Abstract
In this work, a chain of reactions has been proposed as a new heterogeneous technique, based on the use of natural treated clays as an environmentally friendly catalysts for the synthesis of poly(1,4-bis(methacryloyl)piperazine). We first synthesized the monomer; 1,4-bis(methacryloyl)piperazine (NBMP) in bulk (without solvent) by the condensation of heterocyclic secondary amines piperazine with methacrylic anhydride catalyzed by maghnite-H+ at room temperature during 2 h. After that, we have polymerized anionically the obtained NBMP in an ice bath using anionic catalyst maghnite-Na+ at 0°C, the reaction took place in 24 h. The poly(1,4-bis(methacryloyl)piperazine) and NBMP structure was characterized and confirmed by infrared spectroscopy, 1H and 13C nuclear magnetic resonance spectroscopies. Thermal properties of the polymer were determined using thermogravimetric analysis. The yield of the reaction was 72% and 59% for the monomer and polymer synthesis respectively. The effect of the weight content of the catalyst on the reaction yield was studied. A polymerization mechanism has been suggested showing the role of maghnite as a catalyst during the reaction courses.
1 Introduction
Heterogeneous catalysis occupies a very important place in different industrial syntheses, where transformation of the raw reagents into chemicals and commercial fuels can be carried out in an efficient, economical and environmental friendly way. Heterogeneous catalysts are currently used in many industrial sectors such as the oil, pharmaceutical, food and automobile industries [1]. The ease of separating and recovering them from the reaction medium provides the main parameter for them to be preferred to other homogeneous homologs. In addition, they are pretty active (you can run them at higher temperatures as they are more stable) [2]. Nitrogen-containing heterocycles are of considerable importance in the pharmaceutical area; because they are present in many organic compounds with biological activity. Piperazine is one of the most sought heterocyclic compounds for the development of new drug candidates. The piperazine derivatives have pharmacological activity with broad spectrum since they are found as: anticancer [3, 4, 5], antibacterial [6], antifungals [7], anti-malarial [8], antipsychotics, antidepressants, HIV protease inhibitors [9].
Poly(1,4-bis(methacryloyl)piperazine) is a distinct class of functional polymers, which has a variety of applications, is very useful for the preparation of cross-linking agent and their use for preparation of polymer suitable for the separation on of amino acid, peptides, protein and viruses [10, 11, 12, 13]. The synthesis of 1,4-bis(methacryloyl)piperazine could be realized according to different approaches in various solvents and reactants using acryloyl or methacryloyl chloride with triethylamine [11, 12, 13, 14, 15, 16, 17].
The novelty of this study is to design a green process to polymerize 1,4-bis(methacryloyl)piperazine, using a heterogeneous clay catalyst (maghnite-Na+) obtained by a chemical modification of the raw maghnite. The advantage of this new catalyst is in its reusability, ease of separation and handling under mild conditions. Maghnite has acid sites of Brönsted and Lewis type. These active sites responsible for priming chemical reactions can be created by binding high charge density cations such as protons on the surface of layers [18, 19, 20]. Proton-loaded maghnite has been successfully used as a heterogeneous catalyst for many polymerization reactions [21, 22, 23].
2 Experimental
2.1 Materials
All reagents and chemicals were obtained from commercial sources, as they were used without any pre-treatment treatments. Methacrylic anhydride and dichlomethane were purchased from Sigma-Aldrich society in Algeria. The Piperazine and MgSO4 were obtained respectively from ACOROS-ORGANICS and Biochem in France. The raw maghnite was obtained from the ENOF Company located in Maghnia, Algeria. Maghnite-H+ was prepared following the procedure published by Belbachir et al. [18,19]. X-ray diffraction analysis was performed on a D8 Advanced Bruker AXSX diffractometer (Germany). Fourier transform infrared spectroscopy spectra were recorded using a Bruker alpha-PATR No. 9501165 (France) in the range of 400 to 4000 cm-1. The 1H and 13C nuclear magnetic resonance analyzes were performed on a Bruker NMR spectrometer at 300 MHz (Germany), equipped with a probe BB05 mm. Tetramethylsilane (TMS) was used as internal standard and CDCl3 as solvent. TGA analysis was carried out using a Perkin Elmer STA6000 device (USA).
2.2 Preparation of catalysts
Maghnite-H+ and maghnite-Na+ were prepared according to the procedure mentioned in our previous studies [15,16]. 20 g of raw maghnite was milled for 20 min using a ceramic ball mill. It was after dried at 105°C for 2 h. The maghnite was put in an Erlenmeyer flask with 500 mL of distilled water. Then, the mixture was stirred with a magnetic stirrer after adding it to 0.25 M sulfuric acid solution until saturation in 48 h at room temperature. The maghnite-H+ was then washed with water to be free of sulfate ions and then dried at 105°C. The residue of the rinsing water was each time tested by the barium nitrate until the total removal of sulfate ions. The maghnite-Na+ was prepared as follows: The raw maghnite was put in an Erlenmeyer flask with 500 mL of 1 M NaCl solution. The mixture was stirred with a magnetic stirrer until saturation in 24 h at room temperature. The maghnite-Na+ was then washed with water to be free of chloride ions, dried at 105°C and finally stored in a desiccator away from moisture.
2.3 Monomer synthesis
0.86 g of piperazine was mixed with various amounts of maghnite-H+ 0.25 M (3, 5, 10 and 15 %wt) for 30 min. Following that we added 0.2 mol (30 mL) of methacrylic anhydride (with a molar ratio of 2:1 of methacrylic anhydride to piperazine) to the solution. The reaction mixture was cooled to 5°C during 2 h (Figure 1).

Representative scheme for the synthesis of NBMP catalyzed by maghnite-H+.
After 2 h, we filtered the solution recover the clay catalyst, which was transferred to a separating funnel washed thoroughly with a 5% sodium hydroxide solution and extracted with dichloromethane (3×30 mL). The organic layers were combined; dried over anhydrous magnesium sulphate MgSO4 and filtered. After the evaporation of dichloromethane in vacuum, the product was recrystallized in cold diethyl ether; a white crystal solid was obtained.
2.4 Polymer synthesis
The anionic polymerization of synthesized NBMP was carried out in sealed tubes. Each tube contains a mixture of 1 g of NBMP and 0.15 g (15%) of maghnite-Na+. The mixtures were kept in an ice bath at 0°C and stirred with a magnetic stirrer under dry nitrogen for 24 h. The resulting polymer was precipitated in methanol, washed for several times, dried at 40°C in vacuum and weighed (Figure 2).

Representative scheme for the polymerization of NBMP catalyzed by maghnite-Na+.
3 Results and discussion
3.1 IR and XRD analyses of catalysts
IR and XRD analyses of the raw maghnite and those treated are shown in Figures 3 and 4. The FT-IR spectrum (Figure 3) shows the following bands: The broad bands between 3387 and 3625 cm-1 are assigned to AlAlOH coupled by AlMgOH stretching vibrations. The deformation bands at 1641 cm-1, 792 cm-1 and 616 cm-1 are assigned to AlAlOH, AlFe3+OH and AlMgOH, respectively. The bands between 1004 cm-1 and 999 cm-1 are assigned to the Si-O elongation bands out of the plane and Si-O-Si (2 bands) in the plane.
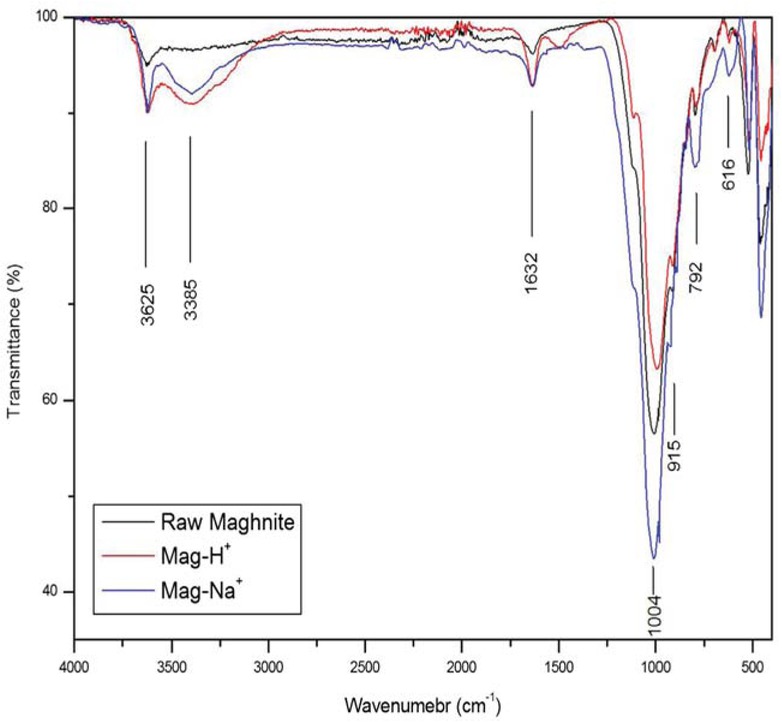
IR spectra of raw maghnite, maghnite sodium and acidic maghnite.

XRD spectra of raw maghnite, maghnite sodium and acidic maghnite.
The X-ray diffractograms (Figure 4) of the raw, sodium and acidic maghnite show that the montmorillonite peak appears at 2θ angle corresponds to 7.05°, 6.96° and 5.67°, respectively. These were calculated by Bragg’s Law, thus indicating the increase in interfoliar distance from 12.52 Å to 12.68 Å for sodium maghnite and to 15.56 Å for acidic maghnite, The intercalation of Na+ and H3O+ ions in the space initially occupied by the Ca2+ and K+ cations led to the swelling of maghnite.
3.2 Characterization of synthesized monomer and polymer
3.2.1 IR spectroscopy
According to Figure 5, the IR spectrum of NBMP exhibits three weak peaks between 2924 and 3055 cm-1 were attributed to C-H symmetrical and asymmetrical stretching on CH2 and CH3 groups, respectively. The strong band at 1716 cm-1 was attributed to amide carbonyl C=O stretching vibration. The strongest bands at 1617 cm-1 and 522 cm-1 corresponded to the C=C stretching vibration. The second strongest and sharp band at 1430 cm-1 was attributed to the C-N stretch vibration. Figure 6 shows the IR spectrum of poly(NBMP), it is identical for the monomer excepting that we observe the increase of the intensity of the bands. Large and intense bands between 2920 and 2952 cm-1 were attributed to C-H symmetrical and asymmetrical stretching on CH2 and CH3 groups, respectively. The disappearance of the intense band at 1617 cm-1 and 522 cm-1 which corresponds to the stretching vibration C=C due to the opening of the double bond allowing the connection between the monomer units.
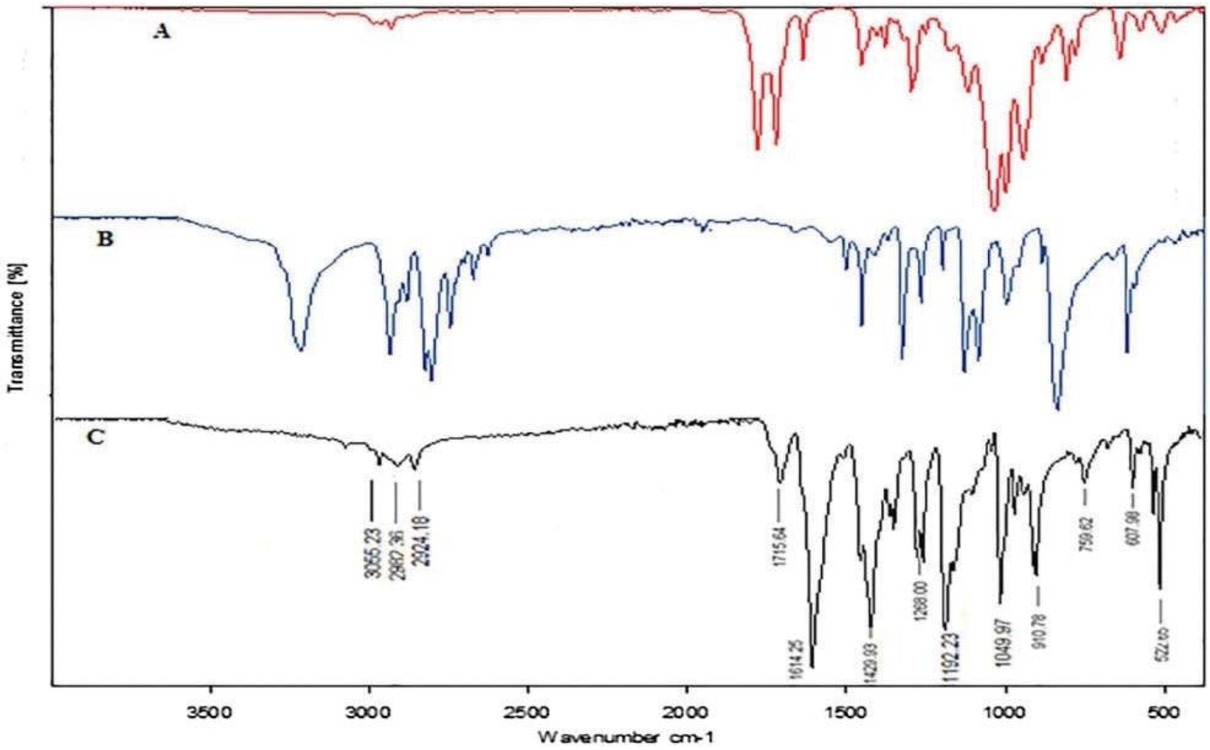
IR spectra of (a) methacrylic anhydride, (b) piperazine and (c) NBMP.
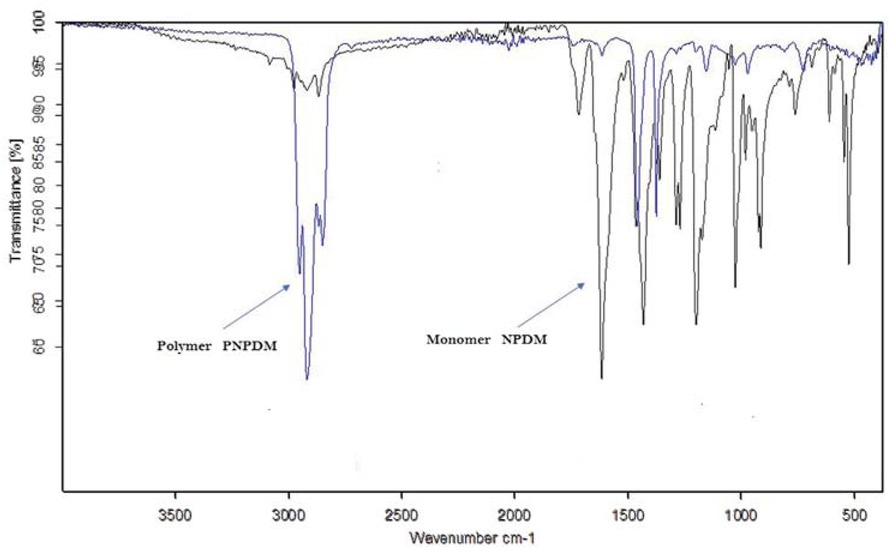
IR spectra of NBMP (black curve) and poly(NBMP) (blue curve).
3.2.2 1H NMR
The 1H NMR spectrum of NBMP and poly(NBMP) and are shown in Figures 7 and 8, respectively. A sharper peak, centered at 1.97 ppm, corresponds to the methyl protons (-CH3), the two peaks of average width between 5.06 and 5.25 ppm are due to methylene protons (=CH2), another broad peak centered at 3.60 ppm corresponds to heterocyclic protons of methylene (N-CH2CH2-N). In Figure 8 corresponding to the 1H NMR spectrum of the polymer, the stronger peak at 0.76 ppm assigns to the methyl proton groups (-CH3), the broad peak between 0.99-1.35 ppm due to methylene protons (-CH2) and the other broad peak at 1.49 ppm corresponding to heterocyclic protons of methylene (N-CH2CH2-N). The small peak at 3.53 ppm is attributed to the hydroxide protons at the end of the polymer chain, the comparison between its integration curve with respect to the others makes it possible to calculate the number-average molecular mass which has been obtained at around 18,000 g/mol.

1H NMR spectrum (CDCl3) of NBMP.
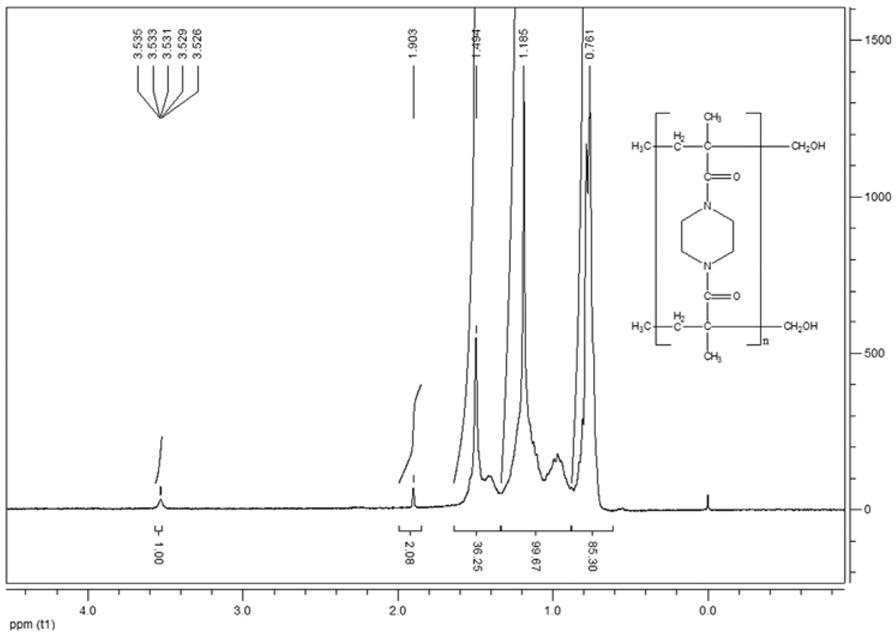
1H NMR spectrum (CDCl3) of poly(NBMP).
3.2.3 13C NMR
Figures 9 and 10 show the 13C NMR spectrum of the obtained monomer and polymer, respectively. This characterization technique was carried out in order to complete the information collected by 1H NMR, for identifying and confirming the molecular structure much more in terms of the nature of the existing carbonic groups. Table 1 summarizes the different chemical shifts observed. We clearly see that the two monomer and polymer spectra generally indicate the same functional groups. Except that in the spectrum of the polymer an additional peak shown at 22 ppm and which corresponds to the group. Except that the two peaks at 116.22 and 139.92 ppm in the monomer spectrum are transformed into two new peaks at 41.55 and 30.05 ppm in the polymer spectrum, they correspond to the -CH2- aliphatic group responsible for the connection between the monomer units, and quaternary carbon resulting from the breaking of the double bond -C=C- existing in the monomer, respectively.

13C NMR spectrum (CDCl3) of NBMP.
13C NMR chemical shift for various carbons of NBMP and poly(NBMP).
| Monomer/Polymer | Attributions | Chemical shift δ (ppm) |
|---|---|---|
| NBMP | –CH3 (a) | 20.49 |
| –CH2– cyclic (b) | 47.02 | |
| =CH2 (c) | 116.22 | |
| –C=C (d) | 139.92 | |
| C=O (e) | 171.40 | |
| Poly(NBMP) | –CH3 (a) | 20.89 |
| –CH2– cyclic (b) | 48.99 | |
| –CH2– Aliphatic (c) | 41.55 | |
| C quaternary (d) | 30.05 | |
| C=O (e) | 177.32 |
3.2.4 Thermal studies
The thermal behavior of the polymer was evaluated by thermogravimetric analysis in air from room temperature. The TGA curve of poly(NBMP) is shown in Figure 11, which clearly indicates that the polymer has degraded in two successive steps. The initial degradation and at the same time the main one, started from 244.90°C to 350°C, which was due to the decomposition of the poly(NBMP) resulting from the weakness of the methyl groups in the polymer chain, this corresponds to a loss of weight 87.07% (4.11 mg). The second degradation step is from 440.38°C to 445.06°C which was attributed to the complete combustion of the residues. This result concludes that the synthesized poly(NBMP) is thermally stable below the temperature of 244°C.
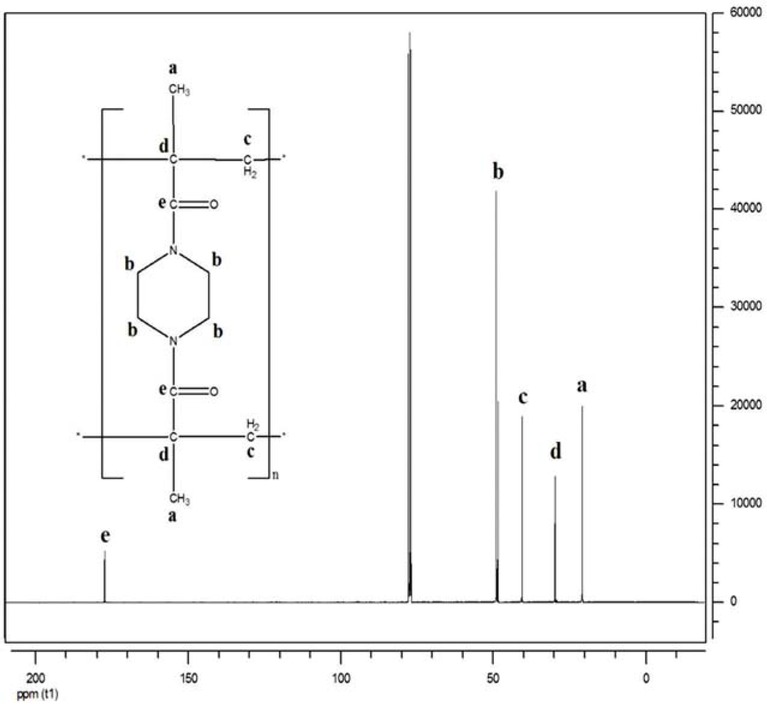
13C NMR spectrum (CDCl3) of poly(NBMP).

TGA thermogram of poly(NBMP).
3.3 Reaction mechanism
According to the results of analysis of the synthesized polymer initiated by maghnite Na+ as a heterogeneous solid catalyst, a reaction mechanism has been proposed in Figure 12. The initiation step is based on the creation of the active center by the fixation of Na+ ions on the methylene groups that open the double bond C=C. In the Propagation step the carbonium ion binds to a methylene group of another monomer unit and the process continues one after one. The chains propagate by the chain-growth polymerization method. The termination step is carried out by the addition of methanol, its molecule is subdivided into two; a CH2OH group binds to the carbonium ions forming one end of the chain, and a proton replaces the Na+ ions and also forms the other end of the chain. The Na+ ions return to the leaves of the clay allowing its use again.

Proposed reaction mechanism of NBMP polymerization.
3.4 Effect of maghnite-H+ and maghnite-Na+ proportion on the yield of the reaction
We can see in Table 2 that the yield of the monomer and polymer synthesis reaction increased with the increase in the amount of catalyst used, and stabilized at a catalyst proportion of 10% and 15% for the monomer and the polymer synthesis reaction respectively. The synthesis of the monomer was obtained with a better yield around 72% of pure product with a selectivity of 100%. The yield of the polymerization reaction reached a threshold of about 59% using maghnite-Na+ as catalyst.
Effect of catalyst amount (maghnite-H+/maghnite-Na+) on conversion of monomer and polymer.
| Monomer/Polymer | Catalyst (weight %) | Yield (%) |
|---|---|---|
| NBMP (at 5°C) | 3 | 40 |
| 5 | 60 | |
| 10 | 72 | |
| 15 | 72 | |
| 20 | 72 | |
| Poly(NBMP) (at 0°C) | 3 | 5 |
| 5 | 30 | |
| 10 | 45 | |
| 15 | 59 | |
| 20 | 59 |
4 Conclusion
In this work, we have shown that 1,4-bis(methacryloyl) piperazine is polymerizable at room temperature, using a non-toxic and recyclable catalyst prepared from natural clay (maghnite-Na+). The yield of the polymerization reaction was 59% using a weight content of 15% of catalyst. The monomer was synthesized by a new green process under conditions consistent with
the principles of green chemistry using maghnite-H+ as catalyst, in which we reacted piperazine and methacrylic anhydride at room temperature for 2 h, the reaction yield was 72%. The molecular structure of the monomer and the polymer was identified by IR, 13C NMR and 13C NMR analyses. The average molecular weight was measured by NMR at 18,000 g/mol. TGA analysis of the polymer obtained showed that it is thermally stable below the temperature of 244°C. In the end, a reaction mechanism has been proposed to show the role of maghnite-Na+ during the various steps of the polymerization reaction.
Acknowledgments
We would like to thank Mr. A. Addou (Laboratory of Polymer Chemistry LCP, University of Oran 1) for the IR analysis. We also thank the head of department, Mrs. H. Hidour (Applied Organic Synthesis Laboratory, University of Es-Senia Oran) for the NMR analysis.
List of Abbreviations
- IR
infrared spectroscopy
- NBMP
1,4-bis(methacryloyl)piperazine
- NMR
nuclear magnetic resonance
- Maghnite
natural Algerian clay
- Maghnite-H+
maghnite treated by acid
- Maghnite-Na+
maghnite treated by NaCl
- poly(NBMP)
poly(1,4-bis(methacryloyl)piperazine)
- TGA
thermogravimetric analysis
- XRD
X-ray diffraction
References
[1] Dumesic J.A., Huber G.W., Boudart M., Principles of Heterogeneous Catalysis. In Handbook of Heterogeneous Catalysis (1st ed.). Wiley-VCH Verlag GmbH & Co. KGaA, 2008.10.1002/9783527610044.hetcat0001Search in Google Scholar
[2] Ong T.C., Verel R., Coperet C., Solid-State NMR: Surface Chemistry Application. In: Encyclopedia of Spectroscopy and Spectrometry (1st ed.). Elsevier, 2017.Search in Google Scholar
[3] Régnier S., Synthèse efficace d’hétérocycles azotés par activation d’amides engendrée par l’anhydride trifluorométhanesulfonique. PhD Thesis, University of Montréal, Montréal, Canada, 2016. https://papyrus.bib.umontreal.ca/xmlui/bitstream/handle/1866/18658/Regnier_Sophie_2016_Memoire.pdf (accessed 04/11/2018).Search in Google Scholar
[4] Haga N., Ishibashi T., Hara A., Abiko Y., Effect of NCO-700, an Inhibitor of Protease, on Myocardial pH Decreased by Coronary Occlusion in Dogs. Pharmacology, 1985, 31, 208-217.10.1159/000138117Search in Google Scholar PubMed
[5] Swindell E.P., Ugolkov A., Freguia C., Dubrovskyi O., Hankins P.L., Yang J., et al., Abstract 4389: Liposomes containing piperazine compounds inhibit tumor growth in a patient-derived xenograft model of glioblastoma multiforme. Cancer Res., 2015, 75, 4389-4389.10.1158/1538-7445.AM2015-4389Search in Google Scholar
[6] Foroumadi A., Ghodsi S., Emami S., Najjari S., Samadi N., Faramarzi M.A., et al., Synthesis and antibacterial activity of new fluoroquinolones containing a substituted N-(phenethyl) piperazine moiety. Bioorg. Med. Chem. Lett., 2006, 16, 3499-3503.10.1016/j.bmcl.2006.03.103Search in Google Scholar PubMed
[7] Upadhayaya R.S., Sinha N., Jain S., Kishore N., Chandra R., Arora S.K., Optically active antifungal azoles: synthesis and antifungal activity of (2R,3S)-2-(2,4-difluorophenyl)-3-(5-{2-[4-aryl-piperazin-1-yl]-ethyl}-tetrazol-2-yl/1-yl)-1-[1,2,4]-triazol-1-yl-butan-2-ol. Bioorgan. Med. Chem., 2004, 12, 2225-2238.10.1016/j.bmc.2004.02.014Search in Google Scholar PubMed
[8] Ryckebusch A., Poulain R.D., Maes L., Debreu-Fontaine M.A., Mouray E., Grellier P., et al., Synthesis and in Vitro and in Vivo Antimalarial Activity of N1-(7-Chloro-4-quinolyl)-1,4-bis(3-aminopropyl)piperazine Derivatives. J. Med. Chem., 2003, 46, 542-557.10.1021/jm020960rSearch in Google Scholar PubMed
[9] Shaquiquzzaman M., Verma G., Marella A., Akhter M., Akhtar W., Faraz Khan M., et al., Piperazine scaffold: A remarkable tool in generation of diverse pharmacological agents. Eur. J. Med. Chem., 2015, 102, 487-529.10.1016/j.ejmech.2015.07.026Search in Google Scholar PubMed
[10] Pirjo K., Novel cross-linking agents and use thereof. Patent WO1995001347, January 1995.Search in Google Scholar
[11] Shundrina I.K., Bukhtoyarova D.A., Russkikh V.V., Parkhomenko A.D., Shelkovnikov V., Synthesis and properties of novel random copolymers made from N-acryloyl piperazine-based monomers and fluoroalkylmethacrylates. Polym. Bull., 2015, 72, 2783-2796.10.1007/s00289-015-1435-zSearch in Google Scholar
[12] Dubois J.L.N., Lavignac N., Poly(amidoamine)s synthesis, characterisation and interaction with BSA. Polym. Chem., 2014, 5, 1586-1592.10.1039/C3PY01121FSearch in Google Scholar
[13] Mauro N., Manfredi A., Degradable Poly(amidoamine) Hydrogels as Scaffolds for In Vitro Culturing of Peripheral Nervous System Cells. Macromol. Biosci., 2012, 13, 332-347.10.1002/mabi.201200354Search in Google Scholar PubMed
[14] Djamila C.D., Meghabar R., Belbachir M., Piperazine Polymerization Catalyzed by Maghnite-H+ Int. J. Environ. An. Ch., 2017, 4, 2680-2391.Search in Google Scholar
[15] Kherroub D.E., Belbachir M., Lamouri S., Green Polymerization of Hexadecamethylcyclooctasiloxane Using an Algerian Proton Exchanged Clay Called Maghnite-H+ Bulletin of Chemical Reaction Engineering & Catalysis, 2018, 13, 36-46.10.9767/bcrec.13.1.993.36-46Search in Google Scholar
[16] Chikh K., Bouhadjar L., Kherroub D.E., Meghabar R., Belbachir M., Synthesis and Characterization of Polyvinyl Alcohol/Na+-MMt Nanocomposite: Effect of Charge Content and CO2 Adsorption Properties. Der Pharma Chemica, 2017, 9, 90-94.Search in Google Scholar
[17] Navjeet K., Dharma K., Montmorillonite: An efficient, heterogeneous and green catalyst for organic synthesis. J. Chem. Pharm. Res., 2012, 4, 991-1015.Search in Google Scholar
[18] Belbachir M., Bensaoula A., Composition and method for catalysis using bentonites. US Patent. No 6: 274,527B1, August 2001.Search in Google Scholar
[19] Belbachir M., Bensaoula A., Composition and method for catalysis using bentonites. US Patent. No 7: 094,823 B2, August 2006,Search in Google Scholar
[20] Kherroub D.E., Belbachir M., Lamouri S., A new approach for the polymerization of tetraphenyltetramethylcyclotetrasiloxane by an environmentally friendly catalyst called Maghnite-H+ Green Process Synth., 2018, 7, 296-305.10.1515/gps-2017-0033Search in Google Scholar
[21] Kherroub D.E., Belbachir M., Lamouri S., Activated bentonite (Maghnite-H+ as green catalyst for ring-opening polymerization of 1,3,5,7-tetravinyltetramethylcyclotetrasiloxane. Res. Chem. Intermediat., 2017, 43, 5841-5856.10.1007/s11164-017-2966-8Search in Google Scholar
[22] Kherroub D.E., Khodja M., Belbachir M., Lamouri S., Bouhadjar L., Boucherdoud A., Maghnite-H+ as Inorganic Acidic Catalyst in Ring Opening Polymerization of Dodecamethylcyclohexasiloxane. Silicon-Neth., 2018, 11, 1165-1173.10.1007/s12633-018-9769-4Search in Google Scholar
[23] Boulaouche T., Kherroub D.E., Khimeche K., Belbachir M., Green strategy for the synthesis of polyurethane by a heterogeneous catalyst based on activated clay. Res. Chem. Intermed., (in press), DOI:10.1007/s11164-019-03810-7.10.1007/s11164-019-03810-7Search in Google Scholar
© 2019 Derkaoui et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering


