Abstract
An in situ Schiff-base condensation between p-phthalaldehyde (PPD) and 1,3,5-tris(4-aminophenyl)benzene (TAPB) or 1,3,5-tris(4-aminophenyl)triazine (TAPT) was actualized in the presence of carbon nanotubes (CNTs), producing imine-linked hyperbranched poly(PPD-TAPB) and poly(PPD-TAPT)-coated CNTs (abbreviated as CNT@HBP-1 and CNT@HBP-2, respectively). Such quasi-1D core–shell heterostructures are interleaved to build robust 3D networks with porous internal channels, which are favorable for efficient electron transport and ion diffusion, exposing active sites, fast redox kinetics, and high electrochemical utilization. When used as Li-ion anodes, both CNT@HBP-1 and CNT@HBP-2 exhibit larger specific capacity, better rate performance, and higher cycling stability compared to their pure polymers. Furthermore, CNT@HBP-2 delivers higher reversible capacities of 351 mA h g−1 at 0.05 A g−1, and 81 mA h g−1 at 1.0 A g−1, respectively, compared to CNT@HBP-1 (335 and 56 mA h g−1). Besides, CNT@HBP-2 retains 268 mA h g−1 over 100 cycles at 0.1 A g−1, and 617 mA h g−1 in the 500th cycles at 0.5 A g−1, respectively, outperforming CNT@HBP-1 (155 and 256 mA h g−1). Further improvements in the electrochemical performance for CNT@HBP-2 relative to CNT@HBP-1 are attributable to the incorporation of additional redox-active triazine units into HBP-2. This work would unlock insights into the rational development of metal-free polymer-based electrodes for rechargeable batteries.
1 Introduction
Lithium-ion batteries (LIBs) have long demonstrated as the main power sources for consumer electronics and electric vehicles [1,2,3]. It is generally known that LIBs are mainly based on transition-metal compounds as cathodes (usually LiFePO4 and LiCoO2) and graphite or Si/C as anodes [4,5,6,7]. However, such electrode materials are suffering from their intrinsically limited Li-storage performance, gradually exhausted mineral resources, and environmental challenges of greenhouse effect and heavy metal pollution [8,9,10]. Therefore, the development of eco-sustainable and cost-efficient materials has become an urgent requirement to seek the future substitutions for the commercially used inorganic electrode materials [11,12,13].
In this context, organic redox polymers as potential electrode alternatives for green LIBs have recently attracted extensive attention due to their merits of high specific capacities, fast redox kinetics, structure designability, resource abundance, low cost, and sustainability and recyclability [14,15,16,17]. In the last years, polymers containing redox-active units of free radicals, organosulfur, imine, carbonyl, and azo groups have been frequently used in organic LIBs [18,19,20]. However, most polymers show high redox potentials (over 2.0 V vs Li/Li+) with their reversible lithiation/delithiation processes in the voltage range of 1.5–3.5 V (vs Li/Li+) [21]. Therefore, such polymers are preferred as cathode materials for LIBs [22,23], and there are few reports on polymer-based anodes so far [24].
The past years has witnessed considerable progress on organic LIBs with polymer electrodes [25]. Nevertheless, there are two challenges that need to be addressed. First, organic polymers are usually electrical insulators which often result in sluggish redox kinetics, and inferior power capability [26]. Generally improved strategies are to combine with electrically conductive additives such as graphene and carbon nanotubes (CNTs) by ex situ physical blending and in situ chemical polymerization methods [27,28]. Another issue involves a low electrochemical utilization (usually less than 50%) of polymers and hence, an inferior practical capacity [29,30]. To alleviate current concerns, it is highly desired but challenging to develop metal-free polymer-based anode materials for sustainable LIBs.
Herein, we report on the fabrication of imine-based hyperbranched polymer-coated CNTs by the in situ Schiff-base condensation reaction of p-phthalaldehyde (PPD) with 1,3,5-tris(4-aminophenyl)benzene (TAPB) and 1,3,5-tris(4-aminophenyl)triazine (TAPT), respectively. This process leads to forming core–shell heterostructures of CNTs encapsulated in hyperbranched poly(PPD-TAPB) and hyperbranched poly(PPD-TAPT), which are accordingly abbreviated as CNT@HBP-1 and CNT@HBP-2, respectively. The thicknesses of the HBP-1 and HBP-2 outer shell are about 18.9 and 19.2 nm, respectively. Both CNT@HBP-1 and CNT@HBP-2 anodes display larger capacities, higher rate, and cycling capabilities in comparison with pure HBP-1 and HBP-2 anodes. Remarkably, the CNT@HBP-2 anode delivers a higher reversible capacity of 351 mA h g−1 at 0.05 A g−1 and retains 617 mA h g−1 over 500 cycles at 0.5 A g−1, compared to CNT@HBP-1 (335 and 256 mA h g−1) due to the presence of additional redox-active triazine sites in HBP-2. This work may provide new insights into the rational crafting of metal-free polymer-based electrodes for rechargeable batteries.
2 Experimental
2.1 Materials
TAPT was synthesized as described elsewhere [31]. CNTs were purchased from Chengdu Organic Chemicals Co. Ltd, CAS, China. Trifluoroacetic acid and PPD were purchased from Shanghai Aladdin Industrial Co. LTD., China. TAPB and 4-aminobenzonitrile were provided by Shanghai Hongyan Bio-medicine Co. Ltd and Shanghai Macklin Biochemical Co., Ltd, China. Dimethyl sulfoxide (DMSO), N-methyl-2-pyrrolidone (NMP), and other chemicals were supplied by Sinopharm Group Chemical Reagent Co. Ltd, China. All chemicals and solvents were used as received.
2.2 Synthesis of hyperbranched polymer-encapsulated CNTs
CNTs (0.066 g) were ultrasonically dispersed into DMSO (30 mL) for 30 min at room temperature. To this stable suspension were then added PPD (0.121 g, 0.9 mmol) and TAPB (0.211 g, 0.6 mmol) or TAPT (0.213 g, 0.6 mmol) under ultrasonic condition for another 5 min. The resultant dispersion was held at 150°C for 12 h under magnetic stirring in an Ar atmosphere. Finally, the final product was collected by filtering and washing the black powder with ethanol to remove free polymers. After vacuum-drying at 80°C for 12 h, CNT/hyperbranched poly(PPD-TAPB) and CNT/hyperbranched poly(PPD-TAPT) composites were obtained and accordingly abbreviated as CNT@HBP-1 and CNT@HBP-2, respectively. As control experiments, pure hyperbranched poly(PPD-TAPB) (HBP-1) and hyperbranched poly(PPD-TAPT) (HBP-2) were also synthesized in the absence of CNTs under identical experimental procedures.
2.3 Material characterization
Fourier transform infrared (FT-IR) spectra were recorded on a Thermo Nicolet NEXUS 470 spectrometer (USA). X-ray diffraction (XRD) analysis was performed on a Bruker D8 Advance (Germany) X-ray diffractometer with a Cu Kα source. Thermogravimetric analysis (TGA) was measured on a Netzsch TG 209 F3 Tarsus analyzer (Germany) at a heating rate of 10°C min−1 under a N2 atmosphere. Raman spectra were collected on a Thermo Scientific DXR spectrometer (USA) with an excitation laser λ = 532 nm. Elemental analysis of C, N, and H was detected by Elementar Vario MICRO Cube (Germany). Transmission electron microscope (TEM, Tecnai G220 S-TWIN, FEI, USA) and scanning electron microscope (SEM, SU8010, Hitachi, Japan) were used to determine material morphologies. Energy dispersive X-ray (EDX) elemental mappings were collected on a scanning transmission electron microscope (STEM, Talos F200X).
2.4 Electrochemical measurements
The working electrode was fabricated by casting a slurry mixture of active materials, acetylene black, and poly(vinylidene fluoride) (ratio: 6:3:1 in weight) in NMP onto the copper foil, followed by drying at 80°C under vacuum for 12 h. The mass loading of active materials was about 1.0 mg cm−2 on each circular electrode slice with a diameter of 10 mm. The as-made anode was assembled into a 2032-type half-cell using the lithium foil as the counter electrode. 1.0 M LiPF6 dissolved in a mixture of ethylene carbonate, dimethyl carbonate, and ethyl–methyl carbonate (ratio: 1:1:1 in volume) was used as the electrolyte. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) data were collected on a Chenhua CHI 660 electrochemical workstation (Shanghai, China) in the range of 0.01 to 3 V. The rate performance and cycling stability were carried out on a Land CT2001A battery testing system (Wuhan, China). All electrochemical measurements were actualized at room temperature.
3 Results and discussion
3.1 Synthesis and structural characteristics
Figure 1 demonstrates chemical structures and synthesis routes of hyperbranched polymers and the composites with CNTs. CNTs are well known to be easily dispersed in polar DMSO due to their well-matched solubility parameters and surface tensions [32,33]. DMSO has also been reported as an excellent reaction medium to synthesize Schiff-base polymers with high yields [34]. Furthermore, phenyl-enriched monomers (PPD, TAPB, and TAPT) and the synchronously-formed conjugated hyperbranched polymers (HBP-1, and HBP-2) enable strong π–π non-covalent interactions with CNTs during the in situ polymerization process [35]. Accordingly, this allows for an intimate contact between CNTs and polymers to build robust core–shell nanostructures.
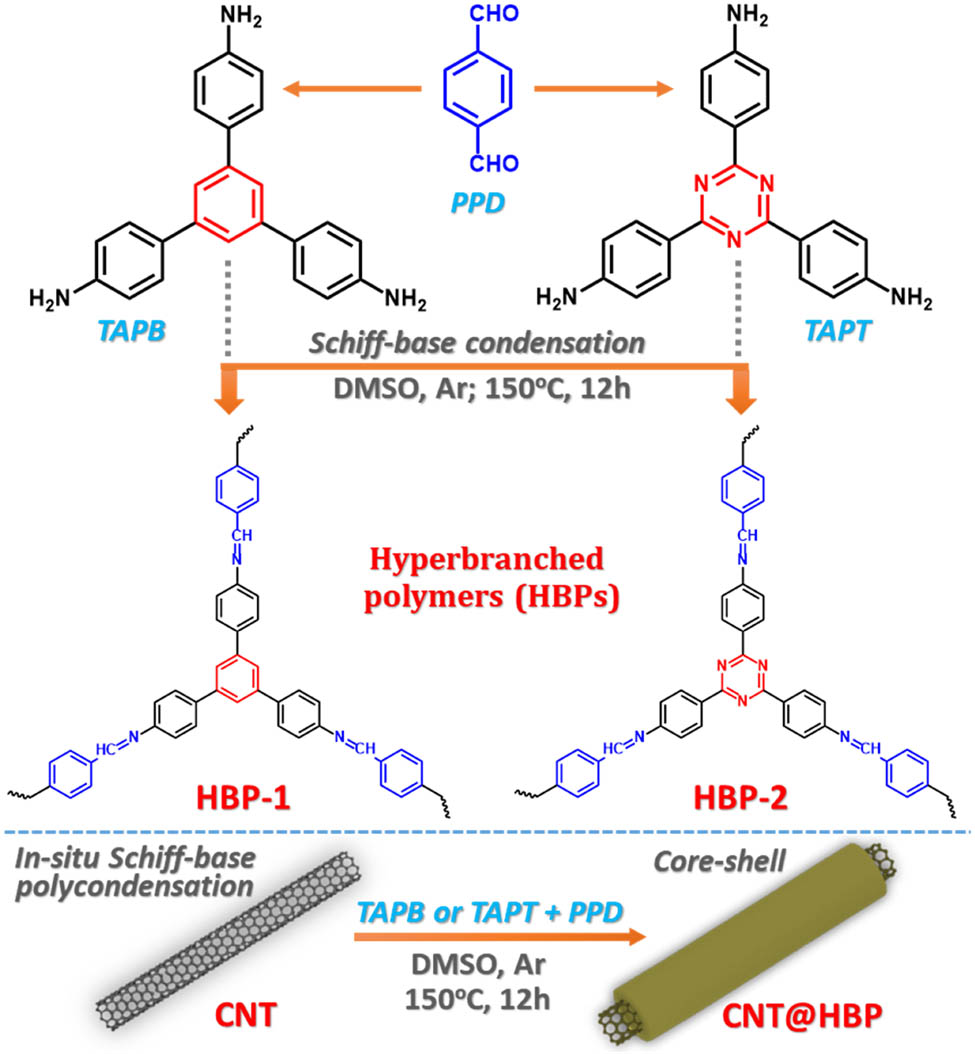
Chemical structures and synthesis routes of hyperbranched polymers (HBP-1 and HBP-2) and the composites with CNTs (CNT@HBP) by Schiff-base condensation.
Figure 2 shows SEM images of HBP-1, HBP-2, CNTs, and their composites. Both HBP-1 and HBP-2 exhibit near-spherical particles with average diameters of 915 and 196 nm, respectively. Bare CNTs have an average diameter of 41 nm. In contrast, CNT@HBP-1 (Figure 2c) and CNT@HBP-2 (Figure 2e and f) display large average diameters of about 77 nm, due to the homogeneous coating of hyperbranched polymers onto the surface of CNTs during the in situ polycondensation process. Apparently, such quasi-1D CNT@HBP-1 and CNT@HBP-2 are intertwined with each other to form 3D networks with porous internal channels, which are favorable for fast charge transport and high electrochemical utilization [36].
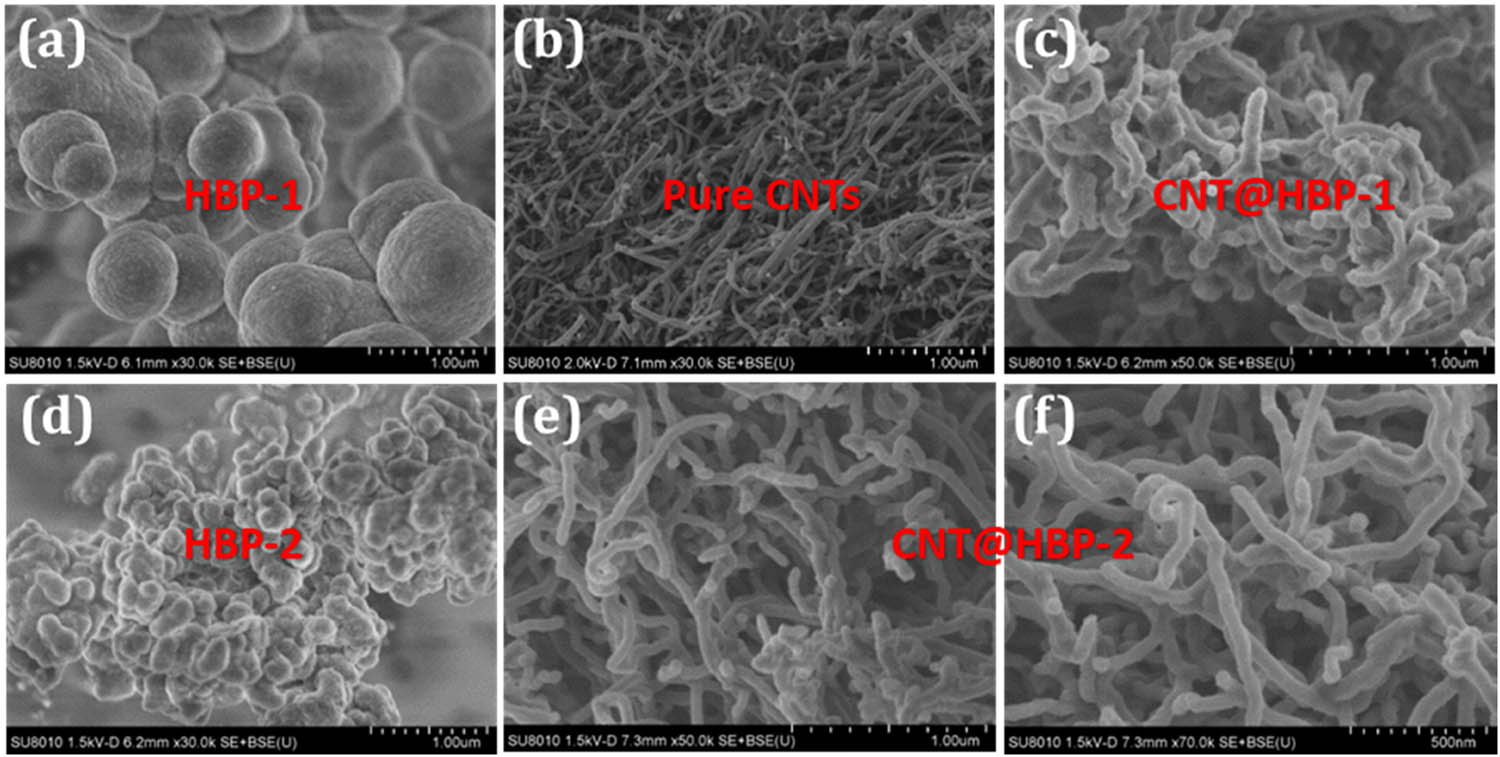
Typical SEM images of (a) HBP-1, (b) CNTs, (c) CNT@HBP-1, (d) HBP-2, and (e and f) CNT@HBP-2.
TEM tests also reveal that HBP-1 (Figure 3a) and HBP-2 (Figure 3d) exhibit near-spherical particles with average diameters of 908 and 186 nm, respectively. In contrast to pure CNTs (Figure 3b), CNT@HBP-1 (Figure 3c) and CNT@HBP-2 (Figure 3e and f) are uniformly covered by polymer shells with average thicknesses of 18.9 and 19.2 nm, respectively. These observations are in good accordance with SEM images, approving the formation of core–shell heterostructures through in situ Schiff-base condensation of organic monomers in the presence of CNTs.
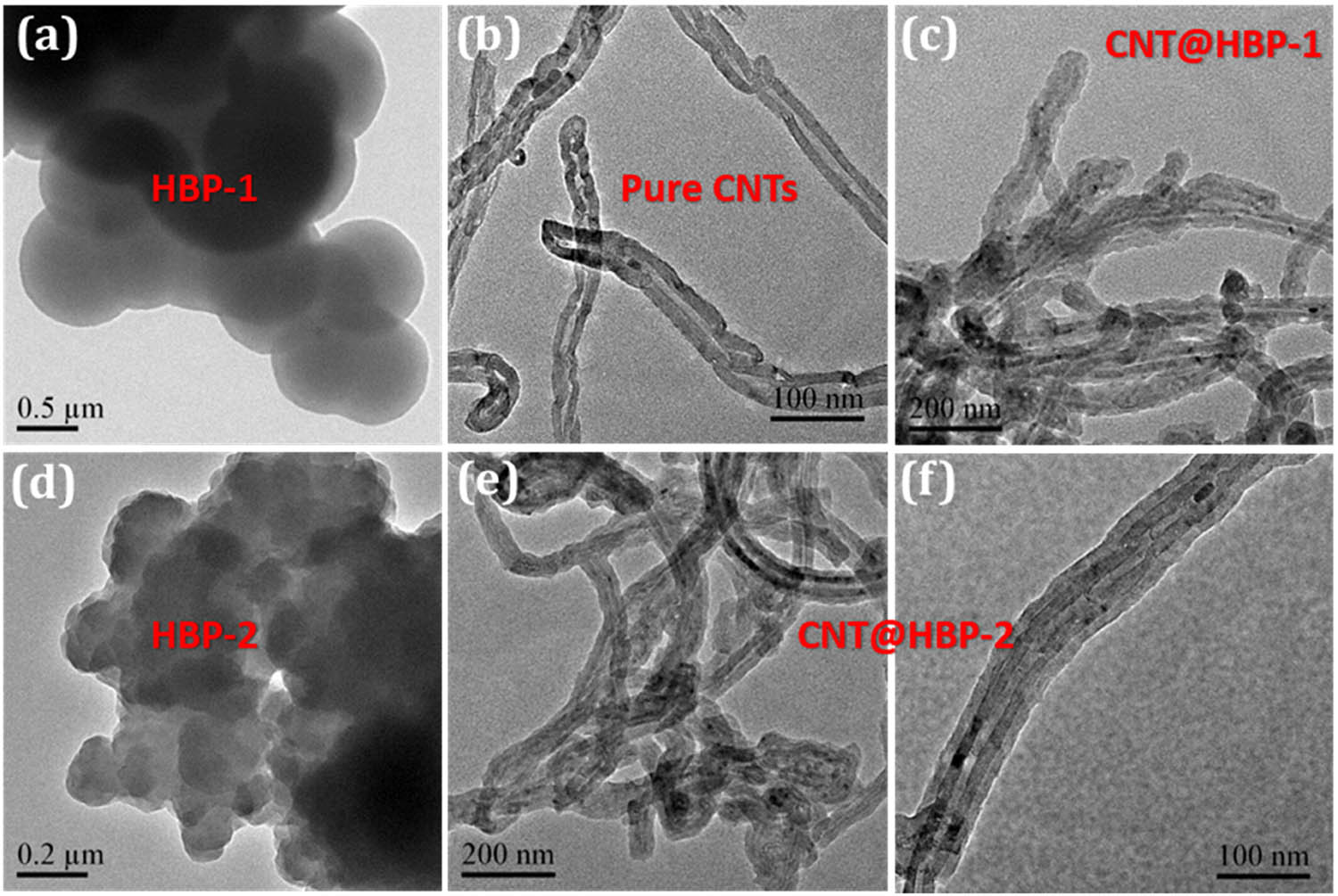
Typical TEM images of (a) HBP-1, (b) CNTs, (c) CNT@HBP-1, (d) HBP-2, and (e and f) CNT@HBP-2.
Further STEM observations disclose that single conductive hollow CNT as an inside axis is fully encapsulated in HBP-1 (Figure 4a) and HBP-2 (Figure 4b) with an evident contrast. The EDX elemental mapping analysis also verifies the presence of high-level elemental N signals in both CNT@HBP-1 and CNT@HBP-2, implying the formation of imine bonds (‒CH═N‒) in the HBP-1 and HBP-2 shells.
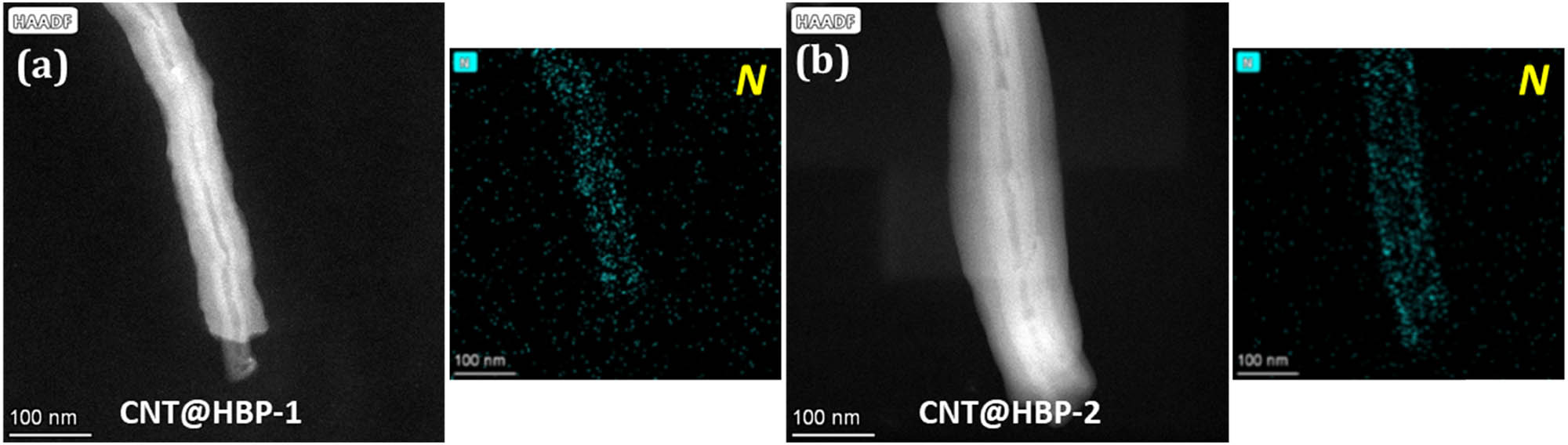
High-angle annular dark-field STEM images and the corresponding EDX elemental mappings of (a) CNT@HBP-1 and (b) CNT@HBP-2.
Chemical structures of the as-synthesized products were further examined by spectroscopy techniques. As shown in the FT-IR spectra (Figure 5a), the characteristic bands in the wavenumber range of 1,700‒1,200 cm−1 suggest the disappearance of aldehyde stretching at 1,690 cm−1 form PPD, and the emergence of C═N stretching at 1,630 cm−1 [37], due to the formation of imine-linked chains in HBP-1, HBP-2, CNT@HBP-1, and CNT@HBP-2. The absorption bands at about 1,510 cm−1 correspond to the characteristic vibration of triazine rings in HBP-2 and CNT@HBP-2 [38]. Raman spectra (Figure 5b) show that two characteristic D and 2D bands for CNTs also appear at ca. 1,330 and 2,660 cm−1 in CNT@HBP-1 and CNT@HBP-2 [39], respectively. The characteristic bands of aromatic C‒H at 1,170 cm−1 for HBP-1 and HBP-2 can be also detected in CNT@HBP-1 and CNT@HBP-2 [37]. Moreover, the coupled characteristic peaks (C═C/C═N) centered at 1,600 cm−1 for polymers and G-band of CNTs are almost completely overlapped. XRD patterns (Figure 5c) demonstrate weak peaks at 2θ = 26° in CNT@HBP-1 and CNT@HBP-2, corresponding to the (002) lattice plane of CNTs. However, polymers and their composites made in our experimental conditions are amorphous states, which are different from crystalline covalent organic frameworks [40]. The coupling of CNTs with polymers obviously increases the thermal stability of the latter (Figure 5d). CNTs exhibit a very tiny loss at 800°C while the major weight loss originates from polymers. The accurate composition was further estimated by the elemental analysis. The theoretical N and C contents are 8.4 and 86.8% for HBP-1, and 16.8 and 79.0% for HBP-2, based on their repeating units. The actual N fractions are 6.1% within CNT@HBP-1 and 12.4% within CNT@HBP-2, respectively. The N signal is not detectable in bare CNTs. Accordingly, it can be calculated that the loading amounts of CNTs are about 27.4% in CNT@HBP-1 and 26.2% in CNT@HBP-2, respectively, by assuming that all N moieties come from imine-linked polymers.
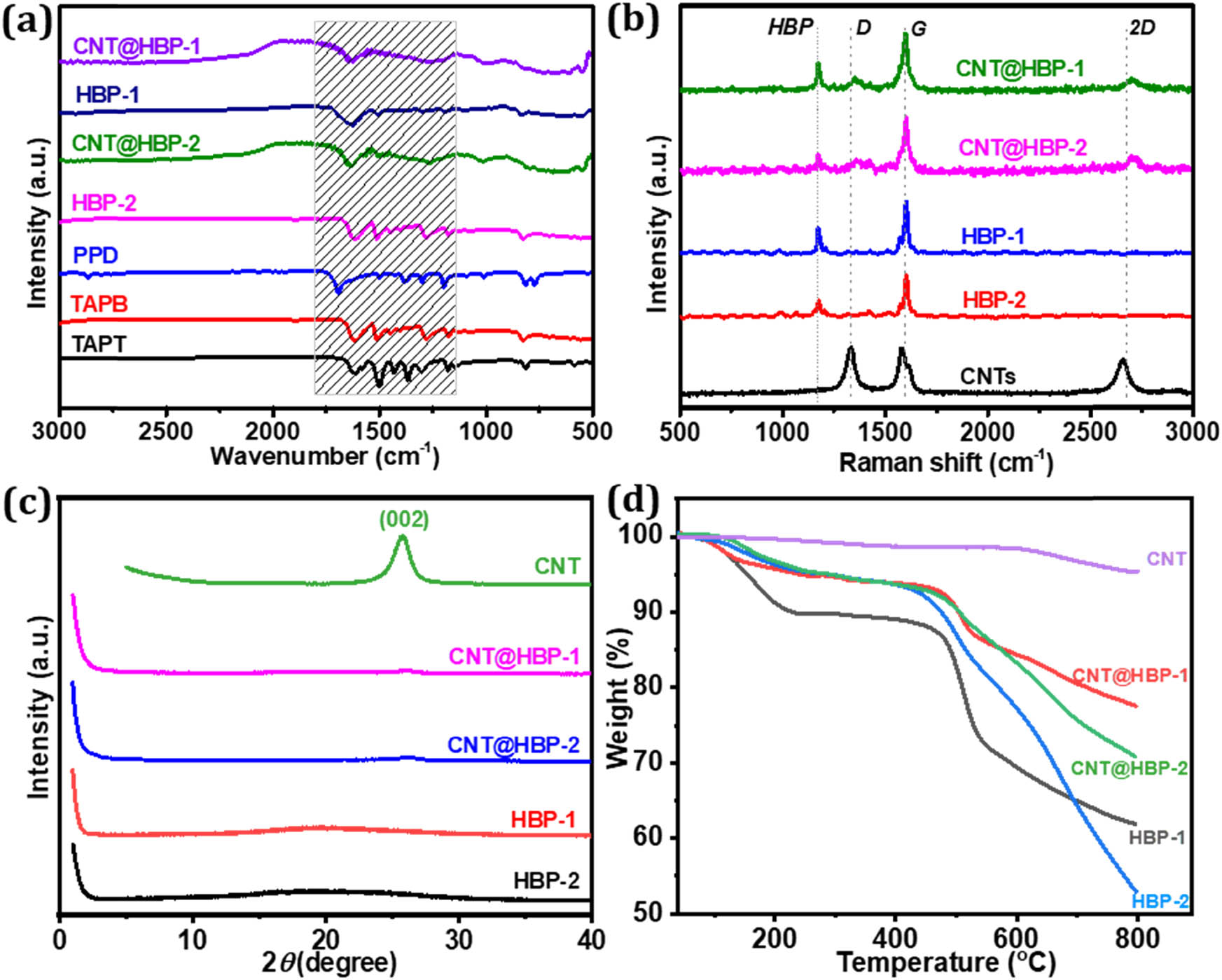
(a) FT-IR spectra, (b) Raman shifts, (c) XRD patterns, and (d) TGA curves of HBP-1, HBP-2, CNTs, CNT@HBP-1, and CNT@HBP-2.
3.2 Lithium-storage performance
Electrochemical performance of anode materials was carefully evaluated in a half-cell using the Li foil as the counter electrode. Figure 6 shows typical CV curves of HBP-1, HBP-2, CNT@HBP-1, and CNT@HBP-2. During the discharge process, the broad cathodic peaks centered at 0.82 V for HBP-1 and 0.77 V for CNT@HBP-1 (Figure 6a), and 0.85 V for HBP-2 and 0.78 V for CNT@HBP-2 (Figure 6b) can be attributed to the insertion of Li+ into the C═N bonds from imine bonds and triazine rings while the strong sharp peaks nearly 0.04 V correspond to the addition of lithium to the C═C bonds from aromatic rings [37,41,42]. During the reverse charge sweep, the anodic peaks centered at about 0.31 V are assigned to the extraction of Li+ from the C═C subunits, while the shoulder peaks appear at around 1.06 V for HBP-1, 1.02 V for CNT@HBP-1, 1.12 V for HBP-2, and 1.03 V for CNT@HBP-2 arising from the delithiation processes [43]. However, it is difficult to distinguish redox peaks of C═N between imine bonds and triazine rings due to their structural similarity [40]. Of note, both HBP-2 and CNT@HBP-2 exhibit the slightly larger polarization compared to HBP-1 and CNT@HBP-1, owing to the incorporation of electron-withdrawing triazine rings with an increased electron affinity [44]. Furthermore, both CNT@HBP-1 and CNT@HBP-2 present the stronger current response than HBP-1 and HBP-2, implying the higher redox activity enabled by conductive inside cores of CNTs [37].
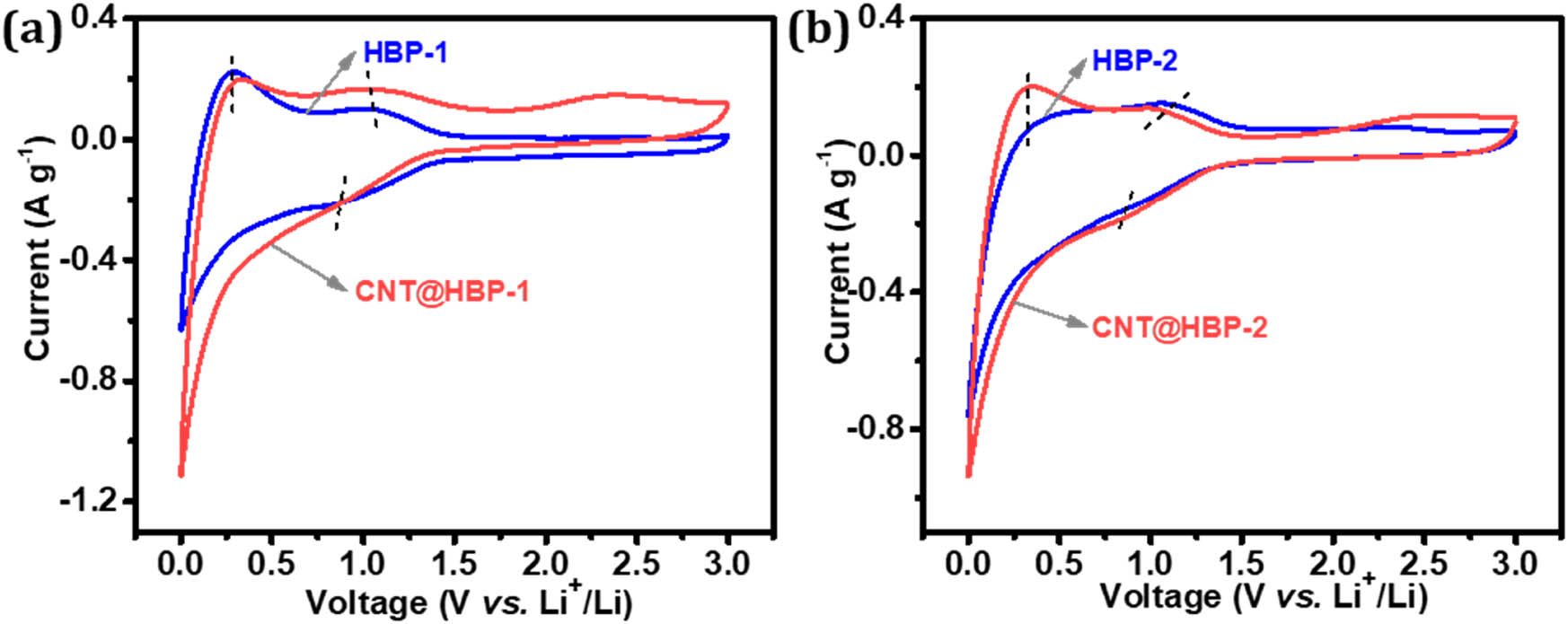
Stable CV curves of (a) HBP-1 and CNT@HBP-1 and (b) HBP-2 and CNT@HBP-2 at a scan rate of 0.5 mV s−1 in the third cycles.
Figure 7 shows discharge/charge curves of HBP-1, HBP-2, CNT@HBP-1, and CNT@HBP-2 in the initial three cycles. The initial discharge (lithiation)/charge (delithiation) capacities are detected to be 309/130 mA h g−1 for HBP-1 (Figure 7a), 1,287/466 mA h g−1 for CNT@HBP-1 (Figure 7b), 1,311/376 mA h g−1 for HBP-2 (Figure 7c), and 1,520/534 mA h g−1 for CNT@HBP-2 (Figure 7d), respectively. The low Coulombic efficiency (29‒42%) and drastic capacity decay in the first cycle are mainly ascribed to the decomposition of electrolyte and the formation of solid electrolyte interface film [45]. The discharge/charge profiles are then overlapped modestly in the subsequent cycles. Notably, the HBP-2-based anodes show much higher capacities compared to the HBP-1 ones, mainly due to the presence of highly redox-active triazine rings [38,46] as well as smaller sizes of HBP-2 relative to HBP-1. Remarkably, the encapsulation of conductive CNTs further improves the redox activity of HBP-1 and HBP-2 associated with the effective exposure of nano-sized outer shells, affording high electrochemical utilization and large specific capacities [28,37].
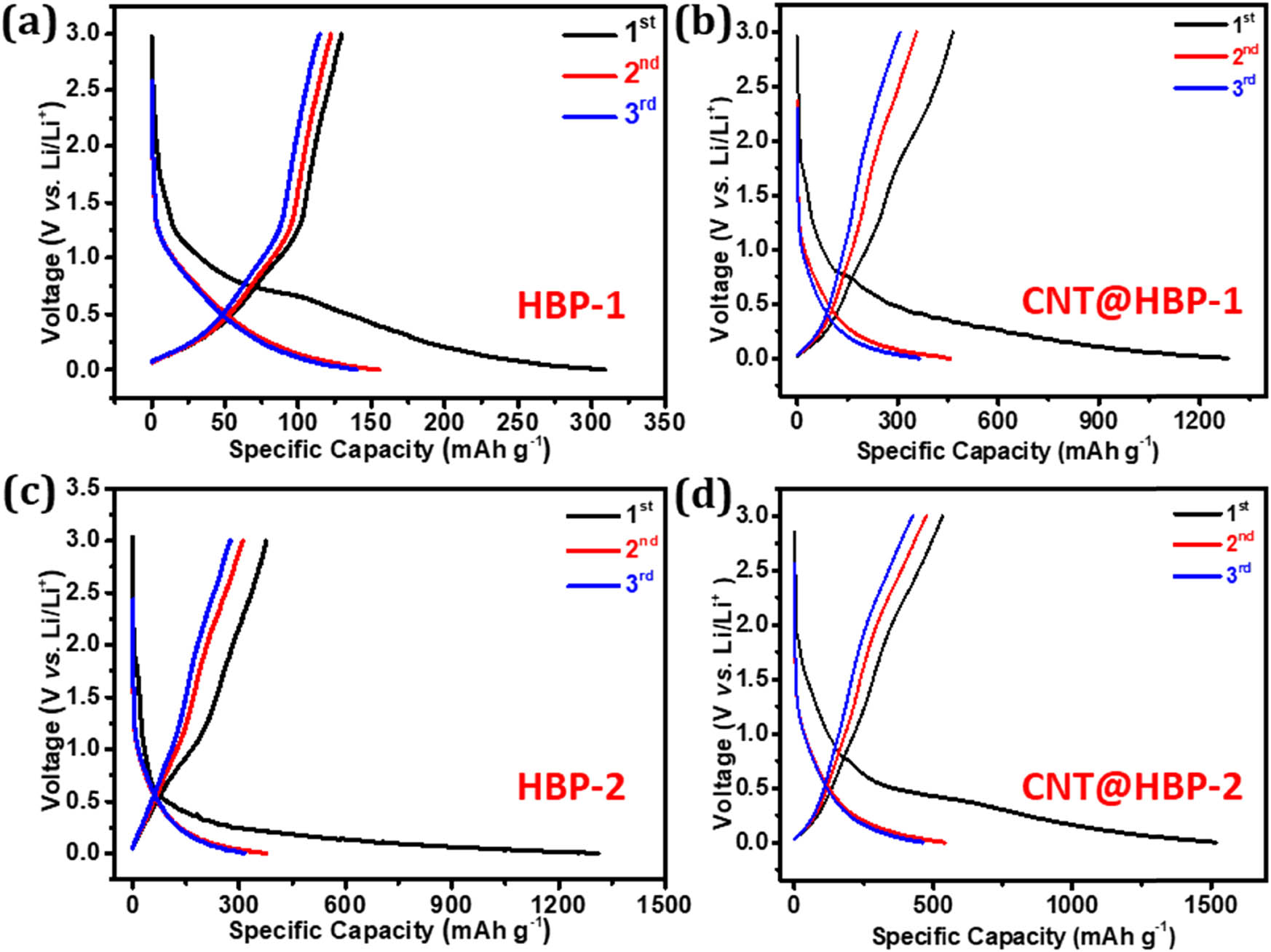
Charge/discharge curves of (a) HBP-1, (b) CNT@HBP-1, (c) HBP-2, and (d) CNT@HBP-2 at a current density of 0.1 A g−1 in the first three cycles.
Rate capabilities of electrode materials were further evaluated by galvanostatic charge–discharge, as shown in Figure 8. The CNT@HBP-1 electrode delivers higher reversible capacities at each rate compared to HBP-1 (Figure 8a). Similarly, the CNT@HBP-2 electrode also has higher specific capacities than HBP-2 with increasing the current density (Figure 8b). In particular, CNT@HBP-1 exhibits an average capacity of 335 mA h g−1 at 0.05 A g−1 and 56 mA h g−1 at 1 A g−1, and maintains 269 mA h g−1 after returning to 0.05 A g−1. Meanwhile, CNT@HBP-2 delivers an average capacity of 351 and 81 mA h g−1 at 0.05 and 1 A g−1, respectively, and retains 288 mA h g−1 after resuming to 0.05 A g−1. The lower capacities of HBP-1 and HBP-2 with respect to CNT@HBP-1 and CNT@HBP-2 mainly originate from the bulk congregating morphologies of pure polymers, leading to the insufficient electrolyte penetration, poor accessibility, and slow ion diffusion [23,47]. Furthermore, the slightly higher rate capability and larger specific capacity of CNT@HBP-2 relative to CNT@HBP-1 and polymers are attributed to the presence of triazine rings and CNTs capable of improving redox activity and electronic conduction during the discharge/charge processes [40,48].
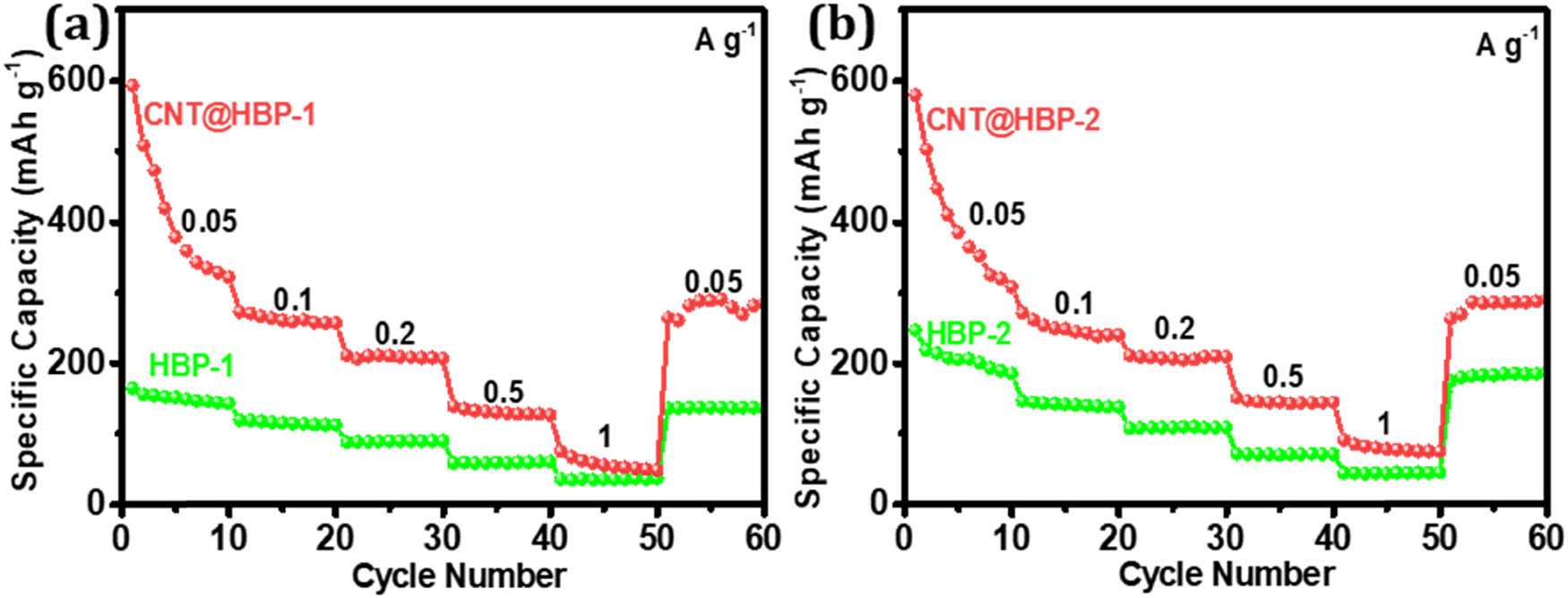
Rate performance of (a) HBP-1 and CNT@HBP-1 and (b) HBP-2 and CNT@HBP-2 at different current densities.
The EIS test was further conducted to evaluate the electrochemical kinetics. As shown in Figure 9a, CNT@HBP-1 (124 Ω) and CNT@HBP-2 (113 Ω) possess the lower charge transfer resistances than HBP-1 (166 Ω) and HBP-2 (147 Ω), because of the efficient electron transport and fast ion diffusion contributed by 3D conductive networks of CNTs. The presence of active triazine rings in HBP-2 also enables a lower resistance of CNT@HBP-2 compared to CNT@HBP-1 [49]. In addition, CNT@HBP-2 also maintains a higher capacity of 268 mA h g−1 after running at 0.1 A g−1 over 100 cycles (Figure 9b), compared to CNT@HBP-1 (155 mA h g−1) and its parent polymer (69 mA h g−1). Their Coulombic efficiencies still remain almost 100%, revealing the high reversibility and cycling stability. Interestingly, the reversible capacities at an enhanced rate of 0.5 A g−1 keep a gradual increase from the 100th to 400th cycles (Figure 9c), possibly due to the electrochemical activation throughout the electrode and superlithiation of aromatic C═C bonds [37,43]. In the prolonged 500th cycle, CNT@HBP-1 and CNT@HBP-2 deliver stable capacities of 617 and 256 mA h g−1, respectively. This implies that the rational integration of triazine and aromatic rings with conductive CNT networks can endow the resulted composite electrodes with abundant active sites, robust electrode structures, and powerful charge transport capability [28,50].
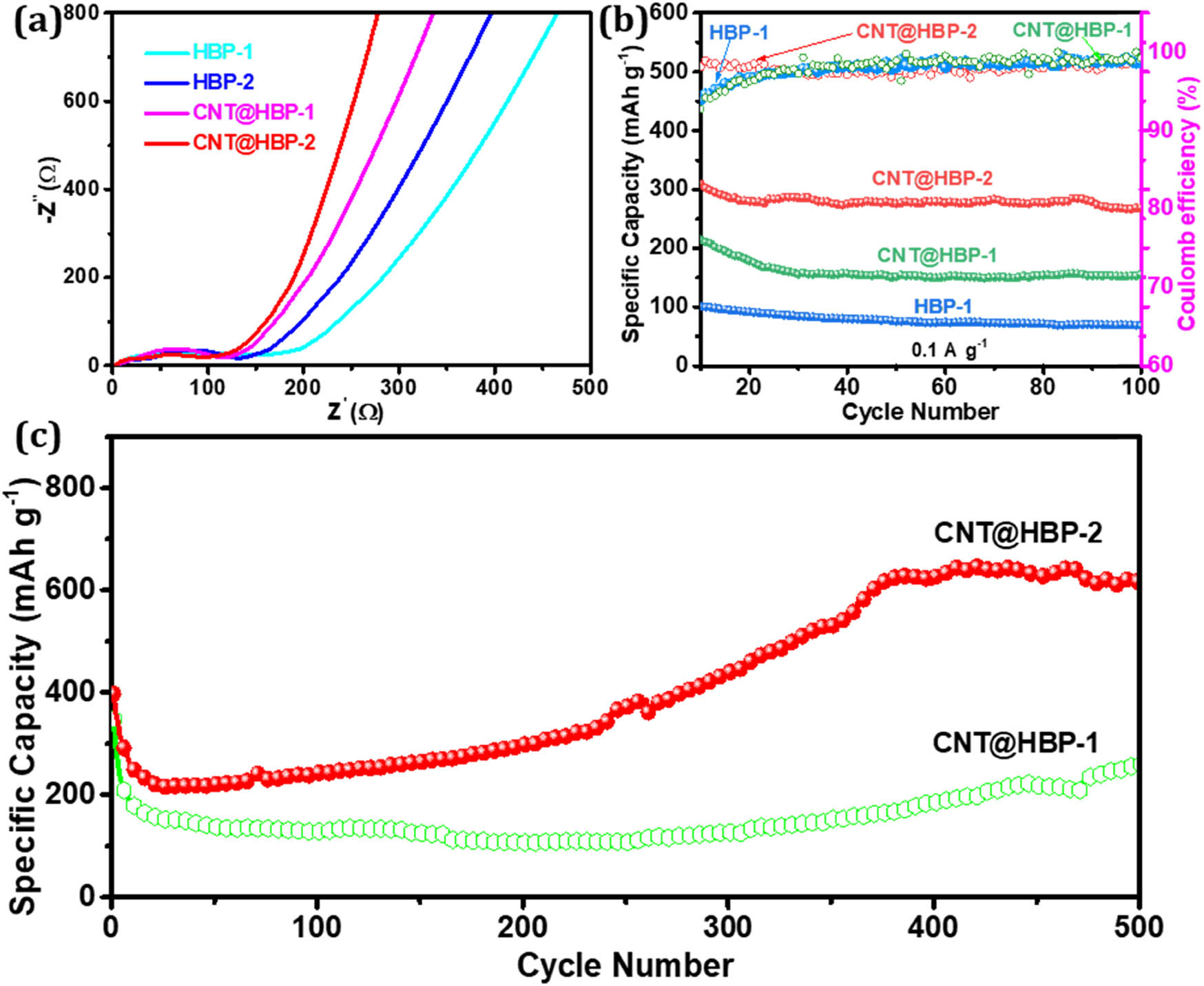
(a) Nyquist plots and (b) cycling stability of HBP-1, HBP-2, CNT@HBP-1, and CNT@HBP-2 at 0.1 A g−1 for 100 cycles, and (c) long-term cycling stability of CNT@HBP-1 and CNT@HBP-2 at 0.5 A g−1 for 500 cycles.
4 Conclusion
Core–shell heterostructures of CNTs and imine-based hyperbranched polymers were successfully fabricated by an in situ Schiff-base condensation between dialdehyde and tris-aminophenyl monomers. The resulted composites were further used as Li-ion anodes, which deliver the larger specific capacity, better rate capability, and higher cycling stability compared to their counterparts of pure polymers. The comprehensively improved electrochemical performance is mainly attributed to the synergistic effects of 3D electrically conductive CNT networks, interconnected porous internal channels, and efficiently exposed active sites. The further improvement in the lithium-storage performance can be also achieved by the incorporation of additional redox-active triazine units into the polymer shell around CNTs. This work would provide a new scenario for the rational crafting of metal-free polymer-based electrodes for the next-generation sustainable batteries.
-
Funding information: This work was financially supported by National Natural Science Foundation of China (52173091), Program for Leading Talents of National Ethnic Affairs Commission of China (MZR21001), and Hubei Provincial Natural Science Foundation of China (2021CFA022).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Dunn B, Kamath H, Tarascon JM. Electrical energy storage for the grid: a battery of choices. Science. 2011;334(6058):928–35.10.1126/science.1212741Search in Google Scholar PubMed
[2] Yang YK, Han CP, Jiang BB, Iocozzia J, He CG, Shi D, et al. Graphene-based materials with tailored nanostructures for energy conversion and storage. Mater Sci Eng R-Rep. 2016;102:1–72.10.1016/j.mser.2015.12.003Search in Google Scholar
[3] Lin YM, Huang JT, Shi LD, Cong GT, Zhu CZ, Xu J. Combining Zn0.76Co0.24s with s-doped graphene as high-performance anode materials for lithium-and sodium-ion batteries. Nanotechnol Rev. 2020;9(1):1227–36.10.1515/ntrev-2020-0091Search in Google Scholar
[4] Zhang JY, Huang ZY, He CG, Zhang JL, Mei P, Han XY, et al. Binary carbon-based additives in LiFePO4 cathode with favorable lithium storage. Nanotechnol Rev. 2020;9(1):934–44.10.1515/ntrev-2020-0071Search in Google Scholar
[5] Yan TT, Zhong SW, Zhou MM, Guo XM, Hu JW, Wang FF, et al. High-efficiency method for recycling lithium from spent LiFePO4 cathode. Nanotechnol Rev. 2020;9(1):1586–93.10.1515/ntrev-2020-0119Search in Google Scholar
[6] Zhao X, Zhao TK, Peng XR, Yang L, Shu Y, Jiang T, et al. In situ synthesis of expanded graphite embedded with amorphous carbon-coated aluminum particles as anode materials for lithium-ion batteries. Nanotechnol Rev. 2020;9(1):436–44.10.1515/ntrev-2020-0033Search in Google Scholar
[7] Goodenough JB. Evolution of strategies for modern rechargeable batteries. Acc Chem Res. 2013;46(5):1053–61.10.1021/ar2002705Search in Google Scholar PubMed
[8] Nayak PK, Yang L, Brehm W, Adelhelm P. From lithium-ion to sodium-ion batteries: advantages, challenges, and surprises. Angew Chem Int Ed Engl. 2018;57(1):102–20.10.1002/anie.201703772Search in Google Scholar PubMed
[9] Palacin MR. Recent advances in rechargeable battery materials: a chemist’s perspective. Chem Soc Rev. 2009;38(9):2565–75.10.1039/b820555hSearch in Google Scholar PubMed
[10] Yang XX, Peng Y, Hou J, Liu YF, Jian X. A review for modified Li composite anode: principle, preparation and challenge. Nanotechnol Rev. 2020;9(1):1610–24.10.1515/ntrev-2020-0120Search in Google Scholar
[11] Chen Z, Li X, Yang C, Cheng K, Tan T, Lv Y, et al. Hybrid porous crystalline materials from metal organic frameworks and covalent organic frameworks. Adv Sci. 2021;8(20):2101883.10.1002/advs.202101883Search in Google Scholar PubMed PubMed Central
[12] Yin X, Sarkar S, Shi S, Huang QA, Zhao H, Yan L, et al. Recent progress in advanced organic electrode materials for sodium‐ion batteries: synthesis, mechanisms, challenges and perspectives. Adv Funct Mater. 2020;30(11):1908445.10.1002/adfm.201908445Search in Google Scholar
[13] Chiluwal S, Rao AM, Podila R. Strategies for improving rechargeable lithium-ion batteries: from active materials to CO2 emissions. Nanotechnol Rev. 2021;10(1):1993–2026.10.1515/ntrev-2021-0114Search in Google Scholar
[14] Zhang Q, Huang Q, Hao SM, Deng S, He Q, Lin Z, et al. Polymers in lithium-sulfur batteries. Adv Sci. 2022;9(2):2103798.10.1002/advs.202103798Search in Google Scholar PubMed PubMed Central
[15] Cui X, Gao LK, Ma R, Wei ZN, Lu CH, Li ZL, et al. Pyrolysis-free covalent organic framework-based materials for efficient oxygen electrocatalysis. J Mater Chem A. 2021;9(37):20985–1004.10.1039/D1TA02795FSearch in Google Scholar
[16] Wang H, Yao CJ, Nie HJ, Wang KZ, Zhong YW, Chen PW, et al. Recent progress in carbonyl-based organic polymers as promising electrode materials for lithium-ion batteries (LIBs). J Mater Chem A. 2020;8(24):11906–22.10.1039/D0TA03321ASearch in Google Scholar
[17] Amin K, Mao L, Wei Z. Recent progress in polymeric carbonyl-based electrode materials for lithium and sodium ion batteries. Macromol Rapid Commun. 2019;40(1):1800565.10.1002/marc.201800565Search in Google Scholar PubMed
[18] Chen Y, Wang C. Designing high performance organic batteries. Acc Chem Res. 2020;53(11):2636–47.10.1021/acs.accounts.0c00465Search in Google Scholar PubMed
[19] Cao S, Li B, Zhu RM, Pang H. Design and synthesis of covalent organic frameworks towards energy and environment fields. Chem Eng J. 2019;355:602–23.10.1016/j.cej.2018.08.184Search in Google Scholar
[20] Zhang Q, Sha ZF, Cui X, Qiu SQ, He CG, Zhang JL, et al. Incorporation of redox-active polyimide binder into LiFePO4 cathode for high-rate electrochemical energy storage. Nanotechnol Rev. 2020;9(1):1350–8.10.1515/ntrev-2020-0092Search in Google Scholar
[21] Zhao Q, Zhu Z, Chen J. Molecular engineering with organic carbonyl electrode materials for advanced stationary and redox flow rechargeable batteries. Adv Mater. 2017;29(48):1607007.10.1002/adma.201607007Search in Google Scholar PubMed
[22] Zhang Q, Cui X, Hao S, Zhang Q, Guo Z, Li H, et al. Chain engineering of carbonyl polymers for sustainable lithium-ion batteries. Mater Today. 2021;50:170–98.10.1016/j.mattod.2021.08.006Search in Google Scholar
[23] Lei S, Dong YY, Dou Y, Zhang XF, Zhang Q, Yang YK. Polymerization-tailored polyimides as cathodes for lithium-ion batteries. Mater Adv. 2021;2(17):5785–90.10.1039/D1MA00554ESearch in Google Scholar
[24] Sha ZF, Qiu SQ, Zhang Q, Huang ZY, Cui X, Yang YK, et al. A facile solvothermal polymerization approach to thermoplastic polymer-based nanocomposites as alternative anodes for high-performance lithium-ion batteries. J Mater Chem A. 2019;7(40):23019–27.10.1039/C9TA08303KSearch in Google Scholar
[25] Lee S, Kwon G, Ku K, Yoon K, Jung SK, Lim HD, et al. Recent progress in organic electrodes for Li and Na rechargeable batteries. Adv Mater. 2018;30(42):1704682.10.1002/adma.201704682Search in Google Scholar PubMed
[26] Liang YL, Tao ZL, Chen J. Organic electrode materials for rechargeable lithium batteries. Adv Energy Mater. 2012;2(7):742–69.10.1002/aenm.201100795Search in Google Scholar
[27] Li H, Zhang X, Wang X, Zhang J, Yang Y. One-pot solvothermal incorporation of graphene into chain-engineered polyquinones for metal-free supercapacitors. Chem Commun. 2020;56(76):11191–4.10.1039/D0CC05310DSearch in Google Scholar
[28] Liu Q, Xiao Z, Cui X, Deng S, He Q, Zhang Q, et al. Conjugated cyclized-polyacrylonitrile encapsulated carbon nanotubes as core–sheath heterostructured anodes with favorable lithium storage. J Mater Chem A. 2021;9(11):6962–70.10.1039/D0TA12243BSearch in Google Scholar
[29] Luo Z, Liu L, Ning J, Lei K, Lu Y, Li F, et al. A microporous covalent-organic framework with abundant accessible carbonyl groups for lithium-ion batteries. Angew Chem Int Ed Engl. 2018;57(30):9443–6.10.1002/anie.201805540Search in Google Scholar PubMed
[30] Zhang Q, He Y, Lin GY, Ma XL, Xiao ZY, Shi DA, et al. In situ growth of polyimide nanoarrays on conductive carbon supports for high-rate charge storage and long-lived metal-free cathodes. J Mater Chem A. 2021;9(17):10652–60.10.1039/D1TA00302JSearch in Google Scholar
[31] Gomes R, Bhanja P, Bhaumik A. A triazine-based covalent organic polymer for efficient CO2 adsorption. Chem Commun. 2015;51(49):10050–3.10.1039/C5CC02147BSearch in Google Scholar PubMed
[32] Yang Y, Xie X, Yang Z, Wang X, Cui W, Yang J, et al. Controlled synthesis and novel solution rheology of hyperbranched poly(urea−urethane)-functionalized multiwalled carbon nanotubes. Macromolecules. 2007;40:5858–67.10.1021/ma0707077Search in Google Scholar
[33] Cheng QH, Debnath S, Gregan E, Byrne HJ. Effect of solvent solubility parameters on the dispersion of single-walled carbon nanotubes. J Phys Chem C. 2008;112(51):20154–8.10.1021/jp8067188Search in Google Scholar
[34] Wang J, Senkovska I, Oschatz M, Lohe MR, Borchardt L, Heerwig A, et al. Imine-linked polymer-derived nitrogen-doped microporous carbons with excellent CO2 capture properties. ACS Appl Mater Interfaces. 2013;5(8):3160–7.10.1021/am400059tSearch in Google Scholar PubMed
[35] Deng S, Zhang Q, Huang Q, Tang D, Mei P, Yang Y. Carbon nanotube-supported polyimide nanoarrays as sulfur host with physical/chemical polysulfide-traps for Li–S batteries. Compos Commun. 2022;29:101019.10.1016/j.coco.2021.101019Search in Google Scholar
[36] Huang ZY, Han XY, Cui X, He CG, Zhang JL, Wang XG, et al. Vertically aligned VS2 on graphene as a 3D heteroarchitectured anode material with capacitance-dominated lithium storage. J Mater Chem A. 2020;8(12):5882–9.10.1039/C9TA13835HSearch in Google Scholar
[37] Lei Z, Yang Q, Xu Y, Guo S, Sun W, Liu H, et al. Boosting lithium storage in covalent organic framework via activation of 14-electron redox chemistry. Nat Commun. 2018;9(1):576.10.1038/s41467-018-02889-7Search in Google Scholar PubMed PubMed Central
[38] Xiao Z, Han J, He H, Zhang X, Xiao J, Han D, et al. A template oriented one-dimensional schiff-base polymer: towards flexible nitrogen-enriched carbonaceous electrodes with ultrahigh electrochemical capacity. Nanoscale. 2021;13(45):19210–7.10.1039/D1NR05618BSearch in Google Scholar PubMed
[39] Yang Y, Wang X, Liu L, Xie X, Yang Z, Li RKY, et al. Structure and photoresponsive behaviors of multiwalled carbon nanotubes grafted by polyurethanes containing azobenzene side chains. J Phys Chem C. 2007;111(30):11231–9.10.1021/jp0728510Search in Google Scholar
[40] Patra BC, Das SK, Ghosh A, Raj KA, Moitra P, Addicoat M, et al. Covalent organic framework based microspheres as an anode material for rechargeable sodium batteries. J Mater Chem A. 2018;6(34):16655–63.10.1039/C8TA04611ESearch in Google Scholar
[41] Wu J, Rui X, Long G, Chen W, Yan Q, Zhang Q. Pushing up lithium storage through nanostructured polyazaacene analogues as anode. Angew Chem Int Ed Engl. 2015;54(25):7354–8.10.1002/anie.201503072Search in Google Scholar PubMed
[42] Man Z, Li P, Zhou D, Zang R, Wang S, Li P, et al. High-performance lithium–organic batteries by achieving 16 lithium storage in poly(imine-anthraquinone). J Mater Chem A. 2019;7(5):2368–75.10.1039/C8TA11230DSearch in Google Scholar
[43] Zhang Z, Zhou Y, Chen P, Zeng S, Nie W, Xu Y. Investigation of capacity increase in schiff-base networks as the organic anode for lithium-ion batteries. ACS Appl Energ Mater. 2021;4(11):12882–91.10.1021/acsaem.1c02569Search in Google Scholar
[44] Ma WY, Zhang C, Gao XM, Shu C, Yan C, Wang F, et al. Structure evolution of azo-fused conjugated microporous polymers for high performance lithium-ion batteries anodes. J Power Sources. 2020;453:227868.10.1016/j.jpowsour.2020.227868Search in Google Scholar
[45] Ortner TS. A granular look at solid electrolyte interfaces in lithium-ion batteries. Comm Chem. 2021;4(1):79.10.1038/s42004-021-00521-2Search in Google Scholar
[46] Bai LY, Gao Q, Zhao YL. Two fully conjugated covalent organic frameworks as anode materials for lithium ion batteries. J Mater Chem A. 2016;4(37):14106–10.10.1039/C6TA06449CSearch in Google Scholar
[47] Zhang Q, Lin G, He Y, Cui X, Yang Y. Chain engineering-tailored microstructures and lithium storage performance of hydrothermally-synthesized linear polyimides. Mater Today Chem. 2020;17:100341.10.1016/j.mtchem.2020.100341Search in Google Scholar
[48] Xiao Z, Song Q, Guo R, Kong D, Zhou S, Huang X, et al. Nitrogen-enriched carbon/CNT composites based on schiff-base networks: ultrahigh N content and enhanced lithium storage properties. Small. 2018;14(12):1703569.10.1002/smll.201703569Search in Google Scholar PubMed
[49] Zhang H, Sun W, Chen X, Wang Y. Few-layered fluorinated triazine-based covalent organic nanosheets for high-performance alkali organic batteries. ACS Nano. 2019;13(12):14252–61.10.1021/acsnano.9b07360Search in Google Scholar PubMed
[50] Wu L, Dai Y, Zeng W, Huang J, Liao B, Pang H. Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium–sulfur batteries. Nanotechnol Rev. 2021;10(1):20–33.10.1515/ntrev-2021-0005Search in Google Scholar
© 2022 Yu Dou et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption
Articles in the same Issue
- Research Articles
- Theoretical and experimental investigation of MWCNT dispersion effect on the elastic modulus of flexible PDMS/MWCNT nanocomposites
- Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques
- Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch/poly(lactic acid) blend bionanocomposites
- Glutathione-loaded non-ionic surfactant niosomes: A new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione
- Relationship between mechano-bactericidal activity and nanoblades density on chemically strengthened glass
- In situ regulation of microstructure and microwave-absorbing properties of FeSiAl through HNO3 oxidation
- Research on a mechanical model of magnetorheological fluid different diameter particles
- Nanomechanical and dynamic mechanical properties of rubber–wood–plastic composites
- Investigative properties of CeO2 doped with niobium: A combined characterization and DFT studies
- Miniaturized peptidomimetics and nano-vesiculation in endothelin types through probable nano-disk formation and structure property relationships of endothelins’ fragments
- N/S co-doped CoSe/C nanocubes as anode materials for Li-ion batteries
- Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight
- Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model
- Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances
- Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment
- Enhancement of the mechanical properties of HDPE mineral nanocomposites by filler particles modulation of the matrix plastic/elastic behavior
- Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films
- Optimization of nano coating to reduce the thermal deformation of ball screws
- Preparation of efficient piezoelectric PVDF–HFP/Ni composite films by high electric field poling
- MHD dissipative Casson nanofluid liquid film flow due to an unsteady stretching sheet with radiation influence and slip velocity phenomenon
- Effects of nano-SiO2 modification on rubberised mortar and concrete with recycled coarse aggregates
- Mechanical and microscopic properties of fiber-reinforced coal gangue-based geopolymer concrete
- Effect of morphology and size on the thermodynamic stability of cerium oxide nanoparticles: Experiment and molecular dynamics calculation
- Mechanical performance of a CFRP composite reinforced via gelatin-CNTs: A study on fiber interfacial enhancement and matrix enhancement
- A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances
- HTR: An ultra-high speed algorithm for cage recognition of clathrate hydrates
- Effects of microalloying elements added by in situ synthesis on the microstructure of WCu composites
- A highly sensitive nanobiosensor based on aptamer-conjugated graphene-decorated rhodium nanoparticles for detection of HER2-positive circulating tumor cells
- Progressive collapse performance of shear strengthened RC frames by nano CFRP
- Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes
- A Galerkin strategy for tri-hybridized mixture in ethylene glycol comprising variable diffusion and thermal conductivity using non-Fourier’s theory
- Simple models for tensile modulus of shape memory polymer nanocomposites at ambient temperature
- Preparation and morphological studies of tin sulfide nanoparticles and use as efficient photocatalysts for the degradation of rhodamine B and phenol
- Polyethyleneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture
- Electrospun nanofibers of Co3O4 nanocrystals encapsulated in cyclized-polyacrylonitrile for lithium storage
- Pitting corrosion induced on high-strength high carbon steel wire in high alkaline deaerated chloride electrolyte
- Formulation of polymeric nanoparticles loaded sorafenib; evaluation of cytotoxicity, molecular evaluation, and gene expression studies in lung and breast cancer cell lines
- Engineered nanocomposites in asphalt binders
- Influence of loading voltage, domain ratio, and additional load on the actuation of dielectric elastomer
- Thermally induced hex-graphene transitions in 2D carbon crystals
- The surface modification effect on the interfacial properties of glass fiber-reinforced epoxy: A molecular dynamics study
- Molecular dynamics study of deformation mechanism of interfacial microzone of Cu/Al2Cu/Al composites under tension
- Nanocolloid simulators of luminescent solar concentrator photovoltaic windows
- Compressive strength and anti-chloride ion penetration assessment of geopolymer mortar merging PVA fiber and nano-SiO2 using RBF–BP composite neural network
- Effect of 3-mercapto-1-propane sulfonate sulfonic acid and polyvinylpyrrolidone on the growth of cobalt pillar by electrodeposition
- Dynamics of convective slippery constraints on hybrid radiative Sutterby nanofluid flow by Galerkin finite element simulation
- Preparation of vanadium by the magnesiothermic self-propagating reduction and process control
- Microstructure-dependent photoelectrocatalytic activity of heterogeneous ZnO–ZnS nanosheets
- Cytotoxic and pro-inflammatory effects of molybdenum and tungsten disulphide on human bronchial cells
- Improving recycled aggregate concrete by compression casting and nano-silica
- Chemically reactive Maxwell nanoliquid flow by a stretching surface in the frames of Newtonian heating, nonlinear convection and radiative flux: Nanopolymer flow processing simulation
- Nonlinear dynamic and crack behaviors of carbon nanotubes-reinforced composites with various geometries
- Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer
- Synthesis and characterization of smart stimuli-responsive herbal drug-encapsulated nanoniosome particles for efficient treatment of breast cancer
- Homotopic simulation for heat transport phenomenon of the Burgers nanofluids flow over a stretching cylinder with thermal convective and zero mass flux conditions
- Incorporation of copper and strontium ions in TiO2 nanotubes via dopamine to enhance hemocompatibility and cytocompatibility
- Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles
- Mechanical properties and microstructure of nano-strengthened recycled aggregate concrete
- Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes
- Tunning matrix rheology and mechanical performance of ultra-high performance concrete using cellulose nanofibers
- Flexible MXene/copper/cellulose nanofiber heat spreader films with enhanced thermal conductivity
- Promoted charge separation and specific surface area via interlacing of N-doped titanium dioxide nanotubes on carbon nitride nanosheets for photocatalytic degradation of Rhodamine B
- Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita
- An implication of magnetic dipole in Carreau Yasuda liquid influenced by engine oil using ternary hybrid nanomaterial
- Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries
- Tunning self-assembled phases of bovine serum albumin via hydrothermal process to synthesize novel functional hydrogel for skin protection against UVB
- A comparative experimental study on damping properties of epoxy nanocomposite beams reinforced with carbon nanotubes and graphene nanoplatelets
- Lightweight and hydrophobic Ni/GO/PVA composite aerogels for ultrahigh performance electromagnetic interference shielding
- Research on the auxetic behavior and mechanical properties of periodically rotating graphene nanostructures
- Repairing performances of novel cement mortar modified with graphene oxide and polyacrylate polymer
- Closed-loop recycling and fabrication of hydrophilic CNT films with high performance
- Design of thin-film configuration of SnO2–Ag2O composites for NO2 gas-sensing applications
- Study on stress distribution of SiC/Al composites based on microstructure models with microns and nanoparticles
- PVDF green nanofibers as potential carriers for improving self-healing and mechanical properties of carbon fiber/epoxy prepregs
- Osteogenesis capability of three-dimensionally printed poly(lactic acid)-halloysite nanotube scaffolds containing strontium ranelate
- Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells
- Preparation and bonding mechanisms of polymer/metal hybrid composite by nano molding technology
- Damage self-sensing and strain monitoring of glass-reinforced epoxy composite impregnated with graphene nanoplatelet and multiwalled carbon nanotubes
- Thermal analysis characterisation of solar-powered ship using Oldroyd hybrid nanofluids in parabolic trough solar collector: An optimal thermal application
- Pyrene-functionalized halloysite nanotubes for simultaneously detecting and separating Hg(ii) in aqueous media: A comprehensive comparison on interparticle and intraparticle excimers
- Fabrication of self-assembly CNT flexible film and its piezoresistive sensing behaviors
- Thermal valuation and entropy inspection of second-grade nanoscale fluid flow over a stretching surface by applying Koo–Kleinstreuer–Li relation
- Mechanical properties and microstructure of nano-SiO2 and basalt-fiber-reinforced recycled aggregate concrete
- Characterization and tribology performance of polyaniline-coated nanodiamond lubricant additives
- Combined impact of Marangoni convection and thermophoretic particle deposition on chemically reactive transport of nanofluid flow over a stretching surface
- Spark plasma extrusion of binder free hydroxyapatite powder
- An investigation on thermo-mechanical performance of graphene-oxide-reinforced shape memory polymer
- Effect of nanoadditives on the novel leather fiber/recycled poly(ethylene-vinyl-acetate) polymer composites for multifunctional applications: Fabrication, characterizations, and multiobjective optimization using central composite design
- Design selection for a hemispherical dimple core sandwich panel using hybrid multi-criteria decision-making methods
- Improving tensile strength and impact toughness of plasticized poly(lactic acid) biocomposites by incorporating nanofibrillated cellulose
- Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity
- The effect of TaC and NbC hybrid and mono-nanoparticles on AA2024 nanocomposites: Microstructure, strengthening, and artificial aging
- Excited-state geometry relaxation of pyrene-modified cellulose nanocrystals under UV-light excitation for detecting Fe3+
- Effect of CNTs and MEA on the creep of face-slab concrete at an early age
- Effect of deformation conditions on compression phase transformation of AZ31
- Application of MXene as a new generation of highly conductive coating materials for electromembrane-surrounded solid-phase microextraction
- A comparative study of the elasto-plastic properties for ceramic nanocomposites filled by graphene or graphene oxide nanoplates
- Encapsulation strategies for improving the biological behavior of CdS@ZIF-8 nanocomposites
- Biosynthesis of ZnO NPs from pumpkin seeds’ extract and elucidation of its anticancer potential against breast cancer
- Preliminary trials of the gold nanoparticles conjugated chrysin: An assessment of anti-oxidant, anti-microbial, and in vitro cytotoxic activities of a nanoformulated flavonoid
- Effect of micron-scale pores increased by nano-SiO2 sol modification on the strength of cement mortar
- Fractional simulations for thermal flow of hybrid nanofluid with aluminum oxide and titanium oxide nanoparticles with water and blood base fluids
- The effect of graphene nano-powder on the viscosity of water: An experimental study and artificial neural network modeling
- Development of a novel heat- and shear-resistant nano-silica gelling agent
- Characterization, biocompatibility and in vivo of nominal MnO2-containing wollastonite glass-ceramic
- Entropy production simulation of second-grade magnetic nanomaterials flowing across an expanding surface with viscidness dissipative flux
- Enhancement in structural, morphological, and optical properties of copper oxide for optoelectronic device applications
- Aptamer-functionalized chitosan-coated gold nanoparticle complex as a suitable targeted drug carrier for improved breast cancer treatment
- Performance and overall evaluation of nano-alumina-modified asphalt mixture
- Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features
- Synthesis of Ag@AgCl modified anatase/rutile/brookite mixed phase TiO2 and their photocatalytic property
- Mechanisms and influential variables on the abrasion resistance hydraulic concrete
- Synergistic reinforcement mechanism of basalt fiber/cellulose nanocrystals/polypropylene composites
- Achieving excellent oxidation resistance and mechanical properties of TiB2–B4C/carbon aerogel composites by quick-gelation and mechanical mixing
- Microwave-assisted sol–gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash
- Pulsed laser-assisted synthesis of nano nickel(ii) oxide-anchored graphitic carbon nitride: Characterizations and their potential antibacterial/anti-biofilm applications
- Effects of nano-ZrSi2 on thermal stability of phenolic resin and thermal reusability of quartz–phenolic composites
- Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit
- Mechanical and heat transfer properties of 4D-printed shape memory graphene oxide/epoxy acrylate composites
- Coupling the vanadium-induced amorphous/crystalline NiFe2O4 with phosphide heterojunction toward active oxygen evolution reaction catalysts
- Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures
- Gray correlation analysis of factors influencing compressive strength and durability of nano-SiO2 and PVA fiber reinforced geopolymer mortar
- Preparation of layered gradient Cu–Cr–Ti alloy with excellent mechanical properties, thermal stability, and electrical conductivity
- Recovery of Cr from chrome-containing leather wastes to develop aluminum-based composite material along with Al2O3 ceramic particles: An ingenious approach
- Mechanisms of the improved stiffness of flexible polymers under impact loading
- Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests
- Review Articles
- Proposed approaches for coronaviruses elimination from wastewater: Membrane techniques and nanotechnology solutions
- Application of Pickering emulsion in oil drilling and production
- The contribution of microfluidics to the fight against tuberculosis
- Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements
- Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy
- Contemporary nano-architectured drugs and leads for ανβ3 integrin-based chemotherapy: Rationale and retrospect
- State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials
- Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications
- A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators
- Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems
- Advances in ZnO: Manipulation of defects for enhancing their technological potentials
- Efficacious nanomedicine track toward combating COVID-19
- A review of the design, processes, and properties of Mg-based composites
- Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes
- Two-dimensional nanomaterial-based polymer composites: Fundamentals and applications
- Recent progress and challenges in plasmonic nanomaterials
- Apoptotic cell-derived micro/nanosized extracellular vesicles in tissue regeneration
- Electronic noses based on metal oxide nanowires: A review
- Framework materials for supercapacitors
- An overview on the reproductive toxicity of graphene derivatives: Highlighting the importance
- Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis
- Research progress of carbon materials in the field of three-dimensional printing polymer nanocomposites
- A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics
- Recent advances in the preparation of PVDF-based piezoelectric materials
- Recent developments in tensile properties of friction welding of carbon fiber-reinforced composite: A review
- Comprehensive review of the properties of fly ash-based geopolymer with additive of nano-SiO2
- Perspectives in biopolymer/graphene-based composite application: Advances, challenges, and recommendations
- Graphene-based nanocomposite using new modeling molecular dynamic simulations for proposed neutralizing mechanism and real-time sensing of COVID-19
- Nanotechnology application on bamboo materials: A review
- Recent developments and future perspectives of biorenewable nanocomposites for advanced applications
- Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery
- 3D printing customized design of human bone tissue implant and its application
- Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications
- A brief review of nanoparticles-doped PEDOT:PSS nanocomposite for OLED and OPV
- Nanotechnology interventions as a putative tool for the treatment of dental afflictions
- Recent advancements in metal–organic frameworks integrating quantum dots (QDs@MOF) and their potential applications
- A focused review of short electrospun nanofiber preparation techniques for composite reinforcement
- Microstructural characteristics and nano-modification of interfacial transition zone in concrete: A review
- Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review
- Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks
- Molecular dynamics application of cocrystal energetic materials: A review
- Synthesis and application of nanometer hydroxyapatite in biomedicine
- Cutting-edge development in waste-recycled nanomaterials for energy storage and conversion applications
- Biological applications of ternary quantum dots: A review
- Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy
- Application of antibacterial nanoparticles in orthodontic materials
- Effect of natural-based biological hydrogels combined with growth factors on skin wound healing
- Nanozymes – A route to overcome microbial resistance: A viewpoint
- Recent developments and applications of smart nanoparticles in biomedicine
- Contemporary review on carbon nanotube (CNT) composites and their impact on multifarious applications
- Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review
- Diamond-like carbon films for tribological modification of rubber
- Layered double hydroxides (LDHs) modified cement-based materials: A systematic review
- Recent research progress and advanced applications of silica/polymer nanocomposites
- Modeling of supramolecular biopolymers: Leading the in silico revolution of tissue engineering and nanomedicine
- Recent advances in perovskites-based optoelectronics
- Biogenic synthesis of palladium nanoparticles: New production methods and applications
- A comprehensive review of nanofluids with fractional derivatives: Modeling and application
- Electrospinning of marine polysaccharides: Processing and chemical aspects, challenges, and future prospects
- Electrohydrodynamic printing for demanding devices: A review of processing and applications
- Rapid Communications
- Structural material with designed thermal twist for a simple actuation
- Recent advances in photothermal materials for solar-driven crude oil adsorption

