Abstract
Ion erosion and carbonization in concrete are the key factors leading to the deterioration of durability. Layered double hydroxides (LDHs) are a kind of functional material with layered structures and ion exchange properties, which can capture a variety of harmful anions in concrete pore solutions. Therefore, LDHs exhibit great potential in improving the durability of concrete as new modified material. This article reviews the recent progress of LDHs. Based on the structural characteristics of LDHs, this work discusses the binding effect of LDHs on Cl−,
1 Introduction
Concrete has become one of the most widely used artificial materials and is widely applied in the construction of civil buildings, bridges, tunnels, ports, coastal projects, and so on [1]. However, concrete structures tend to deteriorate due to reinforcement corrosion, freeze-thaw damage, carbonation, and other durability problems in complex environments (such as marine and severe cold climates) [2,3,4]. These durability problems lead to the premature failure of concrete structures and failure to achieve the expected service life [5]. Therefore, the durability enhancement of concrete structures in complex environments is essential. And this is usually accomplished by incorporating auxiliary cementitious materials, adding modifiers, controlling the content of tricalcium aluminate (C3A) in the cement, etc. In addition, the surface protection of concrete and the cathodic protection of reinforced concrete are also vital methods to improve durability.
At present, these conventional methods to enhance the durability of concrete still have some defects. For example, the concrete protective layer cannot be maintained for a long time and needs to replace periodically. And the long-term use of rust inhibitors is also not reliable. The chloride erosion, carbonation, and sulfate attack caused by the concentration of Cl−,
LDHs are layered materials with the exchange capacity of anions and “structural memory” function [6,7,8,9]. And LDHs have been widely investigated in recent years as catalysts [10,11], ceramic precursors [12], adsorbents [13,14], biological organic nano-hybrid agents [15,16], and scavengers for pollutant metals or anions [17]. LDHs have excellent properties such as high anion exchange capacity, thermal stability, structure memory effect, and large specific surface area, which are ideally suited for capturing anions such as Cl−,
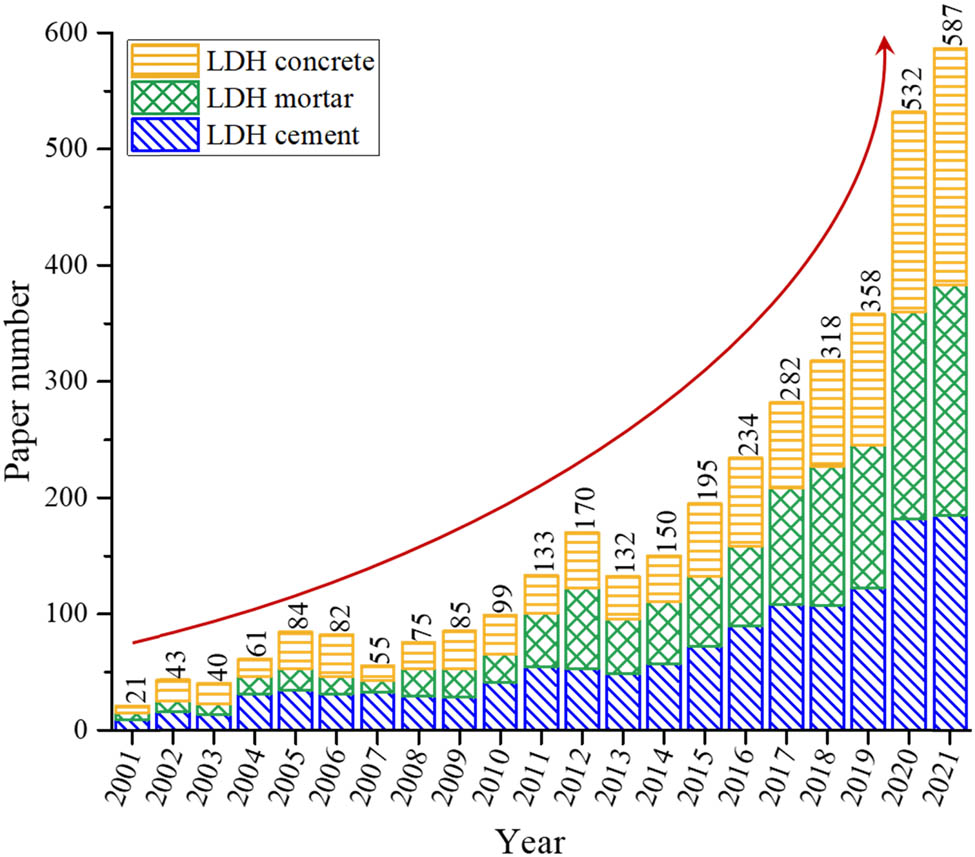
Number of research studies with keywords of LDHs cement, LDHs mortar, and LDHs concrete in the past 20 years (Elsevier database).
2 Chemical structure, ion exchangeability, and memory effect of LDHs
2.1 Chemical structure of LDHs
Some hydration products with layered structures generated in the hydration process of cement, such as the aluminate ferrite mono (AFm) phase, are considered hydrotalcite-like phase, namely LDHs [18]. The stability of AFm phase plays a vital role in controlling the performance of concrete [19]. Chloride ions can interact with cement hydration products of cement to form chloraluminate phase such as Friedel’s salt (3CaO·Al2O3·CaCl2·10H2O) and Kuzel’s salt (3CaO·Al2O3·0.5CaCl2·0.5CaSO4·11H2O) [20]. Furthermore, the addition of admixtures with high Mg content, such as coal gangue [21], calcined dolomite [22,23], blast furnace slag [24,25,26,27,28], alkali activated paste [29], and MgO [30], can promote the formation of LDHs in the process of cement hydration. And the Mg element [28] contained in cement clinker can also produce MgAl–LDHs. The presence of more LDHs gives the cement a more excellent anionic curing ability [31]. Therefore, it has become a promising research trend to enhance the ability of cement-based materials to cure anions (Cl−,
In common, the general formula of LDHs can be expressed as
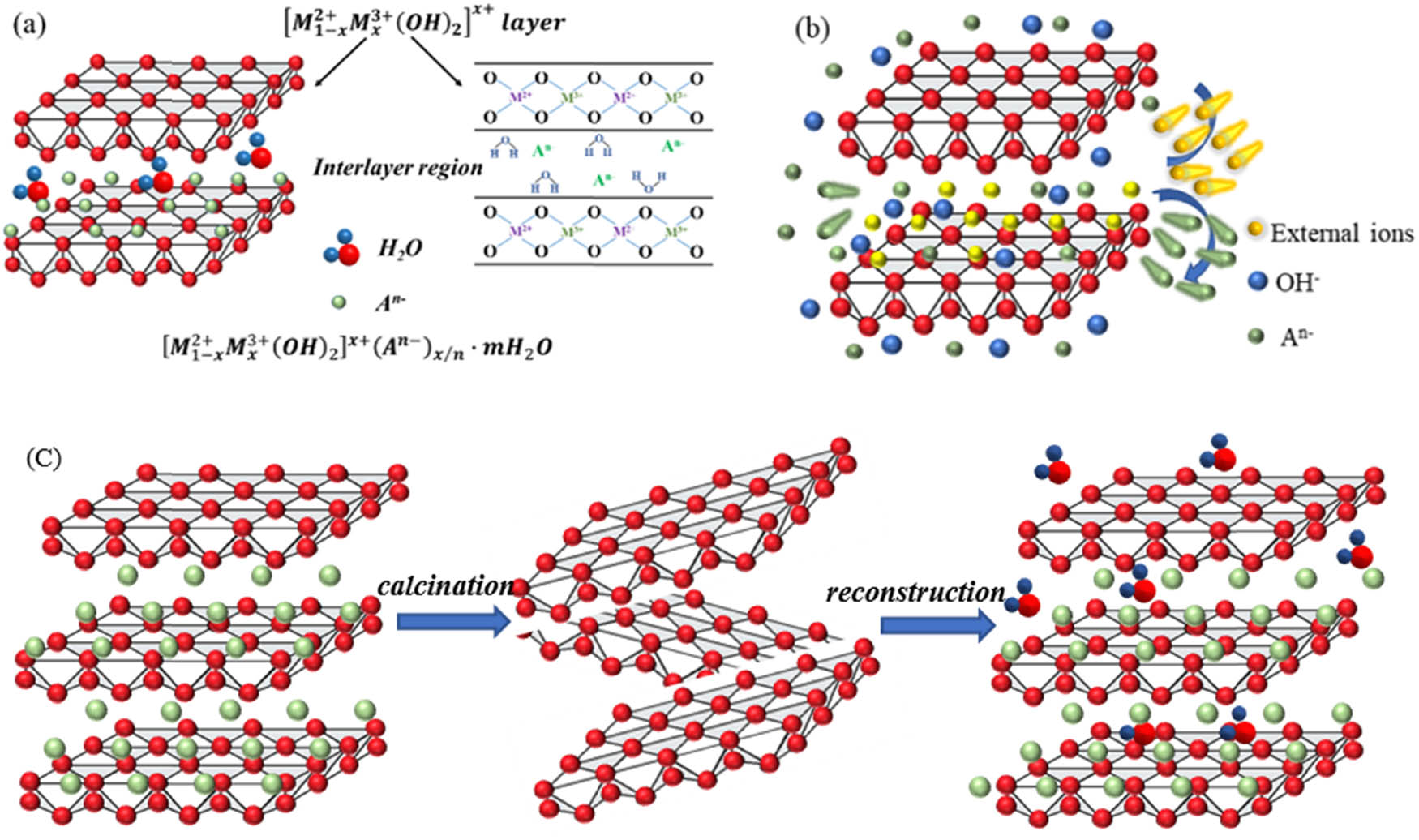
Schematic diagram of a typical structure of LDHs: (a) basic structure, (b) ion exchange of LDHs, and (c) calcination and structure reconstruction of LDHs.
2.2 Ion exchangeability and memory effect of LDHs
The effect of LDHs in cement-based materials depends on their unique chemical structure, which mainly includes the exchangeability of interlayer anions and memory effect.
2.2.1 Exchangeability of the ions between LDHs layers
Anions in the inner layer of LDHs can exchange anions existing in the cement environment based on the inherent nature and unique chemical structures of LDHs. This process is determined by the variation reduction direction of the chemical potential in Gibbs free energy. And the charge balance is maintained during ion exchange to ensure electrical neutrality [9,42,43,44,45,46,47,48,49]. This property is known as the ion-exchangeability of LDHs, as shown in Figure 2b. The ion exchange properties of LDHs can also be used to produce LDHs with the desired anion by placing the LDHs in a solution of the desired anion. Note that anion adsorption depends on many factors, such as temperature [50,51], particle size [52], anion type [52,53], and anion charge [54].
The fundamental mechanism for LDHs to capture anions in cement-based materials lies in the ion exchangeability between their layers. The anions between LDHs layers are connected by weak hydrogen bonds, and the anions between LDHs layers can exchange with other anions, resulting in the change of charge density and electrostatic interaction between the laminate and the interlayer anions, thus changing the layer spacing and allowing LDHs to obtain a new intercalation structure (as shown in Figure 2b). The research indicates that anions carrying higher charges are more likely to enter the interlayer and be trapped, while those holding lower charges are more likely to escape. Therefore, the order of anion exchangeability (i.e., the stability of the new structure) of LDHs is [53]:
2.2.2 Memory effect
Another crucial factor affecting the adsorption of anions by LDHs is the memory effect. When LDHs thermally decompose below 200°C, only the surface adsorbed water and interlayer water are lost, and the lamellar structure is not affected. When LDHs are at temperatures of 250–450°C, the hydroxyl groups of the laminate start to detach gradually, while the interlayer water is further lost [54]. LDHs calcined at 450–600°C will lose the layered structure [55] and form highly active composite metal oxides with high thermal stability, large specific surface area, good basic properties, and small crystal size. It has high anti-sintering stability even under extreme conditions [56]. Research has also shown that this product can reconstruct the original layer structure by rehydration and adsorption of anions. However, Miyata’s work [57] showed that the solid solution deviates into spinel when the calcination temperature is above 800°C, resulting in the non-reconstruction of LDHs. Overall, the calcination temperature is the critical parameter affecting the memory effect [58]. It must be high enough to eliminate most of
As shown in Figure 2c, the calcination products of LDHs are a calcined layered double hydroxide (CLDH) during heating (usually not exceeding 600°C) [55]. CLDHs can adsorb water and anion to the interlayer to balance the positively charged laminate and reconfigure its laminar structure. Based on the structural memory effect of CLDHs, it can work as an anion adsorbent [59], which can adsorb anions from cement-based materials into the interlayer and reconstruct the lamellar structure of LDHs. Due to the increase in specific surface area and pore volume of CLDHs during calcination, their ability to adsorb ions is better than that of LDHs, which gives the cement-based materials a superior erosion resistance.
3 Ion binding effect of LDHs on cement-based materials
Chloride penetration, concrete carbonation, sulfate attack, freeze-thaw damage, and wave impact are the main factors that induce the deterioration of concrete structures. And the chloride penetration and concrete carbonation are the most critical factors leading to reinforcement corrosion. Cl−,
3.1 Binding effect of LDHs on Cl− in cement-based materials
Resistance to chloride penetration is one of the crucial parameters for evaluating the durability of concrete. Chloride ions invade concrete through pores and then react with hydration products of cement and crystallize, thus changing the composition and microstructure of concrete and deteriorating its performance. Several works have shown that [60] chloride ions in concrete pore solution can combine with hydrated calcium silicate (C–S–H) and AFm [60,61,62]. Note that AFm also belongs to LDHs and is an effective chloride adsorbent, which means that LDHs also have the potential to be used as chloride adsorbents for concrete.
Several researchers have investigated the effect of LDHs on the resistance of cement-based materials to chloride penetration by simulating concrete pore (SCP) solutions. Wu’s work [58] found that adding An-LDH to the SCP solution would lead to the replacement of the inhibitor ions in the LDHs layer by chloride ions and thus a decrease in the concentration of free chloride ions in the SCP solution. The corrosion inhibition efficiency of An-LDH for carbon steel in the SCP solution is NO2-LDH > NO3-LDH > C6H5COO-LDH > CrO4-LDH. Research also showed that the two classic hydration products (Mg–Al LDHs, AFm) in alkali-activated slag cement pore solutions have different chloride ion adsorption mechanisms [63]. Surface adsorption is the dominant chloride ion binding mechanism for Mg–Al LDHs, accounting for about 90% of the total chloride ion binding, while the ion exchange accounts for about 10%. In contrast, the surface adsorption of chloride ions by AFM is not significant. Tang et al. [64,65] investigated the effect of CLDHs on the corrosion behavior of reinforcement in a neutralized SCP solution containing chloride ions and found that CLDHs can adsorb some chloride ions and release hydroxide. The release of hydroxide raises the pH of the solution, which can further inhibit the reinforcement corrosion. In simulated carbonated concrete pore solutions (with chloride ions), the corrosion inhibition mechanism of LDHs with nitrate intercalation lies mainly in the exchange of
In addition, the improvement of chloride permeation resistance for concrete by LDHs is related to its dosage and particle size. The appropriate amount of LDHs can refine the pore structure and reduce the pore connectivity, thus decreasing the chloride permeability. On the contrary, the overabundance of LDHs will increase the permeability and porosity of concrete and accelerate the chloride penetration [67]. Current studies have found that the appropriate content of LDHs is within 2% [51,68,69,70]. Moreover, the smaller the particle size of LDHs, the more significant its enhancement of the chloride penetration resistance for concrete, which can attribute to the filling effect of LDHs on the pores [51].
Regarding the comparison between LDHs and some auxiliary cementitious materials for chloride binding ability, researchers concluded that CLDHs > cement > slag > fly ash > silica fume [67,70,71,72]. CLDHs can adsorb chloride ions and moisture through structural memory effect and produce OH− to increase alkalinity during the reconstruction of the laminar structure, which can protect reinforcement very well. In addition, slag has the characteristics of high activity and fast pozzolanic reaction, and the generated C–S(A)–H gel is more favorable for the physical adsorption and chemical binding of chloride, so its adsorption efficiency is higher than that of fly ash and silica fume.
Noted that the binding ability of LDHs to chloride ions was related to the type of laminate metal and the ratio of laminate metals. Research showed that the chloride binding capacity of Zn–Al–LDHs increased with decreasing Zn2+/Al3+, and the best binding capacity occurred when Zn2+/Al3+ was 2. The reason is that the smaller value of Zn2+/Al3+, the more charge of Al3+, and the higher charge density on the stack lead to a better anion adsorption capacity.
Besides, research also showed that the higher the concentration of chloride ions in the external environment, the higher the chloride ion binding rate of LDHs. Therefore, the bound behavior of LDHs to chloride ions can describe by Freundlich or Langmuir adsorption isotherms [73,74]. In the two isotherms, the absorption increases with the concentration of free chloride ions and finally reaches the saturated adsorption state. Note that the ion exchange capacity of LDHs is affected by many factors, such as the type and particle size of LDHs, the concentration of chloride ions in the external environment, etc. But the most critical factor that determines ion exchange is the selectivity order of LDHs (mentioned in Part 2).
3.2 Binding effect of LDHs on
SO
4
2
−
in cement-based materials
It is generally believed that sulfate reacts with the hydration products of cement in concrete to form expansive substances, which makes the hardened concrete crack and causes damage. The damage caused by ettringite is the most common type of sulfate attack. Ettringite can combine with large amounts of crystalline water to produce needle-like/rod-like ettringite crystals. This process creates internal stresses and induces concrete cracking. Cracks exacerbate the diffusion process of harmful substances (corrosive substances, air, moisture, etc.) into the concrete, leading to reinforcement corrosion, structural deterioration, and loss of load-bearing capacity [75,76,77,78,79].
In recent years, a great many studies have shown that LDHs can be used as anionic adsorbents, and the calcined products have the characteristics of uniform structure, large specific surface area, and memory effect. Some scholars have investigated the adsorption effect of LDHs on the aqueous solution, groundwater, high concentration sulfate wastewater, and soil [80,81,82,83]. These researches further support the adsorption mechanism of LDHs and CLDHs on sulfate ions as ion exchange and memory effects, respectively (as shown in Figure 3). LDHs can remove up to 90% of sulfate ions under appropriate conditions [84], which means that LDHs are very promising in the high concentration sulfate treatment.
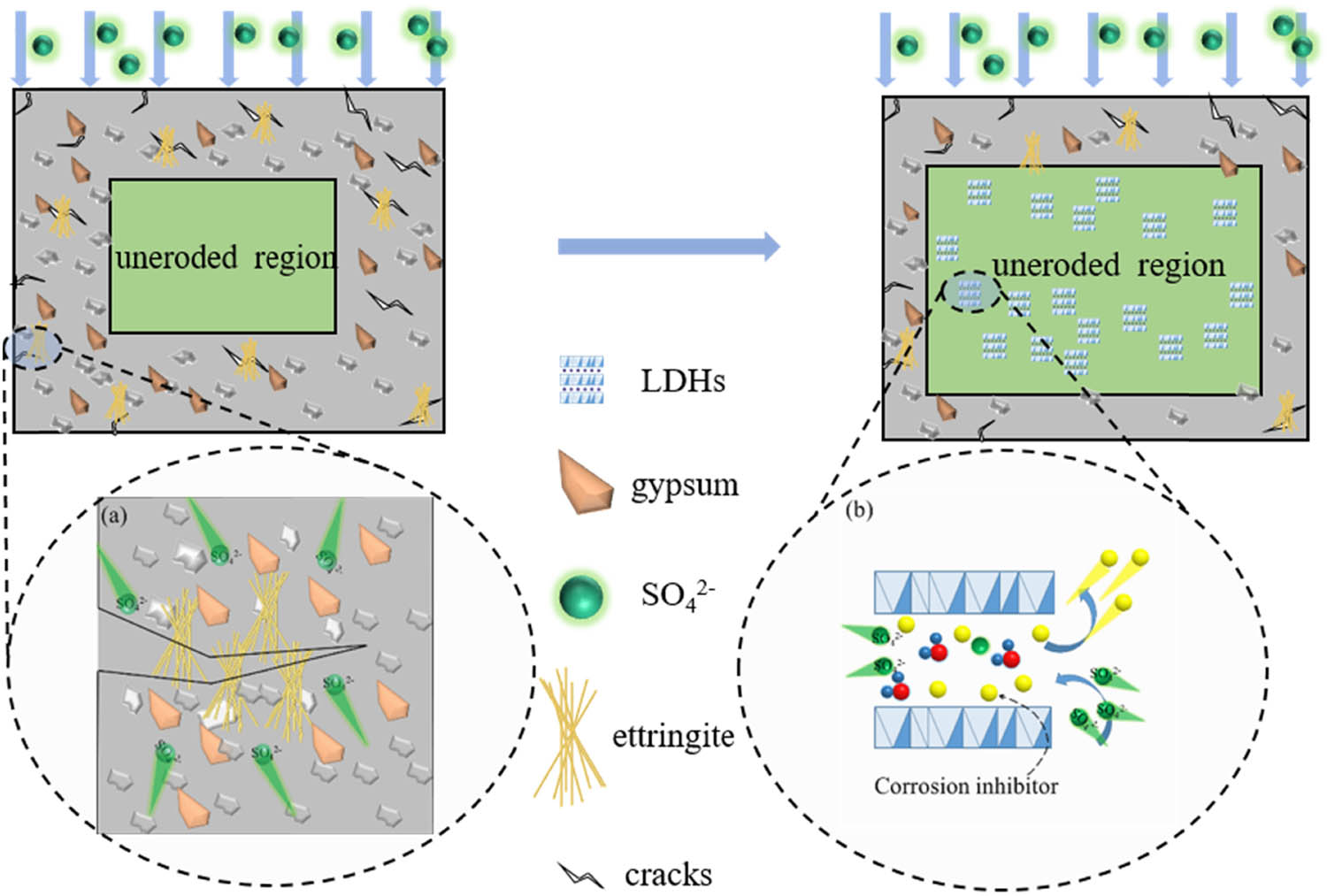
The schematic illustration of sulfate attack induced crack and the modification of LDHs in concrete: (a) the schematic illustration of crack induced by sulfate attack; (b) modification mechanism of LDH on sulfate.
Research showed that CLDHs have higher sulfate ion adsorption efficiency than uncalcined LDHs due to a large interlayer vacancy [85,67,86,87,88]. Chen et al. [87] added Mg–Al–CO3 LDHs and metakaolin (MK) as modifiers to concrete and found that they could adsorb
![Figure 4
Evolutions of corrosion potentials of steel specimens in the solutions: (a) with the Mg–Al LDHs; (b) without the Mg–Al LDHs [85].](/document/doi/10.1515/ntrev-2022-0478/asset/graphic/j_ntrev-2022-0478_fig_004.jpg)
Evolutions of corrosion potentials of steel specimens in the solutions: (a) with the Mg–Al LDHs; (b) without the Mg–Al LDHs [85].
3.3 Binding effect of LDHs on
CO
3
2
−
in cement-based materials
The carbonation of concrete can destroy the passive film of reinforcement and diminish the protection of concrete to reinforcement, thus leading to reinforcement corrosion. Industrial wastes, such as blast furnace slag and fly ash, are already widely used as supplementary cementitious materials for concrete. Research has shown that using fly ash or slag powder to replace 50% or higher proportion of cement to prepare concrete reduced the alkalinity and carbonation resistance of concrete and increased the risk of steel corrosion [90]. The anions between the layers of LDHs can exchange with the anions in the solution [89], and the order of the exchange capacity is
Several researchers have also investigated the binding mechanism of LDHs to
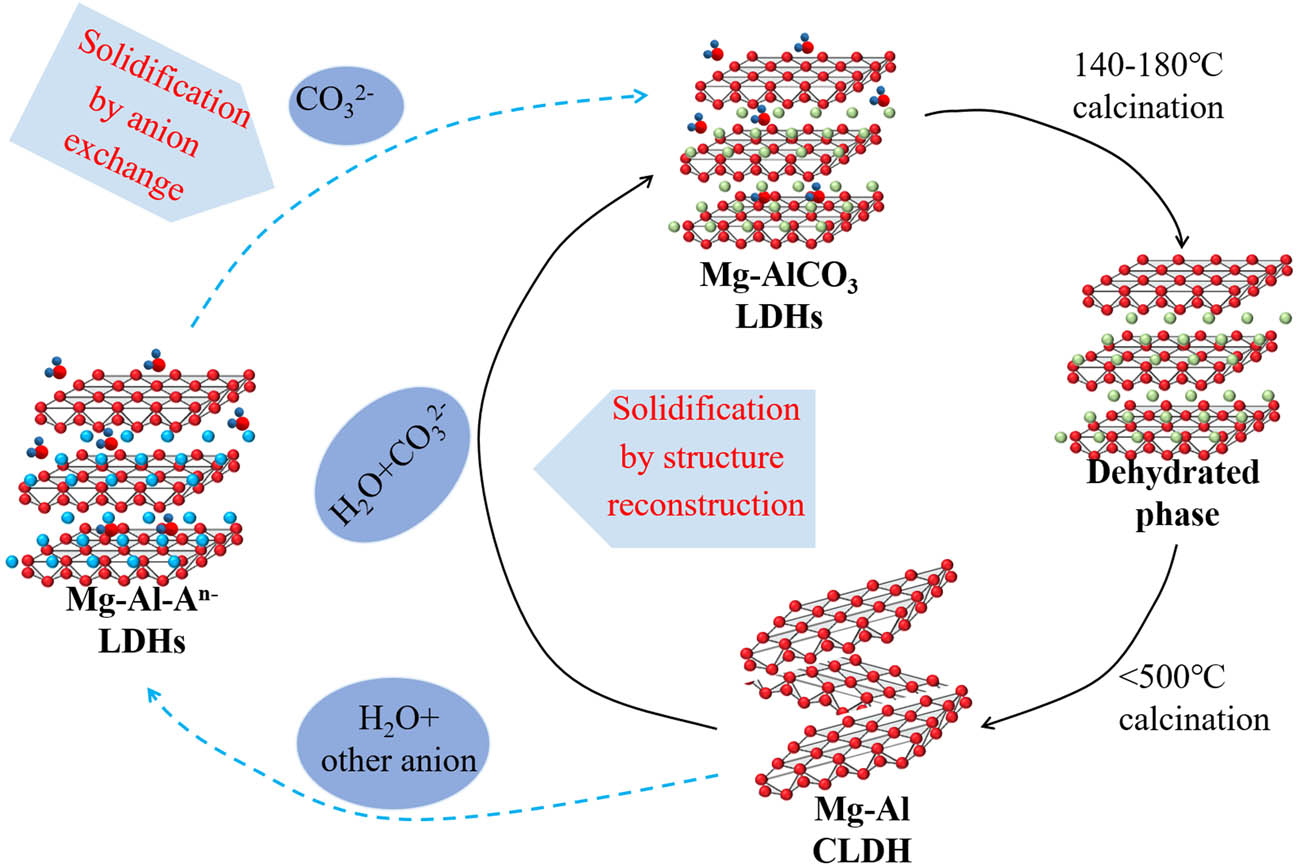
Process of thermal decomposition, structural reconstruction, and
![Figure 6
Carbonation depth of the designed concrete with LDHs materials at different curing days [93].](/document/doi/10.1515/ntrev-2022-0478/asset/graphic/j_ntrev-2022-0478_fig_006.jpg)
Carbonation depth of the designed concrete with LDHs materials at different curing days [93].
4 Effects of LDHs on the physical and mechanical properties of cement-based materials
As previously summarized, the ion exchange properties of LDHs ensure the exchange of interlayer ions with the anions in the pore solution of the cement-based material. Therefore, LDHs can be used to adsorb harmful anions from the pore solution of cement-based materials. However, there are still some concerns about the use of LDHs in cementitious materials: (1) LDHs has a small particle size and large specific surface area, which can fill the pores and exert a “filling effect” and “nucleation effect.” The filling effects caused by the addition of LDHs are often beneficial to the physical and chemical properties of cement-based materials [98]. (2) In general, the incorporation of nanoparticles reduces the energy barrier to liquid-phase precipitation reactions [99]. Therefore, it is necessary to summarize the effect law of LDHs on cement hydration, degree of hydration, and microstructure of cement paste.
4.1 Effect of LDHs on the physical properties of cement-based materials
Research has shown that a larger dose of LDHs and CLDHs makes agglomeration more likely to occur in cement pastes, which can attribute to their large specific surface area and absorbing a large amount of mixed water and some free water [100,101]. LDHs and CLDHs can absorb water from the external environment through surface adsorption and interlayer water storage functions, thus reducing the fluidity of the cement paste. Correspondingly, the water in the cement paste decreases, leading to an increase in the consistency of the cement paste and a decrease in the setting time [102]. On the other hand, the reduction of cement paste fluidity by LDHs also relates to its lamellar structure, which can significantly reduce the fluidity of cement. LDHs are less effective than CLDHs in reducing cement setting time, which can attribute to the presence of water molecules and anions in the interlayer of LDHs, thus making LDHs less absorbent than CLDHs [103]. The specific surface area [104,105] and pore volume of CLDHs increase significantly after calcination, and they can absorb lots of water through physical adsorption and memory effects. Therefore, the incorporation of CLDHs shows a significant influence on the setting time of cement. In addition, the addition of LDHs can bind some sulfate ions (from gypsum), which accelerates the hydration and shortens the setting time (as shown in Figure 7) [106]. Ma et al. [107,108,109] investigated the effect of LDHs–MK on concrete collapse. The results showed that the workability of concrete significantly decreased due to a large amount of water adsorbed by the layered structure of LDH and MK. But the “rolling bearing effect” generated by the combination of ultrafine fly ash and limestone powder can effectively compensate for the loss of concrete workability.
![Figure 7
Effect of LDHs on setting time of cement paste [106].](/document/doi/10.1515/ntrev-2022-0478/asset/graphic/j_ntrev-2022-0478_fig_007.jpg)
Effect of LDHs on setting time of cement paste [106].
4.2 Effect of LDHs on the mechanical properties of cement-based materials
The particle size of LDHs has significant effect on the mechanical properties of cement-based materials. Research has found that the smaller the particle size of LDHs, the higher the early compressive strength, which can be attributed to the improvement of the pore structure by the filling effect [110]. Another work also showed [111] that the smaller the particle size of LDHs, the easier it is to fill the cement pores, thus promoting the hydration reaction of tricalcium silicate (C3S) and increasing the early strength of the cement paste (as shown in Figure 8(d)). Note that with the increase in dosage, LDHs will cover the surface of cement particles, thus hindering the hydration of cement particles and decreasing the growth rate of strength gradually. On the other hand, when incorporating LDHs with large particle size and high crystallinity, the filling effect of LDHs is weaker. When their dosage is low, the “nucleation effect” promotes the local hydration of C3S and generates a large amount of AFm covering the surface of C3S, thus hindering its further hydration and adverse to the early strength. When adding more LDHs, LDHs adsorbed
Table 1 summarizes the relevant research on the effect of incorporation of LDHs on the mechanical properties of cement-based materials. Duan et al. [114] found that incorporating 1% O-LDHs increased the compressive strength while 1% C-LDHs was detrimental, but the dosage and type of LDHs had a less significant impact on the compressive strength. Li et al. [102] pointed out that 1–2% of LDHs can greatly improve the compressive strength of C30 concrete. Because LDHs have a unique layered structure and a large specific surface area, which can absorb water through surface adsorption and reduce the water-cement ratio [9]. In addition, LDHs can fill in pores and reduce the content of harmful pores, thus improving the pore structure and increasing the compressive strength of concrete. But excessive LDHs will make the aggregate cannot cement effectively, which is detrimental to the concrete strength.
Effect of LDHs on compressive strength of cement-based materials
| Parameters of LDHs | Material type | Compressive strength | Age | Ref. | ||
|---|---|---|---|---|---|---|
| Type of LDHs | Preparation methods | Dosage | ||||
| Calcined Mg–Al CO3 | Co-precipitation method | 2% | Concrete | Loss reduced by 5% | 28 days | [89] |
| Calcined Mg–Al CO3 | SNAS method | 1% | UHPC | +8.5% | 56 days | [67] |
| 2% | −6.8% | |||||
| Mg–Al NO3 | Co-precipitation method | 2% | AAFS | −5% | 3 days | [70] |
| +1% | 28 days | |||||
| Calcined Mg–Al CO3 | — | 4% | Seawater–sea sand concrete | +11% | 28 days | [71] |
| Calcined Mg–Al CO3 | Co-precipitation method | 3% | Cement | +40% | 3 days | [106] |
| +3% | 28 days | |||||
| Li–Al CO3 | Solvothermal method | 3% | CSA cement | +19% | 28 days | [110] |
| Ca–Al NO3 | Co-precipitation method | 0.1% | Cement | +27.7% | 1 days | [111] |
| −2.4% | 28 days | |||||
| Mg–Al CO3 | Co-precipitation method | 1% | SAC concrete | +5% | 28 days | [114] |
| Calcined Mg–Al CO3 | 1% | 6.5% | ||||
| Calcined Mg–Al CO3 | — | 2% | Concrete | +25.3% | 28 days | [102] |
| 4% | +10.3% | |||||
| Mg–Al CO3 | — | 5 kg/m3 | Concrete | +13.8% | 28 days | [119] |
| Li–Al | SNAS method | 2% | CSA cement | +98.4% | 1 day | [120] |
| +25.9% | 60 days | |||||
| Ca–Al | SNAS method | 2% | Mortar | +49% | 1 day | [121] |
| +6% | 28 days | |||||
Note: SNAS method is to separate nucleation and aging steps.
A variety of supplementary cementitious materials are used in the concrete structure in the project to improve the performance of concrete and save energy [116,117,118]. Scholars have studied the mechanical properties of LDHs with different supplementary cementitious materials. Liu et al. [70] studied the effects of MgO, LDHs, and CLDHs content on alkali-activated fly ash slag (AAFS) and slag blends and found that the addition of MgO and CLDHs increased the compressive strength of AAFS mainly due to the refinement of mesopores and the decrease of porosity, while LDHs caused minor effects on the compressive strength. Chen et al. [67] prepared ultrahigh-performance concrete (UHPC) using cement, fly ash, and silica fume as raw materials with the addition of CLDHs. They found that adding 1% CLDHs increased the compressive strength of the specimens from 145 to 157 MPa after curing for 56 days. And when the dosage of CLDHs was 2%, the compressive strength decreased due to the increased harmful pores. Qiao et al. [71] investigated the impact of incorporating Mg–Al–CLDH on the mechanical properties of seawater marine sand concrete containing fly ash and slag. On the one hand, CLDHs combined part of chloride ions through structural reconstruction, which weakened the early strength effect of chloride ions; on the other hand, as an ultrafine powder, CLDHs can fill pores and improve the pore structure, thus enhancing the latter strength of seawater marine sand concrete.
In summary, the laminate metal, particle size, crystallinity, and dosage of LDHs all affect the mechanical properties of cement-based materials. The smaller the particle size of LDHs, the more significant the positive effect on strength. The mechanism is that LDHs can fill the pores and improve the pore structure of cement-based materials. The effect mechanism of crystallinity on mechanical properties is that LDHs can act as seeds. And the incorporation of LDHs can provide nucleation points for cement hydration products and promote early hydration, thus improving the strength of cement-based materials. The dosage of LDHs has a significant effect on the strength of concrete-based materials. The high dosage can cause the agglomeration of particles, which is detrimental to strength development; 2% is a safe amount of LDHs in cement-based materials.
4.3 Effect of LDHs on hydration and hardening properties of cement-based materials
The hydration mechanism of cementitious materials is the basis for its application. The hydration mechanism and the hardening process differ for different cementitious materials. The current research focuses on the effect of LDHs on the hydration of CSA cement. The precipitation-dissolution equilibrium exists in the aqueous solution of LDHs, and the dissolved ions in CSA cement paste vary with the laminate elements of LDHs. Different metal ions have various effects on the hydration process of CSA cement. For example, Li+ has a significant role in promoting the hydration process of CSA cement, but Zn2+ retards the setting of cement [120]. In the early hydration stage of cement, Zn2+ ions can combine with Ca2+ to form Zn–Ca complexes such as Ca(Zn(OH3)2)·2H2O, leading to a decrease in the concentration of Ca2+ and OH− in the pore solution, thus delaying the hydration of cement. However, there is also research showing that Li–Al, Mg–Al, Zn–Mg–Al, and Zn–Mg–Al LDHs contribute to the hydration of CSA cement. It can attribute to the fact that LDHs can also act as seeds, reducing the amount of nucleation during the formation of hydration products and thus accelerating the hydration process. Therefore, the promotion of hydration of CSA cement by LDHs may be the result of the combination of crystalline nucleation and interlayer metal ions [106,122].
Recently, Xu et al. [121] found that the addition of CaAl–LDHs accelerates the formation of hydration products (especially for C–S–H), thus increasing the early strength. Guan et al. [123] found that the early hydration heat rate and total heat release rate of CSA cement clinker increased with the increasing of LiAl–LDHs dosage. The addition of LiAl–LDHs accelerated the early hydration, shortened the setting time of CSA cement paste, and no new phase formed in this hydration system. At the same time, Li–Al–LDHs can work as a nucleation site for hydration products, reducing the energy barrier of the hydration product precipitation process, thus accelerating the hydration process and increasing the hydration products [124]. Based on previous research, Ke et al. [8] used CLDHs to control the reaction kinetics of sodium carbonate-activated slag cement. The results showed that adding 10% CLDHs could significantly accelerate the reaction kinetics. Because adding CLDHs accelerated the consumption of carbonate and increased the pH value, thus promoting the dissolution of slag and reducing the water–cement ratio. In summary, the synergistic effects of interlayer metal ions and nucleation effects of LDHs promote (or delay) cement hydration. Adding LDHs to cement-based materials cannot form new hydration products but increases the number of hydration products. The nucleation effect of LDHs reduces the precipitation energy barrier of hydration products and promotes the hydration of cement (Figure 9), leading to a more compact microstructure of hydration products.
![Figure 9
Hydration heat flow and total heat released from cement pastes with LDHs [106].](/document/doi/10.1515/ntrev-2022-0478/asset/graphic/j_ntrev-2022-0478_fig_009.jpg)
Hydration heat flow and total heat released from cement pastes with LDHs [106].
5 Durability of LDHs modified concrete materials
Concrete often suffers from chloride attack, sulfate attack, reinforcement corrosion, and carbonation during service. LDHs have the effect of capturing Cl−,
Effect of LDHs on the durability of concrete
| Type of LDHs | Test indicator | Durability performance | Ref. |
|---|---|---|---|
| Ca–Al–NO3 | D RCM | 1% of LDHs is the best content to improve the chloride transport resistance and the D RCM is reduced by 25% | [51] |
| Calcined Mg–Al | D RCM | UHPC with 1% C-LDHs has the densest structure and the best resistance to chloride ion penetration | [67] |
| Mg–Al–NO3 and Calcined Mg–Al | D RCM | LDHs and CLDHs can improve the chloride resistance of AAFS and CLDHs show the highest chloride binding capacity | [70] |
| Calcined Mg–Al | Expansion of cement mortar | Addition of 1% CLDHs reduced the expansion rate of cement mortar by 15%, and the addition of 2% CLDHs reduced the expansion rate by 25% | [86] |
| Calcined Mg–Al | Carbonation depth | Carbonation depth of cement mortar with 2% LDHs decreased by 62.3% compared with that without LDHs | [87] |
| Calcined Mg–Al | Carbonation depth | Carbonation depth of concrete with CLDHs at 42days is 30% lower than that of the reference sample | [91] |
| C-LDHs | Carbonation depth | When 4% C-LDHs are added to the concrete, the carbonation depth is reduced by more than 50% compared with the reference sample | [93] |
| Mg–Al LDHs | Carbonation depth | Carbonation depth decreased with the addition of LDHs, indicating the improvement effect of LDHs on carbonation resistance of concrete | [94] |
| O-LDHs and C-LDHs | Carbonation depth | Both O-LDHs and C-LDHs can enhance the carbonation resistance of concrete at all ages | [114] |
Note: D RCM is chloride migration coefficient.
The experimental results from Shui’s work [93] demonstrate that the carbonation depth is more than 50% lower than that of the reference sample when CLDHs are added to concrete by about 4%. Ma et al. [91] concluded that incorporating 2% CLDHs into concrete resulted in a 30% lower carbonation depth at 42 days than the reference sample. LDHs can release the anti-corrosive anions from its layers and improve the pH of the pore solution by adsorbing Cl−,
Note that most works focus on the ability and mechanism of anion adsorption by LDHs under different conditions [125,126,127]. However, the harmful anions remain inside the concrete after being adsorbed by LDHs. LDHs only convert the free ions into bound ions, temporarily limiting the migration of ions in the concrete. The corrosive anions immobilized by LDHs are likely to release as free ions with environmental changes during the long service of concrete. Therefore, it is worth considering how to prevent the re-release of aggressive anions immobilized by LDHs during the long-term use of concrete.
6 Corrosion inhibition effect of LDHs on reinforcement in reinforced concrete
Reinforcement corrosion is a crucial problem leading to the deterioration of the durability of reinforced concrete structures. The main factors inducing reinforcement corrosion are the increased concentration of free chloride ions and the decrease in pH in the concrete pore solution (especially at the interface between reinforcement and concrete). The reinforcement corrosion in concrete has caused enormous economic losses and serious accidents, so it is essential to find an effective way to delay the reinforcement corrosion. Adding corrosion inhibitors to concrete is an excellent, economical, and widely applicable corrosion prevention measure. However, most commercial corrosion inhibitors have the disadvantage of single function and environmental unfriendliness. Researchers have paid great attention to developing more effective and green protection against corrosion for reinforcement [128,129], and incorporating LDHs into concrete is promising. Most works on the use of LDHs in concrete have been devoted to delaying the corrosion of reinforcement, either in chloride eroded [130] or in carbonated concrete [63,131,132]. And most research has been conducted on SCP solutions [43,47,58,133], with little research on mortars [47,134] and concrete [135]. Researchers usually also investigate the compatibility and mechanical properties of the mortar or concrete containing LDHs [48,92,99,104].
Current research prefers to develop modified LDHs (MHT) that can effectively alleviate reinforcement corrosion and explore the modification mechanism to enhance the corrosion inhibition of LDHs [16,17]. Research has shown that the hydroxyl ions embedded in MHT can exchange with free chloride ions in the concrete pore solution, thus reducing the free chloride concentration [32]. In addition, some inhibitory organic anions embedded in LDHs may release automatically when chloride ions invade [33,42]. This inhibition effect increased the threshold of chloride-induced corrosion and reduced the corrosion rate. The electrochemical potential was weaker in the simulated concrete pore solution containing MHT than in the solution without MHT. In summary, ion exchange between chloride ions and LDHs embedded groups (e.g., methyl para-aminobenzoate and nitrite) in the simulated concrete solution reduced the number of free chloride ions [45,64,65,85,137] and released anionic groups with anti-corrosive properties [58,66,138], which increased the pH of concrete [47] and the transport resistance to the aggressive ion, thus protecting reinforcement from corrosion (the mechanism shown in Figure 10(b)).
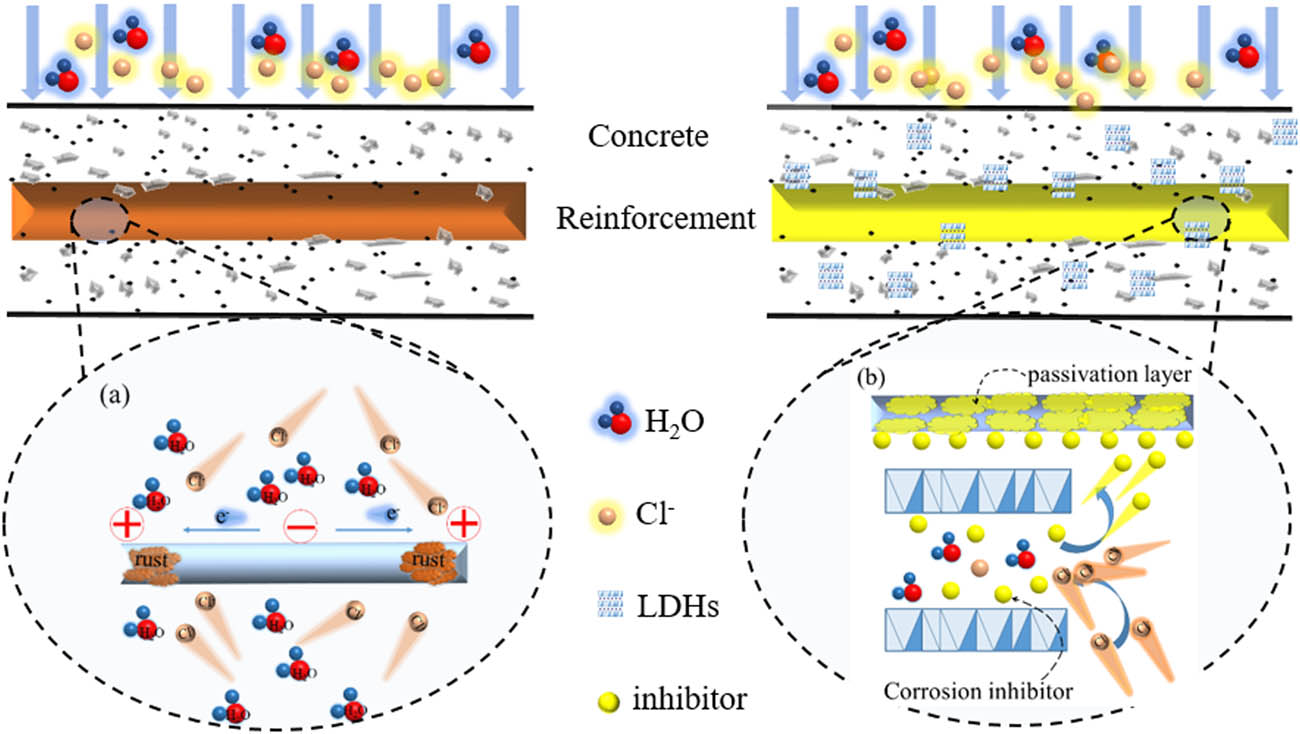
Chloride ions corrosion of reinforcement and corrosion inhibition mechanism of modified LDHs: (a) reinforcement corrosion induced by chloride ions; (b) dual-role mechanism of modified LDHs in reinforced concrete exposed to chloride ions.
Tang et al. [65] investigated the protective effect of CLDHs on reinforcement in SCP solutions and found that CLDHs can effectively adsorb Cl− and raise the pH. Yang et al. [136] prepared modified Mg–Al–LDHs doped with pAB (para-aminobenzoate) and NO2 and incorporated it into cement mortar. The results showed that adding 5% Modified hydrotalcites-para-aminobenzoate (MHT-pAB) to the mortar can significantly improve the resistance to chloride ion diffusion. Thus, the longer service life of reinforced mortar/concrete structures may obtain by using MHT-pAB. Tian et al. [45] suggested that Zn–Al–NO2 LDHs are available as a corrosion inhibitor for reinforcement in chloride-attacked concrete. And the Zn–Al–NO2 LDHs exhibited better chloride ion inhibition than NaNO2.
Although the corrosion rate due to carbonation is much lower than that induced by chloride ingress, the combined chloride ingress and carbonation will make the corrosion process more complex. And the corrosion risk due to the combined effect is much higher than that induced by the two causes alone [2,51]. Xu et al. [66] investigated the hindering effect of Mg–Al–NO2–LDHs on reinforcement corrosion caused by carbonation, chloride attack, and coupling two. The results showed that the inhibition of reinforcement corrosion by Mg–Al–NO2–LDHs under carbonation was better than that under chloride intrusion and the coupling. The inhibition mechanism for carbonation is mainly attributed to the increased alkalinity and
Summary of reinforced concrete with LDHs
| Type of LDHs | Type of samples | Results | Ref. |
|---|---|---|---|
| Zn–Al–NO2 | Steel reinforcements | Compared with NaNO2, Zn–Al–NO2 LDHs increased the chloride threshold value from 0.15 to 0.25 M | [45] |
| MHT-pAB, MHT-NO2 | Reinforcement in cement mortar | MHT-pAB to replace 5% mass of cement or with 20% MHT-pAB or MHT-NO2 by mass of cement coating of the reinforcing steel can prevent chloride-induced corrosion in concrete | [138] |
| Mg–Al–NO2 | Carbon steel in SCCP solution and mortar | MgAl–LDHs–NO2 is able to provide the multifunctional corrosion protection for carbon steel, in both SCCP solution and mortar | [47] |
| Mg–Al–NO2 | Steel in carbonated SCP solution | The corrosion inhibition of Mg–Al–NO2 LDHs is mainly attributed to the increase of solution alkalinity and inhibitive
|
[66] |
| Mg–Al–NO3 | Steel in SCP solution | Mg–Al LDHs has a stronger uptake capacity of Cl− than
|
[85] |
| Zn–Al–NO2, Zn–Al–NO3 | Steel rebar in mortar | Compared with reference mortar, the sample containing LDHs presented lower values of chloride in the same condition | [139] |
| Mg–Al–LDHs–OH–PTL | Carbon steel in the carbonated SCP solution | Inhibition efficiency of the multifunctional inhibitor for carbon steel could reach 91.9% when 20 g/L Mg–Al–LDHs–OH–PTL were added in the SCCP solutions | [132] |
| Zn–Al–LDHs–PTL-co | Carbon steel in the SCP solution | Addition of Zn–Al–LDHs–PTL-co could lead to the release of PTL ions into the solution and the decrease of Cl− concentration | [140] |
| CLDHs | Steel in SCP solution | In CLDHs treated SCP solution with Cl−, the pitting potential of carbon steel notably increased, and the surface impedance was much higher, indicating strengthened passivation | [65] |
7 Problems and prospects
LDHs, as layered compounds, can enhance the resistance of cement-based materials to harmful anion attacks and improve durability through their anion exchange and structural reconstruction properties. The current research initially confirms the promising potential of LDHs to enhance concrete durability by adsorbing anions. However, most of them are at the stage of laboratory experiments, and only a few are used in practical engineering. And there are still some urgent problems in LDHs modified cement-based materials.
Modification of LDHs materials. Single LDHs are difficult to further promote in the field of concrete due to the disadvantages of fewer functional groups, poor acid and alkali resistance, and low reusability. Therefore, the construction of functional LDHs by modifying LDHs materials (with calcination, intercalation, surface modification, etc.) is significant for the application of LDHs in concrete.
The influence of LDHs on the hydration process of cement-based materials. Current research on the effects of LDHs on cement concrete focuses on durability properties, while the impact mechanism of LDHs on cement hydration is not clear. The hydration process of cement determines its strength, durability, and many other properties. Revealing the hydration mechanism of cement containing LDHs is crucial for improving the properties of cement-based materials and solving practical engineering problems.
Research on the compatibility of LDHs with additives. The addition of LDHs reduces fluidity and shortens the setting time of cement-based materials. Practical applications often have high requirements for the fluidity of cement-based materials. Therefore, it is an urgent problem to ensure the proper workability of cement-based materials containing LDHs. And the compatibility of LDHs with high-efficiency admixtures needs to be further investigated.
Adsorption effect and structure reconstruction mechanism of LDHs in the complex ionic environment. The effect of LDHs materials used in concrete combined with auxiliary cementitious materials still needs to be further investigated to explore more functions of LDHs materials.
-
Funding information: This work was financially supported by the Joint Fund of National Natural Science Foundation of China (No. U20A201397), the National Natural Science Foundation of China (No. 51908568), the National Key Research and Development Program of China (No. 2019YFC1905104) and (No. 2019YFC1904302), the Natural Science Foundation of Guangdong Province (No. 2019A1515011981), and the State Key Laboratory open fund of Marine Resource Utilization in the South China Sea (Hainan University) (No. 201904).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Mehta PK, Monteiro PJM. Concrete: microstructure, properties and materials. USA: Mc Graw-Hill Education Professional; 2014.Search in Google Scholar
[2] Angst UM. Challenges and opportunities in corrosion of steel in concrete. Mater Struct. 2018;51:4–24.10.1617/s11527-017-1131-6Search in Google Scholar
[3] Zhao JH, Tong LY, Li BE, Chen TH, Wang CP, Yang GQ, et al. Eco-friendly geopolymer materials: A review of performance improvement, potential application and sustainability assessment. J Clean Prod. 2021;307:127085.10.1016/j.jclepro.2021.127085Search in Google Scholar
[4] Yang GQ, Zhao JH, Wang YR. Durability properties of sustainable alkali-activated cementitious materials as marine engineering material: a review. Mater Today Sustain. 2022;17:100099.10.1016/j.mtsust.2021.100099Search in Google Scholar
[5] Cheewaket T, Jaturapitakkul C, Chalee W. Concrete durability presented by acceptable chloride level and chloride diffusion coefficient in concrete: 10-year results in marine site. Mater Struct. 2014;47:1501–11.10.1617/s11527-013-0131-4Search in Google Scholar
[6] Prinetto F, Ghiotti G, Graffin P, Tichit D. Synthesis and characterization of sol-gel Mg/Al and Ni/Al layered double hydroxides and comparison with co-precipitated samples. Microporous Mesoporous Mater. 2000;39:229–47.10.1016/S1387-1811(00)00197-9Search in Google Scholar
[7] Yang WS, Kim YM, Liu PKT, Sahimi M, Taotsis TT. A study by in situ techniques of the thermal evolution of the structure of a Mg-Al-CO3-layered double hydroxide. Chem Eng Sci. 2002;57:2945–53.10.1016/S0009-2509(02)00185-9Search in Google Scholar
[8] Ke XY, Bernal SA, Provis JL. Controlling the reaction kinetics of sodium carbonate-activated slag cements using calcined layered double hydroxides. Cem Concr Res. 2016;81:24–37.10.1016/j.cemconres.2015.11.012Search in Google Scholar
[9] Raki L, Beaudoin JJ, Mitchell L. Layered double hydroxide-like materials: nanocomposites for use in concrete. Cem Concr Res. 2004;34:1717–24.10.1016/j.cemconres.2004.05.012Search in Google Scholar
[10] Fang X, Chen C, Jia H, Li YN, Liu J, Wang YS, et al. Progress in adsorption-enhanced hydrogenation of CO2 on layered double hydroxide (LDH) derived catalysts. J Ind Eng Chem. 2021;95:16–27.10.1016/j.jiec.2020.12.027Search in Google Scholar
[11] Dewangan N, Hui WM, Jayaprakash S, Bawah AR, Poerjoto AJ, Jie T, et al. Recent progress on layered double hydroxide (LDH) derived metal-based catalysts for CO2 conversion to valuable chemicals. Catal Today. 2020;356:490–513.10.1016/j.cattod.2020.06.020Search in Google Scholar
[12] Alejandre A, Medina F, Slargre P, Correig X, Sueiras JE. Preparation and study of Cu-Al mixed oxides via hydrotalcite-like precursors. Chem Mater. 1999;11:939–48.10.1021/cm980500fSearch in Google Scholar
[13] Lei SQ, Wang SN, Gao BX, Zhan YL, Zhao QC, Jin SS, et al. Ultrathin dodecyl-sulfate-intercalated Mg-Al layered double hydroxide nanosheets with high adsorption capability for dye pollution. J Colloid Interface Sci. 2020;577:181–90.10.1016/j.jcis.2020.05.050Search in Google Scholar PubMed
[14] Liu QP, Zhao Y, Jiang Z, Cui Y, Ai N. Computational and experimental studies on the CO2 adsorption of layered double hydroxide intercalated by anionic surfactant. Appl Clay Sci. 2020;190:105556.10.1016/j.clay.2020.105556Search in Google Scholar
[15] Rives V, Arco MD, Martin C. Intercalation of drugs in layered double hydroxides and their controlled release: A review. Appl Clay Sci. 2014;88–89:239–69.10.1016/j.clay.2013.12.002Search in Google Scholar
[16] Mousty C, Kaftan O, Prevot V, Forano C. Alkaline phosphatase biosensors based on layered double hydroxides matrices: role of LDH composition. Sens Actuators B: Chem. 2008;133:442–8.10.1016/j.snb.2008.03.001Search in Google Scholar
[17] Hibino T. Deterioration of anion-adsorption abilities of layered double hydroxides synthesized in agarose gel. Appl Clay Sci. 2020;186:105435.10.1016/j.clay.2019.105435Search in Google Scholar
[18] Ye HL. Autogenous formation and smart behaviors of nitrite- and nitrate-intercalated layered double hydroxides (LDHs) in Portland cement-metakaolin-dolomite blends. Cem Concr Res. 2021;139:106267.10.1016/j.cemconres.2020.106267Search in Google Scholar
[19] Xue JW, Liu SH, Ma XE, Teng YB, Guan XM. Effect of different gypsum dosage on the chloride binding properties of C4AF hydrated paste. Constr Build Mater. 2022;315:125562.10.1016/j.conbuildmat.2021.125562Search in Google Scholar
[20] Zibara H. Binding of external chlorides by cement pastes. Ph.D. thesis. Ottawa, ON, Canada: National Library of Canada = Bibliothèque nationale du Canada; 2001.Search in Google Scholar
[21] Zhang YL, Ling TC. Reactivity activation of waste coal gangue and its impact on the properties of cement-based materials – A review. Constr Build Mater. 2020;234:117424.10.1016/j.conbuildmat.2019.117424Search in Google Scholar
[22] Yoshida M, Koilraj P, Qiu XH, Hirajima T, Keiko S. Sorption of arsenate on MgAl and MgFe layered double hydroxides derived from calcined dolomite. J Environ Chem Eng. 2015;3(3):1614–21.10.1016/j.jece.2015.05.016Search in Google Scholar
[23] Keiko S, Qiu XH, Hosomomi Y, Moriyama S, Hirajima T. Effect of natural dolomite calcination temperature on sorption of borate onto calcined products. Microporous Mesoporous Mater. 2013;171:1–8.10.1016/j.micromeso.2012.12.029Search in Google Scholar
[24] Chen YX, Shui ZH, Chen W, Li Q, Chen GW. Effect of MgO content of synthetic slag on the formation of Mg-Al LDHs and sulfate resistance of slag-fly ash-clinker binder. Constr Build Mater. 2016;125:766–74.10.1016/j.conbuildmat.2016.08.086Search in Google Scholar
[25] Chen W, Brouwers HJH. The hydration of slag, part 1: reaction models for alkali-activated slag. J Mater Sci. 2007;42(2):428–43.10.1007/s10853-006-0873-2Search in Google Scholar
[26] Chen W, Brouwers HJH, Shui ZH. Three-dimensional computer modeling of slag cement hydration. J Mater Sci. 2007;42(23):9595–610.10.1007/s10853-007-1977-zSearch in Google Scholar
[27] Chen W, Brouwers HJH. The hydration of slag, part 2: reaction models for blended cement. J Mater Sci. 2007;42(2):444–64.10.1007/s10853-006-0874-1Search in Google Scholar
[28] Kayali O, Khan MSH, Ahmed MS. The role of hydrotalcite in chloride binding and corrosion protection in concretes with ground granulated blast furnace slag. Cem Concr Compos. 2012;34(8):936–45.10.1016/j.cemconcomp.2012.04.009Search in Google Scholar
[29] Kang XJ, Ye HL. Antimicrobial alkali-activated slag through self-intercalation of benzoate in layered double hydroxides. Cem Concr Compos. 2022;130:104533.10.1016/j.cemconcomp.2022.104533Search in Google Scholar
[30] Hu YR, Li HH, Wang Q, Zhang J, Song Q. Characterization of LDHs prepared with different activity MgO and resisting Cl− attack of concrete in salt lake brine. Constr Build Mater. 2019;229:116921.10.1016/j.conbuildmat.2019.116921Search in Google Scholar
[31] Florea MVA, Brouwers HJH. Chloride binding related to hydration products: part I: ordinary portland cement. Cem Concr Res. 2012;42(2):282–90.10.1016/j.cemconres.2011.09.016Search in Google Scholar
[32] Mallakpour S, Hatami M, Hussain CM. Recent innovations in functionalized layered double hydroxides: Fabrication, characterization, and industrial applications. Adv Colloid Interface Sci. 2020;283:10221.10.1016/j.cis.2020.102216Search in Google Scholar PubMed
[33] Khan AI, Ohare D. Intercalation chemistry of layered double hydroxides: recent developments and applications. J Mater Chem. 2002;12:3191–8.10.1039/B204076JSearch in Google Scholar
[34] Wang Q, O’Hare D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem Rev. 2012;112(7):4124–55.10.1021/cr200434vSearch in Google Scholar PubMed
[35] Zhang J, Yan TT, Yang YS, Sun JL, Lin YJ, Wei M. Zn-Zr-Al oxides derived from hydrotalcite precursors for ethanol conversion to diethyl carbonate. Chin J Catal. 2019;40(4):515–22.10.1016/S1872-2067(19)63318-8Search in Google Scholar
[36] Wang H, Zhang Z, Jing M, Tang S, Liu W. Synthesis of CuNiSn LDHs as highly efficient Fenton catalysts for degradation of phenol. Appl Clay Sci. 2020;186:105433.10.1016/j.clay.2019.105433Search in Google Scholar
[37] Vahdat ZA, Zhao JM, Yahya P, Muhammad JA. Influence of pH value and Zn/Ce cations ratio on the microstructures and corrosion resistance of LDH coating on AZ31. Corros CommunSearch in Google Scholar
[38] Zhu YR, Zhe A, Jing H. Single-atom and small-cluster Pt induced by Sn (IV) sites confined in an LDH lattice for catalytic reforming. J Catal. 2016;314:44–54.10.1016/j.jcat.2016.06.004Search in Google Scholar
[39] Wang J, Lei ZY, Qin H, Zhang LH, Li F. Structure and catalytic property of Li-Al metal oxides from layered double hydroxide precursors prepared via a facile solution route. Ind Eng Chem Res. 2011;50(12):120–8.10.1021/ie2000264Search in Google Scholar
[40] Wei JB, Gao Z, Song YC, Yang WL, Wang J, Li ZS. Solvothermal synthesis of Li-Al layered double hydroxides and their electrochemical performance. Mater Chem Phys. 2013;139(2–3):395–402.10.1016/j.matchemphys.2012.12.005Search in Google Scholar
[41] Fogg AM, Freij AJ, Parkinson GM. Synthesis and anion exchange chemistry of rhombohedral Li/Al layered double hydroxides. Chem Mater. 2002;14(1):232–4.10.1021/cm0105099Search in Google Scholar
[42] Yang Z, Fischer H, Polder R. Modified hydrotalcites as a new emerging class of smart additive of reinforced concrete for anticorrosion applications: a literature review. Mater Corros. 2014;64:1066–74.10.1002/maco.201206915Search in Google Scholar
[43] Zuo JD, Wu B, Luo CY, Dong BQ, Xing F. Preparation of MgAl layered double hydroxides intercalated with nitrite ions and corrosion protection of steel bars in simulated carbonated concrete pore solution. Corros Sci. 2019;152:120–9.10.1016/j.corsci.2019.03.007Search in Google Scholar
[44] Tedim J, Kuznetsova A, Salak AN, Montemor F, Snihirova D, Pilz M, et al. Zn–Al layered double hydroxides as chloride nanotraps in active protective coatings. Corros Sci. 2012;55:1–4.10.1016/j.corsci.2011.10.003Search in Google Scholar
[45] Tian YW, Dong CF, Wang G, Cheng XQ, Li XG. Zn–Al–NO2 layered double hydroxide as a controlled-release corrosion inhibitor for steel reinforcements. Mater Lett. 2019;236:517–20.10.1016/j.matlet.2018.10.177Search in Google Scholar
[46] Poznyak SK, Tedim J, Rodrigues LM, Salak AN, Zheludkevich ML, Dick LFP, et al. Novel inorganic host layered double hydroxides intercalated with guest organic inhibitors for anticorrosion applications. ACS Appl Mater Interfaces. 2009;1:2353–62.10.1021/am900495rSearch in Google Scholar PubMed
[47] Cao YH, Dong SG, Zheng DJ, Wang JJ, Zhang XJ, Du RG, et al. Multifunctional inhibition based on layered double hydroxides to comprehensively control corrosion of carbon steel in concrete. Corros Sci. 2017;126:166–79.10.1016/j.corsci.2017.06.026Search in Google Scholar
[48] Carlino S. The intercalation of carboxylic acids into layered double hydroxides: a critical evaluation and review of the different methods. Solid State Ion. 1997;98:73–84.10.1016/S0167-2738(96)00619-4Search in Google Scholar
[49] Costa FR, Abdel-Goad M, Wagenknecht U, Heinrich G. Nanocomposites based on polyethylene and Mg–Al layered double hydroxide. I. Synthesis and characterization. Polymer. 2005;46:4447–53.10.1016/j.polymer.2005.02.027Search in Google Scholar
[50] Lv L, Sun PD, Gu ZY, Du HG, Pang XJ, Tao XH, et al. Removal of chloride ion from aqueous solution by ZnAl-NO3 layered double hydroxides as anion-exchanger. J Hazard Mater. 2009;161:1444–9.10.1016/j.jhazmat.2008.04.114Search in Google Scholar PubMed
[51] Qu ZY, Yu QL, Brouwers HJH. Relationship between the particle size and dosage of LDHs and concrete resistance against chloride ingress. Cem Concr Res. 2018;105:81–90.10.1016/j.cemconres.2018.01.005Search in Google Scholar
[52] Miyata S. Anion-exchange properties of hydrotalcite-like compounds. Clays Clay Min. 1983;31:305–11.10.1346/CCMN.1983.0310409Search in Google Scholar
[53] Costa DG, Rocha AB, Souza WF, Chiaro SS, Leitao AA. Comparative Structural, thermodynamic and electronic analyses of Zn-Al-A(n–) hydrotalcite-like compounds (A(n–) = Cl−,F−,Br−,OH−,CO32− or NO3−): an ab initio study. Appl Clay Sci. 2012;56:16–22.10.1016/j.clay.2011.11.014Search in Google Scholar
[54] Sato T, Wakabayashi T, Shimada M. Adsorption of various anions by magnesium aluminum oxide (Mg0.7Al0.3O1.15). Ind Eng Chem. 1986;25:89–92.10.1021/i300021a020Search in Google Scholar
[55] Du YB, Evans DG, Sun P, Duan X. Anionic layered materials. Chemistry. 2000;63:20–4 (in Chinese).Search in Google Scholar
[56] Li F, Jiang XR, Evans DG, Duan X. Structure and basicity of mesoporous materials from Mg/Al/In layered double hydroxides prepared by separate nucleation and aging steps method. J Porous Mater. 2005;12:55–63.10.1007/s10934-005-5234-zSearch in Google Scholar
[57] Miyata S. Physico-chemical properties of synthetic hydrotalcites in relation to composition. Clays Clay Miner. 1980;28:50–6.10.1346/CCMN.1980.0280107Search in Google Scholar
[58] Wu B, Zuo JD, Dong BQ, Xing F, Luo CY. Study on the affinity sequence between inhibitor ions and chloride ions in Mg-Al layer double hydroxides and their effects on corrosion protection for carbon steel. Appl Clay Sci. 2019;180:105181.10.1016/j.clay.2019.105181Search in Google Scholar
[59] Zhao Y, Liang J, Li F, Duan X. Kinetic study on the thermal decomposition of layered double hydroxides. J Tsinghua Univ (Sci Technol). 2004;44:149–52 (in Chinese).Search in Google Scholar
[60] Glasser FP, Marchand J, Samson E. Durability of concrete-degradation phenomena involving detrimental chemical reactions. Cem Concr Res. 2008;38:226–46.10.1016/j.cemconres.2007.09.015Search in Google Scholar
[61] Suryavanshi AK, Scantlebury JD, Lyon SB. Mechanism of Friedel’s salt formation in cements rich in tri-calcium aluminate. Cem Concr Res. 1996;26:717–27.10.1016/S0008-8846(96)85009-5Search in Google Scholar
[62] Jones MR, Macphee DE, Chudek JA, Lannegrand R, Talero R, Scrimgeour SN. Studies using 27Al MAS NMR of AFm and AFt phases and the formation of Friedel’s salt. Cem Concr Res. 2003;33(2):177–82.10.1016/S0008-8846(02)00901-8Search in Google Scholar
[63] Ke XY, Bernal SA, Provis JL. Uptake of chloride and carbonate by Mg-Al and Ca-Al layered double hydroxides in simulated pore solutions of alkali-activated slag cement. Cem Concr Res. 2017;100:1–13.10.1016/j.cemconres.2017.05.015Search in Google Scholar
[64] Tang YM, Niu L, Zuo Y. Effect of CLDH on rebar corrosion behavior in chloride contaminated neutral pore solutions. J Electrochem. 2010;16:368–72 (in Chinese).10.61558/2993-074X.3364Search in Google Scholar
[65] Tang YM, Zhao XH, Niu L, Zuo Y. The adsorbing effect of calcined layered double hydroxide for chloride ions in simulated concrete pore solutions. J Wuhan Univ Technology-Mater Sci Ed. 2014;29:278–83.10.1007/s11595-014-0908-5Search in Google Scholar
[66] Xu JX, Wei JF, Ma GX, Tan QP. Effect of MgAl-NO2 LDHs inhibitor on steel corrosion in chloride-free and contaminated simulated carbonated concrete pore solutions. Corros Sci. 2020;176:108940.10.1016/j.corsci.2020.108940Search in Google Scholar
[67] Chen YX, Yu R, Wang XP, Chen J, Shui ZH. Evaluation and optimization of ultra-high performance concrete (UHPC) subjected to harsh ocean environment: towards an application of layered double hydroxides (LDHs). Constr Build Mater. 2018;177:51–62.10.1016/j.conbuildmat.2018.03.210Search in Google Scholar
[68] Hu YR, Li HL, Wang Q, Zhang J, Song Q. Characterization of LDHs prepared with different activity MgO and resisting Cl- attack of concrete in salt lake brine. Constr Build Mater. 2019;229:116921.10.1016/j.conbuildmat.2019.116921Search in Google Scholar
[69] Duan P, Shui ZH, Chen GW. Influence of LDHs on Chloride ion binding in cementitious materials. Key Eng Mater. 2014;599:34–8.10.4028/www.scientific.net/KEM.599.34Search in Google Scholar
[70] Liu T, Chen YX, Yu QL, Fan JF, Brouwers HJH. Effect of MgO, Mg-Al-NO3 LDH and calcined LDH-CO3 on chloride resistance of alkali activated fly ash and slag blends. Constr Build Mater. 2020;250:118865.10.1016/j.conbuildmat.2020.118865Search in Google Scholar
[71] Qiao Z, Zhang L, Wang ZJ, Yang JJ. Influence research of calcined layerd double hydrixide on performance of seawater-seasand concrete. China Concr Cem Products. 2017;10:1–5 (in Chinese).Search in Google Scholar
[72] Yoon S, Moon J, Bae S, Duan XN, Giannelis EP, Monteiro PM. Chloride adsorption by calcined layered double hydroxides in hardened Portland cement paste. Mater Chem Phys. 2014;145:376–86.10.1016/j.matchemphys.2014.02.026Search in Google Scholar
[73] Cai P, Zheng H, Wang C, Ma H, Hu J, Pu Y, et al. Competitive adsorption characteristics of fluoride and phosphate on calcined Mg–Al–CO3 layered double hydroxides. J Hazard Mater. 2012;213:100–8.10.1016/j.jhazmat.2012.01.069Search in Google Scholar PubMed
[74] Novillo C, Guaya D, Allen-Perkins avendaño A, Armijos C, Cortina JL, Cota I. Evaluation of phosphate removal capacity of Mg/Al layered double hydroxides from aqueous solutions. Fuel. 2014;138:72–9.10.1016/j.fuel.2014.07.010Search in Google Scholar
[75] Tian B, Cohen MD. Does gypsum formation during sulfate attack on concrete lead to expansion? Cem Concr Res. 2000;30:117–23.10.1016/S0008-8846(99)00211-2Search in Google Scholar
[76] Monteiro PJM, Kurtis KE. Time to failure for concrete exposed to severe sulfate attack. Cem Concr Res. 2003;33:987–93.10.1016/S0008-8846(02)01097-9Search in Google Scholar
[77] Rozière E, Loukili A, Hachem RE, Grondin F. Durability of concrete exposed to leaching and external sulphate attacks. Cem Concr Res. 2009;39:1188–98.10.1016/j.cemconres.2009.07.021Search in Google Scholar
[78] Hossack AM, Thomas MDA. Evaluation of the effect of tricalcium aluminate content on the severity of sulfate attack in Portland cement and Portland limestone cement mortars. Cem Concr Compos. 2015;56:115–20.10.1016/j.cemconcomp.2014.10.005Search in Google Scholar
[79] Wang JG. Sulfate attack on hardened cement paste. Cem Concr Res. 1994;24:735–42.10.1016/0008-8846(94)90199-6Search in Google Scholar
[80] Tsujimura A, Uchida M, Okuwaki A. Synthesis and sulfate ion-exchange properties of a hydrotalcite-like compound intercalated by chloride ions. J Hazard Mater. 2007;143:582–6.10.1016/j.jhazmat.2006.09.073Search in Google Scholar PubMed
[81] Cheng JY, Yue XP, Cao Y, Zhang Y. Adsorptive removal of sulfate from water by Zn/Al layered double oxide. Chin J Environ Eng. 2014;8:131–7 (in Chinese).Search in Google Scholar
[82] Li DM, Wang HZ, Wang LQ, Zhao ZP. Removal of sulfate from aqueous solution by adsorption of it on layered double hydroxides. Acta Mineralogica Sin. 2007;27:109–14 (in Chinese).Search in Google Scholar
[83] Halajnia A, Oustan S, Najafi N, Khataee AR, Lakzian A. Adsorption-desorption characteristics of nitrate, phosphate and sulfate on Mg-Al layered double hydroxide. Appl Clay Sci. 2013;80–81:305–12.10.1016/j.clay.2013.05.002Search in Google Scholar
[84] Guimarães D, Rocha NCMD, Morais RAPD, Resende ADLBP, Lima RMF, Costa GMD, et al. Precipitation of a layered double hydroxide comprising Mg2+ and Al3 + to remove sulphate ions from aqueous solutions. J Environ Chem Eng. 2019;7:102815.10.1016/j.jece.2018.102815Search in Google Scholar
[85] Xu JX, Tan QP, Mei YJ. Corrosion protection of steel by Mg-Al layered double hydroxides in simulated concrete pore solution: Effect of SO42-. Corros Sci. 2020;163:108223.10.1016/j.corsci.2019.108223Search in Google Scholar
[86] Ma JT, Wang DG, Duan P, Shi YK. Sulfate ions immobilization of calcined layered double hydroxides in hardened cement paste and concrete. J Wuhan Univ Technol-Mater Sci. 2019;34:1400–7.10.1007/s11595-019-2205-9Search in Google Scholar
[87] Chen GW, Shui ZH, Duan P, Chen YX, Chen W, Lin YJ. Combination of LDHs and metakaolin for improving the sulfate ion resistance of concrete. J Wuhan Univ Technol. 2014;36:1–5 (in Chinese).Search in Google Scholar
[88] Yun Y. Experiment on sulfate corrosion resistance of concrete modified by calcined layered double hydroxides. China: North China University of Water Resources and Electric Power; 2018 (in Chinese).Search in Google Scholar
[89] Guo L, Wu YY, Duan P, Zhang ZH. Improving sulfate attack resistance of concrete by using calcined Mg-Al-CO3 LDHs: adsorption behavior and mechanism. Constr Build Mater. 2020;232:117256.10.1016/j.conbuildmat.2019.117256Search in Google Scholar
[90] Zhu WJ, François R, Poon CS, Dai JG. Influences of corrosion degree and corrosion morphology on the ductility of steel reinforcement. Constr Build Mater. 2017;148:297–306.10.1016/j.conbuildmat.2017.05.079Search in Google Scholar
[91] Ma JT, Duan P, Ren DM, Zhou W. Effects of layered double hydroxides incorporation on carbonation resistance of cementitious materials. J Mater Res Technol. 2019;8:292–8.10.1016/j.jmrt.2017.08.014Search in Google Scholar
[92] Ma JT. Research on modification and mechanism of LDHs-MK compound defence system in concrete. Ph.D. Thesis. China: Wuhan University of Technology; 2012 (in Chinese).Search in Google Scholar
[93] Shui ZH, Yu R, Chen YX, Duan P, Ma JT, Wang XP. Improvement of concrete carbonation resistance based on a structure modified layered double hydroxides (LDHs): Experiments and mechanism analysis. Constr Build Mater. 2018;176:228–40.10.1016/j.conbuildmat.2018.04.222Search in Google Scholar
[94] Duan P, Yan CJ, Zhou W. Effects of calcined layered double hydroxides on carbonation of concrete containing fly ash. Constr Build Mater. 2018;160:725–32.10.1016/j.conbuildmat.2017.11.099Search in Google Scholar
[95] Song XF, Zhang JT, Cui HL, Wang X, Le JC. Effect of calcined layered double hydroxides on carbonation resistance of ordinary portland cement and alkali-activated slag cement. Bull Chin Ceram Soc. 2019;38:3379–84 (in Chinese).Search in Google Scholar
[96] Chen W, Zhang SZ, Chen XX, Ma JT. Carbonation and microstructural mechanism of concrete modified with layered double hydroxides. J Wuhan Univ Technol. 2013;35:1–6 (in Chinese).Search in Google Scholar
[97] Chen AJ, Yun Y, Ma JT, Li ChaoYao, WW. Structure reconstruction of calcined layered double hydroxides in cement materials and its carbonation analysis. Bull Chin Ceram Soc. 2017;36:301–5 (in Chinese).Search in Google Scholar
[98] Rao BK, Reddy MAK, Rao AV. Effect of fly ash as cement replacement material and pore filling material in concrete. Mater Today Proc. 2022;53:1775–80.10.1016/j.matpr.2021.11.444Search in Google Scholar
[99] Liu JL, Wang Y. Predicting the chloride diffusion in concrete incorporating fly ash by a multi-scale model. J Clean Prod. 2022;330:129767.10.1016/j.jclepro.2021.129767Search in Google Scholar
[100] Lei WG, Struble LJ. Microstructure and flow behavior of fresh cement paste. J Am Ceram Soc. 1997;80(8):2021–8.10.1111/j.1151-2916.1997.tb03086.xSearch in Google Scholar
[101] Aı̈tcin , PC. Cements of yesterday and today: concrete of tomorrow. Cem Concr Research. 2000;30(9):1349–59.10.1201/9781482265767-21Search in Google Scholar
[102] Li ZG, Liu JP, Yu PC, Zhang X. Exploratory research of layered double hydroxides on physical and mechanical properties of concrete. Build Technol Dev. 2017;44:95–7 (in Chinese).Search in Google Scholar
[103] Duan P. Research on modification mechanism and the application of layered double hydroxides for durability of concrete. Ph.D. Thesis. China: Wuhan University of Technology; 2014 (in Chinese).Search in Google Scholar
[104] Yang DM. Study on scavenging papermaking anionic trash by hydrotalcites and calcined hydrotalcites. Ph.D. Thesis. China: Northeast Forestry University; 2013 (in Chinese).Search in Google Scholar
[105] Zheng YM. Preparation and performance of ZnAl-layered double hydroxides composites and their calcined products. Ph.D. Thesis. China: South China University of Technology; 2013 (in Chinese).Search in Google Scholar
[106] Wu YY, Duan P, Yan CJ. Role of layered double hydroxides in setting, hydration degree, microstructure and compressive strength of cement paste. Appl Clay Sci. 2018;158:123–31.10.1016/j.clay.2018.03.024Search in Google Scholar
[107] Ma JT, Chen L, Chen W, Wang K. Research on the combination of LDHs and MK on improving the chloride ion resistance of concrete. J Wuhan Univ Technol. 2013;35:34–9 (in Chinese).Search in Google Scholar
[108] Li GS, Zhu JP, Gao G, Guo BK, Guan XM, Feng CH. Research progress on influence of 2D nano materials on properties of cement-based materials. Bull Chin Ceram Soc. 2018;37:3460–6 (in Chinese).Search in Google Scholar
[109] Ma JT. Research on modification and mechanism of LDHs-MK compound defence system in concrete. Ph.D. Thesis. China: Wuhan University of Technology; 2012 (in Chinese).Search in Google Scholar
[110] Guan XM, Li HY, Luo SQ, Liu XX, Zhang JW. Influence of LiAl-layered double hydroxides with 3D micro-nano structures on the properties of calcium sulphoaluminate cement clinker. Cem Concr Compos. 2016;70:15–23.10.1016/j.cemconcomp.2016.03.009Search in Google Scholar
[111] Cao L, Guo JT, Tian JH, Xu Y, Hu MM, Wang MY, et al. Preparation of Ca/Al-Layered Double Hydroxide and the influence of their structure on early strength of cement. Constr Build Mater. 2018;184:203–14.10.1016/j.conbuildmat.2018.06.186Search in Google Scholar
[112] Li LY, Xia J, Lin SS. A multi-phase model for predicting the effective diffusion coefficient of chlorides in concrete. Constr Build Mater. 2012;26:295–301.10.1016/j.conbuildmat.2011.06.024Search in Google Scholar
[113] Spiesz P, Brouwers HJH. The apparent and effective chloride migration coefficients obtained in migration tests. Cem Concr Res. 2013;48:116–27.10.1016/j.cemconres.2013.02.005Search in Google Scholar
[114] Duan P, Chen W, Ma JT, Shui ZH. Influence of layered double hydroxides on microstructure and carbonation resistance of sulphoaluminate cement concrete. Constr Build Mater. 2013;48:601–9.10.1016/j.conbuildmat.2013.07.049Search in Google Scholar
[115] Thomas JJ, Jennings HM, Chen JJ. Influence of nucleation seeding on the hydration mechanisms of tricalcium silicate and cement. J Phys Chem C. 2009;113:4327–34.10.1021/jp809811wSearch in Google Scholar
[116] Yang HJ, Usman M, Hanif A. Suitability of liquid crystal display (LCD) glass waste as supplementary cementing material (SCM): Assessment based on strength, porosity, and durability. J Build Eng. 2021;42:102793.10.1016/j.jobe.2021.102793Search in Google Scholar
[117] Gupta S, Chaudhary S. State of the art review on supplementary cementitious materials in India – II: Characteristics of SCMs, effect on concrete and environmental impact. J Clean Prod. 2022;357:131945.10.1016/j.jclepro.2022.131945Search in Google Scholar
[118] Nguyen HA. Enhancement of engineering properties of slag-cement based self-compacting mortar with dolomite powder. J Build Eng. 2019;24:100738.10.1016/j.jobe.2019.100738Search in Google Scholar
[119] Chen L, Chen D, Liu RG, Xu RJ. Physical mechanic and water purification performance of eco-concrete doping with hydrotalcite. Bull Chin Ceram Soc. 2019;38:834–40 (in Chinese).Search in Google Scholar
[120] Li HY, Liu XX, Si HY, Wei S, Zhang BW, Zhang XY, et al. Effect of nano-layered double hydroxides on hydration and hardening of calcium sulphoaluminate cement. J Chin Ceram Soc. 2018;46:887–94 (in Chinese).Search in Google Scholar
[121] Xu SL, Chen ZR, Zhang BW, Yu JH, Zhang FZ, Evans DG. Facile preparation of pure CaAl-layered double hydroxides and their application as a hardening accelerator in concrete. Chem Eng J. 2009;155:881–5.10.1016/j.cej.2009.08.003Search in Google Scholar
[122] Zou DH, Wang K, Li HY, Guan XM. Effect of LiAl-layered double hydroxides on hydration of calcium sulfoaluminate cement at low temperature. Constr Build Mater. 2019;223:910–7.10.1016/j.conbuildmat.2019.07.251Search in Google Scholar
[123] Guan XM, Li HY, Luo SQ, Liu XX, Zhang JW. Influence of LiAl-layered double hydroxides with 3D micro-nano structures on the properties of calcium sulphoaluminate cement clinker. Cem Concr Compos. 2016;70:15–23.10.1016/j.cemconcomp.2016.03.009Search in Google Scholar
[124] Shao HF, Liu CS, Huang Y, Cao XH, Yang HC. Shape control of hydroxyapaptite crystal seed and its effects on the in-situ reinforcement of calcium phosphate cement. J Inorg Mater. 2001;16:933–9.Search in Google Scholar
[125] Dong YC, Kong XR, Luo XS, Wang HT. Adsorptive removal of heavy metal anions from water by layered double hydroxide: a review. Chemosphere. 2022;303:134685.10.1016/j.chemosphere.2022.134685Search in Google Scholar PubMed
[126] Qiu SK, Zhao D, Feng YY, Li MM, Liang XF, Zhang LS, et al. Adsorption performance and mechanism of Ca–Al-LDHs prepared by oyster shell and pop can for phosphate from aqueous solutions. J Environ Manag. 2022;303:114235.10.1016/j.jenvman.2021.114235Search in Google Scholar PubMed
[127] Wang Y, Dai XJ, Zhou Q, Li KL, Feng L, Liao WL, et al. Insights into the role of metal cation substitution on the anionic dye removal performance of CoAl-LDH. Colloids Surf A Physicochem Eng Asp. 2022;636:128139.10.1016/j.colsurfa.2021.128139Search in Google Scholar
[128] Tang F, Wang XY, Xu XJ, Li LD. Phytic acid doped nanoparticles for green anticorrosion coatings. Colloids Surf A Physicochem Eng Asp. 2010;369:101–5.10.1016/j.colsurfa.2010.08.013Search in Google Scholar
[129] Mohamed HA. Eco-friendly zero VOC anticorrosive paints for steel protection. J Appl Polym Sci. 2012;125:1790–5.10.1002/app.36256Search in Google Scholar
[130] Chung CW, Jung HY, Kwon JH, Jang, Kim JH. Use of calcium aluminum-layered double hydroxide to control chloride ion penetration of cement-based materials. J Struct Integr Maint. 2019;4:37–42.10.1080/24705314.2019.1565057Search in Google Scholar
[131] Chen LJ, Chen XX, Chen W. Research on the carbonation of cement paste modified with layered double hydroxides. Appl Mech Mater. 2012;174–177:706–10.10.4028/www.scientific.net/AMM.174-177.706Search in Google Scholar
[132] Cao YH, Zheng DJ, Dong SG, Zhang F, Lin JY, Wang C, et al. A composite corrosion inhibitor of MgAl layered double hydroxides co-intercalated with hydroxide and organic anions for carbon steel in simulated carbonated concrete pore solutions. J Electrochem Soc. 2019;166:3106–13.10.1149/2.0141911jesSearch in Google Scholar
[133] Xu JX, Song YB, Zhao YH, Jiang LH, Mei YJ, Chen P. Chloride removal and corrosion inhibitions of nitrate, nitrite-intercalated Mg-Al layered double hydroxides on steel in saturated calcium hydroxide solution. Appl Clay Sci. 2018;163:129–36.10.1016/j.clay.2018.07.023Search in Google Scholar
[134] Yang ZX, Polder R, Mol JMC. Modified hydrotalcites as chloride scavengers and inhibitor release agents for improved corrosion protection of reinforced concrete. Heron. 2017;62:61–83.Search in Google Scholar
[135] Tatematsu H, Sasaki T. Repair materials system for chloride-induced corrosion of reinforcing bars. Cem Concr Compos. 2003;25:123–9.10.1016/S0958-9465(01)00059-2Search in Google Scholar
[136] Yang ZX, Fischer H, Polder R. Laboratory investigation of the influence of two types of modified hydrotalcites on chloride ingress into cement mortar. Cem Concr Compos. 2015;58:105–13.10.1016/j.cemconcomp.2014.12.016Search in Google Scholar
[137] Chen YX, Shui ZH, Chen W, Chen GW. Chloride binding of synthetic Ca–Al–NO3 LDHs in hardened cement paste. Constr Build Mater. 2015;93:1051–8.10.1016/j.conbuildmat.2015.05.047Search in Google Scholar
[138] Yang ZX, Polder R, Mol JMC, Andrade C. The effect of two types of modified Mg-Al hydrotalcites on reinforcement corrosion in cement mortar. Cem Concr Res. 2017;100:186–202.10.1016/j.cemconres.2017.06.004Search in Google Scholar
[139] Gomes C, Mir ZM, Sampaio R, Bastos AC, Tedim J, Maia F, et al. Use of ZnAl-layered double hydroxide (LDH) to extend the service life of reinforced concrete. Materials. 2020;13:1769–88.10.3390/ma13071769Search in Google Scholar PubMed PubMed Central
[140] Cao YH, Zheng DJ, Luo JS, Zhang F. Insight into the fabrication of ZnAl layered double hydroxides intercalated with organic anions and their corrosion protection of steel reinforced concrete. J Electrochem Soc. 2019;166:617–23.10.1149/2.0361916jesSearch in Google Scholar
© 2022 Mengyi Zhai et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption

![Figure 8
(a) Porosity of LDHs blended cement pastes after 28 days of curing. (b) Compressive strength of cement containing LDHs at 3, 7, and 28 days. (c) Effects of LDHs on the microstructure of cement pastes [106]. (d) Schematic of the hydration process [115].](/document/doi/10.1515/ntrev-2022-0478/asset/graphic/j_ntrev-2022-0478_fig_008.jpg)
