed Central
[66] Wang P, Huang B, Dai Y, Whangbo M-H. Plasmonic photocatalysts: harvesting visible light with noble metal nanoparticles. Phys Chem Chem Phys. 2012;14:9813–25.10.1039/c2cp40823fSearch in Google Scholar PubMed
[67] Marimuthu S, Antonisamy AJ, Malayandi S, Rajendran K, Tsai P-C, Pugazhendhi A, et al. Silver nanoparticles in dye effluent treatment: a review on synthesis, treatment methods, mechanisms, photocatalytic degradation, toxic effects and mitigation of toxicity. J Photochem Photobiol B Biol. 2020;205:111823.10.1016/j.jphotobiol.2020.111823Search in Google Scholar PubMed
[68] Udayabhanu J, Kannan V, Tiwari M, Natesan G, Giovanni B, Perumal V. Nanotitania crystals induced efficient photocatalytic color degradation, antimicrobial and larvicidal activity. J Photochem Photobiol B Biol. 2018;178:496–504.10.1016/j.jphotobiol.2017.12.005Search in Google Scholar PubMed
[69] Massima Mouele ES, Tijani JO, Masikini M, Fatoba OO, Eze CP, Onwordi CT, et al. Spectroscopic measurements of dissolved O3, H2O2 and OH radicals in double cylindrical dielectric barrier discharge technology: treatment of methylene blue dye simulated wastewater. Plasma. 2020;3:59–91.10.3390/plasma3020007Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy
Articles in the same Issue
- Research Articles
- Tension buckling and postbuckling of nanocomposite laminated plates with in-plane negative Poisson’s ratio
- Polyvinylpyrrolidone-stabilised gold nanoparticle coatings inhibit blood protein adsorption
- Energy and mass transmission through hybrid nanofluid flow passing over a spinning sphere with magnetic effect and heat source/sink
- Surface treatment with nano-silica and magnesium potassium phosphate cement co-action for enhancing recycled aggregate concrete
- Numerical investigation of thermal radiation with entropy generation effects in hybrid nanofluid flow over a shrinking/stretching sheet
- Enhancing the performance of thermal energy storage by adding nano-particles with paraffin phase change materials
- Using nano-CaCO3 and ceramic tile waste to design low-carbon ultra high performance concrete
- Numerical analysis of thermophoretic particle deposition in a magneto-Marangoni convective dusty tangent hyperbolic nanofluid flow – Thermal and magnetic features
- Dual numerical solutions of Casson SA–hybrid nanofluid toward a stagnation point flow over stretching/shrinking cylinder
- Single flake homo p–n diode of MoTe2 enabled by oxygen plasma doping
- Electrostatic self-assembly effect of Fe3O4 nanoparticles on performance of carbon nanotubes in cement-based materials
- Multi-scale alignment to buried atom-scale devices using Kelvin probe force microscopy
- Antibacterial, mechanical, and dielectric properties of hydroxyapatite cordierite/zirconia porous nanocomposites for use in bone tissue engineering applications
- Time-dependent Darcy–Forchheimer flow of Casson hybrid nanofluid comprising the CNTs through a Riga plate with nonlinear thermal radiation and viscous dissipation
- Durability prediction of geopolymer mortar reinforced with nanoparticles and PVA fiber using particle swarm optimized BP neural network
- Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa
- Antibacterial effect of novel dental resin composites containing rod-like zinc oxide
- An extended model to assess Jeffery–Hamel blood flow through arteries with iron-oxide (Fe2O3) nanoparticles and melting effects: Entropy optimization analysis
- Comparative study of copper nanoparticles over radially stretching sheet with water and silicone oil
- Cementitious composites modified by nanocarbon fillers with cooperation effect possessing excellent self-sensing properties
- Confinement size effect on dielectric properties, antimicrobial activity, and recycling of TiO2 quantum dots via photodegradation processes of Congo red dye and real industrial textile wastewater
- Biogenic silver nanoparticles of Moringa oleifera leaf extract: Characterization and photocatalytic application
- Novel integrated structure and function of Mg–Gd neutron shielding materials
- Impact of multiple slips on thermally radiative peristaltic transport of Sisko nanofluid with double diffusion convection, viscous dissipation, and induced magnetic field
- Magnetized water-based hybrid nanofluid flow over an exponentially stretching sheet with thermal convective and mass flux conditions: HAM solution
- A numerical investigation of the two-dimensional magnetohydrodynamic water-based hybrid nanofluid flow composed of Fe3O4 and Au nanoparticles over a heated surface
- Development and modeling of an ultra-robust TPU-MWCNT foam with high flexibility and compressibility
- Effects of nanofillers on the physical, mechanical, and tribological behavior of carbon/kenaf fiber–reinforced phenolic composites
- Polymer nanocomposite for protecting photovoltaic cells from solar ultraviolet in space
- Study on the mechanical properties and microstructure of recycled concrete reinforced with basalt fibers and nano-silica in early low-temperature environments
- Synergistic effect of carbon nanotubes and polyvinyl alcohol on the mechanical performance and microstructure of cement mortar
- CFD analysis of paraffin-based hybrid (Co–Au) and trihybrid (Co–Au–ZrO2) nanofluid flow through a porous medium
- Forced convective tangent hyperbolic nanofluid flow subject to heat source/sink and Lorentz force over a permeable wedge: Numerical exploration
- Physiochemical and electrical activities of nano copper oxides synthesised via hydrothermal method utilising natural reduction agents for solar cell application
- A homotopic analysis of the blood-based bioconvection Carreau–Yasuda hybrid nanofluid flow over a stretching sheet with convective conditions
- In situ synthesis of reduced graphene oxide/SnIn4S8 nanocomposites with enhanced photocatalytic performance for pollutant degradation
- A coarse-grained Poisson–Nernst–Planck model for polyelectrolyte-modified nanofluidic diodes
- A numerical investigation of the magnetized water-based hybrid nanofluid flow over an extending sheet with a convective condition: Active and passive controls of nanoparticles
- The LyP-1 cyclic peptide modified mesoporous polydopamine nanospheres for targeted delivery of triptolide regulate the macrophage repolarization in atherosclerosis
- Synergistic effect of hydroxyapatite-magnetite nanocomposites in magnetic hyperthermia for bone cancer treatment
- The significance of quadratic thermal radiative scrutinization of a nanofluid flow across a microchannel with thermophoretic particle deposition effects
- Ferromagnetic effect on Casson nanofluid flow and transport phenomena across a bi-directional Riga sensor device: Darcy–Forchheimer model
- Performance of carbon nanomaterials incorporated with concrete exposed to high temperature
- Multicriteria-based optimization of roller compacted concrete pavement containing crumb rubber and nano-silica
- Revisiting hydrotalcite synthesis: Efficient combined mechanochemical/coprecipitation synthesis to design advanced tunable basic catalysts
- Exploration of irreversibility process and thermal energy of a tetra hybrid radiative binary nanofluid focusing on solar implementations
- Effect of graphene oxide on the properties of ternary limestone clay cement paste
- Improved mechanical properties of graphene-modified basalt fibre–epoxy composites
- Sodium titanate nanostructured modified by green synthesis of iron oxide for highly efficient photodegradation of dye contaminants
- Green synthesis of Vitis vinifera extract-appended magnesium oxide NPs for biomedical applications
- Differential study on the thermal–physical properties of metal and its oxide nanoparticle-formed nanofluids: Molecular dynamics simulation investigation of argon-based nanofluids
- Heat convection and irreversibility of magneto-micropolar hybrid nanofluids within a porous hexagonal-shaped enclosure having heated obstacle
- Numerical simulation and optimization of biological nanocomposite system for enhanced oil recovery
- Laser ablation and chemical vapor deposition to prepare a nanostructured PPy layer on the Ti surface
- Cilostazol niosomes-loaded transdermal gels: An in vitro and in vivo anti-aggregant and skin permeation activity investigations towards preparing an efficient nanoscale formulation
- Linear and nonlinear optical studies on successfully mixed vanadium oxide and zinc oxide nanoparticles synthesized by sol–gel technique
- Analytical investigation of convective phenomena with nonlinearity characteristics in nanostratified liquid film above an inclined extended sheet
- Optimization method for low-velocity impact identification in nanocomposite using genetic algorithm
- Analyzing the 3D-MHD flow of a sodium alginate-based nanofluid flow containing alumina nanoparticles over a bi-directional extending sheet using variable porous medium and slip conditions
- A comprehensive study of laser irradiated hydrothermally synthesized 2D layered heterostructure V2O5(1−x)MoS2(x) (X = 1–5%) nanocomposites for photocatalytic application
- Computational analysis of water-based silver, copper, and alumina hybrid nanoparticles over a stretchable sheet embedded in a porous medium with thermophoretic particle deposition effects
- A deep dive into AI integration and advanced nanobiosensor technologies for enhanced bacterial infection monitoring
- Effects of normal strain on pyramidal I and II 〈c + a〉 screw dislocation mobility and structure in single-crystal magnesium
- Computational study of cross-flow in entropy-optimized nanofluids
- Significance of nanoparticle aggregation for thermal transport over magnetized sensor surface
- A green and facile synthesis route of nanosize cupric oxide at room temperature
- Effect of annealing time on bending performance and microstructure of C19400 alloy strip
- Chitosan-based Mupirocin and Alkanna tinctoria extract nanoparticles for the management of burn wound: In vitro and in vivo characterization
- Electrospinning of MNZ/PLGA/SF nanofibers for periodontitis
- Photocatalytic degradation of methylene blue by Nd-doped titanium dioxide thin films
- Shell-core-structured electrospinning film with sequential anti-inflammatory and pro-neurogenic effects for peripheral nerve repairment
- Flow and heat transfer insights into a chemically reactive micropolar Williamson ternary hybrid nanofluid with cross-diffusion theory
- One-pot fabrication of open-spherical shapes based on the decoration of copper sulfide/poly-O-amino benzenethiol on copper oxide as a promising photocathode for hydrogen generation from the natural source of Red Sea water
- A penta-hybrid approach for modeling the nanofluid flow in a spatially dependent magnetic field
- Advancing sustainable agriculture: Metal-doped urea–hydroxyapatite hybrid nanofertilizer for agro-industry
- Utilizing Ziziphus spina-christi for eco-friendly synthesis of silver nanoparticles: Antimicrobial activity and promising application in wound healing
- Plant-mediated synthesis, characterization, and evaluation of a copper oxide/silicon dioxide nanocomposite by an antimicrobial study
- Effects of PVA fibers and nano-SiO2 on rheological properties of geopolymer mortar
- Investigating silver and alumina nanoparticles’ impact on fluid behavior over porous stretching surface
- Potential pharmaceutical applications and molecular docking study for green fabricated ZnO nanoparticles mediated Raphanus sativus: In vitro and in vivo study
- Effect of temperature and nanoparticle size on the interfacial layer thickness of TiO2–water nanofluids using molecular dynamics
- Characteristics of induced magnetic field on the time-dependent MHD nanofluid flow through parallel plates
- Flexural and vibration behaviours of novel covered CFRP composite joints with an MWCNT-modified adhesive
- Experimental research on mechanically and thermally activation of nano-kaolin to improve the properties of ultra-high-performance fiber-reinforced concrete
- Analysis of variable fluid properties for three-dimensional flow of ternary hybrid nanofluid on a stretching sheet with MHD effects
- Biodegradability of corn starch films containing nanocellulose fiber and thymol
- Toxicity assessment of copper oxide nanoparticles: In vivo study
- Some measures to enhance the energy output performances of triboelectric nanogenerators
- Reinforcement of graphene nanoplatelets on water uptake and thermomechanical behaviour of epoxy adhesive subjected to water ageing conditions
- Optimization of preparation parameters and testing verification of carbon nanotube suspensions used in concrete
- Max-phase Ti3SiC2 and diverse nanoparticle reinforcements for enhancement of the mechanical, dynamic, and microstructural properties of AA5083 aluminum alloy via FSP
- Advancing drug delivery: Neural network perspectives on nanoparticle-mediated treatments for cancerous tissues
- PEG-PLGA core–shell nanoparticles for the controlled delivery of picoplatin–hydroxypropyl β-cyclodextrin inclusion complex in triple-negative breast cancer: In vitro and in vivo study
- Conduction transportation from graphene to an insulative polymer medium: A novel approach for the conductivity of nanocomposites
- Review Articles
- Developments of terahertz metasurface biosensors: A literature review
- Overview of amorphous carbon memristor device, modeling, and applications for neuromorphic computing
- Advances in the synthesis of gold nanoclusters (AuNCs) of proteins extracted from nature
- A review of ternary polymer nanocomposites containing clay and calcium carbonate and their biomedical applications
- Recent advancements in polyoxometalate-functionalized fiber materials: A review
- Special contribution of atomic force microscopy in cell death research
- A comprehensive review of oral chitosan drug delivery systems: Applications for oral insulin delivery
- Cellular senescence and nanoparticle-based therapies: Current developments and perspectives
- Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies
- Micelle-based nanoparticles with stimuli-responsive properties for drug delivery
- Critical assessment of the thermal stability and degradation of chemically functionalized nanocellulose-based polymer nanocomposites
- Research progress in preparation technology of micro and nano titanium alloy powder
- Nanoformulations for lysozyme-based additives in animal feed: An alternative to fight antibiotic resistance spread
- Incorporation of organic photochromic molecules in mesoporous silica materials: Synthesis and applications
- A review on modeling of graphene and associated nanostructures reinforced concrete
- A review on strengthening mechanisms of carbon quantum dots-reinforced Cu-matrix nanocomposites
- Review on nanocellulose composites and CNFs assembled microfiber toward automotive applications
- Nanomaterial coating for layered lithium rich transition metal oxide cathode for lithium-ion battery
- Application of AgNPs in biomedicine: An overview and current trends
- Nanobiotechnology and microbial influence on cold adaptation in plants
- Hepatotoxicity of nanomaterials: From mechanism to therapeutic strategy
- Applications of micro-nanobubble and its influence on concrete properties: An in-depth review
- A comprehensive systematic literature review of ML in nanotechnology for sustainable development
- Exploiting the nanotechnological approaches for traditional Chinese medicine in childhood rhinitis: A review of future perspectives
- Twisto-photonics in two-dimensional materials: A comprehensive review
- Current advances of anticancer drugs based on solubilization technology
- Recent process of using nanoparticles in the T cell-based immunometabolic therapy
- Future prospects of gold nanoclusters in hydrogen storage systems and sustainable environmental treatment applications
- Preparation, types, and applications of one- and two-dimensional nanochannels and their transport properties for water and ions
- Microstructural, mechanical, and corrosion characteristics of Mg–Gd–x systems: A review of recent advancements
- Functionalized nanostructures and targeted delivery systems with a focus on plant-derived natural agents for COVID-19 therapy: A review and outlook
- Mapping evolution and trends of cell membrane-coated nanoparticles: A bibliometric analysis and scoping review
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma
- In situ growth of carbon nanotubes on fly ash substrates
- Structural performance of boards through nanoparticle reinforcement: An advance review
- Reinforcing mechanisms review of the graphene oxide on cement composites
- Seed regeneration aided by nanomaterials in a climate change scenario: A comprehensive review
- Surface-engineered quantum dot nanocomposites for neurodegenerative disorder remediation and avenue for neuroimaging
- Graphitic carbon nitride hybrid thin films for energy conversion: A mini-review on defect activation with different materials
- Nanoparticles and the treatment of hepatocellular carcinoma
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part II
- Highly safe lithium vanadium oxide anode for fast-charging dendrite-free lithium-ion batteries
- Recent progress in nanomaterials of battery energy storage: A patent landscape analysis, technology updates, and future prospects
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part II
- Calcium-, magnesium-, and yttrium-doped lithium nickel phosphate nanomaterials as high-performance catalysts for electrochemical water oxidation reaction
- Low alkaline vegetation concrete with silica fume and nano-fly ash composites to improve the planting properties and soil ecology
- Mesoporous silica-grafted deep eutectic solvent-based mixed matrix membranes for wastewater treatment: Synthesis and emerging pollutant removal performance
- Electrochemically prepared ultrathin two-dimensional graphitic nanosheets as cathodes for advanced Zn-based energy storage devices
- Enhanced catalytic degradation of amoxicillin by phyto-mediated synthesised ZnO NPs and ZnO-rGO hybrid nanocomposite: Assessment of antioxidant activity, adsorption, and thermodynamic analysis
- Incorporating GO in PI matrix to advance nanocomposite coating: An enhancing strategy to prevent corrosion
- Synthesis, characterization, thermal stability, and application of microporous hyper cross-linked polyphosphazenes with naphthylamine group for CO2 uptake
- Engineering in ceramic albite morphology by the addition of additives: Carbon nanotubes and graphene oxide for energy applications
- Nanoscale synergy: Optimizing energy storage with SnO2 quantum dots on ZnO hexagonal prisms for advanced supercapacitors
- Aging assessment of silicone rubber materials under corona discharge accompanied by humidity and UV radiation
- Tuning structural and electrical properties of Co-precipitated and Cu-incorporated nickel ferrite for energy applications
- Sodium alginate-supported AgSr nanoparticles for catalytic degradation of malachite green and methyl orange in aqueous medium
- An environmentally greener and reusability approach for bioenergy production using Mallotus philippensis (Kamala) seed oil feedstock via phytonanotechnology
- Micro-/nano-alumina trihydrate and -magnesium hydroxide fillers in RTV-SR composites under electrical and environmental stresses
- Mechanism exploration of ion-implanted epoxy on surface trap distribution: An approach to augment the vacuum flashover voltages
- Nanoscale engineering of semiconductor photocatalysts boosting charge separation for solar-driven H2 production: Recent advances and future perspective
- Excellent catalytic performance over reduced graphene-boosted novel nanoparticles for oxidative desulfurization of fuel oil
- Special Issue on Advances in Nanotechnology for Agriculture
- Deciphering the synergistic potential of mycogenic zinc oxide nanoparticles and bio-slurry formulation on phenology and physiology of Vigna radiata
- Nanomaterials: Cross-disciplinary applications in ornamental plants
- Special Issue on Catechol Based Nano and Microstructures
- Polydopamine films: Versatile but interface-dependent coatings
- In vitro anticancer activity of melanin-like nanoparticles for multimodal therapy of glioblastoma
- Poly-3,4-dihydroxybenzylidenhydrazine, a different analogue of polydopamine
- Chirality and self-assembly of structures derived from optically active 1,2-diaminocyclohexane and catecholamines
- Advancing resource sustainability with green photothermal materials: Insights from organic waste-derived and bioderived sources
- Bioinspired neuromelanin-like Pt(iv) polymeric nanoparticles for cancer treatment
- Special Issue on Implementing Nanotechnology for Smart Healthcare System
- Intelligent explainable optical sensing on Internet of nanorobots for disease detection
- Special Issue on Green Mono, Bi and Tri Metallic Nanoparticles for Biological and Environmental Applications
- Tracking success of interaction of green-synthesized Carbopol nanoemulgel (neomycin-decorated Ag/ZnO nanocomposite) with wound-based MDR bacteria
- Green synthesis of copper oxide nanoparticles using genus Inula and evaluation of biological therapeutics and environmental applications
- Biogenic fabrication and multifunctional therapeutic applications of silver nanoparticles synthesized from rose petal extract
- Metal oxides on the frontlines: Antimicrobial activity in plant-derived biometallic nanoparticles
- Controlling pore size during the synthesis of hydroxyapatite nanoparticles using CTAB by the sol–gel hydrothermal method and their biological activities
- Special Issue on State-of-Art Advanced Nanotechnology for Healthcare
- Applications of nanomedicine-integrated phototherapeutic agents in cancer theranostics: A comprehensive review of the current state of research
- Smart bionanomaterials for treatment and diagnosis of inflammatory bowel disease
- Beyond conventional therapy: Synthesis of multifunctional nanoparticles for rheumatoid arthritis therapy

