Abstract
Deep eutectic solvents (DESs) are successfully used as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones (DHPMs). The acidity of DESs is the main factor that determines catalytic activity. DESs, based on p-toluene sulfonic acid (PTSA) and choline chloride (ChCl), exhibits the highest catalytic activity. ChCl/2PTSA is suitable for a vast variety of aromatic aldehydes with electron-donating and electron-withdrawing groups, different β-diketonates, and urea or thiourea to obtain the corresponding DHPMs. Furthermore, DESs can be recycled easily after synthesis. The reused DESs achieve catalytic efficiency six times without significant changes. This study will provide a new green catalyst and efficient process for the synthesis of DHPMs.
1 Introduction
In recent years, green synthesis processes actively seeks new solvents to replace common organic solvents that present inherent toxicity and have high volatility. Deep eutectic solvents (DESs), as ionic liquids analogues, have attracted considerable attention from researchers because of their environment performance. They do not only posses the advantages of ionic liquids, such as designability, chemical stability, and low vapor pressure, they also pose many other advantages, such as diversity, low-cost and easily sourced raw materials, and green and simple synthesis without using other organic solvents [1, 2, 3]. Therefore, DESs are good alternatives to volatile organic solvents as green solvents. In recent years, DESs have been widely used in organic reactions [4, 5, 6], materials preparation [7], electrochemistry [8,9], separation process [10,11], and so on. Moreover, as novel reaction media or catalysts, DESs have been widely used in traditional organic synthesis reactions. For example, choline chloride-zinc chloride ([ChCl][ZnCl2]2) based DESs could be used for catalyzing Friedel-Crafts alkylation of electron-rich arenes with aldehydes [12]; Imperato et al. reported the use of carbohydrates, sugar alcohols or citric acid, with urea and inorganic salts based DESs as reaction media for Diels-Alder reaction [13]; The Knoevenagel condensation proceeded smoothly in reusable and cheap DESs (choline chloride/urea) [14]; The enzyme-catalyzed Henry reaction was realized using choline chloride-based DESs as reaction medium [15]; Choline chloride and urea based DESs provided an efficient and convenient method for Perkin reaction [16]; Urea or glycerol with choline chloride were effective solvents/catalysts for Paal-Knorr reactions [17].
3,4-Dihydropyrimidin-2(1H)-ones/thiones (DHPMs) have high pharmacological and therapeutic activities, such as antibacterial, antiviral, antitumor, calcium antagonistic and antihypertensive [18, 19, 20]. Thus, synthesizing DHPMs has attracted considerable attention in the area of synthetic chemistry by using the multicomponent reactions (MCRs) [21]. Generally, DHPMs can be synthesized by reacting β-ketoesters with aldehydes and urea or thiourea [22]. However, the low yield of the Biginelli reaction has limited its applications in substituted aromatic and aliphatic aldehydes. Selecting suitable catalysts has become the key point in solving this limitation. Brønsted acids or Lewis acids, such as HBF4 [23], H3PW12O40 [24], InBr3 [25], ZrCl4 [26], Fe(OTs)3·6H2O [27], and Ce(NO3)3·6H2O [28], have been traditionally used as catalysts. In recent years, some novel catalysts, including organocatalysts [29,30], biocatalysts [31], immobilized urease [32], PS-PEG-SO3H [33], Ti-graftedpolyamidoamine dendritic silica hybrid catalyst [34], halogenated macroporous sulfonic resins [35], polyaniline-supported FeCl3 [36], cobalt supported on alumina catalyst [37], and ionic liquid [38, 39, 40, 41] have attracted great attention for use in the Biginelli reaction. These catalysts, for their uses in accomplishing the Biginelli reaction, have produced varying degrees of success. However, some of these catalysts are harmful to the environment and have other disadvantages, such as sensitive reaction conditions, complex preparation process, and use of hazardous reagents. Recently, the use of environmentally suitable, economically viable, and green catalysts for organic synthesis has gathered interest. Therefore, developing new green catalytic processes with increased efficiency and selectivity is necessary.
Our group designed, synthesized, and applied DESs in previous research. The results showed that the concise control of the acidity of DESs can be modified by changing the hydrogen bond donors (HBDs) and hydrogen bond acceptors (HBAs). Biginelli reaction was occurred under acidic conditions. This finding inspired us to design new acid DESs for the synthesis of DHPMs. The synthesis of DHPMs using DESs have been reported in the literature [42, 43, 44, 45], however, the types of DESs is limited and catalytic activities need to be further improved. Therefore, the development of new types of DESs catalysts with high efficiency is an important subject in the field of synthesis of DHPMs.
In this work, some novel DESs with different acidities were designed, synthesized and applied as powerful and recyclable catalysts and solvents for synthesizing DHPMs. The reaction system based on DESs benefits from the benign reaction conditions, high catalytic activity, easy work-up procedures, feasible reusability and wide substrates tolerance. The specific synthesis was successful and obtained a variety of DHPMs with reduced reaction time and increased yields. Choline chloride (ChCl), tetrabutylammonium bromide (TBAB), tetrabutyl ammonium chloride (TBAC), tetrabutyl ammonium acetate (TBAA), tetraethylammonium chloride (TEAC), and tetraethylammonium bromide (TEAB) were chosen as typical HBAs; and p-toluenesulfonic acid (PTSA), trichloroacetic acid (TCA), monochloroacetic acid (MCA), propionic acid (PA), and ethylene glycol (EG) were chosen as HBDs to control the acidity of DESs, through which different DESs with acidity gradients were synthesized. The above DESs were used as catalysts and solvents for the Biginelli reaction of benzaldehyde, ethyl acetoacetate and urea; moreover, this was selected as the model reaction to optimize the synthesis conditions. Some important parameters, such as system temperature, reaction time, and amount of DESs, as well as the different kinds of aromatic aldehyde, were investigated. Furthermore, the possible catalytic mechanisms were studied.
2 Experimental
2.1 Chemicals and instruments
Chemicals were purchased from Aladdin Reagent Co., Ltd. (Shanghai) and J&K Scientific LTD. 1H and 13C NMR spectra were recorded on a Bruker Ascend 500 spectrometer. FT-IR analyses were carried out on a Nicolet IS-10 using a single reflection ATR cell.
2.2 Synthesis of DESs
Purified HBAs and HBDs were mixed at a certain molar ratio (mostly 1:2 in this research). DESs were prepared by using different processes to meet the requirements from the final products. ChCl and TCA (or MCA) were grinded with a mortar and pestle at room temperature until a homogeneous liquid was formed [46]. ChCl and PA was stirred at room temperature until a homogeneous liquid was formed. The above methods were applied to prevent the formation of ester impurities between ChCl and the acid. The other DESs were synthetized by heating, in which the systems were stirred vigorously with a magnetic stirrer at 80°C for 4h to obtain pure DESs. The details of the synthesis were reported in our previous literature [47]. The properties of the synthesized DESs, which can be of great significance for future industrial applications, were investigated [48]. The FTIR and 1H NMR spectra of DESs were shown in Figures S1-S5 of the Supplementary material.
2.3 General procedure for the synthesis of DHPMs
The mixture of aromatic aldehyde (2.0 mmol), methyl (or ethyl) acetoacetate (2.0 mmol), urea (or thiourea) (3.0 mmol), and DESs were stirred at 70°C for a certain time. Completion of the reaction was monitored by thin layer chromatography. After the completion of the above reaction, the mixtures were cooled to room temperature (25°C). Water was added into the mixtures to separate the DESs and final products. It is worth mentioning that the products were easily separated with high yield and DESs were easily recovered and reused. After adding water to the reaction system, the DESs was dissolved in water, and the final product was separated by filtration due to its insolubility in water. Then the filter cake washed with water repeatedly, and recrystallized in ethanol to obtain the pure products. The DESs contained in filtrate were recovered by evaporating the water and drying under reduced pressure. All the experiments in this study were performed in triplicate to determine its reproducibility, and the experimental errors were within 3%. All the synthesized products were characterized based on their spectra (1H and 13C NMR).
3 Results and discussion
3.1 Selection of DESs
Generally, Biginelli reaction is carried out in acidic conditions. However, the acidity of the reaction process is difficult to control precisely. Fortunately, one of the advantages of DESs is the simple control of its acidity, which can be achieved by selecting the appropriate HBDs. Thus, the selection of HBD is important for the Biginelli reaction process. With the above considerations, a series of DESs was designed and synthesized according to the acidity of the HBDs. In this way, the acidity of DESs can be controlled precisely. ChCl was chosen as the HBA, and PTSA, TCA, MCA, PA, and EG were chosen as HBDs. A series of DESs was prepared and applied as catalysts and solvents for the Biginelli reaction. The selection of DESs was probed, and the results are shown in Figure 1. The HBDs referring to organic acids or alcohols showed greater influence on the reaction process. The catalytic capabilities decreased with reducing acidity of HBDs. ChCl/2PTSA exhibited the highest yield of DHPM product and reached 88% with DESs of 0.4 mmol. Thus, PTSA was more preferred as HBD than the other acids for the specific reaction. Afterward, the influences of different molar ratios of ChCl and PTSA on the yield were discussed. When the molar ratios of ChCl and PTSA were 1:0.5, 1:1, and 1:2, the DHPM yields were 63%, 74%, and 88%, respectively. Thus, the HBA to HBD at a molar ratio of 1:2 was preferable. Moreover, HBAs were observed to have little influence. DESs based on quaternary ammonium salts had the same catalytic activities with those of the ChCl-based DESs. Compared with quaternary ammonium salts, ChCl was cheaper, greener, and easier to biodegrade. Thus, ChCl/2PTSA was chosen as the typical catalyst and solvent in the following experiments.
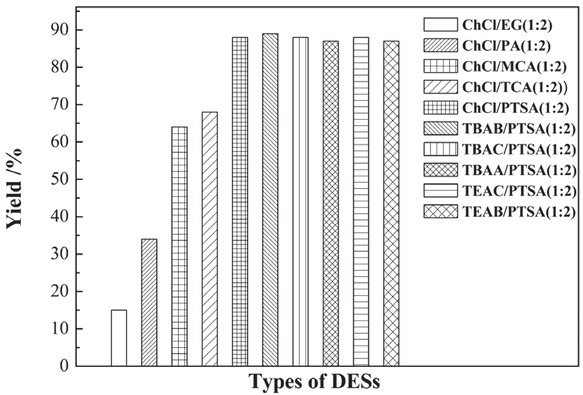
Effect of DESs types on reaction yield. Reaction conditions: benzaldehyde (2.0 mmol), ethyl acetoacetate (2.0 mmol), urea (3.0 mmol), ChCl/2PTSA (0.4 mmol), 70°C, 40 min.
Biginelli reaction can also be catalyzed by PTSA; thus, a comparative study on PTSA and ChCl/2PTSA was conducted. The results demonstrated that the yield of DHPM was 64% with PTSA catalyst under solvent-free condition, which was lower than that of ChCl/2PTSA (88%). Jin et al. reported the use of Biginelli reaction with PTSA as a catalyst in refluxing ethanol [49]. However, the use of the organic solvent in Jin’s study did not meet the requirements of green chemistry. Moreover, DESs can act as solvent and catalyst with high catalytic activity, are environmentally benign, and are easily recycled, which are in accordance with the requirements of green chemistry.
3.2 Optimization of the reaction parameters
The reaction of benzaldehyde and ethyl acetoacetate with urea catalyzed by ChCl/2PTSA was chosen for the synthesis of DHPM. Many factors, such as reaction temperature, DESs dosage, and reaction time, affect the reaction process. Therefore, the above parameters were investigated in detail to optimize the reaction process (Table 1).
Optimization of the reaction conditions with ChCl/2PTAS as catalyst and solvent.a
| Entry | DESs (mmol) | Temp. (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|
| 1 | 0.4 | 50 | 40 | 72 |
| 2 | 0.4 | 60 | 40 | 78 |
| 3 | 0.4 | 70 | 40 | 88 |
| 4 | 0.4 | 80 | 40 | 87 |
| 5 | 0.4 | 90 | 40 | 86 |
| 6 | 0.1 | 70 | 40 | 77 |
| 7 | 0.2 | 70 | 40 | 81 |
| 8 | 0.5 | 70 | 40 | 90 |
| 9 | 0.6 | 70 | 40 | 92 |
| 10 | 0.7 | 70 | 40 | 89 |
| 11 | 0.8 | 70 | 40 | 81 |
| 12 | 1.0 | 70 | 40 | 68 |
| 13 | 0.6 | 70 | 30 | 82 |
| 14 | 0.6 | 70 | 50 | 88 |
| 15 | 0.6 | 70 | 60 | 86 |
a Reaction conditions: benzaldehyde (2.0 mmol), ethyl acetoacetate (2.0 mmol), urea (3.0 mmol). b Isolated yield.
Reaction temperature is important in organic reactions. Moreover, our results showed that high temperature within a certain range (50°C to 70°C) benefitted the organic reaction. When the reaction temperature exceeded 70°C, the yield of DHPM remained unchanged (Table 1, entries 1-5). Thus, 70°C was chosen as the reaction temperature throughout the research.
DES dosage is another factor that influences the reaction process. Table 1 shows that an increase of up to 0.6 mmol strengthened and increased the yield of DHPM. However, when DES dosage continued to increase, the yield decreased gradually and the color of the final product deepened (Table 1, entries 6-12). This finding can be attributed to the fact that very strong acidity is not favorable for the specific reaction. To improve the selectivity of the final product, suitable acidity is necessary. Thus, 0.6 mmol of DES was chosen as the optimal quantity in the following experiments.
Reaction time is an important parameter for the reaction; thus, to determine the reaction more precisely, the effect of reaction time was investigated. Thin layer chromatography was used to monitor the reaction endpoint. At less than 40 min, the yield of DHPM increased tremendously with increasing time. The reaction equillibrium can be achieved in 40 min with an increased yield of up to 92%. The suitable acidity and increased catalytic capability of DESs accounted for the reduced reaction time.
3.3 Biginelli reaction of substituted aldehydes
To widen the scope of the reactions, various substituted aldehydes, β-diketonates, were chosen as raw materials. The reaction was carried out under optimized conditions, i.e. DES loading of 0.6 mmol at 70°C for the synthesis of the corresponding DHPMs. Results are shown in Table 2. The melting points, 1H NMR, and 13C NMR of the products were characterized (see the Supplementary material). As indicated in Table 2, the Biginelli reaction catalyzed by ChCl/2PTSA was suitable for a vast variety of aromatic aldehydes with both electron-donating and electron-withdrawing groups, different β-diketonates, and urea or thiourea to obtain the corresponding DHPMs. Moreover, methyl acetoacetate exhibited the same reactivity as that of ethyl acetoacetate (Table 2, entries 1 and 15; entries 3 and 16; entries 6 and 17; and entries 7 and 18). Furthermore, acetylacetone also had increased reactivity (Table 2, entries 20 and 21). However, the activity of ethyl benzoyl acetate were relatively lower (Table 2, entries 22), and no product was formed in 40 min. Extending the reaction time was favorable in yielding 5-ethoxycarbonyl-4-(2-phenyl)-6-phenyl-3,4-dihydropyrimidin-2(1H)-one (3v), which was 42% in 4 h. The steric hindrance of the benzene ring was the main reason for the low reactivity. In addition, the reactivity of urea was higher than that of thiourea (Table 2, entries 1 and 14; and entries 15 and 19). Meanwhile, the Biginelli reaction of aliphatic aldehyde was achieved with n-butylaldehyde, ethyl acetoacetate, and urea as substrates, and yielded up to 52% in 40 min; the product yield for the reaction of hexaldehyde was 48%. So most aldehydes are suitable for the Biginelli reaction catalyzed by ChCl/2PTSA. Further work on the extension of substrates, such as heterocyclic aldehydes, aliphatic aldehydes, and β-diketonates, is on going.
Substrate scope for the Biginelli reaction in presence of ChCl/2PTAS.a

| Entry | Ar | R1 | R2 | X | Product | Yieldb (%) | |
|---|---|---|---|---|---|---|---|
| 1 | C6H5 | CH3 | OEt | O | 3a | 92 | |
| 2 | 4-CH3C6H4 | CH3 | OEt | O | 3b | 91 | |
| 3 | 4-OCH3C6H4 | CH3 | OEt | O | 3c | 88 | |
| 4 | 4-FC6H4 | CH3 | OEt | O | 3d | 91 | |
| 5 | 4-ClC6H4 | CH3 | OEt | O | 3e | 92 | |
| 6 | 4-BrC6H4 | CH3 | OEt | O | 3f | 88 | |
| 7 | 4-NO2C6H4 | CH3 | OEt | O | 3g | 88 | |
| 8 | 4-OHC6H4 | CH3 | OEt | O | 3h | 90 | |
| 9 | 3-ClC6H4 | CH3 | OEt | O | 3i | 80 | |
| 10 | 2-ClC6H4 | CH3 | OEt | O | 3j | 78 | |
| 11 | 3-BrC6H4 | CH3 | OEt | O | 3k | 92 | |
| 12 | 3-NO2C6H4 | CH3 | OEt | O | 3l | 80 | |
| 13 | 2-OH-3-OCH3C6H3 | CH3 | OEt | O | 3m | 58 | |
| 14 | C6H5 | CH3 | OEt | S | 3n | 80 | |
| 15 | C6H5 | CH3 | OMe | O | 3o | 90 | |
| 16 | 4-OCH3C6H4 | CH3 | OMe | O | 3p | 88 | |
| 17 | 4-BrC6H4 | CH3 | OMe | O | 3q | 90 | |
| 18 | 4-NO2C6H4 | CH3 | OMe | O | 3r | 88 | |
| 19 | C6H5 | CH3 | OMe | S | 3s | 80 | |
| 20 | C6H5 | CH3 | CH3 | O | 3t | 85 | |
| 21 | 4-NO2C6H4 | CH3 | CH3 | O | 3u | 84 | |
| 22 | C6H5 | C6H5 | OEt | O | 3v | – |
a Reaction conditions: aldehyde (2.0 mmol), β-ketoesters (2.0mmol), urea or thiourea (3.0 mmol), ChCl/2PTSA (0.6 mmol), 70°C, 40 min. b Isolated yield.
To show the merit of the present work in comparison with previously reported results in the literature, we summarized some results for the synthesis of DHPMs in Table 3. As shown in Table 3, ChCl/2PTSA has high catalytic activity with the yields of DHPMs are over 78%. Compared with other DESs, the main advantage of ChCl/2PTSA lies in less dosage, thus saving resources and lowering the production cost.
Synthesis of DHPMs using different DESs
| aEntry | DES | DES dosage | Time | Yield (%) | Reference |
|---|---|---|---|---|---|
| 1 | L-(+)-tartaric | 1.5 g | 8-48 h | 70-99 | [42] |
| acid-dimethylurea | |||||
| 2 | ChCl/ClCH2CO2H | 10 mmol | 5-75 min | 70-95 | [43] |
| 3 | ZnCl/Urea | urea:ZnCl2 (3.5:1mmol) | 5-60 min | 76-96 | [44] |
| 4 | ChCl/Urea | 3 mL | 7.5 h | 20 | [45] |
| 5 | ChCl/2PTSA | 0.6 mmol | 40 min | 78-92b | This work |
a Entry 1-3: substrate aldehyde 1 mmol; Entry 4-5: substrate aldehyde 2 mmol. b The reaction yield of 2-hydroxy-3-methoxybenzaldehyde is 58%, but this compound has not been reported in other reference.
3.4 Recycling and reuse of DESs
Synthesizing DESs is low-cost; however, recycling and reusing DESs are also necessary to consider for economic and environmental considerations. Reuse of DESs was carried out by using ChCl/2PTSA to catalyze the model reactions of benzaldehyde, ethyl acetoacetate, and urea at 70°C. Fortunately, DESs was recycled by simply adding water into the reaction mixture after the completion of the reaction. Afterward, by simple filtration, the target compound was obtained. The DESs were obtained by evaporating the water. The recycled DESs was then reused for another cycle. In the next run, the reaction using a mixture of benzaldehyde, ethyl acetoacetate, and urea at stoichiometric ratio was carried out under the same conditions. The results are summarized in Figure 2. The yields were highly stable after six recycling cycles, indicating the potential of DESs for facile recycling.

Reuse of ChCl/2PTSA for Biginelli reaction.
3.5 Reaction mechanism catalyzed by ChCl/2PTSA
The reaction mechanism, which is important for revealing the specific process, was investigated systematically based on the previous mechanism proposed by Kappe [50]. The mechanism for the specific Biginelli reaction with ChCl/2PTSA as catalyst and solvent is shown in Figure 3. The rate-limiting reaction of benzaldehyde and urea catalyzed by ChCl/2PTSA was monitored. The IR spectra of the mixtures with heating treatment were compared with the initial ones. The IR spectra of benzaldehyde, urea, and ChCl/2PTSA significantly changed after heating (Figure 4). The C–H bond stretching vibration peak (2817.9 and 2735.0 cm−1) of benzaldehyde disappeared after heating. Meanwhile, the C=O stretching vibration peak of benzaldehyde (1695.4 cm−1) disappeared gradually by heating, whereas the peak at 1644.2 cm−1 widened. Thus, benzaldehyde can react with urea catalyzed by ChCl/2PTSA to obtain the N-acyl imine intermediate (A). Based on the experiment phenomena, wherein ethyl acetoacetate was added in the benzaldehyde, urea, and ChCl/2PTSA heating mixtures, the final product 3,4-dihydropyrimidin-2(1H)-ones can be obtained immediately, and we speculated that intermediate (B) can be obtained through the reaction of intermediate (A) and the enolized ethyl acetoacetate. Finally, intermediate (B) under went consecutive cyclization and dehydration under the catalytic effect to quickly obtainthe targeted product. From the above investigations, the proposed mechanism obeyed the following scheme (Figure 3).
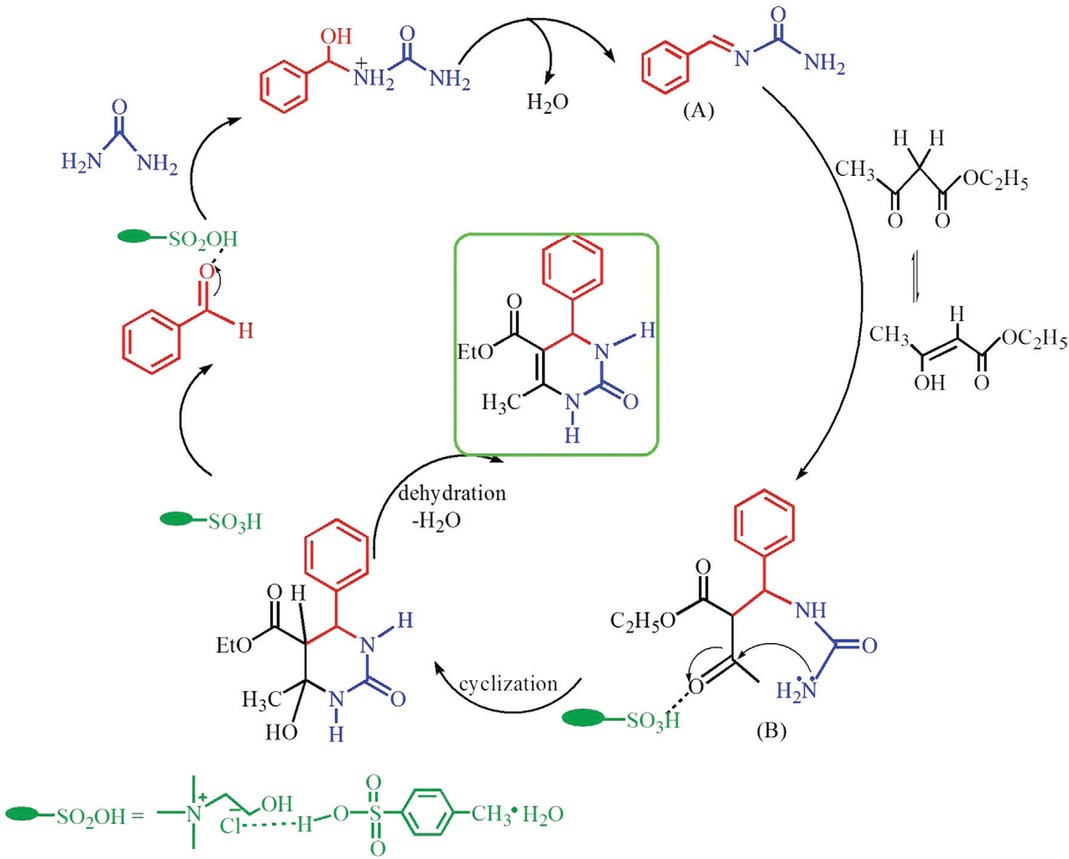
Mechanism of Biginelli reaction in the presence of ChCl/2PTSA.
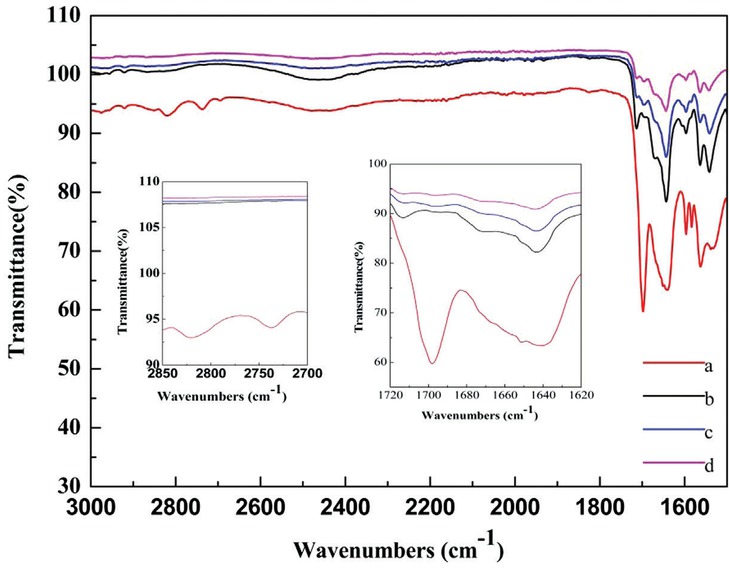
IR spectra of benzaldehyde, urea and ChCl/2PTSA.
Reaction condition: aldehyde (2.0 mmol), urea (3.0 mmol), ChCl/2PTSA (0.6 mmol), 70°C; a: initial mixtures, b: heating for 10 min, c: heating for 20 min, d: heating for 30 min.
4 Conclusions
Biginelli reactions of β-ketoesters with aldehydes and urea or thiourea were achieved in the presence of low-cost and green DESs. Notably, the catalytic performance of DESs was greatly enhanced, which is important from the green chemistry perspective. Under optimal conditions, high yields of DHPMs were obtained. Furthermore, DESs can be recycled and reused by washing with water, indicating a green and simple process. Importantly, the yields did not change after six recycling cycles, thereby demonstrating the potential of DESs for future industrial applications. The reaction systems can be a good alternative to green synthesis of the Biginelli reaction product in mild and solvent free conditions. This specific process provides a green method for the synthesis of DHPMs.
Acknowledgments
Sincere gratitude is extended to National Natural Science Foundation of China (NSFC Grant No. 21546007 and 21133055), Natural Science Foundation of Liaoning Provice of China (Grant No. 20180550078), Dalian Outstanding Scholar Project (Grant No. 2016RJ11), and Dalian Technology Star Project (Grant No. 2016RQ079) for the financial support.
Supplementary material Supplementary material available: The FTIR and 1H NMR spectra of DESs and 1H and 13C NMR spectra for synthetic compounds.
Conflict of interest
Conflict of interest statement: The authors declare to have no conflicts of interest regarding this article.
References
[1] Abbott A.P., Barron J.C., Frisch G., Gurman S., Ryder K.S., Silva A.F., Double layer effects on metal nucleation in deep eutectic solvents. Phys. Chem. Chem. Phys., 2011, 13, 10224-10231.10.1039/c0cp02244fSearch in Google Scholar PubMed
[2] Vigier K.O., Benguerba A., Barrault J., Jérôme F., Conversion of fructose and inulin to 5-hydroxymethylfurfural in sustainable betaine hydrochloride-based media. Green Chem., 2012, 14, 285-289.10.1039/C1GC16236ESearch in Google Scholar
[3] Aroso I.M., Paiva A., Reis R.L., Duarte A.R.C., Natural deep eutectic solvents from choline chloride and betaine-physicochemical properties. J. Mol. Liq., 2017, 241, 654-661.10.1016/j.molliq.2017.06.051Search in Google Scholar
[4] Liu P., Hao J.W., Mo L.P., Zhang Z.H., Recent advances in the application of deep eutectic solvents as sustainable media as well as catalysts in organic reactions. RSC Adv., 2015, 5, 48675-48704.10.1039/C5RA05746ASearch in Google Scholar
[5] Zhang M., Liu Y.H., Shang Z.R., Hu H.C., Zhang Z.H., Supported molybdenum on graphene oxide/Fe3O4 An efficient, magnetically separable catalyst for one-pot construction of spiro-oxindole dihydropyridines in deep eutectic solvent under microwave irradiation. Catal. Commun., 2017, 88, 39-44.10.1016/j.catcom.2016.09.028Search in Google Scholar
[6] Gao G., Wang P., Liu P., Zhang W.H., Mo L.P., Zhang, Z.H., Deep eutectic solvent catalyzed one-pot synthesis of 4,7-dihydro-1H-pyrazolo[3,4-b]pyridine-5-carbonitriles. Chin. J. Org. Chem., 2018, 38, 846-854.10.6023/cjoc201711014Search in Google Scholar
[7] Cooper E.R., Andrews C.D., Wheatley P.S., Webb P.B., Wormald P., Morris R.E., Ionic liquids and eutectic mixtures as solvent and template in synthesis of zeolite analogues. Nature, 2004, 430, 1012-1016.10.1038/nature02860Search in Google Scholar PubMed
[8] Nkuku C.A., LeSuer R.J., Electrochemistry in deep eutectic solvents. J. Phys. Chem. B, 2007, 111, 13271-13277.10.1021/jp075794jSearch in Google Scholar PubMed
[9] Abbott A.P., Ttaib K.E., Frisch G., Ryder K.S., Weston D., The electrodeposition of silver composites using deep eutectic solvents. Phys. Chem. Chem. Phys., 2012, 14, 2443-2449.10.1039/c2cp23712aSearch in Google Scholar PubMed
[10] Li C.P., Li D., Zou S.S., Li Z., Yin J.M., Wang A.L., et al., Extraction desulfurization process of fuels with ammonium-based deep eutectic solvents. Green Chem., 2013, 15, 2793-2799.10.1039/c3gc41067fSearch in Google Scholar
[11] Tang B., Zhang H., Row K.H., Application of deep eutectic solvents in the extraction and separation of target compounds from various samples. J. Sep. Sci., 2015, 38, 1053-1064.10.1002/jssc.201401347Search in Google Scholar
[12] Wang A.L., Xing P.F., Zheng X.L., Cao H.Y., Yang G., Zheng X.F., Deep eutectic solvent catalyzed Friedel-Crafts alkylation of electron-rich arenes with aldehydes. RSC Adv., 2015, 5, 59022-59026.10.1039/C5RA08950FSearch in Google Scholar
[13] Imperato G., Eibler E., Niedermaier J., König B., Low-melting sugar-urea-salt mixtures as solvents for Diels-Alder reactions. Chem. Commun., 2005, 1170-1172.10.1039/B414515ASearch in Google Scholar
[14] Liu S., Ni Y.X., Wei W.J., Qiu F.L., Xu S.L., Ying A.G., Choline chloride and urea based eutectic solvents: effective catalytic systems for the Knoevenagel condensation reactions of substituted acetonitriles. J. Chem. Res., 2014, 38, 186-188.10.3184/174751914X13926483381319Search in Google Scholar
[15] Tian X.M., Zhang S.Q., Zheng L.Y., Enzyme-catalyzed henry reaction in choline chloride-based deep eutectic solvents. J. Microbiol. Biotechnol., 2016, 26, 80-88.10.4014/jmb.1506.06075Search in Google Scholar
[16] Pawar P.M., Jarag K.J., Shankarling G.S., Environmentally benign and energy efficient methodology for condensation: an interesting facet to the classical Perkin reaction. Green Chem., 2011, 13, 2130-2134.10.1039/c0gc00712aSearch in Google Scholar
[17] Handy S., Lavender K., Organic synthesis in deep eutectic solvents: Paal-Knorr reactions, Tetrahedron Lett., 2013, 54, 4377-4379.10.1016/j.tetlet.2013.05.122Search in Google Scholar
[18] Cho H., Ueda M., Shima K., Mizuno A., Hayashimatsu M., Ohnaka Y., et al., Dihydropyrimidines: novel calcium antagonists with potent and long-lasting vasodilative and anti-hypertensive activity. J. Med. Chem., 1989, 32, 2399-2406.10.1021/jm00130a029Search in Google Scholar
[19] Atwal K.S., Rovnyak G.C., O’Reilly B.C., Schwartz J., Substituted 1,4-dihydropyrimidines. 3. Synthesis of selectively functionalized 2-hetero-1,4-dihydropyrimidines. J. Org. Chem., 1989, 54, 5898-5907.10.1021/jo00286a020Search in Google Scholar
[20] Kappe C.O., Fabian W.M.F., Semones M.A., Conformational analysis of 4-aryl-dihydropyrimidine calcium channel modulators. A comparison of ab initio, semiempirical and X-ray crystallographic studies. Tetrahedron, 1997, 53, 2803-2816.10.1016/S0040-4020(97)00022-7Search in Google Scholar
[21] Li X.T., Liu Y.H., Liu X., Zhang Z.H., Meglumine catalyzed one-pot, three-component combinatorial synthesis of pyrazoles bearing a coumarin unit. RSC Adv., 2015, 5, 25625-25633.10.1039/C5RA01677KSearch in Google Scholar
[22] Biginelli P., Synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Gazz. Chim. Ital., 1893, 23, 360-413.Search in Google Scholar
[23] Chen W.Y., Qin S.D., Jin J.R., HBF4-catalyzed Biginelli reaction: one-pot synthesis of dihydropyrimidin-2(1H)-ones under solvent-free conditions. Catal. Commun., 2007, 8, 123-126.10.1016/j.catcom.2006.05.026Search in Google Scholar
[24] Amini M.M., Shaabani A., Bazgir A., Tangstophosphoric acid (H3PW12O40 An efficient and eco-friendly catalyst for the one-pot synthesis of dihydropyrimidin-2(1H)-ones. Catal. Commun., 2006, 7, 843-847.10.1016/j.catcom.2006.02.027Search in Google Scholar
[25] Fu N.Y., Yuan Y.F., Cao Z., Wang S.W., Wang J.T., Peppe C., Indium(III) bromide-catalyzed preparation of dihydropyrimidinones: improved protocol conditions for the Biginelli reaction. Tetrahedron, 2002, 58, 4801-4807.10.1016/S0040-4020(02)00455-6Search in Google Scholar
[26] Reddy C.V., Mahesh M., Raju P.V.K., Babu T.R., Reddy V.V.N., Zirconium(IV) chloride catalyzed one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron Lett., 2002, 43, 2657-2659.10.1016/S0040-4039(02)00280-0Search in Google Scholar
[27] Starcevich J.T., Laughlin T.J., Mohan R.S., Iron(III) tosylate catalyzed synthesis of 3,4-dihydropyrimidin-2(1H)-ones/ thiones via the Biginelli reaction. Tetrahedron Lett., 2013, 54, 983-985.10.1016/j.tetlet.2012.12.032Search in Google Scholar
[28] Adib M., Ghanbary K., Mostofi M., Ganjali M.R., Efficient Ce(NO33·6H2O-catalyzed solvent-free synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Molecules, 2006, 11, 649-654.10.3390/11080649Search in Google Scholar PubMed PubMed Central
[29] Kiyani H., Ghiasi M., Phthalimide-N-sulfonic acid: a new and efficient organocatalyst for the Biginelli reaction under solvent-free conditions. Res. Chem. Intermed., 2015, 41, 6635-6648.10.1007/s11164-014-1766-7Search in Google Scholar
[30] Kiyani H., Ghiasi M., Solvent-free efficient one-pot synthesis of Biginelli and Hantzsch compounds catalyzed by potassium phthalimide as a green and reusable organocatalyst. Res. Chem. Intermed., 2015, 41, 5177-5203.10.1007/s11164-014-1621-xSearch in Google Scholar
[31] Sharma U.K., Sharma N., Kumar R., Sinha A.K., Biocatalysts for multicomponent Biginelli reaction: bovine serum albumin triggered waste-free synthesis of 3,4-dihydropyrimidin-2-(1H)-ones. Amino Acids, 2013, 44, 1031-1037.10.1007/s00726-012-1437-1Search in Google Scholar PubMed
[32] Vargas A.Y., Rojas H.A., Romanelli G.P., Martínez J.J., Synthesis of 1,4-dihydropyrimidines with immobilized urease: effect of method immobilization on magnetic supports. Green Process. Synth., 2017, 6, 377-384.10.1515/gps-2016-0143Search in Google Scholar
[33] Quan Z.J., Da Y.X., Zhang Z., Wang X.C., PS-PEG-SO3H as an efficient catalyst for 3,4-dihydropyrimidones via Biginelli reaction. Catal. Commun., 2009, 10, 1146-1148.10.1016/j.catcom.2008.12.017Search in Google Scholar
[34] Sinija P.S., Sreekumar K., Facile synthesis of pyranopyrazoles and 3,4-dihydropyrimidin-2(1H)-ones by a Ti-grafted polyamidoamine dendritic silica hybrid catalyst via a dual activation route. RSC Adv., 2015, 5, 101776-101788.10.1039/C5RA16723JSearch in Google Scholar
[35] Shen P.F., Xu M.C., Yin D.L., Xie S.A., Zhou C., Li F.D., Halogenated macroporous sulfonic resins as efficient catalysts for the Biginelli reaction. Catal. Commun., 2016, 77, 18-21.10.1016/j.catcom.2016.01.010Search in Google Scholar
[36] Patel H.A., Sawant A.M., Rao V.J., Patel A.L., Bedekar A.V., Polyaniline supported FeCl3 an effective heterogeneous catalyst for Biginelli reaction. Catal. Lett., 2017, 147, 2306-2312.10.1007/s10562-017-2139-9Search in Google Scholar
[37] Khiar C., Tassadit M., Bennini L., Halouane M., González M.J.B., Menad S., et al., Cobalt supported on alumina as green catalyst for Biginelli reaction in mild conditions: effect of catalyst preparation method. Green Process. Synth., 2017, 6, 533-541.10.1515/gps-2016-0149Search in Google Scholar
[38] Chen X.F., Peng Y.Q., Chloroferrate(III) ionic liquid: efficient and recyclable catalyst for solvent-free synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Catal. Lett., 2008, 122, 310-313.10.1007/s10562-007-9377-1Search in Google Scholar
[39] Zhu A.L., Li Q.Q., Li L.J., Wang J.J., One-pot synthesis of 3,4-dihydro-2(H)-pyrimidinones catalyzed by reusable acidic choline-based ionic liquids. Catal. Lett., 2013, 143, 463-468.10.1007/s10562-013-0978-6Search in Google Scholar
[40] Rahman M., Sarkar A., Ghosh M., Majee A., Hajra A., Catalytic application of task specific ionic liquid on the synthesis of benzoquinazolinone derivatives by a multicomponent reaction. Tetrahedron Lett., 2014, 55, 235-239.10.1016/j.tetlet.2013.11.011Search in Google Scholar
[41] Savanur H.M., Kalkhambkar R.G., Aridoss G., Laali K.K., [bmim(SO3H)][OTf]/[bmim][X] and Zn(NTf22/[bmim][X] (X = PF6 and BF4 efficient catalytic systems for the synthesis of tetrahydropyrimidin-ones (-thiones) via the Biginelli reaction. Tetrahedron Lett., 2016, 57, 3029-3035.10.1016/j.tetlet.2016.05.103Search in Google Scholar
[42] Gore S., Baskaran S., Koenig B., Efficient synthesis of 3,4-dihydropyrimidin-2-ones in low melting tartaric acid-urea mixtures, Green Chem., 2011,13, 1009-1013.10.1039/c1gc00009hSearch in Google Scholar
[43] Momeni A.R., Samimi H.A., Vaezzadeh H., Eutectic mixture choline chloride–chloroacetic acid: a new and efficient catalyst for synthesis of 3,4-dihydropyrimidin-2-ones. Chem. Method., 2018, 4, 253-261.Search in Google Scholar
[44] Mahdipour M., Khabazzadeh H., Kermani E.T., Efficient synthesis of dihydropyrimidine and amidoalkyl naphthol derivatives using zinc chloride based deep eutectic systems as solvent & catalyst. J. Sci. I. R. Iran., 2016, 27, 119-127.Search in Google Scholar
[45] Borse B.N., Borude V.S., Shukla S.R., Synthesis of novel dihydropyrimidin-2(1H)-ones derivatives using lipase and their antimicrobial activity. Curr. Chem. Lett., 2012, 1, 59-68.10.5267/j.ccl.2012.3.001Search in Google Scholar
[46] Florindo C., Oliveira F.S., Rebelo L.P.N., Fernandes A.M., Marrucho I.M., Insights into the synthesis and properties of deep eutectic solvents based on cholinium chloride and carboxylic acids. ACS Sustain Chem. Eng., 2014, 2, 2416-2425.10.1021/sc500439wSearch in Google Scholar
[47] Yin J.M., Wang J.P., Li Z., Li D., Yang G., Cui Y.N. et al., Deep desulfurization of fuels based on an oxidation/extraction process with acidic deep eutectic solvents. Green Chem., 2015, 17, 4552-4559.10.1039/C5GC00709GSearch in Google Scholar
[48] Cui Y.N., Li C.P., Yin J.M., Li S.M., Jia Y.P., Bao M., Design, synthesis and properties of acidic deep eutectic solvents based on choline chloride. J. Mol. Liq., 2017, 236, 338-343.10.1016/j.molliq.2017.04.052Search in Google Scholar
[49] Jin T.S., Zhang S.L., Li T.S., p-Toluenesulfonic acid-catalyzed efficient synthesis of dihydropyrimidines: improved high yielding protocol for the Biginelli reaction. Synth. Commun., 2002, 32, 1847-1851.10.1081/SCC-120004068Search in Google Scholar
[50] Kappe C.O., A reexamination of the mechanism of the Biginelli dihydropyrimidine synthesis. Support for an N-acyliminium ion intermediate. J. Org. Chem., 1997, 62, 7201-720410.1021/jo971010uSearch in Google Scholar PubMed
© 2019 Cui et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering


