Abstract
The palladium (Pd)-catalysed reaction has attracted much attention, making Pd the most valuable of the four major precious metals. Several different forms of Pd can be used as a catalyst; nanoparticles (NPs) have the advantage of a high surface area:volume ratio. Since the chemical production of Pd NPs is not environmentally friendly, biological synthesis interest has grown. However, the production mechanism remained unknown in several cases and was recently described for the electroactive bacterium Shewanella oneidensis MR-1. The application of these green synthesised NPs was established in different fields. This review discusses the production pathway and the novel biological-inspired methods to produce tailored biogenic palladium nanoparticles (bio-Pd NPs), with their broad application fields as biogenic nanocatalysts. Two significant applications – reductive bioremediation of persistent organic contaminants and energy-producing microbial fuel cells – are discussed in detail. The current challenges in optimising bio-Pd NPs production and the potential research directions for the complete utilisation of its novel catalytic properties are highlighted.
Graphical abstract
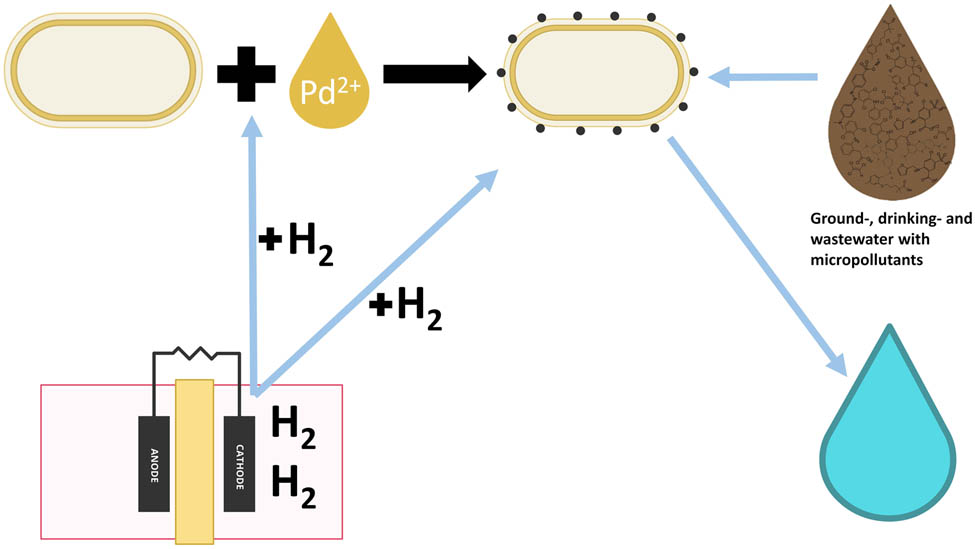
Novel application of bio-Pd NPs
1 Introduction
Palladium (Pd) is a metal belonging to the platinum group metals (PGM), extensively used due to the remarkable properties. Their surfaces are excellent catalysts for chemical reactions involving hydrogen (H2) and oxygen (O2), such as the hydrogenation of (un)saturated organic compounds [1,2,3]. Under suitable conditions, Pd adsorbs more than 900 times its volume of hydrogen [4]. This metal is an essential catalyst for energy conversion, chemical reaction, and abiotic electrochemical processes [5,6]. The Pd is frequently applied in different methods due to its catalytic activity; therefore, it is often used as a catalyst in its nanoparticle (NP) size [7]. Smaller sizes allow a monodisperse distribution of the particles and provide a high surface area that increases the catalytic activity [8]. The NPs can be chemically/electrochemically or biologically produced [6,8,9]. Chemical synthesis of palladium nanoparticles (Pd NPs) requires the use of a series of toxic and expensive agents, whereas strong reductants and stabilisers are required to reduce the metals to zero-valent and as carrier materials [10]. Electrodeposition of Pd is also used and is obtained by using an underpotential deposition condition, e.g. potentials higher than the Nerst one [11,12]. Biological synthesis is less harmful to the environment than chemical production as no secondary toxic wastestream is produced [13]. Furthermore, the presence of the microbial surface serves as the capping agent and stabiliser, and thus no expensive chemicals are needed. This makes the process energy-efficient and cost-effective [14,15,16].
Production of biogenic palladium nanoparticles (bio-Pd NPs) occurs through microorganisms or plants, where they are responsible for the conversion of Pd2+ to Pd0 [16,17,18,19]. Different microorganisms and plants have been used through extensive research to obtain a higher functionality [20,21,22]. When biological carriers are used for the production of bio-Pd NPs, all Pd2+ that is taken up inside or on the biological carrier will be converted to Pd0 [19,23]. For this review article, no difference in terminology is made for bio-Pd NPs inside or outside the cells when production occurs through microorganisms. While the production pathway of most of the microorganisms remains unknown, it was recently unravelled for Shewanella oneidensis MR-1. It was shown that NADH dehydrogenase and hydrogenase enzymes played an important role in the production of bio-Pd NPs, when converting all Pd2+ present in the cell to Pd0 [24,25,26,27]. It was found that Escherichia coli was still viable after exposure to Pd, and hence metabolic active but not culturable [28]. For plants, the biomolecules are responsible for the production, e.g., polyols, proteins, flavones, flavonoids, and tannins [29,30]. Besides these carriers, the potential use of anaerobic granular sludge for production was recently discovered [31,32]. Other than using different biological carriers, the performance of bio-Pd NPs can be tuned through modification in production methods. One of the commonly used techniques is the formation of bimetallic NPs; Pd has been combined with other metals of interest, e.g., gold (Au), silver (Ag), iron (Fe), and ruthenium (Ru) [33,34,35].
The produced bio-Pd NPs are widely utilised to remediate micropollutants in the environment (e.g., pharmaceuticals and antimicrobial agents) [10,36,37]. Several “hard to degrade” micropollutants such as diatrizoate, trichloroethylene, diclofenac, chromate, chlorophenols, and lindane have been efficiently transformed by bio-Pd NPs [10,38,39]. Microbial degradation often stalls or slows down at micropollutant relevant concentration range due to mass transfer limitation across the cell membrane [40,41,42]. Some of the micropollutants are even resistant to microbial enzymes; nevertheless, enzymatic removal of micropollutants can also produce toxic by-products which is not desired [43]. Therefore, oxidative techniques are often used; however, dangerous explosive compounds are needed [44]. Hence, developing alternative sustainable approaches is of utmost importance. The bio-Pd NPs hold promise to overcome the hurdle of micropollutants removal in a sustainable way – (1) reusable, (2) bio origin, environment-friendly production, and (3) high catalytic activity.
A more recent application of bio-Pd NPs can be found in electrochemical systems. The bio-Pd NPs are coated on anodes and used to increase electricity generation in a microbial electrochemical fuel cell (MFC) [45]. The bio-Pd NPs can also be fixed on electrodes to increase the electroconductivity of the electrochemical cell. The electroactive bacteria used for the bio-Pd production and Pd will increase the electro-conductivity. This improved electroconductivity results in an increased electron transfer and thus a higher catalytic activity [46,47].
Some recent reviews have mainly underlined the potential use of green synthesis for metal NP production with their advantages and general applications [48,49,50]. However, the overview of the mechanisms and optimisation of production methods specific for bio-Pd NPs with their novel applications such as in electrochemical systems are missing. A summary of different methods specific to how to increase the catalytic activity of the produced bio-Pd NPs is also present. There is a clear need to acquire a better understanding of the role of bio-Pd NPs in electrochemical cells as the pathway of the microbial production. Hence, this review article focuses on two major topics of bio-Pd NPs: (1) new technologies that have been explored for the production and enhancing the catalytic activity of the bio-Pd NPs and (2) applying bio-Pd NPs in an electrochemical system. First, the mechanisms behind the production of bio-Pd NPs will be discussed (Section 2). In Section 3, the optimisation of its catalytic activity and production methods can be found. Later, the application of electrochemical systems is highlighted (Section 4). A brief discussion on the application of bio-Pd NPs in the treatment of challenging micropollutants is provided in Section 5. In Section 6, the importance of a circular economy for Pd will be briefly discussed. The review will show future research directions which can be crucial for developing highly efficient bio-Pd NPs and, subsequently, an innovative bioremediation method to remove micropollutants.
2 Biological mechanism of bio-Pd production
Different types of microorganisms are capable of producing metal NPs. These NPs are made on the cell wall or within the cell membranes or cytoplasm, depending on the type of bacteria [51]. Although each bacteria is different, bio-Pd synthesis mainly depends on the outer membrane c-type cytochrome and hydrogenase enzyme [10,17,24,25,27]. The c-type cytochrome is responsible for transporting the electrons across the bacterial cell to the metals [52]. At the same time, the hydrogenase contains redox proteins that are important for reducing metals and consequently contribute to the formation of metal particles. Here, the Pd2+ will be converted to Pd0; hence, the formed particles can be found on the cell wall, inside the cell membranes, or cytoplasm [25,51,52,53,54].
The electroactive bacterium, Shewanella oneidensis MR-1, is a well-established microorganism for the production of metal NPs. It has the characteristics to perform the biological dissimilatory metal reduction process (DMR). In this biological process, various metal(loid)s, outside the microorganism, are used as a terminal electron acceptor. Energy is conserved by oxidising an (in)organic matter and reducing a metal [20,54,55,56].
The mechanism used by the microorganisms depends on the type of metal used for NP production [27,55,56,57,58]. The production process of bio-Pd NPs can be in general described in three steps: (1) biological adsorption (biosorption) on the cell surface, (2) bioreduction of Pd2+ to Pd0 in- and outside the cell wall, and (3) autocatalytic reduction, of Pd2+ to Pd0 which is not enzymatic and clusters onto the Pd NPs of the cell wall [10,23,59]. The production of bio-Pd NPs by Shewanella oneidensis MR-1 will be discussed in detail here, due to the general and beneficial character of the microorganisms for the production of bio-Pd NPs. First, biosorption of Pd2+ on the cell wall can occur by ion exchange, electrostatic interaction, complexation, and physical adsorption which was shown in FT-IR analysis by Xu et al. [23]. Independent of the type of biosorption process, the Pd2+ is converted to Pd0, by chemical or autocatalytic conversion [10,52,59]. When ion exchange, electrostatic interaction, or complexation occurs, the functional groups of the cell surface have an essential role and are responsible for attaching the Pd2+ to the cell wall. Nevertheless, it is also possible to have physical adsorption, where the functional groups do not intervene in attaching the Pd2+ to the cell wall [23]. However, besides the biosorption, the cell wall can convert the Pd2+ to Pd0 which was observed in scanning electron microscopic energy-dispersive X-Ray spectroscopy (SEM-EDX) and transmission electron microscopic (TEM) images [23]. This indicates that bioreduction can be achieved through the hydroxyl group present on the cell surface. Interestingly, Yang et al. [26] found that this bioreduction of Pd2+ to Pd0 occurs through the c-type cytochrome on the cell wall, more specific Cym A (such as OmCA and MtrC). Furthermore, the production of bio-Pd NPs inside the cells is also present. Yang et al. [26], suggested that the NADH-dehydrogenase, [FeFe]-hydrogenase (HydA), and [NiFe]-hydrogenase (HyaB) are essential for the bio-Pd production, as they influence the size, distribution, and the number of bio-Pd NPs (Figure 1). These enzymes are mainly responsible for the formation of bio-Pd NPs in- and outside the cell membrane [26]. Whereas [NiFe]-hydrogenase produces hydrogen from formate and oxidises hydrogen, [FeFe]-hydrogenase works together with formate dehydrogenases in the form of formate-hydrogen lyase that generates hydrogen from formate [26]. Dundas et al. [54] stated that the hydrogenase enzymes play an essential role in facilitating the reduction process of Pd2+ by Shewanella oneidensis MR-1. It was observed that this process followed the pseudo-first-order kinetic model [52,54]. Furthermore, it was found that [NiFe]-hydrogenase had greater activity than [FeFe]-hydrogenase; therefore, [NiFe]-hydrogenase is more involved in the synthesis of bio-Pd NPs [26]. Yang et al. [26] stated that bio-Pd on the outer membrane was produced by NADH dehydrogenase, while production in the periplasm was provided by [FeFe]-hydrogenase and [NiFe]-hydrogenase [26]. Deplanche et al. [27] provided evidence that mainly [NiFe]-hydrogenase, localised in the periplasm, is essential for facilitating the production of bio-Pd NPs. This was supported by other researchers as well [10,26,27,52,58,60]. This [NiFe]-hydrogenase enzyme can form and oxidate H2 in a bidirectional function. When oxidation of H2 occurs, electrons are released and used to reduce Pd2+ [22,26]. Anaerobic conditions are often required before reducing Pd2+ as only under these conditions [NiFe]-hydrogenase is expressed [22,60]. This enzyme can release electrons by oxidation of H2, H2 also needs to be added as an electron donor for the conversion [52]. The production of bio-Pd NPs does not only come from [NiFe]-hydrogenase, 50% of the converted Pd2+ comes from other enzymes [52]. The formation of bio-Pd NPs inside the cytoplasm was also found by other microorganisms, e.g., Escherichia coli and Bascillus benzeovorans [22,61]. This was due to the similarities between Ni2+ and Pd2+, according to Deplanche et al. [59]. The Ni2+ is a key component for many metalloenzymes, that are located in the cytoplasm and will be simultaneously transported by the Ni2+ “trafficking system” through the cell membrane. Due to the chemical similarities Pd2+ is transported across the membrane in the same way [59]. The Pd2+ taken up by the cytoplasm is deposited as NPs, however, this cannot substitute for the Ni2+ function [62]. Nevertheless, after the uptake and deposition of the bio-Pd NPs, the metabolic activity could be detected through flow cytometry [35]. Once the Pd2+ is taken up inside the cytoplasm the Pd2+ starts forming Pd0 “seeds”, the formation of these “seeds” results in a loss of cell viability [63]. Besides the biological reduction of Pd2+, the chemical conversion also appears in this production mechanism. The added electron donor can also be used for the chemical reduction of Pd2+ without the use of microorganisms. These chemical-produced NPs are going to attach to the existing bio-Pd NPs, causing particle aggregation [9,59]. Chemically produced NPs have a negative zeta potential and attract the Pd2+, by the van der Waals forces, and finally attach to the newly formed bio-Pd NPs [18,64]. The particle aggregation negatively influences the catalytic activity [9]. The bio-Pd NPs are often detected as NPs in spherical form, where the size can vary abundantly [19]. Besides this, recently it was found that microbes were able to dissolve Pd0 which is an intermediate form of Pd2+ towards Pd NPs during microbial reduction by Shewanella oneidensis MR-1 cells [65]. Furthermore, 2D structured nanomaterials of Pd were also discovered; nevertheless, the 2D structured Pd NPs are not synthesised with biological carriers, but are chemically produced [66].
![Figure 1
Production mechanism of bio-Pd NPs inside and outside the microorganisms: (1) Adsorption of Pd2+ on the cell wall, (2a) conversion of Pd2+ to Pd0 inside the cell, (2b) on the cell wall, and (3) autocatalytic conversion of Pd2+ to Pd0 outside the cell. The figure was inspired by Deplanche et al. [27], Ng et al. [52], and Yang et al. [26].](/document/doi/10.1515/ntrev-2022-0482/asset/graphic/j_ntrev-2022-0482_fig_001.jpg)
Production mechanism of bio-Pd NPs inside and outside the microorganisms: (1) Adsorption of Pd2+ on the cell wall, (2a) conversion of Pd2+ to Pd0 inside the cell, (2b) on the cell wall, and (3) autocatalytic conversion of Pd2+ to Pd0 outside the cell. The figure was inspired by Deplanche et al. [27], Ng et al. [52], and Yang et al. [26].
The presence of metals, like Pd, in microorganisms has a significant impact on the viability of the cells because of the high toxicity. Once the NPs are formed, the bacteria die [31]. However, the toxicity of the metal to the microorganisms is strongly dependent on the type of metal, concentration, and microorganisms. In some cases, the presence of metals can enhance cellular viability which is strongly dependent on the concentration of the metal, by enhancing the decomposition of reactive oxygen species (ROS) [9,67,68,69,70]. When high concentrations of metals are present, microorganisms tend to produce ROS due to the stress caused by the presence of the heavy metals [28,69,71]. It was suggested that Shewanella oneidensis also produces ROS when heavy metals are present, causing damage to essential proteins responsible for transport and respiration inside the cell [71]. The presence of ROS damage proteins which directly reduces the ability of the cells to survive or thrive. Second, it also damages proteins that can release irons in the cultures, resulting in a higher stimulation of ROS production and is more fatal because it occurs when the damaged cells are recovering [71]. The influence of ROS causing inviable cells, due to the presence of Pd was also observed for Bacillus wiedmannii MSM and Escherichia coli [28,69]. It was found that a concentration of 0.07 mM Pd2+ had a positive effect on microbial metabolism [68]. This was due to the exhibited high catalytic activity of the microorganisms towards the production of H2, an essential intermediate for the energy metabolism [68,72]. However, it was found that for a concentration of 1 mM Pd2+, submitted to a glassy carbon-modified electrode with Pd-free Desulfovibrio desulfuricans, interference occurred for the natural enzymatic electron-transfer pathways and catalytic reaction [68]. Chen and Chen [69] found that 900 mg L−1 cells of Bacillus wiedmannii MSM were inhibited at a Pd2+ concentration of 100 mg L−1, while De Windt et al. [9] observed that for 50 mg L−1 Shewanella oneidensis the cells were inviable at a Pd2+ concentration of 125 mg L−1 [9,69]. It was also found that bio-Pd NPs were more toxic for Gram-positive than Gram-negative bacteria [73]. Nevertheless, depending on the type of metal and bacteria biological conversion can still occur independently of the viability of the bacterial cell due to the presence of specific organic functional groups on the cell wall [74]. Joudeh et al. [28] reported that an exposure of Pd to Escherichia coli caused a differential expression of 709 of 3,098 genes. This differential expression also occurred for the functional genes, whereof the highest changes can be found for genes responsible for amino acid transport and metabolism, carbohydrate transport and metabolism, transcription, post-translational modification, protein turnover, and chaperons. Compared to other heavy metals, Pd caused higher protein damage due to cross-linking, disruption of the 3D structures, and allosteric movements. It was found that the microorganism was still metabolic active, and hence viable after exposure to 100 µM sodium tetrachloropalladate, but not culturable. This is due to the downregulation of the repair genes and up-regulation of carbohydrate metabolism genes [28]. However, it was found that higher resistance toward the toxicity of Pd can be solved by genetic modification. Elahian et al. [75] showed that the modification of Pichia pastoris allowed the modified yeast to have a biosorption of 493.35 mg g−1 for Pd. This was obtained due to the overexpression of Cyb5R of the Pichia pastoris cells which is responsible for reinforcing the antioxidant system of the yeast to protect the cells from oxidative stress caused by the presence of Pd [75]. Nevertheless, genetic modification is expensive and labour-intensive.
3 Optimisation and different methods for bio-Pd production
3.1 Production methods of bio-Pd NPs
The production of bio-Pd NPs can be performed by using microorganisms, plants, and anaerobic sludge (Figure 2, Table 1). Microorganisms are often used for production due to their lower cost and environmental sustainability (Table 2) [76]. The use of Shewanella oneidensis and Desulfovibrio desulfuricans are well known for their highly active production of nanocatalysts. However, after the production, an extra washing step is needed to remove the produced H2S which is poisonous to the catalyst [22,77,78]. In contrast, the toxification of the catalyst from H2S can be prevented by using Escherichia coli and Rhodobacter sphaeroides [27,79].
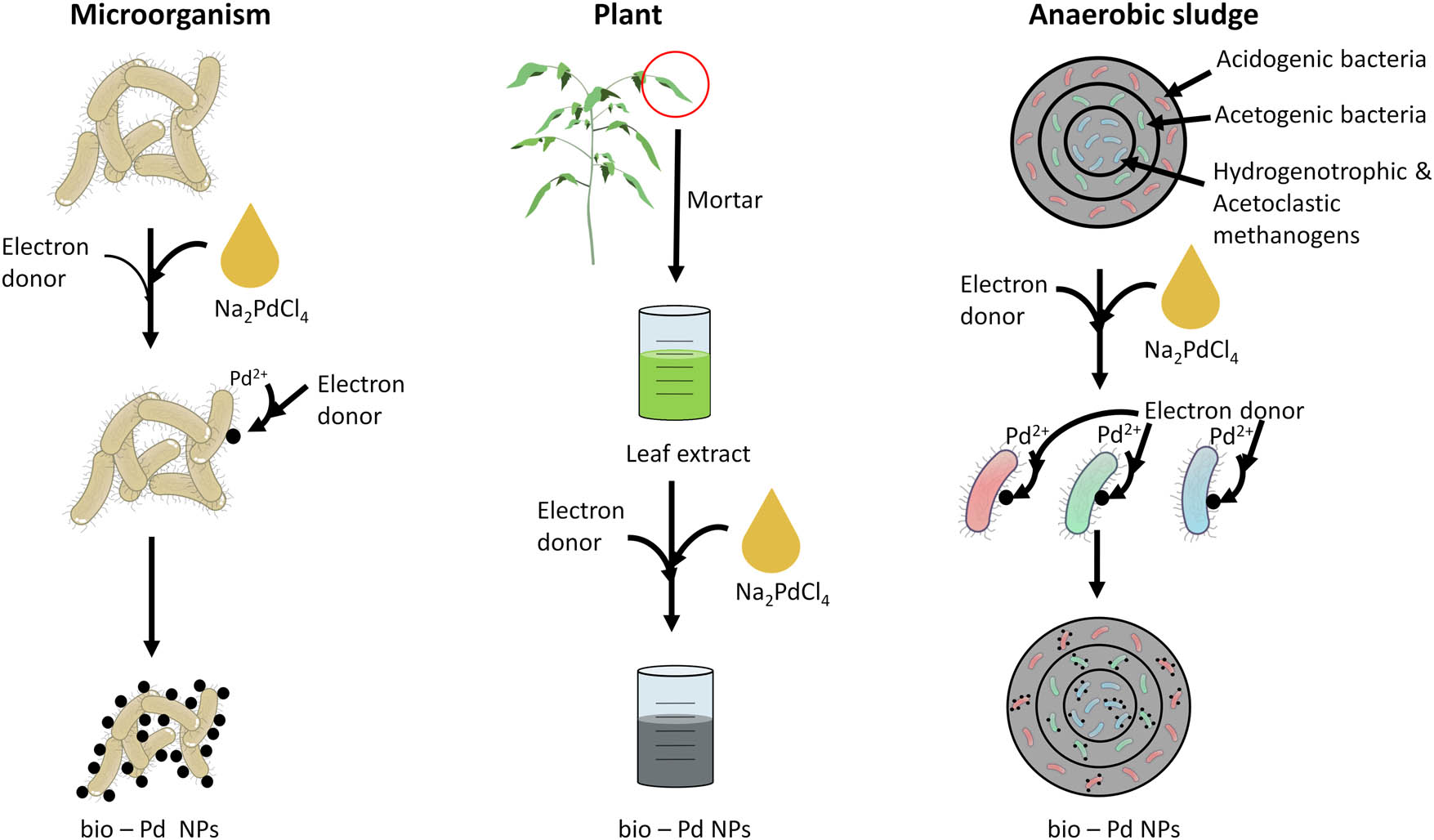
Overview of the production methods for bio-Pd NPs by using microorganisms, plants, and anaerobic sludge.
Overview of the different bio-Pd NPs production methods and mechanisms for microorganisms, plants, and anaerobic sludge
| Category | Place of production | Production mechanisms | Ref. |
|---|---|---|---|
| Microorganism | Production of bio-Pd NPs inside and outside the microbial cells | Synthesis of the bio-Pd NPs inside the cells is mainly established by the NADH-dehydrogenase, [FeFe]-hydrogenase (HydA), and [NiFe]-hydrogenase (HyaB) | [9,26,59,63] |
| Outside the cell, production can be established by (1) by autocatalytic conversion of Pd2+ to Pd0 by the formed bio-Pd0 “seeds,” and (2) through the aggregation of the chemically converted Pd0 | |||
| Plant | Production can occur throughout the plant, in specific parts, or in plant extractions | Bio-Pd NPs synthesis by plants occurs through biomolecules e.g., polyols, proteins, flavones, flavonoids, and tannins | [30,179] |
| Anaerobic sludge | Synthesis of bio-Pd NPs occurs inside the anaerobic sludge | Production of the bio-Pd NPs occurs inside and outside the cells, together with biosorption of Pd2+ on the cell wall. However, the conversion strongly depends on the type of electron donor | [32,86,89,180] |
Overview of used microorganisms and plants for the production of bio-Pd with the corresponding characteristics
| Biological carrier | Name | Type | Advantage (+)/Disadvantage (–) | Ref. |
|---|---|---|---|---|
| Microorganism | Shewanella oneidensis | Gram-negative | + Electroactive bacteria | [9] |
| + Metal-reducer | ||||
| − Washing steps after production | ||||
| Microorganism | Desulfovibrio desulfuricans | Gram-negative | + High hydrogenase activity | [59,166] |
| + Metal-reducer | ||||
| Microorganism | Rhodeobacter sphaeroides | Gram-negative | + No washing steps after production | [79] |
| Microorganism | Escheria coli | Gram-negative | + No washing steps after production | [22] |
| Microorganism | Geobacter sulfurreducens | Gram-negative | + Electroactive bacteria | [167] |
| + Broad variation in metal reduction | ||||
| Microorganism | Cupriavidus necator | Gram-negative | + Small NPs | [168] |
| + Well-suspended Pd0 NPs | ||||
| Microorganism | Serratia sp. | Gram-negative | + Accumulates metal | [59] |
| Microorganism | Bacillus sphaericus | Gram-positive | + High resistance towards metals | [61] |
| Microorganism | Anthrobacter oxydans | Gram-positive | + Production in aerobic conditions | [59,169] |
| + Metal resistant | ||||
| + Accumulates metal | ||||
| Microorganism | Enterococcus faecalis | Gram-postive | + Withstand harsh environmental conditions | [170] |
| + Responsive and accumulation for low concentrations of heavy metals | ||||
| Microorganism | Micrococcus luteus | Gram-positive | + Accumulates high amounts of metals | [59] |
| + Growth in aerobic conditions | ||||
| + Metal-reducer | ||||
| Plant | Pulicaria glutinosa | Whole plant | + Bio-Pd NPs showed catalytic activity towards Suzuki coupling | [171] |
| − Difficult extraction of NPs | ||||
| − Variation in NP sizes | ||||
| Plant | Cinnamon zeylanicum | Bark | + Ultra-low cost production of bio-Pd NPs | [18] |
| Plant | Moringa olifera | Peel extract | + Bio-Pd NPs showed antibacterial activity | [172] |
| Plant | Glycine max | Leaf extract | + Bio-Pd NPs showed activity towards degradation of azo-dyes | [173] |
| + Cost-effective production | ||||
| Plant | Filicium decipiens | Leaf extract | + Bio-Pd NPs showed high antibacterial activity | [82] |
| Plant | Sapium sebiferum | Leaf extract | + Bio-Pd NPs showed high antibacterial activity | [174] |
| + Photocatalytic active bio-Pd NPs | ||||
| Plant | Melia azedarach | Leaf extract | + Bio-Pd NPs showed antibacterial activity | [175] |
| + Bio-Pd NPs also have antifungal activity | ||||
| Plant | Prunus x yedoensis | Leaf extract | + Bio-Pd NPs showed antibacterial activity | [176] |
| Plant | Phoenix dactylifera | Leaf extract | + Bio-Pd NPs showed antibacterial activity | [177] |
| + Bio-Pd NPs showed catalytic activity towards 4-nitrophenol | ||||
| Plant | Phyllanthus emblica | Seed extract | + Bio-Pd NPs showed antibacterial activity | [178] |
The formation of the bio-Pd NPs by Gram-negative or Gram-positive microorganisms is different due to cell wall differences which play an essential role by influencing the metals’ binding onto the surface [59,73]. Various Gram-negative and Gram-positive strains have been tested for the production of different-sized bio-Pd NPs, where a significant difference in size was found. The bio-Pd NPs produced by Gram-negative bacteria tend to outcome small NPs equally distributed around the cell wall. While in contrast, Gram-positive bacteria produce a few large NPs [59]. This difference was not caused by the distinct metabolic activity, but potentially by biosorption. Sorption of Pd2+ was higher for Gram-negative strains than for Gram-positive strains. Resulting in higher concentrations of Pd2+ in the solution for Gram-positive bacteria. Subsequently, more chemical conversions are appearing, hence the generation of larger NPs [59].
Plants are also often used for the production of bio-Pd NPs. Different plants and forms – (e.g., powder or extract) and complete parts of plants (e.g., stem, leaf, root, fruit, flower, and seed) can be used (Table 2) [18,74,80]. They have the capability of accumulating metal ions that can be reduced to NPs [74,81]. However, the use of entire plants is prevented, as this results in the production of NPs of different sizes and shapes throughout the plant. This is caused by the difference in penetration and localisation of the metal ions in the plant due to the diverse reduction capacities of the plant parts [82]. Consequently, complex methods are needed to extract, purify, and increase the recovery of the particles [74,81,82]. Therefore, plant extracts are often preferred in general due to the instant conversion of metals into NPs, absence of penetration, higher recovery, and less variation in size and shape [80,83,84,85]. This is due to the functioning of biomolecules from the extracts as reducing and stabilising agents [29,74,84]. Advanced XRD images have proven that the bio-Pd NPs are crystallographic planes of face-centred cubic bio-Pd NPs. Additional characterisation of the shape and size was also made by TEM and SEM analyses which showed a series of aggregates with oval and spherical NPs being present. The size found for these NPs varied between 10 nm and 35 nm [19]. Nevertheless, production in plant extracts occurs at higher temperatures and pressures in contrast to the synthesis with microorganisms [80,83,84]. However, a higher yield can be obtained because of the higher stability and considerable amount of phytochemicals [49].
A new approach for bio-Pd NPs production can be obtained through anaerobic granular sludge which is mainly used to treat wastewater [32,86]. The benefit of using anaerobic granular sludge is (1) treatment of wastewater, (2) recovery of Pd2+ from wastewater, and (3) the catalytic activity of the NPs. This is without any additional cost and energy [2,38]. However, the reduction of Pd2+ through anaerobic sludge is strongly dependent on the type of electron donor. Hence, different types of electron donors were tested. Nonetheless, only H2 and formate showed a biological and chemical reduction of Pd2+ which was not found for pyruvate, lactate, acetate, and ethanol [86]. A part of the Pd2+ present in the wastewater remained unconverted both biologically and chemically. Besides, the latter two mechanisms, the third mechanism of conversion occurred through biosorption of the metal ion which is the bonding between the Pd2+ and sludge, by chemisorption [86]. Extracellular ligands induced this binding with weak acid organic functional groups associated with organomonometallic complexation [86,87,88]. This biosorption was also caused by the interaction between the organic functional groups on the cell wall (e.g., amine, amide, phosphoryl, and carboxyl groups) and the Pd2+ [86,89,90]. However, an inhibitory effect was also observed on the microbial community due to the exposure of bacteria to Pd. The bio-Pd NPs affected the bacterial and archaeal community as well as the produced total volatile fatty acids [32,86]. When an increase in volatile fatty acids is present, this indicates a failure of the anaerobic sludge, more specifically the inhibition of the microbial community present in the sludge [91,92]. Volatile fatty acids are normally produced and used by the microorganisms present in the anaerobic sludge [91,93]. However, when there is an increase in the volatile acids, this indicates that some of the microorganisms are inhibited, consequently resulting in a decrease in pH and an inhibition of the other microorganisms that are present [94]. Hence, this inhibitory effect on the anaerobic sludge is undesired, as the sludge is responsible for treating the wastewater. This toxic effect is different for each microbial community in the granular sludge due to their characteristics and spatial organisation in the sludge. The bacterial community is present in the outer layer, while the methanogens are in the granular sludge’s core [95]. However, contradictory results were obtained regarding the inhibition effect on the different methanogenic archaeal communities – acetoclastic and hydrogenotrophic methanogens. According to Pat-Espadas et al. [86], the inhibition was stronger for the acetoclastic methanogens than the hydrogenotrophic methanogens. Inhibition of 92% of acetoclastic methanogens occurred at a concentration of 0.96 mg Pd2+ L−1, while 96% inhibition was found for the hydrogenotrophic methanogens at 2.7 mg Pd2+ L−1 [86]. Nonetheless, Quan et al. [32] reported that the hydrogenotrophic methanogens were more sensitive to the bio-Pd NPs than the acetoclastic methanogens. Hence, the difference could be resulted due to the distinct in environmental conditions of the treated wastewater. However, the inhibition of the core microorganisms is a hurdle that cannot be neglected, due to the important functioning of these methanogens.
The disruption of the biological conversion of Pd2+ can also be affected by the binding of Pd onto proteins or by the replacement of essential metals for the function of the proteins. This replaced or bound Pd can disrupt the enzyme function and structure which results in the loss of their essential functional proteins [32,96].
3.2 Enhancing the catalytic activity of bio-Pd NPs
3.2.1 Enhancement by a modified production process of the NPs
Reductions are needed to trigger the well-known catalytic activity of bio-Pd NPs such as dehalogenation or hydrogenation reactions. Therefore, different compounds can be used, such as pyruvate, lactate, acetate, formate, and H2 [46,86]. Based on research, H2 is the most efficient electron donor to activate the dehalogenation or hydrogenation processes by the catalytic activity of the bio-Pd NPs. The Pd adsorbs H2 and converts it into atomic hydrogen which is very reactive as a reduction agent [97,98]. The hydrogen radicals adsorbed onto the NPs contribute significantly to the high catalytic activity of bio-Pd NPs [9,51]. However, the catalytic efficiency of bio-Pd NPs can be influenced by (1) the size and distribution of the NPs on the cell wall, and (2) the activation method of the bio-Pd NPs [20,24].
Small bio-Pd NPs can be obtained without using extreme conditions and toxic chemicals through the presence of the biological carrier [9]. The size of the NPs has a direct influence on the catalytic activity [99]. Therefore, small NPs give a high ratio of surface area:volume which results in high surface energy combined with high reactivity [100,101]. This catalytic activity can be optimised by changing the size and distribution of the NPs. The aforementioned optimisation depends on the Pd:cell dry weight (CDW) ratio which affects the viability of the biological carrier, hence the microorganism [9,62,99]. According to De Windt et al. [9] and Hou et al. [24], a lower Pd:CDW ratio gives a higher biological conversion of Pd2+ into NPs. This results in small and uniformly distributed NPs throughout all the cells. On the contrary, a high ratio gives large NPs due to the abrupt death of cells because of the high Pd2+ concentration, which is toxic, compared to the CDW [9]. Hence, a low ratio is desired when bio-Pd NPs are produced [9,24].
Dehalogenation and hydrogenation by the catalytic activity of bio-Pd NPs occurs mainly through the addition of H2 as an electron donor. This can be optimised by changing the activation method [9,101,102]. Activation agents such as potassium hydroxide (KOH) and sodium hydroxide (NaOH) are often used as bioinorganic catalysts, due to their reaction ability with inert carbon materials that create desired pores in the carbon precursor [103,104]. Optimised catalytic activity of bio-Pd is established by applying KOH as an activation agent. This method is altering the shape of microorganisms and Pd NPs, as the composition of the Pd NPs, by the thermal treatment of KOH. This thermal treatment carbonises the cells and starts the production of porous structures by guiding the reaction between KOH and the carbon [20,103]. Two reaction mechanisms contribute to the formation of the large porous structure: (1) decomposition of functional groups of microorganisms resulting in a high porosity and surface area combined with the release of some volatiles (e.g. H2O, CO2, and CO). Furthermore, (2) the release of H2O and CO2 is contributing as well, through the physical activation of CO2 and steam. The dispersed Pd NPs are spread throughout the carbon hybrid material. This stabilises the Pd NPs and facilitates the diffusion of the target compounds and electron donors to the catalyst’s active sites. The elevated temperature also influences the Pd NPs by increasing the crystallinity of the NPs which showed a substantial effect on the catalytic performance. The advantages of this method are (1) an increase in dispersion of small NPs on the carrier, (2) having more binding sites and surface anchoring groups, and (3) enhancing the catalytic activity by improving the hydrophilicity of the nanostructures [20]. It is also possible to use hydrothermal processes to increase the activity, when producing alloys, such as PdAu alloys which contributes to the heteroatom doping of Pd and Au. To maintain and prevent aggregation from occurring, graphene oxide was used. The benefits of this process are (1) an increase in catalytic activity and (2) higher durability compared to commercial Pd/C NPs [105].
3.2.2 Enhancement through bimetallic NPs production
Another method to increase the bio-Pd NPs activity is by adding a different metal to produce bimetallic NPs [34]. The added value of the second metal lies in the synergistic interaction between the two metals [21,35]. Extended research has been carried out to produce different types of bimetallic NPs, e.g., bio-Pd/Au, bio-Pd/Ru, bio-Pd/Fe, and bio-Pd/Pt [21,27,34,35]. Omajali et al. [35] reported that the addition of Ru would give a higher conversion efficiency and facilitate the catalytic hydrogenation transfer. This hydrogenation transfer is used to synthesis “drop in fuel” precursor (DMF) from upgrading biomass processing waste. The standard procedure for converting 5-(hydroxymethyl) furfural (5-HMF) to 2,5-dimethyl furan (DMF) is generally expensive because of the strict conditions that are needed, high temperature (>260°C) and high pressure, combined with a long reaction time [35]. Hence, the use of bio-Pd/Ru is more environmentally and economically friendly. A combination of bio-Pd with Fe and magnetite (Fe3O4), bio-Pd/Fe@Fe3O4 was also reported by Wei et al. [106]. Here, the magnetite from the core is surrounded by nano zero-valent iron and bio-Pd. This bio-Pd/Fe@Fe3O4 increases the Fenton reaction and hence accelerates the dehalogenation of diclofenac. The Fe on the bio-Pd NPs releases nano-zero-valent iron through oxidation [106,107]. This oxidation will result in Fe2+ that reacts with H2O2 and forms *OH, contributing to the dehalogenation of diclofenac [108]. However, Fe3O4 present in the reaction also functions as a catalyst and reduces the oxidised nano-zero valent ions. A synergistic effect is created between the Pd0 and the nano zero-valent iron. The Pd provides reactive sites for the generation of *OH from H2 and enhances the generation of *OH from H2O2 [106]. Hence, the type of metal used for the production of bimetallic NPs is essential. However, on the contrary, this leads to an extra cost that can increase strongly, depending on the type of metal.
4 Applying bio-Pd in electrochemical systems
4.1 Bio-Pd NPs effect on electrochemical systems
The search for an alternative energy source is emerging as the depletion of fossil fuels increases. Fuel cell technologies present low carbon energy, high energy-density, zero-emission of CO2, and relatively easy production [45,47,109]. Two types of cell technologies are commonly used, hence electrochemical cells: (1) MFC and (2) Microbial Electrolysis Cell (MEC). Lately, research has been focusing on the use of these cells combined with catalysts due to their higher efficiency. One of the commonly used elements for this is Pd [110,111,112]. It has been proven that the use of Pd can enhance the reaction rate, conductivity, and charge transfer, hence the generation of current [46,111]. The bio-Pd NPs have also been applied in electrochemical cells [46].
The MFC is a bio-electrochemical system that uses microorganisms to transform chemical energy into electricity [113,114]. Only electroactive bacteria can be used through the process, as they serve as biocatalysts, hence generating bio-energy [115,116]. These microorganisms transfer electrons, directly (DET) or mediated (MET), to the electrode, by oxidising organic compounds, e.g., glucose, formate, and acetate, which serve as carbon sources [115]. By oxidising the organic compounds, microorganisms make electrons available. This is transferred to the anode, and from there to the cathode through a circuit where they reduce the oxidant [110,117]. Hence, the efficiency of this transfer from chemical to electricity dramatically depends on the anode. The anode is the primary location where the microorganisms attach [47,118]. Therefore, a variety of carbon-based materials enhance bacterial attachment and hence the transfer of electrons [47]. Recently, attention is gained to the use of bio-Pd NPs to cover the anode. This increases the performance of the MFC, by enhancing the generation of current. The combination of bio-Pd NPs with the anode establishes the use of the MFC for electro-oxidation, here, Pd present in the anode is electrocatalytic active [112]. The bio-Pd NPs serve as an electroactive catalyst that enables the degradation of organic compounds which increases the number of electrons [47]. This improves the transfer of electrons and reduction in resistance of charge transfer in the electrochemical systems (Figure 3). It was found that a higher loading of bio-Pd NPs could increase the Coulombic efficiency from 14% (1 mg bio-Pd cm−2) to 31% (2 mg bio-Pd cm−2) compared to the anode without bio-Pd NPs [47]. Matsena et al. [119] also found an increase in bio-energy generation from 31.1 to 59.6%, with 2 to 4 mg bio-Pd cm−2, respectively. This was compared to the electrode without bio-Pd NPs [119].
![Figure 3
Coating anode with bio-Pd in the MFC system to increase the generation of energy. The figure was adapted from Hou et al. [24].](/document/doi/10.1515/ntrev-2022-0482/asset/graphic/j_ntrev-2022-0482_fig_003.jpg)
Coating anode with bio-Pd in the MFC system to increase the generation of energy. The figure was adapted from Hou et al. [24].
Besides, the loading of bio-Pd NPs on the electrode is the type of organic compound also essential for improving the generation of bio-energy. Quan et al. [47] and Matsena et al. [119] reported the inadequate use of glucose to improve bio-energy generation. This is due to the absence of direct electro-oxidation by bio-Pd NPs and hindered utilisation from the microorganisms to generate power [6,47,68,110,119]. Hence, it is important to use a suitable organic compound for the electro- and microbial oxidation. The advantage of having bio-Pd in the electrochemical systems are (1) the presence of microorganisms to oxidise the organic compounds and (2) bio-Pd to catalyse the oxidation of the organic compounds further which improves the energy generation [16,47,119].
While energy is produced in MFC, MEC partially reverses the process using the energy for the production of H2 or degradation of contaminants. The H2 is considered an alternative to fossil fuels [120]. The advantage of this system is that there is no emission of CO2, clean end-products, and has high-energy-density [45,121]. The MEC principles are based on the hydrogen evolution reaction (HER) which is a cathodic reaction that electrochemically split water in the cathode [122]. The HER process is a combination of hydrogen adsorption and desorption from the electrode surface where an optimal level of bonding strength between catalytic surface and hydrogen atoms needs to be achieved aiming not to favour one step over the other. If the bonding between hydrogen and the catalytic surface is weak, adsorption is inadequate, and the overall efficiency would suffer. Besides, if the bonding between hydrogen and the electrocatalytic surface is too strong it is not desorbed from the surface effectively, lowering overall efficiency [123]. To increase this reaction, a catalyst can be used, e.g. platinum and Pd, by coating the metal onto an electrode [124]. The Pd is often used because of having the best properties in hydrogen-storage, under suitable conditions Pd adsorbs more than 900 times its volume of hydrogen [4,111,125]. It also plays an important role in the redox reaction. The use of bio-Pd accelerates the reaction as it serves as a catalyst which increases the HER and, consequently, removes contaminants. This is due to the higher concentration of electron donors which can be used for catalytic activity [24,126]. However, the characteristics of the bio-Pd NPs to achieve a high catalytic activity are different for electrochemical systems and in suspension [9,24]. In electrochemical systems, increased conductivity is obtained by interconnecting all bio-Pd NPs, and hence, smaller NPs in size are not crucial. This results in the activation of every particle in the electrochemical system and thus an enhanced catalytic activity. Therefore, the electrochemical active surface area (ECSA) is an important parameter in conductivity and is directly influencing the catalytic performance [24]. However, the size does play an important role in the catalytic activity of bio-Pd NPs in suspension [9]. The highest catalytic activity is obtained when small NPs are present. This is due to the ratio in size of NPs versus uncovered areas of the cell with Pd [24]. Hou et al. [24] observed the difference in catalytic activity between bio-Pd in electrochemical systems and suspensions. The bio-Pd NPs with a 6:1 Pd:CDW ratio showed the biggest NPs of 54.3 ± 16.4 nm, while 25.8 ± 7.8 nm particles were obtained for the 3:1 ratio. As indicated by De Windt et al. [9], the ratio of Pd:CDW determines the size, extracellular distribution, and coverage of the Pd NPs in the cell. Nevertheless, the highest current densities were obtained due to the high ECSA, and hence, favourable catalytic activity in the electrochemical system was reached with the NPs produced with a 6:1 Pd:CDW ratio [9,24]. However, the highest catalytic activity of bio-Pd in suspension was obtained for a 3:1 Pd:CDW ratio. Hence, high coverage of Pd NPs on cells can negatively impact the catalytic activity depending on the use [24].
4.2 Increasing the electro-conductivity
In the previous Section (4.1), it was mentioned that the presence of bio-Pd NPs in an electrochemical cell would enhance the conductivity and thus the catalytic activity. However, this conductivity is often hindered due to (1) the coating agent used for the fixation of bio-Pd on the electrodes and (2) the limited conductivity of the microorganisms [6,24,46,47]. When bio-Pd NPs are fixed onto the electrode, a restricted increase in conductivity occurs. Only the bio-Pd NPs in contact with the electrode receive the electrons efficiently; this means that the rest, hence the majority, are not electrochemically active [24,127]. Therefore, it is important to overcome this hurdle which is possible by using different conductive materials to promote electron transfer throughout the electrode. It was suggested that carbon nanotubes (CNT) would solve the low increase in conductivity by functioning as an electron bridge between the fixed bio-Pd NPs and the electrode (Figure 4). Here, bio-Pd NPs are trapped between the CNT and form a network between the bio-Pd NPs present throughout the electrode. This network promoted electron transfer and simultaneously increased the current density and reduction of the contaminant [46]. However, it was also found that the use of graphene oxide (GO) during the bio-Pd production can also improve conductivity. A synergistic effect is created between Pd and the reduced GO (rGO), by increasing the nucleation sites to reduce Pd2+ and, hence, available active catalytic sites which established an enhanced electron transfer [125]. When the electrodes containing CNT and rGO were compared, both containing an equivalent amount of Pd and carbon loading, it was observed that 25 times more CNT loading was needed to obtain similar electrocatalytic conductivity as the Pd-cells-rGO electrode. This shows that rGO has a higher conductivity than CNT [46,125]. However, the use of both methods has a disadvantage, hence the extra costs on the conductive materials. Therefore, it was found that a biofilm can be used as a conductive network which can be synthesised by the Geobacter bacteria [126]. This network showed an increase in electrochemical activity and potential from −0.30 to −0.70 V. Compared to the control, an enhanced ECSA and HER were also observed, by 20 and 19.5 times, respectively. Besides, the economical feasibility it also has high stability over time (100 days) even under electrochemical- and mechanical stress due to the inherent filamentous structure of Geobacter [126].
4.3 Advantage of bio-Pd vs electrodeposited Pd vs chemical Pd NPs
The Pd NPs can be made in several different ways, and the most explored methods are through biological, electrochemical, and chemical production [6,8,9]. The Pd is often directly deposited on the electrode, because of the low cost and vital role in the redox reaction [128]. However, the restricted surface area for deposition and limited efficiency, hence transfer of electrons, are disadvantages compared to bio-Pd NPs. The drawbacks found in chemical production are the extreme conditions with the use of several toxic and environmentally unfriendly agents, resulting in high costs. Researchers demonstrated the use of bio-Pd over electrochemically deposited and chemically produced Pd NPs [6,128,129,130].
According to Wu et al. [6], the electron transfer and electric conductivity of the electrochemical cell were enhanced by the presence of bio-Pd NPs compared to electrodeposited Pd NPs. It was shown through the oxidation of formate, that bio-Pd coated on the electrode needed less activation energy and had better electrocatalysis than electrodeposited Pd NPs. It was also observed that a current of more than an order magnitude was needed for the electrodeposited Pd compared to the bio-Pd coated electrode [6]. Nevertheless, the electrodeposition of Pd has its benefits, absence of additives, and cultivation of microorganisms [128]. Besides electrodeposition, chemical production of Pd NPs is also possible; this method has been studied thoroughly, e.g., photochemical, seed-mediated growth, polyol reduction, and thermal decomposition [131]. A combination of the latter methods was shown by Nguyen et al. [130] for the generation of Pd NPs in octahedrons (24 nm), tetrahedrons (22 nm), and cubes (20 nm). Temperatures of 160°C were used together with polyvinylpyrrolidone (PVP) as stabiliser and silver nitrate (AgNO3) to control the sizes of the particles [130]. This shows that the production of chemical Pd NPs requires extreme conditions and is expensive.
5 Use of bio-Pd for bioremediation
Reductive dehalogenation converts the pollutants into less complex compounds that can be further treated by current wastewater treatment systems. The “bioreduction” of pollutants through bio-Pd NPs has also been expanded to remove azo-dyes and persistent antibiotics such as ciprofloxacin, 17β-estradiol, ibuprofen, and sulphamethoxazole [1,2]. Different mechanisms can be responsible for this reduction process. However, hydrogenation and reductive halogenation have been studied the most [13,51,97]. Reductive halogenation of mainly chlorine, hence dechlorination, has been investigated thoroughly, e.g., trichloroethylene and diclofenac [14,97,132].
5.1 Bioreduction of persistent azo-dyes
Increasing urbanisation and industrialisation have led to a tremendous release of emerging compounds like dyes [133]. They are used in many different fields, e.g., paper, cosmetics, agriculture, and pharmaceutical industries [134]. Dyes are structurally stable and have shown high resistance to microbial degradation. It also has a significant impact on the ecosystem and human health through the carcinogenic and mutagenic effects. Furthermore, it disturbs the rate of photosynthesis in the aquatic environment by reducing light penetration, and hence the oxygen levels [133,135]. Therefore, it is crucial to remove dyes before discharge in the environment [136,137]. However, secondary waste products and toxic sludge are currently produced by the removal of azo-dyes; hence, effective and environmentally friendly treatment is essential. Research has been focused on removing azo-dye which consists of an azo (–N═N–) chromophore, the largest class in dyes (Table 3) [133,137,138]. Therefore, bio-Pd NPs have been used in different manners for the removal of these azo dyes. Wang et al. [138] reported the improved removal of the azo-dye through bio-Pd NPs. The presence of bio-Pd NPs resulted in a higher decolourisation of methyl orange compared to viable Klebsiella oxytoca GS-4-08 without Pd. Hence, bio-Pd could be used as (1) a catalytic surface for the chemical reduction, and (2) the NPs would accelerate the extracellular electron transfer needed for the reduction of the azo-dyes [138].
Overview of the micropollutants removed by bio-Pd
| Category | Micropollutant | Removal of micropollutants | Ref. |
|---|---|---|---|
| Pharmaceutical | Diclofenac | Continuous removal in MEC systems, with 57% lower removal efficiency | [97] |
| Pharmaceutical | Ciprofloxacin | According to Martins et al. [1] was the removal negligible as 35 and 49% of ciprofloxacin was removed by active- and non-active catalyst, respectively | [141] |
| Pharmaceutical | Sulfamethoxazole | 85% Removal was accomplished after 24 h, with a removal rate of 0.110 h−1 | [1] |
| Pharmaceutical | Ibuprofen | No removal of the painkiller by bio-Pd produced by D. vulgaris; however, it was removed by the bacterium | [1] |
| Pharmaceutical | 17β-estradiol | Low removal of the compound, 35 and 11%, by catalytic activity and adsorption, respectively | [1] |
| Dyes | Methyl orange | Removal of 75% of the dye in the presence of AQS with bio-Pd | [138] |
| Dyes | Acid blue | Removal over 70% of the dye in the presence of AQS with bio-Pd | [138] |
| Dyes | Reactive black | Removal over 70% of the dye in the presence of AQS with bio-Pd | [138] |
| Dyes | Acid Red | Removal over 70% of the dye in the presence of AQS with bio-Pd | [138] |
| Dyes | Congo Red | Anaerobic sludge containing Pd NPs showed a 2.3 times higher removal compared to non-palladised anaerobic sludge | [2] |
| Dyes | Evans Blue | Anaerobic sludge containing Pd NPs showed a 10.0 times higher removal compared to non-palladised anaerobic sludge | [2] |
| Dyes | Orange II | Anaerobic sludge containing Pd NPs showed 5.1 times higher removal compared to non-palladised anaerobic sludge | [2] |
Another method for removing azo-dyes is with the in-situ produced bio-Pd NPs from granular sludge which was reported by Quan et al. [2]. It appeared that bio-Pd was able to enhance the decolourisation of Congo Red, Evans Blue, and Orange II. Decolourisation was also observed without the presence of an electron donor. This originates from the endogenous metabolism of granular sludge which provided the necessary electrons, H2, and energy. Among all the different tested electron donors, formic acid gave the best removal (99%) in Orange II for bio-Pd from granular sludge due to the similar reactivity of H2 [2,139].
5.2 Degradation of emerging antibiotics
Antibiotics are used worldwide for treating diseases in humans and animals. The pharmaceutical compounds have properties of being highly chemical stable. Hence, they are often not entirely metabolised by the patients, and approximately 70% of all the antibiotics used remain unaltered and excreted. They are released as active metabolites that go to the municipal wastewater systems which are inefficient to treat these pharmaceuticals [1,140,141,142]. Consequently, antibiotics are frequently detected in surface-, sea-, ground-, and drinking water [140,141,142]. Therefore, it is crucial to minimalise the release and accumulation of these pharmaceuticals in the environment, as they are persistent, have a relatively long residence time, and induce resistance in microorganisms [1,143,144]. Among the antibiotics, ciprofloxacin is heavily used and belongs to the fluoroquinolones group [145]. Fluoroquinolones can inhibit DNA replication and have genotoxic and carcinogenic effects on organisms; hence, removal is essential [141,145]. However, treatment of these compounds requires extreme conditions and are hazardous and expensive, e.g., ozone and advanced oxidation process (UV/H2O2, UV/O3, and UV/TiO2) [146,147,148,149]. The bio-Pd NPs offer an environmentally friendly and economically feasible approach (Table 3) [1,141]. Both He et al. [141] and Martins et al. [1] reported the use of bio-Pd NPs for the removal of ciprofloxacin, through different methods.
He et al. [141] observed the influence of the presence of H2 on the removal of ciprofloxacin with bio-Pd NPs. It is speculated that the protonation of the piperazine N atom of ciprofloxacin plays a significant role here [141,150,151,152]. In the absence of H2 28.90% and 32.95% of ciprofloxacin was removed. This removal can be explained by the adsorption of the antibiotic on bio-Pd and the surface of unconverted Pd2+. The unconverted Pd2+ forms a complex with ciprofloxacin molecules and is attributed in this way to the removal of the antibiotic; however, the underlying mechanism using this complex is not discovered yet [141,153]. When H2 was present, the removal of ciprofloxacin was 87.70%, 3.03 times higher than in the absence of H2. This was caused by the sorption of H2 onto the bio-Pd NPs that formed an H2 hybrid, which is a very strong reductant, reacts with the antibiotic, and subsequently degraded the compound [141].
Martins et al. [1] reported the use of Desulfovibrio vulgaris to produce bio-Pd NPs to remove ciprofloxacin. The bio-Pd NPs produced by Desulfovibrio vulgaris did not remove ciprofloxacin efficiently; nevertheless, removal did occur for sulfamethoxazole [1]. This observation of Martins et al. [1] contradicts He et al. [141]. The difference can be explained by the use of different types of microorganisms which can aid in the degradation of the antibiotic. It is also possible that a different pH was used which influenced the solubility and hence the availability for degradation. However, more extensive research needs to be carried out on the use of bio-Pd NPs to remove different types of antibiotics in an environment-friendly way.
6 Metal recovery towards a circular economy
Industrial activities are important for the economy and development, however, they also play a significant role in the pollution of the environment. One of the major sources responsible for pollution is heavy metals which are discharged through wastewater. These heavy metals are highly toxic and have a strong impact on living beings. They accumulate in different parts of the human body due to their nonbiodegradibility, causing undesirable health effects [154]. Therefore, recovery of these heavy metals is important before discharge into the environment. Biological recovery is preferred, due to the effectiveness, low cost, and eco-friendly approach, no increased energy consumption, and addition of chemicals [154,155]. Nowadays, recovery of metals present in wastewater, mines, metal processing plant efflux, and natural mineralised areas can be realised through microorganisms, by the production of NPs. Production of a well-defined size and shape of NPs is highly demanded [156]. However, the use of microorganisms has its difficulties; hence, the toxicity of the heavy metals that has a substantial effect on the viability of the cells [157]. Therefore, microorganisms have evolved genetic and proteomic responses to control metal homeostasis which allows the cells to detoxify the metals. These microbes are called metallophilic bacteria and survive in environments that contain high concentrations of mobile heavy metal ions [156].
The Pd is one of the precious metals that requires to be recovered, due to the price increase which reached in 2019 ca. 45 k€ kg−1, and demand [158]. However, it is expected to keep increasing; therefore, alternative supplies need to be found. Recovery of Pd is also important due to the high environmental burden it has on a per kilogram comparison compared to other non-PGM elements [159,160]. For these reasons, increasing interest has been found in urban mining for the recovery of precious metals, as electronic wastes are increasing and mines are depleting [160]. Depending on the origin of the electronic waste, different treatment methods for the recovery of metals are established. The Pd from urban waste, more specifically printed circuit boards, has been recovered via chemical, pyrometallurgical, or hydrometallurgical treatment. First, the electronic parts that contain Pd will be de-soldered which is alloyed with silver, subsequently, different treatments are used here, and a mixed solution of metals containing Pd is obtained, whereafter further purification steps are needed to derive the Pd from the other metals. The disadvantage of these methods is the use of several chemicals and high temperatures which is expensive and environmentally unfriendly, and this also generates a secondary wastestream that needs to be treated [161,162,163]. A more eco-friendly approach can be obtained through the use of microorganisms, in the production of bio-Pd NPs. Here, microorganisms that are known to have the DMR process are often used [55]. These microbes are able to recover precious metals by reducing the metals and possibly producing NPs. The benefit is the catalytic activity of the NPs that can be used for (1) the removal of micropollutants, (2) recovery of metals, and (3) preventing the discharge of undesired metals into the environment. Recovery of Pd and Au from electronic scrap leachates has been studied, by the use of Desulfobibrio desulfuricans which required a prepalladisation of the microorganisms due to the presence of Cu [164]. Environmental friendly recovery of metals has also been found by the use of Thauera selanatus for selenium oxyanios from water drainage [165]. Nevertheless, more investigation on the recovery of Pd by microorganisms is required.
7 Conclusion and future perspectives
Finding environmentally friendly technologies to remove emerging pollutants because of population growth and intensive industrialisation remains a grant challenge for the twenty-first century. To make the burden of industrial processes as small as possible, both chemical and biological catalysts often play a crucial role. They serve as an activator, creating faster reaction rates and higher efficiencies with lower energy demands. This article reviewed the sustainable production and application of biogenic Pd catalysts developed at the interphase of biology and chemistry and can have broad applicability in various fields like bioremediation, energy generation, and energy storage.
The efficiency, hence the catalytic activity of the bio-Pd NPs, was proved; however, an improvement could still be made in the particles production (1) to overcome the problem of chemical precipitation and concurrent aggregation of Pd NPs during the biogenic production, (2) it is essential to microbially deplete the Pd2+ present in solution when the bio-Pd NPs are synthesised, hence avoiding losses and further processing. Advances are also needed regarding the conductivity of bio-Pd NPs on the electrodes, without the use of expensive fixation chemicals (Nafion, CNT, and graphene oxide). Furthermore, with the increasing prices of Pd over the last years, the use of primary resources of Pd has become an obstacle. Therefore, it is important to have an economically feasible method to produce bio-Pd NPs. The bio-Pd NPs proved their efficiency for the removal of micropollutants and can therefore be used as a polishing step after the secondary treatment. This tertiary treatment poses the possibility of leaching Pd2+ from the detached Pd0 which came from the bio-Pd NPs, as the presence of Pd2+ in the wastewater. This can be solved by incorporating an electrochemical cell in the tertiary treatment. Here, the Pd2+ will be electrodeposited, and the electrochemical system will also be responsible for sustainably producing H2 which is needed for the dehalogenation or hydrogenation by the catalytic activity of the bio-Pd NPs.
Acknowledgments
The authors would like to thank Tim Lacoere for his contribution to the figures and Karen Delbaere for critically reading the manuscript.
-
Funding information: The ELECTRA project has received funding from European Union’s Horizon 2020 research and innovation program under grant agreement No. 826244.
-
Author contributions: Cindy Ka Y Law wrote the manuscript. Luiza Bonin, Bart De Gusseme, Nico Boon and Kankana Kundu reviewed and made suggestion for the manuscript. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Martins M, Mourato C, Sanches S, Noronha JP, Crespo MTB, Pereira IAC. Biogenic platinum and palladium nanoparticles as new catalysts for the removal of pharmaceutical compounds. Water Res. 2017;108:160–8.10.1016/j.watres.2016.10.071Search in Google Scholar PubMed
[2] Quan X, Zhang X, Xu H. In-situ formation and immobilization of biogenic nanopalladium into anaerobic granular sludge enhances azo dyes degradation. Water Res. 2015;78:74–83.10.1016/j.watres.2015.03.024Search in Google Scholar PubMed
[3] Tungler A, Tarnai T, Hegedûs L, Fordor K. Palladium-mediated heterogeneous catalytic hydrogenations: selectivity of liquid-phase reactions for the fine chemicals industry. Platinum Metals Rev. 1998;42(3):108.10.1002/chin.199914331Search in Google Scholar
[4] Dekura S, Kobayashi H, Kusada K, Kitagawa H. Hydrogen in palladium and storage properties of related nanomaterials: Size, shape, alloying, and metal-organic framework coating effects. Chemphyschem. 2019;20(10):1158–76.10.1002/cphc.201900109Search in Google Scholar PubMed
[5] Guo S, Zhang S, Su D, Sun S. Seed-mediated synthesis of core/shell FePtM/FePt (M = Pd, Au) nanowires and their electrocatalysis for oxygen reduction reaction. J Am Chem Soc. 2013;135(37):13879–84.10.1021/ja406091pSearch in Google Scholar PubMed
[6] Wu R, Tian X, Xiao Y, Ulstrup J, Mølager Christensen HE, Zhao F, et al. Selective electrocatalysis of biofuel molecular oxidation using palladium nanoparticles generated on Shewanella oneidensis MR-1. J Mater Chem A. 2018;6(23):10655–62.10.1039/C8TA01318GSearch in Google Scholar
[7] Coy E, Siuzdak K, Pavlenko M, Załęski K, Graniel O, Ziółek M, et al. Enhancing photocatalytic performance and solar absorption by schottky nanodiodes heterojunctions in mechanically resilient palladium coated TiO2/Si nanopillars by atomic layer deposition. Chem Eng J. 2020;392:123702.10.1016/j.cej.2019.123702Search in Google Scholar
[8] Saldan I, Semenyuk Y, Marchuk I, Reshetnyak O. Chemical synthesis and application of palladium nanoparticles. J Mater Sci. 2015;50(6):2337–54.10.1007/s10853-014-8802-2Search in Google Scholar
[9] De Windt W, Boon N, Van den Bulcke J, Rubberecht L, Prata F, Mast J, et al. Biological control of the size and reactivity of catalytic Pd(0) produced by Shewanella oneidensis. Antonie van Leeuwenhoek. 2006;90(4):377–89.10.1007/s10482-006-9088-4Search in Google Scholar PubMed
[10] De Corte S, Hennebel T, De Gusseme B, Verstraete W, Boon N. Bio-palladium: from metal recovery to catalytic applications. Microb Biotechnol. 2012;5(1):5–17.10.1111/j.1751-7915.2011.00265.xSearch in Google Scholar PubMed PubMed Central
[11] Espino-López IE, Romero-Romo M, de Oca-Yemha MGM, Morales-Gil P, Ramírez-Silva MT, Mostany J, et al. Palladium nanoparticles electrodeposition onto glassy carbon from a deep eutectic solvent at 298 K and their catalytic performance toward formic acid oxidation. J Electrochem Soc. 2018;166(1):D3205–11.10.1149/2.0251901jesSearch in Google Scholar
[12] Previdello BAF, Sibert E, Maret M, Soldo-Olivier Y. Palladium electrodeposition onto Pt(100): Two-layer underpotential deposition. Langmuir. 2017;33(9):2087–95.10.1021/acs.langmuir.6b03968Search in Google Scholar PubMed
[13] Hennebel T, De Gusseme B, Boon N, Verstraete W. Biogenic metals in advanced water treatment. Trends Biotechnol. 2009;27(2):90–8.10.1016/j.tibtech.2008.11.002Search in Google Scholar PubMed
[14] De Corte S, Sabbe T, Hennebel T, Vanhaecke L, De Gusseme B, Verstraete W, et al. Doping of biogenic Pd catalysts with Au enables dechlorination of diclofenac at environmental conditions. Water Res. 2012;46(8):2718–26.10.1016/j.watres.2012.02.036Search in Google Scholar PubMed
[15] Ali I, Peng C, Khan ZM, Naz I, Sultan M, Ali M, et al. Overview of microbes based fabricated biogenic nanoparticles for water and wastewater treatment. J Env Manage. 2019;230:128–50.10.1016/j.jenvman.2018.09.073Search in Google Scholar PubMed
[16] Yong P, Rowson NA, Farr JP, Harris IR, Macaskie LE. Bioreduction and biocrystallization of palladium by Desulfovibrio desulfuricans NCIMB 8307. Biotechnol Bioeng. 2002;80(4):369–79.10.1002/bit.10369Search in Google Scholar PubMed
[17] De Windt W, Aelterman P, Verstraete W. Bioreductive deposition of palladium (0) nanoparticles on Shewanella oneidensis with catalytic activity towards reductive dechlorination of polychlorinated biphenyls. Env Microbiol. 2005;7(3):314–25.10.1111/j.1462-2920.2005.00696.xSearch in Google Scholar PubMed
[18] Sathishkumar M, Sneha K, Kwak IS, Mao J, Tripathy SJ, Yun YS. Phyto-crystallization of palladium through reduction process using Cinnamom zeylanicum bark extract. J Hazard Mater. 2009;171(1):400–4.10.1016/j.jhazmat.2009.06.014Search in Google Scholar PubMed
[19] Sahin M, Gubbuk IH. Green synthesis of palladium nanoparticles and investigation of their catalytic activity for methylene blue, methyl orange and rhodamine B degradation by sodium borohydride. React Kinetics Mech Catal. 2022;135:999–1010.10.1007/s11144-022-02185-ySearch in Google Scholar
[20] Xiong L, Zhang X, Huang Y-X, Liu W-J, Chen Y-L, Yu S-S, et al. Biogenic synthesis of Pd-based nanoparticles with enhanced catalytic activity. ACS Appl Nano Mater. 2018;1(4):1467–75.10.1021/acsanm.7b00322Search in Google Scholar
[21] Tuo Y, Liu G, Dong B, Yu H, Zhou J, Wang J, et al. Microbial synthesis of bimetallic PdPt nanoparticles for catalytic reduction of 4-nitrophenol. Env Sci Pollut Res Int. 2017;24(6):5249–58.10.1007/s11356-016-8276-7Search in Google Scholar PubMed
[22] Courtney J, Deplanche K, Rees NV, Macaskie LE. Biomanufacture of nano-Pd(0) by Escherichia coli and electrochemical activity of bio-Pd(0) made at the expense of H2 and formate as electron donors. Biotechnol Lett. 2016;38(11):1903–10.10.1007/s10529-016-2183-3Search in Google Scholar PubMed PubMed Central
[23] Xu H, Tan L, Cui H, Xu M, Xiao Y, Wu H, et al. Characterization of Pd(II) biosorption in aqueous solution by Shewanella oneidensis MR-1. J Mol Liq. 2018;255:333–40.10.1016/j.molliq.2018.01.168Search in Google Scholar
[24] Hou Y-N, Zhang B, Yun H, Yang Z-N, Han J-L, Zhou J, et al. Palladized cells as suspension catalyst and electrochemical catalyst for reductively degrading aromatics contaminants: Roles of Pd size and distribution. Water Res. 2017;125:288–97.10.1016/j.watres.2017.08.055Search in Google Scholar PubMed
[25] Fredrickson JK, Romine MF, Beliaev AS, Auchtung JM, Driscoll ME, Gardner TS, et al. Towards environmental systems biology of Shewanella. Nat Rev Microbiol. 2008;6(8):592–603.10.1038/nrmicro1947Search in Google Scholar PubMed
[26] Yang ZN, Hou YN, Zhang B, Cheng HY, Yong YC, Liu WZ, et al. Insights into palladium nanoparticles produced by Shewanella oneidensis MR-1: Roles of NADH dehydrogenases and hydrogenases. Env Res. 2020;191:110196.10.1016/j.envres.2020.110196Search in Google Scholar PubMed
[27] Deplanche K, Caldelari I, Mikheenko IP, Sargent F, Macaskie LE. Involvement of hydrogenases in the formation of highly catalytic Pd(0) nanoparticles by bioreduction of Pd(II) using Escherichia coli mutant strains. Microbiology. 2010;156(9):2630–40.10.1099/mic.0.036681-0Search in Google Scholar PubMed
[28] Joudeh N, Saragliadis A, Schulz C, Voigt A, Almaas E, Linke D. Transcriptomic response analysis of escherichia coli to palladium stress. Front Microbiol. 2021;12:2840.10.3389/fmicb.2021.741836Search in Google Scholar PubMed PubMed Central
[29] Sarmah M, Neog AB, Boruah PK, Das MR, Bharali P, Bora U. Effect of substrates on catalytic activity of biogenic palladium nanoparticles in C–C cross-coupling reactions. ACS Omega. 2019;4(2):3329–40.10.1021/acsomega.8b02697Search in Google Scholar PubMed PubMed Central
[30] Qazi F, Hussain Z, Tahir MN. Advances in biogenic synthesis of palladium nanoparticles. RSC Adv. 2016;6(65):60277–86.10.1039/C6RA11695GSearch in Google Scholar
[31] Suja E, Nancharaiah YV, Venugopalan VP. Biogenic nanopalladium production by self-immobilized granular biomass: application for contaminant remediation. Water Res. 2014;65:395–401.10.1016/j.watres.2014.08.005Search in Google Scholar PubMed
[32] Quan X, Wang X, Zheng Y, Li W, Chen L, Pei Y. Effects of biogenic nanopalladium precipitation on the performance and microbial community structure of anaerobic granular sludge. Sci Total Environ. 2020;705:135765.10.1016/j.scitotenv.2019.135765Search in Google Scholar PubMed
[33] Sivamaruthi BS, Ramkumar VS, Archunan G, Chaiyasut C, Suganthy N. Biogenic synthesis of silver palladium bimetallic nanoparticles from fruit extract of Terminalia chebula – In vitro evaluation of anticancer and antimicrobial activity. J Drug Delivery Sci Technol. 2019;51:139–51.10.1016/j.jddst.2019.02.024Search in Google Scholar
[34] De Corte S, Hennebel T, Fitts JP, Sabbe T, Bliznuk V, Verschuere S, et al. Biosupported bimetallic Pd-Au nanocatalysts for dechlorination of environmental contaminants. Env Sci Technol. 2011;45(19):8506–13.10.1021/es2019324Search in Google Scholar PubMed
[35] Omajali JB, Gomez-Bolivar J, Mikheenko IP, Sharma S, Kayode B, Al-Duri B, et al. Novel catalytically active Pd/Ru bimetallic nanoparticles synthesized by Bacillus benzeovorans. Sci Rep. 2019;9(1):4715.10.1038/s41598-019-40312-3Search in Google Scholar PubMed PubMed Central
[36] Hoffmann JE. Recovery of platinum-group metals from gabbroic rocks metals from auto catalysts. Jom. 2012;40(6):40–4.10.1007/BF03258173Search in Google Scholar
[37] Hazarika M, Borah D, Bora P, Silva AR, Das P. Biogenic synthesis of palladium nanoparticles and their applications as catalyst and antimicrobial agent. PLoS One. 2017;12(9):e0184936.10.1371/journal.pone.0184936Search in Google Scholar PubMed PubMed Central
[38] Quan X, Wang X, Sun Y, Li W, Chen L, Zhao J. Degradation of diclofenac using palladized anaerobic granular sludge: Effects of electron donor, reaction medium and deactivation factors. J Hazard Mater. 2019;365:155–63.10.1016/j.jhazmat.2018.10.100Search in Google Scholar PubMed
[39] De Gusseme B, Hennebel T, Vanhaecke L, Soetaert M, Desloover J, Wille K, et al. Biogenic palladium enhances diatrizoate removal from hospital wastewater in a microbial electrolysis cell. Env Sci Technol. 2011;45(13):5737–45.10.1021/es200702mSearch in Google Scholar PubMed
[40] Verlicchi P, Galletti A, Masotti L. Management of hospital wastewaters: the case of the effluent of a large hospital situated in a small town. Water Sci Technol. 2010;61(10):2507–19.10.2166/wst.2010.138Search in Google Scholar PubMed
[41] Ehrl BN, Kundu K, Gharasoo M, Marozava S, Elsner M. Rate-limiting mass transfer in micropollutant degradation revealed by isotope fractionation in chemostat. Env Sci Technol. 2019;53(3):1197–205.10.1021/acs.est.8b05175Search in Google Scholar PubMed PubMed Central
[42] Kundu K, Marozava S, Ehrl B, Merl-Pham J, Griebler C, Elsner M. Defining lower limits of biodegradation: atrazine degradation regulated by mass transfer and maintenance demand in Arthrobacter aurescens TC1. ISME J. 2019;13(9):2236–51.10.1038/s41396-019-0430-zSearch in Google Scholar PubMed PubMed Central
[43] Feng S, Hao Ngo H, Guo W, Woong Chang S, Duc Nguyen D, Cheng D, et al. Roles and applications of enzymes for resistant pollutants removal in wastewater treatment. Bioresour Technol. 2021;335:125278.10.1016/j.biortech.2021.125278Search in Google Scholar PubMed
[44] Silva L, Moreira C, Curzio B, da Fonseca F. Micropollutant removal from water by membrane and advanced oxidation processes–A review. J Water Resour Prot. 2017;9:411–31.10.4236/jwarp.2017.95027Search in Google Scholar
[45] Orozco RL, Redwood MD, Yong P, Caldelari I, Sargent F, Macaskie LE. Towards an integrated system for bio-energy: Hydrogen production by Escherichia coli and use of palladium-coated waste cells for electricity generation in a fuel cell. Biotechnol Lett. 2010 Dec;32(12):1837–45.10.1007/s10529-010-0383-9Search in Google Scholar PubMed
[46] Cheng HY, Hou YN, Zhang X, Yang ZN, Xu T, Wang AJ. Activating electrochemical catalytic activity of bio-palladium by hybridizing with carbon nanotube as “e(-) Bridge”. Sci Rep. 2017;7(1):16588.10.1038/s41598-017-16880-7Search in Google Scholar PubMed PubMed Central
[47] Quan X, Sun B, Xu H. Anode decoration with biogenic Pd nanoparticles improved power generation in microbial fuel cells. Electrochim Acta. 2015;182:815–20.10.1016/j.electacta.2015.09.157Search in Google Scholar
[48] Fahmy SA, Preis E, Bakowsky U. Azzazy HME-S. Palladium nanoparticles fabricated by green chemistry: Promising chemotherapeutic, antioxidant and antimicrobial agents. Materials. 2020;13(17):3661.10.3390/ma13173661Search in Google Scholar PubMed PubMed Central
[49] Singh A, Gautam PK, Verma A, Singh V, Shivapriya PM, Shivalkar S, et al. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol Rep (Amst). 2020;25:e00427.10.1016/j.btre.2020.e00427Search in Google Scholar PubMed PubMed Central
[50] Kumari S, Tyagi M, Jagadevan S. Mechanistic removal of environmental contaminants using biogenic nano-materials. Int J Environ Sci Technol. 2019;16(11):7591–606.10.1007/s13762-019-02468-3Search in Google Scholar
[51] Hennebel T, Van Nevel S, Verschuere S, De Corte S, De Gusseme B, Cuvelier C, et al. Palladium nanoparticles produced by fermentatively cultivated bacteria as catalyst for diatrizoate removal with biogenic hydrogen. Appl Microbiol Biotechnol. 2011;91(5):1435–45.10.1007/s00253-011-3329-9Search in Google Scholar PubMed
[52] Ng CK, Cai Tan TK, Song H, Cao B. Reductive formation of palladium nanoparticles by Shewanella oneidensis: role of outer membrane cytochromes and hydrogenases. RSC Adv. 2013;3(44):22498–503.10.1039/c3ra44143aSearch in Google Scholar
[53] Bucking C, Piepenbrock A, Kappler A, Gescher J. Outer-membrane cytochrome-independent reduction of extracellular electron acceptors in Shewanella oneidensis. Microbiology (Read). 2012;158(Pt 8):2144–57.10.1099/mic.0.058404-0Search in Google Scholar PubMed
[54] Dundas CM, Graham AJ, Romanovicz DK, Keitz BK. Extracellular electron transfer by shewanella oneidensis controls palladium nanoparticle phenotype. ACS Synth Biol. 2018;7(12):2726–36.10.1021/acssynbio.8b00218Search in Google Scholar PubMed
[55] Lovley DR. Dissimilatory metal reduction. Annu Rev Microbiol. 1993;47:263–90.10.1146/annurev.mi.47.100193.001403Search in Google Scholar PubMed
[56] Shi L, Richardson DJ, Wang Z, Kerisit SN, Rosso KM, Zachara JM, et al. The roles of outer membrane cytochromes of Shewanella and Geobacter in extracellular electron transfer. Env Microbiol Rep. 2009;1(4):220–7.10.1111/j.1758-2229.2009.00035.xSearch in Google Scholar PubMed
[57] Marshall MJ, Beliaev AS, Dohnalkova AC, Kennedy DW, Shi L, Wang Z, et al. c-Type cytochrome-dependent formation of U(IV) nanoparticles by Shewanella oneidensis. PLoS Biol. 2006;4(9):e268.10.1371/journal.pbio.0040268Search in Google Scholar PubMed PubMed Central
[58] Mikheenko IP, Rousset M, Dementin S, Macaskie LE. Bioaccumulation of palladium by Desulfovibrio fructosivorans wild-type and hydrogenase-deficient strains. Appl Env Microbiol. 2008;74(19):6144–6.10.1128/AEM.02538-07Search in Google Scholar PubMed PubMed Central
[59] Deplanche K, Bennett JA, Mikheenko IP, Omajali J, Wells AS, Meadows RE, et al. Catalytic activity of biomass-supported Pd nanoparticles: Influence of the biological component in catalytic efficacy and potential application in ‘green’ synthesis of fine chemicals and pharmaceuticals. Appl Catal B: Environ. 2014;147:651.10.1016/j.apcatb.2013.09.045Search in Google Scholar
[60] Shi L, Belchik SM, Plymale AE, Heald S, Dohnalkova AC, Sybirna K, et al. Purification and characterization of the [NiFe]-hydrogenase of Shewanella oneidensis MR-1. Appl Env Microbiol. 2011;77(16):5584–90.10.1128/AEM.00260-11Search in Google Scholar PubMed PubMed Central
[61] Omajali JB, Mikheenko IP, Merroun ML, Wood J, Macaskie LE. Characterization of intracellular palladium nanoparticles synthesized by Desulfovibrio desulfuricans and Bacillus benzeovorans. J Nanopart Res. 2015;17:264.10.1007/s11051-015-3067-5Search in Google Scholar PubMed PubMed Central
[62] Gomez-Bolivar J, Mikheenko IP, Macaskie LE, Merroun ML. Characterization of palladium nanoparticles produced by healthy and microwave-injured cells of desulfovibrio desulfuricans and escherichia coli. Nanomaterials (Basel). 2019;9(6):857.10.3390/nano9060857Search in Google Scholar PubMed PubMed Central
[63] Gomez-Bolivar J, Mikheenko IP, Orozco RL, Sharma S, Banerjee D, Walker M, et al. Synthesis of Pd/Ru bimetallic nanoparticles by Escherichia coli and potential as a catalyst for upgrading 5-hydroxymethyl furfural into liquid fuel precursors. Front Microbiol. 2019;10:1276. http://europepmc.org/abstract/MED/31281292.10.3389/fmicb.2019.01276Search in Google Scholar PubMed PubMed Central
[64] Berg JM, Romoser A, Banerjee N, Zebda R, Sayes CM. The relationship between pH and zeta potential of similar to 30 nm metal oxide nanoparticle suspensions relevant to in vitro toxicological evaluations. Nanotoxicology. 2009;3(4):276–83.10.3109/17435390903276941Search in Google Scholar
[65] Zheng Z, Cao H, Meng J, Xiao Y, Ulstrup J, Zhang J, et al. Synthesis and structure of a two-dimensional palladium oxide network on reduced graphene oxide. Nano Lett. 2022;22(12):4854–60.10.1021/acs.nanolett.2c01226Search in Google Scholar PubMed
[66] Xu H, Shang H, Wang C, Du Y. Recent progress of ultrathin 2D Pd-based nanomaterials for fuel cell electrocatalysis. Small. 2021;17(5):2005092.10.1002/smll.202005092Search in Google Scholar PubMed
[67] Ji Z, Zhang H, Liu H, Yaghi OM, Yang P. Cytoprotective metal-organic frameworks for anaerobic bacteria. Proc Natl Acad Sci. 2018;115(42):10582–7.10.1073/pnas.1808829115Search in Google Scholar PubMed PubMed Central
[68] Wu X, Zhao F, Rahunen N, Varcoe JR, Avignone-Rossa C, Thumser AE, et al. A Role for microbial palladium nanoparticles in extracellular electron transfer. Angew Chem Int Ed. 2011;50(2):427–30.10.1002/anie.201002951Search in Google Scholar PubMed
[69] Chen Y, Chen Y. Difference in toxicity of Pd (II) and mechanism of action before and after reduction by Bacillus wiedmannii MSM. Environ Sci Pollut Res. 2021;29:1824–35.10.1007/s11356-021-15736-ySearch in Google Scholar PubMed
[70] Sakimoto KK, Wong AB, Yang P. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production. Science. 2016;351(6268):74–7.10.1126/science.aad3317Search in Google Scholar PubMed
[71] Yin J, Gao H. Stress responses of Shewanella. Int J Microbiology. 2011;2011:863623.10.1155/2011/863623Search in Google Scholar PubMed PubMed Central
[72] Nandi R, Sengupta S. Microbial production of hydrogen: An overview. Crit Rev Microbiol. 1998;24(1):61–84.10.1080/10408419891294181Search in Google Scholar PubMed
[73] Adams CP, Walker KA, Obare SO, Docherty KM. Size-dependent antimicrobial effects of novel palladium nanoparticles. PLoS One. 2014;9(1):e85981.10.1371/journal.pone.0085981Search in Google Scholar PubMed PubMed Central
[74] Vijayaraghavan K, Ashokkumar T. Plant-mediated biosynthesis of metallic nanoparticles: A review of literature, factors affecting synthesis, characterization techniques and applications. J Environ Chem Eng. 2017;5(5):4866–83.10.1016/j.jece.2017.09.026Search in Google Scholar
[75] Elahian F, Heidari R, Charghan VR, Asadbeik E, Mirzaei SA. Genetically modified Pichia pastoris, a powerful resistant factory for gold and palladium bioleaching and nanostructure heavy metal biosynthesis. Artif Cells Nanomed Biotechnol. 2020;48(1):259–65.10.1080/21691401.2019.1699832Search in Google Scholar PubMed
[76] Mughal B, Zaidi S, Zhang X, Hassan SU. Biogenic nanoparticles: Synthesis, characterisation and applications. Appl Sci. 2021;11:2598.10.3390/app11062598Search in Google Scholar
[77] Wu G, Li N, Mao Y, Zhou G, Gao H. Endogenous generation of hydrogen sulfide and its regulation in Shewanella oneidensis. Front Microbiol. 2015;6(374):374.10.3389/fmicb.2015.00374Search in Google Scholar PubMed PubMed Central
[78] Lloyd JR, Yong P, Macaskie LE. Enzymatic recovery of elemental palladium by using sulfate-reducing bacteria. Appl Env Microbiol. 1998;64(11):4607–9.10.1128/AEM.64.11.4607-4609.1998Search in Google Scholar PubMed PubMed Central
[79] Redwood MD, Deplanche K, Baxter-Plant VS, Macaskie LE. Biomass-supported palladium catalysts on Desulfovibrio desulfuricans and Rhodobacter sphaeroides. Biotechnol Bioeng. 2008;99(5):1045–54.10.1002/bit.21689Search in Google Scholar PubMed
[80] Siddiqi KS, Husen A. Green synthesis, characterization and uses of palladium/platinum nanoparticles. Nanoscale Res Lett. 2016;11(1):482.10.1186/s11671-016-1695-zSearch in Google Scholar PubMed PubMed Central
[81] Bali R, Razak N, Lumb A, Harris AT. The synthesis of metallic nanoparticles inside live plants. International Conference on Nanoscience and Nanotechnology, 2006.10.1109/ICONN.2006.340592Search in Google Scholar
[82] Sharmila G, Farzana Fathima M, Haries S, Geetha S, Manoj Kumar N, Muthukumaran C. Green synthesis, characterization and antibacterial efficacy of palladium nanoparticles synthesized using Filicium decipiens leaf extract. J Mol Struct. 2017;1138:35–40.10.1016/j.molstruc.2017.02.097Search in Google Scholar
[83] Md Ishak NAI, Kamarudin SK, Timmiati SN. Green synthesis of metal and metal oxide nanoparticles via plant extracts: an overview. Mater Res Exp. 2019;6(11):112004.10.1088/2053-1591/ab4458Search in Google Scholar
[84] Shaik MR, Ali ZJ, Khan M, Kuniyil M, Assal ME, Alkhathlan HZ, et al. Green synthesis and characterization of palladium nanoparticles using Origanum vulgare L. extract and their catalytic activity. Molecules (Basel, Switz). 2017;22(1):165.10.3390/molecules22010165Search in Google Scholar PubMed PubMed Central
[85] Makarov VV, Love AJ, Sinitsyna OV, Makarova SS. Yaminsky IV, Taliansky ME, et al. “Green” nanotechnologies: synthesis of metal nanoparticles using plants. Acta Nat. 2014;6(1):35–44.10.32607/20758251-2014-6-1-35-44Search in Google Scholar
[86] Pat-Espadas AM, Field JA, Otero-Gonzalez L, Razo-Flores E, Cervantes FJ, Sierra-Alvarez R. Recovery of palladium(II) by methanogenic granular sludge. Chemosphere. 2016;144:745–53.10.1016/j.chemosphere.2015.09.035Search in Google Scholar PubMed
[87] Alibhai KRK, Mehrotra I, Forster CF. Heavy-metal binding to digested-sludge. Water Res. 1985;19(12):1483–8.10.1016/0043-1354(85)90393-8Search in Google Scholar
[88] Gould MS, Genetelli EJ. Heavy-metal complexation behavior in anaerobically digested sludges. Water Res. 1978;12(8):505–12.10.1016/0043-1354(78)90126-4Search in Google Scholar
[89] Rotaru AE, Jiang W, Finster K, Skrydstrup T, Meyer RL. Non-enzymatic palladium recovery on microbial and synthetic surfaces. Biotechnol Bioeng. 2012;109(8):1889–97.10.1002/bit.24500Search in Google Scholar PubMed
[90] Fahmy K, Merroun M, Pollmann K, Raff J, Savchuk O, Hennig C, et al. Secondary structure and Pd(II) coordination in S-layer proteins from Bacillus sphaericus studied by infrared and X-ray absorption spectroscopy. Biophys J. 2006;91(3):996–1007.10.1529/biophysj.105.079137Search in Google Scholar PubMed PubMed Central
[91] Appels L, Baeyens J, Degrève J, Dewil R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog Energy Combust Sci. 2008;34(6):755–81.10.1016/j.pecs.2008.06.002Search in Google Scholar
[92] Franke-Whittle IH, Walter A, Ebner C, Insam H. Investigation into the effect of high concentrations of volatile fatty acids in anaerobic digestion on methanogenic communities. Waste Manag. 2014;34(11):2080–9.10.1016/j.wasman.2014.07.020Search in Google Scholar PubMed PubMed Central
[93] Ahring BK, Schmidt JE, Winther-Nielsen M, Macario AJ, Conway, de Macario E. Effect of medium composition and sludge removal on the production, composition, and architecture of thermophilic (55 degrees C) acetate-utilizing granules from an upflow anaerobic sludge blanket reactor. Appl Environ Microbiol. 1993;59(8):2538–45.10.1128/aem.59.8.2538-2545.1993Search in Google Scholar PubMed PubMed Central
[94] Zaher U, Bouvier J, Steyer JP, Vanrolleghem P. Titrimetric montoring of anaerobic digestion: VFA, alkalinities and more. Proceedings of 10th IWA World Congress on Anaerobic Digestion (AD10); 2004. p. 1Search in Google Scholar
[95] Hulshoff Pol LW, de Castro Lopes SI, Lettinga G, Lens PN. Anaerobic sludge granulation. Water Res. 2004;38(6):1376–89.10.1016/j.watres.2003.12.002Search in Google Scholar PubMed
[96] Vallee BL, Ulmer DD. Biochemical effects of mercury, cadmium, and lead. Annu Rev Biochem. 1972;41(10):91–128.10.1146/annurev.bi.41.070172.000515Search in Google Scholar PubMed
[97] De Gusseme B, Soetaert M, Hennebel T, Vanhaecke L, Boon N, Verstraete W. Catalytic dechlorination of diclofenac by biogenic palladium in a microbial electrolysis cell. Microb Biotechnol. 2012;5(3):396–402.10.1111/j.1751-7915.2011.00325.xSearch in Google Scholar PubMed PubMed Central
[98] Van Nevel S, Hennebel T, Verschuere S, De Corte S, Boon N, Verstraete W. Palladium nanoparticles produced by fermentatively grown bacteria as catalyst for diatrizoate removal with biogenic hydrogen; 2011. p. 185–8.10.1007/s00253-011-3329-9Search in Google Scholar PubMed
[99] Zhou WP, Lewera A, Larsen R, Masel RI, Bagus PS, Wieckowski A. Size effects in electronic and catalytic properties of unsupported palladium nanoparticles in electrooxidation of formic acid. J Phys Chem B. 2006;110(27):13393–8.10.1021/jp061690hSearch in Google Scholar PubMed
[100] Leso V, Iavicoli I. Palladium nanoparticles: Toxicological effects and potential implications for occupational risk assessment. Int J Mol Sci. 2018;19(2):503.10.3390/ijms19020503Search in Google Scholar PubMed PubMed Central
[101] Hennebel T, Simoen H, De Windt W, Verloo M, Boon N, Verstraete W. Biocatalytic dechlorination of trichloroethylene with bio-palladium in a pilot-scale membrane reactor. Biotechnol Bioeng. 2009;102(4):995–1002.10.1002/bit.22138Search in Google Scholar PubMed
[102] Hennebel T, De Corte S, Vanhaecke L, Vanherck K, Forrez I, De Gusseme B, et al. Removal of diatrizoate with catalytically active membranes incorporating microbially produced palladium nanoparticles. Water Res. 2010;44(5):1498–506.10.1016/j.watres.2009.10.041Search in Google Scholar PubMed
[103] Świątkowski A. Industrial carbon adsorbents. In: Dąbrowski A, editor. Studies in surface science and catalysis. Vol. 120. Lublin, Poland: Elsevier; 1999. p. 69–94.10.1016/S0167-2991(99)80549-7Search in Google Scholar
[104] Linares-Solano A, Lillo-Ródenas M, Marco-Lozar J, Kunowsky M. Romero-Anaya A. NaOH and KOH for preparing activated carbons used in energy and environmental applications. Int J Energy Environ Econ. 2012;20:59–91.Search in Google Scholar
[105] Liu J, Zheng Y, Hong Z, Cai K, Zhao F, Han H. Microbial synthesis of highly dispersed PdAu alloy for enhanced electrocatalysis. Sci Adv. 2016;2(9):e1600858.10.1126/sciadv.1600858Search in Google Scholar PubMed PubMed Central
[106] Wei X, Zhu N, Huang X, Kang N, Wu P, Dang Z. Efficient degradation of sodium diclofenac via heterogeneous Fenton reaction boosted by Pd/Fe@Fe3O4 nanoparticles derived from bio-recovered palladium. J Environ Manag. 2020;260:110072.10.1016/j.jenvman.2020.110072Search in Google Scholar PubMed
[107] Huang B, Qi C, Yang Z, Guo Q, Chen W, Zeng G, et al. Pd/Fe3O4 nanocatalysts for highly effective and simultaneous removal of humic acids and Cr(VI) by electro-Fenton with H2O2 in situ electro-generated on the catalyst surface. J Catal. 2017;352:337–50.10.1016/j.jcat.2017.06.004Search in Google Scholar
[108] Xu J, Tang J, Baig SA, Lv X, Xu X. Enhanced dechlorination of 2,4-dichlorophenol by Pd/FeFe3O4 nanocomposites. J Hazard Mater. 2013;244–245:628–36.10.1016/j.jhazmat.2012.10.051Search in Google Scholar PubMed
[109] Aki H, Murata A, Yamamoto S, Kondoh J, Maeda T, Yamaguchi H, et al. Penetration of residential fuel cells and CO mitigation—case studies in Japan by multi-objective models. Int J Hydrog Energy. 2005;30(9):943–52.10.1016/j.ijhydene.2004.11.009Search in Google Scholar
[110] Tahernia M, Mohammadifar M, Feng S, Choi S, editors. Biogenic palladium nanoparticles for improving bioelectricity generation in microbial fuel cells. 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS); Jan. 2020. p. 18–22.10.1109/MEMS46641.2020.9056268Search in Google Scholar
[111] Wang W, Zhang B, He Z. Bioelectrochemical deposition of palladium nanoparticles as catalysts by Shewanella oneidensis MR-1 towards enhanced hydrogen production in microbial electrolysis cells. Electrochim Acta. 2019;318:794–800.10.1016/j.electacta.2019.06.038Search in Google Scholar
[112] Saravanakumar K, MubarakAli D, Kathiresan K, Thajuddin N, Alharbi NS, Chen J. Biogenic metallic nanoparticles as catalyst for bioelectricity production: A novel approach in microbial fuel cells. Mater Sci Eng B. 2016;203:27–34.10.1016/j.mseb.2015.10.006Search in Google Scholar
[113] Chaturvedi V, Verma P. Microbial fuel cell: A green approach for the utilization of waste for the generation of bioelectricity. Bioresour Bioprocess. 2016;3(1):38.10.1186/s40643-016-0116-6Search in Google Scholar
[114] Lovley DR. The microbe electric: conversion of organic matter to electricity. Curr Opin Biotechnol. 2008;19(6):564–71.10.1016/j.copbio.2008.10.005Search in Google Scholar PubMed
[115] Kumar R, Singh L, Zularisam AW. Exoelectrogens: Recent advances in molecular drivers involved in extracellular electron transfer and strategies used to improve it for microbial fuel cell applications. Renew Sustain Energy Rev. 2016;56:1322–36.10.1016/j.rser.2015.12.029Search in Google Scholar
[116] Thapa BS, Kim T, Pandit S, Song YE, Afsharian YP, Rahimnejad M, et al. Overview of electroactive microorganisms and electron transfer mechanisms in microbial electrochemistry. Bioresour Technol. 2022;347:126579.10.1016/j.biortech.2021.126579Search in Google Scholar PubMed
[117] Allen RM, Bennetto HP. Microbial fuel-cells – electricity production from carbohydrates. Appl Biochem Biotechnol. 1993;39(1):27–40.10.1007/BF02918975Search in Google Scholar
[118] Watanabe K. Recent developments in microbial fuel cell technologies for sustainable bioenergy. J Biosci Bioeng. 2008;106(6):528–36.10.1263/jbb.106.528Search in Google Scholar PubMed
[119] Matsena MT, Tichapondwa SM, Chirwa EMN. Synthesis of biogenic palladium nanoparticles using Citrobacter sp. for application as anode electrocatalyst in a microbial fuel cell. Catalysts. 2020;10(8):838.10.3390/catal10080838Search in Google Scholar
[120] Shafiee S, Topal E. When will fossil fuel reserves be diminished? Energy Policy. 2009;37(1):181–9.10.1016/j.enpol.2008.08.016Search in Google Scholar
[121] Abdalla AM, Hossain S, Nisfindy OB, Azad AT, Dawood M, Azad AK. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers Manag. 2018;165:602–27.10.1016/j.enconman.2018.03.088Search in Google Scholar
[122] Seh ZW, Kibsgaard J, Dickens CF, Chorkendorff I, Norskov JK, Jaramillo TF. Combining theory and experiment in electrocatalysis: Insights into materials design. Science. 2017;355(6321):eaad4998.10.1126/science.aad4998Search in Google Scholar PubMed
[123] Safizadeh F, Ghali E, Houlachi G. Electrocatalysis developments for hydrogen evolution reaction in alkaline solutions – A review. Int J Hydrog Energy. 2015;40(1):256–74.10.1016/j.ijhydene.2014.10.109Search in Google Scholar
[124] Yuan H, He Z. Platinum group metal-free catalysts for hydrogen evolution reaction in microbial electrolysis cells. Chem Rec. 2017;17(7):641–52.10.1002/tcr.201700007Search in Google Scholar
[125] Hou Y-N, Sun S-Y, Yang Z-N, Yun H, Zhu T-T, Ma J-F, et al. Shewanella oneidensis MR-1 self-assembled Pd-cells-rGO conductive composite for enhancing electrocatalysis. Environ Res. 2020;184:109317.10.1016/j.envres.2020.109317Search in Google Scholar
[126] Hou Y-N, Liu H, Han J-L, Cai W, Zhou J, Wang A-J, et al. Electroactive biofilm serving as the green synthesizer and stabilizer for in situ fabricating 3D nanopalladium network: An efficient electrocatalyst. ACS Sustain Chem Eng. 2016;4:5392–7.10.1021/acssuschemeng.6b00647Search in Google Scholar
[127] Zhao H, Liu X, Cao Z, Zhan Y, Shi X, Yang Y, et al. Adsorption behavior and mechanism of chloramphenicols, sulfonamides, and non-antibiotic pharmaceuticals on multi-walled carbon nanotubes. J Hazard Mater. 2016;310:235–45.10.1016/j.jhazmat.2016.02.045Search in Google Scholar
[128] Heydari H, Abdolmaleki A, Gholivand MB. Electrodeposition and characterization of palladium nanostructures on stainless steel and application as hydrogen sensor. Ciência e Nat. 2015;37:11.10.5902/2179460X20824Search in Google Scholar
[129] Bradley JC, Ma Z. Contactless electrodeposition of palladium catalysts. Angew Chem Int Ed Engl. 1999;38(11):1663–6.10.1002/(SICI)1521-3773(19990601)38:11<1663::AID-ANIE1663>3.0.CO;2-CSearch in Google Scholar
[130] Nguyen VL, Nguyen DC, Hirata H, Ohtaki M, Hayakawa T, Nogami M. Chemical synthesis and characterization of palladium nanoparticles. Adv Nat Sci Nanosci Nanotechnol. 2010;1(3):035012.10.1088/2043-6262/1/3/035012Search in Google Scholar
[131] Chen HJ, Wei G, Ispas A, Hickey SG, Eychmuller A. Synthesis of palladium nanoparticles and their applications for surface-enhanced Raman scattering and electrocatalysis. J Phys Chem C. 2010;114(50):21976–81.10.1021/jp106623ySearch in Google Scholar
[132] Hennebel T, Verhagen P, Simoen H, Gusseme BD, Vlaeminck SE, Boon N, et al. Remediation of trichloroethylene by bio-precipitated and encapsulated palladium nanoparticles in a fixed bed reactor. Chemosphere. 2009;76(9):1221–5.10.1016/j.chemosphere.2009.05.046Search in Google Scholar
[133] Pandey A, Shukla P, Srivastava P. Remediation of dyes in water using green synthesized nanoparticles (NPs). Int J Plant Environ. 2020 Jan 31;6(1):68–84.10.18811/ijpen.v6i01.08Search in Google Scholar
[134] Khataee AR, Kasiri MB. Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide: Influence of the chemical structure of dyes. J Mol Catal A Chem. 2010;328(1):8–26.10.1016/j.molcata.2010.05.023Search in Google Scholar
[135] Lade H, Kadam A, Paul D, Govindwar S. Biodegradation and detoxification of textile azo dyes by bacterial consortium under sequential microaerophilic/aerobic processes. EXCLI J. 2015;14:158–74.Search in Google Scholar
[136] Selvaraj V, Swarna Karthika T, Mansiya C, Alagar M. An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications. J Mol Struct. 2021;1224:129195.10.1016/j.molstruc.2020.129195Search in Google Scholar
[137] Bansal P, Singh D, Sud D. Photocatalytic degradation of azo dye in aqueous TiO2 suspension: Reaction pathway and identification of intermediates products by LC/MS. Sep Purif Technol. 2010;72(3):357–65.10.1016/j.seppur.2010.03.005Search in Google Scholar
[138] Wang PT, Song YH, Fan HC, Yu L. Bioreduction of azo dyes was enhanced by in-situ biogenic palladium nanoparticles. Bioresour Technol. 2018;266:176–80.10.1016/j.biortech.2018.06.079Search in Google Scholar PubMed
[139] Kopinke F-D, Mackenzie K, Koehler R, Georgi A. Alternative sources of hydrogen for hydrodechlorination of chlorinated organic compounds in water on Pd catalysts. Appl Catal A Gen. 2004;271(1):119–28.10.1016/j.apcata.2004.02.052Search in Google Scholar
[140] Ma Y, Li M, Wu M, Li Z, Liu X. Occurrences and regional distributions of 20 antibiotics in water bodies during groundwater recharge. Sci Total Env. 2015;518–519:498–506.10.1016/j.scitotenv.2015.02.100Search in Google Scholar PubMed
[141] He P, Mao T, Wang A, Yin Y, Shen J, Chen H, et al. Enhanced reductive removal of ciprofloxacin in pharmaceutical wastewater using biogenic palladium nanoparticles by bubbling H2. RSC Adv. 2020;10(44):26067–77.10.1039/D0RA03783DSearch in Google Scholar
[142] Kummerer K. Antibiotics in the aquatic environment–a review–part I. Chemosphere. 2009;75(4):417–34.10.1016/j.chemosphere.2008.11.086Search in Google Scholar PubMed
[143] Ji Y, Ferronato C, Salvador A, Yang X, Chovelon JM. Degradation of ciprofloxacin and sulfamethoxazole by ferrous-activated persulfate: implications for remediation of groundwater contaminated by antibiotics. Sci Total Env. 2014;472:800–8.10.1016/j.scitotenv.2013.11.008Search in Google Scholar PubMed
[144] Gavrilescu M, Demnerova K, Aamand J, Agathos S, Fava F. Emerging pollutants in the environment: present and future challenges in biomonitoring, ecological risks and bioremediation. N Biotechnol. 2015;32(1):147–56.10.1016/j.nbt.2014.01.001Search in Google Scholar PubMed
[145] Malakootian M, Ahmadian M. Removal of ciprofloxacin from aqueous solution by electro-activated persulfate oxidation using aluminum electrodes. Water Sci Technol. 2019;80(3):587–96.10.2166/wst.2019.306Search in Google Scholar PubMed
[146] Anjali R, Shanthakumar S. Insights on the current status of occurrence and removal of antibiotics in wastewater by advanced oxidation processes. J Environ Manage. 2019;246:51–62.10.1016/j.jenvman.2019.05.090Search in Google Scholar PubMed
[147] Mondal SK, Saha AK, Sinha A. Removal of ciprofloxacin using modified advanced oxidation processes: Kinetics, pathways and process optimization. J Clean Prod. 2018;171:1203–14.10.1016/j.jclepro.2017.10.091Search in Google Scholar
[148] Luo Y, Guo W, Ngo HH, Nghiem LD, Hai FI, Zhang J, et al. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci Total Env. 2014;473–474:619–41.10.1016/j.scitotenv.2013.12.065Search in Google Scholar PubMed
[149] Kurt A, Mert BK, Özengin N, Sivrioğlu Ö, Yonar T. Treatment of antibiotics in wastewater using advanced oxidation processes (AOPs). Physico-chemical wastewater treatment and resource recovery; 2017. p. 175.10.5772/67538Search in Google Scholar
[150] Mao Q, Zhou Y, Yang Y, Zhang J, Liang L, Wang H, et al. Experimental and theoretical aspects of biochar-supported nanoscale zero-valent iron activating H2O2 for ciprofloxacin removal from aqueous solution. J Hazard Mater. 2019;380:120848.10.1016/j.jhazmat.2019.120848Search in Google Scholar PubMed
[151] De Witte B, Dewulf J, Demeestere K, De Ruyck M, Van, Langenhove H. Critical points in the analysis of ciprofloxacin by high-performance liquid chromatography. J Chromatogr A. 2007;1140(1–2):126–30.10.1016/j.chroma.2006.11.076Search in Google Scholar PubMed
[152] Dodd MC, Buffle MO, Von Gunten U. Oxidation of antibacterial molecules by aqueous ozone: moiety-specific reaction kinetics and application to ozone-based wastewater treatment. Env Sci Technol. 2006;40(6):1969–77.10.1021/es051369xSearch in Google Scholar PubMed
[153] Li M-F, Liu Y-G, Liu S-B, Shu D, Zeng G-M, Hu X-J, et al. Cu(II)-influenced adsorption of ciprofloxacin from aqueous solutions by magnetic graphene oxide/nitrilotriacetic acid nanocomposite: Competition and enhancement mechanisms. Chem Eng J. 2017;319:219–28.10.1016/j.cej.2017.03.016Search in Google Scholar
[154] Malyan SK, Kumar SS, Singh L, Singh R, Ashok Jadhav D, Kumar V. Chapter 9 - Bioelectrochemical systems for removal and recovery of heavy metals. In: Singh L, Mahapatra DM, Thakur S, editors. Bioremediation, nutrients, and other valuable product recovery. Amsterdam: Elsevier; 2021. p. 185–203.10.1016/B978-0-12-821729-0.00005-1Search in Google Scholar
[155] Kumar M, Pakshirajan K. Continuous removal and recovery of metals from wastewater using inverse fluidized bed sulfidogenic bioreactor. J Clean Prod. 2021;284:124769.10.1016/j.jclepro.2020.124769Search in Google Scholar
[156] Patel A, Enman J, Gulkova A, Guntoro PI, Dutkiewicz A, Ghorbani Y, et al. Integrating biometallurgical recovery of metals with biogenic synthesis of nanoparticles. Chemosphere. 2021;263:128306.10.1016/j.chemosphere.2020.128306Search in Google Scholar PubMed
[157] Giller KE, Witter E, McGrath SP. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: A review. Soil Biol Biochem. 1998;30(10):1389–414.10.1016/S0038-0717(97)00270-8Search in Google Scholar
[158] Cowley A. Johnson Matthey PGM market report, February 2020. London, UK: Johnson Matthey, PLC; 2020. p. 40.Search in Google Scholar
[159] Nuss P, Eckelman MJ. Life cycle assessment of metals: A scientific synthesis. PLOS One. 2014;9(7):e101298.10.1371/journal.pone.0101298Search in Google Scholar PubMed PubMed Central
[160] Sun Z, Xiao Y, Agterhuis H, Sietsma J, Yang Y. Recycling of metals from urban mines – A strategic evaluation. J Clean Prod. 2016;112:2977–87.10.1016/j.jclepro.2015.10.116Search in Google Scholar
[161] Bourgeois D, Lacanau V, Mastretta R, Contino-Pépin C, Meyer D. A simple process for the recovery of palladium from wastes of printed circuit boards. Hydrometallurgy. 2020;191:105241.10.1016/j.hydromet.2019.105241Search in Google Scholar
[162] Fontana D, Pietrantonio M, Pucciarmati S, Torelli GN, Bonomi C, Masi F. Palladium recovery from monolithic ceramic capacitors by leaching, solvent extraction and reduction. J Mater Cycles Waste Manag. 2018;20(2):1199–206.10.1007/s10163-017-0684-3Search in Google Scholar
[163] Bigum M, Brogaard L, Christensen TH. Metal recovery from high-grade WEEE: A life cycle assessment. J Hazard Mater. 2012;207–208:8–14.10.1016/j.jhazmat.2011.10.001Search in Google Scholar PubMed
[164] Creamer NJ, Baxter-Plant VS, Henderson J, Potter M, Macaskie LE. Palladium and gold removal and recovery from precious metal solutions and electronic scrap leachates by Desulfovibrio desulfuricans. Biotechnol Lett. 2006;28(18):1475–84.10.1007/s10529-006-9120-9Search in Google Scholar PubMed
[165] Malunga K, Chirwa E. Recovery of Palladium (II) by biodeposition using a pure culture and a mixed culture. Chem Eng Trans. 2019;74:1519.Search in Google Scholar
[166] Baxter-Plant VS, Mikheenko IP, Macaskie LE. Sulphate-reducing bacteria, palladium and the reductive dehalogenation of chlorinated aromatic compounds. Biodegradation. 2003;14(2):83–90.10.1023/A:1024084611555Search in Google Scholar PubMed
[167] Hernandez-Eligio A, Pat-Espadas AM, Vega-Alvarado L, Huerta-Amparan M, Cervantes FJ, Juarez K. Global transcriptional analysis of Geobacter sulfurreducens under palladium reducing conditions reveals new key cytochromes involved. Appl Microbiol Biotechnol. 2020;104(9):4059–69.10.1007/s00253-020-10502-5Search in Google Scholar PubMed
[168] Bunge M, Sobjerg LS, Rotaru AE, Gauthier D, Lindhardt AT, Hause G, et al. Formation of palladium(0) nanoparticles at microbial surfaces. Biotechnol Bioeng. 2010;107(2):206–15.10.1002/bit.22801Search in Google Scholar PubMed
[169] Wood J, Bodenes L, Bennett J, Deplanche K, Macaskie LE. Hydrogenation of 2-Butyne-1,4-diol using novel bio-palladium catalysts. Ind Eng Chem Res. 2010;49(3):980–8.10.1021/ie900663kSearch in Google Scholar
[170] Ha C, Zhu NW, Shang R, Shi CH, Cui JY, Sohoo I, et al. Biorecovery of palladium as nanoparticles by Enterococcus faecalis and its catalysis for chromate reduction. Chem Eng J. 2016;288:246–54.10.1016/j.cej.2015.12.015Search in Google Scholar
[171] Khan M, Khan M, Kuniyil M, Adil SF, Al-Warthan A, Alkhathlan HZ, et al. Biogenic synthesis of palladium nanoparticles using Pulicaria glutinosa extract and their catalytic activity towards the Suzuki coupling reaction. Dalton Trans. 2014;43(24):9026–31.10.1039/C3DT53554ASearch in Google Scholar
[172] Surendra TV, Roopan SM, Arasu MV, Al-Dhabi NA, Rayalu GM. RSM optimized Moringa oleifera peel extract for green synthesis of M. oleifera capped palladium nanoparticles with antibacterial and hemolytic property. J Photochem Photobiol B, Biol. 2016;162:550–7.10.1016/j.jphotobiol.2016.07.032Search in Google Scholar PubMed
[173] Kumar Petla R, Vivekanandhan S, Misra M, Kumar Mohanty A, Satyanarayana N. Soybean (Glycine max) leaf extract based green synthesis of palladium nanoparticles. J Biomater Nanobiotechnol. 2012;3(1):14–9.10.4236/jbnb.2012.31003Search in Google Scholar
[174] Tahir K, Nazir S, Li B, Ahmad A, Nasir T, Khan AU, et al. Sapium sebiferum leaf extract mediated synthesis of palladium nanoparticles and in vitro investigation of their bacterial and photocatalytic activities. J Photochem Photobiol B, Biol. 2016;164:164–73.10.1016/j.jphotobiol.2016.09.030Search in Google Scholar PubMed
[175] Bhakyaraj K, Kumaraguru S, Gopinath K, Sabitha V, Kaleeswarran PR, Karthika V, et al. Eco-friendly synthesis of palladium nanoparticles using melia azedarach leaf extract and their evaluation for antimicrobial and larvicidal activities. J Clust Sci. 2017;28(1):463–76.10.1007/s10876-016-1114-8Search in Google Scholar
[176] Manikandan V, Velmurugan P, Park J-H, Lovanh N, Seo S-K, Jayanthi P, et al. Synthesis and antimicrobial activity of palladium nanoparticles from Prunus × yedoensis leaf extract. Mater Lett. 2016;185:335–8.10.1016/j.matlet.2016.08.120Search in Google Scholar
[177] Tahir K, Nazir S, Ahmad A, Li B, Ali Shah SA, Khan AU, et al. Biodirected synthesis of palladium nanoparticles using Phoenix dactylifera leaves extract and their size dependent biomedical and catalytic applications. RSC Adv. 2016;6(89):85903–16.10.1039/C6RA11409ASearch in Google Scholar
[178] Dinesh M, Roopan SM, Selvaraj CI, Arunachalam P. Phyllanthus emblica seed extract mediated synthesis of PdNPs against antibacterial, heamolytic and cytotoxic studies. J Photochem Photobiol B Biol. 2017;167:64–71.10.1016/j.jphotobiol.2016.12.012Search in Google Scholar PubMed
[179] Sarmah M, Neog AB, Boruah PK, Das MR, Bharali P, Bora U. Effect of substrates on catalytic activity of biogenic palladium nanoparticles in C–C cross-coupling reactions. ACS Omega. 2019;4(2):3329–40.10.1021/acsomega.8b02697Search in Google Scholar PubMed PubMed Central
[180] Fahmy K, Merroun M, Pollmann K, Raff J, Savchuk O, Hennig C, et al. Secondary structure and Pd(II) coordination in S-layer proteins from Bacillus sphaericus studied by infrared and X-ray absorption spectroscopy. Biophys J. 2006;91(3):996–1007.10.1529/biophysj.105.079137Search in Google Scholar PubMed PubMed Central
© 2022 Cindy Ka Y. Law et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption


![Figure 4
Fixing bio-Pd on an electrode, to increase the conductivity in a 3 – electrode system through CNT, graphene oxide, and biofilm production by Geobacter. The figure was adapted from Hou et al. [126], Cheng et al. [46], and Hou et al. [125].](/document/doi/10.1515/ntrev-2022-0482/asset/graphic/j_ntrev-2022-0482_fig_004.jpg)
