Abstract
This work is focused on the synthesis of organic carbonates from CO2 and ethanol. A parametric study of the synthesis of diethyl carbonate from ethanol is performed in a 100 mL batch reactor. The influence of pressure and temperature is studied and we prove that the presence of water strongly decreases the yield in diethyl carbonate as an equilibrium is quickly reached. One method to improve this yield is to remove water from the reaction mixture to shift the equilibrium toward the formation of carbonates. The chemical methods give good results but separation and regeneration associated steps are prohibitive. For these reasons, a physical technique like pervaporation is chosen to remove water. The study of a pervaporation cell with membrane PERVAP 4100 gives good results for the dehydration of ethanol alone even at low concentrations of water from 0.33 %wt to 0.15 %wt. Twelve experiments on the dehydration of a mixture of ethanol, diethyl carbonate and water are performed. The calculated separation factors show a very good selectivity for water. That means that even in the presence of diethyl carbonate, the membrane has still a selective water permeability.
1 Introduction
With 34 Gt emitted per year, carbon dioxide is the most human emitted greenhouse gases in the world. The elevation of the global temperature can reach up +5°C in 2100 if these emissions are not limited and the global warming effects on the earth could be irreparable [1]. For these reasons, carbon capture and storage is still an important topic [2]. This observation also stimulates a more rational use of carbon atoms, where CO2 becomes a resource to be valued. In a circular economy perspective, a new chemistry using CO2 is developing the reuse of CO2, which becomes a source of carbon [3]. Moreover, considering the rarefaction and the cost of fossil fuels, the use of bio-based reactants has to be done to develop new processes.
In this context, our work is focused on the synthesis of organic carbonates by associating CO2 with ethanol. Because of their low toxicity, organic carbonates have many potential applications like fuel additive, solvent or monomer [4]. Currently, they are produced by using some toxic and harmful components like phosgene. The synthesis via the route “CO2 + alcohol” has proven to be the most interesting one with regard to the environmental aspect and the sequestration of CO2 [5]. Compared to other reactions of CO2 valorisation, organic carbonates represent the most important value added products and the market is around one million tons annually [6]. Figure 1 represents an example of the potential reactions: the synthesis of diethyl carbonate from CO2 and bioethanol.
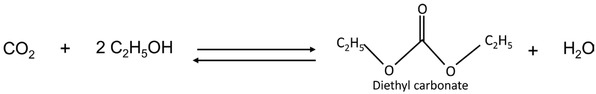
Ethanol carbonation with CO2.
Because of the unfavourable thermodynamics of the reaction, the yield of carbonate is still low [7]. A lot of researches have been done to develop new performant catalysts [7, 8]. Researches began with homogeneous catalysts mainly based on metal like titanium, zirconium and niobium. Nowadays, most of articles are based on heterogeneous catalysts, which are more advantageous for industrial applications. Zirconium oxide and cerium oxide are the most used one. Tomishige et al. were the first to highlight the potential of ZrO2 with a selectivity of 100% [9]. Similar selectivities are obtained with CeO2. The good results obtained are mainly due to the amphoteric properties of these two catalysts. Indeed, both of them have acid and base sites which are essential for the synthesis of carbonates [10]. By changing the shape or the calcination temperature of these catalysts, it is possible to increase the quantity of these sites to improve the yield in carbonates [10, 11]. Some authors improve the yield by adding some Brønsted acid sites on the catalyst by using H3PO4/ZrO2 [12] or H3PW12O4/ZrO2 [13]. Even if new catalysts are developed to improve yields in carbonates, yields are still low because thermodynamic equilibrium exists between reactants and products of the reaction.
A dehydration of the reaction medium can improve the yield. Indeed, by removing molecules of water produced during the reaction, chemical balance promotes reaction product formation and the production of carbonate is improved. The reactive dehydration is the most used method in the literature. A dehydrating agent is added in the reaction mixture to react with water molecules. Recently, the addition of 2-cyanopyridine has been proved to improve the yield in dimethyl carbonate [14]. Even if this dehydration method can improve the yield, it raises some issues like the separation and the recycling of the 2-cyanopyridine and the molecule produced. Beside to this chemical route, a physical dehydration route has to be developed.
Our work involves the use of a physical way to dehydrate called pervaporation. In this separation process, a liquid mixture is circulated on a dense membrane and the downstream side is kept under vacuum [15]. In our case, we used an hydrophilic membrane which separates selectively the water from the reaction mixture. This reputed low energy consuming process is advantageous because no chemical reactive is added, so no additional problem of separation is introduced. The main industrial application of pervaporation is the dehydration of organic solvents, especially the production of anhydrous ethanol for the pharmaceutical industry [16]. A lot of researches have been done to improve the performance of the membrane to dehydrate solvents [17, 18]. Pervaporation is also used in membrane reactors to remove water from the reaction mixture in case of reactions with thermodynamic limitations or if the water inhibits the catalyst [19]. The most developed example is the dehydration of the reaction of esterification [20]. Only few articles present results about the association of the reaction of carbonation with a dense or porous separation membrane [21, 22, 23, 24, 25]. Related experiments prove that this separation method is efficient but needs more studies. For example, Dibenedetto et al. increase the yield in diethyl carbonate from 0.9% to 2.3% by using pervaporation [21]. Kuenen et al. do a modelling on Aspen of a complete process with the catalytic membrane reactor and purification units of the products [23]. They conclude that this process is not viable economically and in term of CO2 emission but they does not have enough data on the kinetics of the reaction and on the efficiency of the membrane separation. In an opposite way, Aresta et al. make an economical study to compare different ways of dehydrating this reaction mixture and they prove that pervaporation can be the most interesting way to dehydrate this kind of reaction mixture in term of energy saving and CO2 emission [24].
In this article, we present the results obtained on the carbonation reaction and on the pervaporation separately. A parametric study has been done on the synthesis of diethyl carbonate (DEC). Some studies deal with the production of DEC from CO2 and ethanol [26] but this synthesis is less studied in the literature than the synthesis of dimethyl carbonate. However, Shulka et al. show in a recent study the growing interest of this molecule and highlight the lack of data for its synthesis [27]. From the previous literature review, an heterogeneous catalyst is needed for the carbonation reaction. In our study, cerium oxide catalyst has been used because of its selectivity, cheapness, availability and low toxicity [28]. For the pervaporation part, a commercial membrane has been tested with different model solutions. These solutions are made of ethanol, DEC and very low amount of water, which can also potentially exist in the reactor. From our knowledge, no data is available on the study of this ternary mixture. The objective is to show the influence of the presence of DEC on the separation.
2 Materials and methods
2.1 Materials
Cerium (IV) oxide with a purity of 99.5% was purchased from Alfa Aesar. This catalyst was calcined at 600°C during 4 h in the air. Absolute ethanol (>99.7%) was purchased from VWR and diethyl carbonate from Alfa Aesar (>99%). Ethanol and diethyl carbonate were used as received.
2.2 Reactor unit
The parametric study of the reaction has been done in a Parr autoclave with a capacity of 100 mL represented in Figure 2. It is composed with a blade agitator, a valve to take samples during the reaction and two valves to add or remove CO2. The temperature is controlled and the pressure is read on a pressure gauge. For a typical experiment, 50 mL of ethanol are filled in the reactor with 2 g of CeO2. A test of pressure is made with nitrogen at 60 bar during 30 min. Then, the reactor is purged three times with CO2. After that, the CO2 is filled at the desired pressure by waiting for its solubilisation in ethanol during 45 min. The temperature is then increased up to the desired temperature. 1 mL of sample is taken at selected times during the reaction. At the end of the reaction, the reactor is cooled down and the pressure is decreased. Samples are diluted in methanol with hexanol as an internal standard and they are analysed by gas chromatography with a FID detector. Table 1 presents the reaction operating conditions used for the experiments.
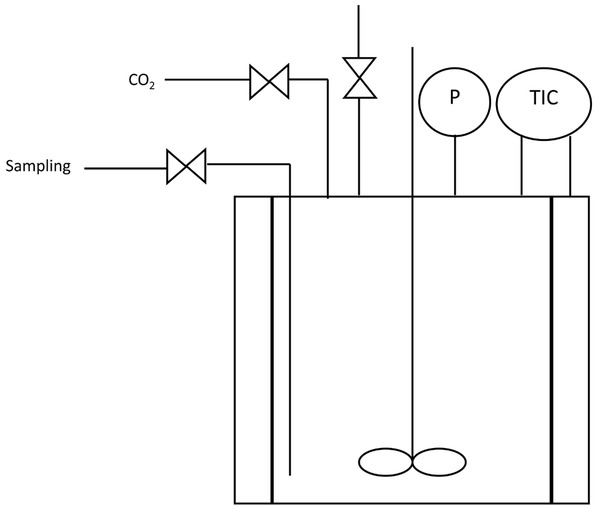
Scheme of the reactor used for the carbonation.
Experimental conditions for reactions of carbonation of ethanol with 2 g of CeO2 during 24 h.
| Experiments | Temperature (°C) | Pressure (bar) | initial water content (%wt) |
|---|---|---|---|
| R1 | 110 | 11 | |
| R2 | 110 | 18 | |
| R3 | 110 | 28 | |
| R4 | 110 | 36 | |
| R5 | 110 | 52 | |
| R6 | 110 | 66 | |
| R7 | 92 | 48 | |
| R8 | 101 | 50 | |
| R9 | 112 | 53 | |
| R10 | 118 | 55 | |
| R11 | 125 | 58 | 0.080 |
| R12 | 125 | 58 | 0.050 |
| R13 | 125 | 58 | 0.163 |
| R14 | 125 | 58 | 0.262 |
| R15 | 125 | 58 | 0.264 |
2.3 Pervaporation experiments
The process used to study the pervaporation is shown schematically in Figure 3. The pervaporation cell and the membrane PERVAP 4100 were supplied from DeltaMem [29]. A porous steel plate is placed in one part of the pervaporation cell, and the membrane is placed on it. The two parts of the cell are then closed and an O-ring in EPDM is used to ensure no leakage between the feed part and the permeate part. The area of the membrane is 0.016 m2. The heated and agitated feed (around 1 L) is circulated at 444 mL/min in the pervaporation cell with a feed pump. A thermometer is placed before the pervaporation cell to control the temperature. The permeate side pressure is maintained at 2 mbar with a vacuum vane pump and the permeate is condensed in two cold traps immersed in liquid nitrogen. For a typical experiment, the feed is heated at the desired temperature. Then it is circulated on the pervaporation cell and the permeate is condensed in a first cold trap. After 1 h of circulation, the steady state is obtained because the temperature in the cell is constant, the permeate is condensed in the second cold trap and the experiment begins. At the end of the experiment, the circulation flow is stopped and the feed is cooled down. The vacuum pump is stopped and the permeate is recovered in the second cold trap. The permeate and the feed are weighted and analysed. The amount of ethanol and DEC are analysed by a gas chromatograph with a FID detector with an accuracy of 2% and the amount of water is determined by a Karl Fischer titration with a coulometric method for concentration below 1 %wt and a volumetric method for higher concentrations. Accuracies of these
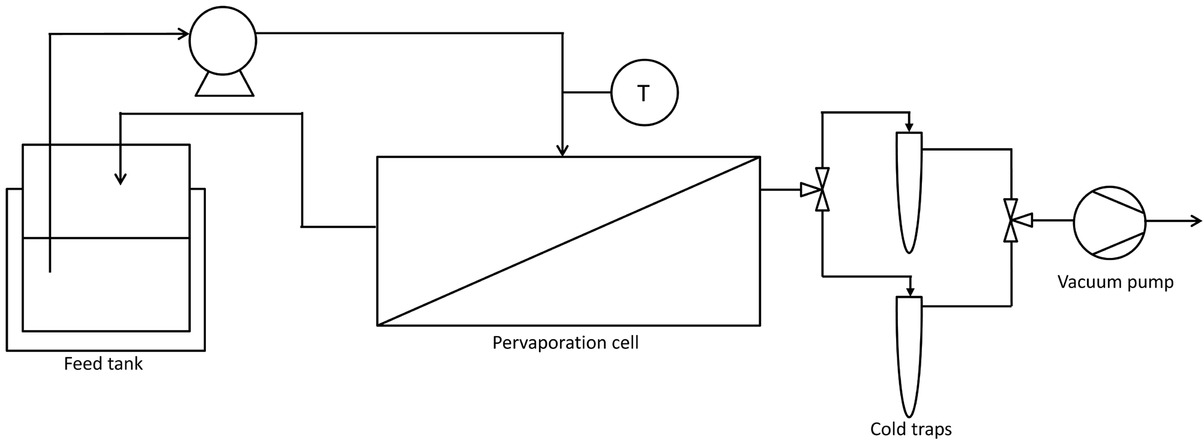
Scheme of the pervaporation process.
two methods are respectively 10% and 2%. The amount of permeate removed by the membrane was below 0.1% of the volume of the feed in order to keep the concentration in the feed almost constant. Table 2 presents the operating conditions used to study the pervaporation process.
Experimental conditions for pervaporation.
| Experiments | Temperature | Time | initial water | initial DEC | |
|---|---|---|---|---|---|
| (°C) | (h) | content (%wt) | content | (%wt) | |
| P1 | 67 | 16 | 0.33 | 0 | |
| P2 | 73 | 16 | 0.33 | 0 | |
| P3 | 77 | 16 | 0.33 | 0 | |
| P4 | 83 | 16 | 0.33 | 0 | |
| P5 | 87 | 16 | 0.33 | 0 | |
| P6 | 87 | 6 | 0.20 | 0 | |
| P7 | 87 | 5 | 0.33 | 0 | |
| P8 | 87 | 6 | 0.20 | 1 | |
| P9 | 87 | 5 | 0.33 | 1 | |
| P10 | 87 | 6 | 0.20 | 3 | |
| P11 | 87 | 5 | 0.33 | 3 | |
| P12 | 87 | 6 | 0.20 | 5 | |
| P13 | 87 | 5 | 0.33 | 5 | |
| P14 | 87 | 6 | 0.20 | 10 | |
| P15 | 87 | 5 | 0.33 | 10 | |
| P16 | 87 | 6 | 0.20 | 15 | |
| P17 | 87 | 5 | 0.33 | 15 | |
3 Results and discussion
3.1 Parametric study of the reaction
Figure 4 represents the evolution of the amount of diethyl carbonate (DEC) as a function of the pressure in the reactor. Reactions are performed at 110°C during 24 h. Between 10 bar and 40 bar, the conversion of ethanol in DEC increases. For pressure higher than 40 bar, the conversion seems to be constant or even to decrease. This behaviour was reported in the literature in the case of the carbonation of methanol [13].
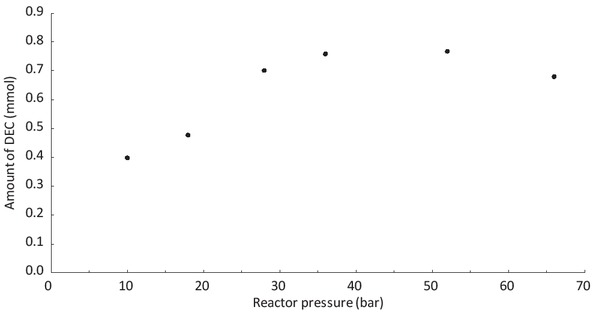
The dependence of the pressure on the amount of DEC produced.
Ethanol: 50 mL, CeO2: 2 g, temperature: 110°C, reaction time: 24 h.
Figure 5 shows the influence of temperature on the conversion of ethanol in DEC. For these reactions, CO2 is introduced until a constant pressure of 24 bar is obtained after dissolution of CO2 in ethanol. Then, the reactor is heated at the desired temperature. Samples have been taken as a function of time to study the evolution of the yield during the time. It can be seen that the conversion seems to increase quickly at the beginning and seems to stabilize after 20 h. This evolution of reaction as a function of time is similar to the case of methanol in literature [30]. The conversion of ethanol increases with the temperature between 95°C and 125°C. This behaviour is also seen in literature for the carbonation of methanol but with higher temperature, the yield may decrease. Indeed, the reaction is exothermic so this decrease can be due to the reaction thermodynamics [31, 32]. Although temperature can improve conversion, the yield of DEC seems to have a limited value.
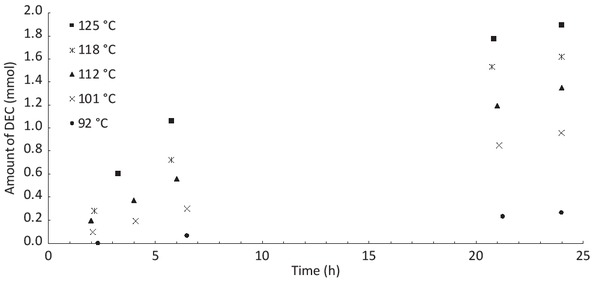
Evolution of amount of DEC produced as a function of time for different reactor temperatures.
Ethanol: 50 mL, CO2: 24 bar, CeO2: 2 g.
The influence of the initial presence of water in the reaction medium has been also tested. The reactions were performed at 125°C with 24 bar of CO2 and different amounts of water were added at the beginning of the reaction. As we can see in Figure 6, the presence of water decreases the yield. Indeed, from 0.050 %wt of water to 0.264 %wt of water, the yield is reduced by half. This conclusion proves that it is necessary to dehydrate the reaction mixture to improve the yield.
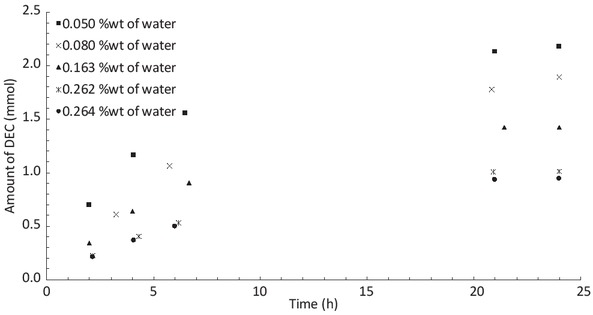
Influence of the initial water concentration on the amount of DEC produced during the reaction.
Ethanol: 50 mL, CeO2: 2 g, CO2: 24 bar, 125°C.
3.2 Dehydration of a ternary mixture ethanol-DEC-water by pervaporation
Classical dehydrations of ethanol by pervaporation are made with amounts of water higher than 1 %wt [17]. In our case, at the end of a typical reaction, the amount of water is lower than 0.30 %wt. To understand the behaviour of the membrane for such low amounts of water, some tests were performed to dehydrate ethanol-water solution with a little amount of water. Figure 7
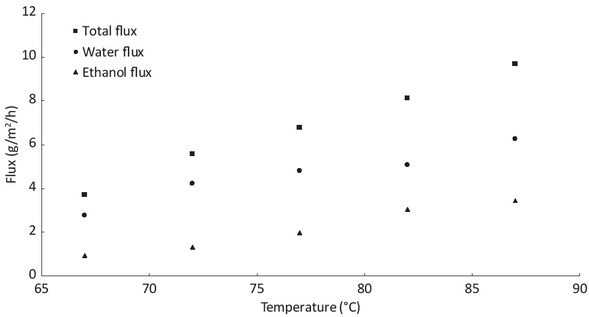
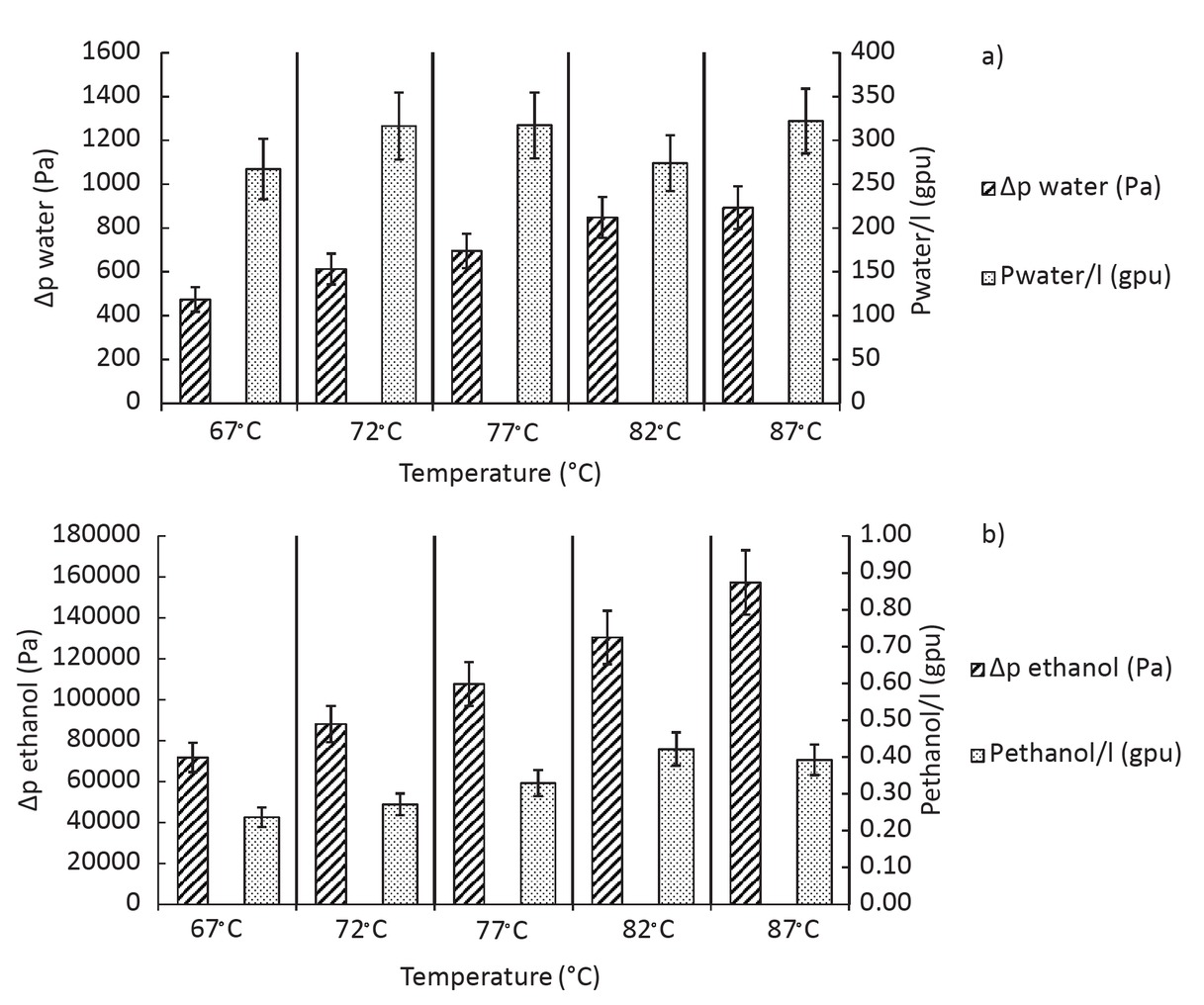
Influence of temperature on the flux for ethanol-water solution with 0.33 %wt of water.
represents results of experiments on the influence of temperature with 0.33 %wt of water in the feed. The flux corresponds to the weight of permeate divided by the time of the experiment and the membrane surface. The accuracy of the calculated flux is around 5%. It can be seen that temperature has an important impact on the flux. Indeed, between 67°C and 87°C the total flux increases from 4 g/m2/h up to 10 g/m2/h. The mass transfer through the membrane can be described by Arrhenius-type laws shown in Eq. 1 [15].
where Ji represents the flux of the component i in g/m2/h, Ai is the pre-exponential factor in g/m2/h, Ei is the activation energy in J/mol. For our case, Figure 8 represents the logarithm of the water and the ethanol flux as a function of the inverse of the temperature. The results show that the mass transfer obeys to an Arrhenius law. The activation energy of water is smaller (37 kJ/mol) than the one of ethanol (69 kJ/mol). We can conclude that the flux increases with the temperature but the selectivity seems to decrease. To understand this phenomenon, we analysed the influence of temperature on the behaviour of the membrane and on the pressure gradient. Indeed, we can described the flux with solution-diffusion model [33]:
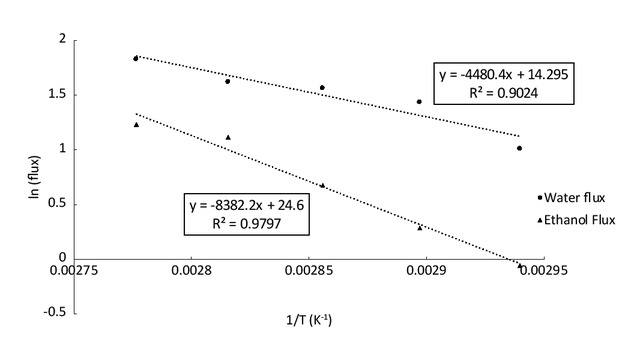
Logarithm of water and ethanol flux against the inverse of temperature.
where Di is the membrane diffusion coefficient and Ki the sorption coefficient of component i. Pi is the permeability, which represents the combination of the two effects of sorption and diffusion. l is the membrane thickness and Pi /l is called the permeance. pi feed and pi permeate represent the pressure of component i respectively in the feed
and in the permeate. With this model, the flux can be represented by the product of the permeance, which is related to the intrinsic properties of the membrane, and the activity gradient, which is the difference of pressure between the two parts of the membrane. We can calculate the two pressures with Eq. 3 and 4:
γi is the activity coefficient (obtained by NRTL model), xi is the liquid molar fraction and pisat is the saturation vapour pressure of the component i in the feed liquid. yi is the vapour molar fraction of component i in the permeate and ppermeate is the pressure in the permeate side. We can calculate the permeance by using Eq. 5:
ji is the molar flux in m3(STP)/m2s and is calculated with Eq. 6:
viG is the molar volume of gas i (0.0224 m3(STP)/mol) and Mi is the molecular weight of component i. In that case, the permeance Pi/l can be expressed in gpu (gas permeation unit, 1 m3/m2 s kPa=1.33 × 108 gpu).
Figure 9 represents the permeance and the pressure gradient of water and ethanol as a function of the temperature between 67°C and 87°C. We can see that for both water and ethanol, the gradient of pressure increases with temperature which is consistent because the partial pressure of each component in the feed increases with temperature. The pressure gradient is 100 times higher for ethanol than for water because the fraction of water is really low (0.33 %wt). But the permeance is 1000 times higher for water than for ethanol that means that the membrane has a good selectivity for water compare to ethanol. The permeance does not seem to be influenced by the temperature. Indeed, the permeance of water is quite constant and the permeance of ethanol increases slightly. The temperature does not seem to influence the intrinsic property of the membrane, so the increase of the water and ethanol flux can be explained only by the increase of the gradient of pressure for this temperature range. The decrease of the selectivity of water with the increase of temperature can be explained by the more important increase of the partial vapour pressure of ethanol in that temperature range than the increase of the partial vapour pressure of water. Indeed, the boiling point of ethanol and water are 78°C and 100°C respectively. Between 67°C and 87°C we are lower than the boiling point of water but the boiling point of ethanol is in this temperature range. For the study of the ternary mixtures, we decided to work at 87°C, which gives better results.
Influence of the temperature on the pressure gradient and the permeance for ethanol-water solution with 0.33 %wt of water.
Figure 10 represents the partial flux, with an accuracy of 6%, obtained for the dehydration of 12 solutions with different compositions of ethanol, DEC (from 0 to 15 %wt) and water (0.20 %wt and 0.33 %wt) detailed in Table 2. The flux for the experiments with 0.33 %wt of water in the feed is higher than the one for 0.20 %wt of water. The amount of water in the permeate is also higher in the case of 0.33 %wt in the feed with 70 %wt. Only 60 %wt of water in the permeate is obtained in the case of 0.20 %wt of water in the feed. The flux of DEC increases with the increase of the amount of DEC in the feed: at 15 %wt of DEC in the feed, we obtained 6% wt in the permeate, which is still low. To compare the selectivity of the membrane between these three components, we calculate selectivity factors defined in Eq. 7.
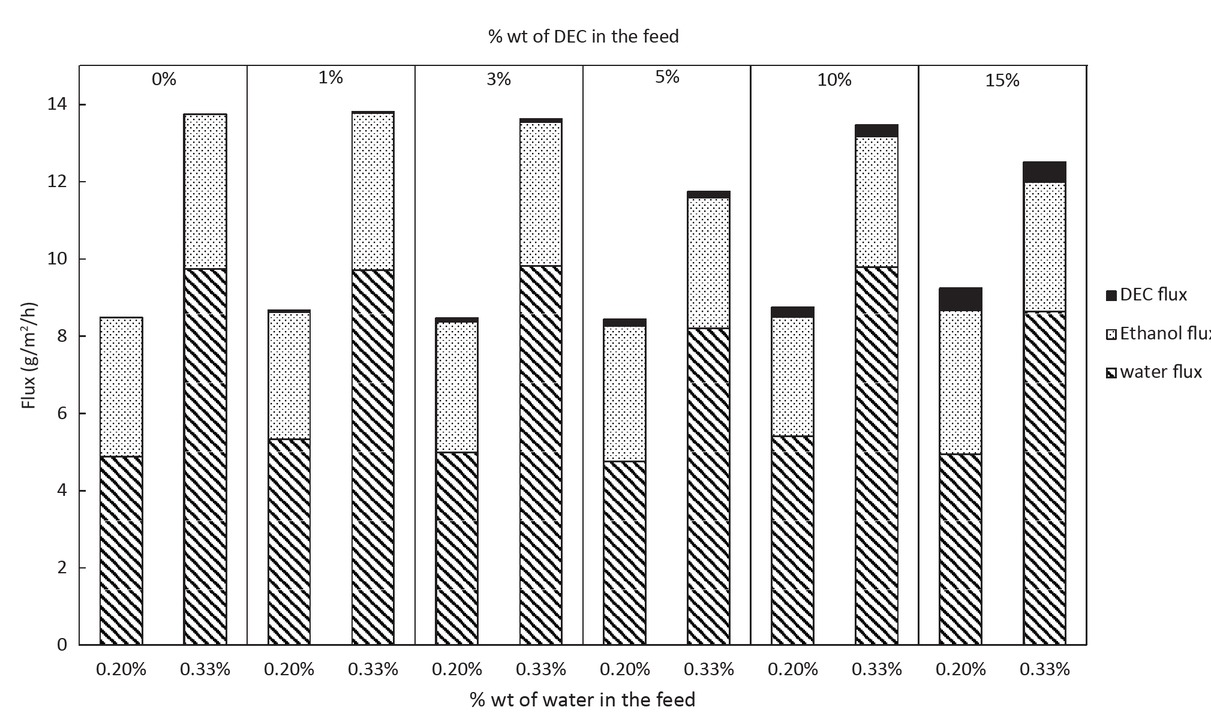
Partial flux of water, ethanol and DEC in function of the amount of water and DEC in the feed.
where x represents the molar fraction of the component in the permeate and in the feed. A represents the component selectively transferred in the permeate, water in our case, and B represents the other component: ethanol or DEC. Table 3 shows the calculated factors for the 12 experiments. We can see that these factors are high, they are in the range of 750 and 1020 for water on ethanol. Water or DEC concentrations do not seem to have a significant impact on these factors. Selectivity
Water selectivity as a function of ethanol and DEC for two amounts of water in the feed: 0.20 %wt and 0.33 %wt and different amounts of DEC: 0 %wt, 1 %wt and 3 %wt.
| % of DEC in the feed | 0.20% of water in the feed | 0.33% of water in the feed | ||
|---|---|---|---|---|
| ∝water/ethanol | ∝water/DEC | ∝water/ethanol | ∝water/DEC | |
| 0% | 860 | 940 | ||
| 1% | 1200 | 961 | 880 | 1630 |
| 3% | 910 | 1130 | 970 | 1440 |
| 5% | 810 | 850 | 940 | 1010 |
| 10% | 990 | 1440 | 970 | 1300 |
| 15% | 750 | 910 | 890 | 1080 |
factors for water on DEC are higher than for water on ethanol especially for the case at 0.33 %wt of water in the feed.
Figure 11 shows the calculated gradients of pressure and permeances for these 12 experiments. Figure 11a presents results obtained for water. The gradient of pressure is higher for the case at 0.33 %wt of water in the feed but the permeance is quite the same for the two cases at 0.20 or 0.33 %wt. So the difference in the flux of water is only due to the difference in the gradient of pressure. The permeance of water seems to decrease slowly with the increase of the amount of DEC in the feed. It can be due to the fact that DEC has a slightly better affinity with the membrane than ethanol. We can see that difference in Figures 11b and 11c the permeance of ethanol is around 0.4 and the one of DEC is higher at 0.6. This difference is quite small so the decrease in water permeance stays negligible for this range of amount of DEC. These results are really interesting and promising for the association of the reaction and the pervaporation because the DEC seems to stay in the feed.
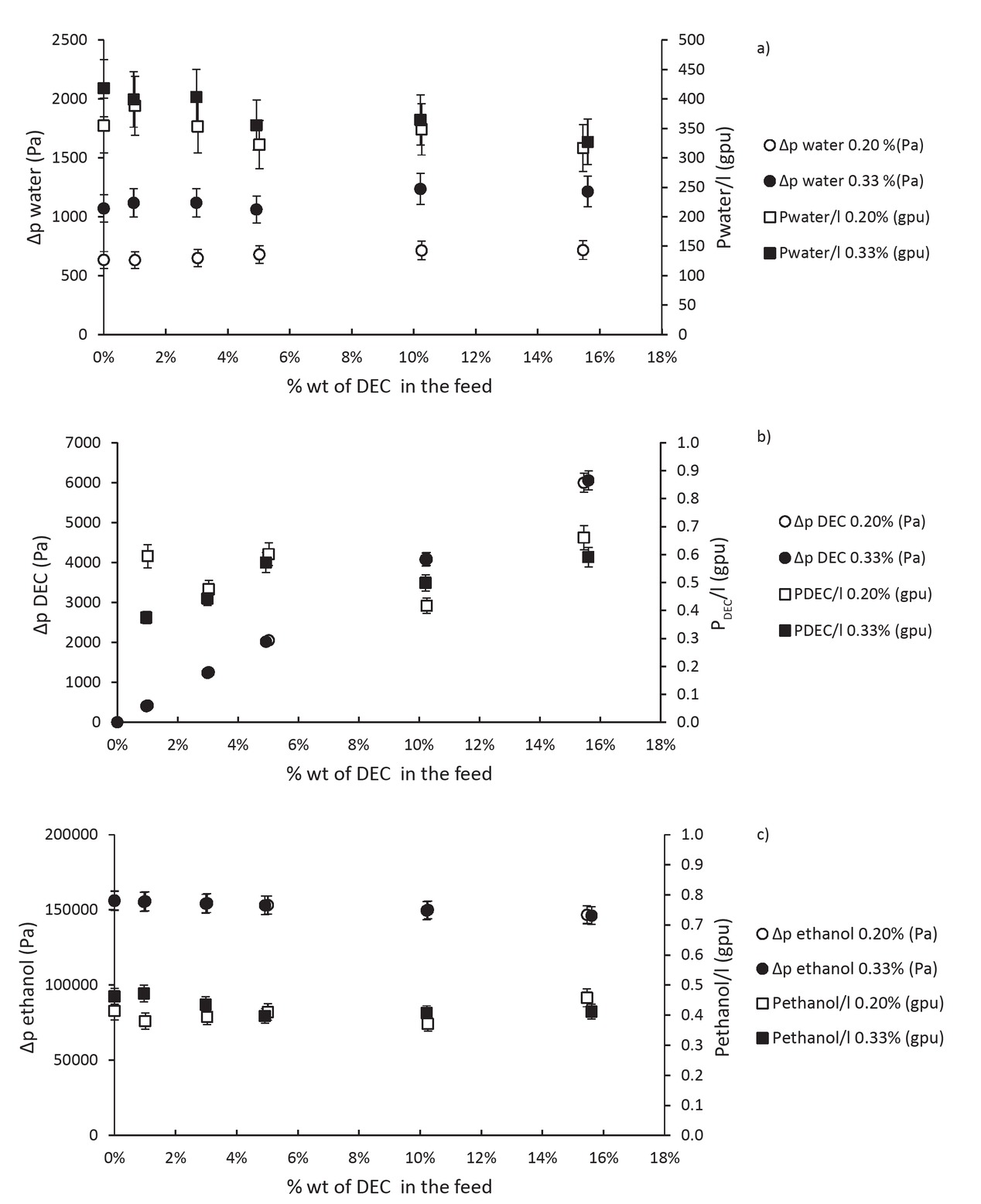
Influence of the amount of DEC on the pressure gradient and permeance of (a) water, (b) DEC and (c) ethanol.
As expected, this first study on the pervaporation of the ternary mixture of ethanol, DEC and water, confirms that the dehydration of the reaction mixture is possible. The objective is now to optimize the surface area of the membrane necessary to improve the yield in DEC significantly. A kinetic model of the reaction and a modelling of the dehydration of the reaction mixture with different compositions are needed to simulate
the circulation loop between the reactor and the pervaporation cell and then, to optimize the surface area of the membrane.
4 Conclusion
A parametric study of the reaction of carbonation of ethanol has been made. The results are quite similar to those obtained in the literature on the synthesis of dimethyl carbonate from methanol. The influence of the presence of water in the production of DEC has been highlighted. This result shows that it is necessary to dehydrate the reaction mixture to improve the yield in DEC. The pervaporation experiments show that it is possible to dehydrate ethanol even with an amount of water below than 0.33 %wt. The temperature and the amount of water in the feed have a strong impact on the separation. Twelve experiments have been performed to simulate the dehydration of a reaction medium at the end of a reaction. The presence of DEC from 0 to 15 %wt in the feed seems to have no impact on the water flux. These results are promising. The continuous extraction of water by pervaporation all along the reaction has to be done to improve the yield in DEC.
Acknowledgements
The authors thank MADNESS project. The MADNESS project has been funded with the support from the European Union with the European Regional Development Fund (ERDF) and from the Regional Council of Normandie. The authors are also grateful for the financial support from ADEME and the Regional Council of Normandie.
List of abbreviations
- A
Pre-exponential factor (g/h/m2)
- D
Membrane diffusion coefficient (m2/s)
- E
Activation energy (J/mol)
- J
Flux (g/h/m2)
- j
Flux (m3(STP)/m2/s)
- K
Sorption coefficient (m3(STP)/m3/ bar)
- l
Membrane thickness (m)
- M
Molecular weight (g/mol)
- P
Permeability (m3(STP)m/m2/s/bar)
- p
Partial pressure (bar)
- psat
Saturation vapour pressure (bar)
- R
Ideal gas constant (J/mol/K)
- T
Temperature (K)
- vG
Molar volume of gas (m3/mol)
- x
Liquid molar fraction (-)
- y
Vapour molar fraction (-)
- α
Selectivity (-)
- γ
Activity coefficient (-)
References
[1] IEA. Energy and Climate Change, 2015.Search in Google Scholar
[2] Leung D.Y.C., Caramanna G., Maroto-Valer M.M., An overview of current status of carbon dioxide capture and storage technologies. Renew. Sust. Energ. Rev., 2014, 39, 426-443.10.1016/j.rser.2014.07.093Search in Google Scholar
[3] Olajire A.A., Valorization of greenhouse carbon dioxide emissions into value-added products by catalytic processes. J. CO2 Util., 2013, 3, 74-92.10.1016/j.jcou.2013.10.004Search in Google Scholar
[4] Huang S., Yan B., Wang S., Ma X., Recent advances in dialkyl carbonates synthesis and applications. Chem. Soc. Rev., 2015, 44, 3079-3116.10.1039/C4CS00374HSearch in Google Scholar
[5] Monteiro J.G.M.-S., Araújo O. de Q.F., Medeiros J.L. de., Sustainability metrics for eco-technologies assessment, part I: preliminary screening. Clean. Technol. Envir., 2009, 11, 209-214.10.1007/s10098-008-0189-9Search in Google Scholar
[6] ADEME. Valorisation chimique du CO2 - Etat des lieux, 2014.Search in Google Scholar
[7] Tamboli A.H., Chaugule A.A., Kim H., Catalytic developments in the direct dimethyl carbonate synthesis from carbon dioxide and methanol. Chem. Eng. J., 2017, 323, 530-544.10.1016/j.cej.2017.04.112Search in Google Scholar
[8] Kindermann N., Jose T., Kleij A.W., Synthesis of Carbonates from Alcohols and CO2 Top. Curr. Chem., 2017, 375, 15.10.1007/978-3-319-77757-3_2Search in Google Scholar
[9] Tomishige K., Sakaihori T., Ikeda Y., Fujimoto K., A novel method of direct synthesis of dimethyl carbonate from methanol and carbon dioxide catalyzed by zirconia. Catal. Lett., 1999, 58, 225-229.10.1023/A:1019098405444Search in Google Scholar
[10] Wang S., Zhao L., Wang W., Zhao Y., Zhang G., Ma X., et al., Morphology control of ceria nanocrystals for catalytic conversion of CO2 with methanol. Nanoscale, 2013, 5, 5582-5588.10.1039/c3nr00831bSearch in Google Scholar PubMed
[11] Tomishige K., Ikeda Y., Sakaihori T., Fujimoto K., Catalytic properties and structure of zirconia catalysts for direct synthesis of dimethyl carbonate from methanol and carbon dioxide. J. Catal., 2000, 192, 355-362.10.1006/jcat.2000.2854Search in Google Scholar
[12] Ikeda Y., Asadullah M., Fujimoto K., Tomishige K., Structure of the Active Sites on H3PO4/ZrO2 Catalysts for Dimethyl Carbonate Synthesis from Methanol and Carbon Dioxide. J. Phys. Chem. B, 2001, 105, 10653-10658.10.1021/jp0121522Search in Google Scholar
[13] Jiang C., Guo Y., Wang C., Hu C., Wu Y., Wang E., Synthesis of dimethyl carbonate from methanol and carbon dioxide in the presence of polyoxometalates under mild conditions. Appl. Catal. A-Gen., 2003, 256, 203-212.10.1016/S0926-860X(03)00400-9Search in Google Scholar
[14] Honda M., Tamura M., Nakagawa Y., Sonehara S., Suzuki K., Fujimoto K., et al., Ceria-Catalyzed Conversion of Carbon Dioxide into Dimethyl Carbonate with 2-Cyanopyridine. ChemSusChem, 2013, 6, 1341-1344.10.1002/cssc.201300229Search in Google Scholar
[15] Jonquières A., Arnal-Hérault C., Babin J., Pervaporation. In: Hoeck E.M.V., Tarabara V.V., Encyclopedia of Membrane Science and Technology, 2013.10.1002/9781118522318.emst092Search in Google Scholar
[16] Jonquières A., Clément R., Lochon P., Néel J., Dresch M., Chrétien B., Industrial state-of-the-art of pervaporation and vapour permeation in the western countries. J. Membrane Sci., 2002, 206, 87-117.10.1016/S0376-7388(01)00768-2Search in Google Scholar
[17] Bolto B., Hoang M, Xie Z., A review of membrane selection for the dehydration of aqueous ethanol by pervaporation. Chem. Eng. Process, 2011, 50, 227-235.10.1016/j.cep.2011.01.003Search in Google Scholar
[18] Chapman P.D., Oliveira T., Livingston A.G., Li K., Membranes for the dehydration of solvents by pervaporation. J. Membrane Sci., 2008, 318, 5-37.10.1016/j.memsci.2008.02.061Search in Google Scholar
[19] Diban N., Aguayo A.T., Bilbao J., Urtiaga A., Ortiz I., Membrane Reactors for in Situ Water Removal: A Review of Applications. Ind. Eng. Chem. Res., 2013, 52, 10342-10354.10.1021/ie3029625Search in Google Scholar
[20] Izák P., Mateus N.M.M., Afonso C.A.M., Crespo J.G., Enhanced esterification conversion in a room temperature ionic liquid by integrated water removal with pervaporation. Sep. Purif. Technol., 2005, 41, 141-145.10.1016/j.seppur.2004.05.004Search in Google Scholar
[21] Dibenedetto A., Aresta M., Angelini A., Ethiraj J., Aresta B.M., Synthesis, Characterization, and Use of NbV/CeIV-Mixed Oxides in the Direct Carboxylation of Ethanol by using Pervaporation Membranes for Water Removal. Chem.-Eur. J., 2012, 18, 10324-10334.10.1002/chem.201201561Search in Google Scholar
[22] Li C.-F., Zhong S.-H., Study on application of membrane reactor in direct synthesis DMC from CO2 and CH3OH over Cu–KF/MgSiO catalyst. Catal. Today, 2003, 82, 83-90.10.1016/S0920-5861(03)00205-0Search in Google Scholar
[23] Kuenen H.J., Mengers H.J., Nijmeijer D.C., van der Ham A.G.J., Kiss A.A., Techno-economic evaluation of the direct conversion of CO2 to dimethyl carbonate using catalytic membrane reactors. Comput. Chem. Eng., 2016, 86, 136-147.10.1016/j.compchemeng.2015.12.025Search in Google Scholar
[24] Aresta M., Dibenedetto A., Dutta A., Energy issues in the utilization of CO2 in the synthesis of chemicals: The case of the direct carboxylation of alcohols to dialkylcarbonates. Catal. Today, 2017, 281, Part 2, 345-351.10.1016/j.cattod.2016.02.046Search in Google Scholar
[25] Wang J., Hao Z., Wohlrab S., Continuous CO2 esterification to diethyl carbonate (DEC) at atmospheric pressure: Application of porous membranes for in-situ H2O removal. Green Chem., 2017, 19, 3595-3600.10.1039/C7GC00916JSearch in Google Scholar
[26] Wang Y., Jia D., Zhu Z., Sun Y., Synthesis of Diethyl Carbonate from Carbon Dioxide, Propylene Oxide and Ethanol over KNO3-CeO2 and KBr-KNO3-CeO2 Catalysts. Catalysts, 2016, 6, 52.10.3390/catal6040052Search in Google Scholar
[27] Shukla K., Srivastava V.C., Diethyl carbonate: critical review of synthesis routes, catalysts used and engineering aspects. RSC Adv., 2016, 6, 32624-32645.10.1039/C6RA02518HSearch in Google Scholar
[28] Yoshida Y., Arai Y., Kado S., Kunimori K., Tomishige K., Direct synthesis of organic carbonates from the reaction of CO2 with methanol and ethanol over CeO2 catalysts. Catal. Today, 2006, 115, 95-101.10.1016/j.cattod.2006.02.027Search in Google Scholar
[29] Yave W., Separation performance of improved PERVAP membrane and its dependence on operating conditions. J. Membrane Sci. Res. (in press), doi:10.22079/jmsr.2018. 88186.1198.10.22079/jmsr.2018.88186.1198.Search in Google Scholar
[30] Santos B.A.V., Pereira C.S.M., Silva V.M.T.M., Loureiro J.M., Rodrigues A.E., Kinetic study for the direct synthesis of dimethyl carbonate from methanol and CO2 over CeO2 at high pressure conditions. Appl. Catal. A-Gen., 2013, 455, 219-226.10.1016/j.apcata.2013.02.003Search in Google Scholar
[31] Kumar P., With P., Chandra Srivastava V., Shukla K., Gläser R., Mani Mishra I., Dimethyl carbonate synthesis from carbon dioxide using ceria–zirconia catalysts prepared using a templating method: characterization, parametric optimization and chemical equilibrium modeling. RSC Adv., 2016, 6, 110235-110246.10.1039/C6RA22643DSearch in Google Scholar
[32] Hofmann H.J., Brandner A., Claus P., Direct Synthesis of Dimethyl Carbonate by Carboxylation of Methanol on Ceria-Based Mixed Oxides. Chem. Eng. Technol., 2012, 35, 2140-2146.10.1002/ceat.201200475Search in Google Scholar
[33] Baker R.W., Wijmans J.G., Huang Y., Permeability, permeance and selectivity: A preferred way of reporting pervaporation performance data. J. Membrane Sci., 2010, 348, 346-352.10.1016/j.memsci.2009.11.022Search in Google Scholar
© 2019 Décultot et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

