Abstract
Biodiesel production from waste cooking oil was obtained using Thermomyces lanuginosus (TL) lipase (E.C.3.1.1.3) anchored on Fe3O4/Au nanoparticles through physical interactions. A remarkable biodiesel yield of ∼90% was obtained without any pre-treatment and at a lipase concentration of 20%, 45°C reaction temperature, 1:6 oil/methanol molar ratio, after 24 h. The immobilized enzyme showed fast kinetic (the biodiesel yield was already of 34.6% after only 3 h) and activity slightly dependent on the length of the acid chains. The effect of the Au NPs sizes was monitored, to study the role of Au conduction centres in facilitating enzymes favourable orientation. The immobilized lipase activity stays above 74% after the first 3 cycles of use. In particular, the produced biodiesel presents an ester content of 97.8% ± 0.21 and a linolenic methyl ester content of 0.53% ± 0.03, in agreement with EN14214 requirements.
1 Introduction
In the last decades, energy consumption has increased significantly because of the change in lifestyles and growth of population. The increase in the energy demand was typically provided by fossil fuel resources, which, otherwise, are limited and cause of serious environmental concerns. Therefore, there is an urgent need for alternative and renewable fuel, such as biodiesel. Biodiesel is a renewable, clean and environmentally friendly fuel derived from vegetable oils and animal fats [1]. However, it has been reported that the cost of the raw materials is about 80% of the total biodiesel production cost [2]. In this context, the cheaper waste cooking oils (WCOs) are potential substitutes for vegetable oils in the production of biodiesel. The use of WCOs, as a secondary raw material, provides the additional advantage to reduce the disposal concerns [3]. The amount of cooking oil produced every year is immense, over 15 million of tons, which, if converted, can satisfy to a large extent the world demand of biodiesel [4]. The production of biodiesel from WCOs allows for a 21% in crude oil saving and 96% in fossil energy saving [5]. Each kilogram of WCOs can be converted into biodiesel with very high yields.
On the other hand, the use of WCOs is challenging, as they basically contain free fatty acid (FFA) and water which make them unsuitable for homogeneous alkaline transesterification catalysis. The typical alkaline catalysts, such as sodium hydroxide (NaOH) and potassium methoxide (KOCH3) [6], incur in saponification reaction in the presence of water [7, 8, 9]. More in general, conventional chemical processes, either from acid or base catalysts, suffer for difficulties in glycerol recovering and removal of inorganic salts, high temperatures, undesirable side reactions [10], and require the use of pretreatments.
Enzymes catalyzed processes have great potential compared to chemical methods [11,12]: intrinsic low environmental impact, no need of pretreatments to remove water, FFAs, etc., high efficiency and conversion of free fatty acids, lower energy consumption, mild conditions during the reactions, product purity and facilitated separation of glycerol are the main advantages of the enzymatic processes [13]. The process of transesterification is affected by different variables. The enzymatic production of biodiesel can be influenced by many aspects such as activity, stability and specificity of the enzyme, alcohol-oil ratio, temperature and water content [11,14,15]. On the other hand, the enzymes can give high biodiesel yields and can exhibit reaction times comparable to those of base catalysts, requiring, in general, higher amounts of catalyst but lower of alcohol [16]. Three major drawbacks still persist the enzyme availability at commercial scale; the enzyme costs; and, when they are surpassed, i.e., in the case of enzyme immobilization, the difficulties to reproduce laboratory experiments in large (i.e. design and up-scaling of the reactor).
Immobilization permits to increase the enzyme time of usage [17,18]. For example, immobilized Thermomyces lanuginosus (TL) lipase on hydrotalcite was found able to catalyze transesterification of WCOs [17]. In particular, the authors observed, after seven cycles, activity retention of 36% at 45°C and of 14% at 55°C. Although, in the paper of Wang et al. [18], MAS1, a laboratory prepared enzyme, showed higher stability and activity, immobilized Thermomyces lanuginosus (TL) lipase on a XAD1180 resin provided similar results to those observed by Yagiz et al. [17]. More recently, magnetic nanomaterials offer in this context a new opportunity [13,19, 20, 21], i.e., higher activities of immobilized enzymes, providing: a high surface area support to anchor high payload of enzymes; a facile separation between the product and the catalyst; reduced diffusion limitation, i.e. easy transition from laboratory to industrial scale and reactor scale-up [14,18,22]. Moreover, physical absorption allows support reusability, whereas maintaining activity [23]. Activity retention, after 5 cycles, of about 58 %, and biodiesel yield of about 20%, after 3 h, were obtained in [19], through the use of Pseudomonas cepacia immobilized on Fe3O4 NPs. Similar results were shown by Km 12 lipase and Burkholderia sp. enzyme immobilized on Fe3O4 NPs [20,21]. Lipase from Candida antarctica, covalently immobilized on magnetic NPs, in the presence of molecular sieves, achieves an activity of 100%, after 96 h [13]. On the other hand, although support plays a key role, it has been little or no studied. Typically, after support choice, the papers focus on the evaluation of the operation parameters effects or enzymes choice.
Here we report, the production of biodiesel from WCOs, using a commercial Thermomyces lanuginosus (TL) lipase directly linked on citric acid and residual oleic acid modified Fe3O4/Au nanoparticles [24], consisting of magnetite NPs supporting smaller Au NPs, through physical adsorption including interfacial activation [23].
Our nanocatalyst, tested on non-pretreated WCOs [25] and without other additives, due to support specificity and favourable interaction with the support, shows a very remarkable combination of properties: excellent activity, also in the presence of high amount of water; stability; recyclability, also of the support because of the physical interaction. It ensures an almost homogeneous catalysis of waste oil. Furthermore, considering the key role of the support, particular attention was devoted to elucidate the role of Au conduction centres in facilitating transfer of electrons and enzymes favourable orientation, through the evaluation of the Au NPs sizes effect.
Moreover, an accurate analysis, which is rarely shown, of the different phases and of the catalyst activity, and a characterization according to EN14214 were performed and reported.
2 Feedstock and experimental
2.1 Materials
All chemicals were analytical grade and acquired from Aldrich Chemical Co.
WCOs were obtained from olive oil (Sigma Aldrich-O1514, analytical grade), after a simulated cooking (temperature of 180°C for 5 h) [25].
2.2 Physicochemical characterization of WCOs
Prior to the biodiesel production, the properties of the WCOs were determined, including: (i) saponification value, (ii) water content, (iii) iodine value and (iv) acid value. The oil properties were analysed in accordance with EN14214. All experiments were run in triplicate and mean values were reported. The psychical properties of WCOs used were collected in Table 1.
Physicochemical properties of WCO. GC-MS configuration. Each value represents the mean of three replicates ± SD.
| Physicochemical properties | ||
|---|---|---|
| Property | Value | Unit |
| Acid value | 1.85 ± 0.08 | (mg KOH/g) |
| Free fatty acid content | 0.93 ± 0.06 | (%) |
| Moisture | 0.08 ± 0.003 | (%) |
| Saponification Index | 182.32 ± 0.55 | (mg KOH/g) |
| Iodine value | 71.67 | (g I2/100g oil) |
2.3 Synthesis of Fe3O4/Au NPs, ligand exchange to obtain hydrophilic NPs, lipase immobilization
The Fe3O4/Au@OA nanoparticles were prepared as previously reported [24]. In particular, two diefferent samples were prepared increasing the amount of Au NPs precursor (HAuCl4) from 42 mg to 62 mg.
For the immobilization, optimized conditions [24] were chosen. In particular, modified NPs were mixed [24] with 10 mL of buffer solution (phosphate buffer 0.1 M, to give at pH = 3.0) with 2 mg of TL (solution ≥ 100,000 U/g from Sigma Aldrich) and shaken at 200 rpm, T = 4°C, for 180 min, obtaining TL immobilized lipase, named NPs@TL in the following. pH 3, lower than isoelectric point of
lipase, was chosen for immobilization, resulting in a more stable enzyme.
2.4 Synthesis of methyl ester
A vessel reactor (25 mL in volume) continuously stirred (200 rpm speed) was used for the methyl ester productions at temperatures of 45°C and 55°C. Three different experiments were performed starting from 1 g of WCOs, in the presence of free or immobilized lipase (5%, 10%, 20% g of enzyme/g of WCO), oil/methanol ratio 1:6. Furthermore, three additional experiments were performed in presence of 10% of enzyme at different oil/methanol ratio, obtained following the procedure described in [24] to give 1:3, 1:6 and 1:18 molar ratio. At an oil/methanol ratio of 6:1 two new experiments were performed in presence of water 10 wt% and 15 wt%. The effect of the cycles number was finally examined at 10% of enzyme (grams of enzyme per grams of WCO) also in presence of water 15 wt%.
Immobilized enzyme was recovered at the end of the transesterification processes under a magnetic field. After few minutes of centrifugation the upper layer of the sample was washed with hot water at a temperature of 60°C and finally dried with anhydrous sodium sulphate to obtain biodiesel.
The oil conversion to methyl ester, i.e. biodiesel yield, was determined as in the following:
The analysis of the fatty acids methyl esters (FAME) produced was carried out by a GC-MS (Thermo Fischer Scientific) equipped with a TraceGOLD™ TG-Polar capillary column (0.25 μm × 0.25 mm × 60 m). GC-MS configuration: initial temperature 120°C for 4 min, rate 1, 6.5°C/min to 170°C, rate 2, 2.75°C/min to 250°C for 9 min. Injector and detector temperatures were set 250°C and 230°C, respectively. Helium was used as the carrier gas. Methanol:BF3 method [26] was used for WCOs derivatization the to obtain composition and times of retention of FAMEs, that were compared with a known concentration FAME mixture and the biodiesel produced. It has been observed that the retention times of the produced biodiesel were almost similar.
EN14214 was used for the methyl ester content evaluation in the produced biodiesel [24]. Measurements were performed in triplicate.
3 Results and discussion
The effect of the oil/methanol molar ratio on the biodiesel yield, obtained by using immobilized lipase at 45°C, and after 24 h of reaction, is shown in Figure 1. Biodiesel yield at an oil methanol ratio of 1:3 results equal to 81.8%. It increases, up to a maximum of 84.5%, for an oil methanol ratio of 1:6. However, further increase in oil/methanol molar ratio results in a lower biodiesel yield, likely due to the inactivation of the lipase exposed to higher concentrations of methanol [22]. Therefore, although the difference in the biodiesel yield obtained at 1:3 and 1:6 oil/methanol molar ratio are limited, a molar ratio of 1:6 was chosen for the further experiments.
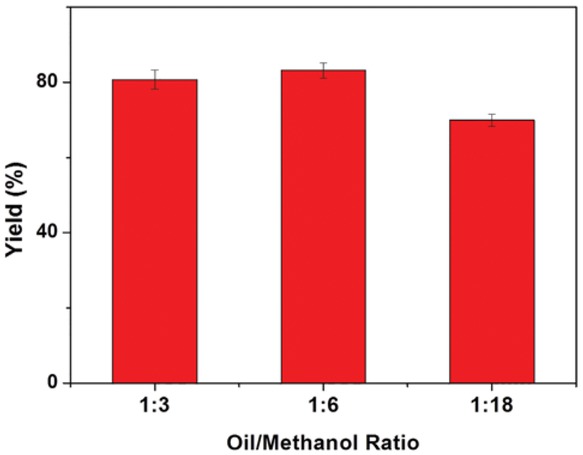
Effect of methanol to oil molar ratio on the free and immobilized lipase for biodiesel production. Immobilization conditions – coupling temperature: 4°C; coupling pH: 3; coupling time: 3 h; lipase amount: 2 mg. Reaction conditions – reaction time: 24 h; reaction temperature: 45°C; lipase concentration: 10%.
Figure 2 shows the effect of enzyme loading on biodiesel yield. Biodiesel yield, after 24 h, increased from 65.42% to 89.53% when enzyme loading ranged from 5 to 20 (g of enzyme/g of WCO). Moreover, after 3 h and 6 h, at 20% enzyme loading, the biodiesel yields was already 34.6% and 70.1%, indicating a fast kinetics for our immobilized TL. Although this is due to the enzyme specificity, the comparison with other literature results [17,18], obtained in similar conditions, suggests a key role of the enzyme interaction with the support, while the quasi-homogeneous catalysis cannot be neglected as well. Enzyme activity is typically slightly affected by water contents. The activity of the immobilized lipase, after 24 h at a methanol/oil molar ratio of 6:1 and at a temperature of 45°C, was also evaluated mixing additional water in the reaction medium. A minor decrease of the enzyme activity was observed, the biodiesel yield reached value up to 76% and 72% at a water content of 10 wt% and 15 wt%, respectively. Moreover, the activity of the immobilized TL, after 24 h methanol/oil = 6:1 M and T = 55°C, results equal to 67%, in agreement with previous observation [23,24], which indicates improved stability of the immobilized enzyme with temperature.
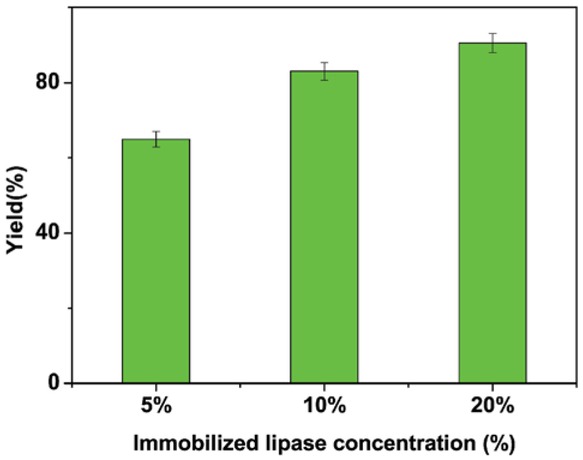
Effect of amount of immobilized lipase used for biodiesel production. Immobilization conditions – coupling temperature: 4°C; coupling pH: 3; coupling time: 3 h; lipase amount: 2 mg. Reaction conditions – reaction time: 24 h; reaction temperature: 45°C; methanol/oil ratio: 6:1 M.
The reusability of immobilized lipase was shown in Figure 3. Relative activity of the immobilized lipase, that is the activity of the immobilized enzyme respect to its best
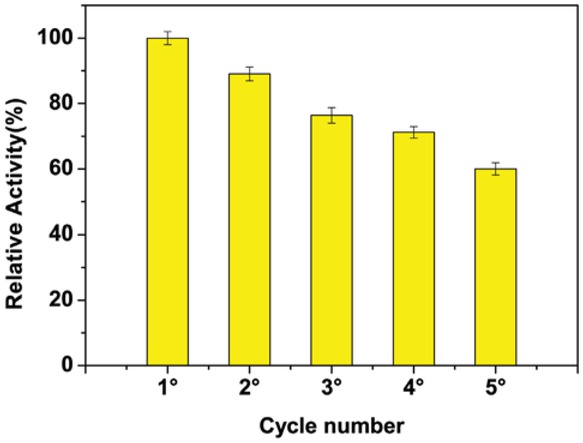
Effect of cycle number on biodiesel production for immobilized lipase. Methanol/oil ratio 6:1 M. Immobilization conditions – coupling temperature: 4°C; coupling pH: 3; coupling time: 3 h; lipase amount: 2 mg. Reaction conditions – reaction time: 24 h; reaction temperature: 45°C; lipase concentration: 10%.
result, after the third reaction was 77.51%. After five cycles, the relative activity in biodiesel production still is higher than 55% and 32% in presence of 15% of water, showing an excellent reusability in the experimental conditions chosen, likely due to the enzyme interaction with the support inducing stability.
As far as the biodiesel production by WCOs is concerned, in Table 2 the main relevant results obtained in the last years are summarized. It is evident that immobilization permits to increase the enzyme time of usage [17,18]. Moreover, nanomaterials offer in this context a new opportunity to obtain higher activities for immobilized enzymes [13,19, 20, 21]. On the other hand, the very remarkable combination of properties showed by our nanocatalyst can be ascribed to a favourable enzyme orientation on the support, and support surface functionality. In particular, the enzyme is anchored thanks to the interaction with citric acid, while the presence of residual oleic acid, which exposes its a-polar tail, not only favours interfacial activation, but also probably protects the enzyme from moisture. Moreover, the heterojunction between magnetite and gold, which have different work functions [27,28], inducing a Fe3O4 surface polarity modification determines an enhanced affinity with citric acid, favouring an increased stability, e.g. strong ionic interaction [29]. To better elucidate the role of Au conduction centres in facilitating electrons transfer [24], the effect of the Au NPs sizes was also monitored. In Table 3, the results obtained by using nanoparticles with different Au NPs sizes were reported. The comparison highlights the role of Au, helping enzymes to assume a favourable orientation and thus an increased enzyme loading and activity.
Comparison of the catalytic abilities of different enzymes for biodiesel production from WCOs.
| Support | Immobilization | Lipase | Activity maintenance (%) | Biodiesel conversion (%) | Maximum biodiesel conversion | Enzyme/ oil | MeOH/ oil | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| at 3 h | at 6 h | (%) | (h) | ||||||||
| Hydrocalc. | Physical ads. | Thermomyces lanuginosus | ~72 after 3 cycles | 36 after 7 cycles | <10ç | <10ç | 92.8 | 105 | 0.04 g/g | 4:1 | [17] |
| XAD1180 resin | - | Thermomyces lanuginosus | - | - | ~5 | ~7 | ~12 | 12 | 80 U/g | 3:1 | [18] |
| XAD1180 resin | - | S. sp. strain W007(MAS 1) | ~82 after 3 cycles | ~66 after 4 cycles | ~42 | ~71 | ~90 | 12 | 80 U/g | 3:1 | [18] |
| Fe3O4 NPs | - | Pseudomo- nas cepacia | ~80 after 3 cycles | 58 after 5 cycles | <20 | <20 | 80 | 72 | 0.4 g/g | 6.6:1 | [19] |
| Fe3O4 NPs | Covalent bond | Km 12 lipase | ~100 after 3 cycles | 80 after 9 cycles | - | ~18 | 71 | 36 | 0.03 g/g | 3:1 | [20] |
| Amino-Fe3O4- SiO2 NPs | Physical ads. | Burkholderia sp. | ~75 after 3 cycles | 54 after 5 cycles | <20$ | <20$ | 91$ | 35 | 0.25 g/g | 6:1 | [21] |
| Fe3O4-silica NPs§ | GPTMS- covalent bond | Candida Antarc. B | ~100§ after 3 cycles | ~62§ after 10 cycles | ~3§ | ~10§ | 100§ | 96 | 0.045 g/g | 9:1 | [13] |
| Fe3O4/Au | Physical interaction | Thermomyces lanuginosus | ~75 after 3 cycles | ~61 after 5 cycles | 34.6 | 70.1 | ~90 | 24 | 0.2 g/g | 6:1 | This paper |
| Fe3O4/Au | Physical interaction | Thermomyces lanuginosus | ~77 after 3 cycles | ~60 after 5 cycles | - | - | ~80 | 24 | 0.1 g/g | 6:1 | This paper |
§ In presence of 70% molecular sieve; ç 10 % yield at 24 h; $ with n-hexane content of 10 wt%.
Effect on size of Au on Fe3O4 for enzyme immobilization and biodiesel production.
| Au NPs (nm) | <2 | 2÷4 |
|---|---|---|
| XRD spectra | 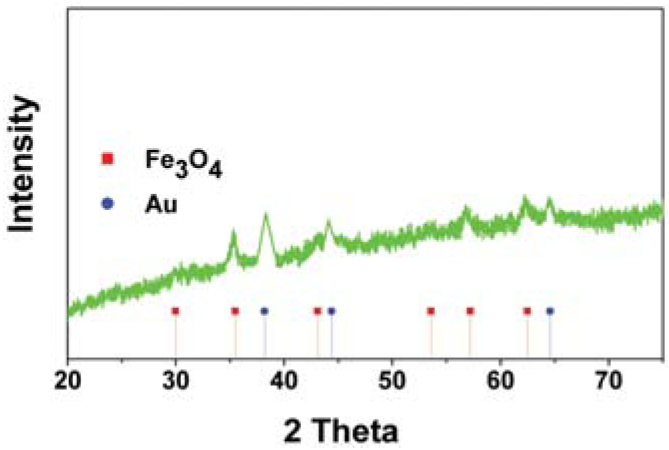 | 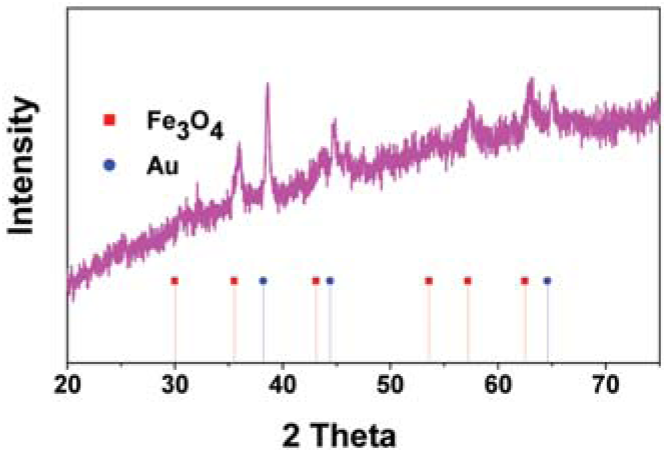 |
| Immobilization efficiency (%) | ~85 | ~90 |
| Biodiesel yield (%)° | ~90 | ~98.5 |
| Re-use* | ~61 | ~66 |
° Biodiesel reaction conditions: time, 24 h; temperature, 45°C; methanol/oil ratio, 6:1 M; Lipase concentration, 20%. * Activity maintenance after 5 cycles.
The efficiency of the biodiesel syntheses was checked by employing GC-MS analyses of derivatized olive oil, derivatized waste cooking oil and biodiesel. The spectra were reported in Figures S1, S2 and 4, respectively.
Fatty acid methyl esters (FAMEs) composition of the biodiesel was identified by comparing with the retention times of a pure FAME standard (C14-C22 standard, Sigma Aldrich) and of derivatized WCOs. The olive oil before cooking simulation consisted of 13 fatty acids, in particular: tetradecanoic acid, palmitic acid, palmitoleate acid, heptadecanoic acid, cis-10-heptadecanoic acid, stearic acid, oleic acid, linoleic acid, eicosanoic acid, linolenic acid, cis-11-eicosanoic acid, behinic acid, and lignoceric acid. The relative composition is reported in Table 4 Column 4°. After cooking simulation (see Table 4 column 5°), new compounds, originated by the cooking process (caprylic acid, octadecanoic acid
Retention times (RT) and area (%) of each fatty acid of olive oil from Sigma Aldrichof waste cooking oil, of biodiesel with of 20% of immobilized lipase concentration.
| Fatty Acid | Time (min) | Area (%) of olive oil from Sigma Aldrich | Area (%) of waste cooking oil | Area (%) of biodiesel with of 20% of immobilized lipase concentration | |
|---|---|---|---|---|---|
| Caprylic Acid | C8:0 | 8.7 | - | 0.20 ± 0.1 | 0.30 ± 0.1 |
| Tetradecanoic Acid | C14:0 | 15.3 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.02 |
| Palmitic Acid | C16:0 | 18.7 | 16.60 ± 0.06 | 18.76 ± 0.07 | 22.43 ± 0.08 |
| Palmitoleate Acid | C16:1 | 19.8 | 2.47 ± 0.03 | 1.60 ± 0.03 | 1.79 ± 0.03 |
| Heptadecanoic Acid | C17:0 | 20.5 | 0.24 ± 0.02 | 0.14 ± 0.02 | 0.16 ± 0.04 |
| Cis-10-Heptadecanoic Acid | C17:1 | 21.6 | 0.31 ± 0.02 | 0.19 ± 0.02 | 0.25 ± 0.03 |
| Stearic Acid | C18:0 | 22.4 | 4.85 ± 0.2 | 4.39 ± 0.3 | 3.11 ± 0.4 |
| Oleic Acid | C18:1 | 23.6 | 63.60 ± 0.2 | 63.32 ± 0.2 | 64.31 ± 0.2 |
| Linoleic Acid | C18:2 | 25.1 | 9.60 ± 0.2 | 7.21 ± 0.1 | 4.19 ± 0.05 |
| Eicosanoic Acid | C20:0 | 26.2 | 0.63 ± 0.05 | 0.55 ± 0.06 | 0.20 ± 0.08 |
| Linolenic Acid | C18:3 | 27.1 | 0.91 ± 0.05 | 0.54 ± 0.03 | 0.37 ± 0.03 |
| Cis-11-Eicosanoic Acid | C20:1 | 27.3 | 0.50 ± 0.02 | 0.59 ± 0.02 | - |
| Behinic Acid | C22:0 | 30.0 | 0.18 ± 0.03 | 0.27 ± 0.03 | - |
| Lignoceric Acid | C24:0 | 33.7 | 0.08 ± 0.01 | 0.06 ± 0.01 | - |
| Octadecanoic Acid 9.10 Epoxy | 35.5 | - | 0.59 ± 0.02 | 0.81 ± 0.03 | |
| Stearic Acid Allyl | C18:1 | 36.1 | - | 0.47 ± 0.04 | 0.63 ± 0.04 |
| 14-Methylhexadecanoic acid | C16:0 | 38.7 | - | 0.47 ± 0.05 | 0.65 ± 0.05 |
| Octadecanoic Acid 15.16 Epoxy | 39.4 | - | 0.62 ± 0.02 | 0.77 ± 0.02 |
9.10 epoxy, stearic acid allyl, 14-methylhexadecanoic acid, octadecanoic acid 15.16 epoxy) were detected. In particular, the palmitic acid increase and the appearance of 14-methylhexadecanoic acid account for the palmitoleate acid decrease. Methylation of stearic acid probably leads to the formation of stearic acid allyl. The appearance of caprylic acid is most likely due to oxidation occurring on the methyl in α position to the double bond [30] in linoleic acid [31], through the formation of a peroxide [32] and decomposition in short-chain products. Octadecanoic acid
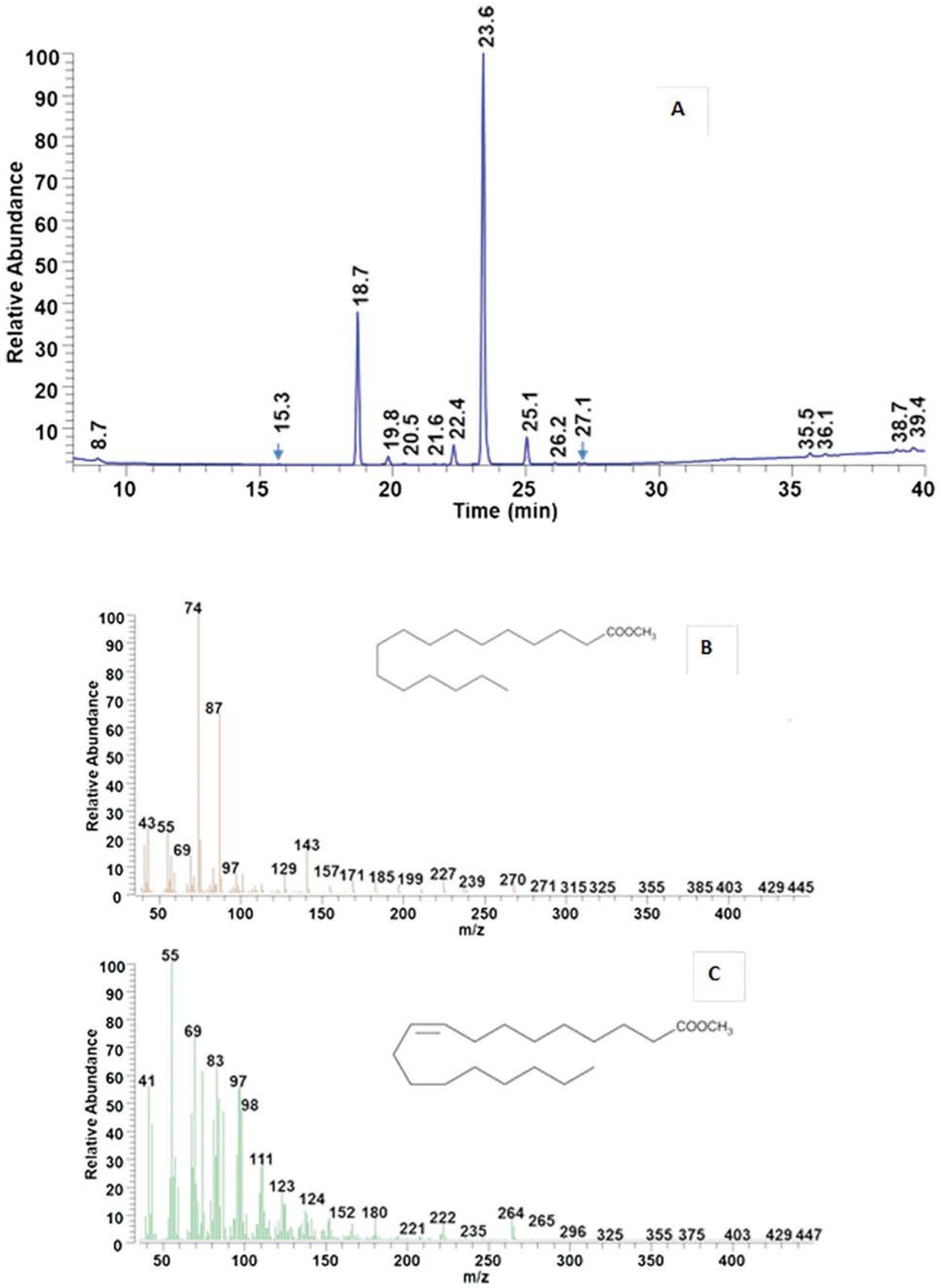
GC spectrum of biodiesel (a). Mass spectra of palmitic acid, methyl ester (b) and oleic acid, methyl ester (c).
9.10 epoxy and octadecanoic acid 15.16 epoxy likely come from linoleic and linolenic acid epoxydation occurring during cooking.
The comparison between the GC-MS of derivatized WCOs and biodiesel (see Table 4 Column 6°) evidences the ability of the nanocatalyst to convert the oil fatty acids into methyl esters. On the other hand, as the length of fatty acid chains increases, a reduction of the catalytic activity was observed. The amounts of octadecanoic acid 9.10 epoxy and octadecanoic acid 15.16 epoxy increases only apparently, as they now weigh on an esterified smaller fraction of the starting oil. In conclusion, the difference between the calculated biodiesel yields and 100%, after 24 h, is due to the presence of unconverted acids, which amount is more pronounced the longer the chains to be converted are.
Biodiesel from waste cooking oil, obtained through the use of our immobilized lipase, after 24 h of synthesis, presents a linolenic methyl ester amount equal to 0.54% ± 0.03, which is in agreement with the EN14214. Ester content, calculated according with the modified method of EN14214, equal to 97.8 ± 0.21, in agreement with the EN14214. The iodine value, calculated according with EN 14214 Annex B, results equal to 66.75 (g iodine/100 g) in agreement with the European standard. The validation through the standard, that is typically a key aspect, is more necessary in this case, given the origin, complexity and variety of the raw material. Although the enzyme activity is very high, also if compared with other literature results [13,17,18], it is lower than that shown on different oils [23,24], likely due to the FFA, water and degraded product content [10,32]. It is worth noticing, that for more degraded oils a pre-treatment may be needed in order to respect the standard. Conversions to biodiesel still higher than 90% can be obtained for longer times, also because of the progressive enrichment in shorter fractions along the cooking. In particular, in Table 5 the results of biodiesel characterization are reported, demonstrating the feasibility of WCOs oil biodiesel as fuel.
Characterization of the Biodiesel from WCOs.
| Fuel properties | unit | value | Biodiesel Standard [33] |
|---|---|---|---|
| Density at 15°C | Kg/m3 | 790 | 878 |
| Viscosity at 40°C | mm2/s | 2.9 | 1.9-6.0 |
| Flash point | °C | > 130 | 100 to 170 |
| Moisture content | ppm | Trace | 0.05% max |
| Cetane number | - | 48-65 | |
| Acid value | mg KOH/g | 0.5 | 0.5 |
| Polyunsaturated double) methyl esters (≥4 | % m/m | 0.0 | 0.0 |
| Methanol content | % m/m | 0.12 | 0.2 |
4 Conclusion
Biodiesel was obtained from WCOs without any pretreatment. In particular, a very high conversion yield, e.g. up to about 90%, was achieved at a lipase loading of
20%, after 24 h. The immobilized enzyme shows a fast kinetic and high activity to form methyl esters (the biodiesel yield was already of 34.6% after only 3 h and of 70.1% after 6 h, at the same operating conditions). The very remarkable combination of properties showed by our nanocatalyst can be ascribed to a favorable enzyme orientation on the support, and support surface functionality. In particular, the enzyme is anchored thanks to the interaction with citric acid, and the presence of residual oleic acid, which exposes its a-polar tail to the medium, not only favors interfacial activation, but also helps enzyme to work in the presence of water. Moreover, the heterojunction between magnetite and gold, inducing a Fe3O4 surface polarity modification, determines an enhanced affinity with citric acid, favoring increased stability, e.g. strong ionic interaction. The comparison between different Au NPs sizes containing catalysts highlights the role of Au, helping enzymes to assume a favourable orientation and thus an increased enzyme loading and activity.
The olive oil before cooking simulation consisted of 13 fatty acids, in particular: tetradecanoic acid, palmitic acid, palmitoleate acid, heptadecanoic acid, cis-10-heptadecanoic acid, stearic acid, oleic acid, linoleic acid, eicosanoic acid, linolenic acid, cis-11-eicosanoic acid, behinic acid, and lignoceric acid. After cooking simulation, new compounds, originated by the cooking process (caprylic acid, octadecanoic acid 9.10 epoxy, stearic acid allyl, 14-methylhexadecanoic acid, octadecanoic acid 15.16 epoxy) were detected. The GC-MS characterization evidences the slight different activity of the enzyme as the length of the chains to be converted increases. The significant activity and stability of the bio-catalyst (activity retention ~60% and above 32% in presence of water, after 5 cycles) can be ascribed to support size and the support enzyme interactions.
Biodiesel from waste cooking oil, obtained through the use of our immobilized lipase, was analysed according to the European Standard, which is, here, a fundamental step because of the origin, complexity and variety of the raw material. In particular, the biodiesel presents an ester content equal to 97.8 ± 0.21 in agreement with the EN14214, all over the characterization demonstrate the feasibility of WCOs oil biodiesel as fuel.
References
[1] Meka P.K., Tripathi V., Singh R.P., Synthesis of biodiesel fuel from safflower oil using various reaction parameters. J. Oleo Sci, 2007, 56, 9-12.10.5650/jos.56.9Suche in Google Scholar PubMed
[2] Narwal S.K., Gupta R., Biodiesel production by transesterification using immobilized lipase. Biotechnol. Lett., 2013, 35, 479-490.10.1007/s10529-012-1116-zSuche in Google Scholar PubMed
[3] Yan J., Zheng X., Li S., A novel and robust recombinant Pichia pastoris yeast whole cell biocatalyst with intracellular over expression of a Thermomyces lanuginosus lipase: Preparation, characterization and application in biodiesel production. Bioresour. Technol., 2014, 151, 43-48.10.1016/j.biortech.2013.10.037Suche in Google Scholar PubMed
[4] Lopresto C., Naccarato S., Albo L., De Paola M., Chakraborty S., Curcio S., et al., Enzymatic transesterification of waste vegetable oil to produce biodiesel. Ecotoxicol. Environ. Saf., 2015, 121, 229-235.10.1016/j.ecoenv.2015.03.028Suche in Google Scholar PubMed
[5] Bobadilla M.C., Lorza R.L., García R.E., Gómez F.S., González E.P.V., An Improvement in Biodiesel Production from Waste Cooking Oil by Applying Thought Multi-Response Surface Methodology Using Desirability Functions. Energies, 2017, 10, 130.10.3390/en10010130Suche in Google Scholar
[6] Shahid E.M., Jamal Y., Production of biodiesel: A technical review. Renew. Sustain. Energy Rev., 2011, 15, 4732-4745.10.1016/j.rser.2011.07.079Suche in Google Scholar
[7] Kulkarni M.G., Dalai A.K., Waste cooking oil an economical source for biodiesel: A review. Ind. Eng. Chem. Res., 2006, 45, 2901-2913.10.1021/ie0510526Suche in Google Scholar
[8] Ghadge S.V., Raheman H., Process optimization for biodiesel production from mahua Madhuca indica oil using response surface methodology. Bioresour. Technol., 2006, 97, 379-384.10.1016/j.biortech.2005.03.014Suche in Google Scholar PubMed
[9] Enweremadu C.C., Barawa M.M., Technical aspects of production and analysis of biodiesel from used cooking oil – A review. Renew. Sustain. Energy Rev., 2009, 13, 2205-2224.10.1016/j.rser.2009.06.007Suche in Google Scholar
[10] Ranganathan S.V., Narasimhan S.L., Muthukumar K., An overview of enzymatic production of biodiesel. Bioresour. Technol., 2008, 99, 3975-3981.10.1016/j.biortech.2007.04.060Suche in Google Scholar PubMed
[11] Padilha G.S., Tambourgi E.B., Alegre R.M., Evaluation of lipase from Burkholderia cepacia immobilized in alginate beads and application in the synthesis of banana flavor (isoamyl acetate). Chem. Eng. Comm., 2018, 205, 23-33.10.1080/00986445.2017.1370707Suche in Google Scholar
[12] Nurcan K., Fife G., Ülkü M., Ayla Ç., Hamdi K., Lipase catalyzed synthesis of oleyl oleate: Optimization by response surface methodology. Chem. Eng. Comm., 2010, 190, 779-796.10.1080/00986440302107Suche in Google Scholar
[13] Mehrasbi M., Mohammadi J., Peyda M., Mohammad M., Covalent immobilization of Candida antarctica lipase on core-shell magnetic nanoparticles for production of biodiesel from waste cooking oil. Ren. Ener., 2017, 101, 593-602.10.1016/j.renene.2016.09.022Suche in Google Scholar
[14] Guldhe A., Singh B., Mutanda T., Permaul K., Bux F., Advances in synthesis of biodiesel via enzyme catalysis: novel and sustainable approaches. Renew. Sustain. Energy Rev., 2015, 41, 1447-1464.10.1016/j.rser.2014.09.035Suche in Google Scholar
[15] Poppe J.K., Fernandez-Lafuente R., Rodrigues R.C., Ayub M.A.Z., Enzymatic reactors for biodiesel synthesis: present status and future prospects. Biotechnol. Adv., 2015, 33, 511-525.10.1016/j.biotechadv.2015.01.011Suche in Google Scholar PubMed
[16] Lam M.K., Lee K.T., Mohamed A.R., Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv., 2010, 28, 500-518.10.1016/j.biotechadv.2010.03.002Suche in Google Scholar PubMed
[17] Yagiz F., Kazan D., Akin A.N., Biodiesel production from waste oils by using lipase immobilized on hydrotalcite and zeolites. Chem. Eng. J., 2007, 134, 262-267.10.1016/j.cej.2007.03.041Suche in Google Scholar
[18] Wang X., Qin X., Li D., Yang B., Wang Y., One step synthesis of high yield biodiesel form waste cooking oils by a novel and highly methanol-tolerant immobilized lipase. Bioresour. Technol., 2017, 235, 18-24.10.1016/j.biortech.2017.03.086Suche in Google Scholar PubMed
[19] Yu C.Y., Huang L.Y., Kuan I.C., Lee S.L., Optimized Production of Biodiesel from Waste Cooking Oil by Lipase Immobilized on Magnetic Nanoparticles. Int. J. Mol. Sci., 2013, 14, 24074-24086.10.3390/ijms141224074Suche in Google Scholar PubMed PubMed Central
[20] Badoei-dalfard A., Malekabadi S., Karami Z., Sargazi G., Magnetic cross-linked enzyme aggregates of Km12 lipase: A stable nanobiocatalyst for biodiesel synthesis from waste cooking oil. Renew. Energ., 2019, 141, 874-882.10.1016/j.renene.2019.04.061Suche in Google Scholar
[21] Liu C., Yuan J., Gao H., Liu C., Biodiesel production from waste cooking oil by immobilized lipase on superparamagnetic Fe3O4 hollow sub-microspheres. Biocatal. Agric. Biotechnol., 2016, 8, 182-188.10.1080/10242422.2016.1265948Suche in Google Scholar
[22] Yücel Y., Biodiesel production from pomace oil by using lipase immobilized onto olive pomace. Bioresour. Technol., 2011, 102, 3977-3980.10.1016/j.biortech.2010.12.001Suche in Google Scholar PubMed
[23] Sarno M., Iuliano M., Polichetti M., Ciambelli P., High activity and selectivity immobilized lipase on Fe3O4 nanoparticles for banana flavour synthesis. Process Biochem., 2017, 56, 98-108.10.1016/j.procbio.2017.02.004Suche in Google Scholar
[24] Sarno M., Iuliano M., Highly active and stable Fe3O4Au nanoparticles supporting lipase catalyst for biodiesel production from waste tomato. Appl. Surf. Sci., 2019, 474, 135-146.10.1016/j.apsusc.2018.04.060Suche in Google Scholar
[25] Brenes M., García A., Dobarganes M.C., Velasco J., Romero C., Influence of Thermal Treatments Simulating Cooking Processes on the Polyphenol Content in Virgin Olive Oil. J. Agric. Food Chem., 2002, 50, 5962-5967.10.1021/jf020506wSuche in Google Scholar PubMed
[26] Hădărugă D.I., Hădărugă N.G., Hermenean A., Riviș A., Pâslaru V., Codina G., Bionanomaterials: Thermal stability of the oleic acid/α-and β-cyclodextrin complexes. Rev. Chim., 2008, 59, 994-998.10.37358/RC.08.9.1955Suche in Google Scholar
[27] Pabisiak T., Winiarski M.J., Ossowski T., Kiejna A., Adsorption of gold subnano-structures on a magnetite(111) surface and their interaction with CO. Phys. Chem. Chem. Phys., 2016, 18, 18169-18179.10.1039/C6CP03222BSuche in Google Scholar
[28] Frey N.A., Phan M.H., Srikanth H., Srinath S., Wang C., Sun S., Interparticle interactions in coupled Au-Fe3O4Au-Fe3O4 nanoparticles. J. Appl. Phys., 2009, 105, 07B5022009.10.1063/1.3056582Suche in Google Scholar
[29] Mohamad N.R., Marzuki N.H.C., Buang N.A., Huyop F., Waha R.A., An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip., 2015, 29, 205-220.10.1080/13102818.2015.1008192Suche in Google Scholar PubMed PubMed Central
[30] Choe E., Min D.B., Chemistry of Deep‐Fat Frying Oils. J. Food Sci., 2007, 5, R77-R86.10.1111/j.1750-3841.2007.00352.xSuche in Google Scholar PubMed
[31] Li X., Li J., Wang Y., Peirang C., Yuanfa L., Effects of frying oils’ fatty acids profile on the formation of polar lipids components and their retention in French fries over deep-frying process. Food Chem., 2017, 237, 98-105.10.1016/j.foodchem.2017.05.100Suche in Google Scholar PubMed
[32] Kowalski R., Gc Analysis Of Changes In The Fatty Acid Composition Of Sunflower And Olive Oils Heated With Quercetin, Caffeic Acid, Protocatechuic Acid, And Butylated Hydroxyanisole. Acta Chromatogr., 2007, 18, 15-23.Suche in Google Scholar
[33] Rushang M.J., Michael J.P., Flow properties of biodiesel fuel blends at low temperatures. Fuel, 2007, 86, 143-151.10.1016/j.fuel.2006.06.005Suche in Google Scholar
© 2019 Sarno and Iuliano, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Artikel in diesem Heft
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

