Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
Abstract
Saponin as a biosurfactant was extracted from Iranian Ziziphus spina-christi leaves using three green extraction methods namely, autoclave, microwave and Bain-Marie heating methods. In this study, three solvents namely, methanol, ethanol and water were used to extract saponin. The results revealed that water, as compared to the methanol and ethanol, is a more suitable solvent to extract saponin from the Z. spina-christi leaves. The obtained results indicated that saponin extraction using autoclave provided more suitable physico-chemical properties along with a better yield. In fact, maximum foam volume (12.56 cm3), color intensity (3.24% absorbance unit [a.u.]) and turbidity (1.39% a.u.) of the extracted solutions was obtained by the autoclave heating method. The high performance liquid chromatography (HPLC) results also illustrated that the amounts of extracted saponin using autoclave, Bain-Marie and microwave heating extraction methods were 14, 8.8 and 1.3 (intensity mV), respectively. The results obtained by HPLC were reconfirmed by Fourier transform infrared (FT-IR) analysis.
1 Introduction
Surfactants are amphiphilic compounds with high potential activity to reduce surface tension between two immiscible fluids [1], [2]. Biosurfactants are biologically produced using numerous microorganisms or isolated from different plants and their derivatives. Biosurfactants have two main functional groups in their structure namely, hydrophobic and hydrophilic parts [3], [4], [5], [6], [7]. As compared to synthesized and commercial surfactants, biosurfactants have several advantages including being environmental friendly, higher surface tension reduction, biodegradability and selectivity in room temperature and normal pH, as well as lower toxicity [3], [5], [6], [7]. Due to their excellent emulsification, foamability, water binding capacity and wetting properties, biosurfactants have been widely used in different industries and products such as cosmetics, food, medicine, pharmaceuticals, and oil and gas production processes [8].
Saponin is one of the most important and applicable biosurfactants which is widely found in different plants, sea cucumber and starfish [9], [10], [11]. In fact, this secondary metabolite is part of the plant defense system against pathogens and herbivores [12]. Saponins are divided into two main categories which are triterpenoids and steroid glycosides attached to one or more sugar chains [13], [14]. This natural biosurfactant which is derived from soapwort has been widely used for centuries as household detergent due to its excellent foamability [10]. Saponins have been extracted from several plants and their products including soy milk, sugar beet, asparagus, strawberry and plum fruits [15], [16], [17]. Ziziphus spina-christi is one of the main sources of saponin which is known as “Sedr” and “Nebeq” in Iran and Arabic countries, respectively [18], [19]. There are around 50 species of Ziziphus distributed in tropical Asia, Africa and America [18], [19]. Several studies have indicated that Z. spina-christi leaves contain flavonoids, tannins, sterols, saponins and triterpenoids. Furthermore, 11 different cyclopeptide alkaloids exist in Z. spina-christi stem and root [20], [21], [22], [23]. In Khuzestan, a southern province of Iran, Z. spina-christi leaves have been widely used as natural detergent and shampoo due to their saponin content [24].
For the extraction of saponins, two different extraction methods namely conventional and green have been developed [25], [26], [27]. Conventional methods including maceration, Soxhlet and reflux extractions are based on using a large amount of chemical solvents. However, in green processes such as ultrasound, microwave and accelerated solvent extractions which are based on water as the solvent, several advantages can be nominated such as lower extraction time with higher efficiency, minimum utilized chemical solvents and energy consumption, as well as prevention of the pollutions [25]. Therefore, the main objectives of the present study were to i) extract saponin from Iranian Z. spina-christi using three different green methods namely, microwave-assisted, autoclave-accelerated and accelerated water extraction and ii) evaluate the physico-chemical properties of the extracted saponin.
2 Materials and methods
2.1 Materials
Z. spina-christi dried leaves were purchased from a local market (Tabriz, Iran). Methanol, ethanol and distilled water as three different solvents were provided from Dr. Mojallali Chemical Complex Co. (Tehran, Iran).
2.2 Extraction of saponin
Z. spina-christi dried leaves were ground using a domestic miller (MX-GX1521, Panasonic, Tokyo, Japan). The provided dried powder was added into the distilled water, methanol and ethanol, as three different solvents, with a ratio of 1:20 g/ml and mixture solutions were exposed to three different extraction methods namely, microwave, autoclave and Bain-Marie. In the microwave-assisted extraction method, the mixture solutions were exposed and heated using a microwave oven (MG-2312W, LG Co., Seoul, South Korea) at a constant power of 800 W for 90 s with a 30 s interval of heating. In the autoclave-accelerated extraction method, the mixture solutions were placed into a laboratory autoclave which was set at a pressure of 15 psi and temperature of 121°C for 15 min. In the water extraction method, the prepared solutions were put into a laboratory Bain-Marie water bath in which temperature was adjusted in 50°C for 24 h. After that, the heated mixture solutions were filtered using No. 1 Whatman filter paper and the filtrates were then put in the laboratory oven at 60°C for 48 h to remove the solvents. Finally, 20 ml of distilled water was added into the solvent-free samples and shaken for 30 s. The samples were then used for the physico-chemical analysis.
2.3 Physico-chemical analysis
In order to identify the main functional groups in the filtrate mixture solutions containing saponin, Fourier transform infrared (FT-IR) measurements was performed. The FT-IR spectra of the provided Z. spina-christi extract were recorded on a Bruker Tensor 27 spectrometer (Ettlingen, Germany) using KBr pellets in the 4000–400 cm−1 region. In order to qualitatively evaluate the extraction yield, the prepared samples were shaken vigorously by hand for 30 s and the volume of the generated foam was measured. The extraction yield of the obtained saponin was then correlated to the foam volume. The amount of the saponin extracted was also monitored by the color intensity and turbidity tests. In fact, a lower concentration of bioactive compounds in aqueous solution leads to lower color intensity and turbidity [28]. In order to measure color intensity and turbidity of the extracts, a Jenway UV-Vis spectrophotometer 6705 (Stone, UK) in a 1 cm optical path quartz cuvette adjusted to wavelengths of 420 nm and 625 nm, respectively, was used. The absorbance unit (% a.u.) was used as unit of both color intensity and turbidity of the extracts. High performance liquid chromatography (HPLC) (Merck Hitachi, L-7100, Darmstadt, Germany) with a C 18 column and IR detector was used to identify and measure saponin in the extracts. The wavelength range was set from 250 nm to 500 nm. The samples were dissolved in HPLC grade methanol and injected into the system. Acetonitrile-water was used as the mobile phase and the sampling rate was 2 (points/second) such that total flow rate was kept at 0.70 ml/min. The amount of saponin in the samples was expressed by intensity mV unit.
2.4 Statistical analysis
Physico-chemical analysis of the prepared extracts was carried out in three replications. Data were interpreted by analysis of variance using Minitab v.16 statistical package (Minitab Inc., PA, USA). Tukey’s comparison test was used to compare the mean values. All comparisons were made at 5% level of significance.
3 Results and discussion
3.1 Solvent selection
According to available data in the literature, water, methanol and ethanol have been extensively utilized in the extraction of saponin from the plant sources [29]. Therefore, these solvents were used in the present study to extract saponin from Z. spina-christi. The results of our study indicated that, as compared to the ethanol and methanol, the extraction of saponin using water provided higher extraction yield. This result was obtained when these three solvents were utilized to extract saponin from its source using a Bain-Marie water bath and then measuring the generated foam volume by shaking the extracts. Figure 1 shows the volume of the generated foam obtained by shaking the saponin extracts. As can be seen, the foam volume is much higher in the case of water as compared to methanol and ethanol. Therefore, water was chosen as the green solvent to extract saponin from Z. spina-christi leaves via microwave, autoclave and Bain-Marie heating methods.

Generated foam by shaking the saponin extract from the Ziziphus spina-christi leaves using (A) ethanol, (B) methanol (C) and water.
3.2 FT-IR analysis of the extracted saponin
In order to identify the main functional groups of the extracted saponin solutions, FT-IR spectra of the saponin extracts obtained using microwave, autoclave and Bain-Marie methods were determined (Figure 2). As clearly observed in Figure 2A, there are two highlighted and sharp peaks centered at 3485.66 cm−1 and 1638.02 cm−1 for the extracted saponin solution by microwave heating. However, the saponin extract obtained using autoclave and Bain-Marie heating methods shows three highlighted peaks centered around 3475 cm−1, 1640 cm−1 and 670 cm−1. The absorption peaks centered around 3475 cm−1 and 1640 cm−1 are related to the hydroxyl group (OH) and amide I (NH) of the existing compounds of the extract, respectively. These results are consistent with the chemical structure of saponin shown in Figure 3. As clearly observed in this figure, saponin has a polyhydroxyl structure containing of the amide I group. The week absorption peak centered around 670 cm−1 in Figure 2B and C could be referred to the ring and skeletal modes of the main components.

Fourier transform infrared (FT-IR) spectrums of the extracted saponin using (A) microwave, (B) autoclave and (C) Bain-Marie heating methods.

Saponin chemical structure.
3.3 Foamability of the extracted saponin
Saponin as a biosurfactant has both hydrophilic and hydrophobic functional groups in its structure which can produce foam in water through simple shaking. In fact, the volume of the generated foam could be well correlated to the saponin concentration: the higher the foam volume, the higher the saponin concentration [3], [4], [5], [6], [7]. As observed in Figure 4, the generated foams of the saponin extracts obtained by the microwave and autoclave heating methods were higher than that obtained by the Bain-Marie method. The statistical analysis did not show significant (p<0.05) difference between foam volumes produced by the saponin extracts using microwave and autoclave extraction methods.

Foam volume generated by shaking the saponin extracts obtained using three different extraction methods. Data are mean values of three replications.
3.4 Color intensity of the extracted saponin
The color intensity of the extracted saponin solutions via three different extraction methods is shown in Figure 5. As clearly observed in this figure, the color intensity of the saponin solutions by the microwave heating method had a minimum value of 2.11 (% a.u.) while the values of the color intensity using the other methods were high. Note that maximum color intensity was obtained for the saponin solution extracted by the autoclave method (3.24% a.u.). This can be explained by the fact that by increasing the extraction time, the yield of saponin extraction from the Z. spina-christi leaves increased, which in turn increased the color intensity of the solution. The obtained results indicated that there were significant (p<0.05) differences between the color intensity value of the saponin solution extracted by the three different methods.
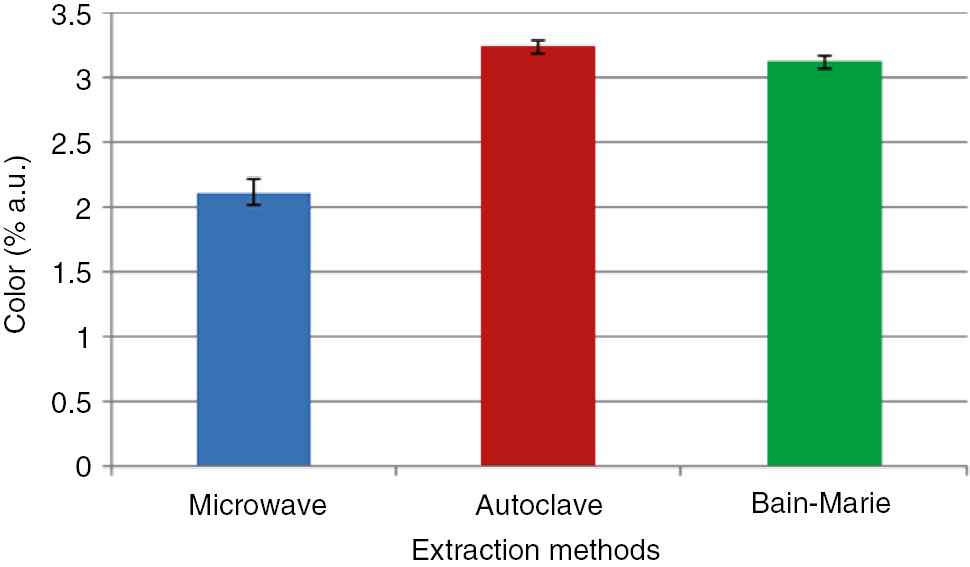
Color intensity of the saponin extracts obtained using three different extraction methods. Data are mean values of three replications.
3.5 Turbidity of the extracted saponin
The turbidity values of the extracted saponin solutions by using microwave, autoclave and Bain-Marie heating methods are illustrated in Figure 6. As clearly observed in this figure, the turbidity values of the extracted samples by microwave, autoclave and Bain-Marie heating methods were 0.69% a.u., 1.39% a.u. and 1.22 % a.u., respectively. The statistical analysis indicated that the turbidity value of the saponin solution obtained by the autoclave heating method was significantly (p<0.05) higher than that of other samples. This can be explained by the fact that in saponin extraction and other unwanted chemical and biochemical reactions, such as caramelization of the sugars and Maillard reactions, could be increased, leading to formation of brown and dark compounds such as melanoidins [30].

Turbidity of the saponin extracts obtained using three different extraction methods. Data are mean values of three replications.
3.6 HPLC chromatogram of the extracted saponin
The HPLC profile of the extracted saponin from Z. spina-christi leaves using the three extraction methods is shown in Figure 7A–C. As observed in Figure 7A, there were three recorded peaks at the retention time range of 2–10 min for the case of the microwave method. However, Figure 7B and C shows five detected peaks in the HPLC chromatogram of autoclave and Bain-Marie methods (Figure 7C). The obtained results reconfirm the turbidity and color intensity analyses of the extracted saponin solutions through the three different methods. In fact, the higher turbidity and color intensity of the extracted saponin solutions using the autoclave and Bain-Marie methods, as compared to those obtained by microwave, could be related to other bioactive compounds being present in the extracted samples. The peak sharpness centered at 2 min (retention time) was related to the extracted saponin. As clearly observed in Figure 7A–C, the highest peak in the 2 min retention time was related to the extract obtained by autoclave (14 intensity mV) and Bain-Marie (8.8 intensity mV) heating methods, respectively.

High performance liquid chromatography (HPLC) chromatograms of the saponin extracts obtained using (A) microwave, (B) autoclave and (C) Bain-Marie heating methods.
4 Conclusions
The green extraction of saponin from Ziziphus spina-christi leaves was studied using a series of simple and low energy consuming methods (microwave, autoclave and Bain-Marie) based on water as the extraction solvent. The results indicated saponin could be extracted efficiently by the hydrothermal methods. The HPLC results revealed that the yield of saponin extraction using autoclave was higher than that obtained using microwave and Bain-Marie heating extraction methods. The achieved results can be easily developed and used to extract lipid-based bioactive compounds with high decomposition temperature from the plants and other natural resources.
Acknowledgments
The authors would like to thank the Food Engineering Research Institute, Sahand University of Technology for material and financial support.
References
[1] Fakruddin Md. J. Pet. Environ. Biotechnol. 2012, 3, 124–129.Search in Google Scholar
[2] Desai JD, Banat IM. Microbiol. Mol. Biol. Rev. 1997, 61, 47–64.10.1128/mmbr.61.1.47-64.1997Search in Google Scholar PubMed PubMed Central
[3] Kumar D, Neo KE, Rub MA. J. Surfactant Deterg. 2016, 19, 101–109.10.1007/s11743-015-1754-ySearch in Google Scholar
[4] Kumar D, Neo KE, Rub MA. Tenside, Surfactants, Deterg. 2016, 53, 168–172.10.3139/113.110422Search in Google Scholar
[5] Paniagua MJ, Rosales A. J. Biorem. Biodegrad. 2015, 6, 273–278.Search in Google Scholar
[6] Onaizi SA, Nasser MS, Al-Lagtah NM. J. Surfactant Deterg. 2016, 19, 645–653.10.1007/s11743-016-1796-9Search in Google Scholar PubMed PubMed Central
[7] Rosen MJ, Mathias JH, Davenport L. Langmuir 1999, 15, 7340–7346.10.1021/la9904096Search in Google Scholar
[8] Gharaei-Fathabad E. Am. J. Drug Discovery Dev. 2011, 1, 58–69.10.3923/ajdd.2011.58.69Search in Google Scholar
[9] Augustin JM, Kuzina V, Anderson SB, Bak S. Phytochem. 2011, 72, 435–457.10.1016/j.phytochem.2011.01.015Search in Google Scholar PubMed
[10] Sparg SG, Light ME, Van Staden J. J. Ethnopharmacol. 2004, 94, 219–243.10.1016/j.jep.2004.05.016Search in Google Scholar PubMed
[11] Demeyer M, De Winter J, Caulier G, Eeckaut I, Flammang P, Gerbaux P. Comp. Biochem. Physiol. B. 2014, 168, 1–11.10.1016/j.cbpb.2013.10.004Search in Google Scholar PubMed
[12] Faizel A, Geelen D. Phytochem. Rev. 2013, 12, 877–893.10.1007/s11101-013-9322-4Search in Google Scholar
[13] Hostettmann K, Marston A, Eds., Saponins. Cambridge University Press: New York, 1995.10.1017/CBO9780511565113Search in Google Scholar
[14] Güçlü-Üstündaĝ Ö, Mazza G. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258.10.1080/10408390600698197Search in Google Scholar
[15] Lai L-R, Hsieh S-C, Huang H-Y, Chou C-C. J. Biosci. Bioeng. 2013, 115, 552–556.10.1016/j.jbiosc.2012.11.022Search in Google Scholar
[16] Ridout CL, Price G, Dijoux M-G, Lavaud C. J. Agric. Food Chem. 1994, 42, 279–282.10.1021/jf00038a010Search in Google Scholar
[17] Vàzquez-Castilla S, Jaramillo-Carmona S, Fuentes-Alventosa SJM, Jiménez-Arauja A, Rodriguez-Across R, Cermeno-Sacristàn P, Espejo-Calvo JA, Guillén-Bejarano R. J. Agric. Food Chem. 2013, 61, 6250–6258.10.1021/jf401462wSearch in Google Scholar
[18] Mukhtar H, Ansari S, Ali M, Naved T. Pharm. Biol. 2004, 42, 508–511.10.3109/13880200490891890Search in Google Scholar
[19] Townsend CC. In Flora in Iraq, Townsend CC, Guest E, Eds., Iraqi Ministry of Agriculture and Agrarian Reform: Baghdad, 1980, Vol. 4, p 411.Search in Google Scholar
[20] Shahat AA, Pieters L, Apers S, Nazeif NM, Abdel-Azim NS, Vanden Berghe D, Vlietink AJ. Phytother. Res. 2001, 15, 593–597.10.1002/ptr.883Search in Google Scholar
[21] Farmani F, Moein M, Amanzadeh A, Kandelous HM, Ehsanour Z, Salimi M. Asian Pac. J. Cancer Prev. 2016, 17, 315–321.10.7314/APJCP.2016.17.1.315Search in Google Scholar
[22] Tschesche R, Khokhar I, Spilles C, von Radloff M. Phytochemistry 1974, 13, 1633.10.1016/0031-9422(74)80352-3Search in Google Scholar
[23] Abdel-galil FM, El-Jissry MA. Phytochemistry 1991, 30, 1348–1349.10.1016/S0031-9422(00)95238-5Search in Google Scholar
[24] Nawwar MAM, Ishak MS, Michael HN, Buddrust J. Phytochemistry 1984, 23, 2110–2111.10.1016/S0031-9422(00)84999-7Search in Google Scholar
[25] Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, Sahena F, Jahurul MHA, Ghafoor K, Norulaini NAN, Omar AKM. J. Food Eng. 2013, 117, 426–436.10.1016/j.jfoodeng.2013.01.014Search in Google Scholar
[26] Wang L, Weller CL. Trends Food Sci. Technol. 2006, 17, 300–312.10.1016/j.tifs.2005.12.004Search in Google Scholar
[27] Heng MY, Tan SN,Yong JWH, Ong ES. TrAC, Trends Anal. Chem. 2013, 50, 1–10.10.1016/j.trac.2013.03.012Search in Google Scholar
[28] Mohammadlou M, Jafarizadeh-Malmiri H, Maghsoudi H. Green Process. Synth. 2017, 6, 31–42.Search in Google Scholar
[29] Cheng TC, Lu JF, Wang JS, Lin LJ, Kuo HI, Chen BH. J. Agric. Food Chem. 2011, 59, 11319–11329.10.1021/jf2018758Search in Google Scholar PubMed
[30] Ahdno H, Jafarizadeh-Malmiri H. Int. J. Food Eng. 11, 651–658.10.1515/ijfe-2015-0093Search in Google Scholar
©2019 Walter de Gruyter GmbH, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering
Articles in the same Issue
- Regular Articles
- Studies on the preparation and properties of biodegradable polyester from soybean oil
- Flow-mode biodiesel production from palm oil using a pressurized microwave reactor
- Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: investigation and optimization of process parameters
- Saccharin: a cheap and mild acidic agent for the synthesis of azo dyes via telescoped dediazotization
- Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system
- Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity
- Ultrasound assisted chemical activation of peanut husk for copper removal
- Room temperature silanization of Fe3O4 for the preparation of phenyl functionalized magnetic adsorbent for dispersive solid phase extraction for the extraction of phthalates in water
- Evaluation of the saponin green extraction from Ziziphus spina-christi leaves using hydrothermal, microwave and Bain-Marie water bath heating methods
- Oxidation of dibenzothiophene using the heterogeneous catalyst of tungsten-based carbon nanotubes
- Calcined sodium silicate as an efficient and benign heterogeneous catalyst for the transesterification of natural lecithin to L-α-glycerophosphocholine
- Synergistic effect between CO2 and H2O2 on ethylbenzene oxidation catalyzed by carbon supported heteropolyanion catalysts
- Hydrocyanation of 2-arylmethyleneindan-1,3-diones using potassium hexacyanoferrate(II) as a nontoxic cyanating agent
- Green synthesis of hydratropic aldehyde from α-methylstyrene catalyzed by Al2O3-supported metal phthalocyanines
- Environmentally benign chemical recycling of polycarbonate wastes: comparison of micro- and nano-TiO2 solid support efficiencies
- Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange
- Production of value-added chemicals from esterification of waste glycerol over MCM-41 supported catalysts
- Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange
- Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens
- Optimization of submerged fermentation conditions to overproduce bioethanol using two industrial and traditional Saccharomyces cerevisiae strains
- Extraction of In3+ and Fe3+ from sulfate solutions by using a 3D-printed “Y”-shaped microreactor
- Foliar-mediated Ag:ZnO nanophotocatalysts: green synthesis, characterization, pollutants degradation, and in vitro biocidal activity
- Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin
- Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract
- Synthesis of high surface area magnesia by using walnut shell as a template
- Controllable biosynthesis of silver nanoparticles using actinobacterial strains
- Green vegetation: a promising source of color dyes
- Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water
- Green synthesis and structural characterization of novel N1-substituted 3,4-dihydropyrimidin-2(1H)-ones
- Biodiesel production from cotton oil using heterogeneous CaO catalysts from eggshells prepared at different calcination temperatures
- Regeneration of spent mercury catalyst for the treatment of dye wastewater by the microwave and ultrasonic spray-assisted method
- Green synthesis of the innovative super paramagnetic nanoparticles from the leaves extract of Fraxinus chinensis Roxb and their application for the decolourisation of toxic dyes
- Biogenic ZnO nanoparticles: a study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight
- Leached compounds from the extracts of pomegranate peel, green coconut shell, and karuvelam wood for the removal of hexavalent chromium
- Enhancement of molecular weight reduction of natural rubber in triphasic CO2/toluene/H2O systems with hydrogen peroxide for preparation of biobased polyurethanes
- An efficient green synthesis of novel 1H-imidazo[1,2-a]imidazole-3-amine and imidazo[2,1-c][1,2,4]triazole-5-amine derivatives via Strecker reaction under controlled microwave heating
- Evaluation of three different green fabrication methods for the synthesis of crystalline ZnO nanoparticles using Pelargonium zonale leaf extract
- A highly efficient and multifunctional biomass supporting Ag, Ni, and Cu nanoparticles through wetness impregnation for environmental remediation
- Simple one-pot green method for large-scale production of mesalamine, an anti-inflammatory agent
- Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies
- A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic
- Effects of acid hydrolysis waste liquid recycle on preparation of microcrystalline cellulose
- Use of deep eutectic solvents as catalyst: A mini-review
- Microwave-assisted synthesis of pyrrolidinone derivatives using 1,1’-butylenebis(3-sulfo-3H-imidazol-1-ium) chloride in ethylene glycol
- Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: Characterization and photo-catalytic activity
- Adsorption optimized of the coal-based material and application for cyanide wastewater treatment
- Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains
- Waste phenolic resin derived activated carbon by microwave-assisted KOH activation and application to dye wastewater treatment
- Direct ethanol production from cellulose by consortium of Trichoderma reesei and Candida molischiana
- Agricultural waste biomass-assisted nanostructures: Synthesis and application
- Biodiesel production from rubber seed oil using calcium oxide derived from eggshell as catalyst – optimization and modeling studies
- Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell
- Assessment of aqueous extract of Gypsophila aretioides for inhibitory effects on calcium carbonate formation
- An environmentally friendly acylation reaction of 2-methylnaphthalene in solvent-free condition in a micro-channel reactor
- Aegle marmelos phytochemical stabilized synthesis and characterization of ZnO nanoparticles and their role against agriculture and food pathogen
- A reactive coupling process for co-production of solketal and biodiesel
- Optimization of the asymmetric synthesis of (S)-1-phenylethanol using Ispir bean as whole-cell biocatalyst
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals, 3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide
- Preconcentration of morphine in urine sample using a green and solvent-free microextraction method
- Extraction of glycyrrhizic acid by aqueous two-phase system formed by PEG and two environmentally friendly organic acid salts - sodium citrate and sodium tartrate
- Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physico-chemical and biological properties
- Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
- Biosynthesis, characterization and anti-microbial activity of silver nanoparticle based gel hand wash
- Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH)
- Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles
- Volcanic ash as reusable catalyst in the green synthesis of 3H-1,5-benzodiazepines
- Green synthesis, anionic polymerization of 1,4-bis(methacryloyl)piperazine using Algerian clay as catalyst
- Selenium supplementation during fermentation with sugar beet molasses and Saccharomyces cerevisiae to increase bioethanol production
- Biosynthetic potential assessment of four food pathogenic bacteria in hydrothermally silver nanoparticles fabrication
- Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides
- Pyrolysis of palm oil using zeolite catalyst and characterization of the boil-oil
- Azadirachta indica leaves extract assisted green synthesis of Ag-TiO2 for degradation of Methylene blue and Rhodamine B dyes in aqueous medium
- Synthesis of vitamin E succinate catalyzed by nano-SiO2 immobilized DMAP derivative in mixed solvent system
- Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology
- Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste
- Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach
- Intelligent modeling and optimization of emulsion aggregation method for producing green printing ink
- Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method
- Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water
- Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil
- Kinetics and the fluidity of the stearic acid esters with different carbon backbones
- Aiming for a standardized protocol for preparing a process green synthesis report and for ranking multiple synthesis plans to a common target product
- Microstructure and luminescence of VO2 (B) nanoparticle synthesis by hydrothermal method
- Optimization of uranium removal from uranium plant wastewater by response surface methodology (RSM)
- Microwave drying of nickel-containing residue: dielectric properties, kinetics, and energy aspects
- Simple and convenient two step synthesis of 5-bromo-2,3-dimethoxy-6-methyl-1,4-benzoquinone
- Biodiesel production from waste cooking oil
- The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation
- Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles
- Microwave-assisted protocol for squalene isolation and conversion from oil-deodoriser distillates
- Denitrification performance of rare earth tailings-based catalysts
- Facile synthesis of silver nanoparticles using Averrhoa bilimbi L and Plum extracts and investigation on the synergistic bioactivity using in vitro models
- Green production of AgNPs and their phytostimulatory impact
- Photocatalytic activity of Ag/Ni bi-metallic nanoparticles on textile dye removal
- Topical Issue: Green Process Engineering / Guest Editors: Martine Poux, Patrick Cognet
- Modelling and optimisation of oxidative desulphurisation of tyre-derived oil via central composite design approach
- CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced
- Organic carbonates synthesis improved by pervaporation for CO2 utilisation
- Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor
- A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate
- Topical on Green Process Engineering

